Form 6-K
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington D.C. 20549
REPORT OF FOREIGN ISSUER
PURSUANT TO RULE 13a-16 OR 15d-16 OF THE
SECURITIES EXCHANGE ACT OF 1934
For the month of April 2010
Commission File Number: 001-33623
WuXi PharmaTech (Cayman) Inc.
288 Fute Zhong Road, Waigaoqiao Free Trade Zone
Shanghai 200131
People’s Republic of China
(Address of principal executive office)
Indicate by check mark whether the registrant files or will file annual reports under cover Form 20-F or Form 40-F.
Form 20-F x Form 40-F ¨
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(1):¨
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(7):¨
Indicate by check mark whether by furnishing the information contained in this Form, the registrant is also thereby furnishing the information to the Commission pursuant to Rule 12g3-2(b) under the Securities Exchange Act of 1934.
Yes ¨ No x
If “Yes” is marked, indicate below the file number assigned to the registrant in connection with Rule 12g3-2(b):82-N/A
WuXi PharmaTech (Cayman) Inc.
Form 6-K
TABLE OF CONTENTS
2
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | | | |
| | WuXi PharmaTech (Cayman) Inc. |
| | |
| | By: | | /S/ GE LI |
| | Name: | | Ge Li |
| | Title: | | Chief Executive Officer |
| | |
Date: April 27, 2010 | | | | |
3
Exhibit 99.1

Dear WuXi AppTec Employees,
Good afternoon and good morning, WuXi AppTec colleagues. I’m pleased to share with you some exciting news. Earlier today, WuXi PharmaTech (Cayman) Inc. and Charles River Laboratories International, Inc., a world-class CRO headquartered in Wilmington, Massachusetts in the United States, announced that our respective boards had reached an agreement for Charles River Laboratories International, Inc. to purchase all the outstanding shares of WuXi PharmaTech (Cayman) Inc. for cash and stock valued at $1.6 billion. WuXi AppTec will become Charles River Laboratories’ new center for global drug discovery services. This is a very exciting time for our company and we could not have reached our leading position in pharmaceutical, biotech and medical device R&D services without all of your hard work and dedication. Thank you!
The combined company will be the world’s premier early-stage CRO, with combined revenues of nearly $1.5 billion in 2009. It will offer its customers an unsurpassed platform of broad, integrated R&D services—from compound synthesis to service biology to animal models for preclinical toxicology and pharmacology to manufacturing to first-in-human clinical trials. The combined company will have leading positions in many of these fast growing and essential verticals and will have the unique advantage of WuXi AppTec’s proven track record of operational excellence in China – something that very few companies can boast.
Charles River Laboratories shares our vision that China will be an exciting growth opportunity in the CRO space and has committed to continued investment in our leading China position. For this reason, WuXi AppTec operations will remain the same and all of our executives and managers are expected to remain in their current positions. Going forward, we will retain our WuXi AppTec name with the notation that we are “a Charles River Laboratories company”. I will remain the Chairman and CEO of WuXi AppTec and will also assume the title of EVP and President of the Global Discovery and China Services business for Charles River Laboratories. Three WuXi board members, including myself, will join the Charles River Laboratories board.
I believe the fit between the two companies is excellent. WuXi AppTec is strong in chemistry, with important additional capabilities in other drug discovery and preclinical laboratory services and in manufacturing, and these capabilities cover small molecules, large molecules, and medical devices. Charles River Laboratories is the world leader in preclinical pharmacology and toxicology. WuXi AppTec is by far the leading China-based contract research organization and superbly positioned to leverage the newfound skills and know-how of Charles River Laboratories to our China operations. Our biopharmaceutical and medical device testing operations in the U.S. will also be important in the new company and strengthen Charles River Laboratories’ current multinational/global operations in the United States, Europe, and Japan.
Through this combination, I’m confident that we will be able to serve our clients even better. We currently share many of the same clients as Charles River Laboratories, but we are often calling on different departments within these global companies. As we share best practices between Charles River Laboratories and WuXi AppTec, I believe we will be able to offer our clients a superior portfolio of services and deepen our relationships. As our customers increasingly look for preferred suppliers of a broad range of early-stage drug research services, I believe we will be in the enviable position of being able to meet their needs with our global and fully integrated model.
| | | | | | | | | | | | |
Shanghai 288 Fute Zhong Road Waigaoqiao Free Trade Zone Shanghai 200131 China Tel: +86 (21) 5046 1111 Fax +86 (21) 5046 1000 | | Tianjin 111 Huanghai Road Tianjin Economic-Technological Development Area (TEDA) Tianjin 300457 China Tel: +86 (22) 5983 1288 Fax +86 (22) 5983 1000 | | Suzhou 1368 Wuzhong Road Wuzhong Science & Technology Park Suzhou 215104 China Tel: +86 (512) 6883 7321 Fax +86 (512) 6883 7303 | | Jinshan 9 Yuegong Road Shanghai Chemical Industrial Park Jinshan District West Shanghai 201507 China Tel: +86 (21) 6725 6015 Fax +86 (21) 6725 6005 | | Philadelphia 4751 League Island Blvd Philadelphia, PA 19112 U S A Tel: +1 (800) 622 8820 +1 (215) 218 5500 Fax +1 (215) 218 5990 | | St. Paul 2540 Executive Drive St Paul, MN 55120 U S A Tel: +1 (888) 794 0077 +1 (651) 675 2000 Fax +1 (651) 675 2005 | | Atlanta 1265 Kennestone Circle Marietta, GA 30066 U S A Tel: +1 (888) 847 6633 +1 (770) 514 0262 Fax +1 (770) 514 0294 |
4

Lastly, I believe the most important aspect of what will drive the success of the new Charles River Laboratories is the similar culture of the two companies. For two companies that started their businesses in cities directly across the world from each other, I was pleasantly surprised to find that our cultures are in fact very similar. We at WuXi AppTec all share the common trait of relentlessly serving our clients in the pursuit of cutting-edge research and development work. We enjoy new challenges and strive to be an innovative force in drug discovery and development. We are all very entrepreneurial. In my visits with Charles River Laboratories, I found these same characteristics at Charles River Laboratories. This shared culture is ultimately what drove the Board and me to proceed with this deal.
So what does this transaction mean for you? Across our organization broadly, it means that WuXi AppTec will be joining forces with Charles River Laboratories and all of our collective efforts and talents will be needed to drive growth for the combined company. For our China operations, this transaction will enable us to realize our goal of offering a complete and best-in-class portfolio of integrated services much sooner than we could have achieved on our own. For our U.S. operations, this transaction will allow us to leverage Charles River Laboratories’ leading businesses and vast resources to accelerate the growth of our businesses. It is a great opportunity across the board, and we are counting on each of you to make the most of the opportunity to help us achieve our goals and objectives. I would also urge all of you to reflect on the enormity and power of the new organization we are creating and then to resume your hard work and dedication. We all firmly believe that outsourcing will transform how R&D will be done in the pharmaceutical, biotechnology and medical device industries, and we should double our efforts in showing the world how the combined company will greatly accelerate the discovery and development of new pharmaceutical and medical device products to benefit patients.
The transaction is targeted to be completed by the 4th quarter of 2010, which is the normal timing for a transaction of this kind. While we diligently work through the process of integrating our two companies in the coming months, know that the senior management of both companies will be carefully considering how to maximize each of your exceptional talents in the combined company with a strong emphasis on your career development. Thank you, and I look forward to working with you to build our combined company from “Good to Great”!
|
| Sincerely, |
|
/s/ Ge Li |
| Ge Li |
Chairman & CEO WuXi AppTec |
| | | | | | | | | | | | |
Shanghai 288 Fute Zhong Road Waigaoqiao Free Trade Zone Shanghai 200131 China Tel: +86 (21) 5046 1111 Fax +86 (21) 5046 1000 | | Tianjin 111 Huanghai Road Tianjin Economic-Technological Development Area (TEDA) Tianjin 300457 China Tel: +86 (22) 5983 1288 Fax +86 (22) 5983 1000 | | Suzhou 1368 Wuzhong Road Wuzhong Science & Technology Park Suzhou 215104 China Tel: +86 (512) 6883 7321 Fax +86 (512) 6883 7303 | | Jinshan 9 Yuegong Road Shanghai Chemical Industrial Park Jinshan District West Shanghai 201507 China Tel: +86 (21) 6725 6015 Fax +86 (21) 6725 6005 | | Philadelphia 4751 League Island Blvd Philadelphia, PA 19112 U S A Tel: +1 (800) 622 8820 +1 (215) 218 5500 Fax +1 (215) 218 5990 | | St. Paul 2540 Executive Drive St Paul, MN 55120 U S A Tel: +1 (888) 794 0077 +1 (651) 675 2000 Fax +1 (651) 675 2005 | | Atlanta 1265 Kennestone Circle Marietta, GA 30066 U S A Tel: +1 (888) 847 6633 +1 (770) 514 0262 Fax +1 (770) 514 0294 |
5
Forward-Looking Statements
Statements herein contain “forward-looking” statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended, and as defined in the Private Securities Litigation Reform Act of 1995. These forward looking statements, including those as to the anticipated benefits of the transaction, are subject to risks and uncertainties including: 1) the possibility that the companies may be unable to obtain stockholder or regulatory approvals required for the combination; 2) problems may arise in successfully integrating the businesses of the two companies; 3) the acquisition may involve unexpected costs; 4) the combined company may not achieve the anticipated transaction benefits; 5) the businesses may suffer as a result of uncertainty surrounding the acquisition; and 6) the industry may be subject to future regulatory or legislative actions and other risks that are described in our 2009 Annual Report on Form 20-F and Charles River Laboratories International, Inc.’s (“Charles River”) 2009 Annual Report on Form 10-K under the caption “Risk Factors”, respectively, each filed with and available on the Securities and Exchange Commission’s website at http://www.sec.gov. We assume no obligation and expressly disclaim any duty to update information contained herein except as required by law.
This document does not constitute an offer of any securities for sale or a solicitation of an offer to buy any securities. The Charles River shares to be issued in the proposed transaction have not been and will not be registered under the Securities Act of 1933, as amended (the “Securities Act”), and may not be offered or sold in the United States absent registration or an applicable exemption from registration requirements. Charles River intends to issue such Charles River shares pursuant to the exemption from registration set forth in Section 3(a)(10) of the Securities Act.
6
Exhibit 99.2

Dear Valued Customers, Collaborative Partners and Friends:
I would like to share with you an important milestone for WuXi AppTec. Today, WuXi AppTec and Charles River Laboratories announced that both boards had reached an agreement to combine the two companies to create the world’s premier early-stage contract research organization. The combined company will be the only CRO to offer a complete range of biopharmaceutical R&D services from compound synthesis through first-in-man clinical testing.
After the closing of the transaction, WuXi will become a subsidiary of Charles River and we expect no changes to our existing operations and leadership. In fact, we firmly believe that combined with the broad portfolio of services offered by Charles River, we will be able to serve you even better. I am very pleased to be joining the company as President of a newly formed business segment of Charles River Laboratories - Global Discovery and China Services. Two other WuXi board members and I will be joining the Charles River board. Our senior management—including our chief operating officer Ed Hu, our chief scientific officer Shuhui Chen, and our chief manufacturing officer Suhan Tang—will also be joining and will have corporate responsibilities in the new segment in addition to their current responsibilities. We anticipate our integration process to be quick and seamless, with a targeted closing by fourth quarter 2010.
Our ambition has always been, and remains, to provide you with the full range of laboratory and manufacturing services that you need to lower the cost and shorten the time of new product discovery and development. Today’s deal further advances that goal.
As you know, WuXi is a leader in chemistry-based services, with growing capabilities in other small-molecule services such as biology, DMPK/ADME, formulation, bioanalytical services, process research, and manufacturing, as well as testing services for medical devices and biologic products. Charles River is a leader in in vivo biology as the leading supplier of research models and a broad range of preclinical services, particularly in pharmacology and toxicology, as well as first-in-man clinical trials. In addition, WuXi has major operations in China and the United States. Charles River has major operations in the United States, Europe, and Japan. Together, the two companies are a strong fit functionally, geographically and culturally.
The one thing we pride ourselves on most at WuXi is our service to you, our customer. We are intensely focused on giving you the highest level of service to help you achieve your goals. With all the time I have spent recently with the people at Charles River, I know that they are similarly customer-focused.
After the transaction closes, we look forward to meeting with you to show you the uniquely broad range of services we can offer you. In the meantime, I can assure you there will be no interruption in our operations, and you can continue to expect the highest-quality services from us. Thank you again for your trust and support.
|
Sincerely, /s/ Ge Li Ge Li Chairman & CEO WuXi AppTec |
| | | | | | | | | | | | |
Shanghai 288 Fute Zhong Road Waigaoqiao Free Trade Zone Shanghai 200131 China Tel: +86 (21) 5046 1111 Fax +86 (21) 5046 1000 | | Tianjin 111 Huanghai Road Tianjin Economic-Technological Development Area (TEDA) Tianjin 300457 China Tel: +86 (22) 5983 1288 Fax +86 (22) 5983 1000 | | Suzhou 1368 Wuzhong Road Wuzhong Science & Technology Park Suzhou 215104 China Tel: +86 (512) 6883 7321 Fax +86 (512) 6883 7303 | | Jinshan 9 Yuegong Road Shanghai Chemical Industrial Park Jinshan District West Shanghai 201507 China Tel: +86 (21) 6725 6015 Fax +86 (21) 6725 6005 | | Philadelphia 4751 League Island Blvd Philadelphia, PA 19112 U S A Tel: +1 (800) 622 8820 +1 (215) 218 5500 Fax +1 (215) 218 5990 | | St. Paul 2540 Executive Drive St Paul, MN 55120 U S A Tel: +1 (888) 794 0077 +1 (651) 675 2000 Fax +1 (651) 675 2005 | | Atlanta 1265 Kennestone Circle Marietta, GA 30066 U S A Tel: +1 (888) 847 6633 +1 (770) 514 0262 Fax +1 (770) 514 0294 |
7
Forward-Looking Statements
Statements herein contain “forward-looking” statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended, and as defined in the Private Securities Litigation Reform Act of 1995. These forward looking statements, including those as to the anticipated benefits of the transaction, are subject to risks and uncertainties including: 1) the possibility that the companies may be unable to obtain stockholder or regulatory approvals required for the combination; 2) problems may arise in successfully integrating the businesses of the two companies; 3) the acquisition may involve unexpected costs; 4) the combined company may not achieve the anticipated transaction benefits; 5) the businesses may suffer as a result of uncertainty surrounding the acquisition; and 6) the industry may be subject to future regulatory or legislative actions and other risks that are described in our 2009 Annual Report on Form 20-F and Charles River Laboratories International, Inc.’s (“Charles River”) 2009 Annual Report on Form 10-K under the caption “Risk Factors”, respectively, each filed with and available on the Securities and Exchange Commission’s website at http://www.sec.gov. We assume no obligation and expressly disclaim any duty to update information contained herein except as required by law.
This document does not constitute an offer of any securities for sale or a solicitation of an offer to buy any securities. The Charles River shares to be issued in the proposed transaction have not been and will not be registered under the Securities Act of 1933, as amended (the “Securities Act”), and may not be offered or sold in the United States absent registration or an applicable exemption from registration requirements. Charles River intends to issue such Charles River shares pursuant to the exemption from registration set forth in Section 3(a)(10) of the Securities Act.
8
Exhibit 99.3
| | |
| Charles River Logo | | WuXi Pharma Logo |
To All WuXi Employees,
I’m sure you have read the recent announcement about our two great companies joining together and are interested in hearing more about the proposed transaction. I’d like to take a moment to share my perspective.
The boards of WuXi PharmaTech and Charles River Laboratories have approved a plan for the two companies to merge to form the world’s premier early-stage contract research organization. Charles River Laboratories will purchase all of the outstanding shares of WuXi PharmaTech, the parent company of WuXi AppTec, for cash and stock totaling $1.6 billion. We expect the transaction to close by the fourth quarter of this year, which is a normal amount of time for a transaction of this nature. The new company will be called Charles River Laboratories, and I will be the company’s Chairman and Chief Executive Officer.
First of all, I want to make clear that everyone at Charles River Laboratories, myself especially, has the highest respect for WuXi AppTec, your performance, and your capabilities. We have seen you grow from a handful of employees and a single laboratory hood in 2000 to become a world-class company, with annual sales of $270 million, strong profitability, and excellent growth prospects.
I welcome you to our new company. The 8,000 employees of Charles River Laboratories and I are eager to get to know you better and to begin working with you to provide the highest level of service to our mutual customers.
WuXi AppTec and Charles River Laboratories are each leaders at what they do. WuXi AppTec is the premier China-based CRO, with important operations in the United States. You are very strong in discovery chemistry, with important capabilities in service biology, DMPK/ADME, formulation, process research, bioanalytical services, research manufacturing, and testing services for the medical device and biotech industries. You are making substantial progress in building new businesses in toxicology and large-scale manufacturing.
Charles River Laboratories has operations in 70 facilities located in 18 countries. We partner with leading pharmaceutical, biotechnology, government, and academic organizations around the world to provide products and services in basic research, discovery, safety and efficacy, clinical trials, and process manufacturing. We operate with two divisions—Research Models and Services, which is the global leader in supply of animal models, and Preclinical Services. Total company sales totaled $1.2 billion in 2009.
9
The boards and management teams will be joining together to realize the full potential of our two organizations. I am very pleased that Dr. Ge Li, the founder and leader of your great company, will serve as President of a new division—Global Discovery and China Services. He and two other WuXi board members will join the Charles River Laboratories board. The rest of your senior management—Edward Hu, Shuhui Chen, Suhan Tang and others—will also assume responsibilities in the new company comparable to their current responsibilities. In this new segment, we will unite WuXi AppTec’s market-leading integrated drug discovery and development platform with Charles River Laboratories’ Discovery and Imaging Services. When combined with our existing Research Models and Services and Preclinical Services businesses, Discovery Services takes us to the next level in building a unique portfolio of products and services that enables us to fully support our clients’ early-stage research efforts.
Together, we will provide our clients a broad portfolio of integrated services that spans from molecule creation to first-in-human testing. WuXi AppTec’s expertise in chemistry and Charles River Laboratories’ expertise inin vivo biology combine to create a partner capable of supporting clients’ drug discovery and early-stage development efforts in ways that no other CRO can.
This transaction brings us closer to our clients with an unmatched global presence. Our footprint will enable us to support clients wherever they choose to work—in North America, Europe or China. As a result of the combination, we will enhance our value as a strategic partner to biopharmaceutical companies by offering more capabilities around the world to support their drug discovery and development.
The power of this unique service portfolio, combined with our global sales force of more than 200 professionals, will help drive revenue growth for the combined company. We believe that the exposure offered through this large and experienced sales force will position WuXi AppTec to capitalize on new opportunities with our global pharmaceutical, biotech, medical device, and academic clients, who will now have access to your services in China and in your existing U.S. sites. And we at Charles River Laboratories will benefit from your customer contacts as well. And finally, becoming an integral part of a larger organization will accelerate WuXi AppTec’s vision of providing integrated discovery and development services in China – including GLP safety assessment services – to the rest of the world.
We believe Charles River Laboratories and WuXi AppTec are an exceptional cultural fit, with both companies focused on employees and clients. We both treat our employees as our most valued assets. We both pride ourselves on providing exceptional service to clients. For WuXi AppTec employees, this transaction will provide you with new opportunities to enhance your professional growth and development in a large company with ample resources.
You have accomplished a lot in WuXi’s ten years of history, and each of you should be very proud of your contributions. Charles River Laboratories’ interest in joining with you to form an even stronger company is tribute to your skill, hard work and dedication. I’m sure that each of you is wondering how you fit into the new Charles River Laboratories. Rest assured that we welcome the opportunity to put all of your talents to work as soon as possible. While it will take a few months to plan the future of our new company, you should continue to perform at a high level. Stay focused on achieving the goals that you, Dr. Li and the rest of your senior management have set. In your day-today work, show us that we made the right decision in choosing to join with you.
10
I look forward to working with all of you in the near future.
|
| Best regards, |
|
/s/ Jim Foster |
| Jim Foster |
Chairman and CEO Charles River Laboratories |
11
Forward-Looking Statements
Statements herein contain “forward-looking” statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended, and as defined in the Private Securities Litigation Reform Act of 1995. These forward looking statements, including those as to the anticipated benefits of the transaction, are subject to risks and uncertainties including: 1) the possibility that the companies may be unable to obtain stockholder or regulatory approvals required for the combination; 2) problems may arise in successfully integrating the businesses of the two companies; 3) the acquisition may involve unexpected costs; 4) the combined company may not achieve the anticipated transaction benefits; 5) the businesses may suffer as a result of uncertainty surrounding the acquisition; and 6) the industry may be subject to future regulatory or legislative actions and other risks that are described in our 2009 Annual Report on Form 20-F and Charles River Laboratories International, Inc.’s (“Charles River”) 2009 Annual Report on Form 10-K under the caption “Risk Factors”, respectively, each filed with and available on the Securities and Exchange Commission’s website at http://www.sec.gov. We assume no obligation and expressly disclaim any duty to update information contained herein except as required by law.
This document does not constitute an offer of any securities for sale or a solicitation of an offer to buy any securities. The Charles River shares to be issued in the proposed transaction have not been and will not be registered under the Securities Act of 1933, as amended (the “Securities Act”), and may not be offered or sold in the United States absent registration or an applicable exemption from registration requirements. Charles River intends to issue such Charles River shares pursuant to the exemption from registration set forth in Section 3(a)(10) of the Securities Act.
12
Exhibit 99.4

13
Exhibit 99.4
Charles river
accelerating drug development. exactly.
WuXi Pharma Tech
Charles River Laboratories and WuXi PharmaTech Acquisition Announcement
Creating the First Fully Integrated, Global Early-Stage CRO
April 26, 2010

14
Forward Looking Statement
Statements herein contain “forward-looking” statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended, and as defined in the Private Securities Litigation Reform Act of 1995. These forward looking statements, including those as to the anticipated benefits of the transaction, are subject to risks and uncertainties including: 1) the possibility that the companies may be unable to obtain stockholder or regulatory approvals required for the combination; 2) problems may arise in successfully integrating the businesses of the two companies; 3) the acquisition may involve unexpected costs; 4) the combined company may not achieve the anticipated transaction benefits; 5) the businesses may suffer as a result of uncertainty surrounding the acquisition; and 6) the industry may be subject to future regulatory or legislative actions and other risks that are described in our 2009 Annual Report on Form 20-F and Charles River Laboratories International, Inc.’s (“Charles River”) 2009 Annual Report on Form 10-K under the caption “Risk Factors”, respectively, each filed with and available on the Securities and Exchange Commission’s website at http://www.sec.gov. We assume no obligation and expressly disclaim any duty to update information contained herein except as required by law.
This document does not constitute an offer of any securities for sale or a solicitation of an offer to buy any securities. The Charles River shares to be issued in the proposed transaction have not been and will not be registered under the Securities Act of 1933, as amended (the “Securities Act”), and may not be offered or sold in the United States absent registration or an applicable exemption from registration requirements. Charles River intends to issue such Charles River shares pursuant to the exemption from registration set forth in Section 3(a)(10) of the Securities Act.
2

15
Transaction Overview
Charles River to acquire WuXi PharmaTech for $1.6B in cash and stock
Combination will create the first and premier early-stage contract research organization (CRO)
Offering a full range of products and services from molecule creation to first-in-human testing
Charles River’s expertise in in vivo biology and WuXi PharmaTech’s expertise in chemistry combine to create a partner capable of supporting clients’ early-stage drug development efforts as no other CRO can
Providing that expertise in North America, Europe and China
Improves both companies’ ability to meet client needs
Exceptional cultural fit between both companies
Focused on clients and employees
WuXi management team remains in place and in charge of combined China operations – to operate under the name WuXi AppTec, a Charles River Laboratories company
3
16
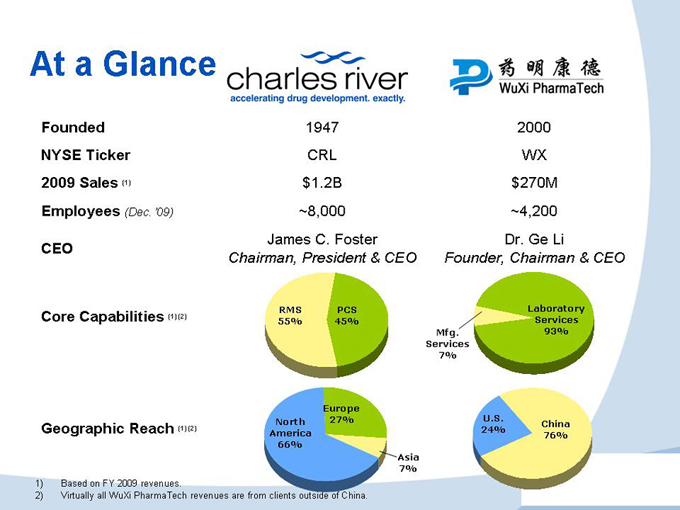
At a Glance
Charles river
Accelerating drug development. exactly.
WuXi Pharma Tech
Founded 1947 2000
NYSE Ticker CRL WX
2009 Sales (1) $1.2B $270M
Employees (Dec. ‘09) ~8,000 ~4,200
CEO
Core Capabilities (1) (2)
Geographic Reach (1) (2)
James C. Foster
Chairman, President & CEO
RMS 55% PCS 45%
Europe North 27% America 66%
Asia 7%
Dr. Ge Li
Founder, Chairman & CEO
Laboratory Services Mfg. 93% Services 7%
U.S.
China 24% 76%
1) Based on FY 2009 revenues.
2) Virtually all WuXi PharmaTech revenues are from clients outside of China.

17
WuXi PharmaTech History
WuXi PharmaTech was founded in Dec. 2000 by Dr. Ge Li and three others
First laboratory space, 7,000 square feet
48 employees by 2001 and chemistry service only
Today: Over 4,000 employees in over 2.0 million square feet of laboratory facilities and manufacturing space
5

18
Charles River Laboratories History
Charles River was founded as one-man company in 1947 by Dr. Henry Foster
First lab with $1,200 worth of rodent cages in a second floor loft overlooking Charles River in Boston
Became an international company in 1966 with the opening of a new research model production facility in France
Listed on NASDAQ in 1968 and subsequently on NYSE in 2000
One-third of sales from outside of the United States by 1983
Jim Foster buys back Charles River in 1999 from Bausch & Lomb
Approximately 8,000 employees worldwide, operating 70 facilities in 16 countries
Massachusetts (2007)
Nevada (2008)
Quebec (2009)
6
19
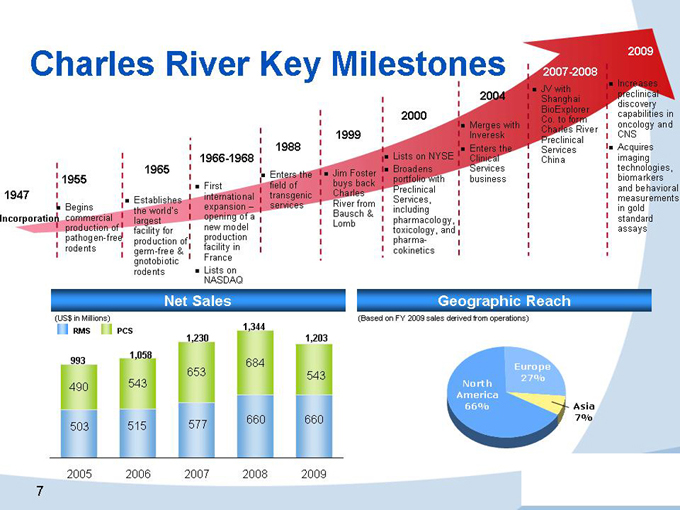
Charles River Key Milestones
1947
Incorporation
1955
Begins commercial production of pathogen-free rodents
1965
Establishes the world’s largest facility for production of germ-free & gnotobiotic rodents
1966-1968
First international expansion – opening of a new model production facility in France
Lists on NASDAQ
1988
Enters the field of transgenic services
1999
Jim Foster buys back Charles River from Bausch & Lomb
2000
Lists on NYSE
Broadens portfolio with Preclinical Services, including pharmacology, toxicology, and pharma-cokinetics
2004
Merges with Inveresk
Enters the Clinical Services business
2007-2008
JV with Shanghai BioExplorer Co. to form Charles River Preclinical Services China
2009
Increases preclinical discovery capabilities in oncology and CNS
Acquires imaging technologies, biomarkers and behavioral measurements in gold standard assays
Net Sales
(US$ in Millions)
RMS PCS
1,230 1,203 1,058 993 1,344 684 653 543 490 543
577 660 660 503 515
2005 2006 2007 2008 2009
Geographic Reach
(Based on FY 2009 sales derived from operations)
Europe 27% North America 66% Asia 7%
7
20
Our New Colleagues
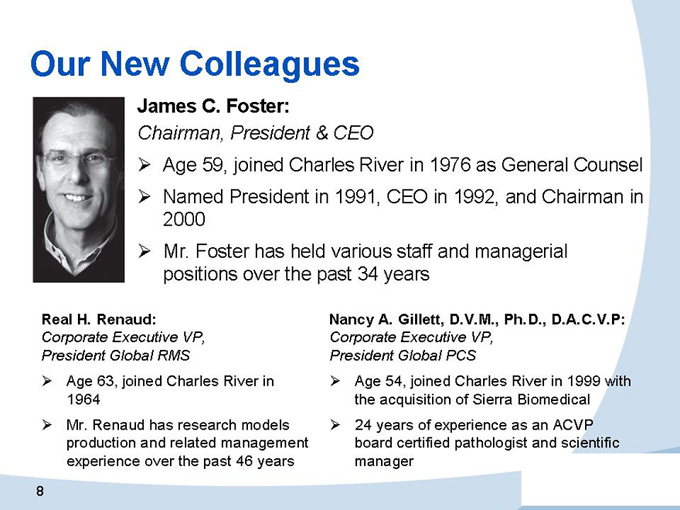
James C. Foster:
Chairman, President & CEO
Age 59, joined Charles River in 1976 as General Counsel
Named President in 1991, CEO in 1992, and Chairman in 2000
Mr. Foster has held various staff and managerial positions over the past 34 years
Real H. Renaud:
Corporate Executive VP, President Global RMS
Age 63, joined Charles River in 1964
Mr. Renaud has research models production and related management experience over the past 46 years
Nancy A. Gillett, D.V.M., Ph.D., D.A.C.V.P:
Corporate Executive VP, President Global PCS
Age 54, joined Charles River in 1999 with the acquisition of Sierra Biomedical
24 years of experience as an ACVP board certified pathologist and scientific manager
8
21
Strategic Rationale
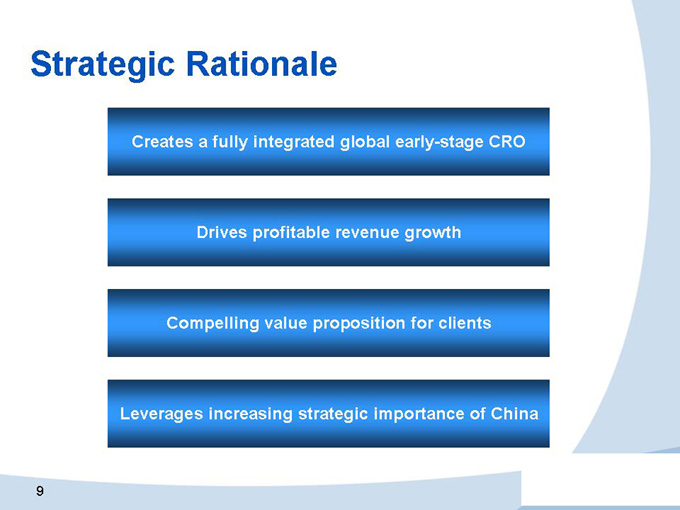
Creates a fully integrated global early-stage CRO
Drives profitable revenue growth
Compelling value proposition for clients
Leverages increasing strategic importance of China
9

22
Shared Corporate Culture
Dedication to exceeding customer expectations
Shared mission to accelerate our clients’ drug development efforts
Scientific expertise
Employee-centric
Focus on market leadership
Commitment to building shareholder value
10

23
Unique Opportunity
Transformational transaction that creates the leading early-stage CRO
Combines leading companies in chemistry and in vivo biology
Provides a larger services platform to support clients’ R&D efforts
Dramatically improves both companies’ ability to meet client needs
Enables pull-through for both companies, upstream and downstream
Provides access for global pharmaceutical companies who view China as the new frontier for drug development
11

24
Unique Opportunity (cont’d)
Larger footprint with more capabilities enables clients to partner with one company from chemistry to man
Strategically more important to drug development efforts of big pharmaceutical companies
Access to Charles River’s global sales force supports expansion of Wizard business globally
Opportunities with mid-tier pharmaceutical and 3,500 academic accounts
Expands management team and scientific leadership
WuXi PharmaTech provides opportunity to drive revenue growth while expanding Charles River’s margins
Creates the only fully integrated, global early-stage CRO
12

25
WuXi PharmaTech Becomes Stronger than Ever
WuXi AppTec management remains intact and in charge of Chinese operations
The first complete and global portfolio of biopharmaceutical R&D services from compound synthesis through first-in-man clinical testing
Accelerate our own plans years ahead of schedule
Offers tremendous career opportunities for each of you
13

26
Our Vision Are Aligned
Strategically partnering with our clients to provide essential products and services that expedite drug development
Charles river
accelerating drug development. exactly.
WuXi Pharma Tech
To provide fully integrated pharmaceutical and medical device R&D services, to improve the success of research and to shorten the time of development by offering customers world-class capabilities and unparalleled capacities
14














