Form 6-K
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington D.C. 20549
REPORT OF FOREIGN ISSUER
PURSUANT TO RULE 13a-16 OR 15d-16 OF
THE SECURITIES EXCHANGE ACT OF 1934
For the month of June 2010
Commission File Number: 001-33623
WuXi PharmaTech (Cayman) Inc.
288 Fute Zhong Road, Waigaoqiao Free Trade Zone
Shanghai 200131
People’s Republic of China
(Address of principal executive office)
Indicate by check mark whether the registrant files or will file annual reports under cover Form 20-F or Form 40-F.
Form 20-F x Form 40-F ¨
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(1): ¨
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(7): ¨
Indicate by check mark whether by furnishing the information contained in this Form, the registrant is also thereby furnishing the information to the Commission pursuant to Rule 12g3-2(b) under the Securities Exchange Act of 1934. Yes ¨ No x
If “Yes” is marked, indicate below the file number assigned to the registrant in connection with Rule 12g3-2(b):82-N/A
WuXi PharmaTech (Cayman) Inc.
Form 6-K
TABLE OF CONTENTS
2
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | | | |
| | WuXi PharmaTech (Cayman) Inc. |
| | |
| | By: | | /S/ GE LI |
| | Name: | | Ge Li |
| | Title: | | Chairman and Chief Executive Officer |
| | |
Date: June 11, 2010 | | | | |
3
Exhibit 99.1

Charles River and
WuXi PharmaTech:
The First Global
Early-Stage CRO
Jefferies 2010
Global Life Sciences
Conference
JUNE 10, 2010
James C. Foster
Chairman, President & CEO
charles river
© 2010 Charles River Laboratories International, Inc.
4

Safe Harbor Statement
This document includes “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995.
Forward-looking statements may be identified by the use of words such as “anticipate,” “believe,” “expect,” “estimate,” “plan,” “outlook,” and “project” and other similar expressions that predict or indicate future events or trends or that are not statements of historical matters. These statements are based on current expectations and beliefs of Charles River Laboratories (“Charles River”) and WuXi PharmaTech (Cayman) Inc (“WuXi”), and involve a number of risks and uncertainties that could cause actual results to differ materially from those stated or implied by the forward-looking statements. Those risks and uncertainties include, but are not limited to: 1) the possibility that the companies may be unable to obtain stockholder or regulatory approvals required for the combination; 2) problems may arise in successfully integrating the businesses of the two companies (including retention of key executives); 3) the acquisition may involve unexpected costs; 4) the combined company may be unable to achieve cost and revenue synergies or achieve potential revenue growth and non-GAAP margin expansion; 5) the businesses may suffer as a result of uncertainty surrounding the acquisition; and 6) the industry may be subject to future regulatory or legislative actions and other risks that are described in Securities and Exchange Commission (“SEC”) reports filed or furnished by Charles River and WuXi.
In addition, these statements include the combined financial outlook of Charles River for 2011 and anticipated use of cash flow, the availability of funding for our customers and the impact of economic and market conditions on them generally, the anticipated strength of our balance sheet, the effects of our 2009 and 2010 cost-saving actions and other actions designed to manage expenses, operating costs and capital spending, and to streamline efficiency, and the ability of the Company to withstand the current market conditions. Forward-looking statements are based on Charles River’s current expectations and beliefs, and involve a number of risks and uncertainties that are difficult to predict and that could cause actual results to differ materially from those stated or implied by the forward-looking statements. Those risks and uncertainties include, but are not limited to: the ability to successfully integrate the businesses we acquire; the ability to successfully develop and commercialize SPC’s technology platform; a decrease in research and development spending, a decrease in the level of outsourced services, or other cost reduction actions by our customers; the ability to convert backlog to sales; special interest groups; contaminations; industry trends; new displacement technologies; USDA and FDA regulations; changes in law; continued availability of products and supplies; loss of key personnel; interest rate and foreign currency exchange rate fluctuations; changes in tax regulation and laws; changes in generally accepted accounting principles; and any changes in business, political, or economic conditions due to the threat of future terrorist activity in the U.S. and other parts of the world, and related U.S. military action overseas. A further description of these risks, uncertainties, and other matters can be found in the Risk Factors detailed in Charles River’s Annual Report on Form 10-K as filed on February 19, 2010 and Quarterly Report on Form 10-Q as filed on April 29, 2010, as well as other filings we make with the SEC.
Because forward-looking statements involve risks and uncertainties, actual results and events may differ materially from results and events currently expected by Charles River and WuXi. Charles River and WuXi assume no obligation and expressly disclaim any duty to update information contained in this filing except as required by law.
Charles River
2
5

Non-GAAP Financial Measures
This presentation includes discussion of non-GAAP financial measures. We believe that the inclusion of these non-GAAP financial measures provides useful information to allow investors to gain a meaningful understanding of our core operating results and future prospects, without the effect of one-time charges, consistent with the manner in which management measures and forecasts the Company’s performance. The non-GAAP financial measures included in this presentation are not meant to be considered superior to or a substitute for results of operations prepared in accordance with GAAP. The company intends to continue to assess the potential value of reporting non-GAAP results consistent with applicable rules and regulations.
Additional Information
This document may be deemed to be solicitation material in respect of the proposed combination of Charles River and WuXi. In connection with the proposed transaction, Charles River has filed a preliminary proxy statement and will file a definitive proxy statement with the SEC. The information contained in the preliminary filing is not complete and may be changed. Before making any voting or investment decisions, investors and security holders are urged to read the definitive proxy statement when it becomes available and any other relevant documents filed with the SEC because they will contain important information. The definitive proxy statement will be mailed to the shareholders of Charles River seeking their approval of the proposed transaction. Charles River’s shareholders will also be able to obtain a copy of the definitive proxy statement free of charge by directing a request to: Charles River Laboratories, 251 Ballardvale Street, Wilmington, MA 01887, Attention: General Counsel. In addition, the preliminary proxy statement is and the definitive proxy statement will be available free of charge at the SEC’s website, www.sec.gov. Shareholders may also access copies of the documents filed with the SEC by Charles River on Charles River’s website at www.criver.com.
Charles River and its directors and executive officers and other members of management and employees may be deemed to be participants in the solicitation of proxies in respect of the proposed transaction. Information regarding Charles River’s directors and executive officers is available in Charles River’s proxy statement for its 2010 annual meeting of shareholders, which was filed with the SEC on March 30, 2010. Information regarding the persons who may, under the rules of the SEC, be considered participants in the solicitation of Charles River shareholders in connection with the proposed transaction is set forth in the preliminary proxy statement filed with the SEC.
This document does not constitute an offer of any securities for sale or a solicitation of an offer to buy any securities. The Charles River shares to be issued in the proposed transaction have not been and will not be registered under the Securities Act of 1933, as amended (the “Securities Act”), and may not be offered or sold in the United States absent registration or an applicable exemption from registration requirements. Charles River intends to issue such Charles River shares pursuant to the exemption from registration set forth in Section 3(a)(10) of the Securities Act.
charles river
3
6

Charles River and WuXi:
A Transformational Combination
The combination creates the first fully integrated global early-stage contract research organization (CRO)
Offers a full range of products and services from molecule creation to first-in-human testing
Leverages increasing strategic importance of China
Drives Charles River (CRL) shareholder value through profitable growth and higher combined margins
Expanded portfolio drives increased sales
Provides compelling value to clients, meeting their needs for early-stage drug development efforts on a global basis
charles river
4
7

Timing of the Acquisition is Key
Critical inflection point in terms of rapid client consolidation and infrastructure reductions
Accelerating need for strategic outsourcing
Clients are reducing the number of strategic partners to those with financial strength, scientific depth, global footprint and an expanding portfolio of services
As client strategies evolve, next 12-24 months are key to gaining significant business pursuant to longer-term contracts
CRL is a stronger organization due to PCS reorganization, sales force realignment, ERP implementation and Six Sigma initiative
PCS business has stabilized
WuXi will achieve its vision of becoming a global integrated service provider on an accelerated basis
charles river
5
8

Charles River Today
A leading in vivo biology company
$1.20B in net sales (FY ‘09)
Unique portfolio of products and services focused on the research and development continuum for new drugs
A multinational company with ~8,000 employees worldwide
~70 facilities in 16 countries
Continuous expansion to support client needs
Client Base
Non-Commercial 16%
Commercial 84%
ROW 34%
North America 66%
Geographic Sales by Facility Location
6 Source: Based on Charles River’s FY 2009 net sales.
charles river
9
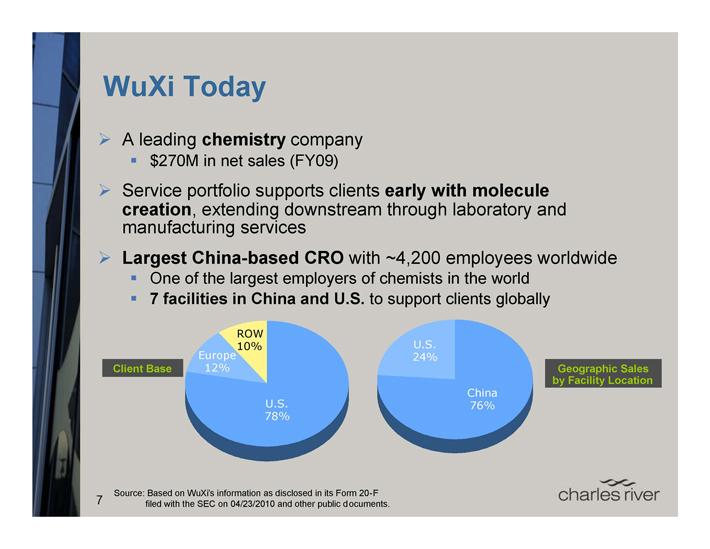
WuXi Today
A leading chemistry company
$270M in net sales (FY09)
Service portfolio supports clients early with molecule creation, extending downstream through laboratory and manufacturing services Largest China-based CRO with ~4,200 employees worldwide
One of the largest employers of chemists in the world
7 facilities in China and U.S. to support clients globally
Client Base
Europe 12%
ROW 10%
U.S. 78%
U.S. 24%
China 76%
Geographic Sales by Facility Location
7 Source: Based on WuXi’s information as disclosed in its Form 20-F filed with the SEC on 04/23/2010 and other public documents.
charles river
10

WuXi Overview
Headquarters
Shanghai, China
Laboratory services, consisting of synthetic and medicinal chemistry, DMPK/ADME, biology, microbiology, in vivo pharmacology, toxicology, pharmaceutical development, analytical services, biopharmaceutical, in vitro and in vivo biocompatibility testing and efficacy testing
Service Offerings
Manufacturing services, focusing on advanced intermediates and active pharmaceutical ingredients (APIs)
Employees
~4,200 employees (~3,800 in China and ~400 in the U.S.)
~2.0M ft2 of lab facilities and manufacturing space
China:
1.0M ft² total research facilities in Shanghai and Tianjin
293K ft² total cGMP* quality process development and manufacturing facilities in Shanghai
Facilities
314K ft² drug safety evaluation center in Suzhou
United States:
209K ft² total three state-of-the-art FDA-registered facilities in St. Paul, MN,
Philadelphia, PA and Atlanta, GA
Over 800 clients worldwide including 100+ for pharmaceutical services and 700+ for biologics and device services
Customers
100% repeat business from top 10 customers
* Current Good Manufacturing Practice
8 Source: Based on WuXi’s information as disclosed in its Form 20-F filed with the SEC on 04/23/2010 and other public documents.
charles river
11
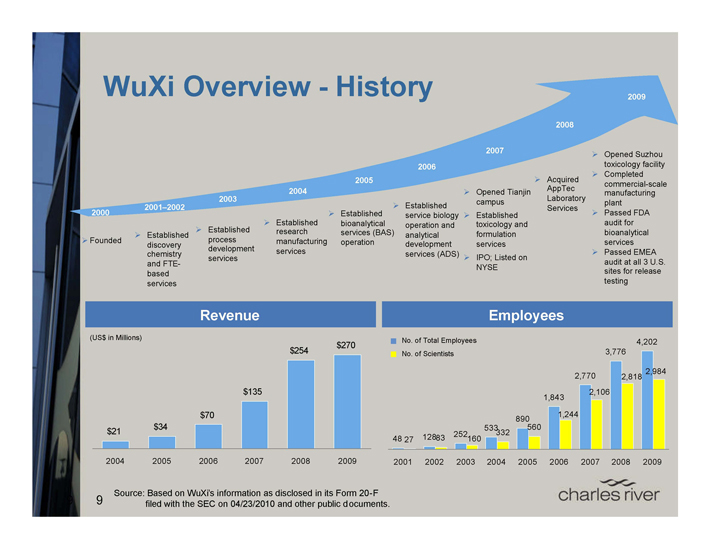
WuXi Overview - History
2000
Founded
2001–2002
Established discovery chemistry and FTE-based services
2003
Established process development services
2004
Established research manufacturing services
2005
Established bioanalytical services (BAS) operation
2006
Established service biology operation and analytical development services (ADS)
2007
Opened Tianjin campus Established toxicology and formulation services IPO; Listed on NYSE
2008
Acquired AppTec Laboratory Services
2009
Opened Suzhou toxicology facility Completed commercial-scale manufacturing plant Passed FDA audit for bioanalytical services Passed EMEA audit at all 3 U.S. sites for release testing
Revenue
(US$ in Millions) $270 $254
$135
$70 $34 $21
2004 2005 2006 2007 2008 2009
Employees
No. of Total Employees
No. of Scientists
4,202 3,776
2,984 2,770 2,818
2,106 1,843
1,244 890 533 560 252 332
48 27 12883 160
2001 2002 2003 2004 2005 2006 2007 2008 2009
Source: Based on WuXi’s information as disclosed in its Form 20-F filed with the SEC on 04/23/2010 and other public documents.
charles river
9
12
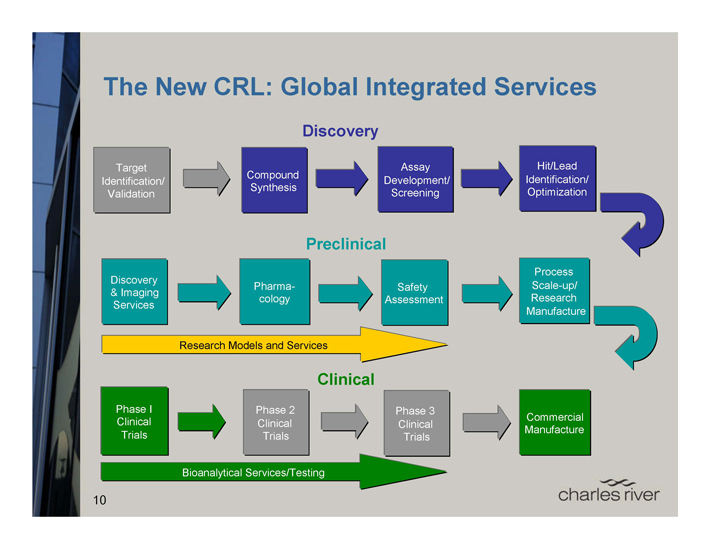
The New CRL: Global Integrated Services
Discovery
Target Assay Hit/Lead Compound Identification/ Synthesis Development/ Identification/ Validation Screening Optimization
Preclinical
Discovery Process Pharma-cology Safety Scale-up/
& Imaging Assessment Research Services Manufacture
Research Models and Services
Clinical
Phase I Phase 2 Phase 3 Commercial Clinical Clinical Clinical Manufacture Trials Trials Trials
Bioanalytical Services/Testing
charles river
10
13
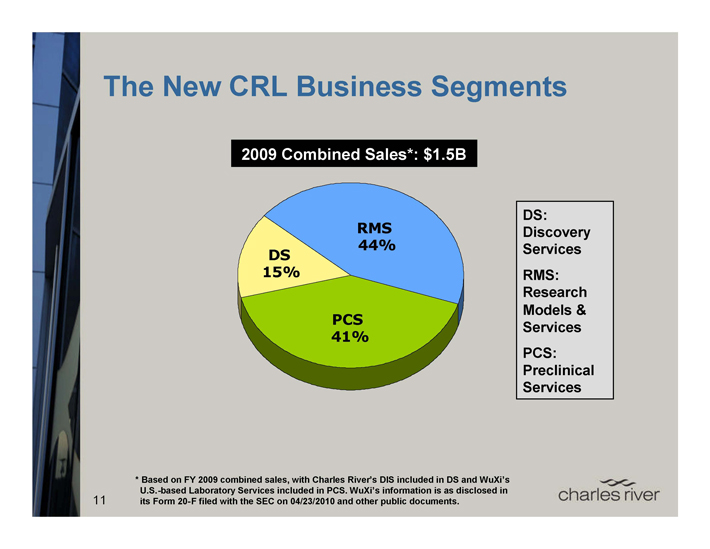
The New CRL Business Segments
2009 Combined Sales*: $1.5B
RMS 44% DS
15%
PCS 41%
DS: Discovery Services
RMS: Research Models & Services
PCS: Preclinical Services
* Based on FY 2009 combined sales, with Charles River’s DIS included in DS and WuXi’s U.S.-based Laboratory Services included in PCS. WuXi’s information is as disclosed in its Form 20-F filed with the SEC on 04/23/2010 and other public documents.
charles river
11
14
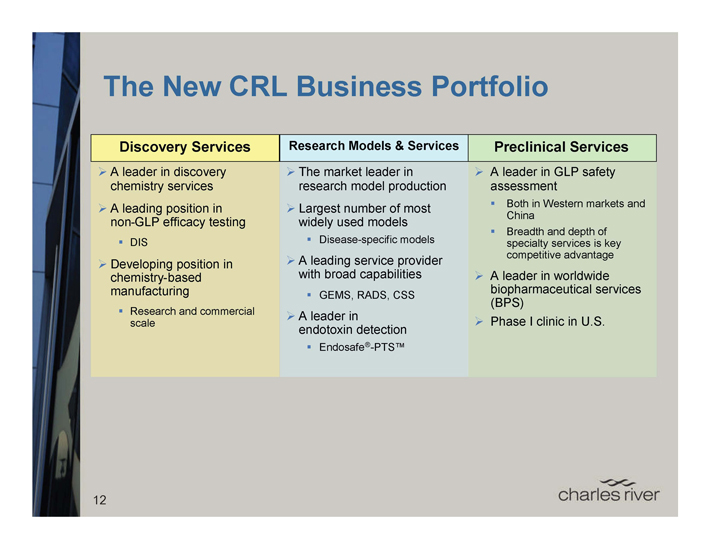
The New CRL Business Portfolio
Discovery Services
A leader in discovery chemistry services
A leading position in non-GLP efficacy testing
• DIS
Developing position in chemistry-based manufacturing
• Research and commercial scale
Research Models & Services
The market leader in research model production
Largest number of most widely used models
• Disease-specific models
A leading service provider with broad capabilities
• GEMS, RADS, CSS
A leader in endotoxin detection
• Endosafe®-PTS™
Preclinical Services
A leader in GLP safety assessment
• Both in Western markets and China
• Breadth and depth of specialty services is key competitive advantage
A leader in worldwide biopharmaceutical services (BPS) Phase I clinic in U.S.
charles river
12
15

Drives Profitable Revenue Growth
Global outsourced discovery chemistry segment is estimated to be at least $2B in 2010, with a mid-teens CAGR over the next 3-5 years*
• Currently ~20-30% outsourced
Growth in Discovery Chemistry and other downstream services accelerated by pharma outsourcing
• Service biology, DMPK/ADME, formulation, process research, bioanalytical chemistry, manufacturing
Emerging opportunity for Manufacturing services
• Active pharmaceutical ingredients (APIs) for clinical trials and commercial-scale manufacturing
Emerging opportunity for GLP safety assessment in China
13 * Source: Citigroup Global Markets, Deutsche Bank and Company estimates. charles river
16
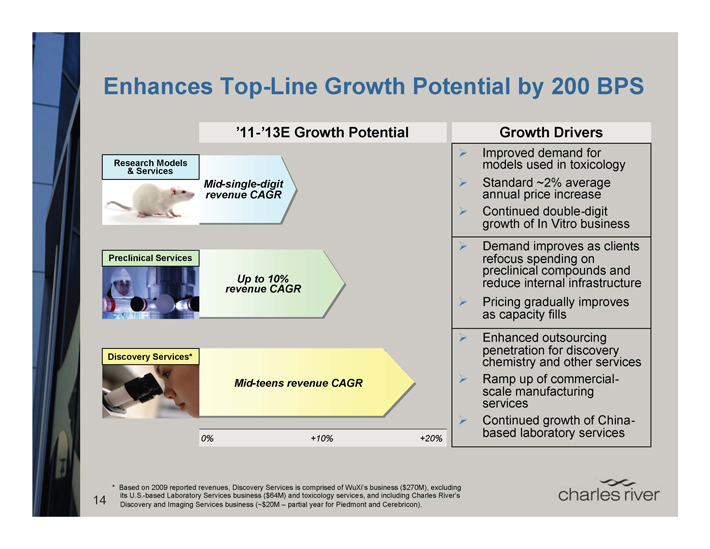
Enhances Top-Line Growth Potential by 200 BPS
’11-’13E Growth Potential
Research Models & Services
Mid-single-digit revenue CAGR
Preclinical Services
Up to 10% revenue CAGR
Discovery Services*
Mid-teens revenue CAGR
0% +10% +20%
Growth Drivers
Improved demand for models used in toxicology Standard ~2% average annual price increase Continued double-digit growth of In Vitro business
Demand improves as clients refocus spending on preclinical compounds and reduce internal infrastructure Pricing gradually improves as capacity fills
Enhanced outsourcing penetration for discovery chemistry and other services Ramp up of commercial-scale manufacturing services Continued growth of China-based laboratory services
14 * Based on 2009 reported revenues, Discovery Services is comprised of WuXi’s business ($270M), excluding its U.S.-based Laboratory Services business ($64M) and toxicology services, and including Charles River’s Discovery and Imaging Services business (~$20M – partial year for Piedmont and Cerebricon).
charles river
17

Revenue Synergies
Cross-selling and combined sales force create incremental opportunity to drive more business upstream and downstream with existing and prospective clients based on unprecedented scope of early-stage portfolio
Increased presence and scale in the emerging China market
Mid-tier and academic accounts where WuXi has limited penetration and CRL has significant share
• Leverage CRL sales force of ~200 professionals to drive strategic outsourcing
Focus on expanding existing client partnerships
• Cross-selling opportunities
These synergies further enhance top-line growth potential
15 charles river
18
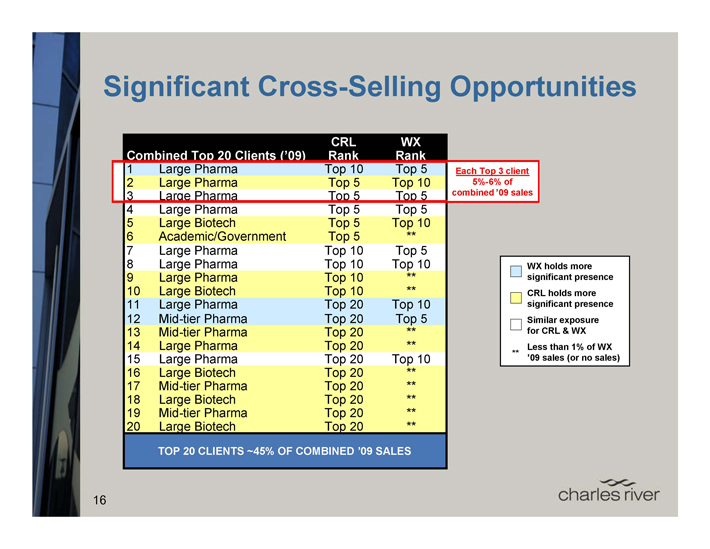
Significant Cross-Selling Opportunities
CRL WX
Combined Top 20 Clients (’09) Rank Rank
1 Large Pharma Top 10 Top 5 Each Top 3 client 5%- 6% of
combined ’09 sales
2 Large Pharma Top 5 Top 10
3 Large Pharma Top 5 Top 5
4 Large Pharma Top 5 Top 5
5 Large Biotech Top 5 Top 10
6 Academic/Government Top 5 **
7 Large Pharma Top 10 Top 5
8 Large Pharma Top 10 Top 10
9 Large Pharma Top 10 **
10 Large Biotech Top 10 **
11 Large Pharma Top 20 Top 10
12 Mid-tier Pharma Top 20 Top 5
13 Mid-tier Pharma Top 20 **
14 Large Pharma Top 20 **
15 Large Pharma Top 20 Top 10
16 Large Biotech Top 20 **
17 Mid-tier Pharma Top 20 **
18 Large Biotech Top 20 **
19 Mid-tier Pharma Top 20 **
20 Large Biotech Top 20 **
TOP 20 CLIENTS ~45% OF COMBINED ’09 SALES
WX holds more significant presence
CRL holds more significant presence
Similar exposure for CRL & WX
** Less than 1% of WX ’09 sales (or no sales)
charles river
16
19

Enthusiastic Client Response
Senior management of CRL and WX each have reviewed the transaction with their respective top 20 pharmaceutical and biotechnology clients
Response has been overwhelmingly positive
Follow-up meetings have been scheduled/initiated
Clients are viewing this transformational combination as a strategic fit for the combined company, which offers them the opportunity to acquire more products and services from a single, larger entity
Clients are requesting a more integrated approach to buying end-to-end services across the drug development continuum
Value of CRL brand seen as an advantage in terms of standardizing products and services in China
charles river
17
20

Leverages Increasing Strategic Importance of China
Supportive of global pharmas who view China as the new frontier for drug development
Pharmas are taking advantage of cost leverage by placing chemistry in China
Lower cost, highly skilled scientists with advanced degrees
Emerging opportunities for chemistry, safety assessment and manufacturing services as clients advance development activities in China
Charles River gains largest early-stage provider in China
Enables clients to choose where to place work
charles river
18
21
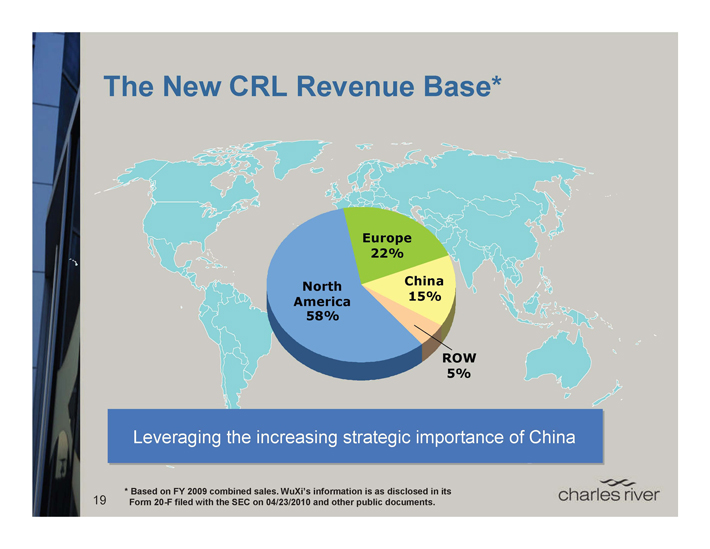
The New CRL Revenue Base*
Europe 22%
China 15%
North America 58%
ROW 5%
Leveraging the increasing strategic importance of China
* Based on FY 2009 combined sales. WuXi’s information is as disclosed in its Form 20-F filed with the SEC on 04/23/2010 and other public documents.
charles river
19
22

2011 Combined Financial Outlook(1)
Revenue(2) At least $1,675M
Non-GAAP Operating Margin % Approx. 22%
Non-GAAP EPS At least $2.70
Does not include the positive impact of expected revenue synergies from cross-selling and combined sales force
Increasingly accretive beyond 2011
(1) Combined financial information is presented as a supplementary non-GAAP financial measure, and is not intended to be superior to or a substitute for any financial measure prepared in accordance with GAAP. Furthermore, combined financial information is not equivalent to pro forma financial information as may be included in any proxy statement Charles River will file with the SEC in connection with this transaction. For example, combined financial information does not give effect to adjustments likely to be found in pro forma financial information such as increased amortization and depreciation. Items excluded from non-GAAP EPS consist of all deal-related costs including repatriation impact.
(2) Reflects weakening of the Euro and British Pound on foreign exchange.
charles river
20
23

2011 General Assumptions(1)
New debt of <$950M
Existing term loan is repaid
Debt amortization begins in 4Q10 (after close)
Expect to accelerate repayment with cash flow
~4.5% average interest rate on new debt
Term A rate expected to be LIBOR plus 275bps
Consolidated CRL non-GAAP tax rate expected to be ~25%-26%
~86-87M CRL fully diluted shares outstanding post-close
Expect to recognize ~$20M of cost synergies
(1) Combined financial information is presented as a supplementary non-GAAP financial measure, and is not intended to be superior to or a substitute for any financial measure prepared in accordance with GAAP. Furthermore, combined financial information is not equivalent to pro forma financial information as may be included in any proxy statement Charles River will file with the SEC in connection with this transaction. For example, combined financial information does not give effect to adjustments likely to be found in pro forma financial information such as increased amortization and depreciation. Items excluded from non-GAAP EPS consist of all deal-related costs including repatriation impact.
charles river
21
24
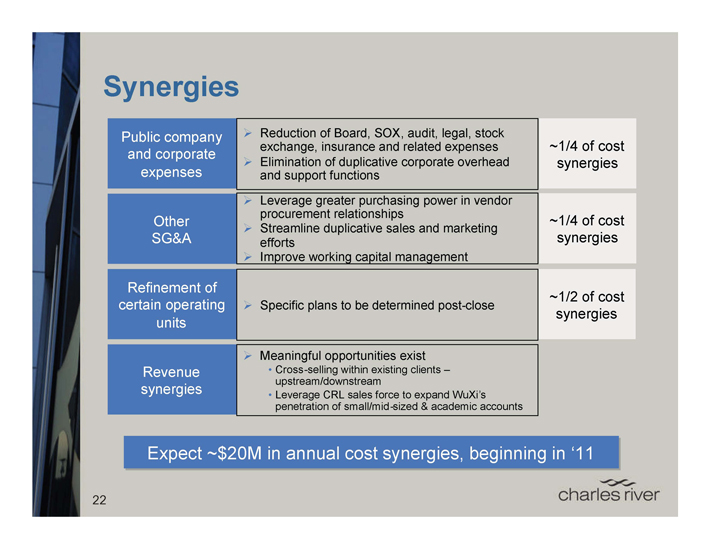
Synergies
Public company and corporate expenses
Reduction of Board, SOX, audit, legal, stock exchange, insurance and related expenses
~1/4 of cost synergies
Elimination of duplicative corporate overhead synergies and support functions
Other SG&A
Leverage greater purchasing power in vendor procurement relationships
~1/4 of cost synergies
Streamline duplicative sales and marketing efforts synergies
Improve working capital management
Refinement of certain operating units
Specific plans to be determined post-close
1/2 of cost synergies
Revenue synergies
Meaningful opportunities exist
Cross -selling within existing clients – upstream/downstream
Leverage CRL sales force to expand WuXi’s penetration of small/mid-sized & academic accounts
Expect ~$20M in annual cost synergies, beginning in ‘11
charles river
22
25
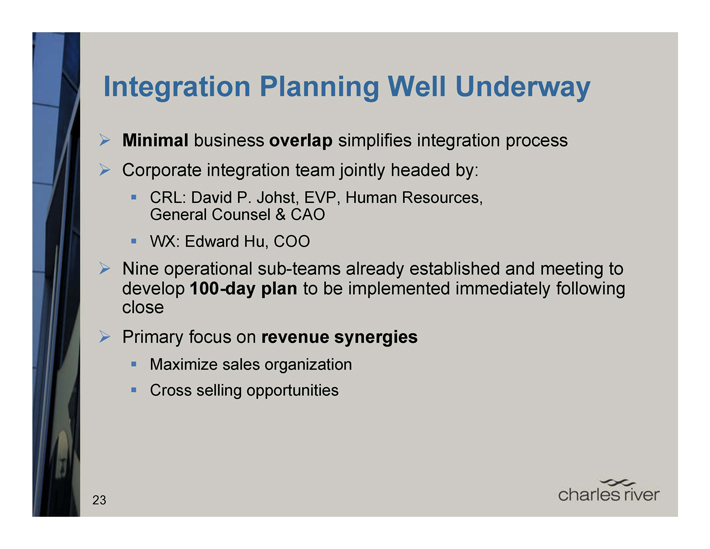
Integration Planning Well Underway
Minimal business overlap simplifies integration process
Corporate integration team jointly headed by:
CRL: David P. Johst, EVP, Human Resources, General Counsel & CAO
WX: Edward Hu, COO
Nine operational sub-teams already established and meeting to develop 100-day plan to be implemented immediately following close
Primary focus on revenue synergies
Maximize sales organization
Cross selling opportunities
charles river
23
26

Management Continuity
Top WuXi executives to join CRL management team (3-year employment agreements)
Ge Li, PhD: Corporate EVP and President, Global Discovery and China Services
Joins CRL Board, along with two additional directors chosen by WuXi
Founded WuXi in 2000; previously helped found Pharmacopeia; PhD in organic chemistry from Columbia University
Edward Hu: Corporate SVP and General Manager, Global Discovery and China Services
11 years of U.S. pharmaceutical/biotech experience; held managerial positions at Merck and Biogen Idec; Chief Operating Officer at Tanox
Shuhui Chen, PhD: Corporate SVP and General Manager, Chemistry Services and Chief Scientific Officer, China
13 years of U.S. pharmaceutical/biotech experience at Vion, Eli Lilly and Bristol-Myers Squibb; PhD in organic chemistry from Yale University
Suhan Tang, PhD: Corporate VP and General Manager, Manufacturing Services
7 years of U.S. pharmaceutical experience at Schering-Plough; PhD in organic chemistry from Columbia University
charles river
24
27

Cultural Fit
WuXi is a client-focused service organization
Excellent client retention
100% retention of top 10 clients (’07-’09)
~90% of WuXi’s sales generated from US and European clients
WuXi shares CRL’s employee-centric culture
Scientific depth and expertise differentiates CRL and WuXi from the competition
WuXi has ~2,000 scientists with advanced degrees
CRL has ~500 scientists with advance degrees
WuXi operates a “Westernized” culture
Most of management team has graduate degrees from U.S. universities and significant work experience at U.S. pharma and biotech companies
NYSE-listed company, foreign filer with the SEC and uses a “Big 4” auditor (Deloitte & Touche)
SOPs and client communications in English
charles river
25
28
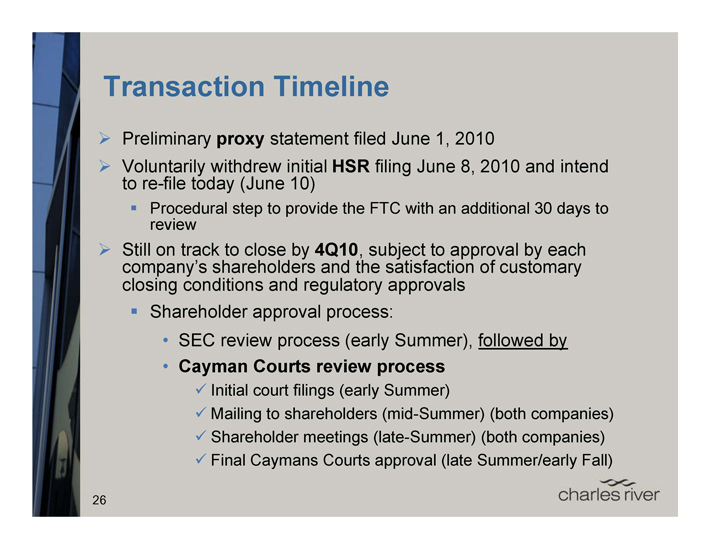
Transaction Timeline
Preliminary proxy statement filed June 1, 2010
Voluntarily withdrew initial HSR filing June 8, 2010 and intend to re-file today (June 10)
Procedural step to provide the FTC with an additional 30 days to review
Still on track to close by 4Q10, subject to approval by each company’s shareholders and the satisfaction of customary closing conditions and regulatory approvals
Shareholder approval process:
SEC review process (early Summer), followed by
Cayman Courts review process
Initial court filings (early Summer)
Mailing to shareholders (mid-Summer) (both companies)
Shareholder meetings (late-Summer) (both companies)
Final Caymans Courts approval (late Summer/early Fall)
charles river
26
29

A Transformational Combination
First fully integrated global early-stage CRO
Overwhelmingly positive response from top 20 clients
Creates opportunities for substantial revenue synergies due to unique portfolio and global footprint
Generates profitable revenue growth, driving long-term return on investment
charles river
27
30

Accelerating Drug Development. Exactly.
CRL
LISTED
NYSE
© 2010 Charles River Laboratories International, Inc.
charles river
31



























