Exhibit 99.1
Targeted Therapeutics for Inflammatory Disease
R&D Day– October 7, 2015
Forward Looking Statements / Safe Harbor
This presentation and the accompanying oral commentary contain “forward-looking” statements that involve substantial risks and uncertainties. All statements other than statements of historical facts contained in this presentation and the accompanying oral commentary, including statements regarding our future financial condition, business strategy and plans and objectives of management for future operations, are forward looking statements. In some cases, you can identify forward-looking statements by terminology such as “believe,” “will,” “may,” “estimate,” “continue,” “anticipate,” “intend,” “should,” “plan,” “might,” “approximately,” “expect,” “predict,” “could,” “potentially” or the negative of these terms or other similar expressions. Forward looking statements appear in a number of places throughout this presentation and the accompanying oral commentary and include statements regarding our intentions, beliefs, projections, outlook, analyses or current expectations concerning, among other things, our ongoing and planned preclinical development and clinical trials, the timing of and our ability to make regulatory filings and obtain and maintain regulatory approvals for AQX-1125 and our future product candidates, our intellectual property position, the degree of clinical utility of AQX-1125 and our future product candidates, particularly in specific patient populations, our ability to develop commercial functions, expectations regarding clinical trial data, our results of operations, cash needs, financial condition, liquidity, prospects, growth and strategies, the industry in which we operate and the trends that may affect the industry or us.
Forward-looking statements involve known and unknown risks, uncertainties, assumptions and other factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. In evaluating these statements, you should specifically consider various factors, including the risks outlined under the caption “Risk Factors” set forth in our Quarterly Report on Form 10-Q for the quarter ended June 30, 2015, which we filed with the Securities and Exchange Commission (“SEC”) on August 6, 2015 and other reports and filings we will make with the SEC from time to time. Forward-looking statements represent our management’s beliefs and assumptions only as of the date of this presentation. Except as required by law, we assume no obligation to update these forward-looking statements publicly, or to update the reasons why actual results could differ materially from those anticipated in the forward-looking statements, even if new information becomes available in the future.
2
Today’s Agenda
8:00AM – Registration & Breakfast
8:30AM – Welcome Remarks—David Main, President & CEO 8:35AM – BPS/IC Prevalence, Diagnosis & Treatment
Dr. Robert Evans, M.D.
Associate Professor of Urology at Wake Forest Baptist Health University
Clinical Instructor at Wake Forest School of Medicine Department of Urology
9:05AM LEADERSHIP Trial Review and Clinical Update
Dr. Stephen Shrewsbury, M.D.
Senior VP of Clinical Development and CMO
9:30AM – Commercial Opportunity—David Main 9:35AM – Q&A Panel & Open Discussion 10:00AM – Event Concludes
3
Focused Strategy and Defined Path
Advancing to pivotal trials Competitive advantage Well capitalized Broad opportunity Independent commercialization
Experienced Management
David Main, President & CEO
INEX Pharmaceuticals, QLT
Stephen Shrewsbury, CMO
Sarepta, MAP, Chiron, Glaxo
Kamran Alam, CFO
Angiotech, AnorMED, PwC
Lloyd Mackenzie, VP Technical Ops
QLT, Inflazyme
David Mitchell, VP Global Regulatory Affairs & QA
AbbVie, Biogen Idec, Bayer Schering
Key Near-Term Milestones
Near-term data, expanded market opportunities and pipeline advancement
ESSIC Meeting (Sept 18, 2015)
KINSHIP Enrollment
R&D Day Complete (Q2/15) (Oct 7, 2015)
LEADERSHIP Top- Initiation of BPS/IC 301 Pivotal
FDA End-of-Phase
Line Results (Q3/15) Trial
2 Meeting (Q4/15)
KINSHIP Top-Line KINSHIP Full Results: Meeting
LEADERSHIP
Results (Q4/15)
Secondary Results • Next Generation Lead Selection (Q3/15) ? 2015 2016
6
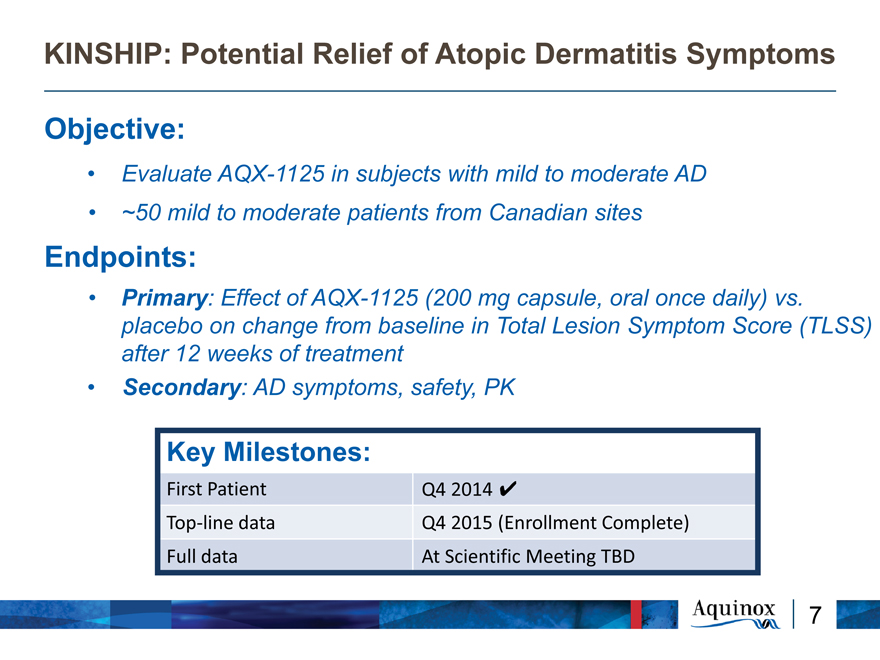
KINSHIP: Potential Relief of Atopic Dermatitis Symptoms
Objective:
Evaluate AQX-1125 in subjects with mild to moderate AD
~50 mild to moderate patients from Canadian sites
Endpoints:
Primary: Effect of AQX-1125 (200 mg capsule, oral once daily) vs. placebo on change from baseline in Total Lesion Symptom Score (TLSS) after 12 weeks of treatment
Secondary: AD symptoms, safety, PK
Key Milestones:
First Patient Q4 2014
Top?line data Q4 2015 (Enrollment Complete) Full data At Scientific Meeting TBD
7
Dr. Robert Evans
Associate Professor of Urology,
Wake Forest Baptist Medical School
Dr. Robert Evans
Associate Professor of Urology, Wake Forest Baptist Medical School
Jefferson Medical College – MD
Private Urology practice ~ 20 years
Associate Professor of Urology 6 years, NC
Co-Director, Continence & Pelvic Pain Center, NC
ICA Physician Award, 2000
GAG Society Urologist of the Year, 2004 & 2008
Key urology publications, 15 since 2002
PI in 6 multicenter urology RCTs in last 2 years
NIDDK/MAPP research (pending) NIH – 2015-17
9
Dr. Stephen Shrewsbury
Senior VP Clinical Development & CMO
10
A Novel First in Class Anti-Inflammatory Therapy
AQX-1125, a SHIP1 activator:
Broad anti-inflammatory potential
Favourable ADME:
Once daily oral administration
T 1/2 21hrs
Dose proportional PK, No food effect
High bioavailability; Not metabolized ~60/40 Liver/Renal elimination as unchanged parent
Well tolerated in 5 completed clinical trials
>360 subjects dosed
11
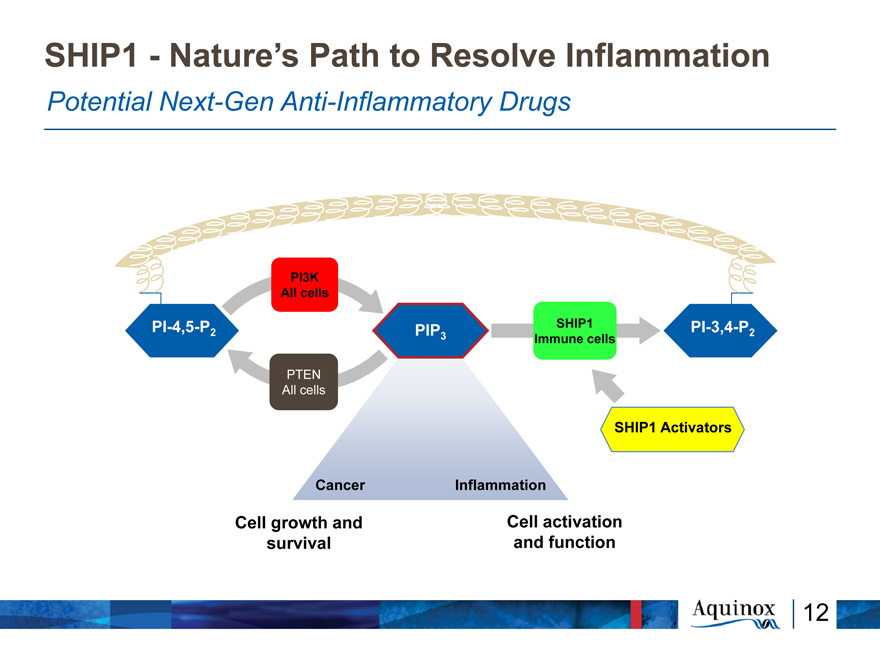
SHIP1—Nature’s Path to Resolve Inflammation
Potential Next-Gen Anti-Inflammatory Drugs
PI3K All cells
PI-4,5-P PIP SHIP1 PI-3,4-P
2 3 2
Immune cells
All
SHIP1 Activators
Cancer Inflammation
Cell growth and Cell activation survival and function
12
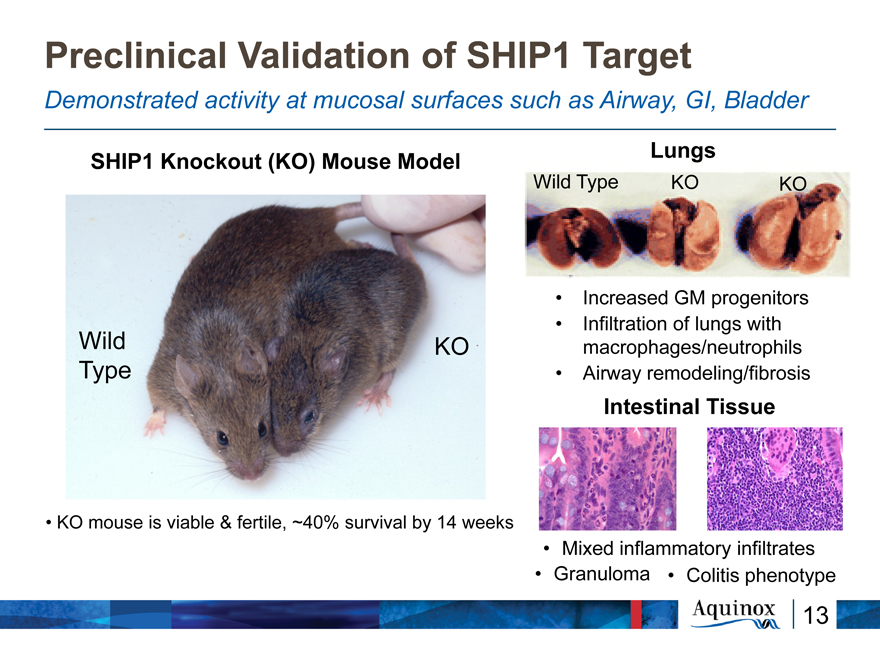
Preclinical Validation of SHIP1 Target
Demonstrated activity at mucosal surfaces such as Airway, GI, Bladder
Lungs SHIP1 Knockout (KO) Mouse Model
Wild Type KO KO
Increased GM progenitors
Infiltration of lungs with Wild KO macrophages/neutrophils Type Airway remodeling/fibrosis
Intestinal Tissue
KO mouse is viable & fertile, ~40% survival by 14 weeks Mixed inflammatory infiltrates
Granuloma Colitis phenotype
13
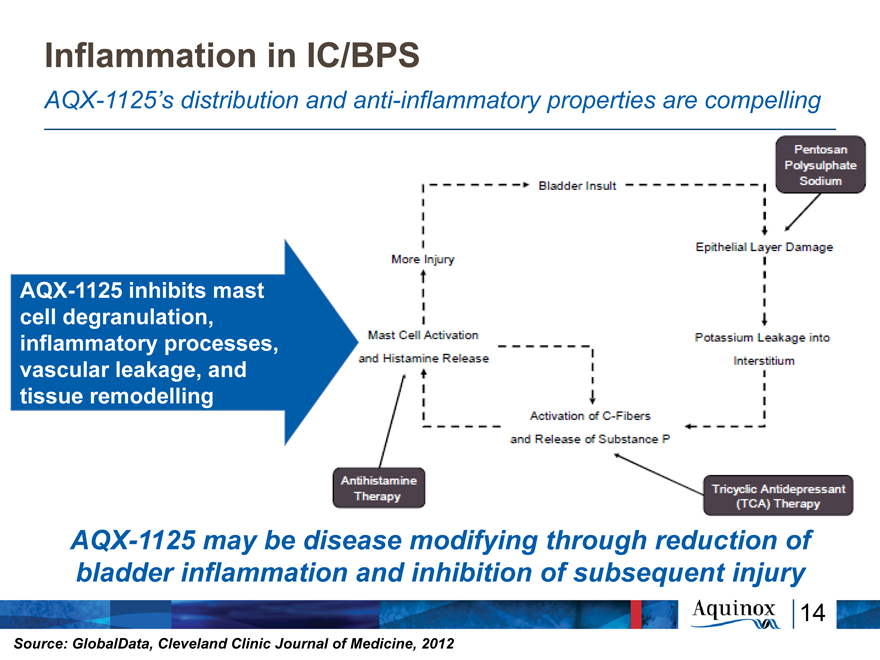
Inflammation in IC/BPS
AQX-1125’s distribution and anti-inflammatory properties are compelling
AQX-1125 inhibits mast cell degranulation, inflammatory processes, vascular leakage, and tissue remodelling
AQX-1125 may be disease modifying through reduction of bladder inflammation and inhibition of subsequent injury
Source: GlobalData, Cleveland Clinic Journal of Medicine, 2012
Bladder Insult More Injury Epithelial Layer Damage Pentosan Polysulphate Sodium
Mast Cell Activation and Histamine Release Potassium Leakage into Interstitium
Activation of C-Fibres and Release of Substance P
Antihistamine Therapy Tricyclic Antidepresent (TCA) Therapy
14
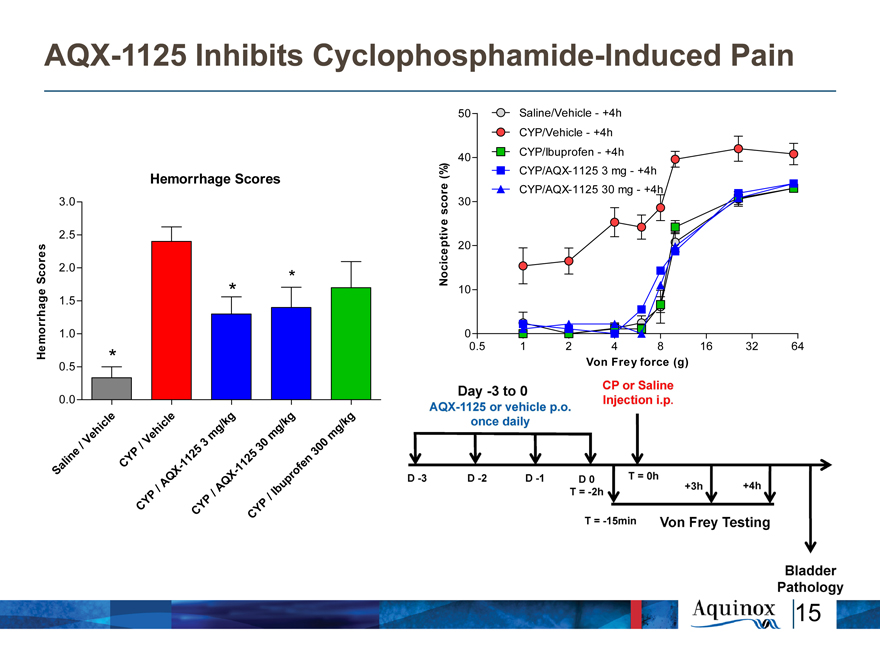
AQX-1125 Inhibits Cyclophosphamide-Induced
Pain
50 Saline/Vehicle—+4h
CYP/Vehicle—+4h
40 CYP/Ibuprofen—+4h
Hemorrhage Scores (%) CYP/AQX-1125 3 mg—+4h
e CYP/AQX-1125 30 mg—+4h
3.0 scor 30
2.5
20
Scores 2.0 * Nociceptive
1.5 * 10
1.0 0
Hemorrhage 0.5 1 2 4 8 16 32 64
0.5 * Von Frey force (g)
0.0
icl e l e / kg / kg k g
h hic g g g/
Ve V e m m m
/ / 5 3 30 0
i ne P 12 5 n 30
al CY —1 1 12 f e
S Q X X— p ro
/ A AQ Ibu
P / /
Y P P
C CY Y
C
Day-3 to 0 AQX-1125 or vehicle p.o. once daily CP or Saline Injection i.p. D-3 D-2 D-1 D0 T=0h T=-2h +3h +4h T=-15 min Von Frey Testing Bladder Pathology
15
AQX-1125 in Bladder Pain Syndrome /
Interstitial Cystitis (BPS/IC)
16
Phase 2 LEADERSHIP Trial: A Comprehensive BPS/IC Trial
Population and Entry Demographics: Sixty-nine female patients across US and Canadian sites with with moderate to severe BPS/IC symptoms:
Mean pain >5/10
Mean BPIC/SS and O’Leary-Sant symptom scores ?19 or ?8 respectively
On background medication (excluding opiates)
Primary Endpoint: 6 weeks treatment with once daily AQX-1125 (200 mg) vs. placebo on reduction of average pain score (11-point numerical rating scale (NRS)) vs. baseline, utilizing eDiaries
Pre-specified Secondary Endpoints:
Difference in the change from baseline in the following:
- Average pain score measured by 11-point NRS recorded at clinic
- Maximum daily pain based on an 11-point NRS recorded by eDiary
- O’Leary-Sant Interstitial Cystitis Symptom Index and Problem Index (ICSI/PI)
- Bladder Pain/Interstitial Cystitis Symptom Score (BPIC-SS)
- Voiding frequency over a 24 hour period
Safety, Pharmacokinetics
17
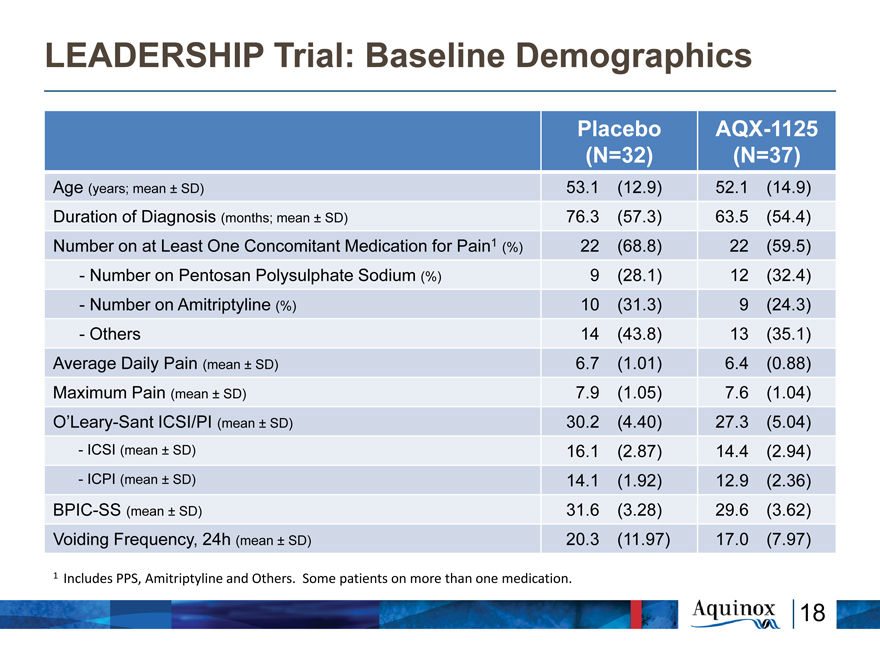
LEADERSHIP Trial: Baseline Demographics
Placebo AQX-1125
(N=32) (N=37)
Age (years; mean ± SD) 53.1 (12.9) 52.1 (14.9)
Duration of Diagnosis (months; mean ± SD) 76.3 (57.3) 63.5 (54.4)
Number on at Least One Concomitant Medication for Pain1 (%) 22 (68.8) 22 (59.5)
- Number on Pentosan Polysulphate Sodium (%) 9 (28.1) 12 (32.4)
- Number on Amitriptyline (%) 10 (31.3) 9 (24.3)
- Others 14 (43.8) 13 (35.1)
Average Daily Pain (mean ± SD) 6.7 (1.01) 6.4 (0.88)
Maximum Pain (mean ± SD) 7.9 (1.05) 7.6 (1.04)
O’Leary-Sant ICSI/PI (mean ± SD) 30.2 (4.40) 27.3 (5.04)
-ICSI (mean ± SD) 16.1 (2.87) 14.4 (2.94)
- ICPI (mean ± SD) 14.1 (1.92) 12.9 (2.36)
BPIC-SS (mean ± SD) 31.6 (3.28) 29.6 (3.62)
Voiding Frequency, 24h (mean ± SD) 20.3 (11.97) 17.0 (7.97)
1 Includes PPS, Amitriptyline and Others. Some patients on more than one medication.
18
LEADERSHIP: Greater Average Daily Pain Reduction
Over 6 Weeks (eDiary)
Average Daily
Pain
(11-Point NRS,
eDiary)
Change from Baseline Plain (eDiary) (Mean +-SE) 0 -1 -2 -3 Baseline 2 Weeks 4 Weeks 6 Weeks p=0.165 p=0.086 p=0.061 Placebo AQX-1125
19
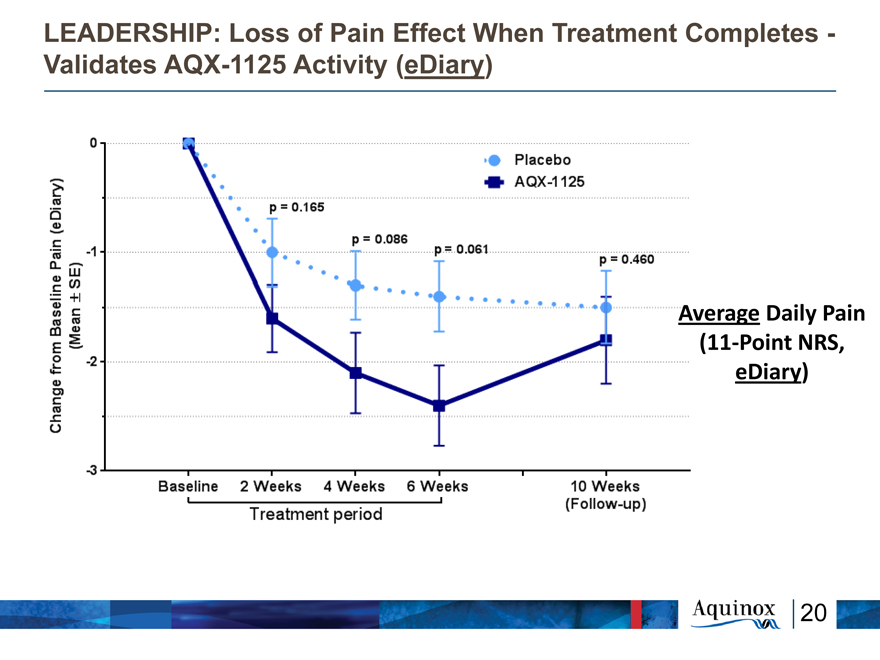
LEADERSHIP: Loss of Pain Effect When Treatment
Completes—
Validates AQX-1125 Activity (eDiary)
Average Daily Pain
(11-Point NRS,
eDiary)
Change from Baseline Plain (eDiary) (Mean +-SE) 0 -1 -2 -3 Baseline 2 Weeks 4 Weeks 6 Weeks p=0.165 p=0.086 p=0.061 Placebo AQX-1125 p=0.460 Treatment period 10 Weeks (Follow-up)
20
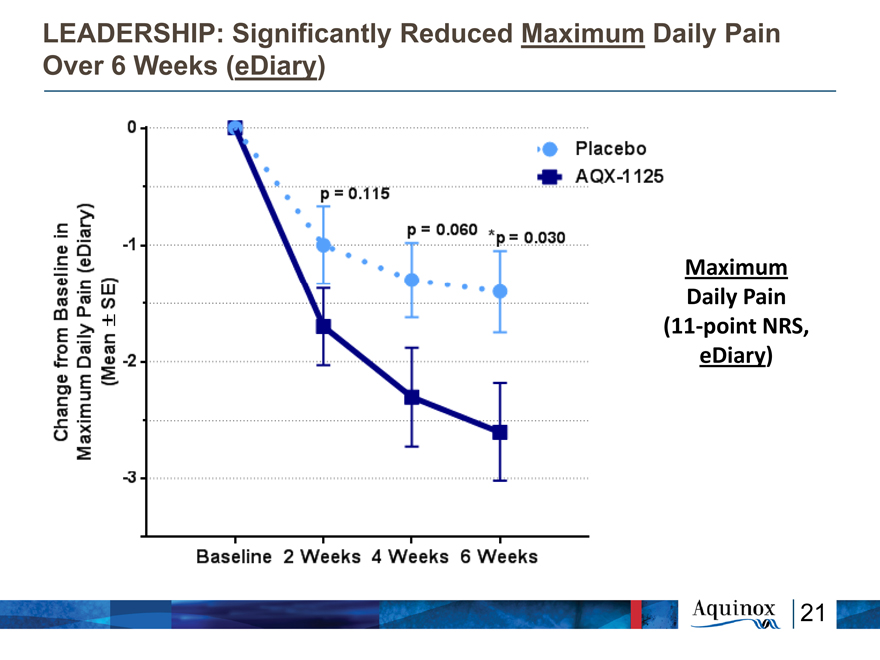
LEADERSHIP: Significantly Reduced Maximum Daily Pain
Over 6 Weeks (eDiary)
Maximum
Daily Pain
(11-point NRS,
eDiary)
Change from Baseline in Maximum Diary Plain (eDiary) (Mean +-SE) 0 -1 -2 -3 Baseline 2 Weeks 4 Weeks 6 Weeks p=0.115 p=0.060 *p=0.030 Placebo AQX-1125
21
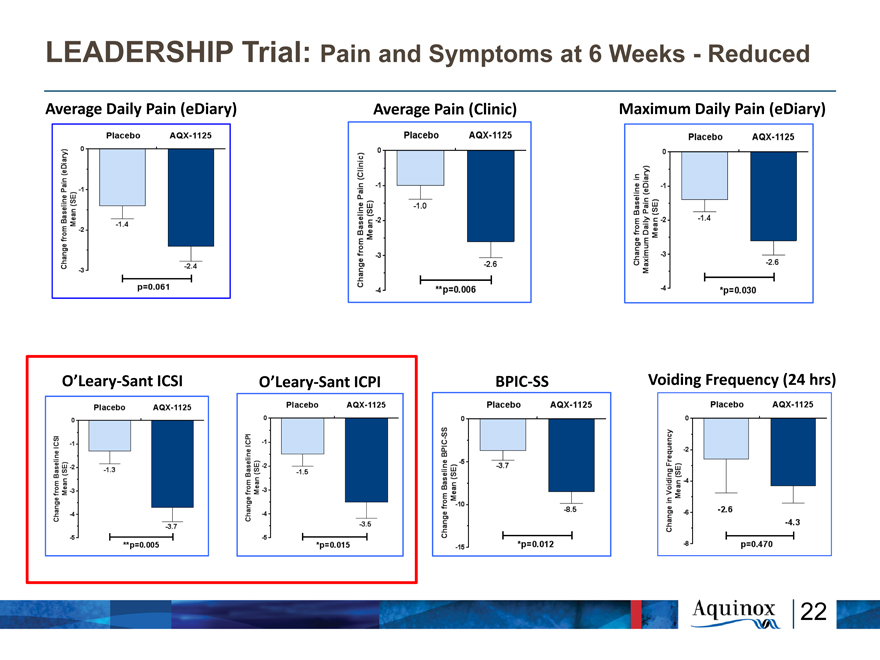
LEADERSHIP Trial: Pain and Symptoms at 6 Weeks—Reduced
Average Daily Pain (eDiary) Average Pain (Clinic) Maximum Daily Pain (eDiary)
O’Leary-Sant ICSI O’Leary-Sant ICPI BPIC-SS Voiding Frequency (24 hrs)
Change from Baseline in Maximum Diary Plain (eDiary) (Mean +-SE) 0 -1 -2 -3 Baseline 2 Weeks 4 Weeks 6 Weeks p=0.115 p=0.060 *p=0.030 Placebo AQX-1125
Change from Baseline Plain (eDiary) (Mean SE) 0 -1 -2 -3 Placebo AQX-1125
-1.4 -2.4 p=0.061
Change from Baseline Plain (clinic) (Mean SE) 0 -1 -2 -3 -4 Placebo AQX-1125
-1.0 -2.6 **p=0.006
Change from Baseline in Maximum Daily Plain (eDiary) (Mean SE) 0 -1 -2 -3 -4 Placebo AQX-1125
-1.4 -2.6 *p=0.030
Change from Baseline ICSI (Mean SE) 0 -1 -2 -3 -4 -5 Placebo AQX-1125
-1.3 -3.7 **p=0.005
Change from Baseline ICPI (Mean SE) 0 -1 -2 -3 -4 -5 Placebo AQX-1125
-1.5 -3.5 *p=0.015
Change from Baseline BPIC-SS (Mean SE) 0 -5 -10 -15 Placebo AQX-1125
-1.5 -3.5 *P=0.012
Change in Voiding Frequency Mean (SE) 0 -2 -4 -6 -8 Placebo AQX-1125 -2.6 -4.3 p=0.470
22
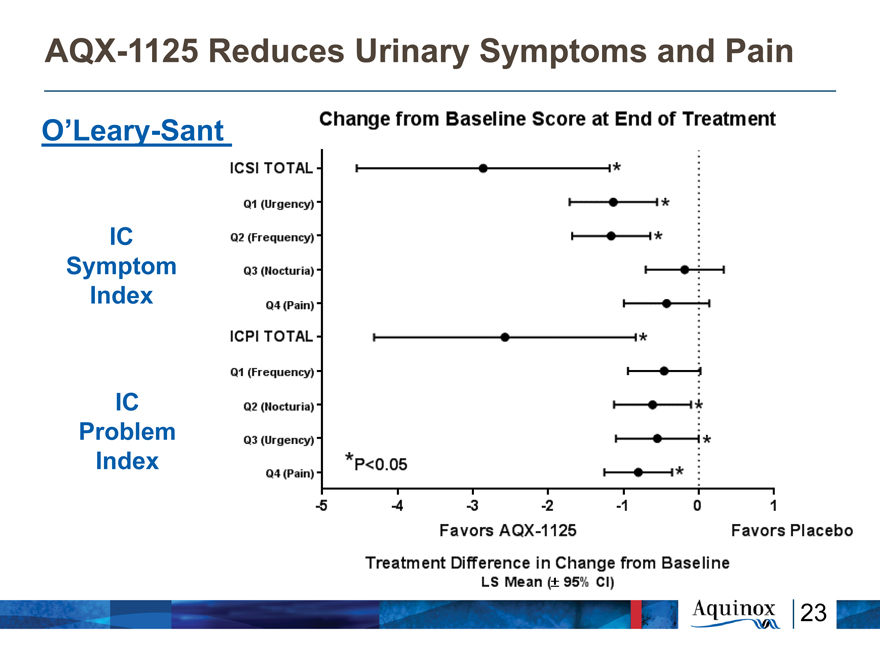
AQX-1125 Reduces Urinary Symptoms and
Pain
O’Leary-Sant
IC
Symptom
Index
IC
Problem
Index
Change from Baseline Score at End of Treatment ICSI TOTAL Q1
Q1 (Urgency) Q2 (Frequency) Q3 (Nocturia) Q4 (Pain) ICPI TOTAL Q1 (Frequency) Q2 (Nocturia) Q3 (Urgency) Q4 (Pain) -5 -4 -3 -2 -1 0 1 Favors AQX-1125 Favors Placebo Treatment Difference in Change from Baseline LS Mean (+-*95% CI) *p<0.05
23
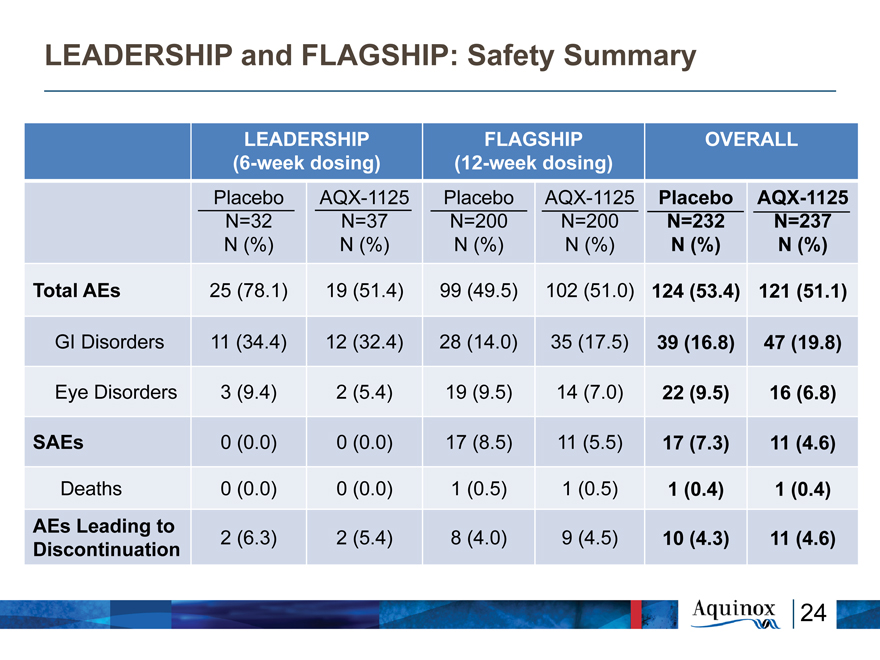
LEADERSHIP and FLAGSHIP: Safety Summary
LEADERSHIP FLAGSHIP OVERALL
(6-week dosing) (12-week dosing)
Placebo AQX-1125 Placebo AQX-1125 Placebo AQX-1125
N=32 N=37 N=200 N=200 N=232 N=237
N (%) N (%) N (%) N (%) N (%) N (%)
Total AEs 25 (78.1) 19 (51.4) 99 (49.5) 102 (51.0) 124 (53.4) 121 (51.1)
GI Disorders 11 (34.4) 12 (32.4) 28 (14.0) 35 (17.5) 39 (16.8) 47 (19.8)
Eye Disorders 3 (9.4) 2 (5.4) 19 (9.5) 14 (7.0) 22 (9.5) 16 (6.8)
SAEs 0 (0.0) 0 (0.0) 17 (8.5) 11 (5.5) 17 (7.3) 11 (4.6)
Deaths 0 (0.0) 0 (0.0) 1 (0.5) 1 (0.5) 1 (0.4) 1 (0.4)
AEs Leading to
Discontinuation 2 (6.3) 2 (5.4) 8 (4.0) 9 (4.5) 10 (4.3) 11 (4.6)
24
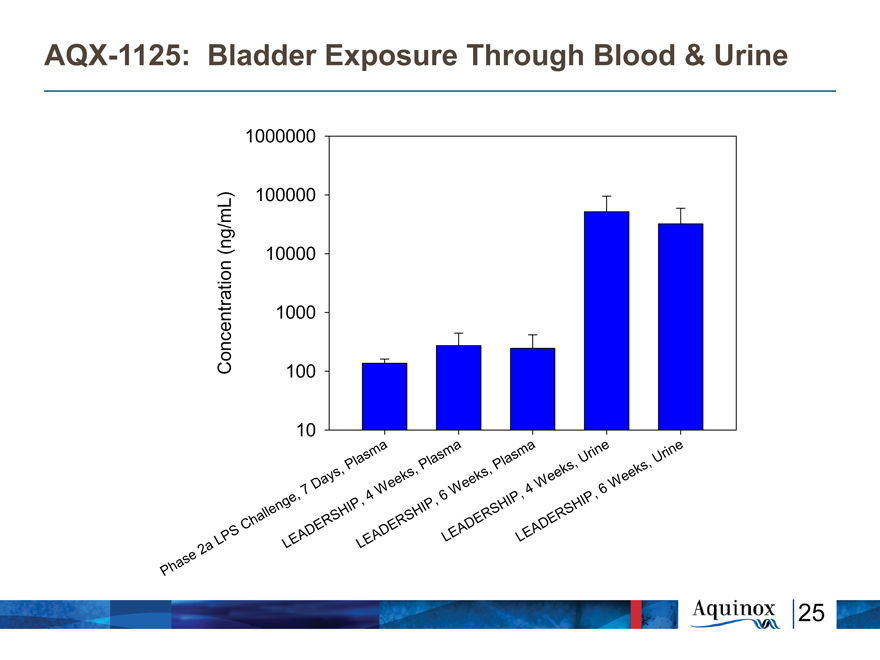
AQX-1125: Bladder Exposure Through Blood & Urine
Concentration (ng/mL)
1000000
100000 10000 1000 100 10
Phase 2a LPS Challenge, 7 Days, Plasma
LEADERSHIP, 4 weeks, Plasma
LEADERSHIP, 6 weeks, Plasma
LEADERSHIP, 4 weeks, Urine
LEADERSHIP, 6 weeks, Urine
25
Exploratory Analyses
26
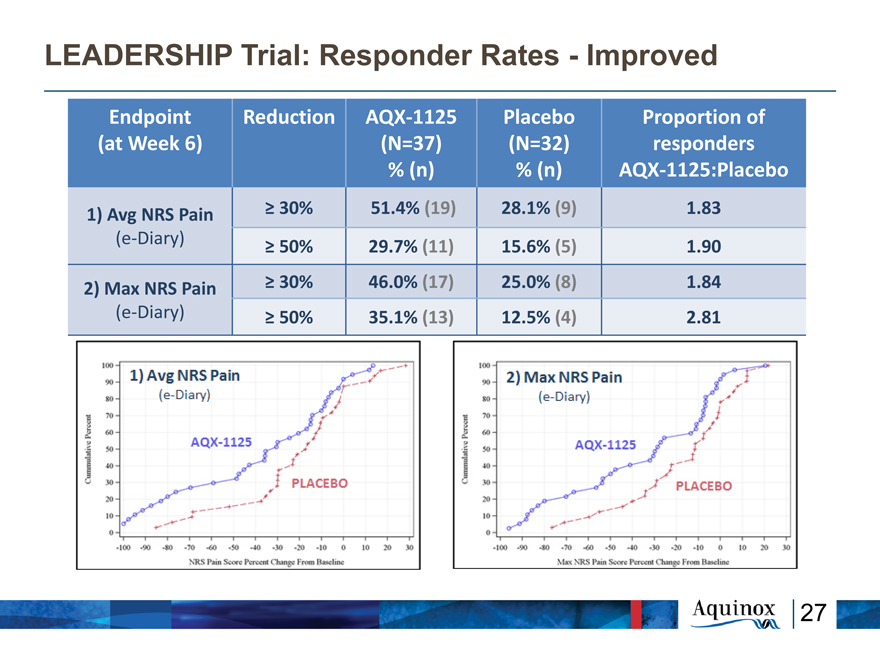
LEADERSHIP Trial: Responder Rates—Improved
Endpoint Reduction AQX?1125 Placebo Proportion of
(at Week 6) (N=37) (N=32) responders
% (n) % (n) AQX?1125:Placebo
1) Avg NRS Pain ? 30% 51.4% (19) 28.1% (9) 1.83
(e?Diary) ? 50% 29.7% (11) 15.6% (5) 1.90
2) Max NRS Pain ? 30% 46.0% (17) 25.0% (8) 1.84
(e?Diary) ? 50% 35.1% (13) 12.5% (4) 2.81
27
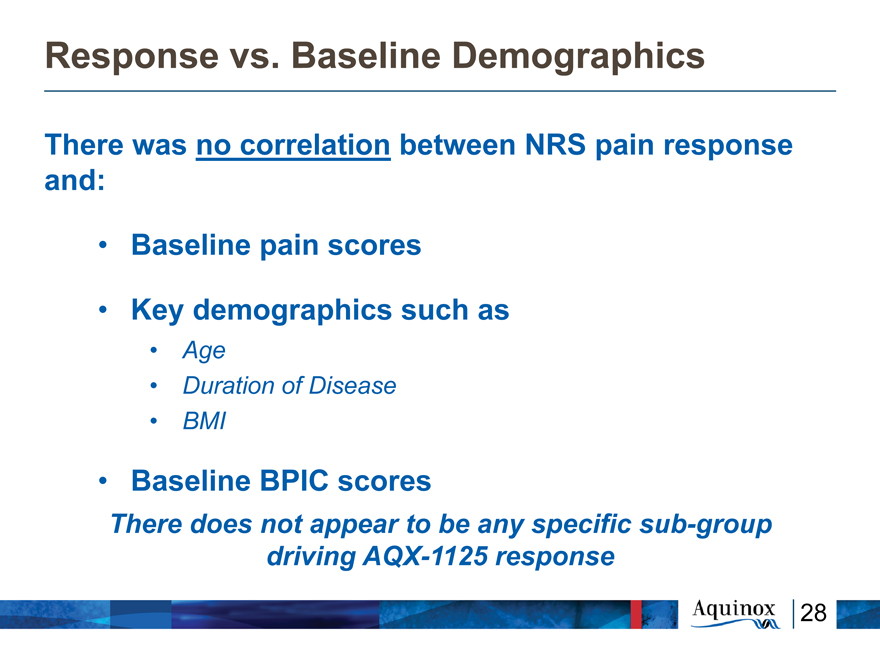
Response vs. Baseline Demographics
There was no correlation between NRS pain response
and:
Baseline pain scores
Key demographics such as
Age
Duration of Disease
BMI
Baseline BPIC scores
There does not appear to be any specific sub-group
driving AQX-1125 response
28
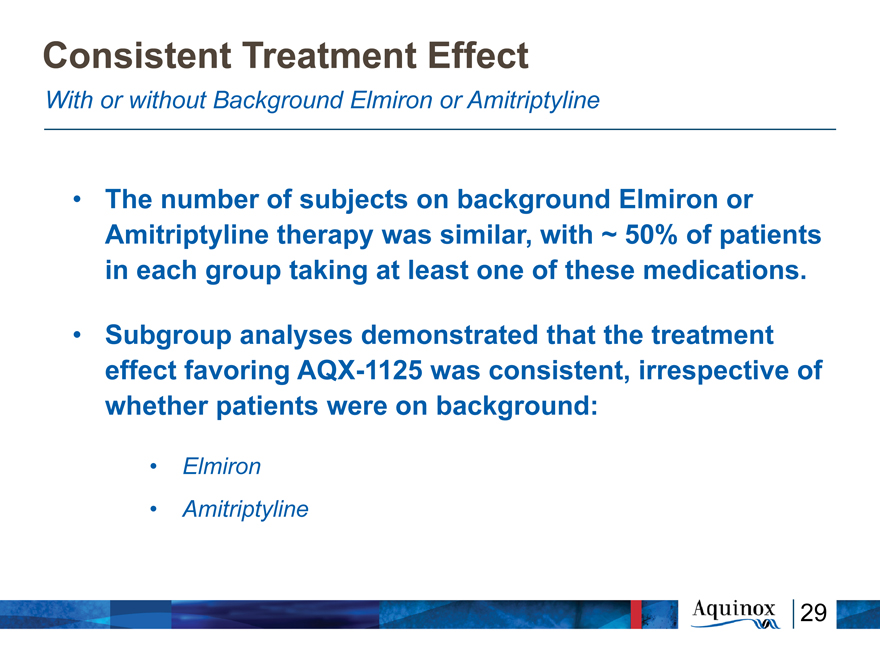
Consistent Treatment Effect
With or without Background Elmiron or Amitriptyline
The number of subjects on background Elmiron or
Amitriptyline therapy was similar, with ~ 50% of
patients in each group taking at least one of these
medications.
Subgroup analyses demonstrated that the treatment
effect favoring AQX-1125 was consistent,
irrespective of whether patients were on
background:
Elmiron
Amitriptyline
29
LEADERSHIP Trial: Lessons Learned – Study
Conduct
Primary Endpoint:
Pain – maximum pain more meaningful for patients
Duration increased from 6 to 12 weeks
Enrollment:
Cystoscopies –scientific data now suggests clinical criteria
more important for defining population than cystoscopic
results
Geographic distribution and proximity of sites to patients
Open label extension important to encourage participation
Anticipate screen failure ~ 50%, patient recruitment strategies
to compensate
Increased communication (via Patient Advocacy) with patients
30
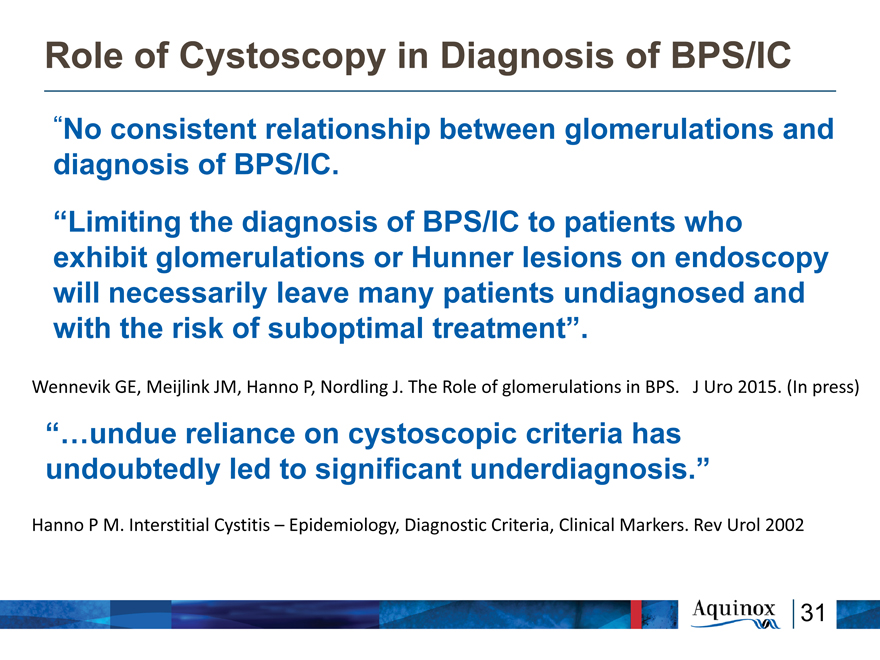
Role of Cystoscopy in Diagnosis of BPS/IC
“No consistent relationship between glomerulations and diagnosis of BPS/IC.
“Limiting the diagnosis of BPS/IC to patients who exhibit glomerulations or Hunner lesions on endoscopy will necessarily leave many patients undiagnosed and with the risk of suboptimal treatment”.
Wennevik GE, Meijlink JM, Hanno P, Nordling J. The Role of glomerulations in BPS. J Uro 2015. (In press)
“…undue reliance on cystoscopic criteria has undoubtedly led to significant underdiagnosis.”
Hanno P M. Interstitial Cystitis – Epidemiology, Diagnostic Criteria, Clinical Markers. Rev Urol 2002
31
Future Development in BPS/IC
32
Proposed BPS/IC Future Development Plans
Q4/15 End of Phase 2 meeting with FDA and
subsequently other agencies:
Agree on endpoints, duration of dosing, safety database requirement
and supportive preclinical/CMC program
2 pivotal trials (Subject to regulatory discussions):
1st—multiple AQX-1125 doses, 12 weeks dosing for efficacy + open-
label extension (OLE) for safety
2nd –Single dose selected from 1st confirmatory trial with same
dosing for efficacy. OLE requirement TBD
Supportive Phase 1 trials:
Bioequivalence (capsules to tablets); Renal & Liver impairment; TQT
Supportive Preclinical:
Standard Carcinogenicity & Reproduction Toxicity studies
33
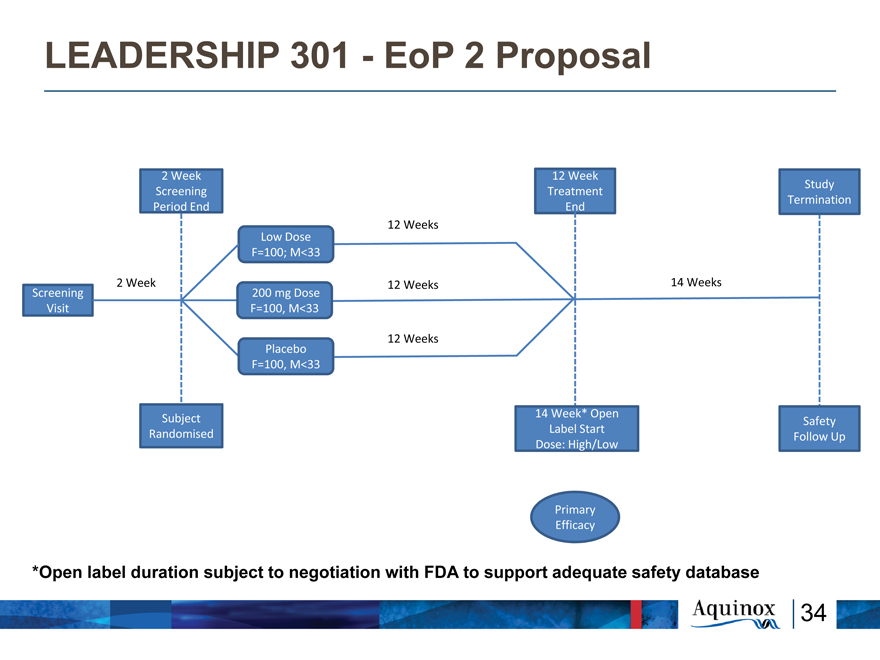
LEADERSHIP 301—EoP 2 Proposal
2 Week 12 Week
Study Screening Treatment Termination Period End End
12 Weeks
Low Dose F=100; M<33
2 Week 12 Weeks 14 Weeks
Screening 200 mg Dose Visit F=100, M<33
Placebo 12 Weeks F=100, M<33
14 Week* Open
Subject Safety Label Start Randomised Follow Up Dose: High/Low
Primary Efficacy
*Open label duration subject to negotiation with FDA to support adequate safety database
34
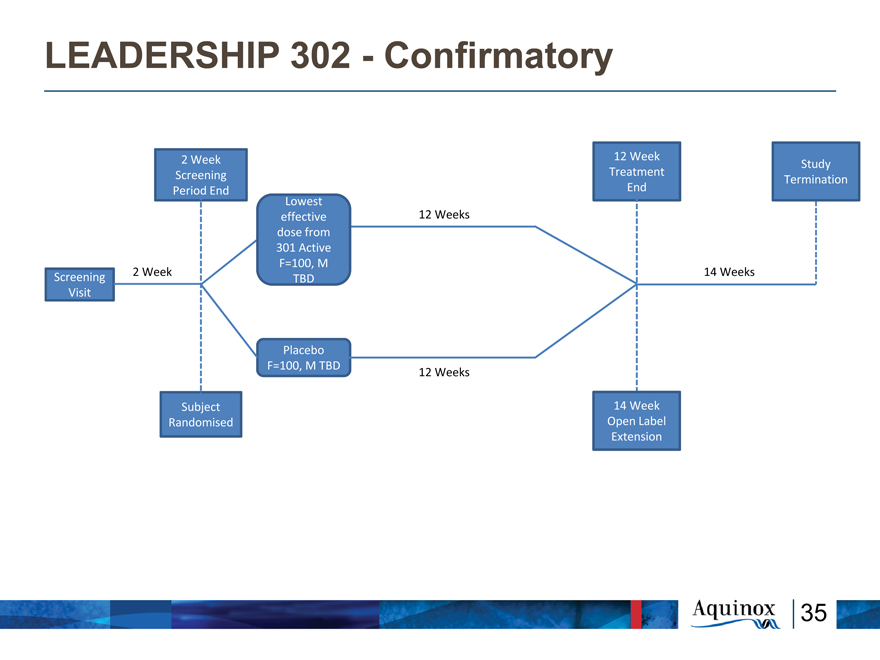
LEADERSHIP 302—Confirmatory
2 Week 12 Week
Study Screening Treatment Termination Period End End Lowest effective 12 Weeks dose from 301 Active F=100, M
2 Week 14 Weeks
Screening TBD Visit
Placebo F=100, M TBD
12 Weeks
Subject 14 Week Randomised Open Label Extension
35
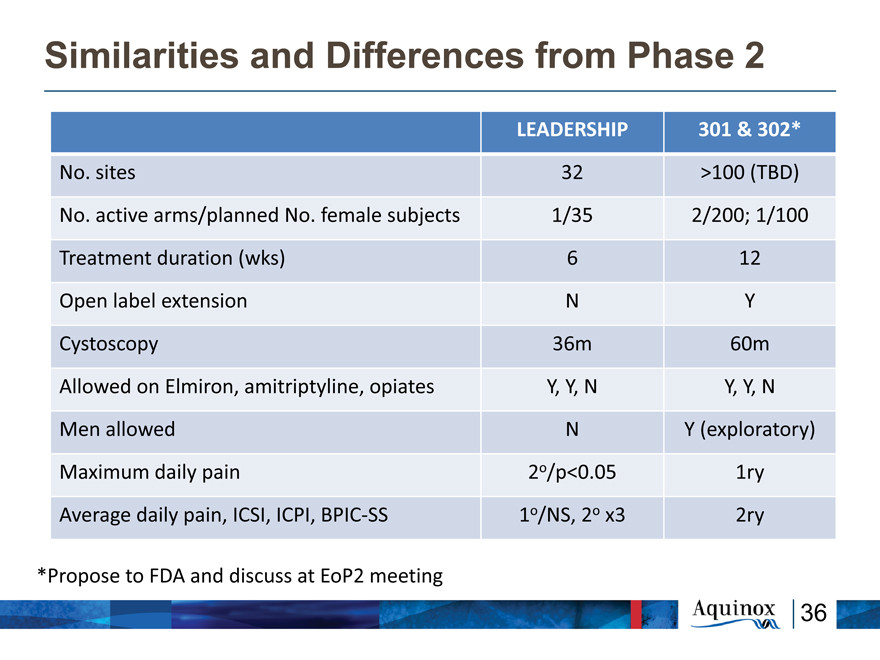
Similarities and Differences from Phase 2
LEADERSHIP 301 & 302*
No. sites 32 >100 (TBD)
No. active arms/planned No. female subjects 1/35 2/200; 1/100
Treatment duration (wks) 6 12
Open label extension N Y
Cystoscopy 36m 60m
Allowed on Elmiron, amitriptyline, opiates Y, Y, N Y, Y, N
Men allowed N Y (exploratory)
Maximum daily pain 2o/p<0.05 1ry
Average daily pain, ICSI, ICPI, BPIC?SS 1o/NS, 2o x3 2ry
*Propose to FDA and discuss at EoP2 meeting
36
Critical Success Factors for 301
12 Week treatment period
Open Label Extension (enrollment/safety)
Less burdensome cystoscopy (window/result)
Broader clinical site coverage
Faster clinical site initiation
Positive data influence on PI and patient
interest
Less restrictive financial constraints
37
BPS/IC Commercial Opportunity
38
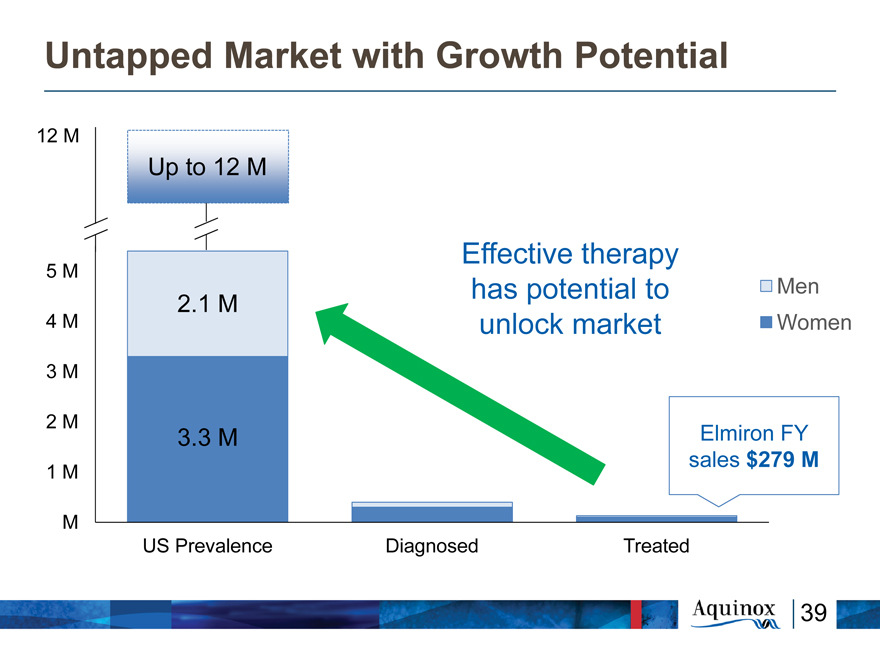
Untapped Market with Growth Potential
12 M
Up to 12 M
Effective therapy
5 M
has potential to Men
2.1 M
4 M unlock market Women
3 M
2 M
3.3 M Elmiron FY sales $279 M
1 M
M
US Prevalence Diagnosed Treated
39
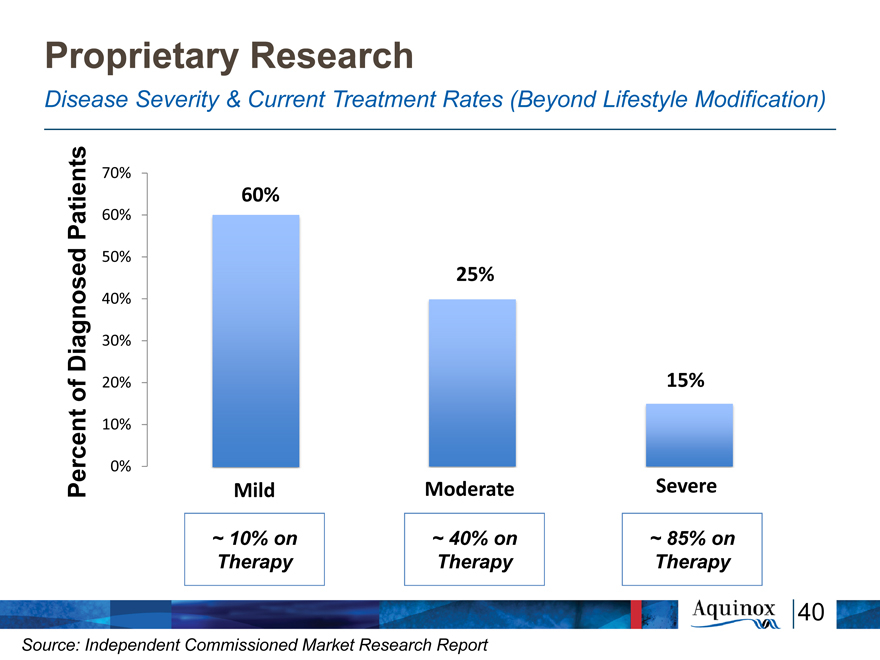
Proprietary Research
Disease Severity & Current Treatment Rates (Beyond Lifestyle Modification)
nts 70%
60%
Patie 60%
ed 50%
25%
40%
Diagnos 30% of 20% 15% rcent 10%
0%
Pe Mild Moderate Severe
~ 10% on ~ 40% on ~ 85% on Therapy Therapy Therapy
40
Source: Independent Commissioned Market Research Report
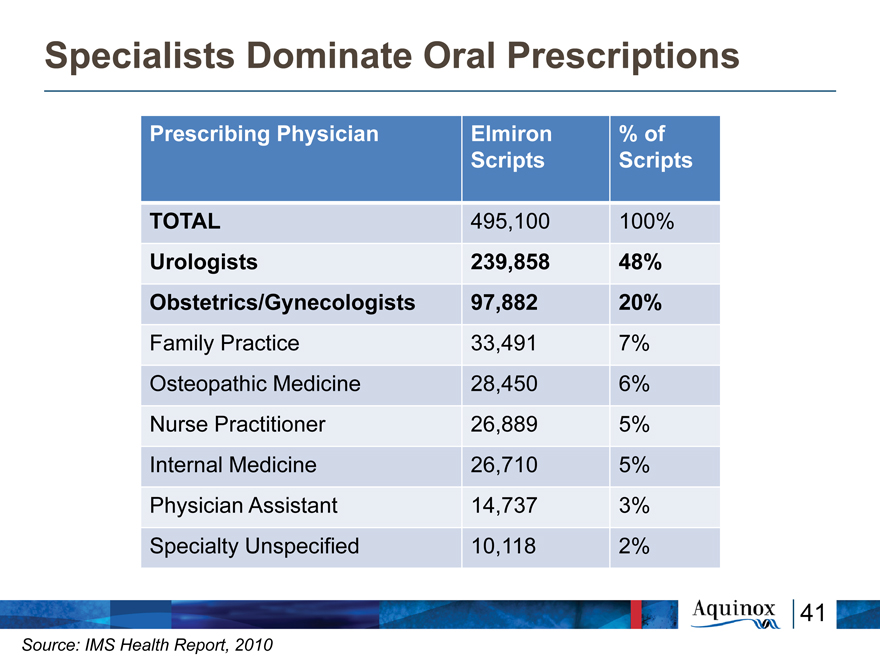
Specialists Dominate Oral Prescriptions
Prescribing Physician Elmiron % of
Scripts Scripts
TOTAL 495,100 100%
Urologists 239,858 48%
Obstetrics/Gynecologists 97,882 20%
Family Practice 33,491 7%
Osteopathic Medicine 28,450 6%
Nurse Practitioner 26,889 5%
Internal Medicine 26,710 5%
Physician Assistant 14,737 3%
Specialty Unspecified 10,118 2%
41
Source: IMS Health Report, 2010
Factors That May Influence Prescribing and
Utilization
Grade A clinical evidence of efficacy
Grade A clinical evidence of safety and
tolerability
Favorable pharmacokinetic properties
Novel, anti-inflammatory mechanism of action
Increased disease awareness
Increased diagnosis rate
Favorable dosing regimen
Long term compliance
42
Clear Path To Independent Commercialization
LEADERSHIP Phase 2 trial: compelling AQX-1125
efficacy and safety profile
High unmet medical need with pent-up demand; no
new treatment options in 20 years
Motivated and engaged patient population
Small, targeted sales force could achieve high market
penetration (urologists, obstetricians &
gynecologists)
Large commercial opportunity, minimal competition
and positive price benchmark
43
Key Near-Term Milestones
Near-term data, expanded market opportunities and pipeline advancement
KINSHIP Enrollment Complete (Q2/15)
LEADERSHIP Top-Line Results (Q3/15)
LEADERSHIP Secondary Results (Q3/15) 2015
ESSIC Meeting (Sept 18, 2015)
R&D Day (Oct 7, 2015)
FDA End-of-Phase
2 Meeting (Q4/15)
KINSHIP Top-Line Results (Q4/15)
Initiation of BPS/IC 301 Pivotal Trial
KINSHIP Full Results: Meeting
Next Generation Lead Selection
2016
44
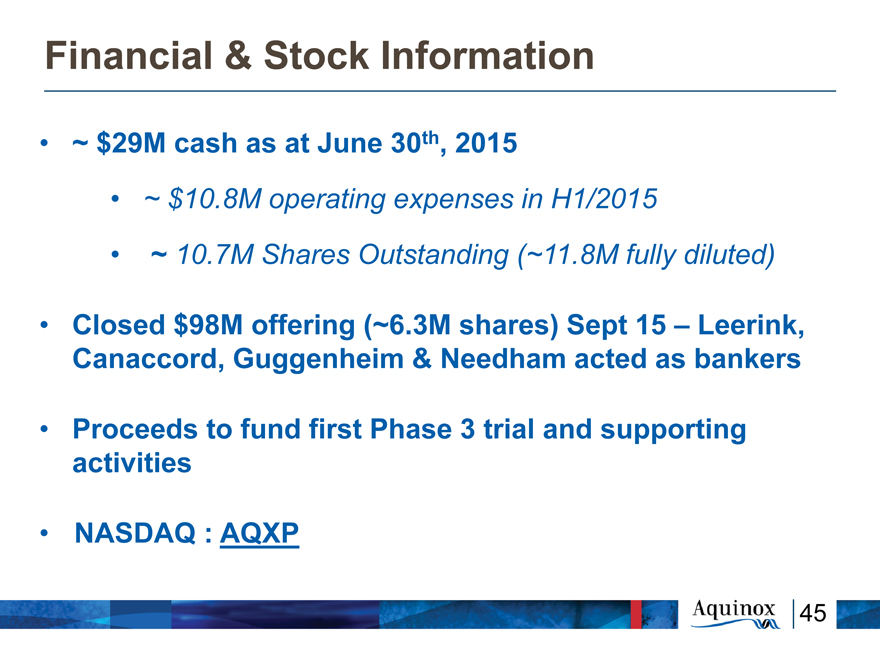
Financial & Stock Information
~ $29M cash as at June 30th, 2015
~ $10.8M operating expenses in H1/2015
~ 10.7M Shares Outstanding (~11.8M fully diluted)
Closed $98M offering (~6.3M shares) Sept 15 –
Leerink, Canaccord, Guggenheim & Needham acted as
bankers
Proceeds to fund first Phase 3 trial and supporting
activities
NASDAQ : AQXP
45
MILLIONS SUFFER, FEW UNDERSTAND
SEPTEMBER IS INTERSTITIAL CYSTITIS / BLADDER PAIN SYNDROME AWARENESS MONTH