
Corporate Presentation August 13, 2013
Corporate Presentation August 14, 2013

Statements under the Private Securities Litigation Reform Act, as amended : With the exception of the historical information contained in this presentation, the matters described herein contain forward - looking statements that involve risks and uncertainties that may individually, mutually, or materially impact the matters herein described, including, but not limited to, Innovus Pharmaceuticals, Inc . ’s (the “Company”) ability to execute its business plan, obtain regulatory approval for products under development, enter into partnering agreements, realize revenue and pursue growth opportunities, some of which are outside the control of the Company . Readers and attendees are cautioned not to place undue reliance on these forward - looking statements as actual results could differ materially from the forward - looking statements contained herein . Attendees are urged to read the risk factors set forth in the Company ’ s most recent annual report on Form 10 - K, subsequent quarterly reports filed on Form 10 - Q and its most recent SEC filings . Company disclaims any intention to update this presentation . Safe - Harbor Statement 2

About Us: Headquartered in La Jolla, California, Innovus Pharmaceuticals, Inc . (“INNV”) is an emerging pharmaceuticals company that delivers innovative and uniquely presented and packaged health solutions through its over - the - counter medicines and consumer and health products . The Company’s management team has a proven track record in product acquisition, development, commercial partnership and commercialization . 3
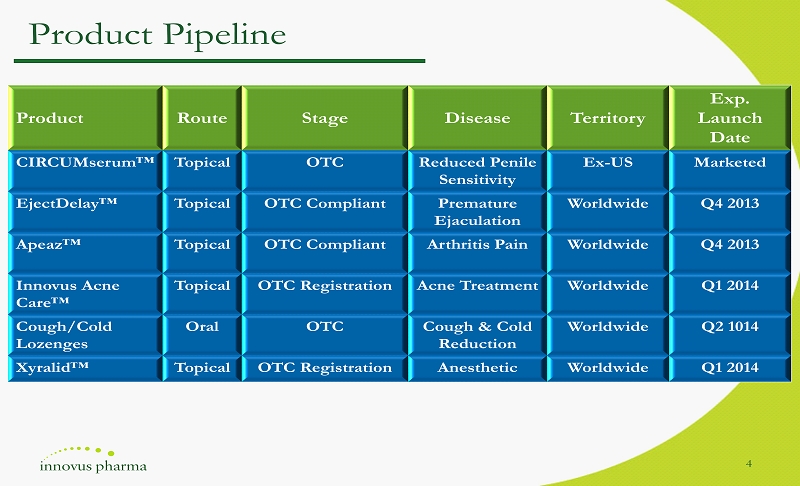
4 Product Route Stage Disease Territory Exp. Launch Date CIRCUMserum ™ Topical OTC Reduced Penile Sensitivity Ex - US Marketed EjectDelay ™ Topical OTC Compliant Premature Ejaculation Worldwide Q4 2013 Apeaz ™ Topical OTC Compliant Arthritis Pain Worldwide Q4 2013 Innovus Acne Care ™ Topical OTC Registration Acne Treatment Worldwide Q1 2014 Cough/Cold Lozenges Oral OTC Cough & Cold Reduction Worldwide Q2 1014 Xyralid ™ Topical OTC Registration Anesthetic Worldwide Q1 2014 Product Pipeline

x Launched CIRCUMserum for Reduced Penile Sensitivity in May 2013 at the AUA for international markets x First commercial ex - US sales of CIRCUMserum occurred in May 2013 • Secure commercial partnerships/distributors in the MENA countries for CIRCUMserum and EjectDelay • Launch of EjectDelay for Premature Ejaculation and Apeaz for Arthritis Pain Relief in the US in Q4 2013 Recent Milestones and Upcoming Expected Milestones 5
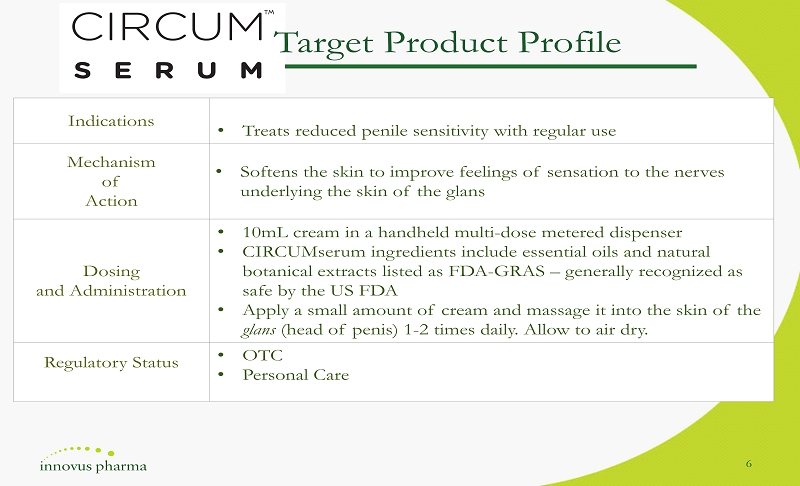
Indications • Treats reduced penile sensitivity with regular use Mechanism of Action • Softens the skin to improve feelings of sensation to the nerves underlying the skin of the glans Dosing and Administration • 10mL cream in a handheld multi - dose metered dispenser • CIRCUMserum in gredients include essential oils and natural botanical extracts listed as FDA - GRAS – generally recognized as safe by the US FDA • Apply a small amount of cream and massage it into the skin of the glans (head of penis) 1 - 2 times daily. Allow to air dry. Regulatory Status • OTC • Personal Care Target Product Profile 6

Reduced Penile Sensitivity • Reduced Penile Sensitivity (“RPS”) refers to the condition of decreased sensation in the penis during sexual activity . • Subjects with RPS report difficulty in stimulating the penis, maintaining erection, and/or achieving orgasm 7

Causes of Reduced Penile Sensitivity RPS is commonly associated with: 1. Age 2. Circumcision whereby exposure of the glans over a period of years leads to excessive keratinization and decreased sexual stimulation 3. Neuropathy associated with diabetes may also account for a higher incidence of RPS in diabetic populations 8
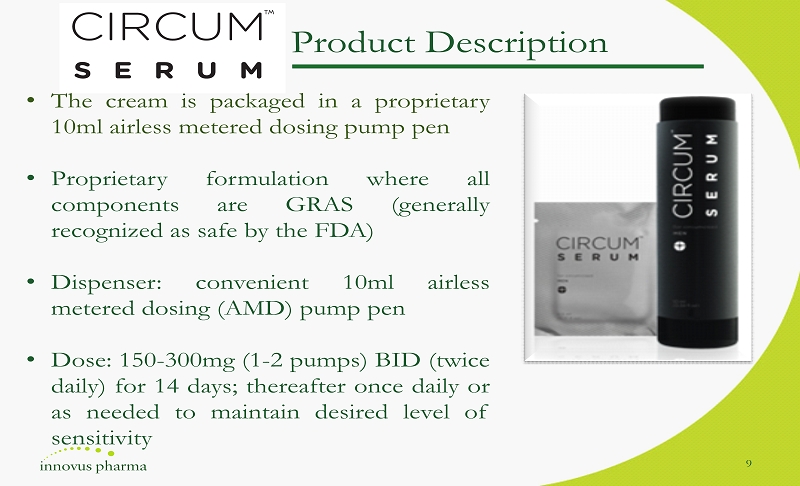
Product Description • The cream is packaged in a proprietary 10 ml airless metered dosing pump pen • Proprietary formulation where all components are GRAS (generally recognized as safe by the FDA) • Dispenser : convenient 10 ml airless metered dosing (AMD) pump pen • Dose : 150 - 300 mg ( 1 - 2 pumps) BID (twice daily) for 14 days ; thereafter once daily or as needed to maintain desired level of sensitivity 9

Clinical Data from Patient Reported Use Patients using CIRCUMserum reported over 70 % increase in penile sensitivity with regular twice a day use for 14 days* 10 Clinical Data * Based on a patient questionnaire of current users conducted by Centric Research Institute
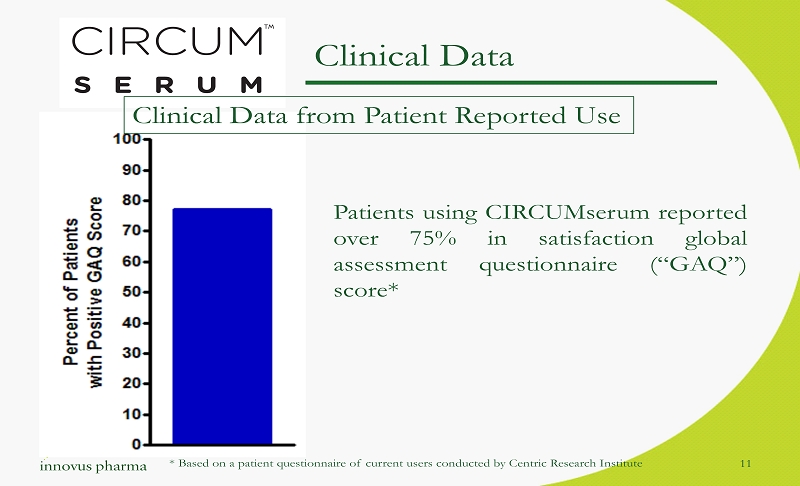
Clinical Data from Patient Reported Use Patients using CIRCUMserum reported over 75 % in satisfaction global assessment questionnaire (“GAQ”) score* 11 Clinical Data * Based on a patient questionnaire of current users conducted by Centric Research Institute
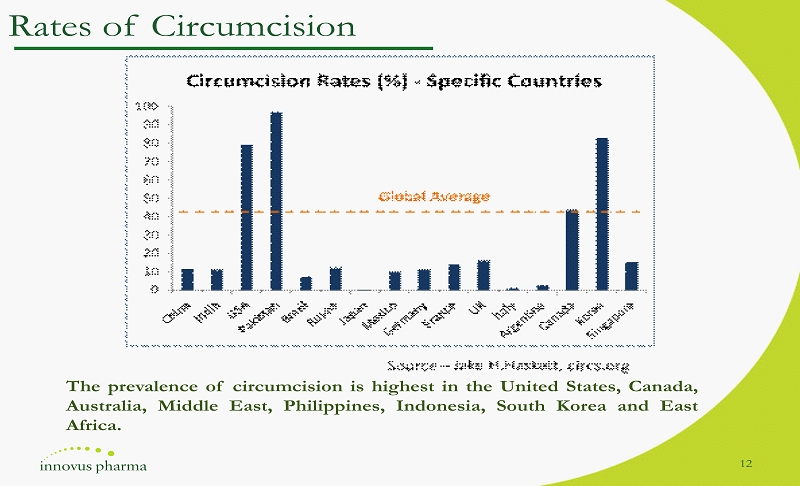
Rates of Circumcision The prevalence of circumcision is highest in the United States, Canada, Australia, Middle East, Philippines, Indonesia, South Korea and East Africa . 12

Market Opportunity • Over 1 Billion circumcised men worldwide • Prevalence: 37% male population are circumcised • Proprietary formulation 13
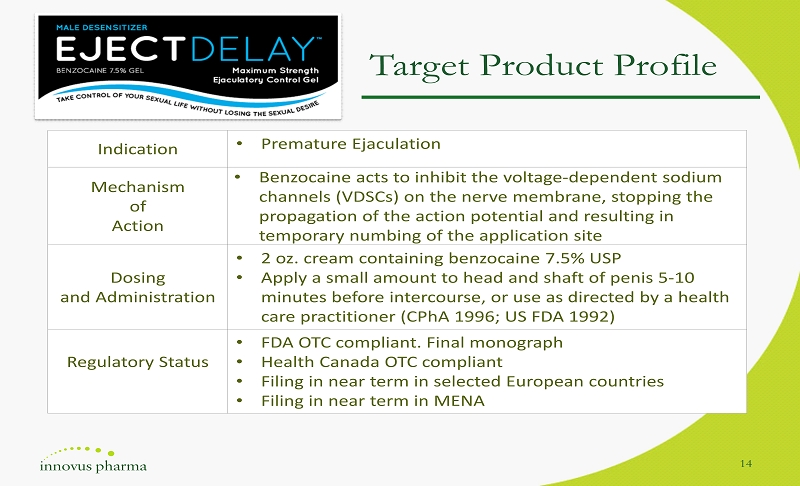
Indication • Premature Ejaculation Mechanism of Action • Benzocaine acts to inhibit the voltage - dependent sodium channels (VDSCs) on the nerve membrane, stopping the propagation of the action potential a nd resulting in temporary numbing of the application site Dosing and Administration • 2 oz. cream containing benzocaine 7.5% USP • Apply a small amount to head and shaft of penis 5 - 10 minutes before intercourse, or use as directed by a health care practitioner ( CPhA 1996; US FDA 1992) Regulatory Status • FDA OTC compliant. Final monograph • Health Canada OTC compliant • Filing in near term in selected European countries • Filing in near term in MENA 14 Target Product Profile
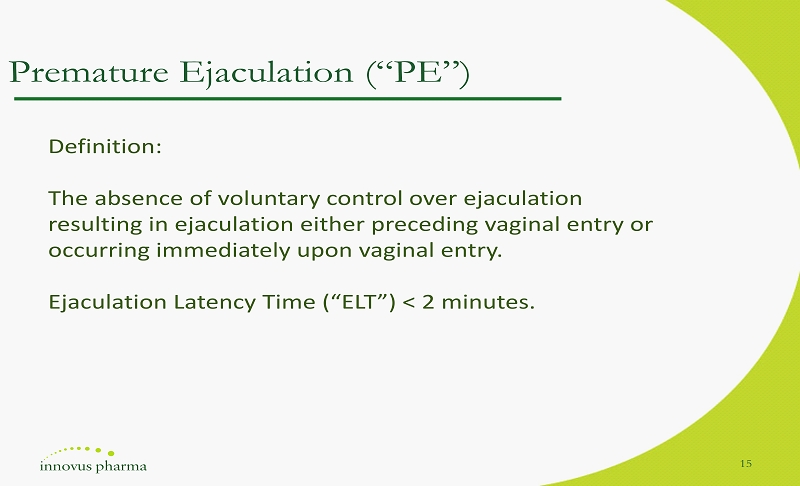
Premature Ejaculation (“PE”) Definition : The absence of voluntary control over ejaculation resulting in ejaculation either preceding vaginal entry or occurring immediately upon vaginal entry . Ejaculation Latency Time (“ELT”) < 2 minutes. 15
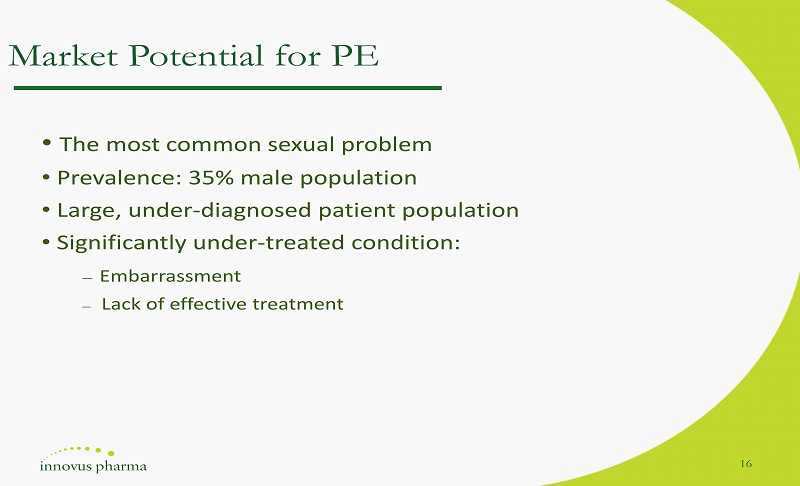
Market Potential for PE • The most common sexual problem • Prevalence: 35% male population • Large, under - diagnosed patient population • Significantly under - treated condition: ― Embarrassment ― Lack of effective treatment 16

Current PE Treatments • ‘ Squeeze’ technique & ‘Stop - Start’ technique ― Disadvantage: hard to follow • Antidepressants targeting psychological factors ― O ff label use ― D isadvantage : various systemic adverse effects • Topical anesthetics ( Lidocaine and Benzocaine) ― Over - the - counter (“OTC”), no need for prescription 17

18 • EjectDelay ™ is a clinically proven non - prescription OTC gel indicated for the treatment of premature ejaculation. • Increases ejaculatory latency time to over 4 minutes as compared to placebo and is applied to the tip of the penis before intercourse. • EjectDelay ™ works by desensitizing the nerves in the penis, allowing a man to improve control of his climax and reducing the urgency to ejaculate without detracting from sexual pleasure. Summary
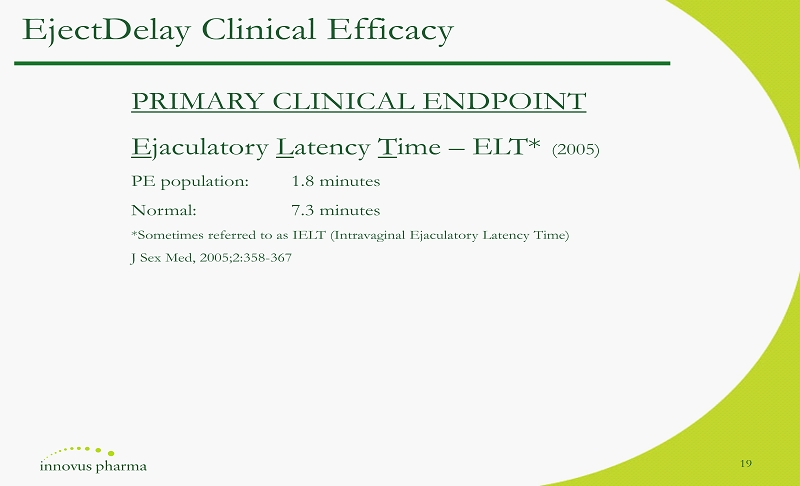
PRIMARY CLINICAL ENDPOINT E jaculatory L atency T ime – ELT* (2005) PE population: 1.8 minutes Normal: 7.3 minutes *Sometimes referred to as IELT ( Intravaginal Ejaculatory Latency Time) J Sex Med, 2005;2:358 - 367 EjectDelay Clinical Efficacy 19
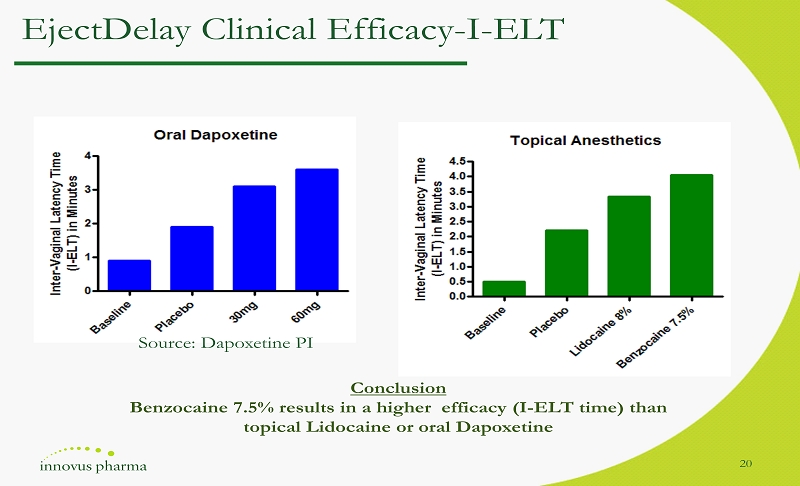
EjectDelay Clinical Efficacy - I - ELT 20 Conclusion Benzocaine 7.5% results in a higher efficacy (I - ELT time) than topical Lidocaine or oral Dapoxetine Source: Dapoxetine PI
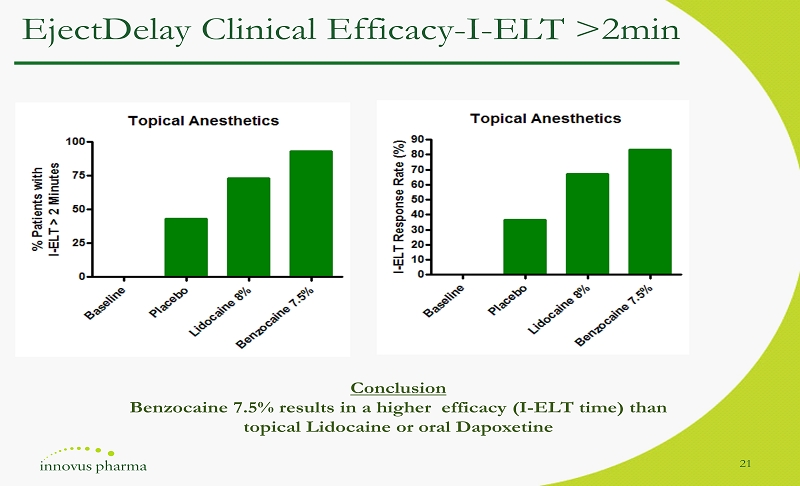
EjectDelay Clinical Efficacy - I - ELT >2min 21 Conclusion Benzocaine 7.5% results in a higher efficacy (I - ELT time) than topical Lidocaine or oral Dapoxetine
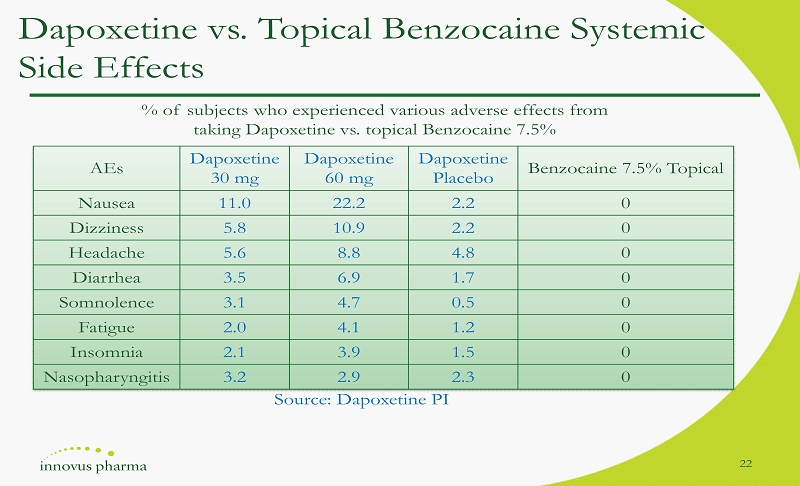
Dapoxetine vs. Topical Benzocaine Systemic Side Effects 22 AEs Dapoxetine 30 mg Dapoxetine 60 mg Dapoxetine Placebo Benzocaine 7.5% Topical Nausea 11.0 22.2 2.2 0 Dizziness 5.8 10.9 2.2 0 Headache 5.6 8.8 4.8 0 Diarrhea 3.5 6.9 1.7 0 Somnolence 3.1 4.7 0.5 0 Fatigue 2.0 4.1 1.2 0 Insomnia 2.1 3.9 1.5 0 Nasopharyngitis 3.2 2.9 2.3 0 % of subjects who experienced various adverse effects from taking Dapoxetine vs. topical Benzocaine 7.5% Source: Dapoxetine PI

Indication • Arthritis pain relief Mechanism of Action • Anti - inflammatory NSAID • Proprietary deep penetrating formulation of Methyl Salicylate 30%, Menthol, Camphor Dosing and Administration • Available in 2 oz. arthritis friendly jars • Apply a small amount of cream no more than 3 - 4 times a day Regulatory Status • FDA OTC compliant. Final monograph • Filing in near term in selected European countries, MENA and Canada Target Product Profile 23

Market Potential for Arthritis • Affects over 21 million people in the US. By 2030, an estimated 67 million Americans aged 18 years or older will have physician - diagnosed arthritis • NSAIDs are the most common treatments used • The estimated U.S. market size for treatment of osteoarthritis is $5+ billion annually 24
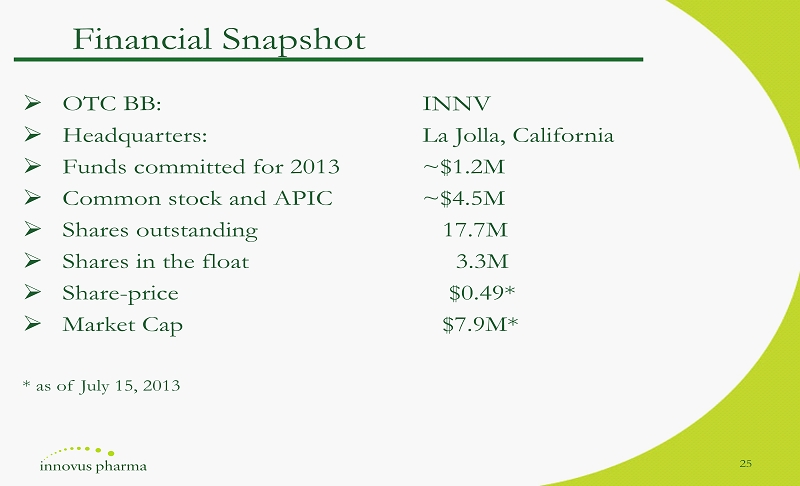
» OTC BB : INNV » Headquarters : La Jolla, California » Funds committed for 2013 ~ $ 1 . 2 M » Common stock and APIC ~ $ 4 . 5 M » Shares outstanding 17 . 7 M » Shares in the float 3 . 3 M » Share - price $ 0 . 49 * » Market Cap $ 7 . 9 M* * as of July 15 , 2013 Financial Snapshot 25

Contact Bassam Damaj, Ph.D. President & CEO or Morgan Brown, MBA, CPA Executive Vice President & CFO +1 858 964 - 5123 bdamaj@innovuspharma.com mbrown@innovuspharma.com 26

























