- EVO Dashboard
- Financials
- Filings
-
Holdings
- Transcripts
- ETFs
-
Insider
- Institutional
- Shorts
-
6-K Filing
Evotec SE (EVO) 6-KCurrent report (foreign)
Filed: 14 May 10, 12:00am
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 6-K
REPORT OF FOREIGN PRIVATE ISSUER
PURSUANT TO RULE 13a-16 OR 15d-16
OF THE SECURITIES EXCHANGE ACT OF 1934
For the periods ended December 31, 2009 and March 31, 2010
Evotec Aktiengesellschaft
(Exact name of registrant as specified in its charter)
Commission File Number: 001-34041
Evotec AG
Schnackenburgallee 114
22525 Hamburg
Germany
(49-40) 56-0810
(Address of Principal Executive Offices)
Indicate by check mark whether the registrant files or will file annual reports under cover Form 20-F or Form 40-F Form 20-F xŸŸŸForm 40-F ¨
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(1): ¨
Note: Regulation S-T Rule 101(b)(1) only permits the submission in paper of a Form 6-K if submitted solely to provide an attached annual report to security holders.
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(7): ¨
Note: Regulation S-T Rule 101(b)(7) only permits the submission in paper of a Form 6-K if submitted to furnish a report or other document that the registrant foreign private issuer must furnish and make public under the laws of the jurisdiction in which the registrant is incorporated, domiciled or legally organized (the registrant’s “home country”), or under the rules of the home country exchange on which the registrant’s securities are traded, as long as the report or other document is not a press release, is not required to be and has not been distributed to the registrant’s security holders, and, if discussing a material event, has already been the subject of a Form 6-K submission or other Commission filing on EDGAR.
Indicate by check mark whether by furnishing the information contained in this Form, the registrant is also thereby furnishing the information to the Commission pursuant to Rule 12g3-2(b) under the Securities Exchange Act of 1934. Yes ¨ No x
If “Yes” is marked, indicate below the file number assigned to the registrant in connection with Rule 12g3-2(b): 82- ¨
Form 6-K
TABLE OF CONTENTS
Item | Page | |
| 3 | ||
| 3 | ||
| 4 | ||
2
On March 25, 2010, EVOTEC AG (Frankfurt Stock Exchange: EVT) (“Evotec”) issued a press release announcing its financial results for the fourth quarter and year ended December 31, 2009 and released its 2009 Annual Report. The press release and the 2009 Annual Report are furnished herewith as Exhibit 99.1 and Exhibit 99.2, respectively, and are incorporated by reference herein.
On May 12, 2010, Evotec issued a press release announcing its financial results for the first quarter ended March 31, 2010 and released its 2010 First Quarter Report. The press release and 2010 First Quarter Report are furnished herewith as Exhibit 99.3 and Exhibit 99.4, respectively, and are incorporated by reference herein.
Pursuant to the requirements of the Securities Exchange Act of 1934, the Registrant has duly caused this Report to be signed on its behalf by the undersigned, thereunto duly authorized.
| Evotec AG | ||
| By: | /s/ Klaus Maleck | |
| Klaus Maleck | ||
| Chief Financial Officer | ||
Date: May 14, 2010
3
Exhibit | Description | |
| 99.1 | Press Release dated March 25, 2010 – Evotec’s 2009 Results: Significant Step towards Sustainability | |
| 99.2 | Evotec AG, Annual Report 2009 | |
| 99.3 | Press Release dated May 12, 2010 – Evotec’s Drug Discovery Business Set for Strong Growth | |
| 99.4 | Evotec AG, First Quarter Report 2010 | |
4
Exhibit 99.1

News release
For further information please contact:
Dr Werner Lanthaler Chief Executive Officer
+49.(0)40.560 81.242 +49.(0)40.560 81.333 Fax werner.lanthaler@evotec.com
Evotec AG Schnackenburgallee 114 22525 Hamburg Germany www.evotec.com | Evotec’s 2009 Results: Significant Step towards Sustainability | |
Hamburg, Germany – 25 March 2010: Evotec AG (Frankfurt Stock Exchange: EVT, TecDAX) today reported financial results and corporate updates for the year ended 31 December 2009.
Major Achievements: | ||
• Growth of business and execution of restructuring programme lead to strong 2009 performance with all financial targets achieved
- Revenues +8%; operating result before exceptional items +57%
- Positive Q4 operating result before exceptional items
- Liquidity position of > € 70 m; exceeding guidance
- Impairment of higher risk programmes reflecting Evotec’s strategy
• Discovery collaborations provide stable basis for future growth
- Several new alliances and contract extensions including Biogen Idec, CHDI (after period-end) and Ono Pharmaceutical
- Strategic capability and capacity expansion through acquisition of Indian RSIPL
• Strong performance in innovative, risk-shared alliances combining high-end technologies and disease know-how
- New alliances with Boehringer Ingelheim, Vifor (after period-end)
- Important milestones demonstrate progress achieved
• Product development alliance with Roche with significant upside but no downside risk; 2011 $ 65 m milestone opportunity
- Successful completion of first-in-man study with EVT 103 (after period-end)
- Positive feedback from the FDA to initiate Phase II with EVT 101 (after period-end)
• Guidance 2010 - accelerated path to profitability and growth
- Revenue growth of at least 15%; strongest February order book ever
- Significantly improved operating result before impairment with profitability no later than 2012
- Liquidity of > € 64 m; cash consumption markedly reduced
1. Operational performance
Growth of business and execution of “Evotec 2012 - Action Plan to Focus and Grow” lead to strong 2009 financial performance
Based on the Action Plan 2012, Evotec decided in March 2009 to streng-then its discovery alliances, creating the central vehicle for growth, and to implement strict cost cutting and restructuring measures. The Company reduced headcount in administrative and clinical development functions, reprioritised its R&D activities, concentrated its operations in Europe and closed the former facility of Renovis, Inc. in South San Francisco. |
Page 1

News Release
These strategic measures are clearly reflected in the financial results for the 2009 fiscal year. Evotec’s discovery alliances are growing significantly and the Company increased and delivered on all financial targets. Total Grouprevenues amounted to EUR 42.7 m, an increase of 8% compared to the same period of the previous year (2008: EUR 39.6 m). The increase is particularly pleasing because, in 2008, Evotec collected three milestones amounting to payments of EUR 8.5 m as part of its strategic alliance with Boehringer Ingelheim, while in 2009 milestone payments from this collaboration added up to EUR 4.0 m. Due to this strong top-line performance and significant reductions in operating expenses (R&D expenses –51%, SG&A cost -16%) Evotec’soperating loss before exceptional items decreased by 57% to EUR 19.6 m (2008: EUR 45.5 m) for the fiscal year and was positive for the fourth quarter amounting to EUR 2.0 m (2008: EUR (10.4) m). The fourth quarter 2009 was the first one in which the impact of Evotec’s strategic restructuring became fully visible. R&D expenses declined by 87% and SG&A cost adjusted for severance payments by 30% compared to the same period of the prior year, a strong basis to develop the Company to profitability.
The Company reduced its R&D cost base significantly through the partner-ing of the EVT 100 compound series to Roche, the discontinuation of EVT 302 due to its failure in smoking cessation, as well as the reduction of early-stage discovery projects. By only supporting the commercially most promising programmes and seeking to aggressively partner those with pharma partners, Evotec de-risked its pipeline substantially. Reflecting this strategy, higher risk programmes were impaired during the year, i.e. EVT 201, EVT 401 and the VR1 programme, leading to total impairment charges of EUR 18.2 m. Including all impairments and EUR 4.8 m of restructuring expenses, Evotec’s 2009operating loss decreased by 42% to EUR 42.3 m (2008: EUR 73.2 m).
With EUR 70.6 m Evotec ended the year 2009 above itsliquidity target of > EUR 65 m.
2. Discovery alliances update
The productivity challenge facing the pharmaceutical industry is set to drive an increase in strategic outsourcing which will likely lead to larger outsourcing contracts favouring bigger partners. Since the industry is highly fragmented there is a leadership gap which Evotec may successfully fill. Due to its scale, attractive growth profile and its strong reputation in the industry Evotec is ideally positioned to take full advantage of these developments. |
Page 2

News Release
Discovery alliances provide stable basis for future growth
Several new alliances and contract extensions
Throughout the year Evotec won new deals with several customers, including Cubist Pharmaceutical, Alios Biopharma and with Biogen Idec. The Company also extended an important collaboration with its strategic partner Ono Pharmaceutical, initiating a new discovery programme on an ion channel target, and a strategic alliance with CHDI (after period-end) to find new treatments for Huntington’s disease. These important contracts clearly demonstrate the value Evotec brings to its alliance partners in the area of drug discovery.
Strategic capability and capacity expansion through acquisition of RSIPL
To best position the Company for growth in the discovery outsourcing market and optimise its cost structure for large alliances, Evotec has initiated an Asian capacity and capability expansion strategy with its purchase, in August 2009, of a 70% controlling stake in the Indian company Research Support International Private Limited (RSIPL). The company was fully integrated as Evotec (India) Private Ltd.
Strong performance in innovative, risk-shared alliances combining high-end technologies and disease know-how
The integration of high-end technologies and disease know-how represents the strategic ‘sweet spot’ in this industry. Evotec has carried on adding to and enhancing its technology platform (e.g. through the addition of the Summit zebrafish screening operations) and therapeutic area focused expertise to become the clear partner of choice for integrated alliances with pharmaceutical companies in which both parties share risk and reward of discovery programmes.
New alliances with Boehringer Ingelheim and Vifor
Based on the successful collaboration to date, Boehringer Ingelheim signed a new, minimum four-year extension of its discovery collaboration with Evotec. Jointly, both companies aim to identify novel therapeutics in innovative disease-focused programmes with a focus on oncology targets. Evotec receives research payments, payments for the achievement of pre-clinical milestones as well as long-term upside through potential clinical milestone achievements and royalties. In January 2010, Evotec also signed a significant integrated alliance with Vifor Pharma to jointly identify a pre-clinical candidate for the treatment of anaemia.
Important milestones demonstrate progress in disease focused alliances
In 2009, the Company achieved two research milestones in its multi-year drug discovery alliance with Boehringer Ingelheim, leading to payments of EUR 4.0 m. Evotec also achieved a milestone with Cardioxyl Pharmaceuticals for the compound, CXL-1020, that was successfully moved into clinical testing in heart failure, and with Ono for the progression of novel protease inhibitors into lead optimisation. |
Page 3

News Release
Product development alliance with Roche with significant upside but no downside risk
In March 2009, Evotec signed a significant alliance with Roche to unleash the commercial upside of the EVT 100 compound family. This is a joint development programme where Evotec is responsible for conducting the clinical Phase II development of EVT 101 in patients with treatment-resistant depression. The costs of that study are fully borne by Roche and so are the development costs of the follow-on molecule EVT 103. Therefore the downside risk for Evotec is zero, while the upside may be significant.
Successful completion of first-in-man study with EVT 103
Early 2010, Evotec completed the clinical part of the first-in-human Phase I study with EVT 103. The compound was safe and very well tolerated after oral single and multiple dose administration, with excellent bioavailability and only a minimal effect of food on the kinetic profile.
Positive feedback from the FDA to initiate Phase II with EVT 101
For EVT 101, the lead compound in the strategic alliance with Roche, Evotec has received approval from the FDA to initiate the Proof-of-Concept study. The study will start recruiting patients in the second quarter of 2010.
If Roche exercises its buy-back option after completion of the Phase II study, Evotec will receive a $65 m payment in exchange for returning the compounds. The total potential value of the deal exceeds $300 m of development and sales performance commercial payments plus potential double-digit commercial payments.
3. Guidance 2010
Revenue growth of at least 15%, significantly improved operating result before impairment with profitability no later than 2012 and liquidity of > EUR 64 m
In 2010, total Group revenues before out-licensing income are expected to grow by at least 15%. These assumptions are based on the strong February 2010 order book of approximately EUR 28 m (2009: EUR 24 m), expected new contracts and contract extensions as well as the achievement of certain milestones.
With the restructuring measures taken in 2009, Evotec has significantly reduced its cost base going forward. The impact of Evotec’s SG&A savings will be fully reflected in the Company’s financial results for the years 2010 and beyond. In addition, Evotec expects research & development (R&D) expenses to decrease considerably from 2009 levels. The Company will focus on key programmes and targets to invest approximately EUR 10 m in R&D in 2010. As a result, Evotec’s Group operating result before impairment is expected to improve significantly over 2009.
The Evotec Group started the year with EUR 71 m of cash, investments and auction rate securities. In 2010, top-line growth and the adjusted cost base are expected to significantly reduce the cash requirements compared to the 2009 fiscal year. Consequently, at constant year-end 2009 currencies, the Company expects to end 2010 with a liquidity of more than EUR 64 m. |
Page 4

News Release
Webcast / Conference Call
Evotec is going to broadcast today’s press & analyst conference in Frankfurt/Main starting at 10.00 am CET (09.00 am GMT/05.00 am EDT) live on the internet. The Management Board of Evotec will inform about the FY 2009 results as well as the status of its discovery alliances and the Company’s development projects. More-over, they will provide an update on the “Evotec 2012 – Action Plan to Focus and Grow” and the business outlook for 2010. The conference will be held in English. To join theaudio webcast and to access thepresentation slides you will find a link on our home pagewww.evotec.com shortly before the event.
For those who prefer to listen to the presentation via phone, please dial:
From Europe: +49.(0)69.2222 7111 (Germany) +44.(0)20.7784 1036 (UK) From the US: +1.718.354 1358 Access Code: 9745589 Presentation: www.equitystory.com Access code: evotec0310
The on-demand version of the webcast will be available on our website:www.evotec.com - Investors - Events - Financial Calendar.
About Evotec AG
Evotec is a leader in the discovery and development of novel small molecule drugs with operational sites in Europe and Asia. The Company has built substantial drug discovery expertise and an industrialised platform that can drive new innovative small molecule compounds into the clinic. In addition, Evotec has built a deep internal knowledge base in the treatment of diseases related to neuroscience, pain, and inflammation. Leveraging these skills and expertise the Company intends to develop best-in-class differentiated therapeutics and deliver superior science-driven discovery alliances with pharmaceutical and biotechnology companies. Evotec has long-term discovery alliances with partners including Boehringer Ingelheim, CHDI, Novartis, Ono Pharmaceutical and Roche. Evotec has product candidates in clinical development and a series of preclinical compounds and development partnerships, including for example a strategic alliance with Roche for the EVT 100 compound family, subtype selective NMDA receptor antagonists for use in treatment-resistant depression. For additional information please go to www.evotec.com.
Forward-Looking Statements
Information set forth in this press release contains forward-looking statements, which involve a number of risks and uncertainties. Such forward-looking statements include, but are not limited to, statements about our financial outlook for 2010 and beyond; our expectation that our current cash, cash equivalents, investments, and operating revenues will be sufficient to fund our planned activities be-yond 2012, and our belief that we are on course to profitability and sustainability by 2012; our expectations regarding the growth of the pharmaceutical outsourcing drug discovery market, the opportunities such growth will provide us, and our ability to take advantage of such market developments and become a leader in this industry |
Page 5

News Release
| in the coming years; our expectations regarding the impacts, and anticipated timing of such impacts, of the “Evotec 2012 – Action Plan to Focus and Grow”; our expectation that our reentry into the German technology TecDAX in 2009 will in-crease liquidity for our shareholders and that our voluntary delisting from NASDAQ and anticipated de-registration with the SEC will streamline our activities and focus the liquidity of Evotec’s stock on one trading platform; our expectations regarding the impact that the recent global financial crisis will have on our company; our expectations and assumptions concerning regulatory, clinical, and business strategies, the progress of our clinical development programmes and timing of the results of our clinical trials, strategic collaborations, and management’s plans, objectives and strategies. These statements are neither promises nor guarantees, but are subject to a variety of risks and uncertainties, many of which are beyond our control, and which could cause actual results to differ materially from those contemplated in these forward-looking statements. In particular, the risks and uncertainties include, among other things: risks that we may be unable to achieve the anticipated benefits of our revised business focus, restructuring, and cost containment measures or recognise the results of such measures within the expected time-frames; risks that we will not achieve the anticipated benefits of our collaborations, partnerships and acquisitions in the timeframes expected, or at all; the risk that we will not achieve the anticipated benefits of our voluntarily delisting from NASDAQ and anticipated de-registration from the SEC; risks that product candidates may fail in the clinic or may not be successfully marketed or manufactured; risks relating to our ability to advance the development of product candidates currently in the pipe-line or in clinical trials; our inability to further identify, develop and achieve commercial success for new products and technologies; the risk that competing products may be more successful; our inability to interest potential partners in our technologies and products; our inability to achieve commercial success for our products and technologies; our inability to protect our intellectual property and the cost of enforcing or defending our intellectual property rights; our failure to comply with regulations relating to our products and product candidates, including FDA requirements; the risk that the FDA may interpret the results of our studies differently than we have; the risk that clinical trials may not result in marketable products; the risk that we may be unable to successfully secure regulatory approval of and market our drug candidates; risks of new, changing and competitive technologies and regulations in the U.S. and internationally; general worldwide economic conditions and related uncertainties; future legislative, regulatory, or tax changes as well as other economic, business and/or competitive factors; and the effect of exchange rate fluctuations on our international operations. The list of risks above is not exhaustive. Our Annual Report on Form 20-F most recently filed with the Securities and Exchange Commission, and other filings and items furnished with the Securities and Exchange Commission, contain additional factors that could impact our businesses and financial performance. We expressly disclaim any obligation or undertaking to release publicly any updates or revisions to any such statements to reflect any change in our expectations or any change in events, conditions or circumstances on which any such statement is based. |
Page 6
Exhibit 99.2

evotec ‘RESEARCH NEVER STOPS’
The world in which we operate
The change in the outsourcing world for innovation in drug discovery
see page 04
Leading the field Growing our drug discovery business and entering the path to sustainability
see page 02
2009 results and outlook in
all details The way we work and our corporate governance
see page 19
ANNUAL REPORT 2009
Evotec AG, Schnackenburgallee 114, 22525 Hamburg (Germany), www.evotec.com
FOCUS AND GROW
2009 was a year of renewal for Evotec
What has altered? And what stays forever
PROMISE
and Deliver
Evotec increased and delivered on all financial targets for the fiscal year 2009. Revenues of € 42.7 m were up 8% on 2008 numbers. R&D and SG&A expenses decreased significantly, by 51% and 16% respectively. With a strong liquidity of € 70.6 m, Evotec started 2010 with a strong financial position.
Evotec reported € 42.7 m in revenues, an increase of 8% compared to the same period of the previous year (2008: € 39.6 m). The revenue performance is the result of continued strong research revenues from Evotec’s discovery alliances, a portion of the upfront payment for the EVT 100 compound family from Roche (€ 3 m) as well as of license and royalty income and milestone payments totalling € 7.2 m from Roche, DeveloGen and Boehringer Ingelheim.
R&D expenses decreased by 51% to € 20.9 m (2008: € 42.5 m). The decrease primarily resulted from the reduction of early discovery programmes, from a focus on fewer, core programmes and from the closure of Evotec’s US site following the implementation of the ‘Evotec 2012 – Action Plan to Focus and Grow’. SG&A expenses decreased by 16% to € 16.7 m (2008: € 20.0 m), primarily as a
consequence of Evotec’s ongoing cost containment measures. The full impact of Evotec’s restructuring on SG&A expenses will be reflected in the financial results for the years 2010 and beyond. continue on page 16
FINANCIAL CALENDAR MEET EVOTEC
25 March 2010 Annual report 2009
12 May 2010 First quarter report 2010
10 June 2010 Annual General Meeting
12 August 2010 Half year report 2010
11 November 2010 Third quarter report 2010

evotec ACTION PLAN
RESEARCH NEVER STOPS
PATH
TO SUSTAINABILITY
The financial results and strategic opportunities of Evotec at the end of the year 2009
The financial results and strategic opportunities of Evotec at the end of the year are supporting the ‘back to the roots’ strategy that the Company adopted in 2009. The Discovery Alliance Business is growing significantly as Evotec continues to optimise its efficiency through technology innovation and global cost leverage. Evotec is on its way to become the leader in high quality drug discovery.
Evotec has de-risked its pipeline significantly by only supporting the commercially most promising programmes. At the same time Evotec has tried to keep the full value potential of its highly promising drug candidates. With this, Evotec has also made a significant step towards sustainability of the Company by substantially growing its revenues and reducing its cost base. With more than € 70 m, the liquidity reserves of the Company are strong, and the tone is set for innovation and growth.
WHAT IS ‘EVOTEC 2012 – ACTION PLAN TO FOCUS AND GROW’?
The 2012 Action Plan was the result of a strategic business review in March 2009. Evotec started the implementation process immediately thereafter in April. The Company evaluated its strengths and weaknesses and made clear decisions regarding its financial resources and strategic direction. The goal was to ensure that all efforts are focused on core differentiated projects and activities capable of delivering the greatest value to stockholders and partners in the near future.
WHAT ARE THE CORE ELEMENTS OF THIS PLAN?
The core element was to strengthen the Discovery Alliance Business to generate the central strategic vehicle for growth. The second goal was to build strategic alliances on selected development projects and to refocus the pipeline on the most valuable assets in order to de-risk the portfolio and reduce R&D cash burn, but without giving away certain significant upsides for shareholders. The third goal was to significantly reduce operating expenses and minimise strategic business risks.
WHAT ARE THE VISIBLE ACHIEVEMENTS OF THIS PLAN TO DATE?
The Alliance Business is working successfully and is about to establish a world class leading position in the discovery outsourcing market. Evotec has excellent collaborations and has throughout 2009 won new deals and reached milestones with e.g. Boehringer Ingelheim, Biogen Idec, Cardioxyl, Ono, Novartis, Roche and others.
CONTENT
Editorial P. 06 Corporate Governance P. 19 Supervisory Board and Management Board P. 86
Financial Calendar P. 01 Outlook P. 49 Supervisory Board Report P. 91
Management Report P. 25 Consolidated Financial Statements P. 51 Imprint P. 03
EVOTEC – ANNUAL REPORT 2009
ACTION PLAN 3
Condensed Key Figures
Evotec AG (IFRS)
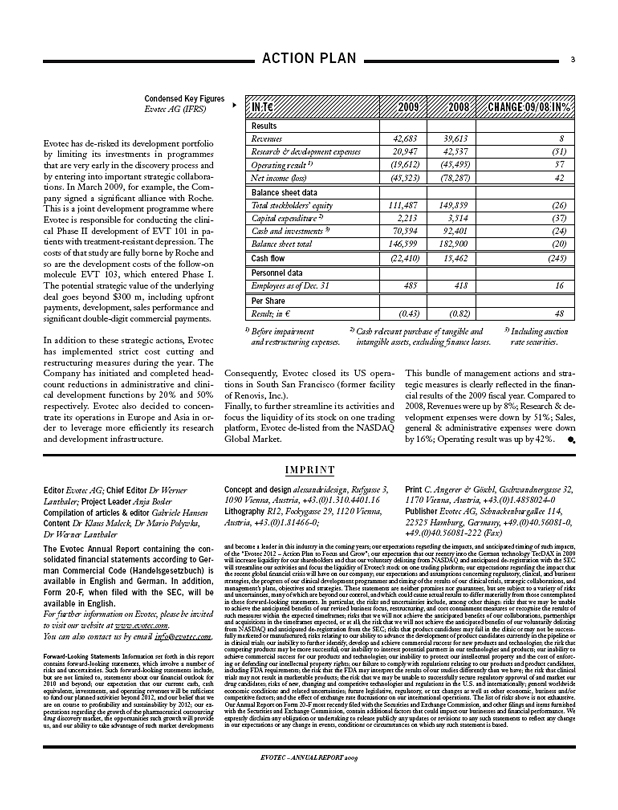
Evotec has de-risked its development portfolio by limiting its investments in programmes that are very early in the discovery process and by entering into important strategic collaborations. In March 2009, for example, the Company signed a significant alliance with Roche. This is a joint development programme where Evotec is responsible for conducting the clinical Phase II development of EVT 101 in patients with treatment-resistant depression. The costs of that study are fully borne by Roche and so are the development costs of the follow-on molecule EVT 103, which entered Phase I. The potential strategic value of the underlying deal goes beyond $300 m, including upfront payments, development, sales performance and significant double-digit commercial payments.
In addition to these strategic actions, Evotec has implemented strict cost cutting and restructuring measures during the year. The Company has initiated and completed head-count reductions in administrative and clinical development functions by 20% and 50% respectively. Evotec also decided to concentrate its operations in Europe and Asia in order to leverage more efficiently its research and development infrastructure.
IN T€ 2009 2008 CHANGE 09/08 IN%
Results
Revenues 42,683 39,613 8
Research & development expenses 20,947 42,537 (51)
Operating result 1) (19,612) (45,495) 57
Net income (loss) (45,523) (78,287) 42
Balance sheet data
Total stockholders’ equity 111,487 149,859 (26)
Capital expenditure 2) 2,213 3,514 (37)
Cash and investments 3) 70,594 92,401 (24)
Balance sheet total 146,599 182,900 (20)
Cash flow (22,410) 15,462 (245)
Personnel data
Employees as of Dec. 31 485 418 16
Per Share
Result; in € (0.43) (0.82) 48
1) Before impairment and restructuring expenses. 2) Cash relevant purchase of tangible and intangible assets, excluding finance leases. 3) Including auction rate securities.
Consequently, Evotec closed its US operation in South San Francisco (former facility of Renovis, Inc.).
Finally, to further streamline its activities and focus the liquidity of its stock on one trading platform, Evotec de-listed from the NASDAQ Global Market.
This bundle of management actions and strategic measures is clearly reflected in the financial results of the 2009 fiscal year. Compared to 2008, Revenues were up by 8%; Research & development expenses were down by 51%; Sales, general & administrative expenses were down by 16%; Operating result was up by 42%.
IMPRINT
Editor Evotec AG; Chief Editor Dr Werner
Lanthaler; Project Leader Anja Bosler
Compilation of articles & editor Gabriele Hansen
Content Dr Klaus Maleck, Dr Mario Polywka, Dr Werner Lanthaler
The Evotec Annual Report containing the consolidated financial statements according to German Commercial Code (Handelsgesetzbuch) is available in English and German. In addition, Form 20-F, when filed with the SEC, will be available in English.
For further information on Evotec, please be invited to visit our website at www.evotec.com.
You can also contact us by email info@evotec.com.
Concept and design alessandridesign, Rufgasse 3, 1090 Vienna, Austria, +43.(0)1.310.4401.16
Lithography R12, Fockygasse 29, 1120 Vienna, Austria, +43.(0)1.81466-0;
Print C. Angerer & Göschl, Gschwandnergasse 32, 1170 Vienna, Austria, +43.(0)1.4858024-0
Publisher Evotec AG, Schnackenburgallee 114, 22525 Hamburg, Germany, +49.(0)40.56081-0, +49.(0)40.56081-222 (Fax)
Forward-Looking Statements Information set forth in this report contains forward-looking statements, which involve a number of risks and uncertainties. Such forward-looking statements include, but are not limited to, statements about our financial outlook for 2010 and beyond; our expectation that our current cash, cash equivalents, investments, and operating revenues will be sufficient to fund our planned activities beyond 2012, and our belief that we are on course to profitability and sustainability by 2012; our expectations regarding the growth of the pharmaceutical outsourcing drug discovery market, the opportunities such growth will provide us, and our ability to take advantage of such market developments and become a leader in this industry in the coming years; our expectations regarding the impacts, and anticipated timing of such impacts, of the “Evotec 2012 – Action Plan to Focus and Grow”; our expectation that our reentry into the German technology TecDAX in 2009 will increase liquidity for our shareholders and that our voluntary delisting from NASDAQ and anticipated de-registration with the SEC will streamline our activities and focus the liquidity of Evotec’s stock on one trading platform; our expectations regarding the impact that the recent global financial crisis will have on our company; our expectations and assumptions concerning regulatory, clinical, and business strategies, the progress of our clinical development programmes and timing of the results of our clinical trials, strategic collaborations, and management’s plans, objectives and strategies. These statements are neither promises nor guarantees, but are subject to a variety of risks and uncertainties, many of which are beyond our control, and which could cause actual results to differ materially from those contemplated in these forward-looking statements. In particular, the risks and uncertainties include, among other things: risks that we may be unable to achieve the anticipated benefits of our revised business focus, restructuring, and cost containment measures or recognise the results of such measures within the expected timeframes; risks that we will not achieve the anticipated benefits of our collaborations, partnerships and acquisitions in the timeframes expected, or at all; the risk that we will not achieve the anticipated benefits of our voluntarily delisting from NASDAQ and anticipated de-registration from the SEC; risks that product candidates may fail in the clinic or may not be successfully marketed or manufactured; risks relating to our ability to advance the development of product candidates currently in the pipeline or in clinical trials; our inability to further identify, develop and achieve commercial success for new products and technologies; the risk that competing products may be more successful; our inability to interest potential partners in our technologies and products; our inability to achieve commercial success for our products and technologies; our inability to protect our intellectual property and the cost of enforcing or defending our intellectual property rights; our failure to comply with regulations relating to our products and product candidates, including FDA requirements; the risk that the FDA may interpret the results of our studies differently than we have; the risk that clinical trials may not result in marketable products; the risk that we may be unable to successfully secure regulatory approval of and market our drug candidates; risks of new, changing and competitive technologies and regulations in the U.S. and internationally; general worldwide economic conditions and related uncertainties; future legislative, regulatory, or tax changes as well as other economic, business and/or competitive factors; and the effect of exchange rate fluctuations on our international operations. The list of risks above is not exhaustive. Our Annual Report on Form 20-F most recently filed with the Securities and Exchange Commission, and other filings and items furnished with the Securities and Exchange Commission, contain additional factors that could impact our businesses and financial performance. We expressly disclaim any obligation or undertaking to release publicly any updates or revisions to any such statements to reflect any change in our expectations or any change in events, conditions or circumstances on which any such statement is based.
EVOTEC – ANNUAL REPORT 2009

evotec MARKET SPACE
THE WORLD IN WHICH WE OPERATE
The drug discovery industry poised for growth
The global pharmaceutical industry is facing a significant productivity challenge. R&D costs have escalated over the years, yet product pipelines are nowhere near producing the returns experienced in earlier decades. Against this industry backdrop, biotech and pharma companies will increasingly turn to outsourcing R&D activities as they are forced to seek greater cost savings and improvements in efficiency. The use of Contract Research Organisations (CROs) allows fixed costs to be converted into variable costs and also provides expertise in selected areas without the client needing to maintain or build internal capabilities and infrastructure. This market we operate in is worth about $ 8bn.
INCREASED OUTSOURCING OF UPSTREAM ACTIVITIES
Outsourcing has been used by the pharmaceutical industry for more than twenty years, mainly for supporting clinical trials or regulatory affairs in a particular country or region. In the current environment, however, we expect companies to increase their outsourcing of upstream activities in the R&D process. For example, Eli Lilly outsources 100% of its pre-clinical GLP work, whereas Merck and Wyeth only outsource 5%. These latter figures may change dramatically during post-merger strategic realignments. Another major driver for business growth in the drug discovery industry will come from mid-size pharma companies and larger biotech companies, who also seek improvements in R&D efficiency.
OUTSOURCING CAN RESOLVE DRUG DISCOVERY BOTTLENECKS
While innovative scientific technologies and approaches are a rich source for the identification of novel drugs, they have and will continue to make drug discovery more complex, dramatically increasing the number of drug targets and active compounds prosecuted. Many companies do not always have sufficient numbers of experienced scientists in-house to advance all of their required programmes and biotechnology companies often do not have the technical staff or technology to process their targets internally. Here is where discovery out-sourcing can help make the drug discovery and development process more efficient.
‘Trying to do everything yourself won’t work. The odds of owning everything and being at the right place at the right time are not high.’
Peter Johnson, Lilly’s Vice President of Corporate Strategic Planning. Wall Street Journal Online, Jan. 2010
DRUG DISCOVERY OUTSOURCING MARKET IS WORTH ABOUT $8BN
The global drug discovery market reached about $5 bn in 2007, an increase of 15% compared to 2006 according to a study from Kalorama Information and this market is expected to experience robust growth and exceed $8 bn in 2010.
TECHNOLOGY LEADERSHIP WILL DRIVE MARKET GROWTH
All stages of drug discovery can be outsourced: target identification, target validation, high-throughput screening (HTS) and lead optimisation. Chemistry and biology are the core scientific disciplines utilised in drug discovery.
Chemistry services represent the largest portion of drug discovery outsourcing accounting for over 40% of all outsourcing revenues in 2007. This market share typically includes preclinical process development and early manufacturing. Other outsourced chemistry activities include the medicinal chemistry required to identify and optimise lead compounds towards clinical candidates. Biology services is the fastest growing segment. New technologies will continue to drive the growth of the biology services market. The biology services segment involves such activities as analysis of biological pathways, target identification and validation, structural analysis of biological targets, protein expression, and assay development. High-throughput screening is an attractive area to outsource as automated HTS systems are expensive to buy and maintain and require specially trained operating personnel.
EVOTEC – ANNUAL REPORT 2009
MARKET SPACE 5
AN OBVIOUS TREND TOWARDS LARGE STRATEGIC ALLIANCES
The productivity challenge facing the pharmaceutical industry is set to drive an increase in strategic outsourcing, which will likely lead to larger outsourcing contracts favouring bigger players, due to lower perceived risk. This will present a challenge for the highly fragmented drug discovery industry, in which the ‘branded’ leaders hold less than 10% market share each.
EVOTEC IS RIGHT IN THE ‘SWEET SPOT’
Evotec is ideally positioned to take full advantage of these market developments, due to its attractive growth and profitability profile, and its strong reputation in the drug discovery industry.
THE PHARMA OUTSOURCING DRUG DISCOVERY MARKET
will grow significantly
Evotec has initiated an Asian capacity and capability expansion strategy with its purchase, in August 2009, of a 70% controlling stake in Research Support International Private Limited (RSIPL), a Mumbai-based company providing organic synthesis services to the global pharma industry.
Evotec will carry on building its technology platform and therapeutic area focused expertise opportunistically, as the integration of technologies and disease know-how represents the strategic ‘sweet spot’ in this industry.
KEY REASONS
for growth
—Pipeline needs of Pharma will keep R&D expenditures at least at current levels, but with a strong demand for increased productivity
—Outsourcing and joint innovation teams lead to higher efficiency in the drug discovery process
—Top-class scientific technology offerings will again become high growth businesses as fixed costs become variable
—Only a few players have built up scientific quality for sustainable innovation alliances
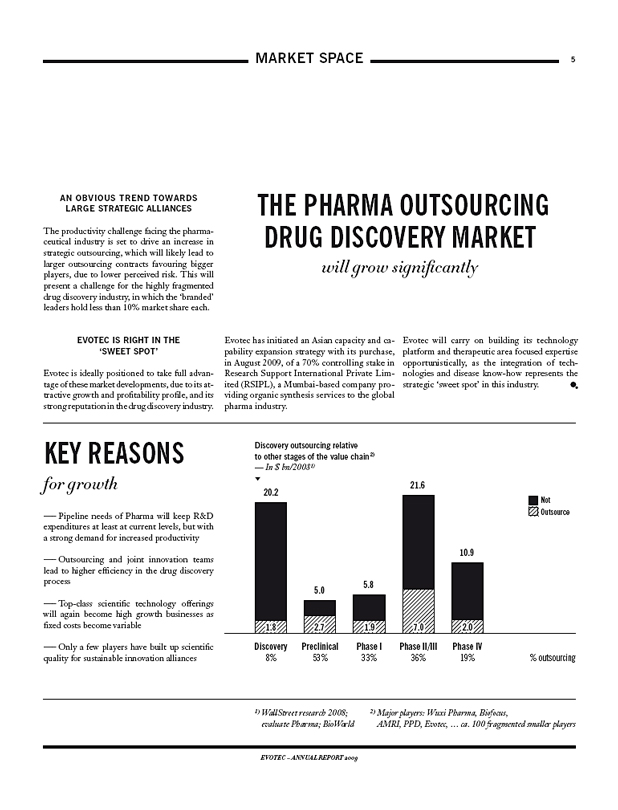
Discovery outsourcing relative to other stages of the value chain2)
— In $ bn/20081)
20.2 5.0 5.8 21.6 10.9
Not Outsource
1.8 2.7 1.9 7.0 2.0
Discovery 8% Preclinical 53% Phase I 33% Phase II/III 36% Phase IV 19% % outsourcing
1) WallStreet research 2008; evaluate Pharma; BioWorld 2) Major players: Wuxi Pharma, Biofocus, AMRI, PPD, Evotec, … ca. 100 fragmented smaller players
EVOTEC – ANNUAL REPORT 2009

evotec EDITORIAL
Dr Werner Lanthaler
in person
If you don’t hear songs like “Nothing else matters …”, probably Werner is on the road!
Dr Werner Lanthaler – he is the Chief Executive Officer of Evotec since March 2009.
From 1995 to 1998 he was Senior Management Consultant at the consulting firm McKinsey & Company. Prior to Evotec he was Chief Financial Officer at Intercell AG for more than eight years. Dr Lanthaler played a pivotal role in many of Intercell’s major corporate milestones including the market approval of the company’s first product and several strategic alliances.
As Chief Executive Officer at Evotec he is responsible for the entire operating business as well as the areas strategy, marketing, communication and investor relations. It is the vision to build a highly innovative biotech company that will make a true difference to help in the fight against diseases with high medical need that motivated Werner to accept this challenging management task. The key element for proper team functioning is communication. Werner’s management style is to communicate and interact as much as possible within the Company and also with partners, shareholders and clients.
Dr Lanthaler holds a doctorate in economics from Vienna University, earned his Master’s degree from Harvard University and holds a degree in Psychology.
DEAR
SHARE– HOLDERS
AND FRIENDS,
First let me thank you for a year of trust, support and also for a year in which Evotec has regained momentum.
YES WE DISCOVER!
In a world where many things change, we know one thing for sure: There is an urgent need to develop new drugs which will increase dramatically in the next few decades. We, at Evotec, are dedicated to build a world-leading pool of innovative technologies and drug discovery and development processes to achieve this goal more efficiently and effectively. This vision is bringing the best scientists and the best pharma and biotech partners together in order to deliver on the promises of discovering better drugs faster. With our strategic programme ‘Evotec 2012 - Action Plan to Focus and Grow’ we have defined and implemented the key strength to truly lead this industry in the next years. 2009 was certainly a year for us in which many things had to be revisited. The first indicators show us that this strategy is already delivering its first successes.
YES WE GROW!
The drug discovery outsourcing market is still immature and the industry is poised for growth. Since it is highly fragmented, there is a leadership gap which we believe Evotec can successfully fill. Before joining Evotec, there was the strategic idea to strengthen the Company’s excellent position and become the driving force behind this growth industry and its consolidation. This also represents an attractive investment case. We still have a lot to do to make this case clearer and better visible to our shareholders, but we will let data and results speak a very clear language to support our growth vision.
The business plan goal is to grow Evotec into a global, sizeable, sustainable, highly innovative and profitable company within 3 - 5 years, with double-digit annual revenue growth.
We see the entrepreneurial chance to make this happen and look forward to a successful 2010 and beyond.
Yours sincerely,
Werner Lanthaler
EVOTEC – ANNUAL REPORT 2009

INDIA 7
WELCOME TO INDIA
Evotec has added Asian capacity in order to optimise its cost structure for large discovery alliances.
Evotec acquired a 70% controlling majority stake in Research Support International Private Limited (RSIPL), a Mumbai-based company providing organic synthesis services to the global pharmaceutical industry, for € 2.4 m in cash. Evotec took full control of the operational business and has a call option for the remaining 30% in the future.
With the RSIPL acquisition, Evotec gained excellent people and facilities and increased its ability to leverage its strategic drug discovery offering: More than 160 highly qualified scientists and seven synthesis laboratories, one analytical laboratory and the existing library synthesis business of the existing joint-venture with DIL have been added to the impressive capabilities of Evotec. These strategic technology and capacity additions further validate Evotec’s goal to become the number one global provider of drug discovery and early development solutions. The aim is to leverage the Company’s global offering with cost efficient scale and enable Evotec to attract large chemistry-based contract work, which is more frequently being outsourced by large pharmaceutical companies.
‘With this acquisition we reinforced our strategic commitment to deliver the highest value, know-how driven services and build the strongest innovation alliances with our customers.’
Dr Mario Polywka, Chief Operating Officer at Evotec
ABOUT RSIPL
Incorporated in 2004, Research Support International Private Limited (RSIPL) was a 100% subsidiary of DIL Ltd (formerly Duphar-Inter-fran Ltd.) providing drug discovery & development services. RSIPL uses its world class infrastructure and facilities to synthesise virtually all types of organic compounds from milligramme to kilogramme scale. The company’s offering includes custom synthesis, process research and development, scale-up and analytical services. RSIPL was conferred the ‘Partner of Choice in
Contract Research - Chemistry Based Services’ award for the year 2007 by Frost & Sullivan in recognition of its experience in offering an organic synthesis services to pharmaceutical and biotech customers worldwide.
INVEST IN ADDITIONAL TECHNOLOGIES – NEW STEPS TO MAKE DRUG DISCOVERY
MORE EFFECTIVE
Evotec anticipates adding to and enhancing its drug discovery offering considerably. As an example, ion channel focused libraries and an electrophysiology screening platform were added in 2009. Evotec signed an agreement with DiscoveRx to access their proprietary technologies (PathHunter™, cAMPHunter™ cell lines and EFC chemilumincescent detection technology). The combination of all these technologies with Evotec’s proprietary technologies and know-how in high-throughput screening means that Evotec can strategically offer unrivalled screening solutions within the drug discovery process for GPCRs, ion channels and other important target classes.
EVOTEC – ANNUAL REPORT 2009

evotec RETROSPECTIVE
SPRING
Highlights
—Strategic business review results in ‘Evotec 2012 – Action Plan to Focus and Grow’
—New alliance with Roche to develop the EVT 100 compound family in treatment-resistant depression
—Successful completion of first Phase I study with EVT 401
—Acquisition of zebrafish screening operations
Lowlights
—Failure of EVT 302 in smoking cessation
—Failure of 2008/2009 partnering process of Evotec’s insomnia drug EVT 201
In March 2009, Evotec appointed Werner Lanthaler as new Chief Executive Officer. In addition Evotec and Roche entered into an agreement for the clinical development of EVT 101 in patients with treatment-resistant depression. This alliance covers the possible development of the entire EVT 100 compound family (NR2B-selective NMDA antagonists) with a total potential deal value exceeding $300 m.
To bring the Company back onto a growth path and to sustainability, the Management Team developed and implemented the ‘Evotec 2012 - Action Plan to Focus and Grow’. The cornerstone of this plan was to re-focus the Company on its key strength, that of its Discovery Alliance Business. In order to enhance the value of its drug discovery offering, Evotec acquired a zebrafish screening operation in the UK and Singapore, expanded its ion channel capabilities, and further added to its electrophysiology screening platform. One unfortunate consequence of this plan was headcount reductions in SG&A and clinical development.
Within its proprietary clinical development programmes, Evotec had to accept the clinical failure of EVT 302, a selective MAO-B inhibitor, in smoking cessation.
In a Phase II quit rate study, the drug candidate failed to demonstrate a significant improvement compared to placebo. Consequently, this programme was terminated. The first Phase I study with 401, an oral P2X7 receptor antagonist, showed a good safety profile. It represents a potentially novel approach to orally treat inflammatory conditions. In partnership with Pfizer, the first small molecule vanilloid receptor 1 (VR1) antagonist that was in Phase I testing to treat pain did not yet meet the optimally required target profile. As a result, Pfizer decided to focus on the development of follow-on antagonists.
A YEAR
SUMMER
Highlights
—New alliances with Cubist, Alios and Biogen Idec
—Milestone payment from Boehringer Ingelheim
—Acquisition of Indian RSIPL (Evotec India)
—Start of Phase I with EVT 103
Lowlights
—Closure of the former facility of Renovis, Inc. in South San Francisco
At the beginning of summer, Evotec signed a number of new alliances: A research agreement on fragment-based drug discovery with Cubist Pharmaceuticals, a high-throughput screening collaboration with Alios Biopharma and a research agreement with Biogen Idec in compound screening. Moreover, a further milestone was successfully achieved in the multi-year drug discovery collaboration with Boehringer Ingelheim.
In August, Evotec acquired a 70% controlling, majority stake of Research Support International Private Limited (RSIPL), India, for €2.4 m to expand capacity and accelerate Evotec’s strategy to become the global, premier partner for drug discovery and early development alliances. On the clinical side, Evotec started a Phase I study with EVT 103, the next generation NR2B-selective NMDA receptor antagonist following EVT 101, planned to enter clinical development in treatment-resistant depression in collaboration with Roche.
The execution of the restructuring programme ‘Evotec 2012 – Action Plan to Focus and Grow’ showed first positive results which are reflected in the financial results for the second quarter. In May, as a further consequence of implementing ‘Evotec 2012 – Action Plan to Focus and Grow’, the Company announced the re-engineering of its research & development operations to more effectively leverage the Discovery Alliance Business. This led to the closure of its South San Francisco facility (ex Renovis) and the concentration of Evotec’s research in Europe.
EVOTEC – ANNUAL REPORT 2009

RETROSPECTIVE 9
FALL
Highlights
— Extension of collaborations with Ono Pharmaceutical and Boehringer Ingelheim
— Compound from Evotec-Cardioxyl collaboration enters the clinic
— Milestone payment from Boehringer Ingelheim
— Evotec shares return to the German technology index TecDAX
Lowlights
— Delay of EVT 101 Phase II start due to FDA safety and toxicology monitoring requests
In October, Evotec announced two development milestones from two important partnerships. One milestone was achieved with Cardioxyl Pharmaceuticals under a collaboration agreement for the compound, CXL-1020, that was successfully moved into clinical testing in heart failure.
The second milestone was achieved with Boehringer Ingelheim and led to a payment of € 2.5 m for the selection of a candidate compound for pre-development studies in a cardiometabolic programme.
Two important contract extensions were also announced in October. Evotec extended the drug discovery collaboration with its strategic partner Ono Pharmaceutical to include an ion channel target. Certainly one of the most important business achievements in 2009 was the extension of the strategic research alliance with Boehringer Ingelheim for at least four
more years with € 15 m in research funding plus milestones and royalties. This collaboration was expanded specifically to build a joint innovation task force in oncology.
Furthermore, Evotec signed an agreement with DiscoveRx to access to their proprietary technologies (PathHunterTM, cAMPHunterTM cell lines and EFC chemiluminescent detection technology).
Finally, Evotec voluntarily de-listed from the NASDAQ and re-entered the German technology index TecDAX. This an important step to regain more visibility for the Company also in the capital markets.
IN
RETROSPECTIVE
WINTER
Highlights
— Milestone payment from Ono Pharmaceutical
— Extension of strategic alliance with CHDI
— Major cooperation agreement with Vifor
— Clearance of FDA for Phase II start with EVT 101
In December 2009, Evotec received a milestone payment from Ono Pharmaceutical for the progression of novel protease inhibitors into lead optimisation. Another important event in the winter was the success of securing a German government research grant of up to € 2.5 m from the BMBF within the Neu2 consortium. The purpose of this grant is to advance research and development activities on the target serine racemase for potential use in neuroprotection.
In January 2010, Evotec announced the extension of a strategic alliance with CHDI, a non-profit organisation. The collaboration is aimed at finding new treatments for
Huntington’s disease and represents one of the largest joint innovation drug discovery alliances within Evotec. It will provide Evotec with up to $ 37.5 m in research funding over the next three years.
Furthermore in January, Evotec signed an agreement with Vifor Pharma. Evotec provides integrated biology, chemistry and preclinical development activities to identify a preclinical candidate for the treatment of anaemia. The collaboration is worth about € 5.5 m.
EVOTEC – ANNUAL REPORT 2009

evotec
PARTNER
UNITE +
Evotec is building integrated alliances with a technology and therapeutic area focus.
CONQUER
We see Evotec as the architect of innovation teams in large alliances designed to develop new drugs.
Both parties in an alliance share innovation, know-how and technology to bring forward best-in-class drugs in areas of highest unmet medical needs. The need for new drugs is urgent in many diseases, getting results faster in today’s very challenging product development environment is Evotec’s mission.
Evotec provides expertise along the full value chain of drug discovery and development. Specifically, Evotec has combined therapeutic area know-how in neurology, pain, inflammation, metabolic diseases and oncology with its world leading drug discovery platform. This, we believe, forms successful innovation teams.
With creative deal structures, but especially with the best and most motivated people, Evotec is confident to deliver on its strategy to be the partner of choice for highly complex and innovative drug discovery franchises. Evotec today is already one of the most innovative outsourcing partners globally, but it is the Company’s clear goal to grow even more aggressively.
CONVINCING TECHNOLOGY, OUTSTANDING QUALITY
and scientific expertise
biogen idec
In 2009, Biogen Idec chose Evotec as a partner to provide assay development and high-throughput screening services against a particular target. Evotec uses its expertise and technologies in protein production, assay development and high-throughput screening to identify hit molecules for Biogen Idec. Further targets may be added to the collaboration as agreed.
A LONG-TERM ALLIANCE
based on success
‘Evotec has continually demonstrated exceptional scientific expertise in support of our research.’
Dr Wolfgang Rettig, Corporate Senior Vice President Research of Boehringer Ingelheim, 2009
Boehringer Ingelheim
To date seven significant milestones have been achieved as part of this long-term agreement. Based on its success, in 2009 this strategic collaboration was extended for a minimum of four more years and has been expanded to specifically include oncology targets.
Evotec and Boehringer Ingelheim started working together in September 2004, initially concentrating their joint research on identifying and developing small molecule therapeutics acting on selected G-Protein Coupled Receptors (GPCRs) with a focus on Central Nervous System diseases. The agreement includes a broad range of services from drug discovery to delivering drug candidates. Boehringer Ingelheim has the global responsibility for all clinical development activities, manufacturing and commercialisation of the compounds identified in the collaboration. Evotec’s success is being rewarded through research payments, preclinical and clinical development related payments and product royalties. In January 2006, the collaboration doubled in size and was extended to the end of 2008. In early 2008, the collaboration was extended for a further twelve months until the end of 2009, when it was extended for at least another four years.
EVOTEC – ANNUAL REPORT 2009

PARTNER 11
Here is just a set of examples of how Evotec is contributing to success in drug discovery partnerships
evotec + Novartis evotec + Boehringer Ingelheim evotec + ONO PHARMACEUTICAL CO., LTD.
evotec + INTERMUNE® evotec + Pfizer evotec + Cardioxyl PHARMACEUTICALS
evotec + Vifor Pharma evotec + Roche evotec + CHDI FOUNDATION, INC.
evotec + spermatech Male contraceptives evotec + biogen idec
WHERE
the sun rises
Ono Pharmaceutical extended an ongoing research collaboration with Evotec and initiated a new discovery collaboration in 2009. This partnership is a good example of Evotec’s ability to grow new relationships into larger alliances based on success. The drug discovery agreement with Ono is now an important part of the portfolios of both companies.
The initial collaboration was signed in early 2008. It applied, amongst other things, Evotec’s proprietary EVOlutionTM platform for fragment-based drug discovery to identify novel and potent compounds against a protease target provided by Ono. The
project has advanced into lead optimisation. Evotec received an upfront payment for access to this industry leading platform, ongoing research funding as well as milestones based on the research progress. Under the new collaboration, Evotec provides integrated drug discovery activities, specifically Evotec’s ion channel drug discovery platform and expertise to discover novel, small molecular weight compounds active against an ion channel target selected by Ono. Evotec will receive research funding and milestone payments.
‘We have a high regard for Evotec’s drug discovery expertise and the capabilities and technologies used in progressing this collaboration.’
Kazuhito Kawabata, Ph.D., Ono, 2009
ONO PHARMACEUTICAL CO., LTD.
THE GENE IS ALREADY
well-known
CHDI FOUNDATION, INC.
In early 2010, Evotec and CHDI Foundation, Inc. extended their collaboration, which was initially signed in 2006, for a further three years to find new treatments for Huntington’s disease. Evotec provides a full range of neurological research activities and expertise to CHDI, including integrated biology and chemistry supported by compound and library management, target validation, screening, computational chemistry, in vitro and in vivo pharmacokinetics and protein crystallography. For this support, Evotec will receive research revenue of up to $ 37.5 m. CHDI is a non-profit organisation with a mission to rapidly discover and develop drugs that delay or slow the progression of Huntington’s disease.
Huntington’s disease is a familial disease caused by a mutation in the huntingtin gene. As a result of carrying the mutation, an individual’s brain cells fail and die leading to cognitive and physical impairments that, over the course of the disease, significantly impair the individual’s quality of life and ultimately cause death. There is currently no way to delay the onset of symptoms or slow the progression of Huntington’s disease.
NEW HOPE FOR
heart failure patients
Cardioxyl PHARMACEUTICALS
Cardioxyl Pharmaceuticals has successfully moved the compound CXL-1020 into clinical testing. CXL-1020 is a novel, proprietary nitroxyl donor currently in development as a potential therapy for acute decompensated heart failure. Evotec has provided medicinal chemistry support to Cardioxyl for over three years. During this time, the compound was developed through lead optimisation and preclinical development, and has now progressed to Phase I/IIa testing in heart failure patients. Meeting this milestone triggered a success payment from Cardioxyl Pharmaceuticals to Evotec.
EVOTEC – ANNUAL REPORT 2009

evotec RESEARCH NEVER STOPS
Dr Mario Polywka
in person
If you hear somebody singing in the corridor,… it is probably Mario!
Dr Mario Polywka – he is the Chief Operating Officer of Evotec since November 2007.
He was one of the co-founders of Oxford Chirality which later became Oxford Asymmetry International (OAI) before it merged with Evotec in 2000. Mario was Chief Executive Officer of OAI during the merger with Evotec. Following the merger he was Chief Operating Officer until 2002. Between 2002 and 2004 he ran a number of spin-out companies in the Oxford area.
As Chief Operating Officer at Evotec, he is responsible for Evotec’s Discovery Alliance Business, managing more than 400 scientists in Abingdon, Hamburg, Mumbai and Singapore. The business collaborates with pharmaceutical and biotech companies in drug discovery. Mario’s secret of a happy and successful workforce is to offer interesting work in a stimulating environment – and this also involves singing and having fun!
Dr Polywka is a Chemist by training, having completed his D. Phil. studies under Professor Steve Davies, at Oxford University, in the field of Mechanistic Organometallic Chemistry, followed by post-doctoral studies on the biosynthesis of Penicillins with Professor Jack Baldwin.
Scientific excellence to fight unmet medical needs
FIN
Evotec has a deep therapeutic knowledge in neurology, pain, inflammation, oncology and metabolic diseases and an exciting pipeline with novel and differentiated compounds in areas of large unmet medical needs. In 2009, Evotec decided to more aggressively create strategic alliances around some of its key assets. With its portfolio of early stage development opportunities, Evotec intends to attract strategic partnerships to progress the development of these assets. The idea is to both de-risk the portfolio, and unleash the commercial upside of its compounds.
RAY OF HOPE FOR DEPRESSION
— EVT 100
One of the most important highlights of the year 2009 was the alliance with Roche in treatment-resistant depression. The agreement covers the development of the EVT 100 compound family (NR2B-selective NMDA antagonists) with a total potential deal value exceeding $ 300 m. The compounds were originally discovered by Roche and licensed to Evotec in 2003 for further development.
Roche has entered into this agreement with Evotec for Phase II clinical development of EVT 101, the most advanced compound, in patients with treatment-resistant depression. Evotec is responsible for conducting a Phase II proof-of-concept study for EVT 101 and Phase I safety and tolerability studies for the follow-on compound EVT 103.
The entire development programme will be funded by Roche. In addition, for the option to buy back rights to the EVT 100 compound family, Roche has paid Evotec an upfront fee of $ 10 m. If Roche exercises its buy-back option after the completion of the Phase II study, Evotec will receive a $ 65 m lump-sum payment from Roche in exchange for returning the compounds. In that case, Evotec is also eligible for further development and sales performance, and scalable double-digit commercial payments.
Preparation of the Phase II study for EVT 101 are ongoing. Evotec expects the study to start in the first half of 2010. The study results are expected in 2011.
The first-in-man Phase I study with EVT 103 started in September. This study was a double-blind, placebo-controlled, randomised ascending dose study in healthy young male subjects that assessed the compound’s safety, tolerability and pharma-cokinetic profile after oral single and multiple dose administration.
In Q1 2010 Evotec announced the successful completion of the clinical part of this study.
‘We believe that EVT 101 has the potential to become an effective new therapy for the high unmet need of these patients.’
Eugene Tierney, Head of CNS at Roche, 2009
Roche
EVOTEC – ANNUAL REPORT 2009

RESEARCH NEVER STOPS 13
DING THE BALANCE
‘We have a solid preclinical pipeline. It’s relatively early but it’s worth the investment. We see options and opportunities but we position this very carefully. We will only let data speak and not sell promises.’
Werner Lanthaler, Chief Executive Officer at Evotec
A CLOSER LOOK AT TREATMENT-RESISTANT
DEPRESSION
It is estimated that over 120 m people suffer from depression and that about one-third of patients treated for major depressive disorder (MDD) do not respond satisfactorily to the first antidepressant pharmacotherapy. Treatment-resistant depression is a term used in clinical psychiatry to describe cases of major depressive disorder that do not respond to adequate courses of at least two antidepressants. There are currently few therapeutic options for TRD, with only very few drugs approved for acute treatment of TRD in the US. There is a need for new treatments and EVT 101 represents one of the few new approaches in clinical development for TRD. According to the National Institute for Mental Health, some of the symptoms include persistent sadness, anxious or ‘empty’ mood, feelings of hopelessness, pessimism or guilt, worthlessness or helplessness, or loss of interest or pleasure in hobbies and activities that were once enjoyed.
PUT TO SLEEP – EVT 201
A past value driver of Evotec has so far failed to deliver its expected commercial success. EVT 201, a partial positive allosteric modulator of GABAA receptors, was tested as a treatment for insomnia in large Phase II clinical trials. Despite very promising scientific data, Evotec
has so far not been in the position to partner this programme. Given the size and investment needed for a decisive Phase III, EVT 201 will not be further developed without a partner.
QUIT SMOKING – QUIT EVT 302
EVT 302 (selective MAO-B inhibitor – smoking cessation & Alzheimer’s disease) failed to demonstrate any significant improvement in the quit rate compared to placebo in a Phase II smoking cessation trial. The company has stopped internal investments but may investigate its potential in the treatment of Alzheimer disease if a suitable partner can be found.
HELP TO IMPROVE LIFE – EVT 401
A first Phase I study with the P2X receptor antagonist EVT 401 was successfully completed. The small molecule drug candidate is a potential novel approach for the oral treatment of inflammatory conditions. EVT 401 was well tolerated and demonstrated the desired pharmacodynamic activity in healthy volunteers. Evotec will focus its efforts on optimising the oral dose formulation, completing the Phase I studies and preparing Phase II studies, if the programme finds an attractive commercial partner.
€ 2.5 M GRANT FOR NEURO-DEGENERATIVE DISEASES RESEARCH
SPONSORED BY THE
Federal Ministry
of Education
and Research
Evotec has been granted up to € 2.5 m in research funds from the Federal Ministry of Education and Research (BMBF) to advance research and development activities on the target serine racemase for potential use in neuropro-tection. The grant will fund the Company’s use of its compound library and fragment-based drug discovery platform to progress the project toward the clinic. The money was awarded as part of Evotec’s involvement in the German government-supported Neu2 consortium. The consortium is focused on developing therapeutics against neurodegenerative diseases including multiple sclerosis. Members include Evotec, MerckSerono, the European ScreeningPort GmbH, Bionamics GmbH, and the University Medical Center Hamburg-Eppendorf.
EVOTEC – ANNUAL REPORT 2009

evotec ZEBRAFISH
POPULAR
Zebrafish jumping out of the aquarium at Evotec Abingdon (UK)
PETS
An example of how Evotec continues to develop its technology portfolio
Evotec acquired the zebrafish screening operations and assets of Summit Corporation plc, including operations in Oxfordshire, UK, and Singapore. Employees and assets associated with the zebrafish business have been transferred. The capability is valuable to drug discovery as it provides important whole organism data about the safety and toxicity of drug-like molecules at an early stage of lead optimisation.
Founded in 2003, Summit built the world’s leading zebrafish capability. The company collaborated with more than 25 pharmaceutical companies worldwide.
The take-over includes a non-exclusive three-year research agreement with Johnson & Johnson signed at the end of 2008 to explore and develop the use of zebrafish screening in drug discovery and development.
THE ZEBRAFISH IS AN IDEAL ORGANISM FOR SMALL MOLECULE STUDIES
Zebrafish have the potential to accelerate and de-risk the drug discovery and development process by reducing attrition rates and lowering the development cost of producing new drugs. The small size and abundance of embryos make zebrafish suitable for screening in a high-throughput manner. Zebrafish are well-characterised model organisms and are used in the screening of potential drug candidates to provide invaluable in vivo safety and efficacy data from the earliest stages of drug discovery and throughout the development process. With a significant genetic similarity to humans and the presence of many vital organs including the heart, brain and liver, the larval zebrafish is highly suitable for screening potential drug candidates for efficacy and safety effects.
New results have been published in the January issue of the journal Science that demonstrates the power of high-throughput behavioural pro-filing in zebrafish to discover and characterise psychotropic drugs and to dissect the pharmacology of complex behaviours.
EVOTEC – ANNUAL REPORT 2009

PEOPLE 15
IRFAN BANDUKWALLA
In August 2009, Evotec acquired a controlling majority stake of Research Support International Private Limited (RSIPL). Within this acquisition, Evotec welcomes Irfan Bandukwalla. He is the General Manager of Evotec India with P&L responsibility for the Indian operations. Before this he was Head of Strategic Planning & Business Development at DIL Ltd. His responsibilities at DIL, amongst others, included establishing, management and business development of RSIPL since its conception in 2004. He is a Chartered Accountant by profession and has about 15 years experience in P&L management, setting up and developing businesses for European multinational organisations in India.
WALTER WENNINGER
Dr Walter Wenninger was elected as new member of the Company’s Supervisory Board at the Annual General Meeting held on 4 June 2009. Dr Wenninger has profound experience in strategic management, research & development and sales & marketing from leading positions in the international pharmaceutical industry. His career includes more than 30 years at Bayer AG where he held top-executive management positions within the life science business in Europe and the United States.
MICHAEL FÄRBER
In November 2009, Michael Färber joined Evotec for a three months internship. He is an advanced student of Molecular Life Science at the University of Luebeck, Germany. Because of the Company’s high reputation as the service provider no 1 for assay development and high-throughput screening he has chosen Evotec for his internship. He felt that his personal interests very much overlap with Evotec’s area of research. And indeed, he had the opportunity to get involved and take an active role in the top-quality research of the Company. He very much enjoyed the high level of responsibility he was given to conduct his own experiments and thereby contributing to the progress of his research project. To share his very positive experience in an inspiring company he highly recommends young students to apply for an internship at Evotec.
PEOPLE
ALEXANDER BÖCKER
In September 2009, Alexander Böcker joined the discovery biology group at Evotec in Hamburg as new Team Leader Discovery Informatics. His job responsibilities range from the statistical evaluation of biological data over the development of chemoinformatic approaches to the implementation and maintenance of software for the analysis and interpretation of biological results. Before this he was working for Boehringer Ingelheim (Canada) Ltd. as Research Scientist where he was responsible for establishing chemoinformatic methodologies. He is a Biologist with additional training in computer science and a Ph.D. in chemistry and has over six years experience in the pharmaceutical and biotech industry.
HEIKE DIEKMANN
Heike Diekmann joined Evotec in May 2009 when the zebrafish unit was acquired from Summit plc. As Principal Scientist, she supervises and develops zebrafish efficacy and disease models. Heike obtained a Ph.D. in biology, with emphasis on developmental biology, at the University of Konstanz, Germany, in 2002. She moved to England in 2005 to work for the zebrafish company Daniolabs in Cambridge, which was acquired by Summit plc in 2007, developing models for neurodegenerative diseases like Alzheimer and Parkinson in zebrafish.
ALEXANDRA RICHENBURG
Alexandra Richenburg works for the HR department in Abingdon. She assists with all aspects of the HR function there and is particularly responsible for training and development. Alexandra began at Evotec in September 2006 as a Scientist in discovery chemistry and worked for just under two years on the CHDI collaboration. In 2008, Alexandra was given the opportunity to transfer to the HR department which she eagerly accepted. The position allows her to utilise her enthusiasm for chemistry but also work even more closely with all the talented employees at Evotec
– Alexandra says it’s sometimes a challenge, but also a real pleasure.
EVOTEC – ANNUAL REPORT 2009
evotec FINANCIAL
PROMISE + DELIVER
Evotec increased and delivered on all financial targets for the fiscal year 2009
continuation cover story
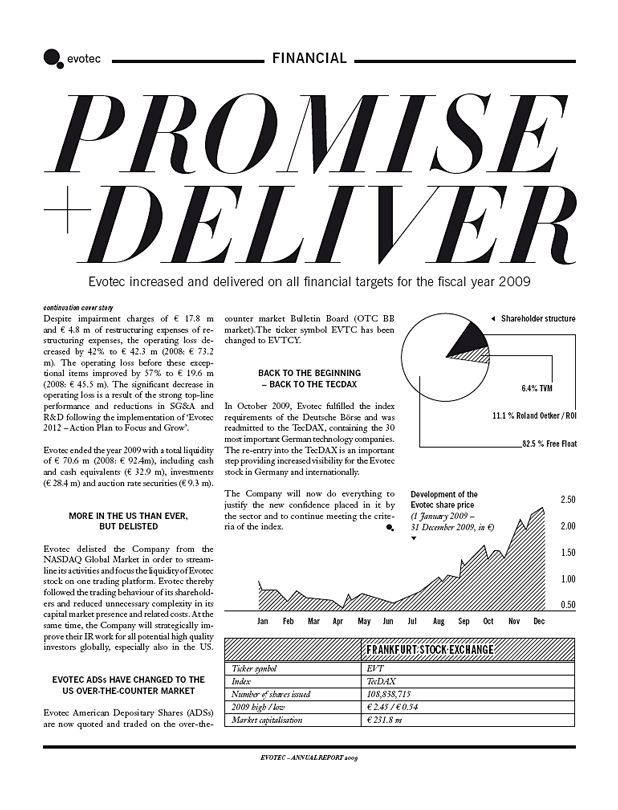
Despite impairment charges of € 17.8 m and € 4.8 m of restructuring expenses of restructuring expenses, the operating loss decreased by 42% to € 42.3 m (2008: € 73.2 m). The operating loss before these exceptional items improved by 57% to € 19.6 m (2008: € 45.5 m). The significant decrease in operating loss is a result of the strong top-line performance and reductions in SG&A and R&D following the implementation of ‘Evotec 2012 – Action Plan to Focus and Grow’.
Evotec ended the year 2009 with a total liquidity of € 70.6 m (2008: € 92.4m), including cash and cash equivalents (€ 32.9 m), investments (€ 28.4 m) and auction rate securities (€ 9.3 m).
MORE IN THE US THAN EVER, BUT DELISTED
Evotec delisted the Company from the NASDAQ Global Market in order to streamline its activities and focus the liquidity of Evotec stock on one trading platform. Evotec thereby followed the trading behaviour of its shareholders and reduced unnecessary complexity in its capital market presence and related costs. At the same time, the Company will strategically improve their IR work for all potential high quality investors globally, especially also in the US.
EVOTEC ADSs HAVE CHANGED TO THE US OVER-THE-COUNTER MARKET
Evotec American Depositary Shares (ADSs) are now quoted and traded on the over-the-counter market Bulletin Board (OTC BB market). The ticker symbol EVTC has been changed to EVTCY.
BACK TO THE BEGINNING
– BACK TO THE TECDAX
In October 2009, Evotec fulfilled the index requirements of the Deutsche Börse and was readmitted to the TecDAX, containing the 30 most important German technology companies. The re-entry into the TecDAX is an important step providing increased visibility for the Evotec stock in Germany and internationally.
The Company will now do everything to justify the new confidence placed in it by the sector and to continue meeting the criteria of the index.
Development of the Evotec share price
(1 January 2009 –
31 December 2009, in €)
Shareholder structure
6.4% TVM
11.1 % Roland Oetker / ROI
82.5 % Free Float
2.50
2.00
1.50
1.00
0.50
Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec
FRANKFURT STOCK EXCHANGE
Ticker symbol EVT
Index TecDAX
Number of shares issued 108,838,715
2009 high / low € 2.45 / € 0.54
Market capitalisation € 231.8 m
EVOTEC – ANNUAL REPORT 2009

OUTSIDE VIEWS 17
Dr Klaus Maleck
in person
If you hear somebody asking why, it’s probably Klaus!
Dr Klaus Maleck – he has been the Chief Financial Officer at Evotec since 2007.
Before joining Evotec, he served as Chief Financial Officer and Vice President Business Development of Bio-GeneriX AG, which he co-founded in 2000. Previously, he worked as a Senior Management Consultant at McKinsey & Co. and as a Genomics Scientist at Novartis, Inc.
As Chief Financial Officer at Evotec he is responsible for the areas of finance, controlling, facility management, IT and IP & Legal, including Compliance Management. To manage all the departments across the world in a successful manner, Klaus is always present and with his strategic and cool-headed way of thinking he ensures that all teams work very well together. Under his leadership, cash and shareholder value generation has prospered.
Dr Maleck holds a Masters degree in biotechnology of the Ecole Supérieure de Biotechnologie in Strasbourg, France and received his Ph.D. in biochemistry from the Max-Planck-Institute in Cologne. In addition, he passed post-graduate training in economics at Ashridge College and Educatis University, where he was awarded an MBA degree.
OUTSIDE
VIEWS
‘The drug discovery alliance with Vifor Pharma announced today, following hot on the heels of the CHDI collaboration (Jan ‘10) and BI deal extension (Nov ‘09), make us increasingly comfortable that Evotec can meet its goal of reaching € 60 m of revenues by 2012.’
Dr Michael Aitkenhead, Piper Jaffray, February 2010
‘We believe that a solid fundament for further business expansion has been established. Looking at the solid revenue basis from current cooperations, the initiated cost reduction programmes, which will lead to further cost savings in the years ahead, the potential for further leveraging of the infrastructure, and the clear management commitment to strive for profitability, we have significantly increased our estimates and now expect the company to achieve profitability by 2012.’
Dr Christian Peter
Sal. Oppenheim, November 2009
‘It takes an average of seven years and $1 bn to move a promising drug candidate to market. Although closer scrutiny of drugs under development is helping to improve efficiency, as less likely candidates are axed earlier in the development process, the speed of development is unlikely to significantly improve in the near term. For this reason, future success for many sponsors will depend on their ability to collaborate with other drug companies, and how well they engage and partner with outside service providers.’
Kenneth I Kaitin
Director of Tufts CSDD, January 2010
‘Not long ago, a big pharmaceutical company wouldn’t have considered farming out the development of a compound found in-house. But expiring patents on top-selling drugs and high-profile failures in finding their replacements have pushed the biggest drug makers to ‘externalise’ much of their R&D.’
Peter Tollman, Boston Consulting Group, Wall Street Journal Online, January 2010
EVOTEC – ANNUAL REPORT 2009

CORPORATE GOVERNANCE 19
CORPORATE GOVERNANCE REPORT
Evotec takes its Corporate Governance responsibilities very seriously
As a consequence of its shares’ listing at the Frankfurt Stock Exchange, its SEC registration and its international shareholder base, the Company recognizes not only German but also international Corporate Governance standards, in particular those laid out in the Sarbanes-Oxley Act for non-US companies and NASDAQ Corporate Governance rules, insofar as German law does not explicitly stipulate otherwise. Evotec’s Management and Supervisory Boards are convinced that complying with rigorous Corporate Governance standards is of great benefit to the Company. Thus Evotec regularly reviews and enhances its Corporate Governance practices.
DECLARATION OF COMPLIANCE WITH THE GERMAN CORPORATE GOVERNANCE CODE
The German Corporate Governance Code as amended on 18 June 2009 (the ‘Code’) sets forth substantial legal requirements for the management and supervision of listed German companies. The rules are based to a large extent on internationally recognised standards for sound and responsible company management.
The general key principles of sound Corporate Governance are: observance of shareholder and employee interests, effective cooperation between the Management Board and the Supervisory Board and open and transparent communication.
With three exceptions, Evotec complies with all recommendations of the Code and the majority of the Code’s suggestions. In December 2009, Evotec’s Management Board and Supervisory Board declared in accordance with Section 161 of the German Stock Corporation Act (AktG):
“Evotec AG has complied in 2009 with the recommendations of the Governmental Commission on the German Corporate Governance Code (the ‘Code’) as published in the official section of the electronic Federal Gazette and intends to comply in the future with the recommendations of the Code with the following exceptions:
— The stock option programmes in place are based on binding resolutions of several Annual General Meetings. While the exercise of these options requires an increase of the share price, the exercise is not related to other relevant comparison parameters as recommended in Section 4.2.3 of the Code. This decision is based on the lack of relevant comparison benchmarks in the field of German Biotech when the stock option programmes were created.
— The Company’s D&O insurance includes a reasonable deductible for members of the Management Board and for members of the Supervisory Board as foreseen by the version of the Code in force before the new version was published on 5 August 2009. For members of the Management Board the deductible will be aligned with Section 3.8 of the Code in due time in accordance with the regulations of the recent Act on the Appropriateness of Management Board Compensation (VorstAG). For members of the Supervisory Board, the Company decided to stay with the current reasonable deductible and to decide at a later point in time about a possible increase as recommended by Section 3.8 of the Code when further information is gathered and the Company has a broader understanding of the corporate practice.
— The Chairman of the Supervisory Board is a member of the committee which handles contracts with members of the Management Board (Remuneration and Nomination Committee), but not the Chairman of this committee as recommended by Section 5.2 of the Code. This enables Evotec to have a further Supervisory Board member involved more deeply in the governance of the Company.”
The current Declaration of Compliance with the German Corporate Governance Code and the declarations of the past five years can be found on Evotec’s website (www.evotec.com) in the section ‘Investors > Corporate Governance’.
TWO-TIER MANAGEMENT AND CONTROL SYSTEM: MANAGEMENT BOARD AND SUPERVISORY BOARD
According to the German Stock Corporation Act (AktG) a two-tier system with clear separation of ‘management’ through the Management Board (‘Vorstand’) and ‘control’ through the Supervisory Board (‘Aufsichtsrat’) is mandatory for German stock corporations.
EVOTEC – ANNUAL REPORT 2009
evotec CORPORATE GOVERNANCE
Tenures and Composition of Supervisory Board Committees*
END OF TENURE 1) AUDIT COMMITTEE REMUNERATION AND NOMINATION COMMITTEE
Dr Flemming Ørnskov, Chairman 2014 … X
Dr Corey Goodman, Vice Chairman 2014 4) … X 3)
Dr Hubert Birner 2014 X (Chair) …
Dr Peter Fellner 2014 … X
Mary Tanner 2014 X …
Dr Walter Wenninger 2) 2014 X X (Chair)
John Walker 3) … X (Chair) 3) …
1) Following the Annual Shareholder Meeting
2) Elected by the Annual Shareholder Meeting on 4 June 09
3) Tenure ended with the Annual Shareholder Meeting on 4 June 09
4) Resigned effective 31 January 2010
* Information on the professional affiliations of Supervisory Board members can be found on page 86
The two boards work closely together to achieve long-term and sustainable growth for the Company and to create shareholder value. They agree on the Company’s strategy and on business transactions that are significant. The Annual Shareholders’ Meeting (‘Hauptversammlung’) is the company body representing the interests of the shareholders.
MANAGEMENT BOARD
(‘Vorstand’)
Evotec’s Management Board is responsible for the day-to-day operations and is supported by the Management Team. In its business operations and decisions the Management Board acts on behalf of the Company and works towards its progress with the objective of sustainable creation of value, thus taking into account the interests of the shareholders, the employees and other stakeholders. The Management Board is appointed by the Supervisory Board.
In accordance with a suggestion of the Code, new members are appointed for up to three years. Members of Evotec’s Management Board have not accepted more than a total of three Supervisory Board mandates in non-Group listed companies. Information on the mandates and professional affiliations of the members of the Management Board can be found on page 87. The Company’s rules of internal procedure assign functional duties and responsibilities to the Management Board members.
SUPERVISORY BOARD
(‘Aufsichtsrat’)
Evotec’s Supervisory Board consists of six independent members who, in accordance with the Code’s recommendations, are appointed on the basis of their qualification, work experience, independence and considering their diversity. No former member of the Management Board is a member of the Supervisory Board. The Supervisory Board appoints Management Board members considering the diversity of the Management Board, provides advice to the Management Board and oversees its activities. It consults regularly with the Management Board and is thus informed at all times about the business and strategic situation of the Company as well as its risk environment. In addition, the Supervisory Board plays a key role in decisions of fundamental importance.
Business activities of fundamental importance requiring approval of the Supervisory Board include:
— the strategic and operational direction of the Company;
— budgeting and significant deviations from budgets;
— significant changes in the drug development pipeline;
— investments (including in-licensing) in excess of € 2.5 m;
— establishing and acquiring companies or changing the Group structure;
— business contracts outside the Company’s ordinary course of business that have significantly different risk profiles;
— out-licensing contracts worth in excess of € 5 m;
— granting loans or liens, providing guarantees, issuing bonds or any measures of capital acquisitions;
— buying or selling real estate property and
— establishing new business operations or significantly revising existing business operations. The Supervisory Board has its own internal rules of procedure (see www.evotec.com; ‘Investors > Corporate Governance > Policies and Charters’) and complies with the Code’s suggestion to hold occasional separate discussions.
With two exceptions, the Supervisory Board was not aware of any potential conflict of interests among any of its members during the year 2009. In the case of these exceptions, two Supervisory Board members disclosed a potential conflict to the Supervisory Board in relation to two independent strategic opportunities for the Company. The respective Supervisory Board members did not participate in the deliberations of the Supervisory Board concerning these opportunities. The Company decided not to pursue both opportunities.
Information on the professional affiliations of board members and on related party transactions can be found on pages 86 and 81.
WORK IN SUPERVISORY BOARD COMMITTEES IN ACCORDANCE WITH GOVERNANCE CODE
A significant proportion of the Supervisory Board’s work is conducted in committees of the Supervisory Board. From among its members, Evotec’s Supervisory Board has, pursuant to the German Stock Corporation Act (AktG) and the recommendations of the Code, established an Audit Committee and a Remuneration and Nomination Committee. Members of both committees are appointed in accordance with the Code.
EVOTEC – ANNUAL REPORT 2009
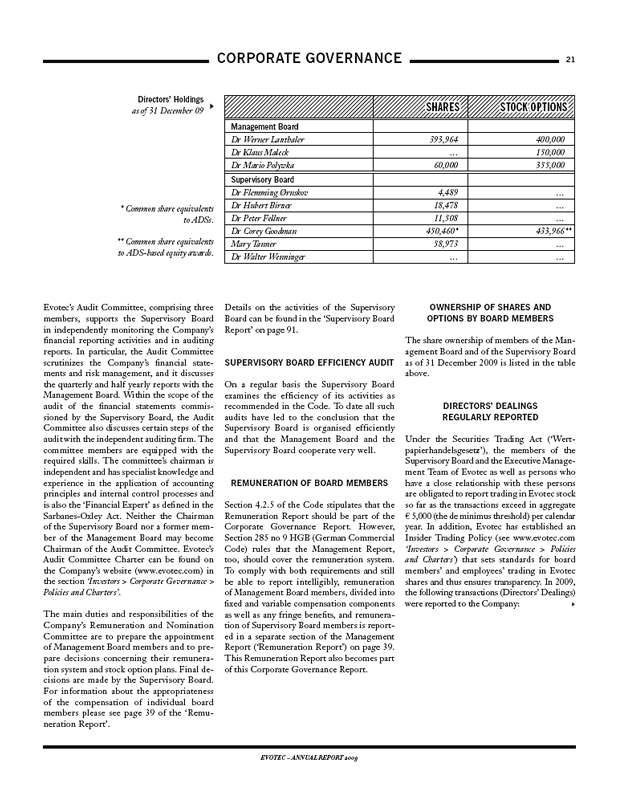
CORPORATE GOVERNANCE 21
Directors’ Holdings as of 31 December 09
* Common share equivalents to ADSs.
** Common share equivalents to ADS-based equity awards.
SHARES STOCK OPTIONS
Management Board
Dr Werner Lanthaler 393,964 400,000
Dr Klaus Maleck … 150,000
Dr Mario Polywka 60,000 355,000
Supervisory Board
Dr Flemming Ørnskov 4,489 …
Dr Hubert Birner 18,478 …
Dr Peter Fellner 11,508 …
Dr Corey Goodman 450,460* 433,966**
Mary Tanner 58,973 …
Dr Walter Wenninger … …
Evotec’s Audit Committee, comprising three members, supports the Supervisory Board in independently monitoring the Company’s financial reporting activities and in auditing reports. In particular, the Audit Committee scrutinizes the Company’s financial statements and risk management, and it discusses the quarterly and half yearly reports with the Management Board. Within the scope of the audit of the financial statements commissioned by the Supervisory Board, the Audit Committee also discusses certain steps of the audit with the independent auditing firm. The committee members are equipped with the required skills. The committee’s chairman is independent and has specialist knowledge and experience in the application of accounting principles and internal control processes and is also the ‘Financial Expert’ as defined in the Sarbanes-Oxley Act. Neither the Chairman of the Supervisory Board nor a former member of the Management Board may become Chairman of the Audit Committee. Evotec’s Audit Committee Charter can be found on the Company’s website (www.evotec.com) in the section ‘Investors > Corporate Governance > Policies and Charters’.
The main duties and responsibilities of the Company’s Remuneration and Nomination Committee are to prepare the appointment of Management Board members and to prepare decisions concerning their remuneration system and stock option plans. Final decisions are made by the Supervisory Board. For information about the appropriateness of the compensation of individual board members please see page 39 of the ‘Remuneration Report’.
Details on the activities of the Supervisory Board can be found in the ‘Supervisory Board Report’ on page 91.
SUPERVISORY BOARD EFFICIENCY AUDIT
On a regular basis the Supervisory Board examines the efficiency of its activities as recommended in the Code. To date all such audits have led to the conclusion that the Supervisory Board is organised efficiently and that the Management Board and the Supervisory Board cooperate very well.
REMUNERATION OF BOARD MEMBERS
Section 4.2.5 of the Code stipulates that the Remuneration Report should be part of the Corporate Governance Report. However, Section 285 no 9 HGB (German Commercial Code) rules that the Management Report, too, should cover the remuneration system. To comply with both requirements and still be able to report intelligibly, remuneration of Management Board members, divided into fixed and variable compensation components as well as any fringe benefits, and remuneration of Supervisory Board members is reported in a separate section of the Management Report (‘Remuneration Report’) on page 39. This Remuneration Report also becomes part of this Corporate Governance Report.
OWNERSHIP OF SHARES AND OPTIONS BY BOARD MEMBERS
The share ownership of members of the Management Board and of the Supervisory Board as of 31 December 2009 is listed in the table above.
DIRECTORS’ DEALINGS REGULARLY REPORTED
Under the Securities Trading Act (‘Wert-papierhandelsgesetz’), the members of the Supervisory Board and the Executive Management Team of Evotec as well as persons who have a close relationship with these persons are obligated to report trading in Evotec stock so far as the transactions exceed in aggregate € 5,000 (the de minimus threshold) per calendar year. In addition, Evotec has established an Insider Trading Policy (see www.evotec.com
‘Investors > Corporate Governance > Policies and Charters’) that sets standards for board members’ and employees’ trading in Evotec shares and thus ensures transparency. In 2009, the following transactions (Directors’ Dealings) were reported to the Company:
EVOTEC – ANNUAL REPORT 2009
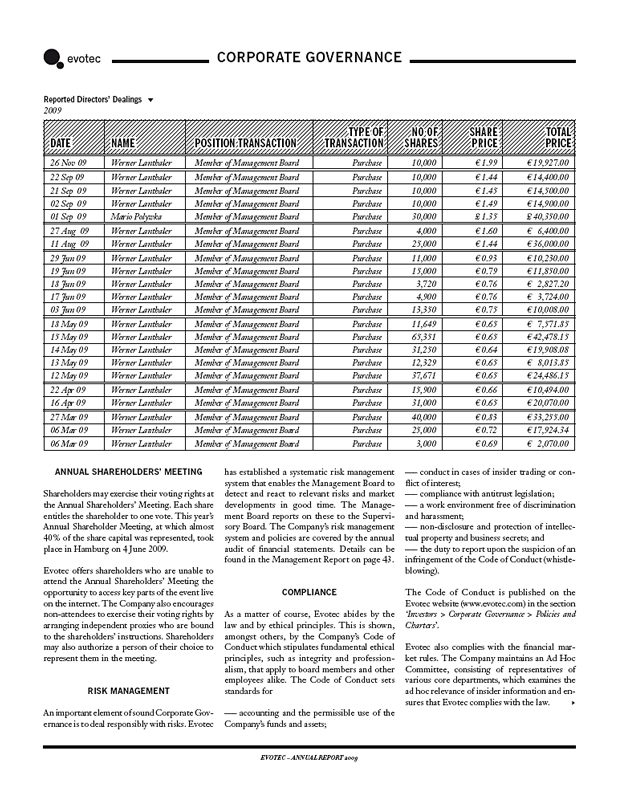
evotec CORPORATE GOVERNANCE
Reported Directors’ Dealings
2009
DATE NAME POSITION TRANSACTION TYPE OF TRANSACTION NO OF SHARES SHARE PRICE TOTAL PRICE
26 Nov 09 Werner Lanthaler Member of Management Board Purchase 10,000 € 1.99 € 19,927.00
22 Sep 09 Werner Lanthaler Member of Management Board Purchase 10,000 € 1.44 € 14,400.00
21 Sep 09 Werner Lanthaler Member of Management Board Purchase 10,000 € 1.45 € 14,500.00
02 Sep 09 Werner Lanthaler Member of Management Board Purchase 10,000 € 1.49 € 14,900.00
01 Sep 09 Mario Polywka Member of Management Board Purchase 30,000 £ 1.35 £ 40,350.00
27 Aug 09 Werner Lanthaler Member of Management Board Purchase 4,000 € 1.60 € 6,400.00
11 Aug 09 Werner Lanthaler Member of Management Board Purchase 25,000 € 1.44 € 36,000.00
29 Jun 09 Werner Lanthaler Member of Management Board Purchase 11,000 € 0.93 € 10,230.00
19 Jun 09 Werner Lanthaler Member of Management Board Purchase 15,000 € 0.79 € 11,850.00
18 Jun 09 Werner Lanthaler Member of Management Board Purchase 3,720 € 0.76 € 2,827.20
17 Jun 09 Werner Lanthaler Member of Management Board Purchase 4,900 € 0.76 € 3,724.00
03 Jun 09 Werner Lanthaler Member of Management Board Purchase 13,350 € 0.75 € 10,008.00
18 May 09 Werner Lanthaler Member of Management Board Purchase 11,649 € 0.65 € 7,571.85
15 May 09 Werner Lanthaler Member of Management Board Purchase 65,351 € 0.65 € 42,478.15
14 May 09 Werner Lanthaler Member of Management Board Purchase 31,250 € 0.64 € 19,908.08
13 May 09 Werner Lanthaler Member of Management Board Purchase 12,329 € 0.65 € 8,013.85
12 May 09 Werner Lanthaler Member of Management Board Purchase 37,671 € 0.65 € 24,486.15
22 Apr 09 Werner Lanthaler Member of Management Board Purchase 15,900 € 0.66 € 10,494.00
16 Apr 09 Werner Lanthaler Member of Management Board Purchase 31,000 € 0.65 € 20,070.00
27 Mar 09 Werner Lanthaler Member of Management Board Purchase 40,000 € 0.83 € 33,255.00
06 Mar 09 Werner Lanthaler Member of Management Board Purchase 25,000 € 0.72 € 17,924.34
06 Mar 09 Werner Lanthaler Member of Management Board Purchase 3,000 € 0.69 € 2,070.00
ANNUAL SHAREHOLDERS’ MEETING
Shareholders may exercise their voting rights at the Annual Shareholders’ Meeting. Each share entitles the shareholder to one vote. This year’s Annual Shareholder Meeting, at which almost 40% of the share capital was represented, took place in Hamburg on 4 June 2009.
Evotec offers shareholders who are unable to attend the Annual Shareholders’ Meeting the opportunity to access key parts of the event live on the internet. The Company also encourages non-attendees to exercise their voting rights by arranging independent proxies who are bound to the shareholders’ instructions. Shareholders may also authorize a person of their choice to represent them in the meeting.
RISK MANAGEMENT
An important element of sound Corporate Governance is to deal responsibly with risks. Evotec
has established a systematic risk management system that enables the Management Board to detect and react to relevant risks and market developments in good time. The Management Board reports on these to the Supervisory Board. The Company’s risk management system and policies are covered by the annual audit of financial statements. Details can be found in the Management Report on page 43.
COMPLIANCE
As a matter of course, Evotec abides by the law and by ethical principles. This is shown, amongst others, by the Company’s Code of Conduct which stipulates fundamental ethical principles, such as integrity and professionalism, that apply to board members and other employees alike. The Code of Conduct sets standards for
— accounting and the permissible use of the Company’s funds and assets;
— conduct in cases of insider trading or conflict of interest;
— compliance with antitrust legislation;
— a work environment free of discrimination and harassment;
— non-disclosure and protection of intellectual property and business secrets; and
— the duty to report upon the suspicion of an infringement of the Code of Conduct (whistle-blowing).
The Code of Conduct is published on the Evotec website (www.evotec.com) in the section
‘Investors > Corporate Governance > Policies and Charters’.
Evotec also complies with the financial market rules. The Company maintains an Ad Hoc Committee, consisting of representatives of various core departments, which examines the ad hoc relevance of insider information and ensures that Evotec complies with the law.
EVOTEC – ANNUAL REPORT 2009

CORPORATE GOVERNANCE 23
Evotec’s Compliance Programme is overseen by the Company’s Compliance Officer, functioning as an independent and objective body that reviews and evaluates compliance issues/ concerns within the organisation.
AUDIT OF FINANCIAL STATEMENTS
On a regular basis, Evotec provides financial and business information to its shareholders and other interested parties by publishing its annual consolidated financial statements and quarterly reports. As an incorporated company whose registered head office is located within the European Union, Evotec must prepare and publish consolidated financial statements in accordance with the International Financial Reporting Standards (IFRS) whilst observing Section 315a HGB (German Commercial Code). The financial statements of the Evotec Group and the financial statements of Evotec AG are audited by the audit firm and the Supervisory Board. The audit firm is appointed by the shareholders at the Annual Shareholders’ Meeting and commissioned by the Supervisory Board. It participates at the Supervisory Board’s deliberations on the financial statements and reports the most significant results of its audit.
EQUITY INVESTEES AND STOCK OPTION PLANS
A list of substantial equity investees as well as details on the Company’s stock option plans can be found in the section ‘Consolidated Financial Statements’ on pages 69 and 74.
INVESTOR RELATIONS / TRANSPARENCY
Evotec informs its shareholders, financial analysts, the media and the public on a regular basis about its progress. In doing so, the Company complies with all requirements of the Code regarding transparency, timeliness, openness and shareholder equality. Evotec is committed to fair disclosure of information and its communication is governed by a Company Disclosure Policy. It is a prime concern of the Company that all relevant target groups receive the same information at the same time, and this implies communicating
in both English and German. The Company’s publications are available on its website (www. evotec.com, section ‘Investors’).
The ‘Investors’ section of Evotec’s website maintains information such as news releases, the financial calendar containing the publication dates of the financial statements, Investor Relations conferences, annual and quarterly reports, other regulatory news and regularly updated corporate governance information. This section of the website also includes the Articles of Association, the Rules of Procedure of the Supervisory Board, the Audit Committee Charter, the Code of Conduct, the Insider Trading Policy and all declarations of compliance.
The website also contains information about the NASDAQ Corporate Governance Rules. Evotec voluntarily complies with a large proportion of the NASDAQ rules, deviating only where German law stipulates otherwise. The exemptions can be found on the Introduction page of the section ‘Investors > Corporate Governance’ under the link ‘NASDAQ Corporate Governance Disclosure’ at www.evotec.com.
Evotec places great emphasis on a continuous dialogue with financial analysts and investors. It conducts at least one analyst meeting every year and telephone conferences when quarterly financial results are published, while ensuring that no stakeholder receives preferential information. In 2009 management presented the Company at 6 national and international investor conferences as well as at 8 road shows in key financial centres.
EVOTEC – ANNUAL REPORT 2009

MANAGEMENT
REPORT
2009
CONTENT
26 Operations and Business Environment
30 Financial Report
37 Non-Financial Performance Indicators
39 Remuneration Report
41 Information pursuant to section 315 paragraph 4 of the German Commercial Code
43 Risk Management and Risk Report
48 Post-Balance Sheet Events
49 Outlook

26 Operations and Business Environment
OPERATIONS AND BUSINESS ENVIRONMENT
ORGANISATIONAL STRUCTURE AND BUSINESS ACTIVITIES
GROUP STRUCTURE
Evotec AG is a publicly listed stock corporation operating under German law. The Company has its headquarters in Hamburg, Germany and operating subsidiaries in Abingdon, UK, North Potomac, Maryland, USA, Thane, India and Singapore and employed 485 people at the end of 2009.
Evotec, through adoption of IFRS 8, reports as one business segment in accordance with its management approach.
PRODUCTS AND SERVICES
Evotec is a drug discovery and development company focused on providing integrated and innovative drug discovery services and alliances to the pharmaceutical and biotechnology industry. In addition, Evotec has a small number of its own drug candidates at various stages of development either partnered or available for partnering.
Through its Discovery Alliance Business, Evotec provides innovative and integrated solutions to the pharmaceutical and biotechnology industry from target to clinical development through a range of capabilities and capacities. This includes early-stage assay development and screening, fragment-based drug discovery through to medicinal chemistry and in vivo pharmacology. Evotec’s partners include, among others, Biogen Idec, Boehringer Ingelheim, CHDI, Novartis, and Ono Pharmaceutical. In exchange for access to its integrated discovery offerings Evotec receives contractual service fees and ongoing FTE-based research payments and, in certain circumstances, up-front technology access fees, and milestone and royalty payments related to the achievement of certain research, development and sales milestones.
In its proprietary projects, Evotec is developing new treatments for diseases related to neuroscience, pain and inflammation. Evotec is progressing three drug candidates through clinical development: EVT 101 and 103, subtype selective NMDA receptor antagonists, partnered with Roche, in clinical development for Treatment Resistant Depression and EVT 401, a P2X7 antagonist for the treatment of inflammatory diseases. Evotec’s proprietary late-stage preclinical research programmes focus on H3 antagonists for the treatment of cognitive disorders and narcolepsy, B1 antagonists for the treatment of neuropathic pain and inflammation, as well as antagonists for the purinergic receptor P2X3 for the treatment of inflammation and urinary incontinence. Evotec seeks to selectively partner or publicly fund these assets for further development.
SIGNIFICANT CORPORATE DEVELOPMENT EVENTS
In March 2009, Evotec published its ‘Evotec 2012 – Action Plan to Focus and Grow’. With this initiative, the Company communicated its refocused strategy to invest into and grow its core Discovery Alliances Business. In addition, Evotec decided to concentrate its pipeline R&D on the most valuable assets and significantly reduce its SG&A expenses. With these measures, reducing headcount initially by approximately 50 employees, Evotec de-risked its business model substantially and laid the grounds to secure liquidity beyond 2012.
Furthermore, in March, Evotec partnered the subtype-specific NMDA program EVT 101/103 with Roche for development in treatment-resistant depression. Besides substantial development, sales performance and future scalable double-digit commercial payments, this deal included the funding of related clinical development costs and thus substantially reduced the total R&D cost burden.
In April, the selective MAO-B inhibitor, EVT 302, which was in clinical development for smoking cessation, failed to show a significant improvement against placebo in a Phase II quit rate study. As a consequence of this failure, the Company discontinued clinical development of EVT 302 for smoking cessation.
In May, as a further result of implementing ‘Evotec 2012 – Action Plan to Focus and Grow’, the Company announced the re-engineering of its research operations, including the closure of its South San Francisco facility (Renovis), to more effectively leverage the Discovery Alliance Business. This further re-structuring resulted in the loss of an additional 45 employees and is expected to lead to € 10 m of savings from 2010 onwards.
Additionally in May, Evotec acquired the zebrafish operations and assets of Summit Corporation against £ 0.5 m in cash. The acquisition added to the Company’s early discovery technologies critical to maintaining a differentiated discovery alliance platform. With this addition, Evotec added 16 scientists in Abingdon, UK and Singapore.
In August, the Company acquired a 70% controlling stake of the Indian company, Research Support International Private Limited, for € 2.4 m in cash. Included in the purchase price is an earn-out due in 2010 amounting to approximately € 0.8 m. Evotec has a call option to purchase the remaining 30% in the event of a change-of-control. The acquisition strengthens Evotec’s chemistry capacity and offering and added 164 headcount through the facility in Thane, India.
EVOTEC – ANNUAL REPORT 2009

Operations and Business Environment 27
In November, Evotec published its intention to voluntarily delist its American Depository Shares from NASDAQ Global Market and to concentrate its share trading on its home market in Germany. Evotec filed a form 25 with the NASDAQ on 20 November 2009, and the delisting became effective on 30 November 2009. Evotec thereby followed the trading behaviour of its shareholders and intends to reduce unnecessary complexity in its capital market presence and related costs. Evotec’s reentry into the German technology index TecDAX in October 2009 is expected to support this decision and to further increase liquidity for Evotec’s shareholders though increased visibility of the stock.
GROUP MANAGEMENT AND SUPERVISION
As required by the German Stock Corporation Act (Aktiengesetz), Evotec AG has a two-tier board system consisting of the Evotec Management Board (Vorstand) and the Evotec Supervisory Board (Aufsichtsrat). The Management Board is responsible for managing Evotec and representing the Company in its dealings with third parties, while the Supervisory Board appoints and dismisses the members of the Evotec Management Board and oversees the management of the Company. German law prohibits the Supervisory Board from making management decisions.
The Evotec Supervisory Board consists of six members – as provided in the current Articles of Association – all of whom are elected by the shareholders by a simple majority of the votes cast at a shareholders’ meeting. The Supervisory Board appoints a chairman and one or more vice-chairmen from among its members. The members of the Supervisory Board are elected for five years and may be re-elected. The term of the current members of the Evotec Supervisory Board will expire at the end of the annual general shareholders’ meeting held in the year 2014.
Under Evotec’s Articles of Association, the Supervisory Board determines the size of the Management Board, which must have at least one member under the German Stock Corporation Act. The statutory maximum term for members of the Management Board is five years, but Evotec’s current practice is to limit the terms to three years. Management Board members may be reappointed and may be dismissed with good cause prior to the termination of their terms of office. Dr Werner Lanthaler was appointed to the position of President & Chief Executive Officer in March 2009, succeeding Jörn Aldag who left the Company in December 2008. Dr Lanthaler had previously served as Chief Financial Officer of Intercell AG in Vienna, Austria, where he was also responsible for business development and marketing. Dr Klaus Maleck, Chief Financial Officer, and Dr Mario Polywka, Chief Operating Officer, continued in their management positions. Information regarding the remuneration of Evotec’s Management Board and Supervisory Board can be found in the ‘Remuneration Report’ on page 39 of this Management Report.
DECLARATION OF CORPORATE MANAGEMENT
More information on Company management practices can be found in the Company’s “Declaration of Corporate Management” according to section 289a HGB on Evotec’s website at www.evotec.com, Investors Corporate Governance.
CORPORATE PERFORMANCE MEASURES, OBJECTIVES AND STRATEGY
Management’s objective is to systematically and continuously increase the value of the Company. Management has in the past years successfully transformed Evotec from a small technology provider into a focused discovery and development company. Non-core research services and the discovery instruments business were divested for cash. Today the Company has integrated into its cash generative discovery alliance business disease know-how, in particular in neuroscience, pain and inflammation, and a number of drug candidates at various stages of development that are either partnered or available for partnering.
GROWTH STRATEGY AND NON-FINANCIAL PERFORMANCE MEASURES
According to ‘Evotec 2012 – Action Plan to Focus and Grow’, Evotec’s strategy is to build a sustainable, profitable drug discovery alliance business. Consequently, Evotec focuses on high value, revenue generating partnerships with pharmaceutical and biotechnology companies. Additionally, Evotec will continue to fund a limited number of its internal pipeline products to a position where they become partnerable. The key elements and related non-financial performance measures of Evotec’s strategy are as follows:
Focus and grow the Discovery Alliance Business
Evotec has built substantial drug discovery expertise and an industrialized platform that can assist pharmaceutical and biotechnology partners drive new innovative small molecule compounds into the clinic. This expertise covers the entire spectrum of discovery and is applicable to targets across multiple therapeutic indications. Its capabilities include high-throughput and high-content screening, medicinal chemistry, fragment-based drug discovery, in vivo pharmacology, as well as an extensive series of in vitro ADMET profiling assays. In addition, Evotec has built a deep internal knowledge base in various therapeutic areas. Leveraging these skills and expertise, the Company intends to organically grow its Discovery Alliance Business through strategic partnerships with pharmaceutical and biotechnology companies. Evotec will also seek to expand its technology and capabilities in offering an integrated drug discovery platform in areas that complement its current operations. The order book at the beginning of the year is strong, indicating potential further revenue growth in 2010 over 2009.
January Order book (continuing business)
in T€ 2007 2008 2009 2010
Sales order book January 17,695 18,643 24,201* 27,290
Year end revenues w/o milestones 32,885 31,109 38,303 -
Year end revenues 32,885 39,613 42,683 -
* Sales order book February
EVOTEC – ANNUAL REPORT 2009

28 Operations and Business Environment
Establish corporate collaborations to assist in the development and commercialisation of Evotec’s pipeline products
Focusing on its third party research, Evotec has significantly reduced its internal proprietary pipeline research and development activities. The Company aims to selectively partner its current pipeline products that address major markets with pharmaceutical companies with the financial strength for the more expensive Phase II / III trials. The Company intends to out-license drug candidates to pharmaceutical companies for upfront and milestone payments, as well as for royalties on the future sale of drugs.
In March 2009, Evotec entered into a collaboration with Roche for the development of the EVT 100 series of NMDA antagonists. These assets are being clinically developed for Treatment Resistant Depression. The deal included a USD 10 m upfront payment and an option for Roche to buy back rights to the entire EVT 100 programme with a potential deal value exceeding USD 300 m in development and sales performance commercial payments plus potential scalable double-digit commercial payments.
FINANCIAL CONTROL CRITERIA
As described above, Evotec has embarked on a strategy of focusing on its core Discovery Alliance Business. The restructuring through implementation of ‘Evotec 2012 – Action Plan to Focus and Grow’ has significantly reduced cash burn in 2009 and is expected to lead to a path of sustainability. The reduced spending in proprietary R&D and a continuous tight watch on SG&A expenses have resulted in a significant change in the structure of Evotec’s income statement. Despite restructuring expenses and impairment of net book values of acquired intangible assets, operating loss was significantly reduced. Liquidity (cash, investments and auction rate securities) at the end of 2009 amounted to € 70.6 m. Evotec’s goal is to reach operative profitability and to generate cash by 2012. The Company believes that the strong growth anticipated in its Discovery Alliance Business and its drive toward sustainable profitability, are the basis for future success and shareholder value creation.
For Evotec, cash forecasts, including the definition of minimum cash levels, the monitoring of contract research revenues and milestones, and operational cash flow are critical to short and mid-term financial performance. Value analysis based on discounted cash flow models are the most important financial control criteria for Evotec’s investment decisions and development projects.
Management engages in monthly financial reviews with a strong emphasis on key financial performance drivers such as revenues, order book status and gross margins as well as careful cost analysis (SG&A, R&D expenses) to measure its performance against its financial targets. Evotec’s performance against its 2009 financial targets is explained in detail below.
RESEARCH AND DEVELOPMENT
Despite some major set-backs, Evotec’s pipeline has made progress during the year. Various discovery and preclinical studies for clinical and preclinical projects were conducted. This section provides a short summary of Evotec’s proprietary 2009 clinical pipeline:
— EVT 101: Preparations for the start of a Phase II proof-of-concept study in Treatment-Resistant Depression. The study is anticipated to start during the first half of 2010.
— EVT 103: Start of Phase I studies.
— EVT 201: Project for treatment of insomnia on hold.
— EVT 302: Completion of a Phase II proof-of-concept quit rate study in Smoking Cessation. EVT 302 failed to demonstrate any significant improvement compared to placebo.
EVT 401: Successful completion of the first Phase I study with the P2X7 antagonist.
GENERAL MARKET AND HEALTHCARE SUMMARY
GENERAL ECONOMIC AND HEALTHCARE REVIEW
After the crash of the financial markets in 2008, the real economy suffered from a strong recession in 2009. Following the governments’ rescue efforts including multi-billion dollar bail outs, the banking systems normalised although financing remained tight. The economic environment reflects the financial shock with high public debt levels and low interest rates. Interest rates in the US are close to zero (FED interest rates: 0.25%), in Europe the ECB inter-banking interest rates are at 1%. Despite this cheap money, capital for riskier investments is not readily available. Changes in raw material prices do not materially influence Evotec’s industry.
While in the first quarter stock markets were still depressed, positive early indicators, underpinned by massive governmental support programmes, helped to re-establish trust in the markets in early summer. Over the year, stock markets across the globe gained 25%, with especially strong developments in the BRIC countries. The German DAX Index closed the year up by 23.8% in line with other European stock markets (FTSE 100: 22.1%, CAC40: 22.3%, IBEX: 29.8%). The Dow Jones Industrial Average improved by 18.8%, while the NASDAQ Composite saw a substantial 43.9% increase over the past year. The biotech indices, starting from a low level, improved too. NASDAQ Biotech Composite improved by 15.6%, which was less than the general market development due to financial distresses.
Although the revenues of the pharmaceutical industry were not affected by this economic downturn, the aftermaths were manifold, especially for biotech companies, as refinancing became challenging and R&D budgets were cut across the industry. Because of ongoing issues over patent expiries and shallow pipelines nearly all the top pharma companies have publicly stated that their growth strategies include both organic R&D and opportunistic M&A of entire companies and individual projects. Looking forward, most sector analysts see little impact on pharma’s medium term R&D spending plans. Importantly for Evotec, these R&D
EVOTEC – ANNUAL REPORT 2009

Operations and Business Environment 29
strategies will increasingly include outsourced R&D on both a fee-for-service and a collaborative/co-development basis in order to increase efficacy and reduce fixed costs.
The regulatory environment continues to be challenging with the FDA focusing specifically on safety and relevance over efficacy. It appears the FDA is concluding that the risk of approval is only justified if a drug meets an unmet medical need or if it provides a well-defined benefit over existing therapies. This means that in the near future Phase II proof-of-concept trials may be required to routinely include comparative trials if there is a treatment already available.
EXCHANGE RATE DEVELOPMENT
The world’s banks’ short-term interest rate cuts had a major impact on currencies, including the US Dollar, Sterling and Euro. Sterling kept its historic low against the Euro, staying close at parity, after the Bank of England’s interest rate cut in January. Investors seeking a safe haven from the havoc that has been overwhelming the world markets flocked first to US treasuries, creating a steep rise in the US Dollar. In the second half of 2009, carry trade impacted the exchange rate to the opposite, and the US Dollar lost its strength against the Euro. While the lower US Dollar to Euro exchange rate negatively impacted Evotec’s financial position the weak UK Sterling was in Evotec’s favour. The Company generates approximately 40 to 50% of its revenues in Dollars and has a strong cost base in the UK. In addition, the currency translation of substantial liquid assets held in US Dollars into Euros negatively impacted on Evotec’s reported liquidity position during the year. At year-end, however, the rise of the US Dollar in the last quarter had a positive liquidity impact. Evotec is one of the very few European small cap biotech companies with a healthy liquidity position and believes this to be a competitive advantage in building the Company and shareholder value. Hence, especially in this difficult market environment, Evotec will continue to operate as capital-efficiently as possible, to assess the funding of its R&D activities and capital investments carefully and to balance this against cash flow from revenue-bearing business to assure that Evotec’s cash will be sufficient to develop the Company to sustainability.
MANAGEMENTS’ GENERAL ASSESSMENT OF BUSINESS PERFORMANCE
Due to a strong top-line performance with significant milestones achieved and important new contracts or contract extensions signed, a reduction in R&D and SG&A expenses, together with favourable exchange rate effects, the Company exceeded its financial targets for the year (see details below). The successful partnering of the EVT 100 compound family with Roche also meant that the most valuable pipeline asset is now funded by a third party under favourable deal terms. Evotec remains well financed and is on course to profitability and sustainability by 2012 at the latest.
EVOTEC – ANNUAL REPORT 2009

30 Financial Report
FINANCIAL REPORT
The 2009 and 2008 results are not fully comparable. The major difference results from the acquisition of a 70% majority stake of Research Support International Private Limited (RSIPL), Thane, India, on 31 August 2009 and of Renovis, Inc., South San Francisco, California, USA on 2 May 2008 respectively.
Consequently, the operating results of RSIPL from the period 1 September 2009 through 31 December 2009 are included in the accompanying consolidated income statement for the year ended 31 December 2009. The assets and liabilities of RSIPL at 31 December 2009 are included in the accompanying consolidated statement of financial position. In addition, the operating results of Renovis Inc. were included in the accompanying consolidated income statement from 2 May 2008 onwards.
For further discussion on the RSIPL acquisition and selected pro-forma financial results see Note 3 of the Consolidated Financial Statements.
Condensed Income Statement
2008 2009
Revenues T€ 39,613 42,683
Gross Margin% 44.5 43.2
— R&D Expenses T€ 42,537 20,947
— SG&A Expenses T€ 19,950 16,695
— Amortisation and impairment T€ 28,136 18,293
— Restructuring expenses T€ 132 4,849
— Other operating expenses (income) T€ 91 (64)
Operating loss T€ (73,210) (42,299)
Net loss attributable to shareholders of Evotec T€ (78,287) (45,523)
COMPARISON OF 2009 FINANCIAL RESULTS WITH FORECAST
ORIGINAL TARGETS EXCEEDED
Evotec’s financial guidance for the full year 2009, as stated in the ‘Outlook’ of the 2008 annual report, was for total Group revenues of € 34 m to € 36 m. R&D expenses were expected to decrease significantly to a range of € 32 m to € 35 m, and to be further offset by research funding obtained from collaborations and license agreements. Removing any impairment charges, the 2009 operating result was expected to improve over the 2008 result. Liquidity (cash and investments, including auction rate securities) was anticipated to be sufficient to fund the Company’s operations for at least three years and was targeted, as described in the report of the first quarter, to exceed € 65 m at the end of 2009.
Evotec increased its revenue target at the time of its second quarter reporting and adjusted the anticipated annual R&D expenses at the beginning of the fourth quarter 2009.
Due to a solid overall performance in its discovery alliances and the partnership of the EVT 100 compound series with Roche, revenues were expected to exceed € 40 m. R&D expenses were expected to be lower than € 25 m due to reduced discovery spending following the closure of the Renovis site and the reduction of clinical activities. In addition, Evotec repeatedly confirmed its liquidity guidance despite two cash acquisitions and restructuring expenses.
Evotec ended the year with a very successful financial performance compared to guidance. The final results for the fiscal year 2009 were € 42.7 m of revenues, € 20.9 m of R&D expenses and € 70.6 m of liquidity.
The revised guidance was fully achieved and the original financial objectives (as stated in the ‘Outlook’ of the 2008 annual report) were well surpassed. As expected, the operating result before impairment charges improved over 2008.
Performance against Forecasts
Forecast March 09 Forecast Aug 09 Final Results
Revenues €34-36m >€40m €42.7m
R&D expenses €32-35m <€25m* €20.9m
Operating result before impairm./restruct. Improved over 2008 Improved over 2008 Improved over 2008: €(19.6)m
Liquidity 3 year runway >€65m €70.6m
* Guidance for R&D expenses was altered at the beginning of the fourth quarter
RESULTS OF OPERATIONS
REVENUES
STRONG PERFORMANCE ON THE BACK OF MAJOR RESEARCH ALLIANCES
Evotec Group revenues amounted to € 42.7 m, 8% above last year’s level (2008: € 39.6 m) due to a strong performance of the Company’s discovery alliances, the out-licensing of the EVT 100 compound family to Roche, license and royalty income from Develogen and Roche as well as additional revenues from the acquired RSIPL business. The increase over the previous year is particularly pleasing because, in 2008, Evotec collected three milestones amounting to payments of € 8.5 m as part of its strategic alliance with Boehringer Ingelheim, while in 2009 milestone payments added up to a lower € 4.0 m. The recognised portion of the Roche upfront payment amounted to € 3.0 m (2008: € 0.0 m) and license and royalty income was € 3.2 m (2008: € 1.5 m). Total revenues from Evotec’s drug discovery alliances before upfront, milestone, license and royalty income increased by 9% over 2008.
EVOTEC – ANNUAL REPORT 2009
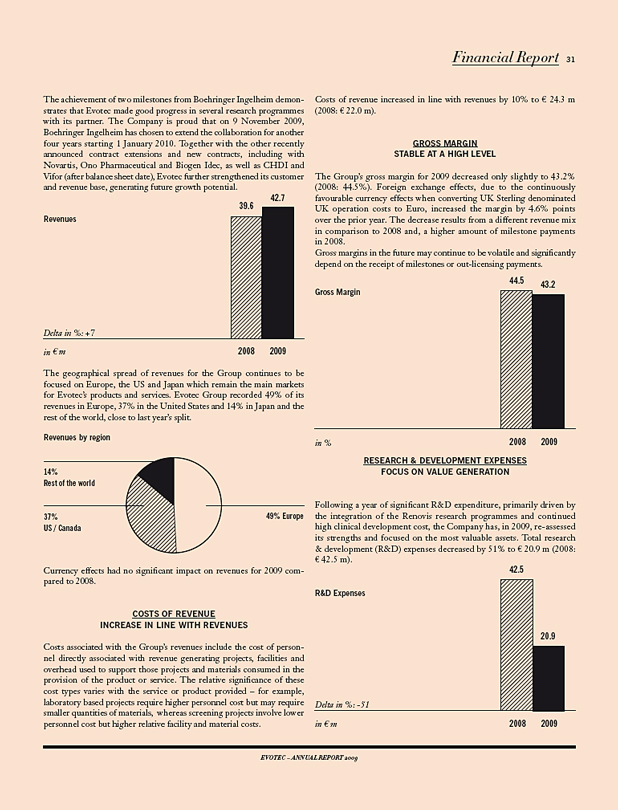
Financial Report 31
The achievement of two milestones from Boehringer Ingelheim demonstrates that Evotec made good progress in several research programmes with its partner. The Company is proud that on 9 November 2009, Boehringer Ingelheim has chosen to extend the collaboration for another four years starting 1 January 2010. Together with the other recently announced contract extensions and new contracts, including with Novartis, Ono Pharmaceutical and Biogen Idec, as well as CHDI and Vifor (after balance sheet date), Evotec further strengthened its customer and revenue base, generating future growth potential.
42.7
39.6
Revenues
Delta in %: +7
in € m 2008 2009
The geographical spread of revenues for the Group continues to be focused on Europe, the US and Japan which remain the main markets for Evotec’s products and services. Evotec Group recorded 49% of its revenues in Europe, 37% in the United States and 14% in Japan and the rest of the world, close to last year’s split.
Revenues by region
14%
Rest of the world
37% US / Canada 49% Europe
Currency effects had no significant impact on revenues for 2009 compared to 2008.
COSTS OF REVENUE
INCREASE IN LINE WITH REVENUES
Costs associated with the Group’s revenues include the cost of personnel directly associated with revenue generating projects, facilities and overhead used to support those projects and materials consumed in the provision of the product or service. The relative significance of these cost types varies with the service or product provided – for example, laboratory based projects require higher personnel cost but may require smaller quantities of materials, whereas screening projects involve lower personnel cost but higher relative facility and material costs.
Costs of revenue increased in line with revenues by 10% to € 24.3 m (2008: € 22.0 m).
GROSS MARGIN
STABLE AT A HIGH LEVEL
The Group’s gross margin for 2009 decreased only slightly to 43.2% (2008: 44.5%). Foreign exchange effects, due to the continuously favourable currency effects when converting UK Sterling denominated UK operation costs to Euro, increased the margin by 4.6% points over the prior year. The decrease results from a different revenue mix in comparison to 2008 and, a higher amount of milestone payments in 2008.
Gross margins in the future may continue to be volatile and significantly depend on the receipt of milestones or out-licensing payments.
44.5
43.2
Gross Margin
in % 2008 2009
RESEARCH & DEVELOPMENT EXPENSES
FOCUS ON VALUE GENERATION
Following a year of significant R&D expenditure, primarily driven by the integration of the Renovis research programmes and continued high clinical development cost, the Company has, in 2009, re-assessed its strengths and focused on the most valuable assets. Total research & development (R&D) expenses decreased by 51% to € 20.9 m (2008: € 42.5 m).
42.5
R&D Expenses
20.9
Delta in %: -51
in € m 2008 2009
EVOTEC – ANNUAL REPORT 2009
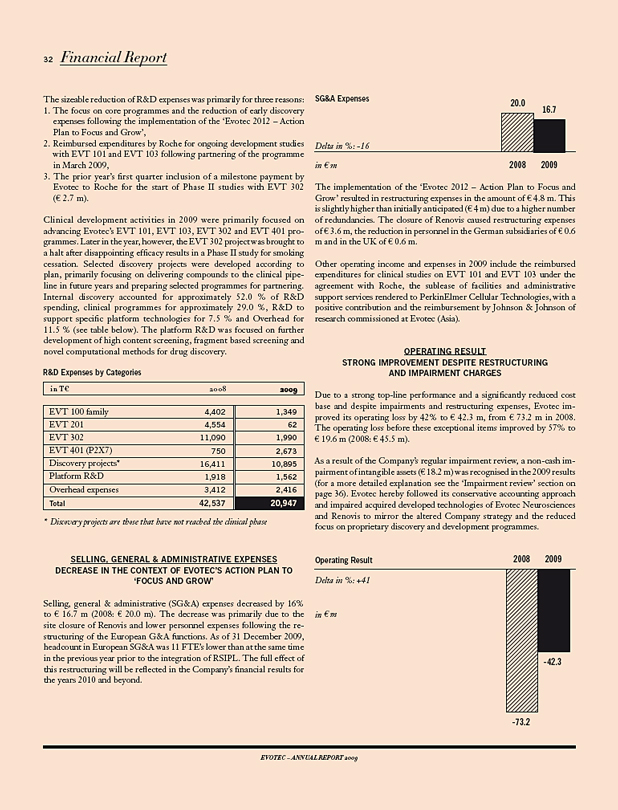
32 Financial Report
The sizeable reduction of R&D expenses was primarily for three reasons:
1. The focus on core programmes and the reduction of early discovery expenses following the implementation of the ‘Evotec 2012 – Action Plan to Focus and Grow’,
2. Reimbursed expenditures by Roche for ongoing development studies with EVT 101 and EVT 103 following partnering of the programme in March 2009,
3. The prior year’s first quarter inclusion of a milestone payment by Evotec to Roche for the start of Phase II studies with EVT 302 (€ 2.7 m).
Clinical development activities in 2009 were primarily focused on advancing Evotec’s EVT 101, EVT 103, EVT 302 and EVT 401 programmes. Later in the year, however, the EVT 302 project was brought to a halt after disappointing efficacy results in a Phase II study for smoking cessation. Selected discovery projects were developed according to plan, primarily focusing on delivering compounds to the clinical pipeline in future years and preparing selected programmes for partnering. Internal discovery accounted for approximately 52.0 % of R&D spending, clinical programmes for approximately 29.0 %, R&D to support specific platform technologies for 7.5 % and Overhead for 11.5 % (see table below). The platform R&D was focused on further development of high content screening, fragment based screening and novel computational methods for drug discovery.
R&D Expenses by Categories
in T€ 2008 2009
EVT 100 family 4,402 1,349
EVT 201 4,554 62
EVT 302 11,090 1,990
EVT 401 (P2X7) 750 2,673
Discovery projects* 16,411 10,895
Platform R&D 1,918 1,562
Overhead expenses 3,412 2,416
Total 42,537 20,947
* Discovery projects are those that have not reached the clinical phase
SELLING, GENERAL & ADMINISTRATIVE EXPENSES
DECREASE IN THE CONTEXT OF EVOTEC’S ACTION PLAN TO ‘FOCUS AND GROW’
Selling, general & administrative (SG&A) expenses decreased by 16% to € 16.7 m (2008: €20.0 m). The decrease was primarily due to the site closure of Renovis and lower personnel expenses following the restructuring of the European G&A functions. As of 31 December 2009, headcount in European SG&A was 11 FTE’s lower than at the same time in the previous year prior to the integration of RSIPL. The full effect of this restructuring will be reflected in the Company’s financial results for the years 2010 and beyond.
SG&A Expenses
20.0
16.7
Delta in %: -16
in €m 2008 2009
The implementation of the ‘Evotec 2012 – Action Plan to Focus and Grow’ resulted in restructuring expenses in the amount of € 4.8 m. This is slightly higher than initially anticipated (€ 4 m) due to a higher number of redundancies. The closure of Renovis caused restructuring expenses of € 3.6 m, the reduction in personnel in the German subsidiaries of € 0.6 m and in the UK of € 0.6 m.
Other operating income and expenses in 2009 include the reimbursed expenditures for clinical studies on EVT 101 and EVT 103 under the agreement with Roche, the sublease of facilities and administrative support services rendered to PerkinElmer Cellular Technologies, with a positive contribution and the reimbursement by Johnson & Johnson of research commissioned at Evotec (Asia).
OPERATING RESULT
STRONG IMPROVEMENT DESPITE RESTRUCTURING AND IMPAIRMENT CHARGES
Due to a strong top-line performance and a significantly reduced cost base and despite impairments and restructuring expenses, Evotec improved its operating loss by 42% to € 42.3 m, from € 73.2 m in 2008. The operating loss before these exceptional items improved by 57% to € 19.6 m (2008: € 45.5 m).
As a result of the Company’s regular impairment review, a non-cash impairment of intangible assets (€ 18.2 m) was recognised in the 2009 results (for a more detailed explanation see the ‘Impairment review’ section on page 36). Evotec hereby followed its conservative accounting approach and impaired acquired developed technologies of Evotec Neurosciences and Renovis to mirror the altered Company strategy and the reduced focus on proprietary discovery and development programmes.
Operating Result 2008 2009
Delta in %: +41
in €m
-42.3
-73.2
EVOTEC – ANNUAL REPORT 2009
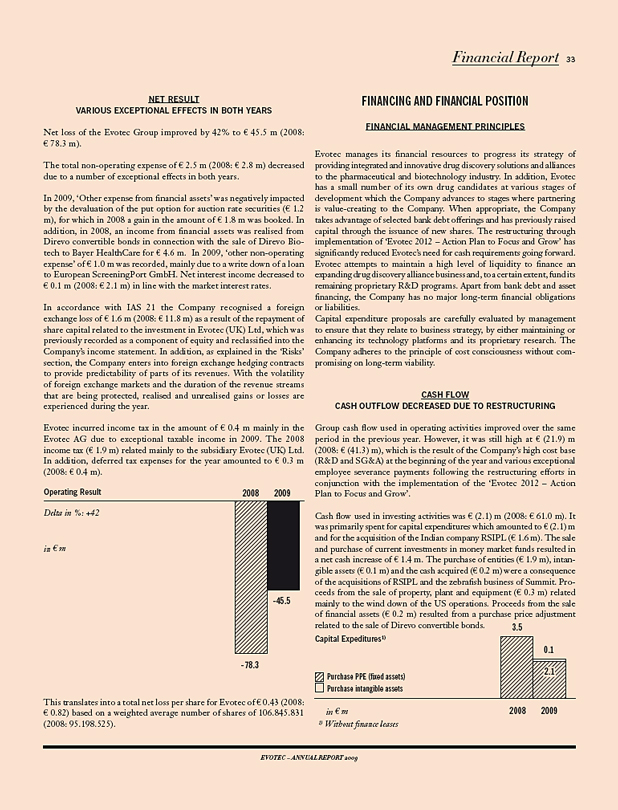
Financial Report 33
NET RESULT
VARIOUS EXCEPTIONAL EFFECTS IN BOTH YEARS
Net loss of the Evotec Group improved by 42% to € 45.5 m (2008: € 78.3 m).
The total non-operating expense of € 2.5 m (2008: € 2.8 m) decreased due to a number of exceptional effects in both years.
In 2009, ‘Other expense from financial assets’ was negatively impacted by the devaluation of the put option for auction rate securities (€ 1.2 m), for which in 2008 a gain in the amount of € 1.8 m was booked. In addition, in 2008, an income from financial assets was realised from Direvo convertible bonds in connection with the sale of Direvo Bio-tech to Bayer HealthCare for € 4.6 m. In 2009, ‘other non-operating expense’ of € 1.0 m was recorded, mainly due to a write down of a loan to European ScreeningPort GmbH. Net interest income decreased to € 0.1 m (2008: € 2.1 m) in line with the market interest rates.
In accordance with IAS 21 the Company recognised a foreign exchange loss of € 1.6 m (2008: € 11.8 m) as a result of the repayment of share capital related to the investment in Evotec (UK) Ltd, which was previously recorded as a component of equity and reclassified into the Company’s income statement. In addition, as explained in the ‘Risks’ section, the Company enters into foreign exchange hedging contracts to provide predictability of parts of its revenues. With the volatility of foreign exchange markets and the duration of the revenue streams that are being protected, realised and unrealised gains or losses are experienced during the year.
Evotec incurred income tax in the amount of € 0.4 m mainly in the Evotec AG due to exceptional taxable income in 2009. The 2008 income tax (€ 1.9 m) related mainly to the subsidiary Evotec (UK) Ltd. In addition, deferred tax expenses for the year amounted to € 0.3 m (2008: € 0.4 m).
Operating Result 2008 2009
Delta in %: +42
in € m -45.5 -78.3
This translates into a total net loss per share for Evotec of € 0.43 (2008: € 0.82) based on a weighted average number of shares of 106.845.831 (2008: 95.198.525).
FINANCING AND FINANCIAL POSITION
FINANCIAL MANAGEMENT PRINCIPLES
Evotec manages its financial resources to progress its strategy of providing integrated and innovative drug discovery solutions and alliances to the pharmaceutical and biotechnology industry. In addition, Evotec has a small number of its own drug candidates at various stages of development which the Company advances to stages where partnering is value-creating to the Company. When appropriate, the Company takes advantage of selected bank debt offerings and has previously raised capital through the issuance of new shares. The restructuring through implementation of ‘Evotec 2012 – Action Plan to Focus and Grow’ has significantly reduced Evotec’s need for cash requirements going forward. Evotec attempts to maintain a high level of liquidity to finance an expanding drug discovery alliance business and, to a certain extent, fund its remaining proprietary R&D programs. Apart from bank debt and asset financing, the Company has no major long-term financial obligations or liabilities.
Capital expenditure proposals are carefully evaluated by management to ensure that they relate to business strategy, by either maintaining or enhancing its technology platforms and its proprietary research. The Company adheres to the principle of cost consciousness without compromising on long-term viability.
CASH FLOW
CASH OUTFLOW DECREASED DUE TO RESTRUCTURING
Group cash flow used in operating activities improved over the same period in the previous year. However, it was still high at € (21.9) m (2008: € (41.3) m), which is the result of the Company’s high cost base (R&D and SG&A) at the beginning of the year and various exceptional employee severance payments following the restructuring efforts in conjunction with the implementation of the ‘Evotec 2012 – Action Plan to Focus and Grow’.
Cash flow used in investing activities was € (2.1) m (2008: € 61.0 m). It was primarily spent for capital expenditures which amounted to € (2.1) m and for the acquisition of the Indian company RSIPL (€ 1.6 m). The sale and purchase of current investments in money market funds resulted in a net cash increase of €1.4 m. The purchase of entities (€ 1.9 m), intangible assets (€ 0.1 m) and the cash acquired (€ 0.2 m) were a consequence of the acquisitions of RSIPL and the zebrafish business of Summit. Proceeds from the sale of property, plant and equipment (€ 0.3 m) related mainly to the wind down of the US operations. Proceeds from the sale of financial assets (€ 0.2 m) resulted from a purchase price adjustment related to the sale of Direvo convertible bonds.
Capital Expeditures1)
3.5
0.1
2.1
Purchase PPE (fixed assets)
Purchase intangible assets
in €m 2008 2009
1) Without finance leases
EVOTEC – ANNUAL REPORT 2009
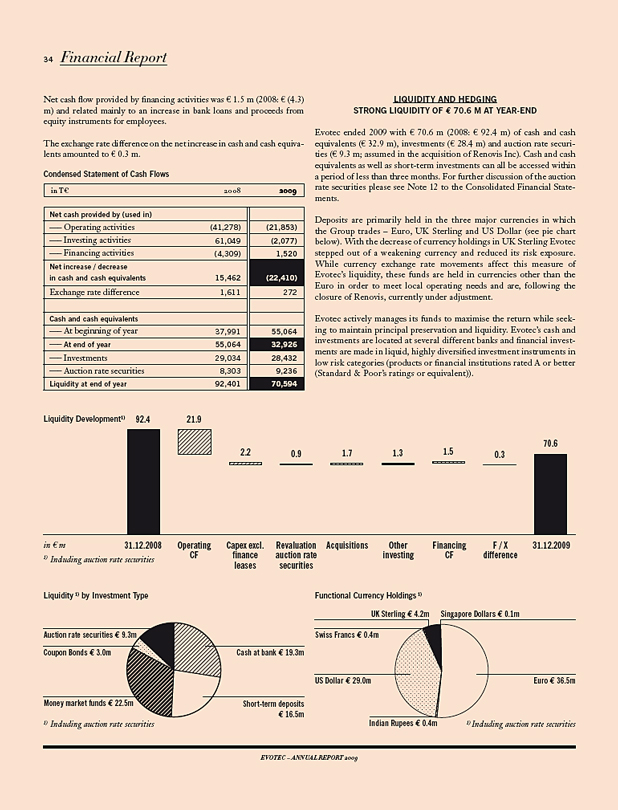
34 Financial Report
Net cash flow provided by financing activities was € 1.5 m (2008: € (4.3) m) and related mainly to an increase in bank loans and proceeds from equity instruments for employees.
The exchange rate difference on the net increase in cash and cash equivalents amounted to € 0.3 m.
Condensed Statement of Cash Flows
in T€ 2008 2009
Net cash provided by (used in)
— Operating activities (41,278) (21,853)
— Investing activities 61,049 (2,077)
— Financing activities (4,309) 1,520
Net increase / decrease in cash and cash equivalents 15,462 (22,410)
Exchange rate difference 1,611 272
Cash and cash equivalents
— At beginning of year 37,991 55,064
— At end of year 55,064 32,926
— Investments 29,034 28,432
— Auction rate securities 8,303 9,236
Liquidity at end of year 92,401 70,594
LIQUIDITY AND HEDGING
STRONG LIQUIDITY OF € 70.6 M AT YEAR-END
Evotec ended 2009 with € 70.6 m (2008: € 92.4 m) of cash and cash equivalents (€ 32.9 m), investments (€ 28.4 m) and auction rate securities (€ 9.3 m; assumed in the acquisition of Renovis Inc). Cash and cash equivalents as well as short-term investments can all be accessed within a period of less than three months. For further discussion of the auction rate securities please see Note 12 to the Consolidated Financial Statements.
Deposits are primarily held in the three major currencies in which the Group trades – Euro, UK Sterling and US Dollar (see pie chart below). With the decrease of currency holdings in UK Sterling Evotec stepped out of a weakening currency and reduced its risk exposure. While currency exchange rate movements affect this measure of Evotec’s liquidity, these funds are held in currencies other than the Euro in order to meet local operating needs and are, following the closure of Renovis, currently under adjustment.
Evotec actively manages its funds to maximise the return while seeking to maintain principal preservation and liquidity. Evotec’s cash and investments are located at several different banks and financial investments are made in liquid, highly diversified investment instruments in low risk categories (products or financial institutions rated A or better (Standard & Poor’s ratings or equivalent)).
Liquidity Development1) 92.4 21.9
70.6
2.2 0.9 1.7 1.3 1.5 0.3
in €m 31.12.2008 Operating CF Capex excl. finance leases Revaluation auction rate securities Acquisitions Other Financing CF F / X difference 31.12.2009
1) Including auction rate securities
Liquidity 1) by Investment Type
Auction rate securities € 9.3m
Coupon Bonds € 3.0m
Money market funds € 22.5m
1) Including auction rate securities
Cash at bank € 19.3m
Short-term deposits €16.5m
Functional Currency Holdings 1)
UK Sterling € 4.2m
Singapore Dollars € 0.1m
Swiss Francs € 0.4m
US Dollar € 29.0m
Euro € 36.5m
Indian Rupees €0.4m
1) Including auction rate securities
EVOTEC – ANNUAL REPORT 2009
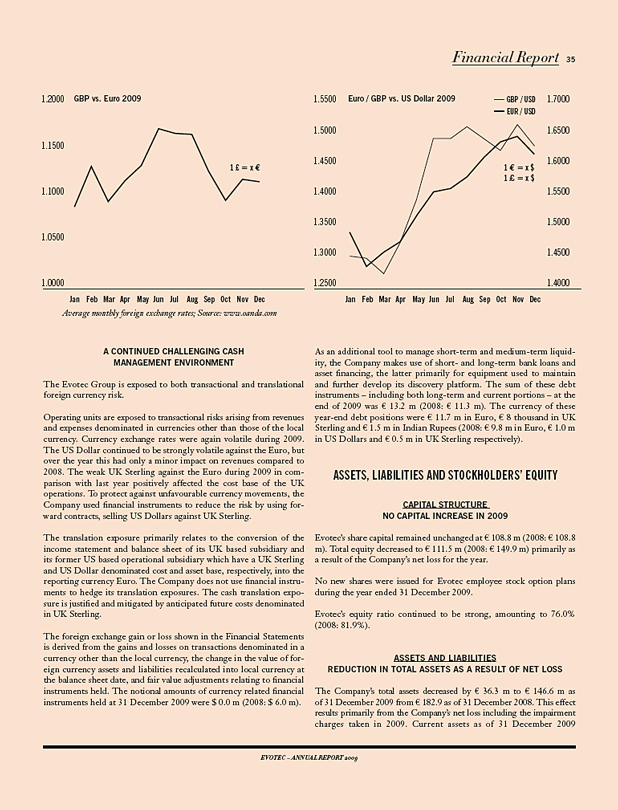
Financial Report 35
1.2000 GBP vs. Euro 2009
1.1500
1 £ = x €
1.1000
1.0500
1.0000
Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec
Average monthly foreign exchange rates; Source: www.oanda.com
A CONTINUED CHALLENGING CASH MANAGEMENT ENVIRONMENT
The Evotec Group is exposed to both transactional and translational foreign currency risk.
Operating units are exposed to transactional risks arising from revenues and expenses denominated in currencies other than those of the local currency. Currency exchange rates were again volatile during 2009. The US Dollar continued to be strongly volatile against the Euro, but over the year this had only a minor impact on revenues compared to 2008. The weak UK Sterling against the Euro during 2009 in comparison with last year positively affected the cost base of the UK operations. To protect against unfavourable currency movements, the Company used financial instruments to reduce the risk by using forward contracts, selling US Dollars against UK Sterling.
The translation exposure primarily relates to the conversion of the income statement and balance sheet of its UK based subsidiary and its former US based operational subsidiary which have a UK Sterling and US Dollar denominated cost and asset base, respectively, into the reporting currency Euro. The Company does not use financial instruments to hedge its translation exposures. The cash translation exposure is justified and mitigated by anticipated future costs denominated in UK Sterling.
The foreign exchange gain or loss shown in the Financial Statements is derived from the gains and losses on transactions denominated in a currency other than the local currency, the change in the value of foreign currency assets and liabilities recalculated into local currency at the balance sheet date, and fair value adjustments relating to financial instruments held. The notional amounts of currency related financial instruments held at 31 December 2009 were $ 0.0 m (2008: $ 6.0 m).
1.5500 Euro / GBP vs. US Dollar 2009 GBP / USD 1.7000
EUR / USD
1.5000 1.6500
1.4500 1.6000
1 € = x $
1 £ = x $
1.4000 1.5500
1.3500 1.5000
1.3000 1.4500
1.2500 1.4000
Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec
As an additional tool to manage short-term and medium-term liquidity, the Company makes use of short- and long-term bank loans and asset financing, the latter primarily for equipment used to maintain and further develop its discovery platform. The sum of these debt instruments – including both long-term and current portions – at the end of 2009 was € 13.2 m (2008: € 11.3 m). The currency of these year-end debt positions were € 11.7 m in Euro, € 8 thousand in UK Sterling and € 1.5 m in Indian Rupees (2008: € 9.8 m in Euro, € 1.0 m in US Dollars and € 0.5 m in UK Sterling respectively).
ASSETS, LIABILITIES AND STOCKHOLDERS’ EQUITY
CAPITAL STRUCTURE
NO CAPITAL INCREASE IN 2009
Evotec’s share capital remained unchanged at € 108.8 m (2008: € 108.8 m). Total equity decreased to € 111.5 m (2008: € 149.9 m) primarily as a result of the Company’s net loss for the year.
No new shares were issued for Evotec employee stock option plans during the year ended 31 December 2009.
Evotec’s equity ratio continued to be strong, amounting to 76.0% (2008: 81.9%).
ASSETS AND LIABILITIES
REDUCTION IN TOTAL ASSETS AS A RESULT OF NET LOSS
The Company’s total assets decreased by € 36.3 m to € 146.6 m as of 31 December 2009 from € 182.9 as of 31 December 2008. This effect results primarily from the Company’s net loss including the impairment charges taken in 2009. Current assets as of 31 December 2009
EVOTEC – ANNUAL REPORT 2009

36 Financial Report
decreased by € 24.1 m to € 69.0 m primarily as a result of cash used in operations as well as decrease in taxes receivable.
In 2009, current liabilities increased by € 4.6 m to € 26.4 m primarily as a result of an increase in current maturities of long-term loans and deferred revenues partially offset by smaller decreases in trade accounts payable, provisions and current income taxes.
Total non-current liabilities decreased by € 2.5 m to € 8.7 m at 31 December 2009. The increase primarily results from deferred revenues linked to the upfront payment for the EVT 100 compound series from Roche.
Condensed Statement of Financial Position
in T€ 2008 2009
Cash, cash equivalents and investments 84,098 58,358
Trade account receivables 2,531 4,510
Inventories 2,139 2,425
Other current assets 4,310 3,664
Property, plant and equipment 18,468 19,162
Intangible assets and goodwill 60,455 45,567
Other non-current assets1) 10,899 12,913
Total assets 182,900 146,599
Trade account payable 7,191 5,235
Current provisions 6,859 4,858
Other current liabilities 7,776 16,352
Long-term liabilities 9,752 6,690
Deferred tax liabilities 1,463 1,977
Total stockholders’ equity 149,859 111,487
Total liabilities and stockholders’ equity 182,900 146,599
1) Including auction rate securities
Working Capital Calculation
in T€ 2008 2) 2009
Trade account receivables 2,531 4,510
Inventories 2,139 2,425
Other current assets 3,449 3,664
Assets 8,119 10,599
Trade account payable 7,191 5,235
Current provisions 6,859 4,858
Other current liabilities1) 7,776 7,036
Liabilities 21,826 17,129
Working Capital (13,707) (6,530)
D Working Capital 7,177
1) Excluding loans and finance leases
2) 2008: Including acquired Renovis assets
OFF-BALANCE-SHEET FINANCING
The Company is not involved in any off-balance sheet financing transactions.
IMPAIRMENT REVIEW
The Company performed its annual regular review of tangible and intangible assets for potential impairment in accordance with IFRS during the final quarter of 2009.
A review of previously impaired fixed assets resulted in an unimpairment of € 0.4 m being recognised against certain operational assets due to improved asset utilisation in one of the two remaining buildings at the Abingdon site.
The goodwill that originated from the acquisition of Renovis Inc was impaired by € 48 thousand as consequence of the restructuring and closure of its South San Francisco facility. The company additionally performed an impairment analysis of the goodwill attributable to the laboratory-based Abingdon operations and concluded that no need for an impairment existed.
The Company performed an impairment review of the intangible assets acquired from Evotec Neurosciences (ENS) in 2005 and an impairment charge of € 4.4 m was taken against EVT 201, as a result of the continuously difficult partnering situation. The Company additionally performed an impairment analysis of the Renovis intangible assets and concluded that the VR1 programme and EVT 401 both had to be impaired. The VR1 programme was impaired in the first quarter (€ 6.6 m) due to the delay in its collaboration with Pfizer and further impaired by € 0.4 m in the fourth quarter. The latter is not a result of scientific results but due to changes in capital market conditions. Despite the development of EVT 401 according to plan, management decided to impair its value by € 6.8 m as a consequence of uncertainties regarding its mechanism of action, as revealed by the termination of a related development programme by AstraZeneca.
EVOTEC – ANNUAL REPORT 2009

Non-Financial Performance Indicators 37
NON-FINANCIAL PERFORMANCE INDICATORS
A number of important non-financial performance measures relating to Evotec’s growth objectives, are described in detail in the section “Growth Strategy and Non-Financial Performance Measures” on page 27 of this Management Report.
INTELLECTUAL PROPERTY
Evotec actively manages its own patent portfolio from the very early stage of an invention. Evotec seeks, when appropriate, patent protection for its technologies, product candidates, and other proprietary information.
Evotec reviews its patent portfolio regularly and decides whether to maintain or withdraw its patent applications and patents based on the importance of such intellectual property for its strategy. Reflecting the diligent review of the patent portfolio, as of 31 December 2009, Evotec had more than 110 (2008: 130) patent and utility model families under its full control. All of these are on file, or pending through national and/ or foreign applications such as patent applications filed under the Patent Cooperation Treaty, or applications filed with the United States Patent Office, the European Patent Office, or the Japanese Patent Office.
Supporting its discovery alliance business, Evotec owns a patent estate for detection and other platform technologies. Furthermore Evotec has developed a number of biological assays, eg. methods to measure the chemical or biological activity of any combination of targets and compounds, which are patent protected.
Evotec also pursues selected own discovery projects and thereby has a pipeline of drug candidates that have the potential to provide compounds for partnering. With this in mind, Evotec monitors the research activities and results of in-house research in order to identify potentially patentable drug candidate series. Numerous patent applications have been filed so far for such series. In addition, pursuant to agreements with Roche, Evotec has exclusively in-licensed several drug candidates, including the EVT 100 family, EVT 201 and EVT 302. These are protected by diverse composition of matter patent families as well as their therapeutic use in major countries worldwide.
Furthermore, with its deep knowledge in CNS-related diseases, Evotec has established a solid position in the identification and validation of molecular targets involved in Alzheimer’s disease and other neuro-degenerative diseases. Over the past few years, Evotec has built a patent portfolio that covers the use of such targets for diagnostic and drug discovery purposes.
INFORMATION TECHNOLOGY
In 2009, Evotec continued with the rationalisation of its IT infrastructure, implementing virtual servers and increasing its centralised network storage. The new infrastructure provides the Company with a more resilient, flexible and easy to manage IT environment and will result in cost savings on server hardware in the future.
All material Group IT procedures and policies were internally checked for SOX compliance. In addition, the infrastructure of the acquired businesses (RSIPL and Summit’s zebrafish unit) was integrated into the Company’s network.
PROCUREMENT AND FACILITY MANAGEMENT
Procurement processes were harmonised across Evotec’s European sites backed by the newly established ERP system. Through the process of targeted strategic cost review the procurement functions identified significant cost benefits across 2009 in line with the cost efficiency process led by the Board. An emphasis on the procurement process as part of significant contract discussions and capital expenditure plans meant that significant savings were identified both with new suppliers as well as on existing supplier terms. Procurement sought to maximise the quality of the goods and services obtained by Evotec but to ensure that these were provided on favourable terms and mindful of managing ongoing supplier risk and the ethical expectations of the Group. In Hamburg, stocks were reduced despite growing business, resulting in a decrease of bound capital and administrative activities.
As a percentage of revenues, procurement costs decreased from 15.9% in 2008 to 13.4% in 2009.
A change in the facility management provider for the Abingdon site in 2009 is the result of a careful review of the requirements of the business to maintain a quality and functioning facility at a competitive cost. Facility costs in Hamburg decreased significantly over the last years, and we expect to see this in the UK following the change to a new management provider.
Evotec was protected from the fluctuations in energy prices by having annually awarded energy contracts with fixed pricing for most of its supply. Energy costs relative to revenues remained stable at 1.5% in 2008 and 1.6% in 2009.
EVOTEC – ANNUAL REPORT 2009
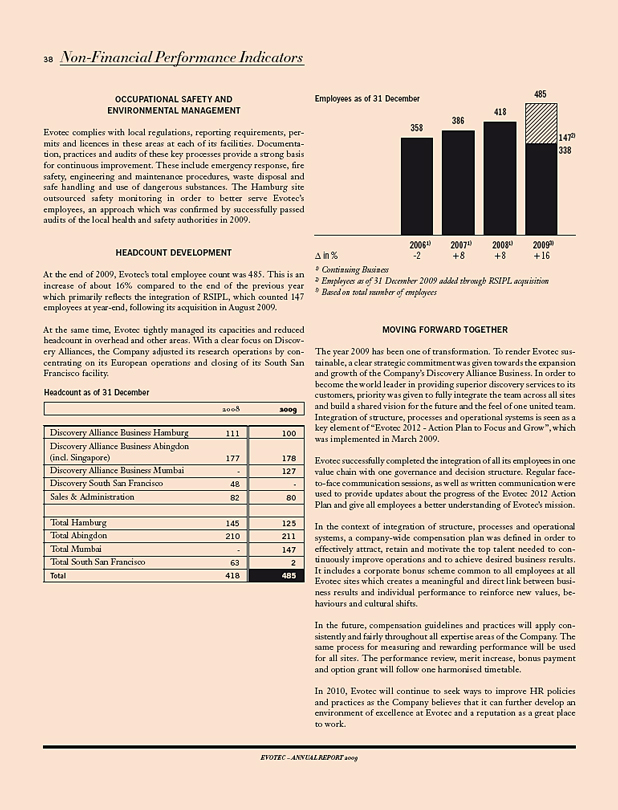
38 Non-Financial Performance Indicators
OCCUPATIONAL SAFETY AND ENVIRONMENTAL MANAGEMENT
Evotec complies with local regulations, reporting requirements, permits and licences in these areas at each of its facilities. Documentation, practices and audits of these key processes provide a strong basis for continuous improvement. These include emergency response, fire safety, engineering and maintenance procedures, waste disposal and safe handling and use of dangerous substances. The Hamburg site outsourced safety monitoring in order to better serve Evotec’s employees, an approach which was confirmed by successfully passed audits of the local health and safety authorities in 2009.
HEADCOUNT DEVELOPMENT
At the end of 2009, Evotec’s total employee count was 485. This is an increase of about 16% compared to the end of the previous year which primarily reflects the integration of RSIPL, which counted 147 employees at year-end, following its acquisition in August 2009.
At the same time, Evotec tightly managed its capacities and reduced headcount in overhead and other areas. With a clear focus on Discovery Alliances, the Company adjusted its research operations by concentrating on its European operations and closing of its South San Francisco facility.
Headcount as of 31 December
2008 2009
Discovery Alliance Business Hamburg 111 100
Discovery Alliance Business Abingdon (incl. Singapore) 177 178
Discovery Alliance Business Mumbai - 127
Discovery South San Francisco 48 -
Sales & Administration 82 80
Total Hamburg 145 125
Total Abingdon 210 211
Total Mumbai - 147
Total South San Francisco 63 2
Total 418 485
Employees as of 31 December
485 358 386 418
1472) 338
20061) -2 20071) +8 20081) +8 20093) +16
D in %
1) Continuing Business
2) Employees as of 31 December 2009 added through RSIPL acquisition
3) Based on total number of employees
MOVING FORWARD TOGETHER
The year 2009 has been one of transformation. To render Evotec sustainable, a clear strategic commitment was given towards the expansion and growth of the Company’s Discovery Alliance Business. In order to become the world leader in providing superior discovery services to its customers, priority was given to fully integrate the team across all sites and build a shared vision for the future and the feel of one united team. Integration of structure, processes and operational systems is seen as a key element of “Evotec 2012 - Action Plan to Focus and Grow”, which was implemented in March 2009.
Evotec successfully completed the integration of all its employees in one value chain with one governance and decision structure. Regular face-to-face communication sessions, as well as written communication were used to provide updates about the progress of the Evotec 2012 Action Plan and give all employees a better understanding of Evotec’s mission.
In the context of integration of structure, processes and operational systems, a company-wide compensation plan was defined in order to effectively attract, retain and motivate the top talent needed to continuously improve operations and to achieve desired business results. It includes a corporate bonus scheme common to all employees at all Evotec sites which creates a meaningful and direct link between business results and individual performance to reinforce new values, behaviours and cultural shifts.
In the future, compensation guidelines and practices will apply consistently and fairly throughout all expertise areas of the Company. The same process for measuring and rewarding performance will be used for all sites. The performance review, merit increase, bonus payment and option grant will follow one harmonised timetable.
In 2010, Evotec will continue to seek ways to improve HR policies and practices as the Company believes that it can further develop an environment of excellence at Evotec and a reputation as a great place to work.
EVOTEC – ANNUAL REPORT 2009

Remuneration Report 39
REMUNERATION REPORT
The Remuneration Report describes the Company’s remuneration structure and provides information about the payments to the board members in accordance with the requirements of the Corporate Governance Codex (the ‘Code’). It is part of both the Consolidated Financial Statements and the Corporate Governance Report.
REMUNERATION OF THE MANAGEMENT BOARD
The total compensation of the individual members of the Management Board is determined at an appropriate amount based on a performance assessment and is oriented towards sustainable growth of Evotec. Criteria for determining the appropriateness of compensation comprise the tasks of the individual member of the Management Board, their personal performance, the economic situation, the performance and outlook of Evotec as well as the common level of compensation taking into account Evotec’s peer companies and the compensation structure in place in other areas of the Company.
In 2009, fixed and variable remuneration of active members of the Management Board totalled T€ 1,201, of which the variable part amounted to T€ 395.
Fixed remuneration includes base salaries, contributions to personal pension plans, premiums for accident and accidental death insurances as well as the benefit derived from the use of company cars.
Variable remuneration is determined by a bonus scheme that is designed every year by the Remuneration and Nomination Committee of the Supervisory Board and is subsequently approved by the Supervisory Board. The variable portion of the remuneration paid out in 2009, payable upon the achievement of certain strategic targets for the business year 2008, was based on several criteria. For Dr Mario Polywka and Dr Klaus Maleck, 90% was based on the achievement of five defined corporate milestones and 10% on the achievement of personal objectives. In addition, Dr Mario Polywka and Dr Klaus Maleck were granted an extra bonus as compensation and incentive for jointly leading the Company during the period of vacancy of the CEO position until Dr Werner Lanthaler joined Evotec in March 2009.
The variable portion of the remuneration to be paid out in 2010 depends on the achievement of certain strategic targets for the business year 2009. For Dr Werner Lanthaler it will be based on the achievement of five sets of corporate milestones. For Dr Klaus Maleck and Dr Mario Polywka it will be based on these corporate milestones for 90 % of their bonus and for the remaining 10% on the achievement of personal objectives.
In addition to their fixed and variable remuneration, the members of the Management Board received a total of 700,000 stock options in 2009 under the Company’s stock option plans. The options granted in 2009 are subject to the stipulations of the Option Plans 2007 and 2008, respectively and may be exercised after three years if the conditions of these plans are met.
Remuneration of the Management Board 2009
Fixed Remuneration in T€
Variable Remuneration in T€
Stock Options in pcs
Fair Values Options granted in T€
Dr Werner Lanthaler 295 - 500,000 287
Dr Klaus Maleck 243 74 100,000 123
Dr Mario Polywka 268 321* 100,000 123
Total 806 395 700,000 533
* Including a onetime retention bonus of T€ 225
The contracts of the Management Board members contain a change-of-control clause that would allow them, in the event of a takeover of the Company, to terminate their current contracts. Such a change-of-control occurs when a new investor assumes more than 30% of the shares of the Company. Upon contract termination the Management Board members Dr Maleck and Dr Polywka are entitled to severance payments of one year’s base salary plus bonus, calculated on the basis of the prior year’s remuneration, while Dr Lanthaler is entitled to two years base salary, but no more than the total remuneration due for the remaining term of the contract.
When Jörn Aldag resigned from the Company’s Management Board effective 31 December 2008, he and Evotec entered into a non-competition agreement exceeding the regular duration of his service agreement. They also agreed upon a lump-sum payment equivalent to the remuneration that Jörn Aldag would have received had his contract expired and not been terminated. No further severance payments were agreed. The exit agreement stipulated gross total payments of T€ 2,022 to Jörn Aldag. These payments consisted of T€ 573 fixed and T€ 805 variable remuneration for the period until his contract would have expired, if not terminated, as well as T€ 644 for the non-competition agreement. Of this sum, T€ 1,700 were paid in early 2009 and the remaining T€ 322 in early 2010. The total amount was accrued in 2008.
Apart from the payments to Jörn Aldag, no payments were made in 2009 to any former Management Board member.
The Company has made a provision for pension for one former Management Board member amounting to T€ 105. No further such provisions are due for other former Management Board members or their surviving dependents.
EVOTEC – ANNUAL REPORT 2009

40 Remuneration Report
REMUNERATION OF THE SUPERVISORY BOARD
The general principles of Supervisory Board remuneration are set forth in the Company’s Articles of Association by the Annual Shareholder Meeting.
The members of Evotec’s Supervisory Board are entitled to fixed and performance-based payments. In accordance with the recommendations of the Corporate Governance Code, Chair and Deputy Chair positions on the Supervisory Board, as well as the chair positions and membership on committees are considered when determining the remuneration of individual members. Every Supervisory Board member receives T€ 15 per year, with the Chair receiving three times that amount and the Deputy Chair twice that amount. Members of Supervisory Board committees additionally receive T€ 3.75 per year, with the chairperson receiving T€ 10.
In addition to the fixed remuneration and in accordance with the suggestions of the Code, the members of the Supervisory Board receive payments tied to the Company’s long-term performance, in the form of Evotec shares. Ordinary members of the Supervisory Board receive shares valued at T€ 10 (Chair three times, Deputy Chair twice this amount) and Committee Chairs receive additional shares valued at T€ 10. This share-based remuneration serves as a further incentive for Supervisory Board members to focus on the Evotec share price. In addition, if Evotec shareholders are paid a dividend, every Supervisory Board member will receive an extra T€ 0.5 for every cent that the dividend per share exceeds € 0.15.
For their contributions in 2009, the individual members of the Evotec Supervisory Board receive the following compensation:
Remuneration of the Supervisory Board 2009
T€
Cash Remuneration
Value of Share based Remuneration
Total
Dr Flemming Ørnskov 51.4 34.2 85.6
Dr Corey Goodman 25.2 15.8 41.0
Dr Hubert Birner 28.7 20.0 48.7
Dr Peter Fellner 18.8 10.0 28.8
Mary Tanner 18.8 10.0 28.8
Dr Walter Wenninger 1) 16.5 11.5 28.0
John Walker 2) 10.6 8.5 19.1
Total 170.0 110.0 280.0
1) Elected by the Annual Shareholder Meeting on 4 June 2009
2) Tenure ended with the Annual Shareholder Meeting on 4 June 2009
After his resignation from the Supervisory Board in August 2008, Professor Dr Heinz Riesenhuber entered into a two-year consultancy agreement with Evotec. Thus the Company is able to call upon Professor Dr Riesenhuber’s knowledge and expertise of the Company’s business activities and its business environment. The agreed compensation amounted to T€ 22.5 in the first 12 months and to T€ 25.0 in the last 12 months of the agreement.
For the period from December 2008 to June 2009, the Company had entered into a consultancy agreement with flemmingo GmbH, a consultancy firm led by Evotec’s Supervisory Board Chairman Dr Flemming Ørnskov. Reaching well beyond Dr Ørnskov’s duties as Chairman of the Supervisory Board, flemmingo GmbH consulted and represented the Company in discussions and negotiations with potential industry partners about partnering of Evotec’s drug candidates. The firm also provided Evotec consulting services related to the finalisation of a strategic project. With the approval of the Supervisory Board the consultancy firm received T€ 25 per month for its services.
Dr Corey Goodman, member of Evotec’s Supervisory Board until 31 January 2010, was a member of the Company’s Scientific Advisory Board until the end of 2009. For his activities on the Scientific Advisory Board, the Company, with the approval of the Supervisory Board, entered into an agreement with Dr Goodman which entitled him to a yearly payment of T€ 10 plus T€ 2 per participation in a full day physical meeting of the Scientific Advisory Board.
There are currently no further consultancy agreements between Evotec and current or former members of the Supervisory Board.
DIRECTORS AND OFFICERS LIABILITY INSURANCE
(D&O INSURANCE)
Evotec has procured directors and officers liability insurance coverage for its Management and Supervisory Board members, its senior management and the directors of its subsidiaries, at a cost to the Company of T€ 180 in 2009. For the Management and Supervisory Board members an appropriately sized deductible was agreed upon.
EVOTEC – ANNUAL REPORT 2009

Information section 315 para 4 41
INFORMATION PURSUANT TO SECTION 315 PARAGRAPH 4 OF THE
GERMAN COMMERCIAL CODE
Evotec’s management focuses on value creation. To that degree, any change-of-control or takeover offer that realises some of the embedded value of the Company for the benefit of current shareholders is carefully analysed with regard to the synergies proposed and the future value creation claimed. A change in control will generally have occurred if, as a result of any takeover, exchange or other transfer, a single shareholder or a group of shareholders acting in concert acquires more than 30% of the outstanding voting rights in Evotec or, if as a result of a merger or reverse merger the shareholders of Evotec from the effective date of such transaction cease to own more than 30% of the outstanding voting shares in the merged entity. Evotec has no specific takeover-defence measures in place.
COMPOSITION OF CAPITAL STOCK AND VOTING RIGHTS
As of 31 December 2009 the capital stock of Evotec AG amounted to
€ 108,838,715.00 and was divided into 108,838,715 non-par value shares. All shares are bearer shares and have the same voting rights. Management is not aware of any restriction of the voting rights or the right to transfer. Existing stock option schemes do not allow for immediate vesting or additional issuance in the case of a takeover offer. Also, no binding lock-up agreements have been made with any shareholder, and neither stock loans, nor pre-emptive stock purchase rights are known to the Company. The Company does not control voting rights of any shares owned by employees. No shareholder holds the right to have representatives on the Company’s Supervisory Board, or is restricted or bound to specific votes at annual shareholder meetings.
SHAREHOLDINGS EXCEEDING 10% OF VOTING RIGHTS
In September 2009, Evotec was notified by its shareholder Roland Oetker that he, via ROI Verwaltungsgesellschaft mbH, Königsallee 20, 40212 Düsseldorf, Germany, owned 11.07% of the shares of the Company. The Company is not aware of any other direct or indirect shareholdings in its share capital exceeding 10% of its capital.
BOARD STRUCTURE
The board structure of Evotec is explained in detail in the section ‘Group Management and Supervision’ on page 27 of this Management Report. Pursuant to section 6 of the Company’s Articles of Association, the Management Board shall consist of one or more members which are appointed and dismissed by the Supervisory Board pursuant to section 84 paragraph 1 of the German Stock Corporation Act (Aktiengesetz).
AUTHORISATION OF MANAGEMENT BOARD TO ISSUE SHARES
The shareholders have provided the Management Board with the following authorisations to issue new shares or conversion rights: 1. Authorised Capital: Pursuant to section 5 paragraph 4 of the Articles of Association the Management Board, with the approval of the Supervisory Board, is authorised to increase the Company’s share capital by up to € 21,733,878.00 in one or more tranches by 27 August 2013 by issuing new shares against cash or non-cash consideration. Any shares to be issued on this basis will be subject to the statutory subscription rights of Evotec’s shareholders. Given the approval of the Supervisory Board, the Management Board may, however, exclude the pre-emptive rights of its shareholders on one or several occasions under certain, well defined conditions.
2. Conditional Capital: The shareholders created conditional capital in the total amount of € 10,826,681.00, divided into 10,826,681 ordinary bearer shares with no par value. The Management Board, with the approval of the Supervisory Board, is authorised to increase the share capital only to the extent that holders of stock options, awarded by Evotec on the basis of the shareholders’ resolutions from
7 June 1999, 26 June 2000, 18 June 2001, 7 June 2005, 30 May 2007 or
28 August 2008 exercise their rights to subscribe for the new shares. Currently, after certain holders of stock options have exercised 227,301 stock options in the past and new shares have been issued from this conditional capital accordingly, an amount of 10,599,380 remains for new shares to be issued from this conditional capital as further outlined in section 5 paragraphs 5 to 11 of the Articles of Association.
Evotec AG has not issued any convertible bonds or option debentures in the last three years and none are currently outstanding.
EVOTEC – ANNUAL REPORT 2009

42 Information section 315 para 4
AUTHORISATION OF MANAGEMENT TO REPURCHASE STOCK
As of 31 December 2009, and as authorised by the last shareholder meeting, the Company is authorised to acquire by 3 December 2010 stock of the Company amounting to a mathematical share in the capital stock of altogether up to € 500,000.00 for the purpose of Supervisory Board compensation and to fulfil subscription rights that were and/or are granted within the framework of stock option programmes. To the extent that treasury stock is to be transferred to members of the Management Board such a decision is to be made by the Supervisory Board. In both cases, shareholders’ subscription rights shall be excluded and stock may not be acquired for trading purposes.
AMENDMENT TO THE COMPANY’S ARTICLES OF ASSOCIATION
Any amendment to the Company’s Articles of Association requires a shareholder resolution. According to section 15 of the Articles, the shareholder resolution amending the Company’s Articles of Association requires an affirmative vote of at least three quarters of the Company’s share capital present in a general shareholders’ meeting.
CHANGE-OF-CONTROL PROVISIONS
The Management Board of Evotec AG has only customary change-of- control rights. Their individual contracts contain a change-of-control clause which would allow management to terminate their current contracts in the event of a change-of-control. The resulting severance entitlement is equal to 24 months of base salary but no more than the total compensation due for the remaining term of the contract for Dr Werner Lanthaler and one year’s base salary plus bonus for Dr Klaus Maleck and Dr Mario Polywka. The remuneration of the Management Board is reported in more detail in Note 36 (e) to the Consolidated Financial Statements and in the ‘Remuneration Report’ on page 39 of this Management Report.
EVOTEC – ANNUAL REPORT 2009

Risk Management and Risk Report 43
RISK MANAGEMENT AND RISK REPORT
RISK AND OPPORTUNITY MANAGEMENT SYSTEM
Evotec is aware that it needs to regularly monitor and limit the risk exposure associated with its business and to successfully capture new business opportunities in order to secure the generation of shareholder value. Evotec therefore places substantial emphasis on risk management as an ongoing management task. Evotec employs a comprehensive risk management policy and risk management system which forms an integral part of the Group’s management processes and complies with the legal requirements. Evotec’s risk management system assesses permanently and in a systematic manner all significant company activities and identifies, analyses and values risks. It documents and communicates these centrally to the Group Risk Manager and the executive board (Vorstand). Despite the suitable and functional system as illustrated in the following, there cannot be an absolute certainty to identify and manage all possible risks. The system’s efficacy is verified on a continuous basis and regularly reported to the Audit Committee. Beside the formal risk management policy as explained in the following, the risk management system is based upon Evotec’s general guidelines of corporate management and the code of conduct, as described in the Declaration of Corporate Management.
According to the Company’s risk management policy Evotec engages in businesses only when this is in line with its strategy and with risks common within the industry, and when adequate reward potential is offered. At least once a year the Management Board defines the Group’s specific affinity to financial risk in accordance with the prevailing business and financial condition, including in particular the definition of minimum cash levels and milestones critical to short and mid-term financial performance. Management engages in monthly financial reviews with a strong emphasis on cash and cash forecasts, and key financial performance drivers such as revenues, order book status and gross margins, as well as careful cost analysis (SG&A, R&D expenses). Currency exposures are reduced through natural hedges and hedging vehicles. It is Company policy not to speculate on foreign exchange movements, but to manage the risks arising from underlying business activities, for example, to gain foreign exchange certainty against the value of signed customer contracts. Financial investments are made in low risk categories (products or financial institutions rated A or better (Standard & Poor’s ratings)). As a consequence of the financial crisis the Management Board has further increased its attention on mitigating financial risks. It is therefore directly involved in all decisions concerning financial assets and manages all businesses and transactions considered to be material for the Company, as reinforced by a revised set of Company policies.
To cover other risks associated with the Company’s business, including those that would not have a short-term financial impact, Evotec performs regular commercial project portfolio reviews. Strict application of project and investment approval processes, legal contract review procedures and signing authorities are also standardized procedures. In addition, the Company emphasizes its IT security throughout the Group and reviews its insurance coverage regularly. Compliance with the regulatory environment, for example environment, health and safety, has a high priority at all operational sites of the Group and corresponding training programmes are in place. All these measures and procedures, as well as further controls, were adapted and implemented in line with the Evotec SOX compliance activities (see page 44). The Company also takes its Corporate Governance responsibilities very seriously. A declaration according to Section 161 AktG was made by the Management Board and the Supervisory Board of the Company. This declaration regarding the Company’s compliance with the Corporate Governance Codex is accessible to the shareholders on Evotec’s website.
Evotec’s risk management system is regularly reviewed by the Group’s Compliance Officer, the Management Board and the Audit Committee of the Supervisory Board in order to adjust to changing environments, risk profiles and business opportunities.
Since April 2007, an upgraded system operates which comprises the following elements:
i) a Risk Early Detection System to identify risks as early as possible, to precisely describe them, to quantify their potential damage value and their probability of occurrence (wherever possible) and to report them to the competent management in a timely fashion, and
ii) a Risk Prevention System to monitor the risks incurred and/or the development of measures and systems to prevent potential risks from occurring.
The Risk Early Detection System shall identify and report risks as early as possible so as to allow management to deal with them from their very onset. It consists of the following two kinds of reports: Through Internal Ad Hoc Notifications, any risks, that are either outside the normal course of business or might have a material impact on the Company’s financial performance, are raised and reported to the Group Risk Manager as they emerge by the manager concerned together with a summary and assessment of the specific risk and the counter measures to be taken. The Group Risk Manager reports the received Internal Ad Hoc Notifications to the Chief Financial Officer. Moreover, on a quarterly basis, responsible line managers forward Periodical Risk Reports which (i) give an update on the risks described in an interim Internal Ad Hoc Notification (if any), (ii) report about any other material risk that has occurred even when beneath the pre-defined thresholds, and (iii) monitor the success of any measure taken to deal with the previously reported risks. The Group Risk Manager evaluates and summarizes the various risk sheets into a quarterly report for the Management Board,
EVOTEC – ANNUAL REPORT 2009

44 Risk Management and Risk Report
which also includes a cash stress test to examine whether Evotec could bear the cash effect of all captured risks should they fully materialize in parallel. As of today, Evotec has always passed this cash stress test.
All regular internal reports and meeting minutes that could be of relevance to important risk categories are formally included in the Company’s risk management system (Risk Prevention System). This procedure increases general alertness to risk and risk management, and also emphasizes the principle of risk prevention across the Group.
INTERNAL CONTROLS OVER FINANCIAL REPORTING
Section 289 para 5 of German Commercial Code (HGB) requires the Management Board to take responsibility for adhering to and reporting on an internal control system for reliable financial reporting. The internal control system is part of the risk management system and primarily secures the preparation of financial statements according to regulatory and legal requirements. It comprises all the principles, processes and measures that are applied to secure effective, economical and proper accounting and compliance with the pertinent legal provisions. Evotec satisfies both German Commercial Code requirements as well as Section 404 of SOX requirements as Evotec shares are registered with the SEC under the Securities Exchange Act of 1934, as amended and is therefore required to comply with all SEC stipulations. Evotec will satisfy SOX requirements at least until its shares will have been deregistered with the SEC.
In this context, Evotec’s Management Board is required to annually assess the effectiveness of internal controls over financial reporting. The effectiveness of Evotec’s internal controls over financial reporting will be audited by its independent registered public accounting firm for the first time for the full-year 2009 (SOX Audit). From internal assessments no material weaknesses were identified and detected deficiencies were remediated immediately. The audit of consolidated financial statements by the Group auditor, in particular, constitutes the key non-process- related monitoring measures with regard to Group accounting. The Audit Committee of the Supervisory Board is informed regularly and discusses the auditing activities.
Evotec uses the Committee of Sponsoring Organizations of the Tread-way Commission’s ‘Internal Control Integrated Framework’ (COSO framework) as the basis for its internal controls over financial reporting. Using the COSO framework, Evotec’s financial reporting control system is based upon: — the control environment; — risk evaluation; — control activities; — information and communication pathways; and — monitoring of the internal control systems
Evotec’s internal control system is designed to ensure compliance with applicable accounting standards over its financial reporting. The system is based upon various automated and manual, preventive and detective controls, segregation of financial related duties as well as the adherence to Evotec’s policies (such as the Four-Eyes-Principle for approval of
all its expenditures and its financial reporting). Such controls include the utilisation of IT-based solutions that define distinctive entry and approval levels and grant respective limited access (in particular the system IFS).
In addition, Evotec includes external specialists, for example to value share-based compensation or to derive deferred taxes.
Specific risks related to Group accounting may arise, for example, from the conclusion of unusual or complex business transactions, in particular at a critical point in time at the end of the financial year. Business transactions not processed by means of routine operations also entail latent risks. The discretion necessarily granted to employees for the recognition and measurement of assets and liabilities may result in further Group accounting-related risks.
The internal control measures, however, aimed at securing proper and reliable Group accounting ensure that business transactions are fully recorded in a timely manner in accordance with the legal provisions and the Articles of Association. The control operations also ensure that accounting records provide reliable and comprehensible information.
Evotec therefore trusts that the measures implemented significantly reduce the risk of harmful influences to the financial reporting.
With the organisational, control and monitoring structures established by Evotec, the internal control and risk management system enables company-specific facts to be captured, processed and recognised in full and properly presented in the consolidated financial statements. However, due to the very nature of business activity, discretionary decision-making, faulty checks, criminal acts and other circumstances cannot be ruled out and will restrict the efficacy and reliability of the internal control and risk management system, so that even group-wide application of the systems cannot guarantee the accurate, complete and timely recording of facts in Group accounting. If necessary, Evotec adjusts its risk management system swiftly to any changes in the respective environment.
RISKS
Evotec is exposed to different risks which are relevant to many business functions. The business, financial condition and results of Evotec may be materially adversely affected by each of these risks. If not stated differently, the risks mentioned below are unchanged in comparison to 2008.
RISKS INHERENT TO DRUG DISCOVERY ALLIANCES
Evotec’s discovery alliance platform is well established within the industry, and has generated a growing revenue stream over the last years. A satisfied customer basis, increased efficiency and superior service quality allow Evotec to generate value through its leveraged research platform and positive gross margin contributions. Evotec considers that these risks could be assessed as medium and remain in the main unchanged in comparison to the previous year.
EVOTEC – ANNUAL REPORT 2009

Risk Management and Risk Report 45
However, business specific risks need to be managed:
The market environment is marked by pricing pressures originating from funding restrictions of some biotechnology customers and from evolving and strengthening competition in individual drug discovery disciplines in low cost countries. Therefore, firm cost management, continuous enhancement of capabilities and technologies, careful market positioning and sales from high-value results-based contracts are mandatory for Evotec. In addition, Evotec continues to explore ways to capture some of the cost advantages in countries like India, as exemplified in the acquisition of a 70% controlling majority stake of the Indian company Research Support International Private Limited (RSIPL).
Evotec intends to employ increasing parts of its capacity for results-based deals, with the goal to keep a higher share of value creation. In return, there are scientific and technical delivery risks in the shorter term which can expose Evotec’s financial performance and in particular the collaborations business margins to the possible failure or delay of certain milestone payments. Detailed project reporting has been established within Evotec to control the risk of milestone achievements. Overall, this value strategy has been validated to date with only a few customers, and the experiences might not be transferable to other customers and contracts.
Even when exhibiting a steady revenue stream, fluctuating capacity utilisation and resource allocation between different parts of the business can significantly impair profitability, unless these are carefully and flexibly adjusted. In addition, dependence on individual larger customer contracts needs to be carefully monitored. In 2009, the largest volume generated with one single customer was 21.3% of total revenues.
Some of the service contracts contain scientific or technical delivery risks, which can be only partly mitigated with high quality project work. It is an explicit goal of Evotec to grow the business to leverage such risks through the required scale.
Evotec’s past success builds in part on customer recognition and branding. It is therefore of utmost importance to maintain, this good reputation and avoid any negative impact on its branding. Evotec has protected its trade name in all active countries and has increased its awareness to strengthen and protect its global market position.
RISKS INHERENT TO PROPRIETARY DRUG DISCOVERY AND DEVELOPMENT
Despite a stronger strategic focus on drug discovery alliances Evotec engages in selective proprietary discovery and development activities that promise significant returns when such programmes are successful, but also carry high scientific and financial risk, concentrated on few individual projects. Today, Evotec has no commercial drug products and there is no assurance that Evotec or its strategic partners will successfully develop and commercialize potential drugs. Significant returns are only expected to materialize when successful research leads to upfront and milestone payments and potential royalties from future drug sales are received. Evotec expects to achieve significant payments when any one of the drug candidates is either out-licensed to a pharmaceutical or biotechnology company, or when Evotec decides to partner the drug whilst still retaining some marketing rights. Evotec trusts that the associated risks have to be assessed as medium/high but remain more or less unchanged in comparison to the previous year or slightly decreased as Evotec is now increasingly focussed on drug discovery alliances than proprietary drug discovery. The associated risks are those inherent to the biotechnology and drug development industry in general:
Evotec acts carefully and responsibly to prove that clinical product candidates are safe and effective for human use and approvable by regulatory agencies. Drug discovery and development, however, is expensive, time consuming and subject to a high degree of failure. At each stage there is an inherent risk that developments need to be aborted or delayed due to unpredictable results. The rate of failure is highest the earlier the stage of a programme. However, the cost of failure tends to be higher the later the stage of development and preclinical studies and early clinical trials involving limited numbers of patients may not accurately predict the results obtained in later stage clinical testing. A number of companies have suffered significant setbacks in late-stage clinical trials even after achieving promising results in earlier development activities. Even if Evotec identifies promising compounds to valuable targets, or in-licenses or otherwise acquires promising projects or drug candidates, any resulting internal R&D project could experience delays or even fail and it could take several years before the Company could sell or license any drug candidates, if at all.
Research and Development activities, the approval and marketing of a pharmaceutical product are subject to extensive regulation by the US FDA, the European Medicines Agency (EMA, formerly known as EMEA) and similar regulatory agencies elsewhere. The approval of the relevant authorities is required before a product can be tested in humans and later sold in a given market. The regulatory approval process is intensive, time-consuming and the timing of receipt of regulatory approval is difficult to predict. The authorities can deny their approval for various reasons. In the recent past the regulatory environment has become less predictable, in particular in the US. Therefore, even if the further development of Evotec’s drug candidates is successful, regulatory approval might not be received, might be restricted to certain geographical regions or indications, later withdrawn or significantly delayed which could significantly impact the receipt of product revenues, if any. It can be very hard to predict potential regulatory difficulties and their influence on further discovery and development of a proprietary drug candidate. Evotec seeks early discussions with the regulatory bodies at all stages of development to ensure that investments are in conformity with legal and ethical requirements.
Evotec depends on external contract research organizations (CROs), and independent clinical investigators to conduct certain preclinical studies and clinical trials. Despite their contractual rights, Evotec cannot control the amount of time and resources that the CROs devote to such programmes. The programmes may therefore not be diligent, careful or timely, and there may be mistakes in the conduct of these studies. For example, failure to enrol patients for clinical trials may cause delays in developing Evotec’s product candidates.
The use of any of Evotec’s product candidates in clinical trials may expose Evotec to product liability claims in excess of Evotec’s limited insurance coverage, although it is diligently assessed for each trial. As of today, Evotec is not aware of any pending threats of product liability claims. In 2009, Evotec has refocused its internal R&D activities on the most
EVOTEC – ANNUAL REPORT 2009

46 Risk Management and Risk Report
valuable assets to decrease its risk exposure. At present, the Company will not engage in building up a more extensive pipeline, but will concentrate its efforts on bringing proprietary products from its existing portfolio to important value inflection points. In addition, Evotec intends to partner or publicly fund proprietary programmes to share the risk wherever possible.
MERGER AND ACQUISITIONS
Evotec’s market position is well established and Evotec is known for its first class services by its customers. However, the Company pursues ambitious goals regarding its growth rate through both internal organic growth development and opportunistic acquisitions of financially rewarding and suiting service capacities and capabilities. In 2009, this was exemplified in the acquisition of a 70% controlling majority stake of the Indian company Research Support International Private Limited (RSIPL). However, such merger and acquisition activities go along with specific risks that need to be managed.
The acquisition of RSIPL bears the risk that the integration of RSIPL into the Evotec group may be difficult and expensive to achieve. The merger will present challenges to Evotec’s management including the integration of RSIPL’s operations and personnel. In addition, the merger may present special risks including possible unanticipated liabilities, unanticipated costs, diversion of management attention and loss of personnel. Evotec may not be able to integrate RSIPL into its operations or successfully manage the operations acquired in the merger. So far, there is no indication that any material agreement between RSIPL and a third party, will be terminated. Nonetheless, following the merger, if Evotec’s management is not able to implement a business plan that effectively integrates RSIPL’s operations, the anticipated benefits of the merger may not be realised which may adversely affect the price of Evotec ordinary shares.
COMMERCIAL RISKS
The commercial risks inherent to Evotec’s drug discovery alliance platform are described in detail in the section ‘Risks inherent to drug discovery alliances’ on page 44 of this Risk Report. Regarding other commercial risk Evotec trusts that the associated risks could be assessed as medium and are unchanged in comparison to the previous year. Those risks are: Although Evotec has adjusted its strategy in 2009 and now focuses its activities on drug discovery alliances, the Company continues to be engaged in a selected number of active drug discovery and development programmes that it intends to license to pharmaceutical companies for clinical development and commercialisation. The market environment and competitive landscape for licensing and licensed projects or individual drug candidates, in general or for individual treatments, might change while engaging in individual projects. The timing and commercial values of, or financial proceeds from partnering individual projects could therefore deviate significantly from earlier projections, for better or worse. During 2009 the out-licensing market in general became increasingly challenging with the impact of the financial crisis hitting the biotech industry.
Evotec’s ongoing efforts to serve as an innovative source of drug candidates to the pharmaceutical industry makes it dependent on individual larger out-licensing or partnering events and hence on individual, typically larger customers. The total amount of payments and the split of these payments obtained in a future out-licensing agreement are unknown and depend on many factors, such as degree of innovation and IP position as well as on external factors not within the control of the Company. In addition, the reliance on corporate partners is subject to additional risks. For example, Evotec’s collaboration partner may not devote sufficient time and resources to the development, introduction and marketing of Evotec’s products or may not pursue further development and commercialisation of the products resulting from the collaboration. To control this risk to the extent possible detailed project reporting is established within Evotec and stipulated in any collaboration agreement.
Even if drug products are approved and commercialized by Evotec or its license partner, hospitals, physicians or patients may conclude that Evotec’s products are less safe or less effective or otherwise less attractive than existing drugs. In addition, Evotec’s competitors may achieve product commercialisation or patent protection earlier than Evotec and/or develop new products that could be more effective or less costly, or seem more cost-effective, than Evotec’s products.
Evotec’s financial planning is not based on any partnering of product candidates or product commercialisation and is solid even in the absence of such an event.
FINANCING AND OTHER FINANCIAL RISKS
Evotec is currently well financed and assesses the financial associated risks to be low/medium remaining unchanged or slightly decreased in comparison to the previous year.
In March 2009 Evotec initiated a restructuring process to concentrate on drug discovery alliances, focus its R&D activities on core value programmes and to significantly reduce its operating costs. Evotec also decided to engage into strategic high-value license agreements for selected drug candidates with pharmaceutical partners that might provide sizeable payments by such a partner, and to expand its indication focus beyond neuroscience, pain and inflammation. With this new strategic focus, Evotec de-risked its business model further with the aim to eventually become sustainable.
Notwithstanding, expenditures on internal discovery and development programmes and other costs, as well as reduced revenues, might negatively impact Evotec’s short- to mid-term profitability and cash reserves. To actively address any related risk, Evotec’s management defined minimum liquidity levels and prepared a scenario planning to safeguard its cash position. As a result of the restructuring measures, even without a significant licensing agreement for one of its clinical or preclinical assets, Evotec believes that existing liquidity reserves are sufficient under the risk management plan to cope with all cumulated, identified risk implications and that those reserves are a strong basis to develop the Company to sustainability.
Evotec is currently well financed and has no plans or necessity to raise capital in the near- to mid-term. However, the option to increase capital may always be considered. This might be the case if new opportunities
EVOTEC – ANNUAL REPORT 2009

Risk Management and Risk Report 47
arise in terms of M&A and in-licensing requiring additional financing. The Company does not intend to engage in projects unless appropriate funding is allocated or secured.
Evotec has not had any relationships with unconsolidated entities or financial partnerships, such as entities often referred to as structured finance or special purpose entities, which would have been established for the purpose of facilitating off-balance sheet arrangements or other contractually narrow or limited purposes. Therefore, Evotec is not materially exposed to any financing, liquidity, market or credit risk that could arise if it had been engaged in these relationships.
The general risk of losing a significant amount of cash in cash investments is continuously mitigated by spreading the investments across different types of instruments and with different counterparties. Evotec monitors its investments and the counterparties on an ongoing basis.
INTELLECTUAL PROPERTY RISKS
The intellectual property (IP) associated risks could be assessed as low and remaining stable in relation to the previous year.
Evotec is dependent on patents and proprietary technology, both its own and those licensed from others, and puts a high emphasis on patent protection and patent monitoring. The Company’s success depends in part on its ability, and the ability of its licensors, to obtain patent protection for technologies, processes and product candidates, to preserve trade secrets, to defend patents against third parties seeking to invalidate such patents, and to reinforce rights against infringing parties. Any disputes could result in sizeable additional expenses, project delays and absorption of management attention, and in a dramatic reduction of project values or even in full project abandonment.
Evotec holds licenses granted by Roche for the EVT 100 compound family, EVT 201 and EVT 302, and by other parties related to certain of Evotec’s preclinical research projects. Any termination of these licenses could result in the loss of significant rights and could harm Evotec’s ability to commercialise its drug candidates. In addition, Evotec must rely on Roche to enforce its rights and obligations to assert, prosecute and defend intellectual property relating to the EVT 100 compound family, EVT 201 and EVT 302.
DEPENDENCE ON KEY PERSONNEL
Evotec, like many biotechnology companies, is highly dependent on the key members of its management and scientific staff. The loss of any of Evotec’s key employees or key consultants could impede the achievement of Evotec’s research and development objectives. However, Evotec has set up its management such that the Company’s knowledge is shared amongst key employees. Furthermore, recruiting and retaining qualified scientific personnel to perform research and development work in the future is critical to Evotec’s success. If Evotec is unable to attract and to retain personnel on acceptable terms despite its strong corporate culture and industry leadership position, this may delay Evotec’s development efforts or otherwise harm its business.
Among other benefits, Evotec has granted stock options as a method of attracting and retaining key employees. Due to significant fluctuations in the trading price of Evotec ordinary shares within the last years, a considerable portion of the stock options held by Evotec employees have exercise prices that are higher than the current trading price of Evotec’s shares. If Evotec is unable to offer competitive remuneration including stock options that provide sufficient incentives, Evotec may be unable to retain its existing employees and attract additional qualified candidates.
In the recent past, Evotec has not encountered difficulties in attracting and retaining qualified employees. Therefore, such risk could be assessed as low and is in comparison to the previous year unchanged.
CURRENCY RISKS
Evotec’s business is affected by fluctuations in foreign exchange rates between the US Dollar, UK Sterling and the Euro. Currency exchange movements impact Evotec’s reported liquidity through the translation of liquid assets held in US Dollars or UK Sterling into Euros. A portion of the funds are held in currencies other than the Euro in order to meet local operating needs.
With a significant proportion of sales denominated in US Dollars and UK Sterling, currency exposure creates a risk to Evotec’s profitability. The Company manages this exposure through either natural hedges with same currency expenses or through active hedging techniques during service contract work. Additionally, opportunities to reduce liquidity risk by converting excess US Dollar into Euro are taken when opportune. However, the currency associated risks have to be considered as medium and remain unchanged in comparison to the previous year.
RISKS ASSOCIATED WITH FINANCIAL CRISIS
The financial turmoil witnessed during the last year has not significantly impacted Evotec’s operations. Evotec’s cash holdings are invested at several different banks, in liquid, highly diversified investment instruments. Evotec has invested parts of its cash reserves in auction rate securities for which no active market making exists. A bank repurchase warranty, however, secures Evotec’s investment.
Evotec’s customers are generally financially stable pharmaceutical companies, foundations and larger biotech companies and we do not foresee an increased level of accounts receivables or a drop of future revenues due to this crisis. The financial crisis also hit the pharmaceutical industry, but especially the biotech industry, and led to an increasingly challenging partnering market and financial weakness of a few of our smaller customers.
Although Evotec is well financed and anticipates no need to raise capital in the short-term, Evotec is more cautious than ever to further conserve its liquidity and strengthen its business performance. Nonetheless, the associated risks have to be assessed as medium and in comparison to the previous year unchanged.
EVOTEC – ANNUAL REPORT 2009

48 Post-Balance Sheet Events
OTHER RISKS
Other risks, such as IT risks, environmental risks, and risks involving production and procurement are not considered to be significant and remain stable in relation to the previous year.
Surviving business risks of divested businesses, Evotec Technologies and the Chemical Development Business, are limited to customary guarantees given to the acquirer as well as the risk of terminating existing sublease agreements with Evotec AG. The Company believes that these are limited and existing precautionary measures are sufficient. Evotec does not foresee any material warranty of future liability claims.
MANAGEMENT ASSESSMENT OF RISK SITUATION
Management believes that although the risks in any drug discovery and development business are significant, the Company has great opportunities to create long-term value that outweigh the foreseeable risks. Following its restructuring, Evotec has further reduced its risk exposure and mitigates its business risk through multiple customer contracts. With highly competitive research collaborations business, a significantly reduced cost structure, a pipeline of development candidates partnered or available for partnering, supported by substantial financing and adequate risk and opportunity management systems, Evotec is well prepared to deliver on its strategy and to develop the Company to sustainability.
POST-BALANCE
SHEET EVENTS
There are no material events to be reported.
EVOTEC – ANNUAL REPORT 2009

Outlook 49
OUTLOOK
Information set forth in this section contains forward-looking statements. These statements are neither promises nor guarantees, but are subject to a variety of risks and uncertainties, many of which are beyond our control, and which could cause actual results to differ materially from those contemplated in these forward-looking statements.
EXPECTED GENERAL MARKET AND HEALTHCARE DEVELOPMENT
ECONOMIC DEVELOPMENT
The global economy is in the early stages of a recovery following the sharpest contraction for many decades, but progress is uneven. Economists are forecasting a rosier 2010 for global finances than the year 2009 has been. But they warn that the economic recovery remains fragile and will not be led by the world’s advanced industrialised nations.
THE MARKET FOR DRUG DISCOVERY ALLIANCES
The global drug discovery market is expected to experience robust growth, exceeding $8 bn in 2010 and reaching $14 bn in 2013.
The global pharmaceutical industry continues to face a significant R&D productivity challenge. There is increasing pressure for pharmaceutical companies to develop new drug compounds due to the near-term loss of patent protection for many drug products. And there is also pressure to reduce the time and money spent in drug discovery in order to bring drugs to market faster and more affordably.
While innovative scientific technologies and approaches are a rich source for the identification of novel drugs, they have and will continue to make drug discovery more complex, dramatically increasing the number of drug targets and active compounds pursued. Biotechnology companies often lack the technical staff or technology to process their targets internally. Those companies as well as pharmaceutical companies increasingly turn to outsourcing R&D activities which provides them with expertise in required areas without the need to build additional infrastructure and capabilities internally. This is where discovery out-sourcing solutions, such as those provided by Evotec, can help make the drug discovery and development process more efficient.
The overall outsourcing trend in the pharmaceutical industry is pointing to larger strategic research contracts favouring big alliance partners due to lower perceived risk. This presents a challenge for the highly fragmented drug discovery industry. With its strong experience and track record in drug discovery and striving to continuously add capabilities and scale, Evotec is ideally positioned to take full advantage of these market developments.
TRENDS IN RESEARCH & DEVELOPMENT
In terms of proprietary research and development of novel drug compounds, experts believe that sufficient capital resources remain a critical competitive advantage for biotechnology companies as funding availability will continue to be limited for the coming years. Hence, in line with Evotec’s approach, many companies across the globe are expected to continue to cut non-core programmes and focus on a few high-value assets.
BUSINESS DIRECTION AND STRATEGY
Evotec aims to further expand its position as a leading global drug discovery alliance partner, building on the competitive advantages and reputation established over the prior years.
Evotec’s competitive advantage over the major Western discovery service providers is based on its highly integrated capability in drug discovery, from high-throughput screening and structure-based drug design for the identification of novel starting points, through to expert medicinal chemistry and in vivo pharmacology. The Company’s competitive advantage over Asian and Eastern Europe providers is its access to an innovative high-throughput screening platform, its strong track record and expertise in medicinal chemistry and its experienced scientists with many years of drug discovery project management. Evotec is possibly the most experienced player in this area, with a 16 year track record of providing drug discovery alliances to the pharmaceutical and biotechnology industry.
Evotec is combining those capabilities and skills with the cost advantage of Asian and Eastern Europe providers. To this end, the Company acquired its first Asian capacity and capability with the purchase, in August 2009, of a 70% controlling stake in Research Support International Private Limited (RSIPL), a Mumbai-based company, providing organic synthesis services to the global pharmaceutical industry. The integration of RSIPL allows Evotec to leverage its innovative drug discovery offering with complementary capabilities and cost efficiencies, and also enables the Company to attract sizable chemistry-based contract work which is more frequently being outsourced by large pharmaceutical companies.
Evotec is expanding its offering from target identification straight through to the Investigational New Drug (IND) Application, stays at the forefront of drug discovery and addresses the significant market need with experience, innovation and scale. In its collaborations, the Company will continue to work with an increasing number of alliance partners on a wide variety of disease areas and target classes. Evotec will also continue to leverage its integrated capability and implement new integrated risk-sharing deals, in which the Company manages complete target-to-IND programmes and both parties share the potential upside of drug development success.
EVOTEC – ANNUAL REPORT 2009

50 Outlook
EXPECTED RESEARCH AND DEVELOPMENT
Historically, Evotec has developed a pipeline of products both clinical and preclinical in neuroscience, pain and inflammation. During 2009, Evotec has substantially reduced the level of funding of its proprietary products as it decided to focus on its core business as a discovery alliance partner. However, the Company continues to develop a selected number of projects internally which are expected to form the basis of larger strategic alliances going forward. In 2009, for example, the EVT 100 series has been partnered with Roche and is now being developed in Treatment-Resistant Depression. Roche funds but Evotec manages the clinical development of EVT101 into Phase II and of EVT 103 through Phase I. In 2010, Evotec expects to further develop its H3 antagonist for narcolepsy and cognition through the preclinical phase. Other assets such as EVT 401 and preclinical programmes will only be progressed if suitably partnered and funded.
FINANCIAL OUTLOOK FOR 2010 AND 2011
In 2010 and 2011, total Group revenues before out-licensing income are expected to grow by at least 15% per year. These assumptions are based on the current order book, expected new contracts and contract extensions as well as the achievement of certain milestones.
On this basis, 2010 and 2011, gross margins are expected to be in line with those achieved in 2009. They will, however, continue to be somewhat volatile, as they are dependent upon contributions from high margin milestone payments.
With the restructuring measures taken in 2009, Evotec has significantly reduced its cost base going forward. The impact of Evotec’s SG&A savings will be fully reflected in the Company’s results for the years 2010 and beyond. No further significant headcount reductions are planned.
Evotec expects research & development (R&D) expenses to decrease considerably from 2009 levels. The Company will focus on key programmes and targets to invest less than € 10 m in R&D in 2010 and 2011. A significant decrease in R&D expenses was made in 2009, following the partnering of the EVT 100 compound series to Roche and the failure of EVT 302 in smoking cessation, as well as the implementation of the ‘Evotec 2012-Action Plan to Focus and Grow’ which was only partially reflected in the results for the 2009 fiscal year.
As a result, Evotec’s Group operating result before impairment for the years 2010 and 2011 is expected to improve significantly over 2009.
The Evotec Group started the year with € 71 m of cash, investments and auction rate securities. In 2010, top-line growth and the adjusted cost base are expected to significantly reduce the cash requirements compared to the 2009 fiscal year. Consequently, at constant year-end 2009 currencies, the Company expects to end 2010 with a liquidity of more than € 60 m, excluding any potential cash outflow for M&A or similar transactions.
DIVIDENDS
Future payment of dividends is dependent upon Evotec’s financial situation and liquidity requirements, the general market conditions, and statutory, tax and regulatory requirements. Evotec currently intends to retain any potential future profits and to re-invest into the Company. Consequently, dividend payments are not foreseen in the near- to mid-term.
OPPORTUNITIES
Evotec operates in a market which has excellent growth opportunities. The need to improve efficiency forces pharmaceutical and biotechnology companies to continuously increase their investment in R&D and to outsource drug discovery and development. There is an obvious trend towards larger contracts in a full-service outsourcing model with increased opportunities for an alliance partner, such as Evotec, offering integrated drug discovery capabilities and project management from hit identification to IND application. Evotec has also lined up many opportunities for pharmaceutical companies to opt into its proprietary drug candidate programmes. In addition, the option for Roche to buy back rights to the entire EVT 100 compound series could potentially result in a substantial net cash inflow for Evotec within the next 24 months.
GENERAL STATEMENT OF EXPECTED DEVELOPMENT
Evotec has executed important transactions to strengthen its business. The Company is well positioned to bring expertise and real value to the pharmaceutical and biotechnology industry, addressing the industry’s growing demand for innovative drugs.
Management’s core strategy for Evotec is to continue to focus on its alliance business to lead the path to profitability and sustainability, to expand base business capabilities through strategic investments in platform technologies, know-how and disease expertise, and to partner with more strategic customers.
By focusing on and expanding its highly profitable alliances, the Company has the opportunity to build significant long-term value for its shareholders.
EVOTEC – ANNUAL REPORT 2009

CONSOLIDATED FINANCIAL STATEMENTS
(IFRS) 2009
52 Consolidated statement of financial position
54 Consolidated income statement
55 Consolidated statements of comprehensive income
56 Consolidated statements of cash flows
58 Consolidated statements of changes in stockholders’ equity
60 Consolidated fixed asset movement schedule
62 Notes
86 Supervisory Board and Management Board
88 Auditor’s Report
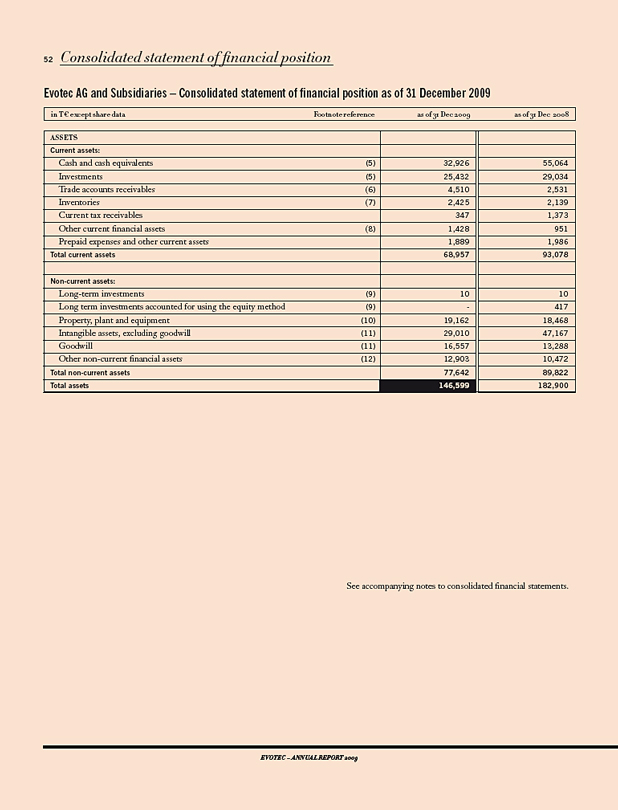
52 Consolidated statement of financial position
Evotec AG and Subsidiaries – Consolidated statement of financial position as of 31 December 2009
in T€ except share data
ASSETS
Current assets:
Cash and cash equivalents Investments Trade accounts receivables Inventories Current tax receivables Other current financial assets
Prepaid expenses and other current assets
Total current assets
Non-current assets:
Long-term investments
Long term investments accounted for using the equity method Property, plant and equipment Intangible assets, excluding goodwill Goodwill Other non-current financial assets
Total non-current assets Total assets
Footnote reference
(5) (5)
(6) (7)
(8)
(9) (9) (10) (11) (11) (12)
as of 31 Dec 2009
32,926 25,432 4,510 2,425 347 1,428 1,889
68,957
10 - 19,162 29,010 16,557 12,903
77,642 146,599
as of 31 Dec 2008
55,064 29,034 2,531 2,139 1,373 951 1,986
93,078
10 417 18,468 47,167 13,288 10,472 89,822 182,900
See accompanying notes to consolidated financial statements.
EVOTEC – ANNUAL REPORT 2009
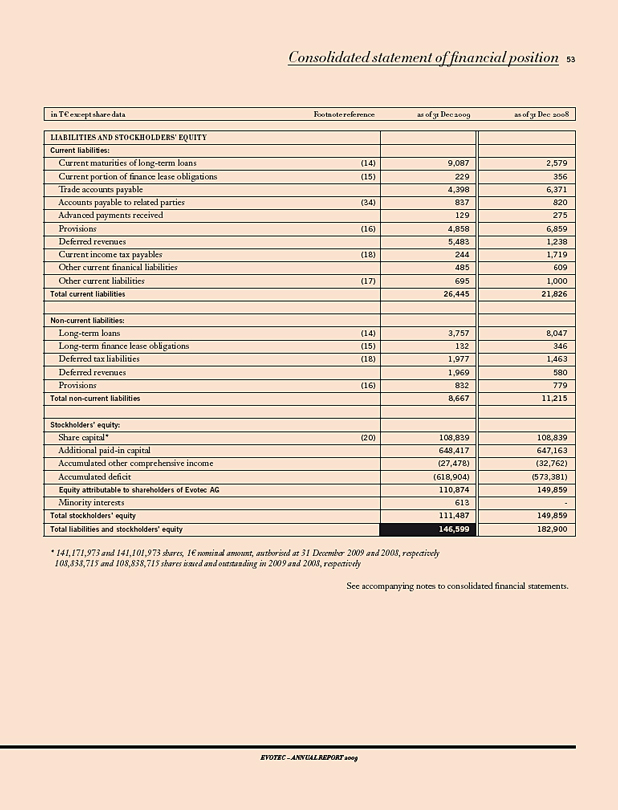
Consolidated statement of financial position 53
in T€ except share data
LIABILITIES AND STOCKHOLDERS’ EQUITY
Current liabilities:
Current maturities of long-term loans Current portion of finance lease obligations Trade accounts payable Accounts payable to related parties Advanced payments received Provisions Deferred revenues Current income tax payables Other current finanical liabilities Other current liabilities
Total current liabilities
Non-current liabilities: Long-term loans
Long-term finance lease obligations Deferred tax liabilities Deferred revenues Provisions
Total non-current liabilities
Stockholders’ equity:
Share capital*
Additional paid-in capital
Accumulated other comprehensive income Accumulated deficit
Equity attributable to shareholders of Evotec AG
Minority interests
Total stockholders’ equity
Total liabilities and stockholders’ equity
Footnote reference
(14) (15)
(34) (16) (18) (17)
(14) (15) (18)
(16)
(20)
as of 31 Dec 2009
9,087 229 4,398 837 129 4,858 5,483 244 485 695
26,445
3,757 132 1,977 1,969 832
8,667
108,839 648,417 (27,478) (618,904)
110,874
613
111,487 146,599
as of 31 Dec 2008
2,579 356 6,371 820 275 6,859 1,238 1,719 609 1,000
21,826
8,047 346 1,463 580 779
11,215
108,839 647,163 (32,762) (573,381)
149,859
-
149,859 182,900
* 141,171,973 and 141,101,973 shares, 1€ nominal amount, authorised at 31 December 2009 and 2008, respectively 108,838,715 and 108,838,715 shares issued and outstanding in 2009 and 2008, respectively
See accompanying notes to consolidated financial statements.
EVOTEC – ANNUAL REPORT 2009
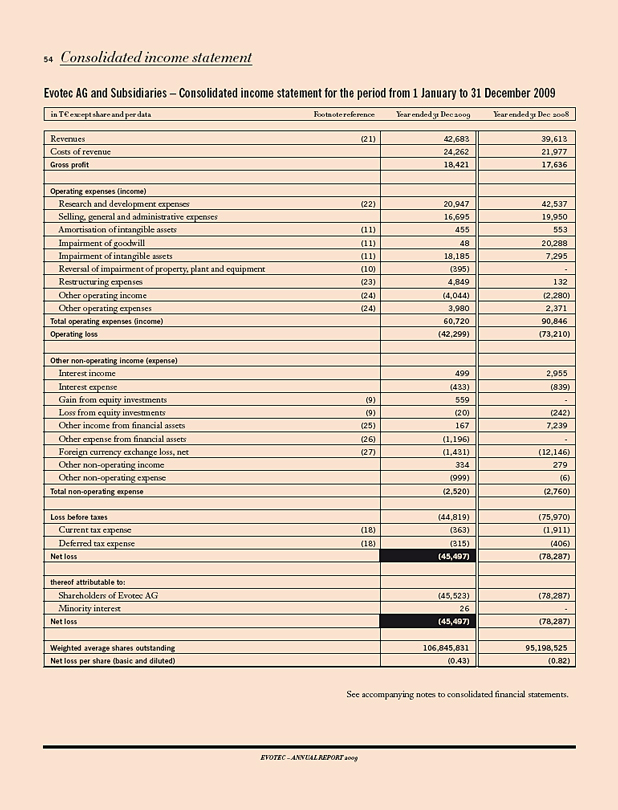
54 Consolidated income statement
Evotec AG and Subsidiaries – Consolidated income statement for the period from 1 January to 31 December 2009
in T€ except share and per data
Revenues Costs of revenue Gross profit
Operating expenses (income)
Research and development expenses Selling, general and administrative expenses Amortisation of intangible assets Impairment of goodwill Impairment of intangible assets
Reversal of impairment of property, plant and equipment Restructuring expenses Other operating income Other operating expenses
Total operating expenses (income) Operating loss
Other non-operating income (expense)
Interest income Interest expense
Gain from equity investments Loss from equity investments Other income from financial assets Other expense from financial assets Foreign currency exchange loss, net Other non-operating income Other non-operating expense
Total non-operating expense
Loss before taxes
Current tax expense Deferred tax expense
Net loss
thereof attributable to:
Shareholders of Evotec AG Minority interest
Net loss
Weighted average shares outstanding Net loss per share (basic and diluted)
Footnote reference
(21)
(22)
(11) (11) (11) (10) (23) (24) (24)
(9) (9) (25) (26) (27)
(18) (18)
Year ended 31 Dec 2009
42,683
24,262
18,421
20,947
16,695
455
48
18,185
(395)
4,849
(4,044)
3,980
60,720
(42,299)
499
(433)
559
(20)
167
(1,196)
(1,431)
334
(999)
(2,520)
(44,819)
(363)
(315)
(45,497)
(45,523)
26
(45,497)
106,845,831
(0.43)
Year ended 31 Dec 2008
39,613 21,977 17,636
42,537 19,950 553 20,288 7,295 -132 (2,280) 2,371
90,846 (73,210)
2,955 (839) - (242) 7,239 - (12,146) 279 (6)
(2,760)
(75,970) (1,911) (406)
(78,287)
(78,287) - (78,287)
95,198,525 (0.82)
See accompanying notes to consolidated financial statements.
EVOTEC – ANNUAL REPORT 2009
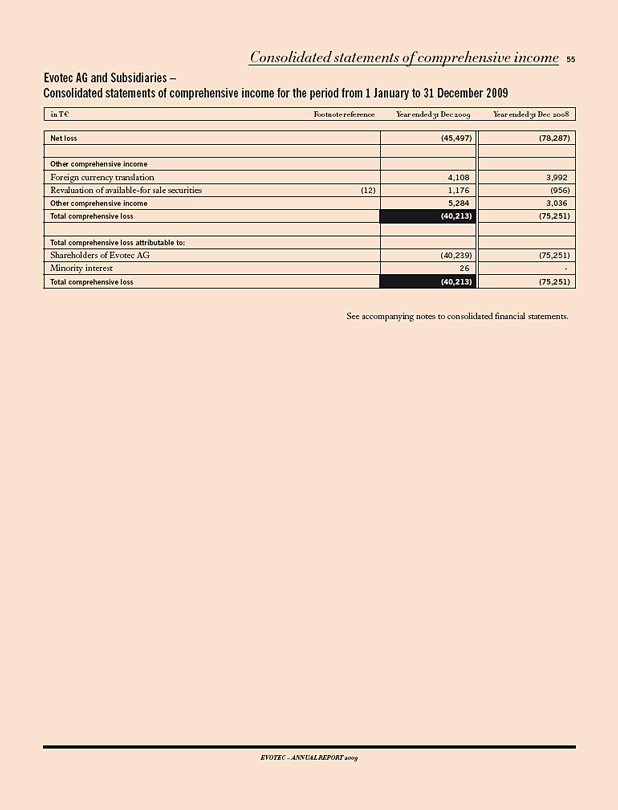
Consolidated statements of comprehensive income 55
Evotec AG and Subsidiaries –
Consolidated statements of comprehensive income for the period from 1 January to 31 December 2009
in T€
Net loss
Other comprehensive income Foreign currency translation
Revaluation of available-for sale securities
Other comprehensive income Total comprehensive loss
Total comprehensive loss attributable to: Shareholders of Evotec AG
Minority interest
Total comprehensive loss
Footnote reference
(12)
Year ended 31 Dec 2009
(45,497)
4,108
1,176
5,284
(40,213)
(40,239)
26
(40,213)
Year ended 31 Dec 2008
(78,287)
3,992
(956)
3,036
(75,251)
(75,251)
-
(75,251)
See accompanying notes to consolidated financial statements.
EVOTEC – ANNUAL REPORT 2009
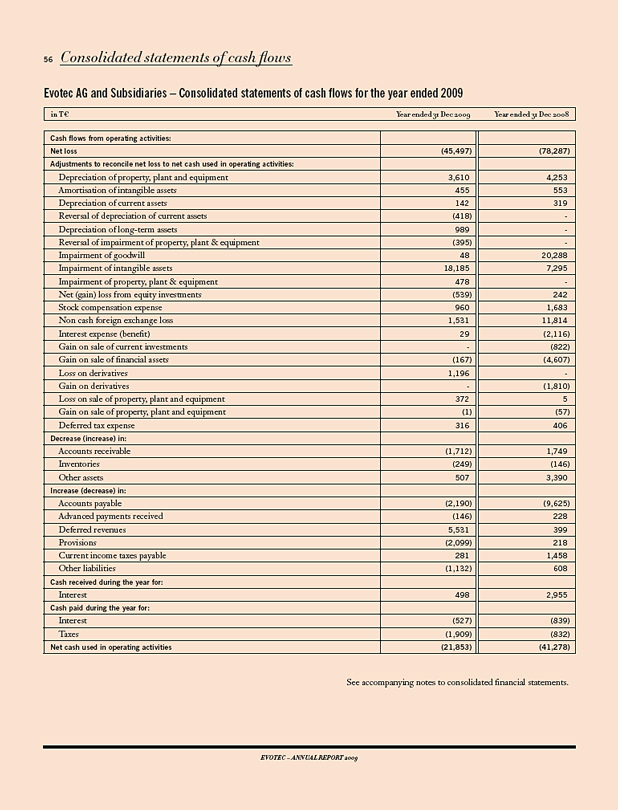
56 Consolidated statements of cash flows
Evotec AG and Subsidiaries – Consolidated statements of cash flows for the year ended 2009
in T€
Cash flows from operating activities: Net loss
Adjustments to reconcile net loss to net cash used in operating activities:
Depreciation of property, plant and equipment Amortisation of intangible assets Depreciation of current assets Reversal of depreciation of current assets Depreciation of long-term assets Reversal of impairment of property, plant & equipment Impairment of goodwill Impairment of intangible assets Impairment of property, plant & equipment Net (gain) loss from equity investments Stock compensation expense Non cash foreign exchange loss Interest expense (benefit) Gain on sale of current investments Gain on sale of financial assets Loss on derivatives Gain on derivatives Loss on sale of property, plant and equipment Gain on sale of property, plant and equipment Deferred tax expense
Decrease (increase) in:
Accounts receivable Inventories Other assets
Increase (decrease) in:
Accounts payable
Advanced payments received Deferred revenues Provisions Current income taxes payable Other liabilities
Cash received during the year for:
Interest
Cash paid during the year for:
Interest Taxes
Net cash used in operating activities
Year ended 31 Dec 2009
(45,497)
3,610
455
142
(418)
989
(395)
48
18,185
478
(539)
960
1,531
29
-
(167)
1,196
-
372
(1)
316
(1,712)
(249)
507
(2,190)
(146)
5,531
(2,099)
281
(1,132)
498
(527)
(1,909)
(21,853)
Year ended 31 Dec 2008
(78,287)
4,253
553
319
-
-
-
20,288
7,295
-
242
1,683
11,814
(2,116)
(822)
(4,607)
-
(1,810)
5
(57)
406
1,749
(146)
3,390
(9,625)
228
399
218
1,458
608
2,955
(839)
(832)
(41,278)
See accompanying notes to consolidated financial statements.
EVOTEC – ANNUAL REPORT 2009
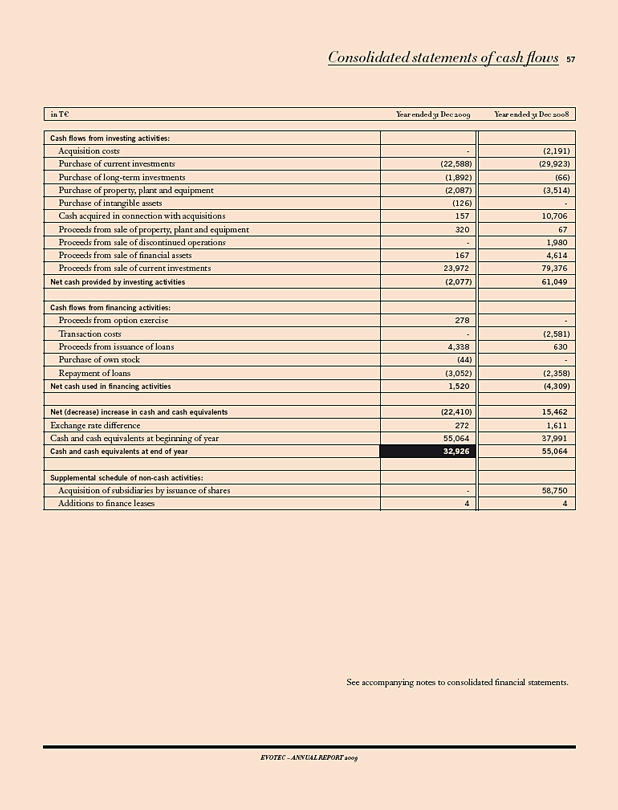
Consolidated statements of cash flows 57
in T€
Cash flows from investing activities:
Acquisition costs
Purchase of current investments Purchase of long-term investments Purchase of property, plant and equipment Purchase of intangible assets Cash acquired in connection with acquisitions Proceeds from sale of property, plant and equipment Proceeds from sale of discontinued operations Proceeds from sale of financial assets Proceeds from sale of current investments
Net cash provided by investing activities
Cash flows from financing activities:
Proceeds from option exercise Transaction costs Proceeds from issuance of loans Purchase of own stock Repayment of loans
Net cash used in financing activities
Net (decrease) increase in cash and cash equivalents
Exchange rate difference
Cash and cash equivalents at beginning of year Cash and cash equivalents at end of year
Supplemental schedule of non-cash activities:
Acquisition of subsidiaries by issuance of shares Additions to finance leases
Year ended 31 Dec 2009
-
(22,588)
(1,892)
(2,087)
(126)
157
320
-
167
23,972
(2,077)
278
-
4,338
(44)
(3,052)
1,520
(22,410)
272
55,064
32,926
-
4
Year ended 31 Dec 2008
(2,191)
(29,923)
(66)
(3,514)
-
10,706
67
1,980
4,614
79,376
61,049
-
(2,581)
630
-
(2,358)
(4,309)
15,462
1,611
37,991
55,064
58,750
4
See accompanying notes to consolidated financial statements.
EVOTEC - ANNUAL REPORT 2009

58 Consolidated statements of changes in stockholders’ equity
Evotec AG and Subsidiaries – Consolidated statements of changes in stockholders’ equity for the year ended 2009
Share Capital
in T€ except share data
Balance at 1 January 2008
Capital increase
Stock option plan
Transfer of treasury shares
Total comprehensive income (loss)
Balance at 31 December 2008
Exercised options from shares in trust
Stock option plan
Purchase of treasury shares
Transfer of treasury shares
Minority interests through acquisition of RSIPL
Total comprehensive income (loss) Balance at 31 December 2009
Footnote reference
(20)
(19)
(19)
(19)
(3)
Shares
73,868,447
34,970,268
-
-
-
108,838,715
-
-
-
-
-
-
108,838,715
Amount
73,868
34,971
-
-
-
108,839
-
-
-
-
-
-
108,839
Additional paid-in capital
627,676
17,804 1,683
-
-
647,163
278
976
-
-
-
-
648,417
Treasury shares
(99)
-
-
99
-
-
-
-
(44)
44
-
-
-
See accompanying notes to consolidated financial statements.
EVOTEC – ANNUAL REPORT 2009
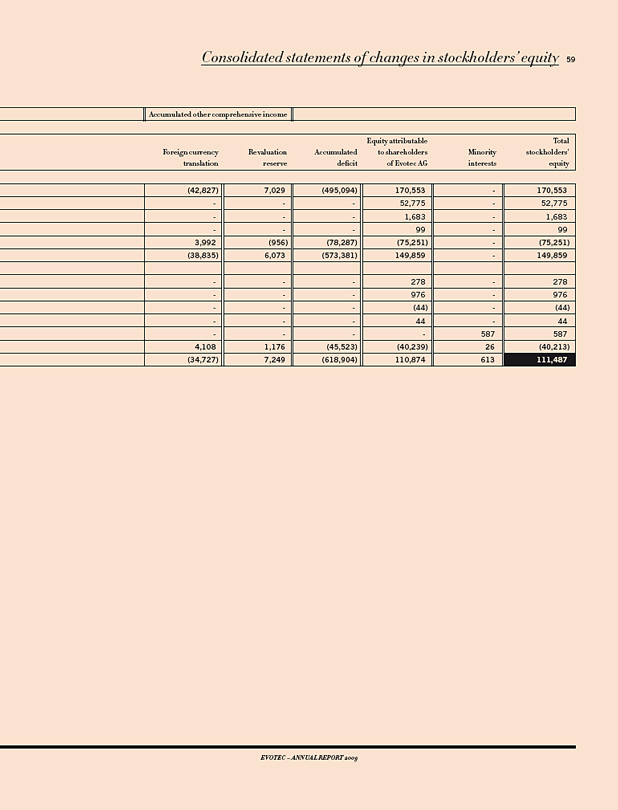
Consolidated statements of changes in stockholders’ equity 59
Accumulated other comprehensive income
Foreign currency translation Revaluation reserve Accumulated deficit Equity attributable to shareholders of Evotec AG Minority interests Total stockholders’ equity
(42,827) 7,029 (495,094) 170,553 - 170,553
- - - 52,775 - 52,775
- - - 1,683 - 1,683
- - - 99 - 99
3,992 (956) (78,287) (75,251) - (75,251)
(38,835) 6,073 (573,381) 149,859 - 149,859
- - - 278 - 278
- - - 976 - 976
- - - (44) - (44)
- - - 44 - 44
- - - - 587 587
4,108 1,176 (45,523) (40,239) 26 (40,213)
(34,727) 7,249 (618,904) 110,874 613 111,487
EVOTEC – ANNUAL REPORT 2009
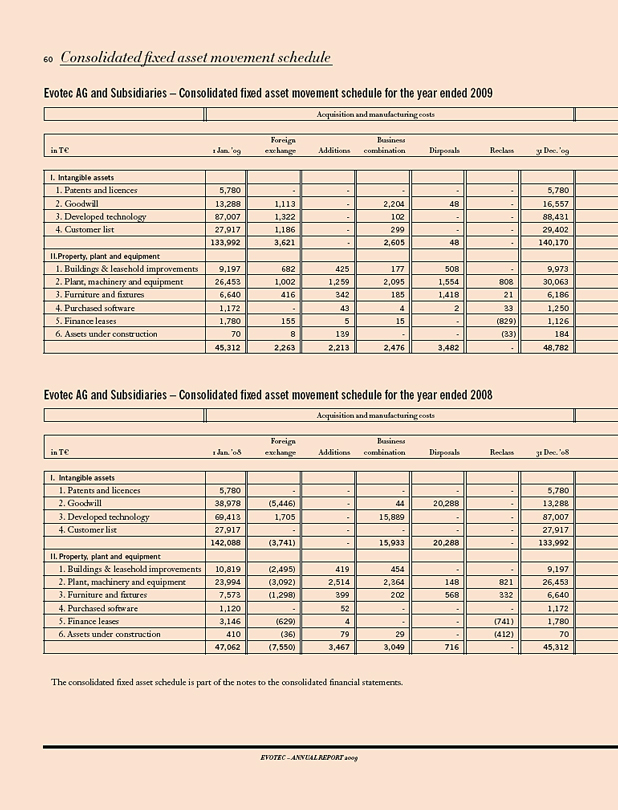
60 Consolidated fixed asset movement schedule
Evotec AG and Subsidiaries – Consolidated fixed asset movement schedule for the year ended 2009
Acquisition and manufacturing costs
in T€ 1 Jan. ’09 Foreign exchange Additions Business combination Disposals Reclass 31 Dec. ’09
I. Intangible assets
1. Patents and licences 5,780 - - - - - 5,780
2. Goodwill 13,288 1,113 - 2,204 48 - 16,557
3. Developed technology 87,007 1,322 - 102 - - 88,431
4. Customer list 27,917 1,186 - 299 - - 29,402
133,992 3,621 - 2,605 48 - 140,170
II. Property, plant and equipment
1. Buildings & leasehold improvements 9,197 682 425 177 508 - 9,973
2. Plant, machinery and equipment 26,453 1,002 1,259 2,095 1,554 808 30,063
3. Furniture and fixtures 6,640 416 342 185 1,418 21 6,186
4. Purchased software 1,172 - 43 4 2 33 1,250
5. Finance leases 1,780 155 5 15 - (829) 1,126
6. Assets under construction 70 8 139 - - (33) 184
45,312 2,263 2,213 2,476 3,482 - 48,782
Evotec AG and Subsidiaries – Consolidated fixed asset movement schedule for the year ended 2008
Acquisition and manufacturing costs
in T€ 1 Jan. ’08 Foreign exchange Additions Business combination Disposals Reclass 31 Dec. ’08
I. Intangible assets
1. Patents and licences 5,780 - - - - - 5,780
2. Goodwill 38,978 (5,446) - 44 20,288 - 13,288
3. Developed technology 69,413 1,705 - 15,889 - - 87,007
4. Customer list 27,917 - - - - - 27,917
142,088 (3,741) - 15,933 20,288 - 133,992
II. Property, plant and equipment
1. Buildings & leasehold improvements 10,819 (2,495) 419 454 - - 9,197
2. Plant, machinery and equipment 23,994 (3,092) 2,514 2,364 148 821 26,453
3. Furniture and fixtures 7,573 (1,298) 399 202 568 332 6,640
4. Purchased software 1,120 - 52 - - - 1,172
5. Finance leases 3,146 (629) 4 - - (741) 1,780
6. Assets under construction 410 (36) 79 29 - (412) 70
47,062 (7,550) 3,467 3,049 716 - 45,312
The consolidated fixed asset schedule is part of the notes to the consolidated financial statements.
EVOTEC – ANNUAL REPORT 2009
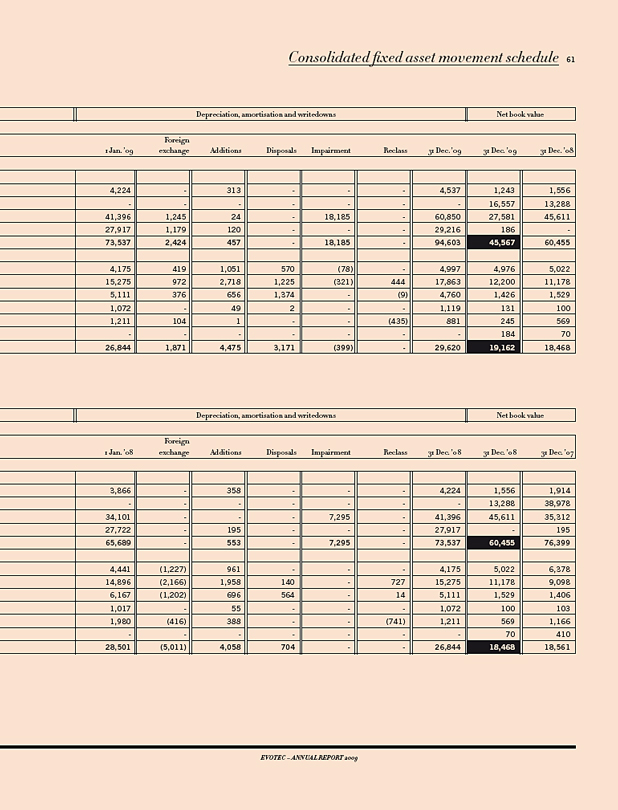
Consolidated fixed asset movement schedule 61
Depreciation, amortisation and writedowns Net book value
1 Jan. ’09 Foreign exchange Additions Disposals Impairment Reclass 31 Dec. ’09 31 Dec. ’09 31 Dec. ’08
4,224 - 313 - - - 4,537 1,243 1,556
- - - - - - - 16,557 13,288
41,396 1,245 24 - 18,185 - 60,850 27,581 45,611
27,917 1,179 120 - - - 29,216 186 -
73,537 2,424 457 - 18,185 - 94,603 45,567 60,455
4,175 419 1,051 570 (78) - 4,997 4,976 5,022
15,275 972 2,718 1,225 (321) 444 17,863 12,200 11,178
5,111 376 656 1,374 - (9) 4,760 1,426 1,529
1,072 - 49 2 - - 1,119 131 100
1,211 104 1 - - (435) 881 245 569
- - - - - - - 184 70
26,844 1,871 4,475 3,171 (399) - 29,620 19,162 18,468
Depreciation, amortisation and writedowns Net book value
1 Jan. ’08 Foreign exchange Additions Disposals Impairment Reclass 31 Dec. ’08 31 Dec. ’08 31 Dec. ’07
3,866 - 358 - - - 4,224 1,556 1,914
- - - - - - - 13,288 38,978
34,101 - - - 7,295 - 41,396 45,611 35,312
27,722 - 195 - - - 27,917 - 195
65,689 - 553 - 7,295 - 73,537 60,455 76,399
4,441 (1,227) 961 - - - 4,175 5,022 6,378
14,896 (2,166) 1,958 140 - 727 15,275 11,178 9,098
6,167 (1,202) 696 564 - 14 5,111 1,529 1,406
1,017 - 55 - - - 1,072 100 103
1,980 (416) 388 - - (741) 1,211 569 1,166
- - - - - - - 70 410
28,501 (5,011) 4,058 704 - - 26,844 18,468 18,561
EVOTEC – ANNUAL REPORT 2009

62 Notes
Evotec AG and Subsidiaries
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEAR 2009
(1) Business description and basis of presentation
Evotec AG, Schnackenburgallee 114, 22525 Hamburg, Germany and subsidiaries (“Evotec” or the “Company”) is a drug discovery and development company focused on providing integrated and innovative drug discovery services and alliances to the pharmaceutical and biotechnology industry. In addition, Evotec has a small number of its own drug candidates at various stages of development either partnered or available for partnering. Evotec provides innovative and integrated solutions to the pharmaceutical and biotechnology industry from target to clinical development through a range of capabilities and capacities, including early-stage assay development and screening, fragment-based drug discovery through to medicinal chemistry and in vivo pharmacology. In its proprietary projects, Evotec is developing new treatments for diseases related to neuroscience, pain and inflammation.
Evotec was founded on 8 December 1993 as EVOTEC BioSystems GmbH. Evotec completed an initial public offering in Germany on 10 November 1999 on Frankfurt Stock Exchange under the trading symbol “EVT”. On 5 May 2008, Evotec became listed on the NASDAQ Global Market in the US under the trading symbol “EVTC”. Effective
30 November 2009 Evotec voluntarily delisted from the NASDAQ
Global Market in the US.
All amounts herein are shown in thousands of Euro (T€), unless indicated otherwise. The Euro is the functional currency of the Company. On 25 February 2010 the Management Board authorised the consolidated financial statements for issue.
(2) Summary of significant accounting policies
The consolidated financial statements have been prepared in accordance with International Financial Reporting Standards (IFRS) and its interpretations as issued by the International Accounting Standards Board (IASB) as adopted by the European Union (EU), as well as the additional requirements of German commercial law pursuant to § 315a par. 1 HGB (German Commercial Law). The consolidated financial statements have been prepared on the historical cost basis except for derivative financial instruments as well as available-for-sale financial instruments, which are measured at fair value. The following is a summary of significant accounting policies followed in the preparation of the accompanying consolidated financial statements.
PRINCIPLES OF CONSOLIDATION
The consolidated financial statements include the accounts of Evotec and all companies which are under its control. All intercompany transactions and balances have been eliminated in consolidation.
Investments where Evotec does not have a controlling interest, but is in a position to influence the operating or capital decisions of the investee are accounted for under the equity method.
In connection with the acquisition of Renovis, Inc. in 2008 (Note 3), the Company issued 3,060,473 shares to a trust as replacement for share-based compensation arrangements. Those shares are included in the consolidated financial statements in accordance with SIC 12.
CASH AND CASH EQUIVALENTS
The Company considers all highly liquid short-term investments with original maturities of three months or less to be cash equivalents.
NON-DERIVATIVE FINANCIAL INSTRUMENTS
Non-derivative financial instruments consist of certain long-term and short-term investments, trade accounts and other receivables, cash and cash equivalents, loans, finance lease obligations, trade accounts and other payables. These instruments are recognised if Evotec becomes party to the contractual provisions of the financial instrument. Evotec accounts for financial assets at settlement date.
Financial assets are derecognised if either the rights to the cash flows arising from the instrument have expired or substantially all risk and rewards attributable to the instrument have been transferred. Financial liabilities are derecognised if the obligations have expired or have been discharged or cancelled.
At initial recognition, non-derivative financial instruments are measured at fair value plus transaction costs unless the financial instruments are classified at fair value through profit and loss. The Company does not have any non-derivative financial instruments classified at fair value
EVOTEC – ANNUAL REPORT 2009

Notes 63
through profit and loss. The subsequent measurement of the financial instruments at Evotec depends on the designation of the financial instruments to the following categories as defined in IAS 39:
— Loans and receivables
Loans and receivables are non-derivative financial assets with fixed or determinable payments that are not quoted in an active market. Financial instruments of this category are measured at amortised cost using the effective interest method less any impairment losses. Loans and receivables include trade accounts and other receivables.
— Available-for-sale financial assets
Evotec’s long-term and short-term investments, unless accounted for under the equity method in accordance with IAS 28 or as held-to-maturity investments, are classified as available-for-sale financial assets. Available-for-sale financial assets are measured at fair value at the balance sheet date or, if this value cannot be determined, at cost. Unrealised gains and losses resulting from changes in fair value are reported in equity, net of any tax effect. Changes in fair value are not recognised in the income statement until the asset is sold or until an impairment loss is recorded. Investments that qualify as equity instruments are measured at cost if their fair value cannot be determined based on quoted prices or by reference to the current fair value of comparable instruments, or by using appropriate pricing models (in cases where cash flows are volatile or cannot be reliably determined).
— Held-to-maturity investments
Held-to-maturity investments are non-derivative financial assets with fixed maturity and fixed or determinable payments that are quoted in an active market. If Evotec has the intent and ability to hold long-term and short-term investments to maturity, those assets are classified as held-to-maturity. Held-to-maturity financial assets are initially measured at fair value plus transaction costs. Subsequent to the initial recognition held-to-maturity investments are measured at amortised cost using the effective interest method less any impairment losses.
DERIVATIVE FINANCIAL INSTRUMENTS
The Company uses foreign currency derivative financial instruments to hedge its exposure to foreign exchange risks. The Company entered into an agreement, where the Company received the right (“Put Option”) to sell financial assets, which is considered to be a derivative and is measured at fair value through profit and loss in accordance with IAS 39. In accordance with its treasury policy, the Company does not hold or issue derivative financial instruments for trading purposes.
Derivative financial instruments are recognised initially and subsequent to initial recognition at fair value. Accounting for the change in fair value of derivatives depends on whether they are designated as hedging instruments and qualify as part of a hedge relationship under IAS 39. If these conditions are not met, even if there is an economic hedge relationship with an underlying transaction, changes in fair value of the derivatives are recognised directly in the income statement.
Evotec’s foreign currency derivative financial instruments are economic hedges, however, they are not accounted for as hedges in accordance with IAS 39. Therefore, all changes in the fair value of the foreign currency derivative financial instruments are recognised in foreign currency exchange gains and losses.
BASIS FOR DETERMINING FAIR VALUES OF FINANCIAL INSTRUMENTS
The following summarises the significant methods and assumptions used in estimating the fair values of financial instruments.
The fair value of financial assets at fair value through profit or loss and available-for-sale financial assets is determined by reference to their quoted bid price at the reporting date unless the available-for-sale financial assets are unquoted equity instruments or financial assets without an active market.
Unquoted equity instruments are measured at cost. Available-for-sale financial assets without an active market are estimated using a valuation technique based on assumptions that are not supported by prices from observable markets.
The fair value of forward exchange contracts is based on their listed market price, if available. If a listed market price is not available, then the fair value is estimated by discounting the difference between the contractual forward price and the current forward price for the residual maturity of the contract using a risk-free interest rate. Unless otherwise reported, the fair values of financial instruments equal the carrying amounts.
INVENTORIES
In accordance with IAS 2, inventories are valued at the lower of cost or net realisable value, with cost being generally determined on the basis of an average method. Net realisable value is the estimated selling price in the ordinary course of business, less the estimated costs of completion and selling expenses. Costs consist of purchased component costs and manufacturing costs, which are comprised of direct material and labour costs and systematic allocated costs. Costs are removed from inventories to costs of revenue based on specific identification.
PROPERTY, PLANT AND EQUIPMENT
Property, plant and equipment acquisitions, including leasehold improvements, are recorded at cost less any vendor rebates. Leased property, plant and equipment meeting certain criteria are capitalised and the present value of the related lease payments are recorded as a liability. Depreciation of property, plant and equipment, which includes depreciation of assets under finance leases, is calculated using the straight-line method over the estimated useful lives of the assets. Depreciation of leasehold improvements is calculated using the straight-line method over the shorter of the related lease term or the estimated useful life. The useful lives are as follows:
Years
Buildings and leasehold improvements 6-35 Plant, machinery and equipment 3-20 Furniture and fixtures 3-15 Computer equipment and software 3-5 Assets under finance lease 3-5
EVOTEC – ANNUAL REPORT 2009

64 Notes
The depreciation period and method is reviewed at each balance sheet date. Differences from previous estimates are accounted for as a change in an accounting estimate in accordance with IAS 8. The costs included in property, plant and equipment related to assets under construction are not depreciated until the assets are placed into service by the Company. Upon sale or retirement, the costs and the related accumulated depreciation are removed from the respective accounts, and any gain or loss is included in other operating income and expense. Maintenance and repairs are expensed as incurred.
INTANGIBLE ASSETS, EXCLUDING GOODWILL
Intangible assets, excluding goodwill, consist of separately identified intangible assets such as developed technologies, customer lists and patents, which were acquired in business combinations, purchased licenses and patents.
Intangible assets with definite useful lives are recorded at cost and are amortised using the straight-line method over the estimated useful lives of the assets:
Years
Developed technologies 3-5 Customer list 2-5 Patents and licenses 15 or shorter life
Developed technologies acquired in the business combinations with ENS Holdings, Inc. (ENS) and Renovis, Inc. are not amortised until the intangible assets are likely to generate benefits and tested for impairment at least annually.
The amortisation period and method is reviewed at each balance sheet date.
GOODWILL
Goodwill acquired in a business combination is recognised as an asset representing any value attributed to items that cannot be individually identified and separately recognised at the acquisition date. The Company recognises separately the acquired identifiable assets, liabilities and contingent liabilities at the acquisition date. Goodwill is measured at cost less accumulated impairment losses until 2008. With the early adoption of the revised IFRS 3 starting 1 January 2009, the Company measures the goodwill at acquisition-date fair value plus the acquisition-date fair value of Evotec’s previously held equity interests in this business combination.
The Company’s goodwill results mainly from its acquisition of Oxford Asymmetry International plc. in October 2000. Additional goodwill acquired in a business combination has arisen from the acquisition of ENS in May 2005 and from the acquisition of Renovis, Inc. in May 2008, which was fully impaired in 2009. The balance sheet as of 31 December 2009 includes an additional goodwill acquired in a business combination from the acquisition of Research Support International Private Limited (RSIPL) in August 2009.
REVENUE RECOGNITION
Revenue is recognised when it is probable that the economic benefits associated with the transaction will flow to the Company based upon the performance requirements of the respective agreements.
Revenues generated from contracted services are recognised as the services are rendered. Payments for contracted services are generally paid in advance and recorded as deferred revenue until earned. Deliverable kind of contracted services are recorded as revenue upon delivery if the Company has received a customer order, the price is determinable and collectibility is reasonably assured. The Company assesses collectibility based on a number of factors, including past transaction history with the customer and the customer’s credit-worthiness. Payments for deliverable kind of contracted services are generally paid in advance and recorded as advanced payments received. Revenue from compound access fees is recognised rateably over the related forecasted service period.
Revenue under long-term collaborative agreements includes, but is not limited to, the following:
1. Research Payments – revenue from research payments finances both direct costs incurred in connection with the Company’s ongoing research and development activities and indirect costs incurred as part of an allocation of certain other administrative expenses. Revenue from research payments is recognised rateably over the related forecasted research period as services are provided.
2. Success Payments – revenue contingent upon the attainment of certain milestones is recognised in the period the milestone is successfully achieved. This typically occurs when the Company’s contract partner agrees that the requirements stipulated in the agreement have been met.
The Company has entered into multiple-element contracts and carefully determined whether the different revenue-generating elements are sufficiently separable and whether there exists sufficient evidence of their fair values to separately account for some or all of the individual elements of the contracts. Only if an element is considered to meet these criteria it represents a separate unit of accounting. The Company has no refund obligations included in their service agreements.
Under the terms of various contractual arrangements, Evotec receives royalty payments, which are incremental to the other company’s respective product sales. Royalty income of T€ 2,165 in 2009 and T€ 810 in 2008 is included in revenue. Additionally the Company might receive sales milestones. Such income is included in revenue in the amount of T€ 1,000 in 2009 and T€ 0 in 2008.
INTEREST INCOME AND EXPENSE
Interest is recorded as expense or income in the period to which it relates. The interest expense component of finance lease payments is recognised in the income statement using the effective interest rate method. Interest income and expense are recognised in the income statement as it occurs, using the effective interest method.
Evotec has no qualifying assets according to the revised IAS 23 and therefore does not capitalise interest expenses.
EVOTEC – ANNUAL REPORT 2009

Notes 65
INCOME TAXES
Income taxes comprise the current taxes on income in the individual countries as well as the deferred taxes. Income taxes are recorded in the income statement except for those items recorded directly in stockholders’ equity.
Under the liability method, deferred tax assets and liabilities are recognised for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases as well as for tax loss carry forwards. Deferred tax assets and liabilities are measured using tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be realised or settled based on enacted or substantially enacted tax rates.
The effect on deferred tax assets and liabilities of a change in tax rates is recognised in the period that includes the date of enactment or substantial enactment. In assessing the recoverability of deferred tax assets, management considers whether it is probable that some portion or all of the deferred tax assets will not be realised. Deferred tax assets are not recognised to the extent that it is not probable that the related tax benefit will be realised.
RESEARCH AND DEVELOPMENT
Research and development costs that are generated for internal projects are capitalised or expensed depending on whether the expenditure incurred falls under the classifications of research or development expenditure given by IAS 38. When it is not certain that research and development projects will generate probable future economic benefits to the Company, such costs are expensed as incurred. Those projects which are expected to generate probable future economic benefits are capitalised as an intangible asset and amortised if all criteria set out in IAS 38 are met. This principle is also used for the accounting of developed software. However, the software included in property, plant and equipment consists only of purchased software. Evotec did not capitalise any research and development costs in 2009 and 2008.
Research and development costs that are acquired in a business combination are capitalised when those research and development projects are expected to generate probable future economic benefits to the Company. Research and development costs acquired in a business combination are not amortised until they are likely to generate benefits.
The Company receives grants from government authorities for the support of specific research and development projects. The grants are requested when qualifying expenses have been incurred and are recognised as a reduction of research and development expense when they are received. No grants were received for capitalised development expenditures. The amounts recognised as a reduction of the Company’s research and development expense were T€ 11 in 2009 and T€ 20 in 2008. Under the terms of the grants, governmental agencies generally have the right to audit qualifying expenses submitted by the Company.
TRANSLATION OF FOREIGN CURRENCY DENOMINATED TRANSACTIONS AND FOREIGN OPERATIONS
The assets and liabilities of foreign subsidiaries with functional currencies other than the Euro are translated into Euro using period-end exchange rates, while the revenues and expenses of such subsidiaries are translated using rates of the date of the transaction during the period. Gains or losses resulting from translating foreign functional currency financial statements are reported as a separate component of stockholders’ equity.
Transactions in foreign currencies are translated into Euro using the foreign exchange rate ruling at the date of the transaction. Assets and liabilities denominated in foreign currencies at the balance sheet date are translated into Euro using period-end exchange rates. Gains or losses resulting from foreign currency denominated transactions are included in other non-operating income and expense.
IMPAIRMENT OF LONG-LIVED ASSETS AND GOODWILL
The Company reviews long-lived assets (property, plant and equipment and intangible assets including goodwill) for impairment, to estimate the value in use or the fair value less cost to sell, in accordance with IAS 36. An impairment review is performed annually for intangible assets with indefinite useful lives, intangible assets not yet available for use and goodwill, or whenever events or changes in circumstances indicate that the carrying amount of an asset or a group of assets may not be recoverable. In line with our policy concerning the impairment of intangible assets with indefinite useful lives and goodwill, the Company carried out an impairment test in the fourth quarter of 2009 and 2008 (see Note 11).
An impairment loss is recognised if the carrying amount of an asset (or a group of assets when considering a cash generating unit) exceeds its recoverable amount which is the higher of its fair value less costs to sell or value in use. The value in use for an asset or cash generating unit is calculated by estimating the net present value of future cash flows arising from that asset or cash generating unit. The discount rate used to calculate the value in use is determined to reflect the risks inherent for each asset or cash generating unit. The evaluation of the net cash flow of the further use is based on a mid range or where applicable long range forecast. Considerable management judgement is necessary to estimate discounted future cash flows.
Any impairment is reported as a separate component of operating expenses in the consolidated income statement. An impairment of tangible assets and intangible assets excluding goodwill is reversed if there has been a change in the estimates used to determine the value in use leading to an increase in value for a previously impaired asset. It is reversed only to the extent that the asset’s carrying amount does not exceed the carrying amount that would have been determined, net of depreciation or amortisation, if no impairment loss had been previously recognised. Impairments of goodwill are not reversed.
STOCK COMPENSATION
The Company applies the provisions of IFRS 2 in accounting for options granted under its stock option plan. Compensation cost from the issuance of employee stock options is measured using the fair value method
EVOTEC – ANNUAL REPORT 2009

66 Notes
at the measurement date and is charged straight-line to expenses over the vesting period in which the employee renders services.
PENSION AND SIMILAR OBLIGATIONS
The Company’s net obligation for defined benefit and other post-retirement benefit plans have been calculated using the projected unit credit method. Actuarial gains and losses are recognised using the 10% corridor.
Service cost and interest costs for pensions and other postretirement obligations are recognised as an expense in operating result.
The Company’s obligations for contributions to defined contribution plans are recognised as expense as incurred.
PROVISIONS
Provisions are recognised when the Company has a present obligation as a result of a past event which will result in a probable outflow of economic benefits that can be reasonably estimated. The amount recognised represents the best estimate of the settlement amount of the present obligation as of the balance sheet date. Expected reimbursements of third parties are not offset, but recorded as a separate asset if it is virtually certain that the reimbursements will be received. Where the effect of the time value of money is material, provisions are discounted using a risk adjusted market rate.
Provisions for restructuring costs are recognised when the Company has a detailed formal plan for the restructuring and has notified the affected parties.
A provision for onerous contracts is recognised when the expected benefits to be derived by the Group from a contract are lower than the unavoidable costs of meeting its obligations under the contract. The Company accrues for estimated losses from legal actions or claims, including legal expenses, when events exist that make the realisation of the losses or expenses probable and they can be reasonably estimated.
NET LOSS PER SHARE
Basic net loss per share is calculated by dividing the net loss by the weighted-average number of common shares outstanding for the period, without consideration for common stock equivalents. Diluted net loss per share is computed by dividing the net loss by the weighted-average number of common share and share equivalents outstanding for the period determined using the treasury-stock method. For purposes of this calculation, stock options are considered to be common stock equivalents and are only included in the calculation of diluted net loss per share when their effect is dilutive. There are no dilutive shares in 2009 and 2008 as a result of net losses. Anti-dilutive common stock equivalents consist of 1,661,450 and 0 stock options in 2009 and 2008, respectively.
USE OF ESTIMATES
The preparation of the accompanying consolidated financial statements requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent
assets and liabilities as of the date of the main financial statements and the reported amounts of revenues and expenses during the reporting period. Main estimates and assumptions affect acquisitions (Note 3), impairment testing (Note 11), provisions (Note 16), measurement of compensation expenses (Note 19) and the recognition of deferred tax assets (Note 18). Actual results could differ from management’s estimates. In addition, changes in the current economic conditions and other events could also have a significant effect on reported amounts.
RECENT PRONOUNCEMENTS
Evotec has early adopted the revised version of IFRS 3 “Business Combinations” and an amended version of IAS 27 “Consolidated and Separate Financial Statements” for all business combinations occurring in 2009. The change in accounting policy is applied prospectively. This accounting change impacted Evotec’s income statement in the amount of T€ 460 due to the gain recognised from remeasuring Evotec’s previously held equity interests and not capitalised transaction costs. All of the following IFRS pronouncements that were issued by the IASB and the IFRIC and were not effective as of 31 December 2009, have not been applied in the preparation of the consolidated financial statements as of 31 December 2009.
In January 2009, the IASB issued IFRIC 18 “Transfer of Assets from Customers”, which has been endorsed on 27 November 2009 by the EU. IFRIC 18 provides guidance on how to account for and recognise revenue for agreements in which an entity receives from a customer assets or cash to acquire assets in order to subsequently provide services. The regulations are effective for annual periods beginning on or after
1 November 2009, with earlier application permitted. This interpretation does not have any impact on the Company’s consolidated financial statements.
On 12 November 2009, the IASB issued IFRS 9 “Financial Instruments” which is not yet endorsed by the EU as the first step in its project to replace IAS 39 “Financial Instruments: Recognition and Measurement”. IFRS 9 introduces new requirements for classifying and measuring financial assets that must be applied starting 1 January 2013, with early adoption permitted. The Company is currently evaluating the effect of IFRS 9.
On 26 November 2009, the IASB issued “Prepayments of a Minimum Funding Requirement” (Amendments to IFRIC 14) which is not yet endorsed by the EU. The amendments correct an unintended consequence of IFRIC 14 IAS 19 – “The Limit on a Defined Benefit Asset, Minimum Funding Requirements and their Interaction”. Without the amendments, in some circumstances entities are not permitted to recognise some voluntary prepayments for minimum funding contributions as an asset. This was not intended when IFRIC 14 was issued, and the amendments correct the problem. The amendments are effective for annual periods beginning 1 January 2011, with earlier application permitted. The amendments must be applied retrospectively to the earliest comparative period presented. Evotec does not expect that the adoption will have a material impact on the consolidated financial statements.
On 18 June 2009, the IASB issued amendments to IFRS 2 “Share-based Payment” which is not yet endorsed by the EU that clarify the accounting for group cash-settled share-based payment transactions. The amendments clarify how an individual subsidiary in a group should account for some share-based payment arrangements in its own financial
EVOTEC – ANNUAL REPORT 2009

Notes 67
statements. In these arrangements, the subsidiary receives goods or services from employees or suppliers but its parent or another entity in the group must pay those suppliers. The amendments are effective for annual periods beginning on or after 1 January 2010 and must be applied retrospectively. Evotec does not expect that the adoption will have a material impact on the consolidated financial statements. On 4 November 2009, the IASB issued a revised version of IAS 24 “Related Party Disclosures” which is not yet endorsed by the EU. The objective of IAS 24 is to ensure that an entity’s financial statements contain the disclosures necessary to draw attention to the possibility that its financial position and profit or loss may have been affected by the existence of related parties and by transactions and outstanding balances with such parties. Effective date is 1 January 2011. Evotec does not expect that the adoption will have a material impact on the consolidated financial statements.
In July 2008, the IASB issued “Eligible Hedged Items – Amendment to IAS 39 Financial Instruments: Recognition and Measurement” which has been endorsed on 15 September 2009 by the EU. The amendment clarifies how the existing principles underlying hedge accounting should be applied in two particular situations – the designation of inflation in a financial hedged item and the designation of a one sided risk in a hedged item. The application of the amendment is compulsory for the fiscal years beginning on or after 1 July 2009 and has to be applied retrospectively, earlier application is permitted. Evotec does not expect that the adoption will have a material impact on the consolidated financial statements.
In November 2008, the IFRIC issued IFRIC 17 “Distributions of Non-cash Assets to Owners” which has been endorsed on 26 November 2009 by the EU. The application of the interpretation is compulsory for financial years beginning on or after 1 July 2009, while earlier application is permitted. Evotec does not expect that the adoption will have a material impact on the consolidated financial statements.
The IASB issued various other pronouncements.
(3) Acquisitions
The Company acquired 70% of the shares in Research Support International Private Limited, Thane, India (RSIPL) including its 51% share in Evotec-RSIL Limited, India (Evotec-RSIL). RSIPL is a company providing drug discovery and development services. This acquisition represents an important capacity expansion for Evotec. It adds a complementary drug discovery operation and capability in the field of science-driven chemistry work in India and efficiently increases Evotec’s ability to deliver services to its partners on a global scale. The acquisition was effective as of 31 August 2009. The purchase price of T€ 2,373 in cash includes a potential earn-out which was calculated based on estimated future revenues and will be paid in 2010. The Company decided to early adopt the revised version of IFRS 3 “Business Combinations” and the amended version of IAS 27.
At 31 August 2009 the Company recognised a gain of T€ 559 to adjust its carrying amount of the investment in Evotec-RSIL which used to be accounted for under the equity method to its fair value according to the revised version of IFRS 3. The fair values of the assets and liabilities acquired from RSIPL were estimated based on the recognised amounts as of the date of the acquisition. Fair value adjustments have been recorded for a customer list in the amount of T€ 103 which has been
estimated based on discounted cash flow modelling. The resulting goodwill from the acquisition amounts to T€ 2,204. The net loss of Evotec for the twelve months ended December 2009 included a net income of T€ 59 from RSIPL and Evotec-RSIL as well as revenues of T€ 1,244.
in T€ 31 Aug. 2009 carrying amount 31 Aug. 2009 fair value
Cash and cash equivalents 137 137
Trade accounts receivable 277 277
Inventories 69 69
Other current assets 465 465
Property, plant and equipment 2,454 2,454
Customer list - 103
Loans (504) (504)
Provisions (74) (74)
Current liabilities (1,145) (1,145)
Deferred tax liabilities (52) (70)
Net assets acquired 1,627 1,712
Minority interests - (587)
At equity investment Evotec-RSIL - (956)
Goodwill - 2,204
Cost of acquisition - 2,373
Plus transaction costs - 98
Less cash and cash equivalents acquired - (137)
Less earn out - (748)
Cash outflow from acquisition - 1,586
The following unaudited pro-forma information is based on the assumption that the investment in RSIPL occurred as of 1 January 2008:
in T€ 2009 2008
Pro-forma revenues 43,810 41,721
Pro-forma net loss (45,385) (78,221)
Pro-forma basic and diluted loss per share (0,42) (0,82)
In 2008, the Company acquired in a share-for-share transaction 100% of the shares in Renovis, Inc., South San Francisco, US, a company operating in the field of drug discovery and development with a focus on pain and inflammation. This acquisition was effective as of 2 May 2008. Evotec issued 34,970,268 shares to acquire the underlying shares, outstanding options and restricted stock units held by Renovis employees. The purchase price of T€ 58,625 comprises the fair value of the shares issued for common stock of € 1.68 per share which was based on the stock price of Evotec at the date of acquisition as well as the fair values determined for the shares issued for equity based compensation plans as of the date of acquisition. The relating transaction costs amounted to T€ 3,249.
The fair values of the assets and liabilities acquired from Renovis were estimated based on the recognised amounts as of the date of the acquisition.
EVOTEC – ANNUAL REPORT 2009

68 Notes
Fair value adjustments have been recorded for developed technologies in the amount of T€ 15,889 which have been estimated based on net present value modelling and for certain non-current financial assets in the amount of T€ (280). Additionally, deferred revenues in the amount of T€ 178 were reversed because no future obligation relates to those amounts. The resulting goodwill amounts to T€ 44. The net loss of Evotec for the twelve months ended December 2008 included a net loss of T€ 7,739 from Renovis.
in T€ 2 May 2008 carrying amount 2 May 2008 fair value
Cash and cash equivalents 10,706 10,706
Investments 25,333 25,333
Prepaid and other current assets 861 861
Property, plant and equipment 3,045 3,045
Developed technologies - 15,889
Other non-current financial assets 8,805 8,525
Current liabilities (5,251) (5,073)
Non-current liabilities (706) (706)
Net assets acquired 42,793 58,580
Goodwill - 44
Cost of acquisition - 58,624
Less cash and cash equivalents acquired - (10,706)
Less fair values of shares issued - (55,375)
Less transaction costs - (3,249)
Cash inflow (-) from acquisition - (10,706)
The following unaudited pro-forma information is based on the assumption that the investment in Renovis, Inc. occurred as of 1 January 2008:
in T€ 2008
Pro-forma revenues 40,391
Pro-forma net loss (57,429)
Pro-forma basic and diluted loss per share (0.60)
(4) Use restrictions on the Company’s technology
Evotec was subject to certain restrictions concerning technologies arising in the course of its cooperation with Novartis.
Pursuant to its agreement with Novartis, Evotec was obligated to pay royalties equal to 5% of the qualifying revenue to Novartis for a period of ten years. This obligation ended on 16 March 2008. The Company recorded no related royalty expenses in 2009 or 2008.
(5) Cash and cash equivalents and investments
On 31 December 2009 and 2008, an amount of T€ 13,928 and T€ 10,343, respectively, of cash and cash equivalents was pledged as security. Investments in mutual funds, which invest in debt instruments to manage the fund investors’ liquidity, including debt instruments with a maturity beyond three months, are reported as current investments and carried at cost that approximates their fair value. Those investments are classified as available-for-sale financial assets.
(6) Trade accounts receivables
The Company has assessed the non-payment risk of all trade accounts receivables which resulted in an allowance of T€ 88 and T€ 0 in 2009 and 2008, respectively. There are no use restrictions on trade accounts receivable.
The aging of trade accounts receivables at the year-end was:
in T€ 31 Dec.’09 31 Dec.’08
Not past due 3,355 1,697
Past due 0-30 days 590 514
Past due 31-120 days 364 99
More than 120 days 201 221
Total trade accounts receivables 4,510 2,531
(7) Inventories
Inventories consist of the following:
in T€ 31 Dec.’09 31 Dec.’08
Raw materials 1,897 1,723
Work-in-progress 528 416
Total inventories 2,425 2,139
Raw materials consist of biological materials and substances as well as chemicals. Work-in-progress in 2009 and in 2008 consists of costs incurred on customer projects which were not completed at year end. The Company carries an allowance on raw materials of T€ 802 and T€ 1,220, included in the amounts above, as of 31 December 2009 and 2008, respectively. An allowance on work-in-progress in the amount of T€ 27 and T€ 28 as of 31 December 2009 and 2008, respectively is included in the amounts above. A reversal of previously written down inventories was recognised in 2009 in the amount of T€ 418 on raw materials.
EVOTEC – ANNUAL REPORT 2009

Notes 69
(8) Other current financial assets
Other current financial assets as of 31 December 2009 mainly consist of accrued revenues.
(9) Long-term investments
Long-term investments consist of the following:
in T€ 31 Dec.’09 31 Dec.’08
European ScreeningPort GmbH, Hamburg 10 10
Evotec RSIL Ltd., Maharashtra (Thane), India - 417
Total long-term investments 10 427
As of 31 December 2009 the Company’s carrying amount of the investment in Evotec RSIL Ltd. (Evotec RSIL), Maharashtra, India amounted to T€ 0 due to the acquisition of RSIPL and the resulting full consolidation of Evotec RSIL from 31 August 2009 onwards (31 December 2008: T€ 417). At 31 August 2009, the Company recognised a gain of T€ 559 to adjust its carrying amount of the investment to its fair value according to the revised IFRS 3. The share of the net loss of Evotec RSIL which was accounted for under the equity method of accounting amounted to T€ 20 in the first eight months 2009 (2008: T€ 242).
In 2007, Evotec founded together with the City of Hamburg the European ScreeningPort GmbH (ESP), Hamburg, with an ownership of 19.9% interest. As of 31 December 2009 and 2008 the carrying amount of the investment is T€ 10. This investment is classified as available-for-sale financial asset.
The long-term investments of Evotec continue to have losses and, therefore, do not have undistributed profits.
In 2009 and 2008, the Company recorded revenues in the amount of T€ 597 and T€ 30, respectively with ESP. Additionally the Company has agreed to grant ESP a loan in the amount of T€ 1,500 of which T€ 989 is drawn. The drawn portion of the loan was fully written down in 2009. Services and materials were purchased in 2009 and 2008 from Evotec RSIL in the amount of T€ 462 and T€ 134, respectively. No further material transactions with investments of the Company were recorded.
(10) Property, plant and equipment
With respect to the development of property, plant and equipment, please refer to the consolidated fixed asset movement schedule. The main additions in 2009 and 2008 relate to upgrades of our screening facility and analytical equipment. Upon completion of the assets under construction, costs are transferred into their respective fixed assets classification. Depreciation expense amounted to T€ 4,475 and T€ 4,058 in 2009 and 2008, respectively.
Laboratory premises in Abingdon, United Kingdom were tested for impairment. During the asset impairment review, as permitted under IAS 36, management estimated the asset impairment using a method based on the physical usage of the laboratory premises. This has resulted in a partial reversal of T€ 395 of previously recognised assets impairment. In 2008 the Company concluded that no impairment, nor reversal of impairment, was necessary.
The net book values included in the fixed assets, which are held under finance leases, relate to plant and machinery as well as fixture and fittings of T€ 231 and T€ 14 as of 31 December 2009 and T€ 533 and T€ 36 as of 31 December 2008, respectively. The related depreciation amounts to T€ 217 and T€ 4 in 2009, T€ 376 and T€ 12 in 2008, respectively.
(11) Other intangible assets and goodwill
With respect to the development of intangible assets and goodwill please refer to the consolidated fixed asset movement schedule.
Intangible assets consist of developed technologies, customer list and acquired patent and licenses.
The main additions in 2009 relate to intangible assets acquired in the business combination with RSIPL with effective date 31 August 2009, amounting to T€ 103. In 2008 the main additions related to intangible assets acquired in the business combination with Renovis, Inc. with effective date 2 May 2008, amounting to T€ 15,889. Amortisation expense of intangible assets amounted to T€ 457 in 2009 and T€ 553 in 2008. The customer lists acquired through the acquisition of ENS in 2005 were fully amortised in 2008.
The developed technologies acquired in a business combination are not amortised until they are likely to generate benefits.
The USD denominated developed technologies from the acquisition of Renovis, Inc. were tested for impairment on the annual designated test date of October 2009 and 2008. The impairment test is based on a discounted cash flow model by using the assumptions of a Long Range Plan (LRP) for 15 to 20 years to determine a value for the cash generating projects. The discount rate considering the risks and rewards of the activities used in the impairment test was 10.9% and 13.3% in 2009 and 2008, respectively. As a result of that test, the Company concluded that an impairment in the amount of T€ 13,799 and T€ 0 at 31 December 2009 and 2008, respectively, was deemed necessary. The carrying amount at 31 December 2009 and 2008 amounted to T€ 3,874 and T€ 17,594, respectively.
The developed technologies from the acquisition of ENS Holdings, Inc. with a carrying amount of T€ 23,631 and T€ 28,017 at 31 December 2009 and 2008, respectively, were tested for impairment on the annual designated test date October 2009. The impairment test is based on a discounted cash flow model by using the assumptions of a Long Range Plan (LRP) for 15 to 20 years to determine a value for the cash generating projects. The discount rate considering the risks and rewards of the activities used in the impairment test was 10.9% and 14.0% in 2009 and 2008, respectively. As a result of that test, the Company concluded that an impairment is deemed necessary in the amount of T€ 4,386. In 2008, the impairment test resulted in an impairment of T€ 7,295.
For the purpose of impairment testing, goodwill is allocated to Evotec’s operating divisions which represent the lowest level within the Company at which the goodwill is monitored for internal management purposes. Goodwill from the acquisition of Oxford Asymmetry International plc has been tested for impairment on the annual designated test date October 2009. The impairment test is based on a discounted cash flow model
EVOTEC – ANNUAL REPORT 2009

70 Notes
by using the assumptions of the Mid Range Plan for 2010 to 2014. The discount rate considering the risks and rewards of the activities used in the impairment test was 9.51% and 9.1% in 2009 and 2008, respectively. As a result of that test, the Company concluded that no impairment was due for the goodwill carried as of that date. In 2008, the impairment review resulted in an impairment of T€ 20,288. The carrying amount at 31 December 2009 and 2008 amounted to T€ 13,825 and T€ 12,778, respectively.
In May 2005 the Company acquired ENS Holdings, Inc. which resulted in goodwill in the amount of T€ 461 which is also the carrying amount at 31 December 2009 and 2008. The Company has tested the cash generating unit for impairment on the annual designated test date October 2009. The impairment test is based on a discounted cash flow model by using the assumptions of the Long Range Plan (LRP) for 15 to 20 years. The discount rate considering the risks and rewards of the activities used in the impairment test was 10.9% and 14.0% in 2009 and 2008, respectively. As a result of this test, the Company concluded that no impairment has to be recorded in 2009 and 2008.
In May 2008 the Company acquired Renovis, Inc. which resulted in a goodwill denominated in USD in the amount of T€ 44 and a carrying amount at 31 December 2009 and 2008 of T€ 0 and T€ 49, respectively. The Company has tested the cash generating unit for impairment on the annual designated test date October 2009. The impairment test is based on a discounted cash flow model by using the assumptions of the Long Range Plan (LRP) for 15 to 20 years. The discount rate considering the risks and rewards of the activities used in the impairment test was 10.9% and 13.3% in 2009 and 2008, respectively. As a result of this test, the Company concluded that an impairment in the amount of T€ 48 and T€ 0 has to be recorded in 2009 and 2008, respectively.
In August 2009 the Company acquired Research Support International Private Ltd. (RSIPL) which resulted in goodwill in the amount of T€ 2,204 and a carrying amount at 31 December 2009 of T€ 2,271. The Company has tested the cash generating unit for impairment on the annual designated test date October 2009. The impairment test is based on a discounted cash flow model by using the assumptions of the Mid Range Plan for 2010 to 2013. The discount rate considering the risks and rewards of the activities used in the impairment test was 13.0% in 2009. As a result of this test, the Company concluded that no impairment has to be recorded in 2009.
The total amount of foreign exchange differences related to goodwill denominated in a foreign currency amounted to T€ 1,113 and T€ 5,446 in 2009 and 2008, respectively and are recorded directly in equity.
(12) Other non-current financial assets
Other non-current financial assets as of 31 December 2009 consist primarily of auction rate securities (“ARSs”) in the amount of T€ 9,236 and Put Options related to these ARSs in the amount of T€ 550 (31 December 2008: T€ 8,303 and T€ 1,726, respectively) as well as Coupon Bonds in the amount of T€ 3,000 which are categorised as level 1 in the fair value hierarchy.
The other non-current financial assets are classified as follows:
in T€ 31 Dec.’09 31 Dec.’08
Held-to-maturity investments 3,000 -
Financial assets designated at fair value
through profit or loss 550 1,726
Available-for-sale financial assets 9,236 8,303
Receivables 117 443
12,903 10,472
The ARSs were acquired as part of the Renovis acquisition and are classified as available-for-sale securities which are measured at fair value with unrealised gains and losses reported as a component of “Accumulated other comprehensive income” in stockholders’ equity. The ARSs the Company holds are debt instruments issued by U.S. municipalities with substantially all being AAA rated or equivalent, backed by pools of student loans and largely guaranteed by the U.S. Department of Education. These instruments are debt securities with long-term maturities with various interest rates historically resetting through auctions generally once a month. Since mid-February 2008, liquidity issues in the global credit markets have resulted in the failure of auctions of all of the ARSs the Company holds. To date all interest payable on the ARSs have been paid when due, however, due to the illiquidity of the ARSs there is not a ready market for the purchase and sale of these instruments. Due to the illiquidity of the ARSs, the Company engaged in 2009 an external valuation group to come up with a valuation analysis. The external valuation group utilised a discounted cash flow (“DCF”) model to derive an estimate of fair value of these securities at 31 December 2009, as did Evotec in 2008. This is categorised as a level 2 in the fair value hierarchy. The discounted cash flow model includes estimates with respect to the amount and timing of future interest payments, projections of interest rates, and the rate of return required by investors to own such securities given the current liquidity risk associated with the ARSs. As a result, the Company recorded an unrealised gain of T€ 1,186 in 2009 and an unrealised loss of T€ 956 in the period May to December 2008 related to ARS investments of T€ 9,803 and T€ 10,110 (par value), respectively. In 2009 ARSs with a par value of T€ 117 matured. The fair value of the ARSs as of the date of acquisition amounted to T€ 8,361. As of 31 December 2009 and 2008 the fair value amounted to T€ 9,236 and T€ 8,303, respectively, including foreign exchange differences. In November 2008, the Company entered into an agreement, where the Company received the right (“Put Option”) to sell its ARSs back to the investment firm that sold the ARSs to Renovis at par, at its discretion, anytime during the period from 30 June 2010 through 2 July 2012. In accordance with IAS 39, the Put Option is considered to be a derivative and is measured at fair value with gains or losses recorded in the income statement at each period end. The Company engaged in 2009 an external valuation group to come up with a valuation analysis. The external valuation group utilised a DCF model to measure the Put Option, recorded in 2009 expense of T€ 1,196 and a corresponding other non-current financial asset of T€ 550 measured with the exchange rate as of 31 December 2009. In 2008 the income amounted to T€ 1,810 and the corresponding other non-current financial asset amounted to T€ 1,726.
In November 2009, the Company acquired Coupon Bonds, which are classified as held-to-maturity investments. The Coupon Bonds carry an interest rate of 1.5% per annum and mature in November 2012.
EVOTEC – ANNUAL REPORT 2009

Notes 71
(13) Discontinued operations
In the third quarter 2007, the Company signed an agreement with Aptuit, Inc. for the sale of Evotec (Scotland) Ltd. as well as a part of Evotec (UK) Ltd. This sale was completed in the fourth quarter 2007. A purchase price adjustment based on a working capital adjustment equivalent to T€ 382, was settled by the Company in the first quarter of 2008. In 2006, the Company signed a purchase agreement for the sale of Evotec Technologies GmbH, Duesseldorf, for T€ 24,147. This purchase became effective as of 1 January 2007. The last portion of the purchase price in the amount of T€ 1,980 was received in 2008.
(14) Long-term loans
In 2009, Evotec entered into a T€ 3,500 loan agreement with a Bank. This loan is outstanding at 31 December 2009 and carries a variable interest rate of 1.15% over EURIBOR per annum. The loan is repayable in total on 30 June 2010. This loan has a cash collateral in the same amount.
In 2007, the Company entered into a T€ 3,000 loan agreement with a bank of which T€ 3,000 is outstanding at 31 December 2009 and 2008. This loan carries a variable interest rate of 1.15% over six month EURI-BOR per annum and is repayable in total on 10 December 2010. This loan has a cash collateral in the same amount.
On 19 December 2007, EVOTEC NeuroSciences GmbH (ENS GmbH) entered into a T€ 3,000 loan agreement with a bank of which T€ 3,000 is outstanding at 31 December 2009 and 2008. The loan carries a variable interest rate of 1.2% over six months EURIBOR per annum and is repayable in one bullet payment at maturity at the end of 2012. This loan has a cash collateral in the amount of T€ 3,000.
Further ENS GmbH has entered in 2006 into a T€ 5,000 loan agreement with a bank of which T€ 1,875 is outstanding at 31 December 2009 (2008: T€ 3,125). This loan carries a fixed interest rate of 5.4% per annum and is repayable in semi-annual instalments of T€ 625 ending on 30 June 2011. ENS GmbH has pledged potential future cash flows from commercialisation of certain assets vis-à-vis the bank to secure repayment of the loan.
Due to the acquisition of Evotec (India) the Company assumed two loans denominated in INR with an Indian Bank. These loans are repayable in monthly instalments until 10 August 2010 and 2011 respectively. These loans carry an interest rate of 13.75% per annum and are secured with a pledge on existing plant and machinery as well as other fixed assets. Further Evotec (India) entered into a loan agreement with a German bank located in India, which carries an interest rate of 10% per annum and is repayable on demand. The total balance outstanding as per 31 December 2009 is T€ 1,469.
With the acquisition of Renovis, Inc., the Company assumed a long-term loan agreement with a US-based financing company. This loan was originally repayable in monthly instalments until 30 October 2010, but was repaid as part of the wind down of Renovis in full on 1 June 2009. The interest rates were variable. The outstanding balance as per the end of 2009 and 2008 was T€ 0 and T€ 987, respectively.
Throughout the year 2009 and 2008, Evotec met all covenants with regard to liquidity of Evotec under the various loan agreements described above.
The annual maturities of these debts are as follows:
T€
2010 9,087
2011 757
2012 3,000
2013 -
Total 12,844
Non-current loans and borrowings:
in T€ 2009 2008
Secured bank loans 9,844 5,047
Unsecured bank loans 3,000 3,000
12,844 8,047
Current portion of loans and borrowings:
in T€ 2009 2008
Current portion of secured bank loans 6,087 2,579
Current portion of unsecured bank loans 3,000 -
9,087 2,579
The currency structure of loans is as follows at 31 December 2009: T€ 11,375 in Euro, T€ 0 in USD, T€ 0 in GBP and T€ 1,469 in INR (31 December 2008: T€ 9,125 in Euro, T€ 987 in USD, T€ 514 in GBP, T€ 0 in INR). The Evotec interest rates are 26% fixed rates and the rest on a variable interest rate basis (2008: 31% fixed rates).
The Company maintains lines of credit totalling T€ 128 and T€ 2,182 to finance its short-term capital requirements, of which the entire balance is available as of 31 December 2009 and 31 December 2008, respectively. These lines of credit provide for borrowings at various interest rates and have various expiration dates as well as no stated expiration date. The fair value of the long-term loans with fixed interest rates amount to T€ 1,935 as of 31 December 2009. As of 31 December 2008 the fair value of the long-term loans was equal to the notional amounts.
(15) Finance lease obligations
Liabilities under finance leases are recognised as financial obligations and the leased assets are capitalised. These assets consist of laboratory equipment. The Company is obligated under finance leases of T€ 361 and T€ 702 as of 31 December 2009 and 2008, respectively that expire at various dates during the next five years.
Those finance leases include property, plant and equipment. The future minimum lease payments under finance leases are as follows:
EVOTEC – ANNUAL REPORT 2009

72 Notes
in T€ Capital Interest Total
2010 229 13 242
2011 104 4 108
2012 28 1 29
2013 - - -
Total principal payable on finance leases 361 18 379
The split into current and non-current finance lease obligations is as follows:
in T€ 2009 2008
Current portion of finance lease liabilities 229 356
Non-current portion of finance lease liabilities 132 346
361 702
The fair value of the long-term finance lease obligation is equal to the notional amounts as of 31 December 2009 and as of 31 December 2008, respectively.
(16) Provisions
The provisions consist of the following:
in T€ 31 Dec.’09 31 Dec.’08
Bonus accruals 2,295 2,734
Severance payments 414 1,750
Accrued vacation 539 842
Accrued lease expenses 671 929
Other provisions 1,771 1,383
Total provisions 5,690 7,638
The following table summarises the provisions recorded during 2009:
in T€ 1 January ’09 Business combination Consumption Disposal Foreign exchange Additions 31 Dec. ’09
Personnel expenses 3,576 64 3,291 129 57 2,557 2,834
Severance payments 1,750 - 1,409 7 (2) 82 414
Accrued lease expenses 929 - 320 - 62 - 671
Other provisions 1,383 - 820 277 (3) 1,488 1,771
Total 7,638 64 5,840 413 114 4,127 5,690
The provision for personnel expenses consists of bonus accruals and accrued vacation. The provision for severance payments relate mainly to the exit agreement the Company entered into with Jörn Aldag in 2008. Other provisions mainly consist of provisions relating to an earn out in the context of the acquisition of RSIPL (T€ 748) as well as a provision for the Supervisory Board remuneration in the amount of T€ 280 (2008: T€ 198) and other provisions with an individual amount under T€ 280 (2008: T€ 198). The provision for personnel costs may differ from the actual amounts due to the fact, that the actual percentage of the variable portion of the remuneration may differ from the estimates. The actual consumption of the accrued lease expenses may vary from the estimated if the lease period changes.
An amount of T€ 832 as per 31 December 2009 (2008: T€ 779) is expected to be paid after one year and therefore is shown under non-current provisions. This amount mainly derives from accrued lease expenses. The fair values of those non-current liabilities as of 31 December 2009 amount to T€ 582 (2008: T€ 514).
(17) Other current liabilities
In 2008, the other current liabilities mainly consist of the fair value of foreign currency contracts as of 31 December 2008. As of 31 December 2009, no foreign currency contracts existed.
(18) Income taxes
Income taxes comprise the current taxes (paid or owed) on income in the individual countries as well as the deferred taxes. For the calculation of current taxes, tax rates are used which are applicable on the balance sheet date. For the deferred taxes tax rates are used which for the expected period of reversion are enacted or substantively enacted at the balance sheet date.
Loss before income taxes is attributable to the following geographic regions for the years ended 31 December 2009 and 2008:
Years ended
in T€ 31 Dec.’09 31 Dec.’08
Germany (19,496) (56,480)
Foreign (25,323) (19,490)
Total (44,819) (75,970)
EVOTEC – ANNUAL REPORT 2009
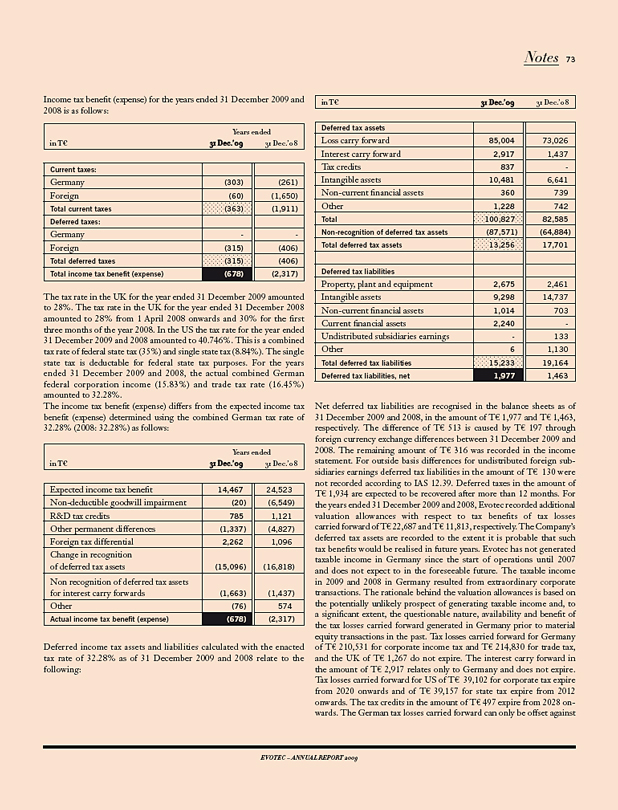
Notes 73
Income tax benefit (expense) for the years ended 31 December 2009 and 2008 is as follows:
Years ended
in T€ 31 Dec.’09 31 Dec.’08
Current taxes:
Germany (303) (261)
Foreign (60) (1,650)
Total current taxes (363) (1,911)
Deferred taxes:
Germany - -
Foreign (315) (406)
Total deferred taxes (315) (406)
Total income tax benefit (expense) (678) (2,317)
The tax rate in the UK for the year ended 31 December 2009 amounted to 28%. The tax rate in the UK for the year ended 31 December 2008 amounted to 28% from 1 April 2008 onwards and 30% for the first three months of the year 2008. In the US the tax rate for the year ended 31 December 2009 and 2008 amounted to 40.746%. This is a combined tax rate of federal state tax (35%) and single state tax (8.84%). The single state tax is deductable for federal state tax purposes. For the years ended 31 December 2009 and 2008, the actual combined German federal corporation income (15.83%) and trade tax rate (16.45%) amounted to 32.28%.
The income tax benefit (expense) differs from the expected income tax benefit (expense) determined using the combined German tax rate of 32.28% (2008: 32.28%) as follows:
Years ended
in T€ 31 Dec.’09 31 Dec.’08
Expected income tax benefit 14,467 24,523
Non-deductible goodwill impairment (20) (6,549)
R&D tax credits 785 1,121
Other permanent differences (1,337) (4,827)
Foreign tax differential 2,262 1,096
Change in recognition of deferred tax assets (15,096) (16,818)
Non recognition of deferred tax assets for interest carry forwards (1,663) (1,437)
Other (76) 574
Actual income tax benefit (expense) (678) (2,317)
Deferred income tax assets and liabilities calculated with the enacted tax rate of 32.28% as of 31 December 2009 and 2008 relate to the following:
in T€ 31 Dec.’09 31 Dec.’08
Deferred tax assets
Loss carry forward 85,004 73,026
Interest carry forward 2,917 1,437
Tax credits 837 -
Intangible assets 10,481 6,641
Non-current financial assets 360 739
Other 1,228 742
Total 100,827 82,585
Non-recognition of deferred tax assets (87,571) (64,884)
Total deferred tax assets 13,256 17,701
Deferred tax liabilities
Property, plant and equipment 2,675 2,461
Intangible assets 9,298 14,737
Non-current financial assets 1,014 703
Current financial assets 2,240 -
Undistributed subsidiaries earnings - 133
Other 6 1,130
Total deferred tax liabilities 15,233 19,164
Deferred tax liabilities, net 1,977 1,463
Net deferred tax liabilities are recognised in the balance sheets as of 31 December 2009 and 2008, in the amount of T€ 1,977 and T€ 1,463, respectively. The difference of T€ 513 is caused by T€ 197 through foreign currency exchange differences between 31 December 2009 and 2008. The remaining amount of T€ 316 was recorded in the income statement. For outside basis differences for undistributed foreign subsidiaries earnings deferred tax liabilities in the amount of T€ 130 were not recorded according to IAS 12.39. Deferred taxes in the amount of T€ 1,934 are expected to be recovered after more than 12 months. For the years ended 31 December 2009 and 2008, Evotec recorded additional valuation allowances with respect to tax benefits of tax losses carried forward of T€ 22,687 and T€ 11,813, respectively. The Company’s deferred tax assets are recorded to the extent it is probable that such tax benefits would be realised in future years. Evotec has not generated taxable income in Germany since the start of operations until 2007 and does not expect to in the foreseeable future. The taxable income in 2009 and 2008 in Germany resulted from extraordinary corporate transactions. The rationale behind the valuation allowances is based on the potentially unlikely prospect of generating taxable income and, to a significant extent, the questionable nature, availability and benefit of the tax losses carried forward generated in Germany prior to material equity transactions in the past. Tax losses carried forward for Germany of T€ 210,531 for corporate income tax and T€ 214,830 for trade tax, and the UK of T€ 1,267 do not expire. The interest carry forward in the amount of T€ 2,917 relates only to Germany and does not expire. Tax losses carried forward for US of T€ 39,102 for corporate tax expire from 2020 onwards and of T€ 39,157 for state tax expire from 2012 onwards. The tax credits in the amount of T€ 497 expire from 2028 onwards. The German tax losses carried forward can only be offset against
EVOTEC – ANNUAL REPORT 2009

74 Notes
an amount of 60% of future taxable income after exceeding a fully deductible amount of T€ 1,000 per year.
No deferred tax assets are set up for the US tax losses carried forward and the tax credits. No deferred tax assets are recorded for the German interest carry forward and for T€ 204,481 corporate income tax losses carry forward and T€ 208,780 of trade tax losses carry forward. Deferred taxes are accounted for as tax expenses or income in the statements of operations unless they relate to items included in equity in which case they are accounted for as part of equity.
(19) Stock-based compensation
The shareholders’ meeting on 7 June 1999 established a stock option plan (“Option Plan 1999”) and authorised the granting of stock options for up to 1,466,600 shares. The plan is subject to certain restrictions regarding the number of stock awards that may be granted in a year and the allocation of the grants to members of the Management Board, other key management personnel and all other employees. The annual shareholders’ meeting in 2000 and 2001 provided for the authorisation of additional 949,000 and 1,129,600 stock options, respectively. Under the terms of the plan, each option entitles the holder to purchase one share of the Company’s stock within ten years of the grant date at a set strike price. For all options granted in 1999, the strike price was the price of the initial public offering of € 13.00 (€ 6.50 after stock split). Options granted in 2000 and 2001 can be exercised at a strike price equal to the closing price of the shares or at a strike price equal to the closing price of the shares plus 5% on the trading day before the option was granted. Options have a graded vesting: a maximum of one-third of which can be exercised at the earliest after two years, a maximum of further two-thirds after three years and all remaining awarded options after four years. Options can only be exercised within certain specified two weeks periods starting on the third day after one of the following events: (i) release of the quarterly results, (ii) annual press conference on the financial statements, or (iii) annual shareholders’ meeting of the Company. The options can only be exercised if the stock price exceeds the strike price by at least 5%.
The terms of the stock option plan further provide that a grant of options is allowed if the average closing price of the Company’s stock has increased by at least 30% when comparing the last quarter of the last business year before the grant with the last quarter of the preceding year. The Supervisory Board, however, has the authority to override this restriction and to authorise the granting of options to employees if such a decision is considered necessary for the interests of the Company. The shareholders’ meetings on 7 June 2005, 30 May 2007 and 28 August 2008 established new stock option plans (“Option Plan 2005, 2007 and 2008”) and authorised the granting of stock options for up to 1,741,481, 2,140,000 and 3,400,000 shares in 2005, 2007 and 2008, respectively. The plans are subject to certain restrictions regarding the number of stock awards that may be granted in a year and the allocation of the grants to members of the Management Board, other key management personnel and all other employees. Within one calendar year, no more than 40% of options from the Option Plan 2005 and 2007 and not more than 50% of options from the Option Plan 2008 shall be granted. Each option entitles the holder to purchase one share of the Company’s stock at a strike price equal to the price of one share at the time of the grant of the option. Options can be exercised after a vesting period of three years after the date of their grant but no later than six years after the respective grant. The Option Plan 2005, 2007 and 2008 stipulates an exercise hurdle of a 33% price increase against the share price at the time of granting. The option holder may exercise his options only if this hurdle is achieved on the day three years after the respective date of granting. In case the hurdle is not achieved, the same increase after four or five years, respectively, would make the options exercisable.
The shareholders’ meeting on 4 June 2009 decided to change the exercise periods of the options under the Option Plan 2005, 2007 and 2008 to be generally exercisable throughout the year. Options cannot be exercised during certain specified three weeks periods ending on the day of the following events: (i) annual shareholders’ meeting of the Company, (ii) annual press conference on the financial statements, or (iii) release of the quarterly results. The options under the Option Plan 2005, 2007 and 2008 used to be exercisable within the specific two weeks period relevant also to the other option programs.
Through the acquisition of ENS Holdings, Inc. in 2005, the Company acquired a stock option plan under which shares in the amount of 323,749 were granted on the date of consolidation 26 May 2005. Under the terms of the plan, each share which has to be treated as an option entitles the holder to receive one share of the Evotec AG’s stock until April or November 2014 at a set strike price of zero. The corresponding new shares are being held in escrow and are released by an individually set amount every quarter as well as on achievement of individual milestones.
Through the acquisition of Renovis, Inc. in 2008, the Company assumed the former equity instruments issued under the original Renovis stock option plan (Renovis Plan) which included options in the amount of 508,038 and restricted stock units (RSU’s) in the amount of 913,106. As part of the acquisition accounting these equity instruments were remeasured on the date of acquisition, 2 May 2008. The original terms of the equity instruments did not change upon assumption by the Company and under the terms of the Renovis Plan each option entitles the holder to purchase two shares of the Company’s stock at a strike price equal to the share price of one share of Renovis at the time of the grant of the option. The options generally vest at the rate of 1/48 per months. Additionally, under the Renovis Plan, each RSU entitles the holder to receive one share of the Company’s stock at no cost. The RSU’s vest monthly from one year to three years. The corresponding new shares are being held in trust and are released according to the relevant agreements. In 2009 199,600 new shares held in trust were exercised, which resulted in a cash inflow in the amount of T€ 278. In 2009, stock options in the amount of 165,321 held by employees of the Company continue to be valid after termination of the relating employment. In 2008, stock options in the amount of 957,820 (see also Note 36 (e)) held by employees of the Company continue to be valid after termination of the relating employment. Those transactions were recognised as accelerated vesting.
A summary of the status of the plans as of 31 December 2009 and 2008, and the changes during the years then ended is presented as follows:
EVOTEC – ANNUAL REPORT 2009
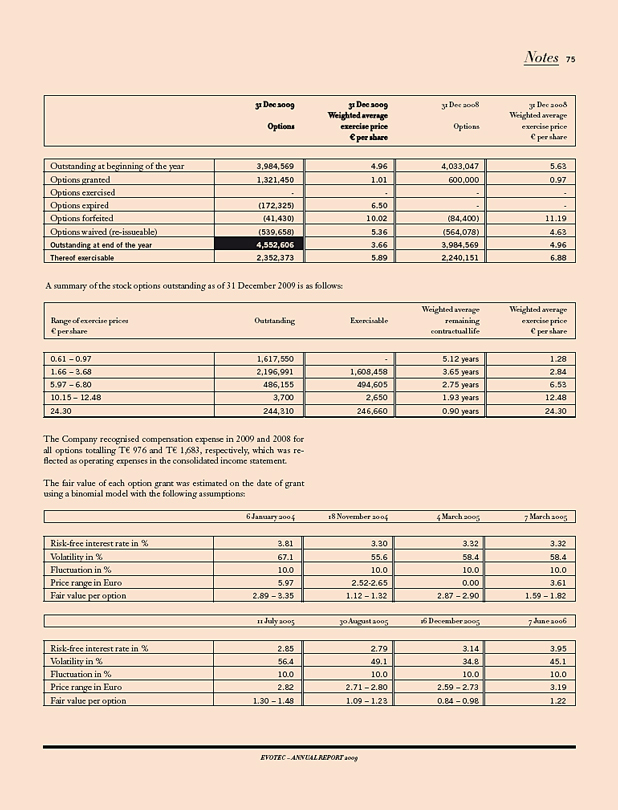
Notes 75
31 Dec 2009 31 Dec 2009 31 Dec 2008 31 Dec 2008
Weighted average Weighted average
Options exercise price Options exercise price
€ per share € per share
Outstanding at beginning of the year 3,984,569 4.96 4,033,047 5.63
Options granted 1,321,450 1.01 600,000 0.97
Options exercised - - - -
Options expired (172,325) 6.50 - -
Options forfeited (41,430) 10.02 (84,400) 11.19
Options waived (re-issueable) (539,658) 5.36 (564,078) 4.63
Outstanding at end of the year 4,552,606 3.66 3,984,569 4.96
Thereof exercisable 2,352,373 5.89 2,240,151 6.88
A summary of the stock options outstanding as of 31 December 2009 is as follows:
Weighted average Weighted average
Range of exercise prices Outstanding Exercisable remaining exercise price
€ per share contractual life € per share
0.61 – 0.97 1,617,550 - 5.12 years 1.28
1.66 – 3.68 2,196,991 1,608,458 3.65 years 2.84
5.97 – 6.80 486,155 494,605 2.75 years 6.53
10.15 – 12.48 3,700 2,650 1.93 years 12.48
24.30 244,310 246,660 0.90 years 24.30
The Company recognised compensation expense in 2009 and 2008 for all options totalling T€ 976 and T€ 1,683, respectively, which was reflected as operating expenses in the consolidated income statement.
The fair value of each option grant was estimated on the date of grant using a binomial model with the following assumptions:
6 January 2004 18 November 2004 4 March 2005 7 March 2005
Risk-free interest rate in % 3.81 3.30 3.32 3.32
Volatility in % 67.1 55.6 58.4 58.4
Fluctuation in % 10.0 10.0 10.0 10.0
Price range in Euro 5.97 2.52-2.65 0.00 3.61
Fair value per option 2.89 – 3.35 1.12 – 1.32 2.87 – 2.90 1.59 – 1.82
11 July 2005 30 August 2005 16 December 2005 7 June 2006
Risk-free interest rate in % 2.85 2.79 3.14 3.95
Volatility in % 56.4 49.1 34.8 45.1
Fluctuation in % 10.0 10.0 10.0 10.0
Price range in Euro 2.82 2.71 – 2.80 2.59 – 2.73 3.19
Fair value per option 1.30 – 1.48 1.09 – 1.23 0.84 – 0.98 1.22
EVOTEC – ANNUAL REPORT 2009
76 Notes
6 November 2006 29 May 2007 17 December 2007 17 October 2008
Risk-free interest rate in % 3.68 4.39 4.19 3.44
Volatility in % 50.5 42.4 42.7 55.0
Fluctuation in % 10.0 5.0 15.0 0.0
Price range in Euro 3.49 – 3.66 3.50 – 3.68 2.64 0.97
Fair value per option 1.47 – 1.73 1.35 – 1.55 0.91 0.47
6 March 2009 22 May 2009 3 December 2009
Risk-free interest rate in % 2.61 2.89 2.67
Volatility in % 64.0 65.0 64.0
Fluctuation in % 0.0 10.0 -
Price range in Euro 0.61 0.71 2.17
Fair value per option 0.41 0.39 1.23
The expected dividend yield is zero, the expected remaining life is 6 years in all models.
(20) Stockholders’ equity
On 31 December 2009, there are 108,838,715 shares issued and outstanding with a nominal amount of Euro 1 per share including equity instruments acquired in the business combination with Renovis held in trust. Furthermore, authorised but unissued shares consist of a conditional capital (bedingtes Kapital) of 10,599,380 shares available with respect to the stock option plan and an authorised capital (genehmigtes Kapital), of 21,733,878 shares.
At the annual shareholders’ meeting on 29 May 2007, the Management Board of the Company was authorised to issue up to 36,849,564 shares for cash or contributions in kind.
Effective 2 May 2008, the Company increased its stockholders’ equity by issuing 34,970,268 new shares against contributions in kind out of the authorised capital (genehmigtes Kapital) to be used as consideration for the acquisition of Renovis, Inc. The price per share amounted to € 1.68. At the annual shareholders’ meeting on 28 August 2008, the Management Board of the Company was authorised to issue up to 21,733,878 shares for cash or contributions in kind. Under German law, the shareholders of a stock corporation may empower the Management Board, with the approval of the Supervisory Board, to issue shares in a specified aggregate nominal value not exceeding 50% of the issued share capital at the time of the shareholder vote, in the form of approved capital (genehmigtes Kapital). The authorisation expires on 27 August 2013.
From an economic perspective, Evotec’s subsidiary Renovis, Inc. owns 1,646,772 of Evotec’s shares, representing 1.51% of Evotec’s nominal capital: In the course of the merger between Renovis, Inc. and Evotec AG, certain options and deferred stock units (DSUs) held by Renovis employees were transformed into Renovis shares. These shares were delivered into an irrevocable Company Trust for the benefit of the Renovis employees (under the conditions of the original agreements entered into when these options or DSUs were granted). At Closing of the merger, the Renovis shares delivered into the Company Trust were exchanged in Evotec American Depository receipts (ADRs), whereby one ADR represents two Evotec shares. Upon valid exercise of options/DSUs by Renovis employees, the Company Trust delivers the respective number of ADRs to these employees. The Trust Agreement between Renovis, Inc. and the Trustee provides that once all obligations of the Trust to deliver ADRs under the option agreements or the DSU agreements are satisfied or otherwise extinguished (e.g. due to an expiry of exercise periods or non-occurrence/ discontinuance of exercise conditions), any ADRs held by the Company Trust shall be delivered to Renovis, Inc. or Evotec AG, as instructed by Renovis, Inc. Therefore ADRs held by the Company Trust are treated as economically owned by Renovis, Inc. Legal ownership of these ADRs will be acquired by Renovis, Inc. or Evotec AG, respectively, once all rights of Renovis employees will have been satisfied or extinguished and the shares remaining in the Company Trust will have been delivered to either Renovis, Inc. or Evotec AG according to instruction by Renovis, Inc. That acquisition of legal ownership will not require a consideration, section 71 paragraph 1 number 4.
(21) Revenues
Revenues include in 2009 milestone payments amounting to T€ 4,000 (2008: T€ 8,500). Also included are royalty and license income in the amount of T€ 3,165 in 2009 (2008: T€ 1,460).
(22) Research and Development
Research and development expenses mainly relate to discovery projects amounting to T€ 10,895 as well as clinical development activities in the amount of T€ 6,074 in 2009 (T€ 16,411 and T€ 20,796 in 2008, respectively). The decline is mainly due to the focus on core programmes and the reduction of early discovery expenses.
EVOTEC – ANNUAL REPORT 2009

Notes 77
(23) Restructuring Expenses
Restructuring expense mainly include personnel related expenses amounting to T€ 3,805 from the restructuring efforts in 2009.
(24) Other operating and income expense
Other operating income as well as other operating expense in 2009 mainly relate to reimbursed expenditures in the context of the collaboration with Roche for the development of the EVT 100 series and amounted to T€ 2,835 each (2008: T€ 0).
(25) Other income from financial assets
Due to the sale of Direvo Biotech to Bayer HealthCare in September 2008 the Direvo convertible bonds, which the Company received as part of the consideration of the sale of the equity holding in Direvo in May 2007, were sold resulting in an income from financial assets amounting to T€ 4,607 in 2008. Additionally, income from the valuation of the put option related to auction rate securities in the amount of T€ 1,810 and profit on the sale of investments in the amount of T€ 822 were recorded in 2008.
(26) Other expense from financial assets
In 2009 other expense from financial assets mainly consist of expenses from the valuation of the put option related to auction rate securities in the amount of T€ 1,196.
(27) Foreign currency exchange loss (net)
In accordance with IAS 21 the Company recognised a foreign exchange loss of T€ 1,674 and T€ 11,814 as a result of the reduction of the capital reserve of one subsidiary, paid to Evotec AG in 2009 and 2008, respectively. This is deemed to be a repayment of share capital resulting in the cumulative foreign exchange losses related to the investment in this subsidiary, which were previously recorded as a component of equity, being reclassified into the Company’s income statement in 2009 and 2008, respectively.
(28) Segment information
Evotec decided to early adopt IFRS 8 “Operating segments” as of 1 January 2008. IFRS 8 was issued in November 2006 and replaces IAS
14 “Segment Reporting”. Pursuant to IFRS 8, reporting on the financial performance of the segments has to be prepared in accordance with the so-called management approach. The internal organisation as well as the management reporting does not identify several segments from
1 January 2008 onwards. The allocation of resources and the internal evaluation of Evotec’s performance by the management are for the entire Evotec group. Following the adoption of IFRS 8 Evotec does not report segment information.
(29) Financial instruments
The fair value of cash and cash equivalents, investments, trade accounts receivable and trade accounts payable approximate their carrying values in the consolidated financial statements due to their short-term nature. Financial assets are accounted for at the settlement date. The credit risk in connection with failures by counterparties to discharge their obligations is assessed by the Company to be immaterial. The fair value of debt varies from the carrying amount, if there is a difference between the underlying interest rate to the market interest rate. The fair value is then determined using an appropriate market interest rate. The Company is exposed to interest rate risk through variable interest-bearing loans and finance lease liabilities. These interest rate risks are deemed not to be significant.
The Company periodically enters into derivative transactions including foreign currency forward contracts. The objective of these transactions is to reduce the risk of exchange rate fluctuations of the Company’s foreign currency denominated cash flows. Evotec does not enter into derivative transactions for trading or speculative purposes. As of 31 December 2009, the Company held US Dollar forward contracts with Euro equivalent notional amounts of T€ 0 and a fair value of T€ 0 (2008: T€ 4,257 and T€ 618, respectively). Foreign currency contracts are carried at fair value which is determined using quoted market prices or discounted cash flows. The maturity for all foreign currency contracts held by the Company is short-term. The fair value of the foreign currency contracts is included in current liabilities on 31 December 2008. Gains and losses from the fair value accounting related to foreign currency derivatives are included in other non-operating income and expense and amounted to T€ 0 and T€ 758 for the years ended 31 December 2009 and 2008, respectively. The maximum exposure to credit risk for trade receivables including related parties at the year end by geographic region was:
Years ended
in T€ 31 Dec.’09 31 Dec.’08
Germany 1,117 250
United Kingdom 52 199
Rest of Europe 1,082 496
United States 1,340 1,572
Rest of the world 919 14
4,510 2,531
EVOTEC – ANNUAL REPORT 2009

78 Notes
Average rate Reporting date rate
2009 2008 2009 2008
USD 0.71916 0.68341 0.6977 0.7095
GBP 1.12297 1.25968 1.1113 1.0272
CHF 0.66245 0.63064 0.6723 0.6720
CURRENCY RISKS
The Company is in connection with all financial instruments recorded at 31 December 2009 and 2008 significantly exposed to currency risks associated with the US Dollar and UK Sterling due to financial instruments held in currencies which are not the functional currency of Evotec. The subsidiaries of Evotec AG located in UK, US and in India, are additionally exposed to the currency risks associated with the Euro in relation to their functional currency. If the Euro had gained (lost) 10 percent against the US Dollar at 31 December 2009 the effect on net loss would have been T€ 592 higher (lower) (31 December 2008: T€ 49 higher (lower)). Shareholders’ equity is impacted in the same amount. If the Euro had gained (lost) 10 percent against the UK Sterling at 31 December 2009 the effect on net loss would have been T€ 90 higher (lower) (31 December 2008: T€ 552 higher (lower)). Shareholders’ equity is impacted in the same amount.
INTEREST RATE RISKS
The Company is exposed to interest rate risks in Germany, India, UK and US due to current investments as well as loans and finance leases. Financial instruments with fixed interest rates are not subject to interest rate risks and therefore are not included in the sensitivity analysis. Financial instruments with variable interest rates as of 31 December 2009 are included in the sensitivity analysis for the period of their existence. If the interest rate had been 100 basis points higher (lower) at 31 December 2009 the effect on net loss would have been T€ 474 higher (lower) (31 December 2008: T€ 358 higher (lower)). Shareholders’ equity is impacted in the same amount.
The fair values of the long-term loans and finance leases as of 31 December 2009 would have been T€ 66 lower (higher) (31 December 2008: T€ 131 lower (higher)) if the relating interest rate used for determining fair values had been 100 basis points higher (lower) at 31 December 2009 and 2008, respectively.
OTHER PRICE RISKS
The Company is not exposed to any price risks associated to their financial instruments.
The Management Board has overall responsibility for the establishment and oversight of the Company’s management framework. The Management Board has established a Risk Management, which is responsible for developing and monitoring the risk management policies. The Risk Management reports regularly to the Management Board on its activities.
(30) Risks
LIQUIDITY RISKS
Based upon the Company’s current financial plan it expects that its current cash and cash equivalents, short-term and long-term investments, together with its operating revenues will be sufficient to fund its planned activities beyond of 2012. The Company’s future cash requirements will depend on various factors, including its success in developing Evotec’s pipeline projects, its ability to partner the Company’s projects with collaborators, increasing sales of both existing and new services, expenses associated with sales growth as well as competition and the general economic situation. Expenditures on internal development programs or potential acquisitions of technologies or intellectual property rights are likely to reduce the Company’s short-to mid-term profitability and cash reserves. The Company intends to reduce part of this financial exposure by entering into early stage collaboration agreements, to the degree possible and advisable while trying to maximise returns. Additionally, in the past, the Company has raised cash through capital increases. The Company does not intend to engage in projects or project phases unless appropriate funding is allocated or secured.
The Company conducts clinical trials, which have a risk of failure. A clinical trial failure may have a negative impact on the Company’s financial position, results of operations and cash flows.
The Company has important collaborations with pharmaceutical and biotechnology companies. Any termination of such collaborations or failure to achieve contracted milestones would likely have an adverse impact on the Company’s financial position, results of operations and cash flows. With a high proportion of sales denominated in US Dollar, currency exposure creates a risk to our profitability, in particular relative to the UK Sterling with the respect to the subsidiaries in the United Kingdom. A weakening of the US Dollar when accompanied by a relative strengthening of the UK Sterling against the Euro will reduce revenues and profitability and constitutes a significant risk to the Company’s financial situation. The Company has entered into certain hedging activities to help mitigate the impact of the currency fluctuations on its results of operations before taxation.
CAPITAL MANAGEMENT
Evotec actively manages its funds to primarily ensure liquidity and principal preservation while seeking to maximise returns. Evotec’s cash and short-term investments are located at several different banks and financial investments are made in liquid, highly diversified investment instruments in low risk categories (products or financial institutions rated A or better (Standard & Poor’s ratings or equivalent)).
EVOTEC – ANNUAL REPORT 2009

Notes 79
The following table shows the total assets, equity as well as equity ratio and net financial assets:
Years ended
in T€ 31 Dec.’09 31 Dec.’08
Total assets 146,599 182,900
Equity 111,487 149,859
Equity ratio (in %) 76,0 81,9
Net cash 19,721 43,736
To manage short-term and medium-term liquidity, the Company makes use of long-term bank loans and asset financing to maintain and further develop its discovery platform. The minimum level of cash as collateral for this purpose is T€ 13,625 (31 December 2008: T€ 9,875). The sum of these debt instruments – including both long-term and current portions – at the end of 2009 was T€ 13,205 (2008: T€ 11,328).
Evotec remains well financed with an equity ratio of 76.0% (31 December 2008: 81.9%) and currently has no plans or necessity to raise capital in the near to mid-term. However, the option to increase capital may always be considered if new opportunities arise in terms of M&A and in-licensing which requires additional financing.
No capital requirements are stipulated in Evotec’s statutes. The Company has obligations to issue shares out of the conditional capital relating to the exercise of stock options on the basis of miscellaneous stock option plans. Please refer with regard to the authorised capital and the conditional capital to Note 20.
CREDIT RISKS
The Company has exposure to credit risks primarily with respect to its trade accounts receivables and its short-term and long-term investment which primarily invest in debt instruments. The Company performs ongoing credit evaluations of its customers’ financial condition and maintains an appropriate allowance for uncollectible accounts receivable based upon the expected collectibility of all accounts receivable. The Company’s accounts receivables are generally unsecured and are not backed by collateral from its customers. At 31 December 2009, one customer accounted for 16% of trade receivables. In the prior year, one customer accounted for 44% of all trade accounts receivables. Concentrations of credit risks with respect to trade accounts receivables are limited by a number of geographically diverse customers and the Company’s monitoring procedures.
The Company has further expanded its customer base. However, the two largest customers of Evotec combined represent more than 40% of the group revenues in 2009 and more than 53% in 2008. A termination of these business relations could have adverse impacts on the Company’s financial results.
At 31 December 2009, the Company had a guarantee outstanding of T€ 190 (31 December 2008: T€ 190) related to securing certain payment obligations of the European ScreeningPort GmbH. The Company has guaranteed the European ScreeningPort a further loan facility in the amount of T€ 511. Other guarantees outstanding at 31 December 2009 amounted to T€ 417 (31 December 2008: T€ 472).
MARKET RISKS
The global economic downturn and the changing regulatory environment are the dominant factors influencing the Company’s macro environment. 2009 has been widely considered as one of the largest economic downturns in the global economy. While Evotec does not intend to raise capital via the equity market in the near-term it is uncertain as to when the financing cycle might improve.
The regulatory environment has become more challenging over the past several years. It appears that the FDA is concluding that the risk of approval is only justified if a drug meets an unmet need or if it provides a well-defined benefit over existing therapies. For biotech companies, including Evotec, this means that they need to demonstrate that there is clear reason for compounds to exist and that companies cannot leave comparative efficacy and reimbursement considerations to a future pharmaceutical partner.
The market environment is marked by pricing pressures, originating from funding restrictions of some biotechnology customers and from evolving and strengthening competition in individual drug discovery disciplines in low cost countries. Therefore, firm cost management, continuous enhancement of capabilities and technologies, careful market positioning and sales from high value results-based contracts are mandatory. In addition, Evotec continues to explore ways to capture some of the cost advantages in countries like India, as exemplified by the acquisition of the majority stake in RSIPL (Thane).
The market environment and competitive landscape for licensing and licensed projects or individual drug candidates, as well as the regulatory and reimbursement environment, in general or for individual treatments, might change while engaging in individual projects. The timing and commercial values of or financial proceeds from partnering individual projects could therefore deviate significantly from earlier projections.
EVOTEC – ANNUAL REPORT 2009
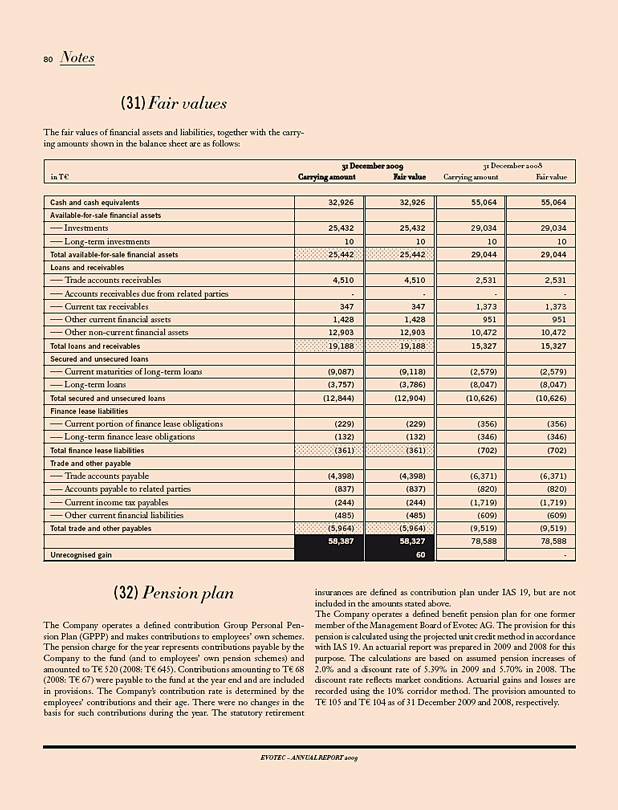
80 Notes
(31) Fair values
The fair values of financial assets and liabilities, together with the carrying amounts shown in the balance sheet are as follows:
31 December 2009 31 December 2008
in T€ Carrying amount Fair value Carrying amount Fair value
Cash and cash equivalents 32,926 32,926 55,064 55,064
Available-for-sale financial assets
— Investments 25,432 25,432 29,034 29,034
— Long-term investments 10 10 10 10
Total available-for-sale financial assets 25,442 25,442 29,044 29,044
Loans and receivables
— Trade accounts receivables 4,510 4,510 2,531 2,531
— Accounts receivables due from related parties - - - -
— Current tax receivables 347 347 1,373 1,373
— Other current financial assets 1,428 1,428 951 951
— Other non-current financial assets 12,903 12,903 10,472 10,472
Total loans and receivables 19,188 19,188 15,327 15,327
Secured and unsecured loans
— Current maturities of long-term loans (9,087) (9,118) (2,579) (2,579)
— Long-term loans (3,757) (3,786) (8,047) (8,047)
Total secured and unsecured loans (12,844) (12,904) (10,626) (10,626)
Finance lease liabilities
— Current portion of finance lease obligations (229) (229) (356) (356)
— Long-term finance lease obligations (132) (132) (346) (346)
Total finance lease liabilities (361) (361) (702) (702)
Trade and other payable
— Trade accounts payable (4,398) (4,398) (6,371) (6,371)
— Accounts payable to related parties (837) (837) (820) (820)
— Current income tax payables (244) (244) (1,719) (1,719)
— Other current financial liabilities (485) (485) (609) (609)
Total trade and other payables (5,964) (5,964) (9,519) (9,519)
58,387 58,327 78,588 78,588
Unrecognised gain 60 -
(32) Pension plan
The Company operates a defined contribution Group Personal Pension Plan (GPPP) and makes contributions to employees’ own schemes. The pension charge for the year represents contributions payable by the Company to the fund (and to employees’ own pension schemes) and amounted to T€ 520 (2008: T€ 645). Contributions amounting to T€ 68 (2008: T€ 67) were payable to the fund at the year end and are included in provisions. The Company’s contribution rate is determined by the employees’ contributions and their age. There were no changes in the basis for such contributions during the year. The statutory retirement insurances are defined as contribution plan under IAS 19, but are not included in the amounts stated above.
The Company operates a defined benefit pension plan for one former member of the Management Board of Evotec AG. The provision for this pension is calculated using the projected unit credit method in accordance with IAS 19. An actuarial report was prepared in 2009 and 2008 for this purpose. The calculations are based on assumed pension increases of 2.0% and a discount rate of 5.39% in 2009 and 5.70% in 2008. The discount rate reflects market conditions. Actuarial gains and losses are recorded using the 10% corridor method. The provision amounted to T€ 105 and T€ 104 as of 31 December 2009 and 2008, respectively.
EVOTEC – ANNUAL REPORT 2009

Notes 81
Total expense for the period for the defined benefit plan amounted to T€ 1 (2008: income of T€ 3) and consist of the following:
Years ended
in T€ 31 Dec.’09 31 Dec.’08
Pension liability beginning of the year 104 107
Interest cost 5 5
Amortisation of actuarial losses (4) (8)
Pension payments - -
Pension liability year end 105 104
(33) Commitments and contingencies
(a) OPERATING LEASE OBLIGATIONS
The Company leases office and laboratory space and other equipment under operating leases in accordance with IAS 17. The longest of these obligations extends through 2023. Certain leases contain rent increases, rent holidays and renewal options. The total rents due under these leases are recognised on a straight-line basis over the lease term. The future minimum lease payments under non-cancellable operating leases are approximately as follows:
T€
2010 3,926
2011 3,392
2012 3,129
2013 3,050
2014 1,267
Thereafter 12,386
Total 27,150
The majority of operating leases is related to rent expenses for facilities. The rent expense for such leases amounted to T€ 3,246 and T€ 3,993 for the years ended 31 December 2009 and 2008, respectively.
(b) OTHER COMMITMENTS AND CONTINGENCIES
The Company has entered into consultancy contracts. During 2009 and 2008, expenses under consultancy contracts totalled T€ 339 and T€ 243, respectively. The future minimum payments associated with long-term consultant and other miscellaneous long-term commitments total approximately T€ 371 and T€ 180 at 31 December 2009 and 2008, respectively.
As discussed in Note 4, the Company has certain commitments resulting from the amendments to its agreements with its technology funding partners.
The Company has, in the sale and purchase agreement for all the shares in Evotec Technologies GmbH, provided certain guarantees customary for such agreements. No current liabilities from this guarantee exist as of 31 December 2009.
The Company has licensed or acquired certain third party intellectual property for use in its business. Under these agreements, the Company is required to pay milestones, dependent on development progress and/or royalties and milestones dependent on present and future net income or on sublicensing fees received from third parties.
The Company is not aware of any litigation as of 31 December 2009.
(34) Related party transactions
According to IAS 24 the Company discloses related party transactions where Supervisory Board members and Management Team members of the Company have significant influence on companies Evotec works with in the ordinary course of business (the figures reflect the total group): Peer Schatz is Chief Executive Officer of Qiagen N.V. and was Vice Chairman of the Supervisory Board of Evotec until 28 August 2008. From affiliates controlled by Qiagen N.V. the Company bought products in the amount of T€ 40 in 2008. The amount of payables to those affiliates on 31 December 2008 including VAT amounts to T€ 1. Dr Peter Fellner is Non-Executive Chairman (until November 2009 Executive Chairman) of Vernalis plc, Winnersh, UK, with whom the Company entered into a service agreement in the ordinary course of business. Related revenues in 2009 amounted to T€ 0 and T€ 0 in 2008, respectively, and the accounts receivables amounted to T€ 0 and T€ 0 as of 31 December 2009 and 2008, respectively.
The spouse of Mary Tanner was Vice Chairman of Lehman Brothers, Inc. (Lehman). Lehman was representing and advising the Company with respect to the acquisition of Renovis, Inc. (since 2007). The relating capitalised expenses amounted to T€ 0 and T€ 2,316 in 2009 and 2008. The amount of the related payables was T€ 837 and T€ 819 as of 31 December 2009 and 2008.
The Company entered into a consultancy agreement with Dr Flemming Ørnskov outside the scope of his Supervisory Board activities with the approval of the full Supervisory Board, which ended in June 2009. The relating expenses amounted to T€ 150 and T€ 13 in 2009 and 2008, respectively, with relating payables as of 31 December 2009 and 2008 in the amount of T€ 0 and T€ 13, respectively.
The Evotec AG has recorded no revenues with related parties in 2009 and 2008. Subsidiaries of Evotec AG recorded no revenues with related parties in 2009 and 2008.
Administrative services provided by the Company to Management Board or Supervisory Board members for their private purposes, if any are reimbursed to the Company at cost.
Accounts payable to related parties
Years ended
in T€ 31 Dec.’09 31 Dec.’08
Lehman Brothers Inc. 837 819
Qiagen N.V. (until August 2008) - 1
837 820
EVOTEC – ANNUAL REPORT 2009

82 Notes
(35) Personnel expenses and cost of material
The personnel expenses of the Company amounted to T€ 27,421 of which T€ 16,701 relate to personnel expenses in India, the UK and US (2008: T€ 28,861 and T€ 17,440, respectively). Thereof expenses for the statutory retirement insurance amounted to T€ 1,911 of which T€ 1,313 relate to expenses in India, the UK and US (2008: T€ 1,959 and T€ 1,328, respectively).
Cost of materials amounted to T€ 16,691, thereof T€ 2,041 are cost of materials in India, the UK and US (2008: T€ 33,161 and T€ 3,096, respectively).
(36) Other disclosures
The following additional disclosures are required by German law in accordance with the European Directives on Accounting and the Corporate Governance Codex. Those disclosures include the continuing and the discontinued operations.
(a) NUMBER OF EMPLOYEES
The average number of persons employed by the Company in 2009 was 442 (2008: 410).
(b) REMUNERATION OF THE AUDITOR
In 2009, remunerations, shown as expenses, to KPMG AG Wirtschaftsprüfungsgesellschaft and other KPMG companies totalled T€ 489 (2008: T€ 716) broken down into auditing of financial statements (T€ 304; 2008: T€ 553), tax consultancy (T€ 38; 2008: T€ 69), other attestation services (T€ 130; 2008: T€ 88) as well as other services
(T€ 17; 2008: T€ 6).
(c) CORPORATE GOVERNANCE CODEX
A declaration according to § 161 AktG was made by the Management Board and the Supervisory Board of the Company. This declaration regarding the Company’s compliance with the Corporate Governance Codex is accessible to the shareholders on Evotec’s website.
(d) CONSOLIDATED SUBSIDIARIES AND EQUITY INVESTEES
Information below is as per the statutory financial statements as of 31 December 2009 prepared in accordance with IFRS or the respective local generally accepted accounting principles.
The Group investments in subsidiaries, associated companies and other investments are not hedged as those currency positions are considered to be long-term in nature.
2009 Company’s voting interest % 2009 Net income / (loss) T€ 2009 Equity T€
Subsidiaries
Evotec (UK) Ltd., Abingdon, UK 100.0 2,209 17,213
ENS Holdings, Inc., Wilmington, Delaware, US (unaudited) 100.0 (38) 25,183
EVOTEC NeuroSciences GmbH, Hamburg (unaudited) 100.0 (12,946) (106,336)
Evotec Neurosciences AG, Zurich, CH (unaudited) 100.0 27 287
Evotec (India) Private Limited, Thane, India (unaudited) 70.0 63 1,807
Evotec RSIL Ltd., Thane, India (unaudited) 70.0 (90) 619
Renovis, Inc., South San Francisco, California, US (unaudited) 100.0 (11,910) 27,620
Evotec Inc., Wilmington, Delaware, US (unaudited) 100.0 16 193
Oxford Asymmetry Employee Shares Trust Ltd., Abingdon, UK (unaudited) 100.0 - 3
Evotec (Asia) Pte. Ltd., Singapore (unaudited) 100.0 131 123
Other Investments
European ScreeningPort GmbH, Hamburg (unaudited) 19.9 (513) (705)
EVOTEC – ANNUAL REPORT 2009
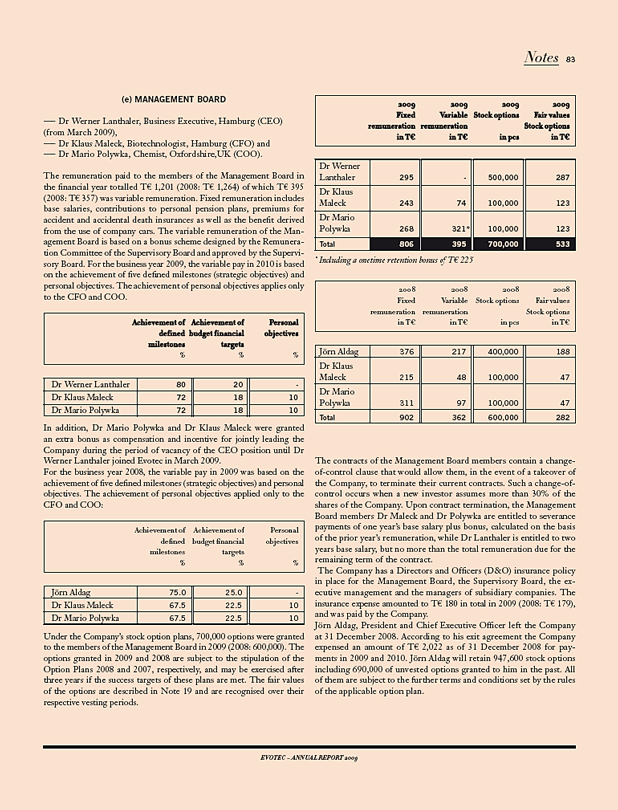
Notes 83
(e) MANAGEMENT BOARD
— Dr Werner Lanthaler, Business Executive, Hamburg (CEO) (from March 2009),
— Dr Klaus Maleck, Biotechnologist, Hamburg (CFO) and
— Dr Mario Polywka, Chemist, Oxfordshire, UK (COO).
The remuneration paid to the members of the Management Board in the financial year totalled T€ 1,201 (2008: T€ 1,264) of which T€ 395 (2008: T€ 357) was variable remuneration. Fixed remuneration includes base salaries, contributions to personal pension plans, premiums for accident and accidental death insurances as well as the benefit derived from the use of company cars. The variable remuneration of the Management Board is based on a bonus scheme designed by the Remuneration Committee of the Supervisory Board and approved by the Supervisory Board. For the business year 2009, the variable pay in 2010 is based on the achievement of five defined milestones (strategic objectives) and personal objectives. The achievement of personal objectives applies only to the CFO and COO.
Achievement of defined milestones % Achievement of budget financial targets % Personal objectives %
Dr Werner Lanthaler 80 20 -
Dr Klaus Maleck 72 18 10
Dr Mario Polywka 72 18 10
In addition, Dr Mario Polywka and Dr Klaus Maleck were granted an extra bonus as compensation and incentive for jointly leading the Company during the period of vacancy of the CEO position until Dr Werner Lanthaler joined Evotec in March 2009.
For the business year 2008, the variable pay in 2009 was based on the achievement of five defined milestones (strategic objectives) and personal objectives. The achievement of personal objectives applied only to the CFO and COO:
Achievement of defined milestones % Achievement of budget financial targets % Personal objectives %
Jörn Aldag 75.0 25.0 -
Dr Klaus Maleck 67.5 22.5 10
Dr Mario Polywka 67.5 22.5 10
Under the Company’s stock option plans, 700,000 options were granted to the members of the Management Board in 2009 (2008: 600,000). The options granted in 2009 and 2008 are subject to the stipulation of the Option Plans 2008 and 2007, respectively, and may be exercised after three years if the success targets of these plans are met. The fair values of the options are described in Note 19 and are recognised over their respective vesting periods.
2009 Fixed remuneration in T€ 2009 Variable remuneration in T€ 2009 Stock options in pcs 2009 Fair values Stock options in T€
Dr Werner
Lanthaler 295 - 500,000 287
Dr Klaus Maleck 243 74 100,000 123
Dr Mario Polywka 268 321* 100,000 123
Total 806 395 700,000 533
* Including a onetime retention bonus of T€ 225
2008 Fixed remuneration in T€ 2008 Variable remuneration in T€ 2008 Stock options in pcs 2008 Fair values Stock options in T€
Jörn Aldag 376 217 400,000 188
Dr Klaus Maleck 215 48 100,000 47
Dr Mario Polywka 311 97 100,000 47
Total 902 362 600,000 282
The contracts of the Management Board members contain a change-of-control clause that would allow them, in the event of a takeover of the Company, to terminate their current contracts. Such a change-of-control occurs when a new investor assumes more than 30% of the shares of the Company. Upon contract termination, the Management Board members Dr Maleck and Dr Polywka are entitled to severance payments of one year’s base salary plus bonus, calculated on the basis of the prior year’s remuneration, while Dr Lanthaler is entitled to two years base salary, but no more than the total remuneration due for the remaining term of the contract.
The Company has a Directors and Officers (D&O) insurance policy in place for the Management Board, the Supervisory Board, the executive management and the managers of subsidiary companies. The insurance expense amounted to T€ 180 in total in 2009 (2008: T€ 179), and was paid by the Company.
Jörn Aldag, President and Chief Executive Officer left the Company at 31 December 2008. According to his exit agreement the Company expensed an amount of T€ 2,022 as of 31 December 2008 for payments in 2009 and 2010. Jörn Aldag will retain 947,600 stock options including 690,000 of unvested options granted to him in the past. All of them are subject to the further terms and conditions set by the rules of the applicable option plan.
EVOTEC – ANNUAL REPORT 2009
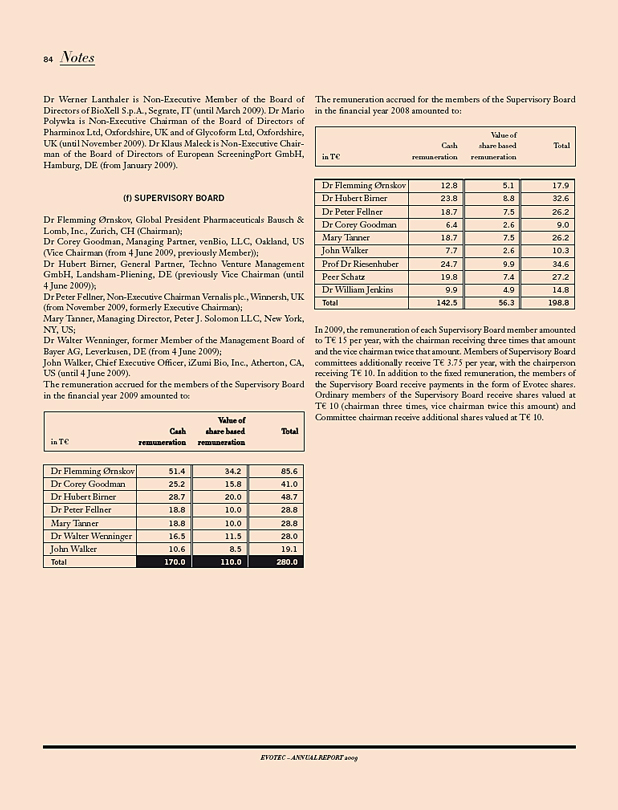
84 Notes
Dr Werner Lanthaler is Non-Executive Member of the Board of Directors of BioXell S.p.A., Segrate, IT (until March 2009). Dr Mario Polywka is Non-Executive Chairman of the Board of Directors of Pharminox Ltd, Oxfordshire, UK and of Glycoform Ltd, Oxfordshire, UK (until November 2009). Dr Klaus Maleck is Non-Executive Chairman of the Board of Directors of European ScreeningPort GmbH, Hamburg, DE (from January 2009).
(f) SUPERVISORY BOARD
Dr Flemming Ørnskov, Global President Pharmaceuticals Bausch & Lomb, Inc., Zurich, CH (Chairman); Dr Corey Goodman, Managing Partner, venBio, LLC, Oakland, US (Vice Chairman (from 4 June 2009, previously Member)); Dr Hubert Birner, General Partner, Techno Venture Management GmbH, Landsham-Pliening, DE (previously Vice Chairman (until 4 June 2009));
Dr Peter Fellner, Non-Executive Chairman Vernalis plc., Winnersh, UK (from November 2009, formerly Executive Chairman); Mary Tanner, Managing Director, Peter J. Solomon LLC, New York, NY, US; Dr Walter Wenninger, former Member of the Management Board of Bayer AG, Leverkusen, DE (from 4 June 2009); John Walker, Chief Executive Officer, iZumi Bio, Inc., Atherton, CA, US (until 4 June 2009).
The remuneration accrued for the members of the Supervisory Board in the financial year 2009 amounted to:
in T€ Cash remuneration Value of share based remuneration Total
Dr Flemming Ørnskov 51.4 34.2 85.6
Dr Corey Goodman 25.2 15.8 41.0
Dr Hubert Birner 28.7 20.0 48.7
Dr Peter Fellner 18.8 10.0 28.8
Mary Tanner 18.8 10.0 28.8
Dr Walter Wenninger 16.5 11.5 28.0
John Walker 10.6 8.5 19.1
Total 170.0 110.0 280.0
The remuneration accrued for the members of the Supervisory Board in the financial year 2008 amounted to:
in T€ Cash remuneration Value of share based remuneration Total
Dr Flemming Ørnskov 12.8 5.1 17.9
Dr Hubert Birner 23.8 8.8 32.6
Dr Peter Fellner 18.7 7.5 26.2
Dr Corey Goodman 6.4 2.6 9.0
Mary Tanner 18.7 7.5 26.2
John Walker 7.7 2.6 10.3
Prof Dr Riesenhuber 24.7 9.9 34.6
Peer Schatz 19.8 7.4 27.2
Dr William Jenkins 9.9 4.9 14.8
Total 142.5 56.3 198.8
In 2009, the remuneration of each Supervisory Board member amounted to T€ 15 per year, with the chairman receiving three times that amount and the vice chairman twice that amount. Members of Supervisory Board committees additionally receive T€ 3.75 per year, with the chairperson receiving T€ 10. In addition to the fixed remuneration, the members of the Supervisory Board receive payments in the form of Evotec shares. Ordinary members of the Supervisory Board receive shares valued at T€ 10 (chairman three times, vice chairman twice this amount) and Committee chairman receive additional shares valued at T€ 10.
EVOTEC – ANNUAL REPORT 2009

Notes 85
In 2008, the remuneration for the chairman of the Supervisory Board was twice, for the vice chairman was one and a half the amount of the remuneration for the Supervisory Board members. The additional remuneration for a member of a Supervisory Board Committee amounted to T€ 3.8, for the chairman of those Committee’s to T€ 7.5. In addition to the fixed remuneration, the members of the Supervisory Board received payments in the form of Evotec shares. Ordinary members of the Supervisory Board received shares valued at T€ 7.5 (chairman twice and vice chairman one and a half the amount).
The total remuneration accrued for the Supervisory Board members in 2009 totalled T€ 280 (2008: T€ 198.8). The Company has a Directors and Officers (D&O) insurance policy in place for the Management Board, the Supervisory Board, the executive management and the managers of subsidiary companies. The insurance expense amounted to T€ 180 in total in 2009 (2008: T€ 179), and was paid by the Company. The Supervisory Board and their additional memberships in supervisory boards and memberships in comparable governing bodies of enterprises according to § 125 par. 1 third sentence of the AktG are listed at the end of this report.
(g) SCIENTIFIC ADVISORY COMMITTEE
The remuneration paid in 2009 to the Scientific Advisory Board amounts to T€ 86 (2008: T€ 39).
(37) Subsequent events
No subsequent events with material impact on the consolidated financial statements of the Company occurred after 31 December 2009.
EVOTEC – ANNUAL REPORT 2009
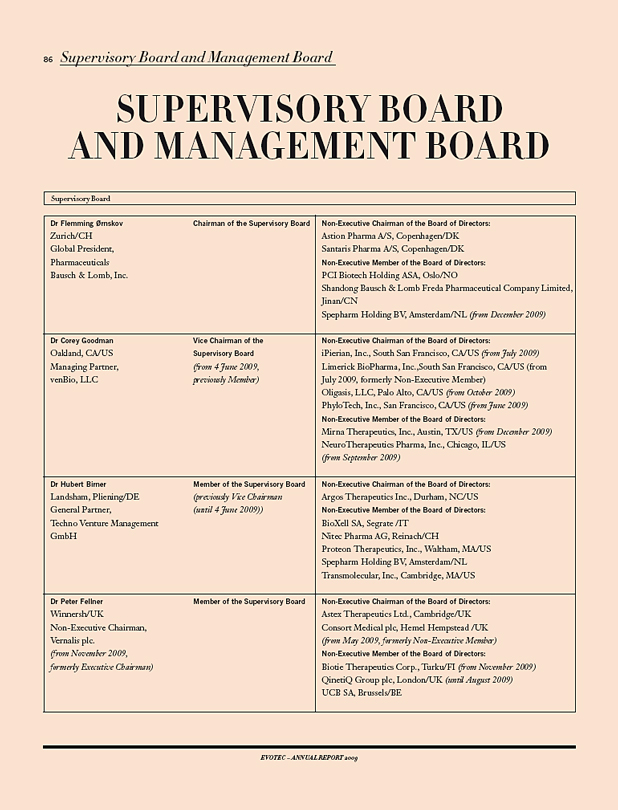
86 Supervisory Board and Management Board
SUPERVISORY BOARD AND MANAGEMENT BOARD
Supervisory Board
Dr Flemming Ørnskov Zurich/CH Global President, Pharmaceuticals Bausch & Lomb, Inc.
Dr Corey Goodman Oakland, CA/US Managing Partner, venBio, LLC
Dr Hubert Birner Landsham, Pliening/DE General Partner, Techno Venture Management GmbH
Dr Peter Fellner
Winnersh/UK
Non-Executive Chairman,
Vernalis plc.
(from November 2009,
formerly Executive Chairman)
Chairman of the Supervisory Board
Vice Chairman of the Supervisory Board (from 4 June 2009, previously Member)
Member of the Supervisory Board (previously Vice Chairman (until 4 June 2009))
Member of the Supervisory Board
Non-Executive Chairman of the Board of Directors: Astion Pharma A/S, Copenhagen/DK Santaris Pharma A/S, Copenhagen/DK Non-Executive Member of the Board of Directors: PCI Biotech Holding ASA, Oslo/NO Shandong Bausch & Lomb Freda Pharmaceutical Company Limited, Jinan/CN Spepharm Holding BV, Amsterdam/NL (from December 2009)
Non-Executive Chairman of the Board of Directors: iPierian, Inc., South San Francisco, CA/US (from July 2009) Limerick BioPharma, Inc., South San Francisco, CA/US (from July 2009, formerly Non-Executive Member) Oligasis, LLC, Palo Alto, CA/US (from October 2009) PhyloTech, Inc., San Francisco, CA/US (from June 2009) Non-Executive Member of the Board of Directors: Mirna Therapeutics, Inc., Austin, TX/US (from December 2009) NeuroTherapeutics Pharma, Inc., Chicago, IL/US (from September 2009)
Non-Executive Chairman of the Board of Directors: Argos Therapeutics Inc., Durham, NC/US Non-Executive Member of the Board of Directors: BioXell SA, Segrate /IT Nitec Pharma AG, Reinach/CH Proteon Therapeutics, Inc., Waltham, MA/US Spepharm Holding BV, Amsterdam/NL Transmolecular, Inc., Cambridge, MA/US
Non-Executive Chairman of the Board of Directors: Astex Therapeutics Ltd., Cambridge/UK Consort Medical plc, Hemel Hempstead /UK (from May 2009, formerly Non-Executive Member) Non-Executive Member of the Board of Directors: Biotie Therapeutics Corp., Turku/FI (from November 2009) QinetiQ Group plc, London/UK (until August 2009) UCB SA, Brussels/BE
EVOTEC – ANNUAL REPORT 2009
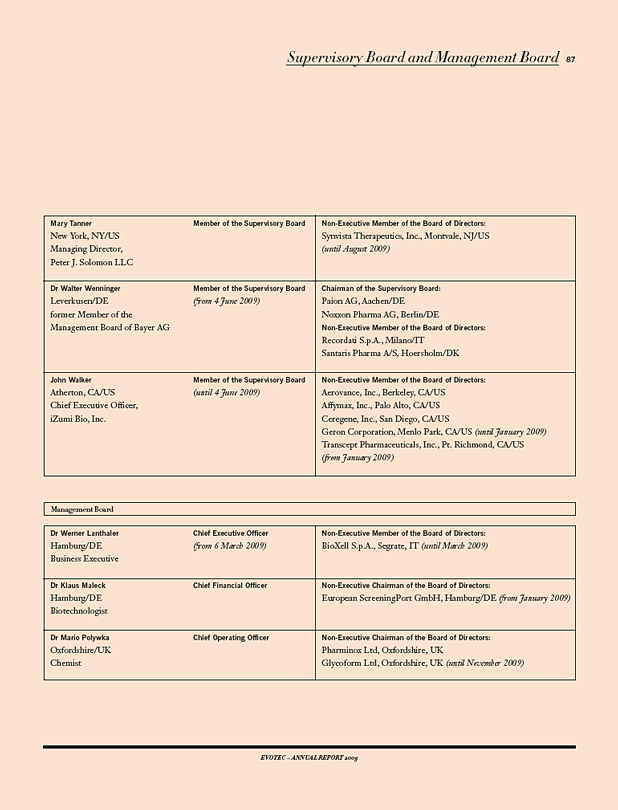
Supervisory Board and Management Board 87
Mary Tanner New York, NY/US Managing Director, Peter J. Solomon LLC
Dr Walter Wenninger Leverkusen/DE former Member of the Management Board of Bayer AG
John Walker Atherton, CA/US Chief Executive Officer, iZumi Bio, Inc.
Member of the Supervisory Board
Member of the Supervisory Board (from 4 June 2009)
Member of the Supervisory Board (until 4 June 2009)
Non-Executive Member of the Board of Directors: Synvista Therapeutics, Inc., Montvale, NJ/US (until August 2009)
Chairman of the Supervisory Board: Paion AG, Aachen/DE Noxxon Pharma AG, Berlin/DE Non-Executive Member of the Board of Directors: Recordati S.p.A., Milano/IT Santaris Pharma A/S, Hoersholm/DK
Non-Executive Member of the Board of Directors: Aerovance, Inc., Berkeley, CA/US Affymax, Inc., Palo Alto, CA/US Ceregene, Inc., San Diego, CA/US Geron Corporation, Menlo Park, CA/US (until January 2009) Transcept Pharmaceuticals, Inc., Pt. Richmond, CA/US (from January 2009)
Management Board
Dr Werner Lanthaler Hamburg/DE Business Executive
Dr Klaus Maleck Hamburg/DE Biotechnologist
Dr Mario Polywka Oxfordshire/UK Chemist
Chief Executive Officer (from 6 March 2009)
Chief Financial Officer
Chief Operating Officer
Non-Executive Member of the Board of Directors: BioXell S.p.A., Segrate, IT (until March 2009)
Non-Executive Chairman of the Board of Directors: European ScreeningPort GmbH, Hamburg/DE (from January 2009)
Non-Executive Chairman of the Board of Directors: Pharminox Ltd, Oxfordshire, UK Glycoform Ltd, Oxfordshire, UK (until November 2009)
EVOTEC – ANNUAL REPORT 2009

88 Auditor’s Report
AUDITOR’S REPORT
We have rendered the Auditor’s Report in German, which was translated as follows: “Auditor’s Report
We have audited the consolidated financial statements prepared by the Evotec AG, Hamburg, comprising the consolidated statement of financial position, the consolidated income statement, the consolidated statement of comprehensive income, the consolidated statements of changes in stockholder’s equity, the consolidated statement of cash flows and the notes to the consolidated financial statements, together with the Group management report for the business year from 1 January to 31 December 2009. The preparation of the consolidated financial statements and the Group management report in accordance with IFRSs, as adopted by EU and the additional requirements of German commercial law pursuant to section 315a par. 1 HGB (Handelsgesetzbuch „German Commercial Code”) are the responsibility of the parent company’s management. Our responsibility is to express an opinion on the consolidated financial statements and on the Group management report based on our audit. We conducted our audit of the consolidated financial statements in accordance with section 317 HGB [„Handelsgesetzbuch”: „German Commercial Code”] and German generally accepted standards for the audit of financial statements promulgated by the Institut der Wirtschaftsprüfer [Institute of Public Auditors in Germany] (IDW). Those standards require that we plan and perform the audit such that misstatements materially affecting the presentation of the net assets, financial position and results of operations in the consolidated financial statements in accordance with the applicable financial reporting framework and in the Group management report are detected with reasonable assurance. Knowledge of the business activities and the economic and legal environment of the Group and expectations as to possible misstatements are taken into account in the determination of audit procedures. The effectiveness of the accounting-related internal control system and the evidence supporting the disclosures in the consolidated financial statements and the Group management report are examined primarily on a test basis within the framework of the audit. The audit includes assessing the annual financial statements of those entities included in consolidation, the determination of entities to be included in consolidation, the accounting and consolidation principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements and Group management report. We believe that our audit provides a reasonable basis for our opinion.
Our audit has not led to any reservations.
In our opinion, based on the findings of our audit, the consolidated financial statements comply with IFRSs, as adopted by the EU, and the additional requirements of German commercial law pursuant to section 315a par. 1 HGB and give a true and fair view of the net assets, financial position and results of operations of the Group in accordance with these requirements. The Group management report is consistent with the consolidated financial statements and as a whole provides a suitable view of the Group’s position and suitably presents the opportunities and risks of future development.”
Hamburg, 25 February 2010
KPMG AG
Wirtschaftsprüfungsgesellschaft
Kniese
German Public Auditor (Wirtschaftsprüfer)
Boßow
German Public Auditor (Wirtschaftsprüfer)
EVOTEC – ANNUAL REPORT 2009

RESPONSIBILITY STATEMENT 89
RESPONSIBILITY
STATEMENT
To the best of our knowledge, and in accordance with the applicable reporting principles, the Consolidated Financial Statements give a true and fair view of the assets, liabilities, financial position and financial results of the Group, and the Group Management Report includes a fair review of the development and performance of the business and the position of the Group, together with a description of the principal opportunities and risks associated with the expected development of the Group.
Evotec AG The Management Board
Hamburg, 25 February 2010
Dr Werner Lanthaler
Chief Executive Officer
Dr Klaus Maleck
Chief Financial Officer
Dr Mario Polywka
Chief Operating Officer
EVOTEC – ANNUAL REPORT 2009

SUPERVISORY BOARD REPORT 91
SUPERVISORY BOARD
REPORT
Dr Flemming Ørnskov
Chairman of the Supervisory Board
The primary task of the Supervisory Board is to regularly supervise and provide advice to the Management Board on the management of the enterprise.
In the course of 2009, the Supervisory Board convened for five formal meetings and held various telephone conferences to discuss the operational and strategic developments of Evotec AG as well as the appointment of the new Chief Executive Officer of the Company. The Audit Committee convened separately for four telephone conferences and the Remuneration and Nomination Committee convened for three physical meetings.
The Management Board also provided continuous updates to the Supervisory Board through regular verbal and written reports that included in depth analysis of the status of operations. The information provided included written monthly management reports with extensive coverage of the Company’s financial figures for the previous month, accompanied by detailed comments and explanatory text. In addition, the Chairman of the Supervisory Board and the Chief Executive Officer as well as other members of the Management Board discussed current and ongoing topics via numerous conference calls, carried out whenever appropriate.
Further to business updates, the status of the Company’s Discovery Alliance Business and its proprietary programs and regular standard agenda items, the Supervisory Board discussed at its physical meetings the following subjects in detail:
— In March, the Supervisory Board discussed the situation of the Company and its strategic direction. Furthermore, the Supervisory Board discussed and approved the 2008 annual financial statements in the presence of the auditors.
— In June, the Supervisory Board held in-depth discussions of the strategy implications of the Action Plan 2012. Following the Company’s
Annual General Meeting, the Supervisory Board decided on the composition of the Audit Committee and of the Remuneration and Nomination Committee.
— In a telephone conference in July, the Supervisory Board approved management’s decision to proceed with the acquisition of the Indian company RSIPL.
— In September, the Supervisory Board discussed the status of the Company’s Discovery Alliance Business, the status of clinical projects and respective business development activities as well as strategic opportunities.
— In December, the Board focused on the budget for the year 2010 which was approved, and discussed the mid range plan and the strategic growth opportunities of the Discovery Alliance Business.
The financial statements and the management report for Evotec AG for the year 2009, as well as the consolidated financial statements together with the consolidated management report of the Evotec Group, were audited by KPMG Deutsche Treuhandgesellschaft Aktiengesellschaft Wirtschaftsprüfungsgesellschaft, Hamburg. The auditors issued an unqualified audit opinion.
In preparation of the Supervisory Board meeting on March 11, 2010, the Auditors presented outline and course of the 2009 audit, audit findings, and other topics to the Audit Committee. The Audit Committee used this information as a guideline for its own evaluation of the statements and reports. The auditors participated in the 2010 March meeting of the full Supervisory Board and presented a comprehensive report on the audit and their observations. The Supervisory Board examined both the financial statements and the consolidated financial statements prepared by the Management Board based on its own judgement, taking into account the Audit Committee’s input as well as information on key topics provided by the auditors. Following this, the Supervisory Board approved the financial statements and the consolidated financial statements for the year 2009.
With two exceptions, the Supervisory Board was not aware of any potential conflict of interests among any of its members during the year 2009. In the case of these exceptions, two Supervisory Board members disclosed a potential conflict to the Supervisory Board in relation to two independent strategic opportunities for the Company. The respective Supervisory Board members did not participate in the deliberations of the Supervisory Board concerning these opportunities. The Company decided not to pursue both opportunities.
With effect of the day of the Company’s Annual General Meeting on June 4, 2009, John Walker stepped down from his position on the Supervisory Board. In the Annual General Meeting, Dr Walter Wenninger was elected in his place. Furthermore, Dr Corey Goodman resigned from his position as member of the Supervisory Board effective January 31, 2010. We thank both John Walker and Dr Corey Goodman for their valuable contribution to Evotec AG following its merger with Renovis, Inc.
Following Jörn Aldag’s resignation from his position as Chief Executive Officer of Evotec in December 2008, Dr Klaus Maleck, Chief Financial Officer, and Dr Mario Polywka, Chief Operating Officer, jointly lead the Company during the transition period until March 6, 2009, when Dr Werner Lanthaler was appointed the Company’s new Chief Executive Officer.
The Supervisory Board thanks the Management Board and the Company’s employees for their hard work during the year and wishes them ongoing success for 2010.
Hamburg, March 11, 2010
The Supervisory Board Dr Flemming Ørnskov
EVOTEC – ANNUAL REPORT 2009

evotec
COMPANY PRESENTATION
Creating integrated drug discovery innovation alliances

EVOTEC BUSINESS MODEL
We create innovation alliances to discover drugs
DISCOVERY AND TECHNOLOGY PLATFORM
DISCOVERY ALLIANCES WITH THE TOP PHARMA AND BIOTECH COMPANIES
PRODUCT FRANCHISES
Strong track record after >15 years of drug discovery history
— >100 lead series
— 20 preclinical candidates
— 16 clinical compounds
Leading technology platforms
— Assay development and screening
— Fragment-based drug discovery
— Comp. chem./structural based drug discovery
— Medicinal chemistry
— Early ADMET and zebrafish
evotec + Boehringer Ingelheim
evotec + NOVARTIS
evotec + Roche
evotec + ONO
evotec + CHDI
evotec + biogen idec
evotec + pfizer
Core expertise with clinical candidates in
— Treatment-resistant depression
— Others
Wealth of preclinical assets for partnering or own development
OUR VALUE PROPOSITION
Expand highly profitable and cash generating discovery alliance business into globally leading Contract Research Organisation
Finance innovative R&D through
— Profits of Discovery Alliance Business
— Development partnerships
CA. 500 EVOTEC EMPLOYEES WORLDWIDE
Global reach for global projects
Hamburg & Berlin, Germany ~125 employees
— Screening
— HTS
— NMR
— in vitro & in vivo biology
Abingdon, UK ~210 employees
— Medicinal chemistry
— Zebrafish screening
— ADMET
— Structural biology
OPERATIONS & SALES REPRESENTATION
SALES REPRESENTATION (SAN FRANCISCO, BOSTON, TOKYO)
Thane, India 150 employees
— Library synthesis and management
— Medicinal chemistry support
— Development chemistry
Singapore
— Zebrafish screening

HIGH VALUE TECHNOLOGY/ INDICATION. ALLIANCES
Building joint innovation partnerships
SCREENING
HIT-TO-LEAD
LEAD OPTIMISATION
PRECLINICAL DEVELOPMENT
CLINICAL DEVELOPMENT
— Assay development and screening
— (u)HTS
— High content screening
— Electrophysiology
— In silico screening technologies
— Fragment-based drug discovery
— Medicinal chemistry
— Hit expansion
— Library design
— High throughput chemistry
— Protein-ligand crystallography
— In vitro biology
— Early ADMET
— Medicinal chemistry
— In vitro biology
— Disease biology and target class expertise
— Computational chemistry and structure-based drug design
— In silico ADMET
— Custom synthesis
— Analytical development
— Process R&D
— Large scale synthesis
— In vivo pharmacology
— Clinical alliances
— Clinical project management
1 + 2
B1 – ANTAGONIST PROGRAMME
P2X3 – ANTAGONIST PROGRAMME
H3 – ANTAGONIST PROGRAMME
P2X7 – ANTAGONIST PROGRAMME
— Strong preclinical validation
— Potentially orally active
Potential indications
— Neuropathic pain
— Strong clinical / preclinical validation
Potential indications
— Pain, urinary incontinence
— Robust oral activity
— Multiple candidates meet clinical entry hurdle
Potential indications
— Narcolepsy, cognitive impairment
— Clinical stage compound
Potential indications
— Inflammation
1 TECHNOLOGIES + 2 INDICATIONS
TECHNOLOGY AND SCIENTIFIC LEADERSHIP FOR NUMEROUS TARGET CLASSES AND NON EXCLUSIVE INDICATION FRANCHISES
CNS / Oncology / Huntington’s disease / Inflammation Metabolic disease Others Ion channels GPCR’s Kinases . . .
STRATEGY FOR MARKET LEADERSHIP
With few but fast moves a market leadership position can be established
BUILD UNIQUE HIGH-END capabilities
LEVERAGE GLOBAL capacities
EXPAND UNIQUE customer RELATIONS
Integrate services for “One Stop Shop” PoC offering
— Build disease core competencies, e.g. cancer, CNS, inflammation
— Build more strategic capacity and product offerings with European, US, Indian and/or Chinese players
— Define global centers of excellence in drug discovery offerings, e.g. HTS, medicinal chemistry…
— Build strategic integrated projects with Pharma & big Biotech
— Build new alliances with clinical CRO’s
— Offer new risk/reward sharing deal structures and alliances
Exhibit 99.3
|
NEWS RELEASE 12 May 2010 | |
| ‘RESEARCH NEVER STOPS’ |
info@evotec.com | www.evotec.com
|
For further information, please contact
Dr Werner Lanthaler Chief Executive Officer +49.(0)40.560 81-242 +49.(0)40.560 81-333 Fax werner.lanthaler@evotec.com
Evotec AG Schnackenburgallee 114 22525 Hamburg (Germany)
| Evotec’s Drug Discovery Business Set for Strong Growth
EXCELLENT START INTO 2010: REVENUES +19% AND STRONG DECREASE OF OPERATING LOSS BY 93%
Hamburg, Germany – 12 May 2010: Evotec AG (Frankfurt Stock Exchange: EVT, TecDAX) today reported financial results and corporate updates for the first quarter of 2010.
RECENT HIGHLIGHTS:
— Sustainability of business clearly visible
— Discovery alliances growing significantly, revenues +19%
— Strong decrease of operating loss by 93% to € 1.5 m
— Cash burn markedly reduced: liquidity at € 67 m
— Increased revenues from long-term collaborations
— New multi-year strategic alliance with Genentech (after period-end)
— Collaboration with CHDI to fight Huntington’s Disease extended for another three years
— Good progress with proprietary pipeline projects; increased third-party funding
— Positive FDA feedback to initiate Phase II with EVT 101; study to start in Q2 2010
— Successful completion of first-in-man study with EVT 103
— BMBF grant for H3 receptor antagonist programme (after period-end)
— Financial guidance for 2010 confirmed
— At least 15% revenue growth and >€ 64m liquidity at year-end
1. OPERATIONAL PERFORMANCE
Sustainability of business clearly visible: revenues +19%; operating loss -93% at € 1.5 m
The effects of Evotec’s ‘Action Plan 2012’ are now clearly reflected in the financial results for the first quarter 2010. Revenues from the Company’s discovery alliances grew significantly. Although Q1 revenues in 2009 and 2010 did not include any milestone payments from collaborations, total Grouprevenues increased by 19% to € 9.8 m (2009: € 8.2 m).Gross margin remained strong at 37.9% (2009: 36.2%). R&D expenses decreased by 83% to € 1.7 m (2009: € 10.3 m), and SG&A expenses by 30% to € 3.4 m (2009: € 4.8 m). The strong decrease in R&D was supported by the fact that the costs for the development of the EVT 100 compound family are now fully borne by Roche, while they were still included in Evotec’s R&D expenses for the majority of the first quarter 2009. Due to this strong top-line performance and the significant reduction in operating expenses as well as exceptional items in Q1 2009, Evotec’soperating loss for the first quarter decreased by 93% to € 1.5 m (2009: € 20.2 m).Net loss decreased by 94% to € 1.2 m (2009: |
€ 21.8 m). On this basis,liquidity including cash and cash equivalents, investments and auction rate securities at the end of March remained very strong at € 66.8 m (31 Dec 2009: € 70.6 m). Going forward, milestone achievements are expected to further enhance Evotec’s financial performance; a strong basis to develop the Company to profitability latest in 2012.
2. DISCOVERY ALLIANCES UPDATE
Increased revenues from long-term collaborations
Due to its scale, integration of drug discovery technologies and disease know-how, as well as its strong reputation in the industry, Evotec is ideally positioned to take advantage of the increase in strategic drug discovery outsourcing. As a partner of choice for integrated alliances with pharmaceutical companies, Evotec further increased its share in revenues from long-term collaborations in the first quarter.
New multi-year strategic alliance with Genentech (after period-end)
On 10 May 2010, Evotec announced a new multi-year integrated drug discovery alliance withGenentech. Jointly, both companies aim to identify novel therapeutics against one or several targets.
Collaboration with CHDI to fight Huntington’s Disease extended for another three years
In January 2010, Evotec announced the extension of its collaboration withCHDI Foundation, Inc.(CHDI) through the end of 2012. The collaboration represents one of the largest drug discovery alliances within Evotec and will provide the Company with up to a total of US$ 37.5 m in research funding over three years. Evotec has been providing research and innovation support to CHDI since 2006.
In the first quarter 2010, Evotec also signed a significant collaboration withVifor Pharma to jointly identify a pre-clinical candidate for the treatment of anaemia and announced collaborations or extensions withCubist Pharmaceuticals andActive Biotech.
3. STATUS OF CLINICAL PROGRAMMES AND PARTNERING OF ASSETS
Proprietary R&D increasingly funded by external partners
Evotec is focusing its proprietary programmes on fewer core assets, aggressively seeking strategic alliances to progress their development and to capture their commercial potential. The EVT 100 programme is partnered with Roche for development in treatment-resistant depression. The costs for the EVT 101 and EVT 103 studies are now fully borne by Roche, significantly reducing Evotec’s R&D expenses and risk profile.
Good progress with EVT 101 and EVT 103 in Roche alliance
Early in 2010, Evotec completed the clinical part of the first-in-human Phase I study with EVT 103. The compound was safe and very well tolerated after single and multiple oral dose administration, with excellent bioavailability and only a minimal effect of food on the kinetic profile.
For EVT 101, the lead compound, Evotec received approval from the FDA to initiate the Phase II Proof-of-Concept study in treatment resistant depression. The study will start recruiting patients in the |
EVOTEC – NEWS RELEASE
2
second quarter of 2010. If Roche exercises its buy-back option after completion of the Phase II trial, Evotec will receive a $65 m payment in exchange for the assignment of all rights.
New BMBF grant for H3 receptor antagonist programme (after period-end)
On 27 April 2010 Evotec announced it will receive up to € 1.5 m funding from the German Federal Ministry of Education and Research (BMBF) to advance its H3 receptor antagonist programme through Phase I clinical studies. H3 receptor antagonists have potential in a number of CNS indications, including excessive fatigue associated with conditions such as multiple sclerosis.
4. GUIDANCE
Financial guidance for 2010 confirmed
The Company confirms all financial targets for the fiscal year 2010 published on 25 March 2010: Total Group revenues before out-licensing income are expected to grow by at least 15%. These assumptions are based on the strong order book of approximately € 30 m at the end of March 2010 (2009: € 24 m), expected new contracts and contract extensions as well as the achievement of certain milestones.
With the restructuring measures taken in 2009, Evotec has significantly reduced its cost base. SG&A expenses will decrease due to cost reductions in all parts of the Group. In addition, Evotec expects R&D expenses to decrease considerably from 2009 levels. The Company will focus on key programmes and targets to invest approximately € 10 m in R&D in 2010. As a result, Evotec’s Group operating result before impairment is expected to improve significantly over 2009.
Top-line growth and the adjusted cost base are expected to significantly reduce the cash requirements compared to the 2009 fiscal year. Consequently, at constant year-end 2009 currencies, the Company expects to end 2010 with a liquidity of more than € 64 m.
CONFERENCE CALL
The Company is going to hold a conference call to discuss the results as well as to provide an update on its performance:
| ||||||
| Conference call details | ||||||
Date: Time: |
Wednesday, 12 May 2010 09.30 a.m. CEST 08.30 a.m. BST 03.30 a.m. US time (East Coast) | |||||
From Europe: |
+49.(0)69.9897 2631 (Germany) +44.(0)20.7138 0814 (UK) | |||||
From the US: Access Code: | +1.718.354 1359 8395457 | |||||
A simultaneous slide presentation for participants dialing in via phone is available atwww.equitystory.com, password: evotec0510.
Webcast details
To join the audio webcast and to access the presentation slides you will find a link on our home pagewww.evotec.com shortly before the event.
A replay of the conference call will be available for 24 hours and can be accessed in Europe by dialing +49.(0)69.2222 2236 (Germany) or +44.(0)20.7111 1244 (UK) and in the US by dialing +1.347.366 9565. The access code is 8395457#. The on-demand version of the webcast will be available on our website:www.evotec.com/Investors/Finance. | ||||||
EVOTEC – NEWS RELEASE
3
ABOUT EVOTEC AG
Evotec is a leader in the discovery and development of novel small molecule drugs with operational sites in Europe and Asia. The Company has built substantial drug discovery expertise and an industrialised platform that can drive new innovative small molecule compounds into the clinic. In addition, Evotec has built a deep internal knowledge base in the treatment of diseases related to neuroscience, pain, oncology and inflammation. Leveraging these skills and expertise the Company intends to develop best-in-class differentiated therapeutics and deliver superior science-driven discovery alliances with pharmaceutical and biotechnology companies. Evotec has long-term discovery alliances with partners including Boehringer Ingelheim, CHDI, Novartis, Ono Pharmaceutical and Roche. Evotec has product candidates in clinical development and a series of preclinical compounds and development partnerships, including for example a strategic alliance with Roche for the EVT 100 compound family, subtype selective NMDA receptor antagonists for use in treatment-resistant depression. For additional information please go towww.evotec.com.
FORWARD-LOOKING STATEMENTS
Information set forth in this press release contains forward-looking statements, which involve a number of risks and uncertainties. Such forward-looking statements include, but are not limited to, statements about our 2010 financial outlook and our expected financial results in future quarters, our ability to deliver on our liquidity guidance, our belief that we are on course to profitability in 2012, our expectations and assumptions concerning regulatory, clinical and business strategies, the progress of our clinical development programs and timing of the commencement and results of our clinical trials, strategic collaborations and management’s plans, objectives and strategies. These statements are neither promises nor guarantees, but are subject to a variety of risks and uncertainties, many of which are beyond our control, and which could cause actual results to differ materially from those contemplated in these forward-looking statements. In particular, the risks and uncertainties include, among other things; risks that product candidates may fail in the clinic or may not be successfully marketed or manufactured; the risk that we will not achieve the anticipated benefits of our collaborations, partnerships and acquisitions in the timeframes expected, or at all; risks relating to our ability to advance the development of product candidates currently in the pipeline or in clinical trials; our inability to further identify, develop and achieve commercial success for new products and technologies; the risk that competing products may be more successful; our inability to interest potential partners in our technologies and products; our inability to achieve commercial success for our products and technologies; our inability to protect our intellectual property and the cost of enforcing or defending our intellectual property rights; our failure to comply with regulations relating to our products and product candidates, including FDA requirements; the risk that the FDA may interpret the results of our studies differently than we have; the risk that clinical trials may not result in marketable products; the risk that we may be unable to successfully secure regulatory approval of and market our drug candidates; and risks of new, changing and competitive technologies and regulations in the U.S. and internationally.
The list of risks above is not exhaustive. Our most recent Annual Report on Form 20-F, filed with the Securities and Exchange Commission, and other documents filed with, or furnished to the Securities and Exchange Commission, contain additional factors that could impact our businesses and financial performance. We expressly disclaim any obligation or undertaking to release publicly any updates or revisions to any such statements to reflect any change in our expectations or any change in events, conditions or circumstances on which any such statement is based. |
EVOTEC – NEWS RELEASE
4
Exhibit 99.4
 | ‘RESEARCH NEVER STOPS’ |
| QUARTERLY REPORT | Evotec AG,Schnackenburgallee 114, 22525 Hamburg (Germany), www.evotec.com |
For further information, please contact
Dr Werner Lanthaler Chief Executive Officer +49.(0)40.560 81-242 +49.(0)40.560 81-333 Fax werner.lanthaler@evotec.com
| I. Management Report of the First Quarter 2010
EVOTEC’S DRUG DISCOVERY BUSINESS SET FOR STRONG GROWTH – EXCELLENT START INTO 2010: REVENUES +19% AND STRONG DECREASE OF OPERATING LOSS BY 93%
Recent highlights:
— Sustainability of business clearly visible
- Discovery alliances growing significantly, revenues +19%
- Strong decrease of operating loss by 93% to € 1.5m
- Cash burn markedly reduced: liquidity at € 67m
— Increased revenues from long-term collaborations
- New multi-year strategic alliance with Genentech (after period-end)
- Collaboration with CHDI to fight Huntington’s Disease extended for another three years
— Good progress with proprietary pipeline projects; increased third party funding
- Positive FDA feedback to initiate Phase II with EVT 101; study to start in Q2 2010
- Successful completion of first-in-man study with EVT 103
- BMBF grant for H3 receptor antagonist programme (after period-end)
— Financial guidance for 2010 confirmed
- At least 15% revenue growth and >€ 64m liquidity at year-end |
1. OPERATIONAL PERFORMANCE
Sustainability of business clearly visible: revenues +19%; operating loss -93% at € 1.5 m
The effects of Evotec’s ‘Action Plan 2012’ are now clearly reflected in the financial results for the first quarter 2010. Revenues from the Company’s discovery alliances grew significantly. Although Q1 revenues in 2009 and 2010 did not include any milestone payments from collaborations, total Group revenues increased by 19% to € 9.8 m (2009: € 8.2 m). R&D expenses declined by 83%, and SG&A costs by 30%. Due to this strong top-line performance and the significant reduction in operating expenses as well as exceptional items in Q1 2009, Evotec’s operating loss for the first quarter decreased by 93% to € 1.5 m (2009: € 20.2 m). On this basis, liquidity including cash and cash equivalents, investments and auction rate securities at the end of March remained strong at € 66.8 m. Going forward, milestone achievements are expected to further enhance Evotec’s financial performance; a strong basis to develop the Company to profitability latest in 2012.
2. DISCOVERY ALLIANCES UPDATE
Increased revenues from long-term collaborations
Due to its scale, integration of drug discovery technologies and disease know-how, as well as its strong reputation in the industry, Evotec is ideally positioned to take advantage of the increase in strategic drug discovery outsourcing. As a partner of choice for integrated alliances with pharmaceutical companies, Evotec further increased its share in revenues from long-term collaborations in the first quarter.
New multi-year strategic alliance with Genentech (after period-end)
On 10 May 2010, Evotec announced a new multi-year integrated drug discovery alliance with Genentech. Jointly, both companies aim to identify novel therapeutics against one or several targets.
Collaboration with CHDI to fight Huntington’s Disease extended for another three years
In January 2010, Evotec announced the extension of its collaboration withCHDI Foundation, Inc.(CHDI) through the end of 2012. The collaboration represents one of the largest drug discovery alliances within Evotec and will provide the Company with up to a total of US$ 37.5 m in research funding over three years. Evotec has been providing research and innovation support to CHDI since 2006.
In the first quarter 2010, Evotec also signed a significant collaboration withVifor Pharma to jointly identify a pre-clinical candidate for the treatment of anaemia and announced collaborations or extensions withCubist Pharmaceuticals andActive Biotech. |
EVOTEC – REPORT AS OF FIRST QUARTER 2010
2
3. STATUS OF CLINICAL PROGRAMMES AND PARTNERING OF ASSETS
Proprietary R&D increasingly funded by external partners
Evotec is focusing its proprietary programmes on fewer core assets, aggressively seeking strategic alliances to progress their development and to capture their commercial potential. The EVT 100 programme is partnered with Roche for development in treatment-resistant depression. The costs for the EVT 101 and EVT 103 studies are now fully borne by Roche, significantly reducing Evotec’s R&D expenses and risk profile.
Good progress with EVT 101 and EVT 103 in Roche alliance
Early in 2010, Evotec completed the clinical part of the first-in-human Phase I study with EVT 103. The compound was safe and very well tolerated after single and multiple oral dose administration, with excellent bioavailability and only a minimal effect of food on the kinetic profile.
For EVT 101, the lead compound, Evotec received approval from the FDA to initiate the Phase II Proof-of-Concept study in treatment resistant depression. The study will start recruiting patients in the second quarter of 2010. If Roche exercises its buy-back option after completion of the Phase II trial, Evotec will receive a $65 m payment in exchange for the assignment of all rights.
New BMBF grant for H3 receptor antagonist programme (after period-end)
On 27 April 2010 Evotec announced it will receive up to € 1.5 m funding from the German Federal Ministry of Education and Research (BMBF) to advance its H3 receptor antagonist programme through Phase I clinical studies. H3 receptor antagonists have potential in a number of CNS indications, including excessive fatigue associated with conditions such as multiple sclerosis.
4. GUIDANCE
Financial guidance for 2010 confirmed
The Company confirms all financial targets for the fiscal year 2010 published on 25 March 2010: Total Group revenues before out-licensing income are expected to grow by at least 15%. SG&A expenses will decrease due to cost reductions in all parts of the Group. In addition, the Company will focus on key programmes and targets to invest approximately € 10 m in R&D. As a result, Evotec’s Group operating result in the absence of impairment charges is expected to improve significantly over 2009. On this basis, the Company expects to end 2010 with a liquidity of more than € 64 m at constant year-end 2009 currencies. |
EVOTEC – REPORT AS OF FIRST QUARTER 2010
3
A. REPORT ON THE FINANCIAL SITUATION AND RESULTS
1. RESULTS
| ||||
| Revenues | Evotec’srevenues for the first quarter 2010 amounted to € 9.8 m, an increase of 19% compared to the same period of the previous year (2009: € 8.2 m). At constant 2009 exchange rates, revenues were € 10.0 m (+22%). Growth was driven by strong contributions from the drug discovery alliances, with additional contributions from the acquired business Evotec (India) Private Ltd., as well as a higher portion recognised from the upfront payment for the EVT 100 compound family from Roche compared to Q1 2009. Q1 2010 revenues did not include any milestone payments from collaborations.
Geographically, 38% of Evotec’s revenues were generated from Europe, 39% from the US, and 23% from Japan and the Rest of the World. This compares to 38%, 49% and 13%, respectively, in the same period of the previous year. The higher contribution of Japanese revenues to Group revenues reflects the expansion of the strategic long-term partnership with Ono Pharmaceutical.
| |||
| Operating cost structure | Costs of revenue for the first quarter 2010 amounted to € 6.1 m (2009: € 5.3 m) yielding a stronggross margin of 37.9% (2009: 36.2%). At constant 2009 currencies, gross margin would have been even higher at 40.1%. The increase over 2009 is attributable primarily to a higher amount of the upfront payment from Roche recognised in the quarter.
Gross margins in the future may be somewhat volatile, and the receipt of potential milestone or out-licensing payments may further enhance Evotec’s financial results.
R&D expenditure for the first quarter 2010 decreased by 83% to € 1.7 m (2009: € 10.3 m). The decrease primarily resulted from a focus on fewer core programmes, from the reduction of early discovery programmes, and from the closure of Evotec’s US site following the implementation of the ‘Evotec 2012 – Action Plan to Focus and Grow’. In addition, costs for the development of the EVT 100 compound family are now fully borne by Roche, while they were still included in Evotec’s R&D expenses for the majority of the first quarter 2009.
SG&A expenses for the first quarter 2010 decreased markedly by 30% to € 3.4 m (2009: € 4.8 m), reflecting the full impact of Evotec’s restructuring and cost saving measures.
Other operating income and expenses resulted primarily from the reimbursement of expenses incurred for the clinical programmes with EVT 101 and EVT 103 by Roche.
| |||
| Financial results | Evotec’soperating loss for the first quarter 2010 decreased by 93% to € 1.5 m (2009: € 20.2 m). This decrease in operating loss is mainly a result of the Company’s strong top-line performance as well as its significant reductions in operating expenses mentioned above. In addition, the first quarter of the prior year included an impairment charge of € 6.6 m for the VR1 (vanilloid receptor 1) antagonist programme and € 1.4 m of restructuring expenses. The operating loss before these exceptional items improved by 88%.
Net loss decreased by 94% to € 1.2 m (2009: € 21.8 m).Loss per share for the first quarter 2010 was € 0.01 (2009: € 0.21).
|
EVOTEC – REPORT AS OF FIRST QUARTER 2010
4
2. FINANCING AND FINANCIAL POSITION
| ||||
| Cash flow and liquidity | Based on a significantly lower net loss than in the first quarter 2009,cash flow used in operating activities for the first quarter 2010 improved significantly to € (5.2) m from € (17.9) m in 2009. Besides net loss, cash outflow was mainly driven by regular bonus payments and prepaid expenses such as payments for insurances and maintenance contracts due at the beginning of each year: Cash outflow is expected to decline over the next quarters.
“Adjustments to reconcile net loss to net cash used in operating activities” included mainly amortisation (€ 0.1 m) and depreciation (€ 1.0 m).
Cash flow from investing activities was almost exclusively from transactions involving investments in money market funds. It resulted in a net cash decrease and respective increase in investments of € 5.1 m. Capital expenditures amounted to € 0.3 m.
Cash flow from financing activities was € (0.4) million and related mainly to the repayment of bank loans and purchase of our own stock.
Liquidity, which includes cash and cash equivalents (€ 24.8 m), investments (€ 32.2 m) and auction rate securities1 (€ 9.8 m), at the end of March 2010 amounted to € 66.8 m (31 December 2009: € 70.6 m).
3. ASSETS AND LIABILITIES
Other current financial assets increased to € 2.3 m due to not yet charged expenses for the EVT 100 series to Roche. Prepaid expenses and other current assets increased to € 3.0 m as a result of prepaid expenses such as payments for insurances and maintenance contracts due at the beginning of each year.
Trade accounts payable increased to € 5.6 m, mainly as a result of accrued outstanding invoices for clinical trials for the EVT 100 series which will be reimbursed by Roche. Provisions decreased to € 1.6 m. The decrease is mainly the consequence of regular bonuses paid in March. Non-current deferred revenues decreased to € 1.1 m mainly due to recognition of the first quarter revenue portion of the Roche upfront payment for the EVT 100 compound family.
More details and all further material changes of assets and liabilities during the first quarter 2010 are described in the Notes to the Unaudited Condensed Consolidated Interim Financial Statements.
Evotec’s capital structure is unchanged compared to the end of 2009. The total number of ordinary shares outstanding as of the date of this report is 108,838,715.
Evotec’s equity ratio as of 31 March 2010 continued to be high at 78.3% (31 December 2009: 76.0%).
4. HUMAN RESOURCES
Employees
At the end of March 2010, following restructuring (-83 people) and the subsequent strategic acquisition of RSIPL and the Zebrafish operation (+180 people) in 2009, 466 people were employed within the Evotec Group, a decrease of 19 compared to the end of December 2009 (485 employees). The decrease in the first quarter 2010 is due to adjustments made during the integration of acquired new businesses and a decreased focus on early research.
|
| 1 | For further discussion of the auction rate securities please see Note 5 to the Unaudited Condensed Consolidated Interim Financial Statements. |
EVOTEC – REPORT AS OF FIRST QUARTER 2010
5
Stock-based compensation
During the first quarter of 2010 no options were granted or exercised. As of 31 March 2010, the total number of options available for future exercise amounted to 4,747,156 (approximately 4% of shares in issue). Options have been accounted for under IFRS 2 using the fair value method at the measurement date.
In connection with the acquisition of Renovis, Evotec issued shares to a trust. These shares were meant to replace outstanding options and similar share-based compensation arrangements for Renovis employees. Of those issued shares, Evotec released 33,522 in the first quarter of 2010 from this trust, which, by the end of March, had approximately 1,486,468 remaining unreleased Evotec shares.
Shareholdings of the Boards of Evotec AG
| ||||||||
| Number of shares | Share options | |||||||
Management Board | ||||||||
Dr Werner Lanthaler | 414,494 | 500,000 | ||||||
Dr Klaus Maleck | 0 | 250,000 | ||||||
Dr Mario Polywka | 60,000 | 455,000 | ||||||
Supervisory Board | ||||||||
Dr Flemming Ornskov | 15,513 | 0 | ||||||
Dr Hubert Birner | 27,897 | 0 | ||||||
Dr Peter Fellner | 14,727 | 0 | ||||||
Mary Tanner | 62,192 | 0 | ||||||
Dr Walter Wenninger | 5,419 | 0 | ||||||
| 31 March 2010 | ||||||||
Pursuant to §15a of the German Securities Trading Act (Wertpapierhandelsgesetz), the above table lists separately for each member of our Management and Supervisory Board, the number of Company shares held, and rights for such shares granted to each board member as of 31 March 2010.
B. Risks and Opportunities Report
The risks and opportunities described in Evotec’s 2009 Annual Report remain unchanged.
C. Important events after the end of the first quarter 2010
There are no material events to be reported.
D. Outlook
| ||||||||
| 2010 financial guidance confirmed | The Company confirms all financial targets for the fiscal year 2010 published on 25 March 2010.
Total Group revenues before out-licensing income are expected to grow by at least 15%. These assumptions are based on the strong order book of approximately € 30 m at the end of March 2010 (2009: € 24 m), expected new contracts and contract extensions as well as the achievement of certain milestones.
| |||||||
EVOTEC – REPORT AS OF FIRST QUARTER 2010
6
With the restructuring measures taken in 2009, Evotec has significantly reduced its cost base. SG&A expenses will decrease due to cost reductions in all parts of the Group. In addition, Evotec expects R&D expenses to decrease considerably from 2009 levels. The Company will focus on key programmes and targets to invest approximately € 10 m in R&D in 2010. As a result, Evotec’s Group operating result before impairment is expected to improve significantly over 2009.
Top-line growth and the adjusted cost base are expected to significantly reduce the cash requirements compared to the 2009 fiscal year. Consequently, at constant year-end 2009 currencies, the Company expects to end 2010 with a liquidity of more than € 64 m.
Note: The 2009 and 2010 results are not fully comparable. The major difference results from the acquisition of Research Support International Private Limited on 31 August 2009. The operating results of RSIPL from the period 1 January 2010 through 31 March 2010 are included in the accompanying consolidated interim statements of operation for the three months ended 31 March 2010. |
EVOTEC – REPORT AS OF FIRST QUARTER 2010
7
II. Consolidated Interim Financial Statements
Evotec AG and Subsidiaries –
Condensed consolidated interim income statement for the period from 1 January to 31 March 2010
in T€ except share and per share data | Three months ended 31 March 2010 | Three months ended 31 March 2009 | ||||
Revenue | 9.841 | 8.238 | ||||
Costs of revenue | 6.112 | 5.259 | ||||
Gross profit | 3.729 | 2.979 | ||||
Operating expenses (income) | ||||||
Research and development expenses | 1.734 | 10.319 | ||||
Selling, general and administrative expenses | 3.350 | 4.793 | ||||
Amortisation of intangible assets | 129 | 78 | ||||
Impairment of intangible assets | — | 6.630 | ||||
Restructuring expenses | — | 1.444 | ||||
Other operating income | (910 | ) | (220 | ) | ||
Other operating expenses | 900 | 184 | ||||
Total operating expenses | 5.203 | 23.228 | ||||
Operating loss | (1.474 | ) | (20.249 | ) | ||
Other non-operating income (expense) | ||||||
Interest income | 84 | 247 | ||||
Interest expense | (101 | ) | (164 | ) | ||
Gain (loss) from equity investments | — | 15 | ||||
Other expenses from financial assets | (12 | ) | (590 | ) | ||
Other income from financial assets | 2 | 167 | ||||
Foreign currency exchange gain (loss), net | 117 | (1.271 | ) | |||
Other non-operating expense | (34 | ) | — | |||
Other non-operating income | 60 | 12 | ||||
Total non-operating income (expense) | 116 | (1.584 | ) | |||
Loss before taxes | (1.358 | ) | (21.833 | ) | ||
Current tax expense | (52 | ) | (4 | ) | ||
Deferred tax income (expense) | 163 | (10 | ) | |||
Net loss | (1.247 | ) | (21.847 | ) | ||
thereof attributable to: | ||||||
Shareholders of Evotec AG | (1.284 | ) | (21.847 | ) | ||
Non-controlling interest | 37 | — | ||||
Net loss | (1.247 | ) | (21.847 | ) | ||
Weighted average shares outstanding | 107.335.773 | 106.564.331 | ||||
Net loss per share (basic and diluted) | (0,01 | ) | (0,21 | ) | ||
EVOTEC – REPORT AS OF FIRST QUARTER 2010
8
Evotec AG and Subsidiaries –
Consolidated statements of comprehensive income for the period from 1 January to 31 March 2010
in T€ | Three months ended 31 March 2010 | Three months ended 31 March 2009 | ||||
Net loss | (1.247 | ) | (21.847 | ) | ||
Other comprehensive income | ||||||
Foreign currency translation | 2.846 | 6.945 | ||||
Revaluation of available-for-sale securities | 17 | 619 | ||||
Other comprehensive income | 2.863 | 7.564 | ||||
Total comprehensive income (loss) | 1.616 | (14.283 | ) | |||
Total comprehensive income (loss) attributable to: | ||||||
Shareholders of Evotec AG | 1.579 | (14.283 | ) | |||
Non-controlling interest | 37 | — | ||||
Total comprehensive income (loss) | 1. 616 | (14.283 | ) | |||
EVOTEC – REPORT AS OF FIRST QUARTER 2010
9
Evotec AG and Subsidiaries –
Consolidated interim statement of financial position as of 31 March 2010
in T€ except share data | as of 31 March 2010 | as of 31 Dec 2009 | ||||
ASSETS | ||||||
Current assets: | ||||||
Cash and cash equivalents | 24.840 | 32.926 | ||||
Investments | 29.149 | 25.432 | ||||
Trade accounts receivables | 3.201 | 4.510 | ||||
Inventories | 3.012 | 2.425 | ||||
Current tax receivables | 619 | 347 | ||||
Other current financial assets | 2.331 | 1.428 | ||||
Prepaid expenses and other current assets | 2.967 | 1.889 | ||||
Total current assets | 66.119 | 68.957 | ||||
Non-current assets: | ||||||
Long-term investments | 10 | 10 | ||||
Property, plant and equipment | 18.922 | 19.162 | ||||
Intangible assets, excluding goodwill | 29.144 | 29.010 | ||||
Goodwill | 16.915 | 16.557 | ||||
Auction rate securities | 9.783 | 9.236 | ||||
Other non-current financial assets | 3.639 | 3.667 | ||||
Total non-current assets | 78.413 | 77.642 | ||||
Total assets | 144.532 | 146.599 | ||||
LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||
Current liabilities: | ||||||
Current maturities of long-term loans | 9.087 | 9.087 | ||||
Current portion of finance lease obligations | 189 | 229 | ||||
Trade accounts payable | 5.587 | 4.398 | ||||
Accounts payable to related parties | 837 | 837 | ||||
Advanced payments received | 460 | 129 | ||||
Provisions | 1.595 | 4.858 | ||||
Deferred revenues | 4.433 | 5.483 | ||||
Current income tax payables | 205 | 244 | ||||
Other current financial liabilities | 472 | 485 | ||||
Other current liabilities | 942 | 695 | ||||
Total current liabilities | 23.807 | 26.445 | ||||
Non-current liabilities: | ||||||
Long-term loans | 3.616 | 3.757 | ||||
Long-term finance lease obligations | 108 | 132 | ||||
Deferred tax liabilities | 1.834 | 1.977 | ||||
Deferred revenues | 1.131 | 1.969 | ||||
Provisions | 825 | 832 | ||||
Total non-current liabilities | 7.514 | 8.667 | ||||
Stockholders’ equity: | ||||||
Share capital | 108.839 | 108.839 | ||||
Additional paid-in capital | 648.525 | 648.417 | ||||
Accumulated other comprehensive income | (24.615 | ) | (27.478 | ) | ||
Accumulated deficit | (620.188 | ) | (618.904 | ) | ||
Equity attributable to shareholders of Evotec AG | 112.561 | 110.874 | ||||
Non-controlling interests | 650 | 613 | ||||
Total stockholders’ equity | 113.211 | 111.487 | ||||
Total liabilities and stockholders’ equity | 144.532 | 146.599 | ||||
EVOTEC – REPORT AS OF FIRST QUARTER 2010
10
Evotec AG and Subsidiaries –
Condensed consolidated interim statement of financial position as of 31 March 2010
in T€ | Three months ended 31 March 2010 | Three months ended 31 March 2009 | ||||
Cash flows from operating activities: | ||||||
Net loss | (1.284 | ) | (21.847 | ) | ||
Adjustments to reconcile net loss to net cash used in operating activities | 1.127 | 8.924 | ||||
Change in assets and liabilities | (5.014 | ) | (5.016 | ) | ||
Net cash used in operating activities | (5.171 | ) | (17.939 | ) | ||
Cash flows from investing activities: | ||||||
Purchase of current investments | (21.640 | ) | (5.196 | ) | ||
Purchase of property, plant and equipment | (295 | ) | (107 | ) | ||
Proceeds from sale of property, plant and equipment | — | 1 | ||||
Proceeds from sale of financial assets | 72 | 166 | ||||
Proceeds from sale of current investments | 16.555 | 8.738 | ||||
Net cash provided by (used in) investing activities | (5.308 | ) | 3.602 | |||
Cash flows from financing activities: | ||||||
Proceeds from option exercise | 41 | — | ||||
Proceeds from sale of own stock | 11 | — | ||||
Purchase of own stock | (96 | ) | (44 | ) | ||
Repayment of loans | (393 | ) | (941 | ) | ||
Net cash used in financing activities | (437 | ) | (985 | ) | ||
Net decrease in cash and cash equivalents | (10.916 | ) | �� | (15.322 | ) | |
Exchange rate difference | 2.830 | 2.483 | ||||
Cash and cash equivalents at beginning of year | 32.926 | 55.064 | ||||
Cash and cash equivalents at end of the period | 24.840 | 42.225 | ||||
EVOTEC – REPORT AS OF FIRST QUARTER 2010
11
Evotec AG and Subsidiaries –
Consolidated interim statements of changes in stockholders’ equity for the three months ended March 2010
| Share capital | Accumulated other comprehensives income | ||||||||||||||||||||||||
in T€ except share | Shares | Amount | Additional paid-in capital | Treasury shares | Foreign currency translation | Revaluation reserve | Accumulated deficit | Equity attributable to shareholders of Evotec AG | Non-controlling interests | Total stockholders’ equity | |||||||||||||||
Balance at 1 January 2009 | 108.838.715 | 108.839 | 647.163 | — | (38.835 | ) | 6.073 | (573.381 | ) | 149.859 | — | 149.859 | |||||||||||||
Stock option plan | — | — | 369 | — | — | — | — | 369 | — | 369 | |||||||||||||||
Purchase of treasury shares | — | — | — | (44 | ) | — | — | — | (44 | ) | — | (44 | ) | ||||||||||||
Total comprehensive income (loss) | 6.945 | 619 | (21.847 | ) | (14.283 | ) | — | (14.283 | ) | ||||||||||||||||
Balance at 31 March 2009 | 108.838.715 | 108.839 | 647.532 | (44 | ) | (31.890 | ) | 6.692 | (595.228 | ) | 135.901 | — | 135.901 | ||||||||||||
Balance at 1 January 2010 | 108.838.715 | 108.839 | 648.417 | — | (34.727 | ) | 7.249 | (618.904 | ) | 110.874 | 613 | 111.487 | |||||||||||||
Exercised shares from shares in trust | — | — | 41 | — | — | — | — | 41 | — | 41 | |||||||||||||||
Stock option plan | — | — | 67 | — | — | — | — | 67 | — | 67 | |||||||||||||||
Purchase of treasury shares | — | — | — | (96 | ) | — | — | — | (96 | ) | — | (96 | ) | ||||||||||||
Transfer of treasury shares | — | — | — | 85 | — | — | — | 85 | — | 85 | |||||||||||||||
Sales of treasury shares | — | — | — | 11 | — | — | — | 11 | — | 11 | |||||||||||||||
Total comprehensive income (loss) | 2.846 | 17 | (1.284 | ) | 1.579 | 37 | 1.616 | ||||||||||||||||||
Balance at 31 March 2010 | 108.838.715 | 108.839 | 648.525 | — | (31.881 | ) | 7.266 | (620.188 | ) | 112.561 | 650 | 113.211 | |||||||||||||
EVOTEC – REPORT AS OF FIRST QUARTER 2010
12
NOTES TO THE UNAUDITED CONDENSED CONSOLIDATED INTERIM FINANCIAL STATEMENTS
1. BASIS OF PRESENTATION
The accompanying unaudited consolidated interim financial statements of Evotec have been prepared in accordance with International Financial Reporting Standards (IFRS) in conjunction with IAS 34. The accounting policies used to prepare interim information are the same as those used to prepare the audited consolidated financial statements for the year ended 31 December 2009.
The interim consolidated financial statements do not include all of the information and footnotes required under IFRS for complete financial statements according to IAS 1. As a result, these interim financial statements should be read in conjunction with the audited consolidated financial statements and notes thereto for the year ended 31 December 2009.
In the opinion of management, all adjustments, consisting of normal recurring adjustments, considered necessary for a fair presentation have been included.
2. BASIS OF CONSOLIDATION
The basis of consolidation changed. Evotec acquired 70% of the shares of Research Support International Private Limited, India (RSIPL) including its 51% share in Evotec-RSIL Limited, India (Evotec-RSIL) as of 31 August 2009 and from this date onwards RSIPL as well as Evotec-RSIL were fully consolidated. Evotec used to account for Evotec-RSIL Limited under the equity method of accounting until 31 August 2009. Effective 30 April 2009, Evotec acquired 100% of the shares of Summit Asia Pte Limited, Singapore, which was fully consolidated from this date onwards. Therefore the consolidated interim financial statements for 2009 and 2010 are not fully comparable.
3. BASIS OF ESTIMATION
In the consolidated interim financial statements for the three months ended 31 March 2010, the Company has used the same estimation processes as those used to prepare the audited consolidated financial statements for the year ended 31 December 2009.
4. ACQUISITIONS
The Company acquired 70% of the shares in Research Support International Private Limited, Thane, India (RSIPL) including its 51% share in Evotec-RSIL Limited, India (Evotec-RSIL). RSIPL is a company providing drug discovery and development services. The acquisition was effective as of 31 August 2009. The purchase price of T€ 2,373 in cash includes a potential earn-out which was calculated based on estimated future revenues and will be paid in 2010. The Company decided to early adopt the revised version of IFRS 3 “Business Combinations” and the amended version of IAS 27.
At 31 August 2009 the Company recognised a gain of T€ 559 to adjust its carrying amount of the investment in Evotec-RSIL which used to be accounted for under the equity method to its fair value according to the revised version of IFRS 3. The fair values of the assets and liabilities acquired from RSIPL were estimated based on the recognised amounts as of the date of the acquisition. Fair value adjustments have been
|
EVOTEC – REPORT AS OF FIRST QUARTER 2010
13
recorded for a customer list in the amount of T€ 103 which has been estimated based on discounted cash flow modelling. The resulting goodwill from the acquisition amounts to T€ 2,204. The net loss of Evotec for the three months ended 31 March 2010 included a net loss of T€ 122 from RSIPL and Evotec-RSIL as well as revenues of T€ 781.
|
| |||||||||
| 31 August 2009 carrying amount | 31 August 2009 fair value | |||||||||
| T€ | T€ | |||||||||
Cash and cash equivalents | 137 | 137 | ||||||||
Trade accounts receivable | 277 | 277 | ||||||||
Inventories | 69 | 69 | ||||||||
Other current assets | 465 | 465 | ||||||||
Property, plant and equipment | 2,454 | 2,454 | ||||||||
Customer list | — | 103 | ||||||||
Loans | (504 | ) | (504 | ) | ||||||
Provisions | (74 | ) | (74 | ) | ||||||
Current liabilities | (1,145 | ) | (1,145 | ) | ||||||
Deferred tax liabilities | (52 | ) | (70 | ) | ||||||
Net assets acquired | 1,627 | 1,712 | ||||||||
Non-controlling interests | — | (587 | ) | |||||||
At equity investment Evotec-RSIL | — | (956 | ) | |||||||
Goodwill | — | 2,204 | ||||||||
Cost of acquisition | — | 2,373 | ||||||||
Plus transaction costs | — | 98 | ||||||||
Less cash and cash equivalents acquired | — | (137 | ) | |||||||
Less earn out | — | (748 | ) | |||||||
Cash outflow from acquisition | — | 1,586 | ||||||||
The following unaudited pro forma information is based on the assumption that the investment in RSIPL occurred as of 1 January 2009:
|
| |||||||||
| Three months ended 31 March 2009 | ||||||||||
| T€ | ||||||||||
Pro-forma revenues | 8,894 | |||||||||
Pro-forma net loss | (21,802 | ) | ||||||||
Pro-forma basic and diluted loss per share | (0.20 | ) | ||||||||
5. AUCTION RATE SECURITIES
The auction rate securities (ARSs) acquired in the Renovis acquisition are classified as available-for-sale which are measured at fair value with unrealised gains and losses reported as a component of “Reserve” in stockholders’ equity.
Due to the illiquidity of the ARSs, the Company utilised a discounted cash flow (“DCF”) model to derive an estimate of fair value of these securities at 31 March 2010. The discounted cash flow model includes estimates with respect to the amount and timing of future interest payments, projections of interest rates, and the rate of return required by investors to own such securities given the current liquidity risk associated with the ARSs. As a result, the Company recorded an unrealised gain of T€ 17 in the first quarter of 2010. The current valuation represents a discount of approximately 6% on the par value of the auction rate securities.
|
| |||||||||
EVOTEC – REPORT AS OF FIRST QUARTER 2010
14
6. OTHER NON-CURRENT FINANCIAL ASSETS
Other non-current financial assets as of 31 March 2010 consist primarily of Coupon Bonds in the amount of T€ 3,000 and Put Options related to the ARSs in the amount of T€ 573 (31 December 2009: T€ 550). In accordance with IAS 39, the Put Option is considered to be a derivative and is measured at fair value with gains or losses recorded in the income statement at each period end. The Company, using a DCF model to measure the Put Option, recorded expenses of T€ 12 and T€ 590 in the first quarter of 2010 and 2009, respectively.
7. PROVISIONS
The provisions as of 31 March 2010 in comparison to 31 December 2009 mainly decreased due to the payments for bonuses and severance in the first quarter 2010.
8. DEFERRED REVENUES
The deferred revenues as of 31 March 2010 mainly decreased due to the partial recognition of the Roche upfront payment in connection with the option to buy back rights to the entire EVT 100 family of compounds. The revenue recognition of this upfront payment will be spread over the expected duration of the Phase II study with EVT 101.
FORWARD-LOOKING STATEMENTS
Information set forth in this report contains forward-looking statements, which involve a number of risks and uncertainties. Such forward-looking statements include, but are not limited to, statements about our 2010 financial outlook and our expected financial results in future quarters, our ability to deliver on our liquidity guidance, our belief that we are on course to profitability in 2012, our expectations and assumptions concerning regulatory, clinical and business strategies, the progress of our clinical development programmes and timing of the commencement and results of our clinical trials, strategic collaborations and management’s plans, objectives and strategies. These statements are neither promises nor guarantees, but are subject to a variety of risks and uncertainties, many of which are beyond our control, and which could cause actual results to differ materially from those contemplated in these forward-looking statements. In particular, the risks and uncertainties include, among other things: risks that product candidates may fail in the clinic or may not be successfully marketed or manufactured; the risk that we will not achieve the anticipated benefits of our collaborations, partnerships and acquisitions in the timeframes expected, or at all; risks relating to our ability to advance the development of product candidates currently in the pipeline or in clinical trials; our inability to further identify, develop and achieve commercial success for new products and technologies; the risk that competing products may be more successful; our inability to interest potential partners in our technologies and products; our inability to achieve commercial success for our products and technologies; our inability to protect our intellectual property and the cost of enforcing or defending our intellectual property rights; our failure to comply with regulations relating to our products and product candidates, including FDA requirements; the risk that the FDA may interpret the results of our studies differently than we have; the risk that clinical trials may not result in marketable products; the risk that we may be unable to successfully secure regulatory approval of and market our drug candidates; and risks of new, changing and competitive technologies and regulations in the U.S. and internationally.
The list of risks above is not exhaustive. Our most recent Annual Report on Form 20-F, filed with the Securities and Exchange Commission, and other documents filed with, or furnished to the Securities and Exchange Commission, contain additional factors that could impact our businesses and financial performance. We expressly disclaim any obligation or undertaking to release publicly any updates or revisions to any such statements to reflect any change in our expectations or any change in events, conditions or circumstances on which any such statement is based.
|
EVOTEC – REPORT AS OF FIRST QUARTER 2010
15