Exhibit 99.3

GALENICA
Galenica to acquire Relypsa
Vifor Pharma & relypsa
Conference Call, July 21, 2016, 2 p.m. CET
The Galenica Group - Excellence in the healthcare market

GALENICA
Agenda and speakers
Etienne Jornod
Executive Chairman Galenica Group
Jörg Kneubühler
CFO Galenica Group & CEO Galenica Santé
Gianni Zampieri
Vice-CEO Vifor Pharma
Christoph Springer
Head BD & Licensing & Deputy CEO VFMCRP
Jörg Kneubühler: Welcome
Etienne Jornod: A new strategic step in our long-term journey
Jörg Kneubühler: Financial considerations
Gianni Zampieri: Strategic rationale for Vifor Pharma
Christoph Springer: Overview of Relypsa
Etienne Jornod: Summary
Jörg Kneubühler: Q&A session and closing of the conference
21.07.2016 - © Galenica Group
2

GALENICA
A new strategic step in our long-term journey
Etienne Jornod
Executive Chairman
21.07.2016 - © Galenica Group
3

GALENICA
Acquisition of Relypsa and the global rights to Veltassa®
relypsa
Veltassa®
(patiromer) for oral suspension
21.07.2016 - © Galenica Group
4

GALENICA
The transaction
- Tender offer to acquire all issued and outstanding Relypsa shares
- Price per share: USD 32
- Total investment: ~ USD 1.53 billion
- Financing secured by debt commitments from CS
- Affirmation of the Galenica Board of Directors to realise the division
- Separation of the Galenica Group foreseen in 2017
- Partial refinancing planned through equity proceeds to be raised in conjunction with the separation of the Group (i.e. IPO of Galenica Santé or other option)
21.07.2016 - © Galenica Group
5

GALENICA
Take the plunge…
A fully integrated global pharma company
PATENTED
PATENTED
PATENTED
2030
Veltassa®
(patiromer) for oral suspension
21.07.2016 - © Galenica Group
6

GALENICA
Vifor Pharma strengthened for planned stand-alone
- Secure long-term development
- Drive future growth of Vifor Pharma
- Veltassa® & Ferinject®: 2 products with blockbuster potential…
- …and another decade of patent protection until 2030
- Significant US footprint in Vifor Pharma’s core therapeutic areas nephrology and cardiology
- Fully-integrated commercial organisation in the US
- Leverage synergies with existing and future US brands
21.07.2016 - © Galenica Group
7

GALENICA
Strategy
This is our beautiful story which continues
21.07.2016 - © Galenica Group
8

GALENICA
To build our Vifor Pharma castle…
21.07.2016 - © Galenica Group
9
GALENICA
…we have a vision
21.07.2016 - © Galenica Group
10

GALENICA
…and then, we start to work step by step…
21.07.2016 - © Galenica Group
11

GALENICA
Relypsa is a corner stone
to realise the vision of our pharma castle…
21.07.2016 - © Galenica Group
12

GALENICA
…until it becomes the most beautiful one!
Vifor Pharma
Vifor Fresenius Medical Care
Renal Pharma
relypsa
21.07.2016 - © Galenica Group
13

GALENICA
This is exactly what we have been doing with…
Vifor Pharma
21.07.2016 - © Galenica Group
14

GALENICA
The fundament of the vision: from iron, sugar and water, we can dramatically improve the quality of life of a patient
Popeye eats spinach
SPINACH
He has super power!
21.07.2016 - © Galenica Group
15
GALENICA
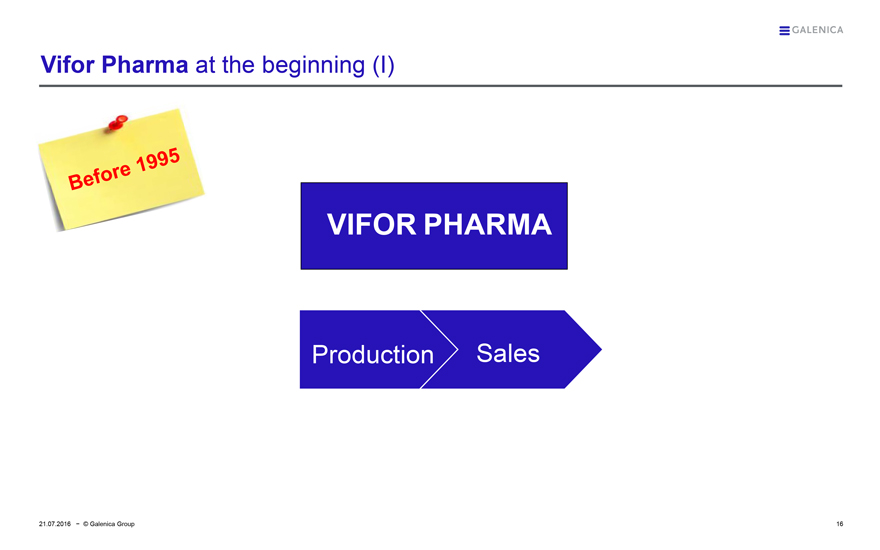
Vifor Pharma at the beginning (I)
Before 1995
VIFOR PHARMA
Production Sales
21.07.2016 - Galenica Group
16
GALENICA
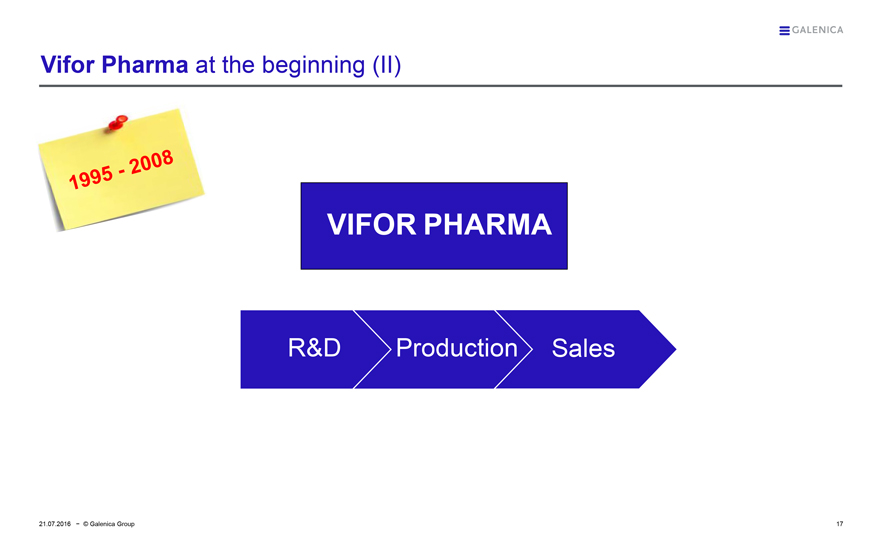
Vifor Pharma at the beginning (II)
1995 - 2008
VIFOR PHARMA
R&D Production Sales
21.07.2016 - Galenica Group
17
GALENICA
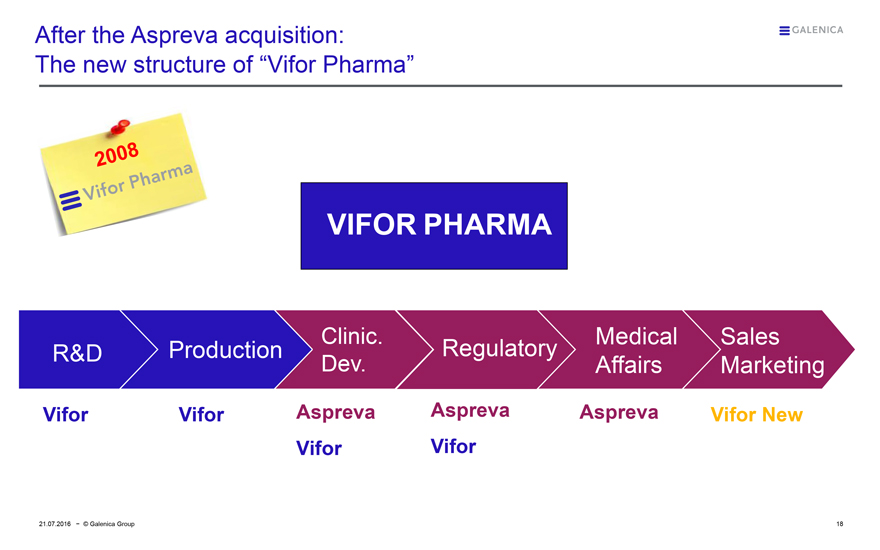
After the Aspreva acquisition: The new structure of “Vifor Pharma”
2008
Vifor Pharma
VIFOR PHARMA
R&D
Production
Clinic. Dev.
Regulatory
Medical Affairs
Sales Marketing
Vifor
Vifor
Aspreva Vifor
Aspreva Vifor
Aspreva
Vifor New
21.07.2016 - Galenica Group
18

GALENICA
Ultimate step: Vifor Pharma sales affiliates and offices
2008 - 2012
- Argentina
- Australia
- Austria
- Belgium
- Canada
- China
- Denmark
- Finland
- France
- Germany
- Ireland
- Italy
- Netherlands
- Norway
- Peru
- Portugal
- Romania
- Russia
- Singapore
- Spain
- Sweden
- Switzerland
- UK
- USA
- UAE
21.07.2016 - Galenica Group
19
GALENICA
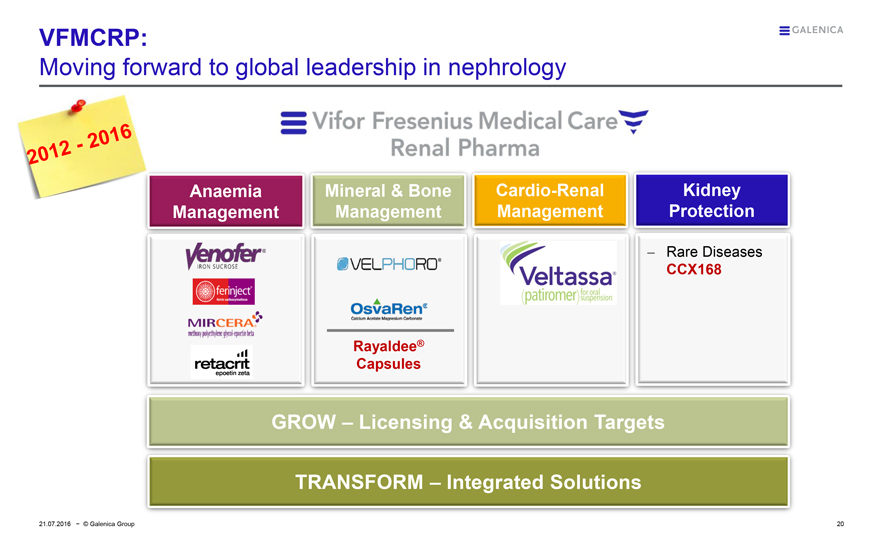
VFMCRP:
Moving forward to global leadership in nephrology
2012 - 2016
Vifor Fresenius Medical Care
Renal Pharma
Anaemia Management
Venofer
IRON SUCROSE
ferinject
ferric carboxymaltose
MIRCERA
Methoxy polyethylene glycol-epoetin beta
Retacrit
epoetin zeta
Mineral & Bone
Management
VELPHORO
OsvaRen
Calcium Acetate Magnesium Carbonate
Rayaldee
Capsules
Cardio-Renal Management
Veltassa
(patiromer) for oral suspension
Kidney Protection
- Rare Diseases
CCX168
GROW – Licensing & Acquisition Targets
TRANSFORM – Integrated Solutions
21.07.2016 - Galenica Group
20

GALENICA
And now!
2016
Relypsa
Veltassa
(patiromer) for oral suspension
21.07.2016 - Galenica Group
21

GALENICA
Let’s build our pharma castle…
21.07.2016 - Galenica Group
22
GALENICA
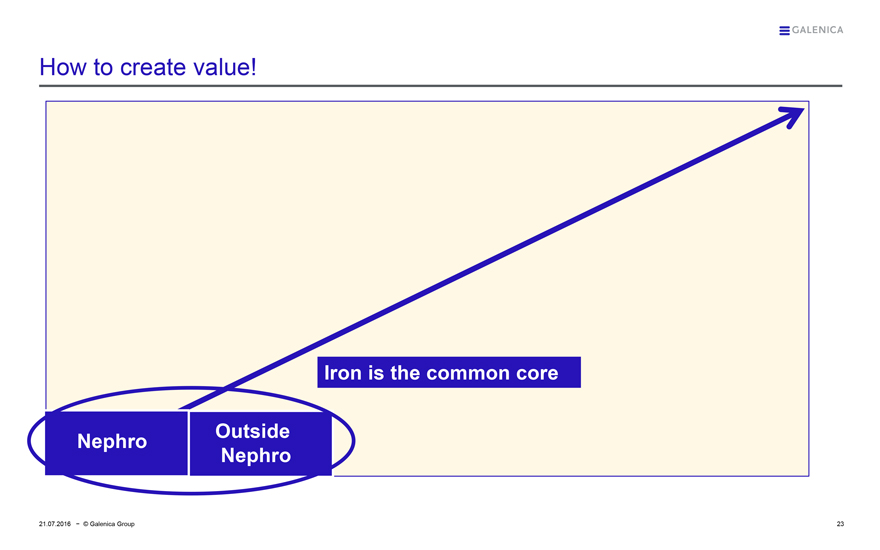
How to create value!
Nephro
Outside Nephro
Iron is the common core
21.07.2016 – © Galenica Group
23
GALENICA
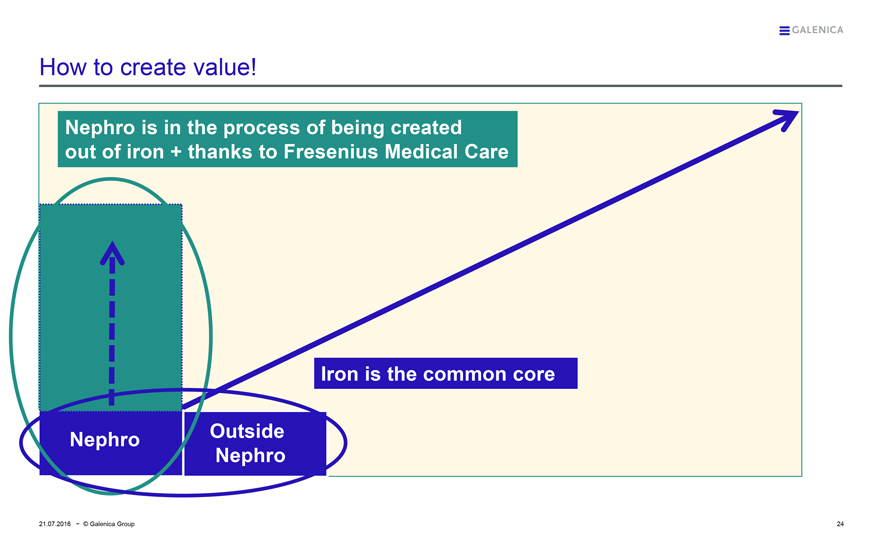
How to create value!
Nephro is in the process of being created out of iron + thanks to Fresenius Medical Care
Nephro
Outside Nephro
Iron is the common core
21.07.2016 – © Galenica Group
24
GALENICA
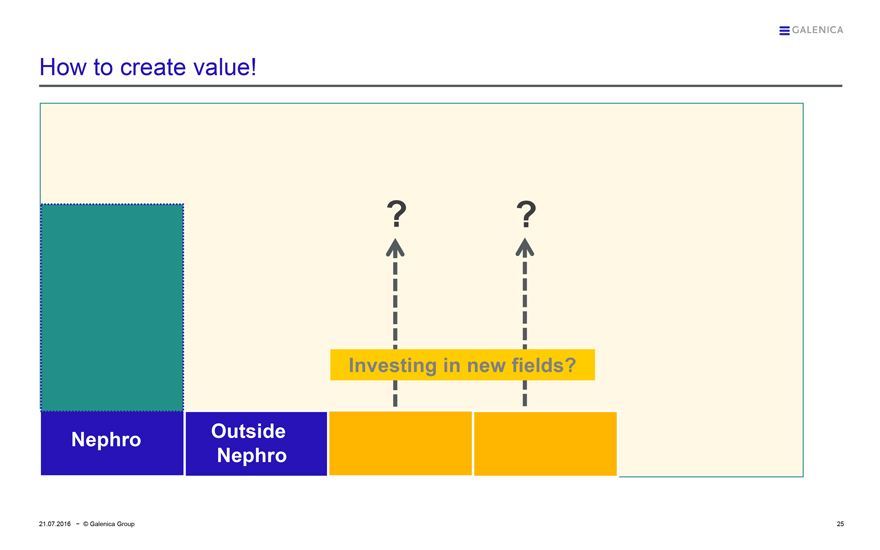
How to create value!
Nephro
Outside Nephro ? ?
Investing in new fields?
21.07.2016 – © Galenica Group
25
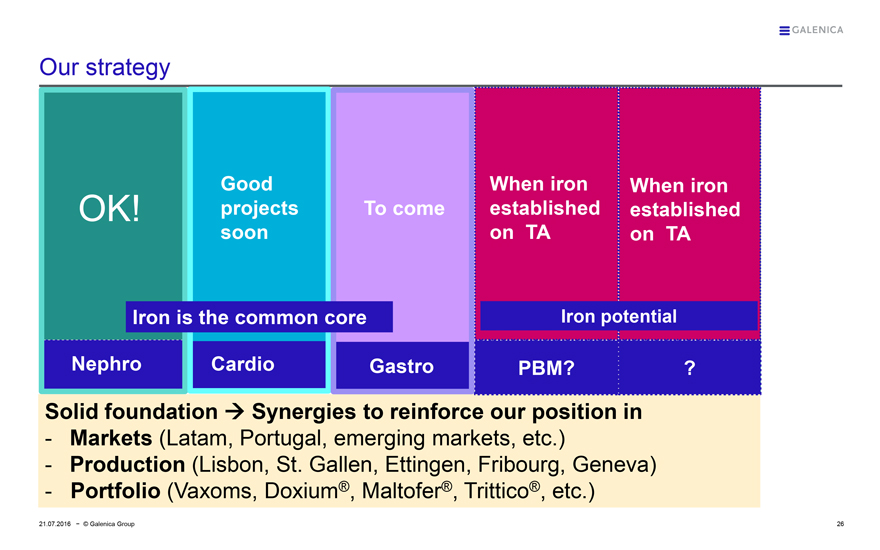
GALENICA
Our strategy
OK!
Good projects soon
To come
When iron established on TA
When iron established on TA
Iron is the common core
Iron potential
Nephro
Cardio
Gastro
PBM?
?
Solid foundationgSynergies to reinforce our position in
- Markets (Latam, Portugal, emerging markets, etc.)
- Production (Lisbon, St. Gallen, Ettingen, Fribourg, Geneva)
- Portfolio (Vaxoms, Doxium®, Maltofer®, Trittico®, etc.)
21.07.2016 – © Galenica Group
26
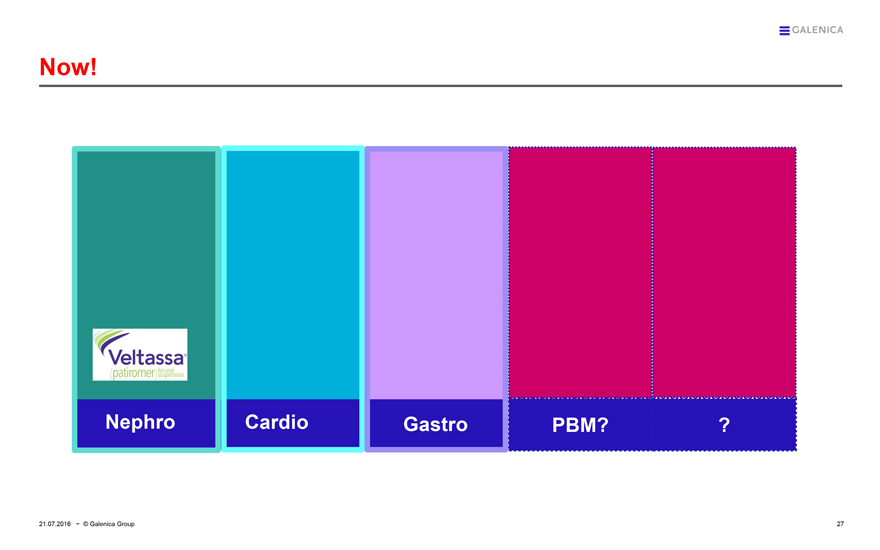
GALENICA
Now!
Veltassa®
(patiromer) for oral suspension
Nephro
Cardio
Gastro
PBM?
?
21.07.2016 – © Galenica Group
27
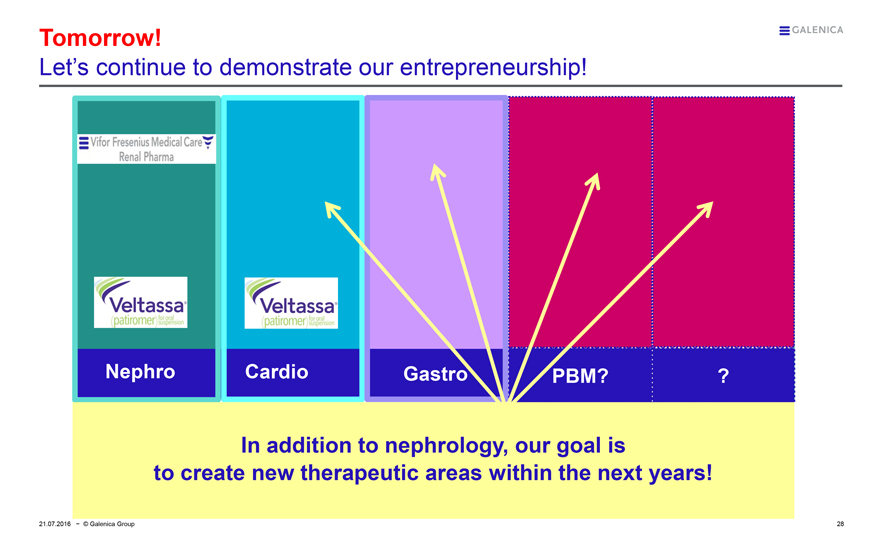
GALENICA
Tomorrow!
Let’s continue to demonstrate our entrepreneurship!
Vifor Fresenius Medical Care
Renal Pharma
Veltassa®
(patiromer) for oral suspension
Veltassa®
(patiromer) for oral suspension
Nephro
Cardio
Gastro
PBM?
?
In addition to nephrology, our goal is to create new therapeutic areas within the next years!
21.07.2016 – © Galenica Group
28

GALENICA
Acquisition of Relypsa
We build on our expertise
1. We have the experience for acquisitions and integrations: Aspreva, VFMCRP, ….
2. We have the US pharma expertise: top executives with US experience
3. We know the target audience (nephrologists and cardiologists) well and how to address them
4. We know how to build new markets for innovative drugs:
g leverage to build the market for chronic hyperkalaemia therapy
5. We have the manufacturing and supply chain expertise:
g securing high volume drugs which will be essential for Veltassa®
21.07.2016 – © Galenica Group
29

GALENICA
NOW!
With the acquisition of Relypsa we are entering into the final stage of the Group separation
21.07.2016 - © Galenica Group
30

GALENICA
NOW!
Intention:
Separation of the Group
into 2 listed companies
in 2017
21.07.2016 - © Galenica Group
31

GALENICA
2 listed companies
Vifor Pharma
Galenica Santé
21.07.2016 - © Galenica Group
32
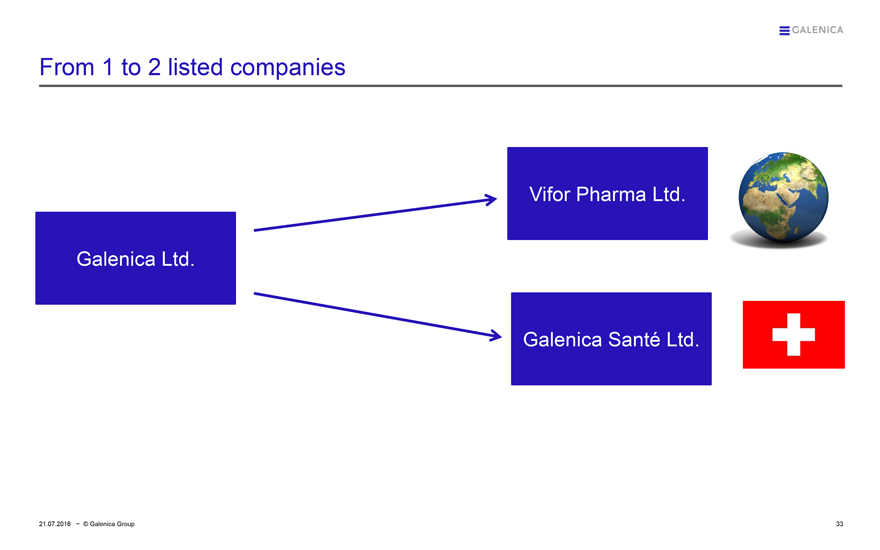
GALENICA
From 1 to 2 listed companies
Galenica Ltd.
Vifor Pharma Ltd.
Galenica Santé Ltd.
21.07.2016 - © Galenica Group
33

GALENICA
Galenica Santé
21.07.2016 - © Galenica Group
34

GALENICA
The largest pharmacy chain in Switzerland
Best locations – 100,000 customers daily – 500 POS
AMAVITA
SUN STORE
coop vitality
MEDI SERVICE
Apotheke Farmacia Pharmacie
Winconcept
Healthy Marketing Solutions
21.07.2016 - © Galenica Group
35

GALENICA
Logistics + Information
Home
INDEX-Produkte
pharmavista
Page Down
21.07.2016 - © Galenica Group
36
GALENICA
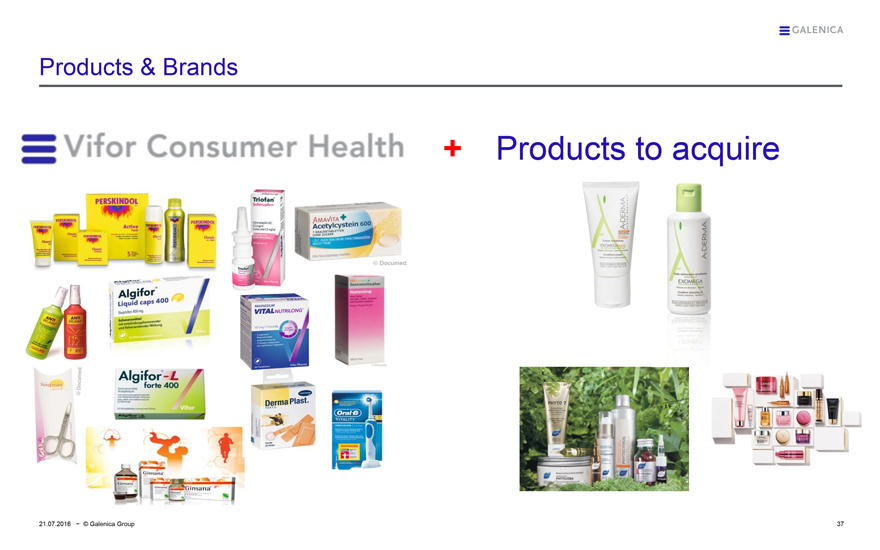
Products & Brands
Vifor Consumer Health + Products to acquire
PERSKINDOL
PERSKINDOL
PERSKINDOL
PERSKINDOL Active 5
PERSKINDOL
PERFORMANCE
PERSKINDOL
Triofan
Triofan
AMAVITA Acetylcystein 600
© Documed
ANTI BRUMM NATUREL
ANTI BRUMM FORTE
Algifor Liquid caps 400
MAGNESIUM VITALNUTRILONG
Dextromethorpan Hustensirup
SUNSTORE
© Documed
Algifor – L forte 400 Vifor
Derma Plast TEXTIL
Oral-B VITALITY
Ginsana
Ginsana
Ginsana
Ginsana
Ginsana
A-DERMA EXOMEGA
A-DERMA EXOMEGA
PHYTO 7
PHYTOJOBA
PHYTO
PHYTOKÉRATINE
PHYTOCITRUS
PHYTO
LIERAC
LIERAC
LIERAC
LIERAC
LIERAC
LIERAC
LIERAC
LIERAC
LIERAC
21.07.2016 – © Galenica Group
37
GALENICA
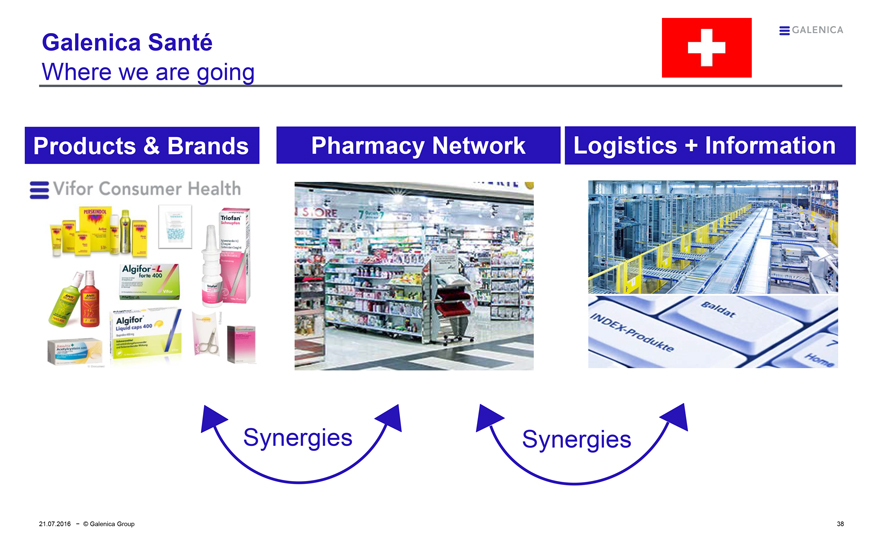
Galenica Santé
Where we are going
Products & Brands
PERSKINDOL
PERSKINDOL
PERSKINDOL
PERSKINDOL Active 5
PERFORMANCE
PERSKINDOL
Triofan Schnupfen
Triofan
Algifor – L forte 400 Vifor
ANTI BRUMM NATUREL
ANTI BRUMM FORTE
Algifor Liquid caps 400
AMAVITA Acetylcystein 600
© Documed
SUNSTORE
© Documed
Vifor Consumer Health
Pharmacy Network
Logistics + Information
galdat
INDEX-Produkte
7 Home
Synergies
Synergies
21.07.2016 – © Galenica Group
38

GALENICA
Galenica Santé
Our ambition
Galenica Santé:
The first choice for health,
beauty and wellbeing.
21.07.2016 – © Galenica Group
39

GALENICA
Galenica Santé
Our responsibility
1. We intend to secure the independence of Galenica Santé on a long-term basis
2. Our mission is to serve all customers in the Swiss healthcare market
3. We want to respect our responsibility in the Swiss healthcare market
4. This responsibility is an important part of the business model of Galenica Santé
5. We are looking for a strategic partner to reach our goal
21.07.2016 – © Galenica Group
40

GALENICA
NOW!
20 years of growth in net profit
have created
trust and share price performance
– © Galenica Group
21.07.2016
41
GALENICA
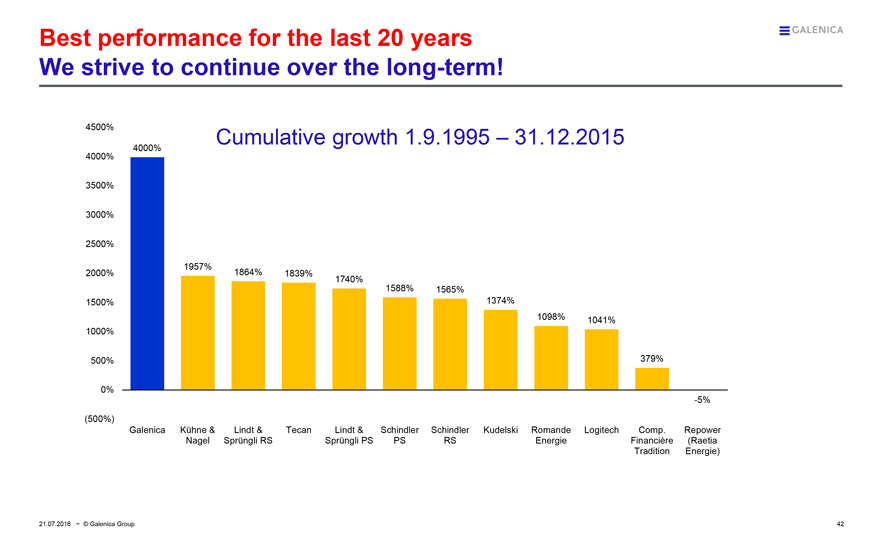
Best performance for the last 20 years
We strive to continue over the long-term!
Cumulative growth 1.9.1995 – 31.12.2015
4000% 1957% 1864% 1839% 1740% 1588% 1565% 1374% 1098% 1041% 379%
4500% 4000% 3500% 3000% 2500% 2000% 1500% 1000% 500% 0%
(500%) -5%
Galenica
Kühne & Nagel
Lindt & Sprüngli RS
Tecan
Lindt & Sprüngli PS
Schindler PS
Schindler RS
Kudelski
Romande Energie
Logitech
Comp. Financière Tradition
Repower (Raetia Energie)
21.07.2016 – © Galenica Group
42
GALENICA
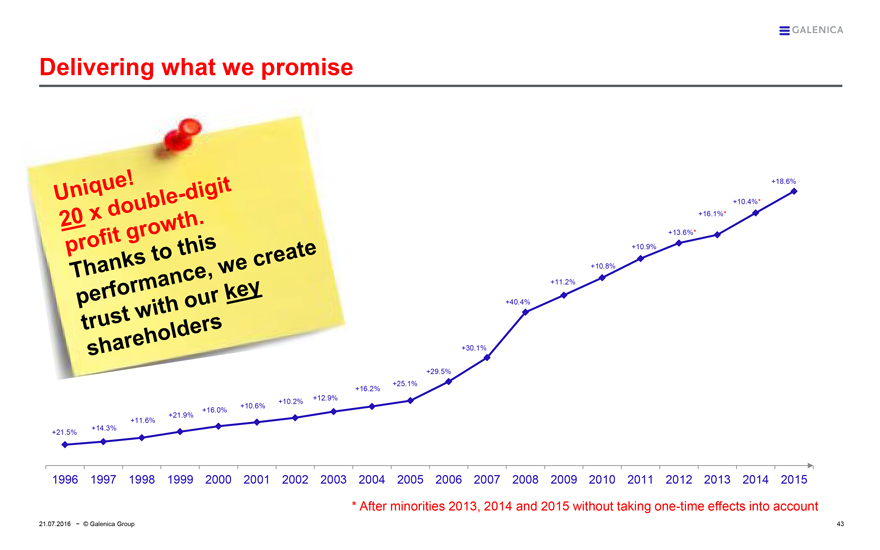
Delivering what we promise
Unique!
20 x double-digit
profit growth.
Thanks to this performance, we create trust with our key shareholders
+21.5% +14.3% +11.6% +21.9% +16.0% +10.6% +10.2% +12.9% +16.2% +25.1% +29.5% +30.1% +40.4% +11.2% +10.8% +10.9% +13.6%* +16.1%* +10.4%* +18.6%
1996 1997 1998 1999 2000 2001 2002 2003 2004 2005 2006 2007 2008 2009 2010 2011 2012 2013 2014 2015
* After minorities 2013, 2014 and 2015 without taking one-time effects into account
21.07.2016 – © Galenica Group
43

GALENICA
Sowing today to harvest tomorrow!
21.07.2016 – © Galenica Group
44
GALENICA
Net profit after tax
We invest now to grow FASTER and LONGER!
21.07.2016 – © Galenica Group 45

GALENICA
Next steps
1. Offer to shareholders
- the Relypsa Board of Directors has resolved to recommend that shareholders accept the offer, once it is commenced
2. Conditions:
- Tender of the majority of all issued and outstanding Relypsa shares
- Expiration of the waiting period under the Hart-Scott-Rodino Antitrust Improvements Act
3. Expected closing of the transaction during Q3 2016
21.07.2016 – © Galenica Group 46

GALENICA
We look forward
to welcoming the Relypsa team that…
relypsa
Veltassa®
(patiromer) for oral suspension
— has developed and launched Veltassa®
— done an excellent job in building a market for chronic hyperkalaemia therapy
— is demonstrating entrepreneurship, team spirit and proven leadership
— brings further expertise and know-how in the cardio- and nephro-space specifically in the US
— has an in-depth market knowledge…
— … which we can leverage for the Veltassa® launch outside the US
21.07.2016 – © Galenica Group 47

GALENICA
Financial considerations
Jörg Kneubühler
CFO Galenica Group
21.07.2016 – © Galenica Group 48
GALENICA
Key terms of transaction
Relypsa
— Tender offer USD 32 per share in cash
— Fully diluted: 47.9 million shares of which 44.8 million basic shares outstanding plus options, RSU’s and warrants
— Implied fully-diluted equity value of the offer approx. USD 1.53 billion
— The agreement is not subject to a financing condition
— Completion anticipated during Q3 2016
— Consolidation expected as of October 2016 -> Guidance
21.07.2016 – © Galenica Group 49
GALENICA
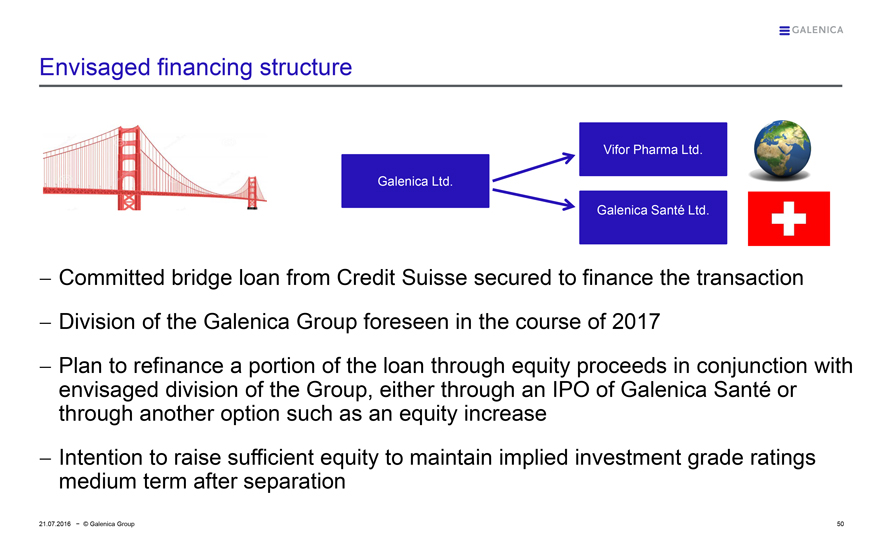
Envisaged financing structure
Galenica Ltd.
Vifor Pharma Ltd.
Galenica Santé Ltd.
— Committed bridge loan from Credit Suisse secured to finance the transaction
— Division of the Galenica Group foreseen in the course of 2017
— Plan to refinance a portion of the loan through equity proceeds in conjunction with envisaged division of the Group, either through an IPO of Galenica Santé or through another option such as an equity increase
— Intention to raise sufficient equity to maintain implied investment grade ratings medium term after separation
21.07.2016 – © Galenica Group 50
GALENICA
Vifor Pharma Guidance 2016
— Former guidance on 2016 EBIT Vifor Pharma raised on a like-for like basis: +10% compared to the previous year.
— Due to significant Veltassa® ramp-up investments, combined 2016 EBIT is expected to reduce by approx. CHF 80 million (incl. depreciation of Intangible Assets PPA).
— Full year 2017 a low triple digit investment is planned in order to drive the continued ramp-up of Veltassa®, continued into 2018, but at a decreasing rate.
— Newly acquired business expected to generate positive EBIT from 2019 onwards, expected to accelerate rapidly up to mid to high three digit numbers in the subsequent years.
Galenica Group: comprehensive guidance update on 9th August 2016 (publication half year results)
21.07.2016 - © Galenica Group 51

GALENICA
Strategic rationale for Vifor Pharma
Gianni Zampieri
Vice-CEO Vifor Pharma
21.07.2016 - © Galenica Group 52
GALENICA
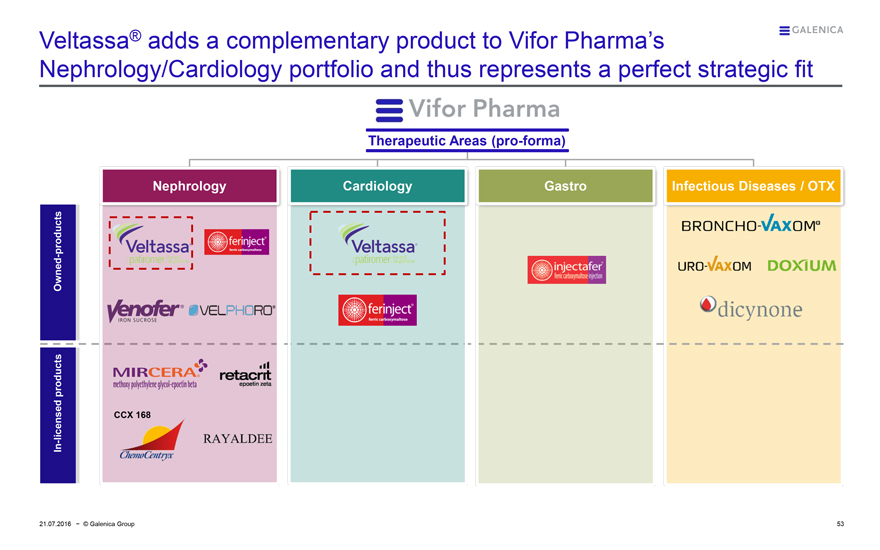
Veltassa® adds a complementary product to Vifor Pharma’s
Nephrology/Cardiology portfolio and thus represents a perfect strategic fit
Vifor Pharma
Therapeutic Areas (pro-forma)
Nephrology Cardiology Gastro Infectious Diseases / OTX
Owned-products
Veltassa®
(patiromer) for oral suspension
ferinject®
ferric carboxymaltose
Venofer®
IRON SUCROSE
VELPHORO®
Veltassa®
(patiromer) for oral suspension
ferinject®
ferric carboxymaltose
injectafer®
ferric carboxymaltose injection
BRONCHO-VAXOM®
URO-VAXOM DOXIUM
dicynone
In-licensed products
MIRCERA®
methoxy polyethylene glycol-epoetin beta
retacrit
epoetin zeta
CCX 168 ChemoCentryx RAYALDEE
21.07.2016 - © Galenica Group 53

5GALENICA
Gain global product rights and access to own US commercial organization
— Gain full global rights to potassium binder Veltassa® (patiromer)
— Opportunity to expand Vifor Pharma’s commercial organization to the US
— Significantly strengthens its presence in the nephrology and cardiology market – a key area of focus for Vifor Pharma
US & Japan Veltassa® (patiromer) rights to be received through the acquisition of Relypsa
Ex-US & Japan existing commercial agreement for Patiromer with VFMCRP since August 2015
21.07.2016 - © Galenica Group 54
GALENICA
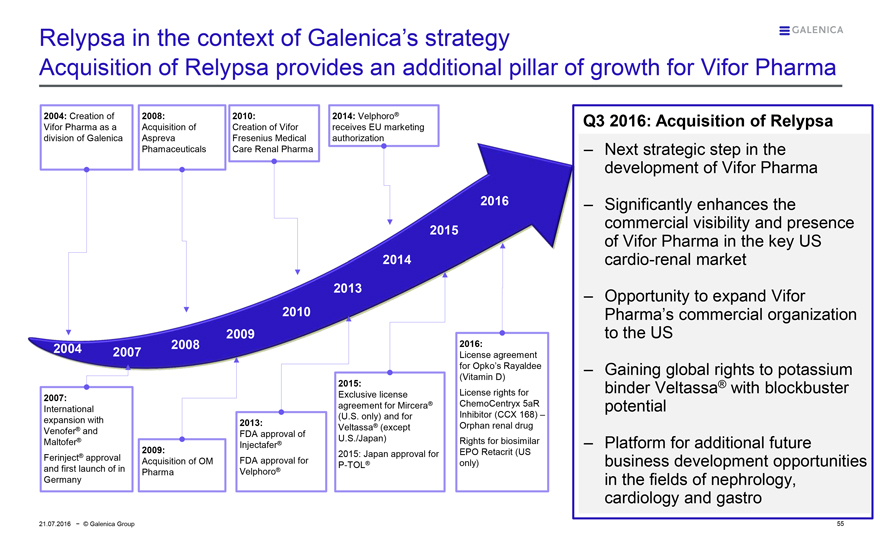
Relypsa in the context of Galenica’s strategy
Acquisition of Relypsa provides an additional pillar of growth for Vifor Pharma
2004: Creation of Vifor Pharma as a division of Galenica
2008: Acquisition of Aspreva Phamaceuticals
2010: Creation of Vifor Fresenius Medical Care Renal Pharma
2014: Velphoro® receives EU marketing authorization
2016
2015
2014
2013
2010
2009
2004 2007 2008
2007: International expansion with Venofer® and Maltofer® Ferinject® approval and first launch of in Germany
2009: Acquisition of OM Pharma
2013: FDA approval of Injectafer® FDA approval for Velphoro®
2015: Exclusive license agreement for Mircera® (U.S. only) and for Veltassa® (except U.S./Japan) 2015: Japan approval for P-TOL®
2016: License agreement for Opko’s Rayaldee (Vitamin D)
License rights for ChemoCentryx 5aR Inhibitor (CCX 168) – Orphan renal drug Rights for biosimilar EPO Retacrit (US only)
Q3 2016: Acquisition of Relypsa
— Next strategic step in the development of Vifor Pharma
— Significantly enhances the commercial visibility and presence of Vifor Pharma in the key US cardio-renal market
— Opportunity to expand Vifor Pharma’s commercial organization to the US
— Gaining global rights to potassium binder Veltassa® with blockbuster potential
— Platform for additional future business development opportunities in the fields of nephrology, cardiology and gastro
21.07.2016 - © Galenica Group 55

GALENICA
Overview of Relypsa
Christoph Springer
Head BD & Licensing & Deputy CEO VFMCRP
21.07.2016 - © Galenica Group 56
GALENICA
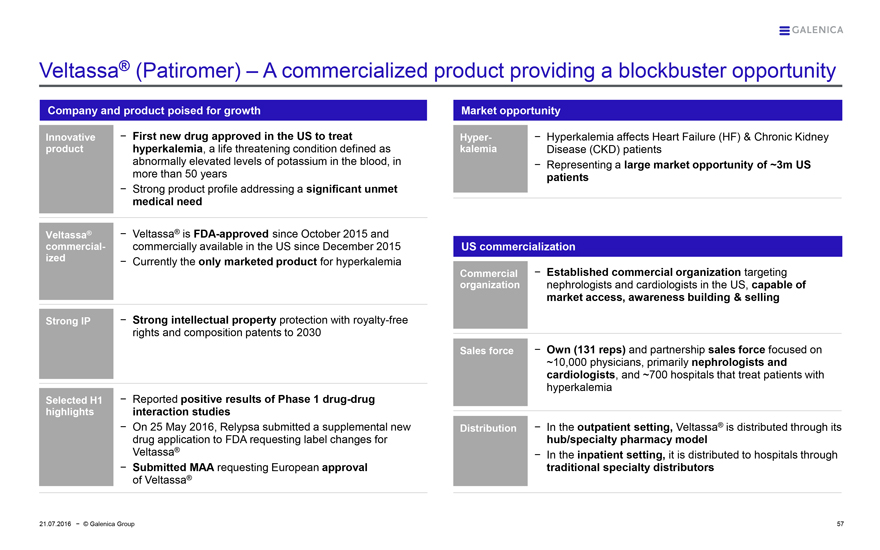
Veltassa® (Patiromer) – A commercialized product providing a blockbuster opportunity
Company and product poised for growth
Innovative product
– First new drug approved in the US to treat hyperkalemia, a life threatening condition defined as abnormally elevated levels of potassium in the blood, in more than 50 years
– Strong product profile addressing a significant unmet medical need
Veltassa® commercialized
– Veltassa® is FDA-approved since October 2015 and commercially available in the US since December 2015
– Currently the only marketed product for hyperkalemia
Strong IP
– Strong intellectual property protection with royalty-free rights and composition patents to 2030
Selected H1 highlights
– Reported positive results of Phase 1 drug-drug interaction studies
– On 25 May 2016, Relypsa submitted a supplemental new drug application to FDA requesting label changes for Veltassa®
– Submitted MAA requesting European approval of Veltassa®
Market opportunity
Hyper-kalemia
– Hyperkalemia affects Heart Failure (HF) & Chronic Kidney Disease (CKD) patients
– Representing a large market opportunity of ~3m US patients
US commercialization
Commercial organization
– Established commercial organization targeting nephrologists and cardiologists in the US, capable of market access, awareness building & selling
Sales force
– Own (131 reps) and partnership sales force focused on ~10,000 physicians, primarily nephrologists and cardiologists, and ~700 hospitals that treat patients with hyperkalemia
Distribution
– In the outpatient setting, Veltassa® is distributed through its hub/specialty pharmacy model
– In the inpatient setting, it is distributed to hospitals through traditional specialty distributors
21.07.2016 - © Galenica Group 57

GALENICA
Vifor Pharma intends to retain Relypsa’s highly experienced leadership team
Executive Management
John A. Orwin
President and CEO
– John joined Relypsa in June of 2013 bringing nearly 25 years of experience in the biotechnology and pharmaceutical industries
– Prior to Relypsa, Mr. Orwin served as chief executive officer and a member of Affymax’s board of directors starting in February 2011
Stephen D. Harrison
Senior Vice President and Chief Scientific Officer
– Stephen joined Relypsa in December 2014 with 20 years’ tenure in biotechnology and pharmaceutical discovery and development. A biochemist and molecular biologist, he is highly published and has extensive experience leading product-driven research organizations at all stages, from target identification to early clinical development
Scott Garland
Senior Vice President and Chief Commercial Officer
– Scott Garland joined Relypsa in October 2014 with over 20 years of biotechnology and pharmaceutical experience
– He has extensive expertise in launching products and building commercial infrastructure to support specialty drugs, including within the nephrology community
Kristine M. Ball
Senior Vice President and Chief Financial Officer
– Kristine M. Ball has served as senior vice president and chief financial officer of Relypsa since November 2012
– Before joining Relypsa, she was an independent consultant, advising start-up life science companies on various strategic and operational business matters
Lance Berman
Senior Vice President and Chief Medical Officer
– Lance joined Relypsa in December 2011 as Senior Vice President, Commercial Strategy and Medical Affairs and was promoted in October 2012 to Senior Vice President and Chief Medical Officer
Wilhelm Stahl
Senior Vice President, Chief Technology Officer
– Wilhelm joined Relypsa in September 2011. Previously, he was a Managing Partner of Rondaxe Enterprises, providing consulting services and strategic advice on CMC aspects of drug development, including supply chain management and strategic business support
21.07.2016 - © Galenica Group 58
GALENICA
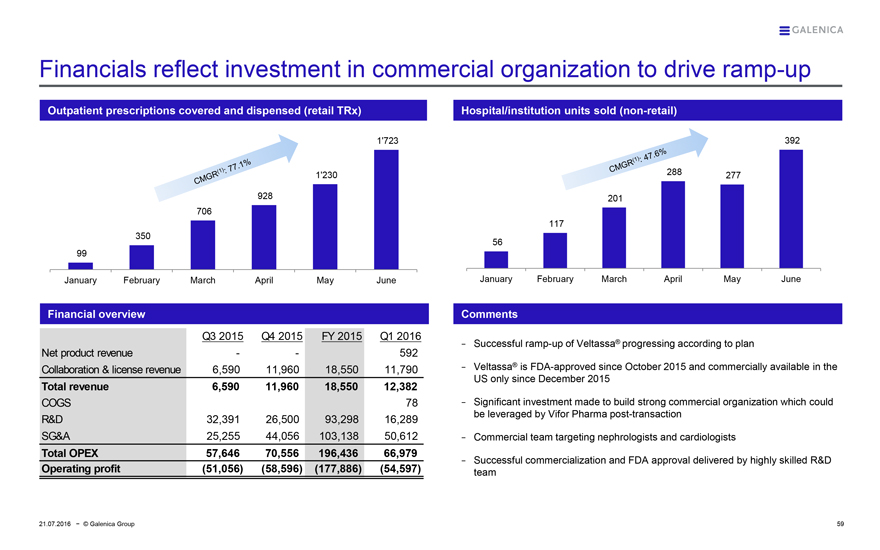
Financials reflect investment in commercial organization to drive ramp-up
Outpatient prescriptions covered and dispensed (retail TRx)
CMGR(1): 77.1%
1’723
1’230
928
706
350
99
January February March April May June
Financial overview
Q3 2015 Q4 2015 FY 2015 Q1 2016
Net product revenue - - 592
Collaboration & license revenue 6,590 11,960 18,550 11,790
Total revenue 6,590 11,960 18,550 12,382
COGS 78
R&D 32,391 26,500 93,298 16,289
SG&A 25,255 44,056 103,138 50,612
Total OPEX 57,646 70,556 196,436 66,979
Operating profit (51,056) (58,596) (177,886) (54,597)
Hospital/institution units sold (non-retail)
CMGR(1): 47.6%
392
288 277
201
117
56
January February March April May June
Comments
- Successful ramp-up of Veltassa® progressing according to plan
- Veltassa® is FDA-approved since October 2015 and commercially available in the US only since December 2015
- Significant investment made to build strong commercial organization which could be leveraged by Vifor Pharma post-transaction
- Commercial team targeting nephrologists and cardiologists
- Successful commercialization and FDA approval delivered by highly skilled R&D team
21.07.2016 - © Galenica Group 59
GALENICA
A new strategic step in our long-term story:
Summary
Etienne Jornod
Executive Chairman
21.07.2016 - © Galenica Group 60
GALENICA
Q&A session
And now we look forward to your questions:
Etienne Jornod Executive Chairman Galenica Group
Jörg Kneubühler CFO Galenica Group & CEO Galenica Santé
Gianni Zampieri Vice-CEO Vifor Pharma
Christoph Springer Head BD & Licensing & Deputy CEO VFMCRP
21.07.2016 - © Galenica Group 61

GALENICA
Galenica Group
Contact information
– Jörg Kneubühler
CFO Galenica Group & CEO Galenica Santé
– Gianni Zampieri
Vice-CEO Vifor Pharma
Through:
– Investor Relations Media Relations
Julien Vignot Christina Hertig
Head Investor Relations Head Corporate Communication
Tel.: +41 58 852 85 29 Tel.: +41 58 852 85 17
Mail: investors@galenica.com Mail: media@galenica.com
21.07.2016 - © Galenica Group 62

GALENICA
Important Safety Information
The Prescribing Information for Veltassa includes a Boxed Warning that Veltassa binds to many other orally administered medications, which could decrease their absorption and reduce their effectiveness. Other oral medications should be administered at least 6 hours before or 6 hours after Veltassa. Doctors should choose Veltassa or the other oral medication if adequate dosing separation is not possible.
Contraindications: Veltassa is contraindicated in patients with a history of a hypersensitivity reaction to Veltassa or any of its components.
Worsening of Gastrointestinal Motility: Use of Veltassa should be avoided in patients with severe constipation, bowel obstruction or impaction, including abnormal post-operative bowel motility disorders, because Veltassa may be ineffective and may worsen gastrointestinal conditions. Patients with a history of bowel obstruction or major gastrointestinal surgery, severe gastrointestinal disorders, or swallowing disorders were not included in clinical studies. Hypomagnesemia: Veltassa binds to magnesium in the colon, which can lead to hypomagnesemia. In clinical studies, hypomagnesemia was reported as an adverse reaction in 5.3 percent of patients treated with Veltassa. Approximately 9 percent of patients in clinical trials developed hypomagnesemia with a serum magnesium value <1.4 mg/dL. Doctors should monitor serum magnesium and consider magnesium supplementation in patients who develop low serum magnesium levels.
Adverse Reactions: The most common adverse reactions (incidence³2 percent) were constipation, hypomagnesemia, diarrhea, nausea, abdominal discomfort and flatulence. Mild to moderate hypersensitivity reactions were reported in 0.3 percent of patients treated with Veltassa and included edema of the lips. For additional Important Safety Information and Veltassa’s full Prescribing Information, please visit www.relypsa.com/veltassa/prescribing-information.
21/07/2016 - © Galenica Group 63

GALENICA
Forward Looking Statements
The statements included in this presentation contain forward-looking statements, which are generally statements that are not historical facts. Forward-looking statements can be identified by the words “expects,” “anticipates,” “believes,” “intends,” “estimates,” “plans,” “will,” “outlook” and similar expressions. Forward-looking statements are based on management’s current plans, estimates, assumptions and projections, speak only as of the date they are made and include without limitation statements regarding the planned completion of the tender offer and the merger, statements regarding the anticipated filings and approvals relating to the tender offer and the merger, statements regarding the expected completion of the tender offer and the merger and statements regarding the ability of Vifor Pharma USA Inc. to complete the tender offer and the merger considering the various closing conditions. Galenica and Relypsa undertake no obligation to update any forward-looking statement in light of new information or future events, except as otherwise required by law. Forward-looking statements involve inherent risks and uncertainties, most of which are difficult to predict and are generally beyond the control of either company, including the following: (a) the occurrence of any event, change or other circumstance that could give rise to the termination of the merger agreement; (b) the inability to complete the transaction due to the failure to satisfy conditions to the transaction; (c) the risk that the proposed transaction disrupts current plans and operations; (d) difficulties or unanticipated expenses in connection with integrating Relypsa into Galenica; (e) the risk that the acquisition does not perform as planned; and (f) potential difficulties in employee retention following the closing of the transaction. Actual results or outcomes may differ materially from those implied by the forward-looking statements as a result of the impact of a number of factors, many of which are discussed in more detail in the public reports of each company filed or to be filed with the SEC or the SIX Swiss Exchange.
21/07/2016 - © Galenica Group 64

GALENICA
Additional Information
This presentation and the description contained herein is for informational purposes only and is not a recommendation, an offer to buy, or the solicitation of an offer to sell any shares of Relypsa’s common stock. The tender offer referenced in this presentation has not commenced. Upon commencement of the tender offer, Galenica and its indirect wholly owned subsidiary, Vifor Pharma USA Inc., will file with the U.S. Securities and Exchange Commission (the “SEC”) a Tender Offer Statement on Schedule TO containing an offer to purchase (the “Offer to Purchase”), a form of letter of transmittal (the “Letter of Transmittal”) and other related documents and, thereafter, Relypsa will file with the SEC a Solicitation/Recommendation Statement on Schedule 14D-9 with respect to the tender offer. Galenica, Vifor Pharma USA Inc. and Relypsa intend to mail these documents to the shareholders of Relypsa. THESE DOCUMENTS, AS EACH MAY BE AMENDED OR SUPPLEMENTED FROM TIME TO TIME, WILL CONTAIN IMPORTANT INFORMATION ABOUT THE TENDER OFFER AND RELYPSA SHAREHOLDERS ARE URGED TO READ THEM CAREFULLY WHEN THEY BECOME AVAILABLE. Shareholders of Relypsa will be able to obtain a free copy of these documents (when they become available) and other documents filed by Relypsa, Galenica or Vifor Pharma USA Inc. with the SEC at the website maintained by the SEC at www.sec.gov. In addition, shareholders of Relypsa may obtain a free copy of these documents (when they become available) by (i) contacting Mackenzie Partners, Inc., the information agent for the tender offer, toll-free at 1-800-322-2885, or call collect +1-212-929-5500 or by email to tenderoffer@mackenziepartners.com or (ii) visiting the “Investors” section of Relypsa’s website at http://investor.relypsa.com.
21/07/2016 - © Galenica Group 65
































































