|
Exhibit 99.4 
|
EXHIBIT 99.4
Trust in excellence
Colin Bond
CFO Vifor Pharma
Vifor Pharma, a company of the Galenica Group
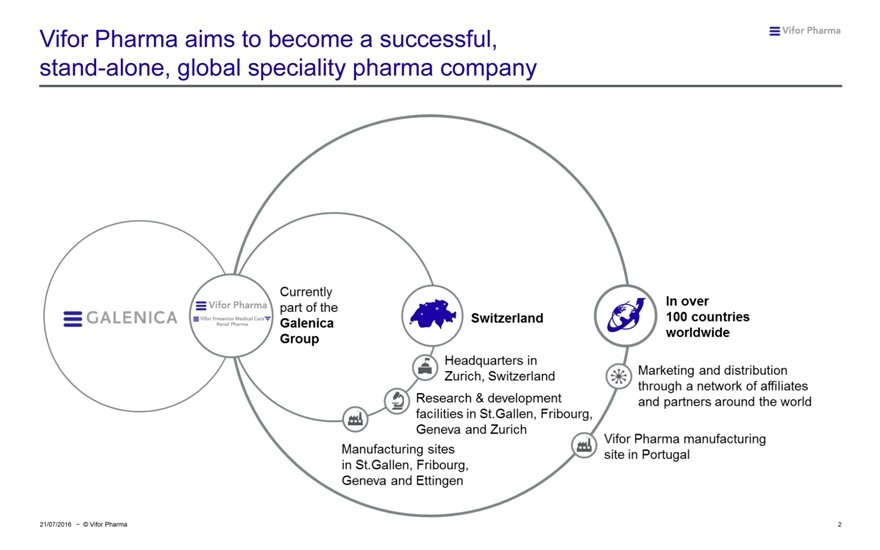
Vifor Pharma aims to become a successful, stand-alone, global speciality pharma company
21/07/2016— © Vifor Pharma 2
VIFOR PHARMA GALENICA CURRENTLY PART OF THE GALENLCA GROUP SWITZERLAND HEADQUARTERS IN ZURICH, SWITZERLAND REASERACH & DEVELOPMENT FACILITIES IN ST. Gallen, Fribourg, geneva and zurich manufacturing sites in st. gallen, Fribourg, geneva and ettingen in over 100 countries worldwide marketing and distribution through a network of affillates and partners around the world vifor pharma manufacturing site in portugal
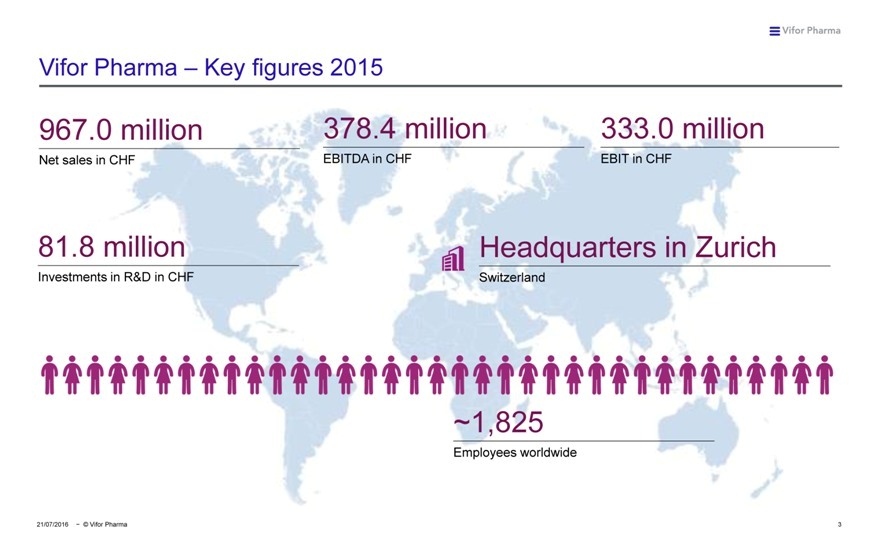
Vifor Pharma – Key figures 2015
967.0 million 378.4 million 333.0 million
Net sales in CHF EBITDA in CHF EBIT in CHF
81.8 million Headquarters in Zurich
Investments in R&D in CHF Switzerland
~1,825
Employees worldwide
21/07/2016— © Vifor Pharma 3
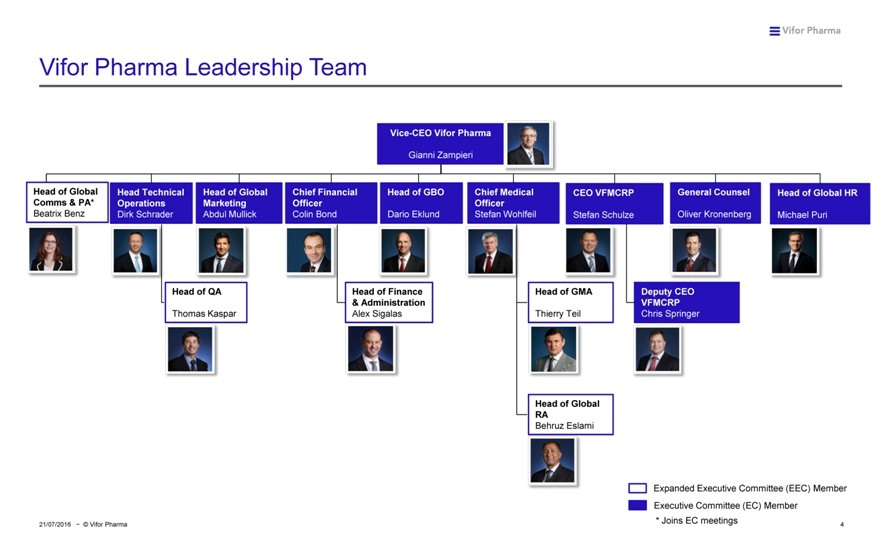
Vifor Pharma Leadership Team
Vice-CEO Vifor Pharma
Gianni Zampieri
Head of Global Head Technical Head of Global Chief Financial Head of GBO Chief Medical CEO VFMCRP General Counsel Head of Global HR Comms & PA* Operations Marketing Officer Officer
Beatrix Benz Dirk Schrader Abdul Mullick Colin Bond Dario Eklund Stefan Wohlfeil Stefan Schulze Oliver Kronenberg Michael Puri
Head of QA Head of Finance Head of GMA Deputy CEO
& Administration VFMCRP
Thomas Kaspar Alex Sigalas Thierry Teil Chris Springer
Head of Global RA
Behruz Eslami
Expanded Executive Committee (EEC) Member Executive Committee (EC) Member
* Joins EC meetings
21/07/2016— © Vifor Pharma 4
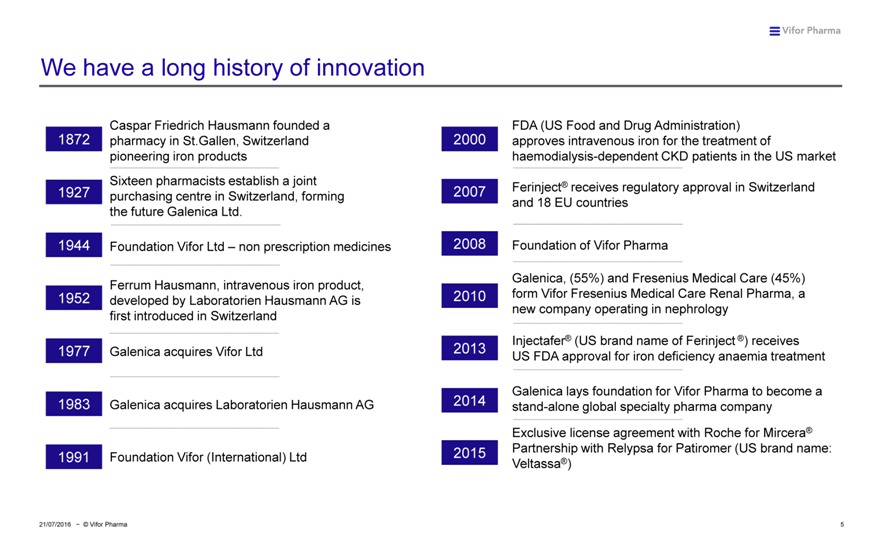
We have a long history of innovation
Caspar Friedrich Hausmann founded a FDA (US Food and Drug Administration) 1872 pharmacy in St.Gallen, Switzerland 2000 approves intravenous iron for the treatment of pioneering iron products haemodialysis-dependent CKD patients in the US market Sixteen pharmacists establish a joint ® 1927 2007 Ferinject receives regulatory approval in Switzerland purchasing centre in Switzerland, forming and 18 EU countries the future Galenica Ltd.
1944 Foundation Vifor Ltd – non prescription medicines 2008 Foundation of Vifor Pharma
Galenica, (55%) and Fresenius Medical Care (45%) Ferrum Hausmann, intravenous iron product, 1952 2010 form Vifor Fresenius Medical Care Renal Pharma, a developed by Laboratorien Hausmann AG is new company operating in nephrology first introduced in Switzerland Injectafer® (US brand name of Ferinject ®) receives 1977 Galenica acquires Vifor Ltd 2013 US FDA approval for iron deficiency anaemia treatment
Galenica lays foundation for Vifor Pharma to become a 1983 Galenica acquires Laboratorien Hausmann AG 2014 stand-alone global specialty pharma company Exclusive license agreement with Roche for Mircera® Partnership with Relypsa for Patiromer (US brand name: 1991 Foundation Vifor (International) Ltd 2015 Veltassa®)
21/07/2016— © Vifor Pharma 5
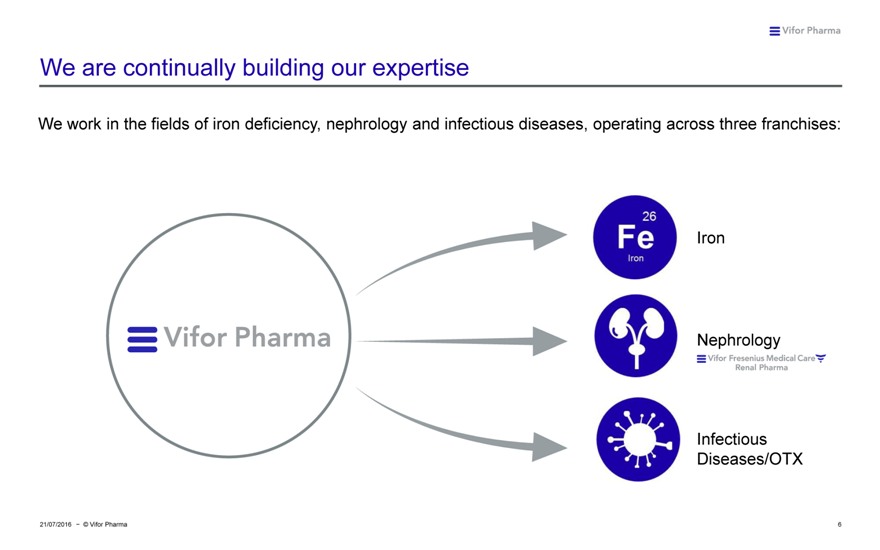
We are continually building our expertise
We work in the fields of iron deficiency, nephrology and infectious diseases, operating across three franchises: Iron Nephrology
Infectious
Diseases/OTX
21/07/2016— © Vifor Pharma 6
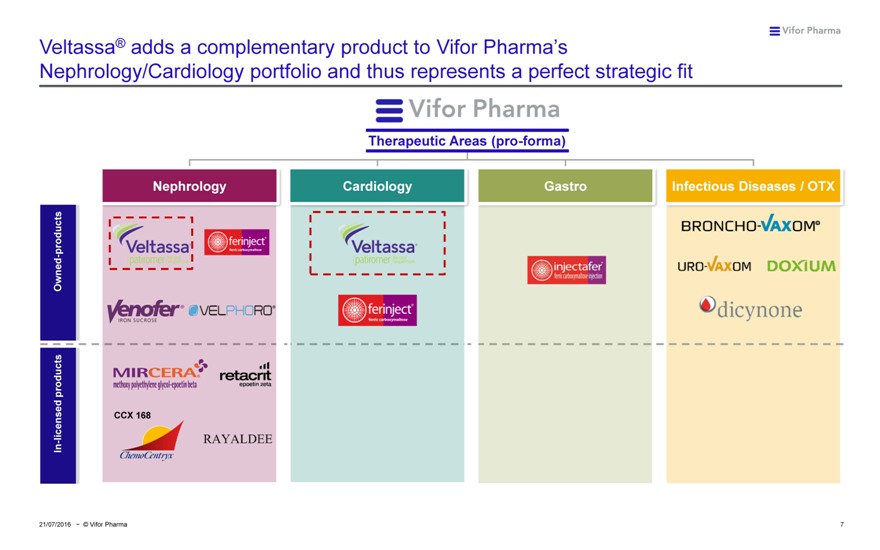
Veltassa® adds a complementary product to Vifor Pharma’s
Nephrology/Cardiology portfolio and thus represents a perfect strategic fit
Therapeutic Areas (pro-forma)
Nephrology Cardiology Gastro Infectious Diseases / OTX
products Owned -
products licensed CCX 168 In -
21/07/2016— © Vifor Pharma 7
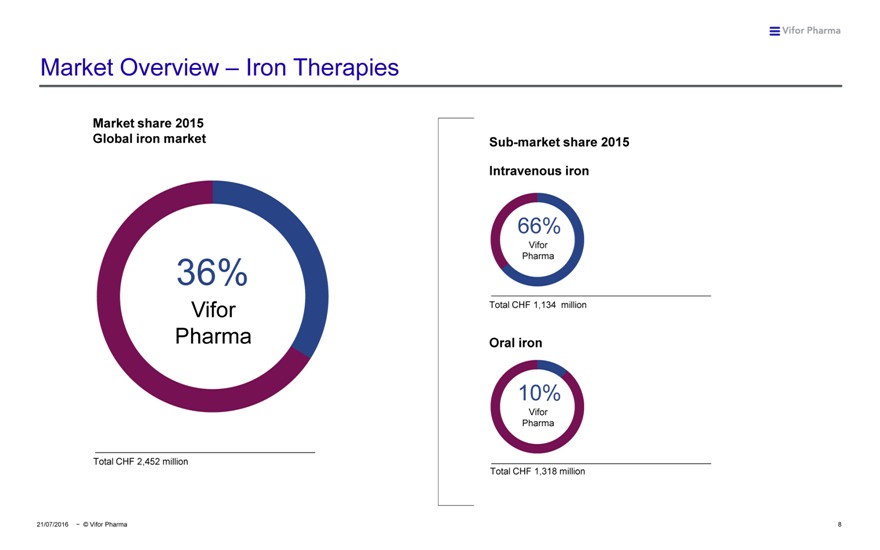
Market Overview – Iron Therapies
Market share 2015
Global iron market Sub-market share 2015 Intravenous iron
66%
Vifor 36% Pharma
Fresenius
Total CHF 1,134 million
Vifor Medical Care
Pharma Oral iron
10%
Vifor Pharma
Total CHF 2,452 million
Total CHF 1,318 million
21/07/2016— © Vifor Pharma 8
We supply leading brands for iron therapy
Intravenous Oral iron iron products product
21/07/2016— © Vifor Pharma 9
We are building a leading portfolio in the field of nephrology with future opportunities in many areas
Nephrology portfolio
21/07/2016— © Vifor Pharma 10
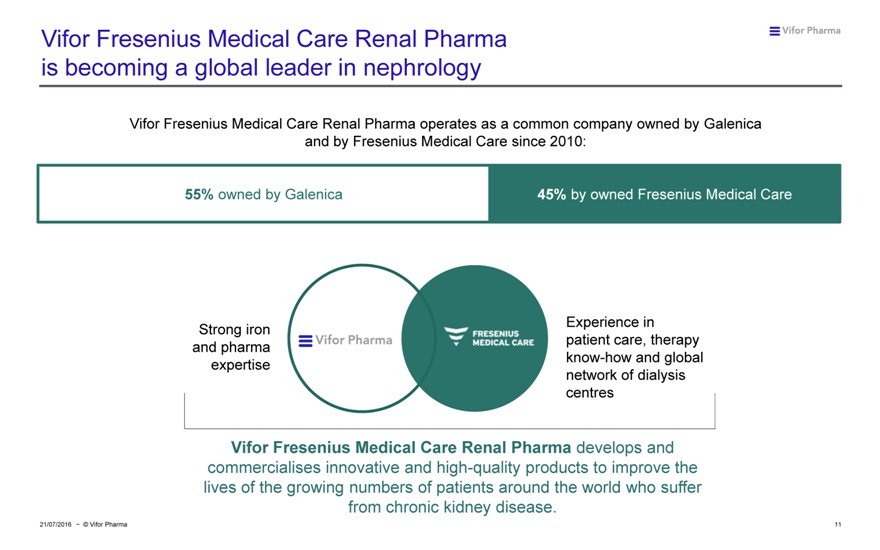
Vifor Fresenius Medical Care Renal Pharma is becoming a global leader in nephrology
Vifor Fresenius Medical Care Renal Pharma operates as a common company owned by Galenica and by Fresenius Medical Care since 2010:
55% owned by Galenica 45% by owned Fresenius Medical Care
Experience in Strong iron patient care, therapy and pharma know-how and global expertise network of dialysis centres
Vifor Fresenius Medical Care Renal Pharma develops and commercialises innovative and high-quality products to improve the lives of the growing numbers of patients around the world who suffer from chronic kidney disease.
21/07/2016— © Vifor Pharma 11
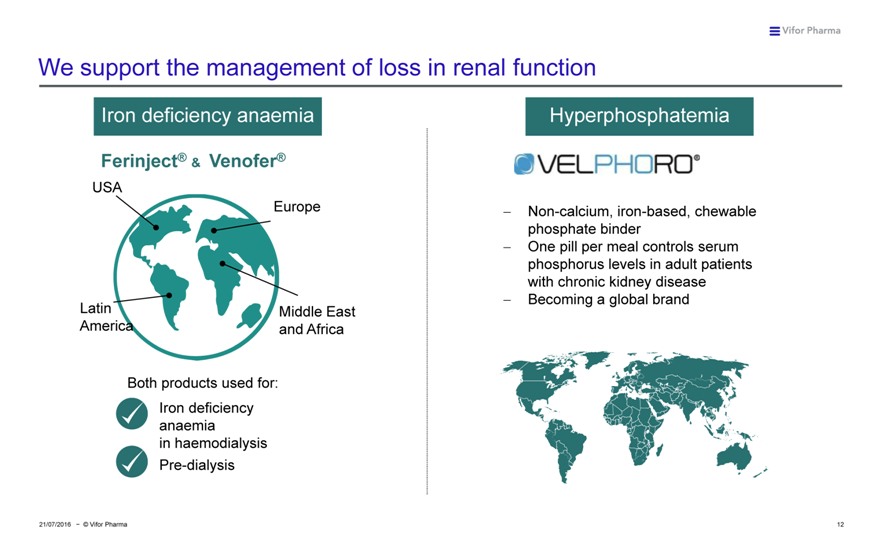
We support the management of loss in renal function
Iron deficiency anaemia Hyperphosphatemia
Ferinject® & Venofer®
USA
Europe ??Non-calcium, iron-based, chewable phosphate binder ??One pill per meal controls serum phosphorus levels in adult patients with chronic kidney disease ??Becoming a global brand Latin Middle East America and Africa
Both products used for: Frese
nius
Iron deficiency Medic anaemia al
Care
in haemodialysis Pre-dialysis
21/07/2016— © Vifor Pharma 12
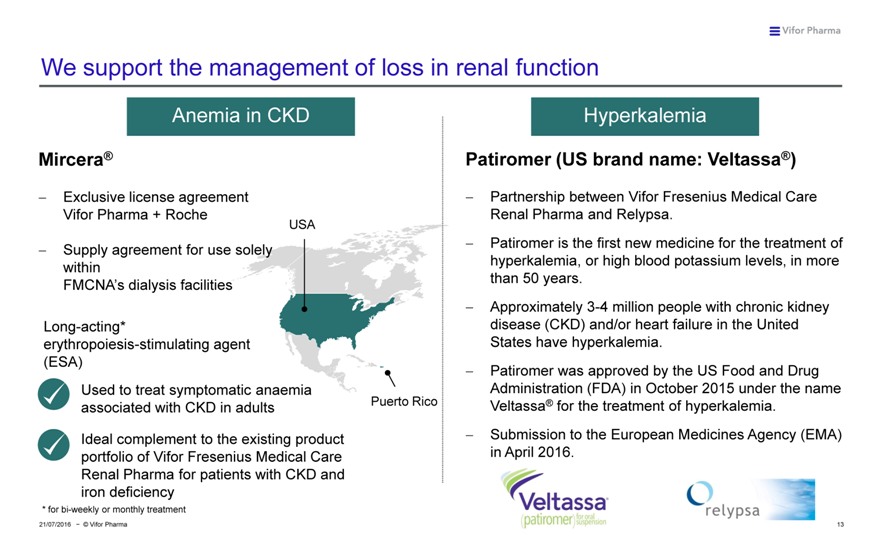
We support the management of loss in renal function
Anemia in CKD Hyperkalemia
Mircera® Patiromer (US brand name: Veltassa®)
??Exclusive license agreement ??Partnership between Vifor Fresenius Medical Care Vifor Pharma + Roche Renal Pharma and Relypsa.
USA
???Patiromer is the first new medicine for the treatment of Supply agreement for use solely hyperkalemia, or high blood potassium levels, in more within than 50 years.
FMCNA’s dialysis facilities
??Approximately 3-4 million people with chronic kidney Long-acting* disease (CKD) and/or heart failure in the United erythropoiesis-stimulating agent States have hyperkalemia.
(ESA)
??Patiromer was approved by the US Food and Drug Used to treat symptomatic anaemia Administration (FDA) in October 2015 under the name associated with CKD in adults Puerto Rico Veltassa® for the treatment of hyperkalemia.
Ideal complement to the existing product ??Submission to the European Medicines Agency (EMA) portfolio of Vifor Fresenius Medical Care in April 2016.
Renal Pharma for patients with CKD and iron deficiency
* for bi-weekly or monthly treatment
21/07/2016— © Vifor Pharma 13
Important Safety Information
The Prescribing Information for Veltassa includes a Boxed Warning that Veltassa binds to many other orally administered medications, which could decrease their absorption and reduce their effectiveness. Other oral medications should be administered at least 6 hours before or 6 hours after Veltassa. Doctors should choose Veltassa or the other oral medication if adequate dosing separation is not possible.
Contraindications: Veltassa is contraindicated in patients with a history of a hypersensitivity reaction to Veltassa or any of its components.
Worsening of Gastrointestinal Motility: Use of Veltassa should be avoided in patients with severe constipation, bowel obstruction or impaction, including abnormal post-operative bowel motility disorders, because Veltassa may be ineffective and may worsen gastrointestinal conditions. Patients with a history of bowel obstruction or major gastrointestinal surgery, severe gastrointestinal disorders, or swallowing disorders were not included in clinical studies. Hypomagnesemia: Veltassa binds to magnesium in the colon, which can lead to hypomagnesemia. In clinical studies, hypomagnesemia was reported as an adverse reaction in 5.3 percent of patients treated with Veltassa. Approximately 9 percent of patients in clinical trials developed hypomagnesemia with a serum magnesium value <1.4 mg/dL. Doctors should monitor serum magnesium and consider magnesium supplementation in patients who develop low serum magnesium levels.
Adverse Reactions: The most common adverse reactions (incidence ?2 percent) were constipation, hypomagnesemia, diarrhea, nausea, abdominal discomfort and flatulence. Mild to moderate hypersensitivity reactions were reported in 0.3 percent of patients treated with Veltassa and included edema of the lips. For additional Important Safety Information and Veltassa’s full Prescribing Information, please visit www.relypsa.com/veltassa/prescribing-information.
21/07/2016— © Vifor Pharma 14
Trust in excellence
Michael Puri Head of Global HR
Vifor Pharma, a company of the Galenica Group
Guided by our values at all times:
Passion, Entrepreneurship, Trust, Respect and Togetherness
21/07/2016— © Vifor Pharma 16
Switzerland
Facts & Figures
A small but well known country in the heart of Europe
21/07/2016— © Vifor Pharma 18
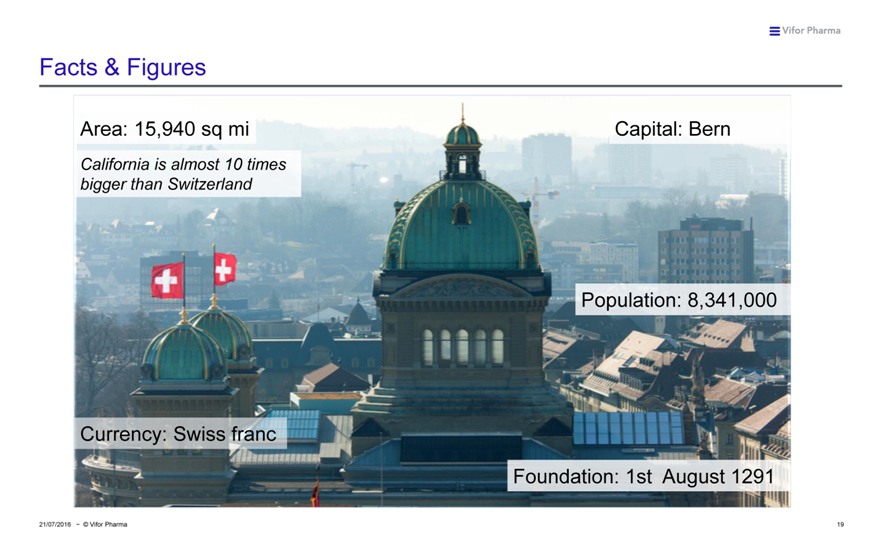
Facts & Figures
Area: 15,940 sq mi Capital: Bern
California is almost 10 times bigger than Switzerland
Population: 8,341,000
Currency: Swiss franc
Foundation: 1st August 1291
21/07/2016— © Vifor Pharma 19
Facts & Figures
4 official languages and a wealth of dialects:
- German: 63.5%
- French: 22.5%
- Italian: 8.1%
- Rhaeto-Rumantsch: 0.5%
Main economic activities:
- Pharma
- Banking
- Insurance
- Micro technology
- Hi-tech
- Biotechnology
21/07/2016— © Vifor Pharma 20
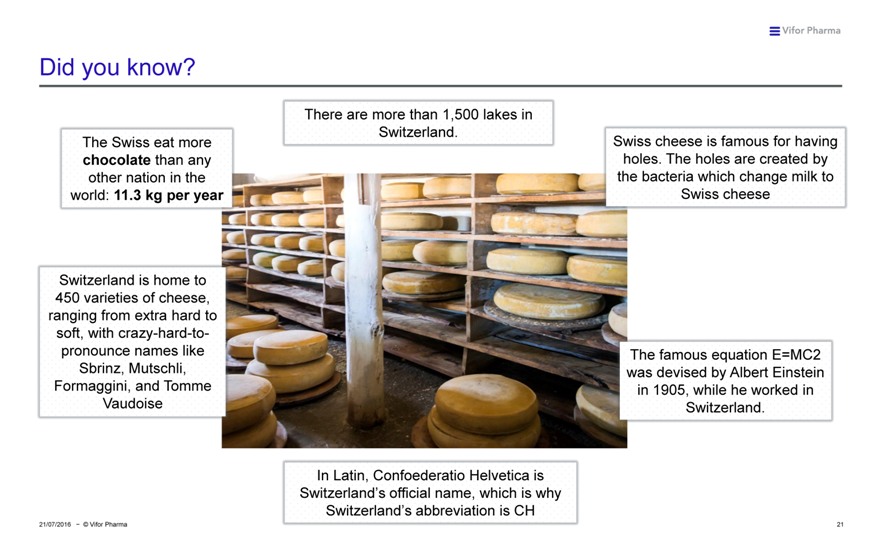
Did you know?
There are more than 1,500 lakes in Switzerland.
The Swiss eat more Swiss cheese is famous for having chocolate than any holes. The holes are created by other nation in the the bacteria which change milk to world: 11.3 kg per year Swiss cheese
Switzerland is home to 450 varieties of cheese, ranging from extra hard to soft, with crazy-hard-to-pronounce names like The famous equation E=MC2 Sbrinz, Mutschli, was devised by Albert Einstein Formaggini, and Tomme in 1905, while he worked in Vaudoise Switzerland.
In Latin, Confoederatio Helvetica is
Switzerland’s official name, which is why Switzerland’s abbreviation is CH
21/07/2016— © Vifor Pharma 21
Thank you!
21/07/2016— © Vifor Pharma 22