- NAYA Dashboard
- Financials
- Filings
-
Holdings
- Transcripts
- ETFs
- Insider
- Institutional
- Shorts
-
PRE 14A Filing
NAYA Biosciences (NAYA) PRE 14APreliminary proxy
Filed: 31 Jan 25, 4:19pm
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
SCHEDULE 14A
Proxy Statement Pursuant to Section 14(a) of the
Securities Exchange Act of 1934
(Amendment No. )
| Filed by the Registrant ☒ | Filed by a Party other than the Registrant ☐ |
Check the appropriate box:
| ☒ | Preliminary Proxy Statement |
| ☐ | Confidential, for Use of the Commission Only (as permitted by Rule 14a-6(e)(2)) |
| ☐ | Definitive Proxy Statement |
| ☐ | Definitive Additional Materials |
| ☐ | Soliciting Material Pursuant to §240.14a-12 |
NAYA BIOSCIENCES, INC.
(Name of Registrant as Specified in Its Charter)
(Name of Person(s) Filing Proxy Statement, if Other Than the Registrant)
Payment of Filing Fee (Check the appropriate box):
| ☒ | No fee required. |
| ☐ | Fee paid previously with preliminary materials. |
| ☐ | Fee computed on table in exhibit required by Item 2(b) per Exchange Act Rules 14a-6(i)(1) and 0-11. |
NAYA Biosciences, Inc.
5582 Broadcast Court
Sarasota, Florida 34240
NOTICE OF 2024 ANNUAL MEETING OF STOCKHOLDERS
To Be Held on March 10, 2025
To the Shareholders of NAYA Biosciences, Inc.:
The 2024 Annual Meeting of Shareholders (the “Annual Meeting”) of NAYA Biosciences, Inc., a Nevada corporation (the “Company,” “NAYA,” “we,” “us,” or “our”), will be held virtually on March 10, 2025 at 12:00 p.m. Eastern Time. The Annual Meeting will be held virtually at www.virtualshareholdermeeting.com/NAYA2024. At the Annual Meeting, the holders of our outstanding common stock will act on the following matters:
| 1. | The issuance, in accordance with Nasdaq Listing Rule 5635(a), of our common stock, upon conversion of our outstanding Series C-1 and C-2 Non-Voting Convertible Preferred Stock, upon conversion of an outstanding 7.0% Senior Secured Convertible Debenture in the principal balance of $3,934,146 due December 11, 2025 (the “Debenture”), and upon settlement of restricted stock units and exercise of stock options issued in exchange for restricted stock units and stock options that were previously granted to certain directors, employees, and consultants of our subsidiary, NAYA Therapeutics, Inc.; | |
| 2. | To elect five directors to the Board of Directors of the Company (the “Board”); | |
| 3. | The approval of an amendment to the Second Amended and Restated 2019 Stock Incentive Plan to increase the number of shares of our common stock available for issuance thereunder to an amount of 8,200,000, equal to approximately 15% of the total of our total issued and outstanding stock, including shares issued upon conversion of our Series C-1 and C-2 Non-Voting Convertible Preferred Stock; | |
| 4. | The approval of an amendment to our Amended and Restated Articles of Incorporation to (1) increase the number of authorized shares of our common stock from 50,000,000 to 100,000,000 after the reverse split below; and (2) effectuate a reverse stock split of our common stock at a ratio ranging from any whole number between 1-for-2 and 1-for-20, as determined by the Board of Directors in its discretion, subject to the Board of Directors’ authority to abandon such amendment; | |
| 5. | The ratification of the appointment of M&K CPAS, PLLC as our independent registered public accounting firm for the fiscal year ended December 31, 2024; | |
| 6. | To approve the adjournment of the Annual Meeting to a later date or time, if necessary, to permit further solicitation and vote of proxies if, based upon the tabulated vote at the time of the Annual Meeting, there are not sufficient votes to approve any of the matters above; and | |
| 7. | To conduct any other business properly brought before the Annual Meeting or any continuation, postponement or adjournment thereof. |
These items of business are more fully described in the Proxy Statement accompanying this Notice. The Annual Meeting will be a completely virtual meeting of shareholders, which will be conducted exclusively on the Internet. The record date for the Annual Meeting is January 24, 2025. Only shareholders of record at the close of business on that date are entitled to notice of and to vote at the meeting or any adjournment thereof.
Important Notice Regarding the Availability of Proxy Materials for the Shareholders’ Meeting
to Be Held Virtually on March 10, 2025 at 12:00 p.m. (ET) virtually via the Internet at www.virtualshareholdermeeting.com/NAYA2024.
The proxy statement and Annual Report on Form 10-K
are available at www.proxyvote.com.
| By Order of the Board of Directors, | |
| /s/ Steven Shum | |
| Steven Shum | |
| Chief Executive Officer and Director | |
| Sarasota, Florida | |
| January *, 2025 |
You are cordially invited to attend the meeting virtually via the Internet. Whether or not you expect to attend the meeting, please complete, date, sign and return the enclosed proxy, or vote by phone or online as instructed in these materials, as promptly as possible in order to ensure your representation at the meeting. A return envelope (which is postage prepaid if mailed in the United States) has been provided for your convenience. Even if you have voted by proxy, you may still vote via the Internet at the meeting. Please note, however, that if your shares are held of record by a broker, bank or other nominee and you wish to vote at the meeting, you must obtain a proxy issued in your name from that record holder.
TABLE OF CONTENTS
| i |

5582 Broadcast Court
Sarasota, Florida 34240
(978) 878-9505
PROXY STATEMENT
2024 ANNUAL MEETING OF STOCKHOLDERS
To Be Held Virtually on March 10, 2025
QUESTIONS AND ANSWERS ABOUT THESE PROXY MATERIALS AND VOTING
| Q. | Why did I receive these proxy materials? |
| A. | We are sending you these proxy materials because our Board is soliciting your proxy to vote at the Annual Meeting. You are invited to attend the Annual Meeting virtually via the Internet to vote on the proposals described in this proxy statement. However, you do not need to attend the Annual Meeting to vote your shares. Instead, you may simply complete, sign and return the enclosed proxy card, or follow the instructions below to submit your proxy by phone or online. We intend to begin mailing this proxy statement, the attached notice of Annual Meeting, and the enclosed proxy card, on or about February 13, 2025 to all shareholders of record entitled to vote at the Annual Meeting. Only shareholders who owned our common stock on January 24, 2025 are entitled to vote at the Annual Meeting. |
| Q. | How do I attend the Annual Meeting? |
| A. | The Annual Meeting will be held on March 10, 2025 at 12:00 p.m. (ET) virtually via the Internet. The Annual Meeting will be a completely virtual meeting of shareholders, which will be conducted exclusively online at www.virtualshareholdermeeting.com/NAYA2024. Information on how to vote at the Annual Meeting is discussed below. |
| 1 |
| Q. | Who can vote at the Annual Meeting? |
| A. | Only shareholders of record as of the close of business on January 24, 2025 (the “Record Date”) will be entitled to notice of, and to vote at, the Annual Meeting. The holders of Series C-1 and C-2 Preferred Stock are not entitled to vote on the matters being considered at the annual meeting. A list of shareholders eligible to vote at the Annual Meeting is available for inspection at any time up to the Annual Meeting. If you would like to inspect the list, please call our Corporate Secretary at (978) 878-9505 to arrange a visit to our offices. |
Shareholder of Record: Shares Registered in Your Name
If on January 24, 2025 your shares were registered directly in your name without Transfer Online, Inc. (“Transfer Online”), then you are a shareholder of record. As a shareholder of record, you may vote via the Internet at the Annual Meeting or vote by proxy. Whether or not you plan to attend the Annual Meeting, we urge you to fill out and return the enclosed proxy card or vote by proxy by phone or online as instructed below to ensure your vote is counted.
Beneficial Owner: Shares Registered in the Name of a Broker or Bank
If on January 24, 2025 your shares were not held in your name, but rather in an account at a brokerage firm, bank, dealer or other similar organization, then you are the beneficial owner of shares held in “street name” and these proxy materials are being forwarded to you by that organization. The organization holding your account is considered to be the shareholder of record for purposes of voting at the Annual Meeting. As a beneficial owner, you have the right to direct your broker or other agent regarding how to vote the shares in your account. You may vote via the Internet at the Annual Meeting or vote by proxy. Whether or not you plan to attend the Annual Meeting, we urge you to fill out and return the enclosed proxy card or vote by proxy by phone or online as instructed below to ensure your vote is counted.
| Q. | What is the purpose of the annual meeting? |
| A. | At the annual meeting, stockholders will consider and vote on the following matters: |
| 1. | The issuance, in accordance with Nasdaq Listing Rule 5635(a), of our common stock upon conversion of our outstanding Series C-1 and C-2 Non-Voting Convertible Preferred Stock, upon conversion of an outstanding 7.0% Senior Secured Convertible Debenture in the principal balance of $3,934,146 due December 11, 2025 (the “Debenture”), and upon settlement of restricted stock units and exercise of stock options issued in exchange for restricted stock units and stock options that were previously granted to certain directors, employees, and consultants of our subsidiary, NAYA Therapeutics, Inc.; |
| 2. | To elect five directors to the Board of Directors of the Company (the “Board”); |
| 3. | The approval of an amendment to the Second Amended and Restated 2019 Stock Incentive Plan to increase the number of shares of our common stock available for issuance thereunder to an amount equal to approximately 15% of our issued and outstanding stock, including shares issued upon conversion of our Series C-1 and C-2 Non-Voting Convertible Preferred Stock; |
| 2 |
| 4. | The approval of an amendment to our Articles of Incorporation to (1) increase the number of authorized shares of our common stock from 50,000,000 to 100,000,000; and (2) effectuate a reverse stock split of our common stock at a ratio ranging from any whole number between 1-for-2 and 1-for- 20, as determined by the Board of Directors in its discretion, subject to the Board of Directors’ authority to abandon such amendment; |
| 5. | The ratification of the appointment of M&K CPAS, PLLC as our independent registered public accounting firm for the fiscal year ended December 31, 2024; | |
| 6. | To approve the adjournment of the Annual Meeting to a later date or time, if necessary, to permit further solicitation and vote of proxies if, based upon the tabulated vote at the time of the Annual Meeting, there are not sufficient votes to approve any of the matters above; and |
| 7. | To conduct any other business properly brought before the Annual Meeting or any continuation, postponement or adjournment thereof. |
As of the date of this proxy statement, we are not aware of any business to come before the meeting other than the items noted above.
| Q. | What if another matter is properly brought before the Annual Meeting? |
| A. | Our Board knows of no other matters that will be presented for consideration at the Annual Meeting. If any other matters are properly brought before the Annual Meeting, the person named as proxy in the proxy card intends to vote the proxies (which confer discretionary authority to vote on such matters) in accordance with his judgment on the matter. |
| Q. | How do I vote? |
| A. | The procedures for voting are: |
The answer depends on whether you own your shares of common stock directly (that is, you hold shares that show your name as the registered shareholder) or if your shares are held in a brokerage account or by another nominee holder.
| Q. | If you own your shares directly (i.e., you are a “registered shareholder”): |
| A. | Your proxy is being solicited directly by us, and you can (1) vote via the Internet at the Annual Meeting, (2) vote by proxy using the enclosed proxy card, (3) vote by proxy online or (4) vote by proxy by phone. Whether or not you plan to attend the Annual Meeting, we urge you to vote by proxy to ensure your vote is counted. You may still attend the Annual Meeting and vote via the Internet even if you have already voted by proxy |
If you wish to vote by mail, please do the following: (i) sign and date the proxy card, (ii) mark the boxes indicating how you wish to vote, and (iii) return the proxy card in the prepaid envelope provided. If you sign your proxy card but do not indicate how you wish to vote, the proxy will vote your shares “FOR” the issuance of our common stock, upon conversion of our outstanding Series C-1 and C-2 Non-Voting Convertible Preferred Stock, “FOR ALL” of the five nominees for director, “FOR” increasing the number of shares of common stock available for issuance under the Plan and authorizing a reverse stock split of INVO’s common stock at a ratio ranging from any whole number between 1-for-2 and 1-for- 20, at the discretion of the Board “FOR” increasing the number of authorized shares, and “FOR” the ratification of M&K as independent auditors for the year ending December 31, 2024, and in his discretion on any other matter that properly comes before the Annual Meeting. Unsigned proxy cards will not be counted.
| 3 |
If you wish to vote over the Internet, go to www.proxyvote.com. Use the Internet to transmit your voting instructions until 11:59 p.m. Eastern Time on March 9, 2025. Have your proxy card in hand when you access the website and follow the instructions to obtain your records and to create an electronic voting instruction form. There may be costs associated with electronic access, such as usage charges from Internet access providers that must be paid by the shareholder. The Internet voting procedures are designed to authenticate a shareholder’s identity to allow a shareholder to vote his, her or its shares and confirm that his, her or its instructions have been properly recorded. Voting over the Internet authorizes the named proxy to vote your shares in the same manner as if you had submitted a validly executed proxy card.
If you wish to vote by telephone, you may vote by calling 1-800-690-6903.
If you wish to vote at the Annual Meeting, go to www.virtualshareholdermeeting.com/NAYA2024 and enter the 16-digit control number provided with your proxy materials. Just prior to the start of the meeting you will need to log back into the meeting site using your control number. Pre-registration is recommended but is not required to attend.
| Q. | If you hold your shares through a broker, bank, or other nominee: |
| A. | If you are the beneficial owner of shares held in street name through a bank, broker or other nominee, you may not vote your shares at the Annual Meeting unless you obtain a “legal proxy” from the bank, broker or nominee that holds your shares, giving you the right to vote the shares at the Annual Meeting. A voting instruction card has been provided to you by your broker, bank or other nominee describing how to vote your shares. If you receive a voting instruction card, you can vote by completing and returning the voting instruction card. Please be sure to mark your voting choices on your voting instruction card before you return it. You may also be able to vote via the Internet or by telephone. Please refer to the instructions provided with your voting instruction card for information about voting. See also “Will my shares be voted if I do not return my proxy?” below. |
| Q. | How many votes do I have? |
| A. | On each matter to be voted upon, you have one vote for each common share you own as of January 24, 2025, provided that common shares that were issued pursuant to the merger (the “Merger”) of INVO Merger Sub Inc., a wholly owned subsidiary of the Company and a Delaware corporation (“Merger Sub”), with and into NAYA Therapeutics, Inc., a Delaware corporation formerly known as NAYA Biosciences, Inc. (“Legacy NAYA”) pursuant to an Amended and Restated Agreement and Plan of Merger by and among the Company, Merger Sub, and Legacy NAYA (the “Merger Agreement”), are not eligible to vote for proposal 1. |
| Q. | Will my shares be voted if I do not return my proxy? |
| A. | If your shares are registered directly in your name, your shares may not be voted if you do not vote by returning your proxy by mail, over the phone or over the Internet before the Annual Meeting or in person at the Annual Meeting. |
If your shares are held in “street name,” your brokerage firm, bank or other nominee may, under certain circumstances, vote your shares if you do not timely return your voting instructions. Brokers, banks or other nominees can vote their customers’ unvoted shares on discretionary matters but cannot vote such shares on non-discretionary matters. If you do not timely return voting instructions to your brokerage firm, bank or other nominee to vote your shares, your brokerage firm, bank or other nominee may, on discretionary matters, either vote your shares or leave your shares unvoted.
| 4 |
| Q. | Who is paying for this proxy solicitation? |
| A. | We will pay for the entire cost of soliciting proxies. We will reimburse brokerage firms and other custodians, nominees and fiduciaries for their reasonable out-of-pocket expenses for forwarding proxy and other materials to our shareholders. Our officers and other employees may solicit proxies in person or by telephone but will receive no special compensation for doing so. |
| Q. | What does it mean if I receive more than one set of proxy materials? |
| A. | If you receive more than one set of proxy materials, your shares may be registered in more than one name or in different accounts. Please follow the voting instructions on the proxy cards in the proxy materials to ensure that all of your shares are voted. |
| Q. | What if I want to change my vote or revoke my proxy? |
| A. | If your shares are registered directly in your name, you may revoke your proxy and change your vote at any time before the Annual Meeting. To do so, you must do one of the following: |
| 1. | Vote over the Internet as instructed above. Only your latest Internet vote is counted. You may not revoke or change your vote over the Internet after 11:59 p.m. Eastern Time on March 9, 2025. | |
| 2. | Sign a new proxy and submit it by mail to Vote Processing, c/o Broadridge, 51 Mercedes Way, Edgewood, NY 11717 who must receive the proxy card no later than March 9, 2025. Only your latest dated proxy will be counted. | |
| 3. | You may attend the Annual Meeting and vote via the Internet. Simply attending the Annual Meeting will not, by itself, revoke your proxy. | |
| 4. | Give our Corporate Secretary written notice before or at the Annual Meeting that you want to revoke your proxy. |
If your shares are held in “street name,” you may submit new voting instructions with a later date by contacting your bank, brokerage firm, or other nominee. You may also vote electronically at the Annual Meeting, which will have the effect of revoking any previously submitted voting instructions, if you obtain a broker’s legal proxy as described in the answer to the question “How do I vote my shares?” above.
| 5 |
| Q. | When are shareholder proposals and director nominations due for the 2025 Annual Meeting? |
| A. | To be considered for inclusion in next year’s proxy materials, shareholder proposals must be submitted in writing in accordance with the procedures in Rule 14a-18 of the Exchange Act to our Corporate Secretary at 5582 Broadcast Court, Sarasota, FL 34240. Since the date of the 2025 annual meeting of stockholders will change by more than 30 days from the anniversary of our 2024 annual meeting, such proposals must be received by us by a reasonable time before we begin to print and send our proxy materials. Upon receipt of any such proposal, we will determine whether or not to include such proposal in the proxy statement and proxy card in accordance with regulations governing the solicitation of proxies. If you wish to nominate an individual for election at, or bring business other than through a shareholder proposal before the 2025 Annual Meeting, you must deliver your notice to our Corporate Secretary at the address above between or mailed and received at the principal executive offices of the corporation not later than the close of business on the sixtieth (60th) day nor earlier than the close of business on the ninetieth (90th) day prior to the first anniversary of the preceding year’s annual meeting; provided, however, that in the event that no annual meeting was held in the previous year or the date of the annual meeting has been changed by more than thirty (30) days from the date contemplated at the time of the previous year’s proxy statement, notice by the stockholder to be timely must be so received not earlier than the close of business on the ninetieth (90th) day prior to such annual meeting and not later than the close of business on the later of the sixtieth (60th) day prior to such annual meeting or, in the event public announcement of the date of such annual meeting is first made by the corporation fewer than seventy (70) days prior to the date of such annual meeting, the close of business on the tenth (10th) day following the day on which public announcement of the date of such meeting is first made by the corporation. Your notice to the Corporate Secretary must set forth information specified in our bylaws, including your name and address and the class and number of our common shares that you beneficially own. If you propose to bring business before an annual meeting other than a director nomination, your notice must also include, as to each matter proposed, the following: (i) a brief description of the business desired to be brought before the annual meeting and the reasons for conducting such business at the annual meeting, (ii) your name and address, (iii) the class and number of shares of the corporation which you beneficially own, (iv) any material interest that you have in your proposal, and (v) any other information that you are required to be provided pursuant to Regulation 14A under the Securities Exchange Act of 1934, as amended (the “Exchange Act”). If you propose to nominate an individual for election as a director, your notice must also include, as to each proposed nominee: (i) her or his name, age, business address and residence address, (ii) her or his principal occupation or employment, (iii) the class and number of shares of our share capital that are owned of record or beneficially by her or him, (iv) the date or dates on which the shares were acquired and the investment intent of the acquisition, and (v) any other information concerning the proposed nominee as would be required to be disclosed in a proxy statement soliciting proxies for the election of such proposed nominee in a contested election (even if a contested election is not involved), or that is otherwise required to be disclosed pursuant to Section 14 of the Exchange Act, and the rules and regulations promulgated under the Exchange Act, including the proposed nominee’s written consent to being named as a nominee and to serving as a director if elected. We may require any proposed nominee to furnish other information as we may reasonably require to determine the eligibility of the proposed nominee to serve as an independent director or that could be material to a reasonable shareholder’s understanding of the independence, or lack of independence, of the proposed nominee. |
The Board strives in its membership profile to have a mix of backgrounds and expertise that enhances the ability of the directors collectively to understand the issues facing the Company and to fulfill the responsibilities of the Board and its committees. It is the policy of the Board that nominees reflect the following characteristics:
| ● | Each director must at all times exhibit high standards of integrity, commitment, and independence of thought and judgment. |
| 6 |
| ● | The Board as a whole will contain a range of talent, skill, and expertise sufficient to provide sound and prudent guidance with respect to all of the Company’s operations and interests, which may include experience at senior levels in public companies, leadership positions in the life sciences, healthcare or public-health fields, science or technology backgrounds and financial expertise. | |
| ● | Each director should exhibit confidence and a willingness to express ideas and engage in constructive discussion with other Board members, Company management, and all relevant persons. | |
| ● | Each director should be willing and able to devote sufficient time, energy, and attention to the affairs of the Company. | |
| ● | Each director should actively participate in the decision-making process, be willing to make difficult decisions in the best interest of the Company and its shareholders, and demonstrate diligence and faithfulness in attending Board and committee meetings. | |
| ● | Each director should be free of any conflict of interest that would impair the director’s ability to fulfill the responsibilities of a Member of the Board. | |
| ● | No director shall be employed by, or serve on the board of, any present or potential competitor of the Company. |
The Board is committed to having diverse individuals from different backgrounds with varying perspectives, professional experience, education and skills serving as members of the Board. The Board believes that a diverse membership with a variety of perspectives and experiences is an important feature of a well-functioning board.
| Q. | How are votes counted? |
| A. | Votes will be counted by the inspector of election appointed for the Annual Meeting, who will separately count votes, broker non-votes and any abstentions for each of the proposals. |
| Q. | What is a Broker Non-Vote |
| A. | A “broker non-vote” occurs when shares held by a broker in “street name” for a beneficial owner are not voted with respect to a proposal because (1) the broker has not received voting instructions from the shareholder who beneficially owns the shares and (2) the broker lacks the authority to vote the shares at their discretion. Broker non-votes will not be included in the tabulation of the voting results of any of the “non-discretionary” proposals and, therefore, will have no effect on such proposals. |
| Q. | What is a quorum? |
| A. | The holders of one-third of the 7,087,796 shares of common stock outstanding as of the Record Date, either present or represented by proxy, constitute a quorum. A quorum is necessary in order to conduct the Annual Meeting. Your shares will be counted towards the quorum only if you submit a valid proxy (or one is submitted on your behalf by your broker, bank or other nominee) or if you vote in person at the Annual Meeting. If you choose to have your shares represented by proxy at the Annual Meeting, you will be considered part of the quorum. Broker non-votes and abstentions will be counted as present for the purpose of establishing a quorum. If a quorum is not present by attendance at the Annual Meeting or represented by proxy, the shareholders present by attendance at the meeting or by proxy may adjourn the Annual Meeting until a quorum is present. |
| 7 |
| Q. | How many shares do the affiliates, directors, and officers of the Company beneficially own, and how do they plan to vote their shares? |
| A. | Directors and executive officers, who, as of the Record Date, had beneficial ownership (or had the right to acquire beneficial ownership within sixty days following the Record Date) of approximately 1.79% of our outstanding voting capital and are expected to vote in favor of the issuance of our common stock upon conversion of our outstanding Series C-1 and C-2 Non-Voting Convertible Preferred Stock and issuing RSUs and Options, to vote in favor of all five directors to the Board of Directors of the Company (the “Board”), increasing the number of shares of common stock available for issuance under the Plan and authorizing a reverse stock split of INVO’s common stock at a ratio ranging from any whole number between 1-for-2 and 1-for- 20, at the discretion of the Board, increasing the number of authorized shares, and, the Ratification of M&K as independent auditor and approve the adjournment of the Annual Meeting to a later date or time, if necessary, to permit further solicitation and vote of proxies if necessary. |
| Q. | How can I find out the results of the voting at the Annual Meeting? |
| A. | Preliminary voting results will be announced at the Annual Meeting. In addition, final voting results will be announced in a current report on Form 8-K that we expect to file within four business days after the Annual Meeting. If final voting results are not available to us in time to file a Form 8-K within four business days after the Annual Meeting, we intend to file a Form 8-K to announce preliminary results and, within four business days after the final results are known to us, file an additional Form 8-K to announce the final results. |
| Q. | What proxy materials are available on the internet? |
| A. | The proxy statement and our Annual Report on Form 10-K are available at www.proxyvote.com, in the “Important Materials” section. |
| Q. | What vote is required to approve each matter, and how are votes counted? |
Proposal 1 – Approval of issuance of Common Shares upon conversion of Series C-1 and C-2 Preferred Stock, Conversion of Debenture, settlement of RSUs, and exercise of Options Proposal
The affirmative vote of the stockholders representing a majority of the votes cast on the matter is required to approve Proposal 1. Shares held in street name by a bank, broker or other nominee who indicate on their proxies that they do not have authority to vote the shares on Proposal 1 will not be counted as votes FOR or AGAINST Proposal 1 and will be treated as broker non-votes. Broker non-votes will have no effect on the voting on Proposal 1. If you vote to ABSTAIN on Proposal 1, your shares will not be voted FOR or AGAINST the proposal and will also not be counted as votes cast of shares voting on Proposal 1. As a result, voting to ABSTAIN will have no effect on the voting on Proposal 1.
Of the shares of our common stock outstanding entitled to vote at the annual meeting, 787,656 shares of common stock were issued in the Legacy NAYA acquisition (as defined below under “Summary of the Transaction”) will not count as votes in favor of Proposal 1 pursuant to the listing rules of the Nasdaq Stock Market.
| 8 |
Proposal 2—Election of Directors
Directors are elected by a plurality of the votes of the shares present in person or represented by proxy and entitled to vote on the election of directors, and which did not abstain. Accordingly, for Proposal 2, the nominees receiving the highest number of votes cast for the number of positions to be filled are elected. Shares represented by executed proxies will be voted, if authority to do so is not withheld, for the election of each of the five nominees named below Broker non-votes will have no effect on the voting on Proposal 2.
You may:
| ● | vote FOR all nominees; | |
| ● | vote FOR one or more nominees and WITHHOLD your vote from the other nominee or nominees; or | |
| ● | WITHHOLD your vote from all nominees. |
Votes that are withheld will not be included in the vote tally for the election of directors and will not affect the vote results.
Proposal 3 – Approval of an Amendment to our 2019 Stock Incentive Plan to Increase the Number of Shares of our Common Stock Available for Issuance Thereunder to 8,200,000 or approximately 15% of the total of NAYA’s total issued and outstanding stock, including shares issued upon conversion of Series C-1 and C-2 Non-Voting Convertible Preferred Stock
The affirmative vote of the stockholders representing a majority of the votes cast on the matter is required to approve Proposal 3. Shares held in street name by a bank, broker or other nominee who indicate on their proxies that they do not have authority to vote the shares on Proposal 3 will not be counted as votes FOR or AGAINST Proposal 3 and will be treated as broker non-votes. Broker non-votes will have no effect on the voting on Proposal 3. If you vote to ABSTAIN on Proposal 3, your shares will not be voted FOR or AGAINST the proposal and will also not be counted as votes cast of shares voting on Proposal 3. As a result, voting to ABSTAIN will have no effect on the voting on Proposal 3.
Proposal 4 – Approval of an Amendment to our Restated Articles of Incorporation, as Amended, to Increase the Number of Authorized Shares of our Common Stock from 50,000,000 to 100,000,000 and authorizing a reverse stock split of NAYA’s common stock at a ratio ranging from any whole number between 1-for-2 and 1-for- 20, at the discretion of the Board.
The affirmative vote of the stockholders representing a majority of the outstanding shares of our common stock entitled to vote on the matter is required to approve Proposal 4. If your shares are held by a bank, broker or other nominee and you do not timely provide voting instructions with respect to your shares, we expect that your bank, broker or other nominee will not have the authority to vote your shares on Proposal 4. Because Proposal 4 requires the affirmative vote of the stockholders representing a majority of the outstanding shares of our common stock entitled to vote on the matter, voting to ABSTAIN will have the same effect as a vote AGAINST Proposal 4.
| 9 |
Proposal 5—Ratification of the Appointment of Independent Registered Public Accounting Firm
The affirmative vote of the stockholders representing a majority of the votes cast at the Annual Meeting on the matter is required ratify M&K CPAS, PLLC as our independent registered public accounting firm for the year ended December 31, 2024. If your shares are held by a bank, broker or other nominee and you do not timely provide voting instructions with respect to your shares, we expect that your bank, broker or other nominee will have the authority to vote your shares on Proposal 5. If you vote to ABSTAIN on Proposal 5, your shares will not be voted FOR or AGAINST the proposal and will also not be counted as votes cast or shares voting on Proposal 5. As a result, voting to ABSTAIN will have no effect on the voting on Proposal 5.
Proposal 6 — Approval of the Adjournment
The affirmative vote of a majority of the voting power present or represented by proxy is required to approve adjournment of the Annual Meeting to solicit additional proxies in favor of proposals 1-5 above. If you vote to ABSTAIN on Proposal 6, your shares will not be voted FOR or AGAINST the proposal and will also not be counted as votes cast of shares voting on Proposal 6. As a result, voting to ABSTAIN will have no effect on the voting on Proposal 6.
| Q. | Who will count the vote? |
| A. | The votes will be counted, tabulated, and certified by Transfer Online, Inc. |
| Q. | How does the board of directors recommend that I vote on the proposals? |
| A. | Our board of directors recommends that you vote: |
FOR the approval of the Series C-1 and C-2 Preferred Stock Conversion Proposal;
FOR the election of all nominees to serve as directors;
FOR the approval of an amendment to our 2019 Stock Incentive Plan to increase the number of shares of our common stock available for issuance thereunder to 15% of the total of NAYA’s issued and outstanding stock and shares issued upon conversion of Series C-1 and C-2 Non-Voting Convertible Preferred Stock;
FOR the approval of an amendment to our Restated Articles of Incorporation, as amended, authorizing a reverse stock split of NAYA’s common stock at a ratio ranging from any whole number between 1-for-2 and 1-for- 20, at the discretion of the Board and to increase the number of authorized shares of our common stock from 50,000,000 to 100,000,000 after such reverse split;
FOR the ratification of the appointment of M&K CPAS, PLLC as our independent registered public accounting firm for the fiscal year ended December 31, 2024.
FOR the adjournment of the Annual Meeting to solicit additional proxies in favor of proposals 1-5.
| Q. | Are there other matters to be voted on at the annual meeting? |
| A. | We do not know of any matters that may come before the annual meeting other than Proposals 1, 2, 3, 4, 5 and 6. If any other matters are properly presented at the annual meeting, the persons named in the accompanying proxy intend to vote or otherwise act in accordance with their judgment on the matter. |
| 10 |
| Q. | Where can I find the voting results? |
| A. | We plan to announce preliminary voting results at the annual meeting and will report final voting results in a Current Report on Form 8-K filed with the SEC within four business days following the date of our annual meeting. |
| Q. | What are the costs of soliciting these proxies? |
| A. | We will bear the cost of soliciting proxies. We are in the process of retaining a proxy solicitor to assist us in the solicitation of proxies for an aggregate fee of approximately $* to $*. In addition to solicitation by mail, our directors, officers, and employees may solicit proxies by telephone, e-mail, facsimile, and in person without additional compensation. We may reimburse brokers or persons holding stock in their names, or in the names of their nominees, for their expenses in sending proxies and proxy material to beneficial owners. |
Implications of Being a Smaller Reporting Company
We are a “smaller reporting company,” meaning that the market value of our stock held by non-affiliates is less than $700.0 million, and our annual revenue was less than $100.0 million during our most recently completed fiscal year. We may continue to be a smaller reporting company if either (i) the market value of our stock held by non-affiliates is less than $250.0 million or (ii) our annual revenue was less than $100.0 million during the most recently completed fiscal year and the market value of our stock held by non-affiliates is less than $700.0 million. As a smaller reporting company, we may rely on exemptions from certain disclosure requirements that are available to smaller reporting companies. Specifically, as a smaller reporting company, we may choose to present only the two most recent fiscal years of audited financial statements in our Annual Report on Form 10-K and smaller reporting companies have reduced disclosure obligations regarding executive compensation.
BACKGROUND AND REASONS FOR THE TRANSACTION
Acquisition of NAYA Therapeutics, Inc. (former name NAYA Biosciences, Inc.) (“LEGACY NAYA”) by INVO Bioscience, Inc. (“LEGACY INVO”).
On October 11, 2024 (the “Effective Time”), LEGACY INVO, Merger Sub, and LEGACY NAYA, entered into the Merger Agreement and consummated the transactions contemplated thereby. Upon the terms and subject to the conditions set forth in the Merger Agreement, Merger Sub merged with and into LEGACY NAYA, with LEGACY NAYA continuing as the surviving corporation and a wholly owned subsidiary of LEGACY INVO. References hereafter to the Company refer to the parent company, LEGACY INVO, or the merged entity, LEGACY NAYA, as context requires.
| 11 |
Historical Background
LEGACY INVO is a commercial-stage fertility company dedicated to expanding the assisted reproductive technology (“ART”) marketplace by making fertility care accessible and inclusive to people around the world. LEGACY INVO’s primary mission is to implement new medical technologies aimed at increasing the availability of affordable, high-quality, patient-centered fertility care. LEGACY INVO’s flagship product is INVOcell, a revolutionary medical device that allows fertilization and early embryo development to take place in vivo within the woman’s body. This treatment solution is the world’s first intravaginal culture technique for the incubation of oocytes and sperm during fertilization and early embryo development. This technique, designated as “IVC”, provides patients a more connected and intimate experience at a more affordable cost in comparison to in vitro fertilization (“IVF”), the other advanced ART treatment. The IVC procedure can deliver comparable results to IVF and is a significantly more effective treatment than intrauterine insemination (“IUI”). LEGACY INVO’s commercialization strategy is focused on the opening of dedicated “INVO Centers” offering the INVOcell and IVC procedure (with three centers in North America now operational) and the acquisition of existing IVF clinics, in addition to continuing to sell their technology solution into existing fertility clinics.
In 2021, the Company began to expand its efforts and transform the company from purely a medical device provider to a more comprehensive medical technology company, including the addition of healthcare services. This expanded effort was highlighted with the initial opening of the company’s first INVO center clinic in Birmingham, Alabama, followed by additional INVO centers opened in Atlanta, Georgia and Monterey Mexico, all in late 2021. In August 2023, the Company completed its first acquisition of an established fertility practice, helping to further accelerate the transformation of the company and accelerate its revenue growth.
In October 2023, the Company then announced its intent to merge with LEGACY NAYA Bioscience, furthering its goals to build a comprehensive healthcare company. The Merger is intended to allow LEGACY NAYA to strengthen LEGACY INVO’s fertility operations through the infusion of new capital to expand LEGACY INVO’s footprint of fertility clinic operations across the United States, as well as advance the development of LEGACY NAYA’s unique clinical stage portfolio of oncology therapeutics. This business combination provides the benefit of having existing revenue generating operations with the fertility business and an ability to further grow those activities from current levels, along with LEGACY NAYA’s potential of a unique cancer-fighting drug platform business that is targeting a multi-billion dollar market opportunity.
The proposed Merger with LEGACY NAYA was the result of a desire and effort to expand beyond fertility into additional medical technology areas and drive added shareholder value. The terms of the business combination were the result of extensive discussions and negotiations between our management team and board and representatives and LEGACY NAYA’s management team and representatives. The following is a brief description of the background of these negotiations, the business combination and related transactions.
| 12 |
Prior to the initial introduction of LEGACY NAYA to LEGACY INVO by one of LEGACY INVO’s current shareholder in late August of 2023, neither LEGACY INVO, nor anyone on its behalf, contacted any prospective target business or had any substantive discussions, formal or otherwise, with respect to a transaction with LEGACY INVO.
LEGACY INVO had significant operating losses and an accumulated deficit of approximately $55 million as of June 30, 2023, shortly before the negotiation began. LEGACY NAYA was incorporated on June 8, 2023 to develop and build a group of agile, disruptive, high-growth companies dedicated to increasing patient access to life-transforming treatments in oncology and fertility and began its operations in July 2023. NAYA acquired certain pre-clinical assets from Cytovia Therapeutics, LLC in October 2023.
Below are the following events that occurred prior to LEGACY INVO’s introduction to LEGACY NAYA:
| ● | In late August 2023, one of LEGACY INVO’s shareholders introduced us to the CEO of Cytovia Therapeutics. | |
| ● | An initial conversation took place between LEGACY INVO’s CEO, Steve Shum, and LEGACY NAYA’s CEO, Daniel Teper, on August 30, 2023. As part of that discussion, Mr. Teper described how Cytovia Therapeutics had an interest in spinning out certain assets in their portfolio into a new company, called NAYA, to merge into an existing public company. Mr. Teper expressed his belief that LEGACY NAYA’s oncology-related intellectual property has significant value and could bring substantial, enhanced value to LEGACY INVO’s current operations. During the discussion, LEGACY INVO expressed a willingness to consider a merger combination. | |
| ● | Following the initial conversation, on August 31, 2023, LEGACY INVO and LEGACY NAYA entered into a mutual non-disclosure agreement. | |
| ● | LEGACY NAYA and LEGACY INVO engaged in further discussion during the first week of September to outline several key details, including the following: |
| ○ | The required capital as part of closing to fund LEGACY INVO for a reasonable period; | |
| ○ | Valuation parameters for both companies; | |
| ○ | Suggested board and senior management changes for the resulting company post-merger; and | |
| ○ | Key conditions for each company in order to close such a transaction. |
| ● | Based on these discussions, on September 8, 2023, LEGACY NAYA delivered a term sheet to LEGACY INVO outlining the proposed merger. | |
| ● | LEGACY INVO’s board of directors met to review the term sheet. LEGACY INVO’s director, Barbara Ryan, offered to speak with Mr. Teper as well as other industry contacts as part of additional board diligence. Director Barbara Ryan separately spoke with Daniel Teper as well as other industry contacts. | |
| ● | On September 15, 2023, Barbara Ryan confirmed to the rest of the LEGACY INVO that her additional diligence was favorable. |
| 13 |
| ● | Following further discussion amongst LEGACY INVO’s board, recommended unanimously by the LEGACY INVO board that if all the specific conditions could be met (as defined in the term sheet), the basic deal structure proposed was favorable for the LEGACY INVO shareholders and the merger should be approved. | |
| ● | Following additional edits by LEGACY INVO, on September 18, 2023, LEGACY INVO and LEGACY NAYA entered into a binding term sheet and engaged in discussions to complete the necessary tasks and closing conditions. On October 22, 2023, LEGACY NAYA and INVO Merger Sub. Inc., a Nevada corporation (“Merger Sub”), a wholly owned subsidiary of LEGACY INVO, entered into an agreement and plan of merger, as amended on October 25, 2023 (the “Original Merger Agreement”). |
Reasons for the Merger
The parties entered into the Merger with the goal of building a company with the following potential advantages:
| ● | LEGACY NAYA will focus on developmental programs aimed at providing innovative and transforming treatments in the areas of oncology, fertility, and regenerative medicine; | |
| ● | each of the Company’s oncology-focused intellectual property can be combined to create a potential streamlined product base; | |
| ● | the Company will be led by an experienced senior management team; and | |
| ● | proceeds from the concurrent and subsequent financing would provide funds for the company’s research and development and operating activities after the closing of the Merger (the “Closing”). |
Interests of the Directors and Executive Officers of Legacy INVO in the Merger
Stockholders should be aware that our current and former directors and executive officers have interests in the Merger that are different from, or in addition to, the interests of our stockholders generally. The members of the board of directors were aware of the different or additional interests and considered these interests, among other matters, in evaluating and negotiating the Merger Agreement. These interests are described in more detail below, and certain of them are quantified in the narrative and the tables below.
Merger Consideration and Exchange Ratio
A third party valuation report was provided to LEGACY NAYA. This report included a detailed analysis of the technology and market opportunity as well as relevant comparables. This analysis was completed in late 2023 and determined a value range between $39.6 million and $133.5 million with a median of approximately $60 million. This valuation report was the starting point for the negotiation of the exchange ratio.
| 14 |
Under the terms of the original Merger Agreement, immediately following the Merger, (a) LEGACY INVO stockholders, lenders and optionees would own approximately 12% of the common stock of NAYA and (b) LEGACY NAYA stockholders, and former lenders would own approximately 88% of the common stock of NAYA. (see proposal 1).
Subsequent Negotiations and Amendments to the Original Merger Agreement
On December 27, 2023, the parties entered into second amendment (“Second Amendment”) to the Original Merger Agreement. Pursuant to the Second Amendment, the parties agreed to extend the End Date to April 30, 2024. The parties further agreed to modify the closing condition for the Interim PIPE from a private offering of shares of LEGACY INVO common stock at a price that is a premium to the market price of the LEGACY INVO common stock in an estimated amount of $5,000,000 or more of gross proceeds to a private offering of LEGACY INVO’s preferred stock at a price per share of $5.00 per share in an amount equal to at least $2,000,000 to LEGACY INVO, plus an additional amount as may be required prior to closing of the Merger to be determined in good faith by the parties to adequately support the LEGACY INVO’s fertility business activities per an agreed forecast, as well as for a period of 12 months post- closing including a catch-up on the LEGACY INVO’s past due accrued payables still outstanding. The parties further agreed to the following schedule (the “Minimum Interim Pipe Schedule”) for the initial $2,000,000: (1) $500,000 no later than December 29, 2023, (2) $500,000 no later than January 19, 2024, (3) $500,000 no later than February 2, 2024, and (4) $500,000 no later than February 16, 2024. The parties also further agreed to modify the covenant of the parties regarding the Interim PIPE to require LEGACY NAYA to consummate the Interim PIPE before the closing of the Merger; provided, however, if the LEGACY INVO did not receive the initial gross proceeds pursuant to the Minimum Interim Pipe Schedule, then LEGACY INVO would be free to secure funding from third parties to make up for short falls on reasonable terms under SEC and Nasdaq regulations.
Effective as of May 1, 2024, the parties entered into third amendment (“Third Amendment”) to the Original Merger Agreement. Pursuant to the Third Amendment, the parties agreed to extend the End Date to June 30, 2024. The parties further agreed to modify the definition of an “Interim PIPE” to mean (a) a sale of shares of the Company’s Series A Preferred Stock pursuant to the Series A Preferred SPA, as amended (“Phase 1”), plus (b) a sale of shares of the Company’s preferred stock at a price per share of $5.00 per share in a private offering, to be consummated prior to the closing of the Merger, resulting in an amount as may be required, to be determined in good faith by the parties to the Merger Agreement, to adequately support LEGACY INVO’s fertility business activities per an agreed forecast of LEGACY INVO as well as for a period of 12 months following the closing, including a catch-up on LEGACY INVO’s past due accrued payables still outstanding (“Phase 2”). The parties agreed that Phase I must be consummated pursuant to the terms of the Series A Preferred SPA and that Phase II must be consummated prior to the closing of the Merger. The parties also confirmed that LEGACY INVO remained free to secure any amount of funding from third parties on any terms LEGACY INVO deems reasonably acceptable under SEC and Nasdaq regulations without the prior written consent of LEGACY NAYA. Under the Third Amendment, LEGACY INVO could terminate the Original Merger Agreement if LEGACY NAYA breached or failed to perform any of its covenants and agreements set forth in the Series A Preferred SPA, as amended.
| 15 |
Effective as of September 12, 2024, the parties entered into a fourth amendment (“Fourth Amendment”) to the previously announced Original Merger Agreement. Pursuant to the Fourth Amendment, the parties agreed to extend the end date (the date by which either the Company or LEGACY INVO could terminate the Merger Agreement, subject to certain exceptions) of the Merger to October 14, 2024. The parties further agreed that the Company would purchase 27,500 shares of LEGACY INVO’s Series A Preferred Stock for $137,500 pursuant to that certain Securities Purchase Agreement, dated as of December 29, 2023, as amended pursuant to an Amendment to Securities Purchase Agreement dated as of May 1, 2024 (as amended, the “Securities Purchase Agreement”) and could purchase up to an additional 72,500 shares of Series A Preferred Stock for an aggregate of $362,500 pursuant to the Securities Purchase Agreement prior to or concurrently with the closing of the Merger. Each party waived prior breaches of the Merger Agreement. Each party also agreed to use its best efforts to consummate the transactions contemplated by the Fourth Amendment, including to negotiate in good faith to amend and restate the Merger Agreement (the “A&R Merger Agreement”) to, among other things, (1) provide that the closing of the Merger shall occur simultaneously or shortly after the execution and delivery of the A&R Merger Agreement, and that the parties shall commit to use its respective best efforts to cause the closing to occur on or about October 1, 2024, but, in any case, no later than October 14, 2024, (2) ensure that the revised structure of the Merger shall be in compliance in all material respects with the applicable current listing and governance rules and regulations of the Nasdaq Stock Market, (3) provide that the aggregate merger consideration to be paid by LEGACY INVO for all of the outstanding shares of the Company’s capital stock shall consist of (a) such number of shares of LEGACY INVO’s common stock as shall represent a number of shares equal to no more than 19.9% of the outstanding shares of the LEGACY INVO’s common stock as of immediately before the effective time of the Merger (the “Common Stock Payment Shares”) and (b) shares of a newly designated Series C Convertible Preferred Stock of LEGACY INVO (the “Parent Preferred Stock Payment Shares”), (4) include an acknowledgment by the parties that the Company shall transfer 85% of the Common Stock Payment Shares to Five Narrow Lane LP, as a secured lender of the Company, (5) provide that LEGACY INVO shall take all action necessary under applicable law to (i) call, give notice of, and hold a meeting of its stockholders as soon as possible after the closing of the Merger, but in any case, no later than 120 days thereafter (the “Stockholder Meeting Deadline”); provided, that, LEGACY INVO acknowledges that, if it receives comments from the Securities and Exchange Commission (“SEC”) on the proxy statement filed in connection with such stockholder meeting and it uses its best efforts to revise and refile the proxy statement to address such comments, the date of such stockholder meeting may be after the Stockholder Meeting Deadline), for the purpose of, among other things, seeking stockholder approval (the “Stockholder Approval”) of the issuance of all shares of the Company’s common stock issuable upon conversion (the “Series C Conversion Shares”) of the Preferred Stock Payment Shares in accordance with the terms of the Certificate of Designations of Series C Convertible Preferred Stock (the “Series C Certificate of Designations”), and (ii) to file with the SEC a proxy statement for the purpose of obtaining the Stockholder Approval, no later than 35 days after the closing of the Merger, (6) provide that, upon receipt of the Stockholder Approval, the Preferred Stock Payment Shares shall be automatically converted into the Series C Conversion Shares at the conversion price in effect as set forth in the Series C Certificate of Designations; provided that, following such conversion, the Series C Conversion Shares shall represent approximately 60.1% of the outstanding shares of LEGACY INVO’s common stock; and (7) provide that, as soon as possible after the closing of the Merger, LEGACY INVO shall file a resale registration statement with the SEC to register the Common Stock Payment Shares and the Series C Conversion Shares in accordance with the terms of a registration rights agreement to be entered into between the parties.
| 16 |
Amended and Restated Merger Agreement
The following is a summary of the material terms of the Amended and Restated Merger Agreement. A copy is incorporated by reference into this proxy statement. It is not intended to provide any other factual information about LEGACY INVO or LEGACY NAYA. The following description does not purport to be complete and is qualified in its entirety by reference to the Merger Agreement. You should refer to the full text of the Merger Agreement for details of the Merger and the terms and conditions of the Merger Agreement.
The Merger Agreement contains representations and warranties that LEGACY INVO, on the one hand, and LEGACY NAYA, on the other hand, have made to one another as of specific dates. These representations and warranties have been made for the benefit of the other parties to the Merger Agreement and may be intended not as statements of fact but rather as a way of allocating the risk to one of the parties if those statements prove to be incorrect. Accordingly, you should not rely on the representations and warranties as current characterizations of factual information about LEGACY INVO or LEGACY NAYA, because they were made as of specific dates, may be intended merely as a risk allocation mechanism and are modified by the disclosure schedules.
On October 11, 2024 (the “Effective Time”), NAYA, Merger Sub, and Legacy NAYA, entered into the Merger Agreement and consummated and the transactions contemplated thereby. Upon the terms and subject to the conditions set forth in the Merger Agreement, Merger Sub merged with and into Legacy NAYA, with Legacy NAYA continuing as the surviving corporation and a wholly owned subsidiary of the Company.
At the Effective Time and as a result of the consummation of the Merger:
| ● | Each share of Class A common stock, par value $0.000001 per share, and Class B common stock, par value $0.000001 per share, of Legacy NAYA (“Legacy NAYA common stock”) outstanding immediately prior to the effective time of the Merger, other than certain excluded shares held by Legacy NAYA as treasury stock or owned by the Company or Merger Sub, automatically converted into the right to receive 118,148 shares of the Company’s common stock and 30,375 shares of the Company’s newly-designated Series C-1 Convertible Preferred Stock (the “Series C-1 Preferred”). The Series C-1 Preferred is not redeemable, has no voting rights, and may not be converted into shares of the Company’s Common Stock unless and until the Company’s stockholders approve the issuance of common stock upon conversion of the Series C-1 Preferred. If the Company’s stockholders approve the issuance of common stock upon conversion of the Series C-1 Preferred, such Series C-1 Preferred will automatically convert into approximately 29,515,315 shares of the Company’s common stock, subject to adjustment if, as a result of such conversion if, after giving effect to the conversion or issuance, any single holder, together with its affiliates, would beneficially own in excess of 19.99% of the Company’s outstanding common stock. A description of the rights, preferences, and privileges of the Series C-1 Preferred are set forth in Item 5.03 below. |
| 17 |
| ● | Certain outstanding debt obligations of Legacy NAYA, including a portion of an amended and restated senior secured convertible debenture issued to Five Narrow Lane LP (“FNL”), with a combined principal balance of $8,575,833 converted into the right to receive 669,508 shares of the Company’s common stock and 8,576 shares of the Company’s newly-designated Series C-2 Convertible Preferred Stock (the “Series C-2 Preferred”). The Company and FNL have agreed that the Company shall issue to FNL a pre-funded common stock purchase warrant to purchase up to 459,508 shares of the Company’s common stock in lieu of 459,508 shares of the aforementioned common stock. The Series C-2 Preferred is only redeemable upon a “Bankruptcy Triggering Event” or a “Change of Control” that occurs 210 days after the closing date of the Merger. The Series C-2 Preferred may not be converted into shares of the Company’s Common Stock unless and until the Company’s stockholders approve the issuance of common stock upon conversion of the Series C-2 Preferred. If the Company’s stockholders approve the issuance of common stock upon conversion of the Series C-2 Preferred, such Series C-2 Preferred will be convertible at the option of the holders into approximately 12,441,607 shares of the Company’s common stock, subject to limitations on beneficial ownership by the holders thereof. A description of the rights, preferences, and privileges of the Series C-2 Preferred are set forth in Item 5.03 below. | |
| ● | The remaining balance of the amended and restated senior secured convertible debenture issued to FNL in the amount of $3,934,146 was exchanged for a 7.0% Senior Secured Convertible Debenture in the principal balance of $3,934,146 due December 11, 2025 (the “Debenture”). A description of the rights, preferences, and privileges of the Debenture are set forth below. | |
| ● | Legacy NAYA has been renamed to “NAYA Therapeutics Inc.” |
In addition, Legacy NAYA stock options shall be converted into Company options to acquire a number of shares of the Company’s common stock equal to the number of shares of Legacy NAYA common stock subject to such Legacy NAYA options multiplied by 8.9108 (the “Exchange Ratio”) (rounded up to the nearest whole share) at an exercise price per share of such Legacy NAYA stock option divided by the Exchange Ratio, and Legacy NAYA restricted stock units shall be converted into Company restricted stock units representing the right to receive a number of shares of the Company’s common stock equal to the number of shares of Legacy NAYA common stock subject to such Legacy NAYA restricted stock unit multiplied by the Exchange Ratio. However, such options may not be exercised for shares of the Company’s common stock and such restricted stock units may not be settled for shares of the Company’s common stock unless and until the Company’s stockholders approve the issuance of common stock upon exercise of such options and settlement of such restricted stock units.
Immediately following the Merger, assuming conversion of the Series C1 and C-2 stock, (a) LEGACY INVO stockholders, certain lenders, option holders, and warrant holders would own approximately 17.75% of the common stock of NAYA on a fully-diluted basis, and (b) LEGACY NAYA stockholders, certain lenders, option holders, and RSU holders would own approximately 82.25% of the common stock of NAYA. Approximately 42.7 million common shares of NAYA will be held by LEGACY NAYA shareholders after conversion of preferred shares (see proposal 1).
In connection with the Merger, Dr. Daniel Teper, Legacy NAYA’s current Chairman and Chief Executive Officer, was appointed President of the Company, and Dr. Teper will remain as Legacy NAYA’s Chief Executive Officer. The combined company will be led by NAYA Chief Executive Officer Steven Shum, NAYA Chief Financial Officer Andrea Goren, and Dr. Teper. In addition, Dr. Teper and Ms. Lyn Falconio were appointed to the Company’s board of directors.
| 18 |
Pursuant to the Merger Agreement, the Company is required to hold a meeting of its stockholders to, among other things, (i) ratify the Merger Agreement and the transactions contemplated thereby, including the Merger, (ii) approve the increase in the amount of authorized shares under the Company’s Second Amended and Restated 2019 Stock Incentive Plan, (iii) approve the issuance of the Company’s common stock issuable upon conversion of the Series C-1 Preferred and Series C-2 Preferred, and (iv) approve an amendment to the Company’s articles of incorporation to (1) increase the number of shares of the Company’s authorized common stock to 100,000,000 shares, and (2) effectuate a reverse stock split of the Company’s common stock at a ratio ranging from any whole number between 1-for-2 and 1-for-20, as determined by the Company’s board of directors in its discretion. discretion. The Company also agreed to take all action necessary to hold the aforementioned stockholder meeting as soon as reasonably practicable.
Pursuant to both the Merger Agreement and the Assignment Agreement described below, the Company has agreed to file a registration statement with the SEC to register for resale the shares of the Company’s common stock issued pursuant to the Merger and the shares of common stock issuable upon exercise or conversion of the Series C-1 Preferred, the Series C-2 Preferred, and the Debenture, as applicable, as soon as practicable but in no event later than 30 days after the Closing Date.
The foregoing description of the Merger Agreement does not purport to be complete and is qualified in its entirety by reference to the Merger Agreement, which is attached hereto as Exhibit 2.1 and is incorporated herein by reference.
The Merger Agreement has been included to provide investors and security holders with information regarding its terms. It is not intended to provide any other factual information about the Company, Merger Sub, or Legacy NAYA. The representations, warranties, and covenants contained in the Merger Agreement were made only for purposes of that agreement and as of specific dates, were solely for the benefit of the parties to the Merger Agreement, may be subject to limitations agreed upon by the contracting parties, including being qualified by confidential disclosures made for the purposes of allocating contractual risk between the parties to the Merger Agreement instead of establishing these matters as facts and may be subject to standards of materiality applicable to the contracting parties that differ from those applicable to investors. Investors should not rely on the representations, warranties, and covenants or any descriptions thereof as characterizations of the actual state of facts or condition of the Company, Merger Sub or Legacy NAYA or any of their respective subsidiaries or affiliates. Moreover, information concerning the subject matter of the representations and warranties may change after the date of the Merger Agreement, which subsequent information may or may not be fully reflected in the Company’s or Legacy NAYA’s public disclosures.
| 19 |
7.0% Senior Secured Convertible Debenture
In connection with the Merger, on October 11, 2024, the Company issued the Debenture to FNL in an exchange of an outstanding note of Legacy NAYA held by FNL. The Debenture carries an interest rate of seven percent (7%) per annum, payable on the first business day of each calendar month commencing November 1, 2024. The maturity date of the Debenture is December 11, 2025 (the “Maturity Date”), at which point the outstanding principal amount, together with any accrued and unpaid interest and other fees, shall be due and payable to the holder of the Debenture.
Conversion. At any time after the Company’s stockholders approve the issuance of any Company common stock upon conversion of the Debenture, the holder of the Debenture will be entitled to convert any portion of the outstanding and unpaid principal amount and accrued interest into shares of Company common stock at a conversion price of $0.93055 per share, subject to adjustment as described therein. The Debenture may not be converted and shares of Company common stock may not be issued upon conversion of the Debenture if, after giving effect to the conversion or issuance, the holder together with its affiliates would beneficially own in excess of 4.99% of the outstanding common stock of the Company.
Prepayment. The Company may not prepay the Debenture without the prior written consent of FNL.
Monthly Redemption. Commencing March 14, 2025 and on the 14th of each month thereafter until the Maturity Date, the Company shall redeem $437,127.24, plus accrued but unpaid interest and other fees, of the principal amount of the Debenture.
Mandatory Redemption. While any portion of the Debenture is outstanding, if the Company receives gross proceeds of more than $3,000,000 from any equity or debt financings (other than a public offering as described herein), the Company shall, at the option of the holder, apply one-third (1/3) of such gross proceeds to the redemption of the principal amount of the Debenture, except that if such equity or debt financing is a public offering of the Company’s securities pursuant to a registration statement on Form S-1, the Company shall, at the option of the holder, apply one hundred percent (100%) of such gross proceeds, not to exceed $500,000, to the redemption of the principal amount of the Debenture. The Company has also agreed that, if it received gross proceeds of more than $2,000,000 from any equity or debt financing, it shall, at the option of the holder, apply $500,000 of such gross proceeds to the redemption of the principal amount of the Debenture.
The Debenture contains events representations, warranties, covenants, and events of default that are customary for similar transactions. Upon an event of default, the Debenture becomes immediately due and payable, and the Borrower is subject to a default rate of interest of 15% per annum and a default sum as stipulated.
The foregoing description of the Debenture does not purport to be complete and is qualified in its entirety by reference to the Debenture.
| 20 |
Joinder Agreement
In connection with the Merger, the Company entered in a joinder agreement (the “Joinder Agreement”) with FNL dated as of October 11, 2024 to a certain securities purchase agreement dated as of January 3, 2024 by and between Legacy NAYA and FNL (the “FNL SPA”) pursuant to which the Company agreed to become a party to the FNL SPA.
The foregoing description of the Joinder Agreement does not purport to be complete and is qualified in its entirety by reference to the Joinder Agreement.
Assignment and Assumption Agreement
In connection with the Merger, on October 11, 2024, the Company entered in an assignment and assumption agreement (the “Assignment Agreement”), pursuant to which the Company agreed to assume the rights, duties, and liabilities of Legacy NAYA under a certain registration rights agreement dated as of September 12, 2024 by and between Legacy NAYA and FNL, pursuant to which the Company agreed to register FNL’s resale of shares of Company common stock issuable upon conversion of the Debenture and the Series C-2 Preferred as well as certain commitment shares issued to FNL in connection with the transactions.
The foregoing description of the Assignment Agreement does not purport to be complete and is qualified in its entirety by reference to the Assignment Agreement.
Second Amendment to Revenue Loan and Security Agreement
On October 11, 2024, the Company entered into a second amendment to Revenue Loan and Security Agreement (the “Second Amendment”) with Decathlon Alpha V, L.P. (“Decathlon”), Steven Shum, and certain subsidiaries of the Company (the “Guarantors”), pursuant to which Decathlon consented to the Merger and Legacy NAYA becoming a subsidiary of the Company. Pursuant to the Second Amendment, Legacy NAYA joined the Revenue Loan and Security Agreement as a Guarantor. The Company agreed to pay down its loan by at least $500,000 and increase its monthly payments by up to $30,000 if the Company closes a private offering of its securities. The Company also agreed to retain an investment banker to pursue a financing or a sale if it fails to meet certain liquidity covenants. The Company also agreed to enter into an intercreditor agreement with Decathlon and Five Narrow Lane LP within 5 business days of the Merger.
The foregoing description of the Second Amendment does not purport to be complete and is qualified in its entirety by reference to the Second Agreement.
In approving the Merger, the board of directors considered the benefits and risks compared to other alternatives, including other potential acquisitions and business development opportunities reviewed by the board. After giving consideration to these and other factors, the board of directors approved the Merger, which it believes better positions the Company for long-term success.
| 21 |
FORWARD-LOOKING STATEMENTS AND RISK FACTOR SUMMARY
The Company believes that some of the information in this proxy statement constitutes forward-looking statements within the definition of the Private Securities Litigation Reform Act of 1995. You can identify these statements by forward-looking words such as “may,” “expect,” “anticipate,” “contemplate,” “believe,” “estimate,” “intends” and “continue” or similar words. You should read statements that contain these words carefully because they:
| ● | discuss future expectations; | |
| ● | contain projections of future results of operations or financial condition; or | |
| ● | state other “forward-looking” information. |
The Company believes it is important to communicate its expectations to its stockholders. However, there may be events in the future that the Company is not able to predict accurately or over which it has no control. The risk factors and cautionary language discussed in this proxy statement provide examples of risks, uncertainties and events that may cause actual results to differ materially from the expectations described in such forward-looking statements, including, among other things:
| ● | The ability to maintain the listing of NAYA’s securities on a national securities exchange following the Merger. | |
| ● | We will need to raise additional capital to meet our business requirements in the future, and such capital raising may be costly or difficult to obtain and could dilute our stockholders’ ownership interests. | |
| ● | The availability and reimbursement levels for our products by government and private payers significantly affect pricing and commercial success. Changes in regulations or pricing controls could negatively impact our business. | |
| ● | Changes in government regulations or the implementation of pricing controls could adversely impact the pricing, reimbursement, and commercial success of our products. | |
| ● | Delays, uncertainties, or failures in obtaining regulatory approvals, particularly from the Food and Drug Administration (“FDA”) or other international regulatory agencies, could significantly impact the development, commercialization, and profitability of our products | |
| ● | The potential liquidity and trading of NAYA’s public securities. | |
| ● | The ability to operate in highly competitive markets, and potential adverse effects of this competition. | |
| ● | Risk of decreased revenues due to pricing pressures. | |
| ● | The ability to attract, motivate and retain qualified employees, including members of its senior management team. | |
| ● | Risk that NAYA is unsuccessful in integrating potential acquired businesses and product lines. |
| 22 |
| ● | The ability to maintain a high level of client service and expand operations. | |
| ● | Risk that NAYA’s products and services fail to interoperate with third-party systems. | |
| ● | The ability to maintain effective controls over disclosure and financial reporting that enable NAYA to comply with regulations and produce accurate financial statements. | |
| ● | Potential issues with NAYA’s product offerings that could cause legal exposure, reputational damage, and an inability to deliver products or services. | |
| ● | Increased risks resulting from NAYA’s international operations. | |
| ● | Potential unauthorized use of NAYA’s products and technology by third parties. | |
| ● | Global economic conditions and the cyclical nature of certain markets NAYA serves. | |
| ● | Exchange rate fluctuations and volatility in global currency markets. | |
| ● | The ability to comply with various trade restrictions, such as sanctions and export controls. | |
| ● | Potential intellectual property infringement claims. | |
| ● | Our success depends on our ability to protect our intellectual property and our proprietary technologies. | |
| ● | Our product candidates may cause undesirable side effects or have other properties that could halt their clinical development, prevent their regulatory approval, limit their commercial potential, or result in significant negative consequences. | |
| ● | The ability to comply with the anti-corruption laws of the United States and various international jurisdictions. | |
| ● | Potential impairment charges related to goodwill, identified intangible assets, and fixed assets. | |
| ● | Potential litigation involving the Company. | |
| ● | Expectations regarding the time during which NAYA will be an “emerging growth company” under the JOBS Act. | |
| ● | Other risks and uncertainties indicated in this proxy statement, including those set forth under the section entitled “Risk Factors.” | |
| ● | The ability to develop new products, improve existing products, and adapt its business model to keep pace with industry trends. |
You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date of this proxy statement.
All forward-looking statements included herein attributable to the Company or any person acting on the Company’s behalf are expressly qualified in their entirety by the cautionary statements contained or referred to in this section. Except to the extent required by applicable laws and regulations, the Company undertakes no obligations to update these forward-looking statements to reflect events or circumstances after the date of this proxy statement or to reflect the occurrence of unanticipated events.
| 23 |
RISK FACTORS
In addition to the other information contained or incorporated by reference into this proxy statement, including the matters addressed in the section entitled “Cautionary Statement Regarding Forward-Looking Statements” beginning on page 22, NAYA stockholders should carefully consider the following risk factors in determining how to vote on the Proposals herein. Stockholders are further advised that the risks described below may not be the only risks we face. Additional risks that we do not yet know of, or that we currently think are immaterial, may also negatively impact our business operations or financial results. Any of the risks and uncertainties set forth in this proxy statement and in the documents incorporated by reference herein, as updated by annual, quarterly and other reports and documents that we file with the SEC and incorporate by reference into this prospectus, could materially and adversely affect our business, results of operations and financial condition, which in turn could materially and adversely affect the value of our securities.
Risks Related to Our Financial Condition and Our Need for Additional Capital
Our financial situation creates doubt whether we will continue as a going concern.
From the inception of our consolidated subsidiaries on January 5, 2007, through September 30, 2024, we had an accumulated net loss of $63.5 million. There can be no assurances that we will be able to achieve a level of revenues adequate to generate sufficient cash flow from operations or additional financing through private placements, public offerings and/or bank financing necessary to support our working capital requirements. To the extent that funds generated from any private placements, public offerings and/or bank financing are insufficient, we will have to raise additional working capital. No assurance can be given that additional financing will be available, or if available, will be on acceptable terms. These conditions raise substantial doubt about our ability to continue as a going concern. If adequate working capital is not available, we may be forced to discontinue operations, which would cause investors to lose their entire investment.
We will need to raise additional funding, which may not be available on acceptable terms, or at all. Failure to obtain this necessary capital when needed may force us to delay, limit or terminate operations.
Without additional funds, we do not expect that our current cash position will be sufficient to fund our current operations for the next 12 months and we do not have sufficient funds to consummate our business plan. Our operating plan may change as a result of many factors currently unknown to us, and we may need to seek additional funds sooner than planned, through public or private equity or debt financings, government or other third-party funding or a combination of these approaches. Raising funds in the current economic environment may present additional challenges. Even if we believe we have sufficient funds for our current or future operating plans, we may seek additional capital if market conditions are favorable or if we have specific strategic considerations.
| 24 |
Any additional fundraising efforts may divert our management from their day-to-day activities, which may adversely affect our ability to develop and commercialize our product candidates. In addition, we cannot guarantee that future financing will be available in sufficient amounts or on terms acceptable to us, if at all. Moreover, the terms of any financing may adversely affect the holdings or the rights of our stockholders and the issuance of additional securities, whether equity or debt, by us, or the possibility of such issuance, may cause the market price of our shares to decline. The sale of additional equity or convertible securities may dilute our existing stockholders. The incurrence of indebtedness would result in increased fixed payment obligations, and we may be required to agree to certain restrictive covenants, such as limitations on our ability to incur additional debt, limitations on our ability to acquire, sell or license intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business. We could also be required to seek funds through arrangements with collaborative partners or otherwise at an earlier stage than otherwise would be desirable and we may be required to relinquish rights to some of our technologies or product candidates or otherwise agree to terms unfavorable to us, any of which may have a material adverse effect on our business, operating results and prospects.
If we are unable to obtain funding on a timely basis, we may be required to significantly curtail, delay or be unable to expand our operations or otherwise capitalize on our business opportunities, as desired, which could materially affect our business, financial condition and results of operations.
Even if we can raise additional funding, we may be required to do so on terms that are dilutive to you.
The capital markets have been unpredictable in the past for unprofitable companies such as ours. In addition, it is generally difficult for development stage companies to raise capital under current market conditions. The amount of capital that a company such as ours is able to raise often depends on variables that are beyond our control. As a result, we may not be able to secure financing on terms attractive to us, or at all. If we are able to consummate a financing arrangement, the amount raised may not be sufficient to meet our future needs. If adequate funds are not available on acceptable terms, or at all, our business, including our results of operations, financial condition and our continued viability will be materially adversely affected.
Our secured debt instruments and preferred stock contain numerous restrictive covenants that limit management’s discretion to operate our business.
In both our secured debt instruments and our certificate of designation for our series C-2 preferred stock, we agreed to certain covenants that place significant restrictions on, among other things, our ability to incur additional indebtedness, to create liens or other encumbrances, to make certain payments and investments, and to sell or otherwise dispose of assets and merge or consolidate with other entities. Any failure to comply with the covenants included in the secured debt instruments could result in an event of default, which could trigger an acceleration of the related debt. If we were unable to repay the debt upon any such acceleration, our senior lenders could seek to foreclose on our assets in an effort to seek repayment under the loans. If our lenders were successful, we would be unable to conduct our business as it is presently conducted and our ability to generate revenues and fund our ongoing operations would be materially adversely affected.
| 25 |
Risks Related to the Acquisition of Legacy NAYA
We may not be able to successfully integrate Legacy NAYA and achieve the benefits expected to result from the acquisition.
The acquisition may present challenges to management, including the integration of the operations, and personnel of NAYA and Legacy NAYA and special risks, including possible unanticipated liabilities, unanticipated integration costs and diversion of management attention.
We cannot assure you that the business of Legacy NAYA and NAYA will be successfully integrated or profitably managed. Even if these businesses are successfully integrated and profitably managed, we cannot assure you that, following the transaction, our business will achieve sales levels, profitability, efficiencies or synergies that justify the acquisition or that the acquisition will result in increased earnings for us in any future period.
Legacy NAYA has a limited operating history and has no products approved for commercial sale, which may make it difficult for you to evaluate the success of Legacy NAYA’s business to date and to assess its future viability.
Legacy NAYA is a clinical stage biotechnology company with a limited operating history upon which we can evaluate Legacy NAYA’s business and prospects. Although the management of Legacy NAYA and its service providers have substantial experience in successfully conducting and completing clinical trials, including large-scale, pivotal clinical trials, obtain marketing approval, manufacturing a clinical or commercial scale product or arranging for a third party to do so on our behalf or conduct sales and marketing activities necessary for successful product commercialization, there is no guarantee that NAYA may be able to successfully advance its pipeline. Typically, it takes about three to six years to develop a new biological drug from the time it enters phase I clinical trials to when it is approved for treating patients, but in many cases it may take longer. Predictions about Legacy NAYA’s future success or viability are highly dependent on sufficient timely financing and the ability of NAYA leadership to execute its development plans and scale-up efficiently its operations.
Risks Related to the Acquisition of Wisconsin Fertility Institute
We may not be able to successfully manage Wisconsin Fertility Institute and to achieve the benefits expected to result from the acquisition.
The acquisition of Wisconsin Fertility Institute (“WFI”) may present challenges to management, including the integration of the operations, and personnel of NAYA and WFI, continued management of the clinic and special risks, including possible unanticipated liabilities, unanticipated integration costs and diversion of management attention.
We cannot assure you that we will successfully integrate or profitably manage WFI’s businesses. Even if we are able to integrate and profitably manage WFI’s business, we cannot assure you that our business will achieve sales levels, profitability, efficiencies or synergies that justify the acquisition or that the acquisition will result in increased earnings for us in any future period.
| 26 |
If we fail to make the required $7.5 million in additional payments required in our acquisition of WFI, our business would be adversely affected.
Following closing of our acquisition of the WFI, we are required to make additional annual payments of approximately $2.5 million each year, for a total of $7.5 million, through 2026, which payments are secured by the sellers having a lien on the assets purchased to acquire WFI. We have not made the first annual payment, and if we are currently in negotiations with the sellers of WFI to revise the terms of the acquisition, including payment terms, and otherwise resolve this payment. If we do not resolve this payment with the sellers of WFI or otherwise negotiate new terms, including payment terms, or if we default on our additional payment obligations to the sellers of WFI, such sellers could exercise their rights and remedies under acquisition agreements, which could include foreclosing on the assets sold to us to acquire WFI. Any such action would have a material adverse effect on our business and prospects.
We may incur additional debt financing to provide the cash proceeds necessary to acquire WFI. If we were unable to service any such debt, our business would be adversely affected.
In order to finance our acquisition of WFI, we secured debt financing and may look to raise additional debt proceeds. The current debt financing requires us to pledge all or substantially all of our assets as collateral. If we were unable to satisfy any such debt obligation or fail to pay such debt obligations in a timely fashion, we would be in default under such debt financing agreement and such lender could exercise its rights and remedies under such debt financing agreements, which could include seizing all of our assets. Any such action would have a material adverse effect on our business and prospects.
Risks Relating to Our Business
Our business has posted net operating losses, has a limited operating history, and needs additional capital to grow and finance its operations.
We have a limited operating history and are essentially an early-stage operation. Our net loss for the nine months ended September 30, 2024 and September 30, 2023 was $5,472,345 and $6,039,830, respectively, and our net loss for the years ended December 31, 2023 and December 31, 2022 was $8,034,612 and $10,892,511, respectively. On a pro-forma basis (based on our historical consolidated financial statements including the historical combined financial statements of WFI, and the historical financial statements of Legacy NAYA, adjusted to give effect to the WFI Acquisition and the Legacy NAYA Merger and related financing transactions), our pro forma net loss for the nine months ended September 30, 2024 was $13,924,954, respectively, and our pro forma net loss for the year ended December 31, 2023 was $10,401,986. Continued net operating losses together with limited working capital make investing in our common stock a high-risk proposal. Our limited operating history may make it difficult for management to provide effective insight into future activities, marketing costs, and customer acquisition and retention. This could lead to NAYA missing targets for achievement of milestones in the therapeutics business and for the achievement of profitability of device sales and INVO Centers, which could negatively affect the value of your investment.
| 27 |
We are subject to risks associated with doing business globally.
Our operations, both inside and outside the United States, are subject to risks inherent in conducting business globally and under the laws, regulations and customs of various jurisdictions and geographies. Our operations outside the United States are subject to special risks and restrictions, including, without limitation: fluctuations in currency values and foreign-currency exchange rates; exchange control regulations; changes in local political or economic conditions; governmental pricing directives; import and trade restrictions; import or export licensing requirements and trade policy; restrictions on the ability to repatriate funds; and other potentially detrimental domestic and foreign governmental practices or policies affecting U.S. companies doing business abroad, including the U.S. Foreign Corrupt Practices Act and the trade sanctions laws and regulations administered by the U.S. Department of the Treasury’s Office of Foreign Assets Control. Acts of terror or war may impair our ability to operate in particular countries or regions and may impede the flow of goods and services between countries. Customers in weakened economies may be unable to purchase our products, or it could become more expensive for them to purchase imported products in their local currency, or sell at competitive prices, and we may be unable to collect receivables from such customers. Further, changes in exchange rates may affect our net earnings, the book value of our assets outside the United States and our stockholders’ equity. Failure to comply with the laws and regulations that affect our global operations could have an adverse effect on our business, financial condition or results of operations.
Failure to comply with the United States Foreign Corrupt Practices Act or similar laws could subject us to penalties and other adverse consequences.
We are subject to the United States Foreign Corrupt Practices Act, which generally prohibits United States companies, including their suppliers, distributors and other commercial partners, from engaging in bribery or other prohibited payments to foreign officials for the purpose of obtaining or retaining business. Corruption, extortion, bribery, pay-offs, theft and other fraudulent practices occur from time-to-time in the countries in which we distribute products. We have adopted formal policies and procedures designed to facilitate compliance with these laws. If our employees or other agents, including our distributors or suppliers, are found to have engaged in such practices, we could suffer severe penalties and other consequences that may have a material adverse effect on our business, financial condition and results of operations.
We are subject to significant domestic and international governmental regulation.
Our business is heavily regulated domestically in the United States and internationally. In the United States the Food and Drug Administration (“FDA”), and other federal, state and local authorities, implement various regulations that subject us to civil and criminal penalties, including cessation of operations and recall of products distributed, in the event we fail to comply. Any such actions could severely curtail our sales and business reputation. In addition, additional restrictive laws, regulations or interpretations could be adopted, making compliance with such regulations more difficult or expensive. While we devote substantial resources to ensure our compliance with laws and regulations, we cannot completely eliminate the risk that we may be found non-compliant with applicable legal and regulatory requirements.
| 28 |
We believe that the healthcare industry will continue to be subject to increased regulation as well as political and legal action, as future proposals to reform the health care system are considered by the U.S. Congress and state legislatures. We do not know of, nor do we have any control over, future changes to health care laws and regulations which may have a significant impact on our business.
We are subject to risks relating to federal and state healthcare fraud, waste, and abuse laws.
We may be subject to healthcare fraud, waste, and abuse regulation and enforcement by the federal government and the governments in the states and foreign countries in which we might conduct our business. Such federal laws generally apply only to entities or individuals that provide items or services for which payment may be made under a federal healthcare program. These laws are subject to extensive and increasing enforcement by numerous federal, state, and local government agencies including the Office of Inspector General, the Department of Justice, the Centers for Medicare & Medicaid Services, and various state authorities. The healthcare laws and regulations that may affect our ability to operate include the following:
| ● | The federal Anti-Kickback Statute (42 U.S.C. § 1320a-7b) (the “AKS”), a criminal statute, makes it illegal for any person or entity to knowingly and willfully, directly or indirectly, solicit, receive, offer, or pay any remuneration that is in exchange for or to induce the referral of business, including the purchase, order, lease of any good, facility, item, or service for which payment may be made under a federal healthcare program, such as Medicare or Medicaid. The term “remuneration” has been broadly interpreted to include anything of value. The Civil Monetary Penalties Law (42 U.S.C. § 1320a-7a) (the “CMPL”) also contains a provision that prohibits the payment of anything of value in return for referrals and provides for the imposition of civil penalties. | |
| ● | Federal false claims and false statement laws, including the federal civil False Claims Act (31 U.S.C. §§ 3729 – 3733), prohibits, among other things, any person or entity from knowingly presenting, or causing to be presented, for payment to, or approval by, federal programs, including Medicare and Medicaid, claims for items or services that are false or fraudulent. |
| 29 |
| ● | Section 1877 of the Social Security Act (42 U.S.C. § 1395nn), commonly referred to as the “Stark Law, prohibits referrals by ordering by a physician of “designated health services,” which include durable medical equipment and supplies as well as inpatient and outpatient hospital services, that are payable, in whole or in part, by Medicare or Medicaid, to an entity in which the physician or the physician’s immediate family member has an investment interest or other financial relationship, subject to several exceptions. Financial relationships that are implicated by the Stark Law can include arrangements ranging from marketing arrangements and consulting agreements to medical director agreements with physicians who order our products. The Stark Law also prohibits billing for services rendered pursuant to a prohibited referral. Several states have enacted laws similar to the Stark Law. These state laws may cover all (not just Medicare and Medicaid) patients. Many federal healthcare reform proposals in the past few years have attempted to expand the Stark Law to cover all patients as well. If we violate the Stark Law, our financial results and operations could be adversely affected. Penalties for violations include denial of payment for the services, significant civil monetary penalties, and exclusion from the Medicare and Medicaid programs; | |
| ● | Section 1877 of the Social Security Act (42 U.S.C. § 1395nn), commonly referred to as the “Stark Law, prohibits referrals by ordering by a physician of “designated health services,” which include durable medical equipment and supplies as well as inpatient and outpatient hospital services, that are payable, in whole or in part, by Medicare or Medicaid, to an entity in which the physician or the physician’s immediate family member has an investment interest or other financial relationship, subject to several exceptions. Financial relationships that are implicated by the Stark Law can include arrangements ranging from marketing arrangements and consulting agreements to medical director agreements with physicians who order our products. The Stark Law also prohibits billing for services rendered pursuant to a prohibited referral. Several states have enacted laws similar to the Stark Law. These state laws may cover all (not just Medicare and Medicaid) patients. Many federal healthcare reform proposals in the past few years have attempted to expand the Stark Law to cover all patients as well. If we violate the Stark Law, our financial results and operations could be adversely affected. Penalties for violations include denial of payment for the services, significant civil monetary penalties, and exclusion from the Medicare and Medicaid programs; | |
| ● | The federal Physician Payments Sunshine Act (42 U.S.C. § 1320a–7h) requires certain manufacturers of drugs, devices, biologics and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program, with specific exceptions, to report annually to the Centers for Medicare & Medicaid Services information related to payments or other transfers of value made to physicians and teaching hospitals, as well as ownership and investment interests held by physicians and their immediate family members. |
| 30 |
At present, our products and services are not reimbursable under any federal healthcare program. If, however, that changes in the future and it were determined that we were not in compliance with these federal fraud, waste, and abuse laws, we would be subject to liability.
Also, as noted above, many states have similar laws and regulations, such as anti-kickback and false claims laws that may be broader in scope and may apply regardless of payor, in addition to items and services reimbursed under Medicaid and other state programs. We may be subject to such laws in Alabama and Georgia due to our joint venture operations in those states. The Georgia State False Medicaid Claims Act (Ga. Code Ann. §§ 49-4-168 – 49-4-168.6), Georgia Medical Assistance Act false statements provision (Ga. Code Ann. §§ 49-4-140 – 49-4-157), and Alabama Medicaid false statements statute (Ala. Code § 22-1-11(a)) contain prohibitions that are analogous to the federal False Claims Act. Alabama law also includes an anti-kickback provision (Ala. Code § 22-1-11(c)) that is analogous to the federal AKS.
The Georgia Patient Self-Referral Act of 1993 (Ga. Code Ann. §§ 43-1B-1 – 43-1B-8) contains prohibitions on self-referral that are similar to those under the Stark Law, however, the Georgia law applies to additional classes of providers, including pharmacists, and is not limited to items or services reimbursable by a federal healthcare program. The Georgia law prohibits health care providers or entities regulated by the law from presenting any claim for payment to any individual, third-party payer, or other entity for a service furnished pursuant to a prohibited referral.
If we are found in violation of applicable laws or regulations, we could suffer severe consequences that would have a material adverse effect on our business, results of operations, financial condition, cash flows, reputation and stock price, including:
| ● | suspension or termination of our participation in federal healthcare programs; | |
| ● | criminal or civil liability, fines, damages or monetary penalties for violations of healthcare fraud and abuse laws, including the federal False Claims Act, CMPL, and AKS; | |
| ● | repayment of amounts received in violation of law or applicable payment program requirements, and related monetary penalties; | |
| ● | mandated changes to our practices or procedures that materially increase operating expenses; | |
| ● | imposition of corporate integrity agreements that could subject us to ongoing audits and reporting requirements as well as increased scrutiny of our business practices; | |
| ● | termination of various relationships or contracts related to our business; and | |
| ● | harm to our reputation which could negatively affect our business relationships, decrease our ability to attract or retain patients and physicians, decrease access to new business opportunities and impact our ability to obtain financing, among other things. |
| 31 |
Responding to lawsuits and other proceedings as well as defending ourselves in such matters would require management’s attention and cause us to incur significant legal expense. It is also possible that criminal proceedings may be initiated against us or individuals in our business in connection with investigations by the federal government.
Additionally, to the extent that our product is sold or our services are provided in a foreign country, we may be subject to similar foreign laws.
We are subject to the requirements of the Health Insurance Portability and Accountability Act of 1996, the Health Information Technology for Economic and Clinical Health Act of 2009 (“HITECH Act”), and related implementing regulations (together, “HIPAA”), and failure to comply, including through a breach of protected health information (“PHI”) could materially harm our business.
HIPAA established comprehensive federal protection for the privacy and security of health information. The HIPAA standards apply to three types of organizations, or “Covered Entities”: (1) health plans, (2) health care clearing houses, and (3) health care providers who conduct certain health care transactions electronically. The HIPAA standards also apply to Covered Entities’ “Business Associates.” Covered Entities and their Business Associates must have in place administrative, physical, and technical standards to guard against the misuse of individually identifiable health information. The HITECH Act promotes the adoption and meaningful use of health information technology. The HITECH Act addresses the privacy and security concerns associated with the electronic transmission of health information, in part, through several provisions that strengthen the civil and criminal enforcement of the HIPAA rules. These laws may impact our business in the future. NAYA is currently a Business Associate of various Covered Entities. Failure to comply with these confidentiality requirements, including via a breach of PHI, may result in penalties and sanctions.
In the ordinary course of our business, we may use, collect, and store sensitive data, including PHI. We face risks relative to protecting this critical information, including loss of access risk, inappropriate disclosure risk, inappropriate modification risk, and the risk of being unable to adequately monitor our controls. Our information technology and infrastructure may be vulnerable to attacks by hackers or viruses or breached due to employee error, malfeasance or other disruptions. Any such breach or interruption could compromise our networks and the information stored there could be accessed by unauthorized parties, publicly disclosed, lost or stolen. Any such access, disclosure or other loss of information could result in legal claims or proceedings, liability under laws that protect the privacy of personal information, such as HIPAA, and regulatory penalties. There is no guarantee that we can continue to protect our systems from breach. Unauthorized access, loss, or dissemination could also disrupt our operations.
| 32 |
The U.S. Office of Civil Rights in the Department of Health and Human Services enforces the HIPAA privacy and security rules and may impose penalties for failure to comply with requirements of HIPAA. Penalties vary significantly depending on factors such as whether failure to comply was due to willful neglect. These penalties include civil monetary penalties of $100 to $50,000 per violation, up to an annual cap of $1,500,000 for identical violations. A person who knowingly obtains or discloses individually identifiable health information in violation of HIPAA may face a criminal penalty of up to $50,000 per violation and up to one-year imprisonment. The criminal penalties increase to $100,000 per violation and up to five-years imprisonment if the wrongful conduct involves false pretenses, and to $250,000 per violation and up to 10-years imprisonment if the wrongful conduct involves the intent to sell, transfer, or use identifiable health information for commercial advantage, personal gain, or malicious harm. The U.S. Department of Justice is responsible for criminal prosecutions under HIPAA. Furthermore, in the event of a breach as defined by HIPAA, there are reporting requirements to the Office of Civil Rights under the HIPAA regulations as well as to affected individuals, and there may also be additional reporting requirements to other state and federal regulators, including the Federal Trade Commission, and to the media. Issuing such notifications can be costly, time and resource intensive, and can generate significant negative publicity. Breaches of HIPAA may also constitute contractual violations, including violation of the Company’s Business Associate contracts with Covered Entities from which the Company receives PHI, that could lead to contractual damages or terminations.
We may not be able to develop or continue our business if we fail to retain key personnel.
We substantially rely upon the efforts and abilities of our executive management and directors. The loss of any of our executive officers and/or directors services could potentially have a material adverse effect on our business, operations, revenues and/or prospects. If one or more of these persons were to become unable or unwilling to continue in their present positions, we may not be able to replace them readily or timely, if at all. We do not maintain key man life insurance on the lives of any of our executive management or directors.
Currency exchange rate fluctuations may affect the results of our operations.
We intend to distribute our INVOcell product internationally with all sales, domestic and international, in U.S. dollars. As a result, our operations could be impacted by fluctuations in currency exchange rates, although we attempt to mitigate such risk by invoicing only in U.S. dollars. In spite of this, our operations may still be negatively impacted by foreign currency exchange rates in the event the U.S. dollar strengthens and the local currency where the product is being sold weakens. In the event such international patients are unable to afford the associated increase costs, international doctors and clinics may not be able to offer the INVOcell and IVC procedure. As we expand our international footprint with joint ventures, these joint ventures will likely have a functional currency based on their location and as a result, if we are required to consolidate these financial results it may create currency fluctuations. Additionally, as an international business we may be susceptible to adverse foreign currency fluctuations unconnected to the U.S. dollar.
We are subject to risks in connection with changes in international, national, and local economic and market conditions.
Our business is subject to risks in connection with changes in international, national and local economic and market conditions, including the effects of global financial crises, effects of terrorist acts, war and global pandemics. Such economic changes could negatively impact infertile people’s ability to pay for fertility treatment around the world.
| 33 |
We anticipate that eventually international sales will account for a meaningful part of our revenue. We will experience additional risks associated with international sales, including:
| ● | political and economic instability; | |
| ● | export controls; | |
| ● | changes in international legal and regulatory requirements; | |
| ● | United States and foreign government policy changes affecting the product marketability; and | |
| ● | changes in tax laws, duties and tariffs. |
Any of these factors could have a material adverse effect on our business, results of operations and financial condition. From 2011 through 2023, we sold products in certain international markets mainly through independent distributors, and we anticipate maintaining a similar sales strategy along with our recent joint venture activity for the foreseeable future. In the event a distributor fails to meet annual sales goals, we may be required to obtain a replacement distributor, which may be costly and difficult to identify. Additionally, a change in our distributors may increase costs, and create a substantial disruption in our operations resulting in a loss of revenue.
Changes in the healthcare industry may require us to decrease the selling price for our products or could result in a reduction in the available market size.
Governmental and private sector initiatives in the U.S. and abroad involving trends toward managed healthcare and cost containment could place an emphasis on our ability to deliver more cost-effective medical therapies. The development of other cost-effective devices could eventually adversely affect the prices and/or sales of our products. Companies in the healthcare industry are subject to various existing and proposed laws and regulations, in both domestic and international markets, regulating healthcare pricing and profitability. Additionally, there have been third-party payer initiatives to challenge the prices associated with medical products, which if successful, could affect our ability to sell products on a competitive basis in the future.
In the United States, there has been a trend of consolidation among healthcare facilities and purchasers of medical devices, allowing such purchasers to limit the number of suppliers from whom they purchase medical products. As result, it is unknown whether such purchasers will decide to stop purchasing our products or demand discounts on our prices. Any pressure to reduce our product prices in response to these industry trends and the decrease in market size could adversely affect our anticipated revenue and profitability of our sales, creating a material adverse effect on our business.
| 34 |
If we are unable to effectively adapt to changes in the healthcare industry, our business may be harmed.
Federal, state, and local legislative bodies frequently pass legislation and promulgate regulations relating to healthcare reform or that affect the healthcare industry. As has been the trend in recent years, it is reasonable to assume that there will continue to be increased government oversight and regulation of the healthcare industry in the future. We cannot predict the ultimate content, timing, or effect of any new healthcare legislation or regulations, nor is it possible at this time to estimate the impact of potential new legislation or regulations on our business. It is possible that future legislation enacted by Congress or state legislatures, or regulations promulgated by regulatory authorities at the federal or state level, could adversely affect our business. It is also possible that the changes to federal healthcare program reimbursements to providers who purchase our products or use our services may serve as precedent to possible changes in other payors’ reimbursement policies in a manner adverse to us. Similarly, changes in private payor reimbursements could lead to adverse changes in federal healthcare programs, which could have a material adverse effect on our business, financial condition, cash flows, and results of operations.
There can be no assurance that we will be able to successfully address changes in the current regulatory environment. Some of the healthcare laws and regulations applicable to us are subject to limited or evolving interpretations, and a review of our business or operations by a court, law enforcement, or a regulatory authority might result in a determination that could have a material adverse effect on us. Furthermore, the healthcare laws and regulations applicable to us may be amended or interpreted in a manner that could have a material adverse effect on our business, financial condition, cash flows and results of operations.
Recent economic trends could adversely affect our financial performance.
Economic downturns and declines in consumption in the healthcare market may affect the levels of both our sales and profitability. If a downturn in economic conditions occurs, or if there is deterioration in financial markets and major economies, our financial performance could be adversely affected. The tightening of credit in financial markets may adversely affect the ability of our customers and suppliers to obtain financing, which could result in a decrease in, or deferrals or cancellations of, the sale of our products and services. In addition, weakening economic conditions may result in a decline in spending for ART and fertility assistance that could adversely affect our business operations and liquidity. We are unable to predict the likely duration and severity of any disruption in the domestic and global financial markets.
Social media platforms present risks and challenges.
The unauthorized use of certain social media vehicles could result in the improper collection and/or dissemination of personally identifiable information causing brand damage and various legal implications. In addition, negative or inaccurate social media posts or comments about us on any social networking site could damage our brand, reputation, and goodwill.
We are susceptible to cybersecurity breaches and cyber-related fraud.
We depend on information technology (“IT”) systems, networks, and services, encompassing internet sites, data hosting and processing facilities, as well as hardware (including laptops and mobile devices), along with software and technical applications and platforms. Some of these are overseen, hosted, supplied, and/or utilized by third parties or their vendors, supporting us in the administration of our business.
| 35 |
The escalation of IT security threats and the increasing sophistication of cyber-crime pose a potential hazard to the security of our IT systems, networks, and services, as well as to the confidentiality, availability, and integrity of our data. Should the IT systems, networks, or service providers we rely on encounter malfunctions or if we experience a loss or disclosure of sensitive information due to various causes such as catastrophic events, power outages, or security breaches, and our business continuity plans fail to address these issues promptly, we could face disruptions in managing operations. This may result in reputational, competitive, and/or business harm, potentially adversely impacting our business operations and financial condition. Furthermore, such incidents could lead to the unauthorized disclosure of critical confidential information, causing financial and reputational damage due to the loss or misappropriation of confidential information belonging to us, our partners, employees, customers, suppliers, or consumers. In such scenarios, significant financial and other resources might be required to rectify the damage caused by a security breach or to repair and replace networks and IT systems.
In addition, in the ordinary course of our business, we may use, collect, and store sensitive data, including personal health information. We face risks relative to protecting this critical information, including loss of access risk, inappropriate disclosure risk, inappropriate modification risk, and the risk of being unable to adequately monitor our controls. Our information technology and infrastructure may be vulnerable to attacks by hackers or viruses or breached due to employee error, malfeasance, or other disruptions. Any such breach or interruption could compromise our networks and the information stored there could be accessed by unauthorized parties, publicly disclosed, lost or stolen. Any such access, disclosure, or other loss of information could result in legal claims or proceedings, liability under laws that protect the privacy of personal information, such as HIPAA, and regulatory penalties. There is no guarantee that we can continue to protect our systems from breach. Unauthorized access, loss, or dissemination could also disrupt our operations.
Risks Related to Our Fertility Business
Our existing INVO Centers were established as joint ventures with medical partners. Future INVO Centers may also be established as joint ventures. These joint ventures will be important to our business. If we are unable to maintain any of these joint ventures, or if they are not successful, our business could be adversely affected.
We have established, and plan to establish additional, entered into, and may enter into additional, joint ventures for the operation of our INVO Centers. Our existing and any future joint ventures may have a number of risks, including that our joint venture partners:
| ● | have significant discretion in determining the efforts and resources that they will apply; | |
| ● | may not perform their obligations as expected; | |
| ● | may dispute the amounts of payments owed; |
| 36 |
| ● | may fail to comply with applicable legal and regulatory requirements regarding the distribution or marketing of our INVOcell product; | |
| ● | may not properly maintain or defend their or our relevant intellectual property rights or may use our proprietary information in such a way as to invite litigation that could jeopardize or invalidate our intellectual property or proprietary information or expose us to potential litigation and liability; | |
| ● | may infringe the intellectual property rights of third parties, which may expose us to litigation and potential liability; | |
| ● | could become involved in a business combination or cessation that could cause them to deemphasize or terminate the development or commercialization of our INVOcell product; and | |
| ● | may seek to terminate our joint venture, which could require us to raise additional capital and to develop new joint venture relationships. |
Additionally, if one of our joint venture partners seeks to terminate its agreement with us, we may find it difficult to attract new joint venture partners and the perception of our INVO Centers in the business and financial communities could be adversely affected.
Our fertility business is subject to significant competition.
The fertility industry is highly competitive and characterized by well entrenched and long-standing practices as well as technological improvements and advancements. New ART services, devices and techniques may be developed that may render the INVOcell obsolete. Competition in the areas of fertility and ART services is largely based on pregnancy rates and other patient outcomes. Accordingly, the ability of our business to compete is largely dependent on our ability to achieve adequate pregnancy rates and patient satisfaction levels. Our business operates in highly competitive areas that are subject to change. New health care providers and medical technology companies entering the market may reduce our and our INVO Centers’ market share, patient volume and growth rates, and could force us to alter our planned pricing and INVO Center service offerings. Additionally, increased competitive pressures may require us to commit more resources to our and our INVO Centers’ marketing efforts, thereby increasing our cost structure and affecting our ability to achieve, or the timing of achieving, profitability. There can be no assurance that we will not be able to compete effectively, nor can there be any assurance that additional competitors will not enter the market. Such competition may make it more difficult for us to enter into additional contracts with fertility clinics or open profitable INVO Centers.
We need to manage growth in our fertility operations, and we may not be successful in implementing our growth strategy.
In order to maximize potential growth in our current and potential markets, we may need to expand the scope of our services in the medical device/bioscience industry. As a result, we plan to continue to improve our INVOcell technology, operating procedures and management information systems. We will also need to effectively train, motivate and manage our employees. Our failure to manage our growth could disrupt our operations and ultimately prevent us from generating revenues at the levels we expect.
| 37 |
Many factors including, but not limited to, increased competition from similar businesses, unexpected costs, costs associated with marketing efforts and maintaining a strong client base may interfere with our ability to expand successfully. Our inability to implement our internal strategy successfully may have a negative impact on our growth, future financial condition, results of operations and/or cash flows.
We may not be successful at managing clinics.
Our management team has limited experience in managing fertility clinics. We seek to retain experienced personnel to provide clinical practice expertise, perform recruitment functions, provide necessary training, and provide day-to-day management of our clinics. We may not be successful in retaining such personnel, integrating such personnel into our operations, or otherwise successfully manage clinics that we have acquired or may acquire in the future.
We face potential liability as a provider of a medical device. These risks may be heightened in the area of artificial reproduction.
The provision of medical devices entails the substantial risk of potential tort injury claims. We currently utilize product liability insurance to provide coverage against potential tort injury claims, as well as customary insurance protection for our INVO Centers. However, there can be no assurance such coverage will provide adequate protection against any potential claims. Furthermore, any claim asserted against us could generate costly legal fees, consume management’s time and resources, and adversely affect our reputation and business, regardless of the merit or eventual outcome of such claim.
There are inherent risks specific to the provision of fertility and ART services. For example, the long-term effects on women of the administration of fertility medication, integral to most fertility and ART services, are of concern to certain physicians and others who fear the medication may prove to be carcinogenic or cause other medical problems. Additionally, any ban or other limitation imposed by the FDA or other foreign regulatory department on fertility medication and services could have a material adverse effect on our business. Any such action would likely adversely affect the value of your investment.
| 38 |
If we fail to maintain adequate quality standards for our products, our reputation and business may be adversely affected and harmed.
Our customers are expecting that our products and services will perform as marketed and in accordance with industrial standards. For our INVOcell device, we rely on third-party manufacturing companies and their packaging processes in connection with the production of our products. Our key INVOcell suppliers, which are located in the U.S. and include NextPhase Medical Devices and Casco Bay Molding, and have been steadfast partners since our company first began and can provide us with virtually an unlimited capability to support our growth objectives, with all manufacturing performed in the New England region of the U.S. However, a failure to maintain product quality standards in accordance with our customers’ expectations could result in the loss of demand for our products. Additionally, delays or quality lapses in our production lines could result in substantial economic losses to us. Although we believe that our current quality control procedures adequately address these risks, there can be no assurance that we will not experience occasional or systemic quality lapses in our manufacturing and service operations. Currently, we have limited manufacturing capabilities as we rely on a single manufacturing provider regarding our production process. In the event our manufacturer is unable to produce an adequate supply of products at appropriate quality levels, our growth could be limited, and our business may be harmed. If we experience significant or prolonged disturbance in our quality standards, our business and reputation may be harmed, which may result in the loss of customers, our inability to participate in future customer product opportunities and reduced revenue and earnings.
We heavily rely on third party package delivery services, and a significant disruption in these services or significant increases in prices may disrupt our ability to import or export materials, increase our costs and negatively affect our ability to achieve and maintain profitability.
We ship our products to our customers through known independent package delivery companies, such as FedEx and UPS. If any third party package delivery providers experience a significant disruption such that any of our products, components or raw materials cannot be delivered in a timely fashion or such that we incur additional shipping costs that we are unable to recoup, our costs may increase and our relationships with certain customers may be adversely affected. In particular, if our third-party package delivery providers increase prices and we are not able to find comparable alternatives or adjust our delivery network, our profitability could be adversely affected.
We will need additional, qualified personnel in order to expand our fertility business. Without additional personnel, we will not be able to expand our fertility business.
Expanding our fertility business requires increasing the number of persons engaged in activities for the sale, marketing, administration and delivery of our products as well as clinical training personnel for proper IVC procedure training. Our ability to attract and hire personnel to fulfil these efforts is dependent on our ability to attract and retain potential employees with the proper background and training matching the skills required for the positions. In addition, we may not be able to attract personnel who will be able to successfully implement our business operations and growth strategy in the manner that we currently anticipate.
| 39 |
Risks Related to the Fertility Industry
The FDA regulatory review process for medical devices is expensive, time-consuming and uncertain, and the failure to obtain and maintain required regulatory clearances and approvals could prevent us from commercializing our products.
Unless an exemption applies, each medical device commercially distributed in the United States requires either FDA clearance of a 510(k) premarket notification, approval of a premarket approval, or issuance of a de novo classification order. The FDA clearance, de novo classification, and approval processes for medical devices are expensive, uncertain and time-consuming.
Future modifications to the INVOcell that was classified through de novo may require a 510(k) clearance. We may make minor changes to the INVOcell without seeking clearance for the modifications if we determine such clearances are not necessary and document the basis for that conclusion. However, the FDA may disagree with our determination or may require additional information, including clinical data, to be submitted before a determination is made, in which case we may be required to delay the introduction and marketing of our modified products, redesign our products, conduct clinical trials to support any modifications, or we may be subject to enforcement actions. In addition, the FDA may not clear such modified INVOcell for the indications that are necessary or desirable for successful commercialization.
There is no assurance that we will be able to obtain the necessary clearances on a timely basis or at all. Further, the FDA may change its policies, adopt additional regulations or revise existing regulations, or take other actions which may impact our ability to modify the INVOcell on a timely basis, and may prevent or delay clearance of future products. Delays in receipt of, or failure to obtain clearances for any product modifications or future products we may develop would result in delayed or no realization of revenue from such products and the viability of our INVO Centers, and in substantial additional costs, which could decrease our profitability.
In addition, we are required to continue to comply with applicable FDA and other regulatory requirements following de novo classification or clearance. The failure to comply with existing or future regulatory requirements could have a material adverse effect on our business.
Improper marketing and promotion or off-label use of our product could lead to investigations and enforcement by governmental bodies including product recalls or market withdrawal, may harm our reputation and business, and could result in product liability suits.
If the FDA or any foreign regulatory entity determines that our promotional materials or training constitute promotion of an off-label use, it could request that we modify our training or promotional materials or subject us to regulatory or enforcement actions. These enforcement actions could include, for example, a warning letter or untitled letter, injunction, seizure, civil fine or criminal penalties. We cannot, however, prevent a physician from using the INVOcell off-label, when in the physician’s independent professional medical judgement, he or she deems it appropriate. There may be increased risk of injury to patients if physicians attempt to use the INVOcell off-label, or the INVOcell may not be as effective, which could harm our reputation.
| 40 |
If we fail to comply with the FDA’s Quality System Regulation (“QSR”) or comparable EU requirements, the FDA or EU competent authorities could take various enforcement actions, including suspending our FDA clearance to market, withdrawal of our EU CE Certificate or halting our manufacturing operations, and our business would suffer.
In the United States, as a manufacturer of a medical device, we are required to demonstrate and maintain compliance with the FDA’s QSR. The QSR covers the methods and documentation of the design, testing, control, manufacturing, labeling, quality assurance, packaging, storage and distribution of medical devices. The FDA enforces the QSR through periodic inspections and unannounced “for cause” inspections. Outside the United States, our products and operations are also required to comply with national requirements where the product is sold and also standards set by industrial standards bodies, such as the International Organization for Standardization. Foreign regulatory bodies may evaluate our products or the testing that our products undergo against these standards. The specific standards, types of evaluation and scope of review differ among foreign regulatory bodies. Our failure to comply with FDA or foreign regulatory agency requirements, or failure to take satisfactory and prompt corrective action in response to an adverse inspection, could result in enforcement actions, including a warning letter, adverse publicity, a shutdown of or restrictions on our manufacturing operations, a recall or seizure of our products, fines, injunctions, civil or criminal penalties, or other sanctions, any of which could cause our business and operating results to suffer.
We are subject to continuing regulation by the FDA, and failure to comply may materially harm our business.
We are subject to Medical Device Reporting (“MDR”) regulations, which require us to report to the FDA if we become aware of information that reasonably suggests our product may have caused or contributed to a death or serious injury or has malfunctioned and the device or a similar device we market would likely cause or contribute to a death or serious injury if the malfunction were to recur. We may fail to report adverse events of which we become aware within the prescribed timeframe. We may also fail to recognize that we have become aware of a reportable adverse event. If we fail to comply with our medical device reporting obligations, the FDA could issue warning letters or untitled letters, take administrative actions, commence criminal prosecution, impose civil monetary penalties, request or require a product recall, seize our products, or delay the clearance of our future products. We must report corrections and removals to the FDA where the correction or removal was initiated to reduce a risk to health posed by the device or to remedy a violation of the Federal Food, Drug, and Cosmetic Act, or FDCA, caused by the device that may present a risk to health.
Our failure to comply with these or other applicable regulatory requirements could result in enforcement actions by the FDA which may include untitled letters, warning letters, fines, injunctions, consent decrees and civil penalties; customer notifications or repair, replacement or refunds; and criminal prosecution.
Our products are generally subject to regulatory requirements in foreign countries in which we sell those products. We will be required to expend significant resources to obtain regulatory approvals or clearances of our products, and there may be delays and uncertainty in obtaining those approvals or clearances.
In order to sell our products in foreign countries, generally we must obtain regulatory approvals and comply with the regulations of those countries. These regulations, including the requirements for approvals or clearances and the time required for regulatory review, vary from country-to-country.
| 41 |
The EU requires that manufacturers certify compliance of medical devices with Council Directive (93/42/EEC) (“MDD”), as amended, and affix the CE mark before selling such devices in member countries of the EU or European Economic Area (“EEA”). The CE mark is an international symbol of adherence to quality assurance standards and compliance with applicable European medical device directives. In order to obtain the authorization to affix the CE mark to products, a manufacturer must certify that its product complies with the applicable directive, which may include a requirement to obtain certification that its processes and products meet certain European quality standards.
In May 2017, the EU adopted Regulation (EU) 2017/745 (“MDR”), which will repeal and replace the MDD with effect from May 26, 2021. Under transitional provisions, medical devices with notified body certificates issued under the MDD prior to May 26, 2021, may continue to be placed on the market for the remaining validity of the certificate, until May 27, 2024, at the latest as long as there have been no significant changes made to the product. After the expiry of any applicable transitional period, only devices that have been CE marked under the MDR may be placed on the market in the EU (or EEA). The MDR includes increasingly stringent requirements in multiple areas, such as pre-market clinical evidence (some of which are now in effect), review of high-risk devices, labeling and post-market surveillance. Under the MDR, pre-market clinical data will now be required to obtain CE Mark approval for high-risk, new and modified medical devices. We believe these new requirements have the potential to be expensive and time-consuming to implement and maintain.
Complying with and obtaining regulatory approval in foreign countries, including compliance with the MDR, have caused and will likely continue to cause us to experience more uncertainty, risk, expense and delay in commercializing products in certain foreign jurisdictions, which could have a material adverse impact on our net sales, market share and operating profits from our international operations.
If third-party payers do not provide adequate coverage and reimbursement for INVOcell and the IVC procedure, we may be unable to generate significant revenue.
Our success in marketing and commercializing INVOcell and the IVC procedure may depend in part on whether private health insurers and other payer organizations provide adequate coverage and reimbursement. If physicians or insurers do not find our clinical data compelling or wish to wait for additional studies, they may choose not to use or provide coverage and reimbursement for INVOcell and the IVC procedure. We cannot provide assurance that data we or others may generate in the future will be consistent with that observed in our existing clinical studies, or that our current or future published clinical evidence will be sufficient to obtain adequate coverage and reimbursement for our products. Moreover, if we cannot obtain adequate coverage for and reimbursement of the cost of our products, we cannot provide assurance that patients will be willing to incur the full cost of INVOcell and the IVC procedure.
| 42 |
Third-party payers, whether foreign or domestic, or governmental or commercial, are developing increasingly sophisticated methods of controlling healthcare costs. In addition, in the United States, no uniform policy of coverage and reimbursement for INVOcell and the procedure exists among third-party payers. Therefore, coverage and reimbursement for INVOcell and the IVC procedure may differ significantly from payer to payer. In addition, payers continually review new technologies for possible coverage and can, without notice, deny coverage for these new products and procedures. As a result, the coverage determination process is often a time-consuming and costly process that will require us to provide scientific and clinical support for the use of INVOcell and the IVC procedure to each payer separately, with no assurance that coverage and adequate reimbursement will be obtained or maintained if obtained.
Reimbursement systems in international markets vary significantly by country and by region within some countries, and reimbursement approvals must be obtained on a country-by-country basis. In many international markets, a product must be approved for reimbursement before it can be approved for sale in that country. Further, many international markets have government-managed healthcare systems that control reimbursement for new devices and procedures. In most markets, there are private insurance systems as well as government-managed systems. If sufficient and timely coverage and reimbursement is not available for our current or future products, in either the United States or internationally, the demand for our products and our revenues may be adversely affected.
We may be subject to risks related to changes in laws regarding abortion, which can affect how a fertility clinic must treat and handle embryos
In June 2022, the U.S. Supreme Court in Dobbs v. Jackson Women’s Health Organization overturned Roe v. Wade by holding that there is no constitutional right to abortion. This ended federal legalization on abortion, bringing the matter back to individual states to determine. Soon after the decision was handed down, several U.S. states adopted laws that drastically limited the availability of abortion, with a number of other states working on or proposing similar restrictions. While we believe these actions are more targeted toward abortions during pregnancy, certain laws may also impact embryos and how excess embryos are handled or implicate fertility procedures and travel reimbursement programs, which may decrease the demand for, or availability of, certain fertility services. Although President Biden issued executive orders and federal agencies have issued guidance intended to protect access to reproductive healthcare services, the enactment of certain state laws restricting abortion care and other changes in laws, or in interpretation of laws through court decisions, affecting fertility benefits may conflict with, and ultimately limit, the covered benefits offered by a company to its employees and the types of fertility treatment services available at provider clinics. We cannot predict the timing or impact of any future rulemaking, executive orders, court decisions or other changes in the law, or in how such laws, once enacted, would be interpreted and enforced. This may negatively impact fertility clinics and their patients operating in those states with more restrictive laws.
| 43 |
Risks Related to Our Therapeutics Business and Industry
Our ability to develop proprietary technology platforms and products and our future growth depend on retaining NAYA Therapeutics’ key personnel and recruiting additional qualified personnel.
NAYA Therapeutics is highly dependent on its co-founder, Chairman and Chief Executive Officer, Dr. Daniel Teper, who may terminate his current employment with us at any time. The loss of the services of Dr. Teper could impede the achievement of our therapeutics research, development and commercialization objectives.
Recruiting and retaining other senior executives, qualified scientific and clinical personnel and, if we progress the development of any of our product candidates, commercialization, manufacturing and sales and marketing personnel, will be critical to our success. The loss of the services of NAYA Therapeutics’ key employees could impede the achievement of our research, development and commercialization objectives and seriously harm our ability to successfully implement our business strategy. Furthermore, replacing key employees may be difficult and may take an extended period of time because of the limited number of individuals in NAYA Therapeutics industry with the breadth of skills and experience required to successfully lead, develop, gain regulatory approval of and commercialize our product candidates. Competition to hire from this limited pool is intense, and we may be unable to hire, train, retain or motivate these key personnel on acceptable terms given the competition among numerous pharmaceutical and biotechnology companies for similar personnel. We also experience competition for the hiring of scientific and clinical personnel from universities and research institutions. In addition, we rely on consultants and advisors, including scientific and clinical advisors, to assist in formulating our research and development and commercialization strategy. Our consultants and advisors may have commitments under consulting or advisory contracts with other entities that may limit their availability to us. If we are unable to continue to attract and retain high-quality personnel, our ability to pursue our growth strategy will be limited.
The regulatory processes that will govern the approval of our product candidates are complex and changes in regulatory requirements could result in delays or discontinuation of development or unexpected costs in obtaining regulatory approval.
Because we are developing novel cellular product candidates that are unique biological entities, the regulatory requirements that it will be subject to are not entirely clear. Regulatory requirements governing gene therapy products and cell therapy products have changed frequently and may continue to change in the future. Moreover, there is substantial, and sometimes uncoordinated, overlap in those responsible for regulation of existing gene therapy products and cell therapy products. Although the FDA and comparable foreign authorities decides whether individual therapy protocols may proceed, related review processes and determinations by other reviewing bodies can impede or delay the initiation of a clinical study, even if the FDA or comparable foreign authorities have reviewed the study and approved its initiation. Conversely, the FDA or comparable foreign authorities can place an IND application or equivalent foreign application or part of the application on clinical hold even if such other entities have provided a favorable review. Furthermore, each clinical trial must be reviewed and approved by an independent IRB or EC at or servicing each institution at which a clinical trial will be conducted. In addition, adverse developments in clinical trials of gene or cell therapy products conducted by others may cause the FDA or comparable foreign regulatory authorities to change the requirements for approval of any of our product candidates. Complex regulatory environments exist in other jurisdictions in which we may consider seeking regulatory approvals for our product candidates, further complicating the regulatory landscape.
| 44 |
The various committees and advisory groups involved in regulatory review, and new or revised guidelines that they promulgate from time to time may lengthen the regulatory review process, require us to perform additional studies, increase our therapeutic development costs, lead to changes in regulatory positions and interpretations, delay or prevent approval and commercialization of our product candidates or lead to significant post-approval limitations or restrictions. Because the regulatory landscape for our placental-derived cell product candidates is new, we may face even more cumbersome and complex regulations than those for more traditional pharmaceutical or biological products. Furthermore, even if our product candidates obtain required regulatory approvals, such approvals may later be withdrawn as a result of changes in regulations or the interpretation of regulations by applicable regulatory authorities. Delay or failure to obtain, or unexpected costs in obtaining, the regulatory approval necessary to bring a potential therapeutic to market could decrease our ability to generate sufficient revenue to maintain our therapeutics business.
We are dependent on the successful clinical development, regulatory approval and subsequent commercialization of our product candidates. If we are not able to obtain required regulatory approvals, we will not be able to commercialize our product candidates and our ability to generate product revenue from therapeutics will be adversely affected.
Our therapeutics business is dependent on our ability to successfully complete development of, obtain regulatory approval for, and, if approved, successfully commercialize our product candidates in a timely manner. We may face unforeseen challenges in its product candidate development strategy, and we can provide no assurances that our product candidate or future clinical trial design will prove to be effective, that we will be able to take advantage of expedited regulatory pathways for any of our product candidates, or that we will ultimately be successful in our future clinical trials. We expect that a substantial portion of our efforts and expenses over the next several years will be devoted to the development of our product candidates, including our lead product candidates, NY—303 and NY-338, in our future clinical trials. Our FLEX-NK™ cell engager antibody platform, including the product candidates derived from our platforms, are in early stages of development and may never be commercialized.
We currently anticipate seeking initial regulatory approvals in the United States and the European Union, but may in the future submit applications for the regulatory approval of one or more of our product candidates to additional foreign regulatory authorities. We have not applied or obtained regulatory approval for any product candidate in the United States or abroad, and it is possible that neither our current product candidates nor any product candidates we may seek to develop in the future will obtain regulatory approval. Neither us nor any of our partners are permitted to market any of our product candidates in the United States or abroad until it receives regulatory approval from FDA or comparable foreign regulatory authorities.
| 45 |
All of our product candidates will require additional clinical and non-clinical development, regulatory review and approval in multiple jurisdictions, substantial investment, access to sufficient commercial manufacturing capacity and significant marketing efforts before they can be successfully commercialized. Prior to obtaining approval to commercialize any product candidate in the United States or abroad, we must demonstrate with substantial evidence from its future well-controlled clinical trials, and to the satisfaction of the FDA or comparable foreign regulatory authorities, that such product candidate is safe and effective for its intended uses. Results from preclinical studies and clinical trials can be interpreted in different ways. Even if we believe that the preclinical or future clinical data for our product candidates are promising, such data may not be sufficient to support approval by the FDA and comparable foreign regulatory authorities. The FDA or comparable foreign regulatory authorities may also require us to conduct additional preclinical studies, assay development or future clinical trials for our product candidates either pre- or post-approval, or it may object to elements of our clinical development program, requiring their alteration. We may also decide to modify clinical protocols or procedures in future clinical trials based on clinical and experimental data.
Of the large number of products in development, only a small percentage successfully complete the FDA or comparable foreign regulatory authorities’ approval processes and are commercialized. The lengthy approval or marketing authorization process as well as the unpredictability of future clinical trial results may result in our failing to obtain regulatory approval or marketing authorization to market our product candidates, which could significantly harm our business, financial condition, results of operations and prospects.
Our product candidates could fail to receive regulatory approval from the FDA or a comparable foreign regulatory authority for many reasons, including, among others:
| ● | disagreement with the design or conduct of any of our future clinical trials; | |
| ● | failure to demonstrate to the satisfaction of regulatory agencies that our product candidates are safe and effective, or have a positive benefit/risk profile for our proposed indication; | |
| ● | failure of future clinical trials to meet the level of statistical significance required for approval; | |
| ● | disagreement with our interpretation of data from preclinical studies or future clinical trials; | |
| ● | the insufficiency of data collected from future clinical trials of our product candidates to support the submission and filing of a BLA or equivalent foreign submission or to obtain regulatory approval; | |
| ● | failure to obtain approval of our therapeutics manufacturing processes or facilities of third-party manufacturers with whom we contract for clinical and commercial supplies or our own therapeutics manufacturing facility; or | |
| ● | changes in the approval policies or regulations that render our preclinical and clinical data insufficient for approval. |
| 46 |
Additionally, any delay in, or termination of, our future clinical trials will delay the submission of a BLA to the FDA or other equivalent applications with other relevant foreign regulatory authorities and, ultimately, our ability to commercialize our product candidates, if approved, and generate product revenue.
Even if we eventually complete clinical testing and receive approval of a BLA, or equivalent foreign marketing application for its product candidates, the FDA or the comparable foreign regulatory authorities may grant approval or other marketing authorization contingent on the performance of costly additional future clinical trials, including post-market clinical trials. The FDA or the comparable foreign regulatory authorities also may approve or authorize marketing a product candidate for a more limited indication or patient population than we originally request, and the FDA or comparable foreign regulatory authorities may not approve or authorize the labeling that we believe is necessary or desirable for the successful commercialization of a product candidate. Any delay in obtaining, or inability to obtain, applicable regulatory approval or other marketing authorization would delay or prevent commercialization of that product candidate and would adversely impact our business and prospects.
Moreover, because all of our product candidates are based on the same FLEX-NK cell engager antibody platform technologies, if any of our product candidates encounter safety or efficacy problems, developmental delays or regulatory issues or other problems, these could impact the development plans for our other product candidates. Our failure to timely complete our future clinical trials, obtain regulatory approval or, if approved, commercialize our product candidates could adversely affect our business, financial condition and results of operations.
Our fully integrated product candidates represent new therapeutic approaches that could result in heightened regulatory scrutiny, delays in clinical development or delays in or our inability to achieve regulatory approval, commercialization, or payor coverage of our product candidates.
Our future success is dependent on the successful development of our product candidates in general and our development product candidates in particular. Because these programs represent a new approach to the treatment of cancer, developing and, if approved, commercializing our product candidates subject us to a number of challenges. Moreover, we cannot be sure that the manufacturing processes used in connection with our product candidates will yield a sufficient supply of satisfactory products that are safe, pure and potent, scalable, or profitable.
Actual or perceived safety issues, including adoption of new therapeutics or novel approaches to treatment, may adversely influence the willingness of subjects to participate in future clinical trials, or if approved by applicable regulatory authorities, of physicians to subscribe to the novel treatment mechanics. The FDA or other comparable foreign regulatory authorities may ask for specific post-market requirements, and additional information informing benefits or risks of our products may emerge at any time prior to or after regulatory approval.
| 47 |
Physicians, hospitals, and third-party payors often are slow to adopt new products, technologies, and treatment practices that require additional upfront costs and training. Physicians may not be willing to undergo training to adopt this novel therapy, may decide the therapy is too complex to adopt without appropriate training or not cost-efficient, and may choose not to administer the therapy. Based on these and other factors, hospitals and payors may decide that the benefits of this new therapy do not or will not outweigh its costs.
Even if any of our product candidates receive marketing approval, we may fail to achieve market acceptance by physicians, patients, third-party payors or others in the medical community necessary for commercial success.
The use of FLEX-NK™ cell engager antibodies as a potential treatment for cancer is a recent development and may not become broadly accepted by physicians, patients, hospitals, cancer treatment centers and others in the medical community. Therefore, even if any of our product candidates receive marketing approval, we may fail to gain market acceptance by physicians, patients, third-party payors and others in the medical community. If any such product candidate does not achieve an adequate level of acceptance, we may not generate significant product revenue and may not become profitable. The degree of market acceptance of any product candidate, if approved for commercial sale, will depend on a number of factors, including but not limited to:
| ● | physicians, hospitals, cancer treatment centers and patients considering our product candidates as a safe and effective treatment; | |
| ● | the cost, efficacy, safety profile, convenience, ease of administration and other potential advantages compared to alternative treatments and therapies; | |
| ● | the willingness of patients to pay out-of-pocket in the absence of coverage and adequate reimbursement by third-party payors and government authorities; | |
| ● | product labeling or product insert requirements of the FDA or other comparable foreign regulatory authorities and any limitations or warning contained in the labeling approved by the FDA or comparable foreign regulatory authorities; | |
| ● | the willingness of the target patient population to try new therapies and of physicians to prescribe these therapies; | |
| ● | the strength of our relationships with patient communities; | |
| ● | the availability of third-party coverage and adequate reimbursement; | |
| ● | the prevalence and severity of any side effects; | |
| ● | the timing of market introduction of our product candidates as well as competitive products; | |
| ● | relative convenience and ease of administration, including as compared to alternative treatments and competitive therapies; and | |
| ● | the effectiveness of our sales and marketing efforts. |
| 48 |
Our efforts to educate physicians, patients, third-party payors and others in the medical community on the benefits of our product candidates may require significant resources and may never be successful. Such efforts may require more resources than are typically required due to the complexity and uniqueness of our product candidates.
Furthermore, the attention to different types of prospective treatments and proposed cures for cancers has historically varied. In recent years, various forms of oncological immunotherapy have been prominent areas for academic and clinical advancement. While FLEX-NK cell engager antibodies have not yet received prominent negative attention from the mainstream media or the scientific press, it is possible that it could, and it is possible that if immunotherapy generally falls out of favor with these key constituencies, whether due to the failure of one or more competitive products or technologies or otherwise, our business, including our ability to conduct future clinical trials and to raise capital, may in turn suffer.
If our product candidates are approved but fail to achieve market acceptance among physicians, patients, hospitals, cancer treatment centers or others in the medical community, we will not be able to generate significant therapeutics revenue. Even if our cell therapies achieve market acceptance, we may not be able to maintain that market acceptance over time if new products or technologies are introduced that are more favorably received than our therapeutics, are more cost effective or render our therapeutics obsolete.
Even if we obtain and maintain approval for our product candidates from the FDA, we may never obtain approval outside the United States, which would limit our market opportunities.
Approval of a product candidate in the United States by the FDA does not ensure approval of such product candidate by regulatory authorities in other countries or jurisdictions, and approval by one foreign regulatory authority does not ensure approval by regulatory authorities in other foreign countries or by the FDA. Sales of our product candidates outside the United States will be subject to foreign regulatory requirements governing clinical trials and marketing approval. Even if the FDA grants marketing approval for a product candidate, comparable foreign regulatory authorities also must approve the manufacturing and marketing of the product candidate in those countries. Approval procedures vary among jurisdictions and can involve requirements and administrative review periods different from, and more onerous than, those in the United States, including additional preclinical studies or clinical trials. In many countries outside the United States, a product candidate must be approved for reimbursement before it can be approved for sale in that country. In some cases, the price that we intend to charge for any product candidates, if approved, is also subject to approval. Obtaining approval for our product candidates in the European Union from the European Commission following the opinion of the EMA, if we choose to submit a marketing authorization application there, would be a lengthy and expensive process. Even if a product candidate is approved, the European Commission may limit the indications for which the product may be marketed, require extensive warnings on the labeling or require expensive and time-consuming additional clinical trials or reporting as conditions of approval. Obtaining foreign regulatory approvals and compliance with foreign regulatory requirements could result in significant delays, difficulties and costs for us and could delay or prevent the introduction of our product candidates in certain countries. We expect the product candidates we develop will be regulated as biologics, and therefore they may be subject to competition sooner than anticipated.
| 49 |
The Biologics Price Competition and Innovation Act of 2009 (“BPCIA”) was enacted as part of the Patient Protection and Affordable Care Act of 2010, as amended by the Health Care and Education Reconciliation Act of 2010 (“ACA”) to establish an abbreviated pathway for the approval of biosimilar and interchangeable biological products. The regulatory pathway establishes legal authority for the FDA to review and approve biosimilar biologics, including the possible designation of a biosimilar as “interchangeable” based on its similarity to an approved biologic. Under the BPCIA, an application for a biosimilar product cannot be approved by the FDA until 12 years after the reference product was approved under a BLA. The law is complex and is still being interpreted and implemented by the FDA. As a result, its ultimate impact, implementation, and meaning are subject to uncertainty. While it is uncertain when processes intended to implement BPCIA may be fully adopted by the FDA, any of these processes could have a material adverse effect on the future commercial prospects for our biological products.
We believe that any of the product candidates we develop that are approved in the United States as a biological product under a BLA should qualify for the 12-year period of exclusivity. However, there is a risk that this exclusivity could be shortened due to congressional action or otherwise, or that the FDA will not consider the subject product candidates to be reference products for competing products, potentially creating the opportunity for generic competition sooner than anticipated. Moreover, the extent to which a biosimilar, once approved, will be substituted for any one of the reference products in a way that is similar to traditional generic substitution for non-biological products is not yet clear, and will depend on a number of marketplace and regulatory factors that are still developing.
The European Union also provides opportunities for market and data exclusivity. In particular, products containing a New Active Substance (“NAS”) (such as a chemical, biological or radiopharmaceutical substance not previously authorized as a medicinal product in the European Union), which have been granted a marketing authorization receive eight years of data exclusivity and an additional two years of market exclusivity. If granted, the data exclusivity period prevents applicants for approval of a biosimilar product from referencing the innovator’s preclinical and clinical trial data contained in the dossier of the reference product in the European Union during a period of eight years from the date on which the reference product was first authorized in the European Union. During the additional two year period of market exclusivity, while an application for marketing authorization of a biosimilar can be submitted, and the innovator’s data referenced no biosimilar product can be marketed until the expiration of the market exclusivity period. The overall 10 year market exclusivity period can be extended to a maximum of eleven years if, during the first eight years of those 10 years, the marketing authorization holder for the innovative product obtains an authorization for one or more new therapeutic indications which, during the scientific evaluation prior to their authorization, are held to bring a significant clinical benefit in comparison with existing therapies.
| 50 |
We believe that any of the product candidates we develop that are approved in the European Union as a biological product should also qualify for the eight years of data exclusivity and 10 years of market exclusivity. However, a biosimilar, once approved, may be substituted for its reference product. The implications of such substitution varies between EU Member States and can, in some Member States, include obligatory substitution in some circumstance. In addition, the approval of a biologic product biosimilar to one of our products could have a material adverse impact on our business as it may be significantly less costly to bring to market and may be priced significantly lower than our products.
Our product candidates are in early stages of development, and therefore will require extensive additional preclinical and clinical testing. Success in preclinical studies or early-stage clinical trials may not be indicative of results in future clinical trials and we cannot assure you that any ongoing, planned or future clinical trials will lead to results sufficient for the necessary regulatory approvals.
Because our product candidates are in early stages of development, they will require extensive preclinical and clinical testing. NY-303 and NY-338 are our only product candidates that have completed preclinical trials. Success in preclinical testing and early-stage clinical trials does not ensure that later clinical trials will generate the same results or otherwise provide adequate data to demonstrate the efficacy and safety of a product candidate. Preclinical studies are primarily designed to test safety and biological activity. Phase II/II oncology clinical trials to study pharmacokinetics and pharmacodynamics and help to understand the preliminary efficacy and side effects of product candidates at various doses and schedules. Success in preclinical studies and early-stage clinical trials does not ensure that later efficacy trials will be successful, nor does it predict final results. Our product candidates may fail to show the desired safety and efficacy in clinical development despite positive results in preclinical studies or even if they successfully advance through early-stage clinical trials.
Further, our novel approaches to address solid and hematological tumors through multispecific antibodies are unproven and as such, the cost and time needed to develop our product candidates is difficult to predict and our efforts may not be successful. If we do not observe favorable results in clinical trials of our product candidates, we may decide to delay or abandon clinical development of such product candidate. Any such delay or abandonment could harm our business, financial condition, results of operations and prospects.
In addition, the design of a clinical trial can determine whether our results will support approval of a product, and flaws in the design of a clinical trial may not become apparent until the clinical trial is well advanced. As an organization, we have limited experience designing clinical trials and may be unable to design and execute a clinical trial to support regulatory approval. Many companies in the pharmaceutical and biotechnology industries have suffered significant setbacks, including failure in late-stage clinical trials even after achieving promising results in preclinical testing and earlier clinical trials. Data obtained from preclinical and clinical activities are subject to varying interpretations, which may delay, limit or prevent regulatory approval.
| 51 |
Further, we cannot predict with any certainty if or when it might submit a Biologics License Application, (“BLA”), or comparable foreign application, for regulatory approval for any of our product candidates or whether any such BLA or comparable foreign application will be accepted for review by the FDA, or comparable foreign authority or whether any BLA or comparable foreign application will be approved upon review. Even if our future clinical trials are completed as planned, it cannot be certain that their results will support the proposed indications. Success in preclinical testing and early clinical trials does not ensure that later clinical trials will be successful, and we cannot be sure that the results of later clinical trials will replicate the results of prior clinical trials and preclinical testing. Our future clinical trial process may fail to demonstrate that our product candidates are safe and effective for their proposed uses. This failure could cause us to abandon a product candidate and may delay development of other product candidates. Any delay in, or termination of, our clinical trials will delay and possibly preclude the filing of any BLAs or comparable foreign application with the FDA and, ultimately, our ability to commercialize our product candidates and generate therapeutic product revenues.
Our approach to the development of product candidates based on our FLEX-NK™ cell engager antibody platform is unproven, and we do not know whether we will be able to develop any products of commercial value, or if competing technological approaches will limit the commercial value of our product candidates or render our platforms obsolete.
We believe that our product candidates represent a novel approach to immune-oncology, and we have concentrated significant research and development efforts to date developing our FLEX-NK™ cell engager antibody platform technology. The product candidates derived from our technologies, including NY-303 and NY-338, have not been extensively tested over any significant period of time. We have not yet succeeded and may never succeed in demonstrating efficacy and safety for any of our product candidates in clinical trials or in obtaining marketing approval therefore.
For example, FLEX-NK cell engager antibody platform technologies are a novel field of development and are subject to particular risks that are difficult to quantify, which could ultimately affect safety, efficacy and our ability to produce products in a reliable and consistent manner. As such, we may be faced with unforeseen delays and setbacks, in addition to the other foreseeable risks and uncertainties associated with developing immune cell therapies.
Any delay or difficulties in the manufacturing and/or clinical supply of NY-303 and NY-338, or any of our other current or future product candidates would adversely affect our therapeutics business and operations.
Advancing product candidates utilizing such novel approaches to immunotherapy creates significant challenges for us, including, among others:
| ● | manufacturing our product candidate to our specifications and in a timely manner to support our future clinical trials, and, if approved, commercialization; | |
| ● | sourcing clinical and, if approved, commercial supplies for the raw materials used to manufacture our product candidates; | |
| ● | enrolling sufficient numbers of patients in future clinical trials; |
| 52 |
| ● | understanding and addressing variability in demand for manufacturing and our impact on capacity utilization of available infrastructure and costs; | |
| ● | submitting applications for and obtaining regulatory approval, as the FDA and other regulatory authorities including comparable foreign authorities have limited experience with commercial development of immunotherapies for cancer and viral associated infectious diseases; and | |
| ● | establishing sales and marketing capabilities, as well as developing a manufacturing process and distribution network to support the commercialization of any approved products. |
We must be able to overcome these challenges in order for it to successfully develop, commercialize and manufacture its product candidates utilizing its novel approaches to address solid and hematological tumors.
Clinical product candidate development involves a lengthy and expensive process and involves uncertain outcomes. We may incur additional costs and encounter substantial delays or difficulties in our therapeutics clinical trials.
We may not commercialize, market, promote or sell any product candidate without obtaining marketing approval from the FDA or other comparable foreign regulatory authority, and it may never receive such approvals. It is impossible to predict when or if any of our product candidates will prove effective or safe in humans and will receive regulatory approval. Before obtaining marketing approval from regulatory authorities for the sale of our product candidates, it must complete preclinical development and then conduct extensive clinical trials to demonstrate the safety and efficacy of our product candidates in humans. Clinical testing is expensive, is difficult to design and implement, can take many years to complete and is uncertain as to outcome.
A failure of one or more clinical trials can occur at any stage of testing. Moreover, preclinical and clinical data are often susceptible to varying interpretations and analyses, and many companies that have believed their product candidates performed satisfactorily in preclinical studies and clinical trials have nonetheless failed to obtain marketing approval of their products. Additionally, preclinical trials for our product candidates involve studying a relatively small patient population, which makes it difficult to predict whether the favorable results observed in such clinical trial will be repeated in larger and more advanced clinical trials.
We may experience numerous unforeseen events prior to, during, or as a result of, clinical trials that could delay or prevent its ability to receive marketing approval or commercialize its product candidates, including, but not limited to the following:
| ● | delays in reaching a consensus with regulatory authorities on the design, location or implementation of our clinical trials; | |
| ● | delays or setbacks in patient enrollment; |
| 53 |
| ● | clinical trials of our product candidates may produce negative or inconclusive results; | |
| ● | the number of patients required for clinical trials for our product candidates may be larger than it anticipates, enrollment in these clinical trials may be slower than it anticipate or may be lower than it anticipates due to challenges in recruiting and enrolling suitable patients that meet the study criteria, participants may drop out of these clinical trials at a higher rate than we anticipate or the duration of these clinical trials may be longer than it anticipates; | |
| ● | imposition of a clinical hold by regulatory authorities as a result of, among other reasons, a serious adverse event, a failure in the chemistry manufacturing and controls requirements, or a failed inspection of our clinical trial operations, trial sites or manufacturing facilities; | |
| ● | occurrence of serious adverse events associated with the product candidate that are viewed to outweigh its potential benefits; and | |
| ● | need to conduct additional clinical trials or abandon product development programs. |
Any inability to successfully complete preclinical and commence and complete clinical development could result in additional costs or impair its ability to generate revenue from future product sales or other sources. In addition, if we make manufacturing or formulation changes to its product candidates, we may need to conduct additional testing to bridge our modified product candidate to earlier versions. Clinical trial delays could also shorten any periods during which we may have the exclusive right to commercialize its product candidates, if approved, or allow our competitors to bring competing products to market before it does, which could impair our ability to successfully commercialize our product candidates.
In addition, the clinical trial requirements of the FDA and other foreign regulatory authorities and the criteria these regulators use to determine the safety and efficacy of a product candidate vary substantially according to the type, complexity, novelty, and intended use and market of the potential products. The regulatory approval process for product candidates such as ours can be more expensive and take longer than for other, better known, or more extensively studied pharmaceutical or other product candidates. Regulatory agencies administering existing or future regulations or legislation may not allow production and marketing of products utilizing gene regulation technology in a timely manner or under technically or commercially feasible conditions. Regulatory action or private litigation could result in expenses, delays or other impediments to our research programs or the future commercialization of resulting products.
Further, if the results of our future clinical trials are inconclusive or if there are safety concerns or serious adverse events associated with our product candidates, it may be delayed in obtaining marketing approval, or not obtain marketing approval at all, obtain approval with labeling that includes significant use or distribution restrictions or safety warnings, and/or have regulatory authorities withdraw or suspend their approval or impose restrictions on distribution in the form of a modified risk evaluation and mitigation strategy (“REMS”) or equivalent steps imposed by foreign authorities among other results. We could also encounter delays if physicians encounter unresolved ethical issues associated with enrolling patients in our future clinical trials of its product candidates in lieu of prescribing existing treatments that have established safety and efficacy profiles.
| 54 |
Additionally, the FDA or comparable foreign authority or an independent institutional review board (“IRB”) or Ethics Committee (“EC”) may also suspend our future clinical trials at any time if it appears that we or our collaborators are failing to conduct a trial in accordance with regulatory requirements, including the FDA’s Good Clinical Practice (“GCP”) regulations, or equivalent foreign rules that it is exposing participants to unacceptable health risks, or if the FDA or comparable foreign authority finds deficiencies in our investigational new drug (“IND”) applications or the conduct of these trials. Therefore, we cannot predict with any certainty the schedule for commencement and completion of future clinical trials. If we experience delays in the commencement or completion of our future clinical trials, or if we terminate a clinical trial prior to completion, the commercial prospects of our product candidates could be negatively impacted, and our ability to generate revenues from our product candidates may be delayed.
If we encounter difficulties in enrolling patients in our future clinical trials, our clinical development activities could be delayed or otherwise adversely affected.
The timely completion of our future clinical trials in part depends on patient enrollment, and as such identifying and qualifying patients to participate in our future clinical trials is critical to our success in therapeutics. We may encounter difficulties in enrolling a sufficient number of eligible patients to participate in its future clinical trials, thereby delaying or preventing development and approval of our product candidates. Even once enrolled, we may be unable to retain a sufficient number of patients to complete any of its trials. There are limited patient pools from which to draw in order to complete our future clinical trials in a timely and cost-effective manner. If any such patient enrolled in our future phase I trials has to drop out due to pre-existing health issues or due to a serious adverse effect, or otherwise dies, and we are not able to recruit additional patients in a timely manner, or at all, our clinical trials could be delayed or otherwise halted. As such, despite diligent planning of our clinical trials and analysis of their feasibility regarding patient recruitment, it may experience difficulties, delays or inability in patient enrollment in our clinical trials for a variety of reasons, including:
| ● | the size and nature of the patient population; | |
| ● | the severity and incidence of the disease under investigation; | |
| ● | the design of the trial and the complexity for patients and clinical sites; | |
| ● | the general health condition of the patient and their immune broadly; | |
| ● | the risk that patients’ general health conditions do not allow the conduct of study/screening procedures (such as leukapheresis), the manufacture of therapeutic product or application of the appropriate standard-of-care treatment or application of the Stupp regimen; |
| 55 |
| ● | the ability to consistently manufacture FLEX-NK™ engager product candidates in sufficient quantities at sufficient activity and/or transduction efficiency to provide a suitable therapeutic dose of FLEX-NK cell engager antibodies; | |
| ● | competing clinical trials for similar therapies, other new therapeutics, new combination treatments, new medicinal products; | |
| ● | clinicians’ and patients’ perceptions as to the potential advantages and side effects of the product candidate being studied in relation to other available therapies, including any new drugs or treatments that may be approved or become standard of care for the indications we are investigating; | |
| ● | the ability to obtain and maintain patient consents due to various reasons; | |
| ● | the risk that enrolled subjects will drop out, develop complications or die before completion of the trial; | |
| ● | the ability to develop and provide appropriate screening, product characterization and release assays; | |
| ● | patients failing to complete a clinical trial or returning for post-treatment follow-up; | |
| ● | our ability to manufacture the requisite materials for a patient and clinical trial; and | |
| ● | inability of clinical sites to enroll patients as health care capacities are required to cope with natural disasters, epidemics or other health system emergencies. |
Our efforts to build relationships with patient communities may not succeed, which could result in delays in patient enrollment in our clinical trials. Any negative results we may report in clinical trials of our product candidates may make it difficult or impossible to recruit and retain patients in other clinical trials of that same product candidate. Delays or failures in planned patient enrollment or retention may result in increased costs, program delays or both, which could have a harmful effect on our ability to develop its product candidates or could render further development impossible. In addition, we may rely on clinical research organizations (“CROs”) and clinical trial sites to ensure proper and timely conduct of our future clinical trials and, while it intends to enter into agreements governing their services, we will be limited in its ability to ensure their actual performance.
| 56 |
We may not be able to file Investigational New Drug Applications to commence future clinical trials on the timelines it expects, and even if it is able to, the FDA or comparable foreign authority may not permit us to proceed.
We expect our pipeline to lead to multiple investigational new drug applications (“INDs”), starting in 2025. We cannot be sure that submission of an IND will result in the FDA or comparable foreign authority allowing testing and clinical trials to begin, or that, once begun, issues will not arise that suspend or terminate such clinical trials. The manufacturing of our product candidates remains an emerging and evolving field. Accordingly, we expect that chemistry, manufacturing and control related topics, including product specifications, will be a focus of IND reviews, which may delay the clearance of INDs or equivalent foreign applications. Additionally, even if such regulatory authorities agree with the design and implementation of the clinical trials set forth in an IND, or equivalent foreign applications or clinical trial application, we cannot guarantee that such regulatory authorities will not change their requirements in the future.
Our product candidates may cause serious adverse events or undesirable side effects or have other properties that may delay or prevent regulatory approval, cause us to suspend or discontinue clinical trials, limit the commercial profile of an approved label, or result in significant negative consequences following marketing approval, if any.
During the conduct of clinical trials, patients report changes in their health, including illnesses, injuries and discomforts, to their doctor. Often, it is not possible to determine whether or not the product candidate being studied caused these conditions. Regulatory authorities may draw different conclusions or require additional testing to confirm these determinations, if they occur. Many times, side effects are only detectable after investigational drugs are tested in large-scale pivotal trials or, in some cases, after they are made available to patients on a commercial scale after approval. If additional clinical experience indicates that any of our product candidates have side effects or cause serious or life threatening side effects, the development of the product candidate may fail or be delayed, or, if the product candidate has received regulatory approval, such approval may be revoked, which would harm our business, prospects, operating results and financial condition.
Undesirable side effects caused by our product candidates, delivery methods or dosage levels could cause us or regulatory authorities to interrupt, delay or halt clinical trials and could result in a more restrictive label or the delay or denial of regulatory approval by the FDA or other comparable foreign regulatory authority. As a result of safety or toxicity issues that we may experience in its clinical trials, we may be placed on clinical hold and not receive approval to market any product candidates, which could prevent it from ever generating revenues or achieving profitability. Results of our trials could reveal an unacceptably high severity and incidence of side effects, or side effects outweighing the benefits of our product candidates. In such an event, our studies could be delayed, suspended or terminated and the FDA or comparable foreign regulatory authorities could order us to cease further development of or deny approval of our product candidates for any or all targeted indications. The drug-related side effects could affect patient recruitment or the ability of enrolled subjects to complete the trial or result in potential product liability claims.
| 57 |
As we start developing its lead product candidates and anticipate initiating clinical trials of our additional product candidates, serious adverse events, (“SAEs”), undesirable or potentially fatal side effects, cytokine release syndrome, viral or bacterial infections, relapse of disease or unexpected characteristics may emerge causing it to abandon these product candidates or limit their development to more narrow uses or subpopulations in which the SAEs or undesirable side effects or other characteristics are less prevalent, less severe or more acceptable from a risk-benefit perspective or in which efficacy is more pronounced or durable. Treatment-related side effects could also affect patient recruitment or the ability of enrolled patients to complete the trial or result in potential product liability claims. In addition, these side effects may not be appropriately recognized or managed by the treating medical staff, and inadequate training in recognizing or managing the potential side effects of our product candidates could result in patient injury or death. Should we observe SAEs in its clinical trials or identify undesirable side effects or other unexpected findings, our trials could be delayed or even terminated and our development programs may be halted entirely.
Additionally, if any of our product candidates receive regulatory approval, and we or others later identify undesirable side effects caused by such product, a number of potentially significant negative consequences could result. The product could face a recall or withdrawal from the market, the FDA could also issue a safety alert about the product or require us to adopt a REMS to ensure that the benefits of treatment with such product candidate outweigh the risks for each potential patient, which may include, among other things, a communication plan to health care practitioners, patient education, extensive patient monitoring or distribution systems and processes that are highly controlled, restrictive and more costly than what is typical for the industry. Our collaborators may also be required to adopt a REMS or engage in similar actions, such as patient education, certification of health care professionals or specific monitoring, if we or others later identify undesirable side effects caused by any product that we develop alone or with collaborators.
Any of these events could diminish the usage or otherwise limit the commercial success of our product candidates and prevent it from achieving or maintaining market acceptance of the affected product candidate, if approved by applicable regulatory authorities.
Interim, “top-line” and preliminary data from our clinical trials that it announces or publishes from time to time may change as more patient data become available and are subject to audit and verification procedures that could result in material changes in the final data.
From time to time, we may publish interim, “top-line” or preliminary data from its clinical trials. Interim, “top-line” or preliminary data from clinical trials that we may complete are subject to the risk that one or more of the clinical outcomes may materially change as patient enrollment continues and more patient data becomes available. Interim, “top-line” and preliminary data also remain subject to audit and verification procedures that may result in the final data being materially different from the preliminary data we previously published. As a result, interim, “top-line,” and preliminary data should be viewed with caution until the final data are available. Differences between interim, “top-line” and preliminary data and final data could significantly harm our business prospects and may cause the trading price of our common stock to fluctuate significantly.
| 58 |
Further, others, including regulatory agencies, may not accept or agree with our assumptions, estimates, calculations, conclusions or analyses or may interpret or weigh the importance of data differently, which could impact the value of the particular program, the future approvability or commercialization of the particular product candidate or product and our business in general. In addition, the information we choose to publicly disclose regarding a particular study or clinical trial is based on what is typically extensive information, and you or others may not agree with what we determine is the material or otherwise appropriate information to include in our disclosure, and any information we determine not to disclose may ultimately be deemed significant with respect to future decisions, conclusions, views, activities or otherwise regarding a particular product candidate or our business. If the interim, “top-line,” or preliminary data that we report differs from actual results, or if others, including regulatory authorities, disagree with the conclusions reached, our ability to obtain approval for and commercialize our product candidates, our business, operating results, prospects or financial condition may be harmed.
We face significant competition from other biotechnology and pharmaceutical companies, which may result in others discovering, developing, or commercializing products before or more successfully than we do.
The clinical and commercial landscape in the indications we are targeting, as well as the field of immune-oncology, is highly competitive. We may face potential competition with respect to our current product candidates and may face competition with respect to any other product candidates that it may seek to develop or commercialize in the future from pharmaceutical and biotechnology companies, academic institutions, government agencies and other public and private research institutions.
Many of our current or potential competitors have greater financial and other resources, larger research and development staffs, and more experienced capabilities in researching, developing and testing products than we do. Many of these companies also have more experience in conducting clinical trials, obtaining FDA and other equivalent foreign regulatory approvals, and manufacturing, marketing and distributing therapeutic products. Smaller or clinical-stage companies like us may successfully compete by establishing collaborative relationships with larger pharmaceutical companies or academic institutions. Mergers and acquisitions in the pharmaceutical and biotechnology industries may result in even more resources being concentrated among a smaller number of our competitors. These competitors also compete with us in recruiting and retaining qualified scientific and management personnel and establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies complementary to, or necessary for, our programs. Our commercial opportunity could be reduced or eliminated if our competitors develop and commercialize products that are safer, more effective, have fewer or less severe side effects, are more convenient, or are less expensive than any products that we may develop. Furthermore, currently approved products could be discovered to have application for treatment of cancer and other diseases, which could give such products significant regulatory and market timing advantages over any of our product candidates. In addition, large pharmaceutical companies or other companies with greater resources or experience than we may choose to forgo therapy opportunities that would have otherwise been complementary to our product development and collaboration plans. Our competitors may succeed in developing, obtaining patent protection for, or commercializing their products more rapidly than us, which could result in our competitors establishing a strong market position before we are able to enter the market. A competing company developing or acquiring rights to a more effective therapeutic product for the same diseases targeted by us, or one that offers significantly lower costs of treatment could render our products noncompetitive or obsolete. We may not be successful in marketing any product candidates we may develop against competitors.
| 59 |
We have provided information, based on publicly available information, relating to many other companies in our industry throughout this proxy statement, including, without limitation, Summit Therapeutics, Keytruda, Bicara Therapeutics, Zenas Biopharma, GSK, Crescent Biopharma, Navigator Medicine, and Janux, as well as larger companies such as Amgen, AstraZeneca, Johnson & Johnson, Merck, Merus, Pfizer, Sanofi, Xencor, and Zymeworks. Our disclosure of the performance, stage of development, capital raising, market capitalizations, and valuations of the foregoing companies is for informational purposes only and is neither indicative nor predicative of our performance, capital raising, market capitalization, or valuation. There are no guarantees that we may secure similar financing, execute our development plans, or achieve comparable performance, capitalization, or market or other valuations.
We face substantial competition from multiple sources, including large and specialty pharmaceutical, biopharmaceutical and biotechnology companies, academic research institutions and governmental agencies and public and private research institutions.
Our competitors compete with it on the level of the technologies employed, or on the level of development of product candidates. In addition, many small biotechnology companies have formed collaborations with large, established companies to (i) obtain support for their research, development and commercialization of products or (ii) combine several treatment approaches to develop longer lasting or more efficacious treatments that may potentially directly compete with our current or future product candidates. We anticipate that we will continue to face increasing competition as new therapies and combinations thereof, technologies, and data emerge within the field of immunotherapy and, furthermore, within the treatment of cancers.
NY-500 may not surpass the current standard of care or that sales of NY-500, if ever approved, will approach or surpass the market leader.
We aim to advance IND filings with the FDA and phase I/IIa clinical trial initiation in 2026 for NY-500, an AI-optimized PD1-VEGF bifunctional antibody, in first line HCC treatment. NY-500 is a tetravalent bifunctional antibody targeting PD-1, a key immune checkpoint targeted by pembrolizumab (Keytruda®, Merck & Co), and VEGF, a vascular endothelial growth factor targeted by bevacizumab (Avastin®, Genentech Roche) which regulates the production of new blood vessels (angiogenesis). There is no guarantee that PD1 x VEGF bifunctional antibodies will surpass the standard of care demonstrated by Keytruda, or at all, and even if it does surpass the standard of care, there is no guarantee that sales in PD1 x VEGF bifunctional antibody products generally, or sales in our product candidate, if ever approved, and if any, will surpass those of Keytruda.
We intend to study our product candidates in patient populations with significant comorbidities, and these patients may also receive treatment with cytotoxic lymphodepletion agents and other immunotherapies, and/or other treatments, and/or other treatments that may result in deaths or serious adverse or unacceptable side effects and require us to abandon or limit our clinical development activities.
Patients treated with our product candidates in clinical trials may also receive treatment with cytotoxic lymphodepletion agents and other immunotherapies and/or other treatments, and may therefore experience side effects or adverse events, including death, that are unrelated to our product candidates. While these side effects or adverse events may be unrelated to our product candidates, they may still affect the success of our clinical studies. The inclusion of critically ill patients in our clinical studies may result in deaths or other adverse medical events due to underlying disease or to other therapies or medications that such patients may receive. Any of these events could prevent us from advancing its product candidates through clinical development, and from obtaining regulatory approval, and would impair our ability to commercialize its product candidates. Any inability to advance our existing product candidates or any other product candidate through clinical development may have a material adverse effect on our business.
| 60 |
We may not identify or discover other product candidates and may fail to capitalize on programs or product candidates that may present a greater commercial opportunity or for which there is a greater likelihood of success.
Our efforts to identify and develop additional product candidates will require substantial technical, financial and human resources, whether or not any product candidates are ultimately identified. We may also broaden the reach of our FLEX-NK™ cell engager antibody platform by selectively in-licensing technologies or product candidates. Alternately, our efforts may initially show promise in identifying potential product candidates, yet fail to yield product candidates for clinical development, approved products or commercial revenues for many reasons, including the following:
| ● | the methodology used may not be successful in identifying potential product candidates; | |
| ● | competitors may develop alternatives that render any product candidates it develops obsolete; | |
| ● | any product candidates it develops may be covered by third parties’ patents or other exclusive rights; | |
| ● | a product candidate may demonstrate harmful side effects or other characteristics that indicate it is unlikely to be effective or otherwise does not meet applicable regulatory criteria; | |
| ● | a product candidate may not be capable of being produced in commercial quantities at an acceptable cost, or at all; and | |
| ● | a product candidate may not be accepted as safe and effective by physicians, patients, the medical community or third-party payors. |
We have limited financial and management resources and, as a result, we may forego or delay pursuit of opportunities with other product candidates or for other indications that later prove to have greater market potential. Our resource allocation decisions may cause us to fail to capitalize on viable commercial products, including attractive or profitable market opportunities. If we do not accurately evaluate the commercial potential or target market for a particular product candidate, we may relinquish valuable rights to that product candidate through collaboration, licensing or other royalty arrangements in circumstances under which it would have been more advantageous for it to retain sole development and commercialization rights to such product candidate. In addition, we may not be successful in replicating our approach to product candidate development for other disease indications. If we are unsuccessful in identifying and developing additional product candidates or are unable to do so, our business may be harmed.
| 61 |
If product liability lawsuits are brought against us, we may incur substantial liabilities and may be required to limit commercialization of our product candidates.
We face an inherent risk of product liability as a result of the future clinical testing of our product candidates and will face an even greater risk if it commercializes any products. For example, we may be sued if our product candidates cause or are perceived to cause injury or are found to be otherwise unsuitable during clinical testing, manufacturing, marketing, or sale. Any such product liability claims may include allegations of defects in manufacturing, defects in design, a failure to warn of dangers inherent in the product, negligence, strict liability or a breach of warranties. Claims could also be asserted under state consumer protection acts. If we cannot successfully defend itself against product liability claims, it may incur substantial liabilities or be required to limit commercialization of its product candidates. Even successful defense would require significant financial and management resources. Regardless of the merits or eventual outcome, liability claims may result in one or more of the following:
| ● | decreased demand for our product candidates; | |
| ● | injury to our reputation; | |
| ● | withdrawal of clinical trial participants; | |
| ● | initiation of investigations by regulators; | |
| ● | costs to defend the related litigation; | |
| ● | a diversion of management’s time and our resources; | |
| ● | substantial monetary awards to trial participants or patients; | |
| ● | product recalls, withdrawals or labeling, marketing or promotional restrictions; | |
| ● | loss of revenue; | |
| ● | exhaustion of any available insurance and our capital resources; and | |
| ● | the inability to commercialize any product candidate. |
Our inability to obtain sufficient product liability insurance at an acceptable cost to protect against potential product liability claims could prevent or inhibit the commercialization of products it develops, alone or with corporate collaborators. Our insurance policies may also have various exclusions, and we may be subject to a product liability claim for which it has no coverage. Assuming we obtain clinical trial insurance for our clinical trials, we may have to pay amounts awarded by a court or negotiated in a settlement that exceeds our coverage limitations or that are not covered by our insurance, and we may not have, or be able to obtain, sufficient capital to pay such amounts. Even if our agreements with any future corporate collaborators entitle it to indemnification against losses, such indemnification may not be available or adequate should any claim arise.
| 62 |
Risks Related to Our Therapeutics Manufacturing
The manufacturing of our product candidates will be very complex. We are subject to a multitude of manufacturing risks, any of which could substantially increase our costs, delay our programs or limit supply of our product candidates.
Historically, engineered antibodies have been particularly difficult to manufacture and Contract Manufacturing Organizations, (“CMOs”), and we have limited experience in the manufacturing of bispecific antibodies to selectively activate NK cells. The process of manufacturing our product candidates is extremely susceptible to product loss due to contamination, equipment failure or improper installation or operation of equipment, vendor or operator error, contamination and inconsistency in yields, variability in product characteristics and difficulties in scaling the production process. Even minor deviations from normal manufacturing processes could result in reduced production yields, product defects and other supply disruptions. If microbial, viral or other contaminations are discovered in our product candidates or in the manufacturing facilities in which our product candidates are made, such manufacturing facilities may need to be closed for an extended period of time to investigate and remedy the contamination in accordance with current good manufacturing practice (“cGMP”).
Any adverse developments affecting manufacturing operations for our product candidates, if any are approved, may result in shipment delays, inventory shortages, lot failures, product withdrawals or recalls, or other interruptions in the supply of our products. We may also have to take inventory write-offs and incur other charges and expenses for products that fail to meet specifications, undertake costly remediation efforts or seek more costly manufacturing alternatives. Furthermore, it is too early to estimate our cost of goods sold. The actual cost to manufacture our product candidates could be greater than we expect because it is early in our development efforts.
We depend on strategic partnerships and collaboration arrangements for the development of our FLEX-NK™ bispecific antibody platform, including Yissum for NKp46 antibodies, INSERM for CD38 antibodies, NCI for GPC3 antibodies, and CytoLynx Therapeutics for development and commercialization in Greater China, and if these arrangements are unsuccessful, this could result in delays and other obstacles in the development, manufacture or commercialization of any of our product candidates.
Our strategy for fully developing and commercializing our therapeutic candidates is dependent upon maintaining our current arrangements and establishing new arrangements with research collaborators, corporate collaborators and other third parties. We currently have corporate and academic collaboration agreements with a number of counterparties including INSERM, NCI, Yissum and CytoLynx Therapeutics. These corporate collaboration agreements provide for, among other things, our need to pay for research funding and significant future payments should certain development, regulatory and commercial milestones be achieved. Under certain of these arrangements, our corporate collaborators are responsible for:
| ● | electing to advance product candidates through preclinical and into clinical development; | |
| ● | conducting clinical development and obtaining required regulatory approvals for product candidates; and | |
| ● | commercializing any resulting products. |
| 63 |
As a result, we may not be able to conduct these corporate collaborations in the manner or on the time schedule it currently contemplates, which may negatively impact our therapeutics business operations.
This lack of control over the research funding for, and the development and commercialization of, certain of our product candidates could cause delays or other difficulties in the development and commercialization of our product candidates, which may prevent completion of research and development activities and intended regulatory filings in a timely fashion, if at all. Because we expect to continue to rely on our current corporate collaborators and to enter into new collaborations in the future, the development and commercialization of any of our product candidates could be substantially delayed, and our ability to receive future funding could be substantially impaired if one or more of our current or future collaborators:
| ● | shifts our priorities and resources away from our collaborations due to a change in business strategies, or a merger, acquisition, sale or downsizing of our company or business unit; | |
| ● | ceases development in therapeutic areas which are the subject of our collaboration; | |
| ● | fails to select a product candidate for advancement into preclinical development, clinical development, or subsequent clinical development into a marketed product; | |
| ● | changes the success criteria for a particular product candidate, thereby delaying or ceasing development of such product candidate; | |
| ● | significantly delays the initiation or conduct of certain activities which could delay our receipt of milestone payments tied to such activities, thereby impacting our ability to fund our own activities; | |
| ● | develops a product candidate that competes, either directly or indirectly, with our product candidates; | |
| ● | does not obtain the requisite regulatory approval of a product candidate; | |
| ● | does not successfully commercialize a product candidate; |
| 64 |
| ● | encounters regulatory, resource or quality issues and are unable to meet demand requirements; | |
| ● | exercises its rights under the agreement to terminate the collaboration, or otherwise withdraws support for, or otherwise impairs development under the collaboration; | |
| ● | disagrees on the research, development or commercialization of a product candidate resulting in a delay in milestones, royalty payments or termination of such product candidate; and | |
| ● | uses our proprietary information or intellectual property in such a way as to jeopardize our rights in such property. |
In addition, the termination of any future strategic partnership or collaboration arrangement may prevent us from receiving any milestone, royalty payment, sharing of profits, and other benefits under such agreement and prevent us from being able to advance our therapeutic products toward commercialization. Furthermore, disagreements with these parties could require or result in litigation or arbitration, which would be time-consuming and expensive. Any of these events could have a material adverse effect on our ability to develop and commercialize any of our therapeutic product candidates and may adversely impact our business, prospects, financial condition, and results of operations.
We rely upon third parties to conduct certain research and development activities and assist us with our preclinical trials and future clinical trials and commercial sale, if approved, of our product candidates. If these third parties do not successfully carry out their contractual duties or meet expected deadlines, it may not be able to timely develop, manufacture, obtain regulatory approval for or commercialize our product candidates.
We utilize independent investigators and collaborators, such as universities, medical institutions, CROs and strategic partners to conduct certain research and future clinical trials as required under our partnership and collaboration agreements. We must negotiate budgets and contracts with CROs and study sites, which may result in delays to our development timelines and increased costs. We will rely on these third parties over the course of our future clinical trials, and we only control certain aspects of their activities. If we or any of these third parties fail to comply with applicable GCP regulations, the clinical data generated in our clinical trials may be deemed unreliable and the FDA or comparable foreign regulatory authorities may require us to perform additional clinical trials before approving our marketing applications. We cannot assure you that, upon inspection, such regulatory authorities will determine that any of our clinical trials comply with the GCP regulations. In addition, our clinical trials must be conducted with biologic product produced under cGMPs and will require many test patients. Our failure or any failure by these third parties to comply with these regulations or to recruit enough patients may require it to repeat clinical trials, which would delay the regulatory approval process. Moreover, our business may be implicated if any of these third parties violates federal or state fraud and abuse or false claims laws and regulations or healthcare privacy and security laws.
| 65 |
Any third parties conducting our clinical trials are not and will not be our employees and, except for remedies available to us under our agreements with such third parties, we cannot control whether or not they devote sufficient time and resources to our ongoing preclinical, clinical and nonclinical programs. These third parties may also have relationships with other commercial entities, including our competitors, for whom they may also be conducting clinical studies or other drug development activities, which could affect their performance. If these third parties do not successfully carry out their contractual duties or obligations or meet expected deadlines, if they need to be replaced or if the quality or accuracy of the clinical data they obtain is compromised due to the failure to adhere to our clinical protocols or regulatory requirements or for other reasons, our clinical trials may be extended, delayed or terminated and we may not be able to complete development of, obtain regulatory approval of or successfully commercialize our product candidates. As a result, our commercial prospects for our product candidates could be harmed, our costs could increase and our ability to generate therapeutics revenue could be delayed.
If any of our relationships with trial sites, or any CRO that it may use in the future, terminates, we may not be able to enter into arrangements with alternative trial sites or CROs or do so on commercially reasonable terms. Switching or adding third parties to conduct our clinical trials involves substantial cost and requires extensive management time and focus. In addition, there is a natural transition period when a new third party commences work. As a result, delays occur, which can materially impact our ability to meet our desired clinical development timelines.
A disruption to our internal or third-party manufacturing operations, or our third-party suppliers’ or manufacturers’ inability to manufacture sufficient quantities of our antibody and cell product candidates at acceptable quality levels or costs, or at all, could materially and adversely affect our business.
We work with several third-party manufacturers and suppliers for the production of our antibody and cell product candidates. We have a long-term relationship with STC Biologics for the manufacturing of our NY-303 and NY-338 products.
Developing manufacturing processes to support clinical trial and commercialization requirements is a difficult and uncertain task, and there are risks associated with scaling to the level required for clinical trials or commercialization, including, among others, cost overruns, potential problems with process scale-out, process reproducibility, stability and purity issues, lot consistency, and timely availability of acceptable reagents and raw materials. If we are unable to scale to the level required for the conduct of clinical trials or commercialization, it may not be able to produce our antibody product candidates in a sufficient quantity to conduct ongoing and planned clinical trials, or to meet demand if any antibody product candidates are approved for commercialization.
| 66 |
We will be dependent and expect to continue to be substantially dependent on our third-party manufacturing facility, STC Biologics, for the production of our antibody product candidates, and we rely, and expect to continue to rely, on other future third parties for the manufacture of certain components and also to manufacture our antibody product candidates, when needed for use in conducting clinical trials. The third-party facilities used to manufacture our antibody and cell product candidates, must be evaluated by the FDA or other foreign regulatory agencies pursuant to inspections that will be conducted after it submits an application to the FDA or other comparable foreign regulatory authorities. If the FDA or a comparable foreign regulatory authority finds deficiencies with, takes enforcement actions against, or does not approve these facilities for the manufacture of our antibody product candidates or if it later finds deficiencies, take enforcement actions, or withdraw any such approval in the future, it may not be able to locate additional or replacement facilities to produce such antibody product candidates or materials in a timely manner and on commercially reasonable terms, or at all. This would significantly impact our ability to develop, obtain regulatory approval for or market our antibody product candidates, if approved, and could considerably delay completion of our clinical trials, product testing and potential regulatory approval of our antibody product candidates. We have not yet caused any antibody product candidates to be manufactured or processed on a commercial scale and may not be able to do so. We intend to make changes as we work to optimize the therapeutics manufacturing process, and we cannot be sure that even minor changes in the process will result in products that are capable or safe and effective.
Because we rely on third parties for the manufacture of certain components and the antibody product candidates themselves, we are required to transfer certain manufacturing process know-how and certain intermediates to third parties, including larger-scale facilities operated by a CMO or by us, to facilitate manufacture of our antibody product candidates for clinical trials and commercialization. Transferring manufacturing testing and processes and know-how is complex and involves review and incorporation of both documented and undocumented processes that may have evolved over time. In addition, transferring production to different facilities may require utilization of new or different processes to meet the specific requirements of a given facility. We and any CMOs or third parties that we engage for manufacturing its antibody product candidates will need to conduct significant development work to transfer these processes and manufacture each of our antibody product candidates for clinical trials and commercialization. In addition, we may be required to demonstrate the comparability of material generated by any CMO or third parties that we engage for manufacturing our antibody product candidates with material previously produced and used in testing. Any inability to manufacture comparable drug product by us or its CMOs could delay the continued development of our antibody product candidates.
Furthermore, certain of the components currently used in manufacturing the antibody product candidates are research-grade only, and it may encounter problems obtaining or achieving adequate quantities and quality of clinical grade materials that meet FDA, the European Medicines Agency (“EMA”), European Union Member State competent authorities’ or other applicable standards or specifications with consistent and acceptable production yields and costs. In addition, if contaminants are discovered in our supply of antibody product candidates or in our manufacturing facilities or those of our third-party suppliers and manufacturers, such manufacturing facilities may need to be closed for an extended period of time to investigate and remedy the contamination. Any such events could delay or prevent our ability to obtain regulatory approval for or commercialize our antibody product candidates, which could adversely affect our business, prospects, financial condition and results of operations.
Further, our reliance on third-party manufacturers entails risks it would not be subject to if it manufactured antibody product candidates, including:
| ● | inability to meet our product specifications and quality requirements consistently; | |
| ● | delay or inability to procure or expand sufficient manufacturing capacity; | |
| ● | issues related to scale-up of manufacturing; |
| 67 |
| ● | costs and validation of new equipment and facilities required for scale-up; | |
| ● | our third-party manufacturers may not be able to execute our manufacturing procedures and other logistical support requirements appropriately; | |
| ● | our third-party manufacturers may fail to comply with cGMP requirements and other inspections by the FDA or other comparable foreign regulatory authorities; | |
| ● | our inability to negotiate manufacturing agreements with third parties under commercially reasonable terms, if at all; | |
| ● | breach, termination or nonrenewal of manufacturing agreements with third parties in a manner or at a time that is costly or damaging to us; | |
| ● | reliance on single sources for reagents and components; | |
| ● | lack of qualified backup suppliers for those components that are currently purchased from a sole or single-source supplier; | |
| ● | our third-party manufacturers may not devote sufficient resources to our antibody product candidates; | |
| ● | we may not own, or may have to share, the intellectual property rights to any improvements made by our third-party manufacturers in the manufacturing process for our antibody product candidates; | |
| ● | operations of our third-party manufacturers or suppliers could be disrupted by conditions unrelated to our business or operations, including the bankruptcy of the manufacturer or supplier; and | |
| ● | carrier disruptions or increased costs that are beyond our control. |
In addition, if we enter into a strategic collaboration with a third party for the commercialization of our current or any future antibody and cell product candidates, we will not be able to control the amount of time or resources that the third party will devote to such efforts. If any strategic collaborator does not commit adequate resources to the marketing and distribution of our current or any future antibody product candidates, it could limit our potential revenues.
Any adverse developments affecting manufacturing operations for our antibody and cell product candidates may result in lot failures, inventory shortages, shipment delays, product withdrawals or recalls or other interruptions in the supply of our drug product which could prevent the administration to patients and delay the development of our antibody product candidates. We may also have to write off inventory, incur other charges and expenses for supply of drug product that fails to meet specifications, undertake costly remediation efforts, or seek more costly manufacturing alternatives.
| 68 |
Any of these events could lead to clinical trial delays or failure to obtain regulatory approval, or impact our ability to successfully commercialize our current or any future antibody product candidates once approved. Some of these events could be the basis for FDA action, including public warnings, injunction, request for recall, seizure, or total or partial suspension of production.
Our product candidates rely on the availability of specialty raw materials, which may not be available to us on acceptable terms or at all.
Our product candidates require many specialty raw materials, some of which are manufactured by small companies with limited resources and experience to deliver raw materials to our specifications. Further, some of our suppliers may not be able to scale-up as we move to clinical trials or commercialization. Accordingly, we may experience delays in receiving, or fail to secure entirely, key raw materials to support clinical or commercial manufacturing. Certain raw materials also require third-party testing, and some of the testing service companies may not have capacity or be able to conduct the testing that we request.
We also face competition for supplies from other cell therapy companies. Such competition may make it difficult for us to secure raw materials or the testing of such materials on commercially reasonable terms or in a timely manner.
Some raw materials are currently available from a single supplier, or a small number of suppliers. We cannot be sure that these suppliers will remain in business or that they will not be purchased by one of our competitors or another company that is not interested in continuing to produce these materials for our intended purpose. In addition, the lead time needed to establish a relationship with a new supplier can be lengthy, and we may experience delays in meeting demand in the event we must switch to a new supplier. The time and effort to qualify a new supplier, including to meet any regulatory requirements for such qualification, could result in additional costs, diversion of resources or reduced manufacturing yields, any of which would negatively impact our operating results. Further, we may be unable to enter into agreements with a new supplier on commercially reasonable terms, which could have a material adverse impact on our business.
If conflicts arise between us and our collaborators or strategic partners, these parties may act in a manner adverse to us and could limit our ability to implement our strategies.
If conflicts arise between us and our corporate or academic collaborators or strategic partners, the other party may act in a manner adverse to us and could limit our ability to implement our strategies. Some of our academic collaborators and strategic partners are conducting multiple product development efforts within each area that is the subject of the collaboration with us. Our collaborators or strategic partners, however, may develop, either alone or with others, products in related fields that are competitive with the products or potential products that are the subject of these collaborations. Competing products, either developed by the collaborators or strategic partners or to which the collaborators or strategic partners have rights, may result in the withdrawal of partner support for our product candidates.
| 69 |
Some of our collaborators or strategic partners could also become our competitors in the future. Our collaborators or strategic partners could develop competing products, preclude us from entering into collaborations with their competitors, fail to obtain timely regulatory approvals, terminate their agreements with us prematurely, or fail to devote sufficient resources to the development and commercialization of products. Any of these developments could harm our therapeutic product development efforts.
Some of our strategic collaborators or partners have the right to terminate their agreements with us, including for our failing to achieve certain milestones or make payments under the agreements, not actively pursuing development of a licensed product, or for materially breaching the agreement and failing to cure such breach within a specific grace period. Our strategic collaborators or partners may also want to discontinue collaborations upon assessing our progress on such development program. If our strategic collaborators or partners terminate their agreements with them or discontinue joint collaborations on a program, we may be required to significantly delay, scale back or discontinue the development or commercialization of one or more of its product candidates or curtail or restructure its operations, any of which could have a material adverse effect on our business and operations.
We may seek to form collaborations in the future with respect to our product candidates, but may not be able to do so, which may cause us to alter our development and commercialization plans.
The advancement of our product candidates and development programs and the potential commercialization of our current and future product candidates will require substantial additional cash to fund expenses. For some of our programs, we may seek to collaborate with pharmaceutical and biotechnology companies to develop and commercialize such product candidates, such as our collaborations with the Hebrew University of Jerusalem for NKp46 antibodies, INSERM for CD38 antibodies, NCI for GPC3 antibodies, and CytoLynx Therapeutics for development and commercialization in Greater China, any of these relationships may require us to incur non-recurring and other charges, increase our near- and long-term expenditures, or disrupt our management and business.
We face significant competition in seeking appropriate strategic partners and the negotiation process is time-consuming and complex. Whether we reach a definitive agreement for other collaborations will depend, among other things, upon its assessment of the collaborator’s resources and expertise, the terms and conditions of the proposed collaboration and the proposed collaborator’s evaluation of a number of factors. Those factors may include the design or results of clinical trials, the progress of our clinical trials, the likelihood of approval by the FDA or comparable regulatory authorities outside the United States, the potential market for the subject product candidate, the costs and complexities of manufacturing and delivering such product candidate to patients, the potential of competing products, the existence of uncertainty with respect to our ownership of technology, which can exist if there is a challenge to such ownership without regard to the merits of the challenge, and industry and market conditions generally. The collaborator may also consider alternative product candidates or technologies for similar indications that may be available to collaborate on and whether such a collaboration could be more attractive than the one for our product candidate. Further, we may not be successful in its efforts to establish a strategic partnership or other alternative arrangements for future product candidates because they may be deemed to be at too early of a stage of development for collaborative effort and third parties may not view them as having the requisite potential to demonstrate safety and efficacy. Any delays in entering into new collaborations or strategic partnership agreements related to any product candidate we develop could delay the development and commercialization of its product candidates.
| 70 |
Our business involves the use of hazardous materials which requires that we, and our third-party manufacturers and suppliers must comply with environmental, health and safety laws and regulations, which can be expensive and restrict or interrupt its business.
Our research and development activities and our third-party manufacturers’ and suppliers’ activities involve the generation, storage, use and disposal of hazardous materials, including the components of its product candidates, such as genetically modified cells, and other hazardous compounds and wastes. We and our third-party manufacturers and suppliers are subject to environmental, health and safety laws and regulations governing, among other matters, the use, manufacture, generation, storage, handling, transportation, discharge and disposal of these hazardous materials and wastes and worker health and safety. In some cases, these hazardous materials and various wastes resulting from their use may be stored at our facilities and our third-party manufacturers’ facilities pending their use and disposal. In addition, we and our third-party manufacturers must supply all necessary documentation in support of a BLA or equivalent foreign application on a timely basis and must comply with the FDA’s applicable regulations, including GLP and cGMP and equivalent foreign provisions. The facilities and quality systems of some or all of our third-party manufacturers and suppliers, as well as any facilities and quality systems it may have in the future, must pass a pre-approval inspection for compliance with the applicable regulations as a condition of regulatory approval of our product candidates or any of our other potential products. In addition, the regulatory authorities may, at any time, audit or inspect a manufacturing facility involved with the preparation of our product candidates or its other potential products or the associated quality systems for compliance with the regulations applicable to the activities being conducted. If these facilities do not pass a pre-approval plant inspection, FDA approval, or comparable foreign approval of the products will not be granted. The regulatory authorities also may, at any time following approval of a product for sale, audit our manufacturing facilities or those of our third-party manufacturers and suppliers. If any such inspection or audit identifies a failure to comply with applicable regulations or if a violation of our product specifications or applicable regulations occurs independent of such an inspection or audit, we or the relevant regulatory authority may require remedial measures that may be costly and/or time-consuming for us or a third party to implement and that may include the temporary or permanent suspension of a clinical trial or commercial sales or the temporary or permanent closure of a facility. Any such remedial measures imposed upon us or third parties with whom it contracts could materially harm our business.
If we or any of our third-party manufacturers fail to maintain regulatory compliance, the FDA, or comparable foreign authority, can impose regulatory sanctions including, among other things, refusal to approve a pending application for a new drug product or biologic product, or revocation or variation of a pre-existing approval.
| 71 |
Additionally, if supply from one approved manufacturer is interrupted, there could be a significant disruption in commercial supply. An alternative manufacturer would need to be qualified and approved through a BLA supplement, or equivalent foreign step, which could result in further delay. The regulatory agencies may also require additional studies if a new manufacturer is relied upon for commercial production. Switching manufacturers may involve substantial costs and is likely to result in a delay in our desired clinical and commercial timelines.
These factors could cause the delay of clinical trials, regulatory submissions, required approvals or commercialization of our product candidates, cause us to incur higher costs and prevent us from commercializing our products successfully.
Furthermore, if our suppliers fail to meet contractual requirements, and we are unable to secure one or more replacement suppliers capable of production at a substantially equivalent cost, our clinical trials may be delayed or we could lose potential revenue.
We cannot eliminate the risk of contamination or injury, which could result in an interruption of our commercialization efforts, research and development efforts and business operations, damages and significant cleanup costs and liabilities under applicable environmental, health and safety laws and regulations. We also cannot guarantee that the safety procedures utilized by our third-party manufacturers for handling and disposing of these materials and wastes generally comply with the standards prescribed by these laws and regulations. We may be held liable for any resulting damages costs or liabilities, which could exceed our resources, and state or federal or other applicable authorities may curtail our use of certain materials and/or interrupt our business operations. Furthermore, environmental, health and safety laws and regulations are complex, change frequently and have tended to become more stringent. We cannot predict the impact of such changes and cannot be certain of our future compliance. Failure to comply with these environmental, health and safety laws and regulations may result in substantial fines, penalties or other sanctions.
We have established a partnership with CytoLynx for the development and commercialization of our NY-303 bispecific antibody in greater China and there are substantial operational, financial, regulatory and political risks with this collaboration.
We are dependent on CytoLynx for the development of our NY-303 bispecific antibody for commercialization in greater China. There are substantial operational, financial, regulatory and political risks with this collaboration. There is no guarantee that CytoLynx can adequately transfer the required know how from NAYA to ensure timely manufacturing and clinical development in China. The China development may require substantial investments and CytoLynx may not be able to raise sufficient capital to fund its operations and therefore we may not realize part of all of the potential $12 million in payments from CytoLynx upon achieving certain developmental milestones, as well as up to $145 million upon CytoLynx reaching certain commercial milestones.
| 72 |
Accordingly, our partnership with CytoLynx is affected significantly by economic, political and legal developments in China. The Chinese economy differs from the economies of most developed countries in many respects, including:
| ● | the degree of government involvement; | |
| ● | the level of development; | |
| ● | the growth rate; | |
| ● | the control of foreign exchange; | |
| ● | access to financing; and | |
| ● | the allocation of resources. |
While the Chinese economy has experienced significant growth in the past 30 years, growth has been uneven, both geographically and among various sectors of the economy. The Chinese economy has also experienced certain adverse effects due to the recent global financial crisis. The Chinese government has implemented various measures to encourage economic growth and guide the allocation of resources. Some of these measures benefit the overall Chinese economy, but may also have a negative effect on us. For example, our operating results and financial condition may be adversely affected by government control over capital investments or changes in tax regulations that are applicable to us, and by government policies or guidance aimed at curtailing the perceived over-capacity of certain industry sectors, such as pharmaceutical companies. The Chinese government has implemented certain measures, including interest rate increases, to control the pace of economic growth. These measures may cause decreased economic activity in China, which could in turn reduce the demand for our products and materially and adversely affect our operating results and financial condition.
The Chinese government also exercises significant control over Chinese economic growth through the allocation of resources, controlling payment of foreign currency-denominated obligations, setting monetary policy and providing preferential treatment to particular industries or companies.
Any adverse change in the economic conditions or government policies in China could have a material and adverse effect on overall economic growth and the level of investments in health industries in China, which in turn could lead to a reduction in demand for our products and consequently have a material and adverse effect on our business.
| 73 |
Risks Related to Our Intellectual Property
Our products incorporate intellectual property rights developed by us that may be difficult to protect or may be found to infringe on the rights of others.
While we currently have U.S. and international patents pending, these potential patents may be challenged, invalidated or circumvented, and ultimately may not be granted. In addition, any rights granted under these potential patents may not provide any competitive advantages. Certain countries, including the United States and in Europe, could place restrictions on the patentability of various medical devices which may materially affect our business and competitive position. Additionally, the laws of some foreign countries, in particular China and India, do not protect our proprietary rights to the same extent or in the same manner as U.S. laws, and we may encounter significant problems in protecting and defending our proprietary rights in these countries. In addition to relying on patent, copyright and trademark laws, we also utilize a combination of trade secrets, confidentiality policies, non-disclosure and other contractual arrangements to protect our intellectual property rights. However, these measures may not be adequate to prevent or deter infringement or other misappropriation. Further, our intellectual property rights may be found to infringe on intellectual property rights of third parties. Moreover, we may not be able to detect unauthorized use or take appropriate and timely steps to establish and enforce our proprietary rights. Existing laws of some countries in which we conduct business offer only limited protection of our intellectual property rights, if at all. As the number of market entrants as well as the complexity of technology in the fertility marketplace increases, the possibility of functional overlap and inadvertent infringement of intellectual property rights also increases.
We may be forced to defend our intellectual property rights from infringement through expensive legal action.
Third parties may in the future assert claims against us alleging infringement on their intellectual property rights. Defending such claims may be expensive, time consuming and divert the efforts of our management and/or technical personnel. Because of litigation, we could be required to pay damages and other compensation, develop non-infringing products or enter into royalty and/or licensing agreements. However, we cannot be certain that any such licenses, will be made available to us on commercially reasonable terms.
We regard our trade secrets, patents and similar intellectual property as important to our operations. To protect our proprietary rights, we rely on intellectual property and trade secret laws, as well as confidentiality and license agreements with certain employees, customers and third parties. No assurance can be given that our intellectual property will not be challenged, invalidated, infringed or circumvented. If necessary, we intend to defend our intellectual property rights from infringement through legal action, which could be very costly and could adversely affect our ability to achieve and maintain profitability. Our limited capital resources could put us at a disadvantage if we are required to take legal action to enforce our intellectual property rights.
Any failure to obtain, maintain, protect, or enforce our intellectual property and proprietary rights, or if the scope of intellectual property protection we obtain are not sufficiently broad, that could impair our ability to compete or protect its proprietary technology and brand.
We rely, and will continue to rely, upon a combination of patents, trade secret protection, and confidentiality agreements to protect the intellectual property related to our proprietary manufacturing methods, proprietary technologies, product candidate development programs, and product candidates. Our success depends in large part on our ability to secure and maintain patent protection in the United States and other countries with respect to our current product candidates and any future product candidates we may develop.
| 74 |
Within NAYA Therapeutics, we own a total of twenty-four (24) pending patent applications in two families. Each family includes one (1) US patent application, one (1) 1 European patent application, and ten (10) patent applications in other foreign jurisdictions. One family based on WO 2022/216744 covers CYT303; while the other family based on WO 2022/216723 covers CYT338. The Kadouche/CNRS multispecific antibody license for CYT303 and CYT338 includes thirteen (13) patents and patent applications, including three (3) granted US patents and one (1) pending US patent applications, two (2) granted European patents and one (1) pending European patent application, and six (6) granted patents in other foreign jurisdictions. We license from Yissum ten (10) pending patent applications for CYT303 and CYT338, which include one (1) US patent application, one (1) European patent application, and eight (8) patent applications in other foreign jurisdictions. We license from INSERM five (5) pending patent applications for CYT338, of which one (1) is a US patent application, one (1) is European, and 3 are in other foreign jurisdictions. Within NAYA Women’s Health, we currently have U.S. and international patents pending.
We seek to protect our proprietary position by filing or collaborating with licensors to file patent applications in the United States and abroad related to our proprietary technologies, development programs, and product candidates. The patent prosecution process is expensive and time-consuming, and we may not be able to file and prosecute all necessary or desirable patent applications at a reasonable cost or in a timely manner. Moreover, the issuance, scope, validity, enforceability, and commercial value of our patent rights are highly uncertain.
Composition of matter patents for biological and pharmaceutical product candidates often provide a strong form of intellectual property protection for those types of products, as such patents provide protection without regard to any method of use. We cannot be certain that the claims in our pending patent applications directed to composition of matter of our product candidates will be considered patentable by the United States Patent and Trademark Office (“USPTO”) or by patent offices in foreign countries. Method of use patents protect the use of a product for the specified method. This type of patent does not prevent a competitor from making and marketing a product that is identical to our product for an indication that is outside the scope of the patented method. Method of manufacturing patents protect the manufacturing process. This type of patent does not prevent a competitor from making and marketing a product that is identical to our product, but manufactured by a method that is outside the scope of the patented manufacturing method. Moreover, even if a competitor’s manufacturing process does infringe or contribute to the infringement of method of manufacturing patents, such infringement is difficult to detect and therefore difficult to prevent or prosecute.
| 75 |
It is also possible that we will fail to identify patentable aspects of our research and development output before it is too late to obtain patent protection. We may not have the right to control the preparation, filing, and prosecution of patent applications or to maintain the rights to patents licensed from or licensed to third parties. Therefore, these patents and patent applications may not be prosecuted and enforced in a manner consistent with the best interests of our business. The patent applications that we own or in-license may fail to result in issued patents with claims that cover our proprietary products and technology, including current product candidates, any future product candidates we may develop, and our FLEX-NK™ cell engager antibody technologies in the United States or in other foreign countries, in whole or in part. Alternately, our existing patents and any future patents we obtain may not be sufficiently broad to prevent others from using our technology or from developing competing products and technologies. It is possible that not all potentially relevant prior art relating to our patents and patent applications have been found, which can prevent a patent from issuing from a pending patent application or can later invalidate or narrow the scope of an issued patent. For example, publications of discoveries in the scientific literature often lag behind the actual discoveries, and patent applications in the United States and other jurisdictions are typically not published until 18 months after filing or, in some cases, not at all. Therefore, we cannot know with certainty whether we were the first to make the inventions claimed in our patents or pending patent applications, or that we were the first to file for patent protection of such inventions. Even if patents do successfully issue and even if such patents cover our current product candidates, any future product candidates we may develop, and FLEX-NK™ cell engager antibody technologies, third parties may challenge their validity, ownership, enforceability, or scope thereof, which may result in such patents being narrowed, invalidated, or held unenforceable or circumvented. Any successful challenge to these patents or any other patents owned by or licensed to us could deprive us of rights necessary for the successful commercialization of any of our product candidates or proprietary technology. In addition, the issuance of a patent does not give us the right to practice the patented invention. Third parties may have blocking patents that could prevent us from marketing our product candidates, if approved, or practicing our own patented technology. Our competitors may be able to circumvent our patents by developing similar or alternative product candidates in a non-infringing manner. Further, if we encounter delays in regulatory approvals, the period of time during which it could market a product candidate and its FLEX-NK™ cell engager antibody platform under patent protection could be reduced. If any of our patents expire or are challenged, invalidated, circumvented, or otherwise limited by third parties prior to the commercialization of our product candidates, and if we do not own or have exclusive rights to other enforceable patents protecting our product candidates or technologies, competitors and other third parties could market products and use processes that are substantially similar, or superior, to ours, and our business would suffer.
If the patent applications we hold or in-license with respect to our product candidates fail to issue, if the validity, breadth, or strength of protection of the resulting patents is threatened, or if the resulting patents fail to provide meaningful exclusivity for any of our current or future product candidates or technology, that could dissuade companies from collaborating with us to develop product candidates, encourage competitors to develop competing products or technologies, and threaten our ability to commercialize future product candidates. Any such outcome could harm our business.
We are party to several intellectual property license agreements which are important to our business, and we expect to enter into additional license agreements in the future. Our existing license agreements impose, and we expect that future license agreements will impose, various diligence, development, and commercialization timelines, milestone payments, royalties and other obligations on us. If we fail to comply with our obligations under these agreements, or if we are subject to a bankruptcy, or, in some cases, under other circumstances, a licensor may have the right to terminate the respective license, in which event we would not be able to market such product candidate(s) covered by such license.
| 76 |
The patent position of biotechnology and pharmaceutical companies is generally highly uncertain, involves complex legal, scientific, and factual questions, and is characterized by the existence of large numbers of patents and frequent litigation based on allegations of patent or other intellectual property infringement, misappropriation, or violation. The standards that the USPTO and its foreign counterparts use to grant patents are not always applied predictably or uniformly. In addition, the laws of jurisdictions outside the United States may not protect our rights to the same extent as the laws of the United States, and many companies have encountered significant problems in protecting and defending such rights in foreign jurisdictions. For example, European patent law restricts the patentability of methods of treatment of the human body more than United States law does. Changes in either the patent laws or interpretation of the patent laws in the United States and other countries may diminish the value of our patents or narrow the scope of our patent protection. Since patent applications in the United States and other jurisdictions are confidential for a period of time after filing, we cannot be certain that we were the first to file for patents covering our inventions. As a result, the issuance, scope, validity, enforceability, and commercial value of our patent rights are highly uncertain. Our pending and future patent applications may not result in the issuance of patents, or may result in the issuance of patents which fail to protect our technology or products, in whole or in part, or which fail to effectively prevent others from commercializing competitive technologies and products.
The issuance of a patent is not conclusive as to its inventorship, ownership, scope, validity, or enforceability, and our owned and licensed patents may be challenged in the courts or patent offices in the U.S. and abroad. Such challenges may result in loss of ownership or exclusivity or in patent claims being narrowed, invalidated, or held unenforceable, in whole or in part, which could limit our ability to stop others from using or commercializing similar or identical technology and products or limit the duration of the patent protection of our technology and products. We may become involved in opposition, derivation, reexamination, inter partes review, post-grant review, or interference proceedings challenging our owned or licensed patent rights. An adverse determination in any such submission, proceeding, or litigation could reduce the scope of, or invalidate, our patent rights, allow third parties to commercialize our technology or products and compete directly with us, without payment to us, or result in our inability to manufacture or commercialize products without infringing third-party patent rights. Thus, even if our patent applications issue as patents, they may not issue in a form that will provide us with meaningful protection, prevent competitors from competing with us, or otherwise provide us with any competitive advantage.
Moreover, patents have a limited lifespan. The term of an individual patent depends on applicable law in the country where the patent is issued. In the U.S., the natural expiration of a patent is generally 20 years from its application filing date or earliest claimed non-provisional filing date. Various extensions may be available; however, the life of a patent, and the protection it affords, is limited. Without patent protection for our current or future product candidates, we may be open to competition from biosimilar versions of such products. Given the amount of time required for the development, testing, and regulatory review of new product candidates, patents protecting such candidates might expire before or shortly after such candidates are commercialized. As a result, our patent portfolio may not provide us with sufficient rights to exclude others from commercializing products similar or identical to our own.
| 77 |
We depend on intellectual property licensed from third parties, and any failure to comply with our obligations under our license agreements or a termination of any of these license agreements could result in the loss of significant rights, which would harm our business.
We have entered into license agreements with third parties and may need to obtain additional licenses from our existing licensors and others to advance our research or allow commercialization of product candidates and technologies we may develop.
In most of our license agreements (and we expect in our future agreements), we have the right under specified conditions to bring any actions against any third party for infringing on the patents we have exclusively licensed. Certain of our license agreements also require us to meet development thresholds and other obligations to maintain the license, including establishing a set timeline for developing and commercializing products. Disputes may arise regarding intellectual property subject to a licensing agreement, including the following:
| ● | the scope of rights granted under the license agreement and other interpretation-related issues; | |
| ● | the extent to which our technology and processes infringe on intellectual property of the licensor that is not subject to the licensing agreement; | |
| ● | the sublicensing of patents and other rights under our collaborative development relationships; | |
| ● | our diligence obligations under the license agreement and what activities satisfy those diligence obligations; | |
| ● | the inventorship and ownership of inventions and know-how resulting from the joint creation or use of intellectual property by us, our licensors, and our partners; and | |
| ● | the priority of invention of patented technology. |
In addition, the agreements under which we currently license intellectual property or technology from third parties are complex, and certain provisions in such agreements may be susceptible to multiple interpretations. The resolution of any contract interpretation disagreement that may arise could narrow what we believe to be the scope of our rights to the relevant intellectual property or technology, or increase what we believe to be our financial or other obligations under the relevant agreement, either of which could have a material adverse effect on our business, financial condition, results of operations, and prospects.
Moreover, if disputes over intellectual property that we have licensed prevent or impair our ability to maintain our current licensing arrangements on commercially acceptable terms, we may be unable to successfully develop and commercialize the affected product candidates, which could have a material adverse effect on our business, financial conditions, results of operations, and prospects.
| 78 |
Our existing license agreements impose (and we expect that future license agreements will impose) various diligence, development, and commercialization timelines, milestone payments, royalties, and other obligations on us. If we fail to comply with our obligations under our license agreements, our licensors may have the right to terminate these license agreements, in which event we might not be able to market any product or technology that is covered by these agreements, which could adversely affect the value of the product candidate being developed under the respective license agreement. Termination of these license agreements or reduction or elimination of our licensed rights may also result in us having to negotiate new or reinstated licenses with less favorable terms.
We may rely on our licensors to file and prosecute patent applications and maintain patents and otherwise protect the intellectual property we license from them. We may have limited control over these activities. For example, we cannot be certain that such activities by these licensors will be conducted in compliance with applicable laws and regulations or will result in valid and enforceable patents or other intellectual property rights. We may have limited control over the manner in which one of our licensors initiate an infringement proceeding against a third party who infringes, misappropriates, or otherwise violates such licensor’s intellectual property rights licensed to us, or defend such intellectual property rights. It is possible that the licensor’s infringement proceeding or defense activities may be less vigorous than if we were to conduct them ourselves.
In spite of our best efforts, our licensors might conclude that we have materially breached our license agreements and might therefore terminate (or seek to terminate) the license agreements, thereby removing our ability to develop and commercialize products and technology covered by these license agreements. If these in-licenses are terminated, or if the underlying patents fail to provide the intended exclusivity, competitors would have the freedom to seek regulatory approval of, and to market, products identical to our own. In addition, we may seek to obtain additional licenses from our licensors and, in connection with obtaining such licenses, we may agree to amend our existing licenses in a manner that may be more favorable to the licensors, including by agreeing to terms that could enable third parties (potentially including our competitors) to receive licenses to a portion of the intellectual property that is subject to its existing licenses. The licensing and acquisition of third-party intellectual property rights is a competitive practice, and companies that may be more established, or have greater resources than we do, may also be pursuing strategies to license or acquire third party intellectual property rights that we may consider necessary or attractive in order to commercialize our product candidates. We may fail to obtain any of these third-party intellectual property rights at a reasonable cost or on reasonable terms, if at all. Any of these events could harm our competitive position, business, financial conditions, results of operations, and prospects.
| 79 |
Third-party claims of intellectual property infringement may prevent or delay our product discovery and development efforts.
Our commercial success depends in part on avoiding the infringement, misappropriation, or other violation of the patents and proprietary rights of third parties. There is a substantial amount of litigation involving patents and other intellectual property rights in the biotechnology and pharmaceutical industries. Numerous U.S. and foreign issued patents and pending patent applications, which are owned by third parties, exist in the fields in which we are developing our product candidates. As the biotechnology and pharmaceutical industries expand and more patents are issued, and as we gain greater visibility and market exposure as a public company, the risk increases that our product candidates may give rise to claims of infringement of the patent rights of others. In addition, many companies in intellectual property-dependent industries, including the biotechnology and pharmaceutical industries, have employed intellectual litigation as a means to gain an advantage over their competitors. Some claimants may have substantially greater resources than we do and may be able to sustain the costs of complex intellectual property litigation to a greater degree and for longer periods of time than we could.
Third parties may assert that we infringe their patents or are otherwise employing their proprietary technology without authorization and may sue us. Because patent applications can take many years to issue, there may be currently pending patent applications that may later result in issued patents that our product candidates may be alleged to infringe. In addition, third parties may obtain patents in the future and claim that our technologies infringe upon these patents. If any third-party patent were held by a court of competent jurisdiction to cover the manufacturing process of any of our product candidates, constructs, or molecules used in or formed during the manufacturing process, or any final therapeutic itself, the holder of any such patent may be able to block our ability to commercialize the therapeutic candidate unless we obtain a license under the applicable patent, or until such patent expires, or it is finally determined to be held not infringed, unpatentable, invalid, or unenforceable. Similarly, if any third-party patent were held by a court of competent jurisdiction to cover aspects of our formulations, processes for manufacture, or methods of use, including combination therapy or patient selection methods, the holder of any such patent may be able to block our ability to develop and commercialize the product candidate unless we obtain a license or until such patent expires or is finally determined to be held not infringed, unpatentable, invalid, or unenforceable. In either case, such a license may not be available on commercially reasonable terms or at all. If we are unable to obtain a necessary license to a third-party patent on commercially reasonable terms, or at all, our ability to commercialize our product candidates may be impaired or delayed, which could in turn significantly harm our business. Moreover, even if we were able to obtain a license, the rights may be nonexclusive, which could result in our competitors gaining access to the same intellectual property.
Parties making claims against us may seek and obtain injunctive or other equitable relief, which could effectively block our ability to further develop and commercialize our product candidates. Defense of these claims, regardless of their merit, would involve substantial litigation expense and would be a substantial diversion of management and employee resources from our business and may impact our reputation. In the event of a successful claim of infringement against us, we may have to pay substantial damages, including treble damages and attorneys’ fees, for willful infringement, obtain one or more licenses from third parties, pay royalties, or redesign our infringing products, which may be impossible or require substantial time and monetary expenditure. We cannot predict whether any such license would be available at all or whether it would be available on commercially reasonable terms if at all. In that event, we would be unable to further develop and commercialize our product candidates, which could harm our business significantly.
| 80 |
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation or administrative proceedings, there is a risk that some of our confidential information could be compromised by disclosure. There could also be public announcements of the results of the hearings, motions, or other interim proceedings or developments, and if securities analysts or investors view these announcements in a negative light, the price of our shares of common stock could be adversely affected. In addition, any uncertainties resulting from the initiation and continuation of any litigation or administrative proceeding could have a material adverse effect on our ability to raise additional funds or otherwise have a material adverse effect on our business, results of operations, financial condition and prospects.
We may be subject to claims asserting that our employees, consultants, or advisors have wrongfully used or disclosed alleged trade secrets of their current or former employers or claims asserting ownership of what we regard as our own intellectual property.
Certain of our employees, consultants, or advisors are currently, or were previously, employed at universities or other biotechnology or pharmaceutical companies, including our competitors or potential competitors. Although we try to ensure that our employees, consultants, and advisors do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that these individuals or that we have used or disclosed intellectual property, including trade secrets or other proprietary information, of any such individual’s current or former employer. Litigation may be necessary to defend against these claims. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel and face increased competition to business. A loss of key research personnel work product could hamper or prevent our ability to commercialize potential technologies and solutions, which could harm our business. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management.
In addition, we may in the future be subject to claims by our former employees, consultants, or advisors asserting an ownership right in our patents or patent applications, as a result of the work they performed on our behalf. Although it is our policy to require our employees and contractors who may be involved in the conception or development of intellectual property to execute agreements assigning such intellectual property to us, we may be unsuccessful in executing such an agreement with each party who, in fact, conceives or develops intellectual property that we regard as our own, and we cannot be certain that our agreements with such parties will be upheld in the face of a potential challenge or that they will not be breached, for which we may not have an adequate remedy. The assignment of intellectual property rights may not be self-executing or the assignment agreements may be breached, and we may be forced to bring claims against third parties, or defend claims that they may bring against us, to determine the ownership of what we regard as our intellectual property.
| 81 |
We may be involved in lawsuits to protect or enforce our patents or the patents of our licensors, which could be expensive, time-consuming and unsuccessful. Further, our issued patents could be found invalid or unenforceable if challenged in court.
Competitors may infringe our intellectual property rights or those of our licensors. To prevent infringement or unauthorized use, we and/or our licensors may be required to file infringement claims, which can be expensive and time-consuming. Further, our licensors may need to file infringement claims, and our licensors may elect not to file such claims. In addition, in a patent infringement proceeding, a court may decide that a patent Legacy NAYA owns or licenses is not valid, is unenforceable and/or is not infringed. If we or any of our licensors or potential future collaborators were to initiate legal proceedings against a third party to enforce a patent directed at one of our product candidates, the defendant could counterclaim that our patent is invalid and/or unenforceable in whole or in part. In a patent litigation, defendant counterclaims alleging invalidity and/or unenforceability are commonplace. Grounds for a validity challenge include an alleged failure to meet any of several statutory requirements, including lack of novelty or written description, obviousness or non-enablement. Grounds for an unenforceability assertion could include an allegation that someone connected with prosecution of the patent intentionally withheld material information from the USPTO or made a misleading statement during prosecution.
If a defendant were to prevail on a legal assertion of invalidity and/or unenforceability, we would lose at least part, and perhaps all, of the patent protection on such product candidate. In addition, if the breadth or strength of protection provided by our patents and patent applications or those of our licensors is threatened, it could dissuade companies from collaborating with us to license, develop, or commercialize current or future product candidates. Such a loss of patent protection would have a material adverse impact on our business.
Even if resolved in our favor, litigation or other legal proceedings relating to our intellectual property rights may cause us to incur significant expenses, and could distract our technical and management personnel from their normal responsibilities. Such litigation or proceedings could result in or substantially increase our operating losses and reduce the resources available for development activities or any future sales, marketing, or distribution activities. We may not have sufficient financial or other resources to conduct such litigation or proceedings adequately. Some of our competitors may be able to sustain the costs of such litigation or proceedings more effectively than we can because of their greater financial resources. Damages or other remedies awarded, if any, may not be commercially meaningful. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could compromise our ability to compete in the marketplace.
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation or other legal proceedings relating to our intellectual property rights, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation or other proceedings.
Changes in U.S. patent law or the patent law of other countries or jurisdictions could diminish the value of patents in general, thereby impairing our ability to protect our current and any future product candidates.
Obtaining and enforcing patents in the biotechnology and pharmaceutical industry is inherently uncertain, due in part to ongoing changes in the patent laws. Depending on decisions by Congress, the federal courts, the USPTO, and comparable institutions in other jurisdictions, the laws and regulations governing patents, and interpretation thereof, could change in unpredictable ways that could weaken our and our licensors’ or collaborators’ ability to obtain new patents or to enforce existing or future patents. For example, in recent years the U.S. Supreme Court and U.S. Court of Appeals for the Federal Circuit have ruled on several patent cases that have been interpreted to have either narrowed the scope of patent protection or weakened the rights of patent owners in certain situations. Therefore, there is increased uncertainty with regard to our and our licensors’ or collaborators’ ability to obtain patents in the future, as well as uncertainty with respect to the value of patents once obtained. Recent patent reform legislation could increase the uncertainties and costs surrounding the prosecution of our and our licensors’ or collaborators’ patent applications and the enforcement or defense of our or our licensors’ or collaborators’ issued patents.
| 82 |
Because of a lower evidentiary standard in USPTO proceedings compared to the evidentiary standard in United States federal courts necessary to invalidate a patent claim, a third party could potentially provide evidence in a USPTO proceeding sufficient for the USPTO to hold a claim invalid even though the same evidence would be insufficient to invalidate the claim if first presented in a district court action. Accordingly, a third party may attempt to use the USPTO proceedings to invalidate our patent claims that would not have been invalidated if first challenged by the third party as a defendant in a district court action.
In addition, the U.S. Supreme Court and U.S. Court of Appeals for the Federal Circuit have ruled on several patent cases in recent years, either narrowing the scope of patent protection available in certain circumstances or weakening the rights of patent owners in certain situations. In addition to increasing uncertainty with regard to our ability to obtain patents in the future, this combination of events has created uncertainty with respect to the value of patents, once obtained. Depending on actions by the U.S. Congress, the federal courts, and the USPTO, the laws and regulations governing patents could change in unpredictable ways that could weaken our ability to obtain new patents or to enforce patents that we own, have licensed, or might obtain in the future. Similarly, changes in patent law and regulations in other countries or jurisdictions, changes in the governmental bodies that enforce them, or changes in how the relevant governmental authority enforces patent laws or regulations may weaken our ability to obtain new patents or to enforce patents that we own or have licensed or that we may obtain in the future.
Patent terms may be inadequate to protect our competitive position on our product candidates for an adequate amount of time. If we or our licensors do not obtain patent term extension for our product candidates, our business may be materially harmed.
Given the amount of time required for the development, testing, and regulatory review of product candidates such as NY-303, patents protecting such candidates might expire before or shortly after such candidates are commercialized. We expect to seek extensions of patent terms in the United States and, if available, in other countries where we have or will obtain patent rights. In the United States, the Drug Price Competition and Patent Term Restoration Act of 1984 permits a patent term extension of up to five years beyond the normal expiration of the patent. However, the extension cannot extend the total patent term beyond 14 years from the date of drug approval, which is limited to the approved indication (or any additional indications approved during the period of extension). Furthermore, only one patent per approved product can be extended and only those claims covering the approved product, a method for using it or a method for manufacturing it may be extended. However, the applicable authorities, including the FDA and the USPTO in the United States, and any comparable regulatory authority in other countries, may not agree with our assessment of whether such extensions are available, and may refuse to grant extensions to our patents, or may grant more limited extensions than we request. If this occurs, the period during which we can enforce our patent rights for the applicable product candidate will be shortened and our competitors may obtain approval to market competing products sooner. Additionally, our competitors may be able to take advantage of our investment in development and clinical trials by referencing our clinical and preclinical data and launch their product earlier than might otherwise be the case.
| 83 |
We may not be able to protect our intellectual property rights throughout the world.
Although we have rights to issued patents and pending patent applications in the United States and certain other countries, filing, prosecuting, and defending patents in all countries throughout the world would be prohibitively expensive, and our intellectual property rights in some countries outside the United States can be less extensive than those in the United States. In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as federal and state laws in the United States. Consequently, we may not be able to prevent third parties from practicing our inventions in all countries outside the United States or from selling or importing products made using our inventions in and into the United States or other jurisdictions. Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products and, further, may export otherwise infringing products to territories where we or our licensors have patent protection but enforcement is not as strong as that in the United States. These products may compete with our product candidates, and our or our licensors’ patents or other intellectual property rights may not be effective or sufficient to prevent them from competing.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of many foreign countries do not favor the enforcement of patents and other intellectual property protection, which could make it difficult for us to stop the infringement of our or our licensors’ patents or marketing of competing products in violation of our proprietary rights. Proceedings to enforce our or our licensors’ patent rights in foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our or our licensors’ patents at risk of being invalidated or interpreted narrowly and our or our licensors’ patent applications at risk of not issuing, and could provoke third parties to assert claims against us. We or our licensors may not prevail in any lawsuits that we or our licensors initiate, and the damages or other remedies awarded, if any, may not be commercially meaningful. Accordingly, our or our licensors’ efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license.
Many countries have compulsory licensing laws under which a patent owner may be compelled to grant licenses to third parties. In addition, many countries limit the enforceability of patents against government agencies or government contractors. In these countries, the patent owner may have limited remedies, which could materially diminish the value of such patent. If we or our licensors are forced to grant a license to third parties with respect to any patents relevant to our business, our competitive position may be impaired, and our business, financial condition, results of operations and prospects may be adversely affected.
| 84 |
We may not identify relevant third-party patents or may incorrectly interpret the relevance, scope, or expiration of a third-party patent, which might adversely affect our ability to develop, manufacture, and eventually market product candidates.
We are developing certain product candidates in highly competitive areas and cannot guarantee that any patent searches or analyses that we may conduct, including the identification of relevant patents or pending patent applications, the scope of patent claims or the expiration of relevant patents, are complete or thorough, nor can we be certain that we have identified each and every third-party patent and pending patent application in the United States and abroad that is or may be relevant to or necessary for the commercialization of our product candidates in any jurisdiction. For example, U.S. patent applications filed before November 29, 2000 and certain U.S. patent applications filed after that date that were not filed outside the United States remain confidential until patents issue. Patent applications in the United States and elsewhere are published approximately 18 months after the earliest filing for which priority is claimed, with such earliest filing date being commonly referred to as the priority date. Therefore, patents or pending patent applications covering our product candidates could have been or may be filed in the future by third parties without our knowledge. Additionally, patents and pending patent applications that have been published can, subject to certain limitations, be later amended in a manner that could cover our product candidates or the manufacturing or use of our product candidates. The scope of a patent claim is determined by an interpretation of the law, the written disclosure in a patent, and the patent’s prosecution history. our interpretation of the relevance or the scope of a patent or a pending patent application may be incorrect, which may negatively impact our ability to market our product candidates. We may incorrectly determine that our product candidates are not covered by a third-party patent or pending patent application or may incorrectly predict whether a third party’s pending application will issue with claims of relevant scope. Our determination of the expiration date of any patent in the United States or abroad that we consider relevant may be incorrect, which may negatively impact our ability to develop and market our product candidates. Our failure to identify and correctly interpret relevant patents or pending patent applications may negatively impact our ability to develop and market our product candidates.
If we fail to identify or correctly interpret relevant patents or pending patent applications or if we are unable to obtain licenses to relevant patents or pending patent applications, we may be subject to infringement claims. We cannot guarantee that we will be able to successfully settle or otherwise resolve such infringement claims. If we fail in any such dispute, in addition to being forced to pay damages, potentially including in the form of future royalties, which may be significant, we may be temporarily or permanently prohibited from commercializing any of our product candidates that are held to be infringing. We might, if possible, also be forced to redesign one or more product candidates so that such candidate no longer infringes the third-party intellectual property rights. Any of these events, even if we were ultimately to prevail, could require us to divert substantial financial and management resources that we would otherwise be able to devote to our business and could adversely affect our business, financial condition, results of operations, and prospects.
| 85 |
Obtaining and maintaining our patent rights depends on compliance with various procedural, document submission, fee payment, and other requirements imposed by government patent agencies, and our patent protection could be reduced or eliminated for noncompliance with these requirements.
The USPTO and various foreign governmental patent agencies require compliance with a number of procedural, documentary, fee payment, and other similar provisions during the patent application process. In addition, periodic maintenance fees, renewal fees, annuity fees, and various other government fees on patents and/or patent applications will have to be paid to the USPTO and various government patent agencies outside the United States over the lifetime of our owned and licensed patents and/or applications and any patent rights we may own or license in the future. We rely on our service providers or our licensors (or their service providers) to pay these fees. We employ reputable law firms and other professionals to help us comply, and we are also dependent on our licensors to take the necessary action to comply with these requirements with respect to our licensed intellectual property. Noncompliance events that could result in abandonment or lapse of a patent or patent application include, but are not limited to, failure to respond to official actions within prescribed time limits, nonpayment of fees, and failure to properly legalize and submit formal documents. If we, our service providers, or our licensors (or their service providers) fail to maintain the patents and patent applications covering our products or technologies, we may not be able to stop a competitor from marketing products that are the same as or similar to our product candidates, which would have an adverse effect on our business. In many cases, an inadvertent lapse can be cured by payment of a late fee or by other means in accordance with the applicable rules. There are situations, however, in which noncompliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. In such an event, potential competitors might be able to enter the market and this circumstance could harm our business.
In addition, if we fail to apply for or otherwise fail to obtain applicable patent term extensions or adjustments, we will have a more limited time during which we can enforce our granted patent rights. In addition, if we are responsible for patent prosecution and maintenance of patent rights in-licensed to us, any of the foregoing could expose us to liability to the applicable patent owner.
If we are unable to protect the confidentiality of our trade secrets, our business and competitive position would be harmed.
In addition to seeking patent and trademark protection for our product candidates, we also rely on trade secrets, including unpatented know-how, technology, and other proprietary information, to maintain our competitive position. We seek to protect our trade secrets, in part, by entering into nondisclosure and confidentiality agreements with parties who have access to them, such as our employees, corporate collaborators, outside scientific collaborators, contract manufacturers, consultants, advisors, and other third parties. We have also entered into confidentiality and invention or patent assignment agreements with our employees, advisors, and consultants. Despite these efforts, any of these parties may breach the agreements and disclose our proprietary information, including our trade secrets. Further, we cannot guarantee that we have entered into such agreements with each party that may have or has had access to our trade secrets or other proprietary information. Monitoring unauthorized uses and disclosures of our intellectual property is difficult, and Legacy NAYA does not know whether the steps we have taken to protect our intellectual property will be effective. In addition, we may not be able to obtain adequate remedies for any such breaches. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret is difficult, expensive, and time-consuming, and the outcome is unpredictable. In addition, some courts inside and outside the United States are less willing or unwilling to protect trade secrets.
| 86 |
Moreover, our competitors may independently develop knowledge, methods, and know-how equivalent to our trade secrets. Competitors could purchase our products and replicate some or all of the competitive advantages we derive from our development efforts for technologies on which we do not have patent protection. If any of our trade secrets were to be lawfully obtained or independently developed by a competitor, we would have no right to prevent them, or those to whom they communicate it, from using that technology or information to compete with us. If any of our trade secrets were to be disclosed to or independently developed by a competitor, our competitive position would be harmed.
We also seek to preserve the integrity and confidentiality of our data and other confidential information by maintaining physical security of our premises and physical and electronic security of our information technology systems. While we have confidence in these individuals, organizations, and systems, agreements or security measures may be breached, and detecting the disclosure or misappropriation of confidential information and enforcing a claim that a party illegally disclosed or misappropriated confidential information is difficult, expensive, and time-consuming, and the outcome is unpredictable. Further, we may not be able to obtain adequate remedies for any breach. In addition, our confidential information may otherwise become known or be independently discovered by competitors, in which case we would have no right to prevent them, or those to whom they communicate it, from using that technology or information to compete with us.
Any trademarks we may obtain may be infringed or successfully challenged, resulting in harm to our business.
We rely and expect to continue to rely on trademarks as one means to distinguish any of our product candidates that are approved for marketing from the products of our competitors. We have a registered trademark for our FLEX-NK™ cell engager antibody platform, but have not yet selected trademarks for our product candidates and have not yet begun the process of applying to register trademarks for our current or any future product candidates. Once we select trademarks and apply to register them, our trademark applications may not be approved. Third parties may oppose our trademark applications or otherwise challenge our use of the trademarks. If our trademarks are successfully challenged, we could be forced to rebrand our products, which could result in loss of brand recognition and could require us to devote resources to advertising and marketing new brands. Our competitors may infringe our trademarks, and we may not have adequate resources to enforce our trademarks.
In addition, any proprietary name we propose to use with our current or any other product candidate in the United States must be approved by the FDA, regardless of whether we have registered it, or applied to register it, as a trademark. The FDA typically conducts a review of proposed product names, including an evaluation of the potential for confusion with other product names. If the FDA objects to any of our proposed proprietary product names, we may be required to expend significant additional resources in an effort to identify a suitable proprietary product name that would qualify under applicable trademark laws, not infringe the existing rights of third parties, and be acceptable to the FDA.
| 87 |
Intellectual property rights do not necessarily address all potential threats to our business.
The degree of future protection afforded by our intellectual property rights are uncertain because intellectual property rights have limitations and may not adequately protect our business. The following examples are illustrative:
| ● | others may be able to make molecules that are similar to our product candidates but that are not covered by the claims of any patents, should they issue, that we own or license; | |
| ● | We or our licensors might not have been the first to make the inventions covered by the issued patents or pending patent applications that we own or license; | |
| ● | We or our licensors might not have been the first to file patent applications covering certain of our inventions; | |
| ● | others may independently develop similar or alternative technologies or duplicate any of our technologies without infringing our intellectual property rights; | |
| ● | it is possible that our pending patent applications will not lead to issued patents; | |
| ● | issued patents that we own or license may not provide us with any competitive advantages or may be held invalid or unenforceable as a result of legal challenges; | |
| ● | Our competitors might conduct research and development activities in the United States and other countries that provide a safe harbor from patent infringement claims for certain research and development activities, as well as in countries where we do not have patent rights, and then use the information learned from such activities to develop competitive drugs for sale in our major commercial markets; | |
| ● | We may not develop additional proprietary technologies that are patentable; and | |
| ● | the patents of others may have an adverse effect on our business. |
Risks Related to Ownership of Shares of our Common Stock
If we are unable to hold a meeting to obtain stockholder approval for the conversion of our Series C-1 and C-2 Preferred Stock, we may be in breach of the terms of the Financing. Since we have not obtained stockholder approval for the conversion of our Series C-1 and C-2 Preferred Stock, the holders of our Series C-2 Preferred Stock will be entitled to received dividends on the stated value of such shares at the rate of 10% per annum payable in shares of the company’s common stock.
Under the terms of the Merger Agreement, we agreed to call and hold a meeting of our stockholders to obtain the requisite approval for the conversion of all outstanding shares of Series C-1 and C-2 Preferred Stock issued in the Merger Agreement and the Financing into shares of our common stock, as required by the Nasdaq listing rules. Commencing on the ninety-first (91st) day after the first issuance of any Series C-2 Preferred, the holders of Series C-2 Preferred shall be entitled to receive dividends on the stated value at the rate of ten percent (10%) per annum, payable in shares of the Company’s common stock, with each payment of a dividend payable in shares of the Company’s common stock at a conversion price of eighty-five percent (85%) of the average of the volume weighted average price of the Company’s common stock for the five (5) trading days before the applicable dividend date. Such dividends shall continue to accrue until paid. Such dividends will not be paid in shares of the Company’s common stock unless and until the Company’s stockholders approve the issuance of common stock upon conversion of the Series C-2 Preferred. The holders of Series C-2 Preferred shall also be entitled to receive a pro-rata portion, on an as-if convertible basis, of any dividends payable on the Company’s common stock.
| 88 |
Furthermore, in connection with the Merger, we issued 38,951 shares of Series C-1 and C-2 Convertible Preferred Stock, which are convertible into an aggregate of 41,956,680 shares of our common stock. Nasdaq Listing Rule 5110(a) provides that a company must apply for initial listing in connection with a transaction whereby a company combines with a non-Nasdaq entity, resulting in a change of control of such company and potentially allowing the non-Nasdaq entity to effectively obtain Nasdaq listing. In determining whether a change of control has occurred, Nasdaq considers all relevant factors including, changes in management, board of directors, voting power, ownership and financing structure of the company. If Nasdaq does not agree with our determination that the Merger and the issuance of shares of our common stock and Series C-1 and C-2 Preferred Stock pursuant to the Merger did not result in a change of control, we will be in violation of Nasdaq Listing Rule 5110(a) and our common stock could be delisted from the Nasdaq Capital Market.
Assuming the conversion of all outstanding Series C-1 and C-2 Preferred Stock, there is a concentration of ownership of our outstanding common stock by one group of affiliated stockholders. If this group chooses to act together, it could exert substantial influence over our business, and the interests of this group may conflict with those of other stockholders.
The Certificate of Designations for the Series C-1 and C-2 Preferred Stock provide that any holder of Series C-1 and C-2 Preferred Stock will not have a right to convert, subject to certain exceptions, the Series C-1 and C-2 Preferred Stock for our common stock if, as a result of such conversion, the holder, together with its affiliates and other attribution parties, would collectively beneficially hold 19.99% of the total number of shares of our common stock then outstanding, subject to decrease of this maximum percentage upon written notice by the holder. Assuming the conversion of all outstanding Series C-1 and C-2 Preferred Stock and without giving effect to the foregoing beneficial ownership limitations on Series C-1 and C-2 Preferred Stock, Five Narrow Lane (“FNL”) would, as of December 31, 2024, own approximately 24.5% of our outstanding common stock. FNL could be able to exert substantial influence over our business. Additionally, the interests of FNL may be different from or conflict with the interests of our other stockholders. This concentration of voting power with FNL could delay, defer, or prevent a change of control, entrench our management and the Board of Directors, or delay or prevent a merger, consolidation, takeover, or other business combination involving us on terms that other stockholders may desire. In addition, conflicts of interest could arise in the future between us, on the one hand, and FNL on the other hand, concerning potential competitive business activities, business opportunities, the issuance of additional securities and other matters.
| 89 |
If we are unable to maintain compliance with all applicable continued listing requirements and standards of Nasdaq, our common stock will be delisted from Nasdaq.
Our common stock is currently listed on Nasdaq. In order to maintain that listing, we must satisfy minimum financial and other continued listing requirements and standards, including those regarding director independence and independent committee requirements, minimum stockholders’ equity, minimum share price, and certain corporate governance requirements.
Minimum Equity Rule
On April 17, 2024, we received a notice from Nasdaq’s Listing Qualifications Staff (the “Staff”) stating that it had determined to delist our securities as a result of us having reported stockholders’ equity, for the period ended December 31, 2023, that was not in compliance with Nasdaq’s Listing Rule 5550(b)(1) (the “Equity Rule”). The Equity Rule requires our stockholders’ equity to meet or exceed $2,500,000. Normally, Nasdaq listed companies may be provided up to 180 calendar days in which to regain compliance with the Equity Rule. However, we were not eligible for such compliance period as we remained under Panel monitoring having regained compliance previously with the Equity Rule on November 22, 2023.
Upon receipt of the delisting notice, we requested a Panel hearing to ask for additional time to regain Equity Rule compliance. At a Panel hearing held on June 6, 2024, we requested an extension until October 14, 2024, which represents the maximum amount of time grantable by the Panel under Nasdaq rules.
On November 4, 2024, we received a notice from the Staff stating that we had determined that we have demonstrated compliance with the equity requirement of the Equity Rule but that we will be subject to a Mandatory Panel Monitor for a period of one year from October 14, 2024.
If we fail to maintain compliance with the Equity Rule our common stock may be delisted from Nasdaq which could have a material adverse effect on our business, financial condition and results of operations.
Minimum Bid Price
On September 18, 2024, we received a letter from the Staff indicating that, based upon the closing bid price of our common stock for the last 34 consecutive business days, we are not currently in compliance with the requirement to maintain a minimum bid price of $1.00 per share for continued listing under Nasdaq Listing Rule 5550(a)(2).
The notice has no immediate effect on the listing of our common stock, and our common stock will continue to trade on Nasdaq.
| 90 |
In accordance with Nasdaq Listing Rule 5810(c)(3)(A), we have been provided an initial period of 180 calendar days, or until May 17, 2025, to regain compliance with the minimum bid price requirement. If at any time before May 17, 2025, the closing bid price of our common stock closes at or above $1.00 per share for a minimum of 10 consecutive business days, Nasdaq will provide written notification that we have achieved compliance with the minimum bid price requirement, and the matter would be resolved. If we do not regain compliance prior to May 17, 2025, then Nasdaq may grant us a second 180 calendar day period to regain compliance, provided we (i) meet the continued listing requirement for market value of publicly-held shares and all other initial listing standards for The Nasdaq Capital Market, other than the minimum closing bid price requirement, and (ii) notify Nasdaq of our intent to cure the deficiency within such second 180 calendar day period, by effecting a reverse stock split, if necessary.
We will continue to monitor the closing bid price of its common stock and will consider implementing available options to regain compliance with the minimum bid price requirement under the Nasdaq Listing Rules. If we do not regain compliance with the minimum bid price requirement within the allotted compliance periods, we will receive a written notification from Nasdaq that our securities are subject to delisting. We would then be entitled to appeal that determination to a Nasdaq hearings panel. There can be no assurance that we will regain compliance during either compliance period, or maintain compliance with the other Nasdaq listing requirements.
There can be no assurance that we will be able to maintain compliance with the continued listing requirements for Nasdaq. If we fail to maintain compliance with any such continued listing requirement, there can also be no assurance that we will be able to regain compliance with any such continued listing requirement in the future or that our common stock will not be delisted from Nasdaq Stock Market in the future.
Annual Shareholder Meeting
On January 10, 2025, we received notice from the Staff advising us that we no longer comply with Nasdaq Listing Rules 5620(a) and 5801(s)(2)(G) that require companies listed on Nasdaq to hold an annual meeting of shareholders (an “ASM”) within twelve months of the fiscal year’s end (the “ASM Rule”). We did not hold an ASM in our fiscal year ended December 31, 2024. The notice has no immediate effect on the listing of our common stock and our common stock continues to trade on Nasdaq under the symbol “NAYA.” We seek to regain compliance with the ASM Rule by holding this Annual Meeting.
Pursuant to the notice, Nasdaq has given us 45 calendar days, or until February 24, 2025, to submit to Nasdaq a plan to regain compliance. If our plan is accepted, Nasdaq may grant an extension of up to 180 calendar days from the fiscal year end, or until June 30, 2025, to evidence compliance.
We plan to timely submit our plan to Nasdaq to regain compliance with the ASM Rule. There can be no assurance that our plan will be accepted or that if it is, we will be able to regain compliance. If our plan to regain compliance is not accepted, or if it is and we do not regain compliance within 180 days from the date of the notice, or if we fail to satisfy another Nasdaq requirement for continued listing, Nasdaq could provide notice that our common stock will become subject to delisting. In such event, Nasdaq rules would permit us to appeal the decision to reject our proposed compliance plan or any delisting determination to a Nasdaq Hearings Panel. The hearing request would stay any suspension or delisting action pending the conclusion of the hearing process and the expiration of any additional extension period granted by the panel following the hearing.
| 91 |
In addition, if our stockholders approve the conversion of our series C-1 preferred stock into common stock, we will be required to meet Nasdaq’s new listing standards, which include stockholder’s equity of at least $5 million, the market value of our unrestricted publicly held shares of at least $15 million, two (2) years of operating history, unrestricted publicly held shares of at least $1 million, and a bid price of at least $4.00. If we do not meet the new listing standards at the time of the conversion of the series C-1 preferred stock, we could be delisted from Nasdaq. There can be no assurance that we will be able to comply with the initial listing requirements for Nasdaq. If we fail comply with any such continued listing requirement, there can also be no assurance that we will be able to regain compliance with any such continued listing requirement in the future or that our common stock will not be delisted from Nasdaq Stock Market in the future.
If we were to be delisted, we would expect our common stock to be traded in the over-the-counter market which could adversely affect the liquidity of our common stock. Additionally, we could face significant material adverse consequences, including:
| ● | a limited availability of market quotations for our common stock; |
| ● | a decreased ability to issue additional securities or obtain additional financing in the future; |
| ● | reduced liquidity for our stockholders; |
| ● | potential loss of confidence by customers, collaboration partners and employees; and |
| ● | loss of institutional investor interest. |
In the event of a delisting, we can provide no assurance that any action taken by us to restore compliance with listing requirements would allow our common stock to become listed again, stabilize the market price or improve the liquidity of our common stock, prevent our common stock from dropping below the Nasdaq minimum bid price requirement, or prevent future non-compliance with Nasdaq’s listing requirements.
Our common stock is subject to risks arising from restrictions on reliance on Rule 144 by shell companies or former shell companies.
Under a SEC rule known as “Rule 144”, a person who has beneficially owned restricted securities of an issuer and who is not an affiliate of that issuer may sell them without registration under the Securities Act provided that certain conditions have been met. However, Rule 144 is unavailable for the resale of securities issued by an issuer that is a shell company or that has been at any time previously a shell company. The SEC defines a shell company as a company that has no or nominal operations and either (i) no or nominal assets, (ii) assets consisting solely of cash and cash equivalents, or (iii) assets consisting of any amount of cash and cash equivalents and nominal other assets. We are a former shell company.
| 92 |
The SEC has provided an exception to this unavailability if and for as long as the following conditions are met: (a) the issuer of the securities that was formerly a shell company has ceased to be a shell company; (b) the issuer of the securities is subject to the reporting requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, as amended; (c) the issuer of the securities has filed all Exchange Act reports and materials required to be filed, as applicable during the preceding 12 months, other than certain Current Reports on Form 8-K; and (d) at least one (1) year has elapsed form the time the issuer filed current comprehensive disclosure with the SEC reflecting its status as an entity that it is not a shell company.
Because of our prior history as a shell company, stockholders who receive our restricted securities will only be able to sell them pursuant to Rule 144 without registration for only as long as we continue to meet the requirements set forth above. No assurance can be given that we will meet these requirements going forward. Furthermore, any non-registered securities we sell in the future or issue will have limited or no liquidity until and unless such securities are registered with the SEC and/or until we comply with the foregoing requirements.
As a result, it may be harder for us to raise funding through the sale of debt or equity securities unless we agree to register such securities with the SEC, which could require us to deploy additional resources. In addition, if we are unable to attract additional capital, it could have an adverse impact on our ability to implement our business plan and/or sustain our operations. Our status as a former “shell company” could prevent us from raising additional funds to develop additional technological advancements, which could cause the value of our securities to decline in value.
Our directors have the right to authorize the issuance of shares of our preferred stock and additional shares of our common stock.
Our directors, within the limitations and restrictions contained in our articles of incorporation and without further action by our shareholders, have the authority to issue shares of preferred stock from time to time in one or more series and to fix the number of shares and the relative conversion and voting rights, and terms of redemption, liquidation preferences and any other preferences, special rights and qualifications of any such series. While we have no intention of issuing shares of preferred stock at the present time, we may seek to raise capital through the sale of our securities and may issue shares of preferred stock in connection with a particular investment. Any issuance of shares of preferred stock could adversely affect the rights of holders of our common stock.
Should we issue additional shares of our common stock, each investor’s ownership interest in our stock would be proportionally reduced.
The indemnification rights provided to our directors, officers and employees may result in substantial expenditures by us and may discourage lawsuits against its directors, officers and employees.
Our articles of incorporation and applicable Nevada law provide for the indemnification of our directors, officers, employees. The foregoing indemnification obligations could result in us incurring substantial expenditures to cover the costs of settlement or damage awards against directors, officers, and employees, which we may be unable to recoup. These provisions and resultant costs may also discourage us from brining a lawsuit against out directors and officers for breaches of their fiduciary duties and may similarly discourage the filing of derivative litigation by our stockholders against our directs or officer even though such actions, if successful, might otherwise benefit us and our stockholders.
| 93 |
Our shares of common stock are thinly traded, and the price may not reflect our value; there can be no assurance that there will be an active market for our shares now or in the future.
We have a trading symbol for our common stock (“NAYA”) and our common stock is currently listed on the Nasdaq Capital Market.
Our shares of common stock are thinly traded, and as such the price, if traded, may not reflect our value. There can be no assurance that there will be an active market for our shares of common stock either now or in the future. The market liquidity will be dependent on, among other things, the perception of our operating business and any steps that our management might take to bring us to the awareness of investors. There can be no assurance given that there will be any awareness generated or, if given, that it will be positive.
Consequently, investors may not be able to liquidate their investment or may be able to liquidate it only at a price that does not reflect the value of the business. If a more active market should develop, the price may be highly volatile. Due to the possibility of our common stock being priced lower than its actual value, many brokerage firms may not be willing to effect transactions in the securities. Even if an investor finds a broker willing to effect a transaction in the shares of our common stock, the combination of brokerage commissions, transfer fees, taxes, if any, and any other selling costs may exceed the selling price.
We do not expect to pay any dividends on our common shares.
To date, we have never declared or paid any dividends to our common stockholders. Our board of directors does not intend to distribute dividends on our common shares in the near future. The declaration, payment, and amount of any future dividends will be made at the discretion of the board of directors, and will depend upon, among other things, the results of our operations, cash flows and financial conditions, operating, and capital requirements, and other factors as the board of directors considers relevant. There is no assurance that future dividends will be paid to common stockholders. If dividends are paid to common stockholders, there is no assurance with respect to the amount of any such dividend.
Our revenue and operating results could fluctuate significantly from quarter to quarter, which may cause our stock price to decline.
Since our inception, we have not generated significant revenue. Our results from year-to-year and from quarter-to-quarter have, and are expected to continue to, vary significantly based on ordering cycles of distributors and partners. As a result, we expect period-to-period comparisons of our operating results may not be meaningful as an indication of our future performance for any future period.
| 94 |
We may have difficulty raising the necessary capital to fund operations and the required $7.5 million in additional payments for the Wisconsin Fertility acquisition because of the thin market and market price volatility for our shares of common stock.
Throughout 2024, there has been a thin market for our shares, and the market price for our shares has been volatile. In recent years, the securities markets in the U.S. and around the world have experienced a high level of price and volume volatility, and the market price of securities of many companies have experienced wide fluctuations that have not necessarily been related to the operations, performances, underlying asset values or prospects of such companies. For these reasons, we expect our shares of common stock may also be subject to volatility resulting from market forces over which we will have no control. The success of our products and services may be dependent upon our ability to obtain additional financing through debt and equity or other means. The thin market for our shares, and the volatility in the market price for our shares, may adversely affect our ability to raise needed additional capital.
An active trading market for our common stock may not be sustained. If an active trading market is not sustained, our ability to raise capital in the future may be impaired.
There is limited history of trading for our common stock. Given the lack of trading history of our common stock, there is a risk that an active trading market for our shares may not be sustained, which could put downward pressure on the market price of our common stock and thereby affect your ability to sell shares you purchased. An inactive trading market for our common stock may also impair our ability to raise capital to continue to fund the operations of the combined companies by selling shares and impair our ability to acquire other companies or technologies by using our shares as consideration.
The trading price of our common stock is highly volatile, which could result in substantial losses for purchasers of our common stock. Securities class action or other litigation involving our company or members of our management team could also substantially harm our business, financial condition and results of operations.
Our stock price is highly volatile. The stock market in general and the market for smaller pharmaceutical and biotechnology companies in particular have experienced extreme volatility that has often been unrelated to the operating performance of particular companies. In addition, if the market for pharmaceutical and biotechnology stocks or the broader stock market continues to experience a loss of investor confidence, the trading price of our common stock could decline for reasons unrelated to our business, financial condition or results of operations. As a result of this volatility, you may not be able to sell your common stock at or above the purchase price and you may lose some or all of your investment. The market price for our common stock may be influenced by many factors, including the following:
| ● | the success of existing or new competitive products or technologies; | |
| ● | regulatory actions with respect to our product candidates or our competitors’ products and product candidates; | |
| ● | announcements by us or our competitors of significant acquisitions, strategic partnerships, joint ventures, collaborations or capital commitments; |
| 95 |
| ● | the timing and results of clinical trials; | |
| ● | commencement or termination of collaborations for our development programs; | |
| ● | failure or discontinuation of any of our development programs; | |
| ● | results of clinical trials of product candidates of our competitors; | |
| ● | regulatory or legal developments in the United States and other countries; | |
| ● | developments or disputes concerning patent applications, issued patents or other proprietary rights; | |
| ● | the recruitment or departure of key personnel; | |
| ● | the level of expenses related to any of our product candidates or clinical development programs; | |
| ● | the results of our efforts to develop additional product candidates or products; | |
| ● | actual or anticipated changes in estimates as to financial results or development timelines; | |
| ● | announcement or expectation of additional financing efforts; | |
| ● | sales of our common stock by us, our insiders or other stockholders; | |
| ● | variations in our financial results or those of companies that are perceived to be similar to us; | |
| ● | changes in estimates or recommendations by securities analysts, if any, that cover us; | |
| ● | changes in the structure of healthcare payment systems; | |
| ● | market conditions in the pharmaceutical and biotechnology sectors; | |
| ● | general economic, industry and market conditions; and | |
| ● | the other factors described in this “Risk Factors” section. |
In the past, securities class action litigation has often been brought against a company following a decline in the market price of its securities. This risk is especially relevant for biopharmaceutical companies, which have experienced significant stock price volatility in recent years.
Related to Market Uncertainties
Unstable market and economic conditions may have serious adverse consequences on our business, financial condition and stock price.
The global credit and financial markets have experienced extreme volatility and disruptions in the past several years, including severely diminished liquidity and credit availability, volatile interest rates, rising and fluctuating inflation rates, reduced corporate profitability, declines in consumer confidence, declines in economic growth, increases in unemployment rates and uncertainty about economic stability. U.S. debt ceiling and budget deficit concerns have increased the possibility of additional credit-rating downgrades and economic slowdowns, or a recession in the United States. Although U.S. lawmakers passed legislation to raise the federal debt ceiling on multiple occasions, ratings agencies have lowered or threatened to lower the long-term sovereign credit rating on the United States. The impact of this or any further downgrades to the U.S. government’s sovereign credit rating or its perceived creditworthiness could adversely affect the U.S. and global financial markets and economic conditions. In addition, inflation rates in the U.S. have recently increased to levels not seen in decades.
| 96 |
We believe that the state of global economic conditions are particularly volatile and uncertain, not only in light of the COVID-19 pandemic and the potential global recession resulting therefrom, but also due to recent global tensions and unexpected shifts in political, legislative and regulatory conditions concerning, among other matters, international trade and taxation, and that an uneven recovery or a renewed global downturn may negatively impact our ability to conduct clinical trials on the scale and timelines anticipated. There can be no assurance that further deterioration in credit and financial markets and confidence in economic conditions will not occur. Our general business strategy may be adversely affected by any such economic downturn, volatile business or political environment or continued unpredictable and unstable market conditions. If the current equity and credit markets deteriorate, it may make obtaining any necessary debt or equity financing more difficult, more costly and more dilutive. For example, as a result of political, social, and economic instability abroad, including as a result of armed conflict, war or threat of war, in particular, the current conflict between Russia and Ukraine, including resulting sanctions, terrorist activity and other security concerns in general, there could be a significant disruption of global financial markets, impairing our ability to raise capital when needed on acceptable terms, if at all. Failure to secure any necessary financing in a timely manner and on favorable terms could have a material adverse effect on our growth strategy, financial performance and stock price and could require us to delay or abandon clinical development plans. In addition, there is a risk that one or more of our current service providers, manufacturers and other partners may not survive an economic downturn, which could directly affect our ability to attain our operating goals on schedule and on budget. To the extent that our profitability and strategies are negatively affected by downturns or volatility in general economic conditions, our business and results of operations may be materially adversely affected.
Our business is affected by macroeconomic conditions, including rising inflation, interest rates and supply chain constraints.
Various macroeconomic factors could adversely affect our business and the results of our operations and financial condition, including changes in inflation, interest rates and overall economic conditions and uncertainties such as those resulting from the current and future conditions in the global financial markets. For instance, rising interest rates have impacted our net income. Recent supply chain constraints have led to higher inflation, which, if sustained, could have a negative impact on our product development and operations. If inflation or other factors were to significantly increase our business costs, our ability to develop our current pipeline and new therapeutic products may be negatively affected. Current capital market conditions, including the impact of inflation, have increased borrowing rates and can be expected to significantly increase our cost of capital as compared to prior periods and could also affect our ability to raise capital on favorable terms, or at all, in order to fund our operations. Similarly, these macroeconomic factors could affect the ability of our third-party suppliers and manufacturers to manufacture clinical trial materials for our product candidates.
| 97 |
General Risk Factors
We are a Smaller Reporting Company, or SRC, and the reduced disclosure requirements applicable to SRCs may make our common stock less attractive to investors.
We are considered a SRC under Rule 12b-2 of the Exchange Act. We are therefore entitled to rely on certain reduced disclosure requirements, such as an exemption from providing selected financial data and executive compensation information. These exemptions and reduced disclosures in our SEC filings due to our status as a smaller reporting company also mean our auditors are not required to review our internal control over financial reporting and may make it harder for investors to analyze our results of operations and financial prospects. We cannot predict if investors will find our common stock less attractive because we may rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our common stock prices may be more volatile. We will remain a smaller reporting company until our public float exceeds $250 million or our annual revenues exceed $100 million with a public float greater than $700 million.
We have broad discretion over the use of our cash and cash equivalents and may not use them effectively.
Our management has broad discretion to use our cash and cash equivalents to fund our operations and could spend these funds in ways that do not improve our results of operations or enhance the value of our common stock. The failure by our management to apply these funds effectively could result in financial losses that could have a material adverse effect on our business, cause the price of our common stock to decline and delay the development of our product candidates. Pending our use to fund operations, we may invest our cash and cash equivalents in a manner that does not produce income or that loses value.
If securities or industry analysts do not publish research or publish inaccurate or unfavorable research about our business, our share price and trading volume could decline.
The trading market for our common stock will be influenced by the research and reports that industry or securities analysts publish about us or our business. If one or more of the analysts who cover us issues an adverse opinion about our company, our stock price would likely decline. If one or more of these analysts ceases research coverage of us or fails to regularly publish reports on us, we could lose visibility in the financial markets, which in turn could cause our stock price or trading volume to decline.
| 98 |
Stockholders may be diluted significantly through our efforts to obtain financing and from issuance of additional shares of our common stock, including such issuances of shares for services.
To satisfy certain financial obligations, we have issued and may continue to issue shares of our common stock and we have incurred and may continue to incur debt, which may be convertible into shares of our common stock. We may attempt to raise capital by selling shares of our common stock, possibly with warrants, which may be issued or exercised at a discount to the market price for our common stock. These actions would result in dilution of the ownership interests of existing shareholders, and may further dilute the common stock book value, and that dilution may be material. Such issuances may also serve to enhance existing management’s ability to control us as the shares may be issued to our officers, directors, new employees, or other related parties.
We are subject to the reporting requirements of U.S. federal securities laws, which can be expensive.
We are a public reporting company and accordingly subject to the information and reporting requirements of the Exchange Act, and other federal securities laws, including compliance with the Sarbanes-Oxley Act of 2002. We are required to prepare and file annual and quarterly reports, proxy statements and other information with the SEC and furnishing audited reports. Compliance with such reporting requirements is both time-consuming and costly for us. We may need to hire additional financial reporting, internal control, and other finance personnel in order to develop and implement appropriate internal controls and reporting procedures.
In addition, the Sarbanes-Oxley Act of 2002 and the Dodd-Frank Wall Street Reform and Consumer Protection Act, as well as rules implemented by the SEC and the securities exchanges, require certain corporate governance practices for public companies. Our management and other personnel have devoted and expect to continue to devote a substantial amount of time to public reporting requirements and corporate governance. These rules and regulations have significantly increased our legal and financial compliance costs and made some activities more time-consuming and costly. If these costs are not offset by increased revenues and improved financial performance, our financial condition and results of operations may be materially adversely affected. These rules and regulations also make it more difficult and more expensive for us to obtain director and officer liability insurance in the future. Additionally, we may be required to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. As a result, it may be more difficult for us to attract and retain qualified personnel to serve on our board of directors or as executive officers.
Failure to comply with internal control attestation requirements could lead to loss of public confidence in our financial statements and negatively impact our stock price.
Pursuant to Section 404 of the Sarbanes-Oxley Act of 2002, we are required to conduct an annual management assessment of the effectiveness of our internal controls over financial reporting. If we fail to timely develop our internal controls, and management is unable to make this assessment, or, once required, if the independent registered public accounting firm cannot timely attest to this assessment, we could be subject to regulatory sanctions. As a result, a loss of public confidence in our financial controls and the reliability of our consolidated financial statements may develop ultimately negatively impacting our stock price and our ability to raise additional capital when and as needed
| 99 |
BUSINESS DESCRIPTION
Company Overview
NAYA Biosciences is a life science portfolio company dedicated to bringing breakthrough treatments to patients in oncology, autoimmune diseases, and fertility. Our hub and spoke model harnesses the shared resources of a parent company and agility of lean strategic franchises, enabling efficient acquisition, development, and partnering of assets as well as optimized return on investment by combining the upside of innovative clinical-stage therapeutics with scalable, profitable commercial revenues.
Our principal operations are focused currently in two divisions:
NAYA Therapeutics
NAYA Therapeutics, Inc. (also referred to as “Legacy NAYA”) carries out our current activities in oncology and autoimmune diseases, including NAYA Therapeutics One (“GPC3 Franchise”), NAYA Therapeutics Two (“CD38 Franchise”), NAYA Biologics, which aims to build an early-stage pipeline of best-in-class multifunctional antibodies (also referred as multispecific antibodies), and NAYA Clinical Intelligence (“NAYA CI”), which aims to optimize through artificial intelligence/ machine learning (“AI/ ML”) the selection and development of clinical candidates for NAYA subsidiaries as well as for external partners.
NAYA’s immediate focus is the clinical development of two bifunctional antibodies (also referred as bispecific antibodies), NY-303, a GPC-3 targeted natural killer (“NK”) engager which has been cleared to recruit patients in a monotherapy safety and efficacy phase I/IIa clinical trial in a hepatocellular carcinoma (“HCC”) patients not responding to first line immunotherapy, and NY-338, a CD38 targeted NK cell engager for the treatment of multiple myeloma and auto-immune diseases. Additionally, NAYA aims to advance NY-500, an AI optimized PD1-VEGF bifunctional antibody, to a phase I/IIa clinical trial in first line treatment of HCC, in 2026, and NY-600, a PSMA FLEX NK™ bifunctional antibody (NY-600), for the treatment of prostate cancer.
We believe that we are uniquely positioned to capitalize on the growing demand for multifunctional antibodies, including immune cell engagers (T-Cell Engagers and NK-Cell Engagers) which bind simultaneously to the tumor cell and to immune cells redirecting immune cells to kill the tumor cells. An increasing number of clinical trials with bifunctional antibodies demonstrate increased efficacy and safety over the legacy monoclonal antibodies, which are currently dominating the oncology and auto-immune disease market.
Additionally, NAYA intends to acquire additional clinical stage assets to further strengthen its therapeutic portfolio. Each asset/strategic franchise will be organized in distinct subsidiaries (spokes).
| 100 |
NAYA Women’s Health
NAYA Women’s Health (also referred to as “Legacy INVO”) includes clinical services that are carried out via its INVO Centers, LLC, a wholly owned subsidiary, which owns all NAYA fertility clinics. NAYA Women’s Health also includes the INVOcell medical device. The U.S. Food and Drug Administration (“FDA”) cleared the INVOcell medical device, which is currently available for commercial use in the United States.
NAYA Portfolio Business Model
According to LEK Consulting Executive Insights (2023), “hub and spoke” business models, which have the potential for alternative funding approaches, have emerged within the biopharma industry. Hub-and-spoke model companies use a centralized portfolio management team (the parent company or “Hub”) that owns and controls a set of subsidiaries (“Spokes”). The subsidiaries remain focused on their asset(s), program(s) and therapeutic area(s), while the parent company provides centralized leadership and resources.
As a key part of this business strategy, the parent seeks to acquire undervalued or shelved assets from larger pharma and/or biotech companies and then spins them out or aggregates them strategically into specific Spokes. Each Spoke has the flexibility of a lean organization supported by centralized resources and the option to be financed in part by the parent company and in part by private capital from institutional investors.
The portfolio, “hub and spoke” approach also potentially allows for risk mitigation for the parent company and its investors. It also provides the optionality - typically within two years - of individual subsidiary exits in the form of spin outs to enable an initial public offering or a merger and acquisition transaction.
This portfolio-based business model has been used by an increasing number of companies, including Roivant (NASDAQ:ROIV), BridgeBio (NASDAQ:BBIO), and Puretech (NASDAQ:PRTC). These companies are significantly larger than us, have a longer operating history, have significantly more capital and other resources available to them. There is no guarantee that we can achieve similar, or any, success using a portfolio-based business model approach. Nevertheless, we believe that we can be successful in adopting and implementing such a business model.
Key elements of our 2025-2026 strategy are expected to include the following value creating milestones:
| ● | Achieve phase I/IIa clinical proof of concept for NY-303, our GPC3-targeting FLEX NK™ bispecific antibody candidate for HCC and other solid tumors, and position it as best-in-class second line monotherapy in HCC patients not responding to first line immunotherapy (Check Point Inhibitors). | |
| ● | Achieve phase I/IIa clinical proof of concept for NY-338, our CD38-targeting FLEX-NK™ bispecific antibody candidate for multiple myeloma, and position it as a Best-In-Class CD38 Therapeutic. |
| 101 |
| ● | Evaluate differentiated profile of NY-338 for the treatment of select autoimmune diseases, a high-growth, underserved market, and advance it into clinical development upon preclinical proof of concept; | |
| ● | Build an early pipeline of best-in-class multifunctional antibodies for IND filing and phase I/IIa clinical trial initiation in 2026, including an AI/ML optimized PD1xVEGF bispecific antibody for the treatment of solid tumors and a PSMA FLEX NK™ bispecific antibody for the treatment of Prostate Cancer. | |
| ● | Acquire for further development at least one clinical asset with phase I/ II data from a large pharma, biotech or international company; | |
| ● | Enter into revenue-generating development partnerships with larger pharmaceutical/biotech companies upon achieving clinical milestones. | |
| ● | Validate a scalable, profitable, commercial business model for NAYA Women’s Health |
The NAYA leadership team includes executives with relevant expertise within our current areas of focus. Additionally, NAYA relies on senior advisors with prior experience at companies such as Ark Investments, Flagship Pioneering, Kymera Therapeutics, Nanobiotix, Novavax, BMS, GSK, Johnson & Johnson, Novartis and Pfizer. If we are successful in raising sufficient capital, we intend to enhance our corporate and operational team to fully execute our growth strategy and achieve value-creating milestones.
NAYA Therapeutics
NAYA Therapeutics is developing and aiming to achieve clinical proof-of-concept for its two NK engager bispecific antibodies for the treatment of select cancers and autoimmune diseases.
Our initial pipeline includes two novel FLEX -NK™ bispecific antibodies acquired from Cytovia. The first is NY-303, which targets a protein expressed on the cell membrane of HCC, called GPC3, and other solid tumors, while predominantly absent in normal tissue, making it a promising new therapeutic target for the treatment of HCC and other solid adult and pediatric tumors. The second is NY-338, for the treatment of multiple myeloma and other autoimmune diseases. These FLEX-NK™ bispecific antibodies are built on a quadrivalent multifunctional antibody platform designed to engage natural killer cells (“NK Cells”) by targeting NKp46 activating receptors using Cytovia’s proprietary FLEX-NK™ technology, licensed by NAYA from Cytovia. The early development pipeline includes a PD1xVEGF bifunctional antibody for solid tumors and a PSMA FLEX-NK™ bispecific antibody for prostate cancer.
| 102 |
We may not be successful in advancing these compounds, see risk factors on page 24 for additional information.
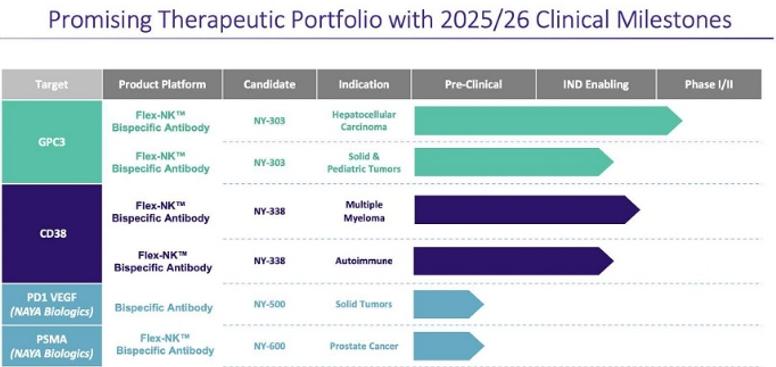
NAYA Therapeutics (Solid Tumors Franchise)
NY-303 (GPC3xNKp46)
GPC3 is an oncofetal protein expressed on adult solid tumors but not on adult healthy cells. GPC3 is expressed in over 70% of hepatocellular carcinoma cancer (HCC) cells according to the American Journal of Pathology and has been shown to correlate with severity of disease and non-response to immunotherapy according to Nature Medicine. GPC3 is also expressed in other adult tumors including ovarian cancer and squamous cell lung cancers, as well as pediatric tumors such as hepatoblastoma.
GPC3 antibodies are being developed in multiple modalities including cell therapy (CAR-T), bispecific antibodies (T-cell engagers and NK-cell engagers), antibody drug conjugates and radio-immuno conjugates.
NAYA is developing NY-303, a first-in-class GPC3-targeted FLEX-NK™ bispecific antibody for the treatment of HCC. NY-303 has been cleared to initiate phase I/IIa clinical trials in 2025. NAYA may expand its franchise strategy in the future to include additional indications and modalities.
Data from preclinical trials on NY-303, developed by the internal R&D team and Contract Research Organizations, is supporting further clinical development and has been presented at leading conferences including the American Academy of Cancer Research, the Society for Immunotherapy of Cancer, the European Society for Medical Oncology, and the American Society of Gene and Cell Therapy, suggesting a differentiated profile with unique characteristics including, but not limited to the following characteristics:
| ● | Redirection and enhancement of NK Cells to kill HCC tumor cells; | |
| ● | Reversal of dysfunction in NK Cells; |
| 103 |
| ● | Increased tumor growth inhibition when combined with endogenous, or natural body-producing peripheral blood or allogeneic NK Cells; | |
| ● | Improved dose-response in HCC tumor models in combination with both induced pluripotent stem cell derived and peripheral blood NK Cells | |
| ● | No significant toxicity at up to 20 times the expected therapeutic dose; | |
| ● | Pharmacokinetics support weekly administration in patients; | |
| ● | Enhanced tumor killing ability and reversal of NK Cells dysfunction in pre-clinical experiments; and | |
| ● | Less likely to induce immune reactions, such as cytokine release syndrome, compared to T-cell engagers. |
Market and Competition
There are several other GPC3-targeting antibodies or cell therapies that are being developed by AstraZeneca, Takeda, Legend Biotech, and Adicet Bio in collaboration with Regeneron. However, NAYA aims to differentiate themselves from the aforementioned companies as the first company to enter clinic trials with a GPC3 targeting NK engager bispecific antibody.
The National Institute of Health (NIH) reported that in 2020, there were 906,000 new diagnosis of hepatocellular carcinoma (HCC), the most frequent form of liver cancer, and 830,000 death of HCC patients.1 According to Polaris Market Research, the market size for liver cancer treatment was $2.44 billion in 2022 and is expected to grow a compounded annual growth rate of 20% to reach $10.48 billion in 2030.2 This market growth is supported by the 2022 approval of new standard of care, Merck’s Keytruda™ as well as a combination of two biological drugs commercialized by Genentech Roche, Tecentriq™ and Avastin™.
Clinical Development Plan
NY-303 has been cleared by the Israeli Ministry of Health and by the Internal Review Boards of leading academic medical centers to enroll patients in a phase I/II a clinical study. This study will assess the safety, pharmacokinetics, biological activity, objective clinical response, and time to progression of NY-303 in monotherapy in patients not responding to standard of care first line immunotherapy. Patient recruitment is expected to start in early 2025, with initial clinical data expected by end of 2025. NAYA intends to expand the NY-303 clinical trials to the United States, Europe, and Asia, pending regulatory approvals, aiming for full clinical proof of concept data in 2026.
1 Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660
2 https://www.researchandmarkets.com/reports/5692310/liver-cancer-market-share-size-trends
| 104 |
NY-500 (PD1xVEGF)
NY-500 is a tetravalent bifunctional antibody targeting PD-1, a key immune checkpoint targeted by pembrolizumab (Keytruda®, Merck & Co), and VEGF, a vascular endothelial growth factor targeted by bevacizumab (Avastin®, Genentech Roche) which regulates the production of new blood vessels (angiogenesis). Synergistic effects of simultaneously targeting PD-1 & VEGF have been shown to improve T-cell infiltration into tumors and enhance immune response while disrupting tumor vasculature. Ivonescimab, a PD-1 x VEGF antibody from Summit Therapeutics, recently outperformed pembrolizumab in a head-to-head lung cancer clinical trial. NY-500 has a differentiated molecular design, leveraging both NAYA’s proprietary FLEX format and AI-optimization, and is expected to enter monotherapy phase I/IIa clinical trials in early 2026 for the treatment of hepatocellular carcinoma (HCC) & other solid tumors.
According to IQVIA, the PD(L)1 market is expected to exceed $50 billion in 2025. The PD-1xVEGF bifunctional field, which is expected to compete directly and potentially replace the legacy PD(L)1 antibodies, is highly competitive. The most advanced product is ivonescimab (Summit Therapeutics) with data showing an improvement in Progression Free Survival over pembrolizumab in a phase III clinical trial in Non-Small-Cell Lung Cancer (NSCLC) in China and anticipating Overall Survival data in 2025. Other competitors include BioNTech (phase II/ III), Merck & Co (phase I in China), Crescent Biopharma (pre-clinical) and Ottimo Pharma (pre-clinical).
There is no guarantee that PD1 x VEGF bifunctional antibodies will surpass the standard of care demonstrated by Keytruda, or at all, and even if it does surpass the standard of care, there is no guarantee that sales in PD1 x VEGF bifunctional antibody products generally, or sales in our product candidate, if ever approved, and if any, will surpass those of Keytruda.
NY-600
NAYA is developing a FLEX NK bispecific antibody targeting PSMA on prostate cancer cells and NKp46 to activate and redirect NK Cells toward cancer cells. NAYA is leveraging its plug and play FLEX NK platform previously used for NY 303 and NY 338. NAYA expects to file an IND and initiate phase I/II clinical trials in metastatic castration resistant prostate cancer (mCRPC). The main competitors are T Cell Engagers, Antibody Drug Conjugates and Radii-immuno therapeutics.
NAYA Therapeutics (Immunotherapy Franchise )
NY-338 (CD38xNKp46)
NY-338 is a CD38-targeted FLEX-NK™ bispecific antibody for the treatment of multiple myeloma and autoimmune diseases. CD38 is a protein with high expression on Multiple myeloma cells and limited expression on normal cells, making it an attractive therapeutic target.
| 105 |
Monoclonal antibodies, or mAb, targeting CD38, such as daratumumab and isatuximab, have shown therapeutic efficacy in Multiple myeloma, both alone and in combination with normal standard of care regimens. However, many patients eventually relapse because of resistance mechanisms, including down regulation of CD38 on tumor cells as well as inhibition of complement dependent cytotoxicity, antibody-dependent cell mediated cytotoxicity and antibody dependent cellular phagocytosis.
We are planning to file an IND NY-338 and initiate phase I/ IIa clinical trials in 2025. Data on NY-338 supporting further clinical development pending regulatory approvals has been presented at leading conferences, including the American Society of Hematology and the American Association of Cancer Research.
According to Professor Ola Landgren, Chair of Myeloma at the Sylvester Comprehensive Cancer Center at the University of Miami, “the synergistic engagement of NK cells through NKp46 greatly enhances the immunotherapeutic effects of FLEX NK bispecific antibodies, reducing NK cell fratricide, maintaining NK cell levels, and enhancing potency including NK cell dysfunction reversal. The data (presented at the American Society of Hematology 2023) supports initiation of clinical trials to evaluate this promising new therapy and makes it a potential best-in-class anti-CD38 therapeutic for multiple myeloma”.
Compared to daratumumab, NY-338 early data suggest:
| ● | 3-fold higher CD38 binding | |
| ● | Higher NK and macrophage cytotoxicity against multiple myeloma; | |
| ● | Minimal NK cell fratricide; | |
| ● | Minimal immune subset depletion; and | |
| ● | Minimal cytokine release; |
Unmet Medical Needs in Multiple Myeloma
Multiple myeloma is the second most common blood cancer worldwide, with high unmet medical need despite significant progress and a rapidly growing market. Bispecific antibodies and off-the-shelf cell therapy have shown significant potential in addressing current limitations and are expected to further grow the addressable market in a substantial way.
Multiple myeloma is a malignant proliferation of plasma cells, or white blood cells capable of secreting immunoglobulin or antibodies, and is the second most common hematological malignancy, with 35,000 new cases/year in the U.S., with around 160,000 patients affected worldwide.3 Myeloma cells prevent normal antibody production leading to the accumulation of abnormal immunoglobulins that compromise the body’s immune response. Remarkable advancements in the understanding of the pathophysiology have revolutionized treatment options and patient outcomes. However, genetic intricacy, instability, and diverse clinical presentations of multiple myeloma remain barriers towards providing a cure, and multiple myeloma is associated with the highest symptom burden and lowest health-related quality of life among patients with hematologic malignancies.
3 https://www.cinj.org/multiple-myeloma-more-common-you-think
| 106 |
The increase in the number of therapeutic alternatives for both newly diagnosed and relapsed/refractory multiple myeloma (“RRMM”) patients has led to multiple therapeutic combinations of chemotherapeutics. Outcomes remain poor for triple-class–refractory patients, defined as disease refractory to proteasome inhibitors, immunomodulatory agents, and monoclonal antibodies (Median Overall Survival 1 year),4 and there is no standard of care.
This has led to the need to develop new drugs with novel mechanisms of action to fill this gap. B-cell maturation antigen (“BCMA”) is now the most widely explored target for CAR-T cell therapies in multiple myeloma, with more than 15 constructs being evaluated for RRMM patients and two FDA approved drugs, idecabtagene vicleucel and ciltacabtagene autoleucel, in addition to belantamab mafodotin, an antibody-drug conjugate targeting BCMA. There is a need for bispecific antibodies to address broader segments of multiple myeloma patients. High cost, product supply, and safety monitoring limit the use of auto CAR-Ts to a small number of patients.
Daratumumab and T-Cell engager bifunctional antibodies are moving to earlier lines of treatment leaving a need for alternative therapies in relapsing resisting patients.
The FDA granted conditional marketing authorization for three T-Cell Engager bifunctional antibodies in 2022 and 2023. The first one, teclistamab (Tecvaily™- J&J), redirects CD3-positive T-cells to BCMA-expressing myeloma cells to induce the killing of tumor cell as monotherapy for the treatment of adult patients with RRMM. T-Cell engagers are a direct competitor to NK cell engagers but might also be combined together.
Competitive Strengths and Weaknesses
Potential strengths of NY-338 include as follows:
| - | NY-338’s competitive strength is that it is expected to be the first CD38-targeting NK engager to enter clinical trials. Enhanced tumor killing ability and reversal of NK Cells dysfunction in pre-clinical experiments; and | |
| - | NY-338 may be positioned, based on the safety and efficacy outcome of phase I/IIa clinical trials, in non-responders to daratumumab and BCMA T-cell engagers, as an alternative or in combination with BCMA T-cell engagers, and eventually as an alternative to daratumumab. |
Potential weaknesses of NY-338 include as follows:
| - | As detailed below, there are several other CD38-targeting and BCMA-targeting antibody candidates in the market that are supported by companies with greater resources; and | |
| - | NY-303 will need to demonstrate a differentiated and improved clinical product profile to support pharma partnering and/or phase II/III clinical development. |
4 https://ashpublications.org/blood/article/142/Supplement%201/3369/502480/Outcomes-of-Triple-Class-Refractory-Multiple
| 107 |
Market and Competition
According to a Delveinsight July 2023 report on the multiple myeloma, the global market size in 2022 for multiple myeloma treatments was $20 billion and is expected to continue to grow significantly with the introduction of new products. The current market leader, CD38 targeting monoclonal antibody, Darzalex ™ (daratumumab) reached $8 billion in global sales in 2022.
Recently the FDA approved bi-specific antibodies, including BCMA targeting Tecvaly™ in 2022 and GPRC5D targeting talquetamab (Talvey ™, J&J) in 2023 from Johnson & Johnson. The new BCMA targeting bispecific antibody from Pfizer, Elrexfio™, was approved in August 2023. Additional bispecific antibodies from Abbvie, Regeneron, and Roche are in early stage of clinical development. However, despite this existing competition, NY-338, is to the best of our knowledge, the first CD38-targeting NK engager to enter clinical trials.
Additional bispecific antibodies from Abbvie, Regeneron, and Roche are in early stage of clinical development. However, NAYA’s NY-338 is the first bispecific antibody to target both NKp46 to redirect NK Cells and CD38, with the potential to demonstrate both efficacy and safety advantages.
NY-338 for Autoimmune Diseases
NAYA intends to explore additional indications including autoimmune diseases as part of its life cycle management strategy for NY-338. Initial clinical proof of concept with other CD38 targeting antibodies such as daratumumab (J&J) and Felzartamab (HI-Bio) have been published in the New England Journal of Medicine and Nature journals. HI-Bio was acquired by Biogen in 2024 following initial phase II clinical proof of concept in antibody-mediated rejection (AMR), IgA nephropathy (IgAN) and primary membranous nephropathy (PMN).
B-cell mediated Autoimmune diseases are susceptible to biologics targeting CD19, CD20, BCMA and CD38.This approach has been validated by biologics approved for rheumatoid arthritis and systemic lupus erythematous, including rituximab, belimumab and anifrolumab. BCMA and CD38 are differentiated from CD19 and CD20 as they target plasma cells in addition to B cells. CD38 uniquely targets type I interferon plasmacytoid dendritic cells (type 1 IFN pDC). This differentiated biology of CD38 as well as the role of NK Cells in specific patient groups will contribute to defining a competitive target product profile for NY-338 in autoimmune diseases.
NAYA plans to conduct pre-clinical studies with NY-338, translational studies to identify best indications and expects to initiate clinical development in late 2025 or early 2026, pending positive data in the dose escalation in multiple myeloma patients and differentiated profile in experimental models of auto-immune diseases.
| 108 |
NY-338 Clinical Development Plan
We expect to file an investigational new drug application for NY-338 with the FDA and initiate a phase I/IIa safety and efficacy clinical trial in 2025 for multiple myeloma patients not responding to earlier line of treatments such as daratumumab (Darzalex™, J&J) and other biologics including BCMA targeting T-Cell engagers.
The phase I will be a dose escalation in patients with relapsed refractory multiple myeloma and will aim to establish safety, pharmacokinetics and a recommended phase II therapeutic dose based on preliminary efficacy (Overall Response Rate, biomarkers, Minimal Residual Disease negativity, time to progression). The phase IIa will expand the evaluation to a larger number of patients treated at therapeutic dose.
NAYA is planning to conduct its phase I/IIa at the Sylvester Comprehensive Cancer Center at the University of Miami and at additional leading academic medical centers.
NAYA plans to initiate a clinical development plan for NY-338 in select auto-immune indications in later 2025. These NY-338 phase I/IIa efforts are subject to clearance by the FDA of an Initial New Drug application.
Key Third-Party Relationships
NAYA has entered into agreements with several key third parties, each of whom plays a critical role in NAYA’s business, including the following:
| ● | Inserm Transfert SA |
Pursuant to a License Agreement between Legacy NAYA and Inserm Transfert SA, dated December 19, 2023 (the “Inserm Agreement”), NAYA was granted an exclusive, worldwide royalty-bearing license under certain patents/patent applications co-owned by Inserm and Université de Paris (now Université Paris Cité), and a non-exclusive transferable, royalty-bearing license, with the right to sublicense under certain know how owned by Inserm for the development and commercialization of CYT338 (now NY-338) in a defined field. The Inserm Agreement can be terminated early either upon the material breach upon one of the parties of the Inserm Agreement, upon either party to the Inserm Agreement being subject to bankruptcy, or upon NAYA failing to meet one of the developmental milestones as described in the Inserm Agreement. Unless terminated earlier, the term of the Inserm Agreement will continue until the last to occur of (i) with respect to a given country – the last valid patent claim covering the product in such country expires or is invalidated, (ii) with respect to a given country – the lapse of ten years from the first commercial sale in such country of a product using intellectual property licensed pursuant to the Inserm Agreement (the “Inserm IP”), or (iii) such time when NAYA no longer continues to generate revenues from the sale of products based on Inserm IP. Under the Inserm Agreement, NAYA is to pay Inserm (a) a sublicense fee of 12% on revenues generated by sub-licensing of Inserm IP; (b) royalties of 2.5% on net sales from the Inserm IP during the term of the Inserm Agreement; and (c) lump sum payments of up to an aggregate of €5.15 million (approximately USD $4.5 million) in preclinical and clinical development and commercial milestones.
| 109 |
| ● | Yissum Research & Development Company of the Hebrew University of Jerusalem (“Yissum”) |
Pursuant to a License Agreement between Legacy NAYA, the University of Rijeka Faculty of Medicine (“Rijeka”), and Yissum (together with Yissum, the “Licensors”) dated December 20, 2023, NAYA was granted an exclusive, worldwide manufacturing, marketing, developing royalty-bearing license to make commercial use of certain patents and patent applications covering two specific anti NKp46 antibodies denoted hNKp46.09 (09) and hNKp46.12 (12) and know how needed in order to develop, manufacture, market, distribute and sell CYT338 (now NY-338) and CYT303 (now NY-303) and or incorporating products known as CYT338 (now NY-338) or CYT303 (now NY-303) in the specified field (the “Yissum License”). The Licensors retain rights to use the licensed technology for their own research and education. NAYA may sublicense only after obtaining Licensors’ written approval of the sublicensee’s identity and key terms, with approval not unreasonably withheld or delayed. They also have the right to license it to other academic and non-profit research organizations for non-commercial research. The Yissum License expires upon the last to occur of (i) with respect to a given country – the date of expiration in such country of the last patent licensed under the Yissum License, (ii) with respect to a given country – the date of expiration of any exclusivity on the Product granted by a regulatory or government body in such country; or (iii) the lapse of twenty (20) years from the date of the first sale by NAYA of a product incorporating the intellectual property licensed to it under the Yissum License (the “Products”). Under the Yissum License, NAYA is to pay Yissum (a) a royalty fee of two percent on net sales of Products, (b) a one-time payment of $1,000,000 upon achieving annual net sales of Products of $100,000,000, (c) a sublicense fee of 10% on revenues generated by NAYA from sublicensing intellectual property covered by the Yissum License, (d) an exit fee of USD $1,000,000 in the case of certain M&A transactions with change of control; and (e) certain additional payments upon reaching various development milestones up to USD $2,250,000.
| ● | Cytovia Therapeutics, Inc. Asset Acquisition |
On October 18, 2023, Legacy NAYA, Cytovia Therapeutics Holdings, Inc., a Delaware corporation (“Holdings”) and Cytovia entered into an Asset Purchase Agreement (the “Asset Purchase Agreement”) for projects CYT303 and the CYT338. The purchase consideration was comprised of 1,363,642 shares of common stock of Legacy NAYA, the assumption of certain liabilities totaling $2,688,745 and a promissory note to Cytovia in the principal amount of $6,000,000, of which $1,000,000 to be paid at the closing of any financing, and $1,000,000 per month for 5 months thereafter.
Payments totaling $1,700,000 were made to Cytovia in January 2024. On May 17, 2024, Legacy NAYA and Cytovia amended the Asset Purchase Agreement. The amended aggregate purchase price consisted of 1,609,098 shares of common stock of Legacy NAYA (approximately 14.3 million shares post-merger on an as-converted basis of C-1 Preferred Shares) and a payment of $1,700,000, which was made by Legacy NAYA in January 2024.
The amended agreement also provides for sublicense fees to be paid to Cytovia at 10% of any gross consideration actually received by NAYA (i) as a fee for sublicensing or selling CYT303 or CYT338 in any indications to any third party or (ii) as payments for development milestones, commercial milestones, or royalties or any other payments under the terms of any such sublicense or asset purchase agreement.
| 110 |
The acquisition agreement also includes, for no further consideration, a sublicense agreement between NAYA and Cytovia, under which Cytovia granted NAYA a non-exclusive sublicense under Cytovia rights in PCT/IB2012/053482 (P-627002-PC) (the “Licensed Technology”), or certain technology jointly owned by Dr. Jean Kadouche and CNRS (The French Center for National Scientific Research) and co-exclusively licensed to Cytovia, for the development and commercialization of CYT303 (now NY-303) and CYT338 (now NY-338). NAYA has expanded the scope of the non-exclusive license to the development of additional multi-functional antibodies for which NAYA will make payments upon achievement pf certain milestones. The agreement terminates upon the expiration of the patent rights to the Licensed Technology.
| ● | CytoLynx Therapeutics |
Additionally, the revenue-generating partnership with CytoLynx Therapeutics (“CytoLynx”) for NY-303 in Greater China will be assigned to NAYA pursuant to the Asset Purchase Agreement. Under the CytoLynx agreement to be assigned by Cytovia to NAYA, Cytovia granted to CytoLynx the rights for the for the development and manufacturing of NY-303 in Mainland China, Hong Kong, Taiwan and Macau. As compensation. Under the agreement, NAYA will be eligible to receive up to $12 million in payments from CytoLynx upon achieving certain developmental milestones, as well as up to $145 million upon CytoLynx reaching certain commercial milestones. Additionally, under the agreement, NAYA will receive royalty fees from CytoLynx based on net sales of the licensed products in the licensed territories (Mainland China, Hong Kong, Taiwan and Macau). The term of the agreement is indefinite until terminated by either party according to the terms of the agreement. The development and commercialization of NY-303 outside of Greater China is not contingent on the success of any of the activities conducted by CytoLynx, and all intellectual property remains owned by NAYA.
NAYA Therapeutics Intellectual Property
NAYA owns a total of twenty-two (22) pending patent applications in two families. Each family includes one (1) US and one (1) European patent application, and nine (9) patent applications in other foreign jurisdictions. One family is based on WO 2022/216744 and covers NY-303, while the other family is based on WO 2022/216723 and covers NY-338.
The Kadouche/CNRS multispecific antibody license with NAYA for NY-303 and NY-338 includes thirteen (13) patents and patent applications, including two (2) granted US patents, two (2) pending US patent applications, two (2) granted European patents, and one (1) pending European patent application, as well as six (6) granted patents in other foreign jurisdictions.
NAYA licenses from Yissum ten (10) pending patent applications for NY-303 and NY-338, which include one (1) US and one (1) European patent application and eight (8) patent applications in other foreign jurisdictions. NAYA licenses from INSERM five (5) pending patent applications for NY-338, which includes one (1) US and one (1) European patent application and three (3) patent applications in other foreign jurisdictions.
| 111 |
NAYA entered into a direct licensing agreement for the use of NY-303 and the necessary intellectual property rights bispecific antibody technology from Dr. Jean Kadouche and CNRS (The French Center for National Scientific Research) fully paid through by Cytovia Therapeutics. Additionally, NAYA entered into a direct licensing agreement to utilize the product-specific NKp46 license from Yissum, the Technology Transfer Company of the Hebrew University of Jerusalem.
NAYA Therapeutics Competitive Landscape
According to international market research firm, Research and Markets, the global bispecific antibodies market is projected to witness over 40% compound annual growth rate and reach over $80 billion by 2030.

The development of bispecific antibodies began when scientists recognized the potential of monoclonal antibodies. This marked the start of a new era in therapeutics in the late 1990s. Bispecific antibodies offer multiple benefits, including dual targeting of different antigens, improved specificity, enhanced targeting ability, reduced dose-limiting toxicities, and the potential for drug-drug or drug-to-protein conjugates. These antibodies provide diversity by targeting two different tumor and/or immune cell antigens or epitopes simultaneously. Several bispecific antibodies. have made their mark in the commercial market, receiving FDA and EMA approval or many more are in clinical development. Among the emerging bispecific antibodies, ivonescimab (Akeso/ Summit Therapeutics) has recently demonstrated the clinical superiority of its bifunctional PD-1/ VEGF antibody over pembrolizumab (Keytruda, Merck), the leading commercial PD-1 blocking monoclonal antibody and Janux Therapeutics’ PSMA targeting T-cell engager has shown, in a phase I clinical trial in heavily pre-treated Prostate Cancer patients, a 50% reduction in Prostate Specific Antigen (“PSA”), a key biomarker of disease progression.
Given the rapid growth of the bispecific antibody market, the competition has increased significantly. There are many companies developing bispecific antibodies including Amgen, AstraZeneca, Johnson & Johnson, Merus, Pfizer, Sanofi, Xencor, Zymeworks.
We believe that NAYA is uniquely positioned to capitalize on the growing demand for multifunctional antibodies as the current and next generation of therapies demonstrate increased efficacy and safety over the legacy monoclonal antibodies, which are currently dominating the oncology and auto-immune disease market.
| 112 |
Recent 2024 transactions, based on publicly available information, all involving early-stage development of bifunctional antibodies for cancer and auto-immune diseases, include:
| - | The initial public offering (“IPO”) of Bicara Therapeutics (NASDAQ:BCAX) which has completed a phase II/IIa in solid tumors with a EGFR x TGF-β bifunctional antibody; | |
| - | The IPO of Zenas Biopharma (NASDAQ:ZBIO) which is in phase II in autoimmune diseases with a phase II CD19 x FcγRIIB bifunctional antibody; | |
| - | The acquisition of Chimagen phase I CD19xCD20 bispecific antibody by GSK; | |
| - | The global licensing of LaNova’s phase I PD1xVEGF bifunctional antibody by Merck; | |
| - | The reverse merger and financing of Crescent Biopharma which is in pre-clinical development for its VEGF x PD1 bifunctional antibody; | |
| - | The Series A financing of Navigator Medicines phase I OX40L x TNF-α bifunctional antibody for autoimmune diseases; and | |
| - | The secondary public financing of JANUX (NASDAQ:JNX) following phase I data availability. |
The performance, capital raising, market capitalizations, and valuations of the foregoing companies are for informational purposes only and are neither indicative nor predicative of the results of NAYA. There are no guarantees that NAYA may secure similar financing, execute its development plans and achieve comparable valuations.
NAYA Biologics
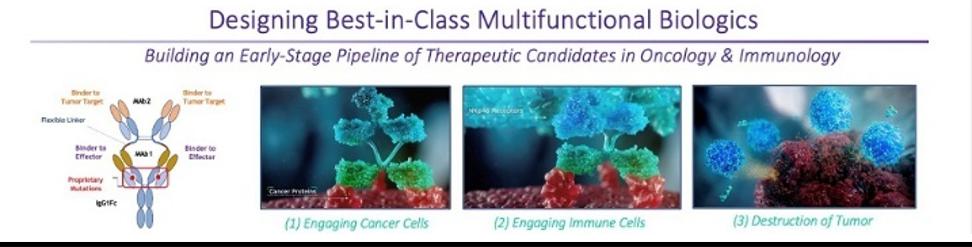
NAYA has entered into a non-exclusive license with Cytovia Therapeutics of its proprietary multifunctional antibody platform, for which NAYA will receive success milestones for each therapeutic candidate. NAYA also collaborates with Lynx Bio (www.lynx.bio) for their multiomics biology platform, MabSilico (www.mabsilico.com) for their AI/ML antibody design and optimization platform and STC biologics (www.stcbiologics), for process development and GMP manufacturing.
The multifunctional antibody platform is expected to be validated through the development of NY-303 and NY-338 as well as other early stage candidates.
| 113 |
The first bifunctional antibody in NAYA Biologics pipeline is developed with the AI/ML support of MAbSilico and targets PD1xVEGF. NAYA aims to file an Initial New Drug application and initiate clinical trials in 2026.
Proprietary design of new candidates will leverage multifunctional scaffold, multiomics biology platform, and AI/ML. Manufacturing process is expected to be validated with high yields for GMP clinical trial batches. Multiple experimental studies, presented at major international conferences such as ASH, AACR, SITC, ESMO, have demonstrated simultaneous attachment to both immune cells and targeted cancer cells, improved & sustained engagement of immune cells in tumor microenvironment through activating receptors, reversal of immune cell dysfunction, a unique mechanism to turn “cold” into “hot” tumors, and synergy with both endogenous & allogeneic immune cells.
NAYA Biologics intends to develop a pipeline of best-in-class therapeutic candidates up to pre-clinical proof of concept, based on a competitive target product profile and GLP manufacturing batch validation. Further development will be undertaken either by an existing or a new NAYA subsidiary (Spoke), or by an external partner through licensing or acquisition.
NAYA Clinical Intelligence (NAYA CI)
NAYA CI aims to optimize the selection and development of clinical candidates for NAYA subsidiaries as well as for external partners. NAYA CI combines the generative AI and graph AI proprietary knowledge of Lynx Analytics (www.lynxanalytics.com) with NAYA Biosciences strategic and clinical biopharma expertise.
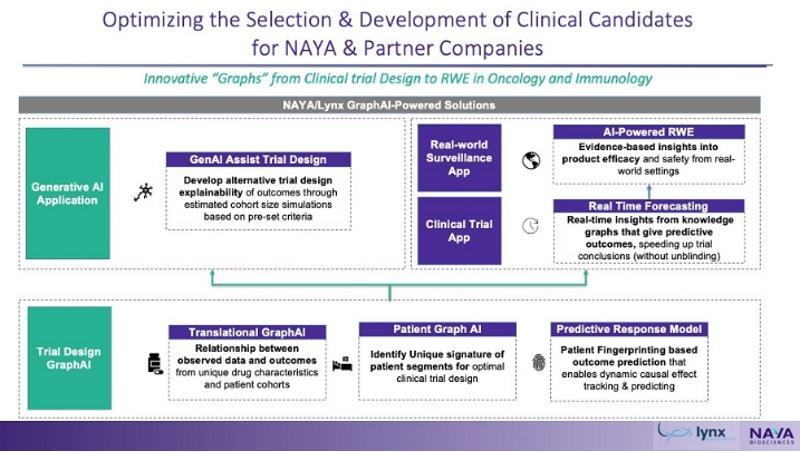
NAYA presented an original abstract – Leveraging Graph AI to analyze the influence of GPC3 Gene expression and NK Cell tumor infiltration on Hepatocellular Carcinoma survival rates - at the 2024 meeting of the Society for Immunotherapy of Cancer (SITC) in Houston, TX.
NAYA CI’s business model is a combination of fee-for-service and scalable subscription fees based on the utilization of each algorithm and application.
NAYA Women’s Health
NAYA aims to advance the future of women’s health with a primary focus on fertility through a growing portfolio of assets dedicated to expanding access, improving outcomes and enhancing the patient experience.
| 114 |
NAYA’s current fertility business is focused on operating fertility-focused clinics. As of the date of this filing, we have two operational INVO Centers, dedicated primarily to offering the intravaginal culture (“IVC”), and an in vitro fertilization (“IVF”) clinic acquired in August 2023. We also continue to engage in the sale and distribution of our INVOcell technology solution into existing independently owned and operated fertility clinics.
Within the fertility market, we are dedicated to expanding assisted reproductive technology (“ART”) by making fertility care more accessible and inclusive. Our flagship product is INVOcell, a revolutionary medical device that allows fertilization and early embryo development to take place in vivo within the woman’s body. This treatment solution is the world’s first intravaginal culture technique for the incubation of oocytes and sperm during fertilization and early embryo development. This technique, designated as “IVC”, provides patients a more natural, intimate and more affordable experience in comparison to other ART treatments. The IVC procedure can deliver comparable results at a lower cost than IVF and is a significantly more effective treatment than intrauterine insemination (“IUI”).
INVO Centers:
On March 10 and June 28, 2021, we established joint ventures to open INVO Centers in Birmingham, Alabama, and Atlanta, Georgia, respectively. We established these clinics to increase use volume for the INVOcell, accelerating the growth and awareness of the IVC procedure and the availability of statistical data supporting its use. These clinics also enabled us to expand our revenue from several hundred dollars per INVOcell to thousands of dollars for each fertility cycle, and to significantly advance our path to profitability. INVO Centers require less investment than traditional IVF clinics and are operationally efficient, making them ideal for underserved secondary markets.
Acquisitions:
On August 10, 2023, we consummated the first acquisition of an existing IVF clinic, the Wisconsin Fertility Institute (“WFI”). As an established and profitable clinic, the closing of the WFI acquisition more than tripled our current annual revenues and became the largest part of our clinic-based operations.
INVOcell:
Our proprietary technology, INVOcell®, is a revolutionary medical device that allows fertilization and early embryo development to take place in vivo within the woman’s body. This treatment solution is the world’s first intravaginal culture technique for the incubation of oocytes and sperm during fertilization and early embryo development and provides patients with a more natural, intimate, and affordable experience in comparison to other ART treatments. We believe the IVC procedure can deliver comparable results at a lower cost than traditional IVF and is a significantly more effective treatment than IUI.
| 115 |
Unlike IVF, where the oocytes and sperm develop into embryos in an expensive laboratory incubator, the INVOcell allows fertilization and early embryo development to take place in the woman’s body. This allows for many benefits in the IVC procedure, including the following:
| ● | Reduces expensive and time-consuming lab procedures, helping clinics and doctors to increase patient capacity and reduce costs; | |
| ● | Provides a natural, stable incubation environment; | |
| ● | Offers a more personal, intimate experience in creating a baby; and | |
| ● | Reduces the risk of errors and wrong embryo transfers. |
In both current utilization of the INVOcell, and in clinical studies, the IVC procedure has demonstrated equivalent pregnancy success and live birth rates as IVF. Below is a summary of the real-world usage data used to support our 510(k) submission to the FDA for expanded usage of INVOcell. This 510(k) was cleared by the FDA in 2023:
| INVO Cycles | INVO Cycles | Conventional IVF | ||||||||||
| Summary Data | Day 5* | Day 3 | Day 5* | |||||||||
| (INSEM & ICSI) | (INSEM & ICSI) | (INSEM & ICSI) | ||||||||||
| Total Cycle Starts | 321 | 450 | Not Avail | |||||||||
| Total Transfers | 240 | 421 | 685 | |||||||||
| Clinical Pregnancies % / Per cycle Start | 42.7 | % | 32.4 | % | Not Avail | |||||||
| Birth Rate % / Per cycle Start | 34.9 | % | 23.8 | % | Not Avail | |||||||
| Clinical Pregnancies % / Per Transfer | 57.1 | % | 34.7 | % | 51.8 | % | ||||||
| Birth Rate % / Per Transfer | 46.8 | % | 25.4 | % | 44.5 | % | ||||||
While INVOcell remains part of our efforts, our commercial and corporate development strategy in the fertility market has expanded to focus more broadly on providing ART services through our operating clinics and other activities. We may also look to further expand our fertility activities by seeking acquisitions of other complimentary and unique products.
Operations:
We operate with a core internal team and outsource certain operational functions in order to help accelerate our efforts as well as reduce internal fixed overhead needs and in-house capital equipment requirements. Our most critical management and leadership functions are carried out by our core management team. We have contracted out the manufacturing, packaging/labeling and sterilization of the INVOcell device to a contract medical manufacturing company that completes final product manufacturing as well as manages the gamma sterilization process at a U.S. Food and Drug Administration (“FDA”) registered contract sterilization facility. We also rely on outside contact firms for most of our clinical development activities. NAYA has entered into a service agreement with Syneos Health, a leading contract commercialization company, to accelerate the growth of INVOcell in the United States.
| 116 |
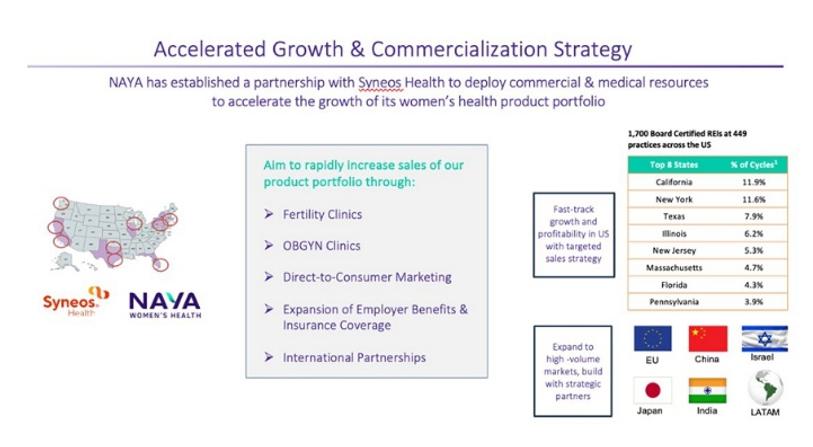
Market Opportunity
The global ART marketplace is a large, multi-billion dollar industry growing at a strong pace in many parts of the world as increased infertility rates, increased patient awareness, acceptance of treatment options, and improving financial incentives such as insurance and governmental assistance continue to drive demand. According to the European Society for Human Reproduction 2020 ART Fact Sheet, one in six couples worldwide experience infertility problems. Additionally, the worldwide market remains vastly underserved as a high percentage of patients in need of care continue to go untreated each year for many reasons, but key among them are capacity constraints and cost barriers. While there have been large increases in the use of IVF, there are still only an estimated 2.6 million ART cycles, including IVF, IUI, and other fertility treatments, performed globally each year, producing around 500,000 babies. This amounts to less than 3% of the infertile couples worldwide being treated and only 1% having a child though IVF. The industry remains capacity constrained which creates challenges in providing access to care to the volume of patients in need and at an affordable price. A survey by “Resolve: The National Infertility Association,” indicates the two main reasons couples do not use IVF is cost and geographical availability (and/or capacity).
In the United States, infertility affects an estimated 10%-15% of the couples of childbearing-age, according to the American Society of Reproductive Medicine (2017). According to the Centers for Disease Control (“CDC”), there are approximately 6.7 million women with impaired fertility. Based on 2021 data from CDC’s National ART Surveillance System (the most recently available data), approximately 413,000 IVF cycles were performed at 453 IVF centers, leaving the U.S. with a large, underserved patient population, similar to most markets around the world.
Competitive Advantages - INVOcell
While our commercial efforts have expanded to clinic services within the ART market, we also continue to believe that our INVOcell device, and the IVC procedure it enables, have the following key advantages:
Lower cost than IVF with equivalent efficacy. The IVC procedure can be offered for less than IVF due to lower cost of supplies, labor, capital equipment and general overhead. The laboratory equipment needed to perform an IVF cycle is expensive and requires ongoing costs as compared to what is required for an IVC cycle. As a result, we also believe INVOcell and the IVC procedure enable a clinic and its laboratory to be more efficient as compared to conventional IVF.
| 117 |
The IVC procedure is currently being offered at several IVF clinics at a price range of $5,000 - $11,000 per cycle and from $4,500 to $7,000 at the existing INVO Centers, thereby making it more affordable than IVF (which tends to average $11,000 to $15,000 per cycle or higher).
Improved efficiency providing for greater capacity and improved access to care and geographic availability. In many parts of the world, including the U.S., IVF clinics tend to be concentrated in higher population centers and are often capacity constrained in terms of how many patients a center can treat, since volume is limited by the number of capital-intensive incubators available in IVF clinic labs. With the significant number of untreated patients along with the growing interest and demand for services, the industry remains challenged to provide sufficient access to care and to do so at an economical price. We believe INVOcell and the IVC procedure it enables can play an important role in helping to address these challenges. We estimate that by adopting the INVOcell, IVF clinics can increase fertility cycle volume by up to 30% without adding to personnel, space and/or equipment costs. Our own INVO Centers also address capacity constraints by adding to the overall ART cycle capacity and doing so with comparable efficacy to IVF outcomes as well as at a lower per cycle price. With our two-pronged strategy (IVF clinics and INVO Centers), in addition to lowering costs, we believe INVOcell and the IVC procedure can address the industry’s key challenges, capacity and cost, and help open up access to care for underserved patients around the world.
Greater patient involvement. With the IVC procedure, the patient uses their own body for fertilization, incubation and early embryo development which creates a greater sense of involvement, comfort and participation. In some cases, this may also free people from barriers related to ethical or religious concerns, or fears of laboratory mix-ups.
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND
RESULTS OF OPERATIONS OF NAYA THERAPEUTICS, INC.
The following discussion and analysis of Legacy NAYA’s financial condition and results of operations should be read together with Legacy NAYA’s audited consolidated financial statements and the related notes for the years ended December 31, 2023 and 2022, and Legacy NAYA’s unaudited condensed consolidated financial statements and related notes for the nine months ended September 30, 2024 and 2023, attached as Annex A and Annex B to this proxy statement, respectively. The unaudited condensed consolidated financial statements for the nine months ended September 30, 2024 and 2023 have not been audited or reviewed by an independent accountant.
The Legacy NAYA Acquisition and Financing Transaction
On October 11, 2024 (the “Effective Time”), LEGACY INVO, Merger Sub, and LEGACY NAYA, entered into the Merger Agreement and consummated the transactions contemplated thereby. Upon the terms and subject to the conditions set forth in the Merger Agreement, Merger Sub merged with and into LEGACY NAYA, with LEGACY NAYA continuing as the surviving corporation and a wholly owned subsidiary of LEGACY INVO. References hereafter to Company refer to the merged entity. The acquisition was intended to qualify for U.S. federal tax purposes as a tax-free reorganization under the provisions of Section 368(a) of the Code.
| 118 |
At the Effective Time and as a result of the consummation of the Merger:
| ● | Each share of Class A common stock, par value $0.000001 per share, and Class B common stock, par value $0.000001 per share, of LEGACY NAYA (“LEGACY NAYA common stock”) outstanding immediately prior to the effective time of the Merger, other than certain excluded shares held by LEGACY NAYA as treasury stock or owned by the Company or Merger Sub, automatically converted into the right to receive 118,148 shares of the Company’s common stock and 30,375 shares of the Company’s newly-designated Series C-1 Convertible Preferred Stock (the “Series C-1 Preferred”). The Series C-1 Preferred is not redeemable, has no voting rights, and may not be converted into shares of the Company’s Common Stock unless and until the Company’s stockholders approve the issuance of common stock upon conversion of the Series C-1 Preferred. If the Company’s stockholders approve the issuance of common stock upon conversion of the Series C-1 Preferred, such Series C-1 Preferred will automatically convert into approximately 29,515,315 shares of the Company’s common stock, subject to adjustment if, as a result of such conversion if, after giving effect to the conversion or issuance, any single holder, together with its affiliates, would beneficially own in excess of 19.99% of the Company’s outstanding common stock. A description of the rights, preferences, and privileges of the Series C-1 Preferred are set forth in Item 5.03 below. | |
| ● | Certain outstanding debt obligations of LEGACY NAYA, including a portion of an amended and restated senior secured convertible debenture issued to Five Narrow Lane LP (“FNL”), with a combined principal balance of $8,575,833 converted into the right to receive 669,508 shares of the Company’s common stock and 8,576 shares of the Company’s newly-designated Series C-2 Convertible Preferred Stock (the “Series C-2 Preferred”). The Series C-2 Preferred is only redeemable upon a “Bankruptcy Triggering Event” or a “Change of Control” that occurs 210 days after the closing date of the Merger. The Series C-2 Preferred may not be converted into shares of the Company’s Common Stock unless and until the Company’s stockholders approve the issuance of common stock upon conversion of the Series C-2 Preferred. If the Company’s stockholders approve the issuance of common stock upon conversion of the Series C-2 Preferred, such Series C-2 Preferred will be convertible at the option of the holders into approximately 12,441,607 shares of the Company’s common stock, subject to limitations on beneficial ownership by the holders thereof. A description of the rights, preferences, and privileges of the Series C-2 Preferred are set forth below. | |
| ● | The remaining balance of the amended and restated senior secured convertible debenture issued to FNL in the amount of $3,934,146 was exchanged for a 7.0% Senior Secured Convertible Debenture in the principal balance of $3,934,146 due December 11, 2025 (the “Debenture”). A description of the rights, preferences, and privileges of the Debenture are set forth below. | |
| ● | The accredited investor note for $500,000 was converted to 500 Series C-2 Convertible Preferred shares. | |
| ● | LEGACY NAYA has been renamed to “NAYA Therapeutics Inc.” |
| 119 |
Overview of Legacy NAYA
Prior to its acquisition by the Company, Legacy NAYA was a privately held clinical stage biopharmaceutical company focused on developing novel therapies for oncology and autoimmune diseases.
Legacy NAYA is developing and aiming to achieve clinical proof-of-concept for its two NK engager bispecific antibodies for the treatment of select cancers and autoimmune diseases. We are currently preparing the initiation of a phase I dose escalation clinical trial for NY-303, our lead GPC3-targeting NK engager bispecific antibody for the treatment of hepatocellular carcinoma and other solid tumors, pending approval from regulatory authorities and hospital internal review boards. Clinical trials for NY-338, our lead CD38-targeting NK engager bispecific antibody for the treatment of multiple myeloma and autoimmune diseases, are expected to initiate in 2025.
Pipeline
Our initial pipeline includes two novel FLEX-NK™ bispecific antibodies acquired from Cytovia Therapeutics, Inc. The first is NY-303, which targets a protein expressed on the cell membrane of hepatocellular carcinoma (“HCC”), called GPC3, and other solid tumors, while predominantly absent in normal tissue, making it a promising new therapeutic target for the treatment of HCC and other solid tumors. The second is NY-338, for the treatment of Multiple myeloma and other autoimmune diseases. These FLEX-NK™ bispecific antibodies are built on a quadrivalent multifunctional antibody platform designed to engage natural killer cells (“NK Cells”) by targeting NKp46 activating receptors using Cytovia’s proprietary FLEX-NK™ technology. We are expecting to initiate two phase I/IIa clinical trials for NY-303 and NY-338 in 2025 with final data readouts in 2026.
Key elements of our strategy include:
| ● | Advancing NY-303, our GPC3-targeting FLEX NK™ bispecific antibody candidate for HCC and other solid tumors, into phase II/IIa clinical trials and towards clinical proof of concept; |
| ● | Advancing NY-338, our CD38-targeting FLEX-NK™ bispecific antibody candidate for multiple myeloma into phase II/IIa clinical trials and towards clinical proof of concept; |
| ● | Evaluate differentiated profile of NY-338 for the treatment of autoimmune diseases include SLE & Lupus Nephritis and advance it into clinical development upon preclinical proof of concept; |
| 120 |
| ● | Acquire for further development a clinical asset with phase II/IIa data from a large pharma, biotech or international company; and |
| ● | Entering into revenue-generating development partnerships with larger pharmaceutical/biotech companies upon achieving clinical milestones. |
Additionally, LEGACY NAYA intends to acquire additional clinical stage assets to further strengthen its therapeutic portfolio. Each asset/strategic franchise, will be organized in distinct subsidiaries (spokes). As of the date of the acquisition, LEGACY NAYA had not completed the development of any of its product candidates, had not generated any revenue from product sales and had never generated an operating profit.
LEGACY NAYA funded its operations primarily with outside debt and capital (e.g., proceeds from loans as well as the sale of stock and simple agreements for future equity, or SAFEs), however, LEGACY NAYA has incurred significant losses since the commencement of its operations.
Components of Legacy NAYA Results of Operations
On October 18, 2023, LEGACY NAYA acquired two assets from Cytovia. Both companies operate under common control and are accounted for as such. The acquisition did not include any other intellectual properties from Cytovia. The results reflect that LEGACY NAYA was a stand-alone entity starting in July 2023, separate from the original common control entity for which the carve out accounting was required.
Indirect research & development costs were allocated based on percentage of research and development full-time equivalent approach. The full-time employees, who worked on the projects have been assigned on a weekly basis to work a defined percentage of their time per project. The assigned time per week per project then was summarized on a monthly basis. The monthly assigned time per employee per project finally defined the average allocation per project CYT303 and CYT338 for salaries and indirect research & development expenses. The allocated expenses related to CYT303 and CYT338 represent 0% and 36% of Cytovia’s expenses for the six months ended June 30, 2024 and 2023, respectively, after which LEGACY NAYA was a stand-alone entity.
Selling, general and administrative expenses were allocated based on percentage of full-time equivalent approach. Allocated percentage is 0% and 10% for the six months ended June 30, 2024 and 2023, respectively, after which LEGACY NAYA was a stand-alone entity. The cost for the rent for the research & development lab has been allocated with the same principle as described above to the two projects CYT303 and CYT338.
Licensing Revenue
As of the date of the Merger, LEGACY NAYA had not generated any revenue from product sales or licenses.
| 121 |
Operating Expenses
Research and Development Expenses
Research and development expenses consist primarily of costs incurred in connection with the research and development of LEGACY NAYA’S product candidates.
Legacy NAYA records research and development expenses when these costs are incurred. Such expenses include:
| ● | employee-related costs, including salaries, related benefits and stock-based compensation expense for employees engaged in research and development functions; | |
| ● | expenses incurred in connection with the clinical development of its product candidates, including under agreements with third parties, such as consultants and contract research organizations, or CROs; | |
| ● | the cost of manufacturing product candidates for use in its clinical trials and preclinical studies, including under agreements with third parties, such as consultants and contract manufacturing organizations, or CMOs; | |
| ● | expenses incurred in connection with the preclinical development of its product candidates, including outsourced professional scientific development services, consulting research fees and payments made under sponsored research arrangements with third parties; | |
| ● | facilities and other expenses, which include direct or allocated expenses for rent and maintenance of facilities; | |
| ● | costs related to compliance with regulatory requirements; and | |
| ● | payments made under third-party licensing agreements. |
General and Administrative Expenses
General and administrative expenses consist primarily of salaries, related benefits and stock-based compensation expenses for personnel in executive, finance and administrative functions. General and administrative expenses also include facilities, insurance and other expenses not otherwise included in research and development expenses, as well as professional fees for legal, patent, consulting, investor and public relations, accounting and audit services.
Other Income (Expense), Net
Other expense includes interest on debt and losses on debt extinguishment.
| 122 |
Results of Operations
Comparison of the Nine Months Ended September 30, 2024 and 2023
STATEMENTS OF OPERATIONS
(UNAUDITED)
| For the Nine Months Ended September 30, 2024 | For the Nine Months Ended September 30, 2023 | |||||||
| Revenue: | ||||||||
| Total revenue | - | - | ||||||
| Operating expenses | ||||||||
| Selling, general and administrative expenses | $ | 2,976,074 | $ | 407,790 | ||||
| Research and development expenses | 176,104 | 939,519 | ||||||
| Total operating expenses | 3,152,078 | 1,347,309 | ||||||
| Loss from operations | (3,152,178 | ) | (1,347,309 | ) | ||||
| Other income (expense) | ||||||||
| Other income | 600,000 | - | ||||||
| Loss on debt extinguishment and penalty | (3,750,000 | ) | - | |||||
| Change in fair value of investment | (865,791 | ) | - | |||||
| Interest income (expense) – related party | (211,270 | ) | 1,035 | |||||
| Interest expense – other | (4,915,053 | ) | - | |||||
| Other income (expense), net | (9,142,114 | ) | 1,035 | |||||
| Net loss before income taxes | (12,294,292 | ) | (1,346,274 | ) | ||||
| Income taxes | - | - | ||||||
| Net loss | $ | (12,294,292 | ) | $ | (1,346,274 | ) | ||
Research and Development Expenses
Research and development expenses decreased in 2024 mainly due to a reduction in R&D payroll and contract research expense as a result of a decrease in available funding. It is expected that R&D expenditures will increase once additional funding is obtained and clinical trials proceed.
Selling, General and Administrative Expenses
General and administrative expenses increased in 2024 mainly due to higher accrued compensation, business development, legal fees and consulting fees and rent.
| 123 |
Other Expense, Net
Other expense increased in 2024 due to interest on new loans in 2024 as well as penalties and losses on debt extinguishment.
Comparison of the Years Ended December 31, 2023 and 2022
STATEMENTS OF OPERATIONS
| Year Ended December 31, 2023 | Year Ended December 31, 2022 | |||||||
| Revenue: | ||||||||
| Total revenue | - | - | ||||||
| Operating expenses | ||||||||
| Selling, general and administrative expenses | $ | 2,159,626 | $ | 1,249,313 | ||||
| Research and development expenses | 975,148 | $ | 8,250,835 | |||||
| Total operating expenses | 3,134,774 | $ | 9,500,148 | |||||
| Loss from operations | (3,134,774 | ) | $ | (9,500,148 | ) | |||
| Other income (expense) | ||||||||
| Interest expense - related party | (144,000 | ) | - | |||||
| Total other income (expense) | (144,000 | ) | - | |||||
| Net loss before income taxes | (3,278,774 | ) | $ | (9,500,148 | ) | |||
| Income taxes | - | - | ||||||
| Net loss | $ | (3,278,774 | ) | $ | (9,500,148 | ) | ||
Research and Development Expenses
Research and development expenses decreased in 2023 mainly due to a reduction in R&D payroll and contract research expense as a result of a decrease in available funding. It is expected that R&D expenditures will increase once additional funding is obtained and clinical trials proceed.
Selling, General and Administrative Expenses
General and administrative expenses increased in 2023 mainly due to higher accrued compensation, business development, legal fees and consulting fees and rent.
Other Expense, Net
Other expense increased in 2023 due to interest on a related party loan.
| 124 |
Liquidity and Capital Resources
Sources of Liquidity
Since its inception, Legacy NAYA has not generated any revenue from product sales and has incurred significant operating losses and negative cash flows from its operations. Legacy NAYA has funded its operations primarily through related party and other debt, as well as SAFE notes and preferred stock. As of September 30, 2023, Legacy NAYA had cash and cash equivalents in the amount of $756,461.
Cash Flows for the Years Ended December 31, 2023 and 2022 and Nine Months Ended September 30, 2024 and 2023
The following table summarizes Legacy NAYA’s sources and uses of cash for each of the periods presented (in thousands):
| Nine Months Ended | Year Ended | |||||||||||||||
| September 30, | December 31, | |||||||||||||||
| 2024 | 2023 | 2023 | 2022 | |||||||||||||
| Net cash flows used in operating activities | $ | (910 | ) | $ | (187 | ) | $ | (270 | ) | $ | - | |||||
| Net cash flows used in investing activities | (1,643 | ) | - | - | - | |||||||||||
| Net cash flows provided by financing activities | 3,221 | 195 | 360 | - | ||||||||||||
| Net (decrease) increase in cash and cash equivalents | $ | 667 | $ | 8 | $ | 89 | $ | - | ||||||||
Operating Activities
For the nine months ended September 30, 2024, net cash used in operating activities consisted of a net loss of $12.3 million, offset by non-cash charges of $4.4 million for amortization of debt discount and $3.8 million loss and penalty on debt extinguishment and a charge of $0.9 million for decrease in fair value of an investment, less $0.6 million other income.
For the nine months ended September 30, 2023, there was a net loss of $1.3 million offset by an increase of $1.3 million in accounts payable and accrued liabilities.
For the year ended December 31, 2023, net cash used in operating activities consisted of a net loss of $3.3 million, offset by an increase of $2.9 million in accounts payable and accrued liabilities.
For the year ended December 31, 2022, net cash used in operating activities consisted of a net loss of $9.5 million, offset by an increase of $9.5 million in accounts payable and accrued liabilities.
Investing Activities
For the nine months ended September 30, 2024, investing activities consisted of an investment in shares of legacy INVO for $1.6 million.
| 125 |
For the nine months ended September 30, 2023, there were no investing activities.
For the years ended December 31, 2023 and 2022, there were no investing activities.
Financing Activities
For the nine months ended September 30, 2024, net cash provided by financing activities consisted of loans for $4.1 million, preferred shares for $0.8 million less loan repayments of $1.7 million.
For the nine months ended September 30, 2023, net cash provided by financing activities consisted of $0.2 million of net proceeds from the issuance of SAFEs.
For the year ended December 31, 2023, net cash provided by financing activities consisted of $0.2 million of net proceeds from the issuance of SAFEs and $0.1 million from related party loans.
For the year ended December 31, 2022, there were no financing activities.
Contractual Obligations and Commitments
Lease Obligations
Legacy NAYA leases space for its office under a short term lease. There are no other lease obligations.
Research and Development and Manufacturing Agreements
Legacy NAYA has entered into contracts in the normal course of business with CROs, CMOs and other third parties for preclinical research studies and testing, clinical trials and manufacturing services. Payments due upon postponement or cancellation consist of payments for services provided and expenses incurred up to the date of cancellation and penalties for postponement or cancellation for select vendors. These potential penalties were also not considered to be contractual obligations or commitments.
License and Collaboration Agreements
Legacy NAYA is required to make certain payments under its license agreements, related to patent expenses, license fees, and assignment fees, as well as milestone and royalty payments upon the achievement of certain development and sales-based events. Legacy NAYA’s licenses agreements are described in more detail in the notes to its audited consolidated financial statements and unaudited condensed consolidated financial statements attached as Annex A and Annex B to this proxy statement, respectively.
Off-Balance Sheet Arrangements
As of September 30, 2024, Legacy NAYA did not have, and did not have during the periods presented, any off-balance sheet arrangements, as defined in the rules and regulations of the SEC.
| 126 |
Critical Accounting Policies and Significant Judgments and Estimates
Legacy NAYA’s consolidated financial statements are prepared in accordance with generally accepted accounting principles in the U.S. The preparation of Legacy NAYA’s consolidated financial statements and related disclosures requires it to make estimates and judgments that affect the reported amounts of assets, liabilities, costs and expenses, and the disclosure of contingent assets and liabilities in its consolidated financial statements. Legacy NAYA based its estimates on historical experience, known trends and events and various other factors that it believes are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Legacy NAYA evaluates its estimates and assumptions on a periodic basis. Actual results may differ from these estimates.
While Legacy NAYA’s significant accounting policies are described in more detail in Note 2 of the notes to its consolidated financial statements attached as Annex A to this proxy statement, Legacy NAYA believes that the following accounting policies are those most critical to the judgments and estimates used in the preparation of its consolidated financial statements.
Research and Development Expenses
As part of the process of preparing Legacy NAYA’s consolidated financial statements, Legacy NAYA is required to estimate its accrued research and development expenses. Legacy NAYA makes estimates of its accrued or prepaid expenses as of each balance sheet date in the consolidated financial statements based on facts and circumstances known to it at that time. Although Legacy NAYA does not expect its estimates to be materially different from amounts actually incurred, its understanding of the status and timing of services performed relative to the actual status and timing of services performed may vary and may result in reporting amounts that are too high or too low in any particular period.
Stock-Based Compensation
Legacy NAYA accounts for stock-based compensation awards in accordance with ASC Topic 718, Compensation—Stock Compensation, or ASC 718. ASC 718 requires all stock-based payments, including grants of stock options and restricted stock, to be recognized in the statements of operations and comprehensive loss based on their fair values. Legacy NAYA measures stock-based awards based on their fair value on the date of grant and recognize compensation expense for those awards over the requisite service period, which is generally the vesting period of the respective award. Generally, Legacy NAYA issues stock options and restricted stock awards with only service-based vesting conditions and record the expense for these awards using the straight-line method.
Legacy NAYA estimates the fair value of each stock option grant at the date of grant using the Black-Scholes option-pricing model and the fair value of each restricted common stock award is estimated on the date of grant based on the fair value of Legacy NAYA’s common stock on that same date. The Black-Scholes option-pricing model requires inputs based on certain subjective assumptions. Changes to these assumptions can materially affect the fair value of stock options and ultimately the amount of stock-based compensation expense recognized in Legacy NAYA’s consolidated financial statements.
Legacy NAYA accounts for stock option and RSU forfeitures during the period in which they occur.
| 127 |
Determination of the Fair Value of Common Stock
As there has been no public market for Legacy NAYA’s common stock as of September 30, 2024, the estimated fair value of its common stock has been determined by its board of directors as of the date of each option or RSU grant, with input from management, considering its most recently available third-party valuation of its common stock as well as its board of directors’ assessment of additional objective and subjective factors that it believed were relevant and which may have changed from the date of the most recent third-party valuation to the date of the grant.
The assumptions underlying these valuations represent Legacy NAYA’s board of directors’ and management’s best estimates, which involve inherent uncertainties and the application of significant judgment. As a result, if factors or expected outcomes change and it uses significantly different assumptions or estimates, its stock-based compensation expense could be materially different.
Investment in Legacy INVO shares
Investments include an investment in the Series A preferred shares of NAYA Biosciences, Inc. (former name INVO Bioscience, Inc. (“LEGACY INVO”) pursuant to a securities purchase agreement. The preferred shares will be marketable upon conversion into publicly traded common shares and therefore are being accounted for as marketable securities at the closing market price on the balance sheet date. As of September 30, 2024, LEGACY NAYA has purchased a total of 328,780 Series A preferred shares of LEGACY INVO at a cost of $1,643,900. The market value of those shares at September 30, 2024 was $778,113 and a reduction in fair value of $865,791 was recorded for the nine months ended September 30, 2024.
Recently Issued Accounting Pronouncements
A description of recently issued accounting pronouncements that may potentially impact Legacy NAYA’s financial position and results of operations is disclosed in the notes to its consolidated financial statements attached as Annex A to this proxy statement.
| 128 |
MATTERS TO BE VOTED ON
Proposal 1: To Approve the Issuance, in accordance with Nasdaq Listing Rule 5635(a), of (1) Our Common Stock, Upon Conversion of Our Outstanding Series C-1 and C-2 Preferred Stock, (2) Our Common Stock, Upon Conversion of the Debenture, and (3) our Common Stock, upon settlement of restricted stock units and exercise of stock options issued in exchange for restricted stock units and stock options that were previously granted to certain directors, employees and consultants of our subsidiary NAYA Therapeutics, Inc.
Overview
As described above, we issued 38,951 shares of Series C-1 and C-2 Preferred Stock in the Merger. In addition, we issued a 7.0% Senior Secured Convertible Debenture in the principal balance of $3,934,146 due December 11, 2025 (the “Debenture”) pursuant to the Merger. We also exchanged restricted stock units and stock options that were previously granted to certain directors, employees and consultants of our subsidiary NAYA Therapeutics, Inc for 1,705,485 of our restricted stock units and 365,580 of our stock options. The shares of common stock underlying these securities cannot be issued unless the stockholders approve the issuance of these shares.
Series C-1 and C-2 Preferred Stock
The Series C-1 and C-2 Preferred Stock is intended to have rights that are generally equivalent to common stock, provided that the Series C-1 and C-2 Preferred Stock do not have the right to vote on most matters (including the election of directors). On January 13, 2025, 4,000 shares of Series C-2 Preferred Stock were redeemed. 36,153,947 shares of common stock (or 53.4% of our outstanding common stock on an as-converted basis) are issuable upon conversion of the above-described Series C-1 and C-2 Preferred Stock, assuming the approval of this Proposal 1 and without taking into account certain beneficial ownership limitations.
Subject to stockholder approval and certain beneficial ownership limitations, each share of Series C-1 and C-2 Preferred Stock is convertible into shares of common stock. This Proposal 1 would provide the necessary approval to permit such conversion.
Shares Issuable Upon Conversion
Set forth below is a table summarizing the issued and outstanding Series C-1 and C-2 Preferred Stock, as well as the number of shares of common stock that are potentially issuable upon conversion of the Series C-1 and C-2 Preferred Stock, without taking into account certain beneficial ownership limitations. The sale into the public market of the underlying common stock could materially and adversely affect the market price of our common stock. See “Risk Factors— Risks Related to Our Common Stock.”
| Preferred Stock | ||||||||
| Issued and | Common Stock | |||||||
| Outstanding | (as converted) | |||||||
| Series C-2 to FNL and other lender | 4,576 | 6,638,632 | ||||||
| Series C-1 | 30,375 | 29,515,315 | ||||||
| Total | 38,951 | 36,153,947 | ||||||
Description of Series C-1 and C-2 Preferred Stock
Series C-1 Preferred
On October 14, 2024, the Company filed with the Nevada Secretary of State a Certificate of Designation of Series C-1 Convertible Preferred Stock (the “Series C-1 Certificate of Designation”) which sets forth the rights, preferences, and privileges of the Series C-1 Preferred Stock. Thirty thousand three hundred seventy five (30,375) shares of Series C-1 Preferred Stock with a stated value of $1,000.00 per share were authorized under the Series C-1 Certificate of Designation.
Each share of Series C-1 Preferred Stock has a stated value of $1,000.00, which is convertible into shares of the Company’s common stock at a conversion price equal to $1.02913 per share, subject to adjustment. The Series C-1 Preferred Stock may not be converted into shares of the Company’s common stock unless and until the Company’s stockholders approve the issuance of common stock upon conversion of the Series C-1 Preferred Stock. Each share of Series C-1 Preferred Stock shall automatically convert into the Company’s common stock if the Company’s stockholders approve the issuance, except that the Company may not effect such conversion if, after giving effect to the conversion or issuance, the holder, together with its affiliates, would beneficially own in excess of 19.99% of the Company’s outstanding common stock.
| 129 |
Commencing on the ninety-first (91st) day after the first issuance of any Series C-1 Preferred Stock, the holders of Series C-1 Preferred Stock shall be entitled to receive dividends on the stated value at the rate of two percent (2%) per annum, payable in shares of the Company’s common stock at the conversion price. Such dividends shall continue to accrue until paid. Such dividends will not be paid in shares of the Company’s common stock unless and until the Company’s stockholders approve the issuance of common stock upon conversion of the Series C-1 Convertible Preferred Stock. The holders of Series C-1 Preferred Stock shall also be entitled to receive a pro-rata portion, on an as-if convertible basis, of any dividends payable on common stock.
The Series C-1 Preferred ranks senior to the Company’s common stock and junior to the Series C-2 Preferred Stock (described below). Subject to the rights of the holders of any senior securities, in the event of any voluntary or involuntary liquidation, dissolution, or winding up, or sale of the Company, each holder of Series C-1 Preferred Stock shall be entitled to receive its pro rata portion of an aggregate payment equal to the amount as would be paid on the Company’s common stock issuable upon conversion of the Series C-1 Preferred Stock, determined on an as-converted basis, without regard to any beneficial ownership limitation.
Other than those rights provided by law, the Series C-1 Preferred Stock has no voting rights. The Series C-1 Preferred Stock is not redeemable.
Series C-2 Preferred Stock
On October 14, 2024, the Company filed with the Nevada Secretary of State a Certificate of Designation of Series C-2 Convertible Preferred Stock (the “Series C-2 Certificate of Designation”) which sets forth the rights, preferences, and privileges of the Series C-2 Preferred Stock. Eight thousand five hundred seventy six (8,576) shares of Series C-2 Preferred Stock with a stated value of $1,000.00 per share were authorized under the Series C-2 Certificate of Designation.
Each share of Series C-2 Preferred Stock has a stated value of $1,000.00, which, along with any additional amounts accrued thereon pursuant to the terms of the Series C-2 Certificate of Designation (collectively, the “Conversion Amount”) is convertible into shares of the Company’s common stock at a conversion price equal to $0.6893 per share, subject to adjustment. The Series C-2 Preferred Stock may not be converted into shares of the Company’s common stock unless and until the Company’s stockholders approve the issuance of common stock upon conversion of the Series C-2 Convertible Preferred Stock. Each share of Series C-2 Preferred Stock shall become convertible into the Company’s common stock at the option of the holder of such Series C-2 Preferred Stock shares if the Company’s stockholders approve the issuance of common stock upon conversion of the Series C-2 Preferred Stock, except that the Company may not effect such conversion if, after giving effect to the conversion or issuance, the holder, together with its affiliates, would beneficially own in excess of 9.99% of the Company’s outstanding common stock.
Commencing on the ninety-first (91st) day after the first issuance of any Series C-2 Preferred Stock, the holders of Series C-2 Preferred Stock shall be entitled to receive dividends on the stated value at the rate of ten percent (10%) per annum, payable in shares of the Company’s common stock, with each payment of a dividend payable in shares of the Company’s common stock at a conversion price of eighty-five percent (85%) of the average of the volume weighted average price of the Company’s common stock for the five (5) trading days before the applicable dividend date. Such dividends shall continue to accrue until paid. Such dividends will not be paid in shares of the Company’s common stock unless and until the Company’s stockholders approve the issuance of common stock upon conversion of the Series C-2 Preferred Stock. The holders of Series C-2 Preferred Stock shall also be entitled to receive a pro-rata portion, on an as-if convertible basis, of any dividends payable on common stock.
The Series C-2 Preferred Stock ranks senior to the Company’s common stock and to the Series C-1 Preferred. Stock Subject to the rights of the holders of any senior securities, in the event of any voluntary or involuntary liquidation, dissolution, or winding up, or sale of the Company, each holder of Series C-2 Preferred Stock shall be entitled to receive its pro rata portion of an aggregate payment equal to the greater of (a) 125% of the Conversion Amount with respect to such shares, and (b) the amount as would be paid on the Company’s common stock issuable upon conversion of the Series C-2 Preferred Stock, determined on an as-converted basis, without regard to any beneficial ownership limitation.
Other than those rights provided by law, the Series C-2 Preferred Stock has no voting rights. The Series C-2 Preferred Stock is only redeemable upon a “Bankruptcy Triggering Event” or a “Change of Control” that occurs 210 days after the closing date of the Merger.
Debenture
Subject to stockholder approval and certain beneficial ownership limitations, the Debenture is convertible into shares of common stock at the conversion price of $0.70 per share, subject to adjustment. This Proposal 1 would provide the necessary approval to permit such conversion.
| 130 |
Shares Issuable Upon Conversion
Set forth below is a table summarizing the number of shares of common stock that are potentially issuable upon conversion of the principal amount of the Debenture, without taking into account certain beneficial ownership limitations. The sale into the public market of the underlying common stock could materially and adversely affect the market price of our common stock. See “Risk Factors— Risks Related to Our Common Stock.”
| Common Stock | ||||||||
| Debenture | (as converted) | |||||||
| Debenture (Principal Only) | $ | 3,934,146 | 5,620,209 | |||||
Description of 7% Senior Secured Convertible Debenture
The Debenture carries an interest rate of seven percent (7%) per annum, payable on the first business day of each calendar month commencing November 1, 2024. The maturity date of the Debenture is December 11, 2025 (the “Maturity Date”), at which point the outstanding principal amount, together with any accrued and unpaid interest and other fees, shall be due and payable to the holder of the Debenture.
Conversion. At any time after the Company’s stockholders approve the issuance of any Company common stock upon conversion of the Debenture, the holder of the Debenture will be entitled to convert any portion of the outstanding and unpaid principal amount and accrued interest into shares of Company common stock at a conversion price of $0.93055 per share, subject to adjustment as described therein. The Debenture may not be converted and shares of Company common stock may not be issued upon conversion of the Debenture if, after giving effect to the conversion or issuance, the holder together with its affiliates would beneficially own in excess of 4.99% of the outstanding common stock of the Company.
Prepayment. The Company may not prepay the Debenture without the prior written consent of FNL.
Monthly Redemption. Commencing March 14, 2025 and on the 14th of each month thereafter until the Maturity Date, the Company shall redeem $437,127.24, plus accrued but unpaid interest and other fees, of the principal amount of the Debenture.
Mandatory Redemption. While any portion of the Debenture is outstanding, if the Company receives gross proceeds of more than $3,000,000 from any equity or debt financings (other than a public offering as described herein), the Company shall, at the option of the holder, apply one-third (1/3) of such gross proceeds to the redemption of the principal amount of the Debenture, except that if such equity or debt financing is a public offering of the Company’s securities pursuant to a registration statement on Form S-1, the Company shall, at the option of the holder, apply one hundred percent (100%) of such gross proceeds, not to exceed $500,000, to the redemption of the principal amount of the Debenture. The Company has also agreed that, if it received gross proceeds of more than $2,000,000 from any equity or debt financing, it shall, at the option of the holder, apply $500,000 of such gross proceeds to the redemption of the principal amount of the Debenture.
The Debenture contains events representations, warranties, covenants, and events of default that are customary for similar transactions. Upon an event of default, the Debenture becomes immediately due and payable, and the Borrower is subject to a default rate of interest of 15% per annum and a default sum as stipulated.
The foregoing description of the Debenture does not purport to be complete and is qualified in its entirety by reference to the Debenture.
Reasons for Stockholder Approval
Our common stock is listed on the Nasdaq Capital Market, and, as such, we are subject to the applicable rules of the Nasdaq Stock Market LLC, including Nasdaq Listing Rule 5635(a), which requires stockholder approval in connection with the acquisition of another company if the Nasdaq-listed company will issue 20% or more of its common stock. Thus, in order to permit (1) the issuance of common stock upon conversion of the Series C-1 and C-2 Preferred Stock, (2) the issuance of common stock upon conversion of the Debenture, and (3) the issuance of common stock upon settlement of restricted stock units and exercise of stock options that were exchanged for restricted stock units and stock options previously granted to certain directors, employees, and consultants of our subsidiary NAYA Therapeutics, Inc., we must first obtain stockholder approval of these issuances.
Beneficial Ownership Limitations
We are not seeking stockholder approval of a potential “change in control” under Nasdaq Listing Rule 5635(b), which generally prohibits Nasdaq-listed companies from issuing common stock to a stockholder in a transaction that would cause the holder to beneficially own 20% or more of the then-outstanding common stock (subject to certain exceptions). Assuming that Proposal 1 is approved, the Series C-1 and C-2 Preferred Stock will continue to have a beneficial ownership conversion limit that would prevent a stockholder from converting such stockholder’s shares if, as a result of such conversion, such stockholder would beneficially own a number of shares above such stockholder’s applicable conversion blocker (which cannot exceed 19.9% of our outstanding common stock).
| 131 |
Vote Required and Board of Directors Recommendation
Stockholder approval of this Proposal 1 requires a “FOR” vote from the stockholders representing a majority of the votes cast on the matter.
Of the shares of our common stock outstanding entitled to vote at the annual meeting, 787,656 shares of common stock were issued in the Legacy NAYA Acquisition (as defined below under “Proposal 1 – Legacy NAYA Acquisition Agreement”) and any votes in favor of Proposal 1 with respect to shares will not count as votes in favor of Proposal 1 pursuant to the listing rules of the Nasdaq Stock Market.
OUR BOARD OF DIRECTORS RECOMMENDS THAT STOCKHOLDERS VOTE
“FOR” THE APPROVAL OF THE SERIES C-1 And C-2 PREFERRED STOCK
CONVERSION PROPOSAL.
Proposal 2: Election of Directors
Our Board currently has seven members, whose terms of office expire at the Annual Meeting. The Board has nominated Dr. Daniel Teper, Dr. Laurent Audoly, Ms. Melissa Fenterstock, Dr. Prakash Raman and Ms. Alexandra Urman for election. Dr. Teper is currently a director of the Company. If elected at the Annual Meeting, each of these nominees will serve until the next annual meeting and until her or his successor has been duly elected and qualified, or, if sooner, until her or his death, resignation or removal. It is the Company’s policy to invite and encourage directors and director nominees to attend the Annual Meeting.
Directors are elected by a plurality of the votes of the shares present in person or represented by proxy and entitled to vote on the election of directors, and which did not abstain. Accordingly, for Proposal 2, the nominees receiving the highest number of votes cast for the number of positions to be filled are elected. Shares represented by executed proxies will be voted, if authority to do so is not withheld, for the election of each of the five nominees named below.
If any of the nominees become unavailable for election as a result of an unexpected occurrence, shares that would have been voted for such nominee will instead be voted for the election of a substitute nominee that the Board proposes. Each person nominated for election has agreed to serve if elected. We have no reason to believe that any of the nominees will be unable to serve.
NOMINEES FOR DIRECTORS
Our directors seek to assemble a Board that, as a whole, possesses the appropriate balance of professional and industry knowledge, financial expertise and high-level management experience necessary to oversee and direct the Company’s business. To that end, the Board has identified and evaluated nominees in the broader context of the Board’s overall composition, with the goal of recruiting members who complement and strengthen the skills of other members and who also exhibit integrity, collegiality, sound business judgment and other qualities that the Board views as critical to its effective functioning. The brief biographies below include information, as of the date of this proxy statement, regarding the specific and particular experience, qualifications, attributes or skills of each director or nominee that led the Board to believe that he or she should continue to serve on the Board. However, each of the members of the Board may have a variety of reasons why he or she believes a particular person would be an appropriate nominee for the Board, and these views may differ from the views of other members.
| 132 |
On the recommendation of the nominating and governance committee, our Board has nominated Dr. Daniel Teper, presently a director, and Dr. Prakash Raman, Dr. Laurent Audoly, Ms. Melissa Fensterstock and Ms. Alexandera Urman who are new director nominees for election. Our Board believes that the election or reelection of each director nominee identified above is advisable and in the best interests of the Company and our shareholders.
Daniel Teper PharmD, MBA. Dr. Teper, 64, is the President of NAYA Biosciences and Founder, Chairman, Chief Executive Officer, Chief Financial Officer, and Director of NAYA Therapeutics since August 2023. He has over 30 years of leadership experience as a biopharma entrepreneur, corporate executive, and management consultant. Previously, Dr. Teper was the Chairman & CEO of Cytovia Therapeutics, from June 2019 to July 2023, where he remains Chairman of the Board. Dr. Teper brings extensive experience. From September 2011 to April 2017, he was the CEO of Immune Pharmaceuticals, which he listed on NASDAQ. He previously served as New York-based Managing Partner (Head of North America) at Bionest Partners, now Accenture, where he advised companies on corporate strategy and business development. He was previously a Partner at ISO Healthcare Group, now Deloitte Monitor, in New York. Dr. Teper helped drive the accelerated growth of Softwatch, a pioneer digital health company, as senior vice president of sales and business development. He also served as global president of Havas Health, advising companies on global launches of major new drugs in multiple disease areas. Dr. Teper started his career at Novartis in Basel and then in the US, where he held management responsibilities in sales and marketing and as head of cardiovascular, new product development. Dr. Teper held general management positions in Europe at GlaxoSmithKline and Sanofi. He was the co-founder and CEO of Wintec Pharma, a European specialty pharmaceutical company focused on anti-infectives and dermatology, which he went on to sell. Dr. Teper co-founded Novagali, an ophthalmology specialty pharma later listed on EuroNext Paris and acquired by Japan’s Santen. He holds a Doctor of Pharmacy degree from Paris XI University and an MBA from INSEAD, where he was the J. Salmon scholar.
Prakash Raman, PhD, 54, serves as a director of Black Diamond Therapeutics, Inc. (NASDAQ:BDTX) since April 2024. Raman has over two decades of biopharmaceutical business development and executive leadership experience, blending his scientific background, program and portfolio management, and strong business development expertise to lead and support biopharma companies. Dr. Raman joined InduPro, Inc. as Chief Executive Officer and Board member in April 2024. Prior to this role, he served as President, Chief Executive Officer, and Board Member at Ribon Therapeutics, a biotech company that was focused on first-in-class small molecule drugs for oncology and immunology targeting the PARP family of enzymes, from February 2022 to March 2024. Ribon Therapeutics filed an Article of Dissolution in the State of Delaware in August 2024 following disappointing clinical results. Before joining Ribon, Dr. Raman served as Senior Partner, Chief Business Development Officer at Flagship Pioneering from October 2019 to February 2022, where he leveraged the platforms and assets in Flagship’s network to generate opportunities for significant value creation. Prior to Flagship, Dr. Raman spent nearly fourteen years at Novartis – from July 2005 to October 2019 – most recently as Vice President, Global Head of Novartis Institutes for Biomedical Research (NIBR) Business Development and Licensing (BD&L). During his tenure at Novartis, Dr. Raman was instrumental in forging key collaborations in immuno-oncology, executing numerous out-licensing opportunities, and guiding the acquisitions of Advanced Accelerator Applications, Endocyte, IFM Tre, and Selexys. Additionally, he led cross-functional drug discovery and early development project teams that successfully advanced compounds to clinical testing in patients. Prior to his time at Novartis, Dr. Raman spent six years as a Senior Scientist at Millennium Pharmaceuticals and two years as a post-doctoral fellow at The Scripps Research Institute. He completed his undergraduate degree at the Indian Institute of Technology, Bombay, and received his Ph.D. in Organic and Medicinal Chemistry from the University of Wisconsin-Madison.
Laurent Audoly, PhD, 52, is a company builder and biopharma executive with over 25 years of industry experience, including Head of R&D in pharma. Since 2023, he has served as the founder, CEO and CSO of PriveBio. Additionally, he has been a Professor at Northeastern University and a Strategic Advisor to the Institute for Experiential AI and Center for Drug Discovery. From 2019 to 2023, Dr. Audoly was the Founder, CEO, President, Chief Scientific Officer of Parthenon Therapeutics, a biotech company operating at the intersection of systems biology, machine learning, molecular pharmacology, and drug development to reprogram immune excluded tumors. From 2017 to 2019, he was the founding CEO, President, and Director of Kymera Therapeutics (NASDAQ: KYMR), where he led the construction of the business, raised over $135 million prior to the company’s IPO, and advanced pharma partnerships with Vertex and Sanofi. Dr. Audoly has served as a member of the Board of Ixaka (2021-2024) and Ermium Therapeutics (2020-2024). Dr. Audoly has led and/or contributed to the identification and development of seven novel medicines (Xeljanz®, Hemangiol®, Javlor®, Renflexis®, Brenzys®, Ontruzant®, Hadlima® - biologics and small molecules). Dr. Audoly served as head of R&D with Fabre Pharmaceuticals, a multinational pharma company, and also co-founder and co-lead for their pharma innovation investment fund. Earlier in his career, he held positions of increasing leadership responsibilities on the business and science fronts at Pfizer, Merck, Astra Zeneca, and Pieris, contributing to the advancement of numerous drug candidates into clinical development across multiple disease areas and modalities. Dr. Audoly is also an inventor, has served on NIH study sections, and has co-authored over 70 papers and patents. He studied chemistry (B.S. honors) at the University of South Florida and pharmacology (Ph.D.) at Vanderbilt University and was the recipient of an American Heart Association Fellowship at Duke University.
Melissa Fensterstock, 39, is an advisor with Material Impact Fund since 2023, where she focuses on frontier technologies, helping early-stage companies translate innovations from academic settings to commercial scale. In 2024, at the request of Material Impact, she served as the interim CEO and as a board member of Nohbo, Inc., a portfolio company of Material Impact that entered into an assignment for the benefit of creditors that is still pending. From 2017 to 2023, Ms. Fensterstock served as the CEO and board member of Lansdowne Labs, a spin-out from MIT and Harvard. From 2018 she has been the owner of Thayer Pond Advisors. Earlier in her career, Ms. Fensterstock worked across the life science sector, running corporate development for a publicly traded biotechnology company, leading consumer product initiatives, and spinning out therapeutic companies from Columbia University and Johns Hopkins University. In 2018 she entered into a consent order with the Federal Trade Commission relating to advertising of a consumer product that was discontinued prior to settlement. The claims at issue and sale of the product were discontinued prior to settlement. As an Expert-in-residence at Digitalis Commons, Ms. Fensterstock advises ARPA-H innovators working on early-stage health technologies. She has also held executive in residence positions at Columbia University, Yale University, and Climate Haven. In addition to her professional roles, Ms. Fensterstock serves as a Trustee of Connecticut Public since 2023 and is a former member of the Milken Young Leadership Circle. Ms. Fensterstock holds a Master in Business Administration from Harvard Business School, a Master of Philosophy in Bioscience Enterprise from the University of Cambridge, and a B.A. in Neuroscience from Johns Hopkins University.
Alexandra Urman, 38, is an Analyst and Portfolio Manager at the intersection of biotechnology and healthcare innovation. Ms. Urman serves as the Senior Vice President of the Florida-Israel Business Accelerator’s Venture Fund. From 2020 to 2024, Ms. Urman served as Genomics Analyst at ARK Investments on ARK’s Genomic Revolution strategy, where she specialized in evaluating cutting-edge technologies. Ms. Urman serves as a board member of Cure Rare Disease and provides strategic guidance as an industry advisor for the Sanford School of Medicine’s SPARK program, Sortium, Diagen, and CLARA. Before joining ARK, Ms. Urman built a strong foundation in clinical cancer research at prestigious institutions, including Memorial Sloan Kettering Cancer Center and Montefiore Medical Center. As a Senior Research Program Manager at IBM Watson Health, she championed the integration of AI in healthcare, earning consecutive Manager’s Choice Awards in 2017 and 2018, as well as the Rookie of the Year Award in 2017. In 2018, Ms. Urman founded her own consulting firm to bridge the gap between emerging technologies and healthcare applications. Ms. Urman has delivered keynote presentations at Forbes and APSG conferences and has been invited as a guest lecturer at New York University and the University of Maryland. Ms. Urman holds a B.A. from McGill University and a Master’s in Epidemiology and Community Health from New York Medical College.
| 133 |
THE BOARD OF DIRECTORS RECOMMENDS
A VOTE “FOR ALL” NOMINEES IN PROPOSAL NO. 2.
Proposal 3: Approval of an Amendment to our 2019 Stock Incentive Plan to Increase the Number of Shares of our Common Stock Available for Issuance Thereunder to 8,200,000 or approximately 15% of the total of NAYA’s total issued and outstanding stock, including shares issued upon conversion of Series C-1 and C-2 Non-Voting Convertible Preferred Stock
Why We Are Requesting Stockholder Approval of an Amendment to the Second Amended and Restated 2019 Stock Incentive Plan
Stockholders will be asked to approve an increase of the amount of authorized shares of NAYA issuable under NAYA’s Second Amended and Restated 2019 Stock Incentive Plan (the “Plan”) to the amount of NAYA’s common stock equal to 8,200,000 or approximately 15% of NAYA’s total issued and outstanding stock, including conversion of C-1 and C-2 Preferred Stock.
The Plan was originally adopted by our Board of Directors on November 14, 2019 and approved by our stockholders on December 16, 2019. The plan was amended and restated on October 12, 2022 by the Second Amended and Restated 2019 Stock Incentive Plan.
The purpose of the Plan is to further align the interests of employees, directors, and non-employee consultants with those of the stockholders by providing incentive compensation opportunities tied to the performance of the common stock and by promoting increased ownership of the common stock by such individuals.
Our Board of Directors has approved an increase the number of shares of common stock authorized for issuance under the Plan to the amount of NAYA’s common stock equal to 15% of NAYA’s total issued and outstanding stock, including conversion of C-1 and C-2 Preferred Stock.
A description of the material terms of the Plan are summarized below. This description is qualified in its entirety by the full text of the Plan, a copy of which is attached to this proxy statement as Annex E.
If stockholder approval of this Proposal 3 is obtained, and subject to adjustment for certain changes in our capitalization, the aggregate number of shares of our common stock that may be issued under the Plan will not exceed 15% of NAYA’s total issued and outstanding stock, including conversion of C-1 and C-2 Preferred Stock (subject to annual increases as set forth Section 4.2 of the Plan).
| 134 |
Vote Required
The stockholders are being asked to approve the increase of shares available for issuance under the Plan. The increase will be approved if more votes are cast in favor of this proposal than are cast against it. Abstentions and broker non-votes on this proposal will have no effect on the outcome.
Description of the Plan
A description of the material terms of Plan is set forth below. The statements made in this Proposal No. 3 concerning the terms and provisions of the Plan are summaries and do not purport to be a complete recitation of the Plan provisions. These statements are qualified in their entirety by express reference to the full text of the Plan, a copy of which is attached to this proxy statement as Annex E and is incorporated by reference herein.
General Purpose
The purpose of the plan is to further align the interests of employees, directors and non-employee consultants with those of the stockholders by providing incentive compensation opportunities tied to the performance of the common stock and by promoting increased ownership of the common stock by such individuals. The plan is also intended to advance the interests of the company and its shareholders by attracting, retaining and motivating key personnel upon whose judgment, initiative and effort the successful conduct of the company’s business is largely dependent. We are permitted to grant awards of stock options, stock awards, and restricted stock awards under the plan. Each type of award is discussed in greater detail below.
ERISA
The plan is not an “employee pension benefit plan” as defined in Section 3(2) of the U.S. Employee Retirement Income Security Act and is not qualified as a profit sharing plan as described in Section 401 of the Internal Revenue Code.
Shares Available
The initial maximum aggregate number of shares of common stock that may be issued and sold under all awards granted under the plan will be 15% of NAYA’s total issued and outstanding stock, including conversion of C-1 and C-2 Preferred Stock. Shares of common stock issued and sold under the plan may be either authorized but unissued shares or shares held in our treasury. The number of shares may be increased each year in the discretion of the plan administrator (described below) through 2029. The annual increase, if any, determined by the plan administrator for each year will be 6% of the total number of shares of outstanding Common Stock as of the end of the preceding year.
| 135 |
To the extent that any award involving the issuance of shares of common stock is forfeited, cancelled, returned to us for failure to satisfy vesting requirements or other conditions of the award, or otherwise terminates without an issuance of shares of common stock being made thereunder, the shares of common stock covered thereby will no longer be counted against the foregoing maximum share limitations and may again be made subject to awards under the plan pursuant to such limitations. Any awards or portions thereof which are settled in cash and not in shares of common stock shall not be counted against the foregoing maximum share limitations.
If there shall occur any change with respect to the outstanding shares of our common stock by reason of any recapitalization, reclassification, stock dividend, extraordinary dividend, stock split, reverse stock split or other distribution with respect to the shares of our common stock, or any merger, reorganization, consolidation, combination, spin-off or other similar corporate change, or any other change affecting our common stock, we may, in the manner and to the extent that it deems appropriate and equitable to the participants in the plan and consistent with the terms of the plan, cause an adjustment to be made in (i) the maximum number of shares available for issuance under the plan, (ii) the number and kind of shares of common stock, or other rights subject to then outstanding awards, (iii) the exercise or base price for each share or other right subject to then outstanding awards, and (iv) any other terms of an award that are affected by the event. However, in the case of incentive stock options, any such adjustments shall, to the extent practicable, be made in a manner consistent with the requirements of Section 424(a) of the Internal Revenue Code.
Administration
The plan shall be administered by a committee comprised of one or more members of our Board, or if no such committee exists, the entire Board.
The committee shall have such powers and authority as may be necessary or appropriate for the committee to carry out its functions as described in the plan. Subject to the express limitations of the plan, the committee shall have authority in its discretion to determine the eligible persons to whom, and the time or times at which, awards may be granted, the number of shares or other rights subject to each award, the exercise, base or purchase price of an award (if any), the time or times at which an award will become vested, exercisable or payable, the performance goals and other conditions of an award, the duration of the award, and all other terms of the award. Subject to the terms of the plan, the committee shall have the authority to amend the terms of an award in any manner that is not inconsistent with the plan, provided that no such action shall adversely affect the rights of a participant with respect to an outstanding award without the participant’s consent. The committee shall also have discretionary authority to interpret the plan, to make factual determinations under the plan, and to make all other determinations necessary or advisable for plan administration, including, without limitation, to correct any defect, to supply any omission or to reconcile any inconsistency in the plan or any award agreement hereunder.
The committee shall have the right, from time to time, to delegate to one or more of our officers the authority of the committee to grant and determine the terms and conditions of awards granted under the plan, subject to the requirements of state law and such other limitations as the committee shall determine. In no event shall any such delegation of authority be permitted with respect to awards to any members of the board or to any eligible person who is subject to Rule 16b-3 under the Securities Exchange Act of 1934, as amended, or Section 162(m) of the Internal Revenue Code.
| 136 |
Eligibility
Participation in the plan is limited to any person which is an employee of ours or any affiliate of ours, or any person to whom an offer of employment with us or one of our affiliates is extended, as determined by the committee, or any person who is a non-employee director, or any person who is consultant to us. The determination of eligibility shall be made by the committee in its sole discretion.
Grant of Stock Awards
A stock award may be granted to any eligible person selected by the committee. The number of shares and other terms of the stock award are specified in each award agreement. The stock award may be granted for past services, in lieu of bonus or other cash compensation, as directors’ compensation or for any other valid purpose as determined by the committee. A stock award granted to an eligible person represents shares of common stock that are issued without restrictions on transfer and other incidents of ownership and free of forfeiture conditions, except as otherwise provided in the plan and the award agreement. The deemed issuance price of shares of common stock subject to each stock award shall not be less than 85 percent of the fair market value of the common stock on the date of the grant. In the case of any person who owns securities possessing more than ten percent of the combined voting power of all classes of securities of the issuer or its parent or subsidiaries possessing voting power, the deemed issuance price of shares of common stock subject to each stock award shall be at least 100 percent of the fair market value of the common stock on the date of the grant. The committee may, in connection with any stock award, require the payment of a specified purchase price. Subject to the foregoing provisions and the applicable award agreement, upon the issuance of the common stock under a stock award, the participant shall have all rights of a stockholder with respect to the shares of common stock, including the right to vote the shares and receive all dividends and other distributions paid or made with respect thereto. The plan does not specify any maximum or minimum amount of shares which may be granted to any person under a stock award.
Grant of Restricted Stock Awards
A restricted stock award may be granted to any eligible person selected by the committee. The number of shares and other terms of the stock award are specified in each award agreement. The deemed issuance price of shares of common stock subject to each restricted stock award shall not be less than 85 percent of the fair market value of the common stock on the date of the grant. In the case of any person who owns securities possessing more than ten percent of the combined voting power of all classes of securities of the issuer or its parent or subsidiaries possessing voting power, the deemed issuance price of shares of common stock subject to each restricted stock award shall be at least 100 percent of the fair market value of the common stock on the date of the grant. The committee may require the payment by the participant of a specified purchase price in connection with any restricted stock award.
| 137 |
The restrictions imposed on shares granted under a restricted stock award shall lapse in accordance with the vesting requirements specified by the committee in the award agreement, provided that the committee may accelerate the vesting of a restricted stock award at any time. Such vesting requirements may be based on the continued service of the participant with the company or its affiliates for a specified time period (or periods) or on the attainment of specified performance goals established by the committee in its discretion. If the vesting requirements of a restricted stock award shall not be satisfied, the award shall be forfeited and the shares of common stock subject to the award shall be returned to the company.
Subject to the foregoing provisions and the applicable award agreement, the participant shall have all rights of a stockholder with respect to the shares granted to the participant under a restricted stock award, including the right to vote the shares and receive all dividends and other distributions paid or made with respect thereto. The committee may provide in an award agreement for the payment of dividends and distributions to the participant at such times as paid to stockholders generally or at the times of vesting or other payment of the restricted stock award.
Grant of Options
A stock option may be granted to any eligible person selected by the Committee. Each stock option shall be designated as an incentive stock option or as a nonqualified stock option. An incentive stock option may only be granted to an eligible person who is considered an employee for purposes of Treasury Regulation §1.421-7(h) with respect to us or any of our affiliates that qualifies as a “subsidiary corporation” with respect to us for purposes of Section 424(f) of the Internal Revenue Code.
The exercise price per share of a stock option shall not be less than 20 percent of the fair market value of the shares of common stock on the date of grant, except that the exercise price per shares of an incentive stock option shall not be less than 100 percent of the fair market value of the shares of common stock on the date of grant, and that the exercise price per shares of an incentive stock option shall not be less than 110 percent of the fair market value in the case of any person who owns securities possessing more than 10 percent of the total combined voting power of all classes of our securities.
The committee shall prescribe the time or times at which, or the conditions upon which, a stock option or portion thereof shall become vested and/or exercisable, and may accelerate the vesting or exercisability of any stock option at any time, provided, however, that any stock option shall vest at the rate of at least twenty percent per year over five years from the date the stock option is granted, subject to reasonable conditions as may be provided for in the award agreement. However, in the case of a stock option granted to officers, non-employee directors, managers or consultants, the stock option may become fully exercisable, subject to reasonable conditions, at any time or during any period established by us. The requirements for vesting and exercisability of a stock option may be based on the continued service of the participant with us or one of our affiliates for a specified time period (or periods) or on the attainment of specified performance goals established by the committee in its discretion.
| 138 |
The committee shall prescribe in an award agreement the period during which a vested stock option may be exercised, provided that the maximum term of a stock option shall be ten years from the date of grant. Except as otherwise provided in the plan or as otherwise may be provided by the committee, no stock option issued to an employee or a non-employee director may be exercised at any time during the term thereof unless the employee or a non-employee director is then in our service or the service of one of our affiliates.
Exercise of Options
Subject to such terms and conditions as shall be specified in an award agreement, a stock option may be exercised in whole or in part at any time during the term thereof by notice in the form required by us, together with payment of the aggregate exercise price therefor and applicable withholding tax. Payment of the exercise price shall be made in the manner set forth in the award agreement, which, unless otherwise provided by the committee, shall be as follows: (i) in cash or by cash equivalent acceptable to the committee, (ii) by payment in shares of our common stock that have been held by the participant for at least six months (or such period as the committee may deem appropriate) valued at the fair market value of such shares on the date of exercise, (iii) through an open-market, broker-assisted sales transaction pursuant to which we are promptly delivered the amount of proceeds necessary to satisfy the exercise price, (iv) by a combination of the methods described above, or (v) by such other method as may be approved by the committee and set forth in the award agreement. In addition to and at the time of payment of the exercise price, the participant shall pay to us the full amount of any and all applicable income tax, employment tax, and other amounts required to be withheld in connection with such exercise, payable under such of the methods described above for the payment of the exercise price as may be approved by the committee and set forth in the award agreement.
Nontransferability
Nonqualified Stock Options. Nonqualified stock options shall be nontransferable except (i) upon the participant’s death, or (ii) for the transfer of all or part of the stock option to a participant’s “family member” (as defined for purposes of the Form S-8 registration statement under the Securities Act of 1933), as may be approved by the committee in its discretion at the time of proposed transfer. The transfer of a nonqualified stock option may be subject to such terms and conditions as the committee may in its discretion impose from time to time. Subsequent transfers of a nonqualified stock option shall be prohibited other than in accordance with the terms set forth herein.
Incentive Stock Options. Incentive stock options shall be nontransferable other than by will or by the laws of descent and distribution, and shall be exercisable during the lifetime of a participant only by such participant.
| 139 |
Termination of Employment
The stock option of any participant whose service with us or one of our affiliates is terminated for any reason shall terminate on the earlier of (A) the date that the stock option expires in accordance with its terms or (B) unless otherwise provided in an award agreement, and except for termination for cause, the expiration of the applicable time period following termination of service, in accordance with the following: (1) twelve months if service ceased due to disability, (2) eighteen months if service ceased at a time when the participant is eligible to elect immediate commencement of retirement benefits at a specified retirement age under a pension plan to which we or any of our affiliates had made contributions, (3) eighteen months if the participant died while in the service of us or any of our affiliates, or (4) three months if service ceased for any other reason. During the foregoing applicable period, except as otherwise specified in the award agreement or in the event service was terminated by the death of the participant, the stock option may be exercised by such participant in respect of the same number of shares of common stock, in the same manner, and to the same extent as if he or she had remained in the continued service of us or any affiliate during the first three months of such period; provided that no additional rights shall vest after such three months. The committee shall have authority to determine in each case whether an authorized leave of absence shall be deemed a termination of service for purposes hereof, as well as the effect of a leave of absence on the vesting and exercisability of a stock option. Unless otherwise provided by the committee, if an entity ceases to be an affiliate of the company or otherwise ceases to be qualified under the plan or if all or substantially all of the assets of an affiliate of the company are conveyed (other than by encumbrance), such cessation or action, as the case may be, shall be deemed for purposes hereof to be a termination of the service.
An award of an incentive stock option may provide that such stock option may be exercised not later than 3 months following termination of employment of the participant with us and all subsidiaries, or not later than one year following a permanent and total disability within the meaning of Section 22(e)(3) of the Internal Revenue Code, as and to the extent determined by the committee to comply with the requirements of Section 422 of the Internal Revenue Code.
Amendment and Termination
The board may at any time and from time to time and in any respect, amend or modify the plan. The board may seek the approval of any amendment or modification by our stockholders to the extent it deems necessary or advisable in its discretion for purposes of compliance with Section 162(m) or Section 422 of the Internal Revenue Code, or exchange or securities market or for any other purpose. No amendment or modification of the plan shall adversely affect any award theretofore granted without the consent of the participant or the permitted transferee of the award. The plan shall terminate on the tenth anniversary of the date of its adoption by the board. The board may, in its discretion and at any earlier date, terminate the plan. Notwithstanding the foregoing, no termination of the plan shall adversely affect any award theretofore granted without the consent of the participant or the permitted transferee of the award.
RESTRICTIONS ON RESALE
Persons ordinarily may publicly resell the shares of common stock issued pursuant to an award granted under the plan without registration under the federal securities laws. However, our affiliates who acquire shares of our common stock pursuant to an award under the plan described herein must ensure that the resale of their shares complies with an available exemption from the registration provisions of the Federal securities law, such as Rule 144 under the Securities Act of 1933, as amended.
| 140 |
Shares granted under any Restricted Stock Award may not be transferred, assigned or subject to any encumbrance, pledge, or charge until all applicable restrictions are removed or have expired, unless otherwise allowed by the committee. Failure to satisfy any applicable restrictions shall result in the subject shares of the restricted stock award being forfeited and returned to us. The committee may require in an award agreement that certificates representing the shares granted under a restricted stock award bear a legend making appropriate reference to the restrictions imposed, and that certificates representing the shares granted or sold under a restricted stock award will remain in the physical custody of an escrow holder until all restrictions are removed or have expired.
We are subject to Section 16(b) of the Securities Exchange Act of 1934, as amended. Section 16(b) allows us to recover any profit realized by any of our officers, directors or 10% stockholders from any purchase and sale, or sale and purchase, of shares of our common stock within any period of less than six months.
FEDERAL INCOME TAX CONSEQUENCES
This section contains a discussion regarding the income tax consequences of the plan under federal income tax law. This discussion is intended only as a broad discussion of the general rules under income tax laws applicable to the issuance of common stock as compensation. Specific situations may be subject to different rules and may result in different tax consequences. You are strongly urged to consult your own personal tax advisor with specific reference to your own tax situation regarding all federal, state and local tax matters in conjunction with the plan and the grant, exercise and ultimate sale of any shares received upon the exercise of options granted pursuant to the plan.
Stock Awards
A recipient of a stock award under the plan will have compensation income upon the receipt of the shares in an amount equal to the fair market value of the shares on the date of the issuance.
Restricted Stock Awards
Unless a recipient files a Section 83(b) election with the Internal Revenue Service within 30 days following the date of grant of a restricted stock award, a recipient of a restricted stock award will not have any taxable income until the award vests. Upon the vesting of the award and receipt by recipient of the not-restricted shares, recipient will have compensation income in an amount equal to the fair market value of the shares on the date of vesting.
If a participant makes an election pursuant to Section 83(b) of the Internal Revenue Code with respect to a restricted stock award, the participant shall file, within 30 days following the date of grant, a copy of such election with us and with the Internal Revenue Service, in accordance with the regulations under Section 83 of the Internal Revenue Code. If a recipient files such a Section 83(b) election, the participant of the restricted stock award will have compensation income on the date of grant in an amount equal to the fair market value of the shares on the date of grant. The committee may provide in an award agreement that the restricted stock award is conditioned upon the participant’s making or refraining from making an election with respect to the award under Section 83(b) of the Internal Revenue Code.
| 141 |
Incentive Stock Options
Grant and Exercise of Incentive Stock Options. In general, a participant realizes no income upon the grant of plan incentive stock options assuming these options qualified as “incentive stock options” under the Internal Revenue Code when they were granted or upon the exercise of incentive stock options. But see, “Alternative Minimum Tax,” below. The amount paid by the participant for the shares of common stock received pursuant to the exercise of incentive stock options will generally constitute his or her basis or cost for tax purposes. The holding period for such common stock generally begins on the date the participant exercises incentive stock options. See below for a discussion of the exceptions to these general rules when the participant uses previously acquired stock of the company to exercise incentive stock options.
Alternative Minimum Tax. Although no current taxable income is realized upon the exercise of incentive stock options, Section 56(b)(3) of the Internal Revenue Code provides that the excess of the fair market value on the date of exercise of the common stock acquired pursuant to such exercise over the option price is an item of tax adjustment. As such, the exercise of incentive stock options may result in the participant being subject to the alternative minimum tax for the year incentive stock options are exercised. The alternative minimum tax is calculated on a taxpayer’s adjusted gross income, subject to special adjustments, plus specified items of tax preference minus specified itemized deductions. The resulting amount is the alternative minimum taxable income.
If the shares are disposed of in a “disqualifying disposition” that is, within one year of exercise or two years from the date of the option grant — in the year in which the incentive stock option is exercised, the maximum amount that will be included as alternative minimum tax income is the gain on the disposition of the incentive stock option stock. In the event there is a disqualifying disposition in a year other than the year of exercise, the income on the disqualifying disposition will not be considered income for alternative minimum tax purposes. In addition, the basis of the incentive stock option stock for determining gain or loss for alternative minimum tax purposes will be the exercise price for the incentive stock option stock increased by the amount that alternative minimum tax income was increased due to the earlier exercise of the incentive stock option. Alternative minimum tax incurred by reason of the exercise of the incentive stock option does not result, for regular income tax purposes, in an increase in basis of the shares acquired upon exercise. The alternative minimum tax attributable to the exercise of an incentive stock option may be applied as a credit against regular tax liability in a subsequent year, subject to certain limitations. The gain recognized upon a sale or exchange of shares acquired through the exercise of the incentive stock options will be limited to the excess of the amount received in the sale or exchange over the fair market value of the shares at the time the incentive stock option was exercised.
The application of the alternative minimum tax for each participant will depend on such participant’s total income and deductions for the year of exercise. As such, the extent to which, if any, the tax adjustment item generated by the exercise of incentive stock options in conjunction with any other tax adjustment items or alternative minimum tax adjustments may result in an alternative minimum tax liability for any participant cannot be determined. Accordingly, each participant should consult his or her own tax counsel to determine the potential impact of the alternative minimum tax on his or her exercise of incentive stock options.
| 142 |
Employment and Holding Requirements of Incentive Stock Options. The Internal Revenue Code requires that the participant remain an employee of ours or one of our subsidiaries at all times during the period beginning on the date that the incentive stock options are granted and ending on the day three months (or one year in the case of permanent and total disability or death) before the date that each incentive stock option is exercised.
In order for an participant exercising incentive stock options to qualify for the income tax free treatment set forth in the preceding section such participant must not dispose of the shares of common stock acquired pursuant to the exercise of incentive stock options within two years from the date the incentive stock options were granted, nor within one year after the exercise of the incentive stock options. If the participant meets these employment and holding requirements, any future gain or loss realized and recognized from the sale or exchange of the common stock should be long term capital gain or loss, if the stock is held as a capital asset. If the participant disposes of the shares of common stock acquired upon exercise of an incentive stock option within two years from the granting of options or one year after the exercise of options, any gain will constitute, in the year of disposition, ordinary compensation income to the extent of the excess of the fair market value of the common stock on its acquisition date over the price paid for it by the participant. Any additional gain will be treated as capital gain. If the participant disposes of the shares of common stock issued upon exercise of an incentive stock option at a loss, such loss will be a capital loss.
For purposes of this section, the transfer of shares of common stock previously acquired by a participant after the participant’s death does not constitute a “disposition.” In addition, the transferee of the shares of common stock is not subject to the holding and employment requirements.
If the recipient disposes of options instead of exercising them, the incentive stock option rules discussed herein have no application. The recipient transferor will recognize either long or short term capital gain or loss and the purchaser will not be subject to any of these rules.
Nonqualified Stock Options
In general, a participant who receives a nonqualified stock option realizes income either at the date of grant or at the date of exercise, but not at both. Unless the nonqualified stock option has a “readily ascertainable fair market value” at the date of grant, the participant recognizes no income on the date of grant and the compensatory aspects are held open until the nonqualified stock option is exercised. In this case, upon exercise, the participant will have compensation income to the extent of the difference between the fair market value of the stock at the time of exercise and the exercise price paid by the participant.
An nonqualified stock option is deemed to have a readily ascertainable fair market value if (a) the nonqualified stock options are actively traded on an established market or (b) the fair market value can be measured with reasonable accuracy, which means that (i) the nonqualified stock options are transferable, (ii) the nonqualified stock options are exercisable immediately in full, (iii) the nonqualified stock options and underlying stock are not subject to restrictions which have a significant effect on the nonqualified stock option’s value and (iv) the fair market value of the option privilege is readily ascertainable.
| 143 |
Exercise of Options Through Use of Previously Acquired Common Stock of the Company
Under the plan, in some circumstances a participant may be allowed to use previously acquired shares of common stock to exercise stock options. Such previously acquired shares of common stock may include common stock acquired pursuant to an earlier partial exercise of options. Generally the Internal Revenue Service recognizes that an exchange of common stock for other common stock does not constitute a taxable disposition of any shares of common stock. The IRS treats such exchanges as two transactions. First, to the extent of the number of previously acquired shares of common stock, a share for share exchange occurs with each new share of common stock succeeding to the cost basis and holding period of the old shares of common stock. Second, the remaining new shares of common stock are deemed acquired at a zero cost with their holding period commencing on the date of acquisition.
The foregoing rules generally apply to the use of previously acquired shares of common stock to acquire shares of common stock under the plan. An participant may use shares of common stock owned at the date options are exercised to acquire shares of common stock upon exercise of the options. However, despite a “carryover” holding period, all of the new shares of common stock are still subject to the holding requirements discussed above. If participant disposes of such common stock acquired pursuant to the exercises of incentive stock options before the later of two years from the granting or one year from exercise, an early disposition occurs first to the extent of the non-carryover shares and then to the extent of the carryover shares.
In addition, if a participant uses shares of common stock acquired through a previous partial exercise of options to acquire new shares of common stock through an exercise of options before the first stock has met the above holding requirements, the first stock will be treated as having been disposed of in an early disposition. Therefore, the participant will have to recognize ordinary compensation to the excess of the fair market value of the first stock on its acquisition dates over its price paid. Despite the early disposition, any excess gain is not recognized, but is deferred and carried over to the second stock. If the first stock is used to acquire other shares of common stock which are not subject to the plan, no early disposition will generally occur and the tax free exchange rules may apply.
Again, you should consult your own tax advisor with regard to the tax treatment applicable in your own tax situation.
Interests of Officers and Directors in this Proposal
Members of our board of directors and our executive officers are eligible to receive awards under the terms of the Plan, including through certain outstanding employment agreements and grants, and they therefore have a substantial interest in Proposal 33.
Required Vote
The affirmative vote of the holders of a majority of the shares of NAYA’s common stock present in person or represented by proxy at the Special Meeting is required to approve Proposal 3.
| 144 |
OUR BOARD OF DIRECTORS RECOMMENDS THAT STOCKHOLDERS VOTE “FOR” APPROVAL OF THE AMENDMENT TO OUR 2019 STOCK INCENTIVE PLAN
Proposal 4: Approval of an Amendment to our Amended and Restated Articles of Incorporation, as Amended, to effectuate a reverse stock split of NAYA’s common stock at a ratio ranging from any whole number between 1-for-2 and 1-for- 20, as determined by the Board of Directors in its discretion, subject to the Board of Directors’ authority to abandon such amendment and after the reverse split to Increase the Number of Authorized Shares of our Common Stock from 50,000,000 to 100,000,000.
Background
We are asking our stockholders to approve an amendment to our Amended and Restated Articles of Incorporation, as amended, to increase the number of authorized shares of our common stock. Our authorized capital stock presently consists of 50,000,000 shares of common stock, $0.00001 par value per share (“common stock”), 1,000,000 shares of which are designated as Series A Preferred Stock, par value $5.00 per share, 1,200,000 shares of which are designated as Series B Preferred Stock, 30,375 shares of which are designated as Series C-1 Convertible Preferred Stock, par value $0.0001 per share, and 8,576 shares of which are designated as Series C-2 Convertible Preferred Stock, par value $0.0001 per share and 97,761,049 of which are designated as preferred stock, par value $0.0001 per share.
As of January 24, 2025, 7,087,796 shares of our common stock were issued and outstanding. In addition, as of January 24, 2025, there were:
| ● | 18,263,442 shares of common stock issuable upon exercise of outstanding warrants and unit purchase options with a weighted average exercise price of $0.95 per share; |
| ● | 11,463,079 shares of common stock issuable upon exercise of prefunded warrants; | |
| ● | 97,992 shares of common stock issuable upon exercise of outstanding options with a weighted average exercise price of $35.20 per share. | |
| ● | 365,580 shares of common stock issuable upon exercise of outstanding options converted from Legacy NAYA options which may not be exercised until shareholder approval; | |
| ● | 1,705,485 shares of common stock issuable upon settlement of outstanding RSUs converted from Legacy NAYA RSUs which may not be settled until shareholder approval; | |
| ● | 5,816,101 shares of common stock issuable upon conversion of outstanding convertible notes with a weighted average conversion price of $0.70 per share; |
| 145 |
| ● | 29,515,222 shares of common stock issuable upon conversion of outstanding series C-1 preferred stock which may not be converted until shareholder approval; | |
| ● | 6,638,632 shares of common stock issuable upon conversion of outstanding series C-2 preferred stock which may not be converted until shareholder approval; | |
| ● | 432,883 shares of common stock reserved for future issuance under the 2019 Stock Incentive Plan. |
Except as otherwise indicated herein, all information in this proxy reflects or assumes that none of the securities above were converted or exercised.
Accordingly, as of January 24, 2025, out of the 50,000,000 shares of common stock presently authorized, 37,429,642 shares are issued and outstanding or reserved for issuance and 12,570,358 shares of common stock remain available for future issuance.
In addition, if Proposal 3 is approved, we will be required to reserve 8,200,000 additional shares of common stock for future issuance under the Second Amended and Restated 2019 Plan.
The approval of Proposal 4 is necessary for the conversion of the Series C-1 and C-2 Preferred Stock into our shares of common Stock and the exchange restricted stock units and stock options that were previously granted to certain directors, employees and consultants of our subsidiary NAYA Therapeutics, Inc. pursuant to Proposal 1 and the reservation of additional shares of common Stock pursuant to Proposal 3 as there is an insufficient number of common stock authorized to permit such conversion without the approval of Proposal 4. Proposal 4 has been considered and approved independently by our board of directors.
Overview of the Proposed Amendment
A copy of the amendment to our Amended and Restated Articles of Incorporation, as amended, is attached as Annex F to this proxy statement. The proposed amendment provides that the section (a) of the Article thereof numbered “IV” of our Amended and Restated Articles of incorporation, as amended, be deleted in its entirety, and replaced with the following:
“(a) At the Effective Time, every * shares of Common Stock, par value of $0.00001 each, issued and outstanding immediately prior to the Effective Time shall, automatically and without any action on the part of the holder(s) thereof, be reverse split into one (1) share of Common Stock (the “Reverse Stock Split”). No fractional shares of Common Stock shall be issued as a result of the Reverse Stock Split and no fractional interest in a share of Common Stock shall be deliverable upon the Reverse Stock Split. Stockholders who otherwise would be entitled to receive fractional shares of Common Stock because they hold certain number of shares not evenly divisible by the Reverse Stock Split ratio will automatically be entitled to receive an additional fraction of a share of Common Stock up to the next whole share. Each certificate that immediately prior to the Effective Time represented shares of Common Stock (“Old Certificates”), shall thereafter represent that number of shares of Common Stock into which the shares of Common Stock represented by the Old Certificate shall have been reverse split, plus any additional fraction of a share of Common Stock to round up to the next whole share. After completion of the Reverse Stock Split, the total number of shares of all classes of stock of which the Corporation shall have the authority to issue is 200,000,000 shares, consisting of (i)100,000,000 shares of Common Stock, $0.00001 par value per share (“Common Stock”), and (ii) 100,000,000 shares of Preferred Stock, $0.00001 par value per share (“Preferred Stock”).”
The proposed amendment, if approved by our stockholders, would become effective upon the filing of a certificate of amendment of our restated Articles of Incorporation, as amended, with the Secretary of State of the State of Nevada. Our board of directors reserves the right, notwithstanding stockholder approval and without further action by our stockholders, to elect not to proceed with the proposed amendment if the board of directors determines that the proposed amendment is no longer in our best interests and the best interests of our stockholders.
If our stockholders approve the proposed amendment, subject to the discretion of our board of directors, we intend to file the certificate of amendment of our restated Articles of Incorporation, as amended, with the Secretary of State of the State of Nevada as soon as practicable after the annual meeting.
| 146 |
Rationale for the Proposed Amendment
Over the past several years, we have used shares of our common stock to, among other things, engage in financings, incentivize and compensate employees and other service providers and for other general corporate purposes. Our board of directors believes that it is in the best interests of our company to increase the number of authorized shares of our common stock in order to give us greater flexibility in considering and planning for potential business needs. The increase in the number of authorized but unissued shares of common stock would enable us, without the expense and delay of seeking stockholder approval, to issue shares from time to time as may be required for proper business purposes.
We anticipate that we may issue additional shares of common stock in the future in connection with one or more of the following:
| ● | financing transactions, such as public or private offerings of common stock or convertible securities; | |
| ● | licenses, partnerships, collaborations and other similar transactions; | |
| ● | our equity incentive plans; | |
| ● | strategic investments and transactions; and | |
| ● | other corporate purposes that have not yet been identified. |
At this time, we do not have any plans, proposals or arrangements, written or oral, to issue any of the proposed additional authorized shares of our common stock for general corporate or any other purposes. However, our board of directors believes that the availability of additional authorized shares of our common stock will afford us needed flexibility in acting upon financing transactions to strengthen our financial position and/or engaging in strategic activities without using cash. Unless required by applicable law or stock exchange rules, no further vote of the holders of common stock will be required with respect to any such transaction.
Potential Effects of the Proposed Amendment
The additional shares of common stock for which authorization is sought would be identical in powers, privileges and rights to the shares of common stock that are now authorized. Holders of common stock do not have preemptive rights to subscribe to additional securities that we may issue.
The issuance of additional shares of common stock may, among other things, have a dilutive effect on earnings per share and on stockholders’ equity and voting rights. Further, future sales of substantial amounts of our common stock, or the perception that these sales might occur, could adversely affect the prevailing market price of our common stock or limit our ability to raise additional capital. Stockholders should recognize that, as a result of this proposal, they will own a smaller percentage of shares relative to the total authorized shares of the company than they presently own.
Our board of directors has not proposed the increase in amount of authorized shares with the intention of discouraging tender offers or takeover attempts. However, the availability of additional authorized shares for issuance may have the effect of discouraging a merger, tender offer, proxy contest or other attempt to obtain control.
| 147 |
Required Vote
The affirmative vote of the stockholders representing a majority of the outstanding shares of our common stock entitled to vote on the matter is required to approve Proposal 4.
OUR BOARD OF DIRECTORS RECOMMENDS THAT STOCKHOLDERS VOTE “FOR” APPROVAL OF THE AMENDMENT TO OUR RESTATED ARTICLES OF INCORPORATION, AS AMENDED, TO AUTHORIZE A REVERSE STOCK SPLIT AT A RATIO RANGING FROM ANY WHOLE NUMBER BETWEEN 1 FOR 2 AND 1 FOR 20 AND TO INCREASE THE NUMBER OF AUTHORIZED SHARES OF OUR COMMON STOCK FROM 50,000,000 TO 100,000,000 AFTER SUCH REVERSE SPLIT.
Proposal 5: Ratification of the Appointment of Independent Registered Public Accounting Firm
The audit committee of the Board has selected M&K as the Company’s independent registered public accounting firm for the fiscal year ending December 31, 2024 and has further directed that management submit the selection of its independent registered public accounting firm for ratification by the shareholders at the Annual Meeting. M&K has audited the Company’s financial statements since September 2019.
Neither the Company’s bylaws nor other governing documents or law require shareholder ratification of the selection of M&K as the Company’s independent registered public accounting firm. However, the Board is submitting the selection of M&K to the shareholders for ratification as a matter of good corporate practice. If the shareholders fail to ratify the selection, the audit committee will reconsider whether or not to retain that firm. Even if the selection is ratified, the audit committee in its discretion may direct the appointment of different independent auditors at any time during the year if they determine that such a change would be in the best interests of the Company and its shareholders.
Required Vote
The affirmative vote of a majority of the votes cast at the Annual Meeting is required to approve Proposal No. 5.
| 148 |
OUR BOARD OF DIRECTORS RECOMMENDS THAT STOCKHOLDERS VOTE “FOR” APPROVAL OF
PRINCIPAL ACCOUNTANT FEES AND SERVICES
The following table represents aggregate fees billed to the Company by M&K.
Fiscal Year Ended December 31, 2023 | Fiscal Year Ended December 31, 2022 | |||||||
| Audit Fees | $ | 71,350 | $ | 61,740 | ||||
| Audit Related Fees | $ | 44,350 | $ | 53,250 | ||||
| Tax Fees | $ | - | $ | - | ||||
| All Other Fees | $ | - | $ | - | ||||
Audit Fees includes fees billed for the fiscal year shown for professional services for the audit of the Company’s annual financial statements, the reviews of the consolidated quarterly financial statements included in each of our quarterly reports on Form 10-Q, and other audit services.
Audit-Related Fees include fees for assurance and related services performed to comply with generally accepted auditing standards and including audit of target acquisition companies and comfort and consent letters in connection with SEC filings and financing transactions.
PRE-APPROVAL POLICIES AND PROCEDURES
Our Board has adopted a procedure for pre-approval of all fees charged by our independent auditors. Under the procedure, the Board approves the engagement letter with respect to audit and review services. Other fees are subject to pre-approval by the Board, or, in the period between meetings, by a designated member of the Board. Any such approval by the designated member is disclosed to the entire Board at the next meeting. The audit fees paid to the auditors with respect to fiscal years 2023 and 2022 were pre-approved by the entire Board.
OUR BOARD OF DIRECTORS RECOMMENDS THAT STOCKHOLDERS VOTE “FOR” THE RATIFICATION OF THE APPOINTMENT OF M&K AS OUR INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM FOR THE FISCAL YEAR ENDED DECEMBER 31, 2024.
Proposal 6: APPROVAL OF THE ADJOURNMENT
The Company is asking stockholders to approve, if necessary, adjournment of the Annual Meeting to solicit additional proxies in favor of the Director Appointment, the Auditor Appointment, and/or the Nasdaq listing rule 5635(d) share issuance authorization. Any adjournment of the Annual Meeting for the purpose of soliciting additional proxies will allow stockholders who have already sent in their proxies to revoke them at any time prior to the time that the proxies are used.
Vote Required
The affirmative vote of a majority of the voting power present or represented by proxy is required to approve the Adjournment proposal. Abstentions represent the voting power present under the Company’s amended and restated bylaws, and accordingly will have the same effect as a vote “AGAINST” the outcome of this Proposal No. 6.
OUR BOARD OF DIRECTORS RECOMMENDS A VOTE FOR THE ADJOURNMENT
| 149 |
BOARD OF DIRECTORS AND CORPORATE GOVERNANCE
INDEPENDENCE OF THE BOARD OF DIRECTORS
The listing rules of Nasdaq require us to maintain a Board comprised of a majority of independent directors, as determined affirmatively by our Board. In addition, the Nasdaq listing rules require that, subject to specified exceptions, each member of our audit, compensation and nominating and governance committees must be independent. Audit committee members and compensation committee members must also satisfy the independence criteria set forth in Rule 10A-3 and Rule 10C-1, respectively, under the Exchange Act. Under the Nasdaq listing rules, a director will only qualify as an “independent director” if, in the opinion of our Board, the director does not have a relationship that would interfere with the exercise of independent judgment in carrying out his or her responsibilities.
Our Board has undertaken a review of the independence of our directors and considered whether any director has a material relationship with us that could compromise his or her ability to exercise independent judgment in carrying out his or her responsibilities. Based upon information requested from and provided by each director concerning his or her background, employment and affiliations, including family relationships, our Board has determined that none of Trent Davis, Mathew Szot, Barbara Ryan and Rebecca Messina, representing four of our five current directors, has a relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director and that they each are an “independent director” as that term is defined under the Nasdaq listing rules. Mr. Shum is not considered independent due to his position as our Chief Executive Officer.
BOARD LEADERSHIP STRUCTURE
The Board does not have a policy regarding the separation of the roles of Chief Executive Officer and Chairman of the Board. The Board believes it is in shareholders’ best interest that such determination be made based on the position and direction of the Company and the membership of the Board.
There are no family relationships among any of our directors and executive officers.
ROLE OF THE BOARD IN RISK OVERSIGHT
One of the key functions of our Board is informed oversight of our risk management process. The Board does not have a standing risk management committee, but rather administers this oversight function directly through the Board as a whole, as well as through various standing committees of our Board that address risks inherent in their respective areas of oversight. In particular, our Board is responsible for monitoring and assessing strategic risk exposure and our audit committee is responsible for considering and discussing our major financial risk exposures and the steps our management has taken to monitor and control these exposures, including guidelines and policies to govern the process by which risk assessment and management is undertaken. The Board monitors compliance with legal and regulatory requirements and the effectiveness of our corporate governance practices, including whether they are successful in preventing illegal or improper liability-creating conduct. Our Board is responsible for overseeing our risk management efforts generally, including (i) the allocation of risk management functions among our Board and its committees, and (ii) assessing and monitoring whether any of our compensation policies and programs has the potential to encourage excessive risk-taking. Our Board periodically reviews its general process for the oversight of risk management.
MEETINGS OF THE BOARD OF DIRECTORS
During 2023, our Board held 14 meetings and in 2024 the Board held 11 meetings. The Board acted by unanimous written consent on 20 occasions in 2023 and in 2024 on 11 occasions.
Each Board member attended 100% of the meetings in 2023 and 2024.
| 150 |
INFORMATION REGARDING COMMITTEES OF THE BOARD OF DIRECTORS
Audit Committee
Our audit committee is currently comprised of Matthew Szot (Chairman), Rebecca Messina and Barbara Ryan. Each of the members of our audit committee is an independent director under the Nasdaq listing rules, satisfies the additional independence criteria for audit committee members and satisfies the requirements for financial literacy under the Nasdaq listing rules and Rule 10A-3 of the Exchange Act, as applicable.
Our Board has also determined that Mr. Szot qualifies as an “audit committee financial expert” within the meaning of the applicable rules and regulations of the Securities and Exchange Commission (“SEC”) and satisfies the financial sophistication requirements of the Nasdaq listing rules.
Our audit committee oversees our corporate accounting and financial reporting process and assists our Board in monitoring our financial systems and our legal and regulatory compliance. Our audit committee also:
| ● | oversees the work of our independent auditors; | |
| ● | approves the engagement, discharge and compensation of our independent auditors; | |
| ● | approves engagements of the independent auditors to render any audit or permissible non-audit services; | |
| ● | reviews the qualifications, independence and performance of the independent auditors; | |
| ● | reviews our financial statements and our critical accounting policies and estimates; | |
| ● | reviews the adequacy and effectiveness of our internal controls; | |
| ● | reviews our policies with respect to risk assessment and risk management; | |
| ● | reviews and monitors our policies and procedures relating to related person transactions; and | |
| ● | reviews and discusses with management and the independent auditors the results of our annual audit, our quarterly financial statements and our publicly filed reports. |
Our audit committee operates under a written charter approved by our Board that satisfies the applicable rules and regulations of the SEC and the listing requirements of Nasdaq. The charter is available on the corporate governance section of our website, which is located at www.nayabiosciences.com. This committee held four meetings in 2023 and six meetings in 2024.
Upon approval of Proposal 2, our Board will appoint three of Dr. Laurent Audoly, Dr. Prakash Raman, Ms. Melissa Fensterstock and Ms. Alexandera Urman as members of our audit committee. Each of these director nominees is an independent director under the Nasdaq listing rules, satisfies the additional independence criteria for audit committee members and satisfies the requirements for financial literacy under the Nasdaq listing rules and Rule 10A-3 of the Exchange Act, as applicable. The Company also believes that Dr. Laurent Audoly qualifies as an “audit committee financial expert” within the meaning of the applicable rules and regulations of the Securities and Exchange Commission (“SEC”) and satisfies the financial sophistication requirements of the Nasdaq listing rules and he will act as chairman of our audit committee.
| 151 |
Report of the Audit Committee of the Board of Directors
The audit committee reviewed, and discussed with management and M&K CPAS, PLLC, the Company’s independent registered public accounting firm, the Company’s audited consolidated financial statements for the fiscal year ended December 31, 2023. The audit committee received, reviewed and discussed (i) the written disclosures and communications from M&K regarding relationships, if any, which might impair M&K’s independence from management and the Company, and (ii) all required communications pertaining to the conduct of the audit, including any difficulties encountered in the course of the audit work, any restrictions on the scope of activities or access to requested information, and any significant disagreements with management. Based on the foregoing, the audit committee recommended to the Board of Directors that the audited financial statements be included in the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2023 and filed with the Securities and Exchange Commission on April 16, 2024, our Annual Report on Form 10-K/A for the fiscal year ended December 31, 2023 filed with the SEC on April 17, 2024, our Annual Report on Form 10-K/A for the fiscal year ended December 31, 2023 filed with the SEC on April 29, 2024, and our Annual Report on Form 10-K/A for the fiscal year ended December 31, 2023 filed with the SEC on November 19, 2024. The report of the audit committee was delivered on April 16, 2024.
The material in this audit committee report is not “soliciting material,” is not deemed “filed” with the Commission and is not to be incorporated by reference in any filing of the Company under the Securities Act or the Exchange Act, whether made before or after the date hereof and irrespective of any general incorporation language in any such filing.
Compensation Committee
Our compensation committee is currently comprised of Ms. Ryan (Chairman), Mr. Davis and Mr. Szot.
The compensation committee oversees our compensation policies, plans and programs, and reviews and determine the compensation to be paid to our executive officers and directors. In addition, the compensation committee has the authority to act on behalf of the Board in fulfilling the Board’s responsibilities with respect to compensation-based and related disclosures in filings as required by the SEC. Our compensation committee operates under a written charter approved by our Board that satisfies the applicable rules and regulations of the SEC and the listing requirements of Nasdaq. The charter is available on the corporate governance section of our website, which is located at www.nayabiosciences.com. This committee held one meeting during 2023 and none during 2024.
Upon approval of Proposal 2, our Board will appoint three of Dr. Prakash Raman, Dr. Laurent Audoly, Ms. Melissa Fensterstock and Ms. Alexandera Urman as members of our compensation committee. Each of these director nominees is an independent director under the Nasdaq listing rules, satisfies the additional independence criteria for compensation committee members.
Nominating and Governance Committee
Our nominating and governance committee is currently comprised of Mr. Davis (Chairman), Mr. Szot and Ms. Ryan.
The nominating and governance committee (i) oversees our corporate governance functions on behalf of the Board; (ii) makes recommendations to the Board regarding corporate governance issues; (iii) identifies and evaluates candidates to serve as our directors consistent with the criteria approved by the Board and reviews and evaluates the performance of the Board; (iv) serves as a focal point for communication between director candidates, non-committee directors and management; (v) selects, or recommends to the Board for selection, director candidates and nominees; and (vi) makes other recommendations to the Board regarding matters relating to our directors. Our nominating and governance committee operates under a written charter approved by our Board that satisfies the applicable rules and regulations of the SEC and the listing requirements of Nasdaq. The charter is available on the corporate governance section of our website, which is located at www.nayabiosciences.com. This committee held one meeting during 2023 and none in 2024.
| 152 |
The nominating and governance committee believes that candidates for director should have certain minimum qualifications, which are discussed above in “Questions and Answers About These Proxy Materials and Voting.” The nominating and governance committee also takes these minimum qualifications into account in identifying and evaluating director nominees, including nominees recommended by shareholders. In identifying director nominees, the nominating and governance committee strives for a diverse mix of backgrounds and expertise that enhances the ability of the directors collectively to understand the issues facing the Company and to fulfill the responsibilities of the Board and its committees.
Upon approval of Proposal 2, our Board will appoint three of Dr. Prakash Raman, Dr. Laurent Audoly, Ms. Melissa Fensterstock and Ms. Alexandera Urman as members of our nominating and governance committee. Each of these director nominees is an independent director under the Nasdaq listing rules, satisfies the additional independence criteria for nominating and governance committee members.
SHAREHOLDER COMMUNICATIONS WITH THE BOARD OF DIRECTORS
Any interested party may communicate in writing with any particular director, any committee of the Board, or the directors as a group, by sending such written communication to our Corporate Secretary at our principal executive offices at 5582 Broadcast Court, Sarasota, FL 34240. Copies of written communications received at such address will be provided to the Board or the relevant director unless such communications are considered, in the reasonable judgment of our Corporate Secretary, to be of a purely marketing nature or inappropriate for submission to the intended recipient(s). The Corporate Secretary or his designee may analyze and prepare a response to the information contained in communications received and may deliver a copy of the communication to other Company staff members or agents who are responsible for analyzing or responding to complaints or requests. Communications concerning potential director nominees submitted by any of our shareholders will be forwarded to the chairman of the nominating and governance committee.
CODE OF CONDUCT AND ETHICS FOR EMPLOYEES, EXECUTIVE OFFICERS AND DIRECTORS
We have adopted a Code of Business Conduct and Ethics (“Code of Conduct”) applicable to all our employees, executive officers and directors. The Code of Conduct is available on our website at www.invobioscience.com, under the “Corporate Governance” heading of the “Investors” section. The nominating and governance committee of our Board is responsible for overseeing the Code of Conduct and must approve any waivers of the Code of Conduct for employees, executive officers and directors. We expect that any amendments to the Code of Conduct, or any waivers of its requirements, will be disclosed on our website.
EXECUTIVE AND DIRECTOR COMPENSATION
Summary Compensation Table
The following Summary Compensation Table sets forth, for the years indicated, all cash compensation paid, distributed or accrued for services, including salary and bonus amounts, rendered in all capacities by the Company’s “named executive officers” for SEC reporting purposes.
| 153 |
SUMMARY COMPENSATION TABLE
| Name and Principal Position | Year | Salary ($) | Bonus ($) | Stock Awards ($) | Option Awards ($) | All other Compensation ($) | Total ($) | |||||||||||||||||||||
| Steven Shum | 2024 | 260,000 | (2) | - | - | - | - | 260,000 | ||||||||||||||||||||
| Chief Executive Officer (1) | 2023 | 201,875 | - | - | 30,800 | (3) | - | 232,675 | ||||||||||||||||||||
| Andrea Goren | 2024 | 215,000 | (4) | - | - | - | - | 215,000 | ||||||||||||||||||||
| Chief Financial Officer | 2023 | 173,750 | - | - | 24,948 | (5) | - | 198,698 | ||||||||||||||||||||
| Dr. Daniel Teper | 2024 | 137,548 | (7) | - | - | - | - | 137,548 | ||||||||||||||||||||
| President (6) | ||||||||||||||||||||||||||||
| Michael Campbell | 2024 | 192,500 | (9) | - | - | - | - | 192,500 | ||||||||||||||||||||
| Chief Operating Officer | 2023 | 220,000 | - | - | 55,002 | (10) | - | 275,002 | ||||||||||||||||||||
| Vice President, Business Development (8) | ||||||||||||||||||||||||||||
| (1) | Mr. Shum did not receive any additional compensation for being a member of the board. |
| (2) | As of December 31, 2024, Mr. Shum deferred $69,540 of his salary. |
| (3) | Amounts reflect the aggregate grant date fair value of the 5,000 shares of common stock underlying the stock option on the date of grant without regards to forfeitures, computed in accordance with ASC 718. This amount does not reflect the actual economic value realized by Mr. Shum. The options issued to Mr. Shum provide for equal quarterly vesting over a 3-year period based on continued employment during that time. |
| (4) | As of December 31, 2024, Mr. Goren deferred $51,948 of his salary. |
| (5) | Amounts reflect the aggregate grant date fair value of the 4,050 shares of common stock underlying the stock option on the date of grant without regards to forfeitures, computed in accordance with ASC 718. This amount does not reflect the actual economic value realized by Mr. Goren. The options issued to Mr. Goren provide for equal quarterly vesting based on continued employment during that time. |
| (6) | Dr. Teper became president of the Company effective October 11, 2024. All amounts for 2024 are from October 11, 2024, through December 31, 2024. |
| (7) | As of December 31, 2024, Dr. Teper deferred $85,548 of his salary. |
| (8) | Effective November 15, 2024, Mr. Campbell retired from the Company, and Mr. Campbell and the Company mutually agreed to terminate his employment agreement. |
| (9) | As of December 31, 2024, Mr. Campbell deferred $194,323 of his salary. |
| (10) | Amounts reflect the aggregate grant date fair value of the 4,150 shares of common stock underlying the stock option on the date of grant without regards to forfeitures, computed in accordance with ASC 718. This amount does not reflect the actual economic value realized by Mr. Campbell. The options issued to Mr. Campbell provide for equal quarterly vesting over a 3-year period based on continued employment during that time. |
| 154 |
Narrative Disclosure to Summary Compensation Table
Except as otherwise described below, there are no compensatory plans or arrangements, including payments to be received from the Company with respect to any named executive officer, that would result in payments to such person because of his resignation, retirement, or other termination of employment with the Company, or our subsidiaries, any change in control, or a change in the person’s responsibilities following a change in control of the Company.
OUTSTANDING EQUITY AWARDS AT END OF 2024
The following table provides information about outstanding stock options issued by the Company held by each of our NEOs as of December 31, 2023. None of our NEOs held any other equity awards from the Company as of December 31, 2023.
| Option Awards | Stock Awards | |||||||||||||||||||||||
| Name | Number of Securities Underlying Unexercised Options (#) Exercisable | Number of Securities Underlying Unexercised Options (#) Unexercisable | Option Exercise Price ($) | Option Expiration Date | Number of Shares of Stock That Has Not Yet Vested | Market Value of Stock that has not Yet Vested | ||||||||||||||||||
| Steve Shum | 15,260 | 1,746 | 7.36-161.39 | 12/05/30-05/17/33 | - | - | ||||||||||||||||||
| Andrea Goren | 15,103 | 1,457 | 7.36-115.20 | 08/10/30-05/17/33 | - | - | ||||||||||||||||||
| Dr. Daniel Teper | - | - | - | - | - | - | ||||||||||||||||||
| Michael Campbell | 18,322 | 1,409 | 7.36-161.39 | 01/17/30-05/17/33 | - | - | ||||||||||||||||||
Employment Agreements
Steven Shum
On October 16, 2019, the Company entered into an employment agreement with Steven Shum (the “Shum Employment Agreement”), pursuant to which Mr. Shum serves as chief executive officer on an at-will basis at an annual base salary of $260,000. The Shum Employment Agreement provided for a performance bonus of $75,000 upon a successful up-listing to the Nasdaq Stock Market, with all other bonuses to be determined by the Board in its sole discretion. In addition to his base salary and performance bonus, Mr. Shum was granted: (i) 625 shares of our common stock and (ii) a three-year option to purchase 10,130 shares of our common stock at an exercise price of $163.20 per share. This option vested monthly over its 3-year term. Pursuant to the Shum Employment Agreement, Mr. Shum is also entitled to customary benefits, including health insurance and participation in employee benefit plans. The Shum Employment Agreement provides that if Mr. Shum is terminated without cause (as defined in the Shum Employment Agreement) or he resigns his employment due to a constructive termination (as defined in the Shum Employment Agreement) then he will be entitled to receive, as severance, (a) 12 month’s base salary continuation, (b) 6 months reimbursement of payments for continuing health coverage, pursuant to COBRA, and (c) continued vesting of his shares for a period of 6 months following such employment termination.
| 155 |
On August 10, 2023, Mr. Shum, voluntarily agreed to temporarily reduce the annual base salary under his employment agreement from $260,000 to $105,000 until further notice, which reduction took effect on August 16, 2023. As of January 1, 2024, the salary for Mr. Shum reverted to the amount reflected in the Shum Employment Agreement.
Andrea Goren
On June 14, 2021, the Company entered into an employment agreement with Andrea Goren (the “Goren Employment Agreement”), pursuant to which Mr. Goren was hired as the Company’s chief financial officer. The Goren Employment Agreement provides for an annual base salary of $215,000 and a target annual incentive bonus of up to 50% of base salary if the Company achieves goals and objectives determined by the Board. In connection with the Goren Employment Agreement, on June 14, 2021 the Company granted Mr. Goren a stock option under the 2019 Plan to purchase 3,625 shares of the Company common stock (the “Goren Option”). The Goren Option vests in equal monthly installments over a 3-year period, has a term of 10 years and can be exercised at a price of $104.10 per share. Also, in connection with the Goren Employment Agreement, as of July 1, 2021, Mr. Goren was granted a restricted stock award for 250 share of Company common stock (the “Goren RSA”). The Goren RSA vested in equal monthly installments over a 12-month period. Mr. Goren is also entitled to customary benefits, including health insurance and participation in employee benefit plans. The Goren Employment Agreement provides that if Mr. Goren terminates the Goren Employment Agreement for “cause” (as defined in the Goren Employment Agreement) or the Company terminates the Goren Employment Agreement without “cause,” then he will continue to receive his base salary for three months after termination and certain insurance benefits for twelve months after termination. The Company may terminate the Goren Employment Agreement without “cause” on 30 days’ notice.
On August 10, 2023, Mr. Goren, voluntarily agreed to temporarily reduce the annual base salary under his employment agreement from $215,000 to $105,000 until further notice, which reduction took effect on August 16, 2023. As of January 1, 2024, the salary for Mr. Goren reverted to the amount reflected in the Goren Employment Agreement.
Michael Campbell
On January 15, 2020, the Company entered into an employment agreement (the “Campbell Employment Agreement”) with Michael Campbell to serve as the Company’s chief operating officer and vice president of business development. The Campbell Employment Agreement provides for an annual base salary of $220,000, and a target annual incentive bonus of up to 50% of base salary if the Company achieves goals and objectives determined by the Board. In connection with the Campbell Employment Agreement, on January 17, 2020, the Company granted Mr. Campbell 1,563 shares of Company common stock, and an option to purchase 6,250 shares of Company common stock (the “Campbell Option”) at an exercise price of $136.8192 per share. One quarter of the Campbell Option vested upon grant, and the remainder vested in monthly increments over a period of two years from the date of grant. Mr. Campbell is also entitled to customary benefits, including health insurance and participation in employee benefit plans. The Campbell Employment Agreement provides that if Mr. Campbell terminates the Campbell Employment Agreement for “cause” (as defined in the Campbell Employment Agreement) or the Company terminates the Campbell Employment Agreement without “cause,” then he will continue to receive his base salary and certain insurance benefits for three months after termination. The Company may terminate the Campbell Employment Agreement without “cause” on 60 days’ notice.
| 156 |
Effective November 15, 2024, Mr. Campbell retired from the Company, and Mr. Campbell and the Company mutually agreed to terminate his employment agreement.
Daniel Teper
Effective as of August 1, 2023, Legacy NAYA entered into an employment agreement with Dr. Daniel Teper (the “Teper Employment Agreement”), pursuant to which Dr. Teper serves as chief executive officer of Legacy NAYA for a period of three years at an initial annual base salary of $624,000, which salary shall be increased annually by an amount equal to the percentage increase of the Consumer Price Index. In addition, Legacy NAYA’s compensation committee shall review Dr. Teper’s salary annually and may further increase the salary following such review in its sole discretion. The Teper Employment Agreement provides for Dr. Teper to be eligible for an annual bonus of up to seventy-five percent of his then applicable salary, which bonus shall be payable in cash and up to 50% in shares of Legacy NAYA common stock at Dr. Teper’s discretion. The salary payable to Dr. Teper may be deferred according to the resources of Legacy NAYA until it closes a round of financing with gross proceeds of at least $10 million. In addition to his base salary and performance bonus, Dr. Teper was granted 500,000 shares of Legacy NAYA’s Class B common stock, and he is eligible to participate in Legacy NAYA’s stock option plan. Pursuant to the Teper Employment Agreement, Dr. Teper is also entitled to customary benefits, including health insurance, participation in employee benefit plans, vacation, and sick time. The Teper Employment Agreement provides that if Dr. Teper is terminated other than for cause (as defined in the Teper Employment Agreement) or he resigns his employment due to a good reason (as defined in the Teper Employment Agreement) then he will be entitled to receive, as severance, (a) the greater of (i) 12 month’s salary for the year in which termination occurred or (ii) the number of months remaining in the term of the agreement plus the amount of the actual bonus earned by Dr. Teper for year prior to his termination and, if the termination occurs in the first year of the term, 100% of the first year target bonus, (b) up to 12 months reimbursement of payments for continuing health coverage, pursuant to COBRA, and (c) immediate vesting of any unvested restricted stock or stock options held by Dr. Teper. The Teper Employment Agreement further provides that, if Dr. Teper’s employment is terminated in connection with a change of control (as defined in the Teper Employment Agreement), he will be entitled to receive (a) an amount equal to two (2) times the sum of (i) his annual salary in effect on the day preceding the change in control termination, plus (ii) an amount equal to the aggregate bonus received by Dr. Teper for the year immediately preceding the change in control termination and, if the termination occurs in the first year of the term, 100% of the first year target bonus, (b) up to 18 months reimbursement of payments for continuing health coverage, pursuant to COBRA, and (c) immediate vesting of any unvested restricted stock or stock options held by Dr. Teper.
POTENTIAL PAYMENTS UPON TERMINATION OR CHANGE IN CONTROL
If Mr. Shum is involuntarily terminated without cause or constructively terminated (in each case, as defined in the Shum Employment Agreement), then he is entitled to 12 months’ severance and continued vesting of his shares for a period of 6-months following termination.
If (i) Mr. Goren terminates his employment agreement for cause, (ii) the Company provides notice not to renew his employment agreement on any anniversary date, or (iii) the Company terminates his employment agreement without cause, then he is entitled to three months’ severance and insurance benefits.
If Dr. Teper’s employment is terminated in connection with a change of control (as defined in the Teper Employment Agreement), he will be entitled to receive (a) an amount equal to two (2) times the sum of (i) his annual salary in effect on the day preceding the change in control termination, plus (ii) an amount equal to the aggregate bonus received by Dr. Teper for the year immediately preceding the change in control termination
The following table sets forth quantitative information with respect to potential payments to be made to either Mr. Shum, Mr. Goren, and Dr. Teper upon termination in various circumstances. The potential payments are based on the terms of each of the employment agreements discussed above. For a more detailed description of the employment agreements, see the “Employment Agreements” section above.
| Name | Potential Payment Upon Termination | |||||||
| ($) | Option Awards (#) | |||||||
| Steven Shum | $ | 260,000 | (1) | 1,746 | (2) | |||
| Andrea Goren | $ | 53,750 | (3) | 1,457 | (4) | |||
| Daniel Teper | $ | 1,248,000 | (5) | - | ||||
| (1) | Mr. Shum is entitled to twelve months’ severance at the then applicable base salary rate. Mr. Shum’s current base salary is $260,000 per annum. |
| (2) | Represents the number of unvested options at December 31, 2024. Mr. Shum’s options vest equally over a 36-month period. At December 31, 2024, there were 12 months remaining in his vesting schedule. The potential payment of shares subject to Mr. Shum’s unvested options will reduce every month as his options vest and the value of his unvested options will be based on our market price at such time. |
| (3) | Mr. Goren is entitled to three months’ severance at the then applicable base salary rate. Mr. Goren’s current base salary is $215,000 per annum. |
| (4) | Represents the number of unvested options at December 31, 2024. Mr. Goren’s options vest equally over a 36-month period. At December 31, 2024, there were 12 months remaining in his vesting schedule. The potential payment of shares subject to Mr. Goren’s unvested options will reduce every month as his options vest and the value of his unvested options will be based on our market price at such time. |
| (5) | Dr. Teper is entitled to two times his annual salary at the then applicable base salary rate. Dr. Teper’s current base salary is $624,000 per annum. |
| 157 |
Disclosure of Equity Awards Based on Material Nonpublic Information: None
Pay Versus Performance
As required by Section 953(a) of the Dodd-Frank Wall Street Reform and Consumer Protection Act of 2010 and Item 402(v) of Regulation S-K, we are providing the following information about the relationship between executive compensation and certain financial performance metrics. The disclosure included in this section is prescribed by SEC rules and does not necessarily align with how we or the Compensation Committee view the link between financial performance and the compensation actually received or realized by our named executive officers. All information provided above under the “Pay Versus Performance” heading will not be deemed to be incorporated by reference into any filing of the Company under the Securities Act of 1933, as amended, or the Exchange Act, whether made before or after the date hereof and irrespective of any general incorporation language in any such filing, except to the extent the Company specifically incorporates such information by reference.
The table below presents information on the compensation of our Chief Executive Officer and other named executive officers in comparison to certain performance metrics for 2024, 2023, and 2022. These metrics are not those that the Compensation Committee uses when setting executive compensation. The use of the term Compensation Actually Paid (“CAP”) is required by the rules and regulations of the SEC, and under such rules, CAP was calculated by adjusting the Summary Compensation Table (“SCT”) Total values for the applicable year as described in the footnotes to the table.
| Year | Summary Compensation Table Total for PEO (1)(2) | Compensation Actually Paid to PEO (3) | Average Summary Compensation Table Total for Non-PEO NEOs (1)(2) | Average Compensation Actually Paid to Non-PEO NEOs (3) | Value of Initial Fixed $100 Investment Based On Total Shareholder Return | Net Income | ||||||||||||||||||
| (a) | (b) | (c) | (d) | (e) | (f) | (g) | ||||||||||||||||||
| 2024 | 260,000 | 256,230 | 181,683 | 179,718 | 10 | -* | ||||||||||||||||||
| 2023 | 232,675 | 202,076 | 222,131 | 200,322 | 16 | (8,034,612 | ) | |||||||||||||||||
| 2022 | 502,001 | 53,053 | 462,913 | (22,302 | ) | 12 | (10,892,511 | ) | ||||||||||||||||
| (1) | The Principal Executive Officer (“PEO”) information reflected in columns (b) and (c) relates to our CEO, Steven Shum. The non-Principal Executive Officer (“non-PEO”) NEOs information reflected in columns (d) and (e) above relates to our CFO Andrea Goren, our President Dr. Daniel Teper, and our COO Michael Campbell. |
| (2) | The amounts shown in this column are the amounts of total compensation reported for Steven Shum or the average total compensation reported for the non-PEO NEOs, as applicable, for each corresponding year in the “Total” column of the Summary Compensation. Please refer to “Executive Compensation—Compensation Tables—Summary Compensation Table.” |
| (3) | The amounts shown have been calculated in accordance with Item 402(v) of Regulation S-K and do not reflect compensation actually realized or received by the Company’s PEO and non-PEO NEOs. In accordance with the requirements of Item 402(v) of Regulation S-K, adjustments were made to Mr. Shum’s total compensation, or the average total compensation of the non-PEO NEOs, as applicable, as described in the tables below. |
| * | Net income for 2024 not available at time of filing. |
| (1) | The Principal Executive Officer (“PEO”) information reflected in columns (b) and (c) relates to our CEO, Steven Shum. The non-Principal Executive Officer (“non-PEO”) NEOs information reflected in columns (d) and (e) above relates to our CFO Andrea Goren, our President Dr. Daniel Teper, and our COO Michael Campbell. |
| (2) | The amounts shown in this column are the amounts of total compensation reported for Steven Shum or the average total compensation reported for the non-PEO NEOs, as applicable, for each corresponding year in the “Total” column of the Summary Compensation. Please refer to “Executive Compensation—Compensation Tables—Summary Compensation Table.” |
| (3) | The amounts shown have been calculated in accordance with Item 402(v) of Regulation S-K and do not reflect compensation actually realized or received by the Company’s PEO and non-PEO NEOs. In accordance with the requirements of Item 402(v) of Regulation S-K, adjustments were made to Mr. Shum’s total compensation, or the average total compensation of the non-PEO NEOs, as applicable, as described in the tables below. |
| * | Net income for 2024 not available at time of filing. |
| 158 |
PEO SCT Total to CAP Reconciliation
| Year | Summary Compensation Total | Less Stock Awards | Less Option Awards | Fair Value Adjustments to SCT Total | CAP | |||||||||||||||
| 2024 | $ | 260,000 | $ | - | $ | - | $ | (3,770 | ) | $ | 256,230 | |||||||||
| 2023 | 232,675 | - | 30,800 | 201 | 202,076 | |||||||||||||||
| 2022 | 502,001 | 72,601 | 169,400 | (206,947 | ) | 53,053 | ||||||||||||||
Average Non-PEO NEOs SCT Total to CAP Reconciliation
| Year | Summary Compensation Total | Less Stock Awards | Less Option Awards | Fair Value Adjustments to SCT Total | CAP | |||||||||||||||
| 2024 | $ | 181,683 | $ | - | $ | - | $ | (1,965 | ) | $ | 179,718 | |||||||||
| 2023 | 222,131 | - | 25,256 | 3,447 | 200,322 | |||||||||||||||
| 2022 | 462,913 | 37,178 | 208,235 | (239,802 | ) | (22,302 | ) | |||||||||||||
PEO Equity Component of CAP
| Year | Fair Value of Current Year Equity Awards at December 31, | Change in Fair Value of Prior Years’ Awards Unvested at December 31, | Change in Fair Value of Current Years’ Awards Vested through the Year Ended December 31, | Change in Fair Value of Prior Years’ Awards Vested through the Year Ended | Equity Value Included in CAP | |||||||||||||||
| (a) | (b) | (c) | (d) | (e) = (a)+(b)+(c)+(d) | ||||||||||||||||
| 2024 | $ | - | $ | (937 | ) | $ | - | $ | (2,833 | ) | $ | (3,770 | ) | |||||||
| 2023 | 4,448 | (4,214 | ) | 5,574 | (5,607 | ) | 201 | |||||||||||||
| 2022 | 14,953 | (159,116 | ) | 31,772 | (94,556 | ) | (206,947 | ) | ||||||||||||
Average Non-PEO NEOs Equity Component of CAP
| Year | Fair Value of Current Year Equity Awards at December 31, | Change in Fair Value of Prior Years’ Awards Unvested at December 31, | Change in Fair Value of Current Years’ Awards Vested through the Year Ended December 31, | Change in Fair Value of Prior Years’ Awards Vested through the Year Ended | Equity Value Included in CAP | |||||||||||||||
| (a) | (b) | (c) | (d) | (e) = (a)+(b)+(c)+(d) | ||||||||||||||||
| 2024 | $ | - | $ | (512 | ) | $ | - | $ | (1,453 | ) | $ | (1,965 | ) | |||||||
| 2023 | 3,648 | (3,293 | ) | 4,570 | (1,478 | ) | 3,447 | |||||||||||||
| 2022 | 12,550 | (191,259 | ) | 38,928 | (100,021 | ) | (239,802 | ) | ||||||||||||
| 159 |
Compensation of Directors
DIRECTOR COMPENSATION TABLE
| Name | Year | Fees earned or paid in cash ($) | Stock awards ($) | Option awards ($) | All other compensation ($) | Total ($) | ||||||||||||||||||
| Trent Davis | 2024 | 42,500 | (1) | - | - | - | 42,500 | |||||||||||||||||
| 2023 | 42,500 | (1) | - | 24,820 | - | 67,320 | ||||||||||||||||||
| Barbara Ryan | 2024 | 52,500 | (2) | - | - | - | 52,500 | |||||||||||||||||
| 2023 | 49,375 | (2) | - | 24,820 | - | 74,195 | ||||||||||||||||||
| Matthew Szot | 2024 | 47,500 | (3) | - | - | - | 47,500 | |||||||||||||||||
| 2023 | 49,375 | (3) | - | 24,820 | - | 74,195 | ||||||||||||||||||
| Rebecca Messina | 2024 | 42,500 | (4) | - | - | - | 42,500 | |||||||||||||||||
| 2023 | 41,250 | (4) | - | 24,820 | - | 66,070 | ||||||||||||||||||
| Jeffrey Segal | 2024 | - | - | - | - | - | ||||||||||||||||||
| Former Director | 2023 | 8,750 | - | - | - | 8,750 | ||||||||||||||||||
| (1) | As of December 31, 2024, Mr. Davis deferred a cumulative total $66,825 of fees earned. |
| (2) | As of December 31, 2024, Ms. Ryan deferred a cumulative total $91,675 of fees earned. |
| (3) | As of December 31, 2024, Mr. Szot deferred a cumulative total $92,325 of fees earned. |
| (4) | As of December 31, 2024, Ms. Messina deferred a cumulative total $74,000 of fees earned. |
Director Compensation Program
Our current director compensation program is designed to align our director compensation program with the long-term interests of our stockholders by implementing a program comprised of cash and equity compensation.
In setting director compensation, we consider the amount of time that directors expend in fulfilling their duties to the Company as well as the skill level and experience required by our board of directors. We also consider board compensation practices at similarly situated companies, while keeping in mind the compensation philosophy of us and the stockholders’ interests. The directors also receive reimbursement for expenses, including reasonable travel expenses to attend board and committee meetings, reasonable outside seminar expenses, and other special board related expenses.
| 160 |
Limitations on Liability and Indemnification
We are a Nevada corporation and generally governed by the Nevada Private Corporations Code, Title 78 of the Nevada Revised Statutes (the “NRS”).
Section 78.138 of the NRS provides that, unless the corporation’s articles of incorporation provide otherwise, a director or officer will not be individually liable unless it is proven that (i) the director’s or officer’s acts or omissions constituted a breach of his or her fiduciary duties, and (ii) such breach involved intentional misconduct, fraud, or a knowing violation of the law. Our articles of incorporation provide the personal liability of our directors is eliminated to the fullest extent permitted under the NRS.
Section 78.7502 of the NRS permits a company to indemnify its directors and officers against expenses, judgments, fines, and amounts paid in settlement actually and reasonably incurred in connection with a threatened, pending, or completed action, suit, or proceeding, if the officer or director (i) is not liable pursuant to NRS 78.138, or (ii) acted in good faith and in a manner the officer or director reasonably believed to be in or not opposed to the best interests of the corporation and, if a criminal action or proceeding, had no reasonable cause to believe the conduct of the officer or director was unlawful. Section 78.7502 of the NRS requires a corporation to indemnify a director or officer that has been successful on the merits or otherwise in defense of any action or suit. Section 78.7502 of the NRS precludes indemnification by the corporation if the officer or director has been adjudged by a court of competent jurisdiction, after exhaustion of all appeals, to be liable to the corporation or for amounts paid in settlement to the corporation, unless and only to the extent that the court determines that in view of all the circumstances, the person is fairly and reasonably entitled to indemnity for such expenses and requires a corporation to indemnify its officers and directors if they have been successful on the merits or otherwise in defense of any claim, issue, or matter resulting from their service as a director or officer.
Section 78.751 of the NRS permits a Nevada company to indemnify its officers and directors against expenses incurred by them in defending a civil or criminal action, suit, or proceeding as they are incurred and in advance of final disposition thereof, upon determination by the stockholders, the disinterested board members, or by independent legal counsel. If so provided in the corporation’s articles of incorporation, bylaws, or other agreement, Section 78.751 of the NRS requires a corporation to advance expenses as incurred upon receipt of an undertaking by or on behalf of the officer or director to repay the amount if it is ultimately determined by a court of competent jurisdiction that such officer or director is not entitled to be indemnified by the company. Section 78.751 of the NRS further permits the company to grant its directors and officers’ additional rights of indemnification under its articles of incorporation, bylaws, or other agreement.
Section 78.752 of the NRS provides that a Nevada company may purchase and maintain insurance or make other financial arrangements on behalf of any person who is or was a director, officer, employee, or agent of the company, or is or was serving at the request of the company as a director, officer, employee, or agent of another company, partnership, joint venture, trust, or other enterprise, for any liability asserted against him and liability and expenses incurred by him in his capacity as a director, officer, employee, or agent, or arising out of his status as such, whether or not the company has the authority to indemnify him against such liability and expenses.
| 161 |
Our articles of incorporation provide for indemnification of our officers and directors to the fullest extent permissible under Nevada General Corporation Law, in accordance with the Company’s Bylaws. Our Bylaws provide for indemnification of our officers and directors to the fullest extent not prohibited by the Nevada; provided however, that the Company may modify the extent of such indemnification by individual contracts with its directors and officers; and provided, further, that the Company shall not be required to indemnify any director or officer in connection with any proceeding (or part thereof) initiated by such person unless (i) such indemnification is expressly required to be made by law; (ii) the proceeding was authorized by the board of directors; (iii) such indemnification is provided by the Company, in its sole discretion, pursuant to the powers vested in the corporation under the Nevada General Corporation Law or; (iv) such indemnification is a result of the enforcement of a contractual right.
SECURITIES AUTHORIZED FOR ISSUANCE UNDER EQUITY COMPENSATION PLANS
The following table shows information regarding our equity compensation plans as of December 31, 2024.
| Plan Category | Number of securities to be issued upon exercise of outstanding options, warrants and rights (a) | Weighted average exercise price of outstanding options, warrants and rights (b) | Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (c) | |||||||||
| Equity compensation plans approved by security holders (1) | 98,289 | (2) | $ | 41.90 | 164,312 | |||||||
| Equity compensation plans not approved by security holders | - | - | - | |||||||||
| Total | 98,289 | $ | 41.90 | 164,312 | ||||||||
(1) 2019 Stock Incentive Plan. On October 3, 2019, our Board adopted the 2019 Stock Incentive Plan (as amended, the “Plan”). The purpose of our Plan is to advance the best interests of the company by providing those persons who have a substantial responsibility for our management and growth with additional incentive and by increasing their proprietary interest in the success of the company, thereby encouraging them to maintain their relationships with us. Further, the availability and offering of stock options and common stock under the plan supports and increases our ability to attract and retain individuals of exceptional talent upon whom, in large measure, the sustained progress, growth and profitability which we depend. As of December 31, 2024 the total number of shares available for the grant of either stock options or compensation stock under the plan, including 20,640 shares approved at our shareholders meeting on October 12, 2022, is 311,049 shares, subject to annual increases of six percent (6%) of the total number of shares of outstanding Common Stock on December 31st of the preceding calendar year.
(2) We granted no shares subject to restricted stock grants under the Plan in the year ended December 31, 2024.
Our Board administers our plan and has full power to grant stock options and common stock, construe and interpret the plan, establish rules and regulations and perform all other acts, including the delegation of administrative responsibilities, it believes reasonable and proper. Any decision made, or action taken, by our Board arising out of or in connection with the interpretation and administration of the plan is final and conclusive.
The Board, in its absolute discretion, may award common stock to employees of, consultants to, and directors of the company, and such other persons as the Board or compensation committee may select, and permit holders of common stock options to exercise such options prior to full vesting therein and hold the common stock issued upon exercise of the option as common stock. Stock options may also be granted by our Board or compensation committee to non-employee directors of the company or other persons who are performing or who have been engaged to perform services of special importance to the management, operation or development of the company.
In the event that our outstanding common stock is changed into or exchanged for a different number or kind of shares or other securities of the company by reason of merger, consolidation, other reorganization, recapitalization, combination of shares, stock split-up or stock dividend, prompt, proportionate, equitable, lawful and adequate adjustment shall be made of the aggregate number and kind of shares subject to stock options which may be granted under the plan.
Our Board may at any time, and from time to time, suspend or terminate the plan in whole or in part or amend it from time to time in such respects as our Board may deem appropriate and in our best interest.
| 162 |
DESCRIPTION OF SECURITIES
The following description of the Company’s capital stock and provisions of its Articles of Incorporation and Bylaws are summaries and are qualified by reference to the Company’s Articles of Incorporation and Bylaws.
General
Our Articles of Incorporation authorizes the issuance of 150,000,000 shares of capital stock, 50,000,000 shares of which are designated as common stock, par value $0.0001 per share, 1,000,000 shares of which are designated as Series A Preferred Stock, par value $5.00 per share, 1,200,000 shares of which are designated as Series B Preferred Stock, 30,375 shares of which are designated as Series C-1 Convertible Preferred Stock, par value $0.0001 per share, and 8,576 shares of which are designated as Series C-2 Convertible Preferred Stock, par value $0.0001 per share and 97,761,049 of which are designated as preferred stock, par value $0.0001 per share. As of January 24, 2025, we have 7,087,796 shares of common stock issued and outstanding and no shares of Series A Preferred Stock issued or outstanding, 1,200,000 shares of Series B Preferred Stock issued but not outstanding, 30,375 shares of Series C-1 Convertible Preferred Stock issued and outstanding, and 4,576 shares of Series C-2 Convertible Preferred Stock issued and outstanding.
Common Stock
Each stockholder of our common stock is entitled to a pro rata share of cash distributions made to stockholders, including dividend payments. The holders of our common stock are entitled to one vote for each share of record on all matters to be voted on by stockholders. There is no cumulative voting with respect to the election of our directors or any other matter. Therefore, the holders of more than 50% of the shares voted for the election of those directors can elect all of the directors. The holders of our common stock are entitled to receive dividends when and if declared by our board of directors from funds legally available therefore. Cash dividends are at the sole discretion of our board of directors. In the event of our liquidation, dissolution or winding up, the holders of common stock are entitled to share ratably in all assets remaining available for distribution to them after payment of our liabilities and after provision has been made for each class of stock, if any, having any preference in relation to our common stockholders of shares of our common stock have no conversion, preemptive or other subscription rights, and there are no redemption provisions applicable to our common stock.
Preferred Stock
The board of directors is authorized, subject to any limitations prescribed by law, without stockholder approval, to issue up to an aggregate of 100,000,000 shares of preferred stock in one or more series and to fix the rights, preferences, privileges and restrictions granted to or imposed upon the preferred stock, including voting rights, dividend rights, conversion rights, redemption privileges and liquidation preferences. The rights of the holders of common stock will be subject to, and may be adversely affected by, the rights of holders of any preferred stock that may be issued in the future. Issuance of preferred stock, while providing flexibility in connection with possible acquisitions and other corporate purposes, could have the effect of delaying, deferring or preventing a change in control of NAYA.
| 163 |
Series A Preferred Stock
On November 20, 2023, the Company filed with the Nevada Secretary of State a Certificate of Designation of Series A Convertible Preferred Stock (the “Series A Certificate of Designation”) which sets forth the rights, preferences, and privileges of the Series A Preferred Stock. One million (1,000,000) shares of Series A Preferred Stock with a stated value of $5.00 per share were authorized under the Series A Certificate of Designation.
Each share of Series A Preferred Stock had a stated value of $5.00, which was convertible into shares of the Company’s common stock at a fixed conversion price equal to $2.20 per share, subject to adjustment. The Company may not effect the conversion of any shares of Series A Preferred Stock if, after giving effect to the conversion or issuance, the holder, together with its affiliates, would beneficially own more than 9.99% of the Company’s outstanding common stock. Moreover, the Company could not effect the conversion of any shares of Series A Preferred Stock if, after giving effect to the conversion or issuance, the holder, together with its affiliates, would have beneficially owned more than 9.99% of the Company’s outstanding common stock. Moreover, the Company could not effect the conversion of any shares of Series A Preferred Stock if, after giving effect to the conversion or issuance, the holder, together with its affiliates, would beneficially own more than 19.99% of the Company’s outstanding common stock unless and until the Company receives the approval required by the applicable rules and regulations of The Nasdaq Stock Market LLC (or any subsequent trading market).
Each share of Series A Preferred Stock would have automatically converted into common stock upon the closing of a merger (the “Merger”) of INVO Merger Sub Inc., a wholly owned subsidiary of the Company and a Delaware corporation (“Merger Sub”), with and into NAYA Therapeutics, Inc., a Delaware corporation formerly known as NAYA Biosciences, Inc. (“Legacy NAYA”) pursuant to an Agreement and Plan of Merger, as amended, by and among the Company, Merger Sub, and Legacy NAYA (the “Merger Agreement”).
The holders of Series A Preferred Stock would have been entitled to receive a pro-rata portion, on an as-if converted basis, of any dividends payable on common stock.
In the event of any voluntary or involuntary liquidation, dissolution, or winding up, or sale of the Company (other than the Merger), each holder of Series A Preferred Stock would have been entitled to receive its pro rata portion of an aggregate payment equal to (i) $5.00, multiplied by (ii) the total number of shares of Series A Preferred Stock issued under the Series A Certificate of Designation.
Other than those rights provided by law, the holders of Series A Preferred Stock did not have any voting rights.
Series B Preferred Stock
On November 20, 2023, the Company filed with the Nevada Secretary of State a Certificate of Designation of Series B Convertible Preferred Stock (the “Series B Certificate of Designation”) which sets forth the rights, preferences, and privileges of the Series B Preferred Stock. One million two hundred (1,200,000) shares of Series B Preferred Stock with a stated value of $5.00 per share were authorized under the Series B Certificate of Designation.
| 164 |
Each share of Series B Preferred Stock had a stated value of $5.00, which was convertible into shares of the Company’s common stock at a fixed conversion price equal to $5.00 per share, subject to adjustment. The Company could not effect the conversion of any shares of Series B Preferred Stock if, after giving effect to the conversion or issuance, the holder, together with its affiliates, would beneficially own more than 19.99% of the Company’s outstanding common stock unless and until the Company receives the approval required by the applicable rules and regulations of Nasdaq (or any subsequent trading market).
Each share of Series B Preferred Stock would have automatically converted into common stock upon the closing of the Merger.
The holders of Series B Preferred Stock were be entitled to receive a pro-rata portion, on an as-if converted basis, of any dividends payable on common stock.
In the event of any voluntary or involuntary liquidation, dissolution, or winding up, or sale of the Company (other than the previously announced merger with NAYA), each holder of Series B Preferred Stock would have been entitled to receive its pro rata portion of an aggregate payment equal to (i) $5.00, multiplied by (ii) the total number of shares of Series B Preferred Stock issued under the Series B Certificate of Designation.
Other than those rights provided by law, the holders of Series B Preferred Stock did not have any voting rights.
Series C-1 Preferred
On October 14, 2024, the Company filed with the Nevada Secretary of State a Certificate of Designation of Series C-1 Convertible Preferred Stock (the “Series C-1 Certificate of Designation”) which sets forth the rights, preferences, and privileges of the Series C-1 Preferred Stock. Thirty thousand three hundred seventy five (30,375) shares of Series C-1 Preferred Stock with a stated value of $1,000.00 per share were authorized under the Series C-1 Certificate of Designation.
Each share of Series C-1 Preferred Stock has a stated value of $1,000.00, which is convertible into shares of the Company’s common stock at a conversion price equal to $1.02913 per share, subject to adjustment. The Series C-1 Preferred Stock may not be converted into shares of the Company’s common stock unless and until the Company’s stockholders approve the issuance of common stock upon conversion of the Series C-1 Preferred Stock. Each share of Series C-1 Preferred Stock shall automatically convert into the Company’s common stock if the Company’s stockholders approve the issuance, except that the Company may not effect such conversion if, after giving effect to the conversion or issuance, the holder, together with its affiliates, would beneficially own in excess of 19.99% of the Company’s outstanding common stock.
Commencing on the ninety-first (91st) day after the first issuance of any Series C-1 Preferred Stock, the holders of Series C-1 Preferred Stock shall be entitled to receive dividends on the stated value at the rate of two percent (2%) per annum, payable in shares of the Company’s common stock at the conversion price. Such dividends shall continue to accrue until paid. Such dividends will not be paid in shares of the Company’s common stock unless and until the Company’s stockholders approve the issuance of common stock upon conversion of the Series C-1 Convertible Preferred Stock. The holders of Series C-1 Preferred Stock shall also be entitled to receive a pro-rata portion, on an as-if convertible basis, of any dividends payable on common stock.
| 165 |
The Series C-1 Preferred ranks senior to the Company’s common stock and junior to the Series C-2 Preferred Stock (described below). Subject to the rights of the holders of any senior securities, in the event of any voluntary or involuntary liquidation, dissolution, or winding up, or sale of the Company, each holder of Series C-1 Preferred Stock shall be entitled to receive its pro rata portion of an aggregate payment equal to the amount as would be paid on the Company’s common stock issuable upon conversion of the Series C-1 Preferred Stock, determined on an as-converted basis, without regard to any beneficial ownership limitation.
Other than those rights provided by law, the Series C-1 Preferred Stock has no voting rights. The Series C-1 Preferred Stock is not redeemable.
Series C-2 Preferred Stock
On October 14, 2024, the Company filed with the Nevada Secretary of State a Certificate of Designation of Series C-2 Convertible Preferred Stock (the “Series C-2 Certificate of Designation”) which sets forth the rights, preferences, and privileges of the Series C-2 Preferred Stock. Eight thousand five hundred seventy six (8,576) shares of Series C-2 Preferred Stock with a stated value of $1,000.00 per share were authorized under the Series C-2 Certificate of Designation.
Each share of Series C-2 Preferred Stock has a stated value of $1,000.00, which, along with any additional amounts accrued thereon pursuant to the terms of the Series C-2 Certificate of Designation (collectively, the “Conversion Amount”) is convertible into shares of the Company’s common stock at a conversion price equal to $0.6893 per share, subject to adjustment. The Series C-2 Preferred Stock may not be converted into shares of the Company’s common stock unless and until the Company’s stockholders approve the issuance of common stock upon conversion of the Series C-2 Convertible Preferred Stock. Each share of Series C-2 Preferred Stock shall become convertible into the Company’s common stock at the option of the holder of such Series C-2 Preferred Stock shares if the Company’s stockholders approve the issuance of common stock upon conversion of the Series C-2 Preferred Stock, except that the Company may not effect such conversion if, after giving effect to the conversion or issuance, the holder, together with its affiliates, would beneficially own in excess of 9.99% of the Company’s outstanding common stock.
Commencing on the ninety-first (91st) day after the first issuance of any Series C-2 Preferred Stock, the holders of Series C-2 Preferred Stock shall be entitled to receive dividends on the stated value at the rate of ten percent (10%) per annum, payable in shares of the Company’s common stock, with each payment of a dividend payable in shares of the Company’s common stock at a conversion price of eighty-five percent (85%) of the average of the volume weighted average price of the Company’s common stock for the five (5) trading days before the applicable dividend date. Such dividends shall continue to accrue until paid. Such dividends will not be paid in shares of the Company’s common stock unless and until the Company’s stockholders approve the issuance of common stock upon conversion of the Series C-2 Preferred Stock. The holders of Series C-2 Preferred Stock shall also be entitled to receive a pro-rata portion, on an as-if convertible basis, of any dividends payable on common stock.
| 166 |
The Series C-2 Preferred Stock ranks senior to the Company’s common stock and to the Series C-1 Preferred. Stock Subject to the rights of the holders of any senior securities, in the event of any voluntary or involuntary liquidation, dissolution, or winding up, or sale of the Company, each holder of Series C-2 Preferred Stock shall be entitled to receive its pro rata portion of an aggregate payment equal to the greater of (a) 125% of the Conversion Amount with respect to such shares, and (b) the amount as would be paid on the Company’s common stock issuable upon conversion of the Series C-2 Preferred Stock, determined on an as-converted basis, without regard to any beneficial ownership limitation.
Other than those rights provided by law, the Series C-2 Preferred Stock has no voting rights. The Series C-2 Preferred Stock is only redeemable upon a “Bankruptcy Triggering Event” or a “Change of Control” that occurs 210 days after the closing date of the Merger.
Options
As of January 24, 2025, we have options to purchase up to 97,992 shares of our common stock issued and outstanding at a weighted average exercise price of $35.20 per share. In addition, we have options to purchase up to 365,580 shares of our common stock issued and outstanding that are not exercisable unless and until our stockholders approve their exercisability.
Restricted Stock Units
As of January 24, 2025, we have 1,705,485 restricted stock units issued and outstanding that cannot be settled for shares of our common stock unless and until our stockholders approve their settlement as provided in Proposal herein.
Unit Purchase Options
As of January 24, 2025, we have unit purchase options to purchase up to 4,645 shares of our common stock at an exercise price of $64.00 per share.
Warrants
As of January 24, 2025, we have warrants to purchase up to 18,263,442 shares of our common stock issued and outstanding at an exercise price between $0.70 and $192.00 per share as well as 11,463,079 prefunded warrants.
| 167 |
Effect of Certain Provisions of our Amended and Restated Articles of Incorporation and Bylaws and the Nevada Anti-Takeover Provisions
Some provisions of Nevada law and our amended and restated articles of incorporation and bylaws contain provisions that could make our acquisition by means of a tender offer, a proxy contest or otherwise, and the removal of incumbent officers and directors more difficult. These provisions, summarized below, are expected to discourage coercive takeover practices and inadequate takeover bids and to promote stability in our management. These provisions are also designed to encourage persons seeking to acquire control of us to first negotiate with our board of directors.
Amended and Restated Articles of Incorporation and Bylaws
Our amended and restated articles of incorporation and bylaws provide for the following:
| ● | Preferred Stock. The ability to authorize preferred stock makes it possible for our board of directors to issue one or more series of preferred stock with voting or other rights or preferences that could impede the success of any attempt to change control of the Company. These and other provisions may have the effect of deferring hostile takeovers or delaying changes in control or management of us. | |
| ● | Requirements for Advance Notification of Stockholder Nominations. Our bylaws establish advance notice procedures with respect to stockholder proposals and the nomination of candidates for election as directors, other than nominations made by or at the direction of the board of directors. | |
| ● | Stockholder Meetings. Our charter documents provide that a special meeting of stockholders may be called only by resolution adopted by the majority board of directors, the chairman of the board of directors or the chief executive officer. | |
| ● | Amendment of Bylaws. Our board of directors have the sole power to amend the bylaws. |
Nevada Anti-Takeover Provision
Section 78.438 of the Nevada Revised Statutes (“NRS”) prohibits a publicly held Nevada corporation from engaging in a business combination with an interested stockholder, generally a person that together with its affiliates owns or within the last two years has owned 10% of the outstanding voting stock, for a period of two years after the date of the transaction in which the person became an interested stockholder, unless the business combination is approved in a prescribed manner, or falls within certain exemptions under the NRS. As a result of these provisions in our charter documents under Nevada law, the price investors may be willing to pay in the future for shares of our common stock may be limited.
| 168 |
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table and notes set forth the beneficial ownership of the common stock of the Company as of January 24, 2025, by each person who was known by the Company to beneficially own more than 5% of the common stock, by each director and named executive officer, and by all directors and executive officers as a group. Beneficial ownership is determined in accordance with the rules of the SEC and includes voting or dispositive power with respect to the securities. Unless otherwise indicated below, to our knowledge, all persons listed below have sole voting and dispositive power with respect to their shares of our common stock, except to the extent authority is shared by spouses under applicable law. Unless otherwise noted, the address of all of the individuals and entities named below is care of NAYA Biosciences, Inc., 5582 Broadcast Court Sarasota, Florida, 34240.
The following table sets forth the beneficial ownership of our common shares as of January 24, 2025 for:
| ● | each person, or group of affiliated persons, who is known by us to beneficially own more than 5% of our common shares; | |
| ● | each of our named executive officers; | |
| ● | each of our directors; and | |
| ● | all of our current executive officers and directors as a group. |
The percentage ownership information is based upon 7,087,796 common shares outstanding as of January 24, 2025. We have determined beneficial ownership in accordance with the rules of the SEC. These rules generally attribute beneficial ownership of securities to persons who possess sole or shared voting power or investment power with respect to those securities. Unless otherwise indicated, the persons or entities identified in this table have sole voting and investment power with respect to all shares shown as beneficially owned by them, subject to applicable community property laws.
| Name and Address of Beneficial Owner (1) | Number of Shares | Percentage of Common Stock | ||||||
| 5% Stockholders: | ||||||||
| Five Narrow Lane LP | 708,074 | (2) | 9.99 | % | ||||
| Intracoastal Capital LLC | 504,478 | (3) | 7.12 | % | ||||
| Officers and Directors: | ||||||||
| Dr. Daniel Teper | 54,179 | (4) | 0.76 | % | ||||
| Andrea Goren | 22,803 | (5) | 0.32 | % | ||||
| Steve Shum | 21,761 | (6) | 0.31 | % | ||||
| Matthew Szot | 7,761 | (7) | 0.11 | % | ||||
| Trent Davis | 7,327 | (8) | 0.10 | % | ||||
| Barbara Ryan | 7,147 | (9) | 0.10 | % | ||||
| Rebecca Messina | 6,217 | (10) | 0.09 | % | ||||
| Lyn Falconio | - | - | % | |||||
| Director Nominees | ||||||||
| Laurent Audoly | - | - | % | |||||
| Melissa Fenterstock | - | - | % | |||||
| Prakash Raman | - | - | % | |||||
| Alexandra Urman | - | - | % | |||||
| All directors, director nominees and executive officers as a group (12 persons) | 127,195 | 1.79 | % | |||||
| (1) | Unless otherwise indicated, the business address of each current director or executive officer is NAYA Biosciences, Inc. 5582 Broadcast Court Sarasota, Florida 34240. |
| (2) | 708, 074 represents the maximum amount of shares that Five Narrow Lane LP can beneficially control under a contractually stipulated 9.99% ownership restriction. The full conversion and/or exercise of Five Narrow Lane LP’s securities would exceed this restriction. |
| (3) | As of the close of business on January 16, 2025, Intracoastal Capital LLC, may been deemed to share beneficial ownership of 504,478 shares of Common Stock, which consisted of (i) 320,478 shares of Common Stock held by Intracoastal and (ii) 184,000 shares of Common Stock issuable upon exercise of a warrant to be issued to Intracoastal. The foregoing excludes 353,000 shares of Common Stock issuable upon exercise of a warrant to be issued to Intracoastal (“Intracoastal Warrant 2”) because Intracoastal Warrant 2 contains a blocker provision under which the holder thereof does not have the right to exercise Intracoastal Warrant 2 to the extent (but only to the extent) that such exercise would result in beneficial ownership by the holder thereof, together with the holder’s affiliates, and any other persons acting as a group together with the holder or any of the holder’s affiliates, of more than 4.99% of the Common Stock. Without such blocker provision, each of the Reporting Persons may have been deemed to have beneficial ownership of 857,478 shares of Common Stock. Mitchell P. Kopin and Daniel B. Asher shall be deemed to share beneficial ownership with Intracoastal. The principal business office of Mr. Kopin and Intracoastal is 245 Palm Trail, Delray Beach, Florida 33483. The principal business office of Mr. Asher is 111 W. Jackson Boulevard, Suite 2000, Chicago, Illinois 60604. |
| (4) | Includes 26,200 shares held by Cytovia Therapeutics Holdings, Inc., of which Dr. Teper is an officer, director, and shareholder. Dr. Teper disclaims beneficial ownership of all such shares held by Cytovia except to the extent of his pecuniary interest therein. Excludes 6,530,759 shares of common stock under Series C-1 Preferred Shares subject to stockholder approval and beneficial ownership limitations. |
| (5) | Includes: 15,208 shares of common stock under options (either presently exercisable or exercisable within 60 days of January 24, 2025). |
| (6) | Includes: 15,339 shares of common stock under options (either presently exercisable or exercisable within 60 days of January 24, 2025). |
| (7) | Includes: 5,801 shares of common stock under options (either presently exercisable or exercisable within 60 days of January 24, 2025). |
| (8) | Includes: 5,645 shares of common stock under options (either presently exercisable or exercisable within 60 days of January 24, 2025). |
| (9) | Includes: 5,567 shares of common stock under options (either presently exercisable or exercisable within 60 days of January 24, 2025). |
| (10) | Includes: 5,067 shares of common stock under options (either presently exercisable or exercisable within 60 days of January 24, 2025). |
| 169 |
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
Related Party Transactions Policy and Procedures
We have adopted a written policy with respect to the review, approval, and ratification of related party transactions. Under the policy, any transactions where the amount involved exceeds the lesser of $120,000 or one percent (1%) of the average of our total assets at year-end for the last two completed fiscal years and in which any related person has or will have a direct or indirect material interest, other than equity and other compensation, termination and other arrangements which are described under the headings “Compensation of Directors” and “Executive and Director Compensation,” is defined as a related party transaction. Any such related party transactions are reviewed and must be approved by the Company’s board of directors.
Certain Related Party Transactions
In the fourth quarter of 2022, the Company issued a series of demand promissory notes in the aggregate principal amount of $550,000 to a related party, JAG, a company in which the Company’s Chief Financial Officer is a beneficiary but does not have any control over its investment decisions with respect to the Company, for an aggregate purchase price of $500,000. The JAG Notes accrue 10% annual interest from their respective dates of issuance. At maturity, the Company agreed to pay outstanding principal, a 10% financing fee and accrued interest. On July 10, 2023, the Company issued an additional demand promissory note in the principal amount of $110,000 to JAG for a purchase price of $100,000.
In consideration for subscribing to the JAG Note for $100,000 dated December 29, 2022, and for agreeing to extend the date on which the other JAG Notes are callable to March 31, 2023, the Company issued JAG a warrant to purchase 17,500 shares of common stock. The warrant may be exercised for a period of five (5) years from issuance at a price of $10.00 per share. On July 10, 2023, JAG agreed to extend the date on which the JAG Notes are callable to September 30, 2023.
In the fourth quarter of 2022, the Company issued demand promissory notes in the aggregate principal amount of $220,000 for an aggregate purchase price of $200,000, of which (1) $100,000 was received from its Chief Executive Officer ($60,000 on November 29, 2022, $15,000 on December 2, 2022, and $25,000 on December 13, 2022) and (2) $100,000 was received from an entity controlled by its Chief Financial Officer ($75,000 on November 29, 2022 and $25,000 on December 13, 2022). These notes accrue 10% annual interest accrues from the date of issuance. These notes are callable with 10 days prior written notice. At maturity, the Company agreed to pay outstanding principal, a 10% financing fee, and accrued interest.
For the nine months ended September 30, 2024, the Company incurred $60,889 in interest related to these demand notes and as of September 30, 2024 the total outstanding balance, including principal and accrued interest, was $1,024,341.
As of September 30, 2024, the Company owed accounts payable to related parties totaling $268,337, primarily related to unpaid employee expense reimbursements and unpaid board fees, and accrued compensation of $640,038, primarily related to deferred wages and accrued paid time off.
Legacy NAYA Related Party Transactions
Legacy NAYA and Cytovia, entered into a loan agreement on August 1, 2023, pursuant to which Cytovia made available to Legacy NAYA a term loan for up to $1,000,000, bearing interest at a rate of 5% per annum. On June 17, 2024, the loan agreement was amended to state that all principal and interest outstanding under the Loan shall be due and payable in full on the date Legacy NAYA receives its next funding.
| 170 |
On October 18, 2023, Legacy NAYA entered into an asset purchase agreement with Cytovia Therapeutics Holdings, Inc. and Cytovia Therapeutics, LLC (collectively, “Cytovia”) to acquire the rights to the two bifunctional antibodies CYT303 and CYT338 (now known as NY-303 and NY-338). The fixed purchase price consists of 818,182 shares of common stock of Legacy NAYA (valued by the parties at approximately $30 million) and a promissory note in the principal amount of $6 million, payable in monthly installments of $1 million per month. In addition, Legacy NAYA agreed to pay an additional $2 million per product if the first patient has begun phase II. These amounts are payable in cash or shares of our common stock at Legacy NAYA’s election, and if the amount is paid in shares, then each share shall be valued at $8.00 per share. Legacy NAYA also agreed to pay an additional $8 million per product at the phase II/IIa data read-out for such product. These amounts are payable in cash or shares of our common stock at Legacy NAYA’s election, and if the amount is paid in shares, then each share shall be valued at $8.00 per share. Legacy NAYA also agreed to assume $2.689 million of liabilities from Cytovia. The parties agreed to enter into an intercompany service agreement, a technology license agreement, and a trademark license agreement for the trademark Flex-NK (TM), detailing sponsored research for which Legacy NAYA would pay Cytovia $6 million, payable over 12 months in the monthly amount of $500,000. The start date of these payments will be mutually agreed between Legacy NAYA and Cytovia. Legacy NAYA and Cytovia closed the transaction contemplated by the asset purchase agreement on October 20, 2023, except that Legacy NAYA issued 1,363,642 shares if its common stock as the stock consideration portion of the purchase price.
On May 17, 2024, Legacy NAYA and Cytovia entered into an amendment to the asset purchase agreement. Pursuant to this amendment, the parties agreed that Legacy NAYA would not assume any liabilities of Cytovia. The parties further agreed that the purchase price would consist of 1,609,098 shares of common stock of Legacy NAYA (valued by the parties at approximately $30 million) and cash of $1.7 million which Legacy NAYA had previously paid in January 2024. The parties also agreed to eliminate the requirement to enter into an intercompany service agreement, a technology license agreement, and a trademark license agreement and further agreed to enter into a sponsored research agreement detailing the sponsored research to be performed by Legacy NAYA and Cytovia, to be mutually agreed on a case-by-case basis. In addition, Cytovia agreed not to develop other bispecific antibodies technologies using the sequence of GPC3 and CD38 and granted to Legacy NAYA the option to sublicense or acquire and develop cell therapy therapeutics. The parties agreed to agree and discuss in good faith appropriate terms for each new indication developed for NY-303 and NY-338 and for each new modality developed for GPC3 or CD37 therapeutics.
OTHER MATTERS
As of the date of this proxy statement, we know of no matter not specifically referred to above as to which any action is expected to be taken at the annual meeting. The persons named as proxies will vote the proxies, insofar as they are not otherwise instructed, regarding such other matters and the transaction of such other business as may be properly brought before the meeting, as seems to them to be in the best interest of our company and our stockholders.
Stockholder Proposals for our 2024 Annual Meeting
While we expect that the date of the 2024 annual meeting of stockholders will change by more than 30 days from the anniversary of our 2023 annual meeting, as of the date of this proxy statement, our board of directors has not determined the date of our 2024 annual meeting. We intend to file a Current Report on Form 8-K disclosing the date of our 2024 annual meeting, and the specific deadlines for timely submission of any stockholder proposal or stockholder director nominations, following our board of directors’ determination of such date. The general qualification requirements and deadlines for stockholder proposals relating to the 2024 annual meeting are set forth below.
| 171 |
Stockholder Proposals Included in Proxy Statement
In order to be considered for inclusion in our proxy statement and proxy card relating to our 2024 annual meeting of stockholders, stockholder proposals must be submitted in accordance with the procedures in Rule 14a-18 of the Exchange Act. Since the date of the 2024 annual meeting of stockholders will change by more than 30 days from the anniversary of our 2023 annual meeting, such proposals must be received by us by a reasonable time before we begin to print and send our proxy materials. Upon receipt of any such proposal, we will determine whether or not to include such proposal in the proxy statement and proxy card in accordance with regulations governing the solicitation of proxies.
Stockholder Proposals Not Included in Proxy Statement
In addition, our by-laws establish an advance notice procedure for nominations for election to our board of directors and other matters that stockholders wish to present for action at an annual meeting other than those to be included in our proxy statement. Since the date of the 2024 annual meeting is more than 30 days before or more than 60 days after the anniversary date of the immediately preceding annual meeting, we must receive other proposals of stockholders (including director nominations) intended to be presented at the 2024 annual meeting of stockholders but not included in the proxy statement no earlier than the close of business 120 calendar days prior to such annual meeting and no later than the close of business on the later of 90 days prior to such annual meeting and 10 days following the day on which notice of the date of such annual meeting was mailed or public announcement of the date of such annual meeting was first made. Stockholders are advised to review our by-laws which also specify requirements as to the form and content of a stockholder’s notice.
In addition to satisfying the advance notice procedure in our by-laws relating to nominations of director candidates, including the earlier notice deadlines set out above, to comply with the SEC’s universal proxy rule, stockholders who intend to solicit proxies in support of director nominees other than the company’s nominees in compliance with Rule 14a-19 under the Exchange Act must also provide notice that sets forth the information required by Rule 14a-19. Since the date of the 2024 annual meeting of stockholder will change by more than 30 calendar days from the date of the annual meeting, the notice required under Rule 14a-19 under the Exchange Act must be provided by the later of 60 calendar days prior to the date of the 2024 annual meeting of stockholders or the 10th calendar day following public announcement by the company of the date of the 2024 annual meeting of stockholders.
Any proposals, notices, or information about proposed director candidates should be sent to NAYA Biosciences, Inc., Attention: Nominating and Corporate Governance Committee, 5582 Broadcast Court, Sarasota, FL 34240.
HEDGING POLICY
The Company does not permit directors, officers or employees to purchase financial instruments (including prepaid variable forward contracts, equity swaps, collars and exchange funds) that are designed to hedge or offset any decrease in the market value of Company securities.
HOUSEHOLDING OF PROXY MATERIALS
The SEC has adopted rules that permit companies and intermediaries (e.g., brokers) to satisfy the delivery requirements for annual meeting materials with respect to two or more shareholders sharing the same address by delivering a single set of annual meeting materials addressed to those shareholders. This process, which is commonly referred to as “householding,” potentially means extra convenience for shareholders and cost savings for companies.
This year, a number of brokers with account holders who are our shareholders will be “householding” the Company’s proxy materials. A single set of Annual Meeting materials will be delivered to multiple shareholders sharing an address unless contrary instructions have been received from the affected shareholders. Once you have received notice from your broker that they will be “householding” communications to your address, “householding” will continue until you are notified otherwise or until you revoke your consent. If, at any time, you no longer wish to participate in “householding” and would prefer to receive a separate set of Annual Meeting materials, please notify your broker or us. Direct your written request to NAYA Biosciences, Inc., Attn: Corporate Secretary, 5582 Broadcast Court, Sarasota, FL 34240. Shareholders who currently receive multiple copies of the Annual Meeting materials at their addresses and would like to request “householding” of their communications should contact their brokers.
| 172 |
ANNEX A
AUDITED CONSOLIDATED FINANCIAL STATEMENTS AND ACCOMPANYING NOTES OF NAYA THERAPEUTICS, INC. (FORMER NAME NAYA BIOSCIENCES, INC.)
TABLE OF CONTENTS
| A-1 |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of NAYA Biosciences, Inc.
Opinion on the Financial Statements
We have audited the accompanying balance sheets of Naya Biosciences, Inc (the Company) as of December 31, 2023 and 2022, and the related statements of operations, stockholders’ deficit, and cash flows for each of the years ended December 31, 2023 and 2022, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2023 and 2022, and the results of its operations and its cash flows for each of the years in the two-year period ended December 31, 2023 in conformity with accounting principles generally accepted in the United States of America.
Going Concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the financial statements, the Company has suffered a net loss from operations and has a net capital deficiency, which raises substantial doubt about its ability to continue as a going concern. Management’s plans regarding those matters are discussed in Note 2. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and the significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe our audits provide a reasonable basis for our opinion.
Critical Audit Matter
The critical audit matter communicated below is a matter arising from the current period audit of the financial statements that was communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of the critical audit matter does not alter in any way our opinion on the financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing separate opinion on the critical audit matter or on the accounts or disclosures to which it relates.
Going Concern
As discussed in Note 2 to the financial statements, the Company had a going concern due to a negative working capital and losses from operations.
Auditing management’s evaluation of a going concern can be a significant judgment given the fact that the Company uses management estimates on future revenues and expenses which are not able to be substantiated.
To evaluate the appropriateness of the going concern, we examined and evaluate the financial information that was the initial cause along with managements’ plans to mitigate the going concern and managements’ disclosure on going concern.
/s/ M&K CPAS, PLLC
We have served as the Company’s auditor since 2023
The Woodlands, TX
July 15, 2024
| A-2 |
NAYA THERAPEUTICS, INC. (FORMER NAME NAYA BIOSCIENCES, INC.)
BALANCE SHEETS
| Year Ended December 31, 2023 | Year Ended December 31, 2022 | |||||||
| ASSETS | ||||||||
| Current Assets | ||||||||
| Cash | $ | 89,302 | - | |||||
| Security deposit | 10,539 | - | ||||||
| Related party advance | 12,000 | - | ||||||
| Total current assets | 111,841 | - | ||||||
| Total Assets | $ | 111,841 | - | |||||
| LIABILITES AND STOCKHOLDER’S DEFICIT | ||||||||
| Current Liabilities | ||||||||
| Accounts payable and accrued liabilities | 4,563,289 | 2,296,034 | ||||||
| Note payable - related party | 6,085,000 | 6,000,000 | ||||||
| SAFE loans | 275,000 | - | ||||||
| Total current liabilities | 10,923,289 | 8,296,034 | ||||||
| Total Liabilities | $ | 10,923,289 | $ | 8,296,034 | ||||
| Commitment and Contingencies (Note 7) | ||||||||
| Stockholders’ Deficit | ||||||||
| Common stock A, par value $0.000001 per share; 50,000,000 shares authorized; 1,363,642 Common A shares issued and outstanding as of December 31, 2023 and December 31, 2022, respectively | 1 | 1 | ||||||
| Common stock B, par value $0.000001 per share; 8,000,000 shares authorized; 1,200,000 Common B Shares issued and outstanding as of December 31, 2023 and December 31, 2022, respectively | 1 | 1 | ||||||
| Additional paid-in capital | 8,207,409 | 7,444,049 | ||||||
| Accumulated deficit | (19,018,859 | ) | (15,740,085 | ) | ||||
| Total stockholders’ deficit | (10,811,448 | ) | (8,296,034 | ) | ||||
| Total Liabilities and Stockholders’ Deficit | $ | 111,841 | - | |||||
The accompanying notes are an integral part of these financial statements.
| A-3 |
NAYA THERAPEUTICS, INC. (FORMER NAME NAYA BIOSCIENCES, INC.)
STATEMENTS OF OPERATIONS
| Year Ended December 31, 2023 | Year Ended December 31, 2022 | |||||||
| Revenue: | ||||||||
| Total revenue | - | - | ||||||
| Operating expenses | ||||||||
| Selling, general and administrative expenses | $ | 2,159,626 | $ | 1,249,313 | ||||
| Research and development expenses | 975,148 | $ | 8,250,835 | |||||
| Total operating expenses | 3,134,774 | $ | 9,500,148 | |||||
| Loss from operations | (3,134,774 | ) | $ | (9,500,148 | ) | |||
| Other income (expense) | ||||||||
| Interest expense - related party | (144,000 | ) | - | |||||
| Total other income (expense) | (144,000 | ) | - | |||||
| Net loss before income taxes | (3,278,774 | ) | $ | (9,500,148 | ) | |||
| Income taxes | - | - | ||||||
| Net loss | $ | (3,278,774 | ) | $ | (9,500,148 | ) | ||
| Net loss per common share: | ||||||||
| Basic and diluted | $ | (1.28 | ) | $ | (3.71 | ) | ||
| Weighted average number of common shares outstanding: | ||||||||
| Basic and diluted | 2,563,642 | 2,563,642 | ||||||
The accompanying notes are an integral part of these financial statements.
| A-4 |
NAYA THERAPEUTICS, INC. (FORMER NAME NAYA BIOSCIENCES, INC.)
STATEMENT OF STOCKHOLDERS’ DEFICIT
| Common Stock A | Common Stock B | Additional Paid-in | Accumulated | |||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Capital | Deficit | Total | ||||||||||||||||||||||
| Balances, December 31, 2021 | 1,363,642 | $ | 1 | 1,200,000 | $ | 1 | $ | (2) | $ | (6,239,937 | ) | $ | (6,239,937 | ) | ||||||||||||||
| Contribution by parent company for liabilities assumed | - | - | - | - | 7,444,051 | - | 7,444,051 | |||||||||||||||||||||
| Net loss | - | - | - | - | - | (9,500,148 | ) | (9,500,148 | ) | |||||||||||||||||||
| Balances, December 31, 2022 | 1,363,642 | $ | 1 | 1,200,000 | $ | 1 | $ | 7,444,049 | (15,740,085 | ) | $ | (8,296,034 | ) | |||||||||||||||
| Imputed interest - related party | - | - | - | - | 144,000 | - | 144,000 | |||||||||||||||||||||
| Contribution by parent company for liabilities assumed | - | - | - | - | 619,360 | - | 619,360 | |||||||||||||||||||||
| Net loss | - | - | - | - | - | (3,278,774 | ) | (3,278,774 | ) | |||||||||||||||||||
| Balances, December 31, 2023 | 1,363,642 | $ | 1 | 1,200,000 | $ | 1 | $ | 8,207,409 | $ | (19,018,859 | ) | $ | (10,811,448 | ) | ||||||||||||||
The accompanying notes are an integral part of these financial statements.
| A-5 |
NAYA THERAPEUTICS, INC. (FORMER NAME NAYA BIOSCIENCES, INC.)
STATEMENTS OF CASH FLOWS
| Years Ended December 31, 2023 | Year Ended December 31, 2022 | |||||||
| Operating activities: | ||||||||
| Net loss | $ | (3,278,774 | ) | $ | (9,500,148 | ) | ||
| Adjustments to reconcile net loss to net cash used in in operations: | ||||||||
| Imputed interest - related party | 144,000 | - | ||||||
| Changes in operating assets and liabilities: | ||||||||
| Related party advance | (12,000 | ) | - | |||||
| Security deposits | (10,539 | ) | - | |||||
| Accounts payable and accrued liabilities | 2,886,615 | 9,500,148 | ||||||
| Net cash used in operating activities | $ | (270,698 | ) | - | ||||
| Financing activities: | ||||||||
| Proceeds from SAFE loans | 275,000 | - | ||||||
| Proceeds from note payable - related party | 85,000 | - | ||||||
| Net cash provided by financing activities | 360,000 | - | ||||||
| Net increase in cash | 89,302 | - | ||||||
| Cash at beginning of period | - | - | ||||||
| Cash at end of period | $ | 89,302 | - | |||||
| Supplemental disclosure of cash flow information: | ||||||||
| Non-cash activities: | ||||||||
| Cash paid for interest | - | - | ||||||
| Cash paid for taxes | - | - | ||||||
| Non-cash financing activities: | ||||||||
| Accounts payable and accrued expenses paid by parent company as contributions | 619,360 | 7,444,051 | ||||||
| Accounts payable converted to payable to parent | - | $ | 465,267 | |||||
The accompanying notes are an integral part of these financial statements.
| A-6 |
NAYA THERAPEUTICS, INC. (FORMER NAME NAYA BIOSCIENCES, INC.)
NOTES TO FINANCIAL STATEMENTS
December 31, 2023 and 2022
Note 1 - Description of Business
Naya Therapeutics, Inc. (former name Naya Biosciences Inc.), or NAYA, (“LEGACY NAYA” or the “Company”) is a Delaware corporation formed June 8, 2023, aiming to develop and build a group of agile, disruptive, high-growth business segments dedicated to increasing patient access to life-transforming treatments in the areas of oncology, fertility, and regenerative medicine.
LEGACY NAYA’s unique capabilities in biology, cell and gene therapy, and artificial intelligence (AI) provide a synergistic platform for the accelerated clinical development and commercialization of these breakthrough treatments.
LEGACY NAYA Oncology aims to achieve clinical proof-of-concept for its two bispecific antibodies acquired from Cytovia Therapeutics, LLC (“Cytovia”), advancing towards breakthrough outcomes for liver & ovarian cancer and multiple myeloma patients. Clinical trials are expected to start in 2024.
LEGACY NAYA Fertility is evaluating the acquisition of product device as well as network of fertility business care.
Note 2- Accounting Policies
Basis of Presentation
On October 18, 2023, LEGACY NAYA acquired two assets from Cytovia. Both companies operate under common control and are accounted for as such. The acquisition did not include any other intellectual properties from Cytovia. As such, the Company has prepared the accompanying financial statements under common control as of and for the years ended December 31, 2023, and December 31, 2022. These financial statements have been prepared in conformity with accounting principles generally accepted in the United States of America (“U.S. GAAP”). The preparation of the Company’s financial statements requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting periods (see Note 4).
In the opinion of management, the Company has made all necessary adjustments, which include normal recurring adjustments, for a fair presentation of the Company’s financial position and results of operations for the periods presented.
Certain reclassifications have been made to prior periods’ data to conform to the current period presentation. These reclassifications had no effect on income (losses) or cash flows.
| A-7 |
Use of Estimates
The preparation of financial statements in conformity with U.S. GAAP required management to make estimates and assumptions that affect the amounts reported in the financial statements. Actual results could differ from those estimates.
Cash and Cash Equivalents
For financial statement presentation purposes, the Company considers time deposits, certificates of deposit and all highly liquid investments with original maturities of three months or less to be cash and cash equivalents. At times, cash and cash equivalents balances exceed amounts insured by the Federal Deposit Insurance Corporation.
Income Taxes
The Company is subject to income taxes in the United States and its domestic tax liabilities are subject to the allocation of expenses in multiple state jurisdictions. The Company uses the asset and liability method to account for income taxes. Under this method, deferred income tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The recoverability of deferred tax assets is evaluated by assessing the adequacy of future expected taxable income from all sources, including taxable income in prior carryback years, reversal of taxable temporary differences, forecasted operating earnings and available tax planning strategies. To the extent the Company does not consider it more-likely-than-not that a deferred tax asset will be recovered, a valuation allowance is established.
Net Loss Per Common Share
Basic loss per share is computed by dividing loss available to common stockholders by the weighted average number of shares of common stock outstanding during the period. Diluted loss per common share is computed using the treasury stock method on the basis of the weighted-average number of shares of common stock plus the dilutive effect of potential shares of common stock outstanding during the period. Dilutive potential shares of common stock include outstanding stock options and restricted shares. The computation of diluted loss per share does not assume conversion, exercise or contingent exercise of securities that would have an anti-dilutive effect on earnings.
Research and Development
Research and development costs are expensed when incurred.
Financial Instruments
The carrying values of current assets and other current liabilities approximate their fair values due to their short-term nature.
| A-8 |
New Accounting Pronouncements
In August 2020, the FASB issued ASU 2020-06, “Debt – Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging – Contracts in Entity’s Own Equity (Subtopic 815-40)” (“ASU 2020-06”). ASU2020-06 simplifies the accounting for certain financial instruments with characteristics of liabilities and equity, including convertible instruments and contracts on an entity’s own equity. The ASU is part of the FASB’s simplification initiative, which aims to reduce unnecessary complexity in U.S. GAAP. The ASU’s amendments are effective for fiscal years beginning after December 15, 2024, and interim periods within those fiscal years. The Company will adopt the provisions of ASU 2020-06 on January 1, 2024, which are not expected to have a material impact on the Company’s financial statements.
In December 2023, the FASB issued ASU No. 2023-09 entitled “Income Taxes (Topic 740): Improvements to Income Tax Disclosures”. This ASU provides guidance related to additional disclosures that will be required related to income taxes. The updated guidance is effective for public entities for fiscal years beginning after December 15, 2024. This ASU is expected to result in additional disclosures in the Company’s financial statements beginning in the year ended December 31, 2025.
Note 3 – Liquidity and Going Concern
Although the Company’s audited financial statements for the years ended December 31, 2023 and December 31, 2022 were prepared under the assumption that it would continue its operations as a going concern, the report of our independent registered public accounting firm that accompanies the financial statements for the years ended December 31, 2023 and December 31, 2022 contains a going concern qualification in which such firm expressed substantial doubt in the Company’s ability to continue as a going concern without additional capital from becoming available, based on the financial statements at that time. Specifically, as noted above, the Company has incurred operating losses since its inception, and the Company expects to continue to incur significant expenses and operating losses for the foreseeable future. These prior losses and expected future losses have had, and will continue to have, an adverse effect on the Company’s financial condition and negatively impact its ability to fund continued operations, obtain additional financing in the future and continue as a going concern. There are no assurances that such financing, if necessary, will be available at all or will be available in sufficient amounts or on reasonable terms. Our financial statements do not include any adjustments that may result from the outcome of this uncertainty. If we are unable to generate additional funds in the future through licensing of the assets, creating revenue streams through upfront and milestone payments, royalties, and commercial milestone payments or funding the Company through equity financing, the Company will be unable to continue operations.
Note 4- Carve-out Accounting
These financial statements are being prepared based on the acquisition of assets as disclosed in note 5. These are the historical results based on common control accounting.
Indirect research & development costs were allocated based on research and development full-time equivalent approach. The full-time employees, who worked on the projects have been assigned on a weekly basis to work a defined percentage of their time per project. The assigned time per week per project then was summarized on a monthly basis. The monthly assigned time per employee per project finally defined the average allocation per project CYT303 and CYT338 for salaries and indirect research & development expenses. The allocated expenses related to CYT303 and CYT338 represent 32% and 36% of Cytovia’s expenses for the years 2022 and 2023 respectively.
| A-9 |
Selling, general and administrative expenses were allocated based on percentage of full-time equivalent approach of general and administrative personnel. Allocated percentages are 12% and 10% for the years 2022 and 2023 respectively.
The cost for the rent for the research & development lab has been allocated with the same principle as described above to the two projects CYT303 and CYT338.
Management believes the assumptions underlying the carve-out combined financial statements, including the assumptions regarding allocation of expenses, are reasonable.
For the portion of the year ended December 31, 2023 following the date LEGACY NAYA was established in June 2023, the financial statements reflect LEGACY NAYA as a stand-alone entity.
Note 5 - Asset Purchase Agreement
On October 18, 2023 LEGACY NAYA, Cytovia Therapeutics Holdings, Inc., a Delaware corporation (“Holdings”) and Cytovia (“Cytovia”; and together with Holdings, the “Sellers”) entered into an Asset Purchase Agreement (the “Asset Purchase Agreement”). The aggregate purchase price for the purchased assets shall be comprised of the (A) closing payments (as provided below), (B) assumption of the certain liabilities totaling $2,688,745
Closing payments will comprise $50,000,000 in common shares of LEGACY NAYA at the closing of the Merger (as such term is defined in note 7) with each share of common stock of LEGACY NAYA to be valued at $36.6665 per share resulting in the issuance of 1,363,642 shares of common stock of LEGACY NAYA which is expected to be converted into shares of common stock of the combined company at the closing of the Merger (as such term is defined in Note 7) (the “Combined Company”) reflecting a value of $5.00 per share of the Combined Company, equaling to 10,000,000 shares: a. $37,500,000 for CYT303 equaling to 7,500,000 shares of the Combined Company; b. $12,500,000 for CYT338 equaling to 2,500,000 shares of the Combined Company.
In addition, on October 20, 2023 LEGACY NAYA issued a Promissory Note to Cytovia (the “Note”) in the principal amount of $6,000,000 for CYT303 of which $750,000 will be paid at the closing of any financing, and $750,000 per month for 5 months and for CYT338: $250,000 will be paid at the will be paid at the closing of any financing and $250,000 per month for 5 months. If LEGACY NAYA or the Combined Company raises more than $30,000,000 in the financing, the full amount of $6,000,000 will be due at the final closing of the financing.
As of March 31, 2024, the outstanding amount per the Note is $4,300,000, as the first payments of $1,000,000, $500,000 and $200,000 were made on January 3, 2024, January 4, 2024, and January 11, 2024 respectively totaling $1,700,000.
See note 11 for amendment of the Asset Purchase Agreement.
| A-10 |
Note 6 -Stockholders Equity
Common Stock
On October 18, 2023, the Company issued 1,363,642 shares of Common A Shares par value 0.000001 to Cytovia, a related party, pursuant to the Asset Purchase Agreement (see Note 5)
On October 13, 2023, the Company issued 1,200,000 of Common B Shares par value 0.000001. These are classified as founders’ shares issued and are shown from the start of the presentation due to common control accounting.
Class A Common Stock and Class B Common Stock shall at all times vote together as one class on all matters (including the election of directors) submitted to a vote or for the consent of the stockholders of the Company. Each holder of shares of Class A Common Stock shall be entitled to one (1) vote for each share of Class A Common Stock held as of the applicable date on any matter that is submitted to a vote or for the consent of the stockholders of the Company. Each holder of shares of Class B Common Stock shall be entitled to ten (10) votes for each share of Class B Common Stock held as of the applicable date on any matter that is submitted to a vote or for the consent of the stockholders of the Company. The number of authorized shares of Common Stock may be increased or decreased (but not below the number of shares thereof then outstanding) by the affirmative vote of the holders of shares of capital stock of the Company representing a majority of the votes represented by all outstanding shares of capital stock of the Company entitled to vote, irrespective of the provisions of Section 242(b)(2) of the General Corporation Law.
Note 7 - Commitment and Contingencies
LEGACY NAYA Merger Agreement
On October 22, 2023, LEGACY NAYA and INVO Merger Sub. Inc., a Nevada corporation (“Merger Sub”), a wholly owned subsidiary of Naya Biosciences, Inc. (former name INVO Bioscience, Inc.), or “LEGACY INVO”, entered into an agreement and plan of merger, as amended on October 25, 2023 (the “Merger Agreement”).
Upon the terms and subject to the conditions set forth in the Merger Agreement, Merger Sub will merge (the “Merger”) with and into LEGACY NAYA, with LEGACY NAYA continuing as the surviving corporation and a wholly owned subsidiary of the Company.
At the effective time and as a result of the Merger, each share of Class A common stock, par value $0.000001 per share, of LEGACY NAYA (the “LEGACY NAYA common stock”) outstanding immediately prior to the effective time of the Merger, other than certain excluded shares held by LEGACY NAYA as treasury stock or owned by the Company or LEGACY INVO, will be converted into the right to receive 7.33333 (subject to adjustment as set forth in the Merger Agreement) shares of a newly designated series of common stock, par value $0.000001 per share, of the Company which shall be entitled to ten (10) votes per each share (“Company Class B common stock”) for a total of approximately 8,150,000 shares of the Company.
Immediately following the effective time of the Merger, Dr. Daniel Teper, LEGACY NAYA’s current chairman and chief executive officer, will be named chairman and chief executive officer of the Combined Company, and the board of directors will be comprised of up to nine (9) directors, of which (i) one shall be Steven Shum, LEGACY INVO’s current chief executive officer, and (ii) eight shall be identified by LEGACY NAYA, of which seven (7) shall be independent directors.
| A-11 |
Pursuant to the original Merger Agreement, the completion of the Merger is subject to satisfaction or waiver of certain customary mutual closing conditions, including (1) the adoption of the Merger Agreement by the stockholders of the Company and LEGACY INVO, (2) the absence of any injunction or other order issued by a court of competent jurisdiction or applicable law or legal prohibition prohibiting or making illegal the consummation of the Merger, (3) the completion of due diligence, (4) the completion of an interim private offering of shares of LEGACY INVO common stock at a price that is a premium to the market price of the LEGACY INVO common stock in an estimated amount of $5,000,000 or more of gross proceeds (the “Interim PIPE”), (5) the completion of a sale of shares of common stock at a target price of $5.00 per share in a private offering resulting in sufficient cash available for Parent for one year of operations, as estimated by LEGACY INVO (6) the aggregate of the liabilities of LEGACY INVO, excluding certain specified liabilities, shall not exceed $5,000,000, (7) the receipt of waivers from any and all holders of warrants (and any other similar instruments) to securities of LEGACY INVO, with respect to any fundamental transaction rights such warrant holders may have under any such warrants, (8) the continued listing of LEGACY INVO common stock on NASDAQ through the effective time of the Merger and the approval for listing on NASDAQ of the shares of LEGACY INVO common stock to be issued in connection with the Merger, the interim private offering, and a private offering of shares of LEGACY INVO common stock at a target price of $5.00 per share (subject to appropriate adjustment in the event of any stock dividend, stock split, combination or other similar recapitalization with respect to the Company common stock) resulting in sufficient cash available for LEGACY INVO for one year of operations, as estimated by LEGACY NAYA, (9) the effectiveness of a registration statement on Form S-4 to be filed by LEGACY INVO pursuant to which the shares of LEGACY INVO’s common stock to be issued in connection with the Merger will be registered with the SEC, and the absence of any stop order suspending such effectiveness or proceeding for the purpose of suspending such effectiveness being pending before or threatened by the SEC, and (10) LEGACY INVO shall have received customary lock-up Agreement from certain of its stockholders. The obligation of each party to consummate the Merger is also conditioned upon (1) the other party having performed in all material respects its obligations under the Merger Agreement and (2) the other party’s representations and warranties in the Merger Agreement being true and correct (subject to certain materiality qualifiers); provided, however, that these conditions, other than with respects to certain representations and warranties, will be deemed waived by LEGACY INVO upon the closing of the interim private offering.
The Merger Agreement contains termination rights for LEGACY INVO and LEGACY NAYA, including, among others: (1) if the consummation of the Merger does not occur on or before December 31, 2023 (the “End Date”) (which has since been extended to April 30, 2024 in a second amendment and then to June 30, 2024 in the third amendment), except that any party whose material breach of the Merger Agreement caused or was the primary contributing factor that resulted in the failure of the Merger to be consummated on or before the End Date, (2) if any governmental authority has enacted any law or order making illegal, permanently enjoining, or otherwise permanently prohibiting the consummation of the Merger, and (3) if the required vote of the stockholders of either the Company or LEGACY NAYA has not been obtained. The Merger Agreement contains additional termination rights for LEGACY NAYA, including, among others: (1) if LEGACY INVO materially breaches its non-solicitation obligations or fails to take all action necessary to hold a stockholder meeting to approve the transactions contemplated by the Merger Agreement, (2) if the aggregate of the liabilities of LEGACY INVO, excluding certain specified liabilities, exceed $5,000,000, (3) if LEGACY NAYA determines that the due diligence contingency will not be satisfied by October 26, 2023, (4) if LEGACY NAYA determines that the Company has experienced a material adverse effect, or (5) LEGACY INVO material breaches any representation, warranty, covenant, or agreement such that the conditions to closing would not be satisfied and such breach is incapable of being cured, unless such breach is caused by LEGACY NAYA’s failure to perform or comply with any of the covenants, agreements, or conditions hereof to be performed or complied with by it prior to the closing.
| A-12 |
If all of LEGACY NAYA’s conditions to closing are satisfied or waived and LEGACY NAYA fails to consummate the Merger, LEGACY NAYA would be required to pay the LEGACY INVO a termination fee of $1,000,000. If all of the Company’s conditions to closing conditions are satisfied or waived and the LEGACY INVO fails to consummate the Merger, LEGACY INVO would be required to pay LEGACY NAYA a termination fee of $1,000,000.
On December 27, 2023, LEGACY NAYA entered into second amendment (“Second Amendment”) to the Merger Agreement. Pursuant to the Second Amendment, the parties agreed to extend the End Date to April 30, 2024. The parties further agreed to modify the closing condition for the Interim PIPE from a private offering of shares of LEGACY INVO common stock at a price that is a premium to the market price of the LEGACY INVO common stock in an estimated amount of $5,000,000 or more of gross proceeds to a private offering of the LEGACY INVO’s preferred stock at a price per share of $5.00 per share in an amount equal to at least $2,000,000 to the LEGACY INVO, plus an additional amount as may be required prior to closing of the Merger to be determined in good faith by the parties to adequately support the LEGACY INVO’s fertility business activities per an agreed forecast, as well as for a period of twelve (12) months post- closing including a catch-up on the LEGACY INVO’s past due accrued payables still outstanding. The parties further agreed to the following schedule (the “Minimum Interim Pipe Schedule”) for the initial $2,000,000: (1) $500,000 no later than December 29, 2023, (2) $500,000 no later than January 19, 2024, (3) $500,000 no later than February 2, 2024, and (4) $500,000 no later than February 16, 2024. The parties also further agreed to modify the covenant of the parties regarding the Interim PIPE to require LEGACY NAYA to consummate the Interim PIPE before the closing of the Merger; provided, however, if the LEGACY INVO does not receive the initial gross proceeds pursuant to the Minimum Interim Pipe Schedule, the LEGACY INVO shall be free to secure funding from third parties to make up for short falls on reasonable terms under SEC and Nasdaq regulations. See Note 11.
Lease
On October 4, 2023, and November 16, 2023, the Company entered into a lease for its corporate headquarters for a twelve-month term as a result they were deemed to be short-term leases. The leases did not include a renewal or purchase option, and thus considered as short-term lease in accordance with ASC 842. The Company elected not to apply the recognition requirements of ASC 842 to short-term leases. Accordingly, the Company instead recognizes lease payments on short-term leases in the period in which the obligation for those payments is incurred. The monthly payments are $6,266.25 and $4,008.75 respectively.
Litigation
LEGACY NAYA is not currently subject to any material legal proceedings; however, it could be subject to legal proceedings and claims from time to time in the ordinary course of its business, or legal proceedings it considered immaterial may in the future become material. Regardless of the outcome, litigation can, among other things, be time consuming and expensive to resolve, and can divert management resources.
Stock Payable
During the fourth quarter of 2023, the Company entered into a media advertising agreement under which the consultant is being paid cash of $250,000 and shares worth $600,000. At December 31, 2023, the shares had not yet been issued and are included in accounts payable and accrued liabilities. See Note 11.
| A-13 |
Note 8 - Related Party
As discussed in Note 4, LEGACY NAYA is, a carve out of Cytovia that remain under common control.
As discussed in Note 5, $6,000,000 of accounts payable that was converted to debt for past expenses incurred by Cytovia is included in the consideration for the two assets purchased from Cytovia: CYT303 and CY338. No gain or loss associated with the conversion of Accounts payable to debt was recorded.
LEGACY NAYA and Cytovia, entered into a loan agreement on August 1, 2023, according to which Cytovia, shall make available to LEGACY NAYA, a term loan of up to $1,000,000 bearing interest rate of 5% per annum. As of December 31, 2023, included in the notes payable – related party is $85,000 due to Cytovia. See Note 9..
An advance to an officer of the Company was made for $12,000 to cover business and travel expenses owed.
Note 9- Debt
Simple Agreement for Future Equity (SAFE)
As of December 31, 2023, LEGACY NAYA has a total of $275,000, simple agreements for equity (“SAFE”) issued to investors giving the right to certain shares of the LEGACY NAYA’s capital stock, subject to the terms as follows: (a) an equity financing before the termination of the SAFE, on the initial closing of such equity financing, the SAFE will automatically convert into the number of shares of safe preferred stock equal to the purchase amount of the SAFE divided by the discount price. The SAFE agreements convert as follows: 1) $80,000 of the SAFE agreement converts into the number of shares of safe preferred stock equal to the purchase amount divided by the lowest price per share of the standard preferred stock sold in the equity financing multiplied by 40% and 2) $195,000 of the SAFE agreement converts into the number of shares of SAFE preferred stock equal to the purchase amount divided by the lowest price per share of the standard preferred stock sold in the equity financing multiplied by 30%.
If there is a liquidity event before the termination of the SAFE, the investor of the SAFE will automatically be entitled to receive a portion of proceeds, due and payable to the investor of the SAFE immediately prior to, or concurrent with, the consummation of such liquidity event, equal to the greater of (i) the purchase amount or (ii) the amount payable on the number of shares of common stock equal to the purchase amount divided by the liquidity price. If any of the Company’s securityholders are given a choice as to the form and amount of proceeds to be received in a liquidity event, the investor will be given the same choice, provided that the investor may not choose to receive a form of consideration that the investor would be ineligible to receive as a result of the investor’s failure to satisfy any requirement or limitation generally applicable to the Company’s securityholders, or under any applicable laws.
Cytovia Notes
LEGACY NAYA and Cytovia, entered into a loan agreement on August 1, 2023, where the Cytovia, shall make available to LEGACY NAYA, a term loan up to $1,000,000 bearing interest rate of 5% per annum. As of December 31, 2023, the outstanding amount for this loan due to Cytovia included in the short-term loan on the balance sheet is $85,000.
| A-14 |
In addition, a Note of $6,000,000 was outstanding as of December 31, 2023 and 2022 see further details disclosed in Note 5. Due to the note having no stated interest rate, interest has been imputed at 12% and is included in additional paid-in capital as a contribution to equity.
NOTE 10 – Income Tax
The Company follows the asset and liability method of accounting for income taxes under ASC 740, “Income Taxes.” Deferred tax assets and liabilities are recognized for the estimated future tax consequences attributable to differences between the financial statements carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that included the enactment date. Valuation allowances are established, when necessary, to reduce deferred tax assets to the amount expected to be realized.
ASC 740 prescribes a recognition threshold and a measurement attribute for the financial statement recognition and measurement of tax positions taken or expected to be taken in a tax return. For those benefits to be recognized, a tax position must be more likely than not to be sustained upon examination by taxing authorities. The Company recognizes accrued interest and penalties related to unrecognized tax benefits as income tax expense. There were no unrecognized tax benefits and no amounts accrued for interest and penalties as of December 31, 2023. The Company is currently not aware of any issues under review that could result in significant payments, accruals or material deviation from its position. The Company is subject to income tax examinations by major taxing authorities since inception. The Company used the separate return method for the preparation of the income tax provision.
For the years ended December 31, 2023 and 2022, there was no income tax provision recorded. The tax benefit was added to the net operating loss to which a full valuation allowance was applied.
The Company has a net operating loss from formation till December 31, 2023 was approximately $1.6 million and the deferred tax asset associated with it was approximately $0.3 million and is fully allowed.
Utilization of NOL and tax credit carryforwards may be subject to a substantial annual limitation due to ownership change limitations that may have occurred or that could occur in the future, as required by the Internal Revenue Code (the “Code”), as amended, as well as similar state provisions. In general, an “ownership change” as defined by the Code results from a transaction or series of transactions over a three-year period resulting in an ownership change of more than 50 percent of the outstanding stock of a company by certain stockholders or public groups.
Note 11 - Subsequent Events
SPA and Debenture Agreement with Five Narrow Lane
On January 3, 2024, LEGACY NAYA entered into a Securities Purchase Agreement (“SPA”), pursuant to which it sold a senior secured debenture (the “Debenture”) in the principal amount of $3,000,000 (the “Principal Amount”), to an accredited investor (the “Investor”). The Debenture accrues interest at 7% per annum on the outstanding principal amount, throughout the term of the Debenture, which has a maturity date of the earlier of (i) April 30, 2024, and (ii) the date of the completion of the Merger under the Merger Agreement. Interest on the Debenture is due monthly on the first business day of every month. LEGACY NAYA may redeem the Debenture upon 10 days’ notice to the Investor. Upon any repayment, LEGACY NAYA will also pay an exit fee equal to 100% of the principal amount of the Debenture.
| A-15 |
In addition, the Investor will receive 42,618 shares of common stock (subject to adjustment for any stock split, stock dividend, reverse stock split or similar event after the date hereof) to be issued to the Investor, upon consummation of the Merger, will be exchanged into such number of shares of LEGACY INVO common stock representing 1.5% of the fully diluted outstanding capital of LEGACY INVO, after giving effect to the consummation of the Merger, but prior to the issuance of shares in the pre-financing capital. In the event of default, 100% of the outstanding principal amount is due as well as 200% of the outstanding principal amount of the Debenture for a total of $9,000,000.
On May 13, 2024, LEGACY NAYA amended the SPA. LEGACY NAYA promised to pay to Five Narrow Lane LP or its registered assigns (the “Holder”), or shall have paid pursuant to the terms thereunder, the principal sum of $9,075,833 on the earlier to occur of the Merger and June 28, 2024 (such earlier date, the “Maturity Date”) or such earlier date as the Debenture is required or permitted to be repaid as provided hereunder, and to pay interest to the Holder on the aggregate unconverted and then outstanding principal amount of the Debenture in accordance with the provisions hereof. Upon the occurrence of an event of default, the outstanding principal amount of the debenture, plus accrued but unpaid interest, and other amounts owing in respect thereof through the date of acceleration, shall become, at the Holder’s election, immediately due and payable in cash. In addition, the interest on the debenture shall accrue at an interest rate equal to the lesser of 15% per annum or the maximum rate permitted under applicable law.
In addition, on May 13, 2024, LEGACY NAYA entered into an additional debenture agreement (“May 2024 Debenture”) with Five Narrow Lane LP. LEGACY NAYA promised to pay to the Holder, or shall have paid pursuant to the terms thereunder, the principal sum of $1,000,000 (or, if lower, the outstanding principal amount of this Debenture as such amount may be modified pursuant to the terms hereof) on Maturity Date or such earlier date as this Debenture is required or permitted to be repaid as provided hereunder, and to pay interest to the Holder on the aggregate unconverted and then outstanding principal amount of this May 2024 Debenture in accordance with the provisions hereof. As of the filing, $700,000 has been received from the debenture. Upon any repayment or redemption by the Company of all or any of the principal amount of the May 2024 Debenture (whether on or prior to the Maturity Date, pursuant to an Optional Redemption, upon acceleration or otherwise), the Company shall pay to the Holder concurrently such repayment or redemption an exit fee in an amount equal to 100% of principal amount of this Debenture being repaid or redeemed. In the event of default, 200% of the outstanding principal is due in addition to the principal.
Simple Agreement for Future Equity (SAFE)
On January 1, 2024, $18,000 of additional SAFE loans were issued by the Company.
GreenBlock Promissory Note
On April 4, 2024, LEGACY NAYA entered into a promissory note (the “GB Note”) of $250,000. LEGACY NAYA promised to pay to the order of GreenBlock Capital, LLC, or its registered assigns, upon the terms set forth below, the principal sum of $500,000. The full amount of principal (including the original issue discount) under the GB Note shall be due on the earlier of: (i) closing of the Merger; or (ii) the six-month anniversary of the date of the GB Note or (iii) the occurrence of an event of default. Commencing five calendar days after the occurrence of any event of default that results in the acceleration of the GB Note, interest on the GB Note shall accrue at the rate of 18% per annum, or such lower maximum amount of interest permitted to be charged under applicable law.
| A-16 |
GreenBlock Consulting Agreement
On April 5, 2024, LEGACY NAYA engaged GreenBlock Capital, LLC, (“Consultant”) to serve as strategic advisor to the Company with respect to the following services: a) advise and assist management throughout the reverse merger process and beyond, including working with the Company’s bankers, lawyers, accounting team, advisors and auditors which make up the deal team, b) advise and assist management on proper corporate governance and other public company “best practices” post-closing of any merger transaction along with other deal team functions as reasonably requested by the Company’s CEO or Board.
For the services provided above over the following 12 months, the Consultant shall receive an issuance of 125,000 shares of Class B common stock of the Company. The shares are issuable within two days of signing.
LEGACY NAYA Merger Agreement, Securities Purchase Agreement
On December 29, 2023, the Company entered into a securities purchase agreement (the “Series A Preferred SPA”) with LEGACY INVO for LEGACY NAYA’s purchase of 1,000,000 shares of the Company’s Series A Preferred Stock at a purchase price of $5.00 per share. The parties agreed that LEGACY NAYA’s purchases will be made in tranches in accordance with the Minimum Interim Pipe Schedule. The Series A Preferred SPA contains customary representations, warranties and covenants of the Company and LEGACY INVO. On January 4, 2024, the Company and LEGACY INVO closed on 100,000 shares of Series A Preferred Stock in the first tranche of this private offering for gross proceeds of $500,000. In addition, the Company and LEGACY NAYA agreed that an additional amount of $96,704 comprised of expenses paid by LEGACY NAYA on behalf of LEGACY INVO would result in an additional 19,340 shares issued.
From April 1, 2024, to April 30, 2024, LEGACY NAYA acquired 25,000 shares of LEGACY INVO Series A preferred stock for gross proceeds of $125,000 per the merger agreement. In addition, expenses totaling $84,700 were paid on behalf of LEGACY INVO and added to the investment for which 16,940 additional shares were issued.
Effective as of May 1, 2024, the Company entered into third amendment (“Third Amendment”) to the Merger Agreement. Pursuant to the Third Amendment, the parties agreed to extend the end date (the date by which either party may terminate the merger agreement) to June 30, 2024. The parties further agreed to modify the definition of an “Interim PIPE”.
Effective as of May 1, 2024, the Company and LEGACY INVO also entered into an Amendment (the “SPA Amendment”) to the Series A Preferred SPA. Pursuant to the SPA Amendment, the parties agreed to an updated closing schedule for LEGACY NAYA’s purchases of LEGACY INVO’s Series A preferred stock at a purchase price of $5.00 per share.
From May 1, 2024, to June 24, 2024, LEGACY NAYA acquired 140,000 shares of LEGACY INVO Series A preferred stock for gross proceeds of $700,000. As of the date of this filing, LEGACY NAYA has purchased a total of 301,280 Series A preferred shares of LEGACY INVO at a cost of $1,506,400.
| A-17 |
Loan Agreement with Cytovia
On May 15, 2024, LEGACY NAYA entered into a loan agreement with Cytovia, under which LEGACY NAYA will make available to Cytovia a term loan of up to $8,000,000 (the “Loan”). Principal amounts outstanding under the Loan shall bear interest at the rate of 7% per annum. Cytovia shall make quarterly payments of accrued interest on the outstanding principal balance of the Loan on the last day of each quarter (the “Interest Payment Date”) starting January 1, 2025, until the Loan is repaid in full. Each payment shall be in the amount of the accrued, but unpaid, interest through the date immediately preceding the date such payment is due.
In addition to quarterly payments of interest, commencing as of June 30, 2026, Cytovia shall make quarterly principal payments on each interest payment date based upon a five year amortization schedule.
On June 17, 2024, LEGACY NAYA Loan Agreement with Cytovia dated August 1, 2023, was amended to state that all principal and interest outstanding under the Loan shall be due and payable in full on the date LEGACY NAYA receives its next funding.
As of the filing date, there were no amounts outstanding under the Loan.
Amendment of Asset Purchase Agreement with Cytovia
On May 17, 2024, LEGACY NAYA amended the Asset Purchase Agreement with Cytovia as follows:
The aggregate purchase price for the purchased assets shall be an amount up to US $60,700,000 (the “Purchase Price”).
Closing payments to Cytovia are comprised of the following:
(a) $59,000,000 (the “Common Stock Consideration”) in common shares of LEGACY NAYA and with each share of common stock of LEGACY NAYA to be valued at $36.6665 per share resulting in the issuance of 1,609,098 shares of common stock of LEGACY NAYA. Each share of LEGACY NAYA is expected to be converted into 7.33333 shares of the Combined Company at the closing of the Merger reflecting a value of $5.00 per share of the Combined Company, to be adjusted for reverse split and/or other merger adjustments
(b) A payment of $1,700,000, which was made by LEGACY NAYA in January 2024.
The amended agreement also provides for sublicense fees to Cytovia at 10% of any gross consideration actually received by LEGACY NAYA (i) as a fee for sublicensing or selling CYT303 or CYT338 in any indications to any third party or (ii) as payments for development milestones, commercial milestones, or royalties or any other payments under the terms of any such sublicense or asset purchase agreement.
Stock Payable
During the fourth quarter of 2023, the Company entered into a media advertising agreement under which the consultant was paid cash of $200,000 and promised shares worth $600,000. On June 24, 2024, the agreement was amended to suspend the last cash payment of $50,000 and to replace the shares with 25,000 restricted stock units of the Company.
| A-18 |
ANNEX B
NAYA THERAPEUTICS, INC. (FORMER NAME NAYA BIOSCIENCES, INC.)
FOR THE NINE MONTH PERIOD ENDED SEPTEMBER 30, 2024
TABLE OF CONTENTS
(UNAUDITED)
| BALANCE SHEETS | B-2 |
| STATEMENTS OF OPERATIONS | B-3 |
| STATEMENTS OF STOCKHOLDERS’ DEFICIT | B-4 |
| STATEMENTS OF CASH FLOWS | B-5 |
| NOTES TO FINANCIAL STATEMENTS | B-6 |
| B-1 |
NAYA THERAPEUTICS, INC. (FORMER NAME NAYA BIOSCIENCES, INC.)
BALANCE SHEETS
| September 30, 2024 | December 31, 2023 | |||||||
| (Unaudited) | (Audited) | |||||||
| ASSETS | ||||||||
| Current Assets | ||||||||
| Cash | $ | 756,461 | $ | 89,302 | ||||
| Investments | 778,113 | - | ||||||
| Prepaid expenses | 33,247 | - | ||||||
| Advance - related party | - | 12,000 | ||||||
| Security deposit | - | 10,539 | ||||||
| Total current assets | 1,567,821 | 111,841 | ||||||
| Total Assets | $ | 1,567,821 | $ | 111,841 | ||||
| LIABILITIES AND STOCKHOLDER’S DEFICIT | ||||||||
| Current Liabilities: | ||||||||
| Accounts payable | $ | 1,631,318 | $ | 3,498,724 | ||||
| Accrued payroll | 1,213,242 | 274,246 | ||||||
| Other accrued liabilities | 383,118 | 790,318 | ||||||
| Notes payable - related party | 290,000 | 6,085,000 | ||||||
| SAFE notes | 38,000 | 275,000 | ||||||
| Notes payable, net of debt discount | 12,523,074 | - | ||||||
| Total current liabilities | 16,078,752 | 10,923,289 | ||||||
| Total Liabilities | $ | 16,078,752 | $ | 10,923,289 | ||||
| Commitment and Contingencies | ||||||||
| Stockholders’ Deficit | ||||||||
| Series A Preferred Stock, par value $0.000001 per share; 50,000 shares authorized; 0 issued and outstanding as of September 30, 2024 and December 31, 2023, respectively | - | - | ||||||
| Class A Common Stock, par value $0.000001 per share; 50,000,000 shares authorized; 1,756,432 and 1,363,642 shares issued and outstanding as of September 30, 2024, and December 31, 2023, respectively | 1 | 1 | ||||||
| Class B Common Stock, par value $0.000001 per share; 8,000,000 shares authorized; 1,367,618 and 1,200,000 shares issued and outstanding as of September 30, 2024, and December 31, 2023, respectively | 1 | 1 | ||||||
| Preferred stock payable | 750,000 | - | ||||||
| Additional paid-in capital | 16,052,218 | 8,207,409 | ||||||
| Accumulated deficit | (31,313,151 | ) | (19,018,859 | ) | ||||
| Total Stockholders’ Deficit | (14,510,931 | ) | (10,811,448 | ) | ||||
| Total Liabilities and Stockholders’ Deficit | $ | 1,567,821 | $ | 111,841 | ||||
The accompanying notes are an integral part of these financial statements.
| B-2 |
NAYA THERAPEUTICS, INC. (FORMER NAME NAYA BIOSCIENCES, INC.)
STATEMENTS OF OPERATIONS
(UNAUDITED)
| For the Nine Months Ended September 30, 2024 | For the Nine Months Ended September 30, 2023 | |||||||
| Revenue: | ||||||||
| Total revenue | - | - | ||||||
| Operating expenses | ||||||||
| Selling, general and administrative expenses | $ | 2,976,074 | $ | 407,790 | ||||
| Research and development expenses | 176,104 | 939,519 | ||||||
| Total operating expenses | 3,152,078 | 1,347,309 | ||||||
| Loss from operations | (3,152,178 | ) | (1,347,309 | ) | ||||
| Other income (expense) | ||||||||
| Other income | 600,000 | - | ||||||
| Loss on debt extinguishment and penalty | (3,750,000 | ) | - | |||||
| Change in fair value of investment | (865,791 | ) | - | |||||
| Interest income (expense) – related party | (211,270 | ) | 1,035 | |||||
| Interest expense – other | (4,915,053 | ) | - | |||||
| Other income (expense), net | (9,142,114 | ) | - | |||||
| Net loss before income taxes | (12,294,292 | ) | (1,346,274 | ) | ||||
| Income taxes | - | - | ||||||
| Net loss | $ | (12,294,292 | ) | $ | (1,346,274 | ) | ||
| Net loss per common share: | ||||||||
| Basic and diluted | $ | (4.33 | ) | $ | (0.53 | ) | ||
| Weighted average number of common shares outstanding: | ||||||||
| Basic and diluted | 2,841,359 | 2,563,642 | ||||||
The accompanying notes are an integral part of these financial statements.
| B-3 |
NAYA THERAPEUTICS, INC. (FORMER NAME NAYA BIOSCIENCES, INC.)
STATEMENT OF STOCKHOLDERS’ DEFICIT
(UNAUDITED)
| Series A Preferred Stock | Common Stock A | Common Stock B | Preferred Stock | Additional Paid-in | Accumulated | |||||||||||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Shares | Amount | Payable | Capital | Deficit | Total | |||||||||||||||||||||||||||||||
| Balances, December 31, 2022 | - | - | 1,363,642 | 1 | 1,200,000 | 1 | $ | 7,444,049 | $ | (15,740,085 | ) | $ | (8,296,034 | ) | ||||||||||||||||||||||||||
| Contribution by parent company for liabilities assumed | (392,711 | ) | (392,711 | ) | ||||||||||||||||||||||||||||||||||||
| Net loss | 1,012,070 | (1,346,274 | ) | (334,204 | ) | |||||||||||||||||||||||||||||||||||
| Balances, September 30, 2023 | - | - | 1,363,642 | 1 | 1,200,000 | 1 | $ | 8,063,408 | $ | (17,086,359 | ) | $ | (9,022,949 | ) | ||||||||||||||||||||||||||
| Balances, December 31, 2023 | - | - | 1,363,642 | 1 | 1,200,000 | 1 | $ | 8,207,409 | $ | (19,018,859 | ) | $ | (10,811,448 | ) | ||||||||||||||||||||||||||
| Imputed interest - related party | 200,200 | 200,200 | ||||||||||||||||||||||||||||||||||||||
| Common stock issued with debenture | 42,618 | 64,353 | 64,353 | |||||||||||||||||||||||||||||||||||||
| Stock based compensation | 25,000 | 125,000 | 324,511 | 324,511 | ||||||||||||||||||||||||||||||||||||
| Amendment of related party | ||||||||||||||||||||||||||||||||||||||||
| Asset Purchase Agreement | 245,456 | 6,988,745 | 6,988,745 | |||||||||||||||||||||||||||||||||||||
| Preferred stock payable | 750,000 | 750,000 | ||||||||||||||||||||||||||||||||||||||
| Conversion of SAFE notes | 122,334 | 267,000 | 267,000 | |||||||||||||||||||||||||||||||||||||
| Net loss | (12,294,292 | ) | (12,294,292 | ) | ||||||||||||||||||||||||||||||||||||
| Balances, September 30, 2024 | - | - | 1,756,432 | 1 | 1,367,618 | 1 | 750,000 | $ | 16,052,218 | $ | (31,313,151 | ) | (14,510,931 | ) | ||||||||||||||||||||||||||
The accompanying notes are an integral part of these financial statements.
| B-4 |
NAYA THERAPEUTICS, INC. (FORMER NAME NAYA BIOSCIENCES, INC.)
STATEMENTS OF CASH FLOWS
(UNAUDITED)
| For the Nine Months Ended September 30, 2024 | For the Nine Months Ended September 30, 2023 | |||||||
| Operating activities: | ||||||||
| Net loss | $ | (12,294,292 | ) | $ | (1,346,274 | ) | ||
| Adjustments to reconcile net loss to net cash used in operations: | ||||||||
| Imputed interest - related party | 200,200 | - | ||||||
| Stock based compensation | 324,511 | - | ||||||
| Amortization of debt discount | 4,353,797 | - | ||||||
| Loss on extinguishment of debt and penalty | 3,750,000 | - | ||||||
| Change in fair value of investment | 865,791 | - | ||||||
| Other income | (600,000 | ) | - | |||||
| Changes in operating assets and liabilities: | ||||||||
| Prepaid expenses and deposits | (22,708 | ) | - | |||||
| Advance – related party | 12,000 | (174,531 | ) | |||||
| Accounts payable and accrued liabilities | 2,500,664 | 1,333,917 | ||||||
| Net cash used in operating activities | (910,037 | ) | (186,888 | ) | ||||
| Investing activities: | ||||||||
| Investment in shares | (1,643,904 | ) | - | |||||
| Net cash used in investing activities | (1,643,904 | ) | - | |||||
| Financing activities: | ||||||||
| Net proceeds from loans | 3,936,100 | - | ||||||
| Proceeds - related party loans | 205,000 | - | ||||||
| Proceeds –preferred shares | 750,000 | |||||||
| Note repayment - related party | (1,700,000 | ) | - | |||||
| Proceeds from SAFE notes | 30,000 | 195,000 | ||||||
| Net cash provided from financing activities | 3,221,100 | 195,000 | ||||||
| Net increase in cash | 667,159 | 8,112 | ||||||
| Cash at beginning of period | 89,302 | - | ||||||
| Cash at end of period | $ | 756,451 | $ | 8,112 | ||||
| Supplemental disclosure of cash flow information: | ||||||||
| Non-cash activities: | ||||||||
| Cash paid for interest | - | |||||||
| Cash paid for taxes | - | - | ||||||
| Non-cash financing activities: | ||||||||
| Accounts payable and accrued expenses paid by parent company as contributions | - | $ | 619,360 | |||||
| Amendment of Asset Purchase Agreement with related party – reduction of accounts payable and accrued liabilities | $ | 6,988,745 | - | |||||
| Commitment shares on loan | $ | 64,353 | - | |||||
| Conversion of SAFE notes to equity | $ | 267,000 | - | |||||
The accompanying notes are an integral part of these financial statements.
| B-5 |
NAYA THERAPEUTICS, INC. (FORMER NAME NAYA BIOSCIENCES, INC.)
NOTES TO FINANCIAL STATEMENTS
(Unaudited)
September 30, 2024
| 1. | Description of Business |
Naya Therapeutics, Inc. (former name Naya Biosciences, Inc.) (“LEGACY NAYA” or the “Company”) is a Delaware corporation formed on June 8, 2023, aiming to develop and build a group of agile, disruptive, high-growth companies dedicated to increasing patient access to life-transforming treatments in oncology and fertility.
LEGACY NAYA’s unique capabilities in biology, cell and gene therapy, and artificial intelligence (AI) provide a synergistic platform for the accelerated clinical development and commercialization of these breakthrough treatments.
LEGACY NAYA Oncology aims to achieve clinical proof-of-concept for its two bispecific antibodies acquired from Cytovia Therapeutics, LLC (“Cytovia”), advancing towards breakthrough outcomes for liver and ovarian cancer and multiple myeloma patients. Clinical trials are expected to start by 2025.
LEGACY NAYA Fertility is evaluating the acquisition of products, devices as well as a network of fertility care businesses.
| 2. | Accounting policies |
Basis of Presentation
On October 18, 2023, LEGACY NAYA acquired two assets from Cytovia. Both companies operate under common control and are accounted for as such. The acquisition did not include any other intellectual properties from Cytovia. As such, the Company has prepared the accompanying financial statements under common control as of and for the nine months ended September 30, 2024 and 2023. The interim financial statements of the Company have been prepared in conformity with accounting principles generally accepted in the United States of America (“U.S. GAAP”). See Note 4.
In the opinion of management, the Company has made all necessary adjustments, which include normal recurring adjustments, for a fair presentation of the Company’s financial position and results of operations for the interim periods presented. Certain information and disclosures normally included in the annual financial statements prepared in accordance with U.S. GAAP have been condensed or omitted. The results for the nine months ended September 30, 2024 and 2023, are not necessarily indicative of the results to be expected for a full year, any other interim periods or any future year or period.
| B-6 |
Use of Estimates
The preparation of financial statements in conformity with U.S. GAAP required management to make estimates and assumptions that affect the amounts reported in the financial statements. Actual results could differ from those estimates.
Cash and Cash Equivalents
For financial statement presentation purposes, the Company considers time deposits, certificates of deposit and all highly liquid investments with original maturities of three months or less to be cash and cash equivalents. At times, cash and cash equivalents balances exceed amounts insured by the Federal Deposit Insurance Corporation.
Investments
Investments include an investment in the Series A preferred shares of NAYA Biosciences, Inc. (former name INVO Bioscience, Inc.) (“LEGACY INVO”) pursuant to a securities purchase agreement. The preferred shares will be marketable upon conversion into publicly traded common shares and therefore are being accounted for as marketable securities at the closing market price on the balance sheet date. See Notes 8 and 10.
Income Taxes
The Company is subject to income taxes in the United States and its domestic tax liabilities are subject to the allocation of expenses in multiple state jurisdictions. The Company uses the asset and liability method to account for income taxes. Under this method, deferred income tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The recoverability of deferred tax assets is evaluated by assessing the adequacy of future expected taxable income from all sources, including taxable income in prior carryback years, reversal of taxable temporary differences, forecasted operating earnings and available tax planning strategies. To the extent the Company does not consider it more-likely-than-not that a deferred tax asset will be recovered, a valuation allowance is established.
Commitment Shares
The Company accounts for commitment shares as either equity-classified or liability-classified instruments based on an assessment of the commitment shares specific terms and applicable authoritative guidance in ASC 480 “Distinguishing Liabilities From Equity” (“ASC 480”) and ASC 815 “Derivatives and Hedging” (“ASC 815”). The assessment considers whether the commitment shares are freestanding financial instruments pursuant to ASC 480, meet the definition of a liability pursuant to ASC 480, and whether the commitment shares meet all of the requirements for equity classification under ASC 815, including whether the shares are indexed to the Company’s own ordinary shares and whether the commitment share holder could potentially require “net cash settlement” in a circumstance outside of the Company’s control, among other conditions for equity classification. This assessment, which requires the use of professional judgment, is conducted at the time of share issuance and as of each subsequent quarterly period end date.
As the commitment shares issued upon the closing of the Company’s January 3, 2024 debenture meet the criteria for equity classification under ASC 815, the commitment shares are classified as equity.
| B-7 |
Research and Development
Research and development costs are expensed when incurred.
Financial Instruments
The carrying values of current assets and current liabilities approximate their fair values due to their short-term nature.
Net Loss Per Common Share
Basic loss per share is computed by dividing loss available to common stockholders by the weighted average number of common shares outstanding during the period. Diluted loss per common share is computed using the treasury stock method on the basis of the weighted-average number of shares of common stock plus the dilutive effect of potential common shares outstanding during the period. Dilutive potential common shares include 40,926 outstanding stock options and 190,926 unvested restricted share units as of September 30, 2024 and 0 at September 30, 2023. The computation of diluted loss per share does not assume conversion, exercise or contingent exercise of securities as the effect would have been anti-dilutive on earnings.
Stock Based Compensation
The Company accounts for stock-based compensation under the provisions of Accounting Standards Codification (“ASC”) subtopic 718-10, Compensation (“ASC 718-10”). This statement requires the Company to measure the cost of employee services received in exchange for an award of equity instruments based on the grant-date fair value of the award. That cost is recognized over the period in which the employee is required to provide service or based on performance goals in exchange for the award, which is usually the vesting period. Under these accounting standards, the fair value of stock options is estimated on the date of grant using the Black-Scholes option pricing model. Forfeitures are recorded in the period in which they occur.
Recent Accounting Pronouncements
In August 2020, the FASB issued ASU 2020-06, “Debt – Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging – Contracts in Entity’s Own Equity (Subtopic 815-40)” (“ASU 2020-06”). ASU2020-06 simplifies the accounting for certain financial instruments with characteristics of liabilities and equity, including convertible instruments and contracts on an entity’s own equity. The ASU is part of the FASB’s simplification initiative, which aims to reduce unnecessary complexity in U.S. GAAP. The ASU’s amendments are effective for fiscal years beginning after December 15, 2024, and interim periods within those fiscal years. The Company adopted the provisions of ASU 2020-06 on January 1, 2024. This did not have a material impact on the Company’s financial statements.
In December 2023, the FASB issued ASU No. 2023-09 entitled “Income Taxes (Topic 740): Improvements to Income Tax Disclosures”. This ASU provides guidance related to additional disclosures that will be required related to income taxes. The updated guidance is effective for public entities for fiscal years beginning after December 15, 2024. This ASU is expected to result in additional disclosures in the Company’s financial statements related to income taxes in 2025.
| B-8 |
| 3. | Liquidity and Going Concern |
Historically, the Company has funded its cash and liquidity needs primarily through debt and equity financings. For the nine months ended September 30, 2024, the Company incurred a net loss of approximately $12.3 million and has an accumulated deficit of approximately $31.3 million as of September 30, 2024. The prior losses and expected future losses have had, and will continue to have, an adverse effect on our financial condition and negatively impact our ability to fund continued operations, obtain additional financing in the future and continue as a going concern. There are no assurances that such financing, if necessary, will be available to us at all or will be available in sufficient amounts or on reasonable terms. Our financial statements do not include any adjustments that may result from the outcome of this uncertainty. If we are unable to generate additional funds in the future through licensing of the assets, creating revenue streams through upfront and milestone payments, royalties, and commercial milestone payments: or funding the Company through equity financing, the Company will be unable to continue operations.
Although the Company’s audited financial statements for the year ended December 31, 2023 were prepared under the assumption that it would continue operations as a going concern, the report of the Company’s independent registered public accounting firm that accompanies the Company’s financial statements for the year ended December 31, 2023 contains a going concern qualification in which such firm expressed substantial doubt about the Company’s ability to continue as a going concern, based on the financial statements at that time. Specifically, as noted above, the Company has incurred significant operating losses, and the Company expects to continue to incur significant expenses and operating losses.
| 4. | Carve-out Accounting |
These financial statements are being prepared based on the acquisition of assets as disclosed in Note 5. These are the historical results based on common control accounting.
The results reflect that LEGACY NAYA was a stand-alone entity starting in July 2023, separate from the original common control entity for which the carve out accounting was required.
Indirect research & development costs were allocated based on percentage of research and development full-time equivalent approach. The full-time employees, who worked on the projects have been assigned on a weekly basis to work a defined percentage of their time per project. The assigned time per week per project then was summarized on a monthly basis. The monthly assigned time per employee per project finally defined the average allocation per project CYT303 and CYT338 for salaries and indirect research & development expenses. The allocated expenses related to CYT303 and CYT338 represent 0% and 36% of Cytovia’s expenses for the six months ended June 30, 2024 and 2023, respectively, after which LEGACY NAYA was a stand-alone entity.
Selling, general and administrative expenses were allocated based on percentage of full-time equivalent approach. Allocated percentage is 0% and 10% for the six months ended June 30, 2024 and 2023, respectively, after which LEGACY NAYA was a stand-alone entity.
The cost for the rent for the research & development lab has been allocated with the same principle as described above to the two projects CYT303 and CYT338.
| B-9 |
| 5. | Asset Purchase Agreement |
On October 18, 2023, LEGACY NAYA, Cytovia Therapeutics Holdings, Inc., a Delaware corporation (“Holdings”) and Cytovia entered into and closed an Asset Purchase Agreement (the “Asset Purchase Agreement”) for projects CYT303 and the CYT338. The aggregate purchase price was comprised of closing payments and the assumption of the certain liabilities totaling $2,688,745.
Closing payments were comprised of the issuance of 1,363,642 shares of common stock of LEGACY NAYA to Cytovia and a promissory note to Cytovia in the principal amount of $6,000,000, of which $1,000,000 will be paid at the closing of any financing, and $1,000,000 per month for 5 months; also if LEGACY NAYA or the Combined Company (as defined) raises more than $30,000,000 in a financing, the full amount of $6,000,000 will be due at the closing of the financing.
Payments totaling $1,700,000 were made to Cytovia in January 2024. On May 17, 2024, LEGACY NAYA and Cytovia amended the Asset Purchase Agreement. The amended aggregate purchase price was comprised of the following:
(a) The issuance of 1,609,098 shares of common stock of LEGACY NAYA.
(b) A payment of $1,700,000, which was made by LEGACY NAYA in January 2024.
The amended agreement also provides for sublicense fees to be paid to Cytovia at 10% of any gross consideration actually received by LEGACY NAYA (i) as a fee for sublicensing or selling CYT303 or CYT338 in any indications to any third party or (ii) as payments for development milestones, commercial milestones, or royalties or any other payments under the terms of any such sublicense or asset purchase agreement. The acquisition agreement also includes for no further consideration a sublicense agreement between LEGACY NAYA and Cytovia, under which Cytovia granted LEGACY NAYA a non-exclusive sublicense under Cytovia rights in PCT/IB2012/053482 (P-627002-PC) (the “Licensed Technology”), or certain technology jointly owned by Dr. Jean Kadouche and CNRS (The French Center for National Scientific Research) and co-exclusively licensed to Cytovia, for the development and commercialization of CYT303 (now NY303) and CYT338 (now NY338). LEGACY NAYA has extended the non-exclusive license to the development of additional multi-functional antibodies for which LEGACY NAYA will make payments upon achievement of certain milestones. The agreement terminates upon the expiration of the patent rights to the Licensed Technology.
These financial statements are being prepared with historical results based on common control accounting. As a result of the amendment to the Asset Purchase Agreement, the remaining note payable of $4,300,000 and accrued liabilities of $2,688,745 were reclassified to additional paid-in capital and the number of shares issued to Cytovia was increased to 1,609,098 shares of Class A Common Stock.
| 6. | Debt |
Debt consisted of the following:
| September 30, 2024 | December 31, 2023 | |||||||
| Related party note payable | $ | 290,000 | $ | 6,085,000 | ||||
| Simple Agreement for Future Equity (SAFE) | 38,000 | 275,000 | ||||||
| Five Narrow Lane debentures | 11,997,530 | - | ||||||
| Accredited investor note | 500,000 | - | ||||||
| Other | 31,100 | - | ||||||
| Less debt discount, accredited investor note | (5,556 | ) | - | |||||
| Total, net of discount | $ | 12,851,074 | $ | 6,360,000 | ||||
| B-10 |
Accrued interest of $547,530 on the Five Narrow Lane debentures was capitalized and is included in the above loan balance at September 30, 2024. Accrued interest of $11,070 for the related party and $13,726 for Five Narrow Lane, respectively, are included in accounts payable and accrued liabilities at September 30, 2024.
Simple Agreement for Future Equity (SAFE Notes)
During 2023, the Company received funding from five investors under simple agreements for future equity (“SAFE notes”) for a total of $275,000, giving the investors the right upon conversion to shares of LEGACY NAYA’s common stock at discounts ranging from 30%-40% from the price of an equity financing or a liquidity event. The SAFE notes provide the investor certain rights upon an equity financing, liquidity event, change in control or dissolution. The SAFE notes have no interest rate or maturity date.
During the nine months ended September 30, 2024, the Company entered into two new SAFE notes for a total of $30,000. During the same period, the Company converted SAFE notes with a value of $267,000 into 122,333 shares of common stock. As of September 30, 2024, SAFE notes with a value of $38,000 remained outstanding.
The SAFE notes were classified as a liability based on an evaluation of the characteristics of the instrument pursuant to ASC 480 as certain redemptions are based upon the occurrence of events that are outside of the control of the Company. The SAFE notes are stated at their fair value which approximates cost.
See Note 10 for subsequent conversions.
Five Narrow Lane LP Securities Purchase Agreement and Debentures
On January 3, 2024, LEGACY NAYA entered into a Securities Purchase Agreement (“SPA”), pursuant to which it sold a senior secured debenture (the “Debenture”) in the principal amount of $3,000,000 to Five Narrow Lane, LP (the “Investor”). The Debenture accrued interest at 7% per annum on the outstanding principal amount throughout the term of the Debenture, which had a maturity date of the earlier of (i) April 30, 2024, and (ii) the date of the completion of the Merger under the Merger Agreement. Interest on the Debenture is due monthly on the first business day of every month. LEGACY NAYA may redeem the Debenture upon 10 days’ notice to the Investor. Upon any repayment, LEGACY NAYA will also pay an exit fee equal to 100% of the principal amount of the Debenture, which has been recorded as an additional $3,000,000 of principal and accounted for as an original issue discount. In the event of default, 100% of the outstanding principal amount is due as well as 200% of the outstanding principal amount of the Debenture for a total of $9,000,000.
In addition, the Investor received 42,618 commitment shares of common stock, which upon consummation of the Merger, will be exchanged into such number of shares of LEGACY INVO common stock representing 1.5% of the fully diluted outstanding capital of LEGACY INVO, after giving effect to the consummation of the Merger, but prior to the issuance of shares in the pre-Merger financing. The commitment shares were issued in January 2024 and were recorded at $1.51 per share fair value based on a valuation of LEGACY NAYA as of December 31, 2023.
| B-11 |
On May 13, 2024, LEGACY NAYA amended the SPA and promised to pay the Investor the principal sum of $9,075,833 (including $75,833 of capitalized interest) on the earlier to occur of the Merger and June 28, 2024. Upon the occurrence of an event of default, the outstanding principal amount of the debenture, plus accrued but unpaid interest, and other amounts owing in respect thereof through the date of acceleration, shall become, at the Holder’s election, immediately due and payable in cash. In addition, the late interest on the Debenture shall accrue at an interest rate equal to the lesser of 15% per annum or the maximum rate permitted under applicable law. On the date of modification, the Company determined that the present value of the cash flows of the modified debt instrument was greater than 10% different from the present value of the remaining cash flows under the original debt instrument and therefore the Company recorded a loss on extinguishment of $3,000,000, representing the default penalty on the original note.
On May 13, 2024, LEGACY NAYA entered into an additional debenture agreement with the Investor for the principal sum of up to $1,000,000 with a maturity date of the earlier of June 28, 2024 or the Trigger Date, as defined, and interest payable at 7%. In May and June 2024, a total of $700,000 was received under the debenture. Upon any repayment or redemption by the Company of all or any of the principal amount of this debenture (whether on or prior to the maturity date, pursuant to an optional redemption, upon acceleration or otherwise), the Company shall pay to the Investor an exit fee in an amount equal to 100% of the principal amount of the debenture, which has been recorded as an additional $700,000 of principal and accounted for as an original issue discount. In the event of default, 200% of the outstanding principal is due in addition to the principal. The Company was in default as of the June 28, 2024 maturity date and therefore incurred a default penalty of $700,000.
Effective September 12, 2024, the Company entered into the following agreements with Five Narrow Lane (“Investor”): an Omnibus Amendment and Agreement, a Senior Secured Convertible Debenture and a Registration Rights Agreement. The Company and the Investor agreed to, among other things:
| ● | Exchange the May 2024 Debentures for a Senior Secured Convertible Debenture due the earlier of the Trigger Date (as defined) and October 14, 2024 in the aggregate principal amount of $11,734,979.48. Following the consummation of the Merger, the portion of the debenture constituting $3,659,146.15 would be exchanged for a Senior Secured Convertible Debenture issued by LEGACY INVO (“LEGACY INVO Debenture”). The portion of this debenture constituting $8,075,833.33 would be converted into shares of Series C Convertible Preferred Stock of LEGACY INVO (the “LEGACY INVO Preferred Stock”). | |
| ● | If any event of default occurs, the outstanding principal amount of this debenture, plus accrued but unpaid interest, and other amounts owing in respect thereof through the date of acceleration, shall become, at the Investor’s election, immediately due and payable in cash. Upon the occurrence of an event of default, the interest rate on this debenture shall accrue at an interest rate equal to the lesser of 15% per annum or the maximum rate permitted under applicable law. | |
| ● | The Company and the Investor agreed to enter into a registration rights agreement pursuant to which the Company agreed to file a resale registration statement with the Securities and Exchange Commission which shall cover the shares of common stock of LEGACY INVO issuable upon the conversion of (i) the LEGACY INVO Debenture and (ii) the shares of LEGACY INVO Preferred Stock. | |
| ● | Following the consummation of the Merger, the Investor would invest $500,000, which shall be repaid using the gross proceeds of a public offering and such principal amount of the LEGACY INVO Debenture shall be increased accordingly. | |
| ● | As consideration for the Investor entering into this agreement, the Investor agreed to fund the $275,000 remaining from the May Debenture. |
| B-12 |
| ● | Provided that the total amount of proceeds raised in a public offering equals or exceeds $5,000,000, the Investor shall purchase $4,000,000, subject to certain terms. | |
| ● | 150,000 shares of the Company’s Common Stock are to be issued to the Investor, representing approximately 5.0% of the fully diluted outstanding capital of LEGACY INVO, after giving effect to the consummation of the Merger, but prior to the issuance of shares in a financing. | |
| ● | Extend the maturity date to the earlier of (a) October 14, 2024, and (b) the date of the consummation of the Merger or the date of termination of the Merger Agreement. |
See Note 10 for subsequent loan amendment.
Cytovia Notes
LEGACY NAYA and Cytovia, entered into a loan agreement on August 1, 2023, pursuant to which Cytovia shall make available to LEGACY NAYA a term loan for up to $1,000,000, bearing interest at a rate of 5% per annum. On June 17, 2024, the loan agreement was amended to state that all principal and interest outstanding under the Loan shall be due and payable in full on the date LEGACY NAYA receives its next funding. As of September 30, 2024, the outstanding amount due to Cytovia was $290,000.
Per the Asset Purchase Agreement, a closing payment of $6,000,000 was part of the consideration for the two assets purchased from Cytovia. A total of $1,700,000 was paid during January 2024. In May 2024, the Asset Purchase Agreement was amended – see Note 5. Due to the note having no stated interest rate, interest on the balance of the note has been imputed at 12% and $200,200 was included in additional paid-in capital as a contribution to equity.
On May 15, 2024, LEGACY NAYA entered into a loan agreement with Cytovia, under which LEGACY NAYA will make available to Cytovia a term loan of up to $8,000,000. Principal amounts outstanding shall bear interest at the rate of 7% per annum. Cytovia shall make quarterly payments of accrued interest starting January 1, 2025, until the loan is repaid in full. In addition to quarterly payments of interest, commencing as of June 30, 2026, Cytovia shall make quarterly principal payments on each interest payment date based upon a five year amortization schedule. As of September 30, 2024, there were no amounts outstanding under this loan.
Accredited Investor Note
On April 4, 2024, the Company received proceeds of $250,000 and entered into a promissory note with an accredited investor for the principal sum of $500,000. The full amount of principal (including the original issue discount) under the note shall be due on the earlier of: (i) closing of the Merger; or (ii) the six-month anniversary of the date of the note or (iii) the occurrence of an event of default. Commencing five calendar days after the occurrence of any event of default that results in the acceleration of the note, interest shall accrue at the rate of 18% per annum, or such lower maximum amount of interest permitted to be charged under applicable law. See Note 10.
| B-13 |
| 7. | Stockholder’s Equity |
Pursuant to the Amended and Restated Certificate of Incorporation of the Company filed on December 14, 2023, the total number of shares of all classes of capital stock which the Company shall have authority to issue is 58,500,000 shares, consisting of: (i) 50,000,000 shares of Class A Common Stock, $0.000001 par value per share (“Class A Common Stock”), (ii) 8,000,000 shares of Class B Common Stock, $0.000001 par value per share (“Class B Common Stock”), and (iii) 500,000 shares of Preferred Stock, $0.000001 par value per share (“Preferred Stock”), of which 50,000 of the authorized shares of Preferred Stock of the Company are designated Series A Preferred Stock.
Preferred Stock
The Board is expressly authorized, subject to any limitations prescribed by the laws of the State of Delaware, by resolution or resolutions adopted from time to time, to provide for the issuance of shares of Preferred Stock in one or more series and by filing a certificate of designation pursuant to the applicable laws of the State of Delaware to establish from time to time the number of shares of Preferred Stock to be included in each such series, to fix the designation, vesting, powers (including voting powers), preferences and relative, participating, optional or other special rights (and the qualifications, limitations or restrictions thereof) of the shares of Preferred Stock of each such series and to increase (but not above the total number of authorized shares of Preferred Stock) or decrease (but not below the number of shares of Preferred Stock of such series then outstanding) the number of shares of Preferred Stock of any such series.
On September 20, 2024, the Company entered into a Securities Purchase Agreement (SPA) and Preferred Stock Purchase Warrant with an accredited investor (“Investor”) under which the Investor intended to purchase preferred shares and warrants for an aggregate purchase price of $750,000. On September 30, 2024, an advance payment of $750,000 was received toward the purchase of the preferred shares and warrants pursuant to the SPA, and is included as Preferred Stock Payable in the accompanying balance sheet.
Common Stock
On October 13, 2023, the Company issued 1,200,000 shares of Class B Common Stock. These shares are considered founders’ shares.
On October 17, 2023, the Company entered into a media advertising agreement under which the consultant was being paid cash of $250,000 and shares worth $600,000 to be settled in cash or shares at a later date with the share price not yet determined. On June 24, 2024, the agreement was amended to suspend the last cash payment of $50,000 and to replace the shares with 25,000 fully vested RSUs of the Company. The shares payable liability that had been recorded in the fourth quarter of 2023 for $600,000 was reversed and the amount is included in Other Income. The $37,750 fair value the 25,000 RSUs is included in Selling, General and Administrative expenses.
On October 18, 2023, the Company issued 1,363,642 shares of Class A Common Stock to Cytovia, a related party, pursuant to the Asset Purchase Agreement. On May 17, 2024, the Asset Purchase Agreement was amended and the number of shares issued to Cytovia was increased to 1,609,098 shares of Class A Common Stock. See Note 5.
On February 8, 2024, the Company issued 42,618 shares of Class B Common Stock to Five Narrow Lane LP. See Note 6.
On April 5, 2024, the Company engaged a consultant to serve as strategic advisor. For the services to be provided over the following 12 months, the consultant received 125,000 shares of Class B Common Stock. The fair value of $188,750 on the date of the consulting agreement is included in Selling, General and Administrative expenses.
| B-14 |
During August 2024, a total of 122,334 shares of Class A common stock were issued upon conversion of SAFE notes. See note 6.
Class A Common Stock and Class B Common Stock shall at all times vote together as one class on all matters (including the election of directors) submitted to a vote or for the consent of the stockholders of the Company. Each holder of shares of Class A Common Stock shall be entitled to one vote and each holder of shares of Class B Common Stock shall be entitled to ten votes for each share held as of the applicable date on any matter that is submitted to a vote or for the consent of the stockholders of the Company. The number of authorized shares of Common Stock may be increased or decreased (but not below the number of shares thereof then outstanding) by the affirmative vote of the holders of shares of capital stock of the Company representing a majority of the votes represented by all outstanding shares of capital stock of the Company entitled to vote, irrespective of the provisions of Section 242(b)(2) of the General Corporation Law. Except as otherwise provided in the Certificate of Incorporation or required by applicable law, shares of Class A Common Stock and Class B Common Stock shall have the same rights and powers, rank equally (including as to dividends and distributions, and upon any liquidation, dissolution or winding up of the corporation), share ratably and be identical in all respects and as to all matters.
Stock-based Compensation
Pursuant to the Company’s Global Equity Incentive Plan (2024) effective January 1, 2024, the Company is authorized to issue 618,692 restricted stock units, stock options or shares to employees, directors, advisory board members and consultants.
Stock option activity for the nine months ended September 30, 2024 is as follows:
| Number of | Weighted Average | |||||||
| Options | Exercise Price | |||||||
| Outstanding, December 31, 2023 | - | - | ||||||
| Granted | 40,926 | $ | 2.00 | |||||
| Exercised | - | - | ||||||
| Forfeited | - | - | ||||||
| Outstanding, September 30, 2024 | 40,926 | 2.00 | ||||||
| Exercisable, September 30, 2024 | 40,926 | $ | 2.00 | |||||
On January 1, 2024, a total of 40,926 options with a term of 10 years were granted to certain Directors of the Company to purchase 40,926 shares of common stock with a vesting schedule of 100% on the first anniversary. Immediately after the Merger (as such term is defined in Note 7) all options convert with the factor 7.33333, subject to adjustment set forth in the Amended and Restated Merger Agreement (see Notes 8 and 10). The amount recorded as the fair value of these options to purchase shares was based on the Black-Scholes model with a fair value of $1.08 per option totaling $44,200 compensation expense. An expense of $21,919 was recorded for the nine months ended September 30, 2024. The fair value of each stock option was estimated using the Black Scholes pricing model, which takes into account as of the grant date: the exercise price ($2.00), the expected life of the stock option (5.5 years), the current value per share $1.51 of the underlying stock, the expected volatility (91.5%), expected dividends (0%) and the risk-free interest rate (5%).
| B-15 |
Restricted stock unit (RSU) activity for the nine months ended September 30, 2024 is as follows:
| Number of | Weighted Average Grant | |||||||
| Unvested Shares | Date Fair Value | |||||||
| Balance as of December 31, 2023 | - | $ | - | |||||
| Granted | 245,496 | 1.51 | ||||||
| Vested | - | - | ||||||
| Forfeited | (54,570 | ) | 1.51 | |||||
| Balance as of September 30, 2024 | 190,926 | $ | 1.51 | |||||
On January 1, 2024, 40,926 Restricted Stock Units (“RSUs”) were granted to certain Directors of the Company, with 100% vesting one year following the grant date. Immediately after the Merger, all RSUs will convert with the factor 7.33333, subject to adjustment set forth in the Amended and Restated Merger Agreement. The fair value of these RSUs was determined based on a valuation as of December 31, 2023, with a fair value of $1.51 per share totaling $61,798 compensation expense, with $30,647 recorded for the nine months ended September 30, 2024.
On January 1, 2024, 40,928 RSUs were granted to an officer of the Company with a vesting schedule of 25% after one year from the grant date, and the remaining 75% of the shares shall continue to vest monthly for a period of two years thereafter. In addition, 13,642 RSUs were granted to the officer of the Company on January 1, 2024, with 100% vesting a year from the grant date. Immediately after the Merger all RSUs convert with the factor 7.33333, subject to adjustment set forth in the Amended and Restated Merger Agreement. The officer left the Company on June 27, 2024 and all of the RSUs were forfeited. Therefore, no compensation expense has been recorded for this grant for the nine months ended September 30, 2024.
During the period July to September 2024, a total of 125,000 RSUs were granted to consultants with vesting periods ranging from 6 to 18 months. Total compensation expense is $188,750, of which $18,875 has been recognized.
Stock compensation expense totaling $324,511 and $0 for the nine months ended September 30, 2024, respectively, is included in the statements of operations within selling, general and administrative expenses. Forfeitures are recognized as they occur. Total unrecognized stock compensation as of September 30, 2024 is $185,535 for RSUs and $11,201 for stock options.
| B-16 |
| 8. | Commitment and Contingencies |
LEGACY NAYA Merger Agreement
On October 22, 2023, LEGACY NAYA and INVO Merger Sub. Inc., a Nevada corporation (“Merger Sub”), a wholly owned subsidiary of LEGACY INVO, entered into an agreement and plan of merger, as amended on October 25, 2023 (the “Merger Agreement”).
Upon the terms and subject to the conditions set forth in the Merger Agreement, Merger Sub would merge (the “Merger”) with and into LEGACY NAYA, with LEGACY NAYA continuing as the surviving corporation and a wholly owned subsidiary of the Company. At the effective time and as a result of the Merger, each share of Class A common stock, par value $0.000001 per share, of LEGACY NAYA outstanding immediately prior to the effective time of the Merger, other than certain excluded shares held by LEGACY NAYA as treasury stock or owned by the Company or LEGACY INVO, would be converted into the right to receive 7.33333 (subject to adjustment as set forth in the Merger Agreement) shares of a newly designated series of common stock, par value $0.000001 per share, of the Company would shall be entitled to ten votes per each share. Immediately following the effective time of the Merger, Dr. Daniel Teper, LEGACY NAYA’s current chairman and chief executive officer, would be named chairman and chief executive officer of the combined company, and the board of directors will be comprised of up to nine directors, of which (i) one would be Steven Shum, LEGACY INVO’s current chief executive officer, and (ii) eight would be identified by LEGACY NAYA, of which seven would be independent directors.
Pursuant to the original Merger Agreement, the completion of the Merger was subject to satisfaction or waiver of certain customary and other mutual closing conditions, including (1) the adoption of the Merger Agreement by the stockholders of the Company and LEGACY INVO, (2) the absence of any injunction or other order issued by a court of competent jurisdiction or applicable law or legal prohibition prohibiting or making illegal the consummation of the Merger, (3) the completion of due diligence, (4) the completion of an interim private offering of shares of LEGACY INVO common stock at a price that is a premium to the market price of the LEGACY INVO common stock in an estimated amount of $5,000,000 or more of gross proceeds (the “Interim PIPE”), (5) the completion of a sale of shares of common stock at a target price of $5.00 per share in a private offering resulting in sufficient cash available for LEGACY INVO for one year of operations, as estimated by LEGACY INVO (6) the aggregate of the liabilities of LEGACY INVO, excluding certain specified liabilities, shall not exceed $5,000,000, (7) the receipt of waivers from any and all holders of warrants (and any other similar instruments) to securities of LEGACY INVO, with respect to any fundamental transaction rights such warrant holders may have under any such warrants, (8) the continued listing of LEGACY INVO common stock on NASDAQ through the effective time of the Merger and the approval for listing on NASDAQ of the shares of LEGACY INVO common stock to be issued in connection with the Merger, the interim private offering, and a private offering of shares of LEGACY INVO common stock at a target price of $5.00 per share resulting in sufficient cash available for LEGACY INVO for one year of operations, as estimated by LEGACY NAYA, (9) the effectiveness of a registration statement on Form S-4 to be filed by LEGACY INVO pursuant to which the shares of LEGACY INVO’s common stock to be issued in connection with the Merger will be registered with the SEC, and the absence of any stop order suspending such effectiveness or proceeding for the purpose of suspending such effectiveness being pending before or threatened by the SEC, and (10) LEGACY INVO shall have received customary lock-up Agreement from certain of its stockholders.
| B-17 |
The Merger Agreement contained termination rights for LEGACY INVO and LEGACY NAYA, including, among others: (1) if the consummation of the Merger does not occur on or before December 31, 2023 (the “End Date”) (which was since been extended to April 30, 2024 in a second amendment, then to June 30, 2024 in the third amendment, and then to October 14, 2024 in the fourth amendment), except that any party whose material breach of the Merger Agreement caused or was the primary contributing factor that resulted in the failure of the Merger to be consummated on or before the End Date, (2) if any governmental authority has enacted any law or order making illegal, permanently enjoining, or otherwise permanently prohibiting the consummation of the Merger, and (3) if the required vote of the stockholders of either the Company or LEGACY NAYA has not been obtained. The Merger Agreement contains additional termination rights for LEGACY NAYA, including, among others: (1) if LEGACY INVO materially breaches its non-solicitation obligations or fails to take all action necessary to hold a stockholder meeting to approve the transactions contemplated by the Merger Agreement, (2) if the aggregate of the liabilities of LEGACY INVO, excluding certain specified liabilities, exceed $5,000,000, (3) if LEGACY NAYA determines that the due diligence contingency will not be satisfied by October 26, 2023, (4) if LEGACY NAYA determines that the Company has experienced a material adverse effect, or (5) LEGACY INVO material breaches any representation, warranty, covenant, or agreement such that the conditions to closing would not be satisfied and such breach is incapable of being cured, unless such breach is caused by LEGACY NAYA’s failure to perform or comply with any of the covenants, agreements, or conditions hereof to be performed or complied with by it prior to the closing.
If all of LEGACY NAYA’s conditions to closing were satisfied or waived and LEGACY NAYA fails to consummate the Merger, LEGACY NAYA would be required to pay the LEGACY INVO a termination fee of $1,000,000. If all of LEGACY NAYA’s conditions to closing were satisfied or waived and LEGACY INVO fails to consummate the Merger, LEGACY INVO would be required to pay LEGACY NAYA a termination fee of $1,000,000.
On December 27, 2023, LEGACY NAYA entered into second amendment (“Second Amendment”) to the Merger Agreement. Pursuant to the Second Amendment, the parties agreed to extend the End Date to April 30, 2024. The parties further agreed to modify the closing condition for the Interim PIPE from a private offering of shares of LEGACY INVO common stock at a price that is a premium to the market price of the LEGACY INVO common stock in an estimated amount of $5,000,000 or more of gross proceeds to a private offering of LEGACY INVO’s preferred stock at a price per share of $5.00 per share in an amount equal to at least $2,000,000 to LEGACY INVO, plus an additional amount as may be required prior to closing of the Merger to be determined in good faith by the parties to adequately support the LEGACY INVO’s fertility business activities per an agreed forecast, as well as for a period of 12 months post- closing including a catch-up on the LEGACY INVO’s past due accrued payables still outstanding. The parties further agreed to the following schedule (the “Minimum Interim Pipe Schedule”) for the initial $2,000,000: (1) $500,000 no later than December 29, 2023, (2) $500,000 no later than January 19, 2024, (3) $500,000 no later than February 2, 2024, and (4) $500,000 no later than February 16, 2024. The parties also further agreed to modify the covenant of the parties regarding the Interim PIPE to require LEGACY NAYA to consummate the Interim PIPE before the closing of the Merger; provided, however, if the LEGACY INVO does not receive the initial gross proceeds pursuant to the Minimum Interim Pipe Schedule, then LEGACY INVO shall be free to secure funding from third parties to make up for short falls on reasonable terms under SEC and Nasdaq regulations.
Effective as of May 1, 2024, the Company entered into third amendment (“Third Amendment”) to the Merger Agreement. Pursuant to the Third Amendment, the parties agreed to extend the End Date to June 30, 2024. The parties further agreed to modify the definition of an “Interim PIPE” to mean (a) a sale of shares of the Company’s Series A Preferred Stock pursuant to the Series A Preferred SPA, as amended (“Phase 1”), plus (b) a sale of shares of the Company’s preferred stock at a price per share of $5.00 per share in a private offering, to be consummated prior to the closing of the Merger, resulting in an amount as may be required, to be determined in good faith by the parties to the Merger Agreement, to adequately support LEGACY INVO’s fertility business activities per an agreed forecast of LEGACY INVO as well as for a period of 12 months following the closing, including a catch-up on LEGACY INVO’s past due accrued payables still outstanding (“Phase 2”). The parties agreed that Phase I must be consummated pursuant to the terms of the Series A Preferred SPA and that Phase II must be consummated prior to the closing of the Merger. The parties also confirmed that LEGACY INVO remains free to secure any amount of funding from third parties on any terms LEGACY INVO deems reasonably acceptable under SEC and Nasdaq regulations without the prior written consent of LEGACY NAYA. Under the Third Amendment, LEGACY INVO may terminate the Merger Agreement if LEGACY NAYA breaches or fails to perform any of its covenants and agreements set forth in the Series A Preferred SPA, as amended.
| B-18 |
Effective as of September 12, 2024, the Company entered into a fourth amendment (“Fourth Amendment”) to the previously announced agreement and plan of merger (the “Merger Agreement”) by and among LEGACY INVO, INVO Merger Sub, Inc. (“Merger Sub”) and the Company.
Pursuant to the Fourth Amendment, the parties agreed to extend the end date (the date by which either the Company or LEGACY INVO may terminate the Merger Agreement, subject to certain exceptions) of the merger contemplated by the Merger Agreement (the “Merger”) to October 14, 2024. The parties further agreed that the Company would purchase 27,500 shares of LEGACY INVO’s Series A Preferred Stock for $137,500 pursuant to that certain Securities Purchase Agreement dated as of December 29, 2023, as amended pursuant to an Amendment to Securities Purchase Agreement dated as of May 1, 2024 (as amended, the “Securities Purchase Agreement”) and could purchase up to an additional 72,500 shares of Series A Preferred Stock for an aggregate of $362,500 pursuant to the Securities Purchase Agreement prior to or concurrently with the closing of the Merger. Each party waived prior breaches of the Merger Agreement.
Each party also agreed to use its best efforts to consummate the transactions contemplated by the Fourth Amendment, including to negotiate in good faith to amend and restate the Merger Agreement (the “A&R Merger Agreement”) to, among other things, (1) provide that the closing of the Merger shall occur simultaneously or shortly after the execution and delivery of the A&R Merger Agreement, and that the parties shall commit to use its respective best efforts to cause the closing to occur on or about October 1, 2024, but, in any case, no later than October 14, 2024, (2) ensure that the revised structure of the Merger shall be in compliance in all material respects with the applicable current listing and governance rules and regulations of the Nasdaq Stock Market, (3) provide that the aggregate merger consideration to be paid by LEGACY INVO for all of the outstanding shares of the Company’s capital stock shall consist of (a) such number of shares of LEGACY INVO’s common stock as shall represent a number of shares equal to no more than 19.9% of the outstanding shares of the LEGACY INVO’s common stock as of immediately before the effective time of the Merger (the “Common Stock Payment Shares”) and (b) shares of a newly designated Series C Convertible Preferred Stock of LEGACY INVO (the “Parent Preferred Stock Payment Shares”), (4) include an acknowledgment by the parties that the Company shall transfer 85% of the Common Stock Payment Shares to Five Narrow Lane LP, as a secured lender of the Company, (5) provide that LEGACY INVO shall take all action necessary under applicable law to (i) call, give notice of, and hold a meeting of its stockholders as soon as possible after the closing of the Merger, but in any case, no later than 120 days thereafter (the “Stockholder Meeting Deadline”; provided, that, LEGACY INVO acknowledges that, if it receives comments from the Securities and Exchange Commission (“SEC”) on the proxy statement filed in connection with such stockholder meeting and it uses its best efforts to revise and refile the proxy statement to address such comments, the date of such stockholder meeting may be after the Stockholder Meeting Deadline), for the purpose of, among other things, seeking stockholder approval (the “Stockholder Approval”) of the issuance of all shares of the Company’s common stock issuable upon conversion (the “Series C Conversion Shares”) of the Preferred Stock Payment Shares in accordance with the terms of the Certificate of Designations of Series C Convertible Preferred Stock (the “Series C Certificate of Designations”), and (ii) to file with the SEC a proxy statement for the purpose of obtaining the Stockholder Approval, no later than 35 days after the closing of the Merger, (6) provide that, upon receipt of the Stockholder Approval, the Preferred Stock Payment Shares shall be automatically converted into the Series C Conversion Shares at the conversion price in effect as set forth in the Series C Certificate of Designations; provided that, following such conversion, the Series C Conversion Shares shall represent approximately 60.1% of the outstanding shares of LEGACY INVO’s common stock; and (7) provide that, as soon as possible after the closing of the Merger, LEGACY INVO shall file a resale registration statement with the SEC to register the Common Stock Payment Shares and the Series C Conversion Shares in accordance with the terms of a registration rights agreement to be entered into between the parties.
| B-19 |
Securities Purchase Agreement
On December 29, 2023, the Company entered into a securities purchase agreement (the “Series A Preferred SPA”) with LEGACY INVO for LEGACY NAYA’s purchase of up to 1,000,000 shares of LEGACY INVO’s Series A Preferred Stock at a purchase price of $5.00 per share. The parties agreed that LEGACY NAYA’s purchases will be made in tranches in accordance with the Minimum Interim Pipe Schedule. The Series A Preferred SPA contains customary representations, warranties and covenants of the Company and LEGACY INVO. On January 4, 2024, the Company and LEGACY INVO closed on 100,000 shares of Series A Preferred Stock in the first tranche of this private offering for gross proceeds of $500,000. In addition, the Company and LEGACY INVO agreed that an additional amount of $96,704 comprised of expenses paid by LEGACY NAYA on behalf of LEGACY INVO would result in an additional 19,340 shares issued.
From April 1, 2024, to April 30, 2024, LEGACY NAYA acquired 25,000 shares of LEGACY INVO Series A preferred stock for gross proceeds of $125,000 per the merger agreement. In addition, expenses totaling $84,700 were paid on behalf of LEGACY INVO and added to the investment for which 16,940 additional shares were issued.
Effective as of May 1, 2024, the Company and LEGACY INVO entered into an Amendment (the “SPA Amendment”) to the Series A Preferred SPA. Pursuant to the SPA Amendment, the parties agreed to an updated closing schedule for LEGACY NAYA’s purchases of LEGACY INVO’s Series A preferred stock at a purchase price of $5.00 per share. From May 1, 2024, to June 24, 2024, LEGACY NAYA acquired 140,000 shares of LEGACY INVO Series A preferred stock for gross proceeds of $700,000 and pursuant to the fourth amendment to the Merger Agreement an additional 27,500 shares were purchased for $137,500. As of September 30, 2024, LEGACY NAYA has purchased a total of 328,780 Series A preferred shares of LEGACY INVO at a cost of $1,643,900. The market value of those shares at September 30, 2024 was $778,113 and a reduction in fair value of $865,791 was recorded for the nine months ended September 30, 2024.
Each share of Series A Preferred has a stated value of $5.00, which is convertible into shares of LEGACY INVO common stock at a fixed conversion price equal to $1.50 per share, subject to adjustment. LEGACY INVO may not effect the conversion of any shares of Series A Preferred if, after giving effect to the conversion or issuance, the holder, together with its affiliates, would beneficially own more than 9.99% of the Company’s outstanding common stock. Moreover, LEGACY INVO may not effect the conversion of any shares of Series A Preferred if, after giving effect to the conversion or issuance, the holder, together with its affiliates, would beneficially own more than 19.99% of the Company’s outstanding common stock unless and until LEGACY INVO receives the approval required by the applicable rules and regulations of The Nasdaq Stock Market LLC (or any subsequent trading market). Each share of Series A Preferred stock shall automatically convert into common stock upon the closing of the Merger. The conversion of the Series A Preferred Stock is contingent on the closing of the Merger.
| B-20 |
The Company as holder of Series A Preferred shall be entitled to receive a pro-rata portion, on an as-if converted basis, of any dividends payable on common stock. In the event of any voluntary or involuntary liquidation, dissolution, or winding up, or sale of LEGACY INVO (other than the Merger), the Company as holder of Series A Preferred shall be entitled to receive its pro rata portion of an aggregate payment equal to (i) $5.00, multiplied by (ii) the total number of shares of Series A Preferred Stock issued under the Series A Certificate of Designation. Other than those rights provided by law, the holders of Series A Preferred shall not have any voting rights.
Leases
In October and November 2023, the Company entered into leases for its corporate headquarters for a twelve-month term. The leases did not include a renewal or purchase option, and therefore are considered short-term in accordance with ASC 842. The Company elected not to apply the recognition requirements of ASC 842 to short-term leases. Accordingly, the Company instead recognizes lease payments on short-term leases in the period in which the obligation for those payments is incurred. The office lease was terminated in September 2024 and an early termination expense of approximately $31,000 was recorded. Rent expense was approximately $117,000 for the nine months ended September 30, 2024.
Licenses
Pursuant to a License Agreement between LEGACY NAYA and Inserm Transfert SA, dated December 19, 2023 (the “Inserm Agreement”), LEGACY NAYA was granted an exclusive worldwide royalty-bearing license under certain patents/patent applications co-owned by Inserm and Université de Paris (now Université Paris Cité), and a non-exclusive transferable, royalty-bearing license, with the right to sublicense under certain know how owned by Inserm for the development and commercialization of CYT338 (now NY338) in a defined field. The Inserm Agreement can be terminated early either upon the material breach upon one of the parties of the Inserm Agreement, upon either party to the Inserm Agreement being subject to bankruptcy, or upon LEGACY NAYA failing to meet one of the developmental milestones as described in the Inserm Agreement. Unless terminated earlier, the term of the Inserm Agreement will continue until the last to occur of (i) with respect to a given country – the last valid patent claim covering the product in such country expires or is invalidated; (ii) with respect to a given country – the lapse of ten years from the first commercial sale in such country of a product using intellectual property licensed pursuant to the Inserm Agreement (the “Inserm IP”), or (iii) such time when LEGACY NAYA no longer continues to generate revenues from the sale of products based on Inserm IP. Under the Inserm Agreement, LEGACY NAYA is to pay Inserm (a) a sublicense fee of 12% on revenues generated by sub-licensing of Inserm IP; (b) royalties of 2.5% on net sales from the Inserm IP during the term of the Inserm Agreement; and (c) lump sum payments of up to an aggregate of €5.15 million in development and commercial milestones.
| B-21 |
Under a License Agreement between LEGACY NAYA, the University of Rijeka Faculty of Medicine (“Rijeka”), and Yissum (together with Yissum, the “Licensors”) dated December 20, 2023, LEGACY NAYA was granted an exclusive, worldwide manufacturing, marketing, developing royalty bearing license to make commercial use of certain patents and patent applications covering two specific anti¬ NKp46 antibodies denoted hNKp46.09 (09) and hNKp46.12 (12) and know how needed in order to develop, manufacture, market, distribute and sell CYT338 (now NY338) and CYT303 (now NY303) and or incorporating products known as CYT338 (now NY338) or CYT303 (now NY303) in the specified field (the “Yissum License”). The Licensors retain rights to use the licensed technology for their own research and education. LEGACY NAYA may sublicense only after obtaining Licensors’ written approval of the sublicensee’s identity and key terms, with approval not unreasonably withheld or delayed They also have the right to license it to other academic and non-profit research organizations for non-commercial research. The Yissum License expires upon the last to occur of: (i) with respect to a given country – the date of expiration in such country of the last patent licensed under the Yissum License; (ii) with respect to a given country – the date of expiration of any exclusivity on the Product granted by a regulatory or government body in such country; or (iii) the lapse of twenty years from the date of the first sale by LEGACY NAYA of a product incorporating the intellectual property licensed to it under the Yissum License (the “Products”). Under the Yissum License, LEGACY NAYA is to pay Yissum (a) a royalty fee of two percent on net sales of Products; (b) a one-time payment of $1,000,000 upon achieving annual net sales of Products of $100,000,000; (c) a sublicense fee of 10% on revenues generated by LEGACY NAYA from sublicensing Intellectual Property covered by the Yissum License; (d) an exit fee of $1,000,000; and (e) certain additional payments upon reaching various development milestones up to US$2.25 million.
The agreement with CytoLynx Therapeutics (“CytoLynx”) for NY-303 in Greater China was assigned to LEGACY NAYA in the Cytovia Acquisition. Under the CytoLynx agreement, Cytovia granted to CytoLynx the rights for the for the development and manufacturing of NY-303 in Mainland China, Hong Kong, Taiwan and Macau. As compensation. Under the agreement, LEGACY NAYA will be eligible to receive up to $12 million in payments from CytoLynx upon achieving certain developmental milestones, as well as up to $145 million upon CytoLynx reaching certain commercial milestones. Additionally, under the agreement, LEGACY NAYA will receive royalty fees from CytoLynx based on net sales of the licensed products in the licensed territories (Mainland China, Hong Kong, Taiwan and Macau). The term of the agreement is indefinite until terminated by either party according to the terms of the agreement.
Litigation
On October 7, 2024, a former employee that had resigned filed a complaint against the Company alleging that she is entitled to severance and other benefits pursuant to a good reason paragraph in her employment agreement. The Company denies the allegations of wrongdoing in the complaint and intends to vigorously defend the matter. Since this case is in an early stage, the Company is unable to predict the ultimate outcome of the matter and cannot reasonably estimate the potential loss or range of loss the Company may incur.
LEGACY NAYA is not currently subject to any other material legal proceedings; however, it could be subject to legal proceedings and claims from time to time in the ordinary course of its business, or legal proceedings it considered immaterial may in the future become material. Regardless of the outcome, litigation can, among other things, be time consuming and expensive to resolve, and can divert management resources.
Stock Payable
During the first quarter of 2024, the Company entered into a public relations consulting service agreement that included $60,000 worth of common stock to be settled in cash or stock on a date that is mutually agreed upon by both parties. This amount is included in Other Accrued Liabilities.
| B-22 |
| 9. | Related Party Transactions |
As of December 31, 2023, the Company had two outstanding loans with Cytovia: $6,000,000 note related to the purchase of two assets from Cytovia and a short-term loan of $285,500. As of September 30, 2024, there was an outstanding loan of $290,000. See Notes 4, 5 and 6.
| 10. | Subsequent events |
SAFE Notes
On October 10, 2024, SAFE notes totaling $38,000 were converted into 12,667 shares of the Company’s common stock.
Amended and Restated Merger Agreement
On October 11, 2024 (the “Effective Time”), LEGACY INVO, Merger Sub, and LEGACY NAYA, entered into the Merger Agreement and consummated the transactions contemplated thereby. Upon the terms and subject to the conditions set forth in the Merger Agreement, Merger Sub merged with and into LEGACY NAYA, with LEGACY NAYA continuing as the surviving corporation and a wholly owned subsidiary of LEGACY INVO. References hereafter to the Company refer to the merged entity.
At the Effective Time and as a result of the consummation of the Merger:
| ● | Each share of Class A common stock, par value $0.000001 per share, and Class B common stock, par value $0.000001 per share, of LEGACY NAYA (“LEGACY NAYA common stock”) outstanding immediately prior to the effective time of the Merger, other than certain excluded shares held by LEGACY NAYA as treasury stock or owned by the Company or Merger Sub, automatically converted into the right to receive 118,148 shares of the Company’s common stock and 30,375 shares of the Company’s newly-designated Series C-1 Convertible Preferred Stock (the “Series C-1 Preferred”). The Series C-1 Preferred is not redeemable, has no voting rights, and may not be converted into shares of the Company’s Common Stock unless and until the Company’s stockholders approve the issuance of common stock upon conversion of the Series C-1 Preferred. If the Company’s stockholders approve the issuance of common stock upon conversion of the Series C-1 Preferred, such Series C-1 Preferred will automatically convert into approximately 29,515,315 shares of the Company’s common stock, subject to adjustment if, as a result of such conversion if, after giving effect to the conversion or issuance, any single holder, together with its affiliates, would beneficially own in excess of 19.99% of the Company’s outstanding common stock. A description of the rights, preferences, and privileges of the Series C-1 Preferred are set forth in Item 5.03 below. | |
| ● | Certain outstanding debt obligations of LEGACY NAYA, including a portion of an amended and restated senior secured convertible debenture issued to Five Narrow Lane LP (“FNL”), with a combined principal balance of $8,575,833 converted into the right to receive 669,508 shares of the Company’s common stock and 8,576 shares of the Company’s newly-designated Series C-2 Convertible Preferred Stock (the “Series C-2 Preferred”). LEGACY NAYA and FNL have agreed that LEGACY NAYA shall issue to FNL a pre-funded common stock purchase warrant to purchase up to 459,508 shares of the Company’s common stock in lieu of 459,508 shares of the aforementioned common stock. The Series C-2 Preferred is only redeemable upon a “Bankruptcy Triggering Event” or a “Change of Control” that occurs 210 days after the closing date of the Merger. The Series C-2 Preferred may not be converted into shares of the Company’s Common Stock unless and until the Company’s stockholders approve the issuance of common stock upon conversion of the Series C-2 Preferred. If the Company’s stockholders approve the issuance of common stock upon conversion of the Series C-2 Preferred, such Series C-2 Preferred will be convertible at the option of the holders into approximately 12,441,607 shares of the Company’s common stock, subject to limitations on beneficial ownership by the holders thereof. A description of the rights, preferences, and privileges of the Series C-2 Preferred are set forth below. |
| B-23 |
| ● | The remaining balance of the amended and restated senior secured convertible debenture issued to FNL in the amount of $3,934,146 was exchanged for a 7.0% Senior Secured Convertible Debenture in the principal balance of $3,934,146 due December 11, 2025 (the “Debenture”). A description of the rights, preferences, and privileges of the Debenture are set forth below. | |
| ● | The accredited investor note for $500,000 was converted to 500 Series C-2 Convertible Preferred shares. See Note 6. | |
| ● | LEGACY NAYA has been renamed to “NAYA Therapeutics Inc.” |
In addition, LEGACY NAYA stock options shall be converted into Company options to acquire a number of shares of the Company’s common stock equal to the number of shares of LEGACY NAYA common stock subject to such LEGACY NAYA options multiplied by 8.9108 (the “Exchange Ratio”) (rounded up to the nearest whole share) at an exercise price per share of such LEGACY NAYA stock option divided by the Exchange Ratio, and LEGACY NAYA restricted stock units shall be converted into Company restricted stock units representing the right to receive a number of shares of the Company’s common stock equal to the number of shares of LEGACY NAYA common stock subject to such LEGACY NAYA restricted stock unit multiplied by the Exchange Ratio. However, such options may not be exercised for shares of the Company’s common stock and such restricted stock units may not be settled for shares of the Company’s common stock unless and until the Company’s stockholders approve the issuance of common stock upon exercise of such options and settlement of such restricted stock units.
In connection with the Merger, Dr. Daniel Teper, LEGACY NAYA’s current Chairman and Chief Executive Officer, was appointed President of the Company, and Dr. Teper will remain as LEGACY NAYA’s Chief Executive Officer. The combined company will be led by LEGACY INVO Chief Executive Officer Steven Shum, LEGACY INVO Chief Financial Officer Andrea Goren, and Dr. Teper. In addition, Dr. Teper and Ms. Lyn Falconio have been appointed to the Company’s board of directors.
Pursuant to the Merger Agreement, the Company is required to hold a meeting of its stockholders to, among other things, (i) ratify the Merger Agreement and the transactions contemplated thereby, including the Merger, (ii) approve the increase in the amount of authorized shares under the Company’s Second Amended and Restated 2019 Stock Incentive Plan, (iii) approve the issuance of the Company’s common stock issuable upon conversion of the Series C-1 Preferred and Series C-2 Preferred, and (iv) approve an amendment to the Company’s articles of incorporation to (1) increase the number of shares of the Company’s authorized common stock to 100,000,000 shares, and (2) effectuate a reverse stock split of the Company’s common stock at a ratio ranging from any whole number between 1-for-2 and 1-for-20, as determined by the Company’s board of directors in its discretion. The Company also agreed to take all action necessary to hold the aforementioned stockholder meeting as soon as reasonably practicable.
| B-24 |
Pursuant to both the Merger Agreement and the Assignment Agreement described below, the Company has agreed to file a registration statement with the SEC to register for resale the shares of the Company’s common stock issued pursuant to the Merger and the shares of common stock issuable upon exercise or conversion of the Series C-1 Preferred, the Series C-2 Preferred, and the Debenture, as applicable, as soon as practicable but in no event later than 30 days after the Closing Date.
7.0% Senior Secured Convertible Debenture
In connection with the Merger, on October 11, 2024, the Company issued the Debenture to FNL in an exchange of an outstanding note of LEGACY NAYA held by FNL. The Debenture carries an interest rate of seven percent (7%) per annum, payable on the first business day of each calendar month commencing November 1, 2024. The maturity date of the Debenture is December 11, 2025 (the “Maturity Date”), at which point the outstanding principal amount, together with any accrued and unpaid interest and other fees, shall be due and payable to the holder of the Debenture.
Conversion. At any time after the Company’s stockholders approve the issuance of any Company common stock upon conversion of the Debenture, the holder of the Debenture will be entitled to convert any portion of the outstanding and unpaid principal amount and accrued interest into shares of Company common stock at a conversion price of $0.93055 per share, subject to adjustment as described therein. The Debenture may not be converted and shares of Company common stock may not be issued upon conversion of the Debenture if, after giving effect to the conversion or issuance, the holder together with its affiliates would beneficially own in excess of 4.99% of the outstanding common stock of the Company.
Prepayment. The Company may not prepay the Debenture without the prior written consent of FNL
Monthly Redemption. Commencing March 14, 2025 and on the 14th of each month thereafter until the Maturity Date, the Company shall redeem $437,127.24, plus accrued but unpaid interest and other fees, of the principal amount of the Debenture.
Mandatory Redemption. While any portion of the Debenture is outstanding, if the Company receives gross proceeds of more than $3,000,000 from any equity or debt financings (other than a public offering as described herein), the Company shall, at the option of the holder, apply one-third (1/3) of such gross proceeds to the redemption of the principal amount of the Debenture, except that if such equity or debt financing is a public offering of the Company’s securities pursuant to a registration statement on Form S-1, the Company shall, at the option of the holder, apply one hundred percent (100%) of such gross proceeds, not to exceed $500,000, to the redemption of the principal amount of the Debenture. The Company has also agreed that, if it received gross proceeds of more than $2,000,000 from any equity or debt financing, it shall, at the option of the holder, apply $500,000 of such gross proceeds to the redemption of the principal amount of the Debenture.
The Debenture contains events representations, warranties, covenants, and events of default that are customary for similar transactions. Upon an event of default, the Debenture becomes immediately due and payable, and the Borrower is subject to a default rate of interest of 15% per annum and a default sum as stipulated.
Joinder Agreement
In connection with the Merger, the Company entered in a joinder agreement (the “Joinder Agreement”) with FNL dated as of October 11, 2024 to a certain securities purchase agreement dated as of January 3, 2024 by and between LEGACY NAYA and FNL (the “FNL SPA”) pursuant to which the Company agreed to become a party to the FNL SPA.
| B-25 |
Assignment and Assumption Agreement
In connection with the Merger, on October 11, 2024, the Company entered in an assignment and assumption agreement (the “Assignment Agreement”), pursuant to which the Company agreed to assume the rights, duties, and liabilities of LEGACY NAYA under a certain registration rights agreement dated as of September 12, 2024 by and between LEGACY NAYA and FNL, pursuant to which the Company agreed to register FNL’s resale of shares of Company common stock issuable upon conversion of the Debenture and the Series C-2 Preferred as well as certain commitment shares issued to FNL in connection with the transactions.
Second Amendment to Revenue Loan and Security Agreement
On October 11, 2024, the Company entered into a second amendment to Revenue Loan and Security Agreement (the “Second Amendment”) with Decathlon Alpha V, L.P. (“Decathlon”), Steven Shum, and certain subsidiaries of the Company (the “Guarantors”), pursuant to which Decathlon consented to the Merger and LEGACY NAYA becoming a subsidiary of the Company. Pursuant to the Second Amendment, LEGACY NAYA joined the Revenue Loan and Security Agreement as a Guarantor. The Company agreed to pay down its loan by at least $500,000 and increase its monthly payments by up to $30,000 if the Company closes a private offering of its securities. The Company also agreed to retain an investment banker to pursue a financing or a sale if it fails to meet certain liquidity covenants. The Company also agreed to enter into an intercreditor agreement with Decathlon and Five Narrow Lane LP within 5 business days of the Merger.
Name Change and Application for Symbol Change
On October 15, 2024, LEGACY INVO changed its corporate name to NAYA Biosciences, Inc., pursuant to an Amendment to Articles of Incorporation filed with the Nevada Secretary of State on October 15, 2024 (the “Name Change”). Pursuant to Nevada law, a stockholder vote was not necessary to effectuate the Name Change.
LEGACY INVO also announced that it intends for its common stock to cease trading under the ticker symbol “INVO” and begin trading under its new ticker symbol, “NAYA”, on the Nasdaq Capital Market, as promptly as possible.
Series C-1 Preferred
The Company’s Articles of Incorporation, as amended, authorizes the Company to issue 100,000,000 shares of preferred stock, $0.0001 par value per share, issuable from time to time in or more series (“Preferred Stock”). On October 14, 2024, the Company filed with the Nevada Secretary of State a Certificate of Designation of Series C-1 Convertible Preferred Stock (the “Series C-1 Certificate of Designation”) which sets forth the rights, preferences, and privileges of the Series C-1 Preferred. Thirty thousand three hundred seventy five (30,375) shares of Series C-1 Preferred with a stated value of $1,000.00 per share were authorized under the Series C-1 Certificate of Designation.
Each share of Series C-1 Preferred has a stated value of $1,000.00, which is convertible into shares of the Company’s common stock (the “Common Stock”) at a conversion price equal to $1.02913 per share, subject to adjustment. The Series C-1 Preferred may not be converted into shares of the Company’s Common Stock unless and until the Company’s stockholders approve the issuance of common stock upon conversion of the Series C-1 Preferred. Each share of Series C-1 Preferred shall automatically convert into the Company’s common stock if the Company’s stockholders approve the issuance, except that the Company may not effect such conversion if, after giving effect to the conversion or issuance, the holder, together with its affiliates, would beneficially own in excess of 19.99% of the Company’s outstanding common stock.
| B-26 |
Commencing on the ninety-first (91st) day after the first issuance of any Series C-1 Preferred, the holders of Series C-1 Preferred shall be entitled to receive dividends on the stated value at the rate of two percent (2%) per annum, payable in shares of the Company’s common stock at the conversion price. Such dividends shall continue to accrue until paid. Such dividends will not be paid in shares of the Company’s common stock unless and until the Company’s stockholders approve the issuance of common stock upon conversion of the Series C-1 Convertible Preferred Stock. The holders of Series C-1 Preferred shall also be entitled to receive a pro-rata portion, on an as-if convertible basis, of any dividends payable on Common Stock.
The Series C-1 Preferred ranks senior to the Company’s common stock and junior to the Series C-2 Preferred. Subject to the rights of the holders of any senior securities, in the event of any voluntary or involuntary liquidation, dissolution, or winding up, or sale of the Company, each holder of Series C-1 Preferred shall be entitled to receive its pro rata portion of an aggregate payment equal to the amount as would be paid on the Company’s common stock issuable upon conversion of the Series C-1 Preferred, determined on an as-converted basis, without regard to any beneficial ownership limitation.
Other than those rights provided by law, the Series C-1 Preferred has no voting rights. The Series C-1 Preferred is not redeemable.
Series C-2 Preferred Stock
On October 14, 2024, the Company filed with the Nevada Secretary of State a Certificate of Designation of Series C-2 Convertible Preferred Stock (the “Series C-2 Certificate of Designation”) which sets forth the rights, preferences, and privileges of the Series C-2 Preferred. Eight thousand five hundred seventy six (8,576) shares of Series C-2 Preferred with a stated value of $1,000.00 per share were authorized under the Series C-2 Certificate of Designation.
Each share of Series C-2 Preferred has a stated value of $1,000.00, which, along with any additional amounts accrued thereon pursuant to the terms of the Series C-2 Certificate of Designation (collectively, the “Conversion Amount”) is convertible into shares of the Company’s common stock (the “Common Stock”) at a conversion price equal to $0.6893 per share, subject to adjustment. The Series C-2 Preferred may not be converted into shares of the Company’s Common Stock unless and until the Company’s stockholders approve the issuance of common stock upon conversion of the Series C-2 Convertible Preferred Stock. Each share of Series C-2 Preferred shall become convertible into the Company’s common stock at the option of the holder of such Series C-2 Preferred shares if the Company’s stockholders approve the issuance of common stock upon conversion of the Series C-2 Preferred, except that the Company may not effect such conversion if, after giving effect to the conversion or issuance, the holder, together with its affiliates, would beneficially own in excess of 9.99% of the Company’s outstanding common stock.
Commencing on the ninety-first (91st) day after the first issuance of any Series C-2 Preferred, the holders of Series C-2 Preferred shall be entitled to receive dividends on the stated value at the rate of ten percent (10%) per annum, payable in shares of the Company’s common stock, with each payment of a dividend payable in shares of the Company’s common stock at a conversion price of eighty-five percent (85%) of the average of the volume weighted average price of the Company’s common stock for the five (5) trading days before the applicable dividend date. Such dividends shall continue to accrue until paid. Such dividends will not be paid in shares of the Company’s common stock unless and until the Company’s stockholders approve the issuance of common stock upon conversion of the Series C-2 Preferred. The holders of Series C-2 Preferred shall also be entitled to receive a pro-rata portion, on an as-if convertible basis, of any dividends payable on Common Stock.
The Series C-2 Preferred ranks senior to the Company’s common stock and to the Series C-1 Preferred. Subject to the rights of the holders of any senior securities, in the event of any voluntary or involuntary liquidation, dissolution, or winding up, or sale of the Company, each holder of Series C-2 Preferred shall be entitled to receive its pro rata portion of an aggregate payment equal to the greater of (a) 125% of the Conversion Amount with respect to such shares, and (b) the amount as would be paid on the Company’s common stock issuable upon conversion of the Series C-2 Preferred, determined on an as-converted basis, without regard to any beneficial ownership limitation.
Other than those rights provided by law, the Series C-2 Preferred has no voting rights. The Series C-2 Preferred is only redeemable upon a “Bankruptcy Triggering Event” or a “Change of Control” that occurs 210 days after the closing date of the Merger.
| B-27 |
ANNEX C
UNAUDITED PRO FORMA CONDENSED COMBINED FINANCIAL INFORMATION AS OF SEPTEMBER 30, 2024 AND FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2024 AND FOR THE YEAR ENDED DECEMBER 31, 2023
NAYA BIOSCIENCES, INC.
UNAUDITED PRO FORMA FINANCIAL STATEMENTS
Legacy NAYA Merger
On October 11, 2024 (the “Effective Time”), the Company, Merger Sub, and Legacy NAYA entered into an Amended and Restated Agreement and Plan of Merger (the “A&R Merger Agreement”) and consummated and the transactions contemplated thereby. Upon the terms and subject to the conditions set forth in the A&R Merger Agreement, Merger Sub merged with and into Legacy NAYA, with Legacy NAYA continuing as the surviving corporation and a wholly owned subsidiary of the Company.
At the Effective Time and as a result of the consummation of the Merger:
● Each share of Class A common stock, par value $0.000001 per share, and Class B common stock, par value $0.000001 per share, of Legacy NAYA (“Legacy NAYA common stock”) outstanding immediately prior to the effective time of the Merger, other than certain excluded shares held by Legacy NAYA as treasury stock or owned by the Company or Merger Sub, automatically converted into the right to receive 118,148 shares of the Company’s common stock and 30,375 shares of the Company’s newly-designated Series C-1 Convertible Preferred Stock (the “Series C-1 Preferred”). The Company and FNL have agreed that the Company NAYA shall issue to FNL a pre-funded common stock purchase warrant to purchase up to 459,508 shares of the Company’s common stock in lieu of 459,508 shares of the aforementioned common stock. The Series C-1 Preferred is not redeemable, has no voting rights, and may not be converted into shares of the Company’s Common Stock unless and until the Company’s stockholders approve the issuance of common stock upon conversion of the Series C-1 Preferred. If the Company’s stockholders approve the issuance of common stock upon conversion of the Series C-1 Preferred, such Series C-1 Preferred will automatically convert into approximately 29,515,315 shares of the Company’s common stock, subject to adjustment if, as a result of such conversion if, after giving effect to the conversion or issuance, any single holder, together with its affiliates, would beneficially own in excess of 19.99% of the Company’s outstanding common stock. A description of the rights, preferences, and privileges of the Series C-1 Preferred are set forth in Item 5.03 below.
| C-1 |
● Certain outstanding debt obligations of Legacy NAYA, including a portion of an amended and restated senior secured convertible debenture issued to Five Narrow Lane LP (“FNL”), with a combined principal balance of $8,575,833 converted into the right to receive 669,508 shares of the Company’s common stock and 8,576 shares of the Company’s newly-designated Series C-2 Convertible Preferred Stock (the “Series C-2 Preferred”). The Series C-2 Preferred is only redeemable upon a “Bankruptcy Triggering Event” or a “Change of Control” that occurs 210 days after the closing date of the Merger. The Series C-2 Preferred may not be converted into shares of the Company’s Common Stock unless and until the Company’s stockholders approve the issuance of common stock upon conversion of the Series C-2 Preferred. If the Company’s stockholders approve the issuance of common stock upon conversion of the Series C-2 Preferred, such Series C-2 Preferred will be convertible at the option of the holders into approximately 12,441,607 shares of the Company’s common stock, subject to limitations on beneficial ownership by the holders thereof.
● The remaining balance of the amended and restated senior secured convertible debenture issued to FNL in the amount of $3,934,146 was exchanged for a 7.0% Senior Secured Convertible Debenture in the principal balance of $3,934,146 due December 11, 2025 (the “Debenture”). A description of the rights, preferences, and privileges of the Debenture are set forth below.
● Legacy NAYA has been renamed “NAYA Therapeutics Inc.”
In addition, Legacy NAYA stock options shall be converted into Company options to acquire a number of shares of the Company’s common stock equal to the number of shares of Legacy NAYA common stock subject to such Legacy NAYA options multiplied by 8.9108 (the “Exchange Ratio”) (rounded up to the nearest whole share) at an exercise price per share of such Legacy NAYA stock option divided by the Exchange Ratio, and Legacy NAYA restricted stock units shall be converted into Company restricted stock units representing the right to receive a number of shares of the Company’s common stock equal to the number of shares of Legacy NAYA common stock subject to such Legacy NAYA restricted stock unit multiplied by the Exchange Ratio. However, such options may not be exercised for shares of the Company’s common stock and such restricted stock units may not be settled for shares of the Company’s common stock unless and until the Company’s stockholders approve the issuance of common stock upon exercise of such options and settlement of such restricted stock units.
Pursuant to the A&R Merger Agreement, the Company is required to hold a meeting of its stockholders to, among other things, (i) ratify the A&R Merger Agreement and the transactions contemplated thereby, including the Merger, (ii) approve the increase in the amount of authorized shares under the Company’s Second Amended and Restated 2019 Stock Incentive Plan, (iii) approve the issuance of the Company’s common stock issuable upon conversion of the Series C-1 Preferred and Series C-2 Preferred, and (iv) approve an amendment to the Company’s articles of incorporation to increase the number of shares of the Company’s authorized common stock to 100,000,000 shares, and (2) effectuate a reverse stock split of the Company’s common stock at a ratio ranging from any whole number between 1-for-2 and 1-for-20, as determined by the Company’s board of directors in its discretion. The Company also agreed to take all action necessary to hold the aforementioned stockholder meeting as soon as reasonably practicable.
Pursuant to both the A&R Merger Agreement and the Assignment Agreement described below, the Company has agreed to file a registration statement with the SEC to register for resale the shares of the Company’s common stock issued pursuant to the Merger and the shares of common stock issuable upon exercise or conversion of the Series C-1 Preferred, the Series C-2 Preferred, and the Debenture, as applicable, as soon as practicable but in no event later than 30 days after the Closing Date.
| C-2 |
7.0% Senior Secured Convertible Debenture
In connection with the Merger, on October 11, 2024, the Company issued the Debenture to FNL in an exchange of an outstanding note of Legacy NAYA held by FNL. The Debenture carries an interest rate of seven percent (7%) per annum, payable on the first business day of each calendar month commencing November 1, 2024. The maturity date of the Debenture is December 11, 2025 (the “Maturity Date”), at which point the outstanding principal amount, together with any accrued and unpaid interest and other fees, shall be due and payable to the holder of the Debenture.
Conversion. At any time after the Company’s stockholders approve the issuance of any Company common stock upon conversion of the Debenture, the holder of the Debenture will be entitled to convert any portion of the outstanding and unpaid principal amount and accrued interest into shares of Company common stock at a conversion price of $0.93055 per share, subject to adjustment as described therein. The Debenture may not be converted and shares of Company common stock may not be issued upon conversion of the Debenture if, after giving effect to the conversion or issuance, the holder together with its affiliates would beneficially own in excess of 4.99% of the outstanding common stock of the Company.
Prepayment. The Company may not prepay the Debenture without the prior written consent of FNL
Monthly Redemption. Commencing March 14, 2025 and on the 14th of each month thereafter until the Maturity Date, the Company shall redeem $437,127.24, plus accrued but unpaid interest and other fees, of the principal amount of the Debenture.
Mandatory Redemption. While any portion of the Debenture is outstanding, if the Company receives gross proceeds of more than $3,000,000 from any equity or debt financings (other than a public offering as described herein), the Company shall, at the option of the holder, apply one-third (1/3) of such gross proceeds to the redemption of the principal amount of the Debenture, except that if such equity or debt financing is a public offering of the Company’s securities pursuant to a registration statement on Form S-1, the Company shall, at the option of the holder, apply one hundred percent (100%) of such gross proceeds, not to exceed $500,000, to the redemption of the principal amount of the Debenture. The Company has also agreed that, if it received gross proceeds of more than $2,000,000 from any equity or debt financing, it shall, at the option of the holder, apply $500,000 of such gross proceeds to the redemption of the principal amount of the Debenture.
The Debenture contains events representations, warranties, covenants, and events of default that are customary for similar transactions. Upon an event of default, the Debenture becomes immediately due and payable, and the Borrower is subject to a default rate of interest of 15% per annum and a default sum as stipulated.
| C-3 |
Wisconsin Fertility Institute Acquisition
On August 10, 2023, the Company, through Wood Violet Fertility LLC, a Delaware limited liability company (“WVF”) and wholly owned subsidiary of INVO Centers, LLC, consummated its acquisition of Wisconsin Fertility Institute (“WFI”) for a combined purchase price of $10 million, of which $2.5 million was paid on the closing date (net cash paid was $2,150,000 after a $350,000 holdback) plus assumption of the inter-company loan owed by WFRSA (as defined below) in the amount of $528,756. The remaining three installments of $2.5 million each will be paid on the subsequent three anniversaries of closing. The sellers have the option to take all or a portion of the final three installments in shares of INVO common stock valued at $125.00, $181.80, and $285.80, for the second, third, and final installments, respectively.
WFI is comprised of (a) a medical practice, Wisconsin Fertility and Reproductive Surgery Associates, S.C., a Wisconsin professional service corporation d/b/a Wisconsin Fertility Institute (“WFRSA”), and (b) a laboratory services company, Fertility Labs of Wisconsin, LLC, a Wisconsin limited liability company (“FLOW”). WFRSA owns, operates and manages WFI’s fertility practice that provides direct treatment to patients focused on fertility, gynecology and obstetrics care and surgical procedures, and employs physicians and other healthcare providers to deliver such services and procedures. FLOW provides WFRSA with related laboratory services.
The Company purchased the non-medical assets of WFRSA and one hundred percent of FLOW’s membership interests. WVF and WFRSA entered into a management services agreement pursuant to which WFRSA outsourced all its non-medical activities to WVF.
Pro Forma Financial Statements
The following unaudited pro forma financial statements are based on the Company’s historical consolidated financial statements including the historical combined financial statements of WFI, and the historical financial statements of Legacy NAYA, adjusted to give effect to the WFI Acquisition and the Legacy NAYA Merger and related financing transactions. The unaudited pro forma statements of operations for the nine months ended September 30, 2024 and the year ended December 31, 2023 give effect to these transactions as if they had occurred on January 1, 2023. The unaudited pro forma balance sheet as of September 30, 2024 gives effect to these transactions as if they had occurred on September 30, 2024.
The unaudited pro forma balance sheet and unaudited statement of operations are presented for informational purposes only and do not purport to be indicative of the combined financial condition that would have resulted if the acquisition would have occurred on January 1, 2023.
The unaudited pro forma financial statements should be read together with the Company’s historical financial statements, which are included in its latest Annual Report on Form 10-K and subsequent Quarterly Reports on Form 10-Q, WFI’s historical financial statements which are included on the and Legacy NAYA’s historical financial statements, which are included in this Form 8-K/A.
| C-4 |
NAYA BIOSCIENCES, INC.
PRO FORMA BALANCE SHEET
(UNAUDITED)
AS OF SEPTEMBER 30, 2024
NAYA (Legacy INVO) September 30, 2024 | Legacy NAYA September 30, 2024 | Pro Forma Adjustments | Pro Forma Balances | |||||||||||||
| ASSETS | ||||||||||||||||
| Current assets | ||||||||||||||||
| Cash | $ | 471,591 | $ | 756,461 | $ | 500,000 | (a) | $ | 1,728,052 | |||||||
| Accounts receivable, net | 257,051 | - | - | 257,051 | ||||||||||||
| Inventory | 241,539 | - | - | 241,539 | ||||||||||||
| Investment in Legacy INVO | - | 778,113 | (778,113 | )(b) | - | |||||||||||
| Prepaid expenses and other current assets | 987,300 | 33,247 | - | 1,020,547 | ||||||||||||
| Total current assets | 1,957,481 | 1,567,821 | (278,113 | ) | 3,247,189 | |||||||||||
| Property and equipment, net | 415,245 | - | - | 415,245 | ||||||||||||
| Lease right of use | 2,343,645 | - | - | 2,343,645 | ||||||||||||
| Intangible assets, net | 3,480,306 | - | - | 3,480,306 | ||||||||||||
| Goodwill | 5,878,986 | - | 35,886,383 | (c) | 41,765,369 | |||||||||||
| Equity investments | 771,826 | - | - | 771,826 | ||||||||||||
| Investment in Legacy NAYA | 2,172,000 | - | (2,172,000 | )(b) | - | |||||||||||
| Total assets | $ | 17,019,489 | $ | 1,567,821 | $ | 33,436,270 | $ | 52,023,580 | ||||||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||||||||||
| Current liabilities | ||||||||||||||||
| Accounts payable and accrued liabilities | $ | 2,655,106 | $ | 2,014,436 | $ | - | 4,669,542 | |||||||||
| Accrued compensation | 640,038 | 1,213,242 | - | 1,853,280 | ||||||||||||
| Notes payable – current portion, net | 837,183 | 12,561,074 | (8,575,833 | )(d) | ||||||||||||
| 310,000 | (a) | 5,132,424 | ||||||||||||||
| Notes payable – related parties, net | 880,000 | 290,000 | - | 1,170,000 | ||||||||||||
| Deferred revenue | 562,402 | - | - | 562,402 | ||||||||||||
| Lease liability, current portion | 230,729 | - | - | 230,729 | ||||||||||||
| Additional payments for acquisition, current portion | 2,500,000 | - | - | 2,500,000 | ||||||||||||
| Other current liabilities | 350,000 | - | 350,000 | |||||||||||||
| Total current liabilities | 8,655,458 | 16,078,752 | (8,265,833 | ) | 16,468,377 | |||||||||||
| Notes payable, net of current portion | 1,134,418 | - | - | 1,134,418 | ||||||||||||
| Lease liability, net of current portion | 2,252,929 | - | - | 2,252,929 | ||||||||||||
| Additional payments for acquisition, net of current portion | 5,000,000 | - | - | 5,000,000 | ||||||||||||
| Total liabilities | 17,042,805 | 16,078,752 | (8,265,833 | ) | 24,855,724 | |||||||||||
| Mezzanine equity | ||||||||||||||||
| NAYA Series C-2 Preferred Stock, $1000.00 par value | - | - | 8,576,000 | (d) | 8,576,000 | |||||||||||
| Stockholders’ equity | ||||||||||||||||
| NAYA Series A Preferred Stock, $5.00 par value | 1,643,904 | - | (1,643,904 | )(b) | - | |||||||||||
| NAYA Series B Preferred Stock, $5.00 par value | 6,000,000 | - | (6,000,000 | )(b) | - | |||||||||||
| NAYA Series C-1 Preferred Stock, $1000.00 par value | - | - | 30,375,000 | (e) | 30,375,000 | |||||||||||
| NAYA Common Stock, $.0001 par value | 391 | - | 33 | (e) | ||||||||||||
| 19 | (a) | 443 | ||||||||||||||
| Legacy NAYA Series A Preferred stock, par value $0.000001 per share | - | - | - | - | ||||||||||||
| Legacy NAYA Class A Common stock, par value $0.000001 per share | - | 1 | (1 | )(e) | - | |||||||||||
| Legacy NAYA Class B Common stock, par value $0.000001 | - | 1 | (1 | )(e) | - | |||||||||||
| Legacy NAYA Preferred Stock Payable | 750,000 | (750,000 | )(e) | - | ||||||||||||
| Additional paid-in capital | 55,873,514 | 16,052,218 | 189,982 | (a) | ||||||||||||
| 5,471,904 | (b) | |||||||||||||||
| (25,830,080 | )(e) | 51,757,539 | ||||||||||||||
| Accumulated deficit | (63,541,125 | ) | (31,313,151 | ) | 31,313,151 | (e) | (63,541,125 | ) | ||||||||
| Total stockholders’ equity | (23,316 | ) | (14,510,931 | ) | 33,126,103 | 18,591,856 | ||||||||||
| Total liabilities and stockholders’ equity | $ | 17,019,489 | $ | 1,567,821 | $ | 33,436,270 | 52,023,580 | |||||||||
| C-5 |
NAYA (Legacy INVO) September 30, 2024 | Legacy NAYA September 30, 2024 | Pro Forma Adjustments | Pro Forma September 30, 2024 | |||||||||||||
| Revenue: | ||||||||||||||||
| Product revenue | $ | 1,418,011 | $ | - | $ | - | $ | 1,418,011 | ||||||||
| Clinic revenue | 15,140 | - | - | 15,140 | ||||||||||||
| Total revenue | 1,433,151 | - | - | 1,433,151 | ||||||||||||
| Operating expenses: | ||||||||||||||||
| Cost of revenue | 988,465 | - | - | 988,465 | ||||||||||||
| Selling, general and administrative | 1,514,593 | 2,976,074 | - | 4,490,667 | ||||||||||||
| Research and development | - | 176,104 | - | 176,104 | ||||||||||||
| Depreciation and amortization | 230,495 | - | - | 230,495 | ||||||||||||
| Total operating expenses | 2,733,553 | 3,152,178 | - | 5,885,731 | ||||||||||||
| Loss from operations | (1,300,402 | ) | (3,152,178 | ) | - | (4,452,580 | ) | |||||||||
| Other income (expense): | ||||||||||||||||
| Gain (loss) from equity method joint ventures | (27,372 | ) | - | - | (27,372 | ) | ||||||||||
| Loss on debt extinguishment | - | (3,750,000 | ) | - | (3,750,000 | ) | ||||||||||
| Other income | - | 600,00 | - | 600,00 | ||||||||||||
| Change in fair value of investment | - | (865,791 | ) | - | (865,791 | ) | ||||||||||
| Interest expense | (273,629 | ) | (5,126,323 | ) | - | (135 | ) | |||||||||
| Total other expense, net | (301,001 | ) | (9,142,114 | ) | - | (9,443,115 | ) | |||||||||
| Net loss before income taxes | (1,601,403 | ) | (12,294,292 | ) | - | (13,895,695 | ) | |||||||||
| Income taxes | 29,259 | - | - | 29,259 | ||||||||||||
| Net loss | $ | (1,630,662 | ) | $ | (12,294,292 | ) | $ | - | $ | (13,924,954 | ) | |||||
| Net loss per common share | ||||||||||||||||
| Basic | (0.42 | ) | (4.33 | ) | - | (2.98 | )(f) | |||||||||
| Diluted | (0.42 | ) | (4.33 | ) | - | (2.98 | )(f) | |||||||||
| Weighted average number of common shares outstanding: | ||||||||||||||||
| Basic | 3,885,985 | 2,841,359 | - | 4,673,641 | (f) | |||||||||||
| Diluted | 3,885,985 | 2,841,359 | - | 4,673,641 | (f) | |||||||||||
| C-6 |
AS OF SEPTEMBER 30, 2024
NAYA BIOSCIENCES, INC.
PRO FORMA STATEMENT OF OPERATIONS
(UNAUDITED)
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2024
NAYA (Legacy INVO) Dec 31, 2023 | WFI For the period January 1, 2023 thru August 9, 2023 | Pro Forma Adjustments | Pro Forma as Adjusted Dec 31, 2023 | Legacy NAYA Dec 31, 2023 | Pro Forma Adjustments | Pro Forma Combined Dec 31, 2023 | ||||||||||||||||||||||
| Revenue: | ||||||||||||||||||||||||||||
| Clinic revenue | $ | 2,862,574 | $ | 3,207,596 | $ | - | $ | 6,070,170 | $ | - | $ | - | $ | 6,070,170 | ||||||||||||||
| Product revenue | 158,001 | - | - | 158,001 | - | - | 158,001 | |||||||||||||||||||||
| Total revenue | 3,020,575 | 3,207,596 | - | 6,228,171 | - | - | 6,228,171 | |||||||||||||||||||||
| Operating expenses: | ||||||||||||||||||||||||||||
| Cost of revenue | 1,934,437 | 1,558,041 | - | 3,492,478 | - | - | 3,492,478 | |||||||||||||||||||||
| Selling, general and administrative | 7,486,454 | 726,763 | - | 8,213,217 | 2,159,626 | - | 10,372,843 | |||||||||||||||||||||
| Research and development | 165,945 | - | - | 165,945 | 975,148 | - | 1,141,093 | |||||||||||||||||||||
| Depreciation and amortization | 200,894 | 10,299 | - | 211,193 | - | 211,193 | ||||||||||||||||||||||
| Total operating expenses | 9,787,730 | 2,295,103 | - | 12,082,833 | 3,134,774 | - | 15,217,607 | |||||||||||||||||||||
| Income (loss) from operations | (6,767,155 | ) | 912,493 | - | (5,854,662 | ) | (3,134,774 | ) | - | (8,989,436 | ) | |||||||||||||||||
| Other income (expense): | ||||||||||||||||||||||||||||
| Loss from equity method investment | (60,270 | ) | - | - | (60,270 | ) | - | - | (60,270 | ) | ||||||||||||||||||
| Impairment from equity method joint venture | (89,794 | ) | (89,794 | ) | (89,794 | ) | ||||||||||||||||||||||
| Loss from debt extinguishment | (163,278 | ) | (163,278 | ) | (163,278 | ) | ||||||||||||||||||||||
| Interest expense - related party | - | - | - | - | (144,000 | ) | - | (144,000 | ) | |||||||||||||||||||
| Interest expense | (925,909 | ) | (47 | ) | - | (925,956 | ) | - | - | (925,956 | ) | |||||||||||||||||
| Foreign currency exchange loss | (420 | ) | - | - | (420 | ) | - | - | (420 | ) | ||||||||||||||||||
| Total other income (expense ) | (1,239,671 | ) | (47 | ) | - | (1,239,718 | ) | (144,000 | ) | - | (1,383,718 | ) | ||||||||||||||||
| Income (loss) before income taxes | (8,006,826 | ) | 912,446 | - | (7,094,380 | ) | (3,278,774 | ) | - | (10,373,154 | ) | |||||||||||||||||
| Income taxes | 27,786 | 1,046 | - | 28,832 | - | - | 28,832 | |||||||||||||||||||||
| Net income (loss) | $ | (8,034,612 | ) | $ | 911,400 | $ | - | $ | (7,123,212 | ) | $ | (3,278,774 | ) | $ | - | $ | (10,401,986 | ) | ||||||||||
| Net loss per common share: | ||||||||||||||||||||||||||||
| Basic | $ | (5.13 | ) | $ | (4.55 | ) | (1.28 | ) | $ | (4.42 | )(f) | |||||||||||||||||
| Diluted | $ | (5.13 | ) | $ | (4.55 | ) | (1.28 | ) | $ | (4.42 | )(f) | |||||||||||||||||
| Weighted average number of common shares outstanding: | ||||||||||||||||||||||||||||
| Basic | 1,565,951 | 1,565,951 | 2,563,642 | 2,353,607 | (f) | |||||||||||||||||||||||
| Diluted | 1,565,951 | 1,565,951 | 2,563,642 | 2,353,607 | (f) | |||||||||||||||||||||||
| C-7 |
NAYA BIOSCIENCES, INC.
PRO FORMA STATEMENT OF OPERATIONS
(UNAUDITED)
FOR THE YEAR ENDED DECEMBER 31, 2023
| C-8 |
NAYA BIOSCIENCES, INC.
NOTES TO FINANCIAL STATEMENTS
Note 1 – Basis of presentation
The Legacy NAYA Merger is accounted for under the acquisition method of accounting in accordance with ASC Topic 805, Business Combinations. As the acquirer for accounting purposes, the Company has estimated the fair value of Legacy NAYA’s assets acquired and liabilities assumed and conformed the accounting policies of Legacy NAYA to its own policies.
Note 2 – Calculation of purchase consideration and preliminary purchase price allocation
The following table summarizes the fair value of purchase consideration that was transferred on the Closing Date:
| Value of 328,148 shares of NAYA common stock issued | $ | 223,961 | ||
| Value of 459,508 prefunded warrants of NAYA issued | 313,614 | |||
| Value of 30,375 shares of NAYA series C-1 preferred stock issued | 20,144,202 | |||
| Value of 8,576 shares NAYA series C-2 preferred stock issued | 8,491,395 | |||
| Total purchase consideration | $ | 29,173,172 |
The value of the common stock and prefunded warrants is based on the closing price of NAYA common stock as of October 10, 2024. The value of the shares of preferred stock is based on the number of common shares on an as converted basis and the closing price of NAYA common stock as of October 10, 2024.
The Company has performed a preliminary valuation analysis of the fair market value of Legacy NAYA’s assets and liabilities. The following table summarizes the preliminary allocation of the purchase price as of September 30, 2024:
| Cash | $ | 756,461 | ||
| Prepaid expenses and other current assets | 33,247 | |||
| Goodwill | 35,886,383 | |||
| Accounts payable and accrued expenses | (3,227,678 | ) | ||
| Notes payable | (4,275,241 | ) | ||
| Total consideration | $ | 29,173,172 |
This preliminary purchase price allocation has been used to prepare pro forma adjustments in the unaudited pro forma balance sheet and income statements. The final purchase price allocation will be determined when NAYA has completed all detailed valuations and necessary calculations, which are expected to be finalized within the next twelve months. The final allocation could differ materially from the preliminary allocation used in the pro forma adjustments. The final allocation may include (i) changes in identifiable net assets, (ii) changes in fair values of property, plant and equipment, and (iii) other changes to assets and liabilities.
Note 3 – Pro forma adjustments
The pro forma adjustments are based on the NAYA’s preliminary estimates and assumptions that are subject to change. The following adjustments have been reflected in the unaudited pro forma condensed combined financial statements:
| (a) | Represents (i) proceeds of $0.5 million from the FNL Debenture after the closing of the Legacy NAYA Merger, and (ii) issuance of 190,000 shares of NAYA common stock upon the conversion of $190,000 in notes payable. | |
| (b) | Represents the cancellation of (i) 328,780 shares of NAYA Preferred Series A and (ii) 1,200,000 shares of NAYA Preferred Series B in connection with the Legacy NAYA Merger. |
| C-9 |
| (c) | Represents the preliminary goodwill associated with the Legacy NAYA Merger as presented in Note 2. Goodwill represents the estimate of the excess of the purchase price over the fair value of the assets acquired and liabilities assumed. | |
| (d) | Represents certain outstanding debt obligations of Legacy NAYA, including a portion of an amended and restated senior secured convertible debenture issued to FNL, with a combined principal balance of $8,575,833 converted into the right to receive 669,508 shares of the Company’s common stock and 8,576 shares of the Company’s Series C-2 Preferred. | |
| (e) | Represents the following equity adjustments associated with the closing of the Legacy NAYA Merger: |
| Legacy NAYA Class A Common Stock | Legacy NAYA Class B Common Stock | Legacy NAYA Preferred Stock | NAYA (Legacy INVO) Common Stock | NAYA (Legacy INVO) Series C-1 Preferred Stock | Additional paid-in | Accumulated | Total stockholders’ | |||||||||||||||||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Payable | Shares | Amount | Shares | Amount | capital | deficit | equity | |||||||||||||||||||||||||||||||||||||
| Elimination of Legacy NAYA’s historical equity | (1,756,432 | ) | (1 | ) | (1,367,618 | ) | (1 | ) | (750,000 | ) | - | - | - | - | (16,052,218 | ) | 31,313,151 | 14,510,931 | ||||||||||||||||||||||||||||||
| Issuance of NAYA stock | - | - | - | - | - | 328,148 | 33 | 30,375 | 30,375,000 | (9,777,862 | ) | - | 20,597,171 | |||||||||||||||||||||||||||||||||||
| Pro forma adjustment | (1,756,432 | ) | (1 | ) | (1,367,618 | ) | (1 | ) | (750,000 | ) | 328,148 | 33 | 30,375 | 30,375,000 | (25,830,080 | ) | 31,313,151 | 35,108,102 | ||||||||||||||||||||||||||||||
(f)
Loss Per Share
Basic loss per share is computed by dividing net loss by the weighted-average number of common shares outstanding. Diluted earnings per share are computed similarly to basic earnings per share except that the denominator is increased to include potentially dilutive securities. The Company’s diluted loss per share is the same as the basic loss per share for the pro forma periods ending September 30, 2024 and December 31, 2023 as the inclusion of any potential shares would have had an anti-dilutive effect due to the Company generating a loss.
| Nine Months Ended September 30, 2024 | Year Ended December 31, 2023 | |||||||
| Pro forma net loss (numerator) | $ | (13,924,954 | ) | (10,401,986 | ) | |||
| Basic and diluted weighted-average number of common shares outstanding as of period end | 3,885,985 | 1,565,951 | ||||||
| NAYA common stock issued as consideration for Legacy NAYA | 328,148 | 328,148 | ||||||
| NAYA prefunded warrants issued as consideration for Legacy NAYA | 459,508 | 459,508 | ||||||
| Total basic and diluted weighted-average number of common shares and prefunded warrants outstanding upon closing of the Legacy NAYA Merger (denominator) | 4,673,641 | 2,353,607 | ||||||
| Basic and diluted net loss per common share | (2.98 | ) | (4.42 | ) | ||||
The Company has excluded the following dilutive securities issued with the closing of the Legacy NAYA Merger from the calculation of fully diluted shares outstanding because the result would have been anti-dilutive:
| NAYA preferred series C-1 shares | 30,375 | |||
| NAYA preferred series C-2 shares | 8,576 | |||
| Total | 38,951 |
| C-10 |
ANNEX D
NAYA Biosciences, Inc.
Amendment to The
Second Amended and Restated
2019 Stock Incentive Plan
This amendment (the “Amendment”) to the Second Amended and Restated 2019 Stock Incentive Plan (the “Plan”) of Naya Biosciences, Inc., a Nevada corporation (the “Company”) is dated as of , 2025 (the “Effective Date”).
WHEREAS, the board of directors of the Company (the “Board”), has adopted, and the stockholders have approved, the Plan.
WHEREAS, pursuant to Section 12.2 of the Plan, the Board holds the authority to amend the Plan, subject to the approval of the stockholders, if required by applicable laws; and
WHEREAS, as of the Effective Date, subject to the approval of the Company’s stockholders, the Board has determined that it is in the best interest of the Company and its stockholders to approve this Amendment to the Plan in order to increase the number of shares of the Company’s Common Stock (as defined in the Plan) reserved for issuance thereunder.
NOW, THEREFORE, as of the Effective Date, subject to approval by the Company’s stockholders, the Plan shall be amended as follows:
Section 4.1 of the Plan shall be deleted in its entirety and the following substituted in lieu thereof:
“4.1 Maximum Share Limitation. Subject to Section 4.2 hereof, the maximum aggregate number of shares of Common Stock that may be issued and sold under all Awards granted under the Plan shall be eight million, two hundred thousand, (8,200,000) shares. Shares of Common Stock issued and sold under the Plan may be either authorized but unissued shares or shares held in the Company’s treasury. To the extent that any Award involving the issuance of shares of Common Stock is forfeited, cancelled, returned to the Company for failure to satisfy vesting requirements or other conditions of the Award, or otherwise terminates without an issuance of shares of Common Stock being made thereunder, the shares of Common Stock covered thereby will no longer be counted against the foregoing maximum share limitations and may again be made subject to Awards under the Plan pursuant to such limitations. Any Awards or portions thereof that are settled in cash and not in shares of Common Stock shall not be counted against the foregoing maximum share limitations.”
[Signature Page Follows]
| D-1 |
IN WITNESS WHEREOF, the undersigned officer certifies that the foregoing Amendment to the Plan was duly adopted by the Board.
| NAYA Biosciences, Inc. | ||
| By: | ||
| Steven Shum | ||
| Chief Executive Officer | ||
| D-2 |
ANNEX E
INVO BIOSCIENCE, INC.
SECOND AMENDED AND RESTATED 2019 STOCK INCENTIVE PLAN
1. Purpose. The purpose of the Second Amended and Restated 2019 Stock Incentive Plan of INVO Bioscience, Inc. is to further align the interests of employees, directors and non-employee Consultants with those of the stockholders by providing incentive compensation opportunities tied to the performance of the Common Stock and by promoting increased ownership of the Common Stock by such individuals. The Plan is also intended to advance the interests of the Company and its stockholders by attracting, retaining and motivating key personnel upon whose judgment, initiative and effort the successful conduct of the Company’s business is largely dependent.
2. Definitions. Wherever the following capitalized terms are used in the Plan, they shall have the meanings specified below:
“Affiliate” means (i) any entity that would be treated as an “affiliate” of the Company for purposes of Rule 12b-2 under the Exchange Act and (ii) any joint venture or other entity in which the Company has a direct or indirect beneficial ownership interest representing at least one-third (1/3) of the aggregate voting power of the equity interests of such entity or one-third (1/3) of the aggregate fair market value of the equity interests of such entity, as determined by the Committee.
“Award” means an award of a Stock Option, Stock Award, or Restricted Stock Award granted under the Plan.
“Award Agreement” means a written or electronic agreement entered into between the Company and a Participant setting forth the terms and conditions of an Award granted to a Participant.
“Board” means the Board of Directors of the Company.
“Code” means the Internal Revenue Code of 1986, as amended.
“Committee” means the Compensation Committee of the Board, or such other committee of the Board appointed by the Board to administer the Plan, or if no such committee exists, the Board.
“Common Stock” means the Company’s common stock, $0.0001 par value per share.
“Company” means INVO Bioscience, Inc., a Nevada corporation.
“Consultant” means any person which is a consultant or advisor to the Company and which is a natural person and who provides bona fide services to the Company which are not in connection with the offer or sale of securities in a capital-raising transaction for the Company, and do not directly or indirectly promote or maintain a market for the Company’s securities.
“Date of Grant” means the date on which an Award under the Plan is made by the Committee, or such later date as the Committee may specify to be the effective date of an Award.
“Disability” means a Participant being considered “disabled” within the meaning of Section 409A(a)(2)(C) of the Code, unless otherwise provided in an Award Agreement.
“Eligible Person” means any person who is an employee of the Company or any Affiliate or any person to whom an offer of employment with the Company or any Affiliate is extended, as determined by the Committee, or any person who is a Non- Employee Director, or any person who is Consultant to the Company.
| E-1 |
“Exchange Act” means the Securities Exchange Act of 1934, as amended.
“Fair Market Value” means the mean between the highest and lowest reported sales prices of the Common Stock on the New York Stock Exchange Composite Tape or, if not listed on such exchange, on any other national securities exchange on which the Company’s common stock is listed or on The Nasdaq Stock Market, or, if not so listed on any other national securities exchange or The Nasdaq Stock Market, then the average of the bid price of the Company’s common stock during the last five trading days on the OTC Bulletin Board immediately preceding the last trading day prior to the date with respect to which the Fair Market Value is to be determined. If the Company’s common stock is not then publicly traded, then the Fair Market Value of the Common Stock shall be the book value of the Company per share as determined on the last day of March, June, September, or December in any year closest to the date when the determination is to be made. For the purpose of determining book value hereunder, book value shall be determined by adding as of the applicable date called for herein the capital, surplus, and undivided profits of the Company, and after having deducted any reserves theretofore established; the sum of these items shall be divided by the number of shares of the Company’s common stock outstanding as of said date, and the quotient thus obtained shall represent the book value of each share of the Company’s common stock.
“Incentive Stock Option” means a Stock Option granted under Section 6 hereof that is intended to meet the requirements of Section 422 of the Code and the regulations thereunder.
“Non-Employee Director” means any member of the Board who is not an employee of the Company.
“Nonqualified Stock Option” means a Stock Option granted under Section 6 hereof that is not an Incentive Stock Option.
“Participant” means any Eligible Person who holds an outstanding Award under the Plan.
“Plan” means the Second Amended and Restated 2019 Stock Incentive Plan of INVO Bioscience, Inc. as set forth herein, as amended from time to time.
“Restricted Stock Award” means a grant of shares of Common Stock to an Eligible Person under Section 8 hereof that is issued subject to such vesting and transfer restrictions as the Committee shall determine and set forth in an Award Agreement.
“Service” means a Participant’s employment with the Company or any Affiliate or a Participant’s service as a Non- Employee Director with the Company, as applicable.
“Stock Award” means a grant of shares of Common Stock to an Eligible Person under Section 7 hereof that are issued free of transfer restrictions and forfeiture conditions.
“Stock Option” means a contractual right granted to an Eligible Person under Section 6 hereof to purchase shares of Common Stock at such time and price, and subject to such conditions, as are set forth in the Plan and the applicable Award Agreement.
3. Administration.
3.1 Committee Members. The Plan shall be administered by a Committee comprised of one or more members of the Board, or if no such committee exists, the Board.
| E-2 |
3.2 Committee Authority. The Committee shall have such powers and authority as may be necessary or appropriate for the Committee to carry out its functions as described in the Plan. Subject to the express limitations of the Plan, the Committee shall have authority in its discretion to determine the Eligible Persons to whom, and the time or times at which, Awards may be granted, the number of shares, units or other rights subject to each Award, the exercise, base or purchase price of an Award (if any), the time or times at which an Award will become vested, exercisable or payable, the performance goals and other conditions of an Award, the duration of the Award, and all other terms of the Award. Subject to the terms of the Plan, the Committee shall have the authority to amend the terms of an Award in any manner that is not inconsistent with the Plan, provided that no such action shall adversely affect the rights of a Participant with respect to an outstanding Award without the Participant’s consent. The Committee shall also have discretionary authority to interpret the Plan, to make factual determinations under the Plan, and to make all other determinations necessary or advisable for Plan administration, including, without limitation, to correct any defect, to supply any omission or to reconcile any inconsistency in the Plan or any Award Agreement hereunder. The Committee may prescribe, amend, and rescind rules and regulations relating to the Plan. The Committee’s determinations under the Plan need not be uniform and may be made by the Committee selectively among Participants and Eligible Persons, whether or not such persons are similarly situated. The Committee shall, in its discretion, consider such factors as it deems relevant in making its interpretations, determinations and actions under the Plan including, without limitation, the recommendations or advice of any officer or employee of the Company or such attorneys, consultants, accountants or other advisors as it may select. All interpretations, determinations and actions by the Committee shall be final, conclusive, and binding upon all parties.
3.3 Delegation of Authority. The Committee shall have the right, from time to time, to delegate to one or more officers of the Company the authority of the Committee to grant and determine the terms and conditions of Awards granted under the Plan, subject to the requirements of state law and such other limitations as the Committee shall determine. In no event shall any such delegation of authority be permitted with respect to Awards to any members of the Board or to any Eligible Person who is subject to Rule 16b-3 under the Exchange Act or Section 162(m) of the Code. The Committee shall also be permitted to delegate, to any appropriate officer or employee of the Company, responsibility for performing certain ministerial functions under the Plan. In the event that the Committee’s authority is delegated to officers or employees in accordance with the foregoing, all provisions of the Plan relating to the Committee shall be interpreted in a manner consistent with the foregoing by treating any such reference as a reference to such officer or employee for such purpose. Any action undertaken in accordance with the Committee’s delegation of authority hereunder shall have the same force and effect as if such action was undertaken directly by the Committee and shall be deemed for all purposes of the Plan to have been taken by the Committee.
4. Shares Subject to the Plan.
4.1 Maximum Share Limitations. Subject to Section 4.2 hereof, the maximum aggregate number of shares of Common Stock that may be issued and sold under all Awards granted under the Plan shall be two million five hundred thousand (2,500,000) shares. Shares of Common Stock issued and sold under the Plan may be either authorized but unissued shares or shares held in the Company’s treasury. To the extent that any Award involving the issuance of shares of Common Stock is forfeited, cancelled, returned to the Company for failure to satisfy vesting requirements or other conditions of the Award, or otherwise terminates without an issuance of shares of Common Stock being made thereunder, the shares of Common Stock covered thereby will no longer be counted against the foregoing maximum share limitations and may again be made subject to Awards under the Plan pursuant to such limitations. Any Awards or portions thereof that are settled in cash and not in shares of Common Stock shall not be counted against the foregoing maximum share limitations.
4.2 Annual Increase. The aggregate number of shares of Common Stock reserved for Awards under the Plan will automatically increase on January 1st of each year, for a period of not more than ten (10) years, commencing on January 1st of the year following the year in which the Effective Date occurs and ending on (and including) January 1, 2029, in an amount equal to six percent (6%) of the total number of shares of outstanding Common Stock on December 31st of the preceding calendar year. Notwithstanding the foregoing, the Board or the Committee may act prior to January 1st of a given year to provide that there will be no January 1st increase for such year or that the increase for such year will be a lesser number of shares of Common Stock than provided herein.
| E-3 |
4.3 Adjustments. In the event of changes in the outstanding Common Stock or in the capital structure of the Company by reason of any stock or extraordinary cash dividend, stock split, reverse stock split, an extraordinary corporate transaction such as any recapitalization, reorganization, merger, consolidation, combination, exchange, or other relevant change in capitalization occurring after the Date of Grant of any Award, Awards granted under the Plan and any Award Agreements, the exercise price of Stock Options, the maximum number of shares of Common Stock subject to all Awards stated in Section 4 hereof will be equitably adjusted or substituted, as to the number, price or kind of consideration. In the case of adjustments made pursuant to this Section 4.3, the Committee shall, in the case of Incentive Stock Options, ensure that any adjustments under this Section 4.3 will not constitute a modification, extension or renewal of the Incentive Stock Options within the meaning of Section 424(h)(3) of the Code and in the case of Nonqualified Stock Options, ensure that any adjustments under this Section 4.3 will not constitute a modification of such Nonqualified Stock Options within the meaning of Section 409A of the Code. Any adjustments made under this Section 4.3 shall be made in a manner which does not adversely affect the exemption provided pursuant to Rule 16b-3 under the Exchange Act. Further, with respect to Awards intended to qualify as “performance-based compensation” under Section 162(m) of the Code, any adjustments or substitutions will not cause the Company to be denied a tax deduction on account of Section 162(m) of the Code. The Company shall give each Participant notice of an adjustment hereunder and, upon notice, such adjustment shall be conclusive and binding for all purposes.
5. Participation and Awards.
5.1 Designations of Participants. All Eligible Persons are eligible to be designated by the Committee to receive Awards and become Participants under the Plan. The Committee has the authority, in its discretion, to determine and designate from time to time those Eligible Persons who are to be granted Awards, the types of Awards to be granted and the number of shares of Common Stock or units subject to Awards granted under the Plan. In selecting Eligible Persons to be Participants and in determining the type and amount of Awards to be granted under the Plan, the Committee shall consider any and all factors that it deems relevant or appropriate.
5.2 Determination of Awards. The Committee shall determine the terms and conditions of all Awards granted to Participants in accordance with its authority under Section 3.2 hereof. An Award may consist of one type of right or benefit hereunder or of two or more such rights or benefits granted in tandem or in the alternative. In the case of any fractional share or unit resulting from the grant, vesting, payment or crediting of dividends or dividend equivalents under an Award, the Committee shall have the discretionary authority to (i) disregard such fractional share or unit, (ii) round such fractional share or unit to the nearest lower or higher whole share or unit, or (iii) convert such fractional share or unit into a right to receive a cash payment. To the extent deemed necessary by the Committee, an Award shall be evidenced by an Award Agreement as described in Section 11.1 hereof.
6. Stock Options.
6.1 Grant of Stock Options. A Stock Option may be granted to any Eligible Person selected by the Committee. Subject to the provisions of Section 6.8 hereof and Section 422 of the Code, each Stock Option shall be designated, in the discretion of the Committee, as an Incentive Stock Option or as a Nonqualified Stock Option.
6.2 Exercise Price. The exercise price per share of a Stock Option shall not be less than 85 percent of the Fair Market Value of the shares of Common Stock on the Date of Grant, provided that the Committee may in its discretion specify for any Stock Option an exercise price per share that is higher than the Fair Market Value on the Date of Grant, except that the price shall not be less than 110 percent of the Fair Market Value in the case of any person who owns securities possessing more than 10 percent of the total combined voting power of all classes of securities of the Company.
6.3 Vesting of Stock Options. The Committee shall in its discretion prescribe the time or times at which, or the conditions upon which, a Stock Option or portion thereof shall become vested and/or exercisable, and may accelerate the vesting or exercisability of any Stock Option at any time, provided, however, that any Stock Option shall vest at the rate of at least twenty percent (20%) per year over five (5) years from the date the Stock Option is granted, subject to reasonable conditions as may be provided for in the Award Agreement. However, in the case of a Stock Option granted to officers, Non-employee Directors, managers or Consultants of the Company, the Stock Option may become fully exercisable, subject to reasonable conditions, at any time or during any period established by the Company. The requirements for vesting and exercisability of a Stock Option may be based on the continued Service of the Participant with the Company or its Affiliates for a specified time period (or periods) or on the attainment of specified performance goals established by the Committee in its discretion.
| E-4 |
6.4 Term of Stock Options. The Committee shall in its discretion prescribe in an Award Agreement the period during which a vested Stock Option may be exercised, provided that the maximum term of a Stock Option shall be ten years from the Date of Grant. Except as otherwise provided in this Section 6 or as otherwise may be provided by the Committee, no Stock Option issued to an employee or a Non-Employee Director of the Company may be exercised at any time during the term thereof unless the employee or a Non-Employee Director Participant is then in the Service of the Company or one of its Affiliates.
6.5 Termination of Service. Subject to Section 6.8 hereof with respect to Incentive Stock Options, the Stock Option of any Participant whose Service with the Company or one of its Affiliates is terminated for any reason shall terminate on the earlier of (A) the date that the Stock Option expires in accordance with its terms or (B) unless otherwise provided in an Award Agreement, and except for termination for cause (as described in Section 10.2 hereof), the expiration of the applicable time period following termination of Service, in accordance with the following: (1) twelve months if Service ceased due to Disability, (2) eighteen months if Service ceased at a time when the Participant is eligible to elect immediate commencement of retirement benefits at a specified retirement age under a pension plan to which the Company or any of its Affiliates had made contributions, (3) eighteen months if the Participant died while in the Service of the Company or any of its Affiliates, or (iv) three months if Service ceased for any other reason. During the foregoing applicable period, except as otherwise specified in the Award Agreement or in the event Service was terminated by the death of the Participant, the Stock Option may be exercised by such Participant in respect of the same number of shares of Common Stock, in the same manner, and to the same extent as if he or she had remained in the continued Service of the Company or any Affiliate during the first three months of such period; provided that no additional rights shall vest after such three months. The Committee shall have authority to determine in each case whether an authorized leave of absence shall be deemed a termination of Service for purposes hereof, as well as the effect of a leave of absence on the vesting and exercisability of a Stock Option. Unless otherwise provided by the Committee, if an entity ceases to be an Affiliate of the Company or otherwise ceases to be qualified under the Plan or if all or substantially all of the assets of an Affiliate of the Company are conveyed (other than by encumbrance), such cessation or action, as the case may be, shall be deemed for purposes hereof to be a termination of the Service.
6.6 Stock Option Exercise; Tax Withholding. Subject to such terms and conditions as shall be specified in an Award Agreement, a Stock Option may be exercised in whole or in part at any time during the term thereof by notice in the form required by the Company, together with payment of the aggregate exercise price therefor and applicable withholding tax. Payment of the exercise price shall be made in the manner set forth in the Award Agreement, unless otherwise provided by the Committee: (i) in cash or by cash equivalent acceptable to the Committee, (ii) by payment in shares of Common Stock that have been held by the Participant for at least six months (or such period as the Committee may deem appropriate, for accounting purposes or otherwise) valued at the Fair Market Value of such shares on the date of exercise, (iii) through an open-market, broker-assisted sales transaction pursuant to which the Company is promptly delivered the amount of proceeds necessary to satisfy the exercise price, (iv) by a combination of the methods described above or (v) by such other method as may be approved by the Committee and set forth in the Award Agreement. In addition to and at the time of payment of the exercise price, the Participant shall pay to the Company the full amount of any and all applicable income tax, employment tax and other amounts required to be withheld in connection with such exercise, payable under such of the methods described above for the payment of the exercise price as may be approved by the Committee and set forth in the Award Agreement.
6.7 Limited Transferability of Nonqualified Stock Options. All Stock Options shall be nontransferable except (i) upon the Participant’s death, in accordance with Section 11.2 hereof or (ii) in the case of Nonqualified Stock Options only, for the transfer of all or part of the Stock Option to a Participant’s “family member” (as defined for purposes of the Form S-8 registration statement under the Securities Act of 1933), as may be approved by the Committee in its discretion at the time of proposed transfer. The transfer of a Nonqualified Stock Option may be subject to such terms and conditions as the Committee may in its discretion impose from time to time. Subsequent transfers of a Nonqualified Stock Option shall be prohibited other than in accordance with Section 11.2 hereof.
| E-5 |
6.8 Additional Rules for Incentive Stock Options.
(a) Eligibility. An Incentive Stock Option may only be granted to an Eligible Person who is considered an employee for purposes of Treasury Regulation §1.421-7(h) with respect to the Company or any Affiliate that qualifies as a “subsidiary corporation” with respect to the Company for purposes of Section 424(f) of the Code.
(b) Termination of Employment. An Award of an Incentive Stock Option may provide that such Stock Option may be exercised not later than 3 months following termination of employment of the Participant with the Company and all subsidiaries, or not later than one year following a permanent and total disability within the meaning of Section 22(e)(3) of the Code, as and to the extent determined by the Committee to comply with the requirements of Section 422 of the Code.
(c) Other Terms and Conditions; Nontransferability. Any Incentive Stock Option granted hereunder shall contain such additional terms and conditions, not inconsistent with the terms of the Plan, as are deemed necessary or desirable by the Committee, which terms, together with the terms of the Plan, shall be intended and interpreted to cause such Incentive Stock Option to qualify as an “incentive stock option” under Section 422 of the Code. An Award Agreement for an Incentive Stock Option may provide that such Stock Option shall be treated as a Nonqualified Stock Option to the extent that certain requirements applicable to “incentive stock options” under the Code shall not be satisfied. An Incentive Stock Option shall by its terms be nontransferable other than by will or by the laws of descent and distribution, and shall be exercisable during the lifetime of a Participant only by such Participant.
(d) Disqualifying Dispositions. If shares of Common Stock acquired by exercise of an Incentive Stock Option are disposed of within two years following the Date of Grant or one year following the transfer of such shares to the Participant upon exercise, the Participant shall, promptly following such disposition, notify the Company in writing of the date and terms of such disposition and provide such other information regarding the disposition as the Company may reasonably require.
6.9 Repricing Prohibited. Subject to the adjustment provisions contained in Section 4.2 hereof, without the prior approval of the Company’s stockholders, evidenced by a majority of votes cast, neither the Committee nor the Board shall cause the cancellation, substitution or amendment of a Stock Option that would have the effect of reducing the exercise price of such a Stock Option previously granted under the Plan, or otherwise approve any modification to such a Stock Option that would be treated as a “repricing” under the then applicable rules, regulations or listing requirements.
7. Stock Awards.
7.1 Grant of Stock Awards. A Stock Award may be granted to any Eligible Person selected by the Committee. A Stock Award may be granted for past services, in lieu of bonus or other cash compensation, as directors’ compensation or for any other valid purpose as determined by the Committee. A Stock Award granted to an Eligible Person represents shares of Common Stock that are issued without restrictions on transfer and other incidents of ownership and free of forfeiture conditions, except as otherwise provided in the Plan and the Award Agreement. The deemed issuance price of shares of Common Stock subject to each Stock Award shall not be less than 85 percent of the Fair Market Value of the Common Stock on the date of the grant. In the case of any person who owns securities possessing more than ten percent of the combined voting power of all classes of securities of the issuer or its parent or subsidiaries possessing voting power, the deemed issuance price of shares of Common Stock subject to each Stock Award shall be at least 100 percent of the Fair Market Value of the Common Stock on the date of the grant. The Committee may, in connection with any Stock Award, require the payment of a specified purchase price.
7.2 Rights as Stockholder. Subject to the foregoing provisions of this Section 7 and the applicable Award Agreement, upon the issuance of the Common Stock under a Stock Award the Participant shall have all rights of a stockholder with respect to the shares of Common Stock, including the right to vote the shares and receive all dividends and other distributions paid or made with respect thereto.
| E-6 |
8. Restricted Stock Awards.
8.1 Grant of Restricted Stock Awards. A Restricted Stock Award may be granted to any Eligible Person selected by the Committee. The deemed issuance price of shares of Common Stock subject to each Restricted Stock Award shall not be less than 85 percent of the Fair Market Value of the Common Stock on the date of the grant. In the case of any person who owns securities possessing more than ten percent of the combined voting power of all classes of securities of the issuer or its parent or subsidiaries possessing voting power, the deemed issuance price of shares of Common Stock subject to each Restricted Stock Award shall be at least 100 percent of the Fair Market Value of the Common Stock on the date of the grant. The Committee may require the payment by the Participant of a specified purchase price in connection with any Restricted Stock Award.
8.2 Vesting Requirements. The restrictions imposed on shares granted under a Restricted Stock Award shall lapse in accordance with the vesting requirements specified by the Committee in the Award Agreement, provided that the Committee may accelerate the vesting of a Restricted Stock Award at any time. Such vesting requirements may be based on the continued Service of the Participant with the Company or its Affiliates for a specified time period (or periods) or on the attainment of specified performance goals established by the Committee in its discretion. If the vesting requirements of a Restricted Stock Award shall not be satisfied, the Award shall be forfeited and the shares of Common Stock subject to the Award shall be returned to the Company.
8.3 Restrictions. Shares granted under any Restricted Stock Award may not be transferred, assigned or subject to any encumbrance, pledge, or charge until all applicable restrictions are removed or have expired, unless otherwise allowed by the Committee. Failure to satisfy any applicable restrictions shall result in the subject shares of the Restricted Stock Award being forfeited and returned to the Company. The Committee may require in an Award Agreement that certificates representing the shares granted under a Restricted Stock Award bear a legend making appropriate reference to the restrictions imposed, and that certificates representing the shares granted or sold under a Restricted Stock Award will remain in the physical custody of an escrow holder until all restrictions are removed or have expired.
8.4 Rights as Stockholder. Subject to the foregoing provisions of this Section 8 and the applicable Award Agreement, the Participant shall have all rights of a stockholder with respect to the shares granted to the Participant under a Restricted Stock Award, including the right to vote the shares and receive all dividends and other distributions paid or made with respect thereto. The Committee may provide in an Award Agreement for the payment of dividends and distributions to the Participant at such times as paid to stockholders generally or at the times of vesting or other payment of the Restricted Stock Award.
8.5 Section 83(b) Election. If a Participant makes an election pursuant to Section 83(b) of the Code with respect to a Restricted Stock Award, the Participant shall file, within 30 days following the Date of Grant, a copy of such election with the Company and with the Internal Revenue Service, in accordance with the regulations under Section 83 of the Code. The Committee may provide in an Award Agreement that the Restricted Stock Award is conditioned upon the Participant’s making or refraining from making an election with respect to the Award under Section 83(b) of the Code.
9. Change in Control.
9.1 Effect of Change in Control. Except to the extent an Award Agreement provides for a different result (in which case the Award Agreement will govern and this Section 9 of the Plan shall not be applicable), notwithstanding anything elsewhere in the Plan or any rules adopted by the Committee pursuant to the Plan to the contrary, if a Triggering Event shall occur within the 12-month period beginning with a Change in Control of the Company, then, effective immediately prior to such Triggering Event, each outstanding Stock Option, to the extent that it shall not otherwise have become vested and exercisable, shall automatically become fully and immediately vested and exercisable, without regard to any otherwise applicable vesting requirement.
| E-7 |
9.2 Definitions
(a) Cause. For purposes of this Section 9, the term “Cause” shall mean a determination by the Committee that a Participant (i) has been convicted of, or entered a plea of nolo contendere to, a crime that constitutes a felony under Federal or state law, (ii) has engaged in willful gross misconduct in the performance of the Participant’s duties to the Company or an Affiliate or (iii) has committed a material breach of any written agreement with the Company or any Affiliate with respect to confidentiality, noncompetition, nonsolicitation or similar restrictive covenant. Subject to the first sentence of Section 9.1 hereof, in the event that a Participant is a party to an employment agreement with the Company or any Affiliate that defines a termination on account of “Cause” (or a term having similar meaning), such definition shall apply as the definition of a termination on account of “Cause” for purposes hereof, but only to the extent that such definition provides the Participant with greater rights. A termination on account of Cause shall be communicated by written notice to the Participant, and shall be deemed to occur on the date such notice is delivered to the Participant.
(b) Change in Control. For purposes of this Section 9, a “Change in Control” shall be deemed to have occurred upon:
(i) the occurrence of an acquisition by any individual, entity or group (within the meaning of Section 13(d)(3) or 14(d)(2) of the Exchange Act) (a “Person”) of beneficial ownership (within the meaning of Rule 13d-3 promulgated under the Exchange Act) of a percentage of the combined voting power of the then outstanding voting securities of the Company entitled to vote generally in the election of directors (the “Company Voting Securities”) (but excluding (1) any acquisition directly from the Company (other than an acquisition by virtue of the exercise of a conversion privilege of a security that was not acquired directly from the Company), (2) any acquisition by the Company or an Affiliate and (3) any acquisition by an employee benefit plan (or related trust) sponsored or maintained by the Company or any Affiliate) (an “Acquisition”) that is thirty percent (30%) or more of the Company Voting Securities;
(ii) at any time during a period of two (2) consecutive years or less, individuals who at the beginning of such period constitute the Board (and any new directors whose election by the Board or nomination for election by the Company’s stockholders was approved by a vote of at least two-thirds (2/3) of the directors then still in office who either were directors at the beginning of the period or whose election or nomination for election was so approved) cease for any reason (except for death, Disability or voluntary retirement) to constitute a majority thereof;
(iii) an Acquisition that is fifty percent (50%) or more of the Company Voting Securities;
(iv) the consummation of a merger, consolidation, reorganization or similar corporate transaction, whether or not the Company is the surviving company in such transaction, other than a merger, consolidation, or reorganization that would result in the Persons who are beneficial owners of the Company Voting Securities outstanding immediately prior thereto continuing to beneficially own, directly or indirectly, in substantially the same proportions, at least fifty percent (50%) of the combined voting power of the Company Voting Securities (or the voting securities of the surviving entity) outstanding immediately after such merger, consolidation or reorganization;
(v) the sale or other disposition of all or substantially all of the assets of the Company;
(vi) the approval by the stockholders of the Company of a complete liquidation or dissolution of the Company; or
(vii) the occurrence of any transaction or event, or series of transactions or events, designated by the Board in a duly adopted resolution as representing a change in the effective control of the business and affairs of the Company, effective as of the date specified in any such resolution.
(c) Constructive Termination. For purposes of this Section 9, a “Constructive Termination” shall mean a termination of employment by a Participant within sixty (60) days following the occurrence of any one or more of the following events without the Participant’s written consent (i) any reduction in position, title (for Vice Presidents or above), overall responsibilities, level of authority, level of reporting (for Vice Presidents or above), base compensation, annual incentive compensation opportunity, aggregate employee benefits or (ii) a request that the Participant’s location of employment be relocated by more than fifty (50) miles. Subject to the first sentence of Section 9.1 hereof, in the event that a Participant is a party to an employment agreement with the Company or any Affiliate (or a successor entity) that defines a termination on account of “Constructive Termination,” “Good Reason” or “Breach of Agreement” (or a term having a similar meaning), such definition shall apply as the definition of “Constructive Termination” for purposes hereof in lieu of the foregoing, but only to the extent that such definition provides the Participant with greater rights. A Constructive Termination shall be communicated by written notice to the Committee, and shall be deemed to occur on the date such notice is delivered to the Committee, unless the circumstances giving rise to the Constructive Termination are cured within five (5) days of such notice.
| E-8 |
(d) Triggering Event. For purposes of this Section 9, a “Triggering Event” shall mean (i) the termination of Service of a Participant by the Company or an Affiliate (or any successor thereof) other than on account of death, Disability or Cause, (ii) the occurrence of a Constructive Termination or (iii) any failure by the Company (or a successor entity) to assume, replace, convert or otherwise continue any Award in connection with the Change in Control (or another corporate transaction or other change effecting the Common Stock) on the same terms and conditions as applied immediately prior to such transaction, except for equitable adjustments to reflect changes in the Common Stock pursuant to Section 4.2 hereof.
9.3 Excise Tax Limit. In the event that the vesting of Awards together with all other payments and the value of any benefit received or to be received by a Participant would result in all or a portion of such payment being subject to the excise tax under Section 4999 of the Code, then the Participant’s payment shall be either (i) the full payment or (ii) such lesser amount that would result in no portion of the payment being subject to excise tax under Section 4999 of the Code (the “Excise Tax”), whichever of the foregoing amounts, taking into account the applicable Federal, state, and local employment taxes, income taxes, and the Excise Tax, results in the receipt by the Participant, on an after-tax basis, of the greatest amount of the payment notwithstanding that all or some portion of the payment may be taxable under Section 4999 of the Code. All determinations required to be made under this Section 9 shall be made by M&K CPAS, PLLC. or any other accounting firm which is the Company’s outside auditor immediately prior to the event triggering the payments that are subject to the Excise Tax (the “Accounting Firm”). The Company shall cause the Accounting Firm to provide detailed supporting calculations of its determinations to the Company and the Participant. All fees and expenses of the Accounting Firm shall be borne solely by the Company. The Accounting Firm’s determinations must be made with substantial authority (within the meaning of Section 6662 of the Code). For the purposes of all calculations under Section 280G of the Code and the application of this Section 9.3, all determinations as to present value shall be made using 120 percent of the applicable Federal rate (determined under Section 1274(d) of the Code) compounded semiannually.
10. Forfeiture Events.
10.1 General. The Committee may specify in an Award Agreement at the time of the Award that the Participant’s rights, payments and benefits with respect to an Award shall be subject to reduction, cancellation, forfeiture or recoupment upon the occurrence of certain specified events, in addition to any otherwise applicable vesting or performance conditions of an Award. Such events shall include, but shall not be limited to, termination of Service for cause, violation of material Company policies, breach of noncompetition, confidentiality or other restrictive covenants that may apply to the Participant, or other conduct by the Participant that is detrimental to the business or reputation of the Company.
10.2 Termination for Cause. Unless otherwise provided by the Committee and set forth in an Award Agreement, if a Participant’s employment with the Company or any Affiliate shall be terminated for cause, the Company may, in its sole discretion, immediately terminate such Participant’s right to any further payments, vesting or exercisability with respect to any Award in its entirety. In the event a Participant is party to an employment (or similar) agreement with the Company or any Affiliate that defines the term “cause,” such definition shall apply for purposes of the Plan. The Company shall have the power to determine whether the Participant has been terminated for cause and the date upon which such termination for cause occurs. Any such determination shall be final, conclusive and binding upon the Participant. In addition, if the Company shall reasonably determine that a Participant has committed or may have committed any act which could constitute the basis for a termination of such Participant’s employment for cause, the Company may suspend the Participant’s rights to exercise any option, receive any payment or vest in any right with respect to any Award pending a determination by the Company of whether an act has been committed which could constitute the basis for a termination for “cause” as provided in this Section 10.2.
| E-9 |
11. General Provisions.
11.1 Award Agreement. To the extent deemed necessary by the Committee, an Award under the Plan shall be evidenced by an Award Agreement in a written or electronic form approved by the Committee setting forth the number of shares of Common Stock or units subject to the Award, the exercise price, base price, or purchase price of the Award, the time or times at which an Award will become vested, exercisable or payable and the term of the Award. The Award Agreement may also set forth the effect on an Award of termination of Service under certain circumstances. The Award Agreement shall be subject to and incorporate, by reference or otherwise, all of the applicable terms and conditions of the Plan, and may also set forth other terms and conditions applicable to the Award as determined by the Committee consistent with the limitations of the Plan. Award Agreements evidencing Incentive Stock Options shall contain such terms and conditions as may be necessary to meet the applicable provisions of Section 422 of the Code. The grant of an Award under the Plan shall not confer any rights upon the Participant holding such Award other than such terms, and subject to such conditions, as are specified in the Plan as being applicable to such type of Award (or to all Awards) or as are expressly set forth in the Award Agreement. The Committee need not require the execution of an Award Agreement by a Participant, in which case, acceptance of the Award by the Participant shall constitute agreement by the Participant to the terms, conditions, restrictions and limitations set forth in the Plan and the Award Agreement as well as the administrative guidelines of the Company in effect from time to time.
11.2 No Assignment or Transfer; Beneficiaries. Except as provided in Section 6.7 hereof, Awards under the Plan shall not be assignable or transferable by the Participant, except by will or by the laws of descent and distribution, and shall not be subject in any manner to assignment, alienation, pledge, encumbrance or charge. Notwithstanding the foregoing, the Committee may provide in the terms of an Award Agreement that the Participant shall have the right to designate a beneficiary or beneficiaries who shall be entitled to any rights, payments or other benefits specified under an Award following the Participant’s death. During the lifetime of a Participant, an Award shall be exercised only by such Participant or such Participant’s guardian or legal representative. In the event of a Participant’s death, an Award may to the extent permitted by the Award Agreement be exercised by the Participant’s beneficiary as designated by the Participant in the manner prescribed by the Committee or, in the absence of an authorized beneficiary designation, by the legatee of such Award under the Participant’s will or by the Participant’s estate in accordance with the Participant’s will or the laws of descent and distribution, in each case in the same manner and to the same extent that such Award was exercisable by the Participant on the date of the Participant’s death.
11.3 Deferrals of Payment. The Committee may in its discretion permit a Participant to defer the receipt of payment of cash or delivery of shares of Common Stock that would otherwise be due to the Participant by virtue of the exercise of a right or the satisfaction of vesting or other conditions with respect to an Award. If any such deferral is to be permitted by the Committee, the Committee shall establish rules and procedures relating to such deferral in a manner intended to comply with the requirements of Section 409A of the Code, including, without limitation, the time when an election to defer may be made, the time period of the deferral and the events that would result in payment of the deferred amount, the interest or other earnings attributable to the deferral and the method of funding, if any, attributable to the deferred amount.
11.4 Rights as Stockholder. A Participant shall have no rights as a holder of shares of Common Stock with respect to any unissued securities covered by an Award until the date the Participant becomes the holder of record of such securities. Except as provided in Section 4.2 hereof, no adjustment or other provision shall be made for dividends or other stockholder rights, except to the extent that the Award Agreement provides for dividend payments or dividend equivalent rights.
11.5 Employment or Service. Nothing in the Plan, in the grant of any Award or in any Award Agreement shall confer upon any Eligible Person any right to continue in the Service of the Company or any of its Affiliates, or interfere in any way with the right of the Company or any of its Affiliates to terminate the Participant’s employment or other service relationship for any reason at any time.
| E-10 |
11.6 Securities Laws. No shares of Common Stock will be issued or transferred pursuant to an Award unless and until all then applicable requirements imposed by Federal and state securities and other laws, rules and regulations and by any regulatory agencies having jurisdiction, and by any exchanges upon which the shares of Common Stock may be listed, have been fully met. As a condition precedent to the issuance of shares pursuant to the grant or exercise of an Award, the Company may require the Participant to take any reasonable action to meet such requirements. The Committee may impose such conditions on any shares of Common Stock issuable under the Plan as it may deem advisable, including, without limitation, restrictions under the Securities Act of 1933, as amended, under the requirements of any exchange upon which such shares of the same class are then listed, and under any blue sky or other securities laws applicable to such shares. The Committee may also require the Participant to represent and warrant at the time of issuance or transfer that the shares of Common Stock are being acquired only for investment purposes and without any current intention to sell or distribute such shares.
11.7 Tax Withholding. The Participant shall be responsible for payment of any taxes or similar charges required by law to be withheld from an Award or an amount paid in satisfaction of an Award, which shall be paid by the Participant on or prior to the payment or other event that results in taxable income in respect of an Award. The Award Agreement may specify the manner in which the withholding obligation shall be satisfied with respect to the particular type of Award.
11.8 Unfunded Plan. The adoption of the Plan and any reservation of shares of Common Stock or cash amounts by the Company to discharge its obligations hereunder shall not be deemed to create a trust or other funded arrangement. Except upon the issuance of Common Stock pursuant to an Award, any rights of a Participant under the Plan shall be those of a general unsecured creditor of the Company, and neither a Participant nor the Participant’s permitted transferees or estate shall have any other interest in any assets of the Company by virtue of the Plan. Notwithstanding the foregoing, the Company shall have the right to implement or set aside funds in a grantor trust, subject to the claims of the Company’s creditors or otherwise, to discharge its obligations under the Plan.
11.9 Other Compensation and Benefit Plans. The adoption of the Plan shall not affect any other share incentive or other compensation plans in effect for the Company or any Affiliate, nor shall the Plan preclude the Company from establishing any other forms of share incentive or other compensation or benefit program for employees of the Company or any Affiliate. The amount of any compensation deemed to be received by a Participant pursuant to an Award shall not constitute includable compensation for purposes of determining the amount of benefits to which a Participant is entitled under any other compensation or benefit plan or program of the Company or an Affiliate, including, without limitation, under any pension or severance benefits plan, except to the extent specifically provided by the terms of any such plan.
11.10 Plan Binding on Transferees. The Plan shall be binding upon the Company, its transferees and assigns, and the Participant, the Participant’s executor, administrator and permitted transferees and beneficiaries.
11.11 Severability. If any provision of the Plan or any Award Agreement shall be determined to be illegal or unenforceable by any court of law in any jurisdiction, the remaining provisions hereof and thereof shall be severable and enforceable in accordance with their terms, and all provisions shall remain enforceable in any other jurisdiction.
11.12 Foreign Jurisdictions. The Committee may adopt, amend and terminate such arrangements and grant such Awards, not inconsistent with the intent of the Plan, as it may deem necessary or desirable to comply with any tax, securities, regulatory or other laws of other jurisdictions with respect to Awards that may be subject to such laws. The terms and conditions of such Awards may vary from the terms and conditions that would otherwise be required by the Plan solely to the extent the Committee deems necessary for such purpose. Moreover, the Board may approve such supplements to or amendments, restatements or alternative versions of the Plan, not inconsistent with the intent of the Plan, as it may consider necessary or appropriate for such purposes, without thereby affecting the terms of the Plan as in effect for any other purpose.
11.13 Substitute Awards in Corporate Transactions. Nothing contained in the Plan shall be construed to limit the right of the Committee to grant Awards under the Plan in connection with the acquisition, whether by purchase, merger, consolidation or other corporate transaction, of the business or assets of any corporation or other entity. Without limiting the foregoing, the Committee may grant Awards under the Plan to an employee or director of another corporation who becomes an Eligible Person by reason of any such corporate transaction in substitution for awards previously granted by such corporation or entity to such person. The terms and conditions of the substitute Awards may vary from the terms and conditions that would otherwise be required by the Plan solely to the extent the Committee deems necessary for such purpose.
| E-11 |
11.14 Governing Law. The Plan and all rights hereunder shall be subject to and interpreted in accordance with the laws of the State of Nevada, without reference to the principles of conflicts of laws, and to applicable Federal securities laws.
11.15 Financial Statements. All Participants shall receive the financial statements of the Company at least annually.
11.16 Performance Based Awards. For purposes of Stock Awards and Restricted Stock Awards granted under the Plan that are intended to qualify as “performance-based” compensation under Section 162(m) of the Code, such Awards shall be granted to the extent necessary to satisfy the requirements of Section 162(m) of the Code.
11.17 Stockholder Approval. The Plan must be approved by the stockholders by a majority of all shares entitled to vote within twelve (12) months after the date the Plan was adopted by the Board. Any Incentive Stock Options granted before stockholder approval is obtained shall be converted into Nonqualified Stock Options if stockholder approval is not obtained within twelve (12) months before or after the Plan was adopted.
12. Effective Date; Amendment and Termination.
12.1 Effective Date. The Plan shall become effective following its adoption by the Board (such date, the “Effective Date”). The term of the Plan shall be ten (10) years from the date of adoption by the Board, subject to Section 12.3 hereof.
12.2 Amendment. The Board may at any time and from time to time and in any respect, amend or modify the Plan. The Board may seek the approval of any amendment or modification by the Company’s stockholders to the extent it deems necessary or advisable in its discretion for purposes of compliance with Section 162(m) or Section 422 of the Code, or exchange or securities market or for any other purpose. No amendment or modification of the Plan shall adversely affect any Award theretofore granted without the consent of the Participant or the permitted transferee of the Award.
12.3 Termination. The Plan shall terminate on the tenth anniversary of the date of its adoption by the Board. The Board may, in its discretion and at any earlier date, terminate the Plan. Notwithstanding the foregoing, no termination of the Plan shall adversely affect any Award theretofore granted without the consent of the Participant or the permitted transferee of the Award.
| E-12 |
ANNEX F
CERTIFICATE OF AMENDMENT TO RESTATED ARTICLES OF INCORPORATION

| F-1 |

| F-2 |
EXHIBIT A
CERTIFICATE OF AMENDMENT
TO THE
AMENDED AND RESTATED
ARTICLES OF INCORPORATION
OF
NAYA BIOSCIENCES, INC.
Upon the filing and effectiveness (the “Effective Time”) pursuant to the Nevada Revised Statutes of this Certificate of Amendment, the Amended and Restated Articles of Incorporation of Naya Biosciences, Inc. shall be amended by changing the section (a) of the Article thereof numbered “IV” so that, as amended Article IV(a) shall be read as follows:
(a) At the Effective Time, every [●] shares of Common Stock, par value of $0.00001 each, issued and outstanding immediately prior to the Effective Time shall, automatically and without any action on the part of the holder(s) thereof, be reverse split into one (1) share of Common Stock (the “Reverse Stock Split”). No fractional shares of Common Stock shall be issued as a result of the Reverse Stock Split and no fractional interest in a share of Common Stock shall be deliverable upon the Reverse Stock Split. Stockholders who otherwise would be entitled to receive fractional shares of Common Stock because they hold certain number of shares not evenly divisible by the Reverse Stock Split ratio will automatically be entitled to receive an additional fraction of a share of Common Stock up to the next whole share. Each certificate that immediately prior to the Effective Time represented shares of Common Stock (“Old Certificates”), shall thereafter represent that number of shares of Common Stock into which the shares of Common Stock represented by the Old Certificate shall have been reverse split, plus any additional fraction of a share of Common Stock to round up to the next whole share. After completion of the Reverse Stock Split, the total number of shares of all classes of stock of which the Corporation shall have the authority to issue is 200,000,000 shares, consisting of (i) 100,000,000 shares of Common Stock, $0.00001 par value per share (“Common Stock”), and (ii) 100,000,000 shares of Preferred Stock, $0.00001 par value per share (“Preferred Stock”).”
IN WITNESS WHEREOF, said corporation has caused this Annex to the Certificate of Amendment to be signed this [●] day of [●], 2025.
| By: | ||
| Authorized Officer | ||
| Title: | ||
| Name: | ||
| Print or Type |
| F-3 |