
A Cancer-Selective Gene Therapy Company Exhibit 99.1

Forward-Looking Statements This presentation contains forward-looking statements about Tocagen Inc. Words such as “believes,” “anticipates,” “plans,” “expects,” “indicates,” “will,” “should,” “intends,” “potential,” “suggests,” “assuming,” “designed” and similar expressions are intended to identify forward‐looking statements. These statements are based on the Company‘s current beliefs and expectations. These forward‐looking statements include statements regarding: the success, cost, timing and potential indications of Tocagen’s product development activities and clinical trials, including ongoing clinical trials of Toca 511 & Toca FC; Tocagen’s ability to obtain and maintain regulatory approval of product candidates, including Toca 511 & Toca FC, in any of the indications for which it plans to develop them, and any related restrictions, limitations, and/or warnings in the label of an approved product candidate; Tocagen’s ability to obtain funding for its operations, including funding necessary to complete the clinical trials of any of our product candidates, including Toca 511 & Toca FC; Tocagen’s plans to research, develop and commercialize its product candidates, including Toca 511 & Toca FC; Tocagen’s ability to attract and retain collaborators with development, regulatory and commercialization expertise; and regulatory developments in the United States and foreign countries. Actual results may differ materially from those expressed or implied in this presentation due to the risk and uncertainties inherent in the company’s business, including, without limitation, risks described in Tocagen’s filings with the Securities and Exchange Commission. You are cautioned not to place undue reliance on these forward‐looking statements, which speak only as of the date hereof, and Tocagen undertakes no obligation to revise or update this presentation to reflect events or circumstances after the date hereof. Further information regarding these and other risks is included under the heading "Risk Factors" in Tocagen's most recent financial report filed with the Securities and Exchange Commission and its other reports, which are available from the SEC's website (www.sec.gov) and on Tocagen’s website under the heading "Investors." All forward‐looking statements are qualified in their entirety by this cautionary statement. This caution is made under the safe harbor provisions of Section 21E of the Private Securities Litigation Reform Act of 1995.

Tocagen At-A-Glance San Diego, California biotechnology company with ~75 employees Founded in 2007 by pioneers of gene therapy Vision: No One Should Die Of Cancer Core technology is a differentiated retroviral replicating vector (RRV) platform Lead program in fully-enrolled pivotal Phase 3 trial under FDA Breakthrough Therapy Designation and EMA PRIME Designation

Marty J. Duvall Chief Executive Officer Mark Foletta EVP & Chief Financial Officer Asha Das, M.D. SVP & Chief Medical Officer Doug Jolly, Ph.D. Founder, EVP, Research and Pharm Development Harry Gruber, M.D. Founder, President Science and Innovation Executive Team with a Proven Track Record
Cancer-Selective Gene Therapy is in Our Name … TO CAncer GENe EXPLORE EXECUTE Achieve POC in other solid tumors with Toca 6 Investigate new opportunities using RRV platform Leverage Phase 1 data: CRs and survival Move Toca 511 & Toca FC to front-line HGG Advance opportunity in key geographies EXPAND BTD and PRIME designations in rHGG Accrual to Toca 5 Phase 3 study in rHGG Commercial launch planning in US

Technology Platform
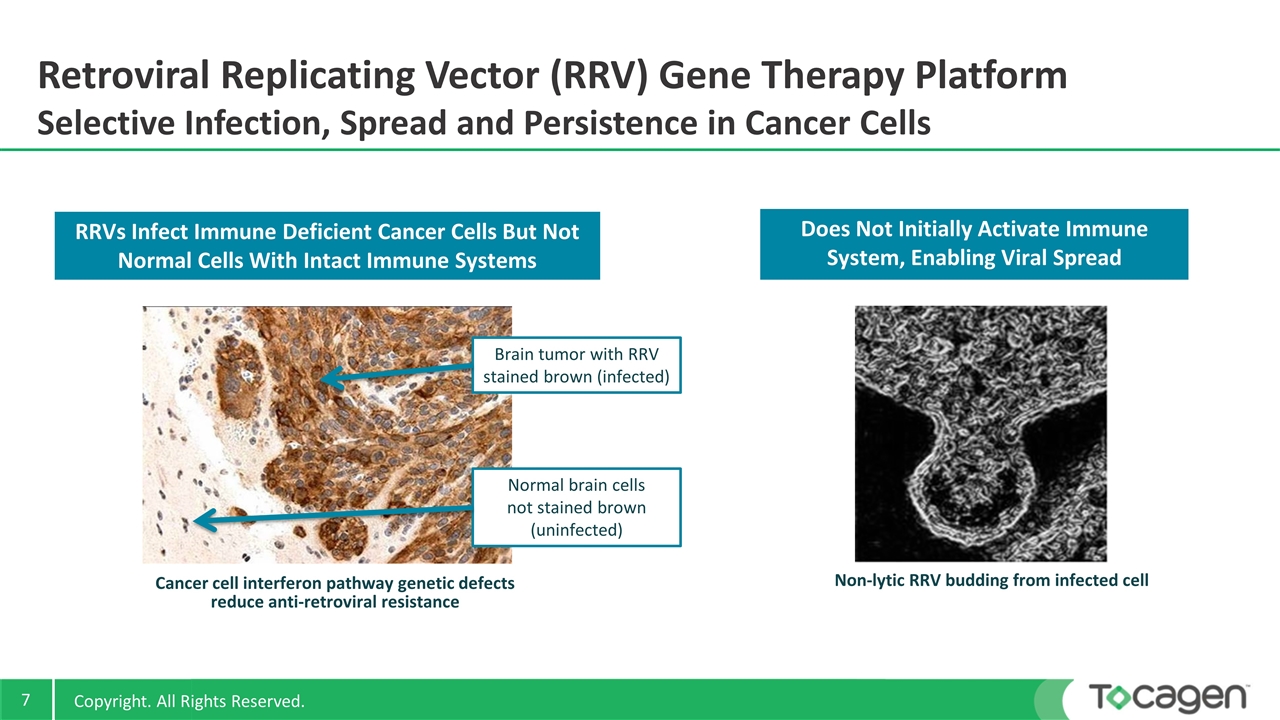
Retroviral Replicating Vector (RRV) Gene Therapy Platform Selective Infection, Spread and Persistence in Cancer Cells RRVs Infect Immune Deficient Cancer Cells But Not Normal Cells With Intact Immune Systems Cancer cell interferon pathway genetic defects reduce anti-retroviral resistance Does Not Initially Activate Immune System, Enabling Viral Spread Brain tumor with RRV stained brown (infected) Normal brain cells not stained brown (uninfected) Non-lytic RRV budding from infected cell
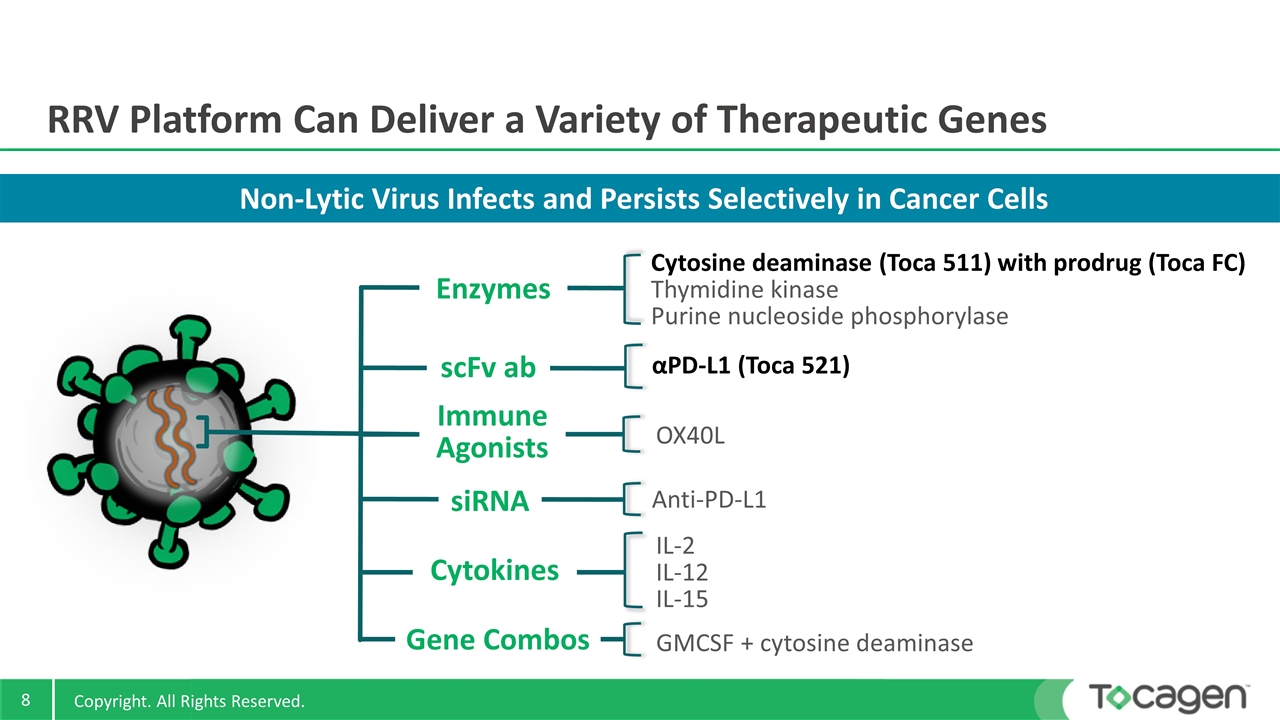
Non-Lytic Virus Infects and Persists Selectively in Cancer Cells RRV Platform Can Deliver a Variety of Therapeutic Genes IL-2 IL-12 IL-15 αPD-L1 (Toca 521) Cytosine deaminase (Toca 511) with prodrug (Toca FC) Thymidine kinase Purine nucleoside phosphorylase Anti-PD-L1 OX40L scFv ab Cytokines Enzymes siRNA Immune Agonists GMCSF + cytosine deaminase Gene Combos
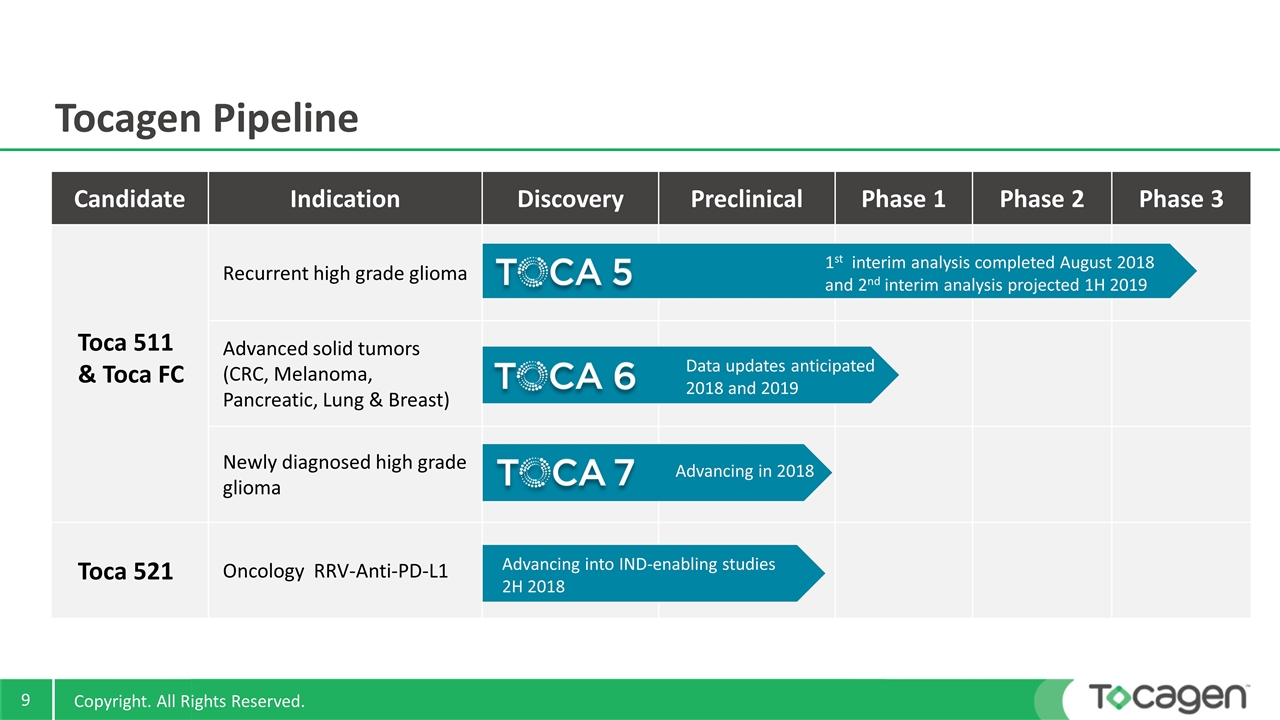
Candidate Indication Discovery Preclinical Phase 1 Phase 2 Phase 3 Toca 511 & Toca FC Recurrent high grade glioma Advanced solid tumors (CRC, Melanoma, Pancreatic, Lung & Breast) Newly diagnosed high grade glioma Toca 521 Oncology RRV-Anti-PD-L1 Tocagen Pipeline 1st interim analysis completed August 2018 and 2nd interim analysis projected 1H 2019 Data updates anticipated 2018 and 2019 Advancing into IND-enabling studies 2H 2018 Advancing in 2018

Lead Program Toca 511 & Toca FC
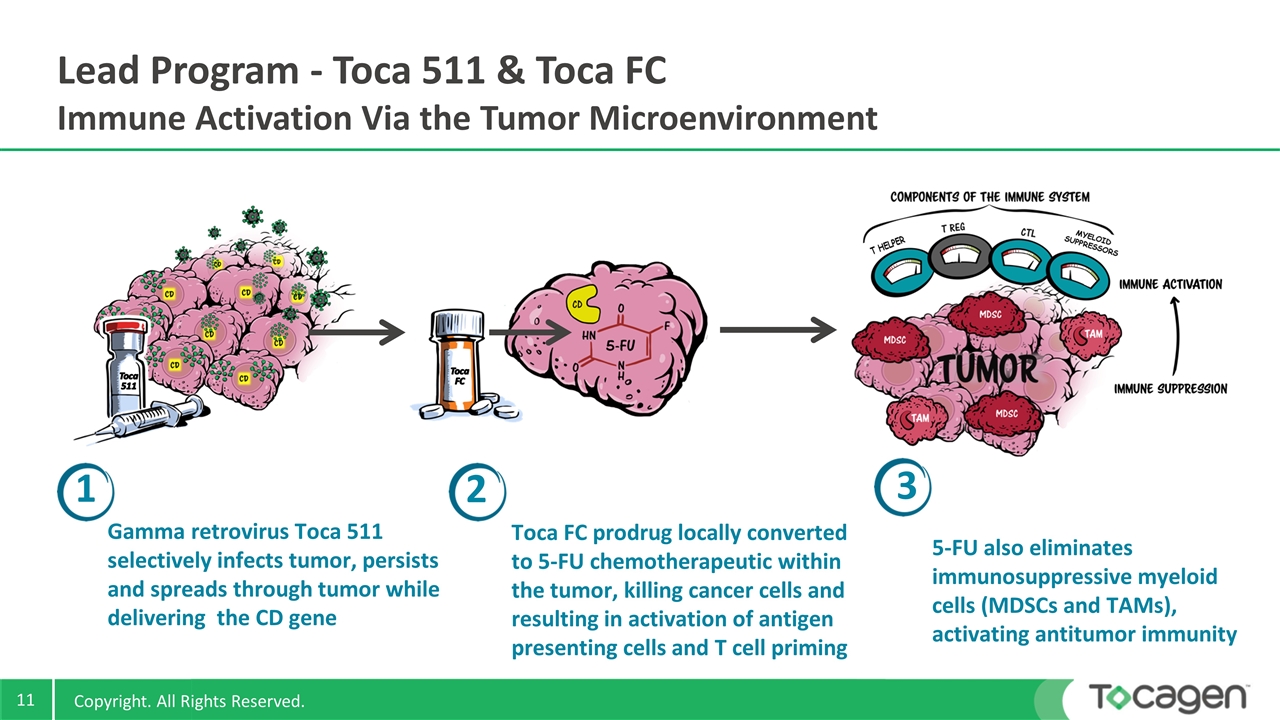
Lead Program - Toca 511 & Toca FC Immune Activation Via the Tumor Microenvironment 5-FU also eliminates immunosuppressive myeloid cells (MDSCs and TAMs), activating antitumor immunity Gamma retrovirus Toca 511 selectively infects tumor, persists and spreads through tumor while delivering the CD gene 1 2 3 Toca FC prodrug locally converted to 5-FU chemotherapeutic within the tumor, killing cancer cells and resulting in activation of antigen presenting cells and T cell priming
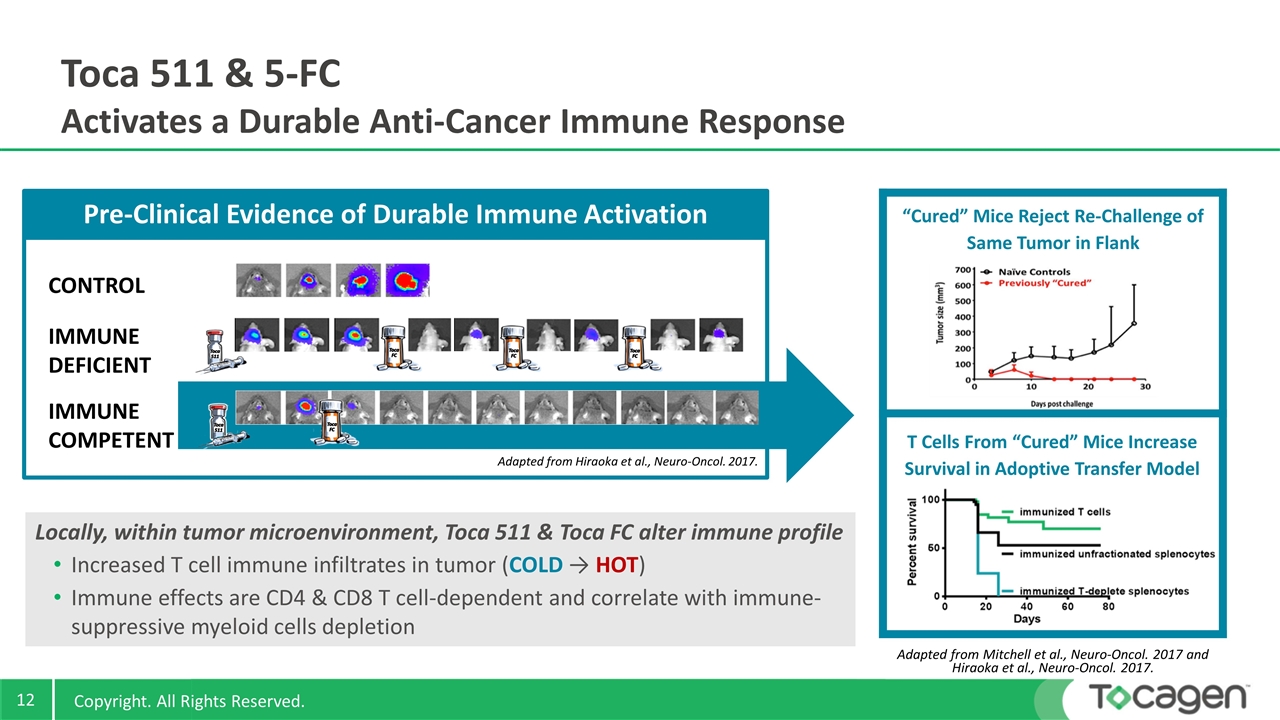
Toca 511 & 5-FC Activates a Durable Anti-Cancer Immune Response Locally, within tumor microenvironment, Toca 511 & Toca FC alter immune profile Increased T cell immune infiltrates in tumor (COLD → HOT) Immune effects are CD4 & CD8 T cell-dependent and correlate with immune-suppressive myeloid cells depletion T Cells From “Cured” Mice Increase Survival in Adoptive Transfer Model “Cured” Mice Reject Re-Challenge of Same Tumor in Flank IMMUNE COMPETENT IMMUNE DEFICIENT CONTROL Adapted from Hiraoka et al., Neuro-Oncol. 2017. Pre-Clinical Evidence of Durable Immune Activation ` Adapted from Mitchell et al., Neuro-Oncol. 2017 and Hiraoka et al., Neuro-Oncol. 2017.
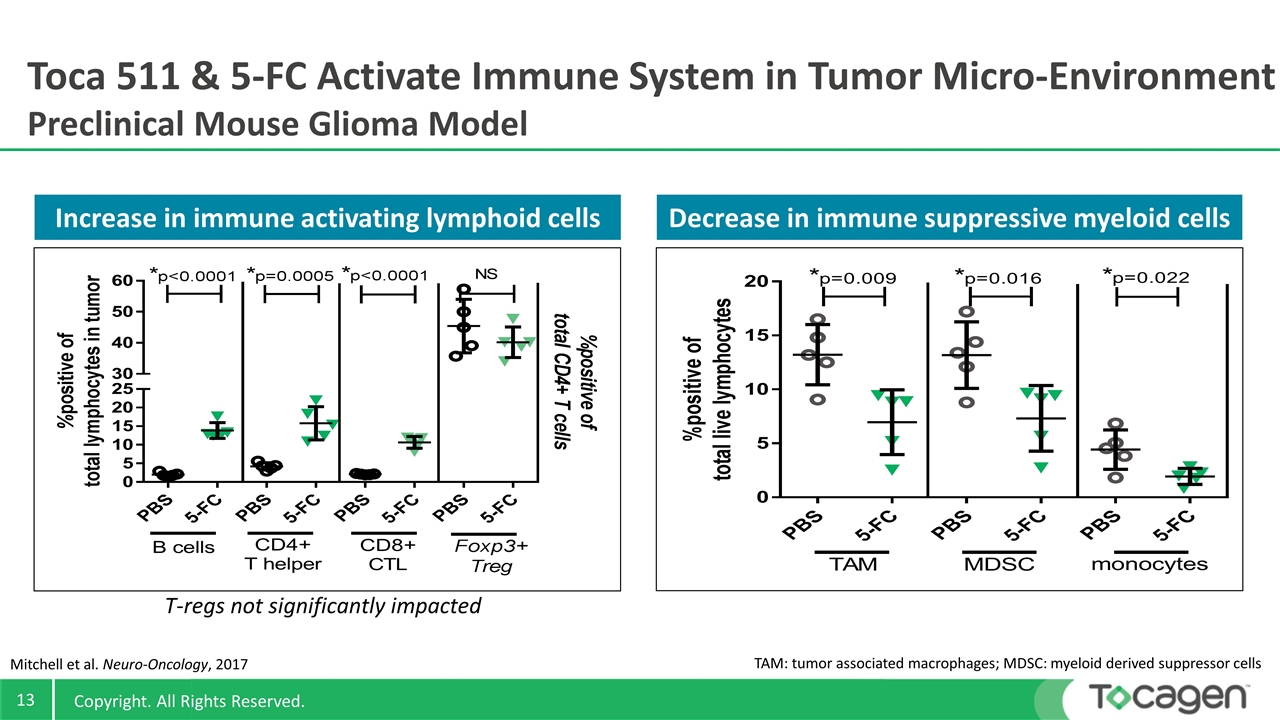
Toca 511 & 5-FC Activate Immune System in Tumor Micro-Environment Preclinical Mouse Glioma Model TAM: tumor associated macrophages; MDSC: myeloid derived suppressor cells Increase in immune activating lymphoid cells Decrease in immune suppressive myeloid cells Mitchell et al. Neuro-Oncology, 2017 T-regs not significantly impacted

Executing in recurrent High Grade Glioma
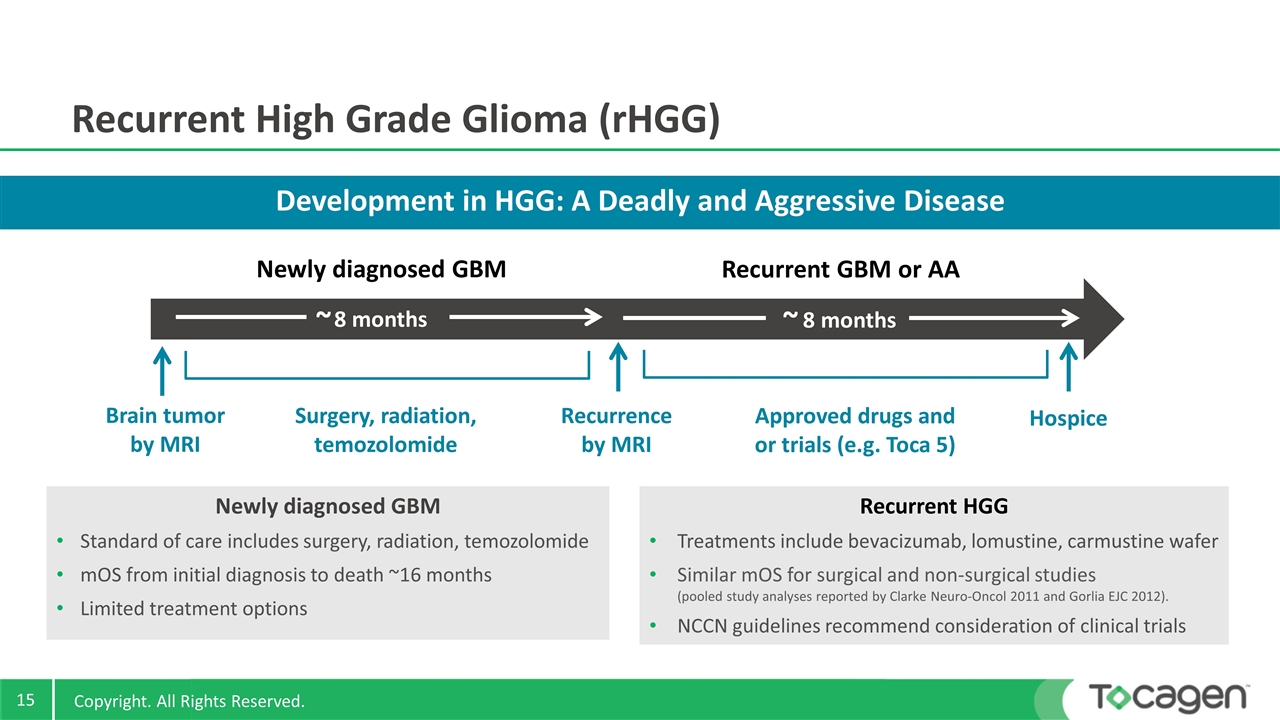
Recurrent High Grade Glioma (rHGG) Newly diagnosed GBM Standard of care includes surgery, radiation, temozolomide mOS from initial diagnosis to death ~16 months Limited treatment options Development in HGG: A Deadly and Aggressive Disease Newly diagnosed GBM Recurrent GBM or AA Brain tumor by MRI Surgery, radiation, temozolomide Recurrence by MRI Approved drugs and or trials (e.g. Toca 5) Hospice 8 months 8 months Recurrent HGG Treatments include bevacizumab, lomustine, carmustine wafer Similar mOS for surgical and non-surgical studies (pooled study analyses reported by Clarke Neuro-Oncol 2011 and Gorlia EJC 2012). NCCN guidelines recommend consideration of clinical trials ~ ~
Key Differentiators for Toca 511 & Toca FC in Brain Cancer Preclinical MOA, CR’s, OS data supported BTD and PRIME designations and peer-reviewed publications Notorious blood brain barrier mitigated with local Toca 511 delivery and precedent for 5-FC to treat brain fungus Multiple mechanisms support robust anti-cancer activity Consistent trial design from Phase 1 to Phase 3 (Toca 5)
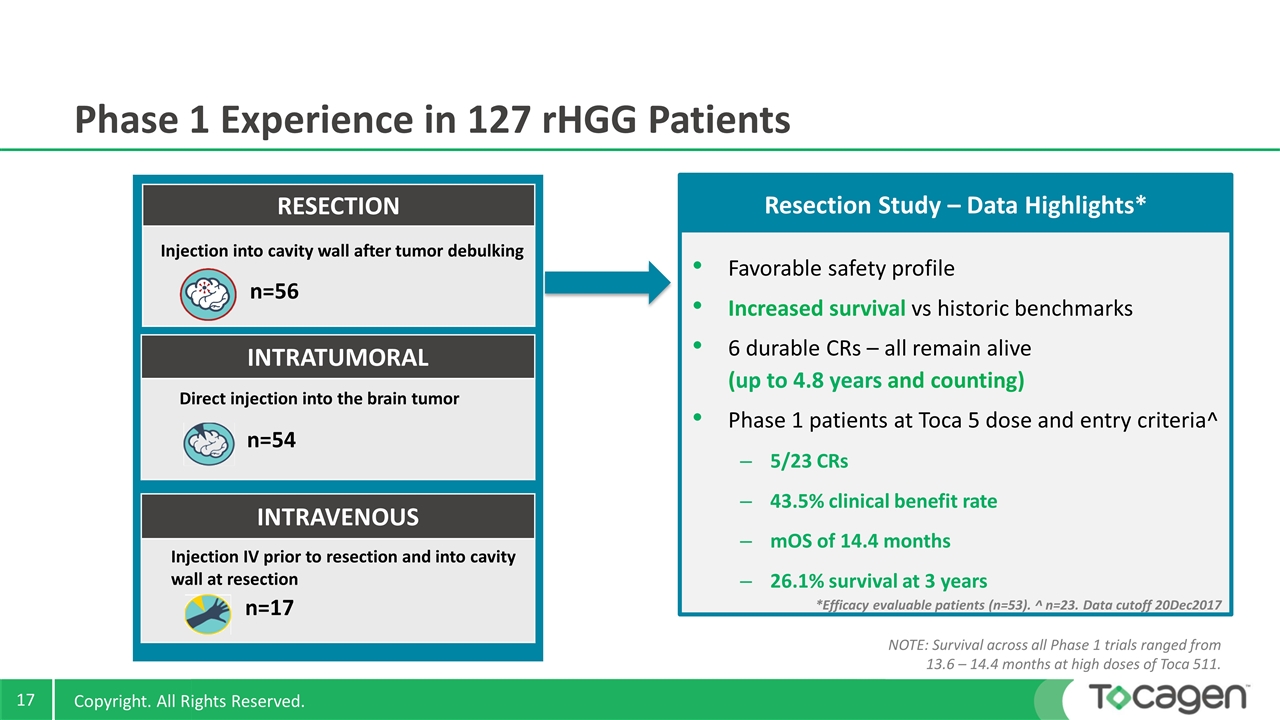
Phase 1 Experience in 127 rHGG Patients RESECTION Injection into cavity wall after tumor debulking n=56 INTRATUMORAL Direct injection into the brain tumor n=54 INTRAVENOUS Injection IV prior to resection and into cavity wall at resection n=17 Favorable safety profile Increased survival vs historic benchmarks 6 durable CRs – all remain alive (up to 4.8 years and counting) Phase 1 patients at Toca 5 dose and entry criteria^ 5/23 CRs 43.5% clinical benefit rate mOS of 14.4 months 26.1% survival at 3 years Resection Study – Data Highlights* Copyright. All Rights Reserved. *Efficacy evaluable patients (n=53). ^ n=23. Data cutoff 20Dec2017 NOTE: Survival across all Phase 1 trials ranged from 13.6 – 14.4 months at high doses of Toca 511.
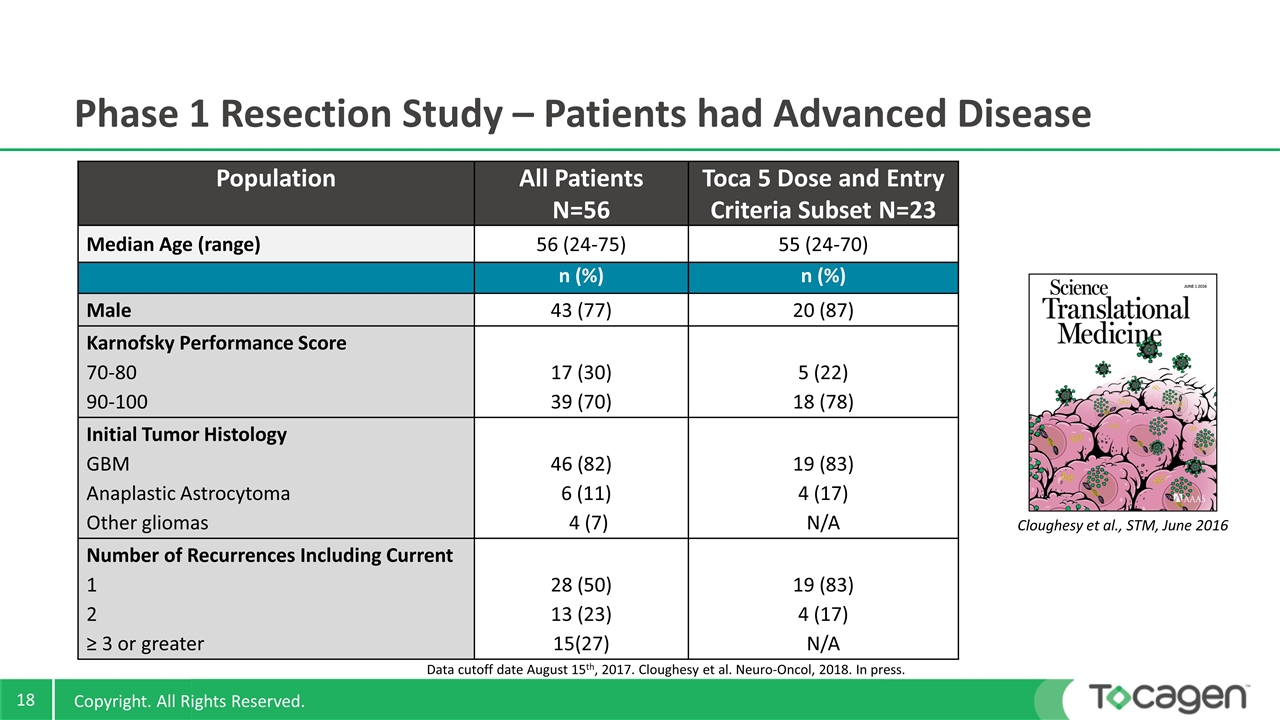
Population All Patients N=56 Toca 5 Dose and Entry Criteria Subset N=23 Median Age (range) 56 (24-75) 55 (24-70) n (%) n (%) Male 43 (77) 20 (87) Karnofsky Performance Score 70-80 90-100 17 (30) 39 (70) 5 (22) 18 (78) Initial Tumor Histology GBM Anaplastic Astrocytoma Other gliomas 46 (82) 6 (11) 4 (7) 19 (83) 4 (17) N/A Number of Recurrences Including Current 1 2 ≥ 3 or greater 28 (50) 13 (23) 15(27) 19 (83) 4 (17) N/A Phase 1 Resection Study – Patients had Advanced Disease Cloughesy et al., STM, June 2016 Data cutoff date August 15th, 2017. Cloughesy et al. Neuro-Oncol, 2018. In press.
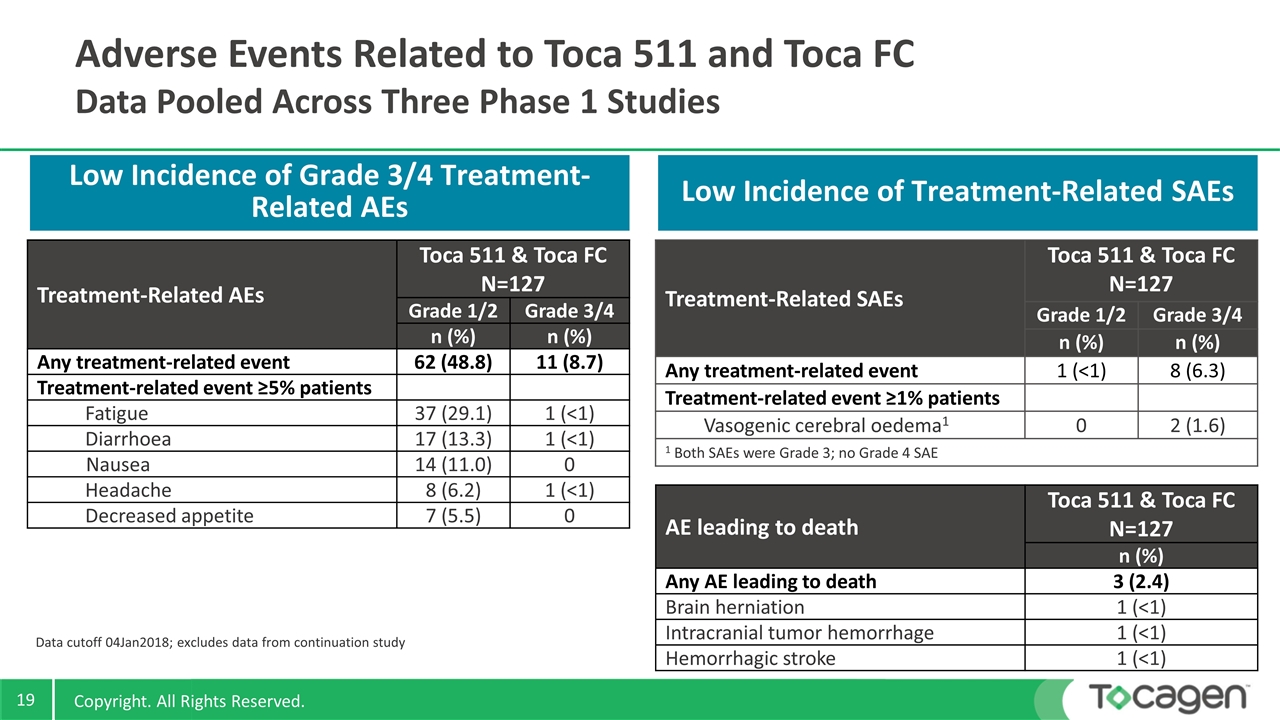
Treatment-Related AEs Toca 511 & Toca FC N=127 Grade 1/2 Grade 3/4 n (%) n (%) Any treatment-related event 62 (48.8) 11 (8.7) Treatment-related event ≥5% patients Fatigue 37 (29.1) 1 (<1) Diarrhoea 17 (13.3) 1 (<1) Nausea 14 (11.0) 0 Headache 8 (6.2) 1 (<1) Decreased appetite 7 (5.5) 0 Data cutoff 04Jan2018; excludes data from continuation study Adverse Events Related to Toca 511 and Toca FC Data Pooled Across Three Phase 1 Studies Low Incidence of Grade 3/4 Treatment-Related AEs Low Incidence of Treatment-Related SAEs AE leading to death Toca 511 & Toca FC N=127 n (%) Any AE leading to death 3 (2.4) Brain herniation 1 (<1) Intracranial tumor hemorrhage 1 (<1) Hemorrhagic stroke 1 (<1) Treatment-Related SAEs Toca 511 & Toca FC N=127 Grade 1/2 Grade 3/4 n (%) n (%) Any treatment-related event 1 (<1) 8 (6.3) Treatment-related event ≥1% patients Vasogenic cerebral oedema1 0 2 (1.6) 1 Both SAEs were Grade 3; no Grade 4 SAE
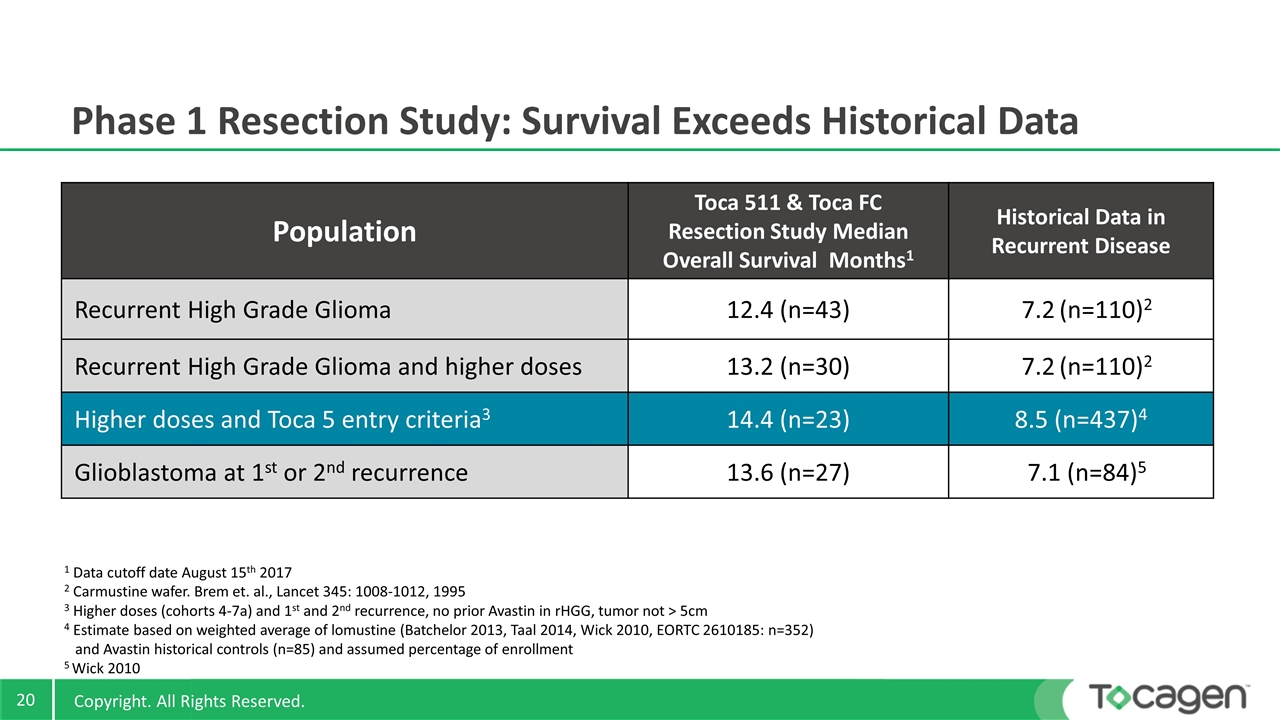
Population Toca 511 & Toca FC Resection Study Median Overall Survival Months1 Historical Data in Recurrent Disease Recurrent High Grade Glioma 12.4 (n=43) 7.2 (n=110)2 Recurrent High Grade Glioma and higher doses 13.2 (n=30) 7.2 (n=110)2 Higher doses and Toca 5 entry criteria3 14.4 (n=23) 8.5 (n=437)4 Glioblastoma at 1st or 2nd recurrence 13.6 (n=27) 7.1 (n=84)5 1 Data cutoff date August 15th 2017 2 Carmustine wafer. Brem et. al., Lancet 345: 1008-1012, 1995 3 Higher doses (cohorts 4-7a) and 1st and 2nd recurrence, no prior Avastin in rHGG, tumor not > 5cm 4 Estimate based on weighted average of lomustine (Batchelor 2013, Taal 2014, Wick 2010, EORTC 2610185: n=352) and Avastin historical controls (n=85) and assumed percentage of enrollment 5 Wick 2010 Phase 1 Resection Study: Survival Exceeds Historical Data
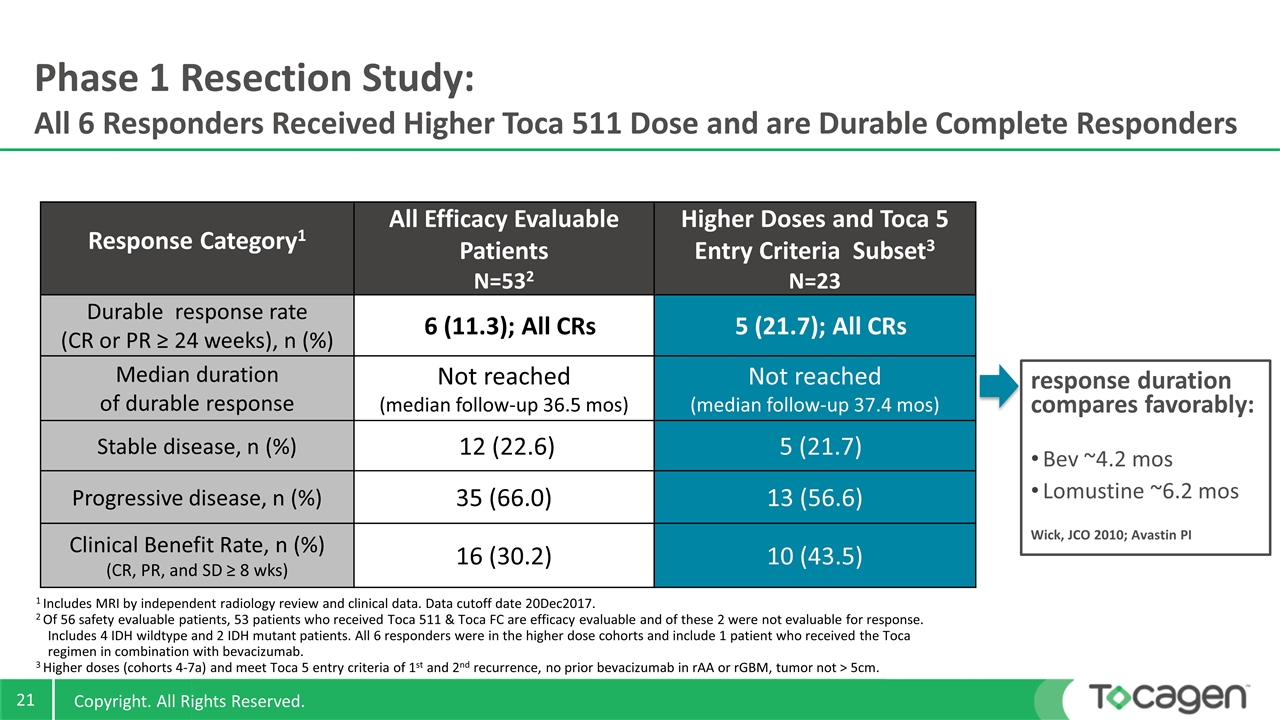
Response Category1 All Efficacy Evaluable Patients N=532 Higher Doses and Toca 5 Entry Criteria Subset3 N=23 Durable response rate (CR or PR ≥ 24 weeks), n (%) 6 (11.3); All CRs 5 (21.7); All CRs Median duration of durable response Not reached (median follow-up 36.5 mos) Not reached (median follow-up 37.4 mos) Stable disease, n (%) 12 (22.6) 5 (21.7) Progressive disease, n (%) 35 (66.0) 13 (56.6) Clinical Benefit Rate, n (%) (CR, PR, and SD ≥ 8 wks) 16 (30.2) 10 (43.5) Phase 1 Resection Study: All 6 Responders Received Higher Toca 511 Dose and are Durable Complete Responders response duration compares favorably: Bev ~4.2 mos Lomustine ~6.2 mos Wick, JCO 2010; Avastin PI 1 Includes MRI by independent radiology review and clinical data. Data cutoff date 20Dec2017. 2 Of 56 safety evaluable patients, 53 patients who received Toca 511 & Toca FC are efficacy evaluable and of these 2 were not evaluable for response. Includes 4 IDH wildtype and 2 IDH mutant patients. All 6 responders were in the higher dose cohorts and include 1 patient who received the Toca regimen in combination with bevacizumab. 3 Higher doses (cohorts 4-7a) and meet Toca 5 entry criteria of 1st and 2nd recurrence, no prior bevacizumab in rAA or rGBM, tumor not > 5cm.
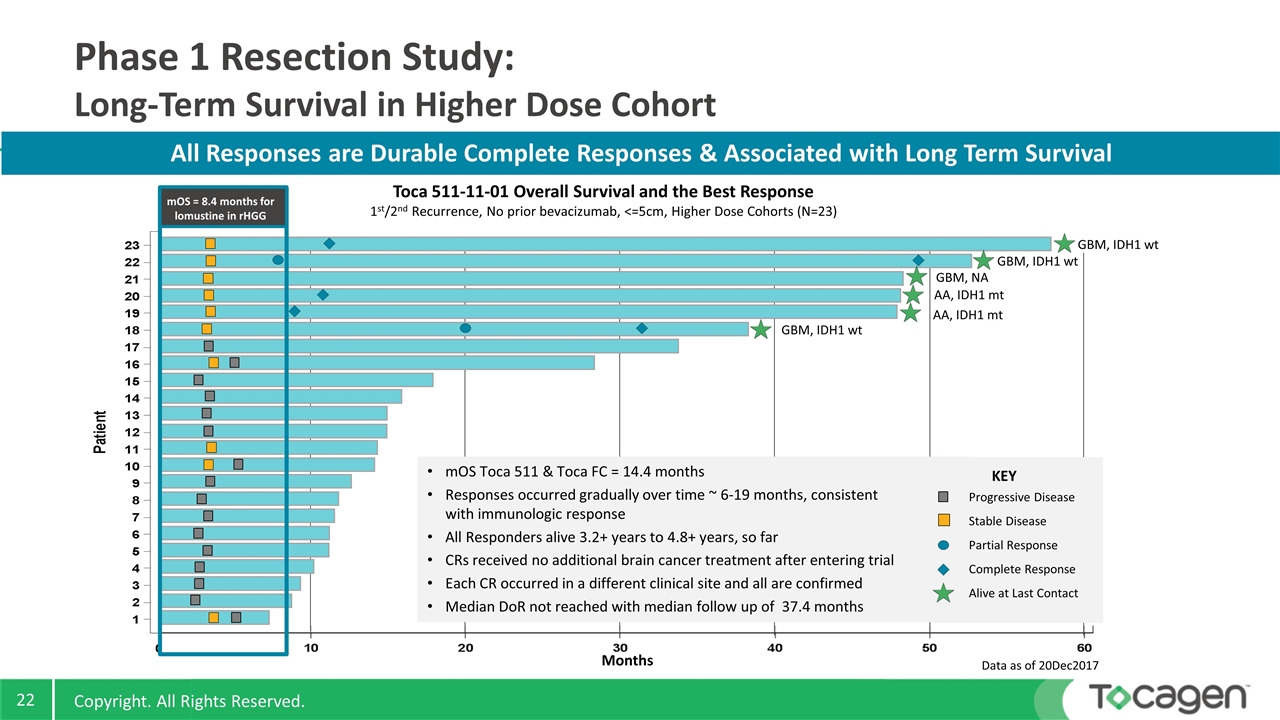
AA, IDH1 mt Phase 1 Resection Study: Long-Term Survival in Higher Dose Cohort All Responses are Durable Complete Responses & Associated with Long Term Survival Toca 511-11-01 Overall Survival and the Best Response 1st/2nd Recurrence, No prior bevacizumab, <=5cm, Higher Dose Cohorts (N=23) Data as of 20Dec2017 mOS Toca 511 & Toca FC = 14.4 months Responses occurred gradually over time ~ 6-19 months, consistent with immunologic response All Responders alive 3.2+ years to 4.8+ years, so far CRs received no additional brain cancer treatment after entering trial Each CR occurred in a different clinical site and all are confirmed Median DoR not reached with median follow up of 37.4 months Progressive Disease Stable Disease Partial Response Complete Response Alive at Last Contact KEY Months GBM, IDH1 wt GBM, NA GBM, IDH1 wt AA, IDH1 mt GBM, IDH1 wt
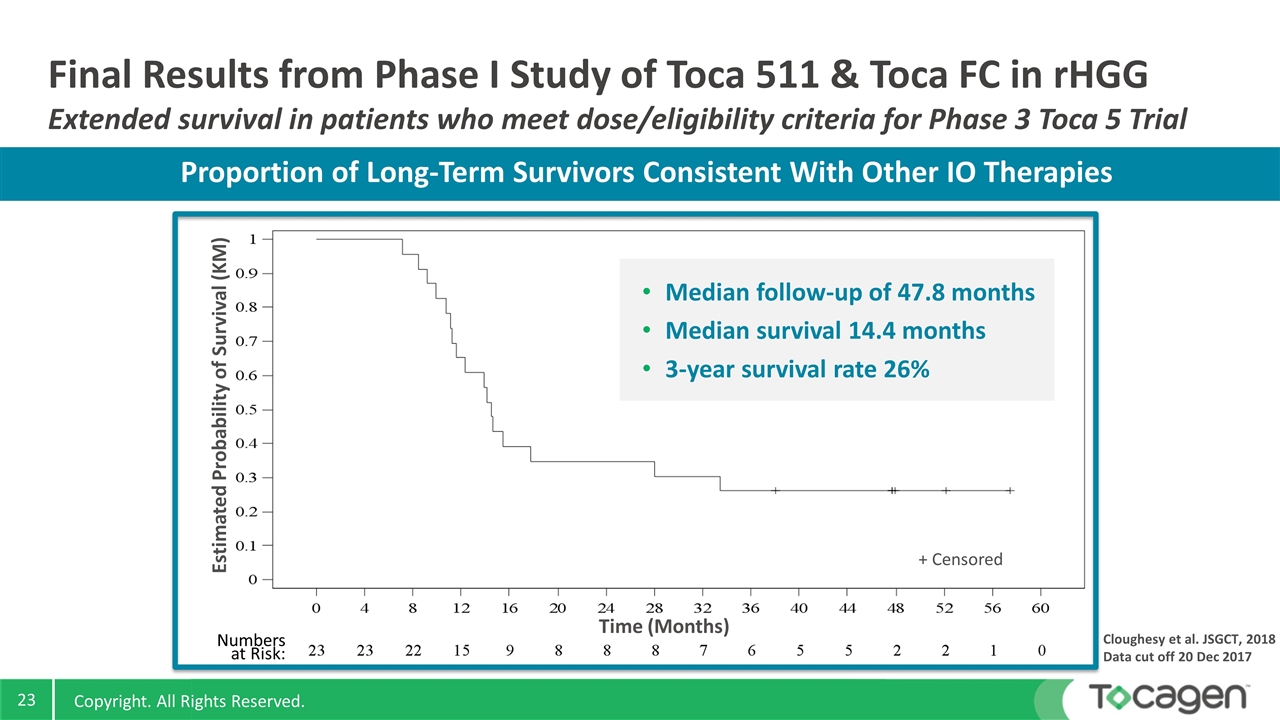
Final Results from Phase I Study of Toca 511 & Toca FC in rHGG Extended survival in patients who meet dose/eligibility criteria for Phase 3 Toca 5 Trial Cloughesy et al. JSGCT, 2018 Data cut off 20 Dec 2017 Estimated Probability of Survival (KM) Time (Months) + Censored Numbers at Risk: Median follow-up of 47.8 months Median survival 14.4 months 3-year survival rate 26% Proportion of Long-Term Survivors Consistent With Other IO Therapies
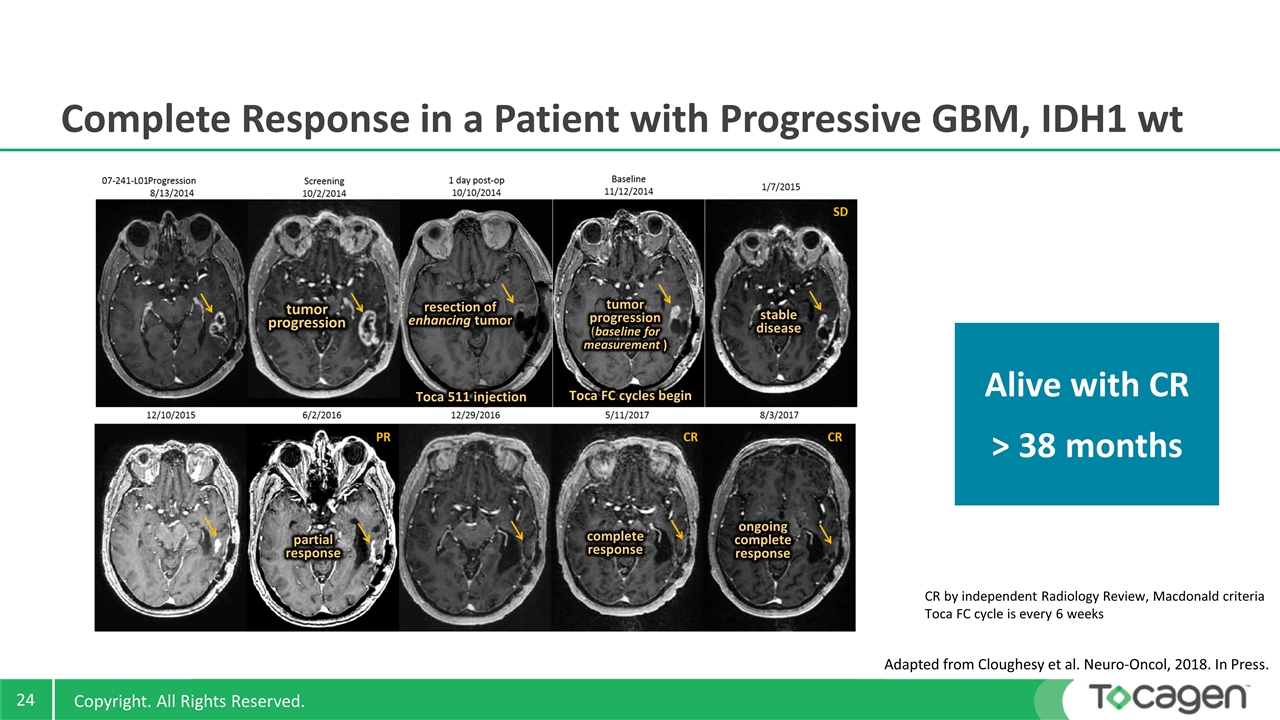
Complete Response in a Patient with Progressive GBM, IDH1 wt Adapted from Cloughesy et al. Neuro-Oncol, 2018. In Press. CR by independent Radiology Review, Macdonald criteria Toca FC cycle is every 6 weeks tumor progression resection of enhancing tumor tumor progression (baseline for measurement ) stable disease partial response complete response ongoing complete response Alive with CR > 38 months Toca FC cycles begin Toca 511 injection
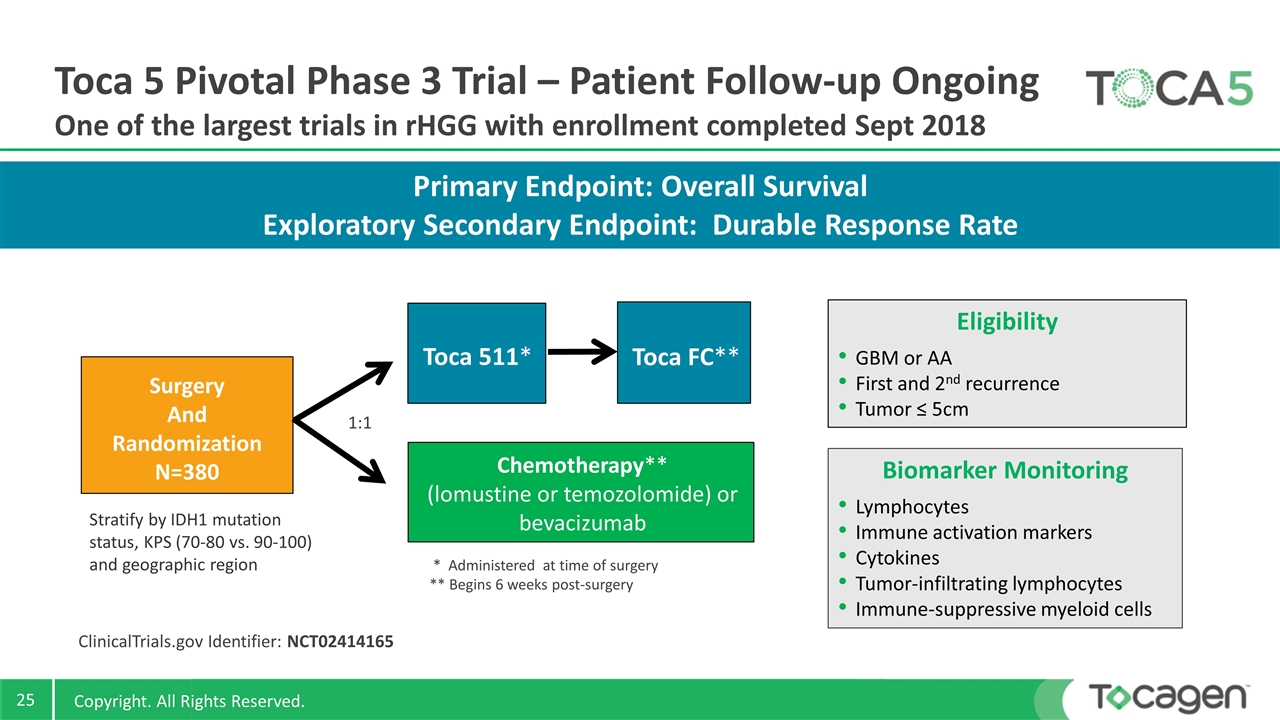
Primary Endpoint: Overall Survival Exploratory Secondary Endpoint: Durable Response Rate Toca 5 Pivotal Phase 3 Trial – Patient Follow-up Ongoing One of the largest trials in rHGG with enrollment completed Sept 2018 Eligibility GBM or AA First and 2nd recurrence Tumor ≤ 5cm ClinicalTrials.gov Identifier: NCT02414165 * Administered at time of surgery ** Begins 6 weeks post-surgery Surgery And Randomization N=380 Toca 511* Toca FC** Chemotherapy** (lomustine or temozolomide) or bevacizumab Stratify by IDH1 mutation status, KPS (70-80 vs. 90-100) and geographic region 1:1 Biomarker Monitoring Lymphocytes Immune activation markers Cytokines Tumor-infiltrating lymphocytes Immune-suppressive myeloid cells
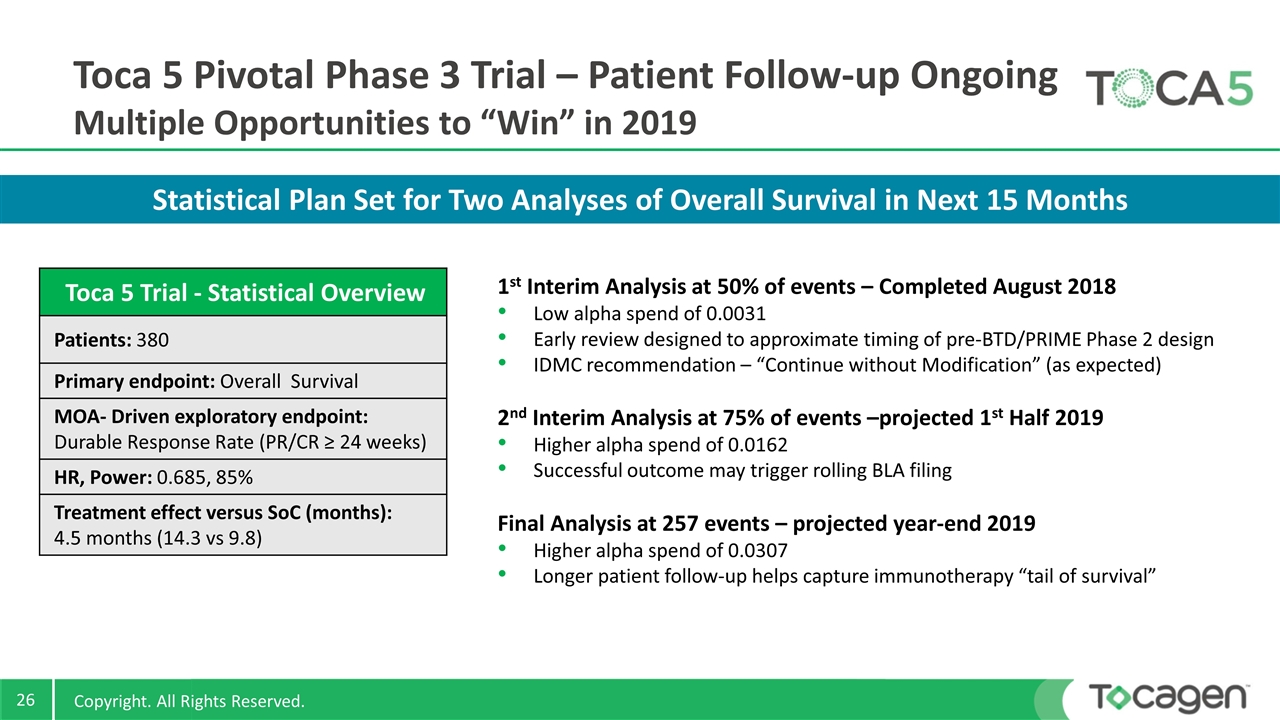
Statistical Plan Set for Two Analyses of Overall Survival in Next 15 Months Toca 5 Pivotal Phase 3 Trial – Patient Follow-up Ongoing Multiple Opportunities to “Win” in 2019 Toca 5 Trial - Statistical Overview Patients: 380 Primary endpoint: Overall Survival MOA- Driven exploratory endpoint: Durable Response Rate (PR/CR ≥ 24 weeks) HR, Power: 0.685, 85% Treatment effect versus SoC (months): 4.5 months (14.3 vs 9.8) 1st Interim Analysis at 50% of events – Completed August 2018 Low alpha spend of 0.0031 Early review designed to approximate timing of pre-BTD/PRIME Phase 2 design IDMC recommendation – “Continue without Modification” (as expected) 2nd Interim Analysis at 75% of events –projected 1st Half 2019 Higher alpha spend of 0.0162 Successful outcome may trigger rolling BLA filing Final Analysis at 257 events – projected year-end 2019 Higher alpha spend of 0.0307 Longer patient follow-up helps capture immunotherapy “tail of survival”
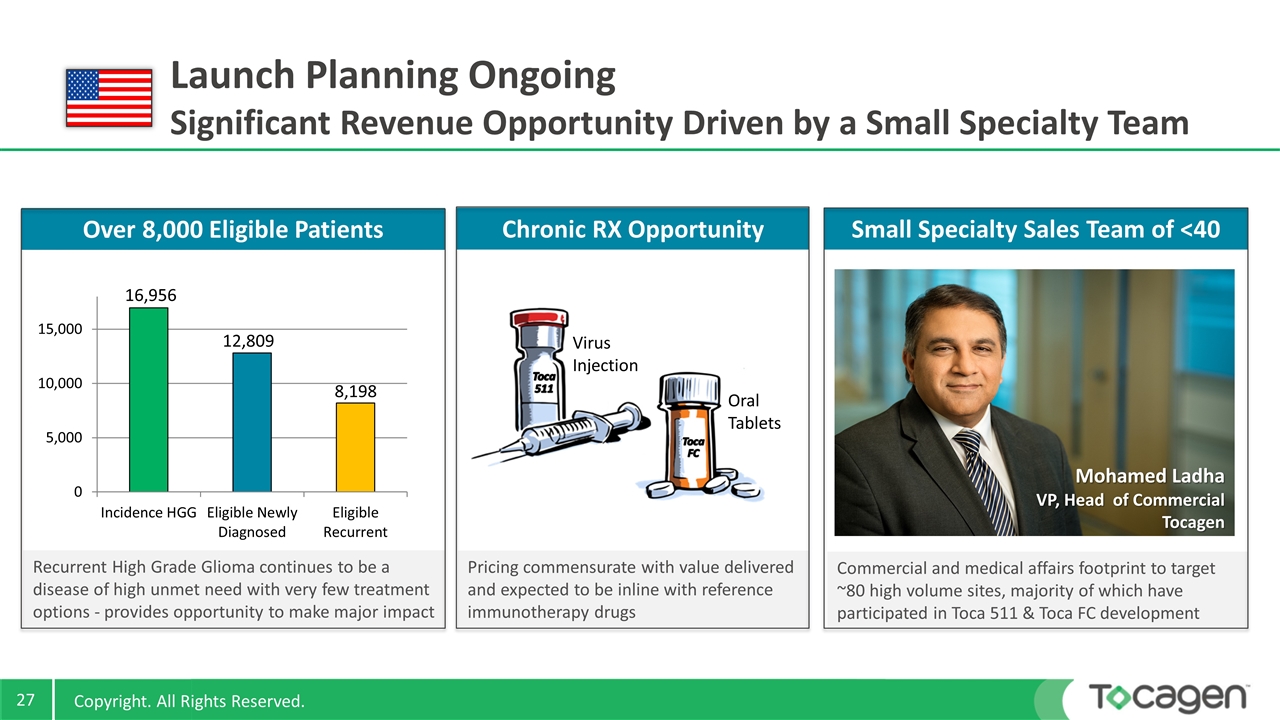
Pricing commensurate with value delivered and expected to be inline with reference immunotherapy drugs Commercial and medical affairs footprint to target ~80 high volume sites, majority of which have participated in Toca 511 & Toca FC development Recurrent High Grade Glioma continues to be a disease of high unmet need with very few treatment options - provides opportunity to make major impact Launch Planning Ongoing Significant Revenue Opportunity Driven by a Small Specialty Team Over 8,000 Eligible Patients Small Specialty Sales Team of <40 Chronic RX Opportunity Virus Injection Oral Tablets Mohamed Ladha VP, Head of Commercial Tocagen

Expanding to realize potential of Toca 511 & Toca FC
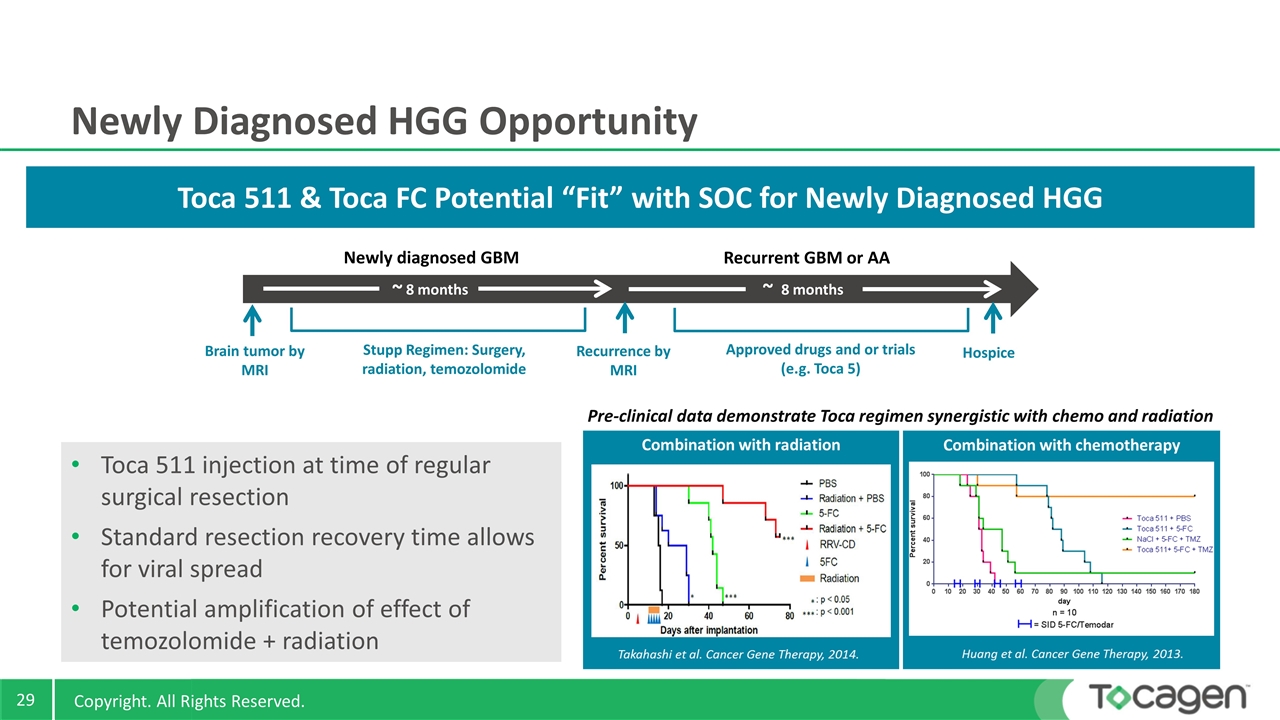
Newly Diagnosed HGG Opportunity Toca 511 injection at time of regular surgical resection Standard resection recovery time allows for viral spread Potential amplification of effect of temozolomide + radiation Newly diagnosed GBM Recurrent GBM or AA Brain tumor by MRI Stupp Regimen: Surgery, radiation, temozolomide Recurrence by MRI Approved drugs and or trials (e.g. Toca 5) Hospice 8 months 8 months Toca 511 & Toca FC Potential “Fit” with SOC for Newly Diagnosed HGG Pre-clinical data demonstrate Toca regimen synergistic with chemo and radiation ~ ~
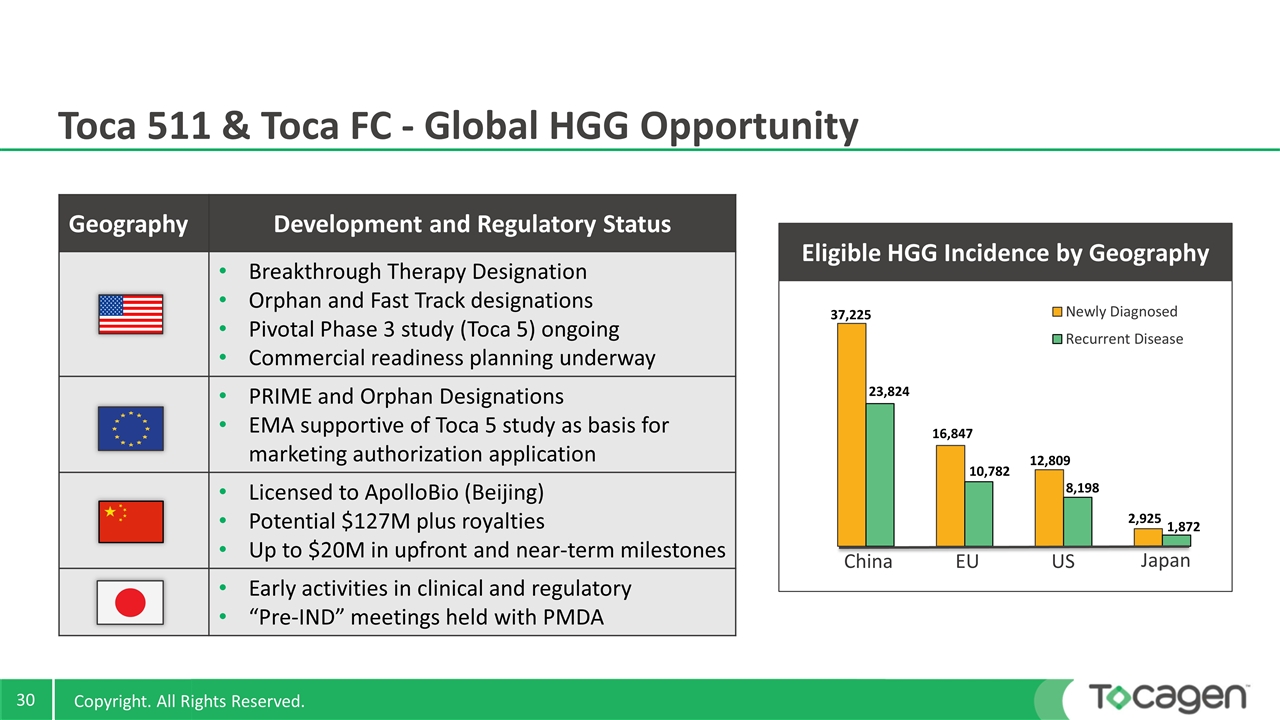
Toca 511 & Toca FC - Global HGG Opportunity Geography Development and Regulatory Status Breakthrough Therapy Designation Orphan and Fast Track designations Pivotal Phase 3 study (Toca 5) ongoing Commercial readiness planning underway PRIME and Orphan Designations EMA supportive of Toca 5 study as basis for marketing authorization application Licensed to ApolloBio (Beijing) Potential $127M plus royalties Up to $20M in upfront and near-term milestones Early activities in clinical and regulatory “Pre-IND” meetings held with PMDA EU US Japan Eligible HGG Incidence by Geography China 12,809 8,198 2,925 1,872

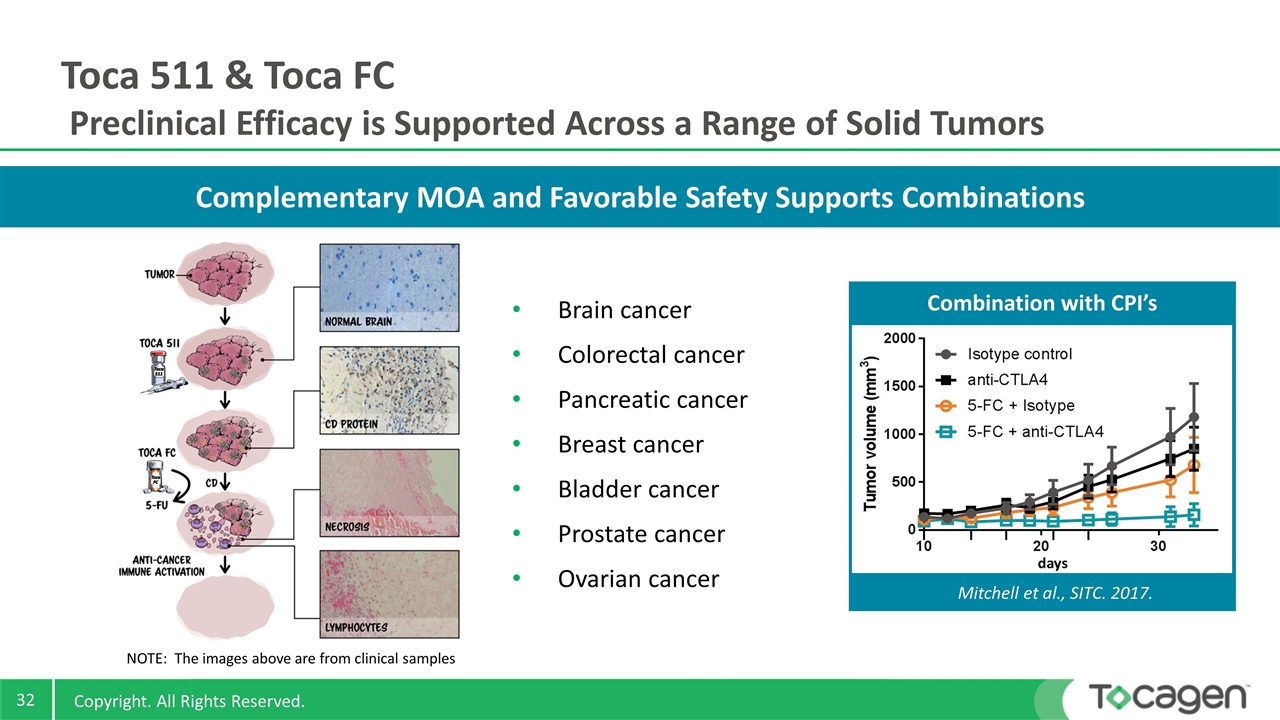
Complementary MOA and Favorable Safety Supports Combinations Brain cancer Colorectal cancer Pancreatic cancer Breast cancer Bladder cancer Prostate cancer Ovarian cancer Toca 511 & Toca FC Preclinical Efficacy is Supported Across a Range of Solid Tumors NOTE: The images above are from clinical samples Combination with CPI’s Mitchell et al., SITC. 2017.
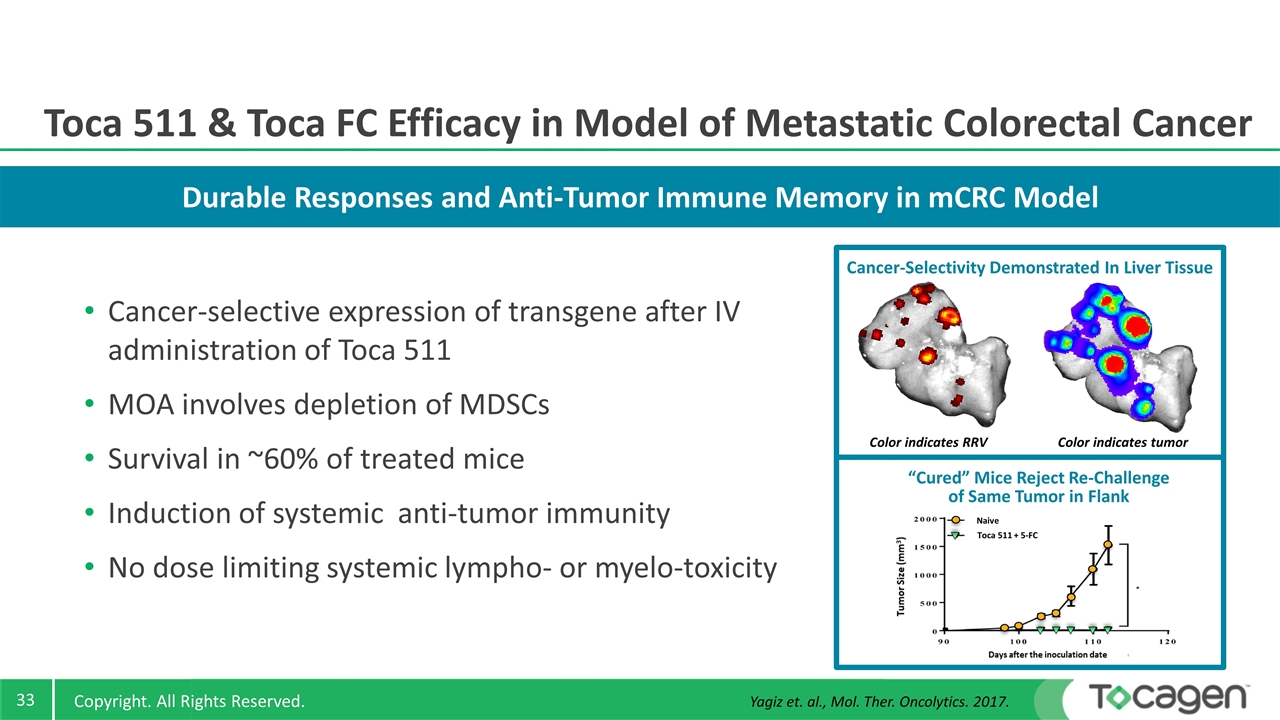
Durable Responses and Anti-Tumor Immune Memory in mCRC Model Cancer-selective expression of transgene after IV administration of Toca 511 MOA involves depletion of MDSCs Survival in ~60% of treated mice Induction of systemic anti-tumor immunity No dose limiting systemic lympho- or myelo-toxicity Toca 511 & Toca FC Efficacy in Model of Metastatic Colorectal Cancer Color indicates tumor Color indicates RRV Cancer-Selectivity Demonstrated In Liver Tissue “Cured” Mice Reject Re-Challenge of Same Tumor in Flank Toca 511 + 5-FC Naive Yagiz et. al., Mol. Ther. Oncolytics. 2017.
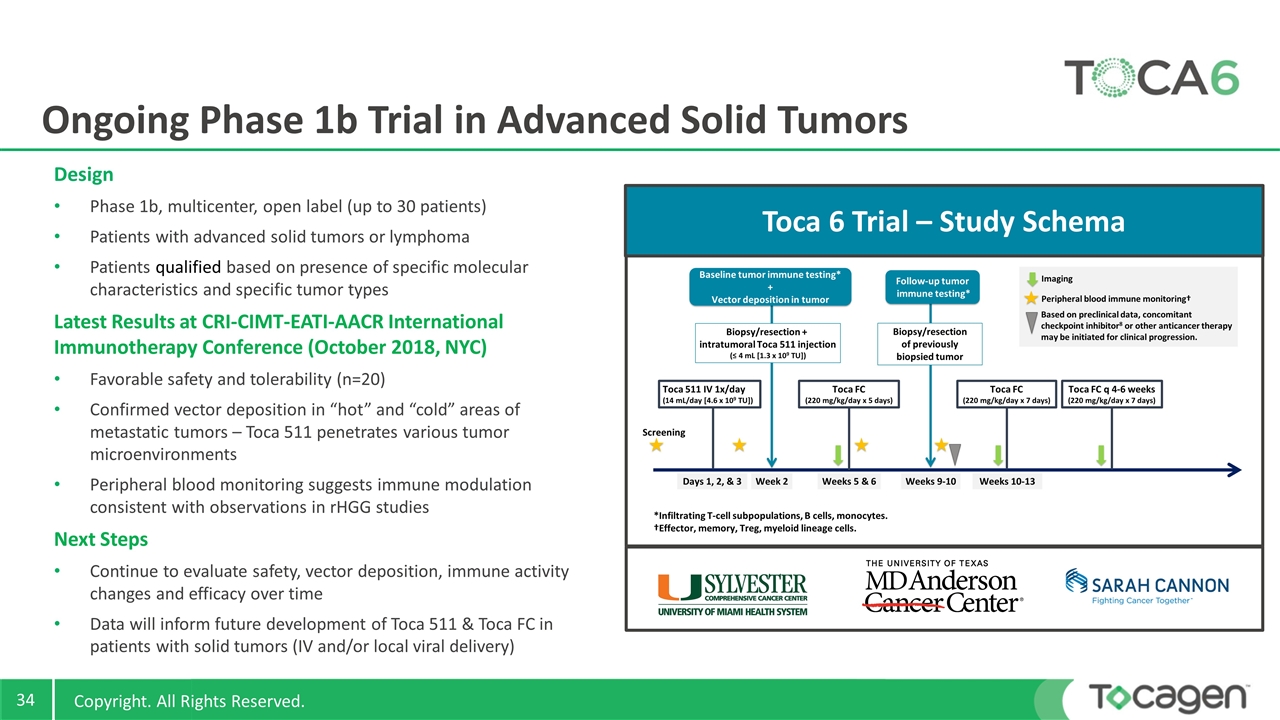
Ongoing Phase 1b Trial in Advanced Solid Tumors Design Phase 1b, multicenter, open label (up to 30 patients) Patients with advanced solid tumors or lymphoma Patients qualified based on presence of specific molecular characteristics and specific tumor types Latest Results at CRI-CIMT-EATI-AACR International Immunotherapy Conference (October 2018, NYC) Favorable safety and tolerability (n=20) Confirmed vector deposition in “hot” and “cold” areas of metastatic tumors – Toca 511 penetrates various tumor microenvironments Peripheral blood monitoring suggests immune modulation consistent with observations in rHGG studies Next Steps Continue to evaluate safety, vector deposition, immune activity changes and efficacy over time Data will inform future development of Toca 511 & Toca FC in patients with solid tumors (IV and/or local viral delivery) Toca 6 Trial – Study Schema
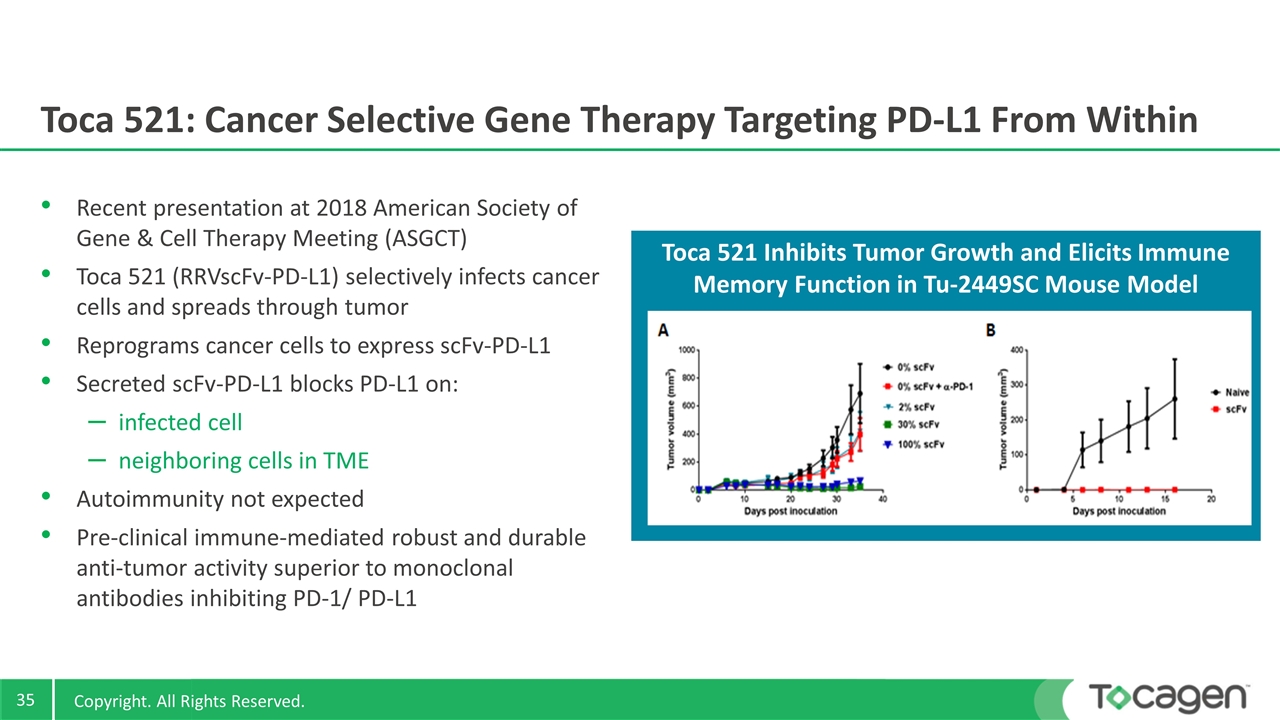
Toca 521 Inhibits Tumor Growth and Elicits Immune Memory Function in Tu-2449SC Mouse Model Toca 521: Cancer Selective Gene Therapy Targeting PD-L1 From Within Recent presentation at 2018 American Society of Gene & Cell Therapy Meeting (ASGCT) Toca 521 (RRVscFv-PD-L1) selectively infects cancer cells and spreads through tumor Reprograms cancer cells to express scFv-PD-L1 Secreted scFv-PD-L1 blocks PD-L1 on: infected cell neighboring cells in TME Autoimmunity not expected Pre-clinical immune-mediated robust and durable anti-tumor activity superior to monoclonal antibodies inhibiting PD-1/ PD-L1

Summary
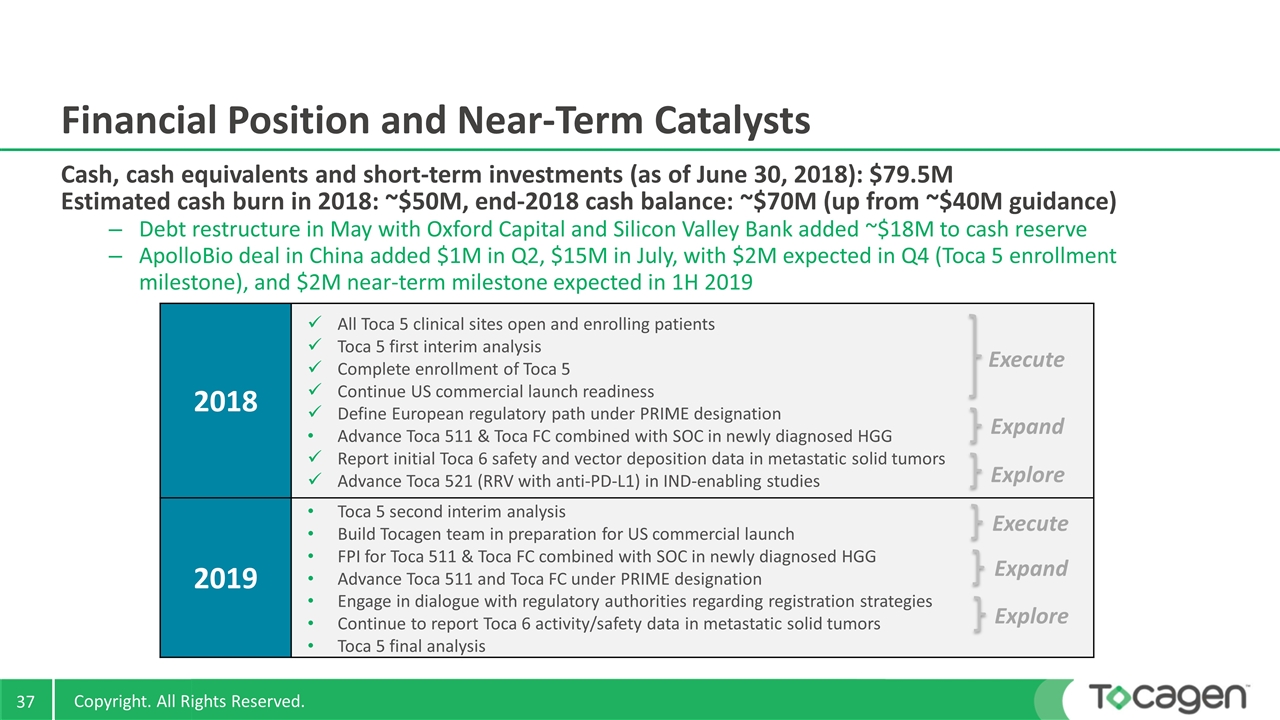
Financial Position and Near-Term Catalysts Cash, cash equivalents and short-term investments (as of June 30, 2018): $79.5M Estimated cash burn in 2018: ~$50M, end-2018 cash balance: ~$70M (up from ~$40M guidance) Debt restructure in May with Oxford Capital and Silicon Valley Bank added ~$18M to cash reserve ApolloBio deal in China added $1M in Q2, $15M in July, with $2M expected in Q4 (Toca 5 enrollment milestone), and $2M near-term milestone expected in 1H 2019 2018 2019 Toca 5 second interim analysis Build Tocagen team in preparation for US commercial launch FPI for Toca 511 & Toca FC combined with SOC in newly diagnosed HGG Advance Toca 511 and Toca FC under PRIME designation Engage in dialogue with regulatory authorities regarding registration strategies Continue to report Toca 6 activity/safety data in metastatic solid tumors Toca 5 final analysis All Toca 5 clinical sites open and enrolling patients Toca 5 first interim analysis Complete enrollment of Toca 5 Continue US commercial launch readiness Define European regulatory path under PRIME designation Advance Toca 511 & Toca FC combined with SOC in newly diagnosed HGG Report initial Toca 6 safety and vector deposition data in metastatic solid tumors Advance Toca 521 (RRV with anti-PD-L1) in IND-enabling studies Execute Expand Explore Execute Expand Explore

Looking Forward to Achieving More Milestone Moments … Thank you to all of the patients and families who have supported our work. Financial support also provided by:





































