
Exhibit 99.2 FORTE BIOSCIENCES FORTE BIOSCIENCES CORPORATE DECK DEC 3, 2024 1

CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS ¡ Certain statements contained in this presentation regarding matters that are not historical facts, are forward-looking statements within the meaning of Section 21E of the Securities and Exchange Act of 1934, as amended, and the Private Securities Litigation Act of 1995, known as the PSLRA. These include statements regarding management’s intention, plans, beliefs, expectations or forecasts for the future, and, therefore, you are cautioned not to place undue reliance on them. No forward-looking statement can be guaranteed, and actual results may differ materially from those projected. Forte Biosciences, Inc. (“we”, the “Company” or “Forte”) undertakes no obligation to publicly update any forward-looking statement, whether as a result of new information, future events or otherwise, except to the extent required by law. We use words such as “anticipates,” “believes,” “plans,” “expects,” “projects,” “intends,” “may,” “will,” “should,” “could,” “estimates,” “predicts,” “potential,” “continue,” “guidance,” and similar expressions to identify these forward-looking statements that are intended to be covered by the safe-harbor provisions of the PSLRA. ¡ Such forward-looking statements are based on our expectations and involve risks and uncertainties; consequently, actual results may differ materially from those expressed or implied in the statements due to a number of factors, including, but not limited to, risks relating to the business and prospects of the Company; Forte’s plans to develop and potentially commercialize its product candidates, including FB102; the risk that results from preclinical studies and early-clinical trails completed by Forte and third parties may not be predictive of results from later-stage clinical trials; the timing of initiation of Forte’s planned clinical trials; the timing of the availability of data from Forte’s clinical trials; the timing of any planned investigational new drug application or new drug application; Forte’s plans to research, develop and commercialize its current and future product candidates; Forte’s projections of the size of the market in certain indications for FB102; the clinical utility, potential benefits and market acceptance of Forte’s product candidates; Forte’s commercialization, marketing and manufacturing capabilities and strategy; developments and projections relating to Forte’s competitors and its industry; the impact of government laws and regulations; Forte’s ability to protect its intellectual property position; Forte’s estimates regarding future revenue, expenses, capital requirements and need for additional financing; and the impact of global events on the Company, the Company’s industry or the economy generally. ¡ We have based these forward-looking statements largely on our current expectations and projections about future events and trends that we believe may affect our financial condition, results of operations, business strategy and financial needs, and these statements represent our views as of the date of this presentation. We may not actually achieve the plans, intentions or expectations disclosed in these forward-looking statements, and you should not place undue reliance on these forward-looking statements. Forward-looking statements are inherently subject to risks and uncertainties, some of which cannot be predicted or quantified. Information regarding certain risks, uncertainties and assumptions may be found in our filings with the Securities and Exchange Commission, including under the caption “Risk Factors” and elsewhere in our Quarterly Report on Form 10-Q for the period ending September 30, 2024 and other filings with the Securities and Exchange Commission. New risk factors emerge from time to time and it is not possible for our management team to predict all risk factors or assess the impact of all factors on the business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in, or implied by, any forward-looking statements. While we may elect to update these forward-looking statements at some point in the future, we specifically disclaim any obligation to do so. These forward-looking statements should not be relied upon as representing our views as of any date subsequent to the date of this presentation. 2
CLINICAL STAGE FB-102 • CD122 is a subunit of the intermediate affinity IL-2/IL-15 receptor expressed on NK cells and T cells and is a subunit of the high affinity IL-2 receptor expressed on Tregs. • FB102 (Forte’s anti-CD122 antibody) is designed to mediate both the IL-2 and the IL-15 induced proliferation and activation of pathogenic NK cells and T cells without effecting the IL-2 induced proliferation of the immune modulating Tregs. • Significant amount of proof-of-concept preclinical data across numerous indications supports “Pipeline-in-a-Product” potential for FB102. • 4 and 13-week NHP tox studies completed • Healthy volunteer SAD/MAD completed • Initiated celiac disease patient study with readout expected in 2Q25 3

FORTE BIOSCIENCES OVERVIEW • Strong Board of Directors comprised of leaders in industry including: • Scott Brun, MD – Former head of Abbvie product development • David Gryska – Former CFO of Incyte and Celgene • Barbera Finck, MD – Led Enbrel development at Immunex and Humira biosimilar development at Coherus • Steve Doberstein, PhD – Former Chief Scientific Officer of Nektar • Steve Kornfeld – Co-Managing Partner of Castle Peak Partners and former Healthcare Sector Team Leader and PM at Franklin Templeton • Shiv Kapoor – Co-Founder of Stonegate Healthcare and biotech veteran with over 25 years of experience in investments and therapeutics development • Rich Vincent – Multiple biotech CFO roles, Deloitte & Touche, CPA 4

EXPERIENCED MANAGEMENT Forte’s management has extensive experience in manufacturing, quality, regulatory and clinical development Paul Wagner, Ph.D., CFA – CEO Tony Riley – Chief Financial Officer Chris Roenfeldt, PMP – Chief Operating Officer Steven Ruhl – Chief Technical Officer Barbara Finck, MD – Senior Medical Clinician 5 5

FB102 CD122 ANTAGONIST MECHANISM 6
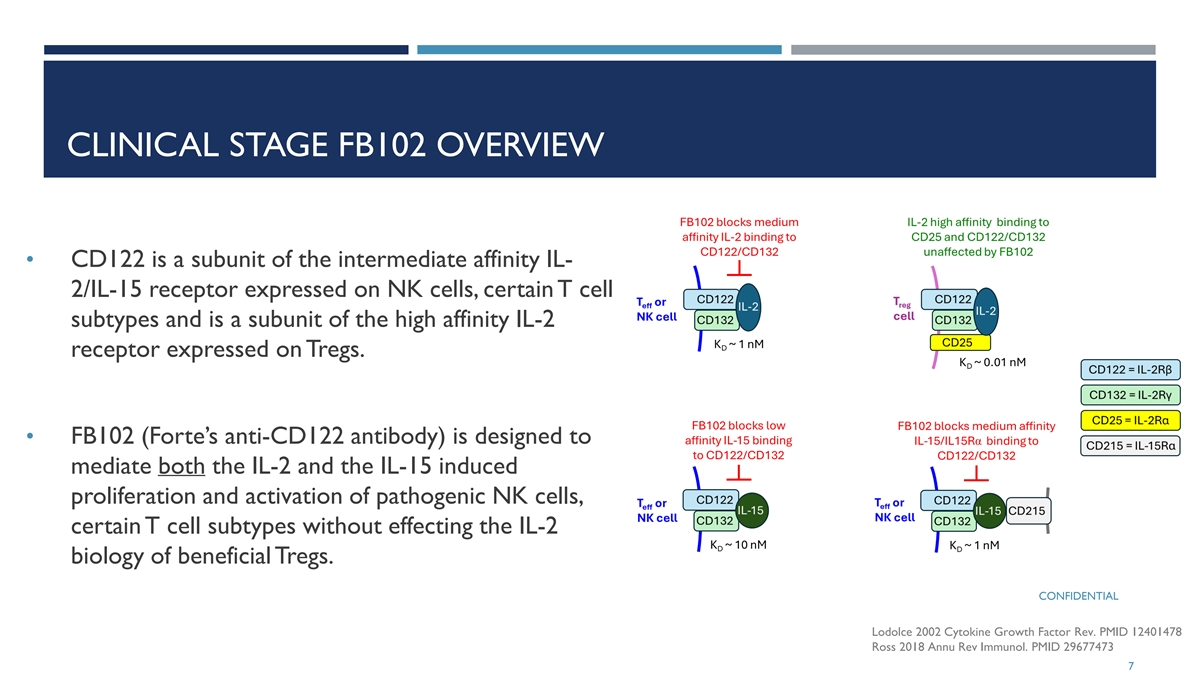
CLINICAL STAGE FB102 OVERVIEW • CD122 is a subunit of the intermediate affinity IL- 2/IL-15 receptor expressed on NK cells, certain T cell subtypes and is a subunit of the high affinity IL-2 receptor expressed on Tregs. • FB102 (Forte’s anti-CD122 antibody) is designed to mediate both the IL-2 and the IL-15 induced proliferation and activation of pathogenic NK cells, certain T cell subtypes without effecting the IL-2 biology of beneficial Tregs. CONFIDENTIAL Lodolce 2002 Cytokine Growth Factor Rev. PMID 12401478 Ross 2018 Annu Rev Immunol. PMID 29677473 7

ADVANCING CLINICAL STAGE FB102 • Phase 1 healthy volunteer SAD/MAD successfully completed and demonstrated a good safety profile. • Forte initiated a celiac disease patient study in 3Q24 and topline data is expected in 2Q25. • Significant amount of proof-of-concept preclinical data across numerous indications supports “Pipeline-in-a-Product” potential for FB102. 8

HIGHLIGHTS OF EXISTING PRECLINICAL ANTI-CD122 PROOF- OF-CONCEPT DATA 9

PARTIAL LIST OF POSITIVE POC ANIMAL DATA FOR ANTI-CD122 HIGHLIGHTS FB102 “PIPELINE-IN-A-PRODUCT” POTENTIAL Disease Species Outcome Reference Celiac disease Mouse Improved IL-15-induced PNAS, 2009 mucosal damage Vitiligo Mouse Enhanced repigmentation Sci Transl Med, 2018 Alopecia areata Mouse Prevented fur loss Nature Med, 2014 Type 1 diabetes Mouse Delayed disease onset JCI Insight, 2018 GVHD Mouse Prolonged survival JN Bio patent, 2015 Skin and kidney transplant Mouse/Monkey Prolonged graft survival in J Clin Invest, 2018 rejection combination with CTLA-4 10

FB102 IN PROOF-OF-CONCEPT AUTOIMMUNE PRECLINICAL DATA 11

GENERATING PRECLINICAL EFFICACY DATA IN A HUMANIZED MOUSE MODEL OF ACUTE GVHD Humanized mouse model of graft vs host disease Aggressive, rapid-onset of GvHD (100% mortality within 30 days) due to both T and NK cell engraftment NCG (immunodeficient) Transplant with human 6 with human IL-15 expression PBMC (5x10 ) Huarte, Immunotherapy 2021 PMID: 34184542. 12
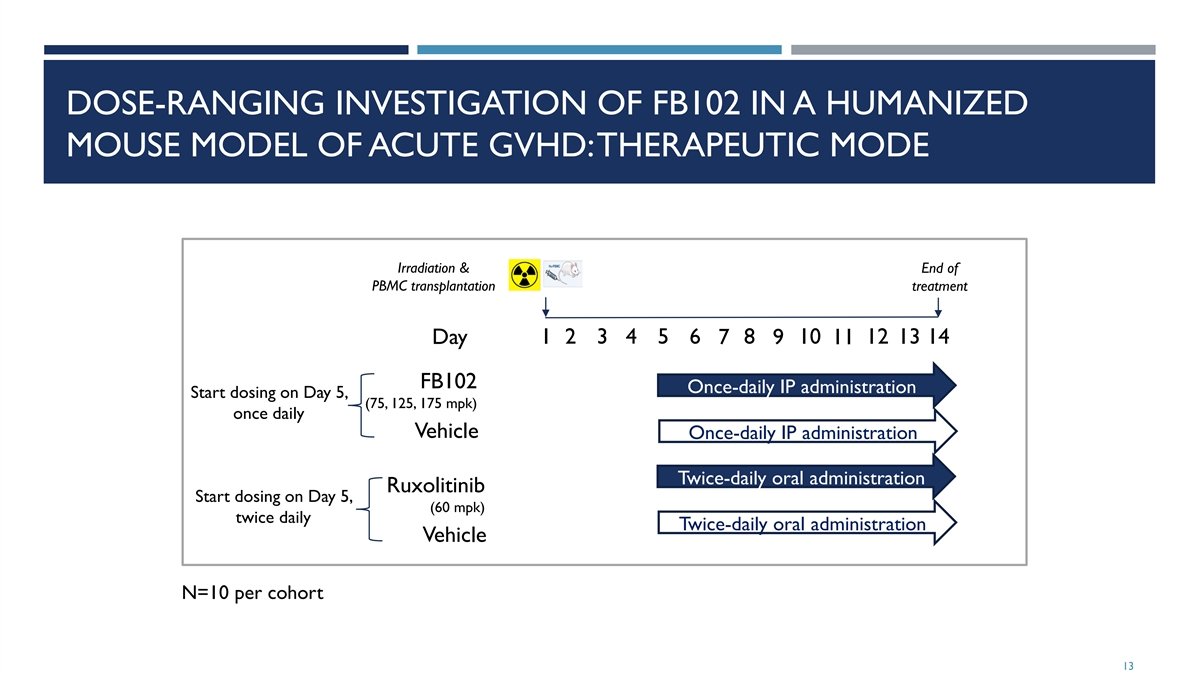
DOSE-RANGING INVESTIGATION OF FB102 IN A HUMANIZED MOUSE MODEL OF ACUTE GVHD: THERAPEUTIC MODE Irradiation & End of PBMC transplantation treatment 1 2 3 4 5 6 8 10 12 13 14 Day 7 9 11 FB102 Once-daily IP administration Start dosing on Day 5, (75, 125, 175 mpk) once daily Vehicle Once-daily IP administration Twice-daily oral administration Ruxolitinib Start dosing on Day 5, (60 mpk) twice daily Twice-daily oral administration Vehicle N=10 per cohort 13

FB102 SURVIVAL BENEFITS: THERAPEUTIC MODEL FB102 Start of daily treatment superior to 1 1 1 1 10 0 0 0 00 0 0 0 0% % % % % FB102(75): 90% aGvHD FB102(175): 90% 8 8 8 8 80 0 0 0 0% % % % % FB102(125): 80% standard of care, 6 6 6 6 60 0 0 0 0% % % % % Ruxolitinib: 60% ruxolitinib, head to head 4 4 4 4 40 0 0 0 0% % % % % 2 2 2 2 20 0 0 0 0% % % % % Vehicle: 0% 0 0 0 0 0% % % % % 1 1 1 1 1 3 3 3 3 3 5 5 5 5 5 7 7 7 7 7 9 9 9 9 9 1 1 1 1 11 1 1 1 1 1 1 1 1 13 3 3 3 3 1 1 1 1 15 5 5 5 5 Study day P=0.0001 for FB102 (75, 175) vs Vehicle on Day 14 P=0.0007 for FB102 (125) vs Vehicle on Day 14 P=0.01 for Ruxolitinib vs Vehicle on Day 14 14

MONO VS COMBINATION THERAPIES WITH FB102, RUXOLITINIB OR CORTICOSTEROIDS Irradiation & End of study PBMC injection (D18) Day 1 5 8 12 15 18 Ruxolitinib Twice-daily oral (60 mpk) FB102+Ruxolitinib Once-daily IP + twice-daily oral (75 mpk+60 mpk) Placebo Once-daily IP + twice-daily oral (Combination of vehicles) N=10 per cohort 15

FB102 SURVIVAL BENEFITS: COMBINATION OF FB102 WITH RUXOLITINIB SIGNIFICANTLY SUPERIOR TO RUXOLITINIB ALONE Start of daily treatment FB102/rux 100% 1 10 00 0% % combo FB102+Rux: 90% significantly 8 8 80 0 0% % % superior to standard of care 6 6 60 0 0% % % single agent, 4 4 40 0 0% % % ruxolitinib, Ruxolitinib: 30% head-to-head 2 2 20 0 0% % % Vehicle: 0% 0 0 0% % % 1 1 1 3 3 3 5 5 5 7 7 7 9 9 9 1 1 11 1 1 1 1 13 3 3 1 1 15 5 5 1 1 17 7 7 Study day p 0.0001 for FB102+Rux vs Vehicle on Day 18 p 0.02 for FB102+Rux vs Ruxolitinib Mono on Day 18 16

IN VIVO NHP AND HUMAN HEALTHY VOLUNTEER SUMMARY 17

FB102 PHASE 1 HEALTHY VOLUNTEER SUMMARY • Phase 1 healthy volunteer SAD/MAD successfully completed and demonstrated a good safety profile. • Significant reductions in NK cell pharmacodynamic marker of FB102 mechanism were observed supporting the in-vitro as well as the NHP data and the mechanism of action of FB102. • Forte initiated a celiac disease patient study in 3Q24 with topline data from this study expected in 2Q25. 18

FB102 INDICATION REVIEW 19
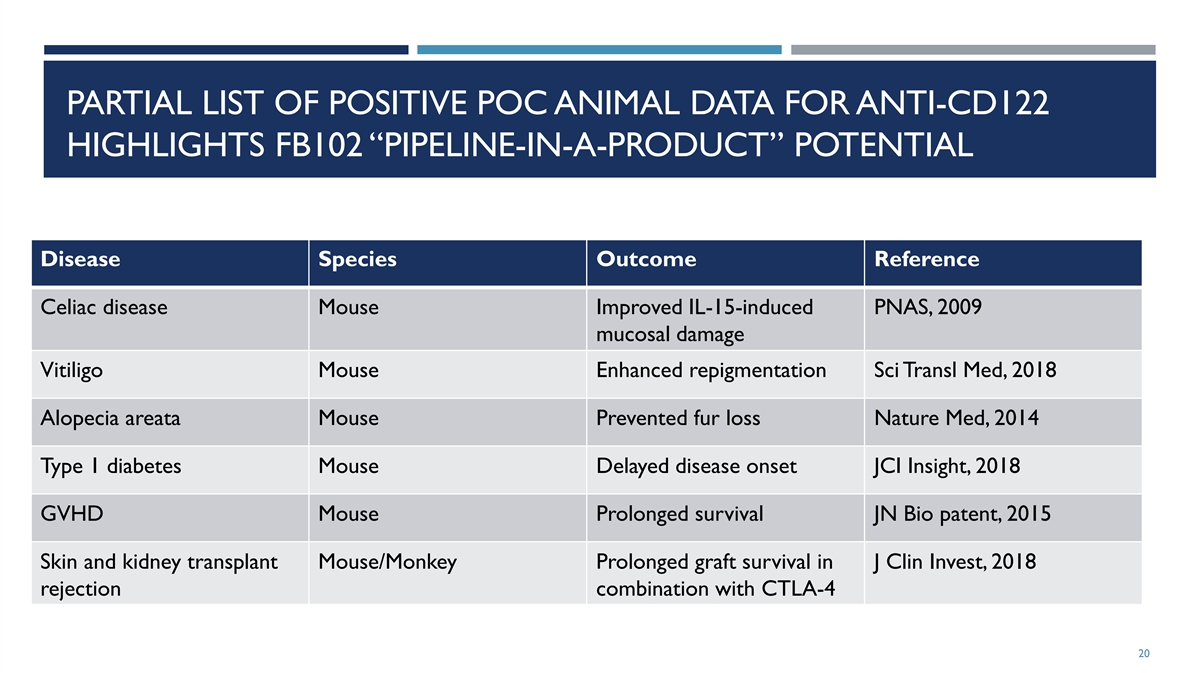
PARTIAL LIST OF POSITIVE POC ANIMAL DATA FOR ANTI-CD122 HIGHLIGHTS FB102 “PIPELINE-IN-A-PRODUCT” POTENTIAL Disease Species Outcome Reference Celiac disease Mouse Improved IL-15-induced PNAS, 2009 mucosal damage Vitiligo Mouse Enhanced repigmentation Sci Transl Med, 2018 Alopecia areata Mouse Prevented fur loss Nature Med, 2014 Type 1 diabetes Mouse Delayed disease onset JCI Insight, 2018 GVHD Mouse Prolonged survival JN Bio patent, 2015 Skin and kidney transplant Mouse/Monkey Prolonged graft survival in J Clin Invest, 2018 rejection combination with CTLA-4 20

CELIAC DISEASE 21

NO APPROVED THERAPIES FOR CELIAC DISEASE; POTENTIAL FOR A SIGNIFICANT MARKET OPPORTUNITY ¡ Celiac disease ¡ Celiac disease is an autoimmune disease that’s triggered by consuming gluten and results in damage to the small intestine ¡ Symptom include diarrhea, fatigue, headaches, anemia, nausea, dermatitis herpetiformis (an itchy skin rash) ¡ Significant patient population does not respond to gluten free diet ¡ Health consequence for not treating include malnourishment, cancer, other autoimmune conditions ¡ Patient Population ¡ Estimated 1:133 in US (2.5 million people) with celiac disease (Fasano, Arch Intern Med. 2003 PMID: 12578508.) ¡ 0.3% to 0.5% of celiac disease patients are non-responsive (Malamut Gastroenterology. 2024 38556189) ¡ No approved treatment options for celiac disease 22

IL-2 AND IL-15 IN CELIAC DISEASE (CED) IL-2 ¡ Clear genetic basis for involvement of IL-2 in CeD ¡ Gluten-induced IL-2 production differentiates true CeD from non-gluten induced GI symptoms ¡ IL-2 strongly correlates with symptom severity and Serum IL-2 peaks within 4 hours after gluten exposure ¡ IL-2 production is followed by increases in intestinal T cells and inflammatory Th-1 type cytokine IFN-γ IL-15 ¡ Clear genetic basis for involvement of IL-15 in CeD ¡ IL-15 levels in intestinal tissue correlate with intestinal damage ¡ IL-15 is overexpressed in gut epithelium and immune cells upon gluten exposure ¡ IL-15Ra is overexpressed in intestinal T cells in patients with CeD ¡ IL-15 induces proliferation and activation of intestinal T cells and inflammatory cytokines IFN- γ and TNF-α ¡ IL-15 activates intestinal cytotoxic CD8+ T cells that kill gut epithelium ¡ IL-15 impairs immunosuppressive and gut-protective activity of CD4+ Tregs and TGF-β 1 - van Heel 2007 Nat Genet. PMID 17558408 23
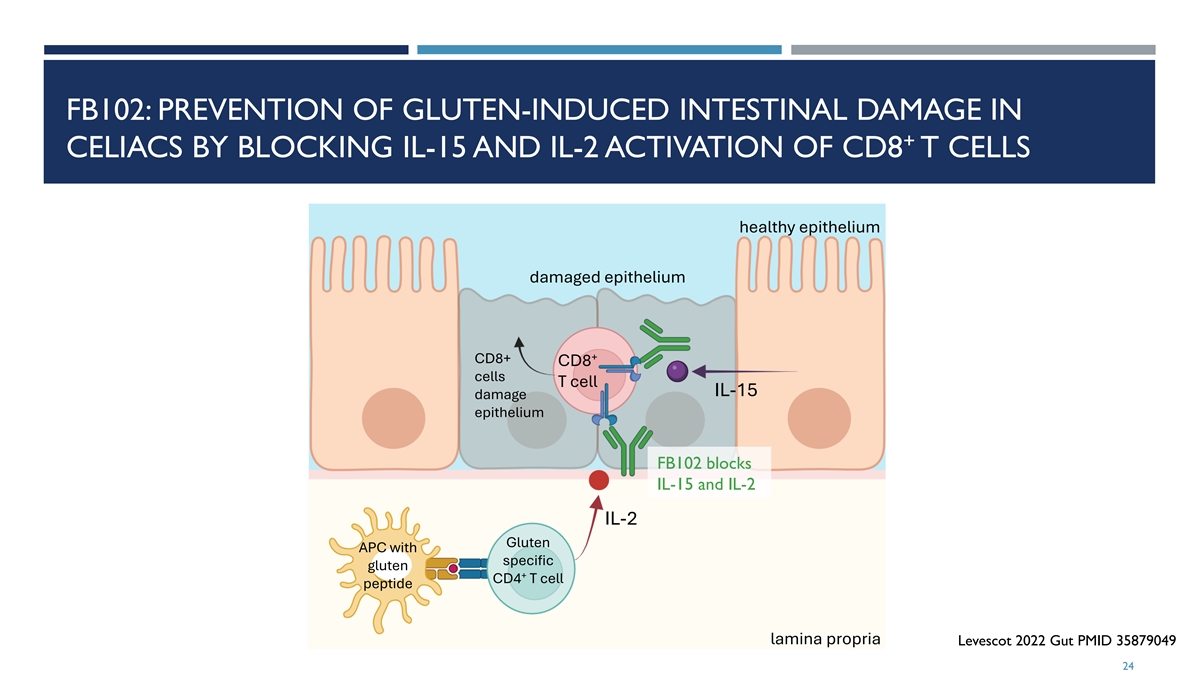
FB102: PREVENTION OF GLUTEN-INDUCED INTESTINAL DAMAGE IN + CELIACS BY BLOCKING IL-15 AND IL-2 ACTIVATION OF CD8 T CELLS healthy epithelium damaged epithelium + CD8+ CD8 cells T cell IL-15 damage epithelium FB102 blocks IL-15 and IL-2 IL-2 Gluten APC with specific gluten + CD4 T cell peptide lamina propria Levescot 2022 Gut PMID 35879049 24

ANTI-CD122 ANTIBODY REVERSES IL-15 INDUCED INTESTINAL DAMAGE IN MICE ¡ Transgenic mice overexpressing IL-15 in the intestine have extensive inflammation and damage to the duodeno- jejunal region with extensive blunting of villi ¡ Extensive influx of NKG2D-expressing CD8+ T cells, and enterocytes express NKG2D ligands, similar to celiac disease ¡ Anti-CD122 antibody treatment for 8 weeks reverses this damage, including reestablishment of normal villus heights Wild-type mice have normal intestine T3:IL-15 transgenic mice have extensive intestinal swelling and distention Anti-CD122 antibody treated T3:IL-15 transgenic mice have normal intestines Yokoyama PNAS 2009 PMID 19805228 25
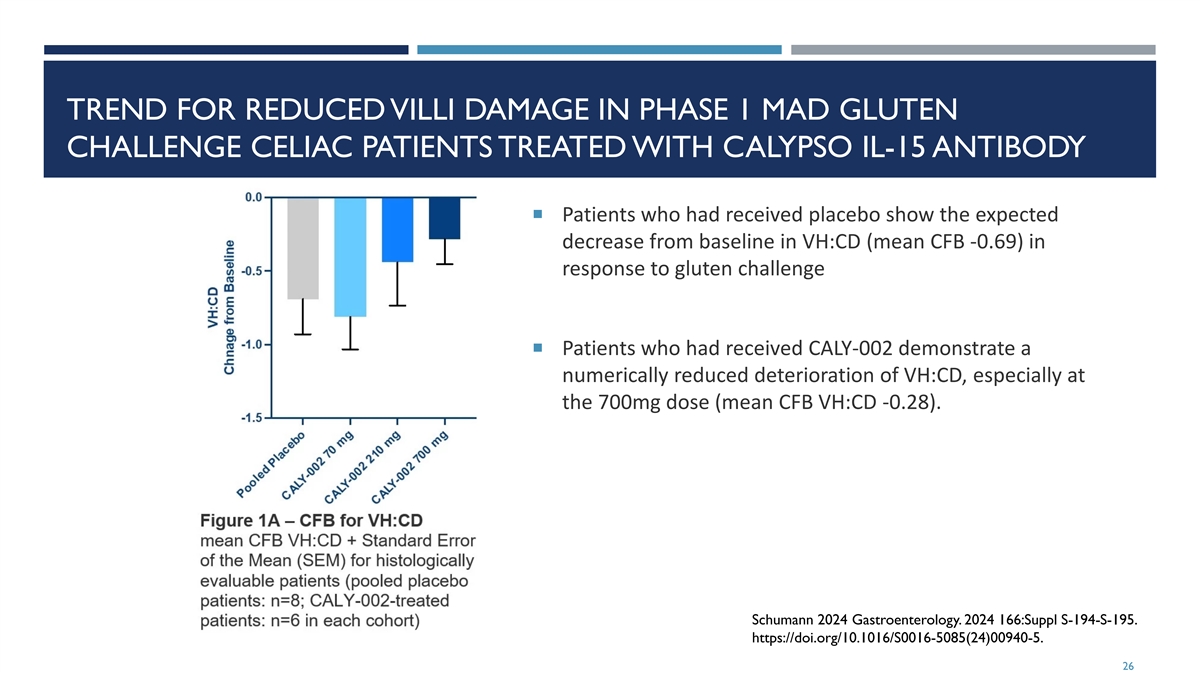
TREND FOR REDUCED VILLI DAMAGE IN PHASE 1 MAD GLUTEN CHALLENGE CELIAC PATIENTS TREATED WITH CALYPSO IL-15 ANTIBODY ¡ Patients who had received placebo show the expected decrease from baseline in VH:CD (mean CFB -0.69) in response to gluten challenge ¡ Patients who had received CALY-002 demonstrate a numerically reduced deterioration of VH:CD, especially at the 700mg dose (mean CFB VH:CD -0.28). Schumann 2024 Gastroenterology. 2024 166:Suppl S-194-S-195. https://doi.org/10.1016/S0016-5085(24)00940-5. 26
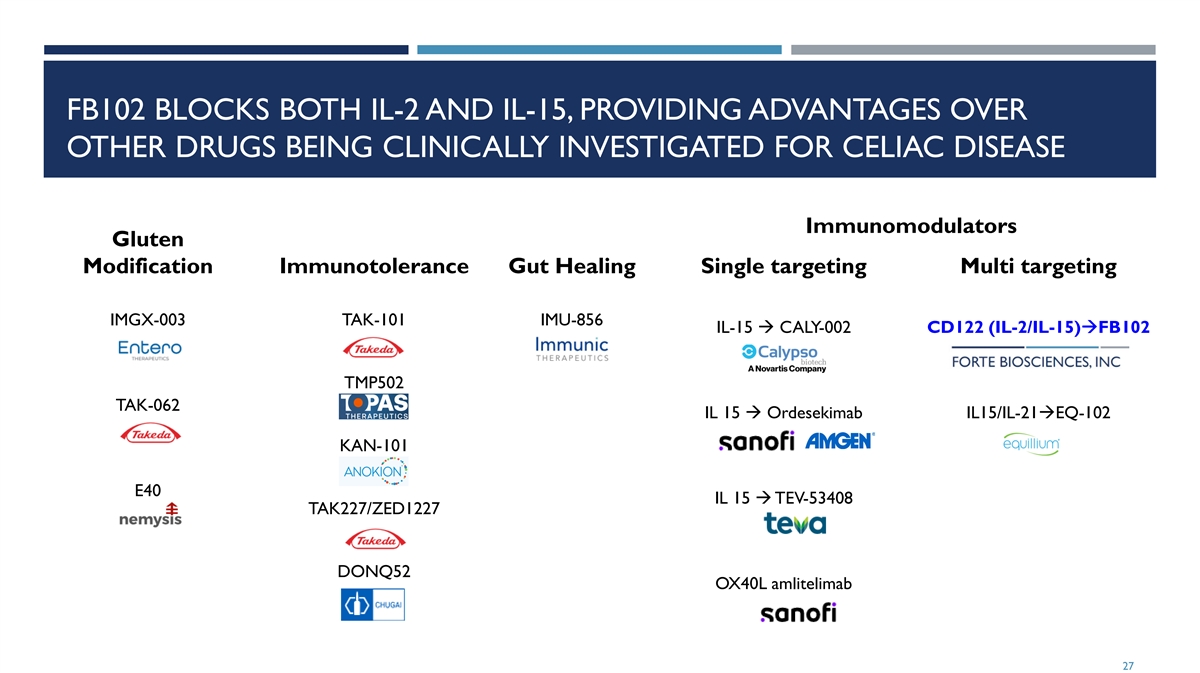
FB102 BLOCKS BOTH IL-2 AND IL-15, PROVIDING ADVANTAGES OVER OTHER DRUGS BEING CLINICALLY INVESTIGATED FOR CELIAC DISEASE Immunomodulators Gluten Modification Immunotolerance Gut Healing Single targeting Multi targeting IMGX-003 TAK-101 IMU-856 IL-15 à CALY-002 CD122 (IL-2/IL-15)àFB102 TMP502 TAK-062 IL 15 à Ordesekimab IL15/IL-21àEQ-102 KAN-101 E40 IL 15 à TEV-53408 TAK227/ZED1227 DONQ52 OX40L amlitelimab 27

CELIAC DISEASE FB102 TRIAL OVERVIEW 28
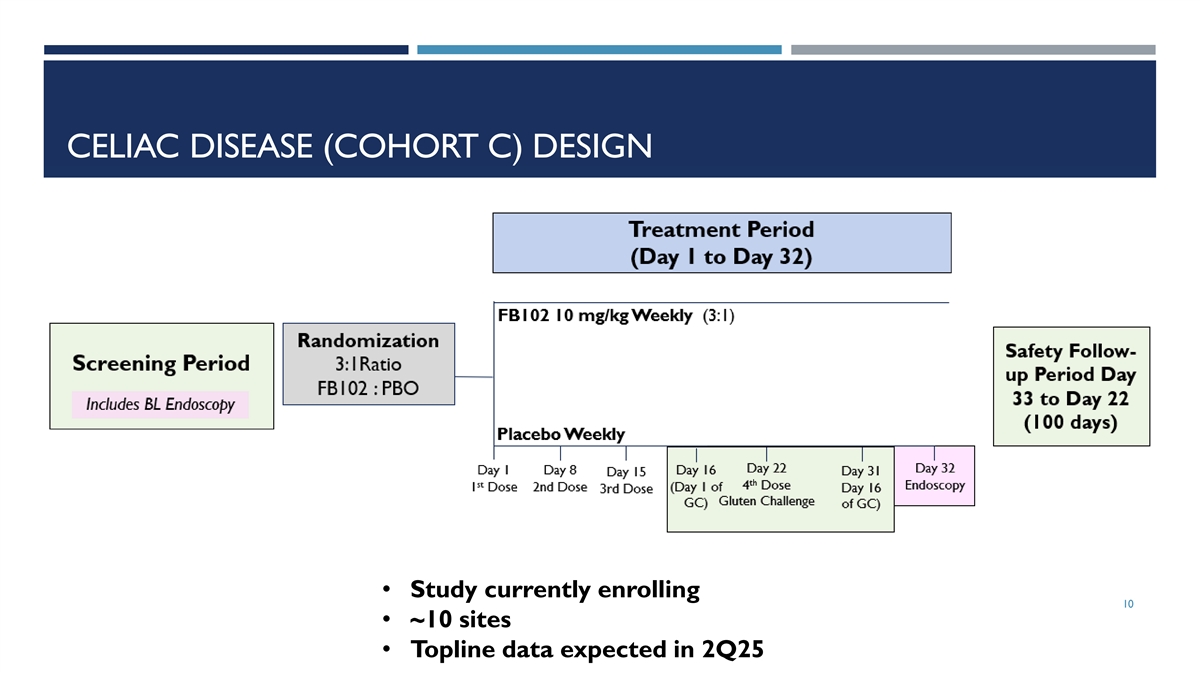
CELIAC DISEASE (COHORT C) DESIGN • Study currently enrolling 10 • ~10 sites • Topline data expected in 2Q25

VITILIGO 30

LARGE UNMET NEED IN VITILIGO PRESENTS A SUBSTANTIAL MARKET OPPORTUNITY ¡ Vitiligo ¡ Vitiligo is an autoimmune disease of the skin driven by pathogenic T cells that kill melanocytes and create white spots. ¡ Vitiligo results in sensitive skin (increasing likelihood of sub burns), eye abnormalities, emotional challenges, and leads to a predisposition of other autoimmune conditions. ¡ Patient Population Amy Deanna / CoverGirl cosmetics ¡ Prevalent in 0.76% of population – 2 Million in US ¡ While JAK inhibitors have demonstrated efficacy in vitiligo, regulatory scrutiny of the JAK class including black box warnings has dampened enthusiasm for this class and as a result there remains a significant unmet need for safe and effective therapies for treating AA and vitiligo https://my.clevelandclinic.org/health/diseases/12419-vitiligo 31
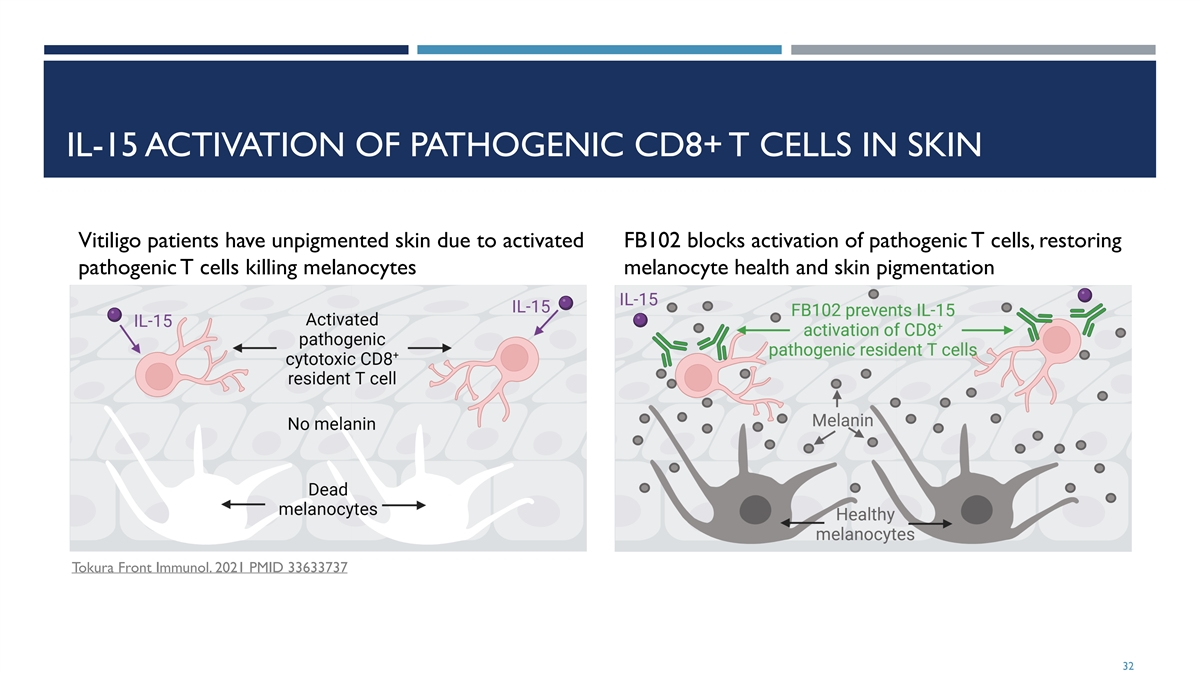
IL-15 ACTIVATION OF PATHOGENIC CD8+ T CELLS IN SKIN Vitiligo patients have unpigmented skin due to activated FB102 blocks activation of pathogenic T cells, restoring pathogenic T cells killing melanocytes melanocyte health and skin pigmentation Tokura Front Immunol. 2021 PMID 33633737 32

IL-2 THERAPY DRIVES VITILIGO Enhanced Survival Associated with Vitiligo Expression during (1) Maintenance Biotherapy for Metastatic Melanoma 1 2 1 1 2 1 Peter D. Boasberg , Dave S.B. Hoon , Lawrence D. Piro , Maureen A. Martin , Akhide Fujimoto , Timothy S. Kristedja , 1 2 1 1 Sandeep Bhachu , Xing Ye , Regina R. Deck and Steven J. O’Day In a large retrospective analysis of 374 metastatic melanoma patients treated with high-dose IL-2, a total of 84 patients (22%) developed treatment-related vitiligo, although in patients with objective clinical responses the incidence of vitiligo was nearly (2) 50% 1) Journal of Investigative Derm. (2006) Vol 126 2) Journal of Clinical Oncology V19(15) 33
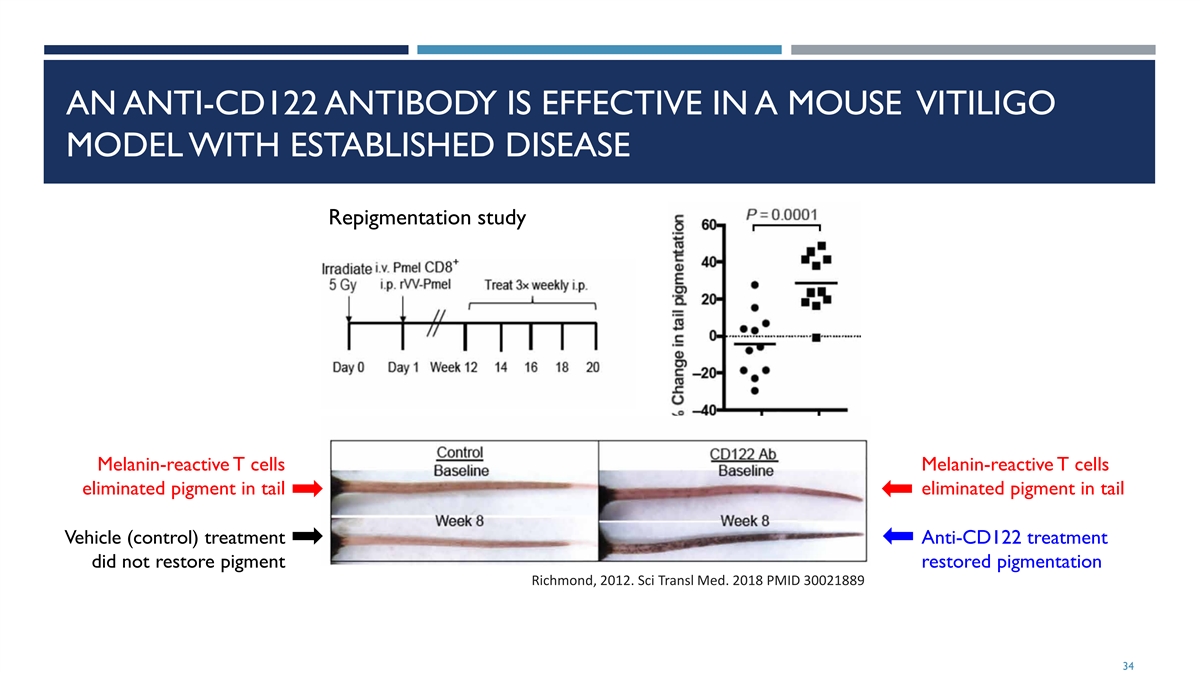
AN ANTI-CD122 ANTIBODY IS EFFECTIVE IN A MOUSE VITILIGO MODEL WITH ESTABLISHED DISEASE Repigmentation study Melanin-reactive T cells Melanin-reactive T cells eliminated pigment in tail eliminated pigment in tail Vehicle (control) treatment Anti-CD122 treatment did not restore pigment restored pigmentation Richmond, 2012. Sci Transl Med. 2018 PMID 30021889 34
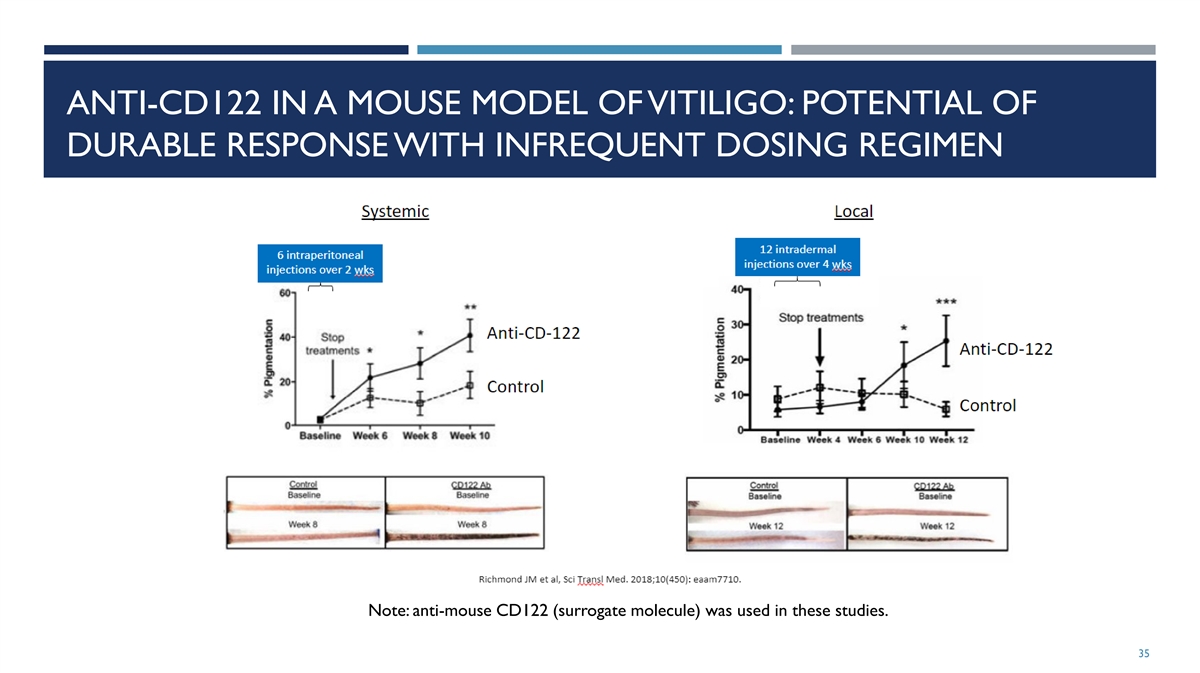
ANTI-CD122 IN A MOUSE MODEL OF VITILIGO: POTENTIAL OF DURABLE RESPONSE WITH INFREQUENT DOSING REGIMEN Note: anti-mouse CD122 (surrogate molecule) was used in these studies. 35
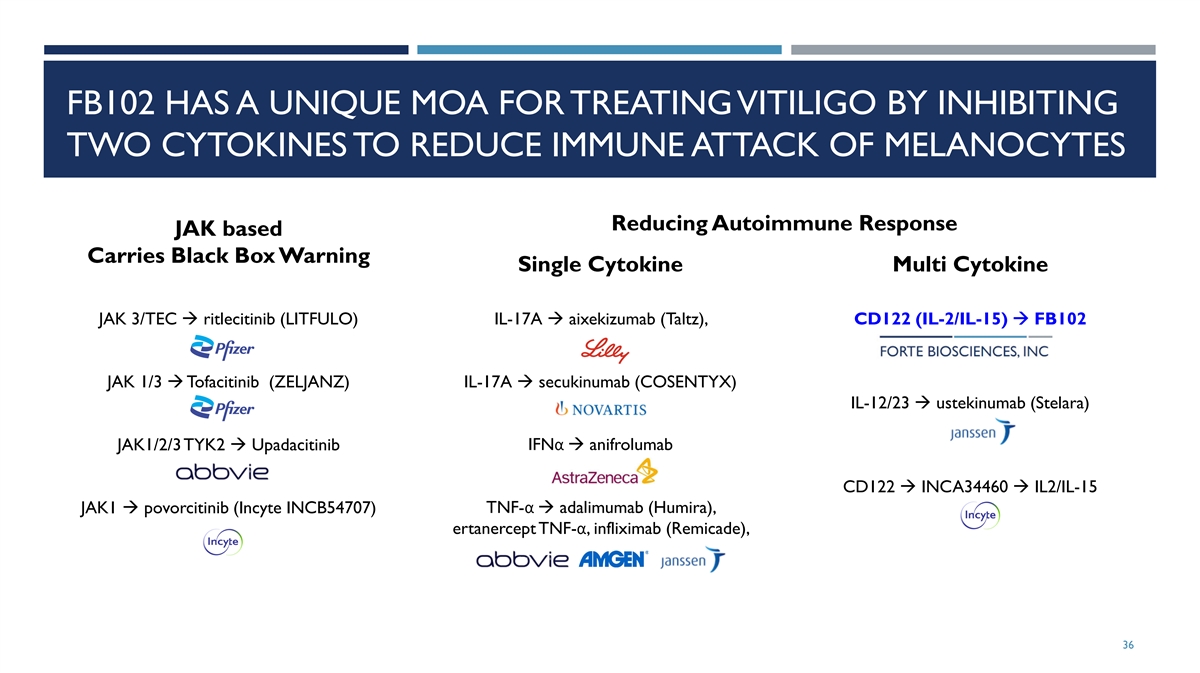
FB102 HAS A UNIQUE MOA FOR TREATING VITILIGO BY INHIBITING TWO CYTOKINES TO REDUCE IMMUNE ATTACK OF MELANOCYTES Reducing Autoimmune Response JAK based Carries Black Box Warning Single Cytokine Multi Cytokine JAK 3/TEC à ritlecitinib (LITFULO) IL-17A à aixekizumab (Taltz), CD122 (IL-2/IL-15) à FB102 JAK 1/3 à Tofacitinib (ZELJANZ) IL-17A à secukinumab (COSENTYX) IL-12/23 à ustekinumab (Stelara) JAK1/2/3 TYK2 à Upadacitinib IFNα à anifrolumab CD122 à INCA34460 à IL2/IL-15 JAK1 à povorcitinib (Incyte INCB54707) TNF-α à adalimumab (Humira), ertanercept TNF-α, infliximab (Remicade), 36

ALOPECIA AREATA 37

LARGE UNMET NEED IN ALOPECIA AREATA PRESENTS A SIGINIFICANT MARKET OPPORTUNITY ¡ Alopecia Areata ¡ Alopecia areata is a common autoimmune disease that occurs when pathogenic T cells attack hair follicles causing hair loss. ¡ Alopecia areata affects both men and women and affects all racial and ethnic groups. ¡ Patient Population ¡ It is estimated that 2% of the global population will experience alopecia areata at some point in their lifetime. ¡ It is estimated that at 700K Americans are currently affected by alopecia areata ¡ While JAK inhibitors have demonstrated efficacy, regulatory scrutiny of the JAK class including black box warnings has dampened enthusiasm for this class and as a result there remains a significant unmet need for safe and effective therapies for treating AA and vitiligo https://www.naaf.org/ https://www.niams.nih.gov/health-topics/alopecia-areata 38

ALOPECIA AREATA (AA) IS DRIVEN BY T LYMPHOCYTES INVADING HAIR FOLLICLES ¡ A healthy hair bulb in anagen phase of the Left: Co-expression of hair follicle cycle is surrounded by an IL-15 and IL-15RA in alopecia areata (AA) but immunosuppressive environment (an not normal control immune privileged space): includes no MHC (NC) class I expression Below: CD122+ CD8+ ¡ Poorly understood environmental factors T cells in follicles from cause immune privilege collapse AA patients ¡ If the space collapses, immune cells attack Xing Nat Med. 2014 PMID: 25129481 pigment expressing cells in the hair bulb ¡ MHC I is strongly overexpressed ¡ Dense accumulation of lymphocytes at hair bulb: 60–80% CD4+ and 20–40% CD8+ T cells Alopecia follicle Healthy follicle - Loss of immune privilege ¡ IL-2 is elevated in AA patients, and IL-15 is - Immune privileged space - Invasion of T and NK cells overexpressed in AA hair follicles - Cytokines (e.g. IL-15, IL-2) (T and NK cells excluded) Bertolini Exp Dermatol. 2020 PMID: 32682334; Ito Clin Dev Immunol. - No MHC expression - MHC expression 2013 PMID: 24151515; Ito 2014 Exp Dermatol. 2014 PMID: 25040075; Xing Nat Med. 2014 PMID: 25129481 - Hair loss 39 Immune privileged

FB102 SHOULD PROTECT HAIR FOLLICLES FROM IMMUNE ATTACK AFTER LOSS OF IMMUNE PRIVILEGE 40
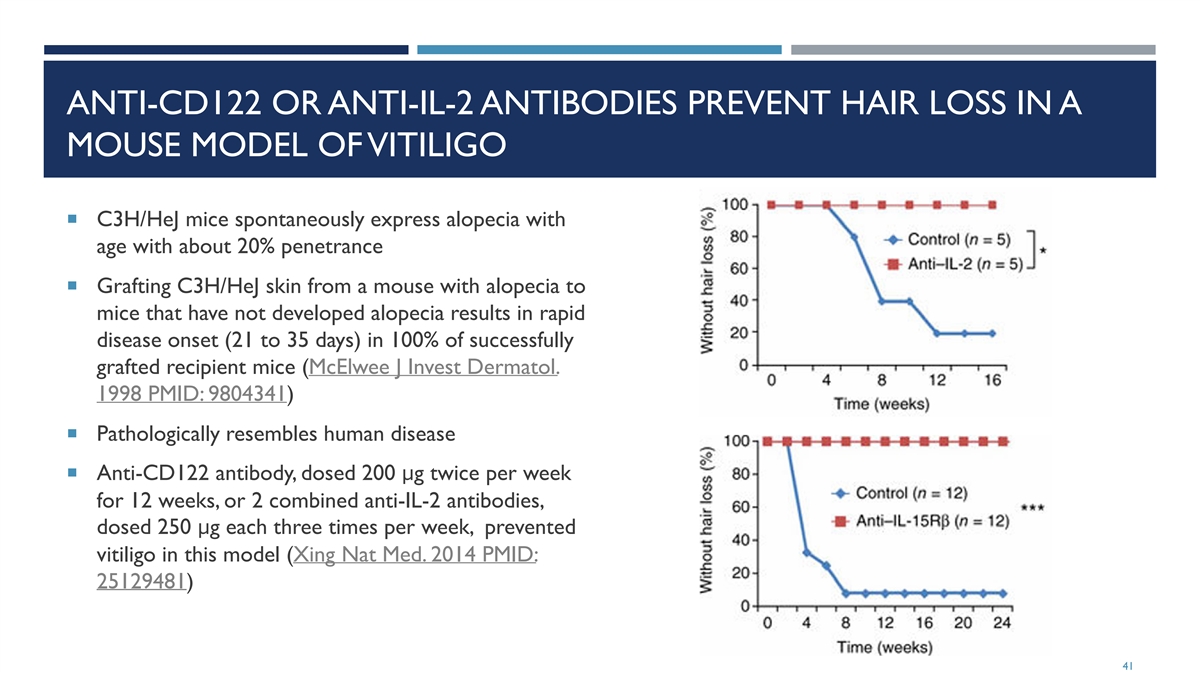
ANTI-CD122 OR ANTI-IL-2 ANTIBODIES PREVENT HAIR LOSS IN A MOUSE MODEL OF VITILIGO ¡ C3H/HeJ mice spontaneously express alopecia with age with about 20% penetrance ¡ Grafting C3H/HeJ skin from a mouse with alopecia to mice that have not developed alopecia results in rapid disease onset (21 to 35 days) in 100% of successfully grafted recipient mice (McElwee J Invest Dermatol. 1998 PMID: 9804341) ¡ Pathologically resembles human disease ¡ Anti-CD122 antibody, dosed 200 μg twice per week for 12 weeks, or 2 combined anti-IL-2 antibodies, dosed 250 μg each three times per week, prevented vitiligo in this model (Xing Nat Med. 2014 PMID: 25129481) 41
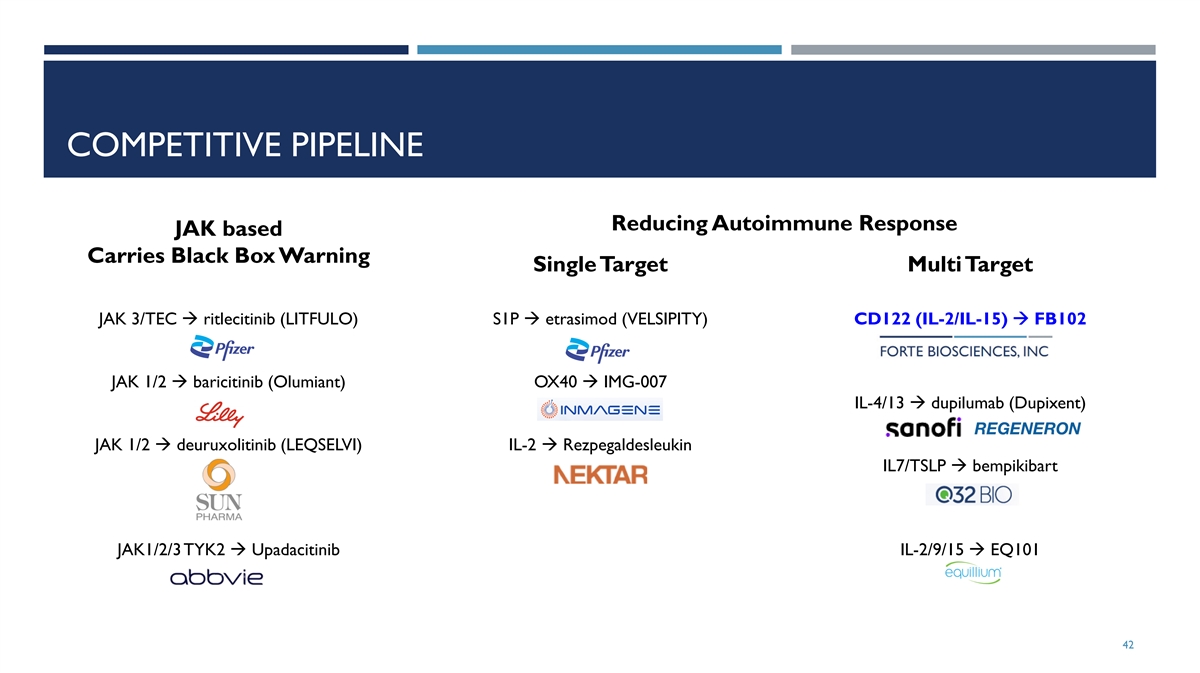
COMPETITIVE PIPELINE Reducing Autoimmune Response JAK based Carries Black Box Warning Single Target Multi Target JAK 3/TEC à ritlecitinib (LITFULO) S1P à etrasimod (VELSIPITY) CD122 (IL-2/IL-15) à FB102 JAK 1/2 à baricitinib (Olumiant) OX40 à IMG-007 IL-4/13 à dupilumab (Dupixent) JAK 1/2 à deuruxolitinib (LEQSELVI) IL-2 à Rezpegaldesleukin IL7/TSLP à bempikibart JAK1/2/3 TYK2 à Upadacitinib IL-2/9/15 à EQ101 42

TYPE 1 DIABETES 43

TYPE 1 DIABETES IS CAUSED BY AUTOREACTIVE T CELLS DESTROYING INSULIN-PRODUCING PANCREATIC BETA CELLS ¡ Type 1 diabetes (T1D) is classified by three stages of progression ¡ Stage 1: at least one diabetes-related autoantibody but has normal blood sugar and no symptoms ¡ Stage 2: at least two diabetes-related autoantibodies and abnormal blood sugar levels but otherwise symptom free ¡ Stage 3: significant beta cell loss has occurred; abnormal blood sugar levels; hemoglobin A1C >6.4%; excessive thirst and urination; blurry vision; fatigue; requires insulin for disease management ¡ Patient Population 1 ¡ 64,000 people diagnosed with type 1 diabetes annually ¡ 2 approved treatment options to delay the onset of type 1 diabetes FB102 may represent a safer way to delay the onset of type 1 diabetes 1 - https://beyondtype1.org/type-1-diabetes-statistics/ 44
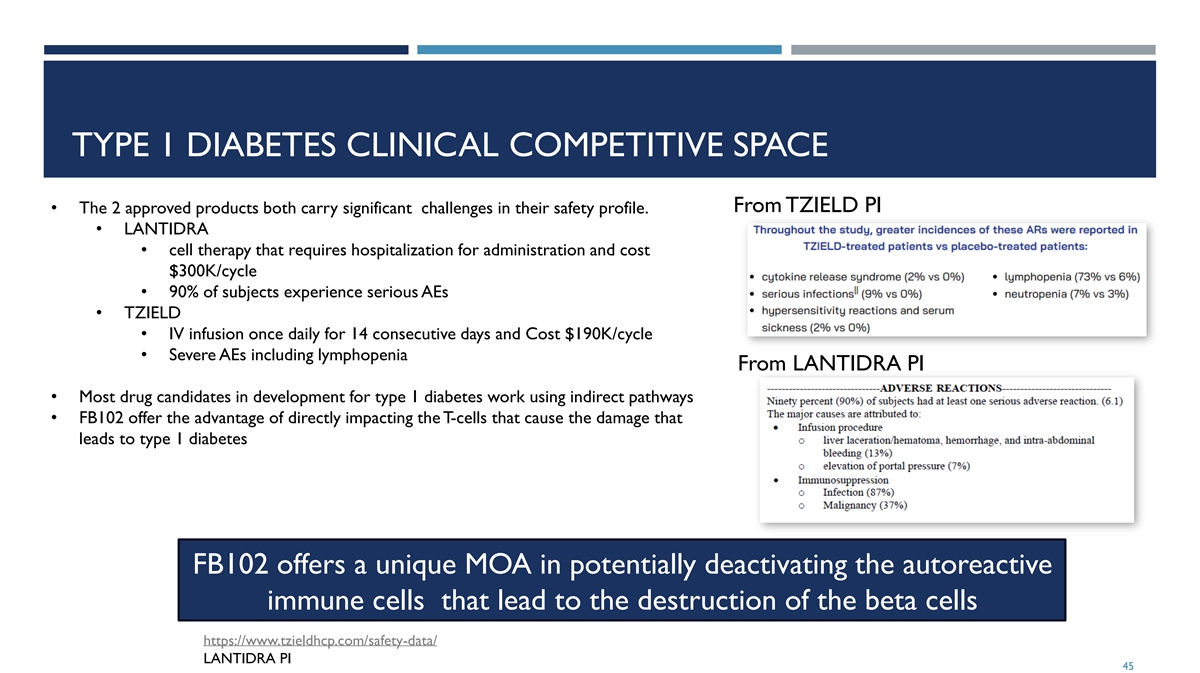
TYPE 1 DIABETES CLINICAL COMPETITIVE SPACE From TZIELD PI • The 2 approved products both carry significant challenges in their safety profile. • LANTIDRA • cell therapy that requires hospitalization for administration and cost $300K/cycle • 90% of subjects experience serious AEs • TZIELD • IV infusion once daily for 14 consecutive days and Cost $190K/cycle • Severe AEs including lymphopenia From LANTIDRA PI • Most drug candidates in development for type 1 diabetes work using indirect pathways • FB102 offer the advantage of directly impacting the T-cells that cause the damage that leads to type 1 diabetes FB102 offers a unique MOA in potentially deactivating the autoreactive immune cells that lead to the destruction of the beta cells https://www.tzieldhcp.com/safety-data/ LANTIDRA PI 45
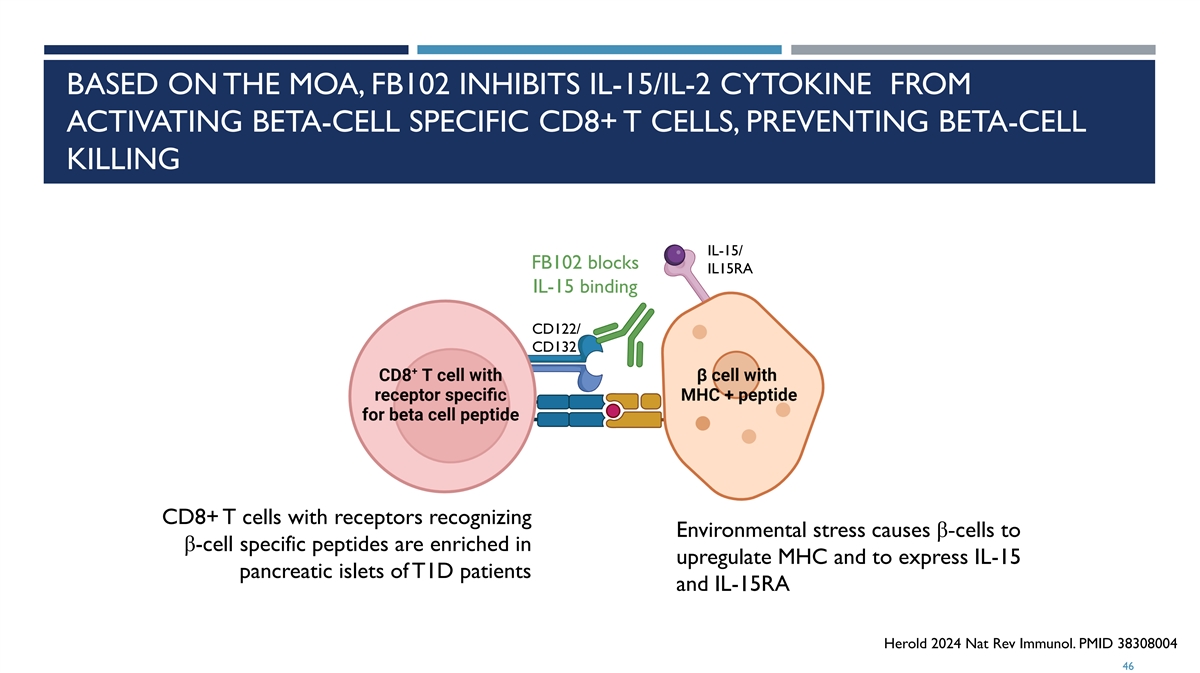
BASED ON THE MOA, FB102 INHIBITS IL-15/IL-2 CYTOKINE FROM ACTIVATING BETA-CELL SPECIFIC CD8+ T CELLS, PREVENTING BETA-CELL KILLING IL-15/ FB102 blocks IL15RA IL-15 binding CD122/ CD132 CD8+ T cells with receptors recognizing Environmental stress causes β-cells to β-cell specific peptides are enriched in upregulate MHC and to express IL-15 pancreatic islets of T1D patients and IL-15RA Herold 2024 Nat Rev Immunol. PMID 38308004 46
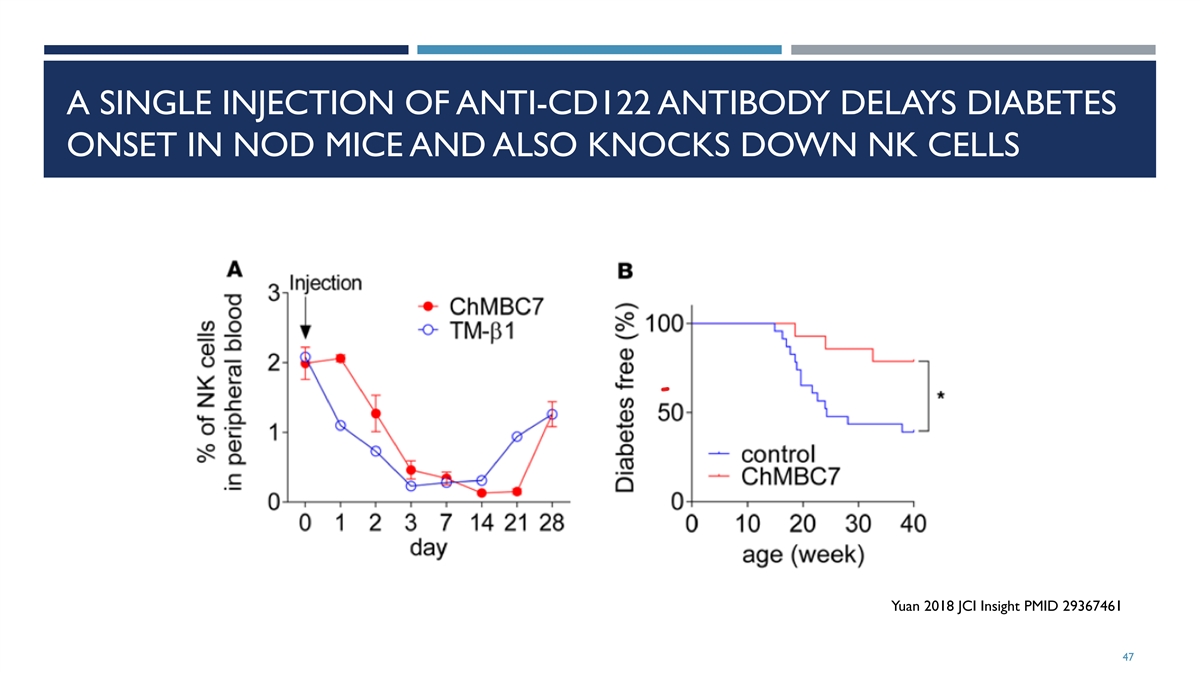
A SINGLE INJECTION OF ANTI-CD122 ANTIBODY DELAYS DIABETES ONSET IN NOD MICE AND ALSO KNOCKS DOWN NK CELLS Yuan 2018 JCI Insight PMID 29367461 47

SUMMARY 48
CLINICAL STAGE FB-102 • CD122 is a subunit of the intermediate affinity IL-2/IL-15 receptor expressed on NK cells and T cells and is a subunit of the high affinity IL-2 receptor expressed on Tregs. • FB102 (Forte’s anti-CD122 antibody) is designed to mediate both the IL-2 and the IL-15 induced proliferation and activation of pathogenic NK cells and T cells without effecting the IL-2 induced proliferation of the immune modulating Tregs. • Significant amount of proof-of-concept preclinical data across numerous indications supports “Pipeline-in-a-Product” potential for FB102. • 4 and 13-week NHP tox studies completed • Healthy volunteer SAD/MAD completed • Initiated celiac disease patient study with readout expected in 2Q25 49
















































