
Targeted Medical Pharma, Inc. Innovators in Pharmaceutical Technology SoCal Bio Presentation – November 2012

Safe Harbor Statement This document does not constitute or form part of an invitation or recommendation to subscribe for or purchase any securities . Targeted Medical Pharma, Inc . (“Targeted Medical Pharma”, “TMP” or “the Company”) shall not have any responsibility for any such violations . This document was prepared exclusively for the benefit and internal use of investors in order to evaluate the feasibility of a possible transaction or transactions and does not carry any right of publication or disclosure to any other party . This document is incomplete without reference to, and should be viewed in conjunction with, the oral briefing provided by TMP, the Company’s public filings and press releases . This presentation may not be used for any other purpose without the prior written consent of Targeted Medical Pharma . In preparing this document we have relied upon and assumed, without independent verification, the accuracy and completeness of all information available from public sources or which was provided to us or otherwise reviewed by us . The information contained in this document has been taken from sources deemed to be reliable . We do not represent that such information is accurate or complete and it should not be relied on as such . Any opinions expressed herein reflect our judgment at this date, all of which are subject to change . We have based the forward - looking statements on our current expectations and projections about future events . These forward - looking statements are subject to known and unknown risks, uncertainties and assumptions about us and with respect to specific factors identified in this presentation and in the Company’s filings with the U . S . Securities Exchange Commission, specifically the reported risk factors, that may cause our actual results, levels of activity, performance or achievements expressed or implied by such forward - looking statements . Actual results in each case could differ materially from those currently anticipated in such statements . Non - GAAP Financial Measures In this presentation, TMP uses the non - GAAP measures adjusted EBITDA and adjusted earnings per share . TMP believes these non - GAAP financial measures are helpful in understanding its past financial performance and its potential future results . They are not meant to be considered in isolation or as a substitute for comparable GAAP measures and should be read in conjunction with the consolidated financial statements prepared in accordance with GAAP . TMP’s management regularly uses these supplemental non - GAAP financial measures internally to understand, manage and evaluate its business and make operating decisions . TMP believes that the use of these non - GAAP measures enhances the ability of investors to compare its results from period to period . Investors should note that adjusted EBIDTA and adjusted earnings per share, as used by TMP, may be calculated differently from, and therefore may not be directly comparable to, similarly titled measures used by TMP’ competitors and other companies . The non - GAAP historical financial measures may exclude amortization of intangible assets, share - based compensation, transaction related costs, non - cash interest expense, loss on extinguishment of debt, and the effect of change in the timing of when certain revenue was recognized . The non - GAAP 2012 financial guidance may excludes amortization of intangible assets, share - based compensation, transaction and integration costs, purchase accounting inventory step - up adjustments and the tax effect of these adjustments to GAAP net income . Trademarks All products named in this presentation are protected under trademark including those not marked “TM” . Safe Harbor Statement, Non - GAAP Financial Measures and Trademarks TARGETED MEDICAL PHARMA 2

Rx - Only Medical Foods Pioneers TARGETED MEDICAL PHARMA 3

Company Snapshot TARGETED MEDICAL PHARMA Specialty pharmaceutical company founded in 1999 (as of October 2012, OTCBB: TRGM) Low - cost development platform A technology that is applicable to multiple diseases A no - side - effect replacement for many pharmaceuticals Nine core proprietary medical food products Strong product pipeline and patent portfolio Low - Cost Pharmaceutical Development Distribution Through Multiple Channels Diversified Reimbursement Sources Reimbursement Operations • CCPI • Billing Services Agreement • Business Associate Agreement • GRAS / GRAE status • Medical Foods • Internally - managed trials • Physicians • Mail Order Pharmacy • Pharma Distributors • Military and VA • Licensing • Nursing Homes • Medicare Part D • Medicaid • Major Insurers • Pharmacy Benefit Managers 4

Must make a disease claim Requires physician prescription and supervision Regulated as drugs prior to 1972 Enteral administration GRAS ingredients Address specific nutritional deficiencies Does not need FDA pre - approval Medical foods are FDA - regulated medications intended for the dietary management of a disease that has distinctive nutritional needs that cannot be met by normal diet alone as defined in the Food and Drug Administration's 1988 Orphan Drug Act Amendments. About Rx - Only Medical Foods TARGETED MEDICAL PHARMA 5

Five - component System Stimulates Uptake and Utilization of Amino Acids Targeted Cellular Technology TARGETED MEDICAL PHARMA 6

Collections Growth TARGETED MEDICAL PHARMA 7 0% 5% 10% 15% 20% 25% Apr-12 May-12 Jun-12 Jul-12 Aug-12 Sep-12 Oct-12 Collections Growth Trend

* First Known Oral Stimulation of Stem Cells Product Rollout and Pipeline TARGETED MEDICAL PHARMA 8 Drug Indication In Development Clinical Trials On Market and Reimbursed Medical Food Co - Packs Multiple disease states x Theramine 90 Fibromyalgia x Sentra PM Sleep Disorders with Depression x Sentra AM Cognitive Disorders / Fatigue x Trepadone 90 Osteoarthritis x GABAdone Sleep Disorders with Anxiety x AppTrim Metabolic Disorders / Obesity x Hypertensa 90 Hypertension x ListerV Viral Infection / Impaired Immunity x Percura* Neuropathy ESS - 1818* Chronic Anemia Pulmona New Asthma STR Nasal Congestion Oral IGF1 Diabetes

The Basis for Low - Cost Research and Development Exemption No safety trials, only efficacy must be demonstrated Registration 60 products registered with the FDA Categorization 2 Categorized as an old drug Regulation 1 Regulated under the FDA’s medical food definition 1 Regulation • Must be administered under physician supervision • Must be labeled for the dietary management of a specific medical disorder • Must be for the dietary management of disease • Must be comprised of Generally Recognized as Safe (GRAS) ingredients • Must meet distinct nutritional needs not met by normal diet • Must adhere to FDA food and safety labeling requirements 2 Categorization • Holds GRAS / GRAE (Generally Recognized as Effective) status • Under the 1962 FDA Amendment • Under the Feb 17, 2012 Federal Register reaffirmation of Covered Drug definition Faster Time to Market TARGETED MEDICAL PHARMA 9

Primary Uses Reduction of Pain and Inflammation Clinical Trial: Theramine® with Naproxen (Theraproxen®) TARGETED MEDICAL PHARMA 10

- 20,000 40,000 60,000 80,000 100,000 120,000 1 2 5 7 17 Effect of ESS on Reticulocyte Count 1 Reticulocytes Stimulation of Red Blood Cell Precursors 1 Effect on one patient in pre - clinical trials. Pre - clinical trials resulted in an 80% response rate. Treatment Initiated Treatment Discontinued Primary Uses Initial Data: ESS - 1818 (Pipelined) TARGETED MEDICAL PHARMA 11

Pharmacoeconomic Analysis of Theramine TARGETED MEDICAL PHARMA 38,500,000 capsules administered with no reported G.I. bleeds or deaths. Use of Theramine Reduces the Cost of Care by >30% 12 Theramine NSAID Product 633,600,000 $ 36,000,000 $ GI Bleed Treatment - 225,000,000 Protective Medications - 432,000,000 Laboratory Testing 1,000,000 75,000,000 Negative GI Workup - 144,000,000 Total 634,600,000 $ 912,000,000 $ Comparative Annual Treatment Cost
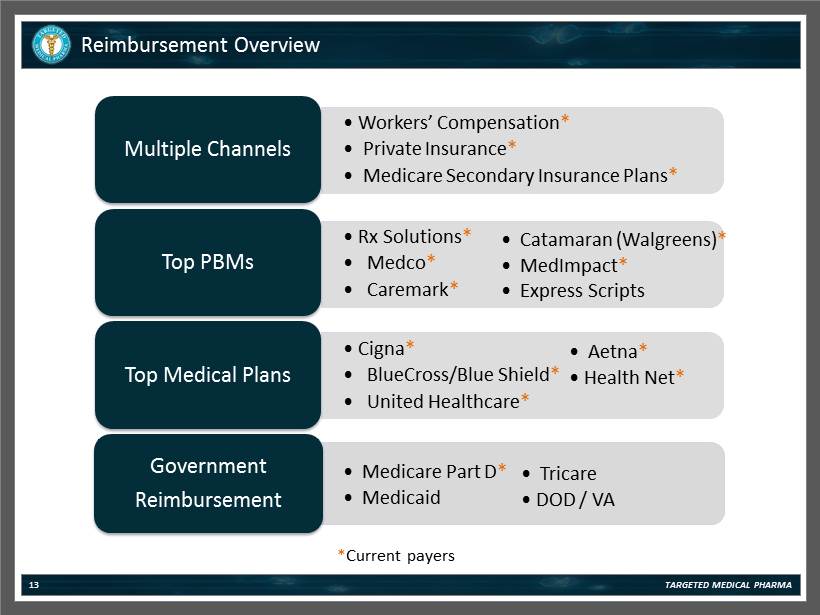
• Workers’ Compensation * • Private Insurance * • Medicare Secondary Insurance Plans * Multiple Channels • Rx Solutions * • Medco * • Caremark * Top PBMs • Cigna * • BlueCross/Blue Shield * • United Healthcare * Top Medical Plans • Catamaran (Walgreens) * • MedImpact * • Express Scripts • Aetna * • Health Net * • Medicare Part D * • Medicaid Government Reimbursement Reimbursement Overview • Tricare • DOD / VA TARGETED MEDICAL PHARMA 13 * Current payers

Intellectual Property Highlights TARGETED MEDICAL PHARMA 特許 Six Issued Patents Four Patents Pending □ 4 USPTO neurotransmitter - specific patents covering Targeted Cellular Technology® (TCT) □ 1 USPTO patent covering the convenience pack presentation □ 1 Japanese patent covering TMP’s Targeted Cellular Technology® □ Stimulation of RBC production* □ Oral Insulin / Insulin Resistance* □ Neuropathy treatment □ Billing methodology for physician dispensing of medication (APPROVED) 1 Targeted Cellular Technology® is a five - component system the stimulates the uptake and utilization of amino acids. It enables efficacy from microgram quantities of amino acids. *First Known Oral Stimulation of Stem Cells 14

Investment Considerations HIGH DEMAND PRODUCTS ADDRESSING LARGE MARKETS 1 GROWING ADOPTION OF MEDICAL FOODS 2 ROBUST PIPELINE 3 STRONG AND VALUABLE INTELLECTUAL PROPERTY PORTFOLIO 4 PROVEN SAFETY AND EFFICACY THROUGH CLINICAL TRIALS 5 LOW SIDE - EFFECT PROFILE 6 LOW - COST DEVELOPMENT PLATFORM / FASTER TIME TO MARKET 7 STRONG REIMBURSEMENT 8 ESTABLISHED SALES 9 STRONG GROWTH POTENTIAL 10 TARGETED MEDICAL PHARMA 15

Contact 2980 Beverly Glen Circle, Suite 301 Los Angeles, CA 90077 Telephone: 310 - 474 - 9809 Fax: 310 - 531 - 7089 investors@tmedpharma.com Brand site: www.ptlcentral.com Company site: www.tmedpharma.com















