
Targeted Medical Pharma, Inc. Innovators in Pharmaceutical Technology September 2013

Safe Harbor Statement This document does not constitute or form part of an invitation or recommendation to subscribe for or purchase any securities of Targeted Medical Pharma, Inc . (“Targeted Medical Pharma”, “TMP” or “the Company”) . This document was prepared exclusively for the benefit and internal use of investors in order to evaluate the feasibility of a possible transaction or transactions and does not carry any right of publication or disclosure to any other party . This document is incomplete without reference to, and should be viewed in conjunction with, the oral briefing provided by TMP, the Company’s public filings with the Securities and Exchange Commission (the “SEC”) and press releases . This presentation may not be used for any other purpose without the prior written consent of Targeted Medical Pharma . In preparing this document we have relied upon and assumed, without independent verification, the accuracy and completeness of all information available from public sources or which was provided to us or otherwise reviewed by us . The information contained in this document has been taken from sources deemed to be reliable . We do not represent that such information is accurate or complete and it should not be relied on as such . Any opinions expressed herein reflect our judgment at this date, all of which are subject to change . We have based the forward - looking statements on our current expectations and projections about future events . These forward - looking statements are subject to known and unknown risks, uncertainties and assumptions about us and with respect to specific factors identified in this presentation and in the Company’s filings with the SEC, specifically the reported risk factors, that may cause our actual results, levels of activity, performance or achievements expressed or implied by such forward - looking statements . Actual results in each case could differ materially from those currently anticipated in such statements . Non - GAAP Financial Measures In this presentation, TMP uses the non - GAAP measures adjusted EBITDA and adjusted earnings per share . TMP believes these non - GAAP financial measures are helpful in understanding its past financial performance and its potential future results . They are not meant to be considered in isolation or as a substitute for comparable GAAP measures and should be read in conjunction with the consolidated financial statements prepared in accordance with GAAP . TMP’s management regularly uses these supplemental non - GAAP financial measures internally to understand, manage and evaluate its business and make operating decisions . TMP believes that the use of these non - GAAP measures enhances the ability of investors to compare its results from period to period . Investors should note that adjusted EBIDTA and adjusted earnings per share, as used by TMP, may be calculated differently from, and therefore may not be directly comparable to, similarly titled measures used by TMP’ competitors and other companies . The non - GAAP historical financial measures may exclude amortization of intangible assets, share - based compensation, transaction related costs, non - cash interest expense, loss on extinguishment of debt, and the effect of change in the timing of when certain revenue was recognized . The non - GAAP 2012 financial guidance may excludes amortization of intangible assets, share - based compensation, transaction and integration costs, purchase accounting inventory step - up adjustments and the tax effect of these adjustments to GAAP net income . Trademarks All products named in this presentation are protected under trademark including those not marked “TM” . Safe Harbor Statement, Non - GAAP Financial Measures and Trademarks TARGETED MEDICAL PHARMA (OTCQB:TRGM) 2

A Biotech Leader in the Rx - Only, Medical Foods Market TARGETED MEDICAL PHARMA (OTCQB:TRGM) 3 3 Core Products Treating Major Disease Markets Pharmaceutical Platforms Applicable to Multiple Diseases Growing Revenue Reimbursed Products, Contract Receivables Low - Cost Product Development No FDA Pre - Approval Required
Company Snapshot TARGETED MEDICAL PHARMA (OTCQB:TRGM) Biotechnology company founded in 1999 (OTCQB : TRGM) Low - cost development platform Ten FDA - regulated, Rx - only products on the market Medical technologies applicable to multiple diseases Safe option for treatment of common medical conditions Robust pipeline and patent portfolio Diversified sales/distribution channels Diversified sources of reimbursement 4
Medical foods are FDA - regulated medications intended for the dietary management of a disease that has distinctive nutritional needs that cannot be met by normal diet alone as defined in the Food and Drug Administration's 1988 Orphan Drug Act Amendments . About Rx - Only Medical Foods TARGETED MEDICAL PHARMA (OTCQB:TRGM) 5 Must make a disease claim Require physician prescription and supervision Do not require FDA pre - approval Enteral administration FDA GRAS/GRAE ingredients (Generally Recognized As Safe and Effective) Address specific nutritional deficiencies

Five - component System for Efficient Uptake and Utilization of Amino Acids Targeted Cellular Technology® TARGETED MEDICAL PHARMA (OTCQB:TRGM) 6 Reduced Attenuation Improved Efficacy Milligram Quantities Amino Acids Are the Precursors to Neurotransmitters >> 5 HTP Choline Arginine Tyrosine GABA Histidine Serotonin Acetylcholine Nitric Oxide Catecholamine GABA Histamine The Five Components of TCT : 1. Neurotransmitter precursor 2. Uptake Stimulator 3. Neuron activator 4. Adenosine brake inhibitor 5. Attenuation inhibitor neurotransmitters neurotransmitters held in storage synapse release binding change in potential Receptor site

Liquid Oral Administration System “Swish and Swallow” TARGETED MEDICAL PHARMA (OTCQB:TRGM) 7 Rapid absorption by mouth with a venous system that by passes portal drainage Absorbed within seconds due to high oral vascularization
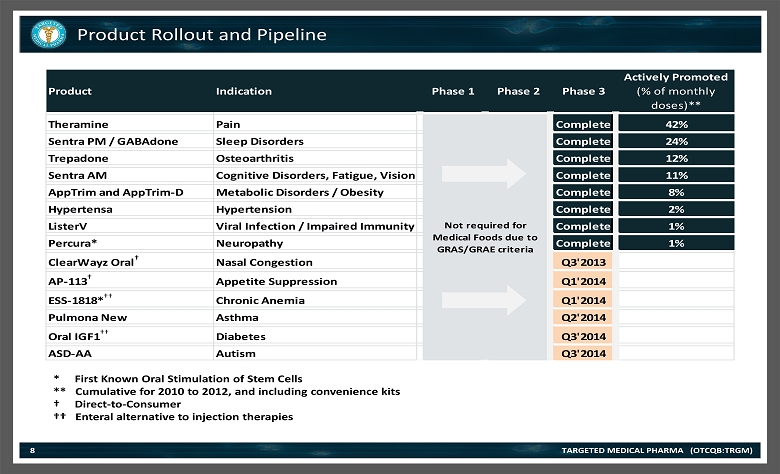
Product Indication Phase 1 Phase 2 Phase 3 Actively Promoted (% of monthly doses)** Theramine Pain Complete 42% Sentra PM / GABAdone Sleep Disorders Complete 24% Trepadone Osteoarthritis Complete 12% Sentra AM Cognitive Disorders, Fatigue, Vision Complete 11% AppTrim and AppTrim-D Metabolic Disorders / Obesity Complete 8% Hypertensa Hypertension Complete 2% ListerV Viral Infection / Impaired Immunity Complete 1% Percura* Neuropathy Complete 1% ClearWayz Oral † Nasal Congestion Q3'2013 AP-113 † Appetite Suppression Q1'2014 ESS-1818* †† Chronic Anemia Q1'2014 Pulmona New Asthma Q2'2014 Oral IGF1 †† Diabetes Q3'2014 ASD-AA Autism Q3'2014 Not required for Medical Foods due to GRAS/GRAE criteria * First Known Oral Stimulation of Stem Cells ** Cumulative for 2010 to 2012, and including convenience kits † Direct - to - Consumer †† Enteral alternative to injection therapies Product Rollout and Pipeline TARGETED MEDICAL PHARMA (OTCQB:TRGM) 8
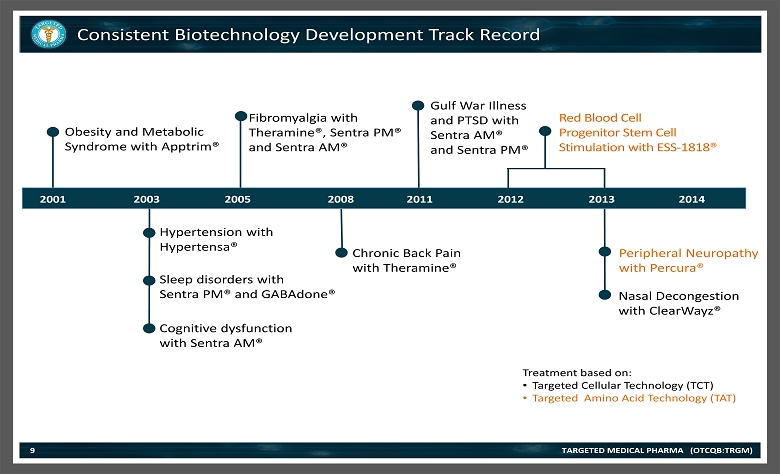
TARGETED MEDICAL PHARMA (OTCQB:TRGM) Consistent Biotechnology Development Track Record 9 Obesity and Metabolic Syndrome with Apptrim ® Hypertension with Hypertensa ® Sleep disorders with Sentra PM® and GABAdone ® Cognitive dysfunction with Sentra AM ® Fibromyalgia with Theramine®, Sentra PM® and Sentra AM ® Chronic Back Pain with Theramine ® Gulf War Illness and PTSD with Sentra AM® and Sentra PM ® Peripheral Neuropathy with Percura ® Red Blood Cell Progenitor Stem Cell Stimulation with ESS - 1818® 2001 2003 2005 2008 2013 2012 2011 Treatment based on: • Targeted Cellular Technology (TCT) • Targeted Amino Acid Technology (TAT) 2014 Nasal Decongestion with ClearWayz ®

SOLUTIONS • Nutritional management of disease • Safe, effective treatments • Reduce dosage of opiates • Improved adherence Medical Foods: Meeting Physician & Patient Demand TARGETED MEDICAL PHARMA (OTCQB:TRGM) 10 NEEDS • Improve patient o utcomes • Reduce negative s ide e ffects • Lower costs of care
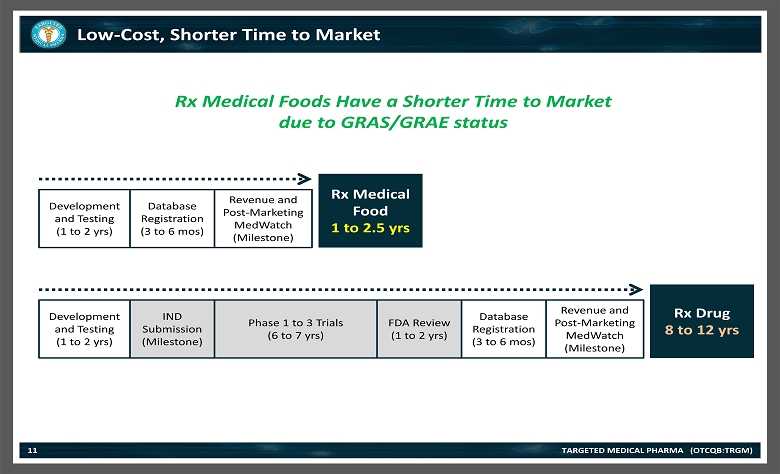
Low - Cost, Shorter Time to Market TARGETED MEDICAL PHARMA (OTCQB:TRGM) 11 Rx Medical Foods Have a Shorter Time to Market due to GRAS/GRAE status Development and Testing (1 to 2 yrs) Database Registration (3 to 6 mos ) Revenue and Post - Marketing MedWatch (Milestone) IND Submission (Milestone) Database Registration (3 to 6 mos ) Revenue and Post - Marketing MedWatch (Milestone) Phase 1 to 3 Trials (6 to 7 yrs) Development and Testing (1 to 2 yrs) FDA Review (1 to 2 yrs) Rx Medical Food 1 to 2.5 yrs Rx Drug 8 to 12 yrs
Two Platform Technologies TARGETED MEDICAL PHARMA (OTCQB:TRGM) 12 Targeted Amino Acid Technology (TAT) Targeted Cellular Technology (TCT)
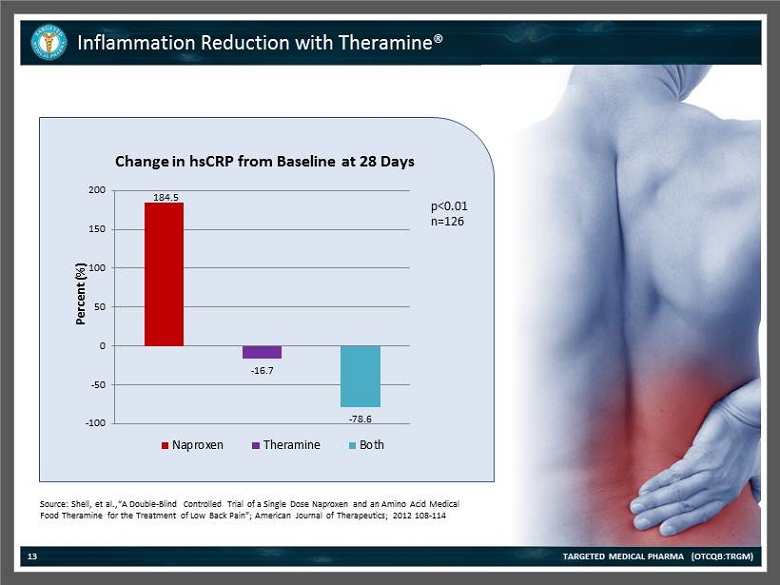
Inflammation Reduction with Theramine® TARGETED MEDICAL PHARMA (OTCQB:TRGM) 13 184.5 - 16.7 - 78.6 -100 -50 0 50 100 150 200 Percent (%) Change in hsCRP from Baseline at 28 Days Naproxen Theramine Both p<0.01 n=126 Source: Shell , et al.,“A Double - Blind Controlled Trial of a Single Dose Naproxen and an Amino Acid Medical Food Theramine for the Treatment of Low Back Pain”; American Journal of Therapeutics; 2012 108 - 114
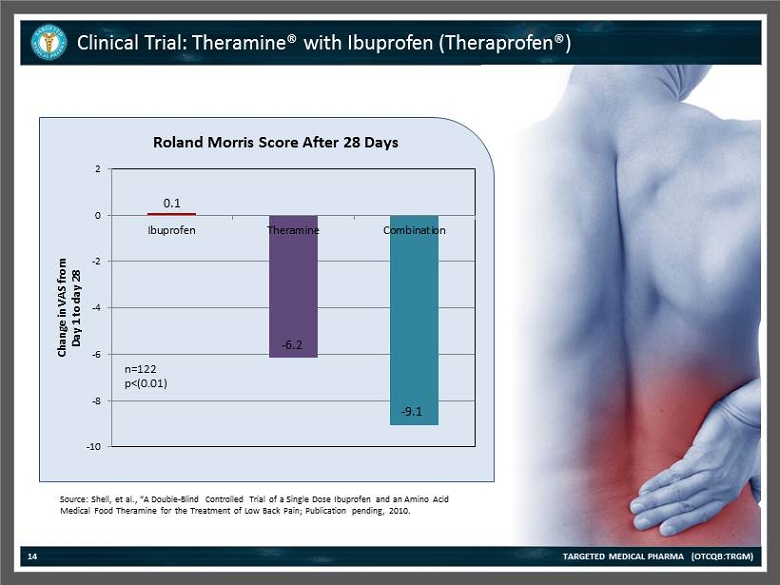
Clinical Trial: Theramine® with Ibuprofen (Theraprofen®) TARGETED MEDICAL PHARMA (OTCQB:TRGM) 14 0.09 - 6.15 - 9.08 -10 -8 -6 -4 -2 0 2 Ibuprofen Theramine Combination Change in VAS from Day 1 to day 28 Roland Morris Score After 28 Days n=122 p<( 0.01 ) - 6.2 - 9.1 0.1 Source: Shell , et al., “A Double - Blind Controlled Trial of a Single Dose Ibuprofen and an Amino Acid Medical Food Theramine for the Treatment of Low Back Pain; Publication pending, 2010.
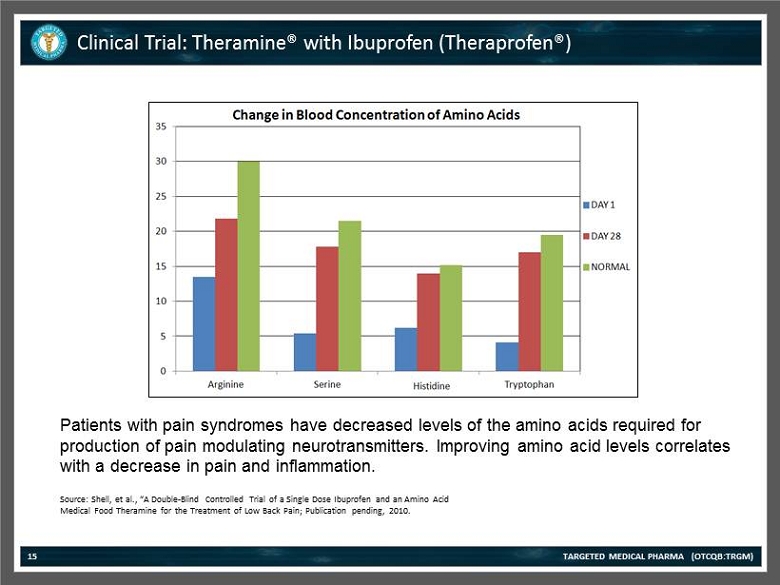
Clinical Trial: Theramine® with Ibuprofen (Theraprofen®) TARGETED MEDICAL PHARMA (OTCQB:TRGM) 15 Source: Shell , et al., “A Double - Blind Controlled Trial of a Single Dose Ibuprofen and an Amino Acid Medical Food Theramine for the Treatment of Low Back Pain; Publication pending, 2010. Patients with pain syndromes have decreased levels of the amino acids required for production of pain modulating neurotransmitters. Improving amino acid levels correlates with a decrease in pain and inflammation.

Pain Reduction with Theramine® TARGETED MEDICAL PHARMA (OTCQB:TRGM) 16 Source: Shell , et al.,“A Double - Blind Controlled Trial of a Single Dose Naproxen and an Amino Acid Medical Food Theramine for the Treatment of Low Back Pain”; American Journal of Therapeutics; 2012 108 - 114 2.95 - 44 - 65 -70 -60 -50 -40 -30 -20 -10 0 10 Percent (%) Percent Reduction of Roland - Morris Pain Index Naproxen Theramine Both * p<0.05 n=126
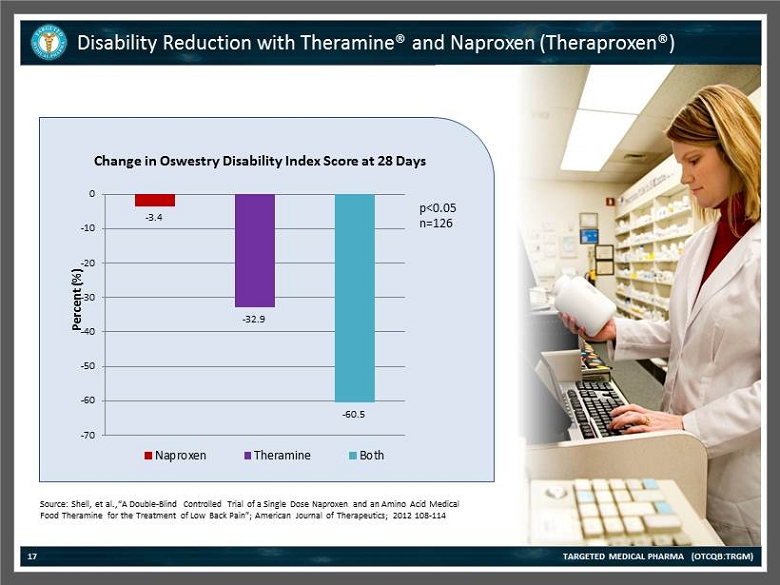
Disability Reduction with Theramine ® and Naproxen ( Theraproxen ®) TARGETED MEDICAL PHARMA (OTCQB:TRGM) 17 Source: Shell , et al.,“A Double - Blind Controlled Trial of a Single Dose Naproxen and an Amino Acid Medical Food Theramine for the Treatment of Low Back Pain”; American Journal of Therapeutics; 2012 108 - 114 - 3.4 - 32.9 - 60.5 -70 -60 -50 -40 -30 -20 -10 0 Percent (%) Change in Oswestry Disability Index Score at 28 Days Naproxen Theramine Both p<0.05 n=126
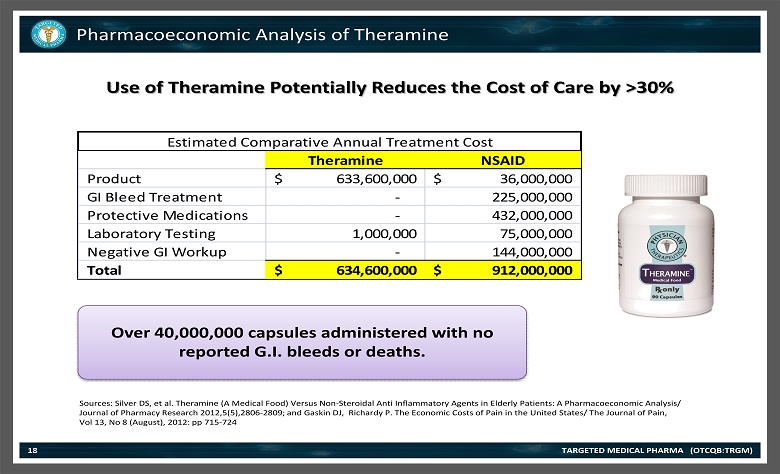
Pharmacoeconomic Analysis of Theramine TARGETED MEDICAL PHARMA (OTCQB:TRGM) Theramine NSAID Product 633,600,000$ 36,000,000$ GI Bleed Treatment - 225,000,000 Protective Medications - 432,000,000 Laboratory Testing 1,000,000 75,000,000 Negative GI Workup - 144,000,000 Total 634,600,000$ 912,000,000$ Estimated Comparative Annual Treatment Cost Over 40,000,000 capsules administered with no reported G.I. bleeds or deaths. Use of Theramine Potentially Reduces the Cost of Care by >30% 18 Sources: Silver DS, et al. Theramine (A Medical Food) Versus Non - Steroidal Anti Inflammatory Agents in Elderly Patients: A Pharmacoeconom ic Analysis/ Journal of Pharmacy Research 2012,5(5),2806 - 2809; and Gaskin DJ, Richardy P. The Economic Costs of Pain in the United States/ The Journal of Pain, Vol 13, No 8 (August), 2012: pp 715 - 724

Clinical Trial: Sentra PM® and Trazodone (Trazamine®) TARGETED MEDICAL PHARMA (OTCQB:TRGM) 19 1 Pittsburgh Sleep Quality Index Source: Shell , et al., “Sentra PM and Trazodone for the management of Sleep Disorders” Journal of Central Nervous System Disease; 2012:4 1.0 2.0 3.9** 6.5** 0 1 2 3 4 5 6 7 10 - Point Scale Sleep Quality (PSQI 1 ) Change from Baseline at Day 14 Placebo Trazodone Sentra PM Both ** p<0.01 n=111
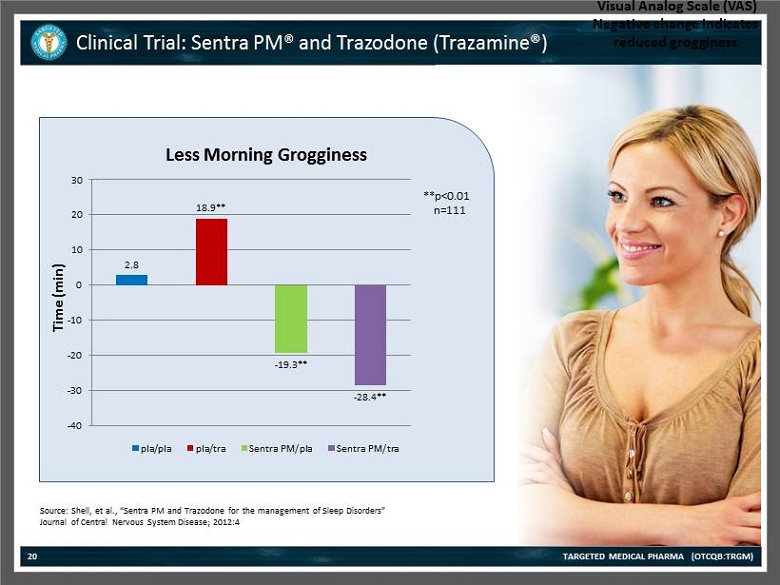
Clinical Trial: Sentra PM® and Trazodone (Trazamine®) TARGETED MEDICAL PHARMA (OTCQB:TRGM) 20 Source: Shell , et al., “Sentra PM and Trazodone for the management of Sleep Disorders” Journal of Central Nervous System Disease; 2012:4 Change in Visual Analog Scale (VAS) Negative change indicates reduced grogginess 2.8 18.9** - 19.3** - 28.4** -40 -30 -20 -10 0 10 20 30 Time (min) Less Morning Grogginess pla/pla pla/tra Sentra PM/pla Sentra PM/tra **p<0.01 n=111
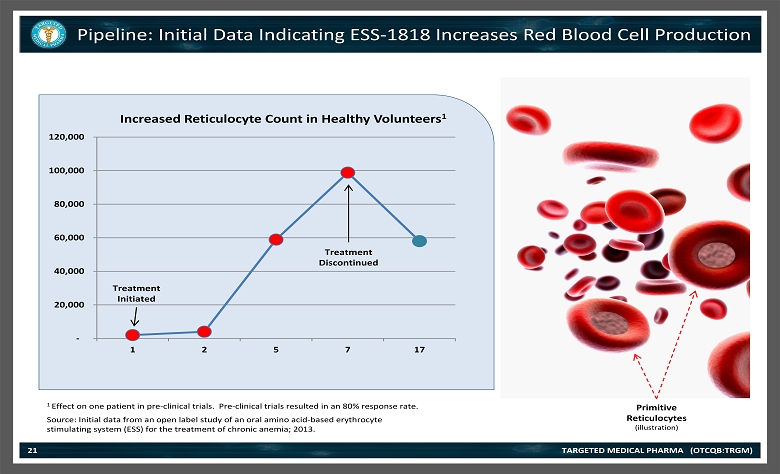
1 Effect on one patient in pre - clinical trials. Pre - clinical trials resulted in an 80% response rate. Pipeline: Initial Data Indicating ESS - 1818 Increases Red Blood Cell Production TARGETED MEDICAL PHARMA (OTCQB:TRGM) 21 Source: Initial data from an open label study of an oral amino acid - based erythrocyte stimulating system (ESS) for the treatment of chronic anemia; 2013. - 20,000 40,000 60,000 80,000 100,000 120,000 1 2 5 7 17 Increased Reticulocyte Count in Healthy Volunteers 1 Treatment Initiated Treatment Discontinued Primitive Reticulocytes (illustration)

Autism Product TARGETED MEDICAL PHARMA (OTCQB:TRGM) 22 0 1 2 3 4 5 1 2 3 4 5 6 7 8 Day Initiation of Treatment Number of Events Involving Anger, Frustration, and Self - Directed Aggressive Behavior Nutritional Deficiency Initial Data Development Status Distribution Channels Anticipated Launch Date All Four Test Subjects have Similarly Responded
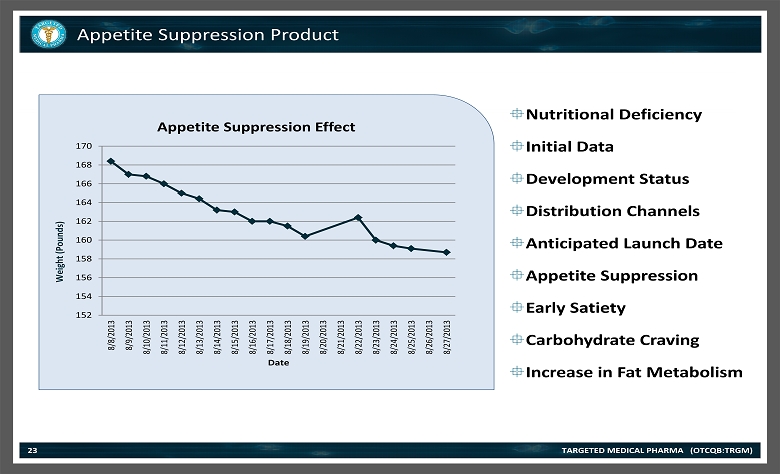
Appetite Suppression Product TARGETED MEDICAL PHARMA (OTCQB:TRGM) 23 Nutritional Deficiency Initial Data Development Status Distribution Channels Anticipated Launch Date Appetite Suppression Early Satiety Carbohydrate Craving Increase in Fat Metabolism 152 154 156 158 160 162 164 166 168 170 8/8/2013 8/9/2013 8/10/2013 8/11/2013 8/12/2013 8/13/2013 8/14/2013 8/15/2013 8/16/2013 8/17/2013 8/18/2013 8/19/2013 8/20/2013 8/21/2013 8/22/2013 8/23/2013 8/24/2013 8/25/2013 8/26/2013 8/27/2013 Weight (Pounds) Date Appetite Suppression Effect

Intellectual Property Highlights TARGETED MEDICAL PHARMA (OTCQB:TRGM) 1 Targeted Cellular Technology® is a five - component system the stimulates the uptake and utilization of amino acids. It enables efficacy from microgram quantities of amino acids. *First Known Oral Stimulation of Stem Cells 24 Seven Patents Issued Three Patents Pending □ 4 USPTO neurotransmitter - specific patents covering Targeted Cellular Technology® (TCT) □ 1 Japanese patent covering TMP’s Targeted Cellular Technology® □ 1 USPTO patent covering the convenience pack presentation □ 1 USPTO Billing methodology for physician dispensing of medication TAT - related patents: □ Stimulation of RBC production* □ Oral Insulin / Insulin Resistance* □ Neuropathy treatment

Innovators in Pharmaceutical Technology Financials and Team

Financial Snapshot TARGETED MEDICAL PHARMA (OTCQB:TRGM) 26 Summary 6 mos ended June 30, 2013 6 mos ended June 30, 2012 QoQ Change $ QoQ Change % Revenue $4.7 million $2.8 million $1.9 million 67% Operating Expenses $5.4 million $4.8 million $0.6 million 12% Shares Outstanding 23.4 million 21.9 million 1.5 million 7% Shares: Fully Diluted 27.3 million 26.2 million 0.7 million 3% Other Information Comments Cash and Cash Equivalents $0.03 million Contract Receivables $16.3 million supported by existing claims Notes Payable $4.0 million, 94% of which are beneficially owned by the Company’s CEO
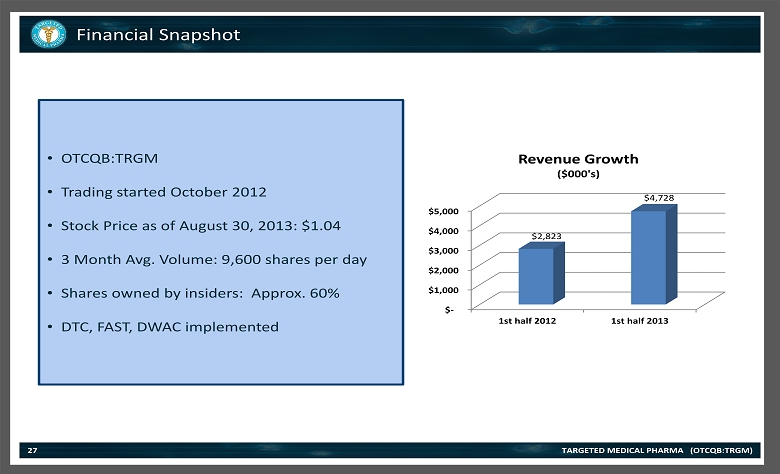
Financial Snapshot TARGETED MEDICAL PHARMA (OTCQB:TRGM) 27 • OTCQB:TRGM • Trading started October 2012 • Stock Price as of August 30, 2013: $1.04 • 3 Month Avg. Volume: 9,600 shares per day • Shares owned by insiders: Approx. 60% • DTC , FAST, DWAC implemented $- $1,000 $2,000 $3,000 $4,000 $5,000 1st half 2012 1st half 2013 $2,823 $4,728 Revenue Growth ($000's)

Shipments : Contract Receivables Collections : Recognized Revenue Contract Receivables are amounts due but not yet recognized as revenue Monthly collections are approximately 1.5% to 2.0% Collected amounts impact bottom - line operating revenue almost directly Contract receivables are constantly rejuvenated by additional shipments $16.3 million as of Dec. 31, 2012 Supported by existing claims. Off - Balance Sheet Asset: Contract Receivables TARGETED MEDICAL PHARMA (OTCQB:TRGM) 28

Improving the Reimbursement Mix TARGETED MEDICAL PHARMA (OTCQB:TRGM) 29 75% 20% 5% 60% 35% 5% 55% 30% 5% 10% 35% 35% 15% 15% 2011 2012 2013 2014 Diversification of Payers Revenue Stability Workers Compensation Private Insurance Medicare Part D DOD / VA
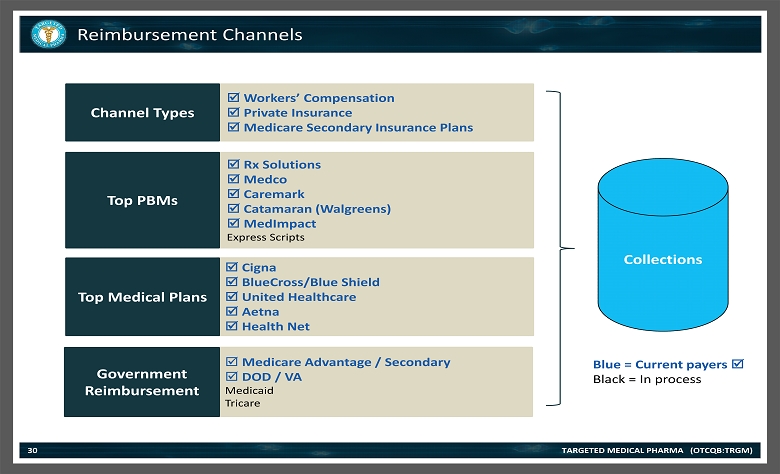
Reimbursement Channels TARGETED MEDICAL PHARMA (OTCQB:TRGM) 30 Medicare Advantage / Secondary DOD / VA Medicaid Tricare Cigna BlueCross/Blue Shield United Healthcare Aetna Health Net Rx Solutions Medco Caremark Catamaran (Walgreens) MedImpact Express Scripts Workers ’ Compensation Private Insurance Medicare Secondary Insurance Plans Top PBMs Top Medical Plans Channel Types Government Reimbursement Blue = Current payers Black = In process
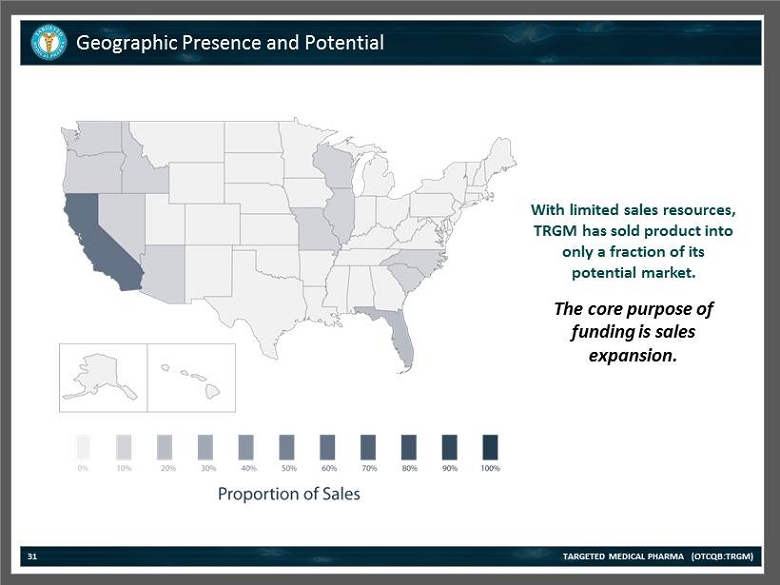
Geographic Presence and Potential TARGETED MEDICAL PHARMA (OTCQB:TRGM) 31 With limited sales resources, TRGM has sold product into only a fraction of its potential market. The core purpose of funding is sales expansion.

Near - Term Catalysts & Goals TARGETED MEDICAL PHARMA (OTCQB:TRGM) 32 Grow sales to physicians with well - insured (PPO) patients • A National Sales Manager was hired in March • A contract was signed to accelerate cash collections • We are contemplating the acquisition of a distributor network Begin sales of an OTC product in Q4’2013 Begin sales of first stem - cell product in Q1’2014 • Stimulation of red b lood c ell production Grow national sales force • HI and WA are performing, next are NV and AZ Bring doctors current on amounts payable to them • Improving practice management and profitability

Key Management TARGETED MEDICAL PHARMA (OTCQB:TRGM) 3 3 David Silver, M.D. President and Chief Operating Officer • University of California Los Angeles - Associate Professor • Cedars - Sinai Medical Center – Clinical Chief of Rheumatology and Dir. of Chronic Pain Rehabilitation Program • Osteoporosis Medical Center - Associate Director • American College of Rheumatology – Scientific Advisory Committee Member • Conducted more then 100 clinical trials • Nationally - recognized expert on pain, fibromyalgia and PTSD • Boston University – BA in Medical Sciences with minor in Economics • Boston University School of Medicine – M.D. • Northwestern University – Residency in Internal Medicine • Cedars Sinai Medical Center – Fellowship in Rheumatology • Board Certified - Internal Medicine and Rheumatology Amir Blachman, MBA VP Strategy and Operations, Chief Compliance Officer, Secretary • PeopleSupport.com (Nasdaq:PSPT) - Director of Operations • Franklin Templeton Mutual Funds (NYSE:BEN ) - Broker Services Supervisor • Israel Air Force – Instructor • University of California Los Angeles – MA Business Administration • University of California Santa Barbara – BA Psychology • University of California Los Angeles – Director Certification Program William Shell, M.D. Co - Founder, CEO and Chief Science Officer • USAF American Soviet Exchange Program – First physician representative in Moscow • Keesler Air Force Base – Director of Coronary Case Unit • Cedars Sinai Medical Center – Dir. of Cardiovascular Biochemistry Research Laboratories • Interactive Medical Technologies - Chairman and Chief Science Officer • Beverly Glen Medical Systems – Chief Science Officer • Board Certified - Internal Medicine and Cardiology • University of California Los Angeles - Associate Professor • Conducted more than 100 clinical trials • Dr. Shell’s innovations have led to 16 issues patents and 6 pending • Published 99 peer - reviewed scientific papers and wrote chapters in 17 books • University of Michigan Medical School – M.D. • National Institutes of Health – Fellow ship under Dr. Eugene Braunwald and member CK - MB team William Horne, CPA Chief Financial Officer • OptimisCorp – Chief Financial Officer • Patient Safety Technologies (OTCBB:PSTX) – CFO and Interim CEO • Alaska Wireless Communications - Managing Member and CFO • Apex Financial Management – CFO and Founding Member • Phoenix Partners VC – Chief Financial Officer • Price Waterhouse, LLP – Accountant • U.S. Marine Corps • Seattle University – BA Business Administration, Magnus Cum Laude
Board of Directors TARGETED MEDICAL PHARMA (OTCQB:TRGM) 34 INDEPENDENT DIRECTORS Maurice J. DeWald Chairman of the Board and of the Compensation Committee KPMG - Regional Managing Partner Serves on the board of director of several healthcare companies Arthur R. Nemiroff Chairman of the Audit Committee BDO USA LLP - Partner City of Hope Medical Center - Director and Audit Committee Member Donald J. Webster Chairman of the Nominating and Corporate Governance Committee Chevron Corporation - General Manager of Procurement NON - INDEPENDENT DIRECTORS William Shell, M.D. CEO and Chief Science Officer David Silver, M.D. President and Chief Operating Officer Kim Giffoni Co - founder and Executive Vice President NutraCorp Scientific – President Giffoni Development Company - CEO

Business Team TARGETED MEDICAL PHARMA (OTCQB:TRGM) 35 Legal Counsel Equities Accounting Investor Relations and Public Relations FDA HEALTHCARE HEALTHCARE SECURITIES AUDITORS TAX ACCOUNTANTS INVESTOR RELATIONS PUBLIC RELATIONS PondelWilkinson MARKET MAKER TRANSFER AGENT
Investment Considerations PRODUCTS HAVE LOW SIDE - EFFECT PROFILE TARGETED MEDICAL PHARMA (OTCQB:TRGM) 36 HIGH DEMAND PRODUCTS ADDRESSING LARGE MARKETS DIVERSIFIED, GROWING SALES GROWING ADOPTION OF MEDICAL FOODS BROAD - REACHING PATENTS AND ROBUST PIPELINE PROVEN SAFETY AND EFFICACY THROUGH CLINICAL TRIALS LOW - COST, HIGH - SPEED PRODUCT DEVELOPMENT PLATFORM TRACK RECORD OF EXCELLENT COST CONTROLS DIVERSIFIED REIMBURSEMENT SOURCES SPECIALTY REIMBURSEMENT OPERATIONS Revenue Growth Revenue Security Profitability Robust Patent Portfolio and Pipeline “Better health through medical foods”

Contact 2980 Beverly Glen Circle, Suite 301 Los Angeles, CA 90077 Telephone: 310 - 474 - 9809 Fax: 310 - 531 - 7089 investors@tmedpharma.com Brand site: www.ptlcentral.com Company site: www.tmedpharma.com




































