UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, DC 20549
FORM 8-K/A
Amendment No. 4
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the
Securities Exchange Act of 1934
Date of report (Date of earliest event reported): May 3, 2011
Emmaus Life Sciences, Inc.
(Exact Name of Registrant as Specified in Its Charter)
| Delaware | 000-53072 | 41-2254389 | ||
(State or Other Jurisdiction of Incorporation) | (Commission File Number) | (IRS Employer Identification No.) |
| 20725 S. Western Avenue, Suite 136, Torrance, CA 90501 |
| (Address, including zip code, of principal executive offices) |
Registrant’s telephone number, including area code 310-214-0065
| EMMAUS HOLDINGS, INC. |
| (Former Name or Former Address, if Changed Since Last Report) |
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
| o | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) | |
| o | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) | |
| o | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) | |
| o | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
EXPLANATORY NOTE
This Amendment No. 4 (this “Amendment No. 4”) on Form 8-K/A amends in its entirety the Current Report on Form 8-K filed by Emmaus Life Sciences, Inc. (“we” or the “Company”) with the Securities and Exchange Commission (the “SEC”) on May 4, 2011 (the “Original Filing”), the Current Report on Form 8-K/A filed by us on July 5, 2011 (“Amendment No. 1”), the Current Report on Form 8-K/A filed by us on August 18, 2011 (“Amendment No. 2) and the Current Report on Form 8-K/A filed by us on October 6, 2011 (“Amendment No. 3”). On October 26, 2011, the Company received a comment letter from the staff of the Securities and Exchange Commission (the “SEC”) on Amendment No. 3. This Amendment No. 4 reflects changes made in response to comments we received from the staff in its comment letter.
Unless indicated otherwise, the disclosures in this Amendment No. 4 continue to describe conditions as of the date of the Original Filing, and the disclosures contained herein have not been updated to reflect events, results or developments that have occurred after the Original Filing, or to modify or update those disclosures affected by subsequent events. Among other things, the Company’s name change from “Emmaus Holdings, Inc.” to “Emmaus Life Sciences, Inc.”, which was effective September 14, 2011, is not reflected in this Amendment No. 4, and forward-looking statements made in the Original Filing have not been revised to reflect events, results or developments that have occurred or facts that have become known to us after the date of the Original Filing, and such forward-looking statements should be read in their historical context. This Amendment No. 4 should be read in conjunction with the Company’s filings made with the SEC subsequent to the Original Filing, including any amendments to those filings.
1
Item 1.01 Entry into a Material Definitive Agreement.
Pursuant to an Agreement and Plan of Merger, dated April 21, 2011 (the “Merger Agreement”), by and among AFH Acquisition IV, Inc. (“AFH IV”), AFH Merger Sub, Inc. (“AFH Merger Sub”), AFH Holding and Advisory, LLC (“AFH Advisory”), and Emmaus Medical, Inc. (“Emmaus Medical”), Emmaus Medical merged with and into AFH Merger Sub with Emmaus Medical continuing as the surviving entity (the “Merger”). Upon the closing of the Merger, AFH IV changed its name from “AFH Acquisition IV, Inc.” to “Emmaus Holdings, Inc.”
Reference is made to Item 2.01 for a description of the Merger Agreement, the Merger and the related transactions. The description of the Merger Agreement is qualified in its entirety by reference to the complete text of the Merger Agreement, which is attached hereto as Exhibit 2.1 and incorporated by reference herein. You are urged to read the entire Merger Agreement and the other exhibits attached hereto.
In connection with the Merger Agreement, we entered into a share cancellation agreement (the “Cancellation Agreement”) pursuant to which the Company’s majority stockholder, AFH Holding canceled 1,827,750 shares of our common stock on the closing date of the Merger.
In connection with the consummation of the Merger, we entered into a Registration Rights Agreement, dated May 3, 2011 (the “Registration Rights Agreement”), for the benefit of certain pre-Merger stockholders of AFH IV (the “Existing AFH IV Stockholders”). Pursuant to the Registration Rights Agreement, the Existing AFH Stockholders will have certain “piggyback” registration rights on registration statements filed after the Merger is consummated other than registration statements (i) filed in connection with any employee stock option or other benefit plan, (ii) for an exchange offer or offering of securities solely to our existing stockholders, (iii) for an offering of debt that is convertible into our equity securities, (iv) for a dividend reinvestment plan or (v) for an offering of our equity securities underwritten by Sunrise Securities Corp. We will bear the expenses incurred in connection with the filing of any such registration statements.
The preceding summaries of the Cancellation Agreement and the Registration Rights Agreement are qualified in their entirety by reference to the complete text of the Cancellation Agreement and the Registration Rights Agreement, which are attached hereto as Exhibit 10.1 and 10.2, respectively, and incorporated by reference herein. You are urged to read the entire Cancellation Agreement and Registration Rights Agreement attached hereto.
Item 2.01 Completion of Acquisition or Disposition of Assets.
OVERVIEW
As used in this report, unless otherwise indicated, the terms “we,” “Company” and “Emmaus” refer to Emmaus Holdings, Inc., a Delaware corporation, formerly known as AFH Acquisition IV, Inc., and its wholly-owned subsidiary Emmaus Medical, and its wholly-owned subsidiaries, Newfield Nutrition Corporation, a Delaware corporation, and Emmaus Medical Japan, Inc., a Japanese corporation.
HISTORY
AFH IV was incorporated in the State of Delaware on September 24, 2007 and was originally organized as a “blank check” shell company to investigate and acquire a target company or business seeking the perceived advantages of being a publicly held corporation.
On May 3, 2011, AFH IV (i) closed a reverse merger transaction, described below, pursuant to which AFH IV became the 100% parent of Emmaus Medical, (ii) assumed the operations of Emmaus Medical and its subsidiaries and (iii) changed its name from “AFH Acquisition IV, Inc.” to “Emmaus Holdings, Inc.”
Emmaus Medical, LLC was organized on December 20, 2000. In October 2003, Emmaus Medical, LLC conducted a reorganization and merged with Emmaus Medical, Inc., a Delaware corporation originally incorporated on September 12, 2003. Through this merger with Emmaus Medical, LLC into Emmaus Medical, Emmaus Medical acquired the exclusive patent rights for a treatment for sickle cell disease (“SCD”).
2
We are engaged in the discovery, development and commercialization of treatments and therapies for rare diseases, an area that management believes has traditionally been underserved by large pharmaceutical companies. We believe that there are attractive niche markets and financial opportunities for companies such as ours that specialize in treatments for rare diseases.
CORPORATE STRUCTURE
The corporate structure of the Company is illustrated as follows:
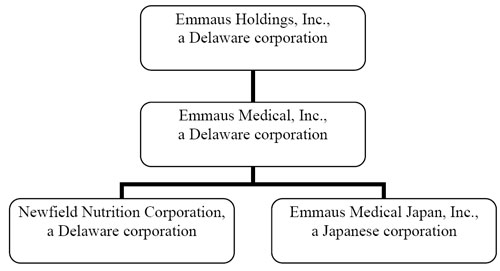
Our principal executive offices and corporate offices are located at 20725 S. Western Avenue, Ste. 136, Torrance, CA 90501-1884. Our telephone number is 310-214-0065.
PRINCIPAL TERMS OF THE MERGER
Upon consummation of the Merger, (i) each outstanding share of Emmaus Medical common stock was exchanged for 29.48548924976 shares of AFH IV common stock, (ii) each outstanding Emmaus Medical option and warrant, which was exercisable for one share of Emmaus Medical common stock, was exchanged for an option or warrant, as applicable, exercisable for 29.48548924976 shares of AFH IV common stock; and (iii) each outstanding convertible note of Emmaus Medical, which was convertible for one share of Emmaus Medical common stock, was exchanged for a convertible note exercisable for 29.48548924976 shares of AFH IV common stock. As a result of the Merger, holders of Emmaus Medical common stock, options, warrants and convertible notes received 20,628,305 shares of our common stock (excluding 47,178 shares held by stockholders who exercised dissenters’ rights in connection with the Merger), options and warrants to purchase an aggregate of 326,507 shares of our common stock, and convertible notes to purchase an aggregate of 270,648 shares of our common stock. Securityholders of Emmaus Medical held 85% of our issued and outstanding common stock on a fully diluted basis upon the closing of the Merger. Immediately after the closing of the Merger, we had 24,378,305 shares of common stock (excluding 47,178 shares held by stockholders who exercised dissenters’ rights), no shares of preferred stock, options to purchase 23,590 shares of common stock, warrants to purchase 302,917 shares of common stock and convertible notes exercisable for 270,648 shares of common stock issued and outstanding.
On May 3, 2011 after the closing of the Merger, AFH IV changed its corporate name from “AFH Acquisition IV, Inc.” to “Emmaus Holdings, Inc.” Our shares of common stock are not currently listed or quoted for trading on any national securities exchange or national quotation system. We intend to apply for the listing of our common stock on the NYSE Amex or the NASDAQ Global Market. We intend to file a listing application with NASDAQ or NYSE Amex at such time as we file a registration statement for a firm commitment public offering of our common stock.
The transactions contemplated by the Merger Agreement were intended to be a “tax-free” reorganization pursuant to the provisions of Sections 351 and/or 368(a) of the Internal Revenue Code of 1986, as amended.
3
The Merger resulted in a change in control of our company from AFH Advisory, which is owned by Mr. Amir F. Heshmatpour, to the former securityholders of Emmaus Medical. In connection with the change in control, the persons set forth below were appointed to our Board of Directors and elected as officers in the positions set forth opposite their names. Mr. Heshmatpour, an officer and director of AFH IV prior to the consummation of the Merger Agreement, resigned from all of his officer positions with AFH IV at the time the transaction was consummated, but continues as a member of our Board of Directors. The appointments of the new officers and directors were effective on the closing of the Merger.
| Name | Position | |
| Yutaka Niihara, M.D., MPH | President and Chief Executive Officer and Director | |
| Willis C. Lee | Chief Operating Officer and Director | |
| Lan T. Tran | Chief Administrative Officer and Corporate Secretary | |
| Yasushi Nagasaki | Chief Financial Officer | |
| Steve Warnecke | Director | |
| Henry A. McKinnell, Jr., Ph.D., | Chairman of the Board | |
| Amir Heshmatpour | Director | |
| Douglas W. Wilmore, M.D. | Director |
Prior to the closing of the Merger, AFH Advisory canceled an aggregate of 1,827,750 shares of AFH IV common stock pursuant to a Share Cancellation Agreement executed in connection with the Merger Agreement. AFH Advisory did not receive any consideration for the cancellation of the shares. The cancellation of the shares was accounted for as a contribution to capital. The number of shares cancelled was determined based on negotiations with AFH Advisory, the majority stockholder of AFH IV, and Emmaus Medical. Emmaus Medical and AFH Advisory negotiated an estimated value of Emmaus Medical and its subsidiaries, an estimated value of the shell company, and the mutually desired capitalization of the company resulting from the Merger. With respect to the determination of the amount of shares cancelled, the value of the shell company was derived primarily from its utility as a public company platform, including its good corporate standing and its timely public reporting status. We did not consider registering our own securities directly as a viable option for accessing the public markets. The services provided by AFH Advisory were not a consideration in determining this aspect of the transaction. Under these circumstances and based on these factors, Emmaus Medical and AFH Advisory agreed upon the number of shares to be cancelled.
We have agreed to pay AFH Advisory $500,000 (the “Shell Cost”) for the identification of AFH Acquisition IV and providing consulting services related to coordinating the Merger, assisting with an offering and managing the interrelationship of legal and accounting activities (the “Services”), and to reimburse AFH Advisory for advancement of expenses on behalf of the Company incurred in connection with the Services, the Merger and an offering, including, without limitation, reasonable expenses of AFH Advisory (the “Transaction Costs”). As of March 31, 2011, AFH Advisory had advanced an aggregate of $192,385 in expenses on our behalf. During the three months ended March 31, 2011, we did not reimburse AFH Advisory for any of the advanced expenses and, therefore, as of March 31, 2011, owed AFH Advisory an aggregate of $692,385, consisting of the $500,000 Shell Cost and $192,385 in advanced expenses.
AFH Advisory may be paid any outstanding Shell Cost and Transaction Costs from the proceeds of a public offering or upon the consummation of any other financing. Alternatively, AFH Advisory may, in its discretion, convert such amount (or any portion thereof) into common stock at a conversion price equal to 75% of the per share price in the public offering (the “Conversion Price”). Additionally, we have agreed to issue warrants to purchase shares of common stock to AFH Advisory upon the closing of a public offering. Such warrants will have a term of 5 years from the date of issuance and will have an exercise price equal to the Conversion Price. The number of shares underlying the warrants will be calculated by dividing the aggregate of the Shell Cost and the total Transaction Costs by the Conversion Price.
4
Shares held by AFH Advisory and certain others will be decreased, at the rate of 1% of the post-offering outstanding common shares, for each $1 million or fraction thereof that the gross proceeds to the Company from the offering are less than $10 million. In the event of such reduction, AFH Advisory will reduce the number of those shares by an appropriate percentage. If an offering cannot be consummated to provide for minimum gross proceeds to the Company of at least $5 million, the Company’s arrangement with AFH Advisory provides that the Company may terminate the offering at its sole and absolute discretion, and if the Company terminates the offering, all shares held by AFH Advisory and certain others will be canceled.
If our arrangement with AFH Advisory is terminated based on mutual agreement of the parties or based on a breach by the Company, AFH Advisory will receive its Transaction Costs incurred as of the date of termination. If the arrangement with AFH Advisory is terminated (i) by either party (without being cured within 30 days) based on identification of information in the course of its due diligence investigation that it deems unsatisfactory, or if the parties are unable to agree to a valuation within 45 days following completion of satisfactory due diligence by an investment bank, or (ii) by the Company if a public offering cannot be consummated to provide for gross proceeds to the Company of at least $10 million or based on a breach by AFH Advisory that is not cured within 30 days, then AFH Advisory will receive reimbursement for 50% of Transaction Costs actually incurred to the date of such termination. If a public offering cannot be consummated with gross proceeds of at least $5 million, and the Company exercises its right to terminate the offering, then the Company will reimburse AFH Advisory an amount equal to 50% of the Shell Cost and 50% of the Transaction Costs incurred as of the date of termination, and AFH Advisory, in its discretion, has the option to be paid any outstanding amounts at the time of termination in cash or to convert such amounts (or any portion thereof) into common stock at a conversion price equal to 75% of the per share price of the shares of common stock sold in the Company’s most recently completed private offering of common stock. In the event of termination by the Company because a public offering cannot be consummated to provide for gross proceeds to the Company of at least $10 million, and if the Company enters into any transaction or a sale of all or substantially all of its assets within 12 months of such termination, AFH Advisory will be entitled to (i) 5% of its holdings (including holdings of certain others) of any securities received by the Company or the Company’s shareholders upon consummation of any business combination or other similar transaction; or (ii) cash or any other consideration it would have received as if it had a 5% ownership interest in the Company immediately prior to the closing of any such transaction.
The Company granted AFH Advisory exclusive rights to act as its advisor in connection with all financings and mergers and acquisitions until November 10, 2012 and the right to appoint two board members to the Company’s board of directors upon the closing of the Merger. In addition, in the event of any merger, stock purchase, asset purchase or similar transaction occurring within one year of the closing of the pbulic offering, AFH Advisory may receive a warrant to purchase shares in an amount to increase AFH Advisory’s total holdings to 10% of the outstanding fully diluted equity of the Company but only to the extent the Company issues securities in connection with such merger, stock purchase, asset purchase or similar transaction.
EMMAUS HOLDINGS, INC.’S BUSINESS
Overview
We are engaged in the discovery, development, and commercialization of innovative and cost-effective treatments and therapies, areas that we believe have traditionally been underserved by large pharmaceutical companies. We believe that there are attractive niche markets and financial opportunities for companies such as ours that specialize in treatments for rare diseases. Over time, we plan to expand our business to include developing and marketing products to treat more common diseases. The primary focus of our business is the late-stage development of the amino acid L-glutamine as a prescription drug for the treatment of sickle cell disease (“SCD”). To a lesser extent, we are also engaged in the marketing and sale of NutreStore® [L-glutamine powder for oral solution] and promotion of Zorbtive® [somatropin (rDNA origin) for injection], as a treatment for short bowel syndrome (“SBS”) and the sale of L-glutamine as a nutritional supplement under the brand name AminoPure®. Since inception, we have generated minimal revenues from the sale and/or promotion of NutreStore®, Zorbtive® and AminoPure®.
Articles referenced in this Current Report can be accessed through PubMed (http://www.ncbi.nlm.nih.gov/pubmed/) or by contacting the publisher directly for a copy of the article.
5
Industry and Market Opportunity
We focus on developing treatments and therapies for rare diseases. Rare diseases, pursuant to the Rare Diseases Act of 2002, defines a “rare disease” as any disease or condition that affects less than 200,000 persons in the United States. In Japan, a rare disease is one that affects fewer than 50,000 persons in Japan. The European Commission on Public Health defines a rare disease as a life-threatening or chronically debilitating disease which is of such low prevalence, namely affecting fewer than 1 in 2,000 people, that special combined efforts are needed to address it.
Sickle Cell Disease
We are currently engaged in a Phase III clinical trial of L-glutamine as a treatment for SCD, for which we hope to obtain approval from the U.S. Food and Drug Administration (“FDA”) in 2013. SCD affects about 70,000 to 100,000 persons in the U.S., according to the section of the Centers for Disease Control’s website regarding SCD, and over 4 million people worldwide as of September 2010.
SCD is an inherited blood disorder that affects red blood cells. Red blood cells contain hemoglobin which allows red blood cells to carry oxygen from the air in the lungs to all parts of the body. Normal red blood cells contain hemoglobin A. In contrast, the bone marrow of people with SCD produces red blood cells with a different form of hemoglobin called hemoglobin S (S stands for sickle). When a person has SCD, rather than remaining round, smooth and flexible, the red blood cells become sickle (crescent) shaped, inflexible, and sticky as they release oxygen to other tissues in the body. These abnormally shaped cells become rigid and lodge in the capillaries when oxygen is released from the cells’ hemoglobin, causing blockages and preventing the normal flow of oxygen to the surrounding tissue. SCD diseased red blood cells also tend to clump together, further impeding circulation.
According to the section of the National Heart, Lung and Blood Institute’s website regarding SCD, normal red blood cells live for about 120 days before they are replaced with new ones. In sharp contrast, sickle-shaped red blood cells are destroyed faster, in about 16 days, and cannot always be replaced quickly. As a result, people with SCD are often anemic. Sickle cell disease includes sickle cell anemia (which results from two hemoglobin S genes), sickle ß-thalassemia (one hemoglobin S and one ß-thalassemia gene), hemoglobin SC disease (one hemoglobin S and one hemoglobin C), and the somewhat rare disease hemoglobin C Harlem. According to the SCD section of the National Heart, Lung and Blood Institute’s website, these hereditary diseases often affect individuals of African American heritage, and increasingly, Hispanic populations. According to the book Hematology in Clinical Practice by Robert S Hillman et al., people of Mediterranean, Middle Eastern, and South East Asian descent are also afflicted with the disease, but to a lesser degree.
The complications of sickle cell disease occur when sickle-shaped red blood cells block veins which then can cause pain in the arms, legs, back and stomach, bones, skin and other parts of the body, as well as long-term organ damage, diminished exercise tolerance, increased stroke and infection rate, and decreased lifespan. According to Hematology in Clinical Practice, “sickle cell crisis” is a broad term that describes several different conditions, particularly aplastic crisis, which is temporary bone marrow aplasia; hemolytic crisis, which is acute red cell destruction, leading to jaundice; and vaso-occlusive crisis, which is severe pain due to infarctions located in the bones, joints, lungs, liver, spleen, kidney, eye, or central nervous system. The acute pain of a sickle cell crisis generally persists for several days, and is usually followed by a dull, aching pain, which generally ends after several weeks, although it may persist between crises. Acute chest syndrome, which can often lead to death, is a particularly serious complication of SCD and is commonly accompanied by infections in the lungs. SCD sufferers often experience shortness of breath and SCD children patients often experience abdominal pain. Pain in the bones is a common symptom due to blockage of blood circulation, which damages bones and the bone marrow (the site of most red blood cell production). Sudden attacks of pain also commonly occur in the fingers and toes and in other bones and joints (known as “hand-foot syndrome.”) The liver may become enlarged, causing great abdominal pain; nausea, low-grade fever, and increasing jaundice can occur when the liver is affected.
6
According to Hematology in Clinical Practice , SCD patients, on average, experience about three sickle cell crises per year that are severe enough to require hospitalization, and their symptoms are treated with nonsteroidal anti-inflammatory drugs (NSAIDs), narcotics (e.g. morphine, codeine and oxycodone), other pain relief medicines, and blood transfusions. SCD patients also require frequent visits to emergency rooms and urgent care facilities. A sickle cell crisis is usually followed by a period of remission. If a patient recovers after a sickle cell crisis, she can resume a relatively normal life between crises. SCD patients also suffer from a variety of other ailments, which according to Hematology in Clinical Practice and the National Heart, Lung and Blood Institute, include stroke, complications from anemia, kidney failure, infection, problems in the genital-urinary tract, liver failure, gallbladder disease, problems in the bones and joints, and other medical complications. Patients who survive infancy are subject to other medical problems, including impaired physical development, gum disease, scarring of the retina, and leg sores.
We have acquired the rights to develop a treatment approach for SCD covered under U.S. Patent No. 5,693,671, entitled “L-glutamine Therapy for Sickle Cell Disease and Thalassemia” issued on December 2, 1997 to Niihara et al. Emmaus Medical is the exclusive worldwide licensee of this patent. The license agreement is effective until the expiration of the patent in 2016. For further information on the key terms of the license agreement, see “Intellectual Property” below. L-glutamine is a conditionally essential amino acid that has long been used as a non-pharmaceutical nutritional supplement. A conditionally essential amino acid is an amino acid that the body can naturally synthesize, but under certain circumstances, the body is unable to synthesize such amino acid and it must be supplied by diet or supplement. Emmaus’ treatment involves SCD patients orally consuming 30 gm/day of pharmaceutical grade L-glutamine for adults, and 0.6 gm/kg of body weight for infants and children, up to 30 gm/day.
In red blood cells, pyridine nucleotides, nicotinamide adenine dinucleotide (NAD) and its reduced form NADH, are the major molecules that regulate and prevent oxidative damage. According to various articles including L-Glutamine Therapy Reduces Endothelial Adhesion of Sickle Red Blood Cells to Human Umbilical Vein Endothelial Cells by Y Niihara et al., published in BMC Blood Disorders in 2005, Oral L-Glutamine Therapy for Sickle Cell Anemia: I. Subjective Clinical Improvement and Favorable Change in Red Cell NAD Redox Potential by Yutaka Niihara et al., published in the American Journal of Hematology in 1998 and Increased Red Cell Glutamine Availability In Sickle Cell Anemia: Demonstration Of Increased Active Transport, Affinity, And Increased Glutamate Level In Intact Red Cells by Yutaka Niihara et al., published in 1997 in the Journal of Laboratory and Clinical Medicine, sickle red blood cells have a significantly increased rate of transport of one of the major precursors of NAD, glutamine. It was proposed that the SCD red blood cell is attempting to improve NAD redox potential by increasing transport of glutamine. Analysis of the chemistry of NAD synthesis in red blood cells has suggested that with glutamine supplementation to sickle red blood cells, the NAD synthesis will further increase, which would prevent the sickle red blood cells from being oxidative damaged, and make the sickle red blood cells less adhesive to small blood vessels, leading to less obstruction or blockage of small blood vessels, which is a major cause of the problems that sickle cell patients face.
Before FDA approval can be granted, we will have to complete the Phase III clinical trial and analyze all of the data collected during the trial. We believe that the Phase III clinical trial trail will be completed by the end of 2012. After we complete the Phase III trial and analyze the data we will submit a New Drug Application (NDA) to the FDA. If the trial is completed by the end of 2012 as anticipated, we believe that we will file the NDA with the FDA in the first half of 2013. After the NDA is submitted, the FDA will have 60 days to decide whether to file the NDA for review. The FDA can refuse to file an application for review that is incomplete for reasons such as missing studies that may be required. In accordance with the Prescription Drug User Fee Act (PDUFA), the FDA’s Center for Drug Evaluation and Research (CDER) expects to review and either approve or issue a comment letter on at least 90 percent of NDAs for standard drugs no later than 10 months after the applications are received. The review goal is six months for priority drugs. Our L-glutamine treatment for SCD has obtained orphan drug and fast track designation as outlined in greater detail below. Based on these designations granted by the FDA, we anticipate that such treatment will qualify as a priority drug.
After reviewing the NDA, the FDA will make a determination as to whether the drug is safe and effective in its proposed use(s), and whether the benefits of the drug outweigh the risks, whether the drug’s proposed labeling (package insert) is appropriate, and what the package insert should contain and whether the methods used in manufacturing the drug and the controls used to maintain the drug’s quality are adequate to preserve the drug’s identity, strength, quality, and purity. Upon completion of the NDA review, the FDA will either approve the application or issue a complete response letter (a letter from the FDA indicating that it cannot approve the application in its present form and informing applicants of changes that must be made before an application can be approved). If FDA approval is granted, we will be able to commercialize the product.
7
The FDA will outline the justification for its decision to reject the NDA in a response letter to us and we will have an opportunity to meet with FDA officials to discuss any deficiencies noted in the letter. At that point, we can choose to ask for a hearing, or correct any deficiencies and submit new information, or we can withdraw the application. Common problems which may delay or prevent the FDA from approving our NDA include unexpected safety issues or the failure to demonstrate a drug’s effectiveness. We may need to conduct additional studies, perhaps studies of more people, different types of people, or for a longer period of time.
Manufacturing issues also may be a reason that approval may be delayed or denied. Drugs must be manufactured in accordance with standards called good manufacturing practices, and the FDA inspects manufacturing facilities before a drug can be approved. If a facility is not ready for inspection, approval can be delayed. We would need to correct any manufacturing deficiencies found before the FDA will approve the NDA.
If FDA approval is not granted, we will not be able to commercialize the product, which will have a material adverse impact on our company. If we must conduct new studies pursuant to the FDA’s review of our NDA, we may not have sufficient funding in order to conduct such additional studies. While we do not expect that the FDA will reject our NDA, we may proceed with having the product approved as a medical food. We will also continue to develop those products in the pipeline, including the cornea technology we are developing with CellSeed, Inc. described below.
Short Bowel Syndrome
We currently sell one prescription pharmaceutical product, NutreStore® [L-glutamine powder for oral solution], in the United States and have the exclusive right to promote another prescription pharmaceutical product, Zorbtive® [somatropin (rDNA origin) for injection], in the United States. Each of these products has received FDA approval to treat SBS.
SBS is a condition affecting people who have had half or more of their small intestine surgically removed or who have a congenital defect or a disease affecting the small intestine, such as Crohn’s disease and inflammatory bowel disease. The small bowel plays a significant role in nutrient absorption and those with SBS experience malnourishment due to an inadequate absorption of nutrients and fluids. As described in AGA Technical Review on Short Bowel Syndrome and Intestinal Transplantation published in Gastroenterology in 2003, Guidelines for management of Patients with Short Bowel published in Gut in 2006 and Mortality and Economics in Short Bowel Syndrome published in Best Practice & Research Clinical Gastroenterology in 2003, some symptoms of SBS include malnutrition, diarrhea, abdominal bloating, fatigue, fat in the stool (steatorrhea), cramping, heartburn, bacterial infections, anemia, depression, gallstones and kidney stones. Complications of SBS include organ failure due to malnourishment or malnutrition which could lead to death.
According to an article titled Long-term Survival and Parenteral Nutrition Dependence in Adult Patients With the Short Bowel Syndrome which was published in 1999 in Gastroenterology and an article titled Management of Complications in Patients Receiving Home Parenteral Nutrition which was published in 2003 in Gastroenterology, the standard treatment for SBS for years has been changes in diet, intravenous (IV) feeding (also called “parenteral nutrition”), vitamin and mineral supplements, and medication to relieve symptoms. Long term IV feeding is expensive, is inconvenient to the patient, and can be harmful to the body.
In 2004, a new and patented treatment regime was approved by the FDA which was covered by US Patent No. 5,288,703 (the “SBS Patent”). This treatment comprises of a man-made human growth hormone (hGH), namely Zorbtive® [somatropin (rDNA origin) for injection] in combination with NutreStore® [L-glutamine powder for oral solution] and a specialized diet.
The treatment is comprised of Zorbtive® and NutreStore® in combination with a specialized diet. Zorbtive® is administered by injection for four weeks, and NutreStore® is orally taken for 16 weeks. Treatment with NutreStore® alone with a specialized diet, Zorbtive® alone with a specialized diet, and Zorbtive® and NutreStore® together with a specialized diet all help the small intestine take in more water, electrolytes and nutrients and reduce the volume and frequency of IV feedings and the problems caused thereby. Study results cited in an article entitled Growth Hormone, Glutamine, and an Optimal Diet Reduces Parenteral Nutrition in Patients With Short Bowel Syndrome. A Prospective, Randomized, Placebo-Controlled, Double-Blind Clinical Trial published in Annals of Surgery in 2005 (the “Ann Surg Article”) and cited in the NutreStore and Zorbtive Full Package Information available on our website show that the
8
treatment has the potential to reduce the mean weekly frequency of intravenous parenteral nutrition from 5.4 days to 1.2 days per week after 4 weeks of treatment, as well to reduce the required weekly volume and caloric content. Published pharmacoeconomic studies, including the Ann Surg Article, an article entitled Mortality and Economics in Short Bowel Syndrome published in 2003 in Best Practice & Research Clinical Gastroenterology and an article titled Economic Implications of Growth Hormone Use in Patients with Short Bowel Syndrome published in Current Medical Research and Opinion in 2006, have shown that when Zorbtive® is used in SBS patients, the average savings (including the costs of Zorbtive®) are about $85,000 over two years over traditional parenteral nutrition treatment. Moreover, when Zorbtive® and NutreStore® are used together, there are superior results, namely a greater reduction in a patient’s requirement for parenteral nutrition, which would lead to even greater cost savings to insurers.
In October 2007, we became the exclusive sublicensee of the SBS Patent for the U.S. market, including the rights to distribute the L-glutamine treatment for SBS under the trademark NutreStore® in the U.S., and commercially launched NutreStore® in June 2008. We were granted this sublicense from Cato Holding Company. EMD Serono, Inc. granted Emmaus the exclusive right to promote Zorbtive® in the United States in December 2008. Internationally, we are in the last stages of seeking approval to market NutreStore® in Hong Kong and have received a Certificate of Free Sale from the FDA to export NutreStore® to Hong Kong.
In December 2010, we were awarded a five-year contract by the U.S. Department of Veterans Affairs for our NutreStore® product. The contract is effective from December 15, 2010 to December 14, 2015. The estimated value of the award is $125,000. Pursuant to the agreement, we agreed to sell Nutrestore® to the Department of Veterans Affairs from time to time during the term of the agreement at a rate of $205.31. The discount pricing that we have offered to the Department of Veterans Affairs under this contract is also extended to the Department of Defense, Public Health Service (Indian Health Service), and U.S. Coast Guard customers. The above description of the agreement with the U.S. Department of Veterans Affairs is qualified in its entirety by reference to the complete text of the agreement, which is attached hereto as Exhibit 10.5 and is incorporated by reference herein. You are urged to read the entire agreement.
AminoPure® Business
We sell L-glutamine as a nutritional supplement under the brand name AminoPure® through our indirect wholly owned subsidiary, Newfield Nutrition Corporation. AminoPure® is made up of pure USP grade L-glutamine that the body needs when a person is under physical exertion, stress, or is sick. Data from scientific research has shown that L-glutamine helps with gastro intestinal health and to support the body’s natural immune response.
AminoPure® is currently sold through retail stores in several states and via importers and distributors in Japan. We have started to export AminoPure® to Taiwan and plan to expand sales of AminoPure® into the Philippines and South Korea in the near future. We added a new distributor, PMAI, a subsidiary of JFC International, Inc., a leading distributor of Japanese foods in Asian-American communities in the United States, in November 2010 to take advantage of its retail outlet network in the United States.
Nutritional supplements are regulated by FDA and we must comply with numerous federal and state laws and regulations related to the AminoPure® product’s testing, manufacture, labeling, packaging, storage, distribution, recordkeeping and reporting. The federal laws and regulations are administered by FDA and are often incorporated by the U.S. States’ individual laws and regulations.
Sales of AminoPure® have steadily increased in recent years. Sales increased 121.2% in the year ended December 31, 2010 as compared to the year ended December 31, 2009 and 78% in the year ended December 31, 2009 as compared to the year ended December 31, 2008. However, despite the increase in sales of AminoPure®, we have generated minimal revenues from the sale of from AminoPure® and NutreStore® since our inception, and the sale of such products are not part of our principal operations.
CellSeed Investment
In January 2009, Emmaus made a strategic investment in CellSeed, Inc. (“CellSeed”), a Japanese company engaged in the research and development, manufacture and sale of temperature-responsive cell culture equipment, which is a cell sheet tissue-engineering platform tool, and application products, as well as cell sheet tissue engineered medical products and application products. Emmaus owns a 3% stake in CellSeed, a public company traded on the JASDAQ NEO market in Tokyo, Japan. In April 2011, we entered into a Joint Research and Development Agreement and an Individual Agreement with CellSeed, described below under the heading “Intellectual Property” to pursue collaborative opportunities with CellSeed.
9
Competitive Strengths
We believe the following strengths contribute to our competitive advantages:
Experienced Management Team
Our senior management team has extensive business experience and knowledge of the healthcare and biotechnology industries. Our President and Chief Executive Officer, Yutaka Niihara, M.D., MPH, has extensive knowledge of our operations and our patents, being one of the initial patentees for the technology for the treatment with SCD. Dr. Niihara has extensive research experience in the field of SCD and other blood diseases, is widely published scientist in the area of SCD and actively treats SCD patients. Members of our senior management team also have significant experience with respect to key aspects of our operations and product candidates.
Strategic Supplier Relationships with Major Suppliers of Pharmaceutical Grade L-Glutamine.
Ajinomoto U.S.A., through its parent company, the Ajinomoto Company in Japan, has provided free of charge L-glutamine for our completed clinical work, including our completed Phase II clinical trials. Ajinomoto is also providing L-glutamine for our Phase III clinical trials without charge. We have supplier relationships with Ajinomoto and the only other major supplier of pharmaceutical grade L-glutamine, Kyowa Hakko U.S.A, the U.S. subsidiary of Kyowa Hakko Kogyo Co., Ltd. We currently source L-glutamine from Kyowa Hakko U.S.A. for our NutreStore® product.
Orphan Drug Act and the Prescription Drug User Fee Act (PDUFA) of 1992 – Federal Subsidies.
We obtained Orphan Drug Designation, which provides numerous advantages, for the L-glutamine therapy for SCD under Application number 01-1459 on August 1, 2001. As a designated orphan drug, pursuant to the Orphan Drug Act (“ODA”) we receive a 50% tax credit for clinical research. Furthermore, our new drug application fee is waived, and upon obtaining FDA approval, we will receive seven years of exclusive marketing rights for the SCD indication, independent of the patent protection. In addition, under the Prescription Drug User Fee Act of 1992 and its amendments, we will not be required to pay the annual establishment fee and product fee, which were $425,600 and $71,520, respectively, for the fiscal year ended December 31, 2009 and $457,200 and $79,720, respectively, for the fiscal year ended December 31, 2010.
Our Strategy
Our goal is to be a specialty pharmaceutical company focused on the development and commercialization of proprietary branded products and product candidates to treat rare diseases. We intend to achieve this goal by:
Maximizing the value of our L-glutamine treatment for SCD
We are currently in phase III clinical trials of our L-glutamine treatment for SCD. We believe our treatment could have advantages over traditional treatments for SCD, including cost savings. We intend to undertake activities to prepare for the commercialization of this treatment. When and if this treatment is approved by the FDA, we intend to commercialize our L-glutamine SCD treatment and may enter into strategic relationships with third parties.
Establishing strategic collaborations
We intend to seek opportunities to enter into strategic collaborations with leading pharmaceutical and biotechnology companies to commercialize our product candidates to drive growth and profitability. We believe that leveraging the capabilities of third parties will allow us to add efficiency to our operations and expand our commercial reach.
10
Expand our collaborative research arrangement with CellSeed
In April 2011, we entered into the Research Agreement and the Individual Agreement with CellSeed. Pursuant to the Research Agreement, the parties formed a relationship regarding the future research and development of cell sheet engineering regenerative medicine products and the future commercialization of such products. Pursuant to the Individual Agreement, CellSeed granted us the exclusive right to manufacture, sell, market and distribute CAOMECS for the cornea in the United States. We intend to work on commercializing the CAOMECS for the cornea and to expand our relationship with CellSeed to develop cell sheets for other types of cells in the future.
Pursuing acquisitions to broaden our drug candidates and product offerings
We will consider strategic acquisitions that will provide us with a broader range of drug candidates and product offerings. When evaluating potential acquisition targets, we will consider factors such as market position, growth potential and earnings prospects and strength and experience of management.
Governmental Regulation of Pharmaceutical and Biotechnology Industries
Regulation by governmental authorities in the U.S. and foreign countries is a significant factor in the development, manufacture, and expected marketing of our drug product candidates and in our ongoing research and development activities. The nature and extent to which such regulation will apply to us will vary depending on the nature of any drug product candidates developed.
In particular, human therapeutic products are subject to rigorous preclinical and clinical testing and other approval procedures of the FDA and similar regulatory authorities in other countries. Various federal and state statutes and regulations also govern or influence research, testing, manufacturing, safety, efficacy, labeling, packaging, storage, distribution and record-keeping related to such products and their marketing. The process of obtaining these approvals and the subsequent compliance with the appropriate federal and state statutes and regulations requires substantial time and financial resources. Any failure by us or our collaborators to obtain, or any delay in obtaining, regulatory approval could adversely affect the marketing of any of our drug product candidates, our ability to receive product revenues, and our liquidity and capital resources.
Before obtaining regulatory approvals for the commercial sale of L-glutamine as a treatment for any of our products under development, we must demonstrate through preclinical studies and clinical trials that the product is safe and efficacious for use in each target indication. The results from preclinical studies and early clinical trials might not be predictive of results that will be obtained in large-scale testing. Our clinical trials might not successfully demonstrate the safety and efficacy of any product candidates or result in marketable products.
In order to clinically test, manufacture, and market products for therapeutic use, we and our third-party collaborators will have to satisfy mandatory procedures and safety and effectiveness standards established by various regulatory bodies. In the U.S., the Public Health Service Act and the Federal Food, Drug, and Cosmetic Act, as amended, and the regulations promulgated thereunder, and other federal and state statutes and regulations govern, among other things, the research, testing, manufacture, labeling, packaging, storage, distribution, record keeping, approval, advertising, and promotion of our current and proposed product candidates. Product development and approval within this regulatory framework takes a number of years and involves the expenditure of substantial resources.
The steps required by the FDA before new drug products may be marketed in the U.S. include:
| ● | completion of preclinical studies; | |
| ● | the submission to the FDA of a request for authorization to conduct clinical trials on an investigational new drug application, or IND, which must become effective before clinical trials may commence; | |
| ● | adequate and well-controlled Phase 1, Phase 2 and Phase 3 clinical trials to establish and confirm the safety and efficacy of a drug candidate; | |
| ● | submission to the FDA of a new drug application, or NDA, for the drug candidate for marketing approval; and | |
| ● | review and approval of the NDA by the FDA before the product may be shipped or sold commercially. |
11
In addition to obtaining FDA approval for each product, each product manufacturing establishment must be registered with the FDA and undergo an inspection prior to the approval of an NDA. Each manufacturing facility and its quality control and manufacturing procedures must also conform and adhere at all times to the FDA’s current good manufacturing practice, or cGMP, regulations. In addition to preapproval inspections, the FDA and other government agencies regularly inspect manufacturing facilities for compliance with these requirements. If, as a result of these inspections, the FDA determines that any equipment, facilities, laboratories or processes do not comply with applicable FDA regulations and conditions of product approval, the FDA may seek civil, criminal, or administrative sanctions and/or remedies against us, including the suspension of the manufacturing operations and market withdrawal of marketed product. Manufacturers must expend substantial time, money and effort in the area of production and quality control to ensure full technical compliance with these standards.
Preclinical testing includes laboratory evaluation and characterization of the safety and efficacy of a drug and its formulation. Preclinical testing results are submitted to the FDA as a part of an IND which must become effective prior to commencement of clinical trials. Clinical trials are typically conducted in three sequential phases following submission of an IND. Phase 1 represents the initial administration of the drug to a small group of humans, either patients or healthy volunteers, typically to test for safety (adverse effects), dosage tolerance, absorption, distribution, metabolism, excretion and clinical pharmacology, and, if possible, to gain early evidence of effectiveness. Phase 2 involves studies in a small sample of the actual intended patient population to assess the efficacy of the drug for a specific indication, to determine dose tolerance and the optimal dose range and to gather additional information relating to safety and potential adverse effects. Once an investigational drug is found to have some efficacy and an acceptable safety profile in the targeted patient population, Phase 3 studies are initiated to further establish clinical safety and efficacy of the therapy in a broader sample of the general patient population, in order to determine the overall risk-benefit ratio of the drug and to provide an adequate basis for any physician labeling. During all clinical studies, we must adhere to Good Clinical Practice, or GCP, standards and applicable human subject protections standards. The results of the research and product development, manufacturing, preclinical studies, clinical studies and related information are submitted in an NDA to the FDA.
The process of completing clinical testing and obtaining FDA approval for a new drug is likely to take a number of years and require the expenditure of substantial resources. If an application is submitted, there can be no assurance that the FDA will review and approve the NDA. Even after initial FDA approval has been obtained, further studies, including post-market studies, might be required to provide additional data on safety and will be required to gain approval for the use of a product as a treatment for clinical indications other than those for which the product was initially tested and approved. Also, the FDA will require post-market reporting and might require surveillance programs to monitor the side effects of the drug. Results of post-marketing programs might limit or expand the further marketing of the products. Further, if there are any modifications to the drug, including changes in indication, manufacturing process, labeling or a change in manufacturing facility, an NDA supplement might be required to be submitted to the FDA prior to or corresponding with that change.
The rate of completion of any clinical trials will be dependent upon, among other factors, the rate of patient enrollment. Patient enrollment is a function of many factors, including the size of the patient population, the nature of the trial, the number of clinical sites, the availability of alternative therapies and drugs, the proximity of patients to clinical sites and the eligibility criteria for the study. Delays in planned patient enrollment might result in increased costs and delays, which could have a material adverse effect on us.
Failure to comply with applicable FDA requirements may result in a number of consequences that could materially and adversely affect us. Failure to adhere to approved trial standards and GCPs in conducting clinical trials could cause the FDA to place a clinical hold on one or more studies which would delay research and data collection necessary for product approval. Noncompliance with GCPs could also have a negative impact on the FDA’s evaluation of an NDA. Failure to adhere to cGMPs and other applicable requirements could result in FDA enforcement action and in civil and criminal sanctions, including but not limited to fines, seizure of product, refusal of the FDA to approve product approval applications, withdrawal of approved applications, and prosecution.
12
Whether or not FDA approval has been obtained, approval of a product by regulatory authorities in foreign countries must be obtained prior to the commencement of marketing of the product in those countries. The requirements governing the conduct of clinical trials and product approvals vary widely from country to country, and the time required for approval might be longer or shorter than that required for FDA approval. Although there are some procedures for unified filings for some European countries, in general, each country at this time has its own procedures and requirements. There can be no assurance that any foreign approvals would be obtained. In most cases, if the FDA has not approved a drug product candidate for sale in the U.S., the drug product candidate may be exported for sale outside of the U.S. only if it has been approved in any one of the following: the European Union, Canada, Australia, New Zealand, Japan, Israel, Switzerland and South Africa. Specific FDA regulations govern this process.
In addition to the regulatory framework for product approvals, we and our collaborative partners must comply with federal, state, and local laws and regulations regarding occupational safety, laboratory practices, the use, handling and disposition of radioactive materials, environmental protection and hazardous substance control, and other local, state, federal and foreign regulation. All facilities and manufacturing processes used by third parties to produce our drug candidates for clinical use in the United States must conform with cGMPs. These facilities and practices are subject to periodic regulatory inspections to ensure compliance with cGMP requirements. Their failure to comply with applicable regulations could extend, delay, or cause the termination of clinical trials conducted for our drug candidates. The impact of government regulation upon us cannot be predicted and could be material and adverse. We cannot accurately predict the extent of government regulation that might result from future legislation or administrative action.
Outside of the United States, we sell AminoPure® in Japan and Taiwan. There are no regulatory requirements in Japan to sell AminoPure® because it is classified as a nutritional supplement product. In Taiwan, we must obtain a Certificate of Free Sale from the FDA and provide it to our distributor. The Certificate of Free Sale is for food, including dietary supplements, and cosmetic products that may be legally marketed in the United States. Once, the Certificate of Free Sale is furnished to our distributor in Taiwan, it is the distributor’s responsibility to comply with local regulations, including but not limited to, obtaining the proper import license. The certificate expires on August 16, 2012. We will submit an application for its renewal 3-6 months before the certificate expires to ensure it does not lapse.
We intend to begin selling Nutrestore® in Hong Kong as soon as we receive the required approvals. We have received a Free Sale Certificate from the FDA in order to sell Nutrestore® in Hong Kong. However, we must also obtain approval from the FDA-equivalent in Hong Kong in order to market Nutrestore® there. Selling NutreStore in Hong Kong requires the approval of the Hong Kong Department of Health. Typically, the process takes approximately six months. We estimate that it will cost a maximum of $10,000 to obtain this approval. In order to obtain such approval, NutreStore first must be registered with Hong Kong Department of Health. The application for registration was originally filed in February 2010 and subsequently re-filed in July 2011. After such product is registered, an application can be submitted to the Hong Kong Department of Health for the necessary approval. We have not yet obtained the requisite approval and do not currently sell Nutrestore® in Hong Kong. We hope to receive the approval in October 2011.
Clinical Trials
We have conducted a number of clinical trials with the goal of obtaining FDA approval to market and sell L-glutamine as a treatment for SCD. The FDA approval process begins with laboratory testing and then moves on to the clinical trial stage. In July 1994, Dr. Niihara started a pilot clinical trial using L-glutamine as an oral supplement to SCD patients. The study showed based on the preclinical data and demonstrated that oral consumption of L-glutamine by SCD patients increases the concentration of the reduced form of NAD, NADH, and its redox status. Clinically, all the patients who participated in the study reported an increase in their energy level and decrease in the severity of chronic pain with treatment.
In a Phase II, 12-week open label study conducted in 1995, Dr. Niihara found a significant decrease in the incidence and severity of sickle cell crisis in selected patients who experience unusually frequent episodes of sickle cell crisis. A Phase II blind clinical trial to assess the reduction of sickle cell crisis and chronic pain, which started in 1997 and was funded by the National Institutes of Health, the results of which were released in January 2003, demonstrated statistically significant reduction of chronic pain, while a strong trend toward significance was observed in the reduction in sickle cell crises. Another Phase II open label clinical trial, which started in 2000 and was funded by the FDA, the results of which were released in April 2009, directed to exercise tolerance, demonstrated that patients on L-glutamine have improved physical stamina with no significant side effects.
13
In April 2009, Emmaus completed an 80 patient Phase II clinical trial funded by the FDA directed to reduce the incidence of sickle cell crisis as its primary indication. The trial took place at the following institutions: the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center (“LABioMed”); Emory University, Atlanta, Georgia; Kaiser Permanente, Bellflower, California; the University of Medicine and Dentistry of New Jersey (Robert Wood Johnson Medical School), New Brunswick, NJ; and the Jacobi Medical Center-North Bronx Healthcare Network, Bronx, New York. This study showed clinical significance for reducing the incidence of sickle cell crisis (more than a 50% reduction in incidence of sickle cell crisis) but due to a higher than expected drop out rate, the statistical significance was not high enough to support Emmaus’ direct submission of a new drug application to the FDA. However, the safety of L-glutamine was well demonstrated in this trial. This Phase II clinical trial was managed by the contract research organization, ClinDatrix, Inc.
In April 2009, the FDA authorized Emmaus to begin a larger Phase III clinical trial directed to study L-glutamine as an experimental agent to reduce sickle cell crisis. Patient enrollment began in mid-2010 and as of May 2011, we have signed contracts with 19 sickle cell study sites across the United States and have enrolled 56 patients. We aim to complete Phase III clinical trial enrollment by the end of 2011. This Phase III trial will include an interim analysis after 24 weeks of the 48-week study period and we expect that it will involve 200+ patients at approximately 30 clinical trial sites around the country. This Phase III clinical trial is managed by CinDatrix.
Status of FDA Approvals and Orphan Drug Designation
The FDA has already approved L-glutamine as a treatment for short bowel syndrome. Accordingly, instead of the more involved approval process of Section 505(b)(1) of the Federal Food, Drug, and Cosmetic Act (“FD&C Act”) that is required for the first medical indication of a drug, Emmaus will proceed under Section 505(b)(2) of the FD&C Act, which provides a more streamlined and easier approval process for subsequent indications of a drug. Consequently, we believe that our Phase III clinical trial directed to reduce sickle cell crises will likely be considered a pivotal study for purposes of applying for FDA marketing approval under Section 505(b)(2). An NDA filed pursuant to Section 505(b)(1) is a “full” NDA that contains all original data produced by a company, while an NDA filed pursuant to Section 505(b)(2) contains slightly less data because a company is able to reference public data or drug approval that is known to the FDA.
The FDA Modernization Act of 1997 codified the FDA’s policy of granting “fast track” review of certain therapies targeting “orphan” indications and other therapies intended to treat severe or life threatening diseases and having potential to address unmet medical needs. Orphan indications are defined by the FDA as having a prevalence of less than 200,000 patients in the U.S. The Orphan Drug Act (“ODA”) provides for the granting of special status to a product to treat a rare disease or condition. The Orphan Drug Designation program provides orphan status to drugs and biologics which are defined as those intended for the safe and effective treatment, diagnosis or prevention of rare diseases/disorders that affect fewer than 200,000 people in the U.S., or drugs for the treatment of diseases/disorders that affect more than 200,000 persons but for which the drug developer is not expected to recover the costs of developing and marketing the drug from the commercialization and sale of such drug.
In order to obtain Orphan Drug designation, we had to submit two copies of a completed, dated, and signed request for designation containing the following:
| ● | A statement requesting orphan drug designation for a rare disease which was identified with specificity; | |
| ● | Our contact information, the generic name of the drug and the name and address of the source of the drug if it is not manufactured by the sponsor; | |
| ● | A description of the rare disease or condition for which the drug is being investigated, the proposed indication or indications for use of the drug, and the reasons why such therapy is needed; |
14
| ● | A description of the drug and a discussion of the scientific rationale for the use of the drug for the rare disease, including all data from nonclinical laboratory studies, clinical investigations, and other relevant data that are available to the sponsor, whether positive, negative, or inconclusive. Copies of pertinent unpublished and published papers are also required; | |
| ● | A summary of the regulatory status and marketing history of the drug in the United States and in foreign countries; and | |
| ● | Documentation, with appended authoritative references, to demonstrate that the disease or condition for which the drug is intended affects fewer than 200,000 people in the United States. |
We applied for Orphan Drug designation June 11, 2001 and were granted orphan drug status on August 1, 2001. There is no expiration date on the status of the Orphan Drug designation. This designation waived the new drug application fee (presently over $1 million) and annual establishment and product fees, provided a 50% tax credit for clinical work, and, if the product is approved, will provide exclusive marketing rights for the SCD indication for seven years. The seven years of exclusivity is measured from the date of the approval of the application or the issuance of the license. Because we were granted Orphan Drug status, the 50% tax credit is absolute and is provided pursuant to the Orphan Drug Act, which provides for tax credits for testing expenses for drugs for rare disease or conditions, and the Internal Revenue Code, which specifically provides a tax credit equal to 50% of the qualified clinical testing expenses for the taxable year.
The waiver of the annual establishment fee is provided for under the FD&C Act, as amended by the Food and Drug Administration Amendments Act of 2007 (the “FDAAA”), which provides that a drug designated as an Orphan Drug shall be exempt from product and establishment fees if the drug meets all of the following conditions: the drug meets the public health requirements contained in the FD&C Act as such requirements are applied to requests for waivers for product and establishment fees; and the drug is owned or licensed and is marketed by a company that had less than $50,000,000 in gross worldwide revenue during the previous year. While our right to a waiver of the product fee and annual establishment fee are not absolute as such waivers are subject to the above conditions, we have qualified for waivers of such fees because the experimental treatment for SCD meets all of the above conditions: (1) L-glutamine treatment for SCD received orphan designation under section 526 of FDC Act on August 1, 2001 and (2) the L-glutamine treatment for SCD is licensed by Emmaus Medical, Inc. whose gross worldwide revenue during the previous 12 months was less than $50,000,000. We believe that we will continue to meet such conditions in the future and receive waivers of the establishment and product fees.
Further, a marketing application for a prescription drug product that has been designated as a drug for a rare disease or condition is not subject to a prescription drug user fee unless the application includes an indication for other than a rare disease or condition.
We have obtained Fast Track designation for the L-glutamine therapy for SCD. Fast Track designation will provide us with many advantages over the normal FDA approval process, including the right to submit modules of the new drug application (NDA) in portions (“rolling submission”), rather than all at once, and the opportunity to have more FDA interaction.
Fast track is a process designed to facilitate the development, and expedite the review of, drugs to treat serious diseases and fill an unmet medical need. The purpose is to get important new drugs to the patient sooner. We assume that since we were granted fast track designation, we will be eligible for some or all the following:
| ● | More frequent meetings with the FDA to discuss the drug’s development plan and ensure collection of appropriate data needed to support drug approval; | |
| ● | More frequent written correspondence from the FDA about such things as the design of the proposed clinical trials; | |
| ● | Eligibility for Accelerated Approval, i.e., approval on an effect on a surrogate, or substitute endpoint reasonably likely to predict clinical benefit; and | |
| ● | Rolling Review, which means that we can submit completed sections of the New Drug Application (NDA) for review by FDA, rather than waiting until every section of the application is completed before the entire application can be reviewed. NDA review usually does not begin until the entire application has been submitted to the FDA. |
15
We assume, based on the fast track designation, that we will receive a Priority Review. A Priority Review is a streamlined review of the NDA and may take up to six months from when an NDA is submitted. If a Priority Review is not granted, the more involved review process would extend the review time to 10-12 months from the filing of the NDA. The primary difference between the Priority Review and the more involved review process is essentially the turnaround time by the FDA. We anticipate completing our clinical trial by the end of 2012 and submitting the NDA for our product in the first half of 2013. If we receive Priority Review of our NDA and no application deficiencies are identified by FDA, we expect that we would receive approval of the NDA in the second half of 2013.
We intend to communicate with the FDA on a regular basis to assure that questions and issues are resolved quickly, which may help lead to earlier drug approval and access by patients.
The expected cost to obtain FDA approval is difficult to estimate based on the dynamic process and unknowns related to FDA’s thinking. Based on our assumption of receiving a Priority Review, we estimate that our costs to obtain FDA approval are approximately $400,000. Actual costs can vary greatly from this figure based on how the FDA review process proceeds. The bulk of these costs would be associated with the consultants retained by us to assist with the FDA application and response process. Should the FDA find deficiencies with our study data, additional studies may be required or if the FDA finds issues with the manufacturing of the product, such issues would need to be resolved prior to the approval of the NDA, the occurrence of either of which would increase the costs dramatically.
Product Sourcing and Packaging
We plan to obtain our pharmaceutical grade L-glutamine from Ajinomoto Company, a Japanese food, amino acid and pharmaceutical company, and from Kyowa Hakko, a Japanese pharmaceutical company. The Ajinomoto Company and Kyowa Hakko together produce the vast majority of pharmaceutical grade L-glutamine approved for sale in the U.S. The manufacture of large quantities of pharmaceutical grade L-glutamine is a complex and expensive undertaking, and is therefore not an easy market for third parties to enter.
Ajinomoto Aminoscience LLC, through its parent company, the Ajinomoto Company, has previously provided L-glutamine to us free of charge for our completed clinical work, including our completed Phase II clinical trials. Ajinomoto is also providing L-glutamine for our Phase III clinical trials without charge. Pursuant to a letter of intent between Emmaus Medical and Ajinomoto, we agreed to purchase or make relevant third party purchases from Ajinomoto all of the L-glutamine will need for our commercial products. Pursuant to the Letter of Intent, we will be permitted to source L-glutamine from third party suppliers of up to 10% of our requirement for L-glutamine on a back-up basis. However, if a third party competitor of Ajinomoto offers us a more favorable pricing on L-glutamine of a similar grade with similar terms and conditions, we may ask Ajinomoto to reconsider its pricing. Although the Letter of Intent contemplates that we will enter into a supply agreement with Ajinomoto, we have not yet entered into the supply agreement.
We currently source L-glutamine from Kyowa Hakko U.S.A. for our NutreStore® product. We do not have an agreement with Kyowa Hakko for the supply of L-glutamine. We purchase L-glutamine from Kyowa Hakko on an as-needed basis pursuant to individual purchase orders.
We eventually plan to enter into exclusive long term supply contracts with these manufacturers for L-glutamine for SCD treatment that will also require that these companies agree not to sell L-glutamine as a nutritional supplement or pharmaceutical for sickle cell disease applications. However, there is no assurance that we will be able to obtain such terms or economically attractive terms for obtaining pharmaceutical grade L-glutamine from these proposed suppliers, or that the suppliers will not experience an interruption in supply that could materially and adversely affect our business.
We expect that the product will be packaged by an FDA approved facility. Anderson Packaging, Inc., of Rockville, Illinois, has handled the packaging for our Phase II and III clinical trials of L-glutamine for SCD and we plan to use the same company for commercial packaging of the product. Anderson Packaging, Inc. packaged L-glutamine for the clinical trials that resulted in the FDA’s marketing approval for L-glutamine for short bowel syndrome using the same dose and packaging protocol as the Company expects to use for treatment of SCD. Prior FDA approval of packaging types and protocols does not guarantee future approval of packaging types and protocols.
16
Sales and Marketing
We have three full time pharmaceutical sales representatives. Our sales representatives conduct weekly teleconferences with management to share current product information and sales strategies and with employees at our headquarters to assist with any immediate patient and physician needs.
As we expand our sales of L-glutamine for SBS and commercialize our L-glutamine treatment for SCD, we intend to increase the size of our sales staff. We intend to employ only sales staff personnel who have experience and training in the U.S. with the sale of prescription pharmaceuticals. Sales representatives will receive continuing training, education and development to ensure that our sales staff has current knowledge of our products as well as the current Compliance Program Guidance for Pharmaceutical Manufacturers published by the U.S. Department of Health and Human Services, Office of Inspector General, the provisions of the Code on Interactions with Healthcare Professionals created by the Pharmaceutical Research and Manufacturers of America (“PhRMA Code”) and the FDA’s regulatory limitations on promotional activities.
After obtaining FDA approval for the SCD indication, we intend to focus our sales and marketing efforts across several different groups, including patients, their physicians and care providers, hospitals and treatment centers, insurance carriers, non-profit associations, and collaborating pharmaceutical companies. Our in-house product specialists and sales representatives will focus on the following tasks as part of our marketing strategy:
| ● | promote our L-glutamine therapy to SCD specialist physicians; | |
| ● | promote awareness of our L-glutamine therapy at all U.S. community-based treatment centers; | |
| ● | develop L-glutamine therapy collateral materials and informational packets to educate patients and physicians and garner industry support; | |
| ● | establish collaborative relationships with non-profit organizations that focus on SCD; and | |
| ● | identify international opportunities for our L-glutamine therapy. |
Our target customers for Zorbtive® and NutreStore® are SBS patients, as well as their local treating medical centers and physicians. Patient and physician awareness of the Zorbtive® and NutreStore® brands will be key to our success. We intend to exhibit at trade shows and other events and maintain websites with current information on Zorbtive® and NutreStore® to strengthen these two brands. In addition, we will continue to place advertisements in medical journals, such as the Journal of Parenteral and Enteral Nutrition (JPEN) and the American Journal of Gastroenterology to raise awareness of Zorbtive® and NutreStore® with healthcare professionals. In addition, we have purchased prescriber data in order to increase our outreach to physicians identified in the data. We will also work with patient support organizations, such as the Oley Foundation and ASPEN, to promote our SBS treatments.
Research and Development
For the years ended December 31, 2010 and 2009, we expended $1.1 million and $0.5 million, respectively, in research and development costs related to our L-glutamine treatment for SCD.
The estimated cost to complete the Phase III clinical trial we are currently conducting for our L-glutamine treatment for SCD is $7.3 million. This estimate is based on our current plan to use 20 to 25 clinical trial sites across the U.S. and assumes that the trial is conducted in accordance with our projected timeline to complete the trial by the end of 2012. Should the trial take longer than expected to complete or we use more trial sites than currently anticipated, our costs related to the Phase III trial will increase.
We believe that the research and development costs for corneal cell sheet technology in U.S. is approximately $3 million in research and development costs. Such estimate includes the cost of obtaining FDA approval for the cornea cell sheets. We have assumed that we will need biologic approval of the FDA for the cornea cell sheets, rather than pharmaceutical approval, and that we will only have to run a small trial here in the U.S. to test the safety and efficacy of the corneal cell sheets because we believe that we will be able to submit, and the FDA will accept, the data regarding corneal cell sheets submitted to the EMEA.
17
Intellectual Property
We rely on a combination of patent, licenses, trademark and trade secret protection and other unpatented proprietary information to protect our intellectual property rights and to maintain and enhance our competitiveness in the pharmaceutical industry. While we do not currently have any patents, we have 2 patent licenses with third parties.
We also rely on unpatented technologies to protect the proprietary nature of our products. We require that our management team and key employees enter into confidentiality agreements that require the employees to assign the rights to any inventions developed by them during the course of their employment with us. All of the confidentiality agreements include non-solicitation provisions that remain effective during the course of employment and for periods following termination of employment.
Licenses and Promotional Rights Agreements
On March 1, 2001, Emmaus Medical became the exclusive worldwide licensee of U.S. Patent No. 5,693,671, entitled “L-glutamine Therapy for Sickle Cell Disease and Thalassemia” (the “SCD Patent”) to develop a treatment for SCD and thalassemia using L-glutamine pursuant to a license agreement. The license agreement is effective until the expiration of the SCD Patent in 2016. Pursuant to the license agreement, we acquired the exclusive right to test, gain governmental approval of, make, have made, use, distribute and sell products (“Licensed Products”) designed for use in carrying out methods covered under the SCD Patent and/or incorporating technical information provided by the licensor or by any of certain doctors affiliated with the licensor. Pursuant to an addendum to the license agreement, we agreed to pay royalties to the licensor during the term of the agreement equal to 4.5% of net sales of Licensed Products in the U.S. until lifetime royalty payments made to the licensor total $100,000, at which time the royalty rate will decrease to 2.5% of net sales of the Licensed Products. No royalties will be paid to the licensor for Licensed Products sold or distributed, or Licensed Methods practiced, on a non-profit basis. Royalty payments are due within 45 days of the end of each fiscal quarter, with the last payment due 45 days after the termination of the agreement. Any payments not made when due accrue interest on and after the due date at a rate equal to the prime interest rate quoted by the Bank of America on the date the payment is due, with interest being compounded on the last day of each calendar quarter.
We are also responsible for paying all fees and costs relating to the maintenance of the SCD Patent. Before any Licensed Products are commercially sold or Licensed Methods are practiced on humans, we are required to obtain comprehensive general liability insurance policies, including product liability insurance coverage in the minimum amount of $5,000,000. If we fail to obtain the required insurance policies, the licensor may terminate the agreement or obtain such insurance at our sole cost and expense. We currently have product liability insurance for NutreStore and also have clinical trial insurance for the SCD study.
The license agreement will terminate upon the expiration of the patent in 2016, or earlier upon a court’s determination that the patent is invalid or unenforceable. If we fail to pay royalties when due and payable, the licensor may terminate the agreement upon ninety (90) days’ written notice, unless we pay all outstanding royalties and interest due, during such 90-day period. The licensor may also terminate the agreement upon our material breach of the agreement upon providing us with 90 days’ written notice. The agreement shall automatically terminate at the end of such 90-day period unless we cure the breach or default during such period.
The above description of the license agreement is qualified in its entirety by reference to the complete text of the agreement, which is attached hereto as Exhibit 10.4 and is incorporated by reference herein. You are urged to read the entire license agreement.
18
In October 2007, Emmaus Medical became the exclusive sublicensee of US Patent No. 5,288,703 (the “SBS Patent”) for the U.S. market, including the rights to distribute the L-glutamine treatment for the treatment of SBS under the trademark NutreStore® in the U.S., and commercially launched NutreStore® in June 2008. Pursuant to the sublicense, as amended by an assignment and transfer agreement, we are required to pay a royalty of 10% of adjusted gross sales of NutreStore® to Cato Holding Company (“Cato”) through 2016. We are also required to pay to Cato Holding Company a royalty of 1% of gross sales of L-glutamine as a treatment for SCD and thalassemia for a period of five years from the date of the first commercial sale of such product as outlined in the Sublicense Agreement attached hereto as Exhibit 10.7. The sublicense is subject to a sublicense that Cato Holding Company holds from Ares Trading, S.A. (the “Ares License”), and if the Ares License is terminated for any reason, then our sublicense with Cato will also terminate. This sublicense was amended in February 2011 and ownership of the NutreStore NDA and Drug Master Files (DMF) containing the proprietary information relating to the manufacturing and packaging specifications of NutreStore product were transferred to us. The sublicense agreement terminates upon the earliest to occur of (i) the expiration or invalidation of the SBS Patent in the U.S., (ii) the voluntary termination by Emmaus Medical at the end of a calendar year upon 180 days’ written notice, (iii) termination by either party upon the other party’s breach of a material provision of the agreement upon 60 days’ notice and opportunity by the breaching party to cure such breach, (iv) by licensor upon 90 days’ written notice to Emmaus Medical for Emmaus Medical’s failure to comply with any applicable law or governmental rule or regulation concerning manufacture, marketing or sale of licensed product and failure to cure such breach within such 90 days period , or (v) the termination, for any reason, of the Ares License. The SBS Patent expires in October 2011 and the sublicense agreement will terminate on the date of expiration. Pursuant to the assignment and transfer agreement, Emmaus Medical agreed to pay royalties to Cato though 2016 on sales of NutreStore®. We agreed to pay royalties after the expiration of the sublicense agreement from 2012 to 2016 in consideration of the transfer of the NDA for Nutrestore® from Cato to us, the transfer of the Nutrestore® related trademarks from Cato to us, the transfer of the Nutrestore Drug Master File Nos. 16633 and 16639 from Cato to us, and the services provided by Cato related to the filing of a new Drug Master File (“DMF”) and for the know-how represented by the Nutrestore NDA and DMFs.
The above description of the sublicense agreement and the assignment and transfer agreement is qualified in its entirety by reference to the complete text of the agreements, which are attached hereto as Exhibits 10.7 and 10.8 respectively, and are incorporated by reference herein. You are urged to read the entire agreements.
EMD Serono, Inc. granted us the exclusive right to promote Zorbtive® in the United States in December 2008 pursuant to a promotional rights agreement executed on March 12, 2008. Pursuant to the agreement, EMD Serono provided Emmaus with the exclusive right to market Zorbtive® in North America during the license period. We use the same sales force to promote and market Zorbtive® and NutreStore®. While we have the exclusive right to promote and market Zorbtive® in the United States, EMD Serono actually sells the product. The promotional rights agreement provides that no royalties are payable from EMD Serono to us until unit sales exceed 16,016. The threshold has not yet been met and we have received no royalties pursuant to the agreement. After the unit sales exceed the threshold, EMD Serono is required to pay us a commission equal to $300,000, plus 35% of annual net sales from unit sales that exceed 16,017 but are less than or equal to 32,032 units; 50% of annual net sales from unit sales that exceed 32,033 but are less than or equal to 80,080 units; and 60% of annual net sales from unit sales that exceed 80,080 units. The parties have provided cross indemnification agreements and terms for maintenance of insurance for potential claims. The agreement terminates upon expiration of U.S. Patent No. 5,288,703, which expires on October 7, 2011.
We do not anticipate that the expiration of the promotional rights agreement or the sublicense agreement with Cato in October 2011 will have a significant impact on the Company. We still intend to sell Nutrestore and promote Zorbtive upon the expiration of the agreement. Based on the financial terms in the promotional rights agreement, we have not received any revenue from the promotion of Zorbtive®. We can continue to sell NutreStore® without the promotional rights agreement. Upon the expiration of the agreements, we currently do not believe there should be significant competition in the marketplace for a generic version of Nutrestore® given the small population of SBS patients (<10,000 adults).
On April 8, 2011, Emmaus Medical entered into a Joint Research and Development Agreement (the “Research Agreement”) and an Individual Agreement (the “Individual Agreement”) with CellSeed. Pursuant to the Research Agreement, the Company and CellSeed formed a relationship regarding the future research and development of cell sheet engineering regenerative medicine products (the “Products”), and the future commercialization of such Products, particularly Emmaus Medical and CellSeed are interested in the joint research and development of Cultured Autologous Oral Mucosal Epithelial Cell-Sheet (“CAOMECS”) for generated medicine of cornea cells, and potentially Cell-Sheets for Cardiac Muscle Regeneration, and Regenerated Cartilage Sheets. The parties will enter into individual agreements for each project or task conducted pursuant to the Research Agreement defining the details of such project. CellSeed will transfer to Emmaus U.S. laboratories the engineering and know-how technology necessary for Emmaus to create cell sheets under each individual agreement. All intellectual property rights created in the course of the Research
19
Agreement and any individual agreement, including rights made jointly by the employees of the Company and CellSeed or made solely by the employee(s) of the other party based on confidential information or intellectual property rights exchanged between the parties, will be owned jointly by the Company and CellSeed. Intellectual property rights related to the Products that are developed solely by one party’s employees independently from confidential information and intellectual property rights of the other party, shall be owned by the party whose employees made such invention, provided however, that such party will grant a worldwide, perpetual, irrevocable, non-exclusive, royalty free, fully paid up, sub-licensable, transferable license of such rights to the other party. Pursuant to the Individual Agreement, CellSeed granted the Company an exclusive right to manufacture, sell, market and distribute CAOMECS for the cornea in the United States CellSeed shall disclose its accumulated information package (the “Package”) for the joint development of CAOMECS to Emmaus Medical. Pursuant to the Research Agreement, the Company agreed to pay CellSeed $8,500,000 (the “Research Agreement Payment”) within 30 days of the completion of all of the following: (i) the execution of the Research Agreement; (ii) the execution of the Individual Agreement; and (iii) CellSeed’s delivery of the Package to Emmaus. Pursuant to the Individual Agreement, the Company agreed to pay $1,500,000 to CellSeed (the “Individual Agreement Payment”) within 30 days of CellSeed’s delivery of the Package to the Company and a royalty to be agreed upon by the parties. Pursuant to the Addendum to the Research Agreement, CellSeed and the Company further acknowledged that all obligations of CellSeed corresponding to the Research Agreement Payment and the Individual Agreement Payment shall be fully discharged by CellSeed’s delivery of the Package, which delivery shall be documented by written confirmation of acceptance by the Company. The Company is obligated to make the Research Agreement Payment and the Individual Agreement Payment only after the Company’s provides its written confirmation of acceptance of the complete Package to CellSeed. The parties will determine the rate at which profits from the net sales of CAOMECS in the United States will be split between the parties. The Individual Agreement will remain in effect until CellSeed’s patents used for the CAOMECS expire in the United States in February 2023 and February 2024, unless terminated earlier by the parties.
Cell sheet technology is a method that allows us to grow and harvest tissue in a sheet form rather than individual cells. Clinical trials to repair damaged cornea and heart muscle using cell sheets have been initiated in Japan and Europe. In Europe, multi-center clinical trials to repair damaged cornea with patients’ own oral mucosal cells are being prepared in consultation with the European Medicines Agency (“EMEA”) using the cell sheet technology. A relatively large single center clinical trial entitled “Multicenter Study of Cultured Autologous Oral Mucosal Epithelial Cell-sheet (CAOMECS) Transplantation to Patients with Total Limbal Stem Cell Deficiency,” was conducted from 2007 to 2011 at the Les Hospices Civils De Lyon in France involving 25 patients with limbal stem cell deficiencies who have suffered loss of vision. The results of the study, which have not yet been published, produced an abundance of positive data indicating improvement in symptoms for patients with “limbal stem cell deficiency” (“LSCD”). Based on this data, management hopes that this treatment can potentially provide a cure for a group of patients diagnosed with LSCD, which was considered incurable. An interim report of the results of the study was presented at the World Cornea Congress in April 2010. CellSeed issued a press release in April 2010 regarding such interim report which can be viewed on CellSeed’s website. The final report for the trial is currently being reviewed by the EMEA. Upon the EMEA’s completion of its review, the results of the completed review will be announced on the EMEA’s website and a summary of the study is anticipated to be announced on CellSeed’s website at that time. It is anticipated that the results of the study will be published in a scientific journal after the EMEA completes its review; however, it is not known in which journal the results will be published. Upon the EMEA’s approval of the product, we intend to apply for FDA approval of the corneal cell sheets, for which we have the exclusive rights to manufacture, sell, market and distribute in the United States pursuant to the Individual Agreement.
Currently, cell sheets for cornea cells and cardiac cells are not being sold. The potential market for the cornea cell sheet products include patients with damaged corneas, which we believe includes a small percentage of the approximate 40,000 corneal transplants in the U.S. each year, which number is from the National Eye Institute section of the National Institutes of Health website. The market for cardiac cell sheets also includes the approximate 3,000 persons who are awaiting heart transplants in the United States, according to the section of the National Heart, Lung and Blood Institute’s website regarding heart transplants.
20
While many large pharmaceutical and biotechnology companies throughout the world, especially in Europe, have contracts with CellSeed to promote this technology, Emmaus is the first company to obtain the marketing and development contracts with CellSeed for this technology in the U.S. The principal steps to develop the corneal and cardiac cell sheets include engaging a manufacturer compliant with applicable cGMP, obtaining FDA approval of the products, training physicians who will use the products and perform procedures with the products and promoting the availability of the products. We believe the cost to develop this the corneal cell sheet technology in U.S. is approximately $13 million, which includes the $10 million in payments we have agreed to make to CellSeed pursuant to the Research Agreement and Individual Agreement and $3 million in research and development costs. Such estimate includes the cost of obtaining FDA approval for the cornea cell sheets. The FDA has different divisions that review different types of products and the type of approval required for a specific product depends on the classification of the product. Biological products, or biologics, include a wide range of things such as vaccines, blood and blood components, allergenics, somatic cells, gene therapy, tissues, and recombinant therapeutic proteins. Biologics can be composed of sugars, proteins, or nucleic acids or complex combinations of these substances, or may be living entities such as cells and tissues. The review and approval process for biologics (“biologic approval”) is handled by the FDA’s Center for Biologics Evaluation and Research (CBER). Pharmaceutical drugs are typically chemically synthesized and have a known structure; whereas, most biologics are complex mixtures that are not easily identified or characterized. The review and approval process for pharmaceuticals (“pharmaceutical approval”) is handled by the FDA’s Center for Drug Evaluation and Research (CDER). Although the review process for biologics and pharmaceuticals is handled by different divisions, the regulatory pathway is virtually the same. We have assumed that we will need biologic approval from the CBER division of the FDA for the cornea cell sheets, rather than pharmaceutical approval from the CDER division, although because the review and approval process for biologics and pharmaceuticals is virtually the same, the type of approval required for the cell sheets should not affect our proposed activities with respect to the cell sheets. We have also assumed that we will only have to run a small trial here in the U.S. to test the safety and efficacy of the corneal cell sheets because we believe that we will be able to submit, and the FDA will accept, the data regarding corneal cell sheets submitted to the EMEA. We estimate that we will need another $2 million to commercialize the corneal cell sheet technology. Due to abundant data available for cornea treatment using this technology, we anticipate it will be two to three years before we can commercialize this product in U.S. For heart treatments, we plan to participate in a multi-center international study within the next two years and anticipate that we will be able to commercialize these products in about five years. We currently do not have an estimate of the estimated costs to develop and commercialize cardiac cell sheets.
Trademarks
We currently own 3 U.S. trademarks, including “Emmaus Medical,” “NutreStore” and “AminoPure” and one Japanese trademark for “AminoPure.”
Our success will depend in part on our ability to obtain patents and preserve other intellectual property rights covering the design and operation of our products. We intend to seek patents on our products when we deem it commercially appropriate. The process of seeking patent protection can be lengthy and expensive, and there can be no assurance that patents will be issued for currently pending or future applications or that our existing patents or any new patents issued will be of sufficient scope or strength or provide meaningful protection or any commercial advantage to us. We may be subject to, or may initiate, litigation or patent office interference proceedings, which may require significant financial and management resources. The failure to obtain necessary licenses or other rights or the advent of litigation arising out of any such intellectual property claims could have a material adverse effect on our operations.
Competition
The development and commercialization of pharmaceutical products is very competitive and characterized by extensive research efforts and rapid technological progress. Competition in our industry occurs on a number of fronts, including developing and bringing new products to market before our competitors, developing new products to provide the same benefits as existing products at lower cost and developing new products to provide benefits superior to those of existing products. We face competition from other pharmaceutical companies, particularly those that provide alternative drugs to treat SCD and SBS, as well as other entities that develop alternative therapies that could limit the market for our L-glutamine product.
We currently face two competing treatments for SCD treatment, one of which being Bristol-Myers Squibb’s Hydroxyurea and the other being bone marrow transplants. Additionally, gene therapy techniques hold promise as a potential treatment for a variety of genetic diseases, including SCD, however, there are currently many questions about the efficacy of gene therapy and when such therapies could become available to treat diseases such as SCD.
21
Presently, the most prevalent therapy for patients with SBS is parenteral nutrition. However, as outlined above, Emmaus’ products NutreStore® and Zorbtive® can be used to reduce the volume and frequency of parenteral nutrition therapy for most patients.
Because L-glutamine is currently sold as a nutritional supplement, there is risk that both of the Company’s pharmaceutical products for treatment of SCD and SBS may experience competition with providers of L-glutamine in nutritional supplement form. In fact, when dealing with a method patent directed to new uses for old compounds, there is always a risk that the medication can be obtained from unauthorized sources, and sold at cut-rate prices, known as the “generic leakage” problem. More generally, generic leakage results when a barrier to competition, e.g. a patent expires or is invalidated, suddenly opening up the formerly price protected (and relatively expensive) product to competition from relatively inexpensive generic products. As a result, consumers will tend to purchase more of the cheaper generic product and less of the expensive product. However, the Company believes generic leakage will not be a major factor for a number of reasons, including but not limited to insurance/reimbursement factors, pricing strategies, regulatory barriers to market entry, distribution mechanisms, FDA-administered market exclusivity protections, intellectual property protections, and other factors inherent in the FDA regulatory differences between pharmaceuticals and nutritional supplements. The FDA-administered market exclusivity protection that we will have is tied to the orphan drug status designation granted by the FDA. This provides for an additional seven (7) years of market exclusivity.
Our competitors may have products that have been approved or are in advanced development and may succeed in developing drugs that are more effective, safer and more affordable or more easily administered than ours or that achieve commercialization sooner than our products.
Employees
As of December 31, 2010, we had 11 employees, 10 of which are full time, as well as two independent sales representatives and four consultants. We have not experienced any work stoppages and we consider our relations with our employees to be good.
Properties
We lease approximately 4,540 square feet of office space at our headquarters at 20725 S. Western Avenue, Ste. 136, Torrance, CA 90501-1884, at a base rent of $5,552 per month. This lease, which was to expire on May 31, 2011, was extended by the parties for an additional term beginning on June 1, 2011 and expiring on May 31, 2012. During the extension period, the monthly rent will be $4,994. In addition, we lease two warehouse suites at 3870 Del Amo Boulevard, Torrance California under two separate leases: Suite 506 (approximately 1,400 square feet) at a base rent of $1,610 per month; and Suite 507 (approximately 1,300 square feet) at a base rent of $1,690 per month. The lease for Suite 506 will expire on August 19, 2011; the lease for Suite 507 will expire on February 28, 2013. Approximately 490 square feet of Suite 506 and 480 square feet of Suite 507 are currently subleased to an unaffiliated entity on a month to month basis. We do not expect to experience any difficulties in renewing our leases, or finding additional or replacement office and warehouse space, at their current or more favorable rates.
The 4,540 square feet office space at 20725 S. Western Ave. is adequate and suitable for our corporate headquarters and we currently use all of such office space. Additionally, the two warehouse facilities at 3870 Del Amo Blvd., a total of 2,700 square feet, are adequate and suitable for the storage and distribution of the sickle cell study medication, study placebo and AminoPure®. Suite 506 at this location is used to store and distribute our SCD study medication and study placebo and we are able to store all study medication and study placebo required at this location. Suite 507 is used to store AminoPure and can store up to a six month supply of AminoPure, which is adequate for operations.
Legal Proceedings
We are not involved in any material legal proceedings outside of the ordinary course of our business.
22
RISK FACTORS
Any investment in our common stock involves a high degree of risk. Investors should carefully consider the risks described below and all of the information contained in this report before deciding whether to purchase our common stock. Our business, financial condition or results of operations could be materially adversely affected by these risks if any of them actually occur. Our shares of common stock are not currently listed or quoted for trading on any national securities exchange or national quotation system. If and when our common stock is traded, the trading price could decline due to any of these risks, and an investor may lose all or part of his or her investment. Some of these factors have affected our financial condition and operating results in the past or are currently affecting us. This Current Report on Form 8-K also contains forward-looking statements that involve risks and uncertainties. Our actual results could differ materially from those anticipated in these forward-looking statements as a result of certain factors, including the risks described below and elsewhere in this report.
We have incurred losses since inception, have limited cash resources and anticipate that we will continue to incur substantial losses for the foreseeable future.
Emmaus Medical is still in the development stage. As of March 31, 2011, we had an accumulated deficit of $13.9 million since our inception in 2000. Our net losses were $3.7 million and $2.6 million for the years ended December 31, 2010 and 2009, respectively, and $1.1 million and $0.7 million for the three months ended March 31, 2011 and 2010, respectively. These losses resulted principally from costs incurred in our research and development programs and from our general and administrative expenses. We have had limited revenue, have sustained significant operating losses, and are likely to sustain operating losses in the foreseeable future. Since inception, we have funded our operations though the private placement of equity securities, convertible notes and loans from stockholders and expect that we will continue to fund our operations through public or private equity or debt financings or other sources, such as strategic partnerships. Such financings may not be available in amounts or on terms acceptable to us, if at all. Our failure to raise capital as and when needed would inhibit our ability to continue operations and implement our business strategies.
We expect to continue to incur significant and increasing negative cash flow and operating losses as we continue our research activities, conduct clinical trials, and seek regulatory approvals for our L-glutamine treatment for SCD. These losses, among other things, have had and will continue to have an adverse effect on our stockholders’ equity, total assets and working capital. Because of the numerous risks and uncertainties associated with drug development, we are unable to predict the extent of any future losses, whether or when we will be able to commercialize our L-glutamine treatment for SCD, or when we will become profitable, if at all. Even if we do achieve profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis.
Our recurring operating losses have raised substantial doubt regarding our ability to continue as a going concern.
Our recurring operating losses raise substantial doubt about our ability to continue as a going concern. As a result, our independent registered public accounting firm included an explanatory paragraph in its report on our financial statements as of and for the year ended December 31, 2010 with respect to this uncertainty. The perception of our ability to continue as a going concern may make it more difficult for us to obtain financing for the continuation of our operations and could result in the loss of confidence by investors, suppliers and employees.
We will require substantial additional funding and may be unable to raise capital when needed, which could force us to delay, reduce or eliminate planned activities or result in our inability to continue as a going concern.
We will require additional capital to pursue planned clinical trials and regulatory approvals, as well as further research and development and marketing efforts for our products and potential products. Our future capital requirements will depend on, and could increase significantly as a result of, many factors, including:
23
| ● | the duration and results of the clinical trials for our various products going forward; | |
| ● | unexpected delays or developments in seeking regulatory approvals; | |
| ● | the time and cost in preparing, filing, prosecuting, maintaining and enforcing patent claims; | |
| ● | other unexpected developments encountered in implementing our business development and commercialization strategies; and | |
| ● | the outcome of litigation, if any, and further arrangements, if any, with collaborators. |
We may attempt to raise additional funds through public or private financings, collaborations with other pharmaceutical companies or financing from other sources. Additional funding may not be available on terms which are acceptable to us. If adequate funding is not available to us on reasonable terms, we may need to delay, reduce or eliminate one or more of our product development programs or obtain funds on terms less favorable than we would otherwise accept. To the extent that additional capital is raised through the sale of equity securities or securities convertible into or exchangeable for equity securities, the issuance of those securities could result in dilution to our stockholders. Moreover, the incurrence of debt financing could result in a substantial portion of our future operating cash flow, if any, being dedicated to the payment of principal and interest on such indebtedness and could impose restrictions on our operations. This could render us more vulnerable to competitive pressures and economic downturns.
If we are unsuccessful in raising additional required funds, we may be required to delay, scale-back or eliminate plans or programs relating to our business. In addition, if we do not meet our payment obligations to third parties as they come due, we may be subject to litigation claims. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to management, and may result in unfavorable results that could further adversely impact our financial condition.
Raising additional capital may cause dilution to our existing stockholders, restrict our operations or require us to relinquish rights.
We may seek additional capital through a combination of private and public equity offerings, debt financings and collaborations and strategic and licensing arrangements. To the extent that we raise additional capital through the sale of equity or convertible debt securities, your ownership interest in us will be diluted, and the terms may include liquidation or other preferences that adversely affect your rights as a stockholder. Debt financing, if available, would result in increased fixed payment obligations and may involve agreements that include covenants limiting or restricting our ability to take specific actions such as incurring debt, making capital expenditures or declaring dividends. If we raise additional funds through collaboration, strategic alliance and licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams or product candidates, or grant licenses on terms that are not favorable to us.
Our business is subject to extensive government regulation, which could cause delays in the development and commercialization of our drug products, impose significant costs on us or provide advantages to our larger competitors.
The FDA and similar agencies in foreign countries impose substantial requirements upon the development, manufacture and marketing of drugs. They require laboratory and clinical testing procedures, manufacturing, labeling, registration, notification, clearance or approval, marketing, distribution, recordkeeping, reporting and promotion, and other costly and time-consuming procedures. Satisfaction of clearance or approval requirements typically takes several years or more and varies substantially from country to country as well as upon the type, complexity and novelty of the therapeutic product.
The effect of government regulation may be to delay marketing of products for a considerable or indefinite period of time, to impose costly procedures upon our activities and to furnish a competitive advantage to larger companies that compete with us. There can be no assurance that the FDA or other regulatory clearance or approval for any products developed by us will be granted on a timely basis, if at all, or, once granted, that clearances or approvals will not be withdrawn or other regulatory actions taken which might limit our ability to market our proposed products. Any such delay in obtaining or failure to obtain such clearance or approvals would adversely affect us, the manufacturing and marketing of the products we intend to develop and our ability to generate product revenue.
24
We cannot assure you that we will be able to complete our clinical trial programs successfully within any specific time period, or if such clinical trials take longer to complete than we project, our ability to execute our current business strategy will be adversely affected.
We do not know if our current clinical trials for our L-glutamine treatment for SCD will be completed on schedule or at all. Even if completed, we do not know if these trials will produce clinically meaningful results sufficient to support an application for marketing approval. Whether or not and how quickly we complete clinical trials is dependent in part upon the rate at which we are able to obtain regulatory clearance to commence clinical trials, engage clinical trial sites and medical investigators, reach agreement on acceptable clinical trial agreement terms or clinical trial protocols with medical investigators or clinical trial sites or institutional review boards and, thereafter, the rate of enrollment of patients, and the rate to collect, clean, lock and analyze the clinical trial database.
Patient enrollment is a function of many factors, including the design of the protocol, the size of the patient population, the proximity of patients to and availability of clinical sites, the eligibility criteria for the study, the perceived risks and benefits of the drug under study and of the control drug, if any, the efforts to facilitate timely enrollment in clinical trials, the patient referral practices of physicians, the existence of competitive clinical trials, and whether existing or new drugs are approved for the indication. If we experience delays in identifying and contracting with sites and/or in patient enrollment/completion in our clinical trial programs, we may incur additional costs and delays in our development programs, and may not be able to complete our clinical trials on a cost-effective or timely basis. Accordingly, we may not be able to complete the clinical trials within an acceptable time frame, if at all. If we or any third party have difficulty obtaining clinical drug materials or enrolling a sufficient number of patients to conduct its clinical trials as planned, or if enrolled patients do not complete the trial as planned, we or a third party may need to delay or terminate ongoing clinical trials, which could negatively affect our business.
Clinical trials often require the enrollment of large numbers of patients, and suitable patients may be difficult to identify and recruit. Our ability to enroll sufficient numbers of patients in our clinical trials depends on many factors, including the size of the patient population, the nature and design of the protocol, the proximity of patients to clinical sites, the eligibility criteria for the trial, competing clinical trials and the availability of approved effective drugs. In addition, patients may withdraw from a clinical trial or be unwilling to follow our clinical trial protocols for a variety of reasons. If we fail to enroll and maintain the number of patients for which the clinical trial was designed, the statistical power of that clinical trial may be reduced which would make it harder to demonstrate that the product candidate being tested in such clinical trial is safe and effective. Additionally, we may not be able to enroll a sufficient number of qualified patients in a timely or cost-effective manner.
The drug development process to obtain FDA approval is very costly and time consuming and if we cannot complete our clinical trials in a cost-effective manner, our results of operations may be adversely affected.
Even with the granting of orphan drug status and fast track designation, the cost associated with the successful development of the L-glutamine treatment for SCD is uncertain. Costs of clinical trials may vary significantly over the life of a project owing but not limited to the following:
| ● | the duration of the clinical trial; | |
| ● | the number of sites included in the trials; | |
| ● | the countries in which the trial is conducted; | |
| ● | the length of time required to enroll eligible patients; | |
| ● | the number of patients that participate in the trials; | |
| ● | the number of doses that patients receive; | |
| ● | the drop-out or discontinuation rates of patients; | |
| ● | per patient trial costs; | |
| ● | potential additional safety monitoring or other studies requested by regulatory agencies; | |
| ● | the duration of patient follow-up; | |
| ● | the efficacy and safety profile of the product candidate; | |
| ● | the costs and timing of obtaining regulatory approvals; and | |
| ● | the costs involved in enforcing or defending patent claims or other intellectual property rights. |
25
If we are unable to control the costs of our clinical trials and conduct our trials in a cost-effective manner, our results of operations may be adversely affected.
We may be required to suspend, repeat or terminate our clinical trials if they do not meet regulatory requirements, the results are negative or inconclusive or adversely affect the necessary human subject protections, or if the trials are not well designed, which may result in significant negative repercussions on our business and financial condition.
We must be evaluated in light of the uncertainties and complexities affecting a development stage company. Our L-glutamine treatment for SCD, which is currently our only product in development, has not yet received regulatory approval for its intended commercial sale. We cannot market a pharmaceutical product in any jurisdiction until it has completed rigorous preclinical testing and clinical trials and passed such jurisdiction’s extensive regulatory approval process. Pre-clinical testing and clinical development are long, expensive and uncertain processes. Data obtained from pre-clinical and clinical tests can be interpreted in different ways, which could delay, limit or prevent regulatory approval. It may take us many years to complete the testing of our products and failure can occur at any stage of this process. We cannot provide assurance that our authorized clinical testing will be completed successfully within any specified time period by us, or without significant additional resources or expertise to those originally expected to be necessary. We cannot provide assurance that such testing will show potential products to be safe and efficacious or that any such product will be approved for a specific indication. Results from early clinical trials may not be indicative of the results that will be obtained in later-stage clinical trials. In addition, negative or inconclusive results from the clinical trials we conduct or adverse medical events could cause us to have to suspend, repeat or terminate the clinical trials. Clinical trials are subject to continuing oversight by governmental regulatory authorities and institutional review boards and must meet the requirements of these authorities and requirements for informed consent and good clinical practices and we cannot guarantee that we will be able to comply with such requirements. We will rely on third parties, such as contract research organizations and/or co-operative groups, to assist us in overseeing and monitoring clinical trials as well as to process the clinical results and manage test requests, which may result in delays or failure to complete trials, if the third parties fail to perform or to meet the applicable standards. A failure by us or such third parties to keep to the terms of a product development program for any particular product candidate or to complete the clinical trials for a product candidate in the envisaged time frame could have a significant negative effect on our business and financial condition.
There are known adverse side effects to our Zorbtive® and Nutrestore® products.
We market and/or sell two prescription pharmaceutical products that have received FDA approval: NutreStore® [L-glutamine powder for oral solution] and Zorbtive® [somatropin (rDNA origin) for injection], as a treatment for SBS. Reported side effects of NutreStore® include, but are not limited to, the urge to empty bowels, gas, abdominal pain, vomiting and hemorrhoids. Common side effects of Zorbtive® include, but are not limited to, muscle and joint pain and fluid retention or swelling. Zorbtive® may also cause serious side effects such as inflammation of the pancreas (pancreatitis), diabetes or other blood sugar problems, pain, numbness or tingling in the wrist and hand, or increased blood pressure in the brain. Any of these known side effects and any associated warning statements or labeling requirements may limit the commercial profile of these products and prevent us from achieving or maintaining market acceptance of such products.
Changes in regulatory requirements and guidance or unanticipated events during our clinical trials may occur, which may result in necessary changes to clinical trial protocols, which could result in increased costs to us, delay our development timeline or reduce the likelihood of successful completion of the clinical trial.
Changes in regulatory requirements and guidance or unanticipated events during our clinical trials may occur, as a result of which we may need to amend clinical trial protocols. If we experience delays in completion of, or if we terminate, any of our clinical trials, the commercial prospects for our L-glutamine treatment for SCD may be harmed and our ability to generate product revenue will be delayed, possibly materially.
26
Even if we are able to develop our L-glutamine treatment for SCD, we may not be able to receive regulatory approval, or if approved, we may not be able to generate significant revenues or successfully commercialize our L-glutamine treatment for SCD, which would adversely affect our financial results and financial condition.
Although our L-glutamine treatment for SCD is in Phase III clinical trials, it will still require regulatory approval before we can market it. We cannot predict the outcome of our Phase III clinical trial of this product and cannot assure you that we will obtain the necessary regulatory approvals. There are many reasons that we may fail in our efforts to develop and commercialize our L-glutamine treatment for SCD and other drug product candidates, including:
| ● | the chance that our preclinical testing or clinical trials could show that our L-glutamine treatment for SCD or other drug product candidates are ineffective an/or cause harmful side effects; | |
| ● | the failure of our drug product candidates to receive necessary regulatory approvals from the FDA or foreign regulatory authorities in a timely manner, or at all; | |
| ● | the failure of our drug product candidates, once approved, to be produced in commercial quantities or at reasonable costs; | |
| ● | physicians’ reluctance to switch from existing treatment methods, including traditional therapy agents, to our products; | |
| ● | the failure of our drug product candidates, once approved, to achieve commercial acceptance; | |
| ● | the introduction of products by our competitors that are more effective or have a different safety profile than our products; | |
| ● | the application of restrictions to our drug product candidates by regulatory or governmental authorities; | |
| ● | the proprietary rights of other parties preventing us or our potential collaborative partners from marketing our drug product candidates; | |
| ● | the possibility that we may not be able to maintain the orphan drug designation or obtain orphan drug exclusivity for our product; and | |
| ● | the possibility that our fast track designation may not actually lead to a faster development or regulatory review or approval process. |
Even if the FDA and other regulatory authorities approve our L-glutamine treatment for SCD or any of our products, the manufacture, packaging, labeling, distribution, marketing and sale of such products will be subject to strict and ongoing regulation. Compliance with such regulation will be expensive and consume substantial financial and management resources. The FDA has the authority to regulate the claims we make in marketing our prescription drug products to ensure that such claims are true, not misleading, supported by scientific evidence and consistent with the labeled use of the drug. Failure to comply with FDA requirements in this regard could result in, among other things, warning letters, suspensions of approvals, seizures or recalls of products, injunctions against a product’s manufacture, distribution, sales and marketing, operating restrictions, civil penalties and criminal prosecutions. Additionally, an approval for a product may be conditioned on our agreement to conduct costly post-marketing follow-up studies to monitor the safety or efficacy of the products. In addition, as a clinical experience with a drug expands after approval because the drug is used by a greater number and more diverse group of patients than during clinical trials, side effects or other problems may be observed after approval that were not observed or anticipated during pre-approval clinical trials. In such a case, a regulatory authority could restrict the indications for which the product may be sold or restrict the distribution channels or revoke the product’s regulatory approval, which could hinder our ability to generate revenues from our products. If we fail to develop and commercialize our drug product candidates as planned, our financial results and financial condition will be adversely affected, we will have to delay or terminate some or all of our research product development programs and may be forced to cease operations.
27
We are subject to various regulations pertaining to healthcare fraud and abuse, violations of which could have a material adverse affect on our business.
We are subject to various federal and state laws pertaining to healthcare fraud and abuse, including inducing, facilitating or encouraging submission of false claims to government programs and prohibitions on the offer or payment or acceptance of kickbacks or other remuneration for the purchase of our products. Specifically, these anti-kickback laws make it illegal for a prescription drug manufacturer to solicit, offer, or pay any remuneration in exchange for purchasing, leasing or ordering any service or items including the purchase or prescribing of a particular drug for which payment may be made under a federal healthcare program. Because of the sweeping language of the federal anti-kickback statute, many potentially beneficial business arrangements would be prohibited if the statute were strictly applied. To avoid this outcome, the Department of Health and Human Services has published regulations, known as “safe harbors,” that identify exceptions or exemptions to the statute’s prohibitions. Arrangements that do not fit within the safe harbors are not automatically deemed to be illegal, but must be evaluated on a case by case basis for compliance with the statute. We seek to comply with anti-kickback statutes and if necessary to fit within one of the defined “safe harbors”; we are unaware of any violations of these laws. However, due to the breadth of the statutory provisions and the absence of uniform guidance in the form of regulations or court decisions, there can be no assurance that our practices will not be challenged under anti-kickback or similar laws. Violations of such restrictions may be punishable by civil or criminal sanctions, including fines and civil monetary penalties, as well as the possibility of exclusion from U.S. federal healthcare programs (including Medicaid and Medicare). Any such violations could have a material adverse effect on our business, financial condition, results or operations and cash flows.
In addition, the FDA has the authority to regulate the claims we make in marketing our prescription drug products to ensure that such claims are true, not misleading, supported by scientific evidence and consistent with the labeled use of the drug. Failure to comply with FDA requirements in this regard could result in, among other things, warning letters, suspensions of approvals, seizures or recalls of products, injunctions against a product’s manufacture, distribution, sales and marketing, operating restrictions, civil penalties and criminal prosecutions. Any of these FDA actions could negatively impact our product sales and profitability.
If the manufacturers upon whom we rely fail to produce in the volumes and quality that we require on a timely basis, or to comply with stringent regulations applicable to pharmaceutical manufacturers, we may face delays in the development and commercialization of, or be unable to meet demand for, our products, if any, and may lose potential revenues.
We do not currently have or intend to develop our own manufacturing capabilities. We intend to enter into various arrangements with contract manufacturers and others to manufacture our products and, thus, will significantly depend upon the subsequent success of these outside parties in performing their manufacturing responsibilities. The manufacture of pharmaceutical products requires significant expertise and capital investment, including the development of advanced manufacturing techniques and process controls. Manufacturers of pharmaceutical products may encounter difficulties in production, including problems with quality control, quality assurance testing, shortages of qualified personnel, and compliance with strictly enforced federal, state and foreign regulations. Our third-party manufacturers and key suppliers may experience manufacturing difficulties due to resource constraints or as a result of labor disputes, unstable political environments at foreign facilities or financial difficulties. If these manufacturers or key suppliers were to encounter any of these difficulties, or otherwise fail to comply with their contractual obligations, our ability to timely launch any potential product candidate, if approved, would be jeopardized.
We currently obtain our pharmaceutical grade L-glutamine from two Japanese companies, which together produce the vast majority of pharmaceutical grade L-glutamine approved for sale in the U.S., and obtain all of our L-glutamine for our NutreStore product from one of these companies. If these suppliers were to experience any manufacturing or production difficulties producing pharmaceutical grade L-glutamine, our ability to complete our clinical trials, to commercialize L-glutamine for the treatment of SCD and to continue to sell NutreStore would be harmed.
28
We intend to enter into long term supply agreements with one or more manufacturers for our products. There can be no assurance that we will be successful in entering into such long term supply contracts, or that such contracts will be at prices that are acceptable to us. Our manufacturing partners may not be able to expand capacity or to produce additional product requirements for us in the event that demand for our products increases. There can be no assurance that we or our manufacturers will be able to continue purchasing products from current suppliers or any other supplier on terms similar to current terms or at all. Any interruption in the availability of certain raw materials or ingredients, or significant increases in the prices paid by us for them, could have a material adverse effect on its business, financial condition, liquidity and operating results.
In addition, all manufacturers and suppliers of pharmaceutical products must comply with applicable current good manufacturing practice, or cGMP, regulations for the manufacture of our products, which are enforced by the FDA through its facilities inspection program. The FDA is likely to conduct inspections of our third party manufacturer and key supplier facilities as part of the Agency’s review of any of our NDAs and its ongoing compliance programs. If our third party manufacturers and key suppliers are not in compliance with cGMP requirements, it may result in a delay of approval for products undergoing regulatory review or the inability to meet market demands for approved, marketed products, particularly if these sites are supplying single source ingredients required for the manufacture of any potential product. These cGMP requirements include quality control, quality assurance and the maintenance of records and documentation, among other items. Furthermore, regulatory qualifications of manufacturing facilities are applied on the basis of the specific facility being used to produce supplies. As a result, if one of the manufacturers that we rely on shifts production from one facility to another, the new facility must go through a complete regulatory qualification and be approved by regulatory authorities prior to being used for commercial supply. Our manufacturers may be unable to comply with these cGMP requirements and with other FDA, state and foreign regulatory requirements. A failure to comply with these requirements may result in fines, product recalls or seizures and related publicity requirements, injunctions, total or partial suspension of production, civil penalties, suspension or withdrawals of previously granted regulatory approvals, warning or untitled letters, refusal to approve pending applications for marketing approval of new products or of supplements to approved applications, import or export bans or restrictions, and criminal prosecution and penalties. Any of these penalties could delay or prevent the promotion, marketing or sale of our products. If the safety of any quantities supplied is compromised due to a third party manufacturer’s or key supplier’s failure to adhere to applicable laws or for other reasons, we may not be able to obtain regulatory approval for or successfully commercialize our products.
The failure of our products to gain market acceptance will hinder our ability to generate revenues from the sale of our products.
Even if our products are approved for commercialization, they may not be successful in the marketplace. Market acceptance of any of our products will depend on a number of factors including, but not limited to: demonstration of clinical efficacy and safety; the prevalence and severity of any adverse side effects; limitations or warnings contained in the product’s approved labeling; availability of alternative treatments for the indications; the advantages and disadvantages of our products relative to current or alternative treatments; the availability of acceptable pricing and adequate third-party reimbursement; and the effectiveness of marketing and distribution methods for the products.
If our products do not gain market acceptance among physicians, patients, treatment centers, healthcare payors and others in the medical community, which may not accept or utilize our products, our ability to generate significant revenues from its products would be limited and our financial conditions will be materially adversely affected. In addition, if we fail to successfully penetrate our core markets and successfully expand our business into new markets, the growth in sales of our products, along with its operating results, could be negatively impacted.
Our ability to successfully penetrate our core markets in which we compete or to successfully expand our business into additional countries in Africa, Europe, Asia or elsewhere is subject to numerous factors, many of which are beyond our control. Our products, if successfully developed, may compete with a number of drugs and therapies currently manufactured and marketed by major pharmaceutical companies. Our products may also compete with new products currently under development by others or with products which may be less expensive than our products. There is no assurance that our efforts to increase market penetration in our core markets and existing geographic markets will be successful. Our failure to do so could have an adverse effect on our operating results.
29
We lack experience in commercializing products, which may have an adverse effect on our business.
We will need to transition from a company with a development focus to a company capable of supporting commercial activities. We may not be successful in such a transition. We have not yet demonstrated an ability to obtain marketing approval for product candidates and have limited experience in commercializing products. As a result, we may not be as successful as companies that have previously obtained marketing approval for drug candidates and have more experience commercially launching drugs.
We are required to make significant payments to CellSeed pursuant to the Research Agreement and the Individual Agreement, and if we cannot make such payments when due, CellSeed may terminate the agreements which will negatively impact our financial condition and our ability to implement our business strategies.
In April 2011, Emmaus Medical entered into the Research Agreement and the Individual Agreement with CellSeed and an addendum to the agreements in August 2011. Pursuant to the Research Agreement, the Company and CellSeed formed a relationship regarding the future research and development of cell sheet engineering regenerative medicine products, and the future commercialization of such products. Pursuant to the Individual Agreement, CellSeed granted us the exclusive right to manufacture, sell, market and distribute CAOMECS for the cornea in the United States and agreed to disclose its accumulated information package for the joint development of CAOMECS to us. Under the Research Agreement, as supplemented by the addendum, we agreed to pay CellSeed $8.5 million within 30 days of the completion of all of the following: (i) the execution of the Research Agreement; (ii) the execution of the Individual Agreement; and (iii) CellSeed’s delivery of the accumulated information package to Emmaus and Emmaus providing written confirmation of its acceptance of the complete accumulated information package. Under the Individual Agreement, we agreed to pay CellSeed $1.5 million within 30 days of CellSeed’s delivery of the accumulated information package to us and a royalty to be agreed upon by the parties. We have had limited revenue, have sustained significant operating losses, and are likely to sustain operating losses in the foreseeable future. As of June 30, 2011, we had cash and cash equivalents of approximately $0.4 million. We anticipate that we will continue to fund our operations through public or private equity or debt financings or other sources, such as strategic partnership agreements. Such financings may not be available in amounts or terms acceptable to us, if at all. Our failure to raise capital as and when needed could impact our ability to pay the amounts required under the Research Agreement and Individual Agreement to CellSeed. If we are unable to pay the amounts required, CellSeed could terminate the agreements which would negatively impact on our ability to pursue our business strategies.
There are various uncertainties related to the research, development and commercialization of cell sheet engineering regenerative medicine products pursuant to the Research Agreement and Individual Agreement which could negatively affect our ability to commercialize such products.
We have historically focused on the research and development of our L-glutamine treatment for SCD and have no experience in the research, development or commercialization of cell sheet regenerative medicine products. Such products would require FDA approval, however, it is uncertain what type of approval the FDA would require for such products or what type of scientific data would be required to provide the safety and efficacy of such products. Such uncertainties could delay our ability to obtain FDA approval for and to commercialize such products. In addition, the research and commercialization of cell sheet regenerative medicine products could be hindered if third party manufacturers of such products all out of compliance with cGMP regulations. Any delay in obtaining FDA approval or the occurrence of problems with third party manufacturers of cell sheet regenerative medicine products would negatively affect our ability to commercialize such products.
Failure to obtain acceptable prices or adequate reimbursement for our products may cause an adverse impact on our results of operations.
Our ability to successfully commercialize our products will depend significantly on our ability to obtain acceptable prices and the availability of reimbursement to the patient from third-party payors, such as governmental and private insurance plans. These third-party payors frequently require companies to provide predetermined discounts from list prices, and they are increasingly challenging the prices charged for pharmaceuticals and other medical products. Our products may not be considered cost-effective, and reimbursement to the patient may not be available or sufficient to allow us or our partners to sell our products on a competitive basis. It may not be possible to negotiate favorable reimbursement rates for our products.
30
Significant uncertainty exists as to the reimbursement status of newly approved healthcare products and third-party payers are increasingly challenging the prices charged for pharmaceuticals and other medical products. In addition, the continuing efforts of third-party payors to contain or reduce the costs of healthcare through various means may limit our commercial opportunity and reduce any associated revenue and profits. For example, in some foreign markets, the pricing or profitability of healthcare products is subject to government control. In other foreign markets, including Africa where the largest population of SCD patients exists, there is limited number of third party payors from which reimbursement can be sought. In the United States, there have been, and we expect there will continue to be, a number of federal and state proposals to implement similar government control, as evidenced by the passing of the Patient Protection and Affordable Care Act and its amendment, the Health Care and Education Reconciliation Act. Such government-adopted reform measures may adversely impact the pricing of healthcare products and services in the United States or internationally and the amount of reimbursement available from governmental agencies or other third party payors. In addition, increasing emphasis on managed care will continue to put pressure on the pricing of pharmaceutical products. Cost control initiatives could decrease the price that we or any current or potential collaborators could receive for any of our products and could adversely affect our profitability. If we fail to obtain acceptable prices or an adequate level of reimbursement for our products, the sales of our products would be adversely affected or there may be no commercially viable market for our products.
If we do not achieve our projected development goals in the time frames we expect and announce, the credibility of our management and our drug products may be adversely affected.
For business planning purposes, we estimate the timing of the accomplishment of various scientific, clinical, regulatory and other product development goals, which we sometimes refer to as milestones. These milestones may include the commencement or completion of preclinical studies and clinical trials and the submission of regulatory filings for our products.
From time to time, we may publicly announce the expected timing of some of these milestones. All of these milestones will be based on a variety of assumptions. The actual timing of these milestones can vary dramatically compared to our estimates, in many cases for reasons beyond our control. If we do not meet these milestones as publicly announced, our stockholders may lose confidence in our ability to meet these milestones and, as a result, the price of our common stock may decline.
If we do not obtain the support of new, and maintain the support of existing, key scientific collaborators, it may be difficult to research other medical indications for L-glutamine other than SCD and to expand our product offerings, which may limit our revenue growth and profitability and could have a material adverse effect on our business, financial condition and operating results.
We will need to establish relationships with additional leading scientists and research institutions in order to develop new products and expand our product offerings and to explore other medical indications for L-glutamine for SCD. We have donated NutreStore to a multicenter study in Canada and the U.S. for the treatment of burn victims with L-glutamine and intend to sponsor a study for the use of NutreStore to treat pediatric SBS patients. Although we have established research collaborations, we cannot assure you that our relationships with our research collaborators will continue or that we will be able to attract additional research partners. If we are not able to maintain existing or establish new scientific relationships to assist in our research and development, we may not be able to successfully develop our drug product candidates or expand our products offerings.
If our competitors succeed in developing products and technologies that are more effective than our own, or if scientific developments change our understanding of the potential scope and utility of our drug product candidates, then our technologies and future drug product candidates may be rendered less competitive.
We face significant competition from industry participants that are pursuing similar technologies that we are pursuing and are developing pharmaceutical products that are competitive with our drug product candidates. Nearly all of our industry competitors have greater capital resources, larger overall research and development staffs and facilities, and a longer history in drug discovery and development, obtaining regulatory approval and pharmaceutical product manufacturing and marketing than we do. With these additional resources, our competitors may be able to respond to the rapid and significant technological changes in the biotechnology and pharmaceutical industries faster than we can.
31
Our future success will depend in large part on our ability to maintain a competitive position with respect to these technologies. Rapid technological development, as well as new scientific developments, may result in our compounds, drug product candidates or processes becoming obsolete before we can recover any of the expenses incurred to develop them. For example, changes in our understanding of the appropriate population of patients who should be treated with a targeted therapy like we are developing may limit the drug’s market potential if it is subsequently demonstrated that only certain subsets of patients should be treated with the targeted therapy.
The use of any of our drug product candidates in clinical trials and in the market may expose us to liability claims.
The nature of our business exposes us to potential liability risks inherent in the testing, manufacturing and marketing of our drug product candidates. While we are in clinical stage testing, our drug product candidates could potentially harm people or allegedly harm people and we may be subject to costly and damaging product liability claims. Some of the patients who participate in clinical trials are already critically ill when they enter a trial. The waivers we obtain may not be enforceable and may not protect us from liability or the costs of product liability litigation. Although we currently carry a $5 million clinical product liability insurance policy, it may not be sufficient to cover future claims. We could be materially and adversely affected if we were required to pay damages or incur defense costs in connection with a claim outside the scope of indemnity or insurance coverage, if the indemnity is not performed or enforced in accordance with its terms, or if our liability exceeds the amount of applicable insurance. In addition, there can be no assurance that insurance will continue to be available on terms acceptable to us, if at all, or that if obtained, the insurance coverage will be sufficient to cover any potential claims or liabilities. Similar risks would exist upon the commercialization or marketing of any products by us or our partners. We currently do not have any clinical or product liability claims or threats of claims filed against us.
The pharmaceutical and biotechnology industries are subject to rapid technological change, and if we fail to keep up with such change, our results of operations and financial condition could be adversely impacted.
Biotechnology and related pharmaceutical technology have undergone and are subject to rapid and significant change. We expect that the technologies associated with biotechnology research and development will continue to develop rapidly. Our failure to keep pace with such rapid change could result in our products becoming obsolete and we may be unable to recoup any expenses incurred with developing such products, which may adversely affect our future revenues and financial condition.
We rely heavily on the founder of Emmaus Medical, Yutaka Niihara, M.D., MPH, our current President and Chief Executive Officer. The loss of his services would have a material adverse effect upon the Company and its business and prospects.
Our success depends, to a significant extent, upon the continued services of Yutaka Niihara, M.D. MPH, who is the founder of Emmaus Medical and our current President and Chief Executive Officer. Since inception, we have been dependent upon Dr. Niihara, who was one of the initial patentees for the technology for treatment of SCD. While Dr. Niihara and the rest of our executive officers are parties to confidentiality agreements that prevent them from soliciting our existing customers or disclosing information deemed confidential to us, we do not have any agreement with Dr. Niihara or any key members of management that would prohibit them from joining our competitors or forming competing companies. In addition, we do not maintain key man life insurance policies on any of our executive officers. If Dr. Niihara, or any key management personnel resign to join a competitor or form a competing company, the loss of such personnel, together with the loss of any customers or potential customers due to such executive’s departure, could materially and adversely affect our business and results of operations.
We are dependent on a technically trained workforce and an inability to retain or effectively recruit such employees could have a material adverse effect on our business, financial condition and results of operations.
Our ability to compete effectively depends largely on our ability to attract and retain certain key personnel, including our clinical, regulatory and scientific staff members. Industry demand for such skilled employees, however, exceeds the number of personnel available, and the competition for attracting and retaining these employees is intense. Because of this intense competition for skilled employees, we may be unable to retain our existing personnel or attract additional qualified employees to keep up with future business needs. If this should happen, our business, operating results and financial condition could be adversely affected.
32
In addition, we intend to hire in-house marketing personnel to promote and market and sell our SCD and SBS treatment products to patients, physicians and treatment centers, and obtain the approval of insurance companies and healthcare payors for reimbursement of the cost of these treatments. We cannot assure you that we will be able to recruit and retain qualified personnel to perform these marketing functions. Our inability to hire and then retain such personnel and scientists could have a materially adverse effect on our business and our projects.
We are dependent on licenses and sublicenses for certain patents for our products. If the licenses of any superior sublicenses terminates or if the if the patents of our licensors are challenged and we are limited in our ability to utilize the licensed patents, we may be unable to develop, out-license, market and sell our products, which would cause a material adverse effect on our business, prospects, financial condition, and operating results.
Our ability to develop products depends on licenses we have obtained to patents for treatment of SCD and SBS. We hold a license as a sublicensee of certain patent rights needed to operate our business. If any of the licenses of any of the superior sublicensees terminate, our license may terminate also.
There can be no assurance that, in the event any claims in such sublicensed patents are challenged, that any court or patent authority would determine that such patent claims are valid and enforceable or sufficiently broad in scope to protect our proprietary rights. Our commercial success will depend, in part, on not infringing patents or proprietary rights of others, and there can be no assurance that the technologies and products used or developed by us will not infringe such rights. Infringement actions could result in litigation and we could incur significant costs and diversion of resources in defending such claims. The party making such claims could secure a judgment awarding substantial damages, as well as injunctive or other equitable relief. Such relief could effectively block our ability to make, use, sell, distribute or market our products and services in such jurisdiction .If such infringement occurs and we are unable to obtain a license from the relevant third party, we will not be able to continue the development, manufacture, use or sale of any such infringing technology or product. There can be no assurance that necessary license to third party technology will be available at all, or on commercially reasonable terms. Our failure to obtain a license to technology that it may require to utilize its technologies or commercialize its products could have a material adverse effect on us. In some cases, litigation or other proceedings may be necessary to defend against or assert claims of infringement, to enforce patents issued to us, to protect trade secrets, know-how or other intellectual property rights owned by us or to determine the scope and validity of the proprietary rights of third parties. Any potential litigation could result in substantial costs to, and diversion of, resources by us and could have a material and adverse impact on us. There can be no assurance that any of our issued or licensed patents would ultimately be held valid or that efforts to defend any of our patents, trade secrets, know-how or other intellectual property rights would be successful. An adverse outcome in any such litigation or proceeding could subject us to significant liabilities, require us to cease using the subject technology or require us to license the subject technology from the third party, all of which could have a material adverse effect on our business.
If we are unable to protect our proprietary technology, we may not be able to compete as effectively and our business and financial prospects may be harmed.
Where appropriate, we seek patent protection for certain aspects of our technology. Patent protection may not be available for some of the drug product candidates we are developing. If we must spend significant time and money protecting our patents, designing around patents held by others or licensing, potentially for large fees, patents or other proprietary rights held by others, our business and financial prospects may be harmed.
We will incur significant ongoing expenses in maintaining our patent portfolio. Should we lack the funds to maintain our patent portfolio or to enforce our rights against infringers, we could be adversely impacted. Even if claims of infringement are without merit, any such action could divert the time and attention of management and impair our ability to access additional capital and/or cost us significant funds to defend.
33
Companies and universities that have licensed product candidates to us for research, clinical development and marketing are sophisticated competitors that could develop similar products to compete with our products which could reduce our future revenues.
Licensing our product candidates from other companies, universities or individuals does not always prevent them from developing non-identical but competitive products for their own commercial purposes, nor from pursuing patent protection in areas that are competitive with us. While we seek patent protection for all of our owned and licensed product candidates, our licensors or assignors who created these product candidates are experienced scientists and business people who may continue to do research and development and seek patent protection in the same areas that led to the discovery of the product candidates that they licensed or assigned to us. By virtue of the previous research that led to the discovery of the drugs or product candidates that they licensed or assigned to us, these companies, universities, or individuals may be able to develop and market competitive products in less time than might be required to develop a product with which they have no prior experience and may reduce our future revenues from such product candidates.
Our success depends on our ability to manage our growth.
If we are able to raise significant additional financing, we expect to continue to grow, which could strain our managerial, operational, financial and other resources. With the addition of our clinical-stage programs and with our plan to in-license and acquire additional clinical-stage product candidates, we will be required to retain experienced personnel in the regulatory, clinical and medical areas over the next several years. Also, as our preclinical pipeline diversifies through the acquisition or in-licensing of new molecules, we will need to hire additional scientists to supplement our existing scientific expertise over the next several years.
Our staff, financial resources, systems, procedures or controls may be inadequate to support our operations and our management may be unable to take advantage of future market opportunities or manage successfully our relationships with third parties if we are unable to adequately manage our anticipated growth and the integration of new personnel.
Our business and results of operations may be negatively impacted by general economic and financial market conditions and such conditions may exacerbate the other risks that affect our business.
The world’s financial markets are currently experiencing significant turmoil, resulting in reductions in available credit, constraints in access to capital, extreme volatility in security prices, rating downgrades of investments and reduced valuations of securities generally. These economic conditions have had, and we expect will continue to have, an adverse impact on the pharmaceutical industries. Our business depends on our ability to raise substantial additional capital and to maintain and enter into new collaborative research, development and commercialization agreements with leading pharmaceutical companies. Current market conditions could impair our ability to raise additional capital when needed for our clinical trials. Recent economic conditions may result in prospective collaborators electing to defer entering into collaborative agreements with us or reduce the amount of discretionary investment that prospective collaborators may have available to invest in our business.
We are unable to predict the likely duration and severity of the current disruption in financial markets and adverse economic conditions in the U.S. and abroad, but the longer the duration the greater risks we face in operating our business. There can be no assurance, therefore, that current economic conditions or worsening economic conditions or a prolonged or recurring recession will not have a significant adverse impact on our operating results.
We may pursue future growth through strategic acquisitions and alliances which may not yield anticipated benefits and may adversely affect our operating results, financial condition and existing business.
We may seek to grow in the future through strategic acquisitions in order to complement and expand our business. The success of our acquisition strategy will depend on, among other things:
| ● | the availability of suitable candidates; | |
| ● | competition from other companies for the purchase of available candidates; |
34
| ● | our ability to value those candidates accurately and negotiate favorable terms for those acquisitions; | |
| ● | the availability of funds to finance acquisitions; | |
| ● | the ability to establish new informational, operational and financial systems to meet the needs of our business; | |
| ● | the ability to achieve anticipated synergies, including with respect to complementary products; and | |
| ● | the availability of management resources to oversee the integration and operation of the acquired businesses. |
If we are not successful in integrating acquired businesses and completing acquisitions in the future, we may be required to reevaluate our acquisition strategy. We also may incur substantial expenses and devote significant management time and resources in seeking to complete acquisitions. Acquired businesses may fail to meet our performance expectations. If we do not achieve the anticipated benefits of an acquisition as rapidly as expected, or at all, investors or analysts may not perceive the same benefits of the acquisition as we do. If these risks materialize, our stock price could be materially adversely affected.
We have adopted an equity incentive plan under which we may grant securities to compensate employees and other services providers, which could result in increased share-based compensation expenses and, therefore, reduce net income.
Under current accounting rules, we would be required to recognize share-based compensation as compensation expense in our statement of operations, based on the fair value of equity awards on the date of the grant, and recognize the compensation expense over the period in which the recipient is required to provide service in exchange for the equity award. We have not made any such grants in the past, and accordingly our results of operations have not contained any share-based compensation charges. The additional expenses associated with share-based compensation may reduce the attractiveness of issuing stock options under an equity incentive plan that we may adopt in the future. If we grant equity compensation to attract and retain key personnel, the expenses associated with share-based compensation may adversely affect our net income. However, if we do not grant equity compensation, we may not be able to attract and retain key personnel or be forced to expend cash or other compensation instead. Furthermore, the issuance of equity awards would dilute the stockholders’ ownership interests in our company.
Our charter documents and our stockholder rights plan may have anti-takeover effects that could prevent a change in control, which may cause our stock price to decline.
Our certificate of incorporation or our bylaws could make it more difficult for a third party to acquire us, even if closing such a transaction would be beneficial to our stockholders. We are authorized to issue up to 20,000,000 shares of preferred stock. This preferred stock may be issued in one or more series, the terms of which may be determined at the time of issuance by our board of directors without further action by stockholders. The terms of any series of preferred stock may include voting rights (including the right to vote as a series on particular matters), preferences as to dividend, liquidation, conversion and redemption rights and sinking fund provisions. No preferred stock is currently outstanding. The issuance of any preferred stock could materially adversely affect the rights of the holders of our common stock, and therefore, reduce the value of our common stock. In particular, specific rights granted to future holders of preferred stock could be used to restrict our ability to merge with, or sell our assets to, a third party and thereby preserve control by the present management.
Our certificate of incorporation and bylaws also contain provisions that could have the effect of discouraging potential acquisition proposals or making a tender offer or delaying or preventing a change in control, including changes a stockholder might consider favorable. In particular, the certificate of incorporation and bylaws, as applicable, among other things:
35
| ● | provide the board of directors with the ability to alter the bylaws without stockholder approval; and | |
| ● | provide that vacancies on the board of directors may be filled by a majority of directors in office, although less than a quorum. |
These provisions are expected to discourage certain types of coercive takeover practices and inadequate takeover bids and to encourage persons seeking to acquire control of our company to first negotiate with its board. These provisions may delay or prevent someone from acquiring or merging with us, which may cause the market price of our common stock to decline.
RISKS RELATED TO OUR CAPITAL STRUCTURE
There is no current trading market for our common stock, and there is no assurance of an established public trading market, which would adversely affect the ability of our investors to sell their securities in the public market.
Our common stock is not currently listed or quoted for trading on any national securities exchange or national quotation system. We intend to apply for the listing of our common stock on the NASDAQ Global Market in the future. There is no guarantee that NASDAQ Global Market, or any other securities exchange or quotation system, will permit our shares to be listed and traded. If we fail to obtain a listing on the NASDAQ Global Market, we may seek listing on the NYSE Amex or quotation on the OTC Bulletin Board. FINRA has enacted changes that limit quotations on the OTC Bulletin Board to securities of issuers that are current in their reports filed with the Securities and Exchange Commission. The effect on the OTC Bulletin Board of these rule changes and other proposed changes cannot be determined at this time. The OTC Bulletin Board is an inter-dealer, over-the-counter market that provides significantly less liquidity than the NASDAQ Global Market and NYSE Amex. Quotes for stocks included on the OTC Bulletin Board are not listed in the financial sections of newspapers as are those for the NASDAQ Stock Market and NYSE Amex. Therefore, prices for securities traded solely on the OTC Bulletin Board may be difficult to obtain and holders of common stock may be unable to resell their securities at or near their original offering price or at any price.
The market price and trading volume of shares of our common stock may be volatile.
When and if a market develops for our securities, the market price of our common stock could fluctuate significantly for many reasons, including reasons unrelated to our specific performance, such as reports by industry analysts, investor perceptions, or announcements by our competitors regarding their own performance, as well as general economic and industry conditions. For example, to the extent that other large companies within our industry experience declines in their share price, our share price may decline as well. Fluctuations in operating results or the failure of operating results to meet the expectations of public market analysts and investors may negatively impact the price of our securities. Quarterly operating results may fluctuate in the future due to a variety of factors that could negatively affect revenues or expenses in any particular quarter, including vulnerability of our business to a general economic downturn; changes in the laws that affect our products or operations; competition; compensation related expenses; application of accounting standards; and our ability to obtain and maintain all necessary government certifications and/or licenses to conduct our business. In addition, when the market price of a company’s shares drops significantly, shareholders could institute securities class action lawsuits against the company. A lawsuit against us could cause us to incur substantial costs and could divert the time and attention of our management and other resources.
Shares eligible for future sale may adversely affect the market price of our common stock, as the future sale of a substantial amount of outstanding stock in the public marketplace could reduce the price of our common stock.
We granted “piggyback” registration rights to certain pre-Merger stockholders of AFH IV. All of the shares included in an effective registration statement may be freely sold and transferred, subject to a lock-up agreement.
Additionally, following the one year anniversary of the filing of this report, the former shareholders of Emmaus Medical may be eligible to sell all or some of our shares of common stock by means of ordinary brokerage transactions in the open market pursuant to Rule 144, promulgated under the Securities Act (“Rule 144”), subject to certain limitations. Under Rule 144, an affiliate stockholder who has satisfied the required holding period may, under certain circumstances, sell within any three-month period a number of securities which does not exceed the greater of 1% of the then outstanding shares of common stock or the average weekly trading volume of the class during the four calendar weeks prior to such sale. As of the closing of the Merger, 1% of our issued and outstanding shares of common stock was approximately 244,237 shares. Non-affiliate stockholders are not subject to volume limitations. Any substantial sale of common stock pursuant to any resale prospectus or Rule 144 may have an adverse effect on the market price of our common stock by creating an excessive supply.
36
Following the Merger, members of our management team have significant influence over us.
Immediately following completion of the Merger, our officers and directors own approximately 50.9% of the outstanding common stock on an undiluted basis. These stockholders, therefore, have a controlling influence in determining the outcome of any corporate transaction or other matters submitted to our stockholders for approval, including mergers, consolidations and the sale of all or substantially all of our assets, election of directors, and other significant corporate actions. These stockholders may also have the power to prevent or cause a change in control. In addition, without the consent of these stockholders, we could be prevented from entering into transactions that could be beneficial to us. The interests of these stockholders may differ from the interests of our other stockholders.
If we fail to maintain effective internal controls over financial reporting, the price of our common stock may be adversely affected.
We are required to establish and maintain appropriate internal controls over financial reporting. Failure to establish those controls, or any failure of those controls once established, could adversely impact our public disclosures regarding our business, financial condition or results of operations. Any failure of these controls could also prevent us from maintaining accurate accounting records and discovering accounting errors and financial frauds. Rules adopted by the SEC pursuant to Section 404 of the Sarbanes-Oxley Act of 2002 require annual assessment of our internal control over financial reporting. The standards that must be met for management to assess the internal control over financial reporting as effective complex, and require significant documentation, testing and possible remediation to meet the detailed standards. We may encounter problems or delays in completing activities necessary to make an assessment of our internal control over financial reporting. If we cannot assess our internal control over financial reporting as effective, investor confidence and share value may be negatively impacted.
In addition, management’s assessment of internal controls over financial reporting may identify weaknesses and conditions that need to be addressed in our internal controls over financial reporting or other matters that may raise concerns for investors. Any actual or perceived weaknesses and conditions that need to be addressed in our internal control over financial reporting or disclosure of management’s assessment of our internal controls over financial reporting may have an adverse impact on the price of our common stock.
We may not be able to achieve the benefits we expect to result from the Merger.
On April 21, 2011, we entered into a Merger Agreement with AFH Merger Sub, AFH Advisory and Emmaus Medical, pursuant to which we (i) exchanged each outstanding share of Emmaus Medical common stock for approximately 29.48548924976 shares of AFH IV common stock, (ii) exchanged each outstanding Emmaus Medical option and warrant, which was exercisable for one share of Emmaus Medical common stock, for an option or warrant, as applicable, exercisable for 29.48548924976 shares of AFH IV common stock and (iii) exchanged each outstanding convertible note of Emmaus Medical, which was convertible for one share of Emmaus Medical common stock, for a convertible note exercisable for 29.48548924976 shares of AFH IV common stock. . As a result of the Merger, Emmaus Medical became our wholly-owned subsidiary and our operations became that of Emmaus Medical.
We may not realize the benefits that we hoped to receive as a result of the Merger, which include:
| ● | access to the capital markets of the United States; | |
| ● | the increased market liquidity expected to result from exchanging stock in a private company for securities of a public company that may eventually be traded; | |
| ● | the ability to use registered securities to make acquisition of assets or businesses; |
37
| ● | increased visibility in the financial community; | |
| ● | enhanced access to the capital markets; | |
| ● | improved transparency of operations; and | |
| ● | perceived credibility and enhanced corporate image of being a publicly traded company. |
There can be no assurance that any of the anticipated benefits of the Merger will be realized with respect to our new business operations. In addition, the attention and effort devoted to achieving the benefits of the Merger and attending to the obligations of being a public company, such as reporting requirements and securities regulations, could significantly divert management’s attention from other important issues, which could materially and adversely affect our operating results or stock price in the future.
We will incur significantly increased costs as a result of operating as a public company, and our management will be required to devote substantial time to compliance efforts.
As a public company, we will incur significant legal, accounting and other expenses that we did not incur as a private company. The Sarbanes-Oxley Act of 2002 and rules subsequently implemented by the SEC. Our management and other personnel will need to devote a substantial amount of time and resources in complying with these requirements. Moreover, these rules and regulations will increase our legal and financial compliance costs and will make some activities more time-consuming and costly, although we are currently unable to estimate these costs with any degree of certainty. These rules and regulations could also make it more difficult for us to attract and retain qualified persons to serve on our board of directors and board committees or as executive officers and more expensive for us to obtain director and officer liability insurance.
Compliance with changing regulation of corporate governance and public disclosure will result in additional expenses.
Changing laws, regulations and standards relating to corporate governance and public disclosure, including the Sarbanes-Oxley Act of 2002 and related SEC regulations, have created uncertainty for public companies and significantly increased the costs and risks associated with accessing the public markets and public reporting. For example, on January 30, 2009, the SEC adopted rules requiring companies to provide their financial statements in interactive data format using the eXtensible Business Reporting Language, or XBRL. We will have to comply with these rules by June 15, 2011. Our management team will need to invest significant management time and financial resources to comply with both existing and evolving standards for public companies, which will lead to increased general and administrative expenses and a diversion of management time and attention from revenue generating activities to compliance activities.
Our common stock may be considered a “penny stock,” and thereby be subject to additional sale and trading regulations that may make it more difficult to sell.
Our common stock, which is not currently listed or quoted for trading, may be considered to be a “penny stock” if it does not qualify for one of the exemptions from the definition of “penny stock” under Section 3a51-1 of the Securities Exchange Act for 1934, as amended (the “Exchange Act”), once, and if, it starts trading. Our common stock may be a “penny stock” if it meets one or more of the following conditions (i) the stock trades at a price less than $5.00 per share; (ii) it is NOT traded on a “recognized” national exchange; (iii) it is NOT quoted on the Nasdaq Capital Market, or even if so, has a price less than $5.00 per share; or (iv) is issued by a company that has been in business less than three years with net tangible assets less than $5 million.
38
The principal result or effect of being designated a “penny stock” is that securities broker-dealers participating in sales of our common stock will be subject to the “penny stock” regulations set forth in Rules 15-2 through 15g-9 promulgated under the Exchange Act. For example, Rule 15g-2 requires broker-dealers dealing in penny stocks to provide potential investors with a document disclosing the risks of penny stocks and to obtain a manually signed and dated written receipt of the document at least two business days before effecting any transaction in a penny stock for the investor’s account. Moreover, Rule 15g-9 requires broker-dealers in penny stocks to approve the account of any investor for transactions in such stocks before selling any penny stock to that investor. This procedure requires the broker-dealer to (i) obtain from the investor information concerning his or her financial situation, investment experience and investment objectives; (ii) reasonably determine, based on that information, that transactions in penny stocks are suitable for the investor and that the investor has sufficient knowledge and experience as to be reasonably capable of evaluating the risks of penny stock transactions; (iii) provide the investor with a written statement setting forth the basis on which the broker-dealer made the determination in (ii) above; and (iv) receive a signed and dated copy of such statement from the investor, confirming that it accurately reflects the investor’s financial situation, investment experience and investment objectives. Compliance with these requirements may make it more difficult and time consuming for holders of our common stock to resell their shares to third parties or to otherwise dispose of them in the market or otherwise.
We do not foresee paying cash dividends in the foreseeable future and, as a result, our investors’ sole source of gain, if any, will depend on capital appreciation, if any.
We do not plan to declare or pay any cash dividends on our shares of common stock in the foreseeable future and currently intend to retain any future earnings for funding growth. As a result, investors should not rely on an investment in our securities if they require the investment to produce dividend income. Capital appreciation, if any, of our shares may be investors’ sole source of gain for the foreseeable future. Moreover, investors may not be able to resell their shares of our common stock at or above the price they paid for them.
39
CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING STATEMENTS
The information contained in this report, including in the documents incorporated by reference into this report, includes some statement that are not purely historical and that are forward-looking statements. Such forward-looking statements include, but are not limited to, statements regarding our and our management’s expectations, hopes, beliefs, intentions or strategies regarding the future, including our financial condition, results of operations, and the expected impact of the Merger on the parties’ individual and combined financial performance. In addition, any statements that refer to projections, forecasts or other characterizations of future events or circumstances, including any underlying assumptions, are forward-looking statements. The words “anticipates,” “believes,” “continue,” “could,” “estimates,” “expects,” “intends,” “may,” “might,” “plans,” “possible,” “potential,” “predicts,” “projects,” “seeks,” “should,” “will,” “would” and similar expressions, or the negatives of such terms, may identify forward-looking statements, but the absence of these words does not mean that a statement is not forward-looking.
The forward-looking statements contained in this report are based on current expectations and beliefs concerning future developments and the potential effects on the parties and the transaction. There can be no assurance that future developments actually affecting us will be those anticipated. These forward-looking statements involve a number of risks, uncertainties (some of which are beyond the parties’ control) or other assumptions that may cause actual results or performance to be materially different from those expressed or implied by these forward-looking statements, including the following:
| ● | our ability to raise additional capital to fund our operations; | |
| ● | our obtaining FDA and other regulatory approval for our drug products; | |
| ● | successful completion of our clinical trials; | |
| ● | our ability to achieve regulatory approval for our L-glutamine treatment for SCD; | |
| ● | our ability to commercialize our L-glutamine treatment for SCD; | |
| ● | our reliance on third party manufacturers for our drug products; | |
| ● | market acceptance of our products; | |
| ● | our dependence on licenses for certain of our products; | |
| ● | our reliance on the expected growth in demand for our products; | |
| ● | exposure to product liability and defect claims; | |
| ● | exposure to intellectual property claims from third parties; | |
| ● | development of a public trading market for our securities; | |
| ● | the cost of complying with current and future governmental regulations and the impact of any changes in the regulations on our operations; and | |
| ● | the other factors referenced in this Current Report, including, without limitation, under the sections entitled “Risk Factors,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” and “Business.” |
These risks and uncertainties, along with others, are also described above under the heading “Risk Factors.” Should one or more of these risks or uncertainties materialize, or should any of the parties’ assumptions prove incorrect, actual results may vary in material respects from those projected in these forward-looking statements. We undertake no obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws.
40
ADDITIONAL DISCLOSURE
For additional information that would be required if the Company were filing a general form for registration of securities on Form 10, see Item 2.02 for “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” and Item 3.03 for a description of the Company’s securities post-Merger and related discussion of market price, all incorporated by reference herein. Required disclosure regarding the change in control of the Company, the impact on its directors, executive officers, control persons and related compensation and beneficial ownership issues are addressed in Item 5.01, incorporated by reference herein. Attention is also directed to Item 9.01, which provides our audited financial statements as of and for the years ended December 31, 2010 and 2009.
Item 2.02 Results of Operations and Financial Condition.
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
MANAGEMENT’S DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read the following discussion and analysis of our financial condition and results of operations together with our consolidated financial statements and the related notes appearing in Item 9.01 of this report. Some of the information contained in this discussion and analysis or set forth elsewhere in this report, including information with respect to our plans and strategy for our business and related financing, includes forward-looking statements that involve risks and uncertainties. You should read the “Risk Factors” section included in Item 2.01 of this report for a discussion of important factors that could cause actual results to differ materially from the results described in or implied by the forward-looking statements contained in the following discussion and analysis.
Company Overview
We develop and commercialize treatments and therapies for rare diseases and are primarily focused on the late-stage development—currently in Phase III clinical trials with the FDA — of the amino acid L-glutamine as a prescription drug for the treatment of SCD. To a lesser extent, we are also engaged in the marketing and sale of NutreStore® [L-glutamine powder for oral solution] and the promotion of Zorbtive® [somatropin (rDNA origin) for injection], which have each received FDA approval, as a treatment for SBS. Our indirect wholly owned subsidiary, Newfield Nutrition Corporation, sells L-glutamine as a nutritional supplement under the brand name AminoPure® through retail stores in multiple states and via importers and distributors in Japan and Taiwan. Since inception, we have generated minimal revenues from the sale and/or promotion of NutreStore®, Zorbtive® and AminoPure®. We also own a minority interest in CellSeed, Inc., a Japanese company listed on the JASDAQ NEO market in Tokyo, engaged in research and development, manufacture and sale of temperature-responsive cell culture equipment. Emmaus Medical, LLC was organized on December 20, 2000. In October 2003, Emmaus Medical, LLC conducted a reorganization and merged with Emmaus Medical, Inc., a Delaware corporation originally incorporated in September 2003.
Our future capital requirements are substantial and may increase beyond our current expectations depending on many factors including: the duration and results of the clinical trials for our various products going forward; unexpected delays or developments in seeking regulatory approvals; the time and cost in preparing, filing, prosecuting, maintaining and enforcing patent claims; other unexpected developments encountered in implementing our business development and commercialization strategies; the outcome of litigation, if any; and further arrangements, if any, with collaborators. Until we can generate a sufficient amount of product revenue, if ever, future cash needs are expected to be financed through public or private equity offerings, debt financings or corporate collaboration and licensing arrangements. As of March 31, 2011, we had an accumulated deficit since inception total $13.9 million and cash and cash equivalents of $1.1 million as of March 31, 2011. Since inception we have had minimal revenues and have had to rely on funding from sales of equity securities and borrowings from officers and stockholders. Currently we estimate we will need approximately $7.3 million to complete our Phase III clinical trial and $400,000 to obtain regulatory approval of L-glutamine as a therapy for SCD. In addition, we have agreed to pay CellSeed an aggregate of $10.0 million pursuant to the Research Agreement and the Individual Agreement and we believe that we will need approximately $3 million for research and development to develop corneal cell sheet technology in the U.S.
41
Recent Highlights
In April 2009, the FDA authorized us to begin a larger Phase III clinical trial directed to study L-glutamine as an experimental agent to reduce sickle cell crisis. Patient enrollment began in mid-2010 and as of February 2011, we have signed contracts with 15 sickle cell study sites across the United States and have enrolled 33 patients. We aim to complete Phase III clinical trial enrollment by the end of 2011.
In October 2010, we formed Emmaus Medical Japan Inc. (“EMJ”) and held approximately 97% of the outstanding shares of EMJ from its formation until March 2011, when we acquired the remaining outstanding shares of EMJ. EMJ is now a wholly owned subsidiary of Emmaus Medical Inc. that markets and sells AminoPure® in Japan and other neighboring regions. EMJ sells AminoPure to retailers and to wholesalers and directly to consumers via direct online internet sales. EMJ also manages our distributors in Japan and may also import other medical products and drugs in the future. The results of EMJ have been included in the consolidated financial statements of the Company since the date of its formation. The aggregate formation cost was $52,500. EMJ recorded approximately $6,000 in net sales in 2010 from the date of its formation through December 31, 2010 and approximately $23,000 in the three months ended March 31, 2011.
The Company has agreed to contribute $15,000 per month to EMJ from January 5, 2011 through June 2011, renewable automatically for six months on equivalent terms if the existing agreement is not updated within one month before expiration. The Company makes the monthly payments in return for consulting services EMJ performs on behalf of the Company including (1) coordinating shareholder briefing meetings in Japan, (2) performing reception duties in Japan for employees and customers of Emmaus Medical, (3) conducting research activities and analysis in conjunction with the raw material market of AminoPure, and (4) providing other services such as translation of documents and creating supporting investor relations documents for Emmaus Medical.
In December 2010, we were awarded a five-year contract by the U.S. Department of Veterans Affairs for our NutreStore® product. In October 2007, we became the exclusive sublicensee of the SBS Patent for the U.S. market, including the rights to distribute the L-glutamine treatment for SBS under the trademark NutreStore, ® in the U.S., and commercially launched NutreStore® in June 2008. Internationally, we are in the last stages of seeking approval to market NutreStore® in Hong Kong and have received a Certificate of Free Sale from the FDA to export NutreStore® to Hong Kong. We entered into an exclusive license agreement with EMD Serono, Inc. to promote Zorbtive® in the United States in December 2008.
In April 2010, we added two additional sales representatives to our sales team, bringing our sales team to a total of three (3) full time pharmaceutical sales representatives. The sales team currently focuses on the marketing of NutreStore® and Zorbtive®, a treatment for SBS.
In March 2011, Emmaus Medical completed a private placement of shares of its common stock in which it sold 9,230 shares of common stock at $125.00 per share for total gross proceeds of $1.2 million.
We sell L-glutamine as a nutritional supplement under the brand name AminoPure® through our wholly-owned subsidiary Newfield Nutrition Corporation. The product is currently sold through retail stores in California and Washington and via importers and distributors in Japan. As part of the growth strategy, Newfield Nutrition is focused on adding additional distributors both domestically and internationally. On November 27, 2010 we added a new distributor in Taiwan.
Financial Overview
Revenue
As noted above, we are still in the development stage. Since our inception in 2000, we have had limited revenue from the sale of NutreStore®, an FDA approved prescription drug to treat SBS, and AminoPure®, a nutritional supplement. We have funded operations principally through debt financings and issuance of common stock. Emmaus’ operations to date have been primarily limited to organizing and staffing, licensing and promoting products for SBS, outsourcing distribution and sales activities, developing clinical trials for sickle cell treatment, establishing manufacturing for products and maintaining and improving its patent portfolio.
42
Currently we generate revenue through sale of NutreStore® [L-glutamine powder for oral solution] as a treatment for SBS as well as AminoPure® as a nutritional supplement In October 2007, Emmaus became the exclusive sublicensee of the SBS Patent for the U.S. market, including the rights to distribute the L-glutamine treatment for SBS under the trademark NutreStore, ® in the U.S., and commercially launched NutreStore® in June 2008. The sublicense requires us to pay a royalty of 10% of adjusted gross sales of NutreStore® to Cato Holding Company (“Cato”), the sublicensor, with a required minimum royalty of $30,000 in 2008 and $70,000 in 2009. There was no required minimum royalty in 2010 and thereafter, other than 10% royalty of adjusted gross sales. EMD Serono, Inc. granted Emmaus the exclusive right to promote and market Zorbtive® in the United States starting in December 2008. We use the same sales force to promote and market Zorbtive® and NutreStore®. The license agreement provides for no royalties to be payable from EMD Serono to us until unit sales exceed 16,016 units. Unit sales have not exceeded this threshold and no royalties have been paid to us to date pursuant to the agreement. After unit sales exceed the threshold, EMD Serono is required to pay us a commission equal to $300,000, plus 35% of annual net sales from unit sales that exceed 16,017 but are less than or equal to 32,032 units; 50% of annual net sales from unit sales that exceed 32,033 but are less than or equal to 80,080 units; and 60% of annual net sales from unit sales that exceed 80,080 units. The agreement terminates upon expiration of U.S. Patent No. 5,288,703, which has an expiration date of October 7, 2011. Management expects that any revenues generated will fluctuate from quarter to quarter as a result of the timing and amount.
Research and Development Expenses
Research and development costs consist of expenditures for new products and technologies, which primarily involve fees paid to the contract research organization (CRO), payroll-related expenses, study site payments, consultants, activities related to regulatory filings, manufacturing development costs and other related supplies. Product candidates in late stages of clinical development generally have higher development costs than those in earlier stages of clinical development, primarily due to the increased size and duration of late stage clinical trials. We plan to increase our research and development expenses for the foreseeable future as we seek to complete development of our most advanced product candidate, the amino acid L-glutamine as a prescription drug for the treatment of SCD. Currently we estimate we will need approximately $7.3 million to complete our Phase III clinical trial.
Expenses related to the Phase III clinical trial are based on estimates of the services received and efforts expended pursuant to contracts with study sites and the CRO that conducts and manages the clinical trial on our behalf. We expect to incur increased research and development expenses as we continue to enroll patients in the Phase III clinical trial for sickle cell disease. The most significant clinical trial expenditures are related to the CRO costs and the payment for study sites. The contract with the CRO is based on time and material whereas the study site agreements are based on per patient costs as well as other pass through costs including but not limited to start-up costs and institutional review board (IRB) fees. The financial terms of these agreements are subject to negotiation and vary from contract to contract and may result in uneven payment flows. Payments under some of these contracts depend on factors such as the successful enrollment of patients and the completion of clinical trial milestones. Management estimates the expenses based on the time period over which the services will be performed and the level of effort to be expended in each period. Although we do not expect the estimated to be materially different from amounts actually incurred, our understanding of the status and timing of services performed relative to the actual status and timing of services performed may vary and result in us reporting amounts that are higher or lower in any particular period.
While we currently are focused on advancing the sickle cell clinical trials, future research and development expenses will depend on any new products or technologies that may be introduced in the pipeline. In addition, we cannot forecast with any degree of certainty which product candidate(s) may be subject to future collaborations, when such arrangements will be secured, if at all, and to what degree such arrangements would affect our development plans and capital requirements.
At this time, due to the inherently unpredictable nature of the drug development process and the interpretation of the regulatory requirements, we are unable to estimate with any certainty the costs we will incur in the continued development of the sickle cell treatment and other clinical programs. Clinical development timelines, the probability of success and development costs can differ materially from expectations. The current estimated cost to complete the Phase III clinical trial is $7.3 million, which is based on the assumption that the total number of trial sites does not increase and we remain on the projected timeline. Should the timeline have to be moved out further than what is planned, there will be an increase in costs associated with the additional time and effort required by the CRO and company staff.
43
The drug development process to obtain FDA approval is very costly and time consuming. Even with the granting of orphan drug status and fast track designation, the successful development of the L-glutamine treatment for SCD is uncertain and subject to a number of risks including those described above under the caption “Risk Factors” in Item 2.01.
The L-glutamine treatment for SCD is investigational in nature and has not received FDA approval. In order to grant marketing approval, the FDA must conclude that the clinical data establishes the safety and efficacy of the L-glutamine treatment for SCD and that the manufacturing processes and controls are adequate. Despite our efforts, the L-glutamine treatment for SCD may not be proven safe and effective in clinical trials, or meet applicable regulatory standards. We are focused on completing the Phase III clinical trial and submitting the new drug application (NDA) to the FDA for consideration. As a result of the uncertainties discussed above, the uncertainty associated with clinical trial enrollment and the risks inherent in the development process, we are unable to determine the duration and completion of costs or when, and to what extent, we will generate revenues from the commercialization and sale of the L-glutamine treatment for SCD. Development timelines, probability of success and development costs vary widely.
In connection with our agreements with CellSeed related to the development of corneal cell sheet technology in U.S., we believe that the cost to develop this technology is approximately $13 million, which includes the $10 million in payments we have agreed to make to CellSeed pursuant to the Joint Research and Development Agreement and Individual Agreement, and $3 million in research and development costs. Such estimate includes the cost of obtaining FDA approval for the cornea cell sheets. We have assumed that we will need biologic approval of the FDA for the cornea cell sheets, rather than pharmaceutical approval, and that we will only have to run a small trial here in the U.S. to test the safety and efficacy of the corneal cell sheets because we believe that we will be able to submit, and the FDA will accept, the data regarding corneal cell sheets submitted to the EMEA.
No research and development costs are associated with the SBS treatment.
General and Administrative Expenses
General and administrative expenses consist principally of salaries and related costs for personnel in executive, finance, business development, information technology, marketing, and legal functions. Other general and administrative expenses include facility costs, patent filing costs, and professional fees for legal, consulting, auditing and tax services.
Inventories
Inventories as of March 31, 2011 of the Company consisted of 69% finished goods and 31% work-in-process and are valued based on first-in, first-out and at the lesser of cost or market value. Work-in-process inventories consist of raw material L-Glutamine for the Company’s products, AminoPure and NutreStore, that has not yet been packaged and labeled for sale.
All of the purchases during the years ended December 31, 2010 and December 31, 2009 for the Company was from two vendors. All of the purchases during the three months ended March 31, 2011 for the Company were from one vendor.
44
Results of Operations
| Three Months Ended March 31, | Year Ended December 31, | From December 20, 2000 (date of inception) to March 31, | ||||||||||||||||||
| 2011 | 2010 | 2010 | 2009 | 2011 | ||||||||||||||||
| (unaudited) (restated) | (unaudited) | (restated) | (unaudited) (restated) | |||||||||||||||||
| Sales | $ | 59,468 | $ | 49,547 | $ | 168,964 | $ | 100,408 | $ | 433,967 | ||||||||||
| Sales Return | (255 | ) | (3,758 | ) | (30,230 | ) | (127 | ) | (30,612 | ) | ||||||||||
| Revenues | 59,213 | 45,789 | 138,734 | 100,281 | 403,355 | |||||||||||||||
| Cost of goods sold | 25,101 | 22,533 | 99,373 | 85,226 | 251,135 | |||||||||||||||
| Scrapped inventory | — | — | 235,537 | — | 235,537 | |||||||||||||||
| Total cost of goods sold | 25,101 | 22,533 | 334,910 | 85,226 | 486,672 | |||||||||||||||
| Gross profit (loss) | 34,112 | 23,256 | (196,176 | ) | 15,055 | (83,317 | ) | |||||||||||||
| Operating expenses | ||||||||||||||||||||
| Research and development | 310,763 | 221,946 | 1,062,031 | 532,351 | 5,210,415 | |||||||||||||||
| Selling | 201,511 | 125,042 | 656,200 | 696,949 | 2,003,719 | |||||||||||||||
| General and administrative | 647,825 | 406,421 | 1,817,728 | 1,300,397 | 6,260,565 | |||||||||||||||
| 1,160,099 | 753,409 | 3,535,959 | 2,529,697 | 13,474,699 | ||||||||||||||||
| Loss from operations | (1,125,987 | ) | (730,153 | ) | (3,732,135 | ) | (2,514,642 | ) | (13,558,016 | ) | ||||||||||
| Other income (expense) | ||||||||||||||||||||
| Interest income | 6,445 | 9,009 | 39,005 | 19,659 | 91,679 | |||||||||||||||
| Interest expense | (11,811 | ) | (11,950 | ) | (59,936 | ) | (71,600 | ) | (401,804 | ) | ||||||||||
| (5,366 | ) | (2,941 | ) | (20,931 | ) | (51,941 | ) | (310,125 | ) | |||||||||||
| Loss before income taxes | (1,131,353 | ) | (733,094 | ) | (3,753,066 | ) | (2,566,583 | ) | (13,868,141 | ) | ||||||||||
| Income taxes (benefit) | 850 | 1,250 | 4,304 | 1,224 | 15,698 | |||||||||||||||
| Net loss | (1,132,203 | ) | (734,344 | ) | (3,757,370 | ) | (2,567,807 | ) | (13,883,839 | ) | ||||||||||
| Loss per share – basic and diluted | (1.63 | ) | (1.12 | ) | (5.63 | ) | (4.02 | ) | ||||||||||||
| Weighted average shares outstanding – basic and diluted | 695,915 | 654,157 | 666,813 | 638,068 | ||||||||||||||||
Three Months Ended March 31, 2011 and 2010
Net Losses. Net losses increased $0.4 million, or 54%, from $0.7 million to $1.1 million for the three months ended March 31, 2010 and 2011, respectively, and we expect to continue to generate losses as we progress toward the commercialization of product candidates. The increase in losses is primarily a result of increased operating expenses as discussed below. As of March 31, 2011, we had an accumulated deficit of approximately $13.9 million. Losses, partially offset by revenue from commercialized products, will continue as we advance our sickle cell treatment toward regulatory approval and potential commercialization. As a result, we anticipate that we will continue to incur net losses and be unprofitable for the foreseeable future. There can be no assurance that we will ever operate at a profit, even if all of our products are commercialized.
Revenues. Revenues increased $13,424, or 29% from $45,789 to $59,213 for the three months ended March 31, 2010 and 2011, respectively. Revenue increased slightly due to increases in the number of units sold of our AminoPure® product.
45
Cost of goods sold. Cost of goods sold increased $2,568 or 11% from $22,533 to $25,101 for the three months ended March 31, 2010 and 2011, respectively. Cost of goods sold increased slightly primarily as a result of increased unit sales of our AminoPure product. No scrapped inventory expense was realized for the three months ended March 31, 2011 and 2010. As a percentage of revenue, cost of goods sold decreased from 45% to 42% for the three months ended March 31, 2010 and 2011, respectively primarily as a result of the increase in sales. All of the purchases during the three months ended March 31, 2011 and 2010 were from one vendor.
Research and Development Expenses. Research and development expenses increased $0.1 million, or 40%, from $0.2 million to $0.3 million for the three months ended March 31, 2010 and 2011, respectively. This increase was primarily due to increased activity in clinical trials. Research and development costs increased in 2011 and consisted of $40, 248 for CRO costs, $39,000 for study site expenses $6,143 for central laboratory costs and $3,549 in pharmacist costs. Our clinical trial activities began to ramp up beginning in March 2010 which contributed to the increase in costs ending March 2011. In 2010, we did not have any active sites. The bulk of payments made in 2010 were deposits to the CRO. As of March 31, 2011, there were 14 sites active and recruiting patients and CRO time and effort increased.
Selling Expenses. Selling expenses increased $0.1 million, or 61%, from $0.1 million to $.2 million for the three months ended March 31, 2010 and 2011, respectively. The increase was primarily due to sales, payroll and travel costs. The selling expense included the cost of distribution, promotion, travel, tradeshows and exhibits for NutreStore®, Zorbtive®, and AminoPure®.
General and Administrative Expenses. General and administrative expenses increased $0.2 million, or 59%, from $.4 million to $0.6 million for the three months ended March 31, 2010 and 2011, respectively. The increase was largely due to an increase in costs related to increased payroll, legal fees and consulting fees. The Company hired additional professional staff in 2011 which resulted in an increase in payroll expenses of $88,672. We also incurred increased legal and consulting expenses of approximately $140,000 in connection with the Merger in 2011.
We anticipate that general and administrative expenses will continue to increase for, among others, the following reasons:
| ● | as a result of increased payroll, expanded infrastructure and higher consulting, legal, accounting and investor relations costs, and director and officer insurance premiums associated with being a public company; | |
| ● | to support research and development activities, which the Company expects to expand as development of our product candidate(s) continue; and | |
| ● | to build a sales and marketing team before we receive regulatory approval of a product candidate in anticipation of commercial launch. |
Years ended December 31, 2010 and 2009
Net Losses. Net losses increased $1.2 million, or 46%, from $2.6 million to $3.8 million for the years ended December 31, 2009 and 2010, respectively. The increase in losses is primarily a result of increased operating expenses as discussed below. As of December 31, 2010, we had an accumulated deficit of approximately $12.8 million. Losses, partially offset by revenue from commercialized products, will continue as we advance our sickle cell treatment toward regulatory approval and potential commercialization. As a result, we anticipate that we will continue to incur net losses and be unprofitable for the foreseeable future. There can be no assurance that we will ever operate at a profit, even if all of our products are commercialized.
Revenues. Revenues increased $38,453, or 38%, from $100,281 to $138,734 for the years ended December 31, 2009 and 2010, respectively. Revenue increased primarily due to increases in the number of units sold of our AminoPure® product.
Cost of Goods Sold. Cost of goods sold increased from $85,226 to $334,910 for the years ended December 31, 2009 and 2010, respectively. Cost of goods sold includes costs for raw material, packaging, testing, shipping and costs related to scrapped inventory. Scrapped inventory expense of $0.2 million was realized for the year ended December 31, 2010 compared to none for the year ended December 31, 2009. This expense referred to the actual value of NutreStore® inventory from 2008 production that had to be destroyed due to its expiration date. Purchases of L-glutamine from two vendors amounted to 48% and 52% of total purchases during the years ended December 31, 2010 and December 31, 2009, respectively.
46
Research and Development Expenses. Research and development expenses increased $0.5 million, or 99%, from $0.5 million to $1.1 million for the years ended December 31, 2009 and 2010, respectively. This increase was primarily due to increases in our CRO costs and study site costs due to the commencement of the Phase III clinical trial of our L-glutamine treatment for SCD. For the year ended December 31, 2009, the CRO was not actively engaged with the Phase III study activities and there were no active sites or pharmacist costs. For the year ended December 31, 2010, the CRO spent a tremendous amount of time with Phase III study related activities and a total of 13 clinical study sites were actively recruiting subjects. Research and development costs increased as follows; $418,005 for CRO costs, $120,986 for study site expenses, $3,425 for central laboratory costs and $8,813 in pharmacist costs.
Selling Expenses. We incurred selling expenses of $0.7 million for the years ended December 31, 2010 and December 31, 2009, respectively. Selling expense included the cost of distribution, promotion, travel, tradeshows and exhibits for NutreStore®, Zorbtive®, and AminoPure®.
General and Administrative Expenses. General and administrative expenses increased $0.5 million, or 40%, from $1.3 million to $1.8 million for the years ended December 31, 2009 and December 31, 2010, respectively. The increase was largely due to an increase in costs related to increased legal fees and consulting fees incurred in 2010. The Company increased its officer and professional staff to support its effort to prepare for the Merger. As a result of this effort, payroll expenses increased $206,100. In addition, legal and consulting expenses increased by $244,331 due to our preparation for the Merger.
Liquidity and Capital Resources
Based on our losses to date, anticipated future revenue and operating expenses and our cash and cash equivalents balance of $1,075,564 as of March 31, 2011, the Company does not appear to have sufficient operating capital without raising additional capital. We incurred losses of $1,132,203 for the three months ended March 31, 2011 and $3,757,370 for the year ended December 31, 2010. We had an accumulated deficit since inception to March 31, 2011 of $13,883,839. Further the Company appears to have inadequate cash and cash equivalents of $1,075,564 as of March 31, 2011 considering that revenues from operations since inception totaled only $403,355 We anticipate that we will continue to incur net losses for the foreseeable future as we incur expenses for the development and commercialization of L-glutamine as a prescription drug for the treatment of sickle cell disease and the expansion of corporate infrastructure, including costs associated with being a public company. As a result, we will seek to fund operations through public or private equity or debt financings or other sources, such as strategic partnership agreements. As part of this effort, we have received many loans from stockholders as discussed below. Additionally, we raised approximately $1.2 million in a private offering of common stock in April 2011. Our failure to raise capital as and when needed would have a negative impact on our financial condition and our ability to pursue our business strategies.
Due to the uncertainty of our ability to meet our current operating and capital expenses, in their report on our audited annual financial statements as of and for the years ended December 31, 2010 and 2009, our independent auditors included an explanatory paragraph regarding concerns about our ability to continue as a going concern. Our financial statements contain additional note disclosures describing the circumstances that led to this disclosure by our independent auditors. There is substantial doubt about our ability to continue as a going concern as the continuation and expansion of our business is dependent upon obtaining further financing, successful and sufficient market acceptance of our products, and, finally, achieving a profitable level of operations.
On April 8, 2011, pursuant to a Research Agreement, we agreed to pay CellSeed $8,500,000 within 30 days of the completion of all of the following: (i) the execution of the Research Agreement; (ii) the execution of the Individual Agreement; and (iii) CellSeed’s delivery of the Package to us. Pursuant to the Individual Agreement, the Company agreed to pay $1,500,000 to CellSeed within 30 days of CellSeed’s delivery of the Package to the Company and a royalty to be agreed upon by the parties. We currently anticipate the delivery of the Package to Emmaus to be completed in the beginning of 2012; although the anticipated date may change as the development process progresses. CellSeed may cancel the agreements if we cannot make the payments when required. In addition, we believe that we will need approximately $3 million for research and development to develop corneal cell sheet technology in the U.S.
47
In addition to the $10 million we have agreed to pay CellSeed, we currently estimate that we will need an additional $7.3 million to complete our Phase III clinical trial and $400,000 to obtain FDA approval for our L-glutamine treatment for SCD. Our current burn rate is approximately $400,000 per month. Our future capital requirements are substantial and may increase beyond our current expectations depending on many factors including: the duration and results of the clinical trials for our various products going forward; unexpected delays or developments in seeking regulatory approvals; the time and cost in preparing, filing, prosecuting, maintaining and enforcing patent claims; other unexpected developments encountered in implementing our business development and commercialization strategies; the outcome of litigation, if any; and further arrangements, if any, with collaborators. We will in part rely on sales of Aminopure for revenus, which we expect to increase. However, until we can generate a sufficient amount of product revenue, if ever, future cash needs are expected to be financed through public or private equity offerings, debt financings or corporate collaboration and licensing arrangements. If we do not receive adequate funding to complete our clinical trials or to obtain FDA approval for our drug, we may have to delay our trial. If we have to delay our trial, we cannot enroll additional subjects which will delay the approval of the study treatment. With respect to our agreements with CellSeed, CellSeed may terminate its agreements with us if we are unable to make payments required under the agreements with CellSeed when due, however, we will work with CellSeed to try to restructure our payment schedule in the event we have insufficient capital to make such payments when due.
The cashflow from operations is not adequate, but, our future capital requirements are substantial and may increase beyond our current expectations depending on many factors including: the duration and results of the clinical trials for our various products going forward; unexpected delays or developments in seeking regulatory approvals; the time and cost in preparing, filing, prosecuting, maintaining and enforcing patent claims; other unexpected developments encountered in implementing our business development and commercialization strategies; the outcome of litigation, if any; and further arrangements, if any, with collaborators. Until we can generate a sufficient amount of product revenue, if ever, future cash needs are expected to be financed through public or private equity offerings, debt financings or corporate collaboration and licensing arrangements. There can be no assurance of the availability of such capital on terms acceptable to the Company.
From inception through March 31, 2011, we have funded consolidated operations through the private placements of approximately $15 million of equity securities, including $1.5 million from bridge notes that have been converted to equity, and approximately $1.6 million in promissory notes. Additionally, we have funded a portion of operations from sales revenues from NutreStore® and AminoPure®. We experienced a $0.8 million increase in cash and cash equivalents from $0.3 million as of December 31, 2010 to $1.1 million as of March 31, 2011. We used $0.8 million of proceeds from notes payable and $1.2 million of proceeds from the issuance of common stock to offset $1.2 million of cash used in operating activities.
For the three months ended March 31, 2011 and during the year ended December 31, 2010, Emmaus Medical borrowed varying amounts pursuant to promissory notes, including certain of its stockholders. As of March 31, 2011 and December 31, 2010, Emmaus Medical’s borrowings totaled $1.6 million and $0.9 million, respectively. Of the $1.6 million of notes outstanding as of March 31, 2011, $1.1 million was due to stockholders and all of the amounts outstanding under the notes as of December 31, 2010 were to stockholders. The notes carry interest from 0% to 10% and except for one note in the principal amount of $500,000, the debt is unsecured. Interest on 0% loans was imputed at the incremental borrowing rate of 6% per annum. The proceeds of the loans provided us with needed working capital. The Company expects to rely on loans in the future, including loans from related parties.
48
The table below lists the outstanding loans as of March 31, 2011 and the material terms of our outstanding loans:
| Lender | Loan Type | Annual Interest Rate | Date of loan | Term of Loan | Principal Loan Amount | Amount Outstanding as of March 31, 2011 (1) | |||||||||||
| Hope Hospice International | Unconvertible | 8 | % | 1/12/11 | 2 years | $ | 200,000 | $ | 203,467 | ||||||||
| Willis C. Lee | Unconvertible | 8 | % | 1/12/11 | 2 years | $ | 100,000 | $ | 101,733 | ||||||||
| Yutaka Niihara | Unconvertible | 6.5 | % | 1/12/09 | Due on demand | $ | 350,000 | $ | 351,201 | ||||||||
| Yutaka Niihara | Unconvertible | 6.5 | % | 4/23/09 | Due on demand | $ | 80,000 | $ | 80,116 | ||||||||
| Daniel Kimbell | Unconvertible | 6.5 | % | 4/27/09 | Due on demand | $ | 20,000 | $ | 20,014 | ||||||||
| Daniel Kimbell | Unconvertible | 6.5 | % | 5/11/09 | Due on demand | $ | 10,000 | $ | 10,042 | ||||||||
| Nami Murakami | Convertible | 0 | % | 8/16/2010 | 5 years | $ | 18,000 | $ | 18,000 | ||||||||
| Makoto Murakami | Convertible | 0 | % | 8/16/2010 | 5 years | $ | 18,000 | $ | 18,000 | ||||||||
| Kazuo Murakami | Convertible | 0 | % | 8/16/2010 | 5 years | $ | 18,000 | $ | 18,000 | ||||||||
| M’s Support Co. Ltd. | Convertible | 0 | % | 8/17/2010 | 5 years | $ | 18,000 | $ | 18,000 | ||||||||
| Yumiko Takemoto | Convertible | 6 | % | 11/23/2010 | 5 years | $ | 2,000 | $ | 2,000 | ||||||||
| Shigeru Matsuda | Convertible | 6.5 | % | 1/12/2009 | 5 years | $ | 246,889 | $ | 253,819 | ||||||||
| Mitsubishi UFJ Capital III, Limited Partnership | Convertible | 10 | % | 3/14/11 | 5 years | $ | 500,000 | $ | 500,000 | ||||||||
| TOTAL | $ | 1,580,889 | $ | 1,594,392 | |||||||||||||
| (1) | Represents the full amount outstanding (inclusive of accrued interest) on each loan as of the date indicated |
Unconvertible Notes
The loans to the Company from Willis Lee and Hope Hospice International are evidenced by promissory notes, copies of which are filed as exhibits to this report as 10.18 and 10.19, respectively. Pursuant to the notes, interest is payable quarterly with the principal being due and payable on the maturity date. If we fail to make any payment when due under the note or we seek relief under the U.S. Bankruptcy Code or suffer an involuntary petition in bankruptcy or receivership that is not vacated within 30 days, the entire balance of the note is due and payable to the holder.
The loans to the Company from Dr. Niihara are evidenced by promissory notes, copies of which are filed as exhibits 10.16 and 10.17 to this report. Pursuant to the notes, interest is payable quarterly with principal and any accrued unpaid interest being due upon demand by the holder. If we fail to make a payment within 10 days after the due date, we must pay an additional late fee of 2% of the late interest payment. If we fail to make any payment when due under the note, breach any condition relating to any security for the note (although the note is unsecured), seek relief under the U.S. Bankruptcy Code or suffer an involuntary petition in bankruptcy or receivership that is not vacated within 30 days, the entire balance of the note is due and payable to the holder.
The loans to the Company from Daniel Kimbell are evidenced by promissory notes, copies of which are filed as exhibits 10.21 and 10.22 to this report. Pursuant to the notes, interest is payable monthly. If we fail to make a payment within 10 days after the due date, we must pay an additional late fee of 2% of the late interest payment. If we fail to make any payment when due under the notes, breach any condition relating to any security for the notes (although the note is unsecured), seek relief under the U.S. Bankruptcy Code or suffer an involuntary petition in bankruptcy or receivership that is not vacated within 30 days, the entire balance of the notes is due and payable to the holder.
Convertible Notes
The loans to the Company from Nami Murakami, Makoto Murakami, Kazuo Murakami and M’s Support Co. Ltd. are evidenced by promissory notes, a form of which is filed as exhibit 4.3 to this report. Pursuant to the notes, interest is payable quarterly with the principal being due and payable on the maturity date. The holder, at his option, may convert the principal amount of the note into common stock of our Company at a conversion price of $3.05 per share. If we fail to make any payment when due under the note or we seek relief under the U.S. Bankruptcy Code or suffer an involuntary petition in bankruptcy or receivership that is not vacated within 30 days, the entire balance of the note is due and payable to the holder.
49
The loan to the Company from Mitsubishi UFJ Capital III, Limited Partnership is evidenced by a promissory note, a copy of which is filed with this report as exhibit 4.2. Pursuant to the note, interest accrues at 10% per annum beginning on January 1, 2012. Interest only payments are due monthly with the principal being due and payable on the maturity date. The holder, at any time during the term of the note but no later than one month after the date our shares of common stock are traded on NASDAQ, may convert the principal amount and any accrued interest owing at the time of such conversion into common stock of the Company at $3.05 per share. Upon the conversion of the note, the holder will also receive a warrant to purchase shares of our common stock in an amount equal to 25% of the number of shares received upon the conversion of the note. The exercise price of the warrants will be $3.05 per share. The principal amount of the note is secured by 73,550 shares of common stock of CellSeed owned by Emmaus Medical. If we fail to make any payment when due under the note, seek relief under the U.S. Bankruptcy Code or suffer an involuntary petition in bankruptcy or receivership that is not vacated within 30 days, the entire balance of the note is due and payable to the holder. In addition, the lender May require us to perform all obligations under the note in the event our shares are not traded on NASDAQ on or prior to December 31, 2011 or that any stockholder is engaged in any antisocial activities.
The loan to the Company from Shigeru Matsuda is evidenced by a promissory note, a copy of which is filed with this report as exhibit 4.4. If requested by the lender, we must make quarterly interest only payments. The lender may also allow interest payments to accrue, pursuant to which the unpaid but accrued interest shall be added to the principal. The entire unpaid principal and any accrued interest thereon shall become immediately due and payable on demand by the holder. The holder, at any time during the term of the note, may convert the principal amount and any accrued interest owing at the time of such conversion into common stock of the Company at $3.05 per share. If we fail to make a payment within 10 days after the due date, we must pay an additional late fee of 2% of the late interest payment. Dr. Niihara and Daniel Kimbell, the former Chief Operating Officer of Emmaus Medical, agreed to be the primary guarantor and secondary guarantor on the note such that in the event we cannot pay the note, Dr. Niihara and Mr. Kimbell, in that order, will make payments due to the lender. If we or the guarantors fail to make any payment due under the terms of the note, or breach any condition relating to any security, security agreement, note, mortgage or lien granted as collateral security for the note, seek relief under the U.S. Bankruptcy Code, or suffer an involuntary petition in bankruptcy or receivership not vacated within 30 days, the entire balance of the note and any interest accrued thereon shall be immediately due and payable to the holder.
The loan to the Company from Yumiko Takemoto is evidenced by a promissory note, a copy of which is filed as exhibit 4.5 to this report. Pursuant to the note, interest is payable quarterly with the principal being due and payable on the maturity date. The holder, at his option, may convert the principal amount of the note into common stock of our Company at a conversion price of $3.05 per share. If we fail to make any payment when due under the note or we seek relief under the U.S. Bankruptcy Code or suffer an involuntary petition in bankruptcy or receivership that is not vacated within 30 days, the entire balance of the note is due and payable to the holder.
Cash Flows
Net cash flows increased $.4 million, or 91%, from $.4 million to $.8 million for the three months ended March 31, 2010 and 2011, respectively. Net cash flows increased $.5 million, or 79% from $(.67 million) to $(.14 million) for the year ended December 31, 2009 and 2010, respectively.
Net cash used in operating activities
Net cash flows used in operating activities increased $.7 million, or 176%, from $0.4 million to $1.1 million for the three months ended March 31, 2010 and 2011, respectively. This increase was primarily due to a $0.4 million increase in operating expenses and an increase of $0.4 million in funds used to decrease accounts payable outstanding. Net cash flows used in operating activities increased $1.5 million, or 67%, from $2.2 million to $3.7 million for the years ended December 31, 2009 and 2010, respectively. This increase was primarily due to a $1.2 million increase in operating expenses and a $.3 million increase in deposits for the CRO.
Net cash used in investing activities
Net cash flows used in investing activities increased from none to $1,494 for the three months ended March 31, 2010 and 2011, respectively for the purchase of property and equipment. Net cash flows used in investing activities decreased $1.1 million from $1.14 million to $.24 million for the years ended December 31, 2009 and 2010, respectively primarily due to the 2010 purchase of $1.1 million of CellSeed stock, which are publicly traded in JASDAQ.
50
Net cash from financing activities
Net cash flows from financing activities increased $1.1 million, or 133%, from $.84 million to $1.96 million for the three months ended March 31, 2010 and 2011, respectively primarily as a result a $.4 million increase in the proceeds from the issuance of common stock and a $.7 million increase in the proceeds from the issuance of notes payable and convertible notes payable Net cash flows from financing activities increased $.9 million, or 33%, from $2.7 million to $3.6 million for the years ended December 31, 2009 and 2010, respectively primarily due to a $1.5 million increase in proceeds from issuances of convertible notes payable and a $.3 million decrease in repayments of lines of credit partially offset by a $.7 million decrease in proceeds from the issuance of notes payable.
A total of $0.1 million of convertible notes payable were converted into common stock during the three months ended March 31, 2011 compared to none for the three months ended March 31, 2010. $1.3 million of convertible notes payable were converted into common stock during the year ended December 31, 2010 compared to none in the year ended December 31, 2009.
Off-Balance-Sheet Arrangements
Since our inception, Emmaus has not engaged in any off-balance sheet arrangements, including the use of structured finance, special purpose entities or variable interest entities.
Critical Accounting Policies
Management’s discussion and analysis of financial condition and results of operations is based on our financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States, or GAAP. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities and expenses. On an ongoing basis, we evaluate these estimates and judgments, including those described below. We base our estimates on our historical experience and on various other assumptions that we believe to be reasonable under the circumstances. These estimates and assumptions form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ materially from these estimates.
While our significant accounting policies are more fully described in Note 2 to our financial statements included at the end of this prospectus, we believe that the following accounting policies are the most critical to aid you in fully understanding and evaluating our reported financial results and affect the more significant judgments and estimates that we use in the preparation of our financial statements.
Revenue recognition
We recognize revenue in accordance with the Securities and Exchange Commission (“SEC”) Staff Accounting Bulletin (“SAB”) No. 101, Revenue Recognition in Financial Statements (“SAB 101”), as amended by Staff Accounting Bulletin No. 104, Revision of Topic 13 (“SAB 104”).
Revenue is recognized when there is persuasive evidence that an arrangement exists, delivery has occurred, the price is fixed and determinable and collection is reasonably assured.
With prior written approval of the Company, product is returnable only by our direct customers for a returned goods credit, if the product meets any of the following criteria:
| A. | Product expiring within six (6) months of the expiration date printed on the package/container that is in the manufacturer’s original package/container and bears the original label. | |
| B. | Expired product that is in the manufacturer’s original package/container and bears the manufacturer’s original label, provided, however, that expired product must be returned within 12 months of the expiration date printed on the package/container. | |
| C. | Product shipped directly by the Company that is damaged in transit, subject to Free on Board (“FOB”) Destination, or material shipped in error by the Company. | |
| D. | Product that is discontinued, withdrawn, or recalled. |
51
Credits will only be issued for full cartons only without any missing packets of product. No credit is issued, nor does the Company accept charges or deductions for administrative, handling, or freight charges associated with the return of product to the Company. No credit is issued for product destroyed by anyone other than the Company. Customers must return the product within 60 days of receiving our written approval for the return or the return will not be issued a credit. The amount of the credit provided for returned product is based on the current wholesale acquisition cost of the returned product less 5%. When product is returned, a credit memo is applied to the customer’s current account balance or applied to future purchases. Credit memos expire one hundred eighty (180) days from date issued.
We estimate our sales return based upon our prior sales and return history. Historically, sales returns have been very nominal. The extraordinary sales returns in the six months ended June 30, 2010 were all related to the same batch of NutreStore product produced in 2008 that expired. We continue to monitor our returns and will adjust our estimates based on our actual sales return experience. Based upon our sales return history we will record a reserve for estimated returns during each reporting period. As of March 31, 2011, no reserve for sales returns has been deemed necessary and accordingly, no reserve has been recorded in the accompanying financial statements.
We are required to pay royalties, which are recognized as expense upon sale of the products. The royalty equivalent to 10% of adjusted gross sales is due to CATO on an annual basis. The 10% royalty is calculated at the end of the year and accrued on an annual basis.
Share-based payments
We recognize compensation cost for share-based compensation awards during the service term of the recipients of the share-based awards. The fair value of share-based is calculated using the Black-Scholes-Merton pricing model. The Black-Scholes-Merton model requires subjective assumptions regarding future stock price volatility and expected time to exercise, which greatly affect the calculated values. The expected term of awards granted is derived from historical data on awards exercised and post-vesting employment termination behavior. The risk-free rate selected to value any particular grant is based on the U.S. Treasury rate that corresponds to the vesting period of the grant effective as of the date of the grant. The expected volatility is based on the historical volatility of the common stock of comparable publicly traded companies. These factors could change, affecting the determination of stock-based awards expense in future periods.
Marketable securities
Securities available-for-sale are recorded at cost and any increases or decreases in fair market value are recorded as unrealized gain or loss, net of taxes in accumulated other comprehensive income. We monitor these investments for impairment and make appropriate reductions in carrying values when necessary.
Item 3.02 Unregistered Sales of Equity Securities.
Shares Issued by the Issuer
On May 3, 2011, pursuant to the terms of the Merger Agreement (i) each outstanding share of Emmaus Medical common stock was exchanged for approximately 29.48548924976 shares of AFH IV common stock, (ii) each outstanding Emmaus Medical option and warrant, which was exercisable for one share of Emmaus Medical common stock, was exchanged for an option or warrant, as applicable, exercisable for 29.48548924976 shares of AFH common stock; and (iii) each outstanding convertible note of Emmaus Medical, which was convertible for one share of Emmaus Medical common stock, was exchanged for a convertible note exercisable for 29.48548924976 shares of AFH IV common stock. As a result of the Merger, holders of Emmaus Medical common stock, options, warrants and convertible notes received 20,628,305 of our shares of common stock (excluding 47,178 shares held by stockholders who exercised dissenters’ rights in connection with the Merger), options and warrants to purchase an aggregate of 326,507 shares of our common stock and convertible notes exercisable for 270,648 shares of our common stock, or 85% of our issued and outstanding common stock on a fully diluted basis.
All of the securities issued pursuant to the Merger Agreement were offered and issued in reliance upon the exemption from registration pursuant to the exemption from registration provided by Section 4(2) of the Securities Act of 1933, as amended (the “Securities Act”) and Rule 506 promulgated thereunder. Each of the securityholders of Emmaus Medical qualified as an “accredited investor,” as defined by Rule 501 under the Securities Act.
52
On April 19, 2011, we sold an aggregate of 577,750 shares of our common stock in a private placement transaction at a purchase price per share equal to $2.00 for aggregate cash proceeds of $1,155,500. The shares were sold to in reliance upon the exemption from registration pursuant to the exemption from registration provided by Section 4(2) of the Securities Act and Rule 506 promulgated thereunder. These securities qualified for exemption under Rule 506 promulgated under Section 4(2) of the Securities Act because the issuance of securities by the Company did not involve a “public offering”. The issuance was not a public offering based upon the following factors: (i) a limited number of securities were issued to a limited number of offerees; (ii) there was no public solicitation; (iii) each offeree was an “accredited investor” as such term is defined by Rule 501 under the Securities Act; and (iv) the investment intent of the offerees.
Securities Issued by Emmaus Medical Prior to the Merger
During the year ended December 31, 2008 prior to the Merger, Emmaus Medical sold an aggregate of 41,613 shares of its common stock to various accredited investors for total proceeds of $2.5 million. During the year ended December 31, 2009 prior to the Merger, Emmaus Medical (a) sold an aggregate of 28,979 shares of its common stock to various accredited investors for total proceeds of $2.2 million; (b) issued warrants to purchase an aggregate 3,863 shares of its common stock at an exercise price of $90 to the purchasers of the common stock in connection with the sale of common stock that expire upon the earlier of five years from the date of issuance or upon the closing of the Company’s initial public offering of common stock; (c) issued a convertible note to Shigeru Matsuda in the principal amount of 20,000,000 Japanese Yen (US$221,895) which was convertible into common stock of Emmaus Medical at $90 per share. During the year ended December 31, 2010 prior to the Merger, Emmaus Medical (a) sold an aggregate of 23,941 shares of its common stock to various accredited investors for total gross proceeds of $2.1 million; (b) issued warrants to purchase an aggregate total of 6,270 shares of its common stock with an exercise price of $90 per share to the purchasers of the common stock in connection with the sale of common stock that expire upon the earlier of five years from the date of issuance or upon the closing of the Company’s initial public offering of common stock; (c) issued 14,511 shares of its common stock upon the conversion of $1,306,000 in convertible notes at a conversion price of $90 per share; (d) issued convertible notes to various accredited investors in the aggregate principal amount of $1,490,030 convertible into common stock of Emmaus Medical at $90.00 per share; and (e) issued options to purchase an aggregate of 800 shares of its common stock, with 200 of such options vesting on June 30, 2010, 200 of such options vesting on September 30, 2010, 200 of such options vesting on December 31, 2011 and 200 of such options vesting on March 31, 2011. Emmaus Medical issued 1,223 shares of common stock upon the conversion of $110,030 of convertible notes in January 2011 prior to the Merger at a conversion price of $90 per share. In March 2011 prior to the Merger, Emmaus Medical (a) sold 9,230 shares of common stock to various accredited investors at $125 per share for total gross proceeds of $1.2 million and (b) issued a convertible note in the principal amount of $500,000 to Mitsubishi UFJ Capital II, Limited Partnership, an accredited investor, convertible into common stock of Emmaus Medical at $90 per share. During the three months ended March 31, 2011, Emmaus Medical granted warrants to its investors and lenders to purchase an aggregate of 200 shares of its common stock at an exercise price of $90 per share to the purchasers of the common stock in connection with the sale of common stock that expire upon the earlier of five years from the date of issuance or upon the closing of the Company’s initial public offering of common stock. In April 2011 prior to the Merger, Emmaus Medical issued 60 shares of common stock upon the exercise of a warrant.
All of the securities issued by Emmaus Medical were sold or issued in reliance upon the exemption from registration provided by Section 4(2) of the Securities Act and Rule 506 promulgated thereunder. These securities qualified for exemption under Rule 506 promulgated under Section 4(2) of the Securities Act because the issuance of shares by the Company did not involve a “public offering.” The issuance was not a public offering based upon the following factors: (i) a limited number of securities were issued to a limited number of offerees; (ii) there was no public solicitation; (iii) each offeree was an “accredited investor” as such term is defined by Rule 501 under the Securities Act; and (iv) the investment intent of the offerees.
53
DESCRIPTION OF SECURITIES – POST- MERGER
Common Stock
We are authorized to issue 100,000,000 shares of common stock, $0.001 par value per share. There are currently 24,378,305 shares of common stock issued and outstanding. Each outstanding share of common stock is entitled to one vote, either in person or by proxy, on all matters that may be voted upon by their holders at meetings of the stockholders.
Holders of our common stock:
| (i) | have equal ratable rights to dividends from funds legally available therefore, if declared by our Board of Directors; | |
| (ii) | are entitled to share ratably in all of the Company’s assets available for distribution to holders of common stock upon our liquidation, dissolution or winding up; | |
| (iii) | do not have preemptive, subscription or conversion rights or redemption or sinking fund provisions; and | |
| (iv) | are entitled to one non-cumulative vote per share on all matters on which stockholders may vote at all meetings of our stockholders. |
The holders of shares of our common stock do not have cumulative voting rights, which means that the holders of more than fifty percent (50%) of outstanding shares voting for the election of directors can elect all of our directors if they so choose and, in such event, the holders of the remaining shares will not be able to elect any of our directors.
At the completion of the Merger, our officers and directors collectively own approximately 50.9% of the outstanding shares of our common stock on an undiluted basis. Accordingly, these stockholders are in a position to control all of our affairs.
Preferred Stock
We may issue up to 20,000,000 shares of our preferred stock, par value $0.001 per share, from time to time in one or more series. No shares of Preferred Stock have been issued.
Our Board of Directors, without further approval of our stockholders, is authorized to fix the dividend rights and terms, conversion rights, voting rights, redemption rights, liquidation preferences and other rights and restrictions relating to any series. Issuances of shares of preferred stock, while providing flexibility in connection with possible financings, acquisitions and other corporate purposes, could, among other things, adversely affect the voting power of the holders of our common stock and prior series of preferred stock then outstanding.
Warrants
Upon consummation of the Merger, each outstanding Emmaus Medical warrant, which was exercisable for one share of Emmaus Medical common stock, was exchanged for a warrant exercisable for 29.48548924976 shares of AFH IV common stock. As a result of the Merger, holders of Emmaus Medical warrants received warrants to purchase an aggregate of 302,917 of our shares at an exercise price of $3.05 per share. The warrants are currently exercisable and expire on the earlier of (1) the date of the closing of our initial public offering of common stock (the “Public Offering Termination”) or (2) five years from their respective dates of issuance. Unless the warrants expire earlier pursuant to the Public Offering Termination, the warrants expire on various dates from June 15, 2014 through November 21, 2015.
Options
Upon consummation of the Merger, each outstanding Emmaus Medical option, which was exercisable for one share of Emmaus Medical common stock, was exchanged for an option exercisable for 29.48548924976 shares of AFH IV common stock. As a result of the Merger, holders of Emmaus Medical options received options to purchase an aggregate of 23,590 of our shares at an exercise price of $3.05 per share. The options are exercisable five years from the date of their original issuance by Emmaus Medical on June 30, 2010 and are fully vested.
54
Convertible Notes
Upon consummation of the Merger, each outstanding convertible note of Emmaus Medical, which was convertible for one share of Emmaus Medical common stock, was exchanged for a convertible note exercisable for 29.48548924976 shares of AFH IV common stock. As a result of the Merger, holders of Emmaus Medical convertible notes received convertible notes exercisable for 270,648 shares of common stock. The convertible notes are convertible at any time into shares of our common stock at $3.05 per share until their maturity date. Of the convertible notes, notes convertible into 83,247 shares mature in January 2014, notes convertible into 23,592 shares mature in August 2015, and notes convertible into 163,809 shares mature in March 2016.
MARKET PRICE OF AND DIVIDENDS ON THE COMPANY’S COMMON STOCK AND RELATED STOCKHOLDER MATTERS
The shares of our common stock are not currently listed or quoted for trading on any national securities exchange or national quotation system. We intend to apply for the listing of our common stock on the NASDAQ Global Market. In order to comply with the corporate governance standards of NASDAQ Global Market, we have added independent directors to our board of directors and formed an audit committee, compensation committee and nominating committee comprised of independent directors. The Company believes that it will meet all initial listing standards of the NASDAQ Global Market.
If and when our common stock is listed or quoted for trading, the price of our common stock will likely fluctuate in the future. The stock market in general has experienced extreme stock price fluctuations in the past few years. In some cases, these fluctuations have been unrelated to the operating performance of the affected companies. Many companies have experienced dramatic volatility in the market prices of their common stock. We believe that a number of factors, both within and outside our control, could cause the price of our common stock to fluctuate, perhaps substantially. Factors such as the following could have a significant adverse impact on the market price of our common stock:
| ● | our financial position and results of operations; | |
| ● | our ability to obtain additional financing and, if available, the terms and conditions of the financing; | |
| ● | the ability of our products to gain market acceptance; | |
| ● | announcements of innovations or new products by us or our competitors; | |
| ● | federal and state regulatory actions and the impact of such requirements on our business; | |
| ● | the development of litigation against us; | |
| ● | changes in estimates of our performance by any securities analysts; | |
| ● | the issuance of new equity securities pursuant to a future offering or acquisition; | |
| ● | competitive developments, including announcements by competitors of new products or services or significant contracts, acquisitions, strategic partnerships, joint ventures or capital commitments; | |
| ● | period-to-period fluctuations in our operating results; | |
| ● | investor perceptions of us; and | |
| ● | general economic and other national conditions. |
Stockholders of Record
As of May 3, 2011, there were 323 stockholders of record of our common stock.
55
Dividends
There were no dividends paid during the years ended December 31, 2010 or 2009.
Derivative Securities
As of the closing of the Merger, we had warrants to purchase 302,917 shares of our common stock, options to purchase 23,590 shares of our common stock and convertible notes convertible into 270,648 shares of our common stock outstanding.
Rule 144
In general, under Rule 144 a person, or persons whose shares are aggregated, who is not deemed to have been one of our affiliates at any time during the 90 days preceding a sale and who has beneficially owned shares of our common stock for at least six months, including the holding period of any prior owner, except if the prior owner was one of our affiliates, would be entitled to sell all of their shares, provided the availability of current public information about our company.
Sales under Rule 144 may also subject to manner of sale provisions and notice requirements and to the availability of current public information about our company. Any substantial sale of common stock pursuant to any resale registration statement or Rule 144 may have an adverse effect on the market price of our common stock by creating an excessive supply.
Because we were a shell company with no operations prior to the close of the Merger, sales of our shares must be compliant with Rule 144(i). Pursuant to Rule 144(i), none of our shares of common stock may be sold under Rule 144 until May 4, 2012, which is 12 months after the filing of this current report on Form 8-K reporting the closing of the Merger. Additionally, stockholders may not sell our shares pursuant to Rule 144 unless at the time of the sale, we have filed all reports, other than reports on Form 8-K, required under the Exchange Act with the SEC for the preceding 12 months.
Registration Rights
In connection with the consummation of the Merger, we entered into the Registration Rights Agreement for the benefit of the Existing AFH IV Stockholders. Pursuant to the Registration Rights Agreement, the Existing AFH IV Stockholders will have certain “piggyback” registration rights on registration statements filed after the Merger is consummated other than registration statements (i) filed in connection with any employee stock option or other benefit plan, (ii) for an exchange offer or offering of securities solely to the Company’s existing stockholders, (iii) for an offering of debt that is convertible into equity securities of the Company; (iv) for a dividend reinvestment plan or (v) for an offering of equity securities of the Company underwritten by Sunrise Securities Corp. The Company will bear the expenses incurred in connection with the filing of any such registration statements.
DELAWARE ANTI-TAKEOVER LAW AND CHARTER AND BYLAW PROVISIONS
We are subject to Section 203 of the Delaware General Corporation Law. This provision generally prohibits a Delaware corporation from engaging in any business combination with any interested stockholder for a period of three years following the date the stockholder became an interested stockholder, unless:
| ● | prior to such date, the Board of Directors approved either the business combination or the transaction that resulted in the stockholder becoming an interested stockholder; | |
| ● | upon consummation of the transaction that resulted in the stockholder becoming an interested stockholder, the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction commenced, excluding for purposes of determining the number of shares outstanding those shares owned by persons who are directors and also officers and by employee stock plans in which employee participants do not have the right to determine confidentially whether shares held subject to the plan will be tendered in a tender or exchange offer; or | |
| ● | on or subsequent to such date, the business combination is approved by the Board of Directors and authorized at an annual meeting or special meeting of stockholders and not by written consent, by the affirmative vote of at least 66 2/3% of the outstanding voting stock that is not owned by the interested stockholder. |
56
Section 203 defines a business combination to include:
| ● | any merger or consolidation involving the corporation and the interested stockholder; | |
| ● | any sale, transfer, pledge or other disposition of 10% or more of the assets of the corporation involving the interested stockholder; | |
| ● | subject to certain exceptions, any transaction that results in the issuance or transfer by the corporation of any stock of the corporation to the interested stockholder; | |
| ● | any transaction involving the corporation that has the effect of increasing the proportionate share of the stock of any class or series of the corporation beneficially owned by the interested stockholder; or | |
| ● | the receipt by the interested stockholder of the benefit of any loans, advances, guarantees, pledges or other financial benefits provided by or through the corporation. |
In general, Section 203 defines an “interested stockholder” as any entity or person beneficially owning 15% or more of the outstanding voting stock of a corporation, or an affiliate or associate of the corporation and was the owner of 15% or more of the outstanding voting stock of a corporation at any time within three years prior to the time of determination of interested stockholder status; and any entity or person affiliated with or controlling or controlled by such entity or person.
Our certificate of incorporation and bylaws contain provisions that could have the effect of discouraging potential acquisition proposals or making a tender offer or delaying or preventing a change in control, including changes a stockholder might consider favorable. In particular, the certificate of incorporation and bylaws, as applicable, among other things:
| ● | provide our board of directors with the ability to alter its bylaws without stockholder approval; and | |
| ● | provide that vacancies on our board of directors may be filled by a majority of directors in office, although less than a quorum. |
Such provisions may have the effect of discouraging a third-party from acquiring us, even if doing so would be beneficial to our stockholders. These provisions are intended to enhance the likelihood of continuity and stability in the composition of our board of directors and in the policies formulated by them, and to discourage some types of transactions that may involve an actual or threatened change in control of our company. These provisions are designed to reduce our vulnerability to an unsolicited acquisition proposal and to discourage some tactics that may be used in proxy fights. We believe that the benefits of increased protection of our potential ability to negotiate with the proponent of an unfriendly or unsolicited proposal to acquire or restructure our company outweigh the disadvantages of discouraging such proposals because, among other things, negotiation of such proposals could result in an improvement of their terms.
However, these provisions could have the effect of discouraging others from making tender offers for our shares that could result from actual or rumored takeover attempts. These provisions also may have the effect of preventing changes in our management.
57
Item 5.01 Changes in Control of Registrant.
OVERVIEW
AFH IV entered into the Merger Agreement with AFH Merger Sub, Inc., AFH Advisory and Emmaus Medical. Pursuant to the Merger Agreement, AFH Merger Sub merged (the “Merger”) with and into Emmaus Medical with Emmaus Medical continuing as the surviving entity (we are the surviving entity of the Merger).
Immediately following the closing of the Merger, the former securityholders of Emmaus Medical beneficially owned approximately 85% of our issued and outstanding common stock on a fully diluted basis and the original AFH IV stockholders owned approximately 15%. We issued no fractional shares in connection with the Merger. Immediately after the closing of the Merger, we had outstanding 24,378,305 (excluding 47,178 shares held by stockholders who exercised dissenters’ rights) shares of common stock, no shares of Preferred Stock, options to purchase to purchase 23,590 shares of our common stock, warrants to purchase 302,917 shares of common stock and convertible notes convertible into 270,648 shares of our commons stock.
The shares of our common stock are not currently listed or quoted for trading on any national securities exchange or national quotation system. We intend to apply for the listing of its common stock on the NASDAQ Global Market.
The shares of our common stock issued to the former stockholders of Emmaus Medical in connection with the Merger were not registered under the Securities Act and, as a result, are “restricted securities” that may not be offered or sold in the United States absent registration or an applicable exemption from registration.
We intend to carry on the business of Emmaus Medical. Our relocated executive offices are located at 20725 S. Western Avenue, Ste. 136, Torrance, CA 90501-1884.
For accounting purposes, the Merger is being treated as a reverse acquisition because the former Emmaus Medical stockholders own a majority of the issued and outstanding shares of common stock of our company immediately following the Merger. Due to the issuance of the 20,628,305 (excluding 47,178 shares held by stockholders who exercised dissenters’ rights) shares of our common stock to the former Emmaus Medical common stockholders, a change in control of our company occurred on May 3, 2011.
At the consummation of the Merger, the board of directors immediately prior to the Merger, which consisted of Amir F. Heshmatpour and Timothy Brasel, appointed Yutaka Niihara, M.D., MPH, Willis C. Lee, Steve Warnecke, Dr. Henry A. McKinnell, Jr., and Douglas W. Wilmore, M.D. to the board of directors of our company. Mr. Brasel resigned as a director and as Vice President of AFH IV. In addition, Amir F. Heshmatpour resigned as President, Secretary and Chief Financial Officer of AFH IV. In addition, concurrent with the closing of the Merger, our board of directors appointed Dr. Niihara as our President and Chief Executive Officer, Mr. Lee as our Chief Operating Officer, Lan T. Tran as our Chief Administrative Officer and Corporate Secretary and Yasushi Nagasaki as our Chief Financial Officer. Dr. McKinnell was appointed Chairman of the Board. For complete information regarding our new officers and directors, refer to “Executive Officers, Directors and Key Employees” under Item 5.01, above.
A copy of the Merger Agreement is filed as Exhibit 2.1 to this Current Report on Form 8-K. The transactions contemplated by the Merger Agreement, as amended, were intended to be a “tax-free” reorganization pursuant to the provisions of Sections 351 and/or 368(a) of the Internal Revenue Code of 1986, as amended.
EXECUTIVE OFFICERS, DIRECTORS AND KEY EMPLOYEES
Prior to the Merger, Amir F. Heshmatpour and Timothy Brasel served as directors of AFH and Mr. Heshmatpour served as President, Secretary and Chief Financial Officer and Mr. Brasel served as the Vice President of AFH. Upon the closing of the Merger, Mr. Heshmatpour and Mr. Brasel resigned from all of their officer positions with the Company. Mr. Brasel also resigned as a director of the Company.
Upon closing of the Merger, the following individuals were named to the board of directors and executive management of our company:
58
| Name | Age | Position | ||
| Yutaka Niihara, M.D., MPH | 51 | President and Chief Executive Officer and Director | ||
| Willis C. Lee | 50 | Chief Operating Officer and Director | ||
| Lan T. Tran | 35 | Chief Administrative Officer and Corporate Secretary | ||
| Yasushi Nagasaki | 43 | Chief Financial Officer | ||
| Steve Warnecke | 54 | Director | ||
| Henry A. McKinnell, Jr., Ph.D. | 68 | Chairman of the Board | ||
| Amir Heshmatpour | 44 | Director | ||
| Douglas W. Wilmore, M.D. | 72 | Director |
All officers of the Company devote at least forty (40) hours per week, the equivalent of a full-time employee, to their positions with Company.
Background of Officers and Directors
The following is a brief summary of the background of each director and executive officer of the Company:
Yutaka Niihara, M.D., MPH, has served as the President and Chief Executive Officer since the closing of the Merger. Dr. Niihara has served as the President, Chief Executive Officer and Chairman of the Board of Emmaus Medical since 2003. Since May 2005, Dr. Niihara has served as the President, Chief Executive Officer and Medical Director of Hope International Hospice. From June 1992 to October 2009, Dr. Niihara served as a physician specialist for Los Angeles County. Dr. Niihara is the principal inventor of the patented L-glutamine therapy for treatment of SCD. Dr. Niihara has been involved in patient care and research for sickle cell disease during most of his career and is a widely published author in the area of sickle cell disease. Dr. Niihara is board-certified by the American Board of Internal Medicine/Medical Oncology and the American Board of Internal Medicine/Hematology. He is licensed to practice medicine in both the U.S. and Japan. Dr. Niihara is a Professor of Medicine at the David Geffen School of Medicine at UCLA. Dr. Niihara received his B.A. in Religion from Loma Linda University in 1982 and obtained his MD degree from the Loma Linda University School of Medicine in 1986 and his MPH degree from the Harvard School of Public Health in 2006. Dr. Niihara is qualified to serve on our Board of Directors due to his knowledge and experience treating SCD, his knowledge of our L-glutamine treatment for SCD and of and his knowledge of our business operations.
Willis C. Lee has served as the Chief Operating Officer and a director of the Company since the closing of the Merger. He has served as the co-Chief Operating Officer and Chief Financial Officer, and as a director of Emmaus Medical since April 2010. Prior to that, he was the Controller at Emmaus from February 2009 to February 2010. From 2004 to 2010, Mr. Lee led worldwide sales and business development of Yield Dynamics product group at MKS Instruments, Inc., a provider of instruments, subsystems, and process control solutions for the semiconductor, flat panel display, solar cell, data storage media, medical equipment, pharmaceutical manufacturing, and energy generation and environmental monitoring industries. Mr. Lee also served as President and Managing Director of Kenos Inc. from January 2004 to December 2008. Mr. Lee held various managerial and senior positions at semiconductor companies such as MicroUnity Systems Engineering, Inc. from August 1995 to July 1996, HPL, Inc. from June 2000 to October 2002, Syntricity, Inc. from November 2002 to April 2004 and also at a healthcare actuarial consulting firm, Reden & Anders from September 1996 to June 2000, which was acquired by United Health Care. Mr. Lee received his B.S. and M.S. in Physics from University of Hawaii (1984) and University of South Carolina (1986) respectively. Mr. Lee’s knowledge of our business and operations and his business, leadership and management experience qualify him to serve as a member of the Company’s board of directors.
Lan T. Tran has served as the Chief Administrative Officer and Corporate Secretary of the Company since the closing of the Merger. She has served as the Co-Chief Operating Officer and Corporate Secretary of Emmaus Medical since April 2010 and as the Chief Compliance Officer of Emmaus Medical since May 2008. Prior to joining Emmaus Medical, Ms. Tran was with LABioMed from September 1999 to April 2008 and held positions of increasing responsibility including Grants and Contracts Trainee from September 1999 to March 2000, Grants and Contracts Officer from April 2000 to August 2004, Associate Director, Pre-Clinical/Clinical Trials Unit from September 2004 to June 2005, Director, Pre-Clinical/Clinical Trials Unit from July 2005 to June 2007, and Assistant Vice President, Research Administration from June 2007 to April 2008. In her position as Director, Pre-Clinical/Clinical Trials Unit and Assistant Vice President, Research Administration, Ms. Tran was part of the executive management team of LABioMed and responsible for all administrative aspects of research in her assigned area at LABioMed, which had a research budget of $61,000,000 in 2008. Ms. Tran holds a B.S. in Psychobiology from UCLA, which was awarded in 1999, and a Masters of Public Health from UCLA which was awarded in 2002.
59
Yasushi Nagasaki was appointed Chief Financial Officer of the Company effective May 2, 2011. From September 2005 until joining our Company, Mr. Nagasaki was the Chief Financial Officer of Hexadyne Corporation, an aerospace and defense supplier. From May 2003 to August 2005, Mr. Nagasaki was the Controller at Upsilon Intertech Corporation. Mr. Nagasaki received a B.A. in Commerce in 1992 from Waseda University and an M.A. in International Policy Studies in 1994 from the Monterey Institute of International Studies.
Steve Warnecke has served as a director since the closing of the Merger. He served as the Chief Financial Officer of Targeted Medical Pharma, Inc.from January 2011 through May 2011. He also has served as chief executive officer of Evolutionary Genomics, Inc., a private company involved in genetic research for agricultural crops) since November 2010. From March 2003 to January 2011, Mr. Warnecke served as a director, Chair of the Audit Committee and Lead Independent Director of Evolving Systems, Inc. (NasdaqCM:EVOL), a provider of software solutions and services to the wireless, wireline and cable markets. From November 2008 to May 2010, Mr. Warnecke served as Chief Financial Officer of Bacterin International, Inc. (BIHI.PK) a company focused on biomaterials research and development and commercialization. From April 2002 to April 2009, he served as Chief Financial Officer of The Children’s Hospital Foundation, a Colorado not-for-profit foundation. Mr. Warnecke also serves as chairman of Children’s Partners Foundation and serves on the board of directors of the Cystic Fibrosis Foundation. In addition, from August 2001 through January 2002, Mr. Warnecke served as Senior Vice President — Strategic Planning for First Data Corp.’s Western Union subsidiary. From August 1999 through June 2001, Mr. Warnecke served as Chief Financial Officer for Denver-based Frontier Airlines. Mr. Warnecke spent the first twenty years of his career, 1979 to 1999, in financial management and chief executive officer positions in the construction industry. He graduated in 1979 from the University of Iowa with a Bachelor of Business Administration degree and passing the C.P.A. exam. Mr. Warnecke is qualified to serve on our Board of Directors due to his knowledge of U.S. GAAP and audit committee functions and his experience serving in various financial management and board of directors roles in U.S. public and private companies.
Henry A. McKinnell, Jr., Ph.D. has served as Chairman of the Board of the Company since the closing of the Merger and as a director of Emmaus Medical since April 2010. Dr. McKinnell served as the Chairman of the Board of Pfizer Inc. (NYSE: PFE), a pharmaceutical company, from May 2001 until his retirement in December 2006. He also served as Chief Executive Officer of Pfizer from January 2001 to July 2006. He served as President of Pfizer from May 1999 to May 2001, and as President of Pfizer Pharmaceuticals Group from January 1997 to April 2001. Dr. McKinnell served as Chief Operating Officer of Pfizer from May 1999 to December 2000 and as Executive Vice President from 1992 to 1999. Since October 1997, Dr. McKinnell has served as a member of the board of directors of Moody’s Corporation (NYSE: MCO), where he serves as the lead independent director and a member of the audit committee, the governance and compensation committee and MIS committee. From 2008 to May 2011, Dr. McKinnell served as a director of Angiotech Pharmaceuticals, Inc. (OTCBB: ANPIQ), and as a member of the audit committee and the governance, nominating and compensation committee. Dr. McKinnell has served as a director of Optimer Pharmaceuticals, Inc. (NasdaqGM: OPTR) since January 2001 and serves as a member of the nominating and corporate governance committee and the science and technology committee. Dr. McKinnell also serves as Chairman of the Board of the Accordia Global Health Foundation. He is Chairman Emeritus of the Connecticut Science Center, and is a member of the Academic Alliance for AIDS Care and Prevention in Africa. He served as director of ExxonMobil Corporation from 2002 to 2007 and John Wiley & Sons until 2005. Dr. McKinnell holds a Bachelor’s Degree in business from the University of British Columbia, and M.B.A. and Ph.D. degrees from the Stanford University Graduate School of Business. We believe that Dr. McKinnell is qualified to serve on our board of directors due to his extensive experience and leadership in the pharmaceutical industry, in addition to his substantial involvement with business and civic organizations and years of experience as an officer and director of publicly traded companies.
60
Amir Heshmatpour has served as a director since the closing of the Merger and served as the President, Secretary and Chief Financial Officer and a director of AFH IV from September 2007 to April 2011 since September 2007. Mr. Heshmatpour has been the Managing Director of AFH Holding & Advisory LLC since July 2003. Prior to that, he took some time off. From 1996 through January 2002, Mr. Heshmatpour served as Chairman and Chief Executive Officer of Metrophone Telecommunications, Inc. Mr. Heshmatpour has a background in venture capital, mergers and acquisitions, investing and corporate finance. Mr. Heshmatpour was the recipient of the Businessman of the Year award in 2003 at the National Republican Congressional Committee. Mr. Heshmatpour currently serves as sole officer and director of AFH Acquisition III, Inc., AFH Acquisition V, Inc., and AFH Acquisition VII, Inc., AFH Acquisition VIII, Inc, AFH Acquisition IX, Inc., AFH Acquisition, X, Inc., AFH Acquisition XI, Inc. and AFH Acquisition XII, Inc., all of which are publicly reporting, non-trading, blank check shell companies. Since October 10, 2007 Mr. Heshmatpour has served as President, Secretary and a member of the board of directors of AFH Holding I, Inc. and AFH Holding II, Inc. Since inception, Mr. Heshmatpour has served as President, Secretary and sole director of AFH Holding III, Inc., AFH Holding V, Inc., AFH Holding VI, Inc. and AFH Holding VII, Inc. Mr. Heshmatpour received a Bachelor of Arts from Pennsylvania State University in 1988. Mr. Heshmatpour is qualified to serve on our Board of Directors due to his operating and leadership experience, knowledge of the financial markets experience as a director of other public companies.
Douglas W. Wilmore, M.D. has served as a director of the Company since the closing of the Merger. Dr. Wilmore has served as a director of Emmaus Medical since 2003. Dr. Wilmore has been retired since 2002. Dr. Wilmore received a B.A. in Biology in 1960 from Washburn University and an M.D. in 1964 from Kansas University. After receiving his medical degree, he worked at the Hospital of the University of Pennsylvania where he received his training in general surgery. While at Penn, Dr. Wilmore worked with the team of investigators who developed total parenteral nutrition, a method of intravenous feeding that is used to support patients throughout the world today. Dr. Wilmore served in the U.S. Army working at the Institute for Surgical Research in San Antonio, Texas from 1971-1979. There he described many of the metabolic derangements associated with major injury and provided methods for resolving the severe catabolic responses that occur following trauma. In 1979 Dr. Wilmore moved to the Harvard Medical School and served as the Frank Sawyer Professor of Surgery and senior staff surgeon at the Brigham and Woman’s Hospital. During this time his laboratory developed modern techniques to measure the amino acid glutamine, described the response of this amino acid in acute illness and evaluated the effects of administering glutamine to seriously ill individuals. This group was the first to demonstrate that glutamine was associated with reduced infection rates in critically ill patients, that glutamine administration improved gut absorption and that this amino acid could restore muscle mass following wasting diseases. The use of glutamine to enhance gut absorption is now approved by the Food and Drug Administration for use in the United States. Wilmore is qualified to serve on our Board of Directors due to his knowledge and experience with L-glutamine and of and his knowledge of our business operations.
Family Relationships
There are no family relationships among any of the officers and directors.
Involvement in Certain Legal Proceedings
There have been no events under any bankruptcy act, no criminal proceedings and no judgments, injunctions, orders or decrees material to the evaluation of the ability and integrity of any director, executive officer, promoter or control person of the Company during the past ten years.
The Company is not aware of any legal proceedings in which any director, nominee, officer or affiliate of the Company, any owner of record or beneficially of more than five percent of any class of voting securities of the Company, or any associate of any such director, nominee, officer, affiliate of the Company, or security holder is a party adverse to the Company or any of its subsidiaries or has a material interest adverse to the Company or any of its subsidiaries.
Board of Directors and Committees and Director Independence
Although our common stock is not currently listed on the NASDAQ Stock Market, we have endeavored to structure our board of directors and board committees to comply with the requirements of the NASDAQ Marketplace Rules. Under NASDAQ Marketplace Rules, a listed company’s board of directors must consist of a majority of independent directors. Certain exceptions are available for this requirement but we do not qualify for any such exception. Currently, our board of directors has determined that each of Steve Warnecke, Henry A. McKinnell, Jr. and Douglas W. Wilmore is an “independent” director as defined by the NASDAQ Marketplace Rules, currently in effect and all applicable rules and regulations of the SEC. All members of the Audit, Compensation and Nominating Committees satisfy the “independence” standards applicable to members of each such committee. The board of directors made this affirmative determination regarding these directors’ independence based on discussions with the directors and on its review of the directors’ responses to a standard questionnaire regarding employment and compensation history; affiliations, family and other relationships; and transactions with the Company. The board of directors considered relationships and transactions between each director or any member of his immediate family and the Company and its subsidiaries and affiliates.
61
Audit Committee
We established our Audit Committee on May 3, 2011. The Audit Committee consists of Dr. McKinnell, Mr. Warnecke and Dr. Wilmore, each of whom is an independent director as defined by the NASDAQ Marketplace Rules. Steve Warnecke, Chairman of the Audit Committee, is an “audit committee financial expert” as defined under Item 407(d) of Regulation S-K. The purpose of the Audit Committee is to represent and assist our board of directors in its general oversight of our accounting and financial reporting processes, audits of the financial statements and internal control and audit functions. The Audit Committee’s responsibilities include:
● | The appointment, replacement, compensation, and oversight of work of the independent auditor, including resolution of disagreements between management and the independent auditor regarding financial reporting, for the purpose of preparing or issuing an audit report or performing other audit, review or attest services. | |
| ● | Reviewing and discussing with management and the independent auditor various topics and events that may have significant financial impact on our company or that are the subject of discussions between management and the independent auditors. |
The board of directors has adopted a written charter for the Audit Committee. A copy of the Audit Committee Charter will be available on our website at www.Emmausmedical.com.
Compensation Committee
We established our Compensation Committee on May 3, 2011. The Compensation Committee consists of Dr. McKinnell, Mr. Warnecke and Dr. Wilmore, each of whom is an independent director as defined by the NASDAQ Marketplace Rules. Dr. McKinnell is the Chairman of the Compensation Committee. The Compensation Committee is responsible for the design, review, recommendation and approval of compensation arrangements for our directors, executive officers and key employees, and for the administration of our equity incentive plans, including the approval of grants under such plans to our employees, consultants and directors. The Compensation Committee also reviews and determines compensation of our executive officers, including our Chief Executive Officer. The board of directors has adopted a written charter for the Compensation Committee. A copy of the Compensation Committee Charter will be available on our website at www.Emmausmedical.com.
Nominating and Corporate Governance Committee
The Nominating and Corporate Governance Committee consists of Dr. McKinnell, Mr. Warnecke and Dr. Wilmore, each of whom is an independent director as defined by the NASDAQ Marketplace Rules. Dr. Wilmore is the Chairman of the Nominating and Corporate Governance Committee. The Nominating and Corporate Governance Committee assists in the selection of director nominees, approves director nominations to be presented for stockholder approval at our annual general meeting, fills any vacancies on our board of directors, considers any nominations of director candidates validly made by stockholders, and reviews and considers developments in corporate governance practices. The board of directors has adopted a written charter for the Nominating and Corporate Governance Committee. A copy of the Nominating and Corporate Governance Committee Charter will be available on our website at www.Emmausmedical.com.
Code of Business Conduct and Ethics
On May 3, 2011, our Board of Directors approved a Code of Conduct and Ethics (the “Code of Ethics”) that applies to all of the directors, officers and employees of the Company. The Code of Ethics addresses, among other things, honesty and ethical conduct, conflicts of interest, compliance with laws, regulations and policies, including disclosure requirements under the federal securities laws, confidentiality, trading on inside information, and reporting of violations of the code. A copy of the Code of Ethics will be available on our website at www.Emmausmedical.com. Requests for copies of the Code of Ethics should be sent in writing to Emmaus Medical, Inc., Attention: Secretary, 20725 S. Western Avenue, Ste. 136, Torrance, CA 90501-1884.
62
EXECUTIVE COMPENSATION
Summary Compensation Table
The following table sets forth information concerning the compensation earned for services rendered to our predecessor and Emmaus Medical for the two fiscal years ended December 31, 2010 of the principal executive officer, in addition to our two most highly compensated officers whose annual compensation exceeded $100,000, and up to two additional individuals, as applicable, for whom disclosure would have been required but for the fact that the individual was not serving as an executive officer of the registrant at the end of the last fiscal year.
| Name and Position | Year | Salary ($) | Total ($) | |||||
| Yutaka Niihara, M.D., MPH | 2010 | 125,000 | 125,000 | |||||
| President and Chief Executive Officer and Director | 2009 | 125,000 | 125,000 | |||||
| Willis C. Lee | 2010 | 119,693 | 119,693 | |||||
| Chief Operating Officer and Director | 2009 | 26,188 | 26,188 | |||||
| Lan T. Tran | 2010 | 104,000 | 104,000 | |||||
| Chief Administrative Officer and | 2009 | 110,500 | 110,500 | |||||
| Corporate Secretary | ||||||||
| Amir F. Heshmatpour (1) | 2010 | — | — | |||||
| Former President, Secretary and Chief | 2009 | — | — | |||||
| Financial Officer | ||||||||
___________________________________
(1) Upon the close of the Merger on May 3, 2011, Mr. Heshmatpour resigned as the President, Secretary and Chief Financial Officer of the Company.
Outstanding Equity Awards at 2010 Fiscal Year End
There were no outstanding equity awards in 2010.
Employment Agreements
We entered into employment agreements with Yutaka Niihara, M.D., MPH, our Chief Executive Officer, Willis C. Lee, our Chief Operating Officer, and Lan T. Tran, our Chief Administrative Officer on April 5, 2011 and with Yasushi Nagasaki, our Chief Financial Officer, on April 8, 2011 (collectively, the “Employment Agreements”). Each of the Employment Agreements has an initial 2-year term, unless terminated earlier. The Employment Agreements for Dr. Niihara, Mr. Lee and Ms. Tran automatically renew for additional one year periods unless the Company or the officer provides notice of non-renewal at least sixty (60) days prior to the expiration of the then current term.
Base Salary, Bonus and Other Compensation. Dr. Niihara’s, Mr. Lee’s, Ms. Tran’s and Mr. Nagasaki’s base salary is $250,000, $180,000, $180,000 and $180,000 per year, respectively, which will be reviewed at least annually. In addition to base salary, the officers are entitled to receive an annual performance bonus based on the officer’s performance for the previous year. The target bonus for 2011 for Dr. Niihara, Mr. Lee and Ms. Tran are $50,000, $25,000 and $20,000, respectively. There is no expected performance bonus for 2011 for Mr. Nagasaki. The officers are also eligible to receive paid vacation, and participate in health and other benefit plans and will be reimbursed for reasonable and necessary business expenses.
63
Equity Compensation. Effective December 31, 2011, and at the end of each calendar year on December 31st or as soon as reasonably practicable after each such December 31st (each a “Grant Date”), the Company will grant non-qualified ten-year stock options with a Black Scholes value of $100,000 to Dr. Niihara, $50,000 to Mr. Lee, and $40,000 to Ms. Tran with an exercise price per share equal to the “Fair Market Value” (as such term is defined in the Company’s 2011 Stock Incentive Plan) on the applicable Grant Date. Mr. Nagasaki will receive a grant of non-qualified ten-year options with a Black Scholes value of $40,000 on December 31, 2012, however, there is no predetermined grant of options to Mr. Nagasaki for 2011. One-third of the options granted to each officer will vest on the first anniversary of the applicable Grant Date, one-third will vest on the second anniversary of the applicable Grant Date and the final one-third will vest on the third anniversary of the applicable Grant Date. Any unvested options will vest immediately upon a change in control (as defined below) or termination of the officer’s employment other than a voluntary termination by the officer or a termination of the officer for cause. In the event that the officer is terminated for any reason other than cause, death or disability or retirement, each option, to the extent that it is exercisable at the time of such termination, shall remain exercisable for the 90 day period following such termination, but in no event following the expiration of its term. In the event that the officer’s employment terminates on account of death, disability or, with respect to any non-qualified stock option, retirement, each option granted that is outstanding and vested as of the date of such termination shall remain exercisable by such officer (or the officer’s legal representatives, heirs or legatees) for the one year period following such termination, but in no event following the expiration of its term.
Severance Compensation. If Dr. Niihara’s, Mr. Lee’s, Ms. Tran’s or Mr. Nagasaki’s employment is terminated for any reason, other than without cause or good reason, each will be entitled to receive his or her base salary prorated through the termination date, any expense reimbursement due and owing for reasonable and necessary business expenses, and accrued vacation benefits (the “Voluntary Termination Benefits”). If termination is due to death or disability of the officer, then such officer will also receive an amount equal to his or her annual performance bonus, and for a termination due to disability only, 6 additional months of his base salary to be paid out over a 6-month period and payment of COBRA benefits of up to 6 months following the termination. If Dr. Niihara is terminated without cause or resigns with good reason (not within 2 years following a change in control), he will receive the Voluntary Termination Benefits and, provided he signs a release of all claims relating to his employment, a severance package equal to one year of his base salary to be paid out over a 12-month period, a pro rata amount of the annual performance bonus for the calendar year in which the termination date occurs based on the achievement of any applicable performance terms or goals for the year, and payment of COBRA benefits of up to 12 months following the termination. If any of Mr. Lee, Ms. Tran or Mr. Nagasaki is terminated without cause or resigns with good reason (not within 2 years following a change in control), he or she will receive the Voluntary Termination Benefits and, provided he or she signs a release of all claims relating to his or her employment, a severance package equal to 6 months of his or her base salary to be paid out over a 6-month period, an amount equal to half of the targeted annual performance bonus, and payment of COBRA benefits of up to 6 months following the termination.
Termination with cause includes a proven act of dishonesty, fraud, embezzlement or misappropriation of Company proprietary information; a conviction of, or plea of nolo contendere to, a felony or a crime involving moral turpitude; willful misconduct which cannot be cured on reasonable notice to the officer; or the officer’s habitual failure or refusal to perform his duties if such failure or refusal is not cured within 20 days after receiving written notice thereof from the board of directors. Good reason includes a reduction of more than 10% (or 25% in the case of Mr. Nagasaki) to the officer’s base salary or other compensation (except as part of a general reduction for all executive employees); a material diminution of the officer’s authority, responsibilities, reporting or job duties (except for any reduction for cause) (and except in Mr. Nagasaki’s case if his position is reduced to Treasurer, Comptroller or Controller during the first 14 months of employment); the Company’s material breach of the Employment Agreement; or, in the case of Dr. Niihara, Mr. Lee and Ms. Tran, a relocation of the business requiring the officer to move or drive to work more than 40 miles from its current location. The officer may terminate the Employment Agreement for good reason if he or she provides written notice to the Company within ninety (90) days of the event constituting good reason and the Company fails to cure the good reason within thirty (30) days after receiving such notice.
64
Change of Control. The Employment Agreements will not be terminated upon a change of control. A change of control means any merger or reorganization where the holders of the Company’s capital stock prior the transaction own fewer than 50% of the shares of capital stock after the transaction, an acquisition of 50% of the voting power of the Company’s outstanding securities by another entity, or a transfer of at least 50% of the fair market value of the Company’s assets. Upon Dr. Niihara’s termination without cause or good reason that occurs within 2 years after a change of control, he will receive the Voluntary Termination Benefits and, provided he signs a release of all claims relating to his employment, a severance package equal to 2 years of his base salary to be paid out over a 12-month period, an amount equal to double the targeted annual performance bonus, payment of COBRA benefits of up to 18 months following the termination; and a one-time cash payment of $3.0 million. Upon Mr. Lee’s or Ms. Tran’s termination without cause or good reason that occurs within 2 years after a change of control or upon Mr. Nagasaki’s termination without cause or good reason that occurs within 1 year after a change of control, he or she will receive the Voluntary Termination Benefits and, provided he or she signs a release of all claims relating to his or her employment, a severance package equal to 1 year of his or her base salary to be paid out over a 12-month period, an amount equal to the full year targeted annual performance bonus, payment of COBRA benefits of up to 12 months following the termination. Mr. Lee and Ms. Tran will also receive a one-time cash payment of $200,000. In addition each officer’s unvested equity awards shall vest upon such termination and the officer will have 36 months, except for Mr. Nagasaki who will have 4 months, in which to sell or exercise such awards (subject to expiration of the term of such options). The officer will also be free from all lock-up or other contractual restrictions upon the free sale of shares upon such termination.
Director Compensation
The following table shows information regarding the compensation earned during the fiscal year ended December 31, 2010 by members of board of directors, except Yutaka Niihara, M.D., MPH and Willis C. Lee, whose compensation for services as a director is included in the Summary Compensation Table above.
| Name | Fees Earned or Paid in Cash ($) | Stock Awards ($) | Option Awards ($) | Non-Equity Incentive Plan Compensation ($) | Change in Pension Value and Nonqualified Deferred Compensation Earnings | All Other Compensation ($) | Total ($) | ||||||||||||
| Steve Warnecke | — | — | — | — | — | — | — | ||||||||||||
| Henry A. McKinnell, Jr., Ph.D. | 3,000 (1) | — | 25,200 (2) | — | — | — | 28,200 | ||||||||||||
| Amir Heshmatpour | — | — | — | — | — | — | — | ||||||||||||
| Douglas W. Wilmore, M.D. | 2,000 (1) | — | 25,200 (2) | — | — | — | 27,200 | ||||||||||||
__________________________________
(1) Represents fees earned for service on the board of directors of Emmaus Medical.
(2) Represents an option to purchase 11,795 shares of common stock of the Company at an exercise price of $3.05. The amount disclosed reflects the grant date fair value in accordance with FASB ASC Topic 718. For assumptions used in the calculation of option awards, see Note 6 to the consolidated financial statements for the years ended December 31, 2010 and 2009 of Emmaus Medical.
Non-officer directors will receive an annual grant of 10,000 options pursuant to the 2011 Stock Incentive Plan. Such grants will vest in equal quarterly installments on the last day of each fiscal quarter. The Chairman of the Board will receive a one-time retainer grant of 10,000 options and each committee chair will receive a one-time retainer grant of 2,000 options. Additionally, non-officer directors will receive compensation of $700 for each in-person board meeting that they attend, $400 for each telephonic board meeting that they attend and $400 for each committee meeting. We expect the board of directors to hold four in-person meetings and two telephonic meetings each calendar year.
65
2011 Stock Incentive Plan
Background
Background
The Emmaus Medical, Inc. 2011 Stock Incentive Plan (the “Incentive Plan”) has been approved by the Company’s Board of Directors and by its stockholders. Stockholder approval of the Incentive Plan enables the Company to satisfy stock exchange listing requirements, and to make awards that qualify as performance-based compensation that is exempt from the deduction limitation set forth under Section 162(m) of the Internal Revenue Code of 1986, as amended (the “Code”). Subject to certain exceptions, Section 162(m) generally limits the corporate income tax deductions to $1,000,000 annually for compensation paid to each of the Chief Executive Officer and the other three highest paid executive officers of the Company required to be reported under the proxy disclosure rules. The Company intends to cause the shares of common stock that will become available for issuance to be registered on a Form S-8 registration statement to be filed with the SEC at the Company’s expense.
The amount and nature of the proposed awards under the Incentive Plan have not yet been determined, although the Incentive Plan permits grants of stock options, stock appreciation rights, or SARs, restricted stock or units, unrestricted stock, deferred stock and performance awards. The Company’s board of directors believes that the Incentive Plan will be an important factor in attracting, retaining and motivating employees, consultants, agents, and directors of the Company and its affiliates, collectively referred to herein as Eligible Persons. Our board of directors believes that the Company needs the flexibility both to have an ongoing reserve of common stock available for future equity-based awards, and to make future awards in a variety of forms.
Pursuant to the Incentive Plan, 3,000,000 shares of common stock will be reserved for future awards to eligible persons, which number has been adjusted for the Reorganization. The following is a summary of the material provisions of the Incentive Plan and is qualified in its entirety by reference to the complete text of the Incentive Plan, a copy of which is attached to this Current Report on Form 8-K as Exhibit 10.2. Capitalized terms used in this summary and not otherwise defined herein will have the meanings ascribed to such terms in the Incentive Plan.
Purpose
The purpose of the Incentive Plan is to attract, retain and motivate select Eligible Persons, and to provide incentives and rewards for superior performance.
Shares Subject to the Incentive Plan
The Incentive Plan provides that no more than 3,000,000 shares of common stock may be issued pursuant to Awards under the Incentive Plan. These shares shall be authorized but unissued shares, or shares that the Company otherwise holds in treasury or in trust. The number of shares available for Awards, as well as the terms of outstanding Awards, is subject to adjustment as provided in the Incentive Plan for stock splits, stock dividends, recapitalizations and other similar events. Shares of common stock that are subject to any Award that expires, or is forfeited, cancelled or otherwise terminated without the issuance of some or all of the shares that are subject to the Award will again be available for subsequent Awards unless such shares are used as payment in connection with any Award or used to satisfy tax obligations with respect to an Award.
The maximum awards that can be granted under the Incentive Plan to a single participant in any calendar year shall be 500,000 shares of common stock in the form of options or SARs, and 500,000 shares of common stock in the form of restricted shares, restricted share units, stock bonus and other stock-based awards.
Administration
Following the consummation of the Merger, either the Company’s Compensation Committee of the Board of Directors or another committee appointed by the Company’s Board of Directors will administer the Incentive Plan. The Compensation Committee of the Company’s Board of Directors and any other committee exercising discretion under the Incentive Plan from time to time are referred to herein as the “Committee.” It is expected that the Compensation Committee of the Company’s Board of Directors will act as the Committee for purposes of the Incentive Plan. To the extent permitted by law, the Committee may authorize one or more persons who are reporting persons for purposes of Rule 16b-3 under the Exchange Act (or other officers) to make Awards to eligible persons who are not reporting persons for purposes of Rule 16b-3 under the Exchange Act (or other officers whom the Company has specifically authorized to make Awards). With respect to decisions involving an Award intended to satisfy the requirements of Section 162(m) of the Code, the Committee is to consist of two or more directors who are “outside directors” for purposes of that Code section. The Committee may delegate administrative functions to individuals who are reporting persons for purposes of Rule 16b-3 of the Exchange Act, officers or employees of the Company or its affiliates.
66
Subject to the terms of the Incentive Plan, the Committee has express authority to determine the Eligible Persons who will receive Awards, the number of shares of common stock, units or dollars to be covered by each Award, and the terms and conditions of Awards. The Committee has broad discretion to prescribe, amend, and rescind rules relating to the Incentive Plan and its administration, to interpret and construe the terms of the Incentive Plan and the terms of all Award agreements, and to take all actions necessary or advisable to administer the Incentive Plan.
Stock awards granted under the Incentive Plan (other than annual director stock grants described below) will have a minimum forfeiture period of at least three years (but such forfeiture periods may lapse in installments). However, performance-based stock awards may have a minimum vesting or forfeiture period of one year. As an exception to these minimum vesting and forfeiture provisions, the Committee has discretion to accelerate the exercisability or vesting of outstanding awards or waive any restrictions applicable to such awards in connection with a participant’s death, disability, retirement, involuntary termination, a change in control or for recruitment. In addition, the Committee will have discretion to award up to 10% of the shares reserved under the Incentive Plan without regard to these minimum vesting or forfeiture periods, primarily for special one-time recognition awards and retention purposes.
The Incentive Plan provides that the Company will indemnify members of the Committee and their delegates against any claims, liabilities or costs arising from the good faith performance of their duties under the Incentive Plan. The Incentive Plan releases these individuals from liability for good faith actions associated with the Incentive Plan’s administration.
Eligibility
The Committee may grant options that are intended to qualify as incentive stock options, or ISOs, only to employees of the Company or its affiliates, and may grant all other Awards to Eligible Persons. The Incentive Plan and the discussion below use the term “Participant” to refer to an Eligible Person who has received an Award.
Types of Awards
Options. Options granted under the Incentive Plan provide Participants with the right to purchase shares of common stock at a predetermined exercise price. The Committee may grant options that are intended to qualify as ISOs or options that are not intended to so qualify, referred to herein as Non-ISOs. The Incentive Plan also provides that ISO treatment may not be available for options that become first exercisable in any calendar year to the extent the value of the underlying shares that are the subject of the option exceeds $100,000 (based upon the fair market value of the shares of common stock on the option grant date).
Share Appreciation Rights (SARs). A share appreciation right generally permits a Participant who receives it to receive, upon exercise, cash and/or shares of common stock equal in value to an amount determined by multiplying (a) the excess of the fair market value, on the date of exercise, of the shares of common stock with respect to which the SAR is being exercised, over the exercise price of the SAR for such shares by (b) the number of shares with respect to which the SARs are being exercised. The Committee may grant SARs in tandem with options or independently of them. SARs that are independent of options may limit the value payable on its exercise to a percentage, not exceeding 100%, of the excess value.
Exercise Price for Options and SARs. The exercise price of ISOs, Non-ISOs, and SARs may not be less than 100% of the fair market value on the grant date of the shares of common stock subject to the Award (110% of fair market value for ISOs granted to employees who, on the grant date, own stock representing more than 10% of the combined voting power of all classes of stock of the Company).
Exercise of Options and SARs. To the extent exercisable in accordance with the agreement granting them, an option or SAR may be exercised in whole or in part, and from time to time during its term, subject to earlier termination relating to a holder’s termination of employment or service. With respect to options, the Committee has the discretion to accept payment of the exercise price in any of several forms (or combination of them), including: cash or check in U.S. dollars, certain shares of common stock, and cashless or “net” exercise under a program the Committee approves. The term over which Participants may exercise options and SARs may not exceed ten years from the date of grant (five years in the case of ISOs granted to employees who, on the grant date, own more than 10% of the combined voting power of all classes of stock of the Company).
67
Unless otherwise provided under the terms of the agreement evidencing a grant, options and SARs that have vested may be exercised during the six-month period after the optionee retires, during the one-year period after the optionee’s termination of service due to death or permanent disability, and during the 90-day period after the optionee’s termination of employment other than for cause (but in no case later than the termination date of the option or SAR). Each option or SAR that remains unexercisable at the time of termination shall be terminated at the time of termination. The agreement evidencing the grant of an option or SAR may, in the discretion of the Committee, set forth additional or different terms and conditions applicable to such grant upon a termination or change in status of the employment or service of the participant. All SARs may be settled in cash or shares of the Company’s stock in the discretion of the Committee.
Restricted Shares, Stock Units, Stock Bonus, and Other Stock-Based Awards. Under the Incentive Plan, the Committee may grant restricted shares that are forfeitable until certain vesting requirements are met, may grant restricted stock units which represent the right to receive shares of common stock after certain vesting requirements are met, and may grant unrestricted shares as to which the Participant’s interest is immediately vested (subject to the exceptions to the minimum vesting requirements described above). The Incentive Plan provides the Committee with discretion to determine the terms and conditions under which a Participant’s interests in such Awards becomes vested, which may include the achievement of financial or other objective performance goals or other objectives.
The performance goals described in the preceding paragraphs may be established by the Committee, in its discretion, based on one or more of the following measures (the “Business Criteria”): (1) return on total stockholder equity; (2) earnings or book value per share of Company Stock; (3) net income (before or after taxes); (4) earnings before all or any interest, taxes, depreciation and/or amortization (“EBIT,” “EBITA” or “EBITDA”); (5) inventory goals; (6) return on assets, capital or investment; (7) market share; (8) cost reduction goals; (9) earnings from continuing operations; (10) levels of expense, costs or liabilities; (11) unit level performance; (12) operating profit; (13) sales or revenues; (14) stock price appreciation; (15) total stockholder return; (16) implementation or completion of critical projects or processes; or (17) any combination of the foregoing. Where applicable, Business Criteria may be expressed in terms of attaining a specified level of the particular criteria or the attainment of a percentage increase or decrease in the particular criteria, and may be applied to one or more of the Company, an affiliate, or a division or strategic business unit of the Company, or may be applied to the performance of the Company relative to a market index, a group of other companies or a combination thereof, all as determined by the Committee. Each of the Business Criteria shall be determined, where applicable and except as otherwise provided by the Committee, in accordance with generally accepted accounting principles and shall be subject to certification by the Committee; provided that the Committee shall have the authority to make equitable adjustments to the Business Criteria in recognition of unusual or non-recurring events affecting the Company or any affiliate or the financial statements of the Company or any affiliate, in response to changes in applicable laws or regulations, or to account for items of gain, loss or expense determined to be extraordinary or unusual in nature or infrequent in occurrence or related to the disposal of a segment of a business or related to a change in accounting principles.
Whenever shares of common stock are delivered pursuant to these Awards, the Participant will be entitled to receive additional shares of common stock equal to the sum of (i) any stock dividends that the Company’s stockholders received between the grant date of the Award and issuance or release of the shares of common stock and (ii) a number of additional shares of common stock equal to the shares of common stock that the Participant could have purchased at Fair Market Value on the payment date of any cash dividends for shares of common stock if the Participant had received such cash dividends between its grant date and its settlement date. However, any dividend equivalents awarded in connection with a grant of any performance-based award will not be payable unless and until the performance conditions applicable to the award have been met, or the award otherwise becomes vested in accordance with the award agreement and the Incentive Plan.
Annual Non-Employee Director Grants. The Incentive Plan provides for annual grants of 10,000 options to non-employee directors (the “Annual Director Award”). Each Annual Director Award will vest in four substantially equal quarterly installments.
68
Clawback of Awards
Unless otherwise provided in an agreement granting an Award, the Company may terminate any outstanding, unexercised, unexpired or unpaid Award, rescind any exercise, payment or delivery pursuant to the Award, or recapture any common stock (whether restricted or unrestricted) or proceeds from the Participant’s sale of shares issued pursuant to the Award in the event of the discovery of the Participant’s fraud or misconduct, or otherwise in connection with a financial restatement.
Income Tax Withholding
As a condition for the issuance of shares pursuant to Awards, the Incentive Plan requires satisfaction of any applicable federal, state, local, or foreign withholding tax obligations that may arise in connection with the award or the issuance of shares.
Transferability
Awards may not be sold, pledged, assigned, hypothecated, transferred or disposed of other than by will or the laws of descent and distribution, except to the extent the Committee permits lifetime transfers in the form of Non-ISOs, Share-settled SARs, Restricted Shares, or Performance Shares to charitable institutions, certain family members or related trusts, or as otherwise approved by the Committee.
Certain Corporate Transactions
The Committee shall equitably adjust the number of shares covered by each outstanding Award, the number of shares that have been authorized for issuance under the Incentive Plan but as to which no Awards have yet been granted or that have been returned to the Incentive Plan upon cancellation, forfeiture or expiration of an Award, and the maximum number of shares that may be granted in any calendar year to individual participants, as well as the price per share covered by each outstanding Award, to reflect any increase or decrease in the number of issued shares resulting from a stock split, reverse stock split, stock dividend, combination, recapitalization or reclassification of the shares, or any other increase or decrease in the number of issued shares effected without receipt of consideration by the Company.
In addition, in the event of a Change in Control (as defined in the Incentive Plan) but subject to the terms of any Award agreements or any employment or other similar agreement between the Company or any of its affiliates and a Participant then in effect, each outstanding Award shall be assumed or a substantially equivalent award shall be substituted by the surviving or successor corporation or a parent or subsidiary of such surviving or successor corporation upon the consummation of the transaction; provided, however, that to the extent outstanding Awards are neither being assumed nor replaced with substantially equivalent Awards by the successor corporation, the Committee may in its sole and absolute discretion and authority, without obtaining the approval or consent of the Company’s stockholders or any Participant with respect to his or her outstanding Awards, take one or more of the following actions: (a) accelerate the vesting of Awards for any period so that Awards shall vest (and, to the extent applicable, become exercisable) as to the shares of common stock that otherwise would have been unvested and provide that repurchase rights of the Company with respect to shares of common stock issued pursuant to an Award shall lapse as to the shares of common stock subject to such repurchase right; (b) arrange or otherwise provide for payment of cash or other consideration to Participants in exchange for the satisfaction and cancellation of outstanding Awards; or (c) terminate all or some Awards upon the consummation of the transaction, provided that the Committee shall provide for vesting such Awards in full as of a date immediately prior to consummation of the Change of Control. To the extent that an Award is not exercised prior to consummation of a transaction in which the Award is not being assumed or substituted, such Award shall terminate upon such consummation.
69
Term of the Incentive Plan; Amendments or Termination
The term of the Incentive Plan is ten years from the date of adoption by the board of directors. The Company’s board of directors may from time to time, amend, alter, suspend, discontinue or terminate the Incentive Plan; provided that no amendment, suspension or termination of the Incentive Plan shall materially and adversely affect Awards already granted. Furthermore, the Incentive Plan specifically prohibits the repricing of stock options or SARs without shareholder approval. For this purpose, a “repricing” means any of the following (or any other action that has the same effect as any of the following): (i) changing the terms of a stock option or SAR to lower its exercise price; (ii) any other action that is treated as a “repricing” under generally accepted accounting principles; and (iii) repurchasing for cash or canceling a stock option or SAR at a time when its exercise price is greater than the fair market value of the underlying stock in exchange for another award, unless the cancellation and exchange occurs in connection with a change in capitalization or similar change. Such cancellation and exchange would be considered a “repricing” regardless of whether it is treated as a “repricing” under generally accepted accounting principles and regardless of whether it is voluntary on the part of the participant.
Expected Tax Consequences
The following is a brief summary of certain U.S. tax consequences of certain transactions under the Incentive Plan. This summary is not intended to be complete and does not describe state or local tax consequences.
U.S. Federal Income Tax Consequences
Under the Code, the Company will generally be entitled to a deduction for federal income tax purposes at the same time and in the same amount as the ordinary income that Participants recognize pursuant to Awards (subject to the Participant’s overall compensation being reasonable, and to the discussion below with respect to Code section 162(m)). For Participants, the expected U.S. federal income tax consequences of Awards are as follows:
Non-ISOs. A Participant will not recognize income at the time a Non-ISO is granted. At the time a Non-ISO is exercised, the Participant will recognize ordinary income in an amount equal to the excess of (a) the fair market value of the shares of common stock issued to the Participant on the exercise date, over (b) the exercise price paid for the shares. At the time of sale of shares acquired pursuant to the exercise of a Non-ISO, the appreciation (or depreciation) in value of the shares after the date of exercise will be treated either as short-term or long-term capital gain (or loss) depending on how long the shares have been held.
ISOs. A Participant will not recognize income upon the grant of an ISO. There are generally no tax consequences to the Participant upon exercise of an ISO (except the amount by which the fair market value of the shares at the time of exercise exceeds the option exercise price is a tax preference item possibly giving rise to an alternative minimum tax). If the shares of common stock are not disposed of within two years from the date the ISO was granted or within one year after the ISO was exercised, any gain realized upon the subsequent disposition of the shares will be characterized as long-term capital gain and any loss will be characterized as long-term capital loss. If both of these holding period requirements are not met, then a “disqualifying disposition” occurs and (a) the Participant recognizes ordinary income gain in the amount by which the fair market value of the shares at the time of exercise exceeded the exercise price for the ISO and (b) any remaining amount realized on disposition (except for certain “wash” sales, gifts or sales to related persons) will be characterized as capital gain or loss.
Share Appreciation Rights. A Participant to whom a SAR is granted will not recognize income at the time of grant of the SAR. Upon exercise of a SAR, the Participant must recognize taxable compensation income in an amount equal to the value of any cash or shares of common stock that the Participant receives.
Restricted Shares. A participant will not be taxed at the date of an award of restricted shares, but will be taxed at ordinary income rates on the fair market value of any restricted shares as of the date that the restrictions lapse, unless the participant, within 30 days after transfer of such restricted shares to the participant, elects under Section 83(b) of the Internal Revenue Code to include in income the fair market value of the restricted shares as of the date of such transfer. If the participant makes the election under Section 83(b), the Company will be entitled to a corresponding deduction. Any disposition of shares after restrictions lapse will be subject to the regular rules governing long-term and short-term capital gains and losses, with the basis for this purpose equal to the fair market value of the shares at the end of the restricted period (or on the date of the transfer of the restricted shares, if the employee elects to be taxed on the fair market value upon such transfer). To the extent dividends are payable during the restricted period under the applicable award agreement, any such dividends will be taxable to the participant at ordinary income tax rates and will be deductible by the Company unless the participant has elected to be taxed on the fair market value of the restricted shares upon transfer, in which case they will thereafter be taxable to the employee as dividends and will not be deductible by the Company.
70
Restricted Units. A participant will normally not recognize taxable income upon an award of restricted units, and the Company will not be entitled to a deduction until the lapse of the applicable restrictions. Upon the lapse of the restrictions and the issuance of the earned shares, the participant will recognize ordinary taxable income in an amount equal to the fair market value of the common stock received and the Company will be entitled to a deduction in the same amount.
Other Stock-Based Awards. Normally, a participant will not recognize taxable income upon the grant of other stock-based awards. Subsequently, when the conditions and requirements for the grants have been satisfied and the payment determined, any cash received and the fair market value of any common stock received will constitute ordinary income to the participant. The Company also will then be entitled to a deduction in the same amount.
Stock Bonus Awards. A Participant will recognize income at the time of grant of unrestricted stock bonus awards in an amount equal to the excess of the market value of the unrestricted shares over any amount the Participant pays for them (in which case subsequent gain or loss would be capital in nature).
Special Tax Provisions. Under certain circumstances, the accelerated vesting, cash-out or accelerated lapse of restrictions on Awards in connection with a change in control of the Company might be deemed an “excess parachute payment” for purposes of the golden parachute tax provisions of Code section 280G, and the Participant may be subject to a 20% excise tax and the Company may be denied a tax deduction. Furthermore, the Company may not be able to deduct the aggregate compensation in excess of $1,000,000 attributable to Awards that are not “performance-based” within the meaning of Code section 162(m) in certain circumstances.
Income Taxes and Deferred Compensation. The Incentive Plan provides that participants are solely responsible and liable for the satisfaction of all taxes and penalties that may arise in connection with Awards (including any taxes arising under Section 409A of the Code), and that the Company will not have any obligation to indemnify or otherwise hold any Participant harmless from any or all of such taxes. Nevertheless, the Incentive Plan authorizes the Committee to grant or to unilaterally modify any Award in a manner that (i) conforms with the requirements of Section 409A of the Code, (ii) that voids any Participant election to the extent it would violate Section 409A of the Code, and (iii) for any distribution election that would violate Section 409A of the Code, to make distributions pursuant to the Award at the earliest to occur of a distribution event that is allowable under Section 409A of the Code or any distribution event that is both allowable under Section 409A of the Code and is elected by the Participant, with the Committee’s consent, in accordance with Section 409A.
General Tax Law Considerations
The preceding paragraphs are intended to be merely a summary of certain important tax law consequences concerning a grant of options under the Incentive Plan and the disposition of shares issued thereunder in existence as of the date of this filing. Special rules may apply to the Company’s officers, directors or greater than ten percent stockholders. Participants in the Incentive Plan should review the current tax treatment with their individual tax advisors at the time of grant, exercise or any other transaction relating to an Award or the underlying shares.
New Plan Benefits
The Committee will grant Awards under the Incentive Plan at its discretion. Consequently, it is not possible to determine at this time the amount or dollar value of Awards to be provided under the Incentive Plan, other than to note that the Committee has not granted Awards that are contingent upon the approval of the Incentive Plan.
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
Emmaus Medical, Inc.
Emmaus Holdings, Inc., Emmaus Medical Inc., Newfield Nutrition Corporation and Emmaus Medical Japan, Inc (“EM Japan”) which are either directly or indirectly wholly-owned subsidiaries of the Company, each have interlocking executive and director positions with us and with each other. Dr. Niihara and Mr. Lee are directors of Newfield Nutrition Corporation. Dr. Niihara is a director of EM Japan. The officers of Emmaus Holdings are also the officers of Emmaus Medical and Newfield Nutrition and hold the same officer positions in Emmaus Medical and Newfield as they do in Emmaus Holdings.
71
AFH Acquisition IV, Inc.
Mr. Heshmatpour, our former President and a director and our original stockholder, is deemed our promoter as this term is defined under the federal securities laws.
Merger and AFH Advisory
Pursuant to the Merger Agreement AFH Merger Sub merged with and into Emmaus Medical with Emmaus Medical continuing as the surviving entity. As a result of the Merger, Emmaus Medical became a wholly-owned subsidiary of the Company. In connection with the Merger, AFH IV changed its corporate name from “AFH Acquisition IV, Inc.” to “Emmaus Holdings, Inc.”
Upon consummation of the Merger, (i) each outstanding share of Emmaus Medical common stock was exchanged for 29.48548924976 shares of AFH IV common stock, (ii) each outstanding Emmaus Medical option and warrant, which was exercisable for one share of Emmaus Medical common stock, was exchanged for an option or warrant, as applicable, exercisable for 29.48548924976 shares of AFH IV common stock and (iii) each outstanding convertible note of Emmaus Medical, which was convertible for one share of Emmaus Medical common stock, was exchanged for a convertible note exercisable for 29.48548924976 shares of AFH IV common stock. As a result of the Merger, Emmaus Medical securityholders received 20,673,714 of our shares of common stock, options and warrants to purchase an aggregate of 326,507 shares of common stock, and convertible notes to purchase an aggregate of 270,648 shares of our common stock, or 85% of our issued and outstanding common stock on a fully diluted basis. Immediately after the closing of the Merger, we had 24,423,714 shares of common stock, no shares of preferred stock, options to purchase 23,590 shares of common stock, warrants to purchase 302,917 shares of common stock and convertible notes convertible into 270,648 shares of our common stock issued and outstanding.
Prior to the closing of the Merger, AFH Advisory canceled an aggregate of 1,827,750 shares of AFH IV. AFH Advisory did not receive any consideration for the cancellation of the shares. The cancellation of the shares was accounted for as a contribution to capital. The number of shares cancelled was determined based on negotiations with AFH Advisory, the sole stockholder of AFH, and Emmaus Medical. Emmaus Medical and AFH Advisory negotiated an estimated value of Emmaus Medical and its subsidiaries, an estimated value of the shell company, and the mutually desired capitalization of the company resulting from the Merger. With respect to the determination of the amount of shares cancelled, the value of the shell company was derived primarily from its utility as a public company platform, including its good corporate standing and its timely public reporting status. We did not consider registering our own securities directly as a viable option for accessing the public markets. The services provided by AFH Advisory were not a consideration in determining this aspect of the transaction. Under these circumstances and based on these factors, Emmaus Medical and AFH Advisory agreed upon the number of shares to be cancelled.
The Merger resulted in a change in control of our company from AFH Advisory, which is owned by Mr. Amir F. Heshmatpour, to the former stockholders of Emmaus Medical. In connection with the change in control, the persons set forth below were appointed to our Board of Directors and elected as officers in the positions set forth opposite their names. Mr. Heshmatpour, an officer and director of AFH IV prior to the consummation of the Merger Agreement, resigned from all officer positions at the time the transaction was consummated, but continues as a member of the Board of Directors. The appointments of the new officers and directors were effective on the Closing Date of the Merger.
| Name | Position | |
| Yutaka Niihara, M.D. | President and Chief Executive Officer and Director | |
| Willis C. Lee | Chief Operating Officer and Director | |
| Lan T. Tran | Chief Administrative Officer and Corporate Secretary | |
| Yasushi Nagasaki | Chief Financial Officer | |
| Steve Warnecke | Director | |
| Henry A. McKinnell, Jr., Ph.D., | Chairman of the Board | |
| Amir Heshmatpour | Director | |
| Douglas W. Wilmore, M.D. | Director |
72
In connection with the Merger, the Company has agreed to pay AFH Holding $500,000 (the “Shell Cost”) for the identification of AFH Acquisition IV and providing consulting services related to coordinating the Merger, assisting with a public offering and managing the interrelationship of legal and accounting activities (the “Services”), and to reimburse AFH Advisory for advancement of expenses on behalf of the Company incurred in connection with the Services, the Merger and a public offering, including, without limitation, reasonable expenses of AFH Advisory (the “Transaction Costs”).
AFH Advisory may be paid any outstanding Shell Cost and Transaction Costs from the proceeds of a public offering or upon the consummation of any other financing. Alternatively, AFH Advisory may, in its discretion, convert such amount (or any portion thereof) into common stock at a conversion price equal to 75% of the per share price in the offering (the “Conversion Price”). Additionally, we have agreed to issue warrants to purchase shares of common stock to AFH Advisory upon the closing of the public offering. Such warrants will have a term of 5 years from the date of issuance and will have an exercise price equal to the Conversion Price. The number of shares underlying the warrants will be calculated by dividing the aggregate of the Shell Cost and the total Transaction Costs by the Conversion Price.
Shares held by AFH Advisory and certain others will be decreased, at the rate of 1% of the post-offering outstanding common shares, for each $1 million or fraction thereof that the gross proceeds to the Company from a public offering are less than $10 million. In the event of such reduction, AFH Advisory will reduce the number of those shares by an appropriate percentage. If an offering cannot be consummated to provide for minimum gross proceeds to the Company of at least $5 million, the Company’s arrangement with AFH Advisory provides that the Company may terminate the offering at its sole and absolute discretion, and if the Company terminates the offering, all shares held by AFH Advisory and certain others will be canceled.
If our arrangement with AFH Advisory is terminated based on mutual agreement of the parties or based on a breach by the Company, AFH Advisory will receive its Transaction Costs incurred as of the date of termination. If the arrangement with AFH Advisory is terminated (i) by either party (without being cured within 30 days) based on identification of information in the course of its due diligence investigation that it deems unsatisfactory, or if the parties are unable to agree to a valuation within 45 days following completion of satisfactory due diligence by an investment bank, or (ii) by the Company if a public offering cannot be consummated to provide for gross proceeds to the Company of at least $10 million or based on a breach by AFH Advisory that is not cured within 30 days, then AFH Advisory will receive reimbursement for 50% of Transaction Costs actually incurred to the date of such termination. If an offering cannot be consummated with gross proceeds of at least $5 million, and the Company exercises its right to terminate the offering, then the Company will reimburse AFH Advisory an amount equal to 50% of the Shell Cost and 50% of the Transaction Costs incurred as of the date of termination, and AFH Advisory, in its discretion, has the option to be paid any outstanding amounts at the time of termination in cash or to convert such amounts (or any portion thereof) into common stock at a conversion price equal to 75% of the per share price of the shares of common stock sold in the Company’s most recently completed private offering of common stock. In the event of termination by the Company because a public offering cannot be consummated to provide for gross proceeds to the Company of at least $10 million, and if the Company enters into any transaction or a sale of all or substantially all of its assets within 12 months of such termination, AFH Advisory will be entitled to (i) 5% of its holdings (including holdings of certain others) of any securities received by the Company or the Company’s shareholders upon consummation of any business combination or other similar transaction; or (ii) cash or any other consideration it would have received as if it had a 5% ownership interest in the Company immediately prior to the closing of any such transaction.
The Company granted AFH Advisory exclusive rights to act as its advisor in connection with all financings and mergers and acquisitions until November 10, 2012 and the right to appoint two board members to the Company’s board of directors upon the closing of the Merger. In addition, in the event of any merger, stock purchase, asset purchase or similar transaction occurring within one year of the closing of the public offering, AFH Advisory may receive a warrant to purchase shares in an amount to increase AFH Advisory’s total holdings to 10% of the outstanding fully diluted equity of the Company but only to the extent the Company issues securities in connection with such merger, stock purchase, asset purchase or similar transaction.
73
Transfer of Private Placement Funds to AFH Advisory
In April 2011, the Company sold an aggregate of 577,750 shares of its common stock in a private placement to various accredited investors at a per share of $2.00 for aggregate cash proceeds equal to $1.2 million. Upon the closing of the private placement, the Company transferred the proceeds from the private placement to AFH Advisory, the Company’s parent company at the time of the private placement.
Loans by Executive Officers and Stockholders
Dr. Niihara, our Chief Executive Officer, made loans to Emmaus on January 12, 2009 and April 23, 2009 in the aggregate principal amounts of $350,000 and $80,000, respectively. The loans each bear interest at a rate of 6.5% per annum. Interest only payments are due monthly. The loans are due on demand by Dr. Niihara. As of the date of this report, the principal amounts outstanding on the January 12, 2009 note and the April 23, 2009 note are $350,000 and $80,000, respectively. During the three months ended March 31, 2011 and the years ended December 31, 2010 and 2009, we made interest payments of $5,687, $22,750 and $20,853 on the January 12, 2009 note. During the three months ended March 31, 2011 and the years ended December 31, 2010 and 2009, we made interest payments of $1,300, $5,200 and $3,467 on the April 23, 2009 note.
Hope International Hospice, Inc., of which Dr. Niihara is the Chief Executive Officer, made a $200,000 loan to Emmaus on January 12, 2011. The loan, which has a term of two years, bears interest at a rate of 8% per annum. Interest only payments are due quarterly. As of the date of this report, the principal amount outstanding on the note and is $200,000. No interest payments were made during the three months ended March 31, 2011.
On January 12, 2011, Willis C. Lee made a two-year loan to Emmaus in the amount of $100,000. The loan bears interest at a rate of 8% per annum. Interest only payments are due quarterly. As of the date of this report, the principal amount outstanding on the note and is $100,000. No interest payments were made during the three months ended March 31, 2011.
The above loans were made to provide Emmaus with needed working capital.
Except as otherwise indicated herein, there have been no related party transactions, or any other transactions or relationships required to be disclosed pursuant to Item 404 of Regulation S-K.
We do not currently have a formal related party approval policy for review and approval of transactions required to be disclosed pursuant to Item 404(a) of Regulation S-K. We expect to adopt such a policy that will identify the types of transactions covered by such policy and the standards to be applied pursuant to such policy. We expect that the Nominating and Corporate Governance Committee will be responsible for applying such policy.
Guarantee by Chief Executive Officer
On January 12, 2009, Emmaus Medical entered into a promissory note with Mr. Shigeru Matsuda for 20 million Japanese Yen, equivalent to $246,889. The loan accrues interest at a rate of 6.5% per annum and the loan may be converted to common stock at $3.05 per share. Dr. Niihara has provided a guarantee on a promissory note entered into with Mr. Matsuda pursuant to section 7 of the note, a copy of which is filed as Exhibit 4.4 hereto.
Policy for Approval of Related Party Transactions
We do not currently have a formal related party approval policy for review and approval of transactions required to be disclosed pursuant to Item 404(a) of Regulation S-K. We expect our board to adopt such a policy in the near future.
74
INDEMNIFICATION OF DIRECTORS AND EXECUTIVE OFFICERS AND LIMITATION OF LIABILITY
Under Section 145 of the General Corporation Law of the State of Delaware, we can indemnify its directors and officers against liabilities they may incur in such capacities, including liabilities under the Securities Act of 1933, as amended (the “Securities Act”). Our Certificate of Incorporation provides for the indemnification, to the fullest extent permitted by Section 145 of the Delaware General Corporation Law, as amended from time to time, of officers, directors, employees and agents of the Company. We may, prior to the final disposition of any proceeding, pay expenses incurred by an officer or director upon receipt of an undertaking by or on behalf of that director or executive officer to repay those amounts if it should be determined ultimately that he or she is not entitled to be indemnified under the bylaws or otherwise. We shall indemnify any officer, director, employee or agent upon a determination that such individual has met the applicable standards of conduct specified in Section 145. In the case of an officer or director, the determination shall be made by (a) a majority vote of directors who are not parties to such proceeding, even though less than a quorum; (b) a committee of such directors designated by majority vote of such directors, even though less than a quorum; (c) if there are no such directors, independent legal counsel in a written opinion or (d) the stockholders.
Our certificate of incorporation provides that, pursuant to Delaware law, our directors shall not be liable for monetary damages for breach of the directors’ fiduciary duty of care to us and our stockholders. This provision in the certificate of incorporation does not eliminate the duty of care, and in appropriate circumstances equitable remedies such as injunctive or other forms of no monetary relief will remain available under Delaware law. In addition, each director will continue to be subject to liability for breach of the director’s duty of loyalty to us or our stockholders, for acts or omissions not in good faith or involving intentional misconduct or knowing violations of the law, for actions leading to improper personal benefit to the director, and for payment of dividends or approval of stock repurchases or redemptions that are unlawful under Delaware law. The provision also does not affect a director’s responsibilities under any other law, such as the federal securities laws or state or federal environmental laws.
We have been advised that in the opinion of the Securities and Exchange Commission, insofar as indemnification for liabilities arising under the Securities Act may be permitted to our directors, officers and controlling persons pursuant to the foregoing provisions, or otherwise, such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable. In the event a claim for indemnification against such liabilities (other than our payment of expenses incurred or paid by its director, officer or controlling person in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, we will, unless in the opinion of our counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question of whether such indemnification by us is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
We maintain officers’ and directors’ liability insurance. We have also entered into indemnification agreements with each of our directors and officers that are, in some cases, broader than the specific indemnification provisions permitted by Delaware law, and that provide additional procedural protection. Such indemnification agreements require us, among other things, to:
| ● | indemnify officers and directors against certain liabilities that may arise because of their status as officers or directors; | |
| ● | advance expenses, as incurred, to officers and directors in connection with a legal proceeding, subject to limited exceptions; or | |
| ● | obtain directors’ and officers’ insurance. |
At present, there is no pending litigation or proceeding involving any of our directors, officers or employees in which indemnification is sought, nor are we aware of any threatened litigation that may result in claims for indemnification.
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT FOLLOWING THE MERGER
Beneficial ownership is determined in accordance with the rules of the SEC. In computing the number of shares beneficially owned by a person and the percentage of ownership of that person, shares of common stock subject to options and warrants held by that person that are currently exercisable or become exercisable within 60 days of the closing of the Merger on May 3, 2011 are deemed outstanding even if they have not actually been exercised. Those shares, however, are not deemed outstanding for the purpose of computing the percentage ownership of any other person.
75
Immediately prior to the closing of the Merger, we had outstanding 5,577,750 shares of common stock, no shares of preferred stock, no options and no warrants to purchase shares of common stock. Immediately after the closing of the Merger, we had 24,423,714 shares of common stock, no shares of preferred stock, warrants to purchase 302,917 shares of our common stock, options to purchase 23,590 of our common stock and convertible notes convertible into 270,648 shares of our common stock issued and outstanding.
The following table sets forth certain information with respect to beneficial ownership of our common stock immediately after the closing of the Merger based on issued and outstanding shares of common stock, by:
| ● | Each person known to be the beneficial owner of 5% or more of our outstanding common stock; | |
| ● | Each executive officer; | |
| ● | Each director; and | |
| ● | All of the executive officers and directors as a group. |
Unless otherwise indicated, the persons and entities named in the table have sole voting and sole investment power with respect to the shares set forth opposite the stockholder’s name, subject to community property laws, where applicable. Unless otherwise indicated, the address of each stockholder listed in the table is c/o Emmaus Medical, Inc. 20725 S. Western Avenue, Ste. 136, Torrance, CA 90501-1884.
Name and Address of Beneficial Owner | Title | Beneficially Owned Post-Merger | Percent of Class (1) | |||||
| Directors and Executive Officers | ||||||||
| Yutaka Niihara, M.D., MPH | President, Chief Executive Officer and Director | 9,573,940 | (2) | 39.3 | % | |||
| Yasushi Nagasaki | Chief Financial Officer | — | — | |||||
| Willis C. Lee | Chief Operating Officer and Director | 176,913 | 0.7 | % | ||||
| Lan T. Tran | Chief Administrative Officer and Corporate Secretary | 23,294 | 0.1 | % | ||||
| Steve Warnecke | Director | 64,000 | 0.3 | % | ||||
| Henry A. McKinnell, Jr., Ph.D. | Chairman of the Board | 11,795 | (3) | * | ||||
| Douglas W. Wilmore, M.D. | Director | 114,995 | (4) | 0.5 | % | |||
| Amir Heshmatpour 9595 Wilshire Blvd, Suite 700 Beverly Hills, CA 90212 | Director | 2,672,250 | (5) | 11.0 | % | |||
| Officers and Directors as a Group (total of 8 persons) | 12,637,187 | (6) | 51.8 | % | ||||
| 5% Holders | ||||||||
AFH Holding & Advisory, LLC (7) 9595 Wilshire Blvd, Suite 700 Beverly Hills, CA 90212 | 2,372,250 | 9.7 | % | |||||
Daniel R. and Yuka I. Kimbell 350 W. Colorado Blvd., Ste. 350 Pasadena, CA 91105 | 2,434,028 | (8) | 9.9 | % |
* Less than 0.1%.
76
| (1) | Each stockholder’s percentage of ownership in the above table is based upon 24,378,305 shares of the Company’s common stock outstanding as of May 3, 2011. |
| (2) | Includes 9,529,711 shares that are held jointly by Yutaka and Soomi Niihara, his wife. Also includes 44,229 shares of common stock for which Dr. Niihara is custodian. Dr. Niihara may be deemed the indirect beneficial owner of these securities since he has sole voting and investment control over the securities |
| (3) | Represents options to purchase 11,795 shares of common stock. |
| (4) | Includes options to purchase 11,795 shares of common stock held by Dr. Wilmore and 103,200 shares of common stock owned by Dr. Wilmore’s spouse over which Dr. Wilmore is deemed to have shared investment and voting power. Dr. Wilmore disclaims beneficial ownership of the shares owned by his spouse. |
| (5) | Represents 2,372,250 shares of common stock owned by AFH Advisory and 300,000 shares of common stock owned by Griffin Ventures LTD (“Griffin”). Mr. Heshmatpour is the sole member of AFH Advisory and the control person of Griffin and has sole voting and investment control over the shares of common stock owned of record by AFH Advisory and Griffin. Accordingly, he may be deemed a beneficial owner of the 2,372,250 shares of common stock owned by AFH Advisory and the 300,000 shares of common stock owned by Griffin. |
| (6) | Includes options to purchase 23,590 shares of common stock. |
| (7) | Mr. Heshmatpour is the managing partner of AFH Advisory and may be deemed to have voting and dispositive controls with respect to these shares. Mr. Heshmatpour disclaims beneficial ownership of any shares in which he does not have a pecuniary interest. |
| (8) | Includes 44,229 shares of common stock held by the holder as custodian. Daniel and Yuka Kimbell may be deemed the indirect beneficial owner of these securities since they have sole voting and investment control over the securities. |
Item 5.02 Departure of Directors or Certain Officers; Election of Directors; Appointment of Certain Officers; Compensatory Arrangements of Certain Officers.
At the consummation of the Merger, the board of directors immediately prior to the Merger, which consisted of Amir F. Heshmatpour and Timothy Brasel, appointed Yutaka Niihara, M.D., MPH, Willis C. Lee, Steve Warnecke, Henry A. McKinnell, and Douglas W. Wilmore, M.D. to the board of directors of our company. Mr. Brasel resigned as a director and as Vice President of AFH IV. In addition, Amir F. Heshmatpour resigned as President, Secretary and Chief Financial Officer of AFH IV. In addition, concurrent with the closing of the Merger, our board of directors appointed Dr. Niihara as our President and Chief Executive Officer, Yasushi Nagasaki as our Chief Financial Officer, Mr. Lee as our Chief Operating Officer and Ms. Tran as our Chief Administrative Officer and Corporate Secretary. Dr. McKinnell was appointed Chairman of the Board. For information regarding our new officers and directors, refer to “Executive Officers, Directors and Key Employees” under Item 5.01, above, which information is incorporated herein by reference.
On May 3, 2011, the Company approved the 2011 Stock Incentive Plan. For information regarding our 2011 Stock Incentive Plan, refer to “2011 Stock Incentive Plan” under Item 5.01, above, which information is incorporated herein by reference.
77
Item 5.03 Amendments to Articles of Incorporation or Bylaws; Change in Fiscal Year.
Immediately after the closing of the Merger, AFH IV changed its corporate name from “AFH Acquisition IV, Inc.” to “Emmaus Holdings, Inc.” by filing the Certificate of Ownership and Merger with the Delaware Secretary of State’s Office on May 3, 2011. AVH IV effected the name change to better reflect the nature of its new business operations following the Merger. The Certificate of Ownership and Merger is attached hereto as Exhibit 3.3. Holders of stock certificates bearing the name “AFH Acquisition IV, Inc.” may continue to hold them and will not be required to exchange them for new certificates or take any other action.
On May 3, 2011, the Company changed its fiscal year end from October 31st to December 31st. The Merger is being accounted for as a recapitalization where Emmaus Medical is considered the accounting acquirer and, therefore, no transition period will be presented in future financial statements.
Item 5.06 Change in Shell Company Status.
Prior to the closing of the Merger, AFH IV was a “shell company” as defined in Rule 405 of the Securities Act and Rule 12b-2 of the Exchange Act. As described in Item 2.01 above, which is incorporated by reference into this Item 5.06, AFH IV ceased being a shell company upon completion of the Merger on May 3, 2011.
Item 9.01 Financial Statements and Exhibits.
(a) Financial Statements of Business Acquired.
FINANCIAL STATEMENTS OF EMMAUS MEDICAL, INC. AND SUBSIDIARIES
The financial statements of Emmaus Medical, Inc. and subsidiaries, as of and for the three months ended March 31, 2011 and as of and for the years ended December 31, 2010 and 2009 are provided below. You are encouraged to review the financial statements and related notes.
78
EMMAUS MEDICAL, INC.
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
| PAGE | ||
| 80 | ||
| 81 | ||
| 82 | ||
| 86 | ||
| 87 | ||
| 113 | ||
| 114 | ||
| 115 | ||
| 116 | ||
| 119 | ||
| 120 |
79
Emmaus Medical, Inc.
(A Development Stage Company)
| As of | ||||||||
| March 31, 2011 | December 31, | |||||||
| (Unaudited) | 2010 | |||||||
| (Restated) | (Restated) | |||||||
| ASSETS | ||||||||
| CURRENT ASSETS | ||||||||
| Cash and cash equivalents | $ | 1,075,564 | $ | 258,676 | ||||
| Accounts receivable | 16,302 | 21,746 | ||||||
| Inventories | 192,911 | 130,573 | ||||||
| Marketable securities | 1,217,103 | 1,674,386 | ||||||
| Prepaid expenses and other current assets | 13,544 | 11,479 | ||||||
| Total Current Assets | 2,515,424 | 2,096,860 | ||||||
| PROPERTY AND EQUIPMENT, net | 89,426 | 94,179 | ||||||
| OTHER ASSETS | ||||||||
| Marketable securities, long-term | 1,217,103 | — | ||||||
| Intangibles, net | 71,281 | 134,880 | ||||||
| Notes receivable | 18,000 | 18,000 | ||||||
| Deposits | 506,515 | 348,408 | ||||||
| Total Other Assets | 1,812,899 | 501,288 | ||||||
| Total Assets | $ | 4,417,749 | $ | 2,692,327 | ||||
| LIABILITIES AND SHAREHOLDERS’ EQUITY | ||||||||
| CURRENT LIABILITIES | ||||||||
| Accounts payable and accrued expenses | $ | 330,633 | $ | 190,107 | ||||
| Notes payable | 460,000 | 460,000 | ||||||
| Convertible notes payable | — | 246,889 | ||||||
| Total current liabilities | 790,633 | 896,996 | ||||||
| LONG-TERM LIABILITIES | ||||||||
| Notes payable | 550,494 | — | ||||||
| Convertible notes payable | 574,000 | 184,030 | ||||||
| Total long-term liabilities | 1,124,494 | 184,030 | ||||||
| Total Liabilities | 1,915,127 | 1,081,026 | ||||||
| SHAREHOLDERS’ EQUITY | ||||||||
| Common stock - par value $0.001 per share, 1,000,000 shares authorized, 701,145 and 690,692 shares issued and outstanding at March 31, 2011 and December 31, 2010, respectively. | 701 | 691 | ||||||
| Additional paid-in capital | 15,083,367 | 13,819,673 | ||||||
| Accumulated other comprehensive income | 1,302,393 | 542,573 | ||||||
| Deficit accumulated during the development stage | (13,883,839 | ) | (12,751,636 | ) | ||||
| Total Shareholders’ Equity | 2,502,622 | 1,611,301 | ||||||
| Total Liabilities & Shareholders’ Equity | $ | 4,417,749 | $ | 2,692,327 | ||||
The accompanying notes are an integral part of these financial statements.
80
Emmaus Medical, Inc.
(A Development Stage Company)
(unaudited)
| Three Months Ended March 31, | From December 20, 2000 (date of inception) to March 31, | |||||||||||
| 2011 | 2010 | 2011 | ||||||||||
| (Restated) | (Restated) | |||||||||||
| SALES | $ | 59,468 | $ | 49,547 | $ | 433,967 | ||||||
| SALES RETURN | (255 | ) | (3,758 | ) | (30,612 | ) | ||||||
| REVENUES | 59,213 | 45,789 | 403,355 | |||||||||
| COST OF GOODS SOLD | ||||||||||||
| Cost of goods sold, net of scrapped inventory | 25,101 | 22,533 | 251,135 | |||||||||
| Scrapped inventory | — | — | 235,537 | |||||||||
| Total cost of goods sold | 25,101 | 22,533 | 486,672 | |||||||||
| GROSS PROFIT (LOSS) | 34,112 | 23,256 | (83,317 | ) | ||||||||
| OPERATING EXPENSES | ||||||||||||
| Research and development | 310,763 | 221,946 | 5,210,415 | |||||||||
| Selling | 201,511 | 125,042 | 2,003,719 | |||||||||
| General and administrative | 647,825 | 406,421 | 6,260,565 | |||||||||
| 1,160,099 | 753,409 | 13,474,699 | ||||||||||
| LOSS FROM OPERATIONS | (1,125,987 | ) | (730,153 | ) | (13,558,016 | ) | ||||||
| OTHER INCOME (EXPENSE) | ||||||||||||
| Interest income | 6,445 | 9,009 | 91,679 | |||||||||
| Interest expense | (11,811 | ) | (11,950 | ) | (401,804 | ) | ||||||
| (5,366 | ) | (2,941 | ) | (310,125 | ) | |||||||
| LOSS BEFORE INCOME TAXES | (1,131,353 | ) | (733,094 | ) | (13,868,141 | ) | ||||||
| INCOME TAXES | 850 | 1,250 | 15,698 | |||||||||
| NET LOSS | (1,132,203 | ) | (734,344 | ) | (13,883,839 | ) | ||||||
| OTHER INCOME | ||||||||||||
| Unrealized holding gain on securities available-for-sale, net of tax | 759,820 | — | 1,302,393 | |||||||||
| COMPREHENSIVE LOSS | $ | (372,383 | ) | $ | (734,344 | ) | $ | (12,581,446 | ) | |||
| NET LOSS PER COMMON SHARE | $ | (1.63 | ) | $ | (1.12 | ) | ||||||
| WEIGHTED AVERAGE COMMON SHARES OUTSTANDING | 695,915 | 654,157 | ||||||||||
The accompanying notes are an integral part of these financial statements.
81
Emmaus Medical, Inc.
(A Development Stage Company)
and for the period from December 20, 2000 (Inception) to March 31, 2011
(unaudited)
| Common stock – par value $0.001 per share, 1,000,000 shares authorized | Additional Paid-in | Accumulated Other Comprehensive | Deficit Accumulated during Development | |||||||||||||||||||||
| Shares | Common stock | Capital | income | Stage | Total | |||||||||||||||||||
| Balance, December 31, 2000 (1) | 425,000 | $ | 425 | $ | 9,175 | — | $ | — | $ | 9,600 | ||||||||||||||
| Net loss | — | — | — | — | (21,942 | ) | (21,942 | ) | ||||||||||||||||
| Balance, December 31, 2001 | 425,000 | 425 | 9,175 | — | (21,942 | ) | (12,342 | ) | ||||||||||||||||
| Net loss | — | — | — | — | (12,464 | ) | (12,464 | ) | ||||||||||||||||
| Balance, December 31, 2002 | 425,000 | 425 | 9,175 | — | (34,406 | ) | (24,806 | ) | ||||||||||||||||
| Constructive distribution of retained loss to Additional Paid-in Capital | — | — | (34,406 | ) | — | 34,406 | — | |||||||||||||||||
| Common stock issued | 25,000 | 25 | 249,975 | — | — | 250,000 | ||||||||||||||||||
| — | ||||||||||||||||||||||||
| Net loss | — | — | — | — | (97,481 | ) | (97,481 | ) | ||||||||||||||||
| Balance, December 31, 2003 | 450,000 | 450 | 224,744 | — | (97,481 | ) | 127,713 | |||||||||||||||||
(1) Reflects recapitalization of members equity of Emmaus Medical, LLC into 425,000 shares of common stock of Emmaus Medical, Inc.
82
Emmaus Medical, Inc.
(A Development Stage Company)
Consolidated Statement of Changes in Shareholders’ Equity (Deficit)
and for the period from December 20, 2000 (Inception) to March 31, 2011 (continued)
(unaudited)
| Common stock – par value $0.001 per share, 1,000,000 shares authorized | Additional Paid-in | Accumulated OtherComprehensive | Deficit Accumulated during Development | |||||||||||||||||||||
| Shares | Common stock | Capital | income | Stage | Total | |||||||||||||||||||
| Balance, December 31, 2003 | 450,000 | $ | 450 | $ | 224,744 | $ | — | $ | (97,481 | ) | $ | 127,713 | ||||||||||||
| Common stock issued | 54,792 | 55 | 648,020 | — | — | 648,075 | ||||||||||||||||||
| Net loss | — | — | — | — | (624,936 | ) | (624,936 | ) | ||||||||||||||||
| Balance, December 31, 2004 | 504,792 | 505 | 872,764 | — | (722,417 | ) | 150,852 | |||||||||||||||||
| Common stock issued | 13,517 | 13 | 328,272 | — | — | 328,285 | ||||||||||||||||||
| Net loss | — | — | — | — | (668,091 | ) | (668,091 | ) | ||||||||||||||||
| Balance, December 31, 2005 | 518,309 | 518 | 1,201,036 | — | (1,390,508 | ) | (188,954 | ) | ||||||||||||||||
| Common stock issued | 17,751 | 18 | 825,022 | — | — | 825,040 | ||||||||||||||||||
| Net loss | — | — | — | — | (759,962 | ) | (759,962 | ) | ||||||||||||||||
| Balance, December 31, 2006 | 536,060 | 536 | 2,026,058 | — | (2,150,470 | ) | (123,876 | ) | ||||||||||||||||
| Common stock issued | 45,588 | 46 | 2,733,814 | — | — | 2,733,860 | ||||||||||||||||||
| Net loss | — | — | — | — | (1,282,212 | ) | (1,282,212 | ) | ||||||||||||||||
| Balance, December 31, 2007 | 581,648 | 582 | 4,759,872 | — | (3,432,682 | ) | 1,327,772 | |||||||||||||||||
83
Emmaus Medical, Inc.
(A Development Stage Company)
Consolidated Statement of Changes in Shareholders’ Equity (Deficit)
and for the period from December 20, 2000 (Inception) to March 31, 2011 (continued)
(unaudited)
| Common stock – par value $0.001 per share, 1,000,000 shares authorized | Additional Paid-in | Accumulated Other Comprehensive | Deficit Accumulated during Development | |||||||||||||||||||||
| Shares | Common stock | Capital | income | Stage | Total | |||||||||||||||||||
| Balance, December 31, 2007 | 581,648 | $ | 582 | $ | 4,759,872 | $ | — | $ | (3,432,682 | ) | $ | 1,327,772 | ||||||||||||
| Common stock issued | 41,613 | 41 | 3,390,650 | — | — | 3,390,691 | ||||||||||||||||||
| Net loss | — | — | — | — | (2,993,777 | ) | (2,993,777 | ) | ||||||||||||||||
| Balance, December 31, 2008 | 623,261 | 623 | 8,150,522 | — | (6,426,459 | ) | 1,724,686 | |||||||||||||||||
| Warrants issued | — | — | 160,000 | — | — | 160,000 | ||||||||||||||||||
| Common stock issued, net of issuance cost of $160,000 | 28,979 | 29 | 2,078,896 | — | — | 2,078,925 | ||||||||||||||||||
| Net loss | — | — | — | — | (2,567,807 | ) | (2,567,807 | ) | ||||||||||||||||
| Balance, December 31, 2009 | 652,240 | 652 | 10,389,418 | — | (8,994,266 | ) | 1,395,804 | |||||||||||||||||
| Warrants issued | — | — | 480,000 | — | — | 480,000 | ||||||||||||||||||
| Common stock issued, net of issuance cost of $480,000 | 23,941 | 24 | 1,644,270 | — | — | 1,644,294 | ||||||||||||||||||
| Conversion of notes payable to common stock | 14,511 | 15 | 1,305,985 | — | — | 1,306,000 | ||||||||||||||||||
| Unrealized gain on securities available for sale | — | — | — | 542,573 | — | 542,573 | ||||||||||||||||||
| Net loss | — | — | — | — | (3,757,370 | ) | (3,757,370 | ) | ||||||||||||||||
| Balance, December 31, 2010 (restated) | 690,692 | 691 | 13,819,673 | 542,573 | (12,751,636 | ) | 1,611,301 | |||||||||||||||||
84
Emmaus Medical, Inc.
(A Development Stage Company)
Consolidated Statement of Changes in Shareholders’ Equity (Deficit)
and for the period from December 20, 2000 (Inception) to March 31, 2011 (continued)
(unaudited)
| Common stock – par value $0.001 per share, 1,000,000 shares authorized | Additional Paid-in | Accumulated OtherComprehensive | Deficit Accumulated during Development | |||||||||||||||||||||
| Shares | Common stock | Capital | income | Stage | Total | |||||||||||||||||||
| Balance, December 31, 2010 (restated) | 690,692 | $ | 691 | $ | 13,819,673 | $ | 542,573 | $ | (12,751,636 | ) | $ | 1,611,301 | ||||||||||||
| Common stock issued, net of issuance cost | 9,230 | 9 | 1,153,665 | — | — | 1,153,674 | ||||||||||||||||||
| Conversion of notes payable to common stock | 1,223 | 1 | 110,029 | — | — | 110,030 | ||||||||||||||||||
| Unrealized gain on securities | — | — | — | 759,820 | — | 759,820 | ||||||||||||||||||
| Net loss | — | — | — | — | (1,132,203 | ) | (1,132,203 | ) | ||||||||||||||||
| Balance, March 31, 2011 (Restated) | 701,145 | $ | 701 | $ | 15,083,367 | $ | 1,302,393 | (13,883,839 | ) | $ | 2,502,622 | |||||||||||||
The accompanying notes are an integral part of these financial statements.
85
Emmaus Medical, Inc.
(A Development Stage Company)
(unaudited)
| March 31, | March 31, | From December 20, 2000 (date of inception) to | ||||||||||
| 2011 | 2010 | March 31, 2011 | ||||||||||
| (Restated) | (Restated) | |||||||||||
| CASH FLOWS FROM OPERATING ACTIVITIES | ||||||||||||
| Net loss | $ | (1,132,203 | ) | $ | (734,344 | ) | $ | (13,883,839 | ) | |||
| Adjustments to reconcile net loss to net cash flows from operating activities | ||||||||||||
| Depreciation and amortization | 69,846 | 70,681 | 776,594 | |||||||||
| Cost of scrapped inventory written off | — | — | 235,537 | |||||||||
| Net changes in operating assets and liabilities, net of acquisition | ||||||||||||
| Accounts receivable | 5,444 | (28,333 | ) | (22,408 | ) | |||||||
| Inventory | (62,338 | ) | (73,000 | ) | (424,438 | ) | ||||||
| Prepaid expenses and other current assets | — | (7,178 | ) | (29,479 | ) | |||||||
| Deposits | (160,172 | ) | (136,105 | ) | (457,944 | ) | ||||||
| Accounts payable and accrued expenses | 140,527 | 495,007 | 270,496 | |||||||||
| Net cash flows used in operating activities | (1,138,896 | ) | (413,272 | ) | (13,535,481 | ) | ||||||
| CASH FLOWS FROM INVESTING ACTIVITIES | ||||||||||||
| Payment towards license | — | — | (750,000 | ) | ||||||||
| Purchases of marketable securities | — | — | (1,131,813 | ) | ||||||||
| Purchases of property and equipment | (1,494 | ) | — | (187,301 | ) | |||||||
| Net cash flows used in investing activities | (1,494 | ) | — | (2,069,114 | ) | |||||||
| CASH FLOWS FROM FINANCING ACTIVITIES | ||||||||||||
| Borrowings from line of credit | — | — | 299,500 | |||||||||
| Repayment of line of credit | — | — | (299,500 | ) | ||||||||
| Proceeds from notes payable issued | 303,604 | 73,606 | 1,046,067 | |||||||||
| Payments of notes payable | — | — | (35,576 | ) | ||||||||
| Proceeds from convertible notes payable issued | 500,000 | — | 1,990,030 | |||||||||
| Proceeds from issuance of common stock | 1,153,674 | 767,249 | 13,668,038 | |||||||||
| Net cash flows from financing activities | 1,957,278 | 840,855 | 16,668,559 | |||||||||
| Net increase in cash and cash equivalents | 816,888 | 427,583 | 1,063,964 | |||||||||
| Cash and cash equivalents, beginning of period | 258,676 | 389,554 | — | |||||||||
| Cash acquired | — | — | 11,600 | |||||||||
| Cash and cash equivalents, end of period | $ | 1,075,564 | $ | 817,137 | $ | 1,075,564 | ||||||
| SUPPLEMENTAL DISCLOSURES OF CASH FLOW ACTIVITIES | ||||||||||||
| Interest paid | $ | 11,811 | $ | — | $ | 101,804 | ||||||
| Income taxes paid | $ | 850 | $ | 1,250 | $ | 15,698 | ||||||
| Non-cash Financing Activities: | ||||||||||||
| Conversion of notes payable to common stock | $ | 110,030 | $ | — | $ | 1,416,030 | ||||||
The accompanying notes are an integral part of these financial statements.
86
Organization - Emmaus Medical, Inc. (the “Company” or “Emmaus Medical”) is engaged in the discovery, development, and commercialization of treatments and therapies for rare diseases. Emmaus Medical is a Delaware corporation originally incorporated on September 12, 2003. Emmaus Medical, LLC was organized on December 20, 2000. In October 2003, Emmaus Medical, LLC conducted a reorganization and merged with Emmaus Medical. As a result of the merger, Emmaus Medical acquired the exclusive patent rights for a treatment for sickle cell disease.
Emmaus Medical, and Emmaus Medical’s subsidiaries, Newfield Nutrition Corporation and Emmaus Medical Japan, Inc., are collectively referred to herein as the “Company.”
Nature of Business - The Company has undertaken the business of developing and commercializing cost-effective treatments and therapies for rare diseases. The Company’s primary business purpose is to continue its late-stage development of the amino acid L-glutamine as a prescription drug for the treatment of sickle cell disease (“SCD”). The Company’s current focus is to complete the Phase 3 clinical trial on SCD that involves over 20 research sites and 200 patients. The Company also sells and/or promotes certain other prescription pharmaceutical drugs, primarily Zorbtive® and NutreStore® that are used in combination as a treatment for short bowel syndrome. Over time, the Company plans to expand its mission to include developing and marketing products for more common diseases.
NOTE 2 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of presentation - The accompanying unaudited consolidated financial statements have been prepared by the Company pursuant to the rules and regulations of the Securities and Exchange Commission. Certain information and footnote disclosures normally included in financial statements prepared in accordance with generally accepted accounting principles have been condensed or omitted in accordance with such rules and regulations. The information furnished in the interim consolidated financial statements includes normal recurring adjustments and reflects all adjustments, which, in the opinion of management, are necessary for a fair presentation of such financial statements. Although management believes the disclosures and information presented are adequate to make the information not misleading, it is suggested that these interim consolidated financial statements be read in conjunction with the Company’s most recent audited consolidated financial statements and notes hereto as of December 31, 2010. Operating results for the three months ended March 31, 2011 are not necessarily indicative of the results that may be expected for the year ending December 31, 2011.
Going concern - The accompanying financial statements have been prepared on the basis that the Company will continue as a going concern. The Company has losses for the three months ended March 31, 2011 totaling $1,132,203 and for the year ended December 31, 2010 totaling $3,757,370 and as well as accumulated deficit since inception amounting to $13,883,839. Further the Company appears to have inadequate cash and cash equivalents of $1,075,564 as of March 31, 2011 considering that revenues from operations since inception totaled only $403,355. As a result, the Company is dependent upon funds from private investors and the support of certain stockholders.
These factors raise substantial doubt about the ability of the Company to continue as a going concern. The financial statements do not include any adjustments that might result from the outcome of these uncertainties. In this regard, Management is planning to raise any necessary additional funds through loans and additional sales of its common stock. There is no assurance that the Company will be successful in raising additional capital.
Recapitalization and change in legal status of entity - In October 2003, Emmaus Medical acquired substantially all of the assets of Emmaus Medical, LLC. The shareholders of Emmaus Medical were substantially the same as the members of Emmaus Medical, LLC. As such, the transaction was accounted for as a transfer of assets between entities under common control pursuant to accounting standards codification 805, Business Combinations.
For a transferred set of activities and assets to be a business, it must contain all of the inputs and processes necessary for it to continue to conduct normal operations after the transferred set of assets is separated from the transferor, which include the ability to sustain a revenue stream by providing its outputs to customers. Emmaus Medical obtained the inputs and processes necessary for normal operations. The transaction has been accounted for as a recapitalization of Emmaus Medical, LLC. Accordingly, the assets were carried over to Emmaus Medical, Inc. at the historical carrying values and the historical operations of those assets owned by Emmaus Medical are presented in the accompanying financial statements as the historical operations of Emmaus Medical, Inc. for all periods presented.
87
The effect of the recapitalization was to retroactively present the stockholders’ equity of Emmaus Medical, Inc. (the surviving entity) to the earliest period presented in the financial statements. This recapitalization had no effect on results of operations for any period presented. Also, concurrent with the recapitalization, Emmaus Medical changed its legal status from a Limited Liability Company to a "C" Corporation. In connection with this change, deficits accumulated in the Limited Liability Company were transferred to additional paid in capital.
Principles of consolidation - The financial statements include the accounts of the Company (and its wholly-owned subsidiary, Emmaus Medical, Inc., and its wholly-owned subsidiaries, Newfield Nutrition Corporation and Emmaus Medical Japan, Inc (“EM Japan”). All significant intercompany transactions have been eliminated.
Development stage company - The Company is a development stage company as defined in accounting principles generally accepted in the United States of America. The company is considered a development stage company because it devotes substantially all of its time to research and development for potential pharmaceutical products and to establish its business and operations. The minimal sales for the period from inception to March 31, 2011 are from NutreStore and the products of its wholly owned subsidiary Newfield Nutrition Corporation which is not considered to be a part of its principal operations.
Use of estimates - Financial statements prepared in accordance with accounting principles generally accepted in the United States require management to make estimates and assumptions that affect the reported amounts of assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Among other things, management has estimated the useful lives of equipment and other assets, along with the variables used to calculate the valuation of stock options and warrants using the Black-Scholes-Merton option valuation model. Actual results could differ from those estimates.
Cash and cash equivalents - Cash and cash equivalents include all short-term securities with original maturities of less than ninety days. The Company maintains its cash and cash equivalents at insured financial institutions, the balances of which may, at times, exceed federally insured limits. Management believes that the risk of loss due to the concentrations is minimal.
Inventories - Inventories of the Company consist of finished goods and work-in-process and are valued based on first-in, first-out and at the lesser of cost or market value. Work-in-process inventories consist of raw material L-Glutamine for the Company’s products, AminoPure and NutreStore, that has not yet been packaged and labeled for sale. As of March 31, 2011, inventories consisted of 69% finished goods and 31% work-in-process. As of December 31, 2010, inventories consisted of 100% finished goods.
All of the purchases during the three months ended March 31, 2011 were from one vendor. The Company made purchases of L-glutamine from two vendors in the year ended December 31, 2010, with one vendor accounting for 48% and the other accounting for 52%, respectively, of total purchases during such year.
Deposits - Carrying value of amounts transferred to third parties for security purposes that are expected to be returned or applied towards payment after one year or beyond the operating cycle, if longer. The deposit amount has increased significantly from 2009. This change can be attributed to the 20% patient site enrollment deposit paid to ClinDatrix for the Phase III clinical trial activities.
Revenue recognition - The Company recognizes revenue in accordance with the Securities and Exchange Commission (“SEC”) Staff Accounting Bulletin (“SAB”) No. 101, Revenue Recognition in Financial Statements (“SAB 101”), as amended by Staff Accounting Bulletin No. 104, Revision of Topic 13 (“SAB 104”).
Revenue is recognized when there is persuasive evidence that an arrangement exists, delivery has occurred, the price is fixed and determinable and collection is reasonably assured.
88
With prior written approval of the Company, product is returnable only by our direct customers for a returned goods credit, if the product meets any of the following criteria:
| A. | Product expiring within six (6) months of the expiration date printed on the package/container that is in the manufacturer’s original package/container and bears the original label. | |
| B. | Expired product that is in the manufacturer’s original package/container and bears the manufacturer’s original label, provided, however, that expired product must be returned within 12 months of the expiration date printed on the package/container. | |
| C. | Product shipped directly by the Company that is damaged in transit, subject to Free on Board (“FOB”) Destination, or material shipped in error by the Company. | |
| D. | Product that is discontinued, withdrawn, or recalled. |
Credits will only be issued for full cartons only without any missing packets of product. No credit is issued, nor does the Company accept charges or deductions for administrative, handling, or freight charges associated with the return of product to the Company. No credit is issued for product destroyed by anyone other than the Company. Customers must return the product within 60 days of receiving our written approval for the return or the return will not be issued a credit. The amount of the credit provided for returned product is based on the current wholesale acquisition cost of the returned product less 5%. When product is returned, a credit memo is applied to the customer’s current account balance or applied to future purchases. Credit memos expire one hundred eighty (180) days from date issued.
The Company estimates its sales return based upon its prior sales and return history. Historically, sales returns have been very nominal. The extraordinary sales returns in the six months ended June 30, 2010 were all related to the same batch of NutreStore product produced in 2008 that expired. The expiry date of the NutreStore product was two years after the date of manufacture but was changed by the Company and is currently four years after the date of manufacture. All products that were sold in 2009 were part of the batch that had a two year expiry period and expired in 2010. All products produced in 2010 and sold from 2010 through 2011 have an expiry date of 2014 and the Company anticipates that the product will be consumed before expiration and not returned. The Company continues to monitor its returns and will adjust its estimates based on its actual sales return experience. Based upon the Company’s sales return history the Company will record a reserve for estimated returns during each reporting period. As of March 31, 2011, no reserve for sales returns has been deemed necessary and accordingly, no reserve has been recorded in the accompanying financial statements.
The Company is currently required to pay royalties to CATO Holding Company on an annual basis, which is recognized as an expense upon sale of the products.
Allowance for doubtful accounts - The Company provides an allowance for uncollectible accounts based upon prior experience and management's assessment of the collectability of existing specific accounts. As of March 31, 2011 and December 31, 2010, management considers all accounts receivable fully collectible.
Advertising cost - Advertising costs are expensed as incurred. Advertising costs for the three months ended March 31, 2011 and 2010 were $12,549 and $6,719 respectively. Advertising costs from inception to March 31, 2011 were approximately $153,000.
Property and equipment - Leaseholds, furniture, and fixtures are recorded at historical cost and depreciated on a straight-line basis over their estimated useful lives of 5 to 7 years. Maintenance and repairs are expensed as incurred, while major additions and improvements are capitalized. Gains and losses on disposition are included in current operations. Equipment is assessed by management, annually, for potential impairment. No impairment exists as of March 31, 2011 and December 31, 2010.
Intangibles - The Company’s intangible assets include license issue fees and patent costs relating to a license agreement (Note 4). These intangible assets are amortized over a period of 3 years, the estimated legal life of the patents and economic life of the License Agreement. The intangible assets are assessed by management, annually, for potential impairment. No impairment exists as of March 31, 2011 and December 31, 2010.
89
Impairment of Long-Lived Assets - In accordance with FASB ASC 360-10-5, Accounting for the Impairment or Disposal of Long-Lived Assets, the Company evaluates the carrying value of its long-lived assets for impairment whenever events or changes in circumstances indicate that such carrying values may not be recoverable. The Company uses its best judgment based on the current facts and circumstances relating to its business when determining whether any significant impairment factors exist. The Company considers the following factors or conditions, among others, that could indicate the need for an impairment review:
| ● | significantly lower performance relative to expected historical or projected future operating results; | |
| ● | market projections; | |
| ● | its ability to obtain patents, including continuation patents, on technology; | |
| ● | significant changes in its strategic business objectives and utilization of the assets; | |
| ● | significant negative industry or economic trends, including legal factors; | |
| ● | potential for strategic partnerships for the development of its patented technology; | |
| ● | changing or implementation of rules regarding manufacture |
If the Company determines that the carrying values of long-lived assets may not be recoverable based upon the existence of one or more of the above indicators of impairment, the Company’s management performs an undiscounted cash flow analysis to determine if impairment exists. If impairment exists, the Company measures the impairment based on the difference between the asset’s carrying amount and its fair value, and the impairment is charged to operations in the period in which the long-lived asset impairment is determined by management.
Based on its analysis, the Company believes that no indicators of impairment of the carrying value of its long-lived assets existed at March 31, 2011 and December 31, 2010.
There can be no assurance, however, that market conditions will not change or demand for the Company’s products will continue or allow the Company to realize the value of its long-lived assets and prevent future impairment.
Research and development - Research and development consist of expenditures for the research and development of new products and technologies, which primarily involve contract research, payroll-related expenses, and other related supplies. Research and development costs are expensed as incurred.
Share-based payments - The Company recognizes compensation cost for share-based compensation awards during the service term of the recipients of the share-based awards. The fair value of share-based is calculated using the Black-Scholes-Merton pricing model. The Black-Scholes-Merton model requires subjective assumptions regarding future stock price volatility and expected time to exercise, which greatly affect the calculated values. The expected term of awards granted is derived from historical data on awards exercised and post-vesting employment termination behavior. The risk-free rate selected to value any particular grant is based on the U.S. Treasury rate that corresponds to the vesting period of the grant effective as of the date of the grant. The expected volatility is based on the historical volatility of the common stock of comparable publicly traded companies. These factors could change, affecting the determination of stock-based awards expense in future periods.
Income taxes - The Company accounts for income taxes under the asset and liability method, wherein deferred tax assets and liabilities are recognized for the future tax consequences attributable to the differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period the enactment occurs. A valuation allowance is provided for certain deferred tax assets if it is more likely than not that the Company will not realize tax assets through future operations.
The Company's short-term and long-term deferred tax liability is based on the calculation of the deferred taxes on the Company's unrealized gain on available-for-sale securities using a 40% effective tax rate based on a 31% federal income tax rate (net of state tax deduction) combined with an 8.84% California state income tax rate. The Company recognizes a deferred tax asset (through changes in the valuation allowance) for the exact amount of the deferred tax liability. The classification of these deferred taxes is concurrent with the classification of investments for which the unrealized gain is derived. For balance sheet presentation, current deferred tax assets and liabilities have been offset and presented as a single amount and non-current deferred tax assets and liabilities within each tax jurisdiction have been offset and presented as a single amount.
90
When tax returns are filed, it is highly probable that some positions taken would be sustained upon examination by the taxing authorities, while others are subject to uncertainty about the merits of the position taken or the amount of the position that would be ultimately sustained. The benefit of a tax position is recognized in the financial statements in the period during which, based on all available evidence, management believes it is more likely than not that the position will be sustained upon examination, including the resolution of appeals or litigation processes, if any. Tax positions taken are not offset or aggregated with other positions. Tax positions that meet the more-likely-than-not recognition threshold are measured as the largest amount of tax benefit that is more than 50 percent likely of being realized upon settlement with the applicable taxing authority. The portion of the benefits associated with tax positions taken that exceeds the amount measured as described above is reflected as a liability for unrecognized tax benefits along with any associated interest and penalties that would be payable to the taxing authorities upon examination. As of March 31, 2011, the Company had no unrecognized tax benefits, and the Company had no positions which, in the opinion of management, would be reversed if challenged by a taxing authority. The Company’s evaluation of tax positions was performed for those tax years which remain open to audit. The Company may from time to time, be assessed interest or penalties by the taxing authorities, although any such assessments historically have been minimal and immaterial to the Company’s financial results. In the event the Company is assessed for interest and/or penalties, such amounts will be classified as income tax expense in the financial statements.
As of March 31, 2011, all federal tax returns since 2008 and state tax returns since 2007 are still subject to adjustment upon audit. No tax returns are currently being examined by taxing authorities.
Comprehensive income (loss) - Comprehensive income (loss) includes net income (loss) and other comprehensive income (loss). The only items of other comprehensive income (loss) for the Company are unrealized gains and losses on securities classified as available-for-sale. When the Company realizes a gain or loss on available-for-sale securities for which an unrealized gain or loss was previously recognized, a corresponding reclassification adjustment is made to remove the unrealized gain or loss from other comprehensive income and reflect the realized gain or loss in current operations.
Marketable securities – In January 2009, Emmaus acquired 147,100 shares of CellSeed stock for 100,028,000 Japanese Yen (equivalent to $1,109,819), at 680 Yen per share. CellSeed’s initial public offering (symbol 7776) occurred on March 16, 2010. As of March 31, 2011 and December 31, 2011, the Company owned 147,100 shares of CellSeed stock. As of March 31, 2011 and December 31, 2010, and, the closing price per share of CellSeed stock on the JASDAQ was 1,371Yen and 921Yen, respectively.
Investment securities as of March 31, 2011 and December 31, 2010 are classified as available-for-sale. Securities available-for-sale are recorded at cost and any increases or decreases in fair market value are recorded as unrealized gain or loss, net of taxes in accumulated other comprehensive income. The Company monitors these investments for impairment and makes appropriate reductions in carrying values when necessary. CellSeed securities are the only marketable security the Company currently carries on its books. The Company’s position in CellSeed is many times the average daily trading volume of the stock on the JASDAQ exchange. Any attempt to sell the Company’s position in a short period of time may have an adverse impact on the price of the stock. During the three months ended March 31, 2011, the Company granted 50% security interest in Cellseed to a lender as discussed in Note 5.
As of March 31, 2011, 50% of the investment in CellSeed is classified as long term asset in the accompanying balance sheet. As discussed in Note 11, the Company entered into a Joint Research and Development Agreement with CellSeed.
Fair value measurements - The Company defines fair value as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. The Company measures fair value under a framework that provides a fair value hierarchy that prioritizes the inputs to valuation techniques used to measure fair value. The hierarchy gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (level 1 measurements) and the lowest priority to unobservable inputs (level 3 measurements). The three levels of the fair value hierarchy are described as follows:
Level 1: Inputs to the valuation methodology are unadjusted quoted prices for identical assets or liabilities in active markets that the Company has the ability to access.
91
Level 2: Inputs to the valuation methodology include:
| · | Quoted prices for similar assets or liabilities in active markets; | |
| · | Quoted prices for identical or similar assets or liabilities in inactive markets; | |
| · | Inputs other than quoted prices that are observable for the asset or liability; | |
| · | Inputs that are derived principally from or corroborated by observable market data by correlation or other means. |
If the asset or liability has a specified (contractual) term, the level 2 input must be observable for substantially the full term of the asset or liability.
Level 3: Inputs to the valuation methodology are unobservable and significant to the fair value measurement.
The assets or liability’s fair value measurement level within the fair value hierarchy is based on the lowest level of any input that is significant to the fair value measurement. Valuation techniques used need to maximize the use of observable inputs and minimize the use of unobservable inputs. The fair value assigned to marketable securities are determined by obtaining quoted prices on nationally recognized securities exchanges, and are classified as Level 1 investments at March 31, 2011. The fair value of the Company’s debt instruments are not materially different from their carrying values as presented.
Net loss per share - In accordance with FASB ASC Topic 260, “Earnings per Share,” the basic loss per common share is computed by dividing net loss available to common stockholders by the weighted average number of common shares outstanding. Dilutive loss per share is computed similar to basic loss per share except that the denominator is increased to include the number of additional common shares that would have been outstanding if the potential common shares had been issued and if the additional common shares were dilutive. As of March 31, 2011 and 2010, there were 20,294 and 4,663 shares of potentially dilutive securities outstanding, respectively. As the Company reported a net loss, none of the potentially dilutive securities were included in the calculation of diluted earnings per share since their effect would be anti-dilutive for that reporting period
NOTE 3 - PROPERTY AND EQUIPMENT
Property and equipment consisted of the following at:
| March 31, 2011 | December 31, 2010 | |||||||
| Equipment | $ | 111,978 | $ | 110,484 | ||||
| Leasehold Improvements | 23,054 | 23,054 | ||||||
| Furniture and Fixtures | 52,269 | 52,269 | ||||||
| 187,301 | 185,807 | |||||||
| Less: accumulated depreciation | (97,875 | ) | (91,628 | ) | ||||
| $ | 89,426 | $ | 94,179 | |||||
During the three months ended March 31, 2011 and 2010, the depreciation expense was $6,247 and $6,508. Depreciation expense from inception to March 31, 2011 was $97,875.
NOTE 4 - INTANGIBLE ASSETS
The Company is licensed to market and sell NutreStore® [L-glutamine powder for oral solution] and promote Zorbtive® [somatropin (rDNA origin) for injection], as a treatment for short bowel syndrome (“SBS”).
Intangible assets consisted of the following at:
| March 31, 2011 | December 31, 2010 | |||||||
| License fees and patent filing costs | $ | 750,000 | $ | 750,000 | ||||
| Less: accumulated amortization | (678,719 | ) | (615,120 | ) | ||||
| $ | 71,281 | $ | 134,880 | |||||
92
During the three months ended March 31, 2011 and 2010, the amortization expense was $63,599 and $63,488. Amortization expense from inception to March 31, 2011 was $678,719. Expected amortization expense for the year ended December 31, 2011 is estimated to be approximately $135,000.
NOTE 5 - NOTES PAYABLE
Notes payable consisted of the following at:
| March 31, 2011 | December 31, 2010 | |||||||
| Note payable to a shareholder, due on demand, interest payable monthly at 6.5% per annum. | ||||||||
| Yutaka Niihara – Note 1 | $ | 350,000 | $ | 350,000 | ||||
| Yutaka Niihara – Note 2 | 80,000 | 80,000 | ||||||
| Daniel Kimbell – Note 1 | 20,000 | 20,000 | ||||||
| Daniel Kimbell – Note 2 | 10,000 | 10,000 | ||||||
| Note Payable to related parties, due 2013, interest payable quarterly at 8% per annum | ||||||||
| Hope International Hospice, Inc. | 200,000 | — | ||||||
| Willis C. Lee | 100,000 | — | ||||||
| Convertible note payable to shareholders, due 2015, 0% interest payable. | 72,000 | 132,030 | ||||||
| Convertible note payable to shareholders, originally due in 2011 but extended by the Lender until 2014, interest payable monthly at 6.5% per annum | 250,494 | 246,889 | ||||||
| Convertible note payable to a bank, due in 2016, interest payable monthly at 10% per annum, beginning January 2012. | 500,000 | — | ||||||
| Convertible notes payable to shareholders, due in 2015, interest payable monthly at 6% per annum. | 2,000 | 52,000 | ||||||
| $ | 1,584,494 | $ | 890,919 | |||||
| Amount due in one year | (460,000 | ) | (706,889 | ) | ||||
| Long term portion of notes payable | $ | 1,124,494 | $ | 184,030 | ||||
As of March 31, 2011 notes payable in the amount of $574,000 were convertible, at option of the lender, into shares of the Company’s common stock at $90.00 per share. During the three months ended March 31, 2011, notes payable in the amount of $110,030 were converted to common stock.
During the three months ended March 31, 2011, convertible notes payable in the amount of $500,000 were issued. Immediately upon conversion of this note, the lender shall receive, without any consideration, warrants to acquire common stock of the Company in such number as equals to twenty-five percent of the number of shares acquired as a result of the conversion. These warrants will be exercisable by giving written notice to the Company. The Company has granted to the lender a security interest in 50% of the Company’s investment in marketable securities as specified in an agreement with the lender.
During the year ended December 31, 2010, convertible notes payable in the amount of $1,490,030 were issued, of which notes payable in the amount of $1,306,000 were converted to common stock.
NOTE 6 - SHAREHOLDERS’ EQUITY
Common stock – During the three months ended March 31, 2011, the Company issued a total of 10,453 shares of the Company’ common stock. The Company issued 9,230 shares for total proceeds of $1,153,674 and converted notes payable in the amount of $110,030 into 1,223 shares of the common stock.
93
During the year ended December 31, 2010, the Company issued a total of 38,452 shares of the Company’s common stock. The Company issued 23,941 shares for total proceeds of $2,124,294 and converted notes payable in the amount of $1,306,000 into 14,511 shares of the common stock.
In March 2011, Emmaus Medical completed a private placement of shares of its common stock in which it sold 9,230 shares of common stock at $125.00 per share for total gross proceeds of $1.2 million.
Stock warrants - During the three months ended March 31, 2011, the Company granted stock warrants to its investors and lenders to purchase an aggregate of 200 shares of the Company’s common stock at an exercise price of $90 per share. During the year ended December 31, 2010, the Company granted stock warrants to purchase an aggregate total of 6,686 shares of the Company’s common stock at an exercise price of $90 per share. The warrants are exercisable and expire on the earlier of (i) five years from their respective date of issuance or (ii) upon the closing of the Company’s initial public offering. The total value of warrants granted during the year ended December 31, 2010 was $480,000 and was recorded against common stock as an issuance cost.
In April 2011, 60 warrants were exercised.
A summary of outstanding warrants at March 31, 2011 and December 31, 2010 is presented below.
| Three months ended March 31, 2011 | ||||
| Warrants outstanding, beginning of year | 10,133 | |||
| Granted | 200 | |||
| Exercised | (60 | ) | ||
| Cancelled, forfeited and expired | — | |||
| Warrants outstanding, end of year | 10,273 | |||
| Outstanding | Exercisable | |||||||||||||||||||||
| Exercise Prices | Total | Weighted Average Remaining Contractual Life (Years) | Weighted Average Exercise Price | Total | Weighted Average Exercise Price | |||||||||||||||||
| During 2011 | ||||||||||||||||||||||
| $90 | 140 | 5.00 | $ | 90 | 140 | $ | 90 | |||||||||||||||
| During 2010 | ||||||||||||||||||||||
| $90 | 6,686 | 4.56 | $ | 90 | 6,686 | $ | 90 | |||||||||||||||
| During 2009 | ||||||||||||||||||||||
| $90 | 3,447 | 3.99 | $ | 90 | 3,447 | $ | 90 | |||||||||||||||
Stock options – Management has valued the options at their date of grant utilizing the Black Scholes Option Pricing Model. Accordingly, the fair value of the underlying shares was determined based on recent transactions by the Company to sell shares to third parties and other factors determined by management to be relevant to the valuation of such shares. The excepted volatility was calculated using the historical volatility of a similar public entity in the industry. In making this determination and finding another similar company, the Company considered the industry, stage of life cycle, size and financial leverage of such other entities. Based on the development stage of the Company, similar companies with enough historical data are not available. The Company was able to find one entity that met the industry criterion and as a result has based its expected volatility off this Company’s historical stock prices for a period similar to the expected term of the option. The risk –free interest rate is based on the implied yield available on U.S. Treasury issues with an equivalent term approximating the expected life of the options depending on the date of the grant and expected life of the options. The expected life of options used was based on the contractual life of the option granted.
94
The Company determined the expected dividend rate based on the assumption and expectation that earnings generated from operations are not expected to be adequate to allow for the payment of dividends in the near future. The following weighted-average assumptions were utilized in the fair value calculations for options granted:
| Expected life | 3 – 5 years | |
| Stock volatility | 100% | |
| Expected dividends | None | |
| Risk-free interest rate | 2% |
The Company had 800 options outstanding to directors of the company exercisable at $90 per share as of March 31, 2011, all of which were vested as of March 31, 2011. These options are exercisable through 2015. The Company recognized $12,600 of compensation expense related to the options for the three months ended March 31, 2011. There is no remaining unrecognized compensation cost related to these options.
NOTE 7 – COMMITMENTS AND CONTINGENCIES
Distribution contract - Cardinal Health Specialty Pharmacy Services is contracted to distribute NutreStore to other wholesale distributors and some independent pharmacies since April 2008. For its service, Emmaus Medical pays monthly commercialization management fee of $7,000, $5,000 with discount.
Operating leases - The Company leased its office space under an operating lease from an unrelated entity under an agreement expiring in May 2011. The rent expense during the three months ended March 31, 2011 and 2010 amounted to $24,475 and $24,425 respectively.
The Company leases its approximately 4,540 square foot headquarters offices in Torrance, CA, at a base rental of $5,552 per month plus $306 per month as its share of common area expenses. The lease was extended to May 31, 2012 at 10% lower lease payment. In addition, the Company leases two office suites in Torrance, California at a base rent of $1,610 per month plus share of common area expenses of $62 per month, at a base rent of $1,690 per month plus share of common area expenses of $57 per month. These leases will expire on August 11, 2011 and February 28, 2013, respectively. Approximately 490 square feet from one office and 1,079 square feet from the other office are currently subleased to an unaffiliated entity on a month to month basis. The Company does not expect to experience any difficulties in renewing its leases, or finding additional or replacement office and warehouse space, at its current or more favorable rates.
Future minimum lease payments under the agreement are as follows:
| March 31, 2011 | $ | 60,920 | ||
| 2012 | 20,280 | |||
| 2013 | 20,280 | |||
| $ | 101,480 |
NOTE 8 - RELATED PARTY TRANSACTIONS
As of March 31, 2011, the Company has loans from its shareholders of $1,084,494 as summarized in Note 5. The debt is unsecured and carries interest rates from 0% to 8%. $824,494 of the loans are convertible to common stock at $90.00 per share. Interest on 0% loans was imputed at the incremental borrowing rate of 6% per annum.
The Company has agreed to contribute $15,000 per month to Emmaus Medical Japan Co., Ltd. (EMJ), its wholly owned subsidiary, from January 5, 2011 through June 2011, renewable automatically for six months on equivalent terms if the existing agreement is not updated within one month before expiration. The Company makes the monthly payments in return for consulting services EMJ performs on behalf of the Company including (1) coordinating shareholder briefing meetings in Japan, (2) reception duties in Japan for employees and customers of the Company, (3) research activities and analysis in conjunction with the raw material market of AminoPure, and (4) services such as translation of documents and creating supporting investor relations documents for the Company. For the three months ended March 31, 2011, the Company paid $46,079 to EMJ. These payments are intercompany transactions and, therefore, they are offset during the consolidation process.
95
NOTE 9 – COMMON STOCK TRANSACTIONS (RESTATED)
The Company engaged in the following stock transactions for the period from inception (December 20, 2000) through March 31, 2011.
| Date | Shares | $/Share | Common stock | Additional Paid-in Capital | Accumulated Other Comprehensive Income | Deficit Accumulated during Development Stage | Total | ||||||||||||||
| Balance, December 31, 2000 | 425,000 | 0.02 | 425 | 9,175 | $ | — | $ | — | 9,600 | ||||||||||||
| Net loss | — | — | — | — | (21,942 | ) | (21,942 | ) | |||||||||||||
| Balance, December 31, 2001 | 425,000 | — | 425 | 9,175 | — | (21,942 | ) | (12,342 | ) | ||||||||||||
| Net loss | — | — | — | — | (12,464 | ) | (12,464 | ) | |||||||||||||
| Balance, December 31, 2002 | 425,000 | — | 425 | 9,175 | — | (34,406 | ) | (24,806 | ) | ||||||||||||
| Constructive distribution of retained loss to Additional Paid-in Capital | — | — | (34,406 | ) | — | 34,406 | — | ||||||||||||||
| Common stock issued | 25,000 | 25 | 249,975 | 250,000 | |||||||||||||||||
| 9/25/03 | 1,000 | 10 | 1 | 9,999 | 10,000 | ||||||||||||||||
| 9/25/03 | 500 | 10 | 1 | 4,999 | 5,000 | ||||||||||||||||
| 9/25/03 | 500 | 10 | 1 | 4,999 | 5,000 | ||||||||||||||||
| 9/25/03 | 500 | 10 | 1 | 4,999 | 5,000 | ||||||||||||||||
| 9/25/03 | 3,500 | 10 | 4 | 34,996 | 35,000 | ||||||||||||||||
| 9/25/03 | 2,000 | 10 | 2 | 19,998 | 20,000 | ||||||||||||||||
| 9/25/03 | 2,500 | 10 | 3 | 24,997 | 25,000 | ||||||||||||||||
| 9/28/03 | 500 | 10 | 1 | 4,999 | 5,000 | ||||||||||||||||
| 9/28/03 | 500 | 10 | 1 | 4,999 | 5,000 | ||||||||||||||||
| 9/28/03 | 500 | 10 | 1 | 4,999 | 5,000 | ||||||||||||||||
| 9/29/03 | 1,000 | 10 | 1 | 9,999 | 10,000 | ||||||||||||||||
| 9/30/03 | 6,000 | 10 | 6 | 59,994 | 60,000 | ||||||||||||||||
| 10/10/03 | 1,000 | 10 | 1 | 9,999 | 10,000 | ||||||||||||||||
| 10/10/03 | 1,000 | 10 | 1 | 9,999 | 10,000 | ||||||||||||||||
| 10/10/03 | 1,000 | 10 | 1 | 9,999 | 10,000 | ||||||||||||||||
96
| Date | Shares | $/Share | Common stock | Additional Paid-in Capital | Accumulated Other Comprehensive Income | Deficit Accumulated during Development Stage | Total | ||||||||||||
| 10/10/03 | 1,000 | 10 | 1 | 9,999 | 10,000 | ||||||||||||||
| 10/10/03 | 1,000 | 10 | 1 | 9,999 | 10,000 | ||||||||||||||
| 10/10/03 | 1,000 | 10 | 1 | 9,999 | 10,000 | ||||||||||||||
| Net loss | — | — | — | — | (97,481 | ) | (97,481 | ) | |||||||||||
| Balance, December 31, 2003 | 450,000 | 450 | 224,744 | — | (97,481 | ) | 127,713 | ||||||||||||
| Common stock issued | 54,800 | 638,000 | |||||||||||||||||
| 4/21/04 | 5,000 | 10 | 5 | 49,995 | 50,000 | ||||||||||||||
| 4/21/04 | 5,000 | 10 | 5 | 49,995 | 50,000 | ||||||||||||||
| 4/21/04 | 5,000 | 10 | 5 | 49,995 | 50,000 | ||||||||||||||
| 4/21/04 | 5,000 | 10 | 5 | 49,995 | 50,000 | ||||||||||||||
| 4/21/04 | 2,500 | 10 | 2 | 24,998 | 25,000 | ||||||||||||||
| 4/21/04 | 2,500 | 10 | 2 | 24,998 | 25,000 | ||||||||||||||
| 4/21/04 | 1,250 | 10 | 1 | 12,499 | 12,500 | ||||||||||||||
| 6/22/04 | 5,000 | 10 | 5 | 49,995 | 50,000 | ||||||||||||||
| 6/22/04 | 3,000 | 10 | 3 | 29,997 | 30,000 | ||||||||||||||
| 6/22/04 | 1,400 | 10 | 1 | 13,999 | 14,000 | ||||||||||||||
| 4/24/04 | 2,500 | 10 | 3 | 24,997 | 25,000 | ||||||||||||||
| 6/22/04 | 2,500 | 10 | 3 | 24,997 | 25,000 | ||||||||||||||
| 7/6/04 | 550 | 10 | — | 5,500 | 5,500 | ||||||||||||||
| 7/6/04 | 550 | 10 | 1 | 5,499 | 5,500 | ||||||||||||||
| 9/21/04 | 3,000 | 2 | 3 | 4,997 | 5,000 | ||||||||||||||
| 4/21/04 | 1,000 | 10 | 1 | 9,999 | 10,000 | ||||||||||||||
| 6/22/04 | 1,250 | 10 | 1 | 12,499 | 12,500 | ||||||||||||||
| 6/22/04 | 5,000 | 10 | 5 | 49,995 | 50,000 | ||||||||||||||
| 12/28/05 | 1,500 | 60 | 2 | 89,998 | A | 90,000 | |||||||||||||
| 4/4/05 | 500 | 10 | 1 | 4,999 | A | 5,000 | |||||||||||||
| 1/31/05 | 500 | 60 | 1 | 29,999 | A | 30,000 | |||||||||||||
| 2/17/05 | 200 | 60 | — | 12,000 | A | 12,000 | |||||||||||||
| 2/24/05 | 100 | 60 | — | 6,000 | A | 6,000 | |||||||||||||
| (Subscriptions Receivable) Collections | 10,075 | 10,075 | |||||||||||||||||
| Net loss | — | — | — | — | (624,936 | ) | (624,936 | ) |
97
| Date | Shares | $/Share | Common stock | Additional Paid-in Capital | Accumulated Other Comprehensive Income | Deficit Accumulated during Development Stage | Total | ||||||||||||||||
| Balance, December 31, 2004 | 504,800 | 505 | 872,764 | — | (722,417 | ) | 150,852 | ||||||||||||||||
| Common stock issued | 13,519 | 811,626 | |||||||||||||||||||||
| 1/31/05 | 1,667 | 60 | 2 | 99,998 | 100,000 | ||||||||||||||||||
| 7/25/05 | 100 | 60 | — | 6,000 | 6,000 | ||||||||||||||||||
| 7/25/05 | 100 | 60 | — | 6,000 | 6,000 | ||||||||||||||||||
| 7/25/05 | 100 | 60 | — | 6,000 | 6,000 | ||||||||||||||||||
| 7/25/05 | 100 | 60 | — | 6,000 | 6,000 | ||||||||||||||||||
| 10/21/05 | 1,000 | 60 | 1 | 59,999 | 60,000 | ||||||||||||||||||
| 12/21/05 | 400 | 60 | — | 24,000 | 24,000 | ||||||||||||||||||
| 7/2/07 | 168 | 60 | — | 10,080 | B | 10,080 | |||||||||||||||||
| 5/9/08 | 9,793 | 60 | 10 | 587,536 | B | 587,546 | |||||||||||||||||
| Rounding | (9 | ) | |||||||||||||||||||||
| 9/21/05 | 100 | 60 | — | 6,000 | B | 6,000 | |||||||||||||||||
| (Subscriptions Receivable) Collections | (483,341 | ) | (483,341 | ) | |||||||||||||||||||
| Net loss | — | — | — | — | (668,091 | ) | (668,091 | ) | |||||||||||||||
| Balance, December 31, 2005 | 518,310 | 518 | 1,201,036 | — | (1,390,508 | ) | (188,954 | ) | |||||||||||||||
| Common stock issued | 17,751 | 1,062,460 | |||||||||||||||||||||
| 7/25/05 | 150 | 60 | — | 9,000 | 9,000 | ||||||||||||||||||
| 12/16/05 | 500 | 60 | 1 | 29,999 | 30,000 | ||||||||||||||||||
| 1/10/06 | 1,000 | 60 | 1 | 59,999 | 60,000 | ||||||||||||||||||
| 1/6/06 | 500 | 60 | 1 | 29,999 | 30,000 | ||||||||||||||||||
| 1/6/06 | 1,000 | 60 | 1 | 59,999 | 60,000 | ||||||||||||||||||
| 1/5/06 | 1,000 | 60 | 1 | 59,999 | 60,000 | ||||||||||||||||||
| 1/6/06 | 1,000 | 60 | 1 | 59,999 | 60,000 | ||||||||||||||||||
| 1/6/06 | 167 | 60 | — | 10,000 | 10,000 | ||||||||||||||||||
| 1/13/06 | 100 | 60 | — | 6,000 | 6,000 | ||||||||||||||||||
| 2/28/06 | 100 | 60 | — | 6,000 | 6,000 | ||||||||||||||||||
| 4/28/06 | 500 | 60 | 1 | 29,999 | 30,000 | ||||||||||||||||||
| 4/28/06 | 500 | 60 | 1 | 29,999 | 30,000 | ||||||||||||||||||
| 4/28/06 | 300 | 60 | — | 18,000 | 18,000 |
98
| Date | Shares | $/Share | Common stock | Additional Paid-in Capital | Accumulated Other Comprehensive Income | Deficit Accumulated during Development Stage | Total | ||||||||||
| 4/28/06 | 100 | 60 | — | 6,000 | 6,000 | ||||||||||||
| 5/1/06 | 100 | 60 | — | 6,000 | 6,000 | ||||||||||||
| 5/9/06 | 500 | 60 | 1 | 29,999 | 30,000 | ||||||||||||
| 5/1/06 | 500 | 60 | 1 | 29,999 | 30,000 | ||||||||||||
| 6/15/06 | 100 | 60 | — | 6,000 | 6,000 | ||||||||||||
| 9/29/06 | 100 | 60 | — | 6,000 | 6,000 | ||||||||||||
| 10/17/06 | 500 | 60 | 1 | 29,999 | 30,000 | ||||||||||||
| 10/13/06 | 500 | 60 | 1 | 29,999 | 30,000 | ||||||||||||
| 10/19/06 | 500 | 60 | 1 | 29,999 | 30,000 | ||||||||||||
| 11/10/06 | 250 | 60 | — | 15,000 | 15,000 | ||||||||||||
| 11/10/06 | 150 | 60 | — | 9,000 | 9,000 | ||||||||||||
| 11/10/06 | 1,000 | 60 | 1 | 59,999 | 60,000 | ||||||||||||
| 11/22/06 | 500 | 60 | 1 | 29,999 | 30,000 | ||||||||||||
| 11/6/06 | 200 | 60 | — | 12,000 | 12,000 | ||||||||||||
| 11/6/06 | 500 | 60 | 1 | 29,999 | 30,000 | ||||||||||||
| 11/22/06 | 500 | 60 | 1 | 29,999 | 30,000 | ||||||||||||
| 11/22/06 | 250 | 60 | — | 15,000 | 15,000 | ||||||||||||
| 12/1/06 | 1,000 | 60 | 1 | 59,999 | 60,000 | ||||||||||||
| 11/27/06 | 100 | 60 | — | 6,000 | 6,000 | ||||||||||||
| 12/6/06 | 334 | 60 | — | 20,040 | 20,040 | ||||||||||||
| 12/6/06 | 150 | 60 | — | 9,000 | 9,000 | ||||||||||||
| 12/6/06 | 250 | 60 | — | 15,000 | 15,000 | ||||||||||||
| 12/18/06 | 500 | 60 | — | 29,999 | 30,000 | ||||||||||||
| 1/24/07 | 500 | 60 | — | 29,999 | 30,000 | ||||||||||||
| 4/10/07 | 500 | 60 | — | 29,999 | 30,000 | ||||||||||||
| 5/31/07 | 100 | 60 | — | 6,000 | 6,000 | ||||||||||||
| 5/29/08 | 500 | 60 | — | 29,999 | 30,000 | ||||||||||||
| 5/29/08 | 500 | 60 | — | 29,999 | 30,000 | ||||||||||||
| 3/31/08 | 150 | 43 | — | 6,420 | 6,420 | ||||||||||||
| 3/10/08 | 100 | 60 | — | 6,000 | 6,000 | ||||||||||||
| Rounding | (5 | ) | |||||||||||||||
| (Subscriptions Receivable) Collections | (237,415 | ) | (237,415 | ) | |||||||||||||
| Net loss | — | — | — | — | (759,962 | ) | (759,962 | ) |
99
| Date | Shares | $/Share | Common stock | Additional Paid-in Capital | Accumulated Other Comprehensive Income | Deficit Accumulated during Development Stage | Total | |||||||||||
| Balance, December 31, 2006 | 536,061 | 536 | 2,026,058 | — | (2,150,470 | ) | (123,876 | ) | ||||||||||
| Common stock issued | 45,587 | 2,680,720 | ||||||||||||||||
| 2/5/07 | 150 | 60 | — | 9,000 | 9,000 | |||||||||||||
| 3/8/07 | 150 | 60 | — | 9,000 | 9,000 | |||||||||||||
| 4/26/07 | 500 | 60 | 1 | 29,999 | 30,000 | |||||||||||||
| 5/3/07 | 500 | 60 | 1 | 29,999 | 30,000 | |||||||||||||
| 5/8/07 | 200 | 60 | — | 12,000 | 12,000 | |||||||||||||
| 5/8/07 | 500 | 60 | 1 | 29,999 | 30,000 | |||||||||||||
| 5/8/07 | 500 | 60 | 1 | 29,999 | 30,000 | |||||||||||||
| 5/11/07 | 300 | 60 | — | 18,000 | 18,000 | |||||||||||||
| 5/11/07 | 150 | 60 | — | 9,000 | 9,000 | |||||||||||||
| 5/11/07 | 150 | 60 | — | 9,000 | 9,000 | |||||||||||||
| 5/14/07 | 330 | 60 | — | 19,800 | 19,800 | |||||||||||||
| 5/11/07 | 150 | 60 | — | 9,000 | 9,000 | |||||||||||||
| 5/18/07 | 500 | 60 | 1 | 29,999 | 30,000 | |||||||||||||
| 5/18/07 | 500 | 60 | 1 | 29,999 | 30,000 | |||||||||||||
| 5/24/07 | 500 | 60 | 1 | 29,999 | 30,000 | |||||||||||||
| 5/31/07 | 1,000 | 60 | 1 | 59,999 | 60,000 | |||||||||||||
| 6/2/07 | 1,668 | 60 | 2 | 100,078 | 100,080 | |||||||||||||
| 6/1/07 | 1,000 | 60 | 1 | 59,999 | 60,000 | |||||||||||||
| 6/1/07 | 1,000 | 60 | 1 | 59,999 | 60,000 | |||||||||||||
| 6/6/07 | 1,000 | 60 | 1 | 59,999 | 60,000 | |||||||||||||
| 6/4/07 | 167 | 60 | — | 10,020 | 10,020 | |||||||||||||
| 6/28/07 | 1,500 | 60 | 2 | 89,998 | 90,000 | |||||||||||||
| 6/15/07 | 200 | 60 | — | 12,000 | 12,000 | |||||||||||||
| 6/14/07 | 250 | 60 | — | 15,000 | 15,000 | |||||||||||||
| 6/15/07 | 150 | 60 | — | 9,000 | 9,000 | |||||||||||||
| 6/15/07 | 200 | 60 | — | 12,000 | 12,000 | |||||||||||||
| 6/15/07 | 500 | 60 | 1 | 29,999 | 30,000 | |||||||||||||
| 6/20/07 | 500 | 60 | 1 | 29,999 | 30,000 | |||||||||||||
| 6/20/07 | 500 | 60 | 1 | 29,999 | 30,000 | |||||||||||||
| 6/28/07 | 335 | 60 | — | 20,100 | 20,100 | |||||||||||||
| 6/20/07 | 500 | 60 | 1 | 29,999 | 30,000 | |||||||||||||
| 6/20/07 | 500 | 60 | 1 | 29,999 | 30,000 | |||||||||||||
| 6/29/07 | 500 | 60 | 1 | 29,999 | 30,000 | |||||||||||||
| 7/2/07 | 500 | 60 | 1 | 29,999 | 30,000 |
100
| Date | Shares | $/Share | Common stock | Additional Paid-in Capital | Accumulated Other Comprehensive Income | Deficit Accumulated during Development Stage | Total | ||||||||||
| 7/2/07 | 200 | 60 | — | 12,000 | 12,000 | ||||||||||||
| 7/2/07 | 1,500 | 60 | 2 | 89,998 | 90,000 | ||||||||||||
| 7/2/07 | 50 | 60 | — | 3,000 | 3,000 | ||||||||||||
| 7/2/07 | 250 | 60 | — | 15,000 | 15,000 | ||||||||||||
| 7/11/07 | 1,000 | 60 | 1 | 59,999 | 60,000 | ||||||||||||
| 7/6/07 | 1,000 | 60 | 1 | 59,999 | 60,000 | ||||||||||||
| 7/11/07 | 1,000 | 60 | 1 | 59,999 | 60,000 | ||||||||||||
| 7/17/07 | 800 | 60 | 1 | 47,999 | 48,000 | ||||||||||||
| 7/16/07 | 5,000 | 60 | 5 | 299,995 | 300,000 | ||||||||||||
| 7/11/07 | 700 | 60 | 1 | 41,999 | 42,000 | ||||||||||||
| 7/18/07 | 900 | 60 | 1 | 53,999 | 54,000 | ||||||||||||
| 7/21/07 | 500 | 60 | 1 | 29,999 | 30,000 | ||||||||||||
| 7/23/07 | 400 | 60 | — | 24,000 | 24,000 | ||||||||||||
| 7/24/07 | 250 | 60 | — | 15,000 | 15,000 | ||||||||||||
| 7/26/07 | 1,000 | 60 | 1 | 59,999 | 60,000 | ||||||||||||
| 7/23/07 | 400 | 60 | — | 24,000 | 24,000 | ||||||||||||
| 7/27/07 | 1,000 | 60 | 1 | 59,999 | 60,000 | ||||||||||||
| 7/27/07 | 500 | 60 | 1 | 29,999 | 30,000 | ||||||||||||
| 7/30/07 | 500 | 60 | 1 | 29,999 | 30,000 | ||||||||||||
| 7/30/07 | 500 | 60 | 1 | 29,999 | 30,000 | ||||||||||||
| 7/31/07 | 900 | 60 | 1 | 53,999 | 54,000 | ||||||||||||
| 8/7/07 | 100 | 60 | — | 6,000 | 6,000 | ||||||||||||
| 8/25/07 | 1,000 | 60 | 1 | 59,999 | 60,000 | ||||||||||||
| 8/25/07 | 500 | 60 | 1 | 29,999 | 30,000 | ||||||||||||
| 8/30/07 | 500 | 60 | — | 29,999 | 30,000 | ||||||||||||
| 10/3/07 | 500 | 60 | — | 29,999 | 30,000 | ||||||||||||
| 10/17/07 | 350 | 61 | — | 21,500 | 21,500 | ||||||||||||
| 11/5/07 | 1,100 | 60 | 1 | 65,999 | 66,000 | ||||||||||||
| 10/22/07 | 167 | 60 | — | 10,020 | 10,020 | ||||||||||||
| 11/28/07 | 500 | 60 | — | 29,999 | 30,000 | ||||||||||||
| 11/15/07 | 180 | 60 | — | 10,800 | 10,800 | ||||||||||||
| 11/15/07 | 180 | 60 | — | 10,800 | 10,800 | ||||||||||||
| 12/15/07 | 500 | 60 | — | 29,999 | 30,000 | ||||||||||||
| 12/5/07 | 1,000 | 5 | 1 | 4,999 | 5,000 | ||||||||||||
| 12/4/07 | 100 | 60 | — | 6,000 | 6,000 | ||||||||||||
| 12/4/07 | 100 | 60 | — | 6,000 | 6,000 | ||||||||||||
| 12/12/07 | 500 | 60 | — | 29,999 | 30,000 | ||||||||||||
| 12/18/07 | 500 | 60 | — | 29,999 | 30,000 | ||||||||||||
| 12/21/07 | 810 | 60 | — | 48,600 | 48,600 |
101
| Date | Shares | $/Share | Common stock | Additional Paid-in Capital | Accumulated Other Comprehensive Income | Deficit Accumulated during Development Stage | Total | |||||||||||
| 12/26/07 | 100 | 60 | — | 6,000 | 6,000 | |||||||||||||
| 12/26/07 | 500 | 60 | — | 29,999 | 30,000 | |||||||||||||
| 12/28/07 | 500 | 60 | — | 29,999 | 30,000 | |||||||||||||
| 12/29/07 | 500 | 60 | — | 29,999 | 30,000 | |||||||||||||
| (Subscriptions Receivable) Collections | 53,149 | 53,149 | ||||||||||||||||
| Rounding | (9 | ) | ||||||||||||||||
| Net loss | — | — | — | — | (1,282,212 | ) | (1,282,212 | ) | ||||||||||
| Balance, December 31, 2007 | 581,648 | — | 582 | 4,759,872 | — | (3,432,682 | ) | 1,327,772 | ||||||||||
| Common stock issued | 41,612 | 2,496,720 | ||||||||||||||||
| 1/4/08 | 500 | 60 | — | 29,999 | 30,000 | |||||||||||||
| 1/4/08 | 500 | 60 | — | 29,999 | 30,000 | |||||||||||||
| 1/4/08 | 500 | 60 | — | 29,999 | 30,000 | |||||||||||||
| 1/4/08 | 500 | 60 | — | 29,999 | 30,000 | |||||||||||||
| 1/9/08 | 600 | 60 | 1 | 35,999 | 36,000 | |||||||||||||
| 1/9/08 | 1,000 | 60 | 1 | 59,999 | 60,000 | |||||||||||||
| 1/9/08 | 500 | 60 | — | 29,999 | 30,000 | |||||||||||||
| 1/10/08 | 500 | 60 | — | 29,999 | 30,000 | |||||||||||||
| 1/11/08 | 500 | 60 | — | 29,999 | 30,000 | |||||||||||||
| 1/11/08 | 500 | 60 | — | 29,999 | 30,000 | |||||||||||||
| 1/15/08 | 500 | 60 | — | 29,999 | 30,000 | |||||||||||||
| 1/15/08 | 5,000 | 60 | 5 | 299,995 | 300,000 | |||||||||||||
| 1/11/08 | 500 | 60 | — | 29,999 | 30,000 | |||||||||||||
| 1/18/08 | 500 | 60 | — | 29,999 | 30,000 | |||||||||||||
| 1/15/08 | 500 | 60 | — | 29,999 | 30,000 | |||||||||||||
| 1/22/08 | 500 | 60 | — | 29,999 | 30,000 | |||||||||||||
| 1/24/08 | 800 | 60 | 1 | 47,999 | 48,000 | |||||||||||||
| 1/24/08 | 500 | 60 | — | 29,999 | 30,000 | |||||||||||||
| 1/24/08 | 500 | 60 | — | 29,999 | 30,000 | |||||||||||||
| 1/24/08 | 500 | 60 | — | 29,999 | 30,000 | |||||||||||||
| 1/28/08 | 500 | 60 | — | 29,999 | 30,000 | |||||||||||||
| 1/29/08 | 800 | 60 | 1 | 47,999 | 48,000 | |||||||||||||
| 1/28/08 | 500 | 60 | 1 | 29,999 | 30,000 | |||||||||||||
| 1/30/08 | 500 | 60 | 1 | 29,999 | 30,000 | |||||||||||||
| 2/7/08 | 600 | 60 | 1 | 35,999 | 36,000 |
102
| Date | Shares | $/Share | Common stock | Additional Paid-in Capital | Accumulated Other Comprehensive Income | Deficit Accumulated during Development Stage | Total | |||||||||||
| 2/1/08 | 500 | 60 | 1 | 29,999 | 30,000 | |||||||||||||
| 2/1/08 | 650 | 60 | 1 | 38,999 | 39,000 | |||||||||||||
| 2/7/08 | 2,500 | 60 | 3 | 149,997 | 150,000 | |||||||||||||
| 2/1/08 | 3,333 | 60 | 3 | 199,977 | 199,980 | |||||||||||||
| 2/25/08 | 500 | 60 | 1 | 29,999 | 30,000 | |||||||||||||
| 2/25/08 | 800 | 60 | 1 | 47,999 | 48,000 | |||||||||||||
| 2/27/08 | 3,334 | 60 | 3 | 200,037 | 200,040 | |||||||||||||
| 3/1/08 | 650 | 60 | 1 | 38,999 | 39,000 | |||||||||||||
| 3/1/08 | 1,000 | 60 | 1 | 59,999 | 60,000 | |||||||||||||
| 2/29/08 | 200 | 60 | 0 | 12,000 | 12,000 | |||||||||||||
| 2/29/08 | 100 | 60 | 0 | 6,000 | 6,000 | |||||||||||||
| 3/3/08 | 500 | 60 | 1 | 29,999 | 30,000 | |||||||||||||
| 2/29/08 | 100 | 60 | 0 | 6,000 | 6,000 | |||||||||||||
| 3/4/08 | 100 | 60 | 0 | 6,000 | 6,000 | |||||||||||||
| 3/4/08 | 350 | 60 | 0 | 21,000 | 21,000 | |||||||||||||
| 3/5/08 | 500 | 60 | 1 | 29,999 | 30,000 | |||||||||||||
| 3/13/08 | 500 | 60 | 1 | 29,999 | 30,000 | |||||||||||||
| 3/10/08 | 500 | 60 | 1 | 29,999 | 30,000 | |||||||||||||
| 3/31/08 | 20 | 60 | 0 | 1,200 | 1,200 | |||||||||||||
| 3/25/08 | 500 | 60 | 1 | 29,999 | 30,000 | |||||||||||||
| 3/31/08 | 500 | 60 | 1 | 29,999 | 30,000 | |||||||||||||
| 4/2/08 | 500 | 60 | 1 | 29,999 | 30,000 | |||||||||||||
| 4/2/08 | 175 | 60 | 0 | 10,500 | 10,500 | |||||||||||||
| 4/7/08 | 4,000 | 60 | 4 | 239,996 | 240,000 | |||||||||||||
| 4/11/08 | 1,000 | 60 | 1 | 59,999 | 60,000 | |||||||||||||
| 4/9/08 | 500 | 60 | 1 | 29,999 | 30,000 | |||||||||||||
| (Subscriptions Receivable) Collections | 893,988 | 893,988 | ||||||||||||||||
| Rounding | 1 | (17 | ) | |||||||||||||||
| Net loss | — | — | — | — | (2,993,777 | ) | (2,993,777 | ) | ||||||||||
| Balance, December 31, 2008 | 623,261 | 623 | 8,150,522 | — | (6,426,459 | ) | 1,724,686 | |||||||||||
| Common stock issued | 28,979 | 2,349,940 | ||||||||||||||||
| 3/28/08 | 2,000 | 60 | 2 | 119,998 | 120,000 |
103
| Date | Shares | $/Share | Common stock | Additional Paid-in Capital | Accumulated Other Comprehensive Income | Deficit Accumulated during Development Stage | Total | ||||||||||
| 3/31/08 | 200 | 60 | 0 | 12,000 | 12,000 | ||||||||||||
| 4/7/08 | 250 | 60 | 0 | 15,000 | 15,000 | ||||||||||||
| 1/12/09 | 650 | 60 | 1 | 38,999 | 39,000 | ||||||||||||
| 1/12/09 | 500 | 60 | — | 29,999 | 30,000 | ||||||||||||
| 1/12/09 | 834 | 60 | 1 | 49,999 | 50,000 | ||||||||||||
| 1/12/09 | 4,167 | 60 | 4 | 249,996 | 250,000 | ||||||||||||
| 3/30/09 | 300 | 90 | 0 | 27,000 | 27,000 | ||||||||||||
| 3/16/09 | 200 | 90 | 0 | 18,000 | 18,000 | ||||||||||||
| 3/16/09 | 400 | 90 | 0 | 36,000 | 36,000 | ||||||||||||
| 3/18/09 | 250 | 90 | 0 | 22,500 | 22,500 | ||||||||||||
| 5/11/09 | 250 | 90 | 0 | 22,500 | 22,500 | ||||||||||||
| 5/15/09 | 250 | 90 | 0 | 22,500 | 22,500 | ||||||||||||
| 5/19/09 | 300 | 90 | 0 | 27,000 | 27,000 | ||||||||||||
| 5/19/09 | 250 | 90 | 0 | 22,500 | 22,500 | ||||||||||||
| 5/19/09 | 250 | 90 | 0 | 22,500 | 22,500 | ||||||||||||
| 5/19/09 | 250 | 90 | 0 | 22,500 | 22,500 | ||||||||||||
| 5/20/09 | 250 | 90 | 0 | 22,500 | 22,500 | ||||||||||||
| 5/22/09 | 250 | 90 | 0 | 22,500 | 22,500 | ||||||||||||
| 6/30/09 | 300 | 90 | 0 | 27,000 | 27,000 | ||||||||||||
| 6/30/09 | 250 | 90 | 0 | 22,500 | 22,500 | ||||||||||||
| 6/25/09 | 250 | 90 | 0 | 22,500 | 22,500 | ||||||||||||
| 6/24/09 | 250 | 90 | 0 | 22,500 | 22,500 | ||||||||||||
| 6/18/09 | 700 | 90 | 1 | 62,999 | 63,000 | ||||||||||||
| 5/21/09 | 250 | 90 | 0 | 22,500 | 22,500 | ||||||||||||
| 5/20/09 | 250 | 90 | 0 | 22,500 | 22,500 | ||||||||||||
| 6/16/09 | 334 | 90 | 0 | 30,060 | 30,060 | ||||||||||||
| 6/29/09 | 1,500 | 90 | 2 | 134,998 | 135,000 | ||||||||||||
| 7/10/09 | 250 | 90 | 0 | 22,500 | 22,500 | ||||||||||||
| 6/25/09 | 250 | 90 | 0 | 22,500 | 22,500 | ||||||||||||
| 7/2/09 | 500 | 90 | 1 | 44,999 | 45,000 | ||||||||||||
| 7/2/09 | 250 | 90 | 0 | 22,500 | 22,500 | ||||||||||||
| 7/27/09 | 250 | 90 | 0 | 22,500 | 22,500 | ||||||||||||
| 7/23/09 | 250 | 90 | 0 | 22,500 | 22,500 | ||||||||||||
| 7/29/09 | 350 | 90 | 0 | 31,500 | 31,500 | ||||||||||||
| 7/31/09 | 1,050 | 90 | 1 | 94,499 | 94,500 | ||||||||||||
| 8/5/09 | 750 | 90 | — | 67,500 | 67,500 | ||||||||||||
| 8/27/09 | 500 | 90 | — | 44,999 | 45,000 | ||||||||||||
| 8/7/09 | 250 | 90 | 0 | 22,500 | 22,500 | ||||||||||||
| 8/21/09 | 700 | 90 | — | 63,000 | 63,000 |
104
| Date | Shares | $/Share | Common stock | Additional Paid-in Capital | Accumulated Other Comprehensive Income | Deficit Accumulated during Development Stage | Total | ||||||||||||
| 9/4/09 | 950 | 90 | 1 | 85,499 | 85,500 | ||||||||||||||
| 9/4/09 | 200 | 90 | 0 | 18,000 | 18,000 | ||||||||||||||
| 9/8/09 | 250 | 90 | 0 | 22,500 | 22,500 | ||||||||||||||
| 9/18/09 | 400 | 90 | 0 | 36,000 | 36,000 | ||||||||||||||
| 7/15/09 | 250 | 90 | 0 | 22,500 | 22,500 | ||||||||||||||
| 7/17/09 | 250 | 90 | 0 | 22,500 | 22,500 | ||||||||||||||
| 7/27/09 | 250 | 90 | 0 | 22,500 | 22,500 | ||||||||||||||
| 9/25/09 | 250 | 90 | 0 | 22,500 | 22,500 | ||||||||||||||
| 1/10/10 | 500 | 90 | 1 | 44,999 | 45,000 | ||||||||||||||
| 12/18/09 | 3,000 | 90 | 3 | 269,997 | 270,000 | ||||||||||||||
| 12/11/09 | 332 | 90 | 0 | 29,880 | 29,880 | ||||||||||||||
| 11/25/09 | 1,112 | 90 | 1 | 99,999 | 100,000 | ||||||||||||||
| (Subscriptions Receivable) Collections | (111,015 | ) | (111,015 | ) | |||||||||||||||
| Rounding | |||||||||||||||||||
| Conversion of notes payable to Common stock | |||||||||||||||||||
| Total | |||||||||||||||||||
| Net loss | — | — | — | — | (2,567,807 | ) | (2,567,807 | ) | |||||||||||
| Balance, December 31, 2009 | 652,240 | 652 | 10,389,417 | — | (8,994,266 | ) | 1,395,804 | ||||||||||||
| Common stock issued | Total | 23,921 | 144 | 3,456,342 | |||||||||||||||
| 11/16/09 | 400 | 90 | 0 | 36,000 | 36,000 | ||||||||||||||
| 1/10/10 | 500 | 90 | 1 | 44,999 | 45,000 | ||||||||||||||
| 2/10/10 | 1,200 | 90 | 1 | 107,999 | 108,000 | ||||||||||||||
| 2/9/10 | 350 | 90 | 0 | 31,500 | 31,500 | ||||||||||||||
| 2/9/10 | 28 | — | 0 | (0 | ) | ||||||||||||||
| 3/23/10 | 1,000 | 90 | — | 90,000 | 90,000 | ||||||||||||||
| 3/23/10 | 1,750 | 90 | 2 | 157,498 | 157,500 | ||||||||||||||
| 3/23/10 | 2,778 | 90 | 3 | 250,017 | 250,020 | ||||||||||||||
| 5/12/10 | 350 | 90 | 0 | 31,500 | 31,500 | ||||||||||||||
| 5/12/10 | 350 | 90 | 0 | 31,500 | 31,500 | ||||||||||||||
| 5/12/10 | 350 | 90 | 0 | 31,500 | 31,500 |
105
| Date | Shares | $/Share | Common stock | Additional Paid-in Capital | Accumulated Other Comprehensive Income | Deficit Accumulated during Development Stage | Total | ||||||||||
| 4/12/10 | 2,000 | 90 | 2 | 179,998 | 180,000 | ||||||||||||
| 5/19/10 | 350 | 90 | 0 | 31,500 | 31,500 | ||||||||||||
| 5/25/10 | 350 | 90 | 0 | 31,500 | 31,500 | ||||||||||||
| 7/15/10 | 3,600 | 90 | 4 | 323,996 | 324,000 | ||||||||||||
| 7/15/10 | 1,111 | 90 | 1 | 99,999 | 100,000 | ||||||||||||
| 7/12/10 | 290 | 90 | 0 | 26,100 | 26,100 | ||||||||||||
| 7/14/10 | 350 | 90 | 0 | 31,500 | 31,500 | ||||||||||||
| 7/12/10 | 250 | 90 | 0 | 22,500 | 22,500 | ||||||||||||
| 10/18/10 | 1,300 | 90 | 1 | 116,999 | 117,000 | ||||||||||||
| 10/20/10 | 350 | 90 | 0 | 31,500 | 31,500 | ||||||||||||
| 10/20/10 | 350 | 90 | 0 | 31,500 | 31,500 | ||||||||||||
| 10/27/10 | 350 | 90 | 0 | 31,500 | 31,500 | ||||||||||||
| 11/10/10 | 1,112 | 90 | 1 | 100,079 | 100,080 | ||||||||||||
| 11/16/10 | 400 | 90 | 0 | 36,000 | 36,000 | ||||||||||||
| 11/17/10 | 350 | 90 | 0 | 31,500 | 31,500 | ||||||||||||
| 11/23/10 | 350 | 90 | 0 | 31,500 | 31,500 | ||||||||||||
| 11/23/10 | 556 | 90 | 1 | 50,039 | 50,040 | ||||||||||||
| 11/24/10 | 250 | 90 | 0 | 22,502 | 22,502 | ||||||||||||
| 11/23/10 | 500 | 90 | 1 | 44,999 | 45,000 | ||||||||||||
| 11/26/10 | 140 | 90 | 0 | 12,600 | 12,600 | ||||||||||||
| 11/9/10 | 556 | 90 | 1 | 49,999 | 50,000 | ||||||||||||
| Conversion of notes payable to Common stock | |||||||||||||||||
| Total | 14,514 | ||||||||||||||||
| 11/9/10 | 1,667 | 90 | 1 | 149,999 | 150,000 | ||||||||||||
| 11/9/10 | 1,667 | 90 | 1 | 149,999 | 150,000 | ||||||||||||
| 11/11/10 | 2,500 | 90 | 3 | 224,997 | 225,000 | ||||||||||||
| 11/12/10 | 1,112 | 90 | 1 | 99,999 | 100,000 | ||||||||||||
| 11/22/10 | 4,445 | 90 | 4 | 399,996 | 400,000 | ||||||||||||
| 11/22/10 | 1,667 | 90 | 2 | 149,998 | 150,000 | ||||||||||||
| 11/23/10 | 500 | 90 | 1 | 44,999 | 45,000 | ||||||||||||
| 11/30/10 | 556 | 90 | 1 | 49,999 | 50,000 | ||||||||||||
| 12/3/10 | 400 | 90 | 0 | 36,000 | 36,000 | ||||||||||||
| (Subscriptions Receivable) Collections | (26,048 | ) | (26,048 | ) | |||||||||||||
| Rounding | 17 |
106
| Date | Shares | $/Share | Common stock | Additional Paid-in Capital | Accumulated Other Comprehensive Income | Deficit Accumulated during Development Stage | Total | |||||||||||
| Unrealized gain on securities available for sale | — | — | — | 542,573 | — | 542,573 | ||||||||||||
| Net loss | — | — | — | — | (3,757,370 | ) | (3,757,370 | ) | ||||||||||
| Balance, December 31, 2010 (restated) | 690,692 | 691 | 13,819,671 | 542,573 | (12,751,636 | ) | 1,611,301 | |||||||||||
| Common stock issued | Total | 9,230 | 125 | 1,153,674 | ||||||||||||||
| 3/18/11 | 2,400 | 125 | 2 | 299,998 | 300,000 | |||||||||||||
| 3/18/11 | 400 | 125 | 1 | 49,999 | 50,000 | |||||||||||||
| 3/18/11 | 200 | 125 | 0 | 25,000 | 25,000 | |||||||||||||
| 3/21/11 | 200 | 125 | 0 | 25,000 | 25,000 | |||||||||||||
| 3/18/11 | 400 | 125 | 1 | 49,999 | 50,000 | |||||||||||||
| 3/18/11 | 400 | 125 | 1 | 49,999 | 50,000 | |||||||||||||
| 3/18/11 | 400 | 125 | 0 | 50,000 | 50,000 | |||||||||||||
| 3/18/11 | 200 | 125 | 0 | 25,000 | 25,000 | |||||||||||||
| 3/18/11 | 200 | 125 | 0 | 25,000 | 25,000 | |||||||||||||
| 3/18/11 | 500 | 125 | 1 | 62,499 | 62,500 | |||||||||||||
| 3/21/11 | 200 | 125 | 0 | 25,000 | 25,000 | |||||||||||||
| 3/21/11 | 290 | 125 | 0 | 36,250 | 36,250 | |||||||||||||
| 3/22/11 | 200 | 125 | 0 | 25,000 | 25,000 | |||||||||||||
| 3/22/11 | 300 | 125 | 0 | 37,500 | 37,500 | |||||||||||||
| 3/22/11 | 200 | 125 | 0 | 25,000 | 25,000 | |||||||||||||
| 3/22/11 | 600 | 125 | 1 | 74,999 | 75,000 | |||||||||||||
| 3/22/11 | 240 | 125 | 0 | 30,000 | 30,000 | |||||||||||||
| 3/23/11 | 500 | 125 | 1 | 62,484 | 62,485 | |||||||||||||
| 3/24/11 | 800 | 125 | 1 | 99,971 | 99,972 | |||||||||||||
| 3/24/11 | 400 | 125 | 0 | 49,985 | 49,985 | |||||||||||||
| 3/25/11 | 200 | 125 | 0 | 24,983 | 24,983 | |||||||||||||
| Conversion of notes payable to Common Stock | ||||||||||||||||||
| Total | 1,223 | |||||||||||||||||
| 1/21/11 | 556 | 90 | — | 50,000 | 50,000 | |||||||||||||
| 1/21/11 | 667 | 90 | 1 | 60,029 | 60,030 | |||||||||||||
| (Subscriptions Receivable) Collections |
107
| Date | Shares | $/Share | Common stock | Additional Paid-in Capital | Accumulated Other Comprehensive Income | Deficit Accumulated during Development Stage | Total | ||||||||||||
| Rounding | |||||||||||||||||||
| Unrealized gain on securities available for sale | — | — | — | 759,820 | — | 759,820 | |||||||||||||
| Net loss | — | — | — | — | (1,132,203 | ) | (1,132,203 | ) | |||||||||||
| Balance, March 31, 2011 (restated) | 701,145 | 701 | 15,083,366 | 1,302,393 | (13,883,839 | ) | $ | 2,502,622 | |||||||||||
NOTE 10 – GEOGRAPHICAL INFORMATION
For the three months ended March 31, 2011 and 2010, the Company earned revenue from Japan in the amount of $23,000 and 32,844, respectively, which represented 39% and 72%, of total revenue by the Company during such periods. The Company did not have any significant currency translation or foreign transaction adjustments during the periods ended March 31, 2011 and 2010
NOTE 11 – SUBSEQUENT EVENTS
Management evaluated subsequent events through November 15, 2011, the date the restated financial statements were issued.
On April 8, 2011, Emmaus Medical entered into a Joint Research and Development Agreement (the “Research Agreement”) and an Individual Agreement (the “Individual Agreement”) with CellSeed, Inc. (“CellSeed”). Pursuant to the Research Agreement, the Company and CellSeed formed a relationship regarding the future research and development of cell sheet engineering regenerative medicine products (the “Products”), and the future commercialization of such Products. The parties will enter into individual agreements for each project or task conducted pursuant to the Research Agreement defining the details of such project. All intellectual property rights created in the course of the Research Agreement and any individual agreement will be owned jointly by the Company and CellSeed. In the event that either the Company or CellSeed becomes the owner of intellectual property rights related to the Products which was developed solely by its employees, then such party will grant a worldwide, perpetual, irrevocable, non-exclusive, royalty free, fully paid up, sub-licensable, transferable license of such rights to the other party. Pursuant to the Individual Agreement, CellSeed granted the Company an exclusive right to manufacture, sell, market and distribute Cultured Autologous Oral Mucosal Epithelial Cell-Sheets (“CAOMECS”) for the cornea in the United States. CellSeed shall disclose its accumulated information package (the “Package”) for the joint development of CAOMECS to Emmaus Medical. Pursuant to the Research Agreement, the Company agreed to pay CellSeed $8,500,000 (the “Research Agreement Payment”) within 30 days of the completion of all of the following: (i) the execution of the Research Agreement; (ii) the execution of the Individual Agreement; and (iii) CellSeed’s delivery of the Package to Emmaus. Pursuant to the Individual Agreement, the Company agreed to pay $1,500,000 to CellSeed (the “Individual Agreement Payment”) within 30 days of CellSeed’s delivery of the Package to the Company and a royalty to be agreed upon by the parties. Pursuant to the Addendum to the Research Agreement, CellSeed and the Company further acknowledged that all obligations of CellSeed corresponding to the Research Agreement Payment and the Individual Agreement Payment shall be fully discharged by CellSeed’s delivery of the Package, which delivery shall be documented by written confirmation of acceptance by the Company. The Company is obligated to make the Research Agreement Payment and the Individual Agreement Payment only after the Company’s provides its written confirmation of acceptance of the complete Package to CellSeed. The parties will determine the rate at which profits from the net sales of CAOMECS in the United States will be split between the parties. The Individual Agreement will remain in effect until CellSeed’s patents used for the CAOMECS expire in the United States in February 2023 and February 2024, unless terminated earlier by the parties.
108
The Individual Agreement will remain in effect until CellSeed’s patents used for the CAOMECS expire in the United States, unless terminated earlier by the parties.
Pursuant to an Agreement and Plan of Merger, dated April 21, 2011 (the “Merger Agreement”), by and among AFH Acquisition IV, Inc. (“AFH IV”), AFH Merger Sub, Inc. (“AFH Merger Sub”), AFH Holding and Advisory, LLC (“AFH Advisory”), and Emmaus Medical, Emmaus Medical merged with and into AFH Merger Sub with Emmaus Medical continuing as the surviving entity (the “Merger”).
Upon the closing of the Merger on May 3, 2011, AFH IV changed its name from “AFH Acquisition IV, Inc.” to “Emmaus Holdings, Inc.”
Upon consummation of the Merger, (i) each outstanding share of Emmaus Medical common stock was exchanged for 29.48548924976 shares of AFH IV common stock, (ii) each outstanding Emmaus Medical option and warrant, which was exercisable for one share of Emmaus Medical common stock, was exchanged for an option or warrant, as applicable, exercisable for 29.48548924976 shares of AFH IV common stock; and (iii) each outstanding convertible note of Emmaus Medical, which was converted for one share of Emmaus Medical common stock, was exchanged for a convertible note exercisable for 29.48548924976 shares of AFH IV common stock.
As a result of the Merger, security holders of Emmaus Medical received 20,628,305 shares of AFH IV common stock, options and warrants to purchase an aggregate of 326,507 shares of AFH IV common stock, and convertible notes to purchase an aggregate of 270,648 shares of AFH IV common stock.
Four shareholders exercised their Dissenters’ rights in connection with the Merger and returned an aggregate of 47,178 shares for an aggregate of $200,000. The shares will be cancelled as of May 3, 2011, the closing date of the Merger.
For accounting purposes, the Merger transaction is being accounted for as a reverse merger. The transaction has been treated as a recapitalization of. Emmaus Medical and its subsidiaries, with Emmaus Holdings, Inc. (the legal acquirer of Emmaus Medical and its subsidiaries) considered the accounting acquiree and Emmaus Medical whose management took control of Emmaus Holdings, Inc. (the legal acquiree of Emmaus Medical) considered the accounting acquirer.
The Company entered into employment agreements with Yutaka Niihara, M.D., MPH, its Chief Executive Officer, Willis C. Lee, its Chief Operating Officer, and Lan T. Tran, its Chief Administrative Officer on April 5, 2011 and with Yasushi Nagasaki, its Chief Financial Officer, on April 8, 2011 (collectively, the “Employment Agreements”). Each of the Employment Agreements has an initial 2-year term, unless terminated earlier. The Employment Agreements for Dr. Niihara, Mr. Lee and Ms. Tran automatically renew for additional one year periods unless the Company or the officer provides notice of non-renewal at least sixty (60) days prior to the expiration of the then current term.
On July 8, 2011, the Company issued a convertible promissory note in the principal amount of $3,240, which bears interest at 8% per annum and matures on July 7, 2012. Upon the lender’s option, the principal amount of the note and any unpaid accrued interest may be converted into shares of common stock of the Company at a conversion price of $3.60 per share. The lender also received a warrant to purchase 225 shares of common stock. The warrant is exercisable for three years and has an exercise price equal to 75% of the fair market value of the Company’s common stock on the date prior to exercise.
On July 7, 2011 and July 11, 2011, Emmaus Medical issued promissory notes each in the principal amount of $300,000 to Yoko Hagiike. The notes bear interest at 10% per annum and all unpaid principal and interest are due upon demand at the lender’s option.
On July 11, 2011, the Company issued a convertible promissory note in the principal amount of $925,200, which bears interest at 8% per annum and matures on July 11, 2012. Upon the lender’s option, the principal amount of the note and any unpaid accrued interest may be converted into shares of common stock of the Company at a conversion price of $3.60 per share. The lender also received a warrant to purchase 64,250 shares of common stock. The warrants will be exercisable until three years from the date of the note and will have an exercise price equal to 75% of the fair market value of the Company’s common stock on the date prior to exercise.
109
On July 21, 2011, Emmaus Medical received a loan from Equities First Holdings, LLC (“Equities First”) pursuant to a loan agreement by and between Emmaus Medical and Equities First dated June 9, 2011. Pursuant to the loan agreement, Equities First agreed to lend to Emmaus Medical funds equal to 70% of the current fair market value of 73,550 shares of CellSeed, Inc. owned by Emmaus Medical for the three consecutive trading days for the CellSeed stock. The loan bears interest at 4.5% and any interest payment not received within ten calendar days is subject to a penalty equal to 7% of the amount due. The loan was funded on July 21, 2011 (the “Closing Date”) in the principal amount of $841,728.27 and matures on the third anniversary of the Closing Date. Prepayment of the principal amount of the loan or any interest due is not permitted. As collateral for the loan, Emmaus Medical pledged 73,550 of its shares of CellSeed (the “Collateral”) pursuant to a Pledge Agreement with Equities First in which Emmaus Medical assigned its right, title and ownership interest in the Collateral. Within five business days of Emmaus Medical’s payment of all of its obligations under the loan agreement, Equities First shall reassign all right, title and ownership interest in identical securities, as defined in Internal Revenue Code Section 1058, to Emmaus Medical and redeliver the Collateral.
In March 2011, AminoPure received a registration approval from Food and Drugs Board under Ghana Ministry of Health. Emmaus contracted East Cantonments Pharmacy Ltd., a wholesale pharmacy distributor, to be its distributor in Ghana and received its first purchase order in May 2011.
On July 31, 2011, the Company agreed to terminate the promotional rights agreement for Zorbtive® with EMD Serono and after that date will no longer promote Zorbtive®.
From August 2011 through October 2011, the Company issued ten one-year convertible notes in the principal amount totaling $2,115,602, which bear interest at 8% per annum and mature on the anniversary date of the note. The principal amount plus the unpaid accrued interest due under each of the convertible notes is convertible into shares of the Company’s common stock at $3.60 per share. In connection with the issuance of the notes totaling $1,987,600, the Company issued three-year warrants to purchase a total of 242,195 shares of our common stock, respectively, at a per share exercise price equal to 75% of the per share fair market value of the Company’s common stock on the date prior to exercise. In connection with the issuance of the note totaling 128,002, the Compant issued three-year warrants to purchase 35,556 shares of its common stock, at a per share exercise price equal to $1.00.
In August 2011, the Company entered into an Addendum to the Joint Research and Development Agreement with CellSeed. Pursuant to the Addendum, CellSeed and the Company further acknowledged that all obligations of CellSeed corresponding to the Research Agreement payment of $8.5 million and the Individual Agreement payment of $1.5 million will be fully discharged by CellSeed’s delivery of the Package, which delivery will be documented by written confirmation of acceptance by the Company. The Company is obligated to make the payments only after the Company provides its written confirmation of acceptance of the complete Package to CellSeed.
On August 31, 2011, the Company issued warrants to purchase 275,482 shares of our common stock at an exercise price of $1.00 per share to a consultant. The warrant is generally exercisable for three years from the issuance date, however, will terminate earlier upon the occurrence of either of the following: (1) FINRA deems the Underwriters’ compensation for a public offering to be unreasonable; or (2) on December 31, 2011 if the Company has not consummated one or more public offerings of securities in the minimum aggregate amount of $5.0 million prior to such date. The warrantholder may not exercise the warrant to the extent that such exercise would cause the holder and its affiliates to be a beneficial owner of more than 9.99% of the Company’s outstanding shares of common stock.
On September 30, 2011, the Company entered into an Amended and Restated Letter of Intent (the “LOI”) with AFH Holding & Advisory LLC (“AFH Advisory”). Pursuant to the LOI, the Company has agreed to pay AFH Advisory $500,000 (the “Shell Cost”) for the identification of AFH Acquisition IV and providing consulting services related to coordinating the Merger, assisting with a public offering and managing the interrelationship of legal and accounting activities (the “Services”), and to reimburse AFH Advisory for advancement of expenses on behalf of the Company incurred in connection with the Services, the Merger and a public offering, including, without limitation, reasonable expenses of AFH Advisory (the “Transaction Costs”).
AFH Advisory may be paid any outstanding Shell Cost and Transaction Costs from the proceeds of a public offering or upon the consummation of any other financing. Alternatively, AFH Advisory may, in its discretion, convert such amount (or any portion thereof) into common stock at a conversion price equal to 75% of the per share price in the public offering (the “Conversion Price”). Additionally, the Company has agreed to issue warrants to purchase shares of common stock to AFH Advisory upon the closing of a public offering. Such warrants will have a term of 5 years from the date of issuance and will have an exercise price equal to the Conversion Price. The number of shares underlying the warrants will be calculated by dividing the aggregate of the Shell Cost and the total Transaction Costs by the Conversion Price.
110
Shares held by AFH Advisory and certain others will be decreased, at the rate of 1% of the post-offering outstanding common shares, for each $1 million or fraction thereof that the gross proceeds to the Company from the offering are less than $10 million. In the event of such reduction, AFH Advisory will reduce the number of those shares by an appropriate percentage. If an offering cannot be consummated to provide for minimum gross proceeds to the Company of at least $5 million, the Company’s arrangement with AFH Advisory provides that the Company may terminate the offering at its sole and absolute discretion, and if the Company terminates the offering, all shares held by AFH Advisory and certain others will be canceled.
If the Company’s arrangement with AFH Advisory is terminated based on mutual agreement of the parties or based on a breach by the Company, AFH Advisory will receive its Transaction Costs incurred as of the date of termination. If the arrangement with AFH Advisory is terminated (i) by either party (without being cured within 30 days) based on identification of information in the course of its due diligence investigation that it deems unsatisfactory, or if the parties are unable to agree to a valuation within 45 days following completion of satisfactory due diligence by an investment bank, or (ii) by the Company if a public offering cannot be consummated to provide for gross proceeds to the Company of at least $10 million or based on a breach by AFH Advisory that is not cured within 30 days, then AFH Advisory will receive reimbursement for 50% of Transaction Costs actually incurred to the date of such termination. If a public offering cannot be consummated with gross proceeds of at least $5 million, and the Company exercises its right to terminate the offering, then the Company will reimburse AFH Advisory an amount equal to 50% of the Shell Cost and 50% of the Transaction Costs incurred as of the date of termination, and AFH Advisory, in its discretion, has the option to be paid any outstanding amounts at the time of termination in cash or to convert such amounts (or any portion thereof) into common stock at a conversion price equal to 75% of the per share price of the shares of common stock sold in the Company’s most recently completed private offering of common stock. In the event of termination by the Company because a public offering cannot be consummated to provide for gross proceeds to the Company of at least $10 million, and if the Company enters into any transaction or a sale of all or substantially all of its assets within 12 months of such termination, AFH Advisory will be entitled to (i) 5% of its holdings (including holdings of certain others) of any securities received by the Company or the Company’s shareholders upon consummation of any business combination or other similar transaction; or (ii) cash or any other consideration it would have received as if it had a 5% ownership interest in the Company immediately prior to the closing of any such transaction.
The Company granted AFH Advisory exclusive rights to act as its advisor in connection with all financings and mergers and acquisitions until November 10, 2012 and the right to appoint two board members to the Company’s board of directors upon the closing of the Merger. In addition, in the event of any merger, stock purchase, asset purchase or similar transaction occurring within one year of the closing of the public offering, AFH Advisory may receive a warrant to purchase shares in an amount to increase AFH Advisory’s total holdings to 10% of the outstanding fully diluted equity of the Company but only to the extent the Company issues securities in connection with such merger, stock purchase, asset purchase or similar transaction.
111
NOTE 12 – RESTATEMENT
The Company had previously restated its previously issued consolidated financial statements to report its deferred tax assets and liabilities related to unrealized gain on available-for-sale securities (the “Initial Restatements”). After further analysis, the Initial Restatements to record the change in the deferred tax asset valuation allowance as a tax benefit were incorrect. Since the change in the valuation allowance is associated with deferred tax recorded for unrealized holding gain on securities available-for-sale, the proper treatment is to record the change in the valuation allowance in other comprehensive income. Accordingly, the Company has restated the consolidated financial statements in the Initial Restatements to properly record the tax impacts associated with the available-for-sale securities in other comprehensive income (the “Second Restatements”). As a result of the Second Restatements, the consolidated financial statements as of and for the three months ended March 31, 2011 are identical to those reported by the Company prior to the Initial Restatements. The effect of the Second Restatements on the results of operations and financial position as of and for the period ended March 31, 2011 were as follows:
| As initially reported | As Restated by Initial Restatement | As Restated by Second Restatement | ||||||||||
| Deferred tax asset | $ | — | $ | 520,957 | $ | — | ||||||
| Total current assets | 2,515,424 | 3,036,381 | 2,515,424 | |||||||||
| Total assets | 4,417,749 | 4,938,706 | 4,417,749 | |||||||||
| Deferred tax liability | — | 520,957 | — | |||||||||
| Total liabilities | 1,915,127 | 2,436,084 | 1,915,127 | |||||||||
| Income tax (benefit) | 850 | (303,078 | ) | 850 | ||||||||
| Unrealized holding gain in securities available-for-sale | 759,820 | 455,892 | 759,820 | |||||||||
| Net loss per common share | (1.63 | ) | (1.19 | ) | (1.63 | ) | ||||||
112
To the Board of Directors and
Stockholders of Emmaus Medical, Inc.
We have audited the accompanying consolidated balance sheets of Emmaus Medical, Inc. (a development stage company) as of December 31, 2010 and 2009, and the related consolidated statements of operations, shareholders’ equity (deficit), and cash flows for each of the years in the two-year period ended December 31, 2010 and for the period of inception (December 20, 2000) through December 31, 2010. Emmaus Medical, Inc.’s management is responsible for these consolidated financial statements. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement. The company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the consolidated financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall consolidated financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Emmaus Medical, Inc. as of December 31, 2010 and 2009, and the results of its operations and its cash flows for each of the years in the two-year period ended December 31, 2010 and for the period since inception (December 20, 2000) through December 31, 2010 in conformity with accounting principles generally accepted in the United States of America.
As discussed in Note 14 to the consolidated financial statements, the 2010 consolidated financial statements were previously incorrectly restated to report the Company’s deferred taxes. Subsequently, the 2010 consolidated financial statements have been further restated to reverse the initial restatement.
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the consolidated financial statements, these conditions raise substantial doubt about its ability to continue as a going concern. Management's plans in regard to these matters are also described in Note 2. The consolidated financial statements do not include any adjustments relating to the recoverability and classification of asset carrying amounts or the amount and classification of liabilities that might result should the Company be unable to continue as a going concern.
| /s/ EFP Rotenberg, LLP | |
| EFP Rotenberg, LLP | |
| Rochester, New York | |
| May 3, 2011 (except for Notes 7, 10, 11, 13 and 14, as to which the date is November 15, 2011.) |
113
Emmaus Medical, Inc.
(A Development Stage Company)
| As of | ||||||||
| December 31, | ||||||||
| 2010 | 2009 | |||||||
| (Restated) | ||||||||
| ASSETS | ||||||||
| CURRENT ASSETS | ||||||||
| Cash and cash equivalents | $ | 258,676 | $ | 389,554 | ||||
| Accounts receivable | 21,746 | 18,022 | ||||||
| Inventories | 130,573 | 227,897 | ||||||
| Marketable securities | 1,674,386 | 1,131,813 | ||||||
| Prepaid expenses and other current assets | 11,479 | 12,982 | ||||||
| Total Current Assets | 2,096,860 | 1,780,268 | ||||||
| PROPERTY AND EQUIPMENT, net | 94,179 | 114,539 | ||||||
| OTHER ASSETS | ||||||||
| Intangibles, net | 134,880 | 388,832 | ||||||
| Notes receivable | 18,000 | 18,000 | ||||||
| Deposits | 348,408 | 865 | ||||||
| Total Other Assets | 501,288 | 407,697 | ||||||
| Total Assets | $ | 2,692,327 | $ | 2,302,504 | ||||
| LIABILITIES AND SHAREHOLDERS’ EQUITY | ||||||||
| CURRENT LIABILITIES | ||||||||
| Accounts payable and accrued expenses | $ | 190,107 | $ | 164,235 | ||||
| Note payable | 460,000 | 460,000 | ||||||
| Convertible notes payable | 246,889 | — | ||||||
| Total current liabilities | 896,996 | 624,235 | ||||||
| LONG-TERM LIABILITIES | ||||||||
| Notes payable | — | — | ||||||
| Convertible notes payable | 184,030 | 282,465 | ||||||
| Total long-term liabilities | 184,030 | 282,465 | ||||||
| Total Liabilities | 1,081,026 | 906,700 | ||||||
| SHAREHOLDERS’ EQUITY | ||||||||
| Common stock – par value $0.001 per share, 1,000,000 shares authorized, 690,692 and 652,240 shares issued and outstanding at December 31, 2010 and 2009, respectively | 691 | 652 | ||||||
| Additional paid-in capital | 13,819,673 | 10,389,418 | ||||||
| Accumulated other comprehensive income | 542,573 | — | ||||||
| Deficit accumulated during the development stage | (12,751,636 | ) | (8,994,266 | ) | ||||
| Total Shareholders’ Equity | 1,611,301 | 1,395,804 | ||||||
| Total Liabilities & Shareholders’ Equity | $ | 2,692,327 | $ | 2,302,504 | ||||
The accompanying notes are an integral part of these financial statements.
114
Emmaus Medical, Inc.
(A Development Stage Company)
| Year Ended December 31, | From December 20, 2000 (date of inception) to December 31, | |||||||||||
| 2010 | 2009 | 2010 | ||||||||||
| (Restated) | (Restated) | |||||||||||
| SALES | $ | 168,964 | $ | 100,408 | 374,499 | |||||||
| SALES RETURN | (30,230 | ) | (127 | ) | (30,357 | ) | ||||||
| REVENUES | $ | 138,734 | $ | 100,281 | $ | 344,142 | ||||||
| COST OF GOODS SOLD | ||||||||||||
| Cost of goods sold, net of scrapped inventory | 99,373 | 85,226 | 226,034 | |||||||||
| Scrapped inventory | 235,537 | — | 235,537 | |||||||||
| Total cost of goods sold | 334,910 | 85,226 | 461,571 | |||||||||
| GROSS PROFIT (LOSS) | (196,176 | ) | 15,055 | (117,429 | ) | |||||||
| OPERATING EXPENSES | ||||||||||||
| Research and development | 1,062,031 | 532,351 | 4,899,652 | |||||||||
| Selling | 656,200 | 696,949 | 1,802,208 | |||||||||
| General and administrative | 1,817,728 | 1,300,397 | 5,612,740 | |||||||||
| 3,535,959 | 2,529,697 | 12,314,600 | ||||||||||
| LOSS FROM OPERATIONS | (3,732,135 | ) | (2,514,642 | ) | (12,432,029 | ) | ||||||
| OTHER INCOME (EXPENSE) | ||||||||||||
| Interest income | 39,005 | 19,659 | 85,234 | |||||||||
| Interest expense | (59,936 | ) | (71,600 | ) | (389,993 | ) | ||||||
| (20,931 | ) | (51,941 | ) | (304,759 | ) | |||||||
| LOSS BEFORE INCOME TAXES | (3,753,066 | ) | (2,566,583 | ) | (12,736,788 | ) | ||||||
| INCOME TAXES | 4,304 | 1,224 | 14,848 | |||||||||
| NET LOSS | (3,757,370 | ) | (2,567,807 | ) | (12,751,636 | ) | ||||||
| OTHER INCOME | ||||||||||||
| Unrealized holding gain on securities available-for-sale, net of tax | 542,573 | — | 542,573 | |||||||||
| COMPREHENSIVE LOSS | $ | (3,214,797 | ) | $ | (2,567,807 | ) | $ | (12,209,063 | ) | |||
| NET LOSS PER COMMON SHARE | $ | (5.63 | ) | $ | (4.02 | ) | ||||||
| WEIGHTED AVERAGE COMMON SHARES OUTSTANDING | 666,813 | 638,068 | ||||||||||
The accompanying notes are an integral part of these financial statements.
115
Emmaus Medical, Inc.
(A Development Stage Company)
and for the period from December 20, 2000 (Inception) to December 31, 2010
| Common stock – par value $0.001 per share, 1,000,000 shares authorized | Additional | Accumulated Other | Deficit Accumulated during | |||||||||||||||||||||
| Shares | Common stock | Paid-in Capital | Comprehensive income | Development Stage | Total | |||||||||||||||||||
| Balance, December 31, 2000 (1) | 425,000 | $ | 425 | $ | 9,175 | — | $ | — | $ | 9,600 | ||||||||||||||
| Net loss | — | — | — | — | (21,942 | ) | (21,942 | ) | ||||||||||||||||
| Balance, December 31, 2001 | �� | 425,000 | 425 | 9,175 | — | (21,942 | ) | (12,342 | ) | |||||||||||||||
| Net loss | — | — | — | — | (12,464 | ) | (12,464 | ) | ||||||||||||||||
| Balance, December 31, 2002 | 425,000 | 425 | 9,175 | — | (34,406 | ) | (24,806 | ) | ||||||||||||||||
Constructive distribution of retained loss to Additional Paid-in Capital | — | — | (34,406 | ) | — | 34,406 | — | |||||||||||||||||
| Common stock issued | 25,000 | 25 | 249,975 | — | — | 250,000 | ||||||||||||||||||
| — | ||||||||||||||||||||||||
| Net loss | — | — | — | — | (97,481 | ) | (97,481 | ) | ||||||||||||||||
| Balance, December 31, 2003 | 450,000 | 450 | 224,744 | — | (97,481 | ) | 127,713 | |||||||||||||||||
(1) Reflects recapitalization of members’ equity of Emmaus Medical, LLC into 425,000 shares of common stock of Emmaus Medical, Inc.
116
Emmaus Medical, Inc.
(A Development Stage Company)
Consolidated Statement of Changes in Shareholders’ Equity (Deficit)
and for the period from December 20, 2000 (Inception) to December 31, 2010 (Continued)
| Common stock – par value $0.001 per share, 1,000,000 shares authorized | Additional | Accumulated Other | Deficit Accumulated during | |||||||||||||||||||||
| Shares | Common stock | Paid-in Capital | Comprehensive income | Development Stage | Total | |||||||||||||||||||
| Balance, December 31, 2003 | 450,000 | 450 | 224,744 | — | (97,481 | ) | 127,713 | |||||||||||||||||
| Common stock issued | 54,792 | 55 | 648,020 | — | — | 648,075 | ||||||||||||||||||
| Net loss | — | — | — | — | (624,936 | ) | (624,936 | ) | ||||||||||||||||
| Balance, December 31, 2004 | 504,792 | 505 | 872,764 | — | (722,417 | ) | 150,852 | |||||||||||||||||
| Common stock issued | 13,517 | 13 | 328,272 | — | — | 328,285 | ||||||||||||||||||
| Net loss | — | — | — | — | (668,091 | ) | (668,091 | ) | ||||||||||||||||
| Balance, December 31, 2005 | 518,309 | 518 | 1,201,036 | — | (1,390,508 | ) | (188,954 | ) | ||||||||||||||||
| Common stock issued | 17,751 | 18 | 825,022 | — | — | 825,040 | ||||||||||||||||||
| Net loss | — | — | — | — | (759,962 | ) | (759,962 | ) | ||||||||||||||||
| Balance, December 31, 2006 | 536,060 | 536 | 2,026,058 | — | (2,150,470 | ) | (123,876 | ) | ||||||||||||||||
| Common stock issued | 45,588 | 46 | 2,733,814 | — | — | 2,733,860 | ||||||||||||||||||
| Net loss | — | — | — | — | (1,282,212 | ) | (1,282,212 | ) | ||||||||||||||||
| Balance, December 31, 2007 | 581,648 | 582 | 4,759,872 | — | (3,432,682 | ) | 1,327,772 | |||||||||||||||||
117
Emmaus Medical, Inc
(A Development Stage Company)
Consolidated Statement of Changes in Shareholders’ Equity (Deficit)
and for the period from December 20, 2000 (Inception) to December 31, 2010 (Continued)
| Common stock – par value $0.001 per share, 1,000,000 shares authorized | Additional | Accumulated Other | Deficit Accumulated during | |||||||||||||||||||||
| Shares | Common stock | Paid-in Capital | Comprehensive income | Development Stage | Total | |||||||||||||||||||
| Balance, December 31, 2007 | 581,648 | 582 | 4,759,872 | — | (3,432,682 | ) | 1,327,772 | |||||||||||||||||
| Common stock issued | 41,613 | 41 | 3,390,650 | — | — | 3,390,691 | ||||||||||||||||||
| Net loss | — | — | — | — | (2,993,777 | ) | (2,993,777 | ) | ||||||||||||||||
| Balance, December 31, 2008 | 23,261 | 623 | 8,150,522 | — | (6,426,459 | ) | 1,724,686 | |||||||||||||||||
| Warrants issued | — | — | 160,000 | — | — | 160,000 | ||||||||||||||||||
| Common stock issued, net of issuance cost of $160,000 | 28,979 | 29 | 2,078,896 | — | — | �� | 2,078,925 | |||||||||||||||||
| Net loss | — | (2,567,807 | ) | (2,567,807 | ) | |||||||||||||||||||
| Balance, December 31, 2009 | 652,240 | 652 | 10,389,418 | — | (8,994,266 | ) | 1,395,804 | |||||||||||||||||
| Warrants issued | — | — | 480,000 | — | — | 480,000 | ||||||||||||||||||
| Common stock issued, net of issuance cost of $480,000 | 23,941 | 24 | 1,644,270 | — | — | 1,644,294 | ||||||||||||||||||
| Conversion of notes payable to common stock | 14,511 | 15 | 1,305,985 | — | — | 1,306,000 | ||||||||||||||||||
| Unrealized gain on securities available for sale | — | — | — | 542,573 | — | 542,573 | ||||||||||||||||||
| Net loss | — | — | — | — | (3,757,370 | ) | (3,757,370 | ) | ||||||||||||||||
| Balance, December 31, 2010 (Restated) | 690,692 | $ | 691 | $ | 13,819,673 | $ | 542,573 | $ | (12,751,636 | ) | $ | 1,611,301 | ||||||||||||
The accompanying notes are an integral part of these financial statements.
118
Emmaus Medical, Inc.
(A Development Stage Company)
| Year Ended December 31, | From December 20, 2000 (date of inception) to December 31, | |||||||||||
| 2010 | 2009 | 2010 | ||||||||||
| (Restated) | (Restated) | |||||||||||
| CASH FLOWS FROM OPERATING ACTIVITIES | ||||||||||||
| Net loss | $ | (3,757,370 | ) | $ | (2,567,807 | ) | $ | (12,751,636 | ) | |||
| Adjustments to reconcile net loss to net cash flows from operating activities | ||||||||||||
| Depreciation and amortization | 280,032 | 281,443 | 706,748 | |||||||||
| Cost of scrapped inventory written off | 235,537 | — | 235,537 | |||||||||
| Net changes in operating assets and liabilities | ||||||||||||
| Accounts receivable | (9,833 | ) | 31,939 | (27,852 | ) | |||||||
| Inventory | (134,203 | ) | 45,644 | (362,100 | ) | |||||||
| Prepaid expenses and other current assets | 1,503 | (3,279 | ) | (29,479 | ) | |||||||
| Deposits | (296,907 | ) | — | (297,772 | ) | |||||||
| Accounts payable and accrued expenses | (34,265 | ) | (2,082 | ) | 129,969 | |||||||
| Net cash flows used in operating activities | (3,715,506 | ) | (2,214,142 | ) | (12,396,585 | ) | ||||||
| CASH FLOWS FROM INVESTING ACTIVITIES | ||||||||||||
| Payment towards license | — | — | (750,000 | ) | ||||||||
| Purchases of marketable securities | — | (1,131,813 | ) | (1,131,813 | ) | |||||||
| Purchases of property and equipment | (5,720 | ) | (10,257 | ) | (185,807 | ) | ||||||
| Net cash flows used in investing activities | (5,720 | ) | (1,142,070 | ) | (2,067,620 | ) | ||||||
| CASH FLOWS FROM FINANCING ACTIVITIES | ||||||||||||
| Borrowings from line of credit | — | — | 299,500 | |||||||||
| Repayment of line of credit | — | (299,500 | ) | (299,500 | ) | |||||||
| Proceeds from notes payable issued | — | 742,463 | 742,463 | |||||||||
| Payments of notes payable | (35,576 | ) | — | (35,576 | ) | |||||||
| Proceeds from convertible notes payable issued | 1,490,030 | — | 1,490,030 | |||||||||
| Proceeds from issuance of common stock | 2,124,294 | 2,238,925 | 12,514,364 | |||||||||
| Net cash flows from financing activities | 3,578,748 | 2,681,888 | 14,711,281 | |||||||||
| Net increase in cash and cash equivalents | (142,478 | ) | (674,324 | ) | 247,076 | |||||||
| Cash and cash equivalents, beginning of period | 389,554 | 1,063,878 | — | |||||||||
| Cash acquired | 11,600 | — | 11,600 | |||||||||
| Cash and cash equivalents, end of period | $ | 258,676 | $ | 389,554 | $ | 258,676 | ||||||
| SUPPLEMENTAL DISCLOSURES OF CASH FLOW ACTIVITIES | ||||||||||||
| Interest paid | $ | 59,936 | $ | 71,600 | $ | 389,993 | ||||||
| Income taxes paid | $ | 4,304 | $ | 1,224 | $ | 14,848 | ||||||
| Non-cash Financing Activities: | ||||||||||||
| Conversion of notes payable to common stock | $ | 1,306,000 | $ | — | $ | 1,306,000 | ||||||
The accompanying notes are an integral part of these financial statements.
119
Organization – Emmaus Medical, Inc. (the “Company” or “Emmaus”) is engaged in the discovery, development, and commercialization of treatments and therapies for rare diseases. Emmaus is a Delaware corporation originally incorporated on September 12, 2003. Emmaus Medical, LLC was organized on December 20, 2000. In October 2003, Emmaus Medical, LLC conducted a reorganization and merged with Emmaus. As a result of the merger, Emmaus acquired the exclusive patent rights for a treatment for sickle cell disease.
Emmaus and its subsidiary, Emmaus Medical, and Emmaus Medical’s subsidiaries, Newfield Nutrition Corporation and Emmaus Medical Japan, Inc., are collectively referred to herein as the “Company.”
Nature of Business – The Company has undertaken the business of developing and commercializing cost effective treatments and therapies for rare diseases. The Company’s primary business purpose is to continue its late-stage development of the amino acid L-glutamine as a prescription drug for the treatment of sickle cell disease (“SCD”). The Company’s current focus is to complete the Phase 3 clinical trial on SCD that involves over 20 research sites and 200 patients.
To a lesser extent, the Company also sells and/or promotes certain other prescription pharmaceutical drugs, namely Zorbtive® and NutreStore®, that are used in combination as a treatment for short bowel syndrome. Through its wholly owned subsidiary, Newfield Nutrition Corporation, the Company sells L-glutamine as a nutritional supplement under the brand name AminoPure® through retail stores in multiple states and via importers and distributors in Japan and Taiwan. Since inception, the Company has generated minimal revenues from the sale and/or promotion of NutreStore®, Zorbtive® and AminoPure®.
NOTE 2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of presentation – The accompanying financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America.
Going concern – The accompanying financial statements have been prepared on the basis that the Company will continue as a going concern. The Company has losses for the years ended December 31, 2010 and 2009 totaling $3,757,370 and $2,567,807, respectively, as well as accumulated deficit since inception amounting to $12,751,636. Further the Company appears to have inadequate cash and cash equivalents of $258,676 as of December 31, 2010 considering that revenues from operations since inception totaled only $344,142. As a result, the Company is dependent upon funds from private investors and the support of certain stockholders.
These factors raise substantial doubt about the ability of the Company to continue as a going concern. The financial statements do not include any adjustments that might result from the outcome of these uncertainties. In this regard, Management is planning to raise any necessary additional funds through loans and additional sales of its common stock. There is no assurance that the Company will be successful in raising additional capital.
Recapitalization and change in legal status of entity – In October 2003, the Company acquired substantially all of the assets of Emmaus Medical, LLC. The shareholders of the Company were substantially the same as the members of Emmaus Medical, LLC. As such, the transaction was accounted for as a transfer of assets between entities under common control pursuant to accounting standards codification 805, Business Combinations.
For a transferred set of activities and assets to be a business, it must contain all of the inputs and processes necessary for it to continue to conduct normal operations after the transferred set of assets is separated from the transferor, which include the ability to sustain a revenue stream by providing its outputs to customers. The Company obtained the inputs and processes necessary for normal operations. The transaction has been accounted for as a recapitalization of Emmaus Medical, LLC. Accordingly, the assets were carried over to Emmaus Medical, Inc. at the historical carrying values and the historical operations of those assets owned by the Company are presented in the accompanying financial statements as the historical operations of Emmaus Medical, Inc. for all periods presented.
The effect of the recapitalization was to retroactively present the stockholders’ equity of Emmaus Medical, Inc. (the surviving entity) to the earliest period presented in the financial statements. This recapitalization had no effect on results of operations for any period presented. Also, concurrent with the recapitalization, the Company changed the legal status from a Limited Liability Company to a “C” Corporation. In connection with this change, deficit accumulated in the Limited Liability Company were transferred to additional paid in capital.
120
Principles of consolidation – The financial statements include the accounts of the Company (and its wholly-owned subsidiaries, Newfield Nutrition Corporation and Emmaus Medical Japan, Inc (“EM Japan”). All significant intercompany transactions have been eliminated.
Development stage company – The Company is a development stage company as defined in accounting principles generally accepted in the United States of America. The company is considered a development stage company because it devotes substantially all of its time to research and development for potential pharmaceutical products and to establish its business and operations. The minimal sales for the period from inception to December 31, 2010 are from NutreStore and the products of its wholly owned subsidiary Newfield Nutrition Corporation which is not considered to be a part of its principal operations.
Use of estimates – Financial statements prepared in accordance with accounting principles generally accepted in the United States require management to make estimates and assumptions that affect the reported amounts of assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Among other things, management has estimated the useful lives of equipment and other assets, along with the variables used to calculate the valuation of stock options and warrants using the Black-Scholes-Merton option valuation model. Actual results could differ from those estimates.
Cash and cash equivalents – Cash and cash equivalents include all short term securities with original maturity of less than ninety days.
The Company maintains its cash and cash equivalents at insured financial institutions, the balances of which may, at times, exceed federally insured limits. Management believes that the risk of loss due to the concentrations is minimal.
Inventories – Inventories of the Company consist of finished goods and work-in-process and are valued based on first-in, first-out and at the lesser of cost or market value. Work-in-process inventories consist of raw material L-Glutamine for the Company’s products, AminoPure and NutreStore, that has not yet been packaged and labeled for sale. As of December 31, 2010 and 2009, inventories consisted of 100% finished goods.
The Company made purchases of L-glutamine from two vendors in the years ended December 31, 2010 and 2009, with one vendor accounting for 48% and the other accounting for 52%, respectively, of total purchases during each of the years.
Deposits - Carrying value of amounts transferred to third parties for security purposes that are expected to be returned or applied towards payment after one year or beyond the operating cycle, if longer. The deposit amount has increased significantly from 2009. This change can be attributed to the 20% patient site enrollment deposit paid to ClinDatrix for the Phase III clinical trial activities.
Revenue recognition – The Company recognizes revenue in accordance with the Securities and Exchange Commission (“SEC”) Staff Accounting Bulletin (“SAB”) No. 101, Revenue Recognition in Financial Statements (“SAB 101”), as amended by Staff Accounting Bulletin No. 104, Revision of Topic 13 (“SAB 104”).
Revenue is recognized when there is persuasive evidence that an arrangement exists, delivery has occurred, the price is fixed and determinable and collection is reasonably assured.
With prior written approval of the Company, product is returnable only by our direct customers for a returned goods credit, if the product meets any of the following criteria:
| A. | Product expiring within six (6) months of the expiration date printed on the package/container that is in the manufacturer’s original package/container and bears the original label. | |
| B. | Expired product that is in the manufacturer’s original package/container and bears the manufacturer’s original label, provided, however, that expired product must be returned within 12 months of the expiration date printed on the package/container. | |
| C. | Product shipped directly by the Company that is damaged in transit, subject to Free on Board (“FOB”) Destination, or material shipped in error by the Company. | |
| D. | Product that is discontinued, withdrawn, or recalled. |
Credits will only be issued for full cartons only without any missing packets of product. No credit is issued, nor does the Company accept charges or deductions for administrative, handling, or freight charges associated with the return of product to the Company. No credit is issued for product destroyed by anyone other than the Company. Customers must return the product within 60 days of receiving our written approval for the return or the return will not be issued a credit. The amount of the credit provided for returned product is based on the current wholesale acquisition cost of the returned product less 5%. When product is returned, a credit memo is applied to the customer’s current account balance or applied to future purchases. Credit memos expire one hundred eighty (180) days from date issued.
121
The Company estimates its sales return based upon its prior sales and return history. Historically, sales returns have been very nominal. The extraordinary sales returns in the six months ended June 30, 2010 were all related to the same batch of NutreStore product produced in 2008 that expired. The expiry date of the NutreStore product was two years after the date of manufacture but was changed by the Company and is currently four years after the date of manufacture. All products that were sold in 2009 were part of the batch that had a two year expiry period and expired in 2010. All products produced in 2010 and sold from 2010 through 2011 have an expiry date of 2014 and the Company anticipates that the product will be consumed before expiration and not returned. The Company continues to monitor its returns and will adjust our estimates based on its actual sales return experience. Based upon the Company’s sales return history the Company will record a reserve for estimated returns during each reporting period. As of December 31, 2010, no reserve for sales returns has been deemed necessary and accordingly, no reserve has been recorded in the accompanying financial statements.
The Company is currently required to pay royalties to CATO Holding Company on an annual basis, which is recognized as an expense upon sale of the products.
Allowance for doubtful accounts – The Company provides an allowance for uncollectible accounts based upon prior experience and management’s assessment of the collectability of existing specific accounts. As of December 31, 2010 and 2009, Management considers all accounts receivable are fully collectible.
Advertising cost – Advertising costs are expensed as incurred. Advertising costs for the year ended December 31, 2010 and 2009 were $52,371 and $68,475 respectively. Advertising costs from inception to December 31, 2010 were approximately $140,000.
Property and equipment – Leaseholds, furniture, and fixtures are recorded at historical cost and depreciated on a straight-line basis over their estimated useful lives of 5 to 7 years. Maintenance and repairs are expensed as incurred, while major additions and improvements are capitalized. Gains and losses on disposition are included in current operations. Equipment is assessed by management, annually, for potential impairment. No impairment exists as of December 31, 2010 and 2009.
Intangibles – The Company’s intangible assets include license issue fees and patent costs relating to a license agreement (Note 4). These intangible assets are amortized over a period of 3 years, the estimated legal life of the patents and economic life of the License Agreement. The intangible assets are assessed by management, annually, for potential impairment. No impairment exists as of December 31, 2010.
Impairment of Long-Lived Assets – In accordance with FASB ASC 360-10-5, Accounting for the Impairment or Disposal of Long-Lived Assets, the Company evaluates the carrying value of its long-lived assets for impairment whenever events or changes in circumstances indicate that such carrying values may not be recoverable. The Company uses its best judgment based on the current facts and circumstances relating to its business when determining whether any significant impairment factors exist. The Company considers the following factors or conditions, among others, that could indicate the need for an impairment review:
| ● | significantly lower performance relative to expected historical or projected future operating results; | |
| ● | market projections; | |
| ● | its ability to obtain patents, including continuation patents, on technology; | |
| ● | significant changes in its strategic business objectives and utilization of the assets; | |
| ● | significant negative industry or economic trends, including legal factors; | |
| ● | potential for strategic partnerships for the development of its patented technology; | |
| ● | changing or implementation of rules regarding manufacture |
If the Company determines that the carrying values of long-lived assets may not be recoverable based upon the existence of one or more of the above indicators of impairment, the Company’s management performs an undiscounted cash flow analysis to determine if impairment exists. If impairment exists, the Company measures the impairment based on the difference between the asset’s carrying amount and its fair value, and the impairment is charged to operations in the period in which the long-lived asset impairment is determined by management. Based on its analysis, the Company believes that no indicators of impairment of the carrying value of its long-lived assets existed at December 31, 2010 and 2009.
122
There can be no assurance, however, that market conditions will not change or demand for the Company’s products will continue or allow the Company to realize the value of its long-lived assets and prevent future impairment
Research and development – Research and development consist of expenditures for the research and development of new products and technologies, which primarily involve contract research, payroll related expenses, and other related supplies. Research and development costs are expensed as incurred.
Share-based payments – The Company recognizes compensation cost for share-based compensation awards during the service term of the recipients of the share-based awards. The fair value of share-based is calculated using the Black-Scholes-Merton pricing model. The Black-Scholes-Merton model requires subjective assumptions regarding future stock price volatility and expected time to exercise, which greatly affect the calculated values. The expected term of awards granted is derived from historical data on awards exercised and post-vesting employment termination behavior. The risk-free rate selected to value any particular grant is based on the U.S. Treasury rate that corresponds to the vesting period of the grant effective as of the date of the grant. The expected volatility is based on the historical volatility of the common stock of comparable publicly traded companies. These factors could change, affecting the determination of stock-based awards expense in future periods.
Income taxes – The Company accounts for income taxes under the asset and liability method, wherein deferred tax assets and liabilities are recognized for the future tax consequences attributable to the differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period the enactment occurs. A valuation allowance is provided for certain deferred tax assets if it is more likely than not that the Company will not realize tax assets through future operations.
The Company's short-term and long-term deferred tax liability is based on the calculation of the deferred taxes on the Company's unrealized gain on available-for-sale securities using a 40% effective tax rate based on a 31% federal income tax rate (net of state tax deduction) combined with an 8.84% California state income tax rate. The Company recognizes a deferred tax asset (through changes in the valuation allowance) for the exact amount of the deferred tax liability. The classification of these deferred taxes is concurrent with the classification of investments for which the unrealized gain is derived. For balance sheet presentation, current deferred tax assets and liabilities have been offset and presented as a single amount and non-current deferred tax assets and liabilities within each tax jurisdiction have been offset and presented as a single amount.
When tax returns are filed, it is highly probable that some positions taken would be sustained upon examination by the taxing authorities, while others are subject to uncertainty about the merits of the position taken or the amount of the position that would be ultimately sustained. The benefit of a tax position is recognized in the financial statements in the period during which, based on all available evidence, management believes it is more likely than not that the position will be sustained upon examination, including the resolution of appeals or litigation processes, if any. Tax positions taken are not offset or aggregated with other positions. Tax positions that meet the more-likely-than-not recognition threshold are measured as the largest amount of tax benefit that is more than 50 percent likely of being realized upon settlement with the applicable taxing authority. The portion of the benefits associated with tax positions taken that exceeds the amount measured as described above is reflected as a liability for unrecognized tax benefits along with any associated interest and penalties that would be payable to the taxing authorities upon examination. As of December 31, 2010, the Company had no unrecognized tax benefits, and the Company had no positions which, in the opinion of management, would be reversed if challenged by a taxing authority. The Company’s evaluation of tax positions was performed for those tax years which remain open to audit. The Company may from time to time, be assessed interest or penalties by the taxing authorities, although any such assessments historically have been minimal and immaterial to the Company’s financial results. In the event the Company is assessed for interest and/or penalties, such amounts will be classified as income tax expense in the financial statements
As of December 31, 2010, all federal tax returns since 2007 and state tax returns since 2006 are still subject to adjustment upon audit. No tax returns are currently being examined by taxing authorities.
Comprehensive income (loss) – Comprehensive income (loss) includes net income (loss) and other comprehensive income (loss). The only items of other comprehensive income (loss) for the Company are unrealized gains and losses on securities classified as available-for-sale. When the Company realizes a gain or loss on available-for-sale securities for which an unrealized gain or loss was previously recognized, a corresponding reclassification adjustment is made to remove the unrealized gain or loss from other comprehensive income and reflect the realized gain or loss in current operations.
Marketable securities – Investment securities as of December 31, 2010 and 2009 are classified as available-for-sale. Securities available-for-sale are recorded at cost and any increases or decreases in fair market value are recorded as unrealized gain or loss, net of taxes in accumulated other comprehensive income. The Company monitors these investments for impairment and makes appropriate reductions in carrying values when necessary.
123
In January 2009, Emmaus made a strategic investment in CellSeed, Inc., a Japanese company engaged in the research and development, manufacture and sale of temperature-responsive cell culture equipment, which is a cell sheet tissue-engineering platform tool, and application products, as well as cell sheet tissue engineered medical products and application products. Emmaus acquired 147,100 shares of CellSeed stock for 100,028,000 Japanese Yen (equivalent to $1,109,819), at 680 Yen per share. As of December 31, 2010 and 2009, Emmaus owned 147,100 shares of CellSeed stock. Emmaus currently owns a 3% stake in CellSeed, Inc., a public company traded on the JASDAQ NEO market in Tokyo, Japan. As of December 31, 2010, the closing price per share of CellSeed stock on the JASDAQ was 921Yen. As of December 31, 2009, Cellseed stock was not yet trading on a JASDAQ and did not have a quoted market price. The Company assessed its investment for impairment using observable market data and determined that no impairment was necessary.
See subsequent events note for details of the April 8, 2011 Joint Research and Development Agreement (the “Research Agreement”) and an Individual Agreement (the “Individual Agreement”) with CellSeed, Inc.
Fair value measurements – The Company defines fair value as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. The Company measures fair value under a framework that provides a fair value hierarchy that prioritizes the inputs to valuation techniques used to measure fair value. The hierarchy gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (level 1 measurements) and the lowest priority to unobservable inputs (level 3 measurements). The three levels of the fair value hierarchy are described as follows:
Level 1: Inputs to the valuation methodology are unadjusted quoted prices for identical assets or liabilities in active markets that the Company has the ability to access.
Level 2: Inputs to the valuation methodology include:
| ● | Quoted prices for similar assets or liabilities in active markets; | |
| ● | Quoted prices for identical or similar assets or liabilities in inactive markets; | |
| ● | Inputs other than quoted prices that are observable for the asset or liability; | |
| ● | Inputs that are derived principally from or corroborated by observable market data by correlation or other means. |
If the asset or liability has a specified (contractual) term, the level 2 input must be observable for substantially the full term of the asset or liability.
Level 3: Inputs to the valuation methodology are unobservable and significant to the fair value measurement.
The assets or liability’s fair value measurement level within the fair value hierarchy is based on the lowest level of any input that is significant to the fair value measurement. Valuation techniques used need to maximize the use of observable inputs and minimize the use of unobservable inputs. The fair value assigned to marketable securities are determined by obtaining quoted prices on nationally recognized securities exchanges, and are classified as Level 1 investments at December 31, 2010. The fair value of the Company’s debt instruments are not materially different from their carrying values as presented.
Net loss per share – In accordance with FASB ASC Topic 260, “Earnings per Share,” the basic loss per common share is computed by dividing net loss available to common stockholders by the weighted average number of common shares outstanding. Dilutive loss per share is computed similar to basic loss per share except that the denominator is increased to include the number of additional common shares that would have been outstanding if the potential common shares had been issued and if the additional common shares were dilutive. As of December 31, 2010 and 2009, there were 15,723 and 4,663 shares of potentially dilutive securities outstanding, respectively. As the Company reported a net loss, none of the potentially dilutive securities were included in the calculation of diluted earnings per share since their effect would be anti-dilutive for that reporting period.
NOTE 3 – PROPERTY AND EQUIPMENT
At December 31, 2010 and 2009 equipment consisted of the following:
| 2010 | 2009 | |||||||
| Equipment | $ | 110,484 | $ | 104,764 | ||||
| Leasehold Improvements | 23,054 | 23,054 | ||||||
| Furniture and Fixtures | 52,269 | 52,269 | ||||||
| 185,807 | 180,087 | |||||||
| Less: accumulated depreciation | (91,628 | ) | (65,548 | ) | ||||
| $ | 94,179 | $ | 114,539 | |||||
124
During the years ended December 31, 2010 and 2009, the depreciation expense was $26,080 and $26,030. Depreciation expense from inception to December 31, 2010 was $91,628.
NOTE 4 – INTANGIBLE ASSETS
The Company is licensed to market and sell NutreStore® [L-glutamine powder for oral solution] and promote Zorbtive® [somatropin (rDNA origin) for injection], as a treatment for short bowel syndrome (“SBS”).
At December 31, 2010 and 2009, intangible assets consisted of the following:
| 2010 | 2009 | |||||||
| License fees and patent filing costs | $ | 750,000 | $ | 750,000 | ||||
| Less: accumulated amortization | (615,120 | ) | (361,168 | ) | ||||
| $ | 134,880 | $ | 388,832 | |||||
During the years ended December 31, 2010 and 2009, the amortization expense was $253,952 and $254,395. Amortization expense from inception to December 31, 2010 was $615,120. Expected amortization expense for the year ended December 31, 2011 is estimated to be approximately $135,000.
NOTE 5 – NOTES PAYABLE
Notes payable at December 31, 2010 and 2009 consisted of the following:
| December 31, 2010 | December 31, 2009 | |||||||
| Note payable to a shareholder, due on demand, interest payable monthly at 6.5% per annum. | ||||||||
| Yutaka Niihara – Note 1 | $ | 350,000 | $ | 350,000 | ||||
| Yutaka Niihara – Note 2 | 80,000 | 80,000 | ||||||
| Daniel Kimbell – Note 1 | 20,000 | 20,000 | ||||||
| Daniel Kimbell – Note 2 | 10,000 | 10,000 | ||||||
| Convertible note payable to shareholders, due 2015, 0% interest payable. Interest was imputed at the incremental borrowing rate of 6% per annum | 132,030 | — | ||||||
| Convertible note payable to shareholders, due in 2011or extendable if Lender wishes, interest payable monthly at 6.5% per annum | 246,889 | 282,465 | ||||||
| Convertible notes payable to shareholders, due in 2015, interest payable monthly at 6% per annum. | 52,000 | — | ||||||
| $ | 890,919 | $ | 742,465 | |||||
| Amount due in one year | (706,889 | ) | (460,000 | ) | ||||
| Long term portion of notes payable | 184,030 | 282,465 | ||||||
As of December 31, 2010 notes payable in the amount of $430,919 were convertible, at option of the lender, into shares of the Company’s common stock at $90.00 per share. During the year ended December 31, 2010, convertible notes payable in the amount of $1,490,030 were issued out of which notes payable in the amount of $1,306,000 were converted to common stock.
NOTE 6 – SHAREHOLDERS’ EQUITY
Common stock – During the year ended December 31, 2010, the Company issued a total of 38,452 shares of the Company’s common stock. The Company issued 23,941 shares for total proceeds of $2,124,294 and converted notes payable in the amount of $1,306,000 into 14,511 shares of the common stock.
125
During the year ended December 31, 2009, the Company issued a total of 28,979 shares of the Company’s common stock for total proceeds of $2,238,925.
Stock Warrants – During the years ended December 31, 2010 and 2009, the Company granted stock warrants to its investors and lenders to purchase an aggregate total of 6,686 shares and 3,447 shares respectively, of the Company’s common stock at an exercise price of $90.00 per share. The warrants are exercisable through 2015, and have contractual lives of five years. The total value of warrants granted during the year ended December 31, 2010 was $480,000 and was recorded against common stock as an issuance cost. The total value of warrants granted during the year ended December 31, 2009 was $160,000 and was recorded against common stock as an issuance cost.
A summary of outstanding warrants at December 31, 2010 and 2009 is presented below.
| Years ended December 31 | Weighted Average | |||||||||||
| 2010 | 2009 | Exercise Price | ||||||||||
| Warrants outstanding, beginning of year | 3,447 | — | $ | 90 | ||||||||
| Granted | 6,686 | 3,447 | 90 | |||||||||
| Cancelled, forfeited and expired | — | — | — | |||||||||
| Warrants outstanding, end of year | 10,133 | 3,447 | $ | 90 | ||||||||
| Outstanding | Exercisable | |||||||||||||||||||||
| Exercise Prices | Total | Weighted Average Remaining Contractual Life (Years) | Weighted Average Exercise Price | Total | Weighted Average Exercise Price | |||||||||||||||||
| During 2010 | ||||||||||||||||||||||
| $90 | 6,686 | 4.56 | $ | 90 | 6,686 | $ | 90 | |||||||||||||||
| During 2009 | ||||||||||||||||||||||
| $90 | 3,447 | 3.99 | $ | 90 | 3,447 | $ | 90 | |||||||||||||||
Stock options – Management has valued the options at their date of grant utilizing the Black Scholes Option Pricing Model. Accordingly, the fair value of the underlying shares was determined based on recent transactions by the Company to sell shares to third parties and other factors determined by management to be relevant to the valuation of such shares. The excepted volatility was calculated using the historical volatility of a similar public entity in the industry. In making this determination and finding another similar company, the Company considered the industry, stage of life cycle, size and financial leverage of such other entities. Based on the development stage of the Company, similar companies with enough historical data are not available. The Company was able to find one entity that met the industry criterion and as a result has based its expected volatility off this Company’s historical stock prices for a period similar to the expected term of the option. The risk –free interest rate is based on the implied yield available on U.S. Treasury issues with an equivalent term approximating the expected life of the options depending on the date of the grant and expected life of the options. The expected life of options used was based on the contractual life of the option granted.
The Company determined the expected dividend rate based on the assumption and expectation that earnings generated from operations are not expected to be adequate to allow for the payment of dividends in the near future. The following weighted-average assumptions were utilized in the fair value calculations for options granted:
| Expected life | 3 – 5 years | |
| Stock volatility | 100% | |
| Expected dividends | None | |
| Risk-free interest rate | 2% |
The Company had 800 options outstanding to directors of the company at $90 per share as of December 31, 2010, 600 of which were vested as of December 31, 2010. These options are exercisable through 2015. The Company recognized $37,800 of compensation expense related to the options for the year ended December 31, 2010.
126
NOTE 7 - INCOME TAXES (RESTATED)
The provision (benefit) for income taxes consists of the following for the year ended December 31:
| 2010 | 2009 | |||||||
| Current | $ | 4,304 | $ | 1,224 | ||||
| Deferred | — | — | ||||||
| $ | 4,304 | $ | 1,224 | |||||
The Company maintains a deferred tax asset in the amount of the deferred tax liability for the unrealized holding gain on its available-for-sale securities. A valuation allowance for the remaining amount of the net deferred tax assets has been recorded as it is more likely than not that these benefits will not be realized through future operations. The change in valuation allowance has been recorded to other comprehensive income as it is associated with the deferred tax recorded for available-for-sale securities.
Deferred tax assets consist of the following as of December 31, 2010 and 2009:
| 2010 | 2009 | |||||||
| Net operating loss carryforward | $ | 4,412,000 | $ | 2,850,000 | ||||
| General business tax credit | 728,000 | 728,000 | ||||||
| 5,140,000 | 3,578,000 | |||||||
| Valuation allowance | (4,922,971 | ) | (3,578,000 | ) | ||||
| $ | 217,029 | $ | — | |||||
Deferred tax liabilities consist of the following as of December 31, 2010 and 2009:
| 2010 | 2009 | |||||||
| Unrealized appreciation on available-for-sale securities | $ | 217,029 | $ | — | ||||
During 2010 and 2009, the valuation allowance increased by $1,344,971 and $1,068,000, respectively.
As of December 31, 2010 and 2009, the Company had net operating loss carryforwards (“NOL”) for federal and state reporting purposes of approximately $11,000,000 and $8,400,000, respectively, which expire in various years through 2030. The Federal and state tax codes provide for restrictive limitations on the annual utilization of NOLs to offset taxable income when the stock ownership of a company significantly changes, as defined. As of December 31, 2010 and 2009, the Company has general business tax credits of $728,000 and $728,000, respectively, for federal tax purposes. The tax credits are available to offset future tax liabilities, if any, through 2019.
NOTE 8 – COMMITMENTS AND CONTINGENCIES
Operating leases – The Company leases its office space under operating leases from unrelated entities. The Company leases approximately 4,540 square feet of office space at a base rent of $5,552 per month. This lease, which expires on May 31, 2011, was extended by the parties for an additional term beginning on June 1, 2011 and expiring on May 31, 2012. During the extension period, the monthly rent will be $4,994. In addition, the Company leases two office suites at 3870 Del Amo Boulevard, Torrance California: Suite 506 (approximately 1,400 square feet) at a base rent of $1,610 per month plus; and Suite 507 (approximately 1,300 square feet) at a base rent of $1,690 per month. The lease for Suite 506 will expire on August 19, 2011; the lease for Suite 507 will expire on February 28, 2013. Approximately 490 square feet of Suite 506 and 480 square feet of Suite 507 are currently subleased to an unaffiliated entity on a month to month basis. The Company does not expect to experience any difficulties in renewing its leases, or finding additional or replacement office and warehouse space, at their current or more favorable rates.
The rent expense during the year ended December 31, 2010 and 2009 amounted to $97,701 and $75,562 respectively.
Cardinal Health Specialty Pharmacy Services is contracted to distribute NutreStore to other wholesale distributors and some independent pharmacies since April 2008. For its service, Emmaus Medical pays monthly commercialization management fee of $7,000, $5,000 with discount. The discount was given based on the low volume of activity. The discount was approved for continuation in June 2011 and will be reviewed in six months to determine whether it will be extended again.
Future minimum lease payments under the agreement are as follows:
| 2011 | $ | 60,920 | ||
| 2012 | 20,280 | |||
| 2013 | 20,280 | |||
| $ | 101,480 |
127
NOTE 9 – RELATED PARTY TRANSACTIONS
As of December 31, 2010, the Company has been loaned a total of $890,919 by shareholders. The debt is unsecured and carries interest rates from 0% to 6.5%. $430,919 of the loans is convertible to common stock at $90 per share. Interest on 0% loans was imputed at the incremental borrowing rate of 6% per annum.
NOTE 10 – NET LOSS PER SHARE (RESTATED)
The “Net loss per share” is disclosed in Note 2 above. Following are the numerators and denominators for the net loss per share:
| 2010 | 2009 | |||||||
| Numerator for the net loss per share: | ||||||||
| Net loss | $ | (3,757,370 | ) | $ | (2,567,807 | ) | ||
| Denominator for the net loss per share: | ||||||||
| Weighted average shares | 666,813 | 638,068 | ||||||
| $ | (5.63 | ) | $ | (4.02 | ) | |||
NOTE 11 – COMMON STOCK TRANSACTIONS (RESTATED)
The Company engaged in the following stock transactions for the period from inception (December 20, 2000) through December 31, 2010.
| Date | Shares | $/Share | Common stock | Additional Paid-in Capital | Accumulated Other Comprehensive Income | Deficit Accumulated during Development Stage | Total | ||||||||||||||||||
| Balance, December 31, 2000 | 425,000 | 0.02 | 425 | 9,175 | $ | — | $ | — | 9,600 | ||||||||||||||||
| Net loss | — | — | — | — | (21,942 | ) | (21,942 | ) | |||||||||||||||||
| Balance, December 31, 2001 | 425,000 | — | 425 | 9,175 | — | (21,942 | ) | (12,342 | ) | ||||||||||||||||
| Net loss | — | — | — | — | (12,464 | ) | (12,464 | ) | |||||||||||||||||
| Balance, December 31, 2002 | 425,000 | — | 425 | 9,175 | — | (34,406 | ) | (24,806 | ) | ||||||||||||||||
| Constructive distribution of retained loss to Additional Paid-in Capita l | — | — | (34,406 | ) | — | 34,406 | — | ||||||||||||||||||
128
| Date | Shares | $/Share | Common stock | Additional Paid-in Capital | Accumulated Other Comprehensive Income | Deficit Accumulated during Development Stage | Total | ||||||||||||||||
| Common stock issued | 25,000 | 25 | 249,975 | 250,000 | |||||||||||||||||||
| 9/25/03 | 1,000 | 10 | 1 | 9,999 | 10,000 | ||||||||||||||||||
| 9/25/03 | 500 | 10 | 1 | 4,999 | 5,000 | ||||||||||||||||||
| 9/25/03 | 500 | 10 | 1 | 4,999 | 5,000 | ||||||||||||||||||
| 9/25/03 | 500 | 10 | 1 | 4,999 | 5,000 | ||||||||||||||||||
| 9/25/03 | 3,500 | 10 | 4 | 34,996 | 35,000 | ||||||||||||||||||
| 9/25/03 | 2,000 | 10 | 2 | 19,998 | 20,000 | ||||||||||||||||||
| 9/25/03 | 2,500 | 10 | 3 | 24,997 | 25,000 | ||||||||||||||||||
| 9/28/03 | 500 | 10 | 1 | 4,999 | 5,000 | ||||||||||||||||||
| 9/28/03 | 500 | 10 | 1 | 4,999 | 5,000 | ||||||||||||||||||
| 9/28/03 | 500 | 10 | 1 | 4,999 | 5,000 | ||||||||||||||||||
| 9/29/03 | 1,000 | 10 | 1 | 9,999 | 10,000 | ||||||||||||||||||
| 9/30/03 | 6,000 | 10 | 6 | 59,994 | 60,000 | ||||||||||||||||||
| 10/10/03 | 1,000 | 10 | 1 | 9,999 | 10,000 | ||||||||||||||||||
| 10/10/03 | 1,000 | 10 | 1 | 9,999 | 10,000 | ||||||||||||||||||
| 10/10/03 | 1,000 | 10 | 1 | 9,999 | 10,000 | ||||||||||||||||||
| 10/10/03 | 1,000 | 10 | 1 | 9,999 | 10,000 | ||||||||||||||||||
| 10/10/03 | 1,000 | 10 | 1 | 9,999 | 10,000 | ||||||||||||||||||
| 10/10/03 | 1,000 | 10 | 1 | 9,999 | 10,000 | ||||||||||||||||||
| Net loss | — | — | — | ��� | (97,481 | ) | (97,481 | ) | |||||||||||||||
| Balance, December 31, 2003 | 450,000 | 450 | 224,744 | — | (97,481 | ) | 127,713 | ||||||||||||||||
| Common stock issued | 54,800 | 638,000 | |||||||||||||||||||||
| 4/21/04 | 5,000 | 10 | 5 | 49,995 | 50,000 | ||||||||||||||||||
| 4/21/04 | 5,000 | 10 | 5 | 49,995 | 50,000 | ||||||||||||||||||
| 4/21/04 | 5,000 | 10 | 5 | 49,995 | 50,000 | ||||||||||||||||||
| 4/21/04 | 5,000 | 10 | 5 | 49,995 | 50,000 | ||||||||||||||||||
| 4/21/04 | 2,500 | 10 | 2 | 24,998 | 25,000 | ||||||||||||||||||
| 4/21/04 | 2,500 | 10 | 2 | 24,998 | 25,000 | ||||||||||||||||||
| 4/21/04 | 1,250 | 10 | 1 | 12,499 | 12,500 | ||||||||||||||||||
| 6/22/04 | 5,000 | 10 | 5 | 49,995 | 50,000 | ||||||||||||||||||
| 6/22/04 | 3,000 | 10 | 3 | 29,997 | 30,000 | ||||||||||||||||||
| 6/22/04 | 1,400 | 10 | 1 | 13,999 | 14,000 | ||||||||||||||||||
| 4/24/04 | 2,500 | 10 | 3 | 24,997 | 25,000 | ||||||||||||||||||
| 6/22/04 | 2,500 | 10 | 3 | 24,997 | 25,000 | ||||||||||||||||||
| 7/6/04 | 550 | 10 | — | 5,500 | 5,500 | ||||||||||||||||||
| 7/6/04 | 550 | 10 | 1 | 5,499 | 5,500 | ||||||||||||||||||
| 9/21/04 | 3,000 | 2 | 3 | 4,997 | 5,000 |
129
| Date | Shares | $/Share | Common stock | Additional Paid-in Capital | Accumulated Other Comprehensive Income | Deficit Accumulated during Development Stage | Total | |||||||||||||||||
| 4/21/04 | 1,000 | 10 | 1 | 9,999 | 10,000 | |||||||||||||||||||
| 6/22/04 | 1,250 | 10 | 1 | 12,499 | 12,500 | |||||||||||||||||||
| 6/22/04 | 5,000 | 10 | 5 | 49,995 | 50,000 | |||||||||||||||||||
| 12/28/05 | 1,500 | 60 | 2 | 89,998 | A | 90,000 | ||||||||||||||||||
| 4/4/05 | 500 | 10 | 1 | 4,999 | A | 5,000 | ||||||||||||||||||
| 1/31/05 | 500 | 60 | 1 | 29,999 | A | 30,000 | ||||||||||||||||||
| 2/17/05 | 200 | 60 | — | 12,000 | A | 12,000 | ||||||||||||||||||
| 2/24/05 | 100 | 60 | — | 6,000 | A | 6,000 | ||||||||||||||||||
| (Subscriptions Receivable) Collections | 10,075 | 10,075 | ||||||||||||||||||||||
| Net loss | — | — | — | — | (624,936 | ) | (624,936 | ) | ||||||||||||||||
| Balance, December 31, 2004 | 504,800 | — | 505 | 872,764 | — | (722,417 | ) | 150,852 | ||||||||||||||||
| Common stock issued | 13,519 | 811,626 | ||||||||||||||||||||||
| 1/31/05 | 1,667 | 60 | 2 | 99,998 | 100,000 | |||||||||||||||||||
| 7/25/05 | 100 | 60 | — | 6,000 | 6,000 | |||||||||||||||||||
| 7/25/05 | 100 | 60 | — | 6,000 | 6,000 | |||||||||||||||||||
| 7/25/05 | 100 | 60 | — | 6,000 | 6,000 | |||||||||||||||||||
| 7/25/05 | 100 | 60 | — | 6,000 | 6,000 | |||||||||||||||||||
| 10/21/05 | 1,000 | 60 | 1 | 59,999 | 60,000 | |||||||||||||||||||
| 12/21/05 | 400 | 60 | — | 24,000 | 24,000 | |||||||||||||||||||
| 7/2/07 | 168 | 60 | — | 10,080 | B | 10,080 | ||||||||||||||||||
| 5/9/08 | 9,793 | 60 | 10 | 587,536 | B | 587,546 | ||||||||||||||||||
| rounding | (9 | ) | ||||||||||||||||||||||
| 9/21/05 | 100 | 60 | — | 6,000 | B | 6,000 | ||||||||||||||||||
| (Subscriptions Receivable) Collections | (483,341 | ) | (483,341 | ) | ||||||||||||||||||||
| Net loss | — | — | — | — | (668,091 | ) | (668,091 | ) | ||||||||||||||||
| Balance, December 31, 2005 | 518,310 | — | 518 | 1,201,036 | — | (1,390,508 | ) | (188,954 | ) | |||||||||||||||
| Common stock issued | 17,751 | 1,062,460 | ||||||||||||||||||||||
| 7/25/05 | 150 | 60 | — | 9,000 | 9,000 |
130
| Date | Shares | $/Share | Common stock | Additional Paid-in Capital | Accumulated Other Comprehensive Income | Deficit Accumulated during Development Stage | Total | ||||||||||
| 12/16/05 | 500 | 60 | 1 | 29,999 | 30,000 | ||||||||||||
| 1/10/06 | 1,000 | 60 | 1 | 59,999 | 60,000 | ||||||||||||
| 1/6/06 | 500 | 60 | 1 | 29,999 | 30,000 | ||||||||||||
| 1/6/06 | 1,000 | 60 | 1 | 59,999 | 60,000 | ||||||||||||
| 1/5/06 | 1,000 | 60 | 1 | 59,999 | 60,000 | ||||||||||||
| 1/6/06 | 1,000 | 60 | 1 | 59,999 | 60,000 | ||||||||||||
| 1/6/06 | 167 | 60 | — | 10,000 | 10,000 | ||||||||||||
| 1/13/06 | 100 | 60 | — | 6,000 | 6,000 | ||||||||||||
| 2/28/06 | 100 | 60 | — | 6,000 | 6,000 | ||||||||||||
| 4/28/06 | 500 | 60 | 1 | 29,999 | 30,000 | ||||||||||||
| 4/28/06 | 500 | 60 | 1 | 29,999 | 30,000 | ||||||||||||
| 4/28/06 | 300 | 60 | — | 18,000 | 18,000 | ||||||||||||
| 4/28/06 | 100 | 60 | — | 6,000 | 6,000 | ||||||||||||
| 5/1/06 | 100 | 60 | — | 6,000 | 6,000 | ||||||||||||
| 5/9/06 | 500 | 60 | 1 | 29,999 | 30,000 | ||||||||||||
| 5/1/06 | 500 | 60 | 1 | 29,999 | 30,000 | ||||||||||||
| 6/15/06 | 100 | 60 | — | 6,000 | 6,000 | ||||||||||||
| 9/29/06 | 100 | 60 | — | 6,000 | 6,000 | ||||||||||||
| 10/17/06 | 500 | 60 | 1 | 29,999 | 30,000 | ||||||||||||
| 10/13/06 | 500 | 60 | 1 | 29,999 | 30,000 | ||||||||||||
| 10/19/06 | 500 | 60 | 1 | 29,999 | 30,000 | ||||||||||||
| 11/10/06 | 250 | 60 | — | 15,000 | 15,000 | ||||||||||||
| 11/10/06 | 150 | 60 | — | 9,000 | 9,000 | ||||||||||||
| 11/10/06 | 1,000 | 60 | 1 | 59,999 | 60,000 | ||||||||||||
| 11/22/06 | 500 | 60 | 1 | 29,999 | 30,000 | ||||||||||||
| 11/6/06 | 200 | 60 | — | 12,000 | 12,000 | ||||||||||||
| 11/6/06 | 500 | 60 | 1 | 29,999 | 30,000 | ||||||||||||
| 11/22/06 | 500 | 60 | 1 | 29,999 | 30,000 | ||||||||||||
| 11/22/06 | 250 | 60 | — | 15,000 | 15,000 | ||||||||||||
| 12/1/06 | 1,000 | 60 | 1 | 59,999 | 60,000 | ||||||||||||
| 11/27/06 | 100 | 60 | — | 6,000 | 6,000 | ||||||||||||
| 12/6/06 | 334 | 60 | — | 20,040 | 20,040 | ||||||||||||
| 12/6/06 | 150 | 60 | — | 9,000 | 9,000 | ||||||||||||
| 12/6/06 | 250 | 60 | — | 15,000 | 15,000 | ||||||||||||
| 12/18/06 | 500 | 60 | — | 29,999 | 30,000 | ||||||||||||
| 1/24/07 | 500 | 60 | — | 29,999 | 30,000 | ||||||||||||
| 4/10/07 | 500 | 60 | — | 29,999 | 30,000 | ||||||||||||
| 5/31/07 | 100 | 60 | — | 6,000 | 6,000 | ||||||||||||
| 5/29/08 | 500 | 60 | — | 29,999 | 30,000 | ||||||||||||
| 5/29/08 | 500 | 60 | — | 29,999 | 30,000 | ||||||||||||
| 3/31/08 | 150 | 43 | — | 6,420 | 6,420 | ||||||||||||
| 3/10/08 | 100 | 60 | — | 6,000 | 6,000 |
131
| Date | Shares | $/Share | Common stock | Additional Paid-in Capital | Accumulated Other Comprehensive Income | Deficit Accumulated during Development Stage | Total | ||||||||||||||||
| Rounding | (5 | ) | |||||||||||||||||||||
| (Subscriptions Receivable) Collections | (237,415 | ) | (237,415 | ) | |||||||||||||||||||
| Net loss | — | — | — | — | (759,962 | ) | (759,962 | ) | |||||||||||||||
| Balance, December 31, 2006 | 536,061 | 536 | 2,026,058 | — | (2,150,470 | ) | (123,876 | ) | |||||||||||||||
| Common stock issued | 45,587 | 2,680,720 | |||||||||||||||||||||
| 2/5/07 | 150 | 60 | — | 9,000 | 9,000 | ||||||||||||||||||
| 3/8/07 | 150 | 60 | — | 9,000 | 9,000 | ||||||||||||||||||
| 4/26/07 | 500 | 60 | 1 | 29,999 | 30,000 | ||||||||||||||||||
| 5/3/07 | 500 | 60 | 1 | 29,999 | 30,000 | ||||||||||||||||||
| 5/8/07 | 200 | 60 | — | 12,000 | 12,000 | ||||||||||||||||||
| 5/8/07 | 500 | 60 | 1 | 29,999 | 30,000 | ||||||||||||||||||
| 5/8/07 | 500 | 60 | 1 | 29,999 | 30,000 | ||||||||||||||||||
| 5/11/07 | 300 | 60 | — | 18,000 | 18,000 | ||||||||||||||||||
| 5/11/07 | 150 | 60 | — | 9,000 | 9,000 | ||||||||||||||||||
| 5/11/07 | 150 | 60 | — | 9,000 | 9,000 | ||||||||||||||||||
| 5/14/07 | 330 | 60 | — | 19,800 | 19,800 | ||||||||||||||||||
| 5/11/07 | 150 | 60 | — | 9,000 | 9,000 | ||||||||||||||||||
| 5/18/07 | 500 | 60 | 1 | 29,999 | 30,000 | ||||||||||||||||||
| 5/18/07 | 500 | 60 | 1 | 29,999 | 30,000 | ||||||||||||||||||
| 5/24/07 | 500 | 60 | 1 | 29,999 | 30,000 | ||||||||||||||||||
| 5/31/07 | 1,000 | 60 | 1 | 59,999 | 60,000 | ||||||||||||||||||
| 6/2/07 | 1,668 | 60 | 2 | 100,078 | 100,080 | ||||||||||||||||||
| 6/1/07 | 1,000 | 60 | 1 | 59,999 | 60,000 | ||||||||||||||||||
| 6/1/07 | 1,000 | 60 | 1 | 59,999 | 60,000 | ||||||||||||||||||
| 6/6/07 | 1,000 | 60 | 1 | 59,999 | 60,000 | ||||||||||||||||||
| 6/4/07 | 167 | 60 | — | 10,020 | 10,020 | ||||||||||||||||||
| 6/28/07 | 1,500 | 60 | 2 | 89,998 | 90,000 | ||||||||||||||||||
| 6/15/07 | 200 | 60 | — | 12,000 | 12,000 | ||||||||||||||||||
| 6/14/07 | 250 | 60 | — | 15,000 | 15,000 | ||||||||||||||||||
| 6/15/07 | 150 | 60 | — | 9,000 | 9,000 | ||||||||||||||||||
| 6/15/07 | 200 | 60 | — | 12,000 | 12,000 | ||||||||||||||||||
| 6/15/07 | 500 | 60 | 1 | 29,999 | 30,000 | ||||||||||||||||||
| 6/20/07 | 500 | 60 | 1 | 29,999 | 30,000 | ||||||||||||||||||
| 6/20/07 | 500 | 60 | 1 | 29,999 | 30,000 |
132
| Date | Shares | $/Share | Common stock | Additional Paid-in Capital | Accumulated Other Comprehensive Income | Deficit Accumulated during Development Stage | Total | |||||||||||||
| 6/28/07 | 335 | 60 | — | 20,100 | 20,100 | |||||||||||||||
| 6/20/07 | 500 | 60 | 1 | 29,999 | 30,000 | |||||||||||||||
| 6/20/07 | 500 | 60 | 1 | 29,999 | 30,000 | |||||||||||||||
| 6/29/07 | 500 | 60 | 1 | 29,999 | 30,000 | |||||||||||||||
| 7/2/07 | 500 | 60 | 1 | 29,999 | 30,000 | |||||||||||||||
| 7/2/07 | 200 | 60 | — | 12,000 | 12,000 | |||||||||||||||
| 7/2/07 | 1,500 | 60 | 2 | 89,998 | 90,000 | |||||||||||||||
| 7/2/07 | 50 | 60 | — | 3,000 | 3,000 | |||||||||||||||
| 7/2/07 | 250 | 60 | — | 15,000 | 15,000 | |||||||||||||||
| 7/11/07 | 1,000 | 60 | 1 | 59,999 | 60,000 | |||||||||||||||
| 7/6/07 | 1,000 | 60 | 1 | 59,999 | 60,000 | |||||||||||||||
| 7/11/07 | 1,000 | 60 | 1 | 59,999 | 60,000 | |||||||||||||||
| 7/17/07 | 800 | 60 | 1 | 47,999 | 48,000 | |||||||||||||||
| 7/16/07 | 5,000 | 60 | 5 | 299,995 | 300,000 | |||||||||||||||
| 7/11/07 | 700 | 60 | 1 | 41,999 | 42,000 | |||||||||||||||
| 7/18/07 | 900 | 60 | 1 | 53,999 | 54,000 | |||||||||||||||
| 7/21/07 | 500 | 60 | 1 | 29,999 | 30,000 | |||||||||||||||
| 7/23/07 | 400 | 60 | — | 24,000 | 24,000 | |||||||||||||||
| 7/24/07 | 250 | 60 | — | 15,000 | 15,000 | |||||||||||||||
| 7/26/07 | 1,000 | 60 | 1 | 59,999 | 60,000 | |||||||||||||||
| 7/23/07 | 400 | 60 | — | 24,000 | 24,000 | |||||||||||||||
| 7/27/07 | 1,000 | 60 | 1 | 59,999 | 60,000 | |||||||||||||||
| 7/27/07 | 500 | 60 | 1 | 29,999 | 30,000 | |||||||||||||||
| 7/30/07 | 500 | 60 | 1 | 29,999 | 30,000 | |||||||||||||||
| 7/30/07 | 500 | 60 | 1 | 29,999 | 30,000 | |||||||||||||||
| 7/31/07 | 900 | 60 | 1 | 53,999 | 54,000 | |||||||||||||||
| 8/7/07 | 100 | 60 | — | 6,000 | 6,000 | |||||||||||||||
| 8/25/07 | 1,000 | 60 | 1 | 59,999 | 60,000 | |||||||||||||||
| 8/25/07 | 500 | 60 | 1 | 29,999 | 30,000 | |||||||||||||||
| 8/30/07 | 500 | 60 | — | 29,999 | 30,000 | |||||||||||||||
| 10/3/07 | 500 | 60 | — | 29,999 | 30,000 | |||||||||||||||
| 10/17/07 | 350 | 61 | — | 21,500 | 21,500 | |||||||||||||||
| 11/5/07 | 1,100 | 60 | 1 | 65,999 | 66,000 | |||||||||||||||
| 10/22/07 | 167 | 60 | — | 10,020 | 10,020 | |||||||||||||||
| 11/28/07 | 500 | 60 | — | 29,999 | 30,000 | |||||||||||||||
| 11/15/07 | 180 | 60 | — | 10,800 | 10,800 | |||||||||||||||
| 11/15/07 | 180 | 60 | — | 10,800 | 10,800 | |||||||||||||||
| 12/15/07 | 500 | 60 | — | 29,999 | 30,000 | |||||||||||||||
| 12/5/07 | 1,000 | 5 | 1 | 4,999 | 5,000 | |||||||||||||||
| 12/4/07 | 100 | 60 | — | 6,000 | 6,000 | |||||||||||||||
| 12/4/07 | 100 | 60 | — | 6,000 | 6,000 | |||||||||||||||
| 12/12/07 | 500 | 60 | — | 29,999 | 30,000 |
133
| Date | Shares | $/Share | Common stock | Additional Paid-in Capital | Accumulated Other Comprehensive Income | Deficit Accumulated during Development Stage | Total | ||||||||||||||||
| 12/18/07 | 500 | 60 | — | 29,999 | 30,000 | ||||||||||||||||||
| 12/21/07 | 810 | 60 | — | 48,600 | 48,600 | ||||||||||||||||||
| 12/26/07 | 100 | 60 | — | 6,000 | 6,000 | ||||||||||||||||||
| 12/26/07 | 500 | 60 | — | 29,999 | 30,000 | ||||||||||||||||||
| 12/28/07 | 500 | 60 | — | 29,999 | 30,000 | ||||||||||||||||||
| 12/29/07 | 500 | 60 | — | 29,999 | 30,000 | ||||||||||||||||||
| (Subscriptions Receivable) Collections | 53,149 | 53,149 | |||||||||||||||||||||
| Rounding | (9 | ) | |||||||||||||||||||||
| Net loss | — | — | — | — | (1,282,212 | ) | (1,282,212 | ) | |||||||||||||||
| Balance, December 31, 2007 | 581,648 | — | 582 | 4,759,872 | — | (3,432,682 | ) | 1,327,772 | |||||||||||||||
| Common stock issued | 41,612 | 2,496,720 | |||||||||||||||||||||
| 1/4/08 | 500 | 60 | — | 29,999 | 30,000 | ||||||||||||||||||
| 1/4/08 | 500 | 60 | — | 29,999 | 30,000 | ||||||||||||||||||
| 1/4/08 | 500 | 60 | — | 29,999 | 30,000 | ||||||||||||||||||
| 1/4/08 | 500 | 60 | — | 29,999 | 30,000 | ||||||||||||||||||
| 1/9/08 | 600 | 60 | 1 | 35,999 | 36,000 | ||||||||||||||||||
| 1/9/08 | 1,000 | 60 | 1 | 59,999 | 60,000 | ||||||||||||||||||
| 1/9/08 | 500 | 60 | — | 29,999 | 30,000 | ||||||||||||||||||
| 1/10/08 | 500 | 60 | — | 29,999 | 30,000 | ||||||||||||||||||
| 1/11/08 | 500 | 60 | — | 29,999 | 30,000 | ||||||||||||||||||
| 1/11/08 | 500 | 60 | — | 29,999 | 30,000 | ||||||||||||||||||
| 1/15/08 | 500 | 60 | — | 29,999 | 30,000 | ||||||||||||||||||
| 1/15/08 | 5,000 | 60 | 5 | 299,995 | 300,000 | ||||||||||||||||||
| 1/11/08 | 500 | 60 | — | 29,999 | 30,000 | ||||||||||||||||||
| 1/18/08 | 500 | 60 | — | 29,999 | 30,000 | ||||||||||||||||||
| 1/15/08 | 500 | 60 | — | 29,999 | 30,000 | ||||||||||||||||||
| 1/22/08 | 500 | 60 | — | 29,999 | 30,000 | ||||||||||||||||||
| 1/24/08 | 800 | 60 | 1 | 47,999 | 48,000 | ||||||||||||||||||
| 1/24/08 | 500 | 60 | — | 29,999 | 30,000 | ||||||||||||||||||
| 1/24/08 | 500 | 60 | — | 29,999 | 30,000 | ||||||||||||||||||
| 1/24/08 | 500 | 60 | — | 29,999 | 30,000 | ||||||||||||||||||
| 1/28/08 | 500 | 60 | — | 29,999 | 30,000 | ||||||||||||||||||
| 1/29/08 | 800 | 60 | 1 | 47,999 | 48,000 | ||||||||||||||||||
| 1/28/08 | 500 | 60 | 1 | 29,999 | 30,000 | ||||||||||||||||||
| 1/30/08 | 500 | 60 | 1 | 29,999 | 30,000 | ||||||||||||||||||
| 2/7/08 | 600 | 60 | 1 | 35,999 | 36,000 | ||||||||||||||||||
| 2/1/08 | 500 | 60 | 1 | 29,999 | 30,000 |
134
| Date | Shares | $/Share | Common stock | Additional Paid-in Capital | Accumulated Other Comprehensive Income | Deficit Accumulated during Development Stage | Total | ||||||||||||||||
| 2/1/08 | 650 | 60 | 1 | 38,999 | 39,000 | ||||||||||||||||||
| 2/7/08 | 2,500 | 60 | 3 | 149,997 | 150,000 | ||||||||||||||||||
| 2/1/08 | 3,333 | 60 | 3 | 199,977 | 199,980 | ||||||||||||||||||
| 2/25/08 | 500 | 60 | 1 | 29,999 | 30,000 | ||||||||||||||||||
| 2/25/08 | 800 | 60 | 1 | 47,999 | 48,000 | ||||||||||||||||||
| 2/27/08 | 3,334 | 60 | 3 | 200,037 | 200,040 | ||||||||||||||||||
| 3/1/08 | 650 | 60 | 1 | 38,999 | 39,000 | ||||||||||||||||||
| 3/1/08 | 1,000 | 60 | 1 | 59,999 | 60,000 | ||||||||||||||||||
| 2/29/08 | 200 | 60 | 0 | 12,000 | 12,000 | ||||||||||||||||||
| 2/29/08 | 100 | 60 | 0 | 6,000 | 6,000 | ||||||||||||||||||
| 3/3/08 | 500 | 60 | 1 | 29,999 | 30,000 | ||||||||||||||||||
| 2/29/08 | 100 | 60 | 0 | 6,000 | 6,000 | ||||||||||||||||||
| 3/4/08 | 100 | 60 | 0 | 6,000 | 6,000 | ||||||||||||||||||
| 3/4/08 | 350 | 60 | 0 | 21,000 | 21,000 | ||||||||||||||||||
| 3/5/08 | 500 | 60 | 1 | 29,999 | 30,000 | ||||||||||||||||||
| 3/13/08 | 500 | 60 | 1 | 29,999 | 30,000 | ||||||||||||||||||
| 3/10/08 | 500 | 60 | 1 | 29,999 | 30,000 | ||||||||||||||||||
| 3/31/08 | 20 | 60 | 0 | 1,200 | 1,200 | ||||||||||||||||||
| 3/25/08 | 500 | 60 | 1 | 29,999 | 30,000 | ||||||||||||||||||
| 3/31/08 | 500 | 60 | 1 | 29,999 | 30,000 | ||||||||||||||||||
| 4/2/08 | 500 | 60 | 1 | 29,999 | 30,000 | ||||||||||||||||||
| 4/2/08 | 175 | 60 | 0 | 10,500 | 10,500 | ||||||||||||||||||
| 4/7/08 | 4,000 | 60 | 4 | 239,996 | 240,000 | ||||||||||||||||||
| 4/11/08 | 1,000 | 60 | 1 | 59,999 | 60,000 | ||||||||||||||||||
| 4/9/08 | 500 | 60 | 1 | 29,999 | 30,000 | ||||||||||||||||||
| (Subscriptions Receivable) Collections | 893,988 | 893,988 | |||||||||||||||||||||
| Rounding | 1 | (17 | ) | ||||||||||||||||||||
| Net loss | — | — | — | — | (2,993,777 | ) | (2,993,777 | ) | |||||||||||||||
| Balance, December 31, 2008 | 623,261 | 623 | 8,150,522 | — | (6,426,459 | ) | 1,724,686 | ||||||||||||||||
| Common stock issued | 28,979 | 2,349,940 | |||||||||||||||||||||
| 3/28/08 | 2,000 | 60 | 2 | 119,998 | 120,000 | ||||||||||||||||||
| 3/31/08 | 200 | 60 | 0 | 12,000 | 12,000 | ||||||||||||||||||
| 4/7/08 | 250 | 60 | 0 | 15,000 | 15,000 | ||||||||||||||||||
| 1/12/09 | 650 | 60 | 1 | 38,999 | 39,000 | ||||||||||||||||||
| 1/12/09 | 500 | 60 | — | 29,999 | 30,000 |
135
| Date | Shares | $/Share | Common stock | Additional Paid-in Capital | Accumulated Other Comprehensive Income | Deficit Accumulated during Development Stage | Total | |||||||||||||
| 1/12/09 | 834 | 60 | 1 | 49,999 | 50,000 | |||||||||||||||
| 1/12/09 | 4,167 | 60 | 4 | 249,996 | 250,000 | |||||||||||||||
| 3/30/09 | 300 | 90 | 0 | 27,000 | 27,000 | |||||||||||||||
| 3/16/09 | 200 | 90 | 0 | 18,000 | 18,000 | |||||||||||||||
| 3/16/09 | 400 | 90 | 0 | 36,000 | 36,000 | |||||||||||||||
| 3/18/09 | 250 | 90 | 0 | 22,500 | 22,500 | |||||||||||||||
| 5/11/09 | 250 | 90 | 0 | 22,500 | 22,500 | |||||||||||||||
| 5/15/09 | 250 | 90 | 0 | 22,500 | 22,500 | |||||||||||||||
| 5/19/09 | 300 | 90 | 0 | 27,000 | 27,000 | |||||||||||||||
| 5/19/09 | 250 | 90 | 0 | 22,500 | 22,500 | |||||||||||||||
| 5/19/09 | 250 | 90 | 0 | 22,500 | 22,500 | |||||||||||||||
| 5/19/09 | 250 | 90 | 0 | 22,500 | 22,500 | |||||||||||||||
| 5/20/09 | 250 | 90 | 0 | 22,500 | 22,500 | |||||||||||||||
| 5/22/09 | 250 | 90 | 0 | 22,500 | 22,500 | |||||||||||||||
| 6/30/09 | 300 | 90 | 0 | 27,000 | 27,000 | |||||||||||||||
| 6/30/09 | 250 | 90 | 0 | 22,500 | 22,500 | |||||||||||||||
| 6/25/09 | 250 | 90 | 0 | 22,500 | 22,500 | |||||||||||||||
| 6/24/09 | 250 | 90 | 0 | 22,500 | 22,500 | |||||||||||||||
| 6/18/09 | 700 | 90 | 1 | 62,999 | 63,000 | |||||||||||||||
| 5/21/09 | 250 | 90 | 0 | 22,500 | 22,500 | |||||||||||||||
| 5/20/09 | 250 | 90 | 0 | 22,500 | 22,500 | |||||||||||||||
| 6/16/09 | 334 | 90 | 0 | 30,060 | 30,060 | |||||||||||||||
| 6/29/09 | 1,500 | 90 | 2 | 134,998 | 135,000 | |||||||||||||||
| 7/10/09 | 250 | 90 | 0 | 22,500 | 22,500 | |||||||||||||||
| 6/25/09 | 250 | 90 | 0 | 22,500 | 22,500 | |||||||||||||||
| 7/2/09 | 500 | 90 | 1 | 44,999 | 45,000 | |||||||||||||||
| 7/2/09 | 250 | 90 | 0 | 22,500 | 22,500 | |||||||||||||||
| 7/27/09 | 250 | 90 | 0 | 22,500 | 22,500 | |||||||||||||||
| 7/23/09 | 250 | 90 | 0 | 22,500 | 22,500 | |||||||||||||||
| 7/29/09 | 350 | 90 | 0 | 31,500 | 31,500 | |||||||||||||||
| 7/31/09 | 1,050 | 90 | 1 | 94,499 | 94,500 | |||||||||||||||
| 8/5/09 | 750 | 90 | — | 67,500 | 67,500 | |||||||||||||||
| 8/27/09 | 500 | 90 | — | 44,999 | 45,000 | |||||||||||||||
| 8/7/09 | 250 | 90 | 0 | 22,500 | 22,500 | |||||||||||||||
| 8/21/09 | 700 | 90 | — | 63,000 | 63,000 | |||||||||||||||
| 9/4/09 | 950 | 90 | 1 | 85,499 | 85,500 | |||||||||||||||
| 9/4/09 | 200 | 90 | 0 | 18,000 | 18,000 | |||||||||||||||
| 9/8/09 | 250 | 90 | 0 | 22,500 | 22,500 | |||||||||||||||
| 9/18/09 | 400 | 90 | 0 | 36,000 | 36,000 | |||||||||||||||
| 7/15/09 | 250 | 90 | 0 | 22,500 | 22,500 | |||||||||||||||
| 7/17/09 | 250 | 90 | 0 | 22,500 | 22,500 | |||||||||||||||
| 7/27/09 | 250 | 90 | 0 | 22,500 | 22,500 |
136
| Date | Shares | $/Share | Common stock | Additional Paid-in Capital | Accumulated Other Comprehensive Income | Deficit Accumulated during Development Stage | Total | ||||||||||||||||
| 9/25/09 | 250 | 90 | 0 | 22,500 | 22,500 | ||||||||||||||||||
| 1/10/10 | 500 | 90 | 1 | 44,999 | 45,000 | ||||||||||||||||||
| 12/18/09 | 3,000 | 90 | 3 | 269,997 | 270,000 | ||||||||||||||||||
| 12/11/09 | 332 | 90 | 0 | 29,880 | 29,880 | ||||||||||||||||||
| 11/25/09 | 1,112 | 90 | 1 | 99,999 | 100,000 | ||||||||||||||||||
| (Subscriptions Receivable) Collections | (111,015 | ) | (111,015 | ) | |||||||||||||||||||
| Rounding | |||||||||||||||||||||||
| Conversion of notes payable to Common stock | |||||||||||||||||||||||
| Total | |||||||||||||||||||||||
| Net loss | — | — | — | — | (2,567,807 | ) | (2,567,807 | ) | |||||||||||||||
| Balance, December 31, 2009 | 652,240 | 652 | 10,389,417 | — | (8,994,266 | ) | 1,395,804 | ||||||||||||||||
| Common stock issued | Total | 23,921 | 144 | 3,456,342 | |||||||||||||||||||
| 11/16/09 | 400 | 90 | 0 | 36,000 | 36,000 | ||||||||||||||||||
| 1/10/10 | 500 | 90 | 1 | 44,999 | 45,000 | ||||||||||||||||||
| 2/10/10 | 1,200 | 90 | 1 | 107,999 | 108,000 | ||||||||||||||||||
| 2/9/10 | 350 | 90 | 0 | 31,500 | 31,500 | ||||||||||||||||||
| 2/9/10 | 28 | — | 0 | (0 | ) | ||||||||||||||||||
| 3/23/10 | 1,000 | 90 | — | 90,000 | 90,000 | ||||||||||||||||||
| 3/23/10 | 1,750 | 90 | 2 | 157,498 | 157,500 | ||||||||||||||||||
| 3/23/10 | 2,778 | 90 | 3 | 250,017 | 250,020 | ||||||||||||||||||
| 5/12/10 | 350 | 90 | 0 | 31,500 | 31,500 | ||||||||||||||||||
| 5/12/10 | 350 | 90 | 0 | 31,500 | 31,500 | ||||||||||||||||||
| 5/12/10 | 350 | 90 | 0 | 31,500 | 31,500 | ||||||||||||||||||
| 4/12/10 | 2,000 | 90 | 2 | 179,998 | 180,000 | ||||||||||||||||||
| 5/19/10 | 350 | 90 | 0 | 31,500 | 31,500 | ||||||||||||||||||
| 5/25/10 | 350 | 90 | 0 | 31,500 | 31,500 | ||||||||||||||||||
| 7/15/10 | 3,600 | 90 | 4 | 323,996 | 324,000 | ||||||||||||||||||
| 7/15/10 | 1,111 | 90 | 1 | 99,999 | 100,000 | ||||||||||||||||||
| 7/12/10 | 290 | 90 | 0 | 26,100 | 26,100 | ||||||||||||||||||
| 7/14/10 | 350 | 90 | 0 | 31,500 | 31,500 | ||||||||||||||||||
| 7/12/10 | 250 | 90 | 0 | 22,500 | 22,500 | ||||||||||||||||||
| 10/18/10 | 1,300 | 90 | 1 | 116,999 | 117,000 | ||||||||||||||||||
| 10/20/10 | 350 | 90 | 0 | 31,500 | 31,500 |
137
| Date | Shares | $/Share | Common stock | Additional Paid-in Capital | Accumulated Other Comprehensive Income | Deficit Accumulated during Development Stage | Total | ||||||||||||||||
| 10/20/10 | 350 | 90 | 0 | 31,500 | 31,500 | ||||||||||||||||||
| 10/27/10 | 350 | 90 | 0 | 31,500 | 31,500 | ||||||||||||||||||
| 11/10/10 | 1,112 | 90 | 1 | 100,079 | 100,080 | ||||||||||||||||||
| 11/16/10 | 400 | 90 | 0 | 36,000 | 36,000 | ||||||||||||||||||
| 11/17/10 | 350 | 90 | 0 | 31,500 | 31,500 | ||||||||||||||||||
| 11/23/10 | 350 | 90 | 0 | 31,500 | 31,500 | ||||||||||||||||||
| 11/23/10 | 556 | 90 | 1 | 50,039 | 50,040 | ||||||||||||||||||
| 11/24/10 | 250 | 90 | 0 | 22,502 | 22,502 | ||||||||||||||||||
| 11/23/10 | 500 | 90 | 1 | 44,999 | 45,000 | ||||||||||||||||||
| 11/26/10 | 140 | 90 | 0 | 12,600 | 12,600 | ||||||||||||||||||
| 11/9/10 | 556 | 90 | 1 | 49,999 | 50,000 | ||||||||||||||||||
| Conversion of notes payable to Common stock | |||||||||||||||||||||||
| Total | 14,514 | — | |||||||||||||||||||||
| 11/9/10 | 1,667 | 90 | 1 | 149,999 | 150,000 | ||||||||||||||||||
| 11/9/10 | 1,667 | 90 | 1 | 149,999 | 150,000 | ||||||||||||||||||
| 11/11/10 | 2,500 | 90 | 3 | 224,997 | 225,000 | ||||||||||||||||||
| 11/12/10 | 1,112 | 90 | 1 | 99,999 | 100,000 | ||||||||||||||||||
| 11/22/10 | 4,445 | 90 | 4 | 399,996 | 400,000 | ||||||||||||||||||
| 11/22/10 | 1,667 | 90 | 2 | 149,998 | 150,000 | ||||||||||||||||||
| 11/23/10 | 500 | 90 | 1 | 44,999 | 45,000 | ||||||||||||||||||
| 11/30/10 | 556 | 90 | 1 | 49,999 | 50,000 | ||||||||||||||||||
| 12/3/10 | 400 | 90 | 0 | 36,000 | 36,000 | ||||||||||||||||||
| (Subscriptions Receivable) Collections | (26,048 | ) | (26,048 | ) | |||||||||||||||||||
| Rounding | 17 | ||||||||||||||||||||||
| Unrealized gain on securities available for sale | — | — | — | 542,573 | — | 542,573 | |||||||||||||||||
| Net loss | — | — | — | — | (3,757,370 | ) | (3,757,370 | ) | |||||||||||||||
| Balance, December 31, 2010 | 690,692 | 691 | 13,819,673 | 542,573 | (12,751,636 | ) | $ | 1,611,301 | |||||||||||||||
NOTE 12 – GEOGRAPHIC INFORMATION
In 2010 and 2009, the Company earned revenue from Japan in the amount of $71,504 and $33,166, respectively, which represented 52% in 2010 and 33% in 2009 of total revenues for the respective periods. The Company did not have any significant currency transaction or foreign translation adjustments during the periods ended December 31, 2010 and 2009.
138
NOTE 13– SUBSEQUENT EVENTS
Management evaluated subsequent events through September 28, 2011, the date the restated financial statements were re-issued.
Hope International Hospice, Inc., of which Dr. Niihara is the Chief Executive Officer, made a $200,000 loan to Emmaus on January 12, 2011, the promissory note is attached as Exhibit 10.19. The loan, which has a term of two years, bears interest at a rate of 8% per annum. Interest only payments are due quarterly. On January 12, 2011, Willis C. Lee made a two-year loan to Emmaus in the amount of $100,000, the promissory note is attached as Exhibit 10.18. The loan bears interest at a rate of 8% per annum. Interest only payments are due quarterly. This loan was repaid by the Company on June 3rd, 2011. The Company also repaid the $80,000 loan made by Dr. Niihara on April 23, 2009, the promissory note is attached as Exhibit 10.17. During the three months ended March 31, 2011, convertible note payable in the amount of $500,000 were issued. Immediately upon conversion of this note, the lender shall receive, without any consideration, warrants to acquire common stock of the Company in such number as equals to twenty-five percent of the number of shares acquired as a result of the conversion. These warrants will be exercisable by giving written notice to the Company. The Company has granted to the lender a security interest in 50% of the Company’s investment in marketable securities as specified in an agreement with the lender.
On April 8, 2011, Emmaus Medical entered into a Joint Research and Development Agreement (the “Research Agreement”) and an Individual Agreement (the “Individual Agreement”) with CellSeed, Inc. (“CellSeed”). Pursuant to the Research Agreement, the Company and CellSeed formed a relationship regarding the future research and development of cell sheet engineering regenerative medicine products (the “Products”), and the future commercialization of such Products. The parties will enter into individual agreements for each project or task conducted pursuant to the Research Agreement defining the details of such project. All intellectual property rights created in the course of the Research Agreement and any individual agreement will be owned jointly by the Company and CellSeed. In the event that either the Company or CellSeed becomes the owner of intellectual property rights related to the Products which was developed solely by its employees, then such party will grant a worldwide, perpetual, irrevocable, non-exclusive, royalty free, fully paid up, sub-licensable, transferable license of such rights to the other party. Pursuant to the Individual Agreement, CellSeed granted the Company an exclusive right to manufacture, sell, market and distribute Cultured Autologous Oral Mucosal Epithelial Cell-Sheets (“CAOMECS”) for the cornea in the United States. CellSeed shall disclose its accumulated information package (the “Package”) for the joint development of CAOMECS to Emmaus Medical. Pursuant to the Research Agreement, the Company agreed to pay CellSeed $8,500,000 (the “Research Agreement Payment”) within 30 days of the completion of all of the following: (i) the execution of the Research Agreement; (ii) the execution of the Individual Agreement; and (iii) CellSeed’s delivery of the Package to Emmaus. Pursuant to the Individual Agreement, the Company agreed to pay $1,500,000 to CellSeed (the “Individual Agreement Payment”) within 30 days of CellSeed’s delivery of the Package to the Company and a royalty to be agreed upon by the parties. Pursuant to the Addendum to the Research Agreement, CellSeed and the Company further acknowledged that all obligations of CellSeed corresponding to the Research Agreement Payment and the Individual Agreement Payment shall be fully discharged by CellSeed’s delivery of the Package, which delivery shall be documented by written confirmation of acceptance by the Company. The Company is obligated to make the Research Agreement Payment and the Individual Agreement Payment only after the Company’s provides its written confirmation of acceptance of the complete Package to CellSeed. The parties will determine the rate at which profits from the net sales of CAOMECS in the United States will be split between the parties. The Individual Agreement will remain in effect until CellSeed’s patents used for the CAOMECS expire in the United States in February 2023 and February 2024, unless terminated earlier by the parties.
Pursuant to an Agreement and Plan of Merger, dated April 21, 2011 (the “Merger Agreement”), by and among AFH Acquisition IV, Inc. (“AFH IV”), AFH Merger Sub, Inc. (“AFH Merger Sub”), AFH Holding and Advisory, LLC (“AFH Advisory”), and Emmaus, Emmaus merged with and into AFH Merger Sub with Emmaus continuing as the surviving entity (the “Merger”). Upon the closing of the Merger on May 3, 2011, AFH IV changed its name from “AFH Acquisition IV, Inc.” to “Emmaus Holdings, Inc.”
Upon consummation of the Merger, (i) each outstanding share of Emmaus common stock was exchanged for 29.48548924976 shares of AFH IV common stock, (ii) each outstanding Emmaus option and warrant, which was exercisable for one share of Emmaus common stock, was exchanged for an option or warrant, as applicable, exercisable for 29.48548924976 shares of AFH IV common stock; and (iii) each outstanding convertible note of Emmaus, which was converted for one share of Emmaus common stock, was exchanged for a convertible note exercisable for 29.48548924976 shares of AFH IV common stock. As a result of the Merger, securityholders of Emmaus received 20,628,305 shares of AFH IV common stock, options and warrants to purchase an aggregate of 326,507 shares of AFH IV common stock, and convertible notes to purchase an aggregate of 270,648 shares of AFH IV common stock.
Four shareholders exercised their Dissenters’ rights in connection with the Merger and returned an aggregate of 47,178 shares for an aggregate of $200,000. The shares will be cancelled as of May 3, 2011, the closing date of the Merger.
139
For accounting purposes, the Merger transaction is being accounted for as a reverse merger. The transaction has been treated as a recapitalization of. Emmaus Medical and its subsidiaries, with Emmaus Holdings, Inc. (the legal acquirer of Emmaus Medical and its subsidiaries) considered the accounting acquiree and Emmaus Medical whose management took control of Emmaus Holdings, Inc. (the legal acquiree of Emmaus Medical) considered the accounting acquirer.
The Company entered into employment agreements with Yutaka Niihara, M.D., MPH, its Chief Executive Officer, Willis C. Lee, its Chief Operating Officer, and Lan T. Tran, its Chief Administrative Officer on April 5, 2011 and with Yasushi Nagasaki, its Chief Financial Officer, on April 8, 2011 (collectively, the “Employment Agreements”). Each of the Employment Agreements has an initial 2-year term, unless terminated earlier. The Employment Agreements for Dr. Niihara, Mr. Lee and Ms. Tran automatically renew for additional one year periods unless the Company or the officer provides notice of non-renewal at least sixty (60) days prior to the expiration of the then current term.
On July 8, 2011, the Company issued a convertible promissory note in the principal amount of $3,240, which bears interest at 8% per annum and matures on July 7, 2012. Upon the lender’s option, the principal amount of the note and any unpaid accrued interest may be converted into shares of common stock of the Company at a conversion price of $3.60 per share. The lender also received a warrant to purchase 225 shares of common stock. The warrant is exercisable for three years and has an exercise price equal to 75% of the fair market value of the Company’s common stock on the date prior to exercise.
On July 7, 2011 and July 11, 2011, Emmaus Medical issued promissory notes each in the principal amount of $300,000 to Yoko Hagiike. The notes bear interest at 10% per annum and all unpaid principal and interest are due upon demand at the lender’s option.
On July 11, 2011, the Company issued a convertible promissory note in the principal amount of $925,200, which bears interest at 8% per annum and matures on July 11, 2012. Upon the lender’s option, the principal amount of the note and any unpaid accrued interest may be converted into shares of common stock of the Company at a conversion price of $3.60 per share. The lender also received a warrant to purchase 64,250 shares of common stock. The warrants will be exercisable until three years from the date of the note and will have an exercise price equal to 75% of the fair market value of the Company’s common stock on the date prior to exercise.
On July 21, 2011, Emmaus Medical received a loan from Equities First Holdings, LLC (“Equities First”) pursuant to a loan agreement by and between Emmaus Medical and Equities First dated June 9, 2011. Pursuant to the loan agreement, Equities First agreed to lend to Emmaus Medical funds equal to 70% of the current fair market value of 73,550 shares of CellSeed, Inc. owned by Emmaus Medical for the three consecutive trading days for the CellSeed stock. The loan bears interest at 4.5% and any interest payment not received within ten calendar days is subject to a penalty equal to 7% of the amount due. The loan was funded on July 21, 2011 (the “Closing Date”) in the principal amount of $841,728.27 and matures on the third anniversary of the Closing Date. Prepayment of the principal amount of the loan or any interest due is not permitted. As collateral for the loan, Emmaus Medical pledged 73,550 of its shares of CellSeed (the “Collateral”) pursuant to a Pledge Agreement with Equities First in which Emmaus Medical assigned its right, title and ownership interest in the Collateral. Within five business days of Emmaus Medical’s payment of all of its obligations under the loan agreement, Equities First shall reassign all right, title and ownership interest in identical securities, as defined in Internal Revenue Code Section 1058, to Emmaus Medical and redeliver the Collateral.
In March 2011, AminoPure received a registration approval from Food and Drugs Board under Ghana Ministry of Health. Emmaus contracted East Cantonments Pharmacy Ltd., a wholesale pharmacy distributor, to be its distributor in Ghana and received its first purchase order in May 2011.
On July 31, 2011, the Company agreed to terminate the promotional rights agreement for Zorbtive® with EMD Serono and after that date will no longer promote Zorbtive®.
From August 2011 through October 2011, the Company issued ten one-year convertible notes in the principal amount totaling $2,115,602, which bear interest at 8% per annum and mature on the anniversary date of the note. The principal amount plus the unpaid accrued interest due under each of the convertible notes is convertible into shares of the Company’s common stock at $3.60 per share. In connection with the issuance of the notes totaling $1,987,600, the Company issued three-year warrants to purchase a total of 242,195 shares of our common stock, respectively, at a per share exercise price equal to 75% of the per share fair market value of the Company’s common stock on the date prior to exercise. In connection with the issuance of the note totaling 128,002, we issued three-year warrants to purchase 35,556 shares of our common stock, at a per share exercise price equal to $1.00.
140
In August 2011, the Company entered into an Addendum to the Joint Research and Development Agreement with CellSeed. Pursuant to the Addendum, CellSeed and the Company further acknowledged that all obligations of CellSeed corresponding to the Research Agreement payment of $8.5 million and the Individual Agreement payment of $1.5 million will be fully discharged by CellSeed’s delivery of the Package, which delivery will be documented by written confirmation of acceptance by the Company. The Company is obligated to make the payments only after the Company provides its written confirmation of acceptance of the complete Package to CellSeed.
On August 31, 2011, the Company issued warrants to purchase 275,482 shares of our common stock at an exercise price of $1.00 per share to a consultant. The warrant is generally exercisable for three years from the issuance date, however, will terminate earlier upon the occurrence of either of the following: (1) FINRA deems the Underwriters’ compensation for the Company’s next public offering to be unreasonable; or (2) on December 31, 2011 if the Company has not consummated one or more public offerings of securities in the minimum aggregate amount of $5.0 million prior to such date. The warrantholder may not exercise the warrant to the extent that such exercise would cause the holder and its affiliates to be a beneficial owner of more than 9.99% of the Company’s outstanding shares of common stock.
On September 30, 2011, the Company entered into an Amended and Restated Letter of Intent (the “LOI”) with AFH Holding & Advisory LLC (“AFH Advisory”). Pursuant to the LOI, the Company has agreed to pay AFH Advisory $500,000 (the “Shell Cost”) for the identification of AFH Acquisition IV and providing consulting services related to coordinating the Merger, assisting with a public offering and managing the interrelationship of legal and accounting activities (the “Services”), and to reimburse AFH Advisory for advancement of expenses on behalf of the Company incurred in connection with the Services, the Merger and a public offering, including, without limitation, reasonable expenses of AFH Advisory (the “Transaction Costs”).
AFH Advisory may be paid any outstanding Shell Cost and Transaction Costs from the proceeds of a public offering or upon the consummation of any other financing. Alternatively, AFH Advisory may, in its discretion, convert such amount (or any portion thereof) into common stock at a conversion price equal to 75% of the per share price in the public offering (the “Conversion Price”). Additionally, the Company has agreed to issue warrants to purchase shares of common stock to AFH Advisory upon the closing of a public offering. Such warrants will have a term of 5 years from the date of issuance and will have an exercise price equal to the Conversion Price. The number of shares underlying the warrants will be calculated by dividing the aggregate of the Shell Cost and the total Transaction Costs by the Conversion Price.
Shares held by AFH Advisory and certain others will be decreased, at the rate of 1% of the post-offering outstanding common shares, for each $1 million or fraction thereof that the gross proceeds to the Company from the offering are less than $10 million. In the event of such reduction, AFH Advisory will reduce the number of those shares by an appropriate percentage. If an offering cannot be consummated to provide for minimum gross proceeds to the Company of at least $5 million, the Company’s arrangement with AFH Advisory provides that the Company may terminate the offering at its sole and absolute discretion, and if the Company terminates the offering, all shares held by AFH Advisory and certain others will be canceled.
If the Company’s arrangement with AFH Advisory is terminated based on mutual agreement of the parties or based on a breach by the Company, AFH Advisory will receive its Transaction Costs incurred as of the date of termination. If the arrangement with AFH Advisory is terminated (i) by either party (without being cured within 30 days) based on identification of information in the course of its due diligence investigation that it deems unsatisfactory, or if the parties are unable to agree to a valuation within 45 days following completion of satisfactory due diligence by an investment bank, or (ii) by the Company if a public offering cannot be consummated to provide for gross proceeds to the Company of at least $10 million or based on a breach by AFH Advisory that is not cured within 30 days, then AFH Advisory will receive reimbursement for 50% of Transaction Costs actually incurred to the date of such termination. If a public offering cannot be consummated with gross proceeds of at least $5 million, and the Company exercises its right to terminate the offering, then the Company will reimburse AFH Advisory an amount equal to 50% of the Shell Cost and 50% of the Transaction Costs incurred as of the date of termination, and AFH Advisory, in its discretion, has the option to be paid any outstanding amounts at the time of termination in cash or to convert such amounts (or any portion thereof) into common stock at a conversion price equal to 75% of the per share price of the shares of common stock sold in the Company’s most recently completed private offering of common stock. In the event of termination by the Company because a public offering cannot be consummated to provide for gross proceeds to the Company of at least $10 million, and if the Company enters into any transaction or a sale of all or substantially all of its assets within 12 months of such termination, AFH Advisory will be entitled to (i) 5% of its holdings (including holdings of certain others) of any securities received by the Company or the Company’s shareholders upon consummation of any business combination or other similar transaction; or (ii) cash or any other consideration it would have received as if it had a 5% ownership interest in the Company immediately prior to the closing of any such transaction.
The Company granted AFH Advisory exclusive rights to act as its advisor in connection with all financings and mergers and acquisitions until November 10, 2012 and the right to appoint two board members to the Company’s board of directors upon the closing of the Merger. In addition, in the event of any merger, stock purchase, asset purchase or similar transaction occurring within one year of the closing of the public offering, AFH Advisory may receive a warrant to purchase shares in an amount to increase AFH Advisory’s total holdings to 10% of the outstanding fully diluted equity of the Company but only to the extent the Company issues securities in connection with such merger, stock purchase, asset purchase or similar transaction.
141
NOTE 14 – RESTATEMENT
The Company had previously restated its previously issued consolidated financial statements to report its deferred tax assets and liabilities related to unrealized gain on available-for-sale securities (the “Initial Restatements”). After further analysis, the Initial Restatements to record the change in the deferred tax asset valuation allowance as a tax benefit were incorrect. Since the change in the valuation allowance is associated with deferred tax recorded for unrealized holding gain on securities available-for-sale, the proper treatment is to record the change in the valuation allowance in other comprehensive income. Accordingly, the Company has restated the consolidated financial statements in the Initial Restatements to properly record the tax impacts associated with the available-for-sale securities in other comprehensive income (the “Second Restatements”). As a result of the Second Restatements, the consolidated financial statements as of and for the year ended December 31, 2011 are identical to those reported by the Company prior to the Initial Restatements. The effect of the Second Restatements on the results of operations and financial position as of and for the year ended December 31, 2011 were as follows:
| As initially reported | As Restated by Initial Restatement | As Restated by Second Restatement | ||||||||||
| Deferred tax asset | $ | — | $ | 217,029 | $ | — | ||||||
| Total current assets | 2,096,860 | 2,313,889 | 2,096,860 | |||||||||
| Total assets | 2,692,327 | 2,909,356 | 2,692,327 | |||||||||
| Deferred tax liability | — | 217,029 | — | |||||||||
| Total liabilities | 1,081,026 | 1,298,055 | 1,081,026 | |||||||||
| Income taxes (benefit) | 4,304 | (212,725 | ) | 4,304 | ||||||||
| Unrealized holding gain on securities available-for-sale | 542,573 | 325,544 | 542,573 | |||||||||
| Net loss per common share | (5.63 | ) | (5.31 | ) | (5.63 | ) | ||||||
142
(b) Pro Forma Financial Statements.
Unaudited Condensed Pro Forma Combined Financial Information
The accompanying unaudited pro forma financial information for the consolidated financial statements for the interim period ended March 31, 2011 and 2010 presents the historical financial information of the accounting acquirer. The pro forma financial information is presented for information purposes only. Such information is based upon the standalone historical results of each company and does not reflect the actual results that would have been reported had the acquisition been completed when assumed, nor is it indicative of the future results of operations for the combined enterprise.
The following represents condensed pro forma revenue and earnings information for the interim period ended March 31, 2011 and 2010 as if the acquisition of AFH Acquisition IV, Inc. and the recapitalization had occurred on the first day of each of the fiscal quarters.
Unaudited, Pro forma Three Months Ended | ||||||||
| March 31, 2011 | March 31, 2010 | |||||||
| Revenues, Net of returns | $ | 59,213 | $ | 45,789 | ||||
| Net Income (Loss) applicable to common shareholders | $ | (1,136,031 | ) | $ | (1,135,713 | ) | ||
| Earnings per share | $ | (.05 | ) | $ | (0.05 | ) | ||
| Weighted Average Shares Outstanding | 24,104,612 | 22,973,025 | ||||||
The unaudited, pro forma information depicted above reflect the impacts of expenses incurred in connection with the recapitalization. There is expected tax effect due to net loss and accumulated retained deficit of Emmaus Medical, Inc.
Unaudited Pro-Forma Financial Information
The accompanying unaudited pro forma financial information for the consolidated financial statements for the years ended December 31, 2010 and 2009 present the historical financial information of the accounting acquirer. The pro forma financial information is presented for information purposes only. Such information is based upon the standalone historical results of each company and does not reflect the actual results that would have been reported had the acquisition been completed when assumed, nor is it indicative of the future results of operations for the combined enterprise.
The following represents condensed pro forma revenue and earnings information for the years ended December 31, 2010 and 2009 as if the acquisition of AFH Acquisition IV, Inc. and the recapitalization had occurred on the first day of each of the fiscal years.
Unaudited, Pro forma Years Ended | ||||||||
| December 31, 2010 | December 31, 2009 | |||||||
| Revenues, , Net of returns | $ | 138,734 | $ | 100,281 | ||||
| Net Income (Loss) applicable to common shareholders | $ | (3,761,198 | ) | $ | (2,975,124 | ) | ||
| Earnings per share | $ | (0.16 | ) | $ | (0.13 | ) | ||
| Weighted Average Shares Outstanding | 23,363,084 | 22,569,647 | ||||||
The unaudited, pro forma information depicted above reflect the impacts of expenses incurred in connection with the recapitalization. There is expected tax effect due to net loss and accumulated retained deficit of Emmaus Medical, Inc.
143
INTRODUCTION TO PRO FORMA CONSOLIDATED FINANCIAL STATEMENTS (UNAUDITED)
On May 3, 2011, the Registrant consummated the acquisition (the “Acquisition”) of all of the shares (the “Shares”) of Emmaus Medical, Inc. (“Emmaus Medical”) from its shareholders under the Agreement and Plan of Merger dated April 21, 2011 among the Registrant, AFH Merger Sub, Inc. a wholly owned subsidiary of the Company, AFH Holding and Advisory, LLC and Emmaus Medical, Inc. Each share of Emmaus Medical’s stock was exchanged for 29.48548924976 shares for the Registrants stock.
As a result of the merger, security holders of Emmaus Medical received an aggregate 20,628,305 of the Registrant’s common stock. At the same time, the Registrant issued 3,750,000 shares of its common stock to the existing shareholders of AFH Merger Sub.
The unaudited pro forma consolidated balance sheet of the Registrant as of April 30, 2011 and is presented as if the acquisition occurred as of date of the Balance Sheet The unaudited pro forma consolidated statement of operations of the Registrant for the interim period ended April 30, 2011 give effect to the acquisition and certain adjustments that are directly attributable to the acquisition as if the transactions occurred as of the earliest date of the period presented.
The unaudited pro forma consolidated statement of operations of the Registrant for the year ended October 31, 2010 give effect to the acquisition and certain adjustments that are directly attributable to the acquisition as if the transactions occurred as of the earliest date of the period presented.
In the opinion of the Registrant and the management of Emmaus Medical, all adjustments and/or disclosures necessary for a fair presentation of the pro forma data have been made. These pro forma consolidated financial statements are presented for illustrative purposes only and are not necessarily indicative of the operating results or the financial position that would have been achieved had the Acquisition actually been consummated as of the dates indicated or of the results that may be obtained in the future.
These pro forma consolidated financial statements and notes thereto should be read in conjunction with the Registrant’s financial statements and the notes thereto as of and for the year ended October 31, 2010 and the interim period ended April 30, 2011.
(A) BASIS OF PRESENTATION
The unaudited pro forma consolidated financial statements have been prepared to reflect stock recapitalization of the Registrant and Emmaus Medical. These unaudited pro forma consolidated financial statements are based on the historical financial statements of the Registrant and Emmaus Medical. The historical financial statements of the Registrant and Emmaus Medical have been prepared in conformity with the accounting principles generally accepted in the United States of America (U.S. GAAP).
144
| Pro Forma Balance Sheet | ||||||||||||||||||
| AFH Acquisition, IV Inc. | Emmaus Medical, Inc. | Pro Forma Adjustments as of closing | Pro Forma Consolidated as of closing | |||||||||||||||
| 4/30/2011 | 3/31/2011 | date | date | |||||||||||||||
| (unaudited) | (unaudited) | (unaudited) | (unaudited) | |||||||||||||||
| ASSETS | ||||||||||||||||||
| Current assets | ||||||||||||||||||
| Cash | $ | 260 | $ | 1,075,564 | $ | (260 | ) | A | $ | 1,075,564 | ||||||||
| Accounts Receivable | — | 16,302 | 16,302 | |||||||||||||||
| Inventories | — | 192,911 | 192,911 | |||||||||||||||
| Marketable Securities | — | 1,217,103 | 1,217,103 | |||||||||||||||
| Prepaid expense | 1,137,874 | 13,544 | (1,137,874 | ) | A | 13,544 | ||||||||||||
| Total current assets | 1,138,134 | 2,515,424 | (1,138,134 | ) | 2,515,424 | |||||||||||||
| Property, plant and equipment, net | — | 89,426 | — | 89,426 | ||||||||||||||
| Other assets | ||||||||||||||||||
| Marketable Securities, Long Term | — | 1,217,103 | 1,217,103 | |||||||||||||||
| Intangibles, Net | — | 71,281 | 71,281 | |||||||||||||||
| Notes receivable | — | 18,000 | 18,000 | |||||||||||||||
| Deposits | — | 506,515 | 506,515 | |||||||||||||||
| Total Other Assets | — | 1,812,899 | — | 1,812,899 | ||||||||||||||
| TOTAL ASSETS | $ | 1,138,134 | $ | 4,417,749 | $ | (1,138,134 | ) | $ | 4,417,749 | |||||||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||||||||||||
| Current liabilities | ||||||||||||||||||
| Accrued liabilities | 863 | 330,633 | (863 | ) | A | |||||||||||||
| 500,000 | D | |||||||||||||||||
| 200,000 | C | 1,030,633 | ||||||||||||||||
| Note payable | 460,000 | 460,000 | ||||||||||||||||
| Total current liabilities | 863 | 790,663 | 699,137 | 1,490,633 | ||||||||||||||
| Long-term liabilities | ||||||||||||||||||
| Notes payable | — | 1,124,494 | 1,124,494 | |||||||||||||||
| Total long-term liabilities | — | 1,124,494 | 1,124,494 | |||||||||||||||
| Total liabilities | 863 | 1,915,127 | 699,137 | 2,615,127 | ||||||||||||||
Shareholders’ equity | ||||||||||||||||||
| Preferred Stock | — | — | ||||||||||||||||
| Common stock | ||||||||||||||||||
| AFH Acquisition IV | 5,578 | 20,628 | B | |||||||||||||||
| (1,828 | ) | E | 24,378 | |||||||||||||||
| Emmaus Medical Inc. | 701 | (1 | ) | C | ||||||||||||||
| (700 | ) | B | — | |||||||||||||||
| Additional paid in capital | 1,174,922 | 15,083,367 | (19,928 | ) | B | |||||||||||||
| (500,000 | ) | D | ||||||||||||||||
| (43,229 | ) | B | ||||||||||||||||
| (199,999 | ) | C | ||||||||||||||||
| 1,828 | E | 15,496,961 | ||||||||||||||||
| Deficit accumulated during development stage | (43,229 | ) | (13,883,839 | ) | 43,229 | B | ||||||||||||
| (1,137,874 | ) | A | ||||||||||||||||
| (260 | ) | A | ||||||||||||||||
| 863 | A | |||||||||||||||||
| (15,021,110 | ) | |||||||||||||||||
| Accumulated other comprehensive income | — | 1,302,393 | — | 1,302,393 | ||||||||||||||
Total shareholders’ equity | 1,137,271 | 2,502,622 | (1,837,271 | ) | 1,802,622 | |||||||||||||
| TOTAL LIABILITIES AND SHAREHOLDERS’ EQUITY (DEFICIT) | $ | 1,138,134 | $ | 4,417,749 | $ | (1,138,134 | ) | $ | 4,417,749 | |||||||||
145
| Notes to Pro forma Adjustments | |||||||
| A | Adjustment to give effect to the distribution of assets and liabilities of AFH Acquisition IV, Inc. prior to the merger. The prepaid expense adjustment represents amounts paid by AFH Acquisition IV to AFH Advisory for consulting services provided by AFH Advisory to AFH Acquisition IV for services associated with the merger. The proceeds received by AFH Holding and Advisory from AFH Acquisition IV may or may not be used by AFH Holding and Advisory to make advance payments for merger transaction costs on behalf of the Company. The Company will be responsible for reimbursing AFH Holding and Advisory for these transaction costs. | ||||||
| B | Adjustment to give effect to the exchange ratio of 1share of Emmaus Medical, Inc. for 29.48548924976 shares of Emmaus Holdings, Inc. resulting in an additional issuance of 20,628,305 shares. | ||||||
| C | Adjustment to give effect to the cancellation of 1,599 pre merger shares in connection with Dissenting Shareholders. This amount is still due and outstanding. | ||||||
| D | Adjustment to give effect to the cost of the acquisition of AFH Acquisition IV, Inc. shell entity, which will be paid by Emmaus to AFH holding and Advisory. This cost is in addition to the merger transaction costs for which Emmaus is responsible for making reimbursement to AFH Holding and Advisory. | ||||||
| E | Adjustment to give effect to the cancellation of 1,827,750 shares of AFH Acquisition IV, Inc. | ||||||
| Reconciliation of Common Stock Outstanding | ||||
| Shares Outstanding April 30, 2011 - Pre Merger | 5,577,750 | |||
| Cancellation of shares per merger agreement | (1,827,750) | |||
| Shares Issued to existing stock holders in connection with share exchange | 20,628,305 | |||
| Shares Outstanding April 30, 2011 - Post Merger | 24,378,305 | |||
146
| Pro Forma Income Statement - Interim | ||||||||||||||||
| AFH Acquisition, IV Inc. | Emmaus Medical, Inc. | |||||||||||||||
| for the three months ended | for the three months ended | Pro Forma adjustments | Pro Forma Consolidated | |||||||||||||
| 4/30/2011 | 3/31/2011 | |||||||||||||||
| (unaudited) | (unaudited) | (unaudited) | ||||||||||||||
| Revenue, Net of returns | $ | — | $ | 59,213 | $ | 59,213 | ||||||||||
| Costs of Goods Sold | — | (25,101 | ) | (25,101 | ) | |||||||||||
| Gross Profit | — | 34,112 | — | 34,112 | ||||||||||||
| Selling, general and administrative expense | (3,703 | ) | (1,160,099 | ) | (1,163,802 | ) | ||||||||||
| Total expenses | (3,703 | ) | (1,160,099 | ) | — | (1,163,802 | ) | |||||||||
| Interest Income | 6,445 | 6,445 | ||||||||||||||
| Interest Expense | (11,811 | ) | (11,811 | ) | ||||||||||||
| Income tax expense | (125 | ) | (850 | ) | (975 | ) | ||||||||||
| Net loss | (3,828 | ) | (1,132,203 | ) | — | (1,136,031 | ) | |||||||||
| Unrealized Gains On Marketable Securities | — | 759,820 | — | 759,820 | ||||||||||||
| Comprehensive loss | $ | $ | (372,383 | ) | $ | — | $ | (376,211 | ) | |||||||
| Basic and diluted net loss per share | $ | (0.00 | ) | $ | (1.63 | ) | $ | — | $ | (0.05 | ) | |||||
| Weighted average shares outstanding | 5,077,899 | 695,915 | — | 24,104,612 | ||||||||||||
Supplemental Footnote Disclosure to Pro forma Statements of Operations
In connection with the merger transaction on May 3, 2011, the Company has incurred expenses in the amount of approximately $288,000. These expenses are for merger transaction costs that have been initially advanced by AFH Holding and Advisory and will be reimbursed by the Company to AFH Holding and Advisory (also see Footnote A to the Pro forma Balance Sheet). These expenses are a one time, non-recurring amount and accordingly are not included as a pro forma adjustment in the accompanying pro forma financial statements.
147
| Pro Forma Income Statement Annual | ||||||||||||||||
AFH Acquisition, IV Inc. for the year ended | Emmaus Medical, Inc. for the year ended | Pro Forma adjustments | Pro Forma Consolidated | |||||||||||||
| 10/31/2010 | 12/31/2010 | |||||||||||||||
| (unaudited) | ||||||||||||||||
| Revenue, Net of returns | $ | — | $ | 138,734 | $ | 138,734 | ||||||||||
| Costs of Goods Sold | — | (334,910 | ) | (334,910 | ) | |||||||||||
| Gross Profit | — | (196,176 | ) | — | (196,176 | ) | ||||||||||
| Selling, general and administrative expense | (3,703 | ) | (3,535,959 | ) | (3,539,662 | ) | ||||||||||
| Total expenses | (3,703 | ) | (3,535,959 | ) | — | (3,539,662 | ) | |||||||||
| Interest Income | 39,005 | 39,005 | ||||||||||||||
| Interest Expense | (59,936 | ) | (59,936 | ) | ||||||||||||
| Income tax (Expense) | (125 | ) | (4,304 | ) | (4,429 | ) | ||||||||||
| Net loss | (3,828 | ) | (3,757,370 | ) | — | (3,761,198 | ) | |||||||||
| Unrealized Gains On Marketable Securities | — | 542,573 | — | 542,573 | ||||||||||||
| Comprehensive loss | $ | — | $ | (3,214,797 | ) | $ | — | $ | (3,218,625 | ) | ||||||
| Basic and diluted net loss per share | $ | (0.00 | ) | $ | (5.63 | ) | $ | — | $ | (0.16 | ) | |||||
| Weighted average shares outstanding | 5,000,000 | 666,813 | — | 23,363,084 | ||||||||||||
Supplemental Footnote Disclosure to Pro forma Statements of Operations
In connection with the merger transaction on May 3, 2011, The Company has incurred expenses in the amount of approximately $288,000. These expenses are for merger transaction costs that have been initially advanced by AFH Holding and Advisory and will be reimbursed by the Company to AFH Holding and Advisory (also see Footnote A to the Pro forma Balance Sheet). These expenses are a one time, non-recurring amount and accordingly are not included as a pro forma adjustment in the accompanying pro forma financial statements.
148
Item 9.01 (d) Exhibits:
| Exhibit No. | Exhibit Description | |
2.1* | Merger Agreement dated as of April 21, 2011 by and among the registrant, AFH Merger Sub, Inc., AFH Holding and Advisory, LLC, and Emmaus Medical, Inc. (incorporated by reference from Exhibit 2.1 to the registrant’s Current Report on Form 8-K filed with the Securities and Exchange Commission on April 25, 2011). | |
3.1* | Certificate of Incorporation (incorporated by reference from Exhibit 3.1 to the registrant’s Form 10-SB filed with the Securities and Exchange Commission on February 1, 2008). | |
| 3.2* | Bylaws (incorporated by reference from Exhibit 3.2 to the registrant’s Form 10-SB filed with the Securities and Exchange Commission on February 1, 2008). | |
| 3.3* | Certificate of Ownership and Merger filed with the Office of Secretary of State of Delaware on May 3, 2011. | |
| 4.1* | Form of Warrant. | |
| 4.2* | Convertible Promissory Note (Cash Interest) dated March 14, 2011. | |
| 4.3* | Form of Convertible Note (No Interest) entered into with the persons indicated in Schedule A attached to the Form of Convertible Note. | |
| 4.4* | Convertible Promissory Note (2-5 Years) dated January 12, 2009. | |
| 4.5 | Convertible Promissory Note (Cash Interest) dated November 23, 2010. | |
| 10.1* | Share Cancellation Agreement dated as of April 21, 2011 by and between the registrant and AFH Holding and Advisory, LLC. | |
| 10.2* | Registration Rights Agreement dated as of May 3, 2011 by and among the registrant and the individuals listed on Schedule A thereto. | |
| 10.3* | Emmaus Holdings, Inc. 2011 Stock Incentive Plan. | |
| 10.3(a)* | Form of Incentive Stock Option Agreement (Time-based and Performance-based Vesting) under the Emmaus Holdings, Inc. 2011 Stock Incentive Plan. | |
| 10.3(b)* | Form of Incentive Stock Option Agreement (Time-based Vesting) under the Emmaus Holdings, Inc. 2011 Stock Incentive Plan. | |
| 10.3(c)* | Form of Non-Qualified Stock Option Agreement (Time-based and Performance-based Vesting) under the Emmaus Holdings, Inc. 2011 Stock Incentive Plan. | |
| 10.3(d)* | Form of Non-Qualified Stock Option Agreement (Time-based Vesting) under the Emmaus Holdings, Inc. 2011 Stock Incentive Plan. | |
| 10.3(e)* | Form of Restricted Stock Agreement (Time-based and Performance-based Vesting) under the Emmaus Holdings, Inc. 2011 Stock Incentive Plan | |
| 10.3(f)* | Form of Restricted Stock Agreement (Time-based Vesting) under the Emmaus Holdings, Inc. 2011 Stock Incentive Plan | |
| 10.4* | License Agreement dated as of March 7, 2001 by and between Harbor-UCLA Research and Education Institute and Orphan Drug International, L.L.C (i.e. Emmaus Medical, Inc.) and corresponding Addendum to the License Agreement entered into December 19, 2003 between Harbor-UCLA Research and Education Institute and Emmaus Medical, Inc. | |
| 10.5* | Federal Supply Schedule Contract Award dated December 8, 2010 by and between the United States Department of Veterans Affairs and Emmaus Medical, Inc. | |
| 10.6* | Office Lease, dated March 12, 2008, by and between Emmaus Medical, Inc. and 20655 S. Western Avenue, LLC. | |
| 10.7* | Sublicense Agreement dated as of October 18, 2007 by and between Cato Holding Company and Emmaus Medical, Inc. | |
| 10.8* | Assignment and Transfer Agreement dated as of February 1, 2011 by and among Cato Holding Company, Nutritional Restart Pharmaceutical Limited Partnership and Emmaus Medical, Inc. | |
| 10.9* | Promotional Rights Agreement effective as of March 12, 2008 by and between Ares Trading S.A. and Emmaus Medical, Inc. | |
| 10.10* | Joint Research and Development Agreement dated as of April 8, 2011 by and between Emmaus Medical, Inc. and CellSeed, Inc. | |
| 10.11* | Individual Agreement dated as of April 8, 2011 by and between Emmaus Medical, Inc. and CellSeed, Inc. |
149
| 10.12* | Employment Agreement dated as of April 5, 2011 by and between Emmaus Medical, Inc. and Yutaka Niihara, M.D., MPH. | |
| 10.13* | Employment Agreement dated as of April 5, 2011 by and between Emmaus Medical, Inc. and Willis C. Lee. | |
| 10.14* | Employment Agreement dated as of April 5, 2011 by and between Emmaus Medical, Inc. and Lan T. Tran. | |
| 10.15* | Employment Agreement dated as of April 8, 2011 by and between Emmaus Medical, Inc. and Yasushi Nagasaki. | |
| 10.16* | Promissory Note dated as of January 12, 2009 by and between Emmaus Medical, Inc. and Yutaka Niihara, M.D., MPH. | |
| 10.17* | Promissory Note dated as of April 23, 2009 by and between Emmaus Medical, Inc. and Yutaka Niihara, M.D., MPH. | |
| 10.18* | Promissory Note dated as of January 12, 2011 by and between Emmaus Medical, Inc. and Willis C. Lee. | |
| 10.19* | Promissory Note dated as of January 12, 2011 by and between Emmaus Medical, Inc. and Hope International Hospice, Inc. | |
| 10.20* | Form of Indemnification Agreement and List of Officers and Directors. | |
| 10.21* | Promissory Note dated as of April 29, 2009 by and between Emmaus Medical, Inc. and Daniel Kimbell. | |
| 10.22* | Promissory Note dated as of May 11, 2009 by and between Emmaus Medical, Inc. and Daniel Kimbell. | |
| 10.23* | Trademark Assignment dated as of February 14, 2011 by and between Cato Research Ltd. and Emmaus Medical, Inc. | |
| 10.24* | Letter of Intent by and between Ajinomoto Company and Emmaus Medical, Inc. | |
| 10.25 | Services Agreement dated as of March 16, 2004 by and between Emmaus Medical, Inc. and ClinDatrix, Inc. | |
| 21.1* | List of Subsidiaries. |
* Previously filed.
150
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Emmaus Life Sciences, Inc. | ||
| Date: November 16, 2011 | ||
| By: | /s/ Yutaka Niihara | |
| Name: | Yutaka Niihara M.D., MPH. | |
| Title: | President and Chief Executive Officer | |
| (principal executive officer and duly authorized officer) | ||
151