First Quarter 2011 Financial Results and Business Update
| GenSpera, Inc. (“GenSpera” or “the Company”) is a biotechnology company developing targeted therapies to treat cancerous tumors. The Company’s novel approach uses a prodrug—an inactive precursor of a drug that converts into its active form at a targeted site—to deliver a potent, cell-killing agent directly to tumors. GenSpera’s prodrugs employ one of two techniques: (1) targeting tumor-associated blood vessels; or (2) targeting tumors directly. In contrast to existing anti-angiogenic drugs, which may only block new blood vessel formation, the Company’s lead prodrug candidate, G-202, attacks existing tumor vasculature, potentially debilitating the tumor’s nutrient supply and causing cancer regression without toxicity to other areas of the body. A Phase I clinical trial with G-202 is ongoing at three major cancer centers to evaluate the prodrug’s safety in patients whose cancers have progressed after prior therapy. GenSpera’s technology can also be used to attack cancer cells directly by targeting the prodrug to enzymes found solely at tumor sites. Using this approach, GenSpera is developing G-115 and G-301 (formerly Ac-GKAFRR-L12ADT), which directly target prostate cancer. The Company owns and controls all rights to G-202, G-115, and G-301, and seeks strategic partnerships to maximize the value of its prodrugs. | GenSpera, Inc. 2511 N Loop 1604 W Suite 204 San Antonio, TX 78258 Phone: (210) 479-8112 Fax: (210) 479-8113 www.genspera.com |
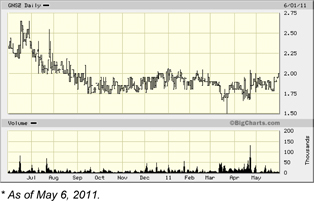
| Ticker (Exchange) | GNSZ (OTC.BB) | |
| Recent Price (06/01/2011) | $1.95 |
| 52-week Range | $1.50 - $2.65 |
| Shares Outstanding* | ~21.4 million |
| Market Capitalization | ~$41.7 million |
| Average 3-month Volume | 16,192 |
| Insider Owners + 5% | 33% |
| Institutional Owners | N/A |
| EPS (Qtr. ended 03/31/2011) | ($0.02) |
| Full-time Employees | 2 |
| n | In early 2011, GenSpera added the Cancer Therapy & Research Center (CTRC) at the University of Texas Health Science Center at San Antonio as the third cancer center in its Phase I clinical program with G-202. The CTRC has significant experience developing cancer therapies and is one of only 65 National Cancer Institute (NCI)-designated cancer centers in the U.S. |
| n | To date, 16 patients have been enrolled and dosed in the Phase I study with G-202. GenSpera seeks to enroll up to 30 patients and expects to complete the trial in the third quarter 2011. |
| n | GenSpera’s technology was developed over 15 years at Johns Hopkins and other global research centers and funded by over $15 million in grants from the U.S. National Institutes of Health, the NCI, and the U.S. Department of Defense, among others. Most recently, the Company was awarded two federal grants totaling $489,000 in 2010 to further its initiatives. |
| n | The Company’s intellectual property portfolio includes nine patents and four pending patent applications. Most recently, GenSpera was issued a patent covering the composition of G-301. |
| n | At March 31, 2011, the Company’s cash position was nearly $6.8 million. Subsequently, in May 2011, GenSpera raised a further $2.2 million through a private placement transaction. Collectively, the funds are being used to support a Phase II clinical program with G-202 as well as preclinical development of G-115. |
PLEASE REFER TO THE EXECUTIVE INFORMATIONAL OVERVIEW® (EIO®), JULY 6, 2010, FOR A FULL COMPANY REPORT. |
| |
| Financial Results and Recent Events |
First Quarter 2011 Financial Results
On May 13, 2011, GenSpera filed financial results for the three months ended March 31, 2011. The Company’s fiscal year ends December 31. In the first quarter 2011 and corresponding 2010 period, GenSpera reported no revenues. The Company does not anticipate revenues during the remainder of 2011 as it continues to develop its prodrug candidates.
For the three months ended March 31, 2011, GenSpera’s general and administrative (G&A) expenses totaled $632,050 versus $395,880 for the year-ago period. The Company primarily attributed the 60% increase to a $248,000 increase in consulting fees (which included $233,000 of stock-based expense), a $62,000 rise in professional and other fees, and an additional $22,000 in travel and entertainment expenses, which were partially offset by an $89,000 reduction in compensation expenses.
Research and development (R&D) expenses for the first quarter 2011 were $421,116 versus $354,065 for the same timeframe in 2010. GenSpera attributed the 19% increase mainly to a $179,000 rise in development expenses due to more advanced clinical trials, which was partially offset by an $114,000 reduction in compensation expense. The Company’s R&D expenses included toxicology and other studies, manufacturing, clinical trials, and compensation and consulting costs.
As well, GenSpera received $244,479 in grant funding during the first quarter 2011 as part of two federal grants awarded in 2010 through the Patient Protection and Affordable Care Act.
Other income for the three months in 2011 totaled $459,968 versus ($1,420,119) for the corresponding 2010 period. This increase in income was primarily the result of a change in fair value of GenSpera’s Warrant derivative liability caused by adjustments in the Company’s stock price and the volatility of its Common Stock during the reporting periods. Change in fair value of derivative liability totaled $453,590 in the three months of 2011 versus ($1,423,492) during the first quarter 2010.
For the first three months of 2011, GenSpera reported a net loss of $348,719, or a loss of $0.02 per share, versus a net loss of $2,170,064, or a loss of $0.14 per share, during the corresponding quarter in 2010.
Subsequently, as of March 31, 2011, the Company’s cash position increased to $6,750,447 versus $3,671,151 at December 31, 2010, due to capital raised during the first quarter 2011. GenSpera reports a current monthly cash burn rate of $390,000, which the Company expects to increase to $760,000 per month in the second half of 2011 as GenSpera increases activities associated with its Phase II clinical program for G-202. Based on these estimates, the Company anticipates that its current cash and expected income may be sufficient for up to 14 months following the close of the first quarter 2011.
Recent Events
An overview of the Company’s recent announcements is provided below, referring the reader to GenSpera’s website (www.genspera.com) for complete press releases.
| n | On May 2, 2011, GenSpera announced that it completed a private placement for roughly $2.2 million, net of fees. Priced at $1.65, each unit included one share of Common Stock and one half of a Common Stock Purchase Warrant. The Warrants have an exercise price of $3.15 with a five-year term and are callable at $6.50, providing certain provisions are met. |
| n | On March 28, 2011, the Company announced that the first patient was dosed at the Cancer Therapy & Research Center (CTRC) at the University of Texas Health Science Center at San Antonio as part of the ongoing Phase I clinical study of G-202. CTRC is the third center to participate in the Phase I safety study with G-202 (summarized on page 6). |
| CRYSTAL RESEARCH ASSOCIATES, LLC | QUARTERLY UPDATE | PAGE 2 |
| n | On March 17, 2011, GenSpera announced that its patent application, entitled “Activation of Peptide Prodrugs by hK2,” was issued by the U.S. Patent and Trademark Office (USPTO) as Patent No. 7,906,477. The patent covers the composition of G-301 (formerly Ac-GKAFRR-L12ADT). G-301 is GenSpera’s second candidate to specifically target prostate cancer. |
| n | On November 30, 2010, the Company announced a plan to expand its Phase I safety trial to include the CTRC. |
| n | On November 10, 2010, GenSpera made a corporate presentation at the 2010 South Florida Biotechnology Dinner Conference at the Deer Creek Country Club in Deerfield Beach, Florida. The event was hosted by LDV Capital Management, a registered investment advisory firm licensed with the state of Florida. As well, the Company announced plans to attend the Lifetech Capital Miami Medical Investors Conference, which was also sponsored by LDV Capital Management, on November 12, 2010, at the JW Marriot Marquis Hotel in Miami, Florida. |
| n | On November 4, 2010, the Company announced that it was awarded two federal grants totaling roughly $489,000 through the Patient Protection and Affordable Care Act, which supports investments in qualifying therapeutic discovery projects. |
| n | On August 17, 2010, GenSpera announced the appointment of Bo Jesper Hansen, M.D., Ph.D. to its Board of Directors. Dr. Hansen replaced John M. Farah, Jr., Ph.D., a Board member since 2008, who resigned due to a change in his employer’s policy regarding outside directorships. |
| | Dr. Hansen is currently executive chairman of Swedish Orphan Biovitrum AB (“Sobi” [SOBI-NASDAQ OMX Stockholm]), a specialty pharmaceutical company focused on treating rare diseases. Dr. Hansen was previously president, chief executive officer (CEO), and chairman of Swedish Orphan International AB, where he took part in growing the company from a small Nordic-focused specialty pharmaceutical company to an international organization with over 60 products in 13 countries across Europe. Swedish Orphan International was acquired by Biovitrum AB in 2010 for approximately $500 million, creating a company with expertise in biological products in addition to specialty pharmaceuticals. Dr. Hansen also serves on the Boards of CMC Contrast AB, MipSalus ApS, TopoTarget A/S (TOPO-NASDAQ OMX), and Zymenex A/S. |
| n | On August 10, 2010, the Company announced that its patent application, entitled “Tissue Specific Prodrugs,” was issued by the USPTO as Patent No. 7,767,648. The patent covers the method of use for G-202. |
| n | On August 3, 2010, GenSpera announced that it had acquired a patent application from Johns Hopkins University and the University of Copenhagen for a medical imaging technology for cancer (overviewed on page 8). |
| CRYSTAL RESEARCH ASSOCIATES, LLC | QUARTERLY UPDATE | PAGE 3 |
GenSpera, Inc. (“GenSpera” or “the Company”) is a development-stage company focused on discovering and developing targeted cancer therapeutics to treat a wide range of solid tumors, including breast, prostate, bladder, and kidney cancers. The Company’s novel prodrug technology combines a potent cytotoxin with a prodrug delivery system that activates the drug only within the tumor. GenSpera’s technology was developed over 15 years at Johns Hopkins and other global research centers and funded by over $15 million in grants from the U.S. National Institutes of Health (NIH), the National Cancer Institute (NCI), and the U.S. Department of Defense, among others. Most recently, the Company was awarded two federal grants totaling $489,000 in 2010 to further its initiatives.
The Company’s business strategy entails developing a series of therapies based on its target-activated prodrug technology platform, identifying potentially attractive drug candidates with solid intellectual property (IP) protection, and then developing these compounds through Phase I/II clinical trials. Once a candidate reaches this stage, the Company intends to license the rights for further development to more established pharmaceutical companies, which could then finalize drug development and market the resulting therapeutic. GenSpera believes that this strategy could maximize the value of its prodrugs as they advance in the clinic. The Company owns and controls all rights to each of its prodrugs.
A Phase I clinical trial evaluating GenSpera’s lead prodrug, G-202, in any type of solid tumor is ongoing at three major cancer centers in the U.S. The Company’s pipeline also includes G-115 and G-301, which are in the preclinical stage and are specifically designed to target prostate cancer. GenSpera aims to obtain Investigational New Drug (IND) approval for G-115 in the second half of 2011.
Cancer
The human body is composed of trillions of cells that constantly grow, divide, and die. For the most part, the cells in the body are healthy and perform their vital functions. However, when healthy cells do not perform properly, they typically self-destruct and are replaced. Cancer cells reproduce uncontrollably, regardless of their abnormalities. While the exact mechanism of transformation that causes normal cells to become cancerous remains unknown, cancer cells are believed to develop due to mutations caused by changed or damaged DNA. Instead of dying when they should, the abnormal cells constantly grow and divide, producing new cells that are not needed by the body.
Once cancerous cells are established, they may rapidly invade surrounding tissues. As cancer cells grow, they require sufficient nutrition, which is initially acquired by feeding off of the body’s systems in a parasitic manner. In order for a tumor to continue to grow beyond the size of roughly a pinhead, its cancer cells recruit blood vessels through a process called tumor angiogenesis. This development enables cancer cells to achieve self-sustainable growth by acquiring their own blood supply, which further fuels the spread of tumors.
Cancer is the second most common cause of death in the U.S. In 2010, the American Cancer Society (ACS) estimated that roughly 1,500 individuals succumb to cancer daily, totaling nearly 570,000 cancer-related deaths annually (Source: the ACS’s Cancer Facts & Figures 2010). The disease also impacts countries on an economic level. The NIH has forecasted that direct medical costs of cancer in the U.S. could exceed $158 billion by 2020.
Traditional chemotherapy involves treating patients with cytotoxins, which are compounds or agents that are toxic to cells. In the early stages of cancer, chemotherapy may be combined with surgery or radiation to improve the efficacy of a treatment regimen. In the later stages of the disease—after the cancer has spread to another region of the body—chemotherapy is often the only treatment option for many forms of cancer. However, traditional chemotherapies have several prominent disadvantages: (1) they affect both healthy and cancer cells indiscriminately, causing negative side effects; (2) they exert their toxic effect when cells divide, which can be ineffective in tumors with cells that divide at a slower rate than cells in normal tissues; and (3) cancer cells may develop a drug resistance after repeated exposure to current chemotherapies, thus limiting the number of times a therapy can be used effectively.
| CRYSTAL RESEARCH ASSOCIATES, LLC | QUARTERLY UPDATE | PAGE 4 |
GenSpera’s Prodrug Chemotherapy
Researchers are investigating prodrug chemotherapy as a technique to deliver higher concentrations of cytotoxic agents to tumors while avoiding the toxicity to healthy tissues of the body. Prodrug technology entails administering an inactive form of a cytotoxin, called a prodrug, to a patient. The prodrug is designed to be activated only through a conversion that occurs exclusively at a tumor site. If successfully developed, GenSpera believes that prodrug therapies could provide an effective therapeutic approach for a broad range of solid tumors caused by breast, prostate, lung, colon, and other cancers. The Company’s prodrug technology involves attaching a targeting/masking agent to an active drug, temporarily making the drug both soluble for intravenous administration and inactive in the bloodstream. Once the compound reaches its target, the masking agent is removed by an enzyme found at the tumor location. With the agent detached, the drug is reactivated and becomes insoluble, precipitating directly into nearby cancer cells. The cancer is killed due to the toxic effects of the activated drug.
GenSpera uses a two-tiered strategy to target its medicine specifically to tumor sites.
| (1) | The Company identifies select enzymes (proteases) that are found at higher levels in tumors relative to other tissues in the body. |
| (2) | Upon identifying these enzymes, GenSpera creates peptides that are recognized predominantly by those enzymes in the tumor and not by enzymes in normal tissues. |
Because enzyme recognition is required to remove the selected peptide (the targeting/masking agent) and activate the cytotoxin, the compound is not expected to cause toxicity in areas of the body other than the targeted site.
GenSpera uses 12ADT—a chemically modified form of the cytotoxin thapsigargin that kills fast-, slow-, and non-dividing cells—as the therapeutic element of its prodrugs, including in its lead candidate, G-202. Thapsigargin is a potent and novel cytotoxin extracted from the plant Thapsia garganica (T. garganica), that is 10- to 100-fold more potent than the NCI’s reference chemotherapeutic agents. 12ADT functions by dramatically raising the level of calcium inside cells, which leads to cell death. The Company believes that 12ADT addresses several issues prevalent with current chemotherapies as it does not appear to trigger the development of resistance to its effects, and it is able to target cells regardless of their rate of division. GenSpera believes that targeting cell death independently of cell division is important for three reasons: (1) it allows the drug to be effective against tumor cells that divide more slowly than normal cells in the body (e.g., prostate cancer); (2) the drug can be effective against the slow-dividing blood vessel cells within solid tumors; and (3) the drug may also kill cancer stem cells, which in general are also slow dividing.
GenSpera’s pipeline includes four prodrug candidates: G-202, G-114, G-115, and G-301 (overviewed in Figure 1). GenSpera uses two approaches in its prodrugs: (1) targeting the blood supply that supports tumor growth; and (2) targeting the tumor directly.
GenSpera, Inc.
CANDIDATES IN DEVELOPMENT
| Product Candidate | | Activating Enzyme | | Indication | | Status |
| | | | | | | |
| G-202 | | Prostate-specific membrane antigen (PSMA) | | Solid tumors | | Ongoing Phase I clinical trial |
| G-114 | | Prostate-specific antigen (PSA) | | Prostate cancers | | Validated efficacy in preclinical animal models (Johns Hopkins University) |
| G-115 | | Prostate-specific antigen (PSA) | | Prostate cancers | | Completed pilot toxicology study |
G-301 (formerly Ac-GKAFRR-L12ADT) | | Human glandular kallikrein 2 (hK2) | | Prostate cancers | | Validated efficacy in preclinical animal models (Johns Hopkins University) |
| CRYSTAL RESEARCH ASSOCIATES, LLC | QUARTERLY UPDATE | PAGE 5 |
Targeting the Blood Supply that Supports Tumor Growth
GenSpera’s lead prodrug candidate, G-202, targets the blood vessels of solid tumors. G-202 is selectively activated within solid tumors by an enzyme present on the tumor blood vessels, thus destroying the existing tumor by ceasing its blood supply. The Company believes that this technique is a dramatic improvement to existing anti-angiogenic drugs, which primarily stop the growth of new blood vessels.
G-202 combines the cytotoxic activity of 12ADT with a specific peptide that masks its activity until it is delivered to the target site. The peptide can only be removed by prostate-specific membrane antigen (PSMA)—a process referred to as the targeted delivery of the active drug. PSMA is expressed in non-cancerous and cancerous prostate tissues, at lower levels in some non-prostate tissues (e.g., kidney), and in the endothelial cells of vasculature associated with non-prostate cancers (Source: Journal of Carcinogenesis 2006, 15[5]:21). Because PSMA is expressed in tumor-associated blood vessels, G-202 and any other PSMA-targeted prodrugs that GenSpera may develop could be able to attack the blood supplies of many different tumor types.
In preclinical testing, G-202 was shown to cause tumor regression in animal models with solid tumors of breast, prostate, bladder, and kidney cancer. G-202’s demonstrated anti-tumor effects in these cancer types further the belief that G-202 may have broad application as a therapy for a variety of human solid tumors due to its ability to selectively target PSMA-producing endothelial cells within tumors. In these studies, the administration of G-202 caused noticeable regression of the tumor—in some cases with no visible re-growth for approximately one month following the last treatment. G-202 was well tolerated at dose levels that caused regression of tumor growth, with no signs of toxicity. At the highest doses, transient weight loss was documented, which quickly recovered after each course of the therapy. The data also indicated that even after repeated dosing cycles, G-202 did not activate drug resistance in tumor cells.
GenSpera believes that its prodrug technology may be able to eliminate cancerous tumors with dosing that is effective for a significant length of time. Further, the Company believes that this is more likely to be achieved in humans than in mice due to the ability to infuse the drug into human patients and an anticipated longer half-life of G-202 in the human bloodstream versus that of laboratory animals. Given its efficacy profile, G-202 could also be effective as a monotherapy, thus reducing the costs and time required to conduct clinical trials.
GenSpera owns and controls all rights to G-202. The Company holds patents covering G-202’s composition of matter as well as its method of use.
Ongoing Phase I Clinical Trial
In 2010, GenSpera initiated a Phase I study with G-202 to evaluate its safety and its pharmacokinetics in cancer patients. To date, the Company has enrolled and dosed 16 patients across three major cancer centers: (1) the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins in Baltimore, Maryland; (2) the University of Wisconsin Carbone Cancer Center in Madison, Wisconsin; and (3) the Cancer Therapy and Research Center (CTRC) at the University of Texas Health Science Center at San Antonio (UTHSCSA) in San Antonio, Texas. Most recently, the CTRC, which has been named a NCI-designated cancer center, was recently added to the Phase I program due to the facility’s experience in cancer drug development, having evaluated over 20 drugs that were eventually approved by the U.S. Food and Drug Administration (FDA).
The open-label, dose-escalation Phase I study could include up to 30 refractory cancer patients—individuals who have relapsed following treatment with other chemotherapies—with any type of solid tumor. GenSpera expects this approach to enhance patient accrual rates and provide the Company with safety data across a range of cancer types. While the primary endpoints of the study are to evaluate the safety, tolerability, and pharmacokinetics of the drug in humans, the design of the trial also allows the collection of efficacy data. GenSpera also has the option to expand the trial to include 18 additional patients in a Phase Ib cohort at the maximum tolerated dose of G-202. Data from this cohort could provide an early signal of potential activity in multiple cancer types.
| CRYSTAL RESEARCH ASSOCIATES, LLC | QUARTERLY UPDATE | PAGE 6 |
Anticipated Phase II Clinical Program
Dependent upon the successful completion of the Phase I study, GenSpera plans to conduct several Phase II clinical trials over 18 months to determine the therapeutic efficacy of G-202 in different tumor types. In particular, the Company is preparing protocol for a Phase II clinical study in castrate-resistant pre-chemotherapy prostate cancer patients in the U.S. as well as at several sites in the UK. GenSpera expects the trial to commence in the third quarter 2011.
Targeting the Tumor Directly
GenSpera also develops prodrugs by pairing cytotoxic derivatives of thapsigargin to peptides that are only removable by enzymes expressed in a particular tumor type. The laboratories of Dr. John T. Isaacs and Dr. Samuel R. Denmeade—who are co-founders of GenSpera presently serving as Scientific Advisory Board members—are working to develop other lead compounds that specifically release thapsigargin within prostate tumors. Biographies for Dr. Isaacs and Dr. Denmeade are provided in GenSpera’s Executive Informational Overview® (EIO®) published on July 6, 2010, on pages 11 and 12, respectively.
The Company’s first approach to targeting the tumor directly couples thapsigargin to peptides that were selectively cleaved by a prostate cancer-specific protease called prostate-specific antigen (PSA), which led to GenSpera’s development candidate, G-115. PSA is active within tumor sites and in normal prostate tissue but is inactive within the bloodstream—characteristics that form the basis for tumor-specific delivery of cytotoxic agents. The Company’s second program entails the delivery of thapsigargin selectively to prostate tumors via human glandular kallikrein 2 (hK2), a distinct prostate cancer-specific protease. The hK2 program has resulted in several molecules with anticancer properties in animal models, and the Company is currently using peptide optimization to identify a legitimate development candidate.
PSA-targeted Prodrugs for Prostate Cancer
Compared to other cancers, prostate cancer has a large proportion of slowly proliferating cells that are resistant to treatment with conventional cytotoxic agents. Current therapies generally attack and destroy rapidly dividing cells, and thus spare cancer cells that divide slowly—a feature that is characteristic of prostate cancers. GenSpera’s technology has a broad range of potential applications, including techniques that pair cytotoxic derivatives of thapsigargin to peptides in order to target prostate tumors directly. As such, GenSpera sought to develop a prodrug candidate using this approach that avoided the negative aspects of current prostate cancer therapies.
To develop a PSA-activated prodrug, GenSpera focused on identifying a derivative of thapsigargin that could be chemically coupled to a PSA-substrate peptide, yet retain all the activity of the parent agent after it was released from the prodrug by PSA. The Company focused on the peptide’s synthetic feasibility, optimization of its activity as a PSA substrate, and its relative stability to cleavage by other proteases that are common in the body. Adhering to these conditions, GenSpera identified an initial PSA-activated prodrug, G-114. However, continued screening and optimization of PSA-cleavable peptides led to the discovery of G-115, an improved prodrug that the Company believes exhibits 10-fold greater activity as a PSA substrate versus G-114. G-114 has been designated as the back-up drug for G-115 due to its significant activity as an anti-tumor agent in animal models of prostate cancer.
G-115 was selected as the lead development candidate in the PSA-targeted prodrug program due to its ability to dramatically inhibit the growth of tumors in animal models of human prostate cancer. It is important to note that G-115 and G-202 are non-competing product candidates; the Company intends to market G-115 to urologists and market G-202 to medical oncologists. GenSpera aims to obtain Investigational New Drug (IND) approval for G-115 in the second half of 2011.
| CRYSTAL RESEARCH ASSOCIATES, LLC | QUARTERLY UPDATE | PAGE 7 |
hK2-targeted Prodrugs for Prostate Cancer
Prostate cancer cells also secrete the protease hK2, which may be used to activate thapsigargin prodrugs. Through the laboratories of Dr. Isaacs and Dr. Denmeade, GenSpera has identified peptides that are selectively cleaved by hK2. The Company has coupled them to 12ADT to generate a family of hK2-activated thapsigargin prodrugs, including G-301 (formerly Ac-GKAFRR-L12ADT), which GenSpera considers to be the most fully characterized prodrug of this species. In 2006, the data obtained with G-301 were published in The Prostate. G-301 demonstrated an anti-tumor effect in animal models of human prostate cancer while it was being administered. However, the prodrug was rapidly cleared from the body and there was a moderately low range of therapeutic doses relative to toxicity. Presently, the Company is focused on developing second-generation hK2-activated thapsigargin prodrugs with improved formulations and increased half-lives.
GenSpera owns and controls all rights to G-301. In March 2011, GenSpera was issued Patent No. 7,906,477, entitled “Activation of Peptide Prodrugs by hK2,” by the U.S. Patent and Trademark Office (USPTO), which covers the composition of G-301.
Thapsigargin’s Potential as a Medical Imaging Agent
GenSpera has also acquired rights to cancer-specific compounds for medical imaging, which the Company believes could improve the ability of oncologists to assess tumor progression as well as the efficacy of cancer therapy. The technology, which GenSpera acquired from Johns Hopkins University and the University of Copenhagen in mid-2010, couples thapsigargin derivatives with patented tumor-targeted peptides. When administered to a patient, the cancer-specific compounds accumulate in tumors, which can then be detected and viewed using standard imaging modalities, such as positron emission tomography (PET) or single photon emission computed tomography (SPECT) scans.
Corporate Information
Incorporated in Delaware in 2003, GenSpera was founded as part of a business development initiative based on technology and IP owned by Johns Hopkins. In early 2004, the IP underlying the Company’s technologies was assigned from Johns Hopkins to its co-inventors—Drs. John T. Isaacs, Samuel R. Denmeade, Soren Brogger Christensen, and Hans Lilja—who in turn awarded an option to license the IP to the Company in return for continued protection of the patent portfolio. GenSpera exercised this option in early 2008 by reimbursing Johns Hopkins for previous patent prosecution costs. The co-inventors, who now comprise the Company’s Scientific Advisory Board, assigned the IP to the Company in April 2008. GenSpera’s activities between 2004 and 2007 were limited to continued prosecution of the relevant patent portfolio.
After filing its Form 10-K in March 2011 without a “going concern” statement and raising $6.2 million to date in 2011, GenSpera believes that its ability to continue supporting its development initiatives financially differentiates the Company from many other biotechnology entities. The funds are being used to support an aggressive Phase II clinical program for G-202 as well as the preclinical development of G-115.
GenSpera’s headquarters are located in San Antonio, Texas, where the Company leases a roughly 850-square foot facility. The Company employs two full-time individuals who serve as executive officers. Under the guidance and direction of key personnel, GenSpera outsources its Good Laboratory Practice (GLP) preclinical development (e.g., toxicology), Good Manufacturing Practice (GMP) manufacturing, and clinical development initiatives to contract research organizations (CROs) and contract manufacturing organizations (CMOs). GenSpera’s Common Stock trades on the Over-the-Counter Bulletin Board (OTC.BB) under the ticker symbol “GNSZ.”
| CRYSTAL RESEARCH ASSOCIATES, LLC | QUARTERLY UPDATE | PAGE 8 |
| n | GenSpera’s novel prodrug technology combines a potent cytotoxin with a prodrug delivery system that activates a drug only at a tumor site. The Company’s technology was developed over 15 years at Johns Hopkins and other global research centers and supported by over $15 million in grant funding. |
| n | Traditional cancer treatments (e.g., surgery or radiation therapy) are localized therapies that lose efficacy once the cancer has spread. Chemotherapeutics are also widely used, but as many are not targeted, their toxic effects harm both healthy tissues and cancer cells. |
| | o | GenSpera believes that its prodrug technology may be more effective for cancer that has spread due to its ability to deliver higher concentrations of cytotoxic agents to tumors while avoiding the toxicity of these higher doses in the rest of the body. |
| n | The Company’s lead prodrug candidate, G-202, is designed to inhibit tumor angiogenesis and attack existing tumor vasculature—stopping growth by depleting the cancer’s nutrient supply and potentially causing tumor regression—versus the current generation of anti-angiogenesis drugs that GenSpera believes only slow or prevent further growth of tumors, but do not cause tumor regression. |
| n | A Phase I clinical trial with G-202 is ongoing at three major cancer centers. The trial is advancing according to a schedule determined in dialog with the U.S. Food and Drug Administration (FDA), with 16 patients enrolled and dosed to date. The study’s primary endpoints include determining the safety, tolerability, and pharmacokinetics of G-202. |
| | o | Most recently, the Cancer Therapy & Research Center (CTRC) at the University of Texas Health Science Center at San Antonio was added to the Phase I program. The CTRC has been named a National Cancer Institute (NCI)-Designated Cancer Center due to the facility’s cancer drug development experience, having evaluated over 20 drugs that eventually received FDA approval. |
| | o | GenSpera has the option to expand the trial to include 18 additional patients in a Phase Ib cohort at the maximum tolerated dose of G-202. Data from this cohort could provide an early signal of potential activity in multiple cancer types. |
| n | Pending successful completion of the Phase I program, the Company plans to initiate several Phase II clinical trials with G-202 in various cancer types. GenSpera is preparing protocol for a Phase II study in castrate-resistant prostate cancer patients who have not yet received chemotherapy. GenSpera expects the trial to commence in the third quarter 2011. |
| n | GenSpera’s second prodrug technology approach attacks cancer cells directly by targeting enzymes found primarily in tumors. G-115, the primary development candidate in this area, targets a protein used by physicians to detect the presence of prostate cancer, called prostate-specific antigen (PSA). The Company aims to obtain an Investigational New Drug (IND) for G-115 in the second half of 2011. |
| n | As well, GenSpera has acquired rights to cancer-specific compounds for medical imaging, which the Company believes could improve the ability of oncologists to assess tumor progression as well as the efficacy of cancer therapy. |
| n | The Company is supported by a management team with extensive experience in identifying oncology treatments and bringing them to the clinic as well as its Scientific Advisory Board, which is composed of members who are all inventors of the technology as well as shareholders. Most recently, GenSpera added Bo Jesper Hansen, M.D., Ph.D. to its Board of Directors, who is experienced in increasing the value of specialty pharmaceutical companies. |
| n | At March 31, 2011, the Company’s cash position was nearly $6.8 million. GenSpera has raised a total of $6.2 million to date in 2011. The Company plans to use the funding to support a Phase II clinical program with G-202 as well as the preclinical development of G-115. |
| CRYSTAL RESEARCH ASSOCIATES, LLC | QUARTERLY UPDATE | PAGE 9 |

Some of the information in this Quarterly Update relates to future events or future business and financial performance. Such statements can only be predictions and the actual events or results may differ from those discussed due to the risks described in GenSpera’s statements on Forms 10-K, 10-Q, 8-K, as well as other forms filed from time to time. The content of this update with respect to GenSpera has been compiled primarily from information available to the public released by GenSpera through news releases, Annual Reports, and U.S. Securities and Exchange Commission (SEC) filings. GenSpera is solely responsible for the accuracy of this information. Information as to other companies has been prepared from publicly available information and has not been independently verified by GenSpera. Certain summaries of activities have been condensed to aid the reader in gaining a general understanding. For more complete information about GenSpera, please refer to the Company’s website at www.genspera.com. Additionally, please refer to GenSpera’s Executive Informational Overview® (EIO®) dated July 6, 2010, and located on Crystal Research Associates’ website at www.crystalra.com for more comprehensive details of GenSpera’s risk factors.
| CRYSTAL RESEARCH ASSOCIATES, LLC | QUARTERLY UPDATE | PAGE 10 |
Intentionally Blank.
| CRYSTAL RESEARCH ASSOCIATES, LLC | QUARTERLY UPDATE | PAGE 11 |
Jeffrey J. Kraws or Karen B. Goldfarb Phone: (609) 306-2274 Fax: (609) 395-9339 Email: eio@crystalra.com Web: www.crystalra.com |
Legal Notes and Disclosures: This Quarterly Update has been prepared by GenSpera, Inc. (“GenSpera” or “the Company”) with the assistance of Crystal Research Associates, LLC (“CRA”) based upon information provided by the Company. CRA has not independently verified such information. In addition, CRA has been compensated by the Company in cash of fifty-eight thousand five hundred U.S. dollars and fifty thousand options/warrants for its services in creating and updating the base report, for updates, and for printing costs.
Some of the information in this report relates to future events or future business and financial performance. Such statements constitute forward-looking information within the meaning of the Private Securities Litigation Act of 1995. Such statements can be only predictions and the actual events or results may differ from those discussed due to, among other things, the risks described in GenSpera’s reports on Forms 10-K, 10-Q, 8-K, and other forms filed with the U.S. Securities and Exchange Commission (SEC) from time to time. The content of this report with respect to GenSpera has been compiled primarily from information available to the public released by the Company. GenSpera is solely responsible for the accuracy of that information. Information as to other companies has been prepared from publicly available information and has not been independently verified by GenSpera or CRA. Certain summaries of scientific activities and outcomes have been condensed to aid the reader in gaining a general understanding. For more complete information about GenSpera, the reader is directed to the Company’s website at www.genspera.com. This report is published solely for information purposes and is not to be construed as an offer to sell or the solicitation of an offer to buy any security in any state. Past performance does not guarantee future performance. Additional information about GenSpera and its public filings, as well as copies of this report, can be obtained in either a paper or electronic format by calling (210) 479-8112.