
Executive Summary December 2011

2 Safe Harbor Statement Safe Harbor Statement Under the Private Securities Litigation Reform Act of 1995: Any statements that are not historical facts are forward - looking statements that involve risks and uncertainties that could cause actual results to differ materially from those in the forward - looking statements, which may include, but are not limited to, factors related to GenSpera’s anticipated growth strategies, future business development, ability to attract and retain new clients, ability to develop new products, expand to other related industries or markets in other geographical locations, and other information detailed from time to time in the filings and future filings with the United States Securities and Exchange Commission. Readers are advised that this information is intended for the use of investment professionals. Anyone interested in obtaining information on GenSpera should contact GenSpera, as set forth above, to receive GenSpera’s most recent financial reports. This presentation was developed by GenSpera and is intended solely for informational purposes and is not to be construed as an offer to sell or the solicitation of an offer to buy the Company’s stock. This profile is based upon information available to the public, as well as other information from sources which management believes to be reliable, but is not guaranteed by the Company as being accurate nor does it purport to be complete. Opinions expressed herein are those of management as of the date of publication and are subject to change without notice.

3 GenSpera - Who We Are Leader in the development of targeted prodrug cancer therapeutics • First in class New Chemical Entity (NCE) – unique biochemical mechanism of action • Potential for “complete kill” – active in both slow and rapidly dividing cells • Prodrug targeted delivery system reduces side effects, maximizes efficacy • Technology originated at Johns Hopkins. GenSpera holds 100% of rights, no milestones or royalties due to third parties • 9 patents issued, 4 patents pending • Phase I clinical trial underway at Johns Hopkins, Univ Wisconsin, CTRC

4 GenSpera’s Breakthrough Technology Traditional chemotherapy features: • Targets cell division • Typified by side effects, drug resistance • Little effect on slow growing tumors or cancer stem cells • Just doesn’t work well enough Our “molecular grenade” features are a major breakthrough: • Kills independent of cell division rate (kills slow - growing tumors and stem cells) • Targeted delivery specifically to tumors (minimize side effects, maximize efficacy) • No development of drug resistance • Complete tumor regression observed in multiple preclinical animal studies • As a “monotherapy,” early clinical trials will be simpler, faster, and less expensive than other chemotherapy trials
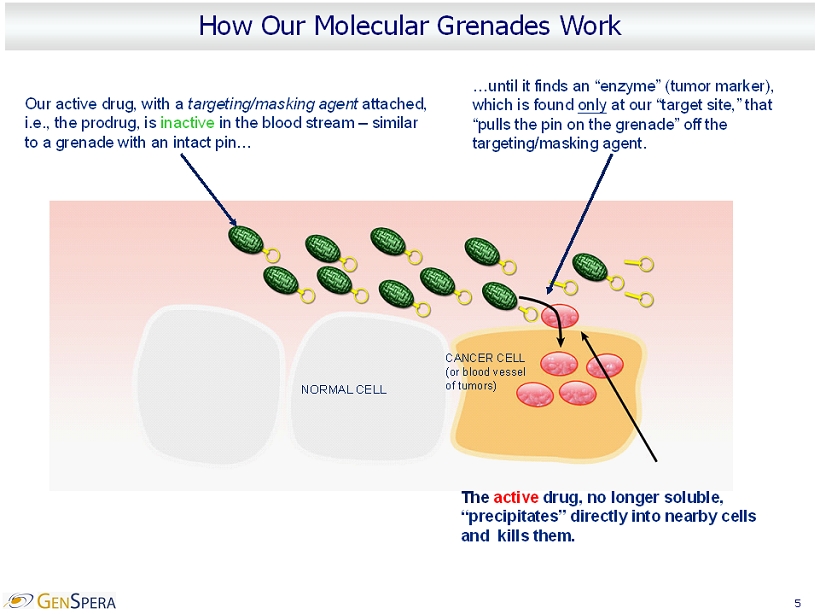
5 How Our Molecular Grenades Work Our active drug, with a targeting/masking agent attached, i.e., the prodrug, is inactive in the blood stream – similar to a grenade with an intact pin… The active drug, no longer soluble, “precipitates” directly into nearby cells and kills them. …until it finds an “enzyme” (tumor marker ), which is found only at our “target site,” that “pulls the pin on the grenade” off the targeting/masking agent. NORMAL CELL CANCER CELL (or blood vessel of tumors)

6 Thapsigargin – A Novel Cytotoxin • Isolated from seeds of Thapsia garganica • 100 kg seeds yields ~1 kg of thapsigargin • Potent inhibitor of ER Ca2+ ATPase pump • Kills cancer cells independent of growth rate • Isolated from seeds of Thapsia garganica • 100 kg seeds yields ~1 kg of thapsigargin • Potent inhibitor of ER Ca2+ ATPase pump • Kills cancer cells independent of growth rate
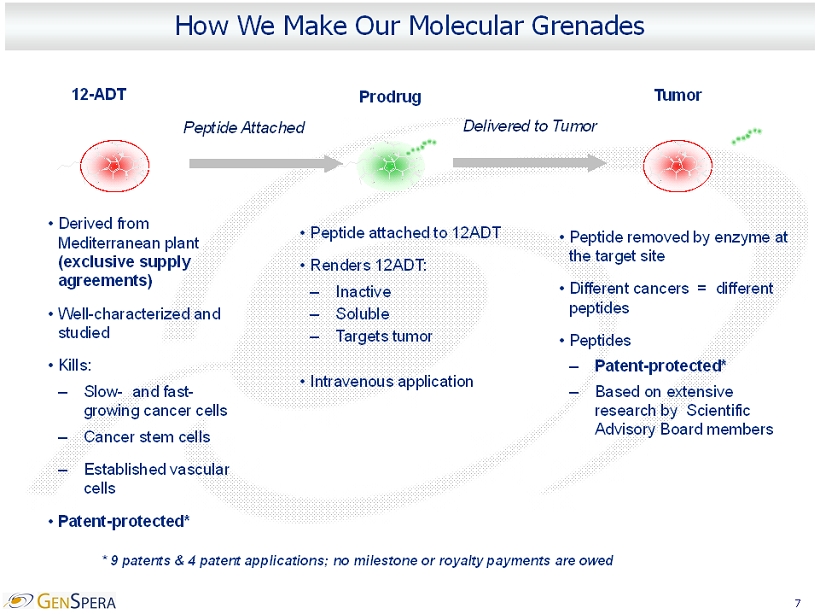
7 How We Make Our Molecular Grenades 12 - ADT • Derived from Mediterranean plant (exclusive supply agreements) • Well - characterized and studied • Kills: – Slow - and fast - growing cancer cells – Cancer stem cells – Established vascular cells • Patent - protected* • Peptide removed by enzyme at the target site • Different cancers = different peptides • Peptides – Patent - protected* – Based on extensive research by Scientific Advisory Board members • Peptide attached to 12ADT • Renders 12ADT : – Inactive – Soluble – Targets tumor • Intravenous application * 9 patents & 4 patent applications; no milestone or royalty payments are owed Peptide Attached Prodrug Delivered to Tumor Tumor
8 Compelling G - 202 Biological Data Efficacy Pharmacology • Complete tumor regression with monotherapy – No need to combine with other drugs – Fewer side effects • Safety margin of 7 to 10 - fold in mouse • No acquired resistance to G - 202 Bioanalytical • In blood can only find intact G - 202 and no released active 12ADT • 12ADT is found at very high levels in tumors, less found in liver and kidneys, not found elsewhere - Targeted Delivery! Toxicology • No effect on liver or cardiovascular system • No effect on bone marrow – no immunosuppression or anemia • Kidney is the affected organ at high doses – dose - dependent, non - cumulative, easily monitored (blood test), and 100% rapidly reversible. May be attenuated via hydration of the patient.

9 Clinical Trial Protocol and Highlights Phase I: Safety, Tolerability, Pharmacokinetics • Open label, all solid tumors with failed treatment - about 30 patients • Dose escalation to Maximum Tolerated Dose • 25 patients dosed, IRB approvals for trial expansion and acceleration • 3 US sites active • Dose - proportional pharmacokinetic data through all seven cohorts Phase Ib Cohort Expansion: Post Chemotherapy Prostate Cancer Patients Planned Phase II Trial: Castrate Resistant, Chemo - naive Prostate Cancer Patients • 3 sites in the US, 2 sites in the UK – O btain possible early signal of activity via PSA response – O btain EMEA trial approval Budget includes 4 concurrent Phase II trials in different tumor types.

10 Achievements and Milestones Recent achievements: • G - 202 exposure in patients is at levels expected to show efficacy • Process improvements in G - 202 manufacture • Second cGMP clinical batch of G - 202 is in use Near - term milestones: • Continued enrollment and dose - escalation in G - 202 Phase I study • Initiate Phase Ib post - chemotherapy prostate cancer study • Initiate Phase II castrate - resistant chemotherapy - naïve prostate cancer study in US and UK

11 GenSpera Team • John T. Isaacs, Ph.D. Professor at the Johns Hopkins School of Medicine, Editor - in - Chief of The Prostate, and on the editorial board of Clinical Cancer Research and Cancer Research. • Samuel R. Denmeade, M.D. Professor at the Johns Hopkins and a Board Certified Medical Oncologist. His laboratory has developed several prodrug therapies that are activated by cancer - specific proteases. • S ø ren Br ø gger Christensen, Ph.D. Professor at the University of Copenhagen. First to elucidate the chemical structure of thapsigargin and is currently engaged in exploring further derivatives of this bioactive compound. • Hans Lilja, M.D., Ph.D. Holds several posts at Memorial Sloan - Kettering Cancer Center and as a Professor at the Lund University. Recognized authority on the biology of prostate specific antigen (PSA) and human kallikrein 2 (hK2). Management & Board • Craig Dionne, Ph.D. President & CEO. Served as VP responsible for many successful oncology drug discovery and development programs at Cephalon, one of the most successful biotechnology companies. • Russell Richerson, Ph.D. COO & Secretary . Over 25 years of senior management experience in the biotechnology industry at companies such as Abbott Laboratories. • Bo Jesper Hansen, M.D., Ph.D. Director. Executive Chairman of Swedish Orphan Biovitrum AB (STO: SOBI). Previously CEO and Chairman of Swedish Orphan International AB Group. Also serves on the boards of CMC AB, MipSalus Aps, TopoTarget A/S (NASDAQ OMX: TOPO ), and Zymenex A/S. • Scott Ogilvie. Director. President and CEO of AFIN International, Inc. Prior career as corporate and securities lawyer with Hill, Farrer & Burrill. Also serves on the board of directors of Neuralstem, Inc. (AMEX:CUR), Derycz Scientific, Inc. (OTCBB:DYSC), and Preferred Voice, Inc. (OTCBD:PRFV). Scientific Advisory Board (inventors of IP and shareholders)
12 Strategy and Value Creation • 15 yrs of research funded by $15M from the Dept of Defense, National Cancer Institute and others – acquired with no royalties due • Raised ~$ 17M since Nov 2007 • Have ~$6M cash - in - hand – adequate to complete Phase I trial and begin Phase II • Trading symbol: OTCBB:GNSZ Financing History • ~21M common shares issued and outstanding • ~12 M currently outstanding options and warrants • Management, inventors, and boards hold approximately 31% of issued shares Business Strategy and Value Creation • Operate with minimal infrastructure requirement • Develop drugs through Phase I/II clinical trials, then out - license or partner in ~2 years • Individual drugs can be worth up to $1B • 2009 – J&J/Cougar • 2009 – Astellas / Medivation • 2011 – Amgen/ Biovex Capital Structure

13 First in class New Chemical Entity with superior activity *** Precisely targeted “molecular grenade” *** Seasoned biotech management *** Business strategy focused on rapidly achieving key valuation milestones with minimal costs GenSpera - Formula for Success

14 Contact Craig A. Dionne, PhD President and CEO cdionne@genspera.com GenSpera, Inc. 2511 N Loop 1604 W, Suite 204 San Antonio, TX 78258 www.genspera.com Phone: (210) 479 - 8112 Fax: (210) 479 - 8113













