
11 – 13 July, 2014, Taipei, Taiwan Clinical Activity of G - 202, a Thapsigargin - Based Prostate Specific Membrane Antigen (PSMA) - Activated Prodrug in Patients with Progressive Hepatocellular Carcinoma (HCC) D Mahalingam , 1 J Neumunaitis, 2 J Sarantopoulos, 1 V Allgood, 3 M Kurman, 3 L Campos 4 1 Institute for Drug Development, Cancer Therapy and Research Center at the University of Texas Health Science Center San Antonio, San Antonio, TX 2 Mary Crowley Cancer Research Center, Dallas, TX 3 GenSpera , Inc., San Antonio, TX 4 Oncology Consultants, PA, Houston, TX

Overview • Pre - clinical and early clinical development G - 202 • Design of the current ongoing phase 2 study in advance HCC following sorafenib failures • Clinical activity and safety of G - 202 in advance HCC – combined phase 1b/2 results

INHIBITION OF SERCA PUMP BY THAPSIGARGIN TG Sarcoplasmic/endoplasmic reticulum Ca 2+ ATP (SERCA) pump transfers Ca2+ from cytosol to the ER lumen.
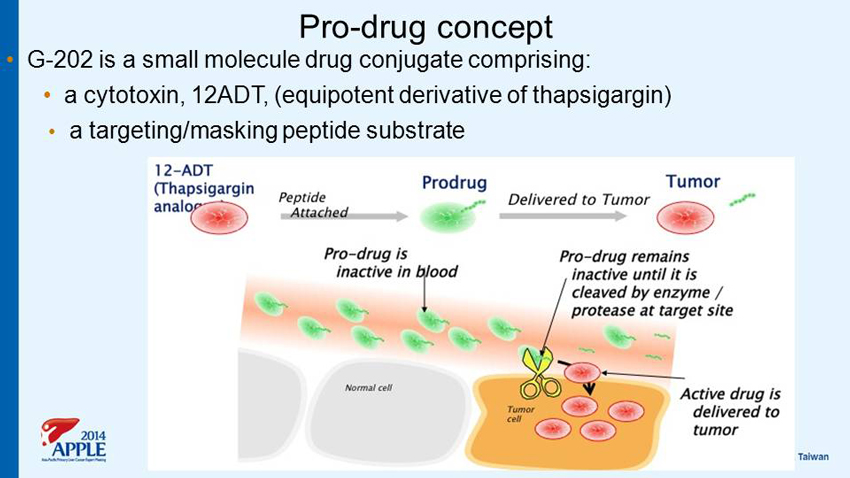
Pro - drug concept • G - 202 is a small molecule drug conjugate comprising: • a cytotoxin, 12ADT, (equipotent derivative of thapsigargin) • a targeting/masking peptide substrate
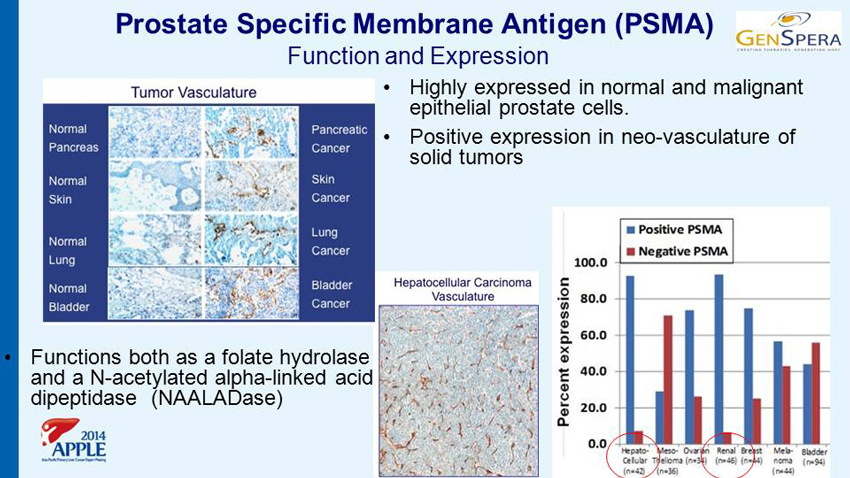
Prostate Specific Membrane Antigen (PSMA) Function and Expression • Highly expressed in normal and malignant epithelial prostate cells. • Positive expression in neo - vasculature of solid tumors • Functions both as a folate hydrolase and a N - acetylated alpha - linked acid dipeptidase (NAALADase)
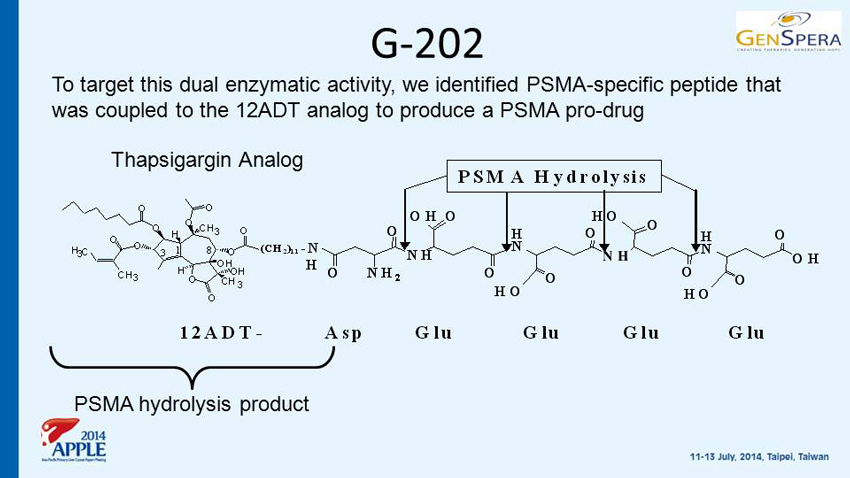
G - 202 O O H OH CH 3 OH O CH 3 O O O O O CH 3 O H 3 C O H 3 8 1 H N N H H N N H H N N H H N N H O O NH 2 O O O H 2 N O HO O OH O HN N N O O H N O (CH 2 ) 11 His (H) Ser- (S) Ser- (S) Lys- (K) Leu- (L) Gln- (Q) Leu- (L)12ADT - PSA Hydrolysis O O H OH CH 3 OH O CH 3 O O O O O CH 3 O H 3 C O H 3 8 1 H N N H H N N H H N N H H N N H O O NH 2 O O O H 2 N O HO O OH O HN N N O O H N O (CH 2 ) 11 His (H) Ser- (S) Ser- (S) Lys- (K) Leu- (L) Gln- (Q) Leu- (L)12ADT - O O H OH CH 3 OH O CH 3 O O O O O CH 3 O H 3 C O H 3 8 1 O O H OH CH 3 CH 3 OH O CH 3 O O O O O CH 3 O H 3 C O H 3 8 1 H N N H H N N H H N N H H N N H O O NH 2 O O O H 2 N O HO O OH O HN N N O O H N O H N N H H N N H H N N H H N N H O O NH 2 O O O H 2 N O HO O OH O HN N N O O H N O (CH 2 ) 11 His (H) Ser- (S) Ser- (S) Lys- (K) Leu- (L) Gln- (Q) Leu- (L)12ADT - PSA Hydrolysis O O H OH CH 3 OH O CH 3 O O O O O CH 3 O H 3 C O H 3 8 1 (CH 2 ) 11 12ADT- NH O O O ONH 2 OH H N N H O HO O H N OH O HO O O HO O NH O O O ONH 2 OH H N N H O HO O H N OH O HO O O HO O -N H Asp Glu Glu Glu Glu PSMA Hydrolysis O O H OH CH 3 OH O CH 3 O O O O O CH 3 O H 3 C O H 3 8 1 O O H OH CH 3 CH 3 OH O CH 3 O O O O O CH 3 O H 3 C O H 3 8 1 (CH 2 ) 11 12ADT- NH O O O ONH 2 OH H N N H O HO O H N OH O HO O O HO O NH O O O ONH 2 OH H N N H O HO O H N OH O HO O O HO O -N H Asp Glu Glu Glu Glu PSMA Hydrolysis PSMA hydrolysis product Thapsigargin Analog To target this dual enzymatic activity, we identified PSMA - specific peptide that was coupled to the 12ADT analog to produce a PSMA pro - drug
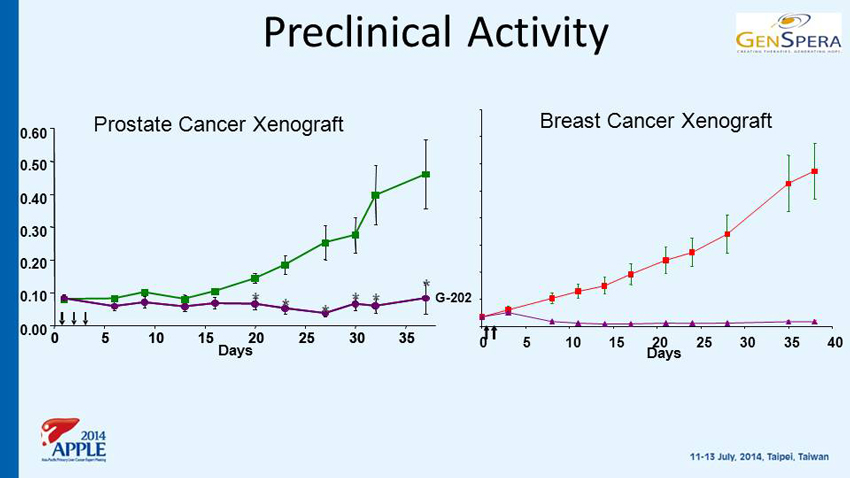
Preclinical Activity Days 0.00 0.10 0.20 0.30 0.40 0.50 0.60 0 5 10 15 20 25 30 35 0 5 10 15 20 25 30 35 G - 202 * * * * * * 0 5 10 15 20 25 30 35 40 Days Breast Cancer Xenograft Prostate Cancer Xenograft

Phase I Clinical Trial of G - 202 • One - hour iv infusion on Days 1, 2 and 3 of 28 - day cycle • 28 patients were treated at 8 dose levels (1.2 – 88 mg/m 2 ) • Protocol - defined MTD was not reached • Infusion - related reactions and creatinine elevations at 88 mg/m 2 led to declaration of 66.8 mg/m 2 as MTD - A modified regimen to reduce infusion - related reactions of G - 202 at 40 mg/m 2 on Day 1 and 66.8 mg/m 2 on Days 2 and 3 was selected for tumor specific expansion. • A further 16 pts were enrolled in the expansion study including 5 with HCC - Prolonged disease stabilization in HCC (9 - 12 months) observed in 2 of 5 patients - One pt completed 12 cycles prior to progressing. - One pt completed 9 cycles, off study due to unrelated event – resumed on G - 202
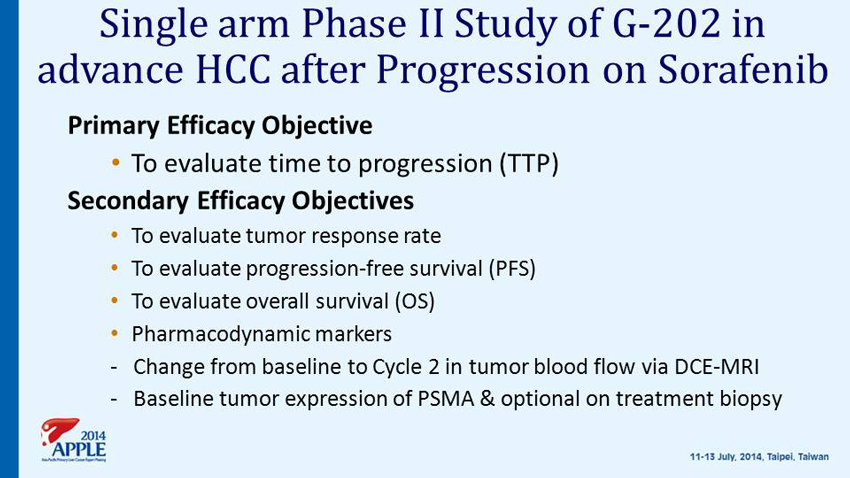
Single arm Phase II Study of G - 202 in advance HCC after Progression on Sorafenib Primary Efficacy Objective • To evaluate time to progression (TTP) Secondary Efficacy Objectives • To evaluate tumor response rate • To evaluate progression - free survival (PFS) • To evaluate overall survival (OS ) • Pharmacodynamic markers - Change from baseline to Cycle 2 in tumor blood flow via DCE - MRI - Baseline tumor expression of PSMA & optional on treatment biopsy
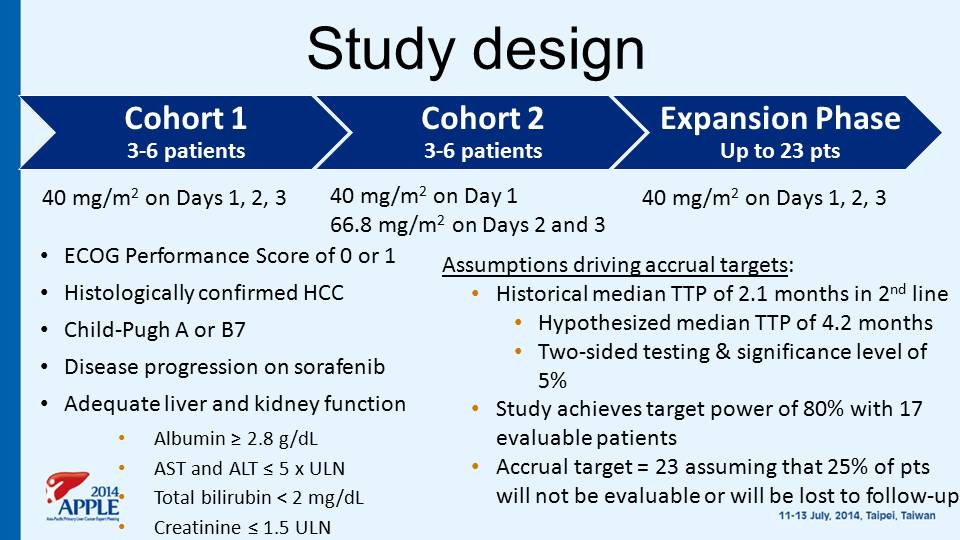
Study design Cohort 1 3 - 6 patients Cohort 2 3 - 6 patients Expansion Phase Up to 23 pts 40 mg/m 2 on Days 1, 2, 3 40 mg/m 2 on Day 1 66.8 mg/m 2 on Days 2 and 3 40 mg/m 2 on Days 1, 2, 3 • ECOG Performance Score of 0 or 1 • Histologically confirmed HCC • Child - Pugh A or B7 • Disease progression on sorafenib • Adequate liver and kidney function • Albumin ≥ 2.8 g/dL • AST and ALT ≤ 5 x ULN • Total bilirubin < 2 mg/dL • Creatinine ≤ 1.5 ULN Assumptions driving accrual targets : • Historical median TTP of 2.1 months in 2 nd line • Hypothesized median TTP of 4.2 months • Two - sided testing & significance level of 5% • Study achieves target power of 80% with 17 evaluable patients • Accrual target = 23 assuming that 25% of pts will not be evaluable or will be lost to follow - up

Interim clinical efficacy and safety results
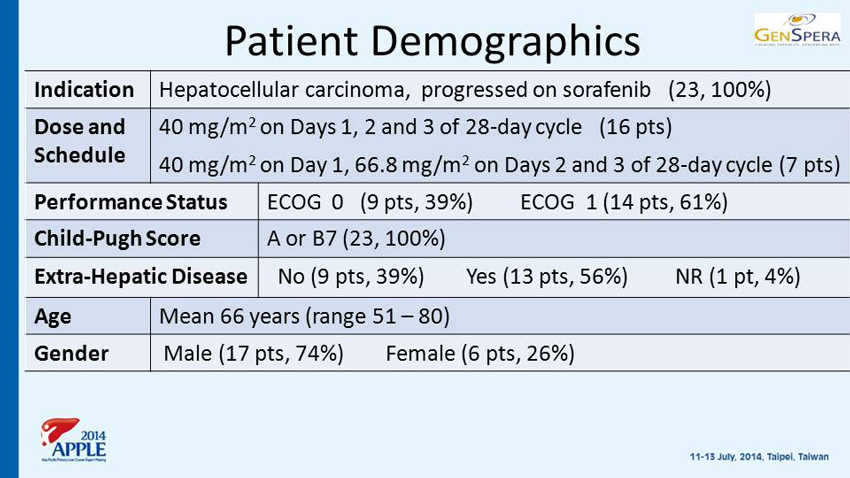
Patient Demographics Indication Hepatocellular carcinoma, progressed on sorafenib (23, 100%) Dose and Schedule 40 mg/m 2 on Days 1, 2 and 3 of 28 - day cycle (16 pts) 40 mg/m 2 on Day 1, 66.8 mg/m 2 on Days 2 and 3 of 28 - day cycle (7 pts) Performance Status ECOG 0 (9 pts, 39%) ECOG 1 (14 pts, 61%) Child - Pugh Score A or B7 (23, 100%) Extra - Hepatic Disease No (9 pts, 39%) Yes (13 pts, 56%) NR (1 pt, 4%) Age Mean 66 years (range 51 – 80) Gender Male (17 pts, 74%) Female (6 pts, 26%)
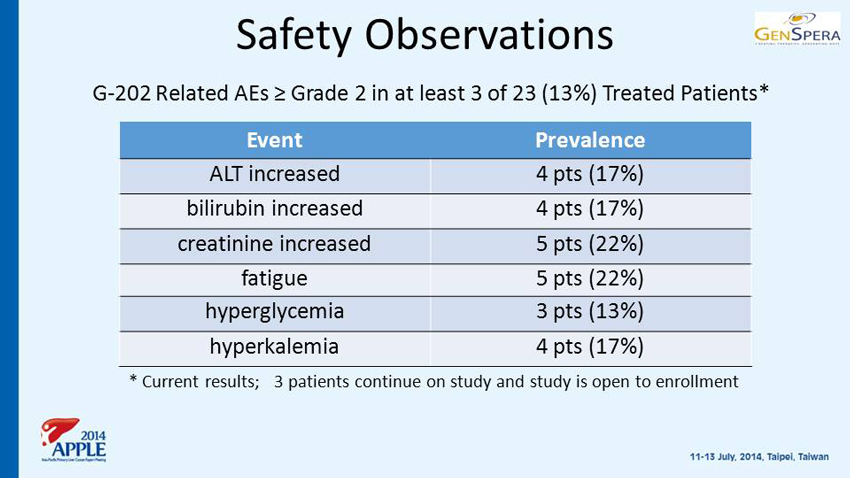
Safety Observations G - 202 Related AEs ≥ Grade 2 in at least 3 of 23 (13%) Treated Patients* Event Prevalence ALT increased 4 pts (17%) bilirubin increased 4 pts (17%) creatinine increased 5 pts (22%) fatigue 5 pts (22%) hyperglycemia 3 pts (13%) hyperkalemia 4 pts (17%) * Current results; 3 patients continue on study and study is open to enrollment
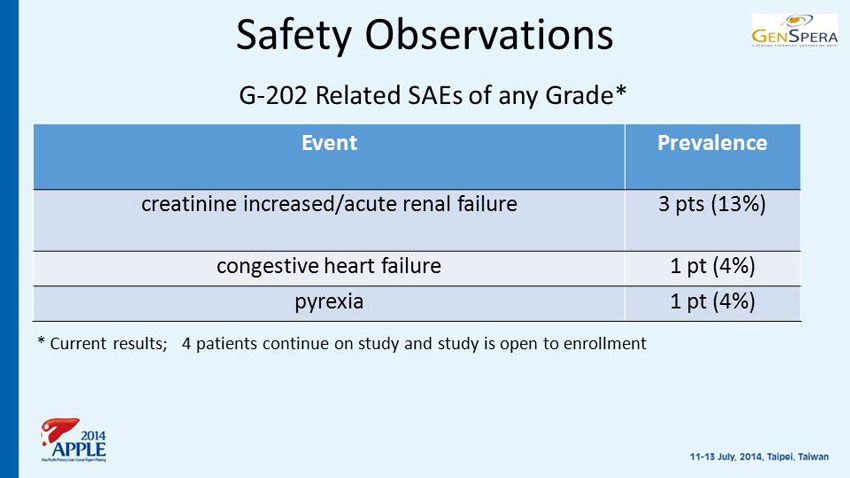
Safety Observations G - 202 Related SAEs of any Grade* Event Prevalence creatinine increased/acute renal failure 3 pts (13%) congestive heart failure 1 pt (4%) pyrexia 1 pt (4%) * Current results; 4 patients continue on study and study is open to enrollment
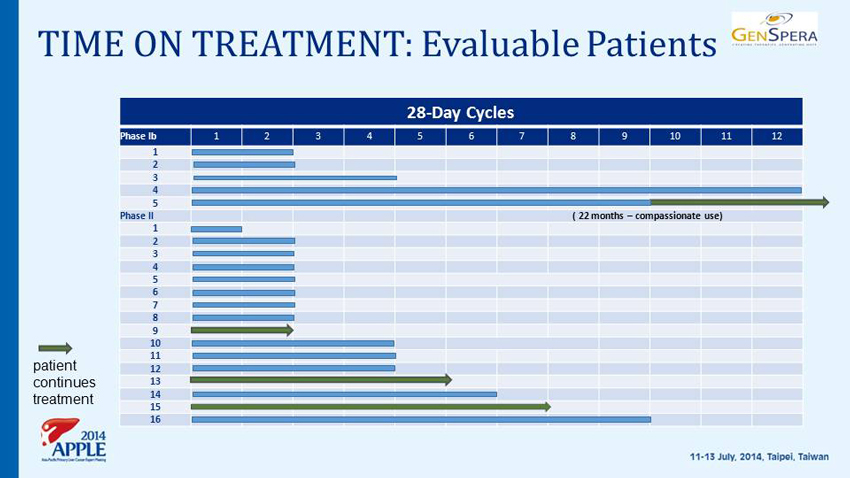
TIME ON TREATMENT: Evaluable Patients 28 - Day Cycles Phase Ib 1 2 3 4 5 6 7 8 9 10 11 12 1 2 3 4 5 Phase II ( 22 months – compassionate use) 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 patient continues treatment
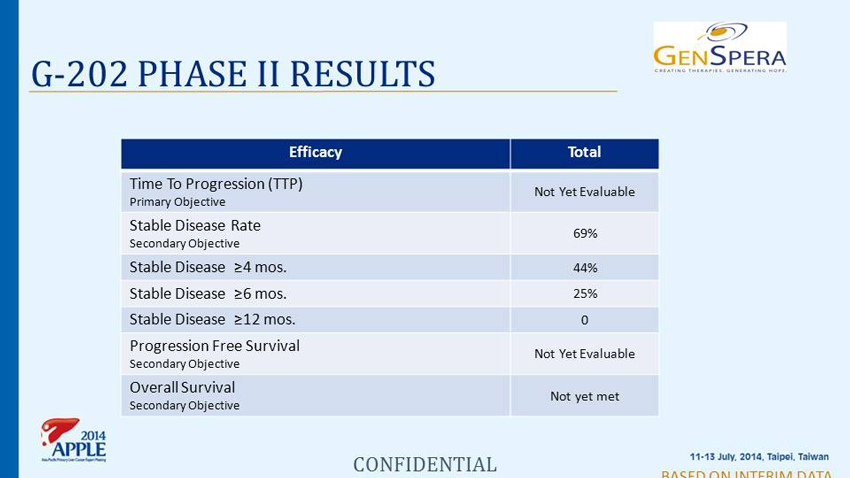
G - 202 PHASE II RESULTS BASED ON INTERIM DATA Efficacy Total Time To Progression (TTP) Primary Objective Not Yet Evaluable Stable Disease Rate Secondary Objective 69% Stable Disease ≥4 mos. 44% Stable Disease ≥6 mos. 25% Stable Disease ≥12 mos. 0 Progression Free Survival Secondary Objective Not Yet Evaluable Overall Survival Secondary Objective Not yet met CONFIDENTIAL
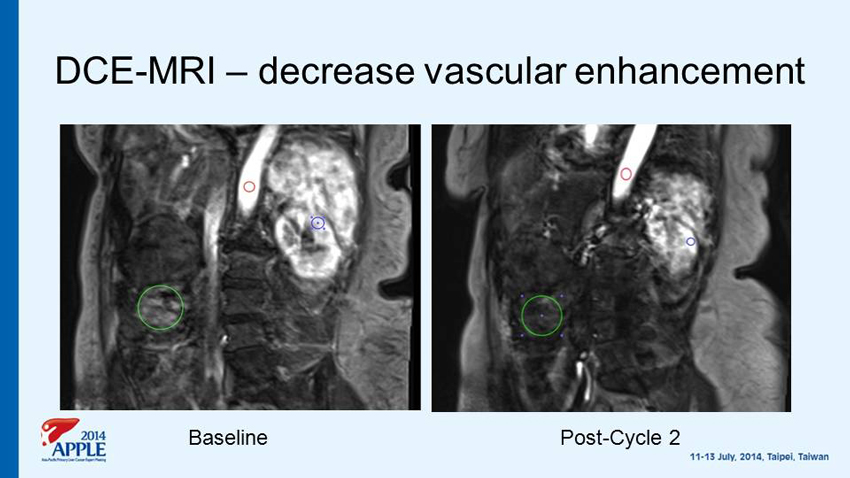
DCE - MRI – decrease vascular enhancement Baseline Post - Cycle 2
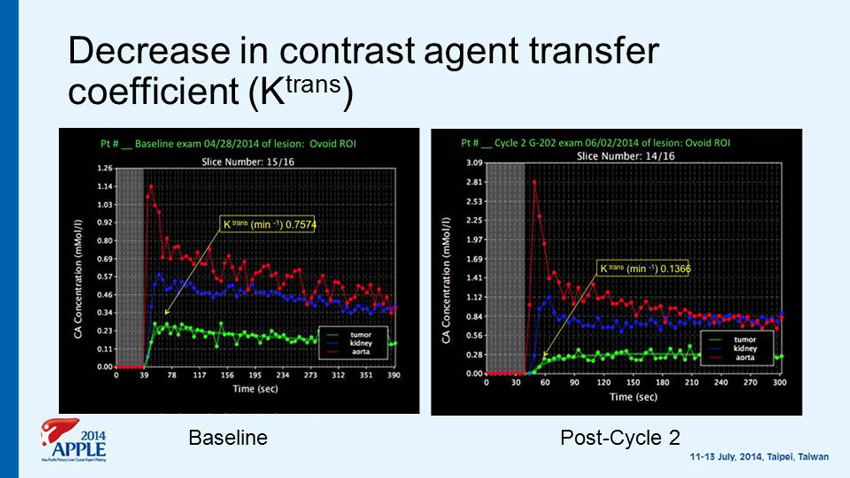
Decrease in contrast agent transfer coefficient (K trans ) Baseline Post - Cycle 2

Conclusions • G - 202 administered iv for 3 consecutive days of a 28 - day cycle is generally well - tolerated. • G - 202 as a single agent promotes disease stabilization (44% pts SD ≥ 4 months) in patients with advanced HCC who have progressed on sorafenib • Studies to evaluate possible effects of G - 202 on tumor vasculature and blood flow metrics are currently ongoing


















