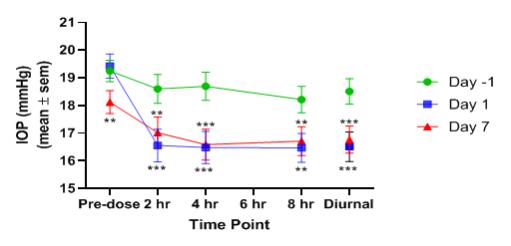
Based on these results, Aerpio plans to further advance its glaucoma program through strategic alternatives such as a partnership or collaboration.
AboutAKB-9778
AKB-9778 binds to and inhibits vascular endothelial protein tyrosine phosphatase(VE-PTP), an important negative regulator of Tie2. Decreased Tie2 activity contributes to vascular instability in many diseases including diabetes.AKB-9778 activates the Tie2 receptor irrespective of extracellular levels of its binding ligands,angiopoietin-1 (agonist) orangiopoietin-2 (antagonist) and may be the most efficient pharmacologic approach to maintain normal Tie2 activation.
About Aerpio Pharmaceuticals
Aerpio Pharmaceuticals, Inc. is a biopharmaceutical company focused on advancingfirst-in-class compounds that activate Tie2 to treat ocular diseases and complications of diabetes. Tie2 is an important regulator of vascular stability, and its down-regulation, through activation of two inhibitorsVE-PTP andAng-2, is found in patients with diabetes and other conditions. The Company’s lead compound,AKB-9778, afirst-in-class small molecule inhibitor ofVE-PTP, is being investigated in an ongoing Phase 1b clinical trial as a topical drop formulation for its therapeutic potential in open-angle glaucoma. In the recently completed Phase 2b study(TIME-2b)AKB-9778 demonstrated the ability to lower proteinuria (as measured by decreasing urinary albumin creatine ratio, UACR) by about 20% replicating a finding in the previous Phase 2 study. The decrease in proteinuria suggests thatAKB-9778 and our other Tie2 activating drug,ARP-1536, may have the potential to improve kidney function in diabetics potentially delaying progression to kidney dialysis. The Company’s second asset,ARP-1536 is a humanized monoclonal antibody observed to activate Tie2 receptors in a dose-dependent manner in preclinical models. Aerpio believesARP-1536 holds potential as a monthly or biweekly systemic therapy to treat diabetic complications, including diabetic nephropathy. The Company’s third asset is a bispecific antibody that binds both VEGF and VE-PTP which inhibits VEGF activation and activates Tie2. This bispecific antibody has the potential to be an improved product for treating wet AMD and diabetic macular edema via intravitreal injection. Finally, the Company has exclusivelyout-licensed its fourth assetAKB-4924 (now called GB004), afirst-in-class small molecule inhibitor of hypoxia-induciblefactor-1 (HIF). GB004 is being developed byAKB-4924’s exclusive licensor, Gossamer Bio, Inc. (Nasdaq: GOSS), in return for an upfront payment of $20 million, future potential development, regulatory, and sales milestones of up to $400 million, and royalties on worldwide net sales. For more information, please visitwww.aerpio.com.
Forward Looking Statements
This press release contains forward-looking statements. Statements in this press release that are not purely historical are forward-looking statements. Such forward-looking statements include, among other things, the Company’s product candidates, includingAKB-9778, and the clinical development and therapeutic potential thereof, its plans to further the development of its glaucoma program, and the intended benefits from its collaboration with Gossamer Bio, Inc. for GB004. Actual results could differ from those projected in any forward-looking statements due to several