
December 20, 2024 Strategic Update Exhibit 99.2

Forward-Looking Statements Certain statements contained in this presentation regarding matters that are not historical facts, are forward-looking statements within the meaning of Section 21E of the Securities and Exchange Act of 1934, as amended, and the Private Securities Litigation Act of 1995, known as the PSLRA. These include statements regarding management’s intention, plans, beliefs, expectations or forecasts for the future, and, therefore, you are cautioned not to place undue reliance on them. Forward-looking statements may include, without limitation, express or implied statements regarding: the timing and completion of the proposed sale of FYARRO to Kaken Pharmaceuticals and the anticipated timing of the closing of the transaction; expectations regarding the timing, closing and completion of a concurrent private financing, including investment amounts from investors, timing of closing, expected proceeds and impact on ownership structure; Aadi’s expected cash position at the closing and cash runway of the company following the sale of FYARRO and private financing; the future operations of Aadi; the development and potential benefits of any of Aadi’s product candidates; anticipated preclinical and clinical development activities and related timelines, including the expected timing for announcement of data and other preclinical and clinical results and potential submission of IND filings for one or more product candidates; and other statements that are not historical fact. No forward-looking statement can be guaranteed, and actual results may differ materially from those projected. Aadi uses words such as “anticipates,” “believes,” “plans,” “expects,” “projects,” “intends,” “may,” “will,” “should,” “could,” “estimates,” “predicts,” “potential,” “continue,” “opportunity,” and similar expressions to identify these forward-looking statements that are intended to be covered by the safe-harbor provisions of the PSLRA. Such forward-looking statements are based on our expectations and involve risks and uncertainties; consequently, actual results may differ materially from those expressed or implied in the statements due to a number of factors, including, but not limited to, (i) the risk that the conditions to the closing of the proposed sale of FYARRO and concurrent private financing are not satisfied, including the failure to timely obtain stockholder approval for the transactions, if at all; (ii) uncertainties as to the timing of the consummation of the proposed transactions and the ability of each of Kaken and Aadi to consummate the proposed sale of FYARRO; (iii) risks related to Aadi’s ability to manage its operating expenses and its expenses associated with the proposed transactions pending the closing; (iv) risks related to the failure or delay in obtaining required approvals from any governmental or quasi-governmental entity necessary to consummate the proposed transactions; (v) unexpected costs, charges or expenses resulting from the transactions; (vii) potential adverse reactions or changes to business relationships resulting from the announcement or completion of the proposed sale of FYARRO, concurrent private financing and in-license of the ADC portfolio; (vii) the uncertainties associated with Aadi’s product candidates, as well as risks associated with the preclinical and clinical development and regulatory approval of product candidates, including potential delays in the completion of preclinical studies and clinical trials; (viii) risks related to the inability of Aadi to obtain sufficient additional capital to continue to advance these product candidates; (ix) uncertainties in obtaining successful preclinical and clinical results for product candidates and unexpected costs that may result therefrom; (x) risks related to the failure to realize any value from product candidates being developed and anticipated to be developed in light of inherent risks and difficulties involved in successfully bringing product candidates to market; (xi) risks associated with the possible failure to realize certain anticipated benefits of the proposed sale of FYARRO, concurrent private financing and in-license of the ADC portfolio, including with respect to future financial and operating results; (xii) the risk that the private financing is not consummated upon the closing. These risks are described in detail under the caption “Risk Factors” in Aadi’s Annual Report on Form 10-K for the fiscal year ended December 31, 2023, including under the caption "Item 1A. Risk Factors," and in Aadi's subsequent Quarterly Reports on Form 10-Q, and other documents filed from time to time with the SEC. Forward-looking statements included in this presentation are based on information available to Aadi as of the date of this presentation. Except as required by law, Aadi undertakes no obligation to revise or update any forward-looking statement, whether as a result of new information, future events or otherwise.

Participants Dave Lennon, PhD President & Chief Executive Officer Scott Giacobello, CPA Chief Financial Officer

Overview of Proposed Transactions
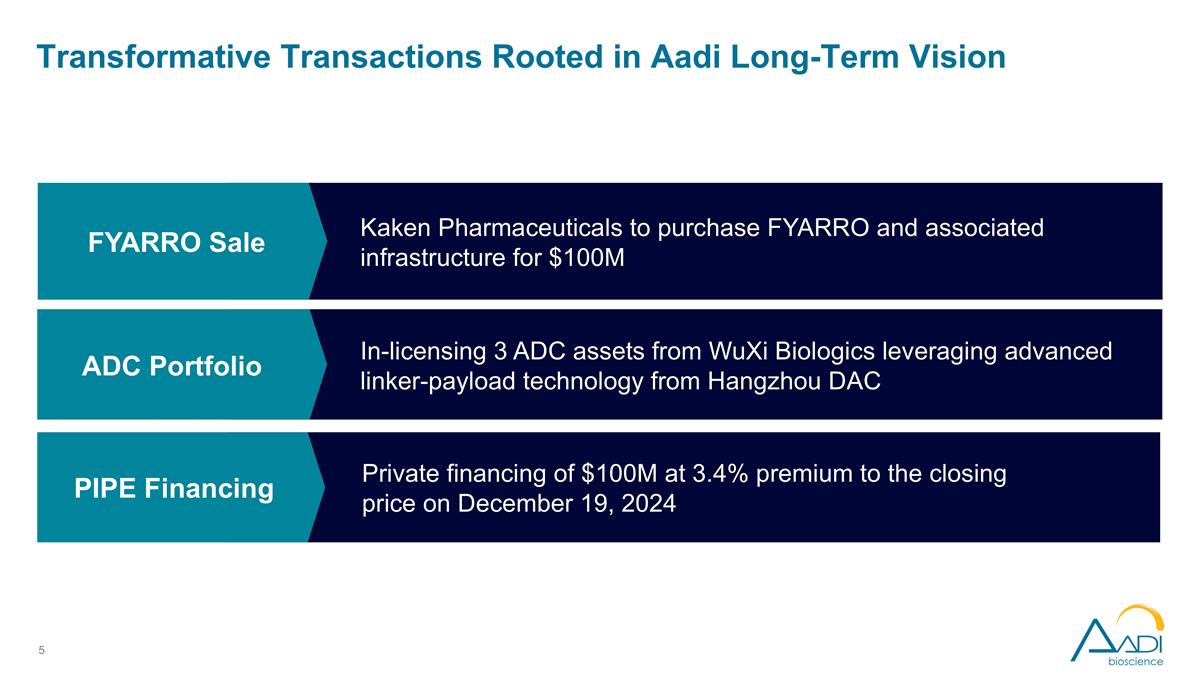
Transformative Transactions Rooted in Aadi Long-Term Vision Pipeline of three ADC portfolio targeting common, high-potential indication cancers Leveraging next wave ADC linker-payload architecture Kaken Pharmaceuticals to purchase FYARRO and associated infrastructure for $100M In-licensing 3 ADC assets from WuXi Biologics leveraging advanced linker-payload technology from Hangzhou DAC Private financing of $100M at 3.4% premium to the closing price on December 19, 2024 FYARRO Sale ADC Portfolio PIPE Financing

Kaken to Purchase FYARRO for $100M to Continue Providing This Important Treatment for Patients with Malignant PEComa Kaken will pay Aadi $100 million in cash at closing, a 4X multiple over the trailing 12 months of sales Kaken to retain associated infrastructure, including the Aadi brand and the majority of Aadi employees who support the FYARRO business Expected to close 1H’25, subject to Aadi stockholder approval and certain closing conditions

FYARRO Expands US Business for Kaken Pharmaceutical, an R&D Driven Pharmaceutical Company Based in Japan The only FDA-approved treatment for advanced malignant PEComa Cumulative sales of $58.3m since launch1 Net sales of $7.2m in Q3 2024 Consistent strong demand across major oncology centers in the US, with a ~90% reorder rate year-to-date R&D driven pharmaceutical company in Japan building sales structure in the U.S. market Acquisition positions Aadi at the center of Kaken’s sales structure in the U.S. market Greatly accelerates the building of a foundation to meet global medical needs Earnings forecast of 88,500 million yen for fiscal year 20242 1. Commercial launch on Feb 22, 2022. Sales as of close of 3Q 2024. 2.Kaken, “Consolidated Financial Results for the Six-Months Period of Fiscal 2024 (Six-Months Period ended September 30, 2024.”

Aadi In-Licensing Portfolio from WuXi Biologics in Dynamic ADC Space Licensing 3 ADC assets from WuXi Biologics utilizing advanced linker-payload from Hangzhou DAC $44M in upfront payments, cumulative development milestone payments of up to $265 million, cumulative commercial milestone payments of up to $540 million, single-digit royalties ADCs directed at promising cancer targets with broad tumor expression and precedent clinical data in high-potential indications IND filings expected in 12-24 months for all three assets
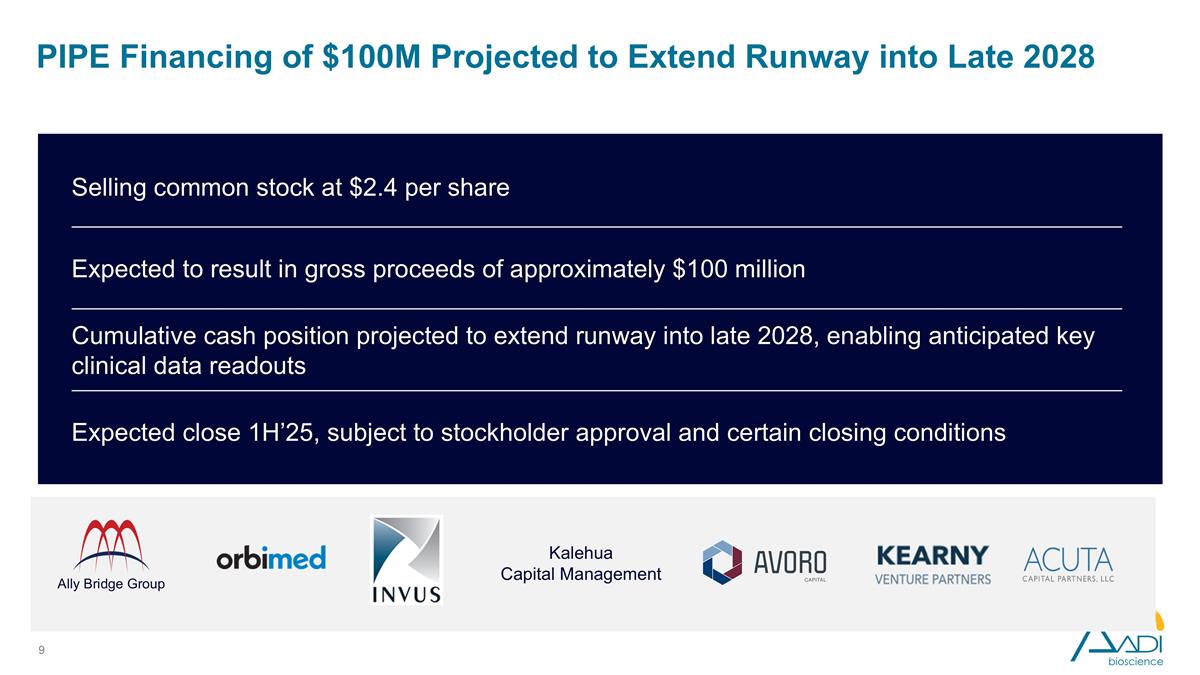
PIPE Financing of $100M Projected to Extend Runway into Late 2028 Expected to result in gross proceeds of approximately $100 million Selling common stock at $2.4 per share Cumulative cash position projected to extend runway into late 2028, enabling anticipated key clinical data readouts Expected close 1H’25, subject to stockholder approval and certain closing conditions Ally Bridge Group Kalehua Capital Management
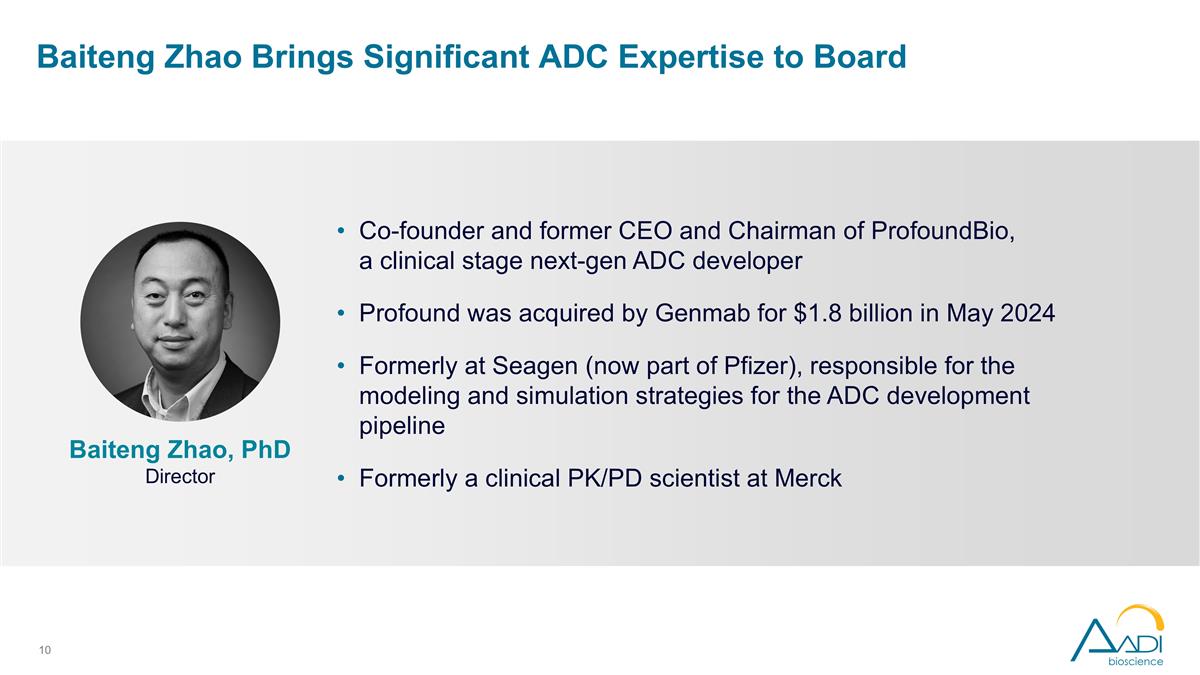
Baiteng Zhao Brings Significant ADC Expertise to Board Co-founder and former CEO and Chairman of ProfoundBio, a clinical stage next-gen ADC developer Profound was acquired by Genmab for $1.8 billion in May 2024 Formerly at Seagen (now part of Pfizer), responsible for the modeling and simulation strategies for the ADC development pipeline Formerly a clinical PK/PD scientist at Merck Baiteng Zhao, PhD Director

Aadi 2.0 Advancing Next Wave ADCs
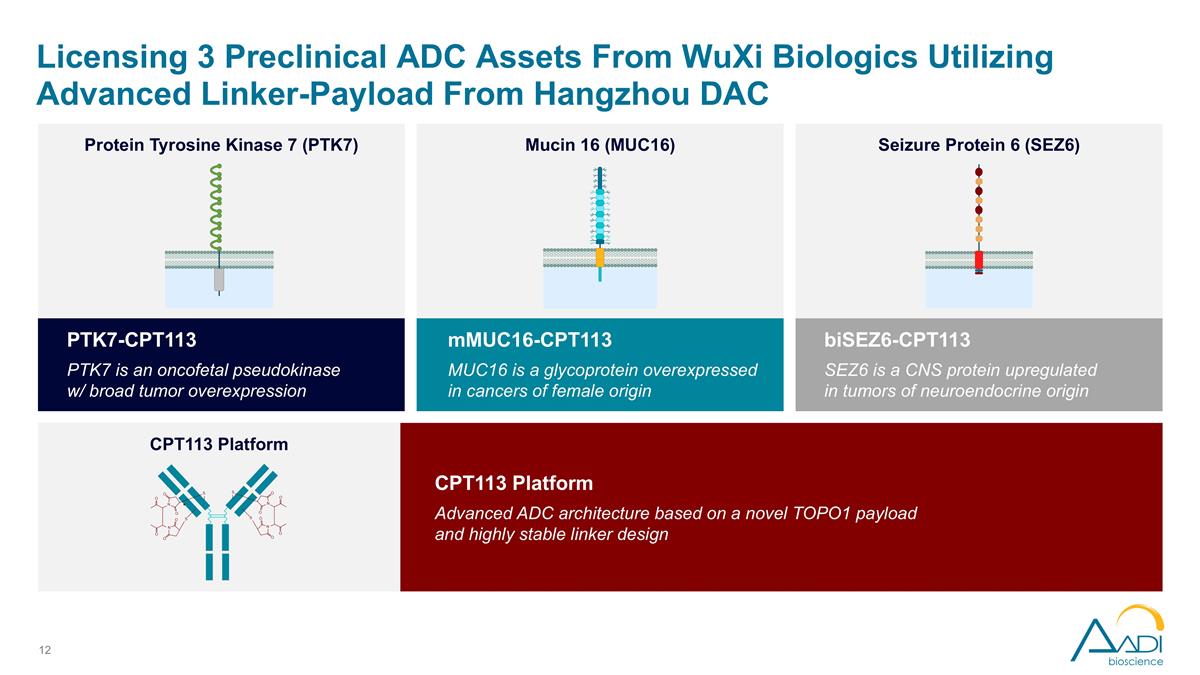
PTK7-CPT113 PTK7 is an oncofetal pseudokinase w/ broad tumor overexpression mMUC16-CPT113 MUC16 is a glycoprotein overexpressed in cancers of female origin biSEZ6-CPT113 SEZ6 is a CNS protein upregulated in tumors of neuroendocrine origin Licensing 3 Preclinical ADC Assets From WuXi Biologics Utilizing Advanced Linker-Payload From Hangzhou DAC CPT113 Platform Advanced ADC architecture based on a novel TOPO1 payload and highly stable linker design Protein Tyrosine Kinase 7 (PTK7) Mucin 16 (MUC16) Seizure Protein 6 (SEZ6) CPT113 Platform
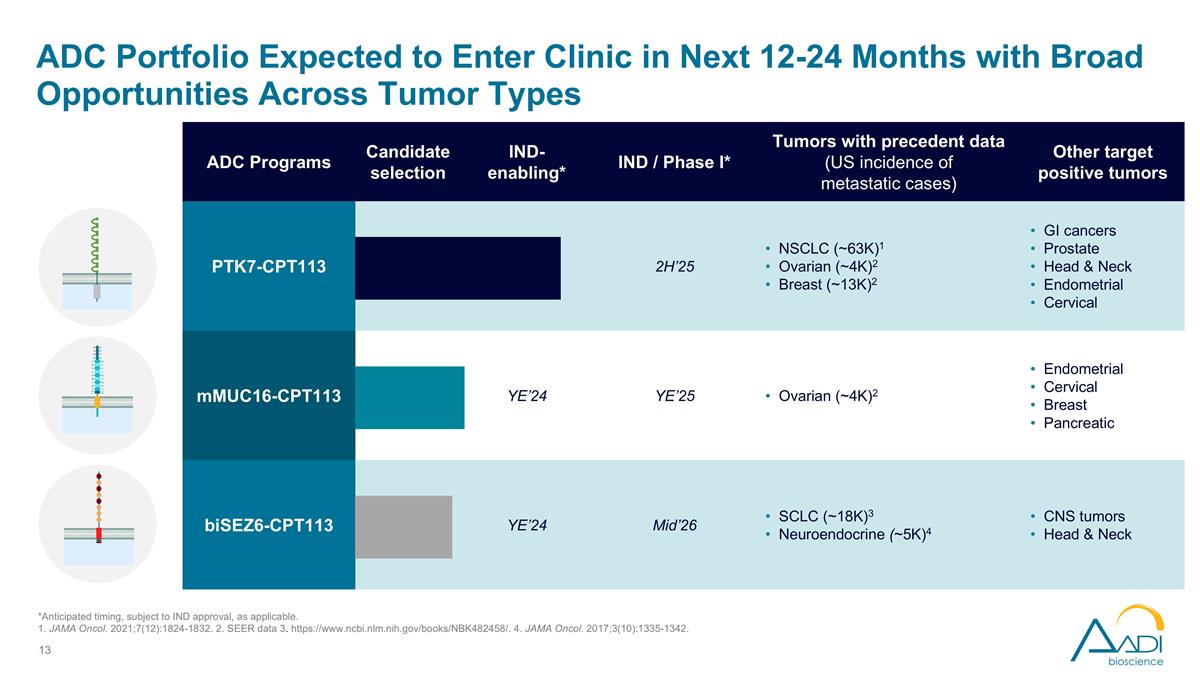
ADC Portfolio Expected to Enter Clinic in Next 12-24 Months with Broad Opportunities Across Tumor Types ADC Programs Candidate selection IND-enabling* IND / Phase I* Tumors with precedent data (US incidence of metastatic cases) Other target positive tumors PTK7-CPT113 2H’25 NSCLC (~63K)1 Ovarian (~4K)2 Breast (~13K)2 GI cancers Prostate Head & Neck Endometrial Cervical mMUC16-CPT113 YE’24 YE’25 Ovarian (~4K)2 Endometrial Cervical Breast Pancreatic biSEZ6-CPT113 YE’24 Mid’26 SCLC (~18K)3 Neuroendocrine (~5K)4 CNS tumors Head & Neck *Anticipated timing, subject to IND approval, as applicable. 1. JAMA Oncol. 2021;7(12):1824-1832. 2. SEER data 3. https://www.ncbi.nlm.nih.gov/books/NBK482458/. 4. JAMA Oncol. 2017;3(10):1335-1342.
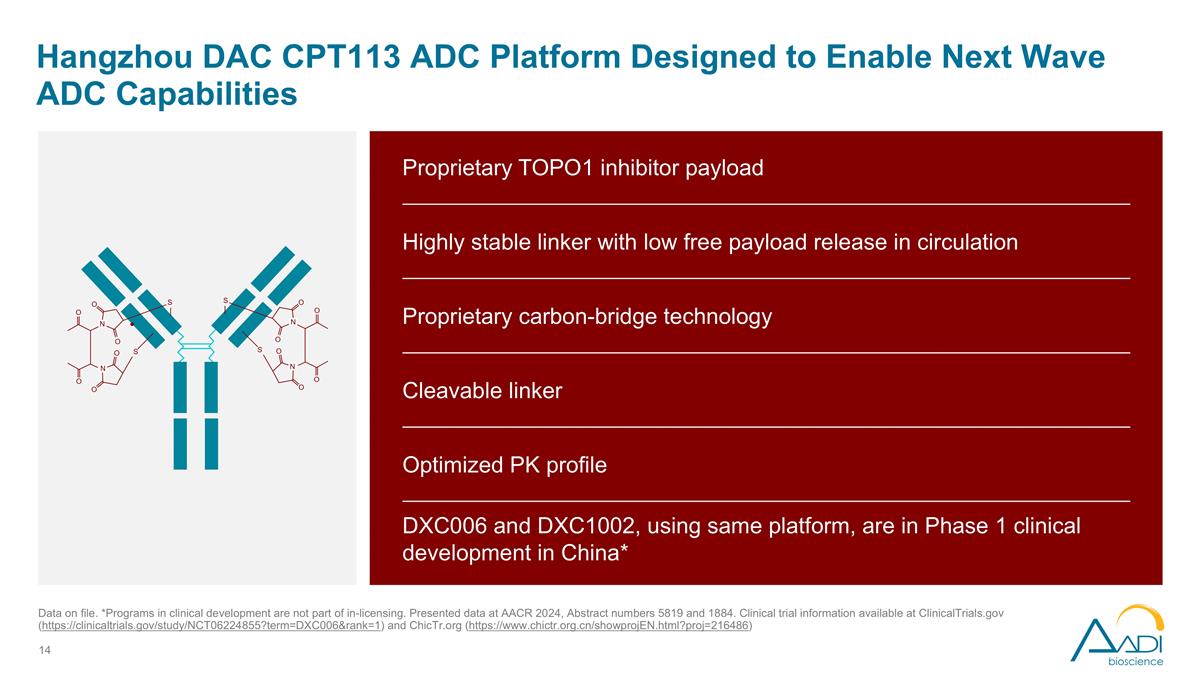
Hangzhou DAC CPT113 ADC Platform Designed to Enable Next Wave ADC Capabilities Data on file. *Programs in clinical development are not part of in-licensing. Presented data at AACR 2024, Abstract numbers 5819 and 1884. Clinical trial information available at ClinicalTrials.gov (https://clinicaltrials.gov/study/NCT06224855?term=DXC006&rank=1) and ChicTr.org (https://www.chictr.org.cn/showprojEN.html?proj=216486) Proprietary TOPO1 inhibitor payload Highly stable linker with low free payload release in circulation Proprietary carbon-bridge technology Cleavable linker Optimized PK profile DXC006 and DXC1002, using same platform, are in Phase 1 clinical development in China*
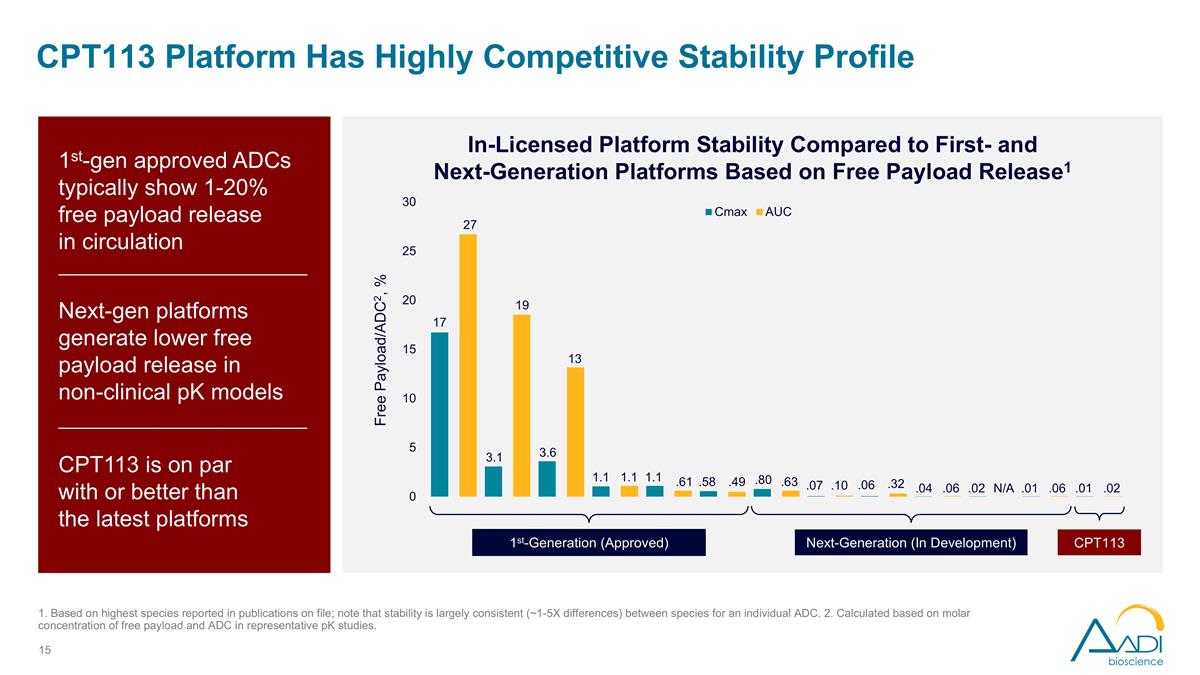
CPT113 Platform Has Highly Competitive Stability Profile In-Licensed Platform Stability Compared to First- and Next-Generation Platforms Based on Free Payload Release1 1st-gen approved ADCs typically show 1-20% free payload release in circulation Next-gen platforms generate lower free payload release in non-clinical pK models CPT113 is on par with or better than the latest platforms 1st-Generation (Approved) Next-Generation (In Development) CPT113 1. Based on highest species reported in publications on file; note that stability is largely consistent (~1-5X differences) between species for an individual ADC. 2. Calculated based on molar concentration of free payload and ADC in representative pK studies. .01 .02 .01 .06 .02 N/A .04 .06 .06 .32 .07 .10 .80 .63 ..58 .49 1.1 .61 1.1 1.1 3.6 13 3.1 19 17 27
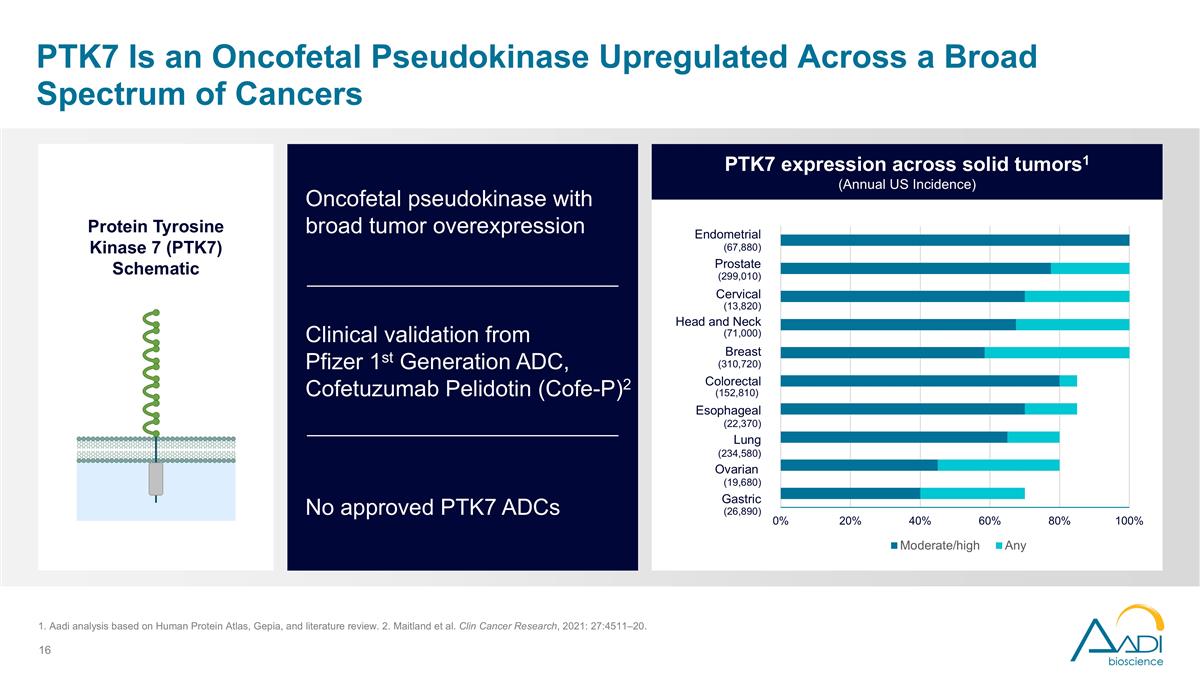
PTK7 Is an Oncofetal Pseudokinase Upregulated Across a Broad Spectrum of Cancers No approved PTK7 ADCs Oncofetal pseudokinase with broad tumor overexpression Clinical validation from Pfizer 1st Generation ADC, Cofetuzumab Pelidotin (Cofe-P)2 PTK7 expression across solid tumors1 (Annual US Incidence) (26,890) (19,680) (234,580) (22,370) (152,810) (310,720) (71,000) (13,820) (299,010) (67,880) Endometrial Prostate Cervical Head and Neck Breast Colorectal Esophageal Lung Ovarian Gastric Protein Tyrosine Kinase 7 (PTK7) Schematic 1. Aadi analysis based on Human Protein Atlas, Gepia, and literature review. 2. Maitland et al. Clin Cancer Research, 2021: 27:4511–20.
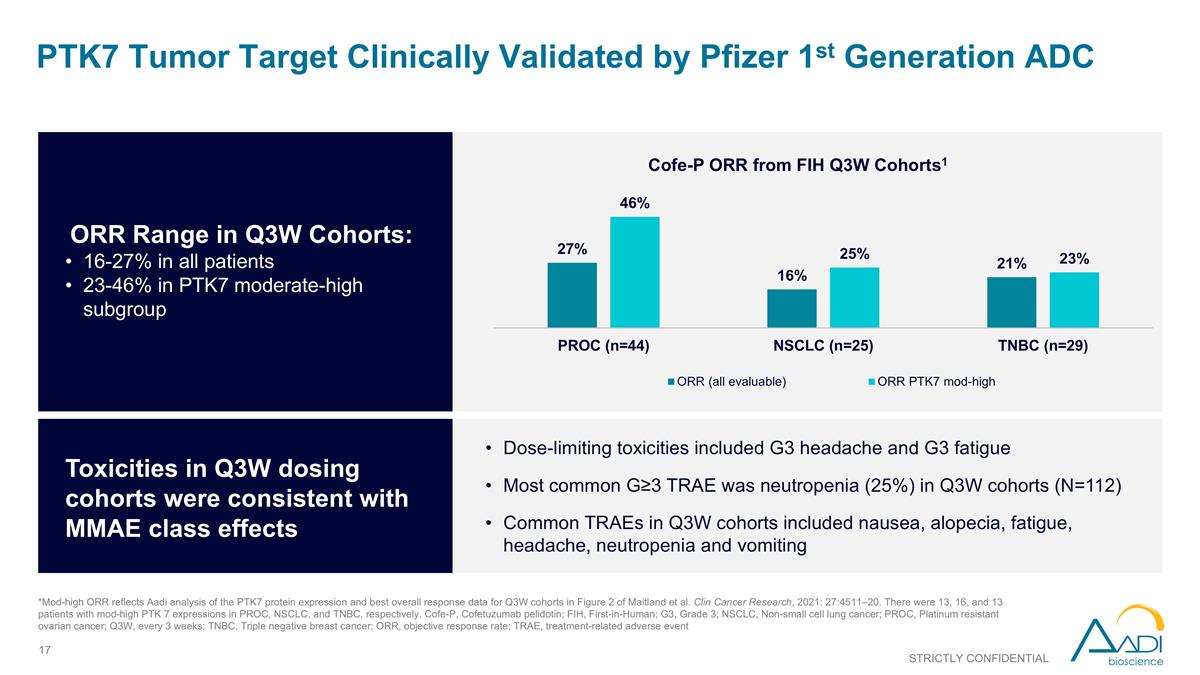
PTK7 Tumor Target Clinically Validated by Pfizer 1st Generation ADC STRICTLY CONFIDENTIAL *Mod-high ORR reflects Aadi analysis of the PTK7 protein expression and best overall response data for Q3W cohorts in Figure 2 of Maitland et al. Clin Cancer Research, 2021: 27:4511–20. There were 13, 16, and 13 patients with mod-high PTK 7 expressions in PROC, NSCLC, and TNBC, respectively. Cofe-P, Cofetuzumab pelidotin; FIH, First-in-Human; G3, Grade 3; NSCLC, Non-small cell lung cancer; PROC, Platinum resistant ovarian cancer; Q3W, every 3 weeks; TNBC, Triple negative breast cancer; ORR, objective response rate; TRAE, treatment-related adverse event ORR Range in Q3W Cohorts: 16-27% in all patients 23-46% in PTK7 moderate-high subgroup Toxicities in Q3W dosing cohorts were consistent with MMAE class effects Dose-limiting toxicities included G3 headache and G3 fatigue Most common G≥3 TRAE was neutropenia (25%) in Q3W cohorts (N=112) Common TRAEs in Q3W cohorts included nausea, alopecia, fatigue, headache, neutropenia and vomiting
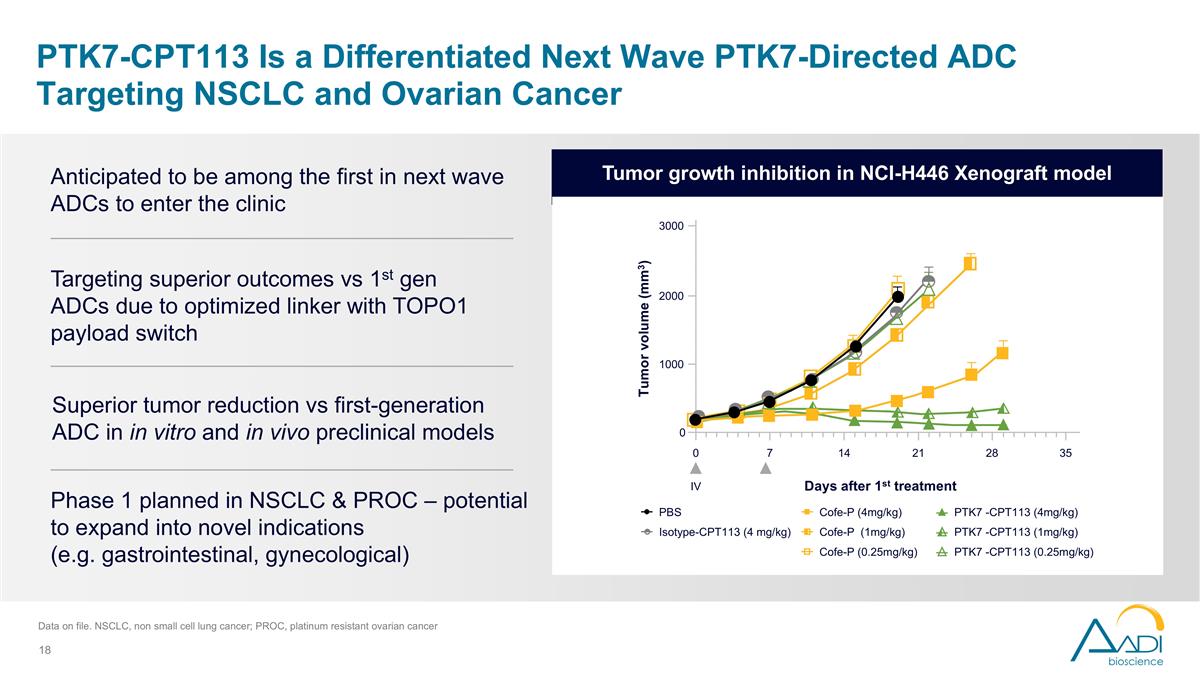
PTK7-CPT113 Is a Differentiated Next Wave PTK7-Directed ADC Targeting NSCLC and Ovarian Cancer Targeting superior outcomes vs 1st gen ADCs due to optimized linker with TOPO1 payload switch Phase 1 planned in NSCLC & PROC – potential to expand into novel indications (e.g. gastrointestinal, gynecological) Anticipated to be among the first in next wave ADCs to enter the clinic Superior tumor reduction vs first-generation ADC in in vitro and in vivo preclinical models Tumor growth inhibition in NCI-H446 Xenograft model Data on file. NSCLC, non small cell lung cancer; PROC, platinum resistant ovarian cancer PBS PTK7 -CPT113 (4mg/kg) Cofe-P (4mg/kg) PTK7 -CPT113 (0.25mg/kg) Cofe-P (0.25mg/kg) Cofe-P (1mg/kg) Isotype-CPT113 (4 mg/kg) PTK7 -CPT113 (1mg/kg) Tumor volume (mm3) Days after 1st treatment 0 0 7 14 21 28 35 1000 3000 2000 IV
MUC16 is a Cleaved Glycoprotein Expressed in Cancers Affecting Women and Contributes to Cancer Pathogenesis 1. Aadi analysis based on Human Protein Atlas, Gepia, and literature review. 2. Liu J, et al. Gynecol Oncol. 2021;163(3):473-480. Clinical validation from Genentech 1st-Gen ADC, DMUC4064A2 Glycoprotein overexpressed in cancers affecting women, as well as lung and pancreatic Shed MUC16 (or CA125) is a widely utilized biomarker for ovarian cancer MUC16 expression across tumor types1 (Annual US Incidence) Mucin 16 (MUC16) Protein Schematic
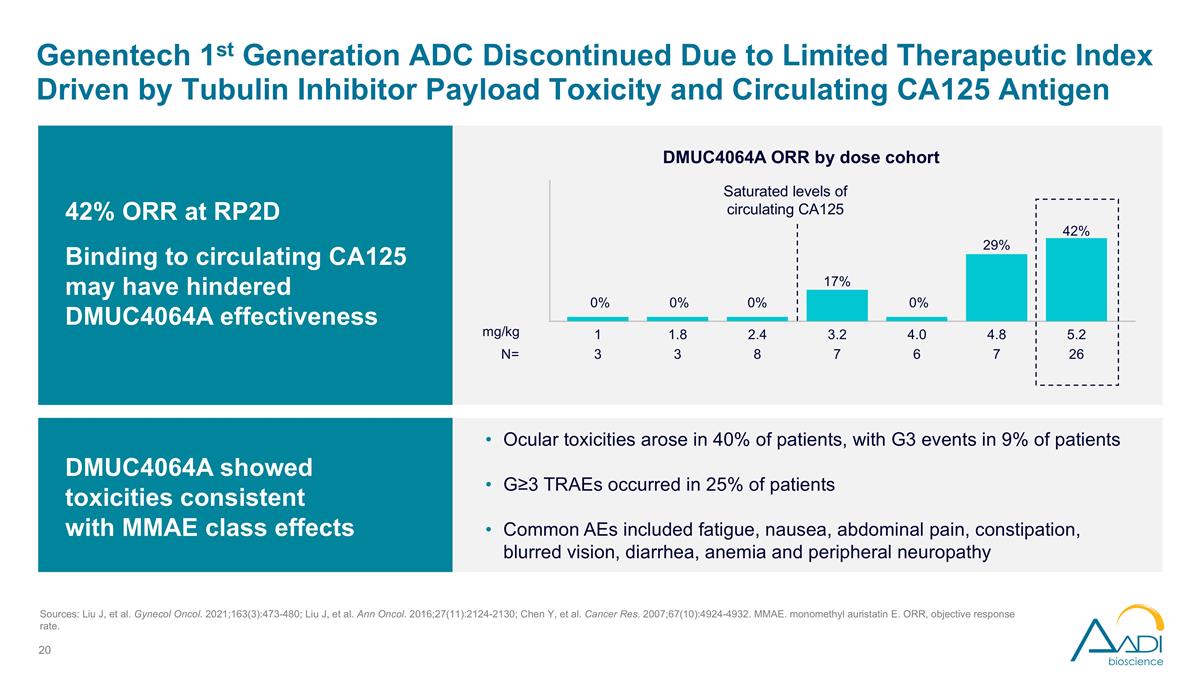
Genentech 1st Generation ADC Discontinued Due to Limited Therapeutic Index Driven by Tubulin Inhibitor Payload Toxicity and Circulating CA125 Antigen DMUC4064A showed toxicities consistent with MMAE class effects Ocular toxicities arose in 40% of patients, with G3 events in 9% of patients G≥3 TRAEs occurred in 25% of patients Common AEs included fatigue, nausea, abdominal pain, constipation, blurred vision, diarrhea, anemia and peripheral neuropathy 42% ORR at RP2D Binding to circulating CA125 may have hindered DMUC4064A effectiveness DMUC4064A ORR by dose cohort N= 3 3 8 7 6 7 26 1 1.8 2.4 3.2 4.0 4.8 5.2 0% 17% 29% 42% Saturated levels of circulating CA125 0% 0% 0% mg/kg Sources: Liu J, et al. Gynecol Oncol. 2021;163(3):473-480; Liu J, et al. Ann Oncol. 2016;27(11):2124-2130; Chen Y, et al. Cancer Res. 2007;67(10):4924-4932. MMAE. monomethyl auristatin E. ORR, objective response rate.
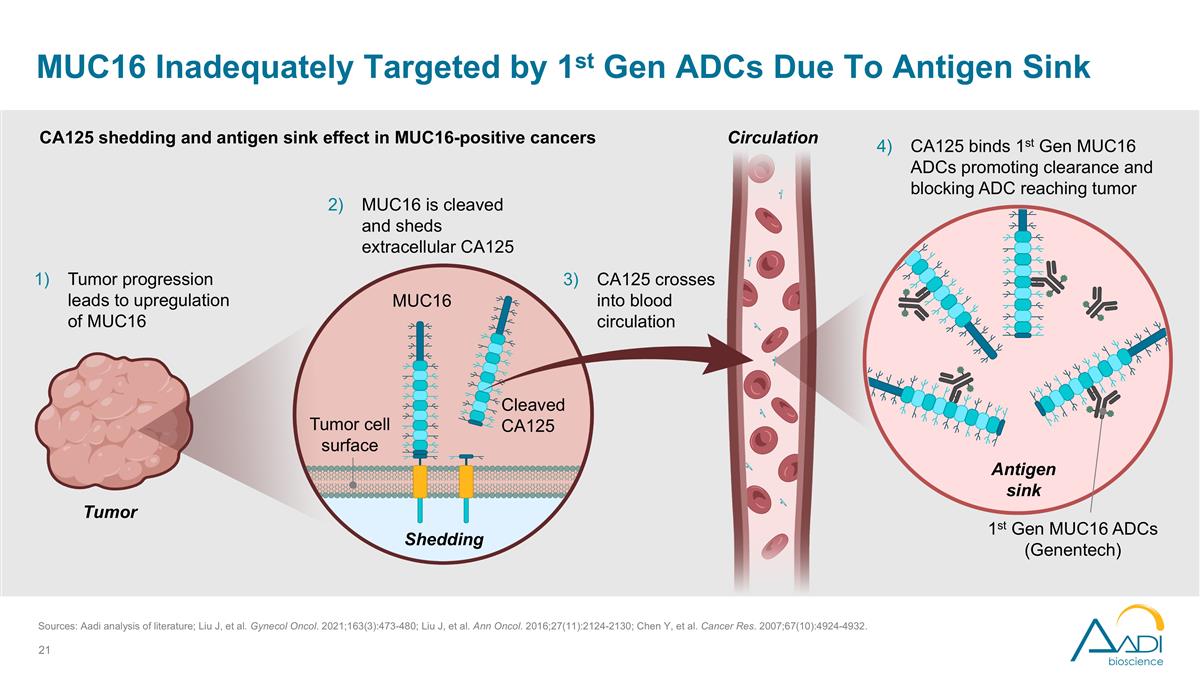
Cleaved CA125 MUC16 Tumor cell surface Tumor Tumor progression leads to upregulation of MUC16 Circulation CA125 shedding and antigen sink effect in MUC16-positive cancers 1st Gen MUC16 ADCs (Genentech) MUC16 is cleaved and sheds extracellular CA125 CA125 crosses into blood circulation CA125 binds 1st Gen MUC16 ADCs promoting clearance and blocking ADC reaching tumor MUC16 Inadequately Targeted by 1st Gen ADCs Due To Antigen Sink Shedding Antigen sink Sources: Aadi analysis of literature; Liu J, et al. Gynecol Oncol. 2021;163(3):473-480; Liu J, et al. Ann Oncol. 2016;27(11):2124-2130; Chen Y, et al. Cancer Res. 2007;67(10):4924-4932.
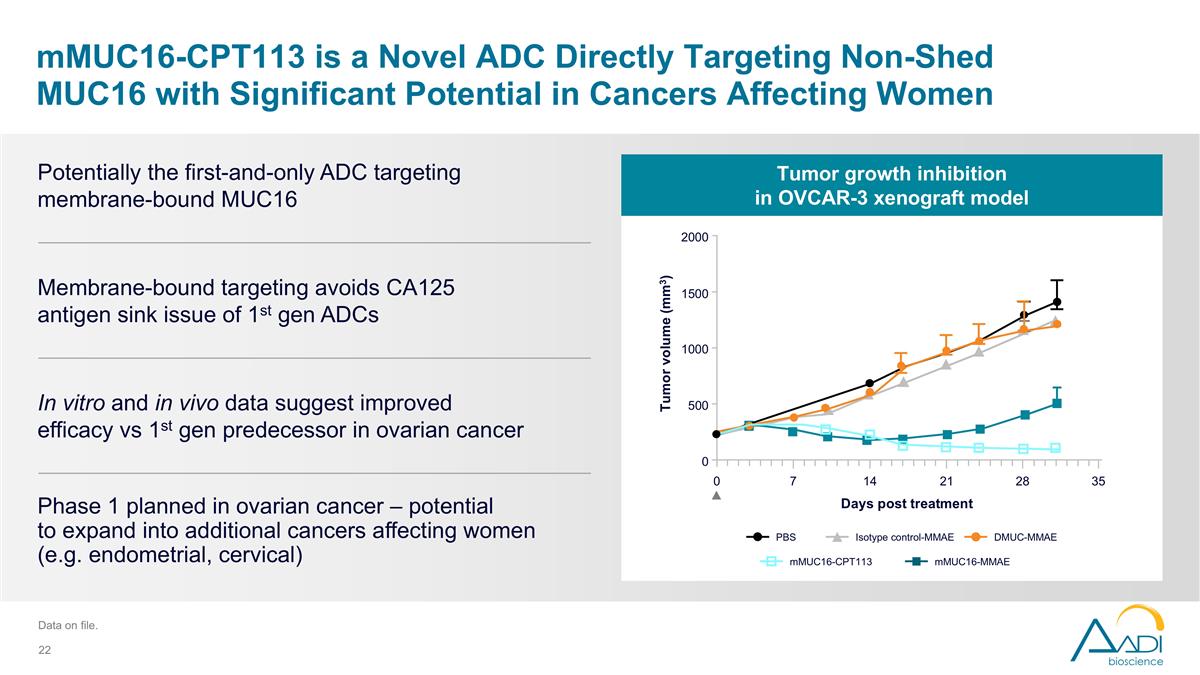
mMUC16-CPT113 is a Novel ADC Directly Targeting Non-Shed MUC16 with Significant Potential in Cancers Affecting Women Membrane-bound targeting avoids CA125 antigen sink issue of 1st gen ADCs Phase 1 planned in ovarian cancer – potential to expand into additional cancers affecting women (e.g. endometrial, cervical) Potentially the first-and-only ADC targeting membrane-bound MUC16 In vitro and in vivo data suggest improved efficacy vs 1st gen predecessor in ovarian cancer Data on file. Tumor growth inhibition in OVCAR-3 xenograft model mMUC16-MMAE mMUC16-CPT113 DMUC-MMAE PBS Isotype control-MMAE Days post treatment 2000 1500 1000 500 0 Tumor volume (mm3) 0 7 14 21 28 35
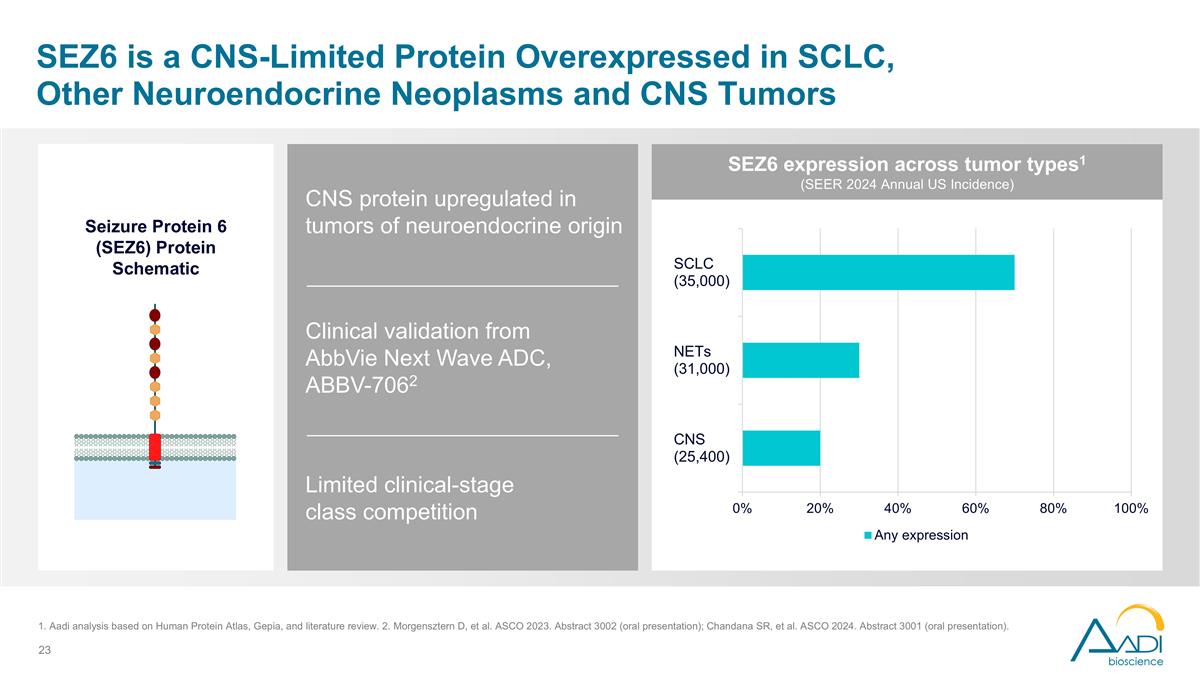
SEZ6 is a CNS-Limited Protein Overexpressed in SCLC, Other Neuroendocrine Neoplasms and CNS Tumors Clinical validation from AbbVie Next Wave ADC, ABBV-7062 Limited clinical-stage class competition CNS protein upregulated in tumors of neuroendocrine origin Seizure Protein 6 (SEZ6) Protein Schematic SEZ6 expression across tumor types1 (SEER 2024 Annual US Incidence) 1. Aadi analysis based on Human Protein Atlas, Gepia, and literature review. 2. Morgensztern D, et al. ASCO 2023. Abstract 3002 (oral presentation); Chandana SR, et al. ASCO 2024. Abstract 3001 (oral presentation).
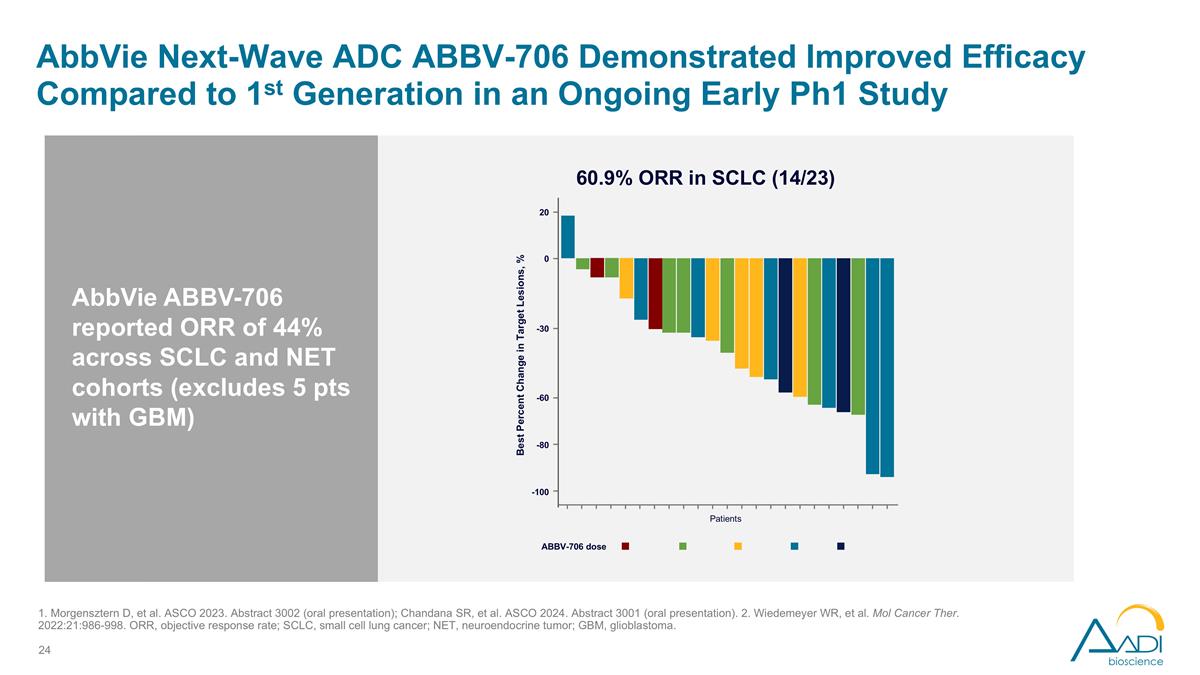
AbbVie Next-Wave ADC ABBV-706 Demonstrated Improved Efficacy Compared to 1st Generation in an Ongoing Early Ph1 Study ABBV-706 dose 60.9% ORR in SCLC (14/23) 20 0 -30 -60 -80 -100 Patients Best Percent Change in Target Lesions, % AbbVie ABBV-706 reported ORR of 44% across SCLC and NET cohorts (excludes 5 pts with GBM) 1. Morgensztern D, et al. ASCO 2023. Abstract 3002 (oral presentation); Chandana SR, et al. ASCO 2024. Abstract 3001 (oral presentation). 2. Wiedemeyer WR, et al. Mol Cancer Ther. 2022:21:986-998. ORR, objective response rate; SCLC, small cell lung cancer; NET, neuroendocrine tumor; GBM, glioblastoma.
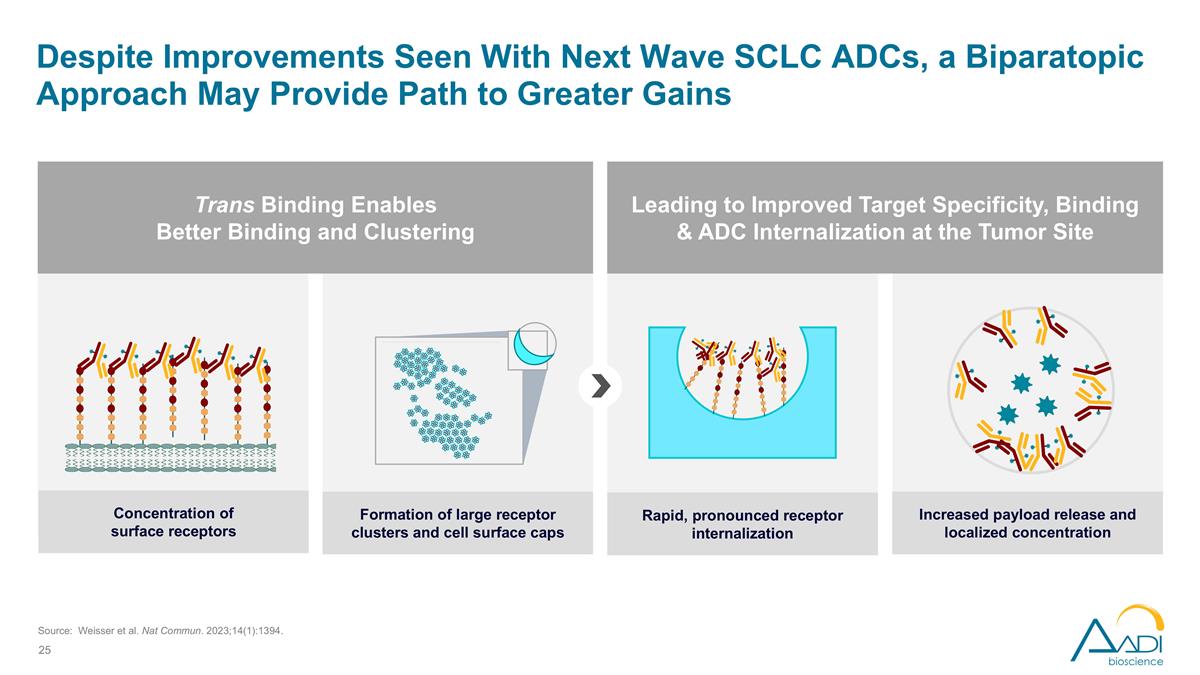
Source: Weisser et al. Nat Commun. 2023;14(1):1394. Despite Improvements Seen With Next Wave SCLC ADCs, a Biparatopic Approach May Provide Path to Greater Gains Trans Binding Enables Better Binding and Clustering Leading to Improved Target Specificity, Binding & ADC Internalization at the Tumor Site Concentration of surface receptors Formation of large receptor clusters and cell surface caps Rapid, pronounced receptor internalization Increased payload release and localized concentration
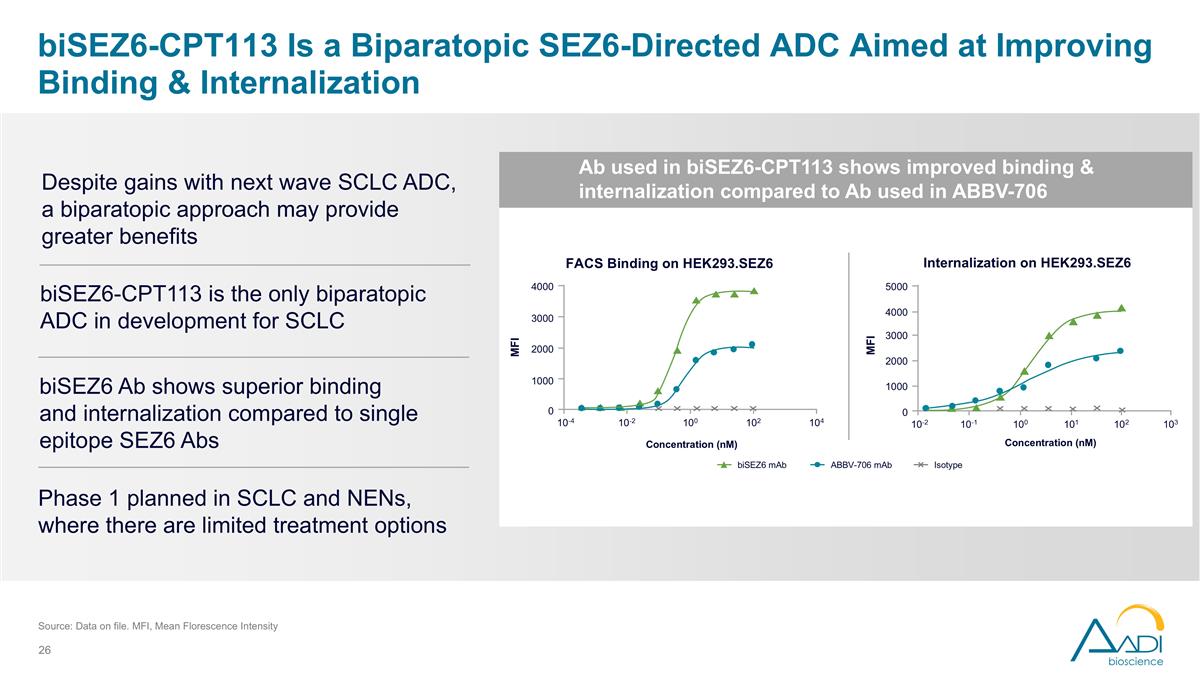
biSEZ6-CPT113 Is a Biparatopic SEZ6-Directed ADC Aimed at Improving Binding & Internalization Source: Data on file. MFI, Mean Florescence Intensity FACS Binding on HEK293.SEZ6 ABBV-706 mAb biSEZ6 mAb Isotype Internalization on HEK293.SEZ6 Concentration (nM) MFI 10-4 4000 3000 2000 1000 0 10-2 100 102 104 MFI Concentration (nM) 10-2 5000 4000 3000 2000 0 10-1 100 101 103 1000 102 Ab used in biSEZ6-CPT113 shows improved binding & internalization compared to Ab used in ABBV-706 biSEZ6-CPT113 is the only biparatopic ADC in development for SCLC Phase 1 planned in SCLC and NENs, where there are limited treatment options Despite gains with next wave SCLC ADC, a biparatopic approach may provide greater benefits biSEZ6 Ab shows superior binding and internalization compared to single epitope SEZ6 Abs
Clinically validated, broadly overexpressed tumor targets combined with next wave ADC linker-payload architecture High-potential indications with anticipated ability to compete Targeting 3 US IND submissions in 12 to 24 months, including lead asset in 2H'25 Experienced team and partners with ability to execute against development goals Post-closing cash expected to fund operations into late 2028, including anticipated key clinical data Transformative Opportunity for Aadi Value-driving potential Patient opportunity Momentum to IND submission Execution-focused Capitalized to clinical data Filing proxy statement and then convening stockholder meeting and distributing proxy materials; closing of transactions expected in the first half of 2025 Next Steps

Questions



























