
Exhibit 99.2 Developing Precision Medicines for the Treatment of Cancer EHA Data Review June 14, 2019Exhibit 99.2 Developing Precision Medicines for the Treatment of Cancer EHA Data Review June 14, 2019

Forward-Looking Statements This presentation contains forward-looking statements. Such statements include, but are not limited to, statements regarding our research, preclinical and clinical development activities, plans and projected timelines for tipifarnib, KO-947 and KO-539, plans regarding regulatory filings, our expectations regarding the relative benefits of our product candidates versus competitive therapies, and our expectations regarding the therapeutic and commercial potential of our product candidates. The words “believe,” “may,” “will,” “estimate,” “promise,” “plan”, “continue,” “anticipate,” “intend,” “expect,” “potential” and similar expressions (including the negative thereof), are intended to identify forward-looking statements. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. Risks that contribute to the uncertain nature of the forward-looking statements include: our preclinical studies and clinical trials may not be successful; the U.S. Food and Drug Administration (FDA) may not agree with our interpretation of the data from clinical trials of our product candidates; we may decide, or the FDA may require us, to conduct additional clinical trials or to modify our ongoing clinical trials; we may experience delays in the commencement, enrollment, completion or analysis of clinical testing for our product candidates, or significant issues regarding the adequacy of our clinical trial designs or the execution of our clinical trials may arise, which could result in increased costs and delays, or limit our ability to obtain regulatory approval; our product candidates may not receive regulatory approval or be successfully commercialized; unexpected adverse side effects or inadequate therapeutic efficacy of our product candidates could delay or prevent regulatory approval or commercialization; and we may not be able to obtain additional financing. Additional risks and uncertainties may emerge from time to time, and it is not possible for Kura’s management to predict all risk factors and uncertainties. All forward-looking statements contained in this presentation speak only as of the date on which they were made. Other risks and uncertainties affecting us are described more fully in our filings with the Securities and Exchange Commission. We undertake no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made.Forward-Looking Statements This presentation contains forward-looking statements. Such statements include, but are not limited to, statements regarding our research, preclinical and clinical development activities, plans and projected timelines for tipifarnib, KO-947 and KO-539, plans regarding regulatory filings, our expectations regarding the relative benefits of our product candidates versus competitive therapies, and our expectations regarding the therapeutic and commercial potential of our product candidates. The words “believe,” “may,” “will,” “estimate,” “promise,” “plan”, “continue,” “anticipate,” “intend,” “expect,” “potential” and similar expressions (including the negative thereof), are intended to identify forward-looking statements. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. Risks that contribute to the uncertain nature of the forward-looking statements include: our preclinical studies and clinical trials may not be successful; the U.S. Food and Drug Administration (FDA) may not agree with our interpretation of the data from clinical trials of our product candidates; we may decide, or the FDA may require us, to conduct additional clinical trials or to modify our ongoing clinical trials; we may experience delays in the commencement, enrollment, completion or analysis of clinical testing for our product candidates, or significant issues regarding the adequacy of our clinical trial designs or the execution of our clinical trials may arise, which could result in increased costs and delays, or limit our ability to obtain regulatory approval; our product candidates may not receive regulatory approval or be successfully commercialized; unexpected adverse side effects or inadequate therapeutic efficacy of our product candidates could delay or prevent regulatory approval or commercialization; and we may not be able to obtain additional financing. Additional risks and uncertainties may emerge from time to time, and it is not possible for Kura’s management to predict all risk factors and uncertainties. All forward-looking statements contained in this presentation speak only as of the date on which they were made. Other risks and uncertainties affecting us are described more fully in our filings with the Securities and Exchange Commission. We undertake no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made.

Agenda for Today’s Call 1) Introduction / Background 2) Data from Positive Phase 2 Trial of Tipifarnib in PTCL – EHA 2019 3) Opportunities to Expand to Additional CXCL12-Driven Indications 4) Upcoming Milestones 5) Q & A 3Agenda for Today’s Call 1) Introduction / Background 2) Data from Positive Phase 2 Trial of Tipifarnib in PTCL – EHA 2019 3) Opportunities to Expand to Additional CXCL12-Driven Indications 4) Upcoming Milestones 5) Q & A 3

Kura Oncology – Key Themes • Targeted therapies remain an essential category for new drug development in oncology • Enhanced clinical benefit in selected patient populations • Displacement of existing therapies and becoming part of the standard of care in a population of high unmet need • Rationale for understanding “exceptional responders” in the clinic • Leverage technology toward comprehensive tumor profiling • Identification of biomarkers to enrich for clinical activity • Improved understanding of mechanisms of sensitivity and resistance • A successful precision medicine based approach permits • Strategies for accelerated development and registration • Label expansion to other biomarker-guided populations • Extension to earlier lines of therapy through displacement or combination 4Kura Oncology – Key Themes • Targeted therapies remain an essential category for new drug development in oncology • Enhanced clinical benefit in selected patient populations • Displacement of existing therapies and becoming part of the standard of care in a population of high unmet need • Rationale for understanding “exceptional responders” in the clinic • Leverage technology toward comprehensive tumor profiling • Identification of biomarkers to enrich for clinical activity • Improved understanding of mechanisms of sensitivity and resistance • A successful precision medicine based approach permits • Strategies for accelerated development and registration • Label expansion to other biomarker-guided populations • Extension to earlier lines of therapy through displacement or combination 4
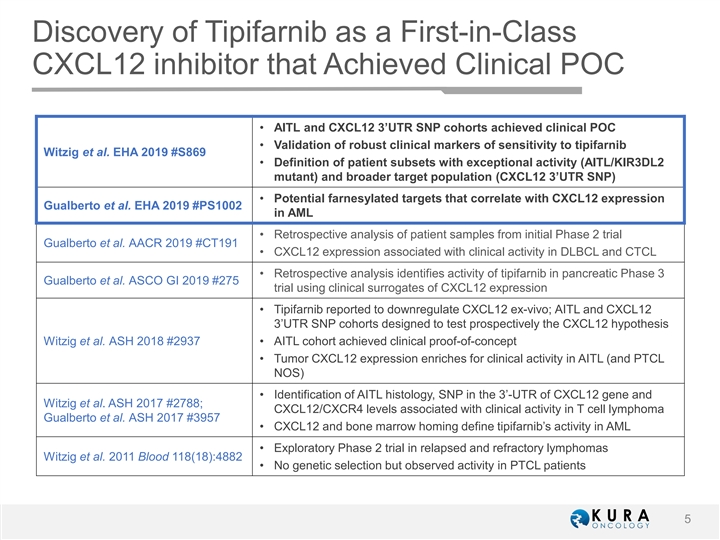
Discovery of Tipifarnib as a First-in-Class CXCL12 inhibitor that Achieved Clinical POC • AITL and CXCL12 3’UTR SNP cohorts achieved clinical POC • Validation of robust clinical markers of sensitivity to tipifarnib Witzig et al. EHA 2019 #S869 • Definition of patient subsets with exceptional activity (AITL/KIR3DL2 mutant) and broader target population (CXCL12 3’UTR SNP) • Potential farnesylated targets that correlate with CXCL12 expression Gualberto et al. EHA 2019 #PS1002 in AML • Retrospective analysis of patient samples from initial Phase 2 trial Gualberto et al. AACR 2019 #CT191 • CXCL12 expression associated with clinical activity in DLBCL and CTCL • Retrospective analysis identifies activity of tipifarnib in pancreatic Phase 3 Gualberto et al. ASCO GI 2019 #275 trial using clinical surrogates of CXCL12 expression • Tipifarnib reported to downregulate CXCL12 ex-vivo; AITL and CXCL12 3’UTR SNP cohorts designed to test prospectively the CXCL12 hypothesis Witzig et al. ASH 2018 #2937 • AITL cohort achieved clinical proof-of-concept • Tumor CXCL12 expression enriches for clinical activity in AITL (and PTCL NOS) • Identification of AITL histology, SNP in the 3’-UTR of CXCL12 gene and Witzig et al. ASH 2017 #2788; CXCL12/CXCR4 levels associated with clinical activity in T cell lymphoma Gualberto et al. ASH 2017 #3957 • CXCL12 and bone marrow homing define tipifarnib’s activity in AML • Exploratory Phase 2 trial in relapsed and refractory lymphomas Witzig et al. 2011 Blood 118(18):4882 • No genetic selection but observed activity in PTCL patients 5Discovery of Tipifarnib as a First-in-Class CXCL12 inhibitor that Achieved Clinical POC • AITL and CXCL12 3’UTR SNP cohorts achieved clinical POC • Validation of robust clinical markers of sensitivity to tipifarnib Witzig et al. EHA 2019 #S869 • Definition of patient subsets with exceptional activity (AITL/KIR3DL2 mutant) and broader target population (CXCL12 3’UTR SNP) • Potential farnesylated targets that correlate with CXCL12 expression Gualberto et al. EHA 2019 #PS1002 in AML • Retrospective analysis of patient samples from initial Phase 2 trial Gualberto et al. AACR 2019 #CT191 • CXCL12 expression associated with clinical activity in DLBCL and CTCL • Retrospective analysis identifies activity of tipifarnib in pancreatic Phase 3 Gualberto et al. ASCO GI 2019 #275 trial using clinical surrogates of CXCL12 expression • Tipifarnib reported to downregulate CXCL12 ex-vivo; AITL and CXCL12 3’UTR SNP cohorts designed to test prospectively the CXCL12 hypothesis Witzig et al. ASH 2018 #2937 • AITL cohort achieved clinical proof-of-concept • Tumor CXCL12 expression enriches for clinical activity in AITL (and PTCL NOS) • Identification of AITL histology, SNP in the 3’-UTR of CXCL12 gene and Witzig et al. ASH 2017 #2788; CXCL12/CXCR4 levels associated with clinical activity in T cell lymphoma Gualberto et al. ASH 2017 #3957 • CXCL12 and bone marrow homing define tipifarnib’s activity in AML • Exploratory Phase 2 trial in relapsed and refractory lymphomas Witzig et al. 2011 Blood 118(18):4882 • No genetic selection but observed activity in PTCL patients 5
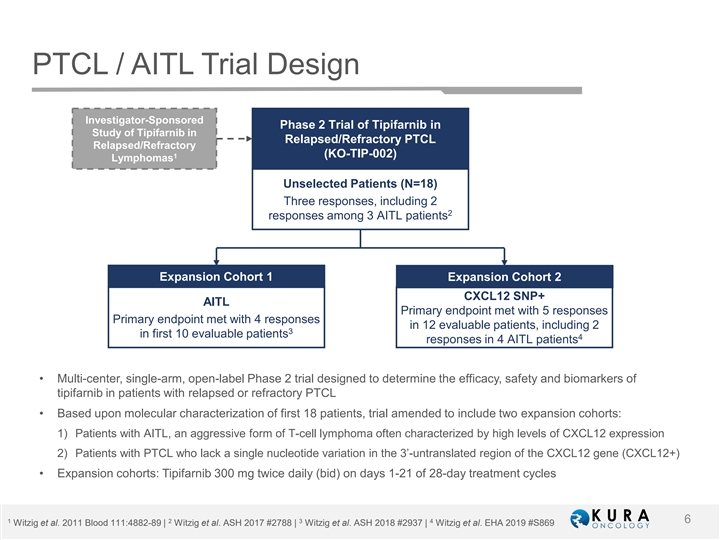
PTCL / AITL Trial Design Investigator-Sponsored Phase 2 Trial of Tipifarnib in Study of Tipifarnib in Relapsed/Refractory PTCL Relapsed/Refractory 1 (KO-TIP-002) Lymphomas Unselected Patients (N=18) Three responses, including 2 2 responses among 3 AITL patients Expansion Cohort 1 Expansion Cohort 2 CXCL12 SNP+ AITL Primary endpoint met with 5 responses Primary endpoint met with 4 responses in 12 evaluable patients, including 2 3 in first 10 evaluable patients 4 responses in 4 AITL patients • Multi-center, single-arm, open-label Phase 2 trial designed to determine the efficacy, safety and biomarkers of tipifarnib in patients with relapsed or refractory PTCL • Based upon molecular characterization of first 18 patients, trial amended to include two expansion cohorts: 1) Patients with AITL, an aggressive form of T-cell lymphoma often characterized by high levels of CXCL12 expression 2) Patients with PTCL who lack a single nucleotide variation in the 3’-untranslated region of the CXCL12 gene (CXCL12+) • Expansion cohorts: Tipifarnib 300 mg twice daily (bid) on days 1-21 of 28-day treatment cycles 1 2 3 4 6 Witzig et al. 2011 Blood 111:4882-89 | Witzig et al. ASH 2017 #2788 | Witzig et al. ASH 2018 #2937 | Witzig et al. EHA 2019 #S869PTCL / AITL Trial Design Investigator-Sponsored Phase 2 Trial of Tipifarnib in Study of Tipifarnib in Relapsed/Refractory PTCL Relapsed/Refractory 1 (KO-TIP-002) Lymphomas Unselected Patients (N=18) Three responses, including 2 2 responses among 3 AITL patients Expansion Cohort 1 Expansion Cohort 2 CXCL12 SNP+ AITL Primary endpoint met with 5 responses Primary endpoint met with 4 responses in 12 evaluable patients, including 2 3 in first 10 evaluable patients 4 responses in 4 AITL patients • Multi-center, single-arm, open-label Phase 2 trial designed to determine the efficacy, safety and biomarkers of tipifarnib in patients with relapsed or refractory PTCL • Based upon molecular characterization of first 18 patients, trial amended to include two expansion cohorts: 1) Patients with AITL, an aggressive form of T-cell lymphoma often characterized by high levels of CXCL12 expression 2) Patients with PTCL who lack a single nucleotide variation in the 3’-untranslated region of the CXCL12 gene (CXCL12+) • Expansion cohorts: Tipifarnib 300 mg twice daily (bid) on days 1-21 of 28-day treatment cycles 1 2 3 4 6 Witzig et al. 2011 Blood 111:4882-89 | Witzig et al. ASH 2017 #2788 | Witzig et al. ASH 2018 #2937 | Witzig et al. EHA 2019 #S869

Tipifarnib in Relapsed or Refractory Angioimmunoblastic T-cell Lymphoma (AITL) and CXCL12+ Peripheral T-cell Lymphoma (PTCL): Preliminary Results from an Open-Label, Phase 2 Study 1 2 3 4 5 6 Thomas Witzig , Lubomir Sokol , Won Seog Kim , Francine Foss , Eric Jacobsen , Fatima de la Cruz Vicente , 7 8 9 10 11 Dolores Caballero , Ranjana Advani , Jose Maria Roncero Vidal , Ana Marin Niebla , Antonia Rodriguez Izquierdo , 12 13 14 15 15 Raquel Oña Navarrete , Maria Jose Terol , Eva Domingo-Domenech , Marta Rodriguez , Miguel Piris , James 16 17 18 18 18 19 Bolognese , Matthew R Janes , Francis Burrows , Linda Kessler , Vishnu Mishra , Robert Curry , Michael 19 19 19 Kurman , Catherine Scholz and Antonio Gualberto 1 11 Mayo Clinic, Rochester, MN USA Hospital Universitario 12 de Octubre, Madrid, Spain 2 12 H. Lee Moffitt Cancer Center & Research Institute, Tampa, FL USA MD Anderson Cancer Center, Madrid, Spain 3 13 Samsung Medical Center, Seoul, South Korea Hospital Clinico Universitario de Valencia, Valencia, Spain 4 14 Yale University School of Medicine, New Haven, CT USA Institut Catala d'Oncologia, Barcelona, Spain 5 15 Dana-Farber Cancer Institute, Boston, MA USA Fundación Jiménez Díaz, Madrid, Spain 6 16 Hospital Universitario Virgen del Rocío, Sevilla, Spain Cytel, Cambridge, MA USA 7 17 Hospital Universitario de Salamanca, Salamanca, Spain Wellspring Biosciences, Inc., San Diego, CA USA 8 18 Stanford University Medical Center, Stanford, CA USA Kura Oncology, Inc., San Diego, CA USA 9 19 Institut Català d'Oncologia, Girona, Spain Kura Oncology, Inc., Cambridge, MA USA 10 Vall D’Hebron Institute of Oncology, Barcelona, Spain th th 24 24 C Cong ongr res ess s of of t the he E Eur uropea opean n H Hem emat atol olog ogy y A As ss soc oci iat ati ion, on, A Abs bst tr rac act t S S869 869Tipifarnib in Relapsed or Refractory Angioimmunoblastic T-cell Lymphoma (AITL) and CXCL12+ Peripheral T-cell Lymphoma (PTCL): Preliminary Results from an Open-Label, Phase 2 Study 1 2 3 4 5 6 Thomas Witzig , Lubomir Sokol , Won Seog Kim , Francine Foss , Eric Jacobsen , Fatima de la Cruz Vicente , 7 8 9 10 11 Dolores Caballero , Ranjana Advani , Jose Maria Roncero Vidal , Ana Marin Niebla , Antonia Rodriguez Izquierdo , 12 13 14 15 15 Raquel Oña Navarrete , Maria Jose Terol , Eva Domingo-Domenech , Marta Rodriguez , Miguel Piris , James 16 17 18 18 18 19 Bolognese , Matthew R Janes , Francis Burrows , Linda Kessler , Vishnu Mishra , Robert Curry , Michael 19 19 19 Kurman , Catherine Scholz and Antonio Gualberto 1 11 Mayo Clinic, Rochester, MN USA Hospital Universitario 12 de Octubre, Madrid, Spain 2 12 H. Lee Moffitt Cancer Center & Research Institute, Tampa, FL USA MD Anderson Cancer Center, Madrid, Spain 3 13 Samsung Medical Center, Seoul, South Korea Hospital Clinico Universitario de Valencia, Valencia, Spain 4 14 Yale University School of Medicine, New Haven, CT USA Institut Catala d'Oncologia, Barcelona, Spain 5 15 Dana-Farber Cancer Institute, Boston, MA USA Fundación Jiménez Díaz, Madrid, Spain 6 16 Hospital Universitario Virgen del Rocío, Sevilla, Spain Cytel, Cambridge, MA USA 7 17 Hospital Universitario de Salamanca, Salamanca, Spain Wellspring Biosciences, Inc., San Diego, CA USA 8 18 Stanford University Medical Center, Stanford, CA USA Kura Oncology, Inc., San Diego, CA USA 9 19 Institut Català d'Oncologia, Girona, Spain Kura Oncology, Inc., Cambridge, MA USA 10 Vall D’Hebron Institute of Oncology, Barcelona, Spain th th 24 24 C Cong ongr res ess s of of t the he E Eur uropea opean n H Hem emat atol olog ogy y A As ss soc oci iat ati ion, on, A Abs bst tr rac act t S S869 869
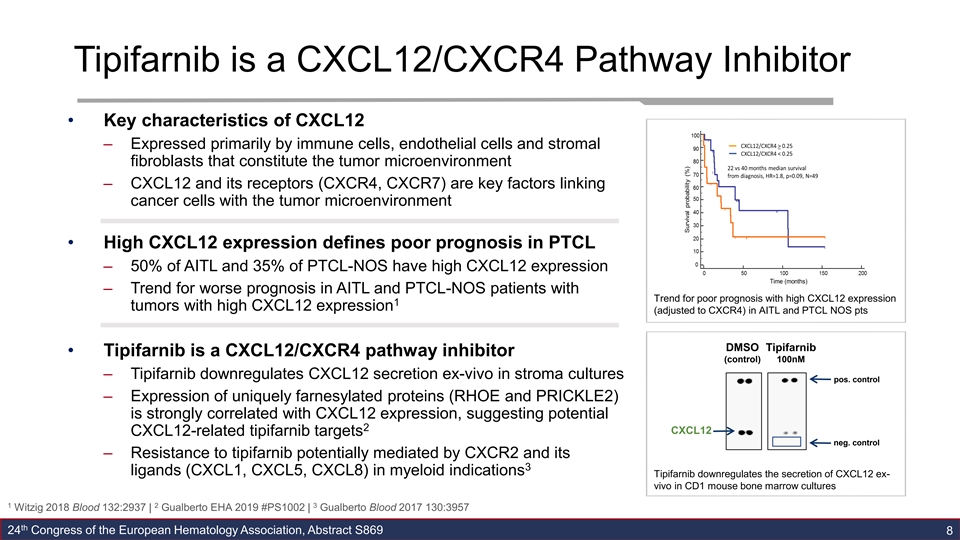
Tipifarnib is a CXCL12/CXCR4 Pathway Inhibitor • Key characteristics of CXCL12 – Expressed primarily by immune cells, endothelial cells and stromal fibroblasts that constitute the tumor microenvironment – CXCL12 and its receptors (CXCR4, CXCR7) are key factors linking cancer cells with the tumor microenvironment • High CXCL12 expression defines poor prognosis in PTCL – 50% of AITL and 35% of PTCL-NOS have high CXCL12 expression – Trend for worse prognosis in AITL and PTCL-NOS patients with Trend for poor prognosis with high CXCL12 expression 1 tumors with high CXCL12 expression (adjusted to CXCR4) in AITL and PTCL NOS pts DMSO Tipifarnib • Tipifarnib is a CXCL12/CXCR4 pathway inhibitor (control) 100nM – Tipifarnib downregulates CXCL12 secretion ex-vivo in stroma cultures pos. control – Expression of uniquely farnesylated proteins (RHOE and PRICKLE2) is strongly correlated with CXCL12 expression, suggesting potential 2 CXCL12 CXCL12-related tipifarnib targets neg. control – Resistance to tipifarnib potentially mediated by CXCR2 and its 3 ligands (CXCL1, CXCL5, CXCL8) in myeloid indications Tipifarnib downregulates the secretion of CXCL12 ex- vivo in CD1 mouse bone marrow cultures 1 2 3 Witzig 2018 Blood 132:2937 | Gualberto EHA 2019 #PS1002 | Gualberto Blood 2017 130:3957 th 24 Congress of the European Hematology Association, Abstract S869 8Tipifarnib is a CXCL12/CXCR4 Pathway Inhibitor • Key characteristics of CXCL12 – Expressed primarily by immune cells, endothelial cells and stromal fibroblasts that constitute the tumor microenvironment – CXCL12 and its receptors (CXCR4, CXCR7) are key factors linking cancer cells with the tumor microenvironment • High CXCL12 expression defines poor prognosis in PTCL – 50% of AITL and 35% of PTCL-NOS have high CXCL12 expression – Trend for worse prognosis in AITL and PTCL-NOS patients with Trend for poor prognosis with high CXCL12 expression 1 tumors with high CXCL12 expression (adjusted to CXCR4) in AITL and PTCL NOS pts DMSO Tipifarnib • Tipifarnib is a CXCL12/CXCR4 pathway inhibitor (control) 100nM – Tipifarnib downregulates CXCL12 secretion ex-vivo in stroma cultures pos. control – Expression of uniquely farnesylated proteins (RHOE and PRICKLE2) is strongly correlated with CXCL12 expression, suggesting potential 2 CXCL12 CXCL12-related tipifarnib targets neg. control – Resistance to tipifarnib potentially mediated by CXCR2 and its 3 ligands (CXCL1, CXCL5, CXCL8) in myeloid indications Tipifarnib downregulates the secretion of CXCL12 ex- vivo in CD1 mouse bone marrow cultures 1 2 3 Witzig 2018 Blood 132:2937 | Gualberto EHA 2019 #PS1002 | Gualberto Blood 2017 130:3957 th 24 Congress of the European Hematology Association, Abstract S869 8
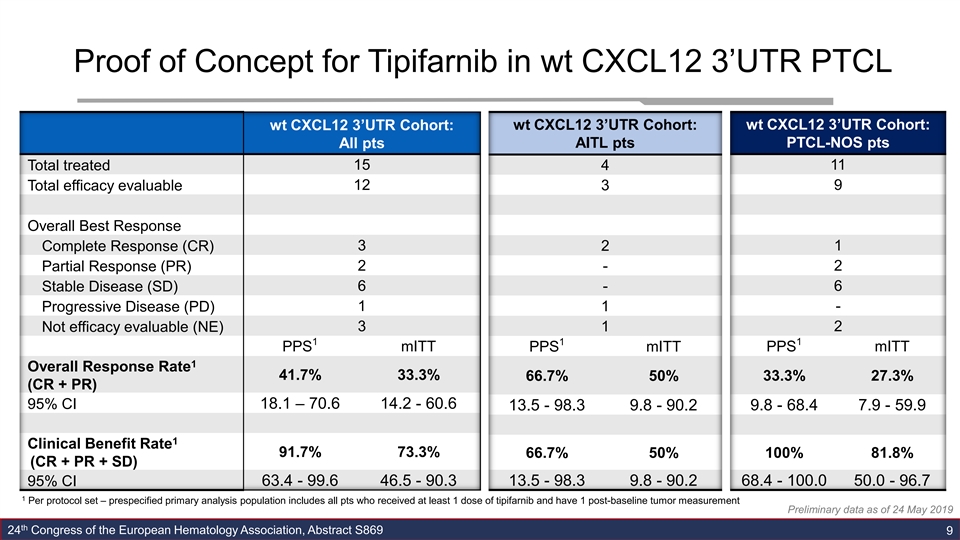
Proof of Concept for Tipifarnib in wt CXCL12 3’UTR PTCL wt CXCL12 3’UTR Cohort: wt CXCL12 3’UTR Cohort: wt CXCL12 3’UTR Cohort: All pts AITL pts PTCL-NOS pts Total treated 15 4 11 12 3 9 Total efficacy evaluable Overall Best Response Complete Response (CR) 3 2 1 Partial Response (PR) 2 - 2 6 - 6 Stable Disease (SD) 1 1 - Progressive Disease (PD) 3 2 Not efficacy evaluable (NE) 1 1 1 1 mITT mITT PPS PPS mITT PPS 1 Overall Response Rate 41.7% 33.3% 66.7% 50% 33.3% 27.3% (CR + PR) 95% CI 18.1 – 70.6 14.2 - 60.6 9.8 - 68.4 7.9 - 59.9 13.5 - 98.3 9.8 - 90.2 1 Clinical Benefit Rate 91.7% 73.3% 66.7% 50% 100% 81.8% (CR + PR + SD) 95% CI 63.4 - 99.6 46.5 - 90.3 13.5 - 98.3 9.8 - 90.2 68.4 - 100.0 50.0 - 96.7 1 Per protocol set – prespecified primary analysis population includes all pts who received at least 1 dose of tipifarnib and have 1 post-baseline tumor measurement Preliminary data as of 24 May 2019 th 24 Congress of the European Hematology Association, Abstract S869 9Proof of Concept for Tipifarnib in wt CXCL12 3’UTR PTCL wt CXCL12 3’UTR Cohort: wt CXCL12 3’UTR Cohort: wt CXCL12 3’UTR Cohort: All pts AITL pts PTCL-NOS pts Total treated 15 4 11 12 3 9 Total efficacy evaluable Overall Best Response Complete Response (CR) 3 2 1 Partial Response (PR) 2 - 2 6 - 6 Stable Disease (SD) 1 1 - Progressive Disease (PD) 3 2 Not efficacy evaluable (NE) 1 1 1 1 mITT mITT PPS PPS mITT PPS 1 Overall Response Rate 41.7% 33.3% 66.7% 50% 33.3% 27.3% (CR + PR) 95% CI 18.1 – 70.6 14.2 - 60.6 9.8 - 68.4 7.9 - 59.9 13.5 - 98.3 9.8 - 90.2 1 Clinical Benefit Rate 91.7% 73.3% 66.7% 50% 100% 81.8% (CR + PR + SD) 95% CI 63.4 - 99.6 46.5 - 90.3 13.5 - 98.3 9.8 - 90.2 68.4 - 100.0 50.0 - 96.7 1 Per protocol set – prespecified primary analysis population includes all pts who received at least 1 dose of tipifarnib and have 1 post-baseline tumor measurement Preliminary data as of 24 May 2019 th 24 Congress of the European Hematology Association, Abstract S869 9
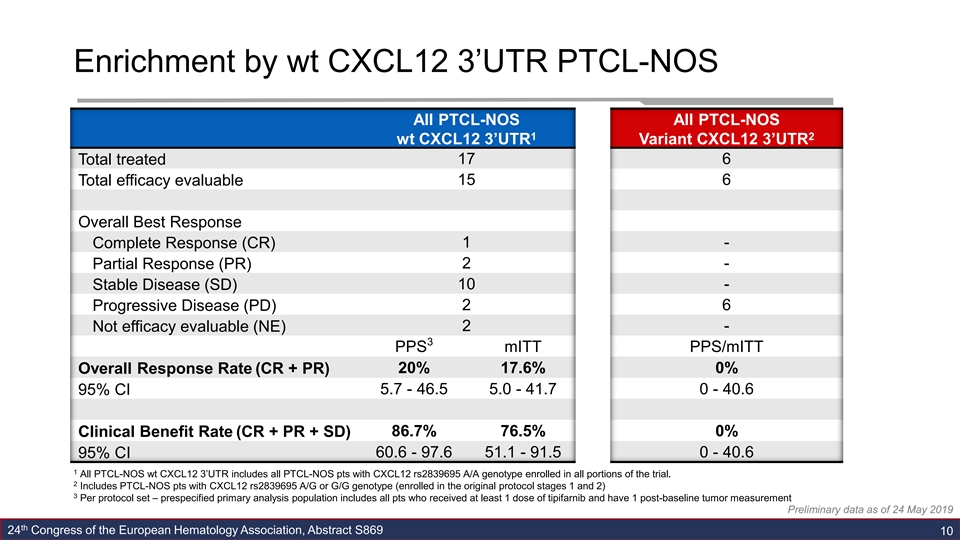
Enrichment by wt CXCL12 3’UTR PTCL-NOS All PTCL-NOS All PTCL-NOS 1 2 wt CXCL12 3’UTR Variant CXCL12 3’UTR 17 6 Total treated 15 6 Total efficacy evaluable Overall Best Response Complete Response (CR) 1 - Partial Response (PR) 2 - 10 - Stable Disease (SD) 2 6 Progressive Disease (PD) 2 - Not efficacy evaluable (NE) 3 PPS mITT PPS/mITT 20% 17.6% 0% Overall Response Rate (CR + PR) 5.7 - 46.5 5.0 - 41.7 0 - 40.6 95% CI 86.7% 76.5% 0% Clinical Benefit Rate (CR + PR + SD) 95% CI 60.6 - 97.6 51.1 - 91.5 0 - 40.6 1 All PTCL-NOS wt CXCL12 3’UTR includes all PTCL-NOS pts with CXCL12 rs2839695 A/A genotype enrolled in all portions of the trial. 2 Includes PTCL-NOS pts with CXCL12 rs2839695 A/G or G/G genotype (enrolled in the original protocol stages 1 and 2) 3 Per protocol set – prespecified primary analysis population includes all pts who received at least 1 dose of tipifarnib and have 1 post-baseline tumor measurement Preliminary data as of 24 May 2019 th 24 Congress of the European Hematology Association, Abstract S869 10Enrichment by wt CXCL12 3’UTR PTCL-NOS All PTCL-NOS All PTCL-NOS 1 2 wt CXCL12 3’UTR Variant CXCL12 3’UTR 17 6 Total treated 15 6 Total efficacy evaluable Overall Best Response Complete Response (CR) 1 - Partial Response (PR) 2 - 10 - Stable Disease (SD) 2 6 Progressive Disease (PD) 2 - Not efficacy evaluable (NE) 3 PPS mITT PPS/mITT 20% 17.6% 0% Overall Response Rate (CR + PR) 5.7 - 46.5 5.0 - 41.7 0 - 40.6 95% CI 86.7% 76.5% 0% Clinical Benefit Rate (CR + PR + SD) 95% CI 60.6 - 97.6 51.1 - 91.5 0 - 40.6 1 All PTCL-NOS wt CXCL12 3’UTR includes all PTCL-NOS pts with CXCL12 rs2839695 A/A genotype enrolled in all portions of the trial. 2 Includes PTCL-NOS pts with CXCL12 rs2839695 A/G or G/G genotype (enrolled in the original protocol stages 1 and 2) 3 Per protocol set – prespecified primary analysis population includes all pts who received at least 1 dose of tipifarnib and have 1 post-baseline tumor measurement Preliminary data as of 24 May 2019 th 24 Congress of the European Hematology Association, Abstract S869 10
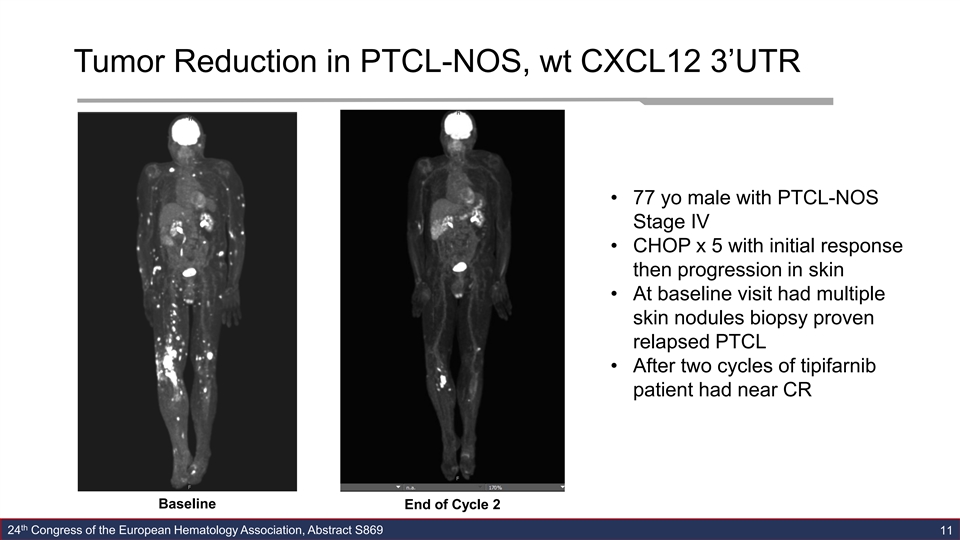
Tumor Reduction in PTCL-NOS, wt CXCL12 3’UTR • 77 yo male with PTCL-NOS Stage IV • CHOP x 5 with initial response then progression in skin • At baseline visit had multiple skin nodules biopsy proven relapsed PTCL • After two cycles of tipifarnib patient had near CR Baseline End of Cycle 2 th 24 Congress of the European Hematology Association, Abstract S869 11Tumor Reduction in PTCL-NOS, wt CXCL12 3’UTR • 77 yo male with PTCL-NOS Stage IV • CHOP x 5 with initial response then progression in skin • At baseline visit had multiple skin nodules biopsy proven relapsed PTCL • After two cycles of tipifarnib patient had near CR Baseline End of Cycle 2 th 24 Congress of the European Hematology Association, Abstract S869 11
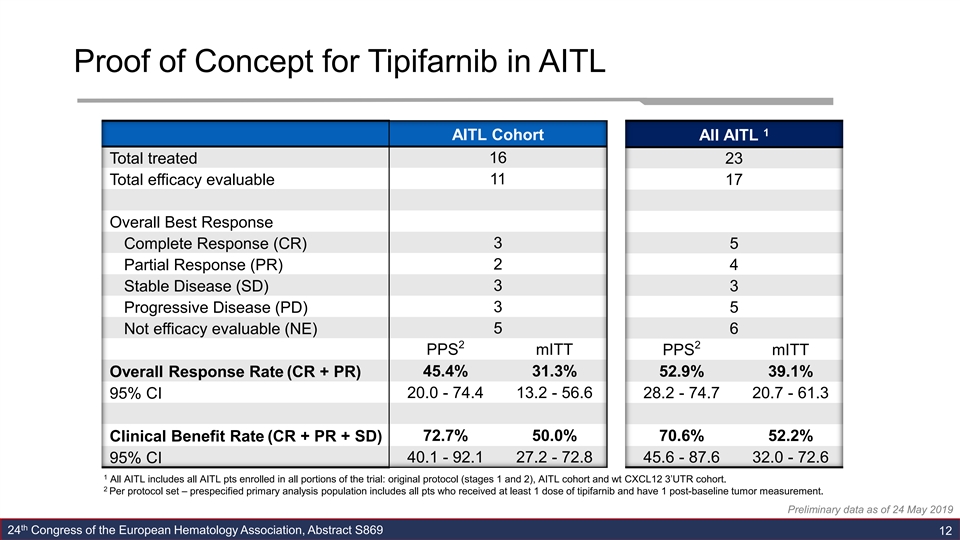
Proof of Concept for Tipifarnib in AITL 1 AITL Cohort All AITL 16 Total treated 23 11 Total efficacy evaluable 17 Overall Best Response 3 Complete Response (CR) 5 Partial Response (PR) 2 4 3 3 Stable Disease (SD) 3 Progressive Disease (PD) 5 Not efficacy evaluable (NE) 5 6 2 2 PPS mITT PPS mITT Overall Response Rate (CR + PR) 45.4% 31.3% 52.9% 39.1% 20.0 - 74.4 13.2 - 56.6 28.2 - 74.7 20.7 - 61.3 95% CI 72.7% 50.0% 70.6% 52.2% Clinical Benefit Rate (CR + PR + SD) 95% CI 40.1 - 92.1 27.2 - 72.8 45.6 - 87.6 32.0 - 72.6 1 All AITL includes all AITL pts enrolled in all portions of the trial: original protocol (stages 1 and 2), AITL cohort and wt CXCL12 3’UTR cohort. 2 Per protocol set – prespecified primary analysis population includes all pts who received at least 1 dose of tipifarnib and have 1 post-baseline tumor measurement. Preliminary data as of 24 May 2019 th 24 Congress of the European Hematology Association, Abstract S869 12Proof of Concept for Tipifarnib in AITL 1 AITL Cohort All AITL 16 Total treated 23 11 Total efficacy evaluable 17 Overall Best Response 3 Complete Response (CR) 5 Partial Response (PR) 2 4 3 3 Stable Disease (SD) 3 Progressive Disease (PD) 5 Not efficacy evaluable (NE) 5 6 2 2 PPS mITT PPS mITT Overall Response Rate (CR + PR) 45.4% 31.3% 52.9% 39.1% 20.0 - 74.4 13.2 - 56.6 28.2 - 74.7 20.7 - 61.3 95% CI 72.7% 50.0% 70.6% 52.2% Clinical Benefit Rate (CR + PR + SD) 95% CI 40.1 - 92.1 27.2 - 72.8 45.6 - 87.6 32.0 - 72.6 1 All AITL includes all AITL pts enrolled in all portions of the trial: original protocol (stages 1 and 2), AITL cohort and wt CXCL12 3’UTR cohort. 2 Per protocol set – prespecified primary analysis population includes all pts who received at least 1 dose of tipifarnib and have 1 post-baseline tumor measurement. Preliminary data as of 24 May 2019 th 24 Congress of the European Hematology Association, Abstract S869 12
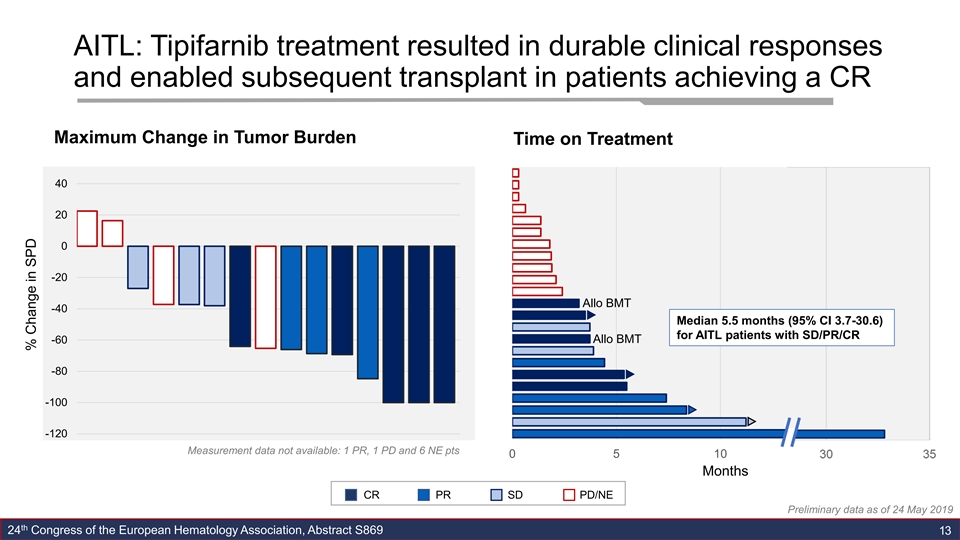
AITL: Tipifarnib treatment resulted in durable clinical responses and enabled subsequent transplant in patients achieving a CR Maximum Change in Tumor Burden Time on Treatment 40 20 0 -20 Allo BMT -40 Median 5.5 months (95% CI 3.7-30.6) for AITL patients with SD/PR/CR Allo BMT -60 -80 -100 -120 Measurement data not available: 1 PR, 1 PD and 6 NE pts 0 5 10 15 20 Months CR PR SD PD/NE Preliminary data as of 24 May 2019 th 24 Congress of the European Hematology Association, Abstract S869 13 3 % Change in SPDAITL: Tipifarnib treatment resulted in durable clinical responses and enabled subsequent transplant in patients achieving a CR Maximum Change in Tumor Burden Time on Treatment 40 20 0 -20 Allo BMT -40 Median 5.5 months (95% CI 3.7-30.6) for AITL patients with SD/PR/CR Allo BMT -60 -80 -100 -120 Measurement data not available: 1 PR, 1 PD and 6 NE pts 0 5 10 15 20 Months CR PR SD PD/NE Preliminary data as of 24 May 2019 th 24 Congress of the European Hematology Association, Abstract S869 13 3 % Change in SPD
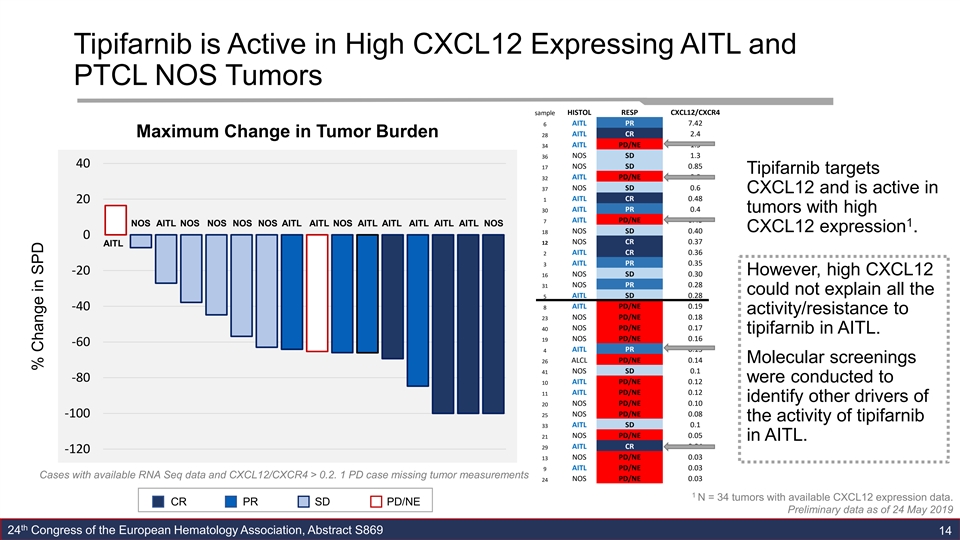
Tipifarnib is Active in High CXCL12 Expressing AITL and PTCL NOS Tumors sample HISTOL RESP CXCL12/CXCR4 6 AITL PR 7.42 Maximum Change in Tumor Burden 28 AITL CR 2.4 AITL PD/NE 1.5 34 NOS SD 1.3 36 40 17 NOS SD 0.85 Tipifarnib targets 32 AITL PD/NE 0.8 NOS SD 0.6 37 CXCL12 and is active in AITL CR 0.48 1 20 AITL PR 0.4 30 tumors with high 7 AITL PD/NE 0.43 NOS AITL NOS NOS NOS NOS AITL AITL NOS AITL AITL AITL AITL AITL NOS 1 CXCL12 expression . NOS SD 0.40 18 0 NOS CR 0.37 12 AITL AITL CR 0.36 2 3 AITL PR 0.35 -20 However, high CXCL12 16 NOS SD 0.30 NOS PR 0.28 31 could not explain all the AITL SD 0.28 5 8 AITL PD/NE 0.19 -40 activity/resistance to 23 NOS PD/NE 0.18 NOS PD/NE 0.17 40 tipifarnib in AITL. NOS PD/NE 0.16 19 -60 AITL PR 0.15 4 26 ALCL PD/NE 0.14 Molecular screenings 41 NOS SD 0.1 -80 were conducted to AITL PD/NE 0.12 10 AITL PD/NE 0.12 11 identify other drivers of 20 NOS PD/NE 0.10 25 NOS PD/NE 0.08 -100 the activity of tipifarnib AITL SD 0.1 33 NOS PD/NE 0.05 21 in AITL. AITL CR 0.04 29 -120 13 NOS PD/NE 0.03 AITL PD/NE 0.03 9 Cases with available RNA Seq data and CXCL12/CXCR4 > 0.2. 1 PD case missing tumor measurements NOS PD/NE 0.03 24 1 N = 34 tumors with available CXCL12 expression data. CR PR SD PD/NE Preliminary data as of 24 May 2019 th 24 Congress of the European Hematology Association, Abstract S869 14 % Change in SPDTipifarnib is Active in High CXCL12 Expressing AITL and PTCL NOS Tumors sample HISTOL RESP CXCL12/CXCR4 6 AITL PR 7.42 Maximum Change in Tumor Burden 28 AITL CR 2.4 AITL PD/NE 1.5 34 NOS SD 1.3 36 40 17 NOS SD 0.85 Tipifarnib targets 32 AITL PD/NE 0.8 NOS SD 0.6 37 CXCL12 and is active in AITL CR 0.48 1 20 AITL PR 0.4 30 tumors with high 7 AITL PD/NE 0.43 NOS AITL NOS NOS NOS NOS AITL AITL NOS AITL AITL AITL AITL AITL NOS 1 CXCL12 expression . NOS SD 0.40 18 0 NOS CR 0.37 12 AITL AITL CR 0.36 2 3 AITL PR 0.35 -20 However, high CXCL12 16 NOS SD 0.30 NOS PR 0.28 31 could not explain all the AITL SD 0.28 5 8 AITL PD/NE 0.19 -40 activity/resistance to 23 NOS PD/NE 0.18 NOS PD/NE 0.17 40 tipifarnib in AITL. NOS PD/NE 0.16 19 -60 AITL PR 0.15 4 26 ALCL PD/NE 0.14 Molecular screenings 41 NOS SD 0.1 -80 were conducted to AITL PD/NE 0.12 10 AITL PD/NE 0.12 11 identify other drivers of 20 NOS PD/NE 0.10 25 NOS PD/NE 0.08 -100 the activity of tipifarnib AITL SD 0.1 33 NOS PD/NE 0.05 21 in AITL. AITL CR 0.04 29 -120 13 NOS PD/NE 0.03 AITL PD/NE 0.03 9 Cases with available RNA Seq data and CXCL12/CXCR4 > 0.2. 1 PD case missing tumor measurements NOS PD/NE 0.03 24 1 N = 34 tumors with available CXCL12 expression data. CR PR SD PD/NE Preliminary data as of 24 May 2019 th 24 Congress of the European Hematology Association, Abstract S869 14 % Change in SPD
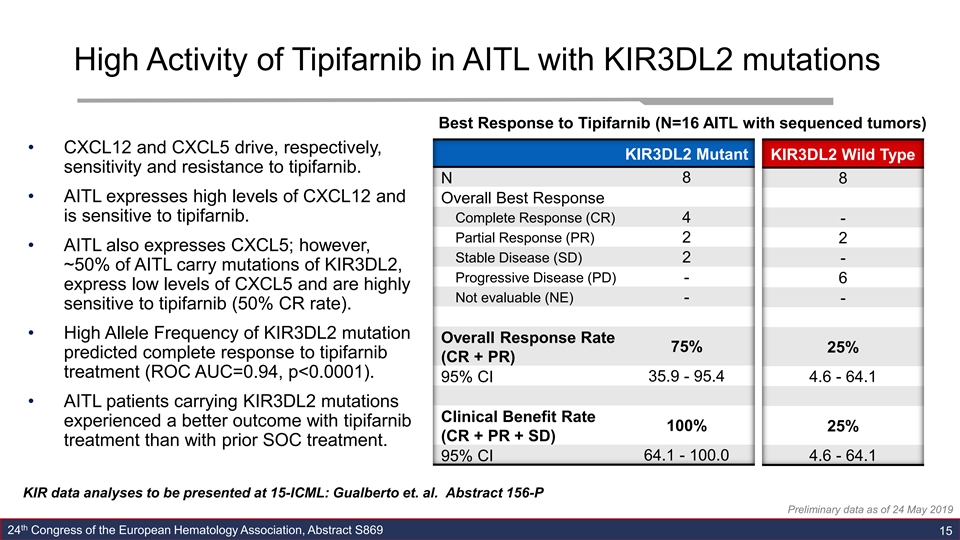
High Activity of Tipifarnib in AITL with KIR3DL2 mutations Best Response to Tipifarnib (N=16 AITL with sequenced tumors) • CXCL12 and CXCL5 drive, respectively, KIR3DL2 Mutant KIR3DL2 Wild Type sensitivity and resistance to tipifarnib. N 8 8 • AITL expresses high levels of CXCL12 and Overall Best Response is sensitive to tipifarnib. Complete Response (CR) 4 - Partial Response (PR) 2 2 • AITL also expresses CXCL5; however, Stable Disease (SD) 2 - ~50% of AITL carry mutations of KIR3DL2, Progressive Disease (PD) - 6 express low levels of CXCL5 and are highly Not evaluable (NE) - - sensitive to tipifarnib (50% CR rate). • High Allele Frequency of KIR3DL2 mutation Overall Response Rate 75% 25% predicted complete response to tipifarnib (CR + PR) treatment (ROC AUC=0.94, p<0.0001). 95% CI 35.9 - 95.4 4.6 - 64.1 • AITL patients carrying KIR3DL2 mutations Clinical Benefit Rate experienced a better outcome with tipifarnib 100% 25% (CR + PR + SD) treatment than with prior SOC treatment. 95% CI 64.1 - 100.0 4.6 - 64.1 KIR data analyses to be presented at 15-ICML: Gualberto et. al. Abstract 156-P Preliminary data as of 24 May 2019 th 24 Congress of the European Hematology Association, Abstract S869 15High Activity of Tipifarnib in AITL with KIR3DL2 mutations Best Response to Tipifarnib (N=16 AITL with sequenced tumors) • CXCL12 and CXCL5 drive, respectively, KIR3DL2 Mutant KIR3DL2 Wild Type sensitivity and resistance to tipifarnib. N 8 8 • AITL expresses high levels of CXCL12 and Overall Best Response is sensitive to tipifarnib. Complete Response (CR) 4 - Partial Response (PR) 2 2 • AITL also expresses CXCL5; however, Stable Disease (SD) 2 - ~50% of AITL carry mutations of KIR3DL2, Progressive Disease (PD) - 6 express low levels of CXCL5 and are highly Not evaluable (NE) - - sensitive to tipifarnib (50% CR rate). • High Allele Frequency of KIR3DL2 mutation Overall Response Rate 75% 25% predicted complete response to tipifarnib (CR + PR) treatment (ROC AUC=0.94, p<0.0001). 95% CI 35.9 - 95.4 4.6 - 64.1 • AITL patients carrying KIR3DL2 mutations Clinical Benefit Rate experienced a better outcome with tipifarnib 100% 25% (CR + PR + SD) treatment than with prior SOC treatment. 95% CI 64.1 - 100.0 4.6 - 64.1 KIR data analyses to be presented at 15-ICML: Gualberto et. al. Abstract 156-P Preliminary data as of 24 May 2019 th 24 Congress of the European Hematology Association, Abstract S869 15
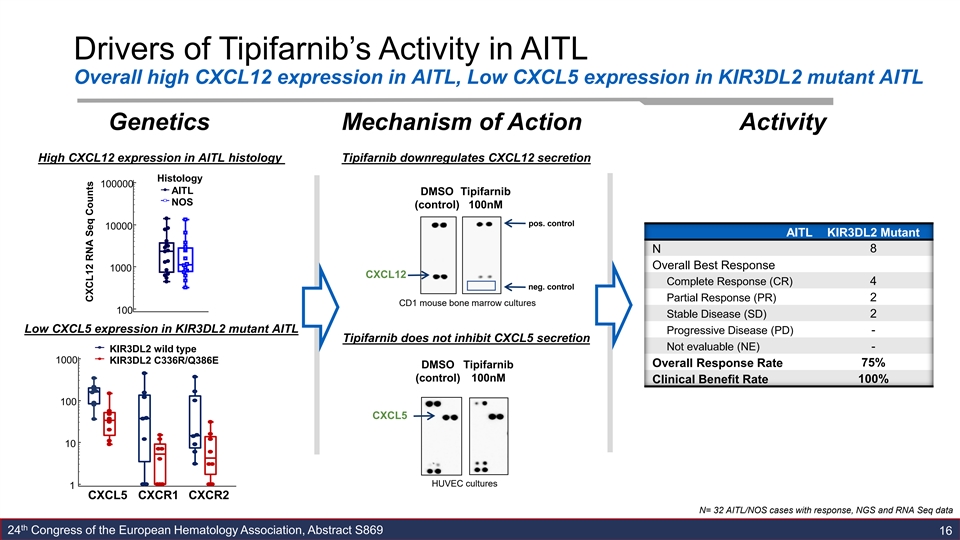
Drivers of Tipifarnib’s Activity in AITL Overall high CXCL12 expression in AITL, Low CXCL5 expression in KIR3DL2 mutant AITL Genetics Mechanism of Action Activity High CXCL12 expression in AITL histology Tipifarnib downregulates CXCL12 secretion Histology 100000 AITL DMSO Tipifarnib NOS (control) 100nM pos. control 10000 AITL KIR3DL2 Mutant 8 N Overall Best Response 1000 CXCL12 Complete Response (CR) 4 neg. control 2 Partial Response (PR) CD1 mouse bone marrow cultures 100 Stable Disease (SD) 2 Low CXCL5 expression in KIR3DL2 mutant AITL Progressive Disease (PD) - Tipifarnib does not inhibit CXCL5 secretion Not evaluable (NE) - KIR3DL2 wild type 1000 KIR3DL2 C336R/Q386E Overall Response Rate 75% DMSO Tipifarnib (control) 100nM Clinical Benefit Rate 100% 100 CXCL5 10 HUVEC cultures 1 CXCL5 CXCR1 CXCR2 N= 32 AITL/NOS cases with response, NGS and RNA Seq data th 24 Congress of the European Hematology Association, Abstract S869 16 CXCL12 RNA Seq CountsDrivers of Tipifarnib’s Activity in AITL Overall high CXCL12 expression in AITL, Low CXCL5 expression in KIR3DL2 mutant AITL Genetics Mechanism of Action Activity High CXCL12 expression in AITL histology Tipifarnib downregulates CXCL12 secretion Histology 100000 AITL DMSO Tipifarnib NOS (control) 100nM pos. control 10000 AITL KIR3DL2 Mutant 8 N Overall Best Response 1000 CXCL12 Complete Response (CR) 4 neg. control 2 Partial Response (PR) CD1 mouse bone marrow cultures 100 Stable Disease (SD) 2 Low CXCL5 expression in KIR3DL2 mutant AITL Progressive Disease (PD) - Tipifarnib does not inhibit CXCL5 secretion Not evaluable (NE) - KIR3DL2 wild type 1000 KIR3DL2 C336R/Q386E Overall Response Rate 75% DMSO Tipifarnib (control) 100nM Clinical Benefit Rate 100% 100 CXCL5 10 HUVEC cultures 1 CXCL5 CXCR1 CXCR2 N= 32 AITL/NOS cases with response, NGS and RNA Seq data th 24 Congress of the European Hematology Association, Abstract S869 16 CXCL12 RNA Seq Counts
Conclusions • The AITL and wt CXCL12 3’UTR cohorts met pre-specified statistical hypotheses supporting proof-of-concept for tipifarnib in PTCL. • Tipifarnib is active in AITL pts and in PTCL-NOS pts with wt CXCL12 3’UTR – AITL: 53% ORR (all subjects, PPS) – PTCL-NOS with wt CXCL12 3’UTR: 20% ORR (all subjects, PPS). • KIR3DL2 and CXCL12 genotype provide robust tools for the selection/stratification of patients: – CXCL12 genotype may enrich for CXCL12 expression and tipifarnib activity, particularly in PTCL-NOS (86.7% Clinical Benefit Rate for PTCL-NOS patients with wt CXCL12 3’UTR). – KIR3DL2 C336R/Q383E mutations may enrich for low CXCL5 expression and anti-tumor activity in AITL (75% ORR, 50% CR rate). – Approximately 50% of AITL carry KIR3DL2 mutations and 70% of PTCL carry reference (wild type) CXCL12 3’UTR rs2839695 sequences. • TEAEs were consistent with the known safety profile of tipifarnib. – Treatment with tipifarnib 300 mg bid days 1-21 every 28-days was generally well tolerated. The majority of Grade ≥ 3 TEAEs were hematological events managed with best supportive care. • These results suggest that further evaluation of tipifarnib in biomarker defined subsets of PTCL and CTCL would be of interest. th 24 Congress of the European Hematology Association, Abstract S869 17Conclusions • The AITL and wt CXCL12 3’UTR cohorts met pre-specified statistical hypotheses supporting proof-of-concept for tipifarnib in PTCL. • Tipifarnib is active in AITL pts and in PTCL-NOS pts with wt CXCL12 3’UTR – AITL: 53% ORR (all subjects, PPS) – PTCL-NOS with wt CXCL12 3’UTR: 20% ORR (all subjects, PPS). • KIR3DL2 and CXCL12 genotype provide robust tools for the selection/stratification of patients: – CXCL12 genotype may enrich for CXCL12 expression and tipifarnib activity, particularly in PTCL-NOS (86.7% Clinical Benefit Rate for PTCL-NOS patients with wt CXCL12 3’UTR). – KIR3DL2 C336R/Q383E mutations may enrich for low CXCL5 expression and anti-tumor activity in AITL (75% ORR, 50% CR rate). – Approximately 50% of AITL carry KIR3DL2 mutations and 70% of PTCL carry reference (wild type) CXCL12 3’UTR rs2839695 sequences. • TEAEs were consistent with the known safety profile of tipifarnib. – Treatment with tipifarnib 300 mg bid days 1-21 every 28-days was generally well tolerated. The majority of Grade ≥ 3 TEAEs were hematological events managed with best supportive care. • These results suggest that further evaluation of tipifarnib in biomarker defined subsets of PTCL and CTCL would be of interest. th 24 Congress of the European Hematology Association, Abstract S869 17
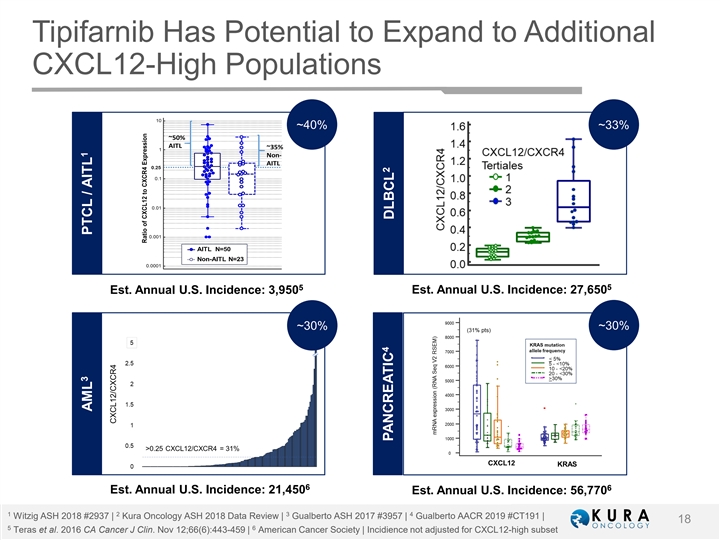
Tipifarnib Has Potential to Expand to Additional CXCL12-High Populations ~40% ~33% 5 5 Est. Annual U.S. Incidence: 3,950 Est. Annual U.S. Incidence: 27,650 ~30% ~30% 6 6 Est. Annual U.S. Incidence: 21,450 Est. Annual U.S. Incidence: 56,770 1 2 3 4 Witzig ASH 2018 #2937 | Kura Oncology ASH 2018 Data Review | Gualberto ASH 2017 #3957 | Gualberto AACR 2019 #CT191 | 18 5 6 Teras et al. 2016 CA Cancer J Clin. Nov 12;66(6):443-459 | American Cancer Society | Incidience not adjusted for CXCL12-high subset 3 1 AML PTCL / AITL 4 2 PANCREATIC DLBCLTipifarnib Has Potential to Expand to Additional CXCL12-High Populations ~40% ~33% 5 5 Est. Annual U.S. Incidence: 3,950 Est. Annual U.S. Incidence: 27,650 ~30% ~30% 6 6 Est. Annual U.S. Incidence: 21,450 Est. Annual U.S. Incidence: 56,770 1 2 3 4 Witzig ASH 2018 #2937 | Kura Oncology ASH 2018 Data Review | Gualberto ASH 2017 #3957 | Gualberto AACR 2019 #CT191 | 18 5 6 Teras et al. 2016 CA Cancer J Clin. Nov 12;66(6):443-459 | American Cancer Society | Incidience not adjusted for CXCL12-high subset 3 1 AML PTCL / AITL 4 2 PANCREATIC DLBCL
Key Takeaways • POC achieved in AITL and wt CXCL12 3’UTR extension cohorts • AITL and related lymphomas represent approximately one-third of PTCL cases • KIR3DL2 and CXCL12 genotype provide additional robust tools for the selection/stratification of PTCL patients • Approximately 50% of AITL carries KIR3DL2 mutations and 70% of PTCL carries reference (wild type) CXCL12 3’UTR rs2839695 sequences • Company believes results support multiple potential pathways to registration in AITL/PTCL and plans to seek regulatory feedback • Potential for CXCL12 variations/mutations to predict clinical activity in additional indications, including DLBCL, AML and pancreatic cancer 19Key Takeaways • POC achieved in AITL and wt CXCL12 3’UTR extension cohorts • AITL and related lymphomas represent approximately one-third of PTCL cases • KIR3DL2 and CXCL12 genotype provide additional robust tools for the selection/stratification of PTCL patients • Approximately 50% of AITL carries KIR3DL2 mutations and 70% of PTCL carries reference (wild type) CXCL12 3’UTR rs2839695 sequences • Company believes results support multiple potential pathways to registration in AITL/PTCL and plans to seek regulatory feedback • Potential for CXCL12 variations/mutations to predict clinical activity in additional indications, including DLBCL, AML and pancreatic cancer 19
Anticipated Milestones & Financial Highlights Phase 1 Program Milestones Status HRAS Initiation of registration-direct1ed trial in HNSCC ü Mutant Additional data from Phase 2 trial in HNSCC and other SCCs 2H 2019 Indications Tipifarnib Patents for tipifarnib in AITL and CXCL12+ PTCL/AML ü Farnesyl Transferase Inhibitor CXCL12 Proof-of-concept in AITL ü Pathway Positive Phase 2 trial in PTCL Indications ü Additional data from Phase 2 trial in CMML 2019 Potential biomarker of activity in squamous cell carcinomas ü KO-947 ERK Inhibitor Data from Phase 1 dose-escalation trial 2019 FDA clearance of IND application ü KO-539 Menin-MLL Inhibitor Initiation of Phase 1 trial Mid-2019 Nasdaq: KURA Financial Shares outstanding: 38.2M basic, 4.3M options* $187.4M Highlights Cash, cash equivalents and short-term investments: $165.5M* 20 * As of March 31, 2019Anticipated Milestones & Financial Highlights Phase 1 Program Milestones Status HRAS Initiation of registration-direct1ed trial in HNSCC ü Mutant Additional data from Phase 2 trial in HNSCC and other SCCs 2H 2019 Indications Tipifarnib Patents for tipifarnib in AITL and CXCL12+ PTCL/AML ü Farnesyl Transferase Inhibitor CXCL12 Proof-of-concept in AITL ü Pathway Positive Phase 2 trial in PTCL Indications ü Additional data from Phase 2 trial in CMML 2019 Potential biomarker of activity in squamous cell carcinomas ü KO-947 ERK Inhibitor Data from Phase 1 dose-escalation trial 2019 FDA clearance of IND application ü KO-539 Menin-MLL Inhibitor Initiation of Phase 1 trial Mid-2019 Nasdaq: KURA Financial Shares outstanding: 38.2M basic, 4.3M options* $187.4M Highlights Cash, cash equivalents and short-term investments: $165.5M* 20 * As of March 31, 2019

Developing Precision Medicines for the Treatment of CancerDeveloping Precision Medicines for the Treatment of Cancer




















