Exhibit 99.1

Tumor Infiltrating Lymphocyte Cell Therapy for Treatment of Solid Tumors April 2021 © 2021, Iovance Biotherapeutics, Inc 1

Forward Looking Statements 2 Certain matters discussed in this presentation are “forward - looking statements” of Iovance Biotherapeutics, Inc, Inc . (hereinafter referred to as the “Company,” “we,” “us,” or “our”) within the meaning of the Private Securities Litigation Reform Act of 1995 (the “PSLRA”) . All such written or oral statements made in this presentation, other than statements of historical fact, are forward - looking statements and are intended to be covered by the safe harbor for forward - looking statements provided by the PSLRA .. Without limiting the foregoing, we may, in some cases, use terms such as “predicts,” “believes,” “potential,” “continue,” “estimates,” “anticipates,” “expects,” “plans,” “intends,” “forecast,” “guidance,” “outlook,” “may,” “could,” “might,” “will,” “should” or other words that convey uncertainty of future events or outcomes and are intended to identify forward - looking statements . Forward - looking statements are based on assumptions and assessments made in light of management’s experience and perception of historical trends, current conditions, expected future developments and other factors believed to be appropriate . Forward - looking statements in this press release are made as of the date of this press release, and we undertake no duty to update or revise any such statements, whether as a result of new information, future events or otherwise . Forward - looking statements are not guarantees of future performance and are subject to risks, uncertainties and other factors, many of which are outside of our control, that may cause actual results, levels of activity, performance, achievements and developments to be materially different from those expressed in or implied by these forward - looking statements . Important factors that could cause actual results, developments and business decisions to differ materially from forward - looking statements are described in the sections titled “Risk Factors“ in our filings with the Securities and Exchange Commission, including our most recent Annual Report on Form 10 - K and Quarterly Reports on Form 10 - Q, and include, but are not limited to, the following substantial known and unknown risks and uncertainties inherent in our business : the effects of the COVID - 19 pandemic ; risks related to the timing of and our ability to successfully develop, submit, obtain and maintain U . S . Food and Drug Administration (“FDA”) or other regulatory authority approval of, or other action with respect to, our product candidates, and our ability to successfully commercialize any product candidates for which we obtain FDA approval ; preliminary and interim clinical results, which may include efficacy and safety results, from ongoing clinical trials may not be reflected in the final analyses of our ongoing clinical trials or subgroups within these trials ; the risk that enrollment may need to be adjusted for our trials and cohorts within those trials based on FDA and other regulatory agency input ; the new version of the protocol which further defines the patient population to include more advanced patients in our cervical cancer trial may have an adverse effect on the results reported to date ; the risk that we may be required to conduct additional clinical trials or modify ongoing or future clinical trials based on feedback from the FDA or other regulatory authorities ; the risk that our interpretation of the results of our clinical trials or communications with the FDA may differ from the interpretation of such results or communications by the FDA ; the acceptance by the market of our product candidates and their potential reimbursement by payors, if approved ; our ability or inability to manufacture our therapies using third party manufacturers or our own facility may adversely affect our potential commercial launch ; the results of clinical trials with collaborators using different manufacturing processes may not be reflected in our sponsored trials ; the risk that unanticipated expenses may decrease our estimated cash balances and increase our estimated capital requirements ; and other factors, including general economic conditions and regulatory developments, not within our control . © 2021, Iovance Biotherapeutics, Inc

Iovance: Developing to commercialize TIL Cell Therapy 400+ Patients Treated with Iovance TIL Using Proprietary Process 3 NASDAQ: IOVA © 2021, Iovance Biotherapeutics, Inc Partners Assets • ~$635M cash (12/31/20) • Global rights to all programs, IP and technology • Iovance manufacturing facility ( i CTC ) • Pivotal programs in metastatic melanoma and advanced cervical cancers • Registration - supporting study in non - small cell lung carcinoma (NSCLC) • Combinations with immune - checkpoint inhibitors in earlier lines • Academic collaborations in new indications Pipeline • Leading cell therapy platform in solid tumors • Clinical data in multiple indications • Consistent GMP manufacturing process across solid tumors • Next gen research in selected and genetically modified TIL Platform

Leading cell therapy company focused on treatment of solid tumors Investment Highlights • Initial focus in post - checkpoint solid tumors • Expansion into combinations and earlier lines of therapy • Five company - sponsored programs in melanoma, cervical, head & neck, NSCLC, and chronic lymphocytic leukemia (CLL) indications • Accelerated path to approval in melanoma and cervical cancer • BLA submission expected 2021 • Melanoma: RMAT, Orphan Drug, and Fast Track • Cervical: BTD, Orphan Drug, and Fast Track • US and EU capacity with contract manufacturers • Iovance Cell Therapy Center ( i CTC ) under construction in Philadelphia • Rapid 22 - day Gen 2 manufacturing with 90%+ success rate • 400+ patients treated with Iovance proprietary process © 2021, Iovance Biotherapeutics, Inc 4 Large market opportunity & strong unmet need Potential to be the first cell therapy approved for solid tumors in melanoma and cervical Efficient & scalable proprietary manufacturing

2020 Accomplishments; Anticipated 2021 Milestones 5 Corporate Goals and Updates Clinical Melanoma : early pivotal Cohort 4 data and updated Cohort 2 data Cervical : last patient dosed in cervical pivotal cohort NSCLC : Moffitt TIL data; registration directed study initiated HNSCC : initial data for TIL + pembrolizumab Cervical : Complete enrollment into Cohort 2, under consideration for inclusion in the BLA NSCLC: Add a new cohort in the basket study; combine TIL + ipilimumab/nivolumab NSCLC : Start patient dosing in IOV - LUN - 202 HNSCC: Expanding the HNSCC TIL + pembrolizumab in basket study (as part of moving TIL in earlier lines); Close C - 145 - 03 HNSCC single therapy Manufacturing Gen 3 process in clinic >90% success rate in >400 patients Melanoma : Initiate administration of 16 - day Gen 3 process in clinic in the basket study Completion of Navy Yard GMP facility ( i CTC ); start clinical manufacturing at i CTC © 2021, Iovance Biotherapeutics, Inc x 2020 2021 x x x x x x Regulatory Agreement with FDA on melanoma Cohort 4 clinical follow up; Cohort 2 supportive Additional work on potency assays BLA : Continue work on potency assays to support submission of a BLA to FDA for lifileucel; additional assay data submitted to FDA x x x x x
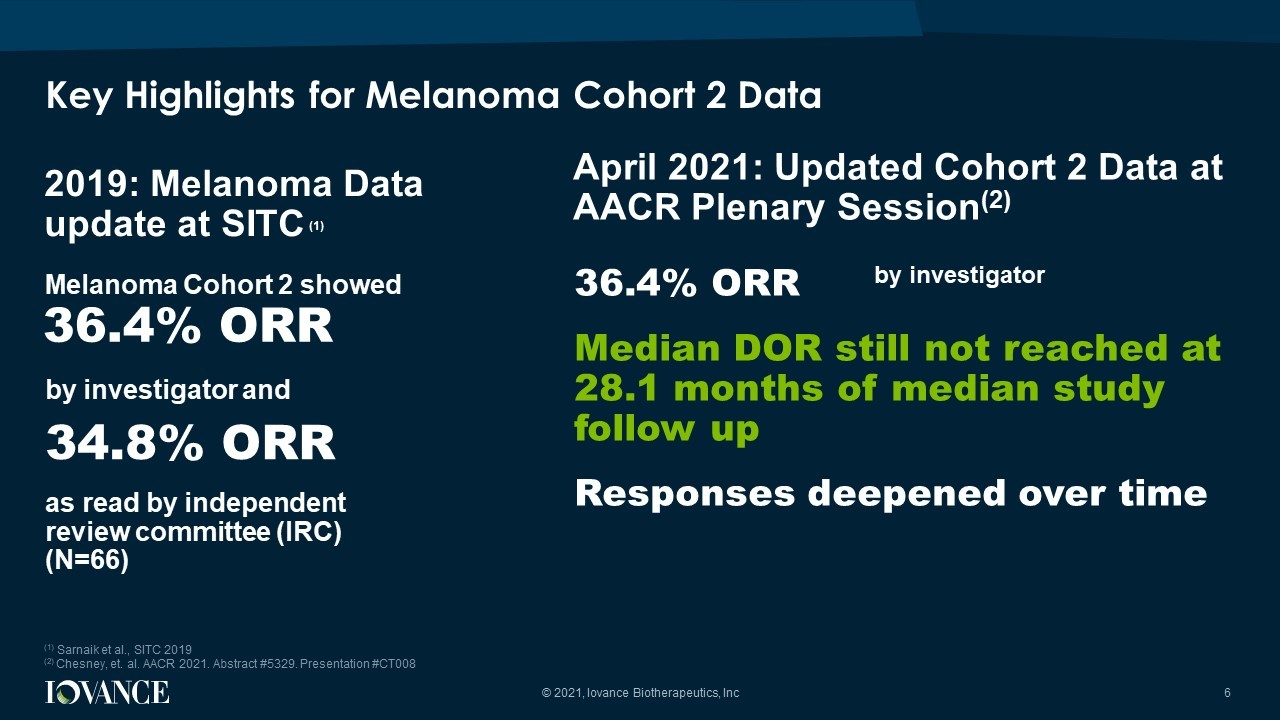
Key Highlights for Melanoma Cohort 2 Data © 2021, Iovance Biotherapeutics, Inc 6 2019: Melanoma Data update at SITC (1) 36.4% ORR 34.8% ORR as read by independent review committee (IRC) (N=66) by investigator and Melanoma Cohort 2 showed 36.4% ORR Median DOR still not reached at 28.1 months of median study follow up Responses deepened over time (1) Sarnaik et al., SITC 2019 (2) Chesney, et. al. AACR 2021. Abstract #5329. Presentation #CT008 April 2021: Updated Cohort 2 Data at AACR Plenary Session (2) by investigator

Tumor Infiltrating Lymphocytes (TIL): Leading Platform for Treatment of Solid Tumors 7 Remove Tumor Sample Expand & Rejuvenate Patient - specific T Cells Lymphodepletion & Infusion (1) Simpson - Abelson et al. , ESMO 2020 • Highly personalized • One - time therapy • Patient’s own immune system amplified and rejuvenated (1) TIL – Unique Mechanism of Action © 2021, Iovance Biotherapeutics, Inc

TIL Mechanism of Action Circulation Migration Peptide Antigen Recognition Lysis (Tumor Killing ) 8 Infusion of tumor - infiltrating lymphocytes (TIL) Tumor Peripheral blood Blood vessel Tumor cell TIL TIL Tumor bed TIL Lysing tumor cell T - cell receptor Tumor antigen peptide MHC - I Chemokine receptor Chemokine TIL IFN Granzyme Perforin TIL Lysing tumor cell © 2021, Iovance Biotherapeutics, Inc
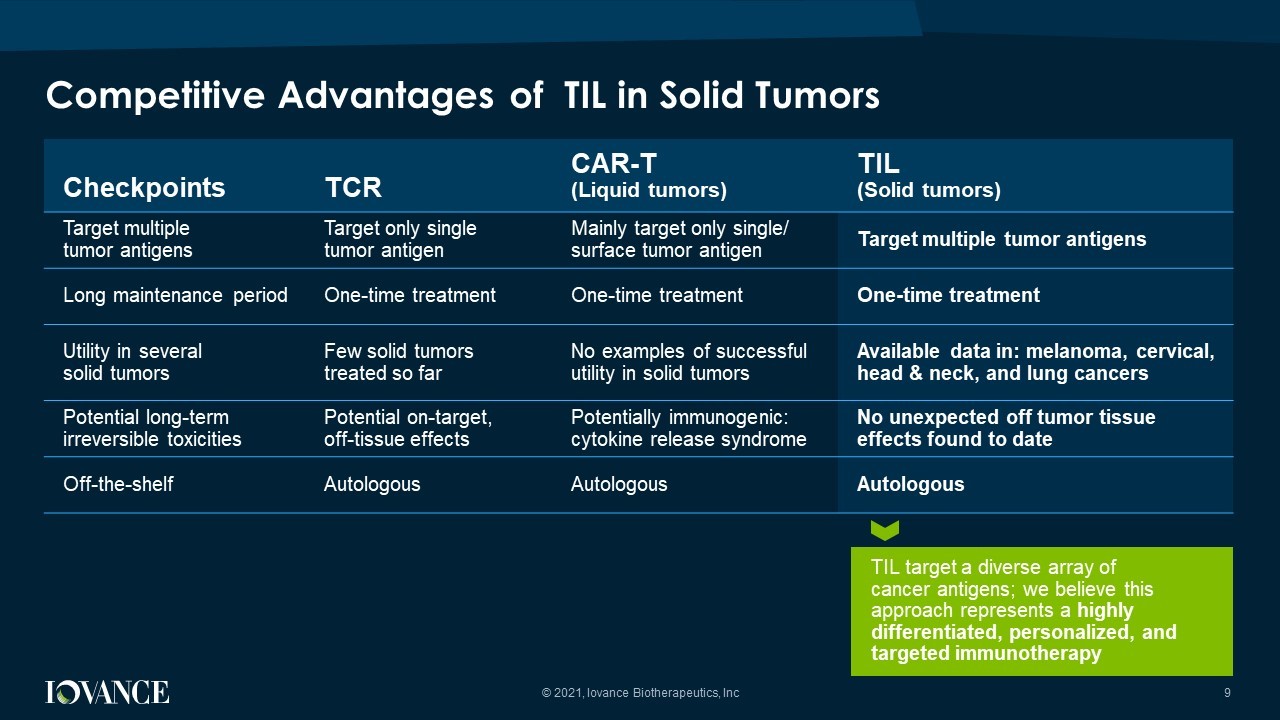
Competitive Advantages of TIL in Solid Tumors Checkpoints TCR CAR - T (Liquid tumors) TIL (Solid tumors) Target multiple tumor antigens Target only single tumor antigen Mainly target only single/ surface tumor antigen Target multiple tumor antigens Long maintenance period One - time treatment One - time treatment One - time treatment Utility in several solid tumors Few solid tumors treated so far No examples of successful utility in solid tumors Available data in: melanoma, cervical, head & neck, and lung cancers Potential l ong - term irreversible toxicities Potential o n - target, off - tissue effects Potentially immunogenic: cytokine release syndrome No unexpected off tumor tissue effects found to date Off - the - shelf Autologous Autologous Autologous TIL target a diverse array of cancer antigens; we believe this approach represents a highly differentiated, personalized, and targeted immunotherapy 9 © 2021, Iovance Biotherapeutics, Inc

Manufacturing Process © 2021, Iovance Biotherapeutics, Inc 10
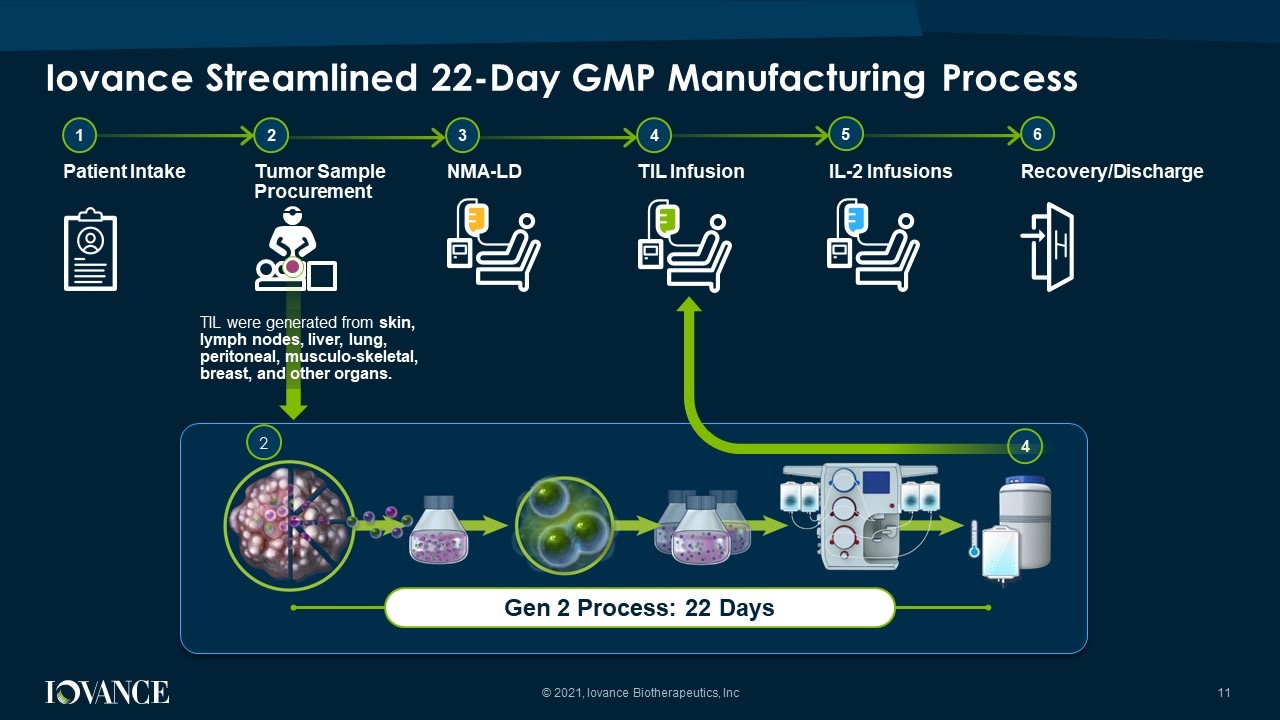
11 Iovance Streamlined 22 - Day GMP Manufacturing Process Gen 2 Process: 22 Days 1 2 Patient Intake Tumor Sample Procurement 3 4 NMA - LD TIL Infusion 5 6 IL - 2 Infusions Recovery/Discharge 4 2 TIL were generated from skin, lymph nodes, liver, lung, peritoneal, musculo - skeletal, breast, and other organs. © 2021, Iovance Biotherapeutics, Inc

Iovance Cell Therapy Center: i CTC © 2021, Iovance Biotherapeutics, Inc. 12 • Build - to - suit custom facility in Philadelphia • ~136,000 ft 2 , $85M investment • First set of clean rooms occupied • Clinical supply planned in 2021 • Commercial GMP planned in 2022 • Significant reduction in COGS expected

First Set of Cleanrooms (Flex Suite) Complete 13 © 2021, Iovance Biotherapeutics, Inc
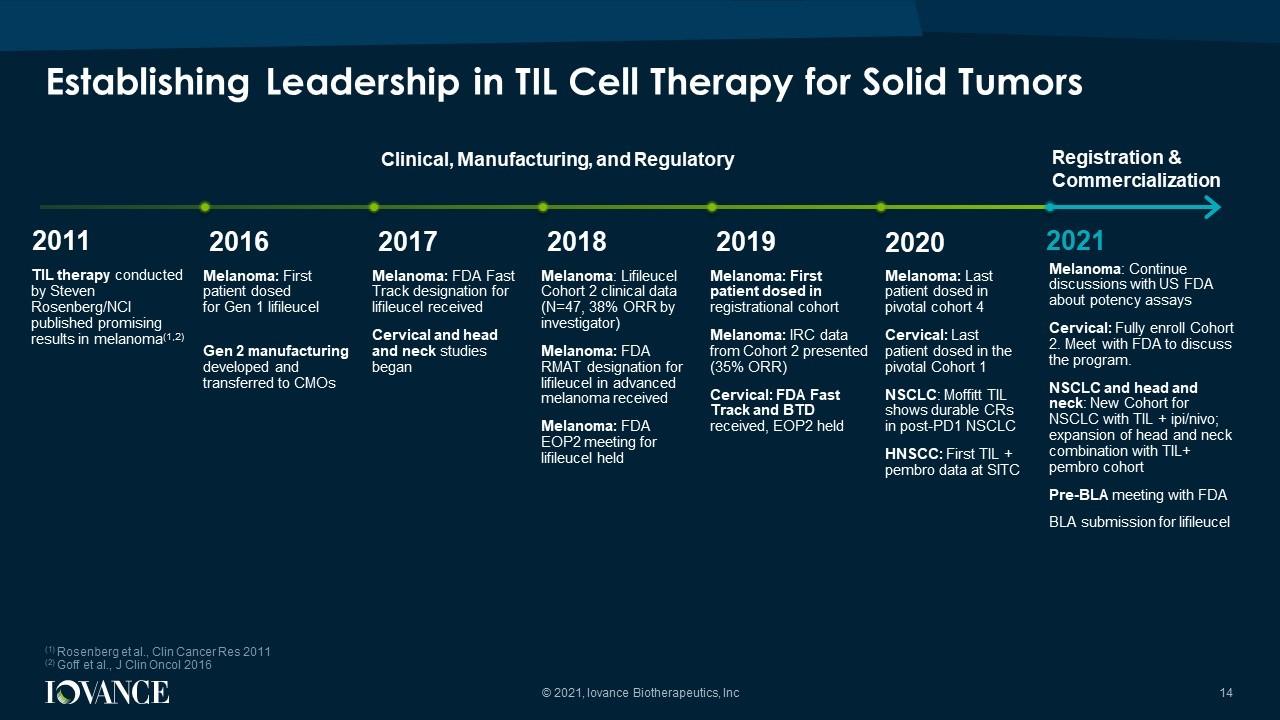
Clinical, Manufacturing, and Regulatory Registration & Commercialization (1) Rosenberg et al., Clin Cancer Res 2011 (2) Goff et al., J Clin Oncol 2016 Melanoma: First patient dosed for Gen 1 l ifileucel Gen 2 manufacturing developed and transferred to CMOs 2016 Melanoma: FDA Fast Track designation for l ifileucel received Cervical and head and neck studies began 2017 Melanoma : L ifileucel Cohort 2 clinical data (N=47, 38% ORR by investigator) Melanoma: FDA RMAT designation for l ifileucel in advanced melanoma received Melanoma: FDA EOP2 meeting for l ifileucel held 2018 Melanoma: Last patient dosed in pivotal cohort 4 Cervical: Last patient dosed in the pivotal Cohort 1 NSCLC : Moffitt TIL shows durable CRs in post - PD1 NSCLC HNSCC: First TIL + pembro data at SITC 2021 2019 Melanoma: First patient dosed in registrational cohort Melanoma: IRC data from Cohort 2 presented (35% ORR) Cervical: FDA Fast Track and BTD received, EOP2 held Establishing Leadership in TIL Cell Therapy for Solid Tumors 14 2020 Melanoma : Continue discussions with US FDA about potency assays Cervical: Fully enroll Cohort 2. Meet with FDA to discuss the program. NSCLC and head and neck : New Cohort for NSCLC with TIL + ipi / nivo ; expansion of head and neck combination with TIL+ pembro cohort Pre - BLA meeting with FDA BLA submission for lifileucel 2011 TIL therapy conducted by Steven Rosenberg/NCI published promising results in melanoma (1,2) © 2021, Iovance Biotherapeutics, Inc
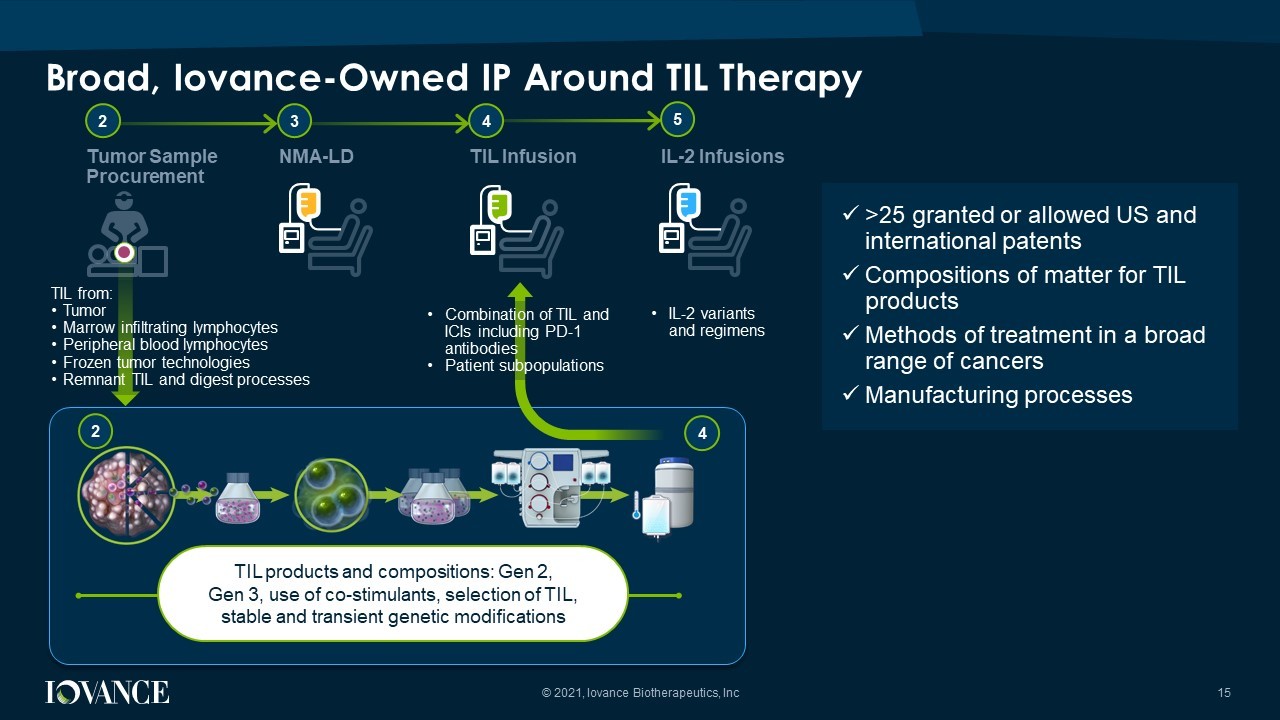
Broad, Iovance - Owned IP Around TIL Therapy 15 © 2021, Iovance Biotherapeutics, Inc TIL products and compositions: Gen 2, Gen 3, use of co - stimulants, selection of TIL, stable and transient genetic modifications 2 Tumor Sample Procurement 3 4 NMA - LD TIL Infusion 5 IL - 2 Infusions 4 2 TIL from: • Tumor • Marrow infiltrating lymphocytes • Peripheral blood lymphocytes • Frozen tumor technologies • Remnant TIL and digest processes • Combination of TIL and ICIs including PD - 1 antibodies • Patient subpopulations x >25 granted or allowed US and international patents x Compositions of matter for TIL products x Methods of treatment in a broad range of cancers x Manufacturing processes • IL - 2 variants and regimens
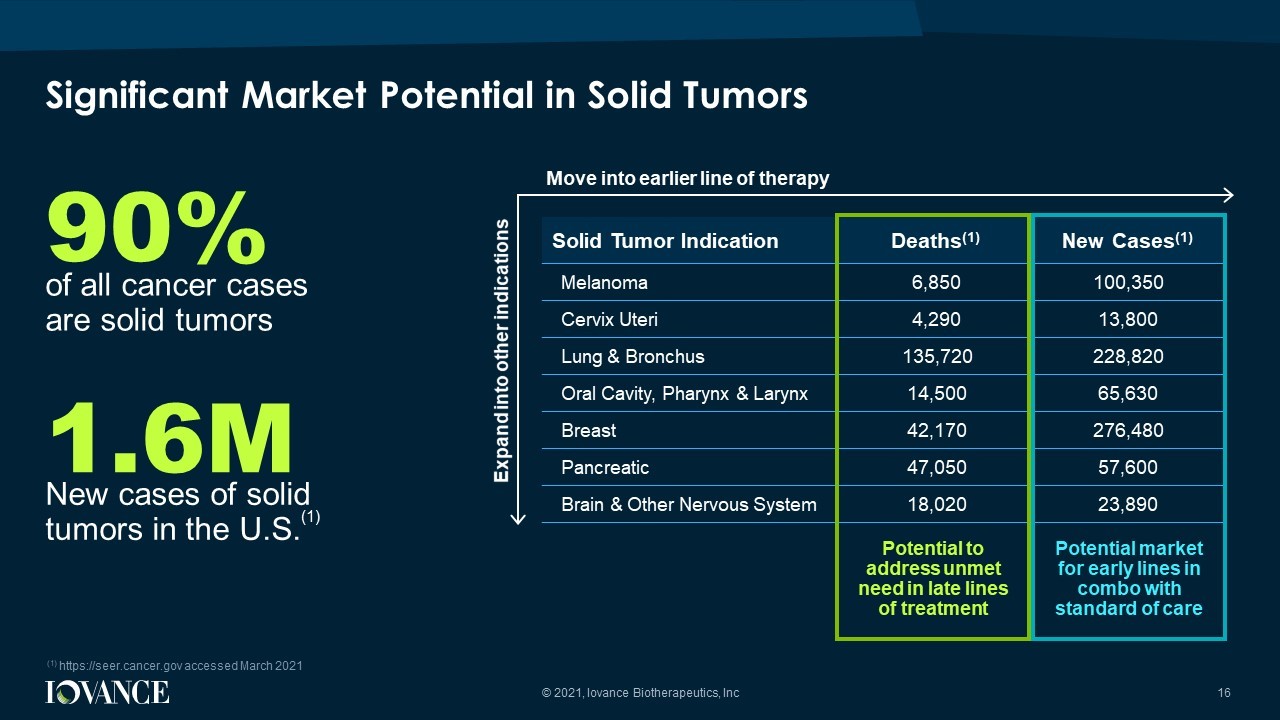
(1) https://seer.cancer.gov accessed March 2021 Solid Tumor Indication Deaths (1) New Cases (1) Melanoma 6,850 100,350 Cervix Uteri 4,290 13,800 Lung & Bronchus 135,720 228,820 Oral Cavity, Pharynx & Larynx 14,500 65,630 Breast 42,170 276,480 Pancreatic 47,050 57,600 Brain & Other Nervous System 18,020 23,890 90% of all cancer cases are solid tumors 1.6M New cases of solid tumors in the U.S. (1) Potential market for early lines in combo with standard of care Potential to address unmet need in late lines of treatment Expand into other indications Move into earlier line of therapy Significant Market Potential in Solid Tumors 16 © 2021, Iovance Biotherapeutics, Inc
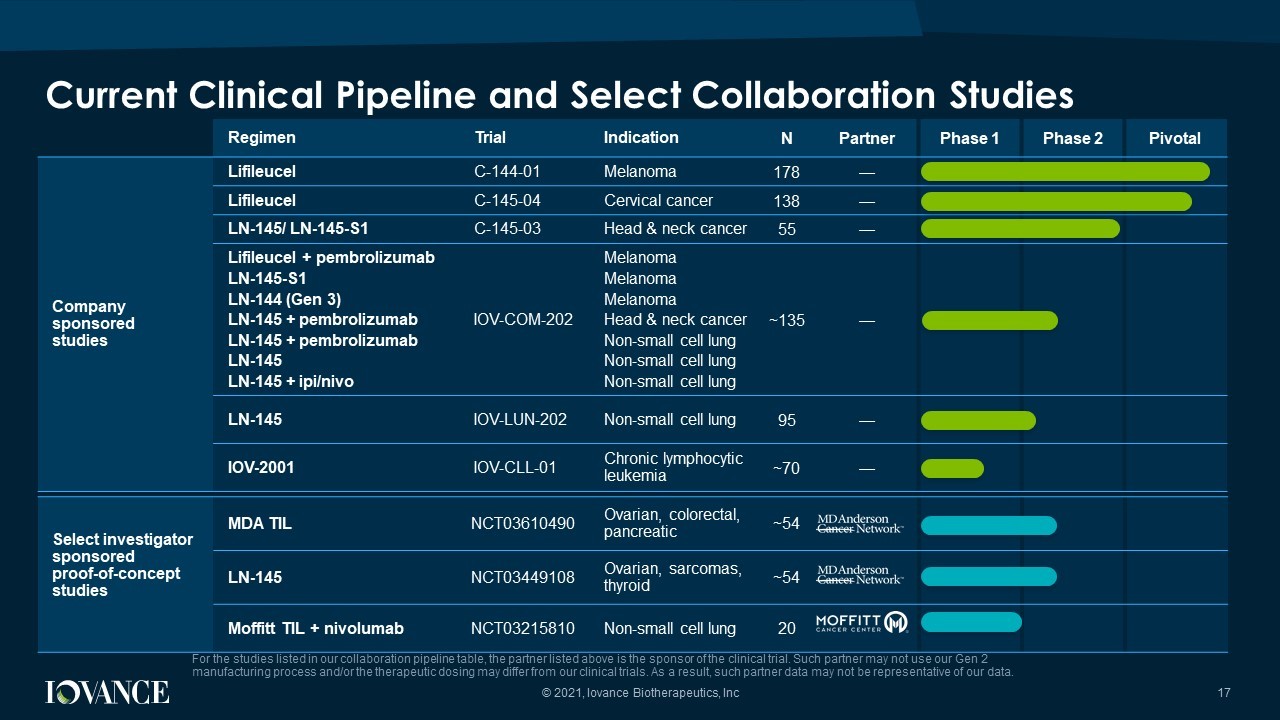
Select investigator sponsored proof - of - concept studies MDA TIL NCT03610490 Ovarian, colorectal, pancreatic ~54 LN - 145 NCT03449108 Ovarian, sarcomas, thyroid ~54 Moffitt TIL + nivolumab NCT03215810 Non - small cell lung 20 Regimen Trial Indication N Partner Phase 1 Phase 2 Pivotal Company sponsored studies Lifileucel C - 144 - 01 Melanoma 178 — Lifileucel C - 145 - 04 Cervical cancer 138 — LN - 145/ LN - 145 - S1 C - 145 - 03 Head & neck cancer 55 — Lifileucel + pembrolizumab LN - 145 - S1 LN - 144 (Gen 3) LN - 145 + pembrolizumab LN - 145 + pembrolizumab LN - 145 LN - 145 + ipi / nivo IOV - COM - 202 Melanoma Melanoma Melanoma Head & neck cancer Non - small cell lung Non - small cell lung Non - small cell lung ~135 — LN - 145 IOV - LUN - 202 Non - small cell lung 95 — IOV - 2001 IOV - CLL - 01 Chronic lymphocytic leukemia ~70 — Current Clinical Pipeline and Select Collaboration Studies For the studies listed in our collaboration pipeline table, the partner listed above is the sponsor of the clinical trial. Su ch partner may not use our Gen 2 manufacturing process and/or the therapeutic dosing may differ from our clinical trials. As a result, such partner data may n ot be representative of our data. © 2021, Iovance Biotherapeutics, Inc 17

Metastatic Melanoma © 2021, Iovance Biotherapeutics, Inc 18

(1) https://seer.cancer.gov accessed March 2021 (2) Keytruda USPI accessed Mar 2021 (4%) and Weber et al., Lancet Oncol 2015 (ICC 10%) (3) Global Burden of Disease Cancer Collaboration, JAMA Oncol 2019 (4) Kirchburger et al., Eur J Cancer 2016 and Goldinger et al., J Clin Oncol 2018 • Estimated 7,000 (1) U.S. patient deaths due to melanoma • Limited options after progression on checkpoint and BRAF/MEK inhibitors Potential Market for Metastatic Melanoma Metastatic Melanoma Facts BRAF/MEK inhibitors for BRAF positive Chemotherapy ORR 4 - 10% (2) OS ~7 - 8 mons (4) New Cases WW each year (3) 309k 100k Diagnoses in U.S. each year (1) 62k 7k Deaths WW each year (3) Deaths in U.S. each year (1) 1 st line: Immuno - therapy Nature has selected TIL to recognize features unique to the tumor not present on normal tissues, which helps make a TIL therapy approach effective compared to other cell therapy strategies for solid tumors. Iovance TIL treatment has a novel mechanism of action, completely separate from those of other treatment options, and has resulted in highly durable responses in patients that have progressed on prior FDA - approved treatment for their metastatic melanoma.” — Dr. Amod Sarnaik Department of Cutaneous Oncology, the Immunology Program and the Melanoma Center of Excellence at Moffitt Cancer Center © 2021, Iovance Biotherapeutics, Inc 19

Endpoints • Primary: Efficacy defined as IRC ORR Study Updates • Mar 2019: Cohort 4 (pivotal trial) FPI • Jan 2020: last patient dosed • Dec 2020: Cohort 2 median DOR not reached at 28.1 months of median study follow up • April 2021: updated cohort 2 data at AACR • Data Extract: 14 Dec 2020 for Cohort 2 C - 144 - 01: Phase 2 Study Design © 2021, Iovance Biotherapeutics, Inc 20 Cohort 1: Non - cryopreserved TIL product (Gen 1) N=30 Closed to enrollment Cohort 2: Cryopreserved TIL product (Gen 2) N=60 Closed to enrollment Cohort 3: TIL re - treatment N=10 Unresectable or metastatic melanoma treated with 1 systemic prior therapy including a PD - 1 blocking antibody and if BRAF V600 mutation positive, a BRAF or BRAF/MEK Cohort 4: Pivotal Cryopreserved TIL product (Gen 2) N=75 Closed to enrollment

C - 144 - 01 Cohort 2 Patient Characteristics C - 144 - 01 Cohort 2 by Investigator (AACR 2021) (1) % is calculated based on number of patients who received prior anti - CTLA - 4 Characteristics Cohort 2, N= 66 Gender, n (%) Female 27 (41) Male 39 (59) Age, years Median 55 Min, Max 20, 79 Prior therapies, n (%) Mean # prior therapies 3.3 anti - PD - 1 / anti - PD - L1 66 (100) anti - CTLA - 4 1 53 (80) BRAFi/MEKi 15 (23) Progressive Disease for at least 1 prior therapy, n (%) Anti - PD - 1 65 (99) Anti - CTLA - 4 41 (77 (1) ) Baseline ECOG score, n (%) 0 37 (56) 1 29 (44) Characteristic Cohort 2, N= 66 BRAF Status, n (%) Mutated V600E or V600K 17 (26) Wild Type 45 (68) Unknown 3 (5) Other 1 (2) Tumor PD - L1 expression , n (%) PD - L1 Positive (TPS ≥ 5%) 23 ( 35) PD - L1 Negative (TPS < 5%) 26 (39) Baseline LDH (U/L) Median 244 1 - 2 times ULN, n (%) 19 (29) > 2 times ULN, n (%) 8 (12) Target Lesions Sum of Diameter (mm) Mean (SD) 106 (71) Min, Max 11, 343 Number of Target and Non - Target Lesions (at Baseline) >3, n (%) 51 (77) Mean (SD) 6 (2.7) Liver and/or Brain Lesions, n (%) 28 (42) Cohort 2 patients have: • 3.3 mean prior therapies, ranging from 1 - 9 • High tumor burden at baseline © 2021, Iovance Biotherapeutics, Inc. 21

• The adverse event profile was consistent with the underlying advanced disease and the safety profile of the NMA - LD and IL - 2 regimens • Median number of IL - 2 doses administered was 6 • Decreasing frequency of AEs over time is reflective of potential benefit of one - time treatment with lifileucel • No new safety risks have been identified for lifileucel during the long - term follow - up Iovance C - 144 - 01 Cohort 2 Safety: Treatment Emergent Adverse Events (≥ 30%) *One death was due to intra - abdominal hemorrhage considered possibly related to TIL, second was due to acute respiratory failure assessed as not related to TIL per Investigator assessment. – Patients with multiple events for a given preferred term are counted only once using the maximum grade under each preferred t erm – Treatment - Emergent Adverse Events refer to all AEs starting on or after the first dose date of TIL up to 30 days Preferred term Cohort 2 (N=66) Any Grade, n (%) Grade 3/4, n (%) Grade 5, n (%) Number of patients reporting at least one Treatment - Emergent AE 66 (100) 64 (97.0) 2 (3.0)* Thrombocytopenia 59 (89.4) 54 (81.8) 0 Chills 53 (80.3) 4 ( 6.1) 0 Anemia 45 (68.2) 37 (56.1) 0 Pyrexia 39 (59.1) 11 (16.7) 0 Neutropenia 37 (56.1) 26 (39.4) 0 Febrile neutropenia 36 (54.5) 36 (54.5) 0 Hypophosphatemia 30 (45.5) 23 (34.8) 0 Leukopenia 28 (42.4) 23 (34.8) 0 Fatigue 26 (39.4) 1 ( 1.5) 0 Hypotension 24 (36.4) 7 (10.6) 0 Lymphopenia 23 (34.8) 21 (31.8) 0 Tachycardia 23 (34.8) 1 ( 1.5) 0 © 2021, Iovance Biotherapeutics, Inc. 22 C - 144 - 01 Cohort 2 (AACR 2021)

• After a median study follow - up of 28.1 months, median DOR was still not reached (range 2.2, 35.2+) • Mean number of TIL cells infused: 27.3 x 10 9 • Responses were demonstrated: • In patients who received prior anti - CTLA - 4 or BRAF/MEK inhibitors • Regardless of BRAF mutational status • Regardless of Tumor PD - L1 expression • In patients with various LDH levels • In patients with various baseline tumor burden • In patients with liver and/or brain lesions • Regardless of time from stop of anti - PD - 1/L1 to TIL infusion C - 144 - 01 Cohort 2 Efficacy (1) Not evaluable (NE) due to not reaching first assessment Response Patients, n=66 N (%) Objective Response Rate 24 (36.4) Complete Response 3 (4.5) Partial Response 21 (31.8) Stable Disease 29 (43.9) Progressive Disease 9 (13.6) Non - Evaluable ( 1) 4 (6.1) Disease Control Rate 53 ( 80.3) Median Duration of Response Not Reached Min, Max (months) 2.2, 35.2+ © 2021, Iovance Biotherapeutics, Inc. 23 C - 144 - 01 Cohort 2 (AACR 2021)
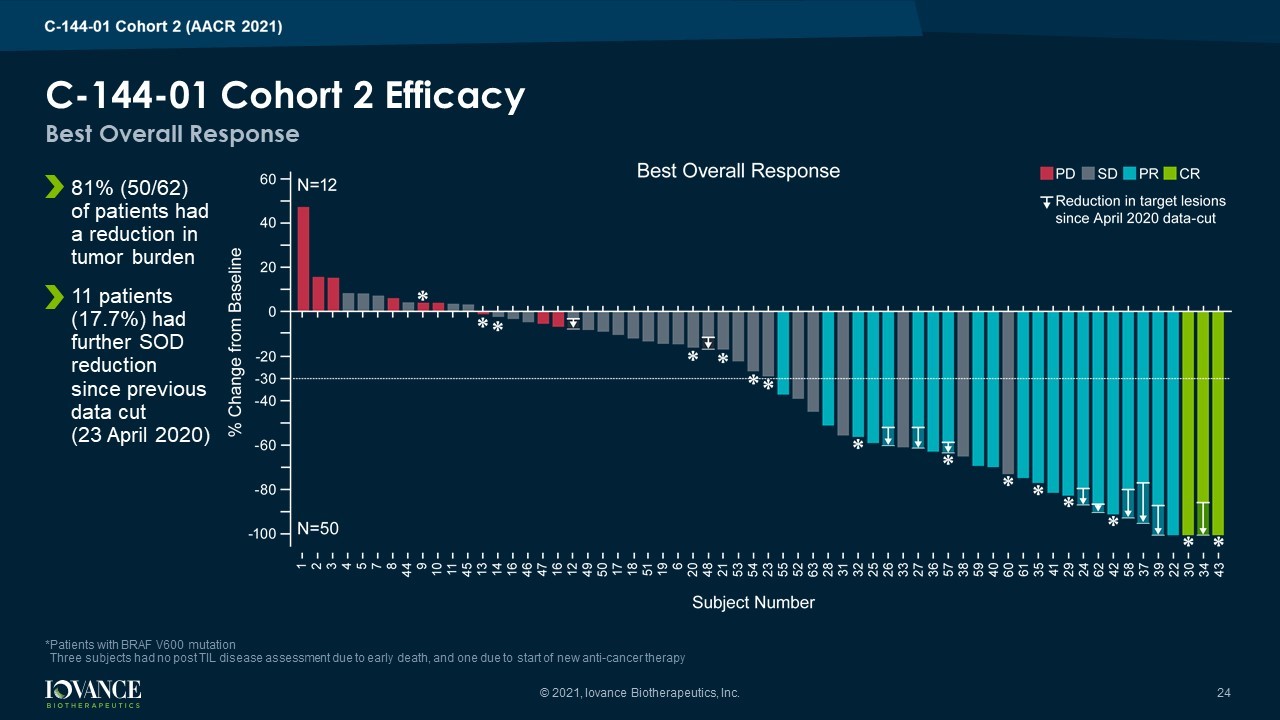
C - 144 - 01 Cohort 2 Efficacy 81% (50/62) of patients had a reduction in tumor burden 11 patients (17.7%) had further SOD reduction since previous data cut (23 April 2020) Best Overall Response *Patients with BRAF V600 mutation Three subjects had no post TIL disease assessment due to early death, and one due to start of new anti - cancer therapy © 2021, Iovance Biotherapeutics, Inc. 24

C - 144 - 01 Cohort 2 Efficacy 79% of responders had received prior ipilimumab One PR converted to CR after 24 months post - lifileucel Time to Response for Evaluable Patients (PR or Better) (1) BOR is best overall response on prior anti - PD - 1 immunotherapy (2) U: unknown (3) Patient 22 BOR is PR © 2021, Iovance Biotherapeutics | Strictly Confidential 25

C - 144 - 01 Cohort 2 Biomarkers Site of Tumor Resection Appropriate amount of TIL was manufactured from tumors regardless of location of resection Total Cell Dose Site of Tumor Resection Target lesion SOD reductions were seen across the range of TIL total cell dose © 2021, Iovance Biotherapeutics, Inc. 26

• In heavily pretreated metastatic melanoma patients who progressed on multiple prior therapies, including anti - PD - 1 and BRAFi/MEKi, if BRAFV600 mutant, lifileucel treatment resulted in: • 36.4% ORR • Median DOR not reached at 28.1 months of median study follow up • Responses deepened over time: • 11 patients (17.7%) demonstrated further reduction in SOD since prior data cut in April 2020 • One patient converted from PR to CR at 24 months post lifileucel infusion • Lifilecuel was successfully manufactured regardless of the organ site of the resected tumor • Target lesion SOD reduction were not associated with CD4 + or CD8 + cell doses • Lifileucel has demonstrated efficacy and durability of response for patients with metastatic melanoma and represents a viable therapeutic option warranting further investigation C - 144 - 01 Cohort 2: Conclusions © 2021, Iovance Biotherapeutics, Inc. 27
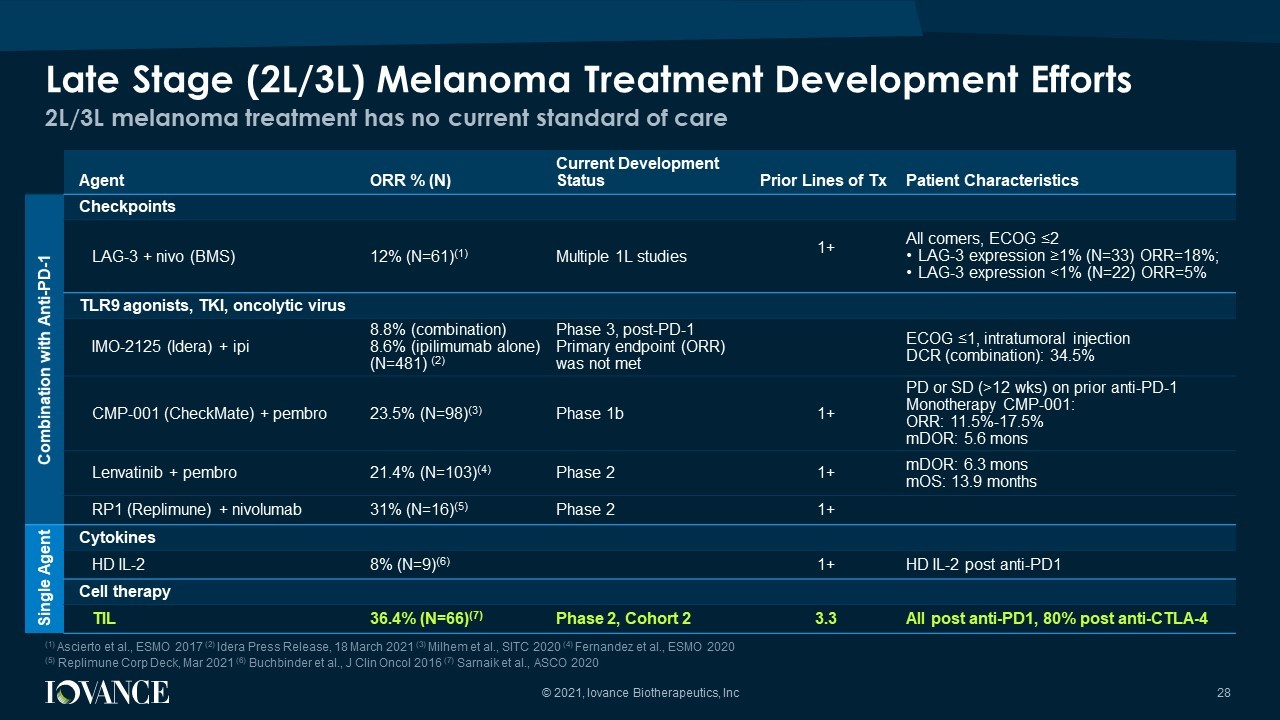
Agent ORR % ( N) Current Development Status Prior Lines of Tx Patient Characteristics Combination with Anti - PD - 1 Checkpoints LAG - 3 + nivo (BMS) 12% (N=61) (1) Multiple 1L studies 1+ All comers, ECOG ≤2 • LAG - 3 expression ≥1% (N=33) ORR=18%; • LAG - 3 expression <1% (N=22) ORR=5% TLR9 agonists, TKI, oncolytic virus IMO - 2125 ( Idera ) + ipi 8.8% (combination) 8.6% (ipilimumab alone) (N=481) (2) Phase 3, post - PD - 1 Primary endpoint (ORR) was not met ECOG ≤1, intratumoral injection DCR (combination): 34.5% CMP - 001 ( CheckMate ) + pembro 23.5% (N=98) (3) Phase 1b 1+ PD or SD (>12 wks ) on prior anti - PD - 1 Monotherapy CMP - 001: ORR: 11.5% - 17.5% mDOR : 5.6 mons Lenvatinib + pembro 21.4% (N=103) (4) Phase 2 1+ mDOR : 6.3 mons mOS : 13.9 months RP1 ( Replimune ) + nivolumab 31% (N=16) (5) Phase 2 1+ Single Agent Cytokines HD IL - 2 8% (N=9) (6) 1+ HD IL - 2 post anti - PD1 Cell therapy TIL 36.4% (N=66) (7) Phase 2, Cohort 2 3.3 All post anti - PD1, 80% post anti - CTLA - 4 2L/3L melanoma treatment has no current standard of care Late Stage (2L/3L) Melanoma Treatment Development Efforts 28 © 2021, Iovance Biotherapeutics, Inc (1) Ascierto et al., ESMO 2017 (2) Idera Press Release, 18 March 2021 (3) Milhem et al., SITC 2020 (4) Fernandez et al., ESMO 2020 (5) Replimune Corp Deck, Mar 2021 (6) Buchbinder et al., J Clin Oncol 2016 (7) Sarnaik et al., ASCO 2020

Cervical Cancer © 2021, Iovance Biotherapeutics, Inc 29

For PD - L1+ patients, post - chemo receiving Keytruda (3) ORR 14.3% Third line patients: ORR 3.4% ( 6) Available Care for chemotherapy in 2L or 3L metastatic cervical patients 3.4 - 13% (4 - 6) New Cases WW each year (1) 601k 14k Diagnoses in U.S. each year (2) 260k 4k Deaths WW each year (1) Deaths in U.S. each year (2) Available care: Chemo - therapy as first line option (1) Global Burden of Disease Cancer Collaboration, JAMA Oncol 2019 (2) https://seer.cancer.gov accessed Mar 2020 (3) Keytruda USPI accessed Mar 2021 (4) Schilder et al., Gynecol Oncol 2005 (5) Weiss et al., Gynecol Oncol 1990 (6) McLachlan et al., Clin Oncol 2017 Potential Market for Cervical Cancer — Amir Jazaeri , M.D. Director of the Gynecologic Cancer Immunotherapy Program in the Department of Gynecologic Oncology and Reproductive Medicine at MD Anderson TIL immunotherapy with lifileucel is literally redefining what is treatable and potentially curable in advanced metastatic chemo - refractory cervical cancer. Patients who only two years ago would be facing hospice as their only alternative now have access to this potentially life extending new treatment. This is the most exciting news in this field in decades.” Cervical Cancer Facts 30 © 2021, Iovance Biotherapeutics, Inc
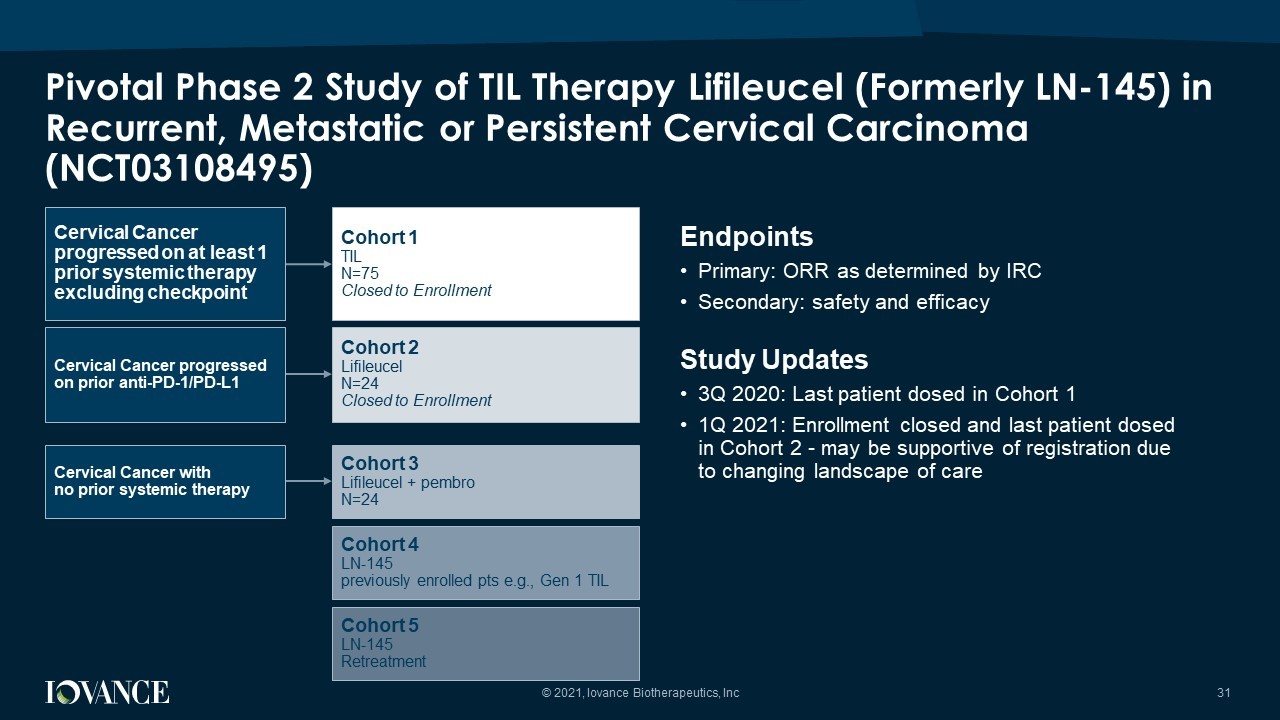
Pivotal Phase 2 Study of TIL Therapy Lifileucel (Formerly LN - 145) in Recurrent, Metastatic or Persistent Cervical Carcinoma (NCT03108495) © 2021, Iovance Biotherapeutics, Inc 31 Endpoints • Primary: ORR as determined by IRC • Secondary: safety and efficacy Study Updates • 3Q 2020: Last patient dosed in Cohort 1 • 1Q 2021: Enrollment closed and last patient dosed in Cohort 2 - may be supportive of registration due to changing landscape of care Cohort 1 TIL N=75 Closed to Enrollment Cohort 2 Lifileucel N=24 Closed to Enrollment Cohort 3 Lifileucel + pembro N=24 Cohort 4 LN - 145 previously enrolled pts e.g., Gen 1 TIL Cohort 5 LN - 145 Retreatment Cervical Cancer progressed on at least 1 prior systemic therapy excluding checkpoint Cervical Cancer progressed on prior anti - PD - 1/PD - L1 Cervical Cancer with no prior systemic therapy

Frequency of AEs over time is reflective of potential benefit of one - time treatment with TIL (lifileucel) Adverse Events Tend to be Early and Transient 32 © 2021, Iovance Biotherapeutics, Inc *The number of AEs is cumulative and represent the total number of patients dosed. Treatment - Emergent Adverse Events refer to all AEs starting on or after the first dose date of TIL up to 30 days. Patients with multiple events for a given preferred term are counted only once using the maximum grade under each preferred term. Safety terms which describe the same medical co ndi tion were combined; * U D G H 7 L P H I U R P 7 , / G R V H 1 X P E H U R I $ ( V 0 00 0 0 0 Adverse Events Over Time (N=27) Preferred Term Any Grade, n (%) Grade 3/4, n (%) Grade 5, n (%) Number of patients reporting at least one Treatment - Emergent AE ** 27 (100) 26 (96.3) 0 Chills 21 (77.8) 0 0 Anemia 15 (55.6) 15 (55.6) 0 Diarrhea 14 (51.9) 2 (7.4) 0 Pyrexia 14 (51.9) 1 (3.7) 0 Thrombocytopenia 14 (51.9) 12 (44.4) 0 Neutropenia 11 (40.7) 8 (29.6) 0 Vomiting 11 (40.7) 1 (3.7) 0 Hypotension 10 (37.0) 4 (14.8) 0 Dyspnea 9 (33.3) 1 (3.7) 0 Febrile neutropenia 9 (33.3) 8 (29.6) 0 Hypoxia 9 (33.3) 3 (11.1) 0 Leukopenia 9 (33.3) 6 (22.2) 0 Hypomagnesemia 8 (29.6) 0 0 Sinus tachycardia 8 (29.6) 0 0 C - 145 - 04: Investigator Assessed Cervical Cancer (ASCO 2019)
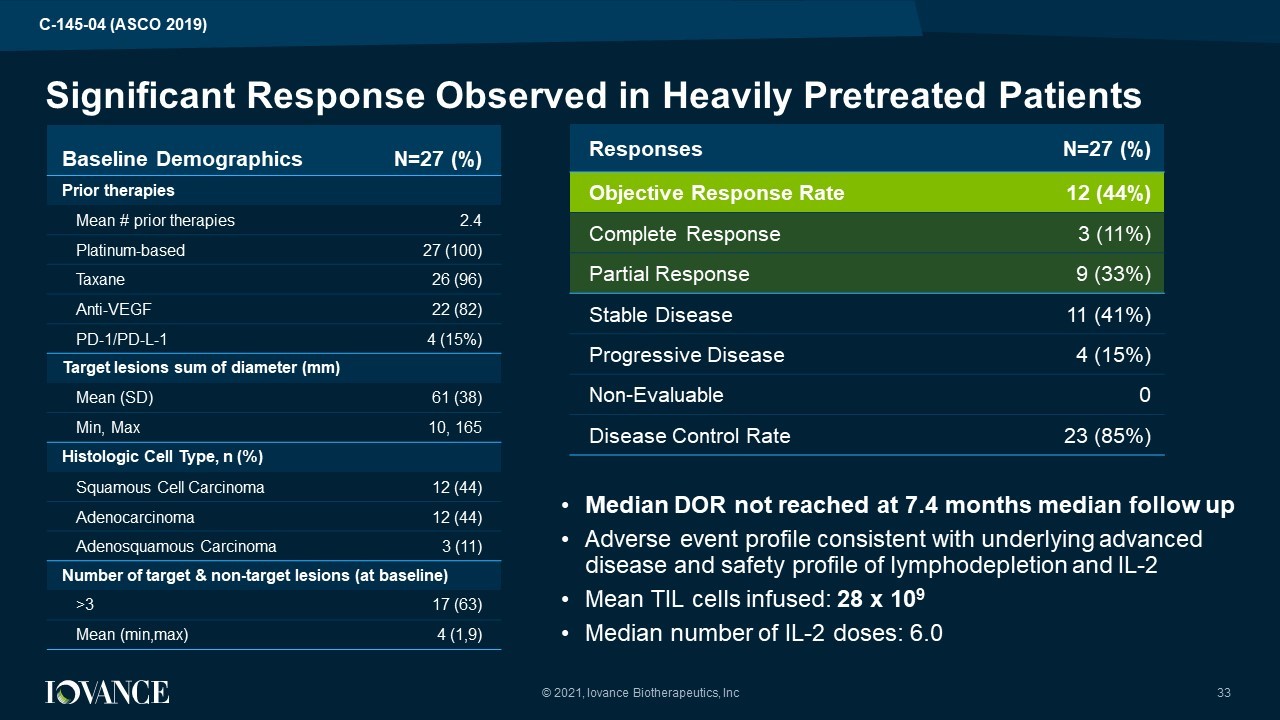
Baseline Demographics N= 27 (%) Prior therapies Mean # prior therapies 2.4 Platinum - based 27 (100) Taxane 26 (96) Anti - VEGF 22 (82) PD - 1/PD - L - 1 4 (15%) Target lesions sum of diameter (mm) Mean (SD) 61 (38) Min, Max 10, 165 Histologic Cell Type, n (%) Squamous Cell Carcinoma 12 (44) Adenocarcinoma 12 (44) Adenosquamous Carcinoma 3 (11) Number of target & non - target lesions (at baseline) >3 17 (63) Mean ( min,max ) 4 (1,9) Significant Response Observed in Heavily Pretreate d Patients © 2021, Iovance Biotherapeutics, Inc 33 Responses N=27 (%) Objective Response Rate 12 (44%) Complete Response 3 (11%) Partial Response 9 (33%) Stable Disease 11 (41%) Progressive Disease 4 (15%) Non - Evaluable 0 Disease Control Rate 23 ( 85%) • Median DOR not reached at 7.4 months median follow up • Adverse event profile consistent with underlying advanced disease and safety profile of lymphodepletion and IL - 2 • Mean TIL cells infused: 28 x 10 9 • Median number of IL - 2 doses: 6.0 C - 145 - 04 (ASCO 2019)
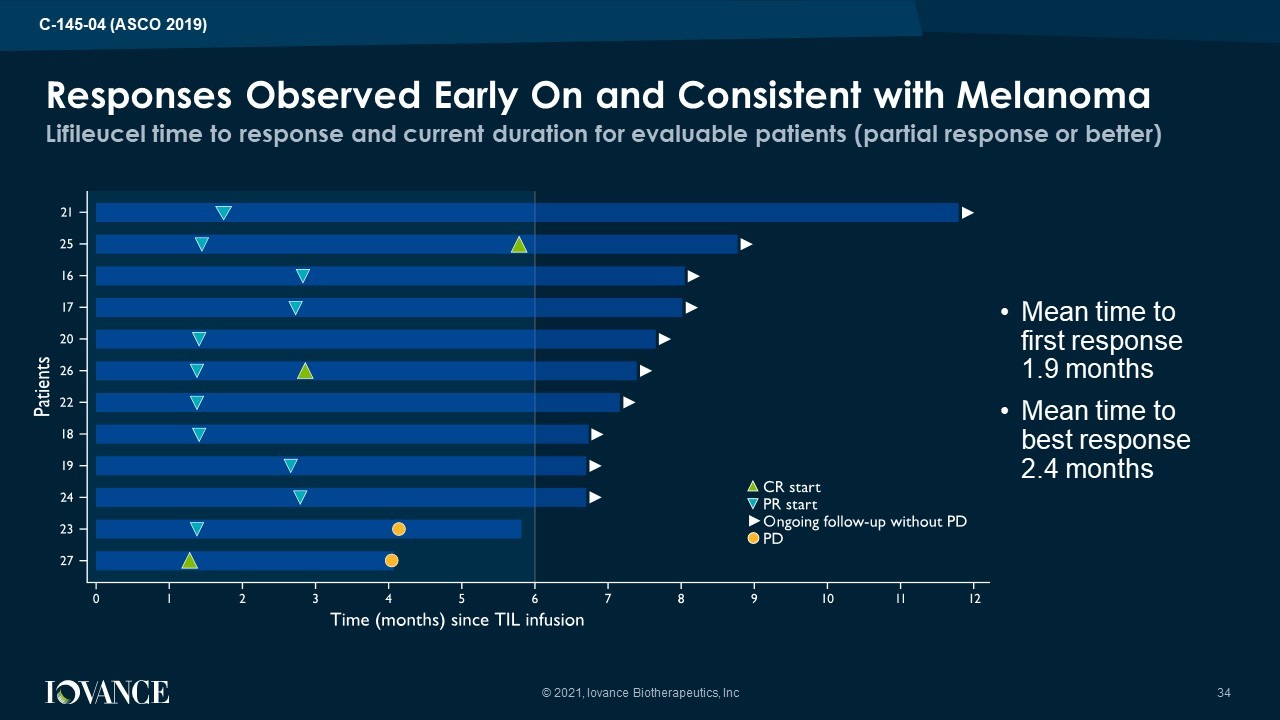
Lifileucel time to response and current duration for evaluable patients (partial response or better) Responses Observed Early On and Consistent with Melanoma • Mean time to first response 1.9 months • Mean time to best response 2.4 months © 2021, Iovance Biotherapeutics, Inc 34 C - 145 - 04 (ASCO 2019)
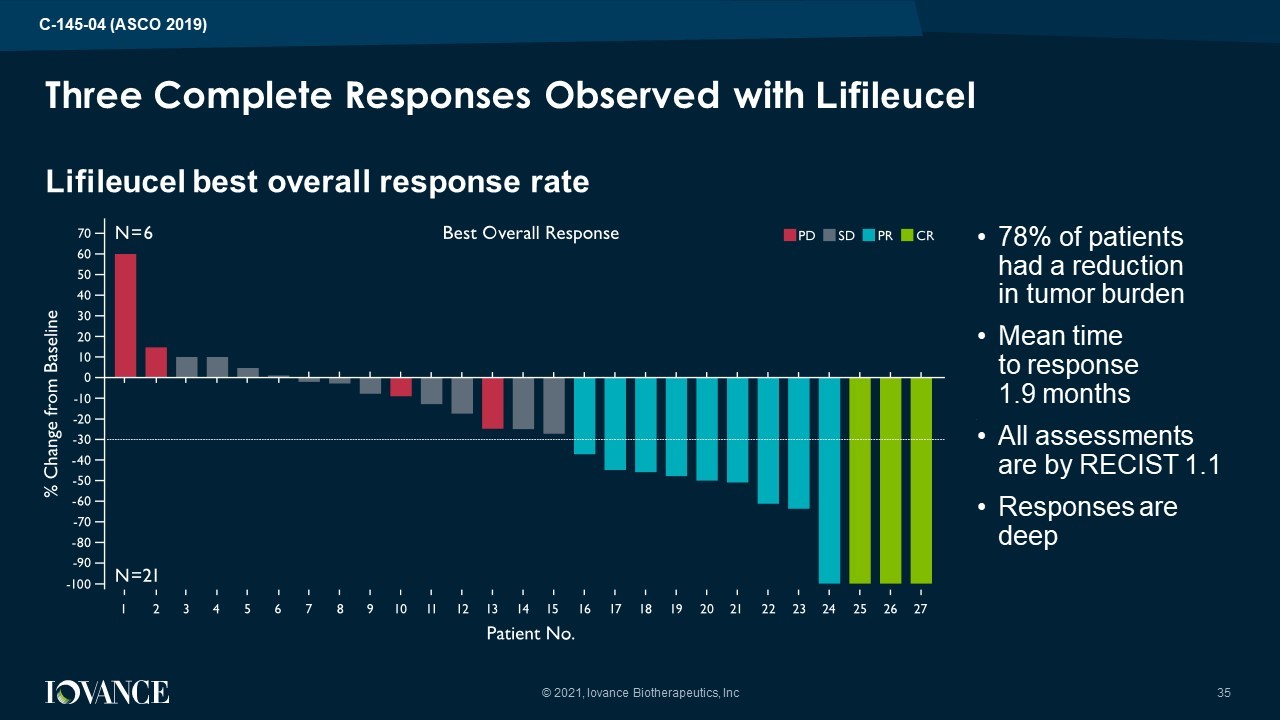
Lifileucel best overall response rate Three Complete Responses Observed with Lifileucel • 78% of patients had a reduction in tumor burden • Mean time to response 1.9 months • All assessments are by RECIST 1.1 • Responses are deep 35 © 2021, Iovance Biotherapeutics, Inc C - 145 - 04 (ASCO 2019)

Agent ORR % ( N) Current Dev Status Prior Line of Tx Patient Characteristics Antibody - drug conjugate tisotumab vedotin (TV) (Genmab/ Seagen ) 24% (N=101) (1) Phase 2 1+ Recurrent or metastatic cervical cancer that progressed on standard therapy ≤2 prior systemic regimens mDOR = 8.3 mons, mOS = 12.1 mons Anti - PD - 1 alone or combination with anti - CTLA4 Balstilimab (Agenus) 14% (N=160) (2) Phase 2 1 + Patients must have relapsed after a platinum - containing doublet administered for treatment of advanced disease, median DOR=15.4 months Balstilimab + Zalifrelimab 22% (N=143) (2) Phase 2 1+ cemiplimab (Regeneron) 10% (N=10) (3) Phase 1 Phase 3 read out 2+ Recurrent or metastatic cervical cancer resistant to, or intolerant of, platinum therapy. Ph 3 mOS 12.0 mons Cell therapies TIL (lifileucel) 44% (N=27) (4) Phase 2 2.4 (mean) mDOR not reached at median study follow up of 7.4 mons Recurrent, metastatic, or persistent cervical carcinoma has no current standard of care Development Efforts in Recurrent, Metastatic or Persistent Cervical Carcinoma © 2021, Iovance Biotherapeutics, Inc 36 (1) Coleman et al., ESMO 2020 (2) O’Malley et al., ESMO 2020 (3) Rischin et al., ESMO 2018 (4) Jazaeri et al., ASCO 2019

HNSCC & NSCLC © 2021, Iovance Biotherapeutics, Inc 37

© 2021, Iovance Biotherapeutics, Inc 38 1A: Melanoma PD - 1/ PD - L1 Naïve LN - 144 + Pembrolizumab N=12 2A:Head and Neck PD - 1/ PD - L1 Naïve LN - 145 + Pembrolizumab N=19 IOV - COM - 202: Global Phase 2 Study of TIL Therapy in Solid Tumors (NCT03645928) 1B: Melanoma ≥ 1 prior systemic therapies LN - 145 - S1 N up to 27 (Simon’s two - stage) 3A: NSCLC PD - 1/ PD - L1 Naïve LN - 145 + Pembrolizumab N=12 3B: NSCLC ≥ 1 prior systemic therapies LN - 145 N=12 1C: Melanoma ≥ 1 prior systemic therapies LN - 144 Gen 3 N up to 27 (Simon’s two - stage) 3C: NSCLC 1 prior systemic therapy LN - 145 + ipi / nivo , N up to 26 (Simon’s two stage) Endpoints • Primary: Efficacy and safety: ORR (RECIST 1.1) assessed by investigator • Secondary: Additional efficacy Study Updates • Additional cohorts 1C and 3C were added in 1Q21 • Sample size for cohort 2A was increased A Phase 2, multicenter study of autologous Tumor Infiltrating Lymphocytes in patients with solid tumors

Head and Neck Squamous Cell Carcinoma (HNSCC) © 2021, Iovance Biotherapeutics, Inc 39

HNSCC Facts Potential Market for Head and Neck Squamous Cell Carcinoma (HNSCC) 40 Abbreviations: ORR, objective response rate; TIL, tumor infiltrating lymphocytes. (1) Global Burden of Disease Cancer Collaboration, JAMA Oncol 2019 (2) https://seer.cancer.gov accessed Mar 2021 (3) Keytruda USPI accessed Mar 2021 and Szturz et al., Ann Transl Med 2020 (4) Vermorken et al., NEJM 2008 (5 ) Bauml et al., J Clin Oncol 2017 © 2021, Iovance Biotherapeutics, Inc — Antonio Jimeno M.D., Ph.D. Professor of Medicine/Oncology and Otolaryngology University of Colorado School of Medicine The majority of patients did experience a tumor shrinkage that in some cases met the criteria for an objective response. It is hard to generalize from such a small cohort, but with that caveat complete responses are relatively rare with PD - 1 inhibition alone based on what has been reported in PD - 1 inhibitor fist - line trials in PD - 1 naïve patients with head and neck carcinoma.” Available Care (NCCN) ORR DOR First Line Anti PD - 1 antibody (3) 16% 22.6 months Anti PD - 1 antibody + Chemo (3) 36% 6.7 months Chemotherapy (EXTREME) (4) 36% 5.6 months Second Line Anti PD - 1 antibody (5) 16% 8 months New Cases WW each year (1) 890k 66k Diagnoses in U.S. each year (2) 507k 15k Deaths WW each year (1) Deaths in U.S. each year (2)
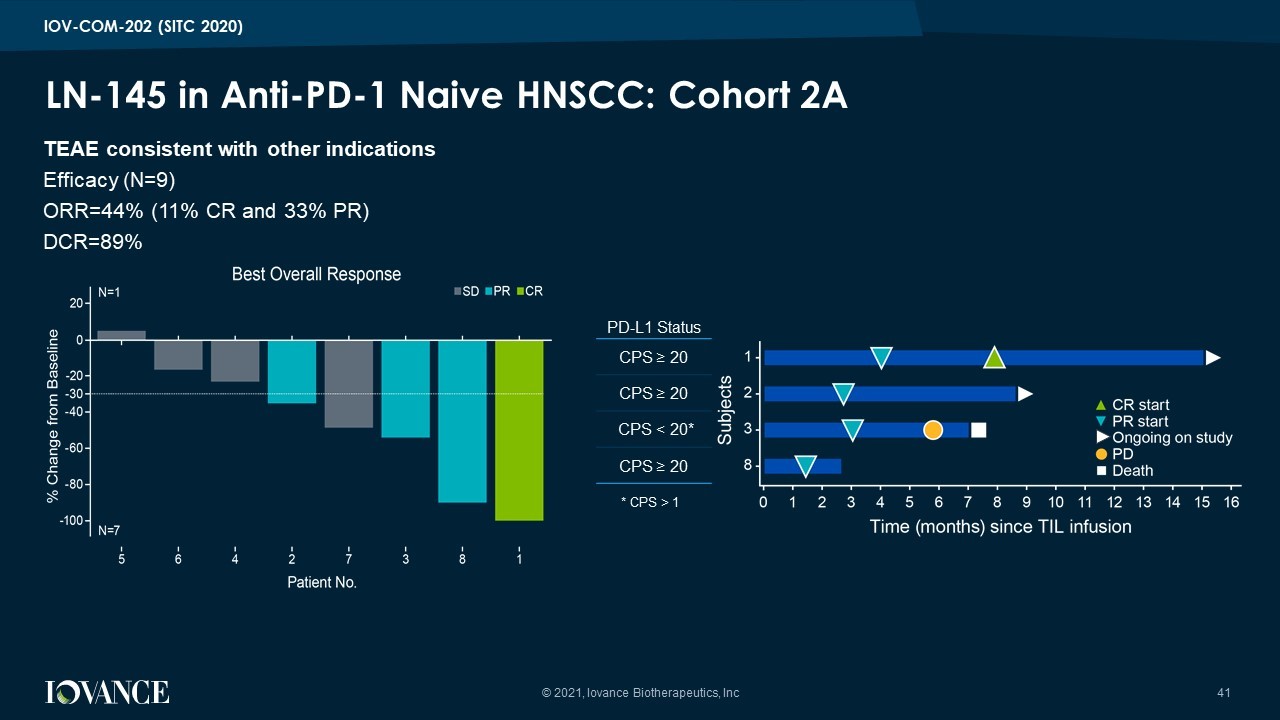
LN - 145 in Anti - PD - 1 Naive HNSCC: Cohort 2A © 2021, Iovance Biotherapeutics, Inc 41 TEAE consistent with other indications Efficacy (N=9) ORR=44% (11% CR and 33% PR) DCR=89% IOV - COM - 202 (SITC 2020) PD - L1 Status CPS ≥ 20 CPS ≥ 20 CPS < 20* CPS ≥ 20 * CPS > 1

Non - Small Cell Lung Cancer (NSCLC) © 2021, Iovance Biotherapeutics, Inc 42

9% ORR for docetaxel in 2L NSCLC following progression on chemo (3) New Cases WW each year (1) 2.1M 229k Diagnoses in U.S. each year (2) 1.8M 136k Deaths WW each year (1) Deaths in U.S. each year (2) Available NSCLC care: Checkpoint Inhibitor + Chemo as first line option (1) Global Burden of Disease Cancer Collaboration, JAMA Oncol 2019 (2) https://seer.cancer.gov accessed Mar 2021 (3) Brahmer et al., NEJM 2015 Addressing a Defined Unmet Need in Second Line NSCLC Potential Market for Non - Small Cell Lung Cancer (NSCLC) 43 — Ben Creelan , M.D.* Thoracic Oncology Program at Moffitt Cancer Center “Despite progression on nivolumab, we did see a decrease in tumor size for many patients, and the ORR was in around one - quarter of patients, and perhaps in a one - third of patients if our unconfirmed PR is confirmed…Clonotype and phenotype analyses suggested good persistence of the transferred TILs — going out to several months.” Lung Cancer Facts We’re excited about carrying TILs further in lung cancer.” * OncLive , AACR 2020, “TIL Therapy Elicits Encouraging Activity in Advanced NSCLC” © 2021, Iovance Biotherapeutics, Inc

Responses N=12 (%) Objective Response Rate 3 (25%) Complete Response 2 (17%) Partial Response 1 (8%) • ORR 25%; • 1 CR is noted in EGFR Δ Ex19 post afatinib , osimertinib , nivolumab • Median DOR not reached; • All 3 responders on TIL were relapsed or refractory to monotherapy Nivo • The TIL CR responses were ongoing • 2/3 responders were PD - L1 low (TPS<5%) Efficacy Data Post Moffitt TIL Infusion © 2021, Iovance Biotherapeutics, Inc 44 Moffitt NSCLC TIL plus Nivo (AACR 2020) (1) (1) Creelan et al., AACR 2020
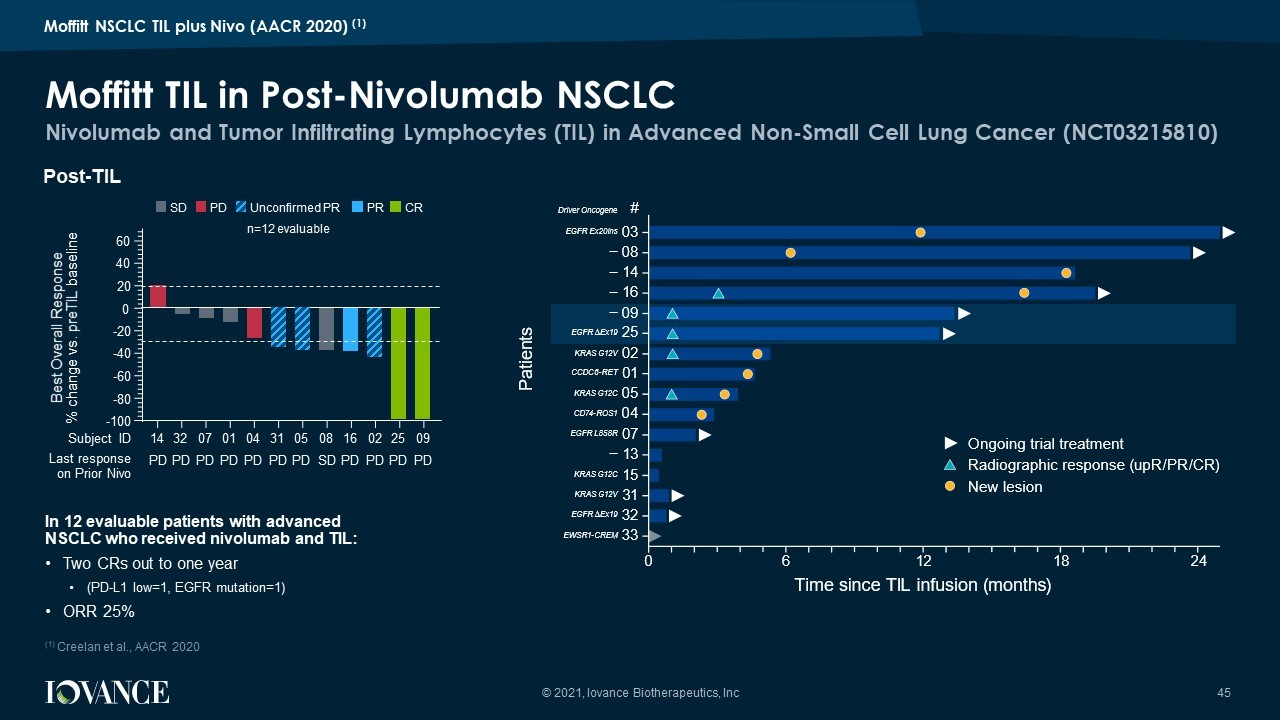
In 12 evaluable patients with advanced NSCLC who received nivolumab and TIL: • Two CRs out to one year • (PD - L1 low=1, EGFR mutation=1) • ORR 25% Nivolumab and Tumor Infiltrating Lymphocytes (TIL) in Advanced Non - Small Cell Lung Cancer (NCT03215810) Moffitt TIL in Post - Nivolumab NSCLC © 2021, Iovance Biotherapeutics, Inc 45 Post - TIL 14 32 07 01 04 31 05 08 16 02 25 09 - 100 - 80 - 60 - 40 - 20 0 20 40 60 Subject ID Best Overall Response % change vs. preTIL baseline PD PD PD PD PD PD PD SD PD PD PD PD Last response on Prior Nivo CR PR Unconfirmed PR SD PD n= 12 evaluable 7 L P H V LQ F H 7 ,/ L Q I X V L R Q P R Q W K V 3 D W L H Q W V 2 Q J R L Q J W U L D O W U H D W P H Q W 5 D G L R J U D S K L F U H V S R Q V H X S 5 5 1 H Z O H V L R Q ' U L Y H U 2 Q F R J H Q H ( * ) 5 ( [ L Q V . 5 $ 6 * ( * ) 5 ( [ . 5 $ 6 * 9 '; 5 ( 7 ( * ) 5 / 5 ' 5 2 6 . 5 $ 6 * . 5 $ 6 * 9 ( * ) 5 ¨ ( [ ( : 6 5 & 5 ( 0 ² ² ² ² Moffitt NSCLC TIL plus Nivo (AACR 2020) (1) (1) Creelan et al., AACR 2020
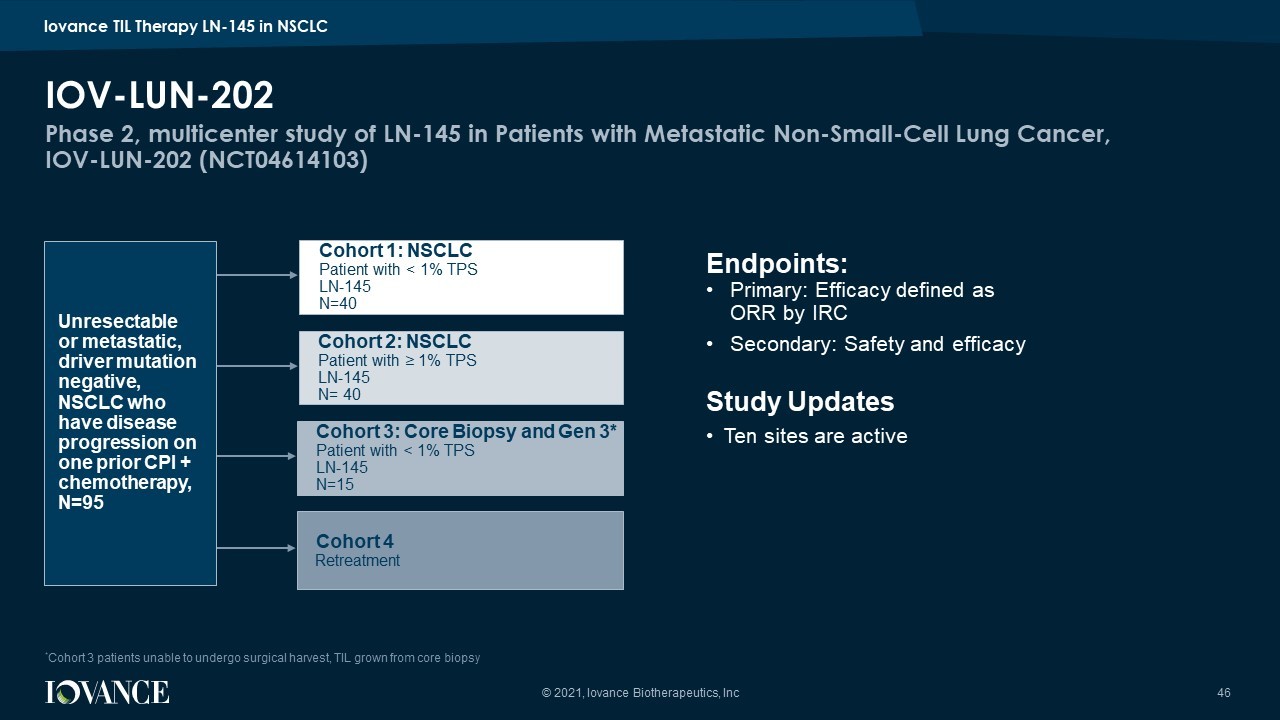
Phase 2, multicenter study of LN - 145 in Patients with Metastatic Non - Small - Cell Lung Cancer, IOV - LUN - 202 (NCT04614103) IOV - LUN - 202 46 Endpoints: • Primary: Efficacy defined as ORR by IRC • Secondary: Safety and efficacy Study Updates • Ten sites are active Cohort 1: NSCLC Patient with < 1% TPS LN - 145 N=40 Cohort 2 : NSCLC Patient with ≥ 1% TPS LN - 145 N= 40 Cohort 3: Core Biopsy and Gen 3* Patient with < 1% TPS LN - 145 N=15 Cohort 4 Retreatment Unresectable or metastatic, driver mutation negative, NSCLC who have disease progression on one prior CPI + chemotherapy, N=95 © 2021, Iovance Biotherapeutics, Inc * Cohort 3 patients unable to undergo surgical harvest, TIL grown from core biopsy Iovance TIL Therapy LN - 145 in NSCLC

Select more potent TIL • Iovance PD - 1 positive selected TIL • PD - 1 positive selected TIL also through collaboration with CHUM Expand the TIL platform into new indications/regimens • IOV - 3001 IL - 2 analog licensed from Novartis: IND enabling studies in 2021 Genetically modify to make a more tumor - reactive TIL • Cellectis TALEN ® collaboration agreement in place to support a clinical program ( 1 ) Research Focus into Next Generation TIL © 2021, Iovance Biotherapeutics, Inc 47 Process optimization • Gen 3 (16 - day) process (COM - 202) • Core biopsy (LUN - 202 study) (1) Ritthipichai et al., ESMO 2020

Iovance Global Reach and Scale © 2021, Iovance Biotherapeutics, Inc 48 Iovance Biotherapeutics has >250 employees • >76% of employees have over 1 year of cell therapy experience • Headquartered in San Carlos, CA • 4 additional offices • Iovance commercial manufacturing facility in Philadelphia, PA San Carlos, CA Philadelphia, PA Zug, CH Tampa, FL Business Office or Subsidiary Iovance Manufacturing Site Corporate Headquarters Amsterdam, NL

December 31, 2020 In millions (unaudited) Common shares outstanding 146.9 Preferred shares outstanding 3.6 (1) Options 12.6 Cash, cash equivalents, short - term investments, restricted cash $635.0 (2) Anticipated cash runway sufficient into 2023 Debt $0 ( 1 ) Preferred shares are shown on an as - converted basis. (2) Includes Restricted Cash of $5.5 million. Well Capitalized in Pursuit of TIL Commercialization © 2021, Iovance Biotherapeutics, Inc 49

Thank You © 2021, Iovance Biotherapeutics, Inc 50

















































