
OrthoPediatrics Corp. Mark Throdahl, CEO March 2019 Fred Hite, CFO

Disclaimer Forward-Looking Statements This presentation includes "forward-looking statements" within the meaning of U.S. federal securities laws. You can identify forward-looking statements by the use of words such as "may," "might," "will," "should," "expect," "plan," "anticipate," "could," "believe," "estimate," "project," "target," "predict," "intend," "future," "goals," "potential,” "objective," "would" and other similar expressions. Forward-looking statements involve risks and uncertainties, many of which are beyond OrthoPediatrics’ control. Important factors could cause actual results to differ materially from those in the forward-looking statements, including, among others, the risks, uncertainties and factors set forth under "Risk Factors" in OrthoPediatrics’ Annual Report on Form 10-K filed with the SEC on March 7, 2019. Forward-looking statements speak only as of the date they are made. OrthoPediatrics assumes no obligation to update forward-looking statements to reflect actual results, subsequent events, or circumstances or other changes affecting such statements except to the extent required by applicable securities laws. Use of Non-GAAP Financial Measures This presentation includes the non-GAAP financial measure of Adjusted EBITDA, which differs from financial measures calculated in accordance with U.S. generally accepted accounting principles (“GAAP”). Adjusted EBITDA in this presentation represents net loss, plus interest expense (income), net plus other expense (income), depreciation and amortization, stock-based compensation expense, accelerated vesting of restricted stock upon our IPO, public company costs and initial public offering costs. Adjusted EBITDA is presented because the Company believes it is a useful indicator of its operating performance. Management uses the measure as a measure of the Company’s operating performance and for planning purposes, including financial projections. The Company believes this measure is useful to investors as supplemental information because it is frequently used by analysts, investors and other interested parties to evaluate companies in its industry. The Company believes Adjusted EBITDA is useful to its management and investors as a measure of comparative operating performance from period to period. Adjusted EBITDA is a non-GAAP financial measure and should not be considered as an alternative to, or superior to, net income or loss as a measure of financial performance or cash flows from operations as a measure of liquidity, or any other performance measure derived in accordance with GAAP, and it should not be construed to imply that the Company’s future results will be unaffected by unusual or non-recurring items. In addition, the measure is not intended to be a measure of free cash flow for management’s discretionary use, as it does not reflect certain cash requirements such as debt service requirements, capital expenditures and other cash costs that may recur in the future. Adjusted EBITDA contains certain other limitations, including the failure to reflect our cash expenditures, cash requirements for working capital needs and other potential cash requirements. In evaluating Adjusted EBITDA, you should be aware that in the future the Company may incur expenses that are the same or similar to some of the adjustments in this presentation. The Company’s presentation of Adjusted EBITDA should not be construed to imply that its future results will be unaffected by any such adjustments. Management compensates for these limitations by primarily relying on the Company’s GAAP results in addition to using Adjusted EBITDA on a supplemental basis. The Company’s definition of this measure is not necessarily comparable to other similarly titled captions of other companies due to different methods of calculation. 2

Highlights Large Market Proprietary Technology Scalable Business Diversified medical device company focused exclusively on pediatric orthopedics Protected market opportunity: $1.1 billion U.S., $2.5 billion globally in 2016 High U.S. procedure concentration: <300 hospitals and ~1,400 surgeons Focused call point: pediatric orthopedic generalists who use our entire product portfolio Broadest product offering: 26 surgical systems specifically designed for children Sustainable competitive advantage: - Comprehensive product offering - Clinical education programs - Surgeon relationships - Experienced sales organization Consistent 20+% growth since inception - FY18 revenue of $57.6 million, up 26% - 24%, 28%, 28%, and 25% revenue growth for 1Q, 2Q, 3Q, and 4Q 2018, respectively Recent financings will, among other things, fund consigned sets and accelerate proven strategy, including any M&A opportunities (~$43M from 12/18 follow-on) 3

OP Today A Company Built on a CAUSE Cause Company Snapshot Improving the lives of children Treated >150,000 patients since inception with orthopedic conditions 26 surgical systems; 5,000+ SKUs; strong pipeline 80 direct employees; >130 focused sales reps* Global sales organization focused on pediatric orthopedic surgeons in 40 countries* 26 issued patents; 35 pending patents* Only non-founding Chief Medical Officer in the industry who is a fellow surgeon Average FDA approval time: < ½ industry average History of stable reimbursement Gideon with CMO Peter Armstrong, M.D., c. 1995. Gideon’s drawing of his girlfriend, 2016. * As of December 31, 2018 4

Our Key Idea Children Are Not Small Adults Superior Clinical Outcomes OP’s Market Impact Re-Purposed Adult Plate OP’s Solution Address orthopedic industry’s lack of focus on product development, clinical education, and sales presence Implants and instruments avoid complications of re-purposed adult products Product development in collaboration with leading pediatric orthopedic surgeons Dedicated sales support attending surgeries Clinical education programs that build brand loyalty Screws Through Screws Parallel To Growth Plate Growth Plate 5

Large and Focused Market OP’S $2.5 Billion Current Addressable Global Market $1.1 Billion U.S. Addressable Market High Concentration of Pediatric Trauma & Deformity and Scoliosis Procedures 3,157 U.S. Sports Hospitals Smart Medicine Implants $116M $299M 38% Procedures Scoliosis (%) $285M 62% Trauma & Deformity $401M 268 U.S. Hospitals Current products target three of the largest categories in Pediatric Orthopedics Pipeline products underway to expand addressable market 6
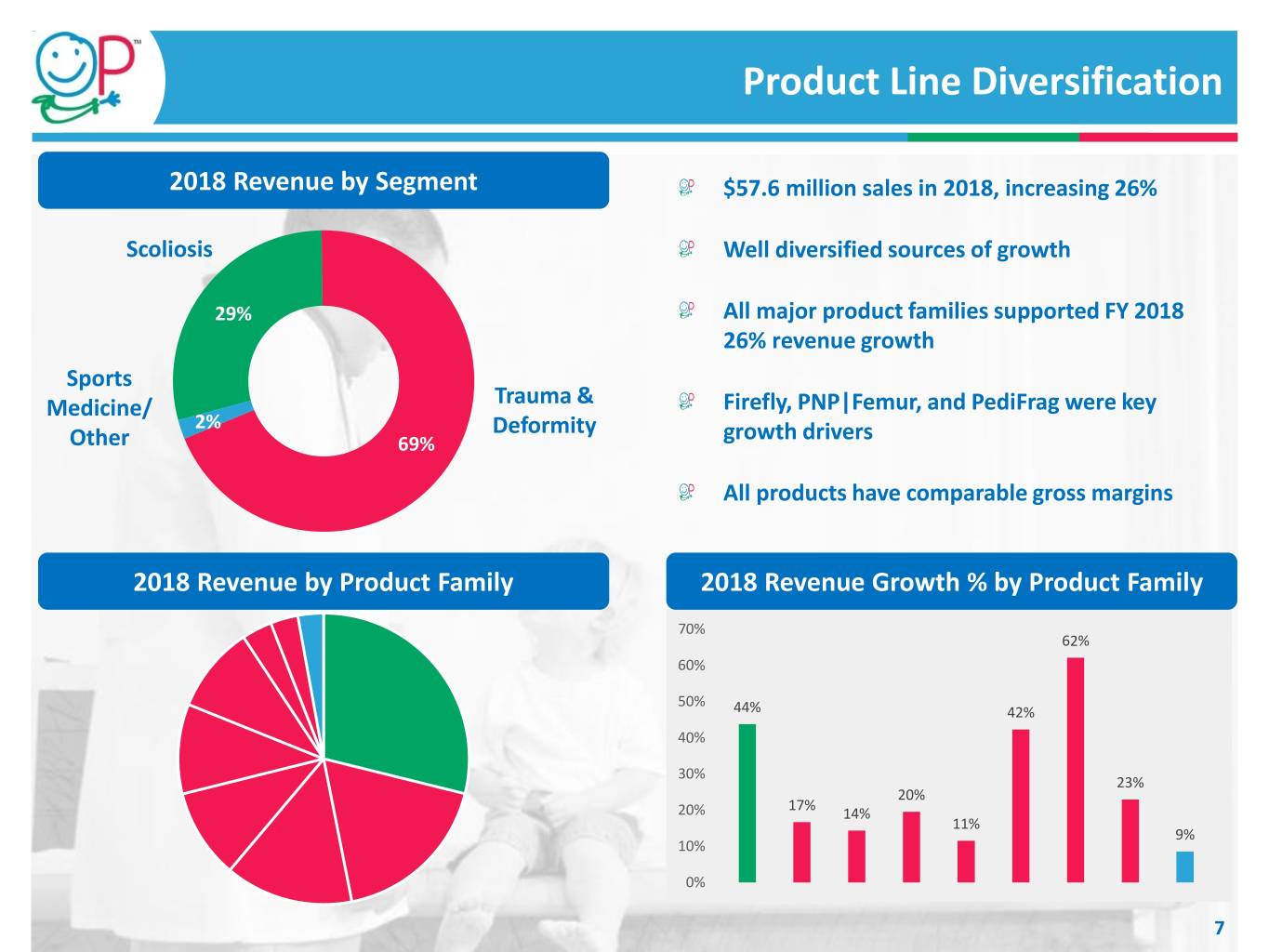
Product Line Diversification 2018 Revenue by Segment $57.6 million sales in 2018, increasing 26% Scoliosis Well diversified sources of growth 29% All major product families supported FY 2018 26% revenue growth Sports Medicine/ Trauma & Firefly, PNP|Femur, and PediFrag were key 2% Deformity Other 69% growth drivers All products have comparable gross margins 2018 Revenue by Product Family 2018 Revenue Growth % by Product Family 70% 62% 60% 50% 44% 42% 40% 30% 23% 20% 17% 20% 14% 11% 9% 10% 0% 7

A Proven Strategy Since 2011 Sales Focus on Teaching Deploy Expand Expand Clinical Institutions and Instrument Addressable Education High Volume Sets Procedures Programs Hospitals Goals Accelerate sales growth Develop novel technologies 8

New Systems & Product Launches (2017-2018) Trauma & Deformity 2017 Launches 2018 Launches Titanium Clavicle Wrist Fusion PediFlex Pediatric Nailing PediPlates® Plate System Plate System Advanced Platform | FEMUR System (First pediatric (First pediatric (Expands into adolescent (Expands physeal specific specific cases) tethering offering) system) system) Scoliosis 2017 Launches 2018 Launches FIREFLY® Pedicle Screw Navigation FIREFLY® RESPONSE 4.5/4.75/5.0mm Guides (Complementary to S2/Alar System RESPONSE Spine System) (Maximizes intraoperative flexibility) Sports Medicine 2017 Launches Medial Patella Femoral Ligament Reconstruction System (Complementary to ACL Reconstruction System) 9

Upcoming New Systems & Product Launches Trauma & Deformity 2019 Expected Product Launches Development Development Development Development Complete Complete In Process In Process Osteogenesis PediFoot Imperfecta Nail PNP | TIbia Cannulated Screws (First pediatric system) System Scoliosis 2019 Product Launches Launched Development Feb’19 In Process Neuromuscular BandLoc DUO System Scoliosis System 10
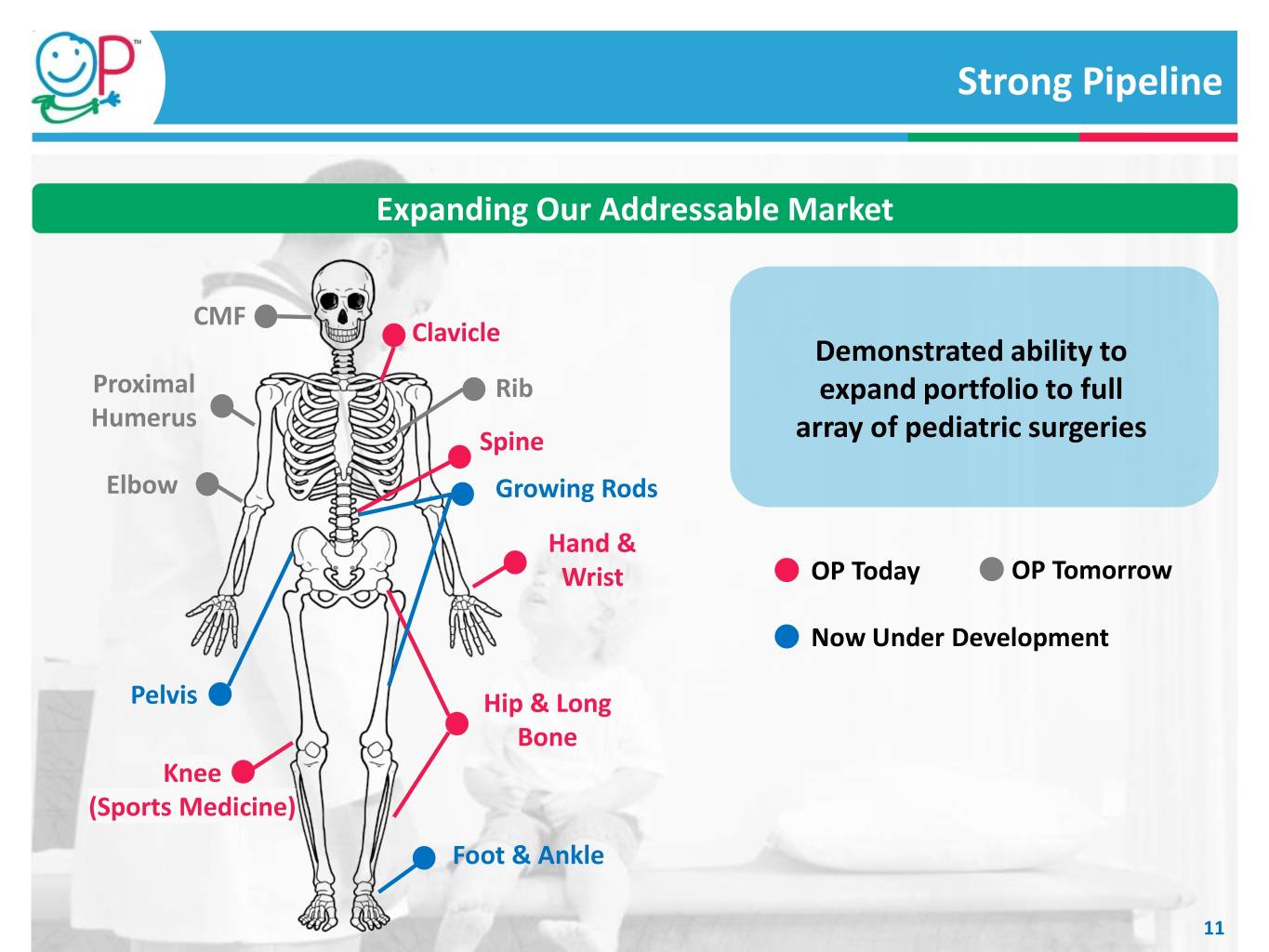
Strong Pipeline Expanding Our Addressable Market CMF Clavicle Demonstrated ability to Proximal Rib expand portfolio to full Humerus Spine array of pediatric surgeries Elbow Growing Rods Hand & Wrist OP Today OP Tomorrow Now Under Development Pelvis Hip & Long Bone Knee (Sports Medicine) Foot & Ankle 11
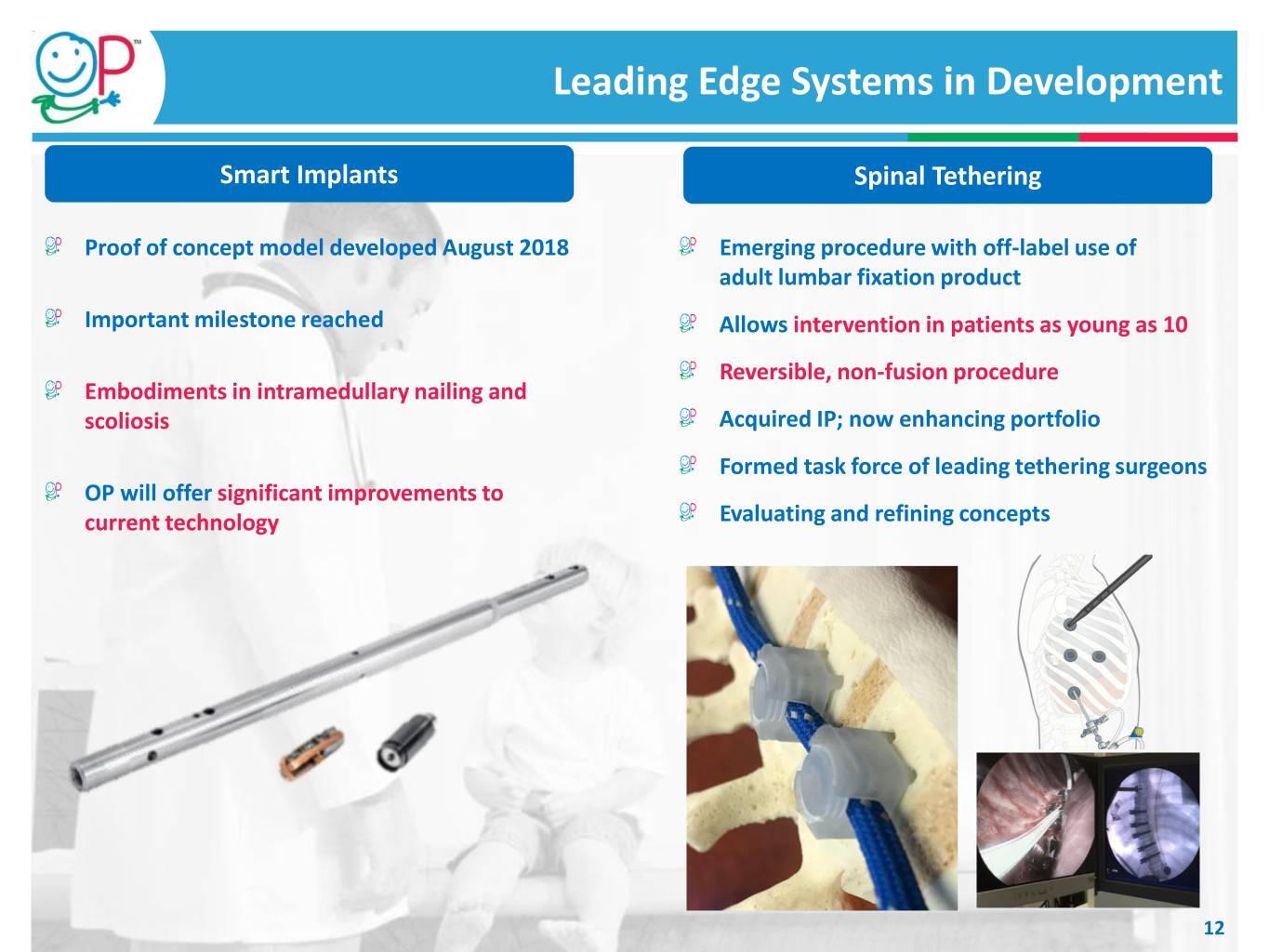
Leading Edge Systems in Development Smart Implants Spinal Tethering Proof of concept model developed August 2018 Emerging procedure with off-label use of adult lumbar fixation product Important milestone reached Allows intervention in patients as young as 10 Reversible, non-fusion procedure Embodiments in intramedullary nailing and scoliosis Acquired IP; now enhancing portfolio Formed task force of leading tethering surgeons OP will offer significant improvements to current technology Evaluating and refining concepts 12

Global Sales Coverage United States International 75% of 2018 25% of 2018 Revenue Revenue 33 Sales Agencies, 6 Sales Agencies most of which are + 35 Stocking exclusive* Distributors* Direct in UK, IRE, AUS, NZ, CAN, BE, NL * Data as of FY 2018 Currently selling to major children’s hospitals in the U.S. and 39 additional countries Converting to agency model in select markets has significantly increased volumes, ASPs, and gross margin Replicate success of sales agency model in UK, IRE, AUS, NZ, CAN, BE, and NL 13

Competitive Landscape New Competitors Would Face Formidable Obstacles Product breadth Surgeon relationships Sales and distribution network Clinical education programs Pediatric brand equity Reputation with pediatric orthopedic societies Dynamic culture “The ship has sailed.” 14

What Does Category Leadership Mean? Surgeon relationships and Broadest, most innovative clinical education product offering Relationships with surgeons who use 12 years’ clinical understanding entire portfolio New product pipeline Major provider of clinical education Pediatric Market Gateway for Leading supporter of surgical societies distributed products and joint Custom instruments product developments Robust organic growth Attractive growth and opportunities margin profile $2.5 billion addressable global market Consistent growth since inception Limited focused competition 74% gross margin in FY 2018 Focused, experienced distribution History of efficient capital Instrument set placements drive growth utilization 15

Financial Review
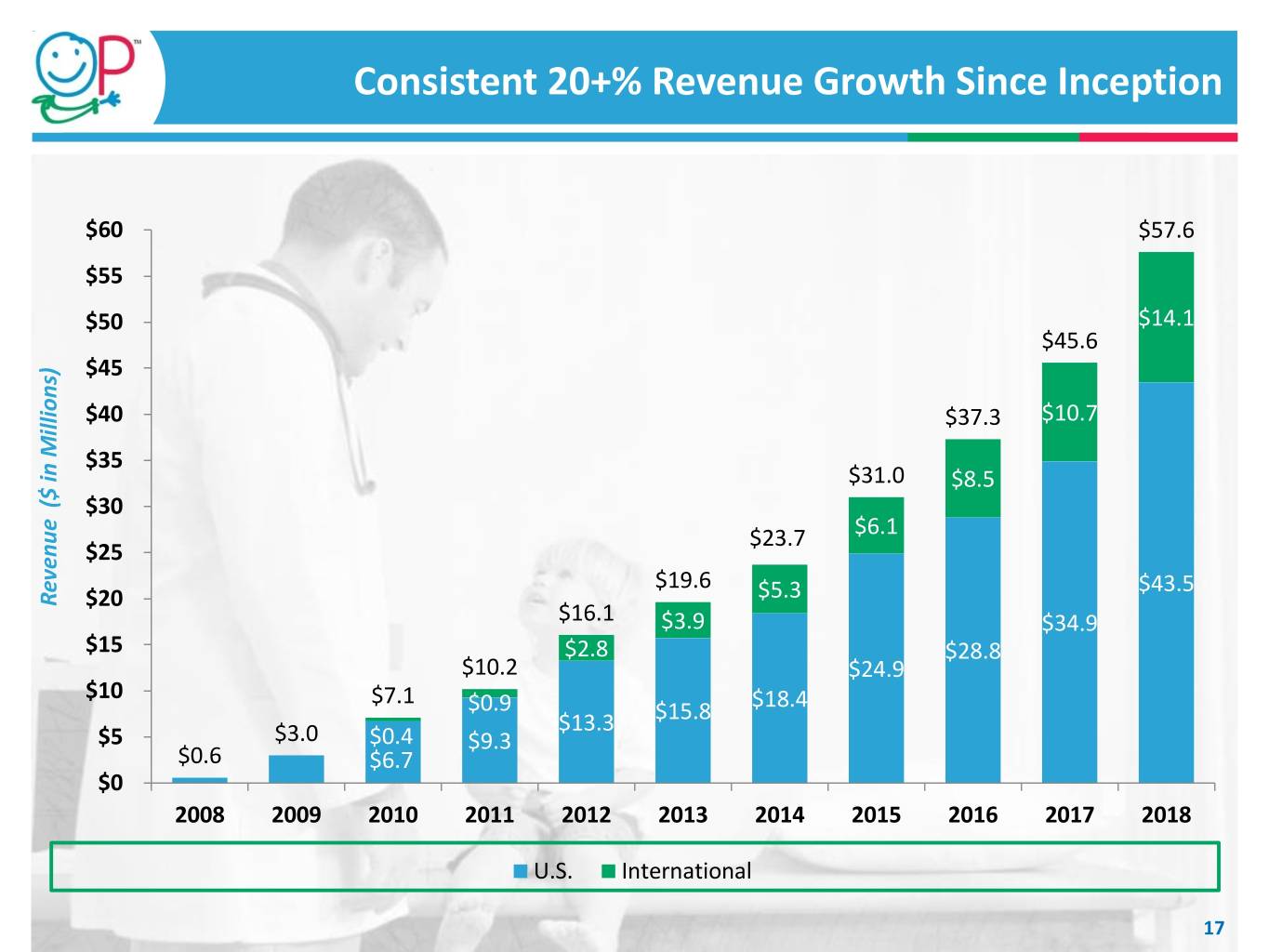
Consistent 20+% Revenue Growth Since Inception $60 $57.6 $55 $50 $14.1 $45.6 $45 $40 $37.3 $10.7 $35 $31.0 $8.5 $30 $23.7 $6.1 $25 $19.6 $5.3 $43.5 Revenue ($ in Millions) ($ Revenue $20 $16.1 $3.9 $34.9 $15 $2.8 $28.8 $10.2 $24.9 $10 $7.1 $0.9 $18.4 $13.3 $15.8 $5 $3.0 $0.4 $9.3 $0.6 $6.7 $0 2008 2009 2010 2011 2012 2013 2014 2015 2016 2017 2018 U.S. International 17

Accelerating Growth 30% 25% 20% 15% 28% 28% 24% 24% 25% Revenue Growth % Revenue 22% 22% 10% 21% 5% 0% 1Q 2017 2Q 2017 3Q 2017 4Q 2017 1Q 2018 2Q 2018 3Q 2018 4Q 2018 18
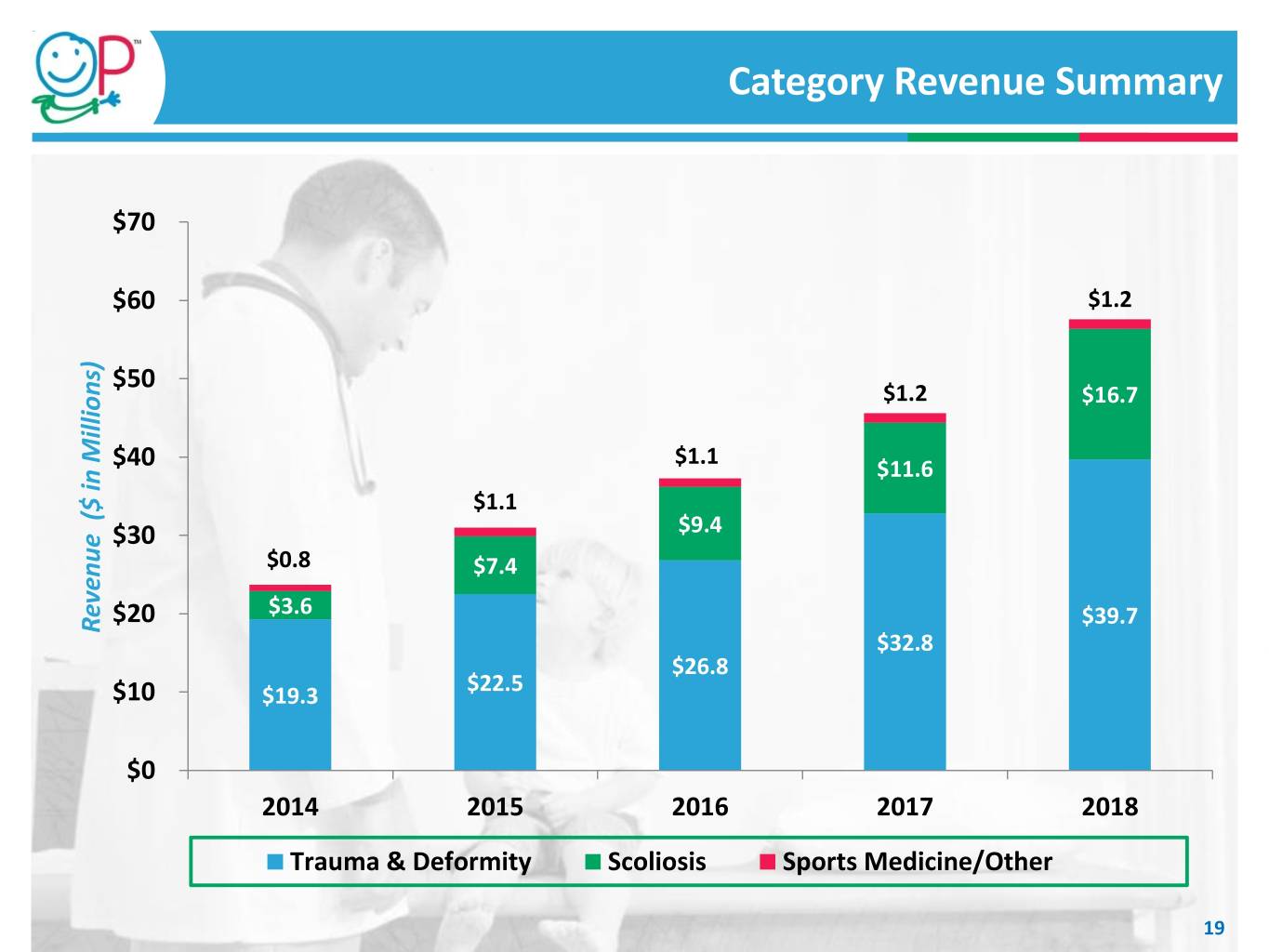
Category Revenue Summary $70 $60 $1.2 $50 $1.2 $16.7 $1.1 $40 $11.6 $1.1 $30 $9.4 $0.8 $7.4 $20 $3.6 $39.7 Revenue Millions) in ($ Revenue $32.8 $26.8 $22.5 $10 $19.3 $0 2014 2015 2016 2017 2018 Trauma & Deformity Scoliosis Sports Medicine/Other 19

Revenue Seasonality Seasonality Drives Stronger Performance in Summer Months and Holiday Periods 30% 27% 27% 27% 26% 25% 26% 26% 26% 25% 25% 22% 21% 21% 20% 15% 10% Revenue as % of Total Year Total of % as Revenue 5% 0% 1Q 2Q 3Q 4Q 1Q 2Q 3Q 4Q 1Q 2Q 3Q 4Q 2016 2016 2016 2016 2017 2017 2017 2017 2018 2018 2018 2018 20
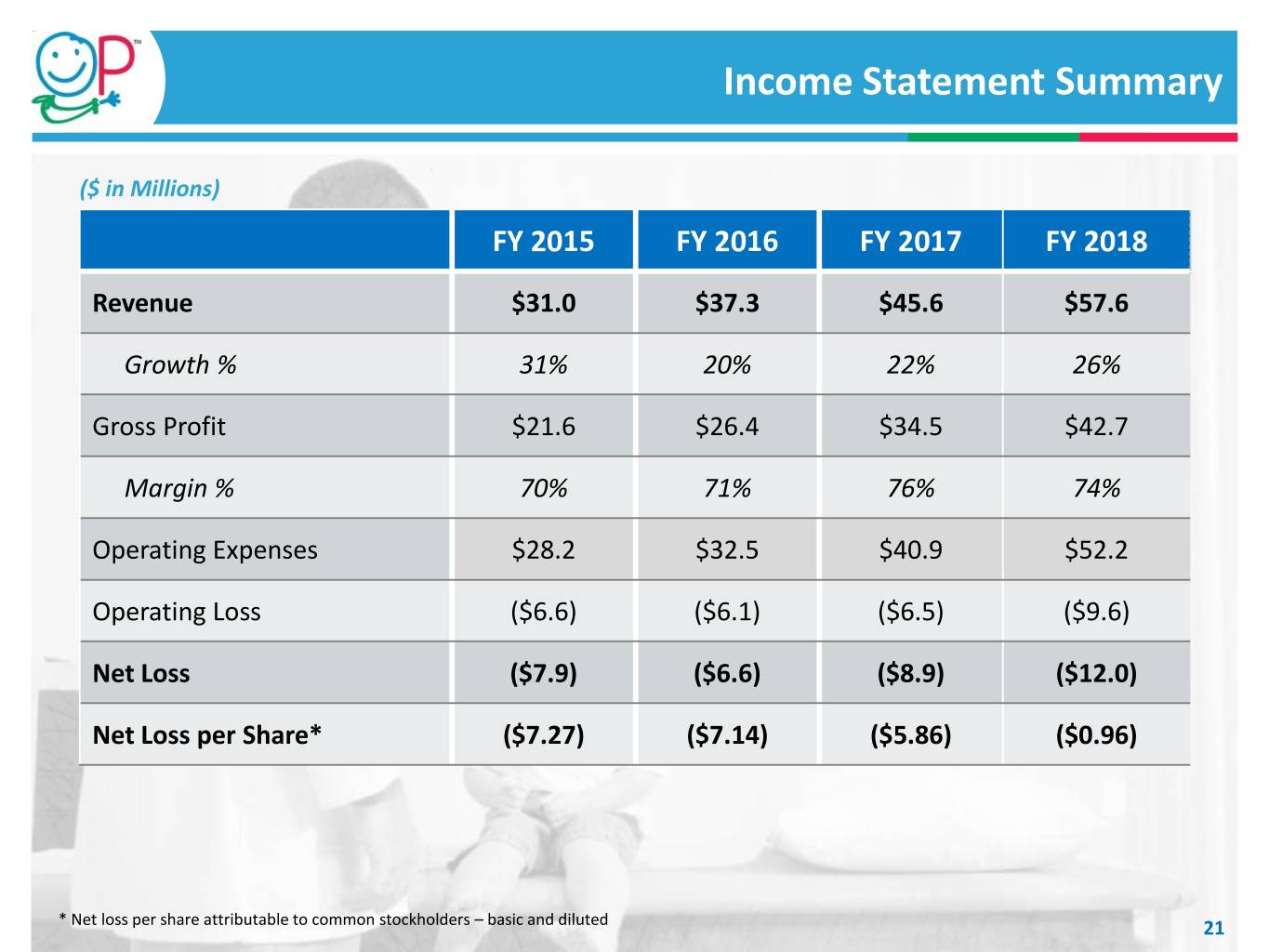
Income Statement Summary ($ in Millions) FY 2015 FY 2016 FY 2017 FY 2018 Revenue $31.0 $37.3 $45.6 $57.6 Growth % 31% 20% 22% 26% Gross Profit $21.6 $26.4 $34.5 $42.7 Margin % 70% 71% 76% 74% Operating Expenses $28.2 $32.5 $40.9 $52.2 Operating Loss ($6.6) ($6.1) ($6.5) ($9.6) Net Loss ($7.9) ($6.6) ($8.9) ($12.0) Net Loss per Share* ($7.27) ($7.14) ($5.86) ($0.96) * Net loss per share attributable to common stockholders – basic and diluted 21

Adjusted EBITDA Reconciliation ($ in Millions) Year Ended December 31, 2017 2018 Net Loss ($8.9) ($12.0) Interest expense 2.5 2.2 Other expense (income) (0.0) 0.2 Depreciation and amortization 2.4 2.9 Stock-based compensation 1.4 1.2 Accelerated vesting of restricted stock 2.0 2.0 Public company costs 0.5 1.4 Non-recurring professional service fees - 2.6 Adjusted EBITDA ($0.1) $0.5 22

Balance Sheet ($ in Millions) As of December 31, 2018 Assets Liabilities Cash $60.7 Accounts Payable $4.0 Accounts Receivable 9.0 Debt 21.3 Inventory 25.7 Accrued Expenses 3.5 Other Current Assets 1.7 All Other Liabilities 1.6 PP&E (net) 12.8 Paid In Capital 197.4 Intangibles 2.2 Accumulated Deficit (net) (115.7) Total Assets $112.1 Total Liabilities / Equity $112.1 Recent financings will, among other things, fund consigned sets and accelerate proven strategy, including any M&A opportunities (~$43M from 12/18 follow-on) 23

Summary Surgeon relationships and Broadest, most innovative clinical education product offering Robust organic growth Attractive growth and opportunities margin profile 24