
AASLD 2019 Review November 11, 2019 Exhibit 99.1

Agenda and Speakers on Today’s Call Agenda Introductions and Safe Harbor Overview of ASMB vision and HBV core inhibitor portfolio Review of HBV data from AASLD ABI-H0731 Final 24 week data from Study 201 and 202 Interim long-term data from Study 211 ABI-H2158 Interim data from initial dosing cohort of Phase 1b study (100 mg) Q&A Speakers Lauren Glaser - SVP IR and Corporate Affairs John McHutchison, AO, MD – CEO and President Richard Colonno, PhD – EVP & CSO Virology Operations

Cautionary Note Regarding Forward-Looking Statements The information in this presentation contains forward-looking statements regarding future events, including statements about the clinical and therapeutic potential of Assembly Biosciences’ HBV-cure program, the therapeutic potential of core inhibitors, including ABI-H0731, ABI-H2158 and ABI-H3733, and the plans, strategies and intentions related to its HBV-cure program and proposed stages to cure. Certain forward-looking statements may be identified by reference to a future period or periods or by use of forward-looking terminology such as “may,” “planned,” “potential,” and “will.” Such forward-looking statements, which are intended to be covered by the safe harbor provisions contained in Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended, are just predictions and are subject to risks and uncertainties that could cause the actual events or results to differ materially. These risks and uncertainties include, among others: the scientific theory for our therapeutics is unproven and novel; outcomes of clinical studies are uncertain; results observed in earlier preclinical and nonclinical studies and early clinical studies may not be predictive of future clinical studies results; and the emergence of unforeseen safety issues. These and other potential risks and uncertainties that could cause actual results to differ from the results predicted are more fully detailed under the heading “Risk Factors” in our Quarterly Report on Form 10-Q for the quarter ended September 30, 2019, filed with the Securities and Exchange Commission (the “SEC”) and any additional reports filed with the SEC following the date of this presentation. It is not possible for Assembly Biosciences management to predict all risks nor can it assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements. In light of these risks, uncertainties and assumptions, the forward-looking events and circumstances discussed in this presentation may not occur and actual results could differ materially and adversely from those anticipated. Any forward-looking statement speaks only as of the date on which it is made, and no obligation to update or revise any forward-looking statement is assumed, whether as a result of new information, future events or otherwise, except as required by law.
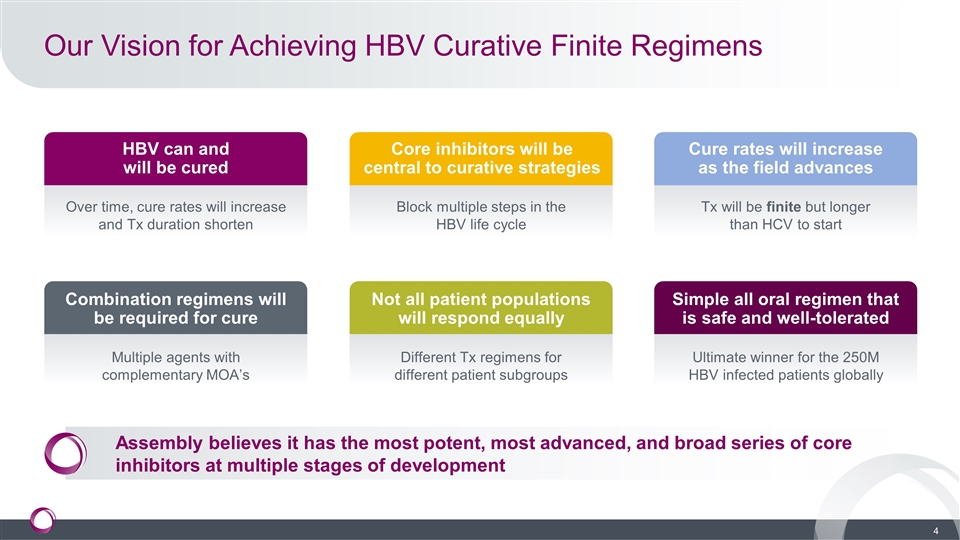
Our Vision for Achieving HBV Curative Finite Regimens HBV can and will be cured Over time, cure rates will increase and Tx duration shorten Core inhibitors will be central to curative strategies Block multiple steps in the HBV life cycle Cure rates will increase as the field advances Tx will be finite but longer than HCV to start Combination regimens will be required for cure Multiple agents with complementary MOA’s Not all patient populations will respond equally Different Tx regimens for different patient subgroups Simple all oral regimen that is safe and well-tolerated Ultimate winner for the 250M HBV infected patients globally Assembly believes it has the most potent, most advanced, and broad series of core inhibitors at multiple stages of development
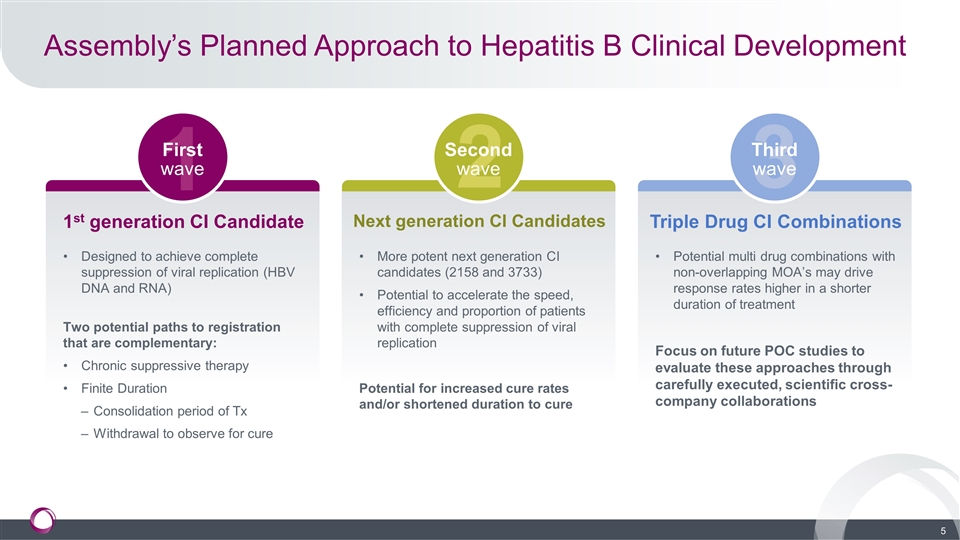
Assembly’s Planned Approach to Hepatitis B Clinical Development 1st generation CI Candidate Designed to achieve complete suppression of viral replication (HBV DNA and RNA) Two potential paths to registration that are complementary: Chronic suppressive therapy Finite Duration Consolidation period of Tx Withdrawal to observe for cure Next generation CI Candidates More potent next generation CI candidates (2158 and 3733) Potential to accelerate the speed, efficiency and proportion of patients with complete suppression of viral replication Potential for increased cure rates and/or shortened duration to cure Triple Drug CI Combinations Potential multi drug combinations with non-overlapping MOA’s may drive response rates higher in a shorter duration of treatment Focus on future POC studies to evaluate these approaches through carefully executed, scientific cross-company collaborations 1 2 3 First wave Second wave Third wave
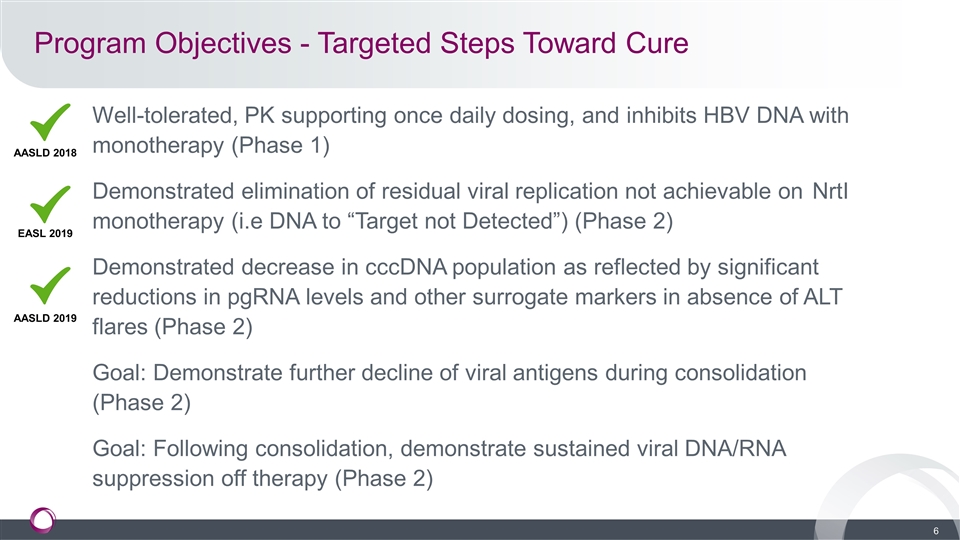
Program Objectives - Targeted Steps Toward Cure Well-tolerated, PK supporting once daily dosing, and inhibits HBV DNA with monotherapy (Phase 1) Demonstrated elimination of residual viral replication not achievable on NrtI monotherapy (i.e DNA to “Target not Detected”) (Phase 2) Demonstrated decrease in cccDNA population as reflected by significant reductions in pgRNA levels and other surrogate markers in absence of ALT flares (Phase 2) Goal: Demonstrate further decline of viral antigens during consolidation (Phase 2) Goal: Following consolidation, demonstrate sustained viral DNA/RNA suppression off therapy (Phase 2) AASLD 2018 EASL 2019 AASLD 2019
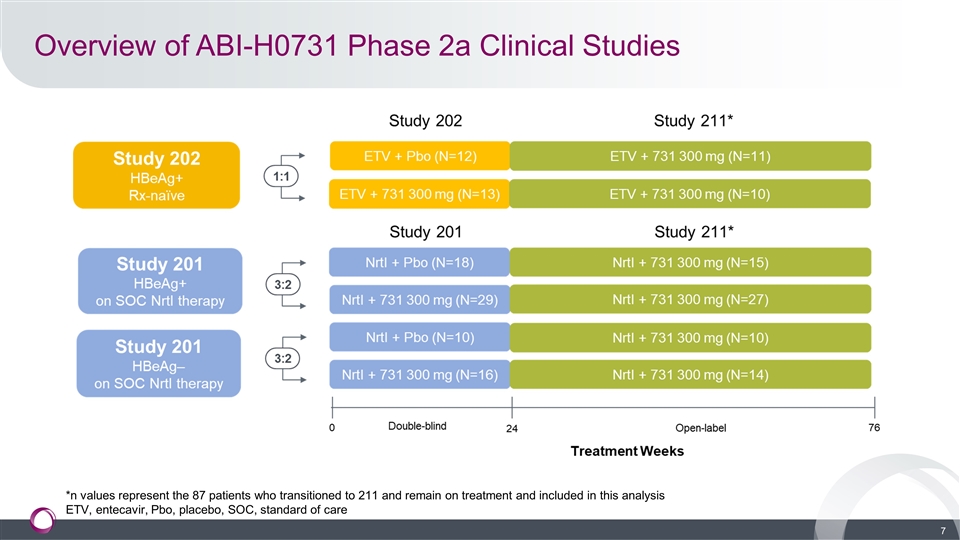
Overview of ABI-H0731 Phase 2a Clinical Studies *n values represent the 87 patients who transitioned to 211 and remain on treatment and included in this analysis ETV, entecavir, Pbo, placebo, SOC, standard of care
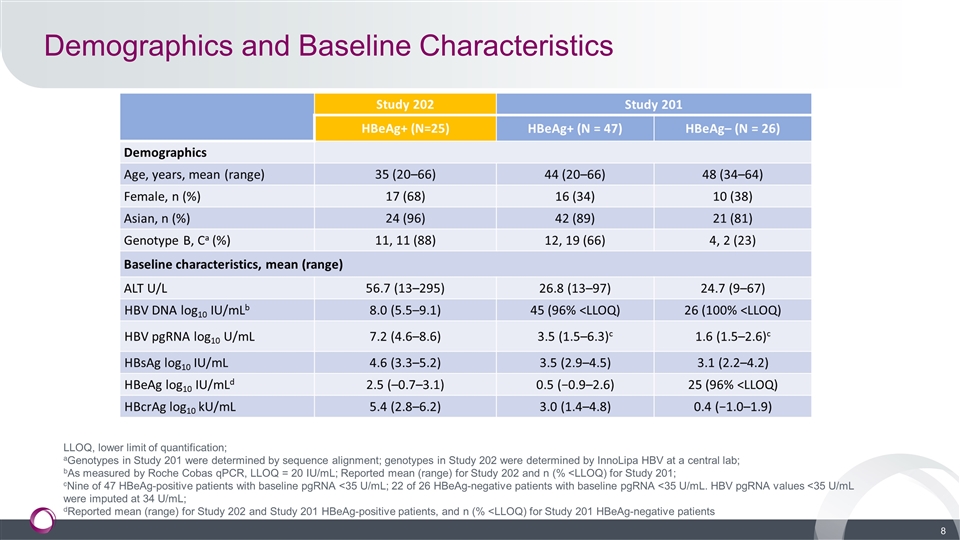
Demographics and Baseline Characteristics LLOQ, lower limit of quantification; aGenotypes in Study 201 were determined by sequence alignment; genotypes in Study 202 were determined by InnoLipa HBV at a central lab; bAs measured by Roche Cobas qPCR, LLOQ = 20 IU/mL; Reported mean (range) for Study 202 and n (% <LLOQ) for Study 201; cNine of 47 HBeAg-positive patients with baseline pgRNA <35 U/mL; 22 of 26 HBeAg-negative patients with baseline pgRNA <35 U/mL. HBV pgRNA values <35 U/mL were imputed at 34 U/mL; dReported mean (range) for Study 202 and Study 201 HBeAg-positive patients, and n (% <LLOQ) for Study 201 HBeAg-negative patients
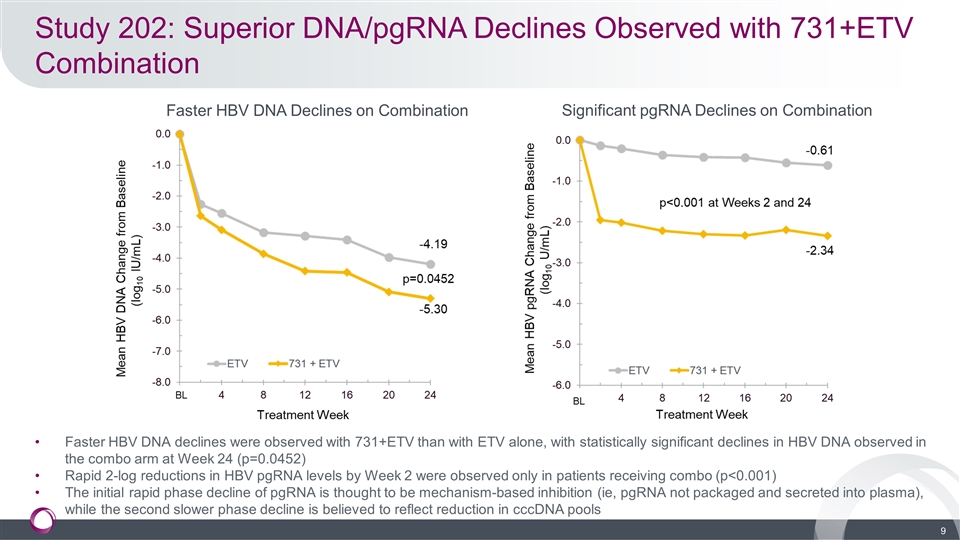
Study 202: Superior DNA/pgRNA Declines Observed with 731+ETV Combination Faster HBV DNA declines were observed with 731+ETV than with ETV alone, with statistically significant declines in HBV DNA observed in the combo arm at Week 24 (p=0.0452) Rapid 2-log reductions in HBV pgRNA levels by Week 2 were observed only in patients receiving combo (p<0.001) The initial rapid phase decline of pgRNA is thought to be mechanism-based inhibition (ie, pgRNA not packaged and secreted into plasma), while the second slower phase decline is believed to reflect reduction in cccDNA pools Faster HBV DNA Declines on Combination Significant pgRNA Declines on Combination
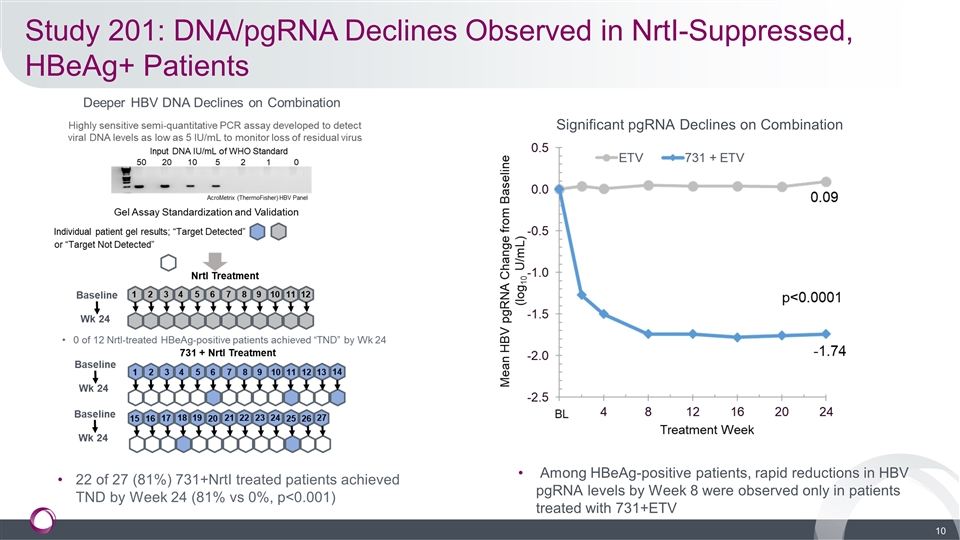
Study 201: DNA/pgRNA Declines Observed in NrtI-Suppressed, HBeAg+ Patients Among HBeAg-positive patients, rapid reductions in HBV pgRNA levels by Week 8 were observed only in patients treated with 731+ETV Significant pgRNA Declines on Combination 22 of 27 (81%) 731+NrtI treated patients achieved TND by Week 24 (81% vs 0%, p<0.001)
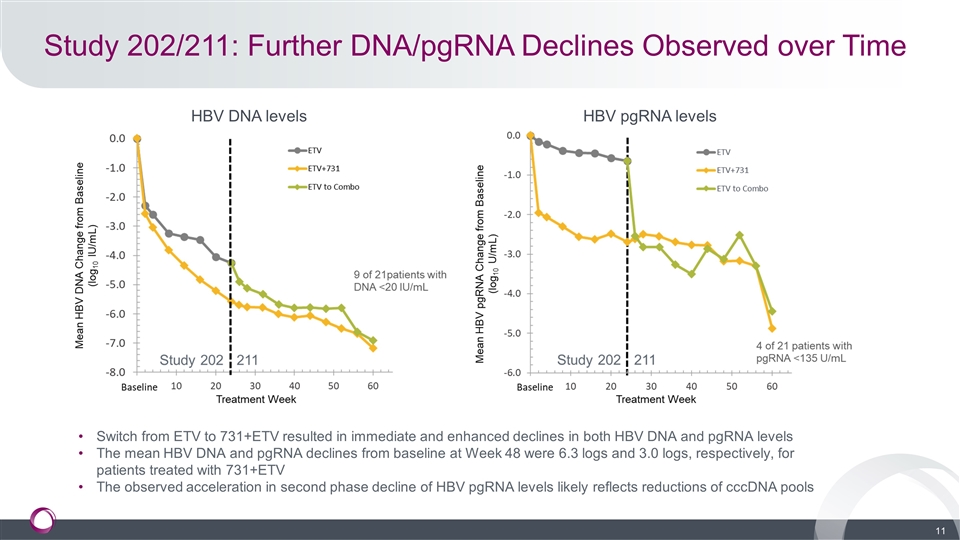
Study 202/211: Further DNA/pgRNA Declines Observed over Time HBV DNA levels HBV pgRNA levels Switch from ETV to 731+ETV resulted in immediate and enhanced declines in both HBV DNA and pgRNA levels The mean HBV DNA and pgRNA declines from baseline at Week 48 were 6.3 logs and 3.0 logs, respectively, for patients treated with 731+ETV The observed acceleration in second phase decline of HBV pgRNA levels likely reflects reductions of cccDNA pools
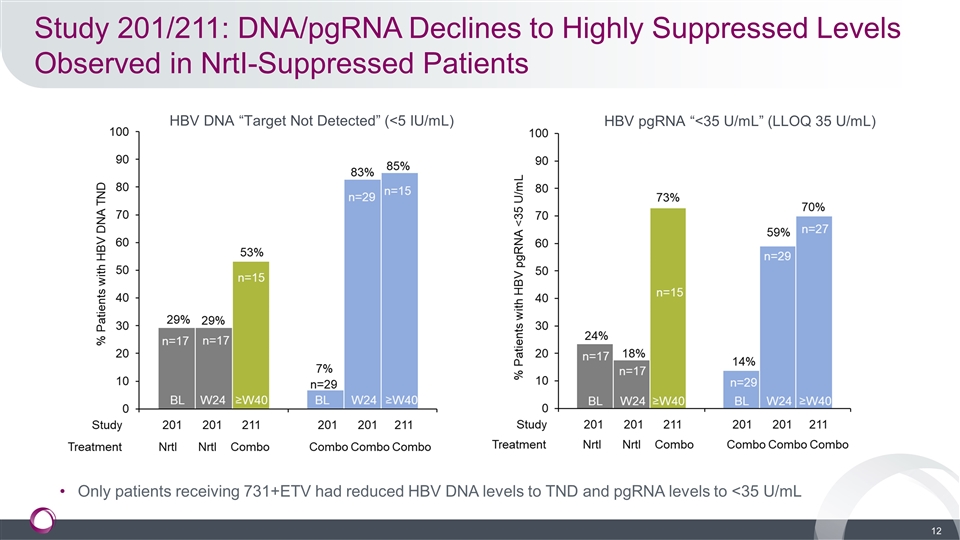
Study 201/211: DNA/pgRNA Declines to Highly Suppressed Levels Observed in NrtI-Suppressed Patients Only patients receiving 731+ETV had reduced HBV DNA levels to TND and pgRNA levels to <35 U/mL HBV DNA “Target Not Detected” (<5 IU/mL) HBV pgRNA “<35 U/mL” (LLOQ 35 U/mL)
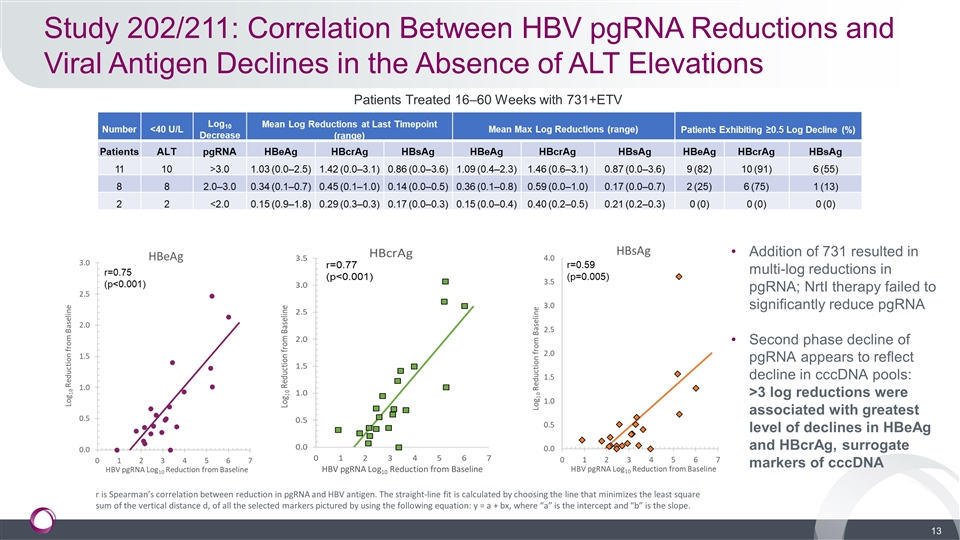
Study 202/211: Correlation Between HBV pgRNA Reductions and Viral Antigen Declines in the Absence of ALT Elevations Addition of 731 resulted in multi-log reductions in pgRNA; NrtI therapy failed to significantly reduce pgRNA Second phase decline of pgRNA appears to reflect decline in cccDNA pools: >3 log reductions were associated with greatest level of declines in HBeAg and HBcrAg, surrogate markers of cccDNA Patients Treated 16–60 Weeks with 731+ETV HBV pgRNA Log10 Reduction from Baseline r is Spearman’s correlation between reduction in pgRNA and HBV antigen. The straight-line fit is calculated by choosing the line that minimizes the least square sum of the vertical distance d, of all the selected markers pictured by using the following equation: y = a + bx, where “a” is the intercept and “b” is the slope.
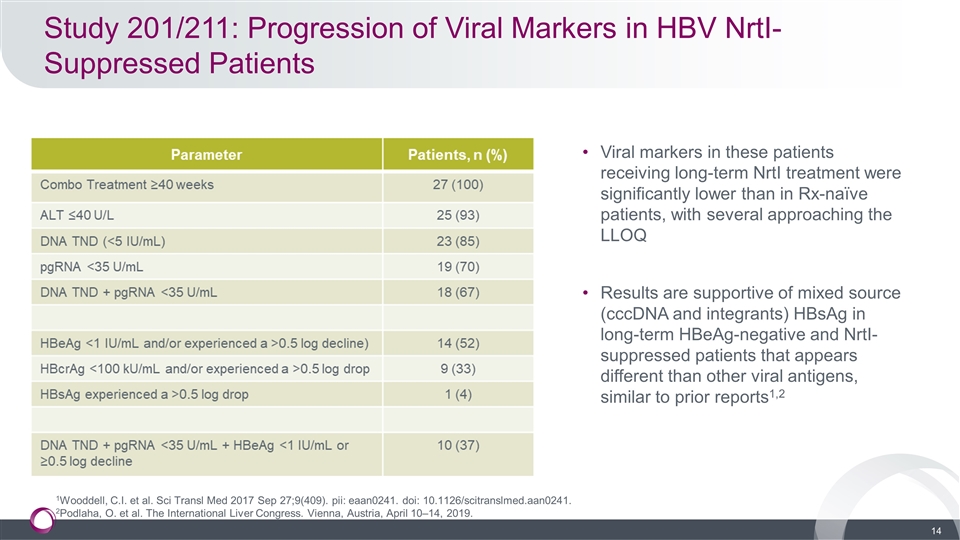
Study 201/211: Progression of Viral Markers in HBV NrtI-Suppressed Patients Viral markers in these patients receiving long-term NrtI treatment were significantly lower than in Rx-naïve patients, with several approaching the LLOQ Results are supportive of mixed source (cccDNA and integrants) HBsAg in long-term HBeAg-negative and NrtI-suppressed patients that appears different than other viral antigens, similar to prior reports1,2 1Wooddell, C.I. et al. Sci Transl Med 2017 Sep 27;9(409). pii: eaan0241. doi: 10.1126/scitranslmed.aan0241. 2Podlaha, O. et al. The International Liver Congress. Vienna, Austria, April 10–14, 2019.
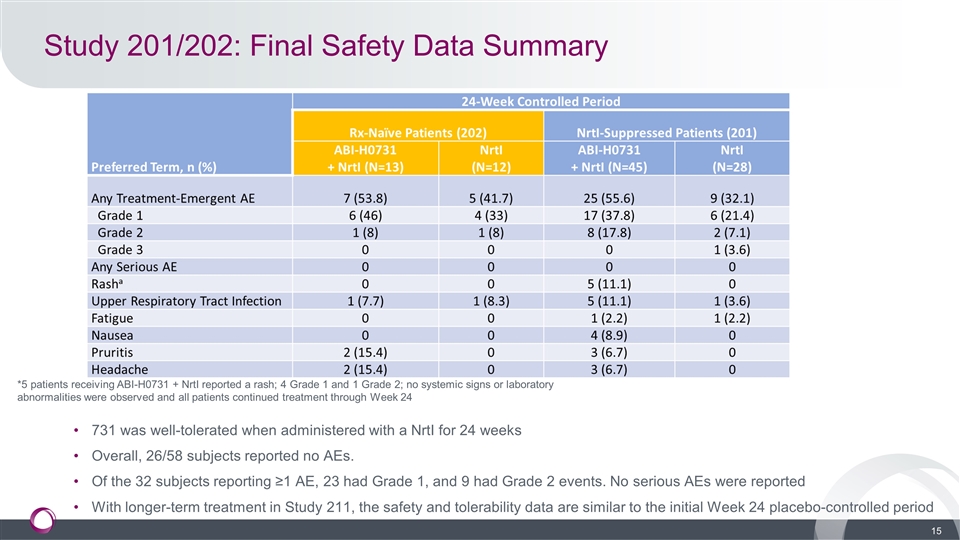
Study 201/202: Final Safety Data Summary 731 was well-tolerated when administered with a NrtI for 24 weeks Overall, 26/58 subjects reported no AEs. Of the 32 subjects reporting ≥1 AE, 23 had Grade 1, and 9 had Grade 2 events. No serious AEs were reported With longer-term treatment in Study 211, the safety and tolerability data are similar to the initial Week 24 placebo-controlled period *5 patients receiving ABI-H0731 + NrtI reported a rash; 4 Grade 1 and 1 Grade 2; no systemic signs or laboratory abnormalities were observed and all patients continued treatment through Week 24
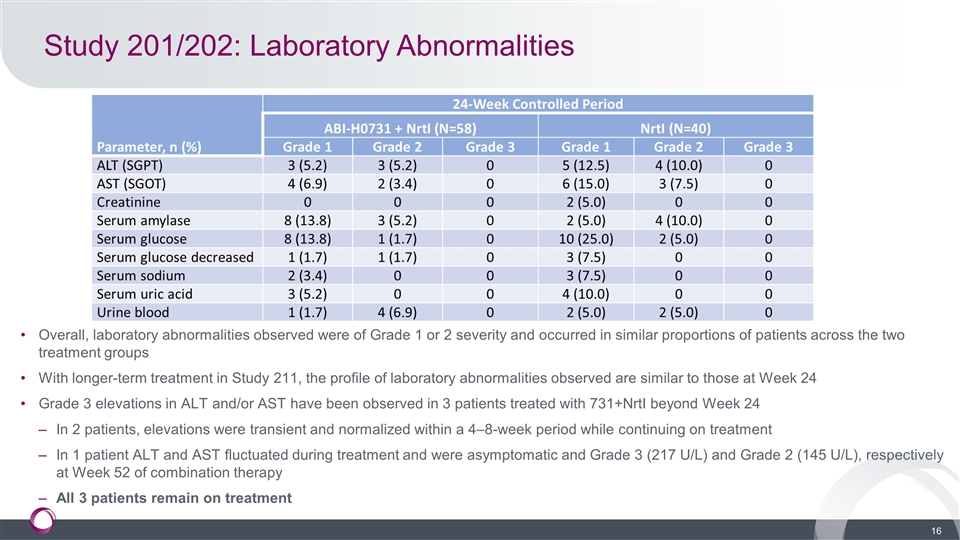
Study 201/202: Laboratory Abnormalities Overall, laboratory abnormalities observed were of Grade 1 or 2 severity and occurred in similar proportions of patients across the two treatment groups With longer-term treatment in Study 211, the profile of laboratory abnormalities observed are similar to those at Week 24 Grade 3 elevations in ALT and/or AST have been observed in 3 patients treated with 731+NrtI beyond Week 24 In 2 patients, elevations were transient and normalized within a 4–8-week period while continuing on treatment In 1 patient ALT and AST fluctuated during treatment and were asymptomatic and Grade 3 (217 U/L) and Grade 2 (145 U/L), respectively at Week 52 of combination therapy All 3 patients remain on treatment

ABI-H0731 Core Inhibitor Program Summary Summary of Interim Data for Phase 2a Studies with ABI-H0731 Well tolerated Combination of 731+NrtI demonstrated superior antiviral activity vs. NrtI monotherapy Faster and deeper declines in HBV DNA observed HBV DNA TND and pgRNA <35 U/mL thresholds only achieved in patients receiving the combination Significant HBV pgRNA (surrogate marker of cccDNA) declines in both studies Second phase declines in pgRNA >3 logs, which is a primary surrogate marker of cccDNA, were strongly associated with reductions in viral antigens, suggesting declining cccDNA pools
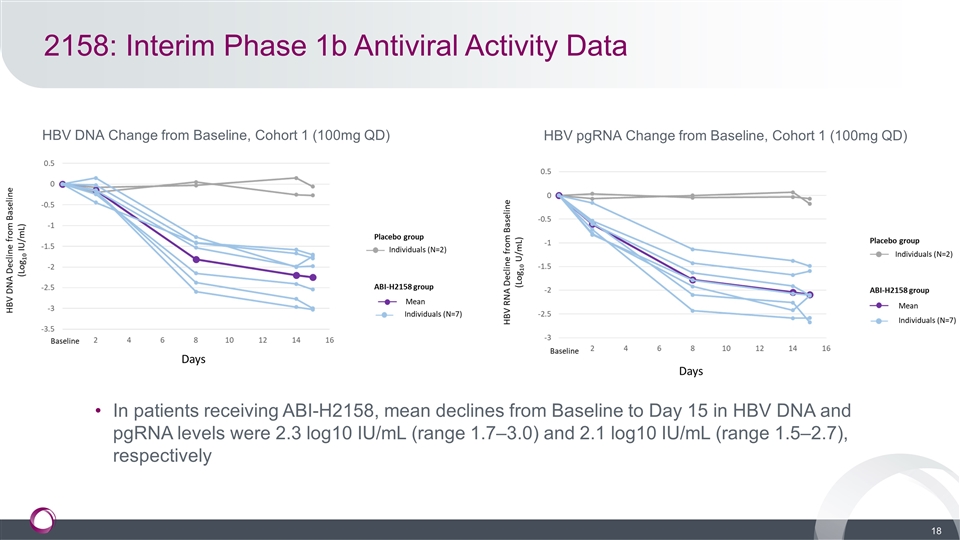
2158: Interim Phase 1b Antiviral Activity Data HBV DNA Change from Baseline, Cohort 1 (100mg QD) HBV pgRNA Change from Baseline, Cohort 1 (100mg QD) In patients receiving ABI-H2158, mean declines from Baseline to Day 15 in HBV DNA and pgRNA levels were 2.3 log10 IU/mL (range 1.7–3.0) and 2.1 log10 IU/mL (range 1.5–2.7), respectively
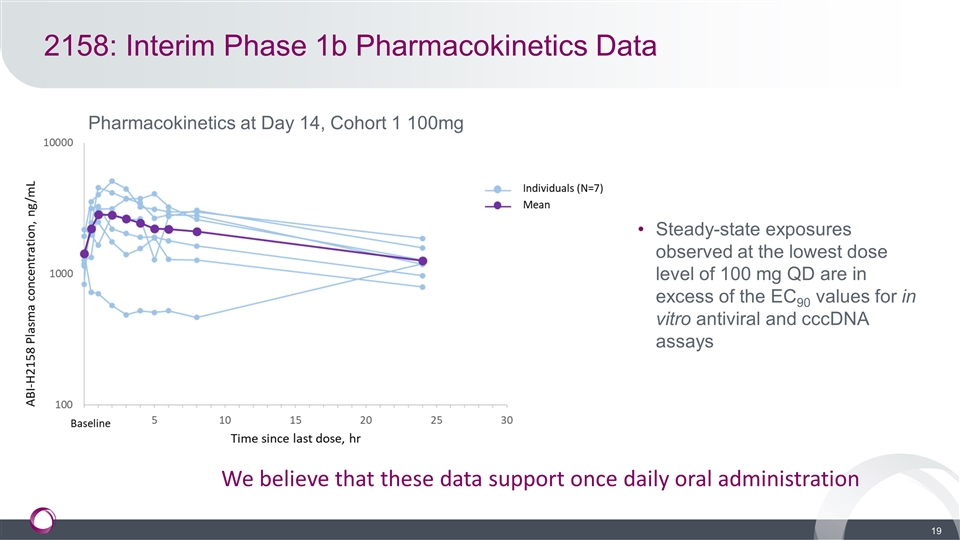
2158: Interim Phase 1b Pharmacokinetics Data Pharmacokinetics at Day 14, Cohort 1 100mg Steady-state exposures observed at the lowest dose level of 100 mg QD are in excess of the EC90 values for in vitro antiviral and cccDNA assays We believe that these data support once daily oral administration
Program Objectives - Targeted Steps Toward Cure Well-tolerated, PK supporting once daily dosing, and inhibits HBV DNA with monotherapy (Phase 1) Demonstrated elimination of residual viral replication not achievable on NrtI monotherapy (i.e DNA to “Target not Detected”) (Phase 2) Demonstrated decrease in cccDNA population as reflected by significant reductions in pgRNA levels and other surrogate markers in absence of ALT flares (Phase 2) Goal: Demonstrate further decline of viral antigens during consolidation (Phase 2) Goal: Following consolidation, demonstrate sustained viral DNA/RNA suppression off therapy (Phase 2) AASLD 2018 EASL 2019 AASLD 2019

Thank you




















