
Not for Product Promotional Use Q3 2024 Results October 31, 2024

Not for Product Promotional UseQ3 2024 Results Forward Looking Statements and Non-GAAP Financial Information 2 This presentation contains statements about Bristol-Myers Squibb Company’s (the “Company”) future financial results, plans, business development strategy, anticipated clinical trials, results and regulatory approvals that constitute forward-looking statements for purposes of the safe harbor provisions under the Private Securities Litigation Reform Act of 1995. All statements that are not statements of historical facts are, or may be deemed to be, forward-looking statements. Actual results may differ materially from those expressed in, or implied by, these statements as a result of various factors, including, but not limited to: (i) new laws and regulations, (ii) our ability to obtain, protect and maintain market exclusivity rights and enforce patents and other intellectual property rights, (iii) our ability to achieve expected clinical, regulatory and contractual milestones on expected timelines or at all, (iv) difficulties or delays in the development and commercialization of new products, (v) difficulties or delays in our clinical trials and the manufacturing, distribution and sale of our products, (vi) adverse outcomes in legal or regulatory proceedings, (vii) risks relating to acquisitions, divestitures, alliances, joint ventures and other portfolio actions and (viii) political and financial instability, including changes in general economic conditions. These and other important factors are discussed in the Company’s most recent annual report on Form 10-K and reports on Forms 10-Q and 8-K. These documents are available on the U.S. Securities and Exchange Commission’s website, on the Company’s website or from Bristol-Myers Squibb Investor Relations. No forward- looking statements can be guaranteed. In addition, any forward-looking statements and clinical data included herein are presented only as of the date hereof. Except as otherwise required by applicable law, the Company undertakes no obligation to publicly update any of the provided information, whether as a result of new information, future events, changed circumstances or otherwise. This presentation includes certain non-generally accepted accounting principles (“GAAP”) financial measures that we use to describe the Company’s performance. The non-GAAP financial measures are provided as supplemental information and are presented because management has evaluated the Company’s financial results both including and excluding the adjusted items or the effects of foreign currency translation, as applicable, and believes that the non-GAAP financial measures presented portray the results of the Company’s baseline performance, supplement or enhance management’s, analysts’ and investors’ overall understanding of the Company’s underlying financial performance and trends and facilitate comparisons among current, past and future periods. This presentation also provides certain revenues and expenses excluding the impact of foreign exchange (“Ex- FX”). We calculate foreign exchange impacts by converting our current-period local currency financial results using the prior period average currency rates and comparing these adjusted amounts to our current-period results. Ex-FX financial measures are not accounted for according to GAAP because they remove the effects of currency movements from GAAP results. The non-GAAP information presented herein provides investors with additional useful information but should not be considered in isolation or as substitutes for the related GAAP measures. Moreover, other companies may define non-GAAP measures differently, which limits the usefulness of these measures for comparisons with such other companies. We encourage investors to review our financial statements and publicly filed reports in their entirety and not to rely on any single financial measure. An explanation of these non-GAAP financial measures and a reconciliation to the most directly comparable financial measure are available on our website at www.bms.com/investors. Also note that a reconciliation of forward-looking non-GAAP measures, including non-GAAP earnings per share (EPS), to the most directly comparable GAAP measures is not provided because comparable GAAP measures for such measures are not reasonably accessible or reliable due to the inherent difficulty in forecasting and quantifying measures that would be necessary for such reconciliation. Namely, we are not, without unreasonable effort, able to reliably predict the impact of accelerated depreciation and impairment charges, legal and other settlements, gains and losses from equity investments and other adjustments. In addition, the Company believes such a reconciliation would imply a degree of precision and certainty that could be confusing to investors. These items are uncertain, depend on various factors and may have a material impact on our future GAAP results.
Chris Boerner, PhD Board Chair and Chief Executive Officer 3 Q3 2024 Results

Q3 2024 Results Not for Product Promotional Use Research & Development Q3 2024 performance 4 Commercial *See “Forward-Looking Statements and Non-GAAP Financial Information” 1. Not an exhaustive list of assets, programs, or indications $4.9 $5.8 $6.0 $6.1 $11.0 $11.9 Q3 2023 Q3 2024 Legacy Growth $ in billions Growth portfolio revenues: +18% or +20% Ex-FX* YoY +129% +80% Achieved multiple clinical & regulatory milestones1 +40% nivolumab + relatlimab HD +143% Re-established presence in Neuroscience

Q3 2024 Results Not for Product Promotional Use 5 Novel first-in-class schizophrenia treatment U.S. Approval: September 26, 2024 Launch now underway First new mechanism in decades • ~1.6M patients treated in the U.S.1 • ~70% of patients not well managed with current treatments Compelling efficacy with proven safety & tolerability profile • Depth & breadth of efficacy across symptom domains • No boxed warning & atypical antipsychotic class warnings & precautions Expansion opportunities2 • Expected Phase 3 data readouts: Adjunctive Schizophrenia (2025) & Alzheimer’s Psychosis (2026) • Planned registrational studies: — Alzheimer’s Agitation, Bipolar I Disorder, Alzheimer’s Cognition, & Autism Spectrum Disorder Irritability *See “Forward-Looking Statements and Non-GAAP Financial Information” 1. DRG – Clarivate, as of July 2023; 2. Subject to positive registrational trials and regulatory approval Anticipate majority of access by 2H 2025*
Q3 2024 Results Not for Product Promotional Use Reshaping the company for long-term sustainable growth 6 *The Company does not reconcile forward-looking non-GAAP measures. See “Forward-Looking Statements and Non-GAAP Financial Information” 1. Refer to Appendix for details Focusing on transformational medicines where we have a competitive advantage Driving operational excellence throughout the organization Strategically allocating capital for long-term growth & returns • Growth portfolio led by Reblozyl, Breyanzi, Camzyos & Opdualag • 8 new oncology registrational trials1 added in past year • Focusing R&D on higher ROI programs • Realizing anticipated internal cost savings of ~$1.5B by YE 2025* • Business development remains a priority • Committed to our dividend Accelerating delivery of important medicines to more patients

Q3 2024 Results Not for Product Promotional Use 7 Near-term milestones build pipeline momentum*1 CAR T in Immunology Extending in Immuno- Oncology CD19 NEX-T Phase 1 data at ACR: November 2024 Pipeline enters catalyst-rich period starting next year2 Subcutaneous nivolumab U.S. FDA PDUFA date: December 29th *See “Forward-Looking Statements and Non-GAAP Financial Information” 1. Subject to positive registrational trials and regulatory approval; 2. Refer to Appendix for details Phase 3 PsA POETYK-PsA-I & II: data by YE Expanding in Immunology
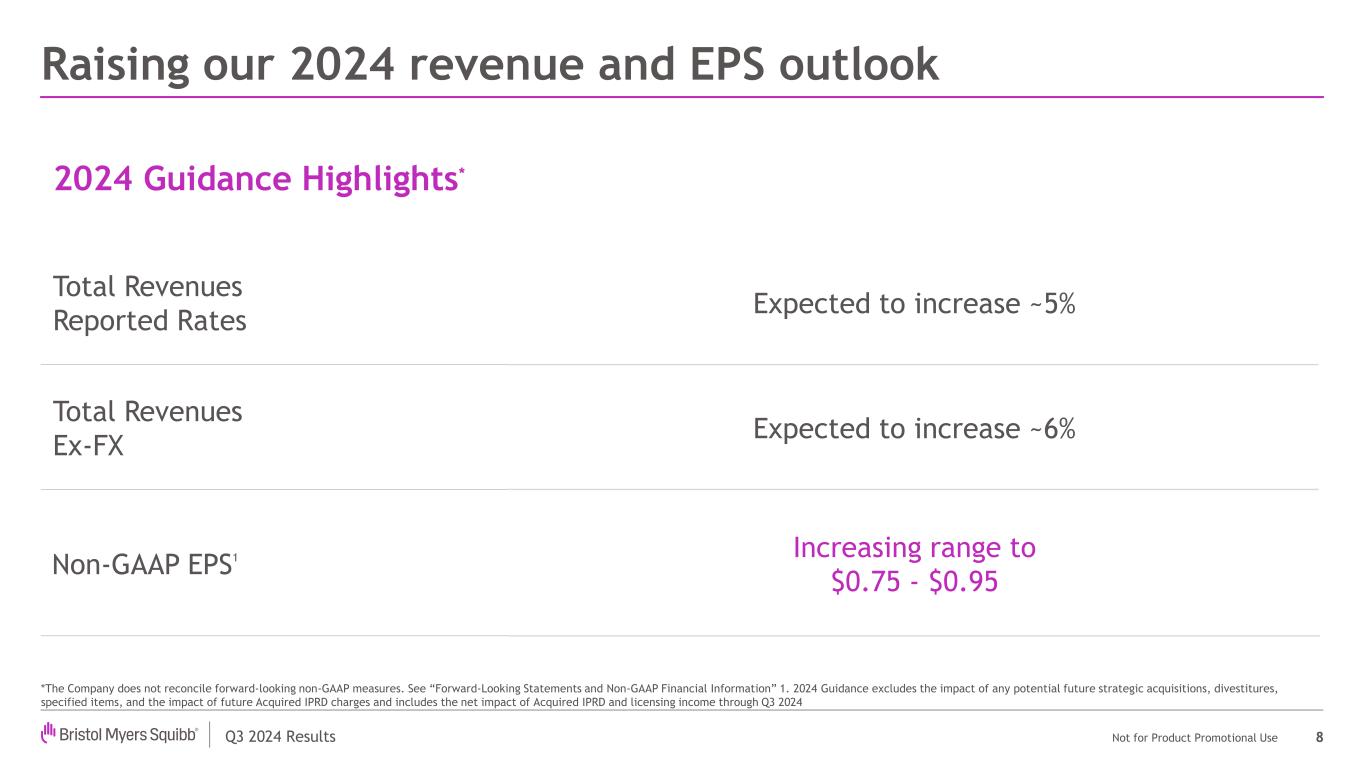
Q3 2024 Results Not for Product Promotional Use 2024 Guidance Highlights* Total Revenues Reported Rates Expected to increase ~5% Total Revenues Ex-FX Expected to increase ~6% Non-GAAP EPS1 Increasing range to $0.75 - $0.95 Raising our 2024 revenue and EPS outlook 8 *The Company does not reconcile forward-looking non-GAAP measures. See “Forward-Looking Statements and Non-GAAP Financial Information” 1. 2024 Guidance excludes the impact of any potential future strategic acquisitions, divestitures, specified items, and the impact of future Acquired IPRD charges and includes the net impact of Acquired IPRD and licensing income through Q3 2024
Q3 2024 Results Not for Product Promotional Use Executing on critical business priorities to build a solid foundation for sustainable growth 9 • Growth Portfolio continues to expand Focusing on commercial execution • Cobenfy U.S. approval: Multi-billion-dollar potential including LCM opportunities Re-established presence in Neuroscience • On track to deliver against productivity initiatives Maintaining P&L discipline • Near-term catalysts: CD19 NEX-T, Sotyktu, & Subcutaneous nivolumab Advancing our pipeline Strengthening the company to deliver long-term value
David Elkins Executive Vice President and Chief Financial Officer 10 Q3 2024 Results
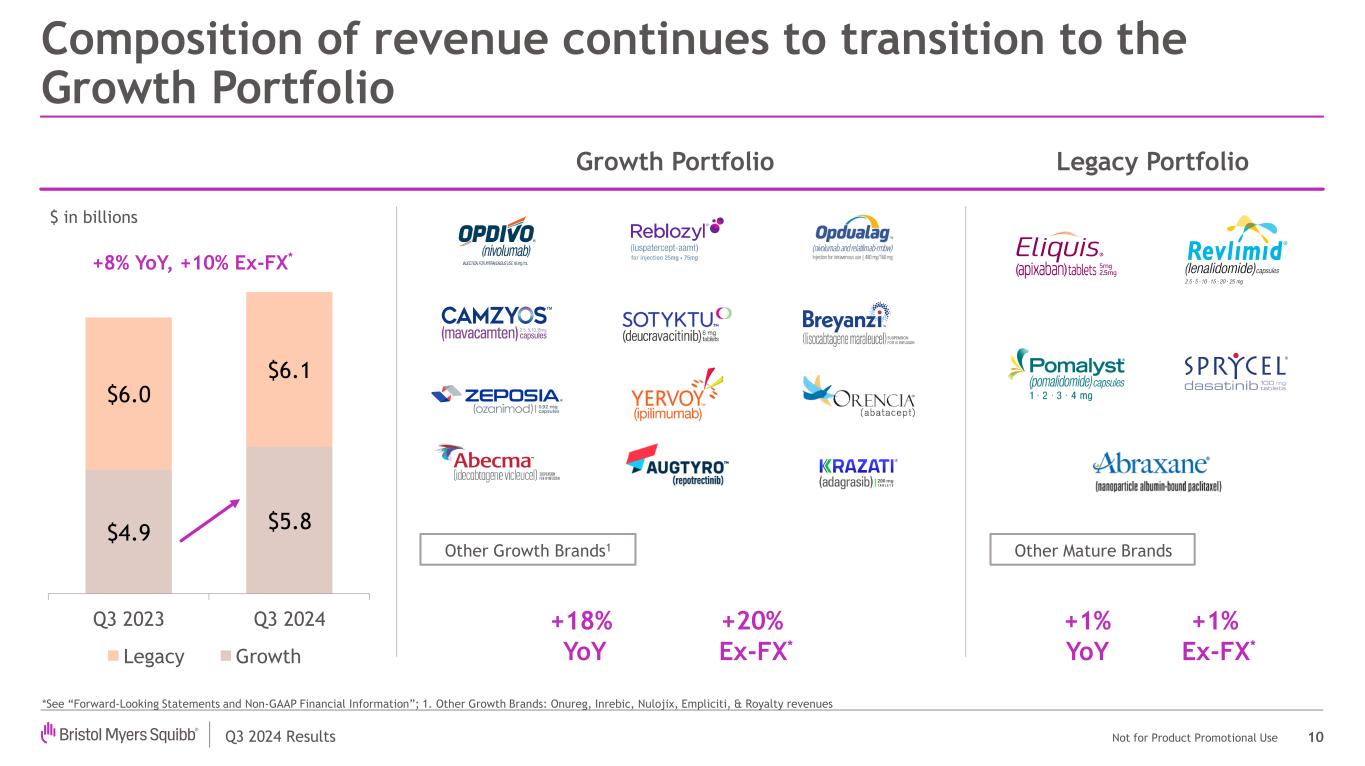
Q3 2024 Results Not for Product Promotional Use Composition of revenue continues to transition to the Growth Portfolio 10 $4.9 $5.8 $6.0 $6.1 Q3 2023 Q3 2024 Legacy Growth Growth Portfolio Other Growth Brands1 Legacy Portfolio Other Mature Brands *See “Forward-Looking Statements and Non-GAAP Financial Information”; 1. Other Growth Brands: Onureg, Inrebic, Nulojix, Empliciti, & Royalty revenues $ in billions +1% Ex-FX* +18% YoY +1% YoY +20% Ex-FX* +8% YoY, +10% Ex-FX*
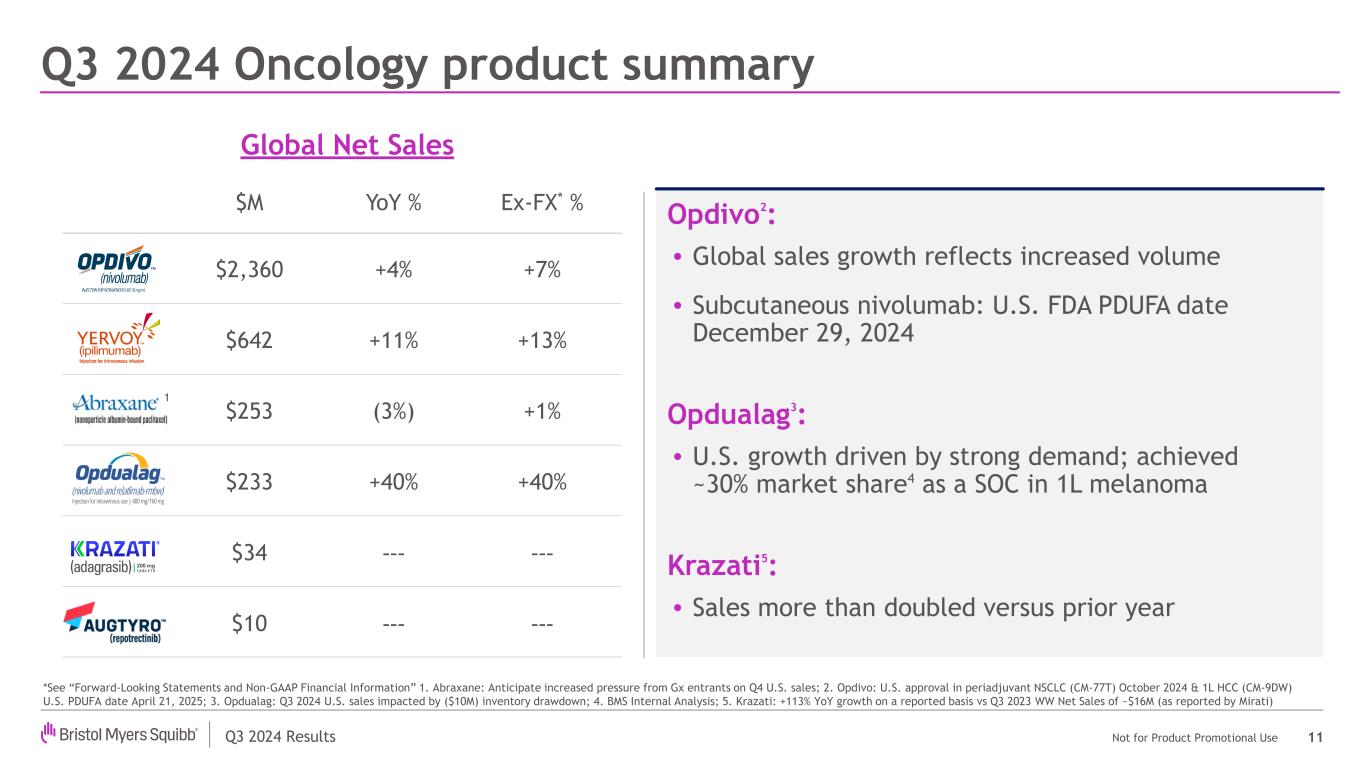
Q3 2024 Results Not for Product Promotional Use Q3 2024 Oncology product summary 11 Opdivo2: • Global sales growth reflects increased volume • Subcutaneous nivolumab: U.S. FDA PDUFA date December 29, 2024 Opdualag3: • U.S. growth driven by strong demand; achieved ~30% market share4 as a SOC in 1L melanoma Krazati5: • Sales more than doubled versus prior year Global Net Sales *See “Forward-Looking Statements and Non-GAAP Financial Information” 1. Abraxane: Anticipate increased pressure from Gx entrants on Q4 U.S. sales; 2. Opdivo: U.S. approval in periadjuvant NSCLC (CM-77T) October 2024 & 1L HCC (CM-9DW) U.S. PDUFA date April 21, 2025; 3. Opdualag: Q3 2024 U.S. sales impacted by ($10M) inventory drawdown; 4. BMS Internal Analysis; 5. Krazati: +113% YoY growth on a reported basis vs Q3 2023 WW Net Sales of ~$16M (as reported by Mirati) $M YoY % Ex-FX* % $2,360 +4% +7% $642 +11% +13% $253 (3%) +1% $233 +40% +40% $34 --- --- $10 --- --- 1
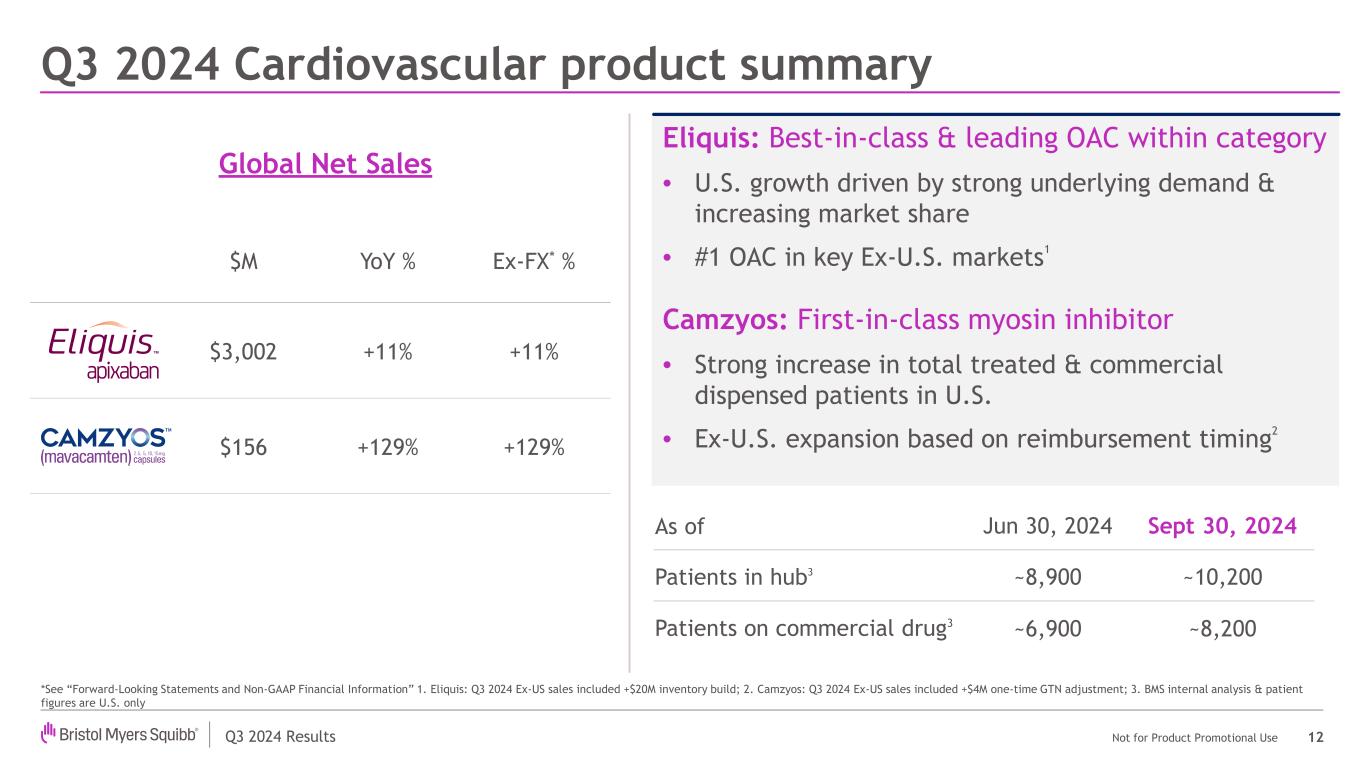
Q3 2024 Results Not for Product Promotional Use Q3 2024 Cardiovascular product summary 12 Eliquis: Best-in-class & leading OAC within category • U.S. growth driven by strong underlying demand & increasing market share • #1 OAC in key Ex-U.S. markets1 Camzyos: First-in-class myosin inhibitor • Strong increase in total treated & commercial dispensed patients in U.S. • Ex-U.S. expansion based on reimbursement timing2 Global Net Sales % $M YoY % Ex-FX* % $3,002 +11% +11% $156 +129% +129% As of Jun 30, 2024 Sept 30, 2024 Patients in hub3 ~8,900 ~10,200 Patients on commercial drug3 ~6,900 ~8,200 *See “Forward-Looking Statements and Non-GAAP Financial Information” 1. Eliquis: Q3 2024 Ex-US sales included +$20M inventory build; 2. Camzyos: Q3 2024 Ex-US sales included +$4M one-time GTN adjustment; 3. BMS internal analysis & patient figures are U.S. only

Q3 2024 Results Not for Product Promotional Use Q3 2024 Hematology product summary 13 Reblozyl: • Strong demand in 1L MDS-associated anemia • Focus on increasing market share in 1L RS negative population • Ex-U.S. growth driven by both European markets3 & recent Japanese approval Breyanzi: • Growth driven by expanded manufacturing capacity & increased demand 1 $M YoY % Ex-FX* % $1,412 (1%) (1%) $898 +3% +3% $447 +80% +81% $290 (44%) (43%) $224 +143% +143% $124 +33% +34% Global Net Sales *See “Forward-Looking Statements and Non-GAAP Financial Information”; 1. Pomalyst: In the EU, generic pomalidomide products entered the market in August 2024; 2. U.S. generic Sprycel launched Sept. 1, 2024; 3. AMNOG six-month free pricing period in Germany ended Sept. 30, 2024 1 2
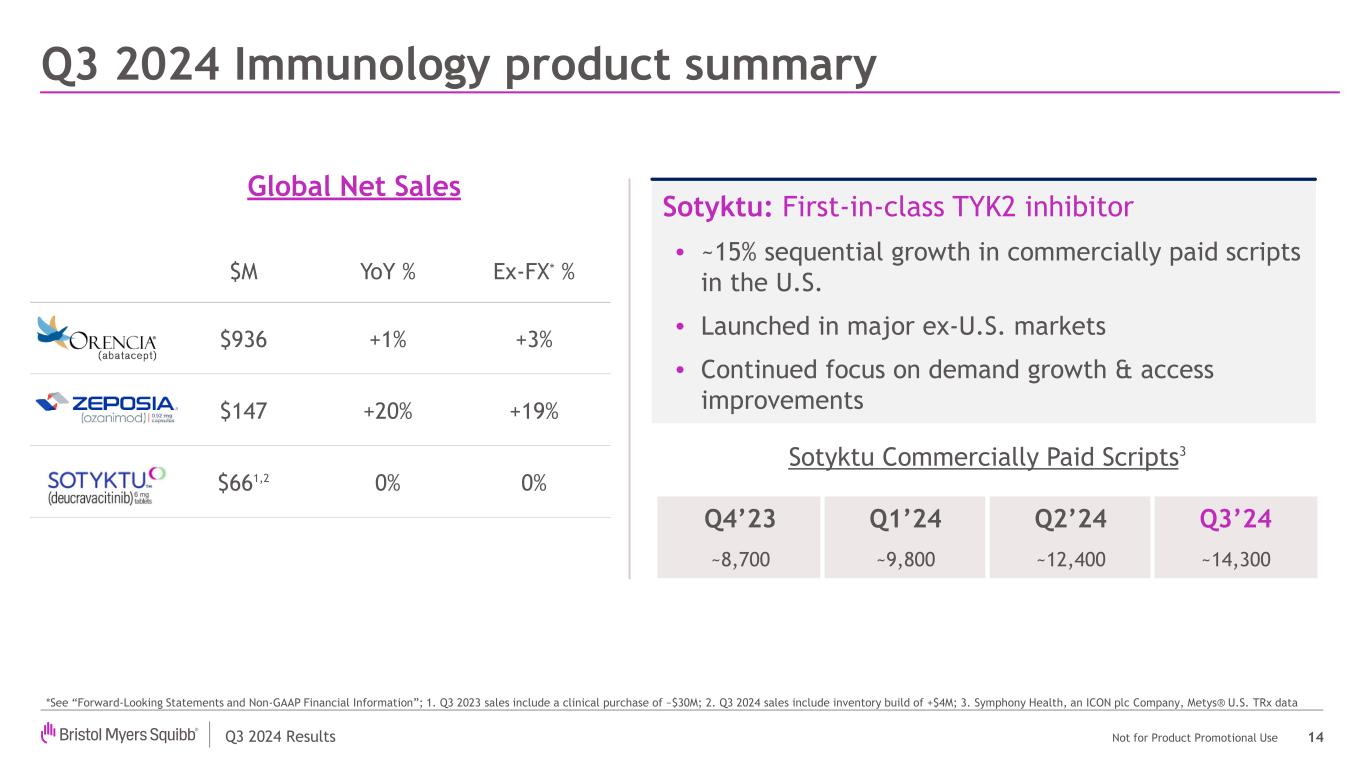
Q3 2024 Results Not for Product Promotional Use Q3 2024 Immunology product summary 14 Sotyktu: First-in-class TYK2 inhibitor • ~15% sequential growth in commercially paid scripts in the U.S. • Launched in major ex-U.S. markets • Continued focus on demand growth & access improvements Global Net Sales *See “Forward-Looking Statements and Non-GAAP Financial Information”; 1. Q3 2023 sales include a clinical purchase of ~$30M; 2. Q3 2024 sales include inventory build of +$4M; 3. Symphony Health, an ICON plc Company, Metys® U.S. TRx data Q4’23 Q1’24 Q2’24 Q3’24 ~8,700 ~9,800 ~12,400 ~14,300 Sotyktu Commercially Paid Scripts3 % $M YoY % Ex-FX* % $936 +1% +3% $147 +20% +19% $661,2 0% 0%
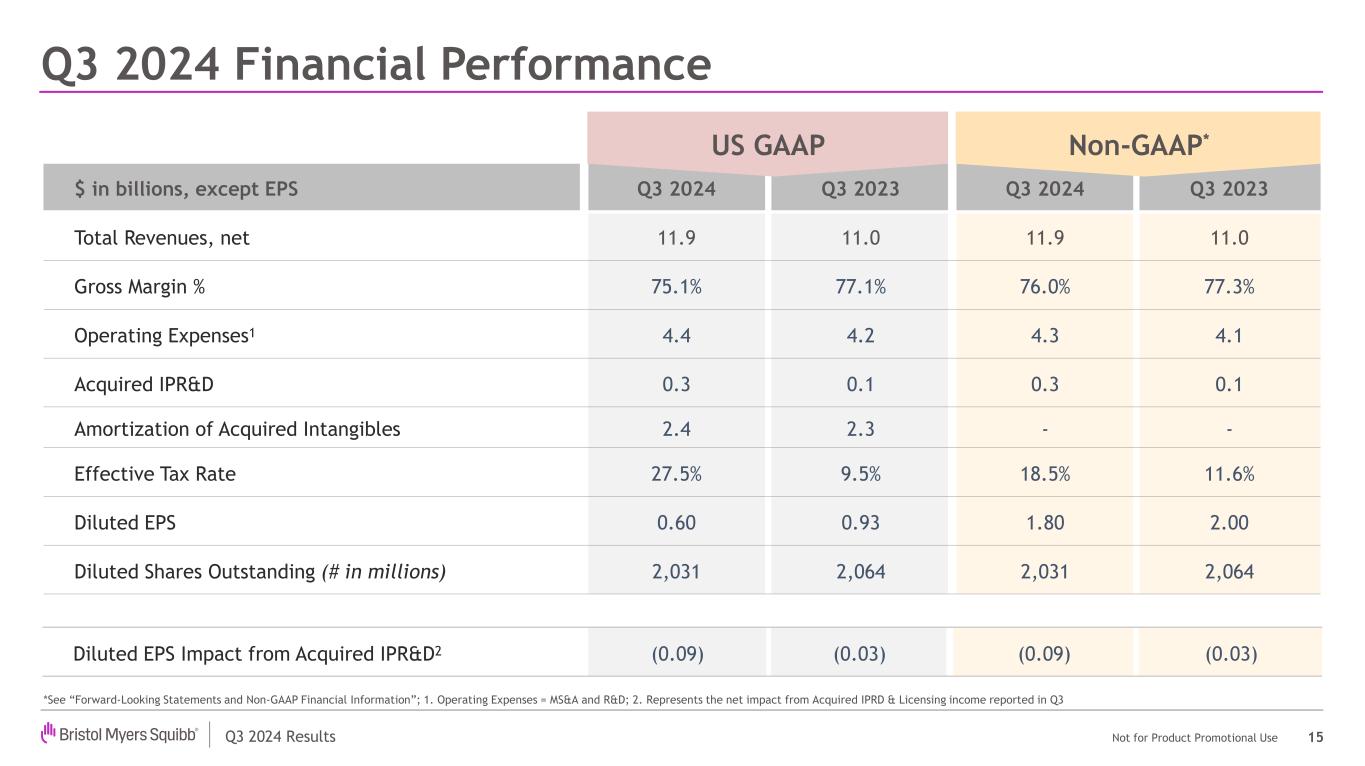
Q3 2024 Results Not for Product Promotional Use US GAAP Non-GAAP $ in billions, except EPS Q3 2024 Q3 2023 Q3 2024 Q3 2023 Total Revenues, net 11.9 11.0 11.9 11.0 Gross Margin % 75.1% 77.1% 76.0% 77.3% Operating Expenses1 4.4 4.2 4.3 4.1 Acquired IPR&D 0.3 0.1 0.3 0.1 Amortization of Acquired Intangibles 2.4 2.3 - - Effective Tax Rate 27.5% 9.5% 18.5% 11.6% Diluted EPS 0.60 0.93 1.80 2.00 Diluted Shares Outstanding (# in millions) 2,031 2,064 2,031 2,064 Q3 2024 Financial Performance 15 US P Non- P* Diluted EPS Impact from Acquired IPR&D2 (0.09) (0.03) (0.09) (0.03) *See “Forward-Looking Statements and Non-GAAP Financial Information”; 1. Operating Expenses = MS&A and R&D; 2. Represents the net impact from Acquired IPRD & Licensing income reported in Q3
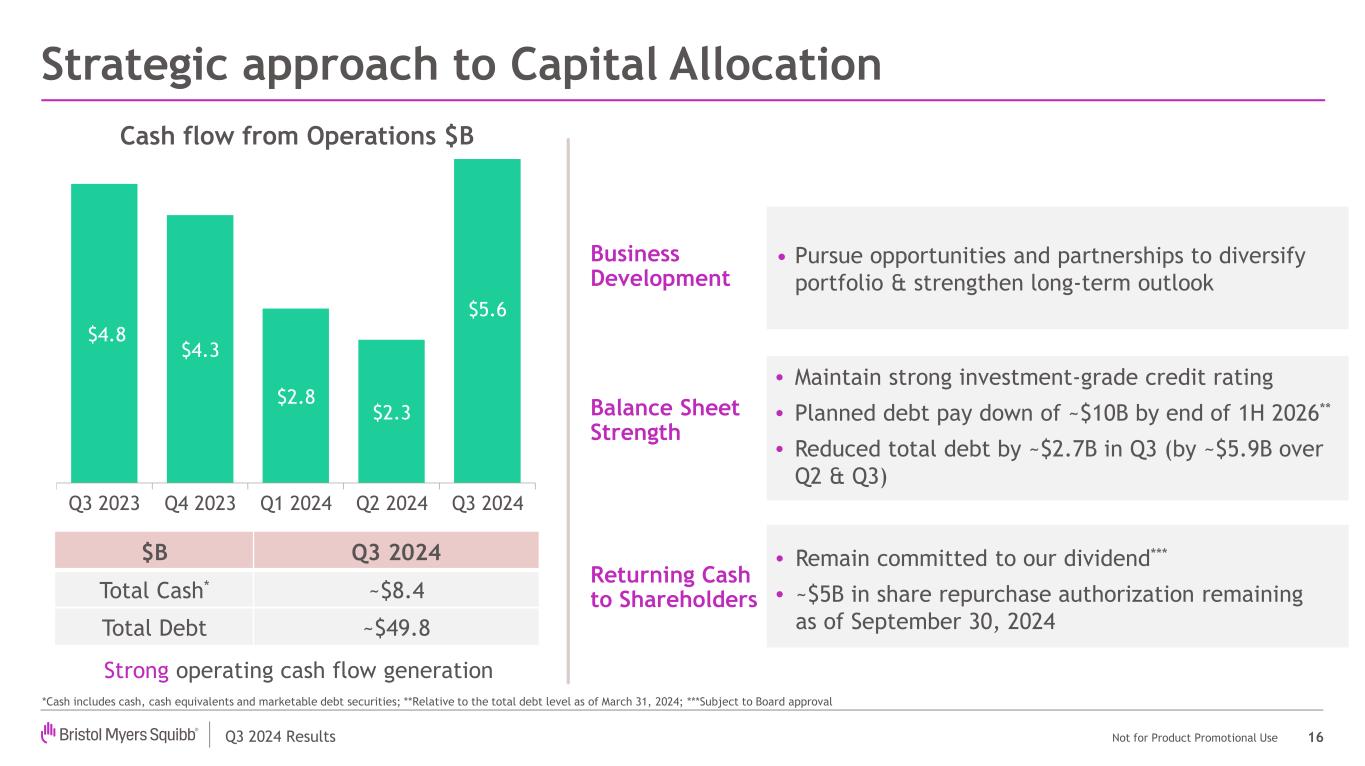
Q3 2024 Results Not for Product Promotional Use Strategic approach to Capital Allocation 16 *Cash includes cash, cash equivalents and marketable debt securities; **Relative to the total debt level as of March 31, 2024; ***Subject to Board approval $B Q3 2024 Total Cash* ~$8.4 Total Debt ~$49.8 • Pursue opportunities and partnerships to diversify portfolio & strengthen long-term outlook • Maintain strong investment-grade credit rating • Planned debt pay down of ~$10B by end of 1H 2026** • Reduced total debt by ~$2.7B in Q3 (by ~$5.9B over Q2 & Q3) Business Development Balance Sheet Strength Returning Cash to Shareholders • Remain committed to our dividend*** • ~$5B in share repurchase authorization remaining as of September 30, 2024 Strong operating cash flow generation $4.8 $4.3 $2.8 $2.3 $5.6 Q3 2023 Q4 2023 Q1 2024 Q2 2024 Q3 2024 Cash flow from Operations $B

Q3 2024 Results Not for Product Promotional Use July (Prior) October (Updated) Total Revenues Reported Rates Upper end of low single-digit range ~5% increase Total Revenues Ex-FX* Upper end of low single-digit range ~6% increase Gross Margin % Between ~74% and ~75% Between ~74.5% and ~75% Operating Expenses1 Low single-digit increase ~4% to ~5% increase Other Income/ (Expense) ~($50M) ~$125M Tax Rate2 ~66% ~60% Diluted EPS2 $0.60 - $0.90 $0.75 - $0.95 Revised 2024 Guidance 17 Non-GAAP* *The Company does not reconcile forward-looking non-GAAP measures. See "Forward-Looking Statements and Non-GAAP Financial Information”; 1. Operating Expenses = MS&A and R&D, excluding Acquired IPR&D and Amortization of acquired intangibles; 2. Includes the net impact of Acquired IPRD and licensing income through Q3 2024. Guidance excludes the impact of any potential future strategic acquisitions, divestitures, specified items, and the impact of future Acquired IPRD charges Key Highlights • Raising FY Revenue & EPS guidance due to strength of results YTD • Gross Margin range narrowed due to sales mix • FY OpEx guidance reflects increased investment in Q4 to support portfolio & pipeline • OIE guidance reflects higher royalty & interest income • Underlying Tax Rate excluding Acquired IPR&D: • Q3 at ~18.8% • FY’24 estimated at ~18%
Q3 2024 Results Not for Product Promotional Use Delivered Solid Performance in Q3 18 Re-established Presence in Neuroscience Driving Sustainable Growth Q3 Performance • Topline growth: +8% or +10% Ex-FX* • Growth portfolio: +18% or +20% Ex-FX* Raising FY 2024 Revenue & EPS Non-GAAP Guidance *See “Forward-Looking Statements and Non-GAAP Financial Information” 1. Refer to Appendix for details • Focusing on transformational medicines • Driving operational excellence • Strategically allocating capital Advancing our Pipeline • 8 new oncology registrational trials added in past year1 • Near-term milestones build pipeline momentum • Cobenfy: First-in-class medicine with multi-billion-dollar potential including LCM opportunities • Launch in schizophrenia now underway; anticipate majority of access by 2H 2025

Chris Boerner, PhD Board Chair, Chief Executive Officer David Elkins Executive VP, Chief Financial Officer Samit Hirawat, MD Executive VP, Chief Medical Officer, Global Drug Development Adam Lenkowsky Executive VP, Chief Commercialization Officer 20 Q3 2024 Results Q&A



















