As filed with the Securities and Exchange Commission on May 14, 2013
Registration No. 333-188255
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
_________________
AMENDMENT NO. 1 TO
FORM S-4
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
UNDER
THE SECURITIES ACT OF 1933
_________________
BIOMET, INC.
(Exact name of registrant as specified in its charter)
_________________
| Indiana | 3842 | 35-1418342 | ||
| (State or other jurisdiction of incorporation or organization) | (Primary Standard Industrial Classification Code Number) | (I.R.S. Employer Identification Number) | ||
(see table of additional registrants below)
56 East Bell Drive Warsaw, Indiana 46582 (574) 267-6639 (Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices) | |
Jody S. Gale Vice President and Associate General Counsel – M&A, Securities & Governance Biomet, Inc. 56 East Bell Drive Warsaw, Indiana 46582 (574) 267-6639 (Address, including zip code, and telephone number, including area code, of agent for service.) | Jeffrey D. Karpf James D. Small Cleary Gottlieb Steen & Hamilton LLP One Liberty Plaza New York, New York 10006 (212) 225-2000 (Copies of all communications, including communications sent to agent for service) |
Approximate date of commencement of proposed sale to the public: As soon as practicable after this registration statement becomes effective.
If the securities being registered on this Form are being offered in connection with the formation of a holding company and there is compliance with General Instruction G, check the following box.
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering.
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering.
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer,” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | Accelerated filer | Non-accelerated filer x | Smaller reporting company |
(Do not check if a smaller reporting company)
If applicable, place an X in the box to designate the appropriate rule provision relied upon in conducting this transaction:
Exchange Act Rule 13e-4(i) (Cross-Border Issuer Tender Offer)
Exchange Act Rule 14d-1(d) (Cross-Border Third-Party Tender Offer)
________________
CALCULATION OF REGISTRATION FEE
Title of each class of securities to be registered | Amount to be registered | Proposed maximum offering price per unit | Proposed maximum aggregate offering price(1) | Amount of registration fee(2)(5) |
| 6.500% Senior Notes due 2020 | $1,825,000,000 | 100% | $1,825,000,000 | $248,930 |
| Guarantees of 6.500% Senior Notes due 2020(3) | (4) | (4) | (4) | (4) |
| 6.500% Senior Subordinated Notes due 2020 | $800,000,000 | 100% | $800,000,000 | $109,120 |
| Guarantees of 6.500% Senior Subordinated Notes due 2020(3) | (4) | (4) | (4) | (4) |
| (1) | Estimated solely for the purpose of calculating the registration fee pursuant to Rule 457(f) under the Securities Act of 1933, as amended. |
| (2) | Calculated pursuant to Rule 457 under the Securities Act. |
| (3) | Each of Biomet, Inc.’s current and future wholly-owned domestic restricted subsidiaries that is a guarantor of Biomet’s senior secured credit facilities jointly, severally and unconditionally guarantees, the 6.500% Senior Notes due 2020 on a senior unsecured basis, and the 6.500% Senior Subordinated Notes due 2020 on a senior subordinated unsecured basis. See inside facing page for table of additional registrant guarantors. |
| (4) | Pursuant to Rule 457(n) under the Securities Act, no separate fee is payable for the registration of the Guarantees. |
| (5) | Previously paid. |
_________________
The registrants hereby amend this registration statement on such date or dates as may be necessary to delay its effective date until the registrants shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until the registration statement shall become effective on such date as the Commission, acting pursuant to said Section 8(a), may determine.
TABLE OF ADDITIONAL REGISTRANT GUARANTORS
| Exact Name of Registrant as Specified in its Charter | State or Other Jurisdiction of Incorporation or Organization | Primary Standard Industrial Classification Code Number | I.R.S. Employer Identification Number | Address, including Zip Code and Telephone Number, including Area Code, of Agent for Service, of Registrant’s Principal Executive Offices | ||||
| Biolectron, Inc. | Delaware | 3842 | 13-2914413 | 399 Jefferson Road Parsippany, NJ 07054 (973) 299-9300 | ||||
| Biomet 3i, LLC | Florida | 3842 | 59-2816882 | 4555 Riverside Drive Palm Beach Gardens, FL 33410 (561) 776-6700 | ||||
| Biomet Biologics, LLC | Indiana | 3842 | 03-04079652 | 56 E. Bell Drive Warsaw, IN 46582 (574) 267-6639 | ||||
| Biomet Europe Ltd. | Delaware | 3842 | 35-1603620 | 56 E. Bell Drive Warsaw, IN 46582 (574) 267-6639 | ||||
| Biomet Fair Lawn, LLC | Indiana | 3842 | 31-1651311 | 20-01 Pollitt Drive Fairlawn, NJ 07410 (201) 797-7300 | ||||
| Biomet International Ltd. | Delaware | 3842 | 35-2046422 | 56 E. Bell Drive Warsaw, IN 46582 (574) 267-6639 | ||||
| Biomet Leasing, Inc. | Indiana | 3842 | 35-2076217 | 56 E. Bell Drive Warsaw, IN 46582 (574) 267-6639 | ||||
| Biomet Manufacturing Corporation | Indiana | 3842 | 35-2074039 | 56 E. Bell Drive Warsaw, IN 46582 (574) 267-6639 | ||||
| Biomet Microfixation, LLC | Florida | 3842 | 59-1692523 | 1520 Tradeport Drive Jacksonville, FL 32218-2482 (904) 741-4400 | ||||
| Biomet Orthopedics, LLC | Indiana | 3842 | 35-2074037 | 56 E. Bell Drive Warsaw, IN 46582 (574) 267-6639 | ||||
| Biomet Sports Medicine, LLC | Indiana | 3842 | 35-1803072 | 56 E. Bell Drive Warsaw, IN 46852 (574) 267-6639 | ||||
| Cross Medical Products, LLC | Delaware | 3842 | 31-0992628 | 181 Technology Drive Irvine, CA 92618 (574) 267-6639 | ||||
| EBI Holdings, LLC | Delaware | 3842 | 22-2407246 | 399 Jefferson Road Parsippany, NJ 07054 (973) 299-9300 | ||||
| EBI, LLC | Indiana | 3842 | 31-1651314 | 399 Jefferson Road Parsippany, NJ 07054 (973) 299-9300 | ||||
| EBI Medical Systems, LLC | Delaware | 3842 | 22-2406619 | 399 Jefferson Road Parsippany, NJ 07054 (973) 299-9300 | ||||
| Exact Name of Registrant as Specified in its Charter | State or Other Jurisdiction of Incorporation or Organization | Primary Standard Industrial Classification Code Number | I.R.S. Employer Identification Number | Address, including Zip Code and Telephone Number, including Area Code, of Agent for Service, of Registrant’s Principal Executive Offices | ||||
| Electro-Biology, LLC | Delaware | 3842 | 22-2278360 | #1 Electro-Biology Boulevard Los Frailes Industrial Park Guaynabo, Puerto Rico 00657 (787) 720-6855 | ||||
| Biomet Florida Services, LLC | Florida | 3842 | 20-0388276 | 1520 Tradeport Drive Jacksonville, FL 32218 (904) 741-4400 | ||||
| Implant Innovations Holdings, LLC | Indiana | 3842 | 35-2088040 | 56 E. Bell Drive Warsaw, IN 46852 (574) 267-6639 | ||||
| Interpore Cross International, LLC | California | 3842 | 33-0818017 | 181 Technology Drive, Irvine, CA 92618 (949) 453-3200 | ||||
| Interpore Spine Ltd. | Delaware | 3842 | 95-3043318 | 181 Technology Drive, Irvine, CA 92618 (949) 453-3200 | ||||
| Kirschner Medical Corporation | Delaware | 3842 | 52-1319702 | 56 E. Bell Drive Warsaw, IN 46852 (574) 267-6639 | ||||
| Biomet Trauma, LLC | Indiana | 3842 | 27-3309062 | 56 E. Bell Drive Warsaw, IN 46582 (574) 267-6639 | ||||
| Biomet U.S. Reconstruction, LLC | Indiana | 3842 | 45-5118007 | 56 E. Bell Drive Warsaw, IN 46582 (574) 267-6639 | ||||
The information in this prospectus is not complete and may be changed. These securities may not be sold until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell or a solicitation of an offer to purchase these securities in any jurisdiction where the offer or sale is not permitted.
SUBJECT TO COMPLETION, DATED MAY 14, 2013
PRELIMINARY PROSPECTUS

OFFERS TO EXCHANGE
$1,825,000,000, aggregate principal amount of our 6.500% Senior Notes due 2020 (the “exchange senior notes”) and $800,000,000 aggregate principal amount of our 6.500% Senior Subordinated Notes due 2020, (the “exchange senior subordinated notes” and together with the exchange senior notes, the “exchange notes”), the issuance of each of which has been registered under the Securities Act of 1933, as amended (the “Securities Act”),
for
$1,825,000,000 of our 6.500% Senior Notes due 2020, (the “original senior notes”, and together with the exchange senior notes, the “Senior Notes”) and $800,000,000 of our 6.500% Senior Subordinated Notes due 2020 (the “original senior subordinated notes” and together with the exchange senior subordinated notes, the “Subordinated Notes” and the original senior subordinated notes with the original senior notes, the “original notes”, and the original notes together with the exchange notes, the “notes”), respectively, that have not been registered under the Securities Act.
for
$1,825,000,000 of our 6.500% Senior Notes due 2020, (the “original senior notes”, and together with the exchange senior notes, the “Senior Notes”) and $800,000,000 of our 6.500% Senior Subordinated Notes due 2020 (the “original senior subordinated notes” and together with the exchange senior subordinated notes, the “Subordinated Notes” and the original senior subordinated notes with the original senior notes, the “original notes”, and the original notes together with the exchange notes, the “notes”), respectively, that have not been registered under the Securities Act.
_________________
THIS EXCHANGE OFFERS WILL EXPIRE AT 5:00 P.M., NEW YORK CITY TIME, ON , 2013 UNLESS EXTENDED BY US.
_________________
The Exchange Offers: • We will exchange all original notes that are validly tendered and not validly withdrawn for an equal principal amount of exchange notes. • The exchange offers expire at 5:00 P.M., New York City time, on , 2013 (such date and time, the “Expiration Date,” unless we extend or terminate either or both exchange offers, in which case the “Expiration Date” will mean the latest date and time to which we extend such exchange offer or exchange offers). We do not currently intend to extend the Expiration Date with respect to either exchange offer. • You may withdraw tenders of original notes at any time prior to the Expiration Date. • The exchange of original notes for exchange notes in the exchange offers generally will not be a taxable event for U.S. federal income tax purposes. • We will not receive any proceeds from the exchange offers. | The Exchange Notes: • The exchange notes are being offered in order to satisfy certain of our obligations under the registration rights agreements entered into in connection with the private offerings of the original notes. • The terms of the exchange notes to be issued in the exchange offers are substantially the same as the terms of the original notes, except that the offer of the exchange notes is registered under the Securities Act, and the exchange notes have no transfer restrictions, rights to additional interest or registration rights. Resales of the Exchange Notes: • The exchange notes may be sold in the over-the-counter market, in negotiated transactions or through a combination of such methods. We do not plan to list the exchange notes on a securities exchange or automated quotation system. |
_________________
Investing in the exchange notes to be issued in the exchange offers involves certain risks. You should consider carefully the risk factors beginning on page 9 of this prospectus before participating in the exchange offers.
Each broker-dealer that receives exchange notes for its own account pursuant to the exchange offers must acknowledge that it will deliver a prospectus in connection with any resale of such exchange notes. The letter of transmittal set forth in Annex A to this prospectus states that by so acknowledging and by delivering a prospectus, a broker-dealer will not be deemed to admit that it is an “underwriter” within the meaning of the Securities Act. This prospectus, as it may be amended or supplemented from time to time, may be used by a broker-dealer in connection with resales of exchange notes received in exchange for original notes where such original notes were acquired by such broker-dealer as a result of market-making activities or other trading activities. We have agreed that, for a period of 90 days after the Expiration Date (as defined herein), we will make this prospectus available to any broker-dealer for use in connection with any such resale. See “Plan of Distribution.”
We are not making an offer to exchange notes in any jurisdiction where the offer is not permitted.
NEITHER THE SECURITIES AND EXCHANGE COMMISSION NOR ANY STATE SECURITIES COMMISSION HAS APPROVED OR DISAPPROVED OF THESE SECURITIES OR PASSED UPON THE ADEQUACY OR ACCURACY OF THIS PROSPECTUS. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENSE.
_________________
The date of this prospectus is , 2013.
You should rely only on the information contained in this prospectus. We have not authorized any person to provide you with any information or represent anything about us or this offering that is not contained in this prospectus. If given or made, any such other information or representation should not be relied upon as having been authorized by us. We are offering to exchange the original notes for the exchange notes only in places where the exchange offers are permitted. You should not assume that the information contained in this prospectus is accurate as of any date other than the date on the front cover of this prospectus.
_______________________________________________
| TABLE OF CONTENTS | |
| Page | |
_______________________________________________
i
WHERE YOU CAN FIND MORE INFORMATION
We and the guarantors have filed with the Securities and Exchange Commission (the “SEC”), a registration statement on Form S-4 under the Securities Act with respect to the notes being offered hereby. This prospectus, which forms a part of the registration statement, does not contain all of the information set forth in the registration statement. For further information with respect to us, the guarantors or the notes, we refer you to the registration statement. We also file annual, quarterly, and current reports and other information with the SEC. The registration statement, such reports and other information can be inspected and copied at the Public Reference Room of the SEC located at Room 1580, 100 F Street, N.E., Washington D.C. 20549. Copies of such materials, including copies of all or any portion of the registration statement, can be obtained from the Public Reference Room of the SEC at prescribed rates. You can call the SEC at 1-800-SEC-0330 to obtain information on the operation of the Public Reference Room. Such materials may also be accessed electronically by means of the SEC’s home page on the Internet (http://www.sec.gov).
Our Internet address is www.biomet.com. There we make available free of charge, on or through the “Investors” section of our Web site, Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities and Exchange Act of 1934, as amended (the “Exchange Act”), as soon as reasonably practicable after we electronically file such material with, or furnish it to, the SEC. The information found on our Web site does not form a part of this prospectus.
Under the terms of the indentures relating to the notes, we have agreed that, whether or not we are required to do so by the rules and regulations of the SEC, for so long as any of the notes remain outstanding, we will furnish to the trustee and holders of the notes the information specified in the indentures. See “Description of Exchange Senior Notes” and “Description of Exchange Senior Subordinated Notes.”
FORWARD-LOOKING STATEMENTS
Some of the statements made under the headings “Summary” and elsewhere in this prospectus contain forward-looking statements within the meaning of U.S. federal securities laws, including Section 27A of the Securities Act and Section 21E of the Exchange Act. Statements that are not historical facts, including statements about our beliefs and expectations, are forward-looking statements. Forward-looking statements include statements generally preceded by, followed by or that include the words “believe,” “could,” “expect,” “forecast,” “intend,” “may,” “anticipate,” “plan,” “predict,” “possibly,” “project,” “potential,” “estimate,” “should,” “will,” or similar expressions. These statements include, but are not limited to, statements related to:
| • | the timing and number of planned new product introductions; |
| • | the effect of anticipated changes in the size, health and activities of the population or on the demand for our products; |
| • | assumptions and estimates regarding the size and growth of certain market categories; |
| • | our ability and intent to expand in key international markets; |
| • | the timing and anticipated outcome of clinical studies; |
| • | assumptions concerning anticipated product developments and emerging technologies; |
| • | the future availability of raw materials; |
| • | the anticipated adequacy of our capital resources to meet the needs of our business; |
| • | our continued investment in new products and technologies; |
| • | the ultimate marketability of products currently being developed; |
ii
| • | our ability to successfully implement new technologies and transition certain manufacturing operations to China; |
| • | our ability to manage working capital and generate adequate cash flows to service outstanding debt; |
| • | our ability to sustain sales and earnings growth; |
| • | our success in achieving timely approval or clearance of our products with domestic and foreign regulatory entities; |
| • | our success in implementing our operational improvement programs; |
| • | the stability of certain foreign economic markets; |
| • | the impact of anticipated changes in the musculoskeletal industry and our ability to react to and capitalize on those changes; |
| • | our ability to successfully implement desired organizational changes; |
| • | our ability to successfully integrate the DePuy Trauma acquisition; |
| • | the impact of our managerial changes; and |
| • | our ability to take advantage of technological advancements. |
Forward-looking statements reflect our current expectations and are not guarantees of performance. These statements are based on our management’s beliefs and assumptions, which in turn are based on currently available information. Important assumptions relating to these forward-looking statements include, among others, assumptions regarding demand for our products, expected pricing levels, raw material costs, the timing and cost of planned capital expenditures, future regulatory reforms affecting the healthcare industry, expected outcomes of pending litigation and regulatory matters, the solvency of our insurers and the ultimate resolution of allocation and coverage issues with those insurers, competitive conditions and general economic conditions. Readers of this prospectus are cautioned that reliance on any forward-looking statement involves risks and uncertainties. Although we believe that the assumptions on which the forward-looking statements contained herein are based are reasonable, any of those assumptions could prove to be inaccurate given the inherent uncertainties as to the occurrence or nonoccurrence of future events. There can be no assurance that the forward-looking statements contained in this prospectus will prove to be accurate. The inclusion of a forward-looking statement in this prospectus should not be regarded as a representation by us that our objectives will be achieved. Forward-looking statements also involve risks and uncertainties, which could cause actual results to differ materially from those projected by any forward-looking statement. Many of these factors are beyond our ability to control or predict and could, among other things, cause actual results to differ from those contained in forward-looking statements made in this prospectus and presented elsewhere by management from time to time. Such factors, among others, may have a material adverse effect upon our business, financial condition, results of operations and cash flows and may include, but are not limited to, factors discussed under the heading “Risk Factors” and the following:
| • | changes in general economic conditions and interest rates; |
| • | changes in the availability of capital and financing sources; |
| • | changes in competitive conditions and prices in our markets; |
| • | changes to the regulatory environment for our products, including national health care reform; |
| • | the effects of incurring or having incurred a substantial amount of indebtedness under the notes, our senior secured credit facilities and our existing notes; |
iii
| • | the effects upon us of complying with the covenants contained in our senior secured credit facilities and the indentures and our existing notes; |
| • | restrictions that the terms and conditions of the notes, our senior secured credit facilities and the existing notes may place on our ability to respond to changes in our business or take certain actions; |
| • | changes in the relationship between supply of and demand for our products; |
| • | fluctuations in costs of raw materials and labor; |
| • | changes in other significant operating expenses; |
| • | decreases in sales of our principal product lines; |
| • | slowdowns or inefficiencies in our product research and development efforts; |
| • | increases in expenditures related to increased government regulation of our business; |
| • | developments adversely affecting our sales activities inside or outside the United States; |
| • | decreases in reimbursement levels by our customers, including certain of our foreign government customers that are experiencing financial distress; |
| • | difficulties in transitioning certain manufacturing operations to China and other locations; |
| • | challenges in effectively implementing restructuring and cost saving initiatives; |
| • | increases in cost-containment efforts from managed care organizations and other third-party payors; |
| • | loss of our key management and other personnel or inability to attract such management and other personnel; |
| • | increases in costs of retaining existing independent sales agents of our products; |
| • | potential future goodwill and/or intangible impairment charges; |
| • | unanticipated expenditures related to litigation; and |
| • | failure to comply with the terms of the DPA (as defined elsewhere in this prospectus). |
There may be other factors of which we are currently unaware or that we deem immaterial that may cause our actual results to differ materially from the expectations we express in our forward-looking statements. Although we believe the assumptions underlying our forward-looking statements are reasonable, any of these assumptions, and, therefore, also the forward-looking statements based on these assumptions could themselves prove to be inaccurate.
Forward-looking statements are based on current plans, estimates, assumptions and projections, and therefore you should not place undue reliance on them. Forward-looking statements speak only as of the date they are made and we undertake no obligation to update them publicly in light of new information or future events.
You should carefully consider the “Risk Factors” and other information included in this prospectus before making any investment decision with respect to the exchange notes. If any of these trends, risks, assumptions or uncertainties actually occurs or continues, our business, financial condition or operating results could be materially adversely affected, the trading prices of the notes could decline and you could lose all or part of your investment. All forward-looking statements attributable to us or persons acting on our behalf are expressly qualified in their entirety by this cautionary statement.
iv
MARKET AND INDUSTRY DATA
In this prospectus, we rely on and refer to information and statistics regarding our industry products and our market share based on revenues in the sectors in which we compete. Where possible, we obtained this information and statistics from third-party sources, such as independent industry publications, government publications or reports by market research firms, including, without limitation, Eurostat, Knowledge Enterprises, Inc., the U.S. Census Bureau, Wall Street research and from company research and trade interviews. In addition, we have supplemented third-party information where necessary with management estimates based on our review of internal surveys, information from our customers and vendors, trade and business organizations and other contacts in markets in which we operate, and our management’s knowledge and experience. However, these estimates are subject to change and are uncertain due to limits on the availability and reliability of primary sources of information and the voluntary nature of the data gathering process. Although we believe that these independent sources and our management’s estimates are reliable as of the date of this prospectus, the information contained in them has not been independently verified, and we cannot assure you as to the accuracy or completeness of such information. As a result, you should be aware that market share and industry data included in this prospectus, and estimates and beliefs based on that data, may not be reliable. We make no representation as to the accuracy or completeness of such information.
TERMS USED IN THIS PROSPECTUS
Unless otherwise noted or indicated by the context, in this prospectus:
The term “guarantors”, as of the date of this prospectus with respect to both the senior notes and the senior subordinated notes, means Biolectron, Inc., Biomet 3i, LLC, Biomet Biologics, LLC, Biomet Europe Ltd., Biomet Fair Lawn, LLC, Biomet Florida Services, LLC, Biomet International Ltd, Biomet Leasing, Inc., Biomet Manufacturing Corporation, Biomet Microfixation, LLC, Biomet Orthopedics, LLC, Biomet Sports Medicine, LLC, Biomet U.S. Reconstruction, LLC, Biomet Trauma, LLC, Cross Medical Products, LLC, EBI Holdings, LLC, EBI, LLC, EBI Medical Systems, LLC, Electro-Biology, LLC, Implant Innovations Holdings, LLC, Interpore Cross International, LLC, Interpore Spine Ltd., and Kirschner Medical Corporation. However, since each of our current and future wholly owned domestic restricted subsidiaries that is a guarantor of our senior secured credit facilities will fully and unconditionally guarantee the exchange senior notes on a senior unsecured basis and the exchange senior subordinated notes on a senior subordinated unsecured basis, the identities of the guarantors may change from time to time without notice. See “Description of Exchange Senior Notes—Guarantees” and “Description of Exchange Senior Subordinated Notes—Guarantees.”
The term “senior notes indenture” refers to the Senior Notes Indenture dated as of August 8, 2012 among Biomet, Inc., the Guarantors listed therein and Wells Fargo Bank, National Association and the First Supplemental Indenture, dated as of October 2, 2012 among Biomet, Inc., the Guarantors listed therein and Wells Fargo Bank, National Association, collectively.
The term “senior subordinated notes indenture” refers to the Senior Subordinated Notes Indenture dated as of October 2, 2012 among Biomet, Inc., the Guarantors listed therein and Wells Fargo Bank, National Association.
The term “indentures” refers to the senior notes indenture and senior subordinated notes indenture, collectively.
The term “senior notes registration rights agreements” refers to each of the registration rights agreements we entered into with the initial purchasers of the original senior notes concurrently with the sales of the original senior notes on August 8, 2012 and October 2, 2012, respectively.
The term “senior subordinated notes registration rights agreement” refers to registration rights agreement we entered into with the initial purchasers of the original senior subordinated notes concurrently with the sale of the original senior subordinated notes on October 2, 2012.
The term “registration rights agreements” refers to the senior notes registration rights agreements and the senior subordinated notes registration rights agreement, collectively.
References to our fiscal years through and including fiscal 2012 are to the twelve months ended on May 31 of such year.
v
SUMMARY
This summary highlights aspects of our business and the exchange offers. You should, however, carefully read the entire prospectus, including the information presented under the section entitled “Risk Factors” and our consolidated financial statements and the notes thereto included elsewhere in this prospectus before making an investment decision. This summary contains forward-looking statements that involve risks and uncertainties. Our actual results may differ significantly from the results discussed in the forward-looking statements as a result of certain factors, including those set forth under “Risk Factors” and “Forward-Looking Statements.”
Unless the context otherwise requires or indicates, references to “Biomet,” “the Company,” “we,” “us” and “our” refer to Biomet, Inc. and its subsidiaries.
Our Company
General
Biomet, Inc., an Indiana corporation incorporated in 1977, is one of the largest orthopedic medical device companies in the United States and worldwide with operations in more than 50 locations throughout the world and distribution in approximately 90 countries. Our principal subsidiaries include Biomet U.S. Reconstruction, LLC; Biomet Orthopedics, LLC; Biomet Manufacturing Corp.; Biomet Europe BV; EBI, LLC; Biomet 3i, LLC; Biomet International Ltd.; Biomet Microfixation, LLC; Biomet Sports Medicine, LLC; Biomet Trauma, LLC; and Biomet Biologics, LLC. We design, manufacture and market a comprehensive range of both surgical and non-surgical products used primarily by orthopedic surgeons and other musculoskeletal medical specialists. We operate in one reportable business segment, musculoskeletal products, which includes the design, manufacture and marketing of products in five major product categories: Large Joint Reconstructive; Sports, Extremities and Trauma (“S.E.T.”); Spine & Bone Healing; Dental; and Other Products. We have three geographic markets: United States, Europe and International.
Corporate Information
Biomet is incorporated in the State of Indiana. Our principal executive offices are located at 56 East Bell Drive, Warsaw, Indiana 46582. Our website address is www.biomet.com. The information on our website is not deemed to be part of this prospectus. For additional information, contact our Corporate Communications department at (574) 372-1514.
Ownership and Corporate Structures
LVB Acquisition, Inc., or “Parent,” owns all of our issued and outstanding capital stock. LVB Acquisition Holding, LLC (“Holding”) owns 97.0% of the issued and outstanding capital stock of Parent. Substantially all the equity interests in Holding are owned, directly or indirectly, by a consortium of private equity funds affiliated with The Blackstone Group, Goldman, Sachs & Co., Kohlberg Kravis Roberts & Co. and TPG Global, LLC (together with its affiliates, “TPG”), and their co-investors (jointly, the “Sponsors”).
1
The Exchange Offers
On August 8, 2012 and October 2, 2012, we completed private offerings of our original notes. We entered into registration rights agreements with the initial purchasers in the private offerings in which we agreed, among other things, to file the registration statement of which this prospectus is a part. The following is a summary of the exchange offers.
| Original Notes | On August 8, 2012, we issued: • $1,000,000,000 aggregate principal amount of 6.500% Senior Notes due 2020. On October 2, 2012, we issued: • $825,000,000 aggregate principal amount of 6.500% Senior Notes due 2020; and • $800,000,000 aggregate principal amount of 6.500% Senior Subordinated Notes due 2020. The proceeds of these issuances were used to purchase or redeem all of our outstanding 10⅜%/11⅛% Senior PIK Toggle Notes due 2017; all of our outstanding 10% Senior Notes due 2017; and all of our outstanding 11⅝% Senior Subordinated Notes due 2017. |
| Exchange Notes Offered in the Exchange Offer | |
| Exchange Senior Notes | 6.500% Senior Notes due 2020. The terms of the exchange senior notes are substantially identical in all material respects to those terms of the original senior notes, except that the transfer restrictions, registration rights and provisions for additional interest relating to the original senior notes do not apply to the exchange senior notes. |
| Exchange Senior Subordinated Notes | 6.500% Senior Subordinated Notes due 2020. The terms of the exchange senior subordinated notes are substantially identical in all material respects to those terms of the original senior subordinated notes, except that the transfer restrictions, registration rights and provisions for additional interest relating to the original senior subordinated notes do not apply to the exchange senior subordinated notes. The exchange notes are summarized in greater detail below under “The Exchange Notes.” |
| Exchange Offers | We are offering to exchange: • up to $1,825 million principal amount of our exchange senior notes for an equal amount of our original senior notes; and • up to $800 million principal amount of our exchange senior subordinated notes for an equal amount of our original senior subordinated notes. We will not pay any accrued and unpaid interest on the original notes that we will acquire in the exchange offers. Instead, interest on the exchange senior notes will accrue from February 1, 2013, the most recent date on which interest has been paid. Interest on the exchange senior subordinated notes will accrue from October 2, 2012, the issue date of the senior subordinated notes, or from April 1, 2013 if the exchange offer in respect thereof is completed after, and interest is paid on, that date. |
| Expiration Date | The exchange offers will expire at 5:00 P.M., New York City time, , 2013, unless we extend or terminate either or both exchange offers, in which case the “Expiration Date” will mean the latest date and time to which we extend such exchange offer or exchange offers. |
Settlement Date | The settlement date of the exchange offers will be as soon as practicable after the respective Expiration Date. |
2
| Conditions to the Exchange Offers | The registration rights agreements do not require us to accept original notes for exchange if the exchange offers or the making of any exchange by a holder of the original notes would violate any applicable law or interpretation of the staff of the SEC or if any legal action has been instituted or threatened that would impair our ability to proceed with the exchange offers. A minimum aggregate principal amount of original notes being tendered is not a condition to the exchange offers. Please read “The Exchange Offers—Conditions to the Exchange Offers” for more information about the conditions to the exchange offers. |
| Procedures for Tendering Original Notes | To participate in the exchange offers, you must follow the automatic tender offer program (“ATOP”) procedures established by The Depository Trust Company (“DTC”) for tendering original notes held in book-entry form. The ATOP procedures require that the exchange agent receive, prior to the Expiration Date, a computer-generated message known as an “agent’s message” that is transmitted through ATOP and that DTC confirms that: • DTC has received instructions to exchange your original notes; and • you agree to be bound by the terms of the letter of transmittal. |
In the alternative, you may properly complete and duly execute a letter of transmittal and transmit it, along with all other documents required by such letter of transmittal, to the exchange agent on or before the Expiration Date at the address provided on the cover page of the letter of transmittal. The form of the letter of transmittal is set forth in Annex A to this prospectus. For more details, please read “The Exchange Offers—Procedures for Tendering,” “The Exchange Offers—Book-Entry Transfer”. If you elect to have original notes exchanged pursuant to these exchange offers, you must properly tender your original notes prior to 5:00 p.m., New York City time, on the respective Expiration Date. All original notes validly tendered and not properly withdrawn will be accepted for exchange. Original notes may be exchanged only in minimum denominations of $1,000 and integral multiples of $1,000 in excess thereof. | |
Withdrawal of Tenders | You may withdraw your tender of original notes at any time prior to the Expiration Date. |
Fees and Expenses | We will bear all expenses related to the exchange offers. Please read “The Exchange Offers—Fees and Expenses.” |
Use of Proceeds | The issuance of the exchange notes will not provide us with any new proceeds. We are making the exchange offers solely to satisfy certain obligations under our registration rights agreements. |
| Consequences of Failure to Exchange Original Notes | If we complete the exchange offers and you do not participate, then: • your original notes will continue to be subject to the existing restrictions upon their transfer; • we will have no further obligations to provide the registration under the Securities Act of those original notes except under certain limited circumstances; and • the liquidity of the market for your original notes could be adversely affected. |
| U.S. Federal Income Tax Considerations | Neither the registration of the original notes pursuant to our obligations under the registration rights agreements nor the U.S. Holder’s receipt of exchange notes in exchange for original notes will constitute a taxable event for U.S. federal income tax purposes. Please read “Certain U.S. Federal Income Tax Considerations.” |
3
| Exchange Agent | We have appointed Wells Fargo Bank, National Association as the exchange agent for the exchange offers. You should direct questions and requests for assistance and requests for additional copies of this prospectus (including the letter of transmittal) to the exchange agent at the following addresses: By Registered and Certified Mail: Wells Fargo Bank, National Association Corporate Trust Operations MAC N9303-121 P.O. Box 1517 Minneapolis, MN 55480 By Overnight Courier or Regular Mail: Wells Fargo Bank, National Association Corporate Trust Operations MAC N9303-121 6th & Marquette Avenue Minneapolis, MN 55479 By Hand Delivery: Wells Fargo Bank, National Association Corporate Trust Services 608 2nd Avenue South Northstar East Building—12th Floor Minneapolis, MN 55402 By Facsimile Transmission: (612) 667-6282 Confirm by Telephone: (800) 344-5128 |
| Resales of the Exchange Notes | Based on interpretations of the staff of the SEC, we believe that you may offer for sale, resell or otherwise transfer the exchange notes that we issue in the exchange offers without complying with the registration and prospectus delivery requirements of the Securities Act if: • you are not a broker-dealer tendering original notes acquired directly from us; • you acquire the exchange notes in the ordinary course of your business; • you are not participating, do not intend to participate, and have no arrangement or undertaking with anyone to participate, in the distribution (within the meaning of the Exchange Act) of the exchange notes issued to you in the exchange offers; and • you are not an “affiliate” of our company, as that term is defined in Rule 405 of the Securities Act. |
If any of these conditions are not satisfied and you transfer any exchange notes issued to you in the exchange offers without delivering a proper prospectus or without qualifying for a registration exemption, you may incur liability under the Securities Act. We will not be responsible for, or indemnify you against, any liability you incur. Any broker-dealer that acquires exchange notes in the exchange offers for its own account in exchange for original notes which it acquired through market-making or other trading activities must acknowledge that it will deliver this prospectus when it resells or transfers any exchange notes issued in the exchange offers. See “Plan of Distribution” for a description of the prospectus delivery obligations of broker-dealers. | |
4
| The Exchange Notes | |
Issuer | Biomet, Inc. |
| Guarantors | Each of our current and future wholly owned domestic restricted subsidiaries that is a guarantor of our senior secured credit facilities will fully and unconditionally guarantee the exchange senior notes on a senior unsecured basis and the exchange senior subordinated notes on a senior subordinated unsecured basis. See “Description of Exchange Senior Notes—Guarantees” and “Description of Exchange Senior Subordinated Notes—Guarantees.” |
| Notes Offered | |
| Exchange Senior Notes | Up to $1,825 million in aggregate principal amount of 6.500% Senior Notes due 2020. The exchange senior notes and the original senior notes will be considered to be a single class for all purposes under the senior notes indenture, including waivers, amendments, redemptions and offers to purchase. |
| Exchange Senior Subordinated Notes | Up to $800 million in aggregate principal amount of 6.500% Senior Subordinated Notes due 2020. The exchange senior subordinated notes and the original senior subordinated notes will be considered to be a single class for all purposes under the senior subordinated notes indenture, including waivers, amendments, redemptions and offers to purchase. |
Maturity Dates | The exchange senior notes will mature on August 1, 2020, and the exchange senior subordinated notes will mature on October 1, 2020. |
Interest Rates | Interest on the exchange senior notes and exchange senior subordinated notes will be payable in cash and will accrue at a rate of 6.500% per annum. |
Interest Payment Dates Exchange Senior Notes Exchange Senior Subordinated Notes | August 1 and February 1, commencing February 1, 2013. Interest will accrue from February 1, 2013. April 1 and October 1, commencing April 1, 2013. Interest will accrue from October 2, 2012, or from April 1, 2013 if the exchange offer in respect thereof is completed after, and interest is paid on, that date. |
5
| Ranking | |
| Exchange Senior Notes | The exchange senior notes will be our senior unsecured obligations and will: • rank pari passu in right of payment with all of our existing and future indebtedness that is not expressly subordinated in right of payment thereto; • be senior in right of payment to any future indebtedness that is expressly subordinated in right of payment thereto (including our existing senior subordinated notes); and • be effectively junior to our and our guarantors’ existing and future secured indebtedness (including the borrowings under our senior secured credit facilities), to the extent of the value of the collateral securing such indebtedness and to all existing and future liabilities of our non-guarantor subsidiaries. Similarly, the guarantees of the exchange senior notes will be the guarantors’ senior unsecured obligations and will: • rank pari passu in right of payment with all existing and future indebtedness of each guarantor that is not expressly subordinated thereto; • be senior in right of payment to any future indebtedness of each guarantor that is expressly subordinated in right of payment thereto; and • be effectively junior to all existing and future secured indebtedness of each guarantor to the extent of the value of the collateral securing such indebtedness. |
| Exchange Senior Subordinated Notes…… | The exchange senior subordinated notes will be our senior subordinated unsecured obligations and will: • rank junior in right of payment with all of our existing and future indebtedness that is not expressly subordinated in right of payment thereto (including the senior notes); • rank pari passu in right of payment to any of our existing and future senior subordinated indebtedness (including the original senior subordinated notes) and other obligations; and • be senior in right of payment to any future subordinated indebtedness and effectively junior to our and our guarantors’ existing and future secured indebtedness (including the borrowings under our senior secured credit facilities), to the extent of the value of the collateral securing such indebtedness and to all existing and future liabilities of our non-guarantor subsidiaries. Similarly, the guarantees of the exchange senior subordinated notes will be the guarantors’ senior subordinated unsecured obligations and: • rank junior in right of payment with all existing and future indebtedness of each guarantor that is not expressly subordinated thereto; • rank pari passu in right of payment to any of our existing and future senior subordinated indebtedness and other obligations; and • be senior in right of payment to any future indebtedness of each guarantor that is expressly subordinated in right of payment thereto and effectively junior to all existing and future secured indebtedness of each guarantor to the extent of the value of the collateral securing such indebtedness. |
6
| Optional Redemption | |
| Exchange Senior Notes | At any time prior to August 1, 2015, we may redeem up to 35% of the aggregate principal amount of the senior notes (including both the original senior notes and the exchange senior notes) with the net proceeds of certain equity offerings at the redemption price set forth in this prospectus, plus accrued and unpaid interest, if any, to the redemption date. At any time prior to August 1, 2015, we may redeem the exchange senior notes, in whole or in part, at our option, at a redemption price equal to 100% of their principal amount plus a “make-whole premium” and accrued and unpaid interest, if any, to the date of redemption. On and after August 1, 2015, we may redeem some or all of the exchange senior notes at any time at the redemption prices set forth in this prospectus plus accrued and unpaid interest, if any, to the date of redemption. See “Description of Exchange Senior Notes—Optional Redemption.” |
| Exchange Senior Subordinated Notes | At any time prior to October 1, 2015, we may redeem up to 40% of the aggregate principal amount of the senior subordinated notes (including both the original senior subordinated notes and the exchange senior subordinated notes) with the net proceeds of certain equity offerings at the redemption price set forth in this prospectus, plus accrued and unpaid interest, if any, to the redemption date. At any time prior to October 1, 2015, we may redeem the exchange senior subordinated notes, in whole or in part, at our option, at a redemption price equal to 100% of their principal amount plus a “make-whole premium” and accrued and unpaid interest, if any, to the date of redemption. On and after October 1, 2015, we may redeem some or all of the exchange senior subordinated notes at any time at the redemption prices set forth herein plus accrued and unpaid interest, if any, to the date of redemption. See “Description of Exchange Senior Subordinated Notes—Optional Redemption.” |
Change of Control | Upon certain change of control events, each holder of exchange notes may require us to purchase all or a portion of such holder’s notes at a purchase price equal to 101% of the principal amount thereof, plus accrued and unpaid interest, if any, to the purchase date. See “Description of Exchange Senior Notes” and “Description of Exchange Senior Subordinated Notes.” |
7
| Certain Covenants | The senior notes indenture and the senior subordinated notes indenture contain covenants that, among other things, limit our ability and the ability of our restricted subsidiaries to: • pay dividends on, redeem or repurchase capital stock or make other restricted payments; • make investments; • incur indebtedness or issue certain equity; • create certain liens; • incur obligations that restrict the ability of our subsidiaries to make dividend or other payments to us; • enter into transactions with our affiliates; • create or designate unrestricted subsidiaries; and • consolidate, merge or transfer all or substantially all of our assets. These covenants are subject to important exceptions and qualifications, which are described under the headings “Description of Exchange Senior Notes” and “Description of Exchange Senior Subordinated Notes” in this prospectus. Certain of these covenants will be suspended if the notes are assigned an investment grade rating by Standard & Poor’s Rating Services (“Standard & Poor’s”) and Moody’s Investors Services, Inc. (“Moody’s”) and no default has occurred and is continuing. If either rating on the notes should subsequently decline to below investment grade or a default occurs and is continuing, the suspended covenants will be reinstated. |
Absence of a Public Market | The exchange notes are new securities for which there currently is no market and we cannot assure you that any public market for the exchange notes will develop or be sustained. |
Listing | We do not intend to list the notes on any securities exchange. |
Governing Law | The notes are governed by, and construed in accordance with, the laws of the State of New York. |
Trustee | Wells Fargo Bank, National Association |
Risk Factors | See “Risk Factors” and the other information in this prospectus for a discussion of some of the factors you should carefully consider before participating in the exchange offers. |
8
RISK FACTORS
Before tendering original notes in the exchange offers and investing in the exchange notes, you should consider carefully each of the following risk factors, as well as other information included in this prospectus. The risks described below are not the only ones facing our company. Additional risks and uncertainties not currently known to us or that we currently deem to be immaterial may also materially and adversely affect our business or results of operations in the future. Any of the following risks could materially adversely affect our business, financial condition or results of operations. We cannot assure you that any of the events discussed in or incorporated by reference into this prospectus will not occur. In such case, you may lose all or part of your original investment in the notes.
Risks Related to Our Business
Our future profitability depends on the success of our principal product lines.
Sales of our large joint reconstructive products accounted for approximately 56% of our net sales for the nine months ended February 28, 2013 and 60% of our net sales for each of the three fiscal years ended May 31, 2012, 2011 and 2010. We expect sales of reconstructive products to continue to account for a significant portion of our aggregate sales. Any event adversely affecting the sale of reconstructive products may, as a result, adversely affect our business, financial condition, results of operations and cash flows.
If we are unable to continue to develop and market new products and technologies in a timely manner, the demand for our products may decrease or our products could become obsolete, and our revenue and profitability may decline.
The market for our products is highly competitive and dominated by a small number of large companies. We are continually engaged in product development, research and improvement efforts. New products and line extensions of existing products represent a significant component of our growth rate. Our ability to continue to grow sales effectively depends on our capacity to keep up with existing or new products and technologies in the musculoskeletal products market. The process of obtaining regulatory approvals to market a medical device, particularly from the U.S. Food and Drug Administration (“FDA”) and certain foreign governmental authorities, can be costly and time consuming and approvals and clearances might not be granted for future products on a timely basis, if at all. On July 29, 2011, the Institute of Medicine (“IoM”) published a report of its review of the 510(k) clearance program to FDA. The IoM report recommended that the FDA pursue a legislative change from the current 510(k) process to an integrated premarket and post-market regulatory framework. It is uncertain if these recommendations will ultimately be pursued. If they are pursued, it is possible we will be required to submit additional clinical and manufacturing information with respect to premarket applications in the future, resulting in increased costs and increased delay in introducing products to the market. Other devices we develop and market fall into a class of products for which the FDA has implemented stringent clinical investigation and Premarket Approval (“PMA”) requirements. The PMA process requires us to provide clinical and laboratory data that establishes that the new medical device is safe and effective. The FDA will approve the new device for commercial distribution if it determines that the data and information in the PMA relating to design, materials, bench and animal testing and human clinical data constitute valid scientific evidence and that there is reasonable assurance that the device is safe and effective for its intended use. In addition, if our competitors’ new products and technologies reach the market before our products, they may gain a competitive advantage or render our products obsolete. The ultimate success of our product development efforts will depend on many factors, including, but not limited to, our ability to create innovative designs and materials, provide innovative surgical techniques, accurately anticipate and meet customers’ needs, commercialize new products in a timely manner, and manufacture and deliver products and instrumentation in sufficient volumes on time. Moreover, research and development efforts may require a substantial investment of time and resources before we are adequately able to determine the commercial viability of a new product, technology, material or other innovation. Even in the event that we are able to successfully develop innovations, they may not produce revenue in excess of the costs of development and may be quickly rendered obsolete as a result of changing customer preferences or the introduction by our competitors of products embodying new technologies or features.
In addition to the impact of the 2.3% excise tax on our results of operations beginning January 1, 2013 following enactment of the Patient Protection and Affordable Health Care Act (H.R. 3590), our business, financial condition, results of operations and cash flows could be significantly and adversely affected if this legislation
9
ultimately results in lower reimbursements for our products or reduced medical procedure volumes or if certain other types of healthcare reform programs are adopted in our key markets.
In the United States, healthcare providers that purchase our products (e.g., hospitals, physicians, dentists and other healthcare providers) generally rely on payments from third-party payors (principally federal Medicare, state Medicaid and private health insurance plans) to cover all or a portion of the cost of our musculoskeletal products. These third-party payors may deny reimbursement if they determine that a device used in a procedure was not in accordance with cost-effective treatment methods, as determined by the third-party payor, or was used for an unapproved indication. Third-party payors may also decline to reimburse for experimental procedures and devices. In the event that third-party payors deny coverage or reduce their current levels of reimbursement, we may be unable to sell certain products on a profitable basis, thereby materially adversely impacting our results of operations. Further, third-party payors are continuing to carefully review their coverage policies with respect to existing and new therapies and can, without notice, deny coverage for treatments that may include the use of our products.
In March 2010, the U.S. Congress adopted and President Obama signed into law comprehensive healthcare reform legislation through the passage of the Patient Protection and Affordable Health Care Act (H.R. 3590) and the Healthcare and Education Reconciliation Act (H.R. 4872). Among other initiatives, these bills impose a 2.3% excise tax on domestic sales of medical devices following December 31, 2012, which is estimated to contribute approximately $20 billion to healthcare reform. The law was upheld by a Supreme Court decision that was announced on June 28, 2012. Various healthcare reform proposals have also emerged at the state level. Outside of the excise tax, which has impacted our results of operations following December 31, 2012, we cannot predict with certainty what healthcare initiatives, if any, will be implemented at the state level, or what the ultimate effect of federal healthcare reform or any future legislation or regulation will have on us. However, an expansion in government’s role in the U.S. healthcare industry may lower reimbursements for our products, reduce medical procedure volumes and adversely affect our business and results of operations, possibly materially.
Outside of the United States, reimbursement systems vary significantly from country to country. In the majority of the international markets in which our products are sold, government-managed healthcare systems mandate the reimbursement rates and methods for medical devices and procedures. If adequate levels of reimbursement from third-party payors outside of the United States are not obtained, international sales of our products may decline. Many foreign markets, including Canada, and some European and Asian countries, have tightened reimbursement rates. Our ability to continue to sell certain products profitably in these markets may diminish if the government-managed healthcare systems continue to reduce reimbursement rates.
Our business, financial condition, results of operations and cash flows could be significantly and negatively affected by substantial government regulations.
Our products are subject to rigorous regulation by the FDA and numerous other federal, state and foreign governmental authorities. Overall, there appears to be a trend toward more stringent regulation throughout the world, and we do not anticipate this trend to dissipate in the near future.
In general, the development, testing, manufacturing and marketing of our products are subject to extensive regulation and review by numerous governmental authorities both in the United States and abroad. The regulatory process requires the expenditure of significant time, effort and expense to bring new products to market. In addition, we are required to implement and maintain stringent reporting, labeling and record keeping procedures. The medical device industry also is subject to a myriad of complex laws and regulations governing Medicare and Medicaid reimbursement and healthcare fraud and abuse laws, with these laws and regulations being subject to interpretation. In many instances, the industry does not have the benefit of significant regulatory or judicial interpretation of these laws and regulations. In certain public statements, governmental authorities have taken positions on issues for which little official interpretation was previously available. Some of these positions appear to be inconsistent with common practices within the industry but have not previously been challenged.
Various federal and state agencies have become increasingly vigilant in recent years in their investigation of various business practices. Governmental and regulatory actions against us can result in various actions that could adversely impact our operations, including:
| • | the recall or seizure of products; |
| • | the suspension or revocation of the authority necessary for the production or sale of a product; |
10
| • | the suspension of shipments from particular manufacturing facilities; |
| • | the imposition of fines and penalties; |
| • | the delay of our ability to introduce new products into the market; |
| • | the exclusion of our products from being reimbursed by federal and state healthcare programs (such as Medicare, Medicaid, Veterans Administration health programs and Civilian Health and Medical Program Uniformed Service (“CHAMPUS”); and |
| • | other civil or criminal sanctions against us. |
Any of these actions, in combination or alone, or even a public announcement that we are being investigated for possible violations of these laws, could have a material adverse effect on our business, financial condition, results of operations and cash flows.
In many of the foreign countries in which we market our products, we are subject to regulations affecting, among other things, clinical efficacy, product standards, packaging requirements, labeling requirements, import/ export restrictions, tariff regulations, duties and tax requirements. Many of the regulations applicable to our devices and products in these countries, such as the European Medical Devices Directive, are similar to those of the FDA. In addition, in many countries the national health or social security organizations require our products to be qualified before they can be marketed with the benefit of reimbursement eligibility. Failure to receive or delays in the receipt of relevant foreign qualifications also could have a material adverse effect on our business, financial condition, results of operations and cash flows.
As both the U.S. and foreign government regulators have become increasingly stringent, we may be subject to more rigorous regulation by governmental authorities in the future. Our products and operations are also often subject to the rules of industrial standards bodies, such as the International Standards Organization (“ISO”). If we fail to adequately address any of these regulations, our business will be harmed.
Certain provisions of the Dodd-Frank Wall Street Reform and Consumer Protection Act currently or in the future will require us to report on “conflict minerals” used in our products and the due diligence plan we put in place to track whether such minerals originate from the Democratic Republic of Congo and adjoining countries. The implementation of these requirements could affect the sourcing and availability of minerals used in certain of our products.
We, like other companies in the orthopedic industry, are involved in ongoing governmental investigations, the results of which may adversely impact our business and results of operations.
In September 2010, we received a Civil Investigative Demand (“CID”) issued by the U.S. Department of Justice Civil Division pursuant to the False Claims Act. The CID requests that we provide documents and testimony related to allegations that we and OtisMed Corp. and Stryker Corp. have violated the False Claims Act relating to the marketing of, and payment submissions for, OtisMed’s OtisKnee™ (a registered trademark of Otis Med) knee replacement system. We have produced responsive documents and are fully cooperating in the investigation. We can make no assurances as to the time or resources that will be needed to devote to this inquiry or its final outcome.
In February 2010, we received a subpoena from the Office of the Inspector General of the U.S. Department of Health and Human Services requesting various documents relating to agreements or arrangements between physicians and our Interpore Cross subsidiary for the period from 1999 through the present and the marketing and sales activities associated with Interpore Cross’ spinal products. We are cooperating with the request of the Office of the Inspector General. We can make no assurances as to the time or resources that will be needed to devote to this inquiry or its final outcome.
In April 2009, we received an administrative subpoena from the U.S. Attorney’s Office for the District of Massachusetts requesting various documents relating primarily to the Medicare reimbursement of and certain business practices related to our EBI subsidiary’s non-invasive bone growth stimulators. It is our understanding that competitors in the non-invasive bone growth stimulation market received similar subpoenas. We received subsequent subpoenas in connection with the investigation in September 2009, June 2010 and February 2011 along with several informal requests for information. We are producing responsive documents and are fully cooperating in
11
the investigation. We can make no assurances as to the time or resources that will be needed to devote to this investigation or its final outcome.
In April 2009, we became aware of a qui tam complaint alleging violations of the federal and various state False Claims Acts filed in the United States District Court for the District of Massachusetts, where it is currently pending. Biomet, Parent and several of our competitors in the non-invasive bone growth stimulation market were named as defendants in this action. The allegations in the complaint are similar in nature to certain categories of requested documents in the above- referenced administrative subpoenas. The U.S. government has not intervened in the action. We are vigorously defending this matter and intend to continue to do so. We can make no assurances as to the time or resources that will be needed to devote to this investigation or its final outcome.
On September 25, 2007, we received a letter from the SEC informing us that it was conducting an informal investigation regarding possible violations of the Foreign Corrupt Practices Act (“FCPA”), in the sale of medical devices in certain foreign countries by companies in the medical devices industry. The FCPA prohibits U.S. companies and their officers, directors, employees, shareholders acting on their behalf and agents from offering, promising, authorizing or making payments to foreign officials for the purpose of obtaining or retaining business abroad or otherwise obtaining favorable treatment and this law requires companies to maintain records which fairly and accurately reflect transactions and to maintain internal accounting controls. In many countries, hospitals and clinics are government-owned and healthcare professionals employed by such hospitals and clinics, with whom we regularly interact, may meet the definition of a foreign official for purposes of the FCPA. If we are found to have violated the FCPA, we may face sanctions including fines, criminal penalties, disgorgement of profits and suspension or debarment of our ability to contract with government agencies or receive export licenses. On November 9, 2007, we received a letter from the Department of Justice (“DOJ”) requesting any information provided to the SEC be provided to the DOJ on a voluntary basis.
On March 26, 2012, Biomet entered into a Deferred Prosecution Agreement (“DPA”) with the DOJ and a Consent to Final Judgment (“Consent Agreement”) with the SEC related to these investigations by the DOJ and the SEC. Pursuant to the DPA, the DOJ has agreed not to prosecute the Company in connection with this matter, provided that the Company satisfies its obligations under the agreement over the next three years. In addition, pursuant to the terms of the DPA, an independent external compliance monitor has been appointed to review the Company’s compliance with the DPA, particularly in relation to the Company’s international sales practices, for at least the first 18 months of the three year term of the DPA. The Company agreed to pay a monetary penalty of $17.3 million to resolve the charges brought by the DOJ. The terms of the DPA and the associated monetary penalty reflect the Company’s full cooperation throughout the investigation.
The Company contemporaneously reached a Consent Agreement with the SEC to settle civil claims related to this matter. As part of the Consent Agreement, Biomet agreed to the SEC’s entry of a Final Judgment requiring Biomet to disgorge profits and pay prejudgment interest in the aggregate amount of $5.6 million.
From time to time, we have been, and may be in the future, the subject of additional investigations. If, as a result of these investigations described above or any additional investigations, we are found to have violated one or more applicable laws, our business, financial condition, results of operations and cash flows could be materially adversely affected. If some of our existing business practices are challenged as unlawful, we may have to modify those practices, which could have a material adverse effect on our business, financial condition, results of operations and cash flows.
Compliance with the terms of the DPA requires cooperation by many employees and others and may divert substantial financial and human resources from our other business activities.
On March 26, 2012, Biomet entered into the DPA with the DOJ related to the DOJ’s FCPA investigation. Pursuant to the Deferred Prosecution Agreement, the DOJ has agreed not to prosecute the Company in connection with this matter, provided that the Company satisfies its obligations under the agreement over the next three years. In addition, pursuant to the terms of the Deferred Prosecution Agreement, an independent external compliance monitor has been appointed to review the Company’s compliance with the Deferred Prosecution Agreement, particularly in relation to the Company’s international sales practices, for at least the first 18 months of the three year term of the Deferred Prosecution Agreement.
Compliance with this agreement requires substantial cooperation of our employees, distributors and sales agents and the healthcare professionals with whom they interact. These efforts not only involve expense, but also require management and other key employees to focus extensively on these matters.
12
We could be subject to further governmental investigations or actions by other third parties as a result of our settlement with the DOJ and the Office of the Inspector General of the U.S. Department of Health and Human Services (“OIG-HHS”).
We are subject to various federal and state laws concerning healthcare fraud and abuse, including false claims laws and anti-kickback laws. Violations of these laws are punishable by criminal and/or civil sanctions, including, in some instances, fines, imprisonment and, within the United States, exclusion from participation in government healthcare programs, including Medicare, Medicaid and Veterans Administration (“VA”) health programs. These laws are administered by, among others, the DOJ, the OIG-HHS and state attorneys general. Many of these agencies have increased their enforcement activities with respect to medical device manufacturers in recent years.
As a result of our settlement with the DOJ and SEC related to the FCPA investigation described above, we may be subject to further governmental investigations by foreign governments or other claims by third parties arising from the conduct subject to the investigation.
We intend to review and take appropriate actions with respect to any such investigations or proceedings; however, we cannot assure you that the costs of defending or fines imposed in resolving those civil or criminal investigations or proceedings would not have a material adverse effect on our financial condition, results of operations and cash flows.
The current global economic uncertainties may adversely affect our results of operations.
Our results of operations could be substantially affected not only by global economic conditions, but also by local operating and economic conditions, which can vary substantially by market. Unfavorable conditions can depress sales in a given market and may result in actions that adversely affect our margins, constrain our operating flexibility or result in charges which are unusual or non-recurring. Certain macroeconomic events, such as the current adverse conditions in the global economy, including most recently with the market disruptions caused by the economic and political challenges facing specific Eurozone countries such as Greece, Ireland, Italy, Portugal, and Spain, could have a more wide-ranging and prolonged impact on the general business environment, which could also adversely affect us. These economic developments could affect us in numerous ways, many of which we cannot predict. Among the potential effects could be an increase in our variable interest rates, an inability to access credit markets should we require external financing, and further impairments of our goodwill and other intangible assets. In addition, it is possible that further deteriorating economic conditions, and resulting federal budgetary concerns, could prompt the federal government to make significant changes in the Medicare program, which could adversely affect our results of operations. We are unable to predict the likely duration and severity of the current disruption in financial markets and adverse economic conditions, or the effects these disruptions and conditions could have on us.
We are subject to cost-containment efforts of group purchasing organizations, which may have a material adverse effect on our financial condition, results of operations and cash flows.
Many customers of our products have joined or developed group purchasing organizations in an effort to contain costs. Group purchasing organizations negotiate pricing arrangements with medical supply manufacturers and distributors, and these negotiated prices are made available to a group purchasing organization’s affiliated hospitals and other members. If we are not one of the providers selected by a group purchasing organization, affiliated hospitals and other members may be less likely to purchase our products, and if the group purchasing organization has negotiated a strict compliance contract for another manufacturer’s products, we may be precluded from making sales to members of the group purchasing organization for the duration of the contractual arrangement. We have observed a trend in accelerating average sales price declines due to bundled purchases through group purchasing organizations. Our failure to respond to the cost-containment efforts of group purchasing organizations may cause us to lose market share to our competitors and could have a material adverse effect on our sales, financial condition, results of operations and cash flows.
We conduct a significant amount of our sales activity outside of the United States, which subjects us to additional business risks and may adversely affect our results due to increased costs.
During the nine months ended February 28, 2013 and the fiscal year ended May 31, 2012, we derived approximately 38% and 40% of our net sales from sales of our products outside of the United States, respectively. We intend to continue to pursue growth opportunities in sales internationally, which could expose us to additional
13
risks associated with international sales and operations. Our international operations are, and will continue to be, subject to a number of risks and potential costs, including:
| • | changes in foreign medical reimbursement policies and programs; |
| • | unexpected changes in foreign regulatory requirements; |
| • | differing local product preferences and product requirements; |
| • | diminished protection of intellectual property in some countries outside of the United States; |
| • | differing payment cycles; |
| • | trade protection measures, import or export licensing requirements and compliance with economic sanctions laws and regulations; |
| • | the application of U.S. and U.K. regulatory and anti-corruption laws to our international operations; |
| • | difficulty in staffing, training and managing foreign operations; |
| • | differing legal regulations and labor relations; |
| • | potentially negative consequences from changes in tax laws (including potential taxes payable on earnings of foreign subsidiaries upon repatriation); and |
| • | political and economic instability. |
In addition, we are subject to risks arising from currency exchange rate fluctuations, which could increase our costs and may adversely affect our results. The U.S. dollar value of our foreign-generated revenues varies with currency exchange rate fluctuations. Measured in local currency, the majority of our foreign-generated revenues were generated in Europe. Significant increases in the value of the U.S. dollar relative to foreign currencies could have a material adverse effect on our results of operations.
Recently, there have been widely publicized concerns with respect to the overall stability of the Euro as a single currency, given the economic and political challenges facing several Eurozone countries, including Greece, Ireland, Italy, Portugal and Spain. The collapse of the Euro as a common European currency, the withdrawal of one or more member countries from the EU or continuing deterioration in the creditworthiness of the Eurozone countries could adversely affect the Company’s revenues, financial condition or results of operations.
Any of these factors may, individually or collectively, have a material adverse effect on our business, financial condition, results of operations and cash flows. In addition, we are subject to risks arising from currency exchange rate fluctuations, which could increase our costs and may adversely affect our results. The U.S. dollar value of our foreign-generated revenues varies with currency exchange rate fluctuations. Measured in local currency, the majority of our foreign-generated revenues were generated in Europe. Significant increases in the value of the U.S. dollar relative to foreign currencies could have a material adverse effect on our results of operations.
We conduct manufacturing operations outside of the United States and are in the process of transitioning certain manufacturing operations to China, which will expose us to additional business risks.
In addition to our principal executive offices, we maintain more than 50 other manufacturing facilities, offices and warehouse facilities in various countries and regions, including Canada, Europe, Asia Pacific and Latin America.
We currently conduct operations in Jinhua, Zhejiang Province, China and Changzhou, Jiangsu Province, China. Our future business strategy may involve the operation of other manufacturing facilities in China. As a result of this initiative, we will be exposed to all the risks inherent in operating in an emerging market like China. In recent years the Chinese economy has undergone various developments, including beginning the transition from a more heavily government influenced-planned economy to a more market- oriented economy. Despite this transition, the
14
Chinese government continues to own significant production assets and exercises significant control over economic growth. Our international operations, including our planned expansion in China, may be subject to greater or new political, legal and economic risks than those faced by our operations in the United States, including such risks as those arising from:
| • | unexpected changes in foreign or domestic legal, regulatory or governmental requirements or approvals, such as those related to taxation, lending, import and tariffs, environmental regulations, land use rights, intellectual property and other matters; |
| • | unexpected increases in taxes, tariffs and other assessments; |
| • | diminished protection of intellectual property; |
| • | trade protection measures and import or export licensing requirements; |
| • | difficulty in staffing, training and managing foreign operations; |
| • | differing legal and labor regulations; |
| • | political and economic instability; and |
| • | operating in a market with a less developed supply chain, transportation and distribution infrastructure. |
Due to these inherent risks, there can be no assurance that we will achieve any anticipated benefits from transitioning manufacturing operations to China and any of these factors may, individually or collectively, have a material adverse effect on our business, financial condition, results of operations and cash flows.
Our business and financial performance may be adversely affected by our inability to effectively implement our global reconstructive product reorganization initiative.
As of the fourth quarter of fiscal year 2011, we commenced a global reconstructive products reorganization program. The program includes the reorganization of our domestic and international reconstructive products corporate structure. Projected costs and savings associated with this program are subject to a variety of risks, including contemplated costs to implement this program may exceed estimates and the loss of skilled employees in connection with this program.
While we expect to continue to implement this program, there can be no assurance that we will be able to do so successfully or that we will realize the projected benefits of this initiative. If we are unable to realize the anticipated benefits and efficiencies of the reorganization program, our business may be adversely affected. Moreover, our continued implementation of our reorganization program may have a material adverse effect on our business, financial condition, results of operations and cash flows.
If pricing pressures cause us to decrease prices for our goods and services and we are unable to compensate for such reductions through product mix and reductions to our expenses, our results of operations will suffer.
We may experience decreasing prices for our goods and services we offer due to pricing pressure exerted by our customers in response to increased cost containment efforts from managed care organizations and other third-party payors and increased market power of our customers as the medical device industry consolidates. If we are unable to offset such price reductions through product mix or reductions in our expenses, our business, financial condition, results of operations and cash flows will be adversely affected.
Quality problems with our manufacturing processes or our goods and services could significantly and adversely affect both our reputation for producing high-quality products and our results of operations.
Our ability to manufacture and supply high-quality goods and services is critical to the marketing success of our goods and services. If we fail to satisfy our ISO quality standards, our reputation could be significantly harmed, resulting in the loss of customers and market share and significantly and adversely affecting our business, financial condition, results of operations and cash flows.
15
Inventory may become obsolete due to shortened product life cycles, reduced product demand or changes in market conditions, resulting in inventory write-downs that may adversely affect our results of operations, possibly materially.
In our industry, inventory is routinely placed at hospitals to provide the healthcare provider with the appropriate product when needed. Because product usage tends to follow a bell curve, larger and smaller sizes of inventory are provided, but infrequently used. In addition, the musculoskeletal market is highly competitive, with new products, raw materials and procedures being introduced continually, which may make those products currently on the market obsolete. We make estimates regarding the future use of these products and provide a provision for excess and obsolete inventory. If actual product life cycles, product demand or market conditions are less favorable than those projected by management, additional inventory write-downs may be required, which would adversely affect our business, financial condition, results of operations and cash flows.
Our business may be harmed as a result of product liability litigation.
Our involvement in the manufacture and sale of medical devices creates exposure to risks of product liability claims, particularly in the United States. In the past, we have received product liability claims relating to our products and anticipate that we will continue to receive claims in the future, some of which could have a material adverse impact on our business. In addition, we could experience a material design or manufacturing failure in our products, a quality system failure, other safety issues or heightened regulatory scrutiny that would warrant a recall of some of our products. Our existing product liability insurance coverage may be inadequate to satisfy liabilities we might incur. Moreover, even if any product liability loss is covered by an insurance policy, these policies have substantial self-insured retentions or deductibles that we remain responsible for. If a product liability claim or series of claims is brought against us for uninsured liabilities or is in excess of our insurance coverage limits, our business could suffer and our financial condition, results of operations and cash flow could be materially adversely impacted.
We have received claims for personal injury associated with our metal-on-metal hip products. The pre-trial management of certain of these claims has been consolidated in a federal court in South Bend, Indiana. Certain other claims are pending in various state courts. The number of claims continues to increase incrementally, we believe due to the negative publicity regarding metal-on-metal hip products generally. We believe we have data that supports the efficacy and safety of our metal-on-metal hip products, and we intend to vigorously defend ourselves in these matters. We currently account for these claims in accordance with our standard product liability accrual methodology on a case by case basis. Management does not believe that the outcome of the currently reported claims will have a material adverse effect on our consolidated financial positions or results of operations. However, we are unable to estimate the impact of future potential claims.
We may be subject to intellectual property litigation and infringement claims, which could cause us to incur significant expenses or prevent us from selling our products.
The musculoskeletal products industry is highly litigious with respect to the enforcement of patents and other intellectual property rights. In some cases, intellectual property litigation may be used to gain a competitive advantage. We have in the past and may in the future become a party to lawsuits involving patents or other intellectual property. A legal proceeding, regardless of the outcome, could put pressure on our financial resources and divert the time, energy and efforts of our management.
A successful claim of patent or other intellectual property infringement against us could adversely affect our growth and results of operations, in some cases materially. From time to time, we receive notices from third parties of potential infringement and receive claims of potential infringement. We may be unaware of intellectual property rights of others that may cover some of our technology. If someone claims that our products infringed their intellectual property rights, any resulting litigation could be costly and time consuming and would divert the attention of management and key personnel from other business issues.
The complexity of the technology involved and the uncertainty of intellectual property litigation increase these risks. Claims of intellectual property infringement also might require us to enter into costly royalty or license agreements. However, we may be unable to obtain royalty or license agreements on terms acceptable to us or at all. We also may be subject to significant damages or an injunction preventing us from manufacturing, selling or using some of our products in the event of a successful claim of patent or other intellectual property infringement. Any of these adverse consequences could have a material adverse effect on our business, financial condition, results of operations and cash flows.
16
In January 2009, Heraeus Kulzer GmbH initiated legal proceedings in Germany against Biomet, Inc. and our subsidiary, Biomet Europe BV, alleging that we and Biomet Europe BV misappropriated Heraeus Kulzer trade secrets when developing our new lines of European bone cements. The lawsuit seeks damages in excess of €30.0 million and injunctive relief to preclude us from producing our current line of European bone cements. On December 20, 2012, the trial court ruled that Biomet did not misappropriate trade secrets and consequently dismissed Biomet, Inc., Biomet Europe BV, Biomet Deutschland GmbH and other defendants from the lawsuit. Biomet Orthopaedics Switzerland GmbH (“Biomet Switzerland”) remains as the only defendant in the lawsuit and as to it the trial court has ruled that Heraeus Kulzer will not be permitted to review certification materials of Biomet Switzerland for purposes of determining whether there is any evidence that would support a claim of trade secret misappropriation by that entity. The trial court’s decision remains subject to appeal by Heraeus Kulzer and we are continuing to vigorously defend this matter. We can make no assurance as to the final outcome of this matter.
On May 3, 2013, Bonutti Skeletal Innovations LLC, a company formed to hold certain patents acquired from Dr. Peter M. Bonutti and an affiliate of patent licensing firm Acacia Research Group LLC, filed suit against us in the U.S. District Court for the Eastern District of Texas, alleging a failure to pay royalties due under a license agreement with Dr. Bonutti , misuse of confidential information and infringement of U.S. Patent Nos. 5,921,986; 6,099,531; 6,423,063; 6,638,279; 6,702,821; 7,070,557; 7,087,073; 7,104,996; 7,708,740; 7,806,896; 7,806,897; 7,828,852; 7,931,690; 8,133,229; and 8,147,514. The lawsuit seeks damages in an amount yet to be determined and injunctive relief. Previous to the filing of this lawsuit, on March 8, 2013 we had filed a complaint for declaratory judgment with the U.S. District Court for the Northern District of Indiana seeking a judgment of non-infringement and invalidity of the patents at issue. We are vigorously defending this matter and believe that our defenses against infringement are valid and meritorious. We can make no assurances as to the time or resources that will be needed to devote to this litigation or its final outcome.
The conditions of the U.S. and international capital markets may adversely affect our ability to draw on our current revolving credit facilities as well as the value of certain of our investments.
We believe that our cash, other liquid assets and operating cash flow, together with available borrowings and potential access to credit and capital markets, will be sufficient to meet our operating expenses, research and development costs and capital expenditures and service our debt requirements as they become due. However, our ongoing ability to meet our substantial debt service and other obligations will be dependent upon our future performance, which will be subject to business, financial and other factors. We will not be able to control many of these factors, such as economic conditions in the markets where we operate and pressure from competitors. We cannot be certain that our cash flow will be sufficient to allow us to pay principal and interest on our debt, support our operations and meet our other obligations. If we do not have enough money, we may be required to refinance all or part of our existing debt, sell assets or borrow more money. We may not be able to do so on terms acceptable to us, if at all. In addition, the terms of existing or future debt agreements may restrict us from pursuing any of these alternatives.
If financial institutions that have extended credit commitments to us are adversely affected by the conditions of the U.S. and international capital markets, they may become unable to fund borrowings under their credit commitments to us, which could have a material adverse impact on our financial condition and our ability to borrow additional funds, if needed, for working capital, capital expenditures, acquisitions, research and development and other corporate purposes.
Loss of our key management and other personnel, or an inability to attract such management and other personnel, could impact our business.
We depend on our senior managers and other key personnel to run our business and on technical experts to develop new products and technologies. The loss of any of these senior managers or other key personnel could adversely affect our operations. Competition for qualified employees is intense, and the loss of qualified employees or an inability to attract, retain and motivate additional highly skilled employees required for the management, operation and expansion of our business could hinder our ability to expand, conduct research and development activities successfully and develop marketable products.
If we fail to retain our existing relationships with our independent sales agents and distributors or establish relationships with different agents and distributors, our results of operations may be negatively impacted.
Our revenues and profitability depend largely on the ability of independent sales agents and distributors to sell our products to customers. Typically, these agents and distributors have developed long-standing relationships
17
with our customers and provide our customers with the necessary training and product support relating to our products. If we fail to retain our existing relationships with these agents and distributors or establish relationships with different agents and distributors, our results of operations may be negatively impacted.
We may record future goodwill and/or intangible impairment charges related to one or more of our reporting units, which could materially adversely impact our results of operations.
We test our goodwill and indefinite lived intangible asset balances as of March 31 of each fiscal year for impairment. We test these balances more frequently if indicators are present or changes in circumstances suggest that impairment may exist. In evaluating the potential for impairment we make assumptions regarding revenue projections, growth rates, cash flows, tax rates and discount rates. These assumptions are uncertain and by nature can vary from actual results. Various future events could have a negative impact on the fair value of our reporting units’ goodwill and indefinite lived intangibles when the annual or interim impairment test is completed. The events include, but are not limited to:
| • | our ability to sustain sales and earnings growth; |
| • | the effect of anticipated changes in the size, health and activities of the population or on the demand for our products; |
| • | our ability and intent to expand in key international markets; |
| • | the timing and anticipated outcome of clinical studies; |
| • | assumptions concerning anticipated product developments and emerging technologies; |
| • | our continued investment in new products and technologies; |
| • | the ultimate marketability of products currently being developed; |
| • | our success in achieving timely approval or clearance of our products with domestic and foreign regulatory entities; and |
| • | the stability of certain foreign economic markets. |
We recorded a goodwill and intangible asset impairment charge of $334.1 million in the third quarter of fiscal year 2013 that was related to our Dental Reconstructive reporting unit, primarily due to declining industry market growth rates in certain European and Asia Pacific markets and corresponding unfavorable margin trends.
We have identified a total of four reporting units with a material amount of goodwill that are at a higher risk of potential failure of step one of the goodwill impairment test in the future. These reporting units include our U.S. Reconstructive reporting unit ($2,973.4 million of goodwill), our International reporting unit ($523.5 million of goodwill), our dental reconstructive reporting unit ($66.3 million of goodwill) and our Europe reporting unit ($299.4 million). The level of excess fair value over carrying value for these higher risk reporting units were each less than 10% for the latest step one impairment test.
A natural or man-made disaster could have a material adverse effect on our business.
We have 14 manufacturing operations located throughout the world. However, a significant portion of our products are produced at and shipped from our facility in Warsaw, Indiana. In the event that this facility is severely damaged or destroyed as a result of a natural or man-made disaster, we would be forced to shift production to our other facilities and/or rely on third-party manufacturers. Our existing business interruption insurance coverage may be inadequate to satisfy liabilities we might incur in such a situation. If a business interruption claim or series of claims is in excess of our insurance coverage limits, or is not otherwise covered in whole or in part by our insurance coverage, our business could suffer and our financial condition, results of operations and cash flow could be materially adversely impacted.
Failure to successfully integrate acquired businesses into our operations or to otherwise successfully execute strategic transactions could adversely affect our business.
18
We may, from time to time, consider and take advantage of selected opportunities to grow by acquiring businesses whose operations or product lines fit well within our existing businesses or whose geographic location or market position would enable us to expand into new markets. Our ability to implement this expansion strategy will, however, depend on whether any suitable businesses are available at suitable valuations, how much money we can spend and maintaining our customer base. Any acquisition that we make could be subject to a number of risks, including failing to discover liabilities of the acquired company for which we may be responsible as a successor owner or operator despite any investigation we may make before the acquisition, our inability to assimilate the operations and personnel of the acquired company, the loss of key personnel in the acquired company and any adverse impact on our financial statements from the amortization of acquired intangible assets or the creation of reserves or write-downs. We may not be able to adequately meet these challenges, and any failure to do so could adversely affect our business, financial condition, results of operations and cash flows. In addition, if we incur additional indebtedness to finance these acquisitions, the related risks we face from our already substantial level of indebtedness could intensify.
On June 15, 2012, we announced the initial closing of the previously announced $280.0 million acquisition of the worldwide trauma business of DePuy Orthopaedics, Inc. During the first and second quarters of fiscal year 2013, subsequent closings in various foreign countries occurred on a staggered basis, with the final closing occurring on December 7, 2012. Our integration of the operations of the acquired business requires significant efforts, including the coordination of complex information technology environments, research and development, sales and marketing, operations, manufacturing and finance.
The integration efforts related to the DePuy Trauma acquisition require significant expenses and involve significant amounts of management’s time that cannot be dedicated to other initiatives. We may not be able to adequately meet these challenges, and any failure to do so could adversely affect our business, financial condition, results of operations and cash flows.
We are increasingly dependent on sophisticated information technology and if we fail to properly maintain the integrity of our data, our business could be adversely affected.
We are increasingly dependent on sophisticated information technology for our products and infrastructure. As a result of technology upgrades, recently enacted regulations, improvements in our system platforms and integration of new business acquisitions, we have been consolidating and integrating the number of systems we operate and have upgraded and expanded our information systems capabilities. Our information systems require an ongoing commitment of significant resources to maintain, protect, and enhance existing systems and keep information technology systems current. In addition, our obligations to protect patient and customer information have increased significantly. Third parties may attempt to hack into our products or systems and may obtain data relating to patients with our products or our proprietary information. If we fail to maintain or protect our information systems and data integrity effectively, we could lose existing customers, have difficulty preventing, detecting, and controlling fraud, have disputes with customers, physicians, and other health care professionals, have regulatory sanctions or penalties imposed, incur expenses or lose revenues as a result of a data privacy breach, or suffer other adverse consequences. There can be no assurance that our process of consolidating the number of systems we operate, upgrading and expanding our information systems capabilities, protecting and enhancing our systems and developing new systems to keep pace with continuing changes in information processing technology will be successful or that additional systems issues will not arise in the future.
Risks Related to Our Indebtedness and the Notes
Our substantial level of indebtedness could materially adversely affect our ability to generate sufficient cash to fulfill our obligations under the notes and any other outstanding indebtedness, our ability to react to changes in our business and our ability to incur additional indebtedness to fund future needs.
We are highly leveraged. As of February 28, 2013, we had total indebtedness of $5,978.4 million (compared to total indebtedness of $5,827.8 million as of May 31, 2012). The following chart shows our level of indebtedness as of February 28, 2013 and May 31, 2012:
19
| (in millions) | February 28, 2013 | May 31, 2012 | |||||
| Debt Instruments | |||||||
| Non-U.S. facility | $ | 2.6 | $ | 3.5 | |||
| Term loan facilities | 3,311.7 | 3,274.3 | |||||
| Cash flow revolving credit facility | — | — | |||||
| Asset-based revolving credit facility | — | — | |||||
| 10% Senior Cash Pay Notes due 2017 | — | 761.0 | |||||
10⅜%/11⅛% Senior PIK Toggle Notes due 2017 | — | 771.0 | |||||
| 11⅝% Senior Subordinated Notes due 2017 | — | 1,015.0 | |||||
| 6.500% Senior Notes due 2020 | 1,825.0 | — | |||||
| 6.500% Senior Subordinated Notes due 2020 | 800.0 | — | |||||
| Premium on notes | 39.1 | 3.0 | |||||
| Total debt | $ | 5,978.4 | $ | 5,827.8 | |||
As of February 28, 2013, we had outstanding approximately $3,311.7 million in aggregate principal amount of indebtedness under our senior secured credit facilities that bears interest at a floating rate.
On August 2, 2012, we entered into an amendment and restatement agreement that amended our existing senior secured credit facilities. The amendment (i) extended the maturity of approximately $1,007.2 million of our U.S. dollar-denominated term loans and approximately €631.3 million of our euro-denominated term loans under the credit facility to July 25, 2017, (ii) refinanced and replaced the previous alternative currency revolving credit commitments under the credit facility with a new class of alternative currency revolving credit commitments in an aggregate amount of $165.0 million and (iii) refinanced and replaced the previous U.S. dollar revolving credit commitments under the credit facility with a new class of U.S. dollar-denominated revolving credit commitments in an aggregate amount of $165.0 million. The new revolving credit commitments will mature on April 25, 2017, except that, if as of December 23, 2014, there is an outstanding aggregate principal amount of non-extended U.S. dollar and euro term loans in excess of $200.0 million, then such revolving credit commitments will mature on December 24, 2014.
The joinder agreement was entered into pursuant to our senior secured credit facility, as amended by the amendment and restatement agreement dated August 2, 2012. By entering into the joinder agreement, the joining lenders party thereto have agreed to extend the maturity of (i) approximately $392.7 million of Biomet’s U.S. dollar-denominated term loans and (ii) approximately €32.9 million of Biomet’s euro-denominated term loans, to July 25, 2017. The term loans extended pursuant to the joinder agreement are on terms identical to the terms loans that were extended pursuant to the prior Amendment. The remaining term loans of the lenders under the senior secured credit facilities who did not elect to extend such loans either pursuant to the August 2 amendment and restatement agreement or the subsequent joinder agreement will continue to mature on March 25, 2015.
On November 14, 2012, we also replaced and refinanced our asset-based revolving credit facility with a new asset-based revolving credit facility that has a U.S. tranche of up to $400.0 million and a European borrower tranche (denominated in euros) of up to the euro-equivalent of $100.0 million. The European borrower tranche is secured by certain foreign assets of European subsidiary borrowers, and the U.S. borrowers under the U.S. tranche guarantee the obligations of any such European subsidiary borrowers (and those guarantees are secured by the current asset collateral that secures the direct obligations of those U.S. borrowers under the U.S. tranche).
In addition, on December 27, 2012, we completed a $730.0 million add-on to our extended U.S. dollar-denominated term loan. The proceeds from the add-on were used to refinance the non-extended U.S. dollar-denominated term B loan, which was net of fees associated with the add-on closing. The terms of the add-on are consistent with the terms in the August 2 amendment.
On August 8, 2012, we completed our offering of $1,000.0 million aggregate principal amount of new senior notes. We used a portion of the proceeds of that offering to fund a tender offer for any and all of our outstanding 10⅜%/11⅛% Senior PIK Toggle Notes due 2017 including related fees and expenses, to redeem the remaining 10⅜%/11⅛% Senior PIK Toggle Notes and to redeem $140.0 million aggregate principal amount of 11⅝% Senior Subordinated Notes due 2017.
20
On October 2, we completed our offering of $825.0 million aggregate principal amount of additional senior notes and $800.0 million aggregate principal amount of our senior subordinated notes. We used the net proceeds of those offerings, together with cash on hand and other sources, to purchase any and all of our 10% Senior Cash Pay Notes due 2017 and $940 million principal amount of outstanding 11⅝% Senior Subordinated Notes due 2017. On November 1, 2012, we purchased and redeemed all remaining outstanding 11⅝% Senior Subordinated Notes using cash on hand and asset-based revolver proceeds.
We have also entered into a series of interest rate swap agreements to fix the interest rates on approximately 60% of the borrowings under our senior secured credit facilities.
Our substantial level of indebtedness increases the possibility that we may be unable to generate cash sufficient to pay, when due, the principal of, interest on or other amounts due in respect of our indebtedness. Our substantial indebtedness, combined with our other financial obligations and contractual commitments, could have important consequences. For example, it could:
| • | make it more difficult for us to satisfy our obligations with respect to our indebtedness, including the notes, and any failure to comply with the obligations under any of our debt instruments, including restrictive covenants, could result in an event of default under the indentures, any other outstanding notes and the agreements governing such other indebtedness; |
| • | require us to dedicate a substantial portion of our cash flow from operations to payments on our indebtedness, thereby reducing funds available for working capital, capital expenditures, acquisitions, research and development and other purposes; |
| • | increase our vulnerability to adverse economic and industry conditions, which could place us at a competitive disadvantage compared to our competitors that have relatively less indebtedness; |
| • | increase the risk we assess with our counterparties which could affect the fair value of our derivative instruments related to our debt facilities noted above; |
| • | limit our flexibility in planning for, or reacting to, changes in our business and the industries in which we operate; |
| • | limit our noteholders’ rights to receive payments under the notes and any other outstanding notes if secured creditors have not been paid; |
| • | limit our ability to borrow additional funds, or to dispose of assets to raise funds, if needed, for working capital, capital expenditures, acquisitions, research and development and other corporate purposes; and |
| • | prevent us from raising the funds necessary to repurchase all notes tendered to us upon the occurrence of certain changes of control, which would constitute a default under the indentures. |
Restrictions imposed by the indentures, our senior secured credit facilities and our other outstanding indebtedness may limit our ability to operate our business and to finance our future operations or capital needs or to engage in other business activities.
The agreements governing our indebtedness, including the indentures, contain various covenants that limit our discretion in the operation of our business and also require us to meet financial maintenance tests and other covenants. The failure to comply with such tests and covenants could have a material adverse effect on us. The agreements governing our indebtedness, including the indentures, restrict our and our restricted subsidiaries’ ability, among other things, to:
| • | incur additional indebtedness; |
| • | pay dividends on our capital stock or redeem, repurchase or retire our capital stock or indebtedness; |
| • | make investments, loans, advances and acquisitions; |
21
| • | create restrictions on the payment of dividends or other amounts to us from our restricted subsidiaries; |
| • | engage in transactions with our affiliates; |
| • | sell assets, including capital stock of our subsidiaries; |
| • | consolidate or merge; |
| • | create liens; and |
| • | enter into sale and lease-back transactions. |
The terms of our senior secured credit facilities also restrict Parent from conducting any business or operations other than, among others, (i) owning Biomet, Inc., (ii) maintaining its legal existence, (iii) performing its obligations with respect to the senior secured credit facilities and the indentures governing the notes, (iv) publicly offering its common stock, (v) financing activities, including the issuance of securities, incurrence of debt, payment of dividends, making contributions to the capital of its subsidiaries and guaranteeing the obligations of its subsidiaries, or (vi) providing indemnification to its officers and directors.
In addition, although the agreements governing our senior secured credit facilities and the indentures governing the notes do not require us to comply with any financial ratio maintenance covenants, if Excess Global Availability (as that term is defined in the asset-based revolving credit facility) is less than 10% of the sum of (1) aggregate commitments under our asset-based revolving credit facility plus (2) the revolving credit commitments under our cash flow credit facility at any time, the fixed charge coverage ratio as of the end of the most recently ended fiscal quarter must be greater than or equal to 1.00 to 1.00. In the event of a default under any of our senior secured credit facilities, the lenders could elect to declare all amounts outstanding under the agreements governing our senior secured credit facilities to be immediately due and payable. If the indebtedness under our senior secured credit facilities, or the notes were to be accelerated, our assets may not be sufficient to repay such indebtedness in full. In particular, noteholders will be paid only if we have assets remaining after we pay amounts due on our secured indebtedness, including our senior secured credit facilities.
We, including our subsidiaries, have the ability to incur substantially more indebtedness, including senior secured indebtedness, and our noteholders’ right to receive payments on each series of notes is effectively junior to the right of lenders who have a security interest in our assets to the extent of the value of those assets.
Our obligations under the notes and our guarantors’ obligations under their guarantees of the notes are unsecured, but our obligations under our senior secured credit facilities and each guarantor’s obligations under its guarantee of our senior secured credit facilities are secured by a security interest in substantially all of our domestic tangible and intangible assets, including the stock of substantially all of our wholly-owned U.S. subsidiaries and a portion of the stock of certain of our non-U.S. subsidiaries. If we are declared bankrupt or insolvent, or if we default under our senior secured credit facilities, the lenders could declare all of the funds borrowed thereunder, together with accrued interest, immediately due and payable. If we were unable to repay such indebtedness, the lenders could foreclose on the pledged assets to the exclusion of holders of the notes, even if an event of default exists at such time under the indentures. Furthermore, if the lenders foreclose and sell the pledged equity interests in any guarantor under the notes, then that guarantor will be released from its guarantee of the notes automatically and immediately upon such sale. In any such event, because the notes are not secured by any of our assets or the equity interests in the guarantors, it is possible that there would be no assets remaining from which noteholders’ claims could be satisfied or, if any assets remained, they might be insufficient to satisfy noteholders’ claims in full.
Subject to the restrictions in our senior secured credit facilities and the indentures, we, including our subsidiaries, may incur significant additional indebtedness. As of February 28, 2013:
| • | we and the guarantors had approximately $330.0 million available for borrowing under our cash flow revolving credit facilities, which, if borrowed, would be senior secured indebtedness; |
| • | we and the guarantors had $465.5 million available for borrowing under our asset-based revolving credit facility, subject to borrowing base limitations, which, if borrowed, would be senior secured indebtedness; |
22
| • | we and the guarantors have the option to incur additional incremental term loans or increase the cash flow revolving credit facilities commitments under our senior secured credit facilities up to an amount that would cause our Senior Secured Leverage Ratio (as defined in our senior secured credit facilities) to be equal to or less than 4.50 to 1.00, which, if borrowed, would be senior secured indebtedness; and |
| • | we and the guarantors have the option to increase the asset-based revolving credit facility commitments under our asset-based revolving credit facility by up to $100.0 million, which, if borrowed, would be senior secured indebtedness. |
Although the terms of our senior secured credit facilities and the indentures contain restrictions on the incurrence of additional indebtedness, these restrictions are subject to a number of important exceptions, and indebtedness incurred in compliance with these restrictions could be substantial. If we and our restricted subsidiaries incur significant additional indebtedness, the related risks that we face could intensify.
We may not be able to generate sufficient cash to service all of our indebtedness, including the notes, and may be forced to take other actions to satisfy our obligations under our indebtedness, which may not be successful.
Our ability to make scheduled payments on or to refinance our debt obligations depends on our financial condition and operating performance, which is subject to prevailing economic and competitive conditions and to certain financial, business and other factors beyond our control. We may not be able to maintain a level of cash flows from operating activities sufficient to permit us to pay the principal, premium, if any, and interest on our indebtedness, including the notes.
If our cash flows and capital resources are insufficient to fund our debt service obligations, we may be forced to reduce or delay investments and capital expenditures or to sell assets, seek additional capital or restructure or refinance our indebtedness, including the notes. Our ability to restructure or refinance our debt will depend on the condition of the capital markets and our financial condition at such time. Any refinancing of our debt could be at higher interest rates and may require us to comply with more onerous covenants, which could further restrict our business operations. The terms of existing or future debt instruments, including the indentures, may restrict us from adopting some of these alternatives. In addition, any failure to make payments of interest and principal on our outstanding indebtedness on a timely basis would likely result in a reduction of our credit rating, which could harm our ability to incur additional indebtedness. In the absence of such operating results and resources, we could face substantial liquidity problems and might be required to dispose of material assets or operations to meet our debt service and other obligations. Our senior secured credit facilities and the indentures restrict our ability to dispose of assets and use the proceeds from the disposition. We may not be able to consummate those dispositions or to obtain the proceeds that we could realize from them and these proceeds may not be adequate to meet any debt service obligations then due. These alternative measures may not be successful and may not permit us to meet our scheduled debt service obligations.
Repayment of our debt, including the notes, is dependent on cash flow generated by our subsidiaries.
Our subsidiaries own a significant portion of our assets and conduct a significant portion of our operations. Accordingly, repayment of our indebtedness, including the notes, is dependent, to a significant extent, on the generation of cash flow by our subsidiaries and their ability to make such cash available to us, by dividend, debt repayment or otherwise. Unless they are guarantors of the notes, our subsidiaries do not have any obligation to pay amounts due on the notes or to make funds available for that purpose. Our subsidiaries may not be able to, or may not be permitted to, make distributions to enable us to make payments in respect of our indebtedness, including the notes. Each subsidiary is a distinct legal entity and, under certain circumstances, legal and contractual restrictions may limit our ability to obtain cash from our subsidiaries. While the indentures limit the ability of our subsidiaries to incur consensual restrictions on their ability to pay dividends or make other intercompany payments to us, these limitations are subject to certain qualifications and exceptions. In the event that we do not receive distributions from our subsidiaries, we may be unable to make required principal and interest payments on our indebtedness, including the notes.
Claims of noteholders will be structurally subordinated to claims of creditors of all our non-U.S. subsidiaries and some of our U.S. subsidiaries because they will not guarantee the notes.
The notes are not guaranteed by any of our non-U.S. subsidiaries or any of our less than wholly-owned U.S. subsidiaries. Accordingly, claims of holders of the notes will be structurally subordinated to the claims of
23
creditors of these non-guarantor subsidiaries, including trade creditors. Therefore, all obligations of our non-guarantor subsidiaries will have to be satisfied before any of the assets of such subsidiaries would be available for distribution, upon a liquidation or otherwise, to us or a guarantor of the notes.
For the nine months ended February 28, 2013 and February 29, 2012, our non-guarantor subsidiaries accounted for $830.4 million, or 37% of our consolidated net sales, and $782.5 million, or 37% of our consolidated net sale, respectively. As of February 28, 2013, our non-guarantor subsidiaries accounted for approximately $2,806.9 million, or 28%, of our consolidated assets and approximately $409.6 million, or 5.3%, of our total consolidated liabilities.
For the fiscal years ended May 31, 2012 and 2011, our non-guarantor subsidiaries accounted for $1,068.3 million, or 38% of our consolidated net sales and $1,015.7 million, or 37% of our consolidated net sales, respectively. As of May 31, 2012 and 2011, our non-guarantor subsidiaries accounted for approximately $2,734.3 million, or 26%, and $3,236.1 million, or 28%, of our consolidated assets, respectively, and approximately $413.1 million, or 5.3%, and $587.9 million, or 7.2%, of our consolidated liabilities, respectively. All amounts are presented after giving effect to intercompany eliminations.
The lenders under our senior secured credit facilities will have the discretion to release any guarantors under these facilities in a variety of circumstances, which will cause those guarantors to be released from their guarantees of the notes.
While any obligations under our senior secured credit facilities remain outstanding, any guarantee of the notes may be released without action by, or consent of, any holder of the notes or the trustee under the indentures, at the discretion of lenders under our senior secured credit facilities, if the related guarantor is no longer a guarantor of obligations under our senior secured credit facilities or any other indebtedness. The lenders under our senior secured credit facilities will have the discretion to release the guarantees under our senior secured credit facilities in a variety of circumstances. Noteholders will not have a claim as a creditor against any subsidiary that is no longer a guarantor of the notes, and the indebtedness and other liabilities, including trade payables, whether secured or unsecured, of those subsidiaries will effectively be senior to claims of noteholders.
If we default on our obligations to pay our other indebtedness, we may not be able to make payments on the notes.
Any default under the agreements governing our indebtedness, including a default under our senior secured credit facilities that is not waived by the required lenders, and the remedies sought by the holders of such indebtedness, could prevent us from paying principal, premium, if any, and interest on the notes and substantially decrease the market value of the notes. If we are unable to generate sufficient cash flow and are otherwise unable to obtain funds necessary to meet required payments of principal, premium, if any, and interest on our indebtedness, or if we otherwise fail to comply with the various covenants, including financial and operating covenants in the instruments governing our indebtedness (including covenants in our senior secured credit facilities and the indentures), we could be in default under the terms of the agreements governing such indebtedness, including our senior secured credit facilities and the indentures.
In the event of such default:
| • | the holders of such indebtedness may be able to cause all of our available cash flow to be used to pay such indebtedness and, in any event, could elect to declare all the funds borrowed thereunder to be due and payable, together with accrued and unpaid interest; |
| • | the lenders under our senior secured credit facilities could elect to terminate their commitments thereunder, cease making further loans and institute foreclosure proceedings against our assets; |
| • | we could be forced into bankruptcy or liquidation; and |
| • | the subordination provisions in senior subordinated notes may prevent us from paying any obligation with respect to such notes. |
If our operating performance declines, we may in the future need to obtain waivers from the required lenders under our senior secured credit facilities to avoid being in default. If we breach our covenants under our
24
senior secured credit facilities and seek a waiver, we may not be able to obtain a waiver from the required lenders. If this occurs, we would be in default under our senior secured credit facilities, the lenders could exercise their rights, as described above, and we could be forced into bankruptcy or liquidation.
Your right to receive payments on the exchange senior subordinated notes will be junior to the rights of the lenders under our senior secured credit facilities and all of our other senior debt (including the exchange senior notes) and any of our future senior indebtedness.
The senior subordinated notes will be general unsecured senior subordinated obligations that will rank junior in right or payment to all of our existing and future senior indebtedness. We may not pay principal, premium, if any, interest or other amounts on account of the notes in the event of a payment default or certain other defaults in respect of certain of our senior indebtedness, including the exchange senior notes and borrowings under our senior secured credit facilities, unless the senior indebtedness has been paid in full or the default has been cured or waived. In addition, in the event of certain other defaults with respect to certain of our senior indebtedness, we may not be permitted to pay any amount on account of the notes for a designated period of time.
Because of the subordination provisions in the indenture governing the senior subordinated notes, in the event of our bankruptcy, liquidation or dissolution, our assets will not be available to pay obligations under the senior subordinated notes until we have made all payments in cash on our senior indebtedness, see “Description of Exchange Senior Subordinated Notes.” Sufficient assets may not remain after all these payments have been made to make any payments on the senior subordinated notes, including payments of principal or interest when due. See “Description of Other Indebtedness” for a description of the outstanding indebtedness that will rank senior to the senior subordinated notes.
We may not be able to repurchase the notes upon a change of control.
Upon the occurrence of specific kinds of change of control events, we will be required to offer to repurchase all outstanding notes at 101% of their principal amount plus accrued and unpaid interest, if any. The source of funds for any such purchase of the notes will be our available cash or cash generated from our subsidiaries’ operations or other sources, including borrowings, sales of assets or sales of equity. We may not be able to repurchase the notes upon a change of control because we may not have sufficient financial resources to purchase all of the notes that are tendered upon a change of control. Further, we will be contractually restricted under the terms of our senior secured credit facilities from repurchasing all of the notes tendered by holders upon a change of control. Accordingly, we may not be able to satisfy our obligations to purchase the notes unless we are able to refinance or obtain waivers under our senior secured credit facilities. Our failure to repurchase the notes upon a change of control would cause a default under the indentures and a cross default under our senior secured credit facilities. Our senior secured credit facilities also provide that a change of control will be a default that permits lenders to accelerate the maturity of borrowings thereunder. Any of our future debt agreements may contain similar provisions.
The trading prices for the notes will be directly affected by many factors, including our credit rating.
Credit rating agencies continually revise their ratings for companies they follow. The condition of the financial and credit markets and prevailing interest rates have fluctuated in the past and are likely to fluctuate in the future. Any such fluctuation may impact the trading price of the notes. In addition, developments in our business and operations could lead to a ratings downgrade which could adversely affect the trading price of the notes, or the trading market for the notes.
An adverse rating of the notes may cause their trading price to fall.
If a rating agency rates the notes, it may assign a rating that is lower than the rating expected by the noteholders. Ratings agencies also may lower ratings on the notes or any of our other debt in the future, or may choose to cease providing ratings on the notes or such other debt. If rating agencies assign a lower than expected rating or reduce, or indicate that they may reduce, their ratings of our debt in the future, the trading price of the notes could significantly decline.
Certain covenants under the indentures will be suspended if and for so long as the notes are rated “investment grade” by both Standard & Poor’s and Moody’s and no default has occurred and is continuing. These covenants restrict, among other things, our and our restricted subsidiaries’ ability to incur or guarantee debt or issue certain stock, pay dividends, make distributions on, or redeem or repurchase, capital stock and enter into
25
transactions with affiliates. Because these restrictions would not apply if the notes are rated investment grade, we would be able to incur additional debt and consummate transactions that may impair our ability to satisfy our obligations with respect to the notes. In addition, we would not have to make certain offers to repurchase the notes. These covenants would be reinstated if the credit ratings assigned to the notes later declined below investment grade or a default occurs and is continuing.
Federal and state fraudulent transfer laws may permit a court to void the notes and the guarantees, subordinate claims in respect of the notes and the guarantees, and require noteholders to return payments received. If this occurs, noteholders may not receive any payments on the notes.
Federal and state fraudulent transfer and conveyance statutes may apply to the issuance of the notes and the incurrence of any guarantees. Under federal bankruptcy law and comparable provisions of state fraudulent transfer or conveyance laws, which may vary from state to state, the notes or guarantees could be voided as a fraudulent transfer or conveyance if (1) we or any of the guarantors, as applicable, issued the notes or incurred the guarantees with the intent of hindering, delaying or defrauding creditors or (2) we or any of the guarantors, as applicable, received less than reasonably equivalent value or fair consideration in return for either issuing the notes or incurring the guarantees and, in the case of (2) only, one of the following is also true at the time thereof:
| • | we or any of the guarantors, as applicable, were insolvent or rendered insolvent by reason of the issuance of the notes or the incurrence of the guarantees; |
| • | the issuance of the notes or the incurrence of the guarantees left us or any of the guarantors, as applicable, with an unreasonably small amount of capital to carry on the business; |
| • | we or any of the guarantors intended to, or believed that we or such guarantor would, incur debts beyond our or such guarantor’s ability to pay such debts as they mature; or |
| • | we or any of the guarantors was a defendant in an action for money damages, or had a judgment for money damages docketed against us or such guarantor if, in either case, after final judgment, the judgment is unsatisfied. |
A court would likely find that we or a guarantor did not receive reasonably equivalent value or fair consideration for the notes or such guarantee if we or such guarantor did not substantially benefit directly or indirectly from the issuance of the notes or the applicable guarantee. As a general matter, value is given for a transfer or an obligation if, in exchange for the transfer or obligation, property is transferred or an antecedent debt is secured or satisfied.
We cannot be certain as to the standards a court would use to determine whether or not we or the guarantors were solvent at the relevant time or, regardless of the standard that a court uses, that the issuance of the guarantees would not be further subordinated to our or any of our guarantors’ other debt. Generally, however, an entity would be considered insolvent if, at the time it incurred indebtedness:
| • | the sum of its debts, including contingent liabilities, was greater than the fair value of all its assets; |
| • | the present fair saleable value of its assets was less than the amount that would be required to pay its probable liability on its existing debts, including contingent liabilities, as they become absolute and mature; or |
| • | it could not pay its debts as they become due. |
If a court were to find that the issuance of the notes or the incurrence of the guarantee was a fraudulent transfer or conveyance, the court could void the payment obligations under the notes or such guarantee or further subordinate the notes or such guarantee to presently existing and future indebtedness of ours or of the related guarantor, or require the holders of the notes to repay any amounts received with respect to such guarantee. In the event of a finding that a fraudulent transfer or conveyance occurred, noteholders may not receive any repayment on the notes. Further, the voidance of the notes could result in an event of default with respect to our and our subsidiaries’ other debt that could result in acceleration of such debt.
26
Although each guarantee entered into by a guarantor will contain a provision intended to limit that guarantor’s liability to the maximum amount that it could incur without causing the incurrence of obligations under its guarantee to be a fraudulent transfer, this provision may not be effective to protect those guarantees from being voided under fraudulent transfer law, or may reduce that guarantor’s obligation to an amount that effectively makes its guarantee worthless.
We are indirectly owned and controlled by the Sponsors, and the Sponsors’ interests as equity holders may conflict with the interests of noteholders as creditors.
We are a subsidiary of Parent, which is controlled by the Sponsors, and, accordingly, the Sponsors have the ability to control our policies and operations. The interests of the Sponsors may not in all cases be aligned with our noteholders’ interests. For example, if we encounter financial difficulties or are unable to pay our debts as they mature, the interests of our equity holders might conflict with our noteholders’ interests. In addition, our equity holders may have an interest in pursuing acquisitions, divestitures, financings or other transactions that, in their judgment, could enhance their equity investments, even though such transactions might involve risks to holders of the notes. Furthermore, the Sponsors may in the future own businesses that directly or indirectly compete with us. The Sponsors also may pursue acquisition opportunities that may be complementary to our business, and as a result, those acquisition opportunities may not be available to us.
Risks Related to the Exchange Offers
The consummation of the exchange offers may not occur.
We are not obligated to complete the exchange offers under certain circumstances. See “The Exchange Offers—Conditions to the Exchange Offers.” Even if the exchange offers are completed, they may not be completed on the schedule described in this prospectus. Accordingly, holders participating in the exchange offers may have to wait longer than expected to receive their exchange notes, during which time those holders of original notes who have tendered original notes in the exchange offer, will not be able to effect transfers of those original notes.
If you fail to exchange the original notes, they will remain subject to transfer restrictions, and it may be harder for you to resell and transfer your original notes.
The original notes were not, and will not be, registered under the Securities Act or under the securities laws of any state. Any original notes that remain outstanding after these exchange offers will continue to be subject to restrictions on their transfer. If you do not exchange your original notes for exchange notes by these exchange offers, or if you do not properly tender your original notes in these exchange offers, you will not be able to resell, offer to resell or otherwise transfer your original notes unless they are registered under the Securities Act or unless you resell them, offer to resell or otherwise transfer them under an exemption from the registration requirements of, or in a transaction not subject to, the Securities Act. After these exchange offers, holders of original notes will not have any further rights to have their original notes exchanged for exchange notes registered under the Securities Act and will not have any right to additional interest in the case of non-registration.
Late deliveries of original notes and failure to follow proper procedures could prevent a holder from exchanging its original notes.
Holders are responsible for complying with all of the procedures of the exchange offers. The issuance of exchange notes in exchange for original notes will only occur upon completion of the procedures described in this prospectus under “The Exchange Offers.” Therefore, holders of original notes who wish to exchange them for exchange notes should allow sufficient time for timely completion of the exchange procedure. Neither we nor the exchange agent are obligated to extend the offer or notify you of any failure to follow the proper procedure.
If you hold your original notes through a broker, dealer, commercial bank, trust company or other nominee, you should keep in mind that such entity may require you to take action with respect to the exchange offers a number of days before the Expiration Date in order for such entity to tender original notes on your behalf at or prior to the Expiration Date.
If you are a broker-dealer or a person that tenders original notes for the purpose of participating in a distribution of the exchange notes, your ability to transfer the exchange notes may be restricted.
27
Broker-dealers that acquired the original notes directly from Biomet, but not as a result of market-making activities or other trading activities, as well as any other person that tenders original notes for the purpose of participating in a distribution of the exchange notes, must comply with all applicable registration and prospectus delivery requirements of the Securities Act in connection with a resale of the exchange notes.
Each broker-dealer that receives exchange notes for its own account pursuant to the exchange offers in exchange for original notes that it acquired as a result of market-making or other trading activities must comply with its prospectus delivery obligations in connection with any resale of the exchange notes. Our obligation to make this prospectus available to broker-dealers is limited. Consequently, we cannot guarantee that a proper prospectus will be available to broker-dealers wishing to resell their exchange notes.
Holders who fail to exchange their original notes may have reduced liquidity after the exchange offers.
As the original notes of any series that are tendered and accepted in the exchange offers will be cancelled, the principal amount of remaining original notes of that series will decrease. This decrease could reduce the liquidity of the trading market for the original notes of that series. We cannot assure you of the liquidity, or even the continuation, of any trading market for the original notes following the completion of the exchange offers.
28
RATIO OF EARNINGS TO FIXED CHARGES
Our ratio of earnings to fixed charges for the nine months ended February 28, 2013 and the years ended May 31, 2012, 2011, 2010, 2009, the periods from July 12, 2007 to May 31, 2008 and June1, 2007 to July 11, 2007 is set forth below.
| Successor | Predecessor | ||||||||||||||||||||||||||
| (in millions) | Nine Months Ended February 28, 2013 | Year Ended May 31, 2012 | Year Ended May 31, 2011 | Year Ended May 31, 2010 | Year Ended May 31, 2009 | Period from July 12, 2007 – May 31, 2008 | Period from June 1, 2007 – July 11, 2007 | ||||||||||||||||||||
| Earnings: | |||||||||||||||||||||||||||
| Earnings (loss) before income taxes …….. | $ | (508.4 | ) | $ | (590.8 | ) | $ | (1,064.6 | ) | $ | (141.7 | ) | $ | (920.4 | ) | $ | (1,194.3 | ) | $ | (81.9 | ) | ||||||
| Add: Fixed charges (per below) …… | $ | 310.8 | $ | 479.8 | $ | 498.9 | $ | 516.4 | $ | 618.9 | $ | 603.1 | $ | 0.3 | |||||||||||||
| Total earnings (loss) ……. | $ | (197.6 | ) | $ | (111.0 | ) | $ | (565.7 | ) | $ | 374.7 | $ | (301.5 | ) | $ | (591.2 | ) | $ | (81.6 | ) | |||||||
Fixed charges: | |||||||||||||||||||||||||||
Interest expense(2)… | $ | 310.8 | $ | 479.8 | $ | 498.9 | $ | 516.4 | $ | 618.9 | $ | 603.1 | $ | 0.3 | |||||||||||||
| Total fixed charges ….. | $ | 310.8 | $ | 479.8 | $ | 498.9 | $ | 516.4 | $ | 618.9 | $ | 603.1 | $ | 0.3 | |||||||||||||
| Ratio of earnings to fixed charges | N/A(1) | N/A(1) | N/A(1) | N/A(1) | N/A(1) | N/A(1) | N/A(1) | ||||||||||||||||||||
| (1) | Earnings were inadequate to cover fixed charges for the nine months ended February 28, 2013 and years ended May 31, 2012, 2011, 2010, 2009, for the period from July 12, 2007 through May 31, 2008 and for the period from June 1, 2007 through July 11, 2007 by $508.4 million, $590.8 million, $1,064.6 million, $141.7 million, $920.4 million, $1,194.3 million and $81.9 million, respectively. |
| (2) | Interest expense includes the amortization of deferred financing costs and bond premium. |
29
THE EXCHANGE OFFERS
General
Concurrently with the sales of the original senior notes on August 8, 2012 and October 2, 2012, we entered into the senior notes registration rights agreements with the initial purchasers of the original senior notes, and concurrently with the sale of the original senior subordinated notes on October 2, 2012, we entered into the senior subordinated notes registration rights agreement with the initial purchasers of the original senior subordinated notes. Those agreements require us to use our commercially reasonable efforts to prepare and file a registration statement under the Securities Act with respect to the exchange notes and, upon the effectiveness of the registration statement, to offer to the holders of the original notes the opportunity to exchange their original notes for a like principal amount of exchange notes.
The registration rights agreements provide that we must (a) use our commercially reasonable efforts to cause the registration statement of which this prospectus is a part with respect to the exchange of the original notes for the exchange notes to be declared effective under the Securities Act and (b) keep the exchange offers open for at least 20 business days (or longer, if required by applicable law) after the date notice of the exchange offers is mailed to holders of the original notes and (c) consummate the exchange offers on or prior to the 360th day (or if the 360th day is not a business day, the first business day thereafter) after the original issue date of the original notes.
Copies of the registration rights agreements have been filed as exhibits to the registration statement of which this prospectus is a part. Following the completion of the exchange offers, holders of original notes not tendered will not have any further registration rights other than as set forth in the paragraphs below, and the original notes will continue to be subject to certain restrictions on transfer.
Resale of the Exchange Notes
We are making the exchange offers in reliance on the position of the staff of the SEC as set forth in interpretive letters addressed to other parties in other transactions. For further information on the SEC’s position, see Exxon Capital Holdings Corporation, available May 13, 1988, Morgan Stanley & Co. Incorporated, available June 5, 1991, Shearman & Sterling, available July 2, 1993, and other interpretive letters to similar effect. We have not sought our own interpretive letter, however, and we cannot assure you that the staff would make a similar determination with respect to the exchange offers as it has in interpretive letters to other parties. Based on these interpretations by the staff, however, we believe that, with the exceptions set forth below, the exchange notes issued in the exchange offers may be offered for resale, resold and otherwise transferred by the holder of exchange notes without further compliance with the registration and prospectus delivery provision of the Securities Act, so long as such holder:
| • | is acquiring the exchange notes in the ordinary course of its business; |
| • | is not participating in, and does not intend to participate in, a distribution of the exchange notes within the meaning of the Securities Act and has no arrangement or understanding with any person to participate in a distribution of the exchange notes within the meaning of the Securities Act; |
| • | is not a broker-dealer who acquired the original notes directly from us; and |
| • | is not an “affiliate” of ours within the meaning of Rule 405 of the Securities Act. |
By tendering your original notes in exchange for exchange notes, you will be required to represent to us that each of the above statements applies to you. If you are participating in or intend to participate in, a distribution of the exchange notes, or have any arrangement or understanding with any person to participate in a distribution of the exchange notes to be acquired in these exchange offers, you may be deemed to have received restricted securities and may not rely on the applicable interpretations of the staff of the SEC. If you are so deemed, you will have to comply with the registration and prospectus delivery requirements of the Securities Act in connection with any secondary resale transaction.
Each broker-dealer that receives exchange notes for its own account in exchange for original notes, where the original notes were acquired by the broker-dealer as a result of market-making activities or other trading
30
activities, must acknowledge that it will deliver a prospectus in connection with any resale of the exchange notes. The letter of transmittal states that by so acknowledging and by delivering a prospectus, a broker-dealer will not be deemed to admit that it is an “underwriter” within the meaning of the Securities Act. So long as we maintain the effectiveness of the registration statement to which this prospectus relates, a broker-dealer may use this prospectus, as it may be amended or supplemented from time to time, in connection with resales of exchange notes received in exchange for original notes which the broker-dealer acquired as a result of market-making or other trading activities. See “Plan of Distribution.”
The exchange offers are not being made to, nor will we accept tenders for exchange from, holders of original notes in any jurisdiction in which the exchange offers or the acceptance of it would not be in compliance with the securities or blue sky laws of such jurisdiction.
Terms of the Exchange Offers
Upon the terms and subject to the conditions set forth in this prospectus and in the letter of transmittal, we will accept any and all original notes validly tendered and not withdrawn prior to 5:00 p.m., New York City time, on , 2013, or such date and time to which we extend either or both of the exchange offers, as the case may be. We will issue $1,000 in principal amount of exchange notes in exchange for each $1,000 principal amount of original notes validly tendered and accepted in the exchange offers.
We will not pay any accrued and unpaid interest on the original notes we acquire in the exchange offers. Instead, interest on the exchange senior notes will accrue from February 1, 2013, the most recent interest payment date from which interest on the original senior notes has previously been paid, and interest on the exchange senior subordinated notes will accrue from October 2, 2012, the issue date of the senior subordinated notes, or from April 1, 2013 if the exchange offer in respect thereof is completed after, and interest is paid on, that date.
The exchange notes will evidence the same debt as the original notes and will be issued under the terms of, and entitled to the benefits of, the applicable indenture relating to the original notes.
The terms of the exchange notes are identical in all material respects to the terms of the original notes, except that:
| (1) | we have registered the exchange notes under the Securities Act and therefore the exchange notes will not bear legends restricting their transfer, and |
| (2) | specified rights under the registration rights agreements, including the provisions providing for payment of additional interest in specified circumstances relating to the exchange offers, will be eliminated for all the notes. |
As of the date of this prospectus: (a) $1,825 million in aggregate principal amount of original senior notes were outstanding, and there was one registered holder, a nominee of DTC and (b) $800 million in aggregate principal amount of original senior subordinated notes were outstanding, and there was one registered holder, a nominee of DTC. Original notes accepted for exchange will be retired and cancelled and not reissued.
Except as described under “Form, Book-Entry Procedures and Transfer,” we will issue the exchange notes in the form of one or more global notes registered in the name of DTC or its nominee, and each beneficial owner’s interest in it will be transferable in book-entry form through DTC. We will conduct the exchange offers in accordance with the applicable requirements of the Exchange Act and the rules and regulations of the SEC promulgated under the Exchange Act.
We will be considered to have accepted validly tendered original notes when, as and if we have given oral or written notice thereof to the exchange agent. The exchange agent will act as agent for the tendering holders for the purpose of receiving the exchange notes from us. If any tendered original notes are not accepted for exchange because of an invalid tender, the occurrence of certain other events set forth under the heading “—Conditions to the Exchange Offers,” any such unaccepted original notes will be returned, without expense, to the tendering holder of those original notes promptly after the Expiration Date.
Holders who tender original notes in the exchange offers will not be required to pay brokerage commissions or fees or, subject to the instructions in the letter of transmittal, transfer taxes with respect to the
31
exchange of original notes in the exchange offers. We will pay all charges and expenses, other than certain applicable taxes, applicable to the exchange offers. See “—Fees and Expenses.”
If we successfully complete the exchange offers, any original notes that holders do not tender or we do not accept in the exchange offers will remain outstanding and continue to accrue interest. The holders of original notes after the exchange offers in general will not have further rights under the registration rights agreements, including registration rights and any rights to additional interest. Holders wishing to transfer the original notes would have to rely on exemptions from the registration requirements of the Securities Act.
Expiration Date; Extensions; Amendments
The Expiration Date shall be 5:00 p.m., New York City time, on , 2013, unless we, in our sole discretion, extend either or both of the exchange offers, in which case the Expiration Date shall be the latest date and time to which such exchange offer or exchange offers are extended. In order to extend the exchange offer or exchange offers, we will notify the exchange agent and each registered holder of any extension by oral or written notice prior to 9:00 a.m., New York City time, on the next business day after the previously scheduled Expiration Date and will also disseminate notice of any extension by press release or other public announcement no later than 9:00 a.m., New York City time on such date. We reserve the right, in our sole discretion:
| • | to delay accepting any original notes, to extend either or both of the exchange offers or, if any of the conditions set forth under “—Conditions to the Exchange Offers” shall not have been satisfied, to terminate either or both of the exchange offers, by giving oral or written notice of that delay, extension or termination to the exchange agent, or |
| • | to amend the terms of either or both of the exchange offers in any manner. |
Procedures for Tendering
To participate in the exchange offers, you must properly tender your original notes to the exchange agent as described below. We will only issue exchange notes in exchange for original notes that you timely and properly tender. Therefore, you should allow sufficient time to ensure timely delivery of the original notes, and you should follow carefully the instructions on how to tender your original notes. It is your responsibility to properly tender your original notes. We have the right to waive any defects. However, we are not required to waive defects, and neither we nor the exchange agent is required to notify you of defects in your tender.
If you have any questions or need help in exchanging your original notes, please contact the exchange agent at the address or telephone numbers set forth below.
All of the original notes were issued in book-entry form, and all of the original notes are currently represented by global certificates registered in the name of Cede & Co., the nominee of DTC. We have confirmed with DTC that the original notes may be tendered using DTC’s automatic tender offer program, or ATOP. The exchange agent will establish an account with DTC for purposes of the exchange offers promptly after the commencement of the exchange offers, and DTC participants may electronically transmit their acceptance of the exchange offers by causing DTC to transfer their original notes to the exchange agent using the ATOP procedures. In connection with the transfer, DTC will send an “agent’s message” to the exchange agent. The agent’s message will state that DTC has received instructions from the participant to tender original notes and that the participant agrees to be bound by the terms of the letter of transmittal.
By using the ATOP procedures to exchange original notes, you will not be required to deliver a letter of transmittal to the exchange agent. However, you will be bound by its terms just as if you had signed it. The form of the letter of transmittal is set forth in Annex A to this prospectus.
Determinations Under the Exchange Offers
We will determine in our sole discretion all questions as to the validity, form, eligibility, time of receipt, acceptance of tendered original notes and withdrawal of tendered original notes. Our determination will be final and binding. We reserve the absolute right to reject any original notes not properly tendered or any original notes our acceptance of which would, in the opinion of our counsel, be unlawful. We also reserve the right to waive any defect, irregularities or conditions of tender as to particular original notes. Our interpretation of the terms and
32
conditions of the exchange offers, including the instructions in the letter of transmittal, will be final and binding on all parties. Unless waived, all defects or irregularities in connection with tenders of original notes must be cured within such time as we shall determine. Although we intend to notify holders of defects or irregularities with respect to tenders of original notes, neither we, the exchange agent nor any other person will incur any liability for failure to give such notification. Tenders of original notes will not be deemed made until such defects or irregularities have been cured or waived. Any original notes received by the exchange agent that are not properly tendered and as to which the defects or irregularities have not been cured or waived will be returned to the tendering holder as soon as practicable after the Expiration Date of the exchange.
When We Will Issue Exchange Notes
In all cases, we will issue exchange notes for original notes that we have accepted for exchange under the exchange offers only after the exchange agent receives, prior to 5:00 p.m., New York City time, on the Expiration Date:
| • | a book-entry confirmation of such number of original notes into the exchange agent’s account at DTC; and |
| • | a properly transmitted agent’s message. |
Return of Original Notes Not Accepted or Exchanged
If we do not accept any tendered original notes for exchange or if original notes are submitted for a greater principal amount than the holder desires to exchange, the unaccepted or non-exchanged original notes will be returned without expense to their tendering holder. Such non-exchanged original notes will be credited to an account maintained with DTC. These actions will occur as promptly as practicable after the expiration or termination of the exchange offers.
Participating Broker-Dealers
Each broker-dealer that receives exchange notes for its own account in exchange for original notes, where those original notes were acquired by such broker-dealer as a result of marketmaking activities or other trading activities, must acknowledge that it will deliver a prospectus in connection with any resale of those exchange notes. See “Plan of Distribution.”
Acceptance of Original Notes for Exchange; Delivery of Exchange Notes Issued in the Exchange Offers
Following completion of the exchange offers, on the settlement date exchange notes to be issued in exchange for original notes in the exchange offers, if consummated, will be delivered in book-entry form.
We will be deemed to accept validly tendered original notes that have not been validly withdrawn as provided in this prospectus when, and if, we give oral or written notice of acceptance to the exchange agent. Subject to the terms and conditions of the exchange offers, delivery of the exchange notes will be made by the exchange agent on the settlement date following receipt of that notice. The exchange agent will act as agent for tendering holders of original notes for the purpose of receiving original notes and transmitting exchange notes as of the settlement date. If any tendered original notes are not accepted for any reason described in the terms and conditions of the exchange offers, such unaccepted original notes will be returned without expense to the tendering holders as promptly as practicable after the expiration or termination of the exchange offers.
Book-Entry Transfer
The participant should transmit its acceptance to DTC, Euroclear or Clearstream, as the case may be, on or prior to the Expiration Date. DTC, Euroclear or Clearstream, as the case may be, will verify the acceptance and then send to the exchange agent confirmation of the book-entry transfer. The confirmation of the book-entry transfer will be deemed to include an agent’s message confirming that DTC, Euroclear or Clearstream, as the case may be, has received an express acknowledgment from the participant that the participant has received and agrees to be bound by the letter of transmittal and that we may enforce the letter of transmittal against such participant. Delivery of exchange notes issued in the exchange offers may be effected through book-entry transfer at DTC, Euroclear or Clearstream, as the case may be. However, the letter of transmittal or facsimile thereof or an agent’s message, with
33
any required signature guarantees and any other required documents, must be transmitted to and received by the exchange agent at the address set forth below under “—Exchange Agent” on or prior to the Expiration Date.
DTC’s ATOP program is the only method of processing exchange offers through DTC. To accept exchange offers through ATOP, participants in DTC must send electronic instructions to DTC through DTC’s communication system. In addition, unless an agent’s message is transmitted in lieu thereof, such tendering participants should deliver a copy of the letter of transmittal to the exchange agent. DTC is obligated to communicate those electronic instructions to the exchange agent through an agent’s message. Any instruction through ATOP, such as an agent’s message, is at your risk and such instruction will be deemed made only when actually received by the exchange agent.
In order for an acceptance of exchange offers through ATOP to be valid, an agent’s message must be transmitted to and received by the exchange agent prior to the Expiration Date. Delivery of instructions to DTC does not constitute delivery to the exchange agent.
Withdrawal of Tenders
Tenders of original notes may be withdrawn at any time prior to 5:00 p.m., New York City time, on the Expiration Date.
For a withdrawal to be effective, you must comply with the appropriate ATOP procedures. Any notice of withdrawal must specify the name and number of the account at DTC to be credited with withdrawn original notes and otherwise comply with the ATOP procedures.
We will determine all questions as to the validity, form, eligibility and time of receipt of a notice of withdrawal. Our determination shall be final and binding on all parties. We will deem any original notes so withdrawn not to have been validly tendered for exchange for purposes of the exchange offers.
Any original notes that have been tendered for exchange but that are not exchanged for any reason will be credited to an account maintained with DTC for the original notes. This return or crediting will take place as soon as practicable after withdrawal, rejection of tender, expiration or termination of the exchange offers. You may retender properly withdrawn original notes by following the procedures described under “—Procedures for Tendering” above at any time on or prior to the Expiration Date.
Conditions to the Exchange Offers
Notwithstanding any other provision of the exchange offers, we may (a) refuse to accept any original notes and return all tendered original notes to the tendering holders, (b) extend the exchange offers and retain all original notes tendered before the expiration of the exchange offers, subject, however, to the rights of holders to withdraw those original notes, or (c) to the extent lawful, waive the unsatisfied conditions with respect to the exchange offers and accept all properly tendered original notes that have not been previously validly withdrawn, if we determine, in our reasonable judgment, that (i) the exchange offers violate applicable law or any applicable interpretation of the staff of the SEC; (ii) an action or proceeding shall have been instituted or threatened in any court or by any governmental agency which might materially impair our ability to proceed with the exchange offers or a material adverse development shall have occurred in any existing action or proceeding with respect to us; or (iii) all governmental approvals that we deem necessary for the consummation of the exchange offers have not been obtained.
The foregoing conditions are for our sole benefit and may be asserted by us regardless of the circumstances giving rise to any such condition or may be waived by us in whole or in part at any time and from time to time. The failure by us at any time to exercise any of the foregoing rights shall not be deemed a waiver of any of those rights and each of those rights shall be deemed an ongoing right which may be asserted at any time and from time to time.
In addition, we will not accept for exchange any original notes tendered, and no exchange notes will be issued in exchange for those original notes, if at such time any stop order shall be threatened or in effect with respect to the registration statement of which this prospectus constitutes a part or with respect to the qualification of the indenture governing the exchange notes under the Trust Indenture Act of 1939, as amended. In any such an event, we are required to use every reasonable effort to obtain the withdrawal of any stop order at the earliest possible time.
34
Effect of Not Tendering
Holders who desire to tender their original notes in exchange for exchange notes should allow sufficient time to ensure timely delivery. Neither the exchange agent nor we are under any duty to give notification of defects or irregularities with respect to the tenders of original notes for exchange.
Original notes that are not tendered or are tendered but not accepted will, following the consummation of the exchange offers, continue to accrue interest and to be subject to the provisions in the indenture regarding the transfer and exchange of the original notes and the existing restrictions on transfer set forth in the legend on the original notes and in the offering circulars relating to the original notes dated July 25, 2012 and September 18, 2012 (in the case of the original senior notes) and September 18, 2012 (in the case of the original senior subordinated notes). After completion of these exchange offers, we will have no further obligation to provide for the registration under the Securities Act of those original notes except in limited circumstances with respect to specific types of holders of original notes and we do not otherwise intend to register the original notes under the Securities Act. In general, original notes, unless registered under the Securities Act, may not be offered or sold except pursuant to an exemption from, or in a transaction not subject to, the Securities Act and applicable state securities laws.
Exchange Agent
Wells Fargo Bank, National Association has been appointed as exchange agent for the exchange offers. Questions, requests for assistance and requests for additional copies of this prospectus or of the letter of transmittal should be directed to the exchange agent addressed as follows:
| By Registered and Certified Mail: | By Overnight Courier or Regular Mail: | By Hand Delivery: |
| Wells Fargo Bank, National Association Corporate Trust Operations MAC N9303-121 P.O. Box 1517 Minneapolis, MN 55480 | Wells Fargo Bank, National Association Corporate Trust Operations MAC N9303-121 6th & Marquette Avenue Minneapolis, MN 55479 | Wells Fargo Bank, National Association Corporate Trust Services 608 2nd Avenue South Northstar East Building—12th Floor Minneapolis, MN 55402 |
By Facsimile Transmission: (612) 667-6282 Confirm by Telephone: (800) 344-5128 | ||
Fees and Expenses
We will bear the expenses of soliciting tenders of the original notes. The principal solicitation is being made by mail and by electronic transmission through DTC. Additional solicitations may, however, be made by e-mail, facsimile transmission, telephone or in person by the exchange agent as well as our officers and other employees and those of our affiliates.
We have not retained any dealer-manager in connection with these exchange offers and will not make any payments to broker-dealers or others soliciting acceptances of the exchange offers. However, we will pay the exchange agent reasonable and customary fees for its services and will reimburse it for its reasonable out-of-pocket expenses.
Tendering holders of original notes will not be required to pay any fee or commission to the exchange agent. If, however, a tendering holder handles the transaction through its commercial bank, broker, dealer, trust company or other institution, that holder may be required to pay brokerage fees or commissions.
Accounting Treatment
We will record the exchange notes at the same carrying value as the original notes, as reflected in our accounting records on the date of the exchange. Accordingly, we will not recognize any gain or loss for accounting
35
purposes as the terms of the exchange notes are substantially identical to those of the original notes. The expenses of the exchange offers will be amortized over the terms of the exchange notes.
Transfer Taxes
Holders who tender their original notes for exchange will not be obligated to pay any transfer taxes in connection with that tender or exchange, except that holders who instruct us to register exchange notes in the name of, or who request that original notes not tendered or not accepted in the exchange offers be returned to, a person other than the registered tendering holder will be responsible for the payment of any applicable transfer tax on those original notes.
Exchange Offers Registration Statement; Additional Interest
Under the registration rights agreements, we have agreed that if:
(1) any change in law or applicable interpretation of the staff of the SEC does not permit us to effect the exchange offers;
(2) for any other reason the exchange offers are not consummated by 360 days from August 8, 2012 for the senior notes, or from October 2, 2012 for the senior subordinated notes, respectively;
(3) any initial purchaser of the original notes named in the Registration Rights Agreement notifies us that:
| (a) | it is prohibited by law or SEC policy from participating in the exchange offers; or |
| (b) | it holds original notes that are or were ineligible to be exchanged in the exchange offers. |
then we will use our commercially reasonable efforts, at our cost, to (a) file as reasonably promptly as practicable a registration statement (the “shelf registration statement”) covering resales of the relevant notes; (b) cause the shelf registration statement to be declared effective under the Securities Act and (c) use our commercially reasonable efforts to keep the shelf registration statement continuously effective until the earliest of (x) the date that is two years from August 8, 2012 (in the case of the senior notes) or October 2, 2012 (in the case of the senior subordinated notes), or (y) such shorter period ending when all registrable securities covered by the initial shelf registration statement have been sold in a manner set forth and as contemplated in the initial shelf registration statement or, if applicable, a subsequent shelf registration statement (the “effectiveness period”).
We will, in the event a shelf registration statement is filed, among other things, provide to each holder for whom such shelf registration statement was filed copies of the prospectus which is a part of the shelf registration statement, notify each such holder when the shelf registration statement has become effective and take certain other actions as are required to permit unrestricted resales of the notes. A holder selling original notes or exchange notes pursuant to the shelf registration statement generally would be required to be named as a selling security holder in the related prospectus and to deliver a prospectus to purchasers, will be subject to certain of the civil liability provisions under the Securities Act in connection with such sales and will be bound by the provisions of the registration rights agreement which are applicable to such holder (including certain indemnification obligations).
Notwithstanding anything to the contrary in the registration rights agreements, at any time, we may delay the filing of any initial shelf registration Statement or delay or suspend the effectiveness thereof, for a reasonable period of time, but not in excess of 45 consecutive days or more than three (3) times during any calendar year (each, a “shelf suspension period”), if our board of directors determines reasonably and in good faith that the filing of any such initial shelf registration statement or the continuing effectiveness thereof would require the disclosure of non-public material information that, in the reasonable judgment of our board of directors, would be detrimental to us if so disclosed or would otherwise materially adversely affect a financing, acquisition, disposition, merger or other material transaction or such action is required by applicable law; provided however that the effectiveness period shall be extended for the number of days of any such shelf suspension period exercised by us.
The registration rights agreements further provides that in the event that either (each such event referred to in clauses (a) through (b), a “registration default”):
| (a) | we have not exchanged exchange notes for all notes validly tendered in accordance with the terms of the exchange offers, or a shelf registration statement has not been declared effective on or prior |
36
to 360 days from August 8, 2012 for the senior notes, and October 2, 2012 for the senior subordinated notes, respectively; or
| (b) | if applicable, a shelf registration statement has been declared effective and such shelf registration statement ceases to be effective at any time during the effectiveness period (other than because of the sale of all of the notes registered thereunder); |
then additional interest shall accrue on the principal amount of the senior notes and/or the senior subordinated notes, as the case may be, at a rate of 0.25% per annum (which rate will be increased by an additional 0.25% per annum for each subsequent 90-day period that such additional interest continues to accrue, provided that the rate at which such additional interest accrues may in no event exceed 1.00% per annum) (such additional interest to be calculated by us) commencing on (x) the 361st day after August 8, 2012 (in the case of the senior notes) or October 2, 2012 (in the case of the senior subordinated notes), in the case of clause (a), or (y) the day such shelf registration statement ceases to be effective, in the case of clause (b); provided, however, that upon the exchange of the exchange notes for all original notes tendered (in the case of clause (a)), or upon the effectiveness of the applicable shelf registration statement which had ceased to remain effective (in the case of clause (b)), additional interest on such notes will cease to accrue.
Other
Participation in these exchange offers is voluntary, and you should carefully consider whether to participate. You are urged to consult your financial and tax advisors in making your own decision as to what action to take.
37
USE OF PROCEEDS
The exchange offers are intended to satisfy certain of our obligations under the registration rights agreements. We will not receive any proceeds from the issuance of the exchange notes in the exchange offers. In exchange for each of the exchange notes, we will receive original notes in like principal amount. We will retire or cancel all of the original notes tendered in the exchange offers. Accordingly, issuance of the exchange notes will not result in any change in our capitalization.
38
CAPITALIZATION
The following table sets forth our cash, cash equivalents and capitalization as of February 28, 2013:
You should read this table in conjunction with “Use of Proceeds,” “Description of Other Indebtedness,” and our consolidated financial statements and the related notes included elsewhere in this prospectus.
| (in millions) | February 28, 2013 | ||
| Cash and cash equivalents | $ | 217.4 | |
| Debt | |||
| Non-U.S. facility | $ | 2.6 | |
| USD Term loan facility | $ | 2,226.7 | |
| EUR Term loan facility (€829.2) | $ | 1,085.0 | |
| USD/EUR Cash flow revolving credit facility | $/€ — | ||
| Asset-based revolving credit facility | — | ||
| 10% senior cash pay notes due 2017 | — | ||
| 10⅜%/ 11⅛% senior PIK toggle notes due 2017 | — | ||
| 11⅝% senior subordinated notes | — | ||
| 6.500% senior notes due 2020 | $ | 1,825.0 | |
| 6.500% senior subordinated notes due 2020 | $ | 800.0 | |
| Premium on notes | $ | 39.1 | |
Total debt | $ | 5,978.4 | |
Shareholder’s equity | $ | 2,266.0 | |
Total capitalization | $ | 8,244.4 | |
39
SELECTED HISTORICAL CONSOLIDATED AND UNAUDITED CONDENSED CONSOLIDATED FINANCIAL AND OTHER DATA
The following table presents our selected historical financial information for the periods and at the dates indicated. The statement of operations data presented below for the years ended May 31, 2012, 2011 and 2010, and balance sheet data as of May 31, 2012 and 2011, were derived from the audited consolidated financial statements of Biomet included in this prospectus. The statement of operations data presented below for the years ended May 31, 2009 and for the periods from July 12, 2007 to May 31, 2008 and from June 1, 2007 to July 11, 2007, and the balance sheet data as of May 31, 2010, 2009 and 2008 were derived from audited consolidated financial statements not included in this prospectus.
The selected historical interim financial data for the nine months ended February 28, 2013 and February 29, 2012, and as of February 28, 2013, have been derived from our unaudited condensed consolidated financial statements included elsewhere in this prospectus. The unaudited financial data presented has been prepared on a basis consistent with our audited consolidated financial statements. In the opinion of management, such unaudited financial data reflect all adjustments, consisting only of normal and recurring adjustments, necessary for a fair presentation of the results for those periods. Certain amounts recorded in previous periods have been reclassified to conform to the current presentation.
The selected historical and unaudited consolidated financial and other data to be read in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our audited and unaudited consolidated financial statements and related notes included elsewhere in this prospectus.
Statement of Operations Data
| (Unaudited) For the Nine Months Ended | |||||||
| (in millions) | February 28, 2013 | February 29, 2012 | |||||
| Net sales | $ | 2,269.0 | $ | 2,098.6 | |||
| Cost of sales | 736.0 | 669.9 | |||||
| Gross profit | 1,533.0 | 1,428.7 | |||||
| Selling, general and administrative expense | 886.7 | 800.9 | |||||
| Research and development expense | 107.2 | 93.2 | |||||
| Amortization | 230.2 | 250.0 | |||||
| Goodwill and intangible assets impairment charge | 334.1 | — | |||||
| Operating income (loss) | (25.2 | ) | 284.6 | ||||
| Interest expense | 310.8 | 363.4 | |||||
| Other (income) expense | 172.4 | 9.3 | |||||
| Loss before income taxes | (508.4 | ) | (88.1 | ) | |||
| Benefit from income taxes | (106.2 | ) | (18.4 | ) | |||
| Net loss | $ | (402.2 | ) | $ | (69.7 | ) | |
40
| Successor | Predecessor | |||||||||||||||||||||
| July 12, 2007 | June 1, 2007 | |||||||||||||||||||||
| Fiscal Year Ended May 31, | to | to | ||||||||||||||||||||
| (in millions) | 2012 | 2011 | 2010 | 2009 | May 31, 2008 | July 11, 2007(1) | ||||||||||||||||
| Net sales | $ | 2,838.1 | $ | 2,732.2 | $ | 2,698.0 | $ | 2,504.1 | $ | 2,134.5 | $ | 248.8 | ||||||||||
| Cost of sales | 894.4 | 838.7 | 819.9 | 828.4 | 814.7 | 102.3 | ||||||||||||||||
| Gross profit | 1,943.7 | 1,893.5 | 1,878.1 | 1,675.7 | 1,319.8 | 146.5 | ||||||||||||||||
Selling, general and administrative expense | 1,053.3 | 1,041.7 | 1,042.3 | 1,003.6 | 1,097.6 | 194.2 | ||||||||||||||||
| Research and development expense | 126.8 | 119.4 | 106.6 | 93.5 | 82.2 | 34.0 | ||||||||||||||||
| In-process research and development | — | — | — | — | 479.0 | — | ||||||||||||||||
| Amortization | 327.2 | 367.9 | 372.6 | 375.8 | 329.3 | 0.5 | ||||||||||||||||
Goodwill and intangible assets impairment charge | 529.8 | 941.4 | — | 551.1 | — | — | ||||||||||||||||
| Operating income (loss) | (93.4 | ) | (576.9 | ) | 356.6 | (348.3 | ) | (668.3 | ) | (82.2 | ) | |||||||||||
| Interest expense | 479.8 | 498.9 | 516.4 | 550.3 | 516.3 | 0.3 | ||||||||||||||||
| Other (income) expense | 17.6 | (11.2 | ) | (18.1 | ) | 21.8 | 9.7 | (0.6 | ) | |||||||||||||
| Loss before income taxes | (590.8 | ) | (1,064.6 | ) | (141.7 | ) | (920.4 | ) | (1,194.3 | ) | (81.9 | ) | ||||||||||
| Benefit from income taxes | (132.0 | ) | (214.8 | ) | (94.1 | ) | (171.2 | ) | (230.1 | ) | (27.3 | ) | ||||||||||
| Net loss | $ | (458.8 | ) | $ | (849.8 | ) | $ | (47.6 | ) | $ | (749.2 | ) | $ | (964.2 | ) | $ | (54.6 | ) | ||||
___________________________________________________
| (1) | The successor and predecessor periods together are not comparable to the preceding Predecessor period presented above due to a new basis of accounting as of the completion of the Offer on July 12, 2007. |
Balance Sheet Data
| (in millions) | (Unaudited) February 28, 2013 | May 31, 2012 | May 31, 2011 | May 31, 2010 | May 31, 2009 | May 31, 2008 | |||||||||||||||||
| Current assets less current liabilities | $ | 1,118.0 | $ | 1,200.8 | $ | 1,079.0 | $ | 786.5 | $ | 756.9 | $ | 785.2 | |||||||||||
| Total assets | 10,001.7 | 10,420.4 | 11,357.0 | 11,969.0 | 12,600.9 | 13,781.8 | |||||||||||||||||
| Total debt | 5,978.4 | 5,827.8 | 6,020.3 | 5,896.5 | 6,212.7 | 6,300.8 | |||||||||||||||||
| Shareholder’s equity | 2,266.0 | 2,682.1 | 3,175.1 | 3,733.5 | 3,840.3 | 4,836.3 | |||||||||||||||||
41
MANAGEMENT’S DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis of our financial condition and results of operations contains forward-looking statements, which are subject to numerous risks and uncertainties, including, but not limited to, those described in “Risk Factors” and “Forward-Looking Statements.” Actual results may differ materially from those contained in any forward-looking statements.
Overview
Executive Overview
Our net sales increased 8% for the nine months ended February 28, 2013 to $2,269.0 million, compared to $2,098.6 million for the nine months ended February 29, 2012, driven primarily by our acquisition of DePuy’s worldwide trauma business (the “Trauma Acquisition”) as described below. Our net sales for the year ended May 31, 2012, increased 4% to $2,838.1 million, compared to $2,732.2 million for the year ended May 31, 2011. The effect of foreign currency fluctuations negatively impacted reported net sales for the nine months ended February 28, 2013 by $37.3 million, with Europe reported net sales negatively impacted by $26.8 million, or 5%, and International reported net sales negatively impacted by $10.5 million, or 4%. For the year ended May 31, 2012, the effect of foreign currency fluctuations positively impacted reported net sales by $15.3 million, with Europe reported net sales positively impacted by $2.9 million and International reporting net sales positively impacted by $12.4 million. The following represents key sales growth statistics for the nine months ended February 28, 2013, in each case compared to the nine months ended February 29, 2012, and for the year ended May 31, 2012 compared to the year ended May 31, 2011, respectively:
| • | Large Joint Reconstructive product sales were flat worldwide and increased 1% in the U.S. for the nine months ended February 28, 2013, and 4% worldwide and 3% in the U.S. for the year ended May 31, 2012. | ||
| • | S.E.T. product sales, including the Trauma Acquisition, increased 67% worldwide and 60% in the U.S. for the nine months ended February 28, 2013, and 13% worldwide and 13% in the U.S. for the year ended May 31, 2012. | ||
| • | Spine & Bone Healing product sales were flat worldwide and in the U.S. for the nine months ended February 28, 2013, and decreased 4% worldwide and 5% in the U.S. for the year ended May 31, 2012. | ||
| • | Dental product sales decreased 5% worldwide and increased 5% in the U.S. for the nine months ended February 28, 2013, and decreased 1% worldwide and increased 8% in the U.S. for the year ended May 31, 2012. | ||
| • | Other product sales increased 1% worldwide and were flat in the U.S. for the nine months ended February 28, 2013, and increased 6% worldwide and increased 1% in the U.S. for the year ended May 31, 2012. | ||
On May 24, 2012, DePuy Orthopaedics, Inc. accepted our binding offer to purchase certain assets representing substantially all of DePuy’s worldwide trauma business, which involves researching, developing, manufacturing, marketing, distributing and selling products to treat certain bone fractures or deformities in the human body. On June 15, 2012, the Company announced the initial closing of the transaction. During the first and second quarters of fiscal year 2013, subsequent closings in various foreign countries occurred on a staggered basis, with the final closing occurring on December 7, 2012.
We have been active in the capital markets during fiscal year 2013. Our objectives included reducing market risk by extending the maturity on the majority of our term loans from March 2015 to July 2017, reducing the cost of our capital structure and retaining access to liquidity through the refinancing of our cash flow and asset-based revolvers.
42
Our Business
We design, manufacture and market a comprehensive range of both surgical and non-surgical products used primarily by orthopedic surgeons and other musculoskeletal medical specialists. We operate in one reportable business segment, musculoskeletal products, which includes the design, manufacture and marketing of products in five major product categories: Large Joint Reconstructive, S.E.T., Spine & Bone Healing, Dental and Other Products. We have three geographic markets: United States, Europe and International. Our current product categories include:
Large Joint Reconstructive Products, which represented 56% of our net sales for the nine months ended February 28, 2013, and 60% of our net sales for the fiscal year ended May 31, 2012, include knees and hips. We also produce some of the associated instruments required by orthopedic surgeons to implant our reconstructive products, as well as bone cements and cement delivery systems. Orthopedic reconstructive implants are used to replace joints that have deteriorated as a result of disease (principally osteoarthritis) or injury. Reconstructive joint surgery involves the modification of the area surrounding the affected joint and the implantation of one or more manufactured components, and may involve the use of bone cement.
S.E.T. Products, which represented 19% of our net sales for the nine months ended February 28, 2013, and 12% of our net sales for the fiscal year ended May 31, 2012, include sports medicine, extremity, and trauma products. Our sports medicine products are used in minimally-invasive orthopedic surgical procedures. Extremity products include reconstructive implants that are used to replace joints, other than hips and knees, that have deteriorated as a result of disease or injury. Our primary reconstructive joint in this product category is the shoulder, but we produce other joints as well. Trauma devices are used for setting and stabilizing damaged bones to support and/or augment the body’s natural healing process. Trauma products include internal fixation devices (such as nails, plates, screws, pins and wires designed to stabilize traumatic bone injuries) and external fixation devices (utilized to stabilize fractures when alternative methods of fixation are not suitable).
Spine & Bone Healing Products, which represented 10% of our net sales for the nine months ended February 28, 2013, and 11% of our net sales for the fiscal year ended May 31, 2012, include spinal fixation systems for cervical, thoracolumbar, deformity correction and spacer applications; implantable and non-invasive electrical stimulation devices for spinal applications; and osteobiologics, including bone substitute materials, as well as allograft services for spinal applications. Bone Healing products include electrical stimulation devices used for trauma indications, offering implantable and non-invasive options to stimulate bone growth, as well as orthopedic support products (also referred to as bracing products).
Dental Products, which represented 8% of our net sales for the nine months ended February 28, 2013, and 9% of our net sales for the fiscal year ended May 31, 2012, include dental reconstructive devices and associated instrumentation that are used for oral rehabilitation through the replacement of teeth and repair of hard and soft tissues. We also offer crown and bridge products.
Other Products, which represented 7% or our net sales for the nine months ended February 28, 2013, and 8% of our net sales for the fiscal year ended May 31, 2012, include microfixation products, autologous therapies, operating room supplies, casting materials, general surgical instruments, wound care products and other surgical products.
We have operations in over 50 locations, distribute our products in approximately 90 countries throughout the world and manage our operations through three geographic markets mentioned above. We are the fourth largest competitor in the U.S. orthopedic reconstructive market and have maintained this position for over a decade. We supply products to over 60% of U.S. hospitals performing joint replacement surgery. In addition, we are a leading provider in manufacturing and marketing of dental reconstructive devices worldwide, electrical stimulation and craniomaxillofacial fields. We have a long history of innovation, engineering quality and successful new product launches.
Opportunities and Challenges
Our results of operations could be substantially affected not only by global economic conditions, but also by local operating and economic conditions, which can vary substantially by market. Unfavorable conditions can depress sales in a given market and may result in actions that adversely affect our margins, constrain our operating flexibility or result in charges which are unusual or non-recurring. Certain macroeconomic events, such as the
43
current adverse conditions in the global economy, could have a more wide-ranging and prolonged impact on the general business environment, which could also adversely affect us.
In the United States, healthcare providers that purchase our products (e.g., hospitals, physicians, dentists and other health care providers) generally rely on payments from third-party payors (principally federal Medicare, state Medicaid and private health insurance plans) to cover all or a portion of the cost of our musculoskeletal products. In March 2010, comprehensive health care reform legislation was enacted through the Patient Protection and Affordable Health Care Act (H.R. 3590) and the Health Care and Education Reconciliation Act (H.R. 4872). Among other initiatives, these bills impose a 2.3% excise tax on domestic sales of medical devices following December 31, 2012, which is estimated to contribute approximately $20 billion to healthcare reform. Various healthcare reform proposals have also emerged at the state level. Except for the excise tax, which has impacted results of operations starting January 1, 2013, we cannot predict with certainty what healthcare initiatives, if any, will be implemented at the state level, or what the ultimate effect of federal health care reform or any future legislation or regulation will have on us. However, an expansion in government’s role in the U.S. healthcare industry may lower reimbursements for our products, may result in incremental pricing pressure, reduce medical procedure volumes and thereby adversely affect our business and results of operations, possibly materially.
Outside the United States, reimbursement systems vary significantly from country to country. If adequate levels of reimbursement from third-party payors outside the United States are not obtained, international sales of our products may decline. Many foreign markets, including Canada and some European and Asian countries, have decreased reimbursement rates. Our ability to continue to sell certain products profitably in these markets may diminish if the government-managed healthcare systems continue to reduce reimbursement rates, which can decrease pricing and procedural volume.
Seasonality
Our business is somewhat seasonal in nature, as many of our products are used in elective procedures, which typically decline during the summer months, particularly in European countries, and the winter holiday season.
44
Results of Operations
For the Nine Months Ended February 28, 2013 Compared to the Nine Months Ended February 29, 2012
| (in millions, except percentages) | Nine Months Ended February 28, 2013 | Percentage of Net Sales | Nine Months Ended February 29, 2012 | Percentage of Net Sales | Percentage Increase/ (Decrease) | |||||||||||
| Net sales | $ | 2,269.0 | 100 | % | $ | 2,098.6 | 100 | % | 8 | % | ||||||
| Cost of sales | 736.0 | 32 | 669.9 | 32 | 10 | |||||||||||
| Gross profit | 1,533.0 | 68 | 1,428.7 | 68 | 7 | |||||||||||
| Selling, general and administrative expense | 886.7 | 39 | 800.9 | 38 | 11 | |||||||||||
| Research and development expense | 107.2 | 5 | 93.2 | 4 | 15 | |||||||||||
| Amortization | 230.2 | 10 | 250.0 | 12 | (8 | ) | ||||||||||
| Goodwill and intangible assets impairment charge | 334.1 | 15 | — | — | * | |||||||||||
| Operating income (loss) | (25.2 | ) | (1 | ) | 284.6 | 14 | * | |||||||||
| Interest expense | 310.8 | 14 | 363.4 | 17 | (14 | ) | ||||||||||
| Other (income) expense | 172.4 | 8 | 9.3 | — | * | |||||||||||
| Other expense, net | 483.2 | 21 | 372.7 | 18 | * | |||||||||||
| Loss before income taxes | (508.4 | ) | (22 | ) | (88.1 | ) | (4 | ) | * | |||||||
| Provision (benefit) from income taxes | (106.2 | ) | (5 | ) | (18.4 | ) | (1 | ) | * | |||||||
| Net loss | $ | (402.2 | ) | (18 | )% | $ | (69.7 | ) | (3 | )% | * | |||||
___________________________________________________
* The percentage change is not as meaningful as the change in the dollar value.
Sales
Net sales were $2,269.0 million for the nine months ended February 28, 2013, and $2,098.6 million for the nine months ended February 29, 2012. The primary driver for the increase in sales was the Trauma Acquisition. The following tables provide net sales by geography and product category:
Geography Sales Summary
| (in millions, except percentages) | Nine Months Ended February 28, 2013 | Percentage of Net Sales | Nine Months Ended February 29, 2012 | Percentage of Net Sales | Percentage Increase/ (Decrease) | |||||||||||
| United States | $ | 1,395.9 | 62 | % | $ | 1,273.8 | 61 | % | 10 | % | ||||||
| Europe | 521.5 | 23 | 520.3 | 25 | — | |||||||||||
International (1) | 351.6 | 15 | 304.5 | 14 | 15 | |||||||||||
| Total | $ | 2,269.0 | 100 | % | $ | 2,098.6 | 100 | % | 8 | % | ||||||
___________________________________________________
(1)International primarily includes Canada, South America, Mexico and the Asia Pacific region.
45
Product Category Summary
| (in millions, except percentages) | Nine Months Ended February 28, 2013 | Percentage of Net Sales | Nine Months Ended February 29, 2012(1) | Percentage of Net Sales | Percentage Increase/ (Decrease) | |||||||||||
| Large Joint Reconstructive | $ | 1,261.1 | 56 | % | $ | 1,259.2 | 60 | % | — | % | ||||||
| Sports, Extremities, Trauma (S.E.T.) | 440.9 | 19 | 263.4 | 13 | 67 | |||||||||||
| Spine & Bone Healing | 224.3 | 10 | 224.9 | 11 | — | |||||||||||
| Dental | 188.5 | 8 | 198.5 | 9 | (5 | ) | ||||||||||
| Other | 154.2 | 7 | 152.6 | 7 | 1 | |||||||||||
| Total | $ | 2,269.0 | 100 | % | $ | 2,098.6 | 100 | % | 8 | % | ||||||
___________________________________________________
| (1) | Certain amounts have been adjusted to conform to the current presentation. The current presentation aligns with how we presently manage and market our products. |
We were affected by large unfavorable currency fluctuations during the first quarter of fiscal year 2013 as compared to the first quarter of fiscal year 2012.
Large Joint Reconstructive
Net sales of large joint reconstructive products for the nine months ended February 28, 2013 were $1,261.1 million, or 56% of consolidated net sales, compared to net sales of $1,259.2 million, or 60% of consolidated net sales, during the nine months ended February 29, 2012. Unfavorable foreign currency translation negatively impacted our large joint reconstructive product sales during the nine month period by $23.4 million. Pricing for knees and hips declined during the nine month period on a year-over-year basis in the low single digit range, which is generally consistent with pricing trends we have experienced over the last few years.
Knee product sales were flat worldwide and increased 1% in the United States during the nine months ended February 28, 2013, compared to the nine months ended February 29, 2012. Unfavorable foreign currency translation negatively impacted our knee sales. Key products during the nine month period ended February 28, 2013 included our Vanguard® SSK 360 Revision System, the Signature™ Personalized Patient Care System, E1® Vitamin E infused bearings and the OSS™ (Orthopaedic Salvage System). Procedure volume and mix growth during the nine month period was partially offset by price pressures.
Hip product sales were flat worldwide and increased 2% in the United States during the nine months ended February 28, 2013, compared to the nine months ended February 29, 2012. Unfavorable foreign currency translation negatively impacted our hip sales. We continued to see strong market demand for our Arcos® Modular Femoral Revision System and our new Taperloc® Complete Hip Stem during the nine month period ended February 28, 2013. In addition, the Microplasty® version of the Taperloc® Complete Hip Stem and the GTS (Global Tissue Sparing) short stem received strong market acceptance. Key acetabular products included the Ringloc®+ cup, E1® and ArCom XL® bearings, as well as our Active Articulation™ Systems that are available with E1® or ArCom XL® liners. In Europe, our Exceed ABT (Advanced Bearing Technologies) System received strong market demand during the nine month period ended February 28, 2013. Procedure volume and mix growth during the nine month period was partially offset by price pressures.
Sales of bone cement and other reconstructive products were flat worldwide and increased 4% in the United States during the nine months ended February 28, 2013, compared to the nine months ended February 29, 2012. Demand for our Cobalt™ MV (Medium Viscosity) and HV (High Viscosity) cements with Gentamicin contributed to our sales in this category. The Optipac® Pre-Packed Cement Mixing System continued to be well received in the European market during the nine months ended February 28, 2013. Demand for our StageOne™ Knee and Modular Hip Cement Spacer Molds continued to increase.
S.E.T.
Worldwide net sales of S.E.T. products for the nine months ended February 28, 2013 were $440.9 million, or 19% of consolidated net sales, representing a 67% increase compared to net sales of $263.4 million, or 13% of consolidated net sales, during the nine months ended February 29, 2012. S.E.T. sales, excluding the Trauma Acquisition, increased 10% worldwide and 12% in the U.S. Trauma Acquisition sales of $150.9 million were excluded in order to provide period-over-period comparability. Unfavorable foreign currency translation negatively impacted our S.E.T. sales by $5.8 million.
46
Sports medicine sales increased 8% worldwide, with a 1% sales increase in the United States, during the nine months ended February 28, 2013, compared to the nine months ended February 29, 2012. The sales increase was primarily driven by strong demand for our JuggerKnot™ brand, which includes soft anchors to repair the shoulder, hand and wrist, and foot and ankle. Additional key products contributing to the sales growth were the TunneLoc® Tibial Fixation Device and the ToggleLoc™ Femoral Fixation Device, both with and without ZipLoop™ Technology and the Repicci II® Resurfacing Knee System.
Extremity product sales increased 18% worldwide, with a 26% sales increase in the United States, during the nine months ended February 28, 2013, compared to the nine months ended February 29, 2012. The increase was driven by strong market demand for our Comprehensive® product lines including our Primary, Reverse and S.R.S. (Segmental Revision System) Shoulder Systems.
Trauma product sales increased 250% worldwide and 242% in the United States, during the nine months ended February 28, 2013, compared to the nine months ended February 29, 2012, driven by $150.9 million of sales related to the Trauma Acquisition. Trauma sales, excluding the Trauma Acquisition, decreased 1% worldwide and increased 3% in the U.S. Key products acquired as a result of the Trauma Acquisition include the DVR® Anatomic Volar Plating Systems, the A.L.P.S.™ Plating Systems, and the AFFIXUS® Hip Fracture Nails.
Spine & Bone Healing
Worldwide net sales of spine & bone healing products for the nine months ended February 28, 2013 were $224.3 million, or 10% of consolidated net sales, compared to net sales of $224.9 million, or 11% of consolidated net sales, for the nine months ended February 29, 2012. Spine & Bone Healing sales were flat during the nine month period primarily due to increased royalty revenue, which was offset by mid-single-digit price erosion, soft volumes due to the general economy, a challenging reimbursement environment for some fusion procedures and a trend toward physician-owned distributorships.
Spine product sales increased 4% worldwide and 5% in the United States during the nine months ended February 28, 2013, compared to the nine months ended February 29, 2012. Price declines in spine hardware continued to be in the mid-single digit range. Spine product sales increased during the nine month period, primarily due to increased royalty revenue. New products and services that contributed to growth during the nine months ended February 28, 2013, included the PlatFORM™ CM, an all natural, osteoconductive material; and Cellentra™ VCBM (Viable Cell Bone Matrix), an allogenic bone graft substitute.
Sales of bone healing products decreased 12% both worldwide and in the United States during the nine months ended February 28, 2013, compared to the nine months ended February 29, 2012. The need for additional clinical and economic data to support reimbursement continued to challenge the non-invasive stimulation business.
Dental
Worldwide net sales of dental products for the nine months ended February 28, 2013 were $188.5 million, or 8% of consolidated net sales, representing a 5% decrease compared to net sales of $198.5 million, or 9% of consolidated net sales, during the nine months ended February 29, 2012. Unfavorable foreign currency translation impacted our dental sales by $4.6 million. Dental sales in the U.S. increased 5% during the nine months ended February 28, 2013. While the U.S. dental market has been stronger than the market in Europe, there was continued softness worldwide as challenging economic conditions persisted. Dental sales were negatively impacted by unfavorable media reports in Japan related to the dental implant industry.
Other
Worldwide net sales of other products for the nine months ended February 28, 2013 were $154.2 million, or 7% of consolidated net sales, representing a 1% increase compared to net sales of $152.6 million, also 7% of consolidated net sales, during the nine months ended February 29, 2012. Our microfixation product sales continued to be strong, driven by continued market acceptance of the iQ® Intelligent Delivery System, the TraumaOne™ Plating System and the SternaLock® Blu Primary Closure System, as well as the Pectus Bar product line. Our microfixation sales growth was partially offset by a decrease in sales of autologous therapies.
Gross Profit
Gross profit for the nine months ended February 28, 2013 increased to $1,533.0 million, as compared to gross profit for the nine months ended February 29, 2012 of $1,428.7 million, or 68% of consolidated net sales for both periods. Gross margins decreased primarily due to product rationalization charges in our global spine and
47
trauma product lines and increased product liability reserves. Product rationalization is related to more focused product offerings for spine through innovative product development and technology acquisition and to product redundancies related to the Trauma Acquisition. Gross margins increased primarily as a result of lower operational restructuring costs, improved geographic and product mix and lower manufacturing and other costs of sales. This increase was partially offset by lower selling prices.
Selling, General and Administrative Expense
Selling, general and administrative expense during the nine months ended February 28, 2013 was $886.7 million, as compared to $800.9 million for the nine months ended February 29, 2012, or 39% and 38% of consolidated net sales, respectively. As a percentage of consolidated net sales, the expense increased due to investment in our sales force related to the Trauma Acquisition and higher stock-based compensation. This increase was partially offset by lower litigation and settlement costs and operational restructuring costs.
Research and Development Expense
Research and development expense during the nine months ended February 28, 2013 was $107.2 million or 5% of consolidated net sales, compared to $93.2 million for the nine months ended February 29, 2012, or 4% of consolidated net sales. The increase in expense was primarily related to the S.E.T. product lines, which includes the Trauma Acquisition, and stock-based compensation. Our principal research and development efforts relate to primary and revision orthopedic reconstructive devices, spinal fixation products, dental reconstructive devices, sports medicine products, resorbable technology, biomaterial products and autologous therapies.
Amortization
Amortization expense for the nine months ended February 28, 2013 was $230.2 million or 10% of consolidated net sales, compared to $250.0 million for the nine months ended February 29, 2012, or 12% of consolidated net sales. This decrease is primarily due to the intangible asset impairment charge taken in the fourth quarter of fiscal year 2012 related to our Dental Reconstructive and Spine & Bone Healing reporting units as well as the impairment charge taken in the third quarter of fiscal year 2013 related to our Dental Reconstructive reporting unit.
Goodwill and Intangible Assets Impairment Charge
During the third quarter of fiscal year 2013, we recorded a $334.1 million goodwill and definite and indefinite-lived intangible assets impairment charge related to our Dental Reconstructive reporting unit, primarily due to declining industry market growth rates in certain European and Asia Pacific markets and corresponding unfavorable margin trends.
Interest Expense
Interest expense was $310.8 million for the nine months ended February 28, 2013, compared to interest expense of $363.4 million for the nine months ended February 29, 2012. The decrease in interest expense was primarily due to lower average interest rates on our term loans and lower bond interest as a result of refinancing activities in fiscal year 2013.
Other (Income) Expense
Other (income) expense was expense of $172.4 million for the nine months ended February 28, 2013, compared to expense of $9.3 million for the nine months ended February 29, 2012. The expense for the nine months ended February 28, 2013 is primarily composed of the loss on retirement of bonds of $155.2 million and the write off of deferred financing fees related to the tender/retirement of the senior notes due 2017 of $17.1 million, while the nine months ended February 29, 2012 included an other-than-temporary impairment loss of $19.3 million related to the Greek bonds.
Provision (Benefit) from Income Taxes
The effective income tax rate was 20.9% for the nine months ended February 28, 2013 compared to 20.9% for the nine months ended February 29, 2012. Primary factors in determining the effective tax rate include the mix of various jurisdictions in which profits are projected to be earned and taxed, as well as assertions regarding the expected repatriation of earnings of our foreign operations. Our effective tax rate for the nine months ended February 28, 2013 was also impacted by a non-deductible goodwill impairment charge of $233.0 million, which was
48
treated as a non-deductible permanent difference and contributed significantly to the effective tax rate being lower than U.S. statutory tax rates. Fluctuations in effective tax rates between comparable periods also reflect the discrete tax benefit or expense of items in continuing operations that represent tax effects not attributable to current-year ordinary income. Discrete items, consisting primarily of the tax benefit associated with the reduction of net deferred tax liabilities due to the impairment of intangible assets, as well as the prospective reduction of the United Kingdom statutory corporate tax rate enacted in July 2012 and finalization of the 2011 income tax returns had the effect of increasing the effective income tax rate by 6.7% in the nine months ended February 28, 2013. Our effective income tax rate for the nine months ended February 29, 2012 increased by 18.7% due to discrete items consisting primarily of the tax benefit associated with the reduction of net deferred tax liabilities due to the prospective reduction of corporate tax rates in Japan and the United Kingdom, restructuring-related adjustments and finalization of the 2010 income tax returns.
For the Year Ended May 31, 2012 Compared to the Year Ended May 31, 2011
| (in millions, except percentages) | Year Ended May 31, 2012 | Percentage of Net Sales | Year Ended May 31, 2011 | Percentage of Net Sales | Percentage Increase/ (Decrease) | |||||||||||
| Net sales | $ | 2,838.1 | 100 | % | $ | 2,732.2 | 100 | % | 4 | % | ||||||
| Cost of sales | 894.4 | 32 | 838.7 | 31 | 7 | |||||||||||
| Gross profit | 1,943.7 | 68 | 1,893.5 | 69 | 3 | |||||||||||
| Selling, general and administrative expense | 1,053.3 | 37 | 1,041.7 | 38 | 1 | |||||||||||
| Research and development expense | 126.8 | 4 | 119.4 | 4 | 6 | |||||||||||
| Amortization | 327.2 | 12 | 367.9 | 13 | (11) | |||||||||||
| Goodwill & intangible assets impairment charge | 529.8 | 19 | 941.4 | 34 | * | |||||||||||
| Operating loss | (93.4 | ) | (3) | (576.9 | ) | (21) | * | |||||||||
| Interest expense | 479.8 | 17 | 498.9 | 18 | (4) | |||||||||||
| Other (income) expense | 17.6 | 1 | (11.2 | ) | — | * | ||||||||||
| Other expense, net | 497.4 | 18 | 487.7 | 18 | 2 | |||||||||||
| Loss before income taxes | (590.8 | ) | (21) | (1,064.6 | ) | (39) | * | |||||||||
| Benefit from income taxes | (132.0 | ) | (5) | (214.8 | ) | (8) | * | |||||||||
| Net loss | $ | (458.8 | ) | (16 | )% | $ | (849.8 | ) | (31 | )% | * | |||||
___________________________________________________
| * | The percentage change is not as meaningful as the change in the dollar value. |
Sales
Net sales were $2,838.1 million for the year ended May 31, 2012, and $2,732.2 million for the year ended May 31, 2011. The following tables provide net sales by geography and product category:
Geography Sales Summary
| (in millions, except percentages) | Year Ended May 31, 2012 | Percentage of Net Sales | Year Ended May 31, 2011 | Percentage of Net Sales | Percentage Increase/ (Decrease) | |||||||||||
| United States | $ | 1,713.3 | 60 | % | $ | 1,659.2 | 61 | % | 3 | % | ||||||
| Europe | 702.7 | 25 | 697.8 | 26 | 1 | |||||||||||
| International(1) | 422.1 | 15 | 375.2 | 13 | 13 | |||||||||||
| Total | $ | 2,838.1 | 100 | % | $ | 2,732.2 | 100 | % | 4 | % | ||||||
___________________________________________________
| (1) | International primarily includes Canada, South America, Mexico, and the Asia Pacific region. |
49
Product Category Summary
| (in millions, except percentages) | Year Ended May 31, 2012 | Percentage of Net Sales | Year Ended May 31, 2011(1) | Percentage of Net Sales | Percentage Increase/ (Decrease) | |||||||||||
| Large Joint Reconstructive | $ | 1,698.8 | 60 | % | $ | 1,630.6 | 60 | % | 4 | % | ||||||
| Sports, Extremities, Trauma (S.E.T.) | 354.4 | 12 | 312.3 | 11 | 13 | |||||||||||
| Spinal & Bone Healing | 314.0 | 11 | 327.4 | 12 | (4) | |||||||||||
| Dental | 267.7 | 9 | 269.5 | 10 | (1) | |||||||||||
| Other | 203.2 | 8 | 192.4 | 7 | 6 | |||||||||||
| Total | $ | 2,838.1 | 100 | % | $ | 2,732.2 | 100 | % | 4 | % | ||||||
___________________________________________________
| (1) | New product categories were adopted in order to more closely represent the way we report sales and market products. Certain amounts have been reclassified to conform to the current presentation. |
Large Joint Reconstructive
Net sales of large joint reconstructive products for the year ended May 31, 2012 were $1,698.8 million, or 60% of net sales, representing a 4% increase compared to net sales of $1,630.6 million, also 60% of net sales, during the year ended May 31, 2011.
Knee product sales increased 3% worldwide and increased 1% in the United States during the year ended May 31, 2012, compared to the year ended May 31, 2011. The worldwide knee sales growth was primarily due to increased sales in Europe and our International countries. Europe knee sales increased primarily due to sales growth of primary and revision components of our Vanguard® Knee, as well as demand for the Orthopaedic Salvage System. Knee sales grew in our International countries principally from increased demand for our Vanguard® Complete Knee System. Worldwide knee sales growth was partially offset by decreased partial knee sales. We believe partial knee sales have declined due to macroeconomic conditions impacting patients and competitive activities with partial knee product offerings in the market place the last several years.
Hip product sales increased 6% worldwide and in the United States during the year ended May 31, 2012, compared to the year ended May 31, 2011. We believe the sales increase was primarily driven by the strong market acceptance of the new Arcos® Modular Femoral Revision System, our Taperloc® Complete Hip Stem, E1® Antioxidant Infused Acetabular Liners and the new Active Articulation™ E1® Hip System. Our worldwide hip sales growth was impacted by the industry-wide erosion of metal-on-metal hip sales.
Sales of bone cement and other reconstructive products increased 5% worldwide and 8% in the United States during the year ended May 31, 2012, compared to the year ended May 31, 2011. Sales of Cobalt™ Bone Cement with Gentamicin, the Optipac™ Pre-packed Vacuum Mixing System (not available in the U.S.) and our StageOne™ Hip and Knee Cement Spacer Molds, particularly the StageOne™ Select Modular Hip Spacer Molds, contributed to our sales growth in the bone cement and other reconstructive product category.
S.E.T.
Worldwide net sales of S.E.T. products for the year ended May 31, 2012 were $354.4 million, or 12% of net sales, representing a 13% increase compared to net sales of $312.3 million, or 11% of net sales, during the year ended May 31, 2011.
Sports medicine sales increased 18% worldwide, with a 12% sales increase in the United States, during the year ended May 31, 2012, compared to the year ended May 31, 2011. The primary contributor of sales growth was the JuggerKnot™ Soft Anchor due to increased volumes from strong market acceptance. During the fourth fiscal quarter, we completed the commercial launch of the JuggerKnot™ Short Soft Anchor used for foot and ankle repair, which also contributed to the growth.
Extremity product sales increased 18% worldwide and 22% in the United States during the year ended May 31, 2012, compared to the year ended May 31, 2011. The Comprehensive® Primary and Reverse Shoulder
50
Systems continued to drive strong sales growth for the extremity product category. During the fourth fiscal quarter we launched a couple of line extensions, including a small base plate for the reverse shoulder and E1® bearings which contributed to our extremity sales.
Trauma product sales decreased 2% worldwide, with a 4% sales decrease in the United States, during the year ended May 31, 2012, compared to the year ended May 31, 2011. External fixation sales declined due to a continued market shift from external fixation to internal fixation products and competitive pressures, partially offset by increased internal fixation sales. The increased internal fixation sales were primarily due to sales growth for the OptiLock® VL Distal Radius Plating System, the OptiLock® Humeral Plating System, and the Phoenix™ Ankle Arthrodesis Nail System.
Spine & Bone Healing
Worldwide net sales of spine & bone healing products for the year ended May 31, 2012 were $314.0 million, or 11% of net sales, representing a 4% decrease compared to net sales of $327.4 million, or 12% of net sales, for the year ended May 31, 2011. We believe the spine market continued to be affected by mid-single-digit price erosion, soft volumes due to the general economy, a challenging reimbursement environment for some fusion procedures, and a trend toward physician-owned distributorships.
Spine product sales decreased 3% both worldwide and in the United States during the year ended May 31, 2012, compared to the year ended May 31, 2011.
Sales of bone healing products decreased 7% both worldwide and in the United States during the year ended May 31, 2012, compared to the year ended May 31, 2011.
Dental
Worldwide net sales of dental products for the year ended May 31, 2012 were $267.7 million, or 9% of net sales, representing a 1% decrease compared to net sales of $269.5 million, or 10% of net sales, during the year ended May 31, 2011. The decreased dental sales were primarily due to weakness in the European market due to the economic uncertainty in the regions where we currently have the largest market share, which were partially offset by sales growth in the U.S. driven, in part, by increased average selling prices.
Other
Worldwide net sales of other products for the year ended May 31, 2012 were $203.2 million, or 8% of net sales, representing a 6% increase compared to net sales of $192.4 million, or 7% of net sales, during the year ended May 31, 2011. Our microfixation product sales increased both worldwide and in the United States during fiscal year 2012, and were partially offset by a decrease in sales of autologous therapies.
Gross Profit
Gross profit for the year ended May 31, 2012 increased to $1,943.7 million, compared to gross profit for the year ended May 31, 2011 of $1,893.5 million, or 68% and 69% of net sales, respectively. Gross profit as a percentage of net sales was slightly down compared to the year ended May 31, 2011 primarily due to a decrease in average selling prices, unfavorable manufacturing variances as production volumes were lower, higher instrument depreciation expense related to new product launches and costs related to the closure of the Swindon, United Kingdom plant that commenced during the second quarter of fiscal 2012, which were partially offset by our ability to leverage fixed costs.
Selling, General and Administrative Expense
Selling, general and administrative expense for the year ended May 31, 2012 and May 31, 2011 was $1,053.3 million and $1,041.7 million, respectively, or 37% and 38% of net sales, respectively. The expense increased during the year ended May 31, 2012 primarily due to costs to implement the restructuring plan that commenced in the first quarter of fiscal 2012 and costs related to settlement of the FCPA investigation as compared to the year ended May 31, 2011, which were partially offset by a legal settlement related to the Heraeus litigation described in “Note 16 —Contingencies” to the consolidated financial statements contained elsewhere in this prospectus.
51
Research and Development Expense
Research and development expense during the year ended May 31, 2012 and May 31, 2011 was $126.8 million and $119.4 million, respectively, or 4% of net sales for both periods. The slight increase in research and development expense for the year ended May 31, 2012 primarily related to our ongoing commitment to increase investment in clinical research and regulatory affairs within our business. Our principal research and development efforts relate to primary and revision large joint reconstructive devices, S.E.T. products, spinal products, dental products, resorbable technologies, biomaterial products and autologous therapies.
Amortization
Amortization expense for the year ended May 31, 2012 was $327.2 million, or 12% of net sales, compared to $367.9 million for the year ended May 31, 2011, or 13% of net sales. This decrease was primarily due to the intangible asset impairment charge taken in the fourth quarter of fiscal 2012 related to our spine & bone healing and dental reconstructive reporting units and the intangible asset impairment charge taken in the fourth quarter of fiscal 2011 related to our Europe business, both described below.
Goodwill and Intangible Assets Impairment Charge
During the fourth quarter of fiscal 2012, we recorded a $529.8 million goodwill and definite and indefinite-lived intangible assets impairment charge primarily related to our spine & bone healing and dental reconstructive reporting units, due primarily to evidence of declining industry market growth rates in certain European and Asia Pacific markets and unfavorable margin trends resulting from changes in product mix in our dental reconstructive reporting unit and growth rate declines as compared to the original purchase accounting assumptions at the time of the Merger for our spine & bone healing reporting unit. During the fourth quarter of fiscal 2011, we recorded a $941.4 million goodwill and definite and indefinite-lived intangible assets impairment charge primarily related to our Europe business due to the continued market slowdown in Europe relative to our original purchase accounting assumptions at the time of the Merger due to the continued financial and credit challenges in some European countries, which continue to impact our sales growth.
Interest Expense
Interest expense was $479.8 million for the year ended May 31, 2012, compared to interest expense of $498.9 million for the year ended May 31, 2011. The change in interest expense was primarily due to a lower average interest rate on our term loan facilities as our interest rate swaps continue to mature, moving more of our term loan facilities from fixed to floating rate debt.
Other (Income) Expense
Other (income) expense was expense of $17.6 million for the year ended May 31, 2012, compared to income of $11.2 million for the year ended May 31, 2011. The decrease is primarily due to an other-than-temporary impairment that was recorded on the Greek bonds of $20.1 million for the year ended May 31, 2012 and $7.1 million of expense was due to revaluation of our foreign cash accounts.
Benefit from Income Taxes
The effective income tax rate was 22.3% for the year ended May 31, 2012 compared to 20.2% for the year ended May 31, 2011. The primary factor in determining the effective tax rate is the mix of various jurisdictions in which profits are projected to be earned and taxed. The effective tax rate was also impacted by non-deductible goodwill impairment. In fiscal 2012 and fiscal 2011, $291.9 million and $422.8 million of goodwill impairment charges, respectively, were treated as non-deductible permanent differences and contributed significantly to the effective tax rate being lower than U.S. statutory tax rates. Other items impacting the effective tax rate for the year ended May 31, 2012 include decreases due to income inclusions related to U.S. anti-deferral provisions and updated assertions regarding the permanent reinvestment of earnings of foreign operations, offset by settlements relating to uncertain tax benefits and changes in statutory tax rates (particularly in the United Kingdom). The May 31, 2011 effective tax rate was decreased due to an increase in valuation allowance relating to state and foreign net operating loss carryforwards and an increase in liabilities for uncertain tax benefits, offset by reductions to the company’s state effective tax rate (primarily due to New Jersey’s change to single-sales factor) as well as the reduction in United Kingdom corporate tax rates.
52
For the Year Ended May 31, 2011 Compared to the Year Ended May 31, 2010
| (in millions, except percentages) | Year Ended May 31, 2011 | Percentage of Net Sales | Year Ended May 31, 2010 | Percentage of Net Sales | Percentage Increase/ (Decrease) | |||||||||||
| Net sales | $ | 2,732.2 | 100 | % | $ | 2,698.0 | 100 | % | 1 | % | ||||||
| Cost of sales | 838.7 | 31 | 819.9 | 30 | 2 | |||||||||||
| Gross profit | 1,893.5 | 69 | 1,878.1 | 70 | 1 | |||||||||||
| Selling, general and administrative expense | 1,041.7 | 38 | 1,042.3 | 39 | — | |||||||||||
| Research and development expense | 119.4 | 4 | 106.6 | 4 | 12 | |||||||||||
| Amortization | 367.9 | 13 | 372.6 | 14 | (1) | |||||||||||
| Goodwill & intangible assets impairment charge | 941.4 | 34 | — | — | * | |||||||||||
| Operating income (loss) | (576.9 | ) | (21) | 356.6 | 13 | * | ||||||||||
| Interest expense | 498.9 | 18 | 516.4 | 19 | (3) | |||||||||||
| Other (income) expense | (11.2 | ) | — | (18.1 | ) | (1) | (38) | |||||||||
| Other expense, net | 487.7 | 18 | 498.3 | 18 | (2) | |||||||||||
| Loss before income taxes | (1,064.6 | ) | (39) | (141.7 | ) | (5) | * | |||||||||
| Benefit from income taxes | (214.8 | ) | (8) | (94.1 | ) | (3) | * | |||||||||
| Net loss | $ | (849.8 | ) | (31 | )% | $ | (47.6 | ) | (2 | )% | * | |||||
___________________________________________________
| * | The percentage change is not as meaningful as the change in the dollar value. |
Sales
Net sales were $2,732.2 million for the year ended May 31, 2011, and $2,698.0 million for the year ended May 31, 2010. The following tables provide net sales by geography and product category:
Geography Sales Summary
| (in millions, except percentages) | Year Ended May 31, 2011 | Percentage of Net Sales | Year Ended May 31, 2010(1) | Percentage of Net Sales | Percentage Increase/ (Decrease) | |||||||||||
| United States | $ | 1,659.2 | 61 | % | $ | 1,644.1 | 61 | % | 1 | % | ||||||
| Europe | 697.8 | 26 | 724.5 | 27 | (4) | |||||||||||
International(2) | 375.2 | 13 | 329.4 | 12 | 14 | |||||||||||
| Total | $ | 2,732.2 | 100 | % | $ | 2,698.0 | 100 | % | 1 | % | ||||||
___________________________________________________
| (1) | Certain amounts have been adjusted to conform to the current presentation. Specifically, International net sales increased, and Europe net sales decreased, $4.3 million for the year ended May 31, 2010. The current presentation aligns with how the Company presently manages and markets its products. |
| (2) | International primarily includes Canada, South America, Mexico and the Asia Pacific region. |
Product Category Summary
53
| (in millions, except percentages) | Year Ended May 31, 2011(1) | Percentage of Net Sales | Year Ended May 31, 2010 (1) | Percentage of Net Sales | Percentage Increase/ (Decrease) | |||||||||||
| Large Joint Reconstructive | $ | 1,630.6 | 60 | % | $ | 1,615.7 | 60 | % | 1 | % | ||||||
| Sports, Extremities, Trauma (S.E.T.) | 312.3 | 11 | 283.7 | 11 | 10 | |||||||||||
| Spine & Bone Healing | 327.4 | 12 | 345.3 | 13 | (5) | |||||||||||
| Dental | 269.5 | 10 | 265.2 | 10 | 2 | |||||||||||
| Other | 192.4 | 7 | 188.1 | 6 | 2 | |||||||||||
| Total | $ | 2,732.2 | 100 | % | $ | 2,698.0 | 100 | % | 1 | % | ||||||
___________________________________________________
| (1) | New product categories were adopted in order to more closely represent the way we currently report sales and market products. Certain amounts have been reclassified to conform to the current presentation. |
Large Joint Reconstructive
Net sales of large joint reconstructive products for the year ended May 31, 2011 were $1,630.6 million, or 60% of net sales, representing a 1% increase compared to net sales of $1,615.7 million, also 60% of net sales, during the year ended May 31, 2010.
Our growth rates for knee and hip product sales were in the low single digits during the year ended May 31, 2011, compared to high single to low double-digit growth rates in prior periods. Certain events, such as the current adverse conditions in the global economy, including high unemployment rates, employed patients’ concerns about taking medical leave during the slow economy, increased deductibles and co-pays and the expiration of COBRA subsidies have contributed to the decelerating growth rates. In addition, the litigious environment in the industry surrounding metal-on-metal hips, as well as our inability to market our Signature™ Personalized Patient Care System to new customers for most of the first three quarters of fiscal 2011, also impacted growth rates. In July 2010, we received a Warning Letter from the FDA regarding the Signature™ Personalized Patient Care system, alleging that we did not have appropriate clearance or approval to market the system in the United States. In September 2010, we met with the FDA and we agreed on a course of corrective action and an additional 510(k) application for our Signature™ Personalized Patient Care System was submitted to the FDA in September 2010. During the FDA’s review of the 510(k), we ceased all promotional activities regarding the system as well as sales to new customers in the United States. The FDA granted the 510(k) clearance in a letter sent to Materialise NV, the manufacturer of the Signature™ system, on February 8, 2011, which resolved the warning letter sent to Biomet in July 2010.
Knee product sales increased 1% worldwide and were flat in the United States during the year ended May 31, 2011, compared to the year ended May 31, 2010. Increased knee sales, including sales growth of primary and revision components of the Vanguard® Knee, along with E1® Antioxidant Infused Tibial Bearings, were partially offset by decreased sales of our partial knee systems.
Hip product sales increased 1% worldwide and in the United States during the year ended May 31, 2011, compared to the year ended May 31, 2010. Strong market acceptance of the new Arcos® Modular Femoral Revision System and sales growth of E1® Antioxidant Infused Acetabular Liners were key contributors to hip sales growth, partially offset by decreased metal-on-metal hip sales.
S.E.T.
Worldwide net sales of S.E.T. products for the year ended May 31, 2011 were $312.3 million, or 11% of net sales, representing a 10% increase compared to net sales of $283.7 million, or 11% of net sales, during the year ended May 31, 2010.
The contributors of our double digit sales growth in sports medicine during the year ended May 31, 2011 primarily consisted of procedure specific devices, including the JuggerKnot™ Soft Anchor, the ComposiTCP™ Interference Screw, the MaxFire™ MarXmen™ Meniscal Repair Device, the ToggleLoc™ Femoral Fixation Device with ZipLoop™ Technology, and the ALLthread™ Knotless Suture Anchor.
Extremity product sales increased 20% worldwide, with a 30% sales increase in the United States, during the year ended May 31, 2011, compared to the year ended May 31, 2010. The Comprehensive® Primary, Reverse and Fracture Shoulder Systems continued to drive strong growth for the extremity product category.
54
Spine & Bone Healing
Worldwide net sales of spine & bone healing products for the year ended May 31, 2011 were $327.4 million, or 12% of net sales, representing a 5% decrease compared to net sales of $345.3 million, or 13% of net sales, for the year ended May 31, 2010. We believe the spine market continued to be affected by mid-single-digit price erosion, the slowdown in volumes due to the general economy, a challenging reimbursement environment for some fusion procedures, and the continued trend toward physician-owned distributorships.
Dental
Worldwide net sales of dental products for the year ended May 31, 2011 were $269.5 million, or 10% of net sales, representing a 2% increase compared to net sales of $265.2 million, also 10% of net sales, during the year ended May 31, 2010. The OSSEOTITE® product line, our flagship dental reconstructive implant system, was a key contributor to our fiscal year dental sales growth.
Other
Worldwide net sales of other products for the year ended May 31, 2011 were $192.4 million, or 7% of net sales, representing a 2% increase compared to net sales of $188.1 million, or 6% of net sales, during the year ended May 31, 2010. Our microfixation product sales grew both worldwide and in the United States during fiscal year 2011, and were partially offset by a decrease in sales of autologous therapies.
Gross Profit
Gross profit for the year ended May 31, 2011 increased to $1,893.5 million compared to gross profit for the year ended May 31, 2010 of $1,878.1 million, or 69% and 70% of net sales, respectively. Gross profit as a percentage of net sales was slightly down due to a decrease in average selling prices compared to the year ended May 31, 2010.
Selling, General and Administrative Expense
Selling, general and administrative expense during the years ended May 31, 2011 and 2010 was $1,041.7 million and $1,042.3 million, respectively, or 38% and 39% of net sales, respectively. The expense was slightly down year over year due to continued cost containment strategies worldwide.
Research and Development Expense
Research and development expense during the years ended May 31, 2011 and 2010 was $119.4 million and $106.6 million, respectively, or 4% of net sales for both periods. This increase in research and development expenses for the year ended May 31, 2011 primarily related to our ongoing commitment to increase investment in clinical research and regulatory affairs within our business. Our principal research and development efforts relate to primary and revision orthopedic reconstructive devices, spinal fixation products, dental reconstructive devices, sports medicine products, resorbable technology, biomaterial products and autologous therapies. Expenses during the year ended May 31, 2011 have primarily been related to the following research and development projects: E1® Antioxidant Infused Technology Tibial bearings (Reconstructive-Knees), Vanguard® SSK 360 Revision System (Reconstructive-Knees), Arcos® Modular Revision Hip System (Reconstructive-Hips), Taperloc® Complete Hip System (Reconstructive-Hips) OrthoPak® and SpinalPak® stimulation platform technologies (Fixation-Stimulation) and iQ® Intelligent Delivery System (Fixation-Craniomaxillofacial).
Amortization
Amortization expense for the year ended May 31, 2011 was $367.9 million or 13% of net sales, compared to $372.6 million for the year ended May 31, 2010, or 14% of net sales. This decrease is primarily due to the accelerated method for amortizing customer relationship intangibles as the value for those relationships is greater at the beginning of their life cycle and the decrease in amortization in the fourth quarter due to the intangible impairment charge taken in the fourth quarter of fiscal 2011 related to our Europe business and described below.
Goodwill and Intangible Assets Impairment Charge
55
During the fourth quarter of fiscal 2011, we recorded a $941.4 million goodwill and definite and indefinite-lived intangible assets impairment charge primarily related to our Europe business due to the continued market slowdown in Europe relative to our original purchase accounting assumptions at the time of the Merger due to the continued financial and credit challenges in some European countries, which continue to impact our sales growth.
Interest Expense
Interest expense was $498.9 million for the year ended May 31, 2011, compared to interest expense of $516.4 million for the year ended May 31, 2010. The decrease in interest expense was primarily due to a lower average interest rate on our outstanding floating rate debt.
Other (Income) Expense
Other (income) expense was income of $11.2 million for the year ended May 31, 2011, compared to income of $18.1 million for the year ended May 31, 2010. The decrease is primarily due to a decrease in currency transaction gains of $5.6 million.
Benefit from Income Taxes
Our effective income tax rate decreased to 20.2% for the year ended May 31, 2011 compared to 66.4% for the year ended May 31, 2010. The fiscal 2011 tax rate is lower than statutory tax rates due to amounts deducted for financial reporting purposes that are not deductible for tax purposes. In fiscal 2011, $422.8 million of the $941.4 million impairment charge taken on the European business unit was a non-deductible permanent difference. This rate also decreased due to an increase in valuation allowance relating to state and foreign net operating loss carryforwards and an increase in uncertain tax benefits, offset by reductions to our state effective tax rate (primarily due to New Jersey’s change to single-sales factor) as well as the reduction in United Kingdom corporate tax rates. The Company’s effective tax rate in fiscal 2010 was higher than statutory rates primarily due to the Company’s mix of profits and losses in certain foreign and domestic jurisdictions, specifically a higher pre-tax loss in the United States as a percent of the total worldwide loss before income taxes.
Liquidity and Capital Resources
For the Nine Months Ended February 28, 2013 and February 29, 2012
The following is a summary of the cash flows by activity for the nine months ended February 28, 2013 and February 29, 2012:
| (in millions) | Nine Months Ended February 28, 2013 | Nine Months Ended February 29, 2012 | |||||
| Net cash from (used in): | |||||||
| Operating activities | $ | 273.8 | $ | 291.3 | |||
| Investing activities | (433.8 | ) | (81.7 | ) | |||
| Financing activities | (130.9 | ) | (28.9 | ) | |||
| Effect of exchange rate changes on cash | 15.9 | (12.5 | ) | ||||
| Change in cash and cash equivalents | $ | (275.0 | ) | $ | 168.2 | ||
For the Nine Months Ended February 28, 2013 Compared to the Nine Months Ended February 29, 2012
Our cash and cash equivalents were $217.4 million as of February 28, 2013 compared to $496.0 million as of February 29, 2012. We generally maintain our cash and cash equivalents and investments in money market funds, corporate bonds and debt instruments. Cash and cash equivalents held outside of the United States were $178.3 million as of February 28, 2013. If we were to repatriate this cash back to the United States, additional tax of up to 35%, the maximum federal tax rate, could be incurred. In addition, we require a certain amount of cash to support on-going operations outside the United States.
Operating Cash Flows
56
Net cash provided by operating activities was $273.8 million for the nine months ended February 28, 2013, compared to $291.3 million for the nine months ended February 29, 2012. Operating cash flows for the nine months ended February 28, 2013 were unfavorably impacted by increased inventory levels due to additional inventory needed to support new product introductions and the Trauma Acquisition and increased accounts receivable due to increased sales and seasonality, partially offset by lower cash paid for interest. Cash generated by operating activities continued to be a source of funds for deleveraging and investing in our growth.
Investing Cash Flows
Net cash used in investing activities was $433.8 million for the nine months ended February 28, 2013 and $81.7 million for the nine months ended February 29, 2012. The investing cash flow decrease was primarily due to the Trauma Acquisition purchase price of $280.0 million and an increase in capital expenditures of $27.0 million during the nine months ended February 28, 2013. Additionally, during the nine months ended February 29, 2012 we received proceeds from the sales/maturities of investments of $42.0 million primarily related to the sale of a time deposit.
Financing Cash Flows
Net cash used in financing activities was $130.9 million for the nine months ended February 28, 2013, compared to cash used in financing activities of $28.9 million for the nine months ended February 29, 2012. The difference was primarily related to the refinancing activities. We received proceeds of $3,396.2 million related to the offerings of our 6.500% senior notes due 2020 and 6.500% senior subordinated notes due 2020 and term loans and tendered or retired $3,423.0 million of senior notes due 2017 and term loans. Additionally, related to the refinancing activities we incurred $77.8 million of fees. The refinancing activities are explained in Note 7, Debt, to the condensed consolidated financial statements contained elsewhere in this prospectus.
For the Years Ended May 31, 2012, 2011 and 2010
The following is a summary of the cash flows by activity for the years ended May 31, 2012, 2011 and 2010:
| (in millions) | Year Ended May 31, 2012 | Year Ended May 31, 2011 | Year Ended May 31, 2010 | ||||||||
| Net cash from (used in): | |||||||||||
| Operating activities | $ | 377.3 | $ | 380.1 | $ | 321.5 | |||||
| Investing activities | (144.0 | ) | (205.0 | ) | (182.0 | ) | |||||
| Financing activities | (38.1 | ) | (51.4 | ) | (159.9 | ) | |||||
| Effect of exchange rate changes on cash | (30.6 | ) | 15.0 | (6.1 | ) | ||||||
| Change in cash and cash equivalents | $ | 164.6 | $ | 138.7 | $ | (26.5 | ) | ||||
Our cash and cash equivalents were $492.4 million as of May 31, 2012 compared to $327.8 million as of May 31, 2011. We generally maintain our cash and cash equivalents and investments in money market funds, corporate bonds and debt instruments. Cash and cash equivalents held outside of the United States were $302.3 million as of May 31, 2012. If we were to repatriate this cash back to the United States, additional tax of up to 35%, the maximum federal tax rate, could be incurred. In addition, we require a certain amount of cash to support on-going operations outside the United States.
Our cash and cash equivalents were $327.8 million as of May 31, 2011 compared to $189.1 million as of May 31, 2010. We maintain our cash and cash equivalents and investments in money market funds, time deposits, corporate bonds and debt instruments. We are exposed to interest rate risk on certain debt instruments.
Operating Cash Flows
Net cash provided by operating activities was $377.3 million for the year ended May 31, 2012, compared to cash flows provided of $380.1 million for the year ended May 31, 2011. Cash generated by operating activities continued to be a source of funds for deleveraging and investing in our growth. The decrease in cash provided by operating activities of $2.8 million was primarily due to an increase in cash paid for taxes due to net operating losses being fully utilized in the United States and an increase in accounts receivable due to increased sales with an increase in days sales outstanding, which was offset by favorability in inventory and accounts payable.
57
Net cash provided by operating activities was $380.1 million for the year ended May 31, 2011, compared to cash flows provided of $321.5 million for the year ended May 31, 2010. Cash generated by operating activities continued to be a source of funds for deleveraging and investing in our growth. The increase in cash provided by operating activities of $58.6 million was primarily due to working capital improvement initiatives and the prior year being negatively impacted by $53.0 million related to a previously disclosed litigation settlement. Net cash provided by operating activities for the year ended May 31, 2011 included a net loss of $849.8 million, offset by non-cash amounts of $1,222.1 million (primarily goodwill and intangible asset impairment charge, depreciation and amortization, and partially offset by deferred income taxes), and cash provided by working capital of $7.8 million. Net cash provided by operating activities for the year ended May 31, 2010 included a net loss of $47.6 million, offset by non-cash amounts of $460.4 million (primarily depreciation and amortization and stock based compensation, partially offset by deferred income taxes), and cash used in working capital of $91.3 million.
Investing Cash Flows
Net cash used in investing activities was $144.0 million for the year ended May 31, 2012 and $205.0 million for the year ended May 31, 2011. The decrease in cash used in investing activities year-over-year was primarily related to the investment in time deposits. During the fiscal year ended May 31, 2011 we invested in $78.7 million in time deposits and received proceeds of $44.3 million also related to the time deposits. During the fiscal year ended May 31, 2012 we received $33.4 million in proceeds related to the time deposits, but did not make any additional investments.
Net cash used in investing activities was $205.0 million for the year ended May 31, 2011 and $182.0 million for the year ended May 31, 2010. Cash generated by operating activities continued to be a source of funds for deleveraging and investing in our growth. Net cash used in investing activities for the years ended May 31, 2011 and 2010 primarily related to capital expenditures of $174.0 million and $186.4 million, respectively, and purchases of investments of $78.7 million and $13.3 million, respectively, partially offset by proceeds from the sale/maturity of investments of $59.3 million and $24.9 million, respectively.
Financing Cash Flows
Net cash used in financing activities was $38.1 million for the year ended May 31, 2012, compared to $51.4 million for the year ended May 31, 2011. The decrease in cash used in financing activities year-over-year was primarily related to a discretionary repurchase of $10.0 million par value of senior cash pay notes for $11.2 million in the fiscal year ended May 31, 2011.
Net cash used in financing activities was $51.4 million for the year ended May 31, 2011, compared to $159.9 million for the year ended May 31, 2010. Net cash used in financing activities for the year ended May 31, 2011 primarily related to required payments under the senior secured credit facilities of $34.8 million and a discretionary repurchase of $10.0 million par value of senior cash pay notes for $11.2 million. Net cash used in financing activities for the year ended May 31, 2010 primarily related to required payments under the senior secured credit facilities of $35.8 million, discretionary payments under the revolving credit facilities of $68.9 million, and discretionary payments under the asset-based revolving credit facility of $65.2 million, partially offset by proceeds under the revolving credit facilities of $20.4 million.
Balance Sheet Metrics
Cash flows from operations are impacted by profitability and changes in operating working capital. Management monitors operating working capital with particular focus on certain metrics, including days sales outstanding (“DSO”) and inventory turns. The following is a summary of our DSO and inventory turns for the nine months ended February 28, 2013 and the fiscal years ended May 31, 2012 and 2011.
___________________________________________________
| February 28, 2013 | May 31, 2012 | May 31, 2011 | ||||||
Days Sales Outstanding(1) | 63.9 | 62.5 | 62.3 | |||||
Inventory Turns (2) | 1.60 | 1.59 | 1.54 | |||||
| (1) | DSO is calculated by dividing the year-over-year average accounts receivable balance by the last twelve months net sales multiplied by 365 days. |
58
| (2) | Inventory turns are calculated by dividing the last twelve months cost of sales by the year-over-year average net inventory balance. |
We use DSO as a measure that places emphasis on how quickly we collect our accounts receivable balances from customers. The increase in DSOs is due to seasonality and increased sales in the last three quarters related to the Trauma Acquisition. We use inventory turns as a measure that places emphasis on how quickly we turn over our inventory. Inventory turns were slightly faster at February 28, 2013 due to the product rationalization, partially offset by integration of Trauma Acquisition inventory. These measures may not be computed the same as similarly titled measures used by other companies.
Our higher DSO when comparing May 31, 2012 to May 31, 2011 is the result of a global slowdown in customer payments, specifically in Europe. We were unable to continue factoring receivables in Spain as we have reached our limit on our current factoring facility, which is causing our DSO to increase. The favorability in inventory turns when comparing May 31, 2012 to May 31, 2011 was primarily driven by continued improvements in our global supply chain and field inventory management.
Non-GAAP Disclosures
We use certain non-GAAP financial measures to evaluate our performance using information that differs from what is required under GAAP. These non-GAAP financial measures may not be comparable to similar measures reported by other companies and should be considered in addition to, and not as a substitute for, or superior to, other measures prepared in accordance with GAAP.
Senior Secured Leverage Ratio
The senior secured leverage ratio provides a measure of our financial ability to meet our debt service obligations. The ratio level determines the interest rate charged on our cash flow revolving credit facilities, and letters of credit fees. In addition to determining the current interest rate on our cash flow revolving credit facilities, the ratio is also used as a benchmark in our credit agreements to determine maximum levels of additional indebtedness we may incur. We believe the directional trend of this ratio provides valuable insight to understanding our operational performance and financial position with respect to our debt obligations
February 28, 2013 and May 31, 2012
| (in millions, except ratios) | February 28, 2013 | May 31, 2012 | |||||
| USD Term Loan | $ | 2,226.7 | $ | 2,234.7 | |||
| EUR Term Loan | 1,085.0 | 1,039.6 | |||||
| Consolidated Senior Secured Debt | 3,311.7 | 3,274.3 | |||||
| Cash and Cash Equivalents | 217.4 | 492.4 | |||||
| Consolidated Senior Secured Debt Net of Cash and Cash Equivalents | $ | 3,094.3 | $ | 2,781.9 | |||
| LTM Adjusted EBITDA | $ | 1,079.1 | $ | 1,031.1 | |||
Senior Secured Leverage Ratio(1) | 2.87 | 2.70 | |||||
___________________________________________________
| (1) | Our senior secured leverage ratio is defined by our credit agreement as total consolidated senior secured debt net of cash and cash equivalents, as defined by our credit agreement, divided by the total of the last twelve months, or “LTM,” Adjusted EBITDA. |
| (2) | The LTM Adjusted EBITDA for February 28, 2013 includes nine months of Adjusted EBITDA during fiscal year 2013 of $801.4 million, plus the last three months of Adjusted EBITDA from fiscal year 2012 of $277.7 million. |
The increase in the senior secured leverage ratio at February 28, 2013 as compared to May 31, 2012 is primarily due to the decrease in cash and cash equivalents, as defined by our credit agreement, and the increase in the debt, partially offset by the increase in LTM Adjusted EBITDA. The cash decrease and the debt increase were driven by the refinancing activities that are explained in Note 7, Debt, to the condensed consolidated financial statements contained elsewhere in this prospectus as well as the impact of the Trauma Acquisition.
59
For the Years Ended May 31, 2012, 2011 and 2010
| (in millions, except ratios) | May 31, 2012 | May 31, 2011 | May 31, 2010 | ||||||||
| USD Term Loan B | $ | 2,234.7 | $ | 2,258.1 | $ | 2,281.5 | |||||
| EUR Term Loan B | 1,039.6 | 1,206.3 | 1,047.3 | ||||||||
| Consolidated Senior Secured Debt | 3,274.3 | 3,464.4 | 3,328.8 | ||||||||
Cash and Cash Equivalents(1) | 492.4 | 360.9 | 189.1 | ||||||||
Consolidated Senior Secured Debt Net of Cash and Cash Equivalents (1) | $ | 2,781.9 | $ | 3,103.5 | $ | 3,139.7 | |||||
| LTM Adjusted EBITDA | $ | 1,031.1 | $ | 1,010.4 | $ | 1,000.0 | |||||
“Run Rate” Cost Savings(2) | — | — | 12.6 | ||||||||
| LTM Adjusted EBITDA, plus cost savings | $ | 1,031.1 | $ | 1,010.4 | $ | 1,012.6 | |||||
Senior Secured Leverage Ratio(3) | 2.7 | 3.1 | 3.1 | ||||||||
___________________________________________________
| (1) | Cash and cash equivalents as defined by the credit agreement includes $33.1 million of time deposits at May 31, 2011. |
| (2) | As defined by the Credit Agreement dated September 25, 2007. |
| (3) | Our senior secured leverage ratio is defined by our credit agreement as total consolidated senior secured debt net of cash and cash equivalents, as defined by our credit agreement, divided by the total of the last twelve months, or “LTM,” Adjusted EBITDA, plus cost savings. |
The decrease in the senior secured leverage ratio at May 31, 2012 as compared to May 31, 2011 is primarily due to the weakening of the euro against the U.S. dollar, debt service payments and an increased Adjusted EBITDA in fiscal year 2012.
The decrease in the senior secured leverage ratio at May 31, 2011 as compared to May 31, 2010 is primarily due to debt service payments and an increase in cash and cash equivalents, partially offset by the strengthening of the euro against the U.S. dollar.
Adjusted EBITDA
We use Adjusted EBITDA, among other measures, to evaluate the performance of our core operations, establish operational goals and forecasts that are used in allocating resources and to evaluate our performance period-over-period, including for incentive program purposes. The term “as adjusted,” a non-GAAP financial measure, refers to financial performance measures that exclude certain income statement line items, such as interest, taxes, depreciation or amortization, other (income) expense and/or exclude certain expenses as defined by our credit agreement, such as restructuring charges, non-cash impairment charges, integration and facilities opening costs or other business optimization expenses, new systems design and implementation costs, certain start-up costs and costs related to consolidation of facilities, certain non-cash charges, advisory fees paid to the private equity owners, certain severance charges, purchase accounting costs, stock-based compensation, litigation costs, acquisition costs and other related charges.
60
For the Nine Months Ended February 28, 2013 and February 29, 2012
Adjusted EBITDA for the nine months ended February 28, 2013 and February 29, 2012 and the three months ended May 31, 2012 is calculated as follows:
___________________________________________________
| (in millions) | Nine Months Ended February 28, 2013 | Nine Months Ended February 29, 2012 | Three Months Ended May 31, 2012(1) | ||||||||
| Operating income (loss) | $ | (25.2 | ) | $ | 284.6 | $ | (378.0 | ) | |||
| Depreciation and amortization | 364.8 | 388.0 | 121.4 | ||||||||
Inventory step-up related to the Trauma Acquisition(2) | 3.3 | — | — | ||||||||
Stock-based compensation expense(3) | 32.3 | 12.2 | 3.8 | ||||||||
Litigation settlements and reserves and other legal fees(4) | 32.4 | 21.3 | (12.7 | ) | |||||||
Trauma Acquisition(2) | 10.3 | — | 4.6 | ||||||||
Operational restructuring and consulting expenses related to operational initiatives (severance, building impairments, abnormal manufacturing variances and other related costs)(5) | 18.5 | 39.8 | 6.0 | ||||||||
Product rationalization charges(6) | 22.7 | — | — | ||||||||
Sponsor fee(7) | 8.2 | 7.5 | 2.8 | ||||||||
Goodwill and intangible assets impairment charge(8) | 334.1 | — | 529.8 | ||||||||
Adjusted EBITDA(9) | $ | 801.4 | $ | 753.4 | $ | 277.7 | |||||
| (1) | The three months ended May 31, 2012 shows the activity from March 1, 2012 to May 31, 2012. |
| (2) | We exclude acquisition-related expenses for the Trauma Acquisition from non-GAAP financial measures that are not reflective of our ongoing operational performance. We further believe this information is useful to investors in that it provides period-over-period comparability. |
| (3) | Stock-based compensation expense is excluded from non-GAAP financial measures primarily because it is a non-cash expense. We believe that excluding this item is useful to investors in that it facilitates comparisons to competitors’ operating results. |
| (4) | We exclude certain litigation-related expenses and settlements from non-GAAP financial measures that are not reflective of our ongoing operational performance. We believe this information is useful to investors in that it provides period-over-period comparability. |
| (5) | Restructuring charges relate principally to employee severance and facility consolidation costs resulting from the closure of facilities and other workforce reductions attributable to our efforts to reduce costs. Operational restructuring charges also include abnormal manufacturing variances related to temporary redundant overhead costs within our plant network as we continue to rationalize and move production to our larger operating locations in order to increase manufacturing efficiency. We exclude these costs from non-GAAP financial measures primarily because they are not reflective of the ongoing operating results and they are not used by management to assess ongoing operational performance. We believe the exclusion of this information in the applicable non-GAAP financial measure is useful to investors in that it provides period-over-period comparability. |
| (6) | We exclude expenses for product rationalization charges from non-GAAP financial measures that are not reflective of our ongoing operational performance. We further believe this information is useful to investors in that it provides period-over-period comparability. |
| (7) | Upon completion of the Merger, we entered into a management services agreement with certain affiliates of the Sponsors, pursuant to which such affiliates of the Sponsors or their successors, assigns, affiliates, officers, employees, and/or representatives and third parties (collectively, the “Managers”) provide management, advisory, and consulting services to us. Pursuant to such agreement, the Managers received a transaction fee equal to 1% of total enterprise value of the Transactions for the services rendered by such entities related to the Transactions upon entering into the agreement, and the Sponsors receive an annual monitoring fee equal to 1% of our annual Adjusted EBITDA (as defined by our credit agreement) as compensation for the services rendered and reimbursement for out-of-pocket expenses incurred by the Managers in connection with the agreement and the Transactions. We exclude these costs from non-GAAP financial measures primarily because they are not reflective of the ongoing operating results and they are not used by management to assess ongoing operational performance. We further believe this information is useful to investors in that it provides period-over-period comparability. |
| (8) | During fiscal 2013, we recorded a $334.1 million goodwill and definite and indefinite-lived intangible asset impairment charge associated with our Dental Reconstructive reporting unit. Also, during fiscal 2012, we recorded a $529.8 million goodwill and definite and indefinite-lived intangible asset impairment charge primarily associated with our dental reconstructive and spine & bone healing reporting units. We exclude this non-cash charge from non-GAAP financial measures because it is not reflective of our ongoing operational performance or liquidity. We believe the exclusion of this information in the applicable non-GAAP financial measure is useful to investors in that it provides period-over-period comparability. |
| (9) | As defined in our credit agreement. |
Adjusted EBITDA growth has historically generally been in line with the growth in net sales and has continued the trend for the three and nine months ended February 28, 2013 as compared to the three and nine months ended February 29, 2012.
61
For the Years Ended May 31, 2012, 2011 and 2010
Adjusted EBITDA for the fiscal years ended May 31, 2012, 2011 and 2010 is calculated as follows:
| (in millions) | Year Ended May 31, 2012 | Year Ended May 31, 2011 | Year Ended May 31, 2010 | ||||||||
| Operating income (loss) | $ | (93.4 | ) | $ | (576.9 | ) | $ | 356.6 | |||
| Depreciation | 182.2 | 181.1 | 175.0 | ||||||||
| Amortization | 327.2 | 367.9 | 372.6 | ||||||||
| Special items adjustments: | |||||||||||
Stock-based compensation expense(1) | 16.0 | 12.7 | 22.4 | ||||||||
Litigation settlements and reserves and other legal fees(2) | 8.6 | 12.5 | 10.7 | ||||||||
DePuy trauma acquisition(3) | 4.6 | ||||||||||
Operational restructuring and consulting expenses related to operational initiatives (severance, building impairments, abnormal manufacturing variances and other related costs)(4) | 45.8 | 61.6 | 43.3 | ||||||||
Sponsor fee(5) | 10.3 | 10.1 | 10.1 | ||||||||
Greece bad debt expense (6) | |||||||||||
Goodwill and intangible assets impairment charge(7) | 529.8 | 941.4 | 9.3 | ||||||||
Adjusted EBITDA(8) | $ | 1,031.1 | $ | 1,010.4 | $ | 1,000.0 | |||||
___________________________________________________
| (1) | Stock-based compensation expense is excluded from non-GAAP financial measures primarily because it is a non-cash expense. We believe that excluding this item is useful to investors in that it facilitates comparisons to competitors’ operating results. |
| (2) | We exclude certain litigation-related expenses and settlements from non-GAAP financial measures that are not reflective of our ongoing operational performance. We believe this information is useful to investors in that it provides period-over-period comparability. |
| (3) | We exclude acquisition-related expenses for the DePuy trauma acquisition from non-GAAP financial measures that are not reflective of the Company’s ongoing operational performance. The Company further believes this information is useful to investors in that it provides period-over-period comparability. |
| (4) | Restructuring charges relate principally to employee severance and facility consolidation costs resulting from the closure of facilities and other workforce reductions attributable to our efforts to reduce costs. Operational restructuring charges also include abnormal manufacturing variances related to temporary redundant overhead costs within our plant network as we continue to rationalize and move production to our larger operating locations in order to increase manufacturing efficiency. We exclude these costs from non-GAAP financial measures primarily because they are not reflective of the ongoing operating results and they are not used by management to assess ongoing operational performance. We believe the exclusion of this information in the applicable non-GAAP financial measure is useful to investors in that it provides period-over-period comparability. |
| (5) | Upon completion of the Merger, we entered into a management services agreement with certain affiliates of the Sponsors, pursuant to which such affiliates of the Sponsors or their successors, assigns, affiliates, officers, employees, and/or representatives and third parties (collectively, the “Managers”) provide management, advisory, and consulting services to us. Pursuant to such agreement, the Managers received a transaction fee equal to 1% of total enterprise value of the Transactions for the services rendered by such entities related to the Transactions upon entering into the agreement, and the Sponsors receive an annual monitoring fee equal to 1% of our annual Adjusted EBITDA (as defined by our credit agreement) as compensation for the services rendered and reimbursement for out-of-pocket expenses incurred by the Managers in connection with the agreement and the Transactions. We exclude these costs from non-GAAP financial measures primarily because they are not reflective of the ongoing operating results and they are not used by management to assess ongoing operational performance. |
| (6) | This charge is related to the proposal the Greek government announced on June 15, 2010 to settle their outstanding debts from 2007 through 2009 primarily by issuing zero-coupon bonds. We exclude this charge from non-GAAP measures primarily because it is not reflective of ongoing operating results. |
| (7) | During fiscal 2012, we recorded at $529.8 million goodwill and definite and indefinite-lived intangible asset impairment charge primarily associated with our dental reconstructive and spine & bone healing reporting units and in fiscal 2011, we recorded a $941.4 million goodwill and definite and indefinite-lived intangible asset impairment charge primarily associated with our Europe reporting unit. We exclude this non-cash charge from non-GAAP financial measures because it is not reflective of our ongoing operational performance or liquidity. We believe the exclusion of this information in the applicable non-GAAP financial measure is useful to investors in that it provides period-over-period comparability |
| (8) | As defined in our credit agreement. |
Adjusted EBITDA growth has historically generally been in line with the growth in net sales. The fall through from net sales to Adjusted EBITDA has slowed due to a decline in gross margin percentage.
Credit Facilities and Notes
Senior Secured Credit Facilities
62
On September 25, 2007, we entered into a credit agreement and related security and other agreements providing for (a) a $2,340.0 million U.S. dollar-denominated term loan facility and a €875.0 million (approximately $1,207.4 million at September 25, 2007) euro-denominated term loan facility and (b) $400.0 million cash flow revolving credit facilities with Bank of America, N.A. as administrative agent and collateral agent. We refer to our term loan facilities and our cash flow revolving credit facilities collectively as the “senior secured credit facilities.”
Our senior secured credit facilities contain a number of covenants that, among other things are subject to certain exceptions, will restrict our ability and the ability of our restricted subsidiaries to: (1) incur additional indebtedness; (2) pay dividends on our capital stock or redeem, repurchase or retire our capital stock or indebtedness; (3) make investments, loans, advances and acquisitions; (4) create restrictions on the payment of dividends or other amounts to us from our restricted subsidiaries; (5) engage in transactions with our affiliates; (6) sell assets, including capital stock of our subsidiaries; (7) consolidate or merge; (8) create liens; and (9) enter into sale and lease-back transactions. The credit agreement governing our senior secured credit facilities does not require us to comply with any financial ratio maintenance covenants. As of February 28, 2013, we were in compliance with our covenants and intend to maintain compliance.
The credit agreement governing our senior secured credit facilities also contains certain customary affirmative covenants and events of default.
On August 2, 2012, we entered into an amendment and restatement agreement that amended our existing senior secured credit facilities. The amendment (i) extended the maturity of approximately $1,007.2 million of our U.S. dollar-denominated term loans and approximately €631.3 million of our euro-denominated term loans under the credit facility to July 25, 2017, (ii) refinanced and replaced the previous alternative currency revolving credit commitments under the credit facility with a new class of alternative currency revolving credit commitments in an aggregate amount of $165.0 million and (iii) refinanced and replaced the previous U.S. dollar revolving credit commitments under the credit facility with a new class of U.S. dollar-denominated revolving credit commitments in an aggregate amount of $165.0 million. The new revolving credit commitments will mature on April 25, 2017, except that, if as of December 23, 2014, there is an outstanding aggregate principal amount of non-extended U.S. dollar and euro term loans in excess of $200.0 million, then such revolving credit commitments will mature on December 24, 2014.
The joinder agreement was entered into pursuant to our senior secured credit facility, as amended by the amendment and restatement agreement dated August 2, 2012. By entering into the joinder agreement, the joining lenders party thereto have agreed to extend the maturity of (i) approximately $392.7 million of Biomet’s U.S. dollar-denominated term loans and (ii) approximately €32.9 million of Biomet’s euro-denominated term loans, to July 25, 2017. The term loans extended pursuant to the joinder agreement are on terms identical to the terms loans that were extended pursuant to the prior Amendment. The remaining term loans of the lenders under the senior secured credit facilities who did not elect to extend such loans either pursuant to the August 2 amendment and restatement agreement or the subsequent joinder agreement will continue to mature on March 25, 2015.
In addition, on December 27, 2012, we completed a $730.0 million add-on to our extended U.S. dollar-denominated term loan. The proceeds from the add-on were used to refinance the non-extended U.S. dollar-denominated term B loan, which was net of fees associated with the add-on closing. The terms of the add-on are consistent with the terms in the August 2 amendment.
Asset-based Revolving Credit Facility
On November 14, 2012, we entered into an asset-based credit agreement and related security and other agreements for a senior secured asset-based revolving credit facility with Bank of America, N.A., as administrative agent and collateral agent. The Credit Agreement provides senior secured financing of up to $500.0 million, subject to borrowing base limitations. Under the Credit Agreement there is (i) a U.S. subfacility in an aggregate principal amount of up to $400 million and (ii) a Dutch subfacility in an aggregate principal amount of up to the Euro equivalent of $100.0 million. We and our wholly-owned domestic subsidiaries are the borrowers under the U.S. subfacility and Biomet GSCC, a Dutch subsidiary, is the borrower under the Dutch subfacility.
The U.S. borrowing base at any time will equal the sum of 85% of eligible accounts receivable and 85% of the net orderly liquidation value of eligible inventory (not to exceed 65% of the borrowing base), less certain reserves and subject to certain limitations on eligible consignment inventory and accounts receivable owed by non-U.S. persons. The asset-based credit agreement includes a $100 million U.S. sublimit for letters of credit under the U.S. subfacility and the euro equivalent of $25.0 million sublimit for letters of credit under the Dutch subfacility.
63
Under the U.S. subfacility there is also a swingline sublimit for same-day borrowings of up to the lesser of (i) $50.0 million and (ii) the aggregate principal amount of the commitments under the U.S. sub-facility. At the closing of the transactions, we borrowed approximately $80.0 million under the U.S. subfacility to repay obligations under our existing asset-based credit agreement entered into on September 25, 2007. As of February 28, 2013 there were no borrowings outstanding under our asset-based credit facility.
Borrowings under the asset-based credit agreement bear interest at a rate per annum dependent upon the average availability of the applicable subfacility as set forth in the following pricing grid:
Average Availability | Adjusted Eurocurrency Rate for Loans and Letter of Credit Fees | Base Rate | ||
≥66 2/3% | 1.75% | 0.75% | ||
<66 2/3% but ≥ 33 1/3% | 2.00% | 1.00% | ||
<33 1/3% | 2.25% | 1.25% | ||
In addition, the we are required to pay a commitment fee of (i) 0.25% per annum if the amount of outstanding loans, unreimbursed letter of credit drawings and undrawn letters of credit under the senior secured asset-based revolving credit facility exceed 50% of the commitment amount, and (ii) if otherwise, 0.375% per annum, on the average daily unused portion of the senior secured asset-based revolving credit facility, payable quarterly in arrears.
The senior secured asset-based revolving credit facility will mature on July 25, 2017; provided, however, that if as of December 23, 2014, there is an outstanding aggregate principal amount of non-extended U.S. dollar and euro term loans in excess of $200 million under our cash flow credit agreement, then the loans under the Credit Agreement will mature on December 24, 2014.
Like our senior secured credit facilities described above, our asset-based revolving credit facility contains a number of covenants that restrict Parent, us and our restricted subsidiaries. The credit agreement governing our asset-based revolving credit facility also contains certain customary affirmative covenants and events of default. As of February 28, 2013, we were in compliance with our covenants and intend to maintain compliance.
Notes
We issued an aggregate of $2,348.0 million of original notes on September 25, 2007 and an aggregate of $217.0 million of original notes on October 16, 2007 (which were issued at a premium above par of $6.0 million). The notes are our unsecured obligations, with $1,550.0 million being our senior obligations (consisting of $775.0 million of senior cash pay notes and $775.0 million of senior PIK toggle notes) and $1,015.0 million being our senior subordinated obligations. All of the notes were issued by Biomet and are guaranteed by each of its existing and future wholly-owned domestic subsidiaries that guarantee our obligations under our senior secured credit facilities. Interest is payable in cash.
On August 8, 2012 Biomet completed its offering of $1.0 billion aggregate principal amount of senior notes. We used the net proceeds of this offering to fund a tender offer for any and all of our outstanding senior toggle notes, including related fees and expenses, and to purchase, redeem, defease or otherwise acquire or retire our outstanding indebtedness. On October 2, we completed our offering of $825.0 million aggregate principal amount of additional senior notes and $800.0 million aggregate principal amount of senior subordinated notes. We used the net proceeds of those offerings, together with cash on hand and other sources, to purchase any and all of our 10% Senior Cash Pay Notes and $940.0 million principal amount of our outstanding 11⅝% Senior Subordinated Notes. On November 1, 2012, we purchased and redeemed all remaining outstanding 11⅝% Senior Subordinated Notes using cash on hand and asset-based revolver proceeds.
The indentures, among other things, limit our and our restricted subsidiaries’ ability to incur additional indebtedness or issue certain preferred stock, pay dividends and make other restricted payments, make certain investments, sell assets, create liens, consolidate, merge or sell all or substantially all of our assets, enter into transactions with affiliates and designate subsidiaries as unrestricted subsidiaries. These covenants are subject to important exceptions during any period of time for which (i) the respective notes have received investment grade ratings from certain specified rating agencies and (ii) no default has occurred and is continuing under the indentures
64
that govern the respective notes. As of February 28, 2013, we were in compliance with our covenants and intend to maintain compliance.
Non-U.S. Facility
As of February 28, 2013, we had a loan in Spain referred to as the non-U.S. facility. During the month of November 2011, ABN AMRO Bank terminated the European revolver facility due to the limited use of the facility. As of February 28, 2013, we had $2.6 million in outstanding borrowings under our non-U.S. facility.
Capital Expenditures and Investments
We maintain our cash and investments in money market funds, certificates of deposit, equity securities and Greek bonds. We are exposed to interest rate risk on our corporate bonds and debt instruments. We see the growth prospects in our markets and intend to invest in an effort to improve our worldwide market position. We expect to spend in excess of $500.0 million over the next two fiscal years for capital expenditures (including instrumentation issued to the field) and research and development costs in an effort to develop products and technologies that further enhance musculoskeletal procedures. Funding of these and other activities is expected to come from currently available funds, cash flows generated from operations, and currently available credit lines.
Contractual Obligations
Summarized in the table below are our long-term obligations and commitments as of February 28, 2013. We have issued notes, entered into senior secured credit facilities, including term loan facilities and cash flow revolving credit facilities, and an asset-based revolving facility, all of which are primarily classified as long-term obligations. There were no borrowings outstanding under our asset-based revolving facility as of February 28 2013. As of February 28, 2013, required principal payments of $33.4 million were due within the next twelve months. Our term loan facilities require payments each year in an amount equal to (x) 0.25% of the product of (i) the aggregate principal amount of all euro-denominated term loans and dollar-denominated term loans outstanding under the original credit agreement on the closing date multiplied by (ii) a fraction, the numerator of which is the aggregate principal amount of euro-denominated term B loans and dollar-denominated term B loans outstanding on August 2, 2012 (after giving effect to certain conversions to occur on or after August 2, 2012 pursuant to the amended and restated credit agreement) and the denominator of which is the aggregate principal amount of all outstanding term loans on August 2, 2012 and (y) 0.25% of the aggregate principal amount of all outstanding euro-denominated term B-1 loans and dollar-denominated term B-1, in each case in equal calendar quarterly installments until maturity of the loan and after giving effect to the application of any prepayments. As of February 28, 2013, required principal payments of $33.4 million were due within the next twelve months.
Our revolving borrowing base available under all debt facilities at February 28, 2013 was $795.5 million, which is net of the borrowing base limitations relating to the asset-based revolving credit facility.
| (in millions) | Total | 2013 | 2014 and 2015 | 2016 and 2017 | 2018 and Thereafter | ||||||||||||||
Contractual obligations(1) | |||||||||||||||||||
| Projected future pension benefit payments | $ | 60.9 | $ | 4.9 | $ | 10.9 | $ | 11.7 | $ | 33.4 | |||||||||
| Long-term debt (including current maturities) | 5,978.4 | 9.4 | 383.6 | 59.8 | 5,525.6 | ||||||||||||||
Interest payments(2) | 2,336.9 | 385.3 | 654.7 | 634.2 | 662.7 | ||||||||||||||
| Material purchase commitments | 125.7 | 41.2 | 48.3 | 26.3 | 9.9 | ||||||||||||||
| Outsourcing contract obligation | 6.0 | 5.5 | 0.5 | — | — | ||||||||||||||
| DePuy trauma acquisition purchase price commitment | 280.0 | 280.0 | — | — | — | ||||||||||||||
| Total contractual obligations | $ | 8,787.9 | $ | 726.3 | $ | 1,098.0 | $ | 732.0 | $ | 6,231.6 | |||||||||
___________________________________________________
| (1) | The total amounts of capital lease obligations and operating lease obligations are not significant. |
| (2) | Amounts include the effect of interest rate swaps currently in place. |
In addition, due to the uncertainty with respect to the timing of future cash flows associated with our unrecognized tax benefits at May 31, 2012, we are unable to make reasonably reliable estimates of the period of
65
cash settlement with the respective taxing authorities. Therefore, $63.0 million of unrecognized tax benefits have been excluded from the contractual obligations table above.
See “Description of Other Indebtedness” and Note 6 to our audited financial statements included elsewhere in this prospectus for more information on our debt offering and amendment of our existing secured senior cash flow credit facility.
We believe that our cash, other liquid assets and operating cash flow, together with available borrowings and potential access to credit and capital markets, will be sufficient to meet our operating expenses, research and development costs, capital expenditures and to service our debt requirements as they become due. However, our ongoing ability to meet our substantial debt service and other obligations will be dependent upon our future performance, which will be subject to business, financial, economic, regulatory and other factors. We will not be able to control many of these factors, such as economic conditions and regulatory changes in the markets where we operate and pressure from competitors. We cannot be certain that our cash flow will be sufficient to allow us to pay principal and interest on our debt, support our operations and meet our other obligations. If we do not have sufficient liquidity, we may be required to refinance all or part of our existing debt, sell assets or borrow more money. We cannot guarantee that we will be able to do so on terms acceptable to us, if at all. In addition, the terms of existing or future debt agreements may restrict us from pursuing any of these alternatives. See “Risk Factors—Risks Related to Our Indebtedness and the Notes.”
Off-Balance Sheet Arrangements
We do not currently have any off-balance sheet arrangements that have or are reasonably likely to have a material current or future effect on our financial condition, results of operations, liquidity, capital expenditures or capital resources.
Critical Accounting Policies and Estimates
Management’s Discussion and Analysis of Financial Condition and Results of Operations are based upon our consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of these financial statements requires management to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses, and related disclosure of contingent assets and liabilities. In management’s opinion, our critical accounting policies include revenue recognition, excess and obsolete inventory, goodwill and intangible assets, legal proceedings and other loss contingencies, and income taxes. For further information, including our significant accounting policies, refer to the audited consolidated financial statements and our unaudited condensed consolidated interim financial statements and, in each case, the notes thereto included elsewhere in this prospectus.
Revenue Recognition
We sell product through four principal channels: (1) direct to healthcare institutions, referred to as direct channel accounts, (2) through stocking distributors and healthcare dealers, (3) indirectly through insurance companies and (4) directly to dental practices and dental laboratories. Sales through the direct and distributor/dealer channels account for a majority of net sales. Through these channels, inventory is consigned to sales agents or customers so that products are available when needed for surgical procedures. Revenue is not recognized upon the placement of inventory into consignment as we retain title and maintain the inventory on the balance sheet; however, it is recognized upon implantation and receipt of proper purchase order and/or purchase requisition documentation. Pricing for products is predetermined by contracts with customers, agents acting on behalf of customer groups or by government regulatory bodies, depending on the market. Price discounts under group purchasing contracts are linked to volume of implant purchases by customer healthcare institutions within a specified group. At negotiated thresholds within a contract buying period, price discounts may increase.
At certain locations we record a contractual allowance that is offset against revenue for each sale to a non-contracted payor so that revenue is recorded at the estimated determinable price at the time of the sale. Those non-contracted payors and insurance companies in some cases do not have contracted rates for products sold, but may have pricing available for certain products through their respective web sites. We will invoice at its list price and establish the contractual allowance to estimate what the non-contracted payor will settle the claim for based on the information available as noted above. At certain locations, revenue is recognized on sales to stocking distributors, healthcare dealers, dental practices and dental laboratories when title to product passes to them, generally upon shipment. Certain subsidiaries allow customers to return product in the event that we terminate the relationship.
66
Under those circumstances, we record an estimated sales return in the period in which constructive notice of termination is given to a distributor. Product returns were not significant for any period presented.
We also maintain a separate allowance for doubtful accounts for estimated losses based on our assessment of the collectability of specific customer accounts and the aging of the accounts receivable. We analyze accounts receivable and historical bad debts, customer concentrations, customer solvency, current economic and geographic trends, and changes in customer payment terms and practices when evaluating the adequacy of our current and future allowance. In circumstances where we are aware of a specific customer’s inability to meet its financial obligations, a specific allowance for bad debt is estimated and recorded, which reduces the recognized receivable to the estimated amount we believe will ultimately be collected. We monitor and analyze the accuracy of the allowance for doubtful accounts estimate by reviewing past collectability and adjust it for future expectations to determine the adequacy of our current and future allowance. Our reserve levels have generally been sufficient to cover credit losses.
Excess and Obsolete Inventory
In our industry, inventory is routinely placed at hospitals to provide the healthcare provider with the appropriate product when needed. Because product usage tends to follow a bell curve, larger and smaller sizes of inventory are provided, but infrequently used. In addition, the musculoskeletal market is highly competitive, with new products, raw materials and procedures being introduced continually, which may make those products currently on the market obsolete. We make estimates regarding the future use of these products, which are used to adjust inventory to the lower of cost or market. If actual product life cycles, product demand or market conditions are less favorable than those projected by management, additional inventory write-downs may be required which would affect future operating results.
Goodwill and Other Intangible Assets
We operate in one reportable segment and evaluate goodwill for impairment at the reporting unit level. We have six identified reporting units for the purpose of testing goodwill for impairment. The reporting units are based on our current administrative organizational structure and the availability of discrete financial information.
During the fourth quarter of fiscal year 2012, we recorded a $529.8 million goodwill and definite and indefinite-lived intangible asset impairment charge primarily associated with our spine & bone healing and dental reconstructive reporting units. As of February 29, 2012, we concluded that certain indicators were present that suggested impairment may exist for our dental reconstructive reporting unit’s goodwill and intangible assets. The indicators of impairment in our dental reconstructive reporting unit included evidence of declining industry market growth rates in certain European and Asia Pacific markets and unfavorable margin trends resulting from change in product mix. The impact of these recent items resulted in management initiating an interim preliminary impairment test as of February 29, 2012. However, the preliminary result of this interim test of impairment for the dental reconstructive reporting unit’s goodwill and intangibles was inconclusive during the third quarter of fiscal year 2012. We finalized impairment test during the fourth quarter of fiscal year 2012. During the annual impairment test, described below, our spine and bone healing reporting unit failed step one. The indicators were primarily due to growth rate declines as compared to prior assumptions.
During the fourth quarter of fiscal year 2011, we recorded a $941.4 million goodwill and definite and indefinite-lived intangible asset impairment charge primarily associated with our Europe reporting unit. As of February 28, 2011, we concluded that certain indicators were present that suggested impairment may exist for our Europe reporting unit’s goodwill and intangibles. The indicators of potential impairment in our Europe reporting unit included:
| • | recent reductions in revenue growth rates for the reporting unit’s knee and hip products; |
| • | recent market pressure resulting in reduced average selling prices of the reporting unit’s products; |
| • | evidence of declining industry market growth rates for many countries; and |
| • | certain European governments actively pursuing healthcare spend restructuring programs. |
67
The impact of these recent items resulted in management initiating an interim preliminary impairment test as of February 28, 2011. However, the preliminary result of this interim test of impairment for the Europe reporting unit’s goodwill and intangibles was inconclusive during the third quarter of fiscal year 2011. We finalized the impairment tests during the fourth quarter of fiscal year 2011.
We used only the income approach, specifically the discounted cash flow method, to determine the fair value of the dental reconstructive, spine & bone healing and Europe reporting units and the associated amount of the impairment charges. This approach calculates fair value by estimating the after-tax cash flows attributable to a reporting unit and then discounting these after-tax cash flows to a present value using a risk-adjusted discount rate. This methodology is consistent with how we estimate the fair value of our reporting units during our annual goodwill and indefinite lived intangible asset impairment tests. In applying the income approach to calculate the fair value of the dental reconstructive, spine & bone healing and Europe reporting units, we used assumptions about future revenue contributions and cost structures. In addition, the application of the income approach for both goodwill and intangibles requires judgment in determining a risk-adjusted discount rate at the reporting unit level. We based this determination on estimates of the weighted-average costs of capital of market participants. We performed a peer company analysis and considered the industry the weighted-average return on debt and equity from a market participant perspective.
To calculate the amount of the impairment charge related to the dental reconstructive, spine & bone healing and Europe reporting units, we allocated the reporting unit’s fair value to all of its assets and liabilities, including certain unrecognized intangible assets, in order to determine the implied fair value of goodwill. This allocation process required judgment and the use of additional valuation assumptions in deriving the individual fair values of our dental reconstructive, spine & bone healing and Europe reporting unit’s assets and liabilities as if the reporting units had been acquired in a business combination.
We also performed our annual assessment for impairment as of March 31, 2012 for all six reporting units. We utilized discount rates ranging from 9.2% to 13.5%. Based on the discount rate used in our most recent test for impairment, if the discount rate increased by 1% the fair value of the consolidated company could be lower by approximately $1.3 billion and a decrease in the discount rate of 1% results in an increase in fair value of $1.8 billion. The step one test also includes assumptions derived from competitor market capitalization and beta values as well as the twenty year Treasury bill rate as of March 31, 2012. The only reporting unit that failed step one and was required to complete a step two analysis was the spine & bone healing reporting unit.
The estimates and assumptions underlying the fair value calculations used in our annual impairment tests are uncertain by their nature and can vary significantly from actual results. Factors that management must estimate include, but are not limited to, industry and market conditions, sales volume and pricing, raw material costs, capital expenditures, working capital changes, cost of capital, royalty rates and tax rates. These factors are especially difficult to predict when global financial markets are volatile. The estimates and assumptions used in our impairment tests are consistent with those we use in our internal planning. These estimates and assumptions may change from period to period. If we use different estimates and assumptions in the future, future impairment charges may occur and could be material.
We have identified a total of four reporting units with a material amount of goodwill that are at a higher risk of potential failure of step one of the goodwill impairment test in the future. These reporting units include our U.S. Reconstructive reporting unit ($2,973.4 million of goodwill), our International reporting unit ($523.5 million of goodwill), our dental reconstructive reporting unit ($66.3 million of goodwill) and our Europe reporting unit ($299.4 million). The level of excess fair value over carrying value for these higher risk reporting units were each less than 10% for the latest step one impairment test.
We recorded a goodwill and intangible asset impairment charge of $334.1 million in the third quarter of fiscal year 2013 that was related to our Dental Reconstructive reporting unit, due to evidence of continued declining industry market growth rates in certain European and Asia Pacific markets and corresponding unfavorable margin trends.
Other Loss Contingencies
We accrue anticipated costs of settlement, damages, and loss of product liability claims based on historical experience or to the extent specific losses are probable and estimable. If the estimate of a probable loss is in a range and no amount within the range is more likely, we accrue the minimum amount of the range. Such estimates and any subsequent changes in estimates may result in adjustments to our operating results in the future. We have self-
68
insured reserves against product liability claims with insurance coverage above the retention limits. There are various other claims, lawsuits and disputes with third parties, investigations and pending actions involving various allegations against it. Product liability claims are routinely reviewed by our insurance carriers and management routinely reviews all claims for purposes of establishing ultimate loss estimates.
Income Taxes
There are inherent risks that could create uncertainties related to our income tax estimates. We adjust estimates based on normal operating circumstances and conclusions related to tax audits. While we do not believe any audit finding could materially affect our financial position, however, there could be a material impact on our consolidated results of operations and cash flows of a given period.
Our operations are subject to the tax laws, regulations and administrative practices of the United States, U.S. state jurisdictions and other countries in which we do business. We must make estimates and judgments in determining the provision for taxes for financial statement purposes. These estimates and judgments occur in the calculation of tax credits, benefits, and deductions, and in the calculation of certain tax assets and liabilities that arise from differences in the timing of recognition of revenue and expense for tax and financial statement purposes, as well as the interest and penalties related to uncertain tax positions. Significant changes in these estimates may result in an increase or decrease to our tax provision in a subsequent period.
The calculation of our tax liabilities involves accounting for uncertainties in the application of complex tax regulations. We recognize liabilities for uncertain tax benefits (“UTBs”) based on a two-step process. We recognize the tax benefit from an UTB only if it is more likely than not that the tax position will be sustained on examination by the taxing authorities based on the technical merits of the position. The amount of UTBs is measured as appropriate for changes in facts and circumstances, such as significant amendments to existing tax law, new regulations or interpretations by the taxing authorities, new information obtained during a tax examination, or resolution of an examination. We believe our estimates for UTBs are appropriate and sufficient for any assessments that may result from examinations of our tax returns. We recognize both accrued interest and penalties, where appropriate, related to UTBs as a component of income tax expense.
Certain items are included in our tax return at different times than they are reflected in our financial statements. Such timing differences create deferred tax assets and liabilities. Deferred tax assets are generally items that can be used as a tax deduction or credit in the tax return in future years but for which we have already recorded the tax benefit in the financial statements. We have recorded valuation allowances against certain of our deferred tax assets, primarily those that have been generated from net operating losses and tax credit carryforwards in certain taxing jurisdictions. In evaluating whether we would more likely than not recover these deferred tax assets, we have not assumed any future taxable income or tax planning strategies in the jurisdictions associated with these carryforwards where history does not support such an assumption. Implementation of tax planning strategies to recover these deferred tax assets or future income generation in these jurisdictions could lead to the reversal of these valuation allowances and a reduction of income tax expense. Deferred tax liabilities are either: (i) a tax expense recognized in the financial statements for which payment has been deferred; or (ii) an expense for which we have already taken a deduction on the tax return, but have not yet recognized the expense in the financial statements.
We have not historically provided for U.S. or additional foreign taxes on the excess of the amount of financial reporting over the tax basis of investments in non-U.S. subsidiaries. A company is not required to recognize a deferred tax liability for the outside basis difference of an investment in a non-U.S. subsidiary or a non-U.S. corporate joint venture that is essentially permanent in duration, unless it becomes apparent that such difference will reverse in the foreseeable future. The excess of financial reporting basis over tax basis of investments in non-U.S. subsidiaries is primarily attributable to the financial restatement of the carrying amount of these investments due to the Merger, adjusted for subsequent accumulation of earnings and losses. It is our practice and intention to continue to permanently reinvest a substantial portion of the reported earnings of our non-U.S. subsidiaries in non-U.S. operations. Currently, there are no plans to divest any of our investments in non-U.S. subsidiaries. It is not practicable to estimate the amount of deferred tax liability related to excess of financial reporting basis over tax basis in these non-U.S. subsidiaries. Our non-U.S. subsidiaries have not accumulated positive reported earnings subsequent to the Merger. However, to the extent it is determined that any amounts of excess cash will be repatriated, we will record a deferred tax liability reflecting the estimated amount of tax that will be payable due to such repatriation. If future events, including material changes in estimates of cash, working capital and long-term investment requirements necessitate repatriation of portions of the earnings currently treated as permanently reinvested, under current tax laws an additional tax provision may be required which could have a material effect on our financial results.
69
INDUSTRY OVERVIEW
We participate in the worldwide orthopedic and dental implant markets, which management estimates to be greater than $40 billion. We believe the global economic uncertainty has impacted the year-over-year market growth rates of the orthopedic reconstructive device industry from the historical rates in the high single digits to recent market growth rates in the flat-to-low single digit range. The orthopedic industry benefits from several favorable factors, including, but not limited to:
Favorable Demographics. An aging population is driving growth in the orthopedic products market. Many conditions that require orthopedic surgery affect people in middle age or later in life. As the baby boomer population ages and life expectancy increases, the elderly will represent a higher percentage of the overall population. According to a 2008 U.S. Census Bureau projection, the U.S. population aged 65 and over is expected to grow from 40 million and 13% of the population in 2010 to 72 million and 19% of the population by 2030. According to a 2011 Eurostat projection, the European population aged 65 and over will grow from 16% of the population in 2010 to 21% of the population in 2030.
Stable Industry Structure. Following a period of consolidation during the late 1990s, over the past twelve years, we, together with Zimmer Holdings, Inc., DePuy, Inc. (a Johnson & Johnson company), Stryker Corporation and Smith & Nephew plc, have constituted over 85% of the orthopedic reconstructive industry’s worldwide revenues. These players have achieved critical components to success, including product innovations and advancements, accumulation of clinical data, regulatory expertise, economies of scale, and sales force and surgeon customer relationships, which have led to minimal market share movement among top players from year to year.
Close Working Relationships with Surgeon Customers. Due to the nature of orthopedic implants, the orthopedic medical device industry is unique with respect to the working relationships between orthopedic device manufacturers and their surgeon customers. As a component of innovation in the industry, some surgeons serve as consultants and are instrumental in the development of new products and the ongoing evaluation and improvement of existing products.
Technological Advancement of Orthopedic Products. Incremental and continuous technological advancement of orthopedic products is expanding the addressable market. Product innovation strives to improve the durability and performance of orthopedic devices and promote less invasive surgery. Examples include bearing surfaces in hips with potential for greater longevity, premium knee systems that allow greater range of motion, and non-cemented hip stems that facilitate minimally invasive hip procedures. As a result of ongoing innovation, we believe that surgeons are increasingly recommending and utilizing implant products for younger patients as well as elderly patients who are remaining healthier and more active than those of past generations.
Favorable Product Mix Shift. Continued product innovation is driving a favorable shift in mix towards premium products that offer enhanced outcomes for patients. Product evolution is also expanding the addressable market to include younger patients who are more likely to require and demand premium and high-performance products.
70
BUSINESS
General
Biomet, an Indiana corporation incorporated in 1977, is one of the largest orthopedic medical device companies in the United States and worldwide with operations in more than 50 locations throughout the world and distribution in approximately 90 countries. Biomet principal subsidiaries include Biomet U.S. Reconstruction, LLC; Biomet Orthopedics, LLC; Biomet Manufacturing Corporation; Biomet Europe BV; EBI, LLC; Biomet 3i, LLC; Biomet International Ltd.; Biomet Microfixation, LLC; Biomet Sports Medicine, LLC; Biomet Trauma, LLC; and Biomet Biologics, LLC. We design, manufacture and market a comprehensive range of both surgical and non-surgical products used primarily by orthopedic surgeons and other musculoskeletal medical specialists. For over 30 years, we have applied advanced engineering and manufacturing technology to the development of highly durable joint replacement systems.
Transactions with the Sponsor Group
On December 18, 2006, Biomet entered into an Agreement and Plan of Merger with LVB Acquisition, LLC, a Delaware limited liability company, which was subsequently converted to a corporation, LVB Acquisition, Inc. (“Parent”), and LVB Acquisition Merger Sub, Inc., an Indiana corporation and a wholly-owned subsidiary of LVB (“Purchaser”), which agreement was amended and restated as of June 7, 2007 and which we refer to as the “Merger Agreement.” Pursuant to the Merger Agreement, on June 13, 2007, Purchaser commenced a cash tender offer (the “Offer”) to purchase all of Biomet’s outstanding common shares, without par value (the “Shares”) at a price of $46.00 per Share (the “Offer Price”). Approximately 82% of the outstanding Shares were tendered to Purchaser in the Offer. At Biomet’s special meeting of shareholders held on September 5, 2007, more than 91% of its shareholders voted to approve the proposed merger, and Parent acquired Biomet on September 25, 2007 through a reverse subsidiary merger with Biomet being the surviving company (the “Merger”). Subsequent to the acquisition, Biomet became a subsidiary of Parent, which is controlled by Holding, an entity controlled by a consortium of private equity funds affiliated with the Sponsors (as defined below) and their co-investors.
The Merger was completed on September 25, 2007 and was financed through:
| • | the proceeds from the initial offering of Biomet’s 10% Senior Notes due 2017, 10⅜%/11⅛% Senior Toggle Notes due 2017, and 11⅝% Senior Subordinated Notes due 2017; |
| • | initial borrowings under senior secured credit facilities and senior unsecured bridge facilities; |
| • | equity investments funded by direct and indirect equity investments from certain investment funds associated with or designated by the Sponsors, or the “Sponsor Funds,” certain investors who have agreed to co-invest with the Sponsor Funds, including investment funds affiliated with certain of the initial purchasers of the original notes, or the “Co-Investors,” and certain of our executive officers and members of our senior management, or the “Management Participants,” who rolled over existing equity interests and/or made cash equity contributions; and |
| • | cash on hand. |
On October 16, 2007, the borrowings under our senior unsecured cash pay bridge facility, our senior unsecured payment-in-kind (“PIK”) option bridge facility and our senior subordinated unsecured bridge facility were repaid with the proceeds from the follow-on offering of equal amounts of additional 10% Senior Notes due 2017, 10⅜%/11⅛% Senior Toggle Notes due 2017 and 11⅝% Senior Subordinated Notes due 2017, respectively.
We refer to these transactions, including the Merger and our payment of any fees and expenses related to these transactions, collectively as the “Transactions.”
In connection with the Transactions, we incurred significant indebtedness and became highly leveraged. In addition, we allocated the purchase price to the fair value of the assets and liabilities of Biomet based on estimated fair value. The purchase accounting adjustments increased the carrying value of our property and equipment, inventory and established intangible assets (such as corporate and product trade names, core and completed technology, and customer relationships), among other things. Subsequent to the Transactions, interest expense and
71
non-cash depreciation and amortization charges have significantly increased. As a result, our successor financial statements subsequent to the Transactions are not comparable to our predecessor financial statements.
Notes Offering and Credit Facility Amendment
On August 2, 2012, we entered into an amendment and restatement agreement that amended our existing senior secured credit facilities. The amendment (i) extended the maturity of approximately $1,007.2 million of our U.S. dollar-denominated term loans and approximately €631.3 million of our euro-denominated term loans under the credit facility to July 25, 2017, (ii) refinanced and replaced the previous alternative currency revolving credit commitments under the credit facility with a new class of alternative currency revolving credit commitments in an aggregate amount of $165.0 million and (iii) refinanced and replaced the previous U.S. dollar revolving credit commitments under the credit facility with a new class of U.S. dollar-denominated revolving credit commitments in an aggregate amount of $165.0 million. The new revolving credit commitments will mature on April 25, 2017, except that, if as of December 23, 2014, there is an outstanding aggregate principal amount of non-extended U.S. dollar and euro term loans in excess of $200.0 million, then such revolving credit commitments will mature on December 24, 2014.
The joinder agreement was entered into pursuant to our senior secured credit facility, as amended by the amendment and restatement agreement dated August 2, 2012. By entering into the joinder agreement, the joining lenders party thereto have agreed to extend the maturity of (i) approximately $392.7 million of Biomet’s U.S. dollar-denominated term loans and (ii) approximately €32.9 million of Biomet’s euro-denominated term loans, to July 25, 2017. The term loans extended pursuant to the joinder agreement are on terms identical to the terms loans that were extended pursuant to the prior Amendment. The remaining term loans of the lenders under the senior secured credit facilities who did not elect to extend such loans either pursuant to the August 2 amendment and restatement agreement or the subsequent joinder agreement will continue to mature on March 25, 2015.
On November 14, 2012, we also replaced and refinanced our asset-based revolving credit facility with a new asset-based revolving credit facility that has a U.S. tranche of up to $400.0 million and a European borrower tranche (denominated in euros) of up to the euro-equivalent of $100.0 million. The European borrower tranche is secured by certain foreign assets of European subsidiary borrowers, and the U.S. borrowers under the U.S. tranche guarantee the obligations of any such European subsidiary borrowers (and those guarantees are secured by the current asset collateral that secures the direct obligations of those U.S. borrowers under the U.S. tranche).
In addition, on December 27, 2012, we completed a $730.0 million add-on to our extended U.S. dollar-denominated term loan. The proceeds from the add-on were used to refinance the non-extended U.S. dollar-denominated term B loan, which was net of fees associated with the add-on closing. The terms of the add-on are consistent with the terms in the August 2 amendment.
On August 8, 2012, we completed our offering of $1,000.0 million aggregate principal amount of our new senior notes. We used a portion of the proceeds of that offering to fund a tender offer for any and all of our outstanding 10⅜%/11⅛% Senior PIK Toggle Notes due 2017 including related fees and expenses, to redeem the remaining 10⅜%/11⅛% Senior PIK Toggle Notes and to redeem $140.0 million aggregate principal amount of 11⅝% Senior Subordinated Notes due 2017.
On October 2, we completed our offering of $825.0 million aggregate principal amount of additional senior notes and $800.0 million aggregate principal amount of our new senior subordinated notes. We used the net proceeds of those offerings, together with cash on hand and other sources, to purchase any and all of our 10% Senior Cash Pay Notes due 2017 and $940 million principal amount of outstanding 115/8% Senior Subordinated Notes due 2017. On November 1, 2012, we purchased and redeemed all remaining outstanding 115/8% Senior Subordinated Notes using cash on hand and asset-based revolver proceeds.
Competitive Strengths
We believe we have a number of competitive strengths that will enable us to further enhance our position in the orthopedic medical device market.
Broad Market Leadership. We believe we are the fourth largest player in the U.S. orthopedic reconstructive market and have maintained this position for over a decade. We have a large presence at U.S. hospitals, supplying products to over 60% of hospitals performing joint replacement surgery. In addition, we are a
72
leading provider of dental reconstructive devices worldwide and maintain market leadership positions in the electrical stimulation and craniomaxillofacial fields.
Strong Relationships with Surgeon Customers. Based on their satisfaction with our products, we enjoy long-standing relationships with our surgeon customers, many of which commence during the surgeons’ residency training programs. Our support of medical education programs provides important training opportunities for orthopedic surgeons early in their careers. Supporting “hands-on” training provides opportunities for residents, fellows and attending surgeons to experience the clinical benefits of our products. Surgeons have historically exhibited limited willingness to switch manufacturers, as successful patient outcomes are related to the practitioners’ familiarity with the procedural characteristics and instrumentation of certain implants.
Consistently Strong Operating Cash Flow Generation. Our business is characterized by consistently strong operating cash flows due to our robust operating history and moderate capital intensity. We have continually increased revenues, with fiscal year 2012 representing our 34th consecutive year of year-over-year net sales growth. Over the last 20 years, from fiscal year 1992 through fiscal year 2012, we increased net sales at a compounded annual growth rate of approximately 12%. We have sustained growth through multiple macro-economic cycles, demonstrating a stable business profile. In addition, we have historically had modest capital expenditures and working capital requirements, providing for strong operating cash flow conversion.
Experienced and Dedicated Management Team. We have a highly experienced management team at both the corporate and operational level. Our team is led by Jeffrey R. Binder, a 21-year veteran of the orthopedic medical device industry, who was appointed President and Chief Executive Officer in February 2007. Daniel P. Florin was appointed Senior Vice President and Chief Financial Officer in June 2007 and brings 21 years of financial officer/controller experience in the medical device industry and five years of public accounting and auditing experience to Biomet. In February 2008, Jon C. Serbousek was appointed President of Biomet Orthopedics and was recently appointed as President, Biomet Biologics, having spent 8 years with Medtronic and 13 years with DePuy, for a total of 25 years in the medical device industry. Adam Johnson was appointed Senior Vice President and President of EBI, LLC, d/b/a Biomet Spine & Bone Healing Technologies in June 2012, having previously served and continuing to serve as President of Biomet Microfixation and brings 13 years of experience in the medical device industry.
Premier Equity Sponsorship. The Blackstone Group, Goldman, Sachs & Co., Kohlberg Kravis Roberts & Co. and TPG are among the most well-known and respected financial sponsors in the world. The Sponsors have made investments in over 950 companies. The Sponsors have considerable experience in the healthcare sector with investments in companies such as Accellent Inc., HCA Inc., IASIS Healthcare Corporation, Quintiles Transnational Corp., DJO Inc. and Vanguard Health Systems, Inc., among others.
Economic Uncertainties
Our results of operations could be substantially affected not only by global economic conditions, but also by local operating and economic conditions, which can vary substantially by market. Unfavorable conditions can depress sales in a given market and may result in actions that adversely affect our margins, constrain our operating flexibility or result in charges which are unusual or non-recurring.
Regulatory and Other Uncertainties
In the United States, healthcare providers that purchase our products (e.g., hospitals, physicians, dentists and other health care providers) generally rely on payments from third-party payors (principally federal Medicare, state Medicaid and private health insurance plans) to cover all or a portion of the cost of our musculoskeletal products. In March 2010, comprehensive health care reform legislation was enacted through the passage of the Patient Protection and Affordable Health Care Act (H.R. 3590) and the Health Care and Education Reconciliation Act (H.R. 4872). Among other initiatives, these laws impose a 2.3% excise tax on domestic sales of medical devices following December 31, 2012, which is estimated to contribute approximately $20 billion to healthcare reform. Various healthcare reform proposals have also emerged at the state level. Outside of the excise tax, which has impacted our results of operations and cash flows following December 31, 2012, we cannot predict with certainty what healthcare initiatives, if any, will be implemented at the state level, or what the ultimate effect of federal health care reform or any future legislation or regulation will have on us. However, an expansion in government’s role in the U.S. healthcare industry may lower reimbursements for our products, reduce medical procedure volumes and adversely affect our business, results of operations and cash flows, possibly materially.
73
Outside the United States, reimbursement systems vary significantly from country to country. If adequate levels of reimbursement from third-party payors outside the United States are not obtained, international sales of our products may decline. Many foreign markets, including Canada and some European and Asian countries, have tightened reimbursement rates. Our ability to continue to sell certain products profitably in these markets may diminish if the government-managed healthcare systems continue to reduce reimbursement rates, which can decrease pricing and procedural volume.
We continue to monitor economic conditions, including the volatility associated with international sovereign economies, and associated impacts on the financial markets and our business, especially in light of the global economic downturn and the European sovereign debt crisis. We believe the credit and economic conditions within Greece, Ireland, Italy, Portugal and Spain, among other members of the European Union, have continued to deteriorate. These conditions have resulted in, and may continue to result in, an increase in the average length of time that it takes to collect on our accounts receivable outstanding in these countries.
As of May 31, 2012, our orthopedic net accounts receivable in these countries totaled over $70.0 million. During fiscal year 2010 we did recognize $9.3 million of expense to adjust our public accounts receivable in Greece to its expected net realizable value based upon the proposal by the Greek government to settle certain past due healthcare liabilities with long-term zero coupon bonds. We currently hold Greek bonds with a fair value of $6.3 million at May 31, 2012. Further, there have been widely publicized concerns with respect to the overall stability of the Euro as a single currency, given the economic and political challenges facing the Eurozone countries described above. The collapse of the Euro as a common European currency, the withdrawal of one or more member countries from the EU or continuing deterioration in the creditworthiness of the Eurozone countries could adversely affect the Company’s revenues, financial condition or results of operations.
Business Strategy
We intend to enhance our position as a leading orthopedic medical device company by pursuing the following strategic initiatives:
Continue to Develop and Launch New Products and Technologies. In May 2009, we launched our New Product Introduction, or NPI, process worldwide. The NPI process is a global portfolio and project management approach that helps bring visibility and control to all commercial aspects of new product development projects. The process breaks each project down into six stages of work and further divides these stages by formal review gates. We have a single database of all of our development projects that is easily filtered and sorted to generate customized project roadmaps that serve as communication tools providing visibility to all functional teams. The database is designed to prioritize and focus the portfolio and also ensure that the workload is properly resourced and managed across the business. Projects are assessed against pre-determined gate criteria. Functional teams, along with the global portfolio review teams, select and prioritize projects that can be adequately resourced and help deliver product category growth targets, satisfy specific hurdle rates and strategic drivers and provide a balanced product portfolio.
Enhance Surgeon Customer Relationships through Product Performance and Innovation. We intend to continue to meet the needs of our surgeon customers and hospital customers by providing clinically successful and innovative products that offer a cost-effective means of treating patients. Our success has been built on responsiveness to the needs of the healthcare community, the clinical performance of our products and our ongoing commitment to continued product innovation.
Expand Our Global Reach. We intend to continue to increase the geographic presence of each of our business categories. We believe there are considerable opportunities for global expansion as healthcare spending increases in international markets—the United States accounted for approximately 60% of the global orthopedic market in 2011. The United States, Europe and Japan totaled more than 80% of the global orthopedic market in 2011, but less than 20% of the world’s 7 billion people live in these three geographic regions. We particularly plan to focus on deepening our position in under-penetrated regions where we believe there are attractive opportunities for growth, including Asia and Latin America, by deploying more resources to capture market opportunities, as well as by leveraging our established worldwide manufacturing facilities and sales force. We believe we can successfully grow our presence in these regions by differentiating ourselves as a provider with a comprehensive portfolio of leading musculoskeletal products.
Focus on Operational Efficiency. We believe we have identified significant opportunities to streamline operations. We believe the historically decentralized nature of our management and decision-making structure
74
creates opportunities to improve operational efficiency as we centralize operations and increase focus, coordination and accountability throughout the organization. Plans include manufacturing footprint optimization, implementation of Six Sigma and Lean Manufacturing, procurement and offshoring initiatives, as well as reduction in overhead expenses. These changes were initiated during fiscal year 2008, continued through fiscal year 2012 and are expected to continue into future fiscal years. We believe these changes will enable us to maximize asset utilization, optimize working capital and increase cash flow, as well as accelerate product development and enhance customer service. During fiscal year 2011, we initiated a reorganization of our global reconstructive product organization to further the alignment and collaboration of our team members across our various businesses, functions and geographies.
Maximize Free Cash Flow. We are focused on maximizing our operating cash flow. Over the last 20 years, we have generated significant operating cash flow due to our business growth, strong operating margins and modest capital expenditure and other cash requirements. These business fundamentals have been supplemented by working capital improvement initiatives, which historically had not been a primary focus area of management. In addition, we have benefited and believe we will continue to benefit from identified cost savings as we enhance operational efficiencies. We plan to use available cash after capital expenditures primarily to reduce leverage, strengthen our balance sheet and make strategic acquisitions.
Products
We operate in one reportable business segment, musculoskeletal products, which includes the design, manufacture and marketing of products in five major categories: Large Joint Reconstructive, Sports, Extremities, Trauma, Spine & Bone Healing, Dental and Other Products. We have three geographic markets: United States, Europe and International.
The following charts set forth our net sales by product category and geographic markets for the fiscal year ended May 31, 2012 and the nine months ended February 28, 2013. We changed our product categories in fiscal year 2012 to more closely represent the way we currently report sales and market our products, and to provide increased reporting transparency. For certain financial information concerning our product categories and geographic markets, see Note 13 to our audited consolidated financial statements for the fiscal year ended May 31, 2012 and Note 13 to our unaudited condensed consolidated interim financial statements included elsewhere herein.
Year Ended May 31, 2012
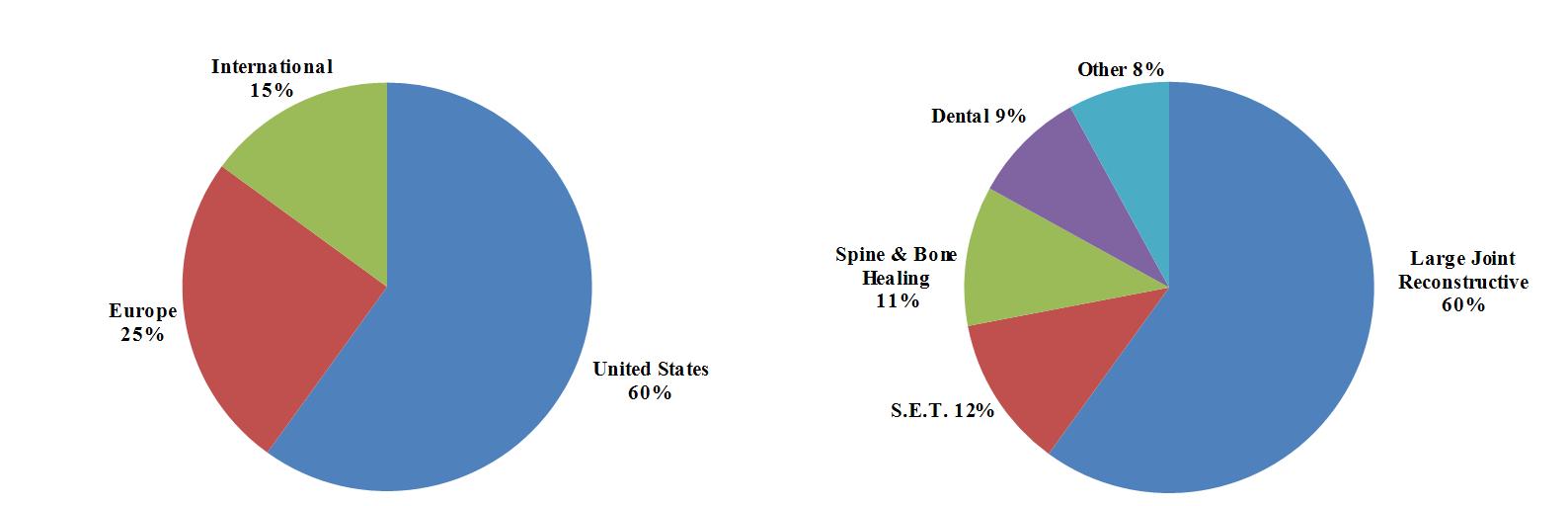
75
Nine Months Ended February 28, 2013
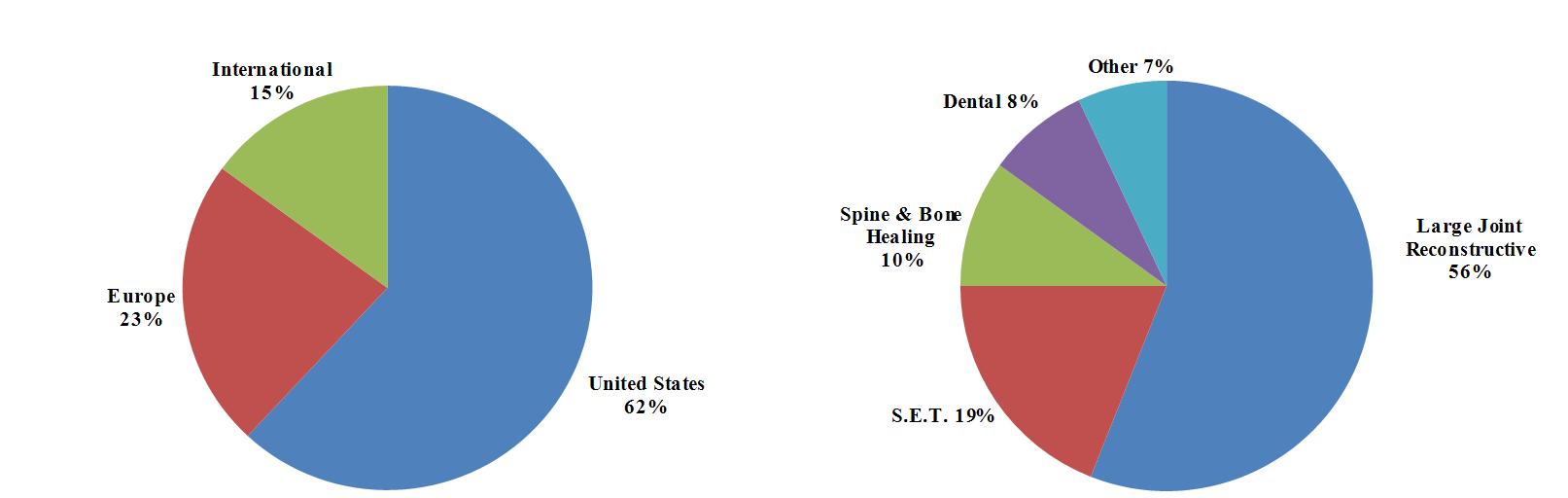
Large Joint Reconstructive Products
Orthopedic reconstructive implants are used to replace joints that have deteriorated as a result of disease (principally osteoarthritis) or injury. Reconstructive joint surgery involves the modification of the area surrounding the affected joint and the implantation of one or more manufactured components, and may involve the use of bone cement. Our primary orthopedic reconstructive joints are knees and hips. We also produce the associated instruments required by orthopedic surgeons to implant our reconstructive products, as well as bone cements and cement delivery systems.
Our PMI® (Patient-Matched Implant) services group designs, manufactures and delivers patient-specific reconstructive devices to orthopedic specialists. We believe this service continues to enhance our reconstructive sales by strengthening our business relationships with orthopedic surgeons and augmenting our reputation as a responsive company committed to excellent product design. In order to assist orthopedic surgeons and their surgical teams in preoperative planning, our PMI® group utilizes a three-dimensional, or 3-D, bone reconstruction imaging system. We use computed tomography, or CT, data to produce 3-D reconstructions for the design and manufacture of patient-matched implants. With this imaging and model-making technology, our PMI® group is able to assist the physician prior to surgery by creating 3-D models. Within strict guidelines, the model is used by engineers, working closely with a surgeon, to create a PMI® design for the actual manufacturing of the implant for a specific patient.
Knee Systems. A total knee replacement typically includes a femoral component, a patellar component, a tibial component and an articulating surface. Total knee replacement may occur as an initial joint replacement procedure or as a revision procedure, which may be required to replace, repair or enhance the initial implant. Partial, traditionally referred to as unicompartmental, knee replacement is an option when only a portion of the knee requires replacement.
Our most comprehensive total knee system, the Vanguard® Complete Knee System, accommodates up to 145 degrees of flexion, provides advanced sizing options and offers full interchangeability of the system’s components to provide for a precise fit for each patient. The Vanguard® Complete Knee System is supported by five instrumentation platforms: Microplasty®, Premier™, Microplasty® Elite, Vanguard® Tensor and Vanguard® Anterior Referencing systems, accommodating a number of workflows and techniques.
At the end of fiscal year 2012, we started the global commercial launch of our newest revision knee offering, the Vanguard® SSK 360 Revision System. This innovative system, which is an extension of our Vanguard® Complete Knee System, is designed to offer optimum stability, while maximizing options for intraoperative flexibility.
Biomet continues to globally lead the patient specific instrument market with the Signature™ System. The Signature™ System uses a patient’s MRI or CT data to deliver patient-specific positioning guides to the surgeon for improved pre-operative planning, custom positioning of the implants, and improved surgical efficiency. Signature Technology is currently utilized for implantation of the Vanguard® Complete Knee System and the Oxford® Partial Knee System. The Signature™ System was developed through a partnership with Materialise NV and we believe this technology will be expanded to other orthopedic applications beyond the knee.
76
During the fiscal year 2012, E1® Antioxidant Infused Technology Tibial Bearings continued to receive strong market acceptance. The E1® technology provides Vitamin E infused highly cross-linked polyethylene, which is designed to offer strength and oxidative stability for implant longevity.
We believe we continue to be the market leader for products accommodating minimally-invasive knee techniques. The Oxford® Partial Knee, which was introduced in the United States during fiscal 2005 and has been commercially available in Europe for 35 years, is currently the only free-floating meniscal bearing unicompartmental knee system approved by the U.S. Food and Drug Administration, or “FDA,” for use in the United States. Our offering of minimally-invasive partial knee systems also includes the Alpina™ Unicompartmental Knee (which is not currently available in the United States); the Vanguard M™ Series Unicompartmental Knee System, a modified version of the Oxford® Partial Knee that incorporates a fixed-bearing tibial component as opposed to a free-floating tibial bearing; and the Repicci II® Knee System.
Hip Systems. A total hip replacement involves the replacement of the head and neck of the femur and the acetabulum and may occur as an initial joint replacement procedure, or as a revision procedure, which may be required to replace, repair or enhance the initial implant. A femoral hip prosthesis consists of a femoral head and stem, which can be cast, forged or wrought, depending on the design and material used. Many of our femoral prostheses utilize our proprietary PPS® Porous Plasma Spray coating, which enables cementless fixation.
Acetabular components include a prosthetic replacement of the socket portion, or acetabulum, of the pelvic bone. Because of variations in human anatomy and differing design preferences among surgeons, we manufacture femoral and acetabular prostheses in a variety of sizes and configurations. We offer a broad array of total hip systems, most of which utilize titanium or cobalt chromium alloy femoral components and our ArCom®, ArComXL® or E1® polyethylene-lined, metal-on-metal or ceramic-on-ceramic acetabular components.
From our broad product platform of hip stem offerings, the Taperloc® Hip System has become our best-selling component. The Taperloc® Stem is marketed for non-cemented use in patients undergoing primary or revision hip replacement surgery as a result of noninflammatory degenerative joint disease. The Taperloc® femoral component is a collarless, flat, wedge-shaped device that is relatively simple to implant and is particularly well-suited for minimally-invasive procedures. During the fourth quarter of fiscal 2011, we initiated the rollout of the Taperloc® Complete stem, which combines the proven clinical data of the Taperloc stem with subtle design changes to better address the fit and biomechanics of patients. We also offer the Taperloc® Microplasty® and Taperloc® Complete Microplasty® stems that address the demand for a minimally-invasive, bone-conserving total hip implant. The shorter length of the Microplasty® Stem, compared to a traditional hip stem, allows for preservation of distal bone, while maintaining proximal femoral bone fixation.
Our comprehensive Microplasty® Minimally Invasive Hip Program includes proprietary products from our broad array of hip implants, as well as a distinctive training program and uniquely-designed instruments for a minimally-invasive approach. Our minimally-invasive hip development efforts have been focused on various surgical approaches, including an anterior supine intermuscular surgical approach.
The Echo® Bi-Metric® stem, which is a cementless press-fit stem for primary total hip procedures, utilizes proven features of the Integral® and Bi-Metric® stems, while integrating new design features to further enhance clinical performance by accommodating a wider range of femoral canals, allowing for increased range of motion, and providing standard and lateralized offset options to restore biomechanics.
In our acetabular portfolio, our M2a-Magnum™ Articulation System incorporates large diameter metal-on-metal components to more closely resemble the natural anatomy, offering joint mechanic restoration designed to improve range of motion and joint stability. We market ArComXL® polyethylene, which is a highly crosslinked polyethylene bearing material based on our proven ArCom® polyethylene. ArComXL® polyethylene has demonstrated excellent wear characteristics without measurable oxidation after accelerated aging. We market acetabular hip liners manufactured from E1® material. Vitamin E is a natural antioxidant and is expected to provide optimal oxidation resistance for the implant bearings used in our total joint replacements.
We introduced our Active Articulation™ E1® System and our Active Articulation™ ArcomXL® System during fiscal 2011. These systems are dual-mobility acetabular systems that are designed to provide the benefits of a large head design, including the potential for increased range of motion and low risk of dislocation.
The Regenerex® Construct unites the proven clinical history of titanium with an enhanced interconnecting pore structure, resulting in an innovative material that provides for biologic fixation and provides design flexibility
77
and solutions for difficult primary and revision procedures. The advanced titanium scaffold structure of the Regenerex® Construct is a continuous three-dimensional matrix comprised of industry-standard Ti-6AL-4V. Titanium is a clinically proven material in the orthopedic market, with optimal biological fixation, and the Regenerex® construct is expected to be the material of choice for porous metal constructs.
We introduced our Arcos® Modular Femoral Revision System in fiscal year 2011, which contributed to our revision hip sales growth for fiscal year 2012. The Arcos® System offers surgeons the ability to select from a range of interchangeable components intraoperatively, using a single set of instruments.
Bone Cements and Accessories, and Other Large Joint Reconstructive Products and Services. We offer a wide range of acrylic bone cements and cementing systems for various clinical applications including primary and revision reconstructive joint procedures. Our broad portfolio of high, medium and low viscosity cements, with or without antibiotics, along with our cementing systems provide solutions for most clinical situations where bone cement is required.
We have broadened the range of our internally developed and manufactured bone cement product offerings with both Cobalt™ HV (High Viscosity) Bone Cement and Cobalt™ MV (Medium Viscosity) Bone Cement, which are particularly well suited for use in minimally-invasive surgery, but may be used in all applicable joint replacement procedures. In addition, we maintain a market leading position in Europe with our Refobacin and Biomet Bone Cement lines. The excellent handling characteristics and high optical contrast of our cements are well suited to the current trends in orthopedic surgery. In the United States, the SoftPac™ monomer packaging offers the only alternative to glass vial packaging, which is inherently less safe due to the necessity to break the glass vial to deliver the monomer. In Europe, we introduced the OptiPac™ pre-loaded, all-in-one bone cement and delivery system during fiscal 2008. OptiPac™ is a closed vacuum mixing system prepacked with both polymer and monomer, which eliminates several steps in the mixing procedure. During fiscal year 2012, the OptiPac™ closed vacuum system continued to receive strong market demand, reinforcing our position as the leader in the European bone cement market. In addition, during fiscal year 2012 we launched OptiPac™ Knee, specifically designed to address partial, hybrid and two-step total knee procedures.
Our portfolio of cementing systems includes the Optivac® Mixing System, which provides mixing and collection under vacuum for optimal porosity reduction. In addition to improving bone cement quality, these systems are also designed to reduce the level of monomer exposure in the operating room and minimize direct contact with the cement, thereby creating a safer working environment.
During fiscal year 2011 we increased focus on strengthening our position in the revision market, including the launch of our StageOne™ Select Hip Cement Spacer Molds, which are single-use molds designed to create a temporary cement spacer for patients undergoing a two-stage revision. Design features of StageOne™ Select Hip Cement Spacer Molds provide the surgeon with more options and help enhance patient fit during the first-stage of a two-stage revision. During fiscal year 2012 we initiated the launch of StageOne™ Select Hip Cement Spacer Molds in Europe. We offer cement spacer mold options for both hip and knee revision procedures.
Sports, Extremities, Trauma (S.E.T.) Devices
Our S.E.T. product category includes sports medicine products, extremity devices, and trauma hardware.
Sports Medicine Products. We manufacture and market a line of arthroscopy products. Arthroscopy is a minimally-invasive orthopedic surgical procedure in which an arthroscope is inserted through a small incision to allow the surgeon direct visualization of the joint. This market is comprised of five product categories: power instruments, manual instruments, visualization products, soft tissue anchors, and procedure-specific instruments and implants.
We market several sports medicine products that feature ZipLoop™ Technology, a weave in which a single strand of braided polyethylene is woven through itself twice in opposite directions. This construct allows the production of innovative products that can vary in length and compression/tension, addressing the individual needs of each patient. Since the surgeon has the ability to vary the length of the implant, this eliminates the need for multiple sizes and requires minimal instrumentation. The technology is now being utilized to repair injuries in the shoulder, elbow, knee and foot and ankle.
In the fourth quarter of fiscal year 2010, we launched the 1.4mm JuggerKnot™ Soft Anchor for labral repair. This product represents the next generation of suture anchor technology, as it is completely suture-based and
78
the first of its kind. The key to a labral repair is to remove the least amount of bone possible, and the smaller anchor diameter allows multiple anchors to be placed without removing large amounts of bone. During fiscal year 2012, we launched four additional sizes of JuggerKnot™ products, including the 1.5mm JuggerKnot™ Soft Anchor for labral repair, the 2.9mm JuggerKnot™ Soft Anchor double loaded for rotator cuff repair, and the 1.0mm JuggerKnot™ Soft Anchor and the 1.4mm JuggerKnot™ Short Soft Anchor for extremity repair.
In the third quarter of fiscal year 2011, we launched the TunneLoc® Tibial Fixation Device. This device has a hands-free tensioner that maintains tension during the insertion of the implant, which we believe is a unique feature. This allows the surgeon to set the tension on the inserter as needed and once locked, the surgeon is able to cycle the knee. In addition, the graft tensioner and inserter eliminate the need for reusable instruments, saving costly preparation time for the surgeon.
Extremity Systems. We offer a variety of shoulder systems including the Absolute® Bi-Polar, Bi-Angular®, Bio-Modular®, Comprehensive®, T.E.S.S., Copeland™, Integrated™ and Mosaic™ Shoulder Systems, as well as uniquely-designed elbow replacement systems.
The Comprehensive® Primary Shoulder System includes the standard and mini length Comprehensive® Primary Stems and the Versa-Dial® Heads, as well as the Hybrid® glenoids.
The Comprehensive® Reverse Shoulder System offers improved intraoperative flexibility and is our first reverse shoulder that will utilize the Comprehensive® platform stems, providing for cemented or cementless use. This system was designed to eliminate scapular notching by incorporating a more anatomic center of rotation utilizing our Versa-Dial® glenospheres.
The T.E.S.S. shoulder system, commercially available in Europe, was developed to provide a less-invasive, bone conserving solution for all shoulder arthroplasty indications. The T.E.S.S. was the first system to introduce the concept of stem-less shoulder arthroplasty to the market.
The Copeland™ Humeral Resurfacing Head was developed to minimize bone removal in shoulder procedures and has approximately 20 years of positive clinical results in the United Kingdom. This system was expanded to include the Copeland™ EAS™ Extended Articular Surface Humeral Resurfacing Head designed to address rotator cuff arthropathy.
Trauma Internal Fixation Devices. Internal fixation devices include products such as intramedullary (IM) nails, plates, screws, pins and wires designed to stabilize traumatic bone injuries. These devices are used by orthopedic surgeons to provide an accurate means of setting and stabilizing fractures and for other acute reconstructive procedures. By holding and stabilizing alignment of the reduced fracture, internal fixation products are intended to aid in the healing process, which may be removed when healing is complete. Internal fixation devices are not intended to replace normal body structures.
Biomet develops, manufactures and distributes innovative products for the internal fixation market. On June 15, 2012, we acquired the worldwide trauma business of DePuy Orthopaedics, Inc. for approximately $280.0 million broadening and deepening our trauma product portfolio. We now offer a complete product line of low-profile, locked periarticular plates and hub-and-spoke mini and small fragment sets, which utilize platform technologies.
The Biomet® DVR® offers a market leading innovative volar approach for treating fractures of the distal radius. Our F.A.S.T. Guide® Technology is designed to improve intraoperative efficiencies and is a platform technology shared in the S3® proximal humeral, elbow and all ALPS mini and small low profile locking plates. All plates, including the POLYAX® distal femoral and proximal tibial periarticular plates, are strengthened by a proprietary type II titanium alloy anodizing process branded TiMAX®.
The Biomet® PTN and Phoenix™ femoral and tibial IM nail product portfolio is now deepened with the addition of AFFIXUS® hip fracture and VersaNail® IM nails, which utilize TiMAX® technology. The AFFIXUS® nail utilizes highly intuitive, efficient, streamlined instrumentation and offers both intraoperative and post operative rotational control/stability of the femoral head, providing a competitive hip fracture solution.
Trauma External Fixation Devices. External fixation devices are used to stabilize fractures when alternative methods of fixation are not suitable, due to a variety of clinical indications, including treatment of open fractures.
79
We offer a complete line of solutions for various segments of the fracture and reconstructive external fixation markets.
Our external fixation products are modular devices intended for use in simple and/or complex fractures of upper extremities, the pelvis and lower extremities. The Biomet® Vision™ Unilateral Fixator is a carbon-based external fixation device intended for use in the treatment of bone conditions including leg lengthening, osteotomies, arthrodesis and fracture fixation addressing periarticular, diaphyseal and other fractures amenable to temporary, or to definitive external fixation measures. This device offers serrated mechanical locks that allow for up to 120 degrees of articulation for controlled fracture reduction and radiolucency for unobstructed radiographic imaging of the fracture site.
The Biomet® Vision™ Pin-to-Bar system offers an MRI/CT safe modality for stabilization of long bone and pelvic fractures. This versatile system allows for independent pin placement and can be used as both temporary and definitive fixation.
Spine and Bone Healing Products
Our spinal products include spinal fixation systems, implantable and non-invasive electrical stimulation devices for spinal applications, orthobiologics (including allograft services). Our bone healing products include implantable and non-invasive electrical stimulation devices for long bone and pelvic fractures, as well as soft goods and bracing products for orthopedic applications. These products and services are primarily marketed in the United States under the Biomet Spine & Bone Healing Technologies trade name.
Spinal Fixation Systems. We market spinal fixation devices for various spinal fusion applications. In the thoracolumbar market segment, we offer the Polaris™ Spinal System, a low profile, top-loading, thoracolumbar system utilizing a Helical Flange® (a registered trademark of Roger P. Jackson) closing mechanism, among other systems. This feature minimizes the potential for cross-threading and seat splay, simplifying the implant closing procedure for the surgeon. The Polaris™ System is available in titanium or stainless steel in 6.35mm or 5.5mm rod diameters, with various screw, hook and rod options. With the 5.5mm diameter rod system, we market titanium, stainless steel and cobalt chrome rod material options. These multiple rod materials and diameters provide surgeons with treatment options for various types of deformity patients. Additionally, the Polaris™ system features the Trivium™ instrumentation permitting direct vertebral body rotation and correction.
We also offer a variety of spacer products for the thoracolumbar market segment. The Solitaire™ Anterior Spinal System is a stand-alone device with numerous implantation options for intraoperative flexibility when performing an Anterior Lumbar Interbody Fusion (ALIF) procedure. This system is available with implants manufactured from titanium or PEEK-OPTIMA® (a registered trademark of Invibio® Limited) polymer, an implant option for increased radiographic fusion assessment. We also offer the ESL®, C-Thru™ and Zyston® interbody spacers. All three of these spacers feature open designs to permit ample space for bone graft placement. The ESL® System has an elliptical shape, offering optimal surface contact with the vertebral body endplates. The Zyston® System is available in straight and curved models to conform to the anterior shape of the adjacent vertebral body. The ESL® and Zyston® spacers are utilized for Posterior Lumbar Interbody Fusion (PLIF) and/or Transforaminal Interbody Fusion (TLIF) procedures. The C-Thru™ spacer is indicated for Cervical Interbody Fusion. All three interbody spacers are available in PEEK-OPTIMA® (a registered trademark of Invibio® Limited) polymer for increased radiographic fusion assessment.
For cervical fixation applications, the open design of the VueLock® Anterior Cervical Plate System provides surgeons with enhanced visualization of the bone graft both during the actual surgical procedure and postoperatively on x-ray. We also offer the C-TEK® Anterior Cervical Plate, which provides a non-constrained, semi-constrained or a completely rigid construct, depending on the surgeon’s preference. Made of titanium, the C-TEK® Plate offers both fixed and variable screws in a wide variety of diameters and lengths, and features a unique locking mechanism to prevent screw back out. The MaxAn® Anterior Cervical Plate System, which incorporates technology developed by Gary K. Michelson, M.D., has a unique design that allows for maximum angulation of the screws. This technology permits the surgeon to utilize a shorter plate, which helps optimize plate placement to potentially prevent impingement of the adjacent healthy disc.
For cervical and upper thoracic procedures, we offer the Altius™ M-INI™ Occipito-Cervico-Thoracic Spinal Fixation System, which features top-loading screws and a 3.5mm rod for maximum strength. This system also incorporates Helical Flange® (a registered trademark of Roger P. Jackson) Locking Technology. Occipital
80
fixation is also available with the Altius™ M-INI™ System, featuring a low-profile plate that is placed independently from the pre-contoured rod.
Minimally-invasive surgery is of growing interest in the practices of many spine surgeons. In the minimally-invasive surgery market, we offer the Ballista® Percutaneous Pedicle Screw Placement System and the AccuVision® Minimally Invasive Access System. These systems address both the mini-open and percutaneous screw placement minimally invasive approaches.
To address the vertebral body compression fracture market, we offer two systems designed for the delivery of materials to weakened bone structures, including the CDV™ and LP2™ Delivery Systems. Through a series of dilating cannulae and various instruments, the systems allow the surgeon to access the anatomy through a percutaneous approach and safely deliver commercially available bone cement under low, controlled pressure. The CDV™ Delivery System offers the ability to biopsy before delivery.
Spine Fusion Stimulation Systems. Spinal fusions are surgical procedures undertaken to establish bony union between adjacent vertebrae. We distribute both non-invasive and implantable electrical stimulation devices that surgeons can use as options to provide an appropriate adjunct to surgical intervention in the treatment of spinal fusion applications. We have assembled extensive preclinical research, documenting the mechanism of action for the technology utilized in our spine fusion stimulation systems.
The SpinalPak® II Spine Fusion Stimulator and Biomet® SpinalPak® Non-Invasive Spine Fusion Stimulator System are noninvasive bone growth stimulators for use as an adjunct electrical treatment to primary lumbar spinal fusion surgery for one or two levels. Both utilize Capacitive Coupling technology that involves the upregulation of factors that modulate bone healing, which may lead to successful fusion incorporation. These devices consist of a small, lightweight generator worn outside the body that is connected to wafer-thin electrodes applied over the fusion site. Both devices are patient-friendly and are designed to optimize compliance with the treatment regimen to help fusion success.
The SpF® Implantable Spine Fusion Stimulator is an established clinical treatment for posterolateral lumbar spine fusions and it is the only implantable spine fusion stimulator on the market, providing a constant dose of electrical stimulation for up to six months. The surgically-implanted SpF® Spine Fusion Stimulator consists of a generator that provides a constant direct current to titanium cathodes placed where bone growth is required. The SpF® Implantable Spine Fusion Stimulator is a Class III device and is indicated as a spinal fusion adjunct that increases the probability of fusion success in one or two levels or three or more levels.
Osteobiologics. The InterGro® DBM (Demineralized Bone Matrix) portfolio includes InterGro® DBM Paste, InterGro® DBM Putty and InterGro® DBM Plus, each providing an osteoconductive and osteoinductive matrix that may be used as an autograft extender in the spine. All InterGro® DBM forms contain human tissue or allograft bone, which has been granulated, demineralized and mixed with lecithin, a natural lipid carrier that is resistant to breakdown by bodily fluids, temperature or aggressive irrigation. InterGro® DBM has the highest DBM content by weight with validated osteoinductivity, and excellent handling and performance characteristics. InterGro® DBM Plus contains InterGro® DBM Paste pre-mixed with Pro Osteon® 500R granules, which provide an osteoconductive scaffold that resorbs in 6-18 months and an interconnected porosity that is similar to cancellous bone that provides continuous pathways for bony ingrowth.
Pro Osteon® 500R and Pro Osteon® 200R are resorbable, biocompatible, and osteoconductive bone graft substitutes made from marine coral, which has a distinct chemical composition and exhibits fully interconnected porosity. The unique pore structure in Pro Osteon® 500R provides continuous pathways for bony ingrowth that are similar to human cancellous bone. The architecture and chemical composition in Pro Osteon® 200R is similar to human bi-cortical bone. Both are a resorbable combination of hydroxyapatite and calcium carbonate that is intended to be replaced with natural bone during the healing process. Pro Osteon® 500R is available in granules and blocks, whereas Pro Osteon® 200R is available in granules.
The Indux™ Cortical Strip, machined from a single piece of human cortical bone, is fully demineralized for optimal osteoinductivity. The design allows for increased osteoinductivity, when compared to demineralized cancellous bone, and its unique cross-hatched texture creates a structure that provides both strength and flexibility. The Indux™ Cortical Strip may be rehydrated with blood, bone marrow aspirate (BMA) or saline solution and then shaped to fit a void or placed in the gutters of the posterolateral spine with local bone, DBM, and/or a bone graft substitute. Rehydration with BMA allows for the introduction of osteogenic cells and completion of the bone growth triad.
81
The Indux™ Cancellous Strip and Sponge are machined from human cancellous bone that is fully demineralized to expose the inherent growth factors and bone morphogenetic proteins that are essential for new bone formation (osteoinductive). The Indux™ Cancellous Strip and Sponge maintain the natural interconnected porosity of cancellous bone providing an ideal scaffold for cellular infiltration and bone formation (osteoconductive). The Indux™ Cancellous Strip and Sponge are available in various shapes and sizes for multiple applications. In addition, they may be rehydrated with blood, bone marrow aspirate (BMA) or saline solution, and they expand to fill the contours of any void, thereby minimizing the space between the graft and the host bone. Rehydration with BMA allows for the introduction of osteogenic cells and completion of the bone growth triad.
PlatFORM™ CM Blocks, PlatFORM™ CM Strips, PlatFORM™ CM Pads and PlatFORM™ Putty are collagen mineral composite matrices processed into either a block, strip, pad or granular form, respectively, for surgical implantation. The principle components of PlatFORM™ CM products are bovine type I collagen and anorganic bovine bone mineral. The mineral particles are dispersed within collagen fibers forming a three-dimensional open porous matrix consisting of bone mineral and collagen. PlatFORM™ CM products are provided as a sterile, dry material that is hydrated with autogenous bone marrow at the point of use. PlatFORM™ CM products are fully resorbed during the natural process of bone formation and remodeling.
Cellentra™ VCBM offers viable osteogenic cells, verified osteoinductivity, and an osteoconductive scaffold and contains at least 250,000 viable cells per cc, including mesenchymal stem cells (MSCs), osteoprogenitor cells and pre‐osteoblasts. The demineralized component of Cellentra™ VCBM provides additional inherent growth factors, including BMP‐2, 4, 7, VEGF, TGF‐ β, PDGF, IGF‐1 and FGF. Additionally, the cancellous bone matrix of Cellentra™ VCBM offers an interconnected trabecular structure for optimal osteoconductivity.
Traditional allografts, derived from donated human tissue, are used in a number of different applications and are available in a variety of forms, including cross-sections, iliac crest wedges, cortical and cancellous chips, granules, and powder. The advantages of traditional allografts include elimination of the need for a second procedure to harvest graft material and, thus, minimization of operating time; minimization of pain, complications, and morbidity; lower supply restrictions than autograft; and availability in various shapes and forms to suit specific anatomical indications.
Precision Machined Allograft Services. Many spinal procedures, in both the lumbar and cervical spine, involve spinal fusion. Surgeons often utilize precision machined allograft spacers to fuse the interbody space. We provide services related to the OsteoStim® Cervical Allograft Spacer for anterior cervical interbody fusions, the OsteoStim® ALIF Allograft Spacer for anterior lumbar interbody fusions and the OsteoStim® PLIF Allograft Spacer for posterior lumbar interbody fusions, depending on the surgical approach. All three systems are lordotic in shape, have serrated teeth on the top and bottom for added stability, are offered in various heights and have specific instrumentation to facilitate implantation.
Motion Preservation Products. In order to address the cervical artificial disc opportunity, we are developing next-generation designs utilizing innovative materials and geometries.
Electrical Stimulation Systems (for use within the appendicular system). Bone growth stimulation is a method of delivering a low level electrical current or ultrasound to a nonunion fracture site to promote bone growth.
The EBI Bone Healing System® is indicated for the treatment of nonunion fractures, failed fusions and congenital pseudarthrosis in the appendicular system. A nonunion is considered to be established when there are no visible progressive signs of healing. The EBI Bone Healing System® utilizes Pulsed Electromagnetic Fields (PEMF) for the treatment of fracture non-unions. Treatment is delivered through an anatomically configured therapeutic treatment coil.
The OrthoPak® 2 Bone Growth Stimulator is indicated for the treatment of an established nonunion acquired secondary to trauma, excluding vertebrae and all flat bones, where the width of the nonunion defect is less than one-half the width of the bone to be treated. The OrthoPak® 2 Bone Growth Stimulator utilizes capacitive coupling technology, which involves the upregulation of growth factors that modulate bone healing. The device consists of a small, lightweight generator worn outside the body that is connected to wafer-thin electrodes applied over the nonunion site.
We also offer an implantable option when bone growth stimulation is required in conjunction with, or subsequent to, surgical intervention. The Biomet® OsteoGen™ surgically implanted bone growth stimulator is
82
indicated for the treatment of long bone nonunions. Specifically, the device is only to be used to treat multiple nonunions or a severely comminuted nonunion where a single cathode cannot span the entire breadth of the nonunion site.
Dental Reconstructive Devices
Through our subsidiary, Biomet 3i, LLC, or Biomet 3i, we develop, manufacture and market products designed to enhance oral rehabilitation through the replacement of teeth and the repair of hard and soft tissues. These products include dental reconstructive devices and related instrumentation, bone substitute materials, regenerative products and materials, as well as crowns and bridges. A dental implant is a small screw, normally constructed of titanium or titanium alloy, which is surgically placed in the bone of the jaw to replace the root of a missing tooth and to provide an anchor for an artificial tooth.
Our historical flagship implant system, the OSSEOTITE® product line, features a micro-roughened surface technology, which allows for early/immediate loading and improved bone integration to the surface of the implant as compared to machined surfaced implants. In fiscal year 2007, we further enhanced implant surface technology with the introduction of the NanoTite™ Implant. The surface features the application of nanometer scale crystals of calcium phosphate to the existing OSSEOTITE® surface. The NanoTite™ Implant was initially introduced in the Certain® Implant configuration, which is an internal connection system that, through the use of the QuickSeat® connection, provides audible and tactile feedback when restorative abutments and ancillary components are seated into the implant. In addition, the 6 / 12 point hex connection design of the Certain® Implant System offers enhanced flexibility in placing the implant when pre-angled abutments are used. The NanoTite™ Certain® Tapered PREVAIL® Implant with integrated platform switching is designed for crestal bone preservation and aesthetic results by limiting hard and soft tissue recession. This is our first tapered geometry implant available commercially that integrates the platform switching concept.
Launched in fiscal year 2011, the OSSEOTITE® 2 Implant is an enhancement to the legacy OSSEOTITE® Implant. With more surface area in direct contact with the osteotomy wall, this implant is designed for greater bone-to-implant contact for primary stability, an important clinical consideration when pursuing more challenging surgical protocols such as immediate loading or immediate extraction and placement cases. Also in fiscal year 2011, the Tapered Certain® Implant manufactured from commercially pure titanium was introduced. Complementing the titanium alloy Tapered Certain® Implant, the commercially pure titanium tapered implant line extension is intended for markets (particularly Europe) where there is a strong preference for implant systems made from this material.
In the site preparation category of the dental product portfolio, we offer our Navigator® Instrumentation for guided surgery, including guided instrumentation for use with our Tapered Implant line. This open architecture instrumentation is designed to interface with the software and surgical guide solutions offered by existing entities in the marketplace. As planning and guide fabrication are based upon computed tomography scans, this may result in more accurate implant placement when combined with the depth and rotational control offered by our instrumentation. As implant placement position can be replicated as planned, this may also provide the opportunity for fabrication of a provisional prosthesis in advance of surgery, thereby allowing for a complete implant restoration in one patient visit.
On the regenerative side of the site preparation portfolio, we have continued to expand and improve our comprehensive bone grafting product and service offering. The portfolio now offers a variety of grafting materials (i.e., allografts, allograft putty, xenografts, and synthetics) and a resorbable collagen membrane, the OsseoGuard® Membrane. We also provide a larger granule size (1000—2000µm) for Endobon® Xenograft Granules. This larger particle size range of bovine-derived particulate bone grafting material is suitable for use in large defects, such as sinus augmentation procedures. In addition, we offer an irradiated version of RegenerOss® Allograft particulate. RegenerOss® Allograft Irradiated material undergoes the same processing as aseptic RegenerOss® Allograft items, with the addition of a step for sterilization.
In our restorative portfolio, we launched the Low Profile Abutment for screw-retained restorations in fiscal year 2011. Screw-retained abutments are designed to provide clearer access to, and retrievability of, single and multiple-unit implant restorations. In addition, certain patient situations may require the benefits of screw-retained restorations such as full mouth reconstruction and immediate loading techniques.
Within Digital Dentistry, we offer our Encode® Impression System patient-specific abutment technology. This technology is an enhancement of the baseline Encode® Abutment offering, allowing us to fabricate an abutment and orient implant body analogs into the proper position in a stone master model. This can enable the complete
83
fabrication of a restoration from one supragingival impression, which is significantly easier than present techniques and a potential opportunity for more general dentists to become involved in implant therapy. The quality of these abutments and the ability to save significant chair time are also potential benefits to experienced restorative dentists. The material choice for Encode® Impression System abutment fabrication also includes Zirconia options for the fabrication of aesthetic, all-ceramic restorations. In fiscal year 2012, the digital dental brand name BellaTek™ was introduced and incorporated into the product portfolio. The impressioning system is now referred to as the BellaTek™ Encode® Impression System and the patient specific definitive abutments are now referred to as BellaTek™ Abutments.
Other Products
We also manufacture and distribute numerous other products, including craniomaxillofacial fixation devices, cardiothoracic fixation devices, autologous therapy products and services, operating room supplies, casting materials, general surgical instruments, wound care products and other surgical products. Our craniomaxillofacial fixation and cardiothoracic products are marketed by our subsidiary, Biomet Microfixation, LLC, or Biomet Microfixation.
Neurosurgical solutions: We offer products used in cranial reconstructive and cranial closure procedures. We focus on providing a complete product offering for complex cases and products for standardized procedures. Products include the HTR®-PMI Hard Tissue Replacement implants for severe cranial defects and the iQ™ Intelligent System for faster screw delivery.
Craniomaxillofacial solutions: We offer plating systems for reconstruction of the face and skull due to tumor and trauma procedures. These products are used by oral surgeons, reconstructive plastic surgeons, and ear, nose and throat surgeons. Products include the TraumaOne™ Plating System, a Total Mandibular Joint Replacement System and Lactosorb® Resorbable Fixation Systems.
Cardiothoracic solutions: We offer devices for sternal closure and chest wall reconstruction. Products include SternaLock® Blu and the Pectus Bar.
SternaLock® Blu is our primary sternal closure system. Cardiothoracic surgeons use our implants to close the sternum after a midline sternotomy or a mini-sternotomy. The system also offers a plating solution for a mini-thoracotomy.
The Pectus Bar is an implant used to correct pectus excavatum, a chest wall deformity. Biomet Microfixation owns the patent for this product, which is commonly used during the Nuss Procedure.
Autologous Therapy Products and Services. We manufacture and market a line of autologous therapy products through our subsidiary, Biomet Biologics, LLC, or Biomet Biologics, including autologous blood processing disposables. Our portfolio is comprised of core technologies including the GPS® III System, the Plasmax® Plasma Concentration System, the BioCUE™ Platelet Concentration System and the Clotalyst® Autologous Serum Collection System.
The GPS® III System is a device that collects platelet concentrate from a small volume of the patient’s blood using a fast, single centrifuge cycle process. The GPS® III System is designed to provide a high percentage of platelet concentrate.
Product Development
Our research and development efforts are essentially divided into two categories: innovative new technology and evolutionary developments. Most of the innovative new technology development efforts are focused on biomaterial products, are managed at the corporate level and take place primarily at our Warsaw, Indiana headquarters. Evolutionary developments are driven primarily by the individual subsidiaries and include product line extensions and improvements.
We continue to aggressively conduct internal research and development efforts to generate new marketable products, technologies and materials. In addition, we believe we are well positioned to take advantage of external acquisition and development opportunities. An important component of our strategy has been the formation of strategic alliances to enhance the development of new musculoskeletal products.
84
For the nine months ended February 28, 2013, we invested $107.2 million, and for the fiscal years 2012, 2011 and 2010, we invested $126.8 million, $119.4 million and $106.6 million, respectively, on research and development. We expect that our research and development investments will continue to increase. Our research and development expenses primarily related to our ongoing commitment to increase investment in clinical research and regulatory affairs within our business. Our principal research and development efforts relate to primary and revision orthopedic reconstructive devices, spinal fixation products, dental reconstructive devices, sports medicine products, resorbable technology, biomaterial products and autologous therapies.
Patents and Trademarks
We believe patents and other intellectual property will continue to be of importance in the musculoskeletal industry. Accordingly, we continue to protect technology developed internally and to acquire intellectual property rights associated with technology developed outside the Company. We enforce our intellectual property rights consistent with our strategic business objectives. We do not believe that we own any single patent or hold any single license (or series of patents or licenses) that is material to our operations, consolidated revenues or earnings.
BIOMET is our principal registered trademark throughout the world, and registrations have been obtained or are in process with respect to various other trademarks associated with our products. Unless otherwise noted in this, all trademarks contained herein are owned by Biomet or one of its affiliates and subsidiaries.
Government Regulation
Most aspects of our business are subject to some degree of government regulation in the countries in which our operations are conducted. It has always been our practice to comply with the regulatory requirements governing our products and operations and to conduct our affairs in an ethical manner. This practice is reflected in our Code of Business Conduct and Ethics, various other compliance policies and through the responsibility of the Audit Committee of the Board of Directors to review our systems of internal control, our process for monitoring compliance with laws and regulations and our process for monitoring compliance with our Code of Business Conduct and Ethics. For some products, and in some areas of the world such as the United States, Canada, Japan and Europe, government regulation is significant and, in general, there appears to be a trend toward more stringent regulation throughout the world, as well as global harmonization of various regulatory requirements. We devote significant time, effort and expense to addressing the extensive government and regulatory requirements applicable to our business. Governmental regulatory actions can result in the recall or seizure of products, suspension or revocation of the authority necessary for the production or sale of a product, and other civil and criminal sanctions. We believe that we are no more or less adversely affected by existing government regulations than are our competitors.
In the United States, the development, testing, marketing and manufacturing of medical devices are regulated under the Medical Device Amendments of 1976 to the Federal Food, Drug and Cosmetic Act, the Safe Medical Devices Act of 1990, the FDA Modernization Act of 1997, the Medical Device User Fee and Modernization Act of 2002, the FDA Amendments Act of 2007, the FDA Safety and Innovation Act of 2012, and additional regulations promulgated by the FDA and various other federal, state and local agencies. In general, these statutes and regulations require that manufacturers adhere to certain standards designed to ensure the safety and efficacy of medical devices and related medical products.
Most of our new device products require the submission of a Premarket Notification, commonly referred to as a 510(k), to the FDA prior to our marketing the product. This process requires us to demonstrate that the device is at least as safe and effective as, or “substantially equivalent” to, a legally marketed device before we can receive an order from the FDA finding substantial equivalence and clearing the new device for commercial distribution in the United States. On July 29, 2011, the Institute of Medicine (IoM) published a report of its review of the 510(k) clearance program to FDA. The IoM report recommended that the FDA pursue a legislative change from the current 510(k) process to an integrated premarket and post-market regulatory framework. It is uncertain if these recommendations will ultimately be pursued. If they are pursued, it is possible we will be required to submit additional clinical and manufacturing information with respect to premarket applications in the future, resulting in increased costs and increased delay in introducing products to the market. Other devices we develop and market fall into a class of products for which the FDA has implemented stringent clinical investigation and Premarket Approval, or PMA, requirements. The PMA process requires us to provide clinical and laboratory data that establishes that the new medical device is safe and effective. The FDA will approve the new device for commercial distribution if it determines that the data and information in the PMA relating to design, materials, bench and animal testing and
85
human clinical data constitute valid scientific evidence and that there is reasonable assurance that the device is safe and effective for its intended use.
There are also various federal healthcare laws that apply when we or customers submit claims for items or services that are reimbursed under Medicare, Medicaid or other federally-funded healthcare programs, including among others: (1) the Anti-Kickback Statute which prohibits offers to pay or receive remuneration of any kind for the purpose of inducing or rewarding referrals of items or services reimbursable by a Federal healthcare program; (2) the False Claims Act, which prohibits the submission of false or otherwise improper claims for payment to a federally-funded health care program; and (3) the Stark law, which prohibits physicians from referring Medicare or Medicaid patients to a provider that bills these programs for the provision of certain designated health services if the physician (or a member of the physician’s immediate family) has a financial relationship with that provider. There are often similar state false claims, anti-kickback and anti-self referral and insurance laws that apply to state-funded Medicaid and other healthcare programs and private third-party payors.
We are subject to various federal and foreign laws that govern our international business practices, particularly with respect to payments to government officials. The U.S. Foreign Corrupt Practices Act, or FCPA, has been used with some frequency to prosecute companies in the United States. The FCPA prohibits U.S. companies and their officers, directors, employees, shareholders acting on their behalf and agents from offering, promising, authorizing or making payments to foreign officials for the purpose of obtaining or retaining business abroad or otherwise obtaining favorable treatment and this law requires companies to maintain records which fairly and accurately reflect transactions and to maintain internal accounting controls. In many countries, hospitals and clinics are government-owned and healthcare professionals employed by such hospitals and clinics, with whom we regularly interact, may meet the definition of a foreign official for purposes of the FCPA. Refer to “Note 16—Contingencies” to our financial statements included elsewhere in this prospectus and “Note 15—Contingencies” to our unaudited condensed consolidated interim financial statements included elsewhere in this prospectus for a description of the outcome of the FCPA investigation of the Company by the SEC and DOJ. On July 1, 2011, the U.K. Bribery Act 2010 became effective, which prohibits active and passive bribery, including commercial bribery, and bribery of a foreign public official for a business purpose. The Act also imposes attribution liability on companies that fail to prevent “associated persons” from committing acts of bribery and includes far-reaching jurisdiction for prosecution.
We are also subject to various federal, state and foreign laws that protect the confidentiality of certain patient health information, including patient medical records, and restrict the use and disclosure of patient health information by healthcare providers. In April 2003, the U.S. Department of Health and Human Services (HHS) published patient privacy rules under the Health Insurance Portability and Accountability Act of 1996 (HIPAA) and, in April 2005, published security rules for protected health information. The HIPAA privacy and security rules govern the use, disclosure and security of protected health information by “Covered Entities,” which include, among others, healthcare providers that submit electronic claims and health plans. In 2009, Congress passed the HITECH Act, which modified certain provisions of the HIPAA privacy and security rules for Covered Entities and their Business Associates, which is anyone that performs a service on behalf of a Covered Entity involving the use or disclosure of protected health information and is not a member of the covered entity’s workforce. Among other things, the HITECH Act provided that Business Associates will now be subject to the same security requirements as Covered Entities, and that with regard to both the security and privacy rule, Business Associates will be subject to direct enforcement by HHS, including civil and criminal liability, just as Covered Entities are. In the past, HIPAA has generally affected us indirectly.
Biomet is generally not a Covered Entity under HIPAA, except for our noninvasive bone growth stimulation business and our health insurance plans. We only operate as a Business Associate to Covered Entities in a limited number of instances. In those cases, the patient data that we receive and analyze may include protected health information. We are committed to maintaining the security and privacy of patients’ health information and believe that we meet the expectations of the HIPAA rules. Some modifications to our systems and policies may be necessary to address requirements for recently enacted state privacy laws, but we believe we have laid the necessary framework for such changes. We believe the ongoing costs and impacts of assuring compliance with the HIPAA privacy and security rules are not material to our business.
We believe that we are well positioned to face the changing international regulatory environment. The International Standards Organization, or the ISO, has an internationally recognized set of standards aimed at ensuring the design and manufacture of quality products. A company that has passed ISO audits and obtained ISO certification applicable to its activity sector is internationally recognized as having quality manufacturing processes. The European Union (EU) legislation requires that medical devices bear a CE mark. The CE mark is a European
86
Union and European Free Trade Association symbol, which indicates that the product adheres to European Medical Device Directives. Compliance with ISO quality systems standards is one of the requirements for placing the CE mark on our products. Each of our principal manufacturing facilities has been certified to ISO 13485:2003. Our products sold in Europe bear the CE mark to the extent required by European law and regulations.
In addition, governmental bodies in the United States and throughout the world have expressed concern about the costs relating to healthcare and, in some cases, have focused attention on the pricing of medical devices. Government regulation regarding pricing of medical devices already exists in some countries and may be expanded in the United States and other countries in the future. We are subject to increasing pricing pressures worldwide as a result of growing regulatory pressures, as well as the expanding predominance of managed care groups and institutional and governmental purchasers. Under Title VI of the Social Security Amendments of 1983, hospitals receive a predetermined amount of Medicare reimbursement for treating a particular patient based upon the patient’s type of illness identified with reference to the patient’s diagnosis under one or more of several hundred diagnosis-related groups. Other factors affecting a specific hospital’s reimbursement rate include the size of the hospital, its teaching status and its geographic location.
While we are unable to predict the extent to which our business may be affected by future regulatory developments, we believe that our substantial experience in dealing with governmental regulatory requirements and restrictions throughout the world, our emphasis on efficient means of distribution and our ongoing development of new and technologically-advanced products should enable us to continue to compete effectively within this increasingly regulated environment.
Sales and Marketing
We have diligently worked to attract and retain qualified, well-trained and motivated sales representatives. The breadth of our product offering and the quality of our sales forces collaborate to create synergies that we believe uniquely position us to continue to efficiently penetrate the musculoskeletal market. In the United States, our products are marketed by a combination of independent third-party distributors, independent commissioned sales agents and direct sales representatives, primarily based on the specific product group being represented. In Europe, our products are promoted by sales representatives employed by subsidiaries, independent third-party distributors, and some independent commissioned sales agents, based primarily on the geographic location. In the rest of the world, we maintain direct selling organizations in eleven countries, as well as independent commissioned sales agents and independent third-party distributors in other key markets. In aggregate, our products are marketed by more than 3,000 sales representatives throughout the world.
Seasonality
Elective surgery-related products are influenced to some degree by seasonal factors, as the number of elective procedures declines during the summer months, particularly in European countries, and the winter holiday season.
Customers
Our customers are the hospitals, surgeons, other physicians and healthcare providers who use our products in the course of their practices. Our business is dependent upon the relationships maintained by our distributors and salespersons with these customers, as well as our ability to design and manufacture products that meet the physicians’ technical requirements at a competitive price.
Inventory and Trade Accounts Receivable
We have inventory located throughout the world with our customers, our distributors and direct salespersons for their use in marketing our products and in filling customer orders. As of May 31, 2012, inventory of approximately $242.5 million was located with these distributors, salespersons and customers. We maintain trade accounts receivable balances based on credit terms that are generally consistent with industry and local market practices.
Distribution
We operate distribution facilities domestically in Warsaw, Indiana; Irvine, California; Palm Beach Gardens, Florida; Parsippany, New Jersey; Jacksonville, Florida; Fair Lawn, New Jersey; and Braintree, Massachusetts, and
87
internationally in Valence, France; Berlin, Germany; Dordrecht, The Netherlands; Hazeldonk, The Netherlands; Valencia, Spain; Bridgend, South Wales; Swindon, England; Tokyo, Japan; Seoul, South Korea; North Ryde, Australia; Jinhua, China; and Changzhou, China. We generally ship our orders via expedited courier service. Our backlog of firm orders is not considered material to understanding our business.
Competition
Our business is highly competitive. Competition within the industry is primarily based on service, clinical results and product design, although price competition is an important factor as healthcare providers continue to be concerned with costs. Major competitors in our five product categories are set forth below by market category.
Large Joint Reconstructive Products
Our large joint orthopedic reconstructive devices compete primarily with those offered by DePuy, Inc. (a Johnson & Johnson company), Smith & Nephew plc, Stryker Orthopaedics (a division of Stryker Corp.) and Zimmer, Inc. (a subsidiary of Zimmer Holdings, Inc.). Management believes these four companies, together with Biomet, have the predominant share of the global large joint orthopedic reconstructive device market. We believe that our prices for large joint orthopedic reconstructive devices are competitive with those in the industry. We believe that our future success will depend upon, among other things, our service and responsiveness to our distributors and orthopedic specialists, the continued strong clinical results of our products, and upon our ability to design and market innovative and technologically-advanced products that meet the needs of the marketplace.
S.E.T. Devices
Our sports medicine products compete primarily in the areas of procedure-specific implants and instruments, manual instruments and power instruments. Our principal competitors include Smith & Nephew Endoscopy (a division of Smith & Nephew plc), Stryker Corp., Linvatec Corp. (a subsidiary of CONMED Corporation), Mitek (a division of Ethicon, a Johnson & Johnson company), Arthrocare Corp. and Arthrex, Inc.
Our extremity devices primarily compete with those offered by DePuy, Inc. (a Johnson & Johnson company), Tornier, Inc., Zimmer, Inc. (a subsidiary of Zimmer Holdings, Inc.), Wright Medical, Exactech and Stryker Orthopaedics (a division of Stryker Corp.)
Our internal and external fixation devices compete with other such devices primarily on the basis of price, ease of application and clinical results. Our internal fixation product lines compete principally with those of DePuy Synthes (a Johnson & Johnson company), Zimmer, Inc. (a subsidiary of Zimmer Holdings, Inc.), Smith & Nephew plc and Stryker Trauma (a division of Stryker Corp.). The principal competitors in the external fixation market are Smith & Nephew plc, Stryker Trauma (a division of Stryker Corp.), DePuy Synthes (a Johnson & Johnson Company), Zimmer, Inc. (a subsidiary of Zimmer Holdings, Inc.) and Orthofix, Inc. (a subsidiary of Orthofix International N.V.).
Spine and Bone Healing Products
Our spinal fixation systems compete with other spinal fixation systems primarily on the basis of breadth of product line, product recognition and price. The principal spinal fixation competitors are Medtronic Sofamor Danek, Inc. (a subsidiary of Medtronic, Inc.), DePuy Synthes (a Johnson & Johnson Company), Stryker Spine (a division of Stryker Corp.), Zimmer Spine (a subsidiary of Zimmer Holdings, Inc.) and others.
Our osteobiologic products compete with other osteobiologics primarily on the basis of breadth of product line, product recognition and price. The principal competitors in osteobiologics are Medtronic Sofamor Danek, Inc. (a subsidiary of Medtronic, Inc.), DePuy Synthes (a Johnson & Johnson Company), Stryker Spine (a division of Stryker Corp.), Zimmer Spine (a subsidiary of Zimmer Holdings, Inc.) and others.
Our electrical stimulation devices primarily compete with those offered by Orthofix, Inc. (a subsidiary of Orthofix International N.V.), DJO, Inc. (formerly ReAble Therapeutics, Inc.) and Smith & Nephew plc. Competition in the electrical stimulation market is on the basis of product design, service, price and success rates of various treatment alternatives.
Dental Reconstructive Devices
88
Our dental reconstructive devices compete in the areas of dental reconstructive implants and related products. The primary competitors in the dental implant market include Nobel Biocare AB, Straumann AG, DENTSPLY International, Inc., and Zimmer Dental (a subsidiary of Zimmer Holdings, Inc.).
Other Products
Our craniomaxillofacial fixation products, specialty surgical instrumentation and neurosurgical cranial flap fixation products compete with those offered by DePuy Synthes (a Johnson & Johnson Company), Stryker Leibinger Micro Implants (a division of Stryker Corp.), KLS-Martin, L.P., Osteomed Corp., Aesculap, Inc., Medtronic, Inc. and Codman & Shurtleff, Inc. (a Johnson & Johnson company).
Raw Materials and Supplies
Our suppliers are a critical element of Biomet’s supply chain. We have established strategic partnerships with key suppliers. This has enabled us to leverage our buying power, establish vendor managed inventory arrangements, enhance product innovation and reduce our risk. Long-term contracts allow us to develop mutually advantageous relationships with our suppliers by providing them with more visibility into our future demand and new product needs. Our Sales, Inventory and Operations Planning (“SIOP”) process balances our inventory position and supply capacity with our forward looking sales plan via an integrated reconciliation process. On a monthly basis, our SIOP process in each business unit reviews demand, supply, and inventory, and identifies potential future capacity or material gaps so that the proper corrective actions can be put in place.
The raw materials used in the manufacture of our orthopedic large joint reconstructive, S.E.T., spine & bone healing and dental devices are principally nonferrous metallic alloys, stainless steel and polyethylene powder. With a few exceptions, none of our raw material requirements are limited to any material extent by critical supply or single origins. The demand for certain raw materials used by us, such as cobalt-chromium alloy and titanium may vary. The primary buyers of these metallic alloys are in the aerospace industry. If the demands of the aerospace industry should increase dramatically, we could experience complications in obtaining these raw materials.
Based on our current relationship with our suppliers, we do not anticipate a material shortage in the foreseeable future. Further, we believe that our inventory of raw materials is sufficient to meet any short-term supply shortages of metallic alloys. The results of our operations are not materially dependent on raw material costs.
Safety stock levels of critical materials are reviewed on a quarterly basis to ensure these stocks are appropriately set. Factors that determine these stock levels include future usage estimates, lead times, forecast accuracy, commodity pricing trends, worldwide market conditions and risk mitigation. In the case of single sourced materials, stock levels are established taking into account potential disruption to supply and, where practical, back-up supply points are identified for contingency.
Environmental Matters
We are subject to various federal, state and local laws and regulations regulating the discharge of materials into the environment and otherwise relating to the protection of the environment. We do not believe that we will be required to spend any material amounts in order to comply with these laws and regulations or that compliance with such laws and regulations will materially affect our capital expenditures, results of operations, financial condition or cash flows.
Employees
As of February 28, 2013, our domestic operations (including Puerto Rico) employed 3,648 persons, of whom 1,747 were engaged in production and 1,901 in research and development, sales, marketing, administrative and clerical efforts. Our international subsidiaries employed 4,687 persons, of whom 2,364 were engaged in production and 2,323 in research and development, sales, marketing, administrative and clerical efforts. None of our principal domestic manufacturing employees are represented by a labor union. The production employees at our Bridgend, South Wales facility are organized. Employees working at the facilities in Berlin, Germany; Valence, France; Swindon, United Kingdom and Valencia, Spain are represented by Workers’ Councils. We believe that our relationship with our employees is satisfactory.
The establishment of our domestic orthopedic reconstructive manufacturing operations in north central Indiana, near other members of the orthopedic industry, provides access to the highly skilled machine operators
89
required for the manufacture of our products. Our European manufacturing locations in South Wales, England, France, Spain, Switzerland and Germany also provide good sources for skilled manufacturing labor. Our Puerto Rican operations principally involve the assembly of purchased components into finished products using a skilled labor force. Our manufacturing operations in Jinhua, Zhejiang Province, and Changzhou, Jiangsu Province, China are growing and currently include approximately 780 persons who are included in the numbers above.
Legal Proceedings
We are involved in various proceedings, legal actions and claims arising in the normal course of business, including proceedings related to product liability, governmental investigations, intellectual property, commercial litigation and other matters. The outcomes of these matters will generally not be known for an extended period of time. In certain of the legal proceedings, the claimants seek damages, as well as other compensatory relief, which could result in the payment of significant claims and settlements. For legal matters for which management has sufficient information to reasonably estimate our future obligations, a liability representing management’s best estimate of the probable cost, or the minimum of the range of probable losses when a best estimate within the range is not known, for the resolution of these legal matters is recorded. The estimates are based on consultation with legal counsel, previous settlement experience and settlement strategies. Our accrual for contingencies at February 28, 2013 and May 31, 2012 of $49.1 million and $25.5 million, respectively, primarily relate to certain product liability claims and the Massachusetts U.S. Department of Justice EBI products investigation.
Other than the Massachusetts U.S. Department of Justice EBI products investigation and certain product liability claims, for which the estimated loss is included in the accrual referenced above, given the relatively early stages of the other governmental investigations and other product liability claims described below, and the complexities involved in these matters, we are unable to estimate a possible loss or range of possible loss for such matters until we know, among other factors, (i) what claims, if any will survive dispositive motion practice, (ii) the extent of the claims, including the size of any potential class, particularly when damages are not specified or are indeterminate, (iii) how the discovery process will affect the litigation, (iv) the settlement posture of the other parties to the litigation and (v) any other factors that may have a material effect on the litigation.
U.S. Department of Justice Consulting Agreement Investigation
On September 27, 2007, we entered into a Deferred Prosecution Agreement with the U.S. Attorney’s Office for the District of New Jersey. The agreement concluded the government’s investigation into whether consulting agreements between the largest orthopedic manufacturers and orthopedic surgeons who use joint reconstruction and replacement products may have violated the federal Anti-Kickback Statute.
Through the agreement, the U.S. Attorney’s Office agreed not to prosecute us in connection with this matter, provided that we satisfied our obligations under the agreement over the 18 months following the date of the Deferred Prosecution Agreement. The agreement called for the appointment of an independent monitor to review Biomet’s compliance with the agreement, particularly in relation to its consulting agreements. On March 27, 2009, the Deferred Prosecution Agreement expired and the complaint was dismissed with prejudice.
As part of the resolution of this matter, we also entered into a Corporate Integrity Agreement with the Office of the Inspector General of the U.S. Department of Health and Human Services. The agreement required us for five years subsequent to September 27, 2007 to continue to adhere to our Code of Business Conduct and Ethics and certain other provisions, including reporting requirements. We submitted our final report under the Corporate Integrity Agreement with OIG-HHS and received confirmation in January 2013 from OIG-HHS that our obligations under the agreement have terminated.
U.S. Department of Justice EBI Products Investigations and Other Matters
In February 2010, we received a subpoena from the Office of the Inspector General of the U.S. Department of Health and Human Services requesting various documents relating to agreements or arrangements between physicians and our Interpore Cross subsidiary for the period from 1999 through the present and the marketing and sales activities associated with Interpore Cross’ spinal products. We are cooperating with the request of the Office of the Inspector General. We can make no assurances as to the time or resources that will be needed to devote to this inquiry or its final outcome.
In April 2009, we received an administrative subpoena from the U.S. Attorney’s Office for the District of Massachusetts requesting various documents relating primarily to the Medicare reimbursement of and certain
90
business practices related to the our EBI subsidiary’s non-invasive bone growth stimulators. It is our understanding that competitors in the non-invasive bone growth stimulation market received similar subpoenas. We received subsequent subpoenas in connection with the investigation in September 2009, June 2010, February 2011 and March 2012 along with several informal requests for information. We have produced responsive documents and are fully cooperating in the investigation.
In April 2009, we became aware of a qui tam complaint alleging violations of the federal and various state False Claims Acts filed in the United States District Court for the District of Massachusetts, where it is currently pending. Biomet, Parent, and several of our competitors in the non-invasive bone growth stimulation market were named as defendants in this action. The allegations in the complaint are similar in nature to certain categories of requested documents in the above-referenced administrative subpoenas. The U.S. government has not intervened in the action. We are vigorously defending this matter and intend to continue to do so.
U.S. Department of Justice Civil Division Investigation
In September 2010, we received a Civil Investigative Demand issued by the U.S. Department of Justice—Civil Division pursuant to the False Claims Act. The CID requests that we provide documents and testimony related to allegations that Biomet, OtisMed Corp. and Stryker Corp. have violated the False Claims Act relating to the marketing of, and payment submissions for, OtisMed’s OtisKneeTM (a registered trademark of OtisMed) knee replacement system. We have produced responsive documents and are fully cooperating in the investigation.
U.S. Securities and Exchange Commission Informal Investigation
On September 25, 2007, we received a letter from the SEC informing us that it was conducting an informal investigation regarding possible violations of the Foreign Corrupt Practices Act in the sale of medical devices in certain foreign countries by companies in the medical devices industry. The Foreign Corrupt Practices Act prohibits U.S. companies and their officers, directors, employees, or shareholders acting on their behalf and agents from offering, promising, authorizing or making payments to foreign officials for the purpose of obtaining or retaining business abroad or otherwise obtaining favorable treatment and this law requires companies to maintain records which fairly and accurately reflect transactions and to maintain internal accounting controls. In many countries, hospitals and clinics are government-owned and healthcare professionals employed by such hospitals and clinics, with whom we regularly interact, may meet the definition of a foreign official for purposes of the Foreign Corrupt Practices Act. On November 9, 2007, we received a letter from the DOJ requesting any information provided to the SEC be provided to the DOJ on a voluntary basis.
On March 26, 2012, we entered into the DPA with the DOJ and a Consent to Final Judgment with the SEC related to these investigations by the DOJ and the SEC. Pursuant to the DPA, the DOJ has agreed not to prosecute us in connection with this matter, provided that we satisfy our obligations under the agreement over the next three years. In addition, pursuant to the terms of the DPA, an independent external compliance monitor has been appointed to review our compliance with the DPA, particularly in relation to our international sales practices, for at least the first 18 months of the three year term of the DPA. We also agreed to pay a monetary penalty of $17.3 million to resolve the charges brought by the DOJ, which was paid in the fourth quarter of fiscal year 2012. The terms of the DPA and the associated monetary penalty reflect our full cooperation throughout the investigation.
We contemporaneously reached a Consent Agreement with the SEC to settle civil claims related to this matter. As part of the Consent Agreement, we agreed to the SEC’s entry of a Final Judgment requiring us to disgorge profits and pay prejudgment interest in the aggregate amount of $5.6 million, which was paid in the fourth quarter of fiscal year 2012.
Product Liability
We have received claims for personal injury associated with its metal-on-metal hip products. The pre-trial management of certain of these claims has been consolidated in a federal court in South Bend, Indiana. Certain other claims are pending in various state courts. We believe the number of claims continues to increase incrementally due to the negative publicity regarding metal-on-metal hip products generally. We believe we have data that supports the efficacy and safety of our metal-on-metal hip products, and we intend to vigorously defend ourselves in these matters. We currently account for these claims in accordance with our standard product liability accrual methodology on a case by case basis. Given the substantial or indeterminate amounts sought in these matters, and the inherent unpredictability of such matters, an adverse outcome in these matters in excess of the
91
amounts included in our accrual for contingencies could have a material adverse effect on our financial condition, results of operations and cash flow.
Future revisions in our estimates of these provisions could materially impact its results of operations and financial position. We use the best information available to determine the level of accrued product liabilities, and we believe our accruals are adequate. We have maintained product liability insurance coverage for a number of years on a claims-made basis. All such insurers have been placed on notice of these claims. To date, the insurance companies have neither accepted nor denied coverage, and an issue may arise as to which policy or policies are to respond. The amounts incurred to date in connection with these claims have not exceeded our self-insured retention(s).
Intellectual Property Litigation
On May 3, 2013, Bonutti Skeletal Innovations LLC, a company formed to hold certain patents acquired from Dr. Peter M. Bonutti and an affiliate of patent licensing firm Acacia Research Group LLC, filed suit against us in the U.S. District Court for the Eastern District of Texas, alleging a failure to pay royalties due under a license agreement with Dr. Bonutti , misuse of confidential information and infringement of U.S. Patent Nos. 5,921,986; 6,099,531; 6,423,063; 6,638,279; 6,702,821; 7,070,557; 7,087,073; 7,104,996; 7,708,740; 7,806,896; 7,806,897; 7,828,852; 7,931,690; 8,133,229; and 8,147,514. The lawsuit seeks damages in an amount yet to be determined and injunctive relief. Previous to the filing of this lawsuit, on March 8, 2013 we had filed a complaint for declaratory judgment with the U.S. District Court for the Northern District of Indiana seeking a judgment of non-infringement and invalidity of the patents at issue. We are vigorously defending this matter and believe that our defenses against infringement are valid and meritorious. We can make no assurances as to the time or resources that will be needed to devote to this litigation or its final outcome.
In January 2009, Heraeus Kulzer GmbH initiated legal proceedings in Germany against us and our subsidiary, Biomet Europe BV, alleging that we and Biomet Europe BV misappropriated Heraeus Kulzer trade secrets when developing our current lines of European bone cements, which were first marketed in 2005. The lawsuit seeks damages in excess of €30 million and injunctive relief to preclude us from producing our current line of European bone cements. On December 20, 2012, the trial court ruled that Biomet did not misappropriate trade secrets and consequently dismissed Biomet, Biomet Europe BV, Biomet Deutschland GmbH and other defendants from the lawsuit. Biomet Orthopaedics Switzerland GmbH (“Biomet Switzerland”) remains as the only defendant in the lawsuit and the trial court has ruled that Heraeus Kulzer will not be permitted to review certification materials of Biomet Switzerland for purposes of determining whether there is any evidence that would support a claim of trade secret misappropriation by that entity. The trial court’s decision remains subject to appeal by Heraeus Kulzer and we are continuing to vigorously defend this matter.
Other Matters
There are various other claims, lawsuits, disputes with third parties, investigations and pending actions involving various allegations against us incident to the operation of our business, principally product liability and intellectual property cases. Each of these matters is subject to various uncertainties, and it is possible that some of these matters may be resolved unfavorably to us. We accrue for losses that are deemed to be probable and subject to reasonable estimate.
Based on the advice of our counsel in these matters, it is unlikely that the resolution of any of these matters and any liabilities in excess of amounts provided will be material to our financial position, results of operations or cash flows.
92
Our Facilities
Our principal executive offices are at 56 East Bell Drive, Warsaw, Indiana. In addition, we maintain more than 50 other manufacturing facilities, offices and warehouse facilities in various countries, including Canada and numerous countries within Europe, Asia Pacific and Latin America. We believe that all of our facilities are adequate, well maintained and suitable for the development, manufacture, distribution and marketing of all our products.
The following is a list of our principal properties as of February 28, 2013:
| FACILITY | LOCATION | SQUARE FEET | OWNED/ LEASED | |||
| Corporate headquarters of Biomet, Inc.; manufacturing, storage and research and development facilities of Biomet Manufacturing Corporation; manufacturing & storage facilities of Biomet Microfixation, LLC; distribution center and offices of Biomet Orthopedics, LLC; distribution center and offices of Biomet Sports Medicine, LLC; distribution center and offices of Biomet Biologics, LLC and distribution center of EBI, LLC | (1) Warsaw, Indiana (2) Warsaw, Indiana (3) Milford, Indiana | 541,699 13,300 54,880 | Owned Leased Leased | |||
| Administrative facility of EBI, LLC and administrative offices of Electro-Biology, LLC | Parsippany, New Jersey | 73,807 | Leased | |||
| Administrative, manufacturing and distribution facility of Biomet Microfixation, LLC | Jacksonville, Florida | 82,500 | Owned | |||
| Office, manufacturing and distribution facility of Biomet 3i, LLC | (1) Palm Beach Gardens, Florida (2) Palm Beach Gardens, Florida (a) | 117,000 69,000 | Owned Owned | |||
| Office, manufacturing and distribution facility of Citra Labs, LLC | Braintree, Massachusetts | 32,150 | Leased | |||
| Manufacturing facility of Biomet Fair Lawn, LLC | Fair Lawn, New Jersey | 40,000 | Owned | |||
| Office and manufacturing facility of Electro-Biology, LLC | Guaynabo, Puerto Rico | 34,700 | Owned | |||
| Office, manufacturing and distribution facilities of Interpore Spine Ltd. | (1) Irvine, California (2) Irvine, California | 36,800 2,700 | Leased Leased | |||
| Office and warehouse facilities of Biomet Europe B.V. | Hazeldonk, The Netherlands | 131,320 | Leased | |||
| Office and research and development facilities for Trauma operations | Miami, Florida | 30,850 | Leased | |||
| Office, manufacturing and warehouse facility of Biomet France Sarl | Valence, France | 86,100 | Owned | |||
| Office, manufacturing and warehouse facilities of Biomet Deutschland GmbH | Berlin, Germany | 49,900 | Owned | |||
| Administrative offices of Biomet Europe B.V. and office and warehouse facility of Biomet Nederland B.V. and Biomet Microfixation Europe B.V. | Dordrecht, The Netherlands | 37,700 | Owned | |||
| Office and manufacturing facility of met 3i Dental Iberica, S.L.. | Valencia, Spain | 69,600 | Owned | |||
| Manufacturing and administrative facilities of Biomet UK Ltd. | (1) Bridgend, South Wales (2) Swindon, England | 111,95654,800 | Owned Owned | |||
| Manufacturing, administrative and warehouse facilities of Zhejiang Biomet | Jinhua, China | 110,000 | Owned | |||
| Manufacturing, administrative and warehouse facilities of Changzhou Biomet | Changzhou, China | 82,000 | Owned | |||
| Administrative office facilities for China operations | Shanghai, China | 6,100 | Leased | |||
| Manufacturing facility for Trauma operations | Le Locle, Switzerland | 115,240 | Leased | |||
93
___________________________________________________
| (a) | Includes 23,000 square feet of space in this facility that is leased to other parties. |
Our properties in Warsaw, Indiana and Palm Beach Gardens, Florida secure our obligations under our senior secured cash flow facilities. We believe our headquarters, manufacturing and other facilities are suitable for their respective uses and are, in all material respects, adequate for our present needs. Our properties are subject to various federal, state, foreign and local laws and regulations regulating their operation. We do not believe that compliance with such laws and regulations will materially affect our financial position or results of operations.
Patents and Trademarks
We believe that patents and other intellectual property will continue to be of importance in the musculoskeletal industry. Accordingly, we continue to protect technology developed internally and to acquire intellectual property rights associated with technology developed externally. We enforce our intellectual property rights consistent with our strategic business objectives. We do not believe that we have any single patent or license (or series of patents or licenses) that is material to our operations. We are not aware of any single patent that, if lost or invalidated, would be material to our consolidated revenues or earnings. We currently have more than 1,300 patents and in excess of 700 pending patent applications.
BIOMET is our principal registered trademark throughout the world, and registrations have been obtained or are in process with respect to various other trademarks associated with our products. Unless otherwise noted in this prospectus, all trademarks contained herein are owned by Biomet Manufacturing Corp., or one of its affiliates.
94
MANAGEMENT
Directors and Executive Officers
The following table sets forth the name, age and position of (1) our directors and (2) our executive officers.
| Name | Age | Position | ||
| Jeffrey R. Binder | 50 | President and Chief Executive Officer, Director | ||
| Jonathan J. Coslet | 48 | Director | ||
| Michael Dal Bello | 41 | Director | ||
| Adrian Jones | 48 | Director | ||
| Max C. Lin | 32 | Director | ||
| Chinh E. Chu | 46 | Director | ||
| Michael Michelson | 62 | Director | ||
| Dane A. Miller, Ph.D. | 67 | Director | ||
| Andrew Y. Rhee | 36 | Director | ||
| Jeffrey K. Rhodes | 38 | Director | ||
| Bareld J. Doedens | 54 | Senior Vice President; President of Biomet 3i, LLC | ||
| Robin T. Bamey | 52 | Senior Vice President, World Wide Operations | ||
| Sujata T. Dayal | 50 | Corporate Vice President and Chief Compliance Officer | ||
| Glenn L. Criser | 49 | Senior Vice President, Quality, Regulatory and Clinical Affairs | ||
| Daniel P. Hann | 58 | Senior Vice President, Business Development | ||
| Daniel P. Florin | 49 | Senior Vice President and Chief Financial Officer | ||
| Adam R. Johnson | 36 | Senior Vice President, President of EBI, LLC and Biomet Microfixation, LLC | ||
| Jon C. Serbousek | 52 | Senior Vice President; President of Biomet Biologics, LLC | ||
| Bradley J. Tandy | 54 | Senior Vice President, General Counsel and Secretary | ||
| Margaret M. Taylor | 56 | Senior Vice President—Human Resources | ||
| Renaat Vermeulen | 56 | Senior Vice President; President of Biomet Europe, Middle East and Africa | ||
| J. Pat Richardson | 53 | Vice President—Corporate Controller | ||
Jeffrey R. Binder has been a director and President and Chief Executive Officer since February 2007. Prior to this appointment, Mr. Binder served as Senior Vice President of Diagnostic Operations of Abbott Laboratories from January 2006 to February 2007. Mr. Binder previously served as President of Abbott Spine from June 2003 to January 2006, and as President and Chief Executive Officer of Spinal Concepts from 2000 to June 2003.
Jonathan J. Coslet has been a director since July 2007. Mr. Coslet has been a Partner of TPG since 1993 and is currently a senior partner and member of the firm’s Executive, Management and Investment Committees. Mr. Coslet serves on the board of directors of IASIS Healthcare Corp., The Neiman Marcus Group, Inc., J. Crew Group, Inc., Petco Animal Supplies, Inc. and Quintiles Transnational Corp.
Michael Dal Bello has been a director since July 2007. Mr. Dal Bello is a Managing Director in the Private Equity Group of The Blackstone Group and has been with Blackstone since 2002. Mr. Dal Bello serves on the board of directors of Alliant, Apria Healthcare Group, Catalent Pharma Solutions, Inc., Emdoen, Team Health Finance LLC and Vanguard Health Systems, Inc.
Adrian Jones has been a director since July 2007. Mr. Jones has been a Managing Director of Goldman, Sachs & Co. since 2002 and has worked at Goldman, Sachs & Co. since 1994. Mr. Jones serves on the board of directors of Dollar General Corporation, Education Management Corporation, HealthMarkets, Inc. and Michael Foods, Inc.
95
Max C. Lin has been a director since 2011. Mr. Lin is a Principal in the health care industry team at Kohlberg Kravis Roberts & Co. L.P. (together with its affiliates, “KKR”). Mr. Lin joined KKR in 2005 and has been involved with the firm’s investments in HCA Holdings, Inc. and The Nielsen Company. Prior to working at KKR, he was with Morgan Stanley in its Financial Sponsors Group.
Chinh E. Chu has been a director since April 2013 and previously served as a director from July 2007 to September 2007. Mr. Chu is a senior managing director of The Blackstone Group, which he joined in 1990. Mr. Chu serves on the board of directors of HealthMarkets, Inc., DJO Global, Bank United, Bayview, Alliant, Catalent and Freescale.
Michael Michelson has been a director since July 2007. Mr. Michelson has been a member of KKR Management LLC, the general partner of KKR & Co. L.P. since October 1, 2009. Previously, he was a member of the limited liability company, which served as the general partner of Kohlberg Kravis Roberts & Co. L.P. He has been employed by KKR since 1981. Mr. Michelson serves on the board of directors of HCA Holdings, Inc.
Dane A. Miller, Ph.D. has been a director since July 2007. Dr. Miller is one of our four founders and served as our President, Chief Executive Officer and a director from 1977 until 2006. Dr. Miller serves on the board of directors of ForeTravel, Inc., the Indiana Economic Development Corporation, the University of Chicago Health Systems and the World Craniofacial Foundation.
Andrew Y. Rhee has been a director since 2009. Mr. Rhee is a Vice President in the Merchant Banking Division of Goldman, Sachs & Co., and has been with Goldman since 1998. Mr. Rhee serves on the board of directors of AssuraMed, Inc. and Drayer Physical Therapy Institute, LLC.
Jeffrey K. Rhodes has been a director since November 2012 and has been a Principal of TPG Global, LLC since 2005 and serves on the board of directors of Immucor, Inc., IMS Health, Surgical Care Affiliates and Par Pharmaceutical Companies. Pursuant to the Amended and Restated Limited Liability Company Operating Agreement of Holding, TPG has the right to nominate two directors to the Parent Board and to the board of directors of Biomet.
Robin T. Barney has been Senior Vice President, World Wide Operations since September 2008. Prior to joining Biomet in 2007, Ms. Barney served as Vice President, Worldwide Operations of DePuy, a Johnson & Johnson company. Ms. Barney joined Johnson & Johnson in 1992 and held various leadership roles within Operations for their Codman & Shurtleff, DePuy Orthopeadics and DePuy Spine units.
Sujata T. Dayal has been Corporate Vice President and Chief Compliance Officer since February 2009. Prior thereto, Ms. Dayal was a Partner at Karmact, LLC, a regulatory and compliance consulting firm from July 2008 to February 2009. Prior thereto, she was an Ethics and Compliance Officer—Pharmaceutical Products, Abbot Laboratories from September 2003 to May 2008.
Glenn L. Criser has been Senior Vice President Global Regulatory, Clinical and Quality of Biomet since September, 2012. Prior thereto, he was Senior Vice President of Quality, Regulatory and Division Counsel of Biomet 3i, LLC from May, 2009 to September, 2012. Prior thereto, Mr. Criser served as Vice President and Division Counsel of Biomet 3i, Inc.. from January, 2000 to May, 2009. Prior thereto, he was the General Counsel of Implant Innovations, Inc. from February, 1997 to January, 2000. Prior to working for Biomet and Implant Innovations, Inc., Mr. Criser was a partner with the law firm of Steel, Hector & Davis.
Bareld J. Doedens has been Senior Vice President; President of Biomet 3i, LLC since February 2013. Prior to that he was Vice President Global CAD/CAM for Sirona Dental Systems from October 2008 to January 2013 and Vice President – Business Development for Sirona Dental Systems from April 2007 through October 2008. Mr. Doedens was the President of EBI, L.P. from 2006 through 2007. He was President of Biomet 3i, Inc. from 1999 through 2005.
Daniel P. Florin has been Senior Vice President and Chief Financial Officer since June 2007. Prior thereto, Mr. Florin served as Vice President and Corporate Controller for Boston Scientific Corporation since 2001. Prior to being appointed as Corporate Controller in 2001, Mr. Florin served in financial leadership positions within Boston Scientific Corporation and its various business units since July 1995.
Daniel P. Hann has been Senior Vice President, Business Development since January 2011. Prior thereto, Mr. Hann served as an independent consultant to Biomet from March 2007 to January 2011. Mr. Hann previously
96
served as Executive Vice President of Administration of Biomet from February 2007 to March 2007, Interim President and Chief Executive Officer of Biomet from March 2006 to February 2007 and as Senior Vice President, General Counsel and Secretary of Biomet from 1989 to March 2006.
Adam R. Johnson has been Senior Vice President; President of EBI, LLC since June 2012 and is currently serving as the President of Biomet Microfixation and has been in that role since August 2007. Mr. Johnson served as the Vice President of Global Marketing for Biomet Microfixation from 2006 until his promotion in August 2007. Prior to that Mr. Johnson was the Director of Global Marketing for RTI Biologics.
Jon C. Serbousek has been Senior Vice President; President of Biomet Biologics since March 2013; and prior thereto served as Group President of Biomet Orthopedics from May 2011 to March 2013 and as Senior Vice President; President of Biomet Orthopedics, LLC from March 2008 to May 2011. For the previous eight years, Mr. Serbousek held diverse general management roles with Medtronic in the areas of Spinal Reconstruction, International, New Technology Development and most recently, worldwide Vice-President and General Manager, Biologics.
Bradley J. Tandy has been Senior Vice President, General Counsel and Secretary since April 2007. Prior thereto, Mr. Tandy served as Senior Vice President, Acting General Counsel and Secretary from January 2007 to April 2007, and Senior Vice President, Acting General Counsel, Secretary and Corporate Compliance Officer from March 2006 to January 2007. Mr. Tandy previously served as Vice President, Assistant General Counsel and Corporate Compliance Officer at Biomet from January 1999 to March 2006.
Margaret M. Taylor has been Senior Vice President, Human Resources since August 2007. Prior thereto, Ms. Taylor served as Vice President of Human Resources for the Diagnostics Division of Abbott Laboratories from April 2000 to August 2007.
Renaat Vermeulen has been Senior Vice President; President of Biomet EMEA since July 2010. Since his arrival at the Company in 1994, Mr. Vermeulen has held many positions of increasing responsibility until his most recent position of Vice President—Sales, Marketing and R&D, Biomet Europe.
J. Pat Richardson has been Vice President and Corporate Controller since October 2012 and has previously held the positions of Vice President – Finance, World Wide Orthopedics Group from July 2011 to October 2012, Vice President – Finance, Financial Planning & Analysis from June 2007 to July 2011 and Vice President – Interim Chief Financial Officer and Treasurer from March 2007 to June 2007. Mr. Richardson has 18 years of financial officer/controller experience and seven years of public accounting and auditing experience. Prior to joining Biomet in March 2007, Mr. Richardson served in financial leadership positions within various Johnson & Johnson business units (Cordis: Vice President, Finance – Cardiology from August 2006 to March 2007 and Group Controller – Cardiology from April 2004 to August 2006; DePuy Orthopaedics: Vice President, Finance – Orthopaedics from June 1997 to April 2004) and held various positions at Ball-Foster Glass Container Co. and was an audit manager at Price Waterhouse.
Board Composition
Our Board of Directors consists of ten directors. Each of our Sponsors has the right to nominate, and have nominated, two directors to serve on our Board of Directors. Following Purchaser’s purchase of the shares tendered in the Offer, the Sponsors jointly appointed Dr. Miller and Jeffrey R. Binder to the Board of Directors in addition to the two directors appointed by each of the Sponsors. Because of their affiliations with the Sponsors and us, none of our directors are independent. For more information regarding the rights of the Sponsors to nominate directors and other related arrangements, see “Certain Relationships and Related Party Transactions—Amended and Restated Limited Liability Company Operating Agreement of Holding.”
Audit Committee
Our Audit Committee is composed of Max C. Lin, Michael Dal Bello, Dane A. Miller, Ph.D., Andrew Rhee and Jeffrey K. Rhodes. In light of our status as a privately held company and the absence of a public listing or trading market for our common stock, our Board has not designated any member of the Audit Committee as an “audit committee financial expert.” Though not formally considered by our Board given that our securities are not traded on any national securities exchange, based upon the listing standards of the NASDAQ National Market, the national securities exchange upon which our common stock was listed prior to the Merger, we do not believe that any of Messrs. Lin, Dal Bello, Rhee or Rhodes would be considered independent because of their relationships with
97
certain affiliates of the Sponsors which hold significant interests in Holding, which owns 97% of our outstanding common stock, and, in the case of Dr. Miller, other relationships with us. See “Certain Relationships and Related Transactions.”
Compensation Committee
Our compensation committee currently consists of Messrs. Coslet, Jones, Michelson and Dal Bello. None of the directors serving on the compensation committee is independent. The compensation committee is responsible for reviewing and approving goals and objectives related to the chief executive officer’s compensation, evaluating the chief executive officer’s performance against these goals and objectives and approving his compensation, approving total compensation for the other senior executive officers, establishing total compensation for the directors and overseeing our general cash-based and equity-based incentive plans.
Compensation Committee Interlocks and Insider Participation
None of our executive officers serve, or in the past fiscal year have served, as a member of the board of directors or compensation committee of any other entity that has executive officers who have served on our board of directors or compensation committee.
Code of Ethics
We have a Code of Business Conduct and Ethics which applies to all employees of Biomet and its subsidiaries and is applicable to all of our directors, officers and team members (the “Code of Conduct”). The Code of Conduct is available on the Corporate Compliance pages of our website at www.biomet.com. To the extent required pursuant to applicable SEC regulations, we intend to post amendments to or waivers of our Code of Conduct (to the extent applicable to our chief executive officer, principal financial officer or principal accounting officer) at this location on our website or report the same on a Current Report on Form 8-K. Our Code of Conduct is available free of charge upon request to our Investor Relations Department at 56 East Bell Drive, Warsaw, IN 46582.
98
EXECUTIVE COMPENSATION
Introduction
Compensation and related matters during the 2012 fiscal year were reviewed and approved by the Compensation Committees of Parent and Biomet which we refer to, collectively or individually as the context requires, as the Compensation Committee.
Compensation Discussion and Analysis
This section includes information regarding, among other things, the overall objectives of our compensation programs and each element of compensation that we provided, in each case with respect to the 2012 fiscal year. The goal of this section is to provide a summary of our executive compensation practices and the decisions that we made during this period concerning the compensation package payable to our executive officers, including the five executives in the Summary Compensation Table. Each of the five executives listed in the Summary Compensation Table is referred to herein as a “named executive officer.” This “Compensation Discussion and Analysis” should be read in conjunction with the detailed tables and narrative descriptions under “Executive Compensation Tables” below.
Compensation Methodology
During the 2012 fiscal year, the Compensation Committee was responsible for administering the compensation and benefit programs for our team members, including our named executive officers. The Compensation Committee annually reviews and evaluates cash compensation and equity award recommendations for our executive officers along with the rationale for such recommendations, as well as summary information regarding the aggregate compensation provided to our executive officers. The Compensation Committee examines these recommendations in relation to our overall objectives and risk profile. Our President and Chief Executive Officer was not a member of the Compensation Committee during the 2012 fiscal year and did not participate in the decisions as to his compensation package.
The most significant development in our executive compensation philosophy following the consummation of the Transactions, including during the 2012 fiscal year, has been a greater emphasis on correlating compensation to long-term equity growth. The Compensation Committee has provided significant equity investment opportunities in Parent tied to financial objectives through (1) offering certain of our employees one-time opportunities to purchase shares of Parent at a purchase price equal to the higher of fair market value and $10.00 per share (subject to the employee’s execution of a Management Stockholders’ Agreement, as described below under “The Elements of Biomet’s Compensation Program—Stock Options and Restricted Stock Units”), (2) granting of options to purchase shares of Parent, and modifying the structure of non-equity awards to provide greater incentives for management performance and (3) granting of restricted stock units of Parent. The philosophy and target levels of each of the other compensation elements, including base salary, perquisites, health and welfare and retirement benefits during the 2012 fiscal year have largely continued to correspond to the levels of such awards, for periods prior to the Transactions. The Compensation Committee’s decisions for the 2012 fiscal year, specifically with respect to merit increases for base salary amounts for the Chief Executive Officer and his reports, including the other named executive officers, were made after considering input from the Sponsors on their general experience of current compensation practices with their respective portfolio companies of similar size and other companies in the orthopedics industry, including Zimmer Holdings, Inc., Stryker Corp. and Medtronic, Inc. This consideration was not made in the context of any benchmarking process. We refer to this group of companies throughout this prospectus as our “informal peer group”, which we use as an anecdotal tool and not for purposes of quantitative benchmarking.
Executive Compensation Philosophy and Objectives
Our executive compensation practices are affected by the highly competitive nature of the orthopedics industry and the location of our executive offices in Warsaw, Indiana. The fact that a number of the leading orthopedic manufacturers in the world have significant operations in and around Warsaw, Indiana means that there are continuing opportunities for experienced orthopedic executives who reside in this area. On the other hand, the fact that Warsaw, Indiana, is a small town in a predominantly rural area can present challenges to attracting executive talent from other industries and parts of the country.
99
Our executive compensation policies and practices during the 2012 fiscal year reflected the compensation philosophies of our founders and were designed to help achieve the superior performance of our executive officers and management team by accomplishing the following goals:
| • | attracting, retaining and rewarding highly qualified and productive persons; |
| • | relating compensation to company, business unit and individual performance; |
| • | encouraging strong performance without incentivizing inappropriate or excessive risk-taking; |
| • | establishing compensation levels that are internally equitable and externally competitive; and |
| • | encouraging an ownership interest and instilling a sense of pride in Biomet. |
This compensation methodology was based upon one of our founding philosophies: equity incentives in the form of stock options and RSUs are an excellent motivation for all team members, including executive officers, and serve to align the interests of team members, management and our equity investors.
Based on these objectives, the compensation package of our executive officers during the 2012 fiscal year was intended to meet each of the following three criteria: (1) market levels competitive with companies of similar size and performance to us; (2) performance based, “at risk” pay that is based on both short and long-term goals; and (3) incentives that are structured to create alignment between our equity investors and executives.
The Elements of Biomet’s Compensation Program
As a result of our compensation philosophies and objectives, the compensation package of our executive officers during the 2012 fiscal year consisted of five primary elements: (1) base salary, (2) non-equity incentive plan awards, (3) stock options and restricted stock units, (4) participation in employee benefit plans, and (5) deferred compensation elections. Consistent with prior fiscal years, our practice during the 2012 fiscal year was to provide total cash compensation (consisting of base salary plus annual cash incentive awards) at amounts we believed to be generally comparable with, or average to, the amounts paid to executives with companies of similar size and performance to us, in each case with responsibilities similar to the responsibilities of our executives.
Base Salary. The Compensation Committee reviewed our performance, the executive officers’ performance, our future objectives and challenges and the current competitive environment and set the base salary for each executive officer at the beginning of the fiscal year. Mr. Binder’s base salary for fiscal year 2012 remained constant by his election. The Chief Executive Officer was given relatively broad latitude by the Compensation Committee to adjust the merit increase percentage upward or downward for his direct reports on the basis of Mr. Binder’s assessment of job performance for the preceding fiscal year. All named executive officer merit increases were deferred in fiscal year 2012. One named executive officer received a base pay adjustment to maintain market competitiveness as identified by quantitative analysis of market data for orthopedic medical device (SIRS®) and global top executive markets (Towers Watson).
Non-equity Incentive Plan. Annual cash incentive awards to our named executive officers for the 2012 fiscal year were paid under the terms of a non-equity incentive plan approved by our Compensation Committee following consummation of the Transactions. The principal objective sought to be achieved by our non-equity incentive plan is to align awards with predetermined objectives and thereby improve performance in specific areas. Payments under the plan are calculated based upon a target percentage of the executive’s base salary determined by position at the Company. Potential payments under the non-equity incentive plan for the 2012 fiscal year could have ranged from 0% to 180% of each named executive officer’s base salary based on corporate, business unit and individual performance with Mr. Binder’s target bonus set at 100% of base salary and the target bonus of each of the other named executive officers set at a range of 60% to 80% of base salary.
For fiscal year 2012, the Compensation Committee chose corporate and business unit incentive metrics that it considered important valuation metrics that would effectively measure our performance. Corporate and business unit criteria for the 2012 fiscal year consisted of (i) adjusted EBITDA, (ii) net sales, (iii) adjusted operating free cash flow as a percentage of net sales (“FCF/Net Sales %”), (iv) value creation, (v) service level and (vi) health hazard evaluation (“HHE”)/field actions targets. For these purposes, adjusted EBITDA is defined as net income/loss before interest expense,
100
income tax, depreciation and amortization, and adjusted for certain expenses as defined by our bank agreement, such as restructuring charges, non-cash impairment charges, integration and facilities opening costs or other business optimization expenses, new systems design and implementation costs, certain start-up costs and costs related to consolidation of facilities, certain non-cash charges, advisory fees paid to the private equity owners, certain severance charges, purchase accounting costs, stock-based compensation and payments, payments to distributors that are not in the ordinary course of business, litigation costs and settlements and other related charges. All adjustments are reviewed and approved by the Compensation Committee. See table below for additional definitions.
The Compensation Committee also established the weighting for each financial metric and approved a grid for each metric to determine the percentage of the target bonus that would be paid in respect of such metric (“percentage payout”) based upon the percentage of target performance actually achieved. Target performance goals for each financial metric were generally established consistent with the Company’s operating plan for the fiscal year 2012.
The following table details the percentage payouts by bonus metric:
Bonus Pay out Percentages (1) | |||
| (percentage of business plan target) | 0% | 200%(2) | |
| Jeffrey R. Binder | |||
| Daniel P. Florin | |||
| Bradley J. Tandy | |||
| Company Adjusted EBITDA | below 95% | 107.5% or greater | |
| Company Sales | below 95% | 107.5% or greater | |
| Company FCF/Company Sales | below 95% | 107.5% or greater | |
| Jon C. Serbousek | |||
| Company Adjusted EBITDA | below 95% | 107.5% or greater | |
| Orthopedics Adjusted EBITDA | below 95% | 107.5% or greater | |
| Orthopedics Sales | below 95% | 107.5% or greater | |
| Global Orthopedics FCF/Global Orthopedic Sales | below 95% | 107.5% or greater | |
| Robin T. Barney | |||
| Company Adjusted EBITDA | below 95% | 107.5% or greater | |
| Company Sales | below 95% | 107.5% or greater | |
| Value Creation | below 95% | 115.0% or greater | |
| Service Level | below 97% | 102.0% or greater | |
| Field Actions | less than a 17% reduction | 66% or greater reduction(3) | |
| Free Cash Flow | below 90% | 120.0% or greater | |
___________________________________________________
| (1) | The payments are calculated based on straight line interpolation from (a) 0%, for performance below the threshold set forth in the 0% bonus payout percentage column above, to 100%, for achievement of 100% of the applicable performance metric, and (b) 100% to 200%, for performance at or above the threshold set forth in the 200% bonus payout percentage column above. |
| (2) | The maximum payout for the service level metric and field actions metric is 120%. |
| (3) | The field actions metric is based on decreasing the level of field actions and as such it is presented differently in the table. |
The Compensation Committee also set quantitative and qualitative individual goals for Mr. Binder for fiscal year 2012, the achievement of which would equal 20% of Mr. Binder’s target bonus. Similarly, Mr. Binder established twenty (20) quarterly, or five goals per fiscal quarter, for each of the other executive officers, including the other named executive officers. The achievement of each such goal at target would equal 1% (or less) of such executive’s officer’s target bonus, or 20% (or less) in the aggregate. The individual performance of Mr. Binder was determined by the Compensation Committee after considering his leadership ability and contributions to the business during the 2012 fiscal year, including by reference to such individual goals. With respect to named executive officers other than the Chief Executive Officer, the Compensation Committee reviewed and approved the Chief Executive Officer’s assessment of their individual performance in determining an individual named executive officer’s performance for fiscal 2012. The Compensation Committee does not consider any one individual goal as material to the determination of any named executive officer’s annual cash award.
101
The Compensation Committee established different weightings for corporate, business unit and individual performance for each named executive officer in recognition of his or her role in driving the Company’s overall performance. The Compensation Committee also retained the authority to reduce or award an additional bonus amount at its discretion (a “leadership/discretionary” award). The Company awarded additional bonus amounts to both Mr. Florin and Mr. Tandy to increase their bonus payouts to 100% of their targets, which amounted to $11,995 and $7,265, respectively. The Company reduced a portion of Mr. Serbosek’s bonus to decrease his bonus payout to 100% of his target, which amounted to $18,381.
The following chart shows the financial metrics and their weighting, targets, actual performance against the targets and resulting payout percentage for each of the Company and business unit performance goals discussed above:
| (in millions, except percentages) | Target Performance(1) | Actual Performance(1) | Financial Metrics Payout | |||||||
| Jeffrey R. Binder | ||||||||||
| Daniel P. Florin | ||||||||||
| Bradley J. Tandy | ||||||||||
| Company Adjusted EBITDA (40%) | $ | 1,028.4 | $ 1,023.6(2) | 38.13 | % | |||||
| Company Sales (25%) | $ | 2,802.2 | $ | 2,785.5 | 23.51 | % | ||||
Company FCF(3)/Company Sales (15%) | 27.0 | % | 27.1 | % | 15.81 | % | ||||
| Total (taking into account weighting) | 77.45 | % | ||||||||
| Jon C. Serbousek | ||||||||||
| Company Adjusted EBITDA (10%) | $ | 1,028.4 | $ 1,023.6(2) | 9.53 | % | |||||
| Global Orthopedics Adjusted EBITDA (30%) | $ | 907.5 | $ | 921.1 | 35.96 | % | ||||
| Global Orthopedics Sales (25%) | $ | 2,049.5 | $ | 2,072.2 | 27.77 | % | ||||
Global Orthopedics FCF(4)/Global Orthopedic Sales (15%) | 36.2 | % | 35.9 | % | 13.54 | % | ||||
| Total (taking into account weighting) | 86.80 | % | ||||||||
| Robin T. Barney | ||||||||||
| Company Adjusted EBITDA (15%) | $ | 1,028.4 | $ 1,023.6(2) | 14.30 | % | |||||
| Company Sales (15%) | $ | 2,802.2 | $ | 2,785.5 | 14.11 | % | ||||
Value Creation(5) (10%) | $ | 134.6 | $ | 154.2 | 19.70 | |||||
Service Level(6) (5%) | $ | 93.6 | $ | 94.6 | 6.00 | % | ||||
Field Actions(7) (5%) | 19-38 | 35 | 5.00 | % | ||||||
Free Cash Flow(8) (15%) | $ | 177.9 | $ | 146.3 | 26.91 | % | ||||
| Total (taking into account weighting) | 86.02 | % | ||||||||
___________________________________________________
| (1) | All dollar targets and actual performance at budget foreign exchange rates except actual Company adjusted EBITDA. |
| (2) | Includes a reduction of $7.5 million due to foreign currency exchange benefits. |
| (3) | Free Cash Flow represents adjusted EBITDA at actual foreign currency rates less capital expenditures at actual foreign currency rates plus or minus the change in working capital less special charges both at actual foreign currency rates. |
| (4) | Free Cash Flow represents adjusted EBITDA at budget foreign currency rates less capital expenditures at budget foreign currency rates plus or minus the change in working capital at budget foreign currency rates. |
| (5) | Value creation is defined as manufacturing cost savings generated through strategic sourcing, cost reductions and plant optimization initiatives. |
| (6) | Service level is defined as the timely order fulfillment ensuring that inventory that is needed is not on back order. |
| (7) | Health hazard evaluations are product evaluation that identifies a potential manufacturing related issue requiring further investigation to determine whether action is necessary. The evaluations may result in no action or a variety of actions such as advisory notices, field corrections or recalls. Field actions do not necessarily reflect a determination that the issue poses a risk to health as field actions are taken for a variety of reasons. |
| (8) | Free Cash Flow represents capital expenditures at budget foreign currency rates plus or minus the change in inventory at budget foreign currency rates. |
102
The following chart shows the weighting assigned to the various corporate, business unit and individual performance goals discussed above as percentage of base salary for each named executive officer:
| Jeffrey R. Binder | Daniel P. Florin | Jon C. Serbousek | Bradley J. Tandy | Robin T. Barney | ||||||||||||||||
| Goals | Target | Max | Target | Max | Target | Max | Target | Max | Target | Max | ||||||||||
| Company Financials | 80 | % | 160 | % | 64 | % | 128 | % | 16 | % | 29 | % | 48 | % | 96 | % | 24 | % | 43 | % |
| Business Unit Financials | — | — | — | — | 48 | % | 86 | % | — | — | 40 | % | 72 | % | ||||||
| Individual Performance Objectives | 20 | % | 20 | % | 16 | % | 16 | % | 16 | % | 29 | % | 12 | % | 12 | % | 16 | % | 29 | % |
| TOTAL | 100 | % | 180 | % | 80 | % | 144 | % | 80 | % | 144 | % | 60 | % | 108 | % | 80 | % | 144 | % |
| Leadership /Discretionary | +/-10% | +/-10% | +/-10% | +/-10% | +/-10% | |||||||||||||||
The chart below includes information about the named executive officers’ 2012 fiscal year non-equity incentive plan target and maximum award opportunities and actual payouts including as a percentage of base salary.
| Non-Equity Incentive Plan Target | Non-Equity Incentive Plan Maximum | Non-Equity Incentive Plan Payout (Paid in July 2012) | |||||||||||||
| % of Base Salary | Amounts ($) | % of Base Salary | Amount ($) | % of Base Salary | Amount ($) | ||||||||||
| Jeffrey R. Binder | 100 | % | $ | 717,036 | 180 | % | $ | 1,290,665 | 96 | % | $ | 687,982 | |||
| Daniel P. Florin | 80 | % | 337,694 | 144 | % | 607,850 | 80 | % | 337,695 | ||||||
| Jon C. Serbousek | 80 | % | 331,065 | 144 | % | 595,917 | 80 | % | 331,065 | ||||||
| Bradley J. Tandy | 60 | % | 238,040 | 108 | % | 428,472 | 60 | % | 238,040 | ||||||
| Robin T. Barney | 80 | % | 252,346 | 144 | % | 454,224 | 85 | % | 267,933 | ||||||
The Compensation Committee and management believe that the metrics for the non-equity incentive plan align well with our objective of relating compensation to company, business unit and individual performance.
Stock Options and Restricted Stock Units. In 2007, the Board of Directors of Parent adopted the LVB Acquisition, Inc. 2007 Management Equity Incentive Plan (the “2007 LVB Plan”), which provides for the grant of non-qualified stock options to purchase shares of common stock of Parent (the “LVB Options”) to our and our affiliates’ key employees, directors, service providers and consultants. Prior to the exchange offer relating to employee options described below, 50% of the LVB Options granted to employees vested based on continued employment, 25% vested based on continued employment and had an exercise price that increased by 10% per annum, and 25% vested based on the achievement of annual adjusted EBITDA-performance criteria established by the Compensation Committee. Following the exchange offer, generally 75% of the LVB Options granted to employees vest based on continued employment and 25% vest based on the achievement of annual adjusted EBITDA-performance criteria established by the Compensation Committee. We have also granted LVB Options to certain of our distributors, which are eligible to vest based on the achievement of specified sales targets.
In May 2009, the Board of Directors of Parent authorized an exchange offer relating to employee options outstanding at May 6, 2009 (including the options held by our named executive officers). Outstanding distributor options were not included in the exchange offer. The exchange offer provided the holders of such options with the opportunity to surrender the options for cancellation in exchange for replacement options, the terms of which were (1) different from the surrendered options with respect to the performance based and accreting exercise price options, and (2) the same as the surrendered options with respect to the time based options. The terms of the performance based and accreting exercise price options were modified in the replacement options as follows:
| • | New Performance Vesting Options (which replaced the surrendered performance based options)—Beginning in fiscal 2010, the remaining unvested options vest ratably over four to six years (depending on the date of grant) instead of the three to five years remaining under the terms of the then outstanding performance based options. The remaining options continue to vest contingent upon the Company achieving certain reduced adjusted EBITDA targets in each of those years (new options granted subsequent to, and not in connection with, the exchange program vest ratably over five years following the grant date contingent upon the Company achieving certain adjusted EBITDA targets with respect to each such year). |
103
| • | New Extended Time Vesting Options (which replaced the surrendered accreting exercise price options)—These options are similar to the then outstanding time based options. The exercise price reverts to $10.00 per share (i.e., the original grant date exercise price before it began accreting) and no longer increases by 10% on an annual basis. The remaining unvested options vest ratably over four to six years (depending on the date of grant) instead of the three to five years remaining under the terms of the then outstanding accreting exercise price options. |
The goal of the exchange offer was to provide employees who elected to participate with new options, the terms of which preserved the original incentive effect of our option program in light of current market-wide economic conditions. Although the Board of Directors of Parent authorized the option exchange program in May 2009, we did not conduct the exchange offer until our 2010 fiscal year. Therefore, the exchange offer is reflected in the 2010 fiscal year compensation tables below and the financial information contained in this prospectus. All of our employees elected to participate in the exchange offer.
Upon termination of a participant’s employment, the 2007 LVB Plan provides that any unvested portion of a participant’s LVB Award will be forfeited, and that the vested portion of his or her LVB Award will expire on the earliest of (1) the date the participant’s employment is terminated for cause, (2) 30 days following the date the participant resigns without good reason, (3) 90 days after the date the participant’s employment is terminated either by us for any reason other than cause, death or disability, or by the participant with good reason, (4) one year after the date the participant’s employment is terminated by reason of death or disability or (5) the tenth anniversary of the grant date of the LVB Award. In no event will any option remain outstanding after the tenth anniversary of the original grant date of such option.
Prior to receiving shares of Parent’s common stock, participants must execute a Management Stockholders’ Agreement, which provides that the shares are subject to certain transfer restrictions, put and call rights, and tag-along and drag-along rights (and, with respect to certain senior members of management, limited registration and preemptive rights).
The Compensation Committee is responsible for administering the 2007 LVB Plan and authorizing the grant of LVB Awards pursuant thereto, and may amend the 2007 LVB Plan (and any LVB Awards) at any time. LVB Awards may not be granted under the 2007 LVB Plan on or after November 16, 2017. When the 2007 LVB Plan became effective, there were 37,520,000 shares of Parent common stock reserved for issuance in connection with LVB Awards to be granted thereunder. Effective December 31, 2010, the 2007 LVB Plan was amended to increase the authorized share pool by 1,000,000 shares. As of May 31, 2012, there were 2,916,750 shares available for issuance under the 2007 LVB Plan.
We do not have a regular program of annual equity grants. The Compensation Committee makes awards to team members in its discretion as it deems necessary or appropriate. While the Company has historically granted stock options as its equity incentives, the Board of Directors and stockholders of Parent adopted and approved a Restricted Stock Unit Plan effective December 31, 2010, for executives and other key team members. In consultation with management, the Compensation Committee determined that such a plan would provide a valuable retention tool in the context of challenging market conditions and the resulting decrease in value of previously granted stock options, while at the same time continuing to align the interests of management and stockholders. In deciding to expand its equity incentives to include restricted stock units (“RSUs”), the Compensation Committee also noted the market trend toward RSUs in light of its need to continue to attract and retain talented people from competitors. The maximum number of shares of common stock, par value $0.01 per share, that may be issued under this plan is 4,000,000, subject to adjustment as described in the plan. Under the terms of the plan, the Compensation Committee may grant participants RSUs, each of which represents the right to receive one share of common stock, subject to certain vesting restrictions and risk of forfeiture. The restricted stock units vest under certain time-vesting and liquidity event conditions. RSUs representing substantially all of the shares available under the plan were granted to recipients in a one-time retention award to participants on February 10, 2011, and as of May 31, 2011, there were 335,000 restricted stock units remaining available for issuance.
The number of RSUs granted to the Chief Executive Officer was determined by the Compensation Committee, which based its determination on the size of the available pool of RSUs and the retention benefit of the award amount. With respect to the other named executive officers and other recipients, the Compensation Committee delegated to the Chief Executive Officer broad latitude to determine the number of RSUs to be granted to such individuals, subject to the final review and approval by the Compensation Committee. The Chief Executive Officer, in consultation with the Senior Vice President—Human Resources, made his determination of the number of RSUs granted to the other named executive officers based on the size of the available pool of RSUs and several subjective factors, including level of responsibility, job performance, importance to the future success of the Company and retention risk.
104
On July 2, 2012, Parent launched a tender offer to eligible employees to exchange all of the stock options and restricted stock units held by such employees for new stock options and restricted stock units. Following the expiration of the tender offer on July 30, 2012, Parent accepted for exchange eligible options to purchase an aggregate of 29,532,500 shares of common stock of Parent and eligible restricted stock units underlying an aggregate of 3,665,000 shares of common stock of Parent. In accordance with the terms and conditions of the tender offer, on July 31, 2012, Parent granted 29,532,500 new options and 10,795,000 new restricted stock units in exchange for the cancellation of such tendered options and restricted stock units.
The new plan offered a one-for-one exchange on the existing options. The new RSUs for the named executive officers is included in the table below:
| New RSU Plan | |||||
| Original RSUs | Time | Performance | |||
| Jeffrey R. Binder | 850,000 | 1,880,000 | 920,000 | ||
| Daniel P. Florin | 175,000 | 380,000 | 185,000 | ||
| Jon C. Serbousek | 175,000 | 240,000 | 120,000 | ||
| Bradley J. Tandy | 110,000 | 230,000 | 110,000 | ||
| Robin T. Barney | 140,000 | 260,000 | 130,000 | ||
The objective of the tender offer was to provide employees who elected to participate with new options and new restricted stock units, the terms of which preserve the original incentive effect of our equity incentive programs in light of market and industry-wide economic conditions. The terms of the new stock options differed in respect to the tendered options principally with respect to:
| • | Exercise Price—The exercise price for the new stock options was lowered to the current fair value of $7.88 per share. |
| • | Vesting Periods—All prior options that were vested as of the completion date of the tender offer remain vested. All time-vesting options which were unvested as of the completion date of the tender offer will continue to vest on the same schedule on which they were originally granted. All unvested replacement extended time vesting options and modified performance options will vest on a schedule which is generally two years longer than the original vesting schedule, but in no case will the vesting schedule be extended past 2017. |
| • | Performance Vesting Threshold—The new modified performance options will vest over the new vesting period if, as of the end of the Company’s most recent fiscal year ending on or prior to such vesting date, Biomet has achieved the EBITDA target for such fiscal year determined by the Compensation Committee of the Board of Directors of the Company on or before the ninetieth (90th) day of such fiscal year and consistent with the Company’s business plan. |
The terms of the new restricted stock units are different from the tendered restricted stock units with respect to the vesting schedule, performance conditions and settlement. The new restricted stock units will be granted subject to either a time-based vesting or a performance-based vesting requirement. Unlike the exchanged restricted stock units, the new restricted stock units will not vest in full on May 31, 2016 regardless of satisfaction of the vesting conditions. In addition, following the termination of employment with the Company, new restricted stock units, whether vested or unvested, will be forfeited if such employee provides services to any of our competitors. In addition, participants holding new restricted stock units will also receive new awards called management dividend awards representing the right to receive a cash payment. Management dividend awards vest on a one-to-one basis with each new time-based restricted stock unit. Vested management dividend awards will be paid by cash distributions promptly following each anniversary of the grant date until the earlier of an initial public offering of the Company or the fifth anniversary of the grant date, subject to withholding taxes. Upon termination of employment for any reason, management dividend awards will be forfeited. The new restricted stock units will be granted under Parent’s 2012 Restricted Stock Unit Plan, which was adopted by Parent on July 31, 2012. The maximum number of shares of common stock, par value $0.01 per share, that may be issued under the 2012 Restricted Stock Unit Plan is 14,000,000, subject to adjustment as described in the Plan.
105
We paid a special bonus amount during the fourth quarter of fiscal year 2012 to our employees who were allocated restricted stock units under Parent’s 2012 Restricted Stock Unit Plan in recognition of the delayed rollout of the Plan.
On March 27, 2013, the Compensation Committee of LVB adopted and approved an amendment to the LVB Acquisition, Inc. 2012 Restricted Stock Unit Plan (the “Amendment”). The Amendment permits certain participants in the LVB Acquisition, Inc. 2012 Restricted Stock Unit Plan to be eligible to elect to receive a cash award with respect to certain of their vested time-based restricted stock units subject to certain conditions, including the satisfaction of certain Company performance thresholds with respect to adjusted EBITDA and unlevered free cash flow. For the initial election period beginning on the second business day following the filing of the Company's Annual Report on Form 10-K for the fiscal year ending May 31, 2013 and subsequent annual election periods occurring thereafter, eligible participants will be able to elect to receive a cash award with respect to up to an aggregate of 40% and 35%, respectively, of their vested time-based restricted stock units subject to the satisfaction of the applicable EBITDA and unlevered free cash flow, determined as follows:
| Performance Threshold: Target EBITDA | ||||
| Percent Achievement of Target EBITDA | <97.5% | 97.5% | 100% | 102.5%+ |
| EBITDA Eligible Percentage (Fiscal Year 2013) | —% | 22.5% | 26.25% | 30% |
| EBITDA Eligible Percentage (Fiscal Years Following Fiscal Year 2013) | —% | 18.75% | 22.5% | 26.25% |
Note: Results between 97.5% - 100% and 100% - 102.5% will be calculated on the basis of straight-line interpolation.
| Performance Threshold: Target Unlevered Free Cash Flow | ||||
| Percent Achievement of Target Unlevered Free Cash Flow | <90.0% | 90% | 100% | 110%+ |
| uFCF Eligible Percentage (Fiscal Year 2013) | —% | 7.5% | 8.75% | 10% |
| uFCF Eligible Percentage (Fiscal Years Following Fiscal Year 2013) | —% | 6.25% | 7.5% | 8.75% |
Note: Results between 90% - 100% and 100% - 110% will be calculated on the basis of straight-line interpolation.
To the extent the Company performance conditions have been satisfied for the applicable fiscal year, eligible participants will be entitled to elect to receive a cash award based on the fair market value of the Parent's common stock on the first day of the applicable election period, payable in three installments over a two-year period, with respect to their vested time-based restricted stock units and such vested time-based restricted stock units will be forfeited upon such election. Payment of the cash award is subject to the participants' continued employment through the payment date (other than with respect to a termination by the Company without cause).
Retirement Plans. During the 2012 fiscal year our executive officers in the U.S. were eligible to participate in our 401(k) plan (the “401(k) Plan”). Each year we, in our sole discretion, may match 100% of each team member’s contributions, up to a maximum amount equal to 6% of the team member’s annual cash compensation. All contributions to the 401(k) Plan are allocated to accounts maintained on behalf of each participating team member and, to the extent vested, are available for distribution to the team member or beneficiary upon retirement, death, disability or termination of service.
During the 2012 fiscal year our European executive officers in certain countries were eligible to participate in a defined contribution plan. Each year we contribute a percentage of employees’ pensionable salaries based on their age at January 1st.
We do not sponsor or maintain any pension plans applicable to our named executive officers.
Deferred Compensation. We maintain the Biomet Deferred Compensation Plan (the “Deferred Compensation Plan”), a non-qualified deferred compensation plan, which is available for our senior management. The Deferred Compensation Plan allows eligible participants to defer pre-tax compensation to reduce current tax liability and assist those team members in their planning for retirement and other long-term savings goals in a tax effective manner. We do not make any contributions to the Deferred Compensation Plan. Under the Deferred Compensation Plan, eligible participants may defer up to 100% of their base salary and annual cash incentive award. Participants receive scheduled distributions from the Deferred Compensation Plan, which are treated as ordinary income subject to federal and state income taxation at the time of distribution. Except in circumstances of hardship, unscheduled withdrawals are not permitted. Amounts contributed to the Deferred Compensation Plan are at the participant’s election and are treated as
106
“deemed investments,” which means that the participants have no ownership interest in the investment alternative selected. The participants’ deferrals and any notional investment gains thereon are reflected on our financial statements and are part of our unsecured general assets. The Deferred Compensation Plan is an unfunded “future promise to pay” by us. Neither Biomet nor the Deferred Compensation Plan record keeper provides any guarantee of investment return. We do not pay above-market interest rates on deferred amounts of compensation. None of our named executive officers participates in the current Deferred Compensation Plan.
Perquisites. We believe that our approach to perquisites has historically been, and continues to be, generally comparable to other companies in our informal peer group discussed above. Our President and Chief Executive Officer and other named executive officers generally have been permitted, when practical and consistent with historical practice, to use company aircraft for business and personal travel for security reasons. On a case by case basis, we have historically reimbursed certain executives for social club dues, offered to provide a travel allowance in connection with Biomet related travel, and offered to provide relocation assistance to certain members of our senior management team who relocate their principal residence at our request. For example, we have historically, at times, provided reimbursement of moving expenses and protection against a loss on the sale of the executive’s home.
Health and Welfare Benefits. Named executive officers have historically received similar benefits to those provided to all other salaried U.S. employees, such as medical, dental, vision, life insurance and disability coverage.
Employment Agreements. We have entered into employment agreements with each of our named executive officers to help ensure the retention of those executives critical to our future success. These agreements contain severance and change in control provisions which provide for potential future compensation depending on the circumstances of their departure from Biomet.
Policy with Respect to Deductibility of Compensation over $1 Million. Section 162(m) of the Code generally limits to $1.0 million the tax deductibility of annual compensation paid by publicly held corporations (as defined in the Code) to certain executives. However, performance based compensation can be excluded from this limit if it meets certain requirements. Prior to the Transactions, Biomet’s Compensation Committee’s policy was historically to consider the impact of Section 162(m) in establishing compensation for our senior executives. However, the committee historically retained the discretion to establish compensation, even if such compensation was not deductible under Section 162(m), if, in the committee’s judgment, such compensation was in our best interest and was reasonably expected to increase shareholder value. Following the Transactions and through the 2012 fiscal year, because we were not a publicly held corporation (as defined in the Code) with publicly held equity, the restrictions of Section 162(m) have not applied to us. During fiscal year 2012, Parent filed a registration statement on Form 10 pursuant to Section 12(g) of the Securities Exchange Act of 1934 because there were more than 500 holders of stock options representing the right to acquire shares of Parent common stock, par value $0.01 per share, as of the end of Parent’s fiscal year ended May 31, 2011, which means that Parent is now a publicly held corporation for purposes of Section 162(m) of the Code. The Compensation Committee will therefore consider the impact of Section 162(m) of the Internal Revenue Code in the design of its compensation strategies going forward. We have determined, however, that we will not necessarily seek to limit executive compensation to amounts deductible under Section 162(m) if we believe such limitation is not in the best interests of our stockholders. While considering the tax implications of its compensation decisions, the Compensation Committee believes its primary focus should be to attract, retain and motivate executives and to align the executives’ interests with those of our stakeholders. Other than with respect to the grandfather period for existing performance based compensation arrangements, until such time as the Compensation Committee or a designated subcommittee is comprised of a majority of outside directors (as defined in the Code), we will not be able to qualify for the exclusions of performance based compensation from the $1 million limit.
Compensation Committee Report
The Compensation Committee has reviewed and discussed the foregoing Compensation Discussion and Analysis with management. Based on such review and discussion, the Compensation Committee recommended to the Board of Directors that the Compensation Discussion and Analysis be included in this prospectus.
Compensation Committee
Jonathan J. Coslet
Adrian Jones
Michael Dal Bello
Michael Michelson
107
Executive Compensation Tables
Summary Compensation Table
The following narrative, tables and footnotes describe the “total compensation” earned during the 2010, 2011 and 2012 fiscal years by our named executive officers. The total compensation presented below does not reflect the actual compensation received by our named executive officers or the target compensation of our named executive officers during the 2010, 2011 and 2012 fiscal years.
The individual components of the total compensation calculation reflected in the Summary Compensation Table with respect to fiscal 2012 are broken out below:
Salary. Base salary earned during the 2012 fiscal year. Refer to “The Elements of Biomet’s Compensation Program—Base Salary” above for further information concerning this element of our compensation program.
Bonus. Each named executive officer earned an annual performance-based cash incentive award as described under “Non-equity Incentive Plan Compensation” below.
Equity-Based Awards. The awards disclosed under the heading “Stock Awards” consist of restricted stock units granted under the Restricted Stock Unit Plan and the awards disclosed under the heading “Option Awards” consist of grants of stock options awarded under the 2007 LVB Plan. For further information about our equity-based award programs, refer to “The Elements of Biomet’s Compensation Program—Stock Options and Restricted Stock Units” above. In addition, details about equity-based awards made during the 2012 fiscal year are included in the Grants of Plan-Based Awards Table below. The dollar amounts for the awards in the Summary Compensation Table below reflect the grant date fair value of award grants made in the fiscal year. The increase in the value of the equity awards in fiscal 2011 is reflective of the fact that grants are determined by number of shares, not dollar amounts and a different valuation for our restricted stock units as compared to our stock options, primarily due to the absence of an exercise price for our restricted stock units. A description of the valuation methodology for our restricted stock units and stock options is included in Note 11, Share-based Compensation and Stock Plans, to our consolidated financial statements for each of the three years in the period ended May 31, 2012 contained elsewhere in this prospectus. The recognized compensation expense of the equity-based awards for financial reporting purposes will likely vary from the actual amount ultimately realized by the named executive officer based on a number of factors. The factors include our actual operating performance, common share price fluctuations, differences from the valuation assumptions used and the timing of exercise or applicable vesting.
Non-equity Incentive Plan Compensation. Our named executive officers earned annual cash incentive awards for the 2012 fiscal year. Refer to “The Elements of Biomet’s Compensation Program—Non-equity Incentive Plan” above for further information concerning this element of our compensation program.
All Other Compensation. The amounts included under the “All Other Compensation” heading represent the sum of: (1) certain perquisites and other personal benefits; (2) Biomet-paid contributions to defined contribution and other retirement plans; (3) Biomet-paid insurance premiums; (4) certain tax reimbursements made by us; and (5) certain other amounts more fully described in footnote (2) to the Summary Compensation Table.
Summary Compensation Table
108
Name and Principal Position(1) | Year | Salary ($) | Stock Awards(1) ($) | Option Awards(1) ($) | Non-Equity Incentive Plan Compensation ($) | All Other Compensation(2) ($) | Total ($) | ||||||||||||
| Jeffrey R. Binder | 2012 | $ | 717,036 | $ | — | $ | — | $ | 687,982 | $ | 689,205 | $ | 2,094,223 | ||||||
| President and Chief Executive Officer | 2011 | 717,036 | 8,500,000 | — | 416,310 | 393,875 | 10,027,221 | ||||||||||||
| 2010 | 696,150 | — | 3,026,988 | 649,949 | 413,218 | 4,786,305 | |||||||||||||
| Daniel P. Florin | 2012 | 422,118 | — | — | 337,695 | 65,876 | 825,689 | ||||||||||||
| Senior Vice President and Chief Financial Officer | 2011 | 422,118 | 1,750,000 | — | 208,054 | 33,216 | 2,413,388 | ||||||||||||
| 2010 | 409,824 | — | 714,420 | 305,280 | 13,063 | 1,442,587 | |||||||||||||
| Jon C. Serbousek | 2012 | 413,831 | — | — | 331,065 | 53,468 | 798,364 | ||||||||||||
| Group President Biomet Orthopedics | 2011 | 413,831 | 1,750,000 | — | 180,066 | 19,430 | 2,363,327 | ||||||||||||
| 2010 | 401,778 | — | 465,423 | 357,686 | 164,358 | 1,389,245 | |||||||||||||
| Bradley J. Tandy Senior Vice President; General Counsel and Secretary | 2012 | 396,733 | — | — | 238,040 | 51,395 | 686,168 | ||||||||||||
| Robin T. Barney Senior Vice President, World Wide Operations | 2012 | 315,433 | — | — | 267,933 | 53,576 | 636,942 | ||||||||||||
| (1) | For each named executive officer listed in the Summary Compensation Table above, the Stock Award’s and Option Award’s value reflects the grant date fair value of grants made in the fiscal year. |
| (2) | The table below presents an itemized account of “All Other Compensation” provided during the 2010, 2011 and 2012 fiscal years. For each named executive officer listed below, the sum of the amounts listed in the columns in the table below reflects the total value included under the “All Other Compensation” heading in the table above. |
| Year | Life Insurance Premiums ($) | Retirement Plan Contributions ($) | Travel Allowance ($)(1) | Personal Use of Company Aircraft ($)(2) | Other ($) | Amounts in Connection with Retirement ($) | ||||||||||||
| Jeffrey R. Binder | 2012 | $ | 176 | $ | 13,200 | $ | 13,000 | $ | 474,829 | $ 188,000(a) | $ | 689,205 | ||||||
| 2011 | 63 | 14,700 | 13,000 | 366,112 | — | 393,875 | ||||||||||||
| 2010 | 63 | — | 13,000 | 400,155 | — | 413,218 | ||||||||||||
| Daniel P. Florin | 2012 | 176 | 14,700 | 13,000 | — | 38,000(a) | 65,876 | |||||||||||
| 2011 | 63 | 14,033 | 13,000 | 6,120 | — | 33,216 | ||||||||||||
| 2010 | 63 | — | 13,000 | — | — | 13,063 | ||||||||||||
| Jon C. Serbousek | 2012 | 176 | 16,292 | 13,000 | — | 24,000(a) | 53,468 | |||||||||||
| 2011 | 63 | 6,367 | 13,000 | — | — (c) | 19,430 | ||||||||||||
| 2010 | 63 | — | 13,000 | 1,295 | 150,000(b) | 164,358 | ||||||||||||
| Bradley J. Tandy | 2012 | 176 | 15,219 | 13,000 | — | 23,000(a) | 51,395 | |||||||||||
| Robin T. Barney | 2012 | 176 | 14,400 | 13,000 | — | 26,000(a) | 53,576 | |||||||||||
| (1) | Represents the cost to us of providing a car allowance to Messrs. Binder, Florin, Serbousek and Tandy and Ms. Barney. |
| (2) | Represents our incremental costs incurred for personal use of our aircraft. This amount is calculated by multiplying the aircraft’s hourly variable operating cost by a trip’s flight time, which includes any flight time used for an empty return flight. Variable operating costs are based on industry standard rates of our variable operating costs, including fuel and oil costs, maintenance and repairs, landing/ramp fees and other miscellaneous variable costs. On certain occasions, a spouse or other family member may accompany one of our named executive officers on a flight. No additional operating cost is incurred in such situations under the foregoing methodology. We do not pay our named executive officers any amounts in connection with taxes on income imputed to them for personal use of our aircraft. |
Pursuant to the employment agreement between us and Mr. Binder, dated June 11, 2008, we agreed to arrange, at our expense, for Mr. Binder to fly once per week to and from Mr. Binder’s Texas home and our headquarters or such other location as may be reasonably specified by us during the term of the employment agreement. We will not provide Mr. Binder with a “gross up” for taxes incurred in connection with these benefits. If, however, Mr. Binder uses a commercial flight and the income imputed in connection with the commercial flight exceeds the amount that would have been imputed to Mr. Binder if he had used our aircraft, we will provide to Mr. Binder a “gross up” for taxes incurred on the amount of such excess. No gross ups were paid for the periods presented. Our incremental costs associated with extending these benefits to Mr. Binder are capped at $500,000 in any twelve-month period. For the purposes of applying this limitation, our incremental cost for commercial flights shall be the cost of Mr. Binder’s tickets, and for flights on Biomet-operated aircraft shall be the incremental per-hour cost associated with Mr. Binder’s flights and other incremental costs related to such flights, such as landing fees, transportation and housing costs of aircrew and other similar costs. The amount that appears under the Personal Use of Company Aircraft heading reflects the amount of this rolling twelve-month allowance that Mr. Binder used during fiscal 2012, 2011 and 2010.
109
During fiscal 2010, pending Mr. Serbousek’s relocation to the Warsaw, Indiana area, we arranged for him to fly, at our expense, between his Tennessee home and our headquarters. Our incremental cost associated with providing this benefit to Mr. Serbousek were calculated as described above with respect to Mr. Binder.
| (a) | We paid a special bonus amount to our employees who were allocated restricted stock units under Parent’s 2012 Restricted Stock Unit Plan in recognition of the delayed rollout of the Plan. |
| (b) | We paid Mr. Serbousek a $150,000 relocation bonus in June 2010. |
| (c) | Also pursuant to Mr. Serbousek’s employment agreement dated March 3, 2008, we agreed to purchase Mr. Serbousek’s prior residence in Tennessee at its appraised value, as determined by an independent appraiser, up to $650,000. As a result of the independent appraisal, we purchased Mr. Serbousek’s prior residence on June 25, 2010 for less than the maximum amount specified above, and Mr. Serbousek has not recognized any gain on the sale of his prior residence to us. As a result, the amount paid by us to Mr. Serbousek is not reflected in the amount shown in the table above for Mr. Serbousek under the “All Other Compensation” heading. In addition, because Mr. Serbousek recognized a loss on the sale of his house, we have not paid any “gross up” amounts to Mr. Serbousek in connection with the sale of his house. |
Grants of Plan-Based Awards Table
During the 2012 fiscal year, we granted cash incentive awards to our named executive officers under our non-equity incentive plan. Information with respect to each of these payments is set forth in the table below. For additional discussion of our non-equity incentive plan, refer to “The Elements of Biomet’s Compensation Program—Non-Equity Incentive Plan.” During the 2012 fiscal year, no grants of equity-based awards were made to our named executive officers.
| Estimated Possible Payouts | Estimated Future Payouts | All Other Stock Awards: Number of Shares of Stock or Units(1)(#) | All Other Option Awards: Number of Securities Underlying Options(1)(#) | Exercise of Base Price of Option Awards ($/Sh) | Grant-Date Fair Value of Stock and Option Awards ($) | |||||||||||||||||||||
| Under Non-Equity Incentive | Under Equity Incentive | |||||||||||||||||||||||||
| Plan Awards | Plan Awards | |||||||||||||||||||||||||
| Name | Grant Date | Threshold ($) | Target ($) | Maximum ($) | Threshold (#) | Target (#) | Maximum (#) | |||||||||||||||||||
| Jeffrey R. Binder | $ | — | $ | 717,036 | $ | 1,290,665 | — | — | — | — | — | $ | — | $ | — | |||||||||||
| Daniel P. Florin | — | 337,694 | 607,850 | — | — | — | — | — | — | — | ||||||||||||||||
| Jon C. Serbousek | — | 331,065 | 595,917 | — | — | — | — | — | — | — | ||||||||||||||||
| Bradley J. Tandy | — | 238,040 | 428,472 | — | — | — | — | — | — | — | ||||||||||||||||
| Robin T. Barney | — | 252,346 | 454,224 | — | — | — | — | — | — | — | ||||||||||||||||
Outstanding Equity Awards at Fiscal Year-End Table
For further information on our equity-based awards and their material terms, refer to “The Elements of Biomet’s Compensation Program—Stock Options and Restricted Stock Units.”
The following table shows the equity awards granted to our named executive officers, which are comprised of stock option awards under the 2007 LVB Plan (vested and unvested) and restricted stock units under the Restricted Stock Unit Plan (vested and unvested) that were outstanding as of the end of the 2012 fiscal year prior to the tender offer in July 2012 described above under “—Stock Options and Restricted Stock Units.”
Outstanding Equity Awards at Fiscal Year End
110
| Name | Number of Securities Underlying Unexercised Options (#) Exercisable(1) | Number of Securities Underlying Unexercised Options (#) Unexercisable(2) | Equity Incentive Plan Awards: Number of Securities Underlying Unexercised Unearned Options (#)(3) | Option Exercise Price ($)(4) | Option Expiration Date(5) | Number of Shares or Units of Stock That Have Not Vested (#) | Market Value of Shares or Units of Stock That Have Not Vested ($)(6) | Equity Incentive Plan Awards: Number of Unearned Shares, Units or Other Rights That Have Not Vested (#) | Equity Incentive Plan Awards: Market or Payout Value of Unearned Shares, Units or Other Rights That Have Not Vested ($) | |||||||||||
| Jeffrey R. Binder | 2,415,000 | 735,000(a) | — | $ | 10.00 | July 11, 2017 | 850,000 | $ | 6,693,750 | — | $ | — | ||||||||
| 577,500 | — | 472,500(b) | 10.00 | July 11, 2017 | — | — | — | — | ||||||||||||
| Daniel P. Florin | 382,376 | 116,374(a) | — | 10.00 | July 11, 2017 | 175,000 | 1,378,125 | — | — | |||||||||||
| 91,438 | — | 74,812(b) | 10.00 | July 11, 2017 | — | — | — | — | ||||||||||||
| 25,500 | 38,250(a) | — | 10.00 | October 5, 2019 | — | — | — | — | ||||||||||||
| 4,250 | — | 17,000(b) | 10.00 | October 5, 2019 | — | — | — | — | ||||||||||||
| Jon C. Serbousek | 484,500 | 153,000(a) | — | 10.00 | May 1, 2018 | 175,000 | 1,378,125 | — | — | |||||||||||
| 76,500 | — | 136,000(b) | 10.00 | May 1,2018 | — | — | — | — | ||||||||||||
| Bradley J. Tandy | 287,500 | 87,500(a) | — | 10.00 | July 11, 2017 | 110,000 | 866,250 | — | — | |||||||||||
| 68,750 | — | 56,250(b) | 10.00 | July 11, 2017 | — | — | — | — | ||||||||||||
| Robin T. Barney | 382,374 | 116,376(a) | — | 10.00 | July 11, 2017 | 140,000 | 1,102,500 | — | — | |||||||||||
| 91,437 | — | 74,813(b) | 10.00 | July 11, 2017 | — | — | — | — | ||||||||||||
| (1) | On an award-by-award basis, reflects the number of common shares underlying unexercised options that are exercisable and that are not reported in Column 3—”Number of Securities Underlying Unexercised Unearned Options.” |
| (2) | On an award-by-award basis, reflects the number of common shares underlying unexercised options that are unexercisable and that are not reported in Column 3—”Number of Securities Underlying Unexercised Unearned Options.” The vesting schedules of the outstanding unvested options are listed below: |
With respect to Mr. Binder, represents the outstanding unvested portion of the time-based option granted on October 5, 2009. The unvested portion is scheduled to vest in increments of 577,500 common shares on July 11, 2012, and 157,500 on July 11, 2013.
With respect to Mr. Florin, represents the outstanding unvested portion of the time-based option granted on October 5, 2009 and October 16, 2009. The unvested portion is scheduled to vest in increments of 91,438 common shares on July 11, 2012, 24,936 on July 11, 2013, and 12,750 on October 1 in each of 2012, 2013 and 2014.
With respect to Mr. Serbousek, represents the outstanding unvested portion of the time-based option granted on October 5, 2009. The unvested portion is scheduled to vest in increments of 119,000 common shares on May 1, 2013, and 34,000 on May 1, 2014.
With respect to Mr. Tandy, represents the outstanding unvested portion of the time-based option granted on October 5, 2009. The unvested portion is scheduled to vest in increments of 68,750 common shares on July 11, 2012 and 18,750 on July 11, 2013.
With respect to Ms. Barney, represents the outstanding unvested portion of the time-based option granted on October 5, 2009. The unvested portion is scheduled to vest in increments of 91,437 common shares on July 11, 2012 and 24,939 on July 11, 2013.
| (3) | Represents, on an award-by-award basis, the total number of common shares underlying unexercised options awarded under any equity incentive plan that have not been earned. Performance awards vest based on our achievement of adjusted EBITDA targets established by the Compensation Committee. |
With respect to Mr. Binder, represents the outstanding unvested portion of the performance-based option granted on October 5, 2009. The unvested portion is eligible to vest in increments of 157,500 common shares on July 11 in each of 2012 and 2013.
With respect to Mr. Florin, represents the outstanding unvested portion of the performance-based option granted on October 5, 2009 and October 16, 2009. The unvested portion is eligible to vest in increments of 91,438 common shares on July 11, 2012, 24,936 common shares on July 11, 2013, and 4,250 common shares on October 1 in each of 2012, 2013 and 2014.
With respect to Mr. Serbousek, represents the outstanding unvested portion of the performance-based option granted on October 5, 2009. The unvested portion is eligible to vest in increments of 34,000 common shares on July 11 in each of 2013 and 2014.
With respect to Mr. Tandy, represents the outstanding unvested portion of the performance-based option granted on October 5, 2009. The unvested portion is eligible to vest in increments of 18,750 common shares on July 11 in each of 2012 and 2013.
With respect to Ms. Barney, represents the outstanding unvested portion of the performance-based option granted on October 5, 2009. The unvested portion is eligible to vest in increments of 24,937 common shares on July 11, 2012 and 24,939 on July 11, 2013.
| (4) | The exercise price, as it was recorded in the applicable stock option award agreement at the time of grant, for each option reported in Columns 1 and 2—”Number of Securities Underlying Unexercised Options” and Column 3—”Number of Securities Underlying Unexercised Unearned Options.” |
| (5) | Represents the tenth year anniversary for each option award reported in Columns 1 and 2—”Number of Securities Underlying Unexercised Options” and Column 3—”Number of Securities Underlying Unexercised Unearned Options.” For information on the vesting schedule of unvested portions of outstanding option awards, see sub-footnotes (a)-(b) of footnote (2), and footnote (3), above. |
| (6) | The market value of shares or units of stock that have not vested is calculated by multiplying the number of shares or units of stock that have not vested by $7.875, which was the fair value of each common share underlying each option or stock unit. |
| (a) | Represents time-based options, which generally vest ratably over 5 years or 6 years for modified accreting exercise price options. |
111
| (b) | Represents performance-based options, which generally vest ratably over 5 years. The performance criteria for options vesting based on the fiscal 2011 and 2012 results did not meet the target and did not vest. |
Option Exercises and Stock Vested Table
During the 2012 fiscal year, no equity-based awards were exercised by, and no stock awards vested to, Biomet’s named executive officers.
Retirement And Non-Qualified Defined Contribution And Deferred Compensation Plans
Non-Qualified Deferred Compensation
Our frozen Deferred Compensation Plan is a non-qualified deferred compensation plan, which was available for members of our senior management. The Plan allowed eligible participants to defer pre-tax compensation to reduce current tax liability and assisted those team members in their plan for retirement and other long-term savings goals in a tax-effective manner. Under the Plan, eligible participants deferred up to 100% of their base salary and annual cash incentive payments, as well as Board fees for non-employee Directors, as applicable. We did not make any contributions to the Plan.
During the 2012 fiscal year, three of our named executive officers had earnings and maintained a balance in a nonqualified deferred compensation plan. The Plan was frozen during the fiscal year 2011, so there were no contributions by either the employee or Biomet.
| Name | Executive Contributions ($) | Registrant Contributions ($) | Aggregate Earnings ($) | Aggregate Withdrawals/ Distributions ($) | Aggregate Balance ($) | ||||||||||
| Jeffrey R. Binder | $ | — | $ | — | $ | (40,602 | ) | $ | — | $ | 435,684 | ||||
| Daniel P. Florin | — | — | — | — | — | ||||||||||
| Jon C. Serbousek | — | — | — | — | — | ||||||||||
| Bradley J. Tandy | — | — | (7,494) | — | 118,649 | ||||||||||
| Robin T. Barney | — | — | 66 | — | 175,307 | ||||||||||
Employment Agreements and Potential Post-Termination Payments
We have employment agreements with each of Messrs. Binder, Florin, Serbousek and Tandy, and Ms. Barney, which agreements contain severance and change in control provisions.
Employment Agreement with Jeffrey R. Binder
On January 14, 2013, the Company entered into an amended and restated employment agreement with Mr. Binder (the “Employment Agreement”), pursuant to which he will continue to serve as President and Chief Executive Officer of the Company and will continue to be appointed to the Company’s Board of Directors and its Executive Committee. The Employment Agreement supersedes the original employment agreement entered into between the Company and Mr. Binder dated as of June 11, 2008 (the “Original Agreement”). The Employment Agreement has an initial three-year term commencing on January 14, 2013 and provides for automatic 12-month extensions on each anniversary of such commencement date, unless either the Company or Mr. Binder gives prior notice of termination.
In addition to the benefits provided in the Original Agreement, the Employment Agreement provides that Mr. Binder would be entitled to certain enhanced severance benefits following certain terminations of employment. If he is terminated by the Company for any reason other than for cause (as defined in the agreement), death or disability (as defined in the agreement), or if Mr. Binder terminates his employment for good reason (as defined in the agreement) or on or after January 1, 2015, with or without good reason (and his employment could not be terminated by the Company for cause at such time), he would be entitled to an amount equal to (a) 2 times his base salary in effect at the date of termination plus (b) 2 times the annual incentive bonus Mr. Binder would have received for the current year if his employment had not been terminated, based on Biomet’s performance to the date of termination extrapolated through the end of the current year.
112
The Employment Agreement also revises the severance to which Mr. Binder would be entitled if his employment is terminated within the two-year period following a change in control. Under the Employment Agreement, if Mr. Binder’s employment is terminated at any time within the two-year period following a change in control either by the Company for any reason other than for cause, death or disability, or by Mr. Binder for good reason or on or after January 1, 2015, with or without good reason (and his employment could not be terminated by the Company for Cause at such time), Mr. Binder will receive an amount equal to (a) 2 times his base salary in effect at the date of termination plus (b) 2 times the annual incentive bonus Mr. Binder would have received for the current year if his employment had not been terminated, based on Biomet’s performance to the date of termination extrapolated through the end of the current year.
In addition, under the Employment Agreement, on or following January 1, 2014, Mr. Binder may terminate his employment for good reason upon the appointment of a successor Chief Executive Officer of the Company by resolution of the Board.
Restricted Stock Unit Grant Agreement
On January 14, 2013, the Company entered into an amended and restated Restricted Stock Unit Grant Agreement with Mr. Binder (the “RSU Agreement”). The RSU Agreement supersedes the original restricted stock grant agreement entered into between the Company and Mr. Binder dated as of July 31, 2012 (the “Original RSU Agreement”). In addition to the terms of the Original RSU Agreement, the RSU Agreement provides that if Mr. Binder is terminated by the Company for any reason other than for cause (as defined in the RSU Agreement), death or disability (as defined in the RSU Agreement), or if Mr. Binder terminates his employment for good reason (as defined in the Employment Agreement) prior to January 1, 2015, any unvested Time-Based Restricted Stock Units that would have vested had Mr. Binder remained employed through January 1, 2015 will satisfy the time-based vesting condition as of the date of his termination.
The RSU Agreement also provides for payment with respect to Mr. Binder’s Management Dividend Awards upon certain terminations. The terminations to which such benefits apply are (a) for periods prior to January 1, 2015, if Mr. Binder’s employment is terminated in any year by the Company other than for cause, death or disability or by him for good reason, and (b) for periods after January 1, 2015, if Mr. Binder’s employment is terminated in any year by the Company without cause or by him for any reason (each an “eligible termination”). In the case of an eligible termination prior to the Management Dividend Award Date in the year of termination, Mr. Binder will be entitled to receive a Management Dividend Award Payment Amount (paid at the same time Management Dividend Award payments are made to other employees for such year) with respect to a number of Management Dividend Awards equal to the number of Time-Based Restricted Stock Units vested and outstanding as of his termination date, regardless of whether he was employed on the Management Dividend Award vesting date(s) or on the Management Dividend Award Payment Date for such year. Mr. Binder would have no entitlement to any Management Dividend Award payment paid in respect of any year subsequent to the year in which his employment terminates.
The RSU Agreement requires that in connection with certain increases and decreases in the numbers of issued and outstanding shares of common stock of the Company, the Board will make adjustments to Mr. Binder’s RSU Agreement that the Board deems appropriate to prevent the enlargement or dilution of rights with respect to the number of shares of common stock available for grant under the 2012 Restricted Stock Unit Plan and the number of shares of common stock subject to restricted stock unit grant agreements. The RSU Agreement also requires that any adjustment made in connection with a cash dividend or distribution will be made in the same manner as the adjustment made to all or substantially all restricted stock units with substantially the same terms and conditions as Mr. Binder’s restricted stock units.
Stock Option Grant Agreement
On January 14, 2013, the Company entered into an amended and restated Stock Option Grant Agreement with Mr. Binder (the “Option Agreement”). The Option Agreement supersedes the original stock option grant agreement entered into between the Company and Mr. Binder dated as of July 31, 2012 (the “Original Option Agreement”).
In addition to the terms of the Original Option Agreement, the Option Agreement provides that if Mr. Binder is terminated by the Company for any reason other than for cause (as defined in the Option Agreement), death or disability (as defined in the Option Agreement), or if Mr. Binder terminates his employment for good reason (as defined in the Employment Agreement) prior to January 1, 2015, any unvested Replacement Extended Time Vesting Options that would have vested had Mr. Binder remained employed through January 1, 2015 will vest on the date of his termination. The Option Agreement also provides that if Mr. Binder terminated his employment without good reason (and his employment
113
could not be terminated by the Company for Cause at such time), he will retain exercise rights on vested stock options until their expiration date as follows: continuously employed through January 1, 2014, retains 70% of vested options; continuously employed through July 1, 2014, retains 85% of vested options; and continuously employed through January 1, 2015, retains 100% of vested options. If the Company terminates Mr. Binder’s employment other than for cause, death or disability, or Mr. Binder terminates for good reason, he will retain 100% of the vested options until their expiration date. The Option Agreement provides that if the Company modifies or offers to employees to modify the expiration date of options granted to employees on substantially the same terms and conditions as applies to Mr. Binder’s option, the expiration date of Mr. Binder’s option will also be modified or eligible for modification.
Employment Agreement with Daniel P. Florin
On February 28, 2008, we entered into an employment agreement with Mr. Florin, our Senior Vice President and Chief Financial Officer. Mr. Florin’s agreement has an initial three-year term that provides for automatic twelve-month extensions, beginning on the first anniversary of the date of the agreement, unless either party gives prior notice of termination. Mr. Florin will receive a base salary at a rate no less than $395,850 per year which shall be increased at our discretion. Mr. Florin will also have the opportunity to earn an annual cash incentive award in an amount no less than 80% of his base salary for on-target performance, with the possibility of exceeding 80% for high achievement. For a further discussion of our non-equity incentive plan, see “The Elements of Biomet’s Compensation Program—Non-equity Incentive Plan.”
The agreement further provides that Mr. Florin could be entitled to certain severance benefits following termination of employment prior to a change in control (as defined in the agreements) or within two years following a change in control. See “—Severance Benefits” below.
Employment Agreement with Jon C. Serbousek
On March 3, 2008, we entered into an employment agreement with Mr. Serbousek, our Senior Vice President and President of Biomet Biologics, LLC. The agreement has an initial three-year term that provides for automatic twelve-month extensions, beginning on the first anniversary of the date of the agreement, unless either party gives prior notice of termination. Mr. Serbousek will receive a base salary at a rate no less than $390,000 per year, which shall be increased at our discretion. Mr. Serbousek will also have the opportunity to earn an annual cash incentive award in an amount no less than 80% of his base salary for on-target performance, with the possibility of exceeding 80% for high achievement. For a further discussion of our non-equity incentive plan, see “The Elements of Biomet’s Compensation Program—Non-equity Incentive Plan.”
The agreement further provides that Mr. Serbousek could be entitled to certain severance benefits following termination of employment prior to a change in control (as defined in the agreement) or within two years of a change in control. See “—Severance Benefits” below.
Employment Agreement with Bradley J. Tandy
On February 28, 2008, we entered into an employment agreement with Mr. Tandy, our Senior Vice President, General Counsel and Secretary. The agreement has an initial three-year term that provides for automatic twelve-month extensions, beginning on the first anniversary of the date of the agreement, unless either party gives prior notice of termination. Mr. Tandy will receive a base salary at a rate no less than $345,050 per year, which shall be increased at our discretion. Mr. Tandy will also have the opportunity to earn an annual cash incentive award in an amount no less than 60% of his base salary for on-target performance, with the possibility of exceeding 60% for high achievement. For a further discussion of our non-equity incentive plan, see “The Elements of Biomet’s Compensation Program—Non-equity Incentive Plan.”
The agreement further provides that Mr. Tandy could be entitled to certain severance benefits following termination of employment prior to a change in control (as defined in his employment agreement) or within two years of a change in control. See “—Severance Benefits” below.
Employment Agreement with Robin T. Barney
On September 2, 2008, we entered into an employment agreement with Ms. Barney, our Senior Vice President of Worldwide Operations. The agreement has an initial three-year term that provides for automatic twelve-month extensions, beginning on the first anniversary of the date of the agreement, unless either party gives prior notice of termination. Ms.
114
Barney will receive a base salary at a rate no less than $275,000 per year, which shall be increased at our discretion. Ms. Barney will also have the opportunity to earn an annual cash incentive award in an amount no less than 80% of her base salary for on-target performance, with the possibility of exceeding 80% for high achievement. For a further discussion of our non-equity incentive plan, see “The Elements of Biomet’s Compensation Program—Non-equity Incentive Plan.”
The agreement further provides that Ms. Barney could be entitled to certain severance benefits following termination of employment prior to a change in control (as defined in her employment agreement) or within two years of a change in control. See “—Severance Benefits” below.
Severance Benefits
Each of our employment agreements with Messrs. Binder, Florin, Serbousek and Tandy, and Ms. Barney contains provisions which entitle the executive to certain severance benefits following termination of employment prior to a change in control (as defined in each of their employment agreements) or within two years following a change in control.
The following summary provides a description of the severance arrangements contained in our employment agreements with Messrs. Binder, Florin, Serbousek and Tandy, and Ms. Barney. Other than with respect to Mr. Binder as described in “Termination Within Two Years Following a Change in Control by Biomet Other Than For Cause, Death or Disability, or by Executive for Good Reason,” the following summary does not discuss the executives’ rights with respect to any equity related awards, as such awards are governed by the applicable terms of the related plan or award agreement.
Termination Prior to a Change in Control by Biomet Other Than For Cause, Death or Disability, or by Executive for Good Reason
With respect to Messrs. Binder, Florin, Serbousek and Tandy, and Ms. Barney, in the event of a termination of the executive’s employment prior to a change in control either (1) by us for any reason other than for “cause” (which generally includes the executive’s failure to substantially perform the executive’s duties, willful misconduct or gross negligence, willful or grossly negligent breach of the executive’s fiduciary duties to Biomet, commission of any felony or other serious crime involving moral turpitude, material breach of any agreement between the executive and Biomet or material breach of our written policies), executive’s death or executive’s disability, or (2) by executive for “good reason” (which generally includes any material diminution in duties and responsibilities (but does not include, in the case of Mr. Serbousek, Mr. Tandy and Ms. Barney, a change in duties and responsibilities that results from becoming a part of a larger organization following a change in control), reduction in base salary or bonus opportunity or relocation of primary work location by more than 50 miles), our employment agreements with Messrs. Binder, Florin, Serbousek and Tandy, and Ms. Barney, provide that such executive would be entitled to the following:
| • | An amount equal to (a) 1.5 times the executive’s base salary in effect at the date of termination (with respect to Messrs. Florin, Serbousek and Tandy, and Ms. Barney, the “Severance Benefit,” and with respect to Mr. Binder, the “Base Component”) plus, with respect to Mr. Binder, (b) 1.5 times the average of (x) the annual cash incentive award earned by Mr. Binder for the preceding fiscal year and (y) the annual cash incentive award Mr. Binder would have received for the current fiscal year had his employment not been terminated, based on Biomet’s performance to the date of termination extrapolated through the end of such fiscal year (the “Bonus Component,” and with respect to Mr. Binder, together with the Base Component, the “Severance Benefit”). The total amount of the Severance Benefit will be paid in equal, ratable installments in accordance with our regular payroll policies over the course of the 18 month non-compete period provided for in the agreement. If Mr. Binder becomes employed by another employer during that period, the Bonus Component will cease and his Severance Benefit will be limited to the Base Component; |
| • | An amount equal to the pro rated portion (based on the percentage of Biomet’s current fiscal year preceding the date on which the executive’s employment is terminated) of the annual cash incentive award the executive would have received for the current fiscal year, based on Biomet’s performance to the date of termination extrapolated through the end of the current fiscal year. The total amount of the pro rated annual cash incentive award will be paid in a lump sum at the time we pay annual cash incentive awards to similarly situated active employees; |
| • | If the executive is eligible for and elects continuation coverage pursuant to COBRA, we will pay the premiums for such coverage (or reimburse the executive for such premiums) until the earlier of (a) the end |
115
of the 18 month period during which, under the employment agreement, the executive agrees not to engage in certain activities in competition with us or (b) the date the executive becomes eligible for coverage under another group plan;
| • | Any “accrued benefits” (as defined in the respective agreement), which generally include any vested compensation deferred by the executive and not yet paid by the Company, any amounts or benefits owing to the executive under the then applicable benefit plans of the Company, and any amounts owing to the executive for reimbursement of expenses properly incurred by the executive; and |
| • | With respect to Mr. Binder, continued payment of Mr. Binder’s company-provided car allowance, if any, for a period of 12 months from the termination date. |
Termination Within Two Years After a Change in Control by Biomet Other Than For Cause, Death or Disability, or by Executive for Good Reason
With respect to Messrs. Binder, Florin, Serbousek and Tandy, and Ms. Barney, in the event of a termination of the executive’s employment within two years after a change in control either (1) by us for any reason other than for cause, executive’s death or executive’s disability, or (2) by executive for good reason, such executive would be entitled to the following:
| • | An amount equal to (a) two times the executive’s base salary in effect at the date of termination plus (b) two times the average of (x) the annual cash incentive award earned by executive for the preceding fiscal year and (y) the annual cash incentive award the executive would have received for the current fiscal year had the executive’s employment not been terminated, based on Biomet’s performance to the date of termination extrapolated through the end of such fiscal year (collectively, the “Change-in-Control Severance Benefit”). The total amount of the Change-in-Control Severance Benefit will be paid in a lump sum as soon as administratively practicable following the termination of the executive’s employment; |
| • | An amount equal to the pro rated portion (based on the percentage of Biomet’s current fiscal year preceding the date on which the executive’s employment is terminated) of the annual cash incentive award the executive would have received for the current fiscal year, based on Biomet’s performance to the date of termination extrapolated through the end of the current year. The total amount of the pro rated annual cash incentive award will be paid in a lump sum at the time we pay annual cash incentive awards to similarly situated active employees; |
| • | If the executive is eligible for and elects continuation coverage pursuant to COBRA, we will pay the premiums for such coverage (or reimburse executive for such premiums) until the earlier of (a) the end of the 18 month period during which, under the employment agreement, the executive agrees not to engage in certain activities in competition with us or (b) the date the executive becomes eligible for coverage under another group plan; |
| • | Any “accrued benefits” (as defined in the respective agreement), which generally include any vested compensation deferred by the executive and not yet paid by the Company, any amounts or benefits owing to the executive under the then applicable benefit plans of the Company, and any amounts owing to the executive for reimbursement of expenses properly incurred by the executive; and |
| • | With respect to Mr. Binder, continued payment of Mr. Binder’s company-provided car allowance, if any, for a period of 12 months from the termination date and immediate vesting of any unvested options held by Mr. Binder as of the date his employment is terminated. |
To receive the severance benefits provided under the agreement, the executive must sign a general release of claims. The agreement contains customary confidentiality, non-competition and non-solicitation provisions. Messrs. Binder’s, Florin’s, Serbousek’s and Tandy’s, and Ms. Barney’s non-competition period is 18 months following the date of termination of employment.
116
Furthermore, in the event that any payments made to Mr. Binder in connection with a termination of employment would be subject to excise taxes under the Code, subject to certain conditions, Biomet will “gross up” his compensation to fully offset such excise taxes.
Termination Due to Death or Disability
If any of Messrs. Binder, Florin, Serbousek or Tandy’s, or Ms. Barney’s employment is terminated due to the executive’s death or disability, the executive is entitled to receive the following:
| • | the executive’s base salary in effect through the date of termination; |
| • | a prorated portion (based on the percentage of our fiscal year preceding the date of termination) of the average of (x) the annual cash incentive award earned by such executive for the preceding year and (y) the annual cash incentive award such executive would have received in the current year if the executive’s employment had not been terminated, based on our performance to the date of termination extrapolated through the end of the then current fiscal year; and |
| • | any “accrued benefits” (as defined in the respective employment agreement). |
Termination With Cause or Without Good Reason
If any of Messrs. Binder, Florin, Serbousek or Tandy’s, or Ms. Barney’s employment is terminated with “cause” or without “good reason” (as defined in the employment agreement) we will pay such executive’s base salary in effect through the termination date and any “accrued benefits” (as defined in the respective employment agreement) when due.
Potential Payments Upon Certain Terminations
This table shows the potential compensation that we would have to pay to certain named executive officers upon a termination of employment—related or unrelated to a change in control—by us without “cause” or by the executive with “good reason” (as defined in the applicable agreements), due to the executive’s death or disability, and by us with “cause” or by the executive without “good reason” (as defined in the applicable agreements). The table excludes certain amounts payable pursuant to plans that are available generally to all salaried employees. In the event of the death or disability of any of the named executive officers listed in the following table, the deceased or disabled named executive officer, or his designated beneficiaries, would receive a payment pursuant to the terms of Biomet-funded life or disability plans, respectively, in addition to the amounts set forth below. The amounts shown assume that termination of employment was effective May 31, 2012. The amounts shown are only estimates of the amounts that would be payable to the executives upon termination of employment and do not reflect tax positions we may take or the accounting treatment of such payments. Actual amounts to be paid can only be determined at the time of separation. Although the calculations are intended to provide reasonable estimates of the potential benefits, they are based on numerous assumptions and do not represent the actual amount an executive would receive if an eligible termination event were to occur.
117
POTENTIAL PAYMENTS UPON TERMINATION OR CHANGE IN CONTROL
Potential Payments Upon Termination or Termination in Connection With a Change in Control
| Termination in Connection with a Change in Control | Termination in Absence of a Change in Control | |||||||||||||||||||||||
| Name of Executive Officer | Termination without Cause or with Good Reason (1) | Termination with Cause or Resignation without Good Reason (2) | Disability (3) | Death (4) | Termination without Cause or with Good Reason (5) | Termination with Cause or Resignation without Good Reason (6) | Disability (7) | Death (8) | ||||||||||||||||
| Jeffrey R. Binder | ||||||||||||||||||||||||
| Estimated Value of Non- Equity Benefits and Accrued Obligations | $ | 3,255,185 | $ | — | $ | 552,146 | $ | 552,146 | $ | 2,620,594 | $ | — | $ | 552,146 | $ | 552,146 | ||||||||
| Estimated Value of Options & Equity Awards | 6,693,750 | — | — | — | — | — | — | — | ||||||||||||||||
| Total | 9,948,935 | — | 552,146 | 552,146 | 2,620,594 | — | 552,146 | 552,146 | ||||||||||||||||
| Daniel P. Florin | ||||||||||||||||||||||||
| Estimated Value of Non- Equity Benefits and Accrued Obligations | 1,743,520 | — | 272,875 | 272,875 | 986,712 | — | 272,875 | 272,875 | ||||||||||||||||
| Estimated Value of Options & Equity Awards | 1,378,125 | — | — | — | — | — | — | — | ||||||||||||||||
| Total | 3,121,645 | — | 272,875 | 272,875 | 986,712 | — | 272,875 | 272,875 | ||||||||||||||||
| Jon C. Serbousek | ||||||||||||||||||||||||
| Estimated Value of Non- Equity Benefits and Accrued Obligations | 1,685,689 | — | 255,566 | 255,566 | 967,651 | — | 255,566 | 255,566 | ||||||||||||||||
| Estimated Value of Options & Equity Awards | 1,378,125 | — | — | — | — | — | — | — | ||||||||||||||||
| Total | 3,063,823 | — | 255,566 | 255,566 | 967,651 | — | 255,566 | 255,566 | ||||||||||||||||
| Bradley J. Tandy | ||||||||||||||||||||||||
| Estimated Value of Non- Equity Benefits and Accrued Obligations | 1,424,975 | — | 188,911 | 188,911 | — | — | 188,911 | 188,911 | ||||||||||||||||
| Estimated Value of Options & Equity Awards | 866,250 | — | — | — | — | — | — | — | ||||||||||||||||
| Total | 2,291,225 | — | 188,911 | 188,911 | 848,786 | — | 188,911 | 188,911 | ||||||||||||||||
| Robin T. Barney | ||||||||||||||||||||||||
| Estimated Value of Non- Equity Benefits and Accrued Obligations | 1,376,631 | — | 234,455 | 234,455 | 750,005 | — | 234,455 | 234,455 | ||||||||||||||||
| Estimated Value of Options & Equity Awards | 1,102,500 | — | — | — | — | — | — | — | ||||||||||||||||
| Total | 2,479,131 | — | 234,455 | 234,455 | 750,005 | — | 234,455 | 234,455 | ||||||||||||||||
(1) With respect to Messrs. Binder, Florin, Serbousek and Tandy, and Ms. Barney:
Non-Equity Benefits and Accrued Obligations represents: (i) an amount equal to (a) two times the executive’s base salary in effect at the date of termination plus (b) two times the average of (x) the annual cash incentive award earned by the executive for the preceding fiscal year and (y) the annual cash incentive award the executive would have received for the current fiscal year had the executive’s employment not been terminated, based on Biomet’s performance to the date of termination extrapolated through the end of such fiscal year; (ii) an amount equal to the pro-rated portion of the annual cash incentive award the executive would have received for the current fiscal year, based on Biomet’s performance to the date of termination extrapolated through the end of the current year; (iii) if the executive is eligible for and elects continuation coverage pursuant to COBRA, the premiums for such coverage until the earlier of (a) the end of the 18-month period during which executive agrees, under the executive’s employment agreement, not to engage in certain activities in competition with us or (b) the date the executive becomes eligible for coverage under another group plan; (iv) any “accrued benefits,” which generally include any vested compensation deferred by the executive and not yet paid by the Company, any amounts or benefits owing to the executive under the then applicable benefit plans of the Company, and any amounts owing to the executive for reimbursement of expenses properly incurred by the executive; and (v) with respect to Mr. Binder, continued payment of Mr. Binder’s company provided car allowance, if any, for a period of 12 months from the termination date.
With respect to Messrs. Binder, Florin, Serbousek and Tandy, and Ms. Barney:
Options and Equity Awards represents the difference between the exercise price and the value of Parent’s common stock on May 31, 2012 with respect to any vested options held by the executive as of May 31, 2012 and the value of their RSUs as of May 31, 2012.
| (2) | With respect to Messrs. Binder, Florin, Serbousek and Tandy, and Ms. Barney: |
Non-Equity Benefits and Accrued Obligations represents (i) base salary in effect through the termination date and (ii) any “accrued benefits” (as defined in the employment agreements), which generally include any vested compensation deferred by the executive and not yet paid by the Company, any amounts or benefits owing to the executive under the then applicable benefit plans of the Company and any amounts owing to the executive for reimbursement of expenses properly incurred by the executive.
| (3) | With respect to Messrs. Binder, Florin, Serbousek and Tandy, and Ms. Barney: |
Non-Equity Benefits and Accrued Obligations represents: (i) the executive’s base salary in effect through date of termination; (ii) a pro-rated portion (based on the percentage of our fiscal year preceding the date of termination) of the average of (x) the annual
118
cash incentive award bonus earned by the executive for the preceding year and (y) the annual cash incentive award the executive would have received in the current year if the executive’s employment had not been terminated, based on our performance to the date of termination extrapolated through the end of the current year; and (iii) any “accrued benefits,” which generally include any vested compensation deferred by the executive and not yet paid by the Company, any amounts or benefits owing to the executive under the then applicable benefit plans of the Company, and any amounts owing to the executive for reimbursement of expenses properly incurred by the executive.
With respect to Messrs. Binder, Florin, Serbousek and Tandy, and Ms. Barney:
Options and Equity Awards represents the difference between the exercise price and the value of Parent’s common stock on May 31, 2012 with respect to any vested options held by the executive as of May 31, 2012.
| (4) | With respect to Messrs. Binder, Florin, Serbousek and Tandy, and Ms. Barney: |
Non-Equity Benefits and Accrued Obligations represents the payments as described in footnote 3 of this table.
With respect to Messrs. Binder, Florin, Serbousek and Tandy, and Ms. Barney:
Options and Equity Awards represents the difference between the exercise price and the value of Parent’s common stock on May 31, 2012 with respect to any vested options held by the executive as of May 31, 2012.
| (5) | With respect to Messrs. Binder, Florin, Serbousek and Tandy, and Ms. Barney: |
Non-Equity Benefits and Accrued Obligations represents: (i) an amount equal to (a) 1.5 times the executive’s base salary in effect at the date of termination plus, with respect to Mr. Binder (b) 1.5 times the average of (x) the annual cash incentive award earned by executive for the preceding fiscal year and (y) the annual cash incentive award the executive would have received for the current fiscal year had the executive’s employment not been terminated, based on Biomet’s performance to the date of termination extrapolated through the end of such fiscal year; (ii) an amount equal to the pro-rated portion (based on the percentage of Biomet’s current fiscal year preceding the date on which executive’s employment is terminated) of the annual cash incentive award the executive would have received for the current fiscal year, based on Biomet’s performance to the date of termination extrapolated through the end of the current year; (iii) if the executive is eligible for and elects continuation coverage pursuant to COBRA, the premiums for such coverage (or reimbursement to the executive for such premiums) until the earlier of (a) the end of the 18-month period during which, under the employment agreement, the executive agrees not to engage in certain activities in competition with us or (b) the date the executive becomes eligible for coverage under another group plan; (iv) any “accrued benefits,” which generally include any vested compensation deferred by the executive and not yet paid by the Company, any amounts or benefits owing to the executive under the then applicable benefit plans of the Company, and any amounts owing to the executive for reimbursement of expenses properly incurred by the executive; and (v) with respect to Mr. Binder, continued payment of Mr. Binder’s company provided car allowance, if any, for a period of 12 months from the termination date and immediate vesting of any unvested options held by Mr. Binder as of the date his employment is terminated.
With respect to Messrs. Binder, Florin, Serbousek and Tandy, and Ms. Barney:
Options and Equity Awards represents the difference between the exercise price and the value of Parent’s common stock on May 31, 2012 with respect to any vested options held by the executive as of May 31, 2012.
| (6) | With respect to Messrs. Binder, Florin, Serbousek and Tandy, and Ms. Barney: |
Non-Equity Benefits and Accrued Obligations represents: (i) base salary in effect through the termination date and (ii) any “accrued benefits,” which generally include any vested compensation deferred by the executive and not yet paid by the Company, any amounts or benefits owing to the executive under the then applicable benefit plans of the Company and any amounts owing to the executive for reimbursement of expenses properly incurred by the executive.
| (7) | With respect to Messrs. Binder, Florin, Serbousek and Tandy, and Ms. Barney: |
Non-Equity Benefits and Accrued Obligations represents: (i) the executive’s base salary in effect through date of termination; (ii) a pro-rated portion (based on the percentage of our fiscal year preceding the date of termination) of the average of (x) the annual cash incentive award earned by the executive for the preceding year and (y) the annual cash incentive award the executive would have received in the current year if the executive’s employment had not been terminated, based on our performance to the date of termination extrapolated through the end of the current year; and (iii) any “accrued benefits,” which generally include any vested compensation deferred by the executive and not yet paid by the Company, any amounts or benefits owing to the executive under the then applicable benefit plans of the Company and any amounts owing to the executive for reimbursement of expenses properly incurred by the executive.
With respect to Messrs. Binder, Florin, Serbousek and Tandy, and Ms. Barney:
Options and Equity Awards represents the difference between the exercise price and the value of Parent’s common stock on May 31, 2012 with respect to any vested options held by the executive as of May 31, 2012.
| (8) | With respect to Messrs. Binder, Florin, Serbousek and Tandy, and Ms. Barney: |
Non-Equity Benefits and Accrued Obligations represents the payments described in footnote 4 of this table.
With respect to Messrs. Binder, Florin, Serbousek and Tandy, and Ms. Barney:
Options and Equity Awards represents the difference between the exercise price and the value of Parent’s common stock on May 31, 2012 with respect to any vested options held by the executive as of May 31, 2012.
Non-Employee Director Compensation and Benefits
Our directors have not received cash retainers, committee fees, or stock option awards for their services as our directors.
119
Business Expenses
The directors are reimbursed for their business expenses related to their attendance at our meetings, including room, meals and transportation to and from Board and committee meetings. On rare occasions, a director’s spouse may accompany a director when traveling on Biomet business. At times, a director may travel to and from our meetings on our corporate aircraft. Directors are also eligible to be reimbursed for attendance at qualified director education programs.
Director and Officer Liability (or D&O) Insurance and Travel Accident Insurance
D&O insurance individually insures our directors and officers against certain losses that they are legally required to bear as a result of their actions while performing duties on our behalf. Our D&O insurance policy does not break out the premium for directors versus officers and, therefore, a dollar amount cannot be assigned to the coverage provided for individual directors.
We also maintain an Aviation Insurance Policy that provides benefits to each director in the event of death or disability (permanent and total) during travel on our corporate aircraft. This policy also covers employees and others while traveling on our corporate aircraft and, therefore, a dollar amount cannot be assigned to the coverage provided for individual directors.
Non-Employee Directors’ Compensation Table
The following table shows information regarding the compensation of our non-employee directors for the 2012 fiscal year. Mr. Binder is not included in the table below because, as President and Chief Executive Officer, disclosure in respect of his compensation is presented in the Summary Compensation Table. Furthermore, as an employee director, Mr. Binder did not receive compensation in his capacity as a director.
Director Compensation
| Name | Fees Earned or Paid in Cash ($) | Stock Awards ($) | Option Awards ($) | Non-Family Incentive Plan Compensation ($) | Change in Pension Value and Nonqualified Deferred Compensation Earnings ($) | All Other Compensation ($) | Total ($) | ||||||||||||||
Jonathon J. Coslet(2) | $ | — | $ | — | $ | — | $ | — | $ | — | $ | — | $ | — | |||||||
Michael Dal Bello(2) | — | — | — | — | — | — | — | ||||||||||||||
Adrian Jones(2) | — | — | — | — | — | — | — | ||||||||||||||
Michael Michelson(2) | — | — | — | — | — | — | — | ||||||||||||||
Dane A. Miller, Ph.D.(1) | — | — | — | — | — | 387,500 | 387,500 | ||||||||||||||
Max C. Lin(2) | — | — | — | — | — | — | — | ||||||||||||||
Jeffrey K. Rhodes(2) | — | — | — | — | — | — | — | ||||||||||||||
David McVeigh(2) | — | — | — | — | — | — | — | ||||||||||||||
Andrew Y. Rhee(2) | — | — | — | — | — | — | — | ||||||||||||||
___________________________________________________
| (1) | On January 14, 2010, the Company entered into a consulting agreement with Dr. Dane A. Miller Ph.D., pursuant to which it will pay Dr. Miller a consulting fee of $0.25 million per fiscal year for Dr. Miller’s consulting services and will reimburse Dr. Miller for out-of-pocket fees and expenses relating to an off-site office and administrative support in an amount of $0.1 million per year. The term of the agreement extends through the earlier of September 1, 2011, an initial public offering or a change of control. The agreement also contains certain restrictive covenants prohibiting Dr. Miller from competing with the Company and soliciting employees of the Company during the term of the agreement and for a period of one year following such term. Dr. Miller received $0.4 million of payment, under the consulting agreement during the year ended May 31, 2012. On September 6, 2011, the Company entered into an amendment to the consulting agreement with Dr. Miller, pursuant to which it agreed to increase the expenses relating to an off-site office and administrative support from $0.1 million per year to $0.15 million per year and extend the term of the agreement through the earlier of September 1, 2013, an initial public offering or a change of control. |
| (2) | Table excludes payments of an annual fee of $2.575 million that was paid to each of our Sponsors (or one or more of their affiliates) pursuant to our management services agreement for the fiscal year ended May 31, 2012 for services provided thereunder by employees of the Sponsors, which, may from time to time include the directors. No such services required substantial time or resources, nor were any employees specifically identified in the agreement as a service provider. Certain of our directors have relationships with the Sponsor entities which received such fees as follows: Messrs. Coslet and Rhodes are partners of TPG Capital; Messrs. Dal Bello and McVeigh are officers of certain affiliates of The Blackstone Group L.P.; Mr. Jones is a Managing Director and Mr. Rhee is a Vice President of Goldman, Sachs & Co.; and Messrs. Michelson and Lin are executives of Kohlberg Kravis Roberts & Co. L.P. None of the directors are compensated directly on the basis of fees received by the |
120
Sponsors under the management services agreement. Please see “Note 18-Related Parties—Management Services Agreement” to our audited financial statements included elsewhere in this prospectus. On October 26, 2012, pursuant to a written consent in lieu of an annual meeting of shareholders, all of the directors of Biomet were removed from the board of directors and a new slate of directors was elected. All of the existing directors of Biomet were re-elected pursuant to such written consent, with the exception of Todd Sisitsky, who was replaced on the board by Jeffrey K. Rhodes.
In addition, the Company has certain other relationships with the Sponsors from time to time, including a consulting engagement with KKR Capstone (a related party of Kohlberg Kravis Roberts & Co) as described under “Note 18—Related Parties” in notes to our audited financial statements included elsewhere in this prospectus. Neither Mr. Michelson nor Mr. Lin is employed by or is a director or officer of KKR Capstone.
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
Holding owns 97.0% of the issued and outstanding capital stock of Parent. All equity interests in Holding are owned, directly or indirectly, by the Sponsor Funds and the Co-Investors.
The following table sets forth information with respect to the ownership of as of February 28, 2013 for (a) each person known by us to own beneficially more than a 5% equity interest in Parent, (b) each member of our board of directors, (c) each of our named executive officers, and (d) all of our executive officers and directors as a group. Biomet has 1,000 shares of common stock outstanding, all of which are owned directly by Parent. Share amounts indicated below reflect beneficial ownership, indirectly through Holding or directly through Parent, by such entities or individuals of these 1,000 shares of Biomet
The amounts and percentages of shares beneficially owned are reported on the basis of SEC regulations governing the determination of beneficial ownership of securities. Under SEC rules, a person is deemed to be a “beneficial owner” of a security if that person has or shares voting power or investment power, which includes the power to dispose of or to direct the disposition of such security. A person is also deemed to be a beneficial owner of any securities of which that person has a right to acquire beneficial ownership within 60 days. Securities that can be so acquired are deemed to be outstanding for purposes of computing such person’s ownership percentage, but not for purposes of computing any other person’s percentage. Under these rules, more than one person may be deemed to be a beneficial owner of the same securities and a person may be deemed to be a beneficial owner of securities as to which such person has no economic interest.
Based solely on its review of the copies of the reports it has received, the Company believes that each of its executive officers and directors has complied with applicable reporting requirements for transactions in Company common stock during the fiscal year ended May 31, 2012, except for late Form 3s filed by its executive officers, directors and beneficial owners of more than 10% of Parent’s common stock.
Except as otherwise indicated in the footnotes below, each of the beneficial owners has, to our knowledge, sole voting and investment power with respect to the indicated shares. Unless otherwise noted, the address of each beneficial owner is c/o Biomet, Inc., 56 East Bell Drive, Warsaw, Indiana 46582.
121
| Name and address of Beneficial Owner | Beneficial Ownership of Biomet Common Shares | Percentage Owned | |
The Blackstone Group(1) | 237.2 | 23.72 | % |
The Goldman Sachs Group, Inc.(2) | 237.2 | 23.72 | % |
KKR Biomet, LLC(3) | 242.9 | 24.29 | % |
TPG Global(4) | 237.2 | 23.72 | % |
Jeffrey R. Binder(5) | 7.4 | 0.74 | % |
Daniel P. Florin(6) | 1.3 | 0.13 | % |
Jon C. Serbousek(7) | 1.3 | 0.13 | % |
Bradley J. Tandy(8) | 1.1 | 0.11 | % |
Robin T. Barney(9) | 1.2 | 0.12 | % |
Jonathan J. Coslet(10) | 0.0 | 0.00 | % |
Michael Dal Bello(11) | 0.0 | 0.00 | % |
Adrian Jones(12) | 0.0 | 0.00 | % |
Max Lin(13) | 0.0 | 0.00 | % |
Chinh E. Chu(11) | 0.0 | 0.00 | % |
Michal Michelson(13) | 0.0 | 0.00 | % |
Dane A. Miller(14) | 21.0 | 2.10 | % |
Andrew Y. Rhee(13) | 0.0 | 0.00 | % |
Jeffrey K. Rhodes(10) | 0.0 | 0.00 | % |
All executive officers and directors as a group (22 persons)(15) | 992.0 | 99.20 | % |
| (1) | The Blackstone Funds beneficially own 1,308,419.15815 membership units of Holding, including (i) 610,123.16500 membership units of Holding held by Blackstone Capital Partners V, L.P., (ii) 97,734.55100 membership units of Holding held by Blackstone Capital Partners V-AC L.P., (iii) 289,050.00000 membership units of Holding held by BCP V-S L.P., (iv) 13,731.75000 membership units of Holding held by Blackstone Family Investment Partnership V L.P., (v) 21,712.55300 membership units of Holding held by Blackstone Family Investment Partnership V-SMD L.P., (vi) 2,291.27315 membership units of Holding held by Blackstone Participation Partnership V L.P., and (vii) 273,775.86600 membership units of Holding held by BCP V Co-Investors L.P., (collectively, the “Blackstone Funds”). |
Blackstone Management Associates V L.L.C is the general partner of each of Blackstone Capital Partners V L.P., Blackstone Capital Partners V-AC L.P., BCP V-S L.P., and BCP V Co-Investors L.P. BMA V L.L.C. is the sole member of Blackstone Management Associates V L.L.C. BCP V Side-By-Side GP L.L.C. is the general partner of Blackstone Family Investment Partnership V L.P. and Blackstone Participation Partnership V L.P. Blackstone Family GP L.L.C. is the general partner of Blackstone Family Investment Partnership V-SMD L.P.
Blackstone Holdings III L.P. is the managing member and the owner of a majority in interest of BMA V L.L.C. and the sole member of BCP V Side-By-Side GP L.L.C. Blackstone Holdings III GP L.P is the general partner of Blackstone Holdings III L.P. The general partner of Blackstone Holdings III GP L.P. is Blackstone Holdings III GP Management L.L.C. The sole member of Blackstone Holdings III GP Management L.L.C. is The Blackstone Group L.P. The general partner of The Blackstone Group L.P. is Blackstone Group Management L.L.C. Blackstone Group Management L.L.C. is wholly owned by Blackstone’s senior managing directors and controlled by its founder, Stephen A. Schwarzman. Blackstone Family GP L.L.C. is wholly owned by Blackstone’s senior managing directors and controlled by its founder, Mr. Schwarzman. Each of such Blackstone entities and Mr. Schwarzman may be deemed to beneficially own the membership units beneficially owned by the Blackstone Funds directly or indirectly controlled by it or him, but each disclaims beneficial ownership of such membership units except to the extent of its or his indirect pecuniary interest therein. The address of Mr. Schwarzman and each of the other entities listed in this footnote is c/o The Blackstone Group L.P., 345 Park Avenue, New York, New York 10154.
| (2) | The Goldman Sachs Group, Inc. beneficially owns 1,308,419.15815 membership units of Holding, including (i) 433,679.15808 membership units of Holding held by GS Capital Partners VI Fund, L.P., (ii) 15,413.18755 membership units of Holding held by GS Capital Partners VI GmbH & Co. KG, (iii) 360,718.75833 membership units of Holding held by GS Capital Partners VI Offshore Fund, L.P., (iv) 119,253.84819 membership units of Holding held by GS Capital Partners VI Parallel, L.P., (v) 61,875.99000 membership units of Holding held by GS LVB Co-Invest, L.P., (vi) 63,137.95000 membership units of Holding held by Goldman Sachs BMET Investors, L.P., (vii) 184,785.45000 membership units of Holding held by Goldman Sachs BMET Investors Offshore Holdings, L.P., (viii) 44,463.81600 membership units of Holding held by GS PEP Bass Holdings, L.L.C., (ix) 6,309.80000 membership units of Holding held by Goldman Sachs Private Equity Partners, 2004-Direct Investment Fund, L.P., (x) 9,013.20000 membership units of Holding held by Goldman Sachs Private Equity Partners, 2005-Direct Investment Fund, L.P., and (xi) 9,768.00000 membership units of Holding held by Goldman Sachs Private Equity Partners IX-Direct Investment Fund, L.P. (collectively, the “GS Entities”) Affiliates of The Goldman Sachs Group, Inc. and Goldman, Sachs & Co. are the general partner, managing limited partner, managing partner or manager of the GS Entities. Goldman, Sachs & Co. is the investment manager for certain of the GS Entities. Goldman, Sachs & Co. is a direct and indirect wholly-owned subsidiary of The Goldman |
122
Sachs Group, Inc. The GS Entities share voting power and dispositive power with respect to the membership units of Holding beneficially owned by them with certain of their respective affiliates. Adrian Jones is a managing director and Andrew Y. Rhee is a vice president of Goldman, Sachs & Co. Each of Mr. Jones, Mr. Rhee and these entities disclaims beneficial ownership of these membership units, except to the extent of their pecuniary interest therein, if any. The address of the GS Entities and The Goldman Sachs Group, Inc. is c/o Goldman, Sachs & Co., 200 West Street, New York, NY 10282.
| (3) | KKR Biomet LLC beneficially owns 1,340,085.82482 membership units of Holding. The address of KKR Biomet, LLC is c/o Kohlberg Kravis Roberts & Co. L.P., 2800 Sand Hill Road, Suite 200, Menlo Park, CA 94025. KKR Biomet LLC is owned by the following entities (with percentage ownership of KKR Biomet LLC): KKR 2006 Fund L.P. (83.4%) (the “KKR 2006 Fund”), KKR PEI Investments, L.P. (11.3%)(“PEI Investments”), 8 North America Investor L.P. (3.6%)(“8 North America”), OPERF Co-Investment, LLC (0.7%)(“OPERF”), and KKR Partners III, L.P. (1.0%)(“KKR Partners III”). |
As the sole general partner of the KKR 2006 Fund and as the manager of OPERF, KKR Associates 2006 L.P. may be deemed to share voting and dispositive power with respect to any membership units beneficially owned by the KKR 2006 Fund and by OPERF but disclaims beneficial ownership of such membership units . As the sole general partner of KKR Associates 2006 L.P., KKR 2006 GP LLC may also be deemed to share voting and dispositive power with respect to any membership units beneficially owned by the KKR 2006 Fund and by OPERF but disclaims beneficial ownership of such membership units.
As the sole general partner of PEI Investments, KKR PEI Associates, L.P. may be deemed to share voting and dispositive power with respect to any membership units beneficially owned by PEI Investments but disclaims beneficial ownership of such membership units . As the sole general partner of KKR PEI Associates, L.P., KKR PEI GP Limited may also be deemed to share voting and dispositive power with respect to any membership units beneficially owned by PEI Investments but disclaims beneficial ownership of such membership units.
As the sole general partner of 8 North America, KKR Associates 8 NA L.P. may be deemed to share voting and dispositive power with respect to the membership units beneficially owned by 8 North America but disclaims beneficial ownership of such membership units. As the sole general partner of KKR Associates 8 NA L.P., KKR 8 NA Limited may be deemed to share voting and dispositive power with respect to the membership units beneficially owned by 8 North America but disclaims beneficial ownership of such membership units.
Each of KKR Fund Holdings L.P. (as the designated member of KKR 2006 GP LLC and the sole shareholder of KKR PEI GP Limited and KKR 8 NA Limited); KKR Fund Holdings GP Limited (as a general partner of KKR Fund Holdings L.P.); KKR Group Holdings L.P. (as a general partner of KKR Fund Holdings L.P. and the sole shareholder of KKR Fund Holdings GP Limited); KKR Group Limited (as the sole general partner of KKR Group Holdings L.P.); KKR & Co. L.P. (as the sole shareholder of KKR Group Limited) and KKR Management LLC (as the sole general partner of KKR & Co. L.P.) may be deemed to share voting and dispositive power with respect to the membership units beneficially owned by the KKR 2006 Fund, OPERF , PEI Investments and 8 North America . KKR Fund Holdings L.P., KKR Fund Holdings GP Limited, KKR Group Holdings L.P., KKR Group Limited, KKR & Co. L.P. and KKR Management LLC disclaim beneficial ownership of such membership units.
As the sole general partner of KKR Partners III, KKR III GP LLC may be deemed to share voting and dispositive power with respect to any membership units beneficially owned by KKR Partners III but disclaims beneficial ownership of such membership units.
As the designated members of KKR Management LLC and the managers of KKR III GP LLC, Henry R. Kravis and George R. Roberts may be deemed to share voting and dispositive power with respect to the membership units beneficially owned by the KKR 2006 Fund, OPERF, 8 North America, PEI Investments and KKR Partners III but disclaim beneficial ownership of such membership units.
| (4) | The TPG Funds (as defined below) beneficially owns 1,308,419.15815 membership units of Holding, including (i) 50,000.00000 membership units held by TPG Partners IV, L.P., a Delaware limited partnership (“TPG Partners IV”), whose general partner is TPG GenPar IV, L.P., a Delaware limited partnership, whose general partner is TPG GenPar IV Advisors, LLC, a Delaware limited liability company, whose sole member is TPG Holdings I, L.P., a Delaware limited partnership (“TPG Holdings”), (ii) 1,015,020.30532 membership units held by TPG Partners V, L.P., a Delaware limited partnership (“TPG Partners V”), whose general partner is TPG GenPar V, L.P., a Delaware limited partnership (“TPG GenPar V”), whose general partner is TPG GenPar V Advisors, LLC, a Delaware limited liability company, whose sole member is TPG Holdings, (iii) 2,655.60483 membership units held by TPG FOF V-A, L.P., a Delaware limited partnership (“TPG FOF A”), whose general partner is TPG GenPar V, (iv) 2,141.61680 membership units held by TPG FOF V-B, L.P., a Delaware limited partnership (“TPG FOF B”), whose general partner is TPG GenPar V, (v) 235,843.63020 membership units held by TPG LVB Co-Invest LLC, a Delaware limited liability company (“TPG Co-Invest I”), whose managing member is TPG GenPar V, (vi) 2,758.00100 membership units held by TPG LVB Co-Invest II LLC, a Delaware limited liability company (“TPG Co-Invest II”, and together with TPG Partners IV, TPG Partners V, TPG FOF A, TPG FOF B and TPG Co-Invest I, the “TPG Funds”), whose managing member is TPG GenPar V. The general partner of TPG Holdings is TPG Holdings I-A, LLC, a Delaware limited liability company, whose sole member is TPG Group Holdings (SBS), L.P., a Delaware limited partnership, whose general partner is TPG Group Holdings (SBS) Advisors, Inc., a Delaware corporation (“TPG Advisors”). David Bonderman and James G. Coulter are directors, officers and sole shareholders of TPG Advisors and may therefore be deemed to be the beneficial owners of the membership units held by the TPG Funds. Messrs. Bonderman and Coulter disclaim beneficial ownership of the shares held by the TPG Funds except to the extent of their pecuniary interest therein. The address of TPG Advisors and Messrs. Bonderman and Coulter is c/o TPG Global, LLC, 301 Commerce Street, Suite 3300, Fort Worth, TX 76102. |
| (5) | Biomet common shares shown as beneficially owned by Mr. Binder reflect an aggregate of the following beneficial ownership of common shares of Parent: (i) 147,500 common shares owned outright and (ii) 3,946,000 shares issuable upon (a) exercise of vested options and options that will vest within 60 days of this filing and (b) settlement of vested RSUs and RSUs that will settle within 60 days of this filing. |
123
| (6) | Biomet common shares shown as beneficially owned by Mr. Florin reflect an aggregate of the following beneficial ownership of common shares of Parent: (i) 60,000 common shares owned outright and (ii) 683,750 shares issuable upon (a) exercise of vested options and options that will vest within 60 days of this filing and (b) settlement of vested RSUs and RSUs that will settle within 60 days of this filing. |
| (7) | Biomet common shares shown as beneficially owned by Mr. Serbousek reflect an aggregate of the following beneficial ownership of common shares of Parent: (i) 15,000 common shares owned outright and (ii) 695,700 shares issuable upon (a) exercise of vested options and options that will vest within 60 days of this filing and (b) settlement of vested RSUs and RSUs that will settle within 60 days of this filing. |
| (8) | Biomet common shares shown as beneficially owned by Mr. Tandy reflect an aggregate of the following beneficial ownership of common shares of Parent: (i) 112,500 common shares owned outright and (ii) 471,000 shares issuable upon (a) exercise of vested options and options that will vest within 60 days of this filing and (b) settlement of vested RSUs and RSUs that will settle within 60 days of this filing. |
| (9) | Biomet common shares shown as beneficially owned by Ms. Barney reflect an aggregate of the following beneficial ownership of common shares of Parent: (i) 55,000 common shares owned outright and (ii) 617,250 shares issuable upon (a) exercise of vested options and options that will vest within 60 days of this filing and (b) settlement of vested RSUs and RSUs that will settle within 60 days of this filing. |
| (10) | Jonathan J. Coslet and Jeffrey K. Rhodes are each partners of TPG Global, LLC, which is an affiliate of the TPG Funds. Neither Mr. Coslet or Mr. Rhodes have voting or investment power over and each disclaim beneficial ownership of the membership units held by the TPG Funds and the Parent common shares held by Holding. The address of Messrs. Coslet and Rhodes is c/o TPG Global, LLC is 301 Commerce Street, Suite 3300, Fort Worth, TX 76102. |
| (11) | Michael Dal Bello and Chinh E. Chu are officers of affiliates of the Blackstone Funds and each such person disclaims beneficial ownership of the membership units held by the Blackstone Funds and the Parent common shares held by Holding. The address of each of Mr. Dal Bello and Mr. Chu is c/o The Blackstone Group, 345 Park Avenue, New York, NY 10154. |
| (12) | Each of Adrian Jones, managing director, and Andrew Y. Rhee, Vice President, may be deemed to be a beneficial owner of the membership units of Holding held by the GS Entities and the Parent common shares held by Holding due to his status with Goldman, Sachs & Co., and each such person disclaims beneficial ownership of any such interests in which he does not have a pecuniary interest. The address of Mr. Jones and Mr. Rhee is c/o Goldman, Sachs & Co., 200 West Street, New York, NY 10282. |
| (13) | Michael M. Michelson and Max C. Lin are executives of Kohlberg Kravis Roberts & Co. L.P. Affiliates of Kohlberg Kravis Roberts & Co. L.P. may be deemed to have beneficial ownership of 1,340,085.82482 membership units of Holdings and/or the Parent common shares held by Holding. Messrs. Michelson and Lin disclaim beneficial ownership of such membership units and common shares. The address of Messrs. Michelson and Lin is c/o Kohlberg Kravis Roberts & Co. L.P., 2800 Sand Hill Road, Suite 200, Menlo Park, CA 94025. |
| (14) | The business address of Dane A. Miller, Ph.D. is 700 Park Avenue, Suite G, Winona Lake, IN 46590. |
| (15) | Reflects beneficial ownership of common shares of Parent and is inclusive of 8,294,200 common shares of Parent issuable upon (a) exercise of vested options and options held by all executive officers and directors as a group that will vest within 60 days of this filing and (b) settlement of vested RSUs and RSUs that will settle within 60 days of this filing held by all executive officers and directors as a group. |
124
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
A description of our Company’s transactions with related persons is included in Note 17 to the audited consolidated financial statements and in Note 16 to the unaudited condensed consolidated interim financial statements, which are including elsewhere in this prospectus.
Pursuant to our Code of Business Conduct and Ethics, all employees and directors (including our named executives) are required to avoid any personal or business influences or relationships that affect their ability to act in the best interests of the Company. If any matter exists that might be or creates the appearance of being a conflict of interest, the matter is required to be referred to our Compliance Department for interpretation and resolution. The Compliance Department reviews all such matters under the standard set forth in our Code of Business Conduct and Ethics as described above and does not approve any related party transaction unless it is in, or not inconsistent with, our best interests and, where applicable, the terms of such transaction are at least as favorable to us as could be obtained from an unrelated third party. As part of the resolution of such matters, the Compliance Department may determine that (i) no actual conflict exists, (ii) a conflict does exist which cannot be remediated, resulting in the cessation of the proposed transaction or arrangement, or (iii) a potential conflict does exist but the risk of the potential conflict can be remediated practically by imposing certain limitations on the affected employees or business transaction to ensure that the conflict does not materialize. Additionally, the LLC Agreement requires that affiliated party transactions involving the Sponsors to be approved by a super-majority of Sponsors not involved in the affiliated party transaction.
Other than as described under this heading, we have not adopted any formal policies or procedures for the review, approval or ratification of related-party transactions that may be required to be reported under the SEC’s disclosure rules. Such transactions, if and when they are proposed or have occurred, have traditionally been (and will continue to be) reviewed by one or more of the Board of Directors, the Audit Committee or the Compensation Committee (other than the directors or committee members involved, if any) on a case-by-case basis, depending on whether the nature of the transaction would otherwise be under the purview of the Audit Committee, Compensation Committee or the Board of Directors.
125
DESCRIPTION OF OTHER INDEBTEDNESS
The description of other indebtedness in this section relates to the terms of the senior secured cash flow facilities and the senior secured asset-based revolving credit facility. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Credit Facilities and Notes” for additional information.
Senior Secured Cash Flow Facilities
On September 25, 2007, we entered into a credit agreement (the “2007 Cash Flow Credit Agreement”) and related security and other agreements providing for (a) a $2,340.0 million U.S. dollar-denominated term loan facility and a €875.0 million (approximately $1,207.4 million at September 25, 2007) euro-denominated term loan facility (collectively, the “Initial Term Facility”) and (b) $400.0 million cash flow revolving credit facilities (the “Initial Revolving Facilities”) with Bank of America, N.A. as administrative agent and collateral agent. We refer to our term loan facilities and our cash flow revolving credit facilities collectively as the “senior secured credit facilities.” The Initial Revolving Facilities include a $100.0 million sub-facility for letters of credit and a $100.0 million sub-capacity for borrowings on same-day notice, referred to as swingline loans.
On August 2, 2012, we entered into an amendment and restatement agreement that amended our existing senior secured credit facilities. The amendment (i) extended the maturity of approximately $1,007.2 million of our U.S. dollar-denominated term loans and approximately €631.3 million of our euro-denominated term loans under the credit facility to July 25, 2017, (ii) refinanced and replaced the previous alternative currency revolving credit commitments under the credit facility with a new class of alternative currency revolving credit commitments in an aggregate amount of $165.0 million and (iii) refinanced and replaced the previous U.S. dollar revolving credit commitments under the credit facility with a new class of U.S. dollar-denominated revolving credit commitments in an aggregate amount of $165.0 million. The new revolving credit commitments will mature on April 25, 2017, except that, if as of December 23, 2014, there is an outstanding aggregate principal amount of non-extended U.S. dollar and euro term loans in excess of $200.0 million, then such revolving credit commitments will mature on December 24, 2014.
The joinder agreement was entered into pursuant to our senior secured credit facility, as amended by the amendment and restatement agreement dated August 2, 2012. By entering into the joinder agreement, the joining lenders party thereto have agreed to extend the maturity of (i) approximately $392.7 million of Biomet’s U.S. dollar-denominated term loans and (ii) approximately €32.9 million of Biomet’s euro-denominated term loans, to July 25, 2017. The term loans extended pursuant to the joinder agreement are on terms identical to the terms loans that were extended pursuant to the prior Amendment. The remaining term loans of the lenders under the senior secured credit facilities who did not elect to extend such loans either pursuant to the August 2 amendment and restatement agreement or the subsequent joinder agreement will continue to mature on March 25, 2015.
In addition, on December 27, 2012, we completed a $730.0 million add-on to our extended U.S. dollar-denominated term loan. The proceeds from the add-on were used to refinance the non-extended U.S. dollar-denominated term B loan, which was net of fees associated with the add-on closing. The terms of the add-on are consistent with the terms in the August 2 amendment.
As of February 28, 2013, we had approximately $3,311.7 million outstanding under the term facilities of which approximately $324.7 million matures on the original maturity date of March 25, 2015 and approximately $2,987.0 million matures on the extend maturity date of July 25, 2017. As of February 28, 2013 we had no balance under the revolving facilities.
The following is a summary of the material terms of the senior secured credit facilities.
Interest Rate and Fees
Borrowings under our senior secured credit facilities bear interest at a rate per annum equal to an applicable margin plus, at our option, either (1) a base rate determined by reference to the higher of (a) the prime rate of Bank of America, N.A., (b) the federal funds effective rate plus 1/2 of 1.00% or (c) a BBA LIBOR rate plus 1.00% or (2) a LIBOR or Eurocurrency rate determined by reference to the cost of funds for deposits in the currency of such borrowing for the interest period relevant to such borrowing adjusted for certain additional costs; provided, that the applicable margins for revolving loans will be subject to (a) a 25 basis point reduction for any quarter if the Senior Secured Leverage Ratio is less than or equal to 2.5 to 1.0 and (b) an additional 25 basis point reduction for any
126
quarter if the Senior Secured Leverage Ratio is less than 2.0 to 1.0. For the extended term loans, the applicable margin is 2.75% with respect to base rate borrowings, 3.75% with respect to Dollar-denominated LIBOR borrowings and 4.00% with respect to Euro-denominated LIBOR borrowings. For revolving loans, the applicable margin is initially up to 2.50% with respect to base rate borrowings and initially up to 3.50% for LIBOR borrowings. Our euro-denominated term loan facility can only be LIBOR-based borrowings. We are entered into a series of interest rate swap agreements which at February 28, 2013 had (1) an aggregate notional amount of $1,410.0 million to fix the interest rates on a portion of the borrowings under the U.S. dollar-denominated term loan facility and (2) an aggregate notional amount of €440.0 million (approximately $575.7 million outstanding at February 28, 2013) to fix the interest rates on a portion of the borrowings under the euro-denominated term loan facility (approximately $1,085.0 million outstanding at February 28, 2013). See “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Quantitative and Qualitative Disclosures about Market Risk—Interest Rate Risk.”
In addition to paying interest on outstanding capital under our cash flow revolving credit facilities, we are required to pay a commitment fee to the lender under the cash flow revolving credit facilities in respect of the unutilized commitments thereunder at an initial rate equal to 0.50% per annum during the first fiscal year after the effectiveness of the Amended and Restated Credit Agreement, subject to reduction to 0.375% per annum if after the first fiscal quarter the senior secured leverage ratio is less than 2.0 to 1.0. We also pay customary letter of credit and agency fees.
Mandatory Repayments
The credit agreement governing our senior secured credit facilities requires us to prepay outstanding term loans, subject to certain exceptions: (1) after our first full fiscal year after the closing date, 50% (which percentage may be reduced to 25% if our senior secured leverage ratio is less than 4.0 to 1.0 and may be further reduced to 0% if our senior secured leverage ratio is less than 3.5 to 1.0) of our annual excess cash flow (as defined in our Amended and Restated Cash Flow Credit Agreement); (2) if our Senior Secured Leverage Ratio is greater than 4.0 to 1.0, 100% (which percentage may be reduced to 50% if our senior secured leverage ratio is less than 4.0 to 1.0 and may be further reduced to 0% if our senior secured leverage ratio is less than 3.0 to 1.0) of the net cash proceeds of certain non-ordinary course asset sales and casualty and condemnation events, if we do not reinvest those proceeds in assets to be used in our business or to make certain other permitted investments; and (3) 100% of the net cash proceeds of debt issuances. No prepayments on the above mentioned debt were required under the credit agreement in the fiscal year ended May 31, 2012.
Voluntary Prepayments
We may voluntarily prepay outstanding loans under our senior secured credit facilities at any time with customary “breakage” costs with respect to LIBOR or Eurocurrency loans. Voluntary prepayments made in the first year after the effectiveness of the Amended and Restated Cash Flow Credit Agreement may be subject to a repricing premium of 1.0% of the loans to be repaid.
Mandatory Payments and Final Maturity
Our term loan facilities require payments each year in an amount equal to (x) 0.25% of the product of (i) the aggregate principal amount of all euro-denominated term loans and dollar-denominated term loans outstanding under the original credit agreement on the closing date multiplied by (ii) a fraction, the numerator of which is the aggregate principal amount of euro-denominated term B loans and dollar-denominated term B loans outstanding on August 2, 2012 (after giving effect to certain conversions to occur on or after August 2, 2012 pursuant to the amended and restated credit agreement) and the denominator of which is the aggregate principal amount of all outstanding term loans on August 2, 2012 and (y) 0.25% of the aggregate principal amount of all outstanding euro-denominated term B-1 loans and dollar-denominated term B-1, in each case in equal calendar quarterly installments until maturity of the loan and after giving effect to the application of any prepayments. The principal amount outstanding under our cash flow revolving credit facilities will be due and payable in full at maturity. The extended term loans under the term loan facility will mature on July 25, 2017. The new revolving credit commitments will mature on April 25, 2017; provided, however, that if as of December 23, 2014, there is an outstanding aggregate principal amount of non-extended Dollar and Euro term loans under the Initial Term Facility in excess of $200.0 million, then such revolving credit commitments will mature on December 24, 2014. The remaining term loans under the Initial Term Facility of the lenders who did not elect to extend such loans will continue to mature on March 25, 2015.
127
Extensions, Refinancings and Incremental Credit Extensions
We may, under the terms of the Amended and Restated Cash Flow Credit Agreement and without further approval from a majority of lenders, (a) extend the revolving commitments and term loans in one or more series of tranches, (b) refinance the revolving facilities and term loans with one or more new facilities with secured or unsecured notes or loans, and (c) issue incremental credit in the form of incremental term loans, revolving commitment increases or additional secured or unsecured notes or loans, so long as at the time of such extension or refinancing, as applicable, and the effectiveness of any incremental commitment or facility, we maintain a senior secured leverage ratio of 4.5 to 1.0.
Guarantees and Security
All obligations under our senior secured credit facilities are unconditionally guaranteed by Parent, and, subject to certain exceptions, each of our existing and future direct and indirect wholly-owned domestic subsidiaries. All obligations under our senior secured credit facilities, and the guarantees of those obligations, are secured, subject to certain exceptions, by substantially all of our assets and the assets of Parent and the subsidiary guarantors, including:
| • | a first-priority pledge of 100% of our capital stock and certain of the capital stock held by us or any subsidiary guarantor (which pledge, in the case of any foreign subsidiary shall be limited to 100% of the non-voting stock (if any) and 65% of the voting stock of such foreign subsidiary), in each case excluding any interests in joint ventures to the extent such a pledge would violate the governing documents thereof; |
| • | a first-priority security interest in, and mortgages on, substantially all other tangible and intangible assets of us, Parent and each subsidiary guarantor, but excluding the collateral described in the following bullet point; and |
| • | a second-priority security interest in personal property of consisting of all accounts receivable (except assets subject to any permitted receivables facility), inventory, cash, deposit accounts and certain related intangible assets and proceeds of the foregoing. |
Certain Covenants and Events of Default
Our senior secured credit facilities contain a number of covenants that, among other things are subject to certain exceptions, will restrict our ability and the ability of our restricted subsidiaries to:
| • | incur additional indebtedness; |
| • | pay dividends on our capital stock or redeem, repurchase or retire our capital stock or indebtedness; |
| • | make investments, loans, advances and acquisitions; |
| • | create restrictions on the payment of dividends or other amounts to us from our restricted subsidiaries; |
| • | engage in transactions with our affiliates; |
| • | sell assets, including capital stock of our subsidiaries; |
| • | consolidate or merge; |
| • | create liens; and |
| • | enter into sale and lease-back transactions. |
128
In addition, the credit agreement governing our senior secured credit facilities does not require us to comply with any financial ratio maintenance covenants. As of February 28, 2013, we were in compliance with our covenants and intend to maintain compliance.
The credit agreement governing our senior secured credit facilities also contains certain customary affirmative covenants and events of default.
Senior Secured Asset-Based Revolving Credit Facility
Overview
On September 25, 2007, we entered into a credit agreement and related security and other agreements for an asset-based revolving credit facility with Bank of America, N.A. as administrative agent and collateral agent. Our asset-based revolving credit facility provides senior secured financing of up to $350.0 million, subject to borrowing base limitations. The borrowing base at any time will equal the sum of 85% of eligible accounts receivable and 85% of the net orderly liquidation value of eligible inventory (not to exceed 65% of the borrowing base), less certain reserves and subject to certain limitations on consigned inventory and accounts receivable owed by non-U.S. persons. Our asset- based revolving credit facility includes a $100.0 million sub-facility for letters of credit and a $35.0 million sub-facility for borrowings on same-day notice, referred to as swingline loans.
On November 14, 2012, we replaced and refinanced its asset-based revolving credit facility with a new asset-based revolving credit facility that has a U.S. tranche of up to $400.0 million and a European borrower tranche denominated in euros of up to the euro-equivalent of $100.0 million. The European borrower tranche is secured by certain foreign assets of European subsidiary borrowers and the U.S. borrowers under the U.S. tranche guarantee the obligations of any such European subsidiary borrowers (and such guarantees are secured by the current assets collateral that secures the direct obligations of such U.S. borrowers under such U.S. tranche).
There were no borrowings outstanding under our asset-based revolving credit facility as of February 28, 2013. As of February 28, 2013, the borrowing base under our asset-based revolving credit facility was $465.5 million, which is net of borrowing base limitations relating to the asset-based revolving credit facility.
The following is a summary of the material terms of the senior secured asset-based revolving credit facility.
Interest Rate and Fees
Borrowings under the asset-based credit agreement bear interest at a rate per annum dependent upon the average availability of the applicable subfacility as set forth in the following pricing grid:
Average Availability | Adjusted Eurocurrency Rate for Loans and Letter of Credit Fees | Base Rate | ||
≥66 2/3% | 1.75% | 0.75% | ||
<66 2/3% but ≥33 1/3% | 2.00% | 1.00% | ||
<33 1/3% | 2.25% | 1.25% | ||
In addition, the we are required to pay a commitment fee of (i) 0.25% per annum if the amount of outstanding loans, unreimbursed letter of credit drawings and undrawn letters of credit under the senior secured asset-based revolving credit facility exceed 50% of the commitment amount, and (ii) if otherwise, 0.375% per annum, on the average daily unused portion of the senior secured asset-based revolving credit facility, payable quarterly in arrears. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Credit Facilities and Notes” for additional information.
Mandatory Repayments
If at any time the aggregate amount of outstanding loans, unreimbursed letter of credit drawings and undrawn letters of credit under our asset-based revolving credit facility exceeds the commitment amount , we will be required to repay outstanding loans or cash collateralize letters of credit in an aggregate amount equal to such excess, with no reduction of the commitment amount. All obligations under our asset-based revolving credit facility are unconditionally guaranteed by Parent.
129
Voluntary Repayments
We may voluntarily reduce the unutilized portion of the commitment amount and repay outstanding loans at any time without premium or penalty, other than customary “breakage” costs with respect to LIBOR or Eurocurrency loans.
Mandatory Payments and Final Maturity
There are no scheduled mandatory payments under our senior secured asset-based revolving credit facility. The senior secured asset-based revolving credit facility will mature on July 25, 2017; provided, however, that if as of December 23, 2014, there is an outstanding aggregate principal amount of non-extended U.S. dollar and euro term loans in excess of $200 million under our cash flow credit agreement, then the loans under the Credit Agreement will mature on December 24, 2014.
Guarantee and Security
The European borrower tranche is secured by certain foreign assets of European subsidiary borrowers and the U.S. borrowers under the U.S. tranche guarantee the obligations of any such European subsidiary borrowers (and such guarantees are secured by the current assets collateral that secures the direct obligations of such U.S. borrowers under such U.S. tranche).
Certain Covenants and Events of Default
Our asset-based revolving credit facility contains a number of covenants that restrict our ability and the ability of our restricted subsidiaries to:
| • | incur additional indebtedness; |
| • | pay dividends on our capital stock or redeem, repurchase or retire our capital stock or indebtedness; |
| • | make investments, loans, advances and acquisitions; |
| • | create restrictions on the payment of dividends or other amounts to us from our restricted subsidiaries; |
| • | engage in transactions with our affiliates; |
| • | sell assets, including capital stock of our subsidiaries; |
| • | consolidate or merge; |
| • | create liens; and |
| • | enter into sale and lease-back transactions. |
The terms of our senior secured credit facilities also restrict Parent from conducting any business or operations other than, among others, (i) owning Biomet, (ii) maintaining its legal existence, (iii) performing its obligations with respect to the senior secured credit facilities and the indentures governing the notes, (iv) publicly offering its common stock, (v) financing activities, including the issuance of securities, incurrence of debt, payment of dividends, making contributions to the capital of its subsidiaries and guaranteeing the obligations of its subsidiaries, or (vi) providing indemnification to its officers and directors.
In addition, although the agreements governing our senior secured credit facilities and the indentures governing the notes do not require us to comply with any financial ratio maintenance covenants, if Excess Global Availability (as such term is defined in the asset-based revolving credit facility) is less than 10% of the sum of (1) aggregate commitments under our asset-based revolving credit facility plus (2) the revolving credit commitments under our cash flow credit facility at any time, the fixed charge coverage ratio as of the end of the most recently ended fiscal quarter must be greater than or equal to 1.00 to 1.00. In the event of a default under any of our senior secured credit facilities, the lenders could elect to declare all amounts outstanding under the agreements governing
130
our senior secured credit facilities to be immediately due and payable. If the indebtedness under our senior secured credit facilities, or the notes were to be accelerated, our assets may not be sufficient to repay such indebtedness in full. In particular, noteholders will be paid only if we have assets remaining after we pay amounts due on our secured indebtedness, including our senior secured credit facilities.
The credit agreement governing our asset-based revolving credit facility also contains certain customary affirmative covenants and events of default. As of February 28, 2013, we were in compliance with our covenants and intend to maintain compliance.
Non-U.S. Facility
As of February 28, 2013, we had a loan in Spain, referred to as the non-U.S. facility with $2.6 million in outstanding borrowings.
131
DESCRIPTION OF EXCHANGE SENIOR NOTES
General
Certain terms used in this description are defined under the subheading “—Certain Definitions.” In this description, (1) the term “Issuer” refers only to Biomet, Inc. and not to any of its subsidiaries and (2) the terms “we,” “our” and “us” each refer to the Issuer and its consolidated Subsidiaries, and (3) the term “senior notes” refers to the original senior notes and the exchange senior notes collectively. Defined terms in this “Description of Exchange Senior Notes” may have different meanings than similar or identical terms used in the “Description of Exchange Senior Subordinated Notes” below. For example, references to “Guarantees” or “Guarantors” in this “Description of Exchange Senior Notes” means guarantees or guarantors of the senior notes, and not guarantees or guarantors of the senior subordinated notes.
The Issuer previously issued $1,000 million aggregate principal amount of original senior notes under an indenture dated as of August 8, 2012 and $825.0 million of original senior notes under a supplemental indenture dated as of October 2, 2012 (collectively, the “senior notes indenture”) among the Issuer, the Guarantors and Wells Fargo Bank, National Association, as trustee (the “Trustee”). The Issuer will issue up to $1,825 million aggregate principal amount of exchange senior notes offered hereby under the senior notes indenture.
The senior notes indenture has been qualified under and is subject to and governed by the Trust Indenture Act of 1939. Except as set forth herein, the terms of the exchange senior notes will be substantially identical in all material respects and include those stated in the senior notes indenture and those made part of the senior notes indenture by reference to the Trust Indenture Act.
The following description is only a summary of the material provisions of the senior notes indenture, does not purport to be complete and is qualified in its entirety by reference to the provisions of the senior notes indenture, including the definitions therein of certain terms used below. We urge you to read the senior notes indenture because it, and not this description, will define your rights as Holders of the senior notes. You may request copies of the senior notes indenture at our address set forth under “Where You Can Find Additional Information.”
Exchange Senior Notes versus Original Senior Notes
The terms of the exchange senior notes are substantially identical in all material respects to the original senior notes except upon completion of the exchange offers, the offer and sale of the exchange senior notes will have been registered under the Securities Act, and the exchange senior notes will be free of any covenants regarding exchange registration rights.
Brief Description of the Exchange Senior Notes
The exchange senior notes:
| • | will be general, unsecured, senior obligations of the Issuer; |
| • | will rank equally in right of payment with all existing and future Senior Indebtedness (including the Senior Credit Facilities) of the Issuer; |
| • | will be effectively subordinated to all Secured Indebtedness of the Issuer (including the Senior Credit Facilities), to the extent of the value of the collateral securing such Secured Indebtedness; |
| • | will be structurally subordinated to all existing and future Indebtedness, claims of holders of Preferred Stock and other liabilities of Subsidiaries of the Issuer that do not guarantee the senior notes; |
| • | will be senior in right of payment to all existing and future Subordinated Indebtedness of the Issuer; and |
| • | will be initially guaranteed on a senior unsecured basis by the Guarantors and will also be guaranteed in the future by each Subsidiary, if any, that guarantees Indebtedness under the CF Credit Facilities. |
132
Guarantees
The Guarantors, as primary obligors and not merely as sureties, will initially jointly and severally, irrevocably and unconditionally, guarantee, on an unsecured senior basis, the full and punctual payment when due, whether at maturity, by acceleration or otherwise, of all obligations of the Issuer under the senior notes indenture and the senior notes, whether for payment of principal of, premium, if any, or interest in respect of the senior notes, expenses, indemnification or otherwise, on the terms set forth in the senior notes indenture by executing the senior notes indenture.
The Guarantors are initially guaranteeing the senior notes and, in the future, each direct and indirect Subsidiary of the Issuer that guarantees Indebtedness under the CF Credit Facilities will guarantee the senior notes. Each of the Guarantees of the senior notes is a general, unsecured, senior obligation of each Guarantor, rank equally in right of payment with all existing and future Senior Indebtedness of such Guarantor (including such Guarantor’s guarantee of the CF Credit Facilities), is effectively subordinated to all Secured Indebtedness of such Guarantor (including such Guarantor’s guarantee of the CF Credit Facilities), to the extent of the value of the collateral securing such Secured Indebtedness, and ranks senior in right of payment to all existing and future Subordinated Indebtedness of such Guarantor (including such Guarantor’s guarantee of the Existing Subordinated Notes and the senior subordinated notes). Each of the Guarantees of the senior notes is structurally subordinated to all existing and future Indebtedness, claims of holders of Preferred Stock and other liabilities of Subsidiaries of each Guarantor that do not guarantee the senior notes.
Not all of the Issuer’s Subsidiaries guarantee the senior notes. In the event of a bankruptcy, liquidation, reorganization or similar proceeding of any of these non-guarantor Subsidiaries, the non-guarantor Subsidiaries will pay the holders of their debt and their trade creditors before they will be able to distribute any of their assets to the Issuer. As a result, all of the existing and future liabilities of our non-guarantor Subsidiaries, including any claims of trade creditors, are effectively senior to the senior notes. For the nine months ended February 28, 2013, the non-guarantor Subsidiaries of the Issuer accounted for approximately $830.4 million, or 37% of the Issuer’s consolidated net sales. As of February 28, 2013, the non-guarantor Subsidiaries of the Issuer accounted for approximately $2,806.9 million, or 28% of the Issuer’s consolidated total assets and approximately $409.6 million, or 5.3% of the Issuer’s consolidated liabilities.
The obligations of each Guarantor under its Guarantee will be limited as necessary to prevent the Guarantee from constituting a fraudulent conveyance under applicable law. This provision may not, however, be effective to protect a Guarantee from being voided under fraudulent transfer law, or may reduce the applicable Guarantor’s obligation to an amount that effectively makes its Guarantee worthless. If a Guarantee was rendered voidable, it could be subordinated by a court to all other indebtedness (including guarantees and other contingent liabilities) of the Guarantor, and, depending on the amount of such indebtedness, a Guarantor’s liability on its Guarantee could be reduced to zero. See “Risk Factors—Risks Related to Our Indebtedness and the Notes—Federal and state fraudulent transfer laws may permit a court to void the notes and the guarantees, subordinate claims in respect of the notes and the guarantees, and require noteholders to return payments received. If this occurs, noteholders may not receive any payments on the notes.”
Any Guarantor that makes a payment under its Guarantee will be entitled upon payment in full of all guaranteed obligations under the senior notes indenture to a contribution from each other Guarantor in an amount equal to such other Guarantor’s pro rata portion of such payment based on the respective net assets of all the Guarantors at the time of such payment determined in accordance with GAAP.
Each Guarantor may consolidate with or merge into or sell all or substantially all of its assets to the Issuer or another Guarantor without limitation or any other Person upon the terms and conditions set forth in the senior notes indenture. See “—Certain Covenants—Merger, Consolidation or Sale of All or Substantially All Assets.”
Each Guarantee by a Guarantor provides by its terms that it will be automatically and unconditionally released and discharged upon:
(1) (a) any sale, exchange or transfer (by merger or otherwise) of (i) the Capital Stock of such Guarantor, after which the applicable Guarantor is no longer a Restricted Subsidiary or (ii) all or substantially all the assets of such Guarantor, in each case if such sale, exchange or transfer is made in compliance with the applicable provisions of the senior notes indenture;
(b) the release or discharge of the guarantee by such Guarantor of Indebtedness under the CF Credit Facilities, or the release or discharge of such other guarantee that resulted in the creation of such Guarantee, except a discharge or release by or as a result of payment under such guarantee;
133
(c) upon the dissolution of such Guarantor; provided that no Default or Event of Default has occurred and is continuing;
(d) the designation of any Restricted Subsidiary that is a Guarantor as an Unrestricted Subsidiary in compliance with the applicable provisions of the senior notes indenture; or
(e) the exercise by the Issuer of its legal defeasance option or covenant defeasance option as described under “Legal Defeasance and Covenant Defeasance” or the discharge of the Issuer’s obligations under the senior notes indenture in accordance with the terms of the senior notes indenture; and
(2) such Guarantor delivering to the Trustee an Officer’s Certificate and an Opinion of Counsel, each stating that all conditions precedent provided for in the senior notes indenture relating to such transaction have been complied with.
Ranking
The payment of the principal of, premium, if any, and interest on the senior notes and the payment of any Guarantee ranks equally in right of payment to all existing and future Senior Indebtedness of the Issuer or the relevant Guarantor, as the case may be, including the obligations of the Issuer and such Guarantor under the Senior Credit Facilities and the senior notes.
The senior notes and the Guarantees are effectively subordinated in right of payment to all of the Issuer’s and the Guarantors’ existing and future Secured Indebtedness to the extent of the value of the collateral securing such Secured Indebtedness. As of February 28, 2013, the Issuer and the Guarantors had $3,311.7 million of Secured Indebtedness outstanding, consisting of borrowings and the related guarantees under the Senior Credit Facilities. As of February 28, 2013, the Issuer had (1) the option to raise additional incremental term loans or incremental cash flow revolving facility commitments under the CF Credit Facilities of up to an amount that would cause our Senior Secured Leverage Ratio (as defined in the CF Credit Facilities) to be equal to or less than 4.50 to 1.00, which, if borrowed, would be Secured Indebtedness and (2) the option to raise additional incremental asset- based revolving credit facility commitments under the ABL Facilities by up to $100.0 million, which, if borrowed, would be Secured Indebtedness.
Although the senior notes indenture contains limitations on the amount of additional Indebtedness that the Issuer and the Issuer’s Restricted Subsidiaries (including the Guarantors) may incur, under certain circumstances the amount of such Indebtedness could be substantial and, in any case, such Indebtedness may be Senior Indebtedness. The senior notes indenture does not limit the amount of additional Indebtedness that the Parent may incur. See “—Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock.”
Paying Agent and Registrar for the Exchange Senior Notes
The Issuer will maintain one or more paying agents for the exchange senior notes. The initial paying agent for the exchange senior notes is the Trustee.
The Issuer also maintains one or more registrars and a transfer agent. The initial registrar and transfer agent with respect to the exchange senior notes is the Trustee. The registrar maintains a register reflecting ownership of the exchange senior notes outstanding from time to time. The registered Holder of an exchange senior note will be treated as the owner of the exchange senior notes for all purposes. The transfer agent will make payments on and facilitate transfer of exchange senior notes on behalf of the Issuer.
The Issuer may change the paying agent, the registrar or the transfer agent without prior notice to the Holders. The Issuer or any of its Subsidiaries may act as a paying agent, registrar or transfer agent.
If the exchange senior notes are listed on an exchange and the rules of such exchange so require, the Issuer will satisfy any requirement of such exchange as to paying agents, registrars and transfer agents and will comply with any notice requirements required under such exchange in connection with any change of paying agent, registrar or transfer agent.
Transfer and Exchange
A Holder may transfer or exchange senior notes in accordance with the senior notes indenture. The registrar and the Trustee may require a Holder to furnish appropriate endorsements and transfer documents in connection with a
134
transfer of exchange senior notes. Holders will be required to pay all taxes due on transfer. The Issuer will not be required to transfer or exchange any senior note selected for redemption or tendered (and not withdrawn) for repurchase in connection with a Change of Control Offer or an Asset Sale Offer. Also, the Issuer will not be required to transfer or exchange any senior note for a period of 15 days before a selection of senior notes to be redeemed.
Principal, Maturity and Interest
The Issuer will issue up to an aggregate principal amount of $1,825.0 million of exchange senior notes in this offering. The exchange senior notes will mature on August 1, 2020. Subject to compliance with the covenant described below under “—Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock,” the Issuer may issue further additional senior notes from time to time after this offering under the senior notes indenture (“Additional Senior Notes”). The exchange senior notes offered by the Issuer and any Additional Senior Notes subsequently issued under the senior notes indenture will be treated as a single class for all purposes under the senior notes indenture, including waivers, amendments, redemptions and offers to purchase, except for certain waivers and amendments. Unless the context requires otherwise, references to “senior notes” for all purposes of the senior notes indenture and this “Description of Exchange Senior Notes” include any Additional Senior Notes that are actually issued. The exchange senior notes will be issued in denominations of $1,000 and any integral multiples of $1,000 in excess of $1,000.
Interest Payments
Interest on the exchange senior notes will accrue at the rate of 6.500% per annum. Interest on the exchange senior notes will be payable semi-annually in arrears on each August 1 and February 1, commencing August 1, 2013 to the Holders of exchange senior notes of record on the immediately preceding July 15 and January 15. Interest on the exchange senior notes will accrue from the most recent date to which interest has been paid or, if no interest has been paid, from and including the Issue Date. Interest on the senior notes will be computed on the basis of a 360-day year comprised of twelve 30-day months.
Additional Interest
Additional Interest may accrue on the exchange senior notes in certain circumstances pursuant to the Senior Notes Registration Rights Agreements, or as described under “Events of Default and Remedies.” All references in the senior notes indenture and this “Description of Exchange Senior Notes,” in any context, to any interest or other amount payable on or with respect to the senior notes shall be deemed to include any Additional Interest payable pursuant to the Senior Notes Registration Rights Agreements and under “Events of Default and Remedies.”
Payment of Principal, Premium and Interest
Payments of principal of, premium, if any, and interest on the exchange senior notes will be payable at the office or agency of the Issuer maintained for such purpose or, at the option of the Issuer, payment of interest may be made by check mailed to the Holders of the exchange senior notes at their respective addresses set forth in the register of Holders; provided that (1) all payments of principal, premium, if any, and interest with respect to the exchange senior notes represented by one or more global notes registered in the name of or held by DTC or its nominee will be made by wire transfer of immediately available funds to the accounts specified by the Holder or Holders thereof and (2) all payments of principal, premium, if any, and interest with respect to certificated exchange senior notes will be made by wire transfer to a U.S. dollar account maintained by the payee with a bank in the United States if such Holder elects payment by wire transfer by giving written notice to the Trustee or the paying agent to such effect designating such account no later than 30 days immediately preceding the relevant due date for payment (or such other date as the Trustee may accept in its discretion). Until otherwise designated by the Issuer, the Issuer’s office or agency will be the office of the Trustee maintained for such purpose.
Mandatory Redemption; Offers to Purchase; Open Market Purchases
The Issuer is not required to make any mandatory redemption or sinking fund payments with respect to the senior notes. However, under certain circumstances, the Issuer may be required to offer to purchase senior notes as described under “Repurchase at the Option of Holders.” The Issuer may at any time and from time to time purchase senior notes in the open market or otherwise.
Optional Redemption
135
Except as set forth below, the Issuer is not entitled to redeem the exchange senior notes at its option prior to August 1, 2015.
At any time prior to August 1, 2015, the Issuer may redeem all or a part of the exchange senior notes, upon notice as described under “—Selection and Notice,” at a redemption price equal to 100% of the principal amount of the exchange senior notes redeemed plus the Applicable Premium as of, plus accrued and unpaid interest, if any, to the date of redemption (the “Redemption Date”), subject to the right of Holders of record on the relevant record date to receive interest due on the relevant interest payment date.
On and after August 1, 2015, the Issuer may redeem the exchange senior notes, in whole or in part, upon notice as described under “—Selection and Notice,” at the redemption prices (expressed as percentages of principal amount of the senior notes to be redeemed) set forth below, plus accrued and unpaid interest, if any, to the Redemption Date, subject to the right of Holders of record on the relevant record date to receive interest due on the relevant interest payment date, if redeemed during the twelve-month period beginning on August 1 of each of the years indicated below:
| Year | Percentage |
| 2015 | 104.875% |
| 2016 | 103.250% |
| 2017 | 101.625% |
| 2018 and thereafter | 100.000% |
In addition, until August 1, 2015, the Issuer may, at its option, redeem up to 35% of the aggregate principal amount of senior notes issued under the senior notes indenture at a redemption price equal to 106.500% of the aggregate principal amount thereof, plus accrued and unpaid interest, if any, to the Redemption Date, subject to the right of Holders of senior notes of record on the relevant record date to receive interest due on the relevant interest payment date, with the net cash proceeds received by it from one or more Equity Offerings; provided that (a) at least 50.0% of the sum of the aggregate principal amount of senior notes originally issued under the senior notes indenture on the Issue Date and any Additional Senior Notes that are issued under the senior notes indenture after the Issue Date remains outstanding immediately after the occurrence of each such redemption; and (b) each such redemption occurs within 180 days of the date of closing of each such Equity Offering.
Notice of any redemption upon any Equity Offering may be given prior to the completion thereof. All redemptions or notices may, at the Issuer’s discretion, be subject to one or more conditions precedent, including, but not limited to, completion of a related Equity Offering. If any senior notes are listed on an exchange, and the rules of such exchange so require, the Issuer will notify the exchange of any such notice of redemption. In addition, the Issuer will notify the exchange of the principal amount of any senior notes outstanding following any partial redemption of the senior notes.
Selection and Notice
If the Issuer is redeeming less than all of the senior notes issued under the senior notes indenture at any time, the Trustee will select the senior notes to be redeemed (1) if the senior notes are listed on an exchange, in compliance with the requirements of such exchange or (2) on a pro rata basis to the extent practicable, or, if the pro rata basis is not practicable for any reason, by lot or by such other method as the Trustee shall deem fair and appropriate. No senior notes of $1,000 or less can be redeemed in part.
Notices of redemption shall be delivered electronically or mailed by first-class mail, postage prepaid, at least 30 but not more than 60 days before the redemption date to each Holder of senior notes at such Holder’s registered address or otherwise in accordance with the procedures of DTC, except that redemption notices may be delivered more than 60 days prior to a redemption date if the notice is issued in connection with a defeasance of the senior notes or a satisfaction and discharge of the senior notes indenture. If any exchange senior note is to be redeemed in part only, any notice of redemption that relates to such exchange senior note shall state the portion of the principal amount thereof that has been or is to be redeemed.
With respect to senior notes represented by certificated notes, the Issuer will issue a new exchange senior note in a principal amount equal to the unredeemed portion of the original senior note in the name of the Holder upon
136
cancellation of the original exchange senior note. Senior notes called for redemption become due on the date fixed for redemption. On and after the Redemption Date, interest ceases to accrue on the senior notes or portions of them called for redemption.
Repurchase at the Option of Holders
Change of Control
The senior notes indenture provides that if a Change of Control occurs, unless the Issuer has previously or concurrently delivered a redemption notice with respect to all the outstanding senior notes as described under “Optional Redemption,” the Issuer will make an offer to purchase all of the senior notes pursuant to the offer described below (the “Change of Control Offer”) at a price in cash (the “Change of Control Payment”) equal to 101% of the aggregate principal amount thereof plus accrued and unpaid interest, if any, to the date of purchase, subject to the right of Holders of the senior notes of record on the relevant record date to receive interest due on the relevant interest payment date. Within 30 days following any Change of Control, the Issuer will deliver notice of such Change of Control Offer electronically or by first-class mail, with a copy to the Trustee, to each Holder of senior notes to the address of such Holder appearing in the security register or otherwise in accordance with the procedures of DTC with the following information:
(1) that a Change of Control Offer is being made pursuant to the covenant entitled “Change of Control,” and that all senior notes properly tendered pursuant to such Change of Control Offer will be accepted for payment by the Issuer;
(2) the purchase price and the purchase date, which will be no earlier than 30 days nor later than 60 days from the date such notice is delivered (the “Change of Control Payment Date”);
(3) that any senior note not properly tendered will remain outstanding and continue to accrue interest;
(4) that unless the Issuer defaults in the payment of the Change of Control Payment, all senior notes accepted for payment pursuant to the Change of Control Offer will cease to accrue interest on the Change of Control Payment Date;
(5) that Holders electing to have any senior notes purchased pursuant to a Change of Control Offer will be required to surrender such senior notes, with the form entitled “Option of Holder to Elect Purchase” on the reverse of such senior notes completed, to the paying agent specified in the notice at the address specified in the notice prior to the close of business on the third Business Day preceding the Change of Control Payment Date;
(6) that Holders will be entitled to withdraw their tendered senior notes and their election to require the Issuer to purchase such senior notes, provided that the paying agent receives, not later than the close of business on the Expiration Date of the Change of Control Offer, a telegram, facsimile transmission or letter setting forth the name of the Holder of the senior notes, the principal amount of senior notes tendered for purchase, and a statement that such Holder is withdrawing its tendered senior notes and its election to have such senior notes purchased;
(7) that Holders whose senior notes are being purchased only in part will be issued new senior notes and such new senior notes will be equal in principal amount to the unpurchased portion of the senior notes surrendered. The unpurchased portion of the senior notes must be equal to at least $1,000 or any integral multiple of $1,000 in excess of $1,000;
(8) if such notice is delivered prior to the occurrence of a Change of Control, stating that the Change of Control Offer is conditional on the occurrence of such Change of Control; and
(9) the other instructions, as determined by the Issuer, consistent with the covenant described hereunder, that a Holder must follow.
The Issuer will comply with the requirements of Rule 14e-1 under the Exchange Act and any other securities laws and regulations thereunder to the extent such laws or regulations are applicable in connection with the repurchase of senior notes pursuant to a Change of Control Offer. To the extent that the provisions of any securities laws or regulations conflict with the provisions of the senior notes indenture, the Issuer will comply with the applicable securities laws and regulations and shall not be deemed to have breached its obligations described in the senior notes indenture by virtue thereof.
On the Change of Control Payment Date, the Issuer will, to the extent permitted by law:
137
(1) accept for payment all senior notes issued by it or portions thereof properly tendered pursuant to the Change of Control Offer;
(2) deposit with the paying agent an amount equal to the aggregate Change of Control Payment in respect of all senior notes or portions thereof so tendered; and
(3) deliver, or cause to be delivered, to the Trustee for cancellation the senior notes so accepted together with an Officer’s Certificate to the Trustee stating that such senior notes or portions thereof have been tendered to and purchased by the Issuer.
The Senior Credit Facilities, and future credit agreements or other agreements relating to Senior Indebtedness to which the Issuer becomes a party may, provide that certain change of control events with respect to the Issuer would constitute a default thereunder (including a Change of Control under the senior notes indenture). If we experience a change of control that triggers a default under the Senior Credit Facilities, we could seek a waiver of such default or seek to refinance the Senior Credit Facilities. In the event we do not obtain such a waiver or refinance the Senior Credit Facilities, such default could result in amounts outstanding under the Senior Credit Facilities being declared due and payable and cause a Qualified Securitization Facility to be wound down.
Our ability to pay cash to the Holders of senior notes following the occurrence of a Change of Control may be limited by our then-existing financial resources. Therefore, sufficient funds may not be available when necessary to make any required repurchases.
The Change of Control purchase feature of the senior notes may in certain circumstances make more difficult or discourage a sale or takeover of us and, thus, the removal of incumbent management. The Change of Control purchase feature is a result of negotiations between the Initial Purchasers and us. After the issue date, we have no present intention to engage in a transaction involving a Change of Control, although it is possible that we could decide to do so in the future. Subject to the limitations discussed below, we could, in the future, enter into certain transactions, including acquisitions, refinancings or other recapitalizations, that would not constitute a Change of Control under the senior notes indenture, but that could increase the amount of Indebtedness outstanding at such time or otherwise affect our capital structure or credit ratings. Restrictions on our ability to incur additional Indebtedness are contained in the covenants described under “—Certain Covenants— Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock” and “—Certain Covenants—Liens.” Such restrictions in the senior notes indenture can be waived only with the consent of the Holders of a majority in principal amount of the senior notes then outstanding. Except for the limitations contained in such covenants, however, the senior notes indenture does not contain any covenants or provisions that may afford Holders of the senior notes protection in the event of a highly leveraged transaction.
The Issuer will not be required to make a Change of Control Offer following a Change of Control if a third party makes the Change of Control Offer in the manner, at the times and otherwise in compliance with the requirements set forth in the senior notes indenture applicable to a Change of Control Offer made by the Issuer and purchases all senior notes validly tendered and not withdrawn under such Change of Control Offer.
Notwithstanding anything to the contrary herein, a Change of Control Offer may be made in advance of a Change of Control, conditional upon such Change of Control, if a definitive agreement is in place for the Change of Control at the time of making of the Change of Control Offer.
The definition of “Change of Control” includes a disposition of all or substantially all of the assets of the Issuer and its Subsidiaries, taken as a whole, to any Person. Although there is a limited body of case law interpreting the phrase “substantially all,” there is no precise established definition of the phrase under applicable law. Accordingly, in certain circumstances there may be a degree of uncertainty as to whether a particular transaction would involve a disposition of “all or substantially all” of the assets of the Issuer and its Subsidiaries, taken as a whole. As a result, it may be unclear as to whether a Change of Control has occurred and whether a Holder of senior notes may require the Issuer to make an offer to repurchase the senior notes as described above.
The provisions under the senior notes indenture relative to the Issuer’s obligation to make an offer to repurchase the senior notes as a result of a Change of Control may be waived or modified with the written consent of the Holders of a majority in principal amount of the senior notes.
Asset Sales
138
The senior notes indenture provides that the Issuer will not, and will not permit any of its Restricted Subsidiaries to, consummate directly or indirectly an Asset Sale, unless:
(1) the Issuer or such Restricted Subsidiary, as the case may be, receives consideration at the time of such Asset Sale at least equal to the fair market value of the assets sold or otherwise disposed of; and
(2) except in the case of a Permitted Asset Swap, at least 75.0% of the consideration therefor received by the Issuer or such Restricted Subsidiary, as the case may be, is in the form of Cash Equivalents; provided that the amount of:
(a) any liabilities (as shown on the Issuer’s or such Restricted Subsidiary’s most recent balance sheet or in the footnotes thereto) of the Issuer or such Restricted Subsidiary, other than liabilities that are by their terms subordinated to the senior notes, that are assumed by the transferee of any such assets and for which the Issuer and all of its Restricted Subsidiaries have been validly released by all creditors in writing;
(b) any securities, notes or other obligations or assets received by the Issuer or such Restricted Subsidiary from such transferee that are converted by the Issuer or such Restricted Subsidiary into cash (to the extent of the cash received) within 180 days following the closing of such Asset Sale; and
(c) any Designated Non-cash Consideration received by the Issuer or such Restricted Subsidiary in such Asset Sale having an aggregate fair market value, taken together with all other Designated Non-cash Consideration received pursuant to this clause (c) that is at that time outstanding, not to exceed the greater of (x) $300.0 million and (y) 3.0% of Total Assets at the time of the receipt of such Designated Non-cash Consideration, with the fair market value of each item of Designated Non-cash Consideration being measured at the time received and without giving effect to subsequent changes in value, shall be deemed to be Cash Equivalents for purposes of this provision and for no other purpose.
Within 450 days after the receipt of any Net Proceeds of any Asset Sale, the Issuer or such Restricted Subsidiary, at its option, may apply the Net Proceeds from such Asset Sale, (1) to permanently reduce:
(a) Obligations under the Senior Credit Facilities, and to correspondingly reduce commitments with respect thereto;
(b) Obligations under Senior Indebtedness that is secured by a Lien, which Lien is permitted by the senior notes indenture, and to correspondingly reduce commitments with respect thereto;
(c) Obligations under other Senior Indebtedness (and to correspondingly reduce commitments with respect thereto), provided that the Issuer shall equally and ratably reduce Obligations under the senior notes as provided under “Optional Redemption” or through open-market purchases (to the extent such purchases are at or above 100% of the principal amount thereof) or by making an offer (in accordance with the procedures set forth below for an Asset Sale Offer) to all Holders to purchase their senior notes at 100% of the principal amount thereof, plus the amount of accrued but unpaid interest, if any, on the amount of senior notes to be repurchased; or
(d) Indebtedness of a Restricted Subsidiary that is not a Guarantor, other than Indebtedness owed to the Issuer or another Restricted Subsidiary;
(2) to make (a) an Investment in any one or more businesses, provided that such Investment in any business is in the form of the acquisition of Capital Stock and results in the Issuer or any of its Restricted Subsidiaries, as the case may be, owning an amount of the Capital Stock of such business such that it constitutes a Restricted Subsidiary, (b) capital expenditures or (c) acquisitions of other assets, in each of (a), (b) and (c), used or useful in a Similar Business; or
(3) to make an Investment in (a) any one or more businesses, provided that such Investment in any business is in the form of the acquisition of Capital Stock and results in the Issuer or any of its Restricted Subsidiaries, as the case may be, owning an amount of the Capital Stock of such business such that it constitutes a Restricted Subsidiary, (b) properties or (c) acquisitions of other assets that, in each of (a), (b) and (c), replace the businesses, properties and/or assets that are the subject of such Asset Sale;
provided that, in the case of clauses (2) and (3) above, a binding commitment shall be treated as a permitted application of the Net Proceeds from the date of such commitment so long as the Issuer or such other Restricted Subsidiary enters into
139
such commitment with the good faith expectation that such Net Proceeds will be applied to satisfy such commitment within 180 days of such commitment (an “Acceptable Commitment”) and, in the event any Acceptable Commitment is later cancelled or terminated for any reason before the Net Proceeds are applied in connection therewith, the Issuer or such Restricted Subsidiary enters into another Acceptable Commitment (a “Second Commitment”) within 180 days of such cancellation or termination; provided, further, that if any Second Commitment is later cancelled or terminated for any reason before such Net Proceeds are applied, then such Net Proceeds shall constitute Excess Proceeds.
Any Net Proceeds from the Asset Sale that are not invested or applied as provided and within the time period set forth in the preceding paragraph will be deemed to constitute “Excess Proceeds.” When the aggregate amount of Excess Proceeds exceeds $75.0 million, the Issuer shall make an offer to all Holders of the senior notes and, if required by the terms of any Indebtedness that is pari passu with the senior notes (“Pari Passu Indebtedness”), to the holders of such Pari Passu Indebtedness (an “Asset Sale Offer”), to purchase the maximum aggregate principal amount of the senior notes and such Pari Passu Indebtedness that is in an amount equal to at least $1,000, that may be purchased out of the Excess Proceeds at an offer price in cash in an amount equal to 100% of the principal amount thereof (or accreted value thereof, if less), plus accrued and unpaid interest, if any, to the date fixed for the closing of such offer, in accordance with the procedures set forth in the senior notes indenture. The Issuer will commence an Asset Sale Offer with respect to Excess Proceeds within ten Business Days after the date that Excess Proceeds exceed $75.0 million by delivering the notice required pursuant to the terms of the senior notes indenture, with a copy to the Trustee. The Issuer may satisfy the foregoing obligations with respect to any Net Proceeds from an Asset Sale by making an Asset Sale Offer with respect to such Net Proceeds prior to the expiration of the relevant 450 days (or such longer period provided above) or with respect to Excess Proceeds of $75.0 million or less.
To the extent that the aggregate amount of senior notes and such Pari Passu Indebtedness tendered pursuant to an Asset Sale Offer is less than the Excess Proceeds, the Issuer may use any remaining Excess Proceeds for general corporate purposes, subject to other covenants contained in the senior notes indenture. If the aggregate principal amount of senior notes or the Pari Passu Indebtedness surrendered by such holders thereof exceeds the amount of Excess Proceeds, the Trustee shall select the senior notes on a pro rata basis and the Issuer shall select such Pari Passu Indebtedness to be purchased on a pro rata basis based on the accreted value or principal amount of the senior notes or such Pari Passu Indebtedness tendered. Upon completion of any such Asset Sale Offer, the amount of Excess Proceeds that resulted in the Asset Sale Offer shall be reset to zero.
Pending the final application of any Net Proceeds pursuant to this covenant, the holder of such Net Proceeds may apply such Net Proceeds temporarily to reduce Indebtedness outstanding under a revolving credit facility or otherwise invest such Net Proceeds in any manner not prohibited by the senior notes indenture.
The Issuer will comply with the requirements of Rule 14e-1 under the Exchange Act and any other securities laws and regulations thereunder to the extent such laws or regulations are applicable in connection with the repurchase of the senior notes pursuant to an Asset Sale Offer. To the extent that the provisions of any securities laws or regulations conflict with the provisions of the senior notes indenture, the Issuer will comply with the applicable securities laws and regulations and shall not be deemed to have breached its obligations described in the senior notes indenture by virtue thereof.
The provisions under the senior notes indenture relative to the Issuer’s obligation to make an offer to repurchase the senior notes as a result of an Asset Sale may be waived or modified with the written consent of the Holders of a majority in principal amount of the senior notes.
Certain Covenants
Set forth below are summaries of certain covenants contained in the senior notes indenture. During any period of time that (i) the senior notes have Investment Grade Ratings from both Rating Agencies and (ii) no Default has occurred and is continuing under the senior notes indenture (the occurrence of the events described in the foregoing clauses (i) and (ii) being collectively referred to as a “Covenant Suspension Event” and the date thereof being referred to as the “Suspension Date”) then, the covenants specifically listed under the following captions in this “Description of Exchange Senior Notes” section of this prospectus will not be applicable to the senior notes (collectively, the “Suspended Covenants”):
(1) “Repurchase at the Option of Holders—Asset Sales”;
(2) “—Limitation on Restricted Payments”;
140
(3) “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”;
(4) clause (4) of the first paragraph of “—Merger, Consolidation or Sale of All or Substantially All Assets”;
(5) “—Transactions with Affiliates”;
(6) “—Dividend and Other Payment Restrictions Affecting Restricted Subsidiaries”; and
(7) “—Limitation on Guarantees of Indebtedness by Restricted Subsidiaries.”
During any period that the foregoing covenants have been suspended, the Issuer may not designate any of its Subsidiaries as Unrestricted Subsidiaries pursuant to the second sentence of the definition of “Unrestricted Subsidiary.”
If and while the Issuer and its Restricted Subsidiaries are not subject to the Suspended Covenants, the senior notes will be entitled to substantially less covenant protection. In the event that the Issuer and its Restricted Subsidiaries are not subject to the Suspended Covenants under the senior notes indenture for any period of time as a result of the foregoing, and on any subsequent date (the “Reversion Date”) one or both of the Rating Agencies withdraw their Investment Grade Rating or downgrade the rating assigned to the senior notes below an Investment Grade Rating, then the Issuer and its Restricted Subsidiaries will thereafter again be subject to the Suspended Covenants under the senior notes indenture with respect to future events. The period of time between the Suspension Date and the Reversion Date is referred to in this “Description of Exchange Senior Notes” as the “Suspension Period.” The Guarantees of the Guarantors will be suspended during the Suspension Period. Additionally, upon the occurrence of a Covenant Suspension Event, the amount of Excess Proceeds from Net Proceeds shall be reset to zero.
Notwithstanding the foregoing, in the event of any such reinstatement, no action taken or omitted to be taken by the Issuer or any of its Restricted Subsidiaries prior to such reinstatement will give rise to a Default or Event of Default under the senior notes indenture with respect to the senior notes; provided that (i) with respect to Restricted Payments made after such reinstatement, the amount available to be made as Restricted Payments will be calculated as though the covenant described above under “—Limitation on Restricted Payments” had been in effect prior to, but not during, the Suspension Period; and (ii) all Indebtedness incurred, or Disqualified Stock issued, during the Suspension Period will be classified to have been incurred or issued pursuant to clause (3) of the second paragraph of “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock.”
There can be no assurance that the senior notes will ever achieve or maintain Investment Grade Ratings.
Limitation on Restricted Payments
The Issuer will not, and will not permit any of its Restricted Subsidiaries to, directly or indirectly: (I) declare or pay any dividend or make any payment or distribution on account of the Issuer’s, or any of its Restricted Subsidiaries’ Equity Interests, including any dividend or distribution payable in connection with any merger or consolidation other than:
(a) dividends or distributions by the Issuer payable solely in Equity Interests (other than Disqualified Stock) of the Issuer; or
(b) dividends or distributions by a Restricted Subsidiary so long as, in the case of any dividend or distribution payable on or in respect of any class or series of securities issued by a Restricted Subsidiary other than a Wholly-Owned Subsidiary, the Issuer or a Restricted Subsidiary receives at least its pro rata share of such dividend or distribution in accordance with its Equity Interests in such class or series of securities;
(II) purchase, redeem, defease or otherwise acquire or retire for value any Equity Interests of the Issuer or any direct or indirect parent company of the Issuer, including in connection with any merger or consolidation;
(III) make any principal payment on, or redeem, repurchase, defease or otherwise acquire or retire for value, in each case, prior to any scheduled repayment, sinking fund payment or maturity, any Subordinated Indebtedness, other than:
(a) Indebtedness permitted under clauses (7) and (8) of the second paragraph of the covenant described under “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”; or
141
(b) the purchase, repurchase or other acquisition of Subordinated Indebtedness purchased in anticipation of satisfying a sinking fund obligation, principal installment or final maturity, in each case due within one year of the date of purchase, repurchase or acquisition; or
(IV) make any Restricted Investment (all such payments and other actions set forth in clauses (I) through (IV) above being collectively referred to as “Restricted Payments”), unless, at the time of such Restricted Payment:
(1) no Default shall have occurred and be continuing or would occur as a consequence thereof;
(2) immediately after giving effect to such transaction on a pro forma basis, the Issuer could incur $1.00 of additional Indebtedness pursuant to the Fixed Charge Coverage Ratio test set forth in the first paragraph of the covenant described under “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock” (the “Fixed Charge Coverage Test”); and
(3) such Restricted Payment, together with the aggregate amount of all other Restricted Payments made by the Issuer and its Restricted Subsidiaries after September 25, 2007 (including Restricted Payments permitted by clauses (1), (2) (with respect to the payment of dividends on Refunding Capital Stock (as defined below) pursuant to clause (b) thereof only), (6)(c), (9) and (14) of the next succeeding paragraph, but excluding all other Restricted Payments permitted by the next succeeding paragraph), is less than the sum of (without duplication):
(a) 50.0% of the Consolidated Net Income of the Issuer for the period (taken as one accounting period) beginning on September 1, 2007 to the end of the Issuer’s most recently ended fiscal quarter for which internal financial statements are available at the time of such Restricted Payment, or, in the case such Consolidated Net Income for such period is a deficit, minus 100.0% of such deficit; plus
(b) 100.0% of the aggregate net cash proceeds and the fair market value of marketable securities or other property received by the Issuer since immediately after September 25, 2007 (other than net cash proceeds to the extent such net cash proceeds have been used to incur Indebtedness or issue Disqualified Stock or Preferred Stock pursuant to clause (12)(a) of the second paragraph of “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”) from the issue or sale of:
(i) (A) Equity Interests of the Issuer, including Treasury Capital Stock (as defined below), but excluding cash proceeds and the fair market value of marketable securities or other property received from the sale of:
(x) Equity Interests to any future, present or former employees, directors, officers, managers, distributors or consultants (or their respective Controlled Investment Affiliates or Immediate Family Members) of the Issuer, any direct or indirect parent company of the Issuer or any of the Issuer’s Subsidiaries after September 25, 2007 to the extent such amounts have been applied to Restricted Payments made in accordance with clause (4) of the next succeeding paragraph; and
(y) Designated Preferred Stock;
and (B) to the extent such net cash proceeds are actually contributed to the Issuer, Equity Interests of any direct or indirect parent company of the Issuer (excluding contributions of the proceeds from the sale of Designated Preferred Stock of such company or contributions to the extent such amounts have been applied to Restricted Payments made in accordance with clause (4) of the next succeeding paragraph); or
(ii) debt securities of the Issuer that have been converted into or exchanged for such Equity Interests of the Issuer;
provided that this clause (b) shall not include the proceeds from (W) Refunding Capital Stock, (X) Equity Interests or convertible debt securities of the Issuer sold to a Restricted Subsidiary, (Y) Disqualified Stock or debt securities that have been converted into Disqualified Stock or (Z) Excluded Contributions; plus
(c) 100.0% of the aggregate amount of cash and the fair market value of marketable securities or other property contributed to the capital of the Issuer following September 25, 2007 (other than net cash proceeds to the extent such net cash proceeds have been used to incur Indebtedness or issue Disqualified Stock or Preferred Stock pursuant to clause (12)(a) of the second paragraph of “—Limitation on Incurrence of Indebtedness and
142
Issuance of Disqualified Stock and Preferred Stock”) (other than by a Restricted Subsidiary and other than any Excluded Contributions); plus
(d) 100.0% of the aggregate amount received in cash and the fair market value of marketable securities or other property received by means of:
(i) the sale or other disposition (other than to the Issuer or a Restricted Subsidiary) of Restricted Investments made by the Issuer or its Restricted Subsidiaries and repurchases and redemptions of such Restricted Investments from the Issuer or its Restricted Subsidiaries (other than by the Issuer or a Restricted Subsidiary) and repayments of loans or advances, and releases of guarantees, which constitute Restricted Investments made by the Issuer or its Restricted Subsidiaries, in each case after September 25, 2007; or
(ii) the sale (other than to the Issuer or a Restricted Subsidiary) of the stock of an Unrestricted Subsidiary or a distribution from an Unrestricted Subsidiary (other than in each case to the extent the Investment in such Unrestricted Subsidiary was made by the Issuer or a Restricted Subsidiary pursuant to clause (7) of the next succeeding paragraph or to the extent such Investment constituted a Permitted Investment) or a dividend from an Unrestricted Subsidiary after September 25, 2007; plus
(e) in the case of the redesignation of an Unrestricted Subsidiary as a Restricted Subsidiary after September 25, 2007, the fair market value of the Investment in such Unrestricted Subsidiary (which, if the fair market value of such Investment shall exceed $125.0 million, shall be determined by the board of directors of the Issuer whose resolution with respect thereto will be delivered to the Trustee) at the time of the redesignation of such Unrestricted Subsidiary as a Restricted Subsidiary, other than to the extent the Investment in such Unrestricted Subsidiary was made by the Issuer or a Restricted Subsidiary pursuant to clause (7) of the next succeeding paragraph or to the extent such Investment constituted a Permitted Investment.
The foregoing provisions do not prohibit:
(1) the payment of any dividend or other distribution or the consummation of any irrevocable redemption within 60 days after the date of declaration of the dividend or other distribution or giving of the redemption notice, as the case may be, if at the date of declaration or notice, the dividend or other distribution or redemption payment would have complied with the provisions of the senior notes indenture;
(2) (a) the redemption, repurchase, retirement or other acquisition of any Equity Interests (“Treasury Capital Stock”) or Subordinated Indebtedness of the Issuer or any Equity Interests of any direct or indirect parent company of the Issuer, in exchange for, or out of the proceeds of the substantially concurrent sale (other than to a Restricted Subsidiary) of, Equity Interests of the Issuer or any direct or indirect parent company of the Issuer to the extent contributed to the Issuer (in each case, other than any Disqualified Stock) (“Refunding Capital Stock”) and (b) if immediately prior to the retirement of Treasury Capital Stock, the declaration and payment of dividend thereon was permitted under clause (6) of this paragraph, the declaration and payment of dividend on the Refunding Capital Stock (other than Refunding Capital Stock the proceeds of which were used to redeem, repurchase, retire or otherwise acquire any Equity Interests of any direct or indirect parent company of the Issuer) in an aggregate amount per year no greater than the aggregate amount of dividends per annum that were declarable and payable on such Treasury Capital Stock immediately prior to such retirement;
(3) the defeasance, redemption, repurchase or other acquisition or retirement of (i) Subordinated Indebtedness of the Issuer or a Guarantor made by exchange for, or out of the proceeds of the substantially concurrent sale of, new Indebtedness of the Issuer or a Guarantor or (ii) Disqualified Stock of the Issuer or a Guarantor made by exchange for, or out of the proceeds of the substantially concurrent sale of, Disqualified Stock of the Issuer or a Guarantor, that, in each case, is incurred in compliance with “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock” so long as:
(a) the principal amount (or accreted value, if applicable) of such new Indebtedness or the liquidation preference of such new Disqualified Stock does not exceed the principal amount of (or accreted value, if applicable), plus any accrued and unpaid interest on, the Subordinated Indebtedness or the liquidation preference of, plus any accrued and unpaid dividends on, the Disqualified Stock being so defeased, redeemed, repurchased, acquired or retired for value, plus the amount of any reasonable premium required to be paid under the terms of the instrument governing the Subordinated Indebtedness or Disqualified Stock being so defeased, redeemed,
143
repurchased, acquired or retired, defeasance costs and any reasonable fees and expenses incurred in connection with the issuance of such new Indebtedness or Disqualified Stock;
(b) such new Indebtedness is subordinated to the senior notes or the applicable Guarantee at least to the same extent as such Subordinated Indebtedness so defeased, redeemed, repurchased, acquired or retired;
(c) such new Indebtedness or Disqualified Stock has a final scheduled maturity date equal to or later than the final scheduled maturity date of the Subordinated Indebtedness or Disqualified Stock being so defeased, redeemed, repurchased, acquired or retired; and
(d) such new Indebtedness or Disqualified Stock has a Weighted Average Life to Maturity equal to or greater than the remaining Weighted Average Life to Maturity of the Subordinated Indebtedness or Disqualified Stock being so defeased, redeemed, repurchased, acquired or retired;
(4) a Restricted Payment to pay for the repurchase, retirement or other acquisition or retirement for value of Equity Interests (other than Disqualified Stock) of the Issuer or any direct or indirect parent company of the Issuer held by any future, present or former (A) employee, director, officer, manager or consultant (or their respective Controlled Investment Affiliates or Immediate Family Members) of the Issuer, any of its Subsidiaries or any of its direct or indirect parent companies pursuant to any management equity plan or stock option plan or any other management or employee benefit plan or agreement, or any stock subscription or shareholder agreement, including any Equity Interest rolled over by management of the Issuer or any direct or indirect parent company of the Issuer in connection with the Transactions; provided that the aggregate amount of Restricted Payments made under this clause (A) does not exceed $20.0 million in the first fiscal year following the Issue Date (which amount shall be increased by $5.0 million each fiscal year thereafter and, if applicable, will be increased to $40.0 million following the consummation of an underwritten public Equity Offering) (with unused amounts in any fiscal year being carried over to succeeding fiscal years subject to a maximum (without giving effect to the following proviso) of $30.0 million in any fiscal year (which shall increase to $60.0 million subsequent to the consummation of an underwritten public Equity Offering)); and (B) distributor (or its respective Controlled Investment Affiliates or Immediate Family Members) of the Issuer, any of its Subsidiaries or any of its direct or indirect parent companies pursuant to any distributor equity plan or agreement; provided that the aggregate amount of Restricted Payments made under this clause (B) does not exceed the greater of (i) $100.0 million and (ii) 1.0% of Total Assets; provided, further, that each of the amounts in any fiscal year under (A) and (B) may be increased by an amount not to exceed:
(a) the cash proceeds from the sale of Equity Interests (other than Disqualified Stock) of the Issuer and, to the extent contributed to the Issuer, Equity Interests of any direct or indirect parent company of the Issuer, in each case to any future, present or former employees, directors, officers, managers, distributors or consultants (or their respective Controlled Investment Affiliates or Immediate Family Members) of the Issuer, any of its Subsidiaries or any of its direct or indirect parent companies that occurs after the Issue Date, to the extent the cash proceeds from the sale of such Equity Interests have not otherwise been applied to the payment of Restricted Payments by virtue of clause (3) of the preceding paragraph; plus
(b) the cash proceeds of key man life insurance policies received by the Issuer or its Restricted Subsidiaries after the Issue Date; less (c) the amount of any Restricted Payments previously made with the cash proceeds described in clauses (a) and (b) of this clause (4);
and provided, further, that cancellation of Indebtedness owing to the Issuer from any future, present or former employees, directors, officers, managers, distributors or consultants of the Issuer (or their respective Controlled Investment Affiliates or Immediate Family Members), any direct or indirect parent company of the Issuer or any of the Issuer’s Restricted Subsidiaries in connection with a repurchase of Equity Interests of the Issuer or any of its direct or indirect parent companies will not be deemed to constitute a Restricted Payment for purposes of this covenant or any other provision of the senior notes indenture;
(5) the declaration and payment of dividends to holders of any class or series of Disqualified Stock of the Issuer or any of its Restricted Subsidiaries or any class or series of Preferred Stock of any Restricted Subsidiary issued in accordance with the covenant described under “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock” to the extent such dividends are included in the definition of “Fixed Charges”;
(6) (a) the declaration and payment of dividends to holders of any class or series of Designated Preferred Stock (other than Disqualified Stock) issued by the Issuer or any of its Restricted Subsidiaries after the Issue Date;
144
(b) the declaration and payment of dividends to any direct or indirect parent company of the Issuer, the proceeds of which will be used to fund the payment of dividends to holders of any class or series of Designated Preferred Stock (other than Disqualified Stock) issued by such parent company after the Issue Date, provided that the amount of dividends paid pursuant to this clause (b) shall not exceed the aggregate amount of cash actually contributed to the Issuer from the sale of such Designated Preferred Stock; or
(c) the declaration and payment of dividends on Refunding Capital Stock that is Preferred Stock in excess of the dividends declarable and payable thereon pursuant to clause (2) of this paragraph;
provided, in the case of each of (a), (b) and (c) of this clause (6), that for the most recently ended four full fiscal quarters for which internal financial statements are available immediately preceding the date of issuance of such Designated Preferred Stock or the declaration of such dividends on Refunding Capital Stock that is Preferred Stock, after giving effect to such issuance or declaration on a pro forma basis, the Issuer would have had a Fixed Charge Coverage Ratio of at least 2.00 to 1.00;
(7) Investments in Unrestricted Subsidiaries taken together with all other Investments made pursuant to this clause (7) that are at the time outstanding, without giving effect to the sale of an Unrestricted Subsidiary to the extent the proceeds of such sale do not consist of cash or marketable securities, not to exceed greater of (a) $300.0 million and (b) 3.0% of Total Assets;
(8) payments made or expected to be made by the Issuer or any Restricted Subsidiary in respect of withholding or similar taxes payable by any future, present or former employee, director, officer, manager, distributor or consultant (or their respective Controlled Investment Affiliates or Immediate Family Members) and any repurchases of Equity Interests deemed to occur upon exercise of stock options or warrants if such Equity Interests represent a portion of the exercise price of such options or warrants;
(9) the declaration and payment of dividends on the Issuer’s common stock (or the payment of dividends to any direct or indirect parent company of the Issuer to fund a payment of dividends on such company’s common stock), following the first public offering of the Issuer’s common stock or the common stock of any direct or indirect parent company of the Issuer after the Issue Date, of up to 6.0% per annum of the net cash proceeds received by or contributed to the Issuer in or from any such public offering, other than public offerings with respect to the Issuer’s common stock registered on Form S-4 or Form S-8 and other than any public sale constituting an Excluded Contribution;
(10) Restricted Payments that are made with Excluded Contributions;
(11) other Restricted Payments in an aggregate amount taken together with all other Restricted Payments made pursuant to this clause (11) not to exceed the greater of (a) $300.0 million and (b) 2.75% of Total Assets;
(12) distributions or payments of Securitization Fees;
(13) any Restricted Payment made in connection with the Transactions and the fees and expenses related thereto or owed to Affiliates, in each case to the extent permitted by the covenant described under “—Transactions with Affiliates”;
(14) the repurchase, redemption or other acquisition or retirement for value of any Subordinated Indebtedness pursuant to the provisions similar to those described under “Repurchase at the Option of Holders—Change of Control” and “Repurchase at the Option of Holders—Asset Sales”; provided that all senior notes validly tendered by Holders in connection with a Change of Control Offer or Asset Sale Offer, as applicable, have been repurchased, redeemed, acquired or retired for value;
(15) the declaration and payment of dividends by the Issuer to, or the making of loans to, any direct or indirect parent company of the Issuer in amounts required for any direct or indirect parent company of the Issuer to pay, in each case without duplication,
(a) franchise and excise taxes and other fees, taxes and expenses required to maintain their corporate existence;
(b) foreign, federal, state and local income taxes, to the extent such income taxes are attributable to the income of the Issuer and its Restricted Subsidiaries and, to the extent of the amount actually received from its
145
Unrestricted Subsidiaries, in amounts required to pay such taxes to the extent attributable to the income of such Unrestricted Subsidiaries; provided that in each case the amount of such payments in any fiscal year does not exceed the amount that the Issuer and its Restricted Subsidiaries would be required to pay in respect of foreign, federal, state and local taxes for such fiscal year were the Issuer, its Restricted Subsidiaries and its Unrestricted Subsidiaries (to the extent described above) to pay such taxes separately from any such parent company;
(c) customary salary, bonus and other benefits payable to employees, directors, officers and managers of any direct or indirect parent company of the Issuer to the extent such salaries, bonuses and other benefits are attributable to the ownership or operation of the Issuer and its Restricted Subsidiaries;
(d) general corporate operating and overhead costs and expenses of any direct or indirect parent company of the Issuer to the extent such costs and expenses are attributable to the ownership or operation of the Issuer and its Restricted Subsidiaries;
(e) fees and expenses other than to Affiliates of the Issuer related to any unsuccessful equity or debt offering of such parent company;
(f) [reserved];
(g) amounts payable pursuant to the Management Fee Agreement, solely to the extent such amounts are not paid directly by the Issuer or its Subsidiaries;
(h) cash payments in lieu of issuing fractional shares in connection with the exercise of warrants, options or other securities convertible into or exchangeable for Equity Interests of the Issuer or any direct or indirect parent company of the Issuer; and
(i) to finance Investments otherwise permitted to be made pursuant to this covenant; provided that (A) such Restricted Payment shall be made substantially concurrently with the closing of such Investment, (B) such direct or indirect parent company shall, immediately following the closing thereof, cause (1) all property acquired (whether assets or Equity Interests) to be contributed to the capital of the Issuer or one of its Restricted Subsidiaries or (2) the merger of the Person formed or acquired into the Issuer or one of its Restricted Subsidiaries (to the extent not prohibited by the covenant “—Merger, Consolidation or Sale of All or Substantially All Assets” below) in order to consummate such Investment, (C) such direct or indirect parent company and its Affiliates (other than the Issuer or a Restricted Subsidiary) receives no consideration or other payment in connection with such transaction except to the extent the Issuer or a Restricted Subsidiary could have given such consideration or made such payment in compliance with the senior notes indenture, (D) any property received by the Issuer shall not increase amounts available for Restricted Payments pursuant to clause (3) of the preceding paragraph and (E) such Investment shall be deemed to be made by the Issuer or such Restricted Subsidiary pursuant to another provision of this covenant (other than pursuant to clause (10) hereof) or pursuant to the definition of “Permitted Investments” (other than clause (9) thereof); and
(16) the distribution, by dividend or otherwise, of shares of Capital Stock of, or Indebtedness owed to the Issuer or a Restricted Subsidiary by, Unrestricted Subsidiaries (other than Unrestricted Subsidiaries, the primary assets of which are Cash Equivalents).
provided that at the time of, and after giving effect to, any Restricted Payment permitted under clauses (11) and (16), no Default shall have occurred and be continuing or would occur as a consequence thereof.
As of the Issue Date, all of the Issuer’s Subsidiaries will be Restricted Subsidiaries. The Issuer will not permit any Unrestricted Subsidiary to become a Restricted Subsidiary except pursuant to the next to the last sentence of the definition of “Unrestricted Subsidiary.” For purposes of designating any Restricted Subsidiary as an Unrestricted Subsidiary, all outstanding Investments by the Issuer and its Restricted Subsidiaries (except to the extent repaid) in the Subsidiary so designated will be deemed to be Restricted Payments in an amount determined as set forth in the penultimate sentence of the definition of “Investments.” Such designation will be permitted only if a Restricted Payment in such amount would be permitted at such time, whether pursuant to the first paragraph of this covenant or under clause (7), (10) or (11) of the second paragraph of this covenant, or pursuant to the definition of “Permitted Investments,” and if such Subsidiary otherwise meets the definition of an Unrestricted Subsidiary. Unrestricted Subsidiaries will not be subject to any of the restrictive covenants set forth in the senior notes indenture.
146
As of February 28, 2013, we have approximately $725.8 million of Restricted Payment capacity under clause (3) of the first paragraph of this covenant and an additional $300.0 million of other Restricted Payments capacity under clause (11) of the second paragraph of this covenant.
Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock
The Issuer will not, and will not permit any of its Restricted Subsidiaries to, directly or indirectly, create, incur, issue, assume, guarantee or otherwise become directly or indirectly liable, contingently or otherwise (collectively, “incur” and collectively, an “incurrence”) with respect to any Indebtedness (including Acquired Indebtedness) and the Issuer will not issue any shares of Disqualified Stock and will not permit any Restricted Subsidiary to issue any shares of Disqualified Stock or Preferred Stock; provided that the Issuer may incur Indebtedness (including Acquired Indebtedness) or issue shares of Disqualified Stock, and, subject to the third paragraph of this covenant, any Restricted Subsidiary may incur Indebtedness (including Acquired Indebtedness), issue shares of Disqualified Stock and issue shares of Preferred Stock, if the Fixed Charge Coverage Ratio for the Issuer’s most recently ended four fiscal quarters for which internal financial statements are available immediately preceding the date on which such additional Indebtedness is incurred or such Disqualified Stock or Preferred Stock is issued would have been at least 2.00 to 1.00, determined on a pro forma basis (including a pro forma application of the net proceeds therefrom), as if the additional Indebtedness had been incurred, or the Disqualified Stock or Preferred Stock had been issued, as the case may be, and the application of proceeds therefrom had occurred at the beginning of such four-quarter period.
The foregoing limitations will not apply to:
(1) the incurrence by the Issuer or any Restricted Subsidiary pursuant to one or more Credit Facilities, including through the issuance and creation of letters of credit and bankers’ acceptances thereunder (with letters of credit and bankers’ acceptances being deemed to have a principal amount equal to the face amount thereof), of: (a) Indebtedness in an aggregate principal amount up to the sum of $3,165.0 million and €835.0 million and (b) Indebtedness in an aggregate principal amount that could be incurred such that at the time of incurrence and after giving effect thereto the Senior Secured Leverage Ratio would not exceed 4.50 to 1.00, determined on a pro forma basis (including a pro forma application of the net proceeds therefrom); provided that in calculating the Senior Secured Leverage Ratio solely for purposes of this clause (1), any unsecured Indebtedness incurred under this clause (1) shall be deemed to be Secured Indebtedness solely for purposes of calculating the Senior Secured Leverage Ratio for this clause (1);
(2) the incurrence by the Issuer and any Guarantor of Indebtedness represented by the senior notes (including any Guarantee) and the exchange notes and related exchange guarantees to be issued in exchange for senior notes and the Guarantees pursuant to the Senior Notes Registration Rights Agreements (but excluding any Additional Notes);
(3) Indebtedness of the Issuer and its Restricted Subsidiaries in existence on the Issue Date (other than Indebtedness described in clauses (1) and (2));
(4) Indebtedness (including Capitalized Lease Obligations) and Disqualified Stock incurred or issued by the Issuer or any Restricted Subsidiary and Preferred Stock issued by any Restricted Subsidiary, to finance the purchase, lease or improvement of property (real or personal) or equipment that is used or useful in a Similar Business, whether through the direct purchase of assets or the Capital Stock of any Person owning such assets in an aggregate principal amount, together with any Refinancing Indebtedness in respect thereof and all other Indebtedness, Disqualified Stock and/or Preferred Stock incurred or issued and outstanding under this clause (4), not to exceed 5.0% of Total Assets (in each case, determined at the date of incurrence) at any time outstanding, so long as such Indebtedness, Disqualified Stock or Preferred Stock is incurred or issued at the date of such purchase, lease or improvement or within 270 days thereafter;
(5) Indebtedness incurred by the Issuer or any of its Restricted Subsidiaries constituting reimbursement obligations with respect to letters of credit issued in the ordinary course of business, including letters of credit in respect of workers’ compensation claims, or other Indebtedness with respect to reimbursement type obligations regarding workers’ compensation claims; provided that upon the drawing of such letters of credit or the incurrence of such Indebtedness, such obligations are reimbursed within 30 days following such drawing or incurrence;
(6) Indebtedness arising from agreements of the Issuer or its Restricted Subsidiaries providing for indemnification, adjustment of purchase price, earnouts or similar obligations, in each case, incurred or assumed in connection with the disposition of any business, assets or a Subsidiary, other than guarantees of Indebtedness incurred by any Person acquiring all or any portion of such business, assets or a Subsidiary for the purpose of financing such acquisition; provided that such Indebtedness is not reflected on the balance sheet of the Issuer, or any of its Restricted
147
Subsidiaries (contingent obligations referred to in a footnote to financial statements and not otherwise reflected on the balance sheet will not be deemed to be reflected on such balance sheet for purposes of this clause (6));
(7) Indebtedness of the Issuer to a Restricted Subsidiary; provided that any such Indebtedness owing to a Restricted Subsidiary that is not a Guarantor is expressly subordinated in right of payment to the senior notes; provided, further, that any subsequent issuance or transfer of any Capital Stock or any other event which results in any such Restricted Subsidiary ceasing to be a Restricted Subsidiary or any other subsequent transfer of any such Indebtedness (except to the Issuer or another Restricted Subsidiary) shall be deemed, in each case, to be an incurrence of such Indebtedness not permitted by this clause;
(8) Indebtedness of a Restricted Subsidiary to the Issuer or another Restricted Subsidiary; provided that if a Guarantor incurs such Indebtedness to a Restricted Subsidiary that is not a Guarantor, such Indebtedness is expressly subordinated in right of payment to the Guarantee of the senior notes of such Guarantor; provided, further, that any subsequent transfer of any such Indebtedness (except to the Issuer or another Restricted Subsidiary) shall be deemed, in each case, to be an incurrence of such Indebtedness not permitted by this clause;
(9) shares of Preferred Stock of a Restricted Subsidiary issued to the Issuer or another Restricted Subsidiary; provided that any subsequent issuance or transfer of any Capital Stock or any other event which results in any such Restricted Subsidiary ceasing to be a Restricted Subsidiary or any other subsequent transfer of any such shares of Preferred Stock (except to the Issuer or another of its Restricted Subsidiaries) shall be deemed, in each case, to be an issuance of such shares of Preferred Stock not permitted by this clause;
(10) Hedging Obligations (excluding Hedging Obligations entered into for speculative purposes) for the purpose of limiting interest rate risk with respect to any Indebtedness permitted to be incurred under the senior notes indenture, exchange rate risk or commodity pricing risk;
(11) obligations in respect of self-insurance and obligations in respect of performance, bid, appeal and surety bonds and completion guarantees and similar obligations provided by the Issuer or any of its Restricted Subsidiaries in the ordinary course of business;
(12) (a) Indebtedness or Disqualified Stock of the Issuer and Indebtedness, Disqualified Stock or Preferred Stock of the Issuer or any Restricted Subsidiary in an aggregate principal amount or liquidation preference up to 100.0% of the net cash proceeds received by the Issuer since immediately after the Issue Date from the issue or sale of Equity Interests of the Issuer or cash contributed to the capital of the Issuer (in each case, other than proceeds of Disqualified Stock or sales of Equity Interests to the Issuer or any of its Subsidiaries) as determined in accordance with clauses (3)(b) and (3)(c) of the first paragraph of “—Limitation on Restricted Payments” to the extent such net cash proceeds or cash have not been applied pursuant to such clauses to make Restricted Payments or to make other Investments, payments or exchanges pursuant to the second paragraph of “—Limitation on Restricted Payments” or to make Permitted Investments (other than Permitted Investments specified in clause (1) or (3) of the definition thereof) and (b) Indebtedness or Disqualified Stock of the Issuer and Indebtedness, Disqualified Stock or Preferred Stock of the Issuer or, subject to the third paragraph of this covenant, any Restricted Subsidiary not otherwise permitted hereunder in an aggregate principal amount or liquidation preference which, when aggregated with the principal amount and liquidation preference of all other Indebtedness, Disqualified Stock and Preferred Stock then outstanding and incurred pursuant to this clause (12)(b), does not at any one time outstanding exceed the greater of (x) $550.0 million and (y) 5.0% of Total Assets (it being understood that any Indebtedness, Disqualified Stock or Preferred Stock incurred pursuant to this clause (12)(b) shall cease to be deemed incurred or outstanding for purposes of this clause (12)(b) but shall be deemed incurred for the purposes of the first paragraph of this covenant from and after the first date on which the Issuer or such Restricted Subsidiary could have incurred such Indebtedness, Disqualified Stock or Preferred Stock under the first paragraph of this covenant without reliance on this clause (12)(b));
(13) the incurrence by the Issuer or any Restricted Subsidiary of Indebtedness, the issuance by the Issuer or any Restricted Subsidiary of Disqualified Stock or the issuance by any Restricted Subsidiary of Preferred Stock which serves to extend, replace, refund, refinance, renew or defease any Indebtedness incurred or Disqualified Stock or Preferred Stock issued as permitted under the first paragraph of this covenant and clauses (2), (3), (4) and (12)(a) above, this clause (13) and clauses (14) and (24) below or any Indebtedness incurred or Disqualified Stock or Preferred Stock issued to so extend, replace, refund, refinance, renew or defease such Indebtedness, Disqualified Stock or Preferred Stock including additional Indebtedness, Disqualified Stock or Preferred Stock incurred to pay premiums (including reasonable tender premiums), defeasance costs and fees in connection therewith (the “Refinancing Indebtedness”) prior to its respective maturity; provided that such Refinancing Indebtedness:
148
(a) has a Weighted Average Life to Maturity at the time such Refinancing Indebtedness is incurred which is not less than the remaining Weighted Average Life to Maturity of, the Indebtedness, Disqualified Stock or Preferred Stock being extended, replaced, refunded, refinanced, renewed or defeased;
(b) to the extent such Refinancing Indebtedness extends, replaces, refunds, refinances, renews or defeases (i) Indebtedness subordinated to the senior notes or any Guarantee thereof, such Refinancing Indebtedness is subordinated to the senior notes or the Guarantee thereof at least to the same extent as the Indebtedness being extended, replaced, refunded, refinanced, renewed or defeased or (ii) Disqualified Stock or Preferred Stock, such Refinancing Indebtedness must be Disqualified Stock or Preferred Stock, respectively; and
(c) shall not include:
(i) Indebtedness, Disqualified Stock or Preferred Stock of a Subsidiary of the Issuer that is not a Guarantor that refinances Indebtedness or Disqualified Stock of the Issuer;
(ii) Indebtedness, Disqualified Stock or Preferred Stock of a Subsidiary of the Issuer that is not a Guarantor that refinances Indebtedness, Disqualified Stock or Preferred Stock of a Guarantor; or
(iii) Indebtedness or Disqualified Stock of the Issuer or Indebtedness, Disqualified Stock or Preferred Stock of a Restricted Subsidiary that refinances Indebtedness, Disqualified Stock or Preferred Stock of an Unrestricted Subsidiary;
and, provided, further, that subclause (a) of this clause (13) will not apply to any extension, replacement, refunding, refinancing, renewal or defeasance of any Indebtedness outstanding under a Credit Facility and Obligations secured by Permitted Liens.
(14) (a) Indebtedness or Disqualified Stock of the Issuer or, subject to the third paragraph of this covenant, Indebtedness, Disqualified Stock or Preferred Stock of a Restricted Subsidiary incurred or issued to finance an acquisition or (b) Indebtedness, Disqualified Stock or Preferred Stock of Persons that are acquired by the Issuer or any Restricted Subsidiary or merged into the Issuer or a Restricted Subsidiary in accordance with the terms of the senior notes indenture; provided that after giving effect to such acquisition or merger, either
(i) the Issuer would be permitted to incur at least $1.00 of additional Indebtedness pursuant to the Fixed Charge Coverage Test, or
(ii) the Fixed Charge Coverage Ratio for the Issuer is equal to or greater than immediately prior to such acquisition or merger;
(15) Indebtedness arising from the honoring by a bank or other financial institution of a check, draft or similar instrument drawn against insufficient funds in the ordinary course of business, provided that such Indebtedness is extinguished within five Business Days of its incurrence;
(16) Indebtedness of the Issuer or any of its Restricted Subsidiaries supported by a letter of credit issued pursuant to the Credit Facilities, in a principal amount not in excess of the stated amount of such letter of credit;
(17) (a) any guarantee by the Issuer or a Restricted Subsidiary of Indebtedness or other obligations of any Restricted Subsidiary so long as the incurrence of such Indebtedness incurred by such Restricted Subsidiary is permitted under the terms of the senior notes indenture, or (b) any guarantee by a Restricted Subsidiary of Indebtedness of the Issuer; provided that such guarantee is incurred in accordance with the covenant described below under “—Limitation on Guarantees of Indebtedness by Restricted Subsidiaries”;
(18) Indebtedness consisting of Indebtedness issued by the Issuer or any of its Restricted Subsidiaries to future, present or former employees, directors, officers, managers, distributors and consultants thereof, their respective Controlled Investment Affiliates or Immediate Family Members, in each case to finance the purchase or redemption of Equity Interests of the Issuer or any direct or indirect parent company of the Issuer to the extent described in clause (4) of the second paragraph under “—Limitation on Restricted Payments”;
(19) customer deposits and advance payments received in the ordinary course of business from customers for goods purchased in the ordinary course of business;
149
(20) Indebtedness owed on a short-term basis of no longer than 30 days to banks and other financial institutions incurred in the ordinary course of business of the Issuer and its Restricted Subsidiaries with such banks or financial institutions that arises in connection with ordinary banking arrangements to manage cash balances of the Issuer and its Restricted Subsidiaries;
(21) Indebtedness incurred by a Restricted Subsidiary in connection with bankers’ acceptances, discounted bills of exchange or the discounting or factoring of receivables for credit management purposes, in each case incurred or undertaken in the ordinary course of business on arm’s length commercial terms on a recourse basis;
(22) Indebtedness of the Issuer or any of its Restricted Subsidiaries consisting of (a) the financing of insurance premiums or (b) take-or-pay obligations contained in supply arrangements in each case, incurred in the ordinary course of business;
(23) the incurrence of Indebtedness of Foreign Subsidiaries of the Issuer or a Restricted Subsidiary of the Issuer in an amount not to exceed at any one time outstanding the greater of (i) $100.0 million and (ii) 5.0% of the Foreign Subsidiary Total Assets (it being understood that any Indebtedness incurred pursuant to this clause (23) shall cease to be deemed incurred or outstanding for the purpose of this clause (23) but shall be deemed incurred for the purposes of the first paragraph of this covenant from and after the first date on which the Issuer or such Restricted Subsidiaries could have incurred such Indebtedness under the first paragraph of this covenant without reliance on this clause (23));
(24) Indebtedness, Disqualified Stock or Preferred Stock of a Restricted Subsidiary incurred to finance or assumed in connection with an acquisition in a principal amount not to exceed $100.0 million in the aggregate at any one time outstanding together with all other Indebtedness, Disqualified Stock and/or Preferred Stock issued under this clause (24) (it being understood that any Indebtedness, Disqualified Stock or Preferred Stock incurred pursuant to this clause (24) shall cease to be deemed incurred or outstanding for purposes of this clause (24) but shall be deemed incurred for the purposes of the first paragraph of this covenant from and after the first date on which such Restricted Subsidiary could have incurred such Indebtedness, Disqualified Stock or Preferred Stock under the first paragraph of this covenant without reliance on this clause (24)); and
(25) Indebtedness of the Issuer or any of its Restricted Subsidiaries undertaken in connection with cash management and related activities with respect to any Subsidiary or joint venture in the ordinary course of business.
Restricted Subsidiaries of the Issuer that are not Guarantors may not incur Indebtedness or Disqualified Stock or Preferred Stock under the first paragraph of this covenant or clause (12)(b), (14)(a) or (24) of the second paragraph of this covenant if, after giving pro forma effect to such incurrence or issuance (including a pro forma application of the net proceeds therefrom), the aggregate amount of Indebtedness and Disqualified Stock and Preferred Stock of Restricted Subsidiaries that are not Guarantors incurred or issued pursuant to the first paragraph of this covenant and clauses (12)(b), (14)(a) and (24) of the second paragraph of this covenant, collectively, would exceed $600.0 million.
For purposes of determining compliance with this covenant:
(1) in the event that an item of Indebtedness, Disqualified Stock or Preferred Stock (or any portion thereof) meets the criteria of more than one of the categories of Permitted Indebtedness, Disqualified Stock or Preferred Stock described in clauses (1) through (25) above or is entitled to be incurred pursuant to the first paragraph of this covenant, the Issuer, in its sole discretion, will classify or reclassify such item of Indebtedness, Disqualified Stock or Preferred Stock (or any portion thereof) and will only be required to include the amount and type of such Indebtedness, Disqualified Stock or Preferred Stock in one of the above clauses or under the first paragraph of this covenant; provided that all Indebtedness outstanding under the Senior Credit Facilities on the Issue Date will be treated as incurred on the Issue Date under clause (1) of the second paragraph above; and
(2) at the time of incurrence, the Issuer will be entitled to divide and classify an item of Indebtedness in more than one of the types of Indebtedness described in the first and second paragraphs above.
Accrual of interest or dividends, the accretion of accreted value, the accretion or amortization of original issue discount and the payment of interest or dividends in the form of additional Indebtedness, Disqualified Stock or Preferred Stock, as the case may be, of the same class will not be deemed to be an incurrence of Indebtedness, Disqualified Stock or Preferred Stock for purposes of this covenant.
150
For purposes of determining compliance with any U.S. dollar-denominated or Euro-denominated, as the case may be, restriction on the incurrence of Indebtedness, the U.S. dollar-equivalent or Euro- equivalent, as the case may be, principal amount of Indebtedness denominated in another currency shall be calculated based on the relevant currency exchange rate in effect on the date such Indebtedness was incurred, in the case of term debt, or first committed, in the case of revolving credit debt; provided that if such Indebtedness is incurred to refinance other Indebtedness denominated in another currency, and such refinancing would cause the applicable U.S. dollar-denominated or Euro-denominated, as the case may be, restriction to be exceeded if calculated at the relevant currency exchange rate in effect on the date of such refinancing, such U.S. dollar-denominated or Euro-denominated, as the case may be, restriction shall be deemed not to have been exceeded so long as the principal amount of such refinancing Indebtedness does not exceed the principal amount of such Indebtedness being refinanced.
The principal amount of any Indebtedness incurred to refinance other Indebtedness, if incurred in a different currency from the Indebtedness being refinanced, shall be calculated based on the currency exchange rate applicable to the currencies in which such respective Indebtedness is denominated that is in effect on the date of such refinancing.
The senior notes indenture provides that the Issuer will not, and will not permit any Guarantor to, directly or indirectly, incur any Indebtedness (including Acquired Indebtedness) that is subordinated or junior in right of payment to any Indebtedness of the Issuer or such Guarantor, as the case may be, unless such Indebtedness is expressly subordinated in right of payment to the senior notes or such Guarantor’s Guarantee to the extent and in the same manner as such Indebtedness is subordinated to other Indebtedness of the Issuer or such Guarantor, as the case may be.
The senior notes indenture does not treat (1) unsecured Indebtedness as subordinated or junior to Secured Indebtedness merely because it is unsecured or (2) Senior Indebtedness as subordinated or junior to any other Senior Indebtedness merely because it has a junior priority with respect to the same collateral.
Liens
The Issuer will not, and will not permit any Guarantor to, directly or indirectly, create, incur, assume or suffer to exist any Lien (except Permitted Liens) that secures Obligations under any Indebtedness or any related Guarantee, on any asset or property of the Issuer or any Guarantor, or any income or profits therefrom, or assign or convey any right to receive income therefrom, unless:
(1) in the case of Liens securing Subordinated Indebtedness, the senior notes and related Guarantees are secured by a Lien on such property, assets or proceeds that is senior in priority to such Liens; and
(2) in all other cases, the senior notes or the Guarantees are equally and ratably secured, except that the foregoing shall not apply to (a) Liens securing the senior notes and the related Guarantees, (b) Liens securing Indebtedness permitted to be incurred under Credit Facilities, including any letter of credit facility relating thereto, that was permitted by the terms of the senior notes indenture to be incurred pursuant to clause (1) of the second paragraph under “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock” and (c) Liens securing Indebtedness permitted to be incurred under the covenant described above under “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”; provided that, with respect to Liens securing Indebtedness permitted under this subclause (c), at the time of incurrence and after giving pro forma effect thereto, the Senior Secured Leverage Ratio would be no greater than 4.50 to 1.00.
Merger, Consolidation or Sale of All or Substantially All Assets
The Issuer may not consolidate or merge with or into or wind up into (whether or not the Issuer is the surviving Person), or sell, assign, transfer, lease, convey or otherwise dispose of all or substantially all of its properties or assets, in one or more related transactions, to any Person unless:
(1) the Issuer is the surviving Person or the Person formed by or surviving any such consolidation or merger (if other than the Issuer) or to which such sale, assignment, transfer, lease, conveyance or other disposition will have been made, is a Person organized or existing under the laws of the United States, any state thereof, the District of Columbia, or any territory thereof (such Person, as the case may be, being herein called the “Successor Company”); provided that in the case where the surviving Person is not a corporation, a co-obligor of the senior notes is a corporation;
(2) the Successor Company, if other than the Issuer, expressly assumes all the obligations of the Issuer under the senior notes pursuant to supplemental indentures or other documents or instruments;
151
(3) immediately after such transaction, no Default exists;
(4) immediately after giving pro forma effect to such transaction and any related financing transactions, as if such transactions had occurred at the beginning of the applicable four-quarter period,
(a) the Successor Company or the Issuer would be permitted to incur at least $1.00 of additional Indebtedness pursuant to the Fixed Charge Coverage Test, or
(b) the Fixed Charge Coverage Ratio for the Issuer would be equal to or greater than the Fixed Charge Coverage Ratio for the Issuer immediately prior to such transaction;
(5) each Guarantor, unless it is the other party to the transactions described above, in which case clause (1)(b) of the second succeeding paragraph shall apply, shall have by supplemental indenture confirmed that its Guarantee shall apply to such Person’s obligations under the senior notes indenture, the senior notes and the Senior Notes Registration Rights Agreements; and
(6) the Issuer shall have delivered to the Trustee an Officer’s Certificate and an Opinion of Counsel, each stating that such consolidation, merger or transfer and such supplemental indentures, if any, comply with the senior notes indenture, and an Opinion of Counsel that such supplemental indenture is the valid and binding obligation of the Issuer and the Guarantors and enforceable in accordance with its terms.
The Successor Company will succeed to, and be substituted for the Issuer under the senior notes indenture, the Guarantees and the senior notes, as applicable. Notwithstanding the immediately preceding clauses (3) and (4),
(1) any Restricted Subsidiary may consolidate with or merge into or transfer all or part of its properties and assets to the Issuer, and
(2) the Issuer may merge with an Affiliate of the Issuer solely for the purpose of reincorporating the Issuer in the United States, the District of Columbia or any territory thereof so long as the amount of Indebtedness of the Issuer and its Restricted Subsidiaries is not increased thereby.
Subject to certain limitations described in the senior notes indenture governing release of a Guarantee upon the sale, disposition or transfer of a Guarantor, no Guarantor will, and the Issuer will not permit any Guarantor to, consolidate or merge with or into or wind up into (whether or not such Guarantor is the surviving Person), or sell, assign, transfer, lease, convey or otherwise dispose of all or substantially all of its properties or assets, in one or more related transactions, to any Person unless:
(1) (a) such Guarantor is the surviving Person or the Person formed by or surviving any such consolidation or merger (if other than such Guarantor) or to which such sale, assignment, transfer, lease, conveyance or other disposition will have been made is a Person organized or existing under the laws of the jurisdiction of organization of such Guarantor, as applicable, or the laws of the United States, any state thereof, the District of Columbia, or any territory thereof (such Person being herein called the “Successor Person”);
(b) the Successor Person, if other than such Guarantor, expressly assumes all the obligations of such Guarantor under the senior notes indenture and such Guarantor’s related Guarantee pursuant to supplemental indentures or other documents or instruments;
(c) immediately after such transaction, no Default exists; and
(d) the Issuer shall have delivered to the Trustee an Officer’s Certificate and an Opinion of Counsel, each stating that such consolidation, merger or transfer and such supplemental indentures, if any, comply with the senior notes indenture, and an Opinion of Counsel that such supplemental indenture is the valid and binding obligation of the Successor Person and enforceable in accordance with its terms; or
(2) the transaction is made in compliance with the covenant described under “Repurchase at the Option of Holders—Asset Sales.”
Subject to certain limitations described in the senior notes indenture, the Successor Person will succeed to, and be substituted for, such Guarantor under the senior notes indenture and such Guarantor’s Guarantee. Notwithstanding the
152
foregoing, any Guarantor may (1) merge into or transfer all or part of its properties and assets to another Guarantor or the Issuer, (2) merge with an Affiliate of the Issuer solely for the purpose of reincorporating the Guarantor in the United States, any state thereof, the District of Columbia or any territory thereof or (3) convert into a corporation, partnership, limited partnership, limited liability corporation or trust organized or existing under the laws of the jurisdiction of organization of such Guarantor.
Transactions with Affiliates
The Issuer will not, and will not permit any of its Restricted Subsidiaries to, make any payment to, or sell, lease, transfer or otherwise dispose of any of its properties or assets to, or purchase any property or assets from, or enter into or make or amend any transaction, contract, agreement, understanding, loan, advance or guarantee with, or for the benefit of, any Affiliate of the Issuer (each of the foregoing, an “Affiliate Transaction”) involving aggregate payments or consideration in excess of $25.0 million, unless:
(1) such Affiliate Transaction is on terms that are not materially less favorable to the Issuer or its relevant Restricted Subsidiary than those that would have been obtained in a comparable transaction by the Issuer or such Restricted Subsidiary with an unrelated Person on an arm’s-length basis; and
(2) the Issuer delivers to the Trustee with respect to any Affiliate Transaction or series of related Affiliate Transactions involving aggregate payments or consideration in excess of $50.0 million, a resolution adopted by the majority of the board of directors of the Issuer approving such Affiliate Transaction and set forth in an Officer’s Certificate certifying that such Affiliate Transaction complies with clause (1) above.
The foregoing provisions will not apply to the following:
(1) transactions between or among the Issuer or any of its Restricted Subsidiaries;
(2) Restricted Payments permitted by the provisions of the senior notes indenture described above under the covenant “—Limitation on Restricted Payments” and the definition of “Permitted Investments”;
(3) the payment of management, consulting, monitoring, advisory and other fees and related expenses pursuant to the Management Fee Agreement (plus any unpaid management, consulting, monitoring, advisory and other fees and related expenses accrued in any prior year) and the termination fees pursuant to the Management Fee Agreement, or any amendment thereto so long as any such amendment is not disadvantageous in the good faith judgment of the board of directors of the Issuer to the Holders when taken as a whole, as compared to the Management Fee Agreement as in effect on the Issue Date;
(4) the payment of reasonable and customary fees paid to, and indemnities provided for the benefit of, current or former employees, directors, officers, managers, distributors or consultants of the Issuer, any of its direct or indirect parent companies or any of its Restricted Subsidiaries;
(5) transactions in which the Issuer or any of its Restricted Subsidiaries, as the case may be, delivers to the Trustee a letter from an Independent Financial Advisor stating that such transaction is fair to the Issuer or such Restricted Subsidiary from a financial point of view or stating that the terms are not materially less favorable to the Issuer or its relevant Restricted Subsidiary with an unrelated Person on an arm’s-length basis;
(6) any agreement as in effect as of the Issue Date, or any amendment thereto (so long as any such amendment is not disadvantageous in the good faith judgment of the board of directors of the Issuer to the Holders when taken as a whole as compared to the applicable agreement as in effect on the Issue Date);
(7) the existence of, or the performance by the Issuer or any of its Restricted Subsidiaries of its obligations under the terms of, any stockholders agreement (including any registration rights agreement or purchase agreement related thereto) to which it is a party as of the Issue Date and any similar agreements which it may enter into thereafter; provided that the existence of, or the performance by the Issuer or any of its Restricted Subsidiaries of obligations under any future amendment to any such existing agreement or under any similar agreement entered into after the Issue Date shall only be permitted by this clause (7) to the extent that the terms of any such amendment or new agreement are not otherwise disadvantageous in the good faith judgment of the board of directors of the Issuer to the Holders when taken as a whole;
153
(8) the Transactions and the payment of all fees and expenses related to the Transactions, in each case as contemplated by the offering circular relating to the original senior notes dated July 25, 2012 and September 18, 2012;
(9) transactions with customers, clients, suppliers, contractors, joint venture partners or purchasers or sellers of goods or services that are Affiliates, in each case in the ordinary course of business and otherwise in compliance with the terms of the senior notes indenture which are fair to the Issuer and its Restricted Subsidiaries, in the reasonable determination of the board of directors of the Issuer or the senior management thereof, or are on terms at least as favorable as might reasonably have been obtained at such time from an unaffiliated party;
(10) the issuance of Equity Interests (other than Disqualified Stock) of the Issuer to any Permitted Holder or to any employee, director, officer, manager, distributor or consultant (or their respective Controlled Investment Affiliates or Immediate Family Members) of the Issuer, any of its direct or indirect parent companies or any of its Restricted Subsidiaries;
(11) sales of accounts receivable, or participations therein, or Securitization Assets or related assets in connection with the ABL Facilities or any Qualified Securitization Facility;
(12) payments by the Issuer or any of its Restricted Subsidiaries to any of the Investors made for any financial advisory, financing, underwriting or placement services or in respect of other investment banking activities, including, without limitation, in connection with acquisitions or divestitures which payments are approved by a majority of the board of directors of the Issuer in good faith;
(13) payments and Indebtedness and Disqualified Stock (and cancellation of any thereof) of the Issuer and its Restricted Subsidiaries and Preferred Stock (and cancellation of any thereof) of any Restricted Subsidiary to any future, current or former employee, director, officer, manager, distributor or consultant (or their respective Controlled Investment Affiliates or Immediate Family Members) of the Issuer, any of its Subsidiaries or any of its direct or indirect parent companies pursuant to any management equity plan or stock option plan or any other management or employee benefit plan or agreement or any stock subscription or shareholder agreement or any distributor equity plan or agreement; and any employment agreements, stock option plans and other compensatory arrangements (and any successor plans thereto) and any supplemental executive retirement benefit plans or arrangements with any such employees, directors, officers, managers, distributors or consultants (or their respective Controlled Investment Affiliates or Immediate Family Members) that are, in each case, approved by the Issuer in good faith;
(14) investments by any of the Investors in securities of the Issuer or any of its Restricted Subsidiaries (and payment of reasonable out-of-pocket expenses incurred by such Investors in connection therewith) so long as (a) the investment is being offered generally to other investors on the same or more favorable terms and (b) the investment constitutes less than 5.0% of the proposed or outstanding issue amount of such class of securities;
(15) payments to or from, and transactions with, any joint venture in the ordinary course of business (including, without limitation, any cash management activities related thereto);
(16) payments by the Issuer (and any direct or indirect parent company thereof) and its Subsidiaries pursuant to tax sharing agreements among the Issuer (and any such parent company) and its Subsidiaries; provided that in each case the amount of such payments in any fiscal year does not exceed the amount that the Issuer, its Restricted Subsidiaries and its Unrestricted Subsidiaries (to the extent of amount received from Unrestricted Subsidiaries) would be required to pay in respect of foreign, federal, state and local taxes for such fiscal year were the Issuer, its Restricted Subsidiaries and its Unrestricted Subsidiaries (to the extent described above) to pay such taxes separately from any such parent entity;
(17) any lease entered into between the Issuer or any Restricted Subsidiary, as lessee and any Affiliate of the Issuer, as lessor, which is approved by a majority of the disinterested members of the board of directors of the Issuer in good faith;
(18) intellectual property licenses in the ordinary course of business; and
(19) any transition services arrangements, supply arrangements and similar arrangements entered into in connection with or in contemplation of dispositions of assets or Equity Interests in any Restricted Subsidiary not otherwise prohibited by the terms of the senior notes indenture that the Issuer determines in good faith are either fair to the Issuer or otherwise on customary terms for such type of arrangements in connection with similar transactions.
154
Dividend and Other Payment Restrictions Affecting Restricted Subsidiaries
The Issuer will not, and will not permit any of its Restricted Subsidiaries that is not a Guarantor to, directly or indirectly, create or otherwise cause or suffer to exist or become effective any consensual encumbrance or consensual restriction on the ability of any such Restricted Subsidiary to:
(1) (a) pay dividends or make any other distributions to the Issuer or any of its Restricted Subsidiaries on its Capital Stock or with respect to any other interest or participation in, or measured by, its profits, or
(b)pay any Indebtedness owed to the Issuer or any of its Restricted Subsidiaries;
(2) make loans or advances to the Issuer or any of its Restricted Subsidiaries; or
(3) sell, lease or transfer any of its properties or assets to the Issuer or any of its Restricted Subsidiaries, except (in each case) for such encumbrances or restrictions existing under or by reason of:
(a) contractual encumbrances or restrictions in effect on the Issue Date, including pursuant to the Senior Credit Facilities and the related documentation, Hedging Obligations;
(b) the senior notes indenture, the senior notes and the guarantees thereof;
(c) purchase money obligations for property acquired in the ordinary course of business and Capital Lease Obligations that impose restrictions of the nature discussed in clause (3) above on the property so acquired;
(d) applicable law or any applicable rule, regulation or order;
(e) any agreement or other instrument of a Person acquired by the Issuer or any of its Restricted Subsidiaries in existence at the time of such acquisition or at the time it merges with or into the Issuer or any of its Restricted Subsidiaries or assumed in connection with the acquisition of assets from such Person (but, in any such case, not created in contemplation thereof), which encumbrance or restriction is not applicable to any Person, or the properties or assets of any Person, other than the Person so acquired and its Subsidiaries, or the property or assets of the Person so acquired and its Subsidiaries;
(f) contracts for the sale of assets, including customary restrictions with respect to a Subsidiary of the Issuer pursuant to an agreement that has been entered into for the sale or disposition of all or substantially all of the Capital Stock or assets of such Subsidiary;
(g) Secured Indebtedness otherwise permitted to be incurred pursuant to the covenants described under “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock” and “—Liens” that limit the right of the debtor to dispose of the assets securing such Indebtedness;
(h) restrictions on cash or other deposits or net worth imposed by customers under contracts entered into in the ordinary course of business;
(i) other Indebtedness, Disqualified Stock or Preferred Stock of Foreign Subsidiaries permitted to be incurred subsequent to the Issue Date pursuant to the provisions of the covenant described under “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”;
(j) customary provisions in joint venture agreements and other similar agreements relating solely to such joint venture;
(k) customary provisions contained in leases, licenses or similar agreements, including with respect to intellectual property and other agreements, in each case, entered into in the ordinary course of business;
(l) restrictions created in connection with any Qualified Securitization Facility that, in the good faith determination of the Issuer are necessary or advisable to effect such Qualified Securitization Facility;
(m) restrictions or conditions contained in any trading, netting, operating, construction, service, supply, purchase, sale or other agreement to which the Issuer or any of its Restricted Subsidiaries is a party entered into
155
in the ordinary course of business; provided that such agreement prohibits the encumbrance of solely the property or assets of the Issuer or such Restricted Subsidiary that are the subject to such agreement, the payment rights arising thereunder or the proceeds thereof and does not extend to any other asset or property of the Issuer or such Restricted Subsidiary or the assets or property of another Restricted Subsidiary; and
(n) any encumbrances or restrictions of the type referred to in clauses (1), (2) and (3) above imposed by any amendments, modifications, restatements, renewals, increases, supplements, refundings, replacements or refinancings of the contracts, instruments or obligations referred to in clauses (a) through (m) above; provided that such amendments, modifications, restatements, renewals, increases, supplements, refundings, replacements or refinancings are, in the good faith judgment of the Issuer, no more restrictive with respect to such encumbrance and other restrictions taken as a whole than those prior to such amendment, modification, restatement, renewal, increase, supplement, refunding, replacement or refinancing.
Limitation on Guarantees of Indebtedness by Restricted Subsidiaries
The Issuer will not permit any of its Wholly-Owned Subsidiaries that are Restricted Subsidiaries (and non-Wholly-Owned Subsidiaries if such non-Wholly-Owned Subsidiaries guarantee other capital markets debt securities of the Issuer or any Guarantor), other than a Guarantor, a Foreign Subsidiary or a Securitization Subsidiary, to guarantee the payment of any Indebtedness of the Issuer or any other Guarantor unless:
(1) such Restricted Subsidiary within 30 days executes and delivers a supplemental indenture to the senior notes indenture providing for a Guarantee by such Restricted Subsidiary, except that with respect to a guarantee of Indebtedness of the Issuer or any Guarantor, if such Indebtedness is by its express terms subordinated in right of payment to the senior notes or such Guarantor’s Guarantee, any such guarantee by such Restricted Subsidiary with respect to such Indebtedness shall be subordinated in right of payment to such Guarantee substantially to the same extent as such Indebtedness is subordinated to the senior notes; and
(2) such Restricted Subsidiary waives and will not in any manner whatsoever claim or take the benefit or advantage of, any rights of reimbursement, indemnity or subrogation or any other rights against the Issuer or any other Restricted Subsidiary as a result of any payment by such Restricted Subsidiary under its Guarantee;
provided that this covenant shall not be applicable to (i) any guarantee of any Restricted Subsidiary that existed at the time such Person became a Restricted Subsidiary and was not incurred in connection with, or in contemplation of, such Person becoming a Restricted Subsidiary and (ii) guarantees of the ABL Facilities by the ABL Financing Entities or of any Qualified Securitization Facility by any Restricted Subsidiary.
Reports and Other Information
Notwithstanding that the Issuer may not be subject to the reporting requirements of Section 13 or 15(d) of the Exchange Act or otherwise report on an annual and quarterly basis on forms provided for such annual and quarterly reporting pursuant to rules and regulations promulgated by the SEC, the senior notes indenture requires the Issuer to file with the SEC from and after the Issue Date,
(1) within 90 days (or any other time period then in effect under the rules and regulations of the Exchange Act with respect to the filing of a Form 10-K by a non-accelerated filer) after the end of each fiscal year, annual reports on Form 10-K, or any successor or comparable form, containing the information required to be contained therein, or required in such successor or comparable form;
(2) within 45 days (or any other time period then in effect under the rules and regulations of the Exchange Act with respect to the filing of a Form 10-Q by a non-accelerated filer) after the end of each of the first three fiscal quarters of each fiscal year, quarterly reports on Form 10-Q containing all quarterly information that would be required to be contained in Form 10-Q, or any successor or comparable form;
(3) promptly from time to time after the occurrence of an event required to be therein reported, such other reports on Form 8-K, or any successor or comparable form; and
(4) any other information, documents and other reports which the Issuer would be required to file with the SEC if it were subject to Section 13 or 15(d) of the Exchange Act;
156
in each case, in a manner that complies in all material respects with the requirements specified in such form; provided that the Issuer shall not be so obligated to file such reports with the SEC if the SEC does not permit such filing, in which event the Issuer will make available such information to prospective purchasers of senior notes, in addition to providing such information to the Trustee and the Holders of the senior notes, in each case within 15 days after the time the Issuer would be required to file such information with the SEC, if it were subject to Sections 13 or 15(d) of the Exchange Act. In addition, to the extent not satisfied by the foregoing, the Issuer will agree that, for so long as any senior notes are outstanding, it will furnish to Holders and to securities analysts and prospective investors, upon their request, the information required to be delivered pursuant to Rule 144A(d)(4) under the Securities Act.
In the event that any direct or indirect parent company of the Issuer becomes a guarantor of the senior notes, the senior notes indenture permits the Issuer to satisfy its obligations in this covenant by furnishing reports of such parent; provided that the same is accompanied, to the extent material to investors in the senior notes, by consolidating financial and other information that explains in reasonable detail the differences between the information in such reports relating to such parent, on the one hand, and the information relating to the Issuer and its consolidated Subsidiaries on a standalone basis, on the other hand. Notwithstanding the foregoing, such requirements shall be deemed satisfied prior to the commencement of the exchange offers or the effectiveness of the shelf registration statement by (1) the filing with the SEC of the registration statement or shelf registration statement (or any other similar registration statement) of the exchange offers, and any amendments thereto, with such financial information that satisfies Regulation S-X of the Securities Act, subject to exceptions consistent with the presentation of financial information in the offering circular relating to the original senior notes dated July 25, 2012 and September 18, 2012, to the extent filed within the time specified above, or (2) by posting on its website and providing to the Trustee within 15 days of the time periods after the Issuer would have been required to file annual and interim reports with the SEC, the financial information (including a “Management’s Discussion and Analysis of Financial Condition and Results of Operations” section) that would be required to be included in such reports, subject to exceptions consistent with the presentation of financial information in this prospectus, to the extent filed within the times specified above.
Notwithstanding anything herein to the contrary, the Issuer will not be deemed to have failed to comply with any of its obligations hereunder for purposes of clause (3) under “Events of Default and Remedies” until 120 days after the date any report hereunder is due.
The Issuer shall use its commercially reasonable efforts, consistent with its judgment as to what is prudent at the time, to participate in quarterly conference calls to discuss operating results and related matters. The Company shall issue a press release which will provide the date and time of any such call and will direct Holders, prospective investors and securities analysts to contact the investor relations office of the Issuer to obtain access to the conference call.
Events of Default and Remedies
The senior notes indenture provides that each of the following is an Event of Default:
(1) default in payment when due and payable, upon redemption, acceleration or otherwise, of principal of, or premium, if any, on the senior notes;
(2) default for 30 days or more in the payment when due of interest or Additional Interest on or with respect to the senior notes;
(3) failure by the Issuer or any Guarantor for 60 days after receipt of written notice given by the Trustee or the Holders of not less than 30.0% in principal amount of the then outstanding senior notes to comply with any of its obligations, covenants or agreements (other than a default referred to in clause (1) or (2) above) contained in the senior notes indenture or the senior notes;
(4) default under any mortgage, indenture or instrument under which there is issued or by which there is secured or evidenced any Indebtedness for money borrowed by the Issuer or any of its Restricted Subsidiaries or the payment of which is guaranteed by the Issuer or any of its Restricted Subsidiaries, other than Indebtedness owed to the Issuer or a Restricted Subsidiary, whether such Indebtedness or guarantee now exists or is created after the issuance of the senior notes, if both:
(a) such default either results from the failure to pay any principal of such Indebtedness at its stated final maturity (after giving effect to any applicable grace periods) or relates to an obligation other than the obligation
157
to pay principal of any such Indebtedness at its stated final maturity and results in the holder or holders of such Indebtedness causing such Indebtedness to become due prior to its stated maturity; and
(b) the principal amount of such Indebtedness, together with the principal amount of any other such Indebtedness in default for failure to pay principal at stated final maturity (after giving effect to any applicable grace periods), or the maturity of which has been so accelerated, aggregate $75.0 million or more at any one time outstanding;
(5) failure by the Issuer or any Significant Subsidiary (or any group of Subsidiaries that together would constitute a Significant Subsidiary) to pay final judgments aggregating in excess of $75.0 million, which final judgments remain unpaid, undischarged and unstayed for a period of more than 60 days after such judgment becomes final, and in the event such judgment is covered by insurance, an enforcement proceeding has been commenced by any creditor upon such judgment or decree which is not promptly stayed;
(6) certain events of bankruptcy or insolvency with respect to the Issuer or any Significant Subsidiary (or any group of Subsidiaries that together would constitute a Significant Subsidiary); or
(7) the Guarantee of any Significant Subsidiary (or any group of Subsidiaries that together would constitute a Significant Subsidiary) shall for any reason cease to be in full force and effect or be declared null and void or any responsible officer of any Guarantor that is a Significant Subsidiary (or the responsible officers of any group of Subsidiaries that together would constitute a Significant Subsidiary), as the case may be, denies that it has any further liability under its Guarantee or gives notice to such effect, other than by reason of the termination of the senior notes indenture or the release of any such Guarantee in accordance with the senior notes indenture.
If any Event of Default (other than of a type specified in clause (6) above) occurs and is continuing under the senior notes indenture, the Trustee or the Holders of at least 30.0% in principal amount of the then total outstanding senior notes may declare the principal, premium, if any, interest and any other monetary obligations on all the then outstanding senior notes to be due and payable immediately.
Upon the effectiveness of such declaration, such principal of and premium, if any, and interest will be due and payable immediately. Notwithstanding the foregoing, in the case of an Event of Default arising under clause (6) of the first paragraph of this section, all outstanding senior notes will become due and payable without further action or notice. The senior notes indenture provides that the Trustee may withhold from the Holders notice of any continuing Default, except a Default relating to the payment of principal, premium, if any, or interest, if it determines that withholding notice is in their interest. In addition, the Trustee has no obligation to accelerate the senior notes if in the best judgment of the Trustee acceleration is not in the best interests of the Holders of the senior notes.
The senior notes indenture provides that the Holders of a majority in aggregate principal amount of the then outstanding senior notes by notice to the Trustee may on behalf of the Holders of all of the senior notes waive any existing Default and its consequences under the senior notes indenture (except a continuing Default in the payment of interest on, premium, if any, or the principal of any senior note held by a non-consenting Holder) and rescind any acceleration with respect to the senior notes and its consequences (except if such rescission would conflict with any judgment of a court of competent jurisdiction). In the event of any Event of Default specified in clause (4) above, such Event of Default and all consequences thereof (excluding any resulting payment default, other than as a result of acceleration of the senior notes) shall be annulled, waived and rescinded, automatically and without any action by the Trustee or the Holders, if within 20 days after such Event of Default arose:
(1) the Indebtedness or guarantee that is the basis for such Event of Default has been discharged;
(2) holders thereof have rescinded or waived the acceleration, notice or action (as the case may be) giving rise to such Event of Default; or
(3) the default that is the basis for such Event of Default has been cured.
Notwithstanding the foregoing, the sole remedy for any breach of our obligation under the senior notes indenture to file periodic or other reports (including pursuant to section 314(a)(1) of the Trust Indenture Act) is the payment of liquidated damages, and the Holders do not have any right under the senior notes indenture to accelerate the maturity of the senior notes as a result of any such breach. If a breach of our obligation under the senior notes indenture to file periodic or other reports (including pursuant to section 314(a)(1) of the Trust Indenture Act) continues for 90 days after
158
notice thereof is given in accordance with the senior notes indenture, we will pay liquidated damages to all the Holders of the senior notes at a rate per annum equal to (i) 0.25% per annum of the principal amount of the senior notes from the 90th day following such notice to but not including the 180th day following such notice (or such earlier date on which the Event of Default relating to the reporting obligations referred to in this paragraph shall have been cured or waived) and (ii) 0.50% per annum of the principal amount of the senior notes from the 180th day following such notice to but not including the 365th day following such notice (or such earlier date on which the Event of Default relating to the reporting obligations referred to in this paragraph shall have been cured or waived). On such 365th day (or earlier, if the Event of Default relating to the reporting obligations referred to in this paragraph shall have been cured or waived prior to such 365th day), such Additional Interest will cease to accrue, and the senior notes will be subject to acceleration as provided above if the Event of Default is continuing. The provisions of the senior notes indenture described in this paragraph will not affect the rights of the Holders of senior notes in the event of the occurrence of any other Event of Default.
Subject to the provisions of the senior notes indenture relating to the duties of the Trustee thereunder, in case an Event of Default occurs and is continuing, the Trustee will be under no obligation to exercise any of the rights or powers under the senior notes indenture at the request or direction of any of the Holders of the senior notes unless the Holders have offered to the Trustee indemnity or security satisfactory to it against any loss, liability or expense. Except to enforce the right to receive payment of principal, premium (if any) or interest when due, no Holder of an exchange senior note may pursue any remedy with respect to the senior notes indenture or the senior notes unless:
(1) such Holder has previously given the Trustee notice that an Event of Default is continuing;
(2) Holders of at least 30.0% in principal amount of the total outstanding senior notes have requested the Trustee to pursue the remedy;
(3) Holders of the senior notes have offered the Trustee security or indemnity satisfactory to it against any loss, liability or expense;
(4) the Trustee has not complied with such request within 60 days after the receipt thereof and the offer of security or indemnity; and
(5) Holders of a majority in principal amount of the total outstanding senior notes have not given the Trustee a direction inconsistent with such request within such 60-day period.
Subject to certain restrictions, under the senior notes indenture the Holders of a majority in principal amount of the total outstanding senior notes are given the right to direct the time, method and place of conducting any proceeding for any remedy available to the Trustee or of exercising any trust or power conferred on the Trustee. The Trustee, however, may refuse to follow any direction that conflicts with law or the senior notes indenture or that the Trustee determines is unduly prejudicial to the rights of any other Holder of a senior note or that would involve the Trustee in personal liability.
The senior notes indenture provides that the Issuer is required to deliver to the Trustee annually a statement regarding compliance with the senior notes indenture, and the Issuer is required, within five Business Days, upon becoming aware of any Default, to deliver to the Trustee a statement specifying such Default.
No Personal Liability of Directors, Officers, Employees and Stockholders
No director, officer, employee, incorporator or stockholder of the Issuer or any Guarantor or any of their parent companies (other than the Issuer and the Guarantors) shall have any liability, for any obligations of the Issuer or the Guarantors under the senior notes, the Guarantees or the senior notes indenture or for any claim based on, in respect of, or by reason of such obligations or their creation. Each Holder by accepting senior notes waives and releases all such liability. The waiver and release are part of the consideration for issuance of the senior notes. Such waiver may not be effective to waive liabilities under the federal securities laws and it is the view of the SEC that such a waiver is against public policy.
Legal Defeasance and Covenant Defeasance
The obligations of the Issuer and the Guarantors under the senior notes indenture will terminate (other than certain obligations) and will be released upon payment in full of all of the senior notes. The Issuer may, at its option and at any time, elect to have all of its obligations discharged with respect to the senior notes and have each Guarantor’s
159
obligation discharged with respect to its Guarantee (“Legal Defeasance”) and cure all then existing Events of Default except for:
(1) the rights of Holders of senior notes to receive payments in respect of the principal of, premium, if any, and interest on the senior notes when such payments are due solely out of the trust created pursuant to the senior notes indenture;
(2) the Issuer’s obligations with respect to senior notes concerning issuing temporary senior notes, registration of such senior notes, mutilated, destroyed, lost or stolen senior notes and the maintenance of an office or agency for payment and money for security payments held in trust;
(3) the rights, powers, trusts, duties and immunities of the Trustee, and the Issuer’s obligations in connection therewith; and
(4) the Legal Defeasance provisions of the senior notes indenture.
In addition, the Issuer may, at its option and at any time, elect to have its obligations and those of each Guarantor released with respect to certain covenants that are described in the senior notes indenture (“Covenant Defeasance”) and thereafter any omission to comply with such obligations shall not constitute a Default with respect to the senior notes. In the event Covenant Defeasance occurs, certain events (not including bankruptcy, receivership, rehabilitation and insolvency events pertaining to the Issuer) described under “Events of Default and Remedies” will no longer constitute an Event of Default with respect to the senior notes.
In order to exercise either Legal Defeasance or Covenant Defeasance with respect to the senior notes:
(1) the Issuer must irrevocably deposit with the Trustee, in trust, for the benefit of the Holders of the senior notes, cash in U.S. dollars, U.S. dollar-denominated Government Securities, or a combination thereof, in such amounts as will be sufficient, in the opinion of a nationally recognized firm of independent public accountants, to pay the principal of, premium, if any, and interest due on the senior notes on the stated maturity date or on the redemption date, as the case may be, of such principal, premium, if any, or interest on such senior notes and the Issuer must specify whether such senior notes are being defeased to maturity or to a particular redemption date;
(2) in the case of Legal Defeasance, the Issuer shall have delivered to the Trustee an Opinion of Counsel reasonably acceptable to the Trustee confirming that, subject to customary assumptions and exclusions,
(a) the Issuer has received from, or there has been published by, the United States Internal Revenue Service a ruling, or
(b) since the issuance of the senior notes, there has been a change in the applicable U.S. federal income tax law, in either case to the effect that, and based thereon such Opinion of Counsel shall confirm that, subject to customary assumptions and exclusions, the Holders of the senior notes will not recognize income, gain or loss for U.S. federal income tax purposes, as applicable, as a result of such Legal Defeasance and will be subject to U.S. federal income tax on the same amounts, in the same manner and at the same times as would have been the case if such Legal Defeasance had not occurred;
(3) in the case of Covenant Defeasance, the Issuer shall have delivered to the Trustee an Opinion of Counsel reasonably acceptable to the Trustee confirming that, subject to customary assumptions and exclusions, the Holders of the senior notes will not recognize income, gain or loss for U.S. federal income tax purposes as a result of such Covenant Defeasance and will be subject to such tax on the same amounts, in the same manner and at the same times as would have been the case if such Covenant Defeasance had not occurred;
(4) no Default (other than that resulting from borrowing funds to be applied to make such deposit and any similar and simultaneous deposit relating to other Indebtedness and, in each case, the granting of Liens in connection therewith) shall have occurred and be continuing on the date of such deposit;
(5) such Legal Defeasance or Covenant Defeasance shall not result in a breach or violation of, or constitute a default under the Senior Credit Facilities or any other material agreement or instrument (other than the senior notes indenture) to which, the Issuer or any Guarantor is a party or by which the Issuer or any Guarantor is bound (other than that resulting from any borrowing of funds to be applied to make the deposit required to effect such Legal Defeasance or
160
Covenant Defeasance and any similar and simultaneous deposit relating to other Indebtedness, and the granting of Liens in connection therewith);
(6) the Issuer shall have delivered to the Trustee an Opinion of Counsel to the effect that, as of the date of such opinion and subject to customary assumptions and exclusions following the deposit, the trust funds will not be subject to the effect of Section 547 of Title 11 of the United States Code;
(7) the Issuer shall have delivered to the Trustee an Officer’s Certificate stating that the deposit was not made by the Issuer with the intent of defeating, hindering, delaying or defrauding any creditors of the Issuer or any Guarantor or others; and
(8) the Issuer shall have delivered to the Trustee an Officer’s Certificate and an Opinion of Counsel (which Opinion of Counsel may be subject to customary assumptions and exclusions) each stating that all conditions precedent provided for or relating to the Legal Defeasance or the Covenant Defeasance, as the case may be, have been complied with.
Satisfaction and Discharge
The senior notes indenture will be discharged and will cease to be of further effect as to all senior notes, when either: (1) all senior notes theretofore authenticated and delivered, except lost, stolen or destroyed senior notes which have been replaced or paid and senior notes for whose payment money has theretofore been deposited in trust, have been delivered to the Trustee for cancellation; or
(2) (a) all senior notes not theretofore delivered to the Trustee for cancellation have become due and payable by reason of the making of a notice of redemption or otherwise, will become due and payable within one year or are to be called for redemption within one year under arrangements satisfactory to the Trustee for the giving of notice of redemption by the Trustee in the name, and at the expense, of the Issuer and the Issuer or any Guarantor have irrevocably deposited or caused to be deposited with the Trustee as trust funds in trust solely for the benefit of the Holders of the senior notes, cash in U.S. dollars, U.S. dollar-denominated Government Securities, or a combination thereof, in such amounts as will be sufficient without consideration of any reinvestment of interest to pay and discharge the entire indebtedness on the senior notes not theretofore delivered to the Trustee for cancellation for principal, premium, if any, and accrued interest to the date of maturity or redemption; provided, that upon any redemption that requires the payment of the Applicable Premium, the amount deposited shall be sufficient for purposes of the senior notes indenture to the extent that an amount is deposited with the Trustee equal to the Applicable Premium calculated as of the date of the notice of redemption, with any deficit as of the date of redemption (any such amount, the “Applicable Premium Deficit”) only required to be deposited with the Trustee on or prior to the date of redemption. Any Applicable Premium Deficit shall be set forth in an Officer’s Certificate delivered to the Trustee simultaneously with the deposit of such Applicable Premium Deficit that confirms that such Applicable Premium Deficit shall be applied toward such redemption;
(b) no Default (other than that resulting from borrowing funds to be applied to make such deposit or any similar and simultaneous deposit relating to other Indebtedness and the granting of Liens in connection therewith) with respect to the senior notes indenture or the senior notes shall have occurred and be continuing on the date of such deposit or shall occur as a result of such deposit and such deposit will not result in a breach or violation of, or constitute a default under the Senior Credit Facilities or any other material agreement or instrument (other than the senior notes indenture) to which the Issuer or any Guarantor is a party or by which the Issuer or any Guarantor is bound (other than resulting from any borrowing of funds to be applied to make such deposit and any similar and simultaneous deposit relating to other Indebtedness and the granting of Liens in connection therewith);
(c) the Issuer has paid or caused to be paid all sums payable by it under the senior notes indenture; and
(d) the Issuer has delivered irrevocable instructions to the Trustee to apply the deposited money toward the payment of the senior notes at maturity or the redemption date, as the case may be.
In addition, the Issuer must deliver an Officer’s Certificate and an Opinion of Counsel to the Trustee stating that all conditions precedent to satisfaction and discharge have been satisfied.
Amendment, Supplement and Waiver
161
Except as provided in the next two succeeding paragraphs, the senior notes indenture, any Guarantee and the senior notes may be amended or supplemented with the consent of the Holders of at least a majority in principal amount of the senior notes then outstanding, including consents obtained in connection with a purchase of, or tender offer or exchange offer for, senior notes, and any existing Default or compliance with any provision of the senior notes indenture or the senior notes issued thereunder may be waived with the consent of the Holders of a majority in principal amount of the then outstanding senior notes, other than senior notes beneficially owned by the Issuer or its Affiliates (including consents obtained in connection with a purchase of or tender offer or exchange offer for the senior notes).
The senior notes indenture provides that, without the consent of each affected Holder of the senior notes, an amendment or waiver may not, with respect to any senior notes held by a non-consenting Holder:
(1) reduce the principal amount of such senior notes whose Holders must consent to an amendment, supplement or waiver;
(2) reduce the principal of or change the fixed final maturity of any such senior note or alter or waive the provisions with respect to the redemption of such senior notes (other than provisions relating to the covenants described above under “Repurchase at the Option of Holders”);
(3) reduce the rate of or change the time for payment of interest on any Note;
(4) waive a Default in the payment of principal of or premium, if any, or interest on the senior notes, except a rescission of acceleration of the senior notes by the Holders of at least a majority in aggregate principal amount of the senior notes and a waiver of the payment default that resulted from such acceleration, or in respect of a covenant or provision contained in the senior notes indenture or any Guarantee which cannot be amended or modified without the consent of all Holders;
(5) make any senior note payable in money other than that stated therein;
(6) make any change in the provisions of the senior notes indenture relating to waivers of past Defaults or the rights of Holders to receive payments of principal of or premium, if any, or interest on the senior notes;
(7) make any change in these amendment and waiver provisions;
(8) impair the right of any Holder to receive payment of principal of, or premium, if any, or interest on such Holder’s senior notes on or after the due dates therefor or to institute suit for the enforcement of any payment on or with respect to such Holder’s senior notes;
(9) make any change to or modify the ranking of the senior notes that would adversely affect the Holders; or
(10) except as expressly permitted by the senior notes indenture, modify the Guarantees of any Significant Subsidiary in any manner adverse to the Holders of the senior notes.
Notwithstanding the foregoing, the Issuer, any Guarantor (with respect to a Guarantee or the senior notes indenture to which it is a party) and the Trustee may amend or supplement the senior notes indenture and any Guarantee or senior notes without the consent of any Holder:
(1) to cure any ambiguity, omission, mistake, defect or inconsistency;
(2) to provide for uncertificated senior notes of such series in addition to or in place of certificated senior notes;
(3) to comply with the covenant relating to mergers, consolidations and sales of assets;
(4) to provide the assumption of the Issuer’s or any Guarantor’s obligations to the Holders;
(5) to make any change that would provide any additional rights or benefits to the Holders or that does not adversely affect the legal rights under the senior notes indenture of any such Holder;
(6) to add covenants for the benefit of the Holders or to surrender any right or power conferred upon the Issuer or any Guarantor;
162
(7) to comply with requirements of the SEC in order to effect or maintain the qualification of the senior notes indenture under the Trust Indenture Act;
(8) to evidence and provide for the acceptance and appointment under the senior notes indenture of a successor Trustee thereunder pursuant to the requirements thereof;
(9) to provide for the issuance of exchange notes or private exchange notes, which are identical to exchange notes except that they are not freely transferable;
(10) to add a guarantor under the senior notes indenture;
(11) to conform the text of the senior notes indenture, Guarantees or the senior notes to any provision of the “Description of Notes” “section of the offering circular relating to the original senior notes to the extent that such provision in such “Description of Notes” section was intended to be a verbatim recitation of a provision of the senior notes indenture, Guarantee or senior notes; or
(12) to make any amendment to the provisions of the senior notes indenture relating to the transfer and legending of senior notes as permitted by the senior notes indenture, including, without limitation, to facilitate the issuance and administration of the senior notes; provided that (a) compliance with the senior notes indenture as so amended would not result in senior notes being transferred in violation of the Securities Act or any applicable securities law and (b) such amendment does not materially and adversely affect the rights of Holders to transfer senior notes.
The consent of the Holders is not necessary under the senior notes indenture to approve the particular form of any proposed amendment. It is sufficient if such consent approves the substance of the proposed amendment.
Notices
Except for notices with respect to the Trustee, notices given by publication or electronic delivery will be deemed given on the first date on which publication is made and notices given by first-class mail, postage prepaid, will be deemed given five calendar days after mailing.
Concerning the Trustee
The senior notes indenture contains certain limitations on the rights of the Trustee thereunder, should it become a creditor of the Issuer, to obtain payment of claims in certain cases, or to realize on certain property received in respect of any such claim as security or otherwise. The Trustee is permitted to engage in other transactions; however, if it acquires any conflicting interest it must eliminate such conflict within 90 days, apply to the SEC for permission to continue or resign.
The senior notes indenture provides that the Holders of a majority in principal amount of the outstanding senior notes will have the right to direct the time, method and place of conducting any proceeding for exercising any remedy available to the Trustee, subject to certain exceptions. The senior notes indenture provides that in case an Event of Default shall occur (which shall not be cured), the Trustee is required, in the exercise of its power, to use the degree of care of a prudent person in the conduct of his own affairs. Subject to such provisions, the Trustee is under no obligation to exercise any of its rights or powers under the senior notes indenture at the request of any Holder of the senior notes, unless such Holder shall have offered to the Trustee security and indemnity satisfactory to it against any loss, liability or expense.
Governing Law
The senior notes indenture, the senior notes and any Guarantee are governed by and construed in accordance with the laws of the State of New York.
Certain Definitions
Set forth below are certain defined terms used in the senior notes indenture. For purposes of the senior notes indenture, unless otherwise specifically indicated, the term “consolidated” with respect to any Person refers to such Person consolidated with its Restricted Subsidiaries, and excludes from such consolidation any Unrestricted Subsidiary as if such Unrestricted Subsidiary were not an Affiliate of such Person. These terms may have different meanings than similar or identical terms used in the senior subordinated notes indenture.
163
“2007 Acquisition” means the transactions pursuant to the Merger Agreement.
“ABL Facilities” means the asset-based revolving credit facilities under the Credit Agreement dated as of September 25, 2007 by and among the Issuer, the lenders party thereto in their capacities as lenders thereunder and Bank of America, N.A., as Administrative Agent, including any guarantees, collateral documents, instruments and agreements executed in connection therewith, and any amendments, supplements, modifications, extensions, renewals, restatements, refundings or refinancings thereof and any indentures or credit facilities or commercial paper facilities with banks or other institutional lenders or investors that replace, refund or refinance any part of the loans, notes, other credit facilities or commitments thereunder, including any such replacement, refunding or refinancing facility or indenture that increases the amount borrowable thereunder or alters the maturity thereof (provided that such increase in borrowings is permitted under “—Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock” above).
“ABL Financing Entity” means the Issuer and certain of its Subsidiaries from time to time named as borrowers or guarantors under the ABL Facilities.
“Acquired Indebtedness” means, with respect to any specified Person,
(1) Indebtedness of any other Person existing at the time such other Person is merged with or into or became a Restricted Subsidiary of such specified Person, including Indebtedness incurred in connection with, or in contemplation of, such other Person merging with or into or becoming a Restricted Subsidiary of such specified Person, and
(2) Indebtedness secured by a Lien encumbering any asset acquired by such specified Person.
“Additional Interest” means all additional interest then owing pursuant to the Senior Notes Registration Rights Agreement.
“Affiliate” of any specified Person means any other Person directly or indirectly controlling or controlled by or under direct or indirect common control with such specified Person. For purposes of this definition, “control” (including, with correlative meanings, the terms “controlling,” “controlled by” and “under common control with”), as used with respect to any Person, shall mean the possession, directly or indirectly, of the power to direct or cause the direction of the management or policies of such Person, whether through the ownership of voting securities, by agreement or otherwise.
“Applicable Premium” means, with respect to any senior note on any Redemption Date, the greater of:
(1) 1.0% of the principal amount of such senior note; and
(2) the excess, if any, of (a) the present value at such Redemption Date of (i) the redemption price of such senior note at August 1, 2015 (such redemption price being set forth in the table appearing above under “Optional Redemption”), plus (ii) all required remaining scheduled interest payments due on such senior note through August 1, 2015 (excluding accrued but unpaid interest to the Redemption Date), computed using a discount rate equal to the Treasury Rate as of such Redemption Date plus 50 basis points; over (b) the principal amount of such senior note.
“Asset Sale” means:
(1) the sale, conveyance, transfer or other disposition, whether in a single transaction or a series of related transactions (including by way of a Sale and Lease-Back Transaction) of property or assets of the Issuer or any of its Restricted Subsidiaries (each referred to in this definition as a “disposition”); or
(2) the issuance or sale of Equity Interests of any Restricted Subsidiary (other than Preferred Stock of Restricted Subsidiaries issued in compliance with the covenant described under “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”), whether in a single transaction or a series of related transactions; in each case, other than:
(a) any disposition of Cash Equivalents or Investment Grade Securities or obsolete or worn out equipment in the ordinary course of business or any disposition of inventory or goods (or other assets) held for sale or no longer used in the ordinary course of business;
164
(b) the disposition of all or substantially all of the assets of the Issuer in a manner permitted pursuant to the provisions described above under “—Certain Covenants—Merger, Consolidation or Sale of All or Substantially All Assets” or any disposition that constitutes a Change of Control pursuant to the senior notes indenture;
(c) the making of any Restricted Payment or Permitted Investment that is permitted to be made, and is made, under the covenant described above under “—Certain Covenants—Limitation on Restricted Payments”;
(d) any disposition of assets or issuance or sale of Equity Interests of any Restricted Subsidiary in any transaction or series of related transactions with an aggregate fair market value of less than $50.0 million;
(e) any disposition of property or assets or the issuance of securities by a Restricted Subsidiary to the Issuer or by the Issuer or a Restricted Subsidiary to a Restricted Subsidiary;
(f) to the extent allowable under Section 1031 of the Internal Revenue Code of 1986, any exchange of like property (excluding any boot thereon) for use in a Similar Business;
(g) the lease, assignment or sub-lease of any real or personal property in the ordinary course of business;
(h) any issuance or sale of Equity Interests in, or Indebtedness or other securities of, an Unrestricted Subsidiary;
(i) foreclosures, condemnation or any similar action on assets or the granting of Liens not prohibited by the senior notes indenture;
(j) sales of accounts receivable, or participations therein, or Securitization Assets or related assets in connection with the ABL Facilities or any Qualified Securitization Facility;
(k) any financing transaction with respect to property built or acquired by the Issuer or any Restricted Subsidiary after the Issue Date, including Sale and Lease-Back Transactions and asset securitizations permitted by the senior notes indenture;
(l) the sale or discount of inventory, accounts receivable or notes receivable in the ordinary course of business or the conversion of accounts receivable to notes receivable;
(m) the licensing or sub-licensing of intellectual property or other general intangibles in the ordinary course of business, other than the licensing of intellectual property on a long-term basis;
(n) any surrender or waiver of contract rights or the settlement, release or surrender of contract rights or other litigation claims in the ordinary course of business;
(o) the unwinding of any Hedging Obligations;
(p) sales, transfers and other dispositions of Investments in joint ventures to the extent required by, or made pursuant to, customary buy/sell arrangements between the joint venture parties set forth in joint venture arrangements and similar binding arrangements; and
(q) the abandonment of intellectual property rights in the ordinary course of business, which in the reasonable good faith determination of the Issuer are not material to the conduct of the business of the Issuer and its Restricted Subsidiaries taken as a whole.
“Business Day” means each day which is not a Legal Holiday.
“Capital Stock” means:
(1) in the case of a corporation, corporate stock;
(2) in the case of an association or business entity, any and all shares, interests, participations, rights or other equivalents (however designated) of corporate stock;
165
(3) in the case of a partnership or limited liability company, partnership or membership interests (whether general or limited); and
(4) any other interest or participation that confers on a Person the right to receive a share of the profits and losses of, or distributions of assets of, the issuing Person but excluding from all of the foregoing any debt securities convertible into Capital Stock, whether or not such debt securities include any right of participation with Capital Stock.
“Capitalized Lease Obligation” means, at the time any determination thereof is to be made, the amount of the liability in respect of a capital lease that would at such time be required to be capitalized and reflected as a liability on a balance sheet (excluding the footnotes thereto) prepared in accordance with GAAP.
“Capitalized Software Expenditures” means, for any period, the aggregate of all expenditures (whether paid in cash or accrued as liabilities) by a Person and its Restricted Subsidiaries during such period in respect of licensed or purchased software or internally developed software and software enhancements that, in conformity with GAAP, are or are required to be reflected as capitalized costs on the consolidated balance sheet of a Person and its Restricted Subsidiaries.
“Cash Equivalents” means:
(1) United States dollars;
(2) (a) Canadian dollars, yen, pounds sterling, euros or any national currency of any participating member state of the EMU; or
(b) in the case of any Foreign Subsidiary that is a Restricted Subsidiary, such local currencies held by it from time to time in the ordinary course of business;
(3) securities issued or directly and fully and unconditionally guaranteed or insured by the U.S. government or any agency or instrumentality thereof the securities of which are unconditionally guaranteed as a full faith and credit obligation of such government with maturities of 24 months or less from the date of acquisition;
(4) certificates of deposit, time deposits and eurodollar time deposits with maturities of 24 months or less from the date of acquisition, bankers’ acceptances with maturities not exceeding one year and overnight bank deposits, in each case with any domestic or foreign commercial bank having capital and surplus of not less than $500.0 million in the case of U.S. banks and $100.0 million (or the U.S. dollar equivalent as of the date of determination) in the case of non-U.S. banks;
(5) repurchase obligations for underlying securities of the types described in clauses (3), (4) and (8) entered into with any financial institution meeting the qualifications specified in clause (4) above;
(6) commercial paper rated at least P-2 by Moody’s or at least A-2 by S&P (or, if at any time neither Moody’s nor S&P shall be rating such obligations, an equivalent rating from another Rating Agency) and in each case maturing within 24 months after the date of creation thereof and Indebtedness or Preferred Stock issued by Persons with a rating of “A” or higher from S&P or “A-2” or higher from Moody’s with maturities of 24 months or less from the date of acquisition;
(7) marketable short-term money market and similar funds having a rating of at least P-2 or A-2 from either Moody’s or S&P, respectively (or, if at any time neither Moody’s nor S&P shall be rating such obligations, an equivalent rating from another Rating Agency);
(8) readily marketable direct obligations issued by any state, commonwealth or territory of the United States or any political subdivision or taxing authority thereof having an Investment Grade Rating from either Moody’s or S&P (or, if at any time neither Moody’s nor S&P shall be rating such obligations, an equivalent rating from another Rating Agency) with maturities of 24 months or less from the date of acquisition;
(9) readily marketable direct obligations issued by any foreign government or any political subdivision or public instrumentality thereof, in each case having an Investment Grade Rating from either Moody’s or S&P (or, if at any time neither Moody’s nor S&P shall be rating such obligations, an equivalent rating from another Rating Agency) with maturities of 24 months or less from the date of acquisition;
166
(10) Investments with average maturities of 12 months or less from the date of acquisition in money market funds rated AAA- (or the equivalent thereof) or better by S&P or Aaa3 (or the equivalent thereof) or better by Moody’s (or, if at any time neither Moody’s nor S&P shall be rating such obligations, an equivalent rating from another Rating Agency); and
(11) investment funds investing at least 90.0% of their assets in securities of the types described in clauses (1) through (10) above.
In the case of Investments by any Foreign Subsidiary that is a Restricted Subsidiary or Investments made in a country outside the United States of America, Cash Equivalents shall also include (a) investments of the type and maturity described in clauses (1) through (8) and clauses (10) and (11) above of foreign obligors, which Investments or obligors (or the parents of such obligors) have ratings described in such clauses or equivalent ratings from comparable foreign rating agencies and (b) other short-term investments utilized by Foreign Subsidiaries that are Restricted Subsidiaries in accordance with normal investment practices for cash management in investments analogous to the foregoing investments in clauses (1) through (11) and in this paragraph.
Notwithstanding the foregoing, Cash Equivalents shall include amounts denominated in currencies other than those set forth in clauses (1) and (2) above, provided that such amounts are converted into any currency listed in clauses (1) and (2) as promptly as practicable and in any event within ten Business Days following the receipt of such amounts.
At any time at which the value, calculated in accordance with GAAP, of all investments of the Issuer and its Restricted Subsidiaries that were deemed, when made, to be Cash Equivalents in accordance with clauses (1) through (11) above exceeds the Indebtedness of the Issuer and its Restricted Subsidiaries, “Cash Equivalents” shall also mean any investment (a “Qualifying Investment”) that satisfies the following two conditions: (a) the Qualifying Investment is of a type described in clauses (1) through (11) of this definition, but has an effective maturity (whether by reason of final maturity, a put option or, in the case of an asset-backed security, an average life) of five years and one month or less from the date of such Qualifying Investment (notwithstanding any provision contained in such clauses (1) through (11) requiring a shorter maturity); and (b) the weighted average effective maturity of such Qualifying Investment and all other investments that were made as Qualifying Investments in accordance with this paragraph, does not exceed two years from the date of such Qualifying Investment.
“CF Credit Facilities” means the term and revolving credit facilities under the Credit Agreement dated as of September 25, 2007 by and among the Issuer, the European subsidiary borrowers party thereto, the lenders party thereto in their capacities as lenders thereunder and Bank of America, N.A., as Administrative Agent, including any guarantees, collateral documents, instruments and agreements executed in connection therewith, and any amendments, supplements, modifications, extensions, renewals, restatements, refundings or refinancings thereof and any indentures or credit facilities or commercial paper facilities with banks or other institutional lenders or investors that replace, refund or refinance any part of the loans, notes, other credit facilities or commitments thereunder, including any such replacement, refunding or refinancing facility or indenture that increases the amount borrowable thereunder or alters the maturity thereof (provided that such increase in borrowings is permitted under “—Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock” above).
“Change of Control” means the occurrence of any of the following:
(1) the sale, lease or transfer, in one or a series of related transactions, of all or substantially all of the assets of the Issuer and its Subsidiaries, taken as a whole, to any Person other than a Permitted Holder; or
(2) the Issuer becomes aware of (by way of a report or any other filing pursuant to Section 13(d) of the Exchange Act, proxy, vote, written notice or otherwise) the acquisition by any Person or group (within the meaning of Section 13(d)(3) or Section 14(d)(2) of the Exchange Act, or any successor provision), including any group acting for the purpose of acquiring, holding or disposing of securities (within the meaning of Rule 13d-5(b)(1) under the Exchange Act), other than one or more Permitted Holders, in a single transaction or in a related series of transactions, by way of merger, consolidation or other business combination or purchase of beneficial ownership (within the meaning of Rule 13d-3 under the Exchange Act, or any successor provision) of 50.0% or more of the total voting power of the Voting Stock of the Issuer or any of its direct or indirect parent companies.
“Consolidated Depreciation and Amortization Expense” means with respect to any Person for any period, the total amount of depreciation and amortization expense of such Person, including the amortization of deferred financing
167
fees, debt issuance costs, commissions, fees and expenses and Capitalized Software Expenditures of such Person and its Restricted Subsidiaries for such period on a consolidated basis and otherwise determined in accordance with GAAP.
“Consolidated Interest Expense” means, with respect to any Person for any period, without duplication, the sum of:
(1) consolidated interest expense of such Person and its Restricted Subsidiaries for such period, to the extent such expense was deducted (and not added back) in computing Consolidated Net Income (including (a) amortization of original issue discount resulting from the issuance of Indebtedness at less than par, (b) all commissions, discounts and other fees and charges owed with respect to letters of credit or bankers acceptances, (c) non-cash interest payments (but excluding any non-cash interest expense attributable to the movement in the mark to market valuation of Hedging Obligations or other derivative instruments pursuant to GAAP), (d) the interest component of Capitalized Lease Obligations, and (e) net payments, if any, made (less net payments, if any, received), pursuant to interest rate Hedging Obligations with respect to Indebtedness, and excluding (t) any expense resulting from the discounting of any Indebtedness in connection with the application of recapitalization accounting or, if applicable, purchase accounting in connection with the 2007 Acquisition or any other acquisition, (u) penalties and interest relating to taxes, (v) any Additional Interest and any “additional interest” with respect to other securities, (w) amortization of deferred financing fees, debt issuance costs, commissions, fees and expenses, (x) any expensing of bridge, commitment and other financing fees, (y) commissions, discounts, yield and other fees and charges (including any interest expense) related to any Qualified Securitization Facility and (z) any accretion of accrued interest on discounted liabilities); plus
(2) consolidated capitalized interest of such Person and its Restricted Subsidiaries for such period, whether paid or accrued; less
(3) interest income of such Person and its Restricted Subsidiaries for such period.
For purposes of this definition, interest on a Capitalized Lease Obligation shall be deemed to accrue at an interest rate reasonably determined by such Person to be the rate of interest implicit in such Capitalized Lease Obligation in accordance with GAAP.
“Consolidated Net Income” means, with respect to any Person for any period, the aggregate of the Net Income of such Person and its Restricted Subsidiaries for such period, on a consolidated basis, and otherwise determined in accordance with GAAP; provided that, without duplication,
(1) any after-tax effect of extraordinary, non-recurring or unusual gains or losses (less all fees and expenses relating thereto) or expenses (including relating to the 2007 Acquisition or any multi-year strategic initiatives, severance, relocation costs and curtailments or modifications to pension and post-retirement employee benefit plans) shall be excluded;
(2) the cumulative effect of a change in accounting principles and changes as a result of the adoption or modification of accounting policies during such period shall be excluded;
(3) any net after-tax gains or losses on disposal of disposed, abandoned or discontinued operations shall be excluded;
(4) any net after-tax effect of gains or losses (less all fees, expenses and charges relating thereto) attributable to asset dispositions or abandonments or the sale or other disposition of any Capital Stock of any Person other than in the ordinary course of business shall be excluded;
(5) the Net Income for such period of any Person that is an Unrestricted Subsidiary shall be excluded, and, solely for the purpose of determining the amount available for Restricted Payments under clause (3)(a) of the first paragraph of “—Certain Covenants—Limitation on Restricted Payments,” the Net Income for such period of any Person that is not a Subsidiary or that is accounted for by the equity method of accounting shall be excluded; provided that Consolidated Net Income of the Issuer shall be increased by the amount of dividends or distributions or other payments that are actually paid in cash (or to the extent converted into cash) to the Issuer or a Restricted Subsidiary thereof in respect of such period;
(6) solely for the purpose of determining the amount available for Restricted Payments under clause (3)(a) of the first paragraph of “—Certain Covenants—Limitation on Restricted Payments,” the Net Income for such period of any
168
Restricted Subsidiary (other than any Guarantor) shall be excluded to the extent that the declaration or payment of dividends or similar distributions by that Restricted Subsidiary of its Net Income is not at the date of determination permitted without any prior governmental approval (which has not been obtained) or, directly or indirectly, by the operation of the terms of its charter or any agreement, instrument, judgment, decree, order, statute, rule, or governmental regulation applicable to that Restricted Subsidiary or its stockholders, unless such restriction with respect to the payment of dividends or similar distributions has been legally waived, provided that Consolidated Net Income of the Issuer will be increased by the amount of dividends or other distributions or other payments actually paid in cash (or to the extent converted into cash) to the Issuer or a Restricted Subsidiary thereof in respect of such period, to the extent not already included therein;
(7) effects of adjustments (including the effects of such adjustments pushed down to the Issuer and its Restricted Subsidiaries) in such Person’s consolidated financial statements pursuant to GAAP resulting from the application of recapitalization accounting or, if applicable, purchase accounting in relation to the Transactions or any consummated acquisition or the amortization or write-off of any amounts thereof, net of taxes, shall be excluded;
(8) any after-tax effect of income (loss) from the early extinguishment of (a) Indebtedness, (b) Hedging Obligations or (c) other derivative instruments shall be excluded;
(9) any impairment charge or asset write-off or write-down, including impairment charges or asset write-offs or write-downs related to intangible assets, long-lived assets, investments in debt and equity securities or as a result of a change in law or regulation, in each case, pursuant to GAAP, and the amortization of intangibles arising pursuant to GAAP shall be excluded;
(10) any non-cash compensation expense recorded from grants of stock appreciation or similar rights, stock options, restricted stock or other rights, and any cash charges associated with the rollover, acceleration, or payout of Equity Interests by management of the Issuer or any of its direct or indirect parent companies in connection with the Transactions, shall be excluded;
(11) any fees, expenses or charges incurred during such period, or any amortization thereof for such period, in connection with any acquisition, Investment, Asset Sale, incurrence or repayment of Indebtedness (including such fees, expenses or charges related to the offering of the senior notes and the other Transactions), issuance of Equity Interests, refinancing transaction or amendment or modification of any debt instrument (including any amendment or other modification of the senior notes and the Credit Facilities) and including, in each case, any such transaction consummated prior to the Issue Date and any such transaction undertaken but not completed, and any charges or non-recurring merger costs incurred during such period as a result of any such transaction, in each case whether or not successful, shall be excluded;
(12) accruals and reserves that are established within twelve months after the closing of any acquisition (including the 2007 Acquisition) that are so required to be established as a result of such acquisition in accordance with GAAP shall be excluded;
(13) to the extent covered by insurance and actually reimbursed, or, so long as the Issuer has made a determination that there exists reasonable evidence that such amount will in fact be reimbursed by the insurer and only to the extent that such amount is (a) not denied by the applicable carrier in writing 180 days and (b) in fact reimbursed within 365 days of the date of the insurable event (with a deduction for any amount so added back to the extent not so reimbursed within such 365-day period), expenses with respect to liability or casualty events or business interruption shall be excluded;
(14) any non-cash compensation expense resulting from the application of Financial Accounting Standards Board Accounting Standards Codification 718 and 505-50, as applicable, shall be excluded; and
(15) the following items shall be excluded:
(a) any net unrealized gain or loss (after any offset) resulting in such period from Hedging Obligations and the application of Financial Accounting Standards Board Accounting Standards Codification 815; and
(b) any net unrealized gain or loss (after any offset) resulting in such period from currency translation gains or losses including those related to currency remeasurements of Indebtedness (including any net loss or gain resulting from Hedging Obligations for currency exchange risk).
169
In addition, to the extent not already included in the Consolidated Net Income of such Person and its Restricted Subsidiaries, notwithstanding anything to the contrary in the foregoing, Consolidated Net Income shall include the amount of proceeds received from business interruption insurance and reimbursements of any expenses and charges that are covered by indemnification or other reimbursement provisions in connection with any Permitted Investment or any sale, conveyance, transfer or other disposition of assets permitted under the senior notes indenture.
Notwithstanding the foregoing, for the purpose of the covenant described under “—Certain Covenants—Limitation on Restricted Payments” only (other than clause (3)(d) of the first paragraph thereof), there shall be excluded from Consolidated Net Income any income arising from any sale or other disposition of Restricted Investments made by the Issuer and its Restricted Subsidiaries, any repurchases and redemptions of Restricted Investments from the Issuer and its Restricted Subsidiaries, any repayments of loans and advances which constitute Restricted Investments by the Issuer or any of its Restricted Subsidiaries, any sale of the stock of an Unrestricted Subsidiary or any distribution or dividend from an Unrestricted Subsidiary, in each case only to the extent such amounts increase the amount of Restricted Payments permitted under such covenant pursuant to clause (3)(d) thereof.
“Contingent Obligations” means, with respect to any Person, any obligation of such Person guaranteeing any leases, dividends or other obligations that do not constitute Indebtedness (“primary obligations”) of any other Person (the “primary obligor”) in any manner, whether directly or indirectly, including, without limitation, any obligation of such Person, whether or not contingent,
(1) to purchase any such primary obligation or any property constituting direct or indirect security therefor;
(2) to advance or supply funds
(a) for the purchase or payment of any such primary obligation, or
(b) to maintain working capital or equity capital of the primary obligor or otherwise to maintain the net worth or solvency of the primary obligor; or
(3) to purchase property, securities or services primarily for the purpose of assuring the owner of any such primary obligation of the ability of the primary obligor to make payment of such primary obligation against loss in respect thereof.
“Controlled Investment Affiliate” means, as to any Person, any other Person, other than any Investor, which directly or indirectly is in control of, is controlled by, or is under common control with such Person and is organized by such Person (or any Person controlling such Person) primarily for making direct or indirect equity or debt investments in the Issuer and/or other companies.
“Credit Facilities” means, with respect to the Issuer or any of its Restricted Subsidiaries, one or more debt facilities, including the Senior Credit Facilities or other financing arrangements (including, without limitation, commercial paper facilities or indentures) providing for revolving credit loans, term loans, letters of credit or other long-term indebtedness, including any notes, mortgages, guarantees, collateral documents, instruments and agreements executed in connection therewith, and any amendments, supplements, modifications, extensions, renewals, restatements or refundings thereof and any indentures or credit facilities or commercial paper facilities that replace, refund or refinance any part of the loans, notes, other credit facilities or commitments thereunder, including any such replacement, refunding or refinancing facility or indenture that increases the amount permitted to be borrowed thereunder or alters the maturity thereof (provided that such increase in borrowings is permitted under “—Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”) or adds Restricted Subsidiaries as additional borrowers or guarantors thereunder and whether by the same or any other agent, lender or group of lenders.
“Default” means any event that is, or with the passage of time or the giving of notice or both would be, an Event of Default.
“Designated Non-cash Consideration” means the fair market value of non-cash consideration received by the Issuer or a Restricted Subsidiary in connection with an Asset Sale that is so designated as Designated Non-cash Consideration pursuant to an Officer’s Certificate, setting forth the basis of such valuation, executed by the principal financial officer of the Issuer, less the amount of Cash Equivalents received in connection with a subsequent sale of or collection on such Designated Non-cash Consideration.
170
“Designated Preferred Stock” means Preferred Stock of the Issuer or any parent company thereof (in each case other than Disqualified Stock) that is issued for cash (other than to a Restricted Subsidiary or an employee stock ownership plan or trust established by the Issuer or any of its Subsidiaries) and is so designated as Designated Preferred Stock, pursuant to an Officer’s Certificate executed by the principal financial officer of the Issuer or the applicable parent company thereof, as the case may be, on the issuance date thereof, the cash proceeds of which are excluded from the calculation set forth in clause (3) of the first paragraph of “—Certain Covenants—Limitation on Restricted Payments.”
“Disqualified Stock” means, with respect to any Person, any Capital Stock of such Person which, by its terms, or by the terms of any security into which it is convertible or for which it is putable or exchangeable, or upon the happening of any event, matures or is mandatorily redeemable (other than solely as a result of a change of control or asset sale) pursuant to a sinking fund obligation or otherwise, or is redeemable at the option of the holder thereof (other than solely as a result of a change of control or asset sale), in whole or in part, in each case prior to the date 91 days after the earlier of the maturity date of the senior notes or the date the senior notes are no longer outstanding; provided that if such Capital Stock is issued to any plan for the benefit of employees of the Issuer or its Subsidiaries or by any such plan to such employees, such Capital Stock shall not constitute Disqualified Stock solely because it may be required to be repurchased by the Issuer or its Subsidiaries in order to satisfy applicable statutory or regulatory obligations; provided, further, that any Capital Stock held by any future, current or former employee, director, officer, manager, distributor or consultant (or their respective Controlled Investment Affiliates or Immediate Family Members), of the Issuer, any of its Subsidiaries, any of its direct or indirect parent companies or any other entity in which the Issuer or a Restricted Subsidiary has an Investment and is designated in good faith as an “affiliate” by the board of directors of the Issuer (or the compensation committee thereof), in each case pursuant to any stock subscription or shareholders’ agreement, management equity plan or stock option plan or any other management or employee benefit plan or agreement or any distributor equity plan or agreement shall not constitute Disqualified Stock solely because it may be required to be repurchased by the Issuer or its Subsidiaries.
“EBITDA” means, with respect to any Person for any period, the Consolidated Net Income of such Person for such period
(1) increased (without duplication) by the following, in each case to the extent deducted (and not added back) in determining Consolidated Net Income for such period:
(a) provision for taxes based on income or profits or capital, including, without limitation, state, franchise and similar taxes, foreign withholding taxes (including any future taxes or other levies which replace or are intended to be in lieu of such taxes and any penalties and interest related to such taxes or arising from tax examinations) and the net tax expense associated with any adjustments made pursuant to clauses (1) through (15) of the definition of “Consolidated Net Income”; plus
(b) Fixed Charges of such Person for such period (including (x) net losses or Hedging Obligations or other derivative instruments entered into for the purpose of hedging interest rate risk, (y) bank fees and (z) costs of surety bonds in connection with financing activities, plus amounts excluded from Consolidated Interest Expense as set forth in clauses (1)(t) through (z) in the definition thereof); plus
(c) Consolidated Depreciation and Amortization Expense of such Person for such period; plus
(d) [reserved]; plus
(e) the amount of any restructuring charges, integration and facilities opening costs or other business optimization expenses (including cost and expenses relating to business optimization programs and new systems design and implementation costs) or accruals or reserves, including any one-time costs incurred in connection with acquisitions after the Issue Date, project start-up costs and costs related to the closure and/or consolidation of facilities; plus
(f) any other non-cash charges, including any write offs or write downs reducing Consolidated Net Income for such period (provided that if any such non-cash charges represent an accrual or reserve for potential cash items in any future period, the cash payment in respect thereof in such future period shall be subtracted from EBITDA to such extent, and excluding amortization of a prepaid cash item that was paid in a prior period); plus
(g) the amount of any minority interest expense consisting of Subsidiary income attributable to minority equity interests of third parties in any non-Wholly Owned Subsidiary; plus
171
(h) the amount of management, monitoring, consulting and advisory fees (including termination fees) and related indemnities and expenses paid or accrued in such period under the Management Fee Agreement or otherwise to the Investors to the extent otherwise permitted under “—Certain Covenants—Transactions with Affiliates”; plus
(i) the amount of “run-rate” cost savings projected by the Issuer in good faith to result from actions either taken or expected to be taken within 12 months after the end of such period (which cost savings shall be subject only to certification by management of the Issuer and calculated on a pro forma basis as though such cost savings had been realized on the first day of such period), net of the amount of actual benefits realized from such actions (it is understood and agreed that “run-rate” means the full recurring benefit that is associated with any action taken or expected to be taken, provided that some portion of such benefit is expected to be realized within 12 months of taking such action) (which adjustments may be incremental to pro forma cost savings, operating improvements, synergies and operating expense adjustments made pursuant to the definition of “Fixed Charge Coverage Ratio”); plus
(j) the amount of loss on sale of receivables, Securitization Assets and related assets to the Securitization Subsidiary in connection with a Qualified Securitization Facility; plus
(k) any costs or expense incurred by the Issuer or a Restricted Subsidiary pursuant to any management equity plan or stock option plan or any other management or employee benefit plan, agreement or any stock subscription or shareholder agreement or any distributor equity plan or agreement, to the extent that such cost or expenses are funded with cash proceeds contributed to the capital of the Issuer or net cash proceeds of an issuance of Equity Interest of the Issuer (other than Disqualified Stock) solely to the extent that such net cash proceeds are excluded from the calculation set forth in clause (3) of the first paragraph under “—Certain Covenants—Limitation on Restricted Payments”; plus
(l) cash receipts (or any netting arrangements resulting in reduced cash expenditures) not representing EBITDA or Consolidated Net Income in any period to the extent non-cash gains relating to such income were deducted in the calculation of EBITDA pursuant to clause (2) below for any previous period and not added back; plus
(m) any net loss from disposed or discontinued operations or from operations expected to be disposed of or discontinued within twelve months after the end of such period; plus
(n) interest income or investment earnings on retiree medical and intellectual property, royalty or license receivables; plus
(o) extraordinary losses and unusual or non-recurring charges (including any unusual or non-recurring operating expenses attributable to the implementation of cost-savings initiatives, severance, retention and relocation costs and curtailments and modifications to pension and post-retirement employee benefit plans); plus
(p) [reserved]; plus
(q) [reserved]; plus
(r) losses on asset sales (other than asset sales made in the ordinary course of business), disposals and abandonments;
(2) decreased (without duplication) by the following, in each case to the extent included in determining Consolidated Net Income for such period:
(a) non-cash gains increasing Consolidated Net Income of such Person for such period, excluding any non-cash gains to the extent they represent the reversal of an accrual or reserve for a potential cash item that reduced EBITDA in any prior period; plus
(b) any non-cash gains with respect to cash actually received in a prior period unless such cash did not increase EBITDA in such prior period; plus
172
(c) any net income from disposed or discontinued operations (excluding held-for-sale discontinued operations) or from operations expected to be disposed of or discontinued within twelve months after the end of such period; plus
(d) extraordinary gains and unusual or non-recurring gains; plus
(e) gains on asset sales (other than asset sales made in the ordinary course of business), disposals and abandonments.
“EMU” means economic and monetary union as contemplated in the Treaty on European Union. “Equity Interests” means Capital Stock and all warrants, options or other rights to acquire Capital Stock, but excluding any debt security that is convertible into, or exchangeable for, Capital Stock.
“Equity Offering” means any public or private sale of common stock or Preferred Stock of the Issuer or any of its direct or indirect parent companies (excluding Disqualified Stock), other than:
(1) public offerings with respect to the Issuer’s or any direct or indirect parent company’s common stock registered on Form S-4 or Form S-8;
(2) issuances to any Subsidiary of the Issuer; and
(3) any such public or private sale that constitutes an Excluded Contribution.
“euro” means the single currency of participating member states of the EMU.
“Exchange Act” means the Securities Exchange Act of 1934, as amended, and the rules and regulations of the SEC promulgated thereunder.
“Excluded Contribution” means net cash proceeds, marketable securities or Qualified Proceeds received by the Issuer from:
(1) contributions to its common equity capital; and
(2) the sale (other than to a Subsidiary of the Issuer or to any management equity plan or stock option plan or any other management or employee benefit plan or agreement or any distributor equity plan or agreement of the Issuer) of Capital Stock (other than Disqualified Stock and Designated Preferred Stock) of the Issuer;
in each case designated as Excluded Contributions pursuant to an Officer’s Certificate executed by the principal financial officer of the Issuer on the date such capital contributions are made or the date such Equity Interests are sold, as the case may be, which are excluded from the calculation set forth in clause (3) of the first paragraph under “—Certain Covenants—Limitation on Restricted Payments.”
“fair market value” means, with respect to any asset or liability, the fair market value of such asset or liability as determined by the Issuer in good faith.
“Fixed Charge Coverage Ratio” means, with respect to any Person for any period, the ratio of EBITDA of such Person for such period to the Fixed Charges of such Person for such period. In the event that the Issuer or any Restricted Subsidiary incurs, assumes, guarantees, redeems, retires or extinguishes any Indebtedness (other than Indebtedness incurred under any revolving credit facility unless such Indebtedness has been permanently repaid and has not been replaced) or issues or redeems Disqualified Stock or Preferred Stock subsequent to the commencement of the period for which the Fixed Charge Coverage Ratio is being calculated but prior to or simultaneously with the event for which the calculation of the Fixed Charge Coverage Ratio is made (the “Fixed Charge Coverage Ratio Calculation Date”), then the Fixed Charge Coverage Ratio shall be calculated giving pro forma effect to such incurrence, assumption, guarantee, redemption, retirement or extinguishment of Indebtedness, or such issuance or redemption of Disqualified Stock or Preferred Stock, as if the same had occurred at the beginning of the applicable four-quarter period.
For purposes of making the computation referred to above, Investments, acquisitions, dispositions, mergers, consolidations and discontinued operations (as determined in accordance with GAAP) that have been made by the Issuer or any of its Restricted Subsidiaries during the four-quarter reference period or subsequent to such reference period and
173
on or prior to or simultaneously with the Fixed Charge Coverage Ratio Calculation Date shall be calculated on a pro forma basis assuming that all such Investments, acquisitions, dispositions, mergers, consolidations and discontinued operations (and the change in any associated fixed charge obligations and the change in EBITDA resulting therefrom) had occurred on the first day of the four-quarter reference period. If since the beginning of such period any Person that subsequently became a Restricted Subsidiary or was merged with or into the Issuer or any of its Restricted Subsidiaries since the beginning of such period shall have made any Investment, acquisition, disposition, merger, consolidation or discontinued operation that would have required adjustment pursuant to this definition, then the Fixed Charge Coverage Ratio shall be calculated giving pro forma effect thereto for such period as if such Investment, acquisition, disposition, merger, consolidation or discontinued operation had occurred at the beginning of the applicable four-quarter period.
For purposes of this definition, whenever pro forma effect is to be given to an Investment, acquisition, disposition, merger or consolidation, the pro forma calculations shall be made in good faith by a responsible financial or accounting officer of the Issuer (and may include, for the avoidance of doubt, cost savings, operating improvements, synergies and operating expense reductions resulting from such Investment, acquisition, merger or consolidation which is being given pro forma effect that have been or are expected to be realized). If any Indebtedness bears a floating rate of interest and is being given pro forma effect, the interest on such Indebtedness shall be calculated as if the rate in effect on the Fixed Charge Coverage Ratio Calculation Date had been the applicable rate for the entire period (taking into account any Hedging Obligations applicable to such Indebtedness). Interest on a Capitalized Lease Obligation shall be deemed to accrue at an interest rate reasonably determined by a responsible financial or accounting officer of the Issuer to be the rate of interest implicit in such Capitalized Lease Obligation in accordance with GAAP. For purposes of making the computation referred to above, interest on any Indebtedness under a revolving credit facility computed on a pro forma basis shall be computed based upon the average daily balance of such Indebtedness during the applicable period except as set forth in the first paragraph of this definition. Interest on Indebtedness that may optionally be determined at an interest rate based upon a factor of a prime or similar rate, a eurocurrency interbank offered rate, or other rate, shall be deemed to have been based upon the rate actually chosen, or, if none, then based upon such optional rate chosen as the Issuer may designate.
“Fixed Charges” means, with respect to any Person for any period, the sum of, without duplication:
(1) Consolidated Interest Expense of such Person for such period;
(2) all cash dividends or other distributions paid (excluding items eliminated in consolidation) on any series of Preferred Stock during such period; and
(3) all cash dividends or other distributions paid (excluding items eliminated in consolidation) on any series of Disqualified Stock during such period.
“Foreign Subsidiary” means, with respect to any Person, any Restricted Subsidiary of such Person that is not organized or existing under the laws of the United States, any state thereof, the District of Columbia, or any territory thereof and any Restricted Subsidiary of such Foreign Subsidiary.
“Foreign Subsidiary Total Assets” means the total assets of the Foreign Subsidiaries, as determined in accordance with GAAP in good faith by the Issuer, without intercompany eliminations.
“GAAP” means generally accepted accounting principles in the United States of America which are in effect on the Issue Date.
“Government Securities” means securities that are:
(1) direct obligations of the United States of America for the timely payment of which its full faith and credit is pledged; or
(2) obligations of a Person controlled or supervised by and acting as an agency or instrumentality of the United States of America the timely payment of which is unconditionally guaranteed as a full faith and credit obligation by the United States of America,
which, in either case, are not callable or redeemable at the option of the issuers thereof, and shall also include a depository receipt issued by a bank (as defined in Section 3(a)(2) of the Securities Act), as custodian with respect to any such Government Securities or a specific payment of principal of or interest on any such Government Securities held by such
174
custodian for the account of the holder of such depository receipt; provided that (except as required by law) such custodian is not authorized to make any deduction from the amount payable to the holder of such depository receipt from any amount received by the custodian in respect of the Government Securities or the specific payment of principal of or interest on the Government Securities evidenced by such depository receipt.
“guarantee” means a guarantee (other than by endorsement of negotiable instruments for collection in the ordinary course of business), direct or indirect, in any manner (including letters of credit and reimbursement agreements in respect thereof), of all or any part of any Indebtedness or other obligations.
“Guarantee” means the guarantee by any Guarantor of the Issuer’s Obligations under the senior notes indenture and the senior notes.
“Guarantor” means each Subsidiary of the Issuer, if any, that Guarantees the senior notes in accordance with the terms of the senior notes indenture.
“Hedging Obligations” means, with respect to any Person, the obligations of such Person under any interest rate swap agreement, interest rate cap agreement, interest rate collar agreement, commodity swap agreement, commodity cap agreement, commodity collar agreement, foreign exchange contract, currency swap agreement or similar agreement providing for the transfer or mitigation of interest rate or currency risks either generally or under specific contingencies.
“Holder” means the Person in whose name a senior note is registered on the registrar’s books.
“Immediate Family Members” means with respect to any individual, such individual’s child, stepchild, grandchild or more remote descendant, parent, stepparent, grandparent, spouse, former spouse, qualified domestic partner, sibling, mother-in-law, father-in-law, son-in-law and daughter-in-law (including adoptive relationships) and any trust, partnership or other bona fide estate-planning vehicle the only beneficiaries of which are any of the foregoing individuals or any private foundation or fund that is controlled by any if the foregoing individuals or any donor-advised fund of which any such individual is the donor.
“Indebtedness” means, with respect to any Person, without duplication:
(1) any indebtedness (including principal and premium) of such Person, whether or not contingent:
(a) in respect of borrowed money;
(b) evidenced by bonds, notes, debentures or similar instruments or letters of credit or bankers’ acceptances (or, without duplication, reimbursement agreements in respect thereof);
(c) representing the balance deferred and unpaid of the purchase price of any property (including Capitalized Lease Obligations) due more than twelve months after such property is acquired, except (i) any such balance that constitutes an obligation in respect of a commercial letter of credit, a trade payable or similar obligation to a trade creditor, in each case accrued in the ordinary course of business and (ii) any earn-out obligations until such obligation becomes a liability on the balance sheet of such Person in accordance with GAAP and if not paid after becoming due and payable;
(d) representing the net obligations under any Hedging Obligations; or
(e) during a Suspension Period only, obligations of the lessee for rental payments in respect of Sale and Lease-back Transactions in an amount equal to the present value of such obligations during the remaining term of the lease using a discount rate equal to the rate of interest implicit in such transaction determined in accordance with GAAP,
if and to the extent that any of the foregoing Indebtedness (other than letters of credit and Hedging Obligations) would appear as a liability upon a balance sheet (excluding the footnotes thereto) of such Person prepared in accordance with GAAP; provided that Indebtedness of any direct or indirect parent of the Issuer appearing upon the balance sheet of the Issuer solely by reason of push-down accounting under GAAP shall be excluded;
(2) to the extent not otherwise included, any obligation by such Person to be liable for, or to pay, as obligor, guarantor or otherwise, on the obligations of the type referred to in clause (1) of a third Person (whether or not such items
175
would appear upon the balance sheet of the such obligor or guarantor), other than by endorsement of negotiable instruments for collection in the ordinary course of business; and
(3) to the extent not otherwise included, the obligations of the type referred to in clause (1) of a third Person secured by a Lien on any asset owned by such first Person, whether or not such Indebtedness is assumed by such first Person;
provided that notwithstanding the foregoing, Indebtedness shall be deemed not to include (a) Contingent Obligations incurred in the ordinary course of business or (b) obligations under or in respect of Qualified Securitization Facilities.
“Independent Financial Advisor” means an accounting, appraisal, investment banking firm or consultant to Persons engaged in Similar Businesses of nationally recognized standing that is, in the good faith judgment of the Issuer, qualified to perform the task for which it has been engaged.
“Initial Purchasers” means Goldman, Sachs & Co., Merrill Lynch, Pierce, Fenner & Smith Incorporated, Barclays Capital Inc., Citigroup Global Markets Inc., J.P. Morgan Securities LLC, Wells Fargo Securities, LLC, HSBC Securities (USA) Inc., ING Financial Markets LLC, Natixis Securities Americas LLC, RBC Capital Markets, LLC, SMBC Nikko Capital Markets Limited and UBS Securities LLC, as the initial purchasers for the 6.500% Senior Notes due 2020.
“Investment Grade Rating” means a rating equal to or higher than Baa3 (or the equivalent) by Moody’s and BBB- (or the equivalent) by S&P, or an equivalent rating by any other Rating Agency.
“Investment Grade Securities” means:
(1) securities issued or directly and fully guaranteed or insured by the United States government or any agency or instrumentality thereof (other than Cash Equivalents);
(2) debt securities or debt instruments with an Investment Grade Rating, but excluding any debt securities or instruments constituting loans or advances among the Issuer and its Subsidiaries;
(3) investments in any fund that invests exclusively in investments of the type described in clauses (1) and (2) which fund may also hold immaterial amounts of cash pending investment or distribution; and
(4) corresponding instruments in countries other than the United States customarily utilized for high quality investments.
“Investments” means, with respect to any Person, all investments by such Person in other Persons (including Affiliates) in the form of loans (including guarantees), advances or capital contributions (excluding accounts receivable, trade credit, advances to customers and distributors, commission, travel and similar advances to employees, directors, officers, managers, distributors and consultants in each case made in the ordinary course of business), purchases or other acquisitions for consideration of Indebtedness, Equity Interests or other securities issued by any other Person and investments that are required by GAAP to be classified on the balance sheet (excluding the footnotes) of the Issuer in the same manner as the other investments included in this definition to the extent such transactions involve the transfer of cash or other property. For purposes of the definition of “Unrestricted Subsidiary” and the covenant described under “—Certain Covenants—Limitation on Restricted Payments”:
(1) “Investments” shall include the portion (proportionate to the Issuer’s equity interest in such Subsidiary) of the fair market value of the net assets of a Subsidiary of the Issuer at the time that such Subsidiary is designated an Unrestricted Subsidiary; provided that upon a redesignation of such Subsidiary as a Restricted Subsidiary, the Issuer shall be deemed to continue to have a permanent “Investment” in an Unrestricted Subsidiary in an amount (if positive) equal to:
(a) the Issuer’s “Investment” in such Subsidiary at the time of such redesignation; less
(b) the portion (proportionate to the Issuer’s Equity Interest in such Subsidiary) of the fair market value of the net assets of such Subsidiary at the time of such redesignation; and
176
(2) any property transferred to or from an Unrestricted Subsidiary shall be valued at its fair market value at the time of such transfer.
The amount of any Investment outstanding at any time shall be the original cost of such Investment, reduced by any dividend, distribution, interest payment, return of capital, repayment or other amount received in cash by the Issuer or a Restricted Subsidiary in respect of such Investment.
“Investors” means The Blackstone Group, Goldman Sachs Capital Partners, Kohlberg Kravis Roberts & Co., TPG Global, LLC and, if applicable, each of their respective Affiliates and funds or partnerships managed by any of them or their respective Affiliates but not including, however, any portfolio companies of any of the foregoing.
“Issue Date” means August 8, 2012, the date of the issuance of the Initial Notes.
“Issuer” means Biomet, Inc., an Indiana corporation (and not any of its Subsidiaries), and its successors.
“Legal Holiday” means a Saturday, a Sunday or a day on which commercial banking institutions are not required to be open in the State of New York or place of payment.
“Lien” means, with respect to any asset, any mortgage, lien (statutory or otherwise), pledge, hypothecation, charge, security interest, preference, priority or encumbrance of any kind in respect of such asset, whether or not filed, recorded or otherwise perfected under applicable law, including any conditional sale or other title retention agreement, any lease in the nature thereof, any option or other agreement to sell or give a security interest in and any filing of or agreement to give any financing statement under the Uniform Commercial Code (or equivalent statutes) of any jurisdiction; provided that in no event shall an operating lease be deemed to constitute a Lien.
“Management Fee Agreement” means the management services agreement, dated as of September 25, 2007 between certain of the management companies associated with the Investors or their advisors, if applicable, and the Issuer.
“Management Stockholders” means the members of management (and their Controlled Investment Affiliates and Immediate Family Members) of the Issuer (or its direct parent) who are holders of Equity Interests of any direct or indirect parent companies of the Issuer on the Issue Date or will become holders of such Equity Interests in connection with the Acquisition.
“Merger Agreement” means the Agreement and Plan of Merger, dated December 18, 2006 (as amended and restated as of June 7, 2007) by and among the Issuer, the Parent and LVB Acquisition Merger Sub, Inc.
“Moody’s” means Moody’s Investors Service, Inc. and any successor to its rating agency business.
“Net Income” means, with respect to any Person, the net income (loss) of such Person, determined in accordance with GAAP and before any reduction in respect of Preferred Stock dividends.
“Net Proceeds” means the aggregate cash proceeds received by the Issuer or any of its Restricted Subsidiaries in respect of any Asset Sale, including any cash received upon the sale or other disposition of any Designated Non-cash Consideration received in any Asset Sale, net of the direct costs relating to such Asset Sale and the sale or disposition of such Designated Non-cash Consideration, including legal, accounting and investment banking fees, payments made in order to obtain a necessary consent or required by applicable law, and brokerage and sales commissions, any relocation expenses incurred as a result thereof, other fees and expenses, including title and recordation expenses, taxes paid or payable as a result thereof (after taking into account any available tax credits or deductions and any tax sharing arrangements), amounts required to be applied to the repayment of principal, premium, if any, and interest on Senior Indebtedness required (other than required by clause (1) of the second paragraph of “Repurchase at the Option of Holders—Asset Sales”) to be paid as a result of such transaction and any deduction of appropriate amounts to be provided by the Issuer or any of its Restricted Subsidiaries as a reserve in accordance with GAAP against any liabilities associated with the asset disposed of in such transaction and retained by the Issuer or any of its Restricted Subsidiaries after such sale or other disposition thereof, including pension and other post-employment benefit liabilities and liabilities related to environmental matters or against any indemnification obligations associated with such transaction.
“Obligations” means any principal, interest (including any interest accruing on or subsequent to the filing of a petition in bankruptcy, reorganization or similar proceeding at the rate provided for in the documentation with respect thereto, whether or not such interest is an allowed claim under applicable state, federal or foreign law), premium,
177
penalties, fees, indemnifications, reimbursements (including reimbursement obligations with respect to letters of credit and banker’s acceptances), damages and other liabilities, and guarantees of payment of such principal, interest, penalties, fees, indemnifications, reimbursements, damages and other liabilities, payable under the documentation governing any Indebtedness.
“Officer” means the Chairman of the board of directors, the Chief Executive Officer, the Chief Financial Officer, the President, any Executive Vice President, Senior Vice President or Vice President, the Treasurer or the Secretary of the Issuer.
“Officer’s Certificate” means a certificate signed on behalf of a Person by an Officer of such Person, who must be the principal executive officer, the principal financial officer, the treasurer or the principal accounting officer of such Person, that meets the requirements set forth in the senior notes indenture.
“Opinion of Counsel” means a written opinion from legal counsel who is acceptable to the Trustee. The counsel may be an employee of or counsel to the Issuer.
“Parent” means LVB Acquisition, Inc., a Delaware corporation and the direct parent of the Issuer.
“Permitted Asset Swap” means the substantially concurrent purchase and sale or exchange of Related Business Assets or a combination of Related Business Assets and Cash Equivalents between the Issuer or any of its Restricted Subsidiaries and another Person; provided that any Cash Equivalents received must be applied in accordance with the covenant described under “Repurchase at the Option of Holders—Asset Sales.”
“Permitted Holders” means each of the Investors, Management Stockholders and any of the direct or indirect parent companies of the Issuer (provided such direct or indirect parent companies of the Issuer have no majority holders other than the Investors, Management Stockholders and any group (within the meaning of Section 13(d)(3) or Section 14(d)(2) of the Exchange Act or any successor provision) of which any of the foregoing are members) and any group (within the meaning of Section 13(d)(3) or Section 14(d)(2) of the Exchange Act or any successor provision) of which any of the foregoing are members; provided that, in the case of such group and without giving effect to the existence of such group or any other group, such Investors, Management Stockholders and any of the direct or indirect parent companies of the Issuer (provided such direct or indirect parent companies of the Issuer have no majority holders other than the Investors, Management Stockholders and any group (within the meaning of Section 13(d)(3) or Section 14(d)(2) of the Exchange Act or any successor provision) of which any of the foregoing are members), collectively, have beneficial ownership of more than 50.0% of the total voting power of the Voting Stock of the Issuer or any of its direct or indirect parent companies. Any Person or group whose acquisition of beneficial ownership constitutes a Change of Control in respect of which a Change of Control Offer is made in accordance with the requirements of the senior notes indenture will thereafter, together with its Affiliates, constitute an additional Permitted Holder.
“Permitted Investments” means:
(1) any Investment in the Issuer or any of its Restricted Subsidiaries;
(2) any Investment in Cash Equivalents or Investment Grade Securities;
(3) any Investment by the Issuer or any of its Restricted Subsidiaries in a Person that is engaged in a Similar Business if as a result of such Investment:
(a) such Person becomes a Restricted Subsidiary; or
(b) such Person, in one transaction or a series of related transactions, is merged or consolidated with or into, or transfers or conveys substantially all of its assets to, or is liquidated into, the Issuer or a Restricted Subsidiary,
and, in each case, any Investment held by such Person; provided that such Investment was not acquired by such Person in contemplation of such acquisition, merger, consolidation or transfer;
(4) any Investment in securities or other assets not constituting Cash Equivalents or Investment Grade Securities and received in connection with an Asset Sale made pursuant to the provisions described under “Repurchase at the Option of Holders—Asset Sales” or any other disposition of assets not constituting an Asset Sale;
178
(5) any Investment existing on the Issue Date or made pursuant to binding commitments in effect on the Issue Date or an Investment consisting of any extension, modification or renewal of any Investment existing on the Issue Date; provided that the amount of any such Investment may be increased (a) as required by the terms of such Investment as in existence on the Issue Date or (b) as otherwise permitted under the senior notes indenture;
(6) any Investment acquired by the Issuer or any of its Restricted Subsidiaries:
(a) in exchange for any other Investment or accounts receivable held by the Issuer or any such Restricted Subsidiary in connection with or as a result of a bankruptcy, workout, reorganization or recapitalization of the issuer of such other Investment or accounts receivable (including any trade creditor or customer); or
(b) in satisfaction of judgments against other Persons; or
(c) as a result of a foreclosure by the Issuer or any of its Restricted Subsidiaries with respect to any secured Investment or other transfer of title with respect to any secured Investment in default;
(7) Hedging Obligations permitted under clause (10) of the covenant described in “—Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”;
(8) any Investment in a Similar Business taken together with all other Investments made pursuant to this clause (8) that are at that time outstanding, not to exceed the greater of (a) $450.0 million and (b) 3.0% of Total Assets;
(9) Investments the payment for which consists of Equity Interests (other than Disqualified Stock) of the Issuer, or any of its direct or indirect parent companies; provided that such Equity Interests will not increase the amount available for Restricted Payments under clause (3) of the first paragraph under the covenant described in “—Certain Covenants—Limitations on Restricted Payments”;
(10) guarantees of Indebtedness permitted under the covenant described in “—Certain Covenants— Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”;
(11) any transaction to the extent it constitutes an Investment that is permitted by and made in accordance with the provisions of the second paragraph of the covenant described under “—Certain Covenants—Transactions with Affiliates” (except transactions described in clauses (2), (5) and (9) of such paragraph);
(12) Investments consisting of purchases and acquisitions of inventory, supplies, material or equipment or the licensing or contribution of intellectual property pursuant to joint marketing arrangements with other Persons;
(13) additional Investments, taken together with all other Investments made pursuant to this clause (13) that are at that time outstanding (without giving effect to the sale of an Unrestricted Subsidiary to the extent the proceeds of such sale do not consist of cash or marketable securities), not to exceed the greater of (a) $450.0 million and (b) 3.0% of Total Assets;
(14) Investments in or relating to a Securitization Subsidiary that, in the good faith determination of the Issuer are necessary or advisable to effect any Qualified Securitization Facility or any repurchase obligation in connection therewith;
(15) advances to, or guarantees of Indebtedness of, employees not in excess of $25.0 million outstanding at any one time, in the aggregate;
(16) loans and advances to employees, directors, officers, managers, distributors and consultants for business-related travel expenses, moving expenses and other similar expenses, in each case incurred in the ordinary course of business or consistent with past practices or to fund such Person’s purchase of Equity Interests of the Issuer or any direct or indirect parent company thereof;
(17) advances, loans or extensions of trade credit in the ordinary course of business by the Issuer or any of its Restricted Subsidiaries;
(18) any Investment in any Subsidiary or any joint venture in connection with intercompany cash management arrangements or related activities arising in the ordinary course of business;
179
(19) Investments consisting of purchases and acquisitions of assets or services in the ordinary course of business;
(20) Investments made in the ordinary course of business in connection with obtaining, maintaining or renewing client contacts and loans or advances made to distributors in the ordinary course of business;
(21) Investments in prepaid expenses, negotiable instruments held for collection and lease, utility and workers compensation, performance and similar deposits entered into as a result of the operations of the business in the ordinary course of business; and
(22) repurchases of the senior notes.
“Permitted Liens” means, with respect to any Person:
(1) pledges or deposits by such Person under workmen’s compensation laws, unemployment insurance, other social security benefits or other insurance related obligations (including, but not limited to, in respect of deductibles, self-insured retention amounts and premiums and adjustments thereto) or good faith deposits in connection with bids, tenders, contracts (other than for the payment of Indebtedness) or leases to which such Person is a party, or deposits to secure public or statutory obligations of such Person or deposits of cash or U.S. government bonds to secure surety or appeal bonds to which such Person is a party, or deposits as security for contested taxes or import duties or for the payment of rent, in each case incurred in the ordinary course of business;
(2) Liens imposed by law, such as carriers’, warehousemen’s and mechanics’ Liens, in each case for sums not yet overdue for a period of more than 30 days or being contested in good faith by appropriate proceedings or other Liens arising out of judgments or awards against such Person with respect to which such Person shall then be proceeding with an appeal or other proceedings for review if adequate reserves with respect thereto are maintained on the books of such Person in accordance with GAAP;
(3) Liens for taxes, assessments or other governmental charges not yet overdue for a period of more than 30 days or not yet payable or subject to penalties for nonpayment or which are being contested in good faith by appropriate proceedings diligently conducted, if adequate reserves with respect thereto are maintained on the books of such Person in accordance with GAAP;
(4) Liens in favor of issuers of performance and surety bonds or bid bonds or with respect to other regulatory requirements or letters of credit issued pursuant to the request of and for the account of such Person in the ordinary course of its business;
(5) minor survey exceptions, minor encumbrances, easements or reservations of, or rights of others for, licenses, rights-of-way, sewers, electric lines, telegraph and telephone lines and other similar purposes, or zoning or other restrictions as to the use of real properties or Liens incidental, to the conduct of the business of such Person or to the ownership of its properties which were not incurred in connection with Indebtedness and which do not in the aggregate materially adversely affect the value of said properties or materially impair their use in the operation of the business of such Person;
(6) Liens securing Indebtedness permitted to be incurred pursuant to clause (4), (12)(b), (13), (23) or (24) of the second paragraph under “—Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”; provided that (a) Liens securing Indebtedness, Disqualified Stock or Preferred Stock permitted to be incurred pursuant to clause (13) relate only to Refinancing Indebtedness that serves to refund or refinance Indebtedness, Disqualified Stock or Preferred Stock incurred under clause (4) or (12)(b) of the second paragraph of “—Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock,” (b) Liens securing Indebtedness permitted to be incurred pursuant to clause (23) extend only to the assets of Foreign Subsidiaries, (c) Liens securing Indebtedness permitted to be incurred pursuant to clause (24) are solely on acquired property or the assets of the acquired entity, as the case may be, and (d) Liens securing Indebtedness, Disqualified Stock or Preferred Stock to be incurred pursuant to clause (4) of the second paragraph under “—Certain Covenants— Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock” extend only to the assets so purchased, leased or improved;
(7) Liens existing on the Issue Date;
180
(8) Liens on property or shares of stock or other assets of a Person at the time such Person becomes a Subsidiary; provided that such Liens are not created or incurred in connection with, or in contemplation of, such other Person becoming such a Subsidiary; provided, further, that such Liens may not extend to any other property or other assets owned by the Issuer or any of its Restricted Subsidiaries;
(9) Liens on property or other assets at the time the Issuer or a Restricted Subsidiary acquired the property or such other assets, including any acquisition by means of a merger or consolidation with or into the Issuer or any of its Restricted Subsidiaries; provided that such Liens are not created or incurred in connection with, or in contemplation of, such acquisition; provided, further, that the Liens may not extend to any other property owned by the Issuer or any of its Restricted Subsidiaries;
(10) Liens securing Indebtedness or other obligations of a Restricted Subsidiary owing to the Issuer or another Restricted Subsidiary permitted to be incurred in accordance with the covenant described under “—Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”;
(11) Liens securing Hedging Obligations; provided that, with respect to Hedging Obligations relating to Indebtedness, such Indebtedness is, and is permitted to be under the senior notes indenture, secured by a Lien on the same property securing such Hedging Obligations;
(12) Liens on specific items of inventory or other goods and proceeds of any Person securing such Person’s obligations in respect of bankers’ acceptances issued or created for the account of such Person to facilitate the purchase, shipment or storage of such inventory or other goods;
(13) leases, subleases, licenses or sublicenses granted to others in the ordinary course of business which do not materially interfere with the ordinary conduct of the business of the Issuer or any of its Restricted Subsidiaries and do not secure any Indebtedness;
(14) Liens arising from Uniform Commercial Code financing statement filings regarding operating leases entered into by the Issuer and its Restricted Subsidiaries in the ordinary course of business;
(15) Liens in favor of the Issuer or any Guarantor;
(16) Liens on equipment of the Issuer or any of its Restricted Subsidiaries granted in the ordinary course of business to the Issuer’s clients;
(17) Liens on accounts receivable, Securitization Assets and related assets incurred in connection with a Qualified Securitization Facility;
(18) Liens to secure any refinancing, refunding, extension, renewal or replacement (or successive refinancing, refunding, extensions, renewals or replacements) as a whole, or in part, of any Indebtedness secured by any Lien referred to in the foregoing clauses (6), (7), (8) and (9); provided that (a) such new Lien shall be limited to all or part of the same property that secured the original Lien (plus improvements on such property), and (b) the Indebtedness secured by such Lien at such time is not increased to any amount greater than the sum of (i) the outstanding principal amount or, if greater, committed amount of the Indebtedness described under clauses (6), (7), (8) and (9) at the time the original Lien became a Permitted Lien under the senior notes indenture, and (ii) an amount necessary to pay any fees and expenses, including premiums, related to such refinancing, refunding, extension, renewal or replacement;
(19) deposits made in the ordinary course of business to secure liability to insurance carriers;
(20) other Liens securing obligations in an aggregate amount at any one time outstanding not to exceed the greater of (a) $100.0 million and (b) 1.0% of Total Assets determined as of the date of incurrence;
(21) Liens securing judgments for the payment of money not constituting an Event of Default under clause (5) under “Events of Default and Remedies” so long as such Liens are adequately bonded and any appropriate legal proceedings that may have been duly initiated for the review of such judgment have not been finally terminated or the period within which such proceedings may be initiated has not expired;
(22) Liens in favor of customs and revenue authorities arising as a matter of law to secure payment of customs duties in connection with the importation of goods in the ordinary course of business;
181
(23) Liens (a) of a collection bank arising under Section 4-210 of the Uniform Commercial Code on items in the course of collection, (b) attaching to commodity trading accounts or other commodity brokerage accounts incurred in the ordinary course of business, and (c) in favor of banking institutions arising as a matter of law encumbering deposits (including the right of set-off) and which are within the general parameters customary in the banking industry;
(24) Liens deemed to exist in connection with Investments in repurchase agreements permitted under “—Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”; provided that such Liens do not extend to any assets other than those that are the subject of such repurchase agreement;
(25) Liens encumbering reasonable customary deposits and margin deposits and similar Liens attaching to commodity trading accounts or other brokerage accounts incurred in the ordinary course of business and not for speculative purposes;
(26) Liens that are contractual rights of set-off (a) relating to the establishment of depository relations with banks not given in connection with the issuance of Indebtedness, (b) relating to pooled deposit or sweep accounts of the Issuer or any of its Restricted Subsidiaries to permit satisfaction of overdraft or similar obligations incurred in the ordinary course of business of the Issuer and its Restricted Subsidiaries or (c) relating to purchase orders and other agreements entered into with customers of the Issuer or any of its Restricted Subsidiaries in the ordinary course of business;
(27) Liens securing obligations owed by the Issuer or any Restricted Subsidiary to any lender under the Senior Credit Facilities or any Affiliate of such a lender in respect of any overdraft and related liabilities arising from treasury, depository and cash management services or any automated clearing house transfers of funds;
(28) during a Suspension Period only, Liens securing Indebtedness (other than Indebtedness that is secured equally and ratably with (or on a basis subordinated to) the senior notes), and Indebtedness represented by Sale and Lease-Back Transactions in an amount not to exceed 15.0% of Total Assets at any one time outstanding;
(29) Liens securing Indebtedness the proceeds of which are used to develop or construct new facilities (or any improvements to existing facilities) or equipment (or any improvements to existing equipment) designed primarily for the purpose of air or water pollution control; provided that such Indebtedness is permitted to be incurred by the terms of the senior notes indenture and such Liens do not extend to any assets of the Issuer or its Restricted Subsidiaries other than the assets developed, constructed or improved with the proceeds of the Indebtedness secured by such Lien;
(30) any encumbrance or restriction (including put and call arrangements) with respect to capital stock of any joint venture or similar arrangement pursuant to any joint venture or similar agreement;
(31) Liens arising out of conditional sale, title retention, consignment or similar arrangements for the sale or purchase of goods entered into by the Issuer or any Restricted Subsidiary in the ordinary course of business;
(32) Liens solely on any cash earnest money deposits made by the Issuer or any of its Restricted Subsidiaries in connection with any letter of intent or purchase agreement permitted;
(33) ground leases in respect of real property on which facilities owned or leased by the Issuer or any of its Subsidiaries are located;
(34) Liens on insurance policies and the proceeds thereof securing the financing of the premiums with respect thereto;
(35) Liens on Capital Stock of an Unrestricted Subsidiary that secure Indebtedness or other obligations of such Unrestricted Subsidiary; and
(36) Liens on the assets of non-guarantor Subsidiaries securing Indebtedness of the Issuer or the Restricted Subsidiaries that were permitted by the terms of the senior notes indenture to be incurred.
For purposes of this definition, the term “Indebtedness” shall be deemed to include interest on such Indebtedness.
182
“Person” means any individual, corporation, limited liability company, partnership, joint venture, association, joint stock company, trust, unincorporated organization, government or any agency or political subdivision thereof or any other entity.
“Preferred Stock” means any Equity Interest with preferential rights of payment of dividends or upon liquidation, dissolution, or winding up.
“Qualified Proceeds” means the fair market value of assets that are used or useful in, or Capital Stock of any Person engaged in, a Similar Business.
“Qualified Securitization Facility” means any Securitization Facility (1) constituting a securitization financing facility that meets the following conditions: (a) the board of directors of the Issuer shall have determined in good faith that such Securitization Facility (including financing terms, covenants, termination events and other provisions) is in the aggregate economically fair and reasonable to the Issuer and the applicable Securitization Subsidiary, (b) all sales and/or contributions of Securitization Assets and related assets to the applicable Securitization Subsidiary are made at fair market value (as determined in good faith by the Issuer) and (c) the financing terms, covenants, termination events and other provisions thereof shall be market terms (as determined in good faith by the Issuer) or (2) constituting a receivables financing facility.
“Rating Agencies” means Moody’s and S&P or if Moody’s or S&P or both shall not make a rating on the senior notes publicly available, a nationally recognized statistical rating agency or agencies, as the case may be, selected by the Issuer which shall be substituted for Moody’s or S&P or both, as the case may be.
“Senior Notes Registration Rights Agreement” means a registration rights agreement with respect to the Original Senior Notes dated as of the Issue Date, among the Issuer, the Guarantors and the Initial Purchasers.
“Related Business Assets” means assets (other than Cash Equivalents) used or useful in a Similar Business, provided that any assets received by the Issuer or a Restricted Subsidiary in exchange for assets transferred by the Issuer or a Restricted Subsidiary shall not be deemed to be Related Business Assets if they consist of securities of a Person, unless upon receipt of the securities of such Person, such Person would become a Restricted Subsidiary.
“Restricted Investment” means an Investment other than a Permitted Investment.
“Restricted Subsidiary” means, at any time, any direct or indirect Subsidiary of the Issuer (including any Foreign Subsidiary) that is not then an Unrestricted Subsidiary; provided that upon an Unrestricted Subsidiary ceasing to be an Unrestricted Subsidiary, such Subsidiary shall be included in the definition of “Restricted Subsidiary.”
“S&P” means Standard & Poor’s, a division of The McGraw-Hill Companies, Inc., and any successor to its rating agency business.
“Sale and Lease-Back Transaction” means any arrangement providing for the leasing by the Issuer or any of its Restricted Subsidiaries of any real or tangible personal property, which property has been or is to be sold or transferred by the Issuer or such Restricted Subsidiary to a third Person in contemplation of such leasing.
“SEC” means the U.S. Securities and Exchange Commission.
“Secured Indebtedness” means any Indebtedness of the Issuer or any of its Restricted Subsidiaries secured by a Lien.
“Securities Act” means the Securities Act of 1933, as amended, and the rules and regulations of the SEC promulgated thereunder.
“Securitization Assets” means the accounts receivable, royalty or other revenue streams and other rights to payment related to the Specified Contract Rights subject to a Qualified Securitization Facility that is a securitization financing facility (and not a receivables financing facility) and the proceeds thereof.
“Securitization Facility” means any of one or more receivables or securitization financing facilities as amended, supplemented, modified, extended, renewed, restated or refunded from time to time, the Obligations of which are non-recourse (except for customary representations, warranties, covenants and indemnities made in connection with such
183
facilities) to the Issuer or any of its Restricted Subsidiaries (other than a Securitization Subsidiary) pursuant to which the Issuer or any of its Restricted Subsidiaries sells or grants a security interest in its accounts receivable or Securitization Assets or assets related thereto to either (a) a Person that is not a Restricted Subsidiary or (b) a Securitization Subsidiary that in turn sells its accounts receivable to a Person that is not a Restricted Subsidiary.
“Securitization Fees” means distributions or payments made directly or by means of discounts with respect to any participation interest issued or sold in connection with, and other fees paid to a Person that is not a Securitization Subsidiary in connection with, any Qualified Securitization Facility.
“Securitization Subsidiary” means any Subsidiary formed for the purpose of, and that solely engages only in one or more Qualified Securitization Facilities and other activities reasonably related thereto.
“Senior Credit Facilities” means the ABL Facilities and the CF Credit Facilities.
“Senior Indebtedness” means:
(1) all Indebtedness of the Issuer or any Guarantor outstanding under the Senior Credit Facilities, the senior notes (and related guarantees) or the senior notes and related Guarantees (including interest accruing on or after the filing of any petition in bankruptcy or similar proceeding or for reorganization of the Issuer or any Guarantor (at the rate provided for in the documentation with respect thereto, regardless of whether or not a claim for post-filing interest is allowed in such proceedings)), and any and all other fees, expense reimbursement obligations, indemnification amounts, penalties, and other amounts (whether existing on the Issue Date or thereafter created or incurred) and all obligations of the Issuer or any Guarantor to reimburse any bank or other Person in respect of amounts paid under letters of credit, acceptances or other similar instruments;
(2) all Hedging Obligations (and guarantees thereof) owing to a Lender (as defined in the Senior Credit Facilities) or any Affiliate of such Lender (or any Person that was a Lender or an Affiliate of such Lender at the time the applicable agreement giving rise to such Hedging Obligation was entered into), provided that such Hedging Obligations are permitted to be incurred under the terms of the senior notes indenture;
(3) any other Indebtedness of the Issuer or any Guarantor permitted to be incurred under the terms of the senior notes indenture, unless the instrument under which such Indebtedness is incurred expressly provides that it is subordinated in right of payment to the senior notes or any related Guarantee; and
(4) all Obligations with respect to the items listed in the preceding clauses (1), (2) and (3); provided that Senior Indebtedness shall not include:
(a) any obligation of such Person to the Issuer or any of its Subsidiaries;
(b) any liability for federal, state, local or other taxes owed or owing by such Person;
(c) any accounts payable or other liability to trade creditors arising in the ordinary course of business;
(d) any Indebtedness or other Obligation of such Person which is subordinate or junior in any respect to any other Indebtedness or other Obligation of such Person; or
(e) that portion of any Indebtedness which at the time of incurrence is incurred in violation of the senior notes indenture.
“Senior Secured Leverage Ratio” means “Senior Secured Leverage Ratio” as defined, together with related definitions, in the CF Credit Facilities as in effect on the Issue Date, provided that the Issuer may elect, pursuant to an Officer’s Certificate delivered to the Trustee to treat all or a portion of a revolving commitment under any Credit Facility as incurred and outstanding Indebtedness at the time such commitments are established and for so long as such revolving commitments remain outstanding. As a result of any such election, any subsequent incurrence of Indebtedness under such revolving commitment shall not be deemed an incurrence of additional Indebtedness or an additional Lien at such subsequent event.
184
“Significant Subsidiary” means any Restricted Subsidiary that would be a “significant subsidiary” as defined in Article 1, Rule 1-02 of Regulation S-X, promulgated pursuant to the Securities Act, as such regulation is in effect on the Issue Date.
“Similar Business” means (1) any business engaged in by the Issuer or any of its Restricted Subsidiaries on the Issue Date, and (2) any business or other activities that are reasonably similar, ancillary, complementary or related to, or a reasonable extension, development or expansion of, the businesses in which the Issuer and its Restricted Subsidiaries are engaged on the Issue Date.
“Specified Contract Rights” means certain intellectual property licenses, agreements or other contracts giving rise to not more than $50.0 million of annual accounts receivable, royalty or other intellectual property revenue streams or other rights to payment.
“Subordinated Indebtedness” means, with respect to the senior notes,
(1) any Indebtedness of the Issuer which is by its terms subordinated in right of payment to the senior notes, and
(2) any Indebtedness of any Guarantor which is by its terms subordinated in right of payment to the Guarantee of such entity of the senior notes.
“Subsidiary” means, with respect to any Person:
(1) any corporation, association, or other business entity (other than a partnership, joint venture, limited liability company or similar entity) of which more than 50.0% of the total voting power of shares of Capital Stock entitled (without regard to the occurrence of any contingency) to vote in the election of directors, managers or trustees thereof is at the time of determination owned or controlled, directly or indirectly, by such Person or one or more of the other Subsidiaries of that Person or a combination thereof or is consolidated under GAAP with such Person at such time; and
(2) any partnership, joint venture, limited liability company or similar entity of which
(a) more than 50.0% of the capital accounts, distribution rights, total equity and voting interests or general or limited partnership interests, as applicable, are owned or controlled, directly or indirectly, by such Person or one or more of the other Subsidiaries of that Person or a combination thereof whether in the form of membership, general, special or limited partnership or otherwise, and
(b) such Person or any Restricted Subsidiary of such Person is a controlling general partner or otherwise controls such entity.
“Total Assets” means the total assets of the Issuer and its Restricted Subsidiaries, determined on a consolidated basis in accordance with GAAP, as shown on the most recent balance sheet of the Issuer or such other Person as may be expressly stated.
“Transactions” means the issuance of the Original Notes and the related transactions described under “Summary—Concurrent Transactions” in the offering circular relating to the Initial Notes dated July 25, 2012 and the offering circular relating to the Additional Notes dated September 18, 2012.
“Treasury Rate” means, as of any Redemption Date, the yield to maturity as of such Redemption Date of United States Treasury securities with a constant maturity (as compiled and published in the most recent Federal Reserve Statistical Release H.15 (519) that has become publicly available at least two Business Days prior to the Redemption Date (or, if such Statistical Release is no longer published, any publicly available source of similar market data)) most nearly equal to the period from the Redemption Date to August 1, 2015; provided that if the period from the Redemption Date to such date is less than one year, the weekly average yield on actually traded United States Treasury securities adjusted to a constant maturity of one year will be used.
“Trust Indenture Act” means the Trust Indenture Act of 1939, as amended (15 U.S.C. §§ 77aaa-77bbbb).
“Unrestricted Subsidiary” means:
185
(1) any Subsidiary of the Issuer which at the time of determination is an Unrestricted Subsidiary (as designated by the Issuer, as provided below); and
(2) any Subsidiary of an Unrestricted Subsidiary.
The Issuer may designate any Subsidiary of the Issuer (including any existing Subsidiary and any newly acquired or newly formed Subsidiary) to be an Unrestricted Subsidiary unless such Subsidiary or any of its Subsidiaries owns any Equity Interests or Indebtedness of, or owns or holds any Lien on, any property of, the Issuer or any Subsidiary of the Issuer (other than solely any Subsidiary of the Subsidiary to be so designated); provided that
(1) any Unrestricted Subsidiary must be an entity of which the Equity Interests entitled to cast at least a majority of the votes that may be cast by all Equity Interests having ordinary voting power for the election of directors or Persons performing a similar function are owned, directly or indirectly, by the Issuer;
(2) such designation complies with the covenants described under “—Certain Covenants—Limitation on Restricted Payments”; and
(3) each of (a) the Subsidiary to be so designated and (b) its Subsidiaries has not at the time of designation, and does not thereafter, create, incur, issue, assume, guarantee or otherwise become directly or indirectly liable with respect to any Indebtedness pursuant to which the lender has recourse to any of the assets of the Issuer or any Restricted Subsidiary.
The Issuer may designate any Unrestricted Subsidiary to be a Restricted Subsidiary; provided that, immediately after giving effect to such designation, no Default shall have occurred and be continuing and either:
(1) the Issuer could incur at least $1.00 of additional Indebtedness pursuant to the Fixed Charge Coverage Test; or
(2) the Fixed Charge Coverage Ratio for the Issuer would be equal to or greater than such ratio for the Issuer immediately prior to such designation, in each case on a pro forma basis taking into account such designation.
Any such designation by the Issuer shall be notified by the Issuer to the Trustee by promptly filing with the Trustee a copy of the resolution of the board of directors of the Issuer or any committee thereof giving effect to such designation and an Officer’s Certificate certifying that such designation complied with the foregoing provisions.
“Voting Stock” of any Person as of any date means the Capital Stock of such Person that is at the time entitled to vote in the election of the board of directors of such Person.
“Weighted Average Life to Maturity” means, when applied to any Indebtedness, Disqualified Stock or Preferred Stock, as the case may be, at any date, the quotient obtained by dividing:
(1) the sum of the products of the number of years from the date of determination to the date of each successive scheduled principal payment of such Indebtedness or redemption or similar payment with respect to such Disqualified Stock or Preferred Stock multiplied by the amount of such payment; by
(2) the sum of all such payments.
“Wholly-Owned Subsidiary” of any Person means a Subsidiary of such Person, 100.0% of the outstanding Equity Interests of which (other than directors’ qualifying shares) shall at the time be owned by such Person or by one or more Wholly-Owned Subsidiaries of such Person.
186
DESCRIPTION OF EXCHANGE SENIOR SUBORDINATED NOTES
General
Certain terms used in this description are defined under the subheading “—Certain Definitions.” In this description, (1) the term “Issuer” refers only to Biomet, Inc. and not to any of its subsidiaries, (2) the terms “we,” “our” and “us” each refer to the Issuer and its consolidated Subsidiaries, and (3) the term “senior subordinated notes” refers to the original senior subordinated notes and the exchange senior subordinated notes collectively. Defined terms in this “Description of Exchange Senior Subordinated Notes” may have different meanings than similar or identical terms used in the “Description of Exchange Senior Notes” above. For example, references to “Guarantees” or “Guarantors” in this “Description of Exchange Senior Subordinated Notes” means guarantees or guarantors of the senior subordinated notes, and not guarantees or guarantors of the senior notes.
The Issuer previously issued $800.0 million aggregate principal amount of original senior subordinated notes on September 18, 2012 under the senior subordinated notes indenture among the Issuer, the Guarantors and Wells Fargo Bank, National Association, as trustee (the “Trustee”). The Issuer will issue up to $800.0 million aggregate principal amount of exchange senior subordinated notes offered hereby under the senior subordinated notes indenture.
The senior subordinated notes indenture has been qualified under and is subject to and governed by the Trust Indenture Act of 1939. Except as set forth herein, the terms of the exchange senior subordinated notes will be substantially identical in all material respects and include those stated in the senior subordinated notes indenture and those made part of the senior subordinated notes indenture by reference to the Trust Indenture Act.
The following description is only a summary of the material provisions of the senior subordinated notes indenture, does not purport to be complete and is qualified in its entirety by reference to the provisions of the senior subordinated notes indenture, including the definitions therein of certain terms used below. We urge you to read the senior subordinated notes indenture because it, and not this description, will define your rights as Holders of the senior subordinated notes. You may request copies of the senior subordinated notes indenture at our address set forth under “Where You Can Find Additional Information.”
Exchange Senior Subordinated Notes versus Original Senior Subordinated Notes
The terms of the exchange senior subordinated notes are substantially identical in all material respects to the original senior subordinated notes except upon completion of the exchange offers, the offer and sale of the exchange senior subordinated notes will have been registered under the Securities Act and the exchange senior subordinated notes will be free of any covenants regarding exchange registration rights.
Brief Description of the Exchange Senior Subordinated Notes
The exchange senior subordinated notes:
| • | will be general, unsecured, senior subordinated obligations of the Issuer; |
| • | will be subordinated in right of payment to all existing and future Senior Indebtedness (including the Senior Credit Facilities and the senior notes) of the Issuer; |
| • | will be structurally subordinated to all existing and future Indebtedness, claims of holders of Preferred Stock and other liabilities of Subsidiaries of the Issuer that do not guarantee the senior subordinated notes; |
| • | will be senior in right of payment to all existing and future Subordinated Indebtedness as defined with respect to the senior subordinated notes of the Issuer; and |
| • | will be initially guaranteed on an unsecured senior subordinated basis by the Guarantors and will also be guaranteed in the future by each Subsidiary, if any, that guarantees Indebtedness under the CF Credit Facilities. |
187
Guarantees
The Guarantors, as primary obligors and not merely as sureties, will initially jointly and severally, irrevocably and unconditionally, guarantee, on an unsecured senior subordinated basis, the full and punctual payment when due, whether at maturity, by acceleration or otherwise, of all obligations of the Issuer under the senior subordinated notes indenture and the senior subordinated notes, whether for payment of principal of, premium, if any, or interest in respect of the senior subordinated notes, expenses, indemnification or otherwise, on the terms set forth in the senior subordinated notes indenture by executing the senior subordinated notes indenture.
The Guarantors are initially guaranteeing the senior subordinated notes and, in the future, each direct and indirect Subsidiary of the Issuer that guarantees Indebtedness under the CF Credit Facilities will guarantee the senior subordinated notes. Each of the Guarantees of the senior subordinated notes is a general, unsecured, senior subordinated obligation of each Guarantor; and is subordinated in right of payment to all existing and future Senior Indebtedness of such Guarantor (including such Guarantor’s guarantee of the CF Credit Facilities and the Senior Notes). Each of the Guarantees of the senior subordinated notes ise structurally subordinated to all existing and future Indebtedness, claims of holders of Preferred Stock and other liabilities of Subsidiaries of each Guarantor that do not guarantee the senior subordinated notes.
Not all of the Issuer’s Subsidiaries guarantee the senior subordinated notes. In the event of a bankruptcy, liquidation, reorganization or similar proceeding of any of these non-guarantor Subsidiaries, the non-guarantor Subsidiaries will pay the holders of their debt and their trade creditors before they will be able to distribute any of their assets to the Issuer. As a result, all of the existing and future liabilities of our non-guarantor Subsidiaries, including any claims of trade creditors, are effectively senior to the senior subordinated notes. For the nine months ended February 28, 2013, the non-guarantor Subsidiaries of the Issuer accounted for approximately $830.4 million, or 37% of the Issuer’s consolidated net sales. As of February 28, 2013, the non-guarantor Subsidiaries of the Issuer accounted for approximately $2,806.9 million, or 28% of the Issuer’s consolidated total assets and approximately $409.6 million or 5.3% of the Issuer’s consolidated liabilities.
The obligations of each Guarantor under its Guarantee will be limited as necessary to prevent the Guarantee from constituting a fraudulent conveyance under applicable law. This provision may not, however, be effective to protect a Guarantee from being voided under fraudulent transfer law, or may reduce the applicable Guarantor’s obligation to an amount that effectively makes its Guarantee worthless. If a Guarantee was rendered voidable, it could be subordinated by a court to all other indebtedness (including guarantees and other contingent liabilities) of the Guarantor, and, depending on the amount of such indebtedness, a Guarantor’s liability on its Guarantee could be reduced to zero. See “Risk Factors—Risks Related to Our Indebtedness and the Notes—Federal and state fraudulent transfer laws may permit a court to void the notes and the guarantees, subordinate claims in respect of the notes and the guarantees and require noteholders to return payments received. If this occurs, noteholders may not receive any payments on the notes.”
Any Guarantor that makes a payment under its Guarantee will be entitled upon payment in full of all guaranteed obligations under the senior subordinated notes indenture to a contribution from each other Guarantor in an amount equal to such other Guarantor’s pro rata portion of such payment based on the respective net assets of all the Guarantors at the time of such payment determined in accordance with GAAP.
Each Guarantor may consolidate with or merge into or sell all or substantially all of its assets to the Issuer or another Guarantor without limitation or any other Person upon the terms and conditions set forth in the senior subordinated notes indenture. See “—Certain Covenants—Merger, Consolidation or Sale of All or Substantially All Assets.”
Each Guarantee by a Guarantor provides by its terms that it will be automatically and unconditionally released and discharged upon:
(1) (a) any sale, exchange or transfer (by merger or otherwise) of (i) the Capital Stock of such Guarantor, after which the applicable Guarantor is no longer a Restricted Subsidiary or (ii) all or substantially all the assets of such Guarantor, in each case if such sale, exchange or transfer is made in compliance with the applicable provisions of the senior subordinated notes indenture;
(b) the release or discharge of the guarantee by such Guarantor of Indebtedness under the CF Credit Facilities, or the release or discharge of such other guarantee that resulted in the creation of such Guarantee, except a discharge or release by or as a result of payment under such guarantee;
188
(c) upon the dissolution of such Guarantor; provided that no Default or Event of Default has occurred and is continuing;
(d) the designation of any Restricted Subsidiary that is a Guarantor as an Unrestricted Subsidiary in compliance with the applicable provisions of the senior subordinated notes indenture; or
(e) the exercise by the Issuer of its legal defeasance option or covenant defeasance option as described under “Legal Defeasance and Covenant Defeasance” or the discharge of the Issuer’s obligations under the senior subordinated notes indenture in accordance with the terms of the senior subordinated notes indenture; and
(2) such Guarantor delivering to the Trustee an Officer’s Certificate and an Opinion of Counsel, each stating that all conditions precedent provided for in the senior subordinated notes indenture relating to such transaction have been complied with.
Ranking
The payment of the principal of, premium, if any, and interest on the senior subordinated notes and the payment of any Guarantee will be subordinated in right of payment to the prior payment in cash in full of all existing and future Senior Indebtedness of the Issuer or the relevant Guarantor, as the case may be, including the obligations of the Issuer and such Guarantor under the Senior Credit Facilities and the Senior Notes.
As of February 28, 2013, the Issuer and the Guarantors had $5,136.7 million of Senior Indebtedness outstanding, (including $1,825.0 million in aggregate principal amount of the Senior Notes and $3,311.7 million of borrowings and the related guarantees under the Senior Credit Facilities). As of February 28, 2013, the Issuer had (1) the option to raise additional incremental term loans or incremental cash flow revolving facility commitments under the CF Credit Facilities of up to an amount that would cause our Senior Secured Leverage Ratio (as defined in the CF Credit Facilities) to be equal to or less than 4.50 to 1.00, which, if borrowed, would be Senior Indebtedness and (2) the option to raise additional incremental asset-based revolving credit facility commitments under the ABL Facilities by up to $100.0 million, which, if borrowed, would be Senior Indebtedness.
Although the senior subordinated notes indenture contains limitations on the amount of additional Indebtedness that the Issuer and the Issuer’s Restricted Subsidiaries (including the Guarantors) may incur, under certain circumstances the amount of such Indebtedness could be substantial and, in any case, such Indebtedness may be Senior Indebtedness. The senior subordinated notes indenture does not limit the amount of additional Indebtedness that the Parent may incur. See “—Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock.”
Paying Agent and Registrar for the Exchange Senior Subordinated Notes
The Issuer will maintain one or more paying agents for the exchange senior subordinated notes. The initial paying agent for the exchange senior subordinated notes is the Trustee.
The Issuer also maintains one or more registrars and a transfer agent. The initial registrar and transfer agent with respect to the exchange senior subordinated notes is the Trustee. The registrar maintains a register reflecting ownership of the exchange senior subordinated notes outstanding from time to time. The registered Holder of an exchange senior subordinated note will be treated as the owner of the exchange senior subordinated note for all purposes. The transfer agent will make payments on and facilitate transfer of exchange senior subordinated notes on behalf of the Issuer.
The Issuer may change the paying agent, the registrar or the transfer agent without prior notice to the Holders. The Issuer or any of its Subsidiaries may act as a paying agent, registrar or transfer agent.
If the exchange senior subordinated notes are listed on an exchange and the rules of such exchange so require, the Issuer will satisfy any requirement of such exchange as to paying agents, registrars and transfer agents and will comply with any notice requirements required under such exchange in connection with any change of paying agent, registrar or transfer agent.
Subordination of the Senior Subordinated Notes
189
Only Indebtedness of the Issuer or a Guarantor that is Senior Indebtedness will rank senior to the senior subordinated notes and the Guarantees in accordance with the provisions of the senior subordinated notes indenture. The senior subordinated notes and Guarantees will rank pari passu in all respects with all other Senior Subordinated Indebtedness of the Issuer and the relevant Guarantor, respectively.
We will agree in the senior subordinated notes indenture that the Issuer and the Guarantors will not incur any Indebtedness that is subordinate or junior in right of payment to the Senior Indebtedness of such Person, unless such Indebtedness is equal in right of payment with the senior subordinated notes or the related Guarantees or is expressly subordinated in right of payment to the Senior Subordinated Indebtedness or the related Guarantees, as the case may be. The senior subordinated notes indenture will not treat (i) unsecured Indebtedness as subordinated or junior to Secured Indebtedness merely because it is unsecured or (ii) Senior Indebtedness as subordinated or junior to any other Senior Indebtedness merely because it has a junior priority with respect to the same collateral.
Neither the Issuer nor any Guarantor will be permitted to pay principal of, premium, if any, or interest on the senior subordinated notes (or pay any other Obligations relating to the senior subordinated notes, including fees, costs, expenses, indemnities and rescission or damage claims) or make any deposit pursuant to the provisions described under “Legal Defeasance and Covenant Defeasance” or “Satisfaction and Discharge” below and may not purchase, redeem or otherwise retire any senior subordinated notes (collectively, “pay the senior subordinated notes”) other than in the form of Permitted Junior Securities if either of the following occurs (a “Payment Default”):
(1) any Obligation on any Designated Senior Indebtedness of the Issuer is not paid in full in cash when due (after giving effect to any applicable grace period); or
(2) any other default on Designated Senior Indebtedness of the Issuer occurs and the maturity of such Designated Senior Indebtedness is accelerated in accordance with its terms;
unless, in either case, the Payment Default has been cured or waived and any such acceleration has been rescinded or such Designated Senior Indebtedness has been discharged or paid in full in cash.
Regardless of the foregoing, the Issuer is permitted to pay the senior subordinated notes if the Issuer and the Trustee receive written notice approving such payment from the Representatives of all Designated Senior Indebtedness with respect to which the Payment Default has occurred and is continuing.
During the continuance of any default (other than a Payment Default) (a “Non-Payment Default”) with respect to any Designated Senior Indebtedness pursuant to which the maturity thereof may be accelerated without further notice (except such notice as may be required to effect such acceleration) or the expiration of any applicable grace periods, the Issuer will not be permitted to pay the senior subordinated notes (except in the form of Permitted Junior Securities) for a period (a “Payment Blockage Period”) commencing upon the receipt by the Trustee (with a copy to the Issuer) of written notice (a “Blockage Notice”) of such Non-Payment Default from the Representative of such Designated Senior Indebtedness specifying an election to effect a Payment Blockage Period and ending 179 days thereafter. The Payment Blockage Period will end earlier if such Payment Blockage Period is terminated:
(1) by written notice to the Trustee and the Issuer from the Person or Persons who gave such Blockage Notice;
(2) because the default giving rise to such Blockage Notice is cured, waived or otherwise no longer continuing; or
(3) because such Designated Senior Indebtedness has been discharged or repaid in full in cash.
Notwithstanding the provisions described above, unless the holders of such Designated Senior Indebtedness or the Representative of such Designated Senior Indebtedness have accelerated the maturity of such Designated Senior Indebtedness, the Issuer and the Guarantors are permitted to resume paying the senior subordinated notes after the end of such Payment Blockage Period. The senior subordinated notes shall not be subject to more than one Payment Blockage Period in any consecutive 360-day period irrespective of the number of defaults with respect to Designated Senior Indebtedness during such period; provided that if any Blockage Notice is delivered to the Trustee by or on behalf of the holders of Designated Senior Indebtedness of the Issuer (other than the holders of Indebtedness under the Senior Credit Facilities), a Representative of holders of Indebtedness under the Senior Credit Facilities may give another Blockage Notice within such period. However, in no event may the total number of days during which any Payment Blockage Period or Periods on the senior subordinated notes is in effect exceed 179 days
190
in the aggregate during any consecutive 360-day period, and there must be at least 181 days during any consecutive 360-day period during which no Payment Blockage Period is in effect. Notwithstanding the foregoing, however, no default that existed or was continuing on the date of delivery of any Blockage Notice to the Trustee will be, or be made, the basis for a subsequent Blockage Notice unless such default has been waived for a period of not less than 90 days (it being acknowledged that any subsequent action, or any breach of any financial covenants during the period after the date of delivery of a Blockage Notice, that, in either case, would give rise to a Non-Payment Default pursuant to any provisions under which a Non-Payment Default previously existed or was continuing shall constitute a new Non-Payment Default for this purpose).
In the event of any payment or distribution of the assets of the Issuer upon a total or partial liquidation or dissolution or reorganization of or similar proceeding relating to the Issuer or its property:
(1) the holders of Senior Indebtedness of the Issuer will be entitled to receive payment in full in cash of such Senior Indebtedness before the Holders of the senior subordinated notes are entitled to receive any payment;
(2) until the Senior Indebtedness of the Issuer is paid in full in cash, any payment or distribution to which Holders of the senior subordinated notes would be entitled but for the subordination provisions of the senior subordinated notes indenture will be made to holders of such Senior Indebtedness as their interests may appear, except that Holders of senior subordinated notes may receive Permitted Junior Securities; and
(3) if a distribution is made to Holders of the senior subordinated notes that, due to the subordination provisions, should not have been made to them, such Holders of the senior subordinated notes will be required to hold it in trust for the holders of Senior Indebtedness of the Issuer and pay it over to them as their interests may appear.
The subordination and payment blockage provisions described above will not prevent a Default from occurring under the senior subordinated notes indenture upon the failure of the Issuer to pay interest or principal with respect to the senior subordinated notes when due by their terms. If payment of the senior subordinated notes is accelerated because of an Event of Default, the Issuer must promptly notify the holders of Designated Senior Indebtedness or the Representative of such Designated Senior Indebtedness of the acceleration. If any Indebtedness under the Senior Credit Facilities is outstanding, no such acceleration will be effective until the earlier of the acceleration of Indebtedness under the Senior Credit Facilities or five Business Days after the Representative under the Senior Credit Facilities receive notice of such acceleration and, thereafter, the Issuer may pay the senior subordinated notes only if the senior subordinated notes indenture otherwise permits payment at that time.
Each Guarantor’s obligations under its Guarantee will be senior subordinated obligations of that Guarantor. As such, the rights of Holders to receive payment pursuant to such Guarantee will be subordinated in right of payment to the rights of holders of Senior Indebtedness of such Guarantor. The terms of the subordination and payment blockage provisions described above with respect to the Issuer’s obligations under the senior subordinated notes apply equally to the obligations of such Guarantor under its Guarantee.
A Holder by its acceptance of senior subordinated notes agrees to be bound by such provisions and authorizes the Trustee, on its behalf, to take such action as may be necessary or appropriate to effectuate the subordination provided for in the senior subordinated notes indenture and appoints the Trustee its attorney-in-fact for such purpose.
By reason of the subordination provisions contained in the senior subordinated notes indenture, in the event of a liquidation or insolvency proceeding, creditors of the Issuer or a Guarantor who are holders of Senior Indebtedness of the Issuer or such Guarantor, as the case may be, may recover more, ratably, than the Holders of the senior subordinated notes, and creditors who are not holders of Senior Indebtedness may recover less, ratably, than holders of Senior Indebtedness and may recover more, ratably, than the Holders of the senior subordinated notes.
The terms of the subordination provisions described above will not apply to payments from money or the proceeds of Government Securities held in trust by the Trustee for the payment of principal of and interest on the senior subordinated notes pursuant to the provisions described under “Legal Defeasance and Covenant Defeasance” or “Satisfaction and Discharge,” if the foregoing subordination provisions were not violated at the time the applicable amounts were deposited in trust pursuant to such provisions.
Transfer and Exchange
191
A Holder may transfer or exchange senior subordinated notes in accordance with the senior subordinated notes indenture. The registrar and the Trustee may require a Holder to furnish appropriate endorsements and transfer documents in connection with a transfer of exchange senior subordinated notes. Holders will be required to pay all taxes due on transfer. The Issuer will not be required to transfer or exchange any senior subordinated note selected for redemption or tendered (and not withdrawn) for repurchase in connection with a Change of Control Offer or an Asset Sale Offer. Also, the Issuer will not be required to transfer or exchange any senior subordinated note for a period of 15 days before a selection of senior subordinated notes to be redeemed.
Principal, Maturity and Interest
The Issuer will issue up to an aggregate principal amount of $800.0 million of exchange senior subordinated notes in this offering. The exchange senior subordinated notes will mature on October 1, 2020. Subject to compliance with the covenant described below under “—Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock,” the Issuer may issue additional senior subordinated notes from time to time after this offering under the senior subordinated notes indenture (“Additional Senior Subordinated Notes”). The exchange senior subordinated notes offered by the Issuer and any Additional Senior Subordinated Notes subsequently issued under the senior subordinated notes indenture will be treated as a single class for all purposes under the senior subordinated notes indenture, including waivers, amendments, redemptions and offers to purchase, except for certain waivers and amendments. Unless the context requires otherwise, references to “senior subordinated notes” for all purposes of the senior subordinated notes indenture and this “Description of Exchange Senior Subordinated Notes” include any Additional Senior Subordinated Notes that are actually issued. The senior subordinated notes will be issued in denominations of $1,000 and any integral multiples of $1,000 in excess of $1,000.
Interest Payments
Interest on the exchange senior subordinated notes will accrue at the rate of 6.500% per annum. Interest on the exchange senior subordinated notes will be payable semi-annually in arrears on each April 1 and October 1, commencing April 1, 2013 (or commencing October 1, 2013 if the exchange offer in respect thereof is completed after April 1, 2013, and interest on the senior subordinated notes has been paid on such date), to the Holders of exchange senior subordinated notes of record on the immediately preceding March 15 and September 15. Interest on the exchange senior subordinated notes will accrue from the most recent date to which interest has been paid or, if no interest has been paid, from and including the Issue Date. Interest on the senior subordinated notes will be computed on the basis of a 360-day year comprised of twelve 30-day months.
Additional Interest
Additional Interest may accrue on the exchange senior subordinated notes in certain circumstances pursuant to the Senior Subordinated Notes Registration Rights Agreement or as described under “Events of Default and Remedies.” All references in the senior subordinated notes indenture and this “Description of Exchange Senior Subordinated Notes,” in any context, to any interest or other amount payable on or with respect to the senior subordinated notes shall be deemed to include any Additional Interest payable pursuant to the Senior Subordinated Notes Registration Rights Agreement and under “Events of Default and Remedies.”
Payment of Principal, Premium and Interest
Payments of principal of, premium, if any, and interest on the exchange senior subordinated notes will be payable at the office or agency of the Issuer maintained for such purpose or, at the option of the Issuer, payment of interest may be made by check mailed to the Holders of the exchange senior subordinated notes at their respective addresses set forth in the register of Holders; provided that (1) all payments of principal, premium, if any, and interest with respect to the exchange senior subordinated notes represented by one or more global notes registered in the name of or held by DTC or its nominee will be made by wire transfer of immediately available funds to the accounts specified by the Holder or Holders thereof and (2) all payments of principal, premium, if any, and interest with respect to certificated exchange senior subordinated notes will be made by wire transfer to a U.S. dollar account maintained by the payee with a bank in the United States if such Holder elects payment by wire transfer by giving written notice to the Trustee or the paying agent to such effect designating such account no later than 30 days immediately preceding the relevant due date for payment (or such other date as the Trustee may accept in its discretion). Until otherwise designated by the Issuer, the Issuer’s office or agency will be the office of the Trustee maintained for such purpose.
192
Mandatory Redemption; Offers to Purchase; Open Market Purchases
The Issuer is not required to make any mandatory redemption or sinking fund payments with respect to the senior subordinated notes. However, under certain circumstances, the Issuer may be required to offer to purchase senior subordinated notes as described under “Repurchase at the Option of Holders.” The Issuer may at any time and from time to time purchase senior subordinated notes in the open market or otherwise.
Optional Redemption
Except as set forth below, the Issuer is not entitled to redeem the exchange senior subordinated notes at its option prior to October 1, 2015.
At any time prior to October 1, 2015, the Issuer may redeem all or a part of the exchange senior subordinated notes, upon notice as described under “—Selection and Notice,” at a redemption price equal to 100.0% of the principal amount of the exchange senior subordinated notes redeemed plus the Applicable Premium as of, plus accrued and unpaid interest, if any, to the date of redemption (the “Redemption Date”), subject to the right of Holders of record on the relevant record date to receive interest due on the relevant interest payment date.
On and after October 1, 2015, the Issuer may redeem the exchange senior subordinated notes, in whole or in part, upon notice as described under “—Selection and Notice,” at the redemption prices (expressed as percentages of principal amount of the senior subordinated notes to be redeemed) set forth below, plus accrued and unpaid interest, if any, to the Redemption Date, subject to the right of Holders of record on the relevant record date to receive interest due on the relevant interest payment date, if redeemed during the twelve-month period beginning on October 1 of each of the years indicated below:
| Year | Percentage |
| 2015 | 103.250% |
| 2016 | 101.625% |
| 2017 and thereafter | 100.000% |
In addition, until October 1, 2015, the Issuer may, at its option, redeem up to 40% of the aggregate principal amount of senior subordinated notes issued under the senior subordinated notes indenture at a redemption price equal to 106.500% of the aggregate principal amount thereof, plus accrued and unpaid interest, if any, to the Redemption Date, subject to the right of Holders of senior subordinated notes of record on the relevant record date to receive interest due on the relevant interest payment date, with the net cash proceeds received by it from one or more Equity Offerings; provided that (a) at least 50.0% of the sum of the aggregate principal amount of senior subordinated notes originally issued under the senior subordinated notes indenture on the Issue Date and any Additional Senior Subordinated Notes that are issued under the senior subordinated notes indenture after the Issue Date remains outstanding immediately after the occurrence of each such redemption; and (b) each such redemption occurs within 180 days of the date of closing of each such Equity Offering.
Notice of any redemption upon any Equity Offering may be given prior to the completion thereof. All redemptions or notices may, at the Issuer’s discretion, be subject to one or more conditions precedent, including, but not limited to, completion of a related Equity Offering. If any senior subordinated notes are listed on an exchange, and the rules of such exchange so require, the Issuer will notify the exchange of any such notice of redemption. In addition, the Issuer will notify the exchange of the principal amount of any senior subordinated notes outstanding following any partial redemption of the senior subordinated notes.
Selection and Notice
If the Issuer is redeeming less than all of the senior subordinated notes issued under the senior subordinated notes indenture at any time, the Trustee will select the senior subordinated notes to be redeemed (1) if the senior subordinated notes are listed on an exchange, in compliance with the requirements of such exchange or (2) on a pro rata basis to the extent practicable, or, if the pro rata basis is not practicable for any reason, by lot or by such other method as the Trustee shall deem fair and appropriate. No senior subordinated notes of $1,000 or less can be redeemed in part.
193
Notices of redemption shall be delivered electronically or mailed by first-class mail, postage prepaid, at least 30 but not more than 60 days before the redemption date to each Holder of senior subordinated notes at such Holder’s registered address or otherwise in accordance with the procedures of DTC, except that redemption notices may be delivered more than 60 days prior to a redemption date if the notice is issued in connection with a defeasance of the senior subordinated notes or a satisfaction and discharge of the senior subordinated notes indenture. If any exchange senior subordinated note is to be redeemed in part only, any notice of redemption that relates to such exchange senior subordinated note shall state the portion of the principal amount thereof that has been or is to be redeemed.
With respect to senior subordinated notes represented by certificated notes, the Issuer will issue a new exchange senior subordinated note in a principal amount equal to the unredeemed portion of the original exchange senior subordinated note in the name of the Holder upon cancellation of the original senior subordinated note. Senior subordinated notes called for redemption become due on the date fixed for redemption. On and after the Redemption Date, interest ceases to accrue on the senior subordinated notes or portions of them called for redemption.
Repurchase at the Option of Holders
Change of Control
The senior subordinated notes indenture provides that if a Change of Control occurs, unless the Issuer has previously or concurrently delivered a redemption notice with respect to all the outstanding senior subordinated notes as described under “Optional Redemption,” the Issuer will make an offer to purchase all of the senior subordinated notes pursuant to the offer described below (the “Change of Control Offer”) at a price in cash (the “Change of Control Payment”) equal to 101% of the aggregate principal amount thereof plus accrued and unpaid interest, if any, to the date of purchase, subject to the right of Holders of the senior subordinated notes of record on the relevant record date to receive interest due on the relevant interest payment date. Within 30 days following any Change of Control, the Issuer will deliver notice of such Change of Control Offer electronically or by first-class mail, with a copy to the Trustee, to each Holder of senior subordinated notes to the address of such Holder appearing in the security register or otherwise in accordance with the procedures of DTC with the following information:
(1) that a Change of Control Offer is being made pursuant to the covenant entitled “Change of Control,” and that all senior subordinated notes properly tendered pursuant to such Change of Control Offer will be accepted for payment by the Issuer;
(2) the purchase price and the purchase date, which will be no earlier than 30 days nor later than 60 days from the date such notice is delivered (the “Change of Control Payment Date”);
(3) that any senior subordinated note not properly tendered will remain outstanding and continue to accrue interest;
(4) that unless the Issuer defaults in the payment of the Change of Control Payment, all senior subordinated notes accepted for payment pursuant to the Change of Control Offer will cease to accrue interest on the Change of Control Payment Date;
(5) that Holders electing to have any senior subordinated notes purchased pursuant to a Change of Control Offer will be required to surrender such senior subordinated notes, with the form entitled “Option of Holder to Elect Purchase” on the reverse of such senior subordinated notes completed, to the paying agent specified in the notice at the address specified in the notice prior to the close of business on the third Business Day preceding the Change of Control Payment Date;
(6) that Holders will be entitled to withdraw their tendered senior subordinated notes and their election to require the Issuer to purchase such senior subordinated notes, provided that the paying agent receives, not later than the close of business on the Expiration Date of the Change of Control Offer, a telegram, facsimile transmission or letter setting forth the name of the Holder of the senior subordinated notes, the principal amount of senior subordinated notes tendered for purchase, and a statement that such Holder is withdrawing its tendered senior subordinated notes and its election to have such senior subordinated notes purchased;
(7) that Holders whose senior subordinated notes are being purchased only in part will be issued new senior subordinated notes and such new senior subordinated notes will be equal in principal amount to the
194
unpurchased portion of the senior subordinated notes surrendered. The unpurchased portion of the senior subordinated notes must be equal to at least $1,000 or any integral multiple of $1,000 in excess of $1,000;
(8) if such notice is delivered prior to the occurrence of a Change of Control, stating that the Change of Control Offer is conditional on the occurrence of such Change of Control; and
(9) the other instructions, as determined by the Issuer, consistent with the covenant described hereunder, that a Holder must follow.
The Issuer will comply with the requirements of Rule 14e-1 under the Exchange Act and any other securities laws and regulations thereunder to the extent such laws or regulations are applicable in connection with the repurchase of senior subordinated notes pursuant to a Change of Control Offer. To the extent that the provisions of any securities laws or regulations conflict with the provisions of the senior subordinated notes indenture, the Issuer will comply with the applicable securities laws and regulations and shall not be deemed to have breached its obligations described in the senior subordinated notes indenture by virtue thereof.
On the Change of Control Payment Date, the Issuer will, to the extent permitted by law:
(1) accept for payment all senior subordinated notes issued by it or portions thereof properly tendered pursuant to the Change of Control Offer;
(2) deposit with the paying agent an amount equal to the aggregate Change of Control Payment in respect of all senior subordinated notes or portions thereof so tendered; and
(3) deliver, or cause to be delivered, to the Trustee for cancellation the senior subordinated notes so accepted together with an Officer’s Certificate to the Trustee stating that such senior subordinated notes or portions thereof have been tendered to and purchased by the Issuer.
The Senior Credit Facilities and the Senior Notes prohibit or limit, and future credit agreements or other agreements relating to Senior Indebtedness to which the Issuer becomes a party may prohibit or limit, the Issuer from purchasing any senior subordinated notes pursuant to this Change of Control covenant. In the event a Change of Control occurs at a time when the Issuer is prohibited from purchasing the senior subordinated notes, the Issuer could seek the consent of its lenders and the holders of Senior Notes to permit the purchase of the senior subordinated notes or could attempt to refinance the indebtedness that contain such prohibition. If the Issuer does not obtain such consent or repay such indebtedness, the Issuer will remain prohibited from purchasing the senior subordinated notes. In such case, the Issuer’s failure to purchase tendered senior subordinated notes would constitute an Event of Default under the senior subordinated notes indenture. If, as a result thereof, a default occurs with respect to any Senior Indebtedness, the subordination provisions in the senior subordinated notes indenture would restrict payments to the Holders of senior subordinated notes under certain circumstances. The Senior Credit Facilities provide that certain change of control events with respect to the Issuer would constitute a default thereunder (including a Change of Control under the senior subordinated notes indenture). If we experience a change of control that triggers a default under the Senior Credit Facilities, we could seek a waiver of such default or seek to refinance the Senior Credit Facilities. In the event we do not obtain such a waiver or refinance the Senior Credit Facilities, such default could result in amounts outstanding under the Senior Credit Facilities being declared due and payable and cause a Qualified Securitization Facility to be wound down.
Our ability to pay cash to the Holders of senior subordinated notes following the occurrence of a Change of Control may be limited by our then-existing financial resources. Therefore, sufficient funds may not be available when necessary to make any required repurchases.
The Change of Control purchase feature of the senior subordinated notes may in certain circumstances make more difficult or discourage a sale or takeover of us and, thus, the removal of incumbent management. The Change of Control purchase feature is a result of negotiations between the initial purchasers and us. After the Issue Date, we have no present intention to engage in a transaction involving a Change of Control, although it is possible that we could decide to do so in the future. Subject to the limitations discussed below, we could, in the future, enter into certain transactions, including acquisitions, refinancings or other recapitalizations, that would not constitute a Change of Control under the senior subordinated notes indenture, but that could increase the amount of Indebtedness outstanding at such time or otherwise affect our capital structure or credit ratings. Restrictions on our ability to incur additional Indebtedness are contained in the covenants described under “—Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock” and “—Certain Covenants—
195
Liens.” Such restrictions in the senior subordinated notes indenture can be waived only with the consent of the Holders of a majority in principal amount of the senior subordinated notes then outstanding. Except for the limitations contained in such covenants, however, the senior subordinated notes indenture will not contain any covenants or provisions that may afford Holders of the senior subordinated notes protection in the event of a highly leveraged transaction.
The Issuer will not be required to make a Change of Control Offer following a Change of Control if a third party makes the Change of Control Offer in the manner, at the times and otherwise in compliance with the requirements set forth in the senior subordinated notes indenture applicable to a Change of Control Offer made by the Issuer and purchases all senior subordinated notes validly tendered and not withdrawn under such Change of Control Offer.
Notwithstanding anything to the contrary herein, a Change of Control Offer may be made in advance of a Change of Control, conditional upon such Change of Control, if a definitive agreement is in place for the Change of Control at the time of making of the Change of Control Offer.
The definition of “Change of Control” includes a disposition of all or substantially all of the assets of the Issuer and its Subsidiaries, taken as a whole, to any Person. Although there is a limited body of case law interpreting the phrase “substantially all,” there is no precise established definition of the phrase under applicable law.
Accordingly, in certain circumstances there may be a degree of uncertainty as to whether a particular transaction would involve a disposition of “all or substantially all” of the assets of the Issuer and its Subsidiaries, taken as a whole. As a result, it may be unclear as to whether a Change of Control has occurred and whether a Holder of senior subordinated notes may require the Issuer to make an offer to repurchase the senior subordinated notes as described above.
The provisions under the senior subordinated notes indenture relative to the Issuer’s obligation to make an offer to repurchase the senior subordinated notes as a result of a Change of Control may be waived or modified with the written consent of the Holders of a majority in principal amount of the senior subordinated notes.
Asset Sales
The senior subordinated notes indenture provides that the Issuer will not, and will not permit any of its Restricted Subsidiaries to, consummate directly or indirectly an Asset Sale, unless:
(1) the Issuer or such Restricted Subsidiary, as the case may be, receives consideration at the time of such Asset Sale at least equal to the fair market value of the assets sold or otherwise disposed of; and
(2) except in the case of a Permitted Asset Swap, at least 75.0% of the consideration therefor received by the Issuer or such Restricted Subsidiary, as the case may be, is in the form of Cash Equivalents; provided that the amount of:
(a) any liabilities (as shown on the Issuer’s or such Restricted Subsidiary’s most recent balance sheet or in the footnotes thereto) of the Issuer or such Restricted Subsidiary, other than liabilities that are by their terms subordinated to the senior subordinated notes, that are assumed by the transferee of any such assets and for which the Issuer and all of its Restricted Subsidiaries have been validly released by all creditors in writing;
(b) any securities, notes or other obligations or assets received by the Issuer or such Restricted Subsidiary from such transferee that are converted by the Issuer or such Restricted Subsidiary into cash (to the extent of the cash received) within 180 days following the closing of such Asset Sale; and
(c) any Designated Non-cash Consideration received by the Issuer or such Restricted Subsidiary in such Asset Sale having an aggregate fair market value, taken together with all other Designated Non-cash Consideration received pursuant to this clause (c) that is at that time outstanding, not to exceed the greater of (x) $300.0 million and (y) 3.0% of Total Assets at the time of the receipt of such Designated Non-cash Consideration, with the fair market value of each item of Designated Non-cash Consideration being measured at the time received and without giving effect to subsequent changes in value, shall be deemed to be Cash Equivalents for purposes of this provision and for no other purpose.
196
Within 450 days after the receipt of any Net Proceeds of any Asset Sale, the Issuer or such Restricted Subsidiary, at its option, may apply the Net Proceeds from such Asset Sale,
(1) to permanently reduce:
(a) Obligations under Senior Indebtedness, and to correspondingly reduce commitments with respect thereto;
(b) Obligations under Senior Subordinated Indebtedness (and to correspondingly reduce commitments with respect thereto); provided that the Issuer shall equally and ratably reduce Obligations under the senior subordinated notes as provided under “Optional Redemption” or through open-market purchases (to the extent such purchases are at or above 100% of the principal amount thereof) or by making an offer (in accordance with the procedures set forth below for an Asset Sale Offer) to all Holders of senior subordinated notes to purchase their senior subordinated notes at 100% of the principal amount thereof, plus the amount of accrued but unpaid interest, if any, on the amount of senior subordinated notes to be repurchased; or
(c) Indebtedness of a Restricted Subsidiary that is not a Guarantor, other than Indebtedness owed to the Issuer or another Restricted Subsidiary;
(2) to make (a) an Investment in any one or more businesses, provided that such Investment in any business is in the form of the acquisition of Capital Stock and results in the Issuer or any of its Restricted Subsidiaries, as the case may be, owning an amount of the Capital Stock of such business such that it constitutes a Restricted Subsidiary, (b) capital expenditures or (c) acquisitions of other assets, in each of (a), (b) and (c), used or useful in a Similar Business; or
(3) to make an Investment in (a) any one or more businesses, provided that such Investment in any business is in the form of the acquisition of Capital Stock and results in the Issuer or any of its Restricted Subsidiaries, as the case may be, owning an amount of the Capital Stock of such business such that it constitutes a Restricted Subsidiary, (b) properties or (c) acquisitions of other assets that, in each of (a), (b) and (c), replace the businesses, properties and/or assets that are the subject of such Asset Sale;
provided that, in the case of clauses (2) and (3) above, a binding commitment shall be treated as a permitted application of the Net Proceeds from the date of such commitment so long as the Issuer or such other Restricted Subsidiary enters into such commitment with the good faith expectation that such Net Proceeds will be applied to satisfy such commitment within 180 days of such commitment (an “Acceptable Commitment”) and, in the event any Acceptable Commitment is later cancelled or terminated for any reason before the Net Proceeds are applied in connection therewith, the Issuer or such Restricted Subsidiary enters into another Acceptable Commitment (a “Second Commitment”) within 180 days of such cancellation or termination; provided, further, that if any Second Commitment is later cancelled or terminated for any reason before such Net Proceeds are applied, then such Net Proceeds shall constitute Excess Proceeds.
Any Net Proceeds from the Asset Sale that are not invested or applied as provided and within the time period set forth in the preceding paragraph will be deemed to constitute “Excess Proceeds.” When the aggregate amount of Excess Proceeds exceeds $75.0 million, the Issuer shall make an offer to all Holders of the senior subordinated notes and, if required by the terms of any Indebtedness that is pari passu with the senior subordinated notes (“Pari Passu Indebtedness”), to the holders of such Pari Passu Indebtedness (an “Asset Sale Offer”), to purchase the maximum aggregate principal amount of the senior subordinated notes and such Pari Passu Indebtedness that is in an amount equal to at least $1,000, that may be purchased out of the Excess Proceeds at an offer price in cash in an amount equal to 100% of the principal amount thereof (or accreted value thereof, if less), plus accrued and unpaid interest, if any, to the date fixed for the closing of such offer, in accordance with the procedures set forth in the senior subordinated notes indenture. The Issuer will commence an Asset Sale Offer with respect to Excess Proceeds within ten Business Days after the date that Excess Proceeds exceed $75.0 million by delivering the notice required pursuant to the terms of the senior subordinated notes indenture, with a copy to the Trustee. The Issuer may satisfy the foregoing obligations with respect to any Net Proceeds from an Asset Sale by making an Asset Sale Offer with respect to such Net Proceeds prior to the expiration of the relevant 450 days (or such longer period provided above) or with respect to Excess Proceeds of $75.0 million or less.
To the extent that the aggregate amount of senior subordinated notes and such Pari Passu Indebtedness tendered pursuant to an Asset Sale Offer is less than the Excess Proceeds, the Issuer may use any remaining Excess
197
Proceeds for general corporate purposes, subject to other covenants contained in the senior subordinated notes indenture. If the aggregate principal amount of senior subordinated notes or the Pari Passu Indebtedness surrendered by such holders thereof exceeds the amount of Excess Proceeds, the Trustee shall select the senior subordinated notes on a pro rata basis and the Issuer shall select such Pari Passu Indebtedness to be purchased on a pro rata basis based on the accreted value or principal amount of the senior subordinated notes or such Pari Passu Indebtedness tendered. Upon completion of any such Asset Sale Offer, the amount of Excess Proceeds that resulted in the Asset Sale Offer shall be reset to zero.
Pending the final application of any Net Proceeds pursuant to this covenant, the holder of such Net Proceeds may apply such Net Proceeds temporarily to reduce Indebtedness outstanding under a revolving credit facility or otherwise invest such Net Proceeds in any manner not prohibited by the senior subordinated notes indenture.
The Issuer will comply with the requirements of Rule 14e-1 under the Exchange Act and any other securities laws and regulations thereunder to the extent such laws or regulations are applicable in connection with the repurchase of the senior subordinated notes pursuant to an Asset Sale Offer. To the extent that the provisions of any securities laws or regulations conflict with the provisions of the senior subordinated notes indenture, the Issuer will comply with the applicable securities laws and regulations and shall not be deemed to have breached its obligations described in the senior subordinated notes indenture by virtue thereof.
The Senior Credit Facilities and the Senior Notes prohibit or limit, and future credit agreements or other agreements relating to Senior Indebtedness to which the Issuer becomes a party may prohibit or limit, the Issuer from purchasing any senior subordinated notes pursuant to this Asset Sales covenant. In the event the Issuer is prohibited from purchasing the senior subordinated notes, the Issuer could seek the consent of its lenders and the holders of the Senior Notes to the purchase of the senior subordinated notes or could attempt to refinance the indebtedness that contain such prohibition. If the Issuer does not obtain such consent or repay such indebtedness, it will remain prohibited from purchasing the senior subordinated notes. In such case, the Issuer’s failure to purchase tendered senior subordinated notes would constitute an Event of Default under the senior subordinated notes indenture. If, as a result thereof, a default occurs with respect to any Senior Indebtedness, the subordination provisions in the senior subordinated notes indenture would restrict payments to the Holders of the senior subordinated notes under certain circumstances.
The provisions under the senior subordinated notes indenture relative to the Issuer’s obligation to make an offer to repurchase the senior subordinated notes as a result of an Asset Sale may be waived or modified with the written consent of the Holders of a majority in principal amount of the senior subordinated notes.
Certain Covenants
Set forth below are summaries of certain covenants contained in the senior subordinated notes indenture. During any period of time that (i) the senior subordinated notes have Investment Grade Ratings from both Rating Agencies and (ii) no Default has occurred and is continuing under the senior subordinated notes indenture (the occurrence of the events described in the foregoing clauses (i) and (ii) being collectively referred to as a “Covenant Suspension Event” and the date thereof being referred to as the “Suspension Date”) then, the covenants specifically listed under the following captions in this “Description of Exchange Senior Subordinated Notes” section of this prospectus will not be applicable to the senior subordinated notes (collectively, the “Suspended Covenants”):
(1) “Repurchase at the Option of Holders—Asset Sales”;
(2) “—Limitation on Restricted Payments”;
(3) “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”;
(4) clause (4) of the first paragraph of “—Merger, Consolidation or Sale of All or Substantially All Assets”;
(5) “—Transactions with Affiliates”;
(6) “—Dividend and Other Payment Restrictions Affecting Restricted Subsidiaries”;
198
(7) “—Limitation on Guarantees of Indebtedness by Restricted Subsidiaries”;
(8) “—Limitation on Layering”; and
(9) “Repurchase at the Option of Holders—Change of Control.”
During any period that the foregoing covenants have been suspended, the Issuer may not designate any of its Subsidiaries as Unrestricted Subsidiaries pursuant to the second sentence of the definition of “Unrestricted Subsidiary.”
If and while the Issuer and its Restricted Subsidiaries are not subject to the Suspended Covenants, the senior subordinated notes will be entitled to substantially less covenant protection. In the event that the Issuer and its Restricted Subsidiaries are not subject to the Suspended Covenants under the senior subordinated notes indenture for any period of time as a result of the foregoing, and on any subsequent date (the “Reversion Date”) one or both of the Rating Agencies withdraw their Investment Grade Rating or downgrade the rating assigned to the senior subordinated notes below an Investment Grade Rating, then the Issuer and its Restricted Subsidiaries will thereafter again be subject to the Suspended Covenants under the senior subordinated notes indenture with respect to future events. The period of time between the Suspension Date and the Reversion Date is referred to in this “Description of Exchange Senior Subordinated Notes” as the “Suspension Period.” The Guarantees of the Guarantors will be suspended during the Suspension Period. Additionally, upon the occurrence of a Covenant Suspension Event, the amount of Excess Proceeds from Net Proceeds shall be reset to zero.
Notwithstanding the foregoing, in the event of any such reinstatement, no action taken or omitted to be taken by the Issuer or any of its Restricted Subsidiaries prior to such reinstatement will give rise to a Default or Event of Default under the senior subordinated notes indenture with respect to the senior subordinated notes; provided that (i) with respect to Restricted Payments made after such reinstatement, the amount available to be made as Restricted Payments will be calculated as though the covenant described above under “—Limitation on Restricted Payments” had been in effect prior to, but not during, the Suspension Period; and (ii) all Indebtedness incurred, or Disqualified Stock issued, during the Suspension Period will be classified to have been incurred or issued pursuant to clause (3) of the second paragraph of “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock.”
There can be no assurance that the senior subordinated notes will ever achieve or maintain Investment Grade Ratings.
Limitation on Restricted Payments
The Issuer will not, and will not permit any of its Restricted Subsidiaries to, directly or indirectly:
(I) declare or pay any dividend or make any payment or distribution on account of the Issuer’s, or any of its Restricted Subsidiaries’ Equity Interests, including any dividend or distribution payable in connection with any merger or consolidation other than:
(a) dividends or distributions by the Issuer payable solely in Equity Interests (other than Disqualified Stock) of the Issuer; or
(b) dividends or distributions by a Restricted Subsidiary so long as, in the case of any dividend or distribution payable on or in respect of any class or series of securities issued by a Restricted Subsidiary other than a Wholly-Owned Subsidiary, the Issuer or a Restricted Subsidiary receives at least its pro rata share of such dividend or distribution in accordance with its Equity Interests in such class or series of securities;
(II) purchase, redeem, defease or otherwise acquire or retire for value any Equity Interests of the Issuer or any direct or indirect parent company of the Issuer, including in connection with any merger or consolidation;
(III) make any principal payment on, or redeem, repurchase, defease or otherwise acquire or retire for value, in each case, prior to any scheduled repayment, sinking fund payment or maturity, any Subordinated Indebtedness, other than:
199
(a) Indebtedness permitted under clauses (7) and (8) of the second paragraph of the covenant described under “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”; or
(b) the purchase, repurchase or other acquisition of Subordinated Indebtedness purchased in anticipation of satisfying a sinking fund obligation, principal installment or final maturity, in each case due within one year of the date of purchase, repurchase or acquisition; or
(IV) make any Restricted Investment
(all such payments and other actions set forth in clauses (I) through (IV) above being collectively referred to as “Restricted Payments”), unless, at the time of such Restricted Payment:
(1) no Default shall have occurred and be continuing or would occur as a consequence thereof;
(2) immediately after giving effect to such transaction on a pro forma basis, the Issuer could incur $1.00 of additional Indebtedness pursuant to the Fixed Charge Coverage Ratio test set forth in the first paragraph of the covenant described under “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock” (the “Fixed Charge Coverage Test”); and
(3) such Restricted Payment, together with the aggregate amount of all other Restricted Payments made by the Issuer and its Restricted Subsidiaries after September 25, 2007 (including Restricted Payments permitted by clauses (1), (2) (with respect to the payment of dividends on Refunding Capital Stock (as defined below) pursuant to clause (b) thereof only), (6)(c), (9) and (14) of the next succeeding paragraph, but excluding all other Restricted Payments permitted by the next succeeding paragraph), is less than the sum of (without duplication):
(a) 50.0% of the Consolidated Net Income of the Issuer for the period (taken as one accounting period) beginning on September 1, 2007 to the end of the Issuer’s most recently ended fiscal quarter for which internal financial statements are available at the time of such Restricted Payment, or, in the case such Consolidated Net Income for such period is a deficit, minus 100.0% of such deficit; plus
(b) 100.0% of the aggregate net cash proceeds and the fair market value of marketable securities or other property received by the Issuer since immediately after September 25, 2007 (other than net cash proceeds to the extent such net cash proceeds have been used to incur Indebtedness or issue Disqualified Stock or Preferred Stock pursuant to clause (12)(a) of the second paragraph of “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”) from the issue or sale of:
(i) (A) Equity Interests of the Issuer, including Treasury Capital Stock (as defined below), but excluding cash proceeds and the fair market value of marketable securities or other property received from the sale of:
(x) Equity Interests to any future, present or former employees, directors, officers, managers, distributors or consultants (or their respective Controlled Investment Affiliates or Immediate Family Members) of the Issuer, any direct or indirect parent company of the Issuer or any of the Issuer’s Subsidiaries after September 25, 2007 to the extent such amounts have been applied to Restricted Payments made in accordance with clause (4) of the next succeeding paragraph; and
(y) Designated Preferred Stock;
and (B) to the extent such net cash proceeds are actually contributed to the Issuer, Equity Interests of any direct or indirect parent company of the Issuer (excluding contributions of the proceeds from the sale of Designated Preferred Stock of such company or contributions to the extent such amounts have been applied to Restricted Payments made in accordance with clause (4) of the next succeeding paragraph); or
200
(ii) debt securities of the Issuer that have been converted into or exchanged for such Equity Interests of the Issuer;
provided that this clause (b) shall not include the proceeds from (W) Refunding Capital Stock, (X) Equity Interests or convertible debt securities of the Issuer sold to a Restricted Subsidiary, (Y) Disqualified Stock or debt securities that have been converted into Disqualified Stock or (Z) Excluded Contributions; plus
(c) 100.0% of the aggregate amount of cash and the fair market value of marketable securities or other property contributed to the capital of the Issuer following September 25, 2007 (other than net cash proceeds to the extent such net cash proceeds have been used to incur Indebtedness or issue Disqualified Stock or Preferred Stock pursuant to clause (12)(a) of the second paragraph of “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”) (other than by a Restricted Subsidiary and other than any Excluded Contributions); plus
(d) 100.0% of the aggregate amount received in cash and the fair market value of marketable securities or other property received by means of:
(i) the sale or other disposition (other than to the Issuer or a Restricted Subsidiary) of Restricted Investments made by the Issuer or its Restricted Subsidiaries and repurchases and redemptions of such Restricted Investments from the Issuer or its Restricted Subsidiaries (other than by the Issuer or a Restricted Subsidiary) and repayments of loans or advances, and releases of guarantees, which constitute Restricted Investments made by the Issuer or its Restricted Subsidiaries, in each case after September 25, 2007; or
(ii) the sale (other than to the Issuer or a Restricted Subsidiary) of the stock of an Unrestricted Subsidiary or a distribution from an Unrestricted Subsidiary (other than in each case to the extent the Investment in such Unrestricted Subsidiary was made by the Issuer or a Restricted Subsidiary pursuant to clause (7) of the next succeeding paragraph or to the extent such Investment constituted a Permitted Investment) or a dividend from an Unrestricted Subsidiary after September 25, 2007; plus
(e) in the case of the redesignation of an Unrestricted Subsidiary as a Restricted Subsidiary after September 25, 2007, the fair market value of the Investment in such Unrestricted Subsidiary (which, if the fair market value of such Investment shall exceed $125.0 million, shall be determined by the board of directors of the Issuer whose resolution with respect thereto will be delivered to the Trustee) at the time of the redesignation of such Unrestricted Subsidiary as a Restricted Subsidiary, other than to the extent the Investment in such Unrestricted Subsidiary was made by the Issuer or a Restricted Subsidiary pursuant to clause (7) of the next succeeding paragraph or to the extent such Investment constituted a Permitted Investment.
The foregoing provisions do not prohibit:
(1) the payment of any dividend or other distribution or the consummation of any irrevocable redemption within 60 days after the date of declaration of the dividend or other distribution or giving of the redemption notice, as the case may be, if at the date of declaration or notice, the dividend or other distribution or redemption payment would have complied with the provisions of the senior subordinated notes indenture;
(2) (a) the redemption, repurchase, retirement or other acquisition of any Equity Interests (“Treasury Capital Stock”) or Subordinated Indebtedness of the Issuer or any Equity Interests of any direct or indirect parent company of the Issuer, in exchange for, or out of the proceeds of the substantially concurrent sale (other than to a Restricted Subsidiary) of, Equity Interests of the Issuer or any direct or indirect parent company of the Issuer to the extent contributed to the Issuer (in each case, other than any Disqualified Stock) (“Refunding Capital Stock”) and (b) if immediately prior to the retirement of Treasury Capital Stock, the declaration and payment of dividend thereon was permitted under clause (6) of this paragraph, the declaration and payment of dividend on the Refunding Capital Stock (other than Refunding Capital Stock the proceeds of which were used to redeem, repurchase, retire or otherwise acquire any Equity
201
Interests of any direct or indirect parent company of the Issuer) in an aggregate amount per year no greater than the aggregate amount of dividends per annum that were declarable and payable on such Treasury Capital Stock immediately prior to such retirement;
(3) the defeasance, redemption, repurchase or other acquisition or retirement of (i) Subordinated Indebtedness of the Issuer or a Guarantor made by exchange for, or out of the proceeds of the substantially concurrent sale of, new Indebtedness of the Issuer or a Guarantor or (ii) Disqualified Stock of the Issuer or a Guarantor made by exchange for, or out of the proceeds of the substantially concurrent sale of, Disqualified Stock of the Issuer or a Guarantor, that, in each case, is incurred in compliance with “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock” so long as:
(a) the principal amount (or accreted value, if applicable) of such new Indebtedness or the liquidation preference of such new Disqualified Stock does not exceed the principal amount of (or accreted value, if applicable), plus any accrued and unpaid interest on, the Subordinated Indebtedness or the liquidation preference of, plus any accrued and unpaid dividends on, the Disqualified Stock being so defeased, redeemed, repurchased, acquired or retired for value, plus the amount of any reasonable premium required to be paid under the terms of the instrument governing the Subordinated Indebtedness or Disqualified Stock being so defeased, redeemed, repurchased, acquired or retired, defeasance costs and any reasonable fees and expenses incurred in connection with the issuance of such new Indebtedness or Disqualified Stock;
(b) such new Indebtedness is subordinated to the senior subordinated notes or the applicable Guarantee at least to the same extent as such Subordinated Indebtedness so defeased, redeemed, repurchased, acquired or retired;
(c) such new Indebtedness or Disqualified Stock has a final scheduled maturity date equal to or later than the final scheduled maturity date of the Subordinated Indebtedness or Disqualified Stock being so defeased, redeemed, repurchased, acquired or retired; and
(d) such new Indebtedness or Disqualified Stock has a Weighted Average Life to Maturity equal to or greater than the remaining Weighted Average Life to Maturity of the Subordinated Indebtedness or Disqualified Stock being so defeased, redeemed, repurchased, acquired or retired;
(4) a Restricted Payment to pay for the repurchase, retirement or other acquisition or retirement for value of Equity Interests (other than Disqualified Stock) of the Issuer or any direct or indirect parent company of the Issuer held by any future, present or former (A) employee, director, officer, manager or consultant (or their respective Controlled Investment Affiliates or Immediate Family Members) of the Issuer, any of its Subsidiaries or any of its direct or indirect parent companies pursuant to any management equity plan or stock option plan or any other management or employee benefit plan or agreement, or any stock subscription or shareholder agreement, including any Equity Interest rolled over by management of the Issuer or any direct or indirect parent company of the Issuer in connection with the Transactions; provided that the aggregate amount of Restricted Payments made under this clause (A) does not exceed $20.0 million in the first fiscal year following the Issue Date (which amount shall be increased by $5.0 million each fiscal year thereafter and, if applicable, will be increased to $40.0 million following the consummation of an underwritten public Equity Offering) (with unused amounts in any fiscal year being carried over to succeeding fiscal years subject to a maximum (without giving effect to the following proviso) of $30.0 million in any fiscal year (which shall increase to $60.0 million subsequent to the consummation of an underwritten public Equity Offering)); and (B) distributor (or its respective Controlled Investment Affiliates or Immediate Family Members) of the Issuer, any of its Subsidiaries or any of its direct or indirect parent companies pursuant to any distributor equity plan or agreement; provided that the aggregate amount of Restricted Payments made under this clause (B) does not exceed the greater of (i) $100.0 million and (ii) 1.0% of Total Assets; provided, further, that each of the amounts in any fiscal year under (A) and (B) may be increased by an amount not to exceed:
(a) the cash proceeds from the sale of Equity Interests (other than Disqualified Stock) of the Issuer and, to the extent contributed to the Issuer, Equity Interests of any direct or indirect parent company of the Issuer, in each case to any future, present or former employees, directors, officers, managers, distributors or consultants (or their respective Controlled Investment Affiliates
202
or Immediate Family Members) of the Issuer, any of its Subsidiaries or any of its direct or indirect parent companies that occurs after the Issue Date, to the extent the cash proceeds from the sale of such Equity Interests have not otherwise been applied to the payment of Restricted Payments by virtue of clause (3) of the preceding paragraph; plus
(b) the cash proceeds of key man life insurance policies received by the Issuer or its Restricted Subsidiaries after the Issue Date; less
(c) the amount of any Restricted Payments previously made with the cash proceeds described in clauses (a) and (b) of this clause (4);
and provided, further, that cancellation of Indebtedness owing to the Issuer from any future, present or former employees, directors, officers, managers, distributors or consultants of the Issuer (or their respective Controlled Investment Affiliates or Immediate Family Members), any direct or indirect parent company of the Issuer or any of the Issuer’s Restricted Subsidiaries in connection with a repurchase of Equity Interests of the Issuer or any of its direct or indirect parent companies will not be deemed to constitute a Restricted Payment for purposes of this covenant or any other provision of the senior subordinated notes indenture;
(5) the declaration and payment of dividends to holders of any class or series of Disqualified Stock of the Issuer or any of its Restricted Subsidiaries or any class or series of Preferred Stock of any Restricted Subsidiary issued in accordance with the covenant described under “ —Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock” to the extent such dividends are included in the definition of “Fixed Charges”;
(6) (a) the declaration and payment of dividends to holders of any class or series of Designated Preferred Stock (other than Disqualified Stock) issued by the Issuer or any of its Restricted Subsidiaries after the Issue Date;
(b) the declaration and payment of dividends to any direct or indirect parent company of the Issuer, the proceeds of which will be used to fund the payment of dividends to holders of any class or series of Designated Preferred Stock (other than Disqualified Stock) issued by such parent company after the Issue Date, provided that the amount of dividends paid pursuant to this clause (b) shall not exceed the aggregate amount of cash actually contributed to the Issuer from the sale of such Designated Preferred Stock; or
(c) the declaration and payment of dividends on Refunding Capital Stock that is Preferred Stock in excess of the dividends declarable and payable thereon pursuant to clause (2) of this paragraph;
provided, in the case of each of (a), (b) and (c) of this clause (6), that for the most recently ended four full fiscal quarters for which internal financial statements are available immediately preceding the date of issuance of such Designated Preferred Stock or the declaration of such dividends on Refunding Capital Stock that is Preferred Stock, after giving effect to such issuance or declaration on a pro forma basis, the Issuer would have had a Fixed Charge Coverage Ratio of at least 2.00 to 1.00;
(7) Investments in Unrestricted Subsidiaries taken together with all other Investments made pursuant to this clause (7) that are at the time outstanding, without giving effect to the sale of an Unrestricted Subsidiary to the extent the proceeds of such sale do not consist of cash or marketable securities, not to exceed greater of (a) $300.0 million and (b) 3.0% of Total Assets;
(8) payments made or expected to be made by the Issuer or any Restricted Subsidiary in respect of withholding or similar taxes payable by any future, present or former employee, director, officer, manager, distributor or consultant (or their respective Controlled Investment Affiliates or Immediate Family Members) and any repurchases of Equity Interests deemed to occur upon exercise of stock options or warrants if such Equity Interests represent a portion of the exercise price of such options or warrants;
(9) the declaration and payment of dividends on the Issuer’s common stock (or the payment of dividends to any direct or indirect parent company of the Issuer to fund a payment of dividends on such company’s common stock), following the first public offering of the Issuer’s common stock or the common stock of any direct or indirect parent company of the Issuer after the Issue Date, of up to 6.0% per annum of
203
the net cash proceeds received by or contributed to the Issuer in or from any such public offering, other than public offerings with respect to the Issuer’s common stock registered on Form S-4 or Form S-8 and other than any public sale constituting an Excluded Contribution;
(10) Restricted Payments that are made with Excluded Contributions;
(11) other Restricted Payments in an aggregate amount taken together with all other Restricted Payments made pursuant to this clause (11) not to exceed the greater of (a) $300.0 million and (b) 2.75% of Total Assets;
(12) distributions or payments of Securitization Fees;
(13) any Restricted Payment made in connection with the Transactions and the fees and expenses related thereto or owed to Affiliates, in each case to the extent permitted by the covenant described under “—Transactions with Affiliates”;
(14) the repurchase, redemption or other acquisition or retirement for value of any Subordinated Indebtedness pursuant to the provisions similar to those described under “Repurchase at the Option of Holders—Change of Control” and “Repurchase at the Option of Holders—Asset Sales”; provided that all senior subordinated notes validly tendered by Holders in connection with a Change of Control Offer or Asset Sale Offer, as applicable, have been repurchased, redeemed, acquired or retired for value;
(15) the declaration and payment of dividends by the Issuer to, or the making of loans to, any direct or indirect parent company of the Issuer in amounts required for any direct or indirect parent company of the Issuer to pay, in each case without duplication,
(a) franchise and excise taxes and other fees, taxes and expenses required to maintain their corporate existence;
(b) foreign, federal, state and local income taxes, to the extent such income taxes are attributable to the income of the Issuer and its Restricted Subsidiaries and, to the extent of the amount actually received from its Unrestricted Subsidiaries, in amounts required to pay such taxes to the extent attributable to the income of such Unrestricted Subsidiaries; provided that in each case the amount of such payments in any fiscal year does not exceed the amount that the Issuer and its Restricted Subsidiaries would be required to pay in respect of foreign, federal, state and local taxes for such fiscal year were the Issuer, its Restricted Subsidiaries and its Unrestricted Subsidiaries (to the extent described above) to pay such taxes separately from any such parent company;
(c) customary salary, bonus and other benefits payable to employees, directors, officers and managers of any direct or indirect parent company of the Issuer to the extent such salaries, bonuses and other benefits are attributable to the ownership or operation of the Issuer and its Restricted Subsidiaries;
(d) general corporate operating and overhead costs and expenses of any direct or indirect parent company of the Issuer to the extent such costs and expenses are attributable to the ownership or operation of the Issuer and its Restricted Subsidiaries;
(e) fees and expenses other than to Affiliates of the Issuer related to any unsuccessful equity or debt offering of such parent company;
(f) [reserved];
(g) amounts payable pursuant to the Management Fee Agreement, solely to the extent such amounts are not paid directly by the Issuer or its Subsidiaries;
(h) cash payments in lieu of issuing fractional shares in connection with the exercise of warrants, options or other securities convertible into or exchangeable for Equity Interests of the Issuer or any direct or indirect parent company of the Issuer; and
204
(i) to finance Investments otherwise permitted to be made pursuant to this covenant; provided that (A) such Restricted Payment shall be made substantially concurrently with the closing of such Investment, (B) such direct or indirect parent company shall, immediately following the closing thereof, cause (1) all property acquired (whether assets or Equity Interests) to be contributed to the capital of the Issuer or one of its Restricted Subsidiaries or (2) the merger of the Person formed or acquired into the Issuer or one of its Restricted Subsidiaries (to the extent not prohibited by the covenant “—Merger, Consolidation or Sale of All or Substantially All Assets” below) in order to consummate such Investment, (C) such direct or indirect parent company and its Affiliates (other than the Issuer or a Restricted Subsidiary) receives no consideration or other payment in connection with such transaction except to the extent the Issuer or a Restricted Subsidiary could have given such consideration or made such payment in compliance with the senior subordinated notes indenture, (D) any property received by the Issuer shall not increase amounts available for Restricted Payments pursuant to clause (3) of the preceding paragraph and (E) such Investment shall be deemed to be made by the Issuer or such Restricted Subsidiary pursuant to another provision of this covenant (other than pursuant to clause (10) hereof) or pursuant to the definition of “Permitted Investments” (other than clause (9) thereof); and
(16) the distribution, by dividend or otherwise, of shares of Capital Stock of, or Indebtedness owed to the Issuer or a Restricted Subsidiary by, Unrestricted Subsidiaries (other than Unrestricted Subsidiaries, the primary assets of which are Cash Equivalents).
provided that at the time of, and after giving effect to, any Restricted Payment permitted under clauses (11) and (16), no Default shall have occurred and be continuing or would occur as a consequence thereof.
As of the Issue Date, all of the Issuer’s Subsidiaries will be Restricted Subsidiaries. The Issuer will not permit any Unrestricted Subsidiary to become a Restricted Subsidiary except pursuant to the next to the last sentence of the definition of “Unrestricted Subsidiary.” For purposes of designating any Restricted Subsidiary as an Unrestricted Subsidiary, all outstanding Investments by the Issuer and its Restricted Subsidiaries (except to the extent repaid) in the Subsidiary so designated will be deemed to be Restricted Payments in an amount determined as set forth in the penultimate sentence of the definition of “Investments.” Such designation will be permitted only if a Restricted Payment in such amount would be permitted at such time, whether pursuant to the first paragraph of this covenant or under clause (7), (10) or (11) of the second paragraph of this covenant, or pursuant to the definition of “Permitted Investments,” and if such Subsidiary otherwise meets the definition of an Unrestricted Subsidiary. Unrestricted Subsidiaries will not be subject to any of the restrictive covenants set forth in the senior subordinated notes indenture.
As of February 28, 2013, we have approximately $725.8 million of Restricted Payment capacity under clause (3) of the first paragraph of this covenant and an additional $300.0 million of other Restricted Payments capacity under clause (11) of the second paragraph of this covenant.
Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock
The Issuer will not, and will not permit any of its Restricted Subsidiaries to, directly or indirectly, create, incur, issue, assume, guarantee or otherwise become directly or indirectly liable, contingently or otherwise (collectively, “incur” and collectively, an “incurrence”) with respect to any Indebtedness (including Acquired Indebtedness) and the Issuer will not issue any shares of Disqualified Stock and will not permit any Restricted Subsidiary to issue any shares of Disqualified Stock or Preferred Stock; provided that the Issuer may incur Indebtedness (including Acquired Indebtedness) or issue shares of Disqualified Stock, and, subject to the third paragraph of this covenant, any Restricted Subsidiary may incur Indebtedness (including Acquired Indebtedness), issue shares of Disqualified Stock and issue shares of Preferred Stock, if the Fixed Charge Coverage Ratio for the Issuer’s most recently ended four fiscal quarters for which internal financial statements are available immediately preceding the date on which such additional Indebtedness is incurred or such Disqualified Stock or Preferred Stock is issued would have been at least 2.00 to 1.00, determined on a pro forma basis (including a pro forma application of the net proceeds therefrom), as if the additional Indebtedness had been incurred, or the Disqualified Stock or Preferred Stock had been issued, as the case may be, and the application of proceeds therefrom had occurred at the beginning of such four-quarter period.
The foregoing limitations will not apply to:
205
(1) (a) the incurrence by the Issuer or any Restricted Subsidiary pursuant to one or more Credit Facilities, including through the issuance and creation of letters of credit and bankers’ acceptances thereunder (with letters of credit and bankers’ acceptances being deemed to have a principal amount equal to the face amount thereof), of: a) Indebtedness in an aggregate principal amount up to the sum of $3,165.0 million and €835.0 million and (b) Indebtedness in an aggregate principal amount that could be incurred such that at the time of incurrence and after giving effect thereto the Senior Secured Leverage Ratio would not exceed 4.50 to 1.00, determined on a pro forma basis (including a pro forma application of the net proceeds therefrom); provided that in calculating the Senior Secured Leverage Ratio solely for purposes of this clause (1), any unsecured Indebtedness incurred under this clause (1) shall be deemed to be Secured Indebtedness solely for purposes of calculating the Senior Secured Leverage Ratio for this clause (1);
(2) the incurrence by the Issuer and any Guarantor of Indebtedness represented by (a) the senior subordinated notes (including any Guarantee) and the exchange notes and related exchange guarantees to be issued in exchange for senior subordinated notes and the Guarantees pursuant to the Senior Subordinated Notes Registration Rights Agreement (but excluding any Additional Senior Subordinated Notes but including Guarantees thereof and exchange notes issued in exchange therefor pursuant to the Senior Subordinated Notes Registration Rights Agreement) and (b) the Senior Notes (including any guarantee thereof) and the exchange notes and related exchange guarantees to be issued in exchange for the Senior Notes and the guarantees thereof pursuant to one or more registration rights agreements (but excluding any additional Senior Notes (as defined in the indentures governing the Senior Notes) but including guarantees thereof and exchange notes issued in exchange therefor pursuant to any such registration rights agreement);
(3) Indebtedness of the Issuer and its Restricted Subsidiaries in existence on the Issue Date (other than Indebtedness described in clauses (1) and (2));
(4) Indebtedness (including Capitalized Lease Obligations) and Disqualified Stock incurred or issued by the Issuer or any Restricted Subsidiary and Preferred Stock issued by any Restricted Subsidiary, to finance the purchase, lease or improvement of property (real or personal) or equipment that is used or useful in a Similar Business, whether through the direct purchase of assets or the Capital Stock of any Person owning such assets in an aggregate principal amount, together with any Refinancing Indebtedness in respect thereof and all other Indebtedness, Disqualified Stock and/or Preferred Stock incurred or issued and outstanding under this clause (4), not to exceed 5.0% of Total Assets (in each case, determined at the date of incurrence) at any time outstanding, so long as such Indebtedness, Disqualified Stock or Preferred Stock is incurred or issued at the date of such purchase, lease or improvement or within 270 days thereafter;
(5) Indebtedness incurred by the Issuer or any of its Restricted Subsidiaries constituting reimbursement obligations with respect to letters of credit issued in the ordinary course of business, including letters of credit in respect of workers’ compensation claims, or other Indebtedness with respect to reimbursement type obligations regarding workers’ compensation claims; provided that upon the drawing of such letters of credit or the incurrence of such Indebtedness, such obligations are reimbursed within 30 days following such drawing or incurrence;
(6) Indebtedness arising from agreements of the Issuer or its Restricted Subsidiaries providing for indemnification, adjustment of purchase price, earnouts or similar obligations, in each case, incurred or assumed in connection with the disposition of any business, assets or a Subsidiary, other than guarantees of Indebtedness incurred by any Person acquiring all or any portion of such business, assets or a Subsidiary for the purpose of financing such acquisition; provided that such Indebtedness is not reflected on the balance sheet of the Issuer, or any of its Restricted Subsidiaries (contingent obligations referred to in a footnote to financial statements and not otherwise reflected on the balance sheet will not be deemed to be reflected on such balance sheet for purposes of this clause (6));
(7) Indebtedness of the Issuer to a Restricted Subsidiary; provided that any such Indebtedness owing to a Restricted Subsidiary that is not a Guarantor is expressly subordinated in right of payment to the senior subordinated notes; provided, further, that any subsequent issuance or transfer of any Capital Stock or any other event which results in any such Restricted Subsidiary ceasing to be a Restricted Subsidiary or any other subsequent transfer of any such Indebtedness (except to the Issuer or another Restricted Subsidiary) shall be deemed, in each case, to be an incurrence of such Indebtedness not permitted by this clause;
(8) Indebtedness of a Restricted Subsidiary to the Issuer or another Restricted Subsidiary; provided that if a Guarantor incurs such Indebtedness to a Restricted Subsidiary that is not a Guarantor, such Indebtedness is
206
expressly subordinated in right of payment to the Guarantee of the senior subordinated notes of such Guarantor; provided, further, that any subsequent transfer of any such Indebtedness (except to the Issuer or another Restricted Subsidiary) shall be deemed, in each case, to be an incurrence of such Indebtedness not permitted by this clause;
(9) shares of Preferred Stock of a Restricted Subsidiary issued to the Issuer or another Restricted Subsidiary; provided that any subsequent issuance or transfer of any Capital Stock or any other event which results in any such Restricted Subsidiary ceasing to be a Restricted Subsidiary or any other subsequent transfer of any such shares of Preferred Stock (except to the Issuer or another of its Restricted Subsidiaries) shall be deemed, in each case, to be an issuance of such shares of Preferred Stock not permitted by this clause;
(10) Hedging Obligations (excluding Hedging Obligations entered into for speculative purposes) for the purpose of limiting interest rate risk with respect to any Indebtedness permitted to be incurred under the senior subordinated notes indenture, exchange rate risk or commodity pricing risk;
(11) obligations in respect of self-insurance and obligations in respect of performance, bid, appeal and surety bonds and completion guarantees and similar obligations provided by the Issuer or any of its Restricted Subsidiaries in the ordinary course of business;
(12) (a) Indebtedness or Disqualified Stock of the Issuer and Indebtedness, Disqualified Stock or Preferred Stock of the Issuer or any Restricted Subsidiary in an aggregate principal amount or liquidation preference up to 100.0% of the net cash proceeds received by the Issuer since immediately after the Issue Date from the issue or sale of Equity Interests of the Issuer or cash contributed to the capital of the Issuer (in each case, other than proceeds of Disqualified Stock or sales of Equity Interests to the Issuer or any of its Subsidiaries) as determined in accordance with clauses (3)(b) and (3)(c) of the first paragraph of “—Limitation on Restricted Payments” to the extent such net cash proceeds or cash have not been applied pursuant to such clauses to make Restricted Payments or to make other Investments, payments or exchanges pursuant to the second paragraph of “—Limitation on Restricted Payments” or to make Permitted Investments (other than Permitted Investments specified in clause (1) or (3) of the definition thereof); and
(b) Indebtedness or Disqualified Stock of the Issuer and Indebtedness, Disqualified Stock or Preferred Stock of the Issuer or, subject to the third paragraph of this covenant, any Restricted Subsidiary not otherwise permitted hereunder in an aggregate principal amount or liquidation preference which, when aggregated with the principal amount and liquidation preference of all other Indebtedness, Disqualified Stock and Preferred Stock then outstanding and incurred pursuant to this clause (12)(b), does not at any one time outstanding exceed the greater of (x) $550.0 million and (y) 5.0% of Total Assets (it being understood that any Indebtedness, Disqualified Stock or Preferred Stock incurred pursuant to this clause (12)(b) shall cease to be deemed incurred or outstanding for purposes of this clause (12)(b) but shall be deemed incurred for the purposes of the first paragraph of this covenant from and after the first date on which the Issuer or such Restricted Subsidiary could have incurred such Indebtedness, Disqualified Stock or Preferred Stock under the first paragraph of this covenant without reliance on this clause (12)(b));
(13) the incurrence by the Issuer or any Restricted Subsidiary of Indebtedness, the issuance by the Issuer or any Restricted Subsidiary of Disqualified Stock or the issuance by any Restricted Subsidiary of Preferred Stock which serves to extend, replace, refund, refinance, renew or defease any Indebtedness incurred or Disqualified Stock or Preferred Stock issued as permitted under the first paragraph of this covenant and clauses (2), (3), (4) and (12)(a) above, this clause (13) and clauses (14) and (24) below or any Indebtedness incurred or Disqualified Stock or Preferred Stock issued to so extend, replace, refund, refinance, renew or defease such Indebtedness, Disqualified Stock or Preferred Stock including additional Indebtedness, Disqualified Stock or Preferred Stock incurred to pay premiums (including reasonable tender premiums), defeasance costs and fees in connection therewith (the “Refinancing Indebtedness”) prior to its respective maturity; provided that such Refinancing Indebtedness:
(a) has a Weighted Average Life to Maturity at the time such Refinancing Indebtedness is incurred which is not less than the remaining Weighted Average Life to Maturity of, the Indebtedness, Disqualified Stock or Preferred Stock being extended, replaced, refunded, refinanced, renewed or defeased;
(b) to the extent such Refinancing Indebtedness extends, replaces, refunds, refinances, renews or defeases (i) Indebtedness subordinated to the senior subordinated notes or any Guarantee thereof, such Refinancing Indebtedness is subordinated to the senior subordinated notes or the Guarantee thereof at least to the same extent as the Indebtedness being extended, replaced, refunded, refinanced, renewed or defeased
207
or (ii) Disqualified Stock or Preferred Stock, such Refinancing Indebtedness must be Disqualified Stock or Preferred Stock, respectively; and
(c) shall not include:
(i) Indebtedness, Disqualified Stock or Preferred Stock of a Subsidiary of the Issuer that is not a Guarantor that refinances Indebtedness or Disqualified Stock of the Issuer;
(ii) Indebtedness, Disqualified Stock or Preferred Stock of a Subsidiary of the Issuer that is not a Guarantor that refinances Indebtedness, Disqualified Stock or Preferred Stock of a Guarantor; or
(iii) Indebtedness or Disqualified Stock of the Issuer or Indebtedness, Disqualified Stock or Preferred Stock of a Restricted Subsidiary that refinances Indebtedness, Disqualified Stock or Preferred Stock of an Unrestricted Subsidiary; and, provided, further, that subclause (a) of this clause (13) will not apply to any extension, replacement, refunding, refinancing, renewal or defeasance of any Indebtedness outstanding under a Credit Facility and Obligations secured by Permitted Liens.
(14) (a) Indebtedness or Disqualified Stock of the Issuer or, subject to the third paragraph of this covenant, Indebtedness, Disqualified Stock or Preferred Stock of a Restricted Subsidiary incurred or issued to finance an acquisition or (b) Indebtedness, Disqualified Stock or Preferred Stock of Persons that are acquired by the Issuer or any Restricted Subsidiary or merged into the Issuer or a Restricted Subsidiary in accordance with the terms of the senior subordinated notes indenture; provided that after giving effect to such acquisition or merger, either
(i) the Issuer would be permitted to incur at least $1.00 of additional Indebtedness pursuant to the Fixed Charge Coverage Test, or
(ii) the Fixed Charge Coverage Ratio for the Issuer is equal to or greater than immediately prior to such acquisition or merger;
(15) Indebtedness arising from the honoring by a bank or other financial institution of a check, draft or similar instrument drawn against insufficient funds in the ordinary course of business, provided that such Indebtedness is extinguished within five Business Days of its incurrence;
(16) Indebtedness of the Issuer or any of its Restricted Subsidiaries supported by a letter of credit issued pursuant to the Credit Facilities, in a principal amount not in excess of the stated amount of such letter of credit;
(17) (a) any guarantee by the Issuer or a Restricted Subsidiary of Indebtedness or other obligations of any Restricted Subsidiary so long as the incurrence of such Indebtedness incurred by such Restricted Subsidiary is permitted under the terms of the senior subordinated notes indenture, or (b) any guarantee by a Restricted Subsidiary of Indebtedness of the Issuer; provided that such guarantee is incurred in accordance with the covenant described below under “—Limitation on Guarantees of Indebtedness by Restricted Subsidiaries”;
(18) Indebtedness consisting of Indebtedness issued by the Issuer or any of its Restricted Subsidiaries to future, present or former employees, directors, officers, managers, distributors and consultants thereof, their respective Controlled Investment Affiliates or Immediate Family Members, in each case to finance the purchase or redemption of Equity Interests of the Issuer or any direct or indirect parent company of the Issuer to the extent described in clause (4) of the second paragraph under “—Limitation on Restricted Payments”;
(19) customer deposits and advance payments received in the ordinary course of business from customers for goods purchased in the ordinary course of business;
(20) Indebtedness owed on a short-term basis of no longer than 30 days to banks and other financial institutions incurred in the ordinary course of business of the Issuer and its Restricted Subsidiaries with such banks or financial institutions that arises in connection with ordinary banking arrangements to manage cash balances of the Issuer and its Restricted Subsidiaries;
208
(21) Indebtedness incurred by a Restricted Subsidiary in connection with bankers’ acceptances, discounted bills of exchange or the discounting or factoring of receivables for credit management purposes, in each case incurred or undertaken in the ordinary course of business on arm’s length commercial terms on a recourse basis;
(22) Indebtedness of the Issuer or any of its Restricted Subsidiaries consisting of (a) the financing of insurance premiums or (b) take-or-pay obligations contained in supply arrangements in each case, incurred in the ordinary course of business;
(23) the incurrence of Indebtedness of Foreign Subsidiaries of the Issuer or a Restricted Subsidiary of the Issuer in an amount not to exceed at any one time outstanding the greater of (i) $100.0 million and (ii) 5.0% of the Foreign Subsidiary Total Assets (it being understood that any Indebtedness incurred pursuant to this clause (23) shall cease to be deemed incurred or outstanding for the purpose of this clause (23) but shall be deemed incurred for the purposes of the first paragraph of this covenant from and after the first date on which the Issuer or such Restricted Subsidiaries could have incurred such Indebtedness under the first paragraph of this covenant without reliance on this clause (23));
(24) Indebtedness, Disqualified Stock or Preferred Stock of a Restricted Subsidiary incurred to finance or assumed in connection with an acquisition in a principal amount not to exceed $100.0 million in the aggregate at any one time outstanding together with all other Indebtedness, Disqualified Stock and/or Preferred Stock issued under this clause (24) (it being understood that any Indebtedness, Disqualified Stock or Preferred Stock incurred pursuant to this clause (24) shall cease to be deemed incurred or outstanding for purposes of this clause (24) but shall be deemed incurred for the purposes of the first paragraph of this covenant from and after the first date on which such Restricted Subsidiary could have incurred such Indebtedness, Disqualified Stock or Preferred Stock under the first paragraph of this covenant without reliance on this clause (24)); and
(25) Indebtedness of the Issuer or any of its Restricted Subsidiaries undertaken in connection with cash management and related activities with respect to any Subsidiary or joint venture in the ordinary course of business.
Restricted Subsidiaries of the Issuer that are not Guarantors may not incur Indebtedness or Disqualified Stock or Preferred Stock under the first paragraph of this covenant or clause (12)(b), (14)(a) or (24) of the second paragraph of this covenant if, after giving pro forma effect to such incurrence or issuance (including a pro forma application of the net proceeds therefrom), the aggregate amount of Indebtedness and Disqualified Stock and Preferred Stock of Restricted Subsidiaries that are not Guarantors incurred or issued pursuant to the first paragraph of this covenant and clauses (12)(b), (14)(a) and (24) of the second paragraph of this covenant, collectively, would exceed $600.0 million.
For purposes of determining compliance with this covenant:
(1) in the event that an item of Indebtedness, Disqualified Stock or Preferred Stock (or any portion thereof) meets the criteria of more than one of the categories of Permitted Indebtedness, Disqualified Stock or Preferred Stock described in clauses (1) through (25) above or is entitled to be incurred pursuant to the first paragraph of this covenant, the Issuer, in its sole discretion, will classify or reclassify such item of Indebtedness, Disqualified Stock or Preferred Stock (or any portion thereof) and will only be required to include the amount and type of such Indebtedness, Disqualified Stock or Preferred Stock in one of the above clauses or under the first paragraph of this covenant; provided that all Indebtedness outstanding under the Senior Credit Facilities on the Issue Date will be treated as incurred on the Issue Date under clause (1) of the second paragraph above; and
(2) at the time of incurrence, the Issuer will be entitled to divide and classify an item of Indebtedness in more than one of the types of Indebtedness described in the first and second paragraphs above.
Accrual of interest or dividends, the accretion of accreted value, the accretion or amortization of original issue discount and the payment of interest or dividends in the form of additional Indebtedness, Disqualified Stock or Preferred Stock, as the case may be, of the same class will not be deemed to be an incurrence of Indebtedness, Disqualified Stock or Preferred Stock for purposes of this covenant.
For purposes of determining compliance with any U.S. dollar-denominated or Euro-denominated, as the case may be, restriction on the incurrence of Indebtedness, the U.S. dollar-equivalent or Euro- equivalent, as the case may be, principal amount of Indebtedness denominated in another currency shall be calculated based on the relevant currency exchange rate in effect on the date such Indebtedness was incurred, in the case of term debt, or first committed, in the case of revolving credit debt; provided that if such Indebtedness is incurred to refinance other
209
Indebtedness denominated in another currency, and such refinancing would cause the applicable U.S. dollar-denominated or Euro-denominated, as the case may be, restriction to be exceeded if calculated at the relevant currency exchange rate in effect on the date of such refinancing, such U.S. dollar-denominated or Euro-denominated, as the case may be, restriction shall be deemed not to have been exceeded so long as the principal amount of such refinancing Indebtedness does not exceed the principal amount of such Indebtedness being refinanced.
The principal amount of any Indebtedness incurred to refinance other Indebtedness, if incurred in a different currency from the Indebtedness being refinanced, shall be calculated based on the currency exchange rate applicable to the currencies in which such respective Indebtedness is denominated that is in effect on the date of such refinancing.
Liens
The Issuer will not, and will not permit any Guarantor to, directly or indirectly, create, incur, assume or suffer to exist any Lien (except Permitted Liens) that secures Obligations under any Indebtedness ranking pari passu with or subordinated to the senior subordinated notes or any related Guarantee, on any asset or property of the Issuer or any Guarantor, or any income or profits therefrom, or assign or convey any right to receive income therefrom, unless:
(1) in the case of Liens securing Subordinated Indebtedness, the senior subordinated notes and related Guarantees are secured by a Lien on such property, assets or proceeds that is senior in priority to such Liens; and
(2) in all other cases, the senior subordinated notes or the Guarantees are equally and ratably secured, except that the foregoing shall not apply to (a) Liens securing the senior subordinated notes and the related Guarantees and (b) Liens securing Senior Indebtedness of the Issuer or any Guarantor.
Merger, Consolidation or Sale of All or Substantially All Assets
The Issuer may not consolidate or merge with or into or wind up into (whether or not the Issuer is the surviving Person), or sell, assign, transfer, lease, convey or otherwise dispose of all or substantially all of its properties or assets, in one or more related transactions, to any Person unless:
(1) the Issuer is the surviving Person or the Person formed by or surviving any such consolidation or merger (if other than the Issuer) or to which such sale, assignment, transfer, lease, conveyance or other disposition will have been made, is a Person organized or existing under the laws of the United States, any state thereof, the District of Columbia, or any territory thereof (such Person, as the case may be, being herein called the “Successor Company”); provided that in the case where the surviving Person is not a corporation, a co-obligor of the senior subordinated notes is a corporation;
(2) the Successor Company, if other than the Issuer, expressly assumes all the obligations of the Issuer under the senior subordinated notes pursuant to supplemental indentures or other documents or instruments;
(3) immediately after such transaction, no Default exists;
(4) immediately after giving pro forma effect to such transaction and any related financing transactions, as if such transactions had occurred at the beginning of the applicable four-quarter period,
(a) the Successor Company or the Issuer would be permitted to incur at least $1.00 of additional Indebtedness pursuant to the Fixed Charge Coverage Test, or
(b) the Fixed Charge Coverage Ratio for the Issuer would be equal to or greater than the Fixed Charge Coverage Ratio for the Issuer immediately prior to such transaction;
(5) each Guarantor, unless it is the other party to the transactions described above, in which case clause (1)(b) of the second succeeding paragraph shall apply, shall have by supplemental indenture confirmed that its Guarantee shall apply to such Person’s obligations under the senior subordinated notes indenture, the senior subordinated notes and the Senior Subordinated Notes Registration Rights Agreement; and
210
(6) the Issuer shall have delivered to the Trustee an Officer’s Certificate and an Opinion of Counsel, each stating that such consolidation, merger or transfer and such supplemental indentures, if any, comply with the senior subordinated notes indenture, and an Opinion of Counsel that such supplemental indenture is the valid and binding obligation of the Issuer and the Guarantors and enforceable in accordance with its terms.
The Successor Company will succeed to, and be substituted for the Issuer under the senior subordinated notes indenture, the Guarantees and the senior subordinated notes, as applicable. Notwithstanding the immediately preceding clauses (3) and (4),
(1) any Restricted Subsidiary may consolidate with or merge into or transfer all or part of its properties and assets to the Issuer, and
(2) the Issuer may merge with an Affiliate of the Issuer solely for the purpose of reincorporating the Issuer in the United States, the District of Columbia or any territory thereof so long as the amount of Indebtedness of the Issuer and its Restricted Subsidiaries is not increased thereby.
Subject to certain limitations described in the senior subordinated notes indenture governing release of a Guarantee upon the sale, disposition or transfer of a Guarantor, no Guarantor will, and the Issuer will not permit any Guarantor to, consolidate or merge with or into or wind up into (whether or not such Guarantor is the surviving Person), or sell, assign, transfer, lease, convey or otherwise dispose of all or substantially all of its properties or assets, in one or more related transactions, to any Person unless:
(1) (a) such Guarantor is the surviving Person or the Person formed by or surviving any such consolidation or merger (if other than such Guarantor) or to which such sale, assignment, transfer, lease, conveyance or other disposition will have been made is a Person organized or existing under the laws of the jurisdiction of organization of such Guarantor, as applicable, or the laws of the United States, any state thereof, the District of Columbia, or any territory thereof (such Person being herein called the “Successor Person”);
(b) the Successor Person, if other than such Guarantor, expressly assumes all the obligations of such Guarantor under the senior subordinated notes indenture and such Guarantor’s related Guarantee pursuant to supplemental indentures or other documents or instruments;
(c) immediately after such transaction, no Default exists; and
(d) the Issuer shall have delivered to the Trustee an Officer’s Certificate and an Opinion of Counsel, each stating that such consolidation, merger or transfer and such supplemental indentures, if any, comply with the senior subordinated notes indenture, and an Opinion of Counsel that such supplemental indenture is the valid and binding obligation of the Successor Person and enforceable in accordance with its terms; or
(2) the transaction is made in compliance with the covenant described under “Repurchase at the Option of Holders—Asset Sales.”
Subject to certain limitations described in the senior subordinated notes indenture, the Successor Person will succeed to, and be substituted for, such Guarantor under the senior subordinated notes indenture and such Guarantor’s Guarantee. Notwithstanding the foregoing, any Guarantor may (1) merge into or transfer all or part of its properties and assets to another Guarantor or the Issuer, (2) merge with an Affiliate of the Issuer solely for the purpose of reincorporating the Guarantor in the United States, any state thereof, the District of Columbia or any territory thereof or (3) convert into a corporation, partnership, limited partnership, limited liability corporation or trust organized or existing under the laws of the jurisdiction of organization of such Guarantor.
Transactions with Affiliates
The Issuer will not, and will not permit any of its Restricted Subsidiaries to, make any payment to, or sell, lease, transfer or otherwise dispose of any of its properties or assets to, or purchase any property or assets from, or enter into or make or amend any transaction, contract, agreement, understanding, loan, advance or guarantee with, or for the benefit of, any Affiliate of the Issuer (each of the foregoing, an “Affiliate Transaction”) involving aggregate payments or consideration in excess of $25.0 million, unless:
211
(1) such Affiliate Transaction is on terms that are not materially less favorable to the Issuer or its relevant Restricted Subsidiary than those that would have been obtained in a comparable transaction by the Issuer or such Restricted Subsidiary with an unrelated Person on an arm’s-length basis; and
(2) the Issuer delivers to the Trustee with respect to any Affiliate Transaction or series of related Affiliate Transactions involving aggregate payments or consideration in excess of $50.0 million, a resolution adopted by the majority of the board of directors of the Issuer approving such Affiliate Transaction and set forth in an Officer’s Certificate certifying that such Affiliate Transaction complies with clause (1) above.
The foregoing provisions will not apply to the following:
(1) transactions between or among the Issuer or any of its Restricted Subsidiaries;
(2) Restricted Payments permitted by the provisions of the senior subordinated notes indenture described above under the covenant “—Limitation on Restricted Payments” and the definition of “Permitted Investments”;
(3) the payment of management, consulting, monitoring, advisory and other fees and related expenses pursuant to the Management Fee Agreement (plus any unpaid management, consulting, monitoring, advisory and other fees and related expenses accrued in any prior year) and the termination fees pursuant to the Management Fee Agreement, or any amendment thereto so long as any such amendment is not disadvantageous in the good faith judgment of the board of directors of the Issuer to the Holders when taken as a whole, as compared to the Management Fee Agreement as in effect on the Issue Date;
(4) the payment of reasonable and customary fees paid to, and indemnities provided for the benefit of, current or former employees, directors, officers, managers, distributors or consultants of the Issuer, any of its direct or indirect parent companies or any of its Restricted Subsidiaries;
(5) transactions in which the Issuer or any of its Restricted Subsidiaries, as the case may be, delivers to the Trustee a letter from an Independent Financial Advisor stating that such transaction is fair to the Issuer or such Restricted Subsidiary from a financial point of view or stating that the terms are not materially less favorable to the Issuer or its relevant Restricted Subsidiary than with an unrelated Person on an arm’s-length basis;
(6) any agreement as in effect as of the Issue Date, or any amendment thereto (so long as any such amendment is not disadvantageous in the good faith judgment of the board of directors of the Issuer to the Holders when taken as a whole as compared to the applicable agreement as in effect on the Issue Date);
(7) the existence of, or the performance by the Issuer or any of its Restricted Subsidiaries of its obligations under the terms of, any stockholders agreement (including any registration rights agreement or purchase agreement related thereto) to which it is a party as of the Issue Date and any similar agreements which it may enter into thereafter; provided that the existence of, or the performance by the Issuer or any of its Restricted Subsidiaries of obligations under any future amendment to any such existing agreement or under any similar agreement entered into after the Issue Date shall only be permitted by this clause (7) to the extent that the terms of any such amendment or new agreement are not otherwise disadvantageous in the good faith judgment of the board of directors of the Issuer to the Holders when taken as a whole;
(8) the Transactions and the payment of all fees and expenses related to the Transactions, in each case as contemplated by the offering circular relating to the original senior subordinated notes dated September 18, 2012;
(9) transactions with customers, clients, suppliers, contractors, joint venture partners or purchasers or sellers of goods or services that are Affiliates, in each case in the ordinary course of business and otherwise in compliance with the terms of the senior subordinated notes indenture which are fair to the Issuer and its Restricted Subsidiaries, in the reasonable determination of the board of directors of the Issuer or the senior management thereof, or are on terms at least as favorable as might reasonably have been obtained at such time from an unaffiliated party;
(10) the issuance of Equity Interests (other than Disqualified Stock) of the Issuer to any Permitted Holder or to any employee, director, officer, manager, distributor or consultant (or their respective Controlled Investment Affiliates or Immediate Family Members) of the Issuer, any of its direct or indirect parent companies or any of its Restricted Subsidiaries;
212
(11) sales of accounts receivable, or participations therein, or Securitization Assets or related assets in connection with the ABL Facilities or any Qualified Securitization Facility;
(12) payments by the Issuer or any of its Restricted Subsidiaries to any of the Investors made for any financial advisory, financing, underwriting or placement services or in respect of other investment banking activities, including, without limitation, in connection with acquisitions or divestitures which payments are approved by a majority of the board of directors of the Issuer in good faith;
(13) payments and Indebtedness and Disqualified Stock (and cancellation of any thereof) of the Issuer and its Restricted Subsidiaries and Preferred Stock (and cancellation of any thereof) of any Restricted Subsidiary to any future, current or former employee, director, officer, manager, distributor or consultant (or their respective Controlled Investment Affiliates or Immediate Family Members) of the Issuer, any of its Subsidiaries or any of its direct or indirect parent companies pursuant to any management equity plan or stock option plan or any other management or employee benefit plan or agreement or any stock subscription or shareholder agreement or any distributor equity plan or agreement; and any employment agreements, stock option plans and other compensatory arrangements (and any successor plans thereto) and any supplemental executive retirement benefit plans or arrangements with any such employees, directors, officers, managers, distributors or consultants (or their respective Controlled Investment Affiliates or Immediate Family Members) that are, in each case, approved by the Issuer in good faith;
(14) investments by any of the Investors in securities of the Issuer or any of its Restricted Subsidiaries (and payment of reasonable out-of-pocket expenses incurred by such Investors in connection therewith) so long as (a) the investment is being offered generally to other investors on the same or more favorable terms and (b) the investment constitutes less than 5.0% of the proposed or outstanding issue amount of such class of securities;
(15) payments to or from, and transactions with, any joint venture in the ordinary course of business (including, without limitation, any cash management activities related thereto);
(16) payments by the Issuer (and any direct or indirect parent company thereof) and its Subsidiaries pursuant to tax sharing agreements among the Issuer (and any such parent company) and its Subsidiaries; provided that in each case the amount of such payments in any fiscal year does not exceed the amount that the Issuer, its Restricted Subsidiaries and its Unrestricted Subsidiaries (to the extent of amount received from Unrestricted Subsidiaries) would be required to pay in respect of foreign, federal, state and local taxes for such fiscal year were the Issuer, its Restricted Subsidiaries and its Unrestricted Subsidiaries (to the extent described above) to pay such taxes separately from any such parent entity;
(17) any lease entered into between the Issuer or any Restricted Subsidiary, as lessee and any Affiliate of the Issuer, as lessor, which is approved by a majority of the disinterested members of the board of directors of the Issuer in good faith;
(18) intellectual property licenses in the ordinary course of business; and
(19) any transition services arrangements, supply arrangements and similar arrangements entered into in connection with or in contemplation of dispositions of assets or Equity Interests in any Restricted Subsidiary not otherwise prohibited by the terms of the senior subordinated notes indenture that the Issuer determines in good faith are either fair to the Issuer or otherwise on customary terms for such type of arrangements in connection with similar transactions.
Dividend and Other Payment Restrictions Affecting Restricted Subsidiaries
The Issuer will not, and will not permit any of its Restricted Subsidiaries that is not a Guarantor to, directly or indirectly, create or otherwise cause or suffer to exist or become effective any consensual encumbrance or consensual restriction on the ability of any such Restricted Subsidiary to:
(1) (a) pay dividends or make any other distributions to the Issuer or any of its Restricted Subsidiaries on its Capital Stock or with respect to any other interest or participation in, or measured by, its profits, or
(b) pay any Indebtedness owed to the Issuer or any of its Restricted Subsidiaries;
213
(2) make loans or advances to the Issuer or any of its Restricted Subsidiaries; or
(3) sell, lease or transfer any of its properties or assets to the Issuer or any of its Restricted Subsidiaries, except (in each case) for such encumbrances or restrictions existing under or by reason of:
(a) contractual encumbrances or restrictions in effect on the Issue Date, including pursuant to the Senior Credit Facilities and the related documentation, Hedging Obligations and the indentures governing the Senior Notes and the related documentation;
(b) the senior subordinated notes indenture, the senior subordinated notes and the guarantees thereof;
(c) purchase money obligations for property acquired in the ordinary course of business and Capital Lease Obligations that impose restrictions of the nature discussed in clause (3) above on the property so acquired;
(d) applicable law or any applicable rule, regulation or order;
(e) any agreement or other instrument of a Person acquired by the Issuer or any of its Restricted Subsidiaries in existence at the time of such acquisition or at the time it merges with or into the Issuer or any of its Restricted Subsidiaries or assumed in connection with the acquisition of assets from such Person (but, in any such case, not created in contemplation thereof), which encumbrance or restriction is not applicable to any Person, or the properties or assets of any Person, other than the Person so acquired and its Subsidiaries, or the property or assets of the Person so acquired and its Subsidiaries;
(f) contracts for the sale of assets, including customary restrictions with respect to a Subsidiary of the Issuer pursuant to an agreement that has been entered into for the sale or disposition of all or substantially all of the Capital Stock or assets of such Subsidiary;
(g) Secured Indebtedness otherwise permitted to be incurred pursuant to the covenants described under “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock” and “—Liens” that limit the right of the debtor to dispose of the assets securing such Indebtedness;
(h) restrictions on cash or other deposits or net worth imposed by customers under contracts entered into in the ordinary course of business;
(i) other Indebtedness, Disqualified Stock or Preferred Stock of Foreign Subsidiaries permitted to be incurred subsequent to the Issue Date pursuant to the provisions of the covenant described under “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”;
(j) customary provisions in joint venture agreements and other similar agreements relating solely to such joint venture;
(k) customary provisions contained in leases, licenses or similar agreements, including with respect to intellectual property and other agreements, in each case, entered into in the ordinary course of business;
(l) restrictions created in connection with any Qualified Securitization Facility that, in the good faith determination of the Issuer are necessary or advisable to effect such Qualified Securitization Facility;
(m) restrictions or conditions contained in any trading, netting, operating, construction, service, supply, purchase, sale or other agreement to which the Issuer or any of its Restricted Subsidiaries is a party entered into in the ordinary course of business; provided that such agreement prohibits the encumbrance of solely the property or assets of the Issuer or such Restricted Subsidiary that are the subject to such agreement, the payment rights arising thereunder or the proceeds thereof and does not extend to any other asset or property of the Issuer or such Restricted Subsidiary or the assets or property of another Restricted Subsidiary; and
(n) any encumbrances or restrictions of the type referred to in clauses (1), (2) and (3) above imposed by any amendments, modifications, restatements, renewals, increases, supplements, refundings, replacements or refinancings of the contracts, instruments or obligations referred to in clauses (a) through (m) above; provided that such amendments, modifications, restatements, renewals, increases, supplements, refundings, replacements or refinancings are, in the good faith judgment of the Issuer, no more restrictive
214
with respect to such encumbrance and other restrictions taken as a whole than those prior to such amendment, modification, restatement, renewal, increase, supplement, refunding, replacement or refinancing.
Limitation on Guarantees of Indebtedness by Restricted Subsidiaries
The Issuer will not permit any of its Wholly-Owned Subsidiaries that are Restricted Subsidiaries (and non-Wholly-Owned Subsidiaries if such non-Wholly-Owned Subsidiaries guarantee other capital markets debt securities of the Issuer or any Guarantor), other than a Guarantor, a Foreign Subsidiary or a Securitization Subsidiary, to guarantee the payment of any Indebtedness of the Issuer or any other Guarantor unless:
(1) such Restricted Subsidiary within 30 days executes and delivers a supplemental indenture to the senior subordinated notes indenture providing for a Guarantee by such Restricted Subsidiary, except that with respect to a guarantee of Indebtedness of the Issuer or any Guarantor:
(a) if the senior subordinated notes or such Guarantor’s Guarantee are subordinated in right of payment to such Indebtedness, the Guarantee under the supplemental indenture shall be subordinated to such Restricted Subsidiary’s guarantee with respect to such Indebtedness substantially to the same extent as the senior subordinated notes are subordinated to such Indebtedness; and
(b) if such Indebtedness is by its express terms subordinated in right of payment to the senior subordinated notes or such Guarantor’s Guarantee, any such guarantee by such Restricted Subsidiary with respect to such Indebtedness shall be subordinated in right of payment to such Guarantee substantially to the same extent as such Indebtedness is subordinated to the senior subordinated notes; and
(2) such Restricted Subsidiary waives and will not in any manner whatsoever claim or take the benefit or advantage of, any rights of reimbursement, indemnity or subrogation or any other rights against the Issuer or any other Restricted Subsidiary as a result of any payment by such Restricted Subsidiary under its Guarantee;
provided that this covenant shall not be applicable to (i) any guarantee of any Restricted Subsidiary that existed at the time such Person became a Restricted Subsidiary and was not incurred in connection with, or in contemplation of, such Person becoming a Restricted Subsidiary and (ii) guarantees of the ABL Facilities by the ABL Financing Entities or of any Qualified Securitization Facility by any Restricted Subsidiary.
Limitation on Layering
The senior subordinated notes indenture provides that the Issuer will not, and will not permit any Guarantor to, directly or indirectly, incur any Indebtedness (including Acquired Indebtedness) that is subordinate in right of payment to any Senior Indebtedness of the Issuer or such other Guarantor, as the case may be, unless such Indebtedness is either:
(1) equal in right of payment with the senior subordinated notes or such Guarantor’s Guarantee of the senior subordinated notes, as the case may be; or
(2) expressly subordinated in right of payment to the senior subordinated notes or such Guarantor’s Guarantee of the senior subordinated notes, as the case may be.
The senior subordinated notes indenture will not treat (1) unsecured Indebtedness as subordinated or junior to Secured Indebtedness merely because it is unsecured or (2) Senior Indebtedness as subordinated or junior to any other Senior Indebtedness merely because it has a junior priority with respect to the same collateral.
Reports and Other Information
Notwithstanding that the Issuer may not be subject to the reporting requirements of Section 13 or 15(d) of the Exchange Act or otherwise report on an annual and quarterly basis on forms provided for such annual and quarterly reporting pursuant to rules and regulations promulgated by the SEC, the senior subordinated notes indenture requires the Issuer to file with the SEC from and after the Issue Date,
(1) within 90 days (or any other time period then in effect under the rules and regulations of the Exchange Act with respect to the filing of a Form 10-K by a non-accelerated filer) after the end of each fiscal year,
215
annual reports on Form 10-K, or any successor or comparable form, containing the information required to be contained therein, or required in such successor or comparable form;
(2) within 45 days (or any other time period then in effect under the rules and regulations of the Exchange Act with respect to the filing of a Form 10-Q by a non-accelerated filer) after the end of each of the first three fiscal quarters of each fiscal year quarterly reports on Form 10-Q containing all quarterly information that would be required to be contained in Form 10-Q, or any successor or comparable form;
(3) promptly from time to time after the occurrence of an event required to be therein reported, such other reports on Form 8-K, or any successor or comparable form; and
(4) any other information, documents and other reports which the Issuer would be required to file with the SEC if it were subject to Section 13 or 15(d) of the Exchange Act;
in each case, in a manner that complies in all material respects with the requirements specified in such form; provided that the Issuer shall not be so obligated to file such reports with the SEC if the SEC does not permit such filing, in which event the Issuer will make available such information to prospective purchasers of senior subordinated notes, in addition to providing such information to the Trustee and the Holders of the senior subordinated notes, in each case within 15 days after the time the Issuer would be required to file such information with the SEC, if it were subject to Sections 13 or 15(d) of the Exchange Act. In addition, to the extent not satisfied by the foregoing, the Issuer will agree that, for so long as any senior subordinated notes are outstanding, it will furnish to Holders and to securities analysts and prospective investors, upon their request, the information required to be delivered pursuant to Rule 144A(d)(4) under the Securities Act.
In the event that any direct or indirect parent company of the Issuer becomes a guarantor of the senior subordinated notes, the senior subordinated notes indenture permits the Issuer to satisfy its obligations in this covenant by furnishing reports of such parent; provided that the same is accompanied, to the extent material to investors in the senior subordinated notes, by consolidating financial and other information that explains in reasonable detail the differences between the information in such reports relating to such parent, on the one hand, and the information relating to the Issuer and its consolidated Subsidiaries on a standalone basis, on the other hand.
Notwithstanding the foregoing, such requirements shall be deemed satisfied prior to the commencement of the exchange offers or the effectiveness of the shelf registration statement by (1) the filing with the SEC of the registration statement or shelf registration statement (or any other similar registration statement) of the exchange offers, and any amendments thereto, with such financial information that satisfies Regulation S-X of the Securities Act, subject to exceptions consistent with the presentation of financial information in the offering circular relating to the original senior subordinated notes dated September 18, 2012, to the extent filed within the time specified above, or (2) by posting on its website and providing to the Trustee within 15 days of the time periods after the Issuer would have been required to file annual and interim reports with the SEC, the financial information (including a “Management’s Discussion and Analysis of Financial Condition and Results of Operations” section) that would be required to be included in such reports, subject to exceptions consistent with the presentation of financial information in this prospectus, to the extent filed within the times specified above.
Notwithstanding anything herein to the contrary, the Issuer will not be deemed to have failed to comply with any of its obligations hereunder for purposes of clause (3) under “Events of Default and Remedies” until 120 days after the date any report hereunder is due.
The Issuer shall use its commercially reasonable efforts, consistent with its judgment as to what is prudent at the time, to participate in quarterly conference calls to discuss operating results and related matters. The Company shall issue a press release which will provide the date and time of any such call and will direct Holders, prospective investors and securities analysts to contact the investor relations office of the Issuer to obtain access to the conference call.
Events of Default and Remedies
The senior subordinated notes indenture provides that each of the following is an Event of Default:
(1) default in payment when due and payable, upon redemption, acceleration or otherwise, of principal of, or premium, if any, on the senior subordinated notes (whether or not prohibited by the subordination provisions of the senior subordinated notes indenture);
216
(2) default for 30 days or more in the payment when due of interest or Additional Interest on or with respect to the senior subordinated notes (whether or not prohibited by the subordination provisions of the senior subordinated notes indenture);
(3) failure by the Issuer or any Guarantor for 60 days after receipt of written notice given by the Trustee or the Holders of not less than 30.0% in principal amount of the then outstanding senior subordinated notes to comply with any of its obligations, covenants or agreements (other than a default referred to in clause (1) or (2) above) contained in the senior subordinated notes indenture or the senior subordinated notes;
(4) default under any mortgage, indenture or instrument under which there is issued or by which there is secured or evidenced any Indebtedness for money borrowed by the Issuer or any of its Restricted Subsidiaries or the payment of which is guaranteed by the Issuer or any of its Restricted Subsidiaries, other than Indebtedness owed to the Issuer or a Restricted Subsidiary, whether such Indebtedness or guarantee now exists or is created after the issuance of the senior subordinated notes, if both:
(a) such default either results from the failure to pay any principal of such Indebtedness at its stated final maturity (after giving effect to any applicable grace periods) or relates to an obligation other than the obligation to pay principal of any such Indebtedness at its stated final maturity and results in the holder or holders of such Indebtedness causing such Indebtedness to become due prior to its stated maturity; and
(b) the principal amount of such Indebtedness, together with the principal amount of any other such Indebtedness in default for failure to pay principal at stated final maturity (after giving effect to any applicable grace periods), or the maturity of which has been so accelerated, aggregate $75.0 million or more at any one time outstanding;
(5) failure by the Issuer or any Significant Subsidiary (or any group of Subsidiaries that together would constitute a Significant Subsidiary) to pay final judgments aggregating in excess of $75.0 million, which final judgments remain unpaid, undischarged and unstayed for a period of more than 60 days after such judgment becomes final, and in the event such judgment is covered by insurance, an enforcement proceeding has been commenced by any creditor upon such judgment or decree which is not promptly stayed;
(6) certain events of bankruptcy or insolvency with respect to the Issuer or any Significant Subsidiary (or any group of Subsidiaries that together would constitute a Significant Subsidiary); or
(7) the Guarantee of any Significant Subsidiary (or any group of Subsidiaries that together would constitute a Significant Subsidiary) shall for any reason cease to be in full force and effect or be declared null and void or any responsible officer of any Guarantor that is a Significant Subsidiary (or the responsible officers of any group of Subsidiaries that together would constitute a Significant Subsidiary), as the case may be, denies that it has any further liability under its Guarantee or gives notice to such effect, other than by reason of the termination of the senior subordinated notes indenture or the release of any such Guarantee in accordance with the senior subordinated notes indenture.
If any Event of Default (other than of a type specified in clause (6) above) occurs and is continuing under the senior subordinated notes indenture, the Trustee or the Holders of at least 30.0% in principal amount of the then total outstanding senior subordinated notes may declare the principal, premium, if any, interest and any other monetary obligations on all the then outstanding senior subordinated notes to be due and payable immediately; provided that so long as any Indebtedness permitted to be incurred under the senior subordinated notes indenture as part of the Senior Credit Facilities or the Senior Notes shall be outstanding, no such acceleration shall be effective until the earlier of:
(1) acceleration of any such Indebtedness under the Senior Credit Facilities or Senior Notes, as the case may be; or
(2) five Business Days after the giving of written notice of such acceleration to the Issuer and the Representative with respect to the Senior Credit Facilities or Senior Notes, as the case may be.
Upon the effectiveness of such declaration, such principal of and premium, if any, and interest will be due and payable immediately. Notwithstanding the foregoing, in the case of an Event of Default arising under clause (6) of the first paragraph of this section, all outstanding senior subordinated notes will become due and payable without further action or notice. The senior subordinated notes indenture provides that the Trustee may withhold from the
217
Holders notice of any continuing Default, except a Default relating to the payment of principal, premium, if any, or interest, if it determines that withholding notice is in their interest. In addition, the Trustee has no obligation to accelerate the senior subordinated notes if in the best judgment of the Trustee acceleration is not in the best interests of the Holders of the exchange senior subordinated notes.
The senior subordinated notes indenture provides that the Holders of a majority in aggregate principal amount of the then outstanding senior subordinated notes by notice to the Trustee may on behalf of the Holders of all of the senior subordinated notes waive any existing Default and its consequences under the senior subordinated notes indenture (except a continuing Default in the payment of interest on, premium, if any, or the principal of any exchange senior subordinated note held by a non-consenting Holder) and rescind any acceleration with respect to the senior subordinated notes and its consequences (except if such rescission would conflict with any judgment of a court of competent jurisdiction). In the event of any Event of Default specified in clause (4) above, such Event of Default and all consequences thereof (excluding any resulting payment default, other than as a result of acceleration of the senior subordinated notes) shall be annulled, waived and rescinded, automatically and without any action by the Trustee or the Holders, if within 20 days after such Event of Default arose:
(1) the Indebtedness or guarantee that is the basis for such Event of Default has been discharged;
(2) holders thereof have rescinded or waived the acceleration, notice or action (as the case may be) giving rise to such Event of Default; or
(3) the default that is the basis for such Event of Default has been cured.
Notwithstanding the foregoing, the sole remedy for any breach of our obligation under the senior subordinated notes indenture to file periodic or other reports (including pursuant to section 314(a)(1) of the Trust Indenture Act) is the payment of liquidated damages, and the Holders do not have any right under the senior subordinated notes indenture to accelerate the maturity of the senior subordinated notes as a result of any such breach. If a breach of our obligation under the senior subordinated notes indenture to file periodic or other reports (including pursuant to section 314(a)(1) of the Trust Indenture Act) continues for 90 days after notice thereof is given in accordance with the senior subordinated notes indenture, we will pay liquidated damages to all the Holders of the senior subordinated notes at a rate per annum equal to (i) 0.25% per annum of the principal amount of the senior subordinated notes from the 90th day following such notice to but not including the 180th day following such notice (or such earlier date on which the Event of Default relating to the reporting obligations referred to in this paragraph shall have been cured or waived) and (ii) 0.50% per annum of the principal amount of the senior subordinated notes from the 180th day following such notice to but not including the 365th day following such notice (or such earlier date on which the Event of Default relating to the reporting obligations referred to in this paragraph shall have been cured or waived). On such 365th day (or earlier, if the Event of Default relating to the reporting obligations referred to in this paragraph shall have been cured or waived prior to such 365th day), such Additional Interest will cease to accrue, and the senior subordinated notes will be subject to acceleration as provided above if the Event of Default is continuing. The provisions of the senior subordinated notes indenture described in this paragraph will not affect the rights of the Holders of senior subordinated notes in the event of the occurrence of any other Event of Default.
Subject to the provisions of the senior subordinated notes indenture relating to the duties of the Trustee thereunder, in case an Event of Default occurs and is continuing, the Trustee will be under no obligation to exercise any of the rights or powers under the senior subordinated notes indenture at the request or direction of any of the Holders of the senior subordinated notes unless the Holders have offered to the Trustee indemnity or security satisfactory to it against any loss, liability or expense. Except to enforce the right to receive payment of principal, premium (if any) or interest when due, no Holder of an exchange senior subordinated note may pursue any remedy with respect to the senior subordinated notes indenture or the senior subordinated notes unless:
(1) such Holder has previously given the Trustee notice that an Event of Default is continuing;
(2) Holders of at least 30.0% in principal amount of the total outstanding senior subordinated notes have requested the Trustee to pursue the remedy;
(3) Holders of the senior subordinated notes have offered the Trustee security or indemnity satisfactory to it against any loss, liability or expense;
218
(4) the Trustee has not complied with such request within 60 days after the receipt thereof and the offer of security or indemnity; and
(5) Holders of a majority in principal amount of the total outstanding senior subordinated notes have not given the Trustee a direction inconsistent with such request within such 60-day period.
Subject to certain restrictions, under the senior subordinated notes indenture the Holders of a majority in principal amount of the total outstanding senior subordinated notes are given the right to direct the time, method and place of conducting any proceeding for any remedy available to the Trustee or of exercising any trust or power conferred on the Trustee. The Trustee, however, may refuse to follow any direction that conflicts with law or the senior subordinated notes indenture or that the Trustee determines is unduly prejudicial to the rights of any other Holder of an exchange senior subordinated note or that would involve the Trustee in personal liability.
The senior subordinated notes indenture provides that the Issuer is required to deliver to the Trustee annually a statement regarding compliance with the senior subordinated notes indenture, and the Issuer is required, within five Business Days, upon becoming aware of any Default, to deliver to the Trustee a statement specifying such Default.
No Personal Liability of Directors, Officers, Employees and Stockholders
No director, officer, employee, incorporator or stockholder of the Issuer or any Guarantor or any of their parent companies (other than the Issuer and the Guarantors) shall have any liability, for any obligations of the Issuer or the Guarantors under the senior subordinated notes, the Guarantees or the senior subordinated notes indenture or for any claim based on, in respect of, or by reason of such obligations or their creation. Each Holder by accepting senior subordinated notes waives and releases all such liability. The waiver and release are part of the consideration for issuance of the senior subordinated notes. Such waiver may not be effective to waive liabilities under the federal securities laws and it is the view of the SEC that such a waiver is against public policy.
Legal Defeasance and Covenant Defeasance
The obligations of the Issuer and the Guarantors under the senior subordinated notes indenture will terminate (other than certain obligations) and will be released upon payment in full of all of the senior subordinated notes. The Issuer may, at its option and at any time, elect to have all of its obligations discharged with respect to the senior subordinated notes and have each Guarantor’s obligation discharged with respect to its Guarantee (“Legal Defeasance”) and cure all then existing Events of Default except for:
(1) the rights of Holders of senior subordinated notes to receive payments in respect of the principal of, premium, if any, and interest on the senior subordinated notes when such payments are due solely out of the trust created pursuant to the senior subordinated notes indenture;
(2) the Issuer’s obligations with respect to senior subordinated notes concerning issuing temporary senior subordinated notes, registration of such senior subordinated notes, mutilated, destroyed, lost or stolen senior subordinated notes and the maintenance of an office or agency for payment and money for security payments held in trust;
(3) the rights, powers, trusts, duties and immunities of the Trustee, and the Issuer’s obligations in connection therewith; and
(4) the Legal Defeasance provisions of the senior subordinated notes indenture.
In addition, the Issuer may, at its option and at any time, elect to have its obligations and those of each Guarantor released with respect to certain covenants that are described in the senior subordinated notes indenture (“Covenant Defeasance”) and thereafter any omission to comply with such obligations shall not constitute a Default with respect to the senior subordinated notes. In the event Covenant Defeasance occurs, certain events (not including bankruptcy, receivership, rehabilitation and insolvency events pertaining to the Issuer) described under “Events of Default and Remedies” will no longer constitute an Event of Default with respect to the senior subordinated notes.
In order to exercise either Legal Defeasance or Covenant Defeasance with respect to the senior subordinated notes:
219
(1) the Issuer must irrevocably deposit with the Trustee, in trust, for the benefit of the Holders of the senior subordinated notes, cash in U.S. dollars, U.S. dollar-denominated Government Securities, or a combination thereof, in such amounts as will be sufficient, in the opinion of a nationally recognized firm of independent public accountants, to pay the principal of, premium, if any, and interest due on the senior subordinated notes on the stated maturity date or on the redemption date, as the case may be, of such principal, premium, if any, or interest on such senior subordinated notes and the Issuer must specify whether such senior subordinated notes are being defeased to maturity or to a particular redemption date;
(2) in the case of Legal Defeasance, the Issuer shall have delivered to the Trustee an Opinion of Counsel reasonably acceptable to the Trustee confirming that, subject to customary assumptions and exclusions,
(a) the Issuer has received from, or there has been published by, the United States Internal Revenue Service a ruling, or
(b) since the issuance of the senior subordinated notes, there has been a change in the applicable U.S. federal income tax law,
in either case to the effect that, and based thereon such Opinion of Counsel shall confirm that, subject to customary assumptions and exclusions, the Holders of the senior subordinated notes will not recognize income, gain or loss for U.S. federal income tax purposes, as applicable, as a result of such Legal Defeasance and will be subject to U.S. federal income tax on the same amounts, in the same manner and at the same times as would have been the case if such Legal Defeasance had not occurred;
(3) in the case of Covenant Defeasance, the Issuer shall have delivered to the Trustee an Opinion of Counsel reasonably acceptable to the Trustee confirming that, subject to customary assumptions and exclusions, the Holders of the senior subordinated notes will not recognize income, gain or loss for U.S. federal income tax purposes as a result of such Covenant Defeasance and will be subject to such tax on the same amounts, in the same manner and at the same times as would have been the case if such Covenant Defeasance had not occurred;
(4) no Default (other than that resulting from borrowing funds to be applied to make such deposit and any similar and simultaneous deposit relating to other Indebtedness and, in each case, the granting of Liens in connection therewith) shall have occurred and be continuing on the date of such deposit;
(5) such Legal Defeasance or Covenant Defeasance shall not result in a breach or violation of, or constitute a default under the Senior Credit Facilities, the Senior Notes, the indenture pursuant to which the Senior Notes were issued or any other material agreement or instrument (other than the senior subordinated notes indenture) to which, the Issuer or any Guarantor is a party or by which the Issuer or any Guarantor is bound (other than that resulting from any borrowing of funds to be applied to make the deposit required to effect such Legal Defeasance or Covenant Defeasance and any similar and simultaneous deposit relating to other Indebtedness, and the granting of Liens in connection therewith);
(6) the Issuer shall have delivered to the Trustee an Opinion of Counsel to the effect that, as of the date of such opinion and subject to customary assumptions and exclusions following the deposit, the trust funds will not be subject to the effect of Section 547 of Title 11 of the United States Code;
(7) the Issuer shall have delivered to the Trustee an Officer’s Certificate stating that the deposit was not made by the Issuer with the intent of defeating, hindering, delaying or defrauding any creditors of the Issuer or any Guarantor or others; and
(8) the Issuer shall have delivered to the Trustee an Officer’s Certificate and an Opinion of Counsel (which Opinion of Counsel may be subject to customary assumptions and exclusions) each stating that all conditions precedent provided for or relating to the Legal Defeasance or the Covenant Defeasance, as the case may be, have been complied with.
Satisfaction and Discharge
The senior subordinated notes indenture will be discharged and will cease to be of further effect as to all senior subordinated notes, when either:
220
(1) all senior subordinated notes theretofore authenticated and delivered, except lost, stolen or destroyed senior subordinated notes which have been replaced or paid and senior subordinated notes for whose payment money has theretofore been deposited in trust, have been delivered to the Trustee for cancellation; or
(2) (a) all senior subordinated notes not theretofore delivered to the Trustee for cancellation have become due and payable by reason of the making of a notice of redemption or otherwise, will become due and payable within one year or are to be called for redemption within one year under arrangements satisfactory to the Trustee for the giving of notice of redemption by the Trustee in the name, and at the expense, of the Issuer and the Issuer or any Guarantor have irrevocably deposited or caused to be deposited with the Trustee as trust funds in trust solely for the benefit of the Holders of the senior subordinated notes, cash in U.S. dollars, U.S. dollar-denominated Government Securities, or a combination thereof, in such amounts as will be sufficient without consideration of any reinvestment of interest to pay and discharge the entire indebtedness on the senior subordinated notes not theretofore delivered to the Trustee for cancellation for principal, premium, if any, and accrued interest to the date of maturity or redemption; provided, that upon any redemption that requires the payment of the Applicable Premium, the amount deposited shall be sufficient for purposes of the senior subordinated notes indenture to the extent that an amount is deposited with the Trustee equal to the Applicable Premium calculated as of the date of the notice of redemption, with any deficit as of the date of redemption (any such amount, the “Applicable Premium Deficit”) only required to be deposited with the Trustee on or prior to the date of redemption. Any Applicable Premium Deficit shall be set forth in an Officer’s Certificate delivered to the Trustee simultaneously with the deposit of such Applicable Premium Deficit that confirms that such Applicable Premium Deficit shall be applied toward such redemption;
(b) no Default (other than that resulting from borrowing funds to be applied to make such deposit or any similar and simultaneous deposit relating to other Indebtedness and the granting of Liens in connection therewith) with respect to the senior subordinated notes indenture or the senior subordinated notes shall have occurred and be continuing on the date of such deposit or shall occur as a result of such deposit and such deposit will not result in a breach or violation of, or constitute a default under the Senior Credit Facilities, the Senior Notes, the indenture pursuant to which the Senior Notes were issued or any other material agreement or instrument (other than the senior subordinated notes indenture) to which the Issuer or any Guarantor is a party or by which the Issuer or any Guarantor is bound (other than resulting from any borrowing of funds to be applied to make such deposit and any similar and simultaneous deposit relating to other Indebtedness and the granting of Liens in connection therewith);
(c) the Issuer has paid or caused to be paid all sums payable by it under the senior subordinated notes indenture; and
(d) the Issuer has delivered irrevocable instructions to the Trustee to apply the deposited money toward the payment of the senior subordinated notes at maturity or the redemption date, as the case may be.
In addition, the Issuer must deliver an Officer’s Certificate and an Opinion of Counsel to the Trustee stating that all conditions precedent to satisfaction and discharge have been satisfied.
Amendment, Supplement and Waiver
Except as provided in the next two succeeding paragraphs, the senior subordinated notes indenture, any Guarantee and the senior subordinated notes may be amended or supplemented with the consent of the Holders of at least a majority in principal amount of the senior subordinated notes then outstanding, including consents obtained in connection with a purchase of, or tender offer or exchange offer for, senior subordinated notes, and any existing Default or compliance with any provision of the senior subordinated notes indenture or the senior subordinated notes issued thereunder may be waived with the consent of the Holders of a majority in principal amount of the then outstanding senior subordinated notes, other than senior subordinated notes beneficially owned by the Issuer or its Affiliates (including consents obtained in connection with a purchase of or tender offer or exchange offer for the senior subordinated notes).
The senior subordinated notes indenture provides that, without the consent of each affected Holder of senior subordinated notes, an amendment or waiver may not, with respect to any senior subordinated notes held by a non-consenting Holder:
(1) reduce the principal amount of such senior subordinated notes whose Holders must consent to an amendment, supplement or waiver;
221
(2) reduce the principal of or change the fixed final maturity of any such senior subordinated note or alter or waive the provisions with respect to the redemption of such senior subordinated notes (other than provisions relating to the covenants described above under “Repurchase at the Option of Holders”);
(3) reduce the rate of or change the time for payment of interest on any senior subordinated note;
(4) waive a Default in the payment of principal of or premium, if any, or interest on the senior subordinated notes, except a rescission of acceleration of the senior subordinated notes by the Holders of at least a majority in aggregate principal amount of the senior subordinated notes and a waiver of the payment default that resulted from such acceleration, or in respect of a covenant or provision contained in the senior subordinated notes indenture or any Guarantee which cannot be amended or modified without the consent of all Holders;
(5) make any senior subordinated note payable in money other than that stated therein;
(6) make any change to the provisions of the senior subordinated notes indenture relating to waivers of past Defaults or the rights of Holders to receive payments of principal of or premium, if any, or interest on the senior subordinated notes;
(7) make any change in these amendment and waiver provisions;
(8) impair the right of any Holder to receive payment of principal of, or premium, if any, or interest on such Holder’s senior subordinated notes on or after the due dates therefor or to institute suit for the enforcement of any payment on or with respect to such Holder’s senior subordinated notes;
(9) make any change in the subordination provisions thereof that would adversely affect the Holders; or
(10) except as expressly permitted by the senior subordinated notes indenture, modify the Guarantees of any Significant Subsidiary in any manner adverse to the Holders of the senior subordinated notes.
Notwithstanding the foregoing, the Issuer, any Guarantor (with respect to a Guarantee or the senior subordinated notes indenture to which it is a party) and the Trustee may amend or supplement the senior subordinated notes indenture and any Guarantee or senior subordinated notes without the consent of any Holder:
(1) to cure any ambiguity, omission, mistake, defect or inconsistency;
(2) to provide for uncertificated senior subordinated notes of such series in addition to or in place of certificated senior subordinated notes;
(3) to comply with the covenant relating to mergers, consolidations and sales of assets;
(4) to provide the assumption of the Issuer’s or any Guarantor’s obligations to the Holders;
(5) to make any change that would provide any additional rights or benefits to the Holders or that does not adversely affect the legal rights under the senior subordinated notes indenture of any such Holder;
(6) to add covenants for the benefit of the Holders or to surrender any right or power conferred upon the Issuer or any Guarantor;
(7) to comply with requirements of the SEC in order to effect or maintain the qualification of the senior subordinated notes indenture under the Trust Indenture Act;
(8) to evidence and provide for the acceptance and appointment under the senior subordinated notes indenture of a successor Trustee thereunder pursuant to the requirements thereof;
(9) to provide for the issuance of exchange notes or private exchange notes, which are identical to exchange notes except that they are not freely transferable;
(10) to add a guarantor under the senior subordinated notes indenture;
222
(11) to conform the text of the senior subordinated notes indenture, Guarantees or the senior subordinated notes to any provision of the “Description of Notes” section of the offering circular relating to the senior subordinated notes dated September 18, 2012 to the extent that such provision in such “Description of Notes” section was intended to be a verbatim recitation of a provision of the senior subordinated notes indenture, Guarantee or senior subordinated notes; or
(12) to make any amendment to the provisions of the senior subordinated notes indenture relating to the transfer and legending of senior subordinated notes as permitted by the senior subordinated notes indenture, including, without limitation to facilitate the issuance and administration of the senior subordinated notes; provided that (a) compliance with the senior subordinated notes indenture as so amended would not result in senior subordinated notes being transferred in violation of the Securities Act or any applicable securities law and (b) such amendment does not materially and adversely affect the rights of Holders to transfer senior subordinated notes.
The consent of the Holders is not necessary under the senior subordinated notes indenture to approve the particular form of any proposed amendment. It is sufficient if such consent approves the substance of the proposed amendment.
Notices
Except for notices with respect to the Trustee, notices given by publication or electronic delivery will be deemed given on the first date on which publication is made and notices given by first-class mail, postage prepaid, will be deemed given five calendar days after mailing.
Concerning the Trustee
The senior subordinated notes indenture contains certain limitations on the rights of the Trustee thereunder, should it become a creditor of the Issuer, to obtain payment of claims in certain cases, or to realize on certain property received in respect of any such claim as security or otherwise. The Trustee is permitted to engage in other transactions; however, if it acquires any conflicting interest it must eliminate such conflict within 90 days, apply to the SEC for permission to continue or resign.
The senior subordinated notes indenture provides that the Holders of a majority in principal amount of the outstanding senior subordinated notes will have the right to direct the time, method and place of conducting any proceeding for exercising any remedy available to the Trustee, subject to certain exceptions. The senior subordinated notes indenture provides that in case an Event of Default shall occur (which shall not be cured), the Trustee is required, in the exercise of its power, to use the degree of care of a prudent person in the conduct of his own affairs. Subject to such provisions, the Trustee is under no obligation to exercise any of its rights or powers under the senior subordinated notes indenture at the request of any Holder of the senior subordinated notes, unless such Holder shall have offered to the Trustee security and indemnity satisfactory to it against any loss, liability or expense.
Governing Law
The senior subordinated notes indenture, the senior subordinated notes and any Guarantee are governed by and construed in accordance with the laws of the State of New York.
Certain Definitions
Set forth below are certain defined terms used in the senior subordinated notes indenture. For purposes of the senior subordinated notes indenture, unless otherwise specifically indicated, the term “consolidated” with respect to any Person refers to such Person consolidated with its Restricted Subsidiaries, and excludes from such consolidation any Unrestricted Subsidiary as if such Unrestricted Subsidiary were not an Affiliate of such Person. These terms may have different meanings than similar or identical terms used in the senior notes indenture.
“2007 Acquisition” means the transactions pursuant to the Merger Agreement.
“ABL Facilities” means the asset-based revolving credit facilities under the Credit Agreement dated as of September 25, 2007 by and among the Issuer, the lenders party thereto in their capacities as lenders thereunder and Bank of America, N.A., as Administrative Agent, including any guarantees, collateral documents, instruments and agreements executed in connection therewith, and any amendments, supplements, modifications, extensions, renewals, restatements, refundings or refinancings thereof and any indentures or credit facilities or commercial
223
paper facilities with banks or other institutional lenders or investors that replace, refund or refinance any part of the loans, notes, other credit facilities or commitments thereunder, including any such replacement, refunding or refinancing facility or indenture that increases the amount borrowable thereunder or alters the maturity thereof (provided that such increase in borrowings is permitted under “—Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock” above).
“ABL Financing Entity” means the Issuer and certain of its Subsidiaries from time to time named as borrowers or guarantors under the ABL Facilities.
“Acquired Indebtedness” means, with respect to any specified Person,
(1) Indebtedness of any other Person existing at the time such other Person is merged with or into or became a Restricted Subsidiary of such specified Person, including Indebtedness incurred in connection with, or in contemplation of, such other Person merging with or into or becoming a Restricted Subsidiary of such specified Person, and
(2) Indebtedness secured by a Lien encumbering any asset acquired by such specified Person.
“Additional Interest” means all additional interest then owing pursuant to the Senior Subordinated Notes Registration Rights Agreement, and as described under “Events of Default and Remedies”.
“Affiliate” of any specified Person means any other Person directly or indirectly controlling or controlled by or under direct or indirect common control with such specified Person. For purposes of this definition, “control” (including, with correlative meanings, the terms “controlling,” “controlled by” and “under common control with”), as used with respect to any Person, shall mean the possession, directly or indirectly, of the power to direct or cause the direction of the management or policies of such Person, whether through the ownership of voting securities, by agreement or otherwise.
“Applicable Premium” means, with respect to any senior subordinated note on any Redemption Date, the greater of:
(1) 1.0% of the principal amount of such senior subordinated note; and
(2) the excess, if any, of (a) the present value at such Redemption Date of (i) the redemption price of such senior subordinated note at October 1, 2015 (such redemption price being set forth in the table appearing above under “Optional Redemption”), plus (ii) all required remaining scheduled interest payments due on such senior subordinated note through October 1, 2015 (excluding accrued but unpaid interest to the Redemption Date), computed using a discount rate equal to the Treasury Rate as of such Redemption Date plus 50 basis points; over (b) the principal amount of such senior subordinated note.
“Asset Sale” means:
(1) the sale, conveyance, transfer or other disposition, whether in a single transaction or a series of related transactions (including by way of a Sale and Lease-Back Transaction) of property or assets of the Issuer or any of its Restricted Subsidiaries (each referred to in this definition as a “disposition”); or
(2) the issuance or sale of Equity Interests of any Restricted Subsidiary (other than Preferred Stock of Restricted Subsidiaries issued in compliance with the covenant described under “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”), whether in a single transaction or a series of related transactions;
in each case, other than:
(a) any disposition of Cash Equivalents or Investment Grade Securities or obsolete or worn out equipment in the ordinary course of business or any disposition of inventory or goods (or other assets) held for sale or no longer used in the ordinary course of business;
(b) the disposition of all or substantially all of the assets of the Issuer in a manner permitted pursuant to the provisions described above under “—Certain Covenants—Merger, Consolidation or Sale of All or
224
Substantially All Assets” or any disposition that constitutes a Change of Control pursuant to the senior subordinated notes indenture;
(c) the making of any Restricted Payment or Permitted Investment that is permitted to be made, and is made, under the covenant described above under “—Certain Covenants—Limitation on Restricted Payments”;
(d) any disposition of assets or issuance or sale of Equity Interests of any Restricted Subsidiary in any transaction or series of related transactions with an aggregate fair market value of less than $50.0 million;
(e) any disposition of property or assets or the issuance of securities by a Restricted Subsidiary to the Issuer or by the Issuer or a Restricted Subsidiary to a Restricted Subsidiary;
(f) to the extent allowable under Section 1031 of the Internal Revenue Code of 1986, any exchange of like property (excluding any boot thereon) for use in a Similar Business;
(g) the lease, assignment or sub-lease of any real or personal property in the ordinary course of business;
(h) any issuance or sale of Equity Interests in, or Indebtedness or other securities of, an Unrestricted Subsidiary;
(i) foreclosures, condemnation or any similar action on assets or the granting of Liens not prohibited by the senior subordinated notes indenture;
(j) sales of accounts receivable, or participations therein, or Securitization Assets or related assets in connection with the ABL Facilities or any Qualified Securitization Facility;
(k) any financing transaction with respect to property built or acquired by the Issuer or any Restricted Subsidiary after the Issue Date, including Sale and Lease-Back Transactions and asset securitizations permitted by the senior subordinated notes indenture;
(l) the sale or discount of inventory, accounts receivable or notes receivable in the ordinary course of business or the conversion of accounts receivable to notes receivable;
(m) the licensing or sub-licensing of intellectual property or other general intangibles in the ordinary course of business, other than the licensing of intellectual property on a long-term basis;
(n) any surrender or waiver of contract rights or the settlement, release or surrender of contract rights or other litigation claims in the ordinary course of business;
(o) the unwinding of any Hedging Obligations;
(p) sales, transfers and other dispositions of Investments in joint ventures to the extent required by, or made pursuant to, customary buy/sell arrangements between the joint venture parties set forth in joint venture arrangements and similar binding arrangements; and
(q) the abandonment of intellectual property rights in the ordinary course of business, which in the reasonable good faith determination of the Issuer are not material to the conduct of the business of the Issuer and its Restricted Subsidiaries taken as a whole.
“Business Day” means each day which is not a Legal Holiday.
“Capital Stock” means:
(1) in the case of a corporation, corporate stock;
(2) in the case of an association or business entity, any and all shares, interests, participations, rights or other equivalents (however designated) of corporate stock;
225
(3) in the case of a partnership or limited liability company, partnership or membership interests (whether general or limited); and
(4) any other interest or participation that confers on a Person the right to receive a share of the profits and losses of, or distributions of assets of, the issuing Person but excluding from all of the foregoing any debt securities convertible into Capital Stock, whether or not such debt securities include any right of participation with Capital Stock.
“Capitalized Lease Obligation” means, at the time any determination thereof is to be made, the amount of the liability in respect of a capital lease that would at such time be required to be capitalized and reflected as a liability on a balance sheet (excluding the footnotes thereto) prepared in accordance with GAAP.
“Capitalized Software Expenditures” means, for any period, the aggregate of all expenditures (whether paid in cash or accrued as liabilities) by a Person and its Restricted Subsidiaries during such period in respect of licensed or purchased software or internally developed software and software enhancements that, in conformity with GAAP, are or are required to be reflected as capitalized costs on the consolidated balance sheet of a Person and its Restricted Subsidiaries.
“Cash Equivalents” means:
(1) United States dollars;
(2) (a) Canadian dollars, yen, pounds sterling, euros or any national currency of any participating member state of the EMU; or
(b) in the case of any Foreign Subsidiary that is a Restricted Subsidiary, such local currencies held by it from time to time in the ordinary course of business;
(3) securities issued or directly and fully and unconditionally guaranteed or insured by the U.S. government or any agency or instrumentality thereof the securities of which are unconditionally guaranteed as a full faith and credit obligation of such government with maturities of 24 months or less from the date of acquisition;
(4) certificates of deposit, time deposits and eurodollar time deposits with maturities of 24 months or less from the date of acquisition, bankers’ acceptances with maturities not exceeding one year and overnight bank deposits, in each case with any domestic or foreign commercial bank having capital and surplus of not less than $500.0 million in the case of U.S. banks and $100.0 million (or the U.S. dollar equivalent as of the date of determination) in the case of non-U.S. banks;
(5) repurchase obligations for underlying securities of the types described in clauses (3), (4) and (8) entered into with any financial institution meeting the qualifications specified in clause (4) above;
(6) commercial paper rated at least P-2 by Moody’s or at least A-2 by S&P (or, if at any time neither Moody’s nor S&P shall be rating such obligations, an equivalent rating from another Rating Agency) and in each case maturing within 24 months after the date of creation thereof and Indebtedness or Preferred Stock issued by Persons with a rating of “A” or higher from S&P or “A-2” or higher from Moody’s with maturities of 24 months or less from the date of acquisition;
(7) marketable short-term money market and similar funds having a rating of at least P-2 or A-2 from either Moody’s or S&P, respectively (or, if at any time neither Moody’s nor S&P shall be rating such obligations, an equivalent rating from another Rating Agency);
(8) readily marketable direct obligations issued by any state, commonwealth or territory of the United States or any political subdivision or taxing authority thereof having an Investment Grade Rating from either Moody’s or S&P (or, if at any time neither Moody’s nor S&P shall be rating such obligations, an equivalent rating from another Rating Agency) with maturities of 24 months or less from the date of acquisition;
(9) readily marketable direct obligations issued by any foreign government or any political subdivision or public instrumentality thereof, in each case having an Investment Grade Rating from either Moody’s or S&P (or, if at any time neither Moody’s nor S&P shall be rating such obligations, an equivalent rating from another Rating Agency) with maturities of 24 months or less from the date of acquisition;
226
(10) Investments with average maturities of 12 months or less from the date of acquisition in money market funds rated AAA- (or the equivalent thereof) or better by S&P or Aaa3 (or the equivalent thereof) or better by Moody’s (or, if at any time neither Moody’s nor S&P shall be rating such obligations, an equivalent rating from another Rating Agency); and
(11) investment funds investing at least 90.0% of their assets in securities of the types described in clauses (1) through (10) above.
In the case of Investments by any Foreign Subsidiary that is a Restricted Subsidiary or Investments made in a country outside the United States of America, Cash Equivalents shall also include (a) investments of the type and maturity described in clauses (1) through (8) and clauses (10) and (11) above of foreign obligors, which Investments or obligors (or the parents of such obligors) have ratings described in such clauses or equivalent ratings from comparable foreign rating agencies and (b) other short-term investments utilized by Foreign Subsidiaries that are Restricted Subsidiaries in accordance with normal investment practices for cash management in investments analogous to the foregoing investments in clauses (1) through (11) and in this paragraph.
Notwithstanding the foregoing, Cash Equivalents shall include amounts denominated in currencies other than those set forth in clauses (1) and (2) above, provided that such amounts are converted into any currency listed in clauses (1) and (2) as promptly as practicable and in any event within ten Business Days following the receipt of such amounts.
At any time at which the value, calculated in accordance with GAAP, of all investments of the Issuer and its Restricted Subsidiaries that were deemed, when made, to be Cash Equivalents in accordance with clauses (1) through (11) above exceeds the Indebtedness of the Issuer and its Restricted Subsidiaries, “Cash Equivalents” shall also mean any investment (a “Qualifying Investment”) that satisfies the following two conditions: (a) the Qualifying Investment is of a type described in clauses (1) through (11) of this definition, but has an effective maturity (whether by reason of final maturity, a put option or, in the case of an asset-backed security, an average life) of five years and one month or less from the date of such Qualifying Investment (notwithstanding any provision contained in such clauses (1) through (11) requiring a shorter maturity); and (b) the weighted average effective maturity of such Qualifying Investment and all other investments that were made as Qualifying Investments in accordance with this paragraph, does not exceed two years from the date of such Qualifying Investment.
“CF Credit Facilities” means the term and revolving credit facilities under the Credit Agreement dated as of September 25, 2007 by and among the Issuer, the European subsidiary borrowers party thereto, the lenders party thereto in their capacities as lenders thereunder and Bank of America, N.A., as Administrative Agent, including any guarantees, collateral documents, instruments and agreements executed in connection therewith, and any amendments, supplements, modifications, extensions, renewals, restatements, refundings or refinancings thereof and any indentures or credit facilities or commercial paper facilities with banks or other institutional lenders or investors that replace, refund or refinance any part of the loans, notes, other credit facilities or commitments thereunder, including any such replacement, refunding or refinancing facility or indenture that increases the amount borrowable thereunder or alters the maturity thereof (provided that such increase in borrowings is permitted under “—Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock” above).
“Change of Control” means the occurrence of any of the following:
(1) the sale, lease or transfer, in one or a series of related transactions, of all or substantially all of the assets of the Issuer and its Subsidiaries, taken as a whole, to any Person other than a Permitted Holder; or
(2) the Issuer becomes aware of (by way of a report or any other filing pursuant to Section 13(d) of the Exchange Act, proxy, vote, written notice or otherwise) the acquisition by any Person or group (within the meaning of Section 13(d)(3) or Section 14(d)(2) of the Exchange Act, or any successor provision), including any group acting for the purpose of acquiring, holding or disposing of securities (within the meaning of Rule 13d-5(b)(1) under the Exchange Act), other than one or more Permitted Holders, in a single transaction or in a related series of transactions, by way of merger, consolidation or other business combination or purchase of beneficial ownership (within the meaning of Rule 13d-3 under the Exchange Act, or any successor provision) of 50.0% or more of the total voting power of the Voting Stock of the Issuer or any of its direct or indirect parent companies.
“Consolidated Depreciation and Amortization Expense” means with respect to any Person for any period, the total amount of depreciation and amortization expense of such Person, including the amortization of deferred
227
financing fees, debt issuance costs, commissions, fees and expenses and Capitalized Software Expenditures of such Person and its Restricted Subsidiaries for such period on a consolidated basis and otherwise determined in accordance with GAAP.
“Consolidated Interest Expense” means, with respect to any Person for any period, without duplication, the sum of:
(1) consolidated interest expense of such Person and its Restricted Subsidiaries for such period, to the extent such expense was deducted (and not added back) in computing Consolidated Net Income (including (a) amortization of original issue discount resulting from the issuance of Indebtedness at less than par, (b) all commissions, discounts and other fees and charges owed with respect to letters of credit or bankers acceptances, (c) non-cash interest payments (but excluding any non-cash interest expense attributable to the movement in the mark to market valuation of Hedging Obligations or other derivative instruments pursuant to GAAP), (d) the interest component of Capitalized Lease Obligations, and (e) net payments, if any, made (less net payments, if any, received), pursuant to interest rate Hedging Obligations with respect to Indebtedness, and excluding (t) any expense resulting from the discounting of any Indebtedness in connection with the application of recapitalization accounting or, if applicable, purchase accounting in connection with the 2007 Acquisition or any other acquisition, (u) penalties and interest relating to taxes, (v) any Additional Interest and any “additional interest” with respect to the Senior Notes or other securities, (w) amortization of deferred financing fees, debt issuance costs, commissions, fees and expenses, (x) any expensing of bridge, commitment and other financing fees, (y) commissions, discounts, yield and other fees and charges (including any interest expense) related to any Qualified Securitization Facility and (z) any accretion of accrued interest on discounted liabilities); plus
(2) consolidated capitalized interest of such Person and its Restricted Subsidiaries for such period, whether paid or accrued; less
(3) interest income of such Person and its Restricted Subsidiaries for such period.
For purposes of this definition, interest on a Capitalized Lease Obligation shall be deemed to accrue at an interest rate reasonably determined by such Person to be the rate of interest implicit in such Capitalized Lease Obligation in accordance with GAAP.
“Consolidated Net Income” means, with respect to any Person for any period, the aggregate of the Net Income of such Person and its Restricted Subsidiaries for such period, on a consolidated basis, and otherwise determined in accordance with GAAP; provided that, without duplication,
(1) any after-tax effect of extraordinary, non-recurring or unusual gains or losses (less all fees and expenses relating thereto) or expenses (including relating to the 2007 Acquisition or any multi-year strategic initiatives, severance, relocation costs and curtailments or modifications to pension and post-retirement employee benefit plans) shall be excluded;
(2) the cumulative effect of a change in accounting principles and changes as a result of the adoption or modification of accounting policies during such period shall be excluded;
(3) any net after-tax gains or losses on disposal of disposed, abandoned or discontinued operations shall be excluded;
(4) any net after-tax effect of gains or losses (less all fees, expenses and charges relating thereto) attributable to asset dispositions or abandonments or the sale or other disposition of any Capital Stock of any Person other than in the ordinary course of business shall be excluded;
(5) the Net Income for such period of any Person that is an Unrestricted Subsidiary shall be excluded, and, solely for the purpose of determining the amount available for Restricted Payments under clause (3)(a) of the first paragraph of “—Certain Covenants—Limitation on Restricted Payments,” the Net Income for such period of any Person that is not a Subsidiary or that is accounted for by the equity method of accounting shall be excluded; provided that Consolidated Net Income of the Issuer shall be increased by the amount of dividends or distributions or other payments that are actually paid in cash (or to the extent converted into cash) to the Issuer or a Restricted Subsidiary thereof in respect of such period;
228
(6) solely for the purpose of determining the amount available for Restricted Payments under clause (3)(a) of the first paragraph of “—Certain Covenants—Limitation on Restricted Payments,” the Net Income for such period of any Restricted Subsidiary (other than any Guarantor) shall be excluded to the extent that the declaration or payment of dividends or similar distributions by that Restricted Subsidiary of its Net Income is not at the date of determination permitted without any prior governmental approval (which has not been obtained) or, directly or indirectly, by the operation of the terms of its charter or any agreement, instrument, judgment, decree, order, statute, rule, or governmental regulation applicable to that Restricted Subsidiary or its stockholders, unless such restriction with respect to the payment of dividends or similar distributions has been legally waived, provided that Consolidated Net Income of the Issuer will be increased by the amount of dividends or other distributions or other payments actually paid in cash (or to the extent converted into cash) to the Issuer or a Restricted Subsidiary thereof in respect of such period, to the extent not already included therein;
(7) effects of adjustments (including the effects of such adjustments pushed down to the Issuer and its Restricted Subsidiaries) in such Person’s consolidated financial statements pursuant to GAAP resulting from the application of recapitalization accounting or, if applicable, purchase accounting in relation to the Transactions or any consummated acquisition or the amortization or write-off of any amounts thereof, net of taxes, shall be excluded;
(8) any after-tax effect of income (loss) from the early extinguishment of (a) Indebtedness, (b) Hedging Obligations or (c) other derivative instruments shall be excluded;
(9) any impairment charge or asset write-off or write-down, including impairment charges or asset write-offs or write-downs related to intangible assets, long-lived assets, investments in debt and equity securities or as a result of a change in law or regulation, in each case, pursuant to GAAP, and the amortization of intangibles arising pursuant to GAAP shall be excluded;
(10) any non-cash compensation expense recorded from grants of stock appreciation or similar rights, stock options, restricted stock or other rights, and any cash charges associated with the rollover, acceleration, or payout of Equity Interests by management of the Issuer or any of its direct or indirect parent companies in connection with the Transactions, shall be excluded;
(11) any fees, expenses or charges incurred during such period, or any amortization thereof for such period, in connection with any acquisition, Investment, Asset Sale, incurrence or repayment of Indebtedness (including such fees, expenses or charges related to the offering of the Senior Notes, the senior subordinated notes and the other Transactions), issuance of Equity Interests, refinancing transaction or amendment or modification of any debt instrument (including any amendment or other modification of the Senior Notes, the senior subordinated notes and the Credit Facilities) and including, in each case, any such transaction consummated prior to the Issue Date and any such transaction undertaken but not completed, and any charges or non-recurring merger costs incurred during such period as a result of any such transaction, in each case whether or not successful, shall be excluded;
(12) accruals and reserves that are established within twelve months after the closing of any acquisition (including the 2007 Acquisition) that are so required to be established as a result of such acquisition in accordance with GAAP shall be excluded;
(13) to the extent covered by insurance and actually reimbursed, or, so long as the Issuer has made a determination that there exists reasonable evidence that such amount will in fact be reimbursed by the insurer and only to the extent that such amount is (a) not denied by the applicable carrier in writing 180 days and (b) in fact reimbursed within 365 days of the date of the insurable event (with a deduction for any amount so added back to the extent not so reimbursed within such 365-day period), expenses with respect to liability or casualty events or business interruption shall be excluded;
(14) any non-cash compensation expense resulting from the application of Financial Accounting Standards Board Accounting Standards Codification 718 and 505-50, as applicable, shall be excluded; and
(15) the following items shall be excluded:
(a) any net unrealized gain or loss (after any offset) resulting in such period from Hedging Obligations and the application of Financial Accounting Standards Board Accounting Standards Codification 815; and
229
(b) any net unrealized gain or loss (after any offset) resulting in such period from currency translation gains or losses including those related to currency remeasurements of Indebtedness (including any net loss or gain resulting from Hedging Obligations for currency exchange risk).
In addition, to the extent not already included in the Consolidated Net Income of such Person and its Restricted Subsidiaries, notwithstanding anything to the contrary in the foregoing, Consolidated Net Income shall include the amount of proceeds received from business interruption insurance and reimbursements of any expenses and charges that are covered by indemnification or other reimbursement provisions in connection with any Permitted Investment or any sale, conveyance, transfer or other disposition of assets permitted under the senior subordinated notes indenture.
Notwithstanding the foregoing, for the purpose of the covenant described under “—Certain Covenants—Limitation on Restricted Payments” only (other than clause (3)(d) of the first paragraph thereof), there shall be excluded from Consolidated Net Income any income arising from any sale or other disposition of Restricted Investments made by the Issuer and its Restricted Subsidiaries, any repurchases and redemptions of Restricted Investments from the Issuer and its Restricted Subsidiaries, any repayments of loans and advances which constitute Restricted Investments by the Issuer or any of its Restricted Subsidiaries, any sale of the stock of an Unrestricted Subsidiary or any distribution or dividend from an Unrestricted Subsidiary, in each case only to the extent such amounts increase the amount of Restricted Payments permitted under such covenant pursuant to clause (3)(d) thereof.
“Contingent Obligations” means, with respect to any Person, any obligation of such Person guaranteeing any leases, dividends or other obligations that do not constitute Indebtedness (“primary obligations”) of any other Person (the “primary obligor”) in any manner, whether directly or indirectly, including, without limitation, any obligation of such Person, whether or not contingent,
(1) to purchase any such primary obligation or any property constituting direct or indirect security therefor;
(2) to advance or supply funds
(a) for the purchase or payment of any such primary obligation, or
(b) to maintain working capital or equity capital of the primary obligor or otherwise to maintain the net worth or solvency of the primary obligor; or
(3) to purchase property, securities or services primarily for the purpose of assuring the owner of any such primary obligation of the ability of the primary obligor to make payment of such primary obligation against loss in respect thereof.
“Controlled Investment Affiliate” means, as to any Person, any other Person, other than any Investor, which directly or indirectly is in control of, is controlled by, or is under common control with such Person and is organized by such Person (or any Person controlling such Person) primarily for making direct or indirect equity or debt investments in the Issuer and/or other companies.
“Credit Facilities” means, with respect to the Issuer or any of its Restricted Subsidiaries, one or more debt facilities, including the Senior Credit Facilities or other financing arrangements (including, without limitation, commercial paper facilities or indentures) providing for revolving credit loans, term loans, letters of credit or other long-term indebtedness, including any notes, mortgages, guarantees, collateral documents, instruments and agreements executed in connection therewith, and any amendments, supplements, modifications, extensions, renewals, restatements or refundings thereof and any indentures or credit facilities or commercial paper facilities that replace, refund or refinance any part of the loans, notes, other credit facilities or commitments thereunder, including any such replacement, refunding or refinancing facility or indenture that increases the amount permitted to be borrowed thereunder or alters the maturity thereof (provided that such increase in borrowings is permitted under “—Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”) or adds Restricted Subsidiaries as additional borrowers or guarantors thereunder and whether by the same or any other agent, lender or group of lenders.
“Default” means any event that is, or with the passage of time or the giving of notice or both would be, an Event of Default.
230
“Designated Non-cash Consideration” means the fair market value of non-cash consideration received by the Issuer or a Restricted Subsidiary in connection with an Asset Sale that is so designated as Designated Non-cash Consideration pursuant to an Officer’s Certificate, setting forth the basis of such valuation, executed by the principal financial officer of the Issuer, less the amount of Cash Equivalents received in connection with a subsequent sale of or collection on such Designated Non-cash Consideration.
“Designated Preferred Stock” means Preferred Stock of the Issuer or any parent company thereof (in each case other than Disqualified Stock) that is issued for cash (other than to a Restricted Subsidiary or an employee stock ownership plan or trust established by the Issuer or any of its Subsidiaries) and is so designated as Designated Preferred Stock, pursuant to an Officer’s Certificate executed by the principal financial officer of the Issuer or the applicable parent company thereof, as the case may be, on the issuance date thereof, the cash proceeds of which are excluded from the calculation set forth in clause (3) of the first paragraph of “—Certain Covenants—Limitation on Restricted Payments.”
“Designated Senior Indebtedness” means:
(1) any Indebtedness outstanding under the Senior Credit Facilities;
(2) the Senior Notes; and
(3) any other Senior Indebtedness permitted under the senior subordinated notes indenture, the principal amount of which is $50.0 million or more and that has been designated by the Issuer as “Designated Senior Indebtedness,” pursuant to an Officer’s Certificate executed by the principal financial officer of the Issuer, which is delivered to the Trustee.
“Disqualified Stock” means, with respect to any Person, any Capital Stock of such Person which, by its terms, or by the terms of any security into which it is convertible or for which it is putable or exchangeable, or upon the happening of any event, matures or is mandatorily redeemable (other than solely as a result of a change of control or asset sale) pursuant to a sinking fund obligation or otherwise, or is redeemable at the option of the holder thereof (other than solely as a result of a change of control or asset sale), in whole or in part, in each case prior to the date 91 days after the earlier of the maturity date of the senior subordinated notes or the date the senior subordinated notes are no longer outstanding; provided that if such Capital Stock is issued to any plan for the benefit of employees of the Issuer or its Subsidiaries or by any such plan to such employees, such Capital Stock shall not constitute Disqualified Stock solely because it may be required to be repurchased by the Issuer or its Subsidiaries in order to satisfy applicable statutory or regulatory obligations; provided, further, that any Capital Stock held by any future, current or former employee, director, officer, manager, distributor or consultant (or their respective Controlled Investment Affiliates or Immediate Family Members), of the Issuer, any of its Subsidiaries, any of its direct or indirect parent companies or any other entity in which the Issuer or a Restricted Subsidiary has an Investment and is designated in good faith as an “affiliate” by the board of directors of the Issuer (or the compensation committee thereof), in each case pursuant to any stock subscription or shareholders’ agreement, management equity plan or stock option plan or any other management or employee benefit plan or agreement or any distributor equity plan or agreement shall not constitute Disqualified Stock solely because it may be required to be repurchased by the Issuer or its Subsidiaries.
“EBITDA” means, with respect to any Person for any period, the Consolidated Net Income of such Person for such period
(1) increased (without duplication) by the following, in each case to the extent deducted (and not added back) in determining Consolidated Net Income for such period:
(a) provision for taxes based on income or profits or capital, including, without limitation, state, franchise and similar taxes, foreign withholding taxes (including any future taxes or other levies which replace or are intended to be in lieu of such taxes and any penalties and interest related to such taxes or arising from tax examinations) and the net tax expense associated with any adjustments made pursuant to clauses (1) through (15) of the definition of “Consolidated Net Income”; plus
(b) Fixed Charges of such Person for such period (including (x) net losses or Hedging Obligations or other derivative instruments entered into for the purpose of hedging interest rate risk, (y) bank fees and (z) costs of surety bonds in connection with financing activities, plus amounts excluded from Consolidated Interest Expense as set forth in clauses (1)(t) through (z) in the definition thereof); plus
231
(c) Consolidated Depreciation and Amortization Expense of such Person for such period; plus
(d) [reserved]; plus
(e) the amount of any restructuring charges, integration and facilities opening costs or other business optimization expenses (including cost and expenses relating to business optimization programs and new systems design and implementation costs) or accruals or reserves, including any one-time costs incurred in connection with acquisitions after the Issue Date, project start-up costs and costs related to the closure and/or consolidation of facilities; plus
(f) any other non-cash charges, including any write offs or write downs reducing Consolidated Net Income for such period (provided that if any such non-cash charges represent an accrual or reserve for potential cash items in any future period, the cash payment in respect thereof in such future period shall be subtracted from EBITDA to such extent, and excluding amortization of a prepaid cash item that was paid in a prior period); plus
(g) the amount of any minority interest expense consisting of Subsidiary income attributable to minority equity interests of third parties in any non-Wholly Owned Subsidiary; plus
(h) the amount of management, monitoring, consulting and advisory fees (including termination fees) and related indemnities and expenses paid or accrued in such period under the Management Fee Agreement or otherwise to the Investors to the extent otherwise permitted under “—Certain Covenants—Transactions with Affiliates”; plus
(i) the amount of “run-rate” cost savings projected by the Issuer in good faith to result from actions either taken or expected to be taken within 12 months after the end of such period (which cost savings shall be subject only to certification by management of the Issuer and calculated on a pro forma basis as though such cost savings had been realized on the first day of such period), net of the amount of actual benefits realized from such actions (it is understood and agreed that “run-rate” means the full recurring benefit that is associated with any action taken or expected to be taken, provided that some portion of such benefit is expected to be realized within 12 months of taking such action) (which adjustments may be incremental to pro forma cost savings, operating improvements, synergies and operating expense adjustments made pursuant to the definition of “Fixed Charge Coverage Ratio”); plus
(j) the amount of loss on sale of receivables, Securitization Assets and related assets to the Securitization Subsidiary in connection with a Qualified Securitization Facility; plus
(k) any costs or expense incurred by the Issuer or a Restricted Subsidiary pursuant to any management equity plan or stock option plan or any other management or employee benefit plan, agreement or any stock subscription or shareholder agreement or any distributor equity plan or agreement, to the extent that such cost or expenses are funded with cash proceeds contributed to the capital of the Issuer or net cash proceeds of an issuance of Equity Interest of the Issuer (other than Disqualified Stock) solely to the extent that such net cash proceeds are excluded from the calculation set forth in clause (3) of the first paragraph under “—Certain Covenants—Limitation on Restricted Payments”; plus
(l) cash receipts (or any netting arrangements resulting in reduced cash expenditures) not representing EBITDA or Consolidated Net Income in any period to the extent non-cash gains relating to such income were deducted in the calculation of EBITDA pursuant to clause (2) below for any previous period and not added back; plus
(m) any net loss from disposed or discontinued operations or from operations expected to be disposed of or discontinued within twelve months after the end of such period; plus
(n) interest income or investment earnings on retiree medical and intellectual property, royalty or license receivables; plus
(o) extraordinary losses and unusual or non-recurring charges (including any unusual or non-recurring operating expenses attributable to the implementation of cost-savings initiatives, severance, retention and relocation costs and curtailments and modifications to pension and post-retirement employee benefit plans); plus
232
(p) [reserved]; plus
(q) [reserved]; plus
(r) losses on asset sales (other than asset sales made in the ordinary course of business), disposals and abandonments;
(2) decreased (without duplication) by the following, in each case to the extent included in determining Consolidated Net Income for such period:
(a) non-cash gains increasing Consolidated Net Income of such Person for such period, excluding any non-cash gains to the extent they represent the reversal of an accrual or reserve for a potential cash item that reduced EBITDA in any prior period; plus
(b) any non-cash gains with respect to cash actually received in a prior period unless such cash did not increase EBITDA in such prior period; plus
(c) any net income from disposed or discontinued operations (excluding held-for-sale discontinued operations) or from operations expected to be disposed of or discontinued within twelve months after the end of such period; plus
(d) extraordinary gains and unusual or non-recurring gains; plus
(e) gains on asset sales (other than asset sales made in the ordinary course of business), disposals and abandonments.
“EMU” means economic and monetary union as contemplated in the Treaty on European Union.
“Equity Interests” means Capital Stock and all warrants, options or other rights to acquire Capital Stock, but excluding any debt security that is convertible into, or exchangeable for, Capital Stock.
“Equity Offering” means any public or private sale of common stock or Preferred Stock of the Issuer or any of its direct or indirect parent companies (excluding Disqualified Stock), other than:
(1) public offerings with respect to the Issuer’s or any direct or indirect parent company’s common stock registered on Form S-4 or Form S-8;
(2) issuances to any Subsidiary of the Issuer; and
(3) any such public or private sale that constitutes an Excluded Contribution.
“euro” means the single currency of participating member states of the EMU.
“Exchange Act” means the Securities Exchange Act of 1934, as amended, and the rules and regulations of the SEC promulgated thereunder.
“Excluded Contribution” means net cash proceeds, marketable securities or Qualified Proceeds received by the Issuer from
(1) contributions to its common equity capital; and
(2) the sale (other than to a Subsidiary of the Issuer or to any management equity plan or stock option plan or any other management or employee benefit plan or agreement or any distributor equity plan or agreement of the Issuer) of Capital Stock (other than Disqualified Stock and Designated Preferred Stock) of the Issuer;
in each case designated as Excluded Contributions pursuant to an Officer’s Certificate executed by the principal financial officer of the Issuer on the date such capital contributions are made or the date such Equity Interests are sold, as the case may be, which are excluded from the calculation set forth in clause (3) of the first paragraph under “—Certain Covenants—Limitation on Restricted Payments.”
233
“fair market value” means, with respect to any asset or liability, the fair market value of such asset or liability as determined by the Issuer in good faith.
“Fixed Charge Coverage Ratio” means, with respect to any Person for any period, the ratio of EBITDA of such Person for such period to the Fixed Charges of such Person for such period. In the event that the Issuer or any Restricted Subsidiary incurs, assumes, guarantees, redeems, retires or extinguishes any Indebtedness (other than Indebtedness incurred under any revolving credit facility unless such Indebtedness has been permanently repaid and has not been replaced) or issues or redeems Disqualified Stock or Preferred Stock subsequent to the commencement of the period for which the Fixed Charge Coverage Ratio is being calculated but prior to or simultaneously with the event for which the calculation of the Fixed Charge Coverage Ratio is made (the “Fixed Charge Coverage Ratio Calculation Date”), then the Fixed Charge Coverage Ratio shall be calculated giving pro forma effect to such incurrence, assumption, guarantee, redemption, retirement or extinguishment of Indebtedness, or such issuance or redemption of Disqualified Stock or Preferred Stock, as if the same had occurred at the beginning of the applicable four-quarter period.
For purposes of making the computation referred to above, Investments, acquisitions, dispositions, mergers, consolidations and discontinued operations (as determined in accordance with GAAP) that have been made by the Issuer or any of its Restricted Subsidiaries during the four-quarter reference period or subsequent to such reference period and on or prior to or simultaneously with the Fixed Charge Coverage Ratio Calculation Date shall be calculated on a pro forma basis assuming that all such Investments, acquisitions, dispositions, mergers, consolidations and discontinued operations (and the change in any associated fixed charge obligations and the change in EBITDA resulting therefrom) had occurred on the first day of the four-quarter reference period. If since the beginning of such period any Person that subsequently became a Restricted Subsidiary or was merged with or into the Issuer or any of its Restricted Subsidiaries since the beginning of such period shall have made any Investment, acquisition, disposition, merger, consolidation or discontinued operation that would have required adjustment pursuant to this definition, then the Fixed Charge Coverage Ratio shall be calculated giving pro forma effect thereto for such period as if such Investment, acquisition, disposition, merger, consolidation or discontinued operation had occurred at the beginning of the applicable four-quarter period.
For purposes of this definition, whenever pro forma effect is to be given to an Investment, acquisition, disposition, merger or consolidation, the pro forma calculations shall be made in good faith by a responsible financial or accounting officer of the Issuer (and may include, for the avoidance of doubt, cost savings, operating improvements, synergies and operating expense reductions resulting from such Investment, acquisition, merger or consolidation which is being given pro forma effect that have been or are expected to be realized). If any Indebtedness bears a floating rate of interest and is being given pro forma effect, the interest on such Indebtedness shall be calculated as if the rate in effect on the Fixed Charge Coverage Ratio Calculation Date had been the applicable rate for the entire period (taking into account any Hedging Obligations applicable to such Indebtedness). Interest on a Capitalized Lease Obligation shall be deemed to accrue at an interest rate reasonably determined by a responsible financial or accounting officer of the Issuer to be the rate of interest implicit in such Capitalized Lease Obligation in accordance with GAAP. For purposes of making the computation referred to above, interest on any Indebtedness under a revolving credit facility computed on a pro forma basis shall be computed based upon the average daily balance of such Indebtedness during the applicable period except as set forth in the first paragraph of this definition. Interest on Indebtedness that may optionally be determined at an interest rate based upon a factor of a prime or similar rate, a eurocurrency interbank offered rate, or other rate, shall be deemed to have been based upon the rate actually chosen, or, if none, then based upon such optional rate chosen as the Issuer may designate.
“Fixed Charges” means, with respect to any Person for any period, the sum of, without duplication:
(1) Consolidated Interest Expense of such Person for such period;
(2) all cash dividends or other distributions paid (excluding items eliminated in consolidation) on any series of Preferred Stock during such period; and
(3) all cash dividends or other distributions paid (excluding items eliminated in consolidation) on any series of Disqualified Stock during such period.
“Foreign Subsidiary” means, with respect to any Person, any Restricted Subsidiary of such Person that is not organized or existing under the laws of the United States, any state thereof, the District of Columbia, or any territory thereof and any Restricted Subsidiary of such Foreign Subsidiary.
234
“Foreign Subsidiary Total Assets” means the total assets of the Foreign Subsidiaries, as determined in accordance with GAAP in good faith by the Issuer, without intercompany eliminations.
“GAAP” means generally accepted accounting principles in the United States of America which are in effect on the Issue Date.
“Government Securities” means securities that are:
(1) direct obligations of the United States of America for the timely payment of which its full faith and credit is pledged; or
(2) obligations of a Person controlled or supervised by and acting as an agency or instrumentality of the United States of America the timely payment of which is unconditionally guaranteed as a full faith and credit obligation by the United States of America,
which, in either case, are not callable or redeemable at the option of the issuers thereof, and shall also include a depository receipt issued by a bank (as defined in Section 3(a)(2) of the Securities Act), as custodian with respect to any such Government Securities or a specific payment of principal of or interest on any such Government Securities held by such custodian for the account of the holder of such depository receipt; provided that (except as required by law) such custodian is not authorized to make any deduction from the amount payable to the holder of such depository receipt from any amount received by the custodian in respect of the Government Securities or the specific payment of principal of or interest on the Government Securities evidenced by such depository receipt.
“guarantee” means a guarantee (other than by endorsement of negotiable instruments for collection in the ordinary course of business), direct or indirect, in any manner (including letters of credit and reimbursement agreements in respect thereof), of all or any part of any Indebtedness or other obligations.
“Guarantee” means the guarantee by any Guarantor of the Issuer’s Obligations under the senior subordinated notes indenture and the senior subordinated notes.
“Guarantor” means each Subsidiary of the Issuer, if any, that Guarantees the senior subordinated notes in accordance with the terms of the senior subordinated notes indenture.
“Hedging Obligations” means, with respect to any Person, the obligations of such Person under any interest rate swap agreement, interest rate cap agreement, interest rate collar agreement, commodity swap agreement, commodity cap agreement, commodity collar agreement, foreign exchange contract, currency swap agreement or similar agreement providing for the transfer or mitigation of interest rate or currency risks either generally or under specific contingencies.
“Holder” means the Person in whose name an senior subordinated note is registered on the registrar’s books.
“Immediate Family Members” means with respect to any individual, such individual’s child, stepchild, grandchild or more remote descendant, parent, stepparent, grandparent, spouse, former spouse, qualified domestic partner, sibling, mother-in-law, father-in-law, son-in-law and daughter-in-law (including adoptive relationships) and any trust, partnership or other bona fide estate-planning vehicle the only beneficiaries of which are any of the foregoing individuals or any private foundation or fund that is controlled by any if the foregoing individuals or any donor-advised fund of which any such individual is the donor.
“Indebtedness” means, with respect to any Person, without duplication:
(1) any indebtedness (including principal and premium) of such Person, whether or not contingent:
(a) in respect of borrowed money;
(b) evidenced by bonds, notes, debentures or similar instruments or letters of credit or bankers’ acceptances (or, without duplication, reimbursement agreements in respect thereof);
(c) representing the balance deferred and unpaid of the purchase price of any property (including Capitalized Lease Obligations) due more than twelve months after such property is acquired, except (i) any
235
such balance that constitutes an obligation in respect of a commercial letter of credit, a trade payable or similar obligation to a trade creditor, in each case accrued in the ordinary course of business and (ii) any earn-out obligations until such obligation becomes a liability on the balance sheet of such Person in accordance with GAAP and if not paid after becoming due and payable;
(d) representing the net obligations under any Hedging Obligations; or
(e) during a Suspension Period only, obligations of the lessee for rental payments in respect of Sale and Lease-back Transactions in an amount equal to the present value of such obligations during the remaining term of the lease using a discount rate equal to the rate of interest implicit in such transaction determined in accordance with GAAP,
if and to the extent that any of the foregoing Indebtedness (other than letters of credit and Hedging Obligations) would appear as a liability upon a balance sheet (excluding the footnotes thereto) of such Person prepared in accordance with GAAP; provided that Indebtedness of any direct or indirect parent of the Issuer appearing upon the balance sheet of the Issuer solely by reason of push-down accounting under GAAP shall be excluded;
(2) to the extent not otherwise included, any obligation by such Person to be liable for, or to pay, as obligor, guarantor or otherwise, on the obligations of the type referred to in clause (1) of a third Person (whether or not such items would appear upon the balance sheet of the such obligor or guarantor), other than by endorsement of negotiable instruments for collection in the ordinary course of business; and
(3) to the extent not otherwise included, the obligations of the type referred to in clause (1) of a third Person secured by a Lien on any asset owned by such first Person, whether or not such Indebtedness is assumed by such first Person;
provided that notwithstanding the foregoing, Indebtedness shall be deemed not to include (a) Contingent Obligations incurred in the ordinary course of business or (b) obligations under or in respect of Qualified Securitization Facilities.
“Independent Financial Advisor” means an accounting, appraisal, investment banking firm or consultant to Persons engaged in Similar Businesses of nationally recognized standing that is, in the good faith judgment of the Issuer, qualified to perform the task for which it has been engaged.
“Investment Grade Rating” means a rating equal to or higher than Baa3 (or the equivalent) by Moody’s and BBB- (or the equivalent) by S&P, or an equivalent rating by any other Rating Agency.
“Investment Grade Securities” means:
(1) securities issued or directly and fully guaranteed or insured by the United States government or any agency or instrumentality thereof (other than Cash Equivalents);
(2) debt securities or debt instruments with an Investment Grade Rating, but excluding any debt securities or instruments constituting loans or advances among the Issuer and its Subsidiaries;
(3) investments in any fund that invests exclusively in investments of the type described in clauses (1) and (2) which fund may also hold immaterial amounts of cash pending investment or distribution; and
(4) corresponding instruments in countries other than the United States customarily utilized for high quality investments.
“Investments” means, with respect to any Person, all investments by such Person in other Persons (including Affiliates) in the form of loans (including guarantees), advances or capital contributions (excluding accounts receivable, trade credit, advances to customers and distributors, commission, travel and similar advances to employees, directors, officers, managers, distributors and consultants in each case made in the ordinary course of business), purchases or other acquisitions for consideration of Indebtedness, Equity Interests or other securities issued by any other Person and investments that are required by GAAP to be classified on the balance sheet (excluding the footnotes) of the Issuer in the same manner as the other investments included in this definition to the extent such transactions involve the transfer of cash or other property. For purposes of the definition of “Unrestricted Subsidiary” and the covenant described under “—Certain Covenants—Limitation on Restricted Payments”:
236
(1) “Investments” shall include the portion (proportionate to the Issuer’s equity interest in such Subsidiary) of the fair market value of the net assets of a Subsidiary of the Issuer at the time that such Subsidiary is designated an Unrestricted Subsidiary; provided that upon a redesignation of such Subsidiary as a Restricted Subsidiary, the Issuer shall be deemed to continue to have a permanent “Investment” in an Unrestricted Subsidiary in an amount (if positive) equal to:
(a) the Issuer’s “Investment” in such Subsidiary at the time of such redesignation; less
(b) the portion (proportionate to the Issuer’s Equity Interest in such Subsidiary) of the fair market value of the net assets of such Subsidiary at the time of such redesignation; and
(2) any property transferred to or from an Unrestricted Subsidiary shall be valued at its fair market value at the time of such transfer.
The amount of any Investment outstanding at any time shall be the original cost of such Investment, reduced by any dividend, distribution, interest payment, return of capital, repayment or other amount received in cash by the Issuer or a Restricted Subsidiary in respect of such Investment.
“Investors” means The Blackstone Group, Goldman Sachs Capital Partners, Kohlberg Kravis Roberts & Co., TPG Global, LLC and, if applicable, each of their respective Affiliates and funds or partnerships managed by any of them or their respective Affiliates but not including, however, any portfolio companies of any of the foregoing.
“Issue Date” means October 2, 2012.
“Issuer” means Biomet, Inc., an Indiana corporation (and not any of its Subsidiaries), and its successors.
“Legal Holiday” means a Saturday, a Sunday or a day on which commercial banking institutions are not required to be open in the State of New York or place of payment.
“Lien” means, with respect to any asset, any mortgage, lien (statutory or otherwise), pledge, hypothecation, charge, security interest, preference, priority or encumbrance of any kind in respect of such asset, whether or not filed, recorded or otherwise perfected under applicable law, including any conditional sale or other title retention agreement, any lease in the nature thereof, any option or other agreement to sell or give a security interest in and any filing of or agreement to give any financing statement under the Uniform Commercial Code (or equivalent statutes) of any jurisdiction; provided that in no event shall an operating lease be deemed to constitute a Lien.
“Management Fee Agreement” means the management services agreement, dated as of September 25, 2007 between certain of the management companies associated with the Investors or their advisors, if applicable, and the Issuer.
“Management Stockholders” means the members of management (and their Controlled Investment Affiliates and Immediate Family Members) of the Issuer (or its direct parent) who are holders of Equity Interests of any direct or indirect parent companies of the Issuer on the Issue Date or will become holders of such Equity Interests in connection with the Acquisition.
“Merger Agreement” means the Agreement and Plan of Merger, dated December 18, 2006 (as amended and restated as of June 7, 2007) by and among the Issuer, the Parent and LVB Acquisition Merger Sub, Inc.
“Moody’s” means Moody’s Investors Service, Inc. and any successor to its rating agency business.
“Net Income” means, with respect to any Person, the net income (loss) of such Person, determined in accordance with GAAP and before any reduction in respect of Preferred Stock dividends.
“Net Proceeds” means the aggregate cash proceeds received by the Issuer or any of its Restricted Subsidiaries in respect of any Asset Sale, including any cash received upon the sale or other disposition of any Designated Non-cash Consideration received in any Asset Sale, net of the direct costs relating to such Asset Sale and the sale or disposition of such Designated Non-cash Consideration, including legal, accounting and investment banking fees, payments made in order to obtain a necessary consent or required by applicable law, and brokerage and sales commissions, any relocation expenses incurred as a result thereof, other fees and expenses, including title
237
and recordation expenses, taxes paid or payable as a result thereof (after taking into account any available tax credits or deductions and any tax sharing arrangements), amounts required to be applied to the repayment of principal, premium, if any, and interest on Senior Indebtedness required (other than required by clause (1) of the second paragraph of “Repurchase at the Option of Holders—Asset Sales”) to be paid as a result of such transaction and any deduction of appropriate amounts to be provided by the Issuer or any of its Restricted Subsidiaries as a reserve in accordance with GAAP against any liabilities associated with the asset disposed of in such transaction and retained by the Issuer or any of its Restricted Subsidiaries after such sale or other disposition thereof, including pension and other post-employment benefit liabilities and liabilities related to environmental matters or against any indemnification obligations associated with such transaction.
“Obligations” means any principal, interest (including any interest accruing on or subsequent to the filing of a petition in bankruptcy, reorganization or similar proceeding at the rate provided for in the documentation with respect thereto, whether or not such interest is an allowed claim under applicable state, federal or foreign law), premium, penalties, fees, indemnifications, reimbursements (including reimbursement obligations with respect to letters of credit and banker’s acceptances), damages and other liabilities, and guarantees of payment of such principal, interest, penalties, fees, indemnifications, reimbursements, damages and other liabilities, payable under the documentation governing any Indebtedness.
“Officer” means the Chairman of the board of directors, the Chief Executive Officer, the Chief Financial Officer, the President, any Executive Vice President, Senior Vice President or Vice President, the Treasurer or the Secretary of the Issuer.
“Officer’s Certificate” means a certificate signed on behalf of a Person by an Officer of such Person, who must be the principal executive officer, the principal financial officer, the treasurer or the principal accounting officer of such Person, that meets the requirements set forth in the senior subordinated notes indenture.
“Opinion of Counsel” means a written opinion from legal counsel who is acceptable to the Trustee. The counsel may be an employee of or counsel to the Issuer.
“Parent” means LVB Acquisition, Inc., a Delaware corporation and the direct parent of the Issuer.
“Permitted Asset Swap” means the substantially concurrent purchase and sale or exchange of Related Business Assets or a combination of Related Business Assets and Cash Equivalents between the Issuer or any of its Restricted Subsidiaries and another Person; provided that any Cash Equivalents received must be applied in accordance with the covenant described under “Repurchase at the Option of Holders—Asset Sales.”
“Permitted Holders” means each of the Investors, Management Stockholders and any of the direct or indirect parent companies of the Issuer (provided such direct or indirect parent companies of the Issuer have no majority holders other than the Investors, Management Stockholders and any group (within the meaning of Section 13(d)(3) or Section 14(d)(2) of the Exchange Act or any successor provision) of which any of the foregoing are members) and any group (within the meaning of Section 13(d)(3) or Section 14(d)(2) of the Exchange Act or any successor provision) of which any of the foregoing are members; provided that, in the case of such group and without giving effect to the existence of such group or any other group, such Investors, Management Stockholders and any of the direct or indirect parent companies of the Issuer (provided such direct or indirect parent companies of the Issuer have no majority holders other than the Investors, Management Stockholders and any group (within the meaning of Section 13(d)(3) or Section 14(d)(2) of the Exchange Act or any successor provision) of which any of the foregoing are members), collectively, have beneficial ownership of more than 50.0% of the total voting power of the Voting Stock of the Issuer or any of its direct or indirect parent companies. Any Person or group whose acquisition of beneficial ownership constitutes a Change of Control in respect of which a Change of Control Offer is made in accordance with the requirements of the senior subordinated notes indenture will thereafter, together with its Affiliates, constitute an additional Permitted Holder.
“Permitted Junior Securities” means:
(1) Equity Interests in the Issuer or any Guarantor or any direct or indirect parent company of the Issuer; or
(2) unsecured debt securities that are subordinated to all Senior Indebtedness (and any debt securities issued in exchange for Senior Indebtedness) to substantially the same extent as, or to a greater extent than, the senior
238
subordinated notes and the related Guarantees are subordinated to Senior Indebtedness under the senior subordinated notes indenture;
provided that the term “Permitted Junior Securities” shall not include any securities distributed pursuant to a plan of reorganization if the Indebtedness under the Senior Credit Facilities is treated as part of the same class as the senior subordinated notes for purposes of such plan of reorganization; provided, further, that to the extent that any Senior Indebtedness of the Issuer outstanding on the date of consummation of any such plan of reorganization is not paid in full in cash on such date, the holders of any such Senior Indebtedness not so paid in full in cash have consented to the terms of such plan of reorganization.
“Permitted Investments” means:
(1) any Investment in the Issuer or any of its Restricted Subsidiaries;
(2) any Investment in Cash Equivalents or Investment Grade Securities;
(3) any Investment by the Issuer or any of its Restricted Subsidiaries in a Person that is engaged in a Similar Business if as a result of such Investment:
(a) such Person becomes a Restricted Subsidiary; or
(b) such Person, in one transaction or a series of related transactions, is merged or consolidated with or into, or transfers or conveys substantially all of its assets to, or is liquidated into, the Issuer or a Restricted Subsidiary,
and, in each case, any Investment held by such Person; provided that such Investment was not acquired by such Person in contemplation of such acquisition, merger, consolidation or transfer;
(4) any Investment in securities or other assets not constituting Cash Equivalents or Investment Grade Securities and received in connection with an Asset Sale made pursuant to the provisions described under “Repurchase at the Option of Holders—Asset Sales” or any other disposition of assets not constituting an Asset Sale;
(5) any Investment existing on the Issue Date or made pursuant to binding commitments in effect on the Issue Date or an Investment consisting of any extension, modification or renewal of any Investment existing on the Issue Date; provided that the amount of any such Investment may be increased (a) as required by the terms of such Investment as in existence on the Issue Date or (b) as otherwise permitted under the senior subordinated notes indenture;
(6) any Investment acquired by the Issuer or any of its Restricted Subsidiaries:
(a) in exchange for any other Investment or accounts receivable held by the Issuer or any such Restricted Subsidiary in connection with or as a result of a bankruptcy, workout, reorganization or recapitalization of the issuer of such other Investment or accounts receivable (including any trade creditor or customer); or
(b) in satisfaction of judgments against other Persons; or
(c) as a result of a foreclosure by the Issuer or any of its Restricted Subsidiaries with respect to any secured Investment or other transfer of title with respect to any secured Investment in default;
(7) Hedging Obligations permitted under clause (10) of the covenant described in “—Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”;
(8) any Investment in a Similar Business taken together with all other Investments made pursuant to this clause (8) that are at that time outstanding, not to exceed the greater of (a) $450.0 million and (b) 3.0% of Total Assets;
(9) Investments the payment for which consists of Equity Interests (other than Disqualified Stock) of the Issuer, or any of its direct or indirect parent companies; provided that such Equity Interests will not increase the
239
amount available for Restricted Payments under clause (3) of the first paragraph under the covenant described in “—Certain Covenants—Limitations on Restricted Payments”;
(10) guarantees of Indebtedness permitted under the covenant described in “—Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”;
(11) any transaction to the extent it constitutes an Investment that is permitted by and made in accordance with the provisions of the second paragraph of the covenant described under “—Certain Covenants—Transactions with Affiliates” (except transactions described in clauses (2), (5) and (9) of such paragraph);
(12) Investments consisting of purchases and acquisitions of inventory, supplies, material or equipment or the licensing or contribution of intellectual property pursuant to joint marketing arrangements with other Persons;
(13) additional Investments, taken together with all other Investments made pursuant to this clause (13) that are at that time outstanding (without giving effect to the sale of an Unrestricted Subsidiary to the extent the proceeds of such sale do not consist of cash or marketable securities), not to exceed the greater of (a) $450.0 million and (b) 3.0% of Total Assets;
(14) Investments in or relating to a Securitization Subsidiary that, in the good faith determination of the Issuer are necessary or advisable to effect any Qualified Securitization Facility or any repurchase obligation in connection therewith;
(15) advances to, or guarantees of Indebtedness of, employees not in excess of $25.0 million outstanding at any one time, in the aggregate;
(16) loans and advances to employees, directors, officers, managers, distributors and consultants for business-related travel expenses, moving expenses and other similar expenses, in each case incurred in the ordinary course of business or consistent with past practices or to fund such Person’s purchase of Equity Interests of the Issuer or any direct or indirect parent company thereof;
(17) advances, loans or extensions of trade credit in the ordinary course of business by the Issuer or any of its Restricted Subsidiaries;
(18) any Investment in any Subsidiary or any joint venture in connection with intercompany cash management arrangements or related activities arising in the ordinary course of business;
(19) Investments consisting of purchases and acquisitions of assets or services in the ordinary course of business;
(20) Investments made in the ordinary course of business in connection with obtaining, maintaining or renewing client contacts and loans or advances made to distributors in the ordinary course of business;
(21) Investments in prepaid expenses, negotiable instruments held for collection and lease, utility and workers compensation, performance and similar deposits entered into as a result of the operations of the business in the ordinary course of business; and
(22) repurchases of the senior subordinated notes.
“Permitted Liens” means, with respect to any Person:
(1) pledges or deposits by such Person under workmen’s compensation laws, unemployment insurance, other social security benefits or other insurance related obligations (including, but not limited to, in respect of deductibles, self-insured retention amounts and premiums and adjustments thereto) or good faith deposits in connection with bids, tenders, contracts (other than for the payment of Indebtedness) or leases to which such Person is a party, or deposits to secure public or statutory obligations of such Person or deposits of cash or U.S. government bonds to secure surety or appeal bonds to which such Person is a party, or deposits as security for contested taxes or import duties or for the payment of rent, in each case incurred in the ordinary course of business;
(2) Liens imposed by law, such as carriers’, warehousemen’s and mechanics’ Liens, in each case for sums not yet overdue for a period of more than 30 days or being contested in good faith by appropriate proceedings
240
or other Liens arising out of judgments or awards against such Person with respect to which such Person shall then be proceeding with an appeal or other proceedings for review if adequate reserves with respect thereto are maintained on the books of such Person in accordance with GAAP;
(3) Liens for taxes, assessments or other governmental charges not yet overdue for a period of more than 30 days or not yet payable or subject to penalties for nonpayment or which are being contested in good faith by appropriate proceedings diligently conducted, if adequate reserves with respect thereto are maintained on the books of such Person in accordance with GAAP;
(4) Liens in favor of issuers of performance and surety bonds or bid bonds or with respect to other regulatory requirements or letters of credit issued pursuant to the request of and for the account of such Person in the ordinary course of its business;
(5) minor survey exceptions, minor encumbrances, easements or reservations of, or rights of others for, licenses, rights-of-way, sewers, electric lines, telegraph and telephone lines and other similar purposes, or zoning or other restrictions as to the use of real properties or Liens incidental, to the conduct of the business of such Person or to the ownership of its properties which were not incurred in connection with Indebtedness and which do not in the aggregate materially adversely affect the value of said properties or materially impair their use in the operation of the business of such Person;
(6) Liens securing Indebtedness permitted to be incurred pursuant to clause (4), (12)(b), (13), (23) or (24) of the second paragraph under “—Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”; provided that (a) Liens securing Indebtedness, Disqualified Stock or Preferred Stock permitted to be incurred pursuant to clause (13) relate only to Refinancing Indebtedness that serves to refund or refinance Indebtedness, Disqualified Stock or Preferred Stock incurred under clause (4) or (12)(b) of the second paragraph of “—Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock,” (b) Liens securing Indebtedness permitted to be incurred pursuant to clause (23) extend only to the assets of Foreign Subsidiaries, (c) Liens securing Indebtedness permitted to be incurred pursuant to clause (24) are solely on acquired property or the assets of the acquired entity, as the case may be, and (d) Liens securing Indebtedness, Disqualified Stock or Preferred Stock to be incurred pursuant to clause (4) of the second paragraph under “—Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock” extend only to the assets so purchased, leased or improved;
(7) Liens existing on the Issue Date;
(8) Liens on property or shares of stock or other assets of a Person at the time such Person becomes a Subsidiary; provided that such Liens are not created or incurred in connection with, or in contemplation of, such other Person becoming such a Subsidiary; provided, further, that such Liens may not extend to any other property or other assets owned by the Issuer or any of its Restricted Subsidiaries;
(9) Liens on property or other assets at the time the Issuer or a Restricted Subsidiary acquired the property or such other assets, including any acquisition by means of a merger or consolidation with or into the Issuer or any of its Restricted Subsidiaries; provided that such Liens are not created or incurred in connection with, or in contemplation of, such acquisition; provided, further, that the Liens may not extend to any other property owned by the Issuer or any of its Restricted Subsidiaries;
(10) Liens securing Indebtedness or other obligations of a Restricted Subsidiary owing to the Issuer or another Restricted Subsidiary permitted to be incurred in accordance with the covenant described under “—Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”;
(11) Liens securing Hedging Obligations; provided that, with respect to Hedging Obligations relating to Indebtedness, such Indebtedness is, and is permitted to be under the senior subordinated notes indenture, secured by a Lien on the same property securing such Hedging Obligations;
(12) Liens on specific items of inventory or other goods and proceeds of any Person securing such Person’s obligations in respect of bankers’ acceptances issued or created for the account of such Person to facilitate the purchase, shipment or storage of such inventory or other goods;
241
(13) leases, subleases, licenses or sublicenses granted to others in the ordinary course of business which do not materially interfere with the ordinary conduct of the business of the Issuer or any of its Restricted Subsidiaries and do not secure any Indebtedness;
(14) Liens arising from Uniform Commercial Code financing statement filings regarding operating leases entered into by the Issuer and its Restricted Subsidiaries in the ordinary course of business;
(15) Liens in favor of the Issuer or any Guarantor;
(16) Liens on equipment of the Issuer or any of its Restricted Subsidiaries granted in the ordinary course of business to the Issuer’s clients;
(17) Liens on accounts receivable, Securitization Assets and related assets incurred in connection with a Qualified Securitization Facility;
(18) Liens to secure any refinancing, refunding, extension, renewal or replacement (or successive refinancing, refunding, extensions, renewals or replacements) as a whole, or in part, of any Indebtedness secured by any Lien referred to in the foregoing clauses (6), (7), (8) and (9); provided that (a) such new Lien shall be limited to all or part of the same property that secured the original Lien (plus improvements on such property), and (b) the Indebtedness secured by such Lien at such time is not increased to any amount greater than the sum of (i) the outstanding principal amount or, if greater, committed amount of the Indebtedness described under clauses (6), (7), (8) and (9) at the time the original Lien became a Permitted Lien under the senior subordinated notes indenture, and (ii) an amount necessary to pay any fees and expenses, including premiums, related to such refinancing, refunding, extension, renewal or replacement;
(19) deposits made in the ordinary course of business to secure liability to insurance carriers;
(20) other Liens securing obligations in an aggregate amount at any one time outstanding not to exceed the greater of (a) $100.0 million and (b) 1.0% of Total Assets determined as of the date of incurrence;
(21) Liens securing judgments for the payment of money not constituting an Event of Default under clause (5) under “Events of Default and Remedies” so long as such Liens are adequately bonded and any appropriate legal proceedings that may have been duly initiated for the review of such judgment have not been finally terminated or the period within which such proceedings may be initiated has not expired;
(22) Liens in favor of customs and revenue authorities arising as a matter of law to secure payment of customs duties in connection with the importation of goods in the ordinary course of business;
(23) Liens (a) of a collection bank arising under Section 4-210 of the Uniform Commercial Code on items in the course of collection, (b) attaching to commodity trading accounts or other commodity brokerage accounts incurred in the ordinary course of business, and (c) in favor of banking institutions arising as a matter of law encumbering deposits (including the right of set-off) and which are within the general parameters customary in the banking industry;
(24) Liens deemed to exist in connection with Investments in repurchase agreements permitted under “—Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”; provided that such Liens do not extend to any assets other than those that are the subject of such repurchase agreement;
(25) Liens encumbering reasonable customary deposits and margin deposits and similar Liens attaching to commodity trading accounts or other brokerage accounts incurred in the ordinary course of business and not for speculative purposes;
(26) Liens that are contractual rights of set-off (a) relating to the establishment of depository relations with banks not given in connection with the issuance of Indebtedness, (b) relating to pooled deposit or sweep accounts of the Issuer or any of its Restricted Subsidiaries to permit satisfaction of overdraft or similar obligations incurred in the ordinary course of business of the Issuer and its Restricted Subsidiaries or (c) relating to purchase orders and other agreements entered into with customers of the Issuer or any of its Restricted Subsidiaries in the ordinary course of business;
242
(27) Liens securing obligations owed by the Issuer or any Restricted Subsidiary to any lender under the Senior Credit Facilities or any Affiliate of such a lender in respect of any overdraft and related liabilities arising from treasury, depository and cash management services or any automated clearing house transfers of funds;
(28) during a Suspension Period only, Liens securing Indebtedness (other than Indebtedness that is secured equally and ratably with (or on a basis subordinated to) the senior subordinated notes), and Indebtedness represented by Sale and Lease-Back Transactions in an amount not to exceed 15.0% of Total Assets at any one time outstanding;
(29) Liens securing Indebtedness the proceeds of which are used to develop or construct new facilities (or any improvements to existing facilities) or equipment (or any improvements to existing equipment) designed primarily for the purpose of air or water pollution control; provided that such Indebtedness is permitted to be incurred by the terms of the senior subordinated notes indenture and such Liens do not extend to any assets of the Issuer or its Restricted Subsidiaries other than the assets developed, constructed or improved with the proceeds of the Indebtedness secured by such Lien;
(30) any encumbrance or restriction (including put and call arrangements) with respect to capital stock of any joint venture or similar arrangement pursuant to any joint venture or similar agreement;
(31) Liens arising out of conditional sale, title retention, consignment or similar arrangements for the sale or purchase of goods entered into by the Issuer or any Restricted Subsidiary in the ordinary course of business;
(32) Liens solely on any cash earnest money deposits made by the Issuer or any of its Restricted Subsidiaries in connection with any letter of intent or purchase agreement permitted;
(33) ground leases in respect of real property on which facilities owned or leased by the Issuer or any of its Subsidiaries are located;
(34) Liens on insurance policies and the proceeds thereof securing the financing of the premiums with respect thereto;
(35) Liens on Capital Stock of an Unrestricted Subsidiary that secure Indebtedness or other obligations of such Unrestricted Subsidiary; and
(36) Liens on the assets of non-guarantor Subsidiaries securing Indebtedness of the Issuer or the Restricted Subsidiaries that were permitted by the terms of the senior subordinated notes indenture to be incurred.
For purposes of this definition, the term “Indebtedness” shall be deemed to include interest on such Indebtedness.
“Person” means any individual, corporation, limited liability company, partnership, joint venture, association, joint stock company, trust, unincorporated organization, government or any agency or political subdivision thereof or any other entity.
“Preferred Stock” means any Equity Interest with preferential rights of payment of dividends or upon liquidation, dissolution, or winding up.
“Qualified Proceeds” means the fair market value of assets that are used or useful in, or Capital Stock of any Person engaged in, a Similar Business.
“Qualified Securitization Facility” means any Securitization Facility (1) constituting a securitization financing facility that meets the following conditions: (a) the board of directors of the Issuer shall have determined in good faith that such Securitization Facility (including financing terms, covenants, termination events and other provisions) is in the aggregate economically fair and reasonable to the Issuer and the applicable Securitization Subsidiary, (b) all sales and/or contributions of Securitization Assets and related assets to the applicable Securitization Subsidiary are made at fair market value (as determined in good faith by the Issuer) and (c) the financing terms, covenants, termination events and other provisions thereof shall be market terms (as determined in good faith by the Issuer) or (2) constituting a receivables financing facility.
243
“Rating Agencies” means Moody’s and S&P or if Moody’s or S&P or both shall not make a rating on the senior subordinated notes publicly available, a nationally recognized statistical rating agency or agencies, as the case may be, selected by the Issuer which shall be substituted for Moody’s or S&P or both, as the case may be.
“Senior Subordinated Notes Registration Rights Agreement” means a registration rights agreement with respect to the Notes dated as of the Issue Date, among the Issuer, the Guarantors and the initial purchasers and any additional registration rights agreements entered into by the Issuer in connection with the issuance of Additional Senior Subordinated Notes.
“Related Business Assets” means assets (other than Cash Equivalents) used or useful in a Similar Business, provided that any assets received by the Issuer or a Restricted Subsidiary in exchange for assets transferred by the Issuer or a Restricted Subsidiary shall not be deemed to be Related Business Assets if they consist of securities of a Person, unless upon receipt of the securities of such Person, such Person would become a Restricted Subsidiary.
“Representative” means any trustee, agent or other representative for an issue of Designated Senior Indebtedness of the Issuer.
“Restricted Investment” means an Investment other than a Permitted Investment.
“Restricted Subsidiary” means, at any time, any direct or indirect Subsidiary of the Issuer (including any Foreign Subsidiary) that is not then an Unrestricted Subsidiary; provided that upon an Unrestricted Subsidiary ceasing to be an Unrestricted Subsidiary, such Subsidiary shall be included in the definition of “Restricted Subsidiary.”
“S&P” means Standard & Poor’s, a division of The McGraw-Hill Companies, Inc., and any successor to its rating agency business.
“Sale and Lease-Back Transaction” means any arrangement providing for the leasing by the Issuer or any of its Restricted Subsidiaries of any real or tangible personal property, which property has been or is to be sold or transferred by the Issuer or such Restricted Subsidiary to a third Person in contemplation of such leasing.
“SEC” means the U.S. Securities and Exchange Commission.
“Secured Indebtedness” means any Indebtedness of the Issuer or any of its Restricted Subsidiaries secured by a Lien.
“Securities Act” means the Securities Act of 1933, as amended, and the rules and regulations of the SEC promulgated thereunder.
“Securitization Assets” means the accounts receivable, royalty or other revenue streams and other rights to payment related to the Specified Contract Rights subject to a Qualified Securitization Facility that is a securitization financing facility (and not a receivables financing facility) and the proceeds thereof.
“Securitization Facility” means any of one or more receivables or securitization financing facilities as amended, supplemented, modified, extended, renewed, restated or refunded from time to time, the Obligations of which are non-recourse (except for customary representations, warranties, covenants and indemnities made in connection with such facilities) to the Issuer or any of its Restricted Subsidiaries (other than a Securitization Subsidiary) pursuant to which the Issuer or any of its Restricted Subsidiaries sells or grants a security interest in its accounts receivable or Securitization Assets or assets related thereto to either (a) a Person that is not a Restricted Subsidiary or (b) a Securitization Subsidiary that in turn sells its accounts receivable to a Person that is not a Restricted Subsidiary.
“Securitization Fees” means distributions or payments made directly or by means of discounts with respect to any participation interest issued or sold in connection with, and other fees paid to a Person that is not a Securitization Subsidiary in connection with, any Qualified Securitization Facility.
“Securitization Subsidiary” means any Subsidiary formed for the purpose of, and that solely engages only in one or more Qualified Securitization Facilities and other activities reasonably related thereto.
“Senior Credit Facilities” means the ABL Facilities and the CF Credit Facilities.
244
“Senior Indebtedness” means:
(1) all Indebtedness of the Issuer or any Guarantor outstanding under the Senior Credit Facilities or Senior Notes and related Guarantees (including interest accruing on or after the filing of any petition in bankruptcy or similar proceeding or for reorganization of the Issuer or any Guarantor (at the rate provided for in the documentation with respect thereto, regardless of whether or not a claim for post-filing interest is allowed in such proceedings)), and any and all other fees, expense reimbursement obligations, indemnification amounts, penalties, and other amounts (whether existing on the Issue Date or thereafter created or incurred) and all obligations of the Issuer or any Guarantor to reimburse any bank or other Person in respect of amounts paid under letters of credit, acceptances or other similar instruments;
(2) all Hedging Obligations (and guarantees thereof) owing to a Lender (as defined in the Senior Credit Facilities) or any Affiliate of such Lender (or any Person that was a Lender or an Affiliate of such Lender at the time the applicable agreement giving rise to such Hedging Obligation was entered into), provided that such Hedging Obligations are permitted to be incurred under the terms of the senior subordinated notes indenture;
(3) any other Indebtedness of the Issuer or any Guarantor permitted to be incurred under the terms of the senior subordinated notes indenture, unless the instrument under which such Indebtedness is incurred expressly provides that it is on a parity with or subordinated in right of payment to the senior subordinated notes or any related Guarantee; and
(4) all Obligations with respect to the items listed in the preceding clauses (1), (2) and (3);
provided that Senior Indebtedness shall not include:
(a) any obligation of such Person to the Issuer or any of its Subsidiaries;
(b) any liability for federal, state, local or other taxes owed or owing by such Person;
(c) any accounts payable or other liability to trade creditors arising in the ordinary course of business;
(d) any Indebtedness or other Obligation of such Person which is subordinate or junior in any respect to any other Indebtedness or other Obligation of such Person; or
(e) that portion of any Indebtedness which at the time of incurrence is incurred in violation of the senior subordinated notes indenture; provided that such Indebtedness shall be deemed not to have been incurred in violation of the senior subordinated notes indenture for purposes of this clause if such Indebtedness consists of Designated Senior Indebtedness, and the holder(s) of such Indebtedness or their agent or representative (i) had no actual knowledge at the time of incurrence that the incurrence of such Indebtedness violated the senior subordinated notes indenture and (ii) shall have received a certificate from an officer of the Issuer to the effect that the incurrence of such Indebtedness does not (or, in the case of a revolving credit facility thereunder, the incurrence of the entire committed amount thereof at the date on which the initial borrowing is made thereunder would not) violate the provisions of the senior subordinated notes indenture.
“Senior Notes” means the Issuer’s $1,825.0 million 6.500% Senior Notes due 2020.
“Senior Subordinated Indebtedness” means:
(1) with respect to the Issuer, Indebtedness which ranks equal in right of payment to the senior subordinated notes issued by the Issuer; and
(2) with respect to any Guarantor, Indebtedness which ranks equal in right of payment to the Guarantee of such Person of the exchange senior subordinated notes
provided that such Indebtedness is not subordinated by its terms in right of payment to any Indebtedness which is not Senior Indebtedness.
“Senior Secured Leverage Ratio” means “Senior Secured Leverage Ratio” as defined, together with related definitions, in the CF Credit Facilities as in effect on August 8, 2012, provided that the Issuer may elect, pursuant to
245
an Officer’s Certificate delivered to the Trustee to treat all or a portion of a revolving commitment under any Credit Facility as incurred and outstanding Indebtedness at the time such commitments are established and for so long as such revolving commitments remain outstanding. As a result of any such election, any subsequent incurrence of Indebtedness under such revolving commitment shall not be deemed an incurrence of additional Indebtedness or an additional Lien at such subsequent event.
“Significant Subsidiary” means any Restricted Subsidiary that would be a “significant subsidiary” as defined in Article 1, Rule 1-02 of Regulation S-X, promulgated pursuant to the Securities Act, as such regulation is in effect on the Issue Date.
“Similar Business” means (1) any business engaged in by the Issuer or any of its Restricted Subsidiaries on the Issue Date, and (2) any business or other activities that are reasonably similar, ancillary, complementary or related to, or a reasonable extension, development or expansion of, the businesses in which the Issuer and its Restricted Subsidiaries are engaged on the Issue Date.
“Specified Contract Rights” means certain intellectual property licenses, agreements or other contracts giving rise to not more than $50.0 million of annual accounts receivable, royalty or other intellectual property revenue streams or other rights to payment.
“Subordinated Indebtedness” means, with respect to the senior subordinated notes,
(1) any Indebtedness of the Issuer which is by its terms subordinated in right of payment to the senior subordinated notes, and
(2) any Indebtedness of any Guarantor which is by its terms subordinated in right of payment to the Guarantee of such entity of the senior subordinated notes.
“Subsidiary” means, with respect to any Person:
(1) any corporation, association, or other business entity (other than a partnership, joint venture, limited liability company or similar entity) of which more than 50.0% of the total voting power of shares of Capital Stock entitled (without regard to the occurrence of any contingency) to vote in the election of directors, managers or trustees thereof is at the time of determination owned or controlled, directly or indirectly, by such Person or one or more of the other Subsidiaries of that Person or a combination thereof or is consolidated under GAAP with such Person at such time; and
(2) any partnership, joint venture, limited liability company or similar entity of which
(a) more than 50.0% of the capital accounts, distribution rights, total equity and voting interests or general or limited partnership interests, as applicable, are owned or controlled, directly or indirectly, by such Person or one or more of the other Subsidiaries of that Person or a combination thereof whether in the form of membership, general, special or limited partnership or otherwise, and
(b) such Person or any Restricted Subsidiary of such Person is a controlling general partner or otherwise controls such entity.
“Total Assets” means the total assets of the Issuer and its Restricted Subsidiaries, determined on a consolidated basis in accordance with GAAP, as shown on the most recent balance sheet of the Issuer or such other Person as may be expressly stated.
“Transactions” means the issuance of the $1,000.0 million in aggregate principal amount of 6.500% Senior Notes due 2020 on August 8, 2012, and the related transactions described under “Summary—Concurrent Transactions” in the prospectus related thereto dated July 25, 2012, the issuance of the $825.0 million in aggregate principal amount of 6.500% Senior Notes due 2020 on October 2, 2012 and the issuance of the $800.0 million in aggregate principal amount of 6.500% Senior Subordinated Notes due 2020 on October 2, 2012 and the related transactions described under “Capitalization” in this offering circular relating to the issuance of the original senior subordinated notes.
“Treasury Rate” means, as of any Redemption Date, the yield to maturity as of such Redemption Date of United States Treasury securities with a constant maturity (as compiled and published in the most recent Federal
246
Reserve Statistical Release H.15 (519) that has become publicly available at least two Business Days prior to the Redemption Date (or, if such Statistical Release is no longer published, any publicly available source of similar market data)) most nearly equal to the period from the Redemption Date to October 1, 2015; provided that if the period from the Redemption Date to such date is less than one year, the weekly average yield on actually traded United States Treasury securities adjusted to a constant maturity of one year will be used.
“Trust Indenture Act” means the Trust Indenture Act of 1939, as amended (15 U.S.C. §§ 77aaa-77bbbb).
“Unrestricted Subsidiary” means:
(1) any Subsidiary of the Issuer which at the time of determination is an Unrestricted Subsidiary (as designated by the Issuer, as provided below); and
(2) any Subsidiary of an Unrestricted Subsidiary.
The Issuer may designate any Subsidiary of the Issuer (including any existing Subsidiary and any newly acquired or newly formed Subsidiary) to be an Unrestricted Subsidiary unless such Subsidiary or any of its Subsidiaries owns any Equity Interests or Indebtedness of, or owns or holds any Lien on, any property of, the Issuer or any Subsidiary of the Issuer (other than solely any Subsidiary of the Subsidiary to be so designated); provided that
(1) any Unrestricted Subsidiary must be an entity of which the Equity Interests entitled to cast at least a majority of the votes that may be cast by all Equity Interests having ordinary voting power for the election of directors or Persons performing a similar function are owned, directly or indirectly, by the Issuer;
(2) such designation complies with the covenants described under “—Certain Covenants—Limitation on Restricted Payments”; and
(3) each of (a) the Subsidiary to be so designated and (b) its Subsidiaries has not at the time of designation, and does not thereafter, create, incur, issue, assume, guarantee or otherwise become directly or indirectly liable with respect to any Indebtedness pursuant to which the lender has recourse to any of the assets of the Issuer or any Restricted Subsidiary.
The Issuer may designate any Unrestricted Subsidiary to be a Restricted Subsidiary; provided that, immediately after giving effect to such designation, no Default shall have occurred and be continuing and either:
(1) the Issuer could incur at least $1.00 of additional Indebtedness pursuant to the Fixed Charge Coverage Test; or
(2) the Fixed Charge Coverage Ratio for the Issuer would be equal to or greater than such ratio for the Issuer immediately prior to such designation, in each case on a pro forma basis taking into account such designation.
Any such designation by the Issuer shall be notified by the Issuer to the Trustee by promptly filing with the Trustee a copy of the resolution of the board of directors of the Issuer or any committee thereof giving effect to such designation and an Officer’s Certificate certifying that such designation complied with the foregoing provisions.
“Voting Stock” of any Person as of any date means the Capital Stock of such Person that is at the time entitled to vote in the election of the board of directors of such Person.
“Weighted Average Life to Maturity” means, when applied to any Indebtedness, Disqualified Stock or Preferred Stock, as the case may be, at any date, the quotient obtained by dividing:
(1) the sum of the products of the number of years from the date of determination to the date of each successive scheduled principal payment of such Indebtedness or redemption or similar payment with respect to such Disqualified Stock or Preferred Stock multiplied by the amount of such payment; by
(2) the sum of all such payments.
247
“Wholly-Owned Subsidiary” of any Person means a Subsidiary of such Person, 100.0% of the outstanding Equity Interests of which (other than directors’ qualifying shares) shall at the time be owned by such Person or by one or more Wholly-Owned Subsidiaries of such Person.
248
FORM, BOOK-ENTRY PROCEDURES AND TRANSFER
General
The original notes were, and the exchange notes will be, issued in fully registered global form. The exchange notes initially will be represented by one or more global certificates without interest coupons (the “global notes”). The global notes will be deposited upon issuance with the trustee as custodian for DTC and registered in the name of DTC or its nominee for credit to the accounts of direct or indirect participants in DTC, as described below under “—Depositary Procedures.”
The global notes will be deposited on behalf of the acquirers of the exchange notes for credit to the respective accounts of the acquirers or to such other accounts as they may direct. Except as described below, the global notes may be transferred, in whole and not in part, only to another nominee of DTC or to a successor of DTC or its nominee. Beneficial interests in the global notes may not be exchanged for exchange notes in certificated form except in the limited circumstances described below under “—Exchange of Book-Entry Notes for Certificated Notes.”
Transfers of beneficial interests in the global notes will be subject to the applicable rules and procedures of DTC and its direct or indirect participants, which may change from time to time.
Depositary Procedures
The following description of the operations and procedures of DTC is provided solely as a matter of convenience. These operations and procedures are solely within the control of the respective settlement systems and are subject to changes by them. We take no responsibility for these operations and procedures and urge investors to contact the systems or their participants directly to discuss these matters.
DTC has advised us that it is:
| • | a limited purpose trust company organized under the New York State Banking Law; |
| • | a “banking organization” within the meaning of the New York State Banking Law; |
| • | a member of the U.S. Federal Reserve System; |
| • | a “clearing corporation” within the meaning of the New York Uniform Commercial Code; and |
| • | a “clearing agency” registered under Section 17A of the Exchange Act. |
DTC was created to hold securities for its participating organizations (collectively, the “participants”) and facilitate the clearance and settlement of transactions in those securities between participants through electronic book-entry changes in accounts of its participants. The participants include securities brokers and dealers, banks, trust companies, clearing corporations and certain other organizations. Access to DTC’s system is also available to other entities such as banks, brokers, dealers and trust companies that clear through or maintain a custodial relationship with a participant, either directly or indirectly (collectively, the “indirect participants”). Persons who are not participants may beneficially own securities held by or on behalf of DTC only through participants or indirect participants. DTC has no knowledge of the identity of beneficial owners of securities held by or on behalf of DTC. DTC’s records reflect only the identity of participants to whose accounts securities are credited. The ownership interests and transfer of ownership interests of each beneficial owner of each security held by or on behalf of DTC are recorded on the records of the participants and indirect participants.
DTC has also advised us that, pursuant to procedures established by DTC, ownership of interests in the global notes will be shown on, and the transfer of ownership of such interest will be effected only through, records maintained by DTC (with respect to the participants) or by the participants and the indirect participants (with respect to other owners of beneficial interests in the global notes).
Investors in the global notes may hold their interests therein directly through DTC if they are participants in such system or indirectly through organizations that are participants or indirect participants in such system. All
249
interests in the global notes will be subject to the procedures and requirements of DTC. The laws of some states require that certain persons take physical delivery of certificates evidencing securities they own. Consequently, the ability to transfer beneficial interests in the global notes to such persons will be limited to that extent. Because DTC can act only on behalf of participants, which in turn act on behalf of indirect participants, the ability of beneficial owners of interests in the global notes to pledge such interests to persons or entities that do not participate in the DTC system, or otherwise take actions in respect of such interests, may be affected by the lack of a physical certificate evidencing such interests.
So long as DTC’s nominee is the registered owner of a global note, that nominee will be considered the sole owner or holder of the notes represented by that global note for all purposes under the indenture. Except as provided below, owners of beneficial interests in a global note:
| • | will not be entitled to have notes represented by the global note registered in their names; |
| • | will not receive or be entitled to receive physical, certificated notes; and |
| • | will not be considered the owners or holders of the notes under the indenture for any purpose, |
including with respect to the giving of any direction, instruction or approval to the trustee under the indenture.
As a result, each investor who owns a beneficial interest in a global note must rely on the procedures of DTC to exercise any rights of a holder of notes under the indenture (and, if the investor is not a participant or an indirect participant in DTC, on the procedures of the DTC participant through which the investor owns its interest).
Payments of principal, premium (if any) and interest with respect to the notes represented by a global note will be made by the trustee to DTC’s nominee as the registered holder of the global note. Neither we nor the trustee will have any responsibility or liability for the payment of amounts to owners of beneficial interests in a global note, for any aspect of the records relating to or payments made on account of those interests by DTC, or for maintaining, supervising or reviewing any records of DTC relating to those interests. Payments by participants and indirect participants in DTC to the owners of beneficial interests in a global note will be governed by standing instructions and customary industry practice and will be the responsibility of those participants or indirect participants and DTC.
Interests in the global notes are expected to be eligible to trade in DTC’s Same-Day Funds Settlement System and secondary market trading activity in such interests will therefore settle in immediately available funds, subject in all cases to the rules and procedures of DTC and its participants.
Subject to compliance with the transfer restrictions applicable to the notes described herein, cross-market transfers between the participants in DTC, on the one hand, and Euroclear or Clearstream participants, on the other hand, will be effected through DTC in accordance with DTC’s rules on behalf of Euroclear or Clearstream, as the case may be, by its respective depositary; however, such cross-market transactions will require delivery of instructions to Euroclear or Clearstream, as the case may be, by the counterparty in such system in accordance with the rules and procedures and within the established deadlines (Brussels time) of such system. Euroclear or Clearstream, as the case may be, will, if the transaction meets its settlement requirements, deliver instructions to its respective depositary to take action to effect final settlement on its behalf by delivering or receiving interests in the relevant global note to DTC, and making or receiving payment in accordance with normal procedures for same-day funds settlement applicable to DTC.
Euroclear participants and Clearstream participants may not deliver instructions directly to the depositories for Euroclear or Clearstream. Transfers between participants in DTC will be effected under DTC’s procedures and will be settled in same-day funds. Transfers between participants in Euroclear or Clearstream will be effected in the ordinary way under the rules and operating procedures of those systems.
DTC, Euroclear and Clearstream have agreed to the above procedures to facilitate transfers of interests in the global notes among participants in their respective settlement systems. However, DTC, Euroclear and Clearstream are not obligated to perform these procedures, and may discontinue or change these procedures at any time. Neither we nor the trustee will have any responsibility for the performance by DTC, Euroclear or Clearstream or their respective participants or indirect participants of their respective obligations under the rules and procedures governing their respective operations.
250
DTC has advised us that it will take any action permitted to be taken by a holder of exchange notes only at the direction of one or more participants to whose account with DTC interests in the global notes are credited and only in respect of such portion of the aggregate principal amount of the exchange notes as to which such participant or participants has or have given such direction.
The information in this section concerning DTC, Euroclear, Clearstream and the book-entry system has been obtained from sources that we believe to be reliable, but we take no responsibility for the accuracy thereof.
Exchange of Book-Entry Notes for Certificated Notes
Notes in physical, certificated form will be issued and delivered to each person that DTC identifies as a beneficial owner of the related notes only if:
| • | DTC notifies us at any time that it is unwilling or unable to continue as depositary for the global notes and a successor depositary is not appointed within 90 days; |
| • | DTC ceases to be registered as a clearing agency under the Exchange Act and a successor depositary is not appointed within 90 days; |
| • | we, at our option, notify the trustee that we elect to cause the issuance of certificated notes; or |
| • | certain other events provided in the indenture should occur, including the occurrence and continuance of an event of default under the indenture followed by a request from holders of the notes as provided in the indenture. |
The indenture permits us to determine at any time and in our sole discretion that notes shall no longer be represented by global securities. DTC has advised us that, under its current practices, it would notify its participants of our request, but will only withdraw beneficial interests from the global security at the request of each DTC participant. We would issue definitive certificates in exchange for any beneficial interests withdrawn.
251
CERTAIN U.S. FEDERAL INCOME TAX CONSIDERATIONS
The following is a summary of certain U.S. federal income tax considerations that may be relevant to persons considering exchanging original notes for exchange notes pursuant to the exchange offer. This summary is based on the Internal Revenue Code of 1986, as amended (the “Code”), administrative pronouncements, judicial decisions and final, temporary and proposed Treasury regulations, in each case as of the date hereof, changes to any of which subsequent to the date of this offering circular may affect the tax consequences described herein, possibly with retroactive effect. This summary is limited to considerations for exchanging holders of original notes that have held the original notes, and will hold the exchange notes, as capital assets (generally, property held for investment), and that acquire exchange notes pursuant to the exchange offer. It does not address tax considerations applicable to investors that may be subject to special tax rules, such as financial institutions, tax-exempt entities, insurance companies, dealers in securities or currencies, traders in securities electing to mark to market, persons that hold or will hold notes as a position in a “straddle” or conversion transaction, or as part of a “synthetic security” or other integrated financial transaction, persons subject to the alternative minimum tax, U.S. expatriates, controlled foreign corporations, passive foreign investment companies, pass-through entities (including partnerships and entities and arrangements classified as partnerships for U.S. federal tax purposes), or U.S. Holders (as defined below) that have a “functional currency” other than the U.S. dollar.
If an entity treated as a partnership for U.S. federal income tax purposes holds notes, the tax treatment of each partner of the partnership generally will depend upon the status of the partner and the activities of the partnership. Prospective holders of exchange notes that are partners in a partnership holding notes should consult their own tax advisors.
As used under this heading “Certain U.S. Federal Income Tax Considerations,” the term “U.S. Holder” means a beneficial owner of an exchange note that is for U.S. federal income tax: (1) an individual who is a citizen or resident of the United States, (2) a U.S. domestic corporation, (3) an estate the income of which is subject to U.S. federal income taxation regardless of its source or (4) a trust if (a) a U.S. court is able to exercise primary supervision over the trust’s administration and one or more “United States persons” (within the meaning of the Code) have the authority to control all of the trust’s substantial decisions, or (b) the trust has a valid election in effect under applicable Treasury regulations to be treated as a “United States person.” As used under this heading “Certain U.S. Federal Income Tax Considerations” the term “Non-U.S. Holder” means a beneficial owner of an exchange note that is an individual, corporation, trust or estate for U.S. federal income tax purposes and is not a U.S. Holder.
This discussion is for general information only and does not consider the effect of any applicable U.S. federal tax laws other than income tax laws (such as U.S. federal estate or gift tax laws) or any foreign, state or local tax laws. Investors should consult their tax advisors in determining the tax consequences to them of holding or exchanging notes, including the application to their particular situation of the United States federal income tax considerations discussed below, as well as the application of other federal tax laws as well as state, local and foreign tax laws.
Tax Consequences to Holders of Participating in the Exchange Offer
An exchange of original notes for exchange notes pursuant to the exchange offers will not be a taxable event for U.S. federal income tax purposes. A U.S. Holder’s initial tax basis in the exchange notes will equal the tax basis in the original notes, and the holding period for the exchange notes will include the holding period for the original notes.
Tax Consequences to U.S. Holders
Payments of Interest
Interest on an exchange note will generally be taxable to a U.S. Holder as ordinary income at the time it is paid or accrued in accordance with the U.S. Holder’s regular method of accounting for U.S. federal income tax purposes.
A portion of the purchase price paid for original notes will include any amounts attributable to interest that accrued prior to the date such original notes were issued (“pre-issuance accrued interest”). We intend to treat such amounts not as part of the purchase price of the notes, but instead effectively as amounts paid to offset an equal amount of the first interest payment made in respect of the notes. Under such treatment, when the first interest
252
payment is made in respect of the notes, any portion of that interest payment attributable to pre-issuance accrued interest will be treated effectively as a nontaxable return of the portion of the purchase price that represents pre-issuance accrued interest to U.S. Holders. References to “interest” throughout the remainder of this discussion should not be read to include any pre-issuance accrued interest or return thereof.
Amortizable Bond Premium
Except as noted below, if a U.S. Holder purchased an original note for an amount (not including any amount paid for pre-issuance accrued interest) that was greater than the principal amount of the original note, the holder will be considered to have purchased the note with amortizable bond premium. With some exceptions, a U.S. Holder may elect to amortize this premium over the remaining term of the exchange note. A U.S. Holder must generally use any amortizable bond premium allocable to an accrual period to offset interest required to be included in income with respect to the exchange note in such accrual period. A U.S. Holder that elects to amortize bond premium with respect to an exchange note must reduce its tax basis in the note by the amount of the premium amortized in any year. An election to amortize bond premium applies to all taxable debt obligations then owned and thereafter acquired by such holder and such election may be revoked only with the consent of the IRS.
Because we may call the exchange notes under certain circumstances at a price in excess of their principal amount, the deduction for amortizable bond premium may be reduced or delayed.
U.S. Holders should consult their own tax advisors regarding the availability of the deduction for amortizable bond premium.
Market Discount
If a U.S. Holder purchased an original note at a price (not including any amount paid for pre-issuance accrued interest) that is lower than its remaining redemption amount, by at least 0.25% of its remaining redemption amount multiplied by the number of remaining whole years to maturity, the U.S. Holder’s exchange note will be considered to have “market discount” in the hands of such U.S. Holder. In such case, gain realized by the U.S. Holder on the disposition of the exchange note generally will be treated as ordinary income to the extent of the market discount that accrued on the note while held by such U.S. Holder. In addition, the U.S. Holder could be required to defer the deduction of a portion of the interest paid on any indebtedness incurred or maintained to purchase or carry the note. In general terms, market discount on a note will be treated as accruing ratably over the term of such note, or, at the election of the holder, under a constant-yield method.
A U.S. Holder may elect to include market discount in income on a current basis as it accrues (on either a ratable or constant-yield basis), in lieu of treating a portion of any gain realized on a sale of an exchange note as ordinary income. If a U.S. Holder elects to include market discount on a current basis, the interest deduction deferral rule described above will not apply. Any such election, if made, applies to all market discount bonds acquired by the taxpayer on or after the first day of the first taxable year to which such election applies and is revocable only with the consent of the IRS.
Sale, Exchange, Redemption and Retirement
Upon the sale, exchange (not including an exchange pursuant to these exchange offers), redemption or retirement of an exchange note, a U.S. Holder generally will recognize gain or loss equal to the difference between the amount realized on the sale, exchange, redemption or retirement (not including amounts attributable to (i) accrued and unpaid interest, which will be taxable as interest income to the extent not previously included in income by the U.S. Holder and (ii) if the sale, exchange or redemption occurs prior to the date the first interest payment is made in respect of the notes, any pre-issuance accrued interest) and the U.S. Holder’s tax basis in such note. As discussed above, a U.S. Holder’s initial tax basis in the exchange notes will equal the tax basis in the original notes. A U.S. Holder’s tax basis in an original note generally will equal the cost of such note to such holder (other than to the extent attributable to any pre-issuance accrued interest), reduced by any bond premium previously amortized by such U.S. Holder and increased by any market discount previously included in income by such U.S. Holder. Such gain or loss recognized by a U.S. Holder generally will constitute long-term capital gain or loss if the U.S. Holder’s holding period for the exchange note (including the holding period for the original note) is more than one year at the time of disposition. Under current law, for certain non-corporate U.S. Holders (including individuals, estates and trusts), net long-term capital gain will be subject to tax at preferential rates. The ability of a U.S. Holder to deduct capital losses may be limited.
253
Tax Consequences to Non-U.S. Holders
Payments of Interest
Under U.S. federal income tax law, and subject to the discussion below concerning backup withholding, no withholding of U.S. federal income tax generally will be required with respect to the payment by us or our paying agent on an exchange note owned by a Non-U.S. Holder of interest that qualifies as portfolio interest. Interest on an exchange note owned by a Non-U.S. Holder will qualify as portfolio interest, provided that (1) such interest is not effectively connected with the conduct of such Non-U.S. Holder’s U.S. trade or business, (2) such Non-U.S. Holder does not actually or constructively own 10% or more of the total combined voting power of all classes of our stock entitled to vote, (3) such Non-U.S. Holder is not a controlled foreign corporation that is related to us actually or constructively through stock ownership, and (4) such Non-U.S. Holder provides a properly completed IRS Form W-8BEN or other applicable IRS Form W-8, signed under penalties of perjury, establishing its status as a Non-U.S. Holder (or satisfies certain documentary evidence requirements for establishing that is it a Non-U.S. Holder).
A Non-U.S. Holder with interest income that does not qualify as portfolio interest will be subject to a 30% U.S. federal withholding tax on payments of interest (or a lower applicable treaty rate, but only if applicable certification and other requirements to claim treaty benefits have been complied with). In the event that tax is withheld solely as a result of a failure to provide certification, a refund claim may generally be filed with the IRS.
Sale, Exchange, Redemption and Retirement
A Non-U.S. Holder will generally not be subject to U.S. federal income tax on any gain realized on the sale, exchange, redemption or retirement of an exchange note unless (1) such gain is effectively connected with the conduct by the Non-U.S. Holder of a trade or business in the United States, which will be taxed as described below or (2) the Non-U.S. Holder is an individual present in the United States for 183 days or more in the taxable year of the sale and certain other conditions are met, in which case such gain (net of certain U.S. source losses) will be taxed at a rate of 30% unless an applicable income tax treaty provides otherwise. As discussed above, a Non-U.S. Holder will not be subject to U.S. federal income tax on an exchange of original notes for exchange notes pursuant to the exchange offer.
Effectively Connected Income
A Non-U.S. Holder generally will be taxed in the same manner as a U.S. Holder with respect to interest income or gain that is effectively connected with its U.S. trade or business and, if required by an applicable income tax treaty, that is attributable to its U.S. “permanent establishment.” In addition, under certain circumstances, effectively connected earnings and profits of a corporate Non-U.S. Holder may be subject to a “branch profits” tax imposed at a 30% rate or at such lower rate as may be specified by an applicable income tax treaty.
Information Reporting and Backup Withholding
The applicable paying agent will generally be required to file information returns with the IRS with respect to payments of interest on, and proceeds from the sale, exchange (not including an exchange pursuant to this exchange offer), redemption or retirement of the exchange notes, made to certain U.S. Holders that are not corporations and cannot otherwise establish an exemption. In addition, such U.S. Holders may be subject to backup withholding tax in respect of such payments if they do not provide their taxpayer identification numbers to the applicable paying agent. Non-U.S. Holders may be required to comply with applicable certification procedures to establish their non-U.S. status in order to avoid the application of such information reporting requirements and backup withholding tax. Backup withholding tax is not an additional tax. Any amounts withheld under the backup withholding rules from a payment to a holder generally will be allowed as a refund or a credit against such holder’s U.S. federal income tax liability, provided that the required procedures are timely followed.
254
PLAN OF DISTRIBUTION
Each broker-dealer that receives exchange notes for its own account pursuant to the exchange offers must acknowledge that it will deliver a prospectus in connection with any resale of the exchange notes. This prospectus, as it may be amended or supplemented from time to time, may be used by a broker-dealer in connection with resales of exchange notes received in exchange for outstanding notes where the outstanding notes are acquired as a result of market-making activities or other trading activities. To the extent any such broker-dealer participates in the exchange offers, we have agreed that for a period of up to 90 days, we will use our commercially reasonable efforts to make this prospectus, as amended or supplemented, available to such broker-dealer for use in connection with any such resale, and will deliver as many additional copies of this prospectus and each amendment or supplement to this prospectus as such broker-dealer may reasonably request.
We will not receive any proceeds from any sale of exchange notes by broker-dealers. Exchange notes received by broker-dealers for their own accounts pursuant to the exchange offers may be sold from time to time in one or more transactions in the over-the-counter market, in negotiated transactions, through the writing of options on the exchange notes or a combination of these methods of resale, at market prices prevailing at the time of resale, at prices related to the prevailing market prices or negotiated prices. Any resale may be made directly to purchasers or to through brokers or dealers who may receive compensation in the form of commissions or concessions from any broker-dealer or the purchasers of any exchange notes. Any broker-dealer that resells exchange notes that were received by it for its own account pursuant to the exchange offers and any broker or dealer that participates in a distribution of the exchange notes may be deemed to be an “underwriter” within the meaning of the Securities Act and any profit on any resale of exchange notes and any commissions or concessions received by these persons may be deemed to be underwriting compensation under the Securities Act. The letter of transmittal states that by acknowledging that it will deliver and by delivering a prospectus, a broker-dealer will not be deemed to admit that it is an “underwriter” within the meaning of the Securities Act.
So long as required pursuant to the terms of the registration rights agreements to make this prospectus available to broker-deals for use in connection with such resales, we will promptly send additional copies of this prospectus and any amendment or supplement to this prospectus to any broker-dealer that requests such documents in the letter of transmittal. We have agreed to pay all expenses incident to the exchange offers and will indemnify the holders of outstanding notes, including any broker-dealers, against certain liabilities, including liabilities under the Securities Act.
255
LEGAL MATTERS
The validity of the exchange notes and the related guarantees offered hereby will be passed upon for us by Cleary Gottlieb Steen & Hamilton LLP, New York, New York. Ice Miller LLP have passed upon certain matters governed by the laws of the state of Indiana, Gunster, Yoakley & Stewart, P.A. have passed upon certain matters governed by the laws of the state of Florida, and Reed Smith LLP have passed upon certain matters governed by the laws of the state of California.
EXPERTS
The consolidated financial statements as of May 31, 2012 and 2011 and for each of the three years in the period ended May 31, 2012 and the related financial statement schedule of valuation and qualifying accounts included in the Prospectus, which is part of this Registration Statement, have been audited by Deloitte & Touche LLP, an independent registered public accounting firm, as stated in their report appearing herein. Such consolidated financial statements and financial statement schedule have been so included in reliance upon the report of such firm given upon their authority as experts in accounting and auditing.
256
INDEX TO FINANCIAL STATEMENTS
| Index | Page Number |
| I. Audited Consolidated Financial Statements for the Three Years Ended May 31, 2012: | |
| Report of Independent Registered Public Accounting Firm | |
| Biomet, Inc. and Subsidiaries Consolidated Balance Sheets as of May 31, 2012 and 2011 | |
| Biomet, Inc. and Subsidiaries Consolidated Statements of Operations and Comprehensive Loss for the years ended May 31, 2012, 2011 and 2010 | |
| Biomet, Inc. and Subsidiaries Consolidated Statements of Shareholder’s Equity for the years ended May 31, 2012, 2011 and 2010 | |
| Biomet, Inc. and Subsidiaries Consolidated Statements of Cash Flows for the years ended May 31, 2012, 2011, and 2010 | |
| Notes to Consolidated Financial Statements | |
| Financial Statement Schedules: | |
| Schedule II—Valuation and Qualifying Accounts | |
| Quarterly Results (Unaudited) | |
| II. Unaudited Condensed Consolidated Financial Statements for the Nine Months Ended February 28, 2013 | |
| Biomet, Inc. and Subsidiaries Condensed Consolidated Balance Sheets as of February 28, 2013 and May 31, 2012. | |
| Biomet, Inc. and Subsidiaries Condensed Consolidated Statements of Operations and Comprehensive Loss for the Nine Months Ended February 28, 2013 and February 29, 2012. | |
| Biomet, Inc. and Subsidiaries Condensed Consolidated Statements of Cash Flows for the Nine Months Ended February 28, 2013 and February 29, 2012. | |
| Notes to Condensed Consolidated Financial Statements (Unaudited) | |
Schedules other than those listed above are omitted because they are not applicable or the required information is shown in the financial statements or notes thereto.
F-1
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Shareholder of Biomet, Inc.
Warsaw, Indiana
We have audited the accompanying consolidated balance sheets of Biomet, Inc. and subsidiaries (the “Company”) as of May 31, 2012 and 2011, and the related consolidated statements of operations and comprehensive loss, shareholder's equity, and cash flows for each of the three years in the period ended May 31, 2012. Our audit also included the financial statement schedule of valuation and qualifying accounts listed in the Index to Financial Statements. These financial statements and financial statement schedule are the responsibility of the Company's management. Our responsibility is to express an opinion on the financial statements and financial statement schedule based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of Biomet, Inc. and subsidiaries as of May 31, 2012 and 2011, and the results of their operations and their cash flows for each of the three years in the period ended May 31, 2012, in conformity with accounting principles generally accepted in the United States of America. Also, in our opinion, such financial statement schedule, when considered in relation to the basic consolidated financial statements taken as a whole, presents fairly in all material respects the information set forth therein.
/s/ DELOITTE & TOUCHE LLP
Indianapolis, Indiana
August 20, 2012
F-2
Biomet, Inc. and Subsidiaries Consolidated Balance Sheets
(in millions, except shares)
| May 31, 2012 | May 31, 2011 | ||||||
| Assets | |||||||
| Current assets: | |||||||
| Cash and cash equivalents | $ | 492.4 | $ | 327.8 | |||
| Accounts receivable, less allowance for doubtful accounts receivables of $36.5 ($38.2 at May 31, 2011) | 491.6 | 480.1 | |||||
| Investments | 2.5 | 41.4 | |||||
| Income tax receivable | 5.0 | 5.4 | |||||
| Inventories | 543.2 | 582.5 | |||||
| Deferred income taxes | 52.5 | 71.5 | |||||
| Prepaid expenses and other | 124.1 | 109.7 | |||||
| Total current assets | 1,711.3 | 1,618.4 | |||||
| Property, plant and equipment, net | 593.6 | 638.4 | |||||
| Investments | 13.9 | 33.1 | |||||
| Intangible assets, net | 3,930.4 | 4,534.4 | |||||
| Goodwill | 4,114.4 | 4,470.1 | |||||
| Other assets | 56.8 | 62.6 | |||||
| Total assets | $ | 10,420.4 | $ | 11,357.0 | |||
| Liabilities & Shareholder’s Equity | |||||||
| Current liabilities: | |||||||
| Current portion of long-term debt | $ | 35.6 | $ | 37.4 | |||
| Accounts payable | 116.2 | 91.1 | |||||
| Accrued interest | 56.5 | 64.1 | |||||
| Accrued wages and commissions | 122.0 | 105.0 | |||||
| Other accrued expenses | 180.2 | 241.8 | |||||
| Total current liabilities | 510.5 | 539.4 | |||||
| Long-term liabilities: | |||||||
| Long-term debt, net of current portion | 5,792.2 | 5,982.9 | |||||
| Deferred income taxes | 1,257.8 | 1,487.6 | |||||
| Other long-term liabilities | 177.8 | 172.0 | |||||
| Total liabilities | 7,738.3 | 8,181.9 | |||||
| Commitments and contingencies | |||||||
| Shareholder’s equity: | |||||||
| Common stock, par value $0.00 per share; 1,000 shares authorized; 1,000 shares issued and outstanding | — | — | |||||
| Contributed and additional paid-in capital | 5,628.8 | 5,614.1 | |||||
| Accumulated deficit | (3,069.6) | (2,610.8) | |||||
| Accumulated other comprehensive income | 122.9 | 171.8 | |||||
| Total shareholder’s equity | 2,682.1 | 3,175.1 | |||||
| Total liabilities and shareholder’s equity | $ | 10,420.4 | $ | 11,357.0 | |||
The accompanying notes are an integral part of the consolidated financial statements.
F-3
Biomet, Inc. and Subsidiaries Consolidated Statements of Operations and Comprehensive Loss.
(in millions)
| For the Year Ended May 31, | |||||||||||
| 2012 | 2011 | 2010 | |||||||||
| Net sales | $ | 2,838.1 | $ | 2,732.2 | $ | 2,698.0 | |||||
| Cost of sales | 894.4 | 838.7 | 819.9 | ||||||||
| Gross profit | 1,943.7 | 1,893.5 | 1,878.1 | ||||||||
| Selling, general and administrative expense | 1,053.3 | 1,041.7 | 1,042.3 | ||||||||
| Research and development expense | 126.8 | 119.4 | 106.6 | ||||||||
| Amortization | 327.2 | 367.9 | 372.6 | ||||||||
| Goodwill and intangible assets impairment charge | 529.8 | 941.4 | — | ||||||||
| Operating income (loss) | (93.4) | (576.9) | 356.6 | ||||||||
| Interest expense | 479.8 | 498.9 | 516.4 | ||||||||
| Other (income) expense | 17.6 | (11.2) | (18.1) | ||||||||
| Other expense, net | 497.4 | 487.7 | 498.3 | ||||||||
| Loss before income taxes | (590.8) | (1,064.6) | (141.7) | ||||||||
| Benefit from income taxes | (132.0) | (214.8) | (94.1) | ||||||||
| Net loss | (458.8) | (849.8) | (47.6) | ||||||||
| Other comprehensive income (loss), net of tax: | |||||||||||
| Change in unrealized holding value on available for sale securities | 4.3 | (6.0) | 1.8 | ||||||||
| Interest rate swap unrealized gain | 13.1 | 19.5 | 11.3 | ||||||||
| Foreign currency related gains (losses) | (62.1) | 264.4 | (96.5) | ||||||||
| Unrecognized actuarial gain (loss) on pension assets | (4.2) | 4.5 | 3.5 | ||||||||
| Net loss | (48.9) | 282.4 | (79.9) | ||||||||
| Comprehensive loss | $ | (507.7 | ) | $ | (567.4 | ) | $ | (127.5 | ) | ||
The accompanying notes are an integral part of the consolidated financial statements.
F-4
Biomet, Inc. and Subsidiaries Consolidated Statements of Shareholder’s Equity.
(in millions, except for the share data)
| Common Shares | Contributed and Additional Paid-in Capital | Accumulated Deficit | Accumulated Other Comprehensive Income (Loss) | Total Shareholder’s Equity | |||||||||||||
| Balance at May 31, 2009 | 1,000 | $ | 5,584.4 | $ | (1,713.4 | ) | $ | (30.7 | ) | $ | 3,840.3 | ||||||
| Net loss | — | (47.6) | — | (47.6) | |||||||||||||
| Change in unrealized holding value on available for sale securities, net of $1.3 tax effect | — | — | 1.8 | 1.8 | |||||||||||||
| Interest rate swap unrealized gain, net of $7.2 tax effect | — | — | 11.3 | 11.3 | |||||||||||||
| Foreign currency related losses | — | — | (96.5) | (96.5) | |||||||||||||
| Unrecognized actuarial gain on pension assets, net of $2.9 tax effect | — | — | 3.5 | 3.5 | |||||||||||||
| Comprehensive loss | — | — | — | (127.5) | |||||||||||||
| Stock-based compensation expense | 22.4 | — | — | 22.4 | |||||||||||||
| Repurchase of LVB Acquisition, Inc. shares | (1.7) | — | — | (1.7) | |||||||||||||
| Balance at May 31, 2010 | 1,000 | 5,605.1 | (1,761.0) | (110.6) | 3,733.5 | ||||||||||||
| Net loss | — | (849.8) | — | (849.8) | |||||||||||||
| Change in unrealized holding value on available for sale securities, net of ($0.9) tax effect | — | — | (6.0) | (6.0) | |||||||||||||
| Interest rate swap unrealized gain, net of $13.6 tax effect | — | — | 19.5 | 19.5 | |||||||||||||
| Foreign currency related gains | — | — | 264.4 | 264.4 | |||||||||||||
| Unrecognized actuarial gain on pension assets, net of $0.2 tax effect | — | — | 4.5 | 4.5 | |||||||||||||
| Comprehensive loss | (567.4) | ||||||||||||||||
| Stock-based compensation expense | 12.7 | — | — | 12.7 | |||||||||||||
| Repurchase of LVB Acquisition, Inc. shares | (3.7) | — | — | (3.7) | |||||||||||||
| Balance at May 31, 2011 | 1,000 | 5,614.1 | (2,610.8) | 171.8 | 3,175.1 | ||||||||||||
| Net loss | — | (458.8) | — | (458.8) | |||||||||||||
| Change in unrealized holding value on available for sale securities | — | — | 4.3 | 4.3 | |||||||||||||
| Interest rate swap unrealized gain, net of $7.8 tax effect | — | — | 13.1 | 13.1 | |||||||||||||
| Foreign currency related losses | — | — | (62.1) | (62.1) | |||||||||||||
| Unrecognized actuarial loss on pension assets, net of $0.8 tax effect | — | — | (4.2) | (4.2) | |||||||||||||
| Comprehensive loss | (507.7) | ||||||||||||||||
| Stock-based compensation expense | 16.0 | — | — | 16.0 | |||||||||||||
| Repurchase of LVB Acquisition, Inc. shares | (1.3) | — | — | (1.3) | |||||||||||||
| Balance at May 31, 2012 | 1,000 | $ | 5,628.8 | $ | (3,069.6 | ) | $ | 122.9 | $ | 2,682.1 | |||||||
The accompanying notes are an integral part of the consolidated financial statements.
F-5
Biomet, Inc. and Subsidiaries Consolidated Statements of Cash Flows.
(in millions)
| For the Year Ended May 31, | |||||||||||
| 2012 | 2011(1) | 2010(1) | |||||||||
| Cash flows provided by (used in) operating activities: | |||||||||||
| Net loss | $ | (458.8 | ) | $ | (849.8 | ) | $ | (47.6 | ) | ||
| Adjustments to reconcile net loss to net cash provided by operating activities: | |||||||||||
| Depreciation and amortization | 509.4 | 549.0 | 547.6 | ||||||||
| Amortization of deferred financing costs | 11.1 | 11.2 | 11.3 | ||||||||
| Stock-based compensation expense | 16.0 | 12.7 | 22.4 | ||||||||
| Recovery of doubtful accounts receivable | (5.3 | ) | (6.2 | ) | (7.0 | ) | |||||
| Realized gain on investments | (2.0 | ) | (4.9 | ) | (4.3 | ) | |||||
| Loss on impairment of investments | 20.1 | — | — | ||||||||
| Goodwill and intangible assets impairment charge | 529.8 | 941.4 | — | ||||||||
| Property, plant and equipment impairment charge | 0.4 | 17.0 | 7.8 | ||||||||
| Deferred income taxes | (204.3 | ) | (271.3 | ) | (120.1 | ) | |||||
| Loss on extinguishment of debt | — | 1.2 | — | ||||||||
| Other | (4.5 | ) | (28.0 | ) | 2.7 | ||||||
| Changes in operating assets and liabilities: | |||||||||||
| Accounts receivable | (36.6 | ) | 14.5 | (5.6 | ) | ||||||
| Inventories | 13.4 | (43.9 | ) | (29.4 | ) | ||||||
| Prepaid expenses | (12.3 | ) | (4.5 | ) | 6.2 | ||||||
| Accounts payable | 28.9 | (0.8 | ) | (9.5 | ) | ||||||
| Income taxes | (29.0 | ) | 46.0 | 9.0 | |||||||
| Accrued interest | (7.6 | ) | (6.1 | ) | (2.9 | ) | |||||
| Accrued expenses and other | 8.6 | 2.6 | (59.1 | ) | |||||||
| Net cash provided by operating activities | 377.3 | 380.1 | 321.5 | ||||||||
| Cash flows provided by (used in) investing activities: | |||||||||||
| Proceeds from sales/maturities of investments | 42.1 | 59.3 | 24.9 | ||||||||
| Purchases of investments | (0.4 | ) | (78.7 | ) | (13.3 | ) | |||||
| Net proceeds from sale of property and equipment | 14.7 | 6.8 | 3.0 | ||||||||
| Capital expenditures | (179.3 | ) | (174.0 | ) | (186.4 | ) | |||||
| Acquisitions, net of cash acquired | (21.1 | ) | (18.4 | ) | (10.2 | ) | |||||
| Net cash used in investing activities | (144.0 | ) | (205.0 | ) | (182.0 | ) | |||||
| Cash flows provided by (used in) financing activities: | |||||||||||
| Debt: | |||||||||||
| Proceeds under European facilities | — | 0.3 | — | ||||||||
| Payments under European facilities | (1.4 | ) | (2.0 | ) | — | ||||||
| Proceeds under revolving credit agreements | — | — | 20.4 | ||||||||
| Payments under revolving credit agreements | — | — | (134.1 | ) | |||||||
| Payments under senior secured credit facilities | (35.4 | ) | (34.8 | ) | (35.8 | ) | |||||
| Repurchases of senior notes | — | (11.2 | ) | (8.7 | ) | ||||||
| Equity: | |||||||||||
| Repurchase of LVB Acquisition, Inc. shares | (1.3 | ) | (3.7 | ) | (1.7 | ) | |||||
| Net cash used in financing activities | (38.1 | ) | (51.4 | ) | (159.9 | ) | |||||
| Effect of exchange rate changes on cash | (30.6 | ) | 15.0 | (6.1 | ) | ||||||
| Increase (decrease) in cash and cash equivalents | 164.6 | 138.7 | (26.5 | ) | |||||||
F-6
| Cash and cash equivalents, beginning of period | 327.8 | 189.1 | 215.6 | ||||||||
| Cash and cash equivalents, end of period | $ | 492.4 | $ | 327.8 | $ | 189.1 | |||||
| Supplemental disclosures of cash flow information: | |||||||||||
| Cash paid during the period for: | |||||||||||
| Interest | $ | 477.1 | $ | 494.1 | $ | 508.6 | |||||
| Income taxes | $ | 95.0 | $ | 42.3 | $ | 29.3 | |||||
| (1) | Certain amounts have been adjusted to conform to the current presentation. |
The accompanying notes are an integral part of the consolidated financial statements.
F-7
Note 1—Summary of Significant Accounting Policies and Nature of Operations.
The accompanying consolidated financial statements include the accounts of Biomet, Inc. and its subsidiaries (individually and collectively with its subsidiaries referred to as “Biomet”, the “Company”, “we”, “us”, or “our”). Biomet is a wholly-owned subsidiary of LVB Acquisition, Inc. (“LVB” or “Parent”). LVB has no other operations beyond its ownership of Biomet. Intercompany accounts and transactions have been eliminated in consolidation.
Transactions with the Sponsor Group
On December 18, 2006, Biomet, Inc. entered into an Agreement and Plan of Merger with LVB Acquisition, LLC, a Delaware limited liability company, which was subsequently converted to a corporation, LVB Acquisition, Inc., and LVB Acquisition Merger Sub, Inc., an Indiana corporation and a wholly-owned subsidiary of Parent (“Purchaser”), which agreement was amended and restated as of June 7, 2007 and which we refer to as the “Merger Agreement.” Pursuant to the Merger Agreement, on June 13, 2007, Purchaser commenced a cash tender offer (the “Offer”) to purchase all of Biomet, Inc.’s outstanding common shares, without par value (the “Shares”) at a price of $46.00 per Share (the “Offer Price”) without interest and less any required withholding taxes. The Offer was made pursuant to Purchaser’s offer to purchase dated June 13, 2007 and the related letter of transmittal, each of which was filed with the SEC on June 13, 2007. In connection with the Offer, Purchaser entered into a credit agreement dated as of July 11, 2007 for a $6,165.0 million senior secured term loan facility (the “Tender Facility”), maturing on June 6, 2008, and pursuant to which it borrowed approximately $4,181.0 million to finance a portion of the Offer and pay related fees and expenses. The Offer expired at midnight, New York City time, on July 11, 2007, with approximately 82% of the outstanding Shares having been tendered to Purchaser. At Biomet, Inc.’s special meeting of shareholders held on September 5, 2007, more than 91% of Biomet, Inc.’s shareholders voted to approve the proposed merger, and Parent acquired Biomet, Inc. on September 25, 2007 through a reverse subsidiary merger with Biomet, Inc. being the surviving company (the “Merger”). Subsequent to the acquisition, Biomet, Inc. became a subsidiary of Parent, which is controlled by LVB Acquisition Holding, LLC, or “Holding”, an entity controlled by a consortium of private equity funds affiliated with The Blackstone Group, Goldman, Sachs & Co., Kohlberg Kravis Roberts & Co., and TPG Global, LLC (each a “Sponsor” and collectively, the “Sponsors”), and certain investors who agreed to co-invest with the Sponsors (the “Co-Investors”). These transactions, including the Merger and the Company’s payment of any fees and expenses related to these transactions, are referred to collectively as the “Transactions.”
General—Biomet, Inc. is the wholly owned subsidiary of LVB. LVB has no other operations beyond its ownership of Biomet. The Company is one of the largest orthopedic medical device companies in the United States and worldwide with operations in over 50 locations throughout the world and distribution in approximately 90 countries. The Company designs, manufactures and markets a comprehensive range of both surgical and non-surgical products used primarily by orthopedic surgeons and other musculoskeletal medical specialists. For over 30 years, the Company has applied advanced engineering and manufacturing technology to the development of highly durable joint replacement systems.
Basis of Presentation—The accompanying consolidated financial statements include the accounts of Biomet and its subsidiaries (individually and collectively referred to as “Biomet” or the “Company”). The consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America.
Products—The Company operates in one reportable business segment, musculoskeletal products, which includes the design, manufacture and marketing of products in five major categories: Large Joint Reconstructive, Sports, Extremities, Trauma (“S.E.T.”), Spine & Bone Healing, Dental and Other Products. The Company has three geographic markets: United States, Europe and International.
Large Joint Reconstructive—Orthopedic reconstructive implants are used to replace joints that have deteriorated as a result of disease (principally osteoarthritis) or injury. Reconstructive joint surgery involves the modification of the area surrounding the affected joint and the implantation of one or more manufactured components, and may involve the use of bone cement. The Company’s large orthopedic reconstructive joints are knees and hips. The Company also produces bone cements and cement delivery systems.
F-8
S.E.T.—The Company manufactures and distributes a number of sports medicine products (used in minimally-invasive orthopedic surgical procedures). Extremity reconstructive implants are used to replace joints other than hips and knees that have deteriorated as a result of disease or injury. The Company’s key reconstructive joint in this product category is the shoulder, but it produces other joints as well. Trauma devices are used for setting and stabilizing bone fractures to support and/or augment the body’s natural healing process. Trauma products include internal fixation devices (such as nails, plates, screws, pins and wires designed to stabilize traumatic bone injuries) and external fixation devices (utilized to stabilize fractures when alternative methods of fixation are not suitable).
Spine & Bone Healing—The Company’s spine products include spinal fixation systems for cervical, thoracolumbar, deformity correction and spacer applications; implantable and non-invasive electrical stimulation devices for spinal applications; and osteobiologics, including bone substitute materials, as well as allograft services for spinal applications. Bone healing products include electrical stimulation devices used for trauma indications, offering implantable and non-invasive options to stimulate bone growth, as well as orthopedic support products (also referred to as bracing products).
Dental—Dental reconstructive devices and associated instrumentation are used for oral rehabilitation through the replacement of teeth and repair of hard and soft tissues. The Company also offers crown and bridge products.
Other—The Company manufactures and distributes a number of other products, including microfixation products, autologous therapies, operating room supplies, casting materials, general surgical instruments, wound care products and other surgical products.
Effect of Foreign Currency—Assets and liabilities of foreign subsidiaries are translated at rates of exchange in effect at the close of their calendar month end. Revenues and expenses are translated at the average exchange rates during the period. Translation gains and losses are accumulated within accumulated other comprehensive income (loss) as a separate component of shareholders’ equity. Foreign currency transaction gains and losses are included in other (income) expense.
Cash and Cash Equivalents—The Company considers all investments that are highly liquid at the date acquired and have original maturities of three months or less to be cash equivalents.
Investments—The Company invests the majority of its excess cash in money market funds. The Company also holds Greek bonds, time deposits, corporate securities, and common stocks. The Company accounts for its investments in equity securities in accordance with guidance issued by the Financial Accounting Standards Board (“FASB”), which requires certain securities to be categorized as trading, available-for-sale or held-to-maturity. The Company also accounts for its investments under guidance for fair value measurements, which establishes a framework for measuring fair value, clarifies the definition of fair value within that framework, and expands disclosures about fair value measurements. Available-for-sale securities are carried at fair value with unrealized gains and losses, net of tax, recorded within accumulated other comprehensive income (loss) as a separate component of shareholders’ equity. The Company has no held-to-maturity investments. Trading securities are carried at fair value with the realized gains and losses, recorded within other (income) expense. The cost of investment securities sold is determined by the specific identification method. Dividend and interest income are accrued as earned. The Company reviews its investments quarterly for declines in fair value that are other-than-temporary. Investments that have declined in market value that are determined to be other-than-temporary are charged to other (income) expense, by writing that investment down to fair value. Investments are classified as short-term for those expected to mature or be sold within twelve months and the remaining portion is classified in long-term investments.
Interest Rate Instruments—The Company uses interest rate swap agreements (cash flow hedges) in both U.S. dollars and euros as a means of fixing the interest rate on portions of its floating-rate debt instruments. As of May 31, 2012, the Company had swap liabilities of $76.2 million, which consisted of $36.0 million short-term, and $41.0 million long-term, partially offset by a $0.8 million credit valuation adjustment. As of May 31, 2011, the Company had swap liabilities of $96.8 million, which consisted of $62.6 million short-term, and $34.8 million long-term, partially offset by a $0.6 million credit valuation adjustment.
Other Comprehensive Income (Loss)—Other comprehensive income (loss) includes net loss, currency translation adjustments, certain derivative-related activity, changes in the value of available-for-sale investments, and changes in prior service cost from pension plans. The Company generally deems its foreign investments to be
F-9
permanent in nature and does not provide for taxes on currency translation adjustments arising from translating the investment in a foreign currency to U.S. dollars. When the Company determines that a foreign investment is no longer permanent in nature, estimated taxes are provided for the related deferred tax liability (asset), if any, resulting from currency translation adjustments. As of May 31, 2012, foreign investments were all permanent in nature.
Concentrations of Credit Risk and Allowance for Doubtful Receivables—The Company provides credit, in the normal course of business, to hospitals, private and governmental institutions and healthcare agencies, insurance providers, dental practices and laboratories, and physicians. The Company maintains an allowance for doubtful receivables based on estimated collection rates and charges actual losses to the allowance when incurred. The determination of estimated collection rates requires management judgment.
Other Loss Contingencies—In accordance with guidance issued by the FASB for contingencies, the Company accrues anticipated costs of settlement, damages, and loss of product liability claims based on historical experience or to the extent specific losses are probable and estimable. If the estimate of a probable loss is in a range and no amount within the range is more likely, the Company accrues the minimum amount of the range. Such estimates and any subsequent changes in estimates may result in adjustments to the Company’s operating results in the future. The Company has self-insured reserves against product liability claims with insurance coverage above the retention limits. There are various other claims, lawsuits and disputes with third parties, investigations and pending actions involving various allegations against it. Product liability claims are routinely reviewed by the Company’s insurance carriers and management routinely reviews all claims for purposes of establishing ultimate loss estimates.
Revenue Recognition—The Company sells product through four principal channels: (1) direct to healthcare institutions, referred to as direct channel accounts, (2) through stocking distributors and healthcare dealers, (3) indirectly through insurance companies and (4) directly to dental practices and dental laboratories. Sales through the direct and distributor/dealer channels account for a majority of net sales. Through these channels, inventory is consigned to sales agents or customers so that products are available when needed for surgical procedures. Revenue is not recognized upon the placement of inventory into consignment as the Company retains title and maintains the inventory on the balance sheet; rather, it is recognized upon implantation and receipt of proper purchase order and/or purchase requisition documentation. Pricing for products is predetermined by contracts with customers, agents acting on behalf of customer groups or by government regulatory bodies, depending on the market. Price discounts under group purchasing contracts are linked to volume of implant purchases by customer healthcare institutions within a specified group. At negotiated thresholds within a contract buying period, price discounts may increase.
At certain locations, the Company records a contractual allowance that is offset against revenue for each sale to a non-contracted payor so that revenue is recorded at the estimated determinable price at the time of the sale. Those non-contracted payors and insurance companies in some cases do not have contracted rates for products sold, but may have pricing available for certain products through their respective web sites. The Company will invoice at its list price and establish the contractual allowance to estimate what the non-contracted payor will settle the claim for based on the information available as noted above. At certain locations, revenue is recognized on sales to stocking distributors, healthcare dealers, dental practices and dental laboratories when title to product passes to them, generally upon shipment. Certain subsidiaries allow customers to return product in the event that the Company terminates the relationship. Under those circumstances, the Company records an estimated sales return in the period in which constructive notice of termination is given to a distributor. Product returns were not significant for any period presented.
The Company also maintains a separate allowance for doubtful accounts for estimated losses based on its assessment of the collectability of specific customer accounts and the aging of the accounts receivable. The Company analyzes accounts receivable and historical bad debts, customer concentrations, customer solvency, current economic and geographic trends, and changes in customer payment terms and practices when evaluating the adequacy of its current and future allowance. In circumstances where the Company is aware of a specific customer’s inability to meet its financial obligations, a specific allowance for bad debt is estimated and recorded, which reduces the recognized receivable to the estimated amount the Company believes will ultimately be collected. The Company monitors and analyzes the accuracy of the allowance for doubtful accounts estimate by reviewing past collectability and adjusts it for future expectations to determine the adequacy of the Company’s current and future allowance. The Company’s reserve levels have generally been sufficient to cover credit losses.
Excess and Obsolete Inventory—In the Company’s industry, inventory is routinely placed at hospitals to provide the healthcare provider with the appropriate product when needed. Because product usage tends to follow a bell curve, larger and smaller sizes of inventory are provided, but infrequently used. In addition, the
F-10
musculoskeletal market is highly competitive, with new products, raw materials and procedures being introduced continually, which may make those products currently on the market obsolete. The Company makes estimates regarding the future use of these products which are used to adjust inventory to the lower of cost or market. If actual product life cycles, product demand or market conditions are less favorable than those projected by management, additional inventory write-downs may be required which would affect future operating results.
Accounting for Shipping and Handling Revenue, Fees and Costs—The Company classifies amounts billed for shipping and handling as a component of net sales. The related shipping and handling fees and costs are included in cost of sales.
Research and Development—Research and development costs are charged to expense as incurred.
Income Taxes—There are inherent risks that could create uncertainties related to the Company’s income tax estimates. The Company adjusts estimates based on normal operating circumstances and conclusions related to tax audits. While the Company does not believe any audit finding could materially affect its financial position, there could be a material impact on its consolidated results of operations and cash flows of a given period.
The Company’s operations are subject to the tax laws, regulations and administrative practices of the United States, U.S. state jurisdictions and other countries in which it does business. The Company must make estimates and judgments in determining the provision for taxes for financial reporting purposes. These estimates and judgments occur in the calculation of tax credits, benefits, and deductions, and in the calculation of certain tax assets and liabilities that arise from differences in the timing of recognition of revenue and expense for tax and financial statement purposes, as well as the interest and penalties related to uncertain tax positions. Significant changes in these estimates may result in an increase or decrease to the Company’s tax provision in a subsequent period.
The calculation of the Company’s tax liabilities involves accounting for uncertainties in the application of complex tax regulations. The Company recognizes liabilities for uncertain tax benefits (“UTBs”) based on a two-step process. The Company recognizes the tax benefit from an UTB only if it is more likely than not that the tax position will be sustained on examination by the taxing authorities based on the technical merits of the position. The amount of UTBs is measured as appropriate for changes in facts and circumstances, such as significant amendments to existing tax law, new regulations or interpretations by the taxing authorities, new information obtained during a tax examination, or resolution of an examination. The Company believes its estimates for UTBs are appropriate and sufficient for any assessments that may result from examinations of its tax returns. The Company recognizes both accrued interest and penalties, where appropriate, related to UTBs as a component of income tax expense.
Certain items are included in the Company’s tax return at different times than they are reflected in its financial statements. Such timing differences create deferred tax assets and liabilities. Deferred tax assets are generally items that can be used as a tax deduction or credit in the tax return in future years but for which the Company has already recorded the tax benefit in the financial statements. The Company has recorded valuation allowances against certain of its deferred tax assets, primarily those that have been generated from net operating losses and tax credit carryforwards in certain taxing jurisdictions. In evaluating whether the Company would more likely than not recover these deferred tax assets, it has not assumed any future taxable income or tax planning strategies in the jurisdictions associated with these carryforwards where history does not support such an assumption. Implementation of tax planning strategies to recover these deferred tax assets or future income generation in these jurisdictions could lead to the reversal of these valuation allowances and a reduction of income tax expense. Deferred tax liabilities are either: (i) a tax expense recognized in the financial statements for which payment has been deferred; or (ii) an expense for which the Company has already taken a deduction on the tax return, but have not yet recognized the expense in the financial statements.
The Company has not historically provided for U.S. or additional foreign taxes on the excess of the amount of financial reporting over the tax basis of investments in non-U.S. subsidiaries. A company is not required to recognize a deferred tax liability for the outside basis difference of an investment in a non-U.S. subsidiary or a non-U.S. corporate joint venture that is essentially permanent in duration, unless it becomes apparent that such difference will reverse in the foreseeable future. The excess of financial reporting basis over tax basis of investments in non-U.S. subsidiaries is primarily attributable to the financial restatement of the carrying amount of these investments due to the Merger, adjusted for subsequent accumulation of earnings and losses. It is the Company’s practice and intention to continue to permanently reinvest a substantial portion of the reported earnings of its non-U.S. subsidiaries in non-U.S. operations. Currently, there are no plans to divest any of the Company’s investments in non-U.S. subsidiaries. It is not practicable to estimate the amount of deferred tax liability related to excess of
F-11
financial reporting basis over tax basis in these non-U.S. subsidiaries. The Company’s non-U.S. subsidiaries have not accumulated positive reported earnings subsequent to the Merger. However, to the extent it is determined that any amounts of excess cash will be repatriated, the Company will record a deferred tax liability reflecting the estimated amount of tax that will be payable due to such repatriation. If future events, including material changes in estimates of cash, working capital and long-term investment requirements necessitate repatriation of portions of the earnings currently treated as permanently reinvested, under current tax laws an additional tax provision may be required which could have a material effect on our financial results.
Goodwill and Other Intangible Assets—The Company operates in one reportable segment and evaluates goodwill for impairment at the reporting unit level. Effective September 1, 2011, in connection with the Company’s global reorganization, the Company made changes to its reporting unit structure. The reorganization eliminated three reporting units (U.S. Orthopedics, Sports Medicine and Biologics) and established a new reporting unit (U.S. Reconstructive). The Company formerly had eight, and now has six, identified reporting units for the purpose of testing goodwill for impairment. The reporting units are based on the Company’s current administrative organizational structure and the availability of discrete financial information.
The Company tests its goodwill and indefinite lived intangible asset balances as of March 31 of each fiscal year for impairment. The Company tests these balances more frequently if indicators are present or changes in circumstances suggest that impairment may exist. In performing the test on goodwill, the Company utilizes the two-step approach prescribed under guidance issued by the FASB for goodwill and other intangible assets. The first step under this guidance requires a comparison of the carrying value of the reporting units, of which the Company has identified six in total, to the fair value of these units. The Company generally uses the income approach to determine the fair value of each reporting unit. This approach calculates fair value by estimating the after-tax cash flows attributable to a reporting unit and then discounting these after-tax cash flows to a present value using a risk-adjusted discount rate. To derive the carrying value of the Company’s reporting units, the Company assigns assets and liabilities, including goodwill, to the reporting units. These would include corporate assets, which relate to a reporting unit’s operations, and would be considered in determining fair value. The Company allocates assets and liabilities not directly related to a specific reporting unit, but from which the reporting unit benefits, based primarily on the respective revenue contribution of each reporting unit. If the carrying value of a reporting unit exceeds its fair value, the Company performs the second step of the goodwill impairment test to measure the amount of impairment loss, if any.
The second step of the goodwill impairment test compares the implied fair value of a reporting unit’s goodwill to its carrying value. If the Company is unable to complete the second step of the test prior to the issuance of its financial statements and an impairment loss is probable and could be reasonably estimated, the Company recognizes its best estimate of the loss in its current period financial statements and discloses that amount as an estimate. The Company then recognizes any adjustment to that estimate in subsequent reporting periods, once the Company has finalized the second step of the impairment test.
The Company determines the fair value of intangible assets using an income based approach to determine the fair value. The approach calculates fair value by estimating the after-tax cash flows attributable to the asset and then discounting these after-tax cash flows to a present value using a risk-adjusted discount rate. The calculated fair value is compared to the carrying value to determine if any impairment exists.
If events or circumstances change, a determination is made by management to ascertain whether property and equipment and finite-lived intangibles have been impaired based on the sum of expected future undiscounted cash flows from operating activities. If the estimated undiscounted net cash flows are less than the carrying amount of such assets, an impairment loss is recognized in an amount necessary to write down the assets to fair value as determined from expected future discounted cash flows.
Management’s Estimates and Assumptions—In preparing the financial statements in accordance with accounting principles generally accepted in the United States of America, management must often make estimates and assumptions that affect the reported amounts of assets, liabilities, revenues, expenses and related disclosures at the date of the financial statements and during the reporting period. Some of those judgments can be subjective and complex. Consequently, actual results could differ from those estimates.
Recent Accounting Pronouncements
Comprehensive Income-In June 2011, the FASB issued an update to Topic 220, Comprehensive Income, which will supersede some of the guidance in Topic 220. This update requires companies to present comprehensive
F-12
income in either one or two consecutive financial statements and eliminates the option under current accounting standards that permits the presentation of other comprehensive income in the statement of changes in equity. Subsequently in December 2011, the FASB issued an additional update to Topic 220 that defers certain disclosure requirements originally included in the June update. In particular, the specific requirement to present items that are reclassified from accumulated other comprehensive income to net income separately with their respective components of net income and other comprehensive income has been deferred. Early adoption is permitted. The Company adopted the provisions of this new guidance in May 2012. The adoption of the new provisions did not have any impact on our financial condition or results of operations.
Goodwill Impairment Testing-In September 2011, the FASB issued Accounting Standards Update (“ASU”) 2011-08, “Intangibles-Goodwill and Other (Topic 350): Testing Goodwill for Impairment” (“ASU 2011-08”). The new guidance is intended to simplify how entities test goodwill for impairment. It includes provisions that permit an entity to first assess qualitative factors in determining whether it is “more likely than not” that the fair value of a reporting unit is less than its carrying amount as a basis for determining whether it is necessary to perform the two-step goodwill impairment test. The more-likely-than-not threshold is defined as having a likelihood of more than 50 percent. The new guidance is effective for annual and interim goodwill impairment tests performed for fiscal years beginning after December 15, 2011. The changes to Topic 350 will be effective for the Company beginning June 1, 2012 and will be applied prospectively. The changes are not expected to have a material impact on the Company’s consolidated financial statements.
Note 2—Inventories.
Inventories are stated at the lower of cost or market, with cost determined under the first-in, first-out method. The Company reviews inventory on hand and writes down excess and slow-moving inventory based on an assessment of future demand and historical experience. Inventories consisted of the following:
| (in millions) | May 31, 2012 | May 31, 2011 | |||||
| Raw materials | $ | 78.3 | $ | 85.0 | |||
| Work-in-process | 42.4 | 44.8 | |||||
| Finished goods | 422.5 | 452.7 | |||||
| Inventories | $ | 543.2 | $ | 582.5 | |||
Note 3—Property, Plant and Equipment
Property, plant and equipment are carried at cost less accumulated depreciation. Depreciation is computed using the straight-line method over the estimated useful life. Depreciation of instruments is included within cost of sales. Related maintenance and repairs are expensed as incurred.
The Company reviews property, plant and equipment for impairment whenever events or changes in circumstances indicate that the carrying value of an asset may not be recoverable. An impairment loss would be recognized when estimated undiscounted future cash flows relating to the asset, or asset group, are less than its carrying value, with the amount of the loss equal to the excess of carrying value of the asset, or asset group, over the estimated fair value.
Useful lives by major product category consisted of the following:
| Useful life | |
| Land improvements | 20 years |
| Buildings and leasehold improvements | 30 years |
| Machinery and equipment | 5-10 years |
| Instruments | 4 years |
F-13
Property, plant and equipment consisted of the following:
| (in millions) | May 31, 2012 | May 31, 2011 | |||||
| Land and land improvements | $ | 40.2 | $ | 43.5 | |||
| Buildings and leasehold improvements | 89.9 | 110.9 | |||||
| Machinery and equipment | 342.3 | 328.6 | |||||
| Instruments | 633.3 | 573.0 | |||||
| Construction in progress | 29.1 | 30.8 | |||||
| Total property, plant and equipment | 1,134.8 | 1,086.8 | |||||
| Accumulated depreciation | (541.2) | (448.4) | |||||
| Total property, plant and equipment, net | $ | 593.6 | $ | 638.4 | |||
The Company recorded a property, plant and equipment impairment charge of $17.0 million during the year ended May 31, 2011, relating to an administrative, manufacturing and distribution facility located in Parsippany, New Jersey. The amount of impairment charge recorded within cost of sales and selling, general and administrative expense was $6.5 million and $10.5 million, respectively. The impairment charge reflects the Company’s change in intended use of this facility.
Note 4—Investments.
At May 31, 2012, the Company’s investment securities were classified as follows:
| Unrealized | |||||||||||||||
| (in millions) | Amortized Cost | Gains | Losses | Fair Value | |||||||||||
| Available-for-sale: | |||||||||||||||
| Equity securities | $ | 0.4 | $ | — | $ | (0.2 | ) | $ | 0.2 | ||||||
| Time deposit | 9.5 | — | — | 9.5 | |||||||||||
| Greek bonds | 6.3 | — | — | 6.3 | |||||||||||
| Total available-for-sale investments | $ | 16.2 | $ | — | $ | (0.2 | ) | $ | 16.0 | ||||||
| Realized | |||||||||||||||
| (in millions) | Amortized Cost | Gains | Losses | Fair Value | |||||||||||
| Trading: | |||||||||||||||
| Equity securities | $ | 0.4 | $ | — | $ | — | $ | 0.4 | |||||||
| Total trading investments | $ | 0.4 | $ | — | $ | — | $ | 0.4 | |||||||
At May 31, 2011, the Company’s investment securities were classified as follows:
| Unrealized | |||||||||||||||
| (in millions) | Amortized Cost | Gains | Losses | Fair Value | |||||||||||
| Available-for-sale: | |||||||||||||||
| Equity securities | $ | 0.5 | $ | 0.1 | $ | (0.2 | ) | $ | 0.4 | ||||||
| Money market funds | 9.5 | — | — | 9.5 | |||||||||||
| Time deposit | 33.1 | — | — | 33.1 | |||||||||||
| Greek bonds | 35.6 | — | (4.5) | 31.1 | |||||||||||
| Other investments | 0.3 | — | — | 0.3 | |||||||||||
| Total available-for-sale investments | $ | 79.0 | $ | 0.1 | $ | (4.7 | ) | $ | 74.4 | ||||||
F-14
| Realized | |||||||||||||||
| (in millions) | Amortized Cost | Gains | Losses | Fair Value | |||||||||||
| Trading: | |||||||||||||||
| Equity securities | $ | 0.1 | $ | — | $ | — | $ | 0.1 | |||||||
| Total trading investments | $ | 0.1 | $ | — | $ | — | $ | 0.1 | |||||||
The Company recorded proceeds on the sales/maturities of investments of $42.1 million, $59.3 million and $24.9 million for the years ended May 31, 2012, 2011 and 2010, respectively. The Company recorded realized gains of $2.0 million, $4.9 million and $4.3 million for the years ended May 31, 2012, 2011 and 2010, respectively, which was included in other (income) expense.
The Company received $45.5 million face value zero coupon bonds in December 2010 from the Greek government as payment for an outstanding accounts receivable balance from calendar years 2007-2009 related to certain government sponsored institutions in a non-cash transaction. Upon receipt, the bonds had a fair value of $33.8 million, with maturity dates of one to three years. The bonds are designated as available-for-sale securities. The Company recorded realized losses of $20.1 million on the Greek bonds related to other-than-temporary impairment for the year ended May 31, 2012, which is included in other (income) expense with no other-than-temporary impairment recorded for the year ended May 31, 2011. The one year bonds matured in December 2011 and the Company received the full par value of approximately $8.4 million. On March 9, 2012 the Greek government finalized the private sector involvement in the Greek debt restructuring. All holders of Greek government bonds were required to exchange the existing bonds to new bonds. The new bonds have maturities ranging from 1 to 30 years. At May 31, 2012 the face value of the bonds was $15.7 million.
The Company reviews impairments to investment securities quarterly to determine if the impairment is “temporary” or “other-than-temporary.” The Company reviews several factors to determine whether losses are other-than-temporary, including but not limited to (1) the length of time each security was in an unrealized loss position, (2) the extent to which fair value was less than cost, (3) the financial condition and near-term prospects of the issuer, and (4) the Company’s intent and ability to hold each security for a period of time sufficient to allow for any anticipated recovery in fair value.
The Company offered a new deferred compensation plan as of January 1, 2011. The investments held by the Company mirror the investment selections of the participants. The investments are held in various equity securities and are considered trading with the realized gain and realized loss being recorded through other (income) expense.
Investment income on available-for-sale securities (included in other (income) expense) consists of the following:
| (in millions) | Year Ended May 31, 2012 | Year Ended May 31, 2011 | Year Ended May 31, 2010 | ||||||||
| Interest income | $ | 0.4 | $ | 0.6 | $ | 0.3 | |||||
| Dividend income | 0.2 | 0.1 | 0.1 | ||||||||
| Net realized gains | 2.0 | 2.6 | 4.3 | ||||||||
| Total investment income | $ | 2.6 | $ | 3.3 | $ | 4.7 | |||||
Note 5—Goodwill and Other Intangible Assets.
The Company operates in one reportable segment and evaluates goodwill for impairment at the reporting unit level. Effective September 1, 2011, in connection with the Company’s global reorganization, the Company made changes to its reporting unit structure. The reorganization eliminated three reporting units (U.S. Orthopedics, Sports Medicine and Biologics) and established a new reporting unit (U.S. Reconstructive). The Company formerly had eight, and now has six, identified reporting units for the purpose of testing goodwill for impairment. The reporting units are based on the Company’s current administrative organizational structure and the availability of discrete financial information.
During the fourth quarter of fiscal year 2012, the Company recorded a $529.8 million goodwill and definite and indefinite-lived intangible asset impairment charge primarily associated with its spine & bone healing and dental reconstructive reporting units. As of February 29, 2012, the Company concluded that certain indicators were present
F-15
that suggested impairment may exist for its dental reconstructive reporting unit’s goodwill and intangible assets. The indicators of impairment in the Company’s dental reconstructive reporting unit included evidence of declining industry market growth rates in certain European and Asia Pacific markets and unfavorable margin trends resulting from change in product mix. The impact of these recent items resulted in management initiating an interim preliminary impairment test as of February 29, 2012. However, the preliminary result of this interim test of impairment for the dental reconstructive reporting unit’s goodwill and intangibles was inconclusive during the third quarter of fiscal year 2012. The Company finalized the impairment test during the fourth quarter of fiscal year 2012. During the annual impairment test, described below, the Company’s spine and bone healing reporting unit failed step one. The indicators were primarily due to growth rate declines as compared to prior assumptions.
During the fourth quarter of fiscal year 2011, the Company recorded a $941.4 million goodwill and definite and indefinite-lived intangible asset impairment charge primarily associated with its Europe reporting unit. As of February 28, 2011, the Company concluded that certain indicators were present that suggested impairment may exist for its Europe reporting unit’s goodwill and intangibles. The indicators of potential impairment in the Company’s Europe reporting unit included:
| • | recent reductions in revenue growth rates for the reporting unit’s knee and hip products; |
| • | recent market pressure resulting in reduced average selling prices of the reporting unit’s products; |
| • | evidence of declining industry market growth rates for many countries; and |
| • | certain European governments actively pursuing healthcare spend restructuring programs. |
The impact of these recent items resulted in management initiating an interim preliminary impairment test as of February 28, 2011. However, the preliminary result of this interim test of impairment for the Europe reporting unit’s goodwill and intangibles was inconclusive during the third quarter of fiscal year 2011. The Company finalized the impairment tests during the fourth quarter of fiscal year 2011.
The Company used only the income approach, specifically the discounted cash flow method, to determine the fair value of the dental reconstructive, spine & bone healing and Europe reporting units and the associated amount of the impairment charges. This approach calculates fair value by estimating the after-tax cash flows attributable to a reporting unit and then discounting these after-tax cash flows to a present value using a risk-adjusted discount rate. This methodology is consistent with how the Company estimates the fair value of its reporting units during its annual goodwill and indefinite lived intangible asset impairment tests. In applying the income approach to calculate the fair value of the dental reconstructive, spine & bone healing and Europe reporting units, the Company used assumptions about future revenue contributions and cost structures. In addition, the application of the income approach for both goodwill and intangibles requires judgment in determining a risk-adjusted discount rate at the reporting unit level. The Company based this determination on estimates of the weighted-average costs of capital of market participants. The Company performed a peer company analysis and considered the industry the weighted-average return on debt and equity from a market participant perspective.
To calculate the amount of the impairment charge related to the dental reconstructive, spine & bone healing and Europe reporting units, the Company allocated the reporting unit’s fair value to all of its assets and liabilities, including certain unrecognized intangible assets, in order to determine the implied fair value of goodwill. This allocation process required judgment and the use of additional valuation assumptions in deriving the individual fair values of the Company’s dental reconstructive, spine & bone healing and Europe reporting unit’s assets and liabilities as if the reporting units had been acquired in a business combination.
The Company determines the fair value of intangible assets using an income based approach to determine the fair value. The approach calculates fair value by estimating the after-tax cash flows attributable to the asset and then discounting these after-tax cash flows to a present value using a risk-adjusted discount rate. The calculated fair value is compared to the carrying value to determine if any impairment exists.
The Company also performed its annual assessment for impairment as of March 31, 2012 for all six reporting units. The Company utilized discount rates ranging from 9.2% to 13.5%. Based on the discount rate used in its most recent test for impairment, if the discount rate increased by 1% the fair value of the consolidated company could be lower by approximately $1.3 billion and a decrease in the discount rate of 1% results in an increase in fair value of $1.8 billion. The step one test also includes assumptions derived from competitor market capitalization and beta values as well as the twenty year Treasury bill rate as of March 31, 2012. The only reporting
F-16
unit that failed step one and was required to complete a step two analysis was the spine & bone healing reporting unit.
The Company tested goodwill of these two reporting units with a carrying value of $597.1 million and under step two recorded an impairment charge of $291.9 million. The implied fair value of the goodwill of these two reporting units was $305.2 million. The Company tested definite-lived intangibles that failed step 1 with a carrying value of $432.4 million and under step two recorded an impairment charge of $229.8 million as the fair value of these definite-lived intangible assets was $202.6 million. The Company tested indefinite-lived intangibles with a carrying value of $75.1 million and under step two took an impairment charge of $8.1 million as the fair value of these indefinite-lived assets was $67.0 million. All of these fair values would be classified as Level 3 in the fair value hierarchy.
The estimates and assumptions underlying the fair value calculations used in the Company’s annual impairment tests are uncertain by their nature and can vary significantly from actual results. Factors that management must estimate include, but are not limited to, industry and market conditions, sales volume and pricing, raw material costs, capital expenditures, working capital changes, cost of capital, royalty rates and tax rates. These factors are especially difficult to predict when global financial markets are volatile. The estimates and assumptions used in its impairment tests are consistent with those the Company use in its internal planning. These estimates and assumptions may change from period to period. If the Company uses different estimates and assumptions in the future, future impairment charges may occur and could be material.
The Company has identified a total of four reporting units with a material amount of goodwill that are at a higher risk of potential failure of step one of the goodwill impairment test in the future. These reporting units include its U.S. Reconstructive reporting unit ($2,971.9 million of goodwill), its International reporting unit ($555.5 million of goodwill), its dental reconstructive reporting unit ($298.6 million of goodwill) and its Europe reporting unit ($223.0 million). The level of excess fair value over carrying value for these higher risk reporting units were each less than 10% for the latest step one impairment test.
The Company uses an accelerated method for amortizing customer relationship intangibles as the value for those relationships is greater at the beginning of their life. The decrease in the net intangible asset balance during fiscal year 2012 is primarily due to the impairment charge, amortization and the weakening of the euro against the U.S. dollar.
The following tables summarize the changes in the carrying amount of goodwill:
| (in millions) | May 31, 2012 | May 31, 2011 | May 31, 2010 | ||||||||
| Beginning of period | $ | 4,470.1 | $ | 4,707.5 | $ | 4,780.5 | |||||
| Goodwill acquired | — | — | — | ||||||||
| Currency translation | (63.8) | 185.4 | (73.0) | ||||||||
| Impairment charge | (291.9) | (422.8) | — | ||||||||
| End of period | $ | 4,114.4 | $ | 4,470.1 | $ | 4,707.5 | |||||
| (in millions) | May 31, 2012 | May 31, 2011 | May 31, 2010 | ||||||||
| Gross carrying amount | $ | 5,324.7 | $ | 5,388.5 | $ | 5,203.1 | |||||
| Accumulated impairment losses | (1,210.3) | (918.4) | (495.6) | ||||||||
| Net carrying amount | $ | 4,114.4 | $ | 4,470.1 | $ | 4,707.5 | |||||
Intangible assets consist of the following at May 31, 2012 and 2011:
F-17
| May 31, 2012 | |||||||||||||||||||||||
| (in millions) | Gross Carrying Amount | Impairment Charge | New Carrying Amount | Accumulated Amortization | Impairment Charge | Net Carrying Amount | |||||||||||||||||
| Core technology | $ | 1,856.1 | $ | (185.7 | ) | $ | 1,670.4 | $ | (457.7 | ) | $ | 74.3 | $ | 1,287.0 | |||||||||
| Completed technology | 594.2 | — | 594.2 | (206.7) | — | 387.5 | |||||||||||||||||
| Product trade names | 184.5 | — | 184.5 | (52.6) | — | 131.9 | |||||||||||||||||
| Customer relationships | 2,666.1 | (306.8) | 2,359.3 | (859.3) | 191.6 | 1,691.6 | |||||||||||||||||
| Non-compete contracts | 4.6 | — | 4.6 | (3.1) | — | 1.5 | |||||||||||||||||
| Sub-total | 5,305.5 | (492.5) | 4,813.0 | (1,579.4) | 265.9 | 3,499.5 | |||||||||||||||||
| Corporate trade names | 323.5 | (11.3) | 312.2 | — | — | 312.2 | |||||||||||||||||
| Currency translation | 147.2 | — | 147.2 | (28.5) | — | 118.7 | |||||||||||||||||
| Total | $ | 5,776.2 | $ | (503.8 | ) | $ | 5,272.4 | $ | (1,607.9 | ) | $ | 265.9 | $ | 3,930.4 | |||||||||
| May 31, 2011 | |||||||||||||||||||||||
| (in millions) | Gross Carrying Amount | Impairment Charge | New Carrying Amount | Accumulated Amortization | Impairment Charge | Net Carrying Amount | |||||||||||||||||
| Core technology | $ | 2,092.6 | $ | (243.1 | ) | $ | 1,849.5 | $ | (416.9 | ) | $ | 53.4 | $ | 1,486.0 | |||||||||
| Completed technology | 664.9 | (70.7) | 594.2 | (183.9) | 21.8 | 432.1 | |||||||||||||||||
| Product trade names | 183.7 | — | 183.7 | (41.0) | — | 142.7 | |||||||||||||||||
| Customer relationships | 2,944.6 | (300.4) | 2,644.2 | (778.5) | 94.5 | 1,960.2 | |||||||||||||||||
| Non-compete contracts | 4.6 | — | 4.6 | (2.1) | — | 2.5 | |||||||||||||||||
| Sub-total | 5,890.4 | (614.2) | 5,276.2 | (1,422.4) | 169.7 | 4,023.5 | |||||||||||||||||
| Corporate trade names | 397.6 | (74.1) | 323.5 | — | — | 323.5 | |||||||||||||||||
| Currency translation | 232.4 | — | 232.4 | (45.0) | — | 187.4 | |||||||||||||||||
| Total | $ | 6,520.4 | $ | (688.3 | ) | $ | 5,832.1 | $ | (1,467.4 | ) | $ | 169.7 | $ | 4,534.4 | |||||||||
The weighted average useful life of the intangibles at May 31, 2012 is as follows:
| Weighted Average Useful Life | |
| Core technology | 17 Years |
| Completed technology | 11 Years |
| Product trade names | 15 Years |
| Customer relationships | 16 Years |
| Non-compete contracts | 3 Years |
| Corporate trade names | Indefinite life |
Expected amortization expense, for the intangible assets stated above, for the years ending May 31, 2013 through 2017 is $305.4 million, $295.1 million, $277.4 million, $268.9 million, and $263.7 million, respectively.
DePuy Trauma Acquisition
On May 24, 2012, DePuy Orthopaedics, Inc. accepted the Company’s binding offer to purchase certain assets representing substantially all of DePuy’s worldwide trauma business, which involves researching, developing, manufacturing, marketing, distributing and selling products to treat certain bone fractures or deformities in the human body, including certain intellectual property assets, and to assume certain liabilities, for approximately $280.0 million in cash. The Company acquired the DePuy worldwide trauma business to strengthen its trauma business and to continue to build a stronger presence in the global trauma market.
On June 15, 2012, the Company announced the initial closing of the transaction, acquiring DePuy’s trauma operations in the U.S., the United Kingdom, Australia, New Zealand and Japan, as well as DePuy’s trauma manufacturing operations in Le Locle, Switzerland. On July 13, 2012, the Company closed in Belgium, France, Germany, Luxembourg, The Netherlands, Portugal, South Africa, Spain (except for 5 hospitals which will be transferred subsequently) and the Switzerland non-manufacturing unit. Subsequent closings for the remaining
F-18
countries will occur on a staggered basis and, in general, are expected to be completed within six months of the initial closing. DePuy affiliates will serve as the Company’s interim distributors in these countries until these operations are fully transitioned to the Company.
The pro forma information required under Accounting Standards Codification 805 is impracticable to include due to different fiscal year ends. The carve out financial statements are not aligned to Biomet’s May 31, 2012 fiscal year end and the complexity of the sales information makes the information unavailable.
F-19
Note 6—Debt.
The senior secured credit facilities and all of the notes are guaranteed by Biomet, Inc., and subject to certain exceptions, each of its existing and future wholly-owned domestic subsidiaries. The asset-based revolving credit facility is guaranteed by the Company and secured, subject to certain exceptions, by a first-priority security interest in substantially all of the Company’s assets and the assets of subsidiary borrowers that consist of all accounts receivable, inventory, cash, deposit accounts, and certain intangible assets. The facilities and notes bear interest at the rates set forth below. Interest is payable in cash. The terms and carrying value of each debt instrument at May 31, 2012 are set forth below:
| (U.S. dollars and euros in millions) | Maturity Date | Interest Rate | Currency | May 31, 2012 | May 31, 2011 | ||||||||
| Debt Instruments | |||||||||||||
| Non-U.S. facility | No Maturity Date | Interest Free | EUR | € | 2.8 | € | 3.9 | ||||||
| $ | 3.5 | $ | 5.6 | ||||||||||
| Term loan facility | March 25, 2015 | LIBOR + 3.00% | USD | $ | 2,234.7 | $ | 2,258.1 | ||||||
| Term loan facility | March 25, 2015 | LIBOR + 3.00% | EUR | € | 835.6 | € | 844.4 | ||||||
| $ | 1,039.6 | $ | 1,206.3 | ||||||||||
| Cash flow revolving credit facility | September 25, 2013 | LIBOR + 2.00% | USD | — | — | ||||||||
| Cash flow revolving credit facility | September 25, 2013 | LIBOR + 2.00% | USD/EUR | $/ € — | $/ € — | ||||||||
| Asset-based revolving credit facility | September 25, 2013 | LIBOR + 1.25% | USD | — | — | ||||||||
| Senior cash pay notes | October 15, 2017 | 10% | USD | $ | 761.0 | $ | 761.0 | ||||||
| Senior PIK toggle notes | October 15, 2017 | 103/8% / 111/8% | USD | $ | 771.0 | $ | 771.0 | ||||||
| Senior subordinated notes | October 15, 2017 | 115/8% | USD | $ | 1,015.0 | $ | 1,015.0 | ||||||
| Premium on notes | $ | 3.0 | $ | 3.3 | |||||||||
| Total debt | $ | 5,827.8 | $ | 6,020.3 | |||||||||
The Company currently elects to use 3-month LIBOR for setting the interest rates on the majority of its U.S. dollar and euro term loans. The 3-month LIBOR rate for the U.S. dollar term loan as of May 31, 2012 was 0.47%. The euro term loan had a 3-month LIBOR rate of 0.72% as of May 31, 2012. The Company’s term loan facilities require payments each year in an amount equal to 1% of the original principal in equal calendar quarterly installments until maturity of the loan on March 25, 2015. Through May 31, 2012, the total amount of required payments under the Company’s term loan facilities was $35.4 million. The cash flow and asset-based revolving credit facilities and the notes do not have terms for mandatory principal pay downs. To calculate the U.S. dollar equivalent on outstanding balances, the Company used a currency conversion rate of 1 euro to $1.2441 and $1.4284, which represents the currency exchange rate from euros to U.S. dollars on May 31, 2012 and May 31, 2011, respectively.
The Company has the option to choose the frequency with which it resets and pays interest on its term loans. The Company currently pays interest on the majority of its term loans and interest rate swaps each calendar quarter. The remaining term loan interest is paid monthly. Interest on the notes is paid semiannually in October and April.
The Company’s revolving borrowing base available under all debt facilities at May 31, 2012 was $713.9 million, which is net of the remaining $22.3 million commitment of the subsidiaries of Lehman Brothers Holding Inc. and borrowing base limitations relating to the asset-based revolving credit facility. During November 2011, ABN AMRO Bank terminated the European revolver facility due to the limited use of the facility.
F-20
As of May 31, 2012, $34.5 million of financing fees related to the Company’s credit agreement remained in long-term assets and continue to be amortized through interest expense over the remaining life of the credit agreement.
Each of Biomet, Inc.’s existing wholly owned domestic subsidiaries fully, unconditionally, jointly, and severally guarantee the senior cash pay and PIK toggle notes on a senior unsecured basis and the senior subordinated notes on a senior subordinated unsecured basis, in each case to the extent such subsidiaries guarantee Biomet, Inc.’s senior secured cash flow facilities. LVB Acquisition, Inc. is neither an issuer nor guarantor of the notes described within this footnote.
As of May 31, 2012 and 2011, short-term borrowings consisted of the following:
| (in millions) | May 31, 2012 | May 31, 2011 | |||||
| Senior secured credit facilities | $ | 34.3 | $ | 35.9 | |||
| Non-U.S. facility | 1.3 | 1.5 | |||||
| Total | $ | 35.6 | $ | 37.4 | |||
Summarized in the table below are the Company’s long-term obligations as of May 31, 2012:
| (in millions) | Total | 2013 | 2014 and 2015 | 2016 and 2017 | 2018 and thereafter | ||||||||||||||
| Long-term debt (including current maturities) | $ | 5,827.8 | $ | 35.6 | $ | 3,240.0 | $ | — | $ | 2,552.2 | |||||||||
The Company currently is restricted in its ability to pay dividends under various covenants of its debt agreements, including its credit facilities and the indentures governing its notes. The Company does not expect for the foreseeable future to pay dividends on its common stock, and did not during fiscal 2012 or fiscal 2011. Any future determination to pay dividends will depend upon, among other factors, its results of operations, financial condition, cash flows, capital requirements, any contractual restrictions and any other considerations the Company’s Board of Directors deems relevant.
Subsequent Events
On August 8, 2012, Biomet, Inc. completed its offering of $1.0 billion aggregate principal amount of new 6.500% senior notes due 2020. The Company expects to use the net proceeds of this offering to fund a tender offer for any and all of its outstanding 103/8% / 111/8% Senior Toggle Notes due 2017, including related fees and expenses, and to purchase, redeem, defease or otherwise acquire or retire its outstanding indebtedness.
On August 2, 2012, the Company entered into an amendment and restatement agreement that amended its existing senior secured credit facilities. The amendment (i) extends the maturity of approximately $1,007.2 million of its U.S. dollar-denominated term loans and approximately €631.3 million of its euro-denominated term loans under the credit facility to July 25, 2017 and (ii) refinances and replaces the existing alternative currency revolving credit commitments under the credit facility with a new class of alternative currency revolving credit commitments in an aggregate amount of $165.0 million and refinances and replaces the existing U.S. dollar revolving credit commitments under the credit facility with a new class of U.S. dollar-denominated revolving credit commitments in an aggregate amount of $165.0 million. The new revolving credit commitments will mature on April 25, 2017, except that if as of December 23, 2014, there is an outstanding aggregate principal amount of non-extended U.S. dollar and euro term loans in excess of $200.0 million, then such revolving credit commitments will mature on December 24, 2014. The remaining term loans of the lenders under the senior secured credit facilities who did not elect to extend such loans will continue to mature on March 25, 2015.
Note 7—Fair Value Measurements.
Assets and Liabilities Measured at Fair Value on a Recurring Basis
Fair value measurements are principally applied to (1) financial assets and liabilities such as marketable equity securities and debt securities, (2) investments in equity and other securities, and (3) derivative instruments consisting of interest rate swaps. These items are marked-to-market at each reporting period to fair value. The
F-21
information in the following paragraphs and tables primarily addresses matters relative to these financial assets and liabilities.
| • | Level 1 – Inputs are quoted prices in active markets for identical assets or liabilities. The Company’s Level 1 assets include money market investments and marketable equity securities. |
| • | Level 2 – Inputs include quoted prices for similar assets or liabilities in active markets, quoted prices for identical or similar assets or liabilities in markets that are not active, and inputs (other than quoted prices) that are observable for the asset or liability, either directly or indirectly. The Company’s Level 2 assets and liabilities primarily include Greek bonds, time deposits, interest rate swaps, pension plan assets (equity securities, debt securities and other) and foreign currency exchange contracts whose value is determined using a pricing model with inputs that are observable in the market or can be derived principally from or corroborated by observable market data. |
| • | Level 3 – Inputs are unobservable for the asset or liability. The Company’s Level 3 assets include other equity investments. See the section below titled Level 3 Valuation Techniques for further discussion of how the Company determines fair value for investments classified as Level 3. |
The following table provides information by level for assets and liabilities that are measured at fair value on a recurring basis at May 31, 2012 and May 31, 2011:
| Fair Value at | Fair Value Measurement Using Inputs Considered as | ||||||||||||||
| (in millions) | May 31, 2012 | Level 1 | Level 2 | Level 3 | |||||||||||
| Assets: | |||||||||||||||
| Money market funds | $ | 303.1 | $ | 303.1 | $ | — | $ | — | |||||||
| Time deposits | 36.3 | — | 36.3 | — | |||||||||||
| Greek bonds | 6.3 | — | 6.3 | — | |||||||||||
| Pension plan assets | 108.7 | — | 108.7 | — | |||||||||||
| Foreign currency exchange contracts | 0.2 | — | 0.2 | — | |||||||||||
| Other | 0.2 | — | — | 0.2 | |||||||||||
| Total assets | $ | 454.8 | $ | 303.1 | $ | 151.5 | $ | 0.2 | |||||||
| Liabilities: | |||||||||||||||
| Interest rate swaps | $ | 76.2 | $ | — | $ | 76.2 | $ | — | |||||||
| Foreign currency exchange contracts | 0.2 | — | 0.2 | — | |||||||||||
| Total liabilities | $ | 76.4 | $ | — | $ | 76.4 | $ | — | |||||||
| Fair Value at | Fair Value Measurement Using Inputs Considered as | ||||||||||||||
| (in millions) | May 31, 2011 | Level 1 | Level 2 | Level 3 | |||||||||||
| Assets: | |||||||||||||||
| Corporate debt securities | $ | 0.3 | $ | — | $ | 0.3 | $ | — | |||||||
| Money market funds | 132.5 | 132.5 | — | — | |||||||||||
| Time deposit | 47.4 | — | 47.4 | — | |||||||||||
| Greek bonds | 31.1 | — | 31.1 | — | |||||||||||
| Pension plan assets | 104.1 | — | 104.1 | — | |||||||||||
| Foreign currency exchange contracts | 0.2 | — | 0.2 | — | |||||||||||
| Other | 0.5 | 0.3 | — | 0.2 | |||||||||||
| Total assets | $ | 316.1 | $ | 132.8 | $ | 183.1 | $ | 0.2 | |||||||
| Liabilities: | |||||||||||||||
| Interest rate swaps | $ | 96.8 | $ | — | $ | 96.8 | $ | — | |||||||
| Foreign currency exchange contracts | 0.1 | — | 0.1 | — | |||||||||||
| Total liabilities | $ | 96.9 | $ | — | $ | 96.9 | $ | — | |||||||
Level 3 Valuation Techniques
F-22
Financial assets are considered Level 3 when their fair values are determined using pricing models, discounted cash flow methodologies or similar techniques and at least one significant model assumption or input is unobservable. Level 3 financial assets also include certain investment securities for which there is limited market activity where the determination of fair value requires significant judgment or estimation. Level 3 investment securities primarily include other equity investments for which there was a decrease in the observation of market pricing. As of May 31, 2012 and May 31, 2011, these securities were valued primarily using internal cash flow valuation that incorporates transaction details such as contractual terms, maturity, timing and amount of future cash flows, as well as assumptions about liquidity and credit valuation adjustments of marketplace participants.
The following table provides a reconciliation of the beginning and ending balances of items measured at fair value on a recurring basis in the tables above that used significant unobservable inputs (Level 3) as of May 31, 2012 and May 31, 2011:
| (in millions) | |||
| Balance at June 1, 2010 | $ | 5.7 | |
| Total net gains included in earnings | 2.6 | ||
| Total unrealized gains included in other comprehensive income | (2.6) | ||
| Total proceeds from sale of Level 3 investments | (5.5) | ||
| Balance at May 31, 2011 | $ | 0.2 | |
| Total net gains included in earnings | — | ||
| Total unrealized gains included in other comprehensive income | — | ||
| Total proceeds from sale of Level 3 investments | — | ||
| Balance at May 31, 2012 | $ | 0.2 | |
The estimated fair value of the Company’s long-term debt, including the current portion, at May 31, 2012 was $6,009.1 million, compared to a carrying value of $5,827.8 million. The fair value of the Company’s traded debt was estimated using quoted market prices for the same or similar instruments. The fair value of the Company’s variable rate term debt was estimated using the carrying value as this debt has rates which approximate market interest rates. In determining the fair values and carrying values, the Company considers the terms of the related debt and excludes the impacts of debt discounts and interest rate swaps.
Assets and Liabilities that are Measured at Fair Value on a Nonrecurring Basis
During the year ended May 31, 2012, the Company measured nonfinancial long-lived assets and liabilities at fair value in conjunction with the impairment of the spine & bone healing and dental reporting units. The Company used the income approach to measure the fair value of the reporting units and related intangible assets. See Note 5 for a full description of key assumptions. The inputs used in the impairment fair value analysis fall within Level 3 due to the significant unobservable inputs used to determine fair value. During the year ended May 31, 2011, the Company measured nonfinancial long-lived assets and liabilities at fair value in conjunction with the impairment of the Europe reporting unit. The Company used the income approach to measure the fair value of the Europe reporting unit and related intangible assets. Please refer to Note 5 for more information. The inputs used in the impairment fair value analysis fall within Level 3 due to the significant unobservable inputs used to determine fair value.
The Company is exposed to certain market risks relating to its ongoing business operations, including foreign currency risk, interest rate risk and commodity price risk. The Company currently manages foreign currency risk and interest rate risk through the use of derivatives.
Note 8—Derivative Instruments and Hedging Activities.
The Company is exposed to certain market risks relating to its ongoing business operations, including foreign currency risk, interest rate risk and commodity price risk. The Company currently manages foreign currency risk and interest rate risk through the use of derivatives.
Derivatives Designated as Hedging Instruments
Foreign Currency Instruments—Certain assets, liabilities and forecasted transactions are exposed to foreign currency risk, primarily the fluctuation of the U.S. dollar against the euro. The Company has hedged a portion of its
F-23
net investment in its European subsidiaries with the issuance of a €875.0 million (approximately $1,207.4 million at September 25, 2007) principal amount euro term loan on September 25, 2007. The Company’s net investment in its European subsidiaries at the hedging date of September 25, 2007 was €1,238.0 million ($1,690.0 million). As of May 31, 2012, the Company’s net investment in European subsidiaries totaled €1,808.9 million ($2,250.4 million) and the outstanding principal balance of the euro term loan was €835.6 million ($1,039.6 million). The difference of €973.3 million ($1,210.8 million) is unhedged as of May 31, 2012. Hedge effectiveness is tested quarterly to determine whether hedge treatment is still appropriate. The Company tests effectiveness on this net investment hedge by determining if the net investment in its European subsidiaries is greater than the outstanding euro-denominated debt balance. Any amount of a derivative instrument designated as a hedge determined to be ineffective is recorded as other (income) expense.
Interest Rate Instruments—The Company uses interest rate swap agreements (cash flow hedges) in both U.S. dollars and euros as a means of fixing the interest rate on portions of its floating-rate debt instruments. As of May 31, 2012, the Company had a swap liability of $76.2 million, which consisted of $36.0 million short-term, and $41.0 million long-term, partially offset by a $0.8 million credit valuation adjustment. As of May 31, 2011, the Company had a swap liability of $96.8 million, which consisted of $62.6 million short-term, and $34.8 million long-term, partially offset by a $0.6 million credit valuation adjustment.
The table below summarizes existing swap agreements:
| (U.S. dollars and euros in millions) | ||||||||||||||||||
| Structure | Currency | Notional Amount | Effective Date | Termination Date | Fair Value at May 31, 2012 Asset (Liability) | Fair Value at May 31, 2011 Asset (Liability) | ||||||||||||
| 4 years | EUR | € | 75.0 | September 25, 2007 | September 25, 2011 | $ | — | $ | (1.7 | ) | ||||||||
| 4 years | EUR | 40.0 | March 25, 2008 | March 25, 2012 | — | (1.4 | ) | |||||||||||
| 5 years | EUR | 230.0 | September 25, 2007 | September 25, 2012 | (3.5 | ) | (13.6 | ) | ||||||||||
| 5 years | EUR | 40.0 | March 25, 2008 | March 25, 2013 | (1.4 | ) | (2.5 | ) | ||||||||||
| 5 years | EUR | 200.0 | September 25, 2012 | September 25, 2017 | (9.5 | ) | — | |||||||||||
| 5 years | EUR | 200.0 | September 25, 2012 | September 25, 2017 | (9.3 | ) | — | |||||||||||
| 4 years | USD | $ | 195.0 | September 25, 2007 | September 25, 2011 | — | (3.1 | ) | ||||||||||
| 4 years | USD | 140.0 | March 25, 2008 | March 25, 2012 | — | (3.0 | ) | |||||||||||
| 5 years | USD | 585.0 | September 25, 2007 | September 25, 2012 | (8.9 | ) | (37.3 | ) | ||||||||||
| 5 years | USD | 190.0 | March 25, 2008 | March 25, 2013 | (4.2 | ) | (9.3 | ) | ||||||||||
| 5 years | USD | 325.0 | December 26, 2008 | December 25, 2013 | (9.0 | ) | (13.3 | ) | ||||||||||
| 5 years | USD | 195.0 | September 25, 2009 | September 25, 2014 | (10.5 | ) | (12.2 | ) | ||||||||||
| 2 years | USD | 190.0 | March 25, 2013 | March 25, 2015 | (1.0 | ) | — | |||||||||||
| 3 years | USD | 270.0 | December 27, 2013 | September 25, 2016 | (3.8 | ) | — | |||||||||||
| 5 years | USD | 350.0 | September 25, 2012 | September 25, 2017 | (8.0 | ) | — | |||||||||||
| 5 years | USD | 350.0 | September 25, 2012 | September 25, 2017 | (7.9 | ) | — | |||||||||||
| Credit valuation adjustment | 0.8 | 0.6 | ||||||||||||||||
| Total interest rate instruments | $ | (76.2 | ) | $ | (96.8 | ) | ||||||||||||
F-24
The interest rate swaps are recorded in other accrued expenses and other long-term liabilities. As a result of cash flow hedge treatment being applied, all unrealized gains and losses related to the derivative instruments are recorded in accumulated other comprehensive income (loss) and are reclassified into operations in the same period in which the hedged transaction affects earnings. Hedge effectiveness is tested quarterly to determine if hedge treatment is still appropriate. The amount of ineffectiveness was not material for any period presented. The tables below summarize the effective portion and ineffective portion of the Company’s interest rate swaps for the years ended May 31, 2012 and 2011:
| (n millions) | ||||||||||||
| Derivatives in cash flow hedging relationship | Year Ended May 31, 2012 | Year Ended May 31, 2011 | Year Ended May 31, 2010 | |||||||||
| Interest rate swaps: | ||||||||||||
| Amount of gain (loss) recognized in OCI | $ | 20.5 | $ | 33.1 | $ | 18.5 | ||||||
| Amount of (gain) loss reclassified from accumulated OCI into interest expense (effective portion) | — | — | — | |||||||||
| Amount (gain) loss recognized in other income (expense) (ineffective portion and amount excluded from effectiveness testing) | — | — | — | |||||||||
As of May 31, 2012, the effective interest rate, including the applicable lending margin, on 57.95% ($1,295.0 million) of the outstanding principal of the Company’s U.S. dollar term loan was fixed at 6.84% through the use of interest rate swaps. The effective interest rate on 32.31% (€270.0 million) of the outstanding principal of the Company’s euro term loan was fixed at 7.36% through the use of interest rate swaps. The remaining unhedged balances of the U.S. dollar and euro term loans had effective interest rates of 3.24% and 3.34%, respectively. As of May 31, 2012 and May 31, 2011, the Company’s effective weighted average interest rate on all outstanding debt, including the interest rate swaps, was 7.80% and 7.96%, respectively.
Derivatives Not Designated as Hedging Instruments
Foreign Currency Instruments—The Company faces transactional currency exposures that arise when it or its foreign subsidiaries enter into transactions, primarily on an intercompany basis, denominated in currencies other than their functional currency. The Company enters into short-term forward currency exchange contracts in order to mitigate the currency exposure related to these intercompany payables and receivables arising from intercompany trade. The Company does not designate these contracts as hedges; therefore, all forward currency exchange contracts are recorded at their fair value each period, with the resulting gains and losses recorded in other (income) expense. Any foreign currency remeasurement gains or losses recognized in a period are generally offset with gains or losses on the forward currency exchange contracts. As of May 31, 2012, the fair value of the Company’s derivatives not designated as hedging instruments on a gross basis were assets of $0.2 million recorded in prepaid expenses and other and liabilities of $0.2 million recorded in other accrued expenses.
Note 9—Retirement and Pension Plans.
The Company has a defined contribution profit sharing plan which covers substantially all of the employees, or team members, within the continental U.S. and allows participants to make contributions by salary reduction pursuant to Section 401(k) of the Internal Revenue Code. The Company currently matches 100% of the team member’s contribution, up to a maximum amount equal to 6% of the team member’s compensation. The amounts expensed under this profit sharing plan for the years ended May 31, 2012, 2011 and 2010 were $11.6 million, $10.9 million and $8.1 million, respectively.
During fiscal year 2012 the Company’s European executive officers in certain countries were eligible to participate in Europe’s defined contribution plan. Each year, in the Company’s sole discretion, the Company may contribute a percentage of employees’ pensionable salaries based on their age at January 1st. The amounts expensed under this profit sharing plan for the years ended May 31, 2012, 2011 and 2010 were $7.2 million, $6.9 million and $5.7 million, respectively.
The Company sponsors various retirement and pension plans, including defined benefit plans, for some of its foreign operations. Many foreign employees are covered by government sponsored programs for which the direct cost to the Company is not significant. Retirement plan benefits are primarily based on the employee’s
F-25
compensation during the last several years before retirement and the employee’s number of years of service for the Company. Some foreign subsidiaries have plans under which funds are deposited with trustees, annuities are purchased under group contracts or reserves are provided. The Company used May 31, 2012 and 2011 as the measurement date for the foreign pension plans.
Net periodic benefit costs for the Company’s defined benefit plans include the following components:
| (in millions) | Year Ended May 31, 2012 | Year Ended May 31, 2011 | Year Ended May 31, 2010 | ||||||||
| Net periodic benefit costs: | |||||||||||
| Service costs | $ | 0.6 | $ | 0.8 | $ | 0.6 | |||||
| Interest costs | 6.3 | 6.8 | 6.9 | ||||||||
| Expected return on plan assets | (5.6 | ) | (5.1 | ) | (3.9 | ) | |||||
| Recognized actuarial losses | 1.6 | 1.1 | 3.3 | ||||||||
| Net periodic benefit costs: | $ | 2.9 | $ | 3.6 | $ | 6.9 | |||||
The following table sets forth information related to the benefit obligation and the fair value of plan assets at May 31, 2012 and 2011 for the Company’s defined benefit retirement plans. The Company maintains no post-retirement medical or other post-retirement plans in the United States.
| (in millions) | May 31, 2012 | May 31, 2011 | |||||
| Change in Benefit Obligation | |||||||
| Projected benefit obligation—beginning of year | $ | 125.3 | $ | 111.6 | |||
| Service costs | 0.6 | 0.8 | |||||
| Interest costs | 6.3 | 6.8 | |||||
| Actuarial (gains)/losses | 10.2 | (7.7 | ) | ||||
| Benefits paid from plan | (5.2 | ) | (2.2 | ) | |||
| Effect of exchange rates | (9.1 | ) | 16.0 | ||||
| Projected benefit obligation—end of year | $ | 128.1 | $ | 125.3 | |||
| Accumulated benefit obligation | $ | 127.2 | $ | 124.2 | |||
| Change in Plan Assets | |||||||
| Plan assets at fair value—beginning of year | $ | 104.1 | $ | 82.1 | |||
| Actual return on plan assets | 10.2 | 6.2 | |||||
| Company contribution | 6.3 | 6.1 | |||||
| Plan participant contribution | — | — | |||||
| Benefits paid from plan | (5.0 | ) | (2.1 | ) | |||
| Effect of exchange rates | (6.9 | ) | 11.8 | ||||
| Plan assets at fair value—end of year | $ | 108.7 | $ | 104.1 | |||
| Unfunded status at end of year | $ | 19.4 | $ | 21.2 | |||
Amounts recognized in the Company’s consolidated balance sheets consist of the following:
| (in millions) | May 31, 2012 | May 31, 2011 | |||||
| Deferred income tax asset | $ | 6.3 | $ | (0.9 | ) | ||
| Employee related obligations | 19.4 | 21.2 | |||||
| Other comprehensive income (loss) | (3.0) | 1.2 | |||||
| Year Ended May 31, 2013 | |||
| Amounts expected to be recognized in Net Periodic Cost in the coming year for the Company’s defined benefit retirement plans (in millions) | |||
| Amortization of net actuarial losses | $ | 1.0 | |
F-26
The weighted-average assumptions in the following table represent the rates used to develop the actuarial present value of the projected benefit obligation for periods presented and also the net periodic benefit cost for the following years.
| Year Ended May 31, 2012 | Year Ended May 31, 2011 | Year Ended May 31, 2010 | ||||||
| Discount rate | 4.57 | % | 5.50 | % | 5.46 | % | ||
| Expected long-term rate of return on plan assets | 4.51 | % | 5.57 | % | 5.54 | % | ||
| Rate increase in compensation levels | 2.58 | % | 2.89 | % | 2.89 | % | ||
The projected future benefit payments from the Company’s defined benefit retirement plans are $4.9 million for fiscal 2013, $5.1 million for fiscal 2014, $5.8 million for fiscal 2015, $5.6 million for fiscal 2016, $6.1 million for fiscal 2017 and $33.4 million for fiscal 2018 to 2021. The Company expects to pay $2.4 million into the plans during fiscal 2013. In certain countries, the funding of pension plans is not a common practice. Consequently, the Company has several pension plans which are not funded.
The Company’s retirement plan asset allocation at May 31, 2012 was 48% to debt securities, 40% to equity securities, and 12% to other. The Company’s retirement plan asset allocation at May 31, 2011 was 48% to debt securities, 40% to equity securities, and 12% to other.
Strategic asset allocations are determined by country, based on the nature of the liabilities and considering demographic composition of the plan participants (average age, years of service and active versus retiree status). The Company’s plans are considered non-mature plans and the long-term strategic asset allocations are consistent with these types of plans. Emphasis is placed on diversifying on a broad basis combined with currency matching the fixed income assets.
Note 10—Accumulated Other Comprehensive Income (Loss).
Other comprehensive income (loss) includes net loss, currency translation adjustments, certain derivative-related activity, changes in the value of available-for-sale investments, and changes in prior service cost from pension plans. The Company generally deems its foreign investments to be essentially permanent in nature and does not provide for taxes on currency translation adjustments arising from translating the investment in a foreign currency to U.S. dollars. When the Company determines that a foreign investment is no longer permanent in nature, estimated taxes are provided for the related deferred tax liability (asset), if any, resulting from currency translation adjustments.
Accumulated other comprehensive income (loss) and the related components are included in the table below:
| (in millions) | May 31, 2012 | May 31, 2011 | |||||
| Unrecognized actuarial gain (loss) on pension assets, net of tax | $ | (3.0 | ) | $ | 1.2 | ||
| Foreign currency translation adjustments | 173.7 | 235.8 | |||||
| Unrealized gain (loss) on interest rate swaps, net of tax | (47.3 | ) | (60.4 | ) | |||
| Unrealized loss on available-for-sale securities, net of tax | (0.5 | ) | (4.8 | ) | |||
| Accumulated other comprehensive income | $ | 122.9 | $ | 171.8 | |||
Note 11—Share-based Compensation and Stock Plans.
The Company expenses all share-based payments to employees and non-employee distributors, including stock options and restricted stock units, based on the grant date fair value over the required award service period using the graded vesting attribution method. For awards with a performance vesting condition, the Company recognizes expense when the performance condition is considered probable to occur. Share-based compensation expense recognized for the years ended May 31, 2012, 2011 and 2010 was $16.0 million, $12.7 million and $22.4 million, respectively.
F-27
Stock Options
The Company grants stock option awards under the LVB Acquisition, Inc. 2007 Management Equity Incentive Plan (the “2007 LVB Plan”). When the 2007 LVB Plan became effective, there were 37,520,000 shares of LVB common stock reserved for issuance in connection with LVB Awards to be granted thereunder. Effective December 31, 2010, the 2007 LVB Plan was amended to increase the authorized share pool by 1,000,000 shares. During the year ended May 31, 2012, stock options were granted with an exercise price of $10.00 and a fair value of the underlying stock of $7.88 on the date of the grant and have 10-year terms. The fair value is determined by taking the average value assigned to the Company on a quarterly basis by its Sponsors, three of which have SEC periodic reporting requirements. Vesting of employee stock options are split into two categories: 1) time based options-75% of option grants generally vesting ratably over 5 years and 2) performance based options-25% of stock option grants generally vesting over 5 years, contingent upon the Company achieving certain Adjusted EBITDA targets in each of those years. As of May 31, 2012, there were 3,768,292 shares available for issuance under the 2007 LVB Plan.
In 2008, the Board of Directors of LVB adopted an addendum to the 2007 LVB Plan, which provides for the grant of leveraged equity awards in LVB under the 2007 LVB Plan (the “LVB Leveraged Awards,” and together with the LVB Options, the “LVB Awards”) to certain of the Company’s European employees. LVB Leveraged Awards permit participants to purchase shares of LVB common stock using the proceeds of non-recourse loans from LVB, which shares remain subject to forfeiture and other restrictions prior to the participant’s repayment of the loan. LVB leveraged award shares outstanding were 504,500 shares, 504,500 shares and 769,500 shares as of May 31, 2012, 2011 and 2010, respectively. All changes to the outstanding shares are due to forfeitures.
Upon termination of a participant’s employment, the 2007 LVB Plan provides that any unvested portion of a participant’s LVB Award will be forfeited, and that the vested portion of his or her LVB Award will expire on the earliest of (1) the date the participant’s employment is terminated for cause, (2) 30 days following the date the participant resigns without good reason, (3) 90 days after the date the participant’s employment is terminated either by us for any reason other than cause, death or disability or by the participant with good reason, (4) one year after the date the participant’s employment is terminated by reason of death or disability, or (5) the tenth anniversary of the grant date of the LVB Award.
In May 2009, the Board of Directors of LVB authorized an exchange offer relating to employee options outstanding at May 6, 2009 (including the options held by the Company’s named executive officers). Outstanding distributor options were not included in the exchange offer. The exchange offer was expected to provide the holders of such options with the opportunity to surrender the options for cancellation in exchange for replacement options, the terms of which were (1) different from the surrendered options with respect to the performance based and accreting exercise price options, and (2) the same as the surrendered options with respect to the time based options. The terms of the performance based and accreting exercise price options were modified in the replacement options as follows:
| • | New Performance Vesting Options (which replaced the surrendered performance based options)—Beginning in fiscal 2010, the remaining unvested options vest ratably over four to six years (depending on the date of grant) instead of the three to five years remaining under the terms of the original performance based options. The remaining options continue to vest contingent upon the Company achieving certain reduced Adjusted EBITDA targets in each of those years. |
| • | New Extended Time Vesting Options (which replaced the surrendered accreting exercise price options)—These options were converted into time vesting options similar to the previously outstanding time based options. The exercise price reverted to $10.00 per share (i.e., the original grant date exercise price before it began accreting) and will no longer increase by 10% on an annual basis. The remaining unvested options vest ratably over four to six years (depending on the date of grant) instead of the three to five years remaining under the terms of the original accreting exercise price options. |
The goal of the exchange offer was to provide employees who elected to participate with new options, the terms of which preserve the original incentive effect of the Company’s option program in light of market-wide economic conditions. In October 2009, the exchange offer was completed with all active employees electing to participate. Beginning July 2009, new option grants subsequent to, and not in connection with the exchange offer, split options into 2 categories: 1) time based options: 75% of option grants generally vesting ratably over 5 years and 2) performance based options: 25% of stock option grants generally vesting over 5 years, contingent upon the Company achieving certain Adjusted EBITDA targets in each of those years.
F-28
Prior to receiving shares of LVB common stock (whether pursuant to the exercise of LVB Options, purchased pursuant to an LVB Leveraged Award or otherwise), participants must execute a Management Stockholders’ Agreement, which provides that the shares are subject to certain transfer restrictions, put and call rights, and tag along and drag along rights (and, with respect to certain senior members of management, limited re-offer registration and preemptive rights).
The following table summarizes stock option activity for the years ended May 31, 2012, 2011 and 2010:
| Stock Options | Weighted Average Exercise Price | ||||
| Outstanding, May 31, 2009 | 32,989,833 | $ | 10.00 | ||
| Granted | 4,296,500 | 10.00 | |||
| Forfeitures | (1,999,833) | 10.00 | |||
| Outstanding, May 31, 2010 | 35,286,500 | $ | 10.00 | ||
| Granted | 2,274,000 | 10.00 | |||
| Forfeitures | (2,535,875) | 10.00 | |||
| Outstanding, May 31, 2011 | 35,024,625 | $ | 10.00 | ||
| Granted | 2,594,500 | 10.00 | |||
| Forfeitures | (2,867,417) | 10.00 | |||
| Outstanding, May 31, 2012 | 34,751,708 | $ | 10.00 | ||
The weighted average fair value of options granted during the years ended May 31, 2012, 2011 and 2010, was $1.76, $3.21 and $3.28, respectively. The Company estimates the fair value of each option primarily using the Black-Scholes option pricing model. Expected volatilities for grants are generally based on historical volatility of the Company’s competitors’ stock. The risk-free rates for periods within the expected life of the option are based on the U.S. Treasury yield curve in effect at the time of grant. As of May 31, 2012, there was approximately $7.8 million of unrecognized share-based compensation expense related to nonvested employee stock options granted under the Company’s plan and is expected to be recognized over a weighted average period of 1.6 years.
The fair value estimates are based on the following weighted average assumptions:
| May 31, 2012 | May 31, 2011 | ||||
| Risk-free interest rate | 0.87 | % | 1.85 | % | |
| Dividend yield | — | — | |||
| Expected volatility | 30.55 | % | 31.58 | % | |
| Expected life in years | 6.0 | 6.0 | |||
The following table summarizes information about outstanding stock options, as of May 31, 2012 and 2011, that were (a) vested and (b) exercisable:
| Outstanding Stock Options Already Vested and Expected to Vest | Options that are Exercisable | ||||||||||||||
| 2012 | 2011 | 2012 | 2011 | ||||||||||||
| Number of outstanding options | 34,751,708 | 35,024,625 | 21,266,528 | 19,488,874 | |||||||||||
| Weighted average remaining contractual life | 6.1 years | 7.1 years | 5.7 years | 6.8 years | |||||||||||
| Weighted average exercise price per share | $ | 10.00 | $ | 10.00 | $ | 10.00 | $ | 10.00 | |||||||
| Intrinsic value | — | — | — | — | |||||||||||
Restricted Stock Units
Effective February 10, 2011, the Board of Directors of LVB adopted and approved a Restricted Stock Unit Plan (the “RSU Plan”). The purpose of the RSU Plan is to provide executives and certain key employees with the opportunity to receive stock-based performance incentives to retain qualified individuals and to align their interests with the interests of the stockholders. The maximum number of shares of common stock, par value $0.01 per share,
F-29
that may be issued under the RSU Plan is 4,000,000, subject to adjustment as described in the RSU Plan. Under the terms of the RSU Plan, the Compensation Committee of the Board of Directors may grant participants restricted stock units each of which represents the right to receive one share of common stock, subject to certain vesting restrictions and risk of forfeiture. Once granted, the restricted stock units will be expensed over the required award service period. The restricted stock units vest under certain time-vesting and liquidity event conditions.
The following table summarizes RSU activity for the years ended May 31, 2012 and 2011:
| RSUs | Weighted Average Grant Date Fair Value | |||||
| Outstanding at June 1, 2010 | — | $ | — | |||
| Granted | 3,835,000 | 10.00 | ||||
| Vested | — | — | ||||
| Forfeited | — | — | ||||
| Outstanding at May 31, 2011 | 3,835,000 | 10.00 | ||||
| Granted | 30,000 | 10.00 | ||||
| Vested | — | — | ||||
| Forfeited | (200,000) | 10.00 | ||||
| Outstanding at May 31, 2012 | 3,665,000 | $ | 10.00 | |||
The restricted stock units are measured at their grant date fair value. The expense is recognized for the restricted stock units ultimately expected to vest, using the straight line method over the service period, which is estimated at approximately five years from the initial grant date for the grants made in the year ended May 31, 2011. As of May 31, 2012, there was approximately $29.3 million of unrecognized share-based compensation expense related to nonvested restricted stock units granted under the RSU Plan and is expected to be recognized over a weighted average period of 4.0 years.
Subsequent Events
On July 2, 2012, LVB launched a tender offer to eligible employees to exchange all of the stock options and restricted stock units held by such employees for new stock options and restricted stock units. Following the expiration of the tender offer on July 30, 2012, LVB accepted for exchange eligible options to purchase an aggregate of 29,532,500 shares of common stock of LVB and eligible restricted stock units underlying an aggregate of 3,665,000 shares of common stock of LVB. In accordance with the terms and conditions of the tender offer, on July 31, 2012, LVB granted 29,532,500 new options and 10,795,000 new restricted stock units in exchange for the cancellation of such tendered options and restricted stock units.
The objective of the tender offer was to provide employees who elected to participate with new options and new restricted stock units, the terms of which preserve the original incentive effect of the Company’s equity incentive programs in light of market and industry-wide economic conditions. The terms of the new stock options differed in respect to the tendered options principally with respect to:
| • | Exercise Price—The exercise price for the new stock options was lowered to the current fair value of $7.88 per share. |
| • | Vesting Periods—All prior options that were vested as of the completion date of the tender offer remain vested. All time-vesting options which were unvested as of the completion date of the tender offer will continue to vest on the same schedule on which they were originally granted. All unvested replacement extended time vesting options and modified performance options will vest on a schedule which is generally two years longer than the original vesting schedule, but in no case will the vesting schedule be extended past 2017. |
| • | Performance Vesting Threshold—The new modified performance options will vest over the new vesting period if, as of the end of the Company’s most recent fiscal year ending on or prior to such vesting date, Biomet, Inc. has achieved the EBITDA target for such fiscal year determined by the Compensation Committee of the Board of Directors of the Company on or before the ninetieth (90th) day of such fiscal year and consistent with the Company’s business plan. |
F-30
The terms of the new restricted stock units are different from the tendered restricted stock units with respect to the vesting schedule, performance conditions and settlement. The new restricted stock units will be granted subject to either a time-based vesting or a performance-based vesting requirement. Unlike the exchanged restricted stock units, the new restricted stock units will not vest in full on May 31, 2016 regardless of satisfaction of the vesting conditions. In addition, following the termination of employment with the Company, new restricted stock units, whether vested or unvested, will be forfeited if such employee provides services to any competitor of the Company. In addition, participants holding new restricted stock units will also receive new awards called management dividend awards representing the right to receive a cash payment. Management dividend awards vest on a one-to-one basis with each new time-based restricted stock unit. Vested management dividend awards will be paid by cash distributions promptly following each anniversary of the grant date until the earlier of an initial public offering of the Company or the fifth anniversary of the grant date, subject to withholding taxes. Upon termination of employment for any reason, management dividend awards will be forfeited. The new restricted stock units will be granted under the Company’s 2012 Restricted Stock Unit Plan, which was adopted by LVB on July 31, 2012. The maximum number of shares of common stock, par value $0.01 per share, that may be issued under the Company’s 2012 Restricted Stock Unit Plan is 14,000,000, subject to adjustment as described in the Plan.
Note 12—Income Taxes
The components of loss before income taxes are as follows:
| (in millions) | Year Ended May 31, 2012 | Year Ended May 31, 2011 | Year Ended May 31, 2010 | ||||||||
| Domestic | $ | (796.1 | ) | $ | (238.2 | ) | $ | (201.7 | ) | ||
| Foreign | 205.3 | (826.4 | ) | 60.0 | |||||||
| Total | $ | (590.8 | ) | $ | (1,064.6 | ) | $ | (141.7 | ) | ||
The income tax benefit is summarized as follows:
| (in millions) | Year Ended May 31, 2012 | Year Ended May 31, 2011 | Year Ended May 31, 2010 | ||||||||
| Current: | |||||||||||
| Federal | $ | (9.5 | ) | $ | (13.3 | ) | $ | 3.9 | |||
| State | 3.0 | 11.1 | 0.9 | ||||||||
| Foreign | 42.6 | 53.9 | 50.2 | ||||||||
| Subtotal | 36.1 | 51.7 | 55.0 | ||||||||
| Deferred: | |||||||||||
| Federal | (83.6 | ) | (43.1 | ) | (98.7 | ) | |||||
| State | (0.9 | ) | (51.2 | ) | (15.7 | ) | |||||
| Foreign | (83.6 | ) | (172.2 | ) | (34.7 | ) | |||||
| Subtotal | (168.1 | ) | (266.5 | ) | (149.1 | ) | |||||
| Total income tax benefit | $ | (132.0 | ) | $ | (214.8 | ) | $ | (94.1 | ) | ||
F-31
A reconciliation of the statutory federal income tax rate to the Company’s U.S. effective tax rate is as follows:
| Year Ended May 31, 2012 | Year Ended May 31, 2011 | Year Ended May 31, 2010 | ||||||
| U.S. statutory income tax rate | (35.0 | )% | (35.0 | )% | (35.0 | )% | ||
| State taxes, net of federal deduction | (0.5 | )% | (0.6 | )% | (8.4 | )% | ||
| Effect of foreign taxes | (1.1 | )% | (2.8 | )% | (19.8 | )% | ||
| Tax credits and other carryovers | 0.1 | % | (0.1 | )% | (4.3 | )% | ||
| Change in liability for uncertain tax positions | (3.7 | )% | 1.7 | % | 9.6 | % | ||
| Adjustment of prior estimates, net of valuation allowance | (4.1 | )% | 5.2 | % | (5.6 | )% | ||
| Goodwill impairment | 17.3 | % | 13.9 | % | — | % | ||
| Change in tax laws and rates | (2.6 | )% | (4.4 | )% | (7.1 | )% | ||
| Nondeductible / nontaxable items | (3.0 | )% | 2.6 | % | 7.0 | % | ||
| Tax on foreign earnings, net of foreign tax credits | 8.9 | % | 0.5 | % | (0.4 | )% | ||
| Other | 1.4 | % | (1.2 | )% | (2.4 | )% | ||
| Effective tax rate | (22.3 | )% | (20.2 | )% | (66.4 | )% | ||
The components of the net deferred income tax assets and liabilities at May 31, 2012 and 2011 are as follows:
| (in millions) | 2012 | 2011 | |||||
| Deferred income tax assets: | |||||||
| Accounts receivable | $ | 22.5 | $ | 19.1 | |||
| Inventories | 62.8 | 47.5 | |||||
| Accrued expenses | 48.9 | 50.1 | |||||
| Tax benefit of net operating losses, tax credits and other carryforwards | 74.9 | 41.5 | |||||
| Future benefit of uncertain tax positions | 12.1 | 20.3 | |||||
| Stock-based compensation | 39.1 | 33.3 | |||||
| Swap liability | 29.0 | 36.9 | |||||
| Other | 0.7 | 33.5 | |||||
| Deferred income tax assets | $ | 290.0 | $ | 282.2 | |||
| Less: Valuation allowance | (45.7 | ) | (38.1 | ) | |||
| Total deferred income tax assets | $ | 244.3 | $ | 244.1 | |||
| Deferred income tax liabilities: | |||||||
| Property, plant, equipment and Intangibles | (1,390.4 | ) | (1,642.0 | ) | |||
| Unremitted foreign earnings | (36.6 | ) | — | ||||
| Other | (22.6 | ) | (18.2 | ) | |||
| Total deferred income tax liabilities | (1,449.6 | ) | (1,660.2 | ) | |||
| Total net deferred income tax liabilities | $ | (1,205.3 | ) | $ | (1,416.1 | ) | |
The Company’s deferred tax assets include federal, state, and foreign net operating loss carryforwards of $5.9 million, $57.1 million ($37.1 million, net of federal benefit) and $4.8 million, respectively. Federal net operating loss carryforwards available are $16.7 million, which begin to expire in 2029. The Company believes it is more likely than not that it will be able to utilize the federal net operating loss carryforwards. The state and foreign net operating loss carryforwards are from various jurisdictions with various carryforward periods.
Deferred tax assets related to tax credits and other carryforwards total $27.1 million as of May 31, 2012. This includes a deferred tax asset for foreign tax credit carryforwards in the amount of $21.3 million, which begin to expire in 2018. The Company believes it is more likely than not that it will be able to utilize the foreign tax credit carryforwards.
As of May 31, 2012, the Company has a $45.7 million valuation allowance against deferred tax assets. This valuation allowance consists of $5.6 million relating to net deferred tax assets for unrealized losses on
F-32
investments and $40.1 million for net deferred tax assets related to state and foreign net operating losses that management believes, more likely than not, will not be realized.
A deferred tax liability is required to be established for the U.S. tax impact of undistributed earnings of non-U.S. subsidiaries unless management asserts that these earnings will be indefinitely reinvested outside the U.S. or will be remitted in a tax-free liquidation. During the fiscal year ended May 31, 2012, the Company accumulated additional cash of $136.7 million at its non-U.S. subsidiaries for which it has no specific plans for permanent reinvestment. This cash is expected to be repatriated to the United States in the form of a taxable distribution. Accordingly, the Company established a deferred tax liability of $36.6 million at May 31, 2012. As of May 31, 2012 and May 31, 2011, all other undistributed earnings of non-U.S. subsidiaries are considered to be permanently reinvested. It is not practicable to estimate the amount of deferred tax liability related to these permanently reinvested earnings.
A reconciliation of the beginning and ending amount of unrecognized tax benefits is as follows:
| (in millions) | May 31, 2012 | May 31, 2011 | May 31, 2010 | ||||||||
| Unrecognized tax benefits, beginning of period | $ | 90.9 | $ | 73.8 | $ | 63.1 | |||||
| Addition based on tax positions related to the current year | 10.9 | 20.0 | 13.8 | ||||||||
| Addition (Reduction) for tax positions of prior periods | (14.8 | ) | 5.2 | (2.7 | ) | ||||||
| Reduction related to settlements with tax authorities | (0.1 | ) | — | (0.2 | ) | ||||||
| Reduction related to lapse of statute of limitations | (23.9 | ) | (8.1 | ) | (0.2 | ) | |||||
| Unrecognized tax benefits, end of period | $ | 63.0 | $ | 90.9 | $ | 73.8 | |||||
Included in the amount of unrecognized tax benefits at May 31, 2012 and 2011 are $61.5 million and $82.9 million, respectively, of tax benefits that would impact the Company’s effective tax rate, if recognized.
The Company recognizes accrued interest and penalties related to unrecognized tax benefits as a component of income tax expense. Related to unrecognized tax benefits noted above, the Company accrued interest of $(1.7) million and $3.1 million during the years ended May 31, 2012 and 2011, respectively. The interest benefit for the year ended May 31, 2012 is primarily due to the reduction in accrued interest from the decrease in unrecognized tax benefits due to the lapse of statute of limitations. As of May 31, 2012 and 2011, the Company has recognized a liability for interest of $10.6 million and $12.3 million, respectively. The Company accrued and recognized an immaterial amount of penalties for the years disclosed.
The Company conducts business globally and, as a result, certain of its subsidiaries file income tax returns in the U.S. federal jurisdiction, and various state and foreign jurisdictions. In the normal course of business, the Company is subject to examinations by taxing authorities throughout the world, including major jurisdictions such as Australia, Canada, France, Germany, Japan, Netherlands, Spain, the United Kingdom and the United States. In addition, certain state and foreign tax returns are under examination by various regulatory authorities. The Company is no longer subject to U.S. federal income tax examinations for the fiscal years prior to and including the year ended May 31, 2002, as well as May 31, 2005 through May 31, 2008.
The Company regularly reviews issues that are raised from ongoing examinations and open tax years to evaluate the adequacy of its liabilities. As the various taxing authorities continue with their audit/examination programs, the Company will adjust its reserves accordingly to reflect these settlements. As of May 31, 2012, the Company does not anticipate a significant change in its worldwide gross liabilities for unrecognized tax benefits within the succeeding twelve months.
Puerto Rico Tax Legislation
On October 25, 2010, the government of Puerto Rico passed legislation that established a new excise tax on the purchases of products manufactured in Puerto Rico, effective January 1, 2011. Puerto Rico has subsequently provided an exemption to the excise tax provided certain employment levels are met. Management anticipates meeting these employment levels and thus expects the Company to be subject to an alternative income tax rather than the excise tax. Management does not expect this new alternative income tax to have a material impact on its financial statements.
United States Tax Legislation
F-33
Congress approved, and President Obama signed into law, The Tax Relief, Unemployment Insurance Reauthorization, and Job Creation Act of 2010, enacted December 17, 2010. This legislation includes temporary extensions of several business tax incentives, including the research and experimentation tax credit, the New Markets Tax Credit, 15-year straight-line cost recovery for qualified leasehold improvements, the exception for active financing income under Subpart F and look-through treatment of payments between related controlled foreign corporations. As a result, these extensions were included, where applicable, in determining the Company’s effective tax rate for the year ended May 31, 2011.
Note 13—Segment Reporting.
The Company operates in one reportable segment, musculoskeletal products, which includes the designing, manufacturing and marketing of large joint reconstructive; sports, extremities and trauma (“S.E.T.”); spine & bone healing; dental and other products. Other products consist primarily of microfixation products, autologous therapies, general instruments and operating room supplies. The Company operates in various geographies. These geographic markets are comprised of the United States, Europe and International. Major markets included in the International geographic market are Canada, South America, Mexico and the Asia Pacific region.
Net sales by product category for the years ended May 31, 2012, 2011 and 2010 were as follows:
| (in millions) | Year Ended May 31, 2012 | Year Ended May 31, 2011(1) | Year Ended May 31, 2010(1) | ||||||||
| Net sales by product: | |||||||||||
| Large Joint Reconstructive | $ | 1,698.8 | $ | 1,630.6 | $ | 1,615.7 | |||||
| Sports, Extremities, Trauma (S.E.T.) | 354.4 | 312.3 | 283.7 | ||||||||
| Spine & Bone Healing | 314.0 | 327.4 | 345.3 | ||||||||
| Dental | 267.7 | 269.5 | 265.2 | ||||||||
| Other | 203.2 | 192.4 | 188.1 | ||||||||
| Total | $ | 2,838.1 | $ | 2,732.2 | $ | 2,698.0 | |||||
| (1) | New product categories were adopted in order to more closely represent the way the Company reports sales and markets products. Certain amounts have been reclassified to conform to the current presentation. |
Net sales by geography for the years ended May 31, 2012, 2011 and 2010 were as follows:
| (in millions) | Year Ended May 31, 2012 | Year Ended May 31, 2011 | Year Ended May 31, 2010(1) | ||||||||
| Net sales by geography: | |||||||||||
| United States | $ | 1,713.3 | $ | 1,659.2 | $ | 1,644.1 | |||||
| Europe | 702.7 | 697.8 | 724.5 | ||||||||
| International(2) | 422.1 | 375.2 | 329.4 | ||||||||
| Total | $ | 2,838.1 | $ | 2,732.2 | $ | 2,698.0 | |||||
| (1) | Certain amounts have been adjusted to conform to the current presentation. Specifically, International net sales increased, and Europe net sales decreased, $4.3 million for the year ended May 31, 2010. The current presentation aligns with how the Company presently manages and markets its products. |
| (2) | International primarily includes Canada, South America, Mexico and the Asia Pacific region. |
F-34
Long-term assets by geography as of May 31, 2012 and 2011 were as follows:
| (in millions) | May 31, 2012 | May 31, 2011 | |||||
| Long-term assets (1) by geography: | |||||||
| United States | $ | 6,817.5 | $ | 7,199.7 | |||
| Europe | 722.7 | 1,233.7 | |||||
| International | 1,098.2 | 1,209.5 | |||||
| Total | $ | 8,638.4 | $ | 9,642.9 | |||
| (1) | Defined as property, plant and equipment, intangibles and goodwill. |
Note 14—Guarantor and Non-guarantor Financial Statements.
Each of Biomet, Inc.’s existing wholly owned domestic subsidiaries fully, unconditionally, jointly, and severally guarantee the senior cash pay and PIK toggle notes on a senior unsecured basis and the senior subordinated notes on a senior subordinated unsecured basis, in each case to the extent such subsidiaries guarantee Biomet, Inc.’s senior secured cash flow facilities. Certain amounts reported in the prior year elimination column have been corrected to more accurately reflect the allocation of intercompany profit between the guarantor and the non-guarantor subsidiaries and to conform to the current period presentation. The Company believes such amounts are immaterial. LVB Acquisition, Inc. is neither an issuer nor guarantor of the notes described in Note 6.
F-35
The following financial information illustrates the composition of the combined guarantor subsidiaries:
CONDENSED CONSOLIDATING BALANCE SHEETS
| May 31, 2012 | |||||||||||||||||||
| (in millions) | Biomet, Inc. | Guarantors | Non-Guarantors | Eliminations | Total | ||||||||||||||
| Assets | |||||||||||||||||||
| Current assets: | |||||||||||||||||||
| Cash and cash equivalents | $ | — | $ | 190.1 | $ | 302.3 | $ | — | $ | 492.4 | |||||||||
| Accounts receivable, net | — | 227.6 | 264.0 | — | 491.6 | ||||||||||||||
| Investments | — | — | 2.5 | — | 2.5 | ||||||||||||||
| Income tax receivable | — | 2.1 | 2.9 | — | 5.0 | ||||||||||||||
| Inventories, net | — | 288.7 | 254.5 | — | 543.2 | ||||||||||||||
| Deferred income taxes | — | 42.3 | 10.2 | — | 52.5 | ||||||||||||||
| Prepaid expenses and other | — | 48.8 | 75.3 | — | 124.1 | ||||||||||||||
| Total current assets | — | 799.6 | 911.7 | — | 1,711.3 | ||||||||||||||
| Property, plant and equipment, net | — | 320.1 | 273.5 | — | 593.6 | ||||||||||||||
| Investments | — | 10.1 | 3.8 | — | 13.9 | ||||||||||||||
| Investment in subsidiaries | 8,562.9 | — | — | (8,562.9 | ) | — | |||||||||||||
| Intangible assets, net | — | 3,239.3 | 691.1 | — | 3,930.4 | ||||||||||||||
| Goodwill | — | 3,271.4 | 843.0 | — | 4,114.4 | ||||||||||||||
| Other assets | — | 45.6 | 11.2 | — | 56.8 | ||||||||||||||
| Total assets | $ | 8,562.9 | $ | 7,686.1 | $ | 2,734.3 | $ | (8,562.9 | ) | $ | 10,420.4 | ||||||||
| Liabilities & Shareholder’s Equity | |||||||||||||||||||
| Current liabilities: | |||||||||||||||||||
| Current portion of long-term debt | $ | 34.3 | $ | — | $ | 1.3 | $ | — | $ | 35.6 | |||||||||
| Accounts payable | — | 71.5 | 44.7 | — | 116.2 | ||||||||||||||
| Accrued interest | 56.5 | — | — | — | 56.5 | ||||||||||||||
| Accrued wages and commissions | — | 69.5 | 52.5 | — | 122.0 | ||||||||||||||
| Other accrued expenses | — | 106.1 | 74.1 | — | 180.2 | ||||||||||||||
| Total current liabilities | 90.8 | 247.1 | 172.6 | — | 510.5 | ||||||||||||||
| Long-term debt | 5,790.0 | — | 2.2 | — | 5,792.2 | ||||||||||||||
| Deferred income taxes | — | 1,065.7 | 192.1 | — | 1,257.8 | ||||||||||||||
| Other long-term liabilities | — | 131.6 | 46.2 | — | 177.8 | ||||||||||||||
| Total liabilities | 5,880.8 | 1,444.4 | 413.1 | — | 7,738.3 | ||||||||||||||
| Shareholder’s equity | 2,682.1 | 6,241.7 | 2,321.2 | (8,562.9 | ) | 2,682.1 | |||||||||||||
| Total liabilities and shareholder’s equity | $ | 8,562.9 | $ | 7,686.1 | $ | 2,734.3 | $ | (8,562.9 | ) | $ | 10,420.4 | ||||||||
F-36
| May 31, 2011 | |||||||||||||||||||
| (in millions) | Biomet, Inc. | Guarantors | Non-Guarantors | Eliminations | Total | ||||||||||||||
| Assets | |||||||||||||||||||
| Current assets: | |||||||||||||||||||
| Cash and cash equivalents | $ | — | $ | 176.4 | $ | 151.4 | $ | — | $ | 327.8 | |||||||||
| Accounts receivable, net | — | 221.6 | 258.5 | — | 480.1 | ||||||||||||||
| Investments | — | 33.4 | 8.0 | — | 41.4 | ||||||||||||||
| Income tax receivable | — | 4.1 | 1.3 | — | 5.4 | ||||||||||||||
| Inventories, net | — | 292.1 | 290.4 | — | 582.5 | ||||||||||||||
| Deferred income taxes | — | 60.3 | 11.2 | — | 71.5 | ||||||||||||||
| Prepaid expenses and other | — | 57.1 | 52.6 | — | 109.7 | ||||||||||||||
| Total current assets | — | 845.0 | 773.4 | — | 1,618.4 | ||||||||||||||
| Property, plant and equipment, net | — | 332.2 | 306.2 | — | 638.4 | ||||||||||||||
| Investments | — | 10.0 | 23.1 | — | 33.1 | ||||||||||||||
| Investment in subsidiaries | 9,253.9 | — | — | (9,253.9 | ) | — | |||||||||||||
| Intangible assets, net | — | 3,416.6 | 1,117.8 | — | 4,534.4 | ||||||||||||||
| Goodwill | — | 3,460.8 | 1,009.3 | — | 4,470.1 | ||||||||||||||
| Other assets | — | 56.3 | 6.3 | — | 62.6 | ||||||||||||||
| Total assets | $ | 9,253.9 | $ | 8,120.9 | $ | 3,236.1 | $ | (9,253.9 | ) | $ | 11,357.0 | ||||||||
| Liabilities & Shareholder’s Equity | |||||||||||||||||||
| Current liabilities: | |||||||||||||||||||
| Current portion of long-term debt | $ | 35.9 | $ | — | $ | 1.5 | $ | — | $ | 37.4 | |||||||||
| Accounts payable | — | 48.1 | 43.0 | — | 91.1 | ||||||||||||||
| Accrued interest | 64.1 | — | — | — | 64.1 | ||||||||||||||
| Accrued wages and commissions | — | 56.7 | 48.3 | — | 105.0 | ||||||||||||||
| Other accrued expenses | — | 153.5 | 88.3 | — | 241.8 | ||||||||||||||
| Total current liabilities | 100.0 | 258.3 | 181.1 | — | 539.4 | ||||||||||||||
| Long-term debt | 5,978.8 | — | 4.1 | — | 5,982.9 | ||||||||||||||
| Deferred income taxes | — | 1,126.1 | 361.5 | — | 1,487.6 | ||||||||||||||
| Other long-term liabilities | — | 130.8 | 41.2 | — | 172.0 | ||||||||||||||
| Total liabilities | 6,078.8 | 1,515.2 | 587.9 | — | 8,181.9 | ||||||||||||||
| Shareholder’s equity | 3,175.1 | 6,605.7 | 2,648.2 | (9,253.9 | ) | 3,175.1 | |||||||||||||
| Total liabilities and shareholder’s equity | $ | 9,253.9 | $ | 8,120.9 | $ | 3,236.1 | $ | (9,253.9 | ) | $ | 11,357.0 | ||||||||
F-37
CONDENSED CONSOLIDATING STATEMENTS OF OPERATIONS AND COMPREHENSIVE INCOME (LOSS)
| Year Ended May 31, 2012 | |||||||||||||||||||
| (in millions) | Biomet, Inc. | Guarantors | Non-Guarantors | Eliminations | Total | ||||||||||||||
| Net sales | $ | — | $ | 1,769.8 | $ | 1,068.3 | $ | — | $ | 2,838.1 | |||||||||
| Cost of sales | — | 491.9 | 402.5 | — | 894.4 | ||||||||||||||
| Gross profit | — | 1,277.9 | 665.8 | — | 1,943.7 | ||||||||||||||
| Goodwill and intangible asset impairment charge | — | 264.3 | 265.5 | — | 529.8 | ||||||||||||||
| Operating expenses | — | 1,023.7 | 483.6 | — | 1,507.3 | ||||||||||||||
| Operating income (loss) | — | (10.1 | ) | (83.3 | ) | — | (93.4 | ) | |||||||||||
| Other (income) expense, net | 477.1 | 3.1 | 17.2 | — | 497.4 | ||||||||||||||
| Income (loss) before income taxes | (477.1 | ) | (13.2 | ) | (100.5 | ) | — | (590.8 | ) | ||||||||||
| Tax expense (benefit) | (181.3 | ) | 86.8 | (37.5 | ) | — | (132.0 | ) | |||||||||||
| Equity in earnings of subsidiaries | (163.0 | ) | — | — | 163.0 | — | |||||||||||||
| Net income (loss) | $ | (458.8 | ) | $ | (100.0 | ) | $ | (63.0 | ) | $ | 163.0 | $ | (458.8 | ) | |||||
| Other comprehensive income (loss) | $ | 13.1 | $ | — | $ | (62.0 | ) | $ | — | $ | (48.9 | ) | |||||||
| Total comprehensive income (loss) | $ | (445.7 | ) | $ | (100.0 | ) | $ | (125.0 | ) | $ | 163.0 | $ | (507.7 | ) | |||||
| Year Ended May 31, 2011 | |||||||||||||||||||
| (in millions) | Biomet, Inc. | Guarantors | Non-Guarantors | Eliminations | Total | ||||||||||||||
| Net sales | $ | — | $ | 1,716.5 | $ | 1,015.7 | $ | — | $ | 2,732.2 | |||||||||
| Cost of sales | — | 399.7 | 439.0 | — | 838.7 | ||||||||||||||
| Gross profit | — | 1,316.8 | 576.7 | — | 1,893.5 | ||||||||||||||
| Goodwill and intangible asset impairment charge | — | — | 941.4 | — | 941.4 | ||||||||||||||
| Operating expenses | — | 1,002.3 | 526.7 | — | 1,529.0 | ||||||||||||||
| Operating income (loss) | — | 314.5 | (891.4 | ) | — | (576.9 | ) | ||||||||||||
| Other (income) expense, net | 493.9 | (9.8 | ) | 3.6 | — | 487.7 | |||||||||||||
| Income (loss) before income taxes | (493.9 | ) | 324.3 | (895.0 | ) | — | (1,064.6 | ) | |||||||||||
| Tax expense (benefit) | (187.2 | ) | 101.0 | (128.6 | ) | — | (214.8 | ) | |||||||||||
| Equity in earnings of subsidiaries | (543.1 | ) | — | — | 543.1 | — | |||||||||||||
| Net income (loss) | $ | (849.8 | ) | $ | 223.3 | $ | (766.4 | ) | $ | 543.1 | $ | (849.8 | ) | ||||||
| Other comprehensive income (loss) | $ | 19.5 | $ | (4.0 | ) | $ | 266.9 | $ | — | $ | 282.4 | ||||||||
| Total comprehensive income (loss) | $ | (830.3 | ) | $ | 219.3 | $ | (499.5 | ) | $ | 543.1 | $ | (567.4 | ) | ||||||
| Year Ended May 31, 2010 | |||||||||||||||||||
| (in millions) | Biomet, Inc. | Guarantors | Non-Guarantors | Eliminations | Total | ||||||||||||||
| Net sales | $ | — | $ | 1,710.4 | $ | 987.6 | $ | — | $ | 2,698.0 | |||||||||
| Cost of sales | — | 405.2 | 414.7 | — | 819.9 | ||||||||||||||
| Gross profit | — | 1,305.2 | 572.9 | — | 1,878.1 | ||||||||||||||
| Operating expenses | — | 996.4 | 525.1 | — | 1,521.5 | ||||||||||||||
| Operating income (loss) | — | 308.8 | 47.8 | — | 356.6 | ||||||||||||||
| Other (income) expense, net | 514.1 | (4.0 | ) | (11.8 | ) | — | 498.3 | ||||||||||||
| Income (loss) before income taxes | (514.1 | ) | 312.8 | 59.6 | — | (141.7 | ) | ||||||||||||
| Tax expense (benefit) | (200.5 | ) | 98.5 | 7.9 | — | (94.1 | ) | ||||||||||||
| Equity in earnings of subsidiaries | 266.0 | — | — | (266.0 | ) | — | |||||||||||||
| Net income (loss) | $ | (47.6 | ) | $ | 214.3 | $ | 51.7 | $ | (266.0 | ) | $ | (47.6 | ) | ||||||
| Other comprehensive income (loss) | $ | 11.3 | $ | 1.8 | $ | (93.0 | ) | $ | — | $ | (79.9 | ) | |||||||
| Total comprehensive income (loss) | $ | (36.3 | ) | $ | 216.1 | $ | (41.3 | ) | $ | (266.0 | ) | $ | (127.5 | ) | |||||
F-38
CONDENSED CONSOLIDATING STATEMENTS OF CASH FLOWS
| Year Ended May 31, 2012 | |||||||||||||||||||
| (in millions) | Biomet, Inc. | Guarantor | Non-Guarantors | Eliminations | Total | ||||||||||||||
| Cash flows provided by (used in) operating activities | $ | (455.6 | ) | $ | 384.7 | $ | 285.2 | $ | 163.0 | $ | 377.3 | ||||||||
| Proceeds from sales/maturities of investments | — | 42.1 | — | — | 42.1 | ||||||||||||||
| Capital expenditures | — | (89.9 | ) | (89.4 | ) | — | (179.3 | ) | |||||||||||
| Other | 492.3 | (323.2 | ) | (12.9 | ) | (163.0 | ) | (6.8 | ) | ||||||||||
| Cash flows provided by (used in) investing activities | 492.3 | (371.0 | ) | (102.3 | ) | (163.0 | ) | (144.0 | ) | ||||||||||
| Payments under senior secured credit facilities | (35.4 | ) | — | — | — | (35.4 | ) | ||||||||||||
| Other | (1.3 | ) | — | (1.4 | ) | — | (2.7 | ) | |||||||||||
| Cash flows used in financing activities | (36.7 | ) | — | (1.4 | ) | — | (38.1 | ) | |||||||||||
| Effect of exchange rate changes on cash | — | — | (30.6 | ) | — | (30.6 | ) | ||||||||||||
| Increase in cash and cash equivalents | — | 13.7 | 150.9 | — | 164.6 | ||||||||||||||
| Cash and cash equivalents, beginning of period | — | 176.4 | 151.4 | — | 327.8 | ||||||||||||||
| Cash and cash equivalents, end of period | $ | — | $ | 190.1 | $ | 302.3 | $ | — | $ | 492.4 | |||||||||
| Year Ended May 31, 2011 | |||||||||||||||||||
| (in millions) | Biomet, Inc. | Guarantor | Non-Guarantors | Eliminations | Total | ||||||||||||||
| Cash flows provided by (used in) operating activities | $ | (844.6 | ) | $ | 432.7 | $ | 244.9 | $ | 543.1 | $ | 380.1 | ||||||||
| Proceeds from sales/maturities of investments | — | 59.3 | — | — | 59.3 | ||||||||||||||
| Purchases of investments | — | (78.7 | ) | — | — | (78.7 | ) | ||||||||||||
| Capital expenditures | — | (81.4 | ) | (92.6 | ) | — | (174.0 | ) | |||||||||||
| Other | 894.3 | (263.0 | ) | (99.8 | ) | (543.1 | ) | (11.6 | ) | ||||||||||
| Cash flows provided by (used in) investing activities | 894.3 | (363.8 | ) | (192.4 | ) | (543.1 | ) | (205.0 | ) | ||||||||||
| Payments under senior secured credit facilities | (34.8 | ) | — | — | — | (34.8 | ) | ||||||||||||
| Other | (14.9 | ) | — | (1.7 | ) | — | (16.6 | ) | |||||||||||
| Cash flows used in financing activities | (49.7 | ) | — | (1.7 | ) | — | (51.4 | ) | |||||||||||
| Effect of exchange rate changes on cash | — | — | 15.0 | — | 15.0 | ||||||||||||||
| Increase in cash and cash equivalents | — | 72.9 | 65.8 | — | 138.7 | ||||||||||||||
| Cash and cash equivalents, beginning of period | — | 103.5 | 85.6 | — | 189.1 | ||||||||||||||
| Cash and cash equivalents, end of period | $ | — | $ | 176.4 | $ | 151.4 | $ | — | $ | 327.8 | |||||||||
F-39
| Year Ended May 31, 2010 | |||||||||||||||||||
| (in millions) | Biomet, Inc. | Guarantor | Non-Guarantors | Eliminations | Total | ||||||||||||||
| Cash flows provided by (used in) operating activities | $ | (40.0 | ) | $ | 457.2 | $ | 170.3 | $ | (266.0 | ) | $ | 321.5 | |||||||
| Capital expenditures | — | (94.7 | ) | (91.7 | ) | — | (186.4 | ) | |||||||||||
| Other | 151.4 | (437.9 | ) | 24.9 | 266.0 | 4.4 | |||||||||||||
| Cash flows provided by (used in) investing activities | 151.4 | (532.6 | ) | (66.8 | ) | 266.0 | (182.0 | ) | |||||||||||
| Payments under revolving credit agreements | (65.2 | ) | — | (68.9 | ) | — | (134.1 | ) | |||||||||||
| Payments under senior secured credit facilities | (35.8 | ) | — | — | — | (35.8 | ) | ||||||||||||
| Other | (10.4 | ) | — | 20.4 | — | 10.0 | |||||||||||||
| Cash flows used in financing activities | (111.4 | ) | — | (48.5 | ) | — | (159.9 | ) | |||||||||||
| Effect of exchange rate changes on cash | — | — | (6.1 | ) | — | (6.1 | ) | ||||||||||||
| Increase (decrease) in cash and cash equivalents | — | (75.4 | ) | 48.9 | — | (26.5 | ) | ||||||||||||
| Cash and cash equivalents, beginning of period | — | 178.9 | 36.7 | — | 215.6 | ||||||||||||||
| Cash and cash equivalents, end of period | $ | — | $ | 103.5 | $ | 85.6 | $ | — | $ | 189.1 | |||||||||
F-40
Note 15—Restructuring
The Company recorded $17.9 million, $10.0 million and $6.2 million in employee severance costs during the years ended May 31, 2012, 2011 and 2010, respectively. The expense during fiscal 2012 resulted primarily from the global reconstructive products reorganization program and the planned closure of the Swindon, United Kingdom manufacturing facility. The expense during fiscal 2011 resulted primarily from the transition of our trauma hardware business from our Parsippany, New Jersey operations to our Warsaw, Indiana-based U.S. Orthopedics division. The expense during fiscal 2010 resulted primarily from the global cost savings program to better manage the Company’s cost base in response to the slowdown in consumer spending which was negatively affecting sales and operating margins that was initiated in fiscal 2009. These restructuring charges were recorded within cost of sales, selling, general and administrative expense, and research and development expense and other accrued expenses. A summary of the severance and benefit costs in the periods presented is as follows:
| (in millions) | Employee Severance and Benefit Costs | ||
| Restructuring Accrual: | |||
| Balance at May 31, 2009 | $ | 5.6 | |
| Costs incurred and charged to expense | 6.2 | ||
| Costs paid or otherwise settled | (8.6 | ) | |
| Non-cash adjustments (1) | (0.4 | ) | |
| Balance at May 31, 2010 | 2.8 | ||
| Costs incurred and charged to expense | 10.0 | ||
| Costs paid or otherwise settled | (7.0 | ) | |
| Non-cash adjustments (1) | 0.1 | ||
| Balance at May 31, 2011 | 5.9 | ||
| Costs incurred and charged to expense | 17.9 | ||
| Costs paid or otherwise settled | (14.2 | ) | |
| Non-cash adjustments (1) | (1.7 | ) | |
| Balance at May 31, 2012 | $ | 7.9 | |
| (1) | Primarily related to foreign currency fluctuations. |
Note 16—Contingencies.
The Company is involved in various proceedings, legal actions and claims arising in the normal course of business, including proceedings related to product liability, governmental investigations, intellectual property, commercial litigation and other matters. The outcomes of these matters will generally not be known for an extended period of time. In certain of the legal proceedings, the claimants seek damages, as well as other compensatory relief, which could result in the payment of significant claims and settlements. For legal matters for which management has sufficient information to reasonably estimate the Company’s future obligations, a liability representing management’s best estimate of the probable cost, or the minimum of the range of probable losses when a best estimate within the range is not known, for the resolution of these legal matters is recorded. The estimates are based on consultation with legal counsel, previous settlement experience and settlement strategies. The Company’s accrual for contingencies at May 31, 2012 and May 31, 2011 of $25.5 million and $30.6 million, respectively, primarily relate to product liability claims, the Massachusetts U.S. Department of Justice EBI products investigation and the Foreign Corrupt Practices Act (“FCPA”) investigation discussed below for which the Company is subject to self-insured limits and has estimated a probable settlement amount, and in the case of the FCPA investigation has settled as described below.
Note 16—Contingencies, Continued.
Based on the advice of the Company’s counsel in these matters, it is unlikely that the resolution of any of these matters and any liabilities in excess of amounts provided will be material to the Company’s financial position, results of operations or cash flows.
F-41
Other than the Massachusetts U.S. Department of Justice EBI products investigation, for which the estimated loss is included in the accrual referenced above, given the relatively early stages of the other governmental investigations described below and the preliminary nature of the trade secret litigation discussed below, and the complexities involved in these matters, the Company is unable to estimate a possible loss or range of possible loss for such matters until the Company knows, among other factors, (i) what claims, if any will survive dispositive motion practice, (ii) the extent of the claims, including the size of any potential class, particularly when damages are not specified or are indeterminate, (iii) how the discovery process will affect the litigation, (iv) the settlement posture of the other parties to the litigation and (v) any other factors that may have a material effect on the litigation.
U.S. Department of Justice Consulting Agreement Investigation
On September 27, 2007, Biomet entered into a Deferred Prosecution Agreement with the U.S. Attorney’s Office for the District of New Jersey. The agreement concluded the government’s investigation into whether consulting agreements between the largest orthopedic manufacturers and orthopedic surgeons who use joint reconstruction and replacement products may have violated the federal Anti-Kickback Statute.
Through the agreement, the U.S. Attorney’s Office agreed not to prosecute Biomet in connection with this matter, provided that Biomet satisfied its obligations under the agreement over the 18 months following the date of the Deferred Prosecution Agreement. The agreement called for the appointment of an independent monitor to review Biomet’s compliance with the agreement, particularly in relation to its consulting agreements. On March 27, 2009, the Deferred Prosecution Agreement expired and the complaint was dismissed with prejudice.
As part of the resolution of this matter, Biomet also entered into a Corporate Integrity Agreement with the Office of the Inspector General of the U.S. Department of Health and Human Services. The agreement requires the Company for five years subsequent to September 27, 2007 to continue to adhere to its Code of Business Conduct and Ethics and certain other provisions, including reporting requirements.
U.S. Department of Justice EBI Products Investigations and Other Matters
In February 2010, Biomet received a subpoena from the Office of the Inspector General of the U.S. Department of Health and Human Services requesting various documents relating to agreements or arrangements between physicians and the Company’s Interpore Cross subsidiary for the period from 1999 through the present and the marketing and sales activities associated with Interpore Cross’ spinal products. Biomet is cooperating with the request of the Office of the Inspector General. The Company can make no assurances as to the time or resources that will be needed to devote to this inquiry or its final outcome.
In April 2009, Biomet received an administrative subpoena from the U.S. Attorney’s Office for the District of Massachusetts requesting various documents relating primarily to the Medicare reimbursement of and certain business practices related to the Company’s EBI subsidiary’s non-invasive bone growth stimulators. It is the Company’s understanding that competitors in the non-invasive bone growth stimulation market received similar subpoenas. The Company received subsequent subpoenas in connection with the investigation in September 2009, June 2010, February 2011 and March 2012 along with several informal requests for information. Biomet has produced responsive documents and is fully cooperating in the investigation.
In April 2009, the Company became aware of a qui tam complaint alleging violations of the federal and various state False Claims Acts filed in the United States District Court for the District of Massachusetts, where it is currently pending. Biomet, Parent, and several of the Company’s competitors in the non-invasive bone growth stimulation market were named as defendants in this action. The allegations in the complaint are similar in nature to certain categories of requested documents in the above-referenced administrative subpoenas. The U.S. government has not intervened in the action. The Company is vigorously defending this matter and intends to continue to do so.
U.S. Department of Justice Civil Division Investigation
In September 2010, Biomet, received a Civil Investigative Demand (“CID”) issued by the U.S. Department of Justice—Civil Division pursuant to the False Claims Act. The CID requests that the Company provide documents and testimony related to allegations that Biomet, OtisMed Corp. and Stryker Corp. have violated the False Claims Act relating to the marketing of, and payment submissions for, OtisMed’s OtisKneeTM (a registered trademark of OtisMed) knee replacement system. The Company has produced responsive documents and is fully cooperating in the investigation.
F-42
U.S. Securities and Exchange Commission Informal Investigation
On September 25, 2007, Biomet received a letter from the SEC informing the Company that it is conducting an informal investigation regarding possible violations of the Foreign Corrupt Practices Act in the sale of medical devices in certain foreign countries by companies in the medical devices industry. The Foreign Corrupt Practices Act prohibits U.S. companies and their officers, directors, employees, or shareholders acting on their behalf and agents from offering, promising, authorizing or making payments to foreign officials for the purpose of obtaining or retaining business abroad or otherwise obtaining favorable treatment and this law requires companies to maintain records which fairly and accurately reflect transactions and to maintain internal accounting controls. In many countries, hospitals and clinics are government-owned and healthcare professionals employed by such hospitals and clinics, with whom the Company regularly interacts, may meet the definition of a foreign official for purposes of the Foreign Corrupt Practices Act. On November 9, 2007, the Company received a letter from the Department of Justice requesting any information provided to the SEC be provided to the Department of Justice on a voluntary basis.
On March 26, 2012, Biomet entered into a Deferred Prosecution Agreement (“DPA”) with the U.S. Department of Justice (“DOJ”) and a Consent to Final Judgment (“Consent Agreement”) with the SEC related to these investigations by the DOJ and the SEC. Pursuant to the DPA, the DOJ has agreed not to prosecute the Company in connection with this matter, provided that the Company satisfies its obligations under the agreement over the next three years. In addition, pursuant to the terms of the DPA, an independent external compliance monitor will be appointed to review the Company’s compliance with the DPA, particularly in relation to the Company’s international sales practices, for at least the first 18 months of the three year term of the DPA. The Company has also agreed to pay a monetary penalty of $17.3 million to resolve the charges brought by the DOJ, which was paid in the fourth fiscal quarter of 2012. The terms of the DPA and the associated monetary penalty reflect the Company’s full cooperation throughout the investigation.
The Company has contemporaneously reached a Consent Agreement with the SEC to settle civil claims related to this matter. As part of the Consent Agreement, Biomet has agreed to the SEC’s entry of a Final Judgment requiring Biomet to disgorge profits and pay prejudgment interest in the aggregate amount of $5.6 million, which was paid in the fiscal fourth quarter of 2012.
Other Matters
In January 2009, Heraeus Kulzer GmbH initiated legal proceedings in Germany against Biomet and its subsidiary, Biomet Europe BV, alleging that the Company and Biomet Europe BV misappropriated Heraeus Kulzer trade secrets when developing its new lines of European bone cements, which were first marketed in 2005. The lawsuit seeks damages in excess of €30 million and injunctive relief to preclude the Company from producing its current line of European bone cements. The Company is vigorously defending this matter and intends to continue to do so.
There are various other claims, lawsuits, disputes with third parties, investigations and pending actions involving various allegations against the Company incident to the operation of its business, principally product liability and intellectual property cases. Each of these matters is subject to various uncertainties, and it is possible that some of these matters may be resolved unfavorably to the Company. The Company accrues for losses that are deemed to be probable and subject to reasonable estimate.
Note 17—Related Parties.
Management Services Agreement
Upon completion of the Transactions, Biomet entered into a management services agreement with certain affiliates of the Sponsors, pursuant to which such affiliates of the Sponsors or their successors assigns, affiliates, officers, employees, and/or representatives and third parties (collectively, the “Managers”) provide management, advisory, and consulting services to the Company. Pursuant to such agreement, the Managers received a transaction fee equal to 1% of total enterprise value of the Transactions for the services rendered by such entities related to the Transactions upon entering into the agreement, and the Sponsors receive an annual monitoring fee equal to 1% of the Company’s annual Adjusted EBITDA (as defined in the credit agreement) as compensation for the services rendered and reimbursement for out-of-pocket expenses incurred by the Managers in connection with the agreement and the Transactions. The Company is required to pay the Sponsors the monitoring fee on a quarterly basis in arrears. The total amount of Sponsor fees was $10.3 million, $10.1 million and $10.1 million for the years ended
F-43
May 31, 2012, 2011 and 2010, respectively. The Company may also pay certain subsequent fees to the Managers for advice rendered in connection with financings or refinancings (equity or debt), acquisitions, dispositions, spin-offs, split-offs, dividends, recapitalizations, an initial underwritten public offering and change of control transactions involving the Company or any of its subsidiaries. The management services agreement includes customary exculpation and indemnification provisions in favor of the Managers and their affiliates.
Amended and Restated Limited Liability Company Operating Agreement of Holding
On September 27, 2007, certain investment funds associated with or designated by the Sponsors (the “Sponsor Funds”) entered into an amended and restated limited liability company operating agreement, or the “LLC Agreement,” in respect of Holding. The LLC Agreement contains agreements among the parties with respect to the election of the Company’s directors and the directors of its parent companies, restrictions on the issuance or transfer of interests in the Company and other corporate governance provisions (including the right to approve various corporate actions).
Pursuant to the LLC Agreement, each of the Sponsors has the right to nominate, and has nominated, two directors to Biomet’s and LVB’s Board of Directors and also is entitled to appoint one non-voting observer to the Board of Directors for so long as such Sponsor remains a member of Holding. In addition to their right to appoint non-voting observers to the Board of Directors, certain of the Sponsor Funds have certain other management rights to the extent that any such Sponsor Fund is required to operate as a “venture capital operating company” as defined in the regulations issued by the U.S. Department of Labor at Section 2510.3-101 of Part 2510 of Chapter XXV, Title 29 of the Code of Federal Regulations, or any successor regulations. Each Sponsor’s right to nominate directors is freely assignable to funds affiliated with such Sponsor, and is assignable to non-affiliates of such Sponsor only if the assigning Sponsor transfers its entire interest in Holding not previously transferred and only with the prior written consent of the Sponsors holding at least 70% of the membership interests in Holding, or “requisite Sponsor consent”. In addition to their rights under the LLC Agreement, the Sponsors may also appoint one or more persons unaffiliated with any of the Sponsors to the Board of Directors. Following Purchaser’s purchase of the Shares tendered in the Offer, the Sponsors jointly appointed Dane A. Miller, Ph.D. and Jeffrey R. Binder to the Board of Directors in addition to the two directors appointed by each of the Sponsors.
Pursuant to the LLC Agreement, each director has one vote for purposes of any Board of Directors action, and all decisions of the Board of Directors require the approval of a majority of the directors designated by the Sponsors. In addition, the LLC Agreement provides that certain major decisions regarding the Company or its parent companies require the requisite Sponsor consent.
The LLC Agreement includes certain customary agreements with respect to restrictions on the issuance or transfer of interests in Biomet and LVB, including preemptive rights, tag-along rights and drag-along rights.
The Co-Investors have also been admitted as members of Holding, both directly and through Sponsor-controlled investment vehicles. Although the Co-Investors are therefore parties to the LLC Agreement, they have no rights with respect to the election of Biomet’s or LVB’s directors or the approval of its corporate actions.
The Sponsors have also caused Holding and Parent to enter into an agreement with the Company obligating the Company and Parent to take all actions necessary to give effect to the corporate governance, preemptive rights, transfer restriction and certain other provisions of the LLC Agreement, and prohibiting the Company and Parent from taking any actions that would be inconsistent with such provisions of the LLC Agreement.
Registration Rights Agreements
The Sponsor Funds and the Co-Investors also entered into a registration rights agreement with Holding, LVB and Biomet upon the closing of the Transactions. Pursuant to this agreement, the Sponsor Funds have the power to cause Holding, LVB and Biomet to register their, the Co-Investors’ and certain other persons’ equity interests under the Securities Act and to maintain a shelf registration statement effective with respect to such interests. The agreement also entitles the Sponsor Funds and the Co-Investors to participate in any future registration of equity interests under the Securities Act that Holding, LVB or Biomet may undertake.
On October 16, 2007, Goldman, Sachs & Co. and the other initial purchasers of the existing senior notes entered into a registration rights agreement with Biomet. Pursuant to this agreement, Biomet is obligated, for the sole benefit of Goldman, Sachs & Co. in connection with its market-making activities with respect to the existing senior notes, to file a registration statement under the Securities Act in a form approved by Goldman, Sachs & Co.
F-44
and to keep such registration statement continually effective for so long as Goldman, Sachs & Co. may be required to deliver a prospectus in connection with transactions in the existing senior notes and to supplement or make amendments to such registration statement as when required by the rules and regulations applicable to such registration statement. On August 8, 2012, Goldman, Sachs & Co. and the other initial purchasers of the new senior notes entered into a registration rights agreement with Biomet providing for similar registration rights with respect to the new senior notes.
Management Stockholders’ Agreements
On September 13, 2007 and November 6, 2007, Holding, LVB and the Sponsor Funds entered into stockholders agreements with certain of the Company’s senior executives and other management stockholders. Pursuant to the terms of the LVB Acquisition, Inc. Management Equity Incentive Plan, LVB Acquisition, Inc. Restricted Stock Unit Plan and LVB Acquisition, Inc. 2012 Restricted Stock Unit Plan, participants who exercise their vested options or settle their vested RSUs are required to become parties to the agreement dated November 6, 2007. The stockholder agreements contain agreements among the parties with respect to restrictions on the transfer and issuance of shares, including preemptive, drag-along, tag-along, and call/put rights.
Consulting Agreements
On January 14, 2010, Biomet entered into a consulting agreement with Dr. Dane A. Miller Ph.D., pursuant to which it will pay Dr. Miller a consulting fee of $0.25 million per fiscal year for Dr. Miller’s consulting services and will reimburse Dr. Miller for out-of-pocket fees and expenses relating to an off-site office and administrative support in an amount of $0.1 million per year. The term of the agreement extends through the earlier of September 1, 2011, an initial public offering or a change of control. The agreement also contains certain restrictive covenants prohibiting Dr. Miller from competing with the Company and soliciting employees of the Company during the term of the agreement and for a period of one year following such term. On September 6, 2011, the Company entered into an amendment to the consulting agreement with Dr. Miller, pursuant to which it agreed to increase the expenses relating to an off-site office and administrative support from $0.1 million per year to $0.15 million per year and extend the term of the agreement through the earlier of September 1, 2013, an initial public offering or a change of control. Dr. Miller received payments under the consulting agreement of $0.4 million, $0.25 million and $0.4 million for the years ended May 31, 2012, 2011 and 2010, respectively.
Indemnification Priority Agreement
On January 11, 2010, Biomet and LVB entered into an indemnification priority agreement with the Sponsors (or certain affiliates designated by the Sponsors) pursuant to which Biomet and LVB clarified certain matters regarding the existing indemnification and advancement of expenses rights provided by Biomet and LVB pursuant to their respective charters and the management services agreement described above. In particular, pursuant to the terms of the indemnification agreement, Biomet acknowledged that as among Biomet, LVB and the Sponsors and their respective affiliates, the obligation to indemnify or advance expenses to any director appointed by any of the Sponsors will be payable in the following priority: Biomet will be the primary source of indemnification and advancement; LVB will be the secondary source of indemnification and advancement; and any obligation of a Sponsor-affiliated indemnitor to indemnify or advance expenses to such director will be tertiary to Biomet’s and, then, LVB obligations. In the event that either Biomet or LVB fails to indemnify or advance expenses to any such director in contravention of its obligations, and any Sponsor-affiliated indemnitor makes any indemnification payment or advancement of expenses to such director on account of such unpaid liability, such Sponsor-affiliated indemnitor will be subrogated to the rights of such director under any such Biomet or LVB indemnification agreement.
Equity Healthcare
Effective January 1, 2009, Biomet entered into an employer health program agreement with Equity Healthcare LLC (“Equity Healthcare”). Equity Healthcare negotiates with providers of standard administrative services for health benefit plans as well as other related services for cost discounts and quality of service monitoring capability by Equity Healthcare. Because of the combined purchasing power of its client participants, Equity Healthcare is able to negotiate pricing terms for providers that are believed to be more favorable than the companies could obtain for themselves on an individual basis.
In consideration for Equity Healthcare’s provision of access to these favorable arrangements and its monitoring of the contracted third parties’ delivery of contracted services to the Company, the Company pays Equity
F-45
Healthcare a fee of $2 per participating employee per month (“PEPM Fee”). As of May 31, 2012, the Company had approximately 3,200 employees enrolled in its health benefit plans in the United States.
Equity Healthcare may also receive a fee (“Health Plan Fees”) from one or more of the health plans with whom Equity Healthcare has contractual arrangements if the total number of employees joining such health plans from participating companies exceeds specified thresholds. If and when Equity Healthcare reaches the point at which the aggregate of its receipts from the PEPM Fee and the Health Plan Fees have covered all of its allocated costs, it will apply the incremental revenues derived from all such fees to (a) reduce the PEPM Fee otherwise payable by the Company; (b) avoid or reduce an increase in the PEPM Fee that might otherwise have occurred on contract renewal; or (c) arrange for additional services to the Company at no cost or reduced cost.
Equity Healthcare is an affiliate of Blackstone, with whom Michael Dal Bello and David McVeigh, members of the Company’s Board of Directors, are affiliated and in which they may have an indirect pecuniary interest.
There were payments of $0.1 million and $0.1 million made during the years ended May 31, 2012 and 2011, respectively, and no payments made during the fiscal year ended May 31, 2010.
Core Trust Purchasing Group Participation Agreement
Effective May 1, 2007, Biomet entered into a 5-year participation agreement (“Participation Agreement”) with Core Trust Purchasing Group, a division of HealthTrust Purchasing Corporation (“CPG”), designating CPG as the Company’s exclusive “group purchasing organization” for the purchase of certain products and services from third party vendors. CPG secures from vendors pricing terms for goods and services that are believed to be more favorable than participants in the group purchasing organization could obtain for themselves on an individual basis. Under the participation agreement, the Company must purchase 80% of the requirements of its participating locations for core categories of specified products and services, from vendors participating in the group purchasing arrangement with CPG or CPG may terminate the contract. In connection with purchases by its participants (including the Company), CPG receives a commission from the vendors in respect of such purchases. The total amount of fees paid to CPG were $0.5 million, $0.2 million and $0.2 million for the years ended May 31, 2012, 2011 and 2010, respectively.
Although CPG is not affiliated with Blackstone, in consideration for Blackstone’s facilitating Biomet’s participation in CPG and monitoring the services CPG provides to the Company, CPG remits a portion of the commissions received from vendors in respect of the Company’s purchases under the Participation Agreement to an affiliate of Blackstone, with whom Michael Dal Bello and David McVeigh, members of the Company’s Board of Directors, are affiliated and in which they may have an indirect pecuniary interest.
Other
Biomet currently holds interest rate swaps with Goldman Sachs. As part of this relationship, the Company receives information from Goldman Sachs that allows it to perform a regression on the swaps as part of its required effectiveness testing on a quarterly basis.
Biomet, Inc. may from time to time, depending upon market conditions, seek to purchase debt securities issued by Biomet or its subsidiaries in open market or privately negotiated transactions or by other means. Biomet understands that its indirect controlling stockholders may from time to time also seek to purchase debt securities issued by the Company or its subsidiaries in open market or privately negotiated transactions or by other means.
The Company engaged Capstone Consulting LLC, a consulting company that works exclusively with KKR and its portfolio companies to provide analysis for certain restructuring initiatives. The Company or its affiliates paid Capstone $1.9 million and $0.7 million during the years ended May 31, 2012 and 2011, respectively, with no payments during the fiscal year ended May 31, 2010.
Capital Contributions and Share Repurchases
At the direction of LVB, Biomet funded the repurchase of common shares of its parent company of $1.3 million, $3.7 million and $1.7 million for the years ended May 31, 2012, 2011 and 2010, respectively, from former employees pursuant to the LVB Acquisition, Inc. Management Stockholders’ Agreement. There were no additional contributions for the years ended May 31, 2012, 2011 and 2010.
F-46
Note 18—Subsequent Events
Biomet 3i Dental Business
On June 4, 2012, the Company announced its decision to pursue strategic exploratory work to separate the Biomet 3i dental business in a tax-free spin-off. Any such transaction would be subject to customary conditions, including receipt of regulatory approvals, an opinion from tax counsel and a favorable ruling from the Internal Revenue Service to ensure the tax-free status of the spin-off, execution of intercompany agreements, further due diligence as appropriate, and final approval by the Board of Directors. There can be no assurance that the evaluation of a potential separation of the Company’s Biomet 3i dental business will result in a separation.
F-47
Financial Statement Schedules
Biomet, Inc. and Subsidiaries Schedule II—Valuation and Qualifying Accounts
For the years ended May 31, 2012, 2011 and 2010:
| (in millions) | Additions | ||||||||||||||
| Description | Balance at Beginning of Period | Charged to Costs and Expenses | Charged to Other Accounts | Deductions | Balance at End of Year | ||||||||||
| Allowance for doubtful receivables: | |||||||||||||||
| For the year ended | |||||||||||||||
| May 31, 2012 | $ | 38.2 | $ | 15.7 | $ (16.2)(B) | $ (1.2)(A) | $ | 36.5 | |||||||
| For the year ended | |||||||||||||||
| May 31, 2011 | $ | 40.6 | $ | 13.8 | $ (12.3)(B) | $ (3.9)(A) | $ | 38.2 | |||||||
| For the year ended | |||||||||||||||
| May 31, 2010 | $ | 48.9 | $ 22.8(C) | $(11.3)(B) (C) | $ (19.8)(A) | $ | 40.6 | ||||||||
Notes:
| (A) | Uncollectible accounts written off. |
| (B) | Primarily effect of foreign currency translation. |
| (C) | For the year ended May 31, 2010, $38.9 million of net accounts receivables related to Greece were reclassified to long-term assets due to the proposal of the Greek government to settle certain debts with the issuance of zero-coupon bonds not expected to be settled in the next twelve months. These net accounts receivables included $8.4 million of Greece allowance for doubtful receivables, which is included above in the effect of foreign currency translation amount, and also included above in the deductions amount. |
F-48
Quarterly Results (Unaudited)
Fiscal 2012
| • | Net loss for the fourth quarter of fiscal 2012 was impacted by a goodwill and intangible asset impairment charge of $529.8 million primarily related to evidence of declining industry market growth rates in certain European and Asia Pacific markets and unfavorable margin trends resulting from change in product mix in our dental reconstructive reporting unit and declining growth rates as compared to the original merger assumptions for our spine & bone healing reporting unit. |
| Quarter ended | |||||||||||||||||||
| (in millions) | August 31, 2011 | November 30, 2011 | February 29, 2012 | May 31, 2012 | Fiscal year ended May 31, 2012 | ||||||||||||||
| Fiscal 2012 | |||||||||||||||||||
| Net sales | $ | 664.6 | $ | 725.1 | $ | 708.9 | $ | 739.5 | $ | 2,838.1 | |||||||||
| Gross profit | 449.3 | 490.2 | 489.2 | 515.0 | 1,943.7 | ||||||||||||||
| Net loss | (39.2 | ) | (14.0 | ) | (16.5 | ) | (389.1 | ) | (458.8 | ) | |||||||||
F-49
Fiscal 2011
| • | Net loss for the fourth quarter of fiscal 2011 was impacted by a goodwill and intangible asset impairment charge of $941.4 million related primarily to the continued market slowdown in Europe relative to our original purchase accounting assumptions at the time of the Merger. |
| Quarter ended | |||||||||||||||||||
| August 31, 2010 | November 30, 2010 | February 28, 2011 | May 31, 2011 | Fiscal year ended May 31, 2011 | |||||||||||||||
| (in millions) | |||||||||||||||||||
| Fiscal 2011 | |||||||||||||||||||
| Net sales | $ | 640.7 | $ | 698.3 | $ | 678.0 | $ | 715.2 | $ | 2,732.2 | |||||||||
| Gross profit | 446.7 | 490.8 | 469.9 | 486.1 | 1,893.5 | ||||||||||||||
| Net loss | (17.8) | (7.6) | (11.6) | (812.8) | (849.8 | ) | |||||||||||||
F-50
Biomet, Inc. and Subsidiaries Condensed Consolidated Balance Sheets
(in millions, except shares)
| (Unaudited) | |||||||
| February 28, 2013 | May 31, 2012 | ||||||
| Assets | |||||||
| Current assets: | |||||||
| Cash and cash equivalents | $ | 217.4 | $ | 492.4 | |||
| Accounts receivable, less allowance for doubtful accounts receivables of $36.0 ($36.5 at May 31, 2012) | 545.9 | 491.6 | |||||
| Investments | — | 2.5 | |||||
| Income tax receivable | 4.4 | 5.0 | |||||
| Inventories | 643.3 | 543.2 | |||||
| Deferred income taxes | 62.1 | 52.5 | |||||
| Prepaid expenses and other | 129.9 | 124.1 | |||||
| Total current assets | 1,603.0 | 1,711.3 | |||||
| Property, plant and equipment, net | 679.4 | 593.6 | |||||
| Investments | 22.1 | 13.9 | |||||
| Intangible assets, net | 3,662.4 | 3,930.4 | |||||
| Goodwill | 3,927.5 | 4,114.4 | |||||
| Other assets | 107.3 | 56.8 | |||||
| Total assets | $ | 10,001.7 | $ | 10,420.4 | |||
| Liabilities & Shareholder's Equity | |||||||
| Current liabilities: | |||||||
| Current portion of long-term debt | $ | 34.5 | $ | 35.6 | |||
| Accounts payable | 87.2 | 116.2 | |||||
| Accrued interest | 43.9 | 56.5 | |||||
| Accrued wages and commissions | 130.4 | 122.0 | |||||
| Other accrued expenses | 189.0 | 180.2 | |||||
| Total current liabilities | 485.0 | 510.5 | |||||
| Long-term liabilities: | |||||||
| Long-term debt, net of current portion | 5,943.9 | 5,792.2 | |||||
| Deferred income taxes | 1,100.9 | 1,257.8 | |||||
| Other long-term liabilities | 205.9 | 177.8 | |||||
| Total liabilities | 7,735.7 | 7,738.3 | |||||
| Commitments and contingencies | |||||||
| Shareholder's equity: | |||||||
| Common stock, without par value; 1,000 shares authorized; 1,000 shares issued and outstanding | — | — | |||||
| Contributed and additional paid-in capital | 5,661.5 | 5,628.8 | |||||
| Accumulated deficit | (3,471.7 | ) | (3,069.6 | ) | |||
| Accumulated other comprehensive income | 76.2 | 122.9 | |||||
| Total shareholder's equity | 2,266.0 | 2,682.1 | |||||
| Total liabilities and shareholder's equity | $ | 10,001.7 | $ | 10,420.4 | |||
The accompanying notes are an integral part of the condensed consolidated financial statements.
F-51
F-52
Biomet, Inc. and Subsidiaries Condensed Consolidated Statements of Operations and Comprehensive Income (Loss)
(in millions)
(Unaudited) For the Nine Months Ended | ||||||||
| February 28, 2013 | February 29, 2012 | |||||||
| Net sales | $ | 2,269.0 | $ | 2,098.6 | ||||
| Cost of sales | 736.0 | 669.9 | ||||||
| Gross profit | 1,533.0 | 1,428.7 | ||||||
| Selling, general and administrative expense | 886.7 | 800.9 | ||||||
| Research and development expense | 107.2 | 93.2 | ||||||
| Amortization | 230.2 | 250.0 | ||||||
| Goodwill and intangible assets impairment charge | 334.1 | — | ||||||
| Operating income (loss) | (25.2 | ) | 284.6 | |||||
| Interest expense | 310.8 | 363.4 | ||||||
| Other (income) expense | 172.4 | 9.3 | ||||||
| Other expense, net | 483.2 | 372.7 | ||||||
| Loss before income taxes | (508.4 | ) | (88.1 | ) | ||||
| Provision (benefit) from income taxes | (106.2 | ) | (18.4 | ) | ||||
| Net loss | (402.2 | ) | (69.7 | ) | ||||
| Other comprehensive income (loss): | ||||||||
| Change in unrealized holding value on available-for-sale securities, net of tax | 3.6 | 4.4 | ||||||
| Interest rate swap unrealized gain (loss), net of tax | 5.9 | 17.4 | ||||||
| Foreign currency related gains (losses) | (56.2 | ) | (26.8 | ) | ||||
| Unrecognized actuarial gain (loss) on pension assets, net of tax | — | (0.3 | ) | |||||
| Other comprehensive income (loss) | (46.7 | ) | (5.3 | ) | ||||
| Comprehensive income (loss) | $ | (448.9 | ) | $ | (75.0 | ) | ||
The accompanying notes are an integral part of the condensed consolidated financial statements.
F-53
Biomet, Inc. and Subsidiaries Condensed Consolidated Statements of Cash Flows
(in millions)
(Unaudited) Nine Months Ended | |||||||
| February 28, 2013 | February 29, 2012(1) | ||||||
| Cash flows provided by (used in) operating activities: | |||||||
| Net loss | $ | (402.2 | ) | $ | (69.7 | ) | |
| Adjustments to reconcile net loss to net cash provided by operating activities: | |||||||
| Depreciation and amortization | 364.8 | 388.0 | |||||
| Amortization and write off of deferred financing costs | 27.3 | 8.3 | |||||
| Stock-based compensation expense | 32.3 | 12.2 | |||||
| Loss on extinguishment of debt | 155.2 | — | |||||
| Recovery of doubtful accounts receivable | (0.4 | ) | (2.6 | ) | |||
| Realized gain on investments | (0.2 | ) | (1.9 | ) | |||
| Goodwill and intangible assets impairment charge | 334.1 | — | |||||
| Loss on impairment of investments | — | 19.3 | |||||
| Deferred income taxes | (165.4 | ) | (120.7 | ) | |||
| Other | 5.9 | (1.6 | ) | ||||
| Changes in operating assets and liabilities, net of acquired assets: | |||||||
| Accounts receivable | (53.1 | ) | (38.4 | ) | |||
| Inventories | (33.6 | ) | 9.6 | ||||
| Prepaid expenses | (7.9 | ) | (1.2 | ) | |||
| Accounts payable | (28.0 | ) | (4.2 | ) | |||
| Income taxes | 5.5 | 19.1 | |||||
| Accrued interest | (12.6 | ) | 61.7 | ||||
| Accrued expenses and other | 52.1 | 13.4 | |||||
| Net cash provided by operating activities | 273.8 | 291.3 | |||||
| Cash flows provided by (used in) investing activities: | |||||||
| Proceeds from sales/maturities of investments | 5.5 | 42.0 | |||||
| Purchases of investments | (6.4 | ) | (0.3 | ) | |||
| Net proceeds from sale of assets | 14.0 | 13.7 | |||||
| Capital expenditures | (149.7 | ) | (122.7 | ) | |||
| Acquisitions, net of cash acquired - Trauma Acquisition | (280.0 | ) | — | ||||
| Other acquisitions, net of cash acquired | (17.2 | ) | (14.4 | ) | |||
| Net cash used in investing activities | (433.8 | ) | (81.7 | ) | |||
| Cash flows provided by (used in) financing activities: | |||||||
| Debt: | |||||||
| Payments under European facilities | (1.0 | ) | (1.1 | ) | |||
| Payments under senior secured credit facilities | (25.2 | ) | (26.6 | ) | |||
| Proceeds under asset based revolver | 80.0 | — | |||||
| Payments under asset based revolver | (80.0 | ) | — | ||||
| Proceeds from senior and senior subordinated notes due 2020 and term loans | 3,396.2 | — | |||||
| Tender/retirement of senior notes due 2017 and term loans | (3,423.0 | ) | — | ||||
| Payment of fees related to refinancing activities | (77.8 | ) | — | ||||
| Equity: | |||||||
| Repurchase of LVB Acquisition, Inc. shares | (0.1 | ) | (1.2 | ) | |||
| Net cash used in financing activities | (130.9 | ) | (28.9 | ) | |||
| Effect of exchange rate changes on cash | 15.9 | (12.5 | ) | ||||
| Increase (decrease) in cash and cash equivalents | (275.0 | ) | 168.2 | ||||
| Cash and cash equivalents, beginning of period | 492.4 | 327.8 | |||||
| Cash and cash equivalents, end of period | $ | 217.4 | $ | 496.0 | |||
| Supplemental disclosures of cash flow information: | |||||||
| Cash paid during the period for: | |||||||
| Interest | $ | 315.5 | $ | 294.0 | |||
| Income taxes | $ | 49.0 | $ | 76.9 | |||
_____________________
| (1) | Certain amounts have been adjusted to conform to the current presentation. |
The accompanying notes are an integral part of the condensed consolidated financial statements.
F-54
Note 1—Basis of Presentation.
The accompanying unaudited condensed consolidated financial statements include the accounts of Biomet, Inc. and its subsidiaries (individually and collectively with its subsidiaries referred to as “Biomet”, the “Company”, “we”, “us” or “our”). Biomet is a wholly owned subsidiary of LVB Acquisition, Inc. ("LVB" and "Parent"). LVB has no other operations beyond its ownership of Biomet. Intercompany accounts and transactions have been eliminated in consolidation.
The unaudited condensed consolidated financial statements have been prepared in accordance with U.S. generally accepted accounting principles (“GAAP”) for condensed financial information, the instructions to Form 10-Q and Article 10 of Regulation S-X. As a result, they do not include all of the information and footnotes required by U.S. GAAP for complete financial statements. In the opinion of management, all adjustments (consisting of normal recurring accruals) considered necessary for a fair presentation of the financial condition, results of operations and cash flows for the periods presented have been included. Operating results for the three and nine months ended February 28, 2013 are not necessarily indicative of the results that may be expected for the fiscal year ending May 31, 2013. For further information, including the Company’s significant accounting policies, refer to the audited consolidated financial statements and notes thereto included in the Company’s Annual Report on Form 10-K for the fiscal year ended May 31, 2012 (the “2012 Form 10-K”).
The May 31, 2012 condensed consolidated balances have been derived from the audited financial statements included in the 2012 Form 10-K.
Recent Accounting Pronouncements
Goodwill Impairment Testing—In September 2011, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) 2011-8, “Intangibles-Goodwill and Other (Topic 350): Testing Goodwill for Impairment” (“ASU 2011-8”). The new guidance is intended to simplify how entities test goodwill for impairment. It includes provisions that permit an entity to first assess qualitative factors in determining whether it is “more likely than not” that the fair value of a reporting unit is less than its carrying amount as a basis for determining whether it is necessary to perform the two-step goodwill impairment test. The more-likely-than-not threshold is defined as having a likelihood of more than 50%. The new guidance is effective for annual and interim goodwill impairment tests performed for fiscal years beginning after December 15, 2011. The changes to Topic 350 were effective for the Company beginning June 1, 2012. The adoption did not have a material impact on the Company’s consolidated financial statements.
Note 2—Acquisition.
Trauma Acquisition
On May 24, 2012, DePuy Orthopaedics, Inc. accepted the Company’s binding offer to purchase certain assets representing substantially all of DePuy’s worldwide trauma business (the “Trauma Acquisition”), which involves researching, developing, manufacturing, marketing, distributing and selling products to treat certain bone fractures or deformities in the human body, including certain intellectual property assets, and to assume certain liabilities, for approximately $280.0 million in cash. The Company acquired the DePuy worldwide trauma business to strengthen its trauma business and to continue to build a stronger presence in the global trauma market. On June 15, 2012, the Company announced the initial closing of the transaction. During the first and second quarters of fiscal year 2013, subsequent closings in various foreign countries occurred on a staggered basis, with the final closing occurring on December 7, 2012.
The Trauma Acquisition net sales for the three and nine months ended February 28, 2013 were $59.4 million and $150.9 million, respectively.
The acquisition has been accounted for as a business combination. The preliminary purchase price was allocated to the acquired assets and liabilities based on the estimated fair value of the acquired assets at the date of acquisition. As of February 28, 2013, the Company recorded a preliminary allocation of the purchase price to acquired tangible and identifiable intangible assets and liabilities assumed based on their fair value at the initial acquisition date. The Company is in the process of obtaining valuations of certain tangible and intangible assets and determining certain employee liabilities. The Company expects to complete the purchase price allocation in fiscal year 2013 after all valuations have been finalized.
F-55
The preliminary purchase price allocation at February 28, 2013 consisted of the following:
| (in millions) | February 28, 2013 | ||
| Inventory | $ | 98.9 | |
| Prepaid expenses and other | 2.1 | ||
| Instruments | 29.2 | ||
| Other property, plant and equipment | 23.3 | ||
| Liabilities assumed | (4.0 | ) | |
| Intangible assets | 70.0 | ||
| Goodwill | 60.5 | ||
| Preliminary purchase price | $ | 280.0 | |
The asset purchase agreement contains a provision requiring an adjustment to the purchase price if the amount of delivered inventory and/or instruments is more or less than the target amount of these items. No adjustments to the purchase price pursuant to this provision has been made. The results of operations of the business have been included subsequent to the respective country closing dates in the accompanying condensed consolidated financial statements. Acquisition-related costs for the three and nine months ended February 28, 2013 were $1.1 million and $10.3 million, respectively, and are recorded in cost of sales and selling, general and administrative expenses. The Company does not expect the goodwill value to be tax deductible.
The pro forma information required under Accounting Standards Codification 805 is impracticable to include due to different fiscal year ends and individual country closings.
F-56
Note 3—Inventories.
Inventories are stated at the lower of cost or market, with cost determined under the first-in, first-out method. The Company reviews inventory on hand and writes down excess and slow-moving inventory based on an assessment of future demand and historical experience. Inventories consisted of the following:
| (in millions) | February 28, 2013 | May 31, 2012 | |||||
| Raw materials | $ | 81.9 | $ | 78.3 | |||
| Work-in-process | 48.1 | 42.4 | |||||
| Finished goods | 513.3 | 422.5 | |||||
| Inventories, net | $ | 643.3 | $ | 543.2 | |||
Note 4—Property, Plant and Equipment.
Property, plant and equipment are carried at cost less accumulated depreciation. Depreciation is computed using the straight-line method over the estimated useful life of the asset. Depreciation of instruments is included within cost of sales. Related maintenance and repairs are expensed as incurred.
The Company reviews property, plant and equipment for impairment whenever events or changes in circumstances indicate that the carrying value of an asset may not be recoverable. An impairment loss would be recognized when estimated undiscounted future cash flows relating to the asset, or asset group, are less than its carrying value, with the amount of the loss equal to the excess of carrying value of the asset, or asset group, over the estimated fair value.
Useful lives by major product category consisted of the following:
| Useful life | |
| Land improvements | 20 years |
| Buildings and leasehold improvements | 30 years |
| Machinery and equipment | 5-10 years |
| Instruments | 4 years |
Property, plant and equipment consisted of the following:
| (in millions) | February 28, 2013 | May 31, 2012 | |||||
| Land and land improvements | $ | 40.8 | $ | 40.2 | |||
| Buildings and leasehold improvements | 102.1 | 89.9 | |||||
| Machinery and equipment | 390.4 | 342.3 | |||||
| Instruments | 780.5 | 633.3 | |||||
| Construction in progress | 35.9 | 29.1 | |||||
| Total property, plant and equipment | 1,349.7 | 1,134.8 | |||||
| Accumulated depreciation | (670.3 | ) | (541.2 | ) | |||
| Total property, plant and equipment, net | $ | 679.4 | $ | 593.6 | |||
F-57
Note 5—Investments.
At February 28, 2013, the Company’s investment securities were classified as follows:
| Amortized | Unrealized | Fair | |||||||||||||
| (in millions) | Cost | Gains | Losses | Value | |||||||||||
| Available-for-sale: | |||||||||||||||
| Equity securities | $ | 0.2 | $ | 0.1 | $ | — | $ | 0.3 | |||||||
| Time deposit | 15.9 | 0.1 | — | 16.0 | |||||||||||
| Greek bonds | 1.1 | 3.8 | — | 4.9 | |||||||||||
| Total available-for-sale investments | $ | 17.2 | $ | 4.0 | $ | — | $ | 21.2 | |||||||
| Amortized | Realized | Fair | |||||||||||||
| Cost | Gains | Losses | Value | ||||||||||||
| Trading: | |||||||||||||||
| Equity securities | $ | 0.8 | $ | 0.1 | $ | — | $ | 0.9 | |||||||
| Total trading investments | $ | 0.8 | $ | 0.1 | $ | — | $ | 0.9 | |||||||
At May 31, 2012, the Company’s investment securities were classified as follows:
| Amortized | Unrealized | Fair | |||||||||||||
| (in millions) | Cost | Gains | Losses | Value | |||||||||||
| Available-for-sale: | |||||||||||||||
| Equity securities | $ | 0.4 | $ | — | $ | (0.2 | ) | $ | 0.2 | ||||||
| Time deposit | 9.5 | — | — | 9.5 | |||||||||||
| Greek bonds | 6.3 | — | — | 6.3 | |||||||||||
| Total available-for-sale investments | $ | 16.2 | $ | — | $ | (0.2 | ) | $ | 16.0 | ||||||
| Amortized | Realized | Fair | |||||||||||||
| Cost | Gains | Losses | Value | ||||||||||||
| Trading: | |||||||||||||||
| Equity securities | $ | 0.4 | $ | — | $ | — | $ | 0.4 | |||||||
| Total trading investments | $ | 0.4 | $ | — | $ | — | $ | 0.4 | |||||||
The Company recorded proceeds on the sales/maturities of investments of $5.5 million for the three and nine months ended February 28, 2013 and $8.3 million and $42.0 million for the three and nine months ended February 29, 2012, respectively. The Company purchased investments of $6.4 million during the nine months ended February 28, 2013 and $0.1 million and $0.3 million for the three and nine months ended February 29, 2012, with no purchases during the three months ended February 28, 2013.
The Company holds Greek bonds which are designated as available-for-sale securities. The bonds have maturities ranging from 1 to 30 years. As of February 28, 2013, the face value of the bonds was $11.2 million. The Company recorded realized losses of $2.8 million and $19.3 million on the Greek bonds related to other-than-temporary impairment for the three and nine months ended February 29, 2012, respectively, which is included in other (income) expense. There was no other-than-temporary impairment for the three and nine months ended February 28, 2013 as fair value was higher than cost.
Note 6—Goodwill and Other Intangible Assets.
The balance of goodwill as of February 28, 2013 and May 31, 2012 was $3,927.5 million and $4,114.4 million, respectively. The change in goodwill is primarily related to the impairment charge described below and foreign currency fluctuations partially offset by the goodwill recorded related to the Trauma Acquisition, which is described in Note 2 – Acquisition.
F-58
The Company operates in one reportable segment and evaluates goodwill for impairment at the reporting unit level. The reporting units are based on the Company's current administrative organizational structure and the availability of discrete financial information.
During the third quarter of fiscal year 2013, the Company recorded a $334.1 million goodwill and definite and indefinite-lived intangible assets impairment charge related to its Dental Reconstructive reporting unit, primarily due to declining industry market growth rates in certain European and Asia Pacific markets and corresponding unfavorable margin trends.
The impairment charge was a result of the finalization of our preliminary impairment work as of November 30, 2012.
The Company used the income approach, specifically the discounted cash flow method, to determine the fair value of the Dental Reconstructive reporting unit and the associated amount of the impairment charges. This approach calculates fair value by estimating the after-tax cash flows attributable to a reporting unit and then discounting these after-tax cash flows to a present value using a risk-adjusted discount rate. This methodology is consistent with how the Company estimates the fair value of its reporting units during its annual goodwill and indefinite lived intangible asset impairment tests. In applying the income approach to calculate the fair value of the Dental Reconstructive reporting unit, the Company used assumptions about future revenue contributions and cost structures. The application of the income approach for both goodwill and intangibles requires judgment in determining a risk-adjusted discount rate at the reporting unit level. The Company based this determination on estimates of the weighted-average costs of capital of market participants. The Company performed a peer company analysis and considered the industry weighted-average return on debt and equity from a market participant perspective.
To calculate the amount of the impairment charge related to the Dental Reconstructive reporting unit, the Company allocated the reporting unit's fair value to all of its assets and liabilities, including certain unrecognized intangible assets, in order to determine the implied fair value of goodwill. This allocation process required judgment and the use of additional valuation assumptions in deriving the individual fair values of the Company's Dental Reconstructive reporting unit's assets and liabilities as if the reporting units had been acquired in a business combination.
The Company determined the fair value of intangible assets using an income based approach to determine the fair value. The approach calculated the fair value by estimating the after-tax cash flows attributable to the asset and then discounting these after-tax cash flows to a present value using a risk-adjusted discount rate. The calculated fair value was compared to the carrying value to determine if any impairment existed.
The Company performs its annual assessment for impairment as of March 31 for all reporting units, or on an interim basis if an event occurs or circumstances change that would more likely than not reduce the fair value of a reporting unit below its carrying amount. The estimates and assumptions underlying the fair value calculations used in the Company’s annual impairment tests are uncertain by their nature and can vary significantly from actual results. Factors that management must estimate include, but are not limited to, industry and market conditions, sales volume and pricing, raw material costs, capital expenditures, working capital changes, cost of capital, and tax rates. These factors are especially difficult to predict when global financial markets are volatile. The estimates and assumptions used in its impairment tests are consistent with those the Company uses in its internal planning. These estimates and assumptions may change from period to period. If the Company uses different estimates and assumptions in the future, impairment charges may occur and could be material.
The Company has identified a total of four reporting units with a material amount of goodwill that are at a higher risk of potential failure of step one of the goodwill impairment test in the future. These reporting units include its U.S. Reconstructive reporting unit ($2,973.4 million of goodwill), its International reporting unit ($523.5 million of goodwill), its dental reconstructive reporting unit ($66.3 million of goodwill) and its Europe reporting unit ($299.4 million). The level of excess fair value over carrying value for these higher risk reporting units were each less than 10% for the latest step one impairment test.
The Company uses an accelerated method for amortizing customer relationship intangibles, as the value for those relationships is greater at the beginning of their life. The accelerated method was calculated using historical customer attrition rates. The remaining finite-lived intangibles are amortized on a straight line basis. The decrease in the net intangible asset balance is primarily due to the impairment charge described below and amortization, partially offset by the intangibles recorded related to the Trauma Acquisition, which is described in Note 2 – Acquisition.
F-59
The following table summarizes the changes in the carrying amount of goodwill:
| (in millions) | February 28, 2013 | ||
| Beginning of period | $ | 4,114.4 | |
| Goodwill acquired | 62.0 | ||
| Currency translation | (15.9 | ) | |
| Impairment charge | (233.0 | ) | |
| End of Period | $ | 3,927.5 | |
Intangible assets consisted of the following at February 28, 2013 and May 31, 2012:
| (in millions) | February 28, 2013 | ||||||||||||||||||||||
| Gross | New | Net | |||||||||||||||||||||
| Carrying | Impairment | Carrying | Accumulated | Impairment | Carrying | ||||||||||||||||||
| Amount | Charge | Amount | Amortization | Charge | Amount | ||||||||||||||||||
| Core technology | $ | 1,699.9 | $ | (39.0 | ) | $ | 1,660.9 | $ | (451.2 | ) | $ | 4.1 | $ | 1,213.8 | |||||||||
| Completed technology | 604.2 | (55.2 | ) | 549.0 | (240.7 | ) | 36.7 | 345.0 | |||||||||||||||
| Product trade names | 192.5 | — | 192.5 | (61.3 | ) | — | 131.2 | ||||||||||||||||
| Customer relationships | 2,387.5 | (46.1 | ) | 2,341.4 | (786.1 | ) | 9.9 | 1,565.2 | |||||||||||||||
| Non-compete contracts | 4.6 | — | 4.6 | (3.8 | ) | — | 0.8 | ||||||||||||||||
| Sub-total | 4,888.7 | (140.3 | ) | 4,748.4 | (1,543.1 | ) | 50.7 | 3,256.0 | |||||||||||||||
| Corporate trade names | 312.2 | (11.5 | ) | 300.7 | — | — | 300.7 | ||||||||||||||||
| Currency translation | 127.3 | — | 127.3 | (21.6 | ) | — | 105.7 | ||||||||||||||||
| Total | $ | 5,328.2 | $ | (151.8 | ) | $ | 5,176.4 | $ | (1,564.7 | ) | $ | 50.7 | $ | 3,662.4 | |||||||||
| (in millions) | May 31, 2012 | ||||||||||||||||||||||
| Gross | New | Net | |||||||||||||||||||||
| Carrying | Impairment | Carrying | Accumulated | Impairment | Carrying | ||||||||||||||||||
| Amount | Charge | Amount | Amortization | Charge | Amount | ||||||||||||||||||
| Core technology | $ | 1,856.1 | $ | (185.7 | ) | $ | 1,670.4 | $ | (457.7 | ) | $ | 74.3 | $ | 1,287.0 | |||||||||
| Completed technology | 594.2 | — | 594.2 | (206.7 | ) | — | 387.5 | ||||||||||||||||
| Product trade names | 184.5 | — | 184.5 | (52.6 | ) | — | 131.9 | ||||||||||||||||
| Customer relationships | 2,666.1 | (306.8 | ) | 2,359.3 | (859.3 | ) | 191.6 | 1,691.6 | |||||||||||||||
| Non-compete contracts | 4.6 | — | 4.6 | (3.1 | ) | — | 1.5 | ||||||||||||||||
| Sub-total | 5,305.5 | (492.5 | ) | 4,813.0 | (1,579.4 | ) | 265.9 | 3,499.5 | |||||||||||||||
| Corporate trade names | 323.5 | (11.3 | ) | 312.2 | — | — | 312.2 | ||||||||||||||||
| Currency translation | 147.2 | — | 147.2 | (28.5 | ) | — | 118.7 | ||||||||||||||||
| Total | $ | 5,776.2 | $ | (503.8 | ) | $ | 5,272.4 | $ | (1,607.9 | ) | $ | 265.9 | $ | 3,930.4 | |||||||||
F-60
The weighted average useful life of the intangibles at February 28, 2013 is as follows:
Weighted Average Useful Life | |
| Core technology | 16 years |
| Completed technology | 10 years |
| Product trade names | 14 years |
| Customer relationships | 15 years |
| Non-compete contracts | 2 years |
| Corporate trade names | Indefinite life |
Expected amortization expense for the intangible assets stated above for the years ending May 31, 2013 through 2017 is $303.8 million, $287.0 million, $270.0 million, $262.0 million, and $257.5 million, respectively.
Note 7—Debt.
The terms and carrying value of each debt instrument at February 28, 2013 and May 31, 2012 are set forth below:
| (U.S. dollars and euros in millions) | Maturity Date | Interest Rate | Currency | February 28, 2013 | May 31, 2012 | ||||||||
| Debt Instruments | |||||||||||||
| European facilities | No Maturity Date | Interest Free | EUR | € | 2.0 | € | 2.8 | ||||||
| $ | 2.6 | $ | 3.5 | ||||||||||
| Term loan facility | March 25, 2015 | LIBOR + 3.00% | USD | $ | 104.6 | $ | 2,234.7 | ||||||
| Term loan facility | July 25, 2017 | LIBOR + 3.75% | USD | $ | 2,122.1 | $ | — | ||||||
| Term loan facility | March 25, 2015 | LIBOR + 3.00% | EUR | € | 168.2 | € | 835.6 | ||||||
| $ | 220.1 | $ | 1,039.6 | ||||||||||
| Term loan facility | July 25, 2017 | LIBOR + 4.00% | EUR | € | 661.0 | € | — | ||||||
| $ | 864.9 | $ | — | ||||||||||
| Cash flow revolving credit facility | April 25, 2017 | LIBOR + 3.50% | USD | $ | — | $ | — | ||||||
| Cash flow revolving credit facility | April 25, 2017 | LIBOR + 3.50% | USD/EUR | $ | — | $ | — | ||||||
| Asset-based revolving credit facility | July 25, 2017 | LIBOR + 1.75% | USD | $ | — | $ | — | ||||||
| Asset-based revolving credit facility | July 25, 2017 | LIBOR + 1.75% | EUR | € | — | € | — | ||||||
| Senior cash pay notes | October 15, 2017 | 10% | USD | $ | — | $ | 761.0 | ||||||
| Senior PIK toggle notes | October 15, 2017 | 10.375% - 11.125% | USD | $ | — | $ | 771.0 | ||||||
| Senior subordinated notes | October 15, 2017 | 11.625% | USD | $ | — | $ | 1,015.0 | ||||||
| Senior notes | August 1, 2020 | 6.500% | USD | $ | 1,825.0 | $ | — | ||||||
| Senior subordinated notes | October 1, 2020 | 6.500% | USD | $ | 800.0 | $ | — | ||||||
| Premium on notes | $ | 39.1 | $ | 3.0 | |||||||||
| Total debt | $ | 5,978.4 | $ | 5,827.8 | |||||||||
The Company has the option to choose the frequency with which it resets and pays interest on its term loans. The Company currently pays interest on the majority of its term loans and interest rate swaps each month. The
F-61
remaining term loan and swap interest is paid quarterly. Interest on the 6.500% senior notes due 2020 is paid semiannually in February and August. Interest on the 6.500% senior subordinated notes due 2020 is paid semiannually in April and October.
The Company currently elects to use 1-month LIBOR for setting the interest rates on 55% of its U.S. dollar-denominated and 95% of its euro-denominated term loans. The 1-month LIBOR rate for the majority of the U.S. dollar-denominated term loan as of February 28, 2013 was 0.20%. The majority of the euro-denominated term loan had a 1-month LIBOR rate of 0.06% as of February 28, 2013. The 3-month LIBOR rate for the U.S. dollar-denominated term loan was 0.31% as of February 28, 2013 and the 3-month LIBOR rate for the euro-denominated term loan was 0.12% as of February 28, 2013. The Company’s term loan facilities require payments each year in an amount equal to (x) 0.25% of the product of (i) the aggregate principal amount of all euro-denominated term loans and dollar-denominated term loans outstanding under the original credit agreement on the closing date multiplied by (ii) a fraction, the numerator of which is the aggregate principal amount of euro-denominated term B loans and dollar-denominated term B loans outstanding on August 2, 2012 (after giving effect to certain conversions to occur on or after August 2, 2012 pursuant to the amended and restated credit agreement) and the denominator of which is the aggregate principal amount of all outstanding term loans on August 2, 2012 and (y) 0.25% of the aggregate principal amount of all outstanding euro-denominated term B-1 loans and dollar-denominated term B-1 loans, in each case in equal calendar quarterly installments until maturity of the loan and after giving effect to the application of any prepayments. Through February 28, 2013, the total amount of required payments under the Company’s term loan facilities was $25.2 million. The cash flow and asset-based revolving credit facilities and the notes do not have terms for mandatory principal paydowns. To calculate the U.S. dollar equivalent on outstanding balances, the Company used a currency conversion rate of 1 euro to $1.3084 and $1.2441, which represents the currency exchange rate from euros to U.S. dollars on February 28, 2013 and May 31, 2012, respectively.
The Company’s revolving borrowing base available under all debt facilities at February 28, 2013 was $795.5 million, which is net of the borrowing base limitations relating to the asset-based revolving credit facility.
As of February 28, 2013, $12.4 million of financing fees related to the Company’s credit agreement remain in long-term assets and continue to be amortized through interest expense over the remaining life of the credit agreement. Additionally, $70.7 million of new financing fees related to the refinancing referenced below are also in long-term assets and will be amortized through interest expense over the remaining lives of the new debt instruments.
Each of Biomet, Inc.’s existing wholly owned domestic subsidiaries fully, unconditionally, jointly, and severally guarantee the 6.500% senior notes due 2020 on a senior unsecured basis and the 6.500% senior subordinated notes due 2020 on a senior subordinated unsecured basis, in each case to the extent such subsidiaries guarantee Biomet, Inc.’s senior secured credit facilities. LVB Acquisition, Inc. is neither an issuer nor guarantor of the notes described within this footnote.
Notes Offerings and Concurrent Tender Offers
On August 8, 2012, Biomet completed its offering of $1,000.0 million aggregate principal amount of new 6.500% senior notes due 2020. Biomet used the net proceeds of that offering to fund a tender offer for any and all of its outstanding 103/8% / 111/8% senior PIK toggle notes due 2017 (“Senior Toggle Notes”) including related fees and expenses, to redeem the remaining Senior Toggle Notes not tendered in the tender offer and to redeem $140.0 million aggregate principal amount of the 115/8% senior subordinated notes due 2017 (“115/8% Senior Subordinated Notes”). Approximately 70% of the Senior Toggle Notes were tendered in August 2012. The remaining Senior Toggle Notes and $140.0 million aggregate principal amount of the 115/8% Senior Subordinated Notes were redeemed in September 2012.
On October 2, 2012, Biomet, Inc. completed its offering of $825.0 million aggregate principal amount of 6.500% senior notes due 2020 as part of a further issuance of 6.500% senior notes due 2020. The Company used the net proceeds of this offering to fund a tender offer for any and all of its 10% senior notes due 2017 (“10% Senior Notes”), including related fees and expenses and to redeem 10% Senior Notes not accepted for purchase in such tender offer. Concurrently with this offering, Biomet also completed an offering of $800.0 million aggregate principal amount of 6.500% senior subordinated notes due 2020. Biomet used the net proceeds of the subordinated notes offering together with cash on hand, to fund a tender offer for up to $800.0 million aggregate principal amount of its 115/8% Senior Subordinated Notes, including related fees and expenses and to redeem 115/8% Senior Subordinated Notes not accepted for purchase in such tender offer, $343.4 million in aggregate principal amount, or approximately 45.12% of the 10% Senior Notes outstanding, were validly tendered and not withdrawn, and $384.2
F-62
million aggregate principal amount, or approximately 43.91% of the 115/8% Senior Subordinated Notes outstanding, were validly tendered and not withdrawn, in each case as of the early tender deadline of October 1, 2020. On November 1, 2012, Biomet retired all outstanding 10% Senior Notes and 115/8% Senior Subordinated Notes not accepted for purchase in the tender offer using cash on hand and asset-based revolver proceeds.
The Company recorded a loss on the retirement of bonds of $155.2 million during the nine months ended February 28, 2013 in other (income) expense, related to the tender/retirement of the Senior Toggle Notes, 10% Senior Notes and 115/8% Senior Subordinated Notes, with no loss recorded during the three months ended February 28, 2013. The Company wrote off deferred financing fees related to the tender/retirement of the Senior Toggle Notes, 10% Senior Notes and 115/8% Senior Subordinated Notes described above and the replacement of the existing cash flow revolvers, asset-based revolver and term loans described below of $3.4 million and $17.1 million during the three and nine months ended February 28, 2013, respectively, in other (income) expense.
Amendment and Restatement Agreement-Senior Secured Credit Facilities
On August 2, 2012, Biomet entered into an amendment and restatement agreement that amended its existing senior secured credit facilities. The amendment (i) extended the maturing of approximately $1,007.2 million of its U.S. dollar-denominated term loans and approximately €631.3 million of its euro-denominated term loans under the credit facility to July 25, 2017 and (ii) refinanced and replaced the then-existing alternative currency revolving credit commitments under the credit facility with a new class of alternative currency revolving credit commitments in an aggregate amount of $165.0 million and refinanced and replaced the then-existing U.S. dollar revolving credit commitments under the credit facility with a new class of U.S. dollar-denominated revolving credit commitments in an aggregate amount of $165.0 million. The new revolving credit commitments will mature on April 25, 2017, except that if as of December 23, 2014, there is an outstanding aggregate principal amount of non-extended U.S. dollar and euro term loans in excess of $200.0 million, then such revolving credit commitments will mature on December 24, 2014. The remaining term loans of the lenders under the senior secured credit facilities who did not elect to extend such loans will continue to mature on March 25, 2015.
Joinder Agreement
On October 4, 2012, LVB, Biomet and certain subsidiaries of Biomet entered into a joinder agreement (the “Joinder”) with Bank of America, N.A., as Administrative Agent, Swing Line Lender and L/C Issuer, each lender from time to time party thereto and each of the other parties identified as an “Extending Term Lender.” The Joinder was entered into pursuant to that certain Credit Agreement, dated as of September 25, 2007, as amended and restated by that certain Amendment and Restatement Agreement dated as of August 2, 2012 (the “Amendment”), by and among Biomet, LVB, certain subsidiaries of Biomet, Bank of America, N.A. and each lender from time to time party thereto. The Amendment, among other things, provides Biomet with the ability to request an extension of the scheduled maturity dates of its existing term loans in one or more series of tranches.
By entering into the Joinder, the joining lenders have agreed to extend the maturity of (i) approximately $392.7 million of Biomet’s U.S. dollar-denominated term loans and (ii) approximately €32.9 million of Biomet’s euro-denominated term loans, to July 25, 2017. The term loans extended pursuant to the Joinder are on terms identical to the terms loans that were extended pursuant to the Amendment. The remaining term loans of the lenders who have not elected to extend their loans will continue to mature on March 25, 2015.
Refinancing of Asset-Based Revolving Credit Facility
On November 14, 2012, Biomet replaced and refinanced its asset-based revolving credit facility with a new asset-based revolving credit facility that has a U.S. tranche of up to $400.0 million and a European borrower tranche denominated in euros of up to the euro-equivalent of $100.0 million. The European borrower tranche is secured by certain foreign assets of European subsidiary borrowers and the U.S. borrowers under the U.S. tranche guarantee the obligations of any such European subsidiary borrowers (and such guarantees are secured by the current assets collateral that secures the direct obligations of such U.S. borrowers under such U.S. tranche).
Refinancing of U.S. dollar-denominated term loan
On December 27, 2012, Biomet completed a $730.0 million add-on to the extended U.S. dollar-denominated term loan. The proceeds from the add-on were used to refinance the non-extended U.S. dollar-denominated term B loan, which was net of fees associated with the add-on closing. The terms of the add-on are consistent with the terms in the Amendment and Restatement Agreement-Senior Secured Credit Facilities explanation above.
Note 8—Fair Value Measurements.
F-63
Assets and Liabilities Measured at Fair Value on a Recurring Basis
Fair value measurements are principally applied to (1) financial assets and liabilities such as marketable equity securities and debt securities, (2) investments in equity and other securities, and (3) derivative instruments consisting of interest rate swaps. These items are marked-to-market at each reporting period to fair value. The information in the following paragraphs and tables primarily addresses matters relative to these financial assets and liabilities.
| • | Level 1 – Inputs are quoted prices in active markets for identical assets or liabilities. The Company’s Level 1 assets include money market investments and marketable equity securities. |
| • | Level 2 – Inputs include quoted prices for similar assets or liabilities in active markets, quoted prices for identical or similar assets or liabilities in markets that are not active, and inputs (other than quoted prices) that are observable for the asset or liability, either directly or indirectly. The Company’s Level 2 assets and liabilities primarily include Greek bonds, time deposits, interest rate swaps, pension plan assets (equity securities, debt securities and other) and foreign currency exchange contracts whose value is determined using a pricing model with inputs that are observable in the market or can be derived principally from or corroborated by observable market data. |
| • | Level 3 – Inputs are unobservable for the asset or liability. The Company’s Level 3 assets include other equity investments. See the section below titled Level 3 Valuation Techniques for further discussion of how the Company determines fair value for investments classified as Level 3. |
The following table provides information by level for assets and liabilities that are measured at fair value on a recurring basis at February 28, 2013 and May 31, 2012:
| Fair Value Measurements | |||||||||||||||
Fair Value at February 28, 2013 | Using Inputs Considered as | ||||||||||||||
| (in millions) | Level 1 | Level 2 | Level 3 | ||||||||||||
| Assets: | |||||||||||||||
| Money market funds | $ | 122.4 | $ | 122.4 | $ | — | $ | — | |||||||
| Time deposits | 16.0 | — | 16.0 | — | |||||||||||
| Greek bonds | 4.9 | — | 4.9 | — | |||||||||||
| Pension plan assets | 128.4 | — | 128.4 | — | |||||||||||
| Foreign currency exchange contracts | 0.2 | — | 0.2 | — | |||||||||||
| Other | 0.3 | 0.2 | — | 0.1 | |||||||||||
| Total assets | $ | 272.2 | $ | 122.6 | $ | 149.5 | $ | 0.1 | |||||||
| Liabilities: | |||||||||||||||
| Interest rate swaps | $ | 66.6 | $ | — | $ | 66.6 | $ | — | |||||||
| Foreign currency exchange contracts | 1.5 | — | 1.5 | — | |||||||||||
| Total liabilities | $ | 68.1 | $ | — | $ | 68.1 | $ | — | |||||||
| Fair Value Measurements | |||||||||||||||
| Fair Value at | Using Inputs Considered as | ||||||||||||||
| (in millions) | May 31, 2012 | Level 1 | Level 2 | Level 3 | |||||||||||
| Assets: | |||||||||||||||
| Money market funds | $ | 303.1 | $ | 303.1 | $ | — | $ | — | |||||||
| Time deposits | 36.3 | — | 36.3 | — | |||||||||||
| Greek bonds | 6.3 | — | 6.3 | — | |||||||||||
| Pension plan assets | 108.7 | — | 108.7 | — | |||||||||||
| Foreign currency exchange contracts | 0.2 | — | 0.2 | — | |||||||||||
| Other | 0.2 | — | — | 0.2 | |||||||||||
| Total assets | $ | 454.8 | $ | 303.1 | $ | 151.5 | $ | 0.2 | |||||||
| Liabilities: | |||||||||||||||
| Interest rate swaps | $ | 76.2 | $ | — | $ | 76.2 | $ | — | |||||||
| Foreign currency exchange contracts | 0.2 | — | 0.2 | — | |||||||||||
| Total liabilities | $ | 76.4 | $ | — | $ | 76.4 | $ | — | |||||||
F-64
Level 3 Valuation Techniques
Financial assets are considered Level 3 when their fair values are determined using pricing models, discounted cash flow methodologies or similar techniques and at least one significant model assumption or input is unobservable. Level 3 financial assets also include certain investment securities for which there is limited market activity where the determination of fair value requires significant judgment or estimation. Level 3 investment securities primarily include other equity investments for which there was a decrease in the observation of market pricing. As of February 28, 2013 and May 31, 2012, these securities were valued primarily using internal cash flow valuation that incorporates transaction details such as contractual terms, maturity, timing and amount of future cash flows, as well as assumptions about liquidity and credit valuation adjustments of marketplace participants.
The estimated fair value of the Company’s long-term debt, including the current portion, at February 28, 2013 was $6,073.5 million, compared to a carrying value of $5,978.4 million. The fair value of the Company’s traded debt was estimated using quoted market prices for the same or similar instruments. The fair value of the Company’s variable rate term debt was estimated using the carrying value as this debt has rates which approximate market interest rates. In determining the fair values and carrying values, the Company considers the terms of the related debt and excludes the impacts of debt discounts and interest rate swaps.
Assets and Liabilities that are Measured at Fair Value on a Nonrecurring Basis
During the three and nine months ended February 28, 2013, the Company measured nonfinancial long-lived assets and liabilities at fair value in conjunction with the impairment of the dental reporting unit. The Company used the income approach to measure the fair value of the reporting unit and related intangible assets. See Note 6 for a full description of key assumptions. The inputs used in the impairment fair value analysis fall within Level 3 due to the significant unobservable inputs used to determine fair value. During the three and nine months ended February 29, 2012, the Company had no significant measurements of assets or liabilities at fair value on a nonrecurring basis subsequent to their initial recognition.
Note 9—Derivative Instruments and Hedging Activities.
The Company is exposed to certain market risks relating to its ongoing business operations, including foreign currency risk, interest rate risk and commodity price risk. The Company currently manages foreign currency risk and interest rate risk through the use of derivatives.
Derivatives Designated as Hedging Instruments
Foreign Currency Instruments—Certain assets, liabilities and forecasted transactions are exposed to foreign currency risk, primarily the fluctuation of the U.S. dollar against the euro. The Company has hedged a portion of its net investment in its European subsidiaries with the issuance of a €875.0 million (approximately $1,207.4 million at September 25, 2007) principal amount euro term loan on September 25, 2007. The Company’s net investment in its European subsidiaries at the hedging date of September 25, 2007 was €1,238.0 million ($1,690.0 million). As of February 28, 2013, the Company’s net investment in European subsidiaries totaled €1,918.2 million ($2,487.3 million) and the outstanding principal balance of the euro term loan was €829.2 million ($1,085.0 million). The difference of €1,089.0 million ($1,402.3 million) is unhedged as of February 28, 2013. Hedge effectiveness is tested quarterly to determine whether hedge treatment is still appropriate. The Company tests effectiveness on this net investment hedge by determining if the net investment in its European subsidiaries is greater than the outstanding euro-denominated debt balance. Any amount of a derivative instrument designated as a hedge determined to be ineffective is recorded as other (income) expense.
Interest Rate Instruments—The Company uses interest rate swap agreements (cash flow hedges) in both U.S. dollars and euros as a means of fixing the interest rate on portions of its floating-rate debt instruments. As of February 28, 2013, the Company had a swap liability of $66.6 million, which consisted of $23.8 million short-term and $44.6 million long-term, partially offset by a $1.8 million credit valuation adjustment. As of May 31, 2012, the Company had a swap liability of $76.2 million, which consisted of $36.0 million short-term and $41.0 million long-term, partially offset by a $0.8 million credit valuation adjustment.
The table below summarizes existing swap agreements at February 28, 2013 and May 31, 2012:
F-65
| (U.S. dollars and euros in millions) | Fair Value at | Fair Value at | ||||||||||||||||
| Notional | February 28, 2013 | May 31, 2012 | ||||||||||||||||
| Structure | Currency | Amount | Effective Date | Termination Date | Asset (Liability) | Asset (Liability) | ||||||||||||
| 5 years | EUR | € | 230.0 | September 25, 2007 | September 25, 2012 | $ | — | $ | (3.5 | ) | ||||||||
| 5 years | EUR | 40.0 | March 25, 2008 | March 25, 2013 | (0.1 | ) | (1.4 | ) | ||||||||||
| 5 years | EUR | 200.0 | September 25, 2012 | September 25, 2017 | (11.9 | ) | (9.5 | ) | ||||||||||
| 5 years | EUR | 200.0 | September 25, 2012 | September 25, 2017 | (11.7 | ) | (9.3 | ) | ||||||||||
| 5 years | USD | $ | 585.0 | September 25, 2007 | September 25, 2012 | — | (8.9 | ) | ||||||||||
| 5 years | USD | 190.0 | March 25, 2008 | March 25, 2013 | (0.4 | ) | (4.2 | ) | ||||||||||
| 5 years | USD | 325.0 | December 26, 2008 | December 25, 2013 | (5.4 | ) | (9.0 | ) | ||||||||||
| 5 years | USD | 195.0 | September 25, 2009 | September 25, 2014 | (8.0 | ) | (10.5 | ) | ||||||||||
| 2 years | USD | 190.0 | March 25, 2013 | March 25, 2015 | (2.1 | ) | (1.0 | ) | ||||||||||
| 3 years | USD | 270.0 | December 27, 2013 | September 25, 2016 | (6.3 | ) | (3.8 | ) | ||||||||||
| 5 years | USD | 350.0 | September 25, 2012 | September 25, 2017 | (11.3 | ) | (8.0 | ) | ||||||||||
| 5 years | USD | 350.0 | September 25, 2012 | September 25, 2017 | (11.2 | ) | (7.9 | ) | ||||||||||
| Credit valuation adjustment | 1.8 | 0.8 | ||||||||||||||||
| Total interest rate instruments | $ | (66.6 | ) | $ | (76.2 | ) | ||||||||||||
The interest rate swaps are recorded in other accrued expenses and other long-term liabilities. As a result of cash flow hedge treatment being applied, all unrealized gains and losses related to the derivative instruments are recorded in accumulated other comprehensive income (loss) and are reclassified into operations in the same period in which the hedged transaction affects earnings. Hedge effectiveness is tested quarterly to determine if hedge treatment is still appropriate. The amount of ineffectiveness was not material for any period presented. The tables below summarize the effective portion and ineffective portion of the Company’s interest rate swaps for the three and nine months ended February 28, 2013 and February 29, 2012:
| (in millions) | Nine Months Ended | |||||||
| Derivatives in cash flow hedging relationship | February 28, 2013 | February 29, 2012 | ||||||
| Interest rate swaps: | ||||||||
| Amount of gain (loss) recognized in OCI | $ | 9.5 | $ | 17.4 | ||||
| Amount of (gain) loss reclassified from accumulated OCI into interest expense (effective portion) | — | — | ||||||
| Amount (gain) loss recognized in other income (expense) (ineffective portion and amount excluded from effectiveness testing) | — | — | ||||||
As of February 28, 2013, the effective interest rate, including the applicable lending margin, on 63.32% ($1,410.0 million) of the outstanding principal of the Company’s U.S. dollar term loan was fixed at 5.83% through the use of interest rate swaps. The effective interest rate on 53.06% (€440.0 million) of the outstanding principal of the Company’s euro term loan was fixed at 5.68% through the use of interest rate swaps. The remaining unhedged balances of the U.S. dollar and euro term loans had effective interest rates of 3.90% and 3.73%, respectively. As of February 28, 2013 and May 31, 2012, the Company’s effective weighted average interest rate on all outstanding debt, including the interest rate swaps, was 6.50% and 7.80%, respectively.
Derivatives Not Designated as Hedging Instruments
Foreign Currency Instruments—The Company faces transactional currency exposures that arise when it or its foreign subsidiaries enter into transactions, primarily on an intercompany basis, denominated in currencies other than their functional currency. The Company enters into short-term forward currency exchange contracts in order to mitigate the currency exposure related to these intercompany payables and receivables arising from intercompany trade. The Company does not designate these contracts as hedges; therefore, all forward currency exchange contracts are recorded at their fair value each period, with the resulting gains and losses recorded in other (income) expense. Any foreign currency remeasurement gains or losses recognized in a period are generally offset with gains or losses on the forward currency exchange contracts. As of February 28, 2013, the fair value of the Company’s derivatives not designated as hedging instruments on a gross basis were assets of $0.2 million recorded in prepaid expenses and other, and liabilities of $1.5 million recorded in other accrued expenses.
F-66
Note 10—Accumulated Other Comprehensive Income (Loss).
Other comprehensive income (loss) includes currency translation adjustments, certain derivative-related activity, changes in the value of available-for-sale investments and changes in pension assets. The Company generally deems its foreign investments to be essentially permanent in nature and does not provide for taxes on currency translation adjustments arising from translating the investment in a foreign currency to U.S. dollars. When the Company determines that a foreign investment is no longer permanent in nature, estimated taxes are provided for the related deferred tax liability (asset), if any, resulting from currency translation adjustments.
Accumulated other comprehensive income (loss) and the related components are included in the table below:
| (in millions) | February 28, 2013 | May 31, 2012 | |||||
| Unrealized gain (loss) on available-for-sale securities, net of tax | $ | 3.1 | $ | (0.5 | ) | ||
| Unrealized gain (loss) on interest rate swaps, net of tax | (41.4 | ) | (47.3 | ) | |||
| Foreign currency translation adjustments | 117.5 | 173.7 | |||||
| Unrecognized actuarial gain (loss) on pension assets, net of tax | (3.0 | ) | (3.0 | ) | |||
| $ | 76.2 | $ | 122.9 | ||||
Note 11—Stock-based Compensation and Stock Plans.
The Company expenses all stock-based payments to employees and non-employee distributors, including stock options, leveraged share awards and restricted stock units, based on the grant date fair value over the required award service period using the graded vesting attribution method. For awards with a performance vesting condition, the Company recognizes expense when the performance condition is considered probable to occur. Stock-based compensation expense recognized was $5.8 million and $3.5 million for the three months ended February 28, 2013 and February 29, 2012 and $32.3 million and $12.2 million for the nine months ended February 28, 2013 and February 29, 2012, respectively. The increase in the expense was related to the modification that is described below.
On July 2, 2012, LVB launched a tender offer to eligible employees to exchange all of the stock options and restricted stock units held by such employees for new stock options and restricted stock units. Following the expiration of the tender offer on July 30, 2012, LVB accepted for exchange eligible options to purchase an aggregate of 29,532,500 shares of common stock of LVB and eligible restricted stock units underlying an aggregate of 3,665,000 shares of common stock of LVB. In accordance with the terms and conditions of the tender offer, on July 31, 2012, LVB granted 29,821,500 new options and 10,795,000 new restricted stock units in exchange for the cancellation of such tendered options and restricted stock units.
The objective of the tender offer was to provide employees who elected to participate with new options and new restricted stock units, the terms of which preserve the original incentive effect of the Company’s equity incentive programs in light of market and industry-wide economic conditions. The terms of the new stock options differed in respect to the tendered options principally with respect to:
| • | Exercise Price—The exercise price for the new stock options was lowered to the current fair value of $7.88 per share. |
| • | Vesting Periods—All prior options that were vested as of the completion date of the tender offer remain vested. All time-vesting options which were unvested as of the completion date of the tender offer will continue to vest on the same schedule on which they were originally granted. All unvested replacement extended time vesting options and modified performance options will vest on a schedule which is generally two years longer than the original vesting schedule, but in no case past 2017. |
| • | Performance Vesting Threshold—The new modified performance options will vest over the new vesting period if, as of the end of the Company’s most recent fiscal year ending on or prior to such vesting date, Biomet, Inc. has achieved the EBITDA target for such fiscal year determined by the Compensation Committee of the Board of Directors of the Company on or before the ninetieth (90th) day of such fiscal year and consistent with the Company’s business plan. |
The terms of the new restricted stock units are different from the tendered restricted stock units with respect to the vesting schedule, performance conditions and settlement. The new restricted stock units are granted subject to either a time-based vesting or a performance-based vesting requirement. Unlike the exchanged restricted stock units, the new restricted stock units do not vest in full on May 31, 2016 regardless of satisfaction of the vesting conditions.
F-67
In addition, following the termination of employment with the Company, new restricted stock units, whether vested or unvested, will be forfeited if such employee provides services to any competitor of the Company. In addition, participants holding new restricted stock units will also receive new awards called management dividend awards representing the right to receive a cash payment. Management dividend awards vest on a one-to-one basis with each new time-based restricted stock unit. Vested management dividend awards will be paid by cash distributions promptly following each anniversary of the grant date until the earlier of an initial public offering of the Company or the fifth anniversary of the grant date, subject to withholding taxes. Upon termination of employment for any reason, management dividend awards will be forfeited. The new restricted stock units were granted under the Company’s 2012 Restricted Stock Unit Plan, which was adopted by LVB on July 31, 2012. The maximum number of shares of common stock, par value $0.01 per share, that may be issued under the Company’s 2012 Restricted Stock Unit Plan is 14,000,000, subject to adjustment as described in the Plan.
On March 27, 2013, the Compensation Committee of LVB approved and adopted an Amended LVB Acquisition, Inc. 2012 Restricted Stock Unit Plan. The amendment permits certain participants in the Plan to be eligible to elect to receive a cash award with respect to their vested time-based restricted stock units subject to certain conditions, including the satisfaction of certain Company performance thresholds with respect to adjusted EBITDA and unlevered free cash flow. To the extent the Company performance conditions have been satisfied for the applicable fiscal year, eligible participants will be entitled to elect to receive a cash award based on the fair market value of the Parent's common stock on the first day of the applicable election period, payable in three installments over a two-year period, with respect to their vested time-based restricted stock units and such vested time-based restricted stock unit will be forfeited upon such election. Payment of the cash award is subject to the participants' continued employment through the payment date (other than with respect to a termination by the Company without cause).
During the second quarter of fiscal year 2013, the distributor options were modified to lower the exercise price to the current fair value of $7.88 per share.
Note 12—Income Taxes.
The Company applies guidance issued by the FASB for uncertainty in income taxes. The Company records the liability for unrecognized tax benefits (“UTBs”) as a long-term liability.
The Company conducts business globally and, as a result, certain of its subsidiaries file income tax returns in the U.S. federal jurisdiction, and various state and foreign jurisdictions. In the normal course of business, the Company is subject to examinations by taxing authorities throughout the world, including major jurisdictions such as Australia, Canada, France, Germany, Japan, the Netherlands, Spain, the United Kingdom and the United States. In addition, certain state and foreign tax returns are under examination by various regulatory authorities. The Company is no longer subject to U.S. federal income tax examinations for the fiscal years prior to and including the year ended May 31, 2008.
The Company regularly reviews issues that are raised from ongoing examinations and open tax years to evaluate the adequacy of its liabilities. As the various taxing authorities continue with their audit/examination programs, the Company will adjust its reserves accordingly to reflect these settlements. As of February 28, 2013, the Company does not anticipate a significant change in its worldwide gross liabilities for unrecognized tax benefits within the succeeding twelve months.
The Company’s effective income tax rates were 9.6% and 20.9% for the three and nine months ended February 28, 2013 compared to (161.9)% and 20.9% for the three and nine months ended February 29, 2012. Primary factors in determining the effective tax rate include the mix of various jurisdictions in which profits are projected to be earned and taxed, as well as assertions regarding the expected repatriation of earnings of the Company's foreign operations. The effective tax rates for the three and nine months ended February 28, 2013 were also impacted by a non-deductible goodwill impairment charge of $233.0 million, which was treated as a non-deductible permanent difference and contributed significantly to the effective tax rate being lower than U.S. statutory tax rates. Fluctuations in effective tax rates between comparable periods also reflect the discrete tax benefit or expense of items in continuing operations that represent tax effects not attributable to current-year ordinary income. Discrete items, consisting primarily of the tax benefit associated with the reduction of net deferred tax liabilities due to the impairment of intangible assets, as well as the prospective reduction of the United Kingdom statutory corporate tax rate enacted in July 2012 and finalization of the 2011 income tax returns had the effect of increasing the effective income tax rates by 9.0% and 6.7%, respectively, in the three and nine months ended February 28, 2013. The effective income tax rates for the three and nine months ended February 29, 2012 increased by 84.4% and 18.7%, respectively, due to discrete items consisting primarily of the tax benefit associated with the
F-68
reduction of net deferred tax liabilities due to the prospective reduction of corporate tax rates in Japan and the United Kingdom, restructuring-related adjustments and finalization of the 2010 income tax returns.
Note 13—Segment Reporting.
The Company operates in one reportable segment, musculoskeletal products, which includes the designing, manufacturing and marketing of large joint reconstructive; sports, extremities and trauma (“S.E.T.”); spine and bone healing; dental; and other products. Other products consist primarily of microfixation products, autologous therapies, general instruments and operating room supplies. The Company operates in various geographies. These geographic markets are comprised of the United States, Europe and International. Major markets included in the International geographic market are Canada, South America, Mexico and the Asia Pacific region.
Net sales by product category for the three and nine months ended February 28, 2013 and February 29, 2012 were as follows:
| Nine Months Ended | |||||||
| (in millions) | February 28, 2013 | February 29, 2012(1) | |||||
| Net sales by product: | |||||||
| Large Joint Reconstructive | $ | 1,261.1 | $ | 1,259.2 | |||
| S.E.T. | 440.9 | 263.4 | |||||
| Spine & Bone Healing | 224.3 | 224.9 | |||||
| Dental | 188.5 | 198.5 | |||||
| Other | 154.2 | 152.6 | |||||
| Total | $ | 2,269.0 | $ | 2,098.6 | |||
______________________
| (1) | Certain amounts have been adjusted to conform to the current presentation. The current presentation aligns with how the Company presently manages and markets its products. |
Net sales by geography for the three and nine months ended February 28, 2013 and February 29, 2012 were as follows:
| Nine Months Ended | |||||||
| (in millions) | February 28, 2013 | February 29, 2012 | |||||
| Net sales by geography: | |||||||
| United States | $ | 1,395.9 | $ | 1,273.8 | |||
| Europe | 521.5 | 520.3 | |||||
International(1) | 351.6 | 304.5 | |||||
| Total | $ | 2,269.0 | $ | 2,098.6 | |||
_____________________
| (1) | International primarily includes Canada, South America, Mexico and the Asia Pacific region. |
Long-term assets by geography as of February 28, 2013 and May 31, 2012 were as follows:
| (in millions) | February 28, 2013 | May 31, 2012 | |||||
Long-term assets (1) by geography: | |||||||
| United States | $ | 6,388.2 | $ | 6,817.5 | |||
| Europe | 893.3 | 722.7 | |||||
| International | 987.8 | 1,098.2 | |||||
| Total | $ | 8,269.3 | $ | 8,638.4 | |||
_____________________
| (1) | Defined as property, plant and equipment, intangibles and goodwill. |
F-69
Note 14—Guarantor and Non-Guarantor Financial Statements.
Each of Biomet’s existing wholly owned domestic subsidiaries fully, unconditionally, jointly, and severally guarantee the senior notes on a senior unsecured basis and the senior subordinated notes on a senior subordinated unsecured basis, in each case to the extent such subsidiaries guarantee Biomet’s senior secured cash flow facilities. Certain amounts reported in the prior year elimination column have been corrected to more accurately reflect the allocation of intercompany profit between the guarantor and the non-guarantor subsidiaries and to conform to the current period presentation. The Company believes such amounts are immaterial. LVB is neither an issuer nor guarantor of the notes described in Note 7.
The following financial information presents the composition of the combined guarantor subsidiaries:
CONDENSED CONSOLIDATING BALANCE SHEETS
| February 28, 2013 | |||||||||||||||||||
| (in millions) | Biomet, Inc. | Guarantors | Non-Guarantors | Eliminations | Total | ||||||||||||||
| Assets | |||||||||||||||||||
| Current assets: | |||||||||||||||||||
| Cash and cash equivalents | $ | — | $ | 39.1 | $ | 178.3 | $ | — | $ | 217.4 | |||||||||
| Accounts receivable, net | — | 270.0 | 275.9 | — | 545.9 | ||||||||||||||
| Income tax receivable | — | 1.3 | 3.1 | — | 4.4 | ||||||||||||||
| Inventories, net | — | 285.6 | 357.7 | — | 643.3 | ||||||||||||||
| Deferred income taxes | — | 47.3 | 14.8 | — | 62.1 | ||||||||||||||
| Prepaid expenses and other | — | 45.5 | 84.4 | — | 129.9 | ||||||||||||||
| Total current assets | — | 688.8 | 914.2 | — | 1,603.0 | ||||||||||||||
| Property, plant and equipment, net | — | 349.7 | 329.7 | — | 679.4 | ||||||||||||||
| Investments | — | 10.6 | 11.5 | — | 22.1 | ||||||||||||||
| Investment in subsidiaries | 8,285.6 | — | — | (8,285.6 | ) | — | |||||||||||||
| Intangible assets, net | — | 2,947.7 | 714.7 | — | 3,662.4 | ||||||||||||||
| Goodwill | — | 3,104.5 | 823.0 | — | 3,927.5 | ||||||||||||||
| Other assets | — | 93.5 | 13.8 | — | 107.3 | ||||||||||||||
| Total assets | $ | 8,285.6 | $ | 7,194.8 | $ | 2,806.9 | $ | (8,285.6 | ) | $ | 10,001.7 | ||||||||
| Liabilities & Shareholder’s Equity | |||||||||||||||||||
| Current liabilities: | |||||||||||||||||||
| Current portion of long-term debt | $ | 33.4 | $ | — | $ | 1.1 | $ | — | $ | 34.5 | |||||||||
| Accounts payable | — | 44.1 | 43.1 | — | 87.2 | ||||||||||||||
| Accrued interest | 43.8 | — | 0.1 | — | 43.9 | ||||||||||||||
| Accrued wages and commissions | — | 70.6 | 59.8 | — | 130.4 | ||||||||||||||
| Other accrued expenses | — | 123.9 | 65.1 | — | 189.0 | ||||||||||||||
| Total current liabilities | 77.2 | 238.6 | 169.2 | — | 485.0 | ||||||||||||||
| Long-term debt | 5,942.4 | — | 1.5 | — | 5,943.9 | ||||||||||||||
| Deferred income taxes | — | 919.9 | 181.0 | — | 1,100.9 | ||||||||||||||
| Other long-term liabilities | — | 148.0 | 57.9 | — | 205.9 | ||||||||||||||
| Total liabilities | 6,019.6 | 1,306.5 | 409.6 | — | 7,735.7 | ||||||||||||||
| Shareholder’s equity | 2,266.0 | 5,888.3 | 2,397.3 | (8,285.6 | ) | 2,266.0 | |||||||||||||
| Total liabilities and shareholder’s equity | $ | 8,285.6 | $ | 7,194.8 | $ | 2,806.9 | $ | (8,285.6 | ) | $ | 10,001.7 | ||||||||
F-70
| May 31, 2012 | |||||||||||||||||||
| (in millions) | Biomet, Inc. | Guarantors | Non-Guarantors | Eliminations | Total | ||||||||||||||
| Assets | |||||||||||||||||||
| Current assets: | |||||||||||||||||||
| Cash and cash equivalents | $ | — | $ | 190.1 | $ | 302.3 | $ | — | $ | 492.4 | |||||||||
| Accounts receivable, net | — | 227.6 | 264.0 | — | 491.6 | ||||||||||||||
| Investments | — | — | 2.5 | — | 2.5 | ||||||||||||||
| Income tax receivable | — | 2.1 | 2.9 | — | 5.0 | ||||||||||||||
| Inventories, net | — | 288.7 | 254.5 | — | 543.2 | ||||||||||||||
| Deferred income taxes | — | 42.3 | 10.2 | — | 52.5 | ||||||||||||||
| Prepaid expenses and other | — | 48.8 | 75.3 | — | 124.1 | ||||||||||||||
| Total current assets | — | 799.6 | 911.7 | — | 1,711.3 | ||||||||||||||
| Property, plant and equipment, net | — | 320.1 | 273.5 | — | 593.6 | ||||||||||||||
| Investments | — | 10.1 | 3.8 | — | 13.9 | ||||||||||||||
| Investment in subsidiaries | 8,562.9 | — | — | (8,562.9 | ) | — | |||||||||||||
| Intangible assets, net | — | 3,239.3 | 691.1 | — | 3,930.4 | ||||||||||||||
| Goodwill | — | 3,271.4 | 843.0 | — | 4,114.4 | ||||||||||||||
| Other assets | — | 45.6 | 11.2 | — | 56.8 | ||||||||||||||
| Total assets | $ | 8,562.9 | $ | 7,686.1 | $ | 2,734.3 | $ | (8,562.9 | ) | $ | 10,420.4 | ||||||||
| Liabilities & Shareholder’s Equity | |||||||||||||||||||
| Current liabilities: | |||||||||||||||||||
| Current portion of long-term debt | $ | 34.3 | $ | — | $ | 1.3 | $ | — | $ | 35.6 | |||||||||
| Accounts payable | — | 71.5 | 44.7 | — | 116.2 | ||||||||||||||
| Accrued interest | 56.5 | — | — | — | 56.5 | ||||||||||||||
| Accrued wages and commissions | — | 69.5 | 52.5 | — | 122.0 | ||||||||||||||
| Other accrued expenses | — | 106.1 | 74.1 | — | 180.2 | ||||||||||||||
| Total current liabilities | 90.8 | 247.1 | 172.6 | — | 510.5 | ||||||||||||||
| Long-term debt | 5,790.0 | — | 2.2 | — | 5,792.2 | ||||||||||||||
| Deferred income taxes | — | 1,065.7 | 192.1 | — | 1,257.8 | ||||||||||||||
| Other long-term liabilities | — | 131.6 | 46.2 | — | 177.8 | ||||||||||||||
| Total liabilities | 5,880.8 | 1,444.4 | 413.1 | — | 7,738.3 | ||||||||||||||
| Shareholder’s equity | 2,682.1 | 6,241.7 | 2,321.2 | (8,562.9 | ) | 2,682.1 | |||||||||||||
| Total liabilities and shareholder’s equity | $ | 8,562.9 | $ | 7,686.1 | $ | 2,734.3 | $ | (8,562.9 | ) | $ | 10,420.4 | ||||||||
F-71
CONDENSED CONSOLIDATING STATEMENTS OF OPERATIONS AND COMPREHENSIVE INCOME (LOSS)
| Nine Months Ended February 28, 2013 | |||||||||||||||||||
| (in millions) | Biomet, Inc. | Guarantors | Non-Guarantors | Eliminations | Total | ||||||||||||||
| Net sales | $ | — | $ | 1,438.6 | $ | 830.4 | $ | — | $ | 2,269.0 | |||||||||
| Cost of sales | — | 551.1 | 184.9 | — | 736.0 | ||||||||||||||
| Gross profit | — | 887.5 | 645.5 | — | 1,533.0 | ||||||||||||||
| Goodwill and intangible asset impairment charge | — | 269.0 | 65.1 | — | 334.1 | ||||||||||||||
| Operating expenses | — | 908.3 | 315.8 | — | 1,224.1 | ||||||||||||||
| Operating income (loss) | — | (289.8 | ) | 264.6 | — | (25.2 | ) | ||||||||||||
| Other (income) expense, net | 479.0 | 5.1 | (0.9 | ) | — | 483.2 | |||||||||||||
| Income (loss) before income taxes | (479.0 | ) | (294.9 | ) | 265.5 | — | (508.4 | ) | |||||||||||
| Tax expense (benefit) | (182.0 | ) | (112.1 | ) | 187.9 | — | (106.2 | ) | |||||||||||
| Equity in earnings of subsidiaries | (105.2 | ) | — | — | 105.2 | — | |||||||||||||
| Net income (loss) | $ | (402.2 | ) | $ | (182.8 | ) | $ | 77.6 | $ | 105.2 | $ | (402.2 | ) | ||||||
| Other comprehensive income (loss) | $ | 5.9 | $ | — | $ | (52.6 | ) | $ | — | $ | (46.7 | ) | |||||||
| Total comprehensive income (loss) | $ | (396.3 | ) | $ | (182.8 | ) | $ | 25.0 | $ | 105.2 | $ | (448.9 | ) | ||||||
| Nine Months Ended February 29, 2012 | |||||||||||||||||||
| (in millions) | Biomet, Inc. | Guarantors | Non-Guarantors | Eliminations | Total | ||||||||||||||
| Net sales | $ | — | $ | 1,316.1 | $ | 782.5 | $ | — | $ | 2,098.6 | |||||||||
| Cost of sales | — | 368.7 | 301.2 | — | 669.9 | ||||||||||||||
| Gross profit | — | 947.4 | 481.3 | — | 1,428.7 | ||||||||||||||
| Operating expenses | — | 763.4 | 380.7 | — | 1,144.1 | ||||||||||||||
| Operating income (loss) | — | 184.0 | 100.6 | — | 284.6 | ||||||||||||||
| Other (income) expense, net | 360.6 | 1.5 | 10.6 | — | 372.7 | ||||||||||||||
| Income (loss) before income taxes | (360.6 | ) | 182.5 | 90.0 | — | (88.1 | ) | ||||||||||||
| Tax expense (benefit) | (115.1 | ) | 69.3 | 27.4 | — | (18.4 | ) | ||||||||||||
| Equity in earnings of subsidiaries | 175.8 | — | — | (175.8 | ) | — | |||||||||||||
| Net income (loss) | $ | (69.7 | ) | $ | 113.2 | $ | 62.6 | $ | (175.8 | ) | $ | (69.7 | ) | ||||||
| Other comprehensive income (loss) | $ | 17.4 | $ | — | $ | (22.7 | ) | $ | — | $ | (5.3 | ) | |||||||
| Total comprehensive income (loss) | $ | (52.3 | ) | $ | 113.2 | $ | 39.9 | $ | (175.8 | ) | $ | (75.0 | ) | ||||||
F-72
CONDENSED CONSOLIDATING STATEMENTS OF CASH FLOWS
| Nine Months Ended February 28, 2013 | |||||||||||||||||||
| (in millions) | Biomet, Inc. | Guarantor | Non-Guarantors | Eliminations | Total | ||||||||||||||
| Cash flows provided by (used in) operating activities | $ | (232.3 | ) | $ | 246.9 | $ | 154.0 | $ | 105.2 | $ | 273.8 | ||||||||
| Capital expenditures | — | (69.4 | ) | (80.3 | ) | — | (149.7 | ) | |||||||||||
| Acquisitions, net of cash acquired - Trauma Acquisition | — | (277.5 | ) | (2.5 | ) | — | (280.0 | ) | |||||||||||
| Other | 354.1 | (50.9 | ) | (202.1 | ) | (105.2 | ) | (4.1 | ) | ||||||||||
| Cash flows provided by (used in) investing activities | 354.1 | (397.8 | ) | (284.9 | ) | (105.2 | ) | (433.8 | ) | ||||||||||
| Proceeds under asset based revolver | 80.0 | — | — | — | 80.0 | ||||||||||||||
| Payments under asset based revolver | (80.0 | ) | — | — | — | (80.0 | ) | ||||||||||||
| Proceeds from senior notes due 2020 and term loans | 3,396.2 | — | — | — | 3,396.2 | ||||||||||||||
| Tender/retirement of senior notes due 2017 and term loans | (3,423.0 | ) | — | — | — | (3,423.0 | ) | ||||||||||||
| Payment of fees related to refinancing activities | (77.8 | ) | — | — | — | (77.8 | ) | ||||||||||||
| Other | (17.2 | ) | (0.1 | ) | (9.0 | ) | — | (26.3 | ) | ||||||||||
| Cash flows used in financing activities | (121.8 | ) | (0.1 | ) | (9.0 | ) | — | (130.9 | ) | ||||||||||
| Effect of exchange rate changes on cash | — | — | 15.9 | — | 15.9 | ||||||||||||||
| Decrease in cash and cash equivalents | — | (151.0 | ) | (124.0 | ) | — | (275.0 | ) | |||||||||||
| Cash and cash equivalents, beginning of period | — | 190.1 | 302.3 | — | 492.4 | ||||||||||||||
| Cash and cash equivalents, end of period | $ | — | $ | 39.1 | $ | 178.3 | $ | — | $ | 217.4 | |||||||||
| Nine Months Ended February 29, 2012 | |||||||||||||||||||
| (in millions) | Biomet, Inc. | Guarantor | Non-Guarantors | Eliminations | Total | ||||||||||||||
| Cash flows provided by (used in) operating activities | $ | 0.2 | $ | 333.1 | $ | 133.8 | $ | (175.8 | ) | $ | 291.3 | ||||||||
| Proceeds from sales/maturities of investments | — | 33.7 | 8.3 | — | 42.0 | ||||||||||||||
| Capital expenditures | — | (60.3 | ) | (62.4 | ) | — | (122.7 | ) | |||||||||||
| Other | 27.6 | (265.1 | ) | 60.7 | 175.8 | (1.0 | ) | ||||||||||||
| Cash flows provided by (used in) investing activities | 27.6 | (291.7 | ) | 6.6 | 175.8 | (81.7 | ) | ||||||||||||
| Cash flows used in financing activities | (27.8 | ) | — | (1.1 | ) | — | (28.9 | ) | |||||||||||
| Effect of exchange rate changes on cash | — | — | (12.5 | ) | — | (12.5 | ) | ||||||||||||
| Increase in cash and cash equivalents | — | 41.4 | 126.8 | — | 168.2 | ||||||||||||||
| Cash and cash equivalents, beginning of period | — | 176.4 | 151.4 | — | 327.8 | ||||||||||||||
| Cash and cash equivalents, end of period | $ | — | $ | 217.8 | $ | 278.2 | $ | — | $ | 496.0 | |||||||||
F-73
Note 15—Contingencies.
The Company is involved in various proceedings, legal actions and claims arising in the normal course of business, including proceedings related to product liability, governmental investigations, intellectual property, commercial litigation and other matters. The outcomes of these matters will generally not be known for an extended period of time. In certain of the legal proceedings, the claimants seek damages, as well as other compensatory relief, which could result in the payment of significant claims and settlements. For legal matters for which management has sufficient information to reasonably estimate the Company’s future obligations, a liability representing management’s best estimate of the probable cost, or the minimum of the range of probable losses when a best estimate within the range is not known, for the resolution of these legal matters is recorded. The estimates are based on consultation with legal counsel, previous settlement experience and settlement strategies. The Company’s accrual for contingencies at February 28, 2013 and May 31, 2012 of $49.1 million and $25.5 million, respectively, primarily relate to certain product liability claims and the Massachusetts U.S. Department of Justice EBI products investigation described below.
Other than the Massachusetts U.S. Department of Justice EBI products investigation and certain product liability claims, for which the estimated loss is included in the accrual referenced above, given the relatively early stages of the other governmental investigations and other product liability claims described below, and the complexities involved in these matters, the Company is unable to estimate a possible loss or range of possible loss for such matters until the Company knows, among other factors, (i) what claims, if any will survive dispositive motion practice, (ii) the extent of the claims, including the size of any potential class, particularly when damages are not specified or are indeterminate, (iii) how the discovery process will affect the litigation, (iv) the settlement posture of the other parties to the litigation and (v) any other factors that may have a material effect on the litigation.
U.S. Department of Justice Consulting Agreement Investigation
On September 27, 2007, Biomet entered into a Deferred Prosecution Agreement with the U.S. Attorney’s Office for the District of New Jersey. The agreement concluded the government’s investigation into whether consulting agreements between the largest orthopedic manufacturers and orthopedic surgeons who use joint reconstruction and replacement products may have violated the federal Anti-Kickback Statute.
Through the agreement, the U.S. Attorney’s Office agreed not to prosecute Biomet in connection with this matter, provided that Biomet satisfied its obligations under the agreement over the 18 months following the date of the Deferred Prosecution Agreement. The agreement called for the appointment of an independent monitor to review Biomet’s compliance with the agreement, particularly in relation to its consulting agreements. On March 27, 2009, the Deferred Prosecution Agreement expired and the complaint was dismissed with prejudice.
As part of the resolution of this matter, Biomet also entered into a Corporate Integrity Agreement with the Office of the Inspector General of the U.S. Department of Health and Human Services. The agreement requires the Company for five years subsequent to September 27, 2007 to continue to adhere to its Code of Business Conduct and Ethics and certain other provisions, including reporting requirements. Biomet submitted its final report under the Corporate Integrity Agreement with the Office of the Inspector General ("OIG-HHS") and received confirmation in January 2013 from OIG-HHS that its obligations under the agreement have terminated.
U.S. Department of Justice EBI Products Investigations and Other Matters
In February 2010, Biomet received a subpoena from the Office of the Inspector General of the U.S. Department of Health and Human Services requesting various documents relating to agreements or arrangements between physicians and the Company’s Interpore Cross subsidiary for the period from 1999 through the present and the marketing and sales activities associated with Interpore Cross’ spinal products. Biomet is cooperating with the request of the Office of the Inspector General. The Company can make no assurances as to the time or resources that will be needed to devote to this inquiry or its final outcome.
In April 2009, Biomet received an administrative subpoena from the U.S. Attorney’s Office for the District of Massachusetts requesting various documents relating primarily to the Medicare reimbursement of and certain business practices related to the Company’s EBI subsidiary’s non-invasive bone growth stimulators. It is the Company’s understanding that competitors in the non-invasive bone growth stimulation market received similar subpoenas. The Company received subsequent subpoenas in connection with the investigation in September 2009, June 2010, February 2011 and March 2012 along with several informal requests for information. Biomet has produced responsive documents and is fully cooperating in the investigation.
F-74
In April 2009, the Company became aware of a qui tam complaint alleging violations of the federal and various state False Claims Acts filed in the United States District Court for the District of Massachusetts, where it is currently pending. Biomet, Parent, and several of the Company’s competitors in the non-invasive bone growth stimulation market were named as defendants in this action. The allegations in the complaint are similar in nature to certain categories of requested documents in the above-referenced administrative subpoenas. The U.S. government has not intervened in the action. The Company is vigorously defending this matter and intends to continue to do so.
U.S. Department of Justice Civil Division Investigation
In September 2010, Biomet received a Civil Investigative Demand (“CID”) issued by the U.S. Department of Justice—Civil Division pursuant to the False Claims Act. The CID requests that the Company provide documents and testimony related to allegations that Biomet, OtisMed Corp. and Stryker Corp. have violated the False Claims Act relating to the marketing of, and payment submissions for, OtisMed’s OtisKnee™ (a registered trademark of OtisMed) knee replacement system. The Company has produced responsive documents and is fully cooperating in the investigation.
U.S. Securities and Exchange Commission (“SEC”) Informal Investigation
On September 25, 2007, Biomet received a letter from the SEC informing the Company that it is conducting an informal investigation regarding possible violations of the Foreign Corrupt Practices Act in the sale of medical devices in certain foreign countries by companies in the medical devices industry. The Foreign Corrupt Practices Act prohibits U.S. companies and their officers, directors, employees, or shareholders acting on their behalf and agents from offering, promising, authorizing or making payments to foreign officials for the purpose of obtaining or retaining business abroad or otherwise obtaining favorable treatment and this law requires companies to maintain records which fairly and accurately reflect transactions and to maintain internal accounting controls. In many countries, hospitals and clinics are government-owned and healthcare professionals employed by such hospitals and clinics, with whom the Company regularly interacts, may meet the definition of a foreign official for purposes of the Foreign Corrupt Practices Act. On November 9, 2007, the Company received a letter from the Department of Justice requesting any information provided to the SEC be provided to the Department of Justice on a voluntary basis.
On March 26, 2012, Biomet entered into a Deferred Prosecution Agreement (“DPA”) with the U.S. Department of Justice (“DOJ”) and a Consent to Final Judgment (“Consent Agreement”) with the SEC related to these investigations by the DOJ and the SEC. Pursuant to the DPA, the DOJ has agreed not to prosecute the Company in connection with this matter, provided that the Company satisfies its obligations under the agreement over the next three years. In addition, pursuant to the terms of the DPA, an independent external compliance monitor has been appointed to review the Company’s compliance with the DPA, particularly in relation to the Company’s international sales practices, for at least the first 18 months of the three year term of the DPA. The Company also agreed to pay a monetary penalty of $17.3 million to resolve the charges brought by the DOJ, which was paid in the fourth quarter of fiscal year 2012. The terms of the DPA and the associated monetary penalty reflect the Company’s full cooperation throughout the investigation.
The Company contemporaneously reached a Consent Agreement with the SEC to settle civil claims related to this matter. As part of the Consent Agreement, Biomet agreed to the SEC’s entry of a Final Judgment requiring Biomet to disgorge profits and pay prejudgment interest in the aggregate amount of $5.6 million, which was paid in the fourth quarter of fiscal year 2012.
Product Liability
The Company has received claims for personal injury associated with its metal-on-metal hip products. The pre-trial management of certain of these claims has been consolidated in a federal court in South Bend, Indiana. Certain other claims are pending in various state courts. The Company believes the number of claims continues to increase incrementally due to the negative publicity regarding metal-on-metal hip products generally. The Company believes it has data that supports the efficacy and safety of its metal-on-metal hip products, and the Company intends to vigorously defend itself in these matters. The Company currently accounts for these claims in accordance with its standard product liability accrual methodology on a case by case basis. Given the substantial or indeterminate amounts sought in these matters, and the inherent unpredictability of such matters, an adverse outcome in these matters in excess of the amounts included in the Company's accrual for contingencies could have a material adverse effect on our financial condition, results of operations and cash flow.
Future revisions in the Company’s estimates of these provisions could materially impact its results of operations and financial position. The Company uses the best information available to determine the level of accrued
F-75
product liabilities, and the Company believes its accruals are adequate. The Company has maintained product liability insurance coverage for a number of years on a claims-made basis. All such insurers have been placed on notice of these claims. To date, the insurance companies have neither accepted nor denied coverage, and an issue may arise as to which policy or policies are to respond. The amounts incurred to date in connection with these claims have not exceeded the Company’s self-insured retention(s).
Other Matters
In January 2009, Heraeus Kulzer GmbH initiated legal proceedings in Germany against Biomet and its subsidiary, Biomet Europe BV, alleging that the Company and Biomet Europe BV misappropriated Heraeus Kulzer trade secrets when developing its current lines of European bone cements, which were first marketed in 2005. The lawsuit seeks damages in excess of €30 million and injunctive relief to preclude the Company from producing its current line of European bone cements. On December 20, 2012, the trial court ruled that Biomet did not misappropriate trade secrets and consequently dismissed Biomet, Biomet Europe BV, Biomet Deutschland GmbH and other defendants from the lawsuit. Biomet Orthopaedics Switzerland GmbH (“Biomet Switzerland”) remains as the only defendant in the lawsuit and the trial court has ruled that Heraeus Kulzer will not be permitted to review certification materials of Biomet Switzerland for purposes of determining whether there is any evidence that would support a claim of trade secret misappropriation by that entity. The trial court’s decision remains subject to appeal by Heraeus Kulzer and the Company is continuing to vigorously defend this matter.
There are various other claims, lawsuits, disputes with third parties, investigations and pending actions involving various allegations against the Company incident to the operation of its business, principally product liability and intellectual property cases. Each of these matters is subject to various uncertainties, and it is possible that some of these matters may be resolved unfavorably to the Company. The Company accrues for losses that are deemed to be probable and subject to reasonable estimate.
Based on the advice of the Company’s counsel in these matters, it is unlikely that the resolution of any of these matters and any liabilities in excess of amounts provided will be material to the Company’s financial position, results of operations or cash flows.
Note 16—Related Parties.
Transactions with the Sponsor Group
On December 18, 2006, Biomet, Inc. entered into an Agreement and Plan of Merger with LVB Acquisition, LLC, a Delaware limited liability company, which was subsequently converted to a corporation, LVB Acquisition, Inc., and LVB Acquisition Merger Sub, Inc., an Indiana corporation and a wholly-owned subsidiary of Parent (“Purchaser”), which agreement was amended and restated as of June 7, 2007 and which we refer to as the “Merger Agreement.” Pursuant to the Merger Agreement, on June 13, 2007, Purchaser commenced a cash tender offer (the “Offer”) to purchase all of Biomet, Inc.’s outstanding common shares, without par value (the “Shares”) at a price of $46.00 per Share (the “Offer Price”) without interest and less any required withholding taxes. The Offer was made pursuant to Purchaser’s offer to purchase dated June 13, 2007 and the related letter of transmittal, each of which was filed with the SEC on June 13, 2007. In connection with the Offer, Purchaser entered into a credit agreement dated as of July 11, 2007 for a $6,165.0 million senior secured term loan facility (the “Tender Facility”), maturing on June 6, 2008, and pursuant to which it borrowed approximately $4,181.0 million to finance a portion of the Offer and pay related fees and expenses. The Offer expired at midnight, New York City time, on July 11, 2007, with approximately 82% of the outstanding Shares having been tendered to Purchaser. At Biomet, Inc.’s special meeting of shareholders held on September 5, 2007, more than 91% of Biomet, Inc.’s shareholders voted to approve the proposed merger, and Parent acquired Biomet, Inc. on September 25, 2007 through a reverse subsidiary merger with Biomet, Inc. being the surviving company (the “Merger”). Subsequent to the acquisition, Biomet, Inc. became a subsidiary of Parent, which is controlled by LVB Acquisition Holding, LLC, or “Holding”, an entity controlled by a consortium of private equity funds affiliated with The Blackstone Group, Goldman, Sachs & Co., Kohlberg Kravis Roberts & Co., and TPG Global, LLC (each a “Sponsor” and collectively, the “Sponsors”), and certain investors who agreed to co-invest with the Sponsors (the “Co-Investors”). These transactions, including the Merger and the Company’s payment of any fees and expenses related to these transactions, are referred to collectively as the “Transactions.”
Management Services Agreement
Upon completion of the Transactions, Biomet entered into a management services agreement with certain affiliates of the Sponsors, pursuant to which such affiliates of the Sponsors or their successors assigns, affiliates, officers, employees, and/or representatives and third parties (collectively, the “Managers”) provide management, advisory, and consulting services to the Company. Pursuant to such agreement, the Managers received a transaction
F-76
fee equal to 1% of total enterprise value of the Transactions for the services rendered by such entities related to the Transactions upon entering into the agreement, and the Sponsors receive an annual monitoring fee equal to 1% of the Company’s annual Adjusted EBITDA (as defined in the credit agreement) as compensation for the services rendered and reimbursement for out-of-pocket expenses incurred by the Managers in connection with the agreement and the Transactions. The Company is required to pay the Sponsors the monitoring fee on a quarterly basis in arrears. The total amount of Sponsor fees was $2.8 million and $2.7 million for the three months ended February 28, 2013 and 2012, respectively, and $8.2 million and $7.5 million for the nine months ended February 28, 2013 and 2012, respectively. The Company may also pay certain subsequent fees to the Managers for advice rendered in connection with financings or refinancings (equity or debt), acquisitions, dispositions, spin-offs, split-offs, dividends, recapitalizations, an initial underwritten public offering and change of control transactions involving the Company or any of its subsidiaries. The management services agreement includes customary exculpation and indemnification provisions in favor of the Managers and their affiliates.
Amended and Restated Limited Liability Company Operating Agreement of Holding
On September 27, 2007, certain investment funds associated with or designated by the Sponsors (the “Sponsor Funds”) entered into an amended and restated limited liability company operating agreement, or the “LLC Agreement,” in respect of Holding. The LLC Agreement contains agreements among the parties with respect to the election of the Company’s directors and the directors of its parent companies, restrictions on the issuance or transfer of interests in the Company and other corporate governance provisions (including the right to approve various corporate actions).
Pursuant to the LLC Agreement, each of the Sponsors has the right to nominate, and has nominated, two directors to Biomet’s and LVB’s Board of Directors and also is entitled to appoint one non-voting observer to the Board of Directors for so long as such Sponsor remains a member of Holding. In addition to their right to appoint non-voting observers to the Board of Directors, certain of the Sponsor Funds have certain other management rights to the extent that any such Sponsor Fund is required to operate as a “venture capital operating company” as defined in the regulations issued by the U.S. Department of Labor at Section 2510.3-101 of Part 2510 of Chapter XXV, Title 29 of the Code of Federal Regulations, or any successor regulations. Each Sponsor’s right to nominate directors is freely assignable to funds affiliated with such Sponsor, and is assignable to non-affiliates of such Sponsor only if the assigning Sponsor transfers its entire interest in Holding not previously transferred and only with the prior written consent of the Sponsors holding at least 70% of the membership interests in Holding, or “requisite Sponsor consent”. In addition to their rights under the LLC Agreement, the Sponsors may also appoint one or more persons unaffiliated with any of the Sponsors to the Board of Directors. Following Purchaser’s purchase of the Shares tendered in the Offer, the Sponsors jointly appointed Dane A. Miller, Ph.D. and Jeffrey R. Binder to the Board of Directors in addition to the two directors appointed by each of the Sponsors.
Pursuant to the LLC Agreement, each director has one vote for purposes of any Board of Directors action, and all decisions of the Board of Directors require the approval of a majority of the directors designated by the Sponsors. In addition, the LLC Agreement provides that certain major decisions regarding the Company or its parent companies require the requisite Sponsor consent.
The LLC Agreement includes certain customary agreements with respect to restrictions on the issuance or transfer of interests in Biomet and LVB, including preemptive rights, tag-along rights and drag-along rights.
The Co-Investors have also been admitted as members of Holding, both directly and through Sponsor-controlled investment vehicles. Although the Co-Investors are therefore parties to the LLC Agreement, they have no rights with respect to the election of Biomet’s or LVB’s directors or the approval of its corporate actions.
The Sponsors have also caused Holding and Parent to enter into an agreement with the Company obligating the Company and Parent to take all actions necessary to give effect to the corporate governance, preemptive rights, transfer restriction and certain other provisions of the LLC Agreement, and prohibiting the Company and Parent from taking any actions that would be inconsistent with such provisions of the LLC Agreement.
Registration Rights Agreement
The Sponsor Funds and the Co-Investors also entered into a registration rights agreement with Holding, LVB and Biomet upon the closing of the Transactions. Pursuant to this agreement, the Sponsor Funds have the power to cause Holding, LVB and Biomet to register their, the Co-Investors’ and certain other persons’ equity interests under the Securities Act and to maintain a shelf registration statement effective with respect to such interests. The agreement also entitles the Sponsor Funds and the Co-Investors to participate in any future registration of equity interests under the Securities Act that Holding, LVB or Biomet may undertake.
F-77
On August 8, 2012 and October 2, 2012, Goldman, Sachs & Co. and the other initial purchasers of the new senior notes and new senior subordinated notes entered into registration rights agreements with Biomet. Pursuant to these agreements, Biomet is obligated, for the sole benefit of Goldman, Sachs & Co. in connection with its market-making activities with respect to the new senior notes and new senior subordinated notes, to file a registration statement under the Securities Act in a form approved by Goldman, Sachs & Co. and to keep such registration statement continually effective for so long as Goldman, Sachs & Co. may be required to deliver a prospectus in connection with transactions in senior and senior subordinated notes due 2020 and to supplement or make amendments to such registration statement as when required by the rules and regulations applicable to such registration statement.
Management Stockholders’ Agreements
On September 13, 2007 and November 6, 2007, Holding, LVB and the Sponsor Funds entered into stockholders agreements with certain of the Company’s senior executives and other management stockholders. Pursuant to the terms of the LVB Acquisition, Inc. Management Equity Incentive Plan, LVB Acquisition, Inc. Restricted Stock Unit Plan and LVB Acquisition, Inc. 2012 Restricted Stock Unit Plan, participants who exercise their vested options or settle their vested restricted stock units are required to become parties to the agreement dated November 6, 2007. The stockholder agreements contain agreements among the parties with respect to restrictions on the transfer and issuance of shares, including preemptive, drag-along, tag-along, and call/put rights.
Consulting Agreements
On January 14, 2010, Biomet entered into a consulting agreement with Dr. Dane A. Miller Ph.D., pursuant to which it will pay Dr. Miller a consulting fee of $0.25 million per fiscal year for Dr. Miller’s consulting services and will reimburse Dr. Miller for out-of-pocket fees and expenses relating to an off-site office and administrative support in an amount of $0.1 million per year. The term of the agreement extends through the earlier of September 1, 2011, an initial public offering or a change of control. The agreement also contains certain restrictive covenants prohibiting Dr. Miller from competing with the Company and soliciting employees of the Company during the term of the agreement and for a period of one year following such term. On September 6, 2011, the Company entered into an amendment to the consulting agreement with Dr. Miller, pursuant to which it agreed to increase the expenses relating to an off-site office and administrative support from $0.1 million per year to $0.15 million per year and extend the term of the agreement through the earlier of September 1, 2013, an initial public offering or a change of control. Dr. Miller received payments under the consulting agreement of $0.1 million and $0.1 million for the three months ended February 28, 2013 and February 29, 2012, respectively, and $0.3 million and $0.3 million for the nine months ended February 28, 2013 and February 29, 2012, respectively.
Indemnification Priority Agreement
On January 11, 2010, Biomet and LVB entered into an indemnification priority agreement with the Sponsors (or certain affiliates designated by the Sponsors) pursuant to which Biomet and LVB clarified certain matters regarding the existing indemnification and advancement of expenses rights provided by Biomet and LVB pursuant to their respective charters and the management services agreement described above. In particular, pursuant to the terms of the indemnification agreement, Biomet acknowledged that as among Biomet, LVB and the Sponsors and their respective affiliates, the obligation to indemnify or advance expenses to any director appointed by any of the Sponsors will be payable in the following priority: Biomet will be the primary source of indemnification and advancement; LVB will be the secondary source of indemnification and advancement; and any obligation of a Sponsor-affiliated indemnitor to indemnify or advance expenses to such director will be tertiary to Biomet’s and, then, LVB obligations. In the event that either Biomet or LVB fails to indemnify or advance expenses to any such director in contravention of its obligations, and any Sponsor-affiliated indemnitor makes any indemnification payment or advancement of expenses to such director on account of such unpaid liability, such Sponsor-affiliated indemnitor will be subrogated to the rights of such director under any such Biomet or LVB indemnification agreement.
Equity Healthcare
Effective January 1, 2009, Biomet entered into an employer health program agreement with Equity Healthcare LLC (“Equity Healthcare”). Equity Healthcare negotiates with providers of standard administrative services for health benefit plans as well as other related services for cost discounts and quality of service monitoring capability by Equity Healthcare. Because of the combined purchasing power of its client participants, Equity Healthcare is able to negotiate pricing terms for providers that are believed to be more favorable than the companies could obtain for themselves on an individual basis.
F-78
In consideration for Equity Healthcare’s provision of access to these favorable arrangements and its monitoring of the contracted third parties’ delivery of contracted services to the Company, the Company pays Equity Healthcare a fee of $2 per participating employee per month (“PEPM Fee”). As of February 28, 2013, the Company had approximately 3,200 employees enrolled in its health benefit plans in the United States.
Equity Healthcare may also receive a fee (“Health Plan Fees”) from one or more of the health plans with whom Equity Healthcare has contractual arrangements if the total number of employees joining such health plans from participating companies exceeds specified thresholds. If and when Equity Healthcare reaches the point at which the aggregate of its receipts from the PEPM Fee and the Health Plan Fees have covered all of its allocated costs, it will apply the incremental revenues derived from all such fees to (a) reduce the PEPM Fee otherwise payable by the Company; (b) avoid or reduce an increase in the PEPM Fee that might otherwise have occurred on contract renewal; or (c) arrange for additional services to the Company at no cost or reduced cost.
Equity Healthcare is an affiliate of Blackstone, with whom Michael Dal Bello and Chinh Chu, members of the Company’s Board of Directors, are affiliated and in which they may have an indirect pecuniary interest.
There were payments of $0.1 million made during both the three and nine months ended February 28, 2013 and payments of $0.1 million made during the nine months ended February 29, 2012 with no payments during the three months ended February 29, 2012.
Core Trust Purchasing Group Participation Agreement
Effective May 1, 2007, Biomet entered into a 5-year participation agreement (“Participation Agreement”) with Core Trust Purchasing Group, a division of HealthTrust Purchasing Corporation (“CPG”), designating CPG as the Company’s exclusive “group purchasing organization” for the purchase of certain products and services from third party vendors. Effective June 1, 2012, Biomet entered into an amendment to extend the term of the Participation Agreement with CPG. CPG secures from vendors pricing terms for goods and services that are believed to be more favorable than participants in the group purchasing organization could obtain for themselves on an individual basis. Under the participation agreement, the Company must purchase 80% of the requirements of its participating locations for core categories of specified products and services, from vendors participating in the group purchasing arrangement with CPG or CPG may terminate the contract. In connection with purchases by its participants (including the Company), CPG receives a commission from the vendors in respect of such purchases. The total amount of fees paid to CPG was $0.3 million and $0.1 million for the three months ended February 28, 2013 and February 29, 2012, respectively, and $0.5 million and $0.3 million for the nine months ended February 28, 2013 and February 29, 2012, respectively.
Although CPG is not affiliated with Blackstone, in consideration for Blackstone’s facilitating Biomet’s participation in CPG and monitoring the services CPG provides to the Company, CPG remits a portion of the commissions received from vendors in respect of the Company’s purchases under the Participation Agreement to an affiliate of Blackstone, with whom Michael Dal Bello and Chinh Chu, members of the Company’s Board of Directors, are affiliated and in which they may have an indirect pecuniary interest.
Refinancing Activities
Goldman Sachs served as a dealer manager and arranger for the refinancing activities explained in Note 7 – Debt and received fees of $0.8 million and $1.3 million during the three and nine months ended February 28, 2013, respectively, for their services. Goldman Sachs also received an underwriting discount of $2.3 million during the first quarter of fiscal year 2013 as one of the initial purchasers of the $1.0 billion aggregate principal amount note offering of 6.50% senior notes due 2020, an underwriting discount of $2.6 million during the second quarter of fiscal year 2013 as of one the initial purchasers of the $825.0 million aggregate principal amount note add-on offering to the 6.50% senior notes due 2020 and an underwriting discount of $2.5 million during the second quarter of fiscal year 2013 as one of the initial purchasers of the $800.0 million aggregate principal amount note offering of the 6.50% senior subordinated notes due 2020 described in Note 7 — Debt.
Other
Biomet currently holds interest rate swaps with Goldman Sachs. As part of this relationship, the Company receives information from Goldman Sachs that allows it to perform a regression on the swaps as part of its required effectiveness testing on a quarterly basis.
Biomet may from time to time, depending upon market conditions, seek to purchase debt securities issued by Biomet or its subsidiaries in open market or privately negotiated transactions or by other means. Biomet
F-79
understands that its indirect controlling stockholders may from time to time also seek to purchase debt securities issued by the Company or its subsidiaries in open market or privately negotiated transactions or by other means.
The Company engaged Capstone Consulting LLC, a consulting company that works exclusively with KKR and its portfolio companies, to provide analysis for certain restructuring initiatives. The Company or its affiliates paid Capstone $0.6 million during the three months ended February 29, 2012 and $2.2 million and $1.7 million during the nine months ended February 28, 2013 and February 29, 2012, respectively, with no payments during the three months ended February 28, 2013.
Capital Contributions and Share Repurchases
At the direction of LVB, Biomet funded the repurchase of common shares of its parent company of $0.1 million during the three months ended February 29, 2012 with no repurchases during the three months ended February 28, 2013, and $0.1 million and $1.2 million for the nine months ended February 28, 2013 and February 29, 2012, respectively, from former employees pursuant to the LVB Acquisition, Inc. Management Stockholders’ Agreement. There were no additional contributions for the three and nine months ended February 28, 2013 and February 29, 2012.
F-80
Annex A
THIS DOCUMENT IS IMPORTANT AND REQUIRES YOUR IMMEDIATE ATTENTION. If you are in any doubt as to the action to be taken, you should immediately consult your broker, bank manager, lawyer, accountant, investment advisor or other professional adviser.
II-1
LETTER OF TRANSMITTAL
Relating to
Biomet, Inc.
Offer to Exchange
Up to $1,825,000,000, aggregate principal amount of Biomet, Inc.’s new 6.500% Senior Notes due 2020 (the “exchange senior notes”) and up to $800,000,000 aggregate principal amount of Biomet, Inc.’s new 6.500% Senior Subordinated Notes due 2020, (the “exchange senior subordinated notes” and together with the exchange senior notes, the “exchange notes”), the issuance of each of which has been registered under the Securities Act of 1933, as amended (the “Securities Act”),
for
any and all of its $1,825,000,000 aggregate principal amount of its 6.500% Senior Notes due 2020 (CUSIP Nos. U0903PAA7, 090613AF7 and U0903PAC3), (the “original senior notes”, and together with the exchange senior notes, the “Senior Notes”) and any and all of its $800,000,000 aggregate principal amount of its 6.500% Senior Subordinated Notes due 2020 (CUSIP Nos. U0903PAB5 and 090613AG5) (the “original senior subordinated notes” and together with the exchange senior subordinated notes, the “Subordinated Notes” and the original senior subordinated notes with the original senior notes, the “original notes”), respectively, that have not been registered under the Securities Act.
for
any and all of its $1,825,000,000 aggregate principal amount of its 6.500% Senior Notes due 2020 (CUSIP Nos. U0903PAA7, 090613AF7 and U0903PAC3), (the “original senior notes”, and together with the exchange senior notes, the “Senior Notes”) and any and all of its $800,000,000 aggregate principal amount of its 6.500% Senior Subordinated Notes due 2020 (CUSIP Nos. U0903PAB5 and 090613AG5) (the “original senior subordinated notes” and together with the exchange senior subordinated notes, the “Subordinated Notes” and the original senior subordinated notes with the original senior notes, the “original notes”), respectively, that have not been registered under the Securities Act.
This document relates to the exchange offers (the “Exchange Offers”) made by Biomet, Inc. (the “Company”) to exchange any and all of its unregistered $1,825,000,000 aggregate principal amount of 6.500% Senior Notes due 2020, (the “original senior notes”) and any and all of its unregistered $800,000,000 aggregate principal amount of 6.500% Senior Subordinated Notes due 2020 (the “original senior subordinated notes” and together with the original senior notes, including the guarantees (as described below), the “original notes”) for new 6.500% Senior Notes due 2020 (the “exchange senior notes”) and new 6.500% Senior Subordinated Notes due 2020, (the “exchange senior subordinated notes” and together with the exchange senior notes, including the guarantees (as described below), the “exchange notes”), respectively, the issuance of each of which has been registered under the Securities Act of 1933, as amended (the “Securities Act”). The exchange notes will initially be guaranteed by certain of Biomet, Inc.’s subsidiaries (as set forth in the Prospectus, collectively the “guarantors”), jointly and severally, irrevocably and unconditionally, the 6.500% Senior Notes due 2020 on a senior unsecured basis, and the 6.500% Senior Subordinated Notes due 2020 on a senior subordinated unsecured basis. The Exchange Offers described in the Prospectus dated , 2013 (the “Prospectus”) and in this letter of transmittal (this “Letter of Transmittal”). All terms and conditions contained in, or otherwise referred to in, the Prospectus are deemed to be incorporated in, and form a part of, this Letter of Transmittal. Therefore you are urged to read carefully the Prospectus and the items referred to therein. The terms and conditions contained in the Prospectus, together with the terms and conditions governing this Letter of Transmittal and the instructions herein, are collectively referred to herein as the “terms and conditions.”
The exchange offers expire at 5:00 P.M., New York City time, on , 2013 (such date and time, the “Expiration Date,” unless we extend or terminate either or both exchange offers, in which case the “Expiration Date” will mean the latest date and time to which we extend such exchange offer or exchange offers). We do not currently intend to extend the Expiration Date with respect to either exchange offer. Tendered original notes may be withdrawn at any time prior to the expiration of the Exchange Offer.
Upon the satisfaction or waiver of the conditions to the acceptance of the original notes set forth in the Prospectus under “The Exchange Offers—Conditions to the Exchange Offers,” the Company will accept for settlement the original notes that have been validly tendered (and not subsequently validly withdrawn). The Company will deliver the exchange notes on a date (the “settlement date”) as soon as practicable after the Expiration Date.
II-2
The Exchange Agent for the Exchange Offers is: Wells Fargo Bank, National Association By Registered & Certified Mail: Wells Fargo Bank, National Association Corporate Trust Operations MAC N9303-121 P.O. Box 1517 Minneapolis, MN 55480 Telephone: (800) 344-5128 By Regular Mail or Overnight Courier: Wells Fargo Bank, National Association Corporate Trust Operations MAC N9303-121 6th & Marquette Avenue Minneapolis, MN 55479 Telephone:(800) 344-512 In Person By Hand Only: Wells Fargo Bank, National Association Corporate Trust Services 608 2nd Avenue South Northstar East Building—12th Floor Minneapolis, MN 55402 Telephone: (800) 344-5128 |
This Letter of Transmittal is to be used by holders of the original notes. Tender of the original notes is to be made using the Automated Tender Offer Program (“ATOP”) of The Depository Trust Company (“DTC”) pursuant to the procedures set forth in the Prospectus under the caption “Description of the Exchange Offer—Procedures for Tendering.” DTC participants that are accepting the offer to exchange original notes for exchange notes in either or both of the Exchange Offers must transmit their acceptance to DTC, which will verify the acceptance and execute a book-entry delivery to the Exchange Agent’s DTC account. DTC will then send a computer-generated message known as an “agent’s message” to the Exchange Agent for its acceptance. For you to validly tender your original notes in the Exchange Offers, the Exchange Agent must receive, prior to the Expiration Date, an agent’s message under the ATOP procedures that confirms that:
| • | DTC has received your instructions to tender your original notes; and |
| • | You agree to be bound by the terms of this Letter of Transmittal. |
By using the ATOP procedures to tender the original notes, you will not be required to deliver this Letter of Transmittal to the Exchange Agent. However, you will be bound by the terms and conditions of, and you will be deemed to have made the acknowledgments and the representations and warranties contained in, this Letter of Transmittal as if you had actually signed it.
The exchange notes will be issued in full exchange for the original notes in the Exchange Offers, if consummated, on the settlement date and will be delivered in book-entry form.
II-3
Please read the accompanying instructions carefully.
Ladies and Gentlemen:
Upon the terms and conditions of the Exchange Offer, the holder of the original notes tendered herewith hereby tenders to the Company the aggregate principal amount of original notes credited by such holder to the Exchange Agent’s account at DTC using ATOP.
The undersigned understands that validly tendered original notes (or defectively tendered original notes with respect to which the Company has waived such defect or caused such defect to be waived) will be considered to have been accepted by the Company when, as and if the Company gives oral or written notice thereof to the Exchange Agent. The undersigned understands that, subject to the terms and conditions, the original notes properly tendered and accepted (and not validly withdrawn) in accordance with the terms and conditions will be exchanged for the exchange notes. The undersigned understands that, under certain circumstances, the Company may not be required to accept any of the original notes tendered (including any such original notes tendered after the Expiration Date). If any tendered original notes are not accepted for any reason described in the terms and conditions of the exchange offers, such unaccepted original notes will be returned without expense to the undersigned’s account at DTC or such other account as designated herein, pursuant to the book-entry transfer procedures described in the Prospectus, as promptly as practicable after the expiration or termination of the Exchange Offer.
By tendering the original notes in the Exchange Offers, the undersigned acknowledges that the Exchange Offers are being made based upon the Company’s understanding of an interpretation by the staff of the Securities and Exchange Commission (the “SEC”) as set forth in no-action letters issued to other parties, including Exxon Capital Holdings Corporation, SEC No-Action Letter (available May 13, 1988), Morgan Stanley & Co. Incorporated, SEC No-Action Letter (available June 5, 1991) and Shearman & Sterling, SEC No-Action Letter (available July 2, 1993), that the exchange notes issued in exchange for the original notes pursuant to the Exchange Offers may be offered for resale, resold and otherwise transferred by each holder thereof (other than a broker-dealer who acquires such exchange notes directly from the Company for resale pursuant to Rule 144A under the Securities Act or any other available exemption under the Securities Act or any such holder that is an “affiliate” of the Company within the meaning of Rule 405 under the Securities Act), without compliance with the registration and prospectus delivery provisions of the Securities Act, provided that such exchange notes are acquired in the ordinary course of such holder’s business and such holder is not engaged in, and does not intend to engage in, a distribution of such exchange notes and has no arrangement with any person to participate in the distribution of such exchange notes. If the undersigned is not a broker-dealer, the undersigned represents that it acquires the exchange notes in the ordinary course of its business, it is not engaged in, and does not intend to engage in, a distribution of the exchange notes and it has no arrangements or understandings with any person to participate in a distribution of the exchange notes. If the undersigned is a broker-dealer that will receive the exchange notes for its own account in exchange for the original notes, it represents that the original notes to be exchanged for the exchange notes were acquired by it as a result of market-making activities or other trading activities and acknowledges that it will deliver a prospectus in connection with any resale of such exchange notes; however, by so acknowledging and by delivering a prospectus, the undersigned will not be deemed to admit that it is an “underwriter” within the meaning of the Securities Act. Any broker-dealer that participates in the Exchange Offers must notify the Company in writing and, if it fails to do so, the Company may not be required to make a prospectus available to such broker-dealer for such deliveries.
Upon agreement to the terms of this Letter of Transmittal pursuant to an agent’s message, the undersigned, or the beneficial holder of the original notes on behalf of which the undersigned has tendered, will, subject to that holder’s ability to withdraw its tender, and subject to the terms and conditions of the Exchange Offers generally, hereby:
| • | irrevocably sell, assign and transfer to or upon the order of the Company or its nominee all right, title and interest in and to, and any and all claims in respect of or arising or having arisen as a result of the undersigned’s status as a holder of, all original notes tendered hereby, such that thereafter it shall have no contractual or other rights or claims in law or equity against the Company or the guarantors or any fiduciary, trustee, fiscal agent or other person connected with the original notes arising under, from or in connection with such original notes; |
| • | waive any and all rights with respect to the original notes tendered hereby, including, without limitation, any existing or past defaults and their consequences in respect of such original notes; and |
| • | release and discharge the Company , the guarantors and Wells Fargo Bank, National Association, as the trustee for the original notes from any and all claims the undersigned may have, now or in the future, |
II-4
arising out of or related to the original notes tendered hereby, including, without limitation, any claims that the undersigned is entitled to receive additional principal or interest payments with respect to the original notes tendered hereby, other than as expressly provided in the Prospectus and in this Letter of Transmittal, or to participate in any redemption or defeasance of the original notes tendered hereby.
The undersigned understands that tenders of the original notes pursuant to the procedures described in the Prospectus and in this Letter of Transmittal and acceptance of such original notes by the Company will, following such acceptance, constitute a binding agreement between the undersigned and the Company and the guarantors upon the terms and conditions.
By tendering the original notes in either or both of the Exchange Offer, the undersigned represents, warrants and agrees that:
| • | it has received and reviewed the Prospectus; |
| • | it is the beneficial owner (as defined below) of, or a duly authorized representative of one or more beneficial owners of, the original notes tendered hereby, and it has full power and authority to execute this Letter of Transmittal; |
| • | the original notes being tendered hereby were owned as of the date of tender, free and clear of any liens, charges, claims, encumbrances, interests and restrictions of any kind, and the Company will acquire good, indefeasible and unencumbered title to such original notes, free and clear of all liens, charges, claims, encumbrances, interests and restrictions of any kind, when the Company accepts the same; |
| • | it will not sell, pledge, hypothecate or otherwise encumber or transfer any original notes tendered hereby from the date of this Letter of Transmittal, and any purported sale, pledge, hypothecation or other encumbrance or transfer will be void and of no effect; |
| • | in evaluating the Exchange Offers and in making its decision whether to participate in the Exchange Offers by tendering its original notes, the undersigned has made its own independent appraisal of the matters referred to in the Prospectus and this Letter of Transmittal and in any related communications and it is not relying on any statement, representation or warranty, express or implied, made to such holder by the Company, the guarantors or the Exchange Agent, other than those contained in the Prospectus, as amended or supplemented through the Expiration Date; |
| • | the execution and delivery of this Letter of Transmittal shall constitute an undertaking to execute any further documents and give any further assurances that may be required in connection with any of the foregoing, in each case on and subject to the terms and conditions described or referred to in the Prospectus; |
| • | the agreement to the terms of this Letter of Transmittal pursuant to an agent’s message shall, subject to the terms and conditions of the Exchange Offer, constitute the irrevocable appointment of the Exchange Agent as its attorney and agent and an irrevocable instruction to such attorney and agent to complete and execute all or any forms of transfer and other documents at the discretion of that attorney and agent in relation to the original notes tendered hereby in favor of the Company or any other person or persons as the Company may direct and to deliver such forms of transfer and other documents in the attorney’s and agent’s discretion and the certificates and other documents of title relating to the registration of such original notes and to execute all other documents and to do all other acts and things as may be in the opinion of that attorney or agent necessary or expedient for the purpose of, or in connection with, the acceptance of the Exchange Offer, and to vest in the Company or their nominees such original notes; |
| • | the terms and conditions of the Exchange Offers shall be deemed to be incorporated in, and form a part of, this Letter of Transmittal, which shall be read and construed accordingly; |
| • | it is acquiring the exchange notes in the ordinary course of its business; |
| • | it is not participating in, and does not intend to participate in, a distribution of the exchange notes within the meaning of the Securities Act and has no arrangement or understanding with any person to participate in a distribution of the exchange notes within the meaning of the Securities Act; |
| • | it is not a broker-dealer who acquired the original notes directly from the Company; and |
| • | it is not an “affiliate” of the Company, within the meaning of Rule 405 of the Securities Act. |
II-5
The representations, warranties and agreements of a holder tendering the original notes shall be deemed to be repeated and reconfirmed on and as of the Expiration Date and the settlement date. For purposes of this Letter of Transmittal, the “beneficial owner” of any original notes means any holder that exercises investment discretion with respect to such original notes.
The undersigned understands that tenders may not be withdrawn at any time after the Expiration Date, except as set forth in the Prospectus, unless either or both of the Exchange Offers are amended with changes to the terms and conditions that are, in the reasonable judgment of the Company, materially adverse to the tendering holders, in which case tenders made in connection with such amended Exchange Offers may be withdrawn under the conditions described in the extension.
If the Exchange Offers are amended in a manner determined by the Company to constitute a material change, the Company will extend the Exchange Offers for a period of two to ten business days, depending on the significance of the amendment and the manner of disclosure to such holders, if the Exchange Offers would otherwise have expired during such two to ten business day period.
All authority conferred or agreed to be conferred in this Letter of Transmittal and every obligation of the undersigned hereunder shall be binding upon the undersigned’s successors, assigns, heirs, executors, administrators, trustees in bankruptcy and legal representatives of the undersigned and shall not be affected by, and shall survive, the death or incapacity of the undersigned.
| | CHECK HERE IF YOU ARE A BROKER-DEALER AND WISH TO RECEIVE 10 ADDITIONAL COPIES OF THE PROSPECTUS AND 10 COPIES OF ANY AMENDMENTS OR SUPPLEMENTS THERETO. |
Name:
Address:
Name of Tendering Institution:
Account Number:
Transaction Code Number:
By crediting the original notes to the Exchange Agent’s account at DTC using ATOP and by complying with applicable ATOP procedures with respect to the Exchange Offer, the participant in DTC confirms on behalf of itself and the beneficial owners of such original notes all provisions of this Letter of Transmittal (including all representations and warranties) applicable to it and such beneficial owner as fully as if it had completed the information required herein and executed and transmitted this Letter of Transmittal to the Exchange Agent.
II-6
INSTRUCTIONS FORMING PART OF
THE TERMS AND CONDITIONS OF THE EXCHANGE OFFER
THE TERMS AND CONDITIONS OF THE EXCHANGE OFFER
1. Book-Entry Confirmations
Any confirmation of a book-entry transfer to the Exchange Agent’s account at DTC of the original notes tendered by book-entry transfer, as well as an agent’s message, and any other documents required by this Letter of Transmittal, must be received by the Exchange Agent at its address set forth on the cover page of this Letter of Transmittal prior to 5:00 p.m., New York City time, on the Expiration Date.
2. Validity of Tenders
The Company will determine in its sole discretion all questions as to the validity, form, eligibility, time of receipt, acceptance of tendered original notes and withdrawal of tendered original notes. The Company’s determination will be final and binding. The Company reserves the absolute right to reject any original notes not properly tendered or any original notes the Company’s acceptance of which would, in the opinion of its counsel, be unlawful. The Company also reserves the right to waive any defect, irregularities or conditions of tender as to particular original notes. The Company’s interpretation of the terms and conditions of the Exchange Offers, including the instructions in this Letter of Transmittal, will be final and binding on all parties. Unless waived, all defects or irregularities in connection with tenders of original notes must be cured within such time as the Company shall determine. Although the Company intends to notify holders of defects or irregularities with respect to tenders of original notes, none of the Company, the guarantors, the Exchange Agent nor any other person will incur any liability for failure to give such notification. Tenders of original notes will not be deemed made until such defects or irregularities have been cured or waived. Any original notes received by the Exchange Agent that are not properly tendered and as to which the defects or irregularities have not been cured or waived will be returned to the tendering holder as soon as practicable after the Expiration Date of the exchange.
3. Waiver of Conditions
The Company reserves the absolute right to waive, in whole or part, at any time or from time to time, any of the conditions to either or both of the Exchange Offers set forth in the Prospectus or in this Letter of Transmittal.
4. No Conditional Tender
No alternative, conditional, irregular or contingent tender of the original notes will be accepted.
5. Request for Assistance or Additional Copies
Requests for assistance or for additional copies of the Prospectus or this Letter of Transmittal may be directed to the Exchange Agent at the address, telephone numbers or fax number set forth on the cover page of this Letter of Transmittal. Holders may also contact their commercial bank, broker, dealer, trust company or other nominee for assistance concerning the Exchange Offer.
6. Withdrawal
Tenders of the original notes may be withdrawn at any time prior to 5:00 p.m., New York City time, on the Expiration Date. For a withdrawal to be effective you must comply with the appropriate ATOP procedures. Any notice of withdrawal must specify the name and number of the account at DTC to be credited with withdrawn original notes and otherwise comply with the ATOP procedures. For more information, see the section of the Prospectus entitled “Description of the Exchange Offers—Withdrawal of Tenders.”
7. Transfer Taxes
Holders who tender their original notes for exchange will not be obligated to pay any transfer taxes in connection with that tender or exchange, except that holders who instruct the Company to register exchange notes in the name of, or who request that original notes not tendered or not accepted in the exchange offers be returned to, a person other than the registered tendering holder will be responsible for the payment of any applicable transfer tax on those original notes.
II-7
IMPORTANT: BY USING THE ATOP PROCEDURES TO TENDER THE ORIGINAL NOTES, A HOLDER OF ORIGINAL NOTES WILL NOT BE REQUIRED TO DELIVER THIS LETTER OF TRANSMITTAL TO THE EXCHANGE AGENT. HOWEVER, SUCH HOLDER WILL BE BOUND BY THE TERMS AND CONDITIONS OF, AND WILL BE DEEMED TO HAVE MADE THE ACKNOWLEDGMENTS AND THE REPRESENTATIONS AND WARRANTIES CONTAINED IN, THIS LETTER OF TRANSMITTAL AS IF SUCH HOLDER HAD ACTUALLY SIGNED IT.
II-8

OFFERS TO EXCHANGE
$1,825,000,000, aggregate principal amount of our 6.500% Senior Notes due 2020 and $800,000,000 aggregate principal amount of our 6.500% Senior Subordinated Notes due 2020, the issuance of each of which has been registered under the Securities Act of 1933,
for
$1,825,000,000 of our 6.500% Senior Notes due 2020 and $800,000,000 of our 6.500% Senior Subordinated Notes due 2020, respectively.
for
$1,825,000,000 of our 6.500% Senior Notes due 2020 and $800,000,000 of our 6.500% Senior Subordinated Notes due 2020, respectively.
_________________
PROSPECTUS
____________________
Until the date that is 90 days from the date of this prospectus, all dealers that effect transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to the dealers’ obligation to deliver a prospectus when acting as underwriters with respect to their unsold allotments or subscriptions.
II-9
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 20. Indemnification of Directors and Officers
California Registrant: Interpore Cross International, LLC is a limited liability company organized under the laws of California.
Interpore Cross International, LLC (“Interpore Cross”) is organized under the laws of the State of California. Section 17155 of the California Beverly-Killea Limited Liability Company Act provides that, except for a breach of a manager’s fiduciary duties of loyalty and care owed to the limited liability company and to its members, the articles of organization or written operating agreement of a California limited liability company may provide for indemnification of any person, including, without limitation, any manager, member, officer, employee, or agent of the limited liability company, against judgments, settlements, penalties, fines, or expenses of any kind incurred as a result of acting in that capacity.
The Limited Liability Agreement of Interpore Cross (the “Interpore Cross LLC Agreement”) provides that to the fullest extent permitted by law, Interpore Cross shall indemnify and hold harmless each of Interpore Spine, Ltd., as the sole member of Interpore Cross, the managers, and any other officers, directors, shareholders, partners, employees, affiliates, representatives, or agents of any of the foregoing, or any officer, employee, representative, or agent of Interpore Cross (each, a “Covered Person”) from and against any and all losses, claims, demands, liabilities, expenses, judgments, fines, settlements, and other amounts arising from any and all claims, demands, actions, suits, or proceedings, civil, criminal, administrative, or investigative (“Claims”), in which each Covered Person may be involved, threatened to be involved, as a party or otherwise, by reason of its management of the affairs of Interpore Cross or which relates to or arises out of Interpore Cross or its property, business, or affairs. Pursuant to the Interpore Cross LLC Agreement, a Covered Person shall not be entitled to indemnification with respect to (i) any Claim with respect to which such Covered Person has engaged in fraud, willful misconduct, bad faith, or gross negligence or (ii) any Claim initiated by such Covered Person unless such Claim (or part thereof) (A) was brought to enforce such Covered Person’s rights to indemnification hereunder or (B) was authorized or consented to by the Board. Expenses incurred by a Covered Person in defending any Claim shall be paid by Interpore Cross in advance of the final disposition of such Claim upon receipt by Interpore Cross of an undertaking by or on behalf of such Covered Person to repay such amount if it shall be ultimately determined that such Covered Person is not entitled to be indemnified by Interpore Cross.
The Interpore Cross LLC Agreement also provides that, notwithstanding any other provisions of the Interpore Cross LLC Agreement, whether express or implied, or any obligation or duty at law or in equity, none of the Covered Persons shall be liable to Interpore Cross or any other person for any act or omission (in relation to Interpore Cross, its property, or the conduct of its business or affairs, the Interpore Cross LLC Agreement, any related document, or any transaction or investment contemplated thereby) taken or omitted by a Covered Person in the reasonable belief that such act or omission is in or is not contrary to the best interests of Interpore Cross and is within the scope of authority granted to such Covered Person by the Interpore Cross LLC Agreement, provided such act or omission does not constitute fraud, willful misconduct, bad faith, or gross negligence.
Delaware Registrants:
(a) Biolectron, Inc., Biomet Europe Ltd., Biomet International Ltd., Interpore Spine Ltd. and Kirschner Medical Corporation are each incorporated under the laws of Delaware.
Section 145 of the Delaware General Corporation Law (the “DGCL”) grants each corporation organized thereunder the power to indemnify any person who is or was a director, officer, employee or agent of a corporation or enterprise, against expenses, including attorneys’ fees, judgments, fines and amounts paid in settlement actually and reasonably incurred by him in connection with any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative, other than an action by or in the right of the corporation, by reason of being or having been in any such capacity, if he acted in good faith in a manner reasonably believed to be in, or not opposed to, the best interests of the corporation, and, with respect to any criminal action or proceeding, had no reasonable cause to believe his conduct was unlawful.
II-10
Section 102(b)(7) of the DGCL enables a corporation in its certificate of incorporation or an amendment thereto to eliminate or limit the personal liability of a director to the corporation or its shareholders for monetary damages for violations of the director’s fiduciary duty of care, except (i) for any breach of the director’s duty of loyalty to the corporation or its shareholders, (ii) for acts or omissions not in good faith or that involve intentional misconduct or a knowing violation of law, (iii) pursuant to Section 174 of the DGCL (providing for liability of directors for unlawful payment of dividends or unlawful stock purchases or redemptions) or (iv) for any transaction from which a director derived an improper personal benefit.
The certificate of incorporation and/or bylaws of the Delaware corporate registrants indemnify, to the fullest extent permitted by law, every director and officer made a party to a proceeding by reason of their position as a director or officer against all liability incurred by such individual in connection with the proceeding, except where the individual failed to meet the standard of conduct for indemnification specified by law. Such indemnification also extends to the payment for reasonable expenses incurred by the director or officer in connection with any proceeding in advance of final disposition thereof, but the bylaws of Interpore Spine, Ltd. and Kirschner Medical Corporation provide that such advancement of expenses is upon receipt of an undertaking by such director or officer to repay such amount if it is ultimately determined that he is not entitled to indemnification. Furthermore, a director or officer who is wholly successful, on the merits or otherwise, in the defense of any such proceeding is entitled to indemnification as a matter of right against reasonable expenses incurred by such individual in connection with such proceeding. The indemnification and advancement of expenses provided for by the certificate of incorporation and/or bylaws of the Delaware corporate registrants is not exclusive of any other rights, by contract or otherwise, relating to indemnification or advance of expenses that such individuals may have against the Delaware corporate registrants.
Neither the certificate of incorporation nor the bylaws of Biomet Europe Ltd. provide for indemnification of directors or officers.
The certificates of incorporation of Biolectron, Inc., Interpore Spine, Ltd. and Kirschner Medical Corp. eliminate their directors’ personal liability to the corporation or its shareholders with respect to acts or omissions in the performance of their duties as director of the corporation, except for personal liability for (i) a breach of the directors’ duties of loyalty to the corporations or their shareholders, (ii) for acts or omissions not in good faith or that involve intentional misconduct or a knowing violation of law, (iii) pursuant to Section 174 of the DGCL or (iv) for any transaction from which a director derived an improper personal benefit.
(b) Cross Medical Products, LLC, EBI Holdings, LLC, EBI Medical Systems, LLC, and Electro-Biology, LLC are each organized as limited liability companies under the laws of Delaware.
Section 18-108 of the Delaware Limited Liability Company Act empowers a Delaware limited liability company to indemnify and hold harmless any member or manager of the limited liability company from and against any and all claims and demands whatsoever.
The operating agreements of each of the Delaware limited liability companies provide for the indemnification to the fullest extent permitted by law of the Members, Managers or any officers, directors, shareholders, partners, employees, affiliates, representatives or agents of any of the foregoing, as well as any officer, employee, representative or agent of the limited liability company (individually, a “Covered Person”, and collectively, “Covered Persons”). Each Covered Person is indemnified against any claims, liabilities, expenses, judgments, settlements or other amounts arising in any proceedings, whether civil, criminal, administrative or investigative in which the Covered Person is involved by reason of its management of the affairs of the limited liability company or which relates to or arises out of the limited liability company or its property, business or affairs. A Covered Person is not entitled to indemnification for such claim if such Covered Person engaged in fraud, willful misconduct, bad faith or gross negligence or if such claim was initiated by such Covered Person (unless the claim was brought to enforce such Covered Person’s right to indemnification or authorized by the Board). The limited liability company must pay expenses incurred by such Covered Person in defending against any claim in advance of the disposition of the claim upon receipt by the limited liability of an undertaking of the Covered Person to repay any amounts advanced if it is ultimately determined that Covered Person is not entitled to indemnification.
Florida Registrants: Biomet 3i, LLC, Biomet Microfixation, LLC and Biomet Florida Services, LLC are each organized as limited liability companies under the laws of Florida.
Section 608.4229(1) of the Florida Limited Liability Company Act provides that a limited liability company may, and shall have the power to, but shall not be required to, indemnify and hold harmless any member or
II-11
manager or other person from and against any and all claims and demands whatsoever. Notwithstanding that provision, indemnification or advancement of expenses shall not be made to or on behalf of any member, manager, managing member, officer, employee, or agent if a judgment or other final adjudication establishes that the actions, or omissions to act, of such member, manager, managing member, officer, employee, or agent were material to the cause of action so adjudicated and constitute any of the following: (a) a violation of criminal law, unless the member, manager, managing member, officer, employee, or agent had no reasonable cause to believe such conduct was unlawful; (b) a transaction from which the member, manager, managing member, officer, employee, or agent derived an improper personal benefit; (c) in the case of a manager or managing member, a circumstance under which the liability provisions of Section 608.426 are applicable; or (d) willful misconduct or a conscious disregard for the best interests of the limited liability company in a proceeding by or in the right of the limited liability company to procure a judgment in its favor or in a proceeding by or in the right of a member.
Article VIII of the operating agreements of each of Biomet 3i, LLC, Biomet Microfixation, LLC and Biomet Florida Services, LLC provides for the limitation of personal liability of the managers and members thereof as follows:
To the fullest extent permitted by law, the Company shall indemnify and hold harmless each member and manager, and any officers, directors, shareholders, partners, employees, affiliates, representatives or agents thereof (“Covered Person”) from and against any and all losses, claims, demands, liabilities, expenses, judgments, fines, settlements and other amounts arising from any and all claims, demands, actions, suits or proceedings, civil, criminal, administrative or investigative (“Claims”), in which the Covered Person may be involved, or threatened to be involved, as a party or otherwise, by reason of its management of the affairs of the Company or which relates to or arises out of the Company or its property, business or affairs. A Covered Person shall not be entitled to indemnification under this Section 8.2 with respect to (i) any Claim with respect to which such Covered Person has engaged in fraud, willful misconduct, bad faith or gross negligence or (ii) any Claim initiated by such Covered Person unless such Claim (or part thereof) (A) was brought to enforce such Covered Person’s rights to indemnification hereunder or (B) was authorized or consented to by the Board. Expenses incurred by a Covered Person in defending any Claim shall be paid by the Company in advance of the final disposition of such Claim upon receipt by the Company of an undertaking by or on behalf of such Covered Person to repay such amount if it shall be ultimately determined that such Covered Person is not entitled to be indemnified by the Company as authorized by this Section 8.2.
Indiana Registrants:
(a) Biomet, Inc., Biomet Leasing Inc. and Biomet Manufacturing Corporation are each incorporated under the laws of Indiana.
Chapter 37 of the Indiana Business Corporation Law provides that a corporation, unless limited by its Articles of Incorporation, is required to indemnify its directors and officers against reasonable expenses incurred in the wholly successful defense of any proceeding to which the director or officer was a party because of serving as a director or officer of the corporation.
Chapter 37 of the Indiana Business Corporation Law also provides that a corporation may voluntarily undertake to provide for indemnification of its directors and, unless otherwise provided in the articles of incorporation, its officers, employees and agents against any and all liability and reasonable expense that may be incurred by them, arising out of any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative and whether formal or informal, in which they may become involved by reason of being or having been a director or officer if (i) such persons acted in good faith, (ii) such persons reasonably believed their actions, in the case of their actions in their official capacity with the corporation, to be in the best interests of the corporation and, in all other cases, to be at least not opposed to its best interests, and (iii) in any criminal action, such persons had reasonable cause to believe their conduct was lawful, or had no reasonable cause to believe that their conduct was unlawful. A corporation may advance or reimburse reasonable expenses incurred by persons entitled to indemnification, in advance of final disposition, if such persons furnish the corporation with a written affirmation of their good faith belief that the applicable standard of conduct was observed, accompanied by a written undertaking to repay the advance if it is ultimately determined that the applicable standards were not met. Unless such persons have been successful in the defense of a proceeding, a corporation may not indemnify such persons unless authorized in the specific case after a determination has been made that indemnification of such persons is permissible in the circumstances because such persons met the standard of conduct set forth under the law.
II-12
The articles of incorporation and/or bylaws of all the Indiana corporate registrants indemnify any directors or officers made a party to a proceeding by reason of their position as director or officer against liability incurred by such persons in connection with the proceeding, except where the persons failed to meet the standard of conduct for indemnification specified by law. Such indemnification extends to the payment for or reimbursement of reasonable expenses incurred by the directors or officers in advance of final disposition of the proceeding. Such indemnification also extends, as a matter of right, to the payment of reasonable expenses incurred by directors or officers who are wholly successful, on the merits or otherwise, in defense of such proceeding.
(b) Biomet Biologics, LLC, Biomet Fair Lawn LLC, EBI, LLC, Biomet Orthopedics, LLC, Biomet Sports Medicine, LLC Biomet Trauma, LLC., Biomet U.S. Reconstruction, LLC and Implant Innovations Holdings, LLC are each organized as limited liability companies under the laws of Indiana.
Chapter 2 of the Indiana Business Flexibility Act provides that, subject to any standards and restrictions set forth in a company’s operating agreement, a limited liability company may indemnify and hold harmless any member, manager, agent or employee from and against any and all claims and demands, unless the action or failure to act for which indemnification is sought constitutes willful misconduct or recklessness.
The operating agreements of all the Indiana limited liability company registrants indemnify, to the fullest extent permitted by law, any member, manager, officer, director, employee or agent of the company, and any officer, director, shareholder, partner, employee, affiliate, representative or agent of any member or manager of the company, from and against any and all losses, claims, demands, liabilities, expenses, judgments, fines, settlements and other amounts arising from any and all claims, demands, actions, suits or proceedings, civil, criminal, administrative or investigative, in which such persons may be involved, or threatened to be involved, as a party or otherwise, by reason of such persons’ management of the affairs of the company or which relates to or arises out of the company or its property, business or affairs. Such indemnification shall not be allowed with respect to (i) any claim with respect to which such persons have engaged in fraud, willful misconduct, bad faith or gross negligence or (ii) any claim initiated by such persons unless such claim (A) was brought to enforce such persons’ rights to indemnification under the operating agreements or (B) was authorized or consented to by the Board of the company. Expenses incurred by such persons in defending any claim shall be paid by the company in advance of the final disposition of such claim upon receipt by the company of an undertaking by or on behalf of such persons to repay such amount if it shall be ultimately determined that such persons were not entitled to be indemnified by the company as authorized by the operating agreements.
Certain Other Arrangements
Biomet, Inc. maintains a directors’ and officers’ liability insurance policy that covers the directors, officers, managers and members of each of the registrants in amounts that Biomet, Inc. believes are customary in its industry, including for liabilities in connection with the registration, offering and sale of the notes.
In addition, pursuant to the Management Services Agreement entered into with certain affiliates of the Sponsors, the Company has agreed to customary exculpation and indemnification provisions for the benefit of the managers and their affiliates.
On January 11, 2010, the Company and LVB Acquisition, Inc. entered into an indemnification priority agreement with the Sponsors (or certain affiliates designated by the Sponsors) pursuant to which the Company and LVB Acquisition, Inc. clarified certain matters regarding the existing indemnification and advancement of expenses rights provided by the Company and LVB Acquisition, Inc. pursuant to their respective charters and the management services agreement described above. In particular, pursuant to the terms of the indemnification agreement, the Company acknowledged that as among the Company, LVB Acquisition, Inc. and the Sponsors and their respective affiliates, the obligation to indemnify or advance expenses to any director appointed by any of the Sponsors will be payable in the following priority: The Company will be the primary source of indemnification and advancement; LVB Acquisition, Inc. will be the secondary source of indemnification and advancement; and any obligation of a Sponsor-affiliated indemnitor to indemnify or advance expenses to such director will be tertiary to the Company’s and, then, LVB Acquisition, Inc. obligations. In the event that either the Company or LVB Acquisition, Inc. fails to indemnify or advance expenses to any such director in contravention of its obligations, and any Sponsor-affiliated indemnitor makes any indemnification payment or advancement of expenses to such director on account of such unpaid liability, such Sponsor-affiliated indemnitor will be subrogated to the rights of such director under any such Company or LVB Acquisition, Inc. indemnification agreement.
Item 21. Exhibits and Financial Statement Schedules.
II-13
(a) Exhibits.
See the Exhibit Index immediately following the signature pages included in this Registration Statement.
(b) Financial Statement Schedules.
None.
Item 22. Undertakings.
(a) Each undersigned registrant hereby undertakes:
(1) To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
(i) To include any prospectus required by Section 10(a)(3) of the Securities Act of 1933;
(ii) To reflect in the prospectus any facts or events arising after the effective date of this registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in this registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than a 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; and
(iii) To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement;
(2) That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(3) To remove from registration by means of a post-effective amendment any of the securities being registered which remain unexchanged at the termination of the offering.
(b) The undersigned registrant hereby undertakes that, for purposes of determining any liability under the Securities Act of 1933, each filing of the registrant’s annual report pursuant to Section 13(a) or Section 15(d) of the Securities Exchange Act of 1934 (and, where applicable, each filing of an employee benefit plan’s annual report pursuant to Section 15(d) of the Securities Exchange Act of 1934) that is incorporated by reference in the registration statement shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(c) Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and persons controlling the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrants of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act of 1933 and will be governed by the final adjudication of such issue.
(d) The undersigned registrant hereby undertakes to respond to requests for information that is incorporated by reference into the prospectus pursuant to Items 4, 10(b), 11, or 13 of this Form, within one business
II-14
day of receipt of such request, and to send the incorporated documents by first class mail or other equally prompt means. This includes information contained in documents filed subsequent to the effective date of the registration statement through the date of responding to the request.
(e) The undersigned registrant hereby undertakes to supply by means of a post-effective amendment all information concerning a transaction, and the company being acquired involved therein, that was not the subject of and included in the registration statement when it became effective.
II-15
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, Biomet, Inc. has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Warsaw, State of Indiana, on the 14th day of May, 2013.
| BIOMET, INC. | ||
| By: | /s/ Daniel P. Florin | |
| Daniel P. Florin | ||
| Senior Vice President and Chief Financial Officer | ||
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
| Signature | Capacity | Date |
| * | ||
| Jeffrey R. Binder | President (Principal Executive Officer) and Chief Executive Officer | May 14, 2013 |
| * | ||
| Daniel P. Florin | Senior Vice President and Chief Financial Officer (Principal Financial Officer) | May 14, 2013 |
| * | ||
| J. Pat Richardson | Vice President – Corporate Controller (Principal Accounting Officer) | May 14, 2013 |
| * | ||
| Chinh E. Chu | Director | May 14, 2013 |
| * | ||
| Jonathan J. Coslet | Director | May 14, 2013 |
| * | ||
| Michael Dal Bello | Director | May 14, 2013 |
| * | ||
| Adrian Jones | Director | May 14, 2013 |
| * | ||
| Michael Michelson | Director | May 14, 2013 |
| * | ||
| Dane A. Miller, Ph.D. | Director | May 14, 2013 |
| * | ||
| Max C. Lin | Director | May 14, 2013 |
| * | ||
| Jeffrey K. Rhodes | Director | May 14, 2013 |
| * | ||
| Andrew Y. Rhee | Director | May 14, 2013 |
| * | The undersigned, by signing his or her name hereto, does execute this Amendment No. 1 to Registration Statement on Form S-4 on behalf of the above-named officers and/or directors and/or managers of the registrant pursuant to the Power of Attorney executed by such officers and/or directors and/or managers on the signature pages to the Registration Statement previously filed on April 30, 2013. |
| /s/ Bradley J. Tandy |
| Bradley J. Tandy |
| Attorney-in-Fact |
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, Biolectron, Inc. has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Warsaw, State of Indiana, on the 14th day of May, 2013.
| BIOLECTRON, INC. | ||
| By: | /s/ Michael T. Hodges | |
| Michael T. Hodges | ||
| Treasurer | ||
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
| Signature | Capacity | Date |
| * | ||
| Adam R. Johnson | President (Principal Executive Officer) | May 14, 2013 |
| /s/ Michael T. Hodges | ||
| Michael T. Hodges | Director and Treasurer (Principal Financial Officer and Principal Accounting Officer) | May 14, 2013 |
| * | ||
| Bradley J. Tandy | Director and Secretary | May 14, 2013 |
| * | ||
| Jeffrey R. Binder | Director | May 14, 2013 |
| * | The undersigned, by signing his or her name hereto, does execute this Amendment No. 1 to Registration Statement on Form S-4 on behalf of the above-named officers and/or directors and/or managers of the registrant pursuant to the Power of Attorney executed by such officers and/or directors and/or managers on the signature pages to the Registration Statement previously filed on April 30, 2013. |
| /s/ Michael T. Hodges |
| Michael T. Hodges |
| Attorney-in-Fact |
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, Biomet 3i, LLC has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Warsaw, State of Indiana, on the 14th day of May, 2013.
| BIOMET 3i, LLC | ||
| By: | /s/ Michael T. Hodges | |
| Michael T. Hodges | ||
| Treasurer | ||
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
| Signature | Capacity | Date |
| * | ||
| Bareld J. Doedens | President (Principal Executive Officer) | May 14, 2013 |
| * | ||
| Jeffrey R. Binder | Manager | May 14, 2013 |
| * | ||
| Bradley J. Tandy | Manager and Secretary | May 14, 2013 |
| * | ||
| Edward G. Sabin | Chief Financial Officer (Principal Financial Officer) | May 14, 2013 |
| /s/ Michael T. Hodges | ||
| Michael T. Hodges | Manager and Treasurer (Principal Accounting Officer) | May 14, 2013 |
| * | The undersigned, by signing his or her name hereto, does execute this Amendment No. 1 to Registration Statement on Form S-4 on behalf of the above-named officers and/or directors and/or managers of the registrant pursuant to the Power of Attorney executed by such officers and/or directors and/or managers on the signature pages to the Registration Statement previously filed on April 30, 2013. |
| /s/ Michael T. Hodges |
| Michael T. Hodges |
| Attorney-in-Fact |
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, Biomet Biologics, LLC has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Warsaw, State of Indiana, on the 14th day of May, 2013.
| BIOMET BIOLOGICS, LLC | ||
| By: | /s/ Michael T. Hodges | |
| Michael T. Hodges | ||
| Treasurer | ||
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
| Signature | Capacity | Date |
| * | ||
| Jon C. Serbousek | President (Principal Executive Officer) | May 14, 2013 |
| * | ||
| Jeffrey R. Binder | Manager | May 14, 2013 |
| * | ||
| Bradley J. Tandy | Manager and Secretary | May 14, 2013 |
| /s/ Michael T. Hodges | ||
| Michael T. Hodges | Manager and Treasurer (Principal Financial Officer and Principal Accounting Officer) | May 14, 2013 |
| * | The undersigned, by signing his or her name hereto, does execute this Amendment No. 1 to Registration Statement on Form S-4 on behalf of the above-named officers and/or directors and/or managers of the registrant pursuant to the Power of Attorney executed by such officers and/or directors and/or managers on the signature pages to the Registration Statement previously filed on April 30, 2013. |
| /s/ Michael T. Hodges |
| Michael T. Hodges |
| Attorney-in-Fact |
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, Biomet Europe Ltd. has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Warsaw, State of Indiana, on the 14th day of May, 2013.
| BIOMET EUROPE LTD. | ||
| By: | /s/ Michael T. Hodges | |
| Michael T. Hodges | ||
| Treasurer | ||
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
| Signature | Capacity | Date |
| * | ||
| Renaat Vermeulen | Managing Director (Principal Executive Officer) | May 14, 2013 |
| /s/ Michael T. Hodges | ||
| Michael T. Hodges | Director, Vice President and Treasurer (Principal Financial Officer and Principal Accounting Officer) | May 14, 2013 |
| * | ||
| Bradley J. Tandy | Director and Secretary | May 14, 2013 |
| * | ||
| Jeffrey R. Binder | Director | May 14, 2013 |
| * | The undersigned, by signing his or her name hereto, does execute this Amendment No. 1 to Registration Statement on Form S-4 on behalf of the above-named officers and/or directors and/or managers of the registrant pursuant to the Power of Attorney executed by such officers and/or directors and/or managers on the signature pages to the Registration Statement previously filed on April 30, 2013. |
| /s/ Michael T. Hodges |
| Michael T. Hodges |
| Attorney-in-Fact |
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, Biomet Fair Lawn LLC has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Warsaw, State of Indiana, on the 14th day of May, 2013.
| BIOMET FAIR LAWN, LLC | ||
| By: | /s/ Michael T. Hodges | |
| Michael T. Hodges | ||
| Treasurer | ||
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
| Signature | Capacity | Date |
| * | ||
| Jeffrey R. Binder | President and Manager (Principal Executive Officer) | May 14, 2013 |
| /s/ Michael T. Hodges | ||
| Michael T. Hodges | Treasurer and Manager (Principal Financial Officer and Principal Accounting Officer) | May 14, 2013 |
| * | ||
| Bradley J. Tandy | Manager and Secretary | May 14, 2013 |
| * | The undersigned, by signing his or her name hereto, does execute this Amendment No. 1 to Registration Statement on Form S-4 on behalf of the above-named officers and/or directors and/or managers of the registrant pursuant to the Power of Attorney executed by such officers and/or directors and/or managers on the signature pages to the Registration Statement previously filed on April 30, 2013. |
| /s/ Michael T. Hodges |
| Michael T. Hodges |
| Attorney-in-Fact |
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, Biomet International Ltd. has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Warsaw, State of Indiana, on the 14th day of May, 2013.
| BIOMET INTERNATIONAL LTD. | ||
| By: | /s/ Michael T. Hodges | |
| Michael T. Hodges | ||
| Treasurer | ||
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
| Signature | Capacity | Date |
| * | ||
| Hadi Saleh | President (Principal Executive Officer) | May 14, 2013 |
| /s/ Michael T. Hodges | ||
| Michael T. Hodges | Director and Treasurer (Principal Financial Officer and Principal Accounting Officer) | May 14, 2013 |
| * | ||
| Bradley J. Tandy | Director and Secretary | May 14, 2013 |
| * | ||
| Jeffrey R. Binder | Director | May 14, 2013 |
| * | The undersigned, by signing his or her name hereto, does execute this Amendment No. 1 to Registration Statement on Form S-4 on behalf of the above-named officers and/or directors and/or managers of the registrant pursuant to the Power of Attorney executed by such officers and/or directors and/or managers on the signature pages to the Registration Statement previously filed on April 30, 2013. |
| /s/ Michael T. Hodges |
| Michael T. Hodges |
| Attorney-in-Fact |
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, Biomet Leasing, Inc. has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Warsaw, State of Indiana, on the 14th day of May, 2013.
| BIOMET LEASING, INC. | ||
| By: | /s/ Bradley J. Tandy | |
| Bradley J. Tandy | ||
| President | ||
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
| Signature | Capacity | Date |
| * | ||
| Bradley J. Tandy | Director and President (Principal Executive Officer) | May 14, 2013 |
| /s/ Michael T. Hodges | ||
| Michael T. Hodges | Director and Treasurer (Principal Financial Officer and Principal Accounting Officer) | May 14, 2013 |
| * | ||
| Jody S. Gale | Secretary | May 14, 2013 |
| * | ||
| Jeffrey R. Binder | Director | May 14, 2013 |
| * | The undersigned, by signing his or her name hereto, does execute this Amendment No. 1 to Registration Statement on Form S-4 on behalf of the above-named officers and/or directors and/or managers of the registrant pursuant to the Power of Attorney executed by such officers and/or directors and/or managers on the signature pages to the Registration Statement previously filed on April 30, 2013. |
| /s/ Michael T. Hodges |
| Michael T. Hodges |
| Attorney-in-Fact |
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, Biomet Manufacturing Corporation has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Warsaw, State of Indiana, on the 14th day of May, 2013.
| BIOMET MANUFACTURING CORPORATION | ||
| By: | /s/ Michael T. Hodges | |
| Michael T. Hodges | ||
| Treasurer | ||
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
| Signature | Capacity | Date |
| * | ||
| Wilber C. Boren, IV | President – S.E.T. (Principal Executive Officer) | May 14, 2013 |
| /s/ Michael T. Hodges | ||
| Michael T. Hodges | Director and Treasurer (Principal Financial Officer and Principal Accounting officer) | May 14, 2013 |
| * | ||
| Bradley J. Tandy | Director and Secretary | May 14, 2013 |
| * | ||
| Jeffrey R. Binder | Director | May 14, 2013 |
| * | The undersigned, by signing his or her name hereto, does execute this Amendment No. 1 to Registration Statement on Form S-4 on behalf of the above-named officers and/or directors and/or managers of the registrant pursuant to the Power of Attorney executed by such officers and/or directors and/or managers on the signature pages to the Registration Statement previously filed on April 30, 2013. |
| /s/ Michael T. Hodges |
| Michael T. Hodges |
| Attorney-in-Fact |
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, Biomet Microfixation, LLC has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Warsaw, State of Indiana, on the 14th day of May, 2013.
| BIOMET MICROFIXATION, LLC | ||
| By: | /s/ Michael T. Hodges | |
| Michael T. Hodges | ||
| Treasurer | ||
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
| Signature | Capacity | Date |
| * | ||
| Adam R. Johnson | President (Principal Executive Officer) | May 14, 2013 |
| * | ||
| Jeffrey R. Binder | Manager | May 14, 2013 |
| * | ||
| Bradley J. Tandy | Manager and Secretary | May 14, 2013 |
| * | ||
| Gary Blackall | Vice President – Finance & Operations (Principal Financial Officer) | May 14, 2013 |
| /s/ Michael T. Hodges | ||
| Michael T. Hodges | Manager and Treasurer (Principal Accounting Officer) | May 14, 2013 |
| * | The undersigned, by signing his or her name hereto, does execute this Amendment No. 1 to Registration Statement on Form S-4 on behalf of the above-named officers and/or directors and/or managers of the registrant pursuant to the Power of Attorney executed by such officers and/or directors and/or managers on the signature pages to the Registration Statement previously filed on April 30, 2013. |
| /s/ Michael T. Hodges |
| Michael T. Hodges |
| Attorney-in-Fact |
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, Biomet Orthopedics, LLC has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Warsaw, State of Indiana, on the 14th day of May, 2013.
| BIOMET ORTHOPEDICS, LLC | ||
| By: | /s/ Michael T. Hodges | |
| Michael T. Hodges | ||
| Treasurer | ||
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
| Signature | Capacity | Date |
| * | ||
| Stuart Kleopfer | President – U.S. Commercial Operations (Principal Executive Officer) | May 14, 2013 |
| * | ||
| Bradley J. Tandy | Manager and Secretary | May 14, 2013 |
| * | ||
| Matthew C. Abernethy | Vice President – Global Finance (Principal Financial Officer) | May 14, 2013 |
| /s/ Michael T. Hodges | ||
| Michael T. Hodges | Manager and Treasurer (Principal Accounting Officer) | May 14, 2013 |
| * | The undersigned, by signing his or her name hereto, does execute this Amendment No. 1 to Registration Statement on Form S-4 on behalf of the above-named officers and/or directors and/or managers of the registrant pursuant to the Power of Attorney executed by such officers and/or directors and/or managers on the signature pages to the Registration Statement previously filed on April 30, 2013. |
| /s/ Michael T. Hodges |
| Michael T. Hodges |
| Attorney-in-Fact |
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, Biomet Sports Medicine, LLC has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Warsaw, State of Indiana, on the 14th day of May, 2013.
| BIOMET SPORTS MEDICINE, LLC | ||
| By: | /s/ Michael T. Hodges | |
| Michael T. Hodges | ||
| Treasurer | ||
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
| Signature | Capacity | Date |
| * | ||
| Wilber C. Boren, IV | President (Principal Executive Officer) | May 14, 2013 |
| * | ||
| Jeffrey R. Binder | Manager | May 14, 2013 |
| * | ||
| Bradley J. Tandy | Manager and Secretary | May 14, 2013 |
| /s/ Michael T. Hodges | ||
| Michael T. Hodges | Manager and Treasurer (Principal Financial Officer and Principal Accounting Officer) | May 14, 2013 |
| * | The undersigned, by signing his or her name hereto, does execute this Amendment No. 1 to Registration Statement on Form S-4 on behalf of the above-named officers and/or directors and/or managers of the registrant pursuant to the Power of Attorney executed by such officers and/or directors and/or managers on the signature pages to the Registration Statement previously filed on April 30, 2013. |
| /s/ Michael T. Hodges |
| Michael T. Hodges |
| Attorney-in-Fact |
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, Cross Medical Products, LLC has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Warsaw, State of Indiana, on the 14th day of May, 2013.
| CROSS MEDICAL PRODUCTS, LLC | ||
| By: | /s/ Michael T. Hodges | |
| Michael T. Hodges | ||
| Treasurer | ||
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
| Signature | Capacity | Date |
| * | ||
| Philip A. Mellinger | General Manager (Principal Executive Officer) | May 14, 2013 |
| * | ||
| Jeffrey R. Binder | Manager | May 14, 2013 |
| * | ||
| Bradley J. Tandy | Manager and Secretary | May 14, 2013 |
| /s/ Michael T. Hodges | ||
| Michael T. Hodges | Manager and Treasurer (Principal Financial Officer and Principal Accounting Officer) | May 14, 2013 |
| * | The undersigned, by signing his or her name hereto, does execute this Amendment No. 1 to Registration Statement on Form S-4 on behalf of the above-named officers and/or directors and/or managers of the registrant pursuant to the Power of Attorney executed by such officers and/or directors and/or managers on the signature pages to the Registration Statement previously filed on April 30, 2013. |
| /s/ Michael T. Hodges |
| Michael T. Hodges |
| Attorney-in-Fact |
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, EBI Holdings, LLC has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Warsaw, State of Indiana, on the 14th day of May, 2013.
| EBI HOLDINGS, LLC | ||
| By: | /s/ Michael T. Hodges | |
| Michael T. Hodges | ||
| Treasurer | ||
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
| Signature | Capacity | Date |
| * | ||
| Adam R. Johnson | President (Principal Executive Officer) | May 14, 2013 |
| * | ||
| Jeffrey R. Binder | Manager | May 14, 2013 |
| * | ||
| Bradley J. Tandy | Manager and Secretary | May 14, 2013 |
| /s/ Michael T. Hodges | ||
| Michael T. Hodges | Manager and Treasurer (Principal Financial Officer and Principal Accounting Officer) | May 14, 2013 |
| * | The undersigned, by signing his or her name hereto, does execute this Amendment No. 1 to Registration Statement on Form S-4 on behalf of the above-named officers and/or directors and/or managers of the registrant pursuant to the Power of Attorney executed by such officers and/or directors and/or managers on the signature pages to the Registration Statement previously filed on April 30, 2013. |
| /s/ Michael T. Hodges |
| Michael T. Hodges |
| Attorney-in-Fact |
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, EBI, LLC has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Warsaw, State of Indiana, on the 14th day of May, 2013.
| EBI, LLC | ||
| By: | /s/ Michael T. Hodges | |
| Michael T. Hodges | ||
| Treasurer | ||
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
| Signature | Capacity | Date |
| * | ||
| Adam R. Johnson | President (Principal Executive Officer) | May 14, 2013 |
| * | ||
| Jeffrey R. Binder | Manager | May 14, 2013 |
| * | ||
| Bradley J. Tandy | Manager and Secretary | May 14, 2013 |
| /s/ Michael T. Hodges | ||
| Michael T. Hodges | Manager and Treasurer (Principal Financial Officer and Principal Accounting Officer) | May 14, 2013 |
| * | The undersigned, by signing his or her name hereto, does execute this Amendment No. 1 to Registration Statement on Form S-4 on behalf of the above-named officers and/or directors and/or managers of the registrant pursuant to the Power of Attorney executed by such officers and/or directors and/or managers on the signature pages to the Registration Statement previously filed on April 30, 2013. |
| /s/ Michael T. Hodges |
| Michael T. Hodges |
| Attorney-in-Fact |
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, EBI Medical Systems, LLC has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Warsaw, State of Indiana, on the 14th day of May, 2013.
| EBI MEDICAL SYSTEMS, LLC | ||
| By: | /s/ Michael T. Hodges | |
| Michael T. Hodges | ||
| Treasurer | ||
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
| Signature | Capacity | Date |
| * | ||
| Adam R. Johnson | President (Principal Executive Officer) | May 14, 2013 |
| * | ||
| Jeffrey R. Binder | Manager | May 14, 2013 |
| * | ||
| Bradley J. Tandy | Manager and Secretary | May 14, 2013 |
| /s/ Michael T. Hodges | ||
| Michael T. Hodges | Manager and Treasurer (Principal Financial Officer and Principal Accounting Officer) | May 14, 2013 |
| * | The undersigned, by signing his or her name hereto, does execute this Amendment No. 1 to Registration Statement on Form S-4 on behalf of the above-named officers and/or directors and/or managers of the registrant pursuant to the Power of Attorney executed by such officers and/or directors and/or managers on the signature pages to the Registration Statement previously filed on April 30, 2013. |
| /s/ Michael T. Hodges |
| Michael T. Hodges |
| Attorney-in-Fact |
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, Electro-Biology, LLC has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Warsaw, State of Indiana, on the 14th day of May, 2013.
| ELECTRO-BIOLOGY, LLC | ||
| By: | /s/ Michael T. Hodges | |
| Michael T. Hodges | ||
| Treasurer | ||
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
| Signature | Capacity | Date |
| * | ||
| Adam R. Johnson | President (Principal Executive Officer) | May 14, 2013 |
| * | ||
| Jeffrey R. Binder | Manager | May 14, 2013 |
| * | ||
| Bradley J. Tandy | Manager and Secretary | May 14, 2013 |
| /s/ Michael T. Hodges | ||
| Michael T. Hodges | Manager and Treasurer (Principal Financial Officer and Principal Accounting Officer) | May 14, 2013 |
| * | The undersigned, by signing his or her name hereto, does execute this Amendment No. 1 to Registration Statement on Form S-4 on behalf of the above-named officers and/or directors and/or managers of the registrant pursuant to the Power of Attorney executed by such officers and/or directors and/or managers on the signature pages to the Registration Statement previously filed on April 30, 2013. |
| /s/ Michael T. Hodges |
| Michael T. Hodges |
| Attorney-in-Fact |
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, Biomet Florida Services, LLC has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Warsaw, State of Indiana, on the 14th day of May, 2013.
| BIOMET FLORIDA SERVICES, LLC | ||
| By: | /s/ Michael T. Hodges | |
| Michael T. Hodges | ||
| Treasurer | ||
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
| Signature | Capacity | Date |
| * | ||
| Adam R. Johnson | President (Principal Executive Officer) | May 14, 2013 |
| * | ||
| Jeffrey R. Binder | Manager | May 14, 2013 |
| * | ||
| Bradley J. Tandy | Manager and Secretary | May 14, 2013 |
| /s/ Michael T. Hodges | ||
| Michael T. Hodges | Manager and Treasurer (Principal Financial Officer and Principal Accounting Officer) | May 14, 2013 |
| * | The undersigned, by signing his or her name hereto, does execute this Amendment No. 1 to Registration Statement on Form S-4 on behalf of the above-named officers and/or directors and/or managers of the registrant pursuant to the Power of Attorney executed by such officers and/or directors and/or managers on the signature pages to the Registration Statement previously filed on April 30, 2013. |
| /s/ Michael T. Hodges |
| Michael T. Hodges |
| Attorney-in-Fact |
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, Implant Innovations Holdings, LLC has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Warsaw, State of Indiana, on the 14th day of May, 2013.
| IMPLANT INNOVATIONS HOLDINGS, LLC | ||
| By: | /s/ Michael T. Hodges | |
| Michael T. Hodges | ||
| Treasurer | ||
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
| Signature | Capacity | Date |
| * | ||
| Bareld J. Doedens | President (Principal Executive Officer) | May 14, 2013 |
| * | ||
| Edward G. Sabin | Senior Vice President – Finance and Administration (Principal Financial Officer) | May 14, 2013 |
| * | ||
| Bradley J. Tandy | Manager and Secretary | May 14, 2013 |
| * | ||
| Jeffrey R. Binder | Manager | May 14, 2013 |
| /s/ Michael T. Hodges | ||
| Michael T. Hodges | Manager and Treasurer (Principal Accounting Officer) | May 14, 2013 |
| * | The undersigned, by signing his or her name hereto, does execute this Amendment No. 1 to Registration Statement on Form S-4 on behalf of the above-named officers and/or directors and/or managers of the registrant pursuant to the Power of Attorney executed by such officers and/or directors and/or managers on the signature pages to the Registration Statement previously filed on April 30, 2013. |
| /s/ Michael T. Hodges |
| Michael T. Hodges |
| Attorney-in-Fact |
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, Interpore Cross International, LLC has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Warsaw, State of Indiana, on the 14th day of May, 2013.
| INTERPORE CROSS INTERNATIONAL, LLC | ||
| By: | /s/ Michael T. Hodges | |
| Michael T. Hodges | ||
| Treasurer | ||
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
| Signature | Capacity | Date |
| * | ||
| Philip A. Mellinger | General Manager (Principal Executive Officer) | May 14, 2013 |
| /s/ Michael T. Hodges | ||
| Michael T. Hodges | Manager and Treasurer (Principal Financial Officer and Principal Accounting Officer) | May 14, 2013 |
| * | ||
| Bradley J. Tandy | Manager and Secretary | May 14, 2013 |
| * | ||
| Jeffrey R. Binder | Manager | May 14, 2013 |
| * | The undersigned, by signing his or her name hereto, does execute this Amendment No. 1 to Registration Statement on Form S-4 on behalf of the above-named officers and/or directors and/or managers of the registrant pursuant to the Power of Attorney executed by such officers and/or directors and/or managers on the signature pages to the Registration Statement previously filed on April 30, 2013. |
| /s/ Michael T. Hodges |
| Michael T. Hodges |
| Attorney-in-Fact |
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, Interpore Spine Ltd. has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Warsaw, State of Indiana, on the 14th day of May, 2013.
| INTERPORE SPINE, LTD. | ||
| By: | /s/ Michael T. Hodges | |
Michael T. Hodges Treasurer | ||
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
| Signature | Capacity | Date |
| * | ||
| Philip A. Mellinger | General Manager (Principal Executive Officer) | May 14, 2013 |
| /s/ Michael T. Hodges | ||
| Michael T. Hodges | Director and Treasurer (Principal Financial Officer and Principal Accounting Officer) | May 14, 2013 |
| * | ||
| Bradley J. Tandy | Director and Secretary | May 14, 2013 |
| * | ||
| Jeffrey R. Binder | Director | May 14, 2013 |
| * | The undersigned, by signing his or her name hereto, does execute this Amendment No. 1 to Registration Statement on Form S-4 on behalf of the above-named officers and/or directors and/or managers of the registrant pursuant to the Power of Attorney executed by such officers and/or directors and/or managers on the signature pages to the Registration Statement previously filed on April 30, 2013. |
| /s/ Michael T. Hodges |
| Michael T. Hodges |
| Attorney-in-Fact |
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, Kirschner Medical Corporation has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Warsaw, State of Indiana, on the 14th day of May, 2013.
| KIRSCHNER MEDICAL CORPORATION | ||
| By: | /s/ Michael T. Hodges | |
| Michael T. Hodges | ||
| Treasurer | ||
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
| Signature | Capacity | Date |
| * | ||
| Adam R. Johnson | President (Principal Executive Officer) | May 14, 2013 |
| /s/ Michael T. Hodges | ||
| Michael T. Hodges | Director and Treasurer (Principal Financial Officer and Principal Accounting Officer) | May 14, 2013 |
| * | ||
| Bradley J. Tandy | Director and Secretary | May 14, 2013 |
| * | ||
| Jeffrey R. Binder | Director | May 14, 2013 |
| * | The undersigned, by signing his or her name hereto, does execute this Amendment No. 1 to Registration Statement on Form S-4 on behalf of the above-named officers and/or directors and/or managers of the registrant pursuant to the Power of Attorney executed by such officers and/or directors and/or managers on the signature pages to the Registration Statement previously filed on April 30, 2013. |
| /s/ Michael T. Hodges |
| Michael T. Hodges |
| Attorney-in-Fact |
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, Biomet Trauma, LLC has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Warsaw, State of Indiana, on the 14th day of May, 2013.
| BIOMET TRAUMA, LLC | ||
| By: | /s/ Michael T. Hodges | |
| Michael T. Hodges | ||
| Treasurer | ||
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
| Signature | Capacity | Date |
| * | ||
| Wilber C. Boren, IV | President (Principal Executive Officer) | May 14, 2013 |
| /s/ Michael T. Hodges | ||
| Michael T. Hodges | Manager and Treasurer (Principal Financial Officer and Principal Accounting Officer) | May 14, 2013 |
| * | ||
| Bradley J. Tandy | Manager and Secretary | May 14, 2013 |
| * | ||
| Jeffrey R. Binder | Manager | May 14, 2013 |
| * | The undersigned, by signing his or her name hereto, does execute this Amendment No. 1 to Registration Statement on Form S-4 on behalf of the above-named officers and/or directors and/or managers of the registrant pursuant to the Power of Attorney executed by such officers and/or directors and/or managers on the signature pages to the Registration Statement previously filed on April 30, 2013. |
| /s/ Michael T. Hodges |
| Michael T. Hodges |
| Attorney-in-Fact |
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, Biomet U.S. Reconstruction, LLC has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Warsaw, State of Indiana, on the 14th day of May, 2013.
| BIOMET U.S. RECONSTRUCTION, LLC | ||
| By: | /s/ Michael T. Hodges | |
| Michael T. Hodges | ||
| Treasurer | ||
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
| Signature | Capacity | Date |
| * | ||
| Jon C. Serbousek | President (Principal Executive Officer) | May 14, 2013 |
| /s/ Michael T. Hodges | ||
| Michael T. Hodges | Manager and Treasurer (Principal Accounting Officer) | May 14, 2013 |
| * | ||
| Bradley J. Tandy | Manager and Secretary | May 14, 2013 |
| * | ||
| Matthew C. Abernethy | Vice President – Global Finance (Principal Financial Officer) | May 14, 2013 |
| * | ||
| Jeffrey R. Binder | Manager | May 14, 2013 |
| * | The undersigned, by signing his or her name hereto, does execute this Amendment No. 1 to Registration Statement on Form S-4 on behalf of the above-named officers and/or directors and/or managers of the registrant pursuant to the Power of Attorney executed by such officers and/or directors and/or managers on the signature pages to the Registration Statement previously filed on April 30, 2013. |
| /s/ Michael T. Hodges |
| Michael T. Hodges |
| Attorney-in-Fact |
EXHIBIT INDEX
| Exhibit No. | Exhibit |
| 2.1 | Agreement and Plan of Merger, dated as of December 18, 2006, amended and restated as of June 7, 2007, among Biomet, Inc., LVB Acquisition, LLC and LVB Acquisition Merger Sub, Inc., incorporated herein by reference to the Company’s Current Report on Form 8-K filed on June 7, 2007 |
| 3.1 | Amended and Restated Articles of Incorporation, incorporated herein by reference to Exhibit 3.1 to the Company’s Current Report on Form 8-K filed on September 25, 2007 |
| 3.2 | Amended and Restated Bylaws, incorporated herein by reference to Exhibit 3.2 to the Company’s Current Report on Form 8-K filed on September 25, 2007 |
| 3.3**** | Amended and Restated Certificate of Incorporation of Biolectron, Inc. |
| 3.4**** | Bylaws of Biolectron, Inc. |
| 3.5**** | Articles of Organization of Biomet 3i, LLC |
| 3.6**** | Limited Liability Company Agreement of Biomet 3i, LLC |
| 3.7**** | Articles of Entity Conversion of Biomet Biologics, LLC |
| 3.8**** | Limited Liability Company Agreement of Biomet Biologics, LLC |
| 3.9**** | Articles of Incorporation of Biomet Europe Ltd. (f/k/a OEC Ltd., Inc.), as amended |
| 3.10**** | Amended and Restated Bylaws of Biomet Europe, Ltd. (f/k/a OEC Ltd., Inc.) |
| 3.11**** | Articles of Entity Conversion of Biomet Fair Lawn LLC |
| 3.12**** | Limited Liability Company Agreement of Biomet Fair Lawn LLC |
| 3.13**** | Certificate of Incorporation of Biomet International Ltd. |
| 3.14**** | Bylaws of Biomet International Ltd |
| 3.15**** | Articles of Incorporation of Biomet Leasing, Inc. |
| 3.16**** | Bylaws of Biomet Leasing, Inc. |
| 3.17**** | Articles of Incorporation of Biomet Manufacturing Corporation |
| 3.18**** | Bylaws of Biomet Manufacturing Corporation |
| 3.19**** | Articles of Organization of Biomet Microfixation, LLC |
| 3.20**** | Limited Liability Company Agreement of Biomet Microfixation, LLC |
| 3.21**** | Articles of Entity Conversion of Biomet Orthopedics, LLC |
| 3.22**** | Limited Liability Company Agreement of Biomet Orthopedics, LLC |
| 3.23**** | Articles of Entity Conversion of Biomet Sports Medicine, LLC |
| 3.24**** | Limited Liability Company Agreement of Biomet Sports Medicine, LLC |
| 3.25**** | Certificate of Formation of Cross Medical Products, LLC |
| 3.26**** | Limited Liability Company Agreement of Cross Medical Products, LLC |
| 3.27**** | Certificate of Formation of EBI Holdings, LLC |
| 3.28**** | Limited Liability Company Agreement of EBI Holdings, LLC |
| 3.29**** | Articles of Entity Conversion of EBI, LLC |
| 3.30**** | Limited Liability Company Agreement of EBI, LLC |
| 3.31**** | Certificate of Formation of EBI Medical Systems, LLC |
| 3.32**** | Limited Liability Company Agreement of EBI Medical Systems, LLC |
| 3.33**** | Certificate of Formation of Electro-Biology, LLC |
| 3.34**** | Limited Liability Company Agreement of Electro-Biology, LLC |
| 3.35**** | Articles of Organization of Biomet Florida Services, LLC |
| 3.36**** | Limited Liability Company Agreement of Biomet Florida Services, LLC |
| 3.37**** | Articles of Entity Conversion of Implant Innovations Holdings, LLC |
| 3.38**** | Limited Liability Company Agreement of Implant Innovations Holdings, LLC |
| 3.39**** | Articles of Organization—Conversion of Interpore Cross International, LLC |
| 3.40**** | Limited Liability Company Agreement of Interpore Cross International, LLC |
| 3.41**** | Amended and Restated Certificate of Incorporation of Interpore Spine Ltd. (f/k/a Interpore International, Inc.) |
| Exhibit No. | Exhibit |
| 3.42**** | Amended and Restated Bylaws of Interpore Spine, Ltd. (f/k/a Interpore International, Inc.) |
| 3.43**** | Certificate of Incorporation of Kirschner Medical Corporation (f/k/a Effner Biomet Corp., f/k/a Kirschner Acquisition Corp.), as amended |
| 3.44**** | Bylaws of Kirschner Medical Corporation |
| 3.45** | Limited Liability Company Agreement of Biomet Trauma, LLC |
| 3.46** | Certificate of Formation of Biomet Trauma, LLC |
| 3.47** | Limited Liability Company Agreement of Biomet U.S. Reconstruction, LLC |
| 3.48** | Certificate of Formation of Biomet U.S. Reconstruction, LLC |
| 4.1 | Senior Notes Indenture, dated as of August 8, 2012, among Biomet, Inc., the Guarantors listed therein and Wells Fargo Bank, National Association, as Trustee, incorporated herein by reference to Exhibit 4.5 to the Company’s Annual Report on Form 10-K filed on August 20, 2012 |
| 4.1.1 | Form of Regulation S Global Note, representing up to $1,000,000,000, 6.500% Senior Notes due 2020, incorporated herein by reference to Exhibit 4.5.1 to the Company’s Annual Report on Form 10-K filed on August 20, 2012 |
| 4.1.2 | Form of Rule 144A Global Note, Certificate No. A-1, representing up to $1,000,000,000, 6.500% Senior Notes due 2020, incorporated herein by reference to Exhibit 4.5.2 to the Company’s Annual Report on Form 10-K filed on August 20, 2012 |
| 4.1.3 | Form of Rule 144A Global Note, Certificate No. A-2, representing up to $1,000,000,000, 6.500% Senior Notes due 2020, incorporated herein by reference to Exhibit 4.5.3 to the Company’s Annual Report on Form 10-K filed on August 20, 2012 |
| 4.2 | Registration Rights Agreement, dated as of August 8, 2012, among Biomet, Inc., the Guarantors listed therein, and Goldman, Sachs & Co., Barclays Capital Inc., J.P. Morgan Securities LLC, Merrill Lynch, Pierce, Fenner & Smith Incorporated, Citigroup Global Markets Inc., Wells Fargo Securities, LLC, HSBC Securities (USA) Inc., ING Financial Markets LLC, Natixis Securities Americas LLC, RBC Capital Markets, LLC, SMBC Nikko Capital Markets Limited, and UBS Securities LLC, incorporated herein by reference to Exhibit 4.6 to the Company’s Annual Report on Form 10-K filed on August 20, 2012 |
| 4.3 | First Supplemental Senior Notes Indenture, dated as of October 2, 2012, among Biomet, Inc., the Guarantors listed therein and Wells Fargo Bank, National Association, as Trustee, incorporated by reference to the Company’s Current Report on Form 8-K filed on October 2, 2012 |
| 4.3.1 | Form of Rule 144A Global Note, Certificate No. A-3, 6.500% Senior Notes due 2020, incorporated by reference to the Company’s Current Report on Form 8-K filed on October 2, 2012 |
| 4.3.2 | Form of Rule 144A Global Note, Certificate No. A-4, 6.500% Senior Notes due 2020, incorporated by reference to the Company’s Current Report on Form 8-K filed on October 2, 2012 |
| 4.3.3 | Form of Regulation S Global Note, Certificate No. S-2, 6.500% Senior Notes due 2020, incorporated by reference to the Company’s Current Report on Form 8-K filed on October 2, 2012 |
| 4.4 | Senior Subordinated Notes Indenture, dated as of October 2, 2012, among Biomet, Inc., the Guarantors listed therein and Wells Fargo Bank, National Association, as Trustee, incorporated by reference to the Company’s Current Report on Form 8-K filed on October 2, 2012 |
| 4.4.1 | Form of Rule 144A Global Note, Certificate No. A-1, 6.500% Senior Subordinated Notes due 2020, incorporated by reference to the Company’s Current Report on Form 8-K filed on October 2, 2012 |
| 4.4.2 | Form of Rule 144A Global Note, Certificate No. A-2, 6.500% Senior Subordinated Notes due 2020, incorporated by reference to the Company’s Current Report on Form 8-K filed on October 2, 2012 |
| 4.4.3 | Form of Regulation S Global Note, Certificate No. S-1, 6.500% Senior Subordinated Notes due 2020, incorporated by reference to the Company’s Current Report on Form 8-K filed on October 2, 2012 |
| 4.5 | Registration Rights Agreement for the 6.500% Senior Subordinated Notes due 2020, dated as of October 2, 2012, Biomet, Inc., the Guarantors listed therein, and Merrill Lynch, Pierce, Fenner & Smith Incorporated, Goldman, Sachs & Co., Barclays Capital Inc., Citigroup Global Markets Inc., J.P. Morgan Securities LLC, Wells Fargo Securities, LLC, HSBC Securities (USA) Inc., ING Financial Markets LLC, Natixis Securities Americas LLC, RBC Capital Markets, LLC, SMBC Nikko Capital Markets Limited and UBS Securities LLC, incorporated by reference to the Company’s Current Report on Form 8-K filed on October 2, 2012 |
| Exhibit No. | Exhibit |
| 4.6 | Registration Rights Agreement for the 6.500% Senior Notes due 2020, dated as of October 2, 2012, Biomet, Inc., the Guarantors listed therein, and Goldman, Sachs & Co., Merrill Lynch, Pierce, Fenner & Smith Incorporated, Barclays Capital Inc., Citigroup Global Markets Inc., J.P. Morgan Securities LLC, Wells Fargo Securities, LLC, HSBC Securities (USA) Inc., ING Financial Markets LLC, Natixis Securities Americas LLC, RBC Capital Markets, LLC, SMBC Nikko Capital Markets Limited and UBS Securities LLC, incorporated by reference to the Company’s Current Report on Form 8-K filed on October 2, 2012 |
| 5.1# | Opinion of Cleary Gottlieb Steen & Hamilton LLP |
| 5.2# | Opinion of Gunster, Yoakley & Stewart, P.A. |
| 5.3# | Opinion of Ice Miller LLP |
| 5.4# | Opinion of Reed Smith LLP |
| 10.1**** | Credit Agreement, dated as of September 25, 2007, among Biomet, Inc., LVB Acquisition, Inc., Bank of America, N.A. and the Other Lenders party thereto |
| 10.1.1**** | Guaranty (Cash Flow), dated as of September 25, 2007, among LVB Acquisition, Inc., Certain Subsidiaries of Biomet, Inc. identified therein, and Bank of America, N.A. |
| 10.1.2**** | Pledge and Security Agreement (Cash Flow), dated as of September 25, 2007, among Biomet, Inc., LVB Acquisition, Inc., Certain Subsidiaries of Biomet, Inc. identified therein, and Bank of America, N.A. |
| 10.1.3**** | Intercreditor Agreement, dated as of September 25, 2007, by and among Bank of America, N.A., as ABL Collateral Agent, and Bank of America, N.A., as CF Collateral Agent |
| 10.1.4**** | Patent Security Agreement, dated as of September 25, 2007, among LVB Acquisition, Inc., Biomet, Inc., Certain Subsidiaries of Biomet, Inc. and Bank of America, N.A. |
| 10.1.5**** | Trademark Security Agreement, dated as of September 25, 2007, among LVB Acquisition, Inc., Biomet, Inc., Certain Subsidiaries of Biomet, Inc. and Bank of America, N.A. |
| 10.2**** | Credit Agreement, dated as of September 25, 2007, among Biomet, Inc., the Several Subsidiary Borrowers Party thereto, LVB Acquisition, Inc., Bank of America, N.A. and the Other Lenders Party thereto |
| 10.2.1 | Joinder to Amendment and Restatement Agreement dated as of October 4, 2012, among Biomet, Inc., LVB Acquisition, Inc., Bank of America, N.A., each lender from time to time party thereto and each of the other parties identified as an “Extending Term Lender” on the signature pages thereto, , incorporated by reference to the Company’s Current Report on Form 8-K filed on October 4, 2012 |
| 10.2.2**** | Guaranty (ABL), dated as of September 25, 2007 between LVB Acquisition, Inc. and Bank of America, N.A. |
| 10.2.3**** | Pledge and Security Agreement (ABL), dated as of September 25, 2007 among Biomet, Inc., LVB Acquisition, Inc., Certain Subsidiaries of Biomet, Inc. identified therein and Bank of America, N.A. |
| 10.3 | ABL Credit Agreement dated as of November 14, 2012, among Biomet, Inc., LVB Acquisition, Inc., Bank of America, N.A., and each of the other parties thereto, incorporated by reference to the Company’s Current Report on Form 8-K filed on November 14, 2012 |
| 10.4**** | Corporate Integrity Agreement, dated as of September 27, 2007, by and between the Office of Inspector General of the Department of Health and Human Services and Biomet, Inc. |
| 10.4.1**** | Settlement Agreement, dated as of September 27, 2007, by and between Biomet, Inc. and the Office of Inspector General of the Department of Health and Human Services |
| 10.5† | Biomet, Inc. Deferred Compensation Plan (Post-409A Plan), incorporated herein by reference to Exhibit 10.2 to the Company’s Quarterly Report on Form 10-Q filed on January 14, 2009 |
| 10.6† | LVB Acquisition Management Stockholders’ Agreement for Senior Executives, dated as of September 13, 2007, by and among LVB Acquisition, Inc. and the stockholders party thereto, incorporated herein by reference to Exhibit 10.5 to the Company’s Annual Report on Form 10-K filed on August 12, 2011 |
| 10.6.1† | LVB Acquisition Management Stockholders’ Agreement, dated as of November 6, 2007, by and among LVB Acquisition, Inc. and the stockholders party thereto, incorporated herein by reference to Exhibit 10.5.1 to the Company’s Annual Report on Form 10-K filed on August 12, 2011 |
| 10.7 | Governance Acknowledgement, dated as of September 25, 2007, by and between LVB Acquisition Holding, LLC, LVB Acquisition, Inc. and Biomet, Inc., incorporated by reference to Exhibit 10.6 to the Company’s Annual Report on Form 10-K filed on August 25, 2010 |
| Exhibit No. | Exhibit |
| 10.8 | Amended and Restated Registration Rights Agreement, dated as of September 27, 2007, by and among LVB Acquisition Holding, LLC, LVB Acquisition, Inc., Biomet, Inc. and the stockholders party thereto, incorporated by reference to Exhibit 10.7 to the Company’s Annual Report on Form 10-K filed on August 25, 2010 |
| 10.8.1 | Indemnification Priority Agreement, dated as of January 11, 2010, among the Company, LVB Acquisition, Inc., The Blackstone Group, L.P., The Goldman Sachs Group, Inc., Kohlberg Kravis Roberts & Co., L.P. and TPC Capital, L.P. incorporated herein by reference to Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q filed on January 14, 2010 |
| 10.9†**** | LVB Acquisition, Inc. 2007 Management Equity Incentive Plan |
| 10.10† | Biomet, Inc. Executive Annual Cash Incentive Plan, effective June 1, 2008, filed as Exhibit 10.26 to the Company’s Annual Report on Form 10-K filed on August 28, 2008 and incorporated herein by reference |
| 10.11.1† | First Amendment to Employment Agreement, dated as of December 31, 2008, by and between Biomet, Inc. and Jeffrey R. Binder, incorporated herein by reference to Exhibit 10.3 to the Company’s Quarterly Report on Form 10-Q filed on January 14, 2009 |
| 10.12† | Employment Agreement, dated as of February 28, 2008, by and among Biomet, Inc. and Daniel P. Florin, filed as Exhibit 10.16 to the Company’s Annual Report on Form 10-K filed on August 28, 2008 and incorporated herein by reference |
| 10.12.1† | First Amendment to Employment Agreement, dated as of December 31, 2008, by and between Biomet, Inc. and Daniel P. Florin, filed as Exhibit 10.4 to the Company’s Quarterly Report on Form 10-Q on January 14, 2009 and incorporated herein by reference |
| 10.15† | Employment Agreement, dated as of March 3, 2008, by and between Biomet, Inc. and Jon Serbousek, filed as Exhibit 10.32 to the Company’s Annual Report on Form 10-K filed on August 21, 2009 and incorporated herein by reference |
| 10.15.1† | First Amendment to Employment Agreement, dated as of December 31, 2008, by and between Biomet, Inc. and Jon Serbousek, filed as Exhibit 10.33 to the Company’s Annual Report on Form 10-K filed on August 21, 2009 and incorporated herein by reference |
| 10.16† | Employment Agreement, dated as of February 28, 2008, by and between Biomet, Inc. and Brad Tandy filed as Exhibit 10.15 to the Company’s Annual Report on Form 10-K filed on August 20, 2012 and incorporated herein by reference |
| 10.16.1† | First Amendment to Employment Agreement, dated as of December 31, 2008, by and between Biomet, Inc. and Bradley J. Tandy filed as Exhibit 10.15.1 to the Company’s Annual Report on Form 10-K filed on August 20, 2012 and incorporated herein by reference |
| 10.17† | Consulting Agreement dated as of January 14, 2010 between Company and Dane A. Miller, Ph. D., filed as Exhibit 10.2 to the Company’s Quarterly Report on Form 10-Q filed on January 14, 2010 and incorporated herein by reference |
| 10.17.1† | First Amendment to Consulting Agreement, dated September 6, 2011 between the Company and Dane A. Miller, Ph. D filed as Exhibit 10.16.1 to the Company’s Annual Report on Form 10-K filed on August 20, 2012 and incorporated herein by reference |
| 10.18 | Indemnification Priority Agreement, dated as of January 11, 2010, among the Company, LVB Acquisition, Inc., The Blackstone Group, L.P., The Goldman Sachs Group, Inc., Kohlberg Kravis Roberts & Co., L.P. and TPG Capital, L.P. filed as Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q filed on January 14, 2010 and incorporated herein by reference |
| 10.19† | Employment Agreement, dated September 2, 2008, by and between Biomet, Inc. and Robin T. Barney filed as Exhibit 10.18 to the Company’s Annual Report on Form 10-K filed on August 20, 2012 and incorporated herein by reference |
| 10.19.1† | First Amendment to Employment Agreement, dated December 31, 2008, by and between Biomet, Inc. and Robin T. Barney filed as Exhibit 10.18.1 to the Company’s Annual Report on Form 10-K filed on August 20, 2012 and incorporated herein by reference |
| 10.21† | LVB Acquisition, Inc. Restricted Stock Unit Plan, filed as Exhibit 10.1 to the Company’s Form 8-K filed on February 15, 2011 and incorporated herein by reference |
| 10.21.1† | LVB Acquisition, Inc. Form Restricted Stock Unit Grant Agreement, filed as Exhibit 10.2 to the Company’s Form 8-K filed on February 15, 2011 and incorporated herein by reference |
| 10.23 | Asset Purchase Agreement, dated April 2, 2012, between Biomet, Inc. and DePuy Orthopaedics, Inc., filed as Exhibit 10.1 to the Company’s Current Report on Form 8-K on April 5, 2012 and incorporated herein by reference |
| 10.23.1 | Amendment No. 1 dated June 1, 2012, between DePuy Orthopaedics, Inc. and Biomet, Inc., to the Asset Purchase Agreement, dated as of April 2, 2012, filed as Exhibit 10.1 to the Company’s Current Report on Form 8-K on June 5, 2012 and incorporated herein by reference |
| Exhibit No. | Exhibit |
| 10.24†# | LVB Acquisition, Inc. 2012 Restricted Stock Unit Plan adopted July 31, 2012 and amended March 27, 2013 |
| 10.24.1† | LVB Acquisition, Inc. 2012 Form Restricted Stock Unit Grant Agreement, filed as Exhibit (d)(2) to the Company’s Schedule TO on July 2, 2012 and incorporated herein by reference |
| 10.25† | Form of Management Equity Incentive Plan Stock Option Grant Agreement, filed as Exhibit (d)(3) to the Company’s Schedule TO on July 2, 2012 and incorporated herein by reference. |
| 10.26 | Amendment and Restatement Agreement dated as of August 2, 2012, among Biomet, Inc., LVB Acquisition, Inc., Bank of America, N.A., and each of the other lenders party thereto, filed as Exhibit 10.1 to the Company’s Current Report on form 8-K on August 6, 2012 and incorporated herein by reference |
| 10.27† | Management Services Agreement dated September 25, 2007, by and among LVB Acquisition Merger Sub, Inc., LVB Acquisition Holding, LLC, LVB Acquisition, Inc., Blackstone Management Partners V L.L.C., Goldman, Sachs & Co., Kohlberg Kravis Roberts & Co. L.P. and TPG Capital, L.P filed as Exhibit 10.26 to the Company’s Annual Report on Form 10-K filed on August 20, 2012 and incorporated herein by reference |
| 10.28† | Biomet, Inc. Executive Annual Cash Incentive Plan, incorporated herein by reference to Exhibit 10.26 to the Company’s Annual Report on Form 10-K filed on August 28, 2008 |
| 12.1# | Computation of Ratio of Earnings to Fixed Charges |
| 21.1# | Subsidiaries of Biomet, Inc. |
| 23.1* | Consent of Deloitte & Touche LLP, Independent Registered Public Accounting Firm |
| 23.2# | Consent of Cleary Gottlieb Steen & Hamilton LLP (included in the opinion filed as Exhibit 5.1) |
| 23.3# | Consent of Gunster, Yoakley & Stewart, P.A. (included in the opinion filed as Exhibit 5.2) |
| 23.4# | Consent of Ice Miller LLP (included in the opinion filed as Exhibit 5.3) |
| 23.5# | Consent of Reed Smith LLP (included in opinion filed as Exhibit 5.4) |
| 25.1# | Form T-1 statement of eligibility under the Trust Indenture Act of 1939, as amended, of Wells Fargo Bank, National Association, as Trustee with respect to the Indenture governing the 6.500% Senior Notes due 2020 and the 6.500% Senior Subordinated Notes due 2020 |
| 101.INS# | XBRL Instance Document |
| 101.SCH# | XBRL Taxonomy Extension Schema Document |
| 101.CAL# | XBRL Taxonomy Extension Calculation Linkbase Document |
| 101.DEF# | XBRL Taxonomy Extension Definition Linkbase Document |
| 101.LAB# | XBRL Taxonomy Extension Label Linkbase Document |
| 101.PRE# | XBRL Taxonomy Extension Presentation Linkbase Document |
| # | Previously filed |
| * | Filed herewith |
| ** | Incorporated by reference to Registration Statement on Form S-1 of Biomet, Inc. filed on September 17, 2012 |
| *** | Incorporated by reference to the Registration Statement on Form S-1 of Biomet, Inc. filed on May 6, 2008 |
| **** | Incorporated by reference to the Registration Statement on Form S-4 of Biomet, Inc. filed on May 6, 2008 |
| † | Management contract or compensatory plan or arrangement |