Table of Contents
As filed with the Securities and Exchange Commission on February 20, 2009
Registration No. 333-150655
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Post-Effective Amendment No. 2
to
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
BIOMET, INC.
(Exact name of registrant as specified in its charter)
(see table of additional registrants)
| Indiana | 3842 | 35-1418342 | ||
(State or other jurisdiction of incorporation or organization) | (Primary Standard Industrial Classification Code Number) | (I.R.S. Employer Identification Number) |
56 East Bell Drive
Warsaw, Indiana 46582
(574) 267-6639
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Bradley J. Tandy
Senior Vice President, General Counsel and Secretary
Biomet, Inc.
56 East Bell Drive
Warsaw, Indiana 46582
(Name, address, including zip code, and telephone number,
including area code, of agent for service)
Approximate date of commencement of proposed sale to the public:
As soon as practicable after this registration statement becomes effective.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. x
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ¨ | Accelerated filer | ¨ | |||
| Non-accelerated filer | x | Smaller reporting company | ¨ | |||
The registrants hereby amend this Registration Statement on such date or dates as may be necessary to delay its effective date until the registrants shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until this Registration Statement shall become effective on such date as the SEC, acting pursuant to said Section 8(a), may determine.
Table of Contents
TABLE OF ADDITIONAL REGISTRANT GUARANTORS
Exact Name of Registrant as Specified in its Charter | State or Other Jurisdiction of Incorporation or Organization | Primary Standard Industrial Classification Code Number | I.R.S. Employer Identification Number | Address, including Zip Code and Telephone Number, for Service, of Registrant’s | ||||
Biolectron, Inc. | Delaware | 3842 | 13-2914413 | 3200 Las Vegas Blvd. Las Vegas, NV 89109 (574) 267-6639 | ||||
Biomet 3i, LLC | Florida | 3842 | 59-2816882 | 4555 Riverside Drive Palm Beach Gardens, FL 33410 (574) 267-6639 | ||||
Biomet Biologics, LLC | Indiana | 3842 | 03-04079652 | 56 E. Bell Drive Warsaw, IN 46582 (574) 267-6639 | ||||
Biomet Europe Ltd. | Delaware | 3842 | 35-1603620 | Toermalijnring 600 3316 LC Dordrecht The Netherlands (574) 267-6639 | ||||
Biomet Fair Lawn, LLC | Indiana | 3842 | 31-1651311 | 20-01 Pollitt Drive Fairlawn, NJ 07410 (574) 267-6639 | ||||
Biomet Holdings Ltd. | Delaware | 3842 | 35-2022857 | 56 E. Bell Drive Warsaw, IN 46582 (574) 267-6639 | ||||
Biomet International Ltd. | Delaware | 3842 | 35-2046422 | 56 E. Bell Drive Warsaw, IN 46582 (574) 267-6639 | ||||
Biomet Leasing, Inc. | Indiana | 3842 | 35-2076217 | 56 E. Bell Drive Warsaw, IN 46582 (574) 267-6639 | ||||
Biomet Manufacturing Corporation | Indiana | 3842 | 35-2074039 | 56 E. Bell Drive Warsaw, IN 46582 (574) 267-6639 | ||||
Biomet Microfixation, LLC | Florida | 3842 | 59-1692523 | 1520 Tradeport Drive Jacksonville, FL 32218-2482 (574) 267-6639 | ||||
Biomet Orthopedics, LLC | Indiana | 3842 | 35-2074037 | 56 E. Bell Drive Warsaw, IN 46582 (574) 267-6639 | ||||
Biomet Sports Medicine, LLC | Indiana | 3842 | 35-1803072 | 56 E. Bell Drive Warsaw, IN 46852 (574) 267-6639 | ||||
Biomet Travel, Inc. | Indiana | 3842 | 56-2284-205 | 56 E. Bell Drive Warsaw, IN 46852 (574) 267-6639 | ||||
Blue Moon Diagnostics, Inc. | Indiana | 3842 | 35-2070282 | 56 E. Bell Drive Warsaw, IN 46852 (574) 267-6639 | ||||
Table of Contents
Exact Name of Registrant as Specified in its Charter | State or Other Jurisdiction of Incorporation or Organization | Primary Standard Industrial Classification Code Number | I.R.S. Employer Identification Number | Address, including Zip Code and Telephone Number, including Area Code, of Agent for Service, of Registrant’s Principal Executive Offices | ||||
Cross Medical Products, LLC | Delaware | 3842 | 31-0992628 | 181 Technology Drive Irvine, CA 92618 (574) 267-6639 | ||||
EBI Holdings, LLC | Delaware | 3842 | 22-2407246 | 100 Interpace Parkway Parsippany, NJ 07054 (574) 267-6639 | ||||
EBI, LLC | Indiana | 3842 | 31-1651314 | 100 Interpace Parkway Parsippany, NJ 07054 (574) 267-6639 | ||||
EBI Medical Systems, LLC | Delaware | 3842 | 22-2406619 | 100 Interpace Parkway Parsippany, NJ 07054 (574) 267-6639 | ||||
Electro-Biology, LLC | Delaware | 3842 | 22-2278360 | 6 Upper Pond Road Parsippany, NJ 07054-01079 (574) 267-6639 | ||||
Biomet Florida Services, LLC | Florida | 3842 | 20-0388276 | 4555 Riverside Drive Palm Beach Gardens, FL 33410 (574) 267-6639 | ||||
Implant Innovations Holdings, LLC | Indiana | 3842 | 35-2088040 | 56 E. Bell Drive Warsaw, IN 46852 (574) 267-6639 | ||||
Interpore Cross International, LLC | California | 3842 | 33-0818017 | 181 Technology Drive, Irvine, CA 92618 (574) 267-6639 | ||||
Interpore Spine Ltd. | Delaware | 3842 | 95-3043318 | 181 Technology Drive, Irvine, CA 92618 (574) 267-6639 | ||||
Kirschner Medical Corporation | Delaware | 3842 | 52-1319702 | 100 Interpace Parkway Parsippany, NJ 07054 (574) 267-6639 | ||||
Table of Contents
The information in this preliminary prospectus is not complete and may be changed. These securities may not be sold until the registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell nor does it seek an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
SUBJECT TO COMPLETION, DATED FEBRUARY 20, 2009
PRELIMINARY PROSPECTUS

$775,000,000 10% Senior Notes due 2017
$775,000,000 10 3/8 %/11 1/8% Senior Toggle Notes due 2017
$1,015,000,000 11 5/8% Senior Subordinated Notes due 2017
NOTES OFFERED
| • | $775 million of our 10% Senior Notes due 2017, which we refer to as the senior cash pay notes. |
• | $775 million of our 10 3/8%/11 1/8% Senior Toggle Notes due 2017, which we refer to as the senior toggle notes. |
• | $1,015 million of our 11 5/8% Senior Subordinated Notes due 2017, which we refer to as the senior subordinated notes. We refer to the senior cash pay notes and the senior toggle notes collectively as the senior notes. We refer to the senior notes and the senior subordinated notes collectively as the notes. |
MATURITY
| • | The notes will mature on October 15, 2017. |
INTEREST
| • | Senior cash pay notes: Interest is payable in cash and accrues at the rate of 10% per annum. |
• | Senior toggle notes: Interest accrues at the rate of 10 3/8% per annum and PIK interest (as defined below) accrues at the rate of 11 1/8% per annum. For any interest period (other than the first initial interest period) through October 15, 2012, we may elect to pay interest on the senior toggle notes (1) entirely in cash, (2) entirely by increasing the principal amount of the toggle notes or issuing new toggle notes, or PIK interest, or (3) 50% in cash and 50% in PIK interest. After October 15, 2012, all interest on the senior toggle notes will be payable in cash. |
• | Senior subordinated notes: Interest is payable in cash and accrues at the rate of 11 5/8% per annum. |
INTEREST PAYMENT DATES
| • | April 15 and October 15, commencing April 15, 2008. |
REDEMPTION
| • | We may redeem some or all of the notes on or after October 15, 2012 at redemption prices described in this prospectus. |
| • | We may also redeem up to 35% of the notes using the proceeds of certain equity offerings completed before October 15, 2010. |
CHANGE OF CONTROL
| • | If we experience a change of control, we must offer to purchase the notes. |
GUARANTEES
| • | Each of our existing and future wholly-owned domestic restricted subsidiaries, which guarantees our senior secured cash flow facilities, will jointly, severally and unconditionally guarantee the senior notes on a senior basis and the senior subordinated notes on a senior subordinated unsecured basis. |
RANKING
| • | The senior notes and the related guarantees are our and the guarantor’s general unsecured indebtedness, rank equally in right of payment to all of our and the guarantors’ existing and future senior unsecured indebtedness and other obligations and rank senior in right of payment to all of our and the guarantors’ existing and future subordinated indebtedness and other obligations. |
| • | The senior subordinated and the related guarantees are our and the guarantor’s general unsecured senior subordinated indebtedness, ranking junior in right of payment to any of our and the guarantors’ existing and future senior indebtedness and other obligations, rank equally in right of payment to all of our and our guarantors’ existing and future senior subordinated indebtedness and other obligations and rank senior in right of payment to any of our and the guarantors’ existing and future subordinated indebtedness and other obligations. |
See “Risk Factors“ beginning on page 13 for a discussion of certain risks that you should consider before investing in the notes.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
This prospectus has been prepared for and may be used by Goldman, Sachs & Co. and any affiliates of Goldman, Sachs & Co. in connection with offers and sales of the notes related to market-making transactions in the notes effected from time to time. Goldman, Sachs & Co. or its affiliates may act as principal or agent in such transactions, including as agent for the counterparty when acting as principal or as agent for both counterparties, and may receive compensation in the form of discounts and commissions, including from both counterparties, when it acts as agents for both. Such sales will be made at prevailing market prices at the time of sale, at prices related thereto or at negotiated prices. We will not receive any proceeds from such sales.
The date of this prospectus is , 2009.
Table of Contents
You should rely only on the information contained or incorporated by reference in this prospectus. We have not authorized any person to provide you with any information or represent anything about us or this offering that is not contained in this prospectus. If given or made, any such other information or representation should not be relied upon as having been authorized by us. This prospectus does not offer to sell nor ask for offers to buy any of the securities in any jurisdiction where it is unlawful, where the person making the offer is not qualified to do so, or to any person who cannot legally be offered the securities. You should not assume that the information contained or incorporated by reference in this prospectus is accurate as of any date other than the date on the front cover of this prospectus or the date of any document incorporated by reference herein.
TABLE OF CONTENTS
| Page | ||
| ii | ||
| ii | ||
| iii | ||
| iv | ||
| iv | ||
| iv | ||
| 1 | ||
| 12 | ||
| 26 | ||
| 27 | ||
| 28 | ||
| 29 | ||
| 32 | ||
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS | 35 | |
| 52 | ||
| 53 | ||
| 67 | ||
| 70 | ||
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT | 90 | |
| 92 | ||
| 93 | ||
| 97 | ||
| 140 | ||
| 184 | ||
CERTAIN MATERIAL UNITED STATES FEDERAL INCOME TAX CONSIDERATIONS | 185 | |
| 187 | ||
| 187 | ||
| 187 | ||
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE | 187 | |
| F-1 |
i
Table of Contents
WHERE YOU CAN FIND MORE INFORMATION
We and the guarantors have filed with the Securities and Exchange Commission, or the SEC, a registration statement on Form S-1 under the Securities Act of 1933, as amended, or the Securities Act, with respect to the notes being offered hereby. This prospectus, which forms a part of the registration statement, does not contain all of the information set forth in the registration statement. For further information with respect to us, the guarantors or the notes, we refer you to the registration statement. Statements contained in this prospectus as to the contents of any contract or other document are not necessarily complete. We file annual, quarterly, and current reports and other information with the SEC. The registration statement, such reports and other information can be inspected and copied at the Public Reference Room of the SEC located at Room 1580, 100 F Street, N.E., Washington D.C. 20549. Copies of such materials, including copies of all or any portion of the registration statement, can be obtained from the Public Reference Room of the SEC at prescribed rates. You can call the SEC at 1-800-SEC-0330 to obtain information on the operation of the Public Reference Room. Such materials may also be accessed electronically by means of the SEC’s home page on the Internet (http://www.sec.gov).
Our Internet address iswww.biomet.com. There we make available free of charge, on or through the financial information section of our Web site, annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities and Exchange Act of 1934, as amended, or the Exchange Act, as soon as reasonably practicable after we electronically file such material with, or furnish it to, the SEC. The information found on our Web site, other than as specifically incorporated by reference into this prospectus, is not part of this prospectus.
Under the terms of the indentures relating to the notes, we have agreed that, whether or nor we are required to do so by the rules and regulations of the SEC, for so long as any of the notes remain outstanding, we will furnish to the trustee and holders of the notes the information specified therein. See “Description of Senior Notes” and “Description of Senior Subordinated Notes.”
This prospectus contains forward-looking statements within the meaning of the U.S. federal securities laws. Statements that are not historical facts, including statements about our beliefs and expectations, are forward-looking statements. Forward-looking statements include statements generally preceded by, followed by or that include the words “believe,” “could,” “expect,” “forecast,” “intend,” “may,” “anticipate,” “plan,” “predict,” “possibly,” “project,” “potential,” “estimate,” “should,” “will” or similar expressions. These statements include, but are not limited to, statements related to:
| • | the timing and number of planned new product introductions; |
| • | the effect of anticipated changes in the size, health and activities of the population or on the demand for our products; |
| • | assumptions and estimates regarding the size and growth of certain market categories; |
| • | our ability and intent to expand in key international markets; |
| • | the timing and anticipated outcome of clinical studies; |
| • | assumptions concerning anticipated product developments and emerging technologies; |
| • | the future availability of raw materials; |
| • | the anticipated adequacy of our capital resources to meet the needs of our business; |
| • | our continued investment in new products and technologies; |
| • | the ultimate marketability of products currently being developed; |
| • | the ability to successfully implement new technologies and transition certain manufacturing operations to China; |
| • | our ability to manage working capital and generate adequate cash flows to service outstanding debt; |
| • | our ability to sustain sales and earnings growth; |
| • | our goals for sales and earnings growth; |
| • | our success in achieving timely approval or clearance of our products with domestic and foreign regulatory entities; |
| • | our success in implementing our value creation and operational improvement programs; |
| • | the stability of certain foreign economic markets; |
| • | the impact of anticipated changes in the musculoskeletal industry and our ability to react to and capitalize on those changes; |
| • | our ability to successfully implement desired organizational changes; |
| • | the impact of our managerial changes; and |
| • | our ability to take advantage of technological advancements. |
Forward-looking statements reflect our current expectations and are not guarantees of performance. These statements are based on our management’s beliefs and assumptions, which in turn are based on currently available information. Important assumptions relating to these forward-looking statements include, among others, assumptions regarding demand for our products, expected pricing levels, raw material costs, the timing and cost of planned capital expenditures, expected outcomes of pending litigation, the solvency
ii
Table of Contents
of our insurers and the ultimate resolution of allocation and coverage issues with those insurers, competitive conditions and general economic conditions. Readers of this prospectus are cautioned that reliance on any forward-looking statement involves risks and uncertainties. Although we believe that the assumptions on which the forward-looking statements contained herein are based are reasonable, any of those assumptions could prove to be inaccurate given the inherent uncertainties as to the occurrence or nonoccurrence of future events. There can be no assurance that the forward-looking statements contained in this prospectus will prove to be accurate. The inclusion of a forward-looking statement in this prospectus should not be regarded as a representation by us that our objectives will be achieved. Forward-looking statements also involve risks and uncertainties, which could cause actual results to differ materially from those contained in any forward-looking statement. Many of these factors are beyond our ability to control or predict and could, among other things, cause actual results to differ from those contained in forward-looking statements made in this prospectus and presented elsewhere by management from time to time. Such factors, among others, may have a material adverse effect upon our business, financial condition and results of operations and may include, but are not limited to, factors discussed under the heading “Risk Factors” and the following:
| • | changes in general economic conditions and interest rates; |
| • | changes in the availability of capital and financing sources; |
| • | changes in competitive conditions and prices in our markets; |
| • | the effects of incurring a substantial amount of indebtedness under our senior secured credit facilities, our senior notes, senior toggle notes and senior subordinated notes; |
| • | the effects upon us of complying with the covenants contained in our senior secured credit facilities and the indentures governing our senior notes, senior toggle notes and senior subordinated notes; |
| • | restrictions that the terms and conditions of our senior secured credit facilities may place on our ability to respond to changes in our business or take certain actions; |
| • | changes in the relationship between supply of and demand for our products; |
| • | fluctuations in costs of raw materials and labor; |
| • | changes in other significant operating expenses; |
| • | decreases in sales of our principal product lines; |
| • | slow downs or inefficiencies in our product research and development efforts; |
| • | increases in expenditures related to increased government regulation of our business; |
| • | developments adversely affecting our sales activities outside the United States; |
| • | decreases in reimbursement levels by our customers; |
| • | difficulties in transitioning certain manufacturing operations to China; |
| • | challenges in effectively implementing restructuring and cost saving initiatives; |
| • | increases in cost-containment efforts by group purchasing organizations; |
| • | loss of our key management and other personnel or inability to attract such management and other personnel; |
| • | increases in costs of retaining existing independent sales agents of our products; |
| • | unanticipated expenditures related to litigation, including litigation related to the Merger, the past stock option grant practices and investigations by the U.S. Department of Justice and the SEC; and |
| • | failure to comply with the terms of the Deferred Prosecution Agreement and Corporate Integrity Agreement. |
We caution you not to place undue reliance on these forward-looking statements that speak only as of the date they were made. We do not undertake any obligation to publicly release any revisions to these forward-looking statements to reflect events or circumstances after the date of this prospectus or to reflect the occurrence of unanticipated events.
In this prospectus, we rely on and refer to information and statistics regarding our industry products and our market share based on revenues in the sectors in which we compete. Where possible, we obtained this information and statistics from third-party sources, such as independent industry publications, government publications or reports by market research firms, including, without limitation, Eurostat, Knowledge Enterprises, Inc., the U.S. Census Bureau, Wall Street research and from company research and trade interviews. In addition, we have supplemented third-party information where necessary with management estimates based on our review of internal surveys, information from our customers and vendors, trade and business organizations and other contacts in markets in which we operate, and our management’s knowledge and experience. However, these estimates are subject to change and are uncertain due to limits on the availability and reliability of primary sources of information and the voluntary nature of the data gathering process. Although we believe that these independent sources and our management’s estimates are reliable as of the date of this prospectus, the information contained in them has not been independently verified, and neither we nor Goldman Sachs & Co. can assure you as to the accuracy or completeness of such information. As a result, you should be aware that market share and industry data included in this prospectus, and estimates and beliefs based on that data, may not be reliable. Neither we nor Goldman Sachs & Co. make any representation as to the accuracy or completeness of such information.
iii
Table of Contents
Numerical figures included in this prospectus have been subject to rounding adjustments.
For purposes of presenting in U.S. dollars the amounts outstanding and the amounts available for borrowing under our senior secured credit facilities, euro-denominated European line of credit and yen-denominated Japanese lines of credit as well as the fair value of the interest rate swap agreements relating to our euro-denominated senior secured term loan facility, in each case as of November 30, 2008, we have used the noon buying rate in New York City for cable transfers in foreign currencies as certified for customs purposes by the Federal Reserve Bank of New York for the euro of €1.00 to $1.2697 and yen of ¥1.00 to $0.009371. These rates are presented for informational purposes and are not the same as the rates that are used for purposes of translating euros or yen into U.S. dollars in our financial statements.
Unless otherwise noted or indicated by the context, in this prospectus:
| • | For periods prior to the Merger, the terms “Biomet,” “Company,” “we,” “us” and “our” refer to Biomet, Inc. as the target corporation and its consolidated subsidiaries, and for periods after the Merger, those terms refer to Biomet, Inc. as the surviving corporation and its consolidated subsidiaries, unless we expressly state otherwise or the context otherwise requires. |
| • | The term “Merger” refers to the merger of LVB Acquisition Merger Sub, Inc., an Indiana corporation and wholly-owned subsidiary of LVB Acquisition, Inc., and the initial issuer of the original notes, with and into Biomet, with Biomet continuing as the surviving corporation after the merger. |
| • | The term “Transactions” refers to the transactions described in the section titled “The Transactions” included elsewhere in this prospectus. |
| • | The term “Sponsors” refers to the investment funds affiliated with The Blackstone Group, Goldman Sachs Capital Partners, Kohlberg Kravis Roberts & Co., or KKR, and TPG Capital, or TPG, that provided the equity investment used to pay a portion of the cash consideration paid as part of the Merger. |
| • | The term “closing date” refers to September 25, 2007, the date of closing of the Merger. |
| • | The term “pro forma” refers to our financial information, as adjusted to give effect to the Transactions on the basis described, and subject to the qualifications expressed, under the heading “Unaudited Pro Forma Condensed Consolidated Financial Data.” |
| • | The term “domestic” refers to the United States and the term “international” refers to all countries other than the United States. |
| • | References to our fiscal years through and including fiscal 2008 are to the twelve months ended on May 31 of such year. |
iv
Table of Contents
This summary contains basic information about us and the notes. Because it is a summary, it does not contain all of the information that is important to you. You should read this entire prospectus carefully, including the section entitled “Risk Factors” and our consolidated financial statements and the notes thereto included elsewhere in this prospectus, before making an investment decision.
Our Company
General
We are one of the largest orthopedic medical device companies in the United States and worldwide with operations in over 50 locations throughout the world and distribution in approximately 90 countries. We design, manufacture and market a comprehensive range of both surgical and non-surgical products used primarily by orthopedic surgeons and other musculoskeletal medical specialists. For over 30 years, we have applied the most advanced engineering and manufacturing technology to the development of highly durable joint replacement systems and minimally invasive surgical procedures. For fiscal 2008 and the six months ended November 30, 2008, we generated net sales of $2,383 million and $1,250 million, respectively.
Products
We operate in one business segment, musculoskeletal products, which includes the design, manufacture and marketing of products in four major market categories: Reconstructive Products, Fixation Devices, Spinal Products and Other Products. We have three reportable geographic markets: United States, Europe and International.
Reconstructive Products. We are a worldwide leader in our principal market category, Reconstructive Products. Primary product offerings include implants and instrumentation for replacing knees and hips as well as extremity joints that have deteriorated due to disease (principally osteoarthritis) or injury. We have been among the fastest growing knee companies in the industry as a result of continued strong demand for our total and partial knee systems. We also believe that our innovative hip product offerings, including our broad platform of bearing options, represent competitive advantages and have led to excellent surgeon acceptance. This market category also includes our dental reconstructive device business, which includes implants and abutments, augmented by a growing line of our other reconstructive products such as regenerative products, accessories and biologics products. The Reconstructive Products category accounted for 74% of our pro forma net sales for fiscal 2008 and 75% of our net sales for the six months ended November 30, 2008.
Fixation Devices. Fixation devices are used for setting and stabilizing damaged bones to support and/or augment the body’s natural healing process. We are a market leader for electrical stimulation devices for trauma indications, offering implantable and non-invasive products to stimulate bone growth. Other products include internal fixation devices (such as nails, plates, screws, pins and wires used to stabilize traumatic bone injuries), external fixation devices (used to stabilize fractures when alternative methods of fixation are not suitable), craniomaxillofacial fixation systems and bone substitute materials. The Fixation Devices category accounted for 10% of our pro forma net sales for fiscal 2008 and 10% of our net sales for the six months ended November 30, 2008.
Spinal Products. Spinal products include devices and instrumentation for repairing defects or wear and tear in the vertebral column. Key products in this category include implantable and non-invasive electrical stimulation devices for spinal indications (used to enhance bone fusion success), spinal fixation systems used to stabilize the spine, bone substitute materials and allograft services used in spinal fusion procedures, as well as motion preservation systems. The Spinal Products category accounted for 8% of our pro forma net sales for fiscal 2008 and 8% of our net sales for the six months ended November 30, 2008.
Other Products. We manufacture and distribute a number of other products, including sports medicine products (used in minimally-invasive orthopedic surgical procedures), orthopedic support products (also referred to as softgoods and bracing products), operating room supplies, casting materials, general surgical instruments, wound care products and other surgical products. The Other Products category accounted for 8% of our pro forma net sales for fiscal 2008 and 7% of our net sales for the six months ended November 30, 2008.
1
Table of Contents
The following charts set forth our pro forma net sales by market category and geographic markets for fiscal 2008.

Industry
We participate in the worldwide orthopedic and dental implant markets, which management estimates to be $30 billion in market size. These markets have shown favorable industry dynamics and internally we estimate that these markets will grow at a compounded annual growth rate of approximately 6% to 9% over the next twenty four months. The orthopedic industry benefits from several favorable factors, including, but not limited to:
Favorable Demographics. An aging population is driving growth in the orthopedic products market. Many conditions that require orthopedic surgery affect people in middle age or later in life. As the baby boomer population ages and life expectancy increases, the elderly will represent a higher percentage of the overall population. According to a 2007 U.S. Census Bureau projection, the U.S. population aged 55 to 74 is expected to grow at approximately three times the average rate of population growth from 51 million and 18% of the population in 2007 to 76 million and 22% of the population by 2027. According to a 2006 Eurostat projection, the European population aged 65 and over will grow at approximately 16 times the average rate of population growth from 77 million and 17% of the population in 2005 to 135 million and 30% of the population by 2025.
Stable Industry Structure. Following a period of consolidation during the late 1990s, over the past nine years, we, together with Zimmer Holdings, Inc., DePuy, Inc. (a Johnson & Johnson company), Stryker Corporation and Smith & Nephew plc, have constituted over 85% of the orthopedic reconstructive industry’s worldwide revenues. These players have achieved critical components to success, including product innovations and advancements, accumulation of clinical data, regulatory expertise, economies of scale, and sales force and surgeon customer relationships, which have led to minimal market share movement among top players from year to year.
Close Working Relationships with Surgeon Customers. Due to the nature of orthopedic implants, the orthopedic medical device industry is unique with respect to the working relationships between orthopedic device manufacturers and their surgeon customers. As a component of innovation in the industry, some surgeons serve as consultants and are instrumental in the development of new products and the ongoing evaluation and improvement of existing products.
Technological Advancement of Orthopedic Products. Incremental and continuous technological advancement of orthopedic products is expanding the addressable market. Product innovation is improving the durability and performance of orthopedic devices and promoting less invasive surgery. Examples include bearing surfaces in hips with potential for greater longevity, premium knee systems that allow greater range of motion, and press-fit hip stems that facilitate minimally invasive hip procedures. As a result of this ongoing innovation, we believe that surgeons are increasingly recommending and utilizing implant products for younger patients as well as elderly patients who are remaining healthier and more active than those of past generations.
Favorable Product Mix Shift. Continued product innovation is driving a favorable shift in mix towards premium products that offer enhanced outcomes for patients. Product evolution is also expanding the addressable market to include younger patients who are more likely to require and demand premium and high-performance products. In addition, the payor mix resulting from the broadening of the patient population to younger patients with private insurance creates a favorable environment due to the fact that joint procedures for non-Medicare payors are generally more profitable for hospitals.
Competitive Strengths
We believe we have a number of competitive strengths that will enable us to further enhance our position in the orthopedic medical device market.
Broad Market Leadership. We are the fourth largest player in the U.S. orthopedic reconstructive market and have maintained this position for over a decade. We have high representation at U.S. hospitals, supplying products to over 60% of hospitals performing joint replacement surgery. In addition, we are the third largest manufacturer and marketer of dental reconstructive products worldwide and maintain leadership positions in the electrical stimulation and craniomaxillofacial fields.
Leading Research and Development Platform. We have a long history of innovation, engineering, quality and successful new product launches. Demonstrating our research and development leadership, we have launched approximately 800 new products in the past nine fiscal years and plan to introduce approximately 100 new products during fiscal 2009.
2
Table of Contents
Strong Relationships with Surgeon Customers. Based on their understanding of and satisfaction with our products, we enjoy long-standing relationships with our surgeon customers, many of which commence during the surgeons’ residency training program. Our support of medical education programs provides important training opportunities for orthopedic surgeons early in their career. In fact, supporting “hands-on” training provides opportunities for residents, fellows and attending surgeons to experience the clinical benefits of our products. Surgeons have historically exhibited limited willingness to switch manufacturers, as successful patient outcomes are related to the practitioners’ familiarity with the procedural characteristics and instrumentation of certain implants. As such, 19 of our top 25 surgeons have been our customers for at least 10 years.
Consistently Strong Operating Cash Flow Generation. Our business is characterized by consistently strong operating cash flows due to our robust operating history and moderate capital intensity. We have continually increased both revenues and profitability, with fiscal 2008 representing our 30th consecutive year of year-over-year net sales growth. Over the last 16 years, from fiscal 1992 to fiscal 2008, we increased net sales at compounded annual growth rate of approximately 15%. We have sustained growth through multiple macro-economic cycles, demonstrating a stable business profile. In addition, we have historically had modest capital expenditures and working capital requirements providing for strong operating cash flow conversion.
Experienced and Dedicated Management Team. We have a highly experienced management team at both the corporate and operational level. Our team is led by Jeffrey R. Binder, a 15-year veteran of the orthopedic medical device industry, who was appointed President and Chief Executive Officer in February 2007. Daniel P. Florin was appointed Senior Vice President and Chief Financial Officer in June 2007 and brings 16 years of financial officer/controller experience in the medical device industry and five years of public accounting and auditing experience to Biomet. Glen A. Kashuba was appointed Senior Vice President and President of Biomet Trauma and Biomet Spine, or BTBS, in April 2007, having previously served as Worldwide President of Cordis Endovascular, a division of Johnson & Johnson. Gregory W. Sasso, who has been with us for 23 years, was appointed Senior Vice President and President of Biomet SBU Operations in June 2007. In February 2008, Jon C. Serbousek was appointed President of Biomet Orthopedics, having spent 21 years in the medical device industry including 8 years with Medtronic and 13 years with DePuy. Even though each of Messrs. Binder, Florin, Kashuba and Serbousek has been with us for less than two years, the members of our senior management team have an average tenure of 13 years with us. Overall, the members of our senior management team have an average tenure of 18 years in the medical device industry. During fiscal 2008 certain members of our management team made a contribution of new equity through cash equity contributions and/or rollover of existing equity interests in the Transactions.
Premier Equity Sponsorship. The Blackstone Group, Goldman Sachs Capital Partners, KKR and TPG are among the most well-known and respected financial sponsors in the world. The Sponsors have made investments in over 950 companies and collectively have more than $125 billion of assets under management. The Sponsors and the Co-Investors (as defined below) contributed approximately $5,387 million of equity in connection with the Transactions, representing 46% of the total funding for the Transactions, as part of one of the largest private equity investments in history. The Sponsors have considerable experience in the healthcare sector with investments in companies such as Accellent Inc., HCA Inc., IASIS Healthcare Corporation, Quintiles Transnational Corp., ReAble Therapeutics, Inc. and Vanguard Health Systems, Inc., among others.
Business Strategy
We intend to enhance our position as a leading orthopedic medical device company by pursuing the following strategic initiatives:
Continue to Develop and Launch New Products and Technologies. We plan to continue to aggressively develop new products, technologies and materials by leveraging our established research and development platform. While we have a strong engineering heritage, we recently have taken steps to enhance our research and development efforts, with the appointment of two global heads charged with coordinating research and development efforts across the organization, which should improve time to market and leverage best technologies and innovations available throughout all business segments and regions. We anticipate that our future research and development investment will be consistent with historical results as a percentage of net sales.
Enhance Surgeon Customer Relationships through Product Performance and Innovation. We intend to continue to meet the demanding needs of our surgeon customers and hospital customers by providing clinically superior and innovative products that offer a cost-effective means of treating patients. Our success has been built on responsiveness to the needs of the health care community, the outstanding clinical performance of our products and our ongoing commitment to continued product innovation.
Expand Our Global Reach. We intend to continue to increase the geographic presence of each of our business categories. There are considerable opportunities for global expansion as healthcare spending increases in international markets—the United States and Canada together accounted for approximately 65% of the global orthopedic market in 2007, but only approximately 5% of the world’s population. We particularly plan to focus on deepening our position in under-penetrated regions with attractive opportunities for growth, including Asia and Latin America, by deploying more resources to capture market opportunities, as well as by leveraging our established worldwide manufacturing facilities and sales force. We believe we can successfully grow our presence in these regions by differentiating ourselves as a provider with a comprehensive portfolio of leading musculoskeletal products.
Focus on Operational Efficiency. We have identified significant opportunities to streamline operations. The historically decentralized nature of our management and decision-making structure creates opportunities to improve operational efficiency as we centralize operations and increase focus, coordination and accountability throughout the organization. Plans include manufacturing footprint optimization, implementation of Six Sigma and Lean Manufacturing, procurement and offshoring initiatives, as well as reduction in overhead expenses. These initiatives will enable us to maximize asset utilization, optimize working capital and increase cash flow, as well as accelerate product development and enhance customer service.
Maximize Operating Cash Flow. We are focused on maximizing our operating cash flow. Over the last 20 years, we have consistently generated significant operating cash flow due to our business growth, strong operating margins and modest capital expenditure and other cash requirements. These solid business fundamentals will be supplemented by recently implemented
3
Table of Contents
initiatives to improve working capital, which historically has not been a focus area of management. In addition, we will benefit from identified cost savings as we enhance operational efficiencies. We plan to use available cash after capital expenditures to reduce leverage and strengthen our balance sheet.
Corporate Information
Biomet is incorporated in the state of Indiana. Our principal executive offices are located at 56 East Bell Drive, Warsaw, Indiana 46581. Our website address is www.biomet.com. The information on our website is not deemed to be part of this prospectus. For additional information, contact our Corporate Communications department at (574) 372-1514.
The Transactions
On December 18, 2006, we entered into an Agreement and Plan of Merger with LVB Acquisition, Inc., or Parent, and LVB Acquisition Merger Sub, Inc., or Purchaser, which agreement was amended and restated as of June 7, 2007 (as may be amended and restated, supplemented or otherwise modified from time to time, the “Merger Agreement”). Pursuant to the Merger Agreement, on June 13, 2007, Purchaser commenced a cash tender offer, or the Offer, to purchase all of our outstanding common shares, without par value, or the Shares, at a price of $46.00 per Share, or the Offer Price, without interest and less any required withholding taxes. The Offer was made pursuant to Purchaser’s offer to purchase dated June 13, 2007 and the related letter of transmittal, each of which was filed with the SEC on June 13, 2007. The Offer expired at 12:00 midnight, New York City time, on July 11, 2007, with approximately 82% of the outstanding Shares having been tendered to Purchaser. At our special meeting of shareholders held on September 5, 2007, more than 91% of our shareholders voted to approve the Merger, and Parent acquired us on September 25, 2007 through a reverse subsidiary merger with Biomet, Inc. being the surviving company. Subsequent to the acquisition, we became a subsidiary of our Parent, which is controlled by LVB Acquisition Holding, LLC, or Holding, an entity controlled by the Sponsors and their Co-Investors.
The Merger was completed on September 25, 2007 and was financed through:
| • | the proceeds from the initial offering of the original notes; |
| • | initial borrowings under our senior secured credit facilities and our senior unsecured bridge facilities; |
| • | equity investments funded by direct and indirect equity investments from certain investment funds associated with or designated by the Sponsors, or the Sponsor Funds, certain investors who have agreed to co-invest with the Sponsor Funds, including investment funds affiliated with certain of the initial purchasers of the original notes, or the Co-Investors, and certain of our executive officers and members of our senior management, or the Management Participants, who rolled over existing equity interests and/or made cash equity contributions; and |
| • | our cash on hand. |
On October 16, 2007, the borrowings under our senior unsecured cash pay bridge facility, our senior unsecured PIK-option bridge facility and our senior subordinated unsecured bridge facility were repaid with the proceeds from the follow-on offering of the equal amounts of the additional original senior cash pay notes, original senior toggle notes and original senior subordinated notes, respectively.
We refer to these transactions, including the Merger and our payment of any fees and expenses related to these transactions, collectively as the “Transactions.” See “Description of Other Indebtedness” for a description of our senior secured credit facilities.
In connection with the Transactions, we incurred significant indebtedness and became highly leveraged. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Liquidity and Capital Resources.” In addition, we allocated the purchase price to the fair value of the assets and liabilities of Biomet based on estimated fair value. The preliminary purchase accounting adjustments increased the carrying value of our property and equipment, inventory and established intangible assets (such as corporate and product names, core and completed technology, customer relationships), among other things. Subsequent to the Transactions, interest expense and non-cash depreciation and amortization charges have significantly increased. As a result, our successor financial statements subsequent to the Transactions are not comparable to our predecessor financial statements.
4
Table of Contents
Ownership and Corporate Structure
The following chart illustrates our ownership and corporate structure after giving effect to the Transactions.
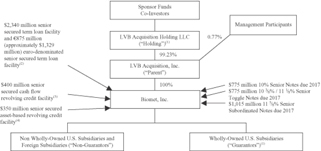
| (1) | The guarantors provide unsecured guarantees of the notes as well as guarantees of and pledges of assets under our senior secured cash flow facilities. The guarantors are co-borrowers and provide pledges of assets under our senior secured asset-based revolving credit facility. Parent guarantees and pledges its assets under our senior secured cash flow facilities and our senior secured asset-based revolving credit facility, in each case as described in more detail under “Description of Other Indebtedness.” |
| (2) | On September 25, 2007, we entered into a $2,340 million U.S. dollar-denominated senior secured term loan facility and a €875 million (approximately $1,329 million) euro-denominated senior secured term loan facility, each with a seven and a half-year maturity. We borrowed the full amount available under our senior secured term loan facilities at the closing of the Transactions to pay a portion of the Transactions. In the third quarter of fiscal 2008, we repaid $6 million of outstanding loans under our U.S. dollar-denominated senior secured term loan facility and $3 million of outstanding loans under our euro-denominated senior secured term loan facility. |
| (3) | On September 25, 2007, we entered into a $400 million senior secured cash flow revolving credit facility with a six-year maturity. We borrowed approximately $131 million under our senior secured cash flow revolving credit facility on or about the closing date of the Transactions to pay a portion of the Transactions. As of November 30, 2008, we had $3,582 million outstanding borrowings under our senior secured cash flow revolving credit facility. |
| (4) | On September 25, 2007, we entered into a $350 million senior secured asset-based revolving credit facility with a six-year maturity. As of November 30, 2008, the borrowing base under our senior secured asset-based revolving credit facility was $321 million. There were borrowings under the asset-based revolver of $165.4 million as of November 30, 2008. |
The Sponsors
The Blackstone Group
The Blackstone Group is a leading global alternative asset manager and provider of financial advisory services. Its alternative asset management businesses include the management of corporate private equity funds, real estate opportunity funds, funds of hedge funds, mezzanine funds, senior debt funds, proprietary hedge funds and closed-end mutual funds. The Blackstone Group also provides various financial advisory services, including mergers and acquisitions advisory, restructuring and reorganization advisory and fund placement services. Its website address ishttp://www.blackstone.com. The information on this website is not deemed part of this prospectus.
Goldman Sachs Capital Partners
Founded in 1869, Goldman, Sachs & Co. is one of the oldest and largest investment banking firms. Goldman Sachs is also a global leader in private corporate equity and mezzanine investing. Established in 1991, the Goldman Sachs Capital Partners family of funds is part of the firm’s Principal Investment Area in the Merchant Banking Division. Goldman Sachs’ Principal Investment Area has formed 14 investment vehicles aggregating $72 billion of capital to date. Significant investments include: ARAMARK, Burger King, CVR Energy, Inc., Education Management Corporation, Hawker Beechcraft, HealthMarkets, Kabel Deutschland, Knight Inc. (formerly known as Kinder Morgan), Polo Ralph Lauren, Prysmian Cables & Systems, VoiceStream Wireless, and YES Network. GS Capital Partners VI is the current primary investment vehicle for Goldman Sachs to make large, privately negotiated equity investments.
KKR
Established in 1976, KKR is a leading global alternative asset manager. The core of the firm’s franchise is sponsoring and managing funds that make private equity investments in North America, Europe, and Asia. Throughout its history, KKR has brought a long-term investment approach to portfolio companies, focusing on working in partnership with management teams and investing for future competitiveness and growth. The firm’s sponsored funds include KKR Private Equity Investors, L.P. (Euronext Amsterdam: KPE), a permanent capital fund that invests in KKR-identified investments; and two credit strategy funds, KKR Financial (NYSE: KFN) and the KKR Strategic Capital Funds, which make investments in debt transactions. KKR has offices in New York, Menlo Park, San Francisco, London, Paris, Hong Kong, Tokyo, Sydney and Beijing.
5
Table of Contents
TPG Capital
TPG Capital is the global buyout group of TPG, a leading private investment firm founded in 1992, with more than $50 billion of assets under management and offices in San Francisco, London, Hong Kong, New York, Minneapolis, Fort Worth, Melbourne, Menlo Park, Moscow, Mumbai, Beijing, Shanghai, Singapore and Tokyo. TPG Capital has extensive experience with global public and private investments executed through leveraged buyouts, recapitalizations, spinouts, joint ventures and restructurings. TPG Capital’s investments span a variety of industries including healthcare, retail/consumer, travel, media and communications, industrials, technology and financial services. Its website address ishttp://www.tpg.com. The information on this website is not deemed part of this prospectus.
6
Table of Contents
Summary of the Terms of the Notes
The following summary contains basic information about the notes and is not intended to be complete. It does not contain all the information that is important to you. For a more complete understanding of the notes, you should read the section of the prospectus entitled “Description of Senior Notes” and “Description of Senior Subordinated Notes.” For purposes of this summary and the “Description of Senior Notes and “Description of Senior Subordinated Notes,” references to “the Company,” “Biomet,” “the issuer,” “we,” “our” and “us” refer only to Biomet, Inc. and not to its subsidiaries.
Issuer | Biomet, Inc. |
Notes Offered
Senior Cash Pay Notes | $775 million in aggregate principal amount of 10% Senior Notes due 2017. |
Senior Toggle Notes | $775 million in aggregate principal amount of 10 3/8%/11 1/8% Senior Toggle Notes due 2017. |
Senior SubordinatedNotes | $1,015 million in aggregate principal amount of 11 5/8% Senior Subordinated Notes due 2017. |
Maturity Dates | The notes will mature on October 15, 2017. |
Interest Rate | Interest on the senior cash pay notes is payable in cash and accrues at a rate of 10% per annum. |
Cash interest on the senior toggle notes accrues at a rate of 10 3/8% per annum, and PIK interest will accrue at a rate of 11 1/8% per annum. The initial interest payment on the senior toggle notes will be payable in cash. For any interest period thereafter through October 15, 2012, we may elect to pay interest on the senior toggle notes (1) entirely in cash, (2) entirely by increasing the principal amount of the toggle notes or issuing new toggle notes, or PIK interest, or (3) 50% in cash interest and 50% in PIK interest. After October 15, 2012, all interest on the senior toggle notes will be payable in cash. If we elect to pay PIK interest, we will increase the principal amount of the senior toggle notes or issue senior toggle notes in an amount equal to the amount of PIK interest for the applicable interest payment period to holders of the senior toggle notes on the relevant record date.
Interest on the senior subordinated notes is payable in cash and accrues at a rate of 11 5/8% per annum.
Interest Payment Dates | April 15 and October 15, commencing April 15, 2008. |
Guarantees | Each of our existing and future wholly-owned domestic restricted subsidiaries has jointly, severally and unconditionally guaranteed the senior notes on a senior unsecured basis and the senior subordinated notes on a senior subordinated unsecured basis, in each case to the extent such subsidiaries guarantee our senior secured cash flow facilities. |
Ranking | The senior notes and the related guarantees are our and the guarantors’ general unsecured senior indebtedness and: |
| • | rank equally in right of payment to all of our and the guarantors’ existing and future indebtedness and other obligations that are not, by their terms, expressly subordinated in right of payment to the senior notes and the related guarantees (including borrowings under our senior secured credit facilities); |
7
Table of Contents
| • | are senior in right of payment to any of our and the guarantors’ existing and future senior subordinated and subordinated indebtedness and other obligations (including the senior subordinated notes and the related guarantees) that are, by their terms, expressly subordinated in right of payment to the senior notes and the related guarantees; and |
| • | are effectively subordinated to all of our and the subsidiary guarantors’ existing and future senior secured indebtedness and other obligations (including borrowings under our senior secured credit facilities) to the extent of the value of the assets securing such indebtedness and other obligations. |
The senior subordinated notes and the related guarantees are our and the guarantors’ general unsecured senior subordinated indebtedness and:
| • | rank junior in right of payment to any of our and the guarantors’ existing and future senior indebtedness and other obligations (including the senior notes and the related guarantees and borrowings under our senior secured credit facilities); |
| • | rank equally in right of payment to all of our and the guarantors’ existing and future senior subordinated indebtedness and other obligations; and |
| • | are senior in right of payment to any of our and the guarantors’ existing and future subordinated indebtedness and other obligations that are, by their terms, expressly subordinated in right of payment to the senior subordinated notes and the related guarantees. |
As of November 30, 2008, we and the guarantors have $3,582 million of senior secured indebtedness outstanding, consisting of borrowings and the related guarantees under our senior secured credit facilities. As of November 30, 2008, we also had:
| • | an additional approximately $400 million of borrowing capacity under our senior secured cash flow revolving facility, which, if borrowed, would be senior secured indebtedness; |
| • | an additional $156 million available for borrowing under our senior secured asset-based revolving credit facility, subject to borrowing base limitations, which, if borrowed, would be senior secured indebtedness; |
| • | the option to raise incremental term loans or increase the cash flow revolving credit facility commitments under our senior secured cash flow facilities of up to an amount that would cause our Senior Secured Leverage Ratio (as defined in our senior secured cash flow facilities) to be equal to or less than 4.50 to 1.00, which, if borrowed, would be senior secured indebtedness; and |
| • | the option to increase the asset-based revolving credit facility commitments under our senior secured asset-based revolving credit facility by up to $100 million, which, if borrowed, would be senior secured indebtedness. |
Optional Redemption | We may redeem the notes, in whole or in part, at any time prior to October 15, 2012 at a price equal to 100% of the aggregate principal amount of the notes plus the applicable “make whole” premium as described in “Description of Senior Notes—Optional Redemption” or in “Description of Senior Subordinated Notes—Optional Redemption,” plus accrued and unpaid interest, if any, to the applicable redemption date. |
We may redeem the notes, in whole or in part, at any time on or after October 15, 2012, at the applicable redemption price specified in “Description of Senior Notes—Optional Redemption” or in “Description of Senior Subordinated Notes—Optional Redemption,” in each case, plus accrued and unpaid interest, if any, to the applicable redemption date.
In addition, we may redeem up to 35% aggregate principal amount of the notes at any time prior to October 15, 2010, with the net cash proceeds from certain equity offerings at the applicable redemption price specified in “Description of Senior Notes—Optional Redemption” or in “Description of Senior Subordinated Notes—Optional Redemption,” in each case, plus accrued and unpaid interest, if any, to the applicable redemption date.
8
Table of Contents
Change of Control | If we experience specific kinds of changes of control, we must offer to repurchase all of the notes at 101% of their principal amount, plus accrued and unpaid interest, if any, to the repurchase date. |
Certain Covenants | The indentures governing the notes, among other things, limit our ability and the ability of our subsidiaries to: |
| • | incur or guarantee additional indebtedness; |
| • | incur liens; |
| • | pay dividends on or make distributions in respect of our capital stock or make other restricted payments; |
| • | make investments; |
| • | consolidate, merge, sell or otherwise dispose of certain assets; and |
| • | enter into transactions with our affiliates. |
These covenants are subject to important exceptions, limitations and qualifications as described in “Description of Senior Notes—Certain Covenants” and “Description of Senior Subordinated Notes—Certain Covenants.”
Risk Factors | See “Risk Factors” and the other information in this prospectus for a discussion of some of the factors you should carefully consider before investing in the notes. |
9
Table of Contents
Summary Historical Consolidated and
Unaudited Pro Forma Condensed Consolidated Financial and Other Data
The following table presents our summary historical and pro forma financial information as of and for the periods presented. The summary historical financial information as of May 31, 2008 and for the periods from June 1, 2007 through July 11, 2007 and from July 12, 2007 through May 31, 2008 have been derived from, and should be read in conjunction with, our audited financial statements included elsewhere in this prospectus. The unaudited summary historical financial information as of and for the six months ended November 30, 2008 and for the period from July 12, 2007 through November 30, 2007 are derived from, and should be read in conjunction with, our unaudited condensed consolidated financial statements included elsewhere in this prospectus, and, except as otherwise described herein, have been prepared on a basis consistent with our annual audited financial statements and, in the opinion of management, include all adjustments consisting of normal recurring accruals considered necessary for a fair presentation of such data. Certain amounts recorded in previous periods have been reclassified to conform to the current presentation.
The Offer for Biomet’s Shares was completed successfully on July 11, 2007. Although Biomet continues as the same legal entity after the Merger, Holding’s cost of acquiring Biomet has been pushed-down to establish a new accounting basis for Biomet. Accordingly, the financial information in the table below for the six months ended November 30, 2007 is presented separately for the period prior to the completion of the Offer (from June 1, 2007 through July 11, 2007, the “Predecessor” or “Predecessor Period”) and the period after the completion of the Offer (from July 12, 2007 through November 30, 2007 and July 12, 2007 through May 31, 2008, the “Successor” or “Successor Periods”), which relate to the accounting periods preceding and succeeding the completion of the Offer. The summary financial information as of and for the Successor Periods are not comparative to the summary financial information for the period June 1, 2007 to July 11, 2007 because of the new basis of accounting resulting from the Merger. Our results of operations for the Predecessor Period and the Successor Periods should not be considered representative of our future results of operations.
In addition, as noted in Note 2 to the audited financial statements included elsewhere in this prospectus, the summary historical financial information for the year ended May 31, 2007 has been prepared on the basis of an April 30 fiscal year for our foreign subsidiaries for financial reporting purposes. Subsequent to the completion of the Offer, we eliminated this one-month lag at our foreign subsidiaries, and therefore, the summary historical financial information as of and for the year ended May 31, 2007 is not comparative to the summary financial information as of and for the Successor Periods, which includes all periods presented subsequent to July 11, 2007, due to the elimination of this one-month lag for financial purposes at our foreign subsidiaries.
The summary unaudited pro forma condensed consolidated statements of operations for the year ended May 31, 2008 is based on our audited financial statements appearing elsewhere in this prospectus and gives effect to the Transactions as if they had occurred on June 1, 2007. See “The Transactions.” The unaudited pro forma condensed consolidated statements of operations should not be considered representative of our future results of operations.
Please refer to “Unaudited Pro Forma Condensed Consolidated Financial Data,” “Selected Historical Consolidated Financial and Other Data,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our financial statements and notes thereto included elsewhere in this prospectus.
| Predecessor | Successor | Successor | Pro Forma | Successor | ||||||||||||||||||
| ($ in millions) | June 1, 2007 through July 11, 2007 | July 12, 2007 through November 30, 2007 | July 12, 2007 through May 31, 2008 | Fiscal Year Ended May 31, 2008 | Six Months Ended November 30, 2008 | |||||||||||||||||
Statements of Operations Data: | ||||||||||||||||||||||
Net sales | $ | 249 | $ | 1,499 | $ | 2,135 | $ | 2,383 | $ | 1,250 | ||||||||||||
Cost of sales | 102 | 614 | 815 | 917 | 377 | |||||||||||||||||
Gross margin | 147 | 885 | 1,320 | 1,466 | 873 | |||||||||||||||||
Selling, general and administrative expense | 194 | 834 | 1098 | 1292 | 508 | |||||||||||||||||
Research and development expense | 34 | 59 | 82 | 116 | 47 | |||||||||||||||||
In-process research and development | — | 479 | 479 | 479 | — | |||||||||||||||||
Amortization | 1 | 227 | 329 | 329.8 | 181 | |||||||||||||||||
Operating income (loss) | (82 | ) | (713 | ) | (668 | ) | (668 | ) | 137 | |||||||||||||
Other income (loss), net | — | (1 | ) | (10 | ) | (9 | ) | (21 | ) | |||||||||||||
Interest expense | — | (372 | ) | (516 | ) | (517 | ) | (280 | ) | |||||||||||||
Income (loss) before income taxes | (82 | ) | (1,086 | ) | (1,194 | ) | (1,276 | ) | (164 | ) | ||||||||||||
Provision (benefit) for income taxes | (27 | ) | (213 | ) | (230 | ) | (257 | (64 | ) | |||||||||||||
Net income (loss) | $ | (55 | ) | $ | (873 | ) | (964 | ) | $ | (1,019 | ) | $ | (100 | ) | ||||||||
10
Table of Contents
| Predecessor | Successor | ||||||||||
| ($ in millions) | Fiscal Year as of May 31, 2007 | Fiscal Year as of May 31, 2008 | Six Months as of November 30, 2008 | ||||||||
| (unaudited) | |||||||||||
Balance Sheet Data (at period end): | |||||||||||
Cash and cash equivalents | $ | 105 | $ | 128 | $ | 97 | |||||
Total current assets | 1,452 | 1,350 | 1,421 | ||||||||
Total assets | 2,458 | 13,782 | 13,602 | ||||||||
Short-term borrowings | 82 | 75 | 42 | ||||||||
Total current liabilities | 346 | 565 | 608 | ||||||||
Total liabilities | 409 | 8,946 | 9,156 | ||||||||
Total shareholders’ equity | $ | 2,049 | $ | 4,836 | $ | 4,446 | |||||
| Predecessor | Successor | Successor | Pro Forma | Successor | |||||||||||||||||
| ($ in millions) | June 1, 2007 through July 11, 2007 | July 12, 2007 through November 2007 | July 12, 2007 through May 31, 2008 | Fiscal Year Ended May 31, 2008 | Six Months Ended November 30, 2008 | ||||||||||||||||
Statements of Cash Flows Data: | |||||||||||||||||||||
Net cash (used in) provided by: | |||||||||||||||||||||
Operating activities | $ | 60 | $ | 1 | $ | 182 | 248.3 | $ | 48 | ||||||||||||
Investing activities | 11 | (11,620 | ) | (11,722 | ) | (11,711 | ) | (95 | ) | ||||||||||||
Financing activities | 1 | 11,525 | 11,489 | 11,483 | 157 | ||||||||||||||||
Other Financial Data: | |||||||||||||||||||||
Depreciation and amortization | $ | 9 | $ | 195 | $ | 472 | 470 | 261 | |||||||||||||
Capital expenditures | (22 | ) | (77 | ) | (168 | ) | (190 | ) | (93 | ) | |||||||||||
Ratio of earnings to fixed charges(1) | — | — | — | — | — | ||||||||||||||||
| (1) | For purposes of computing the ratio of earnings to fixed charges, “earnings” consist of operating income plus other income plus cash dividends received from equity interests, less the equity income recorded. Fixed charges consist of interest expense, including amortization of debt issuance costs and interest capitalized. The interest portion of rental expense is not significant. On a pro forma basis, earnings were inadequate to cover fixed charges for fiscal 2008 by $1,276 million, and on a reported basis, the period from July 12, 2007 through November 30, , from July 12, 2007 through May 31, 2008, and the six months ended November 30, 2008 by $937 million $1,194 million, and $164 million, respectively. Earnings were also inadequate to cover fixed charges for the period from June 1, 2007 through July 11, 2007 by $82 million. |
11
Table of Contents
You should carefully consider the risks described below before investing in the notes. The risks described below are not the only ones facing our company. Additional risks and uncertainties not currently known to us or that we currently deem to be immaterial may also materially and adversely affect our business or results of operations in the future. Any of the following risks could materially adversely affect our business, financial condition or results of operations. In such case, you may lose all or part of your original investment in the notes.
Risks Related to Our Indebtedness and the Notes
Our substantial level of indebtedness could materially adversely affect our ability to generate sufficient cash to fulfill our obligations under the notes, our ability to react to changes in our business and our ability to incur additional indebtedness to fund future needs.
We are highly leveraged. As of November 30, 2008, we had total indebtedness of approximately $6,197 million. The following chart shows our level of indebtedness as of November 30, 2008:
($ in millions) | |||
European Facilities | $ | 45 | |
Senior secured term loan facilities | 3,416 | ||
Senior secured cash flow revolving credit facility | — | ||
Senior secured asset-based revolving credit facility | 165 | ||
Senior cash pay notes | 775 | ||
Senior toggle notes | 775 | ||
Senior subordinated notes | 1,015 | ||
Premium on debt | 5 | ||
Total | $ | 6,196 | |
As of November 30, 2008, we had outstanding approximately $3,582 million in aggregate principal amount of indebtedness under our senior secured credit facilities that would bear interest at a floating rate. Biomet entered into a series of interest rate swap agreements to fix the interest rates on approximately 70% of the borrowings under our senior secured credit facilities. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Quantitative and Qualitative Disclosures about Market Risk—Interest Rate Risk.” An increase of 0.125% in these floating rates would increase our annual interest expense on the borrowings that are not subject to the interest rate swap agreements by approximately $1.4 million. See “Unaudited Pro Forma Condensed Consolidated Financial Data.”
Our substantial level of indebtedness increases the possibility that we may be unable to generate cash sufficient to pay, when due, the principal of, interest on or other amounts due in respect of our indebtedness. Our substantial indebtedness, combined with our other financial obligations and contractual commitments, could have important consequences for our noteholders. For example, it could:
| • | make it more difficult for us to satisfy our obligations with respect to our indebtedness, including the notes, and any failure to comply with the obligations under any of our debt instruments, including restrictive covenants, could result in an event of default under the indentures governing the notes and the agreements governing such other indebtedness; |
| • | require us to dedicate a substantial portion of our cash flow from operations to payments on our indebtedness, thereby reducing funds available for working capital, capital expenditures, acquisitions, research and development and other purposes; |
| • | increase our vulnerability to adverse economic and industry conditions, which could place us at a competitive disadvantage compared to our competitors that have relatively less indebtedness; |
| • | increase the risk we assess with our counterparties which could affect the fair value of our derivative instruments related to our debt facilities noted above; |
| • | limit our flexibility in planning for, or reacting to, changes in our business and the industries in which we operate; |
| • | limit our noteholders’ rights to receive payments under the notes if secured creditors have not been paid; |
| • | limit our ability to borrow additional funds, or to dispose of assets to raise funds, if needed, for working capital, capital expenditures, acquisitions, research and development and other corporate purposes; and |
| • | prevent us from raising the funds necessary to repurchase all notes tendered to us upon the occurrence of certain changes of control, which would constitute a default under the indentures governing the notes. |
Restrictions imposed by the indentures governing the notes, our senior secured credit facilities and our other outstanding indebtedness may limit our ability to operate our business and to finance our future operations or capital needs or to engage in other business activities.
The terms of our senior secured credit facilities and the indentures governing the notes restrict us and our subsidiaries from engaging in specified types of transactions. These covenants restrict our and our restricted subsidiaries’ ability, among other things, to:
| • | incur additional indebtedness; |
| • | pay dividends on our capital stock or redeem, repurchase or retire our capital stock or indebtedness; |
| • | make investments, loans, advances and acquisitions; |
12
Table of Contents
| • | create restrictions on the payment of dividends or other amounts to us from our restricted subsidiaries; |
| • | engage in transactions with our affiliates; |
| • | sell assets, including capital stock of our subsidiaries; |
| • | consolidate or merge; |
| • | create liens; and |
| • | enter into sale and lease-back transactions. |
In addition, although the agreements governing our senior secured credit facilities and the indentures governing the notes do not require us to comply with any financial ratio maintenance covenants, if less than $35 million (plus 10% of any increased commitments thereunder) were available under our senior secured asset-based revolving credit facility at any time, we would not be permitted to borrow any additional amounts under our senior secured asset-based revolving credit facility unless we maintain a certain pro forma ratio of (a) Consolidated EBITDA minus Capital Expenditures minus Cash Taxes to (b) Consolidated Fixed Charges (as such terms are defined in our senior secured asset-based revolving credit facility). In the event of a default under any of our senior secured credit facilities, the lenders could elect to declare all amounts outstanding under the agreements governing our senior secured credit facilities to be immediately due and payable. If the indebtedness under our senior secured credit facilities or the notes were to be accelerated, our assets may not be sufficient to repay such indebtedness in full. In particular, noteholders will be paid only if we have assets remaining after we pay amounts due on our secured indebtedness, including our senior secured credit facilities. See “Description of Other Indebtedness.”
We, including our subsidiaries, will have the ability to incur substantially more indebtedness, including senior secured indebtedness.
Subject to the restrictions in our senior secured credit facilities and the indentures governing the notes, we, including our subsidiaries, may incur significant additional indebtedness. As of November 30, 2008:
| • | we and the guarantors had approximately $400 million available for borrowing under our senior secured cash flow revolving credit facility, which, if borrowed, would be senior secured indebtedness; |
| • | we and the guarantors had $156 million available for borrowing under our senior secured asset-based revolving credit facility, subject to borrowing base limitations, which, if borrowed, would be senior secured indebtedness; |
| • | we and the guarantors have the option to incur additional incremental term loans or increase the cash flow revolving credit facility commitments under our senior secured cash flow facilities up to an amount that would cause our Senior Secured Leverage Ratio (as defined in our senior secured cash flow facilities) to be equal to or less than 4.50 to 1.00, which, if borrowed, would be senior secured indebtedness; |
| • | we and the guarantors have the option to increase the asset-based revolving credit facility commitments under our senior secured asset-based revolving credit facility by up to $100 million, which, if borrowed, would be senior secured indebtedness; and |
| • | we and the guarantors have $27 million available for borrowing under our European credit facilities. |
In addition, under the senior toggle notes, we have the option to elect to pay PIK interest for five years after the closing date for any interest period. In the event we make a PIK interest election in each period in which we are entitled to make such an election, our debt will increase by the amount of such interest.
Although the terms of our senior secured credit facilities and the indentures governing the notes contain restrictions on the incurrence of additional indebtedness, these restrictions are subject to a number of important exceptions, and indebtedness incurred in compliance with these restrictions could be substantial. If we and our restricted subsidiaries incur significant additional indebtedness, the related risks that we face could intensify.
We may not be able to generate sufficient cash to service all of our indebtedness, including the notes, and may be forced to take other actions to satisfy our obligations under our indebtedness, which may not be successful.
Our ability to make scheduled payments on or to refinance our debt obligations depends on our financial condition and operating performance, which is subject to prevailing economic and competitive conditions and to certain financial, business and other factors beyond our control. We may not be able to maintain a level of cash flows from operating activities sufficient to permit us to pay the principal, premium, if any, and interest on our indebtedness, including the notes.
If our cash flows and capital resources are insufficient to fund our debt service obligations, we may be forced to reduce or delay investments and capital expenditures, or to sell assets, seek additional capital or restructure or refinance our indebtedness, including the notes. Our ability to restructure or refinance our debt will depend on the condition of the capital markets and our financial condition at such time. Any refinancing of our debt could be at higher interest rates and may require us to comply with more onerous covenants, which could further restrict our business operations. The terms of existing or future debt instruments and the indentures governing the notes may restrict us from adopting some of these alternatives. In addition, any failure to make payments of interest and principal on our outstanding indebtedness on a timely basis would likely result in a reduction of our credit rating, which could harm our ability to incur additional indebtedness. In the absence of such operating results and resources, we could face substantial liquidity problems and might be required to dispose of material assets or operations to meet our debt service and other obligations. Our senior secured credit facilities and the indentures governing the notes restrict our ability to dispose of assets and use the proceeds from the disposition. We may not be able to consummate those dispositions or to obtain the proceeds that we could realize from them and these proceeds may not be adequate to meet any debt service obligations then due. These alternative measures may not be successful and may not permit us to meet our scheduled debt service obligations.
13
Table of Contents
Your right to receive payments on each series of notes is effectively junior to the right of lenders who have a security interest in our assets to the extent of the value of those assets.
Our obligations under the notes and our guarantors’ obligations under their guarantees of the notes are unsecured, but our obligations under our senior secured credit facilities and each guarantor’s obligations under its guarantee of our senior secured credit facilities are secured by a security interest in substantially all of our domestic tangible and intangible assets, including the stock of substantially all of our wholly-owned U.S. subsidiaries and a portion of the stock of certain of our non-U.S. subsidiaries. If we are declared bankrupt or insolvent, or if we default under our senior secured credit facilities, the lenders could declare all of the funds borrowed thereunder, together with accrued interest, immediately due and payable. If we were unable to repay such indebtedness, the lenders could foreclose on the pledged assets to the exclusion of holders of the notes, even if an event of default exists under the indentures governing the notes at such time. Furthermore, if the lenders foreclose and sell the pledged equity interests in any guarantor under the notes, then that guarantor will be released from its guarantee of the notes automatically and immediately upon such sale. In any such event, because the notes are not secured by any of our assets or the equity interests in the guarantors, it is possible that there would be no assets remaining from which your claims could be satisfied or, if any assets remained, they might be insufficient to satisfy your claims in full. See “Description of Other Indebtedness.”
As of November 30, 2008, we had:
| • | an additional approximately $400 million of borrowing capacity under our senior secured cash flow revolving facility, which, if borrowed, would be senior secured indebtedness; |
| • | an additional $156 million available for borrowing under our senior secured asset-based revolving credit facility, subject to borrowing base limitations, which, if borrowed, would be senior secured indebtedness; |
| • | the option to incur additional incremental term loans or increase the cash flow revolving credit facility commitments under our senior secured cash flow facilities of up to an amount that would cause our Senior Secured Leverage Ratio (as defined in our senior secured cash flow facilities) to be equal to or less than 4.50 to 1.00, which, if borrowed, would be senior secured indebtedness; and |
| • | the option to increase the senior secured asset-based revolving credit facility commitments under our senior secured asset-based revolving credit facility by up to $100 million, which, if borrowed, would be senior secured indebtedness. |
Repayment of our debt, including the notes, is dependent on cash flow generated by our subsidiaries.
Our subsidiaries own a significant portion of our assets and conduct a significant portion of our operations. Accordingly, repayment of our indebtedness, including the notes, is dependent, to a significant extent, on the generation of cash flow by our subsidiaries and their ability to make such cash available to us, by dividend, debt repayment or otherwise. Unless they are guarantors of the notes, our subsidiaries do not have any obligation to pay amounts due on the notes or to make funds available for that purpose. Our subsidiaries may not be able to, or may not be permitted to, make distributions to enable us to make payments in respect of our indebtedness, including the notes. Each subsidiary is a distinct legal entity and, under certain circumstances, legal and contractual restrictions may limit our ability to obtain cash from our subsidiaries. While the indentures governing the notes limit the ability of our subsidiaries to incur consensual restrictions on their ability to pay dividends or make other intercompany payments to us, these limitations are subject to certain qualifications and exceptions. In the event that we do not receive distributions from our subsidiaries, we may be unable to make required principal and interest payments on our indebtedness, including the notes.
Claims of noteholders will be structurally subordinated to claims of creditors of all our non-U.S. subsidiaries and some of our U.S. subsidiaries because they will not guarantee the notes.
The notes are not guaranteed by any of our non-U.S. subsidiaries or any of our less than wholly-owned U.S. subsidiaries. Accordingly, claims of holders of the notes will be structurally subordinated to the claims of creditors of these non-guarantor subsidiaries, including trade creditors. All obligations of our non-guarantor subsidiaries will have to be satisfied before any of the assets of such subsidiaries would be available for distribution, upon a liquidation or otherwise, to us or a guarantor of the notes.
For the periods from June 1, 2007 through July 11, 2007, from July 12, 2007 through May 31, 2008, and the six months ended November 30, 2008 our non-guarantor subsidiaries accounted for approximately $83 million, or 33% of our consolidated net sales, $1,060 million, or 50% of our consolidated net sales, and $469 million, or 38% of our consolidated net sales, for such period, respectively. As of November 30, 2008, our non-guarantor subsidiaries accounted for approximately $3,661 million, or 31% of our consolidated long-term assets. All amounts are presented after giving effect to intercompany eliminations.
The lenders under our senior secured cash flow facilities will have the discretion to release any guarantors under these facilities in a variety of circumstances, which will cause those guarantors to be released from their guarantees of the notes.
While any obligations under our senior secured cash flow facilities remain outstanding, any guarantee of the notes may be released without action by, or consent of, any holder of the notes or the trustee under the indentures governing the notes, at the discretion of lenders under our senior secured cash flow facilities, if the related guarantor is no longer a guarantor of obligations under our senior secured cash flow facilities or any other indebtedness. See “Description of Senior Notes” and “Description of Senior Subordinated Notes.” The lenders under our senior secured cash flow facilities will have the discretion to release the guarantees under our senior secured cash flow facilities in a variety of circumstances. You will not have a claim as a creditor against any subsidiary that is no longer a guarantor of the notes, and the indebtedness and other liabilities, including trade payables, whether secured or unsecured, of those subsidiaries will effectively be senior to claims of noteholders.
14
Table of Contents
Your right to receive payments on the senior subordinated notes is junior to the rights of the lenders under our senior secured credit facilities and all of our other senior debt (including the senior notes) and any of our future senior indebtedness.
The senior subordinated notes are general unsecured senior subordinated obligations that rank junior in right of payment to all of our existing and future senior indebtedness. As of November 30, 2008, we had:
| • | approximately $5,132 million of senior indebtedness outstanding (including $1,550 million in aggregate principal amount of the senior notes and $3,582 million of borrowings under our senior secured credit facilities); |
| • | an additional approximately $400 million of borrowing capacity under our senior secured cash flow revolving credit facility, which, if borrowed, would be senior indebtedness; |
| • | an additional $156 million available for borrowing under our senior secured asset-based revolving credit facility, subject to borrowing base limitations, which, if borrowed, would be senior indebtedness; |
| • | the option to incur additional incremental term loans or increase the cash flow revolving credit facility commitments under our senior secured cash flow facilities of up to an amount that would cause our Senior Secured Leverage Ratio (as defined in our senior secured cash flow facilities) to be equal to or less than 4.50 to 1.00, which, if borrowed, would be senior indebtedness; |
| • | the option to increase the asset-based revolving credit facility commitments under our senior secured asset-based revolving credit facility by up to $100 million, which, if borrowed would be senior indebtedness; and |
| • | an additional $27 million available for borrowing under our European line of credit and Japanese lines of credit, which, if borrowed, would be senior indebtedness. |
In addition, under the senior toggle notes, we will have the option to elect to pay PIK interest for five years after the closing date for any interest period other than the initial interest period. In the event we make a PIK interest election in this period in which we are entitled to make such an election, our debt will increase by the amount of such interest.
We may not pay principal, premium, if any, interest or other amounts on account of the senior subordinated notes in the event of a payment default or certain other defaults in respect of certain of our senior indebtedness, including the senior notes and borrowings under our senior secured credit facilities, unless the senior indebtedness has been paid in full or the default has been cured or waived. In addition, in the event of certain other defaults with respect to certain of our senior indebtedness, we may not be permitted to pay any amount on account of the senior subordinated notes for a designated period of time.
Because of the subordination provisions in the senior subordinated notes, in the event of our bankruptcy, liquidation or dissolution, our assets will not be available to pay obligations under the senior subordinated notes until we have made all payments in cash on our senior indebtedness. Sufficient assets may not remain after all these payments have been made to make any payments on the senior subordinated notes, including payments of principal or interest when due.
If we default on our obligations to pay our other indebtedness, we may not be able to make payments on the notes.
Any default under the agreements governing our indebtedness, including a default under our senior secured credit facilities that is not waived by the required lenders, and the remedies sought by the holders of such indebtedness, could prevent us from paying principal, premium, if any, and interest on the notes and substantially decrease the market value of the notes. If we are unable to generate sufficient cash flow and are otherwise unable to obtain funds necessary to meet required payments of principal, premium, if any, and interest on our indebtedness, or if we otherwise fail to comply with the various covenants, including financial and operating covenants in the instruments governing our indebtedness (including covenants in our senior secured credit facilities and the indentures governing the notes), we could be in default under the terms of the agreements governing such indebtedness, including our senior secured credit facilities and the indentures governing the notes. In the event of such default:
| • | the holders of such indebtedness may be able to cause all of our available cash flow to be used to pay such indebtedness and, in any event, could elect to declare all the funds borrowed thereunder to be due and payable, together with accrued and unpaid interest; |
| • | the lenders under our senior secured credit facilities could elect to terminate their commitments thereunder, cease making further loans and institute foreclosure proceedings against our assets; |
| • | we could be forced into bankruptcy or liquidation; and |
| • | the subordination provisions in the senior subordinated notes may prevent us from paying any obligation with respect to such notes. |
If our operating performance declines, we may in the future need to obtain waivers from the required lenders under our senior secured credit facilities to avoid being in default. If we breach our covenants under our senior secured credit facilities and seek a waiver, we may not be able to obtain a waiver from the required lenders. If this occurs, we would be in default under our senior secured credit facilities, the lenders could exercise their rights, as described above, and we could be forced into bankruptcy or liquidation.
We may not be able to repurchase the notes upon a change of control.
Upon the occurrence of specific kinds of change of control events, we will be required to offer to repurchase all outstanding notes at 101% of their principal amount plus accrued and unpaid interest, if any. The source of funds for any such purchase of the notes will be our available cash or cash generated from our subsidiaries’ operations or other sources, including borrowings, sales of
15
Table of Contents
assets or sales of equity. We may not be able to repurchase the notes upon a change of control because we may not have sufficient financial resources to purchase all of the notes that are tendered upon a change of control. Further, we will be contractually restricted under the terms of our senior secured credit facilities from repurchasing all of the notes tendered by holders upon a change of control. Accordingly, we may not be able to satisfy our obligations to purchase the notes unless we are able to refinance or obtain waivers under our senior secured credit facilities. Our failure to repurchase the notes upon a change of control would cause a default under the indentures governing the notes and a cross default under our senior secured credit facilities. Our senior secured credit facilities also provide that a change of control will be a default that permits lenders to accelerate the maturity of borrowings thereunder. Any of our future debt agreements may contain similar provisions.
The trading prices for the notes will be directly affected by many factors, including our credit rating.
Credit rating agencies continually revise their ratings for companies they follow. The condition of the financial and credit markets and prevailing interest rates have fluctuated in the past and are likely to fluctuate in the future. Any such fluctuation may impact the trading price of the notes. In addition, developments in our business and operations could lead to a ratings downgrade which could adversely affect the trading price of the notes, or the trading market for the notes, to the extent a trading market for the notes develops.
Federal and state fraudulent transfer laws may permit a court to void the notes and the guarantees, subordinate claims in respect of the notes and the guarantees and require noteholders to return payments received. If this occurs, you may not receive any payments on the notes.
Federal and state fraudulent transfer and conveyance statutes may apply to the issuance of the notes and the incurrence of any guarantees. Under federal bankruptcy law and comparable provisions of state fraudulent transfer or conveyance laws, which may vary from state to state, the notes or guarantees could be voided as a fraudulent transfer or conveyance if (1) we or any of the guarantors, as applicable, issued the notes or incurred the guarantees with the intent of hindering, delaying or defrauding creditors or (2) we or any of the guarantors, as applicable, received less than reasonably equivalent value or fair consideration in return for either issuing the notes or incurring the guarantees and, in the case of (2) only, one of the following is also true at the time thereof:
| • | we or any of the guarantors, as applicable, were insolvent or rendered insolvent by reason of the issuance of the notes or the incurrence of the guarantees; |
| • | the issuance of the notes or the incurrence of the guarantees left us or any of the guarantors, as applicable, with an unreasonably small amount of capital to carry on the business; |
| • | we or any of the guarantors intended to, or believed that we or such guarantor would, incur debts beyond our or such guarantor’s ability to pay such debts as they mature; or |
| • | we or any of the guarantors was a defendant in an action for money damages, or had a judgment for money damages docketed against us or such guarantor if, in either case, after final judgment, the judgment is unsatisfied. |
A court would likely find that we or a guarantor did not receive reasonably equivalent value or fair consideration for the notes or such guarantee if we or such guarantor did not substantially benefit directly or indirectly from the issuance of the notes or the applicable guarantee. As a general matter, value is given for a transfer or an obligation if, in exchange for the transfer or obligation, property is transferred or an antecedent debt is secured or satisfied. A debtor will generally not be considered to have received value in connection with a debt offering if the debtor uses the proceeds of that offering to make a dividend payment or otherwise retire or redeem equity securities issued by the debtor.
We cannot be certain as to the standards a court would use to determine whether or not we or the guarantors were solvent at the relevant time or, regardless of the standard that a court uses, that the issuance of the guarantees would not be further subordinated to our or any of our guarantors’ other debt. Generally, however, an entity would be considered insolvent if, at the time it incurred indebtedness:
| • | the sum of its debts, including contingent liabilities, was greater than the fair saleable value of all its assets; |
| • | the present fair saleable value of its assets was less than the amount that would be required to pay its probable liability on its existing debts, including contingent liabilities, as they become absolute and mature; or |
| • | it could not pay its debts as they become due. |
If a court were to find that the issuance of the notes or the incurrence of the guarantee was a fraudulent transfer or conveyance, the court could void the payment obligations under the notes or such guarantee or further subordinate the notes or such guarantee to presently existing and future indebtedness of ours or of the related guarantor, or require the holders of the notes to repay any amounts received with respect to such guarantee. In the event of a finding that a fraudulent transfer or conveyance occurred, you may not receive any repayment on the notes. Further, the voidance of the notes could result in an event of default with respect to our and our subsidiaries’ other debt that could result in acceleration of such debt.
Although each guarantee entered into by a guarantor will contain a provision intended to limit that guarantor’s liability to the maximum amount that it could incur without causing the incurrence of obligations under its guarantee to be a fraudulent transfer, this provision may not be effective to protect those guarantees from being voided under fraudulent transfer law, or may reduce that guarantor’s obligation to an amount that effectively makes its guarantee worthless.
We are indirectly owned and controlled by the Sponsors, and the Sponsors’ interests as equity holders may conflict with yours as a creditor.
We are a subsidiary of Parent and the Sponsors have the ability to control our policies and operations. The interests of the Sponsors may not in all cases be aligned with your interests. For example, if we encounter financial difficulties or are unable to pay our debts as they mature, the interests of our equity holders might conflict with your interests as a noteholder. In addition, our equity holders may have an interest in pursuing acquisitions, divestitures, financings or other transactions that, in their judgment, could
16
Table of Contents
enhance their equity investments, even though such transactions might involve risks to you as a holder of the notes. Furthermore, the Sponsors may in the future own businesses that directly or indirectly compete with us. The Sponsors also may pursue acquisition opportunities that may be complementary to our business, and as a result, those acquisition opportunities may not be available to us. For information concerning our arrangements with the Sponsors following the Transactions, see “Certain Relationships and Related Party Transactions.”
You will be required to pay U.S. federal income tax on the senior toggle notes even if we do not pay cash interest.
None of the interest payments on the senior toggle notes will be qualified stated interest for U.S. federal income tax purposes, even if we never exercise the option to pay PIK interest, because the senior toggle notes provide us with the option to pay cash interest or PIK interest for any interest payment period after the initial interest payment and prior to October 15, 2012. Consequently, the senior toggle notes will be treated as issued with original issue discount for U.S. federal income tax purposes, and U.S. holders will be required to include the original issue discount in gross income on a constant yield to maturity basis, regardless of whether interest is paid currently in cash. See “Certain Material United States Federal Income Tax Considerations.”
Risks Relating to Our Business
Our future profitability depends on the success of our principal product lines.
Sales of our reconstructive products accounted for approximately 75% of our net sales for the six months ended November 30, 2008, 74% of our pro forma net sales for fiscal 2008, and approximately 71% of our net sales for fiscal 2007. We expect sales of reconstructive products to continue to account for a significant portion of our aggregate sales. Any event adversely affecting the sale of reconstructive products may, as a result, adversely affect our business, results of operations and financial condition.
If we are unable to continue to develop and market new products and technologies in a timely manner, the demand for our products may decrease or our products could become obsolete, and our revenue and profitability may decline.
The market for our products is highly competitive and dominated by a small number of large companies. We are continually engaged in product development, research and improvement efforts. New products and line extensions of existing products represent a significant component of our growth rate. Our ability to continue to grow sales effectively depends on our capacity to keep up with existing or new products and technologies in the musculoskeletal products market. The process of obtaining regulatory approvals to market a medical device, particularly from the FDA and certain foreign governmental authorities, can be costly and time consuming and approvals and clearances might not be granted for future products on a timely basis, if at all. Delays in receipt of, or failure to obtain, approvals and clearances for future products could result in delayed realization of product revenues or in substantial additional costs which could have a material adverse effect on our business or results of operations. In addition, if our competitors’ new products and technologies reach the market before our products, they may gain a competitive advantage or render our products obsolete. See “Business—Competition” elsewhere in this prospectus for more information about our competitors. The ultimate success of our product development efforts will depend on many factors, including, but not limited to, our ability to create innovative designs and materials, provide innovative surgical techniques, accurately anticipate and meet customers’ needs, commercialize new products in a timely manner and manufacture and deliver products and instrumentation in sufficient volumes on time.
Moreover, research and development efforts may require a substantial investment of time and resources before we are adequately able to determine the commercial viability of a new product, technology, material or other innovation. Even in the event that we are able to successfully develop innovations, they may not produce revenue in excess of the costs of development and may be quickly rendered obsolete as a result of changing customer preferences or the introduction by our competitors of products embodying new technologies or features.
We and our customers are subject to substantial government regulation and compliance with these regulations can have a material adverse effect on our business.
The medical devices we design, develop, manufacture and market are subject to rigorous regulation by the FDA and numerous other federal, state and foreign governmental authorities. Overall, there appears to be a trend toward more stringent regulation throughout the world, and we do not anticipate this trend to dissipate in the near future.
In general, the development, testing, manufacture and marketing of our products are subject to extensive regulation and review by numerous governmental authorities both in the United States and abroad. The regulatory process requires the expenditure of significant time, effort and expense to bring new products to market. In addition, we are required to implement and maintain stringent reporting, labeling and record keeping procedures. The medical device industry also is subject to a myriad of complex laws governing Medicare and Medicaid reimbursement and health care fraud and abuse laws, with these laws and regulations being very complex and subject to interpretation. In many instances, the industry does not have the benefit of significant regulatory or judicial interpretation of these laws and regulations. In certain public statements, governmental authorities have taken positions on issues for which little official interpretation was previously available. Some of these positions appear to be inconsistent with common practices within the industry but have not previously been challenged.
Various federal and state agencies have become increasingly vigilant in recent years in their investigation of various business practices. Governmental and regulatory actions against us can result in various actions that could adversely impact our operations, including:
| • | the recall or seizure of products; |
| • | the suspension or revocation of the authority necessary for the production or sale of a product; |
| • | the suspension of shipments from particular manufacturing facilities; |
| • | the imposition of fines and penalties; |
17
Table of Contents
| • | the delay of our ability to introduce new products into the market; |
| • | the exclusion of our products from being reimbursed by federal and state health care programs (such as Medicare, Medicaid, Veterans Administration health programs and Civilian Health and Medical Program Uniformed Service, or CHAMPUS); and |
| • | other civil or criminal sanctions against us. |
Any of these actions, in combination or alone, or even a public announcement that we are being investigated for possible violations of these laws, could have a material adverse effect on our business, results of operations and financial condition.
In many of the foreign countries in which we market our products, we are subject to regulations affecting, among other things: clinical efficacy, product standards, packaging requirements, labeling requirements, import/ export restrictions, tariff regulations, duties and tax requirements. Many of the regulations applicable to our devices and products in these countries, such as the European Medical Devices Directive, are similar to those of the FDA. In addition, in many countries the national health or social security organizations require our products to be qualified before they can be marketed with the benefit of reimbursement eligibility. Failure to receive or delays in the receipt of, relevant foreign qualifications also could have a material adverse effect on our business, results of operations and financial condition.
As both the U.S. and foreign government regulators have become increasingly stringent, we may be subject to more rigorous regulation by governmental authorities in the future. Our products and operations are also often subject to the rules of industrial standards bodies, such as the International Standards Organization. If we fail to adequately address any of these regulations, our business will be harmed.
We, like other companies in the orthopedic industry, are involved in ongoing investigations by the U.S. Department of Justice, the results of which may adversely impact our business and results of operations.
In June 2006, we received a federal grand jury subpoena issued at the request of the U.S. Department of Justice, Antitrust Division, requesting documents for the period from January 2001 through June 2006 regarding possible violations of federal criminal law, including possible violations of the antitrust laws, relating to the manufacture and sale of orthopedic implant devices. We are aware of similar subpoenas directed to other companies in the orthopedic industry. We have cooperated and intend to continue to fully cooperate with the Department of Justice investigation. The result of this investigation may not be known for several years. However, the scope of the June 2006 subpoena was narrowed to a specific geographic region and specific product lines. It is our belief that the other orthopedic companies that received similar subpoenas have received similar guidance. It is our belief that the investigation was prompted by an unsolicited e-mail sent by a representative of one of our competitors that proposed a common pricing strategy in connection with a particular hospital. This e-mail was received by an independent sales representative of an independent distributor for Biomet Orthopedics, but it was never transmitted to us. Neither us, the independent distributor, nor the independent sales representative took any action in response to the e-mail, and we believe that no anticompetitive activity took place as a result of it. We require compliance by our employees and our independent distributors with our Code of Business Conduct and Ethics and with applicable antitrust laws. On March 26, 2008, we received a letter from a representative of the Department of Justice, Antitrust Division advising that the Department has closed its grand jury investigation of antitrust and related offenses in the orthopedic implants industry.
We have received complaints in class action lawsuits alleging violations of the Sherman Antitrust Act that raise the same antitrust issues as the U.S. Department of Justice investigation described above. The complaints also named various other companies in the orthopedic industry as defendants. These cases were consolidated under the caption In Re Orthopedic Implant Device Antitrust Litigation, Case No. 1:07-ml-9831-JDT-WTL with the United States District Court Southern District Indianapolis, Indiana Division, and on October 18, 2007 were voluntarily dismissed without prejudice.
In May 2007, we received a subpoena from the U.S. Department of Justice through the U.S. Attorney for the Southern District of West Virginia requesting documents generally relating to a certain number of products manufactured, marketed and sold by the EBI subsidiary for the period from January 1999 through the present. In June 2007, we received a second administrative subpoena from the U.S. Attorney for the Southern District of West Virginia requesting documents relating to a specific physician’s assistant. We understand that the Department of Justice is conducting a civil investigation of EBI’s sales and marketing practices relating to certain spinal products. We are fully cooperating with the request of the Department of Justice. We can make no assurances as to the time or resources that will be needed to devote to this inquiry or its final outcome.
From time to time, we have been, and may be in the future, the subject of additional investigations. If, as a result of these investigations, we are found to have violated one or more applicable laws, our business, results of operations and financial condition could be materially adversely affected. If some of our existing business practices are challenged as unlawful, we may have to change those practices, which could have a material adverse effect on our business, results of operations and financial condition.
If we fail to comply with the terms of the Deferred Prosecution Agreement or the Corporate Integrity Agreement we entered into in September 2007, our results of operation and financial condition could be materially and adversely affected.
As discussed in “Business—Legal Proceedings”, contained elsewhere in the prospectus, on September 27, 2007 we entered into a Deferred Prosecution Agreement with the U.S. Attorney’s Office for the District of New Jersey. The agreement concludes the government’s investigation into whether consulting agreements between the largest orthopedic manufacturers and orthopedic surgeons who use joint reconstruction and replacement products may have violated the federal Anti-Kickback Statute. Through the agreement, the U.S. Attorney’s Office has agreed not to prosecute Biomet, Inc. and our wholly-owned subsidiary Biomet Orthopedics, Inc. in connection with this matter, provided that we satisfy our obligations under the agreement for 18 months subsequent to September 27, 2007. The agreement calls for the appointment of an independent monitor to review our compliance with the agreement, particularly in relation to our consulting agreements. Please see “Business-Legal Proceedings”, for certain regulatory and governmental investigations the outcomes of which could have a material adverse impact on our ability to comply with the terms of the Deferred Prosecution Agreement.
18
Table of Contents
As part of the resolution of this matter, we entered into a $26.9 million settlement with the Department of Justice’s Civil Division and we also entered into a Corporate Integrity Agreement with the Office of the Inspector General of the U.S. Department of Health and Human Services, or OIG-HHS. The agreement requires us for 5 years subsequent to September 27, 2007 to continue to adhere to our Code of Business Conduct and Ethics and certain other provisions, including reporting requirements.
The Deferred Prosecution Agreement imposes a number of continuing obligations on us for the duration of the Deferred Prosecution Agreement, including the obligation not to engage in any criminal conduct, and contains provisions permitting the Department of Justice in certain circumstances in its discretion to pursue remedies against us if we have knowingly and willfully breached any material provisions of the Deferred Prosecution Agreement or engaged in criminal conduct relating to our compliance with health care laws subsequent to September 27, 2007, including excluding us from participation in federal healthcare programs and prosecution against us for violating the federal Anti-Kickback Statute, which would have a material adverse effect on our business results of operation and financial condition. Please see “Business—Legal Proceedings”, for certain ongoing regulatory and governmental investigations involving us.
Compliance with the terms of the Deferred Prosecution Agreement and Corporate Integrity Agreement requires cooperation by many employees and others and may divert substantial financial and human resources from our other business activities.
We are committed to continue to devote sufficient resources to meet our obligations under the Deferred Prosecution Agreement and Corporate Integrity Agreement. Compliance with these agreements requires substantial cooperation of our employees, distributors and sales agents and the healthcare professionals with whom they interact. These efforts not only involve expense, but also require management and other key employees to focus extensively on these matters.
The ongoing informal investigation by the United States Securities and Exchange Commission and the United States Department of Justice regarding potential violations of the Foreign Corrupt Practices Act in the sale of medical devices in a number of foreign countries by companies in the medical device industry could have a material adverse effect on our business, results of operations and financial condition.
On September 25, 2007, we received a letter from the SEC informing us that it is conducting an informal investigation regarding possible violations of the Foreign Corrupt Practices Act in the sale of medical devices in certain foreign countries by companies in the medical devices industry. The Foreign Corrupt Practices Act prohibits U.S. companies and their officers, directors, employees, shareholders acting on their behalf and agents from offering, promising, authorizing or making payments to foreign officials for the purpose of obtaining or retaining business abroad or otherwise obtaining favorable treatment and this law requires companies to maintain records which fairly and accurately reflect transactions and to maintain internal accounting controls. In many countries, hospitals and clinics are government-owned and healthcare professionals employed by such hospitals and clinics, with whom we regularly interact, may meet the definition of a foreign official for purposes of the Foreign Corrupt Practices Act. If we are found to have violated the Foreign Corrupt Practices Act, we may face sanctions including fines, criminal penalties, disgorgement of profits and suspension or debarment of our ability to contract with government agencies or receive export licenses. On November 9, 2007, we received a letter from the Department of Justice requesting any information provided to the SEC be provided to the Department of Justice on a voluntary basis. We intend to fully cooperate with both requests and we are in the process of conducting our own review relating to these matters in certain countries in which we and our distributors conduct business and have met and expect to continue to meet with the SEC and the DOJ to update them on the status of our review.
We could be subject to further governmental investigations or actions by other third parties as a result of our recent settlement with the Department of Justice and OIG-HHS.
As discussed in “Business—Government Regulation”, we are subject to various federal and state laws concerning healthcare fraud and abuse, including false claims laws and anti-kickback laws. Violations of these laws are punishable by criminal and/or civil sanctions, including, in some instances, fines, imprisonment and, within the United States, exclusion from participation in government healthcare programs, including Medicare, Medicaid and Veterans Administration (VA) health programs. These laws are administered by, among others, the U.S. Department of Justice, the Office of Inspector General of the Department of Health and Human Services and state attorneys general. Many of these agencies have increased their enforcement activities with respect to medical device manufacturers in recent years.
As discussed in “Business—Legal Proceedings”, the SEC has commenced an informal investigation into sales by us and other companies of medical devices in foreign countries. In addition, we are in the process of conducting our own review relating to these matters and are also cooperating with the U.S. Department of Justice and one state attorney general. While we believe that the pending state investigation is not likely to have a material adverse effect on our business or financial condition additional claims or investigations by private plaintiffs or other states or governmental agencies are possible. We intend to review and take appropriate actions with respect to any such investigations or proceedings; however, we cannot assure that the costs of defending or fines imposed in resolving those civil or criminal investigations or proceedings would not have a material adverse effect on our financial condition, results of operations and cash flows.
We conduct a significant amount of our sales activity outside of the United States, which subjects us to additional business risks and may cause our profitability to decline due to increased costs.
During the six months ended November 30, 2008 and fiscal 2008, we derived approximately $502 million, or 40% of our net sales, and $976 million, or 41% of our pro forma net sales, respectively, from sales of our products outside of the United States. We intend to continue to pursue growth opportunities in sales internationally, which could expose us to additional risks associated with international sales and operations. Our international operations are, and will continue to be, subject to a number of risks and potential costs, including:
| • | changes in foreign medical reimbursement policies and programs; |
| • | unexpected changes in foreign regulatory requirements; |
| • | differing local product preferences and product requirements; |
19
Table of Contents
| • | diminished protection of intellectual property in some countries outside of the United States; |
| • | trade protection measures and import or export licensing requirements; |
| • | difficulty in staffing, training and managing foreign operations; |
| • | differing legal and labor regulations; |
| • | potentially negative consequences from changes in tax laws; and |
| • | political and economic instability. |
In addition, we are subject to risks arising from currency exchange rate fluctuations, which could increase our costs and may cause our profitability to decline. The U.S. dollar value of our foreign-generated revenues varies with currency exchange rate fluctuations. Measured in local currency, the majority of our foreign-generated revenues were generated in Europe. Significant increases in the value of the U.S. dollar relative to foreign currencies could have a material adverse effect on our results of operations. Our consolidated net sales were not affected during the six months ended November 30, 2008 as a result of the impact of foreign currency translation. Our consolidated net sales were positively affected by approximately 4% during fiscal 2008 as a result of the impact of foreign currency translation.
Any of these factors may, individually or as a group, have a material adverse effect on our business, results of operations and financial condition.
We conduct manufacturing operations outside of the United States and are in the process of transitioning certain manufacturing operations to China, which will expose us to additional business risks.
In addition to our principal executive offices, we maintain more than 50 other manufacturing facilities, offices and warehouse facilities in various countries, including Canada, Europe, Asia Pacific and Latin America.
We currently conduct operations in Jinhua, Zhejiang Province, China and Changzhou, Jiangsu Province, China. Our future business strategy may involve the operation of other manufacturing facilities in China. As a result of this initiative, we will be exposed to all the risks inherent in operating in an emerging market like China where we have not previously operated a manufacturing facility. In recent years the Chinese economy has undergone various developments, including beginning the transition from a more heavily government influenced-planned economy to a more market-oriented economy. Despite this transition, the Chinese government continues to own significant production assets and exercises significant control over economic growth. Our international operations, including our planned expansion in China, may be subject to greater or new political, legal and economic risks than those faced by our operations in the United States, including such risks as those arising from:
| • | unexpected changes in foreign or domestic legal, regulatory or governmental requirements or approvals, such as those related to taxation, lending, import and tariffs, environmental regulations, land use rights, intellectual property and other matters; |
| • | unexpected increases in taxes, tariffs and other assessments; |
| • | diminished protection of intellectual property; |
| • | trade protection measures and import or export licensing requirements; |
| • | difficulty in staffing, training and managing foreign operations; |
| • | differing legal and labor regulations; |
| • | political and economic instability; and |
| • | operating in a market with a less developed supply chain, transportation and distribution infrastructure. |
Due to these inherent risks, there can be no assurance that we will achieve any anticipated benefits from transitioning its manufacturing operations to China and any of these factors may, individually or as a group, have a material adverse effect on our business, results of operations and financial condition.
Our business and financial performance may be adversely affected by our inability to effectively implement restructuring and cost saving initiatives.
Following consummation of the Merger, we commenced plans for a global cost savings program targeting pre-tax savings of $65 million on an annualized basis. The program includes the transition of certain manufacturing facilities to China, the restructuring of our domestic and international corporate structure and improvements to operating processes (including manufacturing footprint optimization, implementation of Six Sigma and Lean Manufacturing, procurement and offshoring initiatives, as well as reduction in overhead expenses). Projected costs and savings associated with these initiatives are subject to a variety of risks, including:
| • | contemplated costs to effect these initiatives may exceed estimates; |
| • | initiatives we are contemplating may require consultation with various employees, labor representatives or regulators, and such consultations may influence the timing, costs and extent of expected savings; |
| • | initiatives will also require close coordination with customers with respect to the transfer of existing business to other company locations, and certain business may not ultimately be retained as a result of possible transition of facilities; |
20
Table of Contents
| • | management changes at various strategic business units, including Biomet Trauma and Biomet Spine, may be unsuccessful in improving or stabilizing our business at those strategic business units; |
| • | the loss of skilled employees in connection with the initiatives; and |
| • | projected savings contemplated under this program may fall short of targets. |
While we have begun and expect to continue to implement these strategies, there can be no assurance that we will be able to do so successfully or that we will realize the projected benefits of these and other restructuring and cost saving initiatives. If we are unable to realize these anticipated cost reductions, our business may be adversely affected. Moreover, our continued implementation of restructuring and cost saving initiatives integration may have a material adverse effect on our business, results of operations and financial condition.
Our business may be harmed as a result of litigation.
Our involvement in the manufacture and sale of medical devices creates exposure to significant risk of product liability claims, particularly in the United States. In the past, we have received product liability claims relating to our products and anticipate that we will continue to receive claims in the future, some of which could have a material adverse impact on our business. In addition, we could experience a material design or manufacturing failure in our products, a quality system failure, other safety issues or heightened regulatory scrutiny that would warrant a recall of some of our products. Our existing product liability insurance coverage may be inadequate to satisfy liabilities we might incur. If a product liability claim or series of claims is brought against us for uninsured liabilities or is in excess of our insurance coverage limits, our business could suffer and our results could be materially adversely impacted.
In addition, the musculoskeletal products industry is highly litigious with respect to the enforcement of patents and other intellectual property rights. In some cases, intellectual property litigation may be used to gain a competitive advantage. We have in the past and may in the future become a party to lawsuits involving patents or other intellectual property. A legal proceeding, regardless of the outcome, could put pressure on our financial resources and divert the time, energy and efforts of our management.
We may be subject to intellectual property litigation and infringement claims, which could cause us to incur significant expenses or prevent us from selling our products.
A successful claim of patent or other intellectual property infringement against us could adversely affect our growth and results of operation, in some cases materially. From time to time, we receive notices from third parties of potential infringement and receive claims of potential infringement. We may be unaware of intellectual property rights of others that may cover some of our technology. If someone claims that our products infringed their intellectual property rights, any resulting litigation could be costly and time consuming and would divert the attention of management and key personnel from other business issues.
The complexity of the technology involved and the uncertainty of intellectual property litigation increase these risks. Claims of intellectual property infringement also might require us to enter into costly royalty or license agreements. However, we may be unable to obtain royalty or license agreements on terms acceptable to us or at all. We also may be subject to significant damages or an injunction preventing us from manufacturing, selling or using some of our products in the event of a successful claim of patent or other intellectual property infringement. Any of these adverse consequences could have a material adverse effect on our business, financial condition and results of operations.
If we are not able to fulfill or otherwise resolve our existing royalty and other payment obligations to consulting surgeons and institutions, our ability to maintain our existing intellectual property rights and obtain future rights may be impaired.
We are reviewing agreements we have entered into with consulting surgeons and institutions and assessing whether we continue those agreements in light of our obligations under the Deferred Prosecution Agreement. If we are not able to continue these agreements, our ability to use the intellectual property covered by those agreements may be adversely affected. In addition, our ability to enter into new agreements with consulting surgeons or institutions for the future development of intellectual property rights may be adversely affected.
Sales may decline if our customers do not receive adequate levels of reimbursement from third-party payors for our products and if certain types of healthcare programs are adopted in our key markets.
In the United States, healthcare providers that purchase our products (e.g., hospitals, physicians, dentists and other health care providers) generally rely on payments from third-party payors (principally federal Medicare, state Medicaid and private health insurance plans) to cover all or a portion of the cost of our musculoskeletal products. These third-party payors may deny reimbursement if they determine that a device used in a procedure was not in accordance with cost-effective treatment methods, as determined by the third-party payor, or was used for an unapproved indication. Third-party payors may also decline to reimburse for experimental procedures and devices. In the event that third-party payors deny coverage or reduce their current levels of reimbursement, we may be unable to sell certain products on a profitable basis, thereby materially adversely impacting our results of operations. Further, third-party payors are continuing to carefully review their coverage policies with respect to existing and new therapies and can, without notice, deny coverage for treatments that may include the use of our products.
In addition, some healthcare providers in the United States have adopted, or are considering the adoption of, a managed care system in which the providers contract to provide comprehensive healthcare for a fixed cost per person. Healthcare providers in a managed care system may attempt to control costs by authorizing fewer elective surgical procedures, including joint reconstructive surgeries, or by requiring the use of the least expensive implant available. In response to these and other pricing pressures, our competitors may lower the prices for their products. We may not be able to match the prices offered by our competitors, thereby adversely impacting our results of operations and future prospects. Further, in the event that the United States considers the adoption of a national healthcare system in which prices are controlled and patient care is managed by the government, such regulation could have a material adverse effect on our business, results of operations and financial condition.
21
Table of Contents
Outside of the United States, reimbursement systems vary significantly from country to country. In the majority of the international markets in which our products are sold, government-managed healthcare systems mandate the reimbursement rates and methods for medical devices and procedures. If adequate levels of reimbursement from third-party payors outside of the United States are not obtained, international sales of our products may decline. Many foreign markets, including Canada, and some European and Asian countries, have tightened reimbursement rates. Our ability to continue to sell certain products profitably in these markets may diminish if the government-managed healthcare systems continue to reduce reimbursement rates.
Our management has identified a material weakness in our internal controls over financial reporting and has concluded that our internal controls over financial reporting was ineffective as of May 31, 2008.
We are required to assess the effectiveness of our internal controls over financial reporting on an annual basis and to include in our annual report on Form 10-K management’s report on that assessment. If there are any material weaknesses in internal control over financial reporting that are identified, then our management is not permitted to conclude in its report that our internal control over financial reporting is effective. This assessment resulted in the identification of a material weakness in our internal controls over financial reporting. Consequently, our management has concluded that our internal controls over financial reporting were not effective as of May 31, 2008. We have identified a material weakness related to multiple control deficiencies in the order to cash process in both the design and operation of controls at one of our subsidiaries, BTBS. A material weakness is defined as a deficiency, or a combination of deficiencies, in internal controls over financial reporting, such that there is a reasonable possibility that a material misstatement of the annual or interim financial statements will not be prevented or detected on a timely basis. Our management is in the process of developing remediation steps to correct the material weakness that was identified. We cannot be certain our remediation efforts will ensure that our management designs, implements and maintains adequate controls over our financial processes and reporting in the future or will be sufficient to address and eliminate the material weakness identified. Our inability to remedy the identified material weakness or any additional deficiencies or material weaknesses could, among other things, cause accounting errors or other inaccuracies in our financial statements or could cause us to fail to file our periodic reports with the SEC in a timely manner or require us to incur additional costs or to divert management resources. Due to its inherent limitations, even effective internal control over financial reporting can provide only reasonable assurance with respect to financial statement preparation and presentations. These limitations may not prevent or detect all misstatements or fraud, regardless of their effectiveness.
The conditions of the U.S. and international capital markets may adversely affect our ability to draw on our current revolving credit facilities as well as the value of certain of our investments.
We believe that our cash, other liquid assets and operating cash flow, together with available borrowings and potential access to credit and capital markets, will be sufficient to meet our operating expenses, research and development costs and capital expenditures and service our debt requirements as they become due. However, our ongoing ability to meet our substantial debt service and other obligations will be dependent upon our future performance, which will be subject to business, financial and other factors. We will not be able to control many of these factors, such as economic conditions in the markets where we operate and pressure from competitors. We cannot be certain that our cash flow will be sufficient to allow us to pay principal and interest on our debt, support our operations and meet our other obligations. If we do not have enough money, we may be required to refinance all or part of our existing debt, sell assets or borrow more money. We cannot guarantee that we will be able to do so on terms acceptable to us, if at all. In addition, the terms of existing or future debt agreements may restrict us from pursuing any of these alternatives.
If other financial institutions that have extended credit commitments to us are adversely affected by the conditions of the U.S. and international capital markets, they may become unable to fund borrowings under their credit commitments to us, which could have a material and adverse impact on our financial condition and our ability to borrow additional funds, if needed, for working capital, capital expenditures, acquisitions, research and development and other corporate purposes.
Similarly, if the current credit conditions of U.S. and international capital markets persist or deteriorate, we may be required to further adjust the fair value of its investments pursuant to mark-to-market rules under SFAS 157, which would result in additional impairment charges and could have a material and adverse impact on our financial condition and results of operations.
The current economic uncertainties may adversely affect our results of operations.
Our results of operations could be substantially affected not only by global economic conditions, but also by local operating and economic conditions, which can vary substantially by market. Unfavorable conditions can depress sales in a given market and may result in actions that adversely affect our margins, constrain our operating flexibility or result in charges which are unusual or non-recurring. Certain macroeconomic events, such as the current crisis in the financial markets, could have a more wide-ranging and prolonged impact on the general business environment, which could also adversely affect us. For example, due to certain patient insurance deductibles restarting on January 1, 2009, it is possible that we could experience a potential adverse impact on sales due to patients deferring procedures because of decreased cash flow.
Historically, we believe that this slowdown due to the uncertain or recessionary environment has been minor to the orthopedic business, however, management is taking precautionary measures to be able to manage expenses more appropriately, if revenues were to decrease below those internally forecasted.
We are subject to cost-containment efforts of group purchasing organizations, which may have a material adverse effect on our results of operations and financial condition.
Many customers of our products have joined group purchasing organizations in an effort to contain costs. Group purchasing organizations negotiate pricing arrangements with medical supply manufacturers and distributors, and these negotiated prices are made available to a group purchasing organization’s affiliated hospitals and other members. If we are not one of the providers selected by a group purchasing organization, affiliated hospitals and other members may be less likely to purchase our products, and if the group purchasing organization has negotiated a strict compliance contract for another manufacturer’s products, we may be precluded from making sales to members of the group purchasing organization for the duration of the contractual arrangement. Our failure to respond to the cost-containment efforts of group purchasing organizations may cause us to lose market share to our competitors and could have a material adverse effect on our sales, results of operations and financial condition.
22
Table of Contents
Loss of our key management and other personnel, or an inability to attract such management and other personnel, could impact our business.
We depend on our senior managers and other key personnel to run our business and on technical experts to develop new products and technologies. The loss of any of these senior managers or other key personnel could adversely affect our operations. Competition for qualified employees is intense, and the loss of qualified employees or an inability to attract, retain and motivate additional highly skilled employees required for the management, operation and expansion of our business could hinder our ability to expand, conduct research and development activities successfully and develop marketable products.
Increased costs of retaining existing independent sales agents of our products have negatively affected our results of operations and if we fail to retain our existing relationships with these independent sales agents or establish relationships with different agents, our results of operations may be negatively impacted.
Our revenues and profitability depend largely on the ability of independent sales agents to sell our products to customers. Typically, these agents have developed long-standing relationships with our customers and provide our customers with the necessary training and product support relating to our products. The average tenure of our independent sales agents within our subsidiary Biomet Orthopedics, LLC, or Biomet Orthopedics, is nine years.
Following the announcement of the Merger Agreement, in an attempt to exploit the uncertainty related to the pending transaction, our direct competitors approached the independent sales agents we work with and offered them incentives to discontinue their existing relationships with us. In an effort to ensure the continuity of our relationships with the independent third-party distributors who represent Biomet Orthopedics, we incurred $39 million in fiscal 2007, $18 million for the period from June 1, 2007 to July 11, 2007 and $31 million for the period from July 12, 2007 to May 31, 2008, which negatively affected our results of operations for these periods. A significant amount of these expenses that were incurred in fiscal 2008 were incurred prior to the end of the first quarter of fiscal 2008. In addition, we and Biomet Orthopedics initiated legal proceedings in Marion County, Indiana against a direct competitor and certain former independent sales agents related to the foregoing. See “Business—Legal Proceedings” elsewhere in this prospectus. If we fail to retain our existing relationships with these agents or establish relationships with different agents, our results of operations may be negatively impacted.
A natural or man-made disaster could have a material adverse effect on our business.
We have approximately 21 manufacturing operations located throughout the world. However, a significant portion of our products are produced at and shipped from our facility in Warsaw, Indiana. In the event that this facility is severely damaged or destroyed as a result of a natural or man-made disaster, we would be forced to shift production to our other facilities and/or rely on third-party manufacturers. Such an event could have a material adverse effect on our business prospects, results of operations and financial condition.
Any expansion or acquisition may prove risky for us.
We may, from time to time, consider and take advantage of selected opportunities to grow by acquiring businesses whose operations or product lines fit well within our existing businesses or whose geographic location or market position would enable us to expand into new markets. Our ability to implement this expansion strategy will, however, depend on whether any suitable businesses are available at suitable valuations, how much money we can spend and maintaining our customer base. Any acquisition that we make could be subject to a number of risks, including, failing to discover liabilities of the acquired company for which we may be responsible as a successor owner or operator despite any investigation we may make before the acquisition, our ability to assimilate the operations and personnel of the acquired company, the loss of key personnel in the acquired company and any impact on our financial statements from the amortization of acquired intangible assets or the creation of reserves or write-downs. We may not be able to adequately meet these challenges, and any failure to do so could adversely affect our business, results of operations and financial condition. In addition, if we incur additional indebtedness to finance these acquisitions, the related risks we face from our already substantial level of indebtedness could intensify.
Risks Relating to the Stock Options Investigation
Our review of historical stock option granting practices and restatement of consolidated financial statements may result in future litigation or regulatory inquiries, which could harm our financial results.
On December 18, 2006 and March 30, 2007, we announced preliminary and updated reports from the Special Committee following the publication of an analyst report suggesting that certain historical stock option grants took place on dates when our stock price was trading at relatively low prices and the filing of two shareholder derivative lawsuits alleging improper “backdating” of options. Based upon the analysis of these reports and relevant accounting literature, including Staff Accounting Bulletin, or SAB, No. 99, our Audit Committee determined on March 30, 2007 that we should amend our Annual Report on Form 10-K for fiscal 2006 and our Quarterly Report on Form 10-Q for the period ended August 31, 2006 to reflect the restatement of the consolidated financial statements reflected therein (fiscal 2004, 2005 and 2006 and periods ended August 31, 2005 and 2006) and related disclosures reflected therein.
On May 25, 2007, our Board of Directors received and discussed the updated findings contained in the Special Committee’s final report, which concluded that:
| • | our written stock option plans were treated by our management, and our Compensation and Stock Option Committee, as formalities concerning the manner in which individual stock option grants were to be approved, resulting in a failure to abide by the terms of the plans; |
23
Table of Contents
| • | we failed to receive appropriate legal or accounting advice from our former general counsel and the chief financial officer related to our stock option program and, as a result, relevant legal and accounting rules were not followed; |
| • | we failed to put in place and implement internal controls to manage our stock option program, including failing to devote sufficient resources to the administration of our stock option program; |
| • | we failed to prepare and maintain appropriate books and records documenting the administration of our stock option program, specifically with regard to the approval of individual stock option grants; |
| • | most options issued by us were dated on dates other than the date of grant of those options, as that date was defined by the stock option plans; |
| • | we engaged in purposeful “opportunistic” dating (and, therefore, pricing) of options; and |
| • | as a result of these deficiencies, certain of our proxy statements were inaccurate. |
Our review of historical stock option granting practices has required us to incur additional expenses for legal, accounting, tax and other professional services, and could in the future adversely affect our business, results of operations, financial condition and cash flows, including by virtue of exposing us to greater risks associated with litigation, regulatory and other governmental proceedings. We have also incurred expenses in connection with certain corrective actions approved by our Compensation and Stock Option Committee with respect to misdated or mispriced options, including (a) payments to compensate certain former holders of options whose option exercise prices we increased to the fair market value of the shares underlying such options on the “measurement date” (as that term is defined in SFAS No. 123(R)) for the options and (b) payments to the Internal Revenue Service, or IRS, on behalf of certain option holders (and reimbursement of one of our executive officers) to cover taxes and penalties payable by such individuals as a result of their exercise of misdated or mispriced options prior to the date we amended such options to bring them into compliance with (and thereby avoid the taxes and penalties imposed under) section 409A of the Internal Revenue Code of 1986, as amended, or the Code, as well as gross-up payments to such individuals for any taxes they incur as a result of such payments. In connection with the closing of the Offer, all outstanding options to purchase Shares under our stock plans, vested or unvested, were cancelled and each option holder was paid an amount in cash equal to the excess, if any, of the Offer Price over the applicable option exercise price for each Share subject to an option, less any required withholding taxes. While we believe that we have made appropriate judgments in determining the correct measurement dates for the approximately 17,000 stock option awards in question, the SEC or other governmental agencies may disagree with the manner in which we have accounted for and reported, or not reported, the financial and other impacts of past stock option grant measurement date errors, and there is a risk that any such inquiry could lead to circumstances in which we may have to further restate our prior financial statements, amend prior SEC filings, or otherwise take other actions not currently contemplated by us. Any such circumstance could also lead to future delays in filing our subsequent SEC reports. We cannot assure you that any future litigation or regulatory action will result in the same conclusions as those reached by the Special Committee. The conduct and resolution of these matters may be time consuming, expensive and distracting from the conduct of our business. Furthermore, if we are subject to adverse findings in any of these matters, we could be required to pay damages, penalties or additional taxes or have other remedies imposed upon us, which could harm our business, results of operations, financial condition and cash flows.
We have been named as a party to a number of shareholder derivative lawsuits relating to our historical stock option grant practices, and we may be named in additional lawsuits in the future. This litigation could become time consuming and expensive and could result in the payment of significant judgments and settlements, which could have a material adverse effect on our results of operations and financial condition.
On September 21, 2006, two shareholder-derivative complaints were filed against certain of our current and former officers and directors in Kosciusko Superior Court I in Kosciusko County, in the State of Indiana. The complaints, captioned Long v. Hann, et al. , and Thorson v. Hann, et al. , alleged violations of state law relating to the issuance of certain stock option awards by Biomet dating back to 1996. Both complaints sought unspecified money damages as well as other equitable and injunctive relief. These two cases were consolidated under the caption In re Biomet, Inc. Derivative Litigation, and on January 19, 2007, plaintiffs filed an amended complaint that made additional allegations based on our December 18, 2006 disclosures related to stock option awards, including allegations that the defendants sought to sell us in order to escape liability for their conduct, and that they did so at a devalued price, thus further breaching their fiduciary duties to shareholders. On February 5, 2008, the court granted the defendants’ motion to dismiss the amended complaint. Plaintiffs appealed the district court’s decision, and on February 13, 2009, the Court of Appeals affirmed the dismissal of the case. Plaintiffs may still seek review of the Court of Appeals’ decision by the Indiana Supreme Court.
Pursuant to Indiana law and provisions of our article of incorporation, we are advancing reasonable expenses, including attorneys’ fees, incurred by our current and former directors and officers in defending these lawsuits.
On May 25, 2007, the Board of Directors received and discussed an updated report from its Special Committee, which concluded that pursuing these shareholder derivative lawsuits was not in our best interests. Under Indiana law, the Special Committee’s determination may be binding on the pending shareholder derivative lawsuits and result in dismissal of these lawsuits.
We cannot, however, predict the outcome of these current lawsuits, nor can we predict the amount of time and expense that will be required to resolve them. There may also be additional lawsuits of this nature filed in the future. Defending the current lawsuits and any additional shareholder derivative lawsuits may become time consuming and expensive, and an unfavorable outcome in any of these cases could have a material adverse effect on our business, results of operations and financial condition.
24
Table of Contents
In addition, the issues arising from our previous retroactive pricing of options may make it more difficult to obtain director and officer insurance coverage in the future. If we are able to obtain this coverage, it could be significantly more costly than in the past, which could have an adverse effect on our financial results and cash flows. As a result of this and related factors, our directors and officers could face increased risks of personal liability in connection with the performance of their duties. Consequently, we may have difficulty attracting and retaining qualified directors and officers, which could adversely affect our business.
25
Table of Contents
On December 18, 2006, we entered into the Merger Agreement with Parent and Purchaser. Pursuant to the Merger Agreement, on June 13, 2007, Purchaser commenced the Offer, to purchase all of our outstanding Shares at the Offer Price without interest and less any required withholding taxes. The Offer was made pursuant to Purchaser’s offer to purchase dated June 13, 2007 and the related letter of transmittal, each of which was filed with the SEC on June 13, 2007. The Offer expired at 12:00 midnight, New York City time, on July 11, 2007, with approximately 82% of the outstanding Shares having been tendered to Purchaser. At our special meeting of shareholders held on September 5, 2007, more than 91% of our shareholders voted to approve the Merger, and Parent acquired us on September 25, 2007 through a reverse subsidiary merger with Biomet, Inc. being the surviving company. Subsequent to the acquisition, we became a subsidiary of Parent, which is controlled by Holding, an entity controlled by the Sponsors and their Co-Investors.
The Merger was completed on September 25, 2007 and was financed through:
| • | the proceeds from the initial offering of the original notes; |
| • | initial borrowings under our senior secured credit facilities and our senior unsecured bridge facilities; |
| • | equity investments funded by direct and indirect equity investments from the Sponsor Funds, the Co-Investors, and the Management Participants, who rolled over existing equity interests and/or made cash equity contributions; and |
| • | our cash on hand. |
On October 16, 2007, the borrowings under our senior unsecured cash pay bridge facility, our senior unsecured PIK-option bridge facility and our senior subordinated unsecured bridge facility were repaid with the proceeds from the follow-on offering of the equal amounts of the additional original senior cash pay notes, original senior toggle notes and original senior subordinated notes, respectively.
We refer to these transactions, including the Merger and our payment of any fees and expenses related to these transactions, collectively as the “Transactions.” See “Description of Other Indebtedness” for a description of our senior secured credit facilities.
In connection with the Transactions, we incurred significant indebtedness and became highly leveraged. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Liquidity and Capital Resources.” In addition, we allocated the purchase price to the fair value of the assets and liabilities of Biomet based on estimated fair value. The preliminary purchase accounting adjustments increased the carrying value of our property and equipment, inventory and established intangible assets (such as corporate and product trade names, core and completed technology and customer relationships), among other things. Subsequent to the Transactions, interest expense and non-cash depreciation and amortization charges have significantly increased. As a result, our successor financial statements subsequent to the Transactions are not comparable to our predecessor financial statements.
26
Table of Contents
This prospectus is delivered in connection with the sale of notes by Goldman, Sachs & Co. and its affiliates in market-making transactions. We will not receive any of the proceeds from such transactions.
27
Table of Contents
The following table sets forth our consolidated cash, cash equivalents and investments and capitalization as of November 30, 2008. You should read the data set forth in the table below in conjunction with “The Transactions,” “Selected Historical Consolidated Financial and Other Data,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” “Description of Other Indebtedness” and our financial statements and the related notes included elsewhere in this prospectus.
| As of November 30, 2008 | |||
| ($ in millions) | |||
Cash, cash equivalents, and short-term investments | $ | 229 | |
Debt: | |||
European facilities(1) | 45 | ||
Japanese lines of credit(2) | — | ||
Senior secured credit facilities: | |||
Term loan facilities(3) | 3,416 | ||
Cash flow revolving credit facility(4) | — | ||
Asset-based revolving credit facility(5) | 165 | ||
Senior cash pay notes | 775 | ||
Senior toggle notes | 775 | ||
Senior subordinated notes | 1,015 | ||
Premium on debt | 5 | ||
Total debt | 6,196 | ||
Shareholder’s equity | 4,322 | ||
Total capitalization | $ | 10,518 | |
| (1) | We have unsecured European credit facilities in the amount of €50 million (approximately $63 million) and a revolving line in Spain. As of November 30, 2008, we had $45 million outstanding borrowings under this credit line. |
| (2) | We have two unsecured Japanese lines of credit in the amount of ¥2.5 billion (approximately $24 million). As of November 30, 2008, there were no outstanding borrowings under these credit lines. |
| (3) | On September 25, 2007, we entered into a $2,340 million U.S. dollar-denominated senior secured term loan facility and a €875 million (approximately $1,329 million) euro-denominated senior secured term loan facility, each with a seven and a half-year maturity. We borrowed the full amount available under our senior secured term loan facilities at the closing of the Transactions to pay a portion of the Transactions. From its inception, we have repaid $24 million of outstanding loans under our U.S. dollar-denominated senior secured term loan facility and $12 million of outstanding loans under our euro-denominated senior secured term loan facility. |
| (4) | On September 25, 2007, we entered into a $400 million senior secured cash flow revolving credit facility with a six-year maturity. We borrowed approximately $131 million under our senior secured cash flow revolving credit facility on or about the closing date of the Transactions to pay a portion of the Transactions. As of November 30, 2008, we had no outstanding borrowings under our senior secured cash flow revolving credit facility. |
| (5) | On September 25, 2007, we entered into a $350 million senior secured asset-based revolving credit facility with a six-year maturity. As of November 30, 2008, the borrowing base under our senior secured asset-based revolving credit facility was $321 million. As of November 30, 2008, we had $165 million of outstanding borrowings under our senior secured asset-based revolving credit facility. |
28
Table of Contents
UNAUDITED PRO FORMA CONDENSED CONSOLIDATED FINANCIAL DATA
The following unaudited pro forma condensed consolidated statements of operations have been developed by applying pro forma adjustments to the historical audited and unaudited consolidated financial statements of Biomet appearing elsewhere in this prospectus. The unaudited pro forma condensed consolidated statements of operations for the fiscal year ended May 31, 2008 gives effect to the Transactions as if they had occurred on June 1, 2007. Assumptions underlying the pro forma adjustments are described in the accompanying notes, which should be read in conjunction with these unaudited pro forma condensed consolidated statements of operations. Although Biomet continues as the same legal entity after the Merger, Holding’s cost of acquiring Biomet has been pushed-down to establish a new accounting basis for Biomet.
The unaudited pro forma adjustments are based upon available information and certain assumptions that we believe are reasonable under the circumstances. The unaudited pro forma condensed consolidated financial data is presented for informational purposes only. The unaudited pro forma condensed consolidated financial data does not purport to represent what our results of operations would have been had the Transactions actually occurred on the dates indicated and they do not purport to project our results of operations for any future period. The unaudited pro forma condensed consolidated financial statements should be read in conjunction with the information contained in the “The Transactions,” “Selected Historical Consolidated Financial and Other Data,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our historical audited and unaudited consolidated financial statements and related notes thereto appearing elsewhere in this prospectus. All pro forma adjustments and their underlying assumptions are described more fully in the notes to our unaudited pro forma condensed consolidated statements of operations.
The Transactions are being accounted for using the purchase method of accounting. The pro forma information presented, including allocations of the purchase price, is based on a determination of the fair values of assets acquired and liabilities assumed, reflecting our consideration of expected future cash flows, market data and comparables, and related tax effects.
Unaudited Pro Forma Condensed Consolidated Statements of Operations
for Fiscal Year Ended May 31, 2008
| June 1, 2007 through July 11, 2007 (Predecessor) | July 12, 2007 through May 31, 2008 (Successor) | Pro Forma Adjustments(a) | Pro Forma Biomet | |||||||||||||||
| ($ in millions) | ||||||||||||||||||
Net sales | $ | 248.8 | $ | 2,134.5 | $ | — | $ | 2,383.3 | ||||||||||
Cost of sales | 102.3 | 814.7 | (186.5 | )(a)(b) | 730.5 | |||||||||||||
Gross margin | 146.5 | 1,319.8 | 186.5 | 1,652.8 | ||||||||||||||
Selling, general and administrative expenses | 194.2 | 1,097.6 | (353.0 | )(a) | 938.8 | |||||||||||||
Research and development expense | 34.0 | 82.2 | (23.0 | )(a) | 93.2 | |||||||||||||
In-process research and development | — | 479.0 | (479.0 | )(a) | — | |||||||||||||
Amortization | 0.5 | 329.3 | 40.1 | (b) | 369.4 | |||||||||||||
Operating income | (82.2 | ) | (668.3 | ) | 1,001.4 | 250.9 | ||||||||||||
Interest expense | (0.3 | ) | (516.3 | ) | (73.3 | )(c) | (589.9 | ) | ||||||||||
Other income (loss), net | 0.6 | (9.7 | ) | — | (9.1 | ) | ||||||||||||
Income (loss) before income taxes | (81.9 | ) | (1,194.3 | ) | 928.1 | (348.1 | ) | |||||||||||
Provision (benefit) for income taxes | (27.3 | ) | (230.1 | ) | 138.8 | (d) | (118.6 | ) | ||||||||||
Net income (loss) | $ | (54.6 | ) | $ | (964.2 | ) | $ | 789.3 | $ | (229.5 | ) | |||||||
See Accompanying Notes to Unaudited Pro Forma Condensed Consolidated Statements of Operations.
29
Table of Contents
Notes to Unaudited Pro Forma Condensed Consolidated Statements of Operations
| (a) | As a result of the Transactions, we recorded certain expenses that have not been included in the pro forma condensed consolidated statements of operations for any period. The items noted below have been excluded from the pro forma condensed consolidated statements of operations as they will not have a recurring impact. |
| For the Fiscal Year Ended May 31, 2008 ($ in millions) | ||||
Write-off of in-process research and development(1) | $ | 479.0 | ||
Amortization of inventory write-up | 160.3 | |||
Value of cash payment to holders of Options at the close of the Offer | 112.0 | |||
Biomet transaction costs(2) | 292.0 | |||
Estimated tax benefit(1) | (182.9 | ) | ||
Total after tax expenses | $ | 860.4 | ||
| (1) | Excludes items that are not tax deductible. |
| (2) | Excludes $87.1 million of costs that are deferred and amortized over the life of the related debt instrument. |
| (b) | Represents adjustments to depreciation and amortization based upon fair values and useful lives. For fiscal year 2008, $1.8 million and $40.1 million reflect step-up depreciation and amortization of acquired intangibles, respectively, for the period from June 1, 2007 through July 11, 2007. |
| Estimated Average Useful Lives | Estimated Fair Value | Depreciation and Amortization Expense Year Ended May 31, 2008 | ||||||
| ($ in millions) | ||||||||
Machinery & Equipment | 3 to 7 years | $ | 334.7 | $ | 83.7 | |||
Buildings and Leasehold Improvement | 10 to 30 years | 188.7 | $ | 12.6 | ||||
| $ | 523.4 | $ | 96.3 | |||||
Less historical depreciation | 88.2 | |||||||
Net adjustment to depreciation | $ | 8.1 | ||||||
Developed Technology and other | 6 to 20 years | $ | 5,896.7 | $ | 370.9 | |||
| (c) | Reflects pro forma interest expense resulting from our capital structure upon consummation of the Transactions, using an assumed three-month LIBOR rate of 5.500% and an assumed three-month Euro currency rate of 4.745% (as of November 30, 2008, the three-month LIBOR rate was 3.76% and the three-month Euro currency rate was 5.16%, respectively): |
| Assumed Interest Rate | Outstanding Indebtedness | Pro Forma Interest Expense Year Ended May 31, 2008 | |||||||
| ($ in millions) | |||||||||
Senior secured credit facilities(1) | 8.033 | % | $ | 3,800.2 | $ | 312.0 | |||
Notes(2) | 10.756 | % | 2,565.0 | 275.9 | |||||
Senior secured cash flow revolving credit facility commitment fee(3) | 0.500 | % | 1.0 | ||||||
Senior secured asset-based revolving credit facility commitment fee(4) | 0.375 | % | 1.0 | ||||||
Total cash interest expense | 589.9 | ||||||||
Amortization of deferred debt issuance costs(5) | 7.6 | ||||||||
Total pro forma interest expense | 597.5 | ||||||||
Less historical interest expense | 7.0 | ||||||||
Net adjustment to interest expense | $ | 590.5 | |||||||
30
Table of Contents
| (1) | Reflects interest on (i) the $2,340.0 million U.S. dollar-denominated senior secured term loan facility, (ii) the €875.0 million ($1,328.9 million) euro-denominated senior secured term loan facility and (iii) $131.3 million of borrowings ($110 million in borrowings in U.S. dollars and €14 million ($21.3 million) in borrowings in euros) drawn under the $400.0 million senior secured cash flow revolving credit facility that is expected to accrue at the estimated weighted average rate of 8.033%, which takes into account the effect of a series of interest rate swap agreements entered into by the Company to fix the interest rates on approximately 56% of the borrowings under these facilities. During fiscal 2008, we repaid $11.6 million of outstanding loans under our U.S. dollar-denominated senior secured term loan facility and $6.0 million of outstanding loans under our euro-denominated senior secured term loan facility. |
| (2) | Reflects interest on the senior notes and senior subordinated notes that is expected to accrue at the estimated weighted average rate of 10.756%. On or prior to the fifth anniversary of the closing date of the Transactions, for any interest period other than the initial interest period, we may elect to pay PIK interest. To the extent we elect to pay PIK interest, the applicable interest rate for such interest period will be increased by an additional 0.75% per annum, and the additional interest expense would increase by $5.9 million for the year ended May 31, 2007 and by $5.2 million for fiscal 2008. |
| (3) | Represents commitment fee of 0.500% on the assumed undrawn balance of the senior secured cash flow revolving credit facility. |
| (4) | Represents commitment fee of 0.375% on the assumed undrawn balance of our senior secured asset-based revolving credit facility. |
| (5) | Represents the $87.1 million of deferred debt issuance costs associated with our senior secured credit facilities and the notes offered hereby amortized over (a) six years for the senior secured cash flow revolving facility and the senior secured asset-based revolving credit facility, (b) seven and a half years for the senior secured term loan facilities, and (c) 10 years for senior notes and senior subordinated notes using the effective interest method. |
| (d) | Represents the tax effect of the taxable pro forma adjustments at an effective rate of 37.0%. |
31
Table of Contents
SELECTED HISTORICAL CONSOLIDATED FINANCIAL AND OTHER DATA
The following table presents our selected historical consolidated financial and other data as of and for the periods presented. The summary historical financial information as of May 31, 2007 and 2008, for each of the years in the three-year period ended May 31, 2008 and for the period June 1, 2007 through July 11, 2007 and from July 12, 2007 to May 31, 2008 have been derived from, and should be read in conjunction with, our audited consolidated financial statements included elsewhere in this prospectus. The selected historical consolidated financial and other data for the years ended May 31, 2004 and 2005 and as of May 31, 2004, 2005 and 2006 have been derived from our audited consolidated financial statements not included in this prospectus. The unaudited summary historical financial information as of and for the six months ended November 30, 2008 and for the period from July 12, 2007 to November 30, 2007 are derived from, and should be read in conjunction with, our unaudited condensed consolidated financial statements included elsewhere in this prospectus, and, except as otherwise described herein, have been prepared on a basis consistent with our annual audited financial statements and, in the opinion of management, include all adjustments consisting of normal recurring accruals considered necessary for a fair presentation of such data. Certain amounts recorded in previous periods have been reclassified to conform to the current presentation.
The Offer for Biomet’s Shares was completed successfully on July 11, 2007. On September 25, 2007, the Merger was complete and 100% of Biomet was owned by the Purchaser. Although Biomet continued as the same legal entity after the Merger, Holding’s cost of acquiring Biomet has been pushed-down to establish a new accounting basis for Biomet. Accordingly, the financial information in the table below for the six months ended November 30, 2007 is presented separately for the period prior to the completion of the Offer (from June 1, 2007 through July 11, 2007, the “Predecessor” or “Predecessor Period”) and the period after the completion of the Offer (from July 12, 2007 through November 30, 2007 and from July 12, 2007 through May 31, 2008, the “Successor” or “Successor Periods”), which relate to the accounting periods preceding and succeeding the completion of the Offer. The summary financial information as of May 31, 2008 and for all other Successor Periods presented, are not comparative to the summary financial information for the period June 1, 2007 through July 11, 2007 because of the new basis of accounting resulting from the Merger. Our results of operations for the Predecessor Period and the Successor Periods should not be considered representative of our future results of operations.
In addition, as noted in Note 2 to the audited financial statements included elsewhere in this prospectus, the summary historical financial information for the year ended May 31, 2007 has been prepared on the basis of an April 30 fiscal year for our foreign subsidiaries for financial reporting purposes. Subsequent to the completion of the Offer, we eliminated this one-month lag at our foreign subsidiaries, and therefore, the summary historical financial information as of and for the year ended May 31, 2007 is not comparative to the summary financial information as of and for the Successor Periods due to the elimination of this one-month lag for financial purposes at our foreign subsidiaries.
The summary historical financial information as of May 31, 2006, 2005 and 2004 has been derived from our audited financial statements not included in this prospectus. Please refer to “Unaudited Pro Forma Condensed Consolidated Financial Data,” “Selected Historical Consolidated Financial and Other Data,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our financial statements and notes thereto included elsewhere in this prospectus. The audited consolidated financial statements for each of the years in the four-year period ended May 31, 2007 have been audited by Ernst & Young LLP, an independent registered public accounting firm. The audited consolidated financial statements for the periods from June 1, 2007 through July 11, 2007 (Predecessor) and from July 12, 2007 through May 31, 2008 (Successor) have been audited by Deloitte & Touche LLP, an independent registered public accounting firm.
As a result of the report from the special committee formed by our Board of Directors, or the Special Committee, to conduct an independent investigation of our past stock option grant practices, and based on the determinations of our Audit Committee, we have restated our consolidated balance sheets as of May 31, 2005 and 2006 and the consolidated statements of operations for the fiscal years ended May 31, 2005 and 2006 to reflect the impact of additional share-based compensation expense and other adjustments described in our Amended Annual Report on Form 10-K/A, which was filed with the SEC on May 29, 2007. The data for the consolidated balance sheets as of May 31, 2003 and 2004 and the consolidated statements of operations for the fiscal year ended May 31, 2003 have also been restated to reflect the impact of additional share-based compensation expense and other adjustments, but such restated data has not been audited and is derived from our books and records. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Overview—Review of Historical Stock Option Grant Practices” for more information relating to the review of our historical stock option grant practices.
32
Table of Contents
| Predecessor | Successor | |||||||||||||||||||||||||||||
| Fiscal Year Ended May 31, | June 1, 2007 through July 11, 2007 | July 12, 2007 through May 31, 2008 | July 12, 2007 through November 30, 2007 | Six Months Ended November 30, 2008 | ||||||||||||||||||||||||||
| ($ in millions), except ratios | 2004 | 2005 | 2006 | 2007 | ||||||||||||||||||||||||||
Statements of Operations Data: | ||||||||||||||||||||||||||||||
Net sales | $ | 1,615 | $ | 1,880 | $ | 2,026 | $ | 2,107 | $ | 249 | $ | 2,135 | $ | 896 | $ | 1,250 | ||||||||||||||
Cost of sales | 462 | 533 | 582 | 642 | 102 | 815 | 351 | 376 | ||||||||||||||||||||||
Gross margin | 1,153 | 1,347 | 1,444 | 1,465 | 147 | 1,320 | 544 | 873 | ||||||||||||||||||||||
Selling, general and administrative expenses | 600 | 697 | 750 | 881 | 194 | 1,098 | 601 | 508 | ||||||||||||||||||||||
Research and development expense | 65 | 80 | 85 | 94 | 34 | 82 | 36 | 47 | ||||||||||||||||||||||
In-process research and | 1 | 26 | — | — | — | 479 | 479 | — | ||||||||||||||||||||||
Other charges/(credits) | — | — | — | — | — | — | — | — | ||||||||||||||||||||||
Amortization | — | — | — | — | 1 | 329 | 138 | �� | 181 | |||||||||||||||||||||
Operating income (loss), net | 487 | 544 | 609 | 490 | (82) | (668) | (708) | 137 | ||||||||||||||||||||||
Other income (loss), net | 18 | 11 | 14 | 21 | — | (10 | ) | 1 | (21 | ) | ||||||||||||||||||||
Interest expense | (4 | (9 | (12 | (9 | — | (516 | ) | (229 | ) | (280 | ) | |||||||||||||||||||
Income (loss) before income taxes | 501 | 546 | 611 | 502 | (82) | (1,194) | (937) | (164) | ||||||||||||||||||||||
Provision (benefit) for income taxes | 174 | 197 | 205 | 166 | (27 | ) | (230 | ) | (153 | ) | (64 | ) | ||||||||||||||||||
Income (loss) before minority interest | 327 | 349 | 406 | 336 | (55) | (964) | (784) | (100) | ||||||||||||||||||||||
Minority interest | 7 | — | — | — | — | — | — | — | ||||||||||||||||||||||
Net income (loss) | $320 | $349 | $406 | $336 | $(55) | $(964) | $(784) | $(100) | ||||||||||||||||||||||
| Predecessor | Successor | ||||||||||||||||||||||
| May 31, | November 30, 2007 | May 31, 2008 | November 30, 2008 | ||||||||||||||||||||
| ($ in millions) | 2004 | 2005 | 2006 | 2007 | |||||||||||||||||||
Balance Sheet Data (at period end): | |||||||||||||||||||||||
Cash and cash equivalents | $ | 159 | $ | 91 | $ | 126 | $ | 105 | $ | 126 | $ | 97 | $ | 229 | |||||||||
Total current assets | 1,123 | 1,192 | 1,299 | 1,452 | 1,373 | 1,421 | 1,415 | ||||||||||||||||
Total assets | 1,790 | 2,115 | 2,283 | 2,458 | 2,358 | 13,602 | 13,011 | ||||||||||||||||
Short-term borrowings | 110 | 282 | 277 | 82 | 100 | 42 | 73 | ||||||||||||||||
Total current liabilities | 312 | 515 | 518 | 346 | 346 | 608 | 466 | ||||||||||||||||
Total liabilities | 338 | 546 | 563 | 409 | 374 | 9,156 | 8,689 | ||||||||||||||||
Total shareholders’ equity | 1,452 | 1,569 | 1,720 | 2,049 | 1,984 | 4,446 | 4,322 | ||||||||||||||||
33
Table of Contents
| Predecessor | Successor | |||||||||||||||||||||||||||||||||
| Fiscal Year Ended May 31, | June 1, 2007 through July 11, 2007 | July 12, 2007 through May 31, 2008 | July 12, 2007 through November 30, 2007 | Six Months Ended November 30, 2008 | ||||||||||||||||||||||||||||||
| ($ in millions, except ratios) | 2004 | 2005 | 2006 | 2007 | ||||||||||||||||||||||||||||||
Statement of Cash Flows Data: | ||||||||||||||||||||||||||||||||||
Net cash provided by/(used in): | ||||||||||||||||||||||||||||||||||
Operating Activities | $ | 386 | $ | 411 | $ | 413 | $ | 440 | $ | 59 | $ | 189 | $ | 1 | $ | 48 | ||||||||||||||||||
Investing Activities | (253 | ) | (301 | ) | (121 | ) | (214 | ) | 11 | (11,722 | ) | (11,620 | ) | (95 | ) | |||||||||||||||||||
Financing Activities | (195 | ) | (98 | ) | (258 | ) | (251 | ) | 1 | 11,482 | 11,525 | 157 | ||||||||||||||||||||||
Other Financial Data: | ||||||||||||||||||||||||||||||||||
Depreciation and amortization | $ | 58 | $ | 70 | $ | 82 | $ | 97 | $ | 9 | $ | 461 | $ | 195 | $ | 261 | ||||||||||||||||||
Capital expenditures | (61 | ) | (97 | ) | (109 | ) | (143 | ) | (22 | ) | (168 | ) | (77 | ) | (93 | ) | ||||||||||||||||||
Ratio of earnings to fixed charges(1) | 126.3 | x | 61.7 | x | 51.9 | x | 56.8 | x | — | — | — | — | ||||||||||||||||||||||
| (1) | For purposes of computing the ratio of earnings to fixed charges, “earnings” consist of operating income plus other income plus cash dividends received from equity interests, less the equity income recorded. Fixed charges consist of interest expense, including amortization of debt issuance costs and interest capitalized. The interest portion of rental expense is not significant. On a pro forma basis, earnings were inadequate to cover fixed charges for the period June 1, 2007 through July 11, 2007, from July 12, 2007 through November 30, 2007, from July 12, 2007 through May 31, 2008, and the six months ended November 30, 2008 by $82 million, $934 million, $1,194 million, and $164 million, respectively. |
34
Table of Contents
MANAGEMENT’S DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis of our financial condition and results of operations includes periods prior to the consummation of the Merger. Accordingly, the following discussion and analysis of historical periods does not reflect the significant impact that the Merger has had on us, including significantly increased leverage and liquidity requirements. You should read the following discussion and analysis of our financial condition and results of operations together with the “Unaudited Pro Forma Condensed Consolidated Financial Data,” “Selected Historical Consolidated Financial and Other Data,” and our historical audited and unaudited consolidated financial statements and related notes appearing elsewhere in this prospectus. The following discussion and analysis of our financial condition and results of operations contains forward-looking statements and involves numerous risks and uncertainties, including, but not limited to, those described in “Risk Factors” and “Forward-Looking Statements” of this prospectus. Actual results may differ materially from those contained in any forward-looking statements.
Overview
Our Business
We design, manufacture and market a comprehensive range of both surgical and non-surgical products used primarily by orthopedic surgeons and other musculoskeletal medical specialists. We operate in one business segment, musculoskeletal products, which includes the design, manufacture and marketing of products in four major market categories: reconstructive products, fixation devices, spinal products and other products. We have three reportable geographic markets: United States, Europe and International.
• | Reconstructive products, which represented 74% and 75% of our net sales for fiscal 2008 and our net sales for the six months ended November 30, 2008, respectively, include knee, hip and extremity joint replacement systems, as well as dental reconstructive implants, bone cements and accessories, the GPS® System and the procedure-specific instrumentation required to implant our reconstructive systems. |
| • | Fixation devices, which represented 10% of our net sales for both fiscal 2008 and our net sales for the six months ended November 30, 2008, include internal and external fixation devices, craniomaxillofacial fixation systems and electrical stimulation devices that do not address the spine. |
| • | Spinal products, which represented 9% and 8% of our net sales for fiscal 2008 and our net sales for the six months ended November 30, 2008, respectively, include electrical stimulation devices addressing the spine, spinal fixation systems and orthobiologics. |
| • | The other product sales category, which represented 7% of our net sales for both fiscal 2008 and our net sales for the six months ended November 30, 2008, respectively, includes sports medicine products, softgoods and bracing products, casting materials, general surgical instruments, operating room supplies and other surgical products. |
Depending on the intended application, we report sales of bone substitute materials in the reconstructive product, fixation device or spinal product category.
We have operations in over 50 locations and distribute our products in approximately 90 countries throughout the world and manage our operations through three reportable geographic markets: United States, Europe and International. We are the fourth largest player in the U.S. orthopedic reconstructive market and have maintained this position for over ten years. We supply products to over 60% of U.S. hospitals performing joint replacement surgery. In addition, we are the third largest manufacturer and marketer of dental reconstructive products worldwide and maintain leadership positions in the electrical stimulation and craniomaxillofacial fields. We have a long history of innovation, engineering, quality and successful new product launches. Demonstrating our research and development leadership, we have launched approximately 800 new products in the past nine fiscal years and plan to introduce approximately 100 new products during fiscal 2009.
The Transactions
On December 18, 2006, we entered into the Merger Agreement with Parent and Purchaser. Pursuant to the Merger Agreement, on June 13, 2007, Purchaser commenced the Offer, to purchase all of our outstanding Shares at the Offer Price without interest and less any required withholding taxes. The Offer was made pursuant to Purchaser’s offer to purchase dated June 13, 2007 and the related letter of transmittal, each of which was filed with the SEC on June 13, 2007. The Offer expired at 12:00 midnight, New York City time, on July 11, 2007, with approximately 82% of the outstanding Shares having been tendered to Purchaser. At our special meeting of shareholders held on September 5, 2007, more than 91% of our shareholders voted to approve the Merger, and Parent acquired us on September 25, 2007 through a reverse subsidiary merger with Biomet, Inc. being the surviving company. Subsequent to the acquisition, we are a wholly owned subsidiary of Parent, which is controlled by Holding, an entity controlled by the Sponsors and their Co-Investors. Parent’s sole asset is 100% of the capital stock of the Company. Accordingly, a separate discussion of Parent’s financial condition and results of operations is not provided since the Company is representative of Parent’s consolidated operations.
The Offer for Biomet’s Shares was completed successfully on July 11, 2007. Although Biomet continued as the same legal entity after the Merger, Holding’s cost of acquiring Biomet has been pushed-down to establish a new accounting basis for Biomet. Accordingly, the financial information in the table below for the nine months ended May 31, 2008 is presented separately for the period prior to the completion of the Offer (from June 1, 2007 through July 11, 2007, the “Predecessor” or “Predecessor Period”) and the period after the completion of the Offer (from July 12, 2007 through May 31, 2008, the “Successor” or “Successor Period”), which relate to the accounting periods preceding and succeeding the completion of the Offer. The financial information as of November 30, 2008 and for the Successor Period are not comparative to the financial information for the period June 1, 2007 through July 11, 2007 because of the new basis of accounting resulting from the Merger. We have prepared our discussion of the results of operations by comparing the results of operations of the Predecessor Period to the historical period from June 1, 2007 through
35
Table of Contents
July 11, 2007. A comparative discussion of the results of operations for the Successor Period has not been provided due to the lack of a comparable period for the Predecessor; however, we have included a brief discussion of the factors that materially affected our results of operations in the Successor Period. Our results of operations for the Predecessor Period and the Successor Period should not be considered representative of our future results of operations.
In connection with the Transactions, we incurred significant indebtedness and became highly leveraged. See “Liquidity and Capital Resources.” In addition, the purchase price paid in connection with the acquisition has been allocated to state the acquired assets and liabilities at fair value.
We allocated the purchase price to the fair value of the assets and liabilities of Biomet based on estimated fair values utilizing generally accepted valuation methodologies. Both assets and liabilities were valued as of July 11, 2007. As noted in the purchase price allocation, in-process research and development projects were acquired. The most significant projects acquired occurred in the hip, knee and spine divisions. We expect to use these products to leverage and build on those products that have been in the market place for a number of years. We expect to launch products from these projects over the next 36 months, subject to regulatory approval. The preliminary purchase accounting adjustments increased the carrying value of our property and equipment, inventory and established intangible assets (such as corporate and product trade names, core and completed technology and customer relationships), among other things. Subsequent to the Transactions, interest expense and non-cash depreciation and amortization charges have significantly increased. As a result, our Successor financial statements subsequent to the Transactions are not comparable to our Predecessor financial statements.
The purchase price allocation was based on information currently available to us, and expectations, assumptions, and valuation methodologies deemed reasonable by our management. No assurance can be given, however, that the underlying assumptions used to estimate expected technology based product revenues, development costs or profitability, or the events associated with such technology, will occur as projected. Certain other fair value estimates require additional information before being finalized, certain intellectual property and other matters, investments, and inventory and instruments associated with brands we are considering to discontinue were also performed. For these reasons, among others, the actual results may vary from the projected results. The final valuation and associated purchase price allocation is expected to be completed as soon as possible, but no later than one year from the completion of the acquisition. To the extent that the estimates need to be adjusted, we will do so.
In addition, as noted in Note 2 of Notes to Consolidated Financial Statements included elsewhere in this prospectus, the summary historical financial information for the year ended May 31, 2007 has been prepared on the basis of an April 30 fiscal year for certain of our foreign subsidiaries for financial reporting purposes. Subsequent to the completion of the Offer, we eliminated this one-month lag at our foreign subsidiaries, and therefore, the summary historical financial information as of and for the year ended May 31, 2007 is not comparative to the summary financial information for the Successor Period due to the elimination of this one-month lag for financial reporting purposes at our foreign subsidiaries. The effect of this one-month lag elimination at our foreign subsidiaries is not considered material to the condensed consolidated financial statements as of and for the Successor Period.
Review of Historical Stock Option Grant Practices
In December 2006, following the publication of an analyst report suggesting that certain of our historical grants of Options took place on dates when our stock price was trading at relatively low prices and the filing of two shareholder derivative lawsuits alleging improper “backdating” of Options, our Board of Directors formed the Special Committee to conduct an independent investigation of our stock option grants for the period from March 1996 to May 2006 and to determine whether we had any claims arising out of any inappropriate stock option backdating and, if so, whether it was in our best interest and the best interest of our shareholders to pursue any such claim.
On December 18, 2006 and March 30, 2007, we announced preliminary reports from the Special Committee. Based upon an analysis of these reports and relevant accounting literature, including SAB No. 99, the Audit Committee determined on March 30, 2007 that we should amend our Annual Report on Form 10-K for fiscal 2006 and our Quarterly Report on Form 10-Q for the period ended August 31, 2006 to reflect the restatement of our consolidated financial statements (fiscal 2004, 2005 and 2006 and periods ended August 31, 2005 and 2006) and related disclosures reflected therein. In light of the Special Committee’s preliminary report discussed below, we announced that our previously issued financial statements and any related reports of our independent registered public accounting firm should not be relied upon. On May 25, 2007, the Board of Directors received and discussed the updated findings contained in the Special Committee’s final report.
The Special Committee’s investigation was based upon review of an extensive collection of physical and electronic documents, interviews of more than two dozen individuals and analysis of approximately 17,000 grants to purchase approximately 17,000,000 Shares on over 500 different grant dates over the 11-year period from March 1996 through May 2006. The Special Committee made the following findings:
| • | our written stock option plans were treated by our management, and our Compensation and Stock Option Committee, as formalities concerning the manner in which individual stock option grants were to be approved, resulting in a failure to abide by the terms of the plans; |
| • | we failed to receive appropriate legal or accounting advice from our former general counsel and the chief financial officer related to our stock option program and, as a result, relevant legal and accounting rules were not followed; |
| • | we failed to put in place and implement internal controls to manage our stock option program, including failing to devote sufficient resources to the administration of our stock option program; |
| • | we failed to prepare and maintain appropriate books and records documenting the administration of our stock option program, specifically with regard to the approval of individual stock option grants; |
| • | most stock options issued by us were dated on dates other than the date of grant of those Options, as that date was defined by the stock option plans; |
| • | we engaged in purposeful “opportunistic” dating (and, therefore, pricing) of Options; and |
| • | as a result of these deficiencies, certain of our proxy statements were inaccurate. |
36
Table of Contents
The Special Committee also reported that members of senior management were aware of the practice of dating Options on a date other than the date on which final action regarding the Option occurred, and that certain members of senior management, namely our chief financial officer and general counsel during the period, were or should have been aware of certain accounting and legal ramifications, respectively, of issuing an Option with an exercise price lower than the fair market value on the date of issuance. The Special Committee also concluded that, based upon the information gathered and reviewed by the Special Committee, the misdating and mispricing of stock option awards was driven by a desire to make the Options more valuable to the employees who received the awards and not to enrich those who managed the stock option program, though the Company’s practice also did inure to the benefit of those who managed the stock option program.
On May 25, 2007, our Board of Directors received and discussed the remedial measures suggested by the Special Committee, which included that:
| • | the procedures for Option approval should be formalized in a manner consistent with the terms of our underlying stock option plans and records of individual stock option awards should be maintained using commercially available software by experienced and qualified personnel; |
| • | the Board of Directors should commit to exercising additional oversight of our management and conduct a thorough review of our governance and internal control practices; |
| • | certain personnel should be removed from the administration of our stock option program and financial reporting function or provided additional oversight and training; |
| • | certain individuals who were our directors or executive officers at the time they received misdated or mispriced awards should disgorge any benefit derived from the exercise of such misdated or mispriced awards and increase the exercise price for those unexercised misdated or mispriced awards; and |
| • | we should take steps to address the tax consequences to employees of our historical stock option granting practices. |
Our Board of Directors continues to thoughtfully consider these recommendations and has either implemented or is in the process of implementing several of the Special Committee’s recommendations.
We have also incurred expenses in connection with certain corrective actions approved by our Compensation and Stock Option Committee with respect to misdated or mispriced Options, including (a) payments to compensate certain former holders of Options whose Option exercise prices we increased to the fair market value of the shares underlying such Options on the “measurement date” (as that term is defined in SFAS No. 123(R)) for the Options and (b) payments to the IRS on behalf of certain Option holders (and reimbursement of one of our executive officers) to cover taxes and penalties payable by such individuals as a result of their exercise of misdated or mispriced Options prior to the date we amended such Options to bring them into compliance with (and thereby avoid the taxes and penalties imposed under) section 409A of the Code, as well as gross-up payments to such individuals for any taxes they incur as a result of such payments. In connection with the closing of the Offer, all outstanding Options to purchase Shares under our stock plans, vested or unvested, were cancelled and each Option holder was paid an amount in cash equal to the excess, if any, of the Offer Price over the applicable Option exercise price for each Share subject to an Option, less any required withholding taxes.
Furthermore, in light of the Special Committee’s findings, on March 30, 2007 Gregory D. Hartman retired as Senior Vice President—Finance, Chief Financial Officer and Treasurer, and Daniel P. Hann retired as our Executive Vice President of Administration and our Director. On February 26, 2007, we announced the appointment of Jeffrey R. Binder as President and Chief Executive Officer and a member of our Board of Directors. On March 30, 2007, we announced the appointment of J. Pat Richardson as Vice President—Finance and Interim Chief Financial Officer and Treasurer, and on May 14, 2007, we announced the appointment of Daniel P. Florin as Senior Vice President and Chief Financial Officer, effective June 5, 2007.
Finally, the Special Committee concluded that pursuit of the claims made in the derivative litigation related to stock option grants would not be in our best interests at this time.
On May 29, 2007, we filed our amended Annual Report on Form 10-K/A for fiscal 2006. On June 4, 2007, we filed our amended Quarterly Report on Form 10-Q/A for the period ended August 31, 2006 and our Quarterly Reports on Form 10-Q for the periods ended November 30, 2006 and February 28, 2007. We have not amended and do not intend to amend any of our previously filed Annual Reports on Form 10-K or Quarterly Reports on Form 10-Q for the periods affected by the restatement other than our amended Annual Report on Form 10-K/A for fiscal 2006 and our amended Quarterly Report on Form 10-Q for the period ended August 31, 2006. Accordingly, our previously issued financial statements affected by the restatement and any related reports of our independent registered public accounting firm should not be relied upon.
Seasonality
Our business is somewhat seasonal in nature, as many of our products are used in elective procedures, which typically decline during the summer months, particularly in European countries.
Results of Operations
We believe the following developments or trends are important in understanding our financial condition, results of operations and cash flows for the Predecessor Period (fiscal years ended 2006 and 2007 and from June 1, 2007 through July 11, 2007) and the Successor Periods (from July 12, 2007 through November 30, 2007, July 12, 2007 through May 31, 2008, and the six months ended November 30, 2008). The growth percentages shown below include the effect of eliminating a one-month reporting lag on July 12, 2007, that was in place during fiscal 2007 at certain foreign subsidiaries. The effect of this one-month lag elimination at our foreign subsidiaries is not considered material to any Successor Period.
37
Table of Contents
Unfavorable conditions in the economy have had an adverse effect on our dental business for the three months ended November 30, 2008 as compared to the prior period principally due to the elective nature of dental implant procedures, which are typically not reimbursed by governmental agencies, as payment is primarily received from dental practices and laboratories. While we have already undertaken and continue to undertake certain operating initiatives in connection with this business, we anticipate that the growth rate of our worldwide dental business will remain flat or have low to mid single digit growth during the current global
recessionary environment, compared to reported double digit growth in the most recent prior year.
Economic Uncertainties
Our results of operations could be substantially affected not only by global economic conditions, but also by local operating and economic conditions, which can vary substantially by market. Unfavorable conditions can depress sales in a given market and may result in actions that adversely affect our margins, constrain our operating flexibility or result in charges which are unusual or non-recurring. Certain macroeconomic events, such as the current crisis in the financial markets, could have a more wideranging and prolonged impact on the general business environment, which could also adversely affect us. For example, due to certain patient insurance deductibles resetting on January 1, 2009, it is possible that we could experience a potential adverse impact on sales due to patients deferring procedures because of decreased cash flow. Historically, we believe that this slowdown due to the uncertain or recessionary environment has been minor to the orthopedic business, however, management is taking precautionary measures to be able to manage expenses more conservatively, if revenues were to decrease below those internally forecasted.
Six Months Ended November 30, 2008 as Compared to the Period July 12, 2007 through November 30, 2007
Our results of operations for the six months ended November 30, 2008 are not comparative to our results of operations for the period June 1, 2007 to July 11, 2007 because of the new basis of accounting resulting from the Merger. Both assets and liabilities were fair valued as of July 11, 2007. On July 11, 2007, 82.4% of the step-up was recorded, which included a $392.8 million in-process research and development (“IPRD”) charge, and a $66.2 million and $132.1 million fair value step up to property, plant, and equipment and inventory, respectively, and then combined with 17.6% of the Predecessor Company. On September 25, 2007 (the “Closing Date”), the remaining fair value step-up of 17.6% was recorded. The additional step-up performed included an increase in the IPRD charge of $86.2 million, increase of the property plant and equipment fair value of $14.2 million, and an increase in the fair value of inventory of $28.2 million. Also, the Tender Facility obtained in connection with the Offer was refinanced on the Closing Date and replaced with senior secured credit facilities, term loan facilities, and cash flow and asset based loan revolvers. On July 12, 2007, we eliminated a one month lag that was in place during the predecessor period at certain non-domestic subsidiaries. The effect of this change is immaterial to the financial results included below.
Unaudited Condensed Consolidated Statements of Operations
| (in millions, except percentages) | The Six Months��Ended November 30, 2008 | Percentage of Net Sales | The Period From July 12 - November 30, 2007 (Successor) | Percentage of Net Sales | ||||||||||
Net sales | $ | 1,249.8 | 100 | % | $ | 895.8 | 100 | % | ||||||
Cost of sales | 376.4 | 30 | 351.4 | 39 | ||||||||||
Gross margin | 873.4 | 70 | 544.4 | 61 | ||||||||||
Selling, general and administrative expense | 508.2 | 41 | 600.5 | 67 | ||||||||||
Research and development expense | 46.9 | 4 | 35.5 | 4 | ||||||||||
In-process research and development | — | — | 479.0 | 54 | ||||||||||
Amortization | 181.3 | 14 | 137.5 | 15 | ||||||||||
Operating income (loss) | 137.0 | 11 | (708.1 | ) | (79 | ) | ||||||||
Interest expense, net | (280.3 | ) | (22 | ) | (229.1 | ) | (26 | ) | ||||||
Other income (expense) | (20.6 | ) | (2 | ) | 0.5 | — | ||||||||
Other expense, net | (300.9 | ) | (24 | ) | (228.6 | ) | (26 | ) | ||||||
Loss before income taxes | (163.9 | ) | (13 | ) | (936.7 | ) | (105 | ) | ||||||
Benefit from income taxes | (64.3 | ) | (5 | ) | (152.5 | ) | (17 | ) | ||||||
Net loss | $ | (99.6 | ) | (8 | )% | $ | (784.2 | ) | (88 | )% | ||||
Sales
Net sales were $1,249.8 million for the six months ended November 30, 2008 and $895.8 million for the period July 12, 2007 through November 30, 2007. The following tables provide net sales by geography and product category:
Geography Sales Summary
| (in millions, except percentages) | The Six Months Ended November 30, 2008 | Percentage of Net Sales | The Period From July 12 - November 30, 2007 (Successor) | Percentage of Net Sales | ||||||||
United States | $ | 747.9 | 60 | % | $ | 527.7 | 59 | % | ||||
Europe | 364.8 | 29 | 268.3 | 30 | ||||||||
International(1) | 137.1 | 11 | 99.8 | 11 | ||||||||
Total | $ | 1,249.8 | 100 | % | $ | 895.8 | 100 | % | ||||
(1) | International primarily includes Canada, South America, Mexico, and the Pacific Rim |
38
Table of Contents
Product Category Summary
| (in millions, except percentages) | The Six Months Ended November 30, 2008 | Percentage of Net Sales | The Period From July 12 - November 30, 2007 (Successor) | Percentage of Net Sales | ||||||||
Reconstructive | $ | 932.6 | 75 | % | $ | 662.5 | 74 | % | ||||
Fixation | 118.3 | 10 | 88.2 | 10 | ||||||||
Spinal | 106.5 | 8 | 79.7 | 9 | ||||||||
Other | 92.4 | 7 | 65.4 | 7 | ||||||||
Total | $ | 1,249.8 | 100 | % | $ | 895.8 | 100 | % | ||||
Reconstructive
Our worldwide sales of reconstructive products continued to be a significant percentage of total net sales. Principal drivers behind the reconstructive product sales were knees, where worldwide demand remained strong for our Oxford® Partial Knee System, as well as the Vanguard™ Complete Knee System. The Vanguard M™ Partial Knee, which is the fixed-bearing version of the Oxford® knee, and the Vanguard PFR™ Patellofemoral Replacement System, which incorporates the technology of the Vanguard™ Complete Knee System, also contributed to our six month growth in knee sales. Hip sales continue to be strong, primarily due to the E-Poly™ Acetabular Liners, conventional and Microplasty™ versions of the Taperloc® Hip System, the M2 a-Magnum™ Acetabular System, and the Regenerex® Ringloc® + Modular Acetabular System. In addition, European sales increased due to the volume growth of the Vanguard™ Complete Knee System, Oxford® Partial Knee System, Aura® Hip Stem, Taperloc® Hip System, the Exceed ABT™ (Advanced Bearing Technologies) Acetabular System, and the T.E.S.S. Shoulder System.
Fixation
Increased sales of fixation products reflected by global growth of the craniomaxillofacial fixation and internal fixation product categories partially offset by decreased sales of electrical stimulation and external fixation products. The TraumaOne™ System contributed to the sales growth for craniomaxillofacial fixation. The Phoenix™ Femoral IM Nailing System, which includes the Phoenix™ Retrograde and Antegrade Femoral Nail components, and the Phoenix™ Tibial Nailing System contributed to internal fixation sales growth.
Spinal
Sales of spinal products increased primarily due to sales of spacer products, led by the C-Thru™ Small Stature PEEK Spacer and Services related to the OsteoStim® Cervical Composite Allograft Implant.
Other
Sales of other products continue to reflect strong global growth in our sports medicine division. Growth drivers for our sports medicine division included the MaxFire™ Meniscal Repair Device, the ToggleLoc™ Femoral Fixation Device with ZipLoop™ Technology, and the Osseofit™ Porous Tissue Matrix™ (Porous Tissue Matrix™ is a trademark of Kensey Nash Corp).
Gross Margin
Gross margin increased as a percentage of net sales to 70% for the six months ended November 30, 2008 compared to 61% for the period July 12, 2007 through November 30, 2007. Gross margin for the period July 12, 2007 through November 30, 2007 was negatively impacted by increased cost of sales in connection with the Merger, including charges for the inventory step-up of $92.3 million and additional depreciation of $5.6 million related to the step-up in property, plant and equipment. Other amounts impacting gross margin were increased consulting expenses related to operational improvement initiatives of $3.3 million for the period July 12, 2007 through November 30, 2007. Excluding these items, gross margin percentage was comparable over the periods presented.
Selling, General and Administrative Expense
Selling, general and administrative expenses were 41% of net sales for the six months ended November 30, 2008, compared to 67% of net sales for the period July 12, 2007 through November 30, 2007. Selling, general and administrative expenses were negatively impacted during the period July 12, 2007 through November 30, 2007 primarily due to (1) the $26.9 million settlement payment to the Department of Justice described in Note 13—Contingencies to the unaudited condensed consolidated financial statements, (2) $27.3 million of distributor fee expense associated with renegotiation of distribution agreements, and (3) $171.6 million of transaction expenses associated with the Merger. Excluding these items, selling, general and administrative expenses as a percentage of net sales were comparable over the periods presented.
39
Table of Contents
Research and Development
Research and development expenses during the six months ended November 30, 2008 were $46.9 million or 4% of net sales, compared to $35.5 million or 4% of net sales during the period July 12, 2007 through November 30, 2007. Expenses decreased as a percentage of net sales by 20 basis points primarily due to terms related to our Deferred Prosecution Agreement affecting our spending prior to the settlement date, which inflated our spending on research and development subsequent to the settlement and payment of the agreed upon amount. Expenses through the six months ended November 30, 2008 have primarily been related to the following research and development projects: T.E.S.S. Long Stem (Reconstructive-Extremities), E-Poly™ Vitamin E stabilized knee bearings (Reconstructive-Knees), OnPoint™ Scope (Fixation), Forerunner™ Plating System (Fixation), Ballista™ Percutaneous Pedicle Screw Placement System (Spine), AccuVision™ Minimally Invasive Spinal Exposure System (Spine), PEEK-OPTIMA® (a trademark of Invibio Ltd.) version of the Solitaire™ Spine System (Spine), and Phoenix™ Ankle Arthrodesis Nail (Fixation-Internal).
In-Process Research & Development
We recorded IPRD charges of $479.0 million during the period July 12, 2007 through November 30, 2007 related to the Merger. We recorded IPRD for the portion of the purchase price representing the value of technologies relating to products that had not received FDA approval or clearance and had no alternative use, excluding the value of core and developed technologies. There were no IPRD charges during the six months ended November 30, 2008.
Amortization
Amortization expense for the six months ended November 30, 2008 was $181.3 million, compared to $137.5 million during the period July 12, 2007 through November 30, 2007. This increase is due to only 82% of the established intangibles being recorded from the Merger Date of July 12, 2007 to the closing date of September 25, 2007 resulting in reduced amortization expense and having 41 less calendar days in the period. On the closing date, an additional 18% step-up of the intangibles was recorded, resulting in higher amortization subsequent to that date.
Interest Expense, net
Interest expense was $281.7 million, partially offset by interest income of $1.4 million, for the six months ended November 30, 2008, compared to $235.8 million, partially offset by interest income of $6.7 million, during the period July 12, 2007 through November 30, 2007. For the six months ended November 30, 2008, interest expense primarily related to interest charges and financing costs related to the debt financings obtained in connection with the Merger. For the period July 12, 2007 through November 30, 2007, interest expense primarily related to interest charges and financing costs on the Tender Facility obtained in connection with the Offer through September 25, 2007, when the Tender Facility was replaced with senior secured credit facilities, term loan facilities, and cash flow and asset based loan revolvers. In addition, interest expense was impacted during the period July 12, 2007 through November 30, 2007 for deferred financing costs of $57.2 million related to the Tender Facility being written off.
Other income (expense) was an expense of $20.6 million for the six months ended November 30, 2008, compared to income of $0.5 million during the period July 12, 2007 through November 30, 2007. Other income (expense) primarily related to write-downs of investments of $5.2 million, and currency transaction losses related to our foreign operations of $10.4 million, primarily due to the weakening Euro compared to the U.S. Dollar.
Provision for Taxes
The effective income tax rate increased to 39.3% for the six months ended November 30, 2008 compared to 16.3% for the period July 12, 2007 through November 30, 2007. This increase was primarily due to the following items incurred in fiscal 2008 that are not deductible for tax purposes: (1) $479.0 million in-process research and development expense related to the Merger, (2) a portion of the $26.9 million Department of Justice settlement, and (3) $51.5 million of merger-related expenses.
For the Period July 12, 2007 through May 31, 2008
| July 12, 2007 through May 31, 2008 (Successor) | Percentage of Net Sales | ||||||
| (in millions, except percentages) | |||||||
Net sales | $ | 2,134.5 | 100 | % | |||
Cost of sales | 814.7 | 38 | |||||
Gross margin | 1,319.8 | 62 | |||||
Selling, general and administrative expenses | 1,097.6 | 51 | |||||
Research and development expense | 82.2 | 4 | |||||
In-process research and development | 479.0 | 23 | |||||
Amortization | 329.3 | 15 | |||||
Operating income (loss) | (668.3 | ) | (31 | ) | |||
Interest expense, net | (516.3 | ) | (24 | ) | |||
Other expense | (9.7 | ) | — | ||||
Other expense, net | (526.0 | ) | (24 | ) | |||
Income (loss) before taxes | (1,194.3 | ) | (55 | ) | |||
Benefit for income taxes | (230.1 | ) | (11 | ) | |||
Net loss | $ | (964.2 | ) | (44 | )% | ||
40
Table of Contents
Net Sales. The following tables provide net sales by geography and product category:
Geography Sales Summary
| July 12, 2007 through May 31, 2008 (Successor) | Percentage of Net Sales | |||||
| (in millions, except percentages) | ||||||
United States | $ | 1,251.4 | 59 | % | ||
Europe | 663.7 | 31 | ||||
International(1) | 219.4 | 10 | ||||
Total | $ | 2,134.5 | 100 | % | ||
(1) | International primarily includes Canada, South America, Mexico, and the Pacific Rim. |
Product Category Summary
| July 12, 2007 through May 31, 2008 (Successor) | Percentage of Net Sales | |||||
| (in millions, except percentages) | ||||||
Reconstructive Products | $ | 1,578.6 | 74 | % | ||
Fixation Devices | 203.2 | 10 | ||||
Spinal Products | 183.1 | 8 | ||||
Other Products | 169.6 | 8 | ||||
Total | $ | 2,134.5 | 100 | % | ||
• | Worldwide sales of reconstructive products continued to be a significant percentage of total sales. European sales continued to grow faster than U.S. sales, primarily due to the positive impact of foreign currency translation. Principal drivers behind the reconstructive products growth are knees, where worldwide demand remained strong for Biomet’s Oxford® Partial Knee System, as well as the Vanguard™ Complete Knee System. Hip sales continued to be strong, primarily due to international sales of the M2 a-Magnum™ Large Metal Articulation System and the Taperloc® Hip System as well as the ReCap® Total Resurfacing System in Europe. In addition, sales of dental reconstructive devices were strong, with the launch of the NanoTite™ Tapered PREVAIL® Implant. |
| • | Sales of fixation and spinal products were lower than expected for the period July 12, 2007 to May 31, 2008 due to the underperformance of the Biomet Trauma and Biomet Spine, or BTBS, division. We have made various changes at the division, including managerial changes and computer system enhancements, among others. The new management team and infrastructure changes at BTBS have allowed us to provide improved focus on the spine and trauma markets and BTBS customers. During the fourth quarter of fiscal 2008, BTBS continued to show signs of stabilization. |
Gross Margin. Gross margin was 62% of net sales during the period July 12, 2007 through May 31, 2008 and was negatively impacted by increased cost of sales due to the inventory step-up of $160.3 million, in connection with the Merger as well as, additional depreciation of $15.0 million related to the step-up in property, plant, and equipment. In addition, stock compensation expense of $2.0 million impacted the period July 12, 2007 through May 31, 2008.
Selling, General and Administrative Expenses. Selling, general and administrative expenses were 51% of net sales during the period July 12, 2007 through May 31, 2008, and were negatively impacted primarily due to (1) $172.0 million of transaction fees associated with the Merger, (2) $26.9 million settlement payment with the Department of Justice described in Note 1 to our consolidated financial statements included elsewhere in this prospectus, (3) $24.0 million of distributor fee expense associated with renegotiation of distribution agreements, and (4) $21.0 million of stock compensation expense.
Research and Development Expenses. Research and development expenditures during the period July 12, 2007 through May 31, 2008 were $82.2 million or 4% of net sales. Investments were primarily on the following research and development projects: Polaris™ 5.5 (Spinal—Spine), Mini BHS (Spinal—Stimulation), E-Poly™ Bearing Surfaces (Reconstructive—Hip and Knee), Comprehensive® Primary Shoulder System (Reconstructive—Extremities), Regenerex® RingLoc® +Modular Acetabular Cup (Reconstructive—Hips) and Regenerex® Tibial Components (Reconstructive—Knees), Signature™ Patient Specific Disposable Knee Instruments (Reconstructive-Knees), TMJ Diagnostic Arthroscope, TMJ Arthrocentesis Convenience Kit, LactoSorb® Fixation System for the Japan Market, Biologic Scaffold Research, NanoTite™ Tapered and Tapered PREVAIL® Implants, Navigator™ Instrumentation System for guided implant placement, ZiReal® Art Ceramic Abutment System, Encod® Complete patient specific products expansion including robotic analog placement, and Acrylic Bone Cement.
In-Process Research & Development (IPRD). We recorded IPRD charges of $479.0 million for the period July 12, 2007 through May 31, 2008 related to the Merger. We recorded IPRD for the portion of the purchase price representing the value of technologies relating to products that have not received FDA approval or clearance and have no alternative use, excluding the value of core and
41
Table of Contents
developed technologies. IPRD projects for Biomet Orthopedics focus on the utilization of new materials, new methods for fabricating existing materials, and new geometries of both new and existing materials to enhance function, durability and bony fixation for orthopedic implant devices primarily focused in the area of partial and total joint replacement. IPRD projects for Biomet Trauma and Biomet Spine (BTBS) are primarily related to addressing unmet needs in the musculoskeletal market utilizing both traditional and new technologies. IPRD projects for Biomet Europe focus primarily on improvements to joint replacement implants, such as wear resistant bearing combinations for hip replacement, total and partial knee prostheses with improved kinematic performance, novel shoulder implants for improved stability and range of motion and development of instrumentation with improved accuracy and ergonomics. IPRD projects for Biomet Biologics focus primarily on producing new devices and applications to use autologous materials for regenerative tissue therapies. IPRD projects for Biomet Sports Medicine focus on the utilization of new technologies, materials and devices to primarily treat soft tissue defects in tendons, ligaments and cartilage. IPRD projects for Biomet 3i focus on the development of intraoral rehabilitation, generally in the area of dental implants, associated components, surgical instrumentation and regenerative therapies necessary for the placement of the implants.
Amortization. Amortization expense during the period from July 12, 2007 through May 31, 2008 was $329.3 million, which relates to the establishment of definite lived intangibles of $6,310.0 million recorded in connection with the Merger.
Interest Expense, net. Interest expense was $516.3 million for the period July 12, 2007 through May 31, 2008, primarily of which relates to interest expense and financing costs related to the debt financings obtained in connection with the Merger of $522.0 million. This was offset by interest income of $5.0 million.
Other income (expense). Other income (expense) was $(9.7) million for the period July 12, 2007 through May 31, 2008, which relates primarily to currency translation adjustments related to our foreign operations.
Provision (Benefit) for Taxes. The effective income tax benefit is 19% for the period July 12, 2007 through May 31, 2008. The rate is lower than the U.S. statutory rates due to the following items not being deductible: (1) $479.0 million IPRD expense related to the Merger, (2) $74.0 million of transaction expenses related to the Merger and (3) a portion of the $26.9 million settlement payment with the Department of Justice described in Note 1 to our consolidated financial statements included elsewhere in this prospectus. These were offset by tax rates in our international locations being lower than in the United States and our plans to have those earnings permanently invested.
Net Loss. A net loss of $964.2, or a negative 44% as a percentage of net sales, was primarily due to the following related to the Transaction: (1) Interest expense, net of $516.3 million, (2) IPRD expense of $479.0 million, (3) additional expense for the step-up in fair value for inventory and property, plant and equipment of $160.3 million and $83.0 million, respectively, and (4) amortization expense related to the newly established intangible assets related to the merger of $329.3 million.
For the Period June 1, 2007 through July 11, 2007
Sales
Net sales were $248.8 million for the period June 1, 2007 through July 11, 2007. The following tables provide net sales by geography and product category:
Geography Sales Summary
| (in millions, except percentages) | The Period From June 1 - July 11, 2007 (Predecessor) | Percentage of Net Sales | ||||
United States | $ | 156.2 | 63 | % | ||
Europe | 70.8 | 28 | ||||
International(1) | 21.8 | 9 | ||||
Total | $ | 248.8 | 100 | % | ||
(1) | International primarily includes Canada, South America, Mexico and the Pacific Rim. |
Product Category Summary
(in millions, except percentages) | The Period From June 1 - July 11, 2007 (Predecessor) | Percentage of Net Sales | ||||
Reconstructive | $ | 178.1 | 71 | % | ||
Fixation | 27.1 | 11 | ||||
Spinal | 24.9 | 10 | ||||
Other | 18.7 | 8 | ||||
Total | $ | 248.8 | 100 | % | ||
42
Table of Contents
Reconstructive
Our worldwide sales of reconstructive products continued to be a significant percentage of total net sales. Principal drivers behind the reconstructive product sales were knees, where worldwide demand remains strong for our Oxford® Partial Knee System, as well as the Vanguard™ Complete Knee System. Hip sales continued to be strong, primarily due to worldwide sales of the M2 a-Magnum™ Acetabular System and the Taperloc® Hip System, as well as strong growth for the ReCap® Total Resurfacing System in Europe. In addition, sales of dental reconstructive devices were strong, with the launch of the NanoTite™ Tapered PREVAIL® Implant.
Fixation and Spinal
Sales of fixation and spinal products were lower than expected for the period June 1, 2007 through July 11, 2007 due to the underperformance of the Biomet Trauma and Biomet Spine, or BTBS, division. We have made various changes at the division, including managerial changes and computer system enhancements, among others. We believe the new management team and infrastructure changes at BTBS will allow us to provide improved focus on the spine and trauma markets and BTBS customers.
Other
Sales of other products include product lines that were sold by the BTBS division and did not meet management expectations during the period June 1, 2007 through July 11, 2007. This poor performance was partly offset by the sales performance of sports medicine products.
Gross Margin
Gross margin was 59% of net sales during the period June 1, 2007 through July 11, 2007, which was negatively impacted by increased cost of sales in connection with the Merger, including $28.0 million of costs in June 2007 to settle in-the-money stock options to employees, as required by the Merger Agreement.
Selling, general and administrative expenses were 78% of net sales during the period June 1, 2007 through July 11, 2007. Selling, general and administrative expenses were negatively impacted during this period due to (1) $61.0 million paid upon the cash-out of outstanding in-the-money stock options of employees, as part of the Merger, (2) $30.0 million of transaction fees associated with the Merger, (3) $18.0 million of distributor fee expense associated with renegotiation of distribution agreements and (4) $2.0 million of additional legal and Merger-related fees.
Research and Development
Research and development expenditures during the period June 1, 2007 through July 11, 2007 were $34.0 million or 14% of net sales, which was impacted by $23.0 million of additional compensation expense upon the cash-out of outstanding in-the-money stock options of employees, as part of the Merger.
Provision for Taxes
The effective income tax rate was 33.3% for the period June 1, 2007 through July 11, 2007. The rate is lower than the U.S. statutory rates due to the tax rates in our international locations being lower than in the United States and our plans to have those earnings permanently invested.
Results of Operations for the Years Ended May 31, 2006 and 2007
For the Year Ended May 31, 2007 Compared to Year Ended May 31, 2006
Net Sales. Net sales in fiscal 2007 were $2,107.4 million, an increase of 4% from fiscal 2006, 2% of the increase in sales related to the positive impact of foreign currency translation.
Product Category Data:
| • | Worldwide sales of reconstructive products increased 9% to $1,503.9 million in fiscal 2007 from $1,379.0 million in fiscal 2006. Factors contributing to this increase include incremental volume as a result of an increase in the overall market size for reconstructive products and favorable product mix (7%) and the impact of foreign currency translation (2%). During fiscal 2007, worldwide dental reconstructive product sales increased 15%, extremity sales increased 14%, knee sales increased 8%, hip sales increased 7% and bone cement and accessory sales were flat. |
| • | Sales of fixation devices decreased 11% to $224.7 million in fiscal 2007 from $251.0 million in fiscal 2006. Decreased volume and product mix accounted for this decrease. Worldwide sales of craniomaxillofacial products, including bone substitutes, increased 2%. Internal fixation devices increased 2%, external fixation devices decreased 13% and electrical stimulation devices decreased 25%. |
| • | Sales of spinal products decreased 7% to $205.8 million in fiscal 2007 from $222.0 million in fiscal 2006. Decreased volume and product mix accounted for this decrease. Worldwide sales of spinal hardware, including orthobiologics, increased 2% while spinal stimulation product sales decreased 21%. During fiscal 2007, BTBS has underperformed against the market and management’s objectives. Results have also been negatively impacted by the implementation of a new computer system at BTBS. However, management changes have been made and progress has been achieved in the computer system implementation, sales support system, the in-sourcing of the manufacture of spinal hardware products and the expansion of the research and development team. |
43
Table of Contents
| • | Sales of our other products were flat at $173.0 million in each of fiscal 2007 and fiscal 2006. Decreased volume and product mix (1%) were offset by the impact of foreign currency translation (1%). Worldwide sales of arthroscopy products increased 10% and general surgical instrumentation increased 3%, while softgoods and bracing products decreased 5%. |
Geographic Markets Data:
| • | Sales in the United States decreased 1% to $1,306.5 million in fiscal 2007 from $1,325.0 million in fiscal 2006. Components of this change were incremental volume and product mix of reconstructive products (5%), offset by decreases in volume of fixation and spinal products (14%). The pricing environment was neutral for fiscal 2007. |
| • | European sales increased 14% to $595.8 million in fiscal 2007 from $521.0 million in fiscal 2006. Components of this increase were incremental volume and product mix (8%) and the impact of foreign currency translation (6%). |
| • | Sales in International increased 14% to $205.1 million in fiscal 2007 from $180.0 million in fiscal 2006. Components of this increase were incremental volume and product mix (13%) and the impact of foreign currency translation (1%). We commenced direct sales of our products in Japan during fiscal 2002 and continued to experience good product acceptance with growth at approximately 22% for fiscal 2007 in local currency. |
Gross Margin. Our gross margin increased 1% to $1,465.1 million in fiscal 2007 from $1,443.6 million in fiscal 2006. Our gross margin percentage decreased to 70% of sales in fiscal 2007 from 71% in fiscal 2006. The components of this change are additional expenses of 1% related to inventory write-downs at our BTBS division and 0.4% from higher growth rates in foreign sales, where gross margins are lower as compared to gross margins on products sold in the United States.
Selling, General and Administrative Expenses. Selling, general and administrative expenses increased 17% to $881.1 million in fiscal 2007 from $750.2 million in fiscal 2006. This increase results from the renewal and re-negotiation of distribution agreements with existing distributors (5%), accounts receivable reserves related to our BTBS division (4%), expenses related to the Merger Agreement and retirement/employment costs associated with changes in executive management (2%), the adoption of SFAS No. 123(R) (2%), increased commission expense on higher sales (4%), and an increase in other marketing and general and administrative expenses (1%). These increases were offset by decreased direct to consumer advertising (1%). As a percentage of sales, selling, general and administrative expenses were 42% in fiscal 2007 compared to 37% in fiscal 2006.
Research and Development Expenses. Research and development expenses increased 11% to $94.4 million in fiscal 2007 from $85.0 million in fiscal 2006. The increase reflects a continued emphasis on new product development and enhancements and additions to our existing product lines and technologies. Also included in the increase is the impact of adopting SFAS No. 123(R) (3%). As a percentage of sales, research and development expenses were 5% in fiscal 2007 and 4% in fiscal 2006.
Operating Income. Operating income decreased 20% to $489.6 million in fiscal 2007 from $608.4 million in fiscal 2006. U.S. operating income decreased 26% to $384.0 million in fiscal 2007 from $520.0 million in fiscal 2006, reflecting a slight decrease in sales and the additional expenses discussed above. European operating income increased 24% to $97.0 million in fiscal 2007 from $78.0 million in fiscal 2006. The growth in Europe operating income reflects solid sales growth and favorable foreign currency exchange rates during fiscal 2007 as compared to fiscal 2006. International operating income decreased 18% to $9.0 million in fiscal 2007 from $11.0 million in fiscal 2006. This decline reflects higher selling expenses due to increased sales and expanding sales forces.
Other Income, Net. Other income, net increased 50% to $21.3 million in fiscal 2007 from $14.3 million in fiscal 2006, while interest expense decreased 25% to $9.3 million in fiscal 2007 from $11.7 million in fiscal 2006. During fiscal 2007, interest expense decreased as borrowings were reduced and investment income increased as our cash and investments increased. To reduce the risk of exchange rate gains and losses on transfer of inventory from domestic sites to international sites, we have lines of credit in both Europe and Japan in local currencies. These lines of credit are used solely to fund inventory purchases, acquisitions, and pay dividends in those local currencies.
Provision (Benefit) for Income Taxes. The provision for income taxes decreased $39.4 million to $165.7 million, or 33% of income before income taxes, for fiscal 2007 from $205.1 million, or 34% of income before income taxes, for fiscal 2006. The effective income tax rate decreased primarily as a result of a higher proportionate share of taxable income in countries where tax rates are lower than in the U.S. and the continued benefit from the Qualified Production Activities Deduction in the United States.
Net Income. The factors mentioned above resulted in a 17% decrease in net income to $335.9 million in fiscal 2007 from $405.9 million in fiscal 2006.
Liquidity and Capital Resources
Cash Flows
The following is a summary of the cash flows by activity for the period from June 1, 2007 through July 11, 2007, from July 12, 2007 through November 30, 2007, from July 12, 2007 through May 31, 2008, and for the six months ended November 30, 2008.
44
Table of Contents
| Predecessor | Successor | |||||||||||||||||
| ($ in millions) | June 1, 2007 through July 11, 2007 | July 12, 2007 through November 30, 2007 | July 12, 2007 through May 31, 2008 | Six Months Ended November 30, 2008 | ||||||||||||||
Statement of Cash Flows Data: | ||||||||||||||||||
Net cash provided by/(used in): | ||||||||||||||||||
Operating Activities | $ | 60 | $ | 1 | $ | 248 | $ | 48 | ||||||||||
Investing Activities | 11 | (11,620 | ) | (11,711 | ) | (95 | ) | |||||||||||
Financing Activities | 1 | 11,525 | 11,483 | 157 | ||||||||||||||
Other Financial Data: | ||||||||||||||||||
Depreciation and amortization | $ | 9 | $ | 195 | $ | 470 | $ | 261 | ||||||||||
Capital expenditures | (22 | ) | (77 | ) | (190 | ) | (93 | ) | ||||||||||
Ratio of earnings to fixed charges(1) | — | — | — | — | ||||||||||||||
Six Months Ended November 30, 2008 Compared to July 12, 2007 through November 30, 2007
Operating Cash Flows
Cash flows provided by operating activities were $47.6 million for the six months ended November 30, 2008 compared to cash used in operating activities of $0.6 million for the period July 12, 2007 through November 30, 2007. Cash generated by operating activities continues to be a source of funds for investing in our growth. Net cash provided by operating activities for the six months ended November 30, 2008 included a net loss of $99.6 million, offset by non-cash amounts of $219.5 million and a use of cash due to working capital needs of $72.3 million, primarily due to increases in accounts receivable and inventory driven by sales growth. Net cash used in operating activities for the period July 12, 2007 through November 30, 2007 primarily related to the following:
| • | a net loss of $(784.2) million, partially offset by non-cash amounts of $522.6 million (primarily IPRD, depreciation and amortization as a result of the Merger), |
| • | a decrease in inventory of $20.5 million, |
| • | an increase in accrued interest of $106.3 million related to the debt financings entered into in connection with the Merger, and |
| • | a $87.1 million increase related to deferred financing costs, |
| • | partially offset by increase in accounts receivable of $29.7 million driven by sales growth. |
Investing Cash Flows
Cash flows used in investing activities were $95.1 million for the six months ended November 30, 2008 and $11,619.5 million for the period July 12, 2007 through November 30, 2007. Cash flows used in investing activities for the six months ended November 30, 2008 primarily related to capital expenditures of $92.9 million and for the period July 12, 2007 through November 30, 2007 primarily related to $11,638.2 million in connection with the acquisition of Biomet, Inc. as discussed in Note 1 to the unaudited condensed consolidated financial statements, and capital expenditures of $76.7 million, partially offset by net proceeds from the sale and purchase of investments of $95.8 million.
Financing Cash Flows
Cash flows provided by financing activities were $157.0 million for the six months ended November 30, 2008, and $11,525.1 million for the period July 12, 2007 through November 30, 2007. Cash flows used in financing activities for the six months ended November 30, 2008 primarily related to proceeds under the revolving credit facilities of $173.9 million, partially offset by payments under the senior secured credit facility of $18.2 million. Cash flows used in financing activities for the period July 12, 2007 through November 30, 2007 primarily related to capital contributions of $5,401.9 million and proceeds from long-term debt of $6,250.7 million in connection with the acquisition of Biomet, Inc. as discussed in Note 1 to the unaudited condensed consolidated financial statements.
July 12, 2007 through May 31, 2008
Our cash and investments were $127.6 million as of May 31, 2008. We maintain our cash and investments in money market funds, certificates of deposit, corporate bonds, debt instruments, auction-rate securities, mortgage-backed securities and equity securities. Our investments are generally liquid and investment grade. We are exposed to interest rate risk on our corporate bonds, debt instruments, fixed rate preferred equity securities and mortgage-backed securities.
Cash Flows from Operating Activities. Cash generated by operating activities continues to be a source of funds for investing in our growth. Net cash generated by operations was $188.9 million for the period July 12, 2007 through May 31, 2008. Cash generation during this period was impacted due to the following Merger-related items:
| • | $26.9 million of investment banking fees, |
45
Table of Contents
| • | $387.3 million paid for interest as a result of the Company’s significant new indebtedness following the Merger; and |
| • | $52.0 million in income taxes payments, being much lower than the expected tax payment had the Merger not occurred. |
Cash Flows from Investing Activities. Net cash used for investing was $11,721.8 million for the period from July 12, 2007 through May 31, 2008. The primary use of cash flows from investing activities for the period from July 12, 2007 through May 31, 2008 was the acquisition of Biomet, Inc. of $11,638.2 million, as discussed in Note 1 to our consolidated financial statements. In addition cash flows from investing activities were negatively impacted by capital expenditures of $167.9 million during this period, partly offset by proceeds from sale of investments of $84.7 million. Capital expenditures in fiscal 2008 included purchases of instruments in the United States of $37.0 million, which were sold to distributors in prior years.
Cash Flows from Financing Activities. Net cash from financing was $11,481.6 million for the period from July 12, 2007 through May 31, 2008. The primary inflow of cash flows from financing activities was for the acquisition of Biomet, Inc. as discussed in Note 1 to our consolidated financial statements, which included equity from Sponsors of $5,387.5 million and new debt facilities of $6,250.7 million. Net payments on credit agreements and deferred financing costs during the period were $291.0 million.
June 1, 2007 through July 11, 2007
Our cash and investments increased to $176.9 million at July 11, 2007, from $105.1 million at May 31, 2007. We maintain our cash and investments in money market funds, certificates of deposit, corporate bonds, debt instruments, auction-rate securities, mortgage-backed securities and equity securities. Our investments are generally liquid and investment grade. We are exposed to interest rate risk on its corporate bonds, debt instruments, fixed rate preferred equity securities and mortgage-backed securities. Net cash from operating activities was $59.4 million for the period June 1, 2007 through July 11, 2007, impacted by payments of $18.0 million to distributors associated with renegotiation of distribution agreements. Net cash provided by investing was $11.0 million primarily due to $42.8 million of proceeds from investing activities, which was partly offset by capital expenditures of $22.0 million for planned improvements to property, plant and equipment.
Debt Issuance and Credit Facilities
Senior Secured Cash Flow Facilities. On September 25, 2007, we entered into a credit agreement and related security and other agreements providing for (a) a $2,340 million U.S. dollar-denominated senior secured term loan facility and a €875 million (approximately $1,329 million at September 25, 2007) euro-denominated senior secured term loan facility and (b) a $400 million senior secured cash flow revolving credit facility with Bank of America, N.A. as administrative agent and collateral agent. We refer to our senior secured term loan facilities and our senior secured cash flow revolving credit facility collectively as the senior secured cash flow facilities.
We borrowed the full amount available under our senior secured term loan facilities on September 25, 2007. As of November 30, 2008 we have repaid $24 million of outstanding loans under our U.S. dollar-denominated senior secured term loan facility and $12 million of outstanding loans under our euro-denominated senior secured term loan facility. The senior secured cash flow revolving credit facility includes a $100 million sub-facility for letters of credit and a $100 million sub-capacity for borrowings on same-day notice, referred to as the swingline loans. We borrowed approximately $131 million under our senior secured cash flow revolving credit facility on September 25, 2007 to pay a portion of the Transactions. As of November 30, 2008, we had no outstanding borrowings under our senior secured cash flow credit facilities.
Borrowings under our senior secured cash flow facilities bear interest at a rate per annum equal to an applicable margin plus, at our option, either (1) a base rate determined by reference to the higher of (a) the prime rate of Bank of America, N.A. and (b) the federal funds effective rate plus 1/2 of 1.00% or (2) a LIBOR or Eurocurrency rate determined by reference to the cost of funds for deposits in the currency of such borrowing for the interest period relevant to such borrowing adjusted for certain additional costs. The initial applicable margin for borrowings under (x) our senior secured term loan facilities is 2.00% with respect to base rate borrowings and 3.00% with respect to LIBOR or Eurocurrency borrowings and (y) our senior secured cash flow revolving credit facility is 1.75% with respect to base rate borrowings and 2.75% with respect to LIBOR or Eurocurrency borrowings. The applicable margin under our senior secured cash flow revolving credit facility may be reduced based on our achievement of certain specified ratios. In connection with our senior secured term loan facilities, Biomet entered into a series of interest rate swap agreements with (1) an aggregate notional amount of $1,760 million to fix the interest rates on a portion of the borrowings under the $2,340 million U.S. dollar-denominated senior secured term loan facility and (2) an aggregate notional amount of €585 million to fix the interest rates on a portion of the borrowings under the €875 million (approximately $1,110 million at November 30, 2008) euro-denominated senior secured term loan facility. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Quantitative and Qualitative Disclosures about Market Risk—Interest Rate Risk.”
Senior Secured Asset-based Revolving Credit Facility. On September 25, 2007, we entered into a credit agreement and related security and other agreements for a senior secured asset-based revolving credit facility with Bank of America, N.A. as administrative agent and collateral agent. Our senior secured asset-based revolving credit facility provides senior secured financing of up to $350 million, subject to borrowing base limitations. The borrowing base at any time will equal the sum of 85% of eligible accounts receivable and 85% of the net orderly liquidation value of eligible inventory (not to exceed 65% of the borrowing base), less certain reserves and subject to certain limitations on consigned inventory and accounts receivable owed by non-U.S. persons. Our senior secured asset-based revolving credit facility includes a $100 million sub-facility for letters of credit and a $35 million sub-facility for borrowings on same-day notice, referred to as the swingline loans. We did not draw on our senior secured asset-based revolving credit facility at the closing of the Transactions. There were drawings of $165 million outstanding as of November 30, 2008. As of November 30, 2008, the borrowing base under our senior secured asset-based revolving credit facility was $321 million.
Borrowings under our senior secured asset-based revolving credit facility bears interest at a rate per annum equal to the applicable margin plus, at our option, either (1) a base rate determined by reference to the higher of (a) the prime rate of Bank of America, N.A. and (b) the federal funds effective rate plus 1/2 of 1.00% or (2) a LIBOR or Eurocurrency rate determined by reference to the cost of funds for deposits in the currency of such borrowing for the interest period relevant to such borrowing
46
Table of Contents
adjusted for certain additional costs. The initial applicable margin for borrowings under our senior secured asset-based revolving credit facility is 0.75% with respect to base rate borrowings and 1.75% with respect to LIBOR or Eurocurrency borrowings. The applicable margin may be reduced based on our achievement of certain specified ratios.
Notes. We issued an aggregate of $2,348 million of original notes on September 25, 2007 and an aggregate of $217 million of original notes on October 16, 2007 (which were issued at a premium above par of approximately $6 million). The notes are our unsecured obligations, with $1,550 million being our senior obligations (consisting of $775 million of senior cash pay notes and $775 million of senior toggle notes) and $1,015 million being our senior subordinated obligations. All of the notes are guaranteed by each of the existing and future wholly-owned domestic subsidiaries that guarantee our obligations under our senior secured cash flow facilities. Interest is payable in cash, except with respect to our ability to elect to pay PIK interest, rather than cash interest, on the senior toggle notes subject to certain exceptions.
The indentures governing the notes, among other things, limit our and our restricted subsidiaries’ ability to incur additional indebtedness or issue certain preferred stock, pay dividends and make other restricted payments, make certain investments, sell assets, create liens, consolidate, merge or sell all or substantially all of our assets, enter into transactions with affiliates and designate subsidiaries as unrestricted subsidiaries. These covenants are subject to important exceptions as described under “Description of Senior Exchange Notes—Certain Covenants” and “Description of Senior Subordinated Exchange Notes—Certain Covenants.”
Unsecured Credit Facilities. As of November 30, 2008, we had (1) a European line of credit in the amount of €50 million (approximately $63 million), (2) two Japanese lines of credit in the amount of ¥2.5 billion (approximately $24 million), and (3) a revolving line in Spain, together called the European credit facilities. Outstanding borrowings under all lines of credit bear interest at a variable rate of the lender’s interbank rate plus an applicable margin, except the Spain line which is interest free, and, accordingly, changes in interest rates impact our cost of financing. As of November 30, 2008, we had $45 million in outstanding borrowings under our European credit facilities.
Future Financing Activities
We believe that our cash, other liquid assets and operating cash flow, together with available borrowings and potential access to credit and capital markets, will be sufficient to meet our operating expenses, research and development costs and capital expenditures and service our debt requirements as they become due. As of November 30, 2008, we had (1) approximately $400 million available for borrowing under our senior secured cash flow revolving credit facility, (2) $326 million available for borrowing under our senior secured asset-based revolving credit facility, (3) the option to incur additional incremental term loans or increase the cash flow revolving credit facility commitments under our senior secured cash flow facilities of up to an amount that would cause our Senior Secured Leverage Ratio (as defined in our senior secured cash flow facilities) to be equal to or less than 4.50 to 1.00, (4) the option to increase the asset-based revolving credit commitments under our senior secured asset-based revolving credit facility by up to $100 million and (5) $171 million available for borrowing under our European and Japanese lines of credit. However, our ongoing ability to meet our substantial debt service and other obligations will be dependent upon our future performance which will be subject to business, financial and other factors. We will not be able to control many of these factors, such as economic conditions in the markets where we operate and pressure from competitors. We cannot be certain that our cash flows will be sufficient to allow us to pay principal and interest on our debt, support our operations and meet our other obligations. If we do not have enough money, we may be required to refinance all or part of our existing debt, sell assets or borrow more money. We cannot guarantee that we will be able to do so on terms acceptable to us, if at all. In addition, the terms of existing or future debt agreements may restrict us from pursuing any of these alternatives.
Capital Expenditures and Investments
We maintain our cash and investments in money market funds, certificates of deposit, corporate bonds, auction-rate securities, debt instruments, mortgage-backed securities and equity securities. We are exposed to interest rate risk on our corporate bonds, debt instruments, fixed rate preferred equity securities and mortgage-backed securities. We are confident about the growth prospects in our markets and intend to invest in an effort to improve our worldwide market position. We expect to spend in excess of $500 million over the next two fiscal years for capital expenditures (including instrumentation issued to the field) and research and development costs in an effort to develop products and technologies that further enhance musculoskeletal procedures. Funding of these and other activities is expected to come from currently available funds, cash flows generated from future operations, and currently available credit lines. We have no off-balance sheet financial arrangements.
Contractual Obligations
Summarized in the table below are our obligations and commitments as of November 30, 2008. We issued the notes and entered into senior secured credit facilities including senior secured term loan facilities and a senior secured cash flow revolving credit facility. Our senior secured term loan facilities amortize each year in an amount equal to 1% in equal quarterly installments for the first seven years and three months. As of November 30, 2008, the amount of principal payments due within the next twelve-month period was $36 million. The remaining short-term balance of $36.5 million is our outstanding balance under the European credit facilities.
47
Table of Contents
| (in millions) | Total | 2009 and 2010 | 2011 and 2012 | 2013 and 2014 | 2015 and Thereafter | ||||||||||
Contractual obligations* | |||||||||||||||
Projected future benefit payments | $ | 28.5 | $ | 11.1 | $ | 17.4 | $ | — | $ | — | |||||
Long-term debt (including current maturities) | 6,196.9 | 106.9 | 69.0 | 69.0 | 5,952.0 | ||||||||||
Interest payments (1) | 4,046.8 | 1,019.6 | 958.7 | 901.8 | 1,166.7 | ||||||||||
Material purchase commitments | 15.3 | 11.1 | 4.2 | — | — | ||||||||||
Outsourcing contract obligation | 30.8 | 10.2 | 13.6 | 7.0 | — | ||||||||||
Total contractual obligations | $ | 10,318.3 | $ | 1,158.9 | $ | 1,062.9 | $ | 977.8 | $ | 7,118.7 | |||||
| * | The total amounts of capital lease obligations, operating lease obligations and purchase obligations are not significant. |
| (1) | Includes interest rate swap liability of $120 million on both our Euro and US Dollar denominated swaps . |
This table reflects cash interest payments that have been calculated assuming the three-month LIBOR rate of 3.76% and Euro currency rate of 5.14% as of November 30, 2008 and do not take into consideration the interest rate swaps that are currently in place or any changes to our hedging program.
In addition, due to the uncertainty with respect to the timing of future cash flows associated with our unrecognized tax benefits at November 30, 2008, Biomet is unable to make reasonably reliable estimates of the period of cash settlement with the respective taxing authority. Therefore, $41 million of unrecognized tax benefits have been excluded from the contractual obligations table above.
Critical Accounting Policies and Estimates
Management’s discussion and analysis of our financial position and results of operations are based upon our consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of these financial statements requires management to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses, and related disclosure of contingent assets and liabilities. Our significant accounting policies are discussed in Note 2 of the Notes to Consolidated Financial Statements and in Note 2 of the Notes to Condensed Consolidated Financial Statements, each included elsewhere in the prospectus. In management’s opinion, our critical accounting policies include revenue recognition, excess and obsolete inventory, goodwill and intangible assets, accrued insurance, stock-based compensation expense, income taxes and valuation of purchased in-process research and development.
Revenue Recognition
We sell product through four principal channels: (1) direct to healthcare institutions, referred to as direct channel accounts, (2) through stocking distributors and healthcare dealers, (3) indirectly through insurance companies and (4) directly to dental practices and dental laboratories. Sales through the direct and distributor/dealer channels account for a majority of net sales. Through these channels, inventory is generally consigned to sales agents or customers so that products are available when needed for surgical procedures. Revenue is not recognized upon the placement of inventory into consignment as we retain title and maintain the inventory on the balance sheet; however, it is recognized upon implantation and receipt of proper purchase order and/or purchase requisition documentation. Pricing for products is generally predetermined by contracts with customers, agents acting on behalf of customer groups or by government regulatory bodies, depending on the market. Price discounts under group purchasing contracts are generally linked to volume of implant purchases by customer healthcare institutions within a specified group. At negotiated thresholds within a contract buying period, price discounts may increase. At certain locations we record a contractual allowance that is offset against revenue for each sale to a non-contracted payor so that revenue is recorded at the estimated determinable price at the time of the sale. At certain locations revenue is recognized on sales to stocking distributors, healthcare dealers, dental practices and dental laboratories when title to product passes to them, generally upon shipment. Certain subsidiaries allow customers to return product in the event that we terminate the relationship. Under those circumstances, we record an estimated sales return in the period in which constructive notice of termination is given to a distributor. Product returns were not significant for any period presented.
Excess and Obsolete Inventory
In our industry, inventory is routinely placed at hospitals to provide the healthcare provider with the appropriate product when needed. Because product usage tends to follow a bell curve, larger and smaller sizes of inventory are provided, but infrequently used. In addition, the musculoskeletal market is highly competitive, with new products, raw materials and procedures being introduced continually, which may obsolete products currently on the market. We must make estimates regarding the future use of these products and provide a provision for excess and obsolete inventory. If actual product life cycles, product demand or market conditions are less favorable than those projected by management, additional inventory write-downs may be required, which would affect future operating results.
Goodwill and Other Intangible Assets
In assessing the recoverability of our intangibles, we must make assumptions regarding estimated future cash flows and other factors to determine the fair value of the respective assets. If these estimates or their related assumptions change in the future, we may be required to record impairment charges for these assets.
48
Table of Contents
Accrued Insurance
As noted in Note 2 of the Notes to Consolidated Financial Statements included elsewhere in this prospectus, we have a self-insured retention against product liability claims with insurance coverage over and above the retention. There are various other claims, lawsuits, disputes with third parties, investigations and pending actions involving various allegations against us. Product liability claims are routinely reviewed by our insurance carrier and management routinely reviews all claims for purposes of establishing ultimate loss estimates. In addition, management must determine the estimated liability for claims incurred, but not reported. Such estimates and any subsequent changes in estimates may result in adjustments to our operating results in the future.
Stock-Based Compensation Expense
On June 1, 2006, we adopted revised SFAS No. 123(R), which requires all share-based payments to be recognized in our financial statements based on their respective grant date fair values. Under this standard, the fair value of each employee stock option is estimated on the date of grant using an option-pricing model that meets certain requirements. We currently use the Black-Scholes option-pricing model to estimate the fair value of our share-based payments. The determination of the fair value of share-based payment awards utilizing the Black-Scholes model is affected by our stock price and a number of assumptions, including expected volatility, expected life, risk-free interest rate and expected dividends. We estimate the expected volatility based on historical volatility of our Shares prior to July 11, 2008 and historical volatility of our competitors stock subsequent to this date. The expected life of the Options is based on historical and other data including life of the Option and vesting period. The risk-free interest rate assumption is the implied yield currently available on zero-coupon U.S. Government issues with a remaining term equal to the expected life of the Options. The dividend yield assumption is based on the historical dividend yield of our Shares. Forfeitures are required to be estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. We will evaluate the assumptions used to value stock-based awards periodically and adjust them if necessary. If factors change and we employ different assumptions, stock-based compensation expense may differ significantly from what we have recorded in the past.
Income Taxes
We record income tax estimates in accordance with SFAS 109, Accounting for Income Taxes, however, there are inherent risks that could create uncertainties related to the estimates. We adjust estimates based on normal operating circumstances and conclusions related to tax audits. We do not believe any audit finding could materially affect its financial position; however there could be a material impact on our consolidated results of operations of a given period.
Effective June 1, 2007, we adopted Financial Accounting Standards Board (FASB) Interpretation 48, Accounting for Uncertainty in Income Taxes—an interpretation of FASB Statement 109 (“FIN 48”). FIN 48 addresses the determination of whether tax benefits claimed or expected to be claimed on a tax return should be recorded in the financial statements. Under FIN 48, the tax benefits from an uncertain tax position may be recognized only if it is more likely than not that the tax position will be sustained upon examination by the taxing authorities, based on the technical merits of the position. FIN 48 also provides guidance on derecognition, classification, interest and penalties on income taxes, accounting in interim periods and requires increased disclosures. FIN 48 requires significant judgment in determining what constitutes an individual tax position as well as assessing the outcome of each tax position.
Valuation of Purchased In-Process Research and Development, Goodwill and Other Intangible Assets
When a business combination occurs, the purchase price is allocated based upon the fair value of tangible assets, in-process research and development, or IPRD, goodwill and intangible assets. We recognize IPRD in business combinations for the portion of the purchase price allocated to the appraised value of in-process technologies, defined as those technologies relating to products that have not received FDA approval and have no alternative future use. The portion assigned to in-process technologies excludes the value of core developed technologies, which are recognized as intangible assets when purchased. Valuations require the use of significant estimates. The amount of the purchase price allocated to IPRD is determined by estimating future cash flows of the technology and discounting net cash flows back to present values. We consider, among other things, the project’s stage of completion, complexity of the work competed as the acquisition date, costs already incurred, projected costs to complete, contribution of core technologies and other acquired assets, expected introduction date and the estimated useful life of the technology. The discount rate used to arrive at a present value as of the date of acquisition is based on the time value of money and medical technology investment risk. Goodwill represents the excess of cost over fair value of identifiable net assets of the business acquired and the amount allocated to IPRD. The methodologies used in arriving at these estimates are in accordance with accepted valuation methods.
Recent Accounting Pronouncements
In December 2007, the Financial Accounting Standards Board (“FASB”) issued SFAS 141R (revised 2007), Business Combinations. SFAS 141R establishes principles and requirements for how the acquirer in a business combination recognizes and measures in its financial statements, the identifiable assets acquired, the liabilities assumed and any noncontrolling interest in the acquiree at the acquisition date at fair value. SFAS 141R determines what information to disclose to enable users of the financial statements to evaluate the nature and financial effects of the business combination. SFAS 141R applies prospectively to business combinations for which the acquisition date is on or after the beginning of the first annual reporting period beginning on or after December 15, 2008. Early adoption is not permitted. We are currently evaluating the effect the adoption of FAS 141R will have on our unaudited condensed consolidated financial statements.
Effective June 1, 2008, we adopted FASB SFAS No. 157, Fair Value Measurements (“SFAS 157”). SFAS 157 establishes a framework for measuring fair value in accordance with generally accepted accounting principles, clarifies the definition of fair value within that framework, and expands disclosures about fair value measurements. SFAS 157 applies whenever other standards require (or permit) assets or liabilities to be measured at fair value, except for the measurement of share-based payments. SFAS 157 does not expand the use of fair value in any new circumstances. On February 12, 2008, the FASB issued FASB Staff Position (“FSP”) FAS 157-2, Effective Date of FASB Statement No. 157 (FSP FAS 157-2). FSP FAS 157-2 defers the implementation of SFAS 157 for certain nonfinancial assets and nonfinancial liabilities. Accordingly, we adopted the required provisions of SFAS 157 at the beginning of fiscal year 2009 and the remaining provisions will be adopted by us at the beginning of fiscal year 2010. The fiscal year 2009
49
Table of Contents
adoption did not result in a material impact to our financial statements (see Note 6). We are currently evaluating the impact of adopting the remaining parts of SFAS 157 in fiscal year 2010 in accordance with FSP FAS No. 157-2. In October 2008, the FASB issued FASB Staff Position No. 157-3, Determining the Fair Value of a Financial Asset When the Market for That Asset is Not Active, which clarifies the application of SFAS 157 in a market that is not active and provides an example to illustrate key considerations in determining fair value of a financial asset when the market for that financial asset is not active.
In February 2007, the FASB issued SFAS 159, Establishing the Fair Value Option for Financial Assets and Liabilities, to permit all entities to choose to elect to measure eligible financial instruments at fair value. SFAS 159 applies to fiscal years beginning after November 15, 2007, with early adoption permitted for an entity that has also elected to apply the provisions of SFAS 157. An entity is prohibited from retrospectively applying SFAS 159, unless it chooses early adoption. On June 1, 2008 we did not elect the fair value option for financial assets and liabilities held at June 1, 2008.
In December 2007, the FASB issued SFAS 160, Noncontrolling Interests in Consolidated Financial Statements – an amendment of ARB 51. SFAS 160 establishes accounting and reporting standards that require noncontrolling interests to be reported as a component of equity, changes in a parent’s ownership interest while the parent retains its controlling interest be accounted for as equity transactions, and any retained noncontrolling equity investment upon the deconsolidation of a subsidiary be initially measured at fair value. This statement is effective for fiscal years, and interim periods within those fiscal years, beginning on or after December 15, 2008. Earlier adoption is prohibited. We do not expect the adoption of SFAS 160 to have a material impact on our consolidated financial statements.
In March 2008, the FASB issued SFAS 161, Disclosures about Derivative Instruments and Hedging Activities-an Amendment of FASB Statement No. 133. This statement requires entities that utilize derivative instruments to provide qualitative disclosures about their objectives and strategies for using such instruments, as well as any details of credit-risk-related contingent features contained within derivatives. It also requires entities to disclose additional information about the amounts and location of derivatives located within the financial statements, how the provisions of SFAS No. 133 have been applied and the impact that hedges have on an entity’s financial position, financial performance and cash flows. This statement is effective for fiscal years, and interim periods within those fiscal years, beginning after November 15, 2008, with early adoption encouraged. We do not expect the adoption of SFAS 161 will have a material impact on our consolidated financial statements.
In May 2008, the FASB issued SFAS No. 162, The Hierarchy of Generally Accepted Accounting Principles. SFAS 162 identifies the sources of accounting principles and the framework for selecting the principles to be used in the preparation of financial statements of nongovernmental entities that are presented in conformity with generally accepted accounting principles in the United States of America. SFAS 162 is effective 60 days following the SEC’s approval of the Public Company Accounting Oversight Board amendments to AICPA Codification of Auditing Standards, AU Section 411, The Meaning of Present Fairly in Conformity with Generally Accepted Accounting Principles. We do not expect the adoption of SFAS 162 will have a material impact on our consolidated financial statements.
In December 2008, the FASB issued FASB Staff Position No. 140-4 and FIN 46(R)-8, Disclosures by Public Entities (Enterprises) about Transfers of Financial Assets and Interests in Variable Interest Entities. FAS 140-4 and FIN 46(R)-8 require additional disclosures about an entity’s involvement with variable interest entities and transfers of financial assets. FAS 140-4 and FIN 46(R)-8 will become effective for our fiscal year beginning June 1, 2009. We are currently evaluating the effect the adoption of FAS 140-4 and FIN 46(R)-8 will have on our consolidated financial statements.
In April 2008, the FASB issued FASB Staff Position No. 142-3, Determination of the Useful Life of Intangible Assets (FSP142-3). FSP 142-3 amends the factors that should be considered in developing renewal or extension assumptions that are used to determine the useful life of a recognized intangible asset under FASB Statement No. 142, Goodwill and Other Intangible Assets, and requires enhanced related disclosures. FSP 142-3 must be applied prospectively to all intangible assets acquired as of and subsequent to fiscal years beginning after December 15, 2008. We are in the process of determining the impact, if any, that the adoption of FSP 142-3 will have on our consolidated financial statements.
In June 2007, the FASB Emerging Issues Task Force issued EITF-07-3, Accounting for Nonrefundable Advance Payments for Goods or Services Received for Use in Future Research and Development Activities. EITF 07-3 provides guidance for entities that may make nonrefundable advance payments for goods or services that will be used in future research and development activities and whether the advance payment should be expensed when the advance payment is made or when the research and development activity has been performed. EITF 07-3 is effective for financial statements issued for fiscal years beginning after December 15, 2007. On June 1, 2008 we adopted EITF 07-3 and the impact was immaterial to our unaudited condensed consolidated financial statements.
In December 2007, the FASB issued EITF 07-1, Accounting for Collaborative Agreements (EITF 07-1). EITF 07-1 provides guidance regarding financial statement presentation and disclosure of collaborative arrangements, as defined, which includes arrangements the Company has entered into regarding development and commercialization of products. EITF 07-1 is effective for the Company as of March 1, 2009. We have not yet completed its evaluation of EITF 07-1, but do not currently believe that adoption will have a material impact on our unaudited condensed consolidated financial statements.
Quantitative and Qualitative Disclosures about Market Risk
In the normal course of business, our operations are exposed to fluctuations in interest rates and foreign currencies. These fluctuations can vary our cost of financing, investment yields and operations.
Interest Rate Risk
Our principal exposure to interest rate risk arises from variable rates associated with our credit facilities. For a description of these facilities, refer to Note 7 “Debt” to the audited financial statements included in this prospectus.
50
Table of Contents
We do not have any investments that would be classified as trading securities under generally accepted accounting principles. Our non-trading investments, excluding cash and cash equivalents, consist of debt securities, equity securities, auction-rate securities and mortgage-backed securities. The debt securities include municipal bonds, with fixed rates, and preferred stocks, which pay quarterly fixed rate dividends. These financial instruments are subject to market risk in that changes in interest rates would impact the market value of such investments. We utilize derivatives to hedge against increases in interest rates, in two areas: 1) one of our investment managers utilized U.S. Treasury bond futures options (futures options) as a protection against the impact of increases in interest rates on the fair value of preferred stocks and 2) interest rate swap agreements.
We mark any outstanding futures options to market and market value changes are recognized in current earnings. The futures options generally have terms ranging from 90 to 180 days. Net realized gains (losses) on sales of futures options aggregated zero for the period June 1, 2007 through July 11, 2007, for the period July 12, 2007 through November 30, 2007, for the period July 12, 2007 through May 31, 2008, for the six months ended November 30, 2008, and $0.1 million for the year ended May 31, 2007. Unrealized gains (losses) on outstanding futures options for the year ended May 31, 2007, for the period June 1, 2007 through July 11, 2007, for the period July 12, 2007, for the period July 12, 2007 through May 31, 2008, and for the six months ended November 30, 2008, were nominal.
On August 7, 2007 and August 17, 2007, we entered into a series of interest rate swap agreements with an aggregate notional amount of $1,890.0 million to fix the interest rates on a portion of the borrowings under the $2,340.0 million U.S. dollar-denominated senior secured term loan facility and during August 2007 and March 2008, we entered into a series of interest rate swap agreements with an aggregate notional amount of €505.0 million to fix the interest rates on a portion of the borrowings under the €875.0 million (approximately $1,329.0 million) euro-denominated senior secured term loan facility. As of November 30, 2008, the fair value of the interest rate swap agreements relating to our U.S. dollar-denominated senior secured term loan facility was approximately a $96.3 million net unrealized loss, and the fair value of the interest rate swap agreements relating to our euro-denominated senior secured term loan facility was approximately a €19.3 million (approximately $24.5 million) net unrealized loss.
Based on the our overall interest rate exposure at November 30, 2008, including variable rate debt, a hypothetical 10 % change in interest rates applied to the fair value of the financial instruments discussed above as of May 31, 2008 and November 30, 2008, respectively, would not have a material impact on earnings, cash flows or fair values of interest rate risk sensitive instruments over a one-year period. Net realized gains (losses) on sales of futures options were nominal for the period from June 1, 2007 through May 31, 2008 and the six months ended November 30, 2008 and there were no outstanding futures options at November 30, 2008.
Foreign Currency Risk
Certain assets, liabilities and forecasted transactions are exposed to foreign currency risk, primarily the fluctuation of the U.S. Dollar against European currencies. We face transactional currency exposures that arise when we or our foreign subsidiaries enter into transactions, generally on an intercompany basis, denominated in currencies other than their local currency. We also face currency exposure that arises from translating the results of our global operations to the U.S. dollar at exchange rates that fluctuate. We have not used financial derivatives to hedge against fluctuations in currency exchange rates, except we have designated our €875.0 million (approximately $1,329.0 million) euro-denominated senior secured term loan facility as a hedge of our net investment in our European subsidiary. Our net investment in our European subsidiary at the hedging date of September 25, 2007 was $1,690.0 million (€1,238.0 million). The difference of €363.0 million between the net investment and debt amount remained unhedged as of May 31, 2008. As a result of cash flow hedge treatment being applied, all gains and losses related to the derivative instrument are included in other comprehensive income. Effectiveness is tested quarterly to determine hedge treatment is still reasonable. We test effectiveness on this net investment hedge by determining that the net investment in our European subsidiaries is greater than the outstanding debt balance. If the hedge is deemed ineffective, gains and losses will be recorded through the statement of operations.
Based on our overall exposure for foreign currency at November 30, 2008, a hypothetical 10% change in foreign currency rates would not have a material impact on our balance sheet, net sales, net income (loss) or cash flows over a one-year period.
51
Table of Contents
We participate in the worldwide orthopedic and dental implant markets, which management estimates to be $30 billion in market size. These markets enjoy favorable industry dynamics and internally we estimate that these markets will grow at a compounded annual growth rate of 6% to 9% over the next twenty four months. The orthopedic industry benefits from several favorable factors, including, but not limited to:
Favorable Demographics. An aging population is driving growth in the orthopedic products market. Many conditions that require orthopedic surgery affect people in middle age or later in life. As the baby boomer population ages and life expectancy increases, the elderly will represent a higher percentage of the overall population. According to a 2007 U.S. Census Bureau projection, the U.S. population aged 55 to 74 is expected to grow at approximately three times the average rate of population growth from 51 million and 18% of the population in 2007 to 76 million and 22% of the population by 2027. According to a 2006 Eurostat projection, the European population aged 65 and over will grow at approximately 16 times the average rate of population growth from 77 million and 17% of the population in 2005 to 135 million and 30% of the population in 2025.
Stable Industry Structure. Following a period of consolidation during the late 1990s, over the past nine years, we, together with Zimmer Holdings, Inc., DePuy, Inc. (a Johnson & Johnson company), Stryker Corporation and Smith & Nephew plc, have constituted over 85% of the orthopedic reconstructive industry’s worldwide revenues. These players have achieved critical components to success, including product innovations and advancements, accumulation of clinical data, regulatory expertise, economies of scale, and salesforce and surgeon customer relationships, which have led to minimal market share movement among top players from year to year.
Close Working Relationships with Surgeon Customers. Due to the nature of orthopedic implants, the orthopedic medical device industry is unique with respect to the working relationships between orthopedic device manufacturers and their surgeon customers. As a component of innovation in the industry, some surgeons serve as consultants and are instrumental in the development of new products and the ongoing evaluation and improvement of existing products.
Technological Advancement of Orthopedic Products. Incremental and continuous technological advancement of orthopedic products is expanding the addressable market. Product innovation is improving the durability and performance of orthopedic devices and promoting less invasive surgery. Examples include bearing surfaces in hips with potential for greater longevity, premium knee systems that allow greater range of motion, and press-fit hip stems that facilitate minimally invasive hip procedures. As a result of this ongoing innovation, we believe that surgeons are increasingly recommending and utilizing implant products for younger patients as well as elderly patients who are remaining healthier and more active than those of past generations.
Favorable Product Mix Shift. Continued product innovation is driving a favorable shift in mix towards premium products that offer enhanced outcomes for patients. Product evolution is also expanding the addressable market to include younger patients who are more likely to require and demand premium and high-performance products. In addition, the payor mix resulting from the broadening of the patient population to younger patients with private insurance creates a favorable environment due to the fact that joint procedures for non-Medicare payors are generally more profitable for hospitals.
52
Table of Contents
General
We are one of the largest orthopedic medical device companies in the United States and worldwide with operations in over 50 locations throughout the world and distribution in approximately 90 countries. We design, manufacture and market a comprehensive range of both surgical and non-surgical products used primarily by orthopedic surgeons and other musculoskeletal medical specialists. For fiscal 2008 and the six months ended November 30, 2008, we generated pro forma net sales of $2,383 million and $1,250 million, respectively.
We operate in one business segment, musculoskeletal products, which includes the design, manufacture and marketing of products in four major market categories: Reconstructive Products, Fixation Devices, Spinal Products and Other Products. We have three reportable geographic markets: United States, Europe and International.
Reconstructive Products. We are a worldwide leader in our principal market category, Reconstructive Products. Primary product offerings include implants and instrumentation for replacing knees and hips as well as extremity joints that have deteriorated due to disease (principally osteoarthritis) or injury. We have been among the fastest growing knee companies in the industry as a result of continued strong demand for our total and partial knee systems. We also believe that our innovative hip product offerings, including our broad platform of bearing options, represent competitive advantages and have led to excellent surgeon acceptance. This market category also includes our dental reconstructive device business, which includes implants and abutments, augmented by a growing line of our other reconstructive products such as regenerative products, accessories and biologics products. The Reconstructive Products category accounted for 74% of our pro forma net sales for fiscal 2008 and 75% of our net sales for the six months ended November 30, 2008.
Fixation Devices. Fixation devices are used for setting and stabilizing damaged bones to support and/or augment the body’s natural healing process. We are a market leader for electrical stimulation devices for trauma indications, offering implantable and non-invasive products to stimulate bone growth. Other products include internal fixation devices (such as nails, plates, screws, pins and wires used to stabilize traumatic bone injuries), external fixation devices (used to stabilize fractures when alternative methods of fixation are not suitable), craniomaxillofacial fixation systems and bone substitute materials. The Fixation Devices category accounted for 10% of our pro forma net sales for fiscal 2008 and 10% of our net sales for the six months ended November 30, 2008.
Spinal Products. Spinal products include devices and instrumentation for repairing defects or wear and tear in the vertebral column. Key products in this category include implantable and non-invasive electrical stimulation devices for spinal indications (used to enhance bone fusion success), spinal fixation systems used to stabilize the spine, bone substitute materials and allograft services used in spinal fusion procedures, as well as motion preservation systems. The Spinal Products category accounted for 8% of our pro forma net sales for fiscal 2008 and 8% of our net sales for the six months ended November 30, 2008.
Other Products. We manufacture and distribute a number of other products, including sports medicine products (used in minimally-invasive orthopedic surgical procedures), orthopedic support products (also referred to as softgoods and bracing products), operating room supplies, casting materials, general surgical instruments, wound care products and other surgical products. The Other Products category accounted for 8% of our pro forma net sales for fiscal 2008 and 7% of our net sales for the six months ended November 30, 2008.
The following charts set forth our net sales by market category and geographic markets for fiscal 2008.

Competitive Strengths
We believe we have a number of competitive strengths that will enable us to further enhance our position in the orthopedic medical device market.
Broad Market Leadership. We are the fourth largest player in the U.S. orthopedic reconstructive market and have maintained this position for over a decade. We have high representation at U.S. hospitals, supplying products to over 60% of hospitals performing joint replacement surgery. In addition, we are the third largest manufacturer and marketer of dental reconstructive products worldwide and maintain leadership positions in the electrical stimulation and craniomaxillofacial fields.
53
Table of Contents
Leading Research and Development Platform. We have a long history of innovation, engineering, quality and successful new product launches. Demonstrating our research and development leadership, we have launched approximately 800 new products in the past nine fiscal years and plan to introduce approximately 100 new products during fiscal 2009.
Strong Relationships with Surgeon Customers. Based on their understanding of and satisfaction with our product, we enjoy long-standing relationships with our surgeon customers, many of which commence during the surgeons’ residency training program. Our support of medical education programs provides important training opportunities for orthopedic surgeons early in their career. In fact, supporting “hands-on” training provides opportunities for residents, fellows and attending surgeons to experience the clinical benefits of our products. Surgeons have historically exhibited limited willingness to switch manufacturers, as successful patient outcomes are related to the practitioners’ familiarity with the procedural characteristics and instrumentation of certain implants. Unfavorable conditions in the economy have had an adverse effect on our dental business for the three months ended November 30, 2008 as compared to the prior period principally due to the elective nature of dental implant procedures, which are typically not reimbursed by governmental agencies, as payment is primarily received from dental practices and laboratories. While we have already undertaken and continue to undertake certain operating initiatives in connection with this business, we anticipate that the growth rate of our worldwide dental business will remain flat or have low to mid single digit growth during the current global recessionary environment, compared to reported double digit growth in the most recent prior year.
Economic Uncertainties
Our results of operations could be substantially affected not only by global economic conditions, but also by local operating and economic conditions, which can vary substantially by market. Unfavorable conditions can depress sales in a given market and may result in actions that adversely affect our margins, constrain our operating flexibility or result in charges which are unusual or non-recurring. Certain macroeconomic events, such as the current crisis in the financial markets, could have a more wide-ranging and prolonged impact on the general business environment, which could also adversely affect us. For example, due to certain patient insurance deductibles resetting on January 1, 2009, it is possible that we could experience a potential adverse impact on sales due to patients deferring procedures because of decreased cash flow.
Historically, we believe that this slowdown due to the uncertain or recessionary environment has been minor to the orthopedic business, however, management is taking precautionary measures to be able to manage expenses more conservatively, if revenues were to decrease below those internally forecasted.
Consistently Strong Operating Cash Flow Generation. Our business is characterized by consistently strong operating cash flows due to our robust operating history and moderate capital intensity. We have continually increased both revenues and profitability, with fiscal 2008 representing our 30th consecutive year of year-over-year net sales and Adjusted EBITDA growth. Over the last 16 years, from fiscal 1992 to fiscal 2008, we increased both net sales and Adjusted EBITDA at compounded annual growth rates of approximately 15%. We have sustained growth through multiple macro-economic cycles, demonstrating a stable business profile. In addition, we have historically had modest capital expenditures and working capital requirements providing for strong operating cash flow conversion.
Experienced and Dedicated Management Team. We have a highly experienced management team at both the corporate and operational level. Our team is led by Jeffrey R. Binder, a 15-year veteran of the orthopedic medical device industry, who was appointed President and Chief Executive Officer in February 2007. Daniel P. Florin was appointed Senior Vice President and Chief Financial Officer in June 2007 and brings 16 years of financial officer/controller experience in the medical device industry and five years of public accounting and auditing experience to Biomet. Glen A. Kashuba was appointed Senior Vice President and President of Biomet Trauma and Biomet Spine, or BTBS, in April 2007, having previously served as Worldwide President of Cordis Endovascular, a division of Johnson & Johnson. Gregory W. Sasso, who has been with us for 23 years, was appointed Senior Vice President and President of Biomet SBU Operations in June 2007. In February 2008, Jon C. Serbousek was appointed President of Biomet Orthopedics, having spent 21 years in the medical device industry including 8 years with Medtronic and 13 years with DePuy. Even though each of Messrs. Binder, Florin, Kashuba and Serbousek has been with us for less than two years, the members of our senior management team have an average tenure of 13 years with us. Overall, the members of our senior management team have an average tenure of 18 years in the medical device industry. During fiscal 2008 certain members of our management team made a contribution of new equity through cash equity contributions and/or rollover of existing equity interests in the Transactions.
Premier Equity Sponsorship. The Blackstone Group, Goldman Sachs Capital Partners, KKR and TPG are among the most well-known and respected financial sponsors in the world. The Sponsors have made investments in over 950 companies and collectively have more than $125 billion of assets under management. The Sponsors and the Co-Investors contributed approximately $5,387 million of equity in connection with the Transactions, representing 46% of the total funding for the Transactions, as part of one of the largest private equity investments in history. The Sponsors have considerable experience in the healthcare sector with investments in companies such as Accellent Inc., HCA Inc., IASIS Healthcare Corporation, Quintiles Transnational Corp., ReAble Therapeutics, Inc. and Vanguard Health Systems, Inc., among others.
Business Strategy
We intend to enhance our position as a leading orthopedic medical device company by pursuing the following strategic initiatives:
Continue to Develop and Launch New Products and Technologies. We plan to continue to aggressively develop new products, technologies and materials by leveraging our established research and development platform. While we have a strong engineering heritage, we recently have taken steps to enhance our research and development efforts, with the appointment of two global heads charged with coordinating research and development efforts across the organization, which should improve time to market and leverage best technologies and innovations available throughout all business segments and regions. We anticipate that our future research and development investment will be consistent with historical results as a percentage of net sales.
Enhance Surgeon Customer Relationships through Product Performance and Innovation. We intend to continue to meet the demanding needs of our surgeon customers and hospital customers by providing clinically superior and innovative products that offer a cost-effective means of treating patients. Our success has been built on responsiveness to the needs of the health care community, the outstanding clinical performance of our products and our ongoing commitment to continued product innovation.
54
Table of Contents
Expand Our Global Reach. We intend to continue to increase the geographic presence of each of our business categories. There are considerable opportunities for global expansion as healthcare spending increases in international markets—the United States and Canada together accounted for approximately 65% of the global orthopedic market in 2007, but only approximately 5% of the world’s population. We particularly plan to focus on deepening our position in under-penetrated regions with attractive opportunities for growth, including Asia and Latin America, by deploying more resources to capture market opportunities, as well as by leveraging our established worldwide manufacturing facilities and salesforce. We believe we can successfully grow our presence in these regions by differentiating ourselves as a provider with a comprehensive portfolio of leading musculoskeletal products.
Focus on Operational Efficiency. We have identified significant opportunities to streamline operations. The historically decentralized nature of our management and decision-making structure creates opportunities to improve operational efficiency as we centralize operations and increase focus, coordination and accountability throughout the organization. Plans include manufacturing footprint optimization, implementation of Six Sigma and Lean Manufacturing, procurement and offshoring initiatives, as well as reduction in overhead expenses. These initiatives will enable us to maximize asset utilization, optimize working capital and increase cash flow, as well as accelerate product development and enhance customer service.
Maximize Operating Cash Flow. We are focused on maximizing our operating cash flow. Over the last 20 years, we have consistently generated significant operating cash flow due to our business growth, strong operating margins and modest capital expenditure and other cash requirements. These solid business fundamentals will be supplemented by recently implemented initiatives to improve working capital, which historically has not been a focus area of management. In addition, we will benefit from identified cost savings as we enhance operational efficiencies. We plan to use available cash after capital expenditures to reduce leverage and strengthen our balance sheet.
Products
Our product portfolio is divided into four market categories: Reconstructive Products, Fixation Devices, Spinal Products and Other Products.
Reconstructive Products
Orthopedic reconstructive implants are used to replace joints that have deteriorated as a result of disease (principally osteoarthritis) or injury. Reconstructive joint surgery involves the modification of the area surrounding the affected joint and the implantation of one or more manufactured components, and may involve the use of bone cement. Our primary orthopedic reconstructive joints are knees, hips and shoulders, but we produce other joints as well. We also produce the associated instruments required by orthopedic surgeons to implant our reconstructive devices, as well as bone cements and cement delivery systems. In addition, dental reconstructive devices and associated instrumentation are used for oral rehabilitation through the replacement of teeth and repair of hard and soft tissues.
Knee Systems. A total knee replacement typically includes a femoral component, a patellar component, a tibial component and an articulating surface. Total knee replacement may occur as an initial joint replacement procedure, or as a revision procedure, which may be required to replace, repair or enhance the initial implant. Partial, or unicompartmental, knee replacement is an option when only a portion of the knee requires replacement.
Our newest and most comprehensive total knee system, the Vanguard™ Complete Knee System, accommodates up to 145 degrees of flexion and offers full interchangeability of the system’s components to provide a precise fit for each patient. The Vanguard™ System may be implanted using our Premier™ Instrumentation for a conventional procedure or our Microplasty® Minimally Invasive Total Knee Instrumentation, which is designed to reduce incision size and surrounding soft tissue disruption, which may provide reduced blood loss, a shortened hospital stay, reduced postoperative pain and less time spent in rehabilitation, as compared to a conventional procedure. During fiscal 2008, we continued the development efforts for the rotating platform version of the Vanguard™ Complete Knee System.
We continue to be a market leader in addressing the increasing demand from practitioners and patients for procedures and products accommodating minimally-invasive knee techniques. The Oxford® Partial Knee, which is a mobile-bearing unicondylar knee that utilizes a minimally-invasive technique, continues to experience strong global sales. The Oxford® Knee, which was introduced in the United States during fiscal 2005, is currently the only free-floating meniscal bearing unicompartmental system approved for use in the United States. Our offering of minimally-invasive partial knee systems also includes the Alpina® Unicompartmental Knee (which is not currently available in the United States); the Vanguard M™ Series Unicompartmental Knee System, a modified version of the Oxford® Knee that incorporates a fixed-bearing tibial component as opposed to a free floating tibial bearing; and the Repicci II® Unicondylar Knee System that is now being distributed by our sports medicine division.
During fiscal 2008, we introduced the Signature™ Personalized Arthritis Care program. The initial introduction was designed specifically for knee procedures. The Signature™ program uses a patient’s MRI data to deliver patient-specific alignment guides to the surgeon for improved pre-operative planning and for implementation during the procedure. The Signature™ program was developed through a partnership with Materialise, a world leader in custom guides for the dental industry, and we believe this technology may be expanded to other orthopedic applications.
Hip Systems. A total hip replacement involves the replacement of the head of the femur and the acetabulum, and may occur as an initial joint replacement procedure, or as a revision procedure, which may be required to replace, repair or enhance the initial implant. A femoral hip prosthesis consists of a femoral head and stem, which can be cast, forged or wrought, depending on the design and material used. Acetabular components include a prosthetic replacement of the socket portion, or acetabulum, of the pelvic bone. Because of variations in human anatomy and differing design preferences among surgeons, we manufacture femoral and acetabular prostheses in a variety of sizes and configurations. We offer a broad array of total hip systems, most of which utilize titanium or
55
Table of Contents
cobalt chromium alloy femoral components and our patented ArCom® , ArComXL® or E-Poly™ polyethylene-lined, metal-on-metal or ceramic-on-ceramic acetabular components. Many of the femoral prostheses utilize our proprietary PPS® porous plasma spray coating, which enables cement-less fixation.
Out of our broad product platform of hip stem offerings, the Taperloc® Hip System has become our best-selling component. The Taperloc® Stem is marketed for non-cemented use in patients undergoing primary or revision hip replacement surgery as a result of noninflammatory degenerative joint disease. The Taperloc® femoral component is a collarless, flat, wedge-shaped implant designed to provide excellent durability and stability in a design that is relatively simple to implant and is particularly well-suited for minimally-invasive procedures. We also offer the Taperloc® Microplasty™ Stem that addresses the demand for a minimally-invasive, bone-conserving total hip implant. The shorter length of the Microplasty™ Stem, compared to a traditional hip stem, allows for preservation of distal bone, while maintaining proximal femoral bone fixation.
Our comprehensive Microplasty™ Minimally Invasive Hip Program includes proprietary products from our broad array of hip products, as well as a distinctive training program and uniquely-designed instruments for a minimally-invasive approach. Our minimally-invasive hip development efforts have been focused on various surgical approaches, including an anterior supine approach, which is an intramuscular surgical approach.
We continue to explore the development of innovative articulation technologies and materials. Our M2a-Taper™ Acetabular System combines a cobalt chromium head with a cobalt chromium liner and has demonstrated a 20- to 100-fold reduction in volumetric wear in simulator studies compared to traditional metal-polyethylene articulation systems. The M2a-Taper™ Acetabular System may be utilized on all of our femoral components and has continued to evolve with the introduction of the M2a-Magnum™ Articulation System, which incorporates larger diameter metal-on-metal components designed to more closely resemble the natural anatomy, offering improved range of motion and joint stability. We introduced the C2a-Taper™ Acetabular System during fiscal 2006, which provides an additional alternative bearing option featuring ceramic-on-ceramic articulation. In addition, we are pursuing the development of a diamond-on-diamond hip articulation system through our relationship with Diamicron, Inc., a global leader in the research, development and manufacture of polycrystalline diamond composite technology for biomedical applications. We continue to market ArComXL®, which is a second-generation highly crosslinked polyethylene bearing material based on our proven ArCom® polyethylene. ArComXL® polyethylene has demonstrated excellent wear characteristics without measurable oxidation after accelerated aging. During fiscal 2007, we received FDA clearance to market acetabular hip liners manufactured from E-Poly™ Highly Crosslinked Polyethylene. We believe our E-Poly™ liners are the world’s first Vitamin E stabilized highly crosslinked polyethylene products to be introduced to the market. Vitamin E is a natural antioxidant and is expected to provide optimal oxidation resistance for the implant bearings used in our total joint replacements.
The ReCap® Total Resurfacing System is a bone-conserving product currently used outside the United States for patients in the early stages of degenerative joint disease, including osteoarthritis, rheumatoid arthritis and avascular necrosis. We commenced a clinical study for the ReCap® Total Resurfacing System in the United States during fiscal 2006 and there were more than 200 patients enrolled in the study as of November 30, 2008. The FDA recently accepted the concept of Biomet including European clinical data to support its U.S. Pre-Market Approval submission, subject to further review of the data after submission. We believe the potential exists to bring this product to the U.S. market during the second half of calendar 2009.
We introduced the Regenerex™ RingLoc®+ Acetabular System during fiscal 2008. The Regenerex™ Construct provides design flexibility and solutions for difficult primary and revision cases. The advanced titanium scaffold structure of the Regenerex™ Construct is a continuous three-dimensional matrix comprised of industry-standard Ti-6AL-4V. Titanium is a clinically proven material in the orthopedic market, with optimal biological fixation, and Regenerex™ is expected to be the material of choice for porous metal constructs.
Extremity Systems. We offer a variety of shoulder systems including the Absolute® Bi-Polar, Bi-Angular®, Bio-Modular®, Comprehensive®, Copeland™, Integrated™ and Mosaic™ Shoulder Systems, as well as uniquely-designed elbow replacement systems.
The Copeland™ Humeral Resurfacing Head was developed to minimize bone removal in shoulder procedures and has approximately 20 years of positive clinical results in the United Kingdom. This system was expanded to include the Copeland™ EAS™ (Extended Articular Surface) Humeral Resurfacing Head designed to address rotator cuff arthropathy.
The first Comprehensive® Primary Shoulder was released at the end of fiscal 2007. This initial release of the new Primary System included the standard and mini length Comprehensive® Primary Stems and the Versa-Dial™ Heads, as well as the hybrid glenoids. The Comprehensive® Primary System was fully released by the end of fiscal 2008.
T.E.S.S.™ Total Evolutive Shoulder System continues to receive strong market acceptance in Europe. The T.E.S.S.™ System, which is only available outside the United States, is a complete shoulder system that can be used in all indications of shoulder arthroplasty.
Dental Reconstructive Devices. Through our subsidiary, Biomet 3i LLC (formerly Implant Innovations, Inc.), or Biomet 3i, we develop, manufacture and market products designed to enhance oral rehabilitation through the replacement of teeth and the repair of hard and soft tissues. These products include dental reconstructive devices and related instrumentation, bone substitute materials and regenerative products and materials. A dental implant is a small screw, normally constructed of titanium or titanium alloy, which is surgically placed in the bone of the jaw to replace the root of a missing tooth and provide an anchor for an artificial tooth.
Biomet 3i’s historical flagship product, the OSSEOTITE® product line, features a patented micro-roughened surface technology, which allows for early loading and improved bone integration to the surface of the implant compared to machined surfaced implants. In fiscal 2007, Biomet 3i further enhanced implant surface technology with the introduction of the NanoTite™ Implant. The surface features the application of nanometer scale crystals of calcium phosphate to the existing OSSEOTITE® surface. This enhancement has been demonstrated via preclinical animal studies to increase the rate and extent of bone integration and results in a mechanical bonding of the host bone to the surface of the implant compared with the OSSEOTITE® surface alone. The NanoTite™ Implant was initially introduced in Certain® Implant configurations, which is an internal connection system that, through the use of the QuickSeat® connection, provides audible and tactile feedback when restorative abutments and ancillary components are seated into the implant.
56
Table of Contents
In addition, the 6/12 point connection design of the Certain® Implant System offers enhanced flexibility in placing the implant where preangled abutments may be used. In fiscal 2008, Biomet 3i continued to build on the strength of the NanoTite Implant line by introducing the NanoTite Certain Tapered Prevail® configuration. This implant is designed to enhance crestal bone preservation as a result of its integration of Platform Switching™—a medialized Implant-Abutment-Junction that has been demonstrated in literature to limit the reformation of soft and hard tissue at the bone crest. This is the first tapered geometry implant available from Biomet 3i that includes the platform switching concept. Other additions for the tapered implant category in fiscal 2008 included a complete set of bone taps for dense bone applications and a 6mm diameter implant with the same implant body design enhancements as implemented for other diameters.
In the site preparation segment of the product portfolio, Biomet 3i completed beta evaluations of its Navigator™ CT Guidance Instrumentation Kits and commercially launched this product during the third quarter of 2008. This open architecture instrumentation is designed to interface with the software and surgical guide solutions offered by existing entities in the marketplace. As planning and guide fabrication are based upon computed tomography scans, this can result in accurate implant placement when combined with the depth and rotational control offered by the Biomet 3i instrumentation. As implant placement position can be replicated as planned, this can also provide the opportunity for fabrication of a provisional prosthesis in advance of surgery thereby allowing for a complete implant restoration in one patient visit. On the regenerative side of the site preparation portfolio, Biomet 3i has bolstered its bone grafting product and service offering. An exclusive agreement was signed with University of Miami Tissue Bank for domestic representation of its dental allograft services. The RegenerOss™ Allograft Putty became available during the third quarter of fiscal 2008. This material features a demineralized bone matrix material in a non-toxic lecithin carrier conveniently offered in a syringe-based delivery system. In the fourth quarter 2008, Biomet 3i introduced Endobon® Xenograft Granules. This bovine-derived particulate bone grafting material is suitable for use in a wide range of dental related bone defects and offers improved handling characteristics and packaging versus some of the competitive products in this category.
During fiscal 2008, Biomet 3i engaged in a limited domestic release of its Encode®Complete patient specific abutment technology. This enhancement of the baseline Encode abutment offering will allow Biomet 3i to fabricate an abutment and orient implant body analogs in the proper position in a stone master model. This can allow for the complete fabrication of a restoration from one supragingival impression—significantly easier than present techniques and a potential opportunity to get more general dentists involved in implant therapy. The quality of these abutments and ability to save significant chair time will also be of potential benefit to more experienced restorative dentists. There was a line extension in fiscal 2008 to the patient-specific CAM StructSURE® bars to include a copy milling capability. This allows a dental laboratory to create a unique design in a resin-based material. This is scanned and milled from titanium where a porcelain finish is later added by the source laboratory. Other restorative product launches in fiscal 2008 included QuickBridge™ provisionalization components and non-hexed UCLA abutments for the Biomet 3i 3.4mm restorative platform.
Other Reconstructive Devices. Our PMI® Patient-Matched Implant services group expeditiously designs, manufactures and delivers one-of-a-kind reconstructive devices to orthopedic specialists. We believe this service continues to enhance our reconstructive sales by strengthening our relationships with orthopedic surgeons and augmenting our reputation as a responsive company committed to excellent product design. In order to assist orthopedic surgeons and their surgical teams in preoperative planning, our PMI® group utilizes a three- dimensional (“3-D”) bone reconstruction imaging system. We use computed tomography (“CT”) data to produce 3-D reconstructions for the design and manufacture of patient-matched implants. With this imaging and model-making technology, our PMI® group is able to assist the physician prior to surgery by creating 3-D models. Within strict deadlines, the model is used by engineers, working closely with the surgeon, to create a PMI® design for the actual manufacturing of the custom implant for the patient.
We are involved in the ongoing development of bone cements and delivery systems. We have broadened the range of our internally developed and manufactured bone cement product offerings. Cobalt™ HV (High Viscosity) Bone Cement, which was introduced in the United States during fiscal 2006, is particularly well suited for use in minimally-invasive surgery, but may be used in all applicable joint replacement procedures. The excellent handling characteristics and high optical contrast of Cobalt™ HV Bone Cement are well suited to the current trends in orthopedic surgery. The patented SoftPac™ monomer packaging offers the only alternative to glass vial packaging, which is inherently less safe due to the necessity to break the glass vial to deliver the monomer. We offer our internally developed and manufactured bone cements with and without antibiotics. In conjunction with antibiotic loaded-bone cement is the use of StageOne™ Cement Spacer Molds. The molds are used in revision surgery following infection as the first stage of a two stage treatment plan. Cobalt™ Bone Cement is marketed in conjunction with our patented Optivac® Vacuum Mixing System. During fiscal 2007, the Fusion™ Vacuum Mixing Bowl was launched to address the open bowl mixing market. In Europe, we introduced the OptiPac™ preloaded bone cement mixing and delivery system during fiscal 2008.
Additional products and services for reconstructive indications include bone substitute materials and services related to allograft material. We also provide services related to the supply of allograft material procured through several tissue bank alliances. Markets addressed by our allograft services include the orthopedic and dental reconstructive market segments, as well as the spinal, craniomaxillofacial and sports medicine segments.
The GPS® III Gravitational Platelet Separation System is a unique device that collects platelet concentrate from a small volume of the patient’s blood using a fast, single spin process, offering a high-quality platelet concentrate that has broad potential applications in the reconstructive and spine markets. The GPS® III System is marketed in conjunction with the Biomet® Rapid Recovery Program, a comprehensive approach to patient education, a minimally-invasive surgical approach and pain management that was developed in conjunction with leading orthopedic surgeons in the United States.
Fixation Devices
Our fixation products include electrical stimulation devices (that do not address the spine), external fixation devices, craniomaxillofacial fixation systems, internal fixation devices and bone substitute materials utilized in fracture fixation applications. Our craniomaxillofacial fixation products are marketed by our subsidiary, Biomet Microfixation, LLC, or Biomet Microfixation. All other fixation products are marketed primarily by Biomet Trauma.
57
Table of Contents
Electrical Stimulation Systems. We are a market leader in the electrical stimulation segment of the fixation market. The FDA has acknowledged our extensive preclinical research documenting the Mechanism of Action for our pulsed electromagnetic field (“PEMF”), capacitative coupling and direct current technologies. The Mechanism of Action for these technologies involves the stimulation of a cascade of bone morphogenic proteins (“BMPs”), as well as angiogenesis, chondrogenesis and osteogenesis.
The EBI Bone Healing System® unit is a non-invasive bone growth stimulation device indicated for the treatment of recalcitrant bone fractures (nonunions), failed fusions and congenital pseudarthrosis that have not healed with conventional surgical and/or non-surgical methods. The non-invasive bone growth stimulation devices sold by us generally provide an alternative to surgical intervention in the management of these bony applications. The EBI Bone Healing System® units produce low-energy PEMF signals that induce weak pulsing currents in living tissues that are exposed to the signals. These pulses, when suitably configured in amplitude, repetition and duration, affect living bone cells to differentiate, migrate and proliferate. The Mechanism of Action behind the PEMF technology involves the stimulation of growth factors involved in normal bone healing. Biomet Trauma’s preclinical research demonstrates that PEMF signals increase a number of growth factors, such as TGF-ß, BMP-2 and BMP-4, which are normal physiological regulators of the various stages of bone healing, including angiogenesis, chondrogenesis and osteogenesis. The EBI Bone Healing System® unit may be utilized over a patient’s cast, incorporated into the cast or worn over the skin.
The OrthoPak® 2 Bone Growth Stimulator, which is indicated for the treatment of recalcitrant (nonunion) fractures, offers a small, lightweight, non-invasive device using capacitive coupling technology. The OrthoPak® 2 device delivers bone growth stimulation through wafer-thin electrodes that add virtually no extra weight on the nonunion site. The Mechanism of Action behind our capacitive coupling stimulation technology involves the stimulation of osteopromotive factors involved in normal bone healing, such as TGF-ß1 and PGE2. The OrthoPak® 2 product provides greater ease of use and enhances access to fracture sites that are normally hard to treat.
We also offer an implantable option when bone growth stimulation is required in conjunction with or subsequent to surgical intervention. The Biomet® OsteoGen™ surgically implanted bone growth stimulator is an adjunct treatment when bone grafting and surgical intervention are required to treat recalcitrant (nonunion) fractures in long bones. The Mechanism of Action behind our direct current stimulation technology involves the stimulation of a number of osteoinductive growth factors including BMP-2, -6 and -7 and the BMP-2 receptor ALK2, which are normal physiological regulators of various stages of bone healing, including chondrogenesis and osteogenesis. In addition, electrochemical reactions at the cathode lower oxygen concentrations and increase pH.
During fiscal 2005, a private company petitioned the FDA to reclassify noninvasive bone growth stimulators from Class III to Class II medical devices. The petition was directed at products, like those described above, that utilize electromagnetic fields to stimulate bone growth. In June 2006, the FDA Advisory Panel recommended that the bone growth stimulator devices remain Class III devices. On January 17, 2007, the FDA published its agreement and sought public comment on the Advisory Panel’s recommendation that bone growth stimulators remain Class III devices. The private company that had petitioned for the down-classification of bone growth stimulators has since formally withdrawn that request. It is our understanding that bone growth stimulators will remain Class III devices.
External Fixation Devices. External fixation is utilized for stabilization of fractures when alternative methods of fixation are not suitable. We offer a complete line of systems that address the various segments of the trauma and reconstructive external fixation marketplace. The DynaFix® and DynaFix® Vision™ Systems are innovative, modular external fixation devices intended for use in complex trauma situations involving upper extremities, the pelvis and lower extremities. The recently introduced Advanced Biomet® Vision FootRing System is a comprehensive external fixation system designed for the treatment of osteotomies, arthrodesis and fracture fixation indications. This system offers expanded indications for both trauma and reconstructive procedures. The simplified, snap-fit application of all fixation components to the Vision™ Ring can be configured into a multitude of constructs ranging from simple fractures to complex reconstruction. The Vision™ FootRing System is made of lightweight, carbon fiber, which is radiolucent and also provides for increased patient comfort. Biomet Trauma also has a full line of external fixation products for certain reconstructive procedures involving limb lengthening, fusion, articulated fixation and deformity correction applications.
Internal Fixation Devices. Our internal fixation devices include products such as nails, plates, screws, pins and wires designed to stabilize traumatic bone injuries. These devices are used by orthopedic surgeons to provide an accurate means of setting and stabilizing fractures and for other reconstructive procedures. They are intended to aid in the healing process and may be removed when healing is complete. Internal fixation devices are not intended to replace normal body structures.
We develop, manufacture and/or distribute innovative products that fit into key segments of the fixation marketplace. Our flagship product used for the treatment of hip fractures is the Biomet® Peritrochanteric Nail System that incorporates an innovative single lag screw to minimize soft tissue impingement. In conjunction with the VHS® * System, the Biomet® Peritrochanteric Nail System offers a choice of options for the treatment of these fractures.
Other innovative nailing products that have been introduced are the Biomet® Pediatric Locking Nail (PLN) and the Biomet WIN™ Flexible Nail that complement our pediatric product line. The PLN, a customizable locking nail, was designed to provide stable fixation of femur fractures in children. The WIN™ Nail is manufactured of titanium alloy and is intended to treat a variety of long bone fractures.
In the area of locked plating designs, the OptiLock® Periarticular Plating System is a unique, pre-contoured plating system designed for fixation of periarticular lower extremity fractures. It incorporates patent-pending Sphere Lock technology that allows the surgeon to utilize locked or unlocked screws in various diameters through any hole in the plate, while incorporating minimally-invasive techniques. The system includes applications for the treatment of proximal tibial, distal femoral and distal tibial/fibular fractures. The release of the system was completed during the first quarter of fiscal 2008 and provides surgeons with a comprehensive system to address a variety of simple and complex periarticular fractures.
* | VHS® is a registered trademark of Implant Distribution Network, Ltd. |
58
Table of Contents
During the third quarter of fiscal 2008, Biomet Trauma introduced the Phoenix Tibial Nailing System, the first in a series of Phoenix Intramedullary Nails to be released this year. Featuring patent-pending CoreLock technology, the nail offers a pre-assembled setscrew that dually locks the proximal oblique screws for enhanced stable fixation and also allows surgeons to utilize up to 5mm of inboard compression for acute fracture reduction. In addition, the nail features a distal bone screw cluster that maximizes the working length of the nail. Surgeon feedback to date continues to be positive with respect to clinical results, implant design and instrumentation.
In the fourth quarter of fiscal 2008, we initiated the clinical evaluation for the remaining modules of the Phoenix Intramedullary Nailing System. Included in this offering are the Phoenix Retrograde Femoral Nail and the Phoenix Antegrade Femoral Nails. Each of these systems offers CoreLock Technology that features embedded set screws that can simultaneously lock bone screw clusters for stable fracture fixation.
During fiscal 2008, we have continued to make innovative improvements in hip fracture, locked plating, external fixation and intramedullary fixation devices to enhance our portfolio of fixation implants for the trauma marketplace.
Craniomaxillofacial Fixation Systems. We manufacture and distribute craniomaxillofacial, neurosurgical, and thoracic titanium and resorbable implants, along with associated surgical instrumentation, principally marketed to craniomaxillofacial, neurosurgical, plastic, ear/nose/throat, pediatric and cardiothoracic surgeons through Biomet Microfixation. We offer HTR-PMI Hard Tissue Replacement for repair of severe cranial defects and bone substitute material for use in craniomaxillofacial and neurosurgical applications. Innovative solutions are also offered for oral and maxillofacial surgeons with an off-the-shelf Total Mandibular Joint Replacement System and other new products for TMJ, including in-office scope systems and arthrocentesis procedure products.
Biomet Microfixation markets the LactoSorb® Fixation System of resorbable plates and screws comprised of a copolymer of poly-L-lactic acid and polyglycolic acid. As a result of its innovative material, the LactoSorb® system is comparable in strength to titanium plating systems at its initial placement and is resorbed within 9 to 15 months after implantation. The LactoSorb® system is especially beneficial in pediatric reconstruction cases by eliminating the need for additional surgery to remove the plates and screws.
Biomet Microfixation rolled out Allogenix™ Plus bone graft material during fiscal 2008. This biomaterial combines the lecithin-based Allogenix™ Demineralized Bone Matrix with ProOsteon® granules, resulting in an improved bone graft material. By combining a scaffold with an osteoinductive source, the need for a second procedure in order to harvest bone chips for use as a scaffold may be eliminated.
Bone Substitute Materials. When presented with a patient demonstrating a bone defect, such as a fractured bone or bone loss due to removal of a tumor, the treating surgeon may remove a portion of bone from the patient at a second site to use as a graft to induce healing at the site of the defect. Bone substitute materials eliminate the pain created at a graft site, as well as the costs associated with this additional surgical procedure. Depending on the specific use of the bone substitute material, it can have reconstructive, fixation or spinal applications. We also have available the InterGro® line of DBM materials (InterGro® Paste, InterGro® Putty and InterGro® Plus). The InterGro® DBM materials use lecithin as a carrier, which is a natural lipid carrier that is resistant to breakdown by bodily fluids, temperature or aggressive irrigation.
Spinal Products
Our spinal products include electrical stimulation devices for spinal applications, spinal fixation systems, bone substitute materials and motion preservation systems, as well as allograft services for spinal applications. These products and services are primarily marketed in the United States under the Biomet Spine tradename.
Spinal Fusion Stimulation Systems. Spinal fusions are surgical procedures undertaken to establish bony union between adjacent vertebrae. We distribute both non-invasive and implantable electrical stimulation units that surgeons can use as options to provide an appropriate adjunct to surgical intervention in the treatment of spinal fusion applications. We have assembled extensive preclinical research documenting the Mechanism of Action for the technology utilized in our spinal fusion stimulation systems.
The SpinalPak® II Spine Fusion Stimulator utilizes capacitive coupling technology to encourage fusion incorporation. The Mechanism of Action behind the capacitive coupling stimulation technology involves the upregulation of osteopromotive factors that modulate normal bone healing, such as TGF- ß1 and PGE2. The unit consists of a small, lightweight generator worn outside the body that is connected to wafer-thin electrodes applied over the fusion site. The SpinalPak® II System is patient friendly, enhancing comfort whether the patient is standing, sitting or reclining, and optimizes compliance with the treatment regimen to enhance fusion success.
The surgically implanted SpF® Spinal Fusion Stimulator consists of a generator that provides a constant direct current to titanium cathodes placed where bone growth is required. The Mechanism of Action behind our direct current stimulation technology involves the upregulation of a number of osteoinductive growth factors including BMP-2, -6 and -7 and the BMP-2 receptor ALK2, which are normal physiological regulators of various stages of bone healing, including chondrogenesis and osteogenesis. The SpF® Stimulator has exhibited a 50% increase in fusion success rates over fusions with autograft alone. In early 2008, we launched the SpF-PLUS Mini Spinal Fusion Stimulator. This new product has the highest current density available, in one-third of the size of the original SpF-PLUS Spinal Fusion Stimulator.
Spinal Fixation Systems. We market spinal fixation products for various spinal fusion applications. Our Synergy™ System, which has been on the market since 1992, is a complete system capable of addressing both degenerative and deformity indications. It is available in both stainless steel and titanium versions, offering 4.75mm and 6.35mm rod diameters, as well as a full complement of screws ranging from 4.0mm to 8.0mm in both fixed and polyaxial styles. The Synergy™ System also contains a full offering of hooks in a wide variety of styles and sizes. A more recent introduction in this market is the Array® Spinal System, which has a single locking setscrew featuring V-Force™ Thread Technology designed to enhance the intraoperative ease of use for the surgeon. During fiscal 2006, we launched the Array® Deformity Spine System, which includes various styles of screws, hooks and rods for scoliosis correction. The most recent product offering in this area is the Polaris™ System, which is a top-loading, inner tightening thoracolumbar system utilizing a patented closing mechanism known as a Helical Flange™.* This feature helps prevent cross
| * | Helical Flange™ is a trademark of the Jackson Group. |
59
Table of Contents
threading and seat splay, simplifying the implant closing procedure for the surgeon. Currently, the Polaris™ System is available in titanium, in 6.35mm and 5.5mm rod diameters, with both fixed and polyaxial screws ranging in size from 4.0mm to 7.0mm. We also market the Structure System, which utilizes various kinds of fixation washers used to secure screws to the vertebral body for an anterior screw/rod construct. In the thoracolumbar fusion area, we market the Biomet® Omega 21™ Spine System. This system features a unique multidirectional coupler and expandable screw. We also market the SpineLink®-II Spinal Fixation System, which addresses many of the inherent limitations of traditional rod and plate systems by linking each spine segment individually for intrasegmental control. Through the use of a modular titanium link and polydirectional screw, this unique system provides an intrasegmental option for spine fixation, enabling the surgeon to tailor the segmental construction to the patient’s anatomy.
We offer a variety of spacer products for the thoracolumbar market segment. The Ionic® Spine Spacer System, for use with the Omega 21™ Spine System or SpineLink® -II Spinal Fixation System, features an open design that allows for optimal bone graft placement and bone ingrowth, along with the additional benefit of excellent postoperative x-ray visualization. The GEO Structure® family features various sizes and shapes, including ovals, straight rectangles and bent rectangles. The Geo Structure® family of products are produced from cast titanium, offering a maximum amount of space inside the implant, with a minimum amount of material, resulting in excellent strength characteristics and imaging capabilities. The Solitaire™ System is a stand-alone device for anterior indications. The TPS™ Telescopic Plate Spacer is a unique implant indicated for trauma and tumor pathologies of the thoracolumbar spine. This implant is designed as a combination of a plate and spacer that is expandable, allowing the surgeon to fit the implant to the defect. We also offer the ESL® (Elliptically Shaped Lumbar) and Ibex™ thoracolumbar spacers. Both of these spacers are endplate-sparing designs, reducing the risk of subsidence. In addition, both the ESL® and Ibex™ Systems are open to permit ample space for bone graft placement and growth. The ESL® System features an elliptical shape offering optimal surface contact with the vertebral body endplates. The Ibex™ implant is curved to conform to the anatomical shape of the vertebral body. In addition, the beveled corners of the Ibex™ implant facilitate ease of use for the surgeon during implantation. The ESL® and Ibex™ thoracolumbar spacers are both available with a PEEK-OPTIMA®** implant option for increased radiographic fusion assessment.
For cervical applications, the VueLock® Anterior Cervical Plate System offers surgeons several important benefits. The open design of the VueLock® System provides surgeons with enhanced visualization of the bone graft both during the actual surgical procedure and postoperatively on x-ray. We also offer the C-TEK® Anterior Cervical Plate System, which offers a non-constrained, semi-constrained or a completely rigid construct, depending on the surgeon’s preference. Made from titanium, the C-TEK® Anterior Cervical Plate System offers both fixed and variable screws in a wide variety of diameters and lengths. This system also features a unique locking mechanism to prevent screw back out. For posterior cervical procedures, we offer the Altius M-INI™ System, which features top loading, inner tightening, polyaxial screws as well as hooks for the cervico-thoracic spine. The Altius M-INI™ System features a 3.5mm rod and a wide variety of screws ranging in diameter from 3.5mm to 4.5mm. This system also incorporates Helical Flange™ Locking Technology. Occipital fixation is also available with the Altius M-INI™ System, featuring a low profile plate that is placed independently from the rod, allowing for easier assembly and less rod contouring.
Minimally-invasive spine surgery is of growing interest in the practice of many spine surgeons. Traditional, open surgical approaches to the spine for discectomy, fusion and fixation have brought with them lengthy postoperative healing and rehabilitation issues. A minimally-invasive approach to spine surgery has demonstrated less morbidity, minimal blood loss and further benefits such as a shorter hospital stay. In the minimally-invasive surgery market, we market the VuePASS™ Portal Access Surgical System. Under direct visualization for a posterior lumbar approach, the VuePASS™ System allows for traditional open techniques through a minimally-invasive cannula access system. This past year we introduced the Ballista™ Percutaneous Pedicle Screw System and the AccuVision™ Minimally Invasive Access System.
To address the vertebral body compression fracture market, we offer two systems designed for the delivery of materials to weakened bone structures, including the CVD™ and LP2™ Delivery Systems. Through a series of dilating cannulae and various instruments, the systems allow the surgeon to access the anatomy through a percutaneous approach and safely deliver high viscosity material under low, controlled pressure. The CVD™ Delivery System offers the ability to biopsy before delivery. During fiscal 2008, we introduced Cobalt™ V Bone Cement for vertebroplasty applications.
Bone Substitute Materials. Traditional spinal fixation surgery includes the use of a spinal fixation device in conjunction with a bone substitute or bone graft material to increase the likelihood of successful bone fusion. Pro Osteon® 200R and Pro Osteon® 500R are bone graft substitutes made from marine coral. Both are a resorbable combination of hydroxyapatite and calcium carbonate that is resorbed and replaced with natural bone during the healing process. Pro Osteon® 200R is available as granules. Pro Osteon® 500R is available in granules and blocks. The Biomet® DBM (Demineralized Bone Matrix) Putty, derived exclusively from human bone, can be used with a variety of substances, such as bone substitute material, machined allograft, autograft and platelet rich plasma, to enhance the surgeon’s treatment options.
During fiscal 2007, Biomet Spine launched PlatFORM DBM, an osteoconductive, osteoinductive and osteogenic matrix. This material consists of freeze-dried flexible and pliable sheets of demineralized bone matrix putty for use as a bone void filler. PlatFORM DBM can be utilized alone or in combination with autologous bone or other forms of allograft and can be rehydrated with bone marrow aspirate for use in posterolateral spine fusions. This matrix has no synthetic additives, eliminating any surgeon concern regarding toxicity of certain carriers currently used in other DBMs.
Precision Machined Allograft. Many spinal fusion procedures, in both the lumbar and cervical spine, involve interbody spinal fusion. Surgeons often utilize precision machined allograft spacers to fuse the interbody space. We provide services related to the OsteoStim® Cervical Allograft Spacer for anterior cervical interbody fusions, the OsteoStim® ALIF Allograft Spacer for anterior lumbar interbody fusions and the OsteoStim® PLIF Allograft Spacer for posterior lumbar interbody fusions, depending on the surgical approach. All three systems are lordotic in shape, have serrated teeth on the top and bottom for added stability, are offered in various heights and have specific instrumentation to facilitate implantation.
* | PEEK-OPTIMA® is a registered trademark of Invibio, Ltd. |
60
Table of Contents
Motion Preservation Products. An Investigational Device Exemption pilot study for the Regain® Disc began in the United States during fiscal 2007. The Regain® Lumbar Artificial Disc is a one-piece pyrocarbon artificial disc nucleus replacement. The pyrocarbon material has a high level of strength, is biocompatible and extremely resistant to wear. In addition, Biomet Spine is developing the Rescue™ Cervical Disc Replacement product and the Min-T™ Lumbar Artificial Disc for total lumbar disc replacement procedures.
Other Products
We also manufacture and distribute several other products, including orthopedic support products (also referred to as soft goods and bracing products), arthroscopy products, operating room supplies, casting materials, general surgical instruments, wound care products and other surgical products. We manufacture and market a line of arthroscopy products through our subsidiary, Biomet Sports Medicine, LLC, or Biomet Sports Medicine.
Arthroscopy Products. Arthroscopy is a minimally-invasive orthopedic surgical procedure in which an arthroscope is inserted through a small incision to allow the surgeon direct visualization of the joint. This market is comprised of five product categories: power instruments, manual instruments, visualization products, soft tissue anchors, and procedure-specific instruments and implants. Our principal products consist of the EZLoc™ Femoral Fixation Device, the Washer Loc™ Tibial Fixation Device, LactoSorb® resorbable arthroscopic fixation products, the ALL Thread™ Suture Anchor, the MaxFire™ Meniscal Repair Device with ZipLoop™ Technology and ToggleLoc™ with ZipLoop™ Technology, and the InnerVue™ Diagnostic Scope system, which utilizes a needle scope to diagnose knee and shoulder conditions in a physician’s office.
Orthopedic Support Products. We distribute a line of orthopedic support products under the Biomet Bracing name, including back braces, knee braces and immobilizers, wrist and forearm splints, cervical collars, shoulder immobilizers, slings, abdominal braces, ankle supports and a variety of other orthopedic splints. Sales of these softgoods and bracing products are assisted by the S.O.S. SM Support-on-Site stock and bill program, which handles the details of product delivery for the healthcare provider.
Product Development
Our research and development efforts are essentially divided into two categories: innovative new technology and evolutionary developments. Most of the innovative new technology development efforts are focused on biomaterial products, and are managed at the corporate level and take place primarily in Warsaw, Indiana. Evolutionary developments are driven primarily by the individual subsidiaries and include product line extensions and improvements.
We continue to aggressively conduct internal research and development efforts to generate new marketable products, technologies and materials. In addition, we are well positioned to take advantage of external acquisition and development opportunities. An important component of our strategy has been the formation of strategic alliances to enhance the development of new musculoskeletal products.
For fiscal 2006 and 2007, the periods from June 1, 2007 through July 11, 2007, from July 12, 2007 through November 30, 2007, and from July 12, 2007 through May 31, 2008, and the six months ended November 30, 2008, we expended approximately $85 million, $94 million, $34 million, $36 million, $82 million, and $23 million, respectively, on research and development. It is expected that ongoing research and development expenses will continue to increase. Our principal research and development efforts relate to our orthopedic reconstructive devices, spinal fixation products, revision orthopedic reconstructive devices, dental reconstructive devices, arthroscopy products, resorbable technology, biomaterial products and autologous therapies.
We have launched approximately 800 new products in the past nine fiscal years and plan to introduce approximately 100 new products during fiscal 2009.
Government Regulation
Most aspects of our business are subject to some degree of government regulation in the countries in which our operations are conducted. It has always been our practice to comply with all regulatory requirements governing our products and operations and to conduct our affairs in an ethical manner. This practice is reflected in our Code of Business Conduct and Ethics and through the responsibility of the Audit Committee of the Board of Directors to review our systems of internal control, our process for monitoring compliance with laws and regulations and our process for monitoring compliance with our Code of Business Conduct and Ethics. For some products, and in some areas of the world such as the United States, Canada, Japan and Europe, government regulation is significant and, in general, there appears to be a trend toward more stringent regulation throughout the world, as well as global harmonization of various regulatory requirements. We devote significant time, effort and expense to addressing the extensive government and regulatory requirements applicable to our business. Governmental regulatory actions can result in the recall or seizure of products, suspension or revocation of the authority necessary for the production or sale of a product, and other civil and criminal sanctions. We believe that we are no more or less adversely affected by existing government regulations than are our competitors.
In the United States, the development, testing, marketing and manufacturing of medical devices are regulated under the Medical Device Amendments of 1976 to the Federal Food, Drug and Cosmetic Act, the Safe Medical Devices Act of 1990, the FDA Modernization Act of 1997, the Medical Device User Fee and Modernization Act of 2002 and additional regulations promulgated by the FDA and various other federal, state and local agencies. In general, these statutes and regulations require that manufacturers adhere to certain standards designed to ensure the safety and efficacy of medical devices and related medical products.
We believe that we are well positioned to face the changing international regulatory environment. The International Standards Organization (“ISO”) has an internationally recognized set of standards aimed at ensuring the design and manufacture of quality products. A company that has passed an ISO audit and obtained ISO certification applicable to its activity sector is internationally
61
Table of Contents
recognized as having quality manufacturing processes. The European Union legislation requires that medical devices bear a CE mark. The CE mark is a European Union and European Free Trade Association symbol, which indicates that the product adheres to European Medical Device Directives. Compliance with ISO quality systems standards is one of the requirements for placing the CE mark on our products. Each of our principal manufacturing facilities has been certified to ISO 13485:2003. Each of our products sold in Europe bears the CE mark, with the exception of custom-made implants that do not require a CE mark. The EU has recently reclassified our total joint products to Class III via Directive 2005/50/EC and we are in the process of complying with this Directive.
In addition, governmental bodies in the United States and throughout the world have expressed concern about the costs relating to healthcare and, in some cases, have focused attention on the pricing of medical devices. Government regulation regarding pricing of medical devices already exists in some countries and may be expanded in the United States and other countries in the future. We are subject to increasing pricing pressures worldwide as a result of growing regulatory pressures, as well as the expanding predominance of managed care groups and institutional and governmental purchasers. Under Title VI of the Social Security Amendments of 1983, hospitals receive a predetermined amount of Medicare reimbursement for treating a particular patient based upon the patient’s type of illness identified with reference to the patient’s diagnosis under one or more of several hundred diagnosis-related groups. Other factors affecting a specific hospital’s reimbursement rate include the size of the hospital, its teaching status and its geographic location.
While we are unable to predict the extent to which our business may be affected by future regulatory developments, we believe that our substantial experience in dealing with governmental regulatory requirements and restrictions throughout the world, our emphasis on efficient means of distribution and our ongoing development of new and technologically-advanced products should enable us to continue to compete effectively within this increasingly regulated environment.
Sales and Marketing
We have diligently worked to attract and retain qualified, well-trained and motivated sales representatives. The breadth of our product offering and the quality of our salesforces collaborate to create synergies that uniquely position us to continue to efficiently penetrate the musculoskeletal market. In the United States, our products are marketed by a combination of independent commissioned sales agents and direct sales representatives, based on the specific product group being represented. In an effort to ensure the continuity of our relationships with the independent third-party distributors who represent Biomet Orthopedics, we incurred $39 million in fiscal 2007, $18 million for the period from June 1, 2007 through July 11, 2007, $30 million for the period from July 12, 2007 through May 31, 2008, and $2.0 million for the six months ended November 30, 2008, which negatively affected our results of operations for these periods. In Europe, our products are promoted by sales representatives employed by subsidiaries, independent third-party distributors, and some independent commissioned sales agents, based primarily on the geographic location. In the rest of the world, we maintain direct selling organizations in ten countries, as well as independent commissioned sales agents and independent third-party distributors in other key markets. In aggregate, our products are marketed by more than 2,700 sales representatives throughout the world.
Elective surgery-related products appear to be influenced to some degree by seasonal factors, as the number of elective procedures declines during the summer months and the winter holiday season.
Our customers are the hospitals, surgeons, other physicians and healthcare providers who use our products in the course of their practices. Our business is dependent upon the relationships maintained by our distributors and salespersons with these customers, as well as our ability to design and manufacture products that meet the physicians’ technical requirements at a competitive price.
We have inventory located throughout the world with our customers, our distributors and direct salespersons for their use in marketing our products and in filling customer orders. As of November 30, 2008, inventory of approximately $99 million was located with these distributors, salespersons and customers.
Competition
Our business is highly competitive. Competition within the industry is primarily based on service, clinical results and product design, although price competition is an important factor as healthcare providers continue to be concerned with costs. Major competitors in our four major market categories are set forth below by market category.
Reconstructive Products
Our orthopedic reconstructive devices compete with those offered by DePuy, Inc. (a subsidiary of Johnson & Johnson), Smith & Nephew plc, Stryker Orthopaedics (a division of Stryker Corp.) and Zimmer, Inc. (a subsidiary of Zimmer Holdings, Inc.). Management believes these four companies, together with Biomet, have the predominant share of the global orthopedic reconstructive device market. We believe that our prices for orthopedic reconstructive devices are competitive with those in the industry. We believe that our future success will depend upon, among other things, our service and responsiveness to our distributors and orthopedic specialists, the continued excellent clinical results of our products, and upon our ability to design and market innovative and technologically-advanced products that meet the needs of the marketplace.
Our dental reconstructive products compete in the areas of dental reconstructive implants and related products. The primary competitors in the dental implant market include Nobel Biocare AB, Straumann AG, Zimmer Dental (a subsidiary of Zimmer Holdings, Inc.) and Astra Tech (part of the AstraZeneca Group).
62
Table of Contents
Fixation Devices
Our electrical stimulation devices primarily compete with those offered by Orthofix, Inc. (a subsidiary of Orthofix International N.V.), DJO Inc. (formerly dj Orthopedics, Inc.) and Smith & Nephew plc. Competition in the electrical stimulation market is on the basis of product design, service, price and success rates of various treatment alternatives.
Our external and internal fixation devices compete with other such devices primarily on the basis of price, ease of application and clinical results. The principal competitors in the external fixation market are Smith & Nephew plc, Stryker Trauma (a division of Stryker Corp.), Synthes, Inc. and Orthofix, Inc. (a subsidiary of Orthofix International N.V.). Our internal fixation product lines compete with those of Synthes, Inc., DePuy, Inc. (a Johnson & Johnson Company), Zimmer, Inc. (a subsidiary of Zimmer Holdings, Inc.), Smith & Nephew plc and Stryker Trauma (a division of Stryker Corp.).
Spinal Products
Our spinal fixation systems compete with other spinal fixation systems primarily on the basis of breadth of product line, product recognition and price. The principal competitors in this area are Medtronic Sofamor Danek, Inc. (a subsidiary of Medtronic, Inc.), DePuy Spine (a Johnson & Johnson Company), Synthes, Inc., Stryker Spine (a division of Stryker Corp.), Zimmer Spine (a subsidiary of Zimmer Holdings, Inc.) and others.
Other Products
Our craniomaxillofacial fixation products, specialty surgical instrumentation and neurosurgical cranial flap fixation products compete with those offered by Synthes, Inc., Stryker Leibinger Micro Implants (a division of Stryker Corp.), KLS-Martin, L.P., Osteomed Corp., Aesculap, Inc., Medtronic, Inc. and Codman (a Johnson & Johnson Company).
Our arthroscopy products compete primarily in the areas of procedure-specific implants and instruments, manual instruments and power instruments. Competitors include Smith & Nephew Endoscopy (a division of Smith & Nephew plc, Stryker Corp, Linvatec Corp. (a subsidiary of CONMED Corporation), Mitek (a division of Ethicon, a Johnson & Johnson Company), Arthrocare Corp. and Arthrex, Inc.
Our orthopedic support products consist primarily of back braces, knee braces and immobilizers, wrist and forearm splints, cervical collars, shoulder immobilizers, slings, abdominal braces and ankle supports that compete with those offered by Orthofix, Inc. (a subsidiary of Orthofix International N.V.), DJO Inc. (formerly dj Orthopedics, Inc.) and Ossur. Competition in the bracing market is on the basis of product design, service and price.
Raw Materials and Supplies
The raw materials used in the manufacture of our orthopedic reconstructive devices are principally nonferrous metallic alloys, stainless steel and polyethylene powder. With the exception of limitations on the supply of polyethylene powder, none of our raw material requirements are limited to any material extent by critical supply or single origins. The demand for certain raw materials used by us, such as cobalt-chromium alloy and titanium may vary. The primary buyers of these metallic alloys are in the aerospace industry. If the demands of the aerospace industry should increase dramatically, we could experience complications in obtaining these raw materials. However, based on our current relationship with our suppliers, we do not anticipate a material shortage in the foreseeable future. Further, we believe that our inventory of raw materials is sufficient to meet any short-term supply shortages of metallic alloys. The results of our operations are not materially dependent on raw material costs.
We purchase all components of our electrical stimulators from approximately 190 outside suppliers, approximately 15 of whom are the single source of supply for the particular product. In most cases, we believe that all components are replaceable with similar components. In the event of a shortage, there are alternative sources of supply available for all components, but some time would likely elapse before our orders could be filled.
Coral is the primary raw material utilized to manufacture certain of our Pro Osteon® products. The coral used in Pro Osteon® products is sourced from two genera located in a variety of geographic locations. Our primary source of coral has historically been the tropical areas of the Pacific and Indian Oceans. Although we obtain our coral from a single source supplier, for which an alternate supplier has not been identified, we believe that we have an adequate supply of coral for the foreseeable future.
We purchase all materials to produce our dental products from approximately 95 suppliers, approximately 87 of whom are the single source of supply for the particular product. We believe that, in the event of a shortage, there are readily available alternative sources of supply for single-source products, and we maintain an inventory of materials sufficient to meet any short-term shortages of supply.
Employees
As of November 30, 2008, our domestic operations (including Puerto Rico) employed 3,899 persons, of whom 1,923 were engaged in production and 1,976 in research and development, sales, marketing, administrative and clerical efforts. Our international subsidiaries employed 3,273 persons, of whom 1,583 were engaged in production and 1,691 in research and development, sales, marketing, administrative and clerical efforts. None of our principal domestic manufacturing employees is represented by a labor union. The production employees at our Bridgend, South Wales facility are organized. Employees working at the facilities in Berlin and Dieburg, Germany; Valence, France; Swindon, United Kingdom; Sjöbo, Sweden; and Valencia, Spain are represented by Workers’ Councils. We believe that our relationship with all of our employees is satisfactory.
63
Table of Contents
The establishment of our domestic orthopedic reconstructive manufacturing operations in north central Indiana, near other members of the orthopedic industry, provides access to the highly skilled machine operators required for the manufacture of Biomet® products. Our European manufacturing locations in South Wales, England, France, Spain, Sweden and Germany also provide good sources for skilled manufacturing labor. Our Puerto Rican operations principally involve the assembly of purchased components into finished products using a skilled labor force.
Legal Proceedings
U.S. Department of Justice EBI Products Investigation
In May 2007, we received a subpoena from the U.S. Department of Justice through the U.S. Attorney for the Southern District of West Virginia requesting documents generally relating to a certain number of products manufactured, marketed and sold by our EBI subsidiary for the period from January 1999 through the present. In June 2007, we received a second administrative subpoena from the U.S. Attorney for the Southern District of West Virginia requesting documents relating to a specific physician’s assistant. We understand that the Department of Justice is conducting a civil investigation of EBI’s sales and marketing practices relating to certain spinal products. We are fully cooperating with the request of the Department of Justice. We can make no assurances as to the time or resources that will be needed to devote to this inquiry or its final outcome.
Litigation Relating to Past Stock Option Grant Practices
On September 21, 2006, two shareholder derivative complaints were filed against certain of our current and former officers and directors in Kosciusko Superior Court I in Kosciusko County, in the State of Indiana. The complaints, captioned Long v. Hann, et al., and Thorson v. Hann, et al., alleged violations of state law relating to the issuance of certain stock option awards by Biomet dating back to 1996. Both complaints sought unspecified money damages as well as other equitable and injunctive relief. These two cases were consolidated under the caption In re Biomet, Inc. Derivative Litigation, and on January 19, 2007, plaintiffs filed an amended complaint that made additional allegations based on our December 18, 2006 disclosures related to stock option awards, including allegations that the defendants sought to sell Biomet in order to escape liability for their conduct, and that they did so at a devalued price, thus further breaching their fiduciary duties to shareholders. On February 16, 2007, defendants filed a motion to dismiss plaintiffs’ amended complaint. On October 11, 2007, after approval of the Company’s sale by its shareholders, the parties filed supplemental briefs on the issue of whether plaintiffs had standing to sue. On February 5, 2008, the court dismissed the case for lack of standing, and plaintiffs’ motion for leave to amend was denied. Plaintiffs appealed the district court’s decision, and on February 13, 2009, the Court of Appeals affirmed the dismissal of the case. Plaintiffs may still seek review of the Court of Appeals’ decision by the Indiana Supreme Court.
U.S. Securities and Exchange Commission Informal Investigation
On September 25, 2007, we received a letter from the SEC informing us that it is conducting an informal investigation regarding possible violations of the Foreign Corrupt Practices Act in the sale of medical devices in certain foreign countries by companies in the medical devices industry. The Foreign Corrupt Practices Act prohibits U.S. companies and their officers, directors, employees, shareholders acting on their behalf and agents from offering, promising, authorizing or making payments to foreign officials for the purpose of obtaining or retaining business abroad or otherwise obtaining favorable treatment and this law requires companies to maintain records which fairly and accurately reflect transactions and to maintain internal accounting controls. In many countries, hospitals and clinics are government-owned and healthcare professionals employed by such hospitals and clinics, with whom we regularly interact, may meet the definition of a foreign official for purposes of the Foreign Corrupt Practices Act. If we are found to have violated the Foreign Corrupt Practices Act, we may face sanctions including fines, criminal penalties, disgorgement of profits and suspension or debarment of our ability to contract with government agencies or receive export licenses. On November 9, 2007, we received a letter from the Department of Justice requesting any information provided to the SEC be provided to the Department of Justice on a voluntary basis. We intend to fully cooperate with both requests and we are in the process of conducting our own review relating to these matters in certain countries in which we and our distributors conduct business.
Massachusetts AG
We received a Civil Investigative Demand (“CID”) issued by the Commonwealth of Massachusetts Office of the Attorney General (“Massachusetts AG”) on or about November 19, 2007. The CID requested documents for the period November 1, 2003 to the present concerning certain physicians and provider groups, including, among other things, documents concerning any contracts or agreements with, and any payments made to, those physicians or provider groups. We have produced documents in response to the CID, and intend to continue to cooperate with the Massachusetts AG. It is not possible at this time to predict the likely outcome of this inquiry or its financial impact should the outcome be adverse to us.
Other Matters
In February 2006, SDGI Holdings, Inc. and Medtronic Sofamor Danek, Inc. (collectively referred to herein as Medtronic) brought an action against EBI subsidiaries and Biomet alleging infringement of seven patents. Specifically, Medtronic alleged that the patents were infringed by certain components of our Vuelock® Anterior Cervical Plate System, as well as instruments and surgical implantation methods associated with our Array® Spinal System. In Fall 2007, Medtronic included similar instruments used with EBI’s Biomet® Omega21™ , Polaris™ , and Synergy™ Spinal Fixation Systems as accused products. We filed a counterclaim seeking a finding of non-infringement of the patents at issue and a finding that certain of the patents are invalid and unenforceable. Effective on December 8, 2008, the parties entered into a confidential settlement agreement, without either party admitting liability, pursuant to which, among other things, the parties exchanged certain cross-licenses to various patents.
Biomet and Biomet Orthopedics initiated legal proceedings on July 17, 2007 against Zimmer US, Inc., or Zimmer, certain of our former distributors and David Montgomery, a former employee who currently works for Zimmer. The thirteen count lawsuit originally filed in Marion County, Indiana and refiled in Hamilton County, Indiana alleges, among other things, that Zimmer and Mr. Montgomery attempted to create an unfair market advantage by engaging in a campaign to misappropriate our confidential
64
Table of Contents
information, to interfere with our contractual relations with distributors and to attempt to buy the assets of most of our distributors (including our surgical instruments) throughout the United States. Further, the lawsuit alleges that the limited number of distributors who accepted Zimmer’s offer are in violation of their contractual obligations to Biomet. Although nearly all of our distributors rejected Zimmer’s offers and have remained with Biomet, and although no amount of money damages can completely compensate Biomet for the losses we have sustained as a result of defendants’ conduct, we are nonetheless seeking to recover compensatory damages that are attributable to financial and other resources spent on signing new agreements with our sales force. To the extent we sustained damages as a result of our former distributors agreeing to purportedly sell their assets to Zimmer, we are seeking to recover lost profits and other damages as well. In addition, we are seeking to recover punitive damages from the defendants. On November 9, 2007, defendants filed a motion to dismiss our complaint. On March 27, 2008, the court denied the motion in its entirety.
In a related matter, we brought suit against a former distributor for Biomet Orthopedics who, in violation of his contractual and other obligations to Biomet under agreements stretching back to 1994, sold the assets of his distributorship to Zimmer in an apparent effort to avoid his contractual obligations to us. The complaint, now pending in federal district court in Indiana, asserts five causes of action that include breach of contract, unjust enrichment and statutory wrongs. Among other things, the complaint seeks injunctive relief and compensatory and punitive damages. On July 16, 2007, a temporary restraining order was entered against this former distributor which subsequently lapsed ten days later. Prior to the filing of the suit described above, this former distributor sued one of his former employees who decided to continue to represent our products in the future as he has for nearly ten years. The suit brought against this employee by our former distributor who sold his assets to Zimmer claims, among other things, that the former employee is violating his non-competition agreement with our former distributor by continuing to sell the same Biomet products the former employee sold while employed by our former distributor. The suit also seeks, among other forms of relief, an injunction and compensatory and punitive damages. In addition, on or about July 3, 2008, Zimmer U.S., Inc. and one of its distributors filed a five count complaint in Tennessee federal court against this same former employee seeking, among other things, injunctive relief, monetary damages, and punitive damages for alleged breach of contract, conspiracy, and other causes of action.
In late 2004 and early 2005, approximately 120 plaintiffs sued Dr. John King in the Circuit Court of Putnam County, West Virginia. Plaintiffs alleged that Dr. King was professionally negligent when he performed surgery on the plaintiffs at Putnam General Hospital in Putnam County, West Virginia between November 2002 and June 2003. In 38 of these lawsuits, plaintiffs alleged that Dr. King had implanted a device manufactured by our EBI subsidiary and EBI was named a party in those 38 lawsuits. Plaintiffs have dismissed or have agreed to dismiss their claims against EBI in 11 cases, leaving EBI as a party in 27 pending lawsuits, all of which relate to EBI’s Ionic Spine Spacer System and its implanted bone stimulator devices, the SpF and OsteoGen. Plaintiffs allege that EBI entered into a joint venture and a civil conspiracy with Dr. King and/or his physician assistant, David McNair. The plaintiffs also allege that EBI failed to warn that its products were not safe for their intended use, that EBI knew that Dr. King was not properly trained or was performing surgeries inappropriately and claims based on strict liability, express and implied breach of warranty and negligent sale. Plaintiffs seek to recover lost income, medical expenses, future medical and life care expenses, damages relating to pain and suffering and punitive and other damages. Dr. King is uninsured in 25 of these 27 cases and has filed bankruptcy.
In July 2007, a Putnam County jury found that Putnam General Hospital had negligently credentialed Dr. King and that the hospital’s conduct in credentialing Dr. King was motivated by fraud, ill will, wantonness, oppressiveness, or by reckless or gross negligence, which allowed the plaintiffs to seek punitive damages against the hospital. In April, May and June of 2008, the hospital and its upstream affiliates and David McNair entered into a confidential settlement of all claims with all but one of the plaintiffs. EBI, Wright Medical Corporation, Wright Medical’s distributor’s employee, Robert Edwards, and Dr. King remain as defendants in the litigation.
The Putnam County Circuit Court revised its case management order with respect to the remaining lawsuits on July 2, 2008 and scheduled a consolidated trial of six plaintiffs for June 1, 2009. We are vigorously defending these matters and intend to continue to do so. An unfavorable outcome in these matters could have a material adverse effect on our financial position, liquidity and results of operations.
There are various other claims, lawsuits, disputes with third parties, investigations, and pending actions involving various allegations against us incidental to the operation of our business, principally product liability and intellectual property cases. Each of these matters is subject to various uncertainties, and it is possible that some of these matters may be resolved unfavorably to Biomet. We accrue for losses that are deemed to be probable and subject to reasonable estimate. Based on the advice of our counsel in these matters, management believes that the ultimate outcome of these matters and any liabilities in excess of amounts provided, except in the immediately preceding paragraph above, will not have a material adverse impact on our unaudited condensed consolidated financial statements taken as a whole.
Our Facilities
Our principal executive offices are at 56 East Bell Drive, Warsaw, Indiana. In addition, we maintain more than 50 other manufacturing facilities, offices and warehouse facilities in various countries, including Canada, Europe, Asia Pacific and Latin America. We believe that all of our facilities are adequate, well maintained and suitable for the development, manufacture, distribution and marketing of all our products. The following is a list of our principal properties as of November 30, 2008:
FACILITY | LOCATION | SQUARE FEET | OWNED/ LEASED | |||
Corporate headquarters of Biomet, Inc.; manufacturing, storage and research and development facilities of Biomet Manufacturing Corp.; distribution center and offices of Biomet Orthopedics, LLC | Warsaw, Indiana | 538,199 | Owned | |||
Administrative, manufacturing and distribution facility of EBI, LLC and administrative offices of Electro-Biology, LLC | (1)Parsippany, New Jersey | 73,450 | Owned | |||
| (2)Parsippany, New Jersey | 213,750 | Owned | ||||
Manufacturing facility of EBI, LLC | Marlow, Oklahoma(b) | 51,500 | Owned | |||
Administrative, manufacturing and distribution facility of Biomet Microfixation, LLC | Jacksonville, Florida | 82,500 | Owned | |||
Office, manufacturing and distribution facility of Biomet 3i, LLC | (1)Palm Beach Gardens, Florida | 117,000 | Owned | |||
| (2) Palm Beach Gardens, Florida (a) | 69,000 | Owned | ||||
Office and manufacturing facilities of Biomet Sports Medicine, LLC | (1) Ontario, California | 35,400 | Owned | |||
| (2) Redding, California | 14,400 | Leased | ||||
Manufacturing facility of Biomet Fair Lawn, LLC | Fair Lawn, New Jersey | 40,000 | Owned | |||
Office and manufacturing facility of Electro-Biology, LLC | Guaynabo, Puerto Rico | 34,700 | Owned | |||
Office, manufacturing and distribution facilities of Interpore Spine Ltd. | (1) Irvine, California | 36,800 | Leased | |||
| (2) Irvine, California | 2,700 | Leased | ||||
Office, manufacturing and warehouse facility of Biomet France Sarl | Valence, France | 86,100 | Owned | |||
Office, manufacturing and warehouse facilities of Biomet Deutschland GmbH | Berlin, Germany | 49,900 | Owned | |||
Administrative offices of Biomet Europe B.V. and office and warehouse facility of Biomet Nederland BV and Biomet Microfixation Europe B.V. | Dordrecht, The Netherlands | 37,700 | Owned | |||
Office and manufacturing facility of Biomet Spain Orthopedics S.L. | Valencia, Spain | 69,600 | Owned | |||
Office, manufacturing and warehouse facilities of Biomet Cementing Technologies AB | Sjobo, Sweden | 24,200 | Owned | |||
Manufacturing and administrative facilities of Biomet UK Ltd. | (1) Bridgend, South Wales | 111,956 | Owned | |||
| (2) Swindon, England | 54,800 | Owned | ||||
Manufacturing, administrative and warehouse facilities of Zhejiang Biomet | Jinhua, China | 110,000 | Owned | |||
Manufacturing, administrative and warehouse facilities of Changzou Biomet | Changzou, China | 82,000 | Owned | |||
| (a) | Includes 23,000 square feet of space in this facility that is leased to other parties. |
| (b) | Currently held as available for sale. |
65
Table of Contents
Patents and Trademarks
We believe that patents and other intellectual property will continue to be of importance in the musculoskeletal industry. Accordingly, we continue to protect technology developed internally and to acquire intellectual property rights associated with technology developed externally. We enforce our intellectual property rights consistent with our strategic business objectives. We do not believe that we have any single patent or license (or series of patents or licenses) that is material to our operations. We are not aware of any single patent that, if lost or invalidated, would be material to our consolidated revenues or earnings. We currently have more than 1,300 patents and in excess of 700 pending patent applications.
BIOMET is our principal registered trademark throughout the world, and registrations have been obtained or are in process with respect to various other trademarks associated with our products. Unless otherwise noted in this prospectus, all trademarks contained herein are owned by Biomet Manufacturing Corp., or one of its affiliates.
66
Table of Contents
Directors and Executive Officers
The following table sets forth the name, age and position of (1) our directors and (2) our executive officers.
Name | Age | Position | ||
Jeffrey R. Binder | 45 | President and Chief Executive Officer, Director | ||
Jonathan J. Coslet | 44 | Director | ||
Michael Dal Bello | 37 | Director | ||
Adrian Jones | 44 | Director | ||
David McVeigh | 41 | Director | ||
Michael Michelson | 57 | Director | ||
Dane A. Miller, Ph.D. | 63 | Director | ||
John Saer | 52 | Director | ||
Todd Sisitsky | 36 | Director | ||
Gregory L. Summe | 52 | Director | ||
Daniel P. Florin | 44 | Senior Vice President and Chief Financial Officer | ||
Glen A. Kashuba | 45 | Senior Vice President; President of Biomet Trauma and Biomet Spine | ||
Gregory W. Sasso | 47 | Senior Vice President; President of Biomet SBU Operations | ||
Steven F. Schiess | 49 | Senior Vice President; President of Biomet 3i, LLC | ||
Jon C. Serbousek | 48 | Senior Vice President; Biomet Orthopedics, LLC | ||
Bradley J. Tandy | 50 | Senior Vice President, General Counsel and Secretary | ||
Peggy Taylor | 52 | Senior Vice President—Human Resources | ||
Roger P. Van Broeck | 60 | Senior Vice President; President of Biomet Europe, Middle East and Africa | ||
Robert E. Durgin | 49 | Senior Vice President—Quality, Regulatory and Clinical Affairs | ||
Robin T. Barney | 47 | Senior Vice President, World Wide Operations | ||
Jacqueline K. Huber | 39 | Corporate Vice President and Chief Compliance Officer |
Jeffrey R. Binder has been a director and President and Chief Executive Officer since February 2007. Prior to this appointment, Mr. Binder served as Senior Vice President of Diagnostic Operations of Abbott Laboratories from January 2006 to February 2007. Mr. Binder previously served as President of Abbott Spine from June 2003 to January 2006, and as President and Chief Executive Officer of Spinal Concepts from 2000 to June 2003.
Jonathan J. Coslet has been a director since July 2007. Mr. Coslet has been a Partner of TPG since 1993 and is currently a senior partner and member of the firm’s Executive, Management and Investment Committees. Mr. Coslet serves on the board of directors of IASIS Healthcare Corp., The Neiman Marcus Group, Inc., J. Crew Group, Inc., PETCO Animal Supplies, Inc. and Quintiles Transnational Corp.
Michael Dal Bello has been a director since July 2007. Mr. Dal Bello has been a Principal in the Private Equity Group of The Blackstone Group since December 2005 and was an Associate in this group from 2002 until December 2005. Prior to joining Blackstone, Mr. Dal Bello received an M.B.A. from Harvard Business School in 2002. Mr. Dal Bello serves on the board of directors of Catalent Pharma Solutions, Inc., Global Tower Partners, Sithe Global Power, LLC, Team Finance LLC and Vanguard Health Systems, Inc.
Adrian Jones has been a director since July 2007. Mr. Jones has been a Managing Director of Goldman, Sachs & Co. since 2002 and has worked at Goldman, Sachs & Co. since 1994. Mr. Jones serves on the board of directors of Burger King Holdings, Inc., Dollar General Corporation, Education Management Corporation, and HealthMarkets, Inc.
David McVeigh has been a director since September 2007. Mr. McVeigh is an executive director at Blackstone in the private equity group. Mr. McVeigh recently joined Blackstone from McKinsey & Company, where he spent 12 years and was a partner. At McKinsey, Mr. McVeigh was one of the leaders of the North American Chemicals practice and the Northeast Energy and Materials practice. Mr. McVeigh serves on the board of directors of Michaels Stores, Inc.
Michael Michelson has been a director since July 2007. Mr. Michelson has been a member of the limited liability company that serves as the general partner of KKR since 1996 and, prior thereto, was a general partner of KKR. Mr. Michelson serves on the board of directors of Accellent Inc., Jazz Pharmaceuticals, Inc. and HCA, Inc.
Dane A. Miller, Ph.D. has been a director since July 2007. Dr. Miller is one of our four founders and served as our President, Chief Executive Officer and a director from 1977 until 2006. Dr. Miller serves on the board of directors of 1st Source Corporation, ForeTravel, Inc., the Indiana Economic Development Corporation, the University of Chicago Health Systems and the World Craniofacial Foundation.
John Saer has been a director since July 2007. Mr. Saer has been an executive of the limited liability company that serves as the general partner of KKR since 2001. Mr. Saer serves on the board of directors of KSL Holdings Corporation and ACTS Corporation.
Todd Sisitsky has been a director since July 2007. Mr. Sisitsky has been a Partner of TPG since 2007. From 2003 until 2007, he was an Investor at TPG. From 2001 until 2003, he was an Investor/Associate at Forstmann Little & Co. Mr. Sisitsky serves on the board of directors of IASIS Healthcare Corp., Fenwal, Inc., Surgical Care Affiliates, and Axcan Pharma.
67
Table of Contents
Gregory L. Summe has been a director since April 2008. Mr. Summe is the Executive Chairman of the Board of PerkinElmer, Inc. and a Senior Advisor to Goldman Sachs Capital Partners. From 1999 until 2008, Mr. Summe was the Chief Executive Officer and Chairman and from 1998 to 2007, he was the President of PerkinElmer, Inc. Mr. Summe also serves on the board of directors of the State Street Corporation and Automatic Data Processing, Inc.
Daniel P. Florin has been Senior Vice President and Chief Financial Officer since June 2007. Prior thereto, Mr. Florin served as Vice President and Corporate Controller for Boston Scientific Corporation since 2001. Prior to being appointed as Corporate Controller in 2001, Mr. Florin served in financial leadership positions within Boston Scientific Corporation and its various business units since July 1995.
Glen A. Kashuba has been Senior Vice President of Biomet, Inc. and President of Biomet Trauma and Biomet Spine since April 2007. Prior thereto, Mr. Kashuba served as Worldwide President of Cordis Endovascular, a division of Johnson & Johnson. Mr. Kashuba had been with Johnson & Johnson since 1998, also holding the positions of Worldwide President of Codman Neuro Science (from December 2002 to November 2005) and U.S. President of DePuy AcroMed, now known as DePuy Spine.
Gregory W. Sasso has been Senior Vice President and President of Biomet SBU Operations since June 2007. Prior thereto, Mr. Sasso served as Senior Vice President—Corporate Development and Communications since June 2006. Prior thereto, he was Vice President—Corporate Development and Communications from April 1997 to June 2006.
Steven F. Schiess has been Senior Vice President and President of Biomet 3i, LLC since January 2007. Prior thereto, he was Vice President and President of Biomet 3i from June 2005 to January 2007. Prior thereto, he was Senior Vice President, Sales and Marketing of Biomet 3i from 2001 to June 2005.
Jon C. Serbousek has been the President of Biomet Orthopedics, LLC since March 2008. For the past eight years, Mr. Serbousek held diverse general management roles with Medtronic in the areas of Spinal Reconstruction, International, New Technology Development and most recently, worldwide Vice-President and General Manager, Biologics.
Bradley J. Tandy has been Senior Vice President, General Counsel and Secretary since April 2007. Prior thereto, Mr. Tandy served as Senior Vice President, Acting General Counsel and Secretary from January 2007 to April 2007, and Senior Vice President, Acting General Counsel, Secretary and Corporate Compliance Officer from March 2006 to January 2007. Mr. Tandy previously served as Vice President, Assistant General Counsel and Corporate Compliance Officer at Biomet, Inc. from January 1999 to March 2006.
Peggy Taylor has been Senior Vice President—Human Resources since August 2007. Prior thereto, Ms. Taylor served as Vice President of Human Resources for Diagnostics Division of Abbott Laboratories from April 2000 to August 2007.
Roger P. Van Broeck has been Vice President since July 2007 and President of Biomet Europe, Middle East and Africa since March 2004. For a brief period during 2007, Mr. Van Broeck also served as President of International Operations. From September 1998 to March 2004, he was Chief Executive Officer of BioMer C.V. and Biomet Merck B.V., Biomet’s joint venture with Merck KGaA (Darmstadt).
Robert E. Durginhas been Senior Vice President, Quality/Regulatory/Clinical Affairs since January 2009. Prior thereto, Mr. Durgin served as Corporate Vice President, Global Quality/Clinical/Regulatory Affairs from June 2007 to January 2009, and Corporate Vice President, Global Regulatory Affairs from May 2006 to June 2007. Mr. Durgin previously served as Vice President, Regulatory Affairs and Quality Assurance from September 2003 to May 2006 and in positions in Biomet’s legal department from June 1998 to September 2003.
Robin T. Barney has been Senior Vice President of Operations since September, 2008. Prior to joining Biomet in 2007, Ms. Barney served as Vice President, Worldwide Operations of DePuy, a Johnson & Johnson company. Ms. Barney joined Johnson & Johnson in 1992 and held various leadership roles within Operations for their Codman & Shurtleff, DePuy Orthopeadics, and DePuy Spine units.
Jacqueline Huber has been Corporate Vice President & Chief Compliance Officer since December 2007. Prior thereto, Ms. Huber was Vice President & Corporate Compliance Officer from January 2007 to December 2007. Prior thereto, Ms. Huber previously served in positions in Biomet’s legal department from June 1996 to January 2007.
Board Composition
Our Board of Directors consists of ten directors. Each of our Sponsors has the right to nominate, and have nominated, two directors to serve on our Board of Directors. Following Purchaser’s purchase of the shares tendered in the Offer, the Sponsors jointly appointed Dr. Miller and Jeffrey R. Binder to the Board of Directors in addition to the two directors appointed by each of the Sponsors. Our Board of Directors presently considers none of our directors to be independent (as independence is defined by Rule 4200 (a) (15) of the NASDAQ Stock Market LLC marketplace rules). As discussed in “-Executive Compensation” below, following the Transactions our common stock was no longer listed on the NASDAQ National Market). For more information regarding the rights of the Sponsors to nominate directors and other related arrangements, see “Certain Relationships and Related Party Transactions—Amended and Restated Limited Liability Company Operating Agreement of LVB Acquisition Holding, LLC.”
Audit Committee
Our audit committee currently consists of Messrs. McVeigh, Saer, Sisitsky and Summe. Our Board of Directors presently considers none of our directors serving on the audit committee to be independent. The audit committee is responsible for assisting our Board of Directors in fulfilling its oversight responsibility relating to the integrity of our financial statements and its financial reporting process, the systems of internal accounting and financial controls, the performance of our independent auditor and internal audit function, and the independent auditor’s qualifications and independence.
68
Table of Contents
Corporate Oversight and Compliance Committee
Our corporate oversight and compliance committee currently consists of Messrs. McVeigh, Miller, Saer, Sisitsky and Summe. None of the directors serving on the corporate oversight and compliance committee is independent. The committee is responsible for assisting the Board in overseeing our compliance with legal and regulatory requirements, our Code of Business Conduct and Ethics and our Fraud and Abuse Compliance Policies.
Compensation Committee
Our compensation committee currently consists of Messrs. Coslet, Jones, Michelson and Dal Bello. Our Board of Directors presently considers none of our directors serving on the compensation committee to be independent. The compensation committee is responsible for reviewing and approving goals and objectives related to the chief executive officer’s compensation, evaluating the chief executive officer’s performance against these goals and objectives and approving his compensation, approving total compensation for the other senior executive officers, establishing total compensation for the directors and overseeing our general cash-based and equity-based incentive plans.
Compensation Committee Interlocks and Insider Participation
None of our executive officers serve, or in the past fiscal year have served, as a member of the board of directors or compensation committee of any other entity that has executive officers who have served on our board of directors or compensation committee.
69
Table of Contents
Introduction
Throughout fiscal 2007 and the first four months of fiscal 2008, we were a public company, with our common stock traded on the NASDAQ National Market. As such, the Compensation Committee of our Board of Directors was responsible for developing, implementing and administering our cash and equity compensation policies. As a result of the Transactions, however, many of our compensation arrangements that had been in place during the 2007 fiscal year and the beginning of the 2008 fiscal year were discontinued in connection with the Transactions. In connection with the Transactions, each member of our Board of Directors (other than Mr. Binder, our President and Chief Executive Officer) serving prior to the Transactions resigned from our Board of Directors and all committees thereof (including our Compensation Committee) and new members of the Board were appointed by our sole shareholder, Parent, on behalf of the Sponsors.
Compensation and related matters during the 2008 fiscal year were reviewed and approved by (1) the Compensation Committee of our Board of Directors with respect to periods prior to consummation of the Transactions and (2) the Compensation Committees of Holding, Parent and our Board of Directors with respect to periods after consummation of the Transactions, which we refer to, collectively, as the Compensation Committee.
Compensation Discussion and Analysis
This section includes information regarding, among other things, the overall objectives of our compensation programs and each element of compensation that we provided, in each case with respect to the 2008 fiscal year. The goal of this section is to provide a summary of our executive compensation practices and the decisions that we made during this period concerning the compensation package payable to our executive officers, including the six executives in the Summary Compensation Table. Each of the six executives listed in the Summary Compensation Table is referred to herein as a “named executive officer.” This “Compensation Discussion and Analysis” should be read in conjunction with the detailed tables and narrative descriptions under “Executive Compensation Tables” below.
Compensation Methodology
During the 2008 fiscal year, the Compensation Committee was responsible for administering the compensation and benefit programs for our team members, including named executive officers. The Compensation Committee annually reviews and evaluates cash compensation and equity award recommendations along with the rationale for such recommendations, as well as summary information regarding the aggregate compensation, provided to our executive officers. The Compensation Committee examines these recommendations in relation to our overall objectives. Our President and Chief Executive Officer was not a member of the Compensation Committee during the 2008 fiscal year and did not participate in the decisions as to his compensation package.
The most significant development in our executive compensation philosophy during the 2008 fiscal year following the consummation of the Transactions has been a greater emphasis on correlating compensation to long-term equity growth. The Compensation Committee has provided significant equity investment opportunities in our Parent tied to financial objectives through grants of options to purchase shares of Parent and has modified the structure of non-equity awards to provide greater incentives for management performance. The Compensation Committee’s decisions for the 2008 fiscal year were made after considering compensation data of an informal peer group of other orthopedic manufacturing companies in our industry and privately owned portfolio companies of the Sponsors. However, the Compensation Committee did not engage in formal benchmarking as part of this informal review in making compensation decisions. In addition, as more fully discussed below, our annual cash bonus program has been redesigned in an effort to more closely align awards to our and our executives’ performance. The philosophy and target levels of each of the other compensation elements, including base salary, perquisites, health and welfare and retirement benefits during the 2008 fiscal year have largely continued to correspond to the levels of such awards compared to our informal peer group for periods prior to the Transactions.
Executive Compensation Philosophy and Objectives
Our executive compensation practices are affected by the highly competitive nature of the orthopedics industry and the location of our executive offices in Warsaw, Indiana. The fact that a number of the leading orthopedic manufacturers in the world have significant operations in and around Warsaw, Indiana means that there are continuing opportunities for experienced orthopedic executives who reside in this area. On the other hand, the fact that Warsaw, Indiana, is a small town in a predominantly rural area can present challenges to attracting executive talent from other industries and parts of the country.
Our executive compensation policies and practices during the 2008 fiscal year reflected the compensation philosophies of our founders and were designed to help achieve the superior performance of our executive officers and management team by accomplishing the following goals:
| • | attracting, retaining and rewarding highly qualified and productive persons; |
| • | relating compensation to both company and individual performance; |
| • | establishing compensation levels that are internally equitable and externally competitive; and |
| • | encouraging an ownership interest and instilling a sense of pride in Biomet. |
This compensation methodology was based upon one of our founding philosophies: equity incentives in the form of stock options are an excellent motivation for all team members, including executive officers, and serve to align the interests of team members, management and our equity investors.
70
Table of Contents
Based on these objectives, the compensation package of our executive officers during the 2008 fiscal year was intended to meet each of the following three criteria: (1) market competitive levels with companies of similar size and performance to us, such as the companies discussed above as our informal peer group; (2) performance based, “at risk” pay that is based on both short and long-term goals; and (3) incentives that are structured to create alignment between our equity investors and executives.
The Elements of Biomet’s Compensation Program
As a result of our compensation philosophies and objectives, the compensation package of our executive officers during the 2008 fiscal year consisted of five primary elements: (1) base salary; (2) non-equity incentive plan awards; (3) stock options and leveraged share awards; (4) participation in employee benefit plans; and (5) deferred compensation elections.
Base Salary. Consistent with prior fiscal years, our practice during the 2008 fiscal year was to provide base salaries at rates that we believed to be comparable with positions of executives in the orthopedics industry and other sponsor backed companies outside of the orthopedics industry, in each case of similar responsibility to our executives and other companies of similar size to us. The Compensation Committee reviewed our performance, the executive officer’s performance, our future objectives and challenges and the current competitive environment and set the base salary for each executive officer at the beginning of the fiscal year. We consider our 2008 base salaries to have been in line with our compensation objectives.
Non-equity Incentive Plan. Annual cash incentive awards to our named executive officers for the 2008 fiscal year were paid under the terms of a non-equity incentive plan approved by our Compensation Committee following consummation of the Transactions. The principal objective sought to be achieved by our non-equity incentive plan is to align awards with predetermined objectives and thereby improve performance in targeted areas. Payments under the plan are calculated based upon a percentage of an executive’s base salary, which are targeted to be competitive with other orthopedic manufacturing companies in our industry and after considering annual cash incentive programs at privately owned portfolio companies of the Sponsors.
Potential payments under the non-equity incentive plan for the 2008 fiscal year could have ranged from 0% to 180% of the an executive’s base salary, as a result of corporate, business unit and individual performance. Greater emphasis for Messrs. Binder, Florin, Kashuba and Richardson was placed on corporate performance, while a more significant factor for Messrs. Van Broeck and Schiess was business unit performance. Corporate and business unit targets for the 2008 fiscal year were EBITDA, net sales and operational objectives (including manufacturing footprint optimization and implementation of Six Sigma, lean manufacturing, and procurement and offshoring initiatives). Individual performance of named executive officers was determined after considering each executive’s leadership ability and contributions to our business during the 2008 fiscal year. With respect to named executive officers other than the Chief Executive Officer, the Compensation Committee also considered the Chief Executive Officer’s assessment of their individual performance in determining an individual named executive officer’s performance.
The chart below includes information about 2008 fiscal year opportunities and actual payout.
| Non-Equity Incentive Plan Target | Non-Equity Incentive Plan Maximum | Non-Equity Incentive Plan Payout (Paid in July 2008) | |||||||||||||
| % of Base Salary | Amount ($) | % of Base Salary | Amount ($) | % of Base Salary | Amount ($) | ||||||||||
Jeffrey R. Binder | 100 | % | 682,500 | 180 | % | 1,228,500 | 131 | % | 840,000 | ||||||
Daniel P. Florin | 80 | % | 321,430 | 144 | % | 578,575 | 89 | % | 356,708 | ||||||
J. Pat Richardson | 60 | % | 155,904 | 108 | % | 280,627 | 68 | % | 176,087 | ||||||
Roger Van Broeck(1) | 80 | % | 328,201 | 144 | % | 590,761 | 68 | % | 278,985 | ||||||
Glen A. Kashuba | 80 | % | 318,178 | 108 | % | 429,540 | 78 | % | 310,223 | ||||||
Steven F. Schiess | 80 | % | 238,968 | 108 | % | 322,607 | 78 | % | 232,086 | ||||||
| (1) | Mr. Van Broeck is employed in the Netherlands and paid in Euros. To calculate the U.S. dollar equivalent for disclosure purposes, we used a currency conversion rate of 1 Euro to $1.5557, which represents the currency exchange rate from Euros to U.S. dollars on May 31, 2008 as published in The Wall Street Journal. |
Since corporate and business unit target performance goals are generally set consistent with our confidential operating plan for the fiscal year, actual performance above our confidential operating plan would generally result in incentive payments above the target level. Conversely, performance below our confidential operating plan would generally result in incentive payments below the target level. The Compensation Committee and management believe that the metrics for the non-equity incentive plan align well with our objective of relating compensation to both company and individual performance. The specific corporate and business unit targets and ranges of acceptable performance set under the non-equity incentive plan are not disclosed because we believe disclosure of this information would cause competitive harm. These targets and ranges of acceptable performance are based on our confidential operating plan for the 2008 fiscal year and, therefore, achievement is substantially uncertain at the time they are set. The targets are intended to be realistic and reasonable, but challenging, in order to drive sustainable growth and individual performance.
Stock Options and Leveraged Share Awards. In 2007, the Board of Parent adopted the LVB Acquisition, Inc. 2007 Management Equity Incentive Plan (the “2007 LVB Plan”), which provides for the grant of non-qualified stock options to purchase shares of common stock of Parent (the “LVB Options”) to our and our affiliates’ key employees, directors, service providers and consultants. Generally, 50% of the LVB Options granted to employees vest based on continued employment, 25% vest based on continued employment and have an exercise price that increases by 10% per annum, and 25% vest based on the achievement of annual EBITDA based performance criteria established by the Board of Parent or a committee appointed by the Board of Parent. We also have granted LVB Options to our distributors, which are expected to be eligible to vest based on the achievement of specified sales targets.
71
Table of Contents
In 2008, the Board of Parent adopted an addendum to the 2007 LVB Plan, which provides the ability to grant leveraged equity awards in Parent under the 2007 LVB Plan (the “LVB Leveraged Awards,” and together with the LVB Options, the “LVB Awards”). LVB Leveraged Awards permit participants to purchase shares of LVB common stock using the proceeds of non recourse loans from Parent, which shares remain subject to forfeiture and other restrictions prior to the participant’s repayment of the loan.
Upon termination of a participant���s employment, the 2007 LVB Plan provides that any unvested portion of a participant’s LVB Award will be forfeited, and that the vested portion of his or her LVB Award will expire on the earlier of (1) the date participant’s employment is terminated for cause, (2) 30 days following the date the participant resigns without good reason, (3) 90 days after the date the participant’s employment is terminated by us for any reason other than cause, death, disability or the participant’s resignation with good reason, (4) one year after the date the participant’s employment is terminated by reason of death or disability or (5) the tenth anniversary of the grant date of the LVB Award.
Prior to receiving shares of LVB common stock (whether pursuant to the exercise of LVB Options, purchased pursuant to an LVB Leveraged Award or otherwise), participants must execute a Management Stockholders’ Agreement, which provides that the shares are subject to certain transfer restrictions, put and call rights, and tag along and drag along rights (and, with respect to certain senior members of management, limited registration and preemptive rights).
There were 37,520,000 shares of LVB common stock reserved for issuance in connection with LVB Awards to be granted pursuant to the 2007 LVB Plan. The Compensation Committee is responsible for administering the 2007 LVB Plan and authorizing the grant of LVB Awards pursuant thereto, and may amend the 2007 LVB Plan (and any LVB Awards) at any time. LVB Awards may not be granted under the 2007 LVB Plan on or after November 16, 2017. Following the Transactions, a total of 28,373,500 LVB Options were granted to employees and distributors under the 2007 LVB Plan during the 2008 fiscal year and 769,500 LVB Leveraged Awards were granted to employees under the 2007 LVB Plan during the 2008 fiscal year. Of the 23,373,500 LVB Options granted during the 2008 fiscal year, 7,245,000 were granted to our named executive officers and of the 769,500 LVB Leveraged Awards granted during the 2008 fiscal year, none were granted to our named executive officers.
Perquisites. We believe that our approach to perquisites has historically been, and continues to be, comparable to other companies in our informal peer group discussed above. Our President and Chief Executive Officer and other named executive officers have historically generally been permitted, when practical, to use company aircraft for business and personal travel for security reasons. On a case by case basis, we have historically reimbursed executives for social club dues or offered to provide a travel allowance in connection with Biomet related travel or relocation assistance to certain members of our senior management team who relocate their principal residence at our request. For example, we have historically, at times, provided reimbursement of moving expenses and protection against a loss on the sale of the executive’s home.
Health and Welfare Benefits. Named executive officers have historically received similar benefits to those provided to all other salaried U.S. employees, such as medical, dental, vision, life insurance and disability coverage.
Post Termination Compensation and Management Continuity Agreements. As described in further detail below, during the 2008 fiscal year, named executive officers were provided arrangements which specified payments in the event the executive’s employment is terminated. The type and amount of payments varied by executive level and the nature of the termination. These severance benefits, which are competitive with the companies discussed above as our informal peer group and general industry practices, are payable if and only if the executive’s employment terminates as specified in the applicable plan document or employment agreement. For more information, refer to “—Employment Agreements and Potential Post Termination Payments.”
Historically, we did not offer management continuity agreements to members of senior management. During the 2007 fiscal year, however, we engaged The Kinsley Group to assist with the preparation of and execution of change in control agreements with members of our senior management team. These agreements were intended to provide for continuity of management in the context of a prospective change in control of Biomet. These agreements were necessary to reinforce and encourage the continued attention and dedication of members of our senior management to their assigned duties without distraction in the face of potentially disturbing circumstances arising from the possibility of a change in control. In addition, we entered into change in control agreements with Messrs. Binder, Florin and Richardson, each of whom joined after the Transactions were announced, to provide such executives’ benefits if the Transactions did not close as expected and a new change in control transaction was consummated. Each of the agreements with Messrs. Binder, Florin and Richardson terminated upon consummation of the Transactions. For further information on the terms of the change in control agreements, refer to “—Employment Agreements and Potential Post Termination Payments—Change in Control Agreements” below.
Retirement Plans. We do not sponsor or maintain any pension plans applicable to our U.S. based named executive officers; however, we do have defined benefit retirement plans for certain of our foreign subsidiaries, discussed herein as our “foreign pension plans,” which covered certain of our overseas employees. One of these foreign pension plans was applicable to Mr. Van Broeck during the 2008 fiscal year and sponsored by Biomet Europe B.V. (“Biomet Europe”). During the 2008 fiscal year Biomet Europe provided all employees, whether salaried or hourly, with the opportunity to build up benefits under pension plans as part of Biomet Europe’s standard conditions for working in the Netherlands in order to provide a level of retirement benefits competitive with European market conditions. The benefits under this foreign pension plan are generally based on years of service and a calculation of the employee’s weighted average final base salary. Detailed explanations of these terms and calculations can be found in the narrative discussion accompanying the Pension Benefits Table in “—Executive Compensation Tables—Retirement And Non-Qualified Defined Contribution And Deferred Compensation Plans—Pension Plans” below. The investment objective is to enable a fixed, guaranteed payout to the employee at the time of the employee’s retirement, except, in the case of Mr. Van Broeck, for a moderate profit sharing provision, which may affect him by providing an additional benefit based on the collective return of the plan assets. The assets covered by the pension plan are managed by independent investment professionals, however, due to the guaranteed payout, policyholders are relatively unaffected by poor performance and affected only by positive investment returns under the profit sharing provision. The net assets of these foreign pension plans did not include any of our common shares as of April 30, 2008 (the same measurement dates used for the 2008 fiscal year with respect to our foreign subsidiaries). For information about Mr. Van Broeck’s pension benefits, refer to the Pension Benefits Table in “—Executive Compensation Tables—Retirement And Non-Qualified Defined Contribution And Deferred Compensation Plans—Pension Plans” below.
72
Table of Contents
In addition, during the 2008 fiscal year our executive officers were eligible to participate in our 401(k) plan (the “401(k) Plan”). All team members residing in the United States who are at least 18 years of age and complete at least 90 days of continuous service or work at least 1,000 hours per year were also eligible during the 2008 fiscal year to participate in the 401(k) Plan. Each year we, in our sole discretion, may match 75% of each team member’s contributions, up to a maximum amount equal to 5% of the team member’s compensation in cash. All contributions to the 401(k) Plan are allocated to accounts maintained on behalf of each participating team member and, to the extent vested, are available for distribution to the team member or beneficiary upon retirement, death, disability or termination of service. The 401(k) Plan generally purchased common shares of Biomet with our matching contribution. Executive officers have also historically participated in our Employee Stock Bonus Plan (the “ESBP”), which was merged into and with our 401(k) Plan during the 2008 fiscal year.
In addition, we maintain The Biomet, Inc. Deferred Compensation Plan (the “Deferred Compensation Plan”), a non-qualified deferred compensation plan, which is available for our senior management. The Deferred Compensation Plan allows eligible participants to defer pre tax compensation to reduce current tax liability and assist those team members in their planning for retirement and other long-term savings goals in a tax effective manner. We do not make any contributions to the Deferred Compensation Plan. Under the Deferred Compensation Plan, eligible participants may defer up to 100% of their base salary and cash bonus payments. Participants received scheduled distributions from the Deferred Compensation Plan are available, which are treated as ordinary income subject to federal and state income taxation at the time of distribution. Except in circumstances of hardship, unscheduled withdrawals are not permitted. Amounts contributed to the Deferred Compensation Plan are at the participant’s election and “deemed investments,” which means that the participants have no ownership interest in the investment alternative selected. The participants’ deferrals and gains are reflected on our financial statements and are our unsecured general assets. The Deferred Compensation Plan is an unfunded “future promise to pay” by us. Neither Biomet nor the Deferred Compensation Plan record keeper provides any guarantee of investment return. We do not pay above market interest rates on deferred amounts of compensation. For more information, refer to “—Executive Compensation Tables—Retirement and Non-Qualified Defined Contribution and Deferred Compensation Plans—Non-Qualified Deferred Compensation” below.
Policy with Respect to Deductibility of Compensation over $1 Million. Section 162(m) of the Code generally limits to $1.0 million the tax deductibility of annual compensation paid to certain executives named in the Summary Compensation Table. However, performance based compensation can be excluded from this limit if it meets certain requirements. Prior to the Transactions, the Compensation Committee’s policy was historically to consider the impact of Section 162(m) in establishing compensation for our senior executives. However, the Compensation Committee historically retained the discretion to establish compensation, even if such compensation was not deductible under Section 162(m), if, in the Compensation Committee’s judgment, such compensation was in our best interest and was reasonably expected to increase shareholder value. Following the Transactions, because we no longer have publicly held equity, the restrictions of Section 162(m) no longer apply.
Compensation Committee Report
The Compensation Committee has reviewed and discussed the foregoing Compensation Discussion and Analysis with management. Based on such review and discussion, the Compensation Committee recommended to the Board of Directors that the Compensation Discussion and Analysis be included in this prospectus.
Compensation Committee
Jonathan J. Coslet
Adrian Jones
Michael Dal Bello
Michael Michelson
Executive Compensation Tables
Summary Compensation Table
The following narrative, tables and footnotes describe the “total compensation” earned during the 2007 and 2008 fiscal years by our named executive officers. The total compensation presented below does not reflect the actual compensation received by our named executive officers or the target compensation of our named executive officers during the 2007 and 2008 fiscal years. The actual value realized by our named executive officers during the 2007 and 2008 fiscal years from long-term incentives (options) is presented in the Option Exercises and Stock Vested Table below.
The individual components of the total compensation calculation reflected in the Summary Compensation Table are broken out below:
Salary. Base salary earned during the 2008 fiscal year. Refer to “—The Elements of Biomet’s Compensation Program—Base Salary” above for further information concerning this element of our compensation program.
Bonus. For the 2008 fiscal year, we did not have any bonus plans applicable to our named executive officers. Each named executive officer, however, earned an annual performance based cash incentive award as described under “—Non-equity Incentive Plan Compensation” below.
Option Awards. The awards disclosed under the heading “Option Awards” consist of grants of stock options awarded under the Biomet, Inc. 1998 Qualified and Non-Qualified Stock Option Plan (the “1998 Plan”) and 2007 LVB Plan. For further information about our stock option programs, refer to “—The Elements of Biomet’s Compensation Program—Stock Options and Leveraged Share Awards” above. In addition, details about option awards made during the 2008 fiscal year are included in the Grant of Plan Based Awards Table below. The dollar amounts for the awards in the Summary Compensation Table below represent the compensation
73
Table of Contents
expense recognized during the 2008 fiscal year under SFAS 123(R) for each named executive officer. The recognized compensation expense of the option awards for financial reporting purposes will likely vary from the actual amount ultimately realized by the named executive officer based on a number of factors. The factors include our actual operating performance, common share price fluctuations, differences from the valuation assumptions used and the timing of exercise or applicable vesting.
Stock Awards. The only equity based compensation that we recognized under SFAS 123(R) with respect to our named executive officers for the 2008 fiscal year was in relation to stock option awards. For information about stock options granted to our named executive officers, see “Option Awards” immediately above.
Non-equity Incentive Plan Compensation. Our named executive officers earned annual incentive bonuses for the 2008 fiscal year. Refer to “—The Elements of Biomet’s Compensation Program—Non-equity Incentive Plan” above for further information concerning this element of our compensation program.
Change in Pension Value and Non-Qualified Deferred Compensation Earnings. We do not sponsor or maintain any pension plans applicable to our U.S. based named executive officers. For Mr. Van Broeck, the change in pension value represents the aggregate change in the actuarial present value of the accumulated benefit under his pension plan sponsored by Biomet Europe from April 30, 2007 to May 31, 2008 (the same measurement dates used for financial statement reporting purposes with respect to our audited financial statements for the 2007 and 2008 fiscal years with respect to our foreign subsidiaries). For information on Mr. Van Broeck’s retirement benefits and certain material features of the pension plan in which he participates, refer to “—The Elements of Biomet’s Compensation Program—Retirement Plans” above and “—Retirement And Non-Qualified Defined Contribution And Deferred Compensation Plans—Pension Plans” below.
None of our named executive officers participated in the Deferred Compensation Plan during the 2008 fiscal year. Furthermore, we do not pay above market or preferential earnings on non-qualified deferred compensation. For information on the Deferred Compensation Plan, refer to “—Compensation Discussion and Analysis—Retirement Plans.”
All Other Compensation. The amounts included under the All Other Compensation heading represent the sum of: (1) certain perquisites and other personal benefits; (2) Biomet paid contributions to retirement plans; (3) Biomet paid insurance premiums; (4) certain tax reimbursements made by us; and (5) certain other amounts more fully described in footnote (2) to the Summary Compensation Table.
SUMMARY COMPENSATION TABLE
Name and Principal Position | Year | Salary ($) | Bonus ($) | Option Awards(1) ($) | Stock Awards(1) ($) | Non-Equity Incentive Plan Compensation ($) | Change in Pension Value and Non-Qualified Deferred Compensation Earnings ($) | All Other Compensation(2) ($) | Total ($) | ||||||||||
Jeffrey R. Binder, | 2008 | 682,500 | — | 4,334,395 | — | 840,000 | — | 1,766,811 | 7,623,706 | ||||||||||
| 2007 | 150,050 | 162,500 | — | — | — | — | 71,858 | 384,408 | |||||||||||
Daniel P. Florin, | 2008 | 401,788 | — | 686,279 | — | 356,708 | — | 13,313 | 1,458,088 | ||||||||||
J. Pat Richardson, | 2008 | 259,840 | — | 340,560 | — | 176,087 | — | 16,863 | 793,350 | ||||||||||
| 2007 | 25,834 | 24,722 | — | — | — | — | 3,788 | 54,344 | |||||||||||
Roger van Broeck, | 2008 | 410,251 | — | 593,399 | — | 278,985 | — | 79,109 | 1,361,744 | ||||||||||
| 2007 | 386,741 | 284,235 | 119,486 | — | — | 77,073 | (3) | 68,311 | 935,846 | ||||||||||
Glen A. Kashuba | 2008 | 397,722 | — | 928,799 | — | 310,223 | — | 13,313 | 1,658,012 | ||||||||||
Steven F. Schiess | 2008 | 298,710 | — | 593,399 | — | 232,086 | — | 13,313 | 1,144,390 | ||||||||||
| (1) | For each named executive officer listed in the Summary Compensation Table above, the value reflects the compensation expense recognized by us during the 2008 and 2007 fiscal years under SFAS 123(R). For information on the full grant-date fair value of awards granted solely during the 2008 and 2007 fiscal years, refer to the Grant of Plan-Based Awards Table below and to footnote (1) of the Grant of Plan-Based Awards Table. |
We use the Black-Scholes option-pricing model to determine the fair value of options to calculate compensation expense. For information about the assumptions used in determining the compensation expense we recognized during the 2008 and 2007 fiscal years, refer to Note 9 to the consolidated financial statements elsewhere in this prospectus.
74
Table of Contents
| (2) | The table below presents an itemized account of “All Other Compensation” provided during the 2007 and 2008 fiscal years. For each named executive officer listed below, the sum of each of the columns reflects the total value included under the All Other Compensation heading in the table above. |
| Year | Life Insurance Premiums ($) | Retirement Plan Contributions ($) | Medical Flex ($) | Travel Allowance ($) | Personal Use of Company Aircraft (a) | Other ($) | ||||||||||
Jeffrey R. Binder | 2008 | 63 | — | 250 | 13,000 | 433,498 | 1,320,000 | (b) | ||||||||
| 2007 | — | — | 146 | — | 63,600 | 8,112 | (c) | |||||||||
Daniel P. Florin | 2008 | 63 | — | 250 | 13,000 | — | — | |||||||||
J. Pat Richardson | 2008 | 63 | — | 250 | 13,000 | — | 3,550 | (d) | ||||||||
| 2007 | — | — | 104 | — | — | 3,684 | (d) | |||||||||
Roger Van Broeck | 2008 | — | 44,960 | — | 28,501 | (e) | — | 5,648 | (f) | |||||||
| 2007 | — | 38,811 | — | 24,621 | (e) | — | 4,879 | (f) | ||||||||
Glen A. Kashuba | 2008 | 63 | — | 250 | 13,000 | — | — | |||||||||
Steven F. Schiess | 2008 | 63 | — | 250 | 13,000 | — | — | |||||||||
| (a) | Our incremental cost for personal use of our aircraft is calculated by multiplying the aircraft’s hourly variable operating cost by a trip’s flight time, which includes any flight time of an empty return flight. Variable operating costs are based on industry standard rates of our variable operating costs, including fuel and oil costs, maintenance and repairs, landing/ramp fees and other miscellaneous variable costs. On certain occasions, a spouse or other family member may accompany one of our named executive officers on a flight. No additional operating cost is incurred in such situations under the foregoing methodology. We do not pay our named executive officers any amounts in connection with taxes on income imputed to them for personal use of our aircraft. |
Pursuant to the employment agreement between us and Mr. Binder, dated February 26, 2007, we agreed to arrange, at our expense, for Mr. Binder to fly once per week to and from Mr. Binder’s Texas home and our headquarters or such other location reasonably specified by us during the term of the employment agreement. We will not provide Mr. Binder with a “gross up” for taxes incurred in connection with these benefits. If, however, Mr. Binder uses a commercial flight and the income imputed in connection with the commercial flight is greater than the amount that would have been imputed to Mr. Binder if he had used our aircraft, we will provide to Mr. Binder a “gross up” for taxes incurred on the incremental income associated with the commercial flight. Our incremental costs associated with extending these benefits to Mr. Binder are capped at $500,000 in any twelve-month period. For the purposes of applying this limitation, our incremental cost for commercial flights shall be the cost of Mr. Binder’s tickets and for flights on Biomet-operated aircraft shall be the incremental per-hour cost associated with Mr. Binder’s flights and other incremental costs related to such flights, such as landing fees, transportation and housing costs of aircrew and other similar costs. The amount that appears under the Personal Use of Company Aircraft heading reflects the amount of this rolling twelve-month allowance that Mr. Binder has used. In addition, pursuant to the employment agreement between us and Mr. Binder dated February 26, 2007, we agreed to purchase Mr. Binder’s prior residence in Illinois at its appraised value, to be determined by an independent appraiser, up to $2,199,000. Furthermore, we agreed to reimburse Mr. Binder for certain capital gains taxes, if any, incurred as a result of the sale of Mr. Binder’s prior residence. As a result of the independent appraisal, we purchased Mr. Binder’s prior residence for significantly less than the maximum amount and Mr. Binder has not recognized any gain on the sale of his prior residence. As a result of our subsequent sale of Mr. Binder’s former residence, the amount paid by us to Mr. Binder is not reflected in the amount shown in the table above for Mr. Binder under the “All Other Compensation” heading. In addition, because Mr. Binder recognized a loss on the sale of his house, we have not paid any “gross up” amounts to Mr. Binder in connection with the sale of his house.
| (b) | Also, pursuant to the employment agreement between us and Mr. Binder dated February 26, 2007, we agreed to reimburse Mr. Binder up to $1,320,000 if Mr. Binder is required to pay his former employer in connection with the termination of his previous employment. On September 21, 2007, we paid $1,320,000 to Mr. Binder in connection with this obligation. |
| (c) | Represents the cost to us of providing temporary housing to Mr. Binder in Warsaw, Indiana. |
| (d) | Represents the cost to us of providing temporary housing to Mr. Richardson in Warsaw, Indiana. |
| (e) | Represents the cost to us of providing a car to Mr. Van Broeck. |
| (f) | Represents the Biomet-paid portion of Mr. Van Broeck’s government mandated health and wellness expense. |
In addition to the foregoing compensation, named executive officers also participated in health and welfare benefit programs, including vacation and medical, dental, prescription drug and disability coverage. These programs are generally available and comparable to those programs provided to all U.S. salaried employees.
| (3) | Mr. Van Broeck is employed in the Netherlands and paid in Euros. To calculate the U.S. dollar equivalent for disclosure purposes, we used a currency conversion rate of 1 Euro to $1.5566, which represents the currency exchange rate from Euros to U.S. dollars on May 31, 2008 as published in The Wall Street Journal. |
75
Table of Contents
Grants of Plan-Based Awards Table
In connection with the Transactions all stock options outstanding under the 1998 Plan and the Biomet, Inc. 2006 Equity Incentive Plan (the “2006 Plan”) (whether held by officers, directors, employees or distributors) were cancelled and the holders thereof became entitled to receive from us an amount equal to the excess, if any, of the $46.00 offer price over the option exercise price for each share subject to the stock option, in each case, less any applicable withholding taxes and without interest and regardless of whether or not the awards were then vested or exercisable. Following consummation of the Transactions, the 2007 LVB Plan was established. For a further discussion of the 2007 LVB Plan, see “—The Elements of Biomet’s Compensation Program—Stock Options and Leveraged Share Awards.” During the 2008 fiscal year, we granted stock options to our named executive officers under the 2007 LVB Plan. Information with respect to each of these awards on a grant-by-grant basis is set forth in the table below. For additional discussion of the 2007 LVB Plan and certain material terms of the stock option awards under this plan, refer to “—The Elements of Biomet’s Compensation Program—Stock Options and Leveraged Share Awards.” All stock option awards to our named executive officers during the 2008 fiscal year were made such that the exercise price of the awards was equal to the fair market value of LVB’s common shares on the date of grant.
During the 2008 fiscal year, we also granted cash incentive awards to our named executive officers under our non-equity incentive plan. Information with respect to each of these payments is set forth in the table below. For additional discussion of our non-equity incentive plan, refer to “The Elements of Biomet’s Compensation Program—Non-Equity Incentive Plan.”
GRANTS OF PLAN-BASED AWARDS
Name | Grant Date | Estimated Possible Payouts Under Non-Equity Incentive Plan Awards | Estimated Future Payouts Under Equity Incentive Plan Awards | All Other Stock Awards: Number of Shares of Stock or Units (#) | All Other Option Awards: Number of Securities Underlying Options (#) | Exercise of Base Price of Option Awards ($/Sh) | Grant-Date Fair Value of Stock and Option Awards (1) ($) | |||||||||||||||
| Threshold ($) | Target ($) | Maximum ($) | Threshold (#) | Target (#) | Maximum (#) | |||||||||||||||||
Jeffrey R. Binder | December 17, 2007 | 3,413 | 682,500 | 1,228,500 | — | — | — | — | — | — | — | |||||||||||
December 17, 2007(2) | — | — | — | — | — | — | — | 2,100,000 | — | 7,770,000 | ||||||||||||
December 17, 2007(3) | — | — | — | — | — | — | — | 1,050,000 | — | 2,373,000 | ||||||||||||
December 17, 2007(4) | — | — | — | — | 1,050,000 | — | — | — | — | 3,885,000 | ||||||||||||
Daniel P. Florin | December 17, 2007 | 2,009 | 321,430 | 578,575 | — | — | — | — | — | — | — | |||||||||||
December 17, 2007(2) | — | — | — | — | — | — | — | 332,500 | — | 1,230,250 | ||||||||||||
December 17, 2007(3) | — | — | — | — | — | — | — | 166,250 | — | 375,725 | ||||||||||||
December 17, 2007(4) | — | — | — | — | 166,250 | — | — | — | — | 615,125 | ||||||||||||
J. Pat Richardson | December 17, 2007 | 1,299 | 155,904 | 280,627 | — | — | — | — | — | — | — | |||||||||||
December 17, 2007(2) | — | — | — | — | — | — | — | 165,000 | 610,500 | |||||||||||||
December 17, 2007(3) | — | — | — | — | — | — | — | 82,500 | — | 186,450 | ||||||||||||
December 17, 2007(4) | — | — | — | — | 82,500 | — | — | — | — | 305,250 | ||||||||||||
Roger van Broeck | December 17, 2007 | 2,051 | 328,201 | 590,761 | — | — | — | — | — | — | — | |||||||||||
December 17, 2007(2) | — | — | — | — | — | — | — | 287,500 | — | 1,063,750 | ||||||||||||
December 17, 2007(3) | — | — | — | — | — | — | — | 143,750 | — | 324,875 | ||||||||||||
December 17, 2007(4) | — | — | — | — | 143,750 | — | — | — | — | 531,875 | ||||||||||||
Glen A. Kashuba | December 17, 2007 | 1,989 | 318,178 | 429,540 | — | — | — | — | — | — | — | |||||||||||
December 17, 2007(2) | — | — | — | — | — | — | — | 450,000 | — | 1,665,000 | ||||||||||||
December 17, 2007(3) | — | — | — | — | — | — | — | 225,000 | — | 508,500 | ||||||||||||
December 17, 2007(4) | — | — | — | — | 225,000 | — | — | — | — | 832,500 | ||||||||||||
Steven F. Schiess | December 17, 2007 | 1,494 | 238,968 | 322,607 | — | — | — | — | — | — | — | |||||||||||
December 17, 2007(2) | — | — | — | — | — | — | — | 287,500 | — | 1,063,750 | ||||||||||||
December 17, 2007(3) | — | — | — | — | — | — | — | 143,750 | — | 324,875 | ||||||||||||
December 17, 2007(4) | — | — | — | — | 143,750 | — | — | — | — | 531,875 | ||||||||||||
| (1) | For each named executive officer listed in the Grant of Plan-Based Awards Table above, the value reflects the full grant-date fair value calculated under SFAS 123(R) solely for awards granted during the 2008 fiscal year. The fair value of the stock option awards for financial reporting purposes likely will vary from the actual amount ultimately realized by the named executive officer based on a number of factors. These factors include our actual operating performance, common share price fluctuations, differences from the valuation assumptions used and the timing of exercise or applicable vesting. See Note 9 to the consolidated financial statements for the assumptions made in determining SFAS 123(R) values. |
| (2) | Represents grants of time-based options, which generally vest ratably over 5 years. For additional discussion of the 2007 LVB Plan and certain material terms of the stock option awards under this plan, refer to “The Elements of Biomet’s Compensation Program—Stock Options and Leveraged Share Awards.” |
| (3) | Represents grants of accreting exercise price options, which have exercise prices that will increase by 10% each year and generally vest ratably over 5 years. For additional discussion of the 2007 LVB Plan and certain material terms of the stock option awards under this plan, refer to “The Elements of Biomet’s Compensation Program—Stock Options and Leveraged Share Awards.” |
| (4) | Represents grants of performance-based options, which generally vest over 5 years, contingent upon the Company achieving certain EBITDA targets in each of those years. For additional discussion of the 2007 LVB Plan and certain material terms of the stock option awards under this plan, refer to “The Elements of Biomet’s Compensation Program—Stock Options and Leveraged Share Awards.” |
76
Table of Contents
Outstanding Equity Awards at Fiscal Year-End Table
As discussed in further detail in “—Grants of Plan Based Awards Table” above, in connection with the Transactions all stock options outstanding under the 1998 Plan and the 2006 Plan (whether held by officers, directors, employees or distributors) were cancelled and the holders thereof became entitled to receive from us an amount equal to the excess, if any, of the $46.00 offer price over the option exercise price for each share subject to the stock option, in each case, less any applicable withholding taxes and without interest and regardless of whether or not the awards were then vested or exercisable. Following consummation of the Transactions, the 2007 LVB Plan was established. For a further discussion of the 2007 LVB Plan, see “The Elements of Biomet’s Compensation Program—Stock Options and Leveraged Share Awards.”
We have historically awarded stock options to members of our senior management and our other team members throughout Biomet. Generally, of the awards listed in the table below, 50% vest based on continued employment, 25% vest based on continued employment and have an exercise price that increases by 10% per annum, and 25% vest based on the achievement of annual EBITDA-based performance criteria established by the Compensation Committee. For information on the vesting schedule of the unvested portions of outstanding equity awards listed below, refer to footnote (2) to the table below. Upon termination of a participant’s employment, the 2007 LVB Plan provides that any unvested portion of a participant’s LVB Award will be forfeited, and that the vested portion of his or her LVB Award will expire on the earlier of (1) the date the participant’s employment is terminated for cause, (2) 30 days following the date the participant resigns without good reason, (3) 90 days after the date the participant’s employment is terminated either by us for any reason other than cause, death, disability or by the participant with good reason, (4) one year after the date the participant’s employment is terminated by reason of death or disability or (5) the tenth anniversary of the grant date of the LVB Award.
For further information on our stock option awards and their material terms, refer to “—The Elements of Biomet’s Compensation Program—Stock Options and Leveraged Share Awards.” For information about stock option awards granted solely during the 2008 fiscal year, refer to “—Grant of Plan-Based Awards Table.”
The following table shows the equity awards granted to our named executive officers, which are comprised solely of stock option awards under the 2007 LVB Plan (vested and unvested), that were outstanding as of the end of the 2008 fiscal year.
OUTSTANDING EQUITY AWARDS AT FISCAL YEAR END
| Option Awards | Stock Awards | ||||||||||||||||||
Name | Number of Securities Underlying Unexercised Options (#) Exercisable(1) | Number of Securities Underlying Unexercised Options (#) Unexercisable(2) | Equity Incentive Plan Awards: Number of Securities Underlying Unexercised Unearned Options(3) (#) | Option Exercise Price (4)($) | Option Expiration Date(5) | Number of Shares or Units of Stock That Have Not Vested (#) | Market Value of Shares or Units of Stock That Have Not Vested ($) | Equity Incentive Plan Awards: Number of Unearned Shares, Units or Other Rights That Have Not Vested (#) | Equity Incentive Plan Awards: Market or Payout Value of Unearned Shares, Units or Other Rights That Have Not Vested ($) | ||||||||||
Jeffrey R. Binder | — | 2,100,000 | (a) | — | 10.00 | July 11, 2017 | — | — | — | — | |||||||||
| — | 1,050,000 | (b) | — | 10.00 | July 11, 2017 | — | — | — | — | ||||||||||
| — | — | 1,050,000 | 10.00 | July 11, 2017 | — | — | — | — | |||||||||||
Daniel P. Florin | — | 332,500 | (a) | — | 10.00 | July 11, 2017 | — | — | — | — | |||||||||
| — | 166,250 | (b) | — | 10.00 | July 11, 2017 | — | — | — | — | ||||||||||
| — | — | 166,250 | 10.00 | July 11, 2017 | — | — | — | — | |||||||||||
J. Pat Richardson | — | 165,000 | (a) | 10.00 | July 11, 2017 | — | — | — | — | ||||||||||
| — | 82,500 | (b) | — | 10.00 | July 11, 2017 | — | — | — | — | ||||||||||
| — | — | 82,500 | 10.00 | July 11, 2017 | — | — | — | — | |||||||||||
Roger van Broeck | — | 287,500 | (a) | — | 10.00 | July 11, 2017 | — | — | — | — | |||||||||
| — | 143,750 | (b) | — | 10.00 | July 11, 2017 | — | — | — | — | ||||||||||
| — | — | 143,750 | 10.00 | July 11, 2017 | — | — | — | — | |||||||||||
Glen A. Kashuba | — | 450,000 | (a) | — | 10.00 | July 11, 2017 | — | — | — | — | |||||||||
| — | 225,000 | (b) | — | 10.00 | July 11, 2017 | — | — | — | — | ||||||||||
| — | — | 225,000 | 10.00 | July 11, 2017 | — | — | — | — | |||||||||||
Steven F. Schiess | — | 287,500 | (a) | — | 10.00 | July 11, 2017 | — | — | — | — | |||||||||
| — | 143,750 | (b) | — | 10.00 | July 11, 2017 | — | — | — | — | ||||||||||
| — | — | 143,750 | 10.00 | July 11, 2017 | — | — | — | — | |||||||||||
77
Table of Contents
| (1) | On an award-by-award basis, the number of common shares underlying unexercised options that are exercisable and that are not reported in Column 3—”Number of Securities Underlying Unexercised Unearned Options.” |
| (2) | On an award-by-award basis, the number of common shares underlying unexercised options that are unexercisable and that are not reported in Column 3—”Number of Securities Underlying Unexercised Unearned Options.” The vesting schedules of the outstanding unvested equity awards are listed below: |
(a) Represents grants of time-based options, which generally vest ratably over 5 years.
With respect to Mr. Binder, represents the outstanding unvested portion of the original option granted on December 4, 2007. The remaining unvested portion of the original award vests in increments of 420,000 Common Shares on July 11, 2008, 2009, 2010, 2011 and 2012.
With respect to Mr. Florin, represents the outstanding unvested portion of the original option granted on December 4, 2007. The remaining unvested portion of the original award vests in increments of 66,500 Common Shares on July 11, 2008, 2009, 2010, 2011 and 2012.
With respect to Mr. Richardson, represents the outstanding unvested portion of the original option granted on December 4, 2007. The remaining unvested portion of the original award vests in increments of 33,000 Common Shares on July 11, 2008, 2009, 2010, 2011 and 2012.
With respect to Van Broeck, represents the outstanding unvested portion of the original option granted on December 4, 2007. The remaining unvested portion of the original award vests in increments of 57,500 Common Shares on July 11, 2008, 2009, 2010, 2011 and 2012.
With respect to Mr. Kashuba, represents the outstanding unvested portion of the original option granted on December 4, 2007. The remaining unvested portion of the original award vests in increments of 90,000 Common Shares on July 11, 2008, 2009, 2010, 2011 and 2012.
With respect to Mr. Schiess, represents the outstanding unvested portion of the original option granted on December 4, 2007. The remaining unvested portion of the original award vests in increments of 57,500 Common Shares on July 11, 2008, 2009, 2010, 2011 and 2012.
(b) Represents grants of performance-based options, which generally vest ratably over 5 years and have an exercise price that increases by 10% per annum.
With respect to Mr. Binder, represents the outstanding unvested portion of the original option granted on December 4, 2007. The remaining unvested portion of the original award vests in increments of 210,000 Common Shares on July 11, 2008, 2009, 2010, 2011 and 2012.
With respect to Mr. Florin, represents the outstanding unvested portion of the original option granted on December 4, 2007. The remaining unvested portion of the original award vests in increments of 33,250 Common Shares on July 11, 2008, 2009, 2010, 2011 and 2012.
With respect to Mr. Richardson, represents the outstanding unvested portion of the original option granted on December 4, 2007. The remaining unvested portion of the original award vests in increments of 16,500 Common Shares on July 11, 2008, 2009, 2010, 2011 and 2012.
With respect to Mr. Van Broeck, represents the outstanding unvested portion of the original option granted on December 4, 2007. The remaining unvested portion of the original award vests in increments of 28,750 Common Shares on July 11, 2008, 2009, 2010, 2011 and 2012.
With respect to Mr. Kashuba, represents the outstanding unvested portion of the original option granted on December 4, 2007. The remaining unvested portion of the original award vests in increments of 45,000 Common Shares on July 11, 2008, 2009, 2010, 2011 and 2012.
With respect to Mr. Schiess, represents the outstanding unvested portion of the original option granted on December 4, 2007. The remaining unvested portion of the original award vests in increments of 28,750 Common Shares on July 11, 2008, 2009, 2010, 2011 and 2012.
| (3) | On an award-by-award basis, the total number of common shares underlying unexercised options awarded under any equity incentive plan that have not been earned. Awards vest over a five-year term based on EBITDA-based criteria established by the Compensation Committee. |
| (4) | The exercise price for each option, as it was recorded in the stock option award at the time of grant, is reported in Columns 1 and 2—”Number of Securities Underlying Unexercised Options” and Column 3—”Number of Securities Underlying Unexercised Unearned Options.” The options have an exercise price equivalent to fair market value on the date of grant. Since our common stock is not currently traded on a national securities exchange, fair market value was determined by the Compensation Committee. |
| (5) | Represents the tenth year anniversary for each option award reported in Columns 1 and 2—”Number of Securities Underlying Unexercised Options” and Column 3—”Number of Securities Underlying Unexercised Unearned Options.” For information on the vesting schedule of unvested portions of outstanding option awards, see sub-footnotes (a)-(f) of footnote (2) above. |
78
Table of Contents
Option Exercises and Stock Vested Table
During the 2008 fiscal year, no option awards were exercised and no stock awards vested applicable to Biomet’s named executive officers.
In connection with the Transactions, however, all stock options outstanding under the 1998 Plan and the 2006 Plan (whether held by officers, directors, employees or distributors) were cancelled and the holders thereof became entitled to receive from us an amount equal to the excess, if any, of the $46.00 offer price over the option exercise price for each share subject to the stock option, in each case, less any applicable withholding taxes and without interest and regardless of whether or not the awards were then vested or exercisable. For a further description of amounts received by named executive officers in connection with the Transactions (including as a result of the cancellation of stock options), see Biomet’s Proxy Statement filed with the SEC on August 8, 2007.
Retirement And Non-Qualified Defined Contribution And Deferred Compensation Plans
Pension Plans
We do not sponsor or maintain any pension plans applicable to our U.S.-based named executive officers. Of our named executive officers, only Mr. Van Broeck, who is based in the Netherlands, participated in a foreign pension plan sponsored by Biomet Europe during the 2008 fiscal year. Biomet Europe offers certain of its employees, whether salaried or hourly, with the opportunity to build up benefits under pension plans as part of Biomet Europe’s standard conditions for working in order to provide a level of retirement benefits competitive with European market conditions. Biomet Europe provides employees with pension benefits beginning after the completion of twelve consecutive months of employment with Biomet Europe. Once this minimum condition is met, however, the employee is credited with accrued time of service for the first twelve months of employment.
Under the foreign pension plan applicable to Mr. Van Broeck during the 2008 fiscal year, the basic contribution was a fixed premium to which he contributed 7% of his annual base salary and Biomet Europe contributed the remainder. Mr. Van Broeck’s bonus was not included for the purposes of pension calculations or contributions. Certain employees have historically been affected by a maximum pensionable salary condition, which imposed a cap on the amount of salary used for calculations that affect certain amounts, such as premiums and benefits. The benefits provided under this foreign pension plan are based on the following formula:
“years of service” × 1.75% × “final salary”
Under this foreign pension plan, “years of service” is calculated on a monthly basis from the date corresponding to the date that the employee first signed a contract with the plan provider providing the underlying coverage, which is meant to correspond to the first day of the employee’s employment at Biomet Europe. The maximum number of years of credited service is 40 years. Biomet Europe does not allow additional years of service credits to be granted to employees under this plan. For the purpose of the benefits formula, the calculation presumes the employee accrues 40 years of credited service and then the value is adjusted downward, if necessary.
In addition, under this foreign pension plan, “final salary” is calculated as the average of the employee’s base salary over the last five calendar years of his or her employment at Biomet Europe.
Benefits under the plan do not provide the employee with a lump sum following retirement. The plan provides for the purchase of an annuity, which in operation provides a monthly retirement allowance. The full benefits are payable only at normal retirement age and the early retirement results in a reduction in benefits. Retirement age under the plan is age 65.
The benefits provided by this foreign pension plan provide a guaranteed payout, which is intended to be based on the targeted annual payout of an annuity purchased at the time of retirement. Mr. Van Broeck joined this plan in 1998, which provides for him to receive a guaranteed annuity on September 1, 2013.
Pension Benefits Table
The following table describes the estimated actuarial present value of accrued pension benefits through the end of the 2008 fiscal year for each of the named executive officers listed in the table. The calculation of actuarial present value is generally consistent with the methodology and assumptions outlined in our audited financial statements, except that the calculation does not assume an average salary increase of 3.0%, a discount rate of 4.9% or an inflation rate of 2.0% because Mr. Van Broeck’s salary is frozen for the purposes of the pension plan and because the payout amount is guaranteed. In addition, the calculation presumes an implied rate of return on the plan assets during the 2008 fiscal year of 4.0%. The expected rate of return on the plan assets is 4.9%, as assumed in conjunction with the preparation of our audited financial statements. The actuarial present value of benefits is calculated in accordance with the following assumptions: (1) assumed retirement age: 65; (2) no pre-retirement decrements; and (3) assumed form of payment: lump sum. The actuarial increase during the 2008 fiscal year of the projected retirement benefits can be found in the “Summary Compensation Table” under the “Change in Pension Value and Non-Qualified Deferred Compensation Earnings” heading (for Mr. Van Broeck, the amount reported under that heading represents actuarial increases in Mr. Van Broeck’s plan).
79
Table of Contents
PENSION BENEFITS
Name | Plan Name | Number of Years of Credited Service (2)(#) | Present Value of Accumulated Benefit (3)($) | Payment During Last Fiscal Year (4)($) | |||||
Roger van Broeck | Biomet Europe Pension Plan | (1) | 10 | 584,226 | 44,960 |
| (1) | Mr. Van Broeck participates in the Biomet Europe Pension Plan, which is sponsored by Biomet Europe. This is the English translation of the plan’s proper name, Biomet Europe Pension Plan. |
| (2) | Mr. Van Broeck’s ten years of accrued service under the Biomet Europe Pension Plan started in 1998 with BioMer C. V., which was a joint venture between Biomet, Inc. and Merck KGaA, and then later with Biomet Europe, the Successor company to BioMer C.V. Prior to 1998, Mr. Van Broeck was with Biomet in different positions in different countries for which he did not carry over any build up of pension benefits to his current pension plan. |
| (3) | For Mr. Van Broeck, represents the actuarial present value of the accumulated benefit under the Biomet Europe Pension plan, which was computed as of April 30, 2007, which is the same pension plan measurement date used for financial statement reporting purposes with respect to our audited financial statements for the fiscal year ended May 31, 2008. For the purposes of the Pension Benefits Table above, to calculate the actuarial present value of Mr. Van Broeck’s accumulated benefit in U.S. dollars, we used a currency conversion rate of 1 Euro to $1.5566. |
| (4) | For Mr. Van Broeck, represents the annual premium contributed to the Biomet Europe Pension Plan after Mr. Van Broeck’s contribution of 7% of his annual base salary. |
Non-Qualified Deferred Compensation
Biomet’s Deferred Compensation Plan is a non-qualified deferred compensation plan, which is available for members of our senior management. The Plan allows eligible participants to defer pre-tax compensation to reduce current tax liability and assist those team members in their plan for retirement and other long-term savings goals in a tax-effective manner. Under the Plan, eligible participants may defer up to 100% of their base salary and bonus payments, as well as Board fees for non-employee Directors, as applicable. We do not make any contributions to the Plan. For further information on the Deferred Compensation Plan, refer to “—The Elements of Biomet’s Compensation Program—Retirement Plans.”
During the 2008 fiscal year, none of Biomet’s named executive officers participated in the Deferred Compensation Plan. We do not pay above-market or preferential earnings on non-qualified deferred compensation.
Employment Agreements and Potential Post-Termination Payments
Of our current named executive officers, we have an employment agreement with Messrs. Binder, Florin, Van Broeck, Kashuba and Schiess and have an offer letter with Mr. Richardson.
On September 20, 2006, we entered into change-in-control agreements with Messrs. Van Broeck and Schiess and other executive officers and their respective employment agreements contain change-in-control and severance provisions that will apply upon expiration of the change-in-control agreements. In addition, our employment agreements with Messrs. Binder, Florin and Kashuba contain change-in-control provisions.
In addition, on September 21, 2006, we adopted the Biomet, Inc. Executive Severance Pay Plan, or the Severance Plan, which provides each of our participating executives with severance benefits in the event of certain terminations of the executive’s employment. The following narrative describes the terms of these various agreements and the Severance Plan.
Employment Agreement with Jeffrey R. Binder
On June 11, 2008, we entered into an amended and restated employment agreement, which we refer to as the employment agreement, with Mr. Binder, our President and Chief Executive Officer. The amended and restated employment agreement supersedes our original employment agreement with Mr. Binder dated as of February 26, 2007, which we refer to as the original employment agreement. Pursuant to the terms of the employment agreement between us and Mr. Binder, the agreement has an initial three-year term that provides for automatic twelve-month extensions, beginning on the first anniversary of the agreement, unless either we or Mr. Binder give prior notice of termination. Mr. Binder will receive a base salary at a rate no less than $650,000 per year, which shall be increased at our discretion. Mr. Binder’s employment agreement provides that he will also have the opportunity to earn an annual cash bonus in an amount no less than 100% of his base salary for on-target performance with the possibility of exceeding 100% for high achievement. For a further discussion of our non-equity incentive plan, see “The Elements of Biomet’s Compensation Program—Non-Equity Incentive Plan.”
Mr. Binder’s employment agreement provides that we will arrange, at our expense, for Mr. Binder to fly once per week to and from his Texas home and our headquarters or such other location reasonably specified by us during the term of the employment agreement. We will not provide Mr. Binder with a “gross up” for taxes incurred in connection with these benefits. If, however, Mr. Binder uses a commercial flight and the income imputed in connection with the commercial flight is greater than the amount that would have been imputed to Mr. Binder if he had used our aircraft, we will provide to Mr. Binder a “gross up” for taxes incurred on the incremental income associated with the commercial flight. Our incremental costs associated with extending these benefits to Mr. Binder are capped at $500,000 in any twelve month period.
Unlike the original employment agreement, our amended and restated employment agreement with Mr. Binder does not provide for annual equity grants, and does not provide for accelerated vesting of the outstanding equity awards that would have vested during the twelve-month period following termination of employment if we terminate Mr. Binder’s employment for any reason other than for cause (as defined in the agreement), death or disability, or Mr. Binder terminates his employment for good reason (as defined in the agreement). The employment agreement provides that, upon any termination of Mr. Binder’s employment, his rights with respect to any equity or equity-related awards will be governed by the applicable terms of the related plan or award agreement. Mr. Binder could be entitled to certain severance benefits following termination of employment prior to a change-in-control (as defined in the agreement) or within two years of a change-in-control. Severance payable to Mr. Binder under such circumstances was previously provided for under the Change in Control Agreement entered into between us and Mr. Binder as of February 26, 2007, which we refer to as the original change in control agreement. The original change in control agreement expired by its terms on July 11, 2007 upon consummation of the “Closing,” as defined in the Merger Agreement.
80
Table of Contents
Under the employment agreement, if Mr. Binder’s employment is terminated at any time within the two-year period following a change in control either by us for any reason other than for cause, death or disability, or by Mr. Binder for good reason, (a) his severance multiple would be increased from 1.5 times base salary and annual incentive bonus to two times base salary and annual incentive bonus and (b) his pro rated bonus for the year of termination of employment would be based on his target annual incentive bonus for such year rather than the actual annual incentive bonus he would have received for such year (as determined based on the Company’s performance to the date of termination of employment, extrapolated through the end of such fiscal year). The employment agreement further provides that if Mr. Binder is subject to the “golden parachute” excise tax under Section 4999 of the Internal Revenue Code as amended, the Company will pay him an additional amount such that he is placed in the same after-tax position as if no excise tax had been imposed. See “—Severance Benefits” below.
Employment Agreements with Roger P. Van Broeck and Steven F. Schiess
On February 1, 2008, we entered into an employment agreement with Mr. Van Broeck, our Vice President and President of Biomet Europe, to become Chairman of the Supervisory Board of Biomet Luxembourg Holding Sarl and Director of Biomet Hong Kong Limited, and on February 28, 2008, we entered into an employment agreement with Steven F. Schiess, our Vice President and President of Biomet 3i, LLC. Both Mr. Van Broeck and Mr. Schiess will be referred to in this section as “Executive.” Both agreements have an initial three-year term that provides for automatic twelve-month extensions, beginning on the first anniversary of the agreement, unless either party gives prior notice of termination. Mr. Van Broeck and Mr. Schiess will receive base salaries at a rate no less than $410,251 and $298,710 per year, respectively, which shall be adjusted at our discretion. Both Mr. Van Broeck and Mr. Schiess will also have the opportunity to earn an annual cash bonus in an amount no less than 80% of his base salary for on-target performance, with the possibility of exceeding 80% for high achievement.
If Messrs. Van Broeck’s or Schiess’ employment is terminated prior to July 11, 2009, his severance benefits will be governed by his change-in-control agreement, dated September 20, 2006. See “—Change-in-Control Agreements,” below.
If we terminate Messrs. Van Broeck or Schiess’ employment after July 11, 2009, the agreement provides that Mr. Van Broeck or Mr. Schiess, as applicable, could be entitled to certain severance benefits following termination of employment prior to a change-in-control (as defined in the agreement) or within two years of a change-in-control. See “—Severance Benefits” below.
Employment Agreements with Daniel P. Florin and Glen A. Kashuba
On February 28, 2008, we entered into employment agreements with Mr. Florin, our Senior Vice President and Chief Financial Officer, and with Mr. Kashuba, our Senior Vice President and President of Biomet Trauma and Biomet Spine. Both Mr. Florin and Mr. Kashuba will be referred to in this section as “Executive.” Both agreements have an initial three-year term that provides for automatic twelve-month extensions, beginning on the first anniversary of the agreement, unless either party gives prior notice of termination. Mr. Florin and Mr. Kashuba will receive a base salary at a rate no less than $395,850 and $397,722 per year, respectively, which shall be increased at our discretion. Executive will also have the opportunity to earn an annual cash bonus in an amount no less than 80% of his base salary for on-target performance, with the possibility of exceeding 80% for high achievement. For a further discussion of our non-equity incentive plan, see “The Elements of Biomet’s Compensation Program—Non-equity Incentive Plan.”
The agreements further provide that Executive could be entitled to certain severance benefits following termination of employment prior to a change-in-control (as defined in the agreements) or within two years of a change-in-control. See “–Severance Benefits” below.
Offer Letter to J. Pat Richardson
On March 30, 2007, we announced the appointment of J. Pat Richardson as Corporate Vice President–Finance and Interim Chief Financial Officer and Treasurer effective April 11, 2007. Pursuant to an offer of employment between us and Mr. Richardson, Mr. Richardson receives, among other benefits, a base salary of $250,000 per year, an opportunity to earn an annual bonus of 60% of base salary for on-target performance, a car allowance, relocation benefits and other customary benefits. In the event that the Merger Agreement was terminated, Mr. Richardson would have been entitled to equity awards issued by the Compensation Committee that are commensurate with his position with us. The options would have been subject to the terms and conditions applicable to options granted under the 2006 Plan, as described in the 2006 Plan and the applicable stock option award. As a result of the Transaction being consummated, Mr. Richardson did not receive this benefit but Mr. Richardson did receive an equity award in Parent following the consummation of the Transaction. For further information, refer to “–Executive Compensation Tables—Grant of Plan-Based Awards Table.” Further, Mr. Richardson’s offer letter provides that if Mr. Richardson is terminated for any reason within the first three years of employment, he is required to repay us his relocation costs. This repayment obligation lapses with respect to 33% of this relocation cost for each year of employment after the date of the agreement.
Severance Benefits Provided Under Employment Agreements
Each of our employment agreements with Messrs. Binder, Florin, Van Broeck, Schiess and Kashuba contains provisions which entitles the executive to certain severance benefits following termination of employment prior to a change of control (as defined in the agreement) or within two years following a change of control.
81
Table of Contents
| • | With respect to Messrs. Van Broeck and Schiess, on September 20, 2006 we entered into change-in-control agreements with Mr. Van Broeck, Mr. Schiess and certain other executive officers. If Messrs. Van Broeck or Schiess’ employment is terminated prior to July 11, 2009, our employment agreements with Messrs. Van Broeck and Schiess provide that the terms of our severance arrangement with Messrs. Van Broeck or Schiess will be governed by our applicable September 20, 2006 change of control agreements with Messrs. Van Broeck or Schiess, as applicable. For a further description of our September 20, 2006 change-in-control agreements with Messrs. Van Broeck and Schiess see “—Change-in-Control Agreements” below. |
| • | With respect to Mr. Richardson, Mr. Richardson’s severance arrangement with us is governed by the Biomet, Inc. Executive Severance Pay Plan discussed in further detail under “—Severance Pay Plan” below. |
The following summary provides a description of the severance arrangements contained in our employment agreements with Messrs. Binder, Florin, Van Broeck, Schiess and Kashuba. Other than with respect to Mr. Binder as described in “—Termination by Biomet Within Two Years Following a Change-in-control Other Than For Cause, Death or Disability or by Executive for Good Reason“ the following summary does not discuss executives’ rights with respect to any equity related awards as such awards are governed by the applicable terms of the related plan or award agreement.
Termination by Biomet Prior to a Change of Control Other Than For Cause, Death or Disability or by Executive for Good Reason
In the event of a termination prior to a change of control (and, with respect to Messrs. Van Broeck and Schiess, after July 11, 2009), for any reason other than for “cause,” which generally includes failure to substantially perform his duties, willful misconduct or gross negligence, willful or grossly negligent breach of his fiduciary duties to Biomet, commission of any felony or other serious crime involving moral turpitude, material breach of any agreement between the executive and Biomet or material breach of our written policies, or due to executive’s death or disability, or if executive terminates his employment prior to a change-in-control for “good reason,” which generally includes any material diminution in duties and responsibilities (but does not include, in the case of Mr. Kashuba, a change in duties and responsibilities that result from becoming a part of a larger organization following a change-in-control), reduction in base salary or bonus opportunity or relocation of primary work location of more than 50 miles, our employment agreements with Messrs. Binder, Florin, Van Broeck, Schiess and Kashuba provide that he would be entitled to the following:
| • | An amount equal to (a) 1.5 times his base salary in effect at the date of termination (with respect to Messrs. Florin, Van Broeck, Schiess and Kashuba, the “Severance Benefit”, with respect to Mr. Binder the “Base Component”) plus, with respect to Mr. Binder, (b) 1.5 times the average of (x) the annual incentive bonus earned by Mr. Binder for the previous fiscal year and (y) the annual incentive bonus Mr. Binder would have received for the current fiscal year had his employment not been terminated, based on Biomet’s performance to the date of termination extrapolated through the end of such fiscal year (the “Bonus Component”, and with respect to Mr. Binder together with the Base Component, the “Severance Benefit”). The total amount of the Severance Benefit will be paid in equal, ratable installments in accordance with our regular payroll policies over the course of the 18 month non-compete period provided for in the agreement. The total amount of the Severance Benefit will be paid in equal, ratable installments in accordance with our regular payroll policies over the course of the 18 month non-compete period provided for in the agreement. If Mr. Binder becomes employed by another employer during that period, the Bonus Component will cease and the Severance Benefit will be limited to the Base Component; |
| • | An amount equal to the pro rated portion (based on the percentage of Biomet’s current fiscal year preceding the date on which the executive’s employment is terminated) of the annual incentive bonus the executive would have received for the current fiscal year, based on Biomet’s performance to the date of termination extrapolated through the end of the current fiscal year. The total amount of the pro rated bonus will be paid in a lump sum at the time we pay annual incentive bonuses to similarly situated active employees; |
| • | If the executive is eligible for and elects continuation coverage pursuant to COBRA, we will pay the premiums for such coverage (or reimburse the executive for such premiums) until the earlier of (a) the end of the 18 month period during which, under the employment agreement, the executive agrees not to engage in certain activities in competition with us or (b) the date the executive becomes eligible for coverage under another group plan; |
| • | Any “accrued benefits” (as defined in the agreement), which generally include any vested compensation deferred by the executive and not yet paid by the Company, any amounts or benefits owing to the executive under the then applicable benefit plans of the Company, and any amounts owing to the executive for reimbursement of expenses properly incurred by the executive; and |
| • | With respect to Mr. Binder, continued payment of Mr. Binder’s company-provided car allowance, if any, for a period of 12 months from the termination date. |
Termination by Biomet Within Two Years After a Change-in-control Other Than For Cause, Death or Disability or by Executive for Good Reason
In the event of a termination within two years after a change of control (and with respect to Messrs. Van Broeck and Schiess after July 11, 2009), for any reason other than for cause or due to the executive’s death or disability, or if the executive terminates his employment within two years after a change-in-control for good reason, he would be entitled to the following:
| • | An amount equal to (a) two times his base salary in effect at the date of termination plus (b) two times the average of (x) the annual incentive bonus earned by executive for the previous fiscal year and (y) the annual incentive bonus executive would have received for the current fiscal year had his employment not been terminated, based on Biomet’s performance to the date of termination extrapolated through the end of such fiscal year (collectively, the “Change-in-control Severance Benefit”). The total amount of the Change-in-control Severance Benefit will be paid as soon as administratively practicable following the termination of executive’s employment; |
82
Table of Contents
| • | An amount equal to the pro rated portion (based on the percentage of Biomet’s current fiscal year preceding the date on which executive’s employment is terminated) of the annual incentive bonus executive would have received for the current fiscal year, based on Biomet’s performance to the date of termination extrapolated through the end of the current year. The total amount of the pro rated bonus will be paid in a lump sum at the time we pay annual incentive bonuses to similarly situated active employees; |
| • | If the executive is eligible for and elects continuation coverage pursuant to COBRA, we will pay the premiums for such coverage (or reimburse Executive for such premiums) until the earlier of (a) the end of the 18 month period during which, under the employment agreement, the executive agrees not to engage in certain activities in competition with us or (b) the date the executive becomes eligible for coverage under another group plan; |
| • | Any “accrued benefits” (as defined in the agreement), which generally include any vested compensation deferred by the executive and not yet paid by the Company, any amounts or benefits owing to the executive under the then applicable benefit plans of the Company, and any amounts owing to the executive for reimbursement of expenses properly incurred by the executive; and |
| • | With respect to Mr. Binder, continued payment of Mr. Binder’s company-provided car allowance, if any, for a period of 12 months from the termination date and immediate vesting of any unvested options held by Mr. Binder as of the date his employment is terminated. |
To receive the severance benefits provided under the agreement, the executive must sign a general release of claims. The agreement contains customary confidentiality, non-competition and non-solicitation provisions. Messrs. Binder, Florin, Van Broeck, Schiess and Kashuba’s non-competition period is 18 months after his termination.
Furthermore, in the event that any payments made to Mr. Binder in connection with a termination of employment would be subject to excise taxes under the Internal Revenue Code, subject to certain conditions, Biomet will “gross up” his compensation to fully offset such excise taxes.
Termination Due to Death or Disability
If Messrs. Binder, Florin, Van Broeck, Schiess or Kashuba’s employment is terminated due to his death or disability (and, with respect to Messrs. Van Broeck and Schiess, after July 11, 2009), he is entitled to receive the following:
| • | His base salary in effect through date of termination; |
| • | A pro-rated portion (based on the percentage of our fiscal year preceding the date of termination) of the average of (x) the annual incentive bonus earned by Executive for the prior year and (y) the annual incentive bonus Executive would have received in the current year if his employment had not been terminated, based on our performance to the date of termination extrapolated through the end of the current year; and |
| • | Any “accrued benefits” (as defined in the agreement). |
Termination With Cause or Without Good Reason
If Messrs. Binder, Florin, Van Broeck, Schiess or Kashuba’s employment is terminated with “cause” or without “good reason” (as defined in the underlying employment agreement) and, with respect to Messrs. Van Broeck and Schiess, after July 11, 2009, we will pay such executive his base salary in effect through the termination date and any “accrued benefits” (as defined in the agreement) when due.
Change-in-Control Agreements
On September 20, 2006, we entered into change-in-control agreements with our then current executive officers, including Messrs. Van Broeck and Schiess. The agreements were intended to provide for continuity of management in the context of a prospective change in control of Biomet, which is generally defined as a change in the majority of the Board, not including any new Board member approved by the majority of the Board, any person becoming the beneficial owner of 20% or more of our outstanding shares, any reorganization, merger, sale of all or substantially all of our assets or similar corporate transaction or approval by the shareholders of our complete liquidation. For additional information, see the change-in-control agreements previously filed with the SEC. Upon a change in control, including as a result of the Transactions, the agreements remain in effect for a period of at least 24 months beyond the month of such change in control. Each agreement provides that during the 24-month period following a change in control, we agree to continue to employ the executive and the executive agrees to remain in our employ.
In addition, prior to consummating the Transactions, we entered into change-in-control agreements with Messrs. Binder and Richardson, each of which terminated by its terms upon consummation of the Transactions. The agreements are intended to provide for continuity of our management in the event of a change in control other than as a result of the consummation of the Transactions, which are exempted from the agreements. The terms of the agreements are substantially the same as the terms of the agreements entered into on September 20, 2006, which are described above.
Under the change-in-control agreements, if, following a change in control, certain executives die or are terminated either by us for any reason other than for “cause,” which is generally defined as willful failure to substantially perform the executive’s duties, willfully engaging in conduct injurious to us or conviction of a felony, or disability, or by the executives for “good reason,” generally defined as any demotion, assignment of duties inconsistent with their title, relocation, any failure to pay or provide benefits to the executive (for more information, please see the agreements on file with the SEC) the executives would be entitled to: (1) a lump sum severance payment equal to two times the sum of the executive’s annual base salary, target bonus (or, in certain circumstances, the executive’s annual bonus earned during a specified time period), our annual contributions to all qualified retirement plans on behalf of the executive and the executive’s total annual car allowance; (2) the executive would receive a payout of his unpaid annual base salary, the higher of the executive’s target bonus for the fiscal year in which termination occurs or the actual bonus paid to the executive for the fiscal year preceding termination and other accrued compensation and benefits through the end of the fiscal year containing the termination date; (3) we would pay the executive a lump sum cash stipend equal to 24 times the monthly premium then charged for family coverage under our medical and dental plans and (4) the executive would receive life insurance and long-term
83
Table of Contents
disability benefits, or the cash equivalent if not available, substantially similar to those that the executive is receiving immediately prior to the notice of termination for a 24-month period after the date of termination. The change-in-control agreements also provide for the reimbursement of outplacement services for a period of 12 months after termination occurs, but not in excess of $25,000.
In the event an “anticipatory termination” (as defined in the agreements) occurs, the executive would receive the same benefits as they would upon a termination without “cause” (as defined in the agreements). The executive is also entitled to receive $25,000 in liquidated damages.
In the event that any payments made to the executives in connection with a change in control and termination of employment would be subject to excise taxes under the Internal Revenue Code, we will “gross up” the executive’s compensation to offset certain of such excise taxes. Severance benefits, other than the life insurance and long-term disability benefits, are generally not subject to mitigation or reduction. To receive the severance benefits provided under the agreements, the executive must sign a general release of claims. In connection with the execution of the agreements, each executive executed a customary confidentiality, non-competition and non-solicitation agreement with us.
Severance Pay Plan
On September 21, 2006, we adopted the Biomet, Inc. Executive Severance Pay Plan for the executives’ party to the change-in-control agreements described above. The Severance Plan provides each of our participating executives with severance benefits in the event of a termination of the executive’s employment unrelated to the executive’s (1) performance of his employment duties or (2) commission of an act or acts outside of the scope of his employment duties that would constitute the basis of a termination for cause under his agreement.
Severance benefits under the Severance Plan generally consist of the following: (1) payment of a pro-rata target bonus (based on the elapsed portion of the year of termination) in a lump sum; (2) continued payment of base salary for 52 weeks plus one week per full year of service with us, up to a maximum of 78 weeks following the termination date; (3) immediate vesting of all of the executive’s outstanding equity awards (stock options and restricted stock); (4) at our expense, continuation of coverage under our health insurance plans pursuant to COBRA for a period not to exceed eighteen months from the termination date; and (5) continuation of any Biomet-provided car allowance for a period of twelve months from the termination date.
As a condition to receiving severance benefits under the Severance Plan, the executive must execute a waiver and release of claims in favor of us and enter into to a customary confidentiality, non-competition and non-solicitation agreement with us. Severance benefits under the Severance Plan are generally intended to be the sole source of severance benefits payable upon a termination of the executive’s employment and are generally not subject to mitigation or reduction. We may amend or terminate the Severance Plan at any time. In the event the executive is entitled to benefits under the change-in-control agreement as a result of a termination of employment, such executive is not entitled to receive benefits under the Severance Plan.
Potential Payments Upon Certain Terminations
This table shows the potential compensation that we would have to pay to certain named executive officers upon a termination by us without “cause” or by the executive with “good reason” (as defined in the applicable agreements) related or unrelated to a change in control, death or disability related or unrelated to a change in control, and termination with “cause” or without “good reason” (as defined in the applicable agreements), related or unrelated to change in control. The table excludes certain amounts payable pursuant to plans that are available generally to all salaried employees. In the event of the death or disability of any of the named executive officers listed in the following table, the deceased or disabled named executive officer, or his designated beneficiaries, would also receive a payment pursuant to the terms of Biomet-funded life or disability plans, respectively, in the addition to the amounts set forth below. The amounts shown assume that termination of employment was effective May 31, 2008. The amounts shown are only estimates of the amounts that would be payable to the executives upon termination of employment and do not reflect tax positions we may take or the accounting treatment of such payments. Actual amounts to be paid can only be determined at the time of separation. Although the calculations are intended to provide reasonable estimates of the potential benefits, they are based on numerous assumptions and do not represent the actual amount an executive would receive if an eligible termination event were to occur.
84
Table of Contents
POTENTIAL PAYMENTS UPON TERMINATION OR CHANGE IN CONTROL
| Termination in Connection with a Change in Control | Termination in Absence of a Change in Control | |||||||||||||||||||||||
Name of Executive Officer | Termination without Cause or with Good Reason(1) | Termination with Cause or without Good Reason(2) | Disability(3) | Death(4) | Termination without Cause or with Good Reason(5) | Termination with Cause or without Good Reason(6) | Disability(7) | Death(8) | ||||||||||||||||
Jeffrey R. Binder(9) | ||||||||||||||||||||||||
Estimated Value of Non-Equity Benefits and Accrued Obligations | $ | 7,984,000 | $ | 8,009,000 | $ | 1,819,504 | $ | 7,959,000 | $ | 2,203,625 | $ | 875,000 | $ | 1,393,750 | $ | 1,393,750 | ||||||||
Estimated Value of Options & Equity Awards | — | — | — | — | — | — | — | — | ||||||||||||||||
Total | 7,984,000 | 7,009,000 | 1,819,504 | 7,959,000 | 3,203,625 | 875,000 | 1,393,750 | 1,393,750 | ||||||||||||||||
Daniel P. Florin(9) | ||||||||||||||||||||||||
Estimated Value of Non-Equity Benefits and Accrued Obligations | 2,291,535 | 2,316,535 | 767,467 | 2,266,535 | 831,285 | 395,850 | 554,190 | 554,190 | ||||||||||||||||
Estimated Value of Options & Equity Awards | — | — | — | — | — | — | — | — | ||||||||||||||||
Total | 2,291,535 | 2,316,535 | 767,467 | 2,266,535 | 831,285 | 395,850 | 554,190 | 554,190 | ||||||||||||||||
J. Pat Richardson(10) | ||||||||||||||||||||||||
Estimated Value of Non-Equity Benefits and Accrued Obligations | 1,156,715 | 1,181,715 | 455,162 | 1,131,715 | 516,179 | 255,199 | 344,120 | 344,120 | ||||||||||||||||
Estimated Value of Options & Equity Awards | — | — | — | — | — | — | — | — | ||||||||||||||||
Total | 1,156,715 | 1,181,715 | 455,162 | 1,131,715 | 516,179 | 255,199 | 344,120 | 344,120 | ||||||||||||||||
Roger Van Broeck(11) | ||||||||||||||||||||||||
Estimated Value of Non-Equity Benefits and Accrued Obligations | 2,380,877 | 2,405,877 | 802,085 | 2,355,877 | 1,074,704 | 410,251 | 716,469 | 716,469 | ||||||||||||||||
Estimated Value of Options & Equity Awards | — | — | — | — | — | — | — | — | ||||||||||||||||
Total | 2,380,877 | 2,405,877 | 802,085 | 2,355,877 | 1,074,704 | 410,251 | 716,469 | 716,469 | ||||||||||||||||
Glen A. Kashuba(9) | ||||||||||||||||||||||||
Estimated Value of Non-Equity Benefits and Accrued Obligations | 2,302,126 | 2,327,126 | 770,944 | 2,277,126 | 835,217 | 397,722 | 556,811 | 556,811 | ||||||||||||||||
Estimated Value of Options & Equity Awards | — | — | — | — | — | — | — | — | ||||||||||||||||
Total | 2,302,126 | 2,327,126 | 770,944 | 2,277,126 | 835,217 | 397,722 | 556,811 | 556,811 | ||||||||||||||||
Steven F. Schiess(11) | ||||||||||||||||||||||||
Estimated Value of Non-Equity Benefits and Accrued Obligations | 1,156,715 | 1,181,715 | 455,162 | 1,131,715 | 516,179 | 255,199 | 344,120 | 344,120 | ||||||||||||||||
Estimated Value of Options & Equity Awards | — | — | — | — | — | — | — | — | ||||||||||||||||
Total | 1,156,715 | 1,181,715 | 455,162 | 1,131,715 | 516,179 | 255,199 | 344,120 | 344,120 | ||||||||||||||||
| (1) | With respect to Messrs. Binder, Florin and Kashuba, |
Non-Equity Benefits and Accrued Obligations represents: (i) an amount equal to (a) two times the executive’s base salary in effect at the date of termination plus (b) two times the average of (x) the annual incentive bonus earned by the executive for the previous fiscal year and (y) the annual incentive bonus the executive would have received for the current fiscal year had his employment not been terminated, based on Biomet’s performance to the date of termination extrapolated through the end of such fiscal year; (ii) an amount equal to the pro-rated portion of the annual incentive bonus the executive would have received for the current fiscal year, based on Biomet’s performance to the date of termination extrapolated through the end of the current year; (iii) if the executive is eligible for and elects continuation coverage pursuant to COBRA, the premiums for such coverage until the earlier of (a) the end of the 18-month period during which executive agrees, under his employment agreement, not to engage in certain activities in competition with us or (b) the date the executive becomes eligible for coverage under another group plan; (iv) any “accrued benefits,” which generally include any vested compensation deferred by the executive and not yet paid by the Company, any amounts or benefits owing to the executive under the then applicable benefit plans of the Company, and any amounts owing to the executive for reimbursement of expenses properly incurred by the executive; and (v) with respect to Mr. Binder, continued payment of Mr. Binder’s company provided car allowance, if any, for a period of 12 months from the termination date.
Options and Equity Awards the difference between the exercise price and the value of LVB’s common stock on May 31, 2008 with respect to any unvested options held by the executive as of May 31, 2008.
For Mr. Richardson,
Non-Equity Benefits and Accrued Obligations represents: (i) a lump-sum payment of a pro-rata target bonus (based on the elapsed portion of the year of termination); (ii) continued payment of base salary for 52 weeks plus one week per full year of service with us, up to a maximum of 78 weeks following the termination date; (iii) at our expense, continuation of coverage under our health insurance plans pursuant to COBRA for a period not to exceed eighteen months from the termination date; and (iv) continuation of any Biomet provided car allowance for a period of twelve months from the termination date.
Options and Equity Awards the difference between the exercise price and the value of LVB’s common stock on May 31, 2008 with respect to any unvested options held by the executive as of May 31, 2008.
For Messrs. Van Broeck and Schiess,
Non-Equity Benefits and Accrued Obligations represents: (i) the sum of (a) the executive’s annual base salary through the end of the fiscal year containing the date of termination, (b) an amount equal to (x) the higher of the target bonus amount or the bonus actually paid to the executive under the Company’s incentive bonus plan for the fiscal year of the Company prior to the date of termination or (y) the target bonus amount payable to the executive under such plan(s) for the fiscal year of the Company which contains the date of termination, whichever of (x) or (y) is higher (the “Target Bonus”), (c) the total contributions made by the Company to all qualified retirement plans on behalf of the executive through the end of the fiscal year containing the date of termination, (d) the total car allowance contributions made by the Company to the executive through the end of the fiscal year containing the date of termination and (e) any accrued vacation or other pay not theretofore paid; (ii) an amount equal to the product of (a) two and (b) the sum of (w) the executive’s annual base salary and (x) the higher of (aa) the Target Bonus and (bb) the highest annual incentive bonus earned by the executive during the last two (2) completed fiscal years of the Company immediately preceding the date of termination, with the product of (1) and (2) reduced by the amounts paid, if any, to the executive pursuant to any other contractual arrangement with the
85
Table of Contents
executive or plan providing coverage to the executive as a result of such termination, (y) the total contributions made by the Company to all qualified retirement plans on behalf of the executive for the calendar year immediately preceding the calendar year in which the Change in Control (as defined in the executive’s respective Change in Control Agreement) occurs and (z) the total car allowance contributions made by the Company to the executive for the calendar year immediately preceding the calendar year in which the Change in Control occurs; (iii) life insurance benefits and long-term disability benefits substantially similar to those that the executive was receiving from the Company immediately prior to the date of termination for 24 months; (iv) if the executive is eligible and so elects, a lump sum cash stipend equal to 24 times the monthly premium then charged to qualified beneficiaries for full family COBRA continuation coverage under the Company’s medical and dental plans; (v) outplacement services the scope and provider of which shall be selected by the executive in his sole discretion; (vi) any other amounts or benefits required to be paid or provided or which the executive is eligible to receive.
Options and Equity Awards represents the difference between the exercise price and the value of LVB’s common stock on May 31, 2008 with respect to any unvested options held by the executive as of May 31, 2008.
| (2) | With respect to Messrs. Binder, Florin, Kashuba and Richardson, |
Non-Equity Benefits and Accrued Obligations represents (i) base salary in effect through the termination date and (ii) any “accrued benefits” (as defined in the agreement), which generally include any vested compensation deferred by the executive and not yet paid by the Company, any amounts or benefits owing to the executive under the then applicable benefit plans of the Company and any amounts owing to the executive for reimbursement of expenses properly incurred by the executive.
For Messrs. Van Broeck and Schiess,
Non-Equity Benefits and Accrued Obligations represents the payments to each executive under an Anticipatory Termination (as defined in their respective Change in Control Agreements), which include the payments described in footnote 1 of this table, with the addition of a $25,000 liquidated damages payment.
| (3) | With respect to Messrs. Binder, Florin and Kashuba, |
Non-Equity Benefits and Accrued Obligations represents: (i) the executive’s base salary in effect through date of termination; (ii) a pro-rated portion (based on the percentage of our fiscal year preceding the date of termination) of the average of (x) the annual incentive bonus earned by the executive for the prior year and (y) the annual incentive bonus the executive would have received in the current year if his employment had not been terminated, based on our performance to the date of termination extrapolated through the end of the current year; and (iii) any “accrued benefits,” which generally include any vested compensation deferred by the executive and not yet paid by the Company, any amounts or benefits owing to the executive under the then applicable benefit plans of the Company, and any amounts owing to the executive for reimbursement of expenses properly incurred by the executive.
For Mr. Richardson,
Non-Equity Benefits and Accrued Obligations represents the payments as described in footnote 1 of this table.
For Messrs. Van Broeck and Schiess,
Non-Equity Benefits and Accrued Obligations represents: (i) disability and other benefits at least equal to the most favorable of those generally provided by the Company to disabled executives and/or their families; (ii) base salary through the end of the fiscal year containing the date of termination; (iii) an amount equal to (x) the higher of the target bonus amount or the bonus actually paid to the executive under the Company’s incentive bonus plan for the fiscal year of the Company prior to the date of termination or (y) the target bonus amount payable to the executive under such plan(s) for the fiscal year of the Company which contains the date of termination, whichever is higher; (iv) the total contributions made by the Company to all qualified retirement plans on behalf of the executive through the end of the fiscal year containing the date of termination; (v) the total car allowance contributions made by the Company to the executive through the end of the fiscal year containing the date of termination; and (vi) any vacation or other pay accrued but not yet paid.
| (4) | With respect to Messrs. Binder, Florin and Kashuba, |
Non-Equity Benefits and Accrued Obligations represents the payments as described in footnote 3 of this table.
For Mr. Richardson,
Non-Equity Benefits and Accrued Obligations represents the payments as described in footnote 1 of this table.
For Messrs. Van Broeck and Schiess,
Non-Equity Benefits and Accrued Obligations represents: (i) a lump-sum payment of a pro-rata target bonus (based on the elapsed portion of the year of termination); (ii) continued payment of base salary for 52 weeks plus one week per full year of service with us, up to a maximum of 78 weeks following the termination date; (iii) at our expense, continuation of coverage under our health insurance plans pursuant to COBRA for a period not to exceed eighteen months from the termination date; and (iv) continuation of any Biomet provided car allowance for a period of twelve months from the termination date.
Options and Equity Awards represents the difference between the exercise price and the value of LVB’s common stock on May 31, 2008 with respect to any unvested options held by the executive as of May 31, 2008.
86
Table of Contents
| (5) | With respect to Messrs. Binder, Florin and Kashuba, |
Non-Equity Benefits and Accrued Obligations represents: (i) an amount equal to (a) two times his base salary in effect at the date of termination plus (b) two times the average of (x) the annual incentive bonus earned by executive for the previous fiscal year and (y) the annual incentive bonus executive would have received for the current fiscal year had his employment not been terminated, based on Biomet’s performance to the date of termination extrapolated through the end of such fiscal year; (ii) an amount equal to the pro-rated portion (based on the percentage of Biomet’s current fiscal year preceding the date on which executive’s employment is terminated) of the annual incentive bonus executive would have received for the current fiscal year, based on Biomet’s performance to the date of termination extrapolated through the end of the current year; (iii) if the executive is eligible for and elects continuation coverage pursuant to COBRA, the premiums for such coverage (or reimburse the executive for such premiums) until the earlier of (a) the end of the 18-month period during which, under the employment agreement, the executive agrees not to engage in certain activities in competition with us or (b) the date the executive becomes eligible for coverage under another group plan; (iv) any “accrued benefits,” which generally include any vested compensation deferred by the executive and not yet paid by the Company, any amounts or benefits owing to the executive under the then applicable benefit plans of the Company, and any amounts owing to the executive for reimbursement of expenses properly incurred by the executive; and (v) with respect to Mr. Binder, continued payment of Mr. Binder’s company provided car allowance, if any, for a period of 12 months from the termination date and immediate vesting of any unvested options held by Mr. Binder as of the date his employment is terminated.
Options and Equity Awards represents the difference between the exercise price and the value of LVB’s common stock on May 31, 2008 with respect to any unvested options held by the executive as of May 31, 2008.
For Mr. Richardson,
Non-Equity Benefits and Accrued Obligations represents the payments discussed in footnote 1 of this table.
For Messrs. Van Broeck and Schiess,
Non-Equity Benefits and Accrued Obligations represents the payments discussed in footnote 1 of this table.
| (6) | With respect to Messrs. Binder, Florin, Kashuba, Richardson, Van Broeck and Schiess, |
Non-Equity Benefits and Accrued Obligations represents (i) base salary in effect through the termination date and (ii) any “accrued benefits,” which generally include any vested compensation deferred by the executive and not yet paid by the Company, any amounts or benefits owing to the executive under the then applicable benefit plans of the Company and any amounts owing to the executive for reimbursement of expenses properly incurred by the executive.
| (7) | For Messrs. Binder, Florin and Kashuba, |
Non-Equity Benefits and Accrued Obligations represents: (i) the executive’s base salary in effect through date of termination; (ii) a pro-rated portion (based on the percentage of our fiscal year preceding the date of termination) of the average of (x) the annual incentive bonus earned by the executive for the prior year and (y) the annual incentive bonus the executive would have received in the current year if his employment had not been terminated, based on our performance to the date of termination extrapolated through the end of the current year; and (iii) any “accrued benefits,” which generally include any vested compensation deferred by the executive and not yet paid by the Company, any amounts or benefits owing to the executive under the then applicable benefit plans of the Company and any amounts owing to the executive for reimbursement of expenses properly incurred by the executive.
Options and Equity Awards represents the difference between the exercise price and the value of LVB’s common stock on May 31, 2008 with respect to any unvested options held by the executive as of May 31, 2008.
For Mr. Richardson,
Non-Equity Benefits and Accrued Obligations represents the payments as described in footnote 1 of this table.
For Messrs. Van Broeck and Schiess,
Non-Equity Benefits and Accrued Obligations represents: (i) disability and other benefits at least equal to the most favorable of those generally provided by the Company to disabled executives and/or their families; (ii) base salary through the end of the fiscal year containing the date of termination; (iii) an amount equal to (x) the higher of the target bonus amount or the bonus actually paid to the executive under the Company’s incentive bonus plan for the fiscal year of the Company prior to the date of termination or (y) the target bonus amount payable to the executive under such plan(s) for the fiscal year of the Company which contains the date of termination, whichever is higher; (iv) the total contributions made by the Company to all qualified retirement plans on behalf of the executive through the end of the fiscal year containing the date of termination; (v) the total car allowance contributions made by the Company to the executive through the end of the fiscal year containing the date of termination; and (vi) any vacation or other accrued but not yet paid pay.
| (8) | With respect to Messrs. Binder, Florin and Kashuba, |
Non-Equity Benefits and Accrued Obligations represents the payments described in footnote 4 of this table.
Options and Equity Awards represents the difference between the exercise price and the value of LVB’s common stock on May 31, 2008 with respect to any unvested options held by the executive as of May 31, 2008.
87
Table of Contents
For Mr. Richardson,
Non-Equity Benefits and Accrued Obligations represents the payments described in footnote 1 of this table.
For Messrs. Van Broeck and Schiess,
Non-Equity Benefits and Accrued Obligations represents the payments described in footnote 1 of this table.
| (9) | The payments described in this table represent payments provided under the executive’s employment agreement and the 2007 LVB Plan. For more information on these employment agreements, refer to “— Employment Agreements and Potential Post-Termination Payments” and “The Elements of Biomet’s Compensation Program—Stock Options and Leveraged Share Awards” above. |
| (10) | The payments described in this table represent payments provided under the executive’s offer letter, the Company’s Severance Pay Plan and the 2007 LVB Plan. For more information on the Company’s Severance Pay Plan, refer to “—Severance Pay Plan” and “—Employment Agreements and Potential Post Termination Payments” and “The Elements of Biomet’s Compensation Program—Stock Options and Leveraged Share Awards”. |
| (11) | The payments described in this table represent payments provided under the executive’s change in control agreement. For more information, refer to “—Change in Control Agreements” and “—Employment Agreements and Potential Post Termination Payments” and “The Elements of Biomet’s Compensation Program—Stock Options and Leveraged Share Awards”. |
Non-Employee Director Compensation and Benefits
In accordance with the provisions of the Merger Agreement, on July 17, 2007 each of Messrs. Jerry L. Ferguson, M. Ray Harroff, Thomas F. Kearns, Jr., Jerry L. Miller, Charles E. Niemier and Niles L. Noblitt and Mses. Sandra A. Lamb and Marilyn Tucker Quayle and September 25, 2007 each of Messrs. C. Scott Harrison, M.D., Kenneth V. Miller and L. Gene Tanner (collectively the “Resigning Directors”), resigned from the Board of Directors and from any committees thereof.
Our compensation package for non-employee directors during the 2008 fiscal year prior to consummation of the Transactions was generally comprised of cash (annual retainers and committee meeting fees) and stock option awards. The annual pay package was designed to attract and retain highly-qualified, independent professionals to represent our shareholders and reflect our position in the industry. Our compensation package is also designed to create alignment between our directors and our shareholders through the use of equity-based awards.
In connection with the Transactions, new members of the Board were appointed by our sole stockholder, Parent, on behalf of the Sponsors, and generally have not received cash retainer or committee fees or stock option awards.
Business Expenses
The directors are reimbursed for their business expenses related to their attendance at our meetings, including room, meals and transportation to and from Board and committee meetings. On rare occasions, a director’s spouse may accompany a director when traveling on Biomet business. At times, a director may travel to and from our meetings on our corporate aircraft. Directors are also eligible to be reimbursed for attendance at qualified director education programs.
Director and Officer Liability Insurance, or D&O, and Travel Accident Insurance
D&O insurance individually insures our directors and officers against certain losses that they are legally required to bear as a result of their actions while performing duties on our behalf. Our D&O insurance policy does not break out the premium for directors versus officers and, therefore, a dollar amount cannot be assigned to the coverage provided for individual directors.
We also maintain an Aviation Insurance Policy that provides benefits to each director in the event of death or disability (permanent and total) during travel on our corporate aircraft. This policy also covers employees and others while traveling on our corporate aircraft and, therefore, a dollar amount cannot be assigned to the coverage provided for individual directors.
Non-Employee Directors’ Compensation Table
The following table shows information regarding the compensation of our non-employee directors for the 2008 fiscal year. Mr. Binder is not included in the table below because, as President and Chief Executive Officer, disclosure in respect of his compensation is presented in the Summary Compensation Table. Furthermore, as an employee director, Mr. Binder did not receive compensation in his capacity as a director.
88
Table of Contents
DIRECTOR COMPENSATION
Name | Fees Earned or Paid in Cash ($)(1) | Stock Awards ($)(2) | Option Awards $(2) | Non-Equity Incentive Plan Compensation ($)(3) | Change in Pension Value and Nonqualified Deferred Compensation Earnings ($)(4) | All Other Compensation ($) | Total ($) | |||||||
Pre-Transaction Directors | ||||||||||||||
Jerry L. Ferguson | 10,800 | — | — | — | — | 14,400 | 25,200 | |||||||
C. Scott Harrison, M.D. | 19,287 | — | — | — | — | — | 19,287 | |||||||
M. Ray Harroff | 7,200 | — | — | — | — | — | 7,200 | |||||||
Thomas F. Kearns, Jr. | 9,600 | — | — | — | — | — | 9,600 | |||||||
Sandra A. Lamb | 14,400 | — | — | — | — | — | 14,400 | |||||||
Jerry L. Miller | 14,400 | — | — | — | — | — | 14,400 | |||||||
Kenneth V. Miller | 22,800 | — | — | — | — | — | 22,800 | |||||||
Niles L. Noblitt | 7,200 | — | — | — | — | — | 7,200 | |||||||
L. Gene Tanner | 18,000 | — | — | — | — | — | 18,000 | |||||||
Marilyn Tucker Quayle | 9,600 | — | — | — | — | — | 9,600 | |||||||
Post-Transaction Directors | ||||||||||||||
Chinh E. Chu(5) | — | — | — | — | — | — | — | |||||||
Jonathan J. Coslet | — | — | — | — | — | — | — | |||||||
Michael Dal Bello | — | — | — | — | — | — | — | |||||||
Sean Fernandes(6) | — | — | — | — | — | — | — | |||||||
Adrian Jones | — | — | — | — | — | — | — | |||||||
Michael Michelson | — | — | — | — | — | — | — | |||||||
Dane Miller, Ph.D.(7) | — | — | — | — | — | 2,100,000 | 2,100,000 | |||||||
John Saer | — | — | — | — | — | — | — | |||||||
Todd Sisitsky | — | — | — | — | — | — | — | |||||||
L. Gene Tanner | — | — | — | — | — | — | — | |||||||
David McVeigh | — | — | — | — | — | — | — | |||||||
Gregory L. Summe | — | — | — | — | — | — | — | |||||||
| (1) | The aggregate dollar amount of all fees earned or paid in cash for services as a director, including annual Board and committee chair retainer fees, and committee meeting fees, in each case including amounts deferred pursuant to director elections. |
| (2) | For each director listed in the Non-Employee Directors’ Compensation Table above, the value reflects the compensation expense we recognized during the 2008 fiscal year under SFAS 123(R). For information concerning the assumptions used in determining the compensation expense we recognized during the 2008 fiscal year, refer Note 9 to the consolidated financial statements included in this prospectus. During the 2008 fiscal year, our non-employee directors agreed to waive their annual grants of option awards. |
| (3) | We do not have a non-equity incentive plan for non-employee directors. |
| (4) | We do not have a pension plan for non-employee directors and do not pay above market or preferential rate on non-qualified deferred compensations for non-employee directors. |
| (5) | Mr. Chu resigned from the Board on January 16, 2008. |
| (6) | Mr. Fernandes resigned from the Board on March 31, 2008. |
| (7) | On May 8, 2006, Biomet, Inc. entered into a Separation, Release and Consultancy Agreement with Dane A. Miller, Ph.D. (the “Miller Agreement”). As previously disclosed in a Current Report on Form 8-K dated May 10, 2006, pursuant to the terms of the Miller Agreement, Mr. Miller received $4,000,000 on October 1, 2006, $500,000 on November 30, 2006 and has received or will receive $500,000 on the last day of each quarter thereafter through the first quarter of fiscal year 2010 as compensation for his consulting services. Also pursuant to the Miller Agreement, Mr. Miller is reimbursed for any out-of-pocket fees and expenses relating to an off-site office and administrative support, in an amount not to exceed $100,000 per year. The Miller Agreement contains certain restrictive covenants prohibiting Mr. Miller from competing with the Company and soliciting employees of the Company during the term of the Miller Agreement. |
89
Table of Contents
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
Parent owns all of our issued and outstanding capital stock. Holding owns 99.23% of Parent and the remaining 0.77% are owned by the Management Participants. All equity interests in Holding are owned, directly or indirectly, by the Sponsor Funds and the Co-Investors.
The following table sets forth information with respect to the ownership of as of November 30, 2008 for (a) each person known by us to own beneficially more than a 5% equity interest in Holdings, (b) each member of our board of directors, (c) each of our named executive officers, and (d) all of our executive officers and directors as a group. Biomet, Inc. has 1,000 shares of common stock outstanding, all of which are owned directly by Parent. Share amounts indicated below reflect beneficial ownership, through Holding, by such entities or individuals of these 1,000 shares of Biomet, Inc.
The amounts and percentages of shares beneficially owned are reported on the basis of SEC regulations governing the determination of beneficial ownership of securities. Under SEC rules, a person is deemed to be a “beneficial owner” of a security if that person has or shares voting power or investment power, which includes the power to dispose of or to direct the disposition of such security. A person is also deemed to be a beneficial owner of any securities of which that person has a right to acquire beneficial ownership within 60 days. Securities that can be so acquired are deemed to be outstanding for purposes of computing such person’s ownership percentage, but not for purposes of computing any other person’s percentage. Under these rules, more than one person may be deemed to be a beneficial owner of the same securities and a person may be deemed to be a beneficial owner of securities as to which such person has no economic interest.
Except as otherwise indicated in the footnotes below, each of the beneficial owners has, to our knowledge, sole voting and investment power with respect to the indicated shares. Unless otherwise noted, the address of each beneficial owner is c/o Biomet, Inc., 56 East Bell Drive, Warsaw, Indiana 46582.
Name and Address of Beneficial Owner | Beneficial Ownership of Biomet Common Shares | Percentage Owned | |||
The Blackstone Group(1) | 242.2 | 24.22 | % | ||
Goldman Sachs Capital Partners(2) | 242.2 | 24.22 | % | ||
KKR Biomet, LLC(3) | 248.1 | 24.81 | % | ||
TPG Capital(4) | 242.2 | 24.22 | % | ||
Jeffrey R. Binder | * | * | |||
Daniel P. Florin | * | * | |||
C. Scott Harrison | — | — | |||
Kenneth V. Miller | — | — | |||
L. Gene Tanner | — | — | |||
Jonathan J. Coslet(5) | 242.2 | 24.22 | % | ||
Michael Dal Bello(6) | 242.2 | 24.22 | % | ||
Adrian Jones(7) | 242.2 | 24.22 | % | ||
David McVeigh(6) | 242.2 | 24.22 | % | ||
Michael Michelson(8) | 248.1 | 24.81 | % | ||
Dane A. Miller(9) | — | — | |||
John Saer(8) | 248.1 | 24.81 | % | ||
Todd Sisitsky(5) | 242.2 | 24.22 | % | ||
Gregory L. Summer(7) | 242.2 | 24.22 | % | ||
All executive officers and directors as a group (14 persons) | 993.0 | 99.30 | % |
| * | Represents less than one percent or one share, as applicable. |
| (1) | Biomet, Inc. shares shown as beneficially owned by The Blackstone Group reflect an aggregate of the following record ownership: (i) 610,133.52800 membership units of Holding held by Blackstone Capital Partners V, L.P., (ii) 97,736.20500 membership units of Holding held by Blackstone Capital Partners V-AC L.P., (iii) 289,050.00000 membership units of Holding held by BCP V-S L.P., (iv) 32,313.00200 membership units of Holding held by Blackstone Family Investment Partnership V L.P., (v) 3,112.96000 membership units of Holding held by Blackstone Family Investment Partnership V-A L.P., (vi) 2,297.59715 membership units of Holding held by Blackstone Participation Partnership V L.P., and (vii) 273,775.86600 membership units of Holding held by BCP V Co-Investors L.P. The address of The Blackstone Group is 345 Park Avenue, New York, NY 10154. |
| (2) | Biomet, Inc. shares shown as beneficially owned by Goldman Sachs Capital Partners reflect an aggregate of the following record ownership: (i) 433,679.15808 membership units of Holding held by GS Capital Partners VI Fund, L.P., (ii) 15,413.18755 membership units of Holding held by GS Capital Partners VI GmbH & Co. KG, (iii) 360,718.75833 membership units of Holding held by GS Capital Partners VI Offshore Fund, L.P., (iv) 119,253.84819 membership units of Holding held by GS Capital Partners VI Parallel, L.P., (v) 61,875.99000 membership units of Holding held by GS LVB Co-Invest, L.P., (vi) 63,137.95000 membership units of Holding held by Goldman Sachs BMET Investors, L.P., (vii) 184,785.45000 membership units of Holding held by Goldman Sachs BMET Investors Offshore Holdings, L.P., (viii) 44,463.81600 membership units of Holding held by GS PEP Bass Holdings, L.L.C., (ix) 6,309.80000 membership units of Holding held by Goldman Sachs Private Equity Partners, 2004-Direct Investment Fund, L.P., (x) 9,013.20000 membership units of Holding held by Goldman Sachs Private Equity Partners, 2005-Direct Investment Fund, L.P., and (xi) 9,768.00000 membership units of Holding held by Goldman Sachs Private Equity Partners IX-Direct Investment Fund, L.P. The address of Goldman Sachs Capital Partners is c/o Goldman, Sachs & Co., 85 Broad Street, New York, NY 10004. |
90
Table of Contents
| (3) | The address of KKR Biomet, LLC is c/o Kohlberg Kravis Roberts & Co. L.P., 2800 Sand Hill Road, Suite 200, Menlo Park, CA 94025. |
| (4) | Biomet, Inc. shares shown as beneficially owned by TPG Capital reflect an aggregate of the following record ownership: (i) 50,000.00000 membership units owned by TPG Partners IV, L.P., (ii) 1,015,020.30532 membership units owned by TPG Partners V, L.P., (iii) 2,655.60483 membership units owned by TPG FOF V-A, L.P., (iv) 2,141.61680 membership units owned by TPG FOF V-B, L.P., (v) 235,843.63020 membership units owned by TPG LVB Co-Invest LLC, (vi) 2,758.00100 membership units owned by TPG LVB Co-Invest II LLC. The address of TPG Capital is 301 Commerce Street, Suite 3300, Fort Worth, TX 76102. |
| (5) | Includes all shares held by TPG Partners IV, L.P., TPG Partners V, L.P., TPG FOF V-A, L.P., TPG FOF V-B, L.P., TPG LVB Co-Invest LLC, and TPG LVB Co-Invest II LLC. Each of Jonathan J. Coslet and Todd Sisitsky may be deemed to be a beneficial owner of these interests due to his status as an employee of TPG Capital, and each such person disclaims beneficial ownership of any such interests in which he does not have a pecuniary interest. The address of each of Mr. Coslet and Mr. Sisitsky is c/o TPG Capital is 301 Commerce Street, Suite 3300, Fort Worth, TX 76102. |
| (6) | Includes all shares held by Blackstone Capital Partners V, L.P., Blackstone Capital Partners V-AC L.P., BCP V-S L.P., Blackstone Family Investment Partnership V L.P., Blackstone Family Investment Partnership V-A L.P., Blackstone Participation Partnership V L.P., and BCP V Co-Investors L.P. Each of Michael Dal Bello and David McVeigh may be deemed to be a beneficial owner of these interests due to his status as an employee of The Blackstone Group, and each such person disclaims beneficial ownership of any such interests in which he does not have a pecuniary interest. The address of each of Mr. Dal Bello and Mr. Mc Veigh is c/o The Blackstone Group is 345 Park Avenue, New York, NY 10154. |
| (7) | Includes all shares held by GS Capital Partners VI Fund, L.P., GS Capital Partners VI GmbH & Co. KG, GS Capital Partners VI Offshore Fund, L.P., GS Capital Partners VI Parallel, L.P., GS LVB Co-Invest, L.P., Goldman Sachs BMET Investors, L.P., Goldman Sachs BMET Investors Offshore Holdings, L.P., GS PEP Bass Holdings, L.L.C., Goldman Sachs Private Equity Partners, 2004-Direct Investment Fund, L.P., Goldman Sachs Private Equity Partners, 2005-Direct Investment Fund, L.P., and Goldman Sachs Private Equity Partners IX-Direct Investment Fund, L.P. Each of Gregory L. Summe and Adrian Jones may be deemed to be a beneficial owner of these interests due to his status as a consultant to or an employee of Goldman, Sachs & Co., and each such person disclaims beneficial ownership of any such interests in which he does not have a pecuniary interest. The address of Mr. Jones is c/o Goldman, Sachs & Co., 85 Broad Street, New York, NY 10004 and the address of Mr. Summe is c/o PerkinElmer, Inc., 940 Winter Street, Waltham, MA 02451. |
| (8) | Includes all shares held by KKR Biomet, LLC. Each of Michael Michelson and John Saer may be deemed to be a beneficial owner of these interests due to his status as an employee of Kohlberg Kravis Roberts & Co. L.P., and each such person disclaims beneficial ownership of any such interests in which he does not have a pecuniary interest. The address of each of Mr. Michelson and Mr. Saer is c/o Kohlberg Kravis Roberts & Co. L.P., 2800 Sand Hill Road, Suite 200, Menlo Park, CA 94025. |
| (9) | The business address of Dane Miller is 700 Park Avenue, Suite G, Winona Lake, IN 46590. |
91
Table of Contents
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
Amended and Restated Limited Liability Company Operating Agreement of Holding
The Sponsor Funds have entered into an amended and restated limited liability company operating agreement, or the LLC Agreement, in respect of Holding. The LLC Agreement contains agreements among the parties with respect to the election of our directors and the directors of our parent companies, restrictions on the issuance or transfer of interests in us and other corporate governance provisions (including the right to approve various corporate actions).
Pursuant to the LLC Agreement, each of the Sponsors has the right to nominate, and have nominated, two directors to our Board of Directors and also are entitled to appoint one non-voting observer to the Board of Directors for so long as such Sponsor remains a member of Holding. In addition to their right to appoint non-voting observers to the Board of Directors, certain of the Sponsor Funds have certain other management rights to the extent that any such Sponsor Fund is required to operate as a “venture capital operating company” as defined in the regulations issued by the U.S. Department of Labor at Section 2510.3-101 of Part 2510 of Chapter XXV, Title 29 of the Code of Federal Regulations, or any successor regulations. Each Sponsor’s right to nominate directors is freely assignable to funds affiliated with such Sponsor, and is assignable to non-affiliates of such Sponsor only if the assigning Sponsor transfers its entire interest in Holding not previously transferred and only with the prior written consent of the Sponsors holding at least 70% of the membership interests in Holding, or Requisite Sponsor Consent. In addition to their rights under the LLC Agreement, the Sponsors may also appoint one or more persons unaffiliated with any of the Sponsors to the Board of Directors. Following Purchaser’s purchase of the Shares tendered in the Offer, the Sponsors jointly appointed Dr. Miller and Jeffrey R. Binder to the Board of Directors in addition to the two directors appointed by each of the Sponsors.
Pursuant to the LLC Agreement, each director has one vote for purposes of any Board of Directors action, and all decisions of the Board of Directors require the approval of a majority of the directors designated by the Sponsors. In addition, the LLC Agreement provides that certain major decisions regarding us or our parent companies require Requisite Sponsor Consent.
The LLC Agreement includes certain customary agreements with respect to restrictions on the issuance or transfer of interests in us, including preemptive rights, tag-along rights and drag-along rights.
The Co-Investors have also been admitted as members of Holding, both directly and through Sponsor controlled investment vehicles. Although the Co-Investors are therefore parties to the LLC Agreement, they have no rights with respect to the election of our directors or the approval of our corporate actions.
The Sponsors have also caused Holding and Parent to enter into a letter agreement with us obligating us and Parent to take all actions necessary to give effect to the corporate governance, preemptive rights, transfer restriction and certain other provisions of the LLC Agreement, and prohibiting us and Parent from taking any actions that would be inconsistent with such provisions of the LLC Agreement.
Registration Rights Agreement
The Sponsor Funds and the Co-Investors also entered into a registration rights agreement with us upon the closing of the Transactions. Pursuant to this agreement, the Sponsor Funds have the power to cause us to register their, the Co-Investors’ and certain other persons’ interests in Biomet under the Securities Act and to maintain a shelf registration statement effective with respect to such interests. The agreement also entitles the Sponsor Funds and the Co-Investors to participate in any future registration of our equity interests under the Securities Act that we may undertake.
Management Services Agreement
Upon completion of the Transactions, we entered into a management services agreement with certain affiliates of the Sponsors, pursuant to which such affiliates of the Sponsors or their successors, assigns, affiliates, officers, employees and/or representatives and third parties (collectively, the “Managers”) provide management, advisory and consulting services to us. Pursuant to such agreement, the Managers will receive a transaction fee equal to 1% of total enterprise value of the Transactions for the services rendered by such entities related to the Transactions upon entering into the agreement, an annual monitoring fee equal to 1% of our annual Adjusted EBITDA as compensation for the services rendered and reimbursement for out-of-pocket expenses incurred by the Managers in connection with the agreement and the Transactions. We are required to pay the sponsors fee on a quarterly basis. In total we paid each of the above sponsors $1.5 million for a total of $6.1 million during fiscal 2008. As of May 31, 2008, the amount payable to the sponsors was $2.3 million. During the 2008 fiscal year we also entered into a consulting agreement with Capstone Consulting, a wholly owned subsidiary of Blackstone, LLC, to perform analysis related to our operational improvement initiative. We paid Capstone Consulting $1.2 million throughout fiscal 2008. We may also pay certain subsequent fees to the Managers for advice rendered in connection with financing or refinancing (equity or debt), acquisition, disposition, spin-off, split-off, dividend, recapitalization, initial underwritten public offering and change of control transactions involving us or any of our subsidiaries. The management services agreement includes customary exculpation and indemnification provisions in favor of the Managers and their affiliates. The Company currently holds interest rate swaps with Goldman Sachs. As part of this relationship, we receive information from Goldman Sachs that allows us to run a regression on the swaps as part of our required effectiveness testing on a quarterly basis.
Related-Party Transactions Review
Our amended and restated articles of incorporation provide that all conflict of interest transactions with our directors, which are transactions with us in which a director has a direct or indirect interest, must be fair to us and must be reviewed and approved by a majority vote of the disinterested members of the Board of Directors or a committee thereof.
92
Table of Contents
DESCRIPTION OF OTHER INDEBTEDNESS
Senior Secured Cash Flow Facilities
Overview
In connection with the Transactions, we entered into a credit agreement and related security and other agreements providing for (1) a $2,340 million U.S. dollar-denominated senior secured term loan facility and a €875 million (approximately $1,329 million) euro-denominated senior secured term loan facility and (2) a $400 million senior secured cash flow revolving credit facility with Bank of America, N.A. as administrative agent and collateral agent. We refer to our senior secured term loan facilities and our senior secured cash flow revolving credit facility collectively as the senior secured cash flow facilities.
We borrowed the full amount available under our senior secured term loan facilities at the closing of the Transactions. To date we have repaid $24 million of outstanding loans under our U.S. dollar-denominated senior secured term loan facility and $12 million of outstanding loans under our euro-denominated senior secured term loan facility. The senior secured cash flow revolving credit facility includes a $100 million sub-facility for letters of credit and a $100 million sub-facility for borrowings on same-day notice, referred to as the swingline loans, and is available in U.S. dollars, euros, British pounds sterling and other currencies to be agreed. We borrowed approximately $131 million under our senior secured cash flow revolving credit facility on or about the closing date of the Transactions to pay a portion of the Transactions. As of November 30, 2008, we had no outstanding borrowings under our senior secured cash flow revolving credit facility.
Our senior secured cash flow facilities provide that we have the right at any time to request an amount of additional term loans or additional revolving credit facility commitments under our senior secured cash flow facilities that would cause our Senior Secured Leverage Ratio (as defined in our senior secured cash flow facilities) to be equal to or less than 4.50 to 1.00. The lenders under these facilities are not under any obligation to provide any such additional loans or commitments, and any additional loans or increase in commitments are subject to customary conditions precedent.
Interest Rate and Fees
Borrowings under our senior secured cash flow facilities bear interest at a rate per annum equal to an applicable margin plus, at our option, either (1) a base rate determined by reference to the higher of (a) the prime rate of Bank of America, N.A. and (b) the federal funds effective rate plus 1/2 of 1.00% or (2) a LIBOR or Eurocurrency rate determined by reference to the costs of funds for deposits in the currency of such borrowing for the interest period relevant to such borrowing adjusted for certain additional costs. The initial applicable margin for borrowings under (x) our senior secured term loan facilities is 2.00% with respect to base rate borrowings and 3.00% with respect to LIBOR or Eurocurrency borrowings and (y) our senior secured cash flow revolving credit facility is 1.75% with respect to base rate borrowings and 2.75% with respect to LIBOR or Eurocurrency borrowings. The applicable margin under our senior secured cash flow revolving credit facility may be reduced based on our achievement of certain specified ratios. In connection with our senior secured cash flow facilities, Purchaser entered into a series of interest swap agreements with (1) an aggregate notional amount of $1,300 million to fix the interest rates on a portion of the borrowings under the $2,340 million U.S. dollar-denominated senior secured term loan facility and (2) an aggregate notional amount of €505 million to fix the interest rates on a portion of the borrowings under the €875 million (approximately $1,329 million) euro-denominated senior secured term loan facility. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Quantitative and Qualitative Disclosures about Market Risk—Interest Rate Risk.”
In addition to paying interest on outstanding principal under our senior secured cash flow facilities, we are required to pay a commitment fee to the lenders under the senior secured cash flow revolving credit facility in respect of the unutilized commitments thereunder at an initial rate equal to 0.50% per annum, subject to reduction based on our achievement of certain specified ratios. We must also pay customary letter of credit and agency fees.
Mandatory Repayments
The credit agreement governing our senior secured cash flow facilities requires us to prepay outstanding term loans, subject to certain exceptions, with: (1) after our first full fiscal year after closing, 50% (which percentage will be reduced to 25% if our Senior Secured Leverage Ratio is less than a specified ratio and will be reduced to 0% if our Senior Secured Leverage Ratio is less than a specified ratio) of our annual excess cash flow (as defined in our senior secured cash flow facilities), (2) if our Senior Secured Leverage Ratio is greater than a specified ratio, 100% (which percentage will be reduced to 50% if our Senior Secured Leverage Ratio is less than a specified ratio and will be reduced to 0% if our Senior Secured Leverage Ratio is less than a specified ratio) of the net cash proceeds of certain non-ordinary course asset sales and casualty and condemnation events, if we do not reinvest those proceeds in assets to be used in our business or to make certain other permitted investments and (3) 100% of the net cash proceeds of any incurrence of debt other than debt permitted under our senior secured cash flow facilities.
Voluntary Repayments
We may voluntarily prepay outstanding loans under our senior secured cash flow facilities at any time without premium or penalty, other than customary “breakage” costs with respect to LIBOR or Eurocurrency loans. However, should we refinance our senior secured term loan facilities within the first anniversary of the closing of the Transactions with new term loans that have applicable margins that are less than the margins for our senior secured term loan facilities, we will pay a prepayment premium equal to 1% of the principal amount of the term loans that are so prepaid.
93
Table of Contents
Amortization and Final Maturity
Our senior secured term loan facilities amortize each year in an amount equal to 1% per annum in equal quarterly installments for the first seven years and three months, with the remaining amounts payable on the date that is seven years and six months from the date of the closing of the Transactions. The principal amount outstanding of the loans under our senior secured cash flow revolving credit facility are due and payable in full at maturity, six years from the date of closing of the Transactions.
Guarantees and Security
All obligations under our senior secured cash flow facilities are unconditionally guaranteed by Parent, and, subject to certain exceptions, each of our existing and future direct and indirect wholly-owned domestic subsidiaries.
All obligations under our senior secured cash flow facilities, and the guarantees of those obligations, are secured, subject to certain exceptions, by substantially all of our assets and the assets of Parent and the subsidiary guarantors, including:
| • | a first-priority pledge of 100% of our capital stock and certain of the capital stock held by us or any subsidiary guarantor (which pledge, in the case of any foreign subsidiary shall be limited to 100% of the non-voting stock (if any) and 65% of the voting stock of such foreign subsidiary), in each case excluding any interests in joint ventures to the extent such a pledge would violate the governing documents thereof; |
| • | a first-priority security interest in, and mortgages on, substantially all other tangible and intangible assets of us, Parent and each subsidiary guarantor, but excluding the collateral described in the following bullet point; and |
| • | a second-priority security interest in personal property of consisting of all accounts receivable (except assets subject to any permitted receivables facility), inventory, cash, deposit accounts and certain related intangible assets and proceeds of the foregoing. |
Certain Covenants and Events of Default
Our senior secured cash flow facilities contain a number of covenants that, among other things and subject to certain exceptions, will restrict our ability and the ability of our restricted subsidiaries to:
| • | incur additional indebtedness; |
| • | pay dividends on our capital stock or redeem, repurchase or retire our capital stock or indebtedness; |
| • | make investments, loans, advances and acquisitions; |
| • | create restrictions on the payment of dividends or other amounts to us from our restricted subsidiaries; |
| • | engage in transactions with our affiliates; |
| • | sell assets, including capital stock of our subsidiaries; |
| • | consolidate or merge; |
| • | create liens; and |
| • | enter into sale and lease-back transactions. |
In addition, the credit agreement governing our senior secured cash flow facilities does not require us to comply with any financial ratio maintenance covenants.
The credit agreement governing our senior secured cash flow facilities also contains certain customary affirmative covenants and events of default.
Senior Secured Asset-Based Revolving Credit Facility
Overview
In connection with the Transactions, we entered into a credit agreement and related security and other agreements for a senior secured asset-based revolving credit facility with Bank of America, N.A. as administrative agent and collateral agent. Our existing and future wholly-owned domestic subsidiaries that guarantee our obligations under our senior secured cash flow facilities will be subsidiary borrowers under this facility.
Our senior secured asset-based revolving credit facility provides senior secured financing of up to $350 million, subject to borrowing base limitations. The borrowing base at any time equals the sum of 85% of eligible accounts receivable and 85% of the net orderly liquidation value of eligible inventory (not to exceed 65% of the borrowing base), less certain reserves and subject to certain limitations on consigned inventory and accounts receivable owed by non-U.S. persons. Our senior secured asset-based revolving credit facility includes a $100 million sub-facility for letters of credit and a $35 million sub-facility for borrowings on same-day notice, referred to as the swingline loans. We do not expect to draw on our senior secured asset-based revolving credit facility at the closing of the Transactions. As of November 30, 2008, the borrowing base under our senior secured asset-based revolving credit facility was $321 million.
Our senior secured asset-based revolving credit facility provides that we have the right at any time to request up to $100 million of additional commitments under this facility. The lenders under this facility are not under any obligation to provide any such additional commitments under this facility, and any increase in commitments will be subject to customary conditions precedent. If we
94
Table of Contents
were to request any such additional commitments and the existing lenders or new lenders were to agree to provide such commitments, the facility size could be increased to up to $450 million, but our ability to borrow under this facility would still be limited by the amount of the borrowing base.
Interest Rate and Fees
Borrowings under our senior secured asset-based revolving credit facility bear interest at a rate per annum equal to the applicable margin plus, at our option, either (1) a base rate determined by reference to the higher of (a) the prime rate of Bank of America, N.A. and (b) the federal funds effective rate plus 1/2 of 1% or (2) a LIBOR or Eurocurrency rate determined by reference to the costs of funds for deposits in the currency of such borrowing for the interest period relevant to such borrowing adjusted for certain additional costs. The initial applicable margin for borrowings under our senior secured asset-based revolving credit facility is 0.750% with respect to base rate borrowings and 1.750% with respect to LIBOR or Eurocurrency borrowings. The applicable margin may be reduced based on our achievement of certain specified ratios.
In addition to paying interest on outstanding principal under our senior secured asset-based revolving credit facility, we are required to pay a commitment fee of 0.375% per annum in respect of the unutilized commitments thereunder. If the average revolving loan utilization thereunder is 50% or more for any applicable period, the commitment fee will be reduced to 0.250% for such period. We must also pay customary letter of credit fees and agency fees.
Mandatory Repayments
If at any time the aggregate amount of outstanding loans, unreimbursed letter of credit drawings and undrawn letters of credit under our senior secured asset-based revolving credit facility exceeds the lesser of (1) the commitment amount and (2) the borrowing base, we will be required to repay outstanding loans or cash collateralize letters of credit in an aggregate amount equal to such excess, with no reduction of the commitment amount. If the aggregate amount available under our senior secured asset-based revolving credit facility and our senior secured cash flow revolving credit facility is less than $75 million plus 10% of any additional commitments under this facility or certain events of default have occurred under our senior secured asset-based revolving credit facility, we are required to repay outstanding loans and cash collateralize letters of credit with the cash we are required to deposit daily in a collection account maintained with the agent under the facility.
Voluntary Repayments
We may voluntarily reduce the unutilized portion of the commitment amount and repay outstanding loans at any time without premium or penalty, other than customary “breakage” costs with respect to LIBOR or Eurocurrency loans.
Amortization and Final Maturity
There is no scheduled amortization under our senior secured asset-based revolving credit facility. The principal amount outstanding of the loans under our senior secured asset-based revolving credit facility is due and payable in full at maturity, six years from the date of closing of the Transactions.
Guarantee and Security
All obligations under our senior secured asset-based revolving credit facility are unconditionally guaranteed by Parent. All obligations under our senior secured asset-based revolving credit facility are secured, subject to certain exceptions, by a first-priority security interest in substantially all of our assets and the assets of the subsidiary borrowers that consist of all accounts receivable, inventory, cash, deposit accounts and certain related intangible assets and proceeds of the foregoing.
Certain Covenants and Events of Default
Our senior secured asset-based revolving credit facility contains a number of covenants that, among other things and subject to certain exceptions, restrict our ability and the ability of our restricted subsidiaries to:
| • | incur additional indebtedness; |
| • | pay dividends on our capital stock or redeem, repurchase or retire our capital stock or indebtedness; |
| • | make investments, loans, advances and acquisitions; |
| • | create restrictions on the payment of dividends or other amounts to us from our restricted subsidiaries; |
| • | engage in transactions with our affiliates; |
| • | sell assets, including capital stock of our subsidiaries; |
| • | consolidate or merge; |
| • | create liens; and |
| • | enter into sale and lease-back transactions. |
The covenants limiting (1) dividends and other restricted payments, (2) investments, loans, advances and acquisitions and (3) prepayments or redemptions of other indebtedness each permit the restricted actions in an unlimited amount, subject to the satisfaction of certain payment conditions, principally that we must have at least $112.5 million plus 15% of any additional commitments under this facility of pro forma excess availability under our senior secured asset-based revolving credit facility and our senior secured cash flow revolving credit facility in the aggregate, and that we must be in pro forma compliance with the fixed charge coverage ratio described in the next paragraph.
95
Table of Contents
Although the credit agreement governing our senior secured asset-based revolving credit facility does not require us to comply with any financial ratio maintenance covenants, if less than $35 million plus 10% of any additional commitments under this facility were available under our senior secured asset-based revolving credit facility at any time, we would not be permitted to borrow any additional amounts unless our pro forma ratio of (a) Consolidated EBITDA minus Capital Expenditures minus Cash Taxes to (b) Fixed Charges (as such terms are defined in the credit agreement and in each case for the most recently ended four quarter period) were at least 1.0 to 1.0.
The credit agreement governing our senior secured asset-based revolving credit facility also contains certain customary affirmative covenants and events of default.
Foreign lines of credit
At November 30, 2008, we had four lines of credit outstanding: (1) a European line of credit in the amount of €50 million (approximately $63 million), (2) two Japanese lines of credit in the amount of ¥2.5 billion (approximately $24 million), and (3) a Spanish open end revolving line of credit. As of November 30, 2008, we had $45 million of outstanding borrowings under our European line of credit and Spain line of credit and there were no outstanding borrowings under our Japanese lines of credit.
96
Table of Contents
General
Certain terms used in this description are defined under the subheading “Certain Definitions.” In this description, (1) the term “Issuer” refers only to LVB Acquisition Merger Sub, Inc. prior to the Acquisition and to Biomet, Inc., as the surviving corporation after the Acquisition, and not to any of their subsidiaries, and (2) the terms “we”, “our” and “us” each refer to the Issuer and its consolidated Subsidiaries assuming completion of the Acquisition.
The Issuer issued (a) $718.8 million aggregate principal amount of the original senior cash pay notes on September 25, 2007 and $56.2 million aggregate principal amount of the original senior cash pay notes on October 16, 2007 and (b) $688.8 million aggregate principal amount of the original senior toggle notes on September 25, 2007 and $86.2 million aggregate principal amount of the original senior toggle notes on October 16, 2007 under an indenture dated as of September 25, 2007 and a supplemental indenture dated as of October 16, 2007 (collectively, the “Indenture”) among the Issuer, the Guarantors and Wells Fargo Bank, National Association, as trustee (the “Trustee”).
The Indenture has been qualified under and is subject to and governed by the Trust Indenture Act of 1939. Except as set forth herein, the terms of the Senior Notes will be substantially identical and include those stated in the Indenture and those made part of the Indenture by reference to the Trust Indenture Act. The Senior Cash Pay Notes and the Senior Toggle Notes are each issued as a separate class, but, except as otherwise provided below, are treated as a single class for all purposes of the Indenture.
The following description is only a summary of the material provisions of the Indenture, does not purport to be complete and is qualified in its entirety by reference to the provisions of the Indenture, including the definitions therein of certain terms used below. We urge you to read the Indenture because it, and not this description, defines your rights as Holders of the Senior Notes. You may request copies of the Indenture at our address set forth under “Where You Can Find Additional Information.”
Brief Description of the Senior Notes
The Senior Notes:
| • | are general, unsecured, senior obligations of the Issuer; |
| • | rank equally in right of payment with all existing and future Senior Indebtedness (including the Senior Credit Facilities) of the Issuer; |
| • | are effectively subordinated to all Secured Indebtedness of the Issuer (including the Senior Credit Facilities), to the extent of the value of the collateral securing such Secured Indebtedness; |
| • | are structurally subordinated to all existing and future Indebtedness, claims of holders of Preferred Stock and other liabilities of Subsidiaries of the Issuer that do not guarantee the Senior Notes; |
| • | are senior in right of payment to all existing and future Subordinated Indebtedness (including the Senior Subordinated Notes) of the Issuer; |
| • | are initially guaranteed on a senior unsecured basis by the Guarantors and will also be guaranteed in the future by each Subsidiary, if any, that guarantees Indebtedness under the CF Credit Facilities; and |
| • | are subject to registration with the SEC pursuant to the Registration Rights Agreement. |
Guarantees
The Guarantors, as primary obligors and not merely as sureties, initially jointly and severally, irrevocably and unconditionally, guarantee, on an unsecured senior basis, the full and punctual payment when due, whether at maturity, by acceleration or otherwise, of all obligations of the Issuer under the Indenture and the Senior Notes, whether for payment of principal of, premium, if any, or interest in respect of the Senior Notes, expenses, indemnification or otherwise, on the terms set forth in the Indenture by executing the Indenture.
The Guarantors initially guarantee the Senior Notes and, in the future, each direct and indirect Subsidiary of the Issuer that guarantees Indebtedness under the CF Credit Facilities will guarantee the Senior Notes. Each of the Guarantees of the Senior Notes is a general, unsecured, senior obligation of each Guarantor, ranks equally in right of payment with all existing and future Senior Indebtedness of such Guarantor (including such Guarantor’s guarantee of the CF Credit Facilities), is effectively subordinated to all Secured Indebtedness of such Guarantor (including such Guarantor’s guarantee of the CF Credit Facilities), to the extent of the value of the collateral securing such Secured Indebtedness, and ranks senior in right of payment to all existing and future Subordinated Indebtedness of such Guarantor (including such Guarantor’s guarantee of the Senior Subordinated Notes). Each of the Guarantees of the Senior Notes is structurally subordinated to all existing and future Indebtedness, claims of holders of Preferred Stock and other liabilities of Subsidiaries of each Guarantor that do not Guarantee the Senior Notes.
Not all of the Issuer’s Subsidiaries guarantee the Senior Notes. In the event of a bankruptcy, liquidation, reorganization or similar proceeding of any of these non-guarantor Subsidiaries, the non-guarantor Subsidiaries will pay the holders of their debt and their trade creditors before they will be able to distribute any of their assets to the Issuer. As a result, all of the existing and future liabilities of our non-guarantor Subsidiaries, including any claims of trade creditors, are effectively senior to the Senior Notes. For the periods from June 1, 2007 through July 11, 2007, from July 12, 2007 through November 30, 2007, from July 12, 2007 through May 31, 2008, and the six months ended November 30, 2008 our non-guarantor subsidiaries accounted for approximately $83 million, or 33% of our consolidated net sales, $373 million, or 42% of our consolidated net sales, $1,060 million, or 50% of our consolidated net sales, and $469 million, or 38% of our consolidated net sales, for such period, respectively. As of November 30, 2008, our non-guarantor subsidiaries accounted for approximately $3,661 million, or 31% of our consolidated long-term assets. All amounts are presented after giving effect to intercompany eliminations.
The obligations of each Guarantor under its Guarantee are limited as necessary to prevent the Guarantee from constituting a fraudulent conveyance under applicable law. This provision may not, however, be effective to protect a Guarantee from being voided
97
Table of Contents
under fraudulent transfer law, or may reduce the applicable Guarantor’s obligation to an amount that effectively makes its Guarantee worthless. If a Guarantee was rendered voidable, it could be subordinated by a court to all other indebtedness (including guarantees and other contingent liabilities) of the Guarantor, and, depending on the amount of such indebtedness, a Guarantor’s liability on its Guarantee could be reduced to zero. See “Risk Factors—Risks Related to Our Indebtedness and the Notes—Federal and state fraudulent transfer laws may permit a court to void the notes and the guarantees, subordinate claims in respect of the notes and the guarantees and require noteholders to return payments received. If this occurs, you may not receive any payments on the notes.”
Any Guarantor that makes a payment under its Guarantee will be entitled upon payment in full of all guaranteed obligations under the Indenture to a contribution from each other Guarantor in an amount equal to such other Guarantor’s pro rata portion of such payment based on the respective net assets of all the Guarantors at the time of such payment determined in accordance with GAAP.
Each Guarantor may consolidate with or merge into or sell all or substantially all its assets to the Issuer or another Guarantor without limitation or any other Person upon the terms and conditions set forth in the Indenture. See “Certain Covenants—Merger, Consolidation or Sale of All or Substantially All Assets.”
Each Guarantee by a Guarantor provides by its terms that it will be automatically and unconditionally released and discharged upon:
(1) (a) any sale, exchange or transfer (by merger or otherwise) of (i) the Capital Stock of such Guarantor, after which the applicable Guarantor is no longer a Restricted Subsidiary or (ii) all or substantially all the assets of such Guarantor, in each case if such sale, exchange or transfer is made in compliance with the applicable provisions of the Indenture;
(b) the release or discharge of the guarantee by such Guarantor of Indebtedness under the CF Credit Facilities, or the release or discharge of such other guarantee that resulted in the creation of such Guarantee, except a discharge or release by or as a result of payment under such guarantee;
(c) the designation of any Restricted Subsidiary that is a Guarantor as an Unrestricted Subsidiary in compliance with the applicable provisions of the Indenture; or
(d) the exercise by the Issuer of its legal defeasance option or covenant defeasance option as described under “Legal Defeasance and Covenant Defeasance” or the discharge of the Issuer’s obligations under the Indenture in accordance with the terms of the Indenture; and
(2) such Guarantor delivering to the Trustee an Officer’s Certificate and an Opinion of Counsel, each stating that all conditions precedent provided for in the Indenture relating to such transaction have been complied with.
Ranking
The payment of the principal of, premium, if any, and interest on the Senior Notes and the payment of any Guarantee rank equally in right of payment to all existing and future Senior Indebtedness of the Issuer or the relevant Guarantor, as the case may be, including the obligations of the Issuer and such Guarantor under the Senior Credit Facilities.
The Senior Notes and the Guarantees are effectively subordinated in right of payment to all of the Issuer’s and the Guarantors’ existing and future Secured Indebtedness to the extent of the value of the collateral securing such Secured Indebtedness. As of November 30, 2008, the Issuer and the Guarantors had $3,582 million of Secured Indebtedness outstanding, consisting of borrowings and the related guarantees under the Senior Credit Facilities. As of November 30, 2008, the Issuer also had (1) an additional approximately $400 million of borrowing capacity under the cash flow revolving credit facility under the CF Credit Facilities, which, if borrowed, would be Secured Indebtedness, (2) an additional $326 million available for borrowing under the ABL Facilities, subject to borrowing base limitations, which, if borrowed, would be Secured Indebtedness, (3) the option to raise additional incremental term loans or incremental cash flow revolving facility commitments under the CF Credit Facilities of up to an amount that would cause our Senior Secured Leverage Ratio (as defined in the CF Credit Facilities) to be equal to or less than 4.50 to 1.00, which, if borrowed, would be Secured Indebtedness and (4) the option to raise additional incremental asset-based revolving credit facility commitments under the ABL Facilities by up to $100 million, which, if borrowed, would be Secured Indebtedness.
Although the Indenture contains limitations on the amount of additional Indebtedness that the Issuer, the Issuer’s Restricted Subsidiaries (including the Guarantors) may incur, under certain circumstances the amount of such Indebtedness could be substantial and, in any case, such Indebtedness may be Senior Indebtedness. The Indenture does not limit the amount of additional Indebtedness that Holdings may incur. See “Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock.”
Paying Agent and Registrar for the Senior Notes
The Issuer maintains one or more paying agents for the Senior Notes. The initial paying agent for the Senior Notes is the Trustee.
The Issuer also maintains one or more registrars and a transfer agent. The initial registrar and transfer agent with respect to the Senior Notes is the Trustee. The registrar maintains a register reflecting ownership of the Senior Notes outstanding from time to time. The registered Holder of a Senior Note is treated as the owner of the Senior Note for all purposes . The transfer agent will make payments on and facilitate transfer of Senior Notes on behalf of the Issuer.
The Issuer may change the paying agent, the registrar or the transfer agent without prior notice to the Holders. The Issuer or any of its Subsidiaries may act as a paying agent, registrar or transfer agent.
If any series of Senior Notes are listed on an exchange and the rules of such exchange so require, the Issuer will satisfy any requirement of such exchange as to paying agents, registrars and transfer agents and will comply with any notice requirements required under such exchange in connection with any change of paying agent, registrar or transfer agent.
98
Table of Contents
Transfer and Exchange
A Holder may transfer or exchange Senior Notes in accordance with the Indenture. The registrar and the Trustee may require a Holder to furnish appropriate endorsements and transfer documents in connection with a transfer of Senior Notes. Holders will be required to pay all taxes due on transfer. The Issuer will not be required to transfer or exchange any Senior Note selected for redemption or tendered (and not withdrawn) for repurchase in connection with a Change of Control Offer or an Asset Sale Offer. Also, the Issuer will not be required to transfer or exchange any Senior Note for a period of 15 days before a selection of Senior Notes to be redeemed.
Principal, Maturity and Interest
The Issuer issued an aggregate principal amount of $775 million of Senior Cash Pay Notes and an aggregate principal amount of $775 million of Senior Toggle Notes. The Senior Notes will mature on October 15, 2017. Subject to compliance with the covenant described below under “Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock,” the Issuer may issue additional Senior Cash Pay Notes and/or Senior Toggle Notes from time to time after this offering under the Indenture (“Additional Senior Notes”); provided that in connection with the payment of PIK Interest (as defined under “—Senior Toggle Notes”), the Issuer is entitled to, without the consent of the Holders (and without regard to any restrictions or limitations set forth under “Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”), increase the outstanding principal amount of the Senior Toggle Notes or issue additional Senior Toggle Notes (the “PIK Notes”) under the Indenture on the same terms and conditions as the Senior Toggle Notes issued on the Issue Date (in each case, the “PIK Payment”). The Senior Notes offered by the Issuer (including any PIK Notes) and any Additional Senior Notes subsequently issued under the Indenture will be treated as a single class for all purposes under the Indenture, including waivers, amendments, redemptions and offers to purchase, except for certain waivers and amendments. Unless the context requires otherwise, references to “Senior Notes” for all purposes of the Indenture and this “Description of Senior Notes” include any Additional Senior Notes and PIK Notes that are actually issued and any increase in the principal amount of the outstanding Senior Toggle Notes (including PIK Notes) as a result of a PIK Payment and references to “principal amount” of the Senior Notes or the Senior Toggle Notes include any increase in the principal amount of the outstanding Senior Toggle Notes (including PIK Notes) as a result of a PIK Payment. The Senior Cash Pay Notes will be issued in denominations of $2,000 and any integral multiples of $1,000 in excess of $2,000. The Senior Toggle Notes will initially be issued in denominations of $2,000 and any integral multiple of $2,000 and, if a PIK Payment is made, in denominations of $1.00 and any integral multiple of $1.00 in excess of $1.00.
Senior Cash Pay Notes
Interest on the Senior Cash Pay Notes accrues at the rate of 10% per annum. Interest on the Senior Cash Pay Notes is payable semi-annually in arrears on each April 15 and October 15, which commenced April 15, 2008 to the Holders of Senior Cash Pay Notes of record on the immediately preceding April 1 and October 1. Interest on the Senior Cash Pay Notes accrues from the most recent date to which interest has been paid or, if no interest has been paid, from and including the Issue Date. Interest on the Senior Cash Pay Notes is computed on the basis of a 360-day year comprised of twelve 30-day months.
Senior Toggle Notes
For any interest period through October 15, 2012, the Issuer may, at its option, elect to pay interest on the Senior Toggle Notes (1) entirely in cash (“Cash Interest”), (2) entirely by increasing the principal amount of the outstanding Senior Toggle Notes or by issuing PIK Notes (“PIK Interest”) or (3) 50.0% as Cash Interest and 50.0% as PIK Interest.
The Issuer must elect the form of interest payment with respect to each interest period by delivering a notice to the Trustee prior to the beginning of each interest period. The Trustee shall promptly deliver a corresponding notice to the Holders. In the absence of such an election for any interest period, interest on the Senior Toggle Notes will be payable in the form of the interest payment for the prior interest period. Interest for the first period commencing on the Issue Date shall be payable in cash. After October 15, 2012, the Issuer will make all interest payments on the Senior Toggle Notes in cash.
Cash Interest on the Senior Toggle Notes accrues at the rate of 10 3/8% per annum and be payable in cash. PIK Interest on the Senior Toggle Notes accrues at the rate of 11 1/8% per annum and be payable (a) with respect to the Senior Toggle Notes represented by one or more global notes registered in the name of, or held by, the Depository Trust Company (“DTC”) or its nominee on the relevant record date, by increasing the principal amount of the outstanding Senior Toggle Notes represented by such global notes by an amount equal to the amount of PIK Interest for the applicable interest period (rounded up to the nearest whole dollar) and (b) with respect to Senior Toggle Notes represented by certificated notes, by issuing PIK Notes in certificated form in an aggregate principal amount equal to the amount of PIK Interest for the applicable interest period (rounded up to the nearest whole dollar) and the Trustee will, at the request of the Issuer, authenticate and deliver such PIK Notes in certificated form for original issuance to the Holders on the relevant record date, as shown by the records of the register. Following an increase in the principal amount of the outstanding Senior Toggle Notes represented by global notes as a result of a PIK Payment, such Senior Toggle Notes will bear interest on such increased principal amount from and after the date of such PIK Payment. Any PIK Notes issued in certificated form will be dated as of the applicable interest payment date and will bear interest from and after such date. All PIK Notes issued pursuant to a PIK Payment will mature on October 15, 2017, and will be governed by, and subject to the terms, provisions and conditions of, the Indenture and shall have the same rights and benefits as the Senior Toggle Notes issued on the Issue Date. Any certificated PIK Notes will be issued with the description “PIK” on the face of such PIK Note.
Interest on the Senior Toggle Notes is payable semi-annually in arrears on each April 15 and October 15 which commenced April 15, 2008, to the Holders of Senior Toggle Notes of record on the immediately preceding April 1 and October 1. Interest on the Senior Toggle Notes accrues from the most recent date to which interest has been paid or, if no interest has been paid, from and including the Issue Date. Interest is computed on the basis of a 360-day year comprised of twelve 30-day months.
99
Table of Contents
Additional Interest
Additional Interest may accrue on the Senior Notes in certain circumstances pursuant to the Registration Rights Agreement or as described under “Events of Default and Remedies.” Any Additional Interest on the Senior Toggle Notes is payable in the same form of payment elected by the Issuer for the payment of interest with respect to the applicable interest period. All references in the Indenture and this “Description of Senior Notes,” in any context, to any interest or other amount payable on or with respect to the Senior Notes shall be deemed to include any Additional Interest payable pursuant to the Registration Rights Agreement and under “Events of Default and Remedies.”
Payment of Principal, Premium and Interest
Cash payments of principal of, premium, if any, and interest on the Senior Notes is payable at the office or agency of the Issuer maintained for such purpose or, at the option of the Issuer, cash payment of interest may be made by check mailed to the Holders of the Senior Notes at their respective addresses set forth in the register of Holders; provided that (1) all cash payments of principal, premium, if any, and interest with respect to the Senior Notes represented by one or more global notes registered in the name of or held by DTC or its nominee will be made by wire transfer of immediately available funds to the accounts specified by the Holder or Holders thereof and (2) all cash payments of principal, premium, if any, and interest with respect to certificated Senior Notes will be made by wire transfer to a U.S. dollar account maintained by the payee with a bank in the United States if such Holder elects payment by wire transfer by giving written notice to the Trustee or the paying agent to such effect designating such account no later than 30 days immediately preceding the relevant due date for payment (or such other date as the Trustee may accept in its discretion). Until otherwise designated by the Issuer, the Issuer’s office or agency is the office of the Trustee maintained for such purpose.
Mandatory Redemption; Offers to Purchase; Open Market Purchases
The Issuer is not required to make any mandatory redemption or sinking fund payments with respect to the Senior Notes. However, under certain circumstances, the Issuer may be required to offer to purchase Senior Notes as described under “Repurchase at the Option of Holders.” The Issuer may at any time and from time to time purchase Senior Notes in the open market or otherwise.
Optional Redemption
Senior Cash Pay Notes
Except as set forth below, the Issuer is not entitled to redeem the Senior Cash Pay Notes at its option prior to October 15, 2012.
At any time prior to October 15, 2012, the Issuer may redeem all or a part of the Senior Cash Pay Notes, upon notice as described under “—Selection and Notice,” at a redemption price equal to 100.0% of the principal amount of the Senior Cash Pay Notes redeemed plus the Applicable Premium as of, plus accrued and unpaid interest, if any, to the date of redemption (the “ Redemption Date”), subject to the right of Holders of record on the relevant record date to receive interest due on the relevant interest payment date.
On and after October 15, 2012, the Issuer may redeem the Senior Cash Pay Notes, in whole or in part, upon notice as described under “—Selection and Notice,” at the redemption prices (expressed as percentages of principal amount of the Senior Cash Pay Notes to be redeemed) set forth below, plus accrued and unpaid interest, if any, to the Redemption Date, subject to the right of Holders of record on the relevant record date to receive interest due on the relevant interest payment date, if redeemed during the twelve-month period beginning on October 15 of each of the years indicated below:
Year | Senior Cash Pay Notes Percentage | ||
2012 | 105.000 | % | |
2013 | 103.333 | % | |
2014 | 101.667 | % | |
2015 and thereafter | 100.000 | % |
In addition, until October 15, 2010, the Issuer may, at its option, redeem up to 35.0% of the aggregate principal amount of Senior Cash Pay Notes issued by under the Indenture at a redemption price equal to 100.0% of the aggregate principal amount thereof, plus a premium equal to the stated interest rate per annum on the Senior Cash Pay Notes, plus accrued and unpaid interest, if any, to the Redemption Date, subject to the right of Holders of Senior Cash Pay Notes of record on the relevant record date to receive interest due on the relevant interest payment date, with the net cash proceeds received by it from one or more Equity Offerings; provided that (a) at least 50.0% of the sum of the aggregate principal amount of Senior Cash Pay Notes originally issued under the Indenture on the Issue Date and any Additional Senior Notes that are Senior Cash Pay Notes issued under the Indenture after the Issue Date remains outstanding immediately after the occurrence of each such redemption; and (b) each such redemption occurs within 180 days of the date of closing of each such Equity Offering.
Notice of any redemption upon any Equity Offering may be given prior to the completion thereof, and any such redemption or notice may, at the Issuer’s discretion, be subject to one or more conditions precedent, including, but not limited to, completion of the related Equity Offering. If any Senior Cash Pay Notes are listed on an exchange, and the rules of such exchange so require, the Issuer will notify the exchange of any such notice of redemption. In addition, the Issuer will notify the exchange of the principal amount of any Senior Cash Pay Notes outstanding following any partial redemption of Senior Cash Pay Notes.
100
Table of Contents
Senior Toggle Notes
Except as set forth below, the Issuer is not entitled to redeem the Senior Toggle Notes at its option prior to October 15, 2012.
At any time prior to October 15, 2012, the Issuer may redeem all or a part of the Senior Toggle Notes, upon notice as described under “—Selection and Notice,” at a redemption price equal to 100.0% of the principal amount of the Senior Toggle Notes redeemed plus the Applicable Premium as of, plus accrued and unpaid interest and, if any, to the Redemption Date, subject to the right of Holders of record on the relevant record date to receive interest due on the relevant interest payment date.
On and after October 15, 2012, the Issuer may redeem the Senior Toggle Notes, in whole or in part, upon notice as described under “—Selection and Notice,” at the redemption prices (expressed as percentages of principal amount of the Senior Toggle Notes to be redeemed) set forth below, plus accrued and unpaid interest, if any, to the Redemption Date, subject to the right of Holders of record on the relevant record date to receive interest due on the relevant interest payment date, if redeemed during the twelve-month period beginning on October 15 of each of the years indicated below:
Year | Senior Toggle Notes Percentage | ||
2012 | 105.188 | % | |
2013 | 103.458 | % | |
2014 | 101.729 | % | |
2015 and thereafter | 100.000 | % |
In addition, until October 15, 2010, the Issuer may, at its option, redeem up to 35.0% of the aggregate principal amount of Senior Toggle Notes issued by it at a redemption price equal to 100.0% of the aggregate principal amount thereof, plus a premium equal to the Cash Interest rate per annum on the Senior Toggle Notes, plus accrued and unpaid interest, if any, to the Redemption Date, subject to the right of Holders of Senior Toggle Notes of record on the relevant record date to receive interest due on the relevant interest payment date, with the net cash proceeds received by it from one or more Equity Offerings; provided that (1) at least 50.0% of the sum of the aggregate principal amount of Senior Toggle Notes originally issued under the Indenture on the Issue Date and any Additional Senior Notes that are Senior Toggle Notes and any PIK Notes issued under the Indenture after the Issue Date remains outstanding immediately after the occurrence of each such redemption; and (2) each such redemption occurs within 180 days of the date of closing of each such Equity Offering.
Notice of any redemption upon any Equity Offering may be given prior to the completion thereof, and any such redemption or notice may, at the Issuer’s discretion, be subject to one or more conditions precedent, including, but not limited to, completion of the related Equity Offering. If any Senior Toggle Notes are listed on an exchange, and the rules of such exchange so require, the Issuer will notify the exchange of any such notice of redemption. In addition, the Issuer will notify the exchange of the principal amount of any Senior Toggle Notes outstanding following any partial redemption of Senior Toggle Notes.
Selection and Notice
If the Issuer is redeeming less than all of a series of the Senior Notes issued under the Indenture at any time, the Trustee will select the Senior Notes to be redeemed (1) if the Senior Notes are listed on an exchange, in compliance with the requirements of such exchange or (2) on a pro rata basis to the extent practicable, or, if the pro rata basis is not practicable for any reason, by lot or by such other method as the Trustee shall deem fair and appropriate. No Senior Notes of $2,000 or less can be redeemed in part.
Notices of redemption shall be delivered electronically or mailed by first-class mail, postage prepaid, at least 30 but not more than 60 days before the redemption date to each Holder of Senior Notes at such Holder’s registered address or otherwise in accordance with the procedures of DTC, except that redemption notices may be delivered more than 60 days prior to a redemption date if the notice is issued in connection with a defeasance of the Senior Notes or a satisfaction and discharge of the Indenture. If any Senior Note is to be redeemed in part only, any notice of redemption that relates to such Senior Note shall state the portion of the principal amount thereof that has been or is to be redeemed.
With respect to Senior Notes represented by certificated notes, the Issuer will issue a senior Note in a principal amount equal to the unredeemed portion of the original Senior Note in the name of the Holder upon cancellation of the original Senior Note. Senior Notes called for redemption become due on the date fixed for redemption. On and after the Redemption Date, interest ceases to accrue on Senior Notes or portions of them called for redemption.
Repurchase at the Option of Holders
Change of Control
The Indenture provides that if a Change of Control occurs, unless the Issuer has previously or concurrently delivered a redemption notice with respect to all the outstanding Senior Notes as described under “Optional Redemption,” the Issuer will make an offer to purchase all of the Senior Notes pursuant to the offer described below (the “Change of Control Offer”) at a price in cash (the “Change of Control Payment”) equal to 101.0% of the aggregate principal amount thereof plus accrued and unpaid interest, if any, to the date of purchase, subject to the right of Holders of the Senior Notes of record on the relevant record date to receive interest due on the relevant interest payment date. Within 30 days following any Change of Control, the Issuer will deliver notice of such Change of Control Offer electronically or by first-class mail, with a copy to the Trustee, to each Holder of Senior Notes to the address of such Holder appearing in the security register or otherwise in accordance with the procedures of DTC with the following information:
(1) that a Change of Control Offer is being made pursuant to the covenant entitled “Change of Control,” and that all Senior Notes properly tendered pursuant to such Change of Control Offer will be accepted for payment by the Issuer;
101
Table of Contents
(2) the purchase price and the purchase date, which will be no earlier than 30 days nor later than 60 days from the date such notice is delivered (the “Change of Control Payment Date”);
(3) that any Senior Note not properly tendered will remain outstanding and continue to accrue interest;
(4) that unless the Issuer defaults in the payment of the Change of Control Payment, all Senior Notes accepted for payment pursuant to the Change of Control Offer will cease to accrue interest on the Change of Control Payment Date;
(5) that Holders electing to have any Senior Notes purchased pursuant to a Change of Control Offer will be required to surrender such Senior Notes, with the form entitled “Option of Holder to Elect Purchase” on the reverse of such Senior Notes completed, to the paying agent specified in the notice at the address specified in the notice prior to the close of business on the third Business Day preceding the Change of Control Payment Date;
(6) that Holders will be entitled to withdraw their tendered Senior Notes and their election to require the Issuer to purchase such Senior Notes, provided that the paying agent receives, not later than the close of business on the expiration date of the Change of Control Offer, a telegram, facsimile transmission or letter setting forth the name of the Holder of the Senior Notes, the principal amount of Senior Notes tendered for purchase, and a statement that such Holder is withdrawing its tendered Senior Notes and its election to have such Senior Notes purchased;
(7) that Holders whose Senior Notes are being purchased only in part will be issued senior Notes and such senior Notes will be equal in principal amount to the unpurchased portion of the Senior Notes surrendered. The unpurchased portion of the Senior Notes must be equal to at least $2,000 or any integral multiple of $1,000 in excess of $2,000 in the case of the Senior Cash Pay Notes and at least $2,000 in the case of the Senior Toggle Notes;
(8) if such notice is delivered prior to the occurrence of a Change of Control, stating that the Change of Control Offer is conditional on the occurrence of such Change of Control; and
(9) the other instructions, as determined by the Issuer, consistent with the covenant described hereunder, that a Holder must follow.
The Issuer will comply with the requirements of Rule 14e-1 under the Exchange Act and any other securities laws and regulations thereunder to the extent such laws or regulations are applicable in connection with the repurchase of Senior Notes pursuant to a Change of Control Offer. To the extent that the provisions of any securities laws or regulations conflict with the provisions of the Indenture, the Issuer will comply with the applicable securities laws and regulations and shall not be deemed to have breached its obligations described in the Indenture by virtue thereof.
On the Change of Control Payment Date, the Issuer will, to the extent permitted by law:
(1) accept for payment all Senior Notes issued by it or portions thereof properly tendered pursuant to the Change of Control Offer;
(2) deposit with the paying agent an amount equal to the aggregate Change of Control Payment in respect of all Senior Notes or portions thereof so tendered; and
(3) deliver, or cause to be delivered, to the Trustee for cancellation the Senior Notes so accepted together with an Officer’s Certificate to the Trustee stating that such Senior Notes or portions thereof have been tendered to and purchased by the Issuer.
The Senior Credit Facilities do, and future credit agreements or other agreements relating to Senior Indebtedness to which the Issuer becomes a party may, provide that certain change of control events with respect to the Issuer would constitute a default thereunder (including a Change of Control under the Indenture). If we experience a change of control that triggers a default under the Senior Credit Facilities, we could seek a waiver of such default or seek to refinance the Senior Credit Facilities. In the event we do not obtain such a waiver or refinance the Senior Credit Facilities, such default could result in amounts outstanding under the Senior Credit Facilities being declared due and payable and cause a Qualified Securitization Facility to be wound down.
Our ability to pay cash to the Holders of Senior Notes following the occurrence of a Change of Control may be limited by our then-existing financial resources. Therefore, sufficient funds may not be available when necessary to make any required repurchases.
The Change of Control purchase feature of the Senior Notes may in certain circumstances make more difficult or discourage a sale or takeover of us and, thus, the removal of incumbent management. The Change of Control purchase feature is a result of negotiations between the Initial Purchasers and us. After the Issue Date, we have no present intention to engage in a transaction involving a Change of Control, although it is possible that we could decide to do so in the future. Subject to the limitations discussed below, we could, in the future, enter into certain transactions, including acquisitions, refinancings or other recapitalizations, that would not constitute a Change of Control under the Indenture, but that could increase the amount of Indebtedness outstanding at such time or otherwise affect our capital structure or credit ratings. Restrictions on our ability to incur additional Indebtedness are contained in the covenants described under “Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock” and “Certain Covenants—Liens.” Such restrictions in the Indenture can be waived only with the consent of the Holders of a majority in principal amount of the Senior Notes then outstanding. Except for the limitations contained in such covenants, however, the Indenture does not contain any covenants or provisions that may afford Holders of the Senior Notes protection in the event of a highly leveraged transaction.
The Issuer is not required to make a Change of Control Offer following a Change of Control if a third party makes the Change of Control Offer in the manner, at the times and otherwise in compliance with the requirements set forth in the Indenture applicable to a Change of Control Offer made by the Issuer and purchases all Senior Notes validly tendered and not withdrawn under such Change of Control Offer.
102
Table of Contents
Notwithstanding anything to the contrary herein, a Change of Control Offer may be made in advance of a Change of Control, conditional upon such Change of Control, if a definitive agreement is in place for the Change of Control at the time of making of the Change of Control Offer.
The definition of “Change of Control” includes a disposition of all or substantially all of the assets of the Issuer and its Subsidiaries, taken as a whole, to any Person. Although there is a limited body of case law interpreting the phrase “substantially all,” there is no precise established definition of the phrase under applicable law. Accordingly, in certain circumstances there may be a degree of uncertainty as to whether a particular transaction would involve a disposition of “all or substantially all” of the assets of the Issuer and its Subsidiaries, taken as a whole. As a result, it may be unclear as to whether a Change of Control has occurred and whether a Holder of Senior Notes may require the Issuer to make an offer to repurchase the Senior Notes as described above.
The provisions under the Indenture relative to the Issuer’s obligation to make an offer to repurchase the Senior Notes as a result of a Change of Control may be waived or modified with the written consent of the Holders of a majority in principal amount of the Senior Notes.
Asset Sales
The Indenture provides that the Issuer will not, and will not permit any of its Restricted Subsidiaries to, consummate directly or indirectly an Asset Sale, unless:
(1) the Issuer or such Restricted Subsidiary, as the case may be, receives consideration at the time of such Asset Sale at least equal to the fair market value of the assets sold or otherwise disposed of; and
(2) except in the case of a Permitted Asset Swap, at least 75.0% of the consideration therefor received by the Issuer or such Restricted Subsidiary, as the case may be, is in the form of Cash Equivalents; provided that the amount of:
(a) any liabilities (as shown on the Issuer’s or such Restricted Subsidiary’s most recent balance sheet or in the footnotes thereto) of the Issuer or such Restricted Subsidiary, other than liabilities that are by their terms subordinated to the Senior Notes, that are assumed by the transferee of any such assets and for which the Issuer and all of its Restricted Subsidiaries have been validly released by all creditors in writing;
(b) any securities, notes or other obligations or assets received by the Issuer or such Restricted Subsidiary from such transferee that are converted by the Issuer or such Restricted Subsidiary into cash (to the extent of the cash received) within 180 days following the closing of such Asset Sale; and
(c) any Designated Non-cash Consideration received by the Issuer or such Restricted Subsidiary in such Asset Sale having an aggregate fair market value, taken together with all other Designated Non-cash Consideration received pursuant to this clause (c) that is at that time outstanding, not to exceed the greater of (x) $300.0 million and (y) 3.0% of Total Assets at the time of the receipt of such Designated Non-cash Consideration, with the fair market value of each item of Designated Non-cash Consideration being measured at the time received and without giving effect to subsequent changes in value,
shall be deemed to be Cash Equivalents for purposes of this provision and for no other purpose.
Within 450 days after the receipt of any Net Proceeds of any Asset Sale, the Issuer or such Restricted Subsidiary, at its option, may apply the Net Proceeds from such Asset Sale,
(1) to permanently reduce:
(a) Obligations under the Senior Credit Facilities, and to correspondingly reduce commitments with respect thereto;
(b) Obligations under Senior Indebtedness that is secured by a Lien, which Lien is permitted by the Indenture, and to correspondingly reduce commitments with respect thereto;
(c) Obligations under the Senior Indebtedness (and to correspondingly reduce commitments with respect thereto), provided that the Issuer shall equally and ratably reduce Obligations under the Senior Notes as provided under “Optional Redemption” or through open-market purchases (to the extent such purchases are at or above 100.0% of the principal amount thereof) or by making an offer (in accordance with the procedures set forth below for an Asset Sale Offer) to all Holders to purchase their Senior Notes at 100.0% of the principal amount thereof, plus the amount of accrued but unpaid interest, if any, on the amount of Senior Notes to be repurchased; or
(d) Indebtedness of a Restricted Subsidiary that is not a Guarantor, other than Indebtedness owed to the Issuer or another Restricted Subsidiary;
(2) to make (a) an Investment in any one or more businesses, provided that such Investment in any business is in the form of the acquisition of Capital Stock and results in the Issuer or any of its Restricted Subsidiaries, as the case may be, owning an amount of the Capital Stock of such business such that it constitutes a Restricted Subsidiary, (b) capital expenditures or (c) acquisitions of other assets, in each of (a), (b) and (c), used or useful in a Similar Business; or
(3) to make an Investment in (a) any one or more businesses, provided that such Investment in any business is in the form of the acquisition of Capital Stock and results in the Issuer or any of its Restricted Subsidiaries, as the case may be, owning an amount of the Capital Stock of such business such that it constitutes a Restricted Subsidiary, (b) properties or (c) acquisitions of other assets that, in each of (a), (b) and (c), replace the businesses, properties and/or assets that are the subject of such Asset Sale;
103
Table of Contents
provided that, in the case of clauses (2) and (3) above, a binding commitment shall be treated as a permitted application of the Net Proceeds from the date of such commitment so long as the Issuer or such other Restricted Subsidiary enters into such commitment with the good faith expectation that such Net Proceeds will be applied to satisfy such commitment within 180 days of such commitment (an “Acceptable Commitment”) and, in the event any Acceptable Commitment is later cancelled or terminated for any reason before the Net Proceeds are applied in connection therewith, the Issuer or such Restricted Subsidiary enters into another Acceptable Commitment (a “Second Commitment”) within 180 days of such cancellation or termination; provided, further, that if any Second Commitment is later cancelled or terminated for any reason before such Net Proceeds are applied, then such Net Proceeds shall constitute Excess Proceeds.
Any Net Proceeds from the Asset Sale that are not invested or applied as provided and within the time period set forth in the preceding paragraph will be deemed to constitute “Excess Proceeds.” When the aggregate amount of Excess Proceeds exceeds $75.0 million, the Issuer shall make an offer to all Holders of the Senior Notes and, if required by the terms of any Indebtedness that is pari passu with the Senior Notes (“Pari Passu Indebtedness”), to the holders of such Pari Passu Indebtedness (an “Asset Sale Offer”), to purchase the maximum aggregate principal amount of the Senior Notes and such Pari Passu Indebtedness that is in an amount equal to at least $2,000, that may be purchased out of the Excess Proceeds at an offer price in cash in an amount equal to 100.0% of the principal amount thereof (or accreted value thereof, if less), plus accrued and unpaid interest, if any, to the date fixed for the closing of such offer, in accordance with the procedures set forth in the Indenture. The Issuer will commence an Asset Sale Offer with respect to Excess Proceeds within ten Business Days after the date that Excess Proceeds exceed $75.0 million by delivering the notice required pursuant to the terms of the Indenture, with a copy to the Trustee. The Issuer may satisfy the foregoing obligations with respect to any Net Proceeds from an Asset Sale by making an Asset Sale Offer with respect to such Net Proceeds prior to the expiration of the relevant 450 days (or such longer period provided above) or with respect to Excess Proceeds of $75.0 million or less.
To the extent that the aggregate amount of Senior Notes and such Pari Passu Indebtedness tendered pursuant to an Asset Sale Offer is less than the Excess Proceeds, the Issuer may use any remaining Excess Proceeds for general corporate purposes, subject to other covenants contained in the Indenture. If the aggregate principal amount of Senior Notes or the Pari Passu Indebtedness surrendered by such holders thereof exceeds the amount of Excess Proceeds, the Trustee shall select the Senior Notes and the Issuer shall select such Pari Passu Indebtedness to be purchased on a pro rata basis based on the accreted value or principal amount of the Senior Notes or such Pari Passu Indebtedness tendered. Upon completion of any such Asset Sale Offer, the amount of Excess Proceeds that resulted in the Asset Sale Offer shall be reset to zero.
Pending the final application of any Net Proceeds pursuant to this covenant, the holder of such Net Proceeds may apply such Net Proceeds temporarily to reduce Indebtedness outstanding under a revolving credit facility or otherwise invest such Net Proceeds in any manner not prohibited by the Indenture.
The Issuer will comply with the requirements of Rule 14e-1 under the Exchange Act and any other securities laws and regulations thereunder to the extent such laws or regulations are applicable in connection with the repurchase of the Senior Notes pursuant to an Asset Sale Offer. To the extent that the provisions of any securities laws or regulations conflict with the provisions of the Indenture, the Issuer will comply with the applicable securities laws and regulations and shall not be deemed to have breached its obligations described in the Indenture by virtue thereof.
The provisions under the Indenture relative to the Issuer’s obligation to make an offer to repurchase the Senior Notes as a result of an Asset Sale may be waived or modified with the written consent of the Holders of a majority in principal amount of the Senior Notes.
Certain Covenants
Set forth below are summaries of certain covenants contained in the Indenture. During any period of time that (i) the Senior Notes have Investment Grade Ratings from both Rating Agencies and (ii) no Default has occurred and is continuing under the Indenture (the occurrence of the events described in the foregoing clauses (i) and (ii) being collectively referred to as a “Covenant Suspension Event”and the date thereof being referred to as the “Suspension Date”) then, the covenants specifically listed under the following captions in this “Description of Senior Notes” section of this prospectus will not be applicable to the Senior Notes (collectively, the “Suspended Covenants”):
“Repurchase at the Option of Holders—Asset Sales”;
“—Limitation on Restricted Payments”;
“—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”;
clause (4) of the first paragraph of “—Merger, Consolidation or Sale of All or Substantially All Assets”;
“—Transactions with Affiliates”;
“—Dividend and Other Payment Restrictions Affecting Restricted Subsidiaries”; and
“—Limitation on Guarantees of Indebtedness by Restricted Subsidiaries.”
During any period that the foregoing covenants have been suspended, the Issuer may not designate any of its Subsidiaries as Unrestricted Subsidiaries pursuant to the second sentence of the definition of “Unrestricted Subsidiary.”
If and while the Issuer and its Restricted Subsidiaries are not subject to the Suspended Covenants, the Senior Notes will be entitled to substantially less covenant protection. In the event that the Issuer and its Restricted Subsidiaries are not subject to the Suspended Covenants under the Indenture for any period of time as a result of the foregoing, and on any subsequent date (the
104
Table of Contents
“Reversion Date”) one or both of the Rating Agencies withdraw their Investment Grade Rating or downgrade the rating assigned to the Senior Notes below an Investment Grade Rating, then the Issuer and its Restricted Subsidiaries will thereafter again be subject to the Suspended Covenants under the Indenture with respect to future events. The period of time between the Suspension Date and the Reversion Date is referred to in this “Description of Senior Notes” as the “Suspension Period.” The Guarantees of the Guarantors will be suspended during the Suspension Period. Additionally, upon the occurrence of a Covenant Suspension Event, the amount of Excess Proceeds from Net Proceeds shall be reset to zero.
Notwithstanding the foregoing, in the event of any such reinstatement, no action taken or omitted to be taken by the Issuer or any of its Restricted Subsidiaries prior to such reinstatement will give rise to a Default or Event of Default under the Indenture with respect to the Senior Notes; provided that (i) with respect to Restricted Payments made after such reinstatement, the amount available to be made as Restricted Payments will be calculated as though the covenant described above under “—Limitation on Restricted Payments” had been in effect prior to, but not during, the Suspension Period; and (ii) all Indebtedness incurred, or Disqualified Stock issued, during the Suspension Period will be classified to have been incurred or issued pursuant to clause (3) of the second paragraph of “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock.”
There can be no assurance that the Senior Notes will ever achieve or maintain Investment Grade Ratings.
Limitation on Restricted Payments
The Issuer will not, and will not permit any of its Restricted Subsidiaries to, directly or indirectly:
(I) declare or pay any dividend or make any payment or distribution on account of the Issuer’s, or any of its Restricted Subsidiaries’ Equity Interests, including any dividend or distribution payable in connection with any merger or consolidation other than:
(a) dividends or distributions by the Issuer payable solely in Equity Interests (other than Disqualified Stock) of the Issuer; or
(b) dividends or distributions by a Restricted Subsidiary so long as, in the case of any dividend or distribution payable on or in respect of any class or series of securities issued by a Restricted Subsidiary other than a Wholly-Owned Subsidiary, the Issuer or a Restricted Subsidiary receives at least its pro rata share of such dividend or distribution in accordance with its Equity Interests in such class or series of securities;
(II) purchase, redeem, defease or otherwise acquire or retire for value any Equity Interests of the Issuer or any direct or indirect parent company of the Issuer, including in connection with any merger or consolidation;
(III) make any principal payment on, or redeem, repurchase, defease or otherwise acquire or retire for value, in each case, prior to any scheduled repayment, sinking fund payment or maturity, any Subordinated Indebtedness, other than:
(a) Indebtedness permitted under clauses (7) and (8) of the second paragraph of the covenant described under “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”; or
(b) the purchase, repurchase or other acquisition of Subordinated Indebtedness purchased in anticipation of satisfying a sinking fund obligation, principal installment or final maturity, in each case due within one year of the date of purchase, repurchase or acquisition; or
(IV) make any Restricted Investment (all such payments and other actions set forth in clauses (I) through (IV) above being collectively referred to as “ Restricted Payments”), unless, at the time of such Restricted Payment:
(1) no Default shall have occurred and be continuing or would occur as a consequence thereof;
(2) immediately after giving effect to such transaction on a pro forma basis, the Issuer could incur $1.00 of additional Indebtedness pursuant to the Fixed Charge Coverage Ratio test set forth in the first paragraph of the covenant described under “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock” (the “Fixed Charge Coverage Test”); and
(3) such Restricted Payment, together with the aggregate amount of all other Restricted Payments made by the Issuer and its Restricted Subsidiaries after the Issue Date (including Restricted Payments permitted by clauses (1), (2) (with respect to the payment of dividends on Refunding Capital Stock (as defined below) pursuant to clause (b) thereof only), (6)(c), (9) and (14) of the next succeeding paragraph, but excluding all other Restricted Payments permitted by the next succeeding paragraph), is less than the sum of (without duplication):
(a) 50.0% of the Consolidated Net Income of the Issuer for the period (taken as one accounting period and including the predecessor) beginning on September 1, 2007 to the end of the Issuer’s most recently ended fiscal quarter for which internal financial statements are available at the time of such Restricted Payment, or, in the case such Consolidated Net Income for such period is a deficit, minus 100.0% of such deficit; plus
(b) 100.0% of the aggregate net cash proceeds and the fair market value of marketable securities or other property received by the Issuer since immediately after the Issue Date (other than net cash proceeds to the extent such net cash proceeds have been used to incur Indebtedness or issue Disqualified Stock or Preferred Stock pursuant to clause (12)(a) of the second paragraph of “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”) from the issue or sale of:
(i) (A) Equity Interests of the Issuer, including Treasury Capital Stock (as defined below), but excluding cash proceeds and the fair market value of marketable securities or other property received from the sale of:
(x) Equity Interests to any future, present or former employees, directors, officers, managers, distributors or consultants (or their respective Controlled Investment Affiliates or Immediate Family Members) of the Issuer, any direct or indirect parent company of the Issuer or any of the Issuer’s Subsidiaries after the Issue Date to the extent such amounts have been applied to Restricted Payments made in accordance with clause (4) of the next succeeding paragraph; and
105
Table of Contents
(y) Designated Preferred Stock;
and (B) to the extent such net cash proceeds are actually contributed to the Issuer, Equity Interests of any direct or indirect parent company of the Issuer (excluding contributions of the proceeds from the sale of Designated Preferred Stock of such company or contributions to the extent such amounts have been applied to Restricted Payments made in accordance with clause (4) of the next succeeding paragraph); or
(ii) debt securities of the Issuer that have been converted into or exchanged for such Equity Interests of the Issuer;
provided that this clause (b) shall not include the proceeds from (W) Refunding Capital Stock, (X) Equity Interests or convertible debt securities of the Issuer sold to a Restricted Subsidiary, (Y) Disqualified Stock or debt securities that have been converted into Disqualified Stock or (Z) Excluded Contributions; plus
(c) 100.0% of the aggregate amount of cash and the fair market value of marketable securities or other property contributed to the capital of the Issuer following the Issue Date (other than net cash proceeds to the extent such net cash proceeds have been used to incur Indebtedness or issue Disqualified Stock or Preferred Stock pursuant to clause (12)(a) of the second paragraph of “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”) (other than by a Restricted Subsidiary and other than any Excluded Contributions); plus
(d) 100.0% of the aggregate amount received in cash and the fair market value of marketable securities or other property received by means of:
(i) the sale or other disposition (other than to the Issuer or a Restricted Subsidiary) of Restricted Investments made by the Issuer or its Restricted Subsidiaries and repurchases and redemptions of such Restricted Investments from the Issuer or its Restricted Subsidiaries (other than by the Issuer or a Restricted Subsidiary) and repayments of loans or advances, and releases of guarantees, which constitute Restricted Investments made by the Issuer or its Restricted Subsidiaries, in each case after the Issue Date; or
(ii) the sale (other than to the Issuer or a Restricted Subsidiary) of the stock of an Unrestricted Subsidiary or a distribution from an Unrestricted Subsidiary (other than in each case to the extent the Investment in such Unrestricted Subsidiary was made by the Issuer or a Restricted Subsidiary pursuant to clause (7) of the next succeeding paragraph or to the extent such Investment constituted a Permitted Investment) or a dividend from an Unrestricted Subsidiary after the Issue Date; plus
(e) in the case of the redesignation of an Unrestricted Subsidiary as a Restricted Subsidiary after the Issue Date, the fair market value of the Investment in such Unrestricted Subsidiary (which, if the fair market value of such Investment shall exceed $125.0 million, shall be determined by the board of directors of the Issuer whose resolution with respect thereto will be delivered to the Trustee) at the time of the redesignation of such Unrestricted Subsidiary as a Restricted Subsidiary, other than to the extent the Investment in such Unrestricted Subsidiary was made by the Issuer or a Restricted Subsidiary pursuant to clause (7) of the next succeeding paragraph or to the extent such Investment constituted a Permitted Investment.
The foregoing provisions will not prohibit:
(1) the payment of any dividend or other distribution or the consummation of any irrevocable redemption within 60 days after the date of declaration of the dividend or other distribution or giving of the redemption notice, as the case may be, if at the date of declaration or notice, the dividend or other distribution or redemption payment would have complied with the provisions of the Indenture;
(2) (a) the redemption, repurchase, retirement or other acquisition of any Equity Interests (“Treasury Capital Stock”) or Subordinated Indebtedness of the Issuer or any Equity Interests of any direct or indirect parent company of the Issuer, in exchange for, or out of the proceeds of the substantially concurrent sale (other than to a Restricted Subsidiary) of, Equity Interests of the Issuer or any direct or indirect parent company of the Issuer to the extent contributed to the Issuer (in each case, other than any Disqualified Stock) (“Refunding Capital Stock”) and (b) if immediately prior to the retirement of Treasury Capital Stock, the declaration and payment of dividend thereon was permitted under clause (6) of this paragraph, the declaration and payment of dividend on the Refunding Capital Stock (other than Refunding Capital Stock the proceeds of which were used to redeem, repurchase, retire or otherwise acquire any Equity Interests of any direct or indirect parent company of the Issuer) in an aggregate amount per year no greater than the aggregate amount of dividends per annum that were declarable and payable on such Treasury Capital Stock immediately prior to such retirement;
(3) the defeasance, redemption, repurchase or other acquisition or retirement of (i) Subordinated Indebtedness of the Issuer or a Guarantor made by exchange for, or out of the proceeds of the substantially concurrent sale of, new Indebtedness of the Issuer or a Guarantor or (ii) Disqualified Stock of the Issuer or a Guarantor made by exchange for, or out of the proceeds of the substantially concurrent sale of, Disqualified Stock of the Issuer or a Guarantor, that, in each case, is incurred in compliance with “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock” so long as:
(a) the principal amount (or accreted value, if applicable) of such new Indebtedness or the liquidation preference of such new Disqualified Stock does not exceed the principal amount of (or accreted value, if applicable), plus any accrued and unpaid interest on, the Subordinated Indebtedness or the liquidation preference of, plus any accrued and unpaid dividends on, the Disqualified Stock being so defeased, redeemed, repurchased, acquired or retired for value, plus the amount of any reasonable premium required to be paid under the terms of the instrument governing the Subordinated Indebtedness or Disqualified Stock being so defeased, redeemed, repurchased, acquired or retired, defeasance costs and any reasonable fees and expenses incurred in connection with the issuance of such new Indebtedness or Disqualified Stock;
106
Table of Contents
(b) such new Indebtedness is subordinated to the Senior Notes or the applicable Guarantee at least to the same extent as such Subordinated Indebtedness so defeased, redeemed, repurchased, acquired or retired;
(c) such new Indebtedness or Disqualified Stock has a final scheduled maturity date equal to or later than the final scheduled maturity date of the Subordinated Indebtedness or Disqualified Stock being so defeased, redeemed, repurchased, acquired or retired; and
(d) such new Indebtedness or Disqualified Stock has a Weighted Average Life to Maturity equal to or greater than the remaining Weighted Average Life to Maturity of the Subordinated Indebtedness or Disqualified Stock being so defeased, redeemed, repurchased, acquired or retired;
(4) a Restricted Payment to pay for the repurchase, retirement or other acquisition or retirement for value of Equity Interests (other than Disqualified Stock) of the Issuer or any direct or indirect parent company of the Issuer held by any future, present or former (A) employee, director, officer, manager or consultant (or their respective Controlled Investment Affiliates or Immediate Family Members) of the Issuer, any of its Subsidiaries or any of its direct or indirect parent companies pursuant to any management equity plan or stock option plan or any other management or employee benefit plan or agreement, or any stock subscription or shareholder agreement, including any Equity Interest rolled over by management of the Issuer or any direct or indirect parent company of the Issuer in connection with the Transactions; provided that the aggregate amount of Restricted Payments made under this clause (A) does not exceed $20.0 million in the first fiscal year following the Issue Date (which amount shall be increased by $5.0 million each fiscal year thereafter and, if applicable, will be increased to $40.0 million following the consummation of an underwritten public Equity Offering) (with unused amounts in any fiscal year being carried over to succeeding fiscal years subject to a maximum (without giving effect to the following proviso) of $30.0 million in any fiscal year (which shall increase to $60.0 million subsequent to the consummation of an underwritten public Equity Offering)); and (B) distributor (or its respective Controlled Investment Affiliates or Immediate Family Members) of the Issuer, any of its Subsidiaries or any of its direct or indirect parent companies pursuant to any distributor equity plan or agreement; provided that the aggregate amount of Restricted Payments made under this clause (B) does not exceed the greater of (i) $100.0 million and (ii) 1.0% of Total Assets; provided, further, that each of the amounts in any fiscal year under (A) and (B) may be increased by an amount not to exceed:
(a) the cash proceeds from the sale of Equity Interests (other than Disqualified Stock) of the Issuer and, to the extent contributed to the Issuer, Equity Interests of any direct or indirect parent company of the Issuer, in each case to any future, present or former employees, directors, officers, managers, distributors or consultants (or their respective Controlled Investment Affiliates or Immediate Family Members) of the Issuer, any of its Subsidiaries or any of its direct or indirect parent companies that occurs after the Issue Date, to the extent the cash proceeds from the sale of such Equity Interests have not otherwise been applied to the payment of Restricted Payments by virtue of clause (3) of the preceding paragraph;plus
(b) the cash proceeds of key man life insurance policies received by the Issuer or its Restricted Subsidiaries after the Issue Date; less
(c) the amount of any Restricted Payments previously made with the cash proceeds described in clauses (a) and (b) of this clause (4);
and provided, further, that cancellation of Indebtedness owing to the Issuer from any future, present or former employees, directors, officers, managers, distributors or consultants of the Issuer (or their respective Controlled Investment Affiliates or Immediate Family Members), any direct or indirect parent company of the Issuer or any of the Issuer’s Restricted Subsidiaries in connection with a repurchase of Equity Interests of the Issuer or any of its direct or indirect parent companies will not be deemed to constitute a Restricted Payment for purposes of this covenant or any other provision of the Indenture;
(5) the declaration and payment of dividends to holders of any class or series of Disqualified Stock of the Issuer or any of its Restricted Subsidiaries or any class or series of Preferred Stock of any Restricted Subsidiary issued in accordance with the covenant described under “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock” to the extent such dividends are included in the definition of “Fixed Charges”;
(6) (a) the declaration and payment of dividends to holders of any class or series of Designated Preferred Stock (other than Disqualified Stock) issued by the Issuer or any of its Restricted Subsidiaries after the Issue Date;
(b) the declaration and payment of dividends to any direct or indirect parent company of the Issuer, the proceeds of which will be used to fund the payment of dividends to holders of any class or series of Designated Preferred Stock (other than Disqualified Stock) issued by such parent company after the Issue Date, provided that the amount of dividends paid pursuant to this clause (b) shall not exceed the aggregate amount of cash actually contributed to the Issuer from the sale of such Designated Preferred Stock; or
(c) the declaration and payment of dividends on Refunding Capital Stock that is Preferred Stock in excess of the dividends declarable and payable thereon pursuant to clause (2) of this paragraph;
provided, in the case of each of (a), (b) and (c) of this clause (6), that for the most recently ended four full fiscal quarters for which internal financial statements are available immediately preceding the date of issuance of such Designated Preferred Stock or the declaration of such dividends on Refunding Capital Stock that is Preferred Stock, after giving effect to such issuance or declaration on a pro forma basis, the Issuer would have had a Fixed Charge Coverage Ratio of at least 2.00 to 1.00;
107
Table of Contents
(7) Investments in Unrestricted Subsidiaries taken together with all other Investments made pursuant to this clause (7) that are at the time outstanding, without giving effect to the sale of an Unrestricted Subsidiary to the extent the proceeds of such sale do not consist of cash or marketable securities, not to exceed greater of (a) $300.0 million and (b) 3.0% of Total Assets;
(8) payments made or expected to be made by the Issuer or any Restricted Subsidiary in respect of withholding or similar taxes payable by any future, present or former employee, director, officer, manager, distributor or consultant (or their respective Controlled Investment Affiliates or Immediate Family Members) and any repurchases of Equity Interests deemed to occur upon exercise of stock options or warrants if such Equity Interests represent a portion of the exercise price of such options or warrants;
(9) the declaration and payment of dividends on the Issuer’s common stock (or the payment of dividends to any direct or indirect parent company of the Issuer to fund a payment of dividends on such company’s common stock), following the first public offering of the Issuer’s common stock or the common stock of any direct or indirect parent company of the Issuer after the Issue Date, of up to 6.0% per annum of the net cash proceeds received by or contributed to the Issuer in or from any such public offering, other than public offerings with respect to the Issuer’s common stock registered on Form S-4 or Form S-8 and other than any public sale constituting an Excluded Contribution;
(10) Restricted Payments that are made with Excluded Contributions;
(11) other Restricted Payments in an aggregate amount taken together with all other Restricted Payments made pursuant to this clause (11) not to exceed the greater of (a) $300.0 million and (b) 2.75% of Total Assets;
(12) distributions or payments of Securitization Fees;
(13) any Restricted Payment made in connection with the Transactions and the fees and expenses related thereto or owed to Affiliates, in each case to the extent permitted by the covenant described under “—Transactions with Affiliates”;
(14) the repurchase, redemption or other acquisition or retirement for value of any Subordinated Indebtedness pursuant to the provisions similar to those described under “Repurchase at the Option of Holders—Change of Control” and “Repurchase at the Option of Holders—Asset Sales”; provided that all Senior Notes validly tendered by Holders in connection with a Change of Control Offer or Asset Sale Offer, as applicable, have been repurchased, redeemed, acquired or retired for value;
(15) the declaration and payment of dividends by the Issuer to, or the making of loans to, any direct or indirect parent company of the Issuer in amounts required for any direct or indirect parent company of the Issuer to pay, in each case without duplication,
(a) franchise and excise taxes and other fees, taxes and expenses required to maintain their corporate existence;
(b) foreign, federal, state and local income taxes, to the extent such income taxes are attributable to the income of the Issuer and its Restricted Subsidiaries and, to the extent of the amount actually received from its Unrestricted Subsidiaries, in amounts required to pay such taxes to the extent attributable to the income of such Unrestricted Subsidiaries; provided that in each case the amount of such payments in any fiscal year does not exceed the amount that the Issuer and its Restricted Subsidiaries would be required to pay in respect of foreign, federal, state and local taxes for such fiscal year were the Issuer, its Restricted Subsidiaries and its Unrestricted Subsidiaries (to the extent described above) to pay such taxes separately from any such parent company;
(c) customary salary, bonus and other benefits payable to employees, directors, officers and managers of any direct or indirect parent company of the Issuer to the extent such salaries, bonuses and other benefits are attributable to the ownership or operation of the Issuer and its Restricted Subsidiaries;
(d) general corporate operating and overhead costs and expenses of any direct or indirect parent company of the Issuer to the extent such costs and expenses are attributable to the ownership or operation of the Issuer and its Restricted Subsidiaries;
(e) fees and expenses other than to Affiliates of the Issuer related to any unsuccessful equity or debt offering of such parent company;
(f) [reserved];
(g) amounts payable pursuant to the Management Fee Agreement, solely to the extent such amounts are not paid directly by the Issuer or its Subsidiaries;
(h) cash payments in lieu of issuing fractional shares in connection with the exercise of warrants, options or other securities convertible into or exchangeable for Equity Interests of the Issuer or any direct or indirect parent company of the Issuer; and
(i) to finance Investments otherwise permitted to be made pursuant to this covenant; provided that (A) such Restricted Payment shall be made substantially concurrently with the closing of such Investment, (B) such direct or indirect parent company shall, immediately following the closing thereof, cause (1) all property acquired (whether assets or Equity Interests) to be contributed to the capital of the Issuer or one of its Restricted Subsidiaries or (2) the merger of the Person formed or acquired into the Issuer or one of its Restricted Subsidiaries (to the extent not prohibited by the covenant “—Merger, Consolidation or Sale of All or Substantially All Assets” below) in order to consummate such Investment, (C) such direct or indirect parent company and its Affiliates (other than the Issuer or a Restricted Subsidiary) receives no consideration or other payment in connection with such transaction except to the extent the Issuer or a Restricted Subsidiary could have given such consideration or made such payment in compliance with the Indenture, (D) any property received by the Issuer shall not increase amounts available for Restricted Payments pursuant to clause (3) of the preceding paragraph and (E) such Investment shall be deemed to be made by the Issuer or such Restricted Subsidiary pursuant to another provision of this covenant (other than pursuant to clause (10) hereof) or pursuant to the definition of “Permitted Investments” (other than clause (9) thereof); and
108
Table of Contents
(16) the distribution, by dividend or otherwise, of shares of Capital Stock of, or Indebtedness owed to the Issuer or a Restricted Subsidiary by, Unrestricted Subsidiaries (other than Unrestricted Subsidiaries, the primary assets of which are Cash Equivalents).
provided that at the time of, and after giving effect to, any Restricted Payment permitted under clauses (11) and (16), no Default shall have occurred and be continuing or would occur as a consequence thereof.
As of the Issue Date, all of the Issuer’s Subsidiaries will be Restricted Subsidiaries. The Issuer will not permit any Unrestricted Subsidiary to become a Restricted Subsidiary except pursuant to the next to the last sentence of the definition of “Unrestricted Subsidiary.” For purposes of designating any Restricted Subsidiary as an Unrestricted Subsidiary, all outstanding Investments by the Issuer and its Restricted Subsidiaries (except to the extent repaid) in the Subsidiary so designated will be deemed to be Restricted Payments in an amount determined as set forth in the penultimate sentence of the definition of “Investments.” Such designation will be permitted only if a Restricted Payment in such amount would be permitted at such time, whether pursuant to the first paragraph of this covenant or under clause (7), (10) or (11) of the second paragraph of this covenant, or pursuant to the definition of “Permitted Investments,” and if such Subsidiary otherwise meets the definition of an Unrestricted Subsidiary. Unrestricted Subsidiaries will not be subject to any of the restrictive covenants set forth in the Indenture.
Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock
The Issuer will not, and will not permit any of its Restricted Subsidiaries to, directly or indirectly, create, incur, issue, assume, guarantee or otherwise become directly or indirectly liable, contingently or otherwise (collectively, “incur” and collectively, an “incurrence”) with respect to any Indebtedness (including Acquired Indebtedness) and the Issuer will not issue any shares of Disqualified Stock and will not permit any Restricted Subsidiary to issue any shares of Disqualified Stock or Preferred Stock; provided that the Issuer may incur Indebtedness (including Acquired Indebtedness) or issue shares of Disqualified Stock, and, subject to the third paragraph of this covenant, any Restricted Subsidiary may incur Indebtedness (including Acquired Indebtedness), issue shares of Disqualified Stock and issue shares of Preferred Stock, if the Fixed Charge Coverage Ratio for the Issuer’s most recently ended four fiscal quarters for which internal financial statements are available immediately preceding the date on which such additional Indebtedness is incurred or such Disqualified Stock or Preferred Stock is issued would have been at least 2.00 to 1.00, determined on a pro forma basis (including a pro forma application of the net proceeds therefrom), as if the additional Indebtedness had been incurred, or the Disqualified Stock or Preferred Stock had been issued, as the case may be, and the application of proceeds therefrom had occurred at the beginning of such four-quarter period.
The foregoing limitations will not apply to:
(1) the incurrence of Indebtedness pursuant to the Senior Credit Facilities by the Issuer or any Restricted Subsidiary and the issuance and creation of letters of credit and bankers’ acceptances thereunder (with letters of credit and bankers’ acceptances being deemed to have a principal amount equal to the face amount thereof) (i) in the case of the CF Credit Facilities, up to the greater of (x) the sum of an aggregate principal amount of $2,340.0 million and an aggregate principal amount of €875.0 million and (y) an aggregate principal amount of Indebtedness outstanding at any one time that does not cause the Senior Secured Leverage Ratio (as defined, together with related definitions, in the CF Credit Facilities in effect on the Issue Date) to exceed 4.50 to 1.00, determined on a pro forma basis (including a pro forma application of the net proceeds therefrom under the CF Credit Facilities) and (ii) in the case of the ABL Facilities, up to an aggregate principal amount of $450.0 million;
(2) the incurrence by the Issuer and any Guarantor of Indebtedness represented by (a) the Senior Notes (including any PIK Notes and any Guarantee) and the exchange notes and related exchange guarantees to be issued in exchange for Senior Notes and the Guarantees pursuant to the Registration Rights Agreement (but excluding any Additional Senior Notes) and (b) the Senior Subordinated Notes (including any guarantee thereof) and the exchange notes and related exchange guarantees to be issued in exchange for the Senior Subordinated Notes and the guarantees thereof pursuant to the Registration Rights Agreement (but excluding any Additional Senior Subordinated Notes (as defined in the indenture governing the Senior Subordinated Notes));
(3) Indebtedness of the Issuer and its Restricted Subsidiaries in existence on the Issue Date (other than Indebtedness described in clauses (1) and (2));
(4) Indebtedness (including Capitalized Lease Obligations) and Disqualified Stock incurred or issued by the Issuer or any Restricted Subsidiary and Preferred Stock issued by any Restricted Subsidiary, to finance the purchase, lease or improvement of property (real or personal) or equipment that is used or useful in a Similar Business, whether through the direct purchase of assets or the Capital Stock of any Person owning such assets in an aggregate principal amount, together with any Refinancing Indebtedness in respect thereof and all other Indebtedness, Disqualified Stock and/or Preferred Stock incurred or issued and outstanding under this clause (4), not to exceed 5.0% of Total Assets (in each case, determined at the date of incurrence) at any time outstanding, so long as such Indebtedness, Disqualified Stock or Preferred Stock is incurred or issued at the date of such purchase, lease or improvement or within 270 days thereafter;
(5) Indebtedness incurred by the Issuer or any of its Restricted Subsidiaries constituting reimbursement obligations with respect to letters of credit issued in the ordinary course of business, including letters of credit in respect of workers’ compensation claims, or other Indebtedness with respect to reimbursement type obligations regarding workers’ compensation claims; provided that upon the drawing of such letters of credit or the incurrence of such Indebtedness, such obligations are reimbursed within 30 days following such drawing or incurrence;
(6) Indebtedness arising from agreements of the Issuer or its Restricted Subsidiaries providing for indemnification, adjustment of purchase price, earnouts or similar obligations, in each case, incurred or assumed in connection with the disposition of any business, assets or a Subsidiary, other than guarantees of Indebtedness incurred by any Person acquiring all or any portion of such business, assets or a Subsidiary for the purpose of financing such acquisition; provided that such Indebtedness is not reflected on the balance sheet of the Issuer, or any of its Restricted Subsidiaries (contingent obligations referred to in a footnote to financial statements and not otherwise reflected on the balance sheet will not be deemed to be reflected on such balance sheet for purposes of this clause (6));
109
Table of Contents
(7) Indebtedness of the Issuer to a Restricted Subsidiary; provided that any such Indebtedness owing to a Restricted Subsidiary that is not a Guarantor is expressly subordinated in right of payment to the Senior Notes; provided, further, that any subsequent issuance or transfer of any Capital Stock or any other event which results in any such Restricted Subsidiary ceasing to be a Restricted Subsidiary or any other subsequent transfer of any such Indebtedness (except to the Issuer or another Restricted Subsidiary) shall be deemed, in each case, to be an incurrence of such Indebtedness not permitted by this clause;
(8) Indebtedness of a Restricted Subsidiary to the Issuer or another Restricted Subsidiary; provided that if a Guarantor incurs such Indebtedness to a Restricted Subsidiary that is not a Guarantor, such Indebtedness is expressly subordinated in right of payment to the Guarantee of the Senior Notes of such Guarantor; provided, further, that any subsequent transfer of any such Indebtedness (except to the Issuer or another Restricted Subsidiary) shall be deemed, in each case, to be an incurrence of such Indebtedness not permitted by this clause;
(9) shares of Preferred Stock of a Restricted Subsidiary issued to the Issuer or another Restricted Subsidiary; provided that any subsequent issuance or transfer of any Capital Stock or any other event which results in any such Restricted Subsidiary ceasing to be a Restricted Subsidiary or any other subsequent transfer of any such shares of Preferred Stock (except to the Issuer or another of its Restricted Subsidiaries) shall be deemed, in each case, to be an issuance of such shares of Preferred Stock not permitted by this clause;
(10) Hedging Obligations (excluding Hedging Obligations entered into for speculative purposes) for the purpose of limiting interest rate risk with respect to any Indebtedness permitted to be incurred under the Indenture, exchange rate risk or commodity pricing risk;
(11) obligations in respect of self-insurance and obligations in respect of performance, bid, appeal and surety bonds and completion guarantees and similar obligations provided by the Issuer or any of its Restricted Subsidiaries in the ordinary course of business;
(12) (a) Indebtedness or Disqualified Stock of the Issuer and Indebtedness, Disqualified Stock or Preferred Stock of the Issuer or any Restricted Subsidiary in an aggregate principal amount or liquidation preference up to 100.0% of the net cash proceeds received by the Issuer since immediately after the Issue Date from the issue or sale of Equity Interests of the Issuer or cash contributed to the capital of the Issuer (in each case, other than proceeds of Disqualified Stock or sales of Equity Interests to the Issuer or any of its Subsidiaries) as determined in accordance with clauses (3)(b) and (3)(c) of the first paragraph of “—Limitation on Restricted Payments” to the extent such net cash proceeds or cash have not been applied pursuant to such clauses to make Restricted Payments or to make other Investments, payments or exchanges pursuant to the second paragraph of “—Limitation on Restricted Payments” or to make Permitted Investments (other than Permitted Investments specified in clause (1) or (3) of the definition thereof) and (b) Indebtedness or Disqualified Stock of the Issuer and Indebtedness, Disqualified Stock or Preferred Stock of the Issuer or, subject to the third paragraph of this covenant, any Restricted Subsidiary not otherwise permitted hereunder in an aggregate principal amount or liquidation preference which, when aggregated with the principal amount and liquidation preference of all other Indebtedness, Disqualified Stock and Preferred Stock then outstanding and incurred pursuant to this clause (12)(b), does not at any one time outstanding exceed the greater of (x) $550.0 million and (y) 5.0% of Total Assets (it being understood that any Indebtedness, Disqualified Stock or Preferred Stock incurred pursuant to this clause (12)(b) shall cease to be deemed incurred or outstanding for purposes of this clause (12)(b) but shall be deemed incurred for the purposes of the first paragraph of this covenant from and after the first date on which the Issuer or such Restricted Subsidiary could have incurred such Indebtedness, Disqualified Stock or Preferred Stock under the first paragraph of this covenant without reliance on this clause (12)(b)); (13) the incurrence by the Issuer or any Restricted Subsidiary of Indebtedness, the issuance by the Issuer or any Restricted Subsidiary of Disqualified Stock or the issuance by any Restricted Subsidiary of Preferred Stock which serves to extend, replace, refund, refinance, renew or defease any Indebtedness incurred or Disqualified Stock or Preferred Stock issued as permitted under the first paragraph of this covenant and clauses (2), (3), (4) and (12)(a) above, this clause (13) and clauses (14) and (24) below or any Indebtedness incurred or Disqualified Stock or Preferred Stock issued to so extend, replace, refund, refinance, renew or defease such Indebtedness, Disqualified Stock or Preferred Stock including additional Indebtedness, Disqualified Stock or Preferred Stock incurred to pay premiums (including reasonable tender premiums), defeasance costs and fees in connection therewith (the “Refinancing Indebtedness”) prior to its respective maturity; provided that such Refinancing Indebtedness:
(a) has a Weighted Average Life to Maturity at the time such Refinancing Indebtedness is incurred which is not less than the remaining Weighted Average Life to Maturity of, the Indebtedness, Disqualified Stock or Preferred Stock being extended, replaced, refunded, refinanced, renewed or defeased;
(b) to the extent such Refinancing Indebtedness extends, replaces, refunds, refinances, renews or defeases (i) Indebtedness subordinated to the Senior Notes or any Guarantee thereof, such Refinancing Indebtedness is subordinated to the Senior Notes or the Guarantee thereof at least to the same extent as the Indebtedness being extended, replaced, refunded, refinanced, renewed or defeased or (ii) Disqualified Stock or Preferred Stock, such Refinancing Indebtedness must be Disqualified Stock or Preferred Stock, respectively; and
(c) shall not include:
(i) Indebtedness, Disqualified Stock or Preferred Stock of a Subsidiary of the Issuer that is not a Guarantor that refinances Indebtedness or Disqualified Stock of the Issuer;
(ii) Indebtedness, Disqualified Stock or Preferred Stock of a Subsidiary of the Issuer that is not a Guarantor that refinances Indebtedness, Disqualified Stock or Preferred Stock of a Guarantor; or
(iii) Indebtedness or Disqualified Stock of the Issuer or Indebtedness, Disqualified Stock or Preferred Stock of a Restricted Subsidiary that refinances Indebtedness, Disqualified Stock or Preferred Stock of an Unrestricted Subsidiary;
and, provided, further, that subclause (a) of this clause (13) will not apply to any extension, replacement, refunding, refinancing, renewal or defeasance of any Indebtedness outstanding under a Credit Facility and Obligations secured by Permitted Liens.
110
Table of Contents
(14) (a) Indebtedness or Disqualified Stock of the Issuer or, subject to the third paragraph of this covenant, Indebtedness, Disqualified Stock or Preferred Stock of a Restricted Subsidiary incurred or issued to finance an acquisition or (b) Indebtedness, Disqualified Stock or Preferred Stock of Persons that are acquired by the Issuer or any Restricted Subsidiary or merged into the Issuer or a Restricted Subsidiary in accordance with the terms of the Indenture; provided that after giving effect to such acquisition or merger, either
(i) the Issuer would be permitted to incur at least $1.00 of additional Indebtedness pursuant to the Fixed Charge Coverage Test, or
(ii) the Fixed Charge Coverage Ratio for the Issuer is equal to or greater than immediately prior to such acquisition or merger;
(15) Indebtedness arising from the honoring by a bank or other financial institution of a check, draft or similar instrument drawn against insufficient funds in the ordinary course of business, provided that such Indebtedness is extinguished within five Business Days of its incurrence;
(16) Indebtedness of the Issuer or any of its Restricted Subsidiaries supported by a letter of credit issued pursuant to the Credit Facilities, in a principal amount not in excess of the stated amount of such letter of credit;
(17) (a) any guarantee by the Issuer or a Restricted Subsidiary of Indebtedness or other obligations of any Restricted Subsidiary so long as the incurrence of such Indebtedness incurred by such Restricted Subsidiary is permitted under the terms of the Indenture, or (b) any guarantee by a Restricted Subsidiary of Indebtedness of the Issuer; provided that such guarantee is incurred in accordance with the covenant described below under “—Limitation on Guarantees of Indebtedness by Restricted Subsidiaries”;
(18) Indebtedness consisting of Indebtedness issued by the Issuer or any of its Restricted Subsidiaries to future, present or former employees, directors, officers, managers, distributors and consultants thereof, their respective Controlled Investment Affiliates or Immediate Family Members, in each case to finance the purchase or redemption of Equity Interests of the Issuer or any direct or indirect parent company of the Issuer to the extent described in clause (4) of the second paragraph under “—Limitation on Restricted Payments”;
(19) customer deposits and advance payments received in the ordinary course of business from customers for goods purchased in the ordinary course of business;
(20) Indebtedness owed on a short-term basis of no longer than 30 days to banks and other financial institutions incurred in the ordinary course of business of the Issuer and its Restricted Subsidiaries with such banks or financial institutions that arises in connection with ordinary banking arrangements to manage cash balances of the Issuer and its Restricted Subsidiaries;
(21) Indebtedness incurred by a Restricted Subsidiary in connection with bankers’ acceptances, discounted bills of exchange or the discounting or factoring of receivables for credit management purposes, in each case incurred or undertaken in the ordinary course of business on arm’s length commercial terms on a recourse basis;
(22) Indebtedness of the Issuer or any of its Restricted Subsidiaries consisting of (a) the financing of insurance premiums or (b) take-or-pay obligations contained in supply arrangements in each case, incurred in the ordinary course of business;
(23) (a) the incurrence of Indebtedness by a Foreign Subsidiary pursuant to (i) the European line of credit in existence on the Issue Date up to an aggregate principal amount of €100.0 million outstanding at any one time and (ii) the Japanese line of credit in existence on the Issue Date up to an aggregate principal amount of ¥4.5 billion outstanding at any one time and the issuance and creation of letters of credit and bankers’ acceptances thereunder (with letters of credit and bankers’ acceptances being deemed to have a principal amount equal to the face amount thereof), and (b) the incurrence of Indebtedness of Foreign Subsidiaries of the Issuer or a Restricted Subsidiary of the Issuer other than Indebtedness described in clause (23)(a) in an amount not to exceed at any one time outstanding and together with any other Indebtedness incurred under this clause (23)(b) the greater of (i) $100.0 million and (ii) 5.0% of the Foreign Subsidiary Total Assets (it being understood that any Indebtedness incurred pursuant to this clause (23) shall cease to be deemed incurred or outstanding for the purpose of this clause (23) but shall be deemed incurred for the purposes of the first paragraph of this covenant from and after the first date on which the Issuer or such Restricted Subsidiaries could have incurred such Indebtedness under the first paragraph of this covenant without reliance on this clause (23));
(24) Indebtedness, Disqualified Stock or Preferred Stock of a Restricted Subsidiary incurred to finance or assumed in connection with an acquisition in a principal amount not to exceed $100.0 million in the aggregate at any one time outstanding together with all other Indebtedness, Disqualified Stock and/or Preferred Stock issued under this clause (24) (it being understood that any Indebtedness, Disqualified Stock or Preferred Stock incurred pursuant to this clause (24) shall cease to be deemed incurred or outstanding for purposes of this clause (24) but shall be deemed incurred for the purposes of the first paragraph of this covenant from and after the first date on which such Restricted Subsidiary could have incurred such Indebtedness, Disqualified Stock or Preferred Stock under the first paragraph of this covenant without reliance on this clause (24)); and
(25) Indebtedness of the Issuer or any of its Restricted Subsidiaries undertaken in connection with cash management and related activities with respect to any Subsidiary or joint venture in the ordinary course of business.
Restricted Subsidiaries of the Issuer that are not Guarantors may not incur Indebtedness or Disqualified Stock or Preferred Stock under the first paragraph of this covenant or clause 12(b), 14(x) or (24) of the second paragraph of this covenant if, after giving pro forma effect to such incurrence or issuance (including a pro forma application of the net proceeds therefrom), the aggregate amount of Indebtedness and Disqualified Stock and Preferred Stock of Restricted Subsidiaries that are not Guarantors incurred or issued pursuant to the first paragraph of this covenant and clauses 12(b), 14(x) and (24) of the second paragraph of this covenant, collectively, would exceed $600.0 million.
111
Table of Contents
For purposes of determining compliance with this covenant:
(1) in the event that an item of Indebtedness, Disqualified Stock or Preferred Stock (or any portion thereof) meets the criteria of more than one of the categories of Permitted Indebtedness, Disqualified Stock or Preferred Stock described in clauses (1) through (25) above or is entitled to be incurred pursuant to the first paragraph of this covenant, the Issuer, in its sole discretion, will classify or reclassify such item of Indebtedness, Disqualified Stock or Preferred Stock (or any portion thereof) and will only be required to include the amount and type of such Indebtedness, Disqualified Stock or Preferred Stock in one of the above clauses or under the first paragraph of this covenant; provided that all Indebtedness outstanding under the CF Credit Facilities on the Issue Date will be treated as incurred on the Issue Date under clause (1) of the second paragraph above; and
(2) at the time of incurrence, the Issuer will be entitled to divide and classify an item of Indebtedness in more than one of the types of Indebtedness described in the first and second paragraphs above.
Accrual of interest or dividends, the accretion of accreted value, the accretion or amortization of original issue discount and the payment of interest or dividends in the form of additional Indebtedness, Disqualified Stock or Preferred Stock, as the case may be, of the same class will not be deemed to be an incurrence of Indebtedness, Disqualified Stock or Preferred Stock for purposes of this covenant.
For purposes of determining compliance with any U.S. dollar-denominated restriction on the incurrence of Indebtedness, the U.S. dollar-equivalent principal amount of Indebtedness denominated in a foreign currency shall be calculated based on the relevant currency exchange rate in effect on the date such Indebtedness was incurred, in the case of term debt, or first committed, in the case of revolving credit debt; provided that if such Indebtedness is incurred to refinance other Indebtedness denominated in a foreign currency, and such refinancing would cause the applicable U.S. dollar-denominated restriction to be exceeded if calculated at the relevant currency exchange rate in effect on the date of such refinancing, such U.S. dollar-denominated restriction shall be deemed not to have been exceeded so long as the principal amount of such refinancing Indebtedness does not exceed the principal amount of such Indebtedness being refinanced.
The principal amount of any Indebtedness incurred to refinance other Indebtedness, if incurred in a different currency from the Indebtedness being refinanced, shall be calculated based on the currency exchange rate applicable to the currencies in which such respective Indebtedness is denominated that is in effect on the date of such refinancing.
The Indenture provides that the Issuer will not, and will not permit any Guarantor to, directly or indirectly, incur any Indebtedness (including Acquired Indebtedness) that is subordinated or junior in right of payment to any Indebtedness of the Issuer or such Guarantor, as the case may be, unless such Indebtedness is expressly subordinated in right of payment to the Senior Notes or such Guarantor’s Guarantee to the extent and in the same manner as such Indebtedness is subordinated to other Indebtedness of the Issuer or such Guarantor, as the case may be.
The Indenture does not treat (1) unsecured Indebtedness as subordinated or junior to Secured Indebtedness merely because it is unsecured or (2) Senior Indebtedness as subordinated or junior to any other Senior Indebtedness merely because it has a junior priority with respect to the same collateral.
Liens
The Issuer will not, and will not permit any Guarantor to, directly or indirectly, create, incur, assume or suffer to exist any Lien (except Permitted Liens) that secures Obligations under any Indebtedness or any related Guarantee, on any asset or property of the Issuer or any Guarantor, or any income or profits therefrom, or assign or convey any right to receive income therefrom, unless:
(1) in the case of Liens securing Subordinated Indebtedness, the Senior Notes and related Guarantees are secured by a Lien on such property, assets or proceeds that is senior in priority to such Liens; and
(2) in all other cases, the Senior Notes or the Guarantees are equally and ratably secured,
except that the foregoing shall not apply to (a) Liens securing the Senior Notes and the related Guarantees, (b) Liens securing Indebtedness permitted to be incurred under Credit Facilities, including any letter of credit facility relating thereto, that was permitted by the terms of the Indenture to be incurred pursuant to clause (1) of the second paragraph under “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock” and (c) Liens securing Indebtedness permitted to be incurred under the covenant described above under “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”; provided that, with respect to Liens securing Indebtedness permitted under this subclause (c), at the time of incurrence and after giving pro forma effect thereto, the Senior Secured Leverage Ratio (as defined, together with related definitions, in the CF Credit Facilities in effect on the Issue Date) would be no greater than 4.50 to 1.00.
Merger, Consolidation or Sale of All or Substantially All Assets
The Issuer may not consolidate or merge with or into or wind up into (whether or not the Issuer is the surviving Person), or sell, assign, transfer, lease, convey or otherwise dispose of all or substantially all of its properties or assets, in one or more related transactions, to any Person unless:
(1) the Issuer is the surviving Person or the Person formed by or surviving any such consolidation or merger (if other than the Issuer) or to which such sale, assignment, transfer, lease, conveyance or other disposition will have been made, is a Person organized or existing under the laws of the United States, any state thereof, the District of Columbia, or any territory thereof (such Person, as the case may be, being herein called the “ Successor Company”); provided that in the case where the surviving Person is not a corporation, a co-obligor of the Senior Notes is a corporation;
112
Table of Contents
(2) the Successor Company, if other than the Issuer, expressly assumes all the obligations of the Issuer under the Senior Notes pursuant to supplemental indentures or other documents or instruments;
(3) immediately after such transaction, no Default exists;
(4) immediately after giving pro forma effect to such transaction and any related financing transactions, as if such transactions had occurred at the beginning of the applicable four-quarter period,
(a) the Successor Company or the Issuer would be permitted to incur at least $1.00 of additional Indebtedness pursuant to the Fixed Charge Coverage Test, or
(b) the Fixed Charge Coverage Ratio for the Issuer would be equal to or greater than the Fixed Charge Coverage Ratio for the Issuer immediately prior to such transaction;
(5) each Guarantor, unless it is the other party to the transactions described above, in which case clause (1)(b) of the second succeeding paragraph shall apply, shall have by supplemental indenture confirmed that its Guarantee shall apply to such Person’s obligations under the Indenture, the Senior Notes and the Registration Rights Agreement; and
(6) the Issuer shall have delivered to the Trustee an Officer’s Certificate and an Opinion of Counsel, each stating that such consolidation, merger or transfer and such supplemental indentures, if any, comply with the Indenture.
The Successor Company will succeed to, and be substituted for the Issuer under the Indenture, the Guarantees and the Senior Notes, as applicable. Notwithstanding the immediately preceding clauses (3) and (4),
(1) any Restricted Subsidiary may consolidate with or merge into or transfer all or part of its properties and assets to the Issuer, and
(2) the Issuer may merge with an Affiliate of the Issuer solely for the purpose of reincorporating the Issuer in the United States, the District of Columbia or any territory thereof so long as the amount of Indebtedness of the Issuer and its Restricted Subsidiaries is not increased thereby.
Subject to certain limitations described in the Indenture governing release of a Guarantee upon the sale, disposition or transfer of a Guarantor, no Guarantor will, and the Issuer will not permit any Guarantor to, consolidate or merge with or into or wind up into (whether or not such Guarantor is the surviving Person), or sell, assign, transfer, lease, convey or otherwise dispose of all or substantially all of its properties or assets, in one or more related transactions, to any Person unless:
(1) (a) such Guarantor is the surviving Person or the Person formed by or surviving any such consolidation or merger (if other than such Guarantor) or to which such sale, assignment, transfer, lease, conveyance or other disposition will have been made is a Person organized or existing under the laws of the jurisdiction of organization of such Guarantor, as applicable, or the laws of the United States, any state thereof, the District of Columbia, or any territory thereof (such Person being herein called the “Successor Person”);
(b) the Successor Person, if other than such Guarantor, expressly assumes all the obligations of such Guarantor under the Indenture and such Guarantor’s related Guarantee pursuant to supplemental indentures or other documents or instruments;
(c) immediately after such transaction, no Default exists; and
(d) the Issuer shall have delivered to the Trustee an Officer’s Certificate and an Opinion of Counsel, each stating that such consolidation, merger or transfer and such supplemental indentures, if any, comply with the Indenture; or
(2) the transaction is made in compliance with the covenant described under “Repurchase at the Option of Holders—Asset Sales.”
Subject to certain limitations described in the Indenture, the Successor Person will succeed to, and be substituted for, such Guarantor under the Indenture and such Guarantor’s Guarantee. Notwithstanding the foregoing, any Guarantor may (1) merge into or transfer all or part of its properties and assets to another Guarantor or the Issuer, (2) merge with an Affiliate of the Issuer solely for the purpose of reincorporating the Guarantor in the United States, any state thereof, the District of Columbia or any territory thereof or (3) convert into a corporation, partnership, limited partnership, limited liability corporation or trust organized or existing under the laws of the jurisdiction of organization of such Guarantor.
Transactions with Affiliates
The Issuer will not, and will not permit any of its Restricted Subsidiaries to, make any payment to, or sell, lease, transfer or otherwise dispose of any of its properties or assets to, or purchase any property or assets from, or enter into or make or amend any transaction, contract, agreement, understanding, loan, advance or guarantee with, or for the benefit of, any Affiliate of the Issuer (each of the foregoing, an “Affiliate Transaction”) involving aggregate payments or consideration in excess of $25.0 million, unless:
(1) such Affiliate Transaction is on terms that are not materially less favorable to the Issuer or its relevant Restricted Subsidiary than those that would have been obtained in a comparable transaction by the Issuer or such Restricted Subsidiary with an unrelated Person on an arm’s-length basis; and
(2) the Issuer delivers to the Trustee with respect to any Affiliate Transaction or series of related Affiliate Transactions involving aggregate payments or consideration in excess of $50.0 million, a resolution adopted by the majority of the board of directors of the Issuer approving such Affiliate Transaction and set forth in an Officer’s Certificate certifying that such Affiliate Transaction complies with clause (1) above.
113
Table of Contents
The foregoing provisions do not apply to the following:
(1) transactions between or among the Issuer or any of its Restricted Subsidiaries;
(2) Restricted Payments permitted by the provisions of the Indenture described above under the covenant “—Limitation on Restricted Payments” and the definition of “Permitted Investments”;
(3) the payment of management, consulting, monitoring, advisory and other fees and related expenses pursuant to the Management Fee Agreement (plus any unpaid management, consulting, monitoring, advisory and other fees and related expenses accrued in any prior year) and the termination fees pursuant to the Management Fee Agreement, or any amendment thereto so long as any such amendment is not disadvantageous in the good faith judgment of the board of directors of the Issuer to the Holders when taken as a whole, as compared to the Management Fee Agreement as in effect on the Issue Date;
(4) the payment of reasonable and customary fees paid to, and indemnities provided for the benefit of, current or former employees, directors, officers, managers, distributors or consultants of the Issuer, any of its direct or indirect parent companies or any of its Restricted Subsidiaries;
(5) transactions in which the Issuer or any of its Restricted Subsidiaries, as the case may be, delivers to the Trustee a letter from an Independent Financial Advisor stating that such transaction is fair to the Issuer or such Restricted Subsidiary from a financial point of view or stating that the terms are not materially less favorable to the Issuer or its relevant Restricted Subsidiary than those that would have been obtained in a comparable transaction by the Issuer or such Restricted Subsidiary with an unrelated Person on an arm’s-length basis;
(6) any agreement as in effect as of the Issue Date, or any amendment thereto (so long as any such amendment is not disadvantageous in the good faith judgment of the board of directors of the Issuer to the Holders when taken as a whole as compared to the applicable agreement as in effect on the Issue Date);
(7) the existence of, or the performance by the Issuer or any of its Restricted Subsidiaries of its obligations under the terms of, any shareholders agreement (including any registration rights agreement or purchase agreement related thereto) to which it is a party as of the Issue Date and any similar agreements which it may enter into thereafter; provided that the existence of, or the performance by the Issuer or any of its Restricted Subsidiaries of obligations under any future amendment to any such existing agreement or under any similar agreement entered into after the Issue Date shall only be permitted by this clause (7) to the extent that the terms of any such amendment or new agreement are not otherwise disadvantageous in the good faith judgment of the board of directors of the Issuer to the Holders when taken as a whole;
(8) the Transactions and the payment of all fees and expenses related to the Transactions, in each case as contemplated by this prospectus;
(9) transactions with customers, clients, suppliers, contractors, joint venture partners or purchasers or sellers of goods or services that are Affiliates, in each case in the ordinary course of business and otherwise in compliance with the terms of the Indenture which are fair to the Issuer and its Restricted Subsidiaries, in the reasonable determination of the board of directors of the Issuer or the senior management thereof, or are on terms at least as favorable as might reasonably have been obtained at such time from an unaffiliated party;
(10) the issuance of Equity Interests (other than Disqualified Stock) of the Issuer to any Permitted Holder or to any employee, director, officer, manager, distributor or consultant (or their respective Controlled Investment Affiliates or Immediate Family Members) of the Issuer, any of its direct or indirect parent companies or any of its Restricted Subsidiaries;
(11) sales of accounts receivable, or participations therein, or Securitization Assets or related assets in connection with the ABL Facilities or any Qualified Securitization Facility;
(12) payments by the Issuer or any of its Restricted Subsidiaries to any of the Investors made for any financial advisory, financing, underwriting or placement services or in respect of other investment banking activities, including, without limitation, in connection with acquisitions or divestitures which payments are approved by a majority of the board of directors of the Issuer in good faith;
(13) payments and Indebtedness and Disqualified Stock (and cancellation of any thereof) of the Issuer and its Restricted Subsidiaries and Preferred Stock (and cancellation of any thereof) of any Restricted Subsidiary to any future, current or former employee, director, officer, manager, distributor or consultant (or their respective Controlled Investment Affiliates or Immediate Family Members) of the Issuer, any of its Subsidiaries or any of its direct or indirect parent companies pursuant to any management equity plan or stock option plan or any other management or employee benefit plan or agreement or any stock subscription or shareholder agreement or any distributor equity plan or agreement; and any employment agreements, stock option plans and other compensatory arrangements (and any successor plans thereto) and any supplemental executive retirement benefit plans or arrangements with any such employees, directors, officers, managers, distributors or consultants (or their respective Controlled Investment Affiliates or Immediate Family Members) that are, in each case, approved by the Issuer in good faith;
(14) investments by any of the Investors in securities of the Issuer or any of its Restricted Subsidiaries (and payment of reasonable out-of-pocket expenses incurred by such Investors in connection therewith) so long as (a) the investment is being offered generally to other investors on the same or more favorable terms and (b) the investment constitutes less than 5.0% of the proposed or outstanding issue amount of such class of securities;
114
Table of Contents
(15) payments to or from, and transactions with, any joint venture in the ordinary course of business (including, without limitation, any cash management activities related thereto);
(16) payments by the Issuer (and any direct or indirect parent company thereof) and its Subsidiaries pursuant to tax sharing agreements among the Issuer (and any such parent company) and its Subsidiaries; provided that in each case the amount of such payments in any fiscal year does not exceed the amount that the Issuer, its Restricted Subsidiaries and its Unrestricted Subsidiaries (to the extent of amount received from Unrestricted Subsidiaries) would be required to pay in respect of foreign, federal, state and local taxes for such fiscal year were the Issuer, its Restricted Subsidiaries and its Unrestricted Subsidiaries (to the extent described above) to pay such taxes separately from any such parent entity;
(17) any lease entered into between the Issuer or any Restricted Subsidiary, as lessee and any Affiliate of the Issuer, as lessor, which is approved by a majority of the disinterested members of the board of directors of the Issuer in good faith; and
(18) intellectual property licenses in the ordinary course of business.
Dividend and Other Payment Restrictions Affecting Restricted Subsidiaries
The Issuer will not, and will not permit any of its Restricted Subsidiaries that is not a Guarantor to, directly or indirectly, create or otherwise cause or suffer to exist or become effective any consensual encumbrance or consensual restriction on the ability of any such Restricted Subsidiary to:
(1) (a) pay dividends or make any other distributions to the Issuer or any of its Restricted Subsidiaries on its Capital Stock or with respect to any other interest or participation in, or measured by, its profits, or
(b) pay any Indebtedness owed to the Issuer or any of its Restricted Subsidiaries;
(2) make loans or advances to the Issuer or any of its Restricted Subsidiaries; or
(3) sell, lease or transfer any of its properties or assets to the Issuer or any of its Restricted Subsidiaries, except (in each case) for such encumbrances or restrictions existing under or by reason of:
(a) contractual encumbrances or restrictions in effect on the Issue Date, including pursuant to the Senior Credit Facilities and the related documentation, Hedging Obligations and the indenture governing the Senior Subordinated Notes and the related documentation;
(b) the Indenture, the Senior Notes and the guarantees thereof;
(c) purchase money obligations for property acquired in the ordinary course of business and Capital Lease Obligations that impose restrictions of the nature discussed in clause (3) above on the property so acquired;
(d) applicable law or any applicable rule, regulation or order;
(e) any agreement or other instrument of a Person acquired by the Issuer or any of its Restricted Subsidiaries in existence at the time of such acquisition or at the time it merges with or into the Issuer or any of its Restricted Subsidiaries or assumed in connection with the acquisition of assets from such Person (but, in any such case, not created in contemplation thereof), which encumbrance or restriction is not applicable to any Person, or the properties or assets of any Person, other than the Person so acquired and its Subsidiaries, or the property or assets of the Person so acquired and its Subsidiaries;
(f) contracts for the sale of assets, including customary restrictions with respect to a Subsidiary of the Issuer pursuant to an agreement that has been entered into for the sale or disposition of all or substantially all of the Capital Stock or assets of such Subsidiary;
(g) Secured Indebtedness otherwise permitted to be incurred pursuant to the covenants described under “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock” and “—Liens” that limit the right of the debtor to dispose of the assets securing such Indebtedness;
(h) restrictions on cash or other deposits or net worth imposed by customers under contracts entered into in the ordinary course of business;
(i) other Indebtedness, Disqualified Stock or Preferred Stock of Foreign Subsidiaries permitted to be incurred subsequent to the Issue Date pursuant to the provisions of the covenant described under “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”;
(j) customary provisions in joint venture agreements and other similar agreements relating solely to such joint venture;
(k) customary provisions contained in leases, licenses or similar agreements, including with respect to intellectual property and other agreements, in each case, entered into in the ordinary course of business;
(l) restrictions created in connection with any Qualified Securitization Facility that, in the good faith determination of the Issuer are necessary or advisable to effect such Qualified Securitization Facility;
115
Table of Contents
(m) restrictions or conditions contained in any trading, netting, operating, construction, service, supply, purchase, sale or other agreement to which the Issuer or any of its Restricted Subsidiaries is a party entered into in the ordinary course of business; provided that such agreement prohibits the encumbrance of solely the property or assets of the Issuer or such Restricted Subsidiary that are the subject to such agreement, the payment rights arising thereunder or the proceeds thereof and does not extend to any other asset or property of the Issuer or such Restricted Subsidiary or the assets or property of another Restricted Subsidiary; and
(n) any encumbrances or restrictions of the type referred to in clauses (1), (2) and (3) above imposed by any amendments, modifications, restatements, renewals, increases, supplements, refundings, replacements or refinancings of the contracts, instruments or obligations referred to in clauses (a) through (m) above; provided that such amendments, modifications, restatements, renewals, increases, supplements, refundings, replacements or refinancings are, in the good faith judgment of the Issuer, no more restrictive with respect to such encumbrance and other restrictions taken as a whole than those prior to such amendment, modification, restatement, renewal, increase, supplement, refunding, replacement or refinancing.
Limitation on Guarantees of Indebtedness by Restricted Subsidiaries
The Issuer will not permit any of its Wholly-Owned Subsidiaries that are Restricted Subsidiaries (and non-Wholly-Owned Subsidiaries if such non-Wholly-Owned Subsidiaries guarantee other capital markets debt securities of the Issuer or any Guarantor), other than a Guarantor, a Foreign Subsidiary or a Securitization Subsidiary, to guarantee the payment of any Indebtedness of the Issuer or any other Guarantor unless:
(1) such Restricted Subsidiary within 30 days executes and delivers a supplemental indenture to the Indenture providing for a Guarantee by such Restricted Subsidiary, except that with respect to a guarantee of Indebtedness of the Issuer or any Guarantor, if such Indebtedness is by its express terms subordinated in right of payment to the Senior Notes or such Guarantor’s Guarantee, any such guarantee by such Restricted Subsidiary with respect to such Indebtedness shall be subordinated in right of payment to such Guarantee substantially to the same extent as such Indebtedness is subordinated to the Senior Notes; and
(2) such Restricted Subsidiary waives and will not in any manner whatsoever claim or take the benefit or advantage of, any rights of reimbursement, indemnity or subrogation or any other rights against the Issuer or any other Restricted Subsidiary as a result of any payment by such Restricted Subsidiary under its Guarantee;
provided that this covenant shall not be applicable to (i) any guarantee of any Restricted Subsidiary that existed at the time such Person became a Restricted Subsidiary and was not incurred in connection with, or in contemplation of, such Person becoming a Restricted Subsidiary and (ii) guarantees of the ABL Facilities by the ABL Financing Entities or of any Qualified Securitization Facility by any Restricted Subsidiary.
Reports and Other Information
Notwithstanding that the Issuer may not be subject to the reporting requirements of Section 13 or 15(d) of the Exchange Act or otherwise report on an annual and quarterly basis on forms provided for such annual and quarterly reporting pursuant to rules and regulations promulgated by the SEC, the Indenture requires the Issuer to file with the SEC (and make available to the Trustee and Holders of the Senior Notes (without exhibits), without cost to any Holder, within 15 days after it files them with the SEC) from and after the Issue Date,
(1) within 90 days (or any other time period then in effect under the rules and regulations of the Exchange Act with respect to the filing of a Form 10-K by a non-accelerated filer) after the end of each fiscal year, annual reports on Form 10-K, or any successor or comparable form, containing the information required to be contained therein, or required in such successor or comparable form;
(2) within 45 days (or any other time period then in effect under the rules and regulations of the Exchange Act with respect to the filing of a Form 10-Q by a non-accelerated filer) after the end of each of the first three fiscal quarters of each fiscal year (commencing with the fiscal quarter ending August 31, 2007), reports on Form 10-Q containing all quarterly information that would be required to be contained in Form 10-Q, or any successor or comparable form;
(3) promptly from time to time after the occurrence of an event required to be therein reported, such other reports on Form 8-K, or any successor or comparable form; and
(4) any other information, documents and other reports which the Issuer would be required to file with the SEC if it were subject to Section 13 or 15(d) of the Exchange Act;
in each case, in a manner that complies in all material respects with the requirements specified in such form; provided that the Issuer shall not be so obligated to file such reports with the SEC if the SEC does not permit such filing, in which event the Issuer will make available such information to prospective purchasers of Senior Notes, in addition to providing such information to the Trustee and the Holders of the Senior Notes, in each case within 15 days after the time the Issuer would be required to file such information with the SEC, if it were subject to Sections 13 or 15(d) of the Exchange Act. In addition, to the extent not satisfied by the foregoing, the Issuer will agree that, for so long as any Senior Notes are outstanding, it will furnish to Holders and to securities analysts and prospective investors, upon their request, the information required to be delivered pursuant to Rule 144A(d)(4) under the Securities Act; provided, further, that any report required to be delivered under clause (2) above prior to the first date of delivery of report pursuant to clause (1) following the Issue Date shall not be required to contain all purchase accounting adjustments relating to the Transactions to the extent it is not practicable to include any such adjustments in such report.
In the event that any direct or indirect parent company of the Issuer becomes a guarantor of the Senior Notes, the Indenture permits the Issuer to satisfy its obligations in this covenant with respect to financial information relating to the Issuer by furnishing financial information relating to such parent; provided that the same is accompanied by consolidating information that explains in reasonable detail the differences between the information relating to such parent, on the one hand, and the information relating to the Issuer and its Restricted Subsidiaries on a standalone basis, on the other hand.
116
Table of Contents
Notwithstanding the foregoing, such requirements shall be deemed satisfied prior to the commencement of the exchange offers or the effectiveness of the shelf registration statement by (1) the filing with the SEC of the exchange offer registration statement or shelf registration statement (or any other similar registration statement), and any amendments thereto, with such financial information that satisfies Regulation S-X of the Securities Act, subject to exceptions consistent with the presentation of financial information in this prospectus, to the extent filed within the time specified above, or (2) by posting on its website and providing to the Trustee within 15 days of the time periods after the Issuer would have been required to file annual and interim reports with the SEC, the financial information (including a “Management’s Discussion and Analysis of Financial Condition and Results of Operations” section) that would be required to be included in such reports, subject to exceptions consistent with the presentation of financial information in this prospectus, to the extent filed within the times specified above.
Notwithstanding anything herein to the contrary, the Issuer will not be deemed to have failed to comply with any of its obligations hereunder for purposes of clause (3) under “Events of Default and Remedies” until 120 days after the date any report hereunder is due.
The Issuer shall use its commercially reasonable efforts, consistent with its judgment as to what is prudent at the time, to participate in quarterly conference calls to discuss operating results and related matters. The Company shall issue a press release which will provide the date and time of any such call and will direct Holders, prospective investors and securities analysts to contact the investor relations office of the Issuer to obtain access to the conference call.
Events of Default and Remedies
The Indenture provides that each of the following is an Event of Default:
(1) default in payment when due and payable, upon redemption, acceleration or otherwise, of principal of, or premium, if any, on the Senior Notes;
(2) default for 30 days or more in the payment when due of interest or Additional Interest on or with respect to the Senior Notes;
(3) failure by the Issuer or any Guarantor for 60 days after receipt of written notice given by the Trustee or the Holders of not less than 30.0% in principal amount of the then outstanding Senior Notes to comply with any of its obligations, covenants or agreements (other than a default referred to in clause (1) or (2) above) contained in the Indenture or the Senior Notes;
(4) default under any mortgage, indenture or instrument under which there is issued or by which there is secured or evidenced any Indebtedness for money borrowed by the Issuer or any of its Restricted Subsidiaries or the payment of which is guaranteed by the Issuer or any of its Restricted Subsidiaries, other than Indebtedness owed to the Issuer or a Restricted Subsidiary, whether such Indebtedness or guarantee now exists or is created after the issuance of the Senior Notes, if both:
(a) such default either results from the failure to pay any principal of such Indebtedness at its stated final maturity (after giving effect to any applicable grace periods) or relates to an obligation other than the obligation to pay principal of any such Indebtedness at its stated final maturity and results in the holder or holders of such Indebtedness causing such Indebtedness to become due prior to its stated maturity; and
(b) the principal amount of such Indebtedness, together with the principal amount of any other such Indebtedness in default for failure to pay principal at stated final maturity (after giving effect to any applicable grace periods), or the maturity of which has been so accelerated, aggregate $75.0 million or more at any one time outstanding;
(5) failure by the Issuer or any Significant Subsidiary (or any group of Subsidiaries that together would constitute a Significant Subsidiary) to pay final judgments aggregating in excess of $75.0 million, which final judgments remain unpaid, undischarged and unstayed for a period of more than 60 days after such judgment becomes final, and in the event such judgment is covered by insurance, an enforcement proceeding has been commenced by any creditor upon such judgment or decree which is not promptly stayed;
(6) certain events of bankruptcy or insolvency with respect to the Issuer or any Significant Subsidiary (or any group of Subsidiaries that together would constitute a Significant Subsidiary); or
(7) the Guarantee of any Significant Subsidiary (or any group of Subsidiaries that together would constitute a Significant Subsidiary) shall for any reason cease to be in full force and effect or be declared null and void or any responsible officer of any Guarantor that is a Significant Subsidiary (or the responsible officers of any group of Subsidiaries that together would constitute a Significant Subsidiary), as the case may be, denies that it has any further liability under its Guarantee or gives notice to such effect, other than by reason of the termination of the Indenture or the release of any such Guarantee in accordance with the Indenture.
If any Event of Default (other than of a type specified in clause (6) above) occurs and is continuing under the Indenture, the Trustee or the Holders of at least 30.0% in principal amount of the then total outstanding Senior Notes may declare the principal, premium, if any, interest and any other monetary obligations on all the then outstanding Senior Notes to be due and payable immediately.
Upon the effectiveness of such declaration, such principal of and premium, if any, and interest will be due and payable immediately. Notwithstanding the foregoing, in the case of an Event of Default arising under clause (6) of the first paragraph of this section, all outstanding Senior Notes will become due and payable without further action or notice. The Indenture provides that the Trustee may withhold from the Holders notice of any continuing Default, except a Default relating to the payment of principal, premium, if any, or interest, if it determines that withholding notice is in their interest. In addition, the Trustee has no obligation to accelerate the Senior Notes if in the best judgment of the Trustee acceleration is not in the best interests of the Holders of the Senior Notes.
117
Table of Contents
The Indenture provides that the Holders of a majority in aggregate principal amount of the then outstanding Senior Notes by notice to the Trustee may on behalf of the Holders of all of the Senior Notes waive any existing Default and its consequences under the Indenture (except a continuing Default in the payment of interest on, premium, if any, or the principal of any Senior Note held by a non-consenting Holder) and rescind any acceleration with respect to the Senior Notes and its consequences (except if such rescission would conflict with any judgment of a court of competent jurisdiction). In the event of any Event of Default specified in clause (4) above, such Event of Default and all consequences thereof (excluding any resulting payment default, other than as a result of acceleration of the Senior Notes) shall be annulled, waived and rescinded, automatically and without any action by the Trustee or the Holders, if within 20 days after such Event of Default arose:
(1) the Indebtedness or guarantee that is the basis for such Event of Default has been discharged; or
(2) holders thereof have rescinded or waived the acceleration, notice or action (as the case may be) giving rise to such Event of Default; or
(3) the default that is the basis for such Event of Default has been cured.
Notwithstanding the foregoing, the sole remedy for any breach of our obligation under the Indenture to file periodic or other reports (including pursuant to section 314(a)(1) of the Trust Indenture Act) shall be the payment of liquidated damages, and the Holders do not have any right under the Indenture to accelerate the maturity of the Senior Notes as a result of any such breach. If a breach of our obligation under the Indenture to file periodic or other reports (including pursuant to section 314(a)(1) of the Trust Indenture Act) continues for 90 days after notice thereof is given in accordance with the Indenture, we will pay liquidated damages to all the Holders of the Senior Notes at a rate per annum equal to (i) 0.25% per annum of the principal amount of the Senior Notes from the 90 th day following such notice to but not including the 180 th day following such notice (or such earlier date on which the Event of Default relating to the reporting obligations referred to in this paragraph shall have been cured or waived) and (ii) 0.50% per annum of the principal amount of the Senior Notes from the 180 th day following such notice to but not including the 365 th day following such notice (or such earlier date on which the Event of Default relating to the reporting obligations referred to in this paragraph shall have been cured or waived). On such 365 th day (or earlier, if the Event of Default relating to the reporting obligations referred to in this paragraph shall have been cured or waived prior to such 365 th day), such Additional Interest will cease to accrue, and the Senior Notes will be subject to acceleration as provided above if the Event of Default is continuing. The provisions of the indenture described in this paragraph do not affect the rights of the Holders of Senior Notes in the event of the occurrence of any other Event of Default.
Subject to the provisions of the Indenture relating to the duties of the Trustee thereunder, in case an Event of Default occurs and is continuing, the Trustee will be under no obligation to exercise any of the rights or powers under the Indenture at the request or direction of any of the Holders of the Senior Notes unless the Holders have offered to the Trustee reasonable indemnity or security against any loss, liability or expense. Except to enforce the right to receive payment of principal, premium (if any) or interest when due, no Holder of a Senior Note may pursue any remedy with respect to the Indenture or the Senior Notes unless:
(1) such Holder has previously given the Trustee notice that an Event of Default is continuing;
(2) Holders of at least 30.0% in principal amount of the total outstanding Senior Notes have requested the Trustee to pursue the remedy;
(3) Holders of the Senior Notes have offered the Trustee reasonable security or indemnity against any loss, liability or expense;
(4) the Trustee has not complied with such request within 60 days after the receipt thereof and the offer of security or indemnity; and
(5) Holders of a majority in principal amount of the total outstanding Senior Notes have not given the Trustee a direction inconsistent with such request within such 60-day period.
Subject to certain restrictions, under the Indenture the Holders of a majority in principal amount of the total outstanding Senior Notes are given the right to direct the time, method and place of conducting any proceeding for any remedy available to the Trustee or of exercising any trust or power conferred on the Trustee. The Trustee, however, may refuse to follow any direction that conflicts with law or the Indenture or that the Trustee determines is unduly prejudicial to the rights of any other Holder of a Senior Note or that would involve the Trustee in personal liability.
The Indenture provides that the Issuer is required to deliver to the Trustee annually a statement regarding compliance with the Indenture, and the Issuer is required, within five Business Days, upon becoming aware of any Default, to deliver to the Trustee a statement specifying such Default.
No Personal Liability of Directors, Officers, Employees and Shareholders
No director, officer, employee, incorporator or shareholder of the Issuer or any Guarantor or any of their parent companies (other than the Issuer and the Guarantors) has any liability, for any obligations of the Issuer or the Guarantors under the Senior Notes, the Guarantees or the Indenture or for any claim based on, in respect of, or by reason of such obligations or their creation. Each Holder by accepting Senior Notes waives and releases all such liability. The waiver and release are part of the consideration for issuance of the Senior Notes. Such waiver may not be effective to waive liabilities under the federal securities laws and it is the view of the SEC that such a waiver is against public policy.
Legal Defeasance and Covenant Defeasance
The obligations of the Issuer and the Guarantors under the Indenture will terminate (other than certain obligations) and will be released upon payment in full of all of the Senior Notes. The Issuer may, at its option and at any time, elect to have all of its obligations discharged with respect to the Senior Notes and have each Guarantor’s obligation discharged with respect to its Guarantee (“ Legal Defeasance”) and cure all then existing Events of Default except for:
(1) the rights of Holders of Senior Notes to receive payments in respect of the principal of, premium, if any, and interest on the Senior Notes when such payments are due solely out of the trust created pursuant to the Indenture;
118
Table of Contents
(2) the Issuer’s obligations with respect to Senior Notes concerning issuing temporary Senior Notes, registration of such Senior Notes, mutilated, destroyed, lost or stolen Senior Notes and the maintenance of an office or agency for payment and money for security payments held in trust;
(3) the rights, powers, trusts, duties and immunities of the Trustee, and the Issuer’s obligations in connection therewith; and
(4) the Legal Defeasance provisions of the Indenture.
In addition, the Issuer may, at its option and at any time, elect to have its obligations and those of each Guarantor released with respect to certain covenants that are described in the Indenture (“ Covenant Defeasance”) and thereafter any omission to comply with such obligations shall not constitute a Default with respect to the Senior Notes. In the event Covenant Defeasance occurs, certain events (not including bankruptcy, receivership, rehabilitation and insolvency events pertaining to the Issuer) described under “Events of Default and Remedies” will no longer constitute an Event of Default with respect to the Senior Notes.
In order to exercise either Legal Defeasance or Covenant Defeasance with respect to the Senior Notes:
(1) the Issuer must irrevocably deposit with the Trustee, in trust, for the benefit of the Holders of the Senior Notes, cash in U.S. dollars, U.S. dollar-denominated Government Securities, or a combination thereof, in such amounts as will be sufficient, in the opinion of a nationally recognized firm of independent public accountants, to pay the principal of, premium, if any, and interest due on the Senior Notes on the stated maturity date or on the redemption date, as the case may be, of such principal, premium, if any, or interest on such Senior Notes and the Issuer must specify whether such Senior Notes are being defeased to maturity or to a particular redemption date;
(2) in the case of Legal Defeasance, the Issuer shall have delivered to the Trustee an Opinion of Counsel reasonably acceptable to the Trustee confirming that, subject to customary assumptions and exclusions,
(a) the Issuer has received from, or there has been published by, the United States Internal Revenue Service a ruling, or
(b) since the issuance of the Senior Notes, there has been a change in the applicable U.S. federal income tax law,
in either case to the effect that, and based thereon such Opinion of Counsel shall confirm that, subject to customary assumptions and exclusions, the Holders of the Senior Notes will not recognize income, gain or loss for U.S. federal income tax purposes, as applicable, as a result of such Legal Defeasance and will be subject to U.S. federal income tax on the same amounts, in the same manner and at the same times as would have been the case if such Legal Defeasance had not occurred;
(3) in the case of Covenant Defeasance, the Issuer shall have delivered to the Trustee an Opinion of Counsel reasonably acceptable to the Trustee confirming that, subject to customary assumptions and exclusions, the Holders of the Senior Notes will not recognize income, gain or loss for U.S. federal income tax purposes as a result of such Covenant Defeasance and will be subject to such tax on the same amounts, in the same manner and at the same times as would have been the case if such Covenant Defeasance had not occurred;
(4) no Default (other than that resulting from borrowing funds to be applied to make such deposit and any similar and simultaneous deposit relating to other Indebtedness and, in each case, the granting of Liens in connection therewith) shall have occurred and be continuing on the date of such deposit;
(5) such Legal Defeasance or Covenant Defeasance shall not result in a breach or violation of, or constitute a default under the Senior Credit Facilities, the Senior Subordinated Notes, the indenture pursuant to which the Senior Subordinated Notes were issued or any other material agreement or instrument (other than the Indenture) to which, the Issuer or any Guarantor is a party or by which the Issuer or any Guarantor is bound (other than that resulting from any borrowing of funds to be applied to make the deposit required to effect such Legal Defeasance or Covenant Defeasance and any similar and simultaneous deposit relating to other Indebtedness, and the granting of Liens in connection therewith);
(6) the Issuer shall have delivered to the Trustee an Opinion of Counsel to the effect that, as of the date of such opinion and subject to customary assumptions and exclusions following the deposit, the trust funds will not be subject to the effect of Section 547 of Title 11 of the United States Code;
(7) the Issuer shall have delivered to the Trustee an Officer’s Certificate stating that the deposit was not made by the Issuer with the intent of defeating, hindering, delaying or defrauding any creditors of the Issuer or any Guarantor or others; and
(8) the Issuer shall have delivered to the Trustee an Officer’s Certificate and an Opinion of Counsel (which Opinion of Counsel may be subject to customary assumptions and exclusions) each stating that all conditions precedent provided for or relating to the Legal Defeasance or the Covenant Defeasance, as the case may be, have been complied with.
Satisfaction and Discharge
The Indenture will be discharged and will cease to be of further effect as to all Senior Notes, when either:
(1) all Senior Notes theretofore authenticated and delivered, except lost, stolen or destroyed Senior Notes which have been replaced or paid and Senior Notes for whose payment money has theretofore been deposited in trust, have been delivered to the Trustee for cancellation; or
119
Table of Contents
(2) (a) all Senior Notes not theretofore delivered to the Trustee for cancellation have become due and payable by reason of the making of a notice of redemption or otherwise, will become due and payable within one year or are to be called for redemption within one year under arrangements satisfactory to the Trustee for the giving of notice of redemption by the Trustee in the name, and at the expense, of the Issuer and the Issuer or any Guarantor have irrevocably deposited or caused to be deposited with the Trustee as trust funds in trust solely for the benefit of the Holders of the Senior Notes, cash in U.S. dollars, U.S. dollar-denominated Government Securities, or a combination thereof, in such amounts as will be sufficient without consideration of any reinvestment of interest to pay and discharge the entire indebtedness on the Senior Notes not theretofore delivered to the Trustee for cancellation for principal, premium, if any, and accrued interest to the date of maturity or redemption; provided , that upon any redemption that requires the payment of the Applicable Premium, the amount deposited shall be sufficient for purposes of the Indenture to the extent that an amount is deposited with the Trustee equal to the Applicable Premium calculated as of the date of the notice of redemption, with any deficit as of the date of redemption (any such amount, the “Applicable Premium Deficit”) only required to be deposited with the Trustee on or prior to the date of redemption. Any Applicable Premium Deficit shall be set forth in an Officer’s Certificate delivered to the Trustee simultaneously with the deposit of such Applicable Premium Deficit that confirms that such Applicable Premium Deficit shall be applied toward such redemption;
(b) no Default (other than that resulting from borrowing funds to be applied to make such deposit or any similar and simultaneous deposit relating to other Indebtedness and the granting of Liens in connection therewith) with respect to the Indenture or the Senior Notes shall have occurred and be continuing on the date of such deposit or shall occur as a result of such deposit and such deposit will not result in a breach or violation of, or constitute a default under the Senior Credit Facilities, the Senior Subordinated Notes, the indenture pursuant to which the Senior Subordinated Notes were issued or any other material agreement or instrument (other than the Indenture) to which the Issuer or any Guarantor is a party or by which the Issuer or any Guarantor is bound (other than resulting from any borrowing of funds to be applied to make such deposit and any similar and simultaneous deposit relating to other Indebtedness and the granting of Liens in connection therewith);
(c) the Issuer has paid or caused to be paid all sums payable by it under the Indenture; and
(d) the Issuer has delivered irrevocable instructions to the Trustee to apply the deposited money toward the payment of the Senior Notes at maturity or the redemption date, as the case may be.
In addition, the Issuer must deliver an Officer’s Certificate and an Opinion of Counsel to the Trustee stating that all conditions precedent to satisfaction and discharge have been satisfied.
Amendment, Supplement and Waiver
Except as provided in the next two succeeding paragraphs, the Indenture, any Guarantee and the Senior Notes may be amended or supplemented with the consent of the Holders of at least a majority in principal amount of the Senior Notes then outstanding, including consents obtained in connection with a purchase of, or tender offer or exchange offer for, Senior Notes, and any existing Default or compliance with any provision of the Indenture or the Senior Notes issued thereunder may be waived with the consent of the Holders of a majority in principal amount of the then outstanding Senior Notes, other than Senior Notes beneficially owned by the Issuer or its Affiliates (including consents obtained in connection with a purchase of or tender offer or exchange offer for the Senior Notes); provided that if any amendment, waiver or other modification would only affect the Senior Cash Pay Notes or the Senior Toggle Notes, only the consent of the holders of at least a majority in principal amount of the then outstanding Senior Cash Pay Notes or Senior Toggle Notes (and not the consent of at least a majority of all Senior Notes), as the case may be, shall be required.
The Indenture provides that, without the consent of each affected Holder of Senior Notes, an amendment or waiver may not, with respect to any Senior Notes held by a non-consenting Holder:
(1) reduce the principal amount of such Senior Notes whose Holders must consent to an amendment, supplement or waiver;
(2) reduce the principal of or change the fixed final maturity of any such Senior Note or alter or waive the provisions with respect to the redemption of such Senior Notes (other than provisions relating to the covenants described above under “Repurchase at the Option of Holders”);
(3) reduce the rate of or change the time for payment of interest on any Senior Note;
(4) waive a Default in the payment of principal of or premium, if any, or interest on the Senior Notes, except a rescission of acceleration of the Senior Notes by the Holders of at least a majority in aggregate principal amount of the Senior Notes and a waiver of the payment default that resulted from such acceleration, or in respect of a covenant or provision contained in the Indenture or any Guarantee which cannot be amended or modified without the consent of all Holders;
(5) make any Senior Note payable in money other than that stated therein;
(6) make any change in the provisions of the Indenture relating to waivers of past Defaults or the rights of Holders to receive payments of principal of or premium, if any, or interest on the Senior Notes;
(7) make any change in these amendment and waiver provisions;
(8) impair the right of any Holder to receive payment of principal of, or premium, if any, or interest on such Holder’s Senior Notes on or after the due dates therefor or to institute suit for the enforcement of any payment on or with respect to such Holder’s Senior Notes;
120
Table of Contents
(9) make any change to or modify the ranking of the Senior Notes that would adversely affect the Holders; or
(10) except as expressly permitted by the Indenture, modify the Guarantees of any Significant Subsidiary in any manner adverse to the Holders of the Senior Notes.
Notwithstanding the foregoing, the Issuer, any Guarantor (with respect to a Guarantee or the Indenture to which it is a party) and the Trustee may amend or supplement the Indenture and any Guarantee or Senior Notes without the consent of any Holder:
(1) to cure any ambiguity, omission, mistake, defect or inconsistency;
(2) to provide for uncertificated Senior Notes of such series in addition to or in place of certificated Senior Notes;
(3) to comply with the covenant relating to mergers, consolidations and sales of assets;
(4) to provide the assumption of the Issuer’s or any Guarantor’s obligations to the Holders;
(5) to make any change that would provide any additional rights or benefits to the Holders or that does not adversely affect the legal rights under the Indenture of any such Holder;
(6) to add covenants for the benefit of the Holders or to surrender any right or power conferred upon the Issuer or any Guarantor;
(7) to comply with requirements of the SEC in order to effect or maintain the qualification of the Indenture under the Trust Indenture Act;
(8) to evidence and provide for the acceptance and appointment under the Indenture of a successor Trustee thereunder pursuant to the requirements thereof;
(9) to provide for the issuance of exchange notes or private exchange notes, which are identical to exchange notes except that they are not freely transferable;
(10) to add a Guarantor under the Indenture;
(11) to conform the text of the Indenture, Guarantees or the Senior Notes to any provision of this “Description of Senior Notes” to the extent that such provision in this “Description of Senior Notes” was intended to be a verbatim recitation of a provision of the Indenture, Guarantee or Senior Notes; or
(12) to make any amendment to the provisions of the Indenture relating to the transfer and legending of Senior Notes as permitted by the Indenture, including, without limitation to facilitate the issuance and administration of the Senior Notes; provided that (a) compliance with the Indenture as so amended would not result in Senior Notes being transferred in violation of the Securities Act or any applicable securities law and (b) such amendment does not materially and adversely affect the rights of Holders to transfer Senior Notes.
The consent of the Holders is not necessary under the Indenture to approve the particular form of any proposed amendment. It is sufficient if such consent approves the substance of the proposed amendment.
Notices
Notices given by publication or electronic delivery will be deemed given on the first date on which publication is made and notices given by first-class mail, postage prepaid, will be deemed given five calendar days after mailing.
Concerning the Trustee
The Indenture contains certain limitations on the rights of the Trustee thereunder, should it become a creditor of the Issuer, to obtain payment of claims in certain cases, or to realize on certain property received in respect of any such claim as security or otherwise. The Trustee is permitted to engage in other transactions; however, if it acquires any conflicting interest it must eliminate such conflict within 90 days, apply to the SEC for permission to continue or resign.
The Indenture provides that the Holders of a majority in principal amount of the outstanding Senior Notes have the right to direct the time, method and place of conducting any proceeding for exercising any remedy available to the Trustee, subject to certain exceptions. The Indenture provides that in case an Event of Default shall occur (which shall not be cured), the Trustee will be required, in the exercise of its power, to use the degree of care of a prudent person in the conduct of his own affairs. Subject to such provisions, the Trustee will be under no obligation to exercise any of its rights or powers under the Indenture at the request of any Holder of the Senior Notes, unless such Holder shall have offered to the Trustee security and indemnity satisfactory to it against any loss, liability or expense.
Governing Law
The Indenture, the Senior Notes and any Guarantee are governed by and construed in accordance with the laws of the State of New York.
121
Table of Contents
Certain Definitions
Set forth below are certain defined terms used in the Indenture. For purposes of the Indenture, unless otherwise specifically indicated, the term “consolidated” with respect to any Person refers to such Person consolidated with its Restricted Subsidiaries, and excludes from such consolidation any Unrestricted Subsidiary as if such Unrestricted Subsidiary were not an Affiliate of such Person.
“ABL Facilities” means the asset-based revolving credit facilities under the Credit Agreement to be entered into as of the Issue Date by and among the Issuer, the lenders party thereto in their capacities as lenders thereunder and Bank of America, N.A., as Administrative Agent, including any guarantees, collateral documents, instruments and agreements executed in connection therewith, and any amendments, supplements, modifications, extensions, renewals, restatements, refundings or refinancings thereof and any indentures or credit facilities or commercial paper facilities with banks or other institutional lenders or investors that replace, refund or refinance any part of the loans, notes, other credit facilities or commitments thereunder, including any such replacement, refunding or refinancing facility or indenture that increases the amount borrowable thereunder or alters the maturity thereof ( provided that such increase in borrowings is permitted under “Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock” above).
“ABL Financing Entity” means the Issuer and certain of its Subsidiaries from time to time named as borrowers or guarantors under the ABL Facilities.
“Acquired Indebtedness” means, with respect to any specified Person,
(1) Indebtedness of any other Person existing at the time such other Person is merged with or into or became a Restricted Subsidiary of such specified Person, including Indebtedness incurred in connection with, or in contemplation of, such other Person merging with or into or becoming a Restricted Subsidiary of such specified Person, and
(2) Indebtedness secured by a Lien encumbering any asset acquired by such specified Person.
“Acquisition” means the transactions contemplated by the Transaction Agreement.
“Additional Interest” means all additional interest then owing pursuant to the Registration Rights Agreement.
“Affiliate” of any specified Person means any other Person directly or indirectly controlling or controlled by or under direct or indirect common control with such specified Person. For purposes of this definition, “control” (including, with correlative meanings, the terms “controlling,” “controlled by” and “under common control with”), as used with respect to any Person, shall mean the possession, directly or indirectly, of the power to direct or cause the direction of the management or policies of such Person, whether through the ownership of voting securities, by agreement or otherwise.
“Applicable Premium” means, with respect to any Senior Cash Pay Note or Senior Toggle Note, as the case may be, on any Redemption Date, the greater of:
(1) 1.0% of the principal amount of such Senior Cash Pay Note or Senior Toggle Note, as the case may be; and
(2) the excess, if any, of (a) the present value at such Redemption Date of (i) the redemption price of such Senior Cash Pay Notes at October 15, 2012 or such Senior Toggle Note at October 15, 2012 as the case may be (each such redemption price being set forth in the table appearing above under “ Optional Redemption”), plus (ii) all required remaining scheduled interest payments due on such Senior Cash Pay Note through October 15, 2012 or such Senior Toggle Note through October 15, 2012, as the case may be (assuming with respect to Senior Toggle Notes, calculated based on the Cash Interest rate) (excluding accrued but unpaid interest to the Redemption Date), computed using a discount rate equal to the Treasury Rate as of such Redemption Date plus 50 basis points; over (b) the principal amount of such Senior Cash Pay Note or Senior Toggle Note, as applicable.
“Asset Sale” means:
(1) the sale, conveyance, transfer or other disposition, whether in a single transaction or a series of related transactions (including by way of a Sale and Lease-Back Transaction) of property or assets of the Issuer or any of its Restricted Subsidiaries (each referred to in this definition as a “disposition”); or
(2) the issuance or sale of Equity Interests of any Restricted Subsidiary (other than Preferred Stock of Restricted Subsidiaries issued in compliance with the covenant described under “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”), whether in a single transaction or a series of related transactions;
in each case, other than:
(a) any disposition of Cash Equivalents or Investment Grade Securities or obsolete or worn out equipment in the ordinary course of business or any disposition of inventory or goods (or other assets) held for sale or no longer used in the ordinary course of business;
(b) the disposition of all or substantially all of the assets of the Issuer in a manner permitted pursuant to the provisions described above under “Certain Covenants—Merger, Consolidation or Sale of All or Substantially All Assets” or any disposition that constitutes a Change of Control pursuant to the Indenture;
(c) the making of any Restricted Payment or Permitted Investment that is permitted to be made, and is made, under the covenant described above under “Certain Covenants—Limitation on Restricted Payments”;
122
Table of Contents
(d) any disposition of assets or issuance or sale of Equity Interests of any Restricted Subsidiary in any transaction or series of related transactions with an aggregate fair market value of less than $50.0 million;
(e) any disposition of property or assets or the issuance of securities by a Restricted Subsidiary to the Issuer or by the Issuer or a Restricted Subsidiary to a Restricted Subsidiary;
(f) to the extent allowable under Section 1031 of the Internal Revenue Code of 1986, any exchange of like property (excluding any boot thereon) for use in a Similar Business;
(g) the lease, assignment or sub-lease of any real or personal property in the ordinary course of business;
(h) any issuance or sale of Equity Interests in, or Indebtedness or other securities of, an Unrestricted Subsidiary;
(i) foreclosures, condemnation or any similar action on assets or the granting of Liens not prohibited by the Indenture;
(j) sales of accounts receivable, or participations therein, or Securitization Assets or related assets in connection with the ABL Facilities or any Qualified Securitization Facility;
(k) any financing transaction with respect to property built or acquired by the Issuer or any Restricted Subsidiary after the Issue Date, including Sale and Lease-Back Transactions and asset securitizations permitted by the Indenture;
(l) the sale or discount of inventory, accounts receivable or notes receivable in the ordinary course of business or the conversion of accounts receivable to notes receivable;
(m) the licensing or sub-licensing of intellectual property or other general intangibles in the ordinary course of business, other than the licensing of intellectual property on a long-term basis;
(n) any surrender or waiver of contract rights or the settlement, release or surrender of contract rights or other litigation claims in the ordinary course of business;
(o) the unwinding of any Hedging Obligations;
(p) sales, transfers and other dispositions of Investments in joint ventures to the extent required by, or made pursuant to, customary buy/sell arrangements between the joint venture parties set forth in joint venture arrangements and similar binding arrangements; and
(q) the abandonment of intellectual property rights in the ordinary course of business, which in the reasonable good faith determination of the Issuer are not material to the conduct of the business of the Issuer and its Restricted Subsidiaries taken as a whole.
“Business Day” means each day which is not a Legal Holiday.
“Capital Stock” means:
(1) in the case of a corporation, corporate stock;
(2) in the case of an association or business entity, any and all shares, interests, participations, rights or other equivalents (however designated) of corporate stock;
(3) in the case of a partnership or limited liability company, partnership or membership interests (whether general or limited); and
(4) any other interest or participation that confers on a Person the right to receive a share of the profits and losses of, or distributions of assets of, the issuing Person but excluding from all of the foregoing any debt securities convertible into Capital Stock, whether or not such debt securities include any right of participation with Capital Stock.
“Capitalized Lease Obligation” means, at the time any determination thereof is to be made, the amount of the liability in respect of a capital lease that would at such time be required to be capitalized and reflected as a liability on a balance sheet (excluding the footnotes thereto) prepared in accordance with GAAP.
“Capitalized Software Expenditures” shall mean, for any period, the aggregate of all expenditures (whether paid in cash or accrued as liabilities) by a Person and its Restricted Subsidiaries during such period in respect of licensed or purchased software or internally developed software and software enhancements that, in conformity with GAAP, are or are required to be reflected as capitalized costs on the consolidated balance sheet of a Person and its Restricted Subsidiaries.
“Cash Equivalents” means:
(1) United States dollars;
(2) (a) Canadian dollars, yen, pounds sterling, euros or any national currency of any participating member state of the EMU; or
(b) in the case of any Foreign Subsidiary that is a Restricted Subsidiary, such local currencies held by it from time to time in the ordinary course of business;
123
Table of Contents
(3) securities issued or directly and fully and unconditionally guaranteed or insured by the U.S. government or any agency or instrumentality thereof the securities of which are unconditionally guaranteed as a full faith and credit obligation of such government with maturities of 24 months or less from the date of acquisition;
(4) certificates of deposit, time deposits and eurodollar time deposits with maturities of 24 months or less from the date of acquisition, bankers’ acceptances with maturities not exceeding one year and overnight bank deposits, in each case with any domestic or foreign commercial bank having capital and surplus of not less than $500.0 million in the case of U.S. banks and $100.0 million (or the U.S. dollar equivalent as of the date of determination) in the case of non-U.S. banks;
(5) repurchase obligations for underlying securities of the types described in clauses (3), (4) and (8) entered into with any financial institution meeting the qualifications specified in clause (4) above;
(6) commercial paper rated at least P-2 by Moody’s or at least A-2 by S&P (or, if at any time neither Moody’s nor S&P shall be rating such obligations, an equivalent rating from another Rating Agency) and in each case maturing within 24 months after the date of creation thereof and Indebtedness or Preferred Stock issued by Persons with a rating of “A” or higher from S&P or “A-2” or higher from Moody’s with maturities of 24 months or less from the date of acquisition;
(7) marketable short-term money market and similar funds having a rating of at least P-2 or A-2 from either Moody’s or S&P, respectively (or, if at any time neither Moody’s nor S&P shall be rating such obligations, an equivalent rating from another Rating Agency);
(8) readily marketable direct obligations issued by any state, commonwealth or territory of the United States or any political subdivision or taxing authority thereof having an Investment Grade Rating from either Moody’s or S&P (or, if at any time neither Moody’s nor S&P shall be rating such obligations, an equivalent rating from another Rating Agency) with maturities of 24 months or less from the date of acquisition;
(9) readily marketable direct obligations issued by any foreign government or any political subdivision or public instrumentality thereof, in each case having an Investment Grade Rating from either Moody’s or S&P (or, if at any time neither Moody’s nor S&P shall be rating such obligations, an equivalent rating from another Rating Agency) with maturities of 24 months or less from the date of acquisition;
(10) Investments with average maturities of 12 months or less from the date of acquisition in money market funds rated AAA- (or the equivalent thereof) or better by S&P or Aaa3 (or the equivalent thereof) or better by Moody’s (or, if at any time neither Moody’s nor S&P shall be rating such obligations, an equivalent rating from another Rating Agency); and
(11) investment funds investing at least 90.0% of their assets in securities of the types described in clauses (1) through (10) above.
In the case of Investments by any Foreign Subsidiary that is a Restricted Subsidiary or Investments made in a country outside the United States of America, Cash Equivalents shall also include (a) investments of the type and maturity described in clauses (1) through (8) and clauses (10) and (11) above of foreign obligors, which Investments or obligors (or the parents of such obligors) have ratings described in such clauses or equivalent ratings from comparable foreign rating agencies and (b) other short-term investments utilized by Foreign Subsidiaries that are Restricted Subsidiaries in accordance with normal investment practices for cash management in investments analogous to the foregoing investments in clauses (1) through (11) and in this paragraph.
Notwithstanding the foregoing, Cash Equivalents shall include amounts denominated in currencies other than those set forth in clauses (1) and (2) above, provided that such amounts are converted into any currency listed in clauses (1) and (2) as promptly as practicable and in any event within ten Business Days following the receipt of such amounts.
At any time at which the value, calculated in accordance with GAAP, of all investments of the Issuer and its Restricted Subsidiaries that were deemed, when made, to be Cash Equivalents in accordance with clauses (1) through (11) above exceeds the Indebtedness of the Issuer and its Restricted Subsidiaries, “Cash Equivalents” shall also mean any investment (a “ Qualifying Investment”) that satisfies the following two conditions: (a) the Qualifying Investment is of a type described in clauses (1) through (11) of this definition, but has an effective maturity (whether by reason of final maturity, a put option or, in the case of an asset-backed security, an average life) of five years and one month or less from the date of such Qualifying Investment (notwithstanding any provision contained in such clauses (1) through (11) requiring a shorter maturity); and (b) the weighted average effective maturity of such Qualifying Investment and all other investments that were made as Qualifying Investments in accordance with this paragraph, does not exceed two years from the date of such Qualifying Investment.
“CF Credit Facilities” means the term and revolving credit facilities under the Credit Agreement to be entered into as of the Issue Date by and among the Issuer, the European subsidiary borrowers party thereto, the lenders party thereto in their capacities as lenders thereunder and Bank of America, N.A., as Administrative Agent, including any guarantees, collateral documents, instruments and agreements executed in connection therewith, and any amendments, supplements, modifications, extensions, renewals, restatements, refundings or refinancings thereof and any indentures or credit facilities or commercial paper facilities with banks or other institutional lenders or investors that replace, refund or refinance any part of the loans, notes, other credit facilities or commitments thereunder, including any such replacement, refunding or refinancing facility or indenture that increases the amount borrowable thereunder or alters the maturity thereof ( provided that such increase in borrowings is permitted under “Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock” above).
“Change of Control” means the occurrence of any of the following:
(1) the sale, lease or transfer, in one or a series of related transactions, of all or substantially all of the assets of the Issuer and its Subsidiaries, taken as a whole, to any Person other than a Permitted Holder; or
124
Table of Contents
(2) the Issuer becomes aware of (by way of a report or any other filing pursuant to Section 13(d) of the Exchange Act, proxy, vote, written notice or otherwise) the acquisition by any Person or group (within the meaning of Section 13(d)(3) or Section 14(d)(2) of the Exchange Act, or any successor provision), including any group acting for the purpose of acquiring, holding or disposing of securities (within the meaning of Rule 13d-5(b)(1) under the Exchange Act), other than one or more Permitted Holders, in a single transaction or in a related series of transactions, by way of merger, consolidation or other business combination or purchase of beneficial ownership (within the meaning of Rule 13d-3 under the Exchange Act, or any successor provision) of 50.0% or more of the total voting power of the Voting Stock of the Issuer or any of its direct or indirect parent companies.
“Co-Investors” means the assignees, if any, of the equity commitments of the Investors on the Issue Date who become holders of Equity Interests in the Issuer (or any of the direct or indirect parent companies of the Issuer) on the Issue Date in connection with the Acquisition.
“Consolidated Depreciation and Amortization Expense” means with respect to any Person for any period, the total amount of depreciation and amortization expense of such Person, including the amortization of deferred financing fees, debt issuance costs, commissions, fees and expenses and Capitalized Software Expenditures of such Person and its Restricted Subsidiaries for such period on a consolidated basis and otherwise determined in accordance with GAAP.
“Consolidated Interest Expense” means, with respect to any Person for any period, without duplication, the sum of:
(1) consolidated interest expense of such Person and its Restricted Subsidiaries for such period, to the extent such expense was deducted (and not added back) in computing Consolidated Net Income (including (a) amortization of original issue discount resulting from the issuance of Indebtedness at less than par, (b) all commissions, discounts and other fees and charges owed with respect to letters of credit or bankers acceptances, (c) non-cash interest payments (but excluding any non-cash interest expense attributable to the movement in the mark to market valuation of Hedging Obligations or other derivative instruments pursuant to GAAP), (d) the interest component of Capitalized Lease Obligations, and (e) net payments, if any, made (less net payments, if any, received), pursuant to interest rate Hedging Obligations with respect to Indebtedness, and excluding (t) any expense resulting from the discounting of any Indebtedness in connection with the application of recapitalization accounting or, if applicable, purchase accounting in connection with the Transactions or any acquisition, (u) penalties and interest relating to taxes, (v) any Additional Interest and any “additional interest” with respect to the Senior Subordinated Notes or other securities, (w) amortization of deferred financing fees, debt issuance costs, commissions, fees and expenses, (x) any expensing of bridge, commitment and other financing fees, (y) commissions, discounts, yield and other fees and charges (including any interest expense) related to any Qualified Securitization Facility and (z) any accretion of accrued interest on discounted liabilities);plus
(2) consolidated capitalized interest of such Person and its Restricted Subsidiaries for such period, whether paid or accrued;less
(3) interest income of such Person and its Restricted Subsidiaries for such period.
For purposes of this definition, interest on a Capitalized Lease Obligation shall be deemed to accrue at an interest rate reasonably determined by such Person to be the rate of interest implicit in such Capitalized Lease Obligation in accordance with GAAP.
“Consolidated Net Income” means, with respect to any Person for any period, the aggregate of the Net Income of such Person and its Restricted Subsidiaries for such period, on a consolidated basis, and otherwise determined in accordance with GAAP; provided that, without duplication,
(1) any after-tax effect of extraordinary, non-recurring or unusual gains or losses (less all fees and expenses relating thereto) or expenses (including relating to the Transactions or any multi-year strategic initiatives, severance, relocation costs and curtailments or modifications to pension and post-retirement employee benefit plans) shall be excluded;
(2) the cumulative effect of a change in accounting principles and changes as a result of the adoption or modification of accounting policies during such period shall be excluded;
(3) any net after-tax gains or losses on disposal of disposed, abandoned or discontinued operations shall be excluded;
(4) any net after-tax effect of gains or losses (less all fees, expenses and charges relating thereto) attributable to asset dispositions or abandonments or the sale or other disposition of any Capital Stock of any Person other than in the ordinary course of business shall be excluded;
(5) the Net Income for such period of any Person that is an Unrestricted Subsidiary shall be excluded, and, solely for the purpose of determining the amount available for Restricted Payments under clause (3)(a) of the first paragraph of “Certain Covenants—Limitation on Restricted Payments,” the Net Income for such period of any Person that is not a Subsidiary or that is accounted for by the equity method of accounting shall be excluded; provided that Consolidated Net Income of the Issuer shall be increased by the amount of dividends or distributions or other payments that are actually paid in cash (or to the extent converted into cash) to the Issuer or a Restricted Subsidiary thereof in respect of such period;
(6) solely for the purpose of determining the amount available for Restricted Payments under clause (3)(a) of the first paragraph of “Certain Covenants—Limitation on Restricted Payments,” the Net Income for such period of any Restricted Subsidiary (other than any Guarantor) shall be excluded to the extent that the declaration or payment of dividends or similar distributions by that Restricted Subsidiary of its Net Income is not at the date of determination permitted without any prior governmental approval (which has not been obtained) or, directly or indirectly, by the operation of the terms of its charter or any agreement, instrument, judgment, decree, order, statute, rule, or governmental regulation applicable to that Restricted Subsidiary or its shareholders, unless such restriction with respect to the payment of dividends or similar distributions has been legally waived, provided that Consolidated Net Income of the Issuer will be increased by the amount of dividends or other distributions or other payments actually paid in cash (or to the extent converted into cash) to the Issuer or a Restricted Subsidiary thereof in respect of such period, to the extent not already included therein;
125
Table of Contents
(7) effects of adjustments (including the effects of such adjustments pushed down to the Issuer and its Restricted Subsidiaries) in such Person’s consolidated financial statements pursuant to GAAP resulting from the application of recapitalization accounting or, if applicable, purchase accounting in relation to the Transactions or any consummated acquisition or the amortization or write-off of any amounts thereof, net of taxes, shall be excluded;
(8) any after-tax effect of income (loss) from the early extinguishment of (a) Indebtedness, (b) Hedging Obligations or (c) other derivative instruments shall be excluded;
(9) any impairment charge or asset write-off or write-down, including impairment charges or asset write-offs or write-downs related to intangible assets, long-lived assets, investments in debt and equity securities or as a result of a change in law or regulation, in each case, pursuant to GAAP, and the amortization of intangibles arising pursuant to GAAP shall be excluded;
(10) any non-cash compensation expense recorded from grants of stock appreciation or similar rights, stock options, restricted stock or other rights, and any cash charges associated with the rollover, acceleration, or payout of Equity Interests by management of the Issuer or any of its direct or indirect parent companies in connection with the Transactions, shall be excluded;
(11) any fees, expenses or charges incurred during such period, or any amortization thereof for such period, in connection with any acquisition, Investment, Asset Sale, incurrence or repayment of Indebtedness (including such fees, expenses or charges related to the offering of the Senior Notes, the Senior Subordinated Notes and the Credit Facilities), issuance of Equity Interests, refinancing transaction or amendment or modification of any debt instrument (including any amendment or other modification of the Senior Notes, the Senior Subordinated Notes and the Credit Facilities) and including, in each case, any such transaction consummated prior to the Issue Date and any such transaction undertaken but not completed, and any charges or non-recurring merger costs incurred during such period as a result of any such transaction, in each case whether or not successful, shall be excluded;
(12) accruals and reserves that are established within twelve months after the Issue Date that are so required to be established as a result of the Transactions (or within twelve months after the closing of any acquisition that are so required to be established as a result of such acquisition) in accordance with GAAP shall be excluded;
(13) to the extent covered by insurance and actually reimbursed, or, so long as the Issuer has made a determination that there exists reasonable evidence that such amount will in fact be reimbursed by the insurer and only to the extent that such amount is (a) not denied by the applicable carrier in writing 180 days and (b) in fact reimbursed within 365 days of the date of the insurable event (with a deduction for any amount so added back to the extent not so reimbursed within such 365 day period), expenses with respect to liability or casualty events or business interruption shall be excluded;
(14) any noncash compensation expense resulting from the application of Statement of Financial Accounting Standards No. 123(R) shall be excluded; and
(15) the following items shall be excluded:
(a) any net unrealized gain or loss (after any offset) resulting in such period from Hedging Obligations and the application of Statement of Financial Accounting Standards No. 133; and
(b) any net unrealized gain or loss (after any offset) resulting in such period from currency translation gains or losses including those related to currency remeasurements of Indebtedness (including any net loss or gain resulting from Hedging Obligations for currency exchange risk).
In addition, to the extent not already included in the Consolidated Net Income of such Person and its Restricted Subsidiaries, notwithstanding anything to the contrary in the foregoing, Consolidated Net Income shall include the amount of proceeds received from business interruption insurance and reimbursements of any expenses and charges that are covered by indemnification or other reimbursement provisions in connection with any Permitted Investment or any sale, conveyance, transfer or other disposition of assets permitted under the Indenture.
Notwithstanding the foregoing, for the purpose of the covenant described under “Certain Covenants—Limitation on Restricted Payments” only (other than clause (3)(d) of the first paragraph thereof), there shall be excluded from Consolidated Net Income any income arising from any sale or other disposition of Restricted Investments made by the Issuer and its Restricted Subsidiaries, any repurchases and redemptions of Restricted Investments from the Issuer and its Restricted Subsidiaries, any repayments of loans and advances which constitute Restricted Investments by the Issuer or any of its Restricted Subsidiaries, any sale of the stock of an Unrestricted Subsidiary or any distribution or dividend from an Unrestricted Subsidiary, in each case only to the extent such amounts increase the amount of Restricted Payments permitted under such covenant pursuant to clause (3)(d) thereof.
“Contingent Obligations” means, with respect to any Person, any obligation of such Person guaranteeing any leases, dividends or other obligations that do not constitute Indebtedness (“primary obligations”) of any other Person (the “ primary obligor”) in any manner, whether directly or indirectly, including, without limitation, any obligation of such Person, whether or not contingent,
(1) to purchase any such primary obligation or any property constituting direct or indirect security therefor;
(2) to advance or supply funds
(a) for the purchase or payment of any such primary obligation, or
126
Table of Contents
(b) to maintain working capital or equity capital of the primary obligor or otherwise to maintain the net worth or solvency of the primary obligor; or
(3) to purchase property, securities or services primarily for the purpose of assuring the owner of any such primary obligation of the ability of the primary obligor to make payment of such primary obligation against loss in respect thereof.
“Controlled Investment Affiliate” means, as to any Person, any other Person, other than any Investor, which directly or indirectly is in control of, is controlled by, or is under common control with such Person and is organized by such Person (or any Person controlling such Person) primarily for making direct or indirect equity or debt investments in the Issuer and/or other companies.
“Credit Facilities” means, with respect to the Issuer or any of its Restricted Subsidiaries, one or more debt facilities, including the Senior Credit Facilities or other financing arrangements (including, without limitation, commercial paper facilities or indentures) providing for revolving credit loans, term loans, letters of credit or other long-term indebtedness, including any notes, mortgages, guarantees, collateral documents, instruments and agreements executed in connection therewith, and any amendments, supplements, modifications, extensions, renewals, restatements or refundings thereof and any indentures or credit facilities or commercial paper facilities that replace, refund or refinance any part of the loans, notes, other credit facilities or commitments thereunder, including any such replacement, refunding or refinancing facility or indenture that increases the amount permitted to be borrowed thereunder or alters the maturity thereof ( provided that such increase in borrowings is permitted under “Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”) or adds Restricted Subsidiaries as additional borrowers or guarantors thereunder and whether by the same or any other agent, lender or group of lenders.
“Default” means any event that is, or with the passage of time or the giving of notice or both would be, an Event of Default.
“Designated Non-cash Consideration” means the fair market value of non-cash consideration received by the Issuer or a Restricted Subsidiary in connection with an Asset Sale that is so designated as Designated Non-cash Consideration pursuant to an Officer’s Certificate, setting forth the basis of such valuation, executed by the principal financial officer of the Issuer, less the amount of Cash Equivalents received in connection with a subsequent sale of or collection on such Designated Non-cash Consideration.
“Designated Preferred Stock” means Preferred Stock of the Issuer or any parent company thereof (in each case other than Disqualified Stock) that is issued for cash (other than to a Restricted Subsidiary or an employee stock ownership plan or trust established by the Issuer or any of its Subsidiaries) and is so designated as Designated Preferred Stock, pursuant to an Officer’s Certificate executed by the principal financial officer of the Issuer or the applicable parent company thereof, as the case may be, on the issuance date thereof, the cash proceeds of which are excluded from the calculation set forth in clause (3) of the first paragraph of “Certain Covenants—Limitation on Restricted Payments.”
“Disqualified Stock” means, with respect to any Person, any Capital Stock of such Person which, by its terms, or by the terms of any security into which it is convertible or for which it is putable or exchangeable, or upon the happening of any event, matures or is mandatorily redeemable (other than solely as a result of a change of control or asset sale) pursuant to a sinking fund obligation or otherwise, or is redeemable at the option of the holder thereof (other than solely as a result of a change of control or asset sale), in whole or in part, in each case prior to the date 91 days after the earlier of the maturity date of the Senior Notes or the date the Senior Notes are no longer outstanding; provided that if such Capital Stock is issued to any plan for the benefit of employees of the Issuer or its Subsidiaries or by any such plan to such employees, such Capital Stock shall not constitute Disqualified Stock solely because it may be required to be repurchased by the Issuer or its Subsidiaries in order to satisfy applicable statutory or regulatory obligations; provided , further , that any Capital Stock held by any future, current or former employee, director, officer, manager, distributor or consultant (or their respective Controlled Investment Affiliates or Immediate Family Members), of the Issuer, any of its Subsidiaries, any of its direct or indirect parent companies or any other entity in which the Issuer or a Restricted Subsidiary has an Investment and is designated in good faith as an “affiliate” by the board of directors of the Issuer (or the compensation committee thereof), in each case pursuant to any stock subscription or shareholders’ agreement, management equity plan or stock option plan or any other management or employee benefit plan or agreement or any distributor equity plan or agreement shall not constitute Disqualified Stock solely because it may be required to be repurchased by the Issuer or its Subsidiaries.
“EBITDA” means, with respect to any Person for any period, the Consolidated Net Income of such Person for such period
(1) increased (without duplication) by the following, in each case to the extent deducted (and not added back) in determining Consolidated Net Income for such period:
(a) provision for taxes based on income or profits or capital, including, without limitation, state, franchise and similar taxes, foreign withholding taxes (including any future taxes or other levies which replace or are intended to be in lieu of such taxes and any penalties and interest related to such taxes or arising from tax examinations) and the net tax expense associated with any adjustments made pursuant to clauses (1) through (15) of the definition of “Consolidated Net Income”;plus
(b) Fixed Charges of such Person for such period (including (x) net losses or Hedging Obligations or other derivative instruments entered into for the purpose of hedging interest rate risk, (y) bank fees and (z) costs of surety bonds in connection with financing activities, plus amounts excluded from Consolidated Interest Expense as set forth in clauses (1)(t) through (z) in the definition thereof);plus
(c) Consolidated Depreciation and Amortization Expense of such Person for such period;plus
(d) [reserved];plus
(e) the amount of any restructuring charges, integration and facilities opening costs or other business optimization expenses (including cost and expenses relating to business optimization programs and new systems design and implementation costs) or accruals or reserves, including any one-time costs incurred in connection with acquisitions after the Issue Date, project start-up costs and costs related to the closure and/or consolidation of facilities; plus
127
Table of Contents
(f) any other non-cash charges, including any write offs or write downs reducing Consolidated Net Income for such period (provided that if any such non-cash charges represent an accrual or reserve for potential cash items in any future period, the cash payment in respect thereof in such future period shall be subtracted from EBITDA to such extent, and excluding amortization of a prepaid cash item that was paid in a prior period);plus
(g) the amount of any minority interest expense consisting of Subsidiary income attributable to minority equity interests of third parties in any non-Wholly Owned Subsidiary;plus
(h) the amount of management, monitoring, consulting and advisory fees (including termination fees) and related indemnities and expenses paid or accrued in such period under the Management Fee Agreement or otherwise to the Investors to the extent otherwise permitted under “Certain Covenants—Transactions with Affiliates”;plus
(i) the amount of “run-rate” cost savings projected by the Issuer in good faith to result from actions either taken or expected to be taken within 12 months after the end of such period (which cost savings shall be subject only to certification by management of the Issuer and calculated on apro forma basis as though such cost savings had been realized on the first day of such period), net of the amount of actual benefits realized from such actions (it is understood and agreed that “run-rate” means the full recurring benefit that is associated with any action taken or expected to be taken,provided that some portion of such benefit is expected to be realized within 12 months of taking such action) (which adjustments may be incremental topro forma cost savings adjustments made pursuant to the definition of “Fixed Charge Coverage Ratio”);plus
(j) the amount of loss on sale of receivables, Securitization Assets and related assets to the Securitization Subsidiary in connection with a Qualified Securitization Facility;plus
(k) any costs or expense incurred by the Issuer or a Restricted Subsidiary pursuant to any management equity plan or stock option plan or any other management or employee benefit plan, agreement or any stock subscription or shareholder agreement or any distributor equity plan or agreement, to the extent that such cost or expenses are funded with cash proceeds contributed to the capital of the Issuer or net cash proceeds of an issuance of Equity Interest of the Issuer (other than Disqualified Stock) solely to the extent that such net cash proceeds are excluded from the calculation set forth in clause (3) of the first paragraph under “Certain Covenants—Limitation on Restricted Payments”;plus
(l) cash receipts (or any netting arrangements resulting in reduced cash expenditures) not representing EBITDA or Consolidated Net Income in any period to the extent non-cash gains relating to such income were deducted in the calculation of EBITDA pursuant to clause (2) below for any previous period and not added back;plus
(m) any net loss from disposed or discontinued operations or from operations expected to be disposed of or discontinued within twelve months after the end of such period;plus
(n) interest income or investment earnings on retiree medical and intellectual property, royalty or license receivables;plus
(o) extraordinary losses and unusual or non-recurring charges (including any unusual or non-recurring operating expenses attributable to the implementation of cost-savings initiatives, severance, retention and relocation costs and curtailments and modifications to pension and post-retirement employee benefit plans);plus
(p) any costs or expenses incurred by the Issuer or a Restricted Subsidiary (whether prior to or following the Issue Date) relating to the Option Accounting Issues, including fees and expenses incurred by the Issuer’s directors, officers, employees and advisors in investigating such Option Accounting Issues and any incremental tax exposure resulting from the resolution of such Option Accounting Issues;plus
(q) expense related to any payments made to distributors prior to the first anniversary of the Issue Date (other than commissions paid in the ordinary course of business);plus
(r) losses on asset sales (other than asset sales made in the ordinary course of business), disposals and abandonments;
(2) decreased (without duplication) by the following, in each case to the extent included in determining Consolidated Net Income for such period:
(a) non-cash gains increasing Consolidated Net Income of such Person for such period, excluding any non-cash gains to the extent they represent the reversal of an accrual or reserve for a potential cash item that reduced EBITDA in any prior period;plus
(b) any non-cash gains with respect to cash actually received in a prior period unless such cash did not increase EBITDA in such prior period;plus
(c) any net income from disposed or discontinued operations or from operations expected to be disposed of or discontinued within twelve months after the end of such period;plus
(d) extraordinary gains and unusual or non-recurring gains;plus
(e) gains on asset sales (other than asset sales made in the ordinary course of business), disposals and abandonments.
128
Table of Contents
“EMU” means economic and monetary union as contemplated in the Treaty on European Union.
“Equity Interests” means Capital Stock and all warrants, options or other rights to acquire Capital Stock, but excluding any debt security that is convertible into, or exchangeable for, Capital Stock.
“Equity Offering” means any public or private sale of common stock or Preferred Stock of the Issuer or any of its direct or indirect parent companies (excluding Disqualified Stock), other than:
(1) public offerings with respect to the Issuer’s or any direct or indirect parent company’s common stock registered on Form S-4 or Form S-8;
(2) issuances to any Subsidiary of the Issuer; and
(3) any such public or private sale that constitutes an Excluded Contribution.
“euro” means the single currency of participating member states of the EMU.
“Exchange Act” means the Securities Exchange Act of 1934, as amended, and the rules and regulations of the SEC promulgated thereunder.
“Excluded Contribution” means net cash proceeds, marketable securities or Qualified Proceeds received by the Issuer from
(1) contributions to its common equity capital; and
(2) the sale (other than to a Subsidiary of the Issuer or to any management equity plan or stock option plan or any other management or employee benefit plan or agreement or any distributor equity plan or agreement of the Issuer) of Capital Stock (other than Disqualified Stock and Designated Preferred Stock) of the Issuer;
in each case designated as Excluded Contributions pursuant to an Officer’s Certificate executed by the principal financial officer of the Issuer on the date such capital contributions are made or the date such Equity Interests are sold, as the case may be, which are excluded from the calculation set forth in clause (3) of the first paragraph under “Certain Covenants—Limitation on Restricted Payments.”
“fair market value” means, with respect to any asset or liability, the fair market value of such asset or liability as determined by the Issuer in good faith.
“Fixed Charge Coverage Ratio” means, with respect to any Person for any period, the ratio of EBITDA of such Person for such period to the Fixed Charges of such Person for such period. In the event that the Issuer or any Restricted Subsidiary incurs, assumes, guarantees, redeems, retires or extinguishes any Indebtedness (other than Indebtedness incurred under any revolving credit facility unless such Indebtedness has been permanently repaid and has not been replaced) or issues or redeems Disqualified Stock or Preferred Stock subsequent to the commencement of the period for which the Fixed Charge Coverage Ratio is being calculated but prior to or simultaneously with the event for which the calculation of the Fixed Charge Coverage Ratio is made (the “ Fixed Charge Coverage Ratio Calculation Date”), then the Fixed Charge Coverage Ratio shall be calculated giving pro forma effect to such incurrence, assumption, guarantee, redemption, retirement or extinguishment of Indebtedness, or such issuance or redemption of Disqualified Stock or Preferred Stock, as if the same had occurred at the beginning of the applicable four-quarter period.
For purposes of making the computation referred to above, Investments, acquisitions, dispositions, mergers, consolidations and discontinued operations (as determined in accordance with GAAP) that have been made by the Issuer or any of its Restricted Subsidiaries during the four-quarter reference period or subsequent to such reference period and on or prior to or simultaneously with the Fixed Charge Coverage Ratio Calculation Date shall be calculated on a pro forma basis assuming that all such Investments, acquisitions, dispositions, mergers, consolidations and discontinued operations (and the change in any associated fixed charge obligations and the change in EBITDA resulting therefrom) had occurred on the first day of the four-quarter reference period. If since the beginning of such period any Person that subsequently became a Restricted Subsidiary or was merged with or into the Issuer or any of its Restricted Subsidiaries since the beginning of such period shall have made any Investment, acquisition, disposition, merger, consolidation or discontinued operation that would have required adjustment pursuant to this definition, then the Fixed Charge Coverage Ratio shall be calculated giving pro forma effect thereto for such period as if such Investment, acquisition, disposition, merger, consolidation or discontinued operation had occurred at the beginning of the applicable four-quarter period.
For purposes of this definition, whenever pro forma effect is to be given to an Investment, acquisition, disposition, merger or consolidation (including the Transactions), the pro forma calculations shall be made in good faith by a responsible financial or accounting officer of the Issuer (and may include, for the avoidance of doubt, cost savings and operating expense reductions resulting from such Investment, acquisition, merger or consolidation (including the Transactions) which is being given pro forma effect that have been or are expected to be realized). If any Indebtedness bears a floating rate of interest and is being given pro forma effect, the interest on such Indebtedness shall be calculated as if the rate in effect on the Fixed Charge Coverage Ratio Calculation Date had been the applicable rate for the entire period (taking into account any Hedging Obligations applicable to such Indebtedness). Interest on a Capitalized Lease Obligation shall be deemed to accrue at an interest rate reasonably determined by a responsible financial or accounting officer of the Issuer to be the rate of interest implicit in such Capitalized Lease Obligation in accordance with GAAP. For purposes of making the computation referred to above, interest on any Indebtedness under a revolving credit facility computed on a pro forma basis shall be computed based upon the average daily balance of such Indebtedness during the applicable period except as set forth in the first paragraph of this definition. Interest on Indebtedness that may optionally be determined at an interest rate based upon a factor of a prime or similar rate, a eurocurrency interbank offered rate, or other rate, shall be deemed to have been based upon the rate actually chosen, or, if none, then based upon such optional rate chosen as the Issuer may designate.
129
Table of Contents
“Fixed Charges” means, with respect to any Person for any period, the sum of, without duplication:
(1) Consolidated Interest Expense of such Person for such period;
(2) all cash dividends or other distributions paid (excluding items eliminated in consolidation) on any series of Preferred Stock during such period; and
(3) all cash dividends or other distributions paid (excluding items eliminated in consolidation) on any series of Disqualified Stock during such period.
“Foreign Subsidiary” means, with respect to any Person, any Restricted Subsidiary of such Person that is not organized or existing under the laws of the United States, any state thereof, the District of Columbia, or any territory thereof and any Restricted Subsidiary of such Foreign Subsidiary.
“Foreign Subsidiary Total Assets” means the total assets of the Foreign Subsidiaries, as determined in accordance with GAAP in good faith by the Issuer, without intercompany eliminations.
“GAAP” means generally accepted accounting principles in the United States of America which are in effect on the Issue Date.
“Government Securities” means securities that are:
(1) direct obligations of the United States of America for the timely payment of which its full faith and credit is pledged; or
(2) obligations of a Person controlled or supervised by and acting as an agency or instrumentality of the United States of America the timely payment of which is unconditionally guaranteed as a full faith and credit obligation by the United States of America,
which, in either case, are not callable or redeemable at the option of the issuers thereof, and shall also include a depository receipt issued by a bank (as defined in Section 3(a)(2) of the Securities Act), as custodian with respect to any such Government Securities or a specific payment of principal of or interest on any such Government Securities held by such custodian for the account of the holder of such depository receipt; provided that (except as required by law) such custodian is not authorized to make any deduction from the amount payable to the holder of such depository receipt from any amount received by the custodian in respect of the Government Securities or the specific payment of principal of or interest on the Government Securities evidenced by such depository receipt.
“guarantee” means a guarantee (other than by endorsement of negotiable instruments for collection in the ordinary course of business), direct or indirect, in any manner (including letters of credit and reimbursement agreements in respect thereof), of all or any part of any Indebtedness or other obligations.
“Guarantee” means the guarantee by any Guarantor of the Issuer’s Obligations under the Indenture and the Senior Notes.
“Guarantor” means each Subsidiary of the Issuer, if any, that Guarantees the Senior Notes in accordance with the terms of the Indenture.
“Hedging Obligations” means, with respect to any Person, the obligations of such Person under any interest rate swap agreement, interest rate cap agreement, interest rate collar agreement, commodity swap agreement, commodity cap agreement, commodity collar agreement, foreign exchange contract, currency swap agreement or similar agreement providing for the transfer or mitigation of interest rate or currency risks either generally or under specific contingencies.
“Holder” means the Person in whose name a Senior Note is registered on the registrar’s books.
“Holdings” means LVB Acquisition, Inc., a Delaware corporation and the direct parent of the Issuer.
“Immediate Family Members” means with respect to any individual, such individual’s child, stepchild, grandchild or more remote descendant, parent, stepparent, grandparent, spouse, former spouse, qualified domestic partner, sibling, mother-in-law, father-in-law, son-in-law and daughter-in-law (including adoptive relationships) and any trust, partnership or other bona fide estate-planning vehicle the only beneficiaries of which are any of the foregoing individuals or any private foundation or fund that is controlled by any if the foregoing individuals or any donor-advised fund of which any such individual is the donor.
“Indebtedness” means, with respect to any Person, without duplication:
(1) any indebtedness (including principal and premium) of such Person, whether or not contingent:
(a) in respect of borrowed money;
(b) evidenced by bonds, notes, debentures or similar instruments or letters of credit or bankers’ acceptances (or, without duplication, reimbursement agreements in respect thereof);
(c) representing the balance deferred and unpaid of the purchase price of any property (including Capitalized Lease Obligations) due more than twelve months after such property is acquired, except (i) any such balance that constitutes an obligation in respect of a commercial letter of credit, a trade payable or similar obligation to a trade creditor, in each case accrued in the ordinary course of business and (ii) any earn-out obligations until such obligation becomes a liability on the balance sheet of such Person in accordance with GAAP and if not paid after becoming due and payable;
130
Table of Contents
(d) representing the net obligations under any Hedging Obligations; or
(e) during a Suspension Period only, obligations of the lessee for rental payments in respect of Sale and Lease-back Transactions in an amount equal to the present value of such obligations during the remaining term of the lease using a discount rate equal to the rate of interest implicit in such transaction determined in accordance with GAAP,
if and to the extent that any of the foregoing Indebtedness (other than letters of credit and Hedging Obligations) would appear as a liability upon a balance sheet (excluding the footnotes thereto) of such Person prepared in accordance with GAAP; provided that Indebtedness of any direct or indirect parent of the Issuer appearing upon the balance sheet of the Issuer solely by reason of push-down accounting under GAAP shall be excluded;
(2) to the extent not otherwise included, any obligation by such Person to be liable for, or to pay, as obligor, guarantor or otherwise, on the obligations of the type referred to in clause (1) of a third Person (whether or not such items would appear upon the balance sheet of the such obligor or guarantor), other than by endorsement of negotiable instruments for collection in the ordinary course of business; and
(3) to the extent not otherwise included, the obligations of the type referred to in clause (1) of a third Person secured by a Lien on any asset owned by such first Person, whether or not such Indebtedness is assumed by such first Person;
provided that notwithstanding the foregoing, Indebtedness shall be deemed not to include (a) Contingent Obligations incurred in the ordinary course of business or (b) obligations under or in respect of Qualified Securitization Facilities.
“Independent Financial Advisor” means an accounting, appraisal, investment banking firm or consultant to Persons engaged in Similar Businesses of nationally recognized standing that is, in the good faith judgment of the Issuer, qualified to perform the task for which it has been engaged.
“Initial Purchasers” means Banc of America Securities LLC, Goldman, Sachs & Co., Lehman Brothers Inc., Merrill Lynch, Pierce, Fenner & Smith Incorporated, Wachovia Capital Markets, LLC and Bear, Stearns & Co. Inc.
“Investment Grade Rating” means a rating equal to or higher than Baa3 (or the equivalent) by Moody’s and BBB- (or the equivalent) by S&P, or an equivalent rating by any other Rating Agency.
“Investment Grade Securities” means:
(1) securities issued or directly and fully guaranteed or insured by the United States government or any agency or instrumentality thereof (other than Cash Equivalents);
(2) debt securities or debt instruments with an Investment Grade Rating, but excluding any debt securities or instruments constituting loans or advances among the Issuer and its Subsidiaries;
(3) investments in any fund that invests exclusively in investments of the type described in clauses (1) and (2) which fund may also hold immaterial amounts of cash pending investment or distribution; and
(4) corresponding instruments in countries other than the United States customarily utilized for high quality investments.
“Investments” means, with respect to any Person, all investments by such Person in other Persons (including Affiliates) in the form of loans (including guarantees), advances or capital contributions (excluding accounts receivable, trade credit, advances to customers and distributors, commission, travel and similar advances to employees, directors, officers, managers, distributors and consultants in each case made in the ordinary course of business), purchases or other acquisitions for consideration of Indebtedness, Equity Interests or other securities issued by any other Person and investments that are required by GAAP to be classified on the balance sheet (excluding the footnotes) of the Issuer in the same manner as the other investments included in this definition to the extent such transactions involve the transfer of cash or other property. For purposes of the definition of “Unrestricted Subsidiary” and the covenant described under “Certain Covenants—Limitation on Restricted Payments”:
(1) “Investments” shall include the portion (proportionate to the Issuer’s equity interest in such Subsidiary) of the fair market value of the net assets of a Subsidiary of the Issuer at the time that such Subsidiary is designated an Unrestricted Subsidiary; provided that upon a redesignation of such Subsidiary as a Restricted Subsidiary, the Issuer shall be deemed to continue to have a permanent “Investment” in an Unrestricted Subsidiary in an amount (if positive) equal to:
(a) the Issuer’s “Investment” in such Subsidiary at the time of such redesignation; less
(b) the portion (proportionate to the Issuer’s Equity Interest in such Subsidiary) of the fair market value of the net assets of such Subsidiary at the time of such redesignation; and
(2) any property transferred to or from an Unrestricted Subsidiary shall be valued at its fair market value at the time of such transfer.
131
Table of Contents
The amount of any Investment outstanding at any time shall be the original cost of such Investment, reduced by any dividend, distribution, interest payment, return of capital, repayment or other amount received in cash by the Issuer or a Restricted Subsidiary in respect of such Investment.
“Investors” means The Blackstone Group, Goldman Sachs Capital Partners, Kohlberg Kravis Roberts & Co, TPG Capital and, if applicable, each of their respective Affiliates and funds or partnerships managed by any of them or their respective Affiliates but not including, however, any portfolio companies of any of the foregoing.
“Issue Date” means September 25, 2007.
“Issuer” means LVB Acquisition Merger Sub, Inc., an Indiana corporation, prior to the Acquisition and Biomet, Inc., an Indiana corporation, as the surviving corporation after the Acquisition (and not to any of their Subsidiaries) and its successors.
“Legal Holiday” means a Saturday, a Sunday or a day on which commercial banking institutions are not required to be open in the State of New York or place of payment.
“Lien” means, with respect to any asset, any mortgage, lien (statutory or otherwise), pledge, hypothecation, charge, security interest, preference, priority or encumbrance of any kind in respect of such asset, whether or not filed, recorded or otherwise perfected under applicable law, including any conditional sale or other title retention agreement, any lease in the nature thereof, any option or other agreement to sell or give a security interest in and any filing of or agreement to give any financing statement under the Uniform Commercial Code (or equivalent statutes) of any jurisdiction; provided that in no event shall an operating lease be deemed to constitute a Lien.
“Management Fee Agreement” means the management services agreement between certain of the management companies associated with the Investors or their advisors, if applicable, and the Issuer.
“Management Shareholders” means the members of management (and their Controlled Investment Affiliates and Immediate Family Members) of the Issuer (or its direct parent) who are holders of Equity Interests of any direct or indirect parent companies of the Issuer on the Issue Date or will become holders of such Equity Interests in connection with the Acquisition.
“Moody’s” means Moody’s Investors Service, Inc. and any successor to its rating agency business.
“Net Income” means, with respect to any Person, the net income (loss) of such Person, determined in accordance with GAAP and before any reduction in respect of Preferred Stock dividends.
“Net Proceeds” means the aggregate cash proceeds received by the Issuer or any of its Restricted Subsidiaries in respect of any Asset Sale, including any cash received upon the sale or other disposition of any Designated Non-cash Consideration received in any Asset Sale, net of the direct costs relating to such Asset Sale and the sale or disposition of such Designated Non-cash Consideration, including legal, accounting and investment banking fees, payments made in order to obtain a necessary consent or required by applicable law, and brokerage and sales commissions, any relocation expenses incurred as a result thereof, other fees and expenses, including title and recordation expenses, taxes paid or payable as a result thereof (after taking into account any available tax credits or deductions and any tax sharing arrangements), amounts required to be applied to the repayment of principal, premium, if any, and interest on Senior Indebtedness required (other than required by clause (1) of the second paragraph of “Repurchase at the Option of Holders—Asset Sales”) to be paid as a result of such transaction and any deduction of appropriate amounts to be provided by the Issuer or any of its Restricted Subsidiaries as a reserve in accordance with GAAP against any liabilities associated with the asset disposed of in such transaction and retained by the Issuer or any of its Restricted Subsidiaries after such sale or other disposition thereof, including pension and other post-employment benefit liabilities and liabilities related to environmental matters or against any indemnification obligations associated with such transaction.
“Obligations” means any principal, interest (including any interest accruing on or subsequent to the filing of a petition in bankruptcy, reorganization or similar proceeding at the rate provided for in the documentation with respect thereto, whether or not such interest is an allowed claim under applicable state, federal or foreign law), premium, penalties, fees, indemnifications, reimbursements (including reimbursement obligations with respect to letters of credit and banker’s acceptances), damages and other liabilities, and guarantees of payment of such principal, interest, penalties, fees, indemnifications, reimbursements, damages and other liabilities, payable under the documentation governing any Indebtedness.
“Officer” means the Chairman of the board of directors, the Chief Executive Officer, the Chief Financial Officer, the President, any Executive Vice President, Senior Vice President or Vice President, the Treasurer or the Secretary of the Issuer.
“Officer’s Certificate” means a certificate signed on behalf of a Person by an Officer of such Person, who must be the principal executive officer, the principal financial officer, the treasurer or the principal accounting officer of such Person, that meets the requirements set forth in the Indenture.
“Opinion of Counsel” means a written opinion from legal counsel who is acceptable to the Trustee. The counsel may be an employee of or counsel to the Issuer or the Trustee.
“Option Accounting Issues” means, with respect to the Issuer and its Subsidiaries, any failure to (i) properly document the measurement date for any stock option grant, (ii) record stock option expense (or other items relating thereto) in accordance with GAAP or (iii) issue stock options in accordance with the terms of any applicable Stock Plan (as defined in the Transaction Agreement), in each case to the extent occurring prior to June 4, 2007.
132
Table of Contents
“Permitted Asset Swap” means the substantially concurrent purchase and sale or exchange of Related Business Assets or a combination of Related Business Assets and Cash Equivalents between the Issuer or any of its Restricted Subsidiaries and another Person; provided that any Cash Equivalents received must be applied in accordance with the covenant described under “Repurchase at the Option of Holders—Asset Sales.”
“Permitted Holders” means each of the Investors, the Co-Investors and Management Shareholders and any group (within the meaning of Section 13(d)(3) or Section 14(d)(2) of the Exchange Act or any successor provision) of which any of the foregoing are members; provided that, in the case of such group and without giving effect to the existence of such group or any other group, such Investors, the Co-Investors and Management Shareholders, collectively, have beneficial ownership of more than 50.0% of the total voting power of the Voting Stock of the Issuer or any of its direct or indirect parent companies. Any Person or group whose acquisition of beneficial ownership constitutes a Change of Control in respect of which a Change of Control Offer is made in accordance with the requirements of the Indenture will thereafter, together with its Affiliates, constitute an additional Permitted Holder.
“Permitted Investments” means:
(1) any Investment in the Issuer or any of its Restricted Subsidiaries;
(2) any Investment in Cash Equivalents or Investment Grade Securities;
(3) any Investment by the Issuer or any of its Restricted Subsidiaries in a Person that is engaged in a Similar Business if as a result of such Investment:
(a) such Person becomes a Restricted Subsidiary; or
(b) such Person, in one transaction or a series of related transactions, is merged or consolidated with or into, or transfers or conveys substantially all of its assets to, or is liquidated into, the Issuer or a Restricted Subsidiary,
and, in each case, any Investment held by such Person; provided that such Investment was not acquired by such Person in contemplation of such acquisition, merger, consolidation or transfer;
(4) any Investment in securities or other assets not constituting Cash Equivalents or Investment Grade Securities and received in connection with an Asset Sale made pursuant to the provisions described under “Repurchase at the Option of Holders—Asset Sales” or any other disposition of assets not constituting an Asset Sale;
(5) any Investment existing on the Issue Date or made pursuant to binding commitments in effect on the Issue Date or an Investment consisting of any extension, modification or renewal of any Investment existing on the Issue Date; provided that the amount of any such Investment may be increased (a) as required by the terms of such Investment as in existence on the Issue Date or (b) as otherwise permitted under the Indenture;
(6) any Investment acquired by the Issuer or any of its Restricted Subsidiaries:
(a) in exchange for any other Investment or accounts receivable held by the Issuer or any such Restricted Subsidiary in connection with or as a result of a bankruptcy, workout, reorganization or recapitalization of the issuer of such other Investment or accounts receivable (including any trade creditor or customer); or
(b) in satisfaction of judgments against other Persons; or
(c) as a result of a foreclosure by the Issuer or any of its Restricted Subsidiaries with respect to any secured Investment or other transfer of title with respect to any secured Investment in default;
(7) Hedging Obligations permitted under clause (10) of the covenant described in “Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”;
(8) any Investment in a Similar Business taken together with all other Investments made pursuant to this clause (8) that are at that time outstanding, not to exceed the greater of (a) $450.0 million and (b) 3.0% of Total Assets;
(9) Investments the payment for which consists of Equity Interests (other than Disqualified Stock) of the Issuer, or any of its direct or indirect parent companies; provided that such Equity Interests will not increase the amount available for Restricted Payments under clause (3) of the first paragraph under the covenant described in “Certain Covenants—Limitations on Restricted Payments”;
(10) guarantees of Indebtedness permitted under the covenant described in “Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”;
(11) any transaction to the extent it constitutes an Investment that is permitted by and made in accordance with the provisions of the second paragraph of the covenant described under “Certain Covenants—Transactions with Affiliates” (except transactions described in clauses (2), (5) and (9) of such paragraph);
(12) Investments consisting of purchases and acquisitions of inventory, supplies, material or equipment or the licensing or contribution of intellectual property pursuant to joint marketing arrangements with other Persons;
133
Table of Contents
(13) additional Investments, taken together with all other Investments made pursuant to this clause (13) that are at that time outstanding (without giving effect to the sale of an Unrestricted Subsidiary to the extent the proceeds of such sale do not consist of cash or marketable securities), not to exceed the greater of (a) $450.0 million and (b) 3.0% of Total Assets;
(14) Investments in or relating to a Securitization Subsidiary that, in the good faith determination of the Issuer are necessary or advisable to effect any Qualified Securitization Facility or any repurchase obligation in connection therewith;
(15) advances to, or guarantees of Indebtedness of, employees not in excess of $25.0 million outstanding at any one time, in the aggregate;
(16) loans and advances to employees, directors, officers, managers, distributors and consultants for business-related travel expenses, moving expenses and other similar expenses, in each case incurred in the ordinary course of business or consistent with past practices or to fund such Person’s purchase of Equity Interests of the Issuer or any direct or indirect parent company thereof;
(17) advances, loans or extensions of trade credit in the ordinary course of business by the Issuer or any of its Restricted Subsidiaries;
(18) any Investment in any Subsidiary or any joint venture in connection with intercompany cash management arrangements or related activities arising in the ordinary course of business;
(19) Investments consisting of purchases and acquisitions of assets or services in the ordinary course of business;
(20) Investments made in the ordinary course of business in connection with obtaining, maintaining or renewing client contacts and loans or advances made to distributors in the ordinary course of business;
(21) Investments in prepaid expenses, negotiable instruments held for collection and lease, utility and workers compensation, performance and similar deposits entered into as a result of the operations of the business in the ordinary course of business; and
(22) repurchases of the Senior Notes.
“Permitted Liens” means, with respect to any Person:
(1) pledges or deposits by such Person under workmen’s compensation laws, unemployment insurance, other social security benefits or other insurance related obligations (including, but not limited to, in respect of deductibles, self insured retention amounts and premiums and adjustments thereto) or good faith deposits in connection with bids, tenders, contracts (other than for the payment of Indebtedness) or leases to which such Person is a party, or deposits to secure public or statutory obligations of such Person or deposits of cash or U.S. government bonds to secure surety or appeal bonds to which such Person is a party, or deposits as security for contested taxes or import duties or for the payment of rent, in each case incurred in the ordinary course of business;
(2) Liens imposed by law, such as carriers’, warehousemen’s and mechanics’ Liens, in each case for sums not yet overdue for a period of more than 30 days or being contested in good faith by appropriate proceedings or other Liens arising out of judgments or awards against such Person with respect to which such Person shall then be proceeding with an appeal or other proceedings for review if adequate reserves with respect thereto are maintained on the books of such Person in accordance with GAAP;
(3) Liens for taxes, assessments or other governmental charges not yet overdue for a period of more than 30 days or not yet payable or subject to penalties for nonpayment or which are being contested in good faith by appropriate proceedings diligently conducted, if adequate reserves with respect thereto are maintained on the books of such Person in accordance with GAAP;
(4) Liens in favor of issuers of performance and surety bonds or bid bonds or with respect to other regulatory requirements or letters of credit issued pursuant to the request of and for the account of such Person in the ordinary course of its business;
(5) minor survey exceptions, minor encumbrances, easements or reservations of, or rights of others for, licenses, rights-of-way, sewers, electric lines, telegraph and telephone lines and other similar purposes, or zoning or other restrictions as to the use of real properties or Liens incidental, to the conduct of the business of such Person or to the ownership of its properties which were not incurred in connection with Indebtedness and which do not in the aggregate materially adversely affect the value of said properties or materially impair their use in the operation of the business of such Person;
(6) Liens securing Indebtedness permitted to be incurred pursuant to clause (4), (12)(b), (13), (23) or (24) of the second paragraph under “Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”; provided that (a) Liens securing Indebtedness, Disqualified Stock or Preferred Stock permitted to be incurred pursuant to clause (13) relate only to Refinancing Indebtedness that serves to refund or refinance Indebtedness, Disqualified Stock or Preferred Stock incurred under clause (4) or (12)(b) of the second paragraph of “Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock,” (b) Liens securing Indebtedness permitted to be incurred pursuant to clause (23) extend only to the assets of Foreign Subsidiaries, (c) Liens securing Indebtedness permitted to be incurred pursuant to clause (24) are solely on acquired property or the assets of the acquired entity, as the case may be, and (d) Liens securing Indebtedness, Disqualified Stock or Preferred Stock to be incurred pursuant to clause (4) of the second paragraph under “Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock” extend only to the assets so purchased, leased or improved;
(7) Liens existing on the Issue Date;
134
Table of Contents
(8) Liens on property or shares of stock or other assets of a Person at the time such Person becomes a Subsidiary; provided that such Liens are not created or incurred in connection with, or in contemplation of, such other Person becoming such a Subsidiary; provided , further , that such Liens may not extend to any other property or other assets owned by the Issuer or any of its Restricted Subsidiaries;
(9) Liens on property or other assets at the time the Issuer or a Restricted Subsidiary acquired the property or such other assets, including any acquisition by means of a merger or consolidation with or into the Issuer or any of its Restricted Subsidiaries; provided that such Liens are not created or incurred in connection with, or in contemplation of, such acquisition; provided , further , that the Liens may not extend to any other property owned by the Issuer or any of its Restricted Subsidiaries;
(10) Liens securing Indebtedness or other obligations of a Restricted Subsidiary owing to the Issuer or another Restricted Subsidiary permitted to be incurred in accordance with the covenant described under “Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”;
(11) Liens securing Hedging Obligations; provided that, with respect to Hedging Obligations relating to Indebtedness, such Indebtedness is, and is permitted to be under the Indenture, secured by a Lien on the same property securing such Hedging Obligations;
(12) Liens on specific items of inventory or other goods and proceeds of any Person securing such Person’s obligations in respect of bankers’ acceptances issued or created for the account of such Person to facilitate the purchase, shipment or storage of such inventory or other goods;
(13) leases, subleases, licenses or sublicenses granted to others in the ordinary course of business which do not materially interfere with the ordinary conduct of the business of the Issuer or any of its Restricted Subsidiaries and do not secure any Indebtedness;
(14) Liens arising from Uniform Commercial Code financing statement filings regarding operating leases entered into by the Issuer and its Restricted Subsidiaries in the ordinary course of business;
(15) Liens in favor of the Issuer or any Guarantor;
(16) Liens on equipment of the Issuer or any of its Restricted Subsidiaries granted in the ordinary course of business to the Issuer’s clients;
(17) Liens on accounts receivable, Securitization Assets and related assets incurred in connection with a Qualified Securitization Facility;
(18) Liens to secure any refinancing, refunding, extension, renewal or replacement (or successive refinancing, refunding, extensions, renewals or replacements) as a whole, or in part, of any Indebtedness secured by any Lien referred to in the foregoing clauses (6), (7), (8) and (9); provided that (a) such new Lien shall be limited to all or part of the same property that secured the original Lien (plus improvements on such property), and (b) the Indebtedness secured by such Lien at such time is not increased to any amount greater than the sum of (i) the outstanding principal amount or, if greater, committed amount of the Indebtedness described under clauses (6), (7), (8) and (9) at the time the original Lien became a Permitted Lien under the Indenture, and (ii) an amount necessary to pay any fees and expenses, including premiums, related to such refinancing, refunding, extension, renewal or replacement;
(19) deposits made in the ordinary course of business to secure liability to insurance carriers;
(20) other Liens securing obligations in an aggregate amount at any one time outstanding not to exceed the greater of (a) $100.0 million and (b) 1.0% of Total Assets determined as of the date of incurrence;
(21) Liens securing judgments for the payment of money not constituting an Event of Default under clause (5) under “Events of Default and Remedies” so long as such Liens are adequately bonded and any appropriate legal proceedings that may have been duly initiated for the review of such judgment have not been finally terminated or the period within which such proceedings may be initiated has not expired;
(22) Liens in favor of customs and revenue authorities arising as a matter of law to secure payment of customs duties in connection with the importation of goods in the ordinary course of business;
(23) Liens (a) of a collection bank arising under Section 4-210 of the Uniform Commercial Code on items in the course of collection, (b) attaching to commodity trading accounts or other commodity brokerage accounts incurred in the ordinary course of business, and (c) in favor of banking institutions arising as a matter of law encumbering deposits (including the right of set-off) and which are within the general parameters customary in the banking industry;
(24) Liens deemed to exist in connection with Investments in repurchase agreements permitted under “Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”; provided that such Liens do not extend to any assets other than those that are the subject of such repurchase agreement;
(25) Liens encumbering reasonable customary deposits and margin deposits and similar Liens attaching to commodity trading accounts or other brokerage accounts incurred in the ordinary course of business and not for speculative purposes;
(26) Liens that are contractual rights of set-off (a) relating to the establishment of depository relations with banks not given in connection with the issuance of Indebtedness, (b) relating to pooled deposit or sweep accounts of the Issuer or any of its Restricted Subsidiaries to permit satisfaction of overdraft or similar obligations incurred in the ordinary course of business of the Issuer and its Restricted Subsidiaries or (c) relating to purchase orders and other agreements entered into with customers of the Issuer or any of its Restricted Subsidiaries in the ordinary course of business;
135
Table of Contents
(27) Liens securing obligations owed by the Issuer or any Restricted Subsidiary to any lender under the Senior Credit Facilities or any Affiliate of such a lender in respect of any overdraft and related liabilities arising from treasury, depository and cash management services or any automated clearing house transfers of funds;
(28) during a Suspension Period only, Liens securing Indebtedness (other than Indebtedness that is secured equally and ratably with (or on a basis subordinated to) the Senior Notes), and Indebtedness represented by Sale and Lease-Back Transactions in an amount not to exceed 15.0% of Total Assets at any one time outstanding;
(29) Liens securing Indebtedness the proceeds of which are used to develop or construct new facilities (or any improvements to existing facilities) or equipment (or any improvements to existing equipment) designed primarily for the purpose of air or water pollutions control; provided that such Indebtedness is permitted to be incurred by the terms of the Indenture and such Liens do not extend to any assets of the Issuer or its Restricted Subsidiaries other than the assets developed, constructed or improved with the proceeds of the Indebtedness secured by such Lien;
(30) any encumbrance or restriction (including put and call arrangements) with respect to capital stock of any joint venture or similar arrangement pursuant to any joint venture or similar agreement;
(31) Liens arising out of conditional sale, title retention, consignment or similar arrangements for the sale or purchase of goods entered into by the Issuer or any Restricted Subsidiary in the ordinary course of business;
(32) Liens solely on any cash earnest money deposits made by the Issuer or any of its Restricted Subsidiaries in connection with any letter of intent or purchase agreement permitted;
(33) ground leases in respect of real property on which facilities owned or leased by the Issuer or any of its Subsidiaries are located;
(34) Liens on insurance policies and the proceeds thereof securing the financing of the premiums with respect thereto;
(35) Liens on Capital Stock of an Unrestricted Subsidiary that secure Indebtedness or other obligations of such Unrestricted Subsidiary; and
(36) Liens on the assets of non-guarantor Subsidiaries securing Indebtedness of the Issuer or the Restricted Subsidiaries that were permitted by the terms of the Indenture to be incurred.
For purposes of this definition, the term “Indebtedness” shall be deemed to include interest on such Indebtedness.
“Person” means any individual, corporation, limited liability company, partnership, joint venture, association, joint stock company, trust, unincorporated organization, government or any agency or political subdivision thereof or any other entity.
“Preferred Stock” means any Equity Interest with preferential rights of payment of dividends or upon liquidation, dissolution, or winding up.
“Qualified Proceeds” means the fair market value of assets that are used or useful in, or Capital Stock of any Person engaged in, a Similar Business.
“Qualified Securitization Facility” means any Securitization Facility (1) constituting a securitization financing facility that meets the following conditions: (a) the board of directors of the Issuer shall have determined in good faith that such Securitization Facility (including financing terms, covenants, termination events and other provisions) is in the aggregate economically fair and reasonable to the Issuer and the applicable Securitization Subsidiary, (b) all sales and/or contributions of Securitization Assets and related assets to the applicable Securitization Subsidiary are made at fair market value (as determined in good faith by the Issuer) and (c) the financing terms, covenants, termination events and other provisions thereof shall be market terms (as determined in good faith by the Issuer) or (2) constituting a receivables financing facility.
“Rating Agencies” means Moody’s and S&P or if Moody’s or S&P or both shall not make a rating on the Senior Notes publicly available, a nationally recognized statistical rating agency or agencies, as the case may be, selected by the Issuer which shall be substituted for Moody’s or S&P or both, as the case may be.
“Registration Rights Agreement” means one or more registration rights agreements with respect to the Notes and the Additional Senior Notes among the Issuer, the Guarantors and the Initial Purchasers.
“Related Business Assets” means assets (other than Cash Equivalents) used or useful in a Similar Business, provided that any assets received by the Issuer or a Restricted Subsidiary in exchange for assets transferred by the Issuer or a Restricted Subsidiary shall not be deemed to be Related Business Assets if they consist of securities of a Person, unless upon receipt of the securities of such Person, such Person would become a Restricted Subsidiary.
“Restricted Investment” means an Investment other than a Permitted Investment.
“Restricted Subsidiary” means, at any time, any direct or indirect Subsidiary of the Issuer (including any Foreign Subsidiary) that is not then an Unrestricted Subsidiary; provided that upon an Unrestricted Subsidiary ceasing to be an Unrestricted Subsidiary, such Subsidiary shall be included in the definition of “Restricted Subsidiary.”
136
Table of Contents
“S&P” means Standard & Poor’s, a division of The McGraw-Hill Companies, Inc., and any successor to its rating agency business.
“Sale and Lease-Back Transaction” means any arrangement providing for the leasing by the Issuer or any of its Restricted Subsidiaries of any real or tangible personal property, which property has been or is to be sold or transferred by the Issuer or such Restricted Subsidiary to a third Person in contemplation of such leasing.
“SEC” means the U.S. Securities and Exchange Commission.
“Secured Indebtedness” means any Indebtedness of the Issuer or any of its Restricted Subsidiaries secured by a Lien.
“Securities Act” means the Securities Act of 1933, as amended, and the rules and regulations of the SEC promulgated thereunder.
“Securitization Assets” means the accounts receivable, royalty or other revenue streams and other rights to payment related to the Specified Contract Rights subject to a Qualified Securitization Facility that is a securitization financing facility (and not a receivables financing facility) and the proceeds thereof.
“Securitization Facility” means any of one or more receivables or securitization financing facilities as amended, supplemented, modified, extended, renewed, restated or refunded from time to time, the Obligations of which are non-recourse (except for customary representations, warranties, covenants and indemnities made in connection with such facilities) to the Issuer or any of its Restricted Subsidiaries (other than a Securitization Subsidiary) pursuant to which the Issuer or any of its Restricted Subsidiaries sells or grants a security interest in its accounts receivable or Securitization Assets or assets related thereto to either (a) a Person that is not a Restricted Subsidiary or (b) a Securitization Subsidiary that in turn sells its accounts receivable to a Person that is not a Restricted Subsidiary.
“Securitization Fees” means distributions or payments made directly or by means of discounts with respect to any participation interest issued or sold in connection with, and other fees paid to a Person that is not a Securitization Subsidiary in connection with, any Qualified Securitization Facility.
“Securitization Subsidiary” means any Subsidiary formed for the purpose of, and that solely engages only in one or more Qualified Securitization Facilities and other activities reasonably related thereto.
“Senior Credit Facilities” means the ABL Facilities and the CF Credit Facilities.
“Senior Indebtedness” means:
(1) all Indebtedness of the Issuer or any Guarantor outstanding under the Senior Credit Facilities or Senior Notes and related Guarantees (including interest accruing on or after the filing of any petition in bankruptcy or similar proceeding or for reorganization of the Issuer or any Guarantor (at the rate provided for in the documentation with respect thereto, regardless of whether or not a claim for post-filing interest is allowed in such proceedings)), and any and all other fees, expense reimbursement obligations, indemnification amounts, penalties, and other amounts (whether existing on the Issue Date or thereafter created or incurred) and all obligations of the Issuer or any Guarantor to reimburse any bank or other Person in respect of amounts paid under letters of credit, acceptances or other similar instruments;
(2) all Hedging Obligations (and guarantees thereof) owing to a Lender (as defined in the Senior Credit Facilities) or any Affiliate of such Lender (or any Person that was a Lender or an Affiliate of such Lender at the time the applicable agreement giving rise to such Hedging Obligation was entered into), provided that such Hedging Obligations are permitted to be incurred under the terms of the Indenture;
(3) any other Indebtedness of the Issuer or any Guarantor permitted to be incurred under the terms of the Indenture, unless the instrument under which such Indebtedness is incurred expressly provides that it is on a parity with or subordinated in right of payment to the Senior Subordinated Notes or any related Guarantee; and
(4) all Obligations with respect to the items listed in the preceding clauses (1), (2) and (3);
provided that Senior Indebtedness shall not include:
(a) any obligation of such Person to the Issuer or any of its Subsidiaries;
(b) any liability for federal, state, local or other taxes owed or owing by such Person;
(c) any accounts payable or other liability to trade creditors arising in the ordinary course of business;
(d) any Indebtedness or other Obligation of such Person which is subordinate or junior in any respect to any other Indebtedness or other Obligation of such Person; or
(e) that portion of any Indebtedness which at the time of incurrence is incurred in violation of the Indenture.
“Senior Subordinated Notes” means the Issuer’s 11 5/8% senior subordinated notes due 2017 issued under the indenture governing the Senior Subordinated Notes.
“Significant Subsidiary” means any Restricted Subsidiary that would be a “significant subsidiary” as defined in Article 1, Rule 1-02 of Regulation S-X, promulgated pursuant to the Securities Act, as such regulation is in effect on the Issue Date.
137
Table of Contents
“Similar Business” means (1) any business engaged in by the Issuer or any of its Restricted Subsidiaries on the Issue Date, and (2) any business or other activities that are reasonably similar, ancillary, complementary or related to, or a reasonable extension, development or expansion of, the businesses in which the Issuer and its Restricted Subsidiaries are engaged on the Issue Date.
“Specified Contract Rights” means certain intellectual property licenses, agreements or other contracts giving rise to not more than $50.0 million of annual accounts receivable, royalty or other intellectual property revenue streams or other rights to payment.
“Subordinated Indebtedness” means, with respect to the Senior Notes,
(1) any Indebtedness of the Issuer which is by its terms subordinated in right of payment to the Senior Notes, and
(2) any Indebtedness of any Guarantor which is by its terms subordinated in right of payment to the Guarantee of such entity of the Senior Notes.
“Subsidiary” means, with respect to any Person:
(1) any corporation, association, or other business entity (other than a partnership, joint venture, limited liability company or similar entity) of which more than 50.0% of the total voting power of shares of Capital Stock entitled (without regard to the occurrence of any contingency) to vote in the election of directors, managers or trustees thereof is at the time of determination owned or controlled, directly or indirectly, by such Person or one or more of the other Subsidiaries of that Person or a combination thereof or is consolidated under GAAP with such Person at such time; and
(2) any partnership, joint venture, limited liability company or similar entity of which
(a) more than 50.0% of the capital accounts, distribution rights, total equity and voting interests or general or limited partnership interests, as applicable, are owned or controlled, directly or indirectly, by such Person or one or more of the other Subsidiaries of that Person or a combination thereof whether in the form of membership, general, special or limited partnership or otherwise, and
(b) such Person or any Restricted Subsidiary of such Person is a controlling general partner or otherwise controls such entity.
“Total Assets” means the total assets of the Issuer and its Restricted Subsidiaries, determined on a consolidated basis in accordance with GAAP, as shown on the most recent balance sheet of the Issuer or such other Person as may be expressly stated.
“Transaction Agreement” means the Agreement and Plan of Merger, dated as of December 18, 2006 (as amended and restated as of June 7, 2007) by and among Biomet, Inc., LVB Acquisition , LLC, and the Issuer, as the same may be amended prior to the Issue Date.
“Transactions” means the transactions contemplated by the Transaction Agreement, the issuance of the Senior Notes and the Senior Subordinated Notes and borrowings under the Senior Credit Facilities as in effect on the Issue Date.
“Treasury Rate” means, as of any Redemption Date, the yield to maturity as of such Redemption Date of United States Treasury securities with a constant maturity (as compiled and published in the most recent Federal Reserve Statistical Release H.15 (519) that has become publicly available at least two Business Days prior to the Redemption Date (or, if such Statistical Release is no longer published, any publicly available source of similar market data)) most nearly equal to the period from the Redemption Date to October 15, 2012 in the case of the Senior Cash Pay Notes and October 15, 2012 in the case of the Senior Toggle Notes; provided that if the period from the Redemption Date to such date is less than one year, the weekly average yield on actually traded United States Treasury securities adjusted to a constant maturity of one year will be used.
“Trust Indenture Act” means the Trust Indenture Act of 1939, as amended (15 U.S.C. §§ 77aaa-777bbbb).
“Unrestricted Subsidiary” means:
(1) any Subsidiary of the Issuer which at the time of determination is an Unrestricted Subsidiary (as designated by the Issuer, as provided below); and
(2) any Subsidiary of an Unrestricted Subsidiary.
The Issuer may designate any Subsidiary of the Issuer (including any existing Subsidiary and any newly acquired or newly formed Subsidiary) to be an Unrestricted Subsidiary unless such Subsidiary or any of its Subsidiaries owns any Equity Interests or Indebtedness of, or owns or holds any Lien on, any property of, the Issuer or any Subsidiary of the Issuer (other than solely any Subsidiary of the Subsidiary to be so designated); provided that
(1) any Unrestricted Subsidiary must be an entity of which the Equity Interests entitled to cast at least a majority of the votes that may be cast by all Equity Interests having ordinary voting power for the election of directors or Persons performing a similar function are owned, directly or indirectly, by the Issuer;
(2) such designation complies with the covenants described under “Certain Covenants—Limitation on Restricted Payments”; and
(3) each of (a) the Subsidiary to be so designated and (b) its Subsidiaries has not at the time of designation, and does not thereafter, create, incur, issue, assume, guarantee or otherwise become directly or indirectly liable with respect to any Indebtedness pursuant to which the lender has recourse to any of the assets of the Issuer or any Restricted Subsidiary.
138
Table of Contents
The Issuer may designate any Unrestricted Subsidiary to be a Restricted Subsidiary; provided that, immediately after giving effect to such designation, no Default shall have occurred and be continuing and either:
(1) the Issuer could incur at least $1.00 of additional Indebtedness pursuant to the Fixed Charge Coverage Test; or
(2) the Fixed Charge Coverage Ratio for the Issuer would be equal to or greater than such ratio for the Issuer immediately prior to such designation, in each case on a pro forma basis taking into account such designation.
Any such designation by the Issuer shall be notified by the Issuer to the Trustee by promptly filing with the Trustee a copy of the resolution of the board of directors of the Issuer or any committee thereof giving effect to such designation and an Officer’s Certificate certifying that such designation complied with the foregoing provisions.
“Voting Stock” of any Person as of any date means the Capital Stock of such Person that is at the time entitled to vote in the election of the board of directors of such Person.
“Weighted Average Life to Maturity” means, when applied to any Indebtedness, Disqualified Stock or Preferred Stock, as the case may be, at any date, the quotient obtained by dividing:
(1) the sum of the products of the number of years from the date of determination to the date of each successive scheduled principal payment of such Indebtedness or redemption or similar payment with respect to such Disqualified Stock or Preferred Stock multiplied by the amount of such payment; by
(2) the sum of all such payments.
“Wholly-Owned Subsidiary” of any Person means a Subsidiary of such Person, 100.0% of the outstanding Equity Interests of which (other than directors’ qualifying shares) shall at the time be owned by such Person or by one or more Wholly-Owned Subsidiaries of such Person.
139
Table of Contents
DESCRIPTION OF SENIOR SUBORDINATED NOTES
General
Certain terms used in this description are defined under the subheading “Certain Definitions.” In this description, (1) the term “Issuer” refers only to LVB Acquisition Merger Sub, Inc. prior to the Acquisition and to Biomet, Inc., as the surviving corporation after the Acquisition, and not to any of their subsidiaries, and (2) the terms “we”, “our” and “us” each refer to the Issuer and its consolidated Subsidiaries assuming completion of the Acquisition.
The Issuer issued $940.7 million aggregate principal amount of the original senior subordinated notes on September 25, 2007 and $74.3 million aggregate principal amount of the original senior subordinated notes on October 16, 2007 under an indenture dated as of September 25, 2007 and a supplemental indenture dated as of October 16, 2007 (collectively, the “Indenture”) among the Issuer, the Guarantors and Wells Fargo Bank, National Association, as trustee (the “Trustee”). The Indenture has been qualified under and is subject to and governed by the Trust Indenture Act of 1939. Except as set forth herein, the terms of the Senior Subordinated Notes will be substantially identical and include those stated in the Indenture and those made part of the Indenture by reference to the Trust Indenture Act.
The following description is only a summary of the material provisions of the Indenture, does not purport to be complete and is qualified in its entirety by reference to the provisions of the Indenture, including the definitions therein of certain terms used below. We urge you to read the Indenture because it, and not this description, will define your rights as Holders of the Senior Subordinated Notes. You may request copies of the Indenture at our address set forth under “Where You Can Find Additional Information.”
Brief Description of the Senior Subordinated Notes
The Senior Subordinated Notes:
| • | are general, unsecured, senior subordinated obligations of the Issuer; |
| • | are subordinated in right of payment to all existing and future Senior Indebtedness (including the Senior Credit Facilities and the Senior Notes) of the Issuer; |
| • | are structurally subordinated to all existing and future Indebtedness, claims of holders of Preferred Stock and other liabilities of Subsidiaries of the Issuer that do not guarantee the Senior Subordinated Notes; |
| • | are senior in right of payment to all existing and future Subordinated Indebtedness (as defined with respect to the Senior Subordinated Notes) of the Issuer; |
| • | are initially guaranteed on an unsecured senior subordinated basis by the Guarantors and will also be guaranteed in the future by each Subsidiary, if any, that guarantees Indebtedness under the CF Credit Facilities; and |
| • | are subject to registration with the SEC pursuant to the Registration Rights Agreement. |
Guarantees
The Guarantors, as primary obligors and not merely as sureties, initially jointly and severally, irrevocably and unconditionally, guarantee, on an unsecured senior subordinated basis, the full and punctual payment when due, whether at maturity, by acceleration or otherwise, of all obligations of the Issuer under the Indenture and the Senior Subordinated Notes, whether for payment of principal of, premium, if any, or interest in respect of the Senior Subordinated Notes, expenses, indemnification or otherwise, on the terms set forth in the Indenture by executing the Indenture.
The Guarantors initially guarantee the Senior Subordinated Notes and, in the future, each direct and indirect Subsidiary of the Issuer that guarantees Indebtedness under the CF Credit Facilities will guarantee the Senior Subordinated Notes. Each of the Guarantees of the Senior Subordinated Notes is a general, unsecured, senior subordinated obligation of each Guarantor, is subordinated in right of payment to all existing and future Senior Indebtedness of such Guarantor (including such Guarantor’s guarantee of the CF Credit Facilities and the Senior Notes). Each of the Guarantees of the Senior Subordinated Notes is structurally subordinated to all existing and future Indebtedness, claims of holders of Preferred Stock and other liabilities of Subsidiaries of each Guarantor that do not Guarantee the Senior Subordinated Notes.
Not all of the Issuer’s Subsidiaries guarantee the Senior Subordinated Notes. In the event of a bankruptcy, liquidation, reorganization or similar proceeding of any of these non-guarantor Subsidiaries, the non-guarantor Subsidiaries will pay the holders of their debt and their trade creditors before they will be able to distribute any of their assets to the Issuer. As a result, all of the existing and future liabilities of our non-guarantor Subsidiaries, including any claims of trade creditors, are effectively senior to the Senior Subordinated Notes. For the periods from June 1, 2007 through July 11, 2007, from July 12, 2007 through November 30, 2007, from July 12, 2007 through May 31, 2008, and the six months ended November 30, 2008 our non-guarantor subsidiaries accounted for approximately $83 million, or 33% of our consolidated net sales, $373 million, or 42% of our consolidated net sales, $1,060 million, or 50% of our consolidated net sales, and $469 million, or 38% of our consolidated net sales, for such period, respectively. As of November 30, 2008, our non-guarantor subsidiaries accounted for approximately $3,661 million, or 31% of our consolidated long-term assets. All amounts are presented after giving effect to intercompany eliminations.
The obligations of each Guarantor under its Guarantee are limited as necessary to prevent the Guarantee from constituting a fraudulent conveyance under applicable law. This provision may not, however, be effective to protect a Guarantee from being voided under fraudulent transfer law, or may reduce the applicable Guarantor’s obligation to an amount that effectively makes its Guarantee worthless. If a Guarantee was rendered voidable, it could be subordinated by a court to all other indebtedness (including guarantees and other contingent liabilities) of the Guarantor, and, depending on the amount of such indebtedness, a Guarantor’s liability on its Guarantee could be reduced to zero. See “Risk Factors—Risks Related to Our Indebtedness and the Notes—Federal and state fraudulent transfer laws may permit a court to void the notes and the guarantees, subordinate claims in respect of the notes and the guarantees and require noteholders to return payments received. If this occurs, you may not receive any payments on the notes.”
140
Table of Contents
Any Guarantor that makes a payment under its Guarantee will be entitled upon payment in full of all guaranteed obligations under the Indenture to a contribution from each other Guarantor in an amount equal to such other Guarantor’s pro rata portion of such payment based on the respective net assets of all the Guarantors at the time of such payment determined in accordance with GAAP.
Each Guarantor may consolidate with or merge into or sell all or substantially all its assets to the Issuer or another Guarantor without limitation or any other Person upon the terms and conditions set forth in the Indenture. See “Certain Covenants—Merger, Consolidation or Sale of All or Substantially All Assets.”
Each Guarantee by a Guarantor provides by its terms that it will be automatically and unconditionally released and discharged upon:
(1) (a) any sale, exchange or transfer (by merger or otherwise) of (i) the Capital Stock of such Guarantor, after which the applicable Guarantor is no longer a Restricted Subsidiary or (ii) all or substantially all the assets of such Guarantor, in each case if such sale, exchange or transfer is made in compliance with the applicable provisions of the Indenture;
(b) the release or discharge of the guarantee by such Guarantor of Indebtedness under the CF Credit Facilities, or the release or discharge of such other guarantee that resulted in the creation of such Guarantee, except a discharge or release by or as a result of payment under such guarantee;
(c) the designation of any Restricted Subsidiary that is a Guarantor as an Unrestricted Subsidiary in compliance with the applicable provisions of the Indenture; or
(d) the exercise by the Issuer of its legal defeasance option or covenant defeasance option as described under “Legal Defeasance and Covenant Defeasance” or the discharge of the Issuer’s obligations under the Indenture in accordance with the terms of the Indenture; and
(2) such Guarantor delivering to the Trustee an Officer’s Certificate and an Opinion of Counsel, each stating that all conditions precedent provided for in the Indenture relating to such transaction have been complied with.
Ranking
The payment of the principal of, premium, if any, and interest on the Senior Subordinated Notes and the payment of any Guarantee are subordinated in right of payment to the prior payment in cash in full of all existing and future Senior Indebtedness of the Issuer or the relevant Guarantor, as the case may be, including the obligations of the Issuer and such Guarantor under the Senior Credit Facilities and the Senior Notes.
As of November 30, 2008, the Issuer and the Guarantors had $5,132 million of Senior Indebtedness outstanding, (including $1,550 million in aggregate principal amount of the Senior Notes and $3,582 million of borrowings under the Senior Credit Facilities). As of November 30, 2008, the Issuer also have had (1) an additional approximately $400 million of borrowing capacity under the cash flow revolving credit facility under the CF Credit Facilities, which, if borrowed, would be Senior Indebtedness, (2) an additional $350 million available for borrowing under the ABL Facilities, subject to borrowing base limitations, which, if borrowed, would be Senior Indebtedness, (3) the option to raise additional incremental term loans or incremental cash flow revolving facility commitments under the CF Credit Facilities of up to an amount that would cause our Senior Secured Leverage Ratio (as defined in the CF Credit Facilities) to be equal to or less than 4.50 to 1.00, which, if borrowed, would be Senior Indebtedness, (4) the option to raise additional incremental asset-based revolving credit facility commitments under the ABL Facilities by up to $100 million, which, if borrowed, would be Senior Indebtedness and (5) an additional $171 million available for borrowing under the existing European line of credit and Japanese lines of credit, which, if borrowed, would be Senior Indebtedness. In addition, under the Senior Toggle Notes we will have the option to elect to pay PIK interest for five years after the closing date for any interest period other than for the initial interest period. In the event we make a PIK interest election in each period in which we are entitled to make such an election, our debt will increase by the amount of such interest.
Although the Indenture contains limitations on the amount of additional Indebtedness that the Issuer, the Issuer’s Restricted Subsidiaries (including the Guarantors) may incur, under certain circumstances the amount of such Indebtedness could be substantial and, in any case, such Indebtedness may be Senior Indebtedness. The Indenture does not limit the amount of additional Indebtedness that Holdings may incur. See “Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock.”
Paying Agent and Registrar for the Senior Subordinated Notes
The Issuer maintains one or more paying agents for the Senior Subordinated Notes. The initial paying agent for the Senior Subordinated Notes is the Trustee.
The Issuer also maintains one or more registrars and a transfer agent. The initial registrar and transfer agent with respect to the Senior Subordinated Notes is the Trustee. The registrar maintains a register reflecting ownership of the Senior Subordinated Notes outstanding from time to time. The registered Holder of a Senior Subordinated Note is treated as the owner of the Senior Subordinated Note for all purposes. The transfer agent makes payments on and facilitates transfer of Senior Subordinated Notes on behalf of the Issuer.
The Issuer may change the paying agent, the registrar or the transfer agent without prior notice to the Holders. The Issuer or any of its Subsidiaries may act as a paying agent, registrar or transfer agent.
141
Table of Contents
If any Senior Subordinated Notes are listed on an exchange and the rules of such exchange so require, the Issuer will satisfy any requirement of such exchange as to paying agents, registrars and transfer agents and will comply with any notice requirements required under such exchange in connection with any change of paying agent, registrar or transfer agent.
Subordination of the Senior Subordinated Notes
Only Indebtedness of the Issuer or a Guarantor that is Senior Indebtedness ranks senior to the Senior Subordinated Notes and the Guarantees in accordance with the provisions of the Indenture. The Senior Subordinated Notes and Guarantees rank pari passu in all respects with all other Senior Subordinated Indebtedness of the Issuer and the relevant Guarantor, respectively.
We agree in the Indenture that the Issuer and the Guarantors will not incur any Indebtedness that is subordinate or junior in right of payment to the Senior Indebtedness of such Person, unless such Indebtedness is equal in right of payment with the Senior Subordinated Notes or the related Guarantees or is expressly subordinated in right of payment to the Senior Subordinated Indebtedness or the related Guarantees, as the case may be. The Indenture does not treat (i) unsecured Indebtedness as subordinated or junior to Secured Indebtedness merely because it is unsecured or (ii) Senior Indebtedness as subordinated or junior to any other Senior Indebtedness merely because it has a junior priority with respect to the same collateral.
Neither the Issuer nor any Guarantor is permitted to pay principal of, premium, if any, or interest on the Senior Subordinated Notes (or pay any other Obligations relating to the Senior Subordinated Notes, including fees, costs, expenses, indemnities and rescission or damage claims) or make any deposit pursuant to the provisions described under “Legal Defeasance and Covenant Defeasance” or “Satisfaction and Discharge” below and may not purchase, redeem or otherwise retire any Senior Subordinated Notes (collectively, “pay the Senior Subordinated Notes”) other than in the form of Permitted Junior Securities if either of the following occurs (a “Payment Default”):
(1) any Obligation on any Designated Senior Indebtedness of the Issuer is not paid in full in cash when due (after giving effect to any applicable grace period); or
(2) any other default on Designated Senior Indebtedness of the Issuer occurs and the maturity of such Designated Senior Indebtedness is accelerated in accordance with its terms;
unless, in either case, the Payment Default has been cured or waived and any such acceleration has been rescinded or such Designated Senior Indebtedness has been discharged or paid in full in cash.
Regardless of the foregoing, the Issuer is permitted to pay the Senior Subordinated Notes if the Issuer and the Trustee receive written notice approving such payment from the Representatives of all Designated Senior Indebtedness with respect to which the Payment Default has occurred and is continuing.
During the continuance of any default (other than a Payment Default) (a “Non-Payment Default”) with respect to any Designated Senior Indebtedness pursuant to which the maturity thereof may be accelerated without further notice (except such notice as may be required to effect such acceleration) or the expiration of any applicable grace periods, the Issuer is not permitted to pay the Senior Subordinated Notes (except in the form of Permitted Junior Securities) for a period (a“Payment Blockage Period”) commencing upon the receipt by the Trustee (with a copy to the Issuer) of written notice (a “Blockage Notice”) of such Non-Payment Default from the Representative of such Designated Senior Indebtedness specifying an election to effect a Payment Blockage Period and ending 179 days thereafter. The Payment Blockage Period will end earlier if such Payment Blockage Period is terminated:
(1) by written notice to the Trustee and the Issuer from the Person or Persons who gave such Blockage Notice;
(2) because the default giving rise to such Blockage Notice is cured, waived or otherwise no longer continuing; or
(3) because such Designated Senior Indebtedness has been discharged or repaid in full in cash.
Notwithstanding the provisions described above, unless the holders of such Designated Senior Indebtedness or the Representative of such Designated Senior Indebtedness have accelerated the maturity of such Designated Senior Indebtedness, the Issuer and the Guarantors are permitted to resume paying the Senior Subordinated Notes after the end of such Payment Blockage Period. The Senior Subordinated Notes shall not be subject to more than one Payment Blockage Period in any consecutive 360-day period irrespective of the number of defaults with respect to Designated Senior Indebtedness during such period; provided that if any Blockage Notice is delivered to the Trustee by or on behalf of the holders of Designated Senior Indebtedness of the Issuer (other than the holders of Indebtedness under the Senior Credit Facilities), a Representative of holders of Indebtedness under the Senior Credit Facilities may give another Blockage Notice within such period. However, in no event may the total number of days during which any Payment Blockage Period or Periods on the Senior Subordinated Notes is in effect exceed 179 days in the aggregate during any consecutive 360-day period, and there must be at least 181 days during any consecutive 360-day period during which no Payment Blockage Period is in effect. Notwithstanding the foregoing, however, no default that existed or was continuing on the date of delivery of any Blockage Notice to the Trustee will be, or be made, the basis for a subsequent Blockage Notice unless such default has been waived for a period of not less than 90 days (it being acknowledged that any subsequent action, or any breach of any financial covenants during the period after the date of delivery of a Blockage Notice, that, in either case, would give rise to a Non-Payment Default pursuant to any provisions under which a Non-Payment Default previously existed or was continuing shall constitute a new Non-Payment Default for this purpose).
In the event of any payment or distribution of the assets of the Issuer upon a total or partial liquidation or dissolution or reorganization of or similar proceeding relating to the Issuer or its property:
(1) the holders of Senior Indebtedness of the Issuer will be entitled to receive payment in full in cash of such Senior Indebtedness before the Holders of the Senior Subordinated Notes are entitled to receive any payment;
142
Table of Contents
(2) until the Senior Indebtedness of the Issuer is paid in full in cash, any payment or distribution to which Holders of the Senior Subordinated Notes would be entitled but for the subordination provisions of the Indenture will be made to holders of such Senior Indebtedness as their interests may appear, except that Holders of Senior Subordinated Notes may receive Permitted Junior Securities; and
(3) if a distribution is made to Holders of the Senior Subordinated Notes that, due to the subordination provisions, should not have been made to them, such Holders of the Senior Subordinated Notes will be required to hold it in trust for the holders of Senior Indebtedness of the Issuer and pay it over to them as their interests may appear.
The subordination and payment blockage provisions described above will not prevent a Default from occurring under the Indenture upon the failure of the Issuer to pay interest or principal with respect to the Senior Subordinated Notes when due by their terms. If payment of the Senior Subordinated Notes is accelerated because of an Event of Default, the Issuer must promptly notify the holders of Designated Senior Indebtedness or the Representative of such Designated Senior Indebtedness of the acceleration. If any Indebtedness under the Senior Credit Facilities is outstanding, no such acceleration will be effective until the earlier of the acceleration of Indebtedness under the Senior Credit Facilities or five Business Days after the Representative under the Senior Credit Facilities receive notice of such acceleration and, thereafter, the Issuer may pay the Senior Subordinated Notes only if the Indenture otherwise permits payment at that time.
Each Guarantor’s obligations under its Guarantee are senior subordinated obligations of that Guarantor. As such, the rights of Holders to receive payment pursuant to such Guarantee are subordinated in right of payment to the rights of holders of Senior Indebtedness of such Guarantor. The terms of the subordination and payment blockage provisions described above with respect to the Issuer’s obligations under the Senior Subordinated Notes apply equally to the obligations of such Guarantor under its Guarantee.
A Holder by its acceptance of Senior Subordinated Notes agrees to be bound by such provisions and authorizes and expressly directs the Trustee, on its behalf, to take such action as may be necessary or appropriate to effectuate the subordination provided for in the Indenture and appoints the Trustee its attorney-in-fact for such purpose.
By reason of the subordination provisions contained in the Indenture, in the event of a liquidation or insolvency proceeding, creditors of the Issuer or a Guarantor who are holders of Senior Indebtedness of the Issuer or such Guarantor, as the case may be, may recover more, ratably, than the Holders of the Senior Subordinated Notes, and creditors who are not holders of Senior Indebtedness may recover less, ratably, than holders of Senior Indebtedness and may recover more, ratably, than the Holders of the Senior Subordinated Notes.
The terms of the subordination provisions described above do not apply to payments from money or the proceeds of Government Securities held in trust by the Trustee for the payment of principal of and interest on the Senior Subordinated Notes pursuant to the provisions described under “Legal Defeasance and Covenant Defeasance” or “Satisfaction and Discharge,” if the foregoing subordination provisions were not violated at the time the applicable amounts were deposited in trust pursuant to such provisions.
Transfer and Exchange
A Holder may transfer or exchange Senior Subordinated Notes in accordance with the Indenture. The registrar and the Trustee may require a Holder to furnish appropriate endorsements and transfer documents in connection with a transfer of Senior Subordinated Notes. Holders are required to pay all taxes due on transfer. The Issuer is not required to transfer or exchange any Senior Subordinated Note selected for redemption or tendered (and not withdrawn) for repurchase in connection with a Change of Control Offer or an Asset Sale Offer. Also, the Issuer is not required to transfer or exchange any Senior Subordinated Note for a period of 15 days before a selection of Senior Subordinated Notes to be redeemed.
Principal, Maturity and Interest
The Issuer issued an aggregate principal amount of $1,015 million of Senior Subordinated Notes. The Senior Subordinated Notes mature on October 15, 2017. Subject to compliance with the covenant described below under “Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock,” the Issuer may issue additional Senior Subordinated Notes from time to time after this offering under the Indenture (“Additional Senior Subordinated Notes”). The Senior Subordinated Notes offered by the Issuer and any Additional Senior Subordinated Notes subsequently issued under the Indenture will be treated as a single class for all purposes under the Indenture, including waivers, amendments, redemptions and offers to purchase, except for certain waivers and amendments. Unless the context requires otherwise, references to “Senior Subordinated Notes” for all purposes of the Indenture and this “Description of Senior Subordinated Notes” include any Additional Senior Subordinated Notes that are actually issued. The Senior Subordinated Notes will be issued in denominations of $2,000 and any integral multiples of $1,000 in excess of $2,000.
Interest Payments
Interest on the Senior Subordinated Notes accrues at the rate of 11 5/8% per annum. Interest on the Senior Subordinated Notes is payable semi-annually in arrears on each April 15 and October 15, commencing April 15, 2008 to the Holders of Senior Subordinated Notes of record on the immediately preceding April 1 and October 1. Interest on the Senior Subordinated Notes accrues from the most recent date to which interest has been paid or, if no interest has been paid, from and including the Issue Date. Interest on the Senior Subordinated Notes is computed on the basis of a 360-day year comprised of twelve 30-day months.
143
Table of Contents
Additional Interest
Additional Interest may accrue on the Senior Subordinated Notes in certain circumstances pursuant to the Registration Rights Agreement or as described under “Events of Default and Remedies”. All references in the Indenture and this “Description of Senior Subordinated Notes,” in any context, to any interest or other amount payable on or with respect to the Senior Subordinated Notes shall be deemed to include any Additional Interest payable pursuant to the Registration Rights Agreement and under “Events of Default and Remedies.”
Payment of Principal, Premium and Interest
Payments of principal of, premium, if any, and interest on the Senior Subordinated Notes is payable at the office or agency of the Issuer maintained for such purpose or, at the option of the Issuer, payment of interest may be made by check mailed to the Holders of the Senior Subordinated Notes at their respective addresses set forth in the register of Holders; provided that (1) all payments of principal, premium, if any, and interest with respect to the Senior Subordinated Notes represented by one or more global notes registered in the name of or held by DTC or its nominee will be made by wire transfer of immediately available funds to the accounts specified by the Holder or Holders thereof and (2) all payments of principal, premium, if any, and interest with respect to certificated Senior Subordinated Notes will be made by wire transfer to a U.S. dollar account maintained by the payee with a bank in the United States if such Holder elects payment by wire transfer by giving written notice to the Trustee or the paying agent to such effect designating such account no later than 30 days immediately preceding the relevant due date for payment (or such other date as the Trustee may accept in its discretion). Until otherwise designated by the Issuer, the Issuer’s office or agency will be the office of the Trustee maintained for such purpose.
Mandatory Redemption; Offers to Purchase; Open Market Purchases
The Issuer is not required to make any mandatory redemption or sinking fund payments with respect to the Senior Subordinated Notes. However, under certain circumstances, the Issuer may be required to offer to purchase Senior Subordinated Notes as described under “Repurchase at the Option of Holders.” The Issuer may at any time and from time to time purchase Senior Subordinated Notes in the open market or otherwise.
Optional Redemption
Except as set forth below, the Issuer is not entitled to redeem the Senior Subordinated Notes at its option prior to October 15, 2012.
At any time prior to October 15, 2012, the Issuer may redeem all or a part of the Senior Subordinated Notes, upon notice as described under “—Selection and Notice,” at a redemption price equal to 100.0% of the principal amount of the Senior Subordinated Notes redeemed plus the Applicable Premium as of, plus accrued and unpaid interest, if any, to the date of redemption (the “Redemption Date”), subject to the right of Holders of record on the relevant record date to receive interest due on the relevant interest payment date.
On and after October 15, 2012, the Issuer may redeem the Senior Subordinated Notes, in whole or in part, upon notice as described under “—Selection and Notice,” at the redemption prices (expressed as percentages of principal amount of the Senior Subordinated Notes to be redeemed) set forth below, plus accrued and unpaid interest, if any, to the Redemption Date, subject to the right of Holders of record on the relevant record date to receive interest due on the relevant interest payment date, if redeemed during the twelve-month period beginning on October 15 of each of the years indicated below:
Year | Percentage | ||
2012 | 105.813 | % | |
2013 | 103.875 | % | |
2014 | 101.938 | % | |
2015 and thereafter | 100.000 | % |
In addition, until October 15, 2010, the Issuer may, at its option, redeem up to 35.0% of the aggregate principal amount of Senior Subordinated Notes issued by under the Indenture at a redemption price equal to 100.0% of the aggregate principal amount thereof, plus a premium equal to the stated interest rate per annum on the Senior Subordinated Notes, plus accrued and unpaid interest, if any, to the Redemption Date, subject to the right of Holders of Senior Subordinated Notes of record on the relevant record date to receive interest due on the relevant interest payment date, with the net cash proceeds received by it from one or more Equity Offerings; provided that (a) at least 50.0% of the sum of the aggregate principal amount of Senior Subordinated Notes originally issued under the Indenture on the Issue Date and any Additional Senior Subordinated Notes that are Senior Subordinated Notes issued under the Indenture after the Issue Date remains outstanding immediately after the occurrence of each such redemption; and (b) each such redemption occurs within 180 days of the date of closing of each such Equity Offering.
Notice of any redemption upon any Equity Offering may be given prior to the completion thereof, and any such redemption or notice may, at the Issuer’s discretion, be subject to one or more conditions precedent, including, but not limited to, completion of the related Equity Offering. If any Senior Subordinated Notes are listed on an exchange, and the rules of such exchange so require, the Issuer will notify the exchange of any such notice of redemption. In addition, the Issuer will notify the exchange of the principal amount of any Senior Subordinated Notes outstanding following any partial redemption of Senior Subordinated Notes.
Selection and Notice
If the Issuer is redeeming less than all of the Senior Subordinated Notes issued under the Indenture at any time, the Trustee will select the Senior Subordinated Notes to be redeemed (1) if the Senior Subordinated Notes are listed on an exchange, in compliance with the requirements of such exchange or (2) on a pro rata basis to the extent practicable, or, if the pro rata basis is not practicable for any reason, by lot or by such other method as the Trustee shall deem fair and appropriate. No Senior Subordinated Notes of $2,000 or less can be redeemed in part.
144
Table of Contents
Notices of redemption shall be delivered electronically or mailed by first-class mail, postage prepaid, at least 30 but not more than 60 days before the redemption date to each Holder of Senior Subordinated Notes at such Holder’s registered address or otherwise in accordance with the procedures of DTC, except that redemption notices may be delivered more than 60 days prior to a redemption date if the notice is issued in connection with a defeasance of the Senior Subordinated Notes or a satisfaction and discharge of the Indenture. If any Senior Subordinated Note is to be redeemed in part only, any notice of redemption that relates to such Senior Subordinated Note shall state the portion of the principal amount thereof that has been or is to be redeemed.
With respect to Senior Subordinated Notes represented by certificated notes, the Issuer will issue a senior Subordinated Note in a principal amount equal to the unredeemed portion of the original Senior Subordinated Note in the name of the Holder upon cancellation of the original Senior Subordinated Note. Senior Subordinated Notes called for redemption become due on the date fixed for redemption. On and after the Redemption Date, interest ceases to accrue on Senior Subordinated Notes or portions of them called for redemption.
Repurchase at the Option of Holders
Change of Control
The Indenture provides that if a Change of Control occurs, unless the Issuer has previously or concurrently delivered a redemption notice with respect to all the outstanding Senior Subordinated Notes as described under “Optional Redemption,” the Issuer will make an offer to purchase all of the Senior Subordinated Notes pursuant to the offer described below (the “Change of Control Offer”) at a price in cash (the “Change of Control Payment”) equal to 101.0% of the aggregate principal amount thereof plus accrued and unpaid interest, if any, to the date of purchase, subject to the right of Holders of the Senior Subordinated Notes of record on the relevant record date to receive interest due on the relevant interest payment date. Within 30 days following any Change of Control, the Issuer will deliver notice of such Change of Control Offer electronically or by first-class mail, with a copy to the Trustee, to each Holder of Senior Subordinated Notes to the address of such Holder appearing in the security register or otherwise in accordance with the procedures of DTC with the following information:
(1) that a Change of Control Offer is being made pursuant to the covenant entitled “Change of Control,” and that all Senior Subordinated Notes properly tendered pursuant to such Change of Control Offer will be accepted for payment by the Issuer;
(2) the purchase price and the purchase date, which will be no earlier than 30 days nor later than 60 days from the date such notice is delivered (the “Change of Control Payment Date”);
(3) that any Senior Subordinated Note not properly tendered will remain outstanding and continue to accrue interest;
(4) that unless the Issuer defaults in the payment of the Change of Control Payment, all Senior Subordinated Notes accepted for payment pursuant to the Change of Control Offer will cease to accrue interest on the Change of Control Payment Date;
(5) that Holders electing to have any Senior Subordinated Notes purchased pursuant to a Change of Control Offer will be required to surrender such Senior Subordinated Notes, with the form entitled “Option of Holder to Elect Purchase” on the reverse of such Senior Subordinated Notes completed, to the paying agent specified in the notice at the address specified in the notice prior to the close of business on the third Business Day preceding the Change of Control Payment Date;
(6) that Holders will be entitled to withdraw their tendered Senior Subordinated Notes and their election to require the Issuer to purchase such Senior Subordinated Notes, provided that the paying agent receives, not later than the close of business on the expiration date of the Change of Control Offer, a telegram, facsimile transmission or letter setting forth the name of the Holder of the Senior Subordinated Notes, the principal amount of Senior Subordinated Notes tendered for purchase, and a statement that such Holder is withdrawing its tendered Senior Subordinated Notes and its election to have such Senior Subordinated Notes purchased;
(7) that Holders whose Senior Subordinated Notes are being purchased only in part will be issued senior Subordinated Notes and such senior Subordinated Notes will be equal in principal amount to the unpurchased portion of the Senior Subordinated Notes surrendered. The unpurchased portion of the Senior Subordinated Notes must be equal to at least $2,000 or any integral multiple of $1,000 in excess of $2,000;
(8) if such notice is delivered prior to the occurrence of a Change of Control, stating that the Change of Control Offer is conditional on the occurrence of such Change of Control; and
(9) the other instructions, as determined by the Issuer, consistent with the covenant described hereunder, that a Holder must follow.
The Issuer will comply with the requirements of Rule 14e-1 under the Exchange Act and any other securities laws and regulations thereunder to the extent such laws or regulations are applicable in connection with the repurchase of Senior Subordinated Notes pursuant to a Change of Control Offer. To the extent that the provisions of any securities laws or regulations conflict with the provisions of the Indenture, the Issuer will comply with the applicable securities laws and regulations and shall not be deemed to have breached its obligations described in the Indenture by virtue thereof.
On the Change of Control Payment Date, the Issuer will, to the extent permitted by law:
(1) accept for payment all Senior Subordinated Notes issued by it or portions thereof properly tendered pursuant to the Change of Control Offer;
145
Table of Contents
(2) deposit with the paying agent an amount equal to the aggregate Change of Control Payment in respect of all Senior Subordinated Notes or portions thereof so tendered; and
(3) deliver, or cause to be delivered, to the Trustee for cancellation the Senior Subordinated Notes so accepted together with an Officer’s Certificate to the Trustee stating that such Senior Subordinated Notes or portions thereof have been tendered to and purchased by the Issuer.
The Senior Credit Facilities and the Senior Notes prohibit or limit, and future credit agreements or other agreements relating to Senior Indebtedness to which the Issuer becomes a party may prohibit or limit, the Issuer from purchasing any Senior Subordinated Notes pursuant to this Change of Control covenant. In the event a Change of Control occurs at a time when the Issuer is prohibited from purchasing the Senior Subordinated Notes, the Issuer could seek the consent of its lenders and the holders of Senior Notes to permit the purchase of the Senior Subordinated Notes or could attempt to refinance the indebtedness that contain such prohibition. If the Issuer does not obtain such consent or repay such indebtedness, the Issuer will remain prohibited from purchasing the Senior Subordinated Notes. In such case, the Issuer’s failure to purchase tendered Senior Subordinated Notes would constitute an Event of Default under the Indenture. If, as a result thereof, a default occurs with respect to any Senior Indebtedness, the subordination provisions in the Indenture would restrict payments to the Holders of Senior Subordinated Notes under certain circumstances. The Senior Credit Facilities will provide that certain change of control events with respect to the Issuer would constitute a default thereunder (including a Change of Control under the Indenture). If we experience a change of control that triggers a default under the Senior Credit Facilities, we could seek a waiver of such default or seek to refinance the Senior Credit Facilities. In the event we do not obtain such a waiver or refinance the Senior Credit Facilities, such default could result in amounts outstanding under the Senior Credit Facilities being declared due and payable and cause a Qualified Securitization Facility to be wound down.
Our ability to pay cash to the Holders of Senior Subordinated Notes following the occurrence of a Change of Control may be limited by our then-existing financial resources. Therefore, sufficient funds may not be available when necessary to make any required repurchases.
The Change of Control purchase feature of the Senior Subordinated Notes may in certain circumstances make more difficult or discourage a sale or takeover of us and, thus, the removal of incumbent management. The Change of Control purchase feature is a result of negotiations between the Initial Purchasers and us. After the Issue Date, we have no present intention to engage in a transaction involving a Change of Control, although it is possible that we could decide to do so in the future. Subject to the limitations discussed below, we could, in the future, enter into certain transactions, including acquisitions, refinancings or other recapitalizations, that would not constitute a Change of Control under the Indenture, but that could increase the amount of Indebtedness outstanding at such time or otherwise affect our capital structure or credit ratings. Restrictions on our ability to incur additional Indebtedness are contained in the covenants described under “Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock” and “Certain Covenants—Liens.” Such restrictions in the Indenture can be waived only with the consent of the Holders of a majority in principal amount of the Senior Subordinated Notes then outstanding. Except for the limitations contained in such covenants, however, the Indenture does not contain any covenants or provisions that may afford Holders of the Senior Subordinated Notes protection in the event of a highly leveraged transaction.
The Issuer will not be required to make a Change of Control Offer following a Change of Control if a third party makes the Change of Control Offer in the manner, at the times and otherwise in compliance with the requirements set forth in the Indenture applicable to a Change of Control Offer made by the Issuer and purchases all Senior Subordinated Notes validly tendered and not withdrawn under such Change of Control Offer.
Notwithstanding anything to the contrary herein, a Change of Control Offer may be made in advance of a Change of Control, conditional upon such Change of Control, if a definitive agreement is in place for the Change of Control at the time of making of the Change of Control Offer.
The definition of “Change of Control” includes a disposition of all or substantially all of the assets of the Issuer and its Subsidiaries, taken as a whole, to any Person. Although there is a limited body of case law interpreting the phrase “substantially all,” there is no precise established definition of the phrase under applicable law.
Accordingly, in certain circumstances there may be a degree of uncertainty as to whether a particular transaction would involve a disposition of “all or substantially all” of the assets of the Issuer and its Subsidiaries, taken as a whole. As a result, it may be unclear as to whether a Change of Control has occurred and whether a Holder of Senior Subordinated Notes may require the Issuer to make an offer to repurchase the Senior Subordinated Notes as described above.
The provisions under the Indenture relative to the Issuer’s obligation to make an offer to repurchase the Senior Subordinated Notes as a result of a Change of Control may be waived or modified with the written consent of the Holders of a majority in principal amount of the Senior Subordinated Notes.
Asset Sales
The Indenture provides that the Issuer will not, and will not permit any of its Restricted Subsidiaries to, consummate directly or indirectly an Asset Sale, unless:
(1) the Issuer or such Restricted Subsidiary, as the case may be, receives consideration at the time of such Asset Sale at least equal to the fair market value of the assets sold or otherwise disposed of; and
(2) except in the case of a Permitted Asset Swap, at least 75.0% of the consideration therefor received by the Issuer or such Restricted Subsidiary, as the case may be, is in the form of Cash Equivalents; provided that the amount of:
(a) any liabilities (as shown on the Issuer’s or such Restricted Subsidiary’s most recent balance sheet or in the footnotes thereto) of the Issuer or such Restricted Subsidiary, other than liabilities that are by their terms subordinated to the Senior Subordinated Notes, that are assumed by the transferee of any such assets and for which the Issuer and all of its Restricted Subsidiaries have been validly released by all creditors in writing;
146
Table of Contents
(b) any securities, notes or other obligations or assets received by the Issuer or such Restricted Subsidiary from such transferee that are converted by the Issuer or such Restricted Subsidiary into cash (to the extent of the cash received) within 180 days following the closing of such Asset Sale; and
(c) any Designated Non-cash Consideration received by the Issuer or such Restricted Subsidiary in such Asset Sale having an aggregate fair market value, taken together with all other Designated Non-cash Consideration received pursuant to this clause (c) that is at that time outstanding, not to exceed the greater of (x) $300.0 million and (y) 3.0% of Total Assets at the time of the receipt of such Designated Non-cash Consideration, with the fair market value of each item of Designated Non-cash Consideration being measured at the time received and without giving effect to subsequent changes in value, shall be deemed to be Cash Equivalents for purposes of this provision and for no other purpose.
Within 450 days after the receipt of any Net Proceeds of any Asset Sale, the Issuer or such Restricted Subsidiary, at its option, may apply the Net Proceeds from such Asset Sale,
(1) to permanently reduce:
(a) Obligations under Senior Indebtedness, and to correspondingly reduce commitments with respect thereto;
(b) Obligations under Senior Subordinated Indebtedness (and to correspondingly reduce commitments with respect thereto); provided that the Issuer shall equally and ratably reduce Obligations under the Senior Subordinated Notes as provided under “Optional Redemption” or through open-market purchases (to the extent such purchases are at or above 100.0% of the principal amount thereof) or by making an offer (in accordance with the procedures set forth below for an Asset Sale Offer) to all Holders of Senior Subordinated Notes to purchase their Senior Subordinated Notes at 100.0% of the principal amount thereof, plus the amount of accrued but unpaid interest, if any, on the amount of Senior Subordinated Notes to be repurchased; or
(c) Indebtedness of a Restricted Subsidiary that is not a Guarantor, other than Indebtedness owed to the Issuer or another Restricted Subsidiary;
(2) to make (a) an Investment in any one or more businesses, provided that such Investment in any business is in the form of the acquisition of Capital Stock and results in the Issuer or any of its Restricted Subsidiaries, as the case may be, owning an amount of the Capital Stock of such business such that it constitutes a Restricted Subsidiary, (b) capital expenditures or (c) acquisitions of other assets, in each of (a), (b) and (c), used or useful in a Similar Business; or
(3) to make an Investment in (a) any one or more businesses, provided that such Investment in any business is in the form of the acquisition of Capital Stock and results in the Issuer or any of its Restricted Subsidiaries, as the case may be, owning an amount of the Capital Stock of such business such that it constitutes a Restricted Subsidiary, (b) properties or (c) acquisitions of other assets that, in each of (a), (b) and (c), replace the businesses, properties and/or assets that are the subject of such Asset Sale;
provided that, in the case of clauses (2) and (3) above, a binding commitment shall be treated as a permitted application of the Net Proceeds from the date of such commitment so long as the Issuer or such other Restricted Subsidiary enters into such commitment with the good faith expectation that such Net Proceeds will be applied to satisfy such commitment within 180 days of such commitment (an “Acceptable Commitment”) and, in the event any Acceptable Commitment is later cancelled or terminated for any reason before the Net Proceeds are applied in connection therewith, the Issuer or such Restricted Subsidiary enters into another Acceptable Commitment (a“Second Commitment”) within 180 days of such cancellation or termination; provided, further, that if any Second Commitment is later cancelled or terminated for any reason before such Net Proceeds are applied, then such Net Proceeds shall constitute Excess Proceeds.
Any Net Proceeds from the Asset Sale that are not invested or applied as provided and within the time period set forth in the preceding paragraph will be deemed to constitute “Excess Proceeds.” When the aggregate amount of Excess Proceeds exceeds $75.0 million, the Issuer shall make an offer to all Holders of the Senior Subordinated Notes and, if required by the terms of any Indebtedness that is pari passu with the Senior Subordinated Notes (“Pari Passu Indebtedness”), to the holders of such Pari Passu Indebtedness (an “Asset Sale Offer”), to purchase the maximum aggregate principal amount of the Senior Subordinated Notes and such Pari Passu Indebtedness that is in an amount equal to $2,000 or an integral multiple of $1,000 in excess of $2,000, that may be purchased out of the Excess Proceeds at an offer price in cash in an amount equal to 100.0% of the principal amount thereof (or accreted value thereof, if less), plus accrued and unpaid interest, if any, to the date fixed for the closing of such offer, in accordance with the procedures set forth in the Indenture. The Issuer will commence an Asset Sale Offer with respect to Excess Proceeds within ten Business Days after the date that Excess Proceeds exceed $75.0 million by delivering the notice required pursuant to the terms of the Indenture, with a copy to the Trustee. The Issuer may satisfy the foregoing obligations with respect to any Net Proceeds from an Asset Sale by making an Asset Sale Offer with respect to such Net Proceeds prior to the expiration of the relevant 450 days (or such longer period provided above) or with respect to Excess Proceeds of $75.0 million or less.
To the extent that the aggregate amount of Senior Subordinated Notes and such Pari Passu Indebtedness tendered pursuant to an Asset Sale Offer is less than the Excess Proceeds, the Issuer may use any remaining Excess Proceeds for general corporate purposes, subject to other covenants contained in the Indenture. If the aggregate principal amount of Senior Subordinated Notes or the Pari Passu Indebtedness surrendered by such holders thereof exceeds the amount of Excess Proceeds, the Trustee shall select the Senior Subordinated Notes and the Issuer shall select such Pari Passu Indebtedness to be purchased on a pro rata basis based on the accreted value or principal amount of the Senior Subordinated Notes or such Pari Passu Indebtedness tendered. Upon completion of any such Asset Sale Offer, the amount of Excess Proceeds that resulted in the Asset Sale Offer shall be reset to zero.
147
Table of Contents
Pending the final application of any Net Proceeds pursuant to this covenant, the holder of such Net Proceeds may apply such Net Proceeds temporarily to reduce Indebtedness outstanding under a revolving credit facility or otherwise invest such Net Proceeds in any manner not prohibited by the Indenture.
The Issuer will comply with the requirements of Rule 14e-1 under the Exchange Act and any other securities laws and regulations thereunder to the extent such laws or regulations are applicable in connection with the repurchase of the Senior Subordinated Notes pursuant to an Asset Sale Offer. To the extent that the provisions of any securities laws or regulations conflict with the provisions of the Indenture, the Issuer will comply with the applicable securities laws and regulations and shall not be deemed to have breached its obligations described in the Indenture by virtue thereof.
The Senior Credit Facilities and the Senior Notes prohibit or limit, and future credit agreements or other agreements relating to Senior Indebtedness to which the Issuer becomes a party may prohibit or limit, the Issuer from purchasing any Senior Subordinated Notes pursuant to this Asset Sales covenant. In the event the Issuer is prohibited from purchasing the Senior Subordinated Notes, the Issuer could seek the consent of its lenders and the holders of the Senior Notes to the purchase of the Senior Subordinated Notes or could attempt to refinance the indebtedness that contain such prohibition. If the Issuer does not obtain such consent or repay such indebtedness, it will remain prohibited from purchasing the Senior Subordinated Notes. In such case, the Issuer’s failure to purchase tendered Senior Subordinated Notes would constitute an Event of Default under the Indenture. If, as a result thereof, a default occurs with respect to any Senior Indebtedness, the subordination provisions in the Indenture would restrict payments to the Holders of the Senior Subordinated Notes under certain circumstances.
The provisions under the Indenture relative to the Issuer’s obligation to make an offer to repurchase the Senior Subordinated Notes as a result of an Asset Sale may be waived or modified with the written consent of the Holders of a majority in principal amount of the Senior Subordinated Notes.
Certain Covenants
Set forth below are summaries of certain covenants contained in the Indenture. During any period of time that (i) the Senior Subordinated Notes have Investment Grade Ratings from both Rating Agencies and (ii) no Default has occurred and is continuing under the Indenture (the occurrence of the events described in the foregoing clauses (i) and (ii) being collectively referred to as a“ Covenant Suspension Event”and the date thereof being referred to as the “Suspension Date ”) then, the covenants specifically listed under the following captions in this “Description of Senior Subordinated Notes” section of this prospectus will not be applicable to the Senior Subordinated Notes (collectively, the “Suspended Covenants ”):
| (1) | “Repurchase at the Option of Holders—Asset Sales”; |
| (2) | “—Limitation on Restricted Payments”; |
| (3) | “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”; |
| (4) | clause (4) of the first paragraph of “—Merger, Consolidation or Sale of All or Substantially All Assets”; |
| (5) | “—Transactions with Affiliates”; |
| (6) | “—Dividend and Other Payment Restrictions Affecting Restricted Subsidiaries”; |
| (7) | “—Limitation on Guarantees of Indebtedness by Restricted Subsidiaries”; |
| (8) | “—Limitation on Layering”; and |
| (9) | “Repurchase at the Option of Holders—Change of Control.” |
During any period that the foregoing covenants have been suspended, the Issuer may not designate any of its Subsidiaries as Unrestricted Subsidiaries pursuant to the second sentence of the definition of “Unrestricted Subsidiary.”
If and while the Issuer and its Restricted Subsidiaries are not subject to the Suspended Covenants, the Senior Subordinated Notes will be entitled to substantially less covenant protection. In the event that the Issuer and its Restricted Subsidiaries are not subject to the Suspended Covenants under the Indenture for any period of time as a result of the foregoing, and on any subsequent date (the“ Reversion Date”) one or both of the Rating Agencies withdraw their Investment Grade Rating or downgrade the rating assigned to the Senior Subordinated Notes below an Investment Grade Rating, then the Issuer and its Restricted Subsidiaries will thereafter again be subject to the Suspended Covenants under the Indenture with respect to future events. The period of time between the Suspension Date and the Reversion Date is referred to in this “Description of Senior Subordinated Notes” as the “Suspension Period.” The Guarantees of the Guarantors will be suspended during the Suspension Period. Additionally, upon the occurrence of a Covenant Suspension Event, the amount of Excess Proceeds from Net Proceeds shall be reset to zero.
Notwithstanding the foregoing, in the event of any such reinstatement, no action taken or omitted to be taken by the Issuer or any of its Restricted Subsidiaries prior to such reinstatement will give rise to a Default or Event of Default under the Indenture with respect to the Senior Subordinated Notes; provided that (i) with respect to Restricted Payments made after such reinstatement, the amount available to be made as Restricted Payments will be calculated as though the covenant described above under “—Limitation on Restricted Payments” had been in effect prior to, but not during, the Suspension Period; and (ii) all Indebtedness incurred, or Disqualified Stock issued, during the Suspension Period will be classified to have been incurred or issued pursuant to clause (3) of the second paragraph of “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock.”
There can be no assurance that the Senior Subordinated Notes will ever achieve or maintain Investment Grade Ratings.
148
Table of Contents
Limitation on Restricted Payments
The Issuer will not, and will not permit any of its Restricted Subsidiaries to, directly or indirectly:
(I) declare or pay any dividend or make any payment or distribution on account of the Issuer’s, or any of its Restricted Subsidiaries’ Equity Interests, including any dividend or distribution payable in connection with any merger or consolidation other than:
(a) dividends or distributions by the Issuer payable solely in Equity Interests (other than Disqualified Stock) of the Issuer; or
(b) dividends or distributions by a Restricted Subsidiary so long as, in the case of any dividend or distribution payable on or in respect of any class or series of securities issued by a Restricted Subsidiary other than a Wholly-Owned Subsidiary, the Issuer or a Restricted Subsidiary receives at least its pro rata share of such dividend or distribution in accordance with its Equity Interests in such class or series of securities;
(II) purchase, redeem, defease or otherwise acquire or retire for value any Equity Interests of the Issuer or any direct or indirect parent company of the Issuer, including in connection with any merger or consolidation;
(III) make any principal payment on, or redeem, repurchase, defease or otherwise acquire or retire for value, in each case, prior to any scheduled repayment, sinking fund payment or maturity, any Subordinated Indebtedness, other than:
(a) Indebtedness permitted under clauses (7) and (8) of the second paragraph of the covenant described under “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”; or
(b) the purchase, repurchase or other acquisition of Subordinated Indebtedness purchased in anticipation of satisfying a sinking fund obligation, principal installment or final maturity, in each case due within one year of the date of purchase, repurchase or acquisition; or
(IV) make any Restricted Investment (all such payments and other actions set forth in clauses (I) through (IV) above being collectively referred to as “Restricted Payments”), unless, at the time of such Restricted Payment:
(1) no Default shall have occurred and be continuing or would occur as a consequence thereof;
(2) immediately after giving effect to such transaction on a pro forma basis, the Issuer could incur $1.00 of additional Indebtedness pursuant to the Fixed Charge Coverage Ratio test set forth in the first paragraph of the covenant described under “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock” (the “Fixed Charge Coverage Test”); and
(3) such Restricted Payment, together with the aggregate amount of all other Restricted Payments made by the Issuer and its Restricted Subsidiaries after the Issue Date (including Restricted Payments permitted by clauses (1), (2) (with respect to the payment of dividends on Refunding Capital Stock (as defined below) pursuant to clause (b) thereof only), (6)(c), (9) and (14) of the next succeeding paragraph, but excluding all other Restricted Payments permitted by the next succeeding paragraph), is less than the sum of (without duplication):
(a) 50.0% of the Consolidated Net Income of the Issuer for the period (taken as one accounting period and including the predecessor) beginning on September 1, 2007 to the end of the Issuer’s most recently ended fiscal quarter for which internal financial statements are available at the time of such Restricted Payment, or, in the case such Consolidated Net Income for such period is a deficit, minus 100.0% of such deficit; plus
(b) 100.0% of the aggregate net cash proceeds and the fair market value of marketable securities or other property received by the Issuer since immediately after the Issue Date (other than net cash proceeds to the extent such net cash proceeds have been used to incur Indebtedness or issue Disqualified Stock or Preferred Stock pursuant to clause (12)(a) of the second paragraph of “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”) from the issue or sale of:
(i) (A) Equity Interests of the Issuer, including Treasury Capital Stock (as defined below), but excluding cash proceeds and the fair market value of marketable securities or other property received from the sale of:
(x) Equity Interests to any future, present or former employees, directors, officers, managers, distributors or consultants (or their respective Controlled Investment Affiliates or Immediate Family Members) of the Issuer, any direct or indirect parent company of the Issuer or any of the Issuer’s Subsidiaries after the Issue Date to the extent such amounts have been applied to Restricted Payments made in accordance with clause (4) of the next succeeding paragraph; and
(y) Designated Preferred Stock;
and (B) to the extent such net cash proceeds are actually contributed to the Issuer, Equity Interests of any direct or indirect parent company of the Issuer (excluding contributions of the proceeds from the sale of Designated Preferred Stock of such company or contributions to the extent such amounts have been applied to Restricted Payments made in accordance with clause (4) of the next succeeding paragraph); or
(ii) debt securities of the Issuer that have been converted into or exchanged for such Equity Interests of the Issuer;
provided that this clause (b) shall not include the proceeds from (W) Refunding Capital Stock,(X) Equity Interests or convertible debt securities of the Issuer sold to a Restricted Subsidiary,(Y) Disqualified Stock or debt securities that have been converted into Disqualified Stock or (Z) Excluded Contributions;plus
149
Table of Contents
(c) 100.0% of the aggregate amount of cash and the fair market value of marketable securities or other property contributed to the capital of the Issuer following the Issue Date (other than net cash proceeds to the extent such net cash proceeds have been used to incur Indebtedness or issue Disqualified Stock or Preferred Stock pursuant to clause (12)(a) of the second paragraph of “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”) (other than by a Restricted Subsidiary and other than any Excluded Contributions);plus
(d) 100.0% of the aggregate amount received in cash and the fair market value of marketable securities or other property received by means of:
(i) the sale or other disposition (other than to the Issuer or a Restricted Subsidiary) of Restricted Investments made by the Issuer or its Restricted Subsidiaries and repurchases and redemptions of such Restricted Investments from the Issuer or its Restricted Subsidiaries (other than by the Issuer or a Restricted Subsidiary) and repayments of loans or advances, and releases of guarantees, which constitute Restricted Investments made by the Issuer or its Restricted Subsidiaries, in each case after the Issue Date; or
(ii) the sale (other than to the Issuer or a Restricted Subsidiary) of the stock of an Unrestricted Subsidiary or a distribution from an Unrestricted Subsidiary (other than in each case to the extent the Investment in such Unrestricted Subsidiary was made by the Issuer or a Restricted Subsidiary pursuant to clause (7) of the next succeeding paragraph or to the extent such Investment constituted a Permitted Investment) or a dividend from an Unrestricted Subsidiary after the Issue Date; plus
(e) in the case of the redesignation of an Unrestricted Subsidiary as a Restricted Subsidiary after the Issue Date, the fair market value of the Investment in such Unrestricted Subsidiary (which, if the fair market value of such Investment shall exceed $125.0 million, shall be determined by the board of directors of the Issuer whose resolution with respect thereto will be delivered to the Trustee) at the time of the redesignation of such Unrestricted Subsidiary as a Restricted Subsidiary, other than to the extent the Investment in such Unrestricted Subsidiary was made by the Issuer or a Restricted Subsidiary pursuant to clause (7) of the next succeeding paragraph or to the extent such Investment constituted a Permitted Investment.
The foregoing provisions will not prohibit:
(1) the payment of any dividend or other distribution or the consummation of any irrevocable redemption within 60 days after the date of declaration of the dividend or other distribution or giving of the redemption notice, as the case may be, if at the date of declaration or notice, the dividend or other distribution or redemption payment would have complied with the provisions of the Indenture;
(2) (a) the redemption, repurchase, retirement or other acquisition of any Equity Interests (“Treasury Capital Stock”) or Subordinated Indebtedness of the Issuer or any Equity Interests of any direct or indirect parent company of the Issuer, in exchange for, or out of the proceeds of the substantially concurrent sale (other than to a Restricted Subsidiary) of, Equity Interests of the Issuer or any direct or indirect parent company of the Issuer to the extent contributed to the Issuer (in each case, other than any Disqualified Stock) (“Refunding Capital Stock”) and (b) if immediately prior to the retirement of Treasury Capital Stock, the declaration and payment of dividend thereon was permitted under clause (6) of this paragraph, the declaration and payment of dividend on the Refunding Capital Stock (other than Refunding Capital Stock the proceeds of which were used to redeem, repurchase, retire or otherwise acquire any Equity Interests of any direct or indirect parent company of the Issuer) in an aggregate amount per year no greater than the aggregate amount of dividends per annum that were declarable and payable on such Treasury Capital Stock immediately prior to such retirement;
(3) the defeasance, redemption, repurchase or other acquisition or retirement of (i) Subordinated Indebtedness of the Issuer or a Guarantor made by exchange for, or out of the proceeds of the substantially concurrent sale of, new Indebtedness of the Issuer or a Guarantor or (ii) Disqualified Stock of the Issuer or a Guarantor made by exchange for, or out of the proceeds of the substantially concurrent sale of, Disqualified Stock of the Issuer or a Guarantor, that, in each case, is incurred in compliance with “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock” so long as:
(a) the principal amount (or accreted value, if applicable) of such new Indebtedness or the liquidation preference of such new Disqualified Stock does not exceed the principal amount of (or accreted value, if applicable), plus any accrued and unpaid interest on, the Subordinated Indebtedness or the liquidation preference of, plus any accrued and unpaid dividends on, the Disqualified Stock being so defeased, redeemed, repurchased, acquired or retired for value, plus the amount of any reasonable premium required to be paid under the terms of the instrument governing the Subordinated Indebtedness or Disqualified Stock being so defeased, redeemed, repurchased, acquired or retired, defeasance costs and any reasonable fees and expenses incurred in connection with the issuance of such new Indebtedness or Disqualified Stock;
(b) such new Indebtedness is subordinated to the Senior Subordinated Notes or the applicable Guarantee at least to the same extent as such Subordinated Indebtedness so defeased, redeemed, repurchased, acquired or retired;
(c) such new Indebtedness or Disqualified Stock has a final scheduled maturity date equal to or later than the final scheduled maturity date of the Subordinated Indebtedness or Disqualified Stock being so defeased, redeemed, repurchased, acquired or retired; and
(d) such new Indebtedness or Disqualified Stock has a Weighted Average Life to Maturity equal to or greater than the remaining Weighted Average Life to Maturity of the Subordinated Indebtedness or Disqualified Stock being so defeased, redeemed, repurchased, acquired or retired;
(4) a Restricted Payment to pay for the repurchase, retirement or other acquisition or retirement for value of Equity Interests (other than Disqualified Stock) of the Issuer or any direct or indirect parent company of the Issuer held by any future, present or former (A) employee, director, officer, manager or consultant (or their respective Controlled Investment Affiliates or Immediate Family Members) of the Issuer, any of its Subsidiaries or any of its direct or indirect parent companies pursuant to any management equity plan or stock option plan or any other management or employee benefit plan or agreement, or any stock subscription or
150
Table of Contents
shareholder agreement, including any Equity Interest rolled over by management of the Issuer or any direct or indirect parent company of the Issuer in connection with the Transactions; provided that the aggregate amount of Restricted Payments made under this clause (A) does not exceed $20.0 million in the first fiscal year following the Issue Date (which amount shall be increased by $5.0 million each fiscal year thereafter and, if applicable, will be increased to $40.0 million following the consummation of an underwritten public Equity Offering) (with unused amounts in any fiscal year being carried over to succeeding fiscal years subject to a maximum (without giving effect to the following proviso) of $30.0 million in any fiscal year (which shall increase to $60.0 million subsequent to the consummation of an underwritten public Equity Offering)); and (B) distributor (or its respective Controlled Investment Affiliates or Immediate Family Members) of the Issuer, any of its Subsidiaries or any of its direct or indirect parent companies pursuant to any distributor equity plan or agreement; provided that the aggregate amount of Restricted Payments made under this clause (B) does not exceed the greater of (i) $100.0 million and (ii) 1.0% of Total Assets; provided, further, that each of the amounts in any fiscal year under (A) and (B) may be increased by an amount not to exceed:
(a) the cash proceeds from the sale of Equity Interests (other than Disqualified Stock) of the Issuer and, to the extent contributed to the Issuer, Equity Interests of any direct or indirect parent company of the Issuer, in each case to any future, present or former employees, directors, officers, managers, distributors or consultants (or their respective Controlled Investment Affiliates or Immediate Family Members) of the Issuer, any of its Subsidiaries or any of its direct or indirect parent companies that occurs after the Issue Date, to the extent the cash proceeds from the sale of such Equity Interests have not otherwise been applied to the payment of Restricted Payments by virtue of clause (3) of the preceding paragraph;plus
(b) the cash proceeds of key man life insurance policies received by the Issuer or its Restricted Subsidiaries after the Issue Date; less
(c) the amount of any Restricted Payments previously made with the cash proceeds described in clauses (a) and (b) of this clause (4);
and provided, further, that cancellation of Indebtedness owing to the Issuer from any future, present or former employees, directors, officers, managers, distributors or consultants of the Issuer (or their respective Controlled Investment Affiliates or Immediate Family Members), any direct or indirect parent company of the Issuer or any of the Issuer’s Restricted Subsidiaries in connection with a repurchase of Equity Interests of the Issuer or any of its direct or indirect parent companies will not be deemed to constitute a Restricted Payment for purposes of this covenant or any other provision of the Indenture;
(5) the declaration and payment of dividends to holders of any class or series of Disqualified Stock of the Issuer or any of its Restricted Subsidiaries or any class or series of Preferred Stock of any Restricted Subsidiary issued in accordance with the covenant described under “ —Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock” to the extent such dividends are included in the definition of “Fixed Charges”;
(6) (a) the declaration and payment of dividends to holders of any class or series of Designated Preferred Stock (other than Disqualified Stock) issued by the Issuer or any of its Restricted Subsidiaries after the Issue Date;
(b) the declaration and payment of dividends to any direct or indirect parent company of the Issuer, the proceeds of which will be used to fund the payment of dividends to holders of any class or series of Designated Preferred Stock (other than Disqualified Stock) issued by such parent company after the Issue Date, provided that the amount of dividends paid pursuant to this clause (b) shall not exceed the aggregate amount of cash actually contributed to the Issuer from the sale of such Designated Preferred Stock; or
(c) the declaration and payment of dividends on Refunding Capital Stock that is Preferred Stock in excess of the dividends declarable and payable thereon pursuant to clause (2) of this paragraph;provided, in the case of each of (a), (b) and (c) of this clause (6), that for the most recently ended four full fiscal quarters for which internal financial statements are available immediately preceding the date of issuance of such Designated Preferred Stock or the declaration of such dividends on Refunding Capital Stock that is Preferred Stock, after giving effect to such issuance or declaration on a pro forma basis, the Issuer would have had a Fixed Charge Coverage Ratio of at least 2.00 to 1.00;
(7) Investments in Unrestricted Subsidiaries taken together with all other Investments made pursuant to this clause (7) that are at the time outstanding, without giving effect to the sale of an Unrestricted Subsidiary to the extent the proceeds of such sale do not consist of cash or marketable securities, not to exceed greater of (a) $300.0 million and (b) 3.0% of Total Assets;
(8) payments made or expected to be made by the Issuer or any Restricted Subsidiary in respect of withholding or similar taxes payable by any future, present or former employee, director, officer, manager, distributor or consultant (or their respective Controlled Investment Affiliates or Immediate Family Members) and any repurchases of Equity Interests deemed to occur upon exercise of stock options or warrants if such Equity Interests represent a portion of the exercise price of such options or warrants;
(9) the declaration and payment of dividends on the Issuer’s common stock (or the payment of dividends to any direct or indirect parent company of the Issuer to fund a payment of dividends on such company’s common stock), following the first public offering of the Issuer’s common stock or the common stock of any direct or indirect parent company of the Issuer after the Issue Date, of up to 6.0% per annum of the net cash proceeds received by or contributed to the Issuer in or from any such public offering, other than public offerings with respect to the Issuer’s common stock registered on Form S-4 or Form S-8 and other than any public sale constituting an Excluded Contribution;
(10) Restricted Payments that are made with Excluded Contributions;
(11) other Restricted Payments in an aggregate amount taken together with all other Restricted Payments made pursuant to this clause (11) not to exceed the greater of (a) $300.0 million and (b) 2.75% of Total Assets;
(12) distributions or payments of Securitization Fees;
151
Table of Contents
(13) any Restricted Payment made in connection with the Transactions and the fees and expenses related thereto or owed to Affiliates, in each case to the extent permitted by the covenant described under “—Transactions with Affiliates”;
(14) the repurchase, redemption or other acquisition or retirement for value of any Subordinated Indebtedness pursuant to the provisions similar to those described under “Repurchase at the Option of Holders—Change of Control” and “Repurchase at the Option of Holders—Asset Sales”; provided that all Senior Subordinated Notes validly tendered by Holders in connection with a Change of Control Offer or Asset Sale Offer, as applicable, have been repurchased, redeemed, acquired or retired for value;
(15) the declaration and payment of dividends by the Issuer to, or the making of loans to, any direct or indirect parent company of the Issuer in amounts required for any direct or indirect parent company of the Issuer to pay, in each case without duplication,
(a) franchise and excise taxes and other fees, taxes and expenses required to maintain their corporate existence;
(b) foreign, federal, state and local income taxes, to the extent such income taxes are attributable to the income of the Issuer and its Restricted Subsidiaries and, to the extent of the amount actually received from its Unrestricted Subsidiaries, in amounts required to pay such taxes to the extent attributable to the income of such Unrestricted Subsidiaries; provided that in each case the amount of such payments in any fiscal year does not exceed the amount that the Issuer and its Restricted Subsidiaries would be required to pay in respect of foreign, federal, state and local taxes for such fiscal year were the Issuer, its Restricted Subsidiaries and its Unrestricted Subsidiaries (to the extent described above) to pay such taxes separately from any such parent company;
(c) customary salary, bonus and other benefits payable to employees, directors, officers and managers of any direct or indirect parent company of the Issuer to the extent such salaries, bonuses and other benefits are attributable to the ownership or operation of the Issuer and its Restricted Subsidiaries;
(d) general corporate operating and overhead costs and expenses of any direct or indirect parent company of the Issuer to the extent such costs and expenses are attributable to the ownership or operation of the Issuer and its Restricted Subsidiaries;
(e) fees and expenses other than to Affiliates of the Issuer related to any unsuccessful equity or debt offering of such parent company;
(f) [reserved];
(g) amounts payable pursuant to the Management Fee Agreement, solely to the extent such amounts are not paid directly by the Issuer or its Subsidiaries;
(h) cash payments in lieu of issuing fractional shares in connection with the exercise of warrants, options or other securities convertible into or exchangeable for Equity Interests of the Issuer or any direct or indirect parent company of the Issuer; and
(i) to finance Investments otherwise permitted to be made pursuant to this covenant; provided that (A) such Restricted Payment shall be made substantially concurrently with the closing of such Investment, (B) such direct or indirect parent company shall, immediately following the closing thereof, cause (1) all property acquired (whether assets or Equity Interests) to be contributed to the capital of the Issuer or one of its Restricted Subsidiaries or (2) the merger of the Person formed or acquired into the Issuer or one of its Restricted Subsidiaries (to the extent not prohibited by the covenant “—Merger, Consolidation or Sale of All or Substantially All Assets” below) in order to consummate such Investment, (C) such direct or indirect parent company and its Affiliates (other than the Issuer or a Restricted Subsidiary) receives no consideration or other payment in connection with such transaction except to the extent the Issuer or a Restricted Subsidiary could have given such consideration or made such payment in compliance with the Indenture, (D) any property received by the Issuer shall not increase amounts available for Restricted Payments pursuant to clause (3) of the preceding paragraph and (E) such Investment shall be deemed to be made by the Issuer or such Restricted Subsidiary pursuant to another provision of this covenant (other than pursuant to clause (10) hereof) or pursuant to the definition of “Permitted Investments” (other than clause (9) thereof); and
(16) the distribution, by dividend or otherwise, of shares of Capital Stock of, or Indebtedness owed to the Issuer or a Restricted Subsidiary by, Unrestricted Subsidiaries (other than Unrestricted Subsidiaries, the primary assets of which are Cash Equivalents).
provided that at the time of, and after giving effect to, any Restricted Payment permitted under clauses (11) and (16), no Default shall have occurred and be continuing or would occur as a consequence thereof.
As of the Issue Date, all of the Issuer’s Subsidiaries are Restricted Subsidiaries. The Issuer will not permit any Unrestricted Subsidiary to become a Restricted Subsidiary except pursuant to the next to the last sentence of the definition of “Unrestricted Subsidiary.” For purposes of designating any Restricted Subsidiary as an Unrestricted Subsidiary, all outstanding Investments by the Issuer and its Restricted Subsidiaries (except to the extent repaid) in the Subsidiary so designated will be deemed to be Restricted Payments in an amount determined as set forth in the penultimate sentence of the definition of “Investments.” Such designation will be permitted only if a Restricted Payment in such amount would be permitted at such time, whether pursuant to the first paragraph of this covenant or under clause (7), (10) or (11) of the second paragraph of this covenant, or pursuant to the definition of “Permitted Investments,” and if such Subsidiary otherwise meets the definition of an Unrestricted Subsidiary. Unrestricted Subsidiaries are be subject to any of the restrictive covenants set forth in the Indenture.
Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock
The Issuer will not, and will not permit any of its Restricted Subsidiaries to, directly or indirectly, create, incur, issue, assume, guarantee or otherwise become directly or indirectly liable, contingently or otherwise (collectively, “incur” and collectively, an “incurrence”) with respect to any Indebtedness (including Acquired Indebtedness) and the Issuer will not issue any shares of Disqualified Stock and will not permit any Restricted Subsidiary to issue any shares of Disqualified Stock or Preferred Stock; provided that the Issuer may incur Indebtedness (including Acquired Indebtedness) or issue shares of Disqualified Stock, and, subject
152
Table of Contents
to the third paragraph of this covenant, any Restricted Subsidiary may incur Indebtedness (including Acquired Indebtedness), issue shares of Disqualified Stock and issue shares of Preferred Stock, if the Fixed Charge Coverage Ratio for the Issuer’s most recently ended four fiscal quarters for which internal financial statements are available immediately preceding the date on which such additional Indebtedness is incurred or such Disqualified Stock or Preferred Stock is issued would have been at least 2.00 to 1.00, determined on a pro forma basis (including a pro forma application of the net proceeds therefrom), as if the additional Indebtedness had been incurred, or the Disqualified Stock or Preferred Stock had been issued, as the case may be, and the application of proceeds therefrom had occurred at the beginning of such four-quarter period.
The foregoing limitations will not apply to:
(1) the incurrence of Indebtedness pursuant to the Senior Credit Facilities by the Issuer or any Restricted Subsidiary and the issuance and creation of letters of credit and bankers’ acceptances thereunder (with letters of credit and bankers’ acceptances being deemed to have a principal amount equal to the face amount thereof) (i) in the case of the CF Credit Facilities, up to the greater of (x) the sum of an aggregate principal amount of $2,340.0 million and an aggregate principal amount of €875.0 million and (y) an aggregate principal amount of Indebtedness outstanding at any one time that does not cause the Senior Secured Leverage Ratio (as defined, together with related definitions, in the CF Credit Facilities in effect on the Issue Date) to exceed 4.50 to 1.00, determined on a pro forma basis (including a pro forma application of the net proceeds therefrom under the CF Credit Facilities) and (ii) in the case of the ABL Facilities, up to an aggregate principal amount of $450.0 million;
(2) the incurrence by the Issuer and any Guarantor of Indebtedness represented by (a) the Senior Subordinated Notes (including any Guarantee) and the exchange notes and related exchange guarantees to be issued in exchange for Senior Subordinated Notes and the Guarantees pursuant to the Registration Rights Agreement (but excluding any Additional Senior Subordinated Notes) and (b) the Senior Notes (including any guarantee thereof) and the exchange notes and related exchange guarantees to be issued in exchange for the Senior Notes and the guarantees thereof pursuant to the Registration Rights Agreement (but excluding any Additional Senior Notes (as defined in the indenture governing the Senior Subordinated Notes));
(3) Indebtedness of the Issuer and its Restricted Subsidiaries in existence on the Issue Date (other than Indebtedness described in clauses (1) and (2));
(4) Indebtedness (including Capitalized Lease Obligations) and Disqualified Stock incurred or issued by the Issuer or any Restricted Subsidiary and Preferred Stock issued by any Restricted Subsidiary, to finance the purchase, lease or improvement of property (real or personal) or equipment that is used or useful in a Similar Business, whether through the direct purchase of assets or the Capital Stock of any Person owning such assets in an aggregate principal amount, together with any Refinancing Indebtedness in respect thereof and all other Indebtedness, Disqualified Stock and/or Preferred Stock incurred or issued and outstanding under this clause (4), not to exceed 5.0% of Total Assets (in each case, determined at the date of incurrence) at any time outstanding, so long as such Indebtedness, Disqualified Stock or Preferred Stock is incurred or issued at the date of such purchase, lease or improvement or within 270 days thereafter;
(5) Indebtedness incurred by the Issuer or any of its Restricted Subsidiaries constituting reimbursement obligations with respect to letters of credit issued in the ordinary course of business, including letters of credit in respect of workers’ compensation claims, or other Indebtedness with respect to reimbursement type obligations regarding workers’ compensation claims; provided that upon the drawing of such letters of credit or the incurrence of such Indebtedness, such obligations are reimbursed within 30 days following such drawing or incurrence;
(6) Indebtedness arising from agreements of the Issuer or its Restricted Subsidiaries providing for indemnification, adjustment of purchase price, earnouts or similar obligations, in each case, incurred or assumed in connection with the disposition of any business, assets or a Subsidiary, other than guarantees of Indebtedness incurred by any Person acquiring all or any portion of such business, assets or a Subsidiary for the purpose of financing such acquisition; provided that such Indebtedness is not reflected on the balance sheet of the Issuer, or any of its Restricted Subsidiaries (contingent obligations referred to in a footnote to financial statements and not otherwise reflected on the balance sheet will not be deemed to be reflected on such balance sheet for purposes of this clause (6));
(7) Indebtedness of the Issuer to a Restricted Subsidiary; provided that any such Indebtedness owing to a Restricted Subsidiary that is not a Guarantor is expressly subordinated in right of payment to the Senior Subordinated Notes; provided, further, that any subsequent issuance or transfer of any Capital Stock or any other event which results in any such Restricted Subsidiary ceasing to be a Restricted Subsidiary or any other subsequent transfer of any such Indebtedness (except to the Issuer or another Restricted Subsidiary) shall be deemed, in each case, to be an incurrence of such Indebtedness not permitted by this clause;
(8) Indebtedness of a Restricted Subsidiary to the Issuer or another Restricted Subsidiary; provided that if a Guarantor incurs such Indebtedness to a Restricted Subsidiary that is not a Guarantor, such Indebtedness is expressly subordinated in right of payment to the Guarantee of the Senior Subordinated Notes of such Guarantor; provided, further, that any subsequent transfer of any such Indebtedness (except to the Issuer or another Restricted Subsidiary) shall be deemed, in each case, to be an incurrence of such Indebtedness not permitted by this clause;
(9) shares of Preferred Stock of a Restricted Subsidiary issued to the Issuer or another Restricted Subsidiary; provided that any subsequent issuance or transfer of any Capital Stock or any other event which results in any such Restricted Subsidiary ceasing to be a Restricted Subsidiary or any other subsequent transfer of any such shares of Preferred Stock (except to the Issuer or another of its Restricted Subsidiaries) shall be deemed, in each case, to be an issuance of such shares of Preferred Stock not permitted by this clause;
(10) Hedging Obligations (excluding Hedging Obligations entered into for speculative purposes) for the purpose of limiting interest rate risk with respect to any Indebtedness permitted to be incurred under the Indenture, exchange rate risk or commodity pricing risk;
(11) obligations in respect of self-insurance and obligations in respect of performance, bid, appeal and surety bonds and completion guarantees and similar obligations provided by the Issuer or any of its Restricted Subsidiaries in the ordinary course of business;
153
Table of Contents
(12) (a) Indebtedness or Disqualified Stock of the Issuer and Indebtedness, Disqualified Stock or Preferred Stock of the Issuer or any Restricted Subsidiary in an aggregate principal amount or liquidation preference up to 100.0% of the net cash proceeds received by the Issuer since immediately after the Issue Date from the issue or sale of Equity Interests of the Issuer or cash contributed to the capital of the Issuer (in each case, other than proceeds of Disqualified Stock or sales of Equity Interests to the Issuer or any of its Subsidiaries) as determined in accordance with clauses (3)(b) and (3)(c) of the first paragraph of “—Limitation on Restricted Payments” to the extent such net cash proceeds or cash have not been applied pursuant to such clauses to make Restricted Payments or to make other Investments, payments or exchanges pursuant to the second paragraph of “—Limitation on Restricted Payments” or to make Permitted Investments (other than Permitted Investments specified in clause (1) or (3) of the definition thereof) and
(b) Indebtedness or Disqualified Stock of the Issuer and Indebtedness, Disqualified Stock or Preferred Stock of the Issuer or, subject to the third paragraph of this covenant, any Restricted Subsidiary not otherwise permitted hereunder in an aggregate principal amount or liquidation preference which, when aggregated with the principal amount and liquidation preference of all other Indebtedness, Disqualified Stock and Preferred Stock then outstanding and incurred pursuant to this clause (12)(b), does not at any one time outstanding exceed the greater of (x) $550.0 million and (y) 5.0% of Total Assets (it being understood that any Indebtedness, Disqualified Stock or Preferred Stock incurred pursuant to this clause (12)(b) shall cease to be deemed incurred or outstanding for purposes of this clause (12)(b) but shall be deemed incurred for the purposes of the first paragraph of this covenant from and after the first date on which the Issuer or such Restricted Subsidiary could have incurred such Indebtedness, Disqualified Stock or Preferred Stock under the first paragraph of this covenant without reliance on this clause (12)(b));
(13) the incurrence by the Issuer or any Restricted Subsidiary of Indebtedness, the issuance by the Issuer or any Restricted Subsidiary of Disqualified Stock or the issuance by any Restricted Subsidiary of Preferred Stock which serves to extend, replace, refund, refinance, renew or defease any Indebtedness incurred or Disqualified Stock or Preferred Stock issued as permitted under the first paragraph of this covenant and clauses (2), (3), (4) and (12)(a) above, this clause (13) and clauses (14) and (24) below or any Indebtedness incurred or Disqualified Stock or Preferred Stock issued to so extend, replace, refund, refinance, renew or defease such Indebtedness, Disqualified Stock or Preferred Stock including additional Indebtedness, Disqualified Stock or Preferred Stock incurred to pay premiums (including reasonable tender premiums), defeasance costs and fees in connection therewith (the “Refinancing Indebtedness”) prior to its respective maturity; provided that such Refinancing Indebtedness:
(a) has a Weighted Average Life to Maturity at the time such Refinancing Indebtedness is incurred which is not less than the remaining Weighted Average Life to Maturity of, the Indebtedness, Disqualified Stock or Preferred Stock being extended, replaced, refunded, refinanced, renewed or defeased;
(b) to the extent such Refinancing Indebtedness extends, replaces, refunds, refinances, renews or defeases (i) Indebtedness subordinated to the Senior Subordinated Notes or any Guarantee thereof, such Refinancing Indebtedness is subordinated to the Senior Subordinated Notes or the Guarantee thereof at least to the same extent as the Indebtedness being extended, replaced, refunded, refinanced, renewed or defeased or (ii) Disqualified Stock or Preferred Stock, such Refinancing Indebtedness must be Disqualified Stock or Preferred Stock, respectively; and
(c) shall not include:
(i) Indebtedness, Disqualified Stock or Preferred Stock of a Subsidiary of the Issuer that is not a Guarantor that refinances Indebtedness or Disqualified Stock of the Issuer;
(ii) Indebtedness, Disqualified Stock or Preferred Stock of a Subsidiary of the Issuer that is not a Guarantor that refinances Indebtedness, Disqualified Stock or Preferred Stock of a Guarantor; or
(iii) Indebtedness or Disqualified Stock of the Issuer or Indebtedness, Disqualified Stock or Preferred Stock of a Restricted Subsidiary that refinances Indebtedness, Disqualified Stock or Preferred Stock of an Unrestricted Subsidiary; and, provided, further , that subclause (a) of this clause (13) will not apply to any extension, replacement, refunding, refinancing, renewal or defeasance of any Indebtedness outstanding under a Credit Facility and Obligations secured by Permitted Liens.
(14) (a) Indebtedness or Disqualified Stock of the Issuer or, subject to the third paragraph of this covenant, Indebtedness, Disqualified Stock or Preferred Stock of a Restricted Subsidiary incurred or issued to finance an acquisition or (b) Indebtedness, Disqualified Stock or Preferred Stock of Persons that are acquired by the Issuer or any Restricted Subsidiary or merged into the Issuer or a Restricted Subsidiary in accordance with the terms of the Indenture; provided that after giving effect to such acquisition or merger, either
(a) the Issuer would be permitted to incur at least $1.00 of additional Indebtedness pursuant to the Fixed Charge Coverage Test, or
(b) the Fixed Charge Coverage Ratio for the Issuer is equal to or greater than immediately prior to such acquisition or merger;
(15) Indebtedness arising from the honoring by a bank or other financial institution of a check, draft or similar instrument drawn against insufficient funds in the ordinary course of business, provided that such Indebtedness is extinguished within five Business Days of its incurrence;
(16) Indebtedness of the Issuer or any of its Restricted Subsidiaries supported by a letter of credit issued pursuant to the Credit Facilities, in a principal amount not in excess of the stated amount of such letter of credit;
(17) (a) any guarantee by the Issuer or a Restricted Subsidiary of Indebtedness or other obligations of any Restricted Subsidiary so long as the incurrence of such Indebtedness incurred by such Restricted Subsidiary is permitted under the terms of the Indenture, or (b) any guarantee by a Restricted Subsidiary of Indebtedness of the Issuer; provided that such guarantee is incurred in accordance with the covenant described below under “—Limitation on Guarantees of Indebtedness by Restricted Subsidiaries”;
154
Table of Contents
(18) Indebtedness consisting of Indebtedness issued by the Issuer or any of its Restricted Subsidiaries to future, present or former employees, directors, officers, managers, distributors and consultants thereof, their respective Controlled Investment Affiliates or Immediate Family Members, in each case to finance the purchase or redemption of Equity Interests of the Issuer or any direct or indirect parent company of the Issuer to the extent described in clause (4) of the second paragraph under “—Limitation on Restricted Payments”;
(19) customer deposits and advance payments received in the ordinary course of business from customers for goods purchased in the ordinary course of business;
(20) Indebtedness owed on a short-term basis of no longer than 30 days to banks and other financial institutions incurred in the ordinary course of business of the Issuer and its Restricted Subsidiaries with such banks or financial institutions that arises in connection with ordinary banking arrangements to manage cash balances of the Issuer and its Restricted Subsidiaries;
(21) Indebtedness incurred by a Restricted Subsidiary in connection with bankers’ acceptances, discounted bills of exchange or the discounting or factoring of receivables for credit management purposes, in each case incurred or undertaken in the ordinary course of business on arm’s length commercial terms on a recourse basis;
(22) Indebtedness of the Issuer or any of its Restricted Subsidiaries consisting of (a) the financing of insurance premiums or (b) take-or-pay obligations contained in supply arrangements in each case, incurred in the ordinary course of business;
(23) (a) the incurrence of Indebtedness by a Foreign Subsidiary pursuant to (i) the European line of credit in existence on the Issue Date up to an aggregate principal amount of €100.0 million outstanding at any one time and (ii) the Japanese line of credit in existence on the Issue Date up to an aggregate principal amount of ¥4.5 billion outstanding at any one time and the issuance and creation of letters of credit and bankers’ acceptances thereunder (with letters of credit and bankers’ acceptances being deemed to have a principal amount equal to the face amount thereof), and (b) the incurrence of Indebtedness of Foreign Subsidiaries of the Issuer or a Restricted Subsidiary of the Issuer other than Indebtedness described in clause (23)(a) in an amount not to exceed at any one time outstanding and together with any other Indebtedness incurred under this clause (23)(b) the greater of (i) $100.0 million and (ii) 5.0% of the Foreign Subsidiary Total Assets (it being understood that any Indebtedness incurred pursuant to this clause (23) shall cease to be deemed incurred or outstanding for the purpose of this clause (23) but shall be deemed incurred for the purposes of the first paragraph of this covenant from and after the first date on which the Issuer or such Restricted Subsidiaries could have incurred such Indebtedness under the first paragraph of this covenant without reliance on this clause (23));
(24) Indebtedness, Disqualified Stock or Preferred Stock of a Restricted Subsidiary incurred to finance or assumed in connection with an acquisition in a principal amount not to exceed $100.0 million in the aggregate at any one time outstanding together with all other Indebtedness, Disqualified Stock and/or Preferred Stock issued under this clause (24) (it being understood that any Indebtedness, Disqualified Stock or Preferred Stock incurred pursuant to this clause (24) shall cease to be deemed incurred or outstanding for purposes of this clause (24) but shall be deemed incurred for the purposes of the first paragraph of this covenant from and after the first date on which such Restricted Subsidiary could have incurred such Indebtedness, Disqualified Stock or Preferred Stock under the first paragraph of this covenant without reliance on this clause (24)); and
(25) Indebtedness of the Issuer or any of its Restricted Subsidiaries undertaken in connection with cash management and related activities with respect to any Subsidiary or joint venture in the ordinary course of business.
Restricted Subsidiaries of the Issuer that are not Guarantors may not incur Indebtedness or Disqualified Stock or Preferred Stock under the first paragraph of this covenant or clause 12(b), 14(x) or (24) of the second paragraph of this covenant if, after giving pro forma effect to such incurrence or issuance (including a pro forma application of the net proceeds therefrom), the aggregate amount of Indebtedness and Disqualified Stock and Preferred Stock of Restricted Subsidiaries that are not Guarantors incurred or issued pursuant to the first paragraph of this covenant and clauses 12(b), 14(x) and (24) of the second paragraph of this covenant, collectively, would exceed $600.0 million.
For purposes of determining compliance with this covenant:
(1) in the event that an item of Indebtedness, Disqualified Stock or Preferred Stock (or any portion thereof) meets the criteria of more than one of the categories of Permitted Indebtedness, Disqualified Stock or Preferred Stock described in clauses (1) through (25) above or is entitled to be incurred pursuant to the first paragraph of this covenant, the Issuer, in its sole discretion, will classify or reclassify such item of Indebtedness, Disqualified Stock or Preferred Stock (or any portion thereof) and will only be required to include the amount and type of such Indebtedness, Disqualified Stock or Preferred Stock in one of the above clauses or under the first paragraph of this covenant; provided that all Indebtedness outstanding under the CF Credit Facilities on the Issue Date will be treated as incurred on the Issue Date under clause (1) of the second paragraph above; and
(2) at the time of incurrence, the Issuer will be entitled to divide and classify an item of Indebtedness in more than one of the types of Indebtedness described in the first and second paragraphs above.
Accrual of interest or dividends, the accretion of accreted value, the accretion or amortization of original issue discount and the payment of interest or dividends in the form of additional Indebtedness, Disqualified Stock or Preferred Stock, as the case may be, of the same class will not be deemed to be an incurrence of Indebtedness, Disqualified Stock or Preferred Stock for purposes of this covenant.
For purposes of determining compliance with any U.S. dollar-denominated restriction on the incurrence of Indebtedness, the U.S. dollar-equivalent principal amount of Indebtedness denominated in a foreign currency shall be calculated based on the relevant currency exchange rate in effect on the date such Indebtedness was incurred, in the case of term debt, or first committed, in the case of revolving credit debt; provided that if such Indebtedness is incurred to refinance other Indebtedness denominated in a foreign
155
Table of Contents
currency, and such refinancing would cause the applicable U.S. dollar-denominated restriction to be exceeded if calculated at the relevant currency exchange rate in effect on the date of such refinancing, such U.S. dollar-denominated restriction shall be deemed not to have been exceeded so long as the principal amount of such refinancing Indebtedness does not exceed the principal amount of such Indebtedness being refinanced.
The principal amount of any Indebtedness incurred to refinance other Indebtedness, if incurred in a different currency from the Indebtedness being refinanced, shall be calculated based on the currency exchange rate applicable to the currencies in which such respective Indebtedness is denominated that is in effect on the date of such refinancing.
Liens
The Issuer will not, and will not permit any Guarantor to, directly or indirectly, create, incur, assume or suffer to exist any Lien (except Permitted Liens) that secures Obligations under any Indebtedness ranking pari passu with or subordinated to the Senior Subordinated Notes or any related Guarantee, on any asset or property of the Issuer or any Guarantor, or any income or profits therefrom, or assign or convey any right to receive income therefrom, unless:
(1) in the case of Liens securing Subordinated Indebtedness, the Senior Subordinated Notes and related Guarantees are secured by a Lien on such property, assets or proceeds that is senior in priority to such Liens; and
(2) in all other cases, the Senior Subordinated Notes or the Guarantees are equally and ratably secured, except that the foregoing shall not apply to (a) Liens securing the Senior Subordinated Notes and the related Guarantees and (b) Liens securing Senior Indebtedness of the Issuer or any Guarantor.
Merger, Consolidation or Sale of All or Substantially All Assets
The Issuer may not consolidate or merge with or into or wind up into (whether or not the Issuer is the surviving Person), or sell, assign, transfer, lease, convey or otherwise dispose of all or substantially all of its properties or assets, in one or more related transactions, to any Person unless:
(1) the Issuer is the surviving Person or the Person formed by or surviving any such consolidation or merger (if other than the Issuer) or to which such sale, assignment, transfer, lease, conveyance or other disposition will have been made, is a Person organized or existing under the laws of the United States, any state thereof, the District of Columbia, or any territory thereof (such Person, as the case may be, being herein called the “Successor Company”); provided that in the case where the surviving Person is not a corporation, a co-obligor of the Senior Subordinated Notes is a corporation;
(2) the Successor Company, if other than the Issuer, expressly assumes all the obligations of the Issuer under the Senior Subordinated Notes pursuant to supplemental indentures or other documents or instruments;
(3) immediately after such transaction, no Default exists;
(4) immediately after giving pro forma effect to such transaction and any related financing transactions, as if such transactions had occurred at the beginning of the applicable four-quarter period,
(a) the Successor Company or the Issuer would be permitted to incur at least $1.00 of additional Indebtedness pursuant to the Fixed Charge Coverage Test, or
(b) the Fixed Charge Coverage Ratio for the Issuer would be equal to or greater than the Fixed Charge Coverage Ratio for the Issuer immediately prior to such transaction;
(5) each Guarantor, unless it is the other party to the transactions described above, in which case clause (1)(b) of the second succeeding paragraph shall apply, shall have by supplemental indenture confirmed that its Guarantee shall apply to such Person’s obligations under the Indenture, the Senior Subordinated Notes and the Registration Rights Agreement; and
(6) the Issuer shall have delivered to the Trustee an Officer’s Certificate and an Opinion of Counsel, each stating that such consolidation, merger or transfer and such supplemental indentures, if any, comply with the Indenture.
The Successor Company will succeed to, and be substituted for the Issuer under the Indenture, the Guarantees and the Senior Subordinated Notes, as applicable. Notwithstanding the immediately preceding clauses (3) and (4),
(1) any Restricted Subsidiary may consolidate with or merge into or transfer all or part of its properties and assets to the Issuer, and
(2) the Issuer may merge with an Affiliate of the Issuer solely for the purpose of reincorporating the Issuer in the United States, the District of Columbia or any territory thereof so long as the amount of Indebtedness of the Issuer and its Restricted Subsidiaries is not increased thereby.
Subject to certain limitations described in the Indenture governing release of a Guarantee upon the sale, disposition or transfer of a Guarantor, no Guarantor will, and the Issuer will not permit any Guarantor to, consolidate or merge with or into or wind up into (whether or not such Guarantor is the surviving Person), or sell, assign, transfer, lease, convey or otherwise dispose of all or substantially all of its properties or assets, in one or more related transactions, to any Person unless:
(1) (a) such Guarantor is the surviving Person or the Person formed by or surviving any such consolidation or merger (if other than such Guarantor) or to which such sale, assignment, transfer, lease, conveyance or other disposition will have been made is a Person organized or existing under the laws of the jurisdiction of organization of such Guarantor, as applicable, or the laws of the United States, any state thereof, the District of Columbia, or any territory thereof (such Person being herein called the “Successor Person”);
156
Table of Contents
(b) the Successor Person, if other than such Guarantor, expressly assumes all the obligations of such Guarantor under the Indenture and such Guarantor’s related Guarantee pursuant to supplemental indentures or other documents or instruments;
(c) immediately after such transaction, no Default exists; and
(d) the Issuer shall have delivered to the Trustee an Officer’s Certificate and an Opinion of Counsel, each stating that such consolidation, merger or transfer and such supplemental indentures, if any, comply with the Indenture; or
(2) the transaction is made in compliance with the covenant described under “Repurchase at the Option of Holders—Asset Sales.”
Subject to certain limitations described in the Indenture, the Successor Person will succeed to, and be substituted for, such Guarantor under the Indenture and such Guarantor’s Guarantee. Notwithstanding the foregoing, any Guarantor may (1) merge into or transfer all or part of its properties and assets to another Guarantor or the Issuer, (2) merge with an Affiliate of the Issuer solely for the purpose of reincorporating the Guarantor in the United States, any state thereof, the District of Columbia or any territory thereof or (3) convert into a corporation, partnership, limited partnership, limited liability corporation or trust organized or existing under the laws of the jurisdiction of organization of such Guarantor.
Transactions with Affiliates
The Issuer will not, and will not permit any of its Restricted Subsidiaries to, make any payment to, or sell, lease, transfer or otherwise dispose of any of its properties or assets to, or purchase any property or assets from, or enter into or make or amend any transaction, contract, agreement, understanding, loan, advance or guarantee with, or for the benefit of, any Affiliate of the Issuer (each of the foregoing, an “Affiliate Transaction”) involving aggregate payments or consideration in excess of $25.0 million, unless:
(1) such Affiliate Transaction is on terms that are not materially less favorable to the Issuer or its relevant Restricted Subsidiary than those that would have been obtained in a comparable transaction by the Issuer or such Restricted Subsidiary with an unrelated Person on an arm’s-length basis; and
(2) the Issuer delivers to the Trustee with respect to any Affiliate Transaction or series of related Affiliate Transactions involving aggregate payments or consideration in excess of $50.0 million, a resolution adopted by the majority of the board of directors of the Issuer approving such Affiliate Transaction and set forth in an Officer’s Certificate certifying that such Affiliate Transaction complies with clause (1) above.
The foregoing provisions will not apply to the following:
(1) transactions between or among the Issuer or any of its Restricted Subsidiaries;
(2) Restricted Payments permitted by the provisions of the Indenture described above under the covenant “—Limitation on Restricted Payments” and the definition of “Permitted Investments”;
(3) the payment of management, consulting, monitoring, advisory and other fees and related expenses pursuant to the Management Fee Agreement (plus any unpaid management, consulting, monitoring, advisory and other fees and related expenses accrued in any prior year) and the termination fees pursuant to the Management Fee Agreement, or any amendment thereto so long as any such amendment is not disadvantageous in the good faith judgment of the board of directors of the Issuer to the Holders when taken as a whole, as compared to the Management Fee Agreement as in effect on the Issue Date;
(4) the payment of reasonable and customary fees paid to, and indemnities provided for the benefit of, current or former employees, directors, officers, managers, distributors or consultants of the Issuer, any of its direct or indirect parent companies or any of its Restricted Subsidiaries;
(5) transactions in which the Issuer or any of its Restricted Subsidiaries, as the case may be, delivers to the Trustee a letter from an Independent Financial Advisor stating that such transaction is fair to the Issuer or such Restricted Subsidiary from a financial point of view or stating that the terms are not materially less favorable to the Issuer or its relevant Restricted Subsidiary than those that would have been obtained in a comparable transaction by the Issuer or such Restricted Subsidiary with an unrelated Person on an arm’s-length basis;
(6) any agreement as in effect as of the Issue Date, or any amendment thereto (so long as any such amendment is not disadvantageous in the good faith judgment of the board of directors of the Issuer to the Holders when taken as a whole as compared to the applicable agreement as in effect on the Issue Date);
(7) the existence of, or the performance by the Issuer or any of its Restricted Subsidiaries of its obligations under the terms of, any shareholders agreement (including any registration rights agreement or purchase agreement related thereto) to which it is a party as of the Issue Date and any similar agreements which it may enter into thereafter; provided that the existence of, or the performance by the Issuer or any of its Restricted Subsidiaries of obligations under any future amendment to any such existing agreement or under any similar agreement entered into after the Issue Date shall only be permitted by this clause (7) to the extent that the terms of any such amendment or new agreement are not otherwise disadvantageous in the good faith judgment of the board of directors of the Issuer to the Holders when taken as a whole;
157
Table of Contents
(8) the Transactions and the payment of all fees and expenses related to the Transactions, in each case as contemplated by this prospectus;
(9) transactions with customers, clients, suppliers, contractors, joint venture partners or purchasers or sellers of goods or services that are Affiliates, in each case in the ordinary course of business and otherwise in compliance with the terms of the Indenture which are fair to the Issuer and its Restricted Subsidiaries, in the reasonable determination of the board of directors of the Issuer or the senior management thereof, or are on terms at least as favorable as might reasonably have been obtained at such time from an unaffiliated party;
(10) the issuance of Equity Interests (other than Disqualified Stock) of the Issuer to any Permitted Holder or to any employee, director, officer, manager, distributor or consultant (or their respective
Controlled Investment Affiliates or Immediate Family Members) of the Issuer, any of its direct or indirect parent companies or any of its Restricted Subsidiaries;
(11) sales of accounts receivable, or participations therein, or Securitization Assets or related assets in connection with the ABL Facilities or any Qualified Securitization Facility;
(12) payments by the Issuer or any of its Restricted Subsidiaries to any of the Investors made for any financial advisory, financing, underwriting or placement services or in respect of other investment banking activities, including, without limitation, in connection with acquisitions or divestitures which payments are approved by a majority of the board of directors of the Issuer in good faith;
(13) payments and Indebtedness and Disqualified Stock (and cancellation of any thereof) of the Issuer and its Restricted Subsidiaries and Preferred Stock (and cancellation of any thereof) of any Restricted Subsidiary to any future, current or former employee, director, officer, manager, distributor or consultant (or their respective Controlled Investment Affiliates or Immediate Family Members) of the Issuer, any of its Subsidiaries or any of its direct or indirect parent companies pursuant to any management equity plan or stock option plan or any other management or employee benefit plan or agreement or any stock subscription or shareholder agreement or any distributor equity plan or agreement; and any employment agreements, stock option plans and other compensatory arrangements (and any successor plans thereto) and any supplemental executive retirement benefit plans or arrangements with any such employees, directors, officers, managers, distributors or consultants (or their respective Controlled Investment Affiliates or Immediate Family Members) that are, in each case, approved by the Issuer in good faith;
(14) investments by any of the Investors in securities of the Issuer or any of its Restricted Subsidiaries (and payment of reasonable out-of-pocket expenses incurred by such Investors in connection therewith) so long as (a) the investment is being offered generally to other investors on the same or more favorable terms and (b) the investment constitutes less than 5.0% of the proposed or outstanding issue amount of such class of securities;
(15) payments to or from, and transactions with, any joint venture in the ordinary course of business (including, without limitation, any cash management activities related thereto);
(16) payments by the Issuer (and any direct or indirect parent company thereof) and its Subsidiaries pursuant to tax sharing agreements among the Issuer (and any such parent company) and its Subsidiaries; provided that in each case the amount of such payments in any fiscal year does not exceed the amount that the Issuer, its Restricted Subsidiaries and its Unrestricted Subsidiaries (to the extent of amount received from Unrestricted Subsidiaries) would be required to pay in respect of foreign, federal, state and local taxes for such fiscal year were the Issuer, its Restricted Subsidiaries and its Unrestricted Subsidiaries (to the extent described above) to pay such taxes separately from any such parent entity;
(17) any lease entered into between the Issuer or any Restricted Subsidiary, as lessee and any Affiliate of the Issuer, as lessor, which is approved by a majority of the disinterested members of the board of directors of the Issuer in good faith; and
(18) intellectual property licenses in the ordinary course of business.
Dividend and Other Payment Restrictions Affecting Restricted Subsidiaries
The Issuer will not, and will not permit any of its Restricted Subsidiaries that is not a Guarantor to, directly or indirectly, create or otherwise cause or suffer to exist or become effective any consensual encumbrance or consensual restriction on the ability of any such Restricted Subsidiary to:
(1) (a) pay dividends or make any other distributions to the Issuer or any of its Restricted Subsidiaries on its Capital Stock or with respect to any other interest or participation in, or measured by, its profits, or
(b) pay any Indebtedness owed to the Issuer or any of its Restricted Subsidiaries;
(2) make loans or advances to the Issuer or any of its Restricted Subsidiaries; or
(3) sell, lease or transfer any of its properties or assets to the Issuer or any of its Restricted Subsidiaries, except (in each case) for such encumbrances or restrictions existing under or by reason of:
(a) contractual encumbrances or restrictions in effect on the Issue Date, including pursuant to the Senior Credit Facilities and the related documentation, Hedging Obligations and the indenture governing the Senior Notes and the related documentation;
158
Table of Contents
(b) the Indenture, the Senior Subordinated Notes and the guarantees thereof;
(c) purchase money obligations for property acquired in the ordinary course of business and Capital Lease Obligations that impose restrictions of the nature discussed in clause (3) above on the property so acquired;
(d) applicable law or any applicable rule, regulation or order;
(e) any agreement or other instrument of a Person acquired by the Issuer or any of its Restricted Subsidiaries in existence at the time of such acquisition or at the time it merges with or into the Issuer or any of its Restricted Subsidiaries or assumed in connection with the acquisition of assets from such Person (but, in any such case, not created in contemplation thereof), which encumbrance or restriction is not applicable to any Person, or the properties or assets of any Person, other than the Person so acquired and its Subsidiaries, or the property or assets of the Person so acquired and its Subsidiaries;
(f) contracts for the sale of assets, including customary restrictions with respect to a Subsidiary of the Issuer pursuant to an agreement that has been entered into for the sale or disposition of all or substantially all of the Capital Stock or assets of such Subsidiary;
(g) Secured Indebtedness otherwise permitted to be incurred pursuant to the covenants described under “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock” and “—Liens” that limit the right of the debtor to dispose of the assets securing such Indebtedness;
(h) restrictions on cash or other deposits or net worth imposed by customers under contracts entered into in the ordinary course of business;
(i) other Indebtedness, Disqualified Stock or Preferred Stock of Foreign Subsidiaries permitted to be incurred subsequent to the Issue Date pursuant to the provisions of the covenant described under “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”;
(j) customary provisions in joint venture agreements and other similar agreements relating solely to such joint venture;
(k) customary provisions contained in leases, licenses or similar agreements, including with respect to intellectual property and other agreements, in each case, entered into in the ordinary course of business;
(l) restrictions created in connection with any Qualified Securitization Facility that, in the good faith determination of the Issuer are necessary or advisable to effect such Qualified Securitization Facility;
(m) restrictions or conditions contained in any trading, netting, operating, construction, service, supply, purchase, sale or other agreement to which the Issuer or any of its Restricted Subsidiaries is a party entered into in the ordinary course of business; provided that such agreement prohibits the encumbrance of solely the property or assets of the Issuer or such Restricted Subsidiary that are the subject to such agreement, the payment rights arising thereunder or the proceeds thereof and does not extend to any other asset or property of the Issuer or such Restricted Subsidiary or the assets or property of another Restricted Subsidiary; and
(n) any encumbrances or restrictions of the type referred to in clauses (1), (2) and (3) above imposed by any amendments, modifications, restatements, renewals, increases, supplements, refundings, replacements or refinancings of the contracts, instruments or obligations referred to in clauses (a) through (m) above; provided that such amendments, modifications, restatements, renewals, increases, supplements, refundings, replacements or refinancings are, in the good faith judgment of the Issuer, no more restrictive with respect to such encumbrance and other restrictions taken as a whole than those prior to such amendment, modification, restatement, renewal, increase, supplement, refunding, replacement or refinancing.
Limitation on Guarantees of Indebtedness by Restricted Subsidiaries
The Issuer will not permit any of its Wholly-Owned Subsidiaries that are Restricted Subsidiaries (and non-Wholly-Owned Subsidiaries if such non-Wholly-Owned Subsidiaries guarantee other capital markets debt securities of the Issuer or any Guarantor), other than a Guarantor, a Foreign Subsidiary or a Securitization Subsidiary, to guarantee the payment of any Indebtedness of the Issuer or any other Guarantor unless:
(1) such Restricted Subsidiary within 30 days executes and delivers a supplemental indenture to the Indenture providing for a Guarantee by such Restricted Subsidiary, except that with respect to a guarantee of Indebtedness of the Issuer or any Guarantor:
(a) if the Senior Subordinated Notes or such Guarantor’s Guarantee are subordinated in right of payment to such Indebtedness, the Guarantee under the supplemental indenture shall be subordinated to such Restricted Subsidiary’s guarantee with respect to such Indebtedness substantially to the same extent as the Senior Subordinated Notes are subordinated to such Indebtedness; and
(b) if such Indebtedness is by its express terms subordinated in right of payment to the Senior Subordinated Notes or such Guarantor’s Guarantee, any such guarantee by such Restricted Subsidiary with respect to such Indebtedness shall be subordinated in right of payment to such Guarantee substantially to the same extent as such Indebtedness is subordinated to the Senior Subordinated Notes; and
(2) such Restricted Subsidiary waives and will not in any manner whatsoever claim or take the benefit or advantage of, any rights of reimbursement, indemnity or subrogation or any other rights against the Issuer or any other Restricted Subsidiary as a result of any payment by such Restricted Subsidiary under its Guarantee;
159
Table of Contents
provided that this covenant shall not be applicable to (i) any guarantee of any Restricted Subsidiary that existed at the time such Person became a Restricted Subsidiary and was not incurred in connection with, or in contemplation of, such Person becoming a Restricted Subsidiary and (ii) guarantees of the ABL Facilities by the ABL Financing Entities or of any Qualified Securitization Facility by any Restricted Subsidiary.
Limitation on Layering
The Indenture provides that the Issuer will not, and will not permit any Guarantor to, directly or indirectly, incur any Indebtedness (including Acquired Indebtedness) that is subordinate in right of payment to any Senior Indebtedness of the Issuer or such other Guarantor, as the case may be, unless such Indebtedness is either:
(1) equal in right of payment with the Senior Subordinated Notes or such Guarantor’s Guarantee of the Senior Subordinated Notes, as the case may be; or
(2) expressly subordinated in right of payment to the Senior Subordinated Notes or such Guarantor’s Guarantee of the Senior Subordinated Notes, as the case may be.
The Indenture does not treat (1) unsecured Indebtedness as subordinated or junior to Secured Indebtedness merely because it is unsecured or (2) Senior Indebtedness as subordinated or junior to any other Senior Indebtedness merely because it has a junior priority with respect to the same collateral.
Reports and Other Information
Notwithstanding that the Issuer may not be subject to the reporting requirements of Section 13 or 15(d) of the Exchange Act or otherwise report on an annual and quarterly basis on forms provided for such annual and quarterly reporting pursuant to rules and regulations promulgated by the SEC, the Indenture requires the Issuer to file with the SEC (and make available to the Trustee and Holders of the Senior Subordinated Notes (without exhibits), without cost to any Holder, within 15 days after it files them with the SEC) from and after the Issue Date,
(1) within 90 days (or any other time period then in effect under the rules and regulations of the Exchange Act with respect to the filing of a Form 10-K by a non-accelerated filer) after the end of each fiscal year, annual reports on Form 10-K, or any successor or comparable form, containing the information required to be contained therein, or required in such successor or comparable form;
(2) within 45 days (or any other time period then in effect under the rules and regulations of the Exchange Act with respect to the filing of a Form 10-Q by a non-accelerated filer) after the end of each of the first three fiscal quarters of each fiscal year (commencing with the fiscal quarter ending August 31, 2007), reports on Form 10-Q containing all quarterly information that would be required to be contained in Form 10-Q, or any successor or comparable form;
(3) promptly from time to time after the occurrence of an event required to be therein reported, such other reports on Form 8-K, or any successor or comparable form; and
(4) any other information, documents and other reports which the Issuer would be required to file with the SEC if it were subject to Section 13 or 15(d) of the Exchange Act;
in each case, in a manner that complies in all material respects with the requirements specified in such form; provided that the Issuer shall not be so obligated to file such reports with the SEC if the SEC does not permit such filing, in which event the Issuer will make available such information to prospective purchasers of Senior Subordinated Notes, in addition to providing such information to the Trustee and the Holders of the Senior Subordinated Notes, in each case within 15 days after the time the Issuer would be required to file such information with the SEC, if it were subject to Sections 13 or 15(d) of the Exchange Act. In addition, to the extent not satisfied by the foregoing, the Issuer agrees that, for so long as any Senior Subordinated Notes are outstanding, it will furnish to Holders and to securities analysts and prospective investors, upon their request, the information required to be delivered pursuant to Rule 144A(d)(4) under the Securities Act; provided, further, that any report required to be delivered under clause (2) above prior to the first date of delivery of report pursuant to clause (1) following the Issue Date shall not be required to contain all purchase accounting adjustments relating to the Transactions to the extent it is not practicable to include any such adjustments in such report.
In the event that any direct or indirect parent company of the Issuer becomes a guarantor of the Senior Subordinated Notes, the Indenture permits the Issuer to satisfy its obligations in this covenant with respect to financial information relating to the Issuer by furnishing financial information relating to such parent; provided that the same is accompanied by consolidating information that explains in reasonable detail the differences between the information relating to such parent, on the one hand, and the information relating to the Issuer and its Restricted Subsidiaries on a standalone basis, on the other hand.
Notwithstanding the foregoing, such requirements shall be deemed satisfied prior to the commencement of the exchange offer or the effectiveness of the shelf registration statement by (1) the filing with the SEC of the exchange offer registration statement or shelf registration statement (or any other similar registration statement), and any amendments thereto, with such financial information that satisfies Regulation S-X of the Securities Act, subject to exceptions consistent with the presentation of financial information in this prospectus, to the extent filed within the time specified above, or (2) by posting on its website and providing to the Trustee within 15 days of the time periods after the Issuer would have been required to file annual and interim reports with the SEC, the financial information (including a “Management’s Discussion and Analysis of Financial Condition and Results of Operations” section) that would be required to be included in such reports, subject to exceptions consistent with the presentation of financial information in this prospectus, to the extent filed within the times specified above.
Notwithstanding anything herein to the contrary, the Issuer will not be deemed to have failed to comply with any of its obligations hereunder for purposes of clause (3) under “Events of Default and Remedies” until 120 days after the date any report hereunder is due.
160
Table of Contents
The Issuer shall use its commercially reasonable efforts, consistent with its judgment as to what is prudent at the time, to participate in quarterly conference calls to discuss operating results and related matters. The Company shall issue a press release which will provide the date and time of any such call and will direct Holders, prospective investors and securities analysts to contact the investor relations office of the Issuer to obtain access to the conference call.
Events of Default and Remedies
The Indenture provides that each of the following is an Event of Default:
(1) default in payment when due and payable, upon redemption, acceleration or otherwise, of principal of, or premium, if any, on the Senior Subordinated Notes (whether or not prohibited by the subordination provisions of the Indenture);
(2) default for 30 days or more in the payment when due of interest or Additional Interest on or with respect to the Senior Subordinated Notes (whether or not prohibited by the subordination provisions of the Indenture);
(3) failure by the Issuer or any Guarantor for 60 days after receipt of written notice given by the Trustee or the Holders of not less than 30.0% in principal amount of the then outstanding Senior Subordinated Notes to comply with any of its obligations, covenants or agreements (other than a default referred to in clause (1) or (2) above) contained in the Indenture or the Senior Subordinated Notes;
(4) default under any mortgage, indenture or instrument under which there is issued or by which there is secured or evidenced any Indebtedness for money borrowed by the Issuer or any of its Restricted Subsidiaries or the payment of which is guaranteed by the Issuer or any of its Restricted Subsidiaries, other than Indebtedness owed to the Issuer or a Restricted Subsidiary, whether such Indebtedness or guarantee now exists or is created after the issuance of the Senior Subordinated Notes, if both:
(a) such default either results from the failure to pay any principal of such Indebtedness at its stated final maturity (after giving effect to any applicable grace periods) or relates to an obligation other than the obligation to pay principal of any such Indebtedness at its stated final maturity and results in the holder or holders of such Indebtedness causing such Indebtedness to become due prior to its stated maturity; and
(b) the principal amount of such Indebtedness, together with the principal amount of any other such Indebtedness in default for failure to pay principal at stated final maturity (after giving effect to any applicable grace periods), or the maturity of which has been so accelerated, aggregate $75.0 million or more at any one time outstanding;
(5) failure by the Issuer or any Significant Subsidiary (or any group of Subsidiaries that together would constitute a Significant Subsidiary) to pay final judgments aggregating in excess of $75.0 million, which final judgments remain unpaid, undischarged and unstayed for a period of more than 60 days after such judgment becomes final, and in the event such judgment is covered by insurance, an enforcement proceeding has been commenced by any creditor upon such judgment or decree which is not promptly stayed;
(6) certain events of bankruptcy or insolvency with respect to the Issuer or any Significant Subsidiary (or any group of Subsidiaries that together would constitute a Significant Subsidiary); or
(7) the Guarantee of any Significant Subsidiary (or any group of Subsidiaries that together would constitute a Significant Subsidiary) shall for any reason cease to be in full force and effect or be declared null and void or any responsible officer of any Guarantor that is a Significant Subsidiary (or the responsible officers of any group of Subsidiaries that together would constitute a Significant Subsidiary), as the case may be, denies that it has any further liability under its Guarantee or gives notice to such effect, other than by reason of the termination of the Indenture or the release of any such Guarantee in accordance with the Indenture.
If any Event of Default (other than of a type specified in clause (6) above) occurs and is continuing under the Indenture, the Trustee or the Holders of at least 30.0% in principal amount of the then total outstanding Senior Subordinated Notes may declare the principal, premium, if any, interest and any other monetary obligations on all the then outstanding Senior Subordinated Notes to be due and payable immediately; provided that so long as any Indebtedness permitted to be incurred under the Indenture as part of the Senior Credit Facilities shall be outstanding, no such acceleration shall be effective until the earlier of:
(1) acceleration of any such Indebtedness under the Senior Credit Facilities; or
(2) five Business Days after the giving of written notice of such acceleration to the Issuer and the Representative with respect to the Senior Credit Facilities.
Upon the effectiveness of such declaration, such principal of and premium, if any, and interest will be due and payable immediately. Notwithstanding the foregoing, in the case of an Event of Default arising under clause (6) of the first paragraph of this section, all outstanding Senior Subordinated Notes will become due and payable without further action or notice. The Indenture provides that the Trustee may withhold from the Holders notice of any continuing Default, except a Default relating to the payment of principal, premium, if any, or interest, if it determines that withholding notice is in their interest. In addition, the Trustee will have no obligation to accelerate the Senior Subordinated Notes if in the best judgment of the Trustee acceleration is not in the best interests of the Holders of the Senior Subordinated Notes.
The Indenture provides that the Holders of a majority in aggregate principal amount of the then outstanding Senior Subordinated Notes by notice to the Trustee may on behalf of the Holders of all of the Senior Subordinated Notes waive any existing Default and its consequences under the Indenture (except a continuing Default in the payment of interest on, premium, if any, or the principal of any Senior Subordinated Note held by a non-consenting Holder) and rescind any acceleration with respect to the Senior Subordinated Notes and its consequences (except if such rescission would conflict with any judgment of a court of competent jurisdiction). In the event of any Event of Default specified in clause (4) above, such Event of Default and all consequences thereof (excluding any resulting payment default, other than as a result of acceleration of the Senior Subordinated Notes) shall be annulled, waived and rescinded, automatically and without any action by the Trustee or the Holders, if within 20 days after such Event of Default arose:
(1) the Indebtedness or guarantee that is the basis for such Event of Default has been discharged; or
161
Table of Contents
(2) holders thereof have rescinded or waived the acceleration, notice or action (as the case may be) giving rise to such Event of Default; or
(3) the default that is the basis for such Event of Default has been cured.
Notwithstanding the foregoing, the sole remedy for any breach of our obligation under the Indenture to file periodic or other reports (including pursuant to section 314(a)(1) of the Trust Indenture Act) shall be the payment of liquidated damages, and the Holders will not have any right under the Indenture to accelerate the maturity of the Senior Subordinated Notes as a result of any such breach. If a breach of our obligation under the Indenture to file periodic or other reports (including pursuant to section 314(a)(1) of the Trust Indenture Act) continues for 90 days after notice thereof is given in accordance with the Indenture, we will pay liquidated damages to all the Holders of the Senior Subordinated Notes at a rate per annum equal to (i) 0.25% per annum of the principal amount of the Senior Subordinated Notes from the 90 th day following such notice to but not including the 180 th day following such notice (or such earlier date on which the Event of Default relating to the reporting obligations referred to in this paragraph shall have been cured or waived) and (ii) 0.50% per annum of the principal amount of the Senior Subordinated Notes from the 180 th day following such notice to but not including the 365 th day following such notice (or such earlier date on which the Event of Default relating to the reporting obligations referred to in this paragraph shall have been cured or waived). On such 365 th day (or earlier, if the Event of Default relating to the reporting obligations referred to in this paragraph shall have been cured or waived prior to such 365 th day), such Additional Interest will cease to accrue, and the Senior Subordinated Notes will be subject to acceleration as provided above if the Event of Default is continuing. The provisions of the indenture described in this paragraph will not affect the rights of the Holders of Senior Subordinated Notes in the event of the occurrence of any other Event of Default.
Subject to the provisions of the Indenture relating to the duties of the Trustee thereunder, in case an Event of Default occurs and is continuing, the Trustee will be under no obligation to exercise any of the rights or powers under the Indenture at the request or direction of any of the Holders of the Senior Subordinated Notes unless the Holders have offered to the Trustee reasonable indemnity or security against any loss, liability or expense. Except to enforce the right to receive payment of principal, premium (if any) or interest when due, no Holder of a Senior Subordinated Note may pursue any remedy with respect to the Indenture or the Senior Subordinated Notes unless:
(1) such Holder has previously given the Trustee notice that an Event of Default is continuing;
(2) Holders of at least 30.0% in principal amount of the total outstanding Senior Subordinated Notes have requested the Trustee to pursue the remedy;
(3) Holders of the Senior Subordinated Notes have offered the Trustee reasonable security or indemnity against any loss, liability or expense;
(4) the Trustee has not complied with such request within 60 days after the receipt thereof and the offer of security or indemnity; and
(5) Holders of a majority in principal amount of the total outstanding Senior Subordinated Notes have not given the Trustee a direction inconsistent with such request within such 60-day period.
Subject to certain restrictions, under the Indenture the Holders of a majority in principal amount of the total outstanding Senior Subordinated Notes are given the right to direct the time, method and place of conducting any proceeding for any remedy available to the Trustee or of exercising any trust or power conferred on the Trustee. The Trustee, however, may refuse to follow any direction that conflicts with law or the Indenture or that the Trustee determines is unduly prejudicial to the rights of any other Holder of a Senior Subordinated Note or that would involve the Trustee in personal liability.
The Indenture provides that the Issuer is required to deliver to the Trustee annually a statement regarding compliance with the Indenture, and the Issuer is required, within five Business Days, upon becoming aware of any Default, to deliver to the Trustee a statement specifying such Default.
No Personal Liability of Directors, Officers, Employees and Shareholders
No director, officer, employee, incorporator or shareholder of the Issuer or any Guarantor or any of their parent companies (other than the Issuer and the Guarantors) shall have any liability, for any obligations of the Issuer or the Guarantors under the Senior Subordinated Notes, the Guarantees or the Indenture or for any claim based on, in respect of, or by reason of such obligations or their creation. Each Holder by accepting Senior Subordinated Notes waives and releases all such liability. The waiver and release are part of the consideration for issuance of the Senior Subordinated Notes. Such waiver may not be effective to waive liabilities under the federal securities laws and it is the view of the SEC that such a waiver is against public policy.
Legal Defeasance and Covenant Defeasance
The obligations of the Issuer and the Guarantors under the Indenture will terminate (other than certain obligations) and will be released upon payment in full of all of the Senior Subordinated Notes. The Issuer may, at its option and at any time, elect to have all of its obligations discharged with respect to the Senior Subordinated Notes and have each Guarantor’s obligation discharged with respect to its Guarantee (“Legal Defeasance”) and cure all then existing Events of Default except for:
(1) the rights of Holders of Senior Subordinated Notes to receive payments in respect of the principal of, premium, if any, and interest on the Senior Subordinated Notes when such payments are due solely out of the trust created pursuant to the Indenture;
162
Table of Contents
(2) the Issuer’s obligations with respect to Senior Subordinated Notes concerning issuing temporary Senior Subordinated Notes, registration of such Senior Subordinated Notes, mutilated, destroyed, lost or stolen Senior Subordinated Notes and the maintenance of an office or agency for payment and money for security payments held in trust;
(3) the rights, powers, trusts, duties and immunities of the Trustee, and the Issuer’s obligations in connection therewith; and
(4) the Legal Defeasance provisions of the Indenture.
In addition, the Issuer may, at its option and at any time, elect to have its obligations and those of each Guarantor released with respect to certain covenants that are described in the Indenture (“Covenant Defeasance”) and thereafter any omission to comply with such obligations shall not constitute a Default with respect to the Senior Subordinated Notes. In the event Covenant Defeasance occurs, certain events (not including bankruptcy, receivership, rehabilitation and insolvency events pertaining to the Issuer) described under “Events of Default and Remedies” will no longer constitute an Event of Default with respect to the Senior Subordinated Notes.
In order to exercise either Legal Defeasance or Covenant Defeasance with respect to the Senior Subordinated Notes:
(1) the Issuer must irrevocably deposit with the Trustee, in trust, for the benefit of the Holders of the Senior Subordinated Notes, cash in U.S. dollars, U.S. dollar-denominated Government Securities, or a combination thereof, in such amounts as will be sufficient, in the opinion of a nationally recognized firm of independent public accountants, to pay the principal of, premium, if any, and interest due on the Senior Subordinated Notes on the stated maturity date or on the redemption date, as the case may be, of such principal, premium, if any, or interest on such Senior Subordinated Notes and the Issuer must specify whether such Senior Subordinated Notes are being defeased to maturity or to a particular redemption date;
(2) in the case of Legal Defeasance, the Issuer shall have delivered to the Trustee an Opinion of Counsel reasonably acceptable to the Trustee confirming that, subject to customary assumptions and exclusions,
(a) the Issuer has received from, or there has been published by, the United States Internal Revenue Service a ruling, or
(b) since the issuance of the Senior Subordinated Notes, there has been a change in the applicable U.S. federal income tax law,
in either case to the effect that, and based thereon such Opinion of Counsel shall confirm that, subject to customary assumptions and exclusions, the Holders of the Senior Subordinated Notes will not recognize income, gain or loss for U.S. federal income tax purposes, as applicable, as a result of such Legal Defeasance and will be subject to U.S. federal income tax on the same amounts, in the same manner and at the same times as would have been the case if such Legal Defeasance had not occurred;
(3) in the case of Covenant Defeasance, the Issuer shall have delivered to the Trustee an Opinion of Counsel reasonably acceptable to the Trustee confirming that, subject to customary assumptions and exclusions, the Holders of the Senior Subordinated Notes will not recognize income, gain or loss for U.S. federal income tax purposes as a result of such Covenant Defeasance and will be subject to such tax on the same amounts, in the same manner and at the same times as would have been the case if such Covenant Defeasance had not occurred;
(4) no Default (other than that resulting from borrowing funds to be applied to make such deposit and any similar and simultaneous deposit relating to other Indebtedness and, in each case, the granting of Liens in connection therewith) shall have occurred and be continuing on the date of such deposit;
(5) such Legal Defeasance or Covenant Defeasance shall not result in a breach or violation of, or constitute a default under the Senior Credit Facilities, the Senior Notes, the indenture pursuant to which the Senior Notes were issued or any other material agreement or instrument (other than the Indenture) to which, the Issuer or any Guarantor is a party or by which the Issuer or any Guarantor is bound (other than that resulting from any borrowing of funds to be applied to make the deposit required to effect such Legal Defeasance or Covenant Defeasance and any similar and simultaneous deposit relating to other Indebtedness, and the granting of Liens in connection therewith);
(6) the Issuer shall have delivered to the Trustee an Opinion of Counsel to the effect that, as of the date of such opinion and subject to customary assumptions and exclusions following the deposit, the trust funds will not be subject to the effect of Section 547 of Title 11 of the United States Code;
(7) the Issuer shall have delivered to the Trustee an Officer’s Certificate stating that the deposit was not made by the Issuer with the intent of defeating, hindering, delaying or defrauding any creditors of the Issuer or any Guarantor or others; and
(8) the Issuer shall have delivered to the Trustee an Officer’s Certificate and an Opinion of Counsel (which Opinion of Counsel may be subject to customary assumptions and exclusions) each stating that all conditions precedent provided for or relating to the Legal Defeasance or the Covenant Defeasance, as the case may be, have been complied with.
Satisfaction and Discharge
The Indenture will be discharged and will cease to be of further effect as to all Senior Subordinated Notes, when either:
(1) all Senior Subordinated Notes theretofore authenticated and delivered, except lost, stolen or destroyed Senior Subordinated Notes which have been replaced or paid and Senior Subordinated Notes for whose payment money has theretofore been deposited in trust, have been delivered to the Trustee for cancellation; or
163
Table of Contents
(2) (a) all Senior Subordinated Notes not theretofore delivered to the Trustee for cancellation have become due and payable by reason of the making of a notice of redemption or otherwise, will become due and payable within one year or are to be called for redemption within one year under arrangements satisfactory to the Trustee for the giving of notice of redemption by the Trustee in the name, and at the expense, of the Issuer and the Issuer or any Guarantor have irrevocably deposited or caused to be deposited with the Trustee as trust funds in trust solely for the benefit of the Holders of the Senior Subordinated Notes, cash in U.S. dollars, U.S. dollar-denominated Government Securities, or a combination thereof, in such amounts as will be sufficient without consideration of any reinvestment of interest to pay and discharge the entire indebtedness on the Senior Subordinated Notes not theretofore delivered to the Trustee for cancellation for principal, premium, if any, and accrued interest to the date of maturity or redemption; provided , that upon any redemption that requires the payment of the Applicable Premium, the amount deposited shall be sufficient for purposes of the Indenture to the extent that an amount is deposited with the Trustee equal to the Applicable Premium calculated as of the date of the notice of redemption, with any deficit as of the date of redemption (any such amount, the “Applicable Premium Deficit”) only required to be deposited with the Trustee on or prior to the date of redemption. Any Applicable Premium Deficit shall be set forth in an Officer’s Certificate delivered to the Trustee simultaneously with the deposit of such Applicable Premium Deficit that confirms that such Applicable Premium Deficit shall be applied toward such redemption;
(b) no Default (other than that resulting from borrowing funds to be applied to make such deposit or any similar and simultaneous deposit relating to other Indebtedness and the granting of Liens in connection therewith) with respect to the Indenture or the Senior Subordinated Notes shall have occurred and be continuing on the date of such deposit or shall occur as a result of such deposit and such deposit will not result in a breach or violation of, or constitute a default under the Senior Credit Facilities, the Senior Notes, the indenture pursuant to which the Senior Notes were issued or any other material agreement or instrument (other than the Indenture) to which the Issuer or any Guarantor is a party or by which the Issuer or any Guarantor is bound (other than resulting from any borrowing of funds to be applied to make such deposit and any similar and simultaneous deposit relating to other Indebtedness and the granting of Liens in connection therewith);
(c) the Issuer has paid or caused to be paid all sums payable by it under the Indenture; and
(d) the Issuer has delivered irrevocable instructions to the Trustee to apply the deposited money toward the payment of the Senior Subordinated Notes at maturity or the redemption date, as the case may be.
In addition, the Issuer must deliver an Officer’s Certificate and an Opinion of Counsel to the Trustee stating that all conditions precedent to satisfaction and discharge have been satisfied.
Amendment, Supplement and Waiver
Except as provided in the next two succeeding paragraphs, the Indenture, any Guarantee and the Senior Subordinated Notes may be amended or supplemented with the consent of the Holders of at least a majority in principal amount of the Senior Subordinated Notes then outstanding, including consents obtained in connection with a purchase of, or tender offer or exchange offer for, Senior Subordinated Notes, and any existing Default or compliance with any provision of the Indenture or the Senior Subordinated Notes issued thereunder may be waived with the consent of the Holders of a majority in principal amount of the then outstanding Senior Subordinated Notes, other than Senior Subordinated Notes beneficially owned by the Issuer or its Affiliates (including consents obtained in connection with a purchase of or tender offer or exchange offer for the Senior Subordinated Notes).
The Indenture provides that, without the consent of each affected Holder of Senior Subordinated Notes, an amendment or waiver may not, with respect to any Senior Subordinated Notes held by a non-consenting Holder:
(1) reduce the principal amount of such Senior Subordinated Notes whose Holders must consent to an amendment, supplement or waiver;
(2) reduce the principal of or change the fixed final maturity of any such Senior Subordinated Note or alter or waive the provisions with respect to the redemption of such Senior Subordinated Notes (other than provisions relating to the covenants described above under “Repurchase at the Option of Holders”);
(3) reduce the rate of or change the time for payment of interest on any Senior Subordinated Note;
(4) waive a Default in the payment of principal of or premium, if any, or interest on the Senior Subordinated Notes, except a rescission of acceleration of the Senior Subordinated Notes by the Holders of at least a majority in aggregate principal amount of the Senior Subordinated Notes and a waiver of the payment default that resulted from such acceleration, or in respect of a covenant or provision contained in the Indenture or any Guarantee which cannot be amended or modified without the consent of all Holders;
(5) make any Senior Subordinated Note payable in money other than that stated therein;
(6) make any change to the provisions of the Indenture relating to waivers of past Defaults or the rights of Holders to receive payments of principal of or premium, if any, or interest on the Senior Subordinated Notes;
(7) make any change in these amendment and waiver provisions;
(8) impair the right of any Holder to receive payment of principal of, or premium, if any, or interest on such Holder’s Senior Subordinated Notes on or after the due dates therefor or to institute suit for the enforcement of any payment on or with respect to such Holder’s Senior Subordinated Notes;
164
Table of Contents
(9) make any change in the subordination provisions thereof that would adversely affect the Holders; or
(10) except as expressly permitted by the Indenture, modify the Guarantees of any Significant Subsidiary in any manner adverse to the Holders of the Senior Subordinated Notes.
Notwithstanding the foregoing, the Issuer, any Guarantor (with respect to a Guarantee or the Indenture to which it is a party) and the Trustee may amend or supplement the Indenture and any Guarantee or Senior Subordinated Notes without the consent of any Holder:
(1) to cure any ambiguity, omission, mistake, defect or inconsistency;
(2) to provide for uncertificated Senior Subordinated Notes in addition to or in place of certificated Senior Subordinated Notes;
(3) to comply with the covenant relating to mergers, consolidations and sales of assets;
(4) to provide the assumption of the Issuer’s or any Guarantor’s obligations to the Holders;
(5) to make any change that would provide any additional rights or benefits to the Holders or that does not adversely affect the legal rights under the Indenture of any such Holder;
(6) to add covenants for the benefit of the Holders or to surrender any right or power conferred upon the Issuer or any Guarantor;
(7) to comply with requirements of the SEC in order to effect or maintain the qualification of the Indenture under the Trust Indenture Act;
(8) to evidence and provide for the acceptance and appointment under the Indenture of a successor Trustee thereunder pursuant to the requirements thereof;
(9) to provide for the issuance of exchange notes or private exchange notes, which are identical to exchange notes except that they are not freely transferable;
(10) to add a Guarantor under the Indenture;
(11) to conform the text of the Indenture, Guarantees or the Senior Subordinated Notes to any provision of this “Description of Senior Subordinated Notes” to the extent that such provision in this “Description of Senior Subordinated Notes” was intended to be a verbatim recitation of a provision of the Indenture, Guarantee or Senior Subordinated Notes; or
(12) to make any amendment to the provisions of the Indenture relating to the transfer and legending of Senior Subordinated Notes as permitted by the Indenture, including, without limitation to facilitate the issuance and administration of the Senior Subordinated Notes; provided that (a) compliance with the Indenture as so amended would not result in Senior Subordinated Notes being transferred in violation of the Securities Act or any applicable securities law and (b) such amendment does not materially and adversely affect the rights of Holders to transfer Senior Subordinated Notes.
The consent of the Holders is not necessary under the Indenture to approve the particular form of any proposed amendment. It is sufficient if such consent approves the substance of the proposed amendment.
Notices
Notices given by publication or electronic delivery is deemed given on the first date on which publication is made and notices given by first-class mail, postage prepaid, is deemed given five calendar days after mailing.
Concerning the Trustee
The Indenture contains certain limitations on the rights of the Trustee thereunder, should it become a creditor of the Issuer, to obtain payment of claims in certain cases, or to realize on certain property received in respect of any such claim as security or otherwise. The Trustee is permitted to engage in other transactions; however, if it acquires any conflicting interest it must eliminate such conflict within 90 days, apply to the SEC for permission to continue or resign.
The Indenture provides that the Holders of a majority in principal amount of the outstanding Senior Subordinated Notes will have the right to direct the time, method and place of conducting any proceeding for exercising any remedy available to the Trustee, subject to certain exceptions. The Indenture provides that in case an Event of Default shall occur (which shall not be cured), the Trustee will be required, in the exercise of its power, to use the degree of care of a prudent person in the conduct of his own affairs. Subject to such provisions, the Trustee will be under no obligation to exercise any of its rights or powers under the Indenture at the request of any Holder of the Senior Subordinated Notes, unless such Holder shall have offered to the Trustee security and indemnity satisfactory to it against any loss, liability or expense.
Governing Law
The Indenture, the Senior Subordinated Notes and any Guarantee are governed by and construed in accordance with the laws of the State of New York.
165
Table of Contents
Certain Definitions
Set forth below are certain defined terms used in the Indenture. For purposes of the Indenture, unless otherwise specifically indicated, the term “consolidated” with respect to any Person refers to such Person consolidated with its Restricted Subsidiaries, and excludes from such consolidation any Unrestricted Subsidiary as if such Unrestricted Subsidiary were not an Affiliate of such Person.
“ABL Facilities” means the asset-based revolving credit facilities under the Credit Agreement to be entered into as of the Issue Date by and among the Issuer, the lenders party thereto in their capacities as lenders thereunder and Bank of America, N.A., as Administrative Agent, including any guarantees, collateral documents, instruments and agreements executed in connection therewith, and any amendments, supplements, modifications, extensions, renewals, restatements, refundings or refinancings thereof and any indentures or credit facilities or commercial paper facilities with banks or other institutional lenders or investors that replace, refund or refinance any part of the loans, notes, other credit facilities or commitments thereunder, including any such replacement, refunding or refinancing facility or indenture that increases the amount borrowable thereunder or alters the maturity thereof ( provided that such increase in borrowings is permitted under “Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock” above).
“ABL Financing Entity” means the Issuer and certain of its Subsidiaries from time to time named as borrowers or guarantors under the ABL Facilities.
“Acquired Indebtedness” means, with respect to any specified Person,
(1) Indebtedness of any other Person existing at the time such other Person is merged with or into or became a Restricted Subsidiary of such specified Person, including Indebtedness incurred in connection with, or in contemplation of, such other Person merging with or into or becoming a Restricted Subsidiary of such specified Person, and
(2) Indebtedness secured by a Lien encumbering any asset acquired by such specified Person.
“Acquisition” means the transactions contemplated by the Transaction Agreement.
“Additional Interest” means all additional interest then owing pursuant to the Registration Rights Agreement.
“Affiliate” of any specified Person means any other Person directly or indirectly controlling or controlled by or under direct or indirect common control with such specified Person. For purposes of this definition, “control” (including, with correlative meanings, the terms “controlling,” “controlled by” and “under common control with”), as used with respect to any Person, shall mean the possession, directly or indirectly, of the power to direct or cause the direction of the management or policies of such Person, whether through the ownership of voting securities, by agreement or otherwise.
“Applicable Premium” means, with respect to any Senior Subordinated Note on any Redemption Date, the greater of:
(1) 1.0% of the principal amount of such Senior Subordinated Note; and
(2) the excess, if any, of (a) the present value at such Redemption Date of (i) the redemption price of such Senior Subordinated Note at October 15, 2012 (such redemption price being set forth in the table appearing above under “Optional Redemption”), plus (ii) all required remaining scheduled interest payments due on such Senior Subordinated Note through October 15, 2012 (excluding accrued but unpaid interest to the Redemption Date), computed using a discount rate equal to the Treasury Rate as of such Redemption Date plus 50 basis points; over (b) the principal amount of such Senior Subordinated Note.
“Asset Sale” means:
(1) the sale, conveyance, transfer or other disposition, whether in a single transaction or a series of related transactions (including by way of a Sale and Lease-Back Transaction) of property or assets of the Issuer or any of its Restricted Subsidiaries (each referred to in this definition as a “disposition”); or
(2) the issuance or sale of Equity Interests of any Restricted Subsidiary (other than Preferred Stock of Restricted Subsidiaries issued in compliance with the covenant described under “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”), whether in a single transaction or a series of related transactions;
in each case, other than:
(a) any disposition of Cash Equivalents or Investment Grade Securities or obsolete or worn out equipment in the ordinary course of business or any disposition of inventory or goods (or other assets) held for sale or no longer used in the ordinary course of business;
(b) the disposition of all or substantially all of the assets of the Issuer in a manner permitted pursuant to the provisions described above under “Certain Covenants—Merger, Consolidation or Sale of All or Substantially All Assets” or any disposition that constitutes a Change of Control pursuant to the Indenture;
(c) the making of any Restricted Payment or Permitted Investment that is permitted to be made, and is made, under the covenant described above under “Certain Covenants—Limitation on Restricted Payments”;
(d) any disposition of assets or issuance or sale of Equity Interests of any Restricted Subsidiary in any transaction or series of related transactions with an aggregate fair market value of less than $50.0 million;
166
Table of Contents
(e) any disposition of property or assets or the issuance of securities by a Restricted Subsidiary to the Issuer or by the Issuer or a Restricted Subsidiary to a Restricted Subsidiary;
(f) to the extent allowable under Section 1031 of the Internal Revenue Code of 1986, any exchange of like property (excluding any boot thereon) for use in a Similar Business;
(g) the lease, assignment or sub-lease of any real or personal property in the ordinary course of business;
(h) any issuance or sale of Equity Interests in, or Indebtedness or other securities of, an Unrestricted Subsidiary;
(i) foreclosures, condemnation or any similar action on assets or the granting of Liens not prohibited by the Indenture;
(j) sales of accounts receivable, or participations therein, or Securitization Assets or related assets in connection with the ABL Facilities or any Qualified Securitization Facility;
(k) any financing transaction with respect to property built or acquired by the Issuer or any Restricted Subsidiary after the Issue Date, including Sale and Lease-Back Transactions and asset securitizations permitted by the Indenture;
(l) the sale or discount of inventory, accounts receivable or notes receivable in the ordinary course of business or the conversion of accounts receivable to notes receivable;
(m) the licensing or sub-licensing of intellectual property or other general intangibles in the ordinary course of business, other than the licensing of intellectual property on a long-term basis;
(n) any surrender or waiver of contract rights or the settlement, release or surrender of contract rights or other litigation claims in the ordinary course of business;
(o) the unwinding of any Hedging Obligations;
(p) sales, transfers and other dispositions of Investments in joint ventures to the extent required by, or made pursuant to, customary buy/sell arrangements between the joint venture parties set forth in joint venture arrangements and similar binding arrangements; and
(q) the abandonment of intellectual property rights in the ordinary course of business, which in the reasonable good faith determination of the Issuer are not material to the conduct of the business of the Issuer and its Restricted Subsidiaries taken as a whole.
“Business Day” means each day which is not a Legal Holiday.
“Capital Stock” means:
(1) in the case of a corporation, corporate stock;
(2) in the case of an association or business entity, any and all shares, interests, participations, rights or other equivalents (however designated) of corporate stock;
(3) in the case of a partnership or limited liability company, partnership or membership interests (whether general or limited); and
(4) any other interest or participation that confers on a Person the right to receive a share of the profits and losses of, or distributions of assets of, the issuing Person but excluding from all of the foregoing any debt securities convertible into Capital Stock, whether or not such debt securities include any right of participation with Capital Stock.
“Capitalized Lease Obligation” means, at the time any determination thereof is to be made, the amount of the liability in respect of a capital lease that would at such time be required to be capitalized and reflected as a liability on a balance sheet (excluding the footnotes thereto) prepared in accordance with GAAP.
“Capitalized Software Expenditures” shall mean, for any period, the aggregate of all expenditures (whether paid in cash or accrued as liabilities) by a Person and its Restricted Subsidiaries during such period in respect of licensed or purchased software or internally developed software and software enhancements that, in conformity with GAAP, are or are required to be reflected as capitalized costs on the consolidated balance sheet of a Person and its Restricted Subsidiaries.
“Cash Equivalents” means:
(1) United States dollars;
(2) (a) Canadian dollars, yen, pounds sterling, euros or any national currency of any participating member state of the EMU; or
(b) in the case of any Foreign Subsidiary that is a Restricted Subsidiary, such local currencies held by it from time to time in the ordinary course of business;
(3) securities issued or directly and fully and unconditionally guaranteed or insured by the U.S. government or any agency or instrumentality thereof the securities of which are unconditionally guaranteed as a full faith and credit obligation of such government with maturities of 24 months or less from the date of acquisition;
167
Table of Contents
(4) certificates of deposit, time deposits and eurodollar time deposits with maturities of 24 months or less from the date of acquisition, bankers’ acceptances with maturities not exceeding one year and overnight bank deposits, in each case with any domestic or foreign commercial bank having capital and surplus of not less than $500.0 million in the case of U.S. banks and $100.0 million (or the U.S. dollar equivalent as of the date of determination) in the case of non-U.S. banks;
(5) repurchase obligations for underlying securities of the types described in clauses (3), (4) and (8) entered into with any financial institution meeting the qualifications specified in clause (4) above;
(6) commercial paper rated at least P-2 by Moody’s or at least A-2 by S&P (or, if at any time neither Moody’s nor S&P shall be rating such obligations, an equivalent rating from another Rating Agency) and in each case maturing within 24 months after the date of creation thereof and Indebtedness or Preferred Stock issued by Persons with a rating of “A” or higher from S&P or “A-2” or higher from Moody’s with maturities of 24 months or less from the date of acquisition;
(7) marketable short-term money market and similar funds having a rating of at least P-2 or A-2 from either Moody’s or S&P, respectively (or, if at any time neither Moody’s nor S&P shall be rating such obligations, an equivalent rating from another Rating Agency);
(8) readily marketable direct obligations issued by any state, commonwealth or territory of the United States or any political subdivision or taxing authority thereof having an Investment Grade Rating from either Moody’s or S&P (or, if at any time neither Moody’s nor S&P shall be rating such obligations, an equivalent rating from another Rating Agency) with maturities of 24 months or less from the date of acquisition;
(9) readily marketable direct obligations issued by any foreign government or any political subdivision or public instrumentality thereof, in each case having an Investment Grade Rating from either Moody’s or S&P (or, if at any time neither Moody’s nor S&P shall be rating such obligations, an equivalent rating from another Rating Agency) with maturities of 24 months or less from the date of acquisition;
(10) Investments with average maturities of 12 months or less from the date of acquisition in money market funds rated AAA- (or the equivalent thereof) or better by S&P or Aaa3 (or the equivalent thereof) or better by Moody’s (or, if at any time neither Moody’s nor S&P shall be rating such obligations, an equivalent rating from another Rating Agency); and
(11) investment funds investing at least 90.0% of their assets in securities of the types described in clauses (1) through (10) above.
In the case of Investments by any Foreign Subsidiary that is a Restricted Subsidiary or Investments made in a country outside the United States of America, Cash Equivalents shall also include (a) investments of the type and maturity described in clauses (1) through (8) and clauses (10) and (11) above of foreign obligors, which Investments or obligors (or the parents of such obligors) have ratings described in such clauses or equivalent ratings from comparable foreign rating agencies and (b) other short-term investments utilized by Foreign Subsidiaries that are Restricted Subsidiaries in accordance with normal investment practices for cash management in investments analogous to the foregoing investments in clauses (1) through (11) and in this paragraph.
Notwithstanding the foregoing, Cash Equivalents shall include amounts denominated in currencies other than those set forth in clauses (1) and (2) above, provided that such amounts are converted into any currency listed in clauses (1) and (2) as promptly as practicable and in any event within ten Business Days following the receipt of such amounts.
At any time at which the value, calculated in accordance with GAAP, of all investments of the Issuer and its Restricted Subsidiaries that were deemed, when made, to be Cash Equivalents in accordance with clauses (1) through (11) above exceeds the Indebtedness of the Issuer and its Restricted Subsidiaries, “Cash Equivalents” shall also mean any investment (a “Qualifying Investment”) that satisfies the following two conditions: (a) the Qualifying Investment is of a type described in clauses (1) through (11) of this definition, but has an effective maturity (whether by reason of final maturity, a put option or, in the case of an asset-backed security, an average life) of five years and one month or less from the date of such Qualifying Investment (notwithstanding any provision contained in such clauses (1) through (11) requiring a shorter maturity); and (b) the weighted average effective maturity of such Qualifying Investment and all other investments that were made as Qualifying Investments in accordance with this paragraph, does not exceed two years from the date of such Qualifying Investment.
“CF Credit Facilities” means the term and revolving credit facilities under the Credit Agreement to be entered into as of the Issue Date by and among the Issuer, the European subsidiary borrowers party thereto, the lenders party thereto in their capacities as lenders thereunder and Bank of America, N.A., as Administrative Agent, including any guarantees, collateral documents, instruments and agreements executed in connection therewith, and any amendments, supplements, modifications, extensions, renewals, restatements, refundings or refinancings thereof and any indentures or credit facilities or commercial paper facilities with banks or other institutional lenders or investors that replace, refund or refinance any part of the loans, notes, other credit facilities or commitments thereunder, including any such replacement, refunding or refinancing facility or indenture that increases the amount borrowable thereunder or alters the maturity thereof ( provided that such increase in borrowings is permitted under “Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock” above).
“Change of Control” means the occurrence of any of the following:
(1) the sale, lease or transfer, in one or a series of related transactions, of all or substantially all of the assets of the Issuer and its Subsidiaries, taken as a whole, to any Person other than a Permitted Holder; or
(2) the Issuer becomes aware of (by way of a report or any other filing pursuant to Section 13(d) of the Exchange Act, proxy, vote, written notice or otherwise) the acquisition by any Person or group (within the meaning of Section 13(d)(3) or Section 14(d)(2) of the Exchange Act, or any successor provision), including any group acting for the purpose of acquiring, holding or disposing of securities (within the meaning of Rule 13d-5(b)(1) under the Exchange Act), other than one or more Permitted Holders, in a single
168
Table of Contents
transaction or in a related series of transactions, by way of merger, consolidation or other business combination or purchase of beneficial ownership (within the meaning of Rule 13d-3 under the Exchange Act, or any successor provision) of 50.0% or more of the total voting power of the Voting Stock of the Issuer or any of its direct or indirect parent companies.
“Co-Investors” means the assignees, if any, of the equity commitments of the Investors on the Issue Date who become holders of Equity Interests in the Issuer (or any of the direct or indirect parent companies of the Issuer) on the Issue Date in connection with the Acquisition.
“Consolidated Depreciation and Amortization Expense” means with respect to any Person for any period, the total amount of depreciation and amortization expense of such Person, including the amortization of deferred financing fees, debt issuance costs, commissions, fees and expenses and Capitalized Software Expenditures of such Person and its Restricted Subsidiaries for such period on a consolidated basis and otherwise determined in accordance with GAAP.
“Consolidated Interest Expense” means, with respect to any Person for any period, without duplication, the sum of:
(1) consolidated interest expense of such Person and its Restricted Subsidiaries for such period, to the extent such expense was deducted (and not added back) in computing Consolidated Net Income (including (a) amortization of original issue discount resulting from the issuance of Indebtedness at less than par, (b) all commissions, discounts and other fees and charges owed with respect to letters of credit or bankers acceptances, (c) non-cash interest payments (but excluding any non-cash interest expense attributable to the movement in the mark to market valuation of Hedging Obligations or other derivative instruments pursuant to GAAP), (d) the interest component of Capitalized Lease Obligations, and (e) net payments, if any, made (less net payments, if any, received), pursuant to interest rate Hedging Obligations with respect to Indebtedness, and excluding (t) any expense resulting from the discounting of any Indebtedness in connection with the application of recapitalization accounting or, if applicable, purchase accounting in connection with the Transactions or any acquisition, (u) penalties and interest relating to taxes, (v) any Additional Interest and any “additional interest” with respect to the Senior Subordinated Notes or other securities, (w) amortization of deferred financing fees, debt issuance costs, commissions, fees and expenses, (x) any expensing of bridge, commitment and other financing fees, (y) commissions, discounts, yield and other fees and charges (including any interest expense) related to any Qualified Securitization Facility and (z) any accretion of accrued interest on discounted liabilities);plus
(2) consolidated capitalized interest of such Person and its Restricted Subsidiaries for such period, whether paid or accrued;less
(3) interest income of such Person and its Restricted Subsidiaries for such period.
For purposes of this definition, interest on a Capitalized Lease Obligation shall be deemed to accrue at an interest rate reasonably determined by such Person to be the rate of interest implicit in such Capitalized Lease Obligation in accordance with GAAP.
“Consolidated Net Income” means, with respect to any Person for any period, the aggregate of the Net Income of such Person and its Restricted Subsidiaries for such period, on a consolidated basis, and otherwise determined in accordance with GAAP; provided that, without duplication,
(1) any after-tax effect of extraordinary, non-recurring or unusual gains or losses (less all fees and expenses relating thereto) or expenses (including relating to the Transactions or any multi-year strategic initiatives, severance, relocation costs and curtailments or modifications to pension and post-retirement employee benefit plans) shall be excluded;
(2) the cumulative effect of a change in accounting principles and changes as a result of the adoption or modification of accounting policies during such period shall be excluded;
(3) any net after-tax gains or losses on disposal of disposed, abandoned or discontinued operations shall be excluded;
(4) any net after-tax effect of gains or losses (less all fees, expenses and charges relating thereto) attributable to asset dispositions or abandonments or the sale or other disposition of any Capital Stock of any Person other than in the ordinary course of business shall be excluded;
(5) the Net Income for such period of any Person that is an Unrestricted Subsidiary shall be excluded, and, solely for the purpose of determining the amount available for Restricted Payments under clause (3)(a) of the first paragraph of “Certain Covenants—Limitation on Restricted Payments,” the Net Income for such period of any Person that is not a Subsidiary or that is accounted for by the equity method of accounting shall be excluded; provided that Consolidated Net Income of the Issuer shall be increased by the amount of dividends or distributions or other payments that are actually paid in cash (or to the extent converted into cash) to the Issuer or a Restricted Subsidiary thereof in respect of such period;
(6) solely for the purpose of determining the amount available for Restricted Payments under clause (3)(a) of the first paragraph of “Certain Covenants—Limitation on Restricted Payments,” the Net Income for such period of any Restricted Subsidiary (other than any Guarantor) shall be excluded to the extent that the declaration or payment of dividends or similar distributions by that Restricted Subsidiary of its Net Income is not at the date of determination permitted without any prior governmental approval (which has not been obtained) or, directly or indirectly, by the operation of the terms of its charter or any agreement, instrument, judgment, decree, order, statute, rule, or governmental regulation applicable to that Restricted Subsidiary or its shareholders, unless such restriction with respect to the payment of dividends or similar distributions has been legally waived, provided that Consolidated Net Income of the Issuer will be increased by the amount of dividends or other distributions or other payments actually paid in cash (or to the extent converted into cash) to the Issuer or a Restricted Subsidiary thereof in respect of such period, to the extent not already included therein;
(7) effects of adjustments (including the effects of such adjustments pushed down to the Issuer and its Restricted Subsidiaries) in such Person’s consolidated financial statements pursuant to GAAP resulting from the application of recapitalization accounting or, if applicable, purchase accounting in relation to the Transactions or any consummated acquisition or the amortization or write-off of any amounts thereof, net of taxes, shall be excluded;
169
Table of Contents
(8) any after-tax effect of income (loss) from the early extinguishment of (a) Indebtedness, (b) Hedging Obligations or (c) other derivative instruments shall be excluded;
(9) any impairment charge or asset write-off or write-down, including impairment charges or asset write-offs or write-downs related to intangible assets, long-lived assets, investments in debt and equity securities or as a result of a change in law or regulation, in each case, pursuant to GAAP, and the amortization of intangibles arising pursuant to GAAP shall be excluded;
(10) any non-cash compensation expense recorded from grants of stock appreciation or similar rights, stock options, restricted stock or other rights, and any cash charges associated with the rollover, acceleration, or payout of Equity Interests by management of the Issuer or any of its direct or indirect parent companies in connection with the Transactions, shall be excluded;
(11) any fees, expenses or charges incurred during such period, or any amortization thereof for such period, in connection with any acquisition, Investment, Asset Sale, incurrence or repayment of Indebtedness (including such fees, expenses or charges related to the offering of the Senior Notes, the Senior Subordinated Notes and the Credit Facilities), issuance of Equity Interests, refinancing transaction or amendment or modification of any debt instrument (including any amendment or other modification of the Senior Notes, the Senior Subordinated Notes and the Credit Facilities) and including, in each case, any such transaction consummated prior to the Issue Date and any such transaction undertaken but not completed, and any charges or non-recurring merger costs incurred during such period as a result of any such transaction, in each case whether or not successful, shall be excluded;
(12) accruals and reserves that are established within twelve months after the Issue Date that are so required to be established as a result of the Transactions (or within twelve months after the closing of any acquisition that are so required to be established as a result of such acquisition) in accordance with GAAP shall be excluded;
(13) to the extent covered by insurance and actually reimbursed, or, so long as the Issuer has made a determination that there exists reasonable evidence that such amount will in fact be reimbursed by the insurer and only to the extent that such amount is (a) not denied by the applicable carrier in writing 180 days and (b) in fact reimbursed within 365 days of the date of the insurable event (with a deduction for any amount so added back to the extent not so reimbursed within such 365 day period), expenses with respect to liability or casualty events or business interruption shall be excluded;
(14) any noncash compensation expense resulting from the application of Statement of Financial Accounting Standards No. 123(R) shall be excluded; and
(15) the following items shall be excluded:
(a) any net unrealized gain or loss (after any offset) resulting in such period from Hedging Obligations and the application of Statement of Financial Accounting Standards No. 133; and
(b) any net unrealized gain or loss (after any offset) resulting in such period from currency translation gains or losses including those related to currency remeasurements of Indebtedness (including any net loss or gain resulting from Hedging Obligations for currency exchange risk).
In addition, to the extent not already included in the Consolidated Net Income of such Person and its Restricted Subsidiaries, notwithstanding anything to the contrary in the foregoing, Consolidated Net Income shall include the amount of proceeds received from business interruption insurance and reimbursements of any expenses and charges that are covered by indemnification or other reimbursement provisions in connection with any Permitted Investment or any sale, conveyance, transfer or other disposition of assets permitted under the Indenture.
Notwithstanding the foregoing, for the purpose of the covenant described under “Certain Covenants—Limitation on Restricted Payments” only (other than clause (3)(d) of the first paragraph thereof), there shall be excluded from Consolidated Net Income any income arising from any sale or other disposition of Restricted Investments made by the Issuer and its Restricted Subsidiaries, any repurchases and redemptions of Restricted Investments from the Issuer and its Restricted Subsidiaries, any repayments of loans and advances which constitute Restricted Investments by the Issuer or any of its Restricted Subsidiaries, any sale of the stock of an Unrestricted Subsidiary or any distribution or dividend from an Unrestricted Subsidiary, in each case only to the extent such amounts increase the amount of Restricted Payments permitted under such covenant pursuant to clause (3)(d) thereof.
“Contingent Obligations” means, with respect to any Person, any obligation of such Person guaranteeing any leases, dividends or other obligations that do not constitute Indebtedness (“primary obligations”) of any other Person (the “primary obligor”) in any manner, whether directly or indirectly, including, without limitation, any obligation of such Person, whether or not contingent,
(1) to purchase any such primary obligation or any property constituting direct or indirect security therefor;
(2) to advance or supply funds
(a) for the purchase or payment of any such primary obligation, or
(b) to maintain working capital or equity capital of the primary obligor or otherwise to maintain the net worth or solvency of the primary obligor; or
170
Table of Contents
(3) to purchase property, securities or services primarily for the purpose of assuring the owner of any such primary obligation of the ability of the primary obligor to make payment of such primary obligation against loss in respect thereof.
“Controlled Investment Affiliate” means, as to any Person, any other Person, other than any Investor, which directly or indirectly is in control of, is controlled by, or is under common control with such Person and is organized by such Person (or any Person controlling such Person) primarily for making direct or indirect equity or debt investments in the Issuer and/or other companies.
“Credit Facilities” means, with respect to the Issuer or any of its Restricted Subsidiaries, one or more debt facilities, including the Senior Credit Facilities or other financing arrangements (including, without limitation, commercial paper facilities or indentures) providing for revolving credit loans, term loans, letters of credit or other long-term indebtedness, including any notes, mortgages, guarantees, collateral documents, instruments and agreements executed in connection therewith, and any amendments, supplements, modifications, extensions, renewals, restatements or refundings thereof and any indentures or credit facilities or commercial paper facilities that replace, refund or refinance any part of the loans, notes, other credit facilities or commitments thereunder, including any such replacement, refunding or refinancing facility or indenture that increases the amount permitted to be borrowed thereunder or alters the maturity thereof ( provided that such increase in borrowings is permitted under “Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”) or adds Restricted Subsidiaries as additional borrowers or guarantors thereunder and whether by the same or any other agent, lender or group of lenders.
“Default” means any event that is, or with the passage of time or the giving of notice or both would be, an Event of Default.
“Designated Non-cash Consideration” means the fair market value of non-cash consideration received by the Issuer or a Restricted Subsidiary in connection with an Asset Sale that is so designated as Designated Non-cash Consideration pursuant to an Officer’s Certificate, setting forth the basis of such valuation, executed by the principal financial officer of the Issuer, less the amount of Cash Equivalents received in connection with a subsequent sale of or collection on such Designated Non-cash Consideration.
“Designated Preferred Stock” means Preferred Stock of the Issuer or any parent company thereof (in each case other than Disqualified Stock) that is issued for cash (other than to a Restricted Subsidiary or an employee stock ownership plan or trust established by the Issuer or any of its Subsidiaries) and is so designated as Designated Preferred Stock, pursuant to an Officer’s Certificate executed by the principal financial officer of the Issuer or the applicable parent company thereof, as the case may be, on the issuance date thereof, the cash proceeds of which are excluded from the calculation set forth in clause (3) of the first paragraph of “Certain Covenants—Limitation on Restricted Payments.”
“Designated Senior Indebtedness” means:
(1) any Indebtedness outstanding under the Senior Credit Facilities;
(2) the Senior Notes; and
(3) any other Senior Indebtedness permitted under the Indenture, the principal amount of which is $50.0 million or more and that has been designated by the Issuer as “Designated Senior Indebtedness.”
“Disqualified Stock” means, with respect to any Person, any Capital Stock of such Person which, by its terms, or by the terms of any security into which it is convertible or for which it is putable or exchangeable, or upon the happening of any event, matures or is mandatorily redeemable (other than solely as a result of a change of control or asset sale) pursuant to a sinking fund obligation or otherwise, or is redeemable at the option of the holder thereof (other than solely as a result of a change of control or asset sale), in whole or in part, in each case prior to the date 91 days after the earlier of the maturity date of the Senior Subordinated Notes or the date the Senior Subordinated Notes are no longer outstanding; provided that if such Capital Stock is issued to any plan for the benefit of employees of the Issuer or its Subsidiaries or by any such plan to such employees, such Capital Stock shall not constitute Disqualified Stock solely because it may be required to be repurchased by the Issuer or its Subsidiaries in order to satisfy applicable statutory or regulatory obligations; provided, further, that any Capital Stock held by any future, current or former employee, director, officer, manager, distributor or consultant (or their respective Controlled Investment Affiliates or Immediate Family Members), of the Issuer, any of its Subsidiaries, any of its direct or indirect parent companies or any other entity in which the Issuer or a Restricted Subsidiary has an Investment and is designated in good faith as an “affiliate” by the board of directors of the Issuer (or the compensation committee thereof), in each case pursuant to any stock subscription or shareholders’ agreement, management equity plan or stock option plan or any other management or employee benefit plan or agreement or any distributor equity plan or agreement shall not constitute Disqualified Stock solely because it may be required to be repurchased by the Issuer or its Subsidiaries.
“EBITDA” means, with respect to any Person for any period, the Consolidated Net Income of such Person for such period
(1) increased (without duplication) by the following, in each case to the extent deducted (and not added back) in determining Consolidated Net Income for such period:
(a) provision for taxes based on income or profits or capital, including, without limitation, state, franchise and similar taxes, foreign withholding taxes (including any future taxes or other levies which replace or are intended to be in lieu of such taxes and any penalties and interest related to such taxes or arising from tax examinations) and the net tax expense associated with any adjustments made pursuant to clauses (1) through (15) of the definition of “Consolidated Net Income”;plus
(b) Fixed Charges of such Person for such period (including (x) net losses or Hedging Obligations or other derivative instruments entered into for the purpose of hedging interest rate risk, (y) bank fees and (z) costs of surety bonds in connection with financing activities, plus amounts excluded from Consolidated Interest Expense as set forth in clauses (1)(t) through (z) in the definition thereof);plus
(c) Consolidated Depreciation and Amortization Expense of such Person for such period;plus
171
Table of Contents
(d) [reserved];plus
(e) the amount of any restructuring charges, integration and facilities opening costs or other business optimization expenses (including cost and expenses relating to business optimization programs and new systems design and implementation costs) or accruals or reserves, including any one-time costs incurred in connection with acquisitions after the Issue Date, project start-up costs and costs related to the closure and/or consolidation of facilities;plus
(f) any other non-cash charges, including any write offs or write downs reducing Consolidated Net Income for such period (provided that if any such non-cash charges represent an accrual or reserve for potential cash items in any future period, the cash payment in respect thereof in such future period shall be subtracted from EBITDA to such extent, and excluding amortization of a prepaid cash item that was paid in a prior period);plus
(g) the amount of any minority interest expense consisting of Subsidiary income attributable to minority equity interests of third parties in any non-Wholly Owned Subsidiary;plus
(h) the amount of management, monitoring, consulting and advisory fees (including termination fees) and related indemnities and expenses paid or accrued in such period under the Management Fee Agreement or otherwise to the Investors to the extent otherwise permitted under “Certain Covenants—Transactions with Affiliates”;plus
(i) the amount of “run-rate” cost savings projected by the Issuer in good faith to result from actions either taken or expected to be taken within 12 months after the end of such period (which cost savings shall be subject only to certification by management of the Issuer and calculated on a pro forma basis as though such cost savings had been realized on the first day of such period), net of the amount of actual benefits realized from such actions (it is understood and agreed that “run-rate” means the full recurring benefit that is associated with any action taken or expected to be taken, provided that some portion of such benefit is expected to be realized within 12 months of taking such action) (which adjustments may be incremental to pro forma cost savings adjustments made pursuant to the definition of “Fixed Charge Coverage Ratio”); plus
(j) the amount of loss on sale of receivables, Securitization Assets and related assets to the Securitization Subsidiary in connection with a Qualified Securitization Facility;plus
(k) any costs or expense incurred by the Issuer or a Restricted Subsidiary pursuant to any management equity plan or stock option plan or any other management or employee benefit plan, agreement or any stock subscription or shareholder agreement or any distributor equity plan or agreement, to the extent that such cost or expenses are funded with cash proceeds contributed to the capital of the Issuer or net cash proceeds of an issuance of Equity Interest of the Issuer (other than Disqualified Stock) solely to the extent that such net cash proceeds are excluded from the calculation set forth in clause (3) of the first paragraph under “Certain Covenants—Limitation on Restricted Payments”;plus
(l) cash receipts (or any netting arrangements resulting in reduced cash expenditures) not representing EBITDA or Consolidated Net Income in any period to the extent non-cash gains relating to such income were deducted in the calculation of EBITDA pursuant to clause (2) below for any previous period and not added back;plus
(m) any net loss from disposed or discontinued operations or from operations expected to be disposed of or discontinued within twelve months after the end of such period;plus
(n) interest income or investment earnings on retiree medical and intellectual property, royalty or license receivables;plus
(o) extraordinary losses and unusual or non-recurring charges (including any unusual or non-recurring operating expenses attributable to the implementation of cost-savings initiatives, severance, retention and relocation costs and curtailments and modifications to pension and post-retirement employee benefit plans);plus
(p) any costs or expenses incurred by the Issuer or a Restricted Subsidiary (whether prior to or following the Issue Date) relating to the Option Accounting Issues, including fees and expenses incurred by the Issuer’s directors, officers, employees and advisors in investigating such Option Accounting Issues and any incremental tax exposure resulting from the resolution of such Option Accounting Issues;plus
(q) expense related to any payments made to distributors prior to the first anniversary of the Issue Date (other than commissions paid in the ordinary course of business);plus
(r) losses on asset sales (other than asset sales made in the ordinary course of business), disposals and abandonments;
(2) decreased (without duplication) by the following, in each case to the extent included in determining Consolidated Net Income for such period:
(a) non-cash gains increasing Consolidated Net Income of such Person for such period, excluding any non-cash gains to the extent they represent the reversal of an accrual or reserve for a potential cash item that reduced EBITDA in any prior period;plus
(b) any non-cash gains with respect to cash actually received in a prior period unless such cash did not increase EBITDA in such prior period;plus
172
Table of Contents
(c) any net income from disposed or discontinued operations or from operations expected to be disposed of or discontinued within twelve months after the end of such period;plus
(d) extraordinary gains and unusual or non-recurring gains;plus
(e) gains on asset sales (other than asset sales made in the ordinary course of business), disposals and abandonments.
“EMU” means economic and monetary union as contemplated in the Treaty on European Union.
“Equity Interests” means Capital Stock and all warrants, options or other rights to acquire Capital Stock, but excluding any debt security that is convertible into, or exchangeable for, Capital Stock.
“Equity Offering” means any public or private sale of common stock or Preferred Stock of the Issuer or any of its direct or indirect parent companies (excluding Disqualified Stock), other than:
(1) public offerings with respect to the Issuer’s or any direct or indirect parent company’s common stock registered on Form S-4 or Form S-8;
(2) issuances to any Subsidiary of the Issuer; and
(3) any such public or private sale that constitutes an Excluded Contribution.
“euro” means the single currency of participating member states of the EMU.
“Exchange Act” means the Securities Exchange Act of 1934, as amended, and the rules and regulations of the SEC promulgated thereunder.
“Excluded Contribution” means net cash proceeds, marketable securities or Qualified Proceeds received by the Issuer from
(1) contributions to its common equity capital; and
(2) the sale (other than to a Subsidiary of the Issuer or to any management equity plan or stock option plan or any other management or employee benefit plan or agreement or any distributor equity plan or agreement of the Issuer) of Capital Stock (other than Disqualified Stock and Designated Preferred Stock) of the Issuer;
in each case designated as Excluded Contributions pursuant to an Officer’s Certificate executed by the principal financial officer of the Issuer on the date such capital contributions are made or the date such Equity Interests are sold, as the case may be, which are excluded from the calculation set forth in clause (3) of the first paragraph under “Certain Covenants—Limitation on Restricted Payments.”
“fair market value” means, with respect to any asset or liability, the fair market value of such asset or liability as determined by the Issuer in good faith.
“Fixed Charge Coverage Ratio” means, with respect to any Person for any period, the ratio of EBITDA of such Person for such period to the Fixed Charges of such Person for such period. In the event that the Issuer or any Restricted Subsidiary incurs, assumes, guarantees, redeems, retires or extinguishes any Indebtedness (other than Indebtedness incurred under any revolving credit facility unless such Indebtedness has been permanently repaid and has not been replaced) or issues or redeems Disqualified Stock or Preferred Stock subsequent to the commencement of the period for which the Fixed Charge Coverage Ratio is being calculated but prior to or simultaneously with the event for which the calculation of the Fixed Charge Coverage Ratio is made (the “Fixed Charge Coverage Ratio Calculation Date”), then the Fixed Charge Coverage Ratio shall be calculated giving pro forma effect to such incurrence, assumption, guarantee, redemption, retirement or extinguishment of Indebtedness, or such issuance or redemption of Disqualified Stock or Preferred Stock, as if the same had occurred at the beginning of the applicable four-quarter period.
For purposes of making the computation referred to above, Investments, acquisitions, dispositions, mergers, consolidations and discontinued operations (as determined in accordance with GAAP) that have been made by the Issuer or any of its Restricted Subsidiaries during the four-quarter reference period or subsequent to such reference period and on or prior to or simultaneously with the Fixed Charge Coverage Ratio Calculation Date shall be calculated on a pro forma basis assuming that all such Investments, acquisitions, dispositions, mergers, consolidations and discontinued operations (and the change in any associated fixed charge obligations and the change in EBITDA resulting therefrom) had occurred on the first day of the four-quarter reference period. If since the beginning of such period any Person that subsequently became a Restricted Subsidiary or was merged with or into the Issuer or any of its Restricted Subsidiaries since the beginning of such period shall have made any Investment, acquisition, disposition, merger, consolidation or discontinued operation that would have required adjustment pursuant to this definition, then the Fixed Charge Coverage Ratio shall be calculated giving pro forma effect thereto for such period as if such Investment, acquisition, disposition, merger, consolidation or discontinued operation had occurred at the beginning of the applicable four-quarter period.
For purposes of this definition, whenever pro forma effect is to be given to an Investment, acquisition, disposition, merger or consolidation (including the Transactions), the pro forma calculations shall be made in good faith by a responsible financial or accounting officer of the Issuer (and may include, for the avoidance of doubt, cost savings and operating expense reductions resulting from such Investment, acquisition, merger or consolidation (including the Transactions) which is being given pro forma effect that have been or are expected to be realized). If any Indebtedness bears a floating rate of interest and is being given pro forma effect, the interest on such Indebtedness shall be calculated as if the rate in effect on the Fixed Charge Coverage Ratio Calculation Date had been the applicable rate for the entire period (taking into account any Hedging Obligations applicable to such Indebtedness). Interest
173
Table of Contents
on a Capitalized Lease Obligation shall be deemed to accrue at an interest rate reasonably determined by a responsible financial or accounting officer of the Issuer to be the rate of interest implicit in such Capitalized Lease Obligation in accordance with GAAP. For purposes of making the computation referred to above, interest on any Indebtedness under a revolving credit facility computed on a pro forma basis shall be computed based upon the average daily balance of such Indebtedness during the applicable period except as set forth in the first paragraph of this definition. Interest on Indebtedness that may optionally be determined at an interest rate based upon a factor of a prime or similar rate, a eurocurrency interbank offered rate, or other rate, shall be deemed to have been based upon the rate actually chosen, or, if none, then based upon such optional rate chosen as the Issuer may designate.
“Fixed Charges” means, with respect to any Person for any period, the sum of, without duplication:
(1) Consolidated Interest Expense of such Person for such period;
(2) all cash dividends or other distributions paid (excluding items eliminated in consolidation) on any series of Preferred Stock during such period; and
(3) all cash dividends or other distributions paid (excluding items eliminated in consolidation) on any series of Disqualified Stock during such period.
“Foreign Subsidiary” means, with respect to any Person, any Restricted Subsidiary of such Person that is not organized or existing under the laws of the United States, any state thereof, the District of Columbia, or any territory thereof and any Restricted Subsidiary of such Foreign Subsidiary.
“Foreign Subsidiary Total Assets” means the total assets of the Foreign Subsidiaries, as determined in accordance with GAAP in good faith by the Issuer, without intercompany eliminations.
“GAAP” means generally accepted accounting principles in the United States of America which are in effect on the Issue Date.
“Government Securities” means securities that are:
(1) direct obligations of the United States of America for the timely payment of which its full faith and credit is pledged; or
(2) obligations of a Person controlled or supervised by and acting as an agency or instrumentality of the United States of America the timely payment of which is unconditionally guaranteed as a full faith and credit obligation by the United States of America,
which, in either case, are not callable or redeemable at the option of the issuers thereof, and shall also include a depository receipt issued by a bank (as defined in Section 3(a)(2) of the Securities Act), as custodian with respect to any such Government Securities or a specific payment of principal of or interest on any such Government Securities held by such custodian for the account of the holder of such depository receipt; provided that (except as required by law) such custodian is not authorized to make any deduction from the amount payable to the holder of such depository receipt from any amount received by the custodian in respect of the Government Securities or the specific payment of principal of or interest on the Government Securities evidenced by such depository receipt.
“guarantee” means a guarantee (other than by endorsement of negotiable instruments for collection in the ordinary course of business), direct or indirect, in any manner (including letters of credit and reimbursement agreements in respect thereof), of all or any part of any Indebtedness or other obligations.
“Guarantee” means the guarantee by any Guarantor of the Issuer’s Obligations under the Indenture and the Senior Subordinated Notes.
“Guarantor” means each Subsidiary of the Issuer, if any, that Guarantees the Senior Subordinated Notes in accordance with the terms of the Indenture.
“Hedging Obligations” means, with respect to any Person, the obligations of such Person under any interest rate swap agreement, interest rate cap agreement, interest rate collar agreement, commodity swap agreement, commodity cap agreement, commodity collar agreement, foreign exchange contract, currency swap agreement or similar agreement providing for the transfer or mitigation of interest rate or currency risks either generally or under specific contingencies.
“Holder” means the Person in whose name a Senior Subordinated Note is registered on the registrar’s books.
“Holdings” means LVB Acquisition, Inc., a Delaware corporation and the direct parent of the Issuer.
“Immediate Family Members” means with respect to any individual, such individual’s child, stepchild, grandchild or more remote descendant, parent, stepparent, grandparent, spouse, former spouse, qualified domestic partner, sibling, mother-in-law, father-in-law, son-in-law and daughter-in-law (including adoptive relationships) and any trust, partnership or other bona fide estate-planning vehicle the only beneficiaries of which are any of the foregoing individuals or any private foundation or fund that is controlled by any if the foregoing individuals or any donor-advised fund of which any such individual is the donor.
“Indebtedness” means, with respect to any Person, without duplication:
(1) any indebtedness (including principal and premium) of such Person, whether or not contingent:
(a) in respect of borrowed money;
174
Table of Contents
(b) evidenced by bonds, notes, debentures or similar instruments or letters of credit or bankers’ acceptances (or, without duplication, reimbursement agreements in respect thereof);
(c) representing the balance deferred and unpaid of the purchase price of any property (including Capitalized Lease Obligations) due more than twelve months after such property is acquired, except (i) any such balance that constitutes an obligation in respect of a commercial letter of credit, a trade payable or similar obligation to a trade creditor, in each case accrued in the ordinary course of business and (ii) any earn-out obligations until such obligation becomes a liability on the balance sheet of such Person in accordance with GAAP and if not paid after becoming due and payable;
(d) representing the net obligations under any Hedging Obligations; or
(e) during a Suspension Period only, obligations of the lessee for rental payments in respect of Sale and Lease-back Transactions in an amount equal to the present value of such obligations during the remaining term of the lease using a discount rate equal to the rate of interest implicit in such transaction determined in accordance with GAAP,
if and to the extent that any of the foregoing Indebtedness (other than letters of credit and Hedging Obligations) would appear as a liability upon a balance sheet (excluding the footnotes thereto) of such Person prepared in accordance with GAAP; provided that Indebtedness of any direct or indirect parent of the Issuer appearing upon the balance sheet of the Issuer solely by reason of push-down accounting under GAAP shall be excluded;
(2) to the extent not otherwise included, any obligation by such Person to be liable for, or to pay, as obligor, guarantor or otherwise, on the obligations of the type referred to in clause (1) of a third Person (whether or not such items would appear upon the balance sheet of the such obligor or guarantor), other than by endorsement of negotiable instruments for collection in the ordinary course of business; and
(3) to the extent not otherwise included, the obligations of the type referred to in clause (1) of a third Person secured by a Lien on any asset owned by such first Person, whether or not such Indebtedness is assumed by such first Person;
provided that notwithstanding the foregoing, Indebtedness shall be deemed not to include (a) Contingent Obligations incurred in the ordinary course of business or (b) obligations under or in respect of Qualified Securitization Facilities.
“Independent Financial Advisor” means an accounting, appraisal, investment banking firm or consultant to Persons engaged in Similar Businesses of nationally recognized standing that is, in the good faith judgment of the Issuer, qualified to perform the task for which it has been engaged.
“Initial Purchasers” means Banc of America Securities LLC, Goldman, Sachs & Co., Lehman Brothers Inc., Merrill Lynch, Pierce, Fenner & Smith Incorporated, Wachovia Capital Markets, LLC and Bear, Stearns & Co. Inc.
“Investment Grade Rating” means a rating equal to or higher than Baa3 (or the equivalent) by Moody’s and BBB- (or the equivalent) by S&P, or an equivalent rating by any other Rating Agency.
“Investment Grade Securities” means:
(1) securities issued or directly and fully guaranteed or insured by the United States government or any agency or instrumentality thereof (other than Cash Equivalents);
(2) debt securities or debt instruments with an Investment Grade Rating, but excluding any debt securities or instruments constituting loans or advances among the Issuer and its Subsidiaries;
(3) investments in any fund that invests exclusively in investments of the type described in clauses (1) and (2) which fund may also hold immaterial amounts of cash pending investment or distribution; and
(4) corresponding instruments in countries other than the United States customarily utilized for high quality investments.
“Investments” means, with respect to any Person, all investments by such Person in other Persons (including Affiliates) in the form of loans (including guarantees), advances or capital contributions (excluding accounts receivable, trade credit, advances to customers and distributors, commission, travel and similar advances to employees, directors, officers, managers, distributors and consultants in each case made in the ordinary course of business), purchases or other acquisitions for consideration of Indebtedness, Equity Interests or other securities issued by any other Person and investments that are required by GAAP to be classified on the balance sheet (excluding the footnotes) of the Issuer in the same manner as the other investments included in this definition to the extent such transactions involve the transfer of cash or other property. For purposes of the definition of “Unrestricted Subsidiary” and the covenant described under “Certain Covenants—Limitation on Restricted Payments”:
(1) “Investments” shall include the portion (proportionate to the Issuer’s equity interest in such Subsidiary) of the fair market value of the net assets of a Subsidiary of the Issuer at the time that such Subsidiary is designated an Unrestricted Subsidiary; provided that upon a redesignation of such Subsidiary as a Restricted Subsidiary, the Issuer shall be deemed to continue to have a permanent “Investment” in an Unrestricted Subsidiary in an amount (if positive) equal to:
(a) the Issuer’s “Investment” in such Subsidiary at the time of such redesignation; less
175
Table of Contents
(b) the portion (proportionate to the Issuer’s Equity Interest in such Subsidiary) of the fair market value of the net assets of such Subsidiary at the time of such redesignation; and
(2) any property transferred to or from an Unrestricted Subsidiary shall be valued at its fair market value at the time of such transfer.
The amount of any Investment outstanding at any time shall be the original cost of such Investment, reduced by any dividend, distribution, interest payment, return of capital, repayment or other amount received in cash by the Issuer or a Restricted Subsidiary in respect of such Investment.
“Investors” means The Blackstone Group, Goldman Sachs Capital Partners, Kohlberg Kravis Roberts & Co, TPG Capital and, if applicable, each of their respective Affiliates and funds or partnerships managed by any of them or their respective Affiliates but not including, however, any portfolio companies of any of the foregoing.
“Issue Date” means September 25, 2007.
“Issuer” means LVB Acquisition Merger Sub, Inc., an Indiana corporation, prior to the Acquisition and Biomet, Inc., an Indiana corporation, as the surviving corporation after the Acquisition (and not to any of their Subsidiaries) and its successors.
“Legal Holiday” means a Saturday, a Sunday or a day on which commercial banking institutions are not required to be open in the State of New York or place of payment.
“Lien” means, with respect to any asset, any mortgage, lien (statutory or otherwise), pledge, hypothecation, charge, security interest, preference, priority or encumbrance of any kind in respect of such asset, whether or not filed, recorded or otherwise perfected under applicable law, including any conditional sale or other title retention agreement, any lease in the nature thereof, any option or other agreement to sell or give a security interest in and any filing of or agreement to give any financing statement under the Uniform Commercial Code (or equivalent statutes) of any jurisdiction; provided that in no event shall an operating lease be deemed to constitute a Lien.
“Management Fee Agreement” means the management services agreement between certain of the management companies associated with the Investors or their advisors, if applicable, and the Issuer.
“Management Shareholders” means the members of management (and their Controlled Investment Affiliates and Immediate Family Members) of the Issuer (or its direct parent) who are holders of Equity Interests of any direct or indirect parent companies of the Issuer on the Issue Date or will become holders of such Equity Interests in connection with the Acquisition.
“Moody’s” means Moody’s Investors Service, Inc. and any successor to its rating agency business.
“Net Income” means, with respect to any Person, the net income (loss) of such Person, determined in accordance with GAAP and before any reduction in respect of Preferred Stock dividends.
“Net Proceeds” means the aggregate cash proceeds received by the Issuer or any of its Restricted Subsidiaries in respect of any Asset Sale, including any cash received upon the sale or other disposition of any Designated Non-cash Consideration received in any Asset Sale, net of the direct costs relating to such Asset Sale and the sale or disposition of such Designated Non-cash Consideration, including legal, accounting and investment banking fees, payments made in order to obtain a necessary consent or required by applicable law, and brokerage and sales commissions, any relocation expenses incurred as a result thereof, other fees and expenses, including title and recordation expenses, taxes paid or payable as a result thereof (after taking into account any available tax credits or deductions and any tax sharing arrangements), amounts required to be applied to the repayment of principal, premium, if any, and interest on Senior Indebtedness required (other than required by clause (1) of the second paragraph of “Repurchase at the Option of Holders—Asset Sales”) to be paid as a result of such transaction and any deduction of appropriate amounts to be provided by the Issuer or any of its Restricted Subsidiaries as a reserve in accordance with GAAP against any liabilities associated with the asset disposed of in such transaction and retained by the Issuer or any of its Restricted Subsidiaries after such sale or other disposition thereof, including pension and other post-employment benefit liabilities and liabilities related to environmental matters or against any indemnification obligations associated with such transaction.
“Obligations” means any principal, interest (including any interest accruing on or subsequent to the filing of a petition in bankruptcy, reorganization or similar proceeding at the rate provided for in the documentation with respect thereto, whether or not such interest is an allowed claim under applicable state, federal or foreign law), premium, penalties, fees, indemnifications, reimbursements (including reimbursement obligations with respect to letters of credit and banker’s acceptances), damages and other liabilities, and guarantees of payment of such principal, interest, penalties, fees, indemnifications, reimbursements, damages and other liabilities, payable under the documentation governing any Indebtedness.
“Officer” means the Chairman of the board of directors, the Chief Executive Officer, the Chief Financial Officer, the President, any Executive Vice President, Senior Vice President or Vice President, the Treasurer or the Secretary of the Issuer.
“Officer’s Certificate” means a certificate signed on behalf of a Person by an Officer of such Person, who must be the principal executive officer, the principal financial officer, the treasurer or the principal accounting officer of such Person, that meets the requirements set forth in the Indenture.
“Opinion of Counsel” means a written opinion from legal counsel who is acceptable to the Trustee. The counsel may be an employee of or counsel to the Issuer or the Trustee.
176
Table of Contents
“Option Accounting Issues” means, with respect to the Issuer and its Subsidiaries, any failure to (i) properly document the measurement date for any stock option grant, (ii) record stock option expense (or other items relating thereto) in accordance with GAAP or (iii) issue stock options in accordance with the terms of any applicable Stock Plan (as defined in the Transaction Agreement), in each case to the extent occurring prior to June 4, 2007.
“Permitted Asset Swap” means the substantially concurrent purchase and sale or exchange of Related Business Assets or a combination of Related Business Assets and Cash Equivalents between the Issuer or any of its Restricted Subsidiaries and another Person; provided that any Cash Equivalents received must be applied in accordance with the covenant described under “Repurchase at the Option of Holders—Asset Sales.”
“Permitted Holders” means each of the Investors, the Co-Investors and Management Shareholders and any group (within the meaning of Section 13(d)(3) or Section 14(d)(2) of the Exchange Act or any successor provision) of which any of the foregoing are members; provided that, in the case of such group and without giving effect to the existence of such group or any other group, such Investors, the Co-Investors and Management Shareholders, collectively, have beneficial ownership of more than 50.0% of the total voting power of the Voting Stock of the Issuer or any of its direct or indirect parent companies. Any Person or group whose acquisition of beneficial ownership constitutes a Change of Control in respect of which a Change of Control Offer is made in accordance with the requirements of the Indenture will thereafter, together with its Affiliates, constitute an additional Permitted Holder.
“Permitted Junior Securities” means:
(1) Equity Interests in the Issuer or any Guarantor or any direct or indirect parent company of the Issuer; or
(2) unsecured debt securities that are subordinated to all Senior Indebtedness (and any debt securities issued in exchange for Senior Indebtedness) to substantially the same extent as, or to a greater extent than, the Senior Subordinated Notes and the related Guarantees are subordinated to Senior Indebtedness under the Indenture;
provided that the term “Permitted Junior Securities” shall not include any securities distributed pursuant to a plan of reorganization if the Indebtedness under the Senior Credit Facilities is treated as part of the same class as the Senior Subordinated Notes for purposes of such plan of reorganization; provided , further , that to the extent that any Senior Indebtedness of the Issuer outstanding on the date of consummation of any such plan of reorganization is not paid in full in cash on such date, the holders of any such Senior Indebtedness not so paid in full in cash have consented to the terms of such plan of reorganization.
“Permitted Investments” means:
(1) any Investment in the Issuer or any of its Restricted Subsidiaries;
(2) any Investment in Cash Equivalents or Investment Grade Securities;
(3) any Investment by the Issuer or any of its Restricted Subsidiaries in a Person that is engaged in a Similar Business if as a result of such Investment:
(a) such Person becomes a Restricted Subsidiary; or
(b) such Person, in one transaction or a series of related transactions, is merged or consolidated with or into, or transfers or conveys substantially all of its assets to, or is liquidated into, the Issuer or a Restricted Subsidiary,
and, in each case, any Investment held by such Person; provided that such Investment was not acquired by such Person in contemplation of such acquisition, merger, consolidation or transfer;
(4) any Investment in securities or other assets not constituting Cash Equivalents or Investment Grade Securities and received in connection with an Asset Sale made pursuant to the provisions described under “Repurchase at the Option of Holders—Asset Sales” or any other disposition of assets not constituting an Asset Sale;
(5) any Investment existing on the Issue Date or made pursuant to binding commitments in effect on the Issue Date or an Investment consisting of any extension, modification or renewal of any Investment existing on the Issue Date; provided that the amount of any such Investment may be increased (a) as required by the terms of such Investment as in existence on the Issue Date or (b) as otherwise permitted under the Indenture;
(6) any Investment acquired by the Issuer or any of its Restricted Subsidiaries:
(a) in exchange for any other Investment or accounts receivable held by the Issuer or any such Restricted Subsidiary in connection with or as a result of a bankruptcy, workout, reorganization or recapitalization of the issuer of such other Investment or accounts receivable (including any trade creditor or customer); or
(b) in satisfaction of judgments against other Persons; or
(c) as a result of a foreclosure by the Issuer or any of its Restricted Subsidiaries with respect to any secured Investment or other transfer of title with respect to any secured Investment in default;
177
Table of Contents
(7) Hedging Obligations permitted under clause (10) of the covenant described in “Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”;
(8) any Investment in a Similar Business taken together with all other Investments made pursuant to this clause (8) that are at that time outstanding, not to exceed the greater of (a) $450.0 million and (b) 3.0% of Total Assets;
(9) Investments the payment for which consists of Equity Interests (other than Disqualified Stock) of the Issuer, or any of its direct or indirect parent companies; provided that such Equity Interests will not increase the amount available for Restricted Payments under clause (3) of the first paragraph under the covenant described in “Certain Covenants—Limitations on Restricted Payments”;
(10) guarantees of Indebtedness permitted under the covenant described in “Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”;
(11) any transaction to the extent it constitutes an Investment that is permitted by and made in accordance with the provisions of the second paragraph of the covenant described under “Certain Covenants—Transactions with Affiliates” (except transactions described in clauses (2), (5) and (9) of such paragraph);
(12) Investments consisting of purchases and acquisitions of inventory, supplies, material or equipment or the licensing or contribution of intellectual property pursuant to joint marketing arrangements with other Persons;
(13) additional Investments, taken together with all other Investments made pursuant to this clause (13) that are at that time outstanding (without giving effect to the sale of an Unrestricted Subsidiary to the extent the proceeds of such sale do not consist of cash or marketable securities), not to exceed the greater of (a) $450.0 million and (b) 3.0% of Total Assets;
(14) Investments in or relating to a Securitization Subsidiary that, in the good faith determination of the Issuer are necessary or advisable to effect any Qualified Securitization Facility or any repurchase obligation in connection therewith;
(15) advances to, or guarantees of Indebtedness of, employees not in excess of $25.0 million outstanding at any one time, in the aggregate;
(16) loans and advances to employees, directors, officers, managers, distributors and consultants for business-related travel expenses, moving expenses and other similar expenses, in each case incurred in the ordinary course of business or consistent with past practices or to fund such Person’s purchase of Equity Interests of the Issuer or any direct or indirect parent company thereof;
(17) advances, loans or extensions of trade credit in the ordinary course of business by the Issuer or any of its Restricted Subsidiaries;
(18) any Investment in any Subsidiary or any joint venture in connection with intercompany cash management arrangements or related activities arising in the ordinary course of business;
(19) Investments consisting of purchases and acquisitions of assets or services in the ordinary course of business;
(20) Investments made in the ordinary course of business in connection with obtaining, maintaining or renewing client contacts and loans or advances made to distributors in the ordinary course of business;
(21) Investments in prepaid expenses, negotiable instruments held for collection and lease, utility and workers compensation, performance and similar deposits entered into as a result of the operations of the business in the ordinary course of business; and
(22) repurchases of the Senior Subordinated Notes.
“Permitted Liens” means, with respect to any Person:
(1) pledges or deposits by such Person under workmen’s compensation laws, unemployment insurance, other social security benefits or other insurance related obligations (including, but not limited to, in respect of deductibles, self insured retention amounts and premiums and adjustments thereto) or good faith deposits in connection with bids, tenders, contracts (other than for the payment of Indebtedness) or leases to which such Person is a party, or deposits to secure public or statutory obligations of such Person or deposits of cash or U.S. government bonds to secure surety or appeal bonds to which such Person is a party, or deposits as security for contested taxes or import duties or for the payment of rent, in each case incurred in the ordinary course of business;
(2) Liens imposed by law, such as carriers’, warehousemen’s and mechanics’ Liens, in each case for sums not yet overdue for a period of more than 30 days or being contested in good faith by appropriate proceedings or other Liens arising out of judgments or awards against such Person with respect to which such Person shall then be proceeding with an appeal or other proceedings for review if adequate reserves with respect thereto are maintained on the books of such Person in accordance with GAAP;
(3) Liens for taxes, assessments or other governmental charges not yet overdue for a period of more than 30 days or not yet payable or subject to penalties for nonpayment or which are being contested in
good faith by appropriate proceedings diligently conducted, if adequate reserves with respect thereto are maintained on the books of such Person in accordance with GAAP;
178
Table of Contents
(4) Liens in favor of issuers of performance and surety bonds or bid bonds or with respect to other regulatory requirements or letters of credit issued pursuant to the request of and for the account of such Person in the ordinary course of its business;
(5) minor survey exceptions, minor encumbrances, easements or reservations of, or rights of others for, licenses, rights-of-way, sewers, electric lines, telegraph and telephone lines and other similar purposes, or zoning or other restrictions as to the use of real properties or Liens incidental, to the conduct of the business of such Person or to the ownership of its properties which were not incurred in connection with Indebtedness and which do not in the aggregate materially adversely affect the value of said properties or materially impair their use in the operation of the business of such Person;
(6) Liens securing Indebtedness permitted to be incurred pursuant to clause (4), (12)(b), (13), (23) or (24) of the second paragraph under “Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”; provided that (a) Liens securing Indebtedness, Disqualified Stock or Preferred Stock permitted to be incurred pursuant to clause (13) relate only to Refinancing Indebtedness that serves to refund or refinance Indebtedness, Disqualified Stock or Preferred Stock incurred under clause (4) or (12)(b) of the second paragraph of “Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock,” (b) Liens securing Indebtedness permitted to be incurred pursuant to clause (23) extend only to the assets of Foreign Subsidiaries, (c) Liens securing Indebtedness permitted to be incurred pursuant to clause (24) are solely on acquired property or the assets of the acquired entity, as the case may be, and (d) Liens securing Indebtedness, Disqualified Stock or Preferred Stock to be incurred pursuant to clause (4) of the second paragraph under “Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock” extend only to the assets so purchased, leased or improved;
(7) Liens existing on the Issue Date;
(8) Liens on property or shares of stock or other assets of a Person at the time such Person becomes a Subsidiary; provided that such Liens are not created or incurred in connection with, or in contemplation of, such other Person becoming such a Subsidiary; provided , further, that such Liens may not extend to any other property or other assets owned by the Issuer or any of its Restricted Subsidiaries;
(9) Liens on property or other assets at the time the Issuer or a Restricted Subsidiary acquired the property or such other assets, including any acquisition by means of a merger or consolidation with or into the Issuer or any of its Restricted Subsidiaries; provided that such Liens are not created or incurred in connection with, or in contemplation of, such acquisition; provided , further, that the Liens may not extend to any other property owned by the Issuer or any of its Restricted Subsidiaries;
(10) Liens securing Indebtedness or other obligations of a Restricted Subsidiary owing to the Issuer or another Restricted Subsidiary permitted to be incurred in accordance with the covenant described under “Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”;
(11) Liens securing Hedging Obligations; provided that, with respect to Hedging Obligations relating to Indebtedness, such Indebtedness is, and is permitted to be under the Indenture, secured by a Lien on the same property securing such Hedging Obligations;
(12) Liens on specific items of inventory or other goods and proceeds of any Person securing such Person’s obligations in respect of bankers’ acceptances issued or created for the account of such Person to facilitate the purchase, shipment or storage of such inventory or other goods;
(13) leases, subleases, licenses or sublicenses granted to others in the ordinary course of business which do not materially interfere with the ordinary conduct of the business of the Issuer or any of its Restricted Subsidiaries and do not secure any Indebtedness;
(14) Liens arising from Uniform Commercial Code financing statement filings regarding operating leases entered into by the Issuer and its Restricted Subsidiaries in the ordinary course of business;
(15) Liens in favor of the Issuer or any Guarantor;
(16) Liens on equipment of the Issuer or any of its Restricted Subsidiaries granted in the ordinary course of business to the Issuer’s clients;
(17) Liens on accounts receivable, Securitization Assets and related assets incurred in connection with a Qualified Securitization Facility;
(18) Liens to secure any refinancing, refunding, extension, renewal or replacement (or successive refinancing, refunding, extensions, renewals or replacements) as a whole, or in part, of any Indebtedness secured by any Lien referred to in the foregoing clauses (6), (7), (8) and (9); provided that (a) such new Lien shall be limited to all or part of the same property that secured the original Lien (plus improvements on such property), and (b) the Indebtedness secured by such Lien at such time is not increased to any amount greater than the sum of (i) the outstanding principal amount or, if greater, committed amount of the Indebtedness described under clauses (6), (7), (8) and (9) at the time the original Lien became a Permitted Lien under the Indenture, and (ii) an amount necessary to pay any fees and expenses, including premiums, related to such refinancing, refunding, extension, renewal or replacement;
(19) deposits made in the ordinary course of business to secure liability to insurance carriers;
(20) other Liens securing obligations in an aggregate amount at any one time outstanding not to exceed the greater of (a) $100.0 million and (b) 1.0% of Total Assets determined as of the date of incurrence;
(21) Liens securing judgments for the payment of money not constituting an Event of Default under clause (5) under “Events of Default and Remedies” so long as such Liens are adequately bonded and any appropriate legal proceedings that may have been duly initiated for the review of such judgment have not been finally terminated or the period within which such proceedings may be initiated has not expired;
179
Table of Contents
(22) Liens in favor of customs and revenue authorities arising as a matter of law to secure payment of customs duties in connection with the importation of goods in the ordinary course of business;
(23) Liens (a) of a collection bank arising under Section 4-210 of the Uniform Commercial Code on items in the course of collection, (b) attaching to commodity trading accounts or other commodity brokerage accounts incurred in the ordinary course of business, and (c) in favor of banking institutions arising as a matter of law encumbering deposits (including the right of set-off) and which are within the general parameters customary in the banking industry;
(24) Liens deemed to exist in connection with Investments in repurchase agreements permitted under “Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”; provided that such Liens do not extend to any assets other than those that are the subject of such repurchase agreement;
(25) Liens encumbering reasonable customary deposits and margin deposits and similar Liens attaching to commodity trading accounts or other brokerage accounts incurred in the ordinary course of business and not for speculative purposes;
(26) Liens that are contractual rights of set-off (a) relating to the establishment of depository relations with banks not given in connection with the issuance of Indebtedness, (b) relating to pooled deposit or sweep accounts of the Issuer or any of its Restricted Subsidiaries to permit satisfaction of overdraft or similar obligations incurred in the ordinary course of business of the Issuer and its Restricted Subsidiaries or (c) relating to purchase orders and other agreements entered into with customers of the Issuer or any of its Restricted Subsidiaries in the ordinary course of business;
(27) Liens securing obligations owed by the Issuer or any Restricted Subsidiary to any lender under the Senior Credit Facilities or any Affiliate of such a lender in respect of any overdraft and related liabilities arising from treasury, depository and cash management services or any automated clearing house transfers of funds;
(28) during a Suspension Period only, Liens securing Indebtedness (other than Indebtedness that is secured equally and ratably with (or on a basis subordinated to) the Senior Subordinated Notes), and Indebtedness represented by Sale and Lease-Back Transactions in an amount not to exceed 15.0% of Total Assets at any one time outstanding;
(29) Liens securing Indebtedness the proceeds of which are used to develop or construct new facilities (or any improvements to existing facilities) or equipment (or any improvements to existing equipment) designed primarily for the purpose of air or water pollutions control; provided that such Indebtedness is permitted to be incurred by the terms of the Indenture and such Liens do not extend to any assets of the Issuer or its Restricted Subsidiaries other than the assets developed, constructed or improved with the proceeds of the Indebtedness secured by such Lien;
(30) any encumbrance or restriction (including put and call arrangements) with respect to capital stock of any joint venture or similar arrangement pursuant to any joint venture or similar agreement;
(31) Liens arising out of conditional sale, title retention, consignment or similar arrangements for the sale or purchase of goods entered into by the Issuer or any Restricted Subsidiary in the ordinary course of business;
(32) Liens solely on any cash earnest money deposits made by the Issuer or any of its Restricted Subsidiaries in connection with any letter of intent or purchase agreement permitted;
(33) ground leases in respect of real property on which facilities owned or leased by the Issuer or any of its Subsidiaries are located;
(34) Liens on insurance policies and the proceeds thereof securing the financing of the premiums with respect thereto;
(35) Liens on Capital Stock of an Unrestricted Subsidiary that secure Indebtedness or other obligations of such Unrestricted Subsidiary; and
(36) Liens on the assets of non-guarantor Subsidiaries securing Indebtedness of the Issuer or the Restricted Subsidiaries that were permitted by the terms of the Indenture to be incurred.
For purposes of this definition, the term “Indebtedness” shall be deemed to include interest on such Indebtedness.
“Person” means any individual, corporation, limited liability company, partnership, joint venture, association, joint stock company, trust, unincorporated organization, government or any agency or political subdivision thereof or any other entity.
“Preferred Stock” means any Equity Interest with preferential rights of payment of dividends or upon liquidation, dissolution, or winding up.
“Qualified Proceeds” means the fair market value of assets that are used or useful in, or Capital Stock of any Person engaged in, a Similar Business.
“Qualified Securitization Facility” means any Securitization Facility (1) constituting a securitization financing facility that meets the following conditions: (a) the board of directors of the Issuer shall have determined in good faith that such Securitization Facility (including financing terms, covenants, termination events and other provisions) is in the aggregate economically fair and reasonable to the Issuer and the applicable Securitization Subsidiary, (b) all sales and/or contributions of Securitization Assets and related assets
180
Table of Contents
to the applicable Securitization Subsidiary are made at fair market value (as determined in good faith by the Issuer) and (c) the financing terms, covenants, termination events and other provisions thereof shall be market terms (as determined in good faith by the Issuer) or (2) constituting a receivables financing facility.
“Rating Agencies” means Moody’s and S&P or if Moody’s or S&P or both shall not make a rating on the Senior Subordinated Notes publicly available, a nationally recognized statistical rating agency or agencies, as the case may be, selected by the Issuer which shall be substituted for Moody’s or S&P or both, as the case may be.
“Registration Rights Agreement” means one or more registration rights agreements with respect to the Notes and the Additional Senior Subordinated Notes among the Issuer, the Guarantors and the Initial Purchasers.
“Related Business Assets” means assets (other than Cash Equivalents) used or useful in a Similar Business, provided that any assets received by the Issuer or a Restricted Subsidiary in exchange for assets transferred by the Issuer or a Restricted Subsidiary shall not be deemed to be Related Business Assets if they consist of securities of a Person, unless upon receipt of the securities of such Person, such Person would become a Restricted Subsidiary.
“Representative” means any trustee, agent or other representative for an issue of Designated Senior Indebtedness of the Issuer.
“Restricted Investment” means an Investment other than a Permitted Investment.
“Restricted Subsidiary” means, at any time, any direct or indirect Subsidiary of the Issuer (including any Foreign Subsidiary) that is not then an Unrestricted Subsidiary; provided that upon an Unrestricted Subsidiary ceasing to be an Unrestricted Subsidiary, such Subsidiary shall be included in the definition of “Restricted Subsidiary.”
“S&P” means Standard & Poor’s, a division of The McGraw-Hill Companies, Inc., and any successor to its rating agency business.
“Sale and Lease-Back Transaction” means any arrangement providing for the leasing by the Issuer or any of its Restricted Subsidiaries of any real or tangible personal property, which property has been or is to be sold or transferred by the Issuer or such Restricted Subsidiary to a third Person in contemplation of such leasing.
“SEC” means the U.S. Securities and Exchange Commission.
“Secured Indebtedness” means any Indebtedness of the Issuer or any of its Restricted Subsidiaries secured by a Lien.
“Securities Act” means the Securities Act of 1933, as amended, and the rules and regulations of the SEC promulgated thereunder.
“Securitization Assets” means the accounts receivable, royalty or other revenue streams and other rights to payment related to the Specified Contract Rights subject to a Qualified Securitization Facility that is a securitization financing facility (and not a receivables financing facility) and the proceeds thereof.
“Securitization Facility” means any of one or more receivables or securitization financing facilities as amended, supplemented, modified, extended, renewed, restated or refunded from time to time, the Obligations of which are non-recourse (except for customary representations, warranties, covenants and indemnities made in connection with such facilities) to the Issuer or any of its Restricted Subsidiaries (other than a Securitization Subsidiary) pursuant to which the Issuer or any of its Restricted Subsidiaries sells or grants a security interest in its accounts receivable or Securitization Assets or assets related thereto to either (a) a Person that is not a Restricted Subsidiary or (b) a Securitization Subsidiary that in turn sells its accounts receivable to a Person that is not a Restricted Subsidiary.
“Securitization Fees” means distributions or payments made directly or by means of discounts with respect to any participation interest issued or sold in connection with, and other fees paid to a Person that is not a Securitization Subsidiary in connection with, any Qualified Securitization Facility.
“Securitization Subsidiary” means any Subsidiary formed for the purpose of, and that solely engages only in one or more Qualified Securitization Facilities and other activities reasonably related thereto.
“Senior Credit Facilities” means the ABL Facilities and the CF Credit Facilities.
“Senior Indebtedness” means:
(1) all Indebtedness of the Issuer or any Guarantor outstanding under the Senior Credit Facilities or Senior Notes and related Guarantees (including interest accruing on or after the filing of any petition in bankruptcy or similar proceeding or for reorganization of the Issuer or any Guarantor (at the rate provided for in the documentation with respect thereto, regardless of whether or not a claim for post-filing interest is allowed in such proceedings)), and any and all other fees, expense reimbursement obligations, indemnification amounts, penalties, and other amounts (whether existing on the Issue Date or thereafter created or incurred) and all obligations of the Issuer or any Guarantor to reimburse any bank or other Person in respect of amounts paid under letters of credit, acceptances or other similar instruments;
(2) all Hedging Obligations (and guarantees thereof) owing to a Lender (as defined in the Senior Credit Facilities) or any Affiliate of such Lender (or any Person that was a Lender or an Affiliate of such Lender at the time the applicable agreement giving rise to such Hedging Obligation was entered into), provided that such Hedging Obligations are permitted to be incurred under the terms of the Indenture;
181
Table of Contents
(3) any other Indebtedness of the Issuer or any Guarantor permitted to be incurred under the terms of the Indenture, unless the instrument under which such Indebtedness is incurred expressly provides that it is on a parity with or subordinated in right of payment to the Senior Subordinated Notes or any related Guarantee; and
(4) all Obligations with respect to the items listed in the preceding clauses (1), (2) and (3);
provided that Senior Indebtedness shall not include:
(a) any obligation of such Person to the Issuer or any of its Subsidiaries;
(b) any liability for federal, state, local or other taxes owed or owing by such Person;
(c) any accounts payable or other liability to trade creditors arising in the ordinary course of business;
(d) any Indebtedness or other Obligation of such Person which is subordinate or junior in any respect to any other Indebtedness or other Obligation of such Person; or
(e) that portion of any Indebtedness which at the time of incurrence is incurred in violation of the Indenture; provided that such Indebtedness shall be deemed not to have been incurred in violation of the Indenture for purposes of this clause if such Indebtedness consists of Designated Senior Indebtedness, and the holder(s) of such Indebtedness or their agent or representative (i) had no actual knowledge at the time of incurrence that the incurrence of such Indebtedness violated the Indenture and (ii) shall have received a certificate from an officer of the Issuer to the effect that the incurrence of such Indebtedness does not (or, in the case of a revolving credit facility thereunder, the incurrence of the entire committed amount thereof at the date on which the initial borrowing is made thereunder would not) violate the provisions of the Indenture.
“Senior Notes” means the Issuer’s 10% Senior Notes due 2017 and the Issuer’s 10 3/8%/11 1/8% Senior Toggle Notes due 2017 (and any increase in the principal amount of such Senior Toggle Notes and any additional Senior Toggle Notes issued, in each case as a result of any PIK Payment (as defined under “Description of Senior Notes—Principal, Maturity and Interest”)) issued under the indenture governing the Senior Notes.
“Senior Subordinated Indebtedness” means:
(1) with respect to the Issuer, Indebtedness which ranks equal in right of payment to the Senior Subordinated Notes issued by the Issuer; and
(2) with respect to any Guarantor, Indebtedness which ranks equal in right of payment to the Guarantee of such Person of the Senior Subordinated Notes;
provided that such Indebtedness is not subordinated by its terms in right of payment to any Indebtedness which is not Senior Indebtedness.
“Significant Subsidiary” means any Restricted Subsidiary that would be a “significant subsidiary” as defined in Article 1, Rule 1-02 of Regulation S-X, promulgated pursuant to the Securities Act, as such regulation is in effect on the Issue Date.
“Similar Business” means (1) any business engaged in by the Issuer or any of its Restricted Subsidiaries on the Issue Date, and (2) any business or other activities that are reasonably similar, ancillary, complementary or related to, or a reasonable extension, development or expansion of, the businesses in which the Issuer and its Restricted Subsidiaries are engaged on the Issue Date.
“Specified Contract Rights” means certain intellectual property licenses, agreements or other contracts giving rise to not more than $50.0 million of annual accounts receivable, royalty or other intellectual property revenue streams or other rights to payment.
“Subordinated Indebtedness” means, with respect to the Senior Subordinated Notes,
(1) any Indebtedness of the Issuer which is by its terms subordinated in right of payment to the Senior Subordinated Notes, and
(2) any Indebtedness of any Guarantor which is by its terms subordinated in right of payment to the Guarantee of such entity of the Senior Subordinated Notes.
“Subsidiary” means, with respect to any Person:
(1) any corporation, association, or other business entity (other than a partnership, joint venture, limited liability company or similar entity) of which more than 50.0% of the total voting power of shares of Capital Stock entitled (without regard to the occurrence of any contingency) to vote in the election of directors, managers or trustees thereof is at the time of determination owned or controlled, directly or indirectly, by such Person or one or more of the other Subsidiaries of that Person or a combination thereof or is consolidated under GAAP with such Person at such time; and
(2) any partnership, joint venture, limited liability company or similar entity of which
(a) more than 50.0% of the capital accounts, distribution rights, total equity and voting interests or general or limited partnership interests, as applicable, are owned or controlled, directly or indirectly, by such Person or one or more of the other Subsidiaries of that Person or a combination thereof whether in the form of membership, general, special or limited partnership or otherwise, and
182
Table of Contents
(b) such Person or any Restricted Subsidiary of such Person is a controlling general partner or otherwise controls such entity.
“Total Assets” means the total assets of the Issuer and its Restricted Subsidiaries, determined on a consolidated basis in accordance with GAAP, as shown on the most recent balance sheet of the Issuer or such other Person as may be expressly stated.
“Transaction Agreement” means the Agreement and Plan of Merger, dated as of December 18, 2006 (as amended and restated as of June 7, 2007) by and among Biomet, Inc., LVB Acquisition, LLC, and the Issuer, as the same may be amended prior to the Issue Date.
“Transactions” means the transactions contemplated by the Transaction Agreement, the issuance of the Senior Notes and the Senior Subordinated Notes and borrowings under the Senior Credit Facilities as in effect on the Issue Date.
“Treasury Rate” means, as of any Redemption Date, the yield to maturity as of such Redemption Date of United States Treasury securities with a constant maturity (as compiled and published in the most recent Federal Reserve Statistical Release H.15 (519) that has become publicly available at least two Business Days prior to the Redemption Date (or, if such Statistical Release is no longer published, any publicly available source of similar market data)) most nearly equal to the period from the Redemption Date to October 15, 2012; provided that if the period from the Redemption Date to such date is less than one year, the weekly average yield on actually traded United States Treasury securities adjusted to a constant maturity of one year will be used.
“Trust Indenture Act” means the Trust Indenture Act of 1939, as amended (15 U.S.C. §§ 77aaa-777bbbb).
“Unrestricted Subsidiary” means:
(1) any Subsidiary of the Issuer which at the time of determination is an Unrestricted Subsidiary (as designated by the Issuer, as provided below); and
(2) any Subsidiary of an Unrestricted Subsidiary.
The Issuer may designate any Subsidiary of the Issuer (including any existing Subsidiary and any newly acquired or newly formed Subsidiary) to be an Unrestricted Subsidiary unless such Subsidiary or any of its Subsidiaries owns any Equity Interests or Indebtedness of, or owns or holds any Lien on, any property of, the Issuer or any Subsidiary of the Issuer (other than solely any Subsidiary of the Subsidiary to be so designated); provided that
(1) any Unrestricted Subsidiary must be an entity of which the Equity Interests entitled to cast at least a majority of the votes that may be cast by all Equity Interests having ordinary voting power for the election of directors or Persons performing a similar function are owned, directly or indirectly, by the Issuer;
(2) such designation complies with the covenants described under “Certain Covenants—Limitation on Restricted Payments”; and
(3) each of (a) the Subsidiary to be so designated and (b) its Subsidiaries has not at the time of designation, and does not thereafter, create, incur, issue, assume, guarantee or otherwise become directly or indirectly liable with respect to any Indebtedness pursuant to which the lender has recourse to any of the assets of the Issuer or any Restricted Subsidiary.
The Issuer may designate any Unrestricted Subsidiary to be a Restricted Subsidiary; provided that, immediately after giving effect to such designation, no Default shall have occurred and be continuing and either:
(1) the Issuer could incur at least $1.00 of additional Indebtedness pursuant to the Fixed Charge Coverage Test; or
(2) the Fixed Charge Coverage Ratio for the Issuer would be equal to or greater than such ratio for the Issuer immediately prior to such designation, in each case on a pro forma basis taking into account such designation.
Any such designation by the Issuer shall be notified by the Issuer to the Trustee by promptly filing with the Trustee a copy of the resolution of the board of directors of the Issuer or any committee thereof giving effect to such designation and an Officer’s Certificate certifying that such designation complied with the foregoing provisions.
“Voting Stock” of any Person as of any date means the Capital Stock of such Person that is at the time entitled to vote in the election of the board of directors of such Person.
“Weighted Average Life to Maturity” means, when applied to any Indebtedness, Disqualified Stock or Preferred Stock, as the case may be, at any date, the quotient obtained by dividing:
(1) the sum of the products of the number of years from the date of determination to the date of each successive scheduled principal payment of such Indebtedness or redemption or similar payment with respect to such Disqualified Stock or Preferred Stock multiplied by the amount of such payment; by
(2) the sum of all such payments.
“Wholly-Owned Subsidiary” of any Person means a Subsidiary of such Person, 100.0% of the outstanding Equity Interests of which (other than directors’ qualifying shares) shall at the time be owned by such Person or by one or more Wholly-Owned Subsidiaries of such Person.
183
Table of Contents
The certificates representing the notes will be issued in fully registered form without interest coupons (the “global notes”). The global notes will be deposited with the relevant trustee as a custodian for DTC, as depositary, and registered in the name of such depositary.
Those who participate in this offering may elect to take physical delivery of their certificates (each a “certificated security”) instead of holding their interests through the global notes (and which are then ineligible to trade through DTC) (collectively referred to herein as the “non-global purchasers”). Upon the transfer of any certificated security initially issued to a non-global purchaser, such certificated security will, unless the transferee requests otherwise or the global notes have previously been exchanged in whole for certificate securities, be exchanged for an interest in the global notes.
The Global Notes
We expect that pursuant to procedures established by DTC (a) upon the issuance of the global notes, DTC or its custodian will credit, on its internal system, the principal amount at maturity of the individual beneficial interests represented by such global notes to the respective accounts of persons who have accounts with such depositary and (b) ownership of beneficial interests in the global notes will be shown on, and the transfer of such ownership will be effected only through, records maintained by DTC or its nominee (with respect to interests of participants) and the records of participants (with respect to interests of persons other than participants). Such accounts initially will be designated by or on behalf of the initial purchasers and ownership of beneficial interests in the global notes will be limited to persons who have accounts with DTC (“participants”) or persons who hold interests through participants. Holders may hold their interests in the global notes directly through DTC if they are participants in such system, or indirectly through organizations which are participants in such system.
So long as DTC, or its nominee, is the registered owner or holder of the notes, DTC or such nominee, as the case may be, will be considered the sole owner or holder of the notes represented by such global notes for all purposes under the indentures. No beneficial owner of an interest in the global notes will be able to transfer that interest except in accordance with DTC’s procedures, in addition to those provided for under the indentures with respect to the notes.
Payments of the principal of, premium (if any), interest (including additional interest) on, the global notes will be made to DTC or its nominee, as the case may be, as the registered owner thereof. None of us, the trustee or any paying agent will have any responsibility or liability for any aspect of the records relating to or payments made on account of beneficial ownership interests in the global notes or for maintaining, supervising or reviewing any records relating to such beneficial ownership interest.
We expect that DTC or its nominee, upon receipt of any payment of principal, premium, if any, interest (including additional interest) on the global notes, will credit participants’ accounts with payments in amounts proportionate to their respective beneficial interests in the principal amount of the global notes as shown on the records of DTC or its nominee. We also expect that payments by participants to owners of beneficial interests in the global notes held through such participants will be governed by standing instructions and customary practice, as is now the case with securities held for the accounts of customers registered in the names of nominees for such customers. Such payments will be the responsibility of such participants.
Transfers between participants in DTC will be effected in the ordinary way through DTC’s same-day funds system in accordance with DTC rules and will be settled in same day funds. If a holder requires physical delivery of a certificated security for any reason, including to sell notes to persons in states which require physical delivery of the notes, or to pledge such securities, such holder must transfer its interest in a global note, in accordance with the normal procedures of DTC and with the procedures set forth in the indentures. DTC has advised us that it will take any action permitted to be taken by a holder of notes (including the presentation of notes for exchange as described below) only at the direction of one or more participants to whose account the DTC interests in the global notes are credited and only in respect of such portion of the aggregate principal amount of notes as to which such participant or participants has or have given such direction. However, if there is an event of default under the indentures, DTC will exchange the global notes for certificated securities, which it will distribute to its participants and which will be legended as set forth under the heading “Notice to Investors” in the final offering memoranda relating to the original notes dated September 25, 2007 and October 11, 2007.
DTC has advised us as follows: DTC is a limited purpose trust company organized under the laws of the State of New York, a member of the Federal Reserve System, a “clearing corporation” within the meaning of the Uniform Commercial Code and a “Clearing Agency” registered pursuant to the provisions of Section 17A of the Exchange Act. DTC was created to hold securities for its participants and facilitate the clearance and settlement of securities transactions between participants through electronic book-entry changes in accounts of its participants, thereby eliminating the need for physical movement of certificates. Participants include securities brokers and dealers, banks, trust companies and clearing corporations and certain other organizations. Indirect access to the DTC system is available to others such as banks, brokers, dealers and trust companies that clear through or maintain a custodial relationship with a participant, either directly or indirectly (“indirect participants”).
Although DTC has agreed to the foregoing procedures in order to facilitate transfers of interests in the global note among participants of DTC, it is under no obligation to perform such procedures, and such procedures may be discontinued at any time. Neither we nor the trustee will have any responsibility for the performance by DTC or its participants or indirect participants of their respective obligations under the rules and procedures governing their operations.
Certificated Securities
Certificated securities shall be issued in exchange for beneficial interests in the global notes (a) if requested by a holder of such interests, (b) if DTC is at any time unwilling or unable to continue as a depositary for the global notes and a successor depositary is not appointed by us within 90 days or (c) there has occurred and is continuing an event of default under the indentures.
184
Table of Contents
CERTAIN MATERIAL UNITED STATES FEDERAL INCOME TAX CONSIDERATIONS
The following is a summary of certain U.S. federal income tax considerations and, in the case of a Non-U.S. Holder (as defined below), certain U.S. federal estate tax considerations, which may be relevant to persons considering the purchase of notes. This summary is based on the Code, administrative pronouncements, judicial decisions and final, temporary and proposed Treasury regulations, in each case as of the date hereof, changes to any of which subsequent to the date of this prospectus may affect the tax consequences described herein, possibly with retroactive effect. This summary deals only with notes that will be held as capital assets (generally, investment property) and, except where otherwise specifically noted, is only addressed to persons who purchase notes for cash in the initial offering at the initial offering price. It does not address tax considerations applicable to investors that may be subject to special tax rules, such as banks, tax-exempt entities, insurance companies, dealers in securities or currencies, traders in securities electing to mark to market, persons that will hold notes as a position in a “straddle” or conversion transaction, or as part of a “synthetic security” or other integrated financial transaction, persons subject to the alternative minimum tax, certain U.S. expatriates, controlled foreign corporations, foreign personal holding companies, passive foreign investment companies, pass-through entities (including partnerships and entities and arrangements classified as partnerships for U.S. federal tax purposes), or U.S. Holders (as defined below) that have a “functional currency” other than the U.S. dollar.
As used under this heading “Certain Material United States Federal Income Tax Considerations,” the term “U.S. Holder” means a beneficial owner of a note that is for U.S. federal income tax or estate tax purposes (as applicable): (1) an individual citizen or resident of the United States, (2) a U.S. domestic corporation, (3) an estate the income of which is subject to U.S. federal income taxation regardless of its source or (4) a trust if (a) a U.S. court is able to exercise primary supervision over the trust’s administration and one or more “United States persons” (within the meaning of the Code) have the authority to control all of the trust’s substantial decisions, or (b) the trust has a valid election in effect under applicable Treasury regulations to be treated as a “United States person.” As used under this heading “Certain Material United States Federal Income Tax Considerations” the term “Non-U.S. Holder” means a beneficial owner of a note that is an individual, corporation, trust or estate for U.S. federal income tax purposes and is not a U.S. Holder. The following summary applies equally to all notes, except where expressly stated otherwise.
Investors should consult their tax advisors in determining the tax consequences to them of holding notes, including the application to their particular situation of the United States federal income tax considerations discussed below, as well as the application of state, local, foreign or other tax laws.
Tax Consequences to U.S. Holders
Payments of Qualified Stated Interest
Payments or accruals of “qualified stated interest” (as defined below) on a note will be taxable to a U.S. Holder as ordinary interest income at the time that such payments are accrued or are received (in accordance with the U.S. Holder’s method of accounting for U.S. federal income tax purposes). The term “qualified stated interest” generally means stated interest that is unconditionally payable in cash or property (other than debt instruments of the issuer) at least annually during the entire term of the note at a single fixed rate of interest, or, subject to certain conditions, based on one or more interest indices. Because we will pay interest in cash unconditionally semiannually with respect to the notes, except the senior toggle notes (discussed below under “—Original Issue Discount”), such interest payments will qualify as qualified stated interest.
Original Issue Discount
For any interest period through October 15, 2012, we may elect to pay interest on the senior toggle notes entirely in cash, entirely in PIK interest or 50% in cash interest and 50% in PIK interest. For U.S. federal income tax purposes, the existence of this option means that none of the interest payments on the senior toggle notes will be qualified stated interest even if we never exercise the option to pay interest in the form of PIK interest. Consequently, the senior toggle notes will be treated as issued with original issue discount, or “OID,” and the U.S. Holder will be required to include such OID in gross income as it accrues, possibly in advance of the receipt of cash attributable to that income.
In general, a U.S. Holder of a note issued with OID, whether such holder uses the cash or the accrual method of tax accounting, will be required to include in ordinary gross income the sum of the “daily portions” of OID on such note for all days during the taxable year that the U.S. Holder owns such note. The daily portions of OID are determined by allocating to each day in any accrual period a ratable portion of the OID allocable to that accrual period. Accrual periods may be of any length and may vary in length over the term of a note with original issue discount, provided that no accrual period is longer than one year and each scheduled payment of principal or interest occurs on either the final day or the first day of an accrual period. The amount of OID on a note with original issue discount allocable to each accrual period is determined by multiplying the “adjusted issue price” (as defined below) of a note with original issue discount at the beginning of the accrual period by the yield to maturity of such note (appropriately adjusted to reflect the length of the accrual period) and subtracting the amount of the qualified stated interest (if any) attributable to the accrual period. The yield to maturity of a note with original issue discount is the discount rate that causes the present value of all principal and interest payments on such note as of its original issue date to equal the issue price of such note. For purposes of determining the yield to maturity of a senior toggle note, a U.S. Holder may assume that we will not exercise the option to pay PIK interest, except in respect of any period in which we actually elect to pay PIK interest. The “adjusted issue price” of a note with original issue discount at the beginning of any accrual period will generally be the sum of its issue price and the amount of OID allocable to all prior accrual periods reduced by the amount of all cash payments (other than payments on qualified stated interest) made with respect to such note in all prior accrual periods.
If we in fact pay the interest in cash on the senior toggle notes, a U.S. Holder will not be required to adjust its OID inclusions. Each payment made in cash under a senior toggle note will be treated first as a payment of any accrued OID that has not been allocated to prior payments and second as a payment of principal (which is not includible in income). A U.S. Holder of a senior toggle note generally will not be required to include separately in income cash payments received on such note to the extent such payments constitute payments of previously accrued OID. If for any interest payment period we exercise our option to pay interest in the form
185
Table of Contents
of PIK interest, a U.S. Holder of a senior toggle note will be required to adjust its OID calculation for future periods by treating the senior toggle note as if it had been retired and then reissued for an amount equal to its adjusted issue price on the date preceding the first date of such future interest payment period, and to re-calculate the yield to maturity of the reissued note by treating the amount of PIK interest paid (and of any prior PIK interest paid) as a payment that will be made on the maturity date of such reissued note.
Purchase, Sale, Exchange and Retirement
A U.S. Holder’s tax basis in a note generally will equal the cost of such note to such holder, increased by any amounts includible in income by the holder as OID and reduced by any cash payments (other than payments of qualified stated interest) made on such note. Subject to the following paragraph, upon the sale, exchange or retirement of a note, a U.S. Holder generally will recognize gain or loss equal to the difference between the amount realized on the sale, exchange or retirement (not including amounts attributable to accrued qualified stated interest) and the U.S. Holder’s tax basis in such note. Such gain or loss recognized by a U.S. Holder generally will be long-term capital gain or loss if the U.S. Holder has held the note for more than one year at the time of disposition. Long-term capital gains recognized by non-corporate holders generally are subject to tax at a reduced rate. The deductibility of capital losses is subject to limitations. Payments attributable to accrued qualified stated interest not previously included in income by a U.S. Holder will be taxable as ordinary income.
Information Reporting and Backup Withholding
Under current U.S. federal income tax law, information reporting requirements apply with respect to payments made to U.S. Holders of principal, interest and OID on (and to the proceeds of sales of) notes unless an exemption exists. In addition, U.S. Holders who are not exempt will be subject to backup withholding tax (currently at a rate of 28%) in respect of such payments if, among other things, they do not provide their correct taxpayer identification numbers to us or our paying agent. All individuals are subject to these requirements. In general, corporations are exempt from these requirements.
Backup withholding tax is not an additional tax and may be credited against a U.S. Holder’s U.S. federal income tax liability (and may entitle the U.S. Holder to a refund), provided that correct information is timely provided to the IRS.
Tax Consequences to Non-U.S. Holders
Tax Consequences
Under U.S. federal income tax law, and subject to the discussion below concerning backup withholding, no withholding of U.S. federal income tax generally will be required with respect to the payment by us or our paying agent on a note owned by a Non-U.S. Holder of interest (including OID, if any) that qualifies as portfolio interest. Interest on a note owned by a Non-U.S. Holder will qualify as portfolio interest, provided that (1) such interest is not effectively connected with the conduct of such U.S. Holder’s U.S. trade or business, (2) such Non-U.S. Holder does not actually or constructively own 10% or more of the total combined voting power of all classes of our stock entitled to vote, (3) such Non-U.S. Holder is not a controlled foreign corporation that is related to us actually or constructively through stock ownership, and (4) such Non-U.S. Holder provides a statement signed under penalties of perjury that includes its name and address and certifies that it is a Non-U.S. Holder in compliance with applicable requirements generally made, under current procedures, on IRS Form W-8BEN (or satisfies certain documentary evidence requirements for establishing that is it a Non-U.S. Holder).
A Non-U.S. Holder with interest income (including OID, if any) that does not qualify as portfolio interest will be subject to a 30% U.S. federal withholding tax unless, under current procedures, it delivers a properly completed IRS Form W-8ECI (stating that interest paid on its notes is not subject to withholding tax because it is effectively connected to its conduct of a trade or business in the U.S.) or IRS Form W-8BEN (claiming an exemption from or reduction in withholding tax under an applicable income tax treaty).
A Non-U.S. Holder will generally not be subject to U.S. federal income tax on any gain realized on the sale, exchange or redemption of a note, unless (1) such gain is effectively connected with the conduct by the Non-U.S. Holder of a trade or business in the United States (and, if an income tax treaty applies, is attributable to a U.S. “permanent establishment”) or (2) in the case of gain realized by an individual holder, the holder is present in the United States for 183 days or more in the taxable year of the retirement or disposition and certain other conditions are met.
A Non-U.S. Holder generally will be taxed in the same manner as a U.S. Holder with respect to interest income (including OID, if any) or gain that is effectively connected with its U.S. trade or business and, if required by an applicable income tax treaty, that is attributable to its U.S. “permanent establishment,” unless an applicable income tax treaty provides otherwise. In addition, under certain circumstances, effectively connected earnings and profits of a corporate Non-U.S. Holder may be subject to a “branch profits” tax imposed at a 30% rate or at such lower rate as may be specified by an applicable income tax treaty.
A note beneficially owned by an individual who at the time of death is not a U.S. citizen or a resident of the U.S. (as specifically defined for U.S. federal estate tax purposes) will generally not be subject to U.S. federal estate tax as a result of such individual’s death, provided that, at the time of such individual’s death, any interest paid on the note would have qualified for an exemption from U.S. federal income tax as “portfolio interest,” as described in more detail above (except that a statement of an individual’s Non-U.S. Holder status, otherwise required to qualify for the “portfolio interest” exemption, is not required for this exemption from U.S. federal estate tax).
Information Reporting and Backup Withholding
U.S. information reporting requirements and backup withholding tax will not apply to payments on a note (and proceeds from the sale of a note) if the beneficial owner (1) certifies its Non-U.S. Holder status under penalties of perjury, generally made, under current procedures, on IRS Form W-8BEN, or satisfies documentary evidence requirements for establishing that it is a Non-U.S. Holder or (2) otherwise establishes an exemption.
186
Table of Contents
This prospectus is to be used by Goldman, Sachs & Co. and its affiliates in connection with offers and sales of the notes in market-making transactions effected from time to time.
Goldman, Sachs & Co. or its affiliates may act as principal or agent in such transactions, including as agent for the counterparty when acting as principal or as agent for both counterparties, and may receive compensation in the form of discounts and commissions, including from both counterparties, when it acts as agents for both. Such sales will be made at prevailing market prices at the time of sale, at price related thereto or at negotiated prices. We will not receive any of the proceeds from such sales.
As of February 20, 2009, entities affiliated with Goldman, Sachs & Co. held approximately 23.68% of our common stock. Pursuant to our shareholders agreement, such entities have a right to designate a specified number of individuals to serve on our Board of Directors. Goldman, Sachs & Co. and its affiliates may in the future engage in commercial and/or investment banking transactions with us and our affiliates. Goldman, Sachs & Co. acted as an initial purchaser in connection with the original sale of the notes on September 25, 2007 and October 16, 2007 and received a customary underwriting discount in connection with each of those transactions. Goldman, Sachs & Co. and its affiliates currently own, and may from time to time trade, the notes for its own account in connection with its principal activities. Such sales may be made pursuant to this prospectus or otherwise pursuant to an applicable exemption from registration. Additionally, in the future Goldman, Sachs & Co. and its affiliates may, from time to time, own notes as a result of their market-making activities.
Goldman, Sachs & Co. has informed us that they do not intend to confirm sales of the securities to any accounts over which they exercise discretionary authority without the prior specific written approval of such transactions by the customer.
We have been advised by Goldman, Sachs & Co. that subject to applicable laws and regulations they currently intend to make a market in the notes. However, Goldman, Sachs & Co. is not obligated to do so, and any such market-making may be interrupted or discontinued at any time without notice. In addition, such market-making activity will be subject to the limits imposed by the Securities Act and the Exchange Act. We cannot assure you that an active trading market will be sustained. See “Risk Factors—Risks related to the notes—Your ability to transfer the notes may be limited by the absence of an active trading market, and there is no assurance that any active trading market will develop for the notes.”
Pursuant to registration rights agreements entered into between us and Goldman, Sachs & Co., we have agreed to indemnify Goldman, Sachs & Co. against certain liabilities, including liabilities under the Securities Act.
The validity of the notes and the related guarantees offered hereby will be passed upon for us by Cleary Gottlieb Steen & Hamilton LLP, New York, New York. Kirkland & Ellis LLP have passed upon certain matters governed by the laws of the state of California, Edwards Angell Palmer & Dodge LLP have passed upon certain matters governed by the laws of the state of Florida and Taft Stettinius & Hollister LLP have passed upon certain matters governed by the laws of the state of Indiana.
The consolidated financial statements as of May 31, 2007 and 2006, and for each of the two years in the period ended May 31, 2007 of Biomet, Inc. and its subsidiaries have been audited by Ernst & Young LLP, independent registered public accounting firm, as stated in their report appearing herein.
The consolidated financial statements as of May 31, 2008, and for the periods June 1, 2007 through July 11, 2007 (predecessor) and July 12, 2007 through May 31, 2008 (successor), included in this prospectus and the related financial statement schedule have been audited by Deloitte & Touche LLP, an independent registered public accounting firm, as stated in their report appearing herein and elsewhere in the Registration Statement (which report expresses an unqualified opinion on the financial statements and financial statement schedule and includes explanatory paragraphs referring to the new basis of accounting resulting from the acquisition of Biomet, Inc. and the adoption of Financial Accounting Standards Board Interpretation No. 48, Accounting for Uncertainty in Income Taxes). Such financial statements and financial statement schedule have been so included in reliance upon the report of such firm given upon their authority as experts in accounting and auditing.
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
Our audit committee approved the dismissal of Ernst & Young LLP (“Ernst & Young”) as our independent registered public accounting firm on January 24, 2008.
The reports of Ernst & Young on our consolidated financial statements as of and for the fiscal years ended May 31, 2007 and 2006, did not contain any adverse opinion or disclaimer of opinion, nor were they qualified or modified as to uncertainty, audit scope or accounting principle, except that the report on our consolidated financial statements as of and for the fiscal years ended May 31, 2006 was modified to indicate that we had restated previously issued financial statements as of May 31, 2006 and 2005 and for each of the three years in the period ended May 31, 2006 to correct our accounting for certain share-based expense and related payroll taxes. During the period from June 1, 2005 through the fiscal year ended May 31, 2007, and through January 24, 2008, there were no (1) disagreements with Ernst & Young on any matter of accounting principles or practices, financial statement disclosure, or auditing scope or procedure, which disagreements, if not resolved to Ernst & Young’s satisfaction, would have caused Ernst & Young to make reference thereto in its report on the financial statements for such years, or (2) reportable events described under Item 304(a)(1)(iv) of Regulation S-K, except for the material weakness reported in our Amended Annual Report on Form 10-K/A, which was
187
Table of Contents
filed with the SEC on May 29, 2007, which indicated that we had ineffective internal control as of May 31, 2006 over financial reporting with respect to the granting, administration and accounting for stock options, namely, we did not maintain effective control over the completeness, valuation, presentation and disclosure of share-based expense. Also on January 24, 2008, our audit committee appointed Deloitte & Touche LLP as its new independent registered public accounting firm. We did not consult with Deloitte & Touche LLP on any matters described in Item 304(a)(2)(i) and Item 304(a)(2)(ii) of Regulation S-K prior to their appointment. The decision to change accountants was approved by our audit committee and ratified by our board of directors.
188
Table of Contents
INDEX TO FINANCIAL STATEMENTS
Index | Page Number | |
Consolidated Financial Statements: | ||
Report of Independent Registered Public Accounting Firm—Successor | F-2 | |
Report of Independent Registered Public Accounting Firm—Predecessor | F-3 | |
Consolidated Balance Sheets as of May 31, 2008 (Successor) and 2007 (Predecessor) | F-4 | |
| F-5 | ||
| F-6 | ||
| F-7 | ||
| F-28 | ||
| F-29 | ||
Condensed Consolidated Financial Statements | ||
Condensed Consolidated Balance Sheets as of November 30, 2008 (unaudited) and May 31, 2008 | F-30 | |
| F-31 | ||
| F-32 | ||
| F-33 | ||
F-1
Table of Contents
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Shareholders of Biomet, Inc.
Warsaw, Indiana
We have audited the consolidated balance sheet of Biomet, Inc. and subsidiaries (“Biomet-successor”) as of May 31, 2008, and the related consolidated statements of operations, shareholders’ equity, and cash flows for the period July 12, 2007 through May 31, 2008. We have also audited the Biomet, Inc. and subsidiaries (“Biomet-predecessor”) consolidated statements of operations, shareholders’ equity and cash flows for the period June 1, 2007 through July 11, 2007. Our audit also included the financial statement schedule as of May 31, 2008 listed in the Index at Item 15. These financial statements and financial statement schedule are the responsibility of the Company’s management. Our responsibility is to express an opinion on the financial statements and financial statement schedules based on our audit.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of Biomet-successor as of May 31, 2008, and the results of their operations and their cash flows for the period July 12, 2007 through May 31, 2008, in conformity with accounting principles generally accepted in the United States of America. Further, in our opinion, the consolidated financial statements for Biomet-predecessor present fairly, in all material respects, the results of their operations and their cash flows for the period June 1, 2007 through July 11, 2007 in conformity with accounting principles generally accepted in the United States of America. Also, in our opinion, such financial statement schedule as of May 31, 2008, when considered in relation to the basic 2008 consolidated financial statements of Biomet-successor taken as a whole, present fairly in all material respects the information set forth therein.
As discussed in Note 1 to the consolidated financial statements, LVB Acquisition, LLC acquired Biomet, Inc. and subsidiaries. The transaction was accounted for as a business combination and the basis of assets and liabilities were adjusted to their estimated fair values. Accordingly, the consolidated financial statements as of and for the successor period ended May 31, 2008 are not comparable with prior periods.
As discussed in Note 2 to the consolidated financial statements, effective June 1, 2007, the Company adopted Financial Accounting Standards Board Interpretation No. 48,Accounting for Uncertainty in Income Taxes.
/s/ Deloitte & Touche LLP
Indianapolis, Indiana
August 28, 2008
F-2
Table of Contents
Report of Independent Registered Public Accounting Firm.
To the Board of Directors and Shareholders of Biomet, Inc.:
We have audited the accompanying consolidated balance sheet of Biomet, Inc. and subsidiaries as of May 31, 2007, and the related consolidated statements of operations, shareholders’ equity, and cash flows for each of the two years in the period ended May 31, 2007. Our audits also included the financial statement schedule listed in the index of Item 15 for the two years in the period ended May 31, 2007. These financial statements and schedule are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements and schedule based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of Biomet, Inc. and subsidiaries at May 31, 2007, and the consolidated results of its operations and its cash flows for each of the two years in the period ended May 31, 2007 in conformity with U.S. generally accepted accounting principles. Also, in our opinion, the related financial statement schedule, when considered in relation to the basic financial statements taken as a whole, presents fairly in all material respects the information set forth therein for the two years in the period ended May 31, 2007.
As discussed in Notes 9 and 8, respectively, to the consolidated financial statements, the Company adopted Statement of Financial Accounting Standards No. 123(R), “Share-Based Payments”, and No. 158, “Employers’ Accounting for Defined Benefit Pension and Postretirement Plans,” an amendment of FASB Statements No. 87, 88, 106, and 132(R), in 2007.
/s/ Ernst & Young LLP
Fort Wayne, Indiana
July 25, 2007, except for Notes 5 and 12, as to which the date is April 29, 2008
F-3
Table of Contents
Biomet, Inc. and Subsidiaries Consolidated Balance Sheets.
(in millions)
| May 31, 2008 (Successor) | May 31, 2007 (Predecessor) | ||||||||
Assets | |||||||||
Current assets: | |||||||||
Cash and cash equivalents | $ | 127.6 | $ | 105.1 | |||||
Short-term investments | — | 125.8 | |||||||
Accounts receivable, net | 486.2 | 498.7 | |||||||
Income tax receivable | 48.8 | — | |||||||
Inventories | 539.7 | 540.4 | |||||||
Deferred income taxes | 100.7 | 136.8 | |||||||
Prepaid expenses and other | 46.7 | 45.0 | |||||||
Total current assets | 1,349.7 | 1,451.8 | |||||||
Property, plant and equipment, net | 640.9 | 427.4 | |||||||
Investments | 41.3 | 43.0 | |||||||
Intangible assets, net | 6,208.2 | 74.6 | |||||||
Other assets | 118.9 | 12.7 | |||||||
Goodwill | 5,422.8 | 448.4 | |||||||
Total assets | $ | 13,781.8 | $ | 2,457.9 | |||||
Liabilities and Shareholders’ Equity | |||||||||
Current liabilities: | |||||||||
Short-term borrowings | $ | 75.4 | $ | 81.8 | |||||
Accounts payable | 83.7 | 68.7 | |||||||
Accrued interest | 80.9 | — | |||||||
Accrued wages and commissions | 79.1 | 80.3 | |||||||
FIN 48 liability | 50.9 | — | |||||||
Other accrued expenses | 194.5 | 115.1 | |||||||
Total current liabilities | 564.5 | 345.9 | |||||||
Long-term liabilities: | |||||||||
Long-term debt | 6,225.4 | — | |||||||
Deferred income taxes | 2,112.5 | 21.2 | |||||||
Employee related obligations | 40.0 | 41.6 | |||||||
Other long-term liabilities | 3.1 | — | |||||||
Total liabilities | 8,945.5 | 408.7 | |||||||
Shareholders’ equity: | |||||||||
Common shares | — | 229.6 | |||||||
Additional paid-in capital | 25.8 | 138.9 | |||||||
Contributed capital | 5,521.9 | — | |||||||
Retained earnings (accumulated deficit) | (964.2 | ) | 1,634.7 | ||||||
Accumulated other comprehensive income | 252.8 | 46.0 | |||||||
Total shareholders’ equity | 4,836.3 | 2,049.2 | |||||||
Total liabilities and shareholders’ equity | $ | 13,781.8 | $ | 2,457.9 | |||||
The accompanying notes are a part of the consolidated financial statements.
F-4
Table of Contents
Biomet, Inc. and Subsidiaries Consolidated Statements of Operations.
(in millions)
| For the Periods | For the Years Ended May 31, | |||||||||||||||||
| 2008 | 2007 | 2006 | ||||||||||||||||
| July 12, 2007 – May 31, 2008 (Successor) | June 1, 2007 – July 11, 2007 (Predecessor) | (Predecessor) | (Predecessor) | |||||||||||||||
Net sales | $ | 2,134.5 | $ | 248.8 | $ | 2,107.4 | $ | 2,025.7 | ||||||||||
Cost of sales | 814.7 | 102.3 | 642.3 | 582.1 | ||||||||||||||
Gross margin | 1,319.8 | 146.5 | 1,465.1 | 1,443.6 | ||||||||||||||
Selling, general and administrative expense | 1,097.6 | 194.2 | 881.1 | 750.2 | ||||||||||||||
Research and development expense | 82.2 | 34.0 | 85.6 | 74.8 | ||||||||||||||
In-process research and development | 479.0 | — | — | — | ||||||||||||||
Amortization | 329.3 | 0.5 | 8.8 | 10.2 | ||||||||||||||
Operating income (loss) | (668.3 | ) | (82.2 | ) | 489.6 | 608.4 | ||||||||||||
Interest expense, net | (516.3 | ) | (0.3 | ) | (9.3 | ) | (11.7 | ) | ||||||||||
Other income (expense) | (9.7 | ) | 0.6 | 21.3 | 14.3 | |||||||||||||
Other income (expense), net | (526.0 | ) | 0.3 | 12.0 | 2.6 | |||||||||||||
Income (loss) before income taxes | (1,194.3 | ) | (81.9 | ) | 501.6 | 611.0 | ||||||||||||
Provision (benefit) for income taxes | (230.1 | ) | (27.3 | ) | 165.7 | 205.1 | ||||||||||||
Net income (loss) | $ | (964.2 | ) | $ | (54.6 | ) | $ | 335.9 | $ | 405.9 | ||||||||
The accompanying notes are a part of the consolidated financial statements.
F-5
Table of Contents
Biomet, Inc. and Subsidiaries Consolidated Statements of Shareholders’ Equity.
(in millions)
| Common Shares | Additional Paid-In Capital | Retained Earnings (Accumulated Deficit) | Accumulated Other Comprehensive Income (Loss) | Total Shareholders’ Equity | |||||||||||||||||||
| Number | Amount | ||||||||||||||||||||||
Balance at June 1, 2005 | 249.9 | $ | 188.2 | $ | 112.3 | $ | 1,245.1 | $ | 23.2 | $ | 1,568.8 | ||||||||||||
Net income | — | — | — | 405.9 | — | 405.9 | |||||||||||||||||
Change in unrealized holding value on investments, net of $0.6 tax effect | — | — | — | — | 1.1 | 1.1 | |||||||||||||||||
Reclassification adjustment for losses included in net income, net of $0.3 tax effect | — | — | — | — | (0.7 | ) | (0.7 | ) | |||||||||||||||
Currency translation adjustments | — | — | — | — | (5.9 | ) | (5.9 | ) | |||||||||||||||
Comprehensive income | — | — | — | — | — | 400.4 | |||||||||||||||||
Exercise of stock options | 1.1 | 23.0 | — | — | — | 23.0 | |||||||||||||||||
Compensation expense | — | — | 2.0 | — | — | 2.0 | |||||||||||||||||
Excess tax benefit from exercise of stock options | — | — | 2.2 | — | — | 2.2 | |||||||||||||||||
Purchase of shares | (6.0 | ) | (4.5 | ) | (1.6 | ) | (209.2 | ) | — | (215.3 | ) | ||||||||||||
Cash dividends | — | — | — | (62.5 | ) | — | (62.5 | ) | |||||||||||||||
Other | — | — | 1.6 | — | — | 1.6 | |||||||||||||||||
Balance at May 31, 2006 | 245.0 | 206.7 | 116.5 | 1,379.3 | 17.7 | 1,720.2 | |||||||||||||||||
Net income | — | — | — | 335.9 | — | 335.9 | |||||||||||||||||
Change in unrealized holding value on investments, net of $0.8 tax effect | — | — | — | — | 1.6 | 1.6 | |||||||||||||||||
Reclassification adjustment for losses included in net income, net of tax effect | — | — | — | — | (0.1 | ) | (0.1 | ) | |||||||||||||||
Currency translation adjustments | — | — | — | — | 43.4 | 43.4 | |||||||||||||||||
Comprehensive income | — | — | — | — | — | 380.8 | |||||||||||||||||
Employee defined benefit plan, net of $6.3 tax effect | — | — | — | — | (16.6 | ) | (16.6 | ) | |||||||||||||||
Exercise of stock options | 0.9 | 23.1 | — | — | — | 23.1 | |||||||||||||||||
Compensation expense | — | — | 17.7 | — | — | 17.7 | |||||||||||||||||
Excess tax benefit from exercise of stock options | — | — | 3.2 | — | — | 3.2 | |||||||||||||||||
Purchase of shares | (0.2 | ) | (0.2 | ) | (0.1 | ) | (7.0 | ) | — | (7.3 | ) | ||||||||||||
Cash dividends | — | — | — | (73.5 | ) | — | (73.5 | ) | |||||||||||||||
Other | — | — | 1.6 | — | — | 1.6 | |||||||||||||||||
Balance at May 31, 2007 | 245.7 | 229.6 | 138.9 | 1,634.7 | 46.0 | 2,049.2 | |||||||||||||||||
Net loss for the period June 1, 2007 to July 11, 2007 | — | — | — | (54.6 | ) | — | (54.6 | ) | |||||||||||||||
Currency translation adjustments | — | — | — | — | (6.6 | ) | (6.6 | ) | |||||||||||||||
Comprehensive loss | — | — | — | — | — | (61.2 | ) | ||||||||||||||||
Adoption of FIN 48 | — | — | — | (9.2 | ) | — | (9.2 | ) | |||||||||||||||
Excess tax benefit from exercise of stock options | — | — | 3.9 | — | — | 3.9 | |||||||||||||||||
Purchase of shares | (1.0 | ) | (2.1 | ) | (0.7 | ) | — | — | (2.8 | ) | |||||||||||||
Effect of merger | (244.7 | ) | (227.5 | ) | (142.1 | ) | (1,570.9 | ) | (39.4 | ) | (1,979.9 | ) | |||||||||||
Net loss for the period July 12, 2007 to May 31, 2008 | — | — | — | (964.2 | ) | — | (964.2 | ) | |||||||||||||||
Change in unrealized holding value on available for sale securities | — | — | — | — | (3.8 | ) | (3.8 | ) | |||||||||||||||
Interest rate swap unrealized loss, net of $(7.2) tax effect | — | — | — | — | (12.1 | ) | (12.1 | ) | |||||||||||||||
Foreign currency related gains | — | — | — | — | 267.1 | 267.1 | |||||||||||||||||
Employee defined benefit plan | — | — | — | — | 1.6 | 1.6 | |||||||||||||||||
Comprehensive loss | — | — | — | — | — | (711.4 | ) | ||||||||||||||||
Contributed capital | — | 5,521.9 | — | — | — | 5,521.9 | |||||||||||||||||
Compensation expense | — | — | 25.8 | — | — | 25.8 | |||||||||||||||||
Balance at May 31, 2008 | — | $ | 5,521.9 | $ | 25.8 | $ | (964.2 | ) | $ | 252.8 | $ | 4,836.3 | |||||||||||
The accompanying notes are a part of the consolidated financial statements.
F-6
Table of Contents
Biomet, Inc. and Subsidiaries Consolidated Statements of Cash Flows.
(in millions)
| For the Periods | For the Years Ended May 31, | |||||||||||||||||
| 2008 | 2007 | 2006 | ||||||||||||||||
| July 12, 2007– May 31, 2008 (Successor) | June 1, 2007– July 11, 2007 (Predecessor) | (Predecessor) | (Predecessor) | |||||||||||||||
Cash flows from (used in) operating activities: | ||||||||||||||||||
Net income (loss) | $ | (964.2 | ) | $ | (54.6 | ) | $ | 335.9 | $ | 405.9 | ||||||||
Adjustments to reconcile net income (loss) to net cash from operating activities: | ||||||||||||||||||
Depreciation and amortization | 461.0 | 9.3 | 97.0 | 82.2 | ||||||||||||||
Amortization of deferred financing costs | 7.7 | — | — | — | ||||||||||||||
Amortization of premium on bonds | (0.4 | ) | — | — | — | |||||||||||||
In-process research and development charge | 479.0 | — | — | — | ||||||||||||||
Stock based compensation expense | 25.8 | — | 17.7 | 2.0 | ||||||||||||||
Inventory step-up related to merger | 160.3 | — | — | — | ||||||||||||||
Provision for inventory obsolescence | 7.7 | — | — | — | ||||||||||||||
Loss on sale of investments, net | — | (7.0 | ) | — | — | |||||||||||||
Deferred income taxes | (27.5 | ) | 76.7 | (61.8 | ) | (4.4 | ) | |||||||||||
Other | — | — | (2.4 | ) | 1.1 | |||||||||||||
Tax benefit from exercise of stock options | — | — | — | 2.2 | ||||||||||||||
Excess tax benefit from exercise of stock options | — | (3.9 | ) | (3.2 | ) | — | ||||||||||||
Changes in operating assets and liabilities, net of effects from acquisitions: | ||||||||||||||||||
Accounts receivable | (14.9 | ) | 5.8 | 22.0 | (31.3 | ) | ||||||||||||
Inventories | 5.7 | (12.0 | ) | 7.9 | (69.7 | ) | ||||||||||||
Prepaid expenses | 25.2 | — | — | — | ||||||||||||||
Accounts payable | 13.4 | (1.6 | ) | 2.8 | 4.0 | |||||||||||||
Accrued (refundable) income taxes | (17.8 | ) | — | 11.6 | — | |||||||||||||
Accrued interest | 80.9 | — | — | — | ||||||||||||||
Other | (53.0 | ) | 46.7 | 12.3 | 21.4 | |||||||||||||
Net cash from operating activities | 188.9 | 59.4 | 439.8 | 413.4 | ||||||||||||||
Cash flows from (used in) investing activities: | ||||||||||||||||||
Net proceeds (purchases) from sale and purchase of investments | 84.7 | 42.8 | (64.7 | ) | (12.8 | ) | ||||||||||||
Capital expenditures | (167.9 | ) | (22.0 | ) | (142.5 | ) | (108.9 | ) | ||||||||||
Acquisitions, net of cash acquired | (0.4 | ) | (9.8 | ) | — | — | ||||||||||||
Acquisition of Biomet, Inc. | (11,638.2 | ) | — | — | — | |||||||||||||
Other | — | — | (6.5 | ) | 1.0 | |||||||||||||
Net cash from (used in) investing activities | (11,721.8 | ) | 11.0 | (213.7 | ) | (120.7 | ) | |||||||||||
Cash flows from (used in) financing activities: | ||||||||||||||||||
Debt: | ||||||||||||||||||
Decrease in short-term borrowings | (51.0 | ) | — | (196.8 | ) | (2.7 | ) | |||||||||||
Proceeds (payments) under amended revolving credit agreement | (134.6 | ) | 0.2 | — | — | |||||||||||||
Payments under senior secured credit facility | (18.3 | ) | — | — | — | |||||||||||||
Proceeds from long-term debt—merger | 6,250.7 | — | — | — | ||||||||||||||
Payment of deferred financing costs | (87.1 | ) | — | — | — | |||||||||||||
Equity: | — | — | — | |||||||||||||||
Capital contributions | 5,521.9 | — | — | — | ||||||||||||||
Issuance of common shares | — | — | 23.1 | 23.0 | ||||||||||||||
Cash dividends | — | — | (73.5 | ) | (62.5 | ) | ||||||||||||
Purchase of common shares | — | (2.8 | ) | (7.3 | ) | (215.3 | ) | |||||||||||
Excess tax benefit from exercise of stock options | — | 3.9 | 3.2 | — | ||||||||||||||
Net cash from (used in) financing activities | 11,481.6 | 1.3 | (251.3 | ) | (257.5 | ) | ||||||||||||
Effect of exchange rate changes on cash | 2.0 | 0.1 | 4.3 | (0.6 | ) | |||||||||||||
Increase (decrease) in cash and cash equivalents | (49.3 | ) | 71.8 | (20.9 | ) | 34.6 | ||||||||||||
Cash and cash equivalents, beginning of period | 176.9 | 105.1 | 126.0 | 91.4 | ||||||||||||||
Cash and cash equivalents, end of period | $ | 127.6 | $ | 176.9 | $ | 105.1 | $ | 126.0 | ||||||||||
Supplemental disclosures of cash flow information: | ||||||||||||||||||
Cash paid during the period for: | ||||||||||||||||||
Interest | $ | 387.3 | $ | — | $ | 9.4 | $ | 11.3 | ||||||||||
Income taxes | $ | 52.0 | $ | — | $ | 188.8 | $ | 216.4 | ||||||||||
The accompanying notes are a part of the consolidated financial statements.
F-7
Table of Contents
Biomet, Inc. and Subsidiaries Notes to Consolidated Financial Statements
Note 1—Merger.
On December 18, 2006, Biomet, Inc. (“Biomet” or “Company”) entered into an Agreement and Plan of Merger with LVB Acquisition, LLC, a Delaware limited liability company (“LVB”), and LVB Acquisition Merger Sub, Inc., an Indiana corporation and a wholly-owned subsidiary of LVB (“Purchaser”), which agreement was amended and restated as of June 7, 2007 (the “Merger Agreement”). LVB is controlled by a consortium of private equity funds: Blackstone Capital Partners V L.P., Goldman Sachs Investments Ltd., KKR 2006 Fund L.P. and Texas Pacific Group (each a “Sponsor” and collectively, the “Sponsors”). The Sponsors, along with other investors contributed $5,387.5 million of equity in connection with the Transactions (as defined below).
Pursuant to the Merger Agreement, on June 13, 2007, Purchaser commenced a cash tender offer (the “Offer” and together with the Merger, the “Transactions”), to purchase all of Biomet’s outstanding common shares, without par value (the “Shares”), at a price of $46.00 per share (the “Offer Price”), without interest and less any required withholding taxes. The Offer expired at 12:00 midnight, New York City time, on July 11, 2007, with 82.4% of the outstanding Shares having been tendered to Purchaser. On July 17, 2007, Purchaser completed its purchase of the tendered shares for $9,319.7 million.
In connection with the closing of the Offer, all outstanding options to purchase shares under Biomet’s stock plans (each an “Option”), vested or unvested, were cancelled and each Option holder was paid an amount in cash equal to the excess, if any, of the Offer Price over the applicable option exercise price for each share subject to an Option, less any required withholding taxes.
In connection with the Offer, Purchaser entered into a credit agreement dated July 11, 2007 (“Merger Date”) for a $6,165.0 million senior secured term loan facility (the “Tender Facility”), maturing on June 6, 2008, and pursuant to which it borrowed $4,181.0 million to finance a portion of the Offer and pay related fees and expenses. Additional financing for the Offer was provided in the form of equity contributions from the Sponsors, which collectively caused $5,197.0 million to be contributed as equity to LVB Acquisition Holding, LLC, (“Holding”), concurrently with the funding of the Tender Facility. Holding, which owned 100% of the outstanding equity interests in LVB at the time of the Offer, contributed such funds to LVB, which in turn contributed such funds to Purchaser.
As a result of Purchaser having acquired 82.4% of the outstanding shares pursuant to the Offer, Biomet called a special meeting of shareholders to vote upon the Merger. At this meeting, LVB and Purchaser voted all of their shares to approve the Merger. At the effective time of the Merger (the “Effective Time”), each share, other than the shares owned by LVB or Purchaser immediately prior to the Effective Time, were cancelled automatically, ceased to exist and converted into the right to receive the Offer Price, without interest and less any required withholding taxes. Additional funds necessary to complete the Merger were funded using equity contributions by certain of Biomet’s directors and equity contribution or rollover of existing equity interests by certain of Biomet’s executive officers and members of Biomet’s senior management, an offering of high-yield debt securities, initial borrowings under Biomet’s new $2,340.0 million senior secured term loan facility, €875 million euro-denominated senior secured term loan facility, $400.0 million senior secured cash flow revolving facility, and $350.0 million asset based revolving credit facility, proceeds from offering of $775.0 million senior cash pay notes due 2017, $775.0 million senior toggle notes due 2017, $1,015.0 million senior subordinated notes due 2017, its cash on hand and additional equity contributions by the Sponsors.
On September 5, 2007, Biomet’s shareholders approved the Merger Agreement and on September 25, 2007, LVB completed the acquisition of Biomet through the merger of Purchaser with and into Biomet. As a result of the Merger, Biomet became a 99.9% subsidiary of LVB. The remaining 0.1% was a purchase of common stock by Company management as a result of the LVB Acquisition Management Shareholder Agreement.
In the Merger, each share, other than the shares owned by LVB or Purchaser immediately prior to the effective time of the Merger, was converted into the right to receive $46.00 per share, without interest and less any required withholding taxes. The aggregate consideration paid by the Purchaser was $11.6 billion, consisting of Company common stock valued at $11.3 billion (100% ownership), $57.0 million of cash, and $344.0 million of fees and expenses.
On June 13, 2007 the Company filed with the Securities and Exchange Commission a Solicitation/Recommendation Statement on Schedule 14D-9 (the “Schedule 14D-9”) pursuant to the Securities Exchange Act of 1934, as amended. In the Schedule 14D-9 the Company disclosed the Board of Directors’ unanimous recommendation that shareholders tender their shares of common stock into the Offer, or otherwise vote to approve the Merger.
The primary reason for the acquisition is to support the Company’s initiative to enhance its position as a leading orthopedic medical device company by pursuing the following strategic initiatives: continue to develop and launch new products and technologies, enhance surgeon customer relationships through product performance and innovation, expand its global reach, focus on operational efficiency and maximize free cash flows.
The Merger was accounted for under the purchase method of accounting pursuant to Statements of Financial Accounting Standards (SFAS) 141, Business Combinations . Accordingly, the effect of the Merger has been included in the Company’s consolidated statement of operations subsequent to the Merger Date, and the respective assets and liabilities have been recorded at their estimated fair values in the Company’s consolidated balance sheet as of the Merger Date, with the excess purchase price recorded as goodwill.
As of July 12, 2007, the effective date of the Merger, the Successor Company began operating under a new basis of accounting for its financial statements. Because of the new basis of accounting, the Predecessor Company’s historical financial information is not comparable to the Successor Company’s financial information for periods after July 11, 2007.
The Company has preliminarily allocated the purchase price to the fair value of the assets and liabilities of Biomet based on estimated fair values utilizing generally accepted valuation methodologies. Both assets and liabilities were valued as of July 11, 2007. On July 11, 2007 82.4% of the step up was recorded and combined with 17.6% of the Predecessor company. On September 25, 2007 the remaining fair value step-up of 17.6% was recorded. See summary below of the allocation of the purchase price:
| (In millions) | ||||
Cash and cash equivalents | $ | 57.0 | ||
Short-term investments | 126.0 | |||
Accounts receivable | 494.0 | |||
Inventories | 714.3 | |||
Deferred tax assets | 60.6 | |||
Prepaids and other assets | 134.4 | |||
Property, plant and equipment | 608.0 | |||
In-process research and development | 479.0 | |||
Intangible assets | 6,304.5 | |||
Goodwill | 5,303.0 | |||
Deferred tax liabilities | (2,184.9 | ) | ||
Other liabilities | (463.0 | ) | ||
Purchase Price | $11,632.9 | |||
F-8
Table of Contents
The preliminary purchase price allocation was based on information currently available to the Company, and expectations, assumptions, and valuation methodologies deemed reasonable by the Company’s management. No assurance can be given, however, that the underlying assumptions used to estimate expected technology based product revenues, development costs or profitability, or the events associated with such technology, will occur as projected. For these reasons, among others, the actual results may vary from the projected results. Goodwill recorded as a result of the Merger is not deductible for income tax purposes. The final valuation and associated purchase price allocation is expected to be completed as soon as possible, but no later than one year from the completion of the acquisition. The primary area of the purchase price allocation that is not yet finalized relates to income taxes. To the extent that the estimates need to be adjusted, the Company will do so.
In-process research and development (IPRD) products are at a stage of development that require further research and development to determine technical feasibility and commercial viability. IPRD valued in the amount of $479.0 million pertains to technology that was not technologically feasible at the date of acquisition and had no future alternative use. The fair value of the IPRD was determined based on the excess earnings method of projected revenues. The fair value was allocated to all business units which involve our four product segments: reconstructive, fixation, spinal, and other products. The significant assumptions made by management and used in the model were revenue projections for each project, project timing, discount rates used, and the related costs to complete each project. The IPRD does not have any alternative future use and did not otherwise qualify for capitalization. As a result, this amount was expensed upon acquisition.
IPRD projects for Biomet Orthopedics focus on the utilization of new materials, new methods for fabricating existing materials, and new geometries of both new and existing materials to enhance function, durability and bony fixation for orthopedic implant devices primarily focused in the area of partial and total joint replacement. IPRD projects also focus in the area of innovative methods for surgically implanting orthopedic implant devices. Orthopedics had 43 projects in development as of July 11, 2007. Certain projects were completed by May 31, 2008, with remaining projects having general anticipated completion dates ranging from the first quarter of fiscal 2009 to the third quarter of fiscal 2010. The estimated costs to complete these IPRD projects for Biomet Orthopedics as of the date of the acquisition were $51.0 million. IPRD projects for Biomet Orthopedics averaged 30% completion as of the acquisition date.
IPRD projects for Biomet Trauma and Biomet Spine (BTBS) are primarily related to addressing unmet needs in the musculoskeletal market utilizing both traditional and new technologies. BTBS had 47 projects in development as of July 11, 2007. Certain projects were completed by May 31, 2008, with remaining projects having general projected completion dates ranging from the first quarter of fiscal 2009 through the second quarter of fiscal 2010. The estimated costs to complete these IPRD projects for BTBS as of the date of the acquisition were $33.0 million. IPRD projects for BTBS averaged 75% completion as of the acquisition date.
IPRD projects for Biomet Europe focus primarily on improvements to joint replacement implants, such as wear resistant bearing combinations for hip replacement, total and partial knee prostheses with improved kinematic performance, novel shoulder implants for improved stability and range of motion and development of instrumentation with improved accuracy and ergonomics. Biomet Europe had 85 projects in development as of July 11, 2007. Certain projects were completed by May 31, 2008, with remaining projects having anticipated completion dates ranging from the first quarter of fiscal 2009 to the second quarter of fiscal 2013. The estimated costs to complete these IPRD projects for Europe as of the date of the acquisition were $15.0 million. IPRD projects for Biomet Europe averaged 50% completion as of the acquisition date.
IPRD projects for Biomet Biologics focus primarily on producing new devices and applications to use autologous materials for regenerative tissue therapies. Biologics had 12 projects in development as of July 11, 2007. Certain projects were completed by May 31, 2008, with remaining projects having anticipated completion dates ranging from the third quarter of fiscal 2009 through the fourth quarter of fiscal 2011. The estimated costs to complete these IPRD projects for Biologics as of the date of the acquisition were $13.0 million. IPRD projects for Biologics averaged 50% completion as of the acquisition date.
IPRD projects for Biomet Sports Medicine focus on the utilization of new technologies, materials and devices to primarily treat soft tissues defects in tendons, ligaments and cartilage. This is accomplished through arthroscopic application of fixation devices and biomaterials. Sports Medicine had 16 projects in development as of July 11, 2007. The estimated costs to complete Sports Medicine’s IPRD as of the date of the acquisition were $1.0 million. The projects averaged 50% completion as of the date of the acquisition and have been fully completed by May 31, 2008.
IPRD projects for Biomet 3i focus on the development of intraoral rehabilitation, generally in the area of dental implants, associated components, surgical instrumentation and regenerative therapies necessary for the placement of the implants. Biomet 3i had 22 projects in development as of July 11, 2007. Certain projects were completed by May 31, 2008, with remaining projects having general projected completion dates ranging from the first quarter of fiscal 2009 through the second quarter of fiscal 2010. The estimated costs to complete Biomet 3i’s IPRD as of the date of the acquisition were $8.0 million. The projects were estimated to be 35% complete as of the acquisition date.
Pro forma Results
The following unaudited pro forma consolidated results of operations have been prepared as if the Merger had occurred at the beginning of each of fiscal year 2008 and 2007. The selected unaudited pro forma consolidated results of operations presented below reflect the purchase method of accounting and have been adjusted for the estimated changes in depreciation and amortization expense on acquired tangible and intangible assets. Interest expense and interest income have been adjusted to coincide with the post acquisition cash and debt balances of the combined Company. Income taxes have also been adjusted to reflect an estimated annual effective tax rate. The pro forma information has not been adjusted for any operating synergies or other anticipated cost savings that may result from the merger. As a result, these unaudited pro forma consolidated results of operations may not be indicative of the historical results that may have been achieved had the companies been combined during the periods presented and is not intended to be a projection of future results.
(in millions, unaudited) | Year Ended May 31, 2008 | Year Ended May 31, 2007 | ||||||||
Revenue | $ | 2,383.3 | $ | 2,107.4 | ||||||
Income before provision (benefit) for income taxes | $ | (1,374.2 | ) | $ | (1,169.4 | ) | ||||
Net income (loss) | $ | (1,081.2 | ) | $ | (736.7 | ) | ||||
The unaudited pro forma consolidated results of operations for the year ended May 31, 2008 includes nonrecurring items including, in-process research and development, financing fees related to the Merger, additional cost of sales due to the inventory step-up, costs to settle in-the-money stock options as a result of the Merger and the tax effect of such items. Goodwill established in the Merger is not tax deductible.
F-9
Table of Contents
Note 2—Summary of Significant Accounting Policies and Nature of Operations.
General—The Company is one of the largest orthopedic medical device companies in the United States and worldwide with operations in over 50 locations throughout the world and distribution in approximately 90 countries. The Company designs, manufactures and markets a comprehensive range of both surgical and non-surgical products used primarily by orthopedic surgeons and other musculoskeletal medical specialists. For approximately 30 years, the Company has applied advanced engineering and manufacturing technology to the development of highly durable joint replacement systems.
Products—The Company operates in one business segment, musculoskeletal products, which include the design, manufacture and marketing of products in four major market categories: Reconstructive Products, Fixation Devices, Spinal Products and Other Products. The Company has three reportable geographic segments: United States, Europe and International.
Reconstructive—Reconstructive products include implants and instrumentation for replacing knees and hips as well as extremity joints that have deteriorated due to disease (principally osteoarthritis) or injury. This category also includes our dental reconstructive business, which includes implants and abutments, augmented by a growing line of our other reconstructive products such as regenerative products, accessories and biologic products.
Fixation—Fixation devices are used for setting and stabilizing damaged bones to support and/or augment the body’s natural healing process. Electrical stimulation devices used in trauma indications offer implantable and non-invasive options to stimulate bone growth. Other products include internal fixation devices (such as nails, plates, screws, pins and wires used to stabilize traumatic bone injuries), external fixation devices (used to stabilize fractures when alternative methods of fixation are not suitable), craniomaxillofacial fixation systems and bone substitute materials.
Spinal—Spinal products include devices and instrumentation for repairing defects or wear and tear in the vertebral column. Key products in this category include implantable and non-invasive electrical stimulation devices for spinal indications (used to enhance bone fusion success), spinal fixation systems used to stabilize the spine, bone substitute materials and allograft services used in spinal fusion procedures, as well as motion preservation systems.
Other—The Company manufactures and distributes a number of other products, including sports medicine products (used in minimally-invasive orthopedic surgical procedures), orthopedic support products (also referred to as softgoods and bracing products), operating room supplies, casting materials, general surgical instruments, wound care products and other surgical products.
Basis of Presentation—The accompanying consolidated financial statements include the accounts of Biomet, Inc. and its subsidiaries (individually and collectively referred to as the “Company” or “Biomet”). The consolidated financial statements include all accounts of Biomet and all of its wholly-owned subsidiaries. The consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America. The Company’s financial position as of May 31, 2008 and results of operations for the period July 12, 2007 through May 31, 2008 are not comparative to the Company’s financial position and results of operations for periods prior to July 12, 2007 because of the new basis of accounting resulting from the Transaction. Minority interest created as a result of the merger for the period July 12, 2007 to September 25, 2007 was not material. Also the Company eliminated a one month reporting lag with its foreign subsidiaries as of the acquisition date.
Translation of Foreign Currency—Assets and liabilities of foreign subsidiaries are translated at rates of exchange in effect at the close of their fiscal period. Revenues and expenses are translated at the weighted average exchange rates during the period. Translation gains and losses are accumulated within other comprehensive income (loss) as a separate component of shareholders’ equity. Foreign currency transaction gains and losses resulting from product transfer between subsidiaries are recorded in cost of goods sold. Other foreign currency exchange gains and losses that do not involve the movement of product and are not material, are included in other income (expense), net.
Cash and Cash Equivalents—The Company considers all highly liquid investments with original maturities of three months or less at the acquisition date to be cash equivalents.
Investments—The Company invests the majority of its excess cash in bank deposits and money market securities. The Company also holds municipal, corporate and mortgage-backed securities, common stocks and auction-rate securities. The Company does not believe it is exposed to any significant credit risk on its cash and cash equivalents or investments. The Company accounts for its investments in debt and equity securities under SFAS 115, Accounting for Certain Investments in Debt and Equity Securities , which requires certain securities to be categorized as trading, available-for-sale or held-to-maturity. Available-for-sale securities are carried at fair value with unrealized gains and losses recorded within other comprehensive income (loss) as a separate component of shareholders’ equity. Held-to-maturity securities are carried at amortized cost. The Company has no trading securities. The cost of investment securities sold is determined by the specific identification method. Dividend and interest income are accrued as earned. The Company reviews its investments quarterly for declines in market value that are other-than-temporary. Investments that have declined in market value that are determined to be other-than-temporary are charged to other income by writing that investment down to market value. Investments are classified as short-term for those expected to mature or be sold within twelve months and the remaining portion is classified in long-term investments.
Risk Management
Foreign Currency Instruments—Certain assets, liabilities and forecasted transactions are exposed to foreign currency risk, primarily the fluctuation of the U.S. Dollar against European currencies. The Company faces transactional currency exposures that arise when its foreign subsidiaries (or the Company itself) enter into transactions, primarily on an intercompany basis, denominated in currencies other than their functional currency. The Company also faces currency exposure that arises from translating the results of its global operations to the U.S. Dollar at exchange rates that have fluctuated from the beginning of the period. The Company has hedged a portion of its net investment in European subsidiaries with the issuance of a €875.0 million principal amount term loan on September 25, 2007. The Company’s net investment in European subsidiaries at the hedging date of September 25, 2007 was $1,690.0 million (€1,238.0 million). As of May 31, 2008 the difference between the net investment and the currently outstanding principal amount of €367.0, remained unhedged. Effectiveness is tested quarterly to determine whether hedge treatment is still appropriate. The Company tests effectiveness on this net investment hedge by determining that the net investment in our European subsidiaries is greater than the outstanding debt balance. Any ineffectiveness is recorded through the income statement.
Interest Rate Instruments—The Company entered into interest rate swap agreements (cash flow hedges) in both U.S. Dollars and Euro on September 25, 2007 and March 25, 2008 as a means of fixing the interest rate on portions of our floating-rate debt instruments. See the table below for existing contracts:
Structure | Currency | Notional Amount | Termination Date | Fair Value at May 31, 2008 Asset (Liability) | |||||||
1 year | Euro | € | 50.0 | September 25, 2008 | $ | 0.1 | |||||
2 year | Euro | 75.0 | September 25, 2009 | 0.8 | |||||||
3 year | Euro | 75.0 | September 25, 2010 | 1.0 | |||||||
| Euro | 50.0 | March 25, 2011 | 1.7 | ||||||||
4 year | Euro | 75.0 | September 25, 2011 | 1.0 | |||||||
| Euro | 40.0 | March 25, 2012 | 1.5 | ||||||||
5 year | Euro | 230.0 | September 25, 2012 | 2.5 | |||||||
| Euro | 40.0 | March 25, 2013 | 1.7 | ||||||||
1 year | USD | $ | 130.0 | September 25, 2008 | (1.0 | ) | |||||
2 year | USD | 195.0 | September 25, 2009 | (4.9 | ) | ||||||
| USD | 150.0 | March 25, 2010 | 2.6 | ||||||||
3 year | USD | 195.0 | September 25, 2010 | (6.0 | ) | ||||||
| USD | 110.0 | March 25, 2011 | 3.1 | ||||||||
4 year | USD | 195.0 | September 25, 2011 | (8.1 | ) | ||||||
| USD | 140.0 | March 25, 2012 | 4.9 | ||||||||
5 year | USD | 585.0 | September 25, 2012 | (27.5 | ) | ||||||
| USD | 190.0 | March 25, 2013 | 7.3 | ||||||||
Total | $ | (19.3 | ) | ||||||||
F-10
Table of Contents
The interest rate swap liability at May 31, 2008 was $19.3 million and is included in other current liabilities. As a result of cash flow hedge treatment being applied, all unrealized gains and losses related to the derivative instrument are included in other comprehensive income. Effectiveness is tested quarterly to determine if hedge treatment is still appropriate. Those unrealized gains and losses recorded in other comprehensive income would be required to be reclassified to the statement of operations if at any time the contracts are deemed ineffective, upon maturity of the contracts, or calling the contracts early. * The Company did not enter into derivative instruments prior to fiscal 2008.
Amount of ineffectiveness recognized in operations was not material for any period presented.
Comprehensive Income—Total comprehensive income combines reported net income (loss) and foreign currency translation adjustments, unrealized appreciation/depreciation of available-for-sale securities, unrealized gain and losses related to the net investment in the Euro term loan, and unrecognized actuarial loss on pension assets and interest rate swap derivatives. Total other comprehensive income (loss) and the related components are included in the table below:
| As of May 31, 2008 (Successor) | As of May 31, 2007 (Predecessor) | |||||||||
Other comprehensive income (loss) (net of tax): | ||||||||||
Unrecognized actuarial gain (loss) on pension assets | $ | 1.6 | $ | (16.6 | ) | |||||
Foreign currency gains | 267.1 | 63.2 | ||||||||
Unrealized loss on interest rate swaps, net of tax | (12.1 | ) | — | |||||||
Unrealized loss on available-for-sale securities | (3.8 | ) | (0.6 | ) | ||||||
Total | $ | 252.8 | $ | 46.0 | ||||||
Concentrations of Credit Risk and Allowance for Doubtful Receivables—The Company provides credit, in the normal course of business, to hospitals, private and governmental institutions and healthcare agencies, insurance providers and physicians. The Company maintains an allowance for doubtful receivables based on estimated collection rates and charges actual losses to the allowance when incurred. The estimated collection rates require management judgment.
Fair Value of Financial Instruments—The carrying amounts of cash and cash equivalents, investments, receivables, short-term borrowings, derivative instruments, and variable and fixed rate debt that meet the definition of a financial instrument approximate fair value.
Other loss contingencies—We have a self-insured retention against product liability claims with insurance coverage over and above the retention. There are various other claims, lawsuits, disputes with third parties, investigations and pending actions involving various allegations against us. Product liability claims are routinely reviewed by our insurance carrier and management routinely reviews all claims for purposes of establishing ultimate loss estimates. In addition, management must determine the estimated liability for claims incurred, but not reported. Such estimates and any subsequent changes in estimates may result in adjustments to our operating results in the future.
Revenue Recognition—The Company sells product through four principle channels: (1) direct to healthcare institutions, referred to as direct channel accounts, (2) through stocking distributors and healthcare dealers, (3) indirectly through insurance companies and (4) directly to dental practices and dental laboratories. Sales through the direct and distributor/dealer channels account for a majority of net sales. Through these channels, inventory is generally consigned to sales agents or customers so that products are available when needed for surgical procedures. Revenue is not recognized upon the placement of inventory into consignment as we retain title and maintain the inventory on our balance sheet; however, it is recognized upon implantation and receipt of proper purchase order and/or purchase requisition documentation. Pricing for products is generally predetermined by contracts with customers, agents acting on behalf of customer groups or by government regulatory bodies, depending on the market. Price discounts under group purchasing contracts are generally linked to volume of implant purchases by customer healthcare institutions within a specified group. At negotiated thresholds within a contract buying period, price discounts may increase. At certain locations the Company records a contractual allowance that is offset against revenue for each sale to a non-contracted payor so that revenue is recorded at the estimated determinable price at the time of the sale. Revenue is recognized on sales to stocking distributors, healthcare dealers, dental practices and dental laboratories when title to product passes to them, generally upon shipment. Certain subsidiaries allow customers to return product in the event that we terminate the relationship. Under those circumstances, we record an estimated sales return in the period in which constructive notice of termination is given to a distributor. Product returns were not significant for the periods June 1, 2007 to July 11, 2007, July 12, 2007 to May 31, 2008, and the years ended May 31, 2007 and 2006.
F-11
Table of Contents
Advertising—Advertising costs are expensed as incurred. Advertising costs included in selling, general and administrative expenses were $0.6 million, $11.5 million, $10.1 million and $15 million, for the period June 1, 2007 to July 11, 2007, for the period July 12, 2007 to May 31, 2008, and for the fiscal years ended May 31, 2007 and 2006, respectively.
Research and Development—Research and development costs are charged to expense as incurred. In-process research and development (IPRD) is recognized in business combinations or asset acquisitions for the portion of the purchase price allocated to the appraised value of in-process technologies, defined as those technologies relating to products that have not received approval of the U.S. Food and Drug Administration and have no alternative future use, consistent with SFAS 2, Accounting for Research and Development Costs , and Financial Accounting Standards Board Interpretation 4, Applicability of SFAS 2 to Business Combinations .
Income Taxes—The Company records income tax estimates in accordance with SFAS 109, Accounting for Income Taxes; however, there are inherent risks that could create uncertainties related to the estimates. The Company adjusts estimates based on normal operating circumstances and conclusions related to tax audits. The Company does not believe any audit finding could materially affect its financial position; however, there could be a material impact on our consolidated results of operations and cash flows of a given period.
Effective June 1, 2007, the Company adopted FASB Interpretation 48, Accounting for Uncertainty in Income Taxes—an interpretation of FASB Statement 109 (“FIN 48”). FIN 48 addresses the determination of whether tax benefits claimed or expected to be claimed on a tax return should be recorded in the financial statements. Under FIN 48, the tax benefits from an uncertain tax position may be recognized only if it is more likely than not that the tax position will be sustained upon examination by the taxing authorities, based on the technical merits of the position. FIN 48 also provides guidance on derecognition, classification, interest and penalties on income taxes, accounting in interim periods and requires increased disclosures. FIN 48 requires significant judgment in determining what constitutes an individual tax position as well as assessing the outcome of each tax position.
Management’s Estimates and Assumptions—In preparing the financial statements in accordance with accounting principles generally accepted in the United States of America, management must often make estimates and assumptions that affect the reported amounts of assets, liabilities, revenues, expenses and related disclosures at the date of the financial statements and during the reporting period. Some of those judgments can be subjective and complex. Consequently, actual results could differ from those estimates.
Change in Accounting Principle—As of the Merger date, the Company eliminated the one-month lag in reporting for certain subsidiaries in Europe and International. The elimination of the one-month lag is considered a change in accounting principle adopted in conjunction with the Merger and was applied prospectively. The effect of the elimination is not considered material to the consolidated financial statements as of May 31, 2008, and for the period from July 12, 2007 to May 31, 2008.
Recent Accounting Pronouncements
SFAS 141R—In December 2007, the Financial Accounting Standards Board (FASB) issued SFAS 141R (revised 2007), Business Combinations. SFAS 141R establishes principles and requirements for how the acquirer in a business combination recognizes and measures in its financial statements, the identifiable assets acquired, the liabilities assumed and any noncontrolling interest in the acquiree at the acquisition date at fair value. SFAS 141R determines what information to disclose to enable users of the financial statements to evaluate the nature and financial effects of the business combination. SFAS 141R applies prospectively to business combinations for which the acquisition date is on or after the beginning of the first annual reporting period beginning on or after December 15, 2008. Early adoption is not permitted.
SFAS 157—In September 2006, the FASB issued SFAS 157, Fair Value Measurements. SFAS 157 defines fair value, establishes a framework for measuring fair value in generally accepted accounting principles and expands disclosures about fair value measurements. SFAS 157 applies under other accounting pronouncements that require or permit fair value measurements and accordingly, does not require any new fair value measurements. SFAS 157 is effective for the Company’s fiscal year beginning June 1, 2008. In February 2008, the FASB issued FASB Staff Position (“FSP”) No. FAS 157-2, “Effective Date of FASB Statement No. 157,” which defers the effective date of Statement 157 for nonfinancial assets and nonfinancial liabilities to fiscal years beginning after November 15, 2008, which for us is our fiscal 2010. The Company does not expect the implementation of SFAS 157 to have a material impact on the consolidated financial statements. The Company does not expect the adoption of SFAS 157 to have a material impact to be immaterial to the consolidated financial statements.
SFAS 159—In February 2007, the FASB issued SFAS 159, Establishing the Fair Value Option for Financial Assets and Liabilities, to permit all entities to choose to elect to measure eligible financial instruments at fair value. SFAS 159 applies to fiscal years beginning after November 15, 2007, with early adoption permitted for an entity that has also elected to apply the provisions of SFAS 157. An entity is prohibited from retrospectively applying SFAS 159, unless it chooses early adoption. On June 1, 2008 the Company will adopt SFAS 159 and expect the impact to be immaterial to the consolidated financial statements.
SFAS 160—In December 2007, the FASB issued SFAS 160, Noncontrolling Interests in Consolidated Financial Statements—an amendment of ARB 51 . SFAS 160 establishes accounting and reporting standards that require noncontrolling interests to be reported as a component of equity, changes in a parent’s ownership interest while the parent retains its controlling interest be accounted for as equity transactions, and any retained noncontrolling equity investment upon the deconsolidation of a subsidiary be initially measured at fair value. This Statement is effective for fiscal years, and interim periods within those fiscal years, beginning on or after December 15, 2008. Earlier adoption is prohibited. The Company does not expect the adoption of SFAS No. 160 to have a material impact on its consolidated financial statements.
SFAS 161—In March 2008, the FASB issued SFAS 161, Disclosures about Derivative Instruments and Hedging Activities—an Amendment of FASB Statement No. 133 . This statement requires entities that utilize derivative instruments to provide qualitative disclosures about their objectives and strategies for using such instruments, as well as any details of credit-risk-related contingent features contained within derivatives. It also requires entities to disclose additional information about the amounts and location of derivatives located within the financial statements, how the provisions of SFAS No. 133 have been applied and the impact that hedges have on an entity’s financial position, financial performance and cash flows. This statement is effective for fiscal years and interim periods beginning after November 15, 2008, with early application encouraged. The Company does not expect the adoption of SFAS 161 to have a material impact on its consolidated financial statements.
Emerging Issues Task Force (EITF) 07-3—In June 2007, the FASB Emerging Issues Task Force issued EITF-07-3 Accounting for Nonrefundable Advance Payments for Goods or Services Received for Use in Future Research and Development Activities . The EITF provides guidance for entities that may make nonrefundable advance payments for goods or services that will be used in future research and development activities and whether the advance payment should be expensed when the advance payment is made or when the research and development activity has been performed. EITF 07-3 is effective for financial statements issued for fiscal years beginning after December 15, 2007. On June 1, 2008 the Company will adopt EITF 07-3 and expect the impact to be immaterial to the consolidated financial statements.
In December 2007, the FASB ratified EITF 07-1,“Accounting for Collaborative Agreements” (“EITF 07-1”). EITF 07-1 provides guidance regarding financial statement presentation and disclosure of collaborative arrangements, as defined, which includes arrangements the Company has entered into regarding development and commercialization of products. EITF 07-1 is effective for the Company as of April 1, 2009. The Company has not yet completed its evaluation of EITF 07-1, but does not currently believe that adoption will have a material impact on its consolidated financial statements.
F-12
Table of Contents
Note 3—Inventories.
Inventories are stated at lower of cost or market, with cost determined under the first-in, first-out method. The Company reviews inventory on hand and writes down excess and slow-moving inventory based on an assessment of future demand and historical experience. Inventories consisted of the following:
| May 31, 2008 (Successor) | May 31, 2007 (Predecessor) | |||||||
Raw materials | $ | 89.6 | $ | 77.7 | ||||
Work-in-process | 57.9 | 70.5 | ||||||
Finished goods | 155.9 | 214.7 | ||||||
Consigned distributor | 236.3 | 177.5 | ||||||
Inventories | $ | 539.7 | $ | 540.4 | ||||
Note 4—Property, Plant and Equipment.
Property, plant and equipment are carried at cost less accumulated depreciation. Depreciation is computed by the straight-line method over the estimated useful lives of 3 to 30 years. Related maintenance and repairs are expensed as incurred. In accordance with SFAS 144, Accounting for the Impairment or Disposal of Long-Lived Assets , the Company reviews property, plant and equipment for impairment whenever events or changes in circumstances indicate that the carrying value of an asset may not be recoverable. An impairment loss would be recognized when estimated undiscounted future cash flows relating to the asset are less than its carrying amount, with the amount of the loss equal to the excess of carrying cost of the asset over fair value. Depreciation on instruments is included within cost of sales. Property, plant and equipment consisted of the following:
| May 31, 2008 (Successor) | May 31, 2007 (Predecessor) | |||||||||
Land and land improvements | $ | 49.3 | $ | 28.2 | ||||||
Buildings and leasehold improvements | 125.5 | 170.2 | ||||||||
Machinery and equipment | 246.6 | 362.3 | ||||||||
Instruments | 323.9 | 221.2 | ||||||||
Construction in progress | 13.5 | — | ||||||||
Total property, plant and equipment | 758.8 | 781.9 | ||||||||
Accumulated depreciation | (117.9 | ) | (354.5 | ) | ||||||
Total property, plant, and equipment, net | $ | 640.9 | $ | 427.4 | ||||||
Note 5—Investments
At May 31, 2008, the Company’s investment securities were classified as follows:
| (Successor) | |||||||||||||
| Amortized Cost | Unrealized | Fair Value | |||||||||||
| Gains | Losses | ||||||||||||
Available-for-sale: | |||||||||||||
Debt securities | $ | 36.3 | $ | — | $ | (3.8 | ) | $ | 32.5 | ||||
Equity securities | 0.7 | 0.1 | — | 0.8 | |||||||||
Mortgage-backed securities | 5.9 | — | (0.1 | ) | 5.8 | ||||||||
Total available-for-sale | 42.9 | 0.1 | (3.9 | ) | 39.1 | ||||||||
Held-to-maturity: | |||||||||||||
Debt securities | 1.5 | — | — | 1.5 | |||||||||
Total held-to-maturity | 1.5 | — | — | 1.5 | |||||||||
Certificates of deposit | 0.7 | — | — | 0.7 | |||||||||
Total | $ | 45.1 | $ | 0.1 | $ | (3.9 | ) | $ | 41.3 | ||||
At May 31, 2007, the Company’s investment securities were classified as follows:
| (Predecessor) | |||||||||||||
| Amortized Cost | Unrealized | Fair Value | |||||||||||
| Gains | Losses | ||||||||||||
Available-for-sale: | |||||||||||||
Debt securities | $ | 128.4 | — | $ | (0.4 | ) | $ | 128.0 | |||||
Equity securities | 9.4 | $ | 0.9 | (0.1 | ) | 10.2 | |||||||
Mortgage-backed securities | 28.9 | — | (1.4 | ) | 27.5 | ||||||||
Total available-for-sale | 166.7 | 0.9 | (1.9 | ) | $ | 165.7 | |||||||
Held-to-maturity: | |||||||||||||
Debt securities | 3.0 | — | (0.1 | ) | 2.9 | ||||||||
Mortgage-backed obligations | 0.1 | — | — | 0.1 | |||||||||
Total held-to-maturity | 3.1 | — | (0.1 | ) | 3.0 | ||||||||
Certificates of deposit | 0.3 | — | — | 0.3 | |||||||||
Total | $ | 170.1 | $ | 0.9 | $ | (2.0 | ) | $ | 169.0 | ||||
F-13
Table of Contents
The Company had auction-rate securities (ARS) which were originally recorded in cash and cash equivalents at May 31, 2007 and 2006 and were reclassified to the appropriate short-term and long-term investment category. These reclassifications also impacted net proceeds (purchases) from the sale and purchase of investments within the investing section of the consolidated statements of cash flows for the years ended May 31, 2007 and 2006.
The net proceeds (purchases) from sales (purchases) of available-for-sale securities were $84.7 million, $42.8 million, $(64.7) million, and $(12.8) million for the periods July 12, 2007 to May 31, 2008, June 1, 2007 to July 11, 2007, and years ended May 31, 2007 and 2006, respectively. There were no sales of held-to-maturity securities for the periods June 1, 2007 to July 11, 2007, July 12, 2007 to May 31, 2008, and years ended May 31, 2007 and 2006. The cost of marketable securities sold is determined by the specific identification method. For the periods July 12, 2007 to May 31, 2008, June 1, 2007 to July 11, 2007, and years ended May 31, 2007 and 2006, net realized gains and (losses) on sales of available-for-sale securities were $0.3 million, $0.1 million, $2.4 million and $0.3 million. The Company’s investment securities at May 31, 2008 include $0.7 million of certificates of deposit, $34.3 million of debt securities, and $5.8 million of mortgage-backed securities, all maturing past one year, and $0.8 million of equity securities.
As of May 31, 2008, the Company held ARSs of $30.8 million. They are AAA rated securities with long-term nominal maturities secured by student loans, which are guaranteed by the U.S. Government. Each of these securities was subject to auction processes for which there were insufficient bidders on the scheduled rollover dates. The Company will not be able to liquidate any of its ARSs until a future auction is successful, a buyer is found outside of the auction process (a secondary market develops) or the notes are redeemed. These ARSs have been classified as long-term available-for-sale securities as of May 31, 2008 because of the inability to predict when the market will stabilize. A significant portion of these ARSs are held by the Company’s captive insurance company as part of their required capital. The securities continue to earn and be paid interest at the maximum contractual rate.
The Company has evaluated these securities for temporary or other-than-temporary impairment at May 31, 2008. In doing so, the Company has considered a variety of factors, including intent, liquidity factors, ability to generate alternative cash, other broker pricing, and internally-generated fair value analysis. Our conclusion is that the securities are temporarily impaired as of May 31, 2008 therefore a temporary impairment charge was taken to other comprehensive income in the amount of $3.2 million at May 31, 2008 leaving a balance of $30.8 million of ARS at May 31, 2008.
The Company reviews its impairments in accordance with SFAS 115, Accounting for Certain Investments in Debt and Equity Securities, Staff Accounting Bulletin Topic 5, Miscellaneous Accounting and Financial Accounting Standards Board Staff Position , SFAS 115-1 and 124-1, The Meaning of Other-Than-Temporary Impairment and Its Application to Certain Investments , to determine if the impairment is “temporary” or “other-than-temporary.” The Company reviews several factors to determine whether the losses are other-than-temporary, including but not limited to (1) the length of time each security was in an unrealized loss position, (2) the extent to which fair value was less than cost, (3) the financial condition and near term prospects of the issuer or insurer and, (4) the Company’s intent and ability to hold each security for a period of time sufficient to allow for any anticipated recovery in fair value.
Investment income (included in other income, net) consists of the following:
| July 12, 2007 to May 31, 2008 (Successor) | June 1, 2007 to July 11, 2007 (Predecessor) | Year Ended May 31, 2007 (Predecessor) | Year Ended May 31, 2006 (Predecessor) | ||||||||||||
Interest income | $ | 5.9 | $ | 1.3 | $ | 8.1 | $ | 6.9 | |||||||
Dividend income | 0.5 | 0.1 | 1.6 | 1.9 | |||||||||||
Net realized gains | (0.2 | ) | 0.6 | 9.1 | 5.6 | ||||||||||
Total | $ | 6.2 | $ | 2.0 | $ | 18.8 | $ | 14.4 | |||||||
The Company follows SFAS 142, Goodwill and Other Intangible Assets. Accordingly, goodwill, indefinite, and finite lived intangible assets are not amortized but are tested for impairment at least annually or more frequently if impairment indicators arise. The balance of goodwill as of May 31, 2008 and May 31, 2007 was $5,422.8 million and $448.4 million, respectively. The Company uses an accelerated method for amortizing customer relationship intangibles as the value for those relationships is greater at the beginning of their life.
The following table summarizes the changes in the carrying amount of goodwill:
(in millions)
| United States | Europe | International | Total | |||||||||
Balance at June 1, 2006 (Predecessor) | $ | 246.0 | $ | 190.7 | $ | 4.7 | $ | 441.4 | ||||
Goodwill acquired | — | 1.9 | — | 1.9 | ||||||||
Currency translation | — | 4.9 | 0.2 | 5.1 | ||||||||
Balance at May 31, 2007 (Predecessor) | 246.0 | 197.5 | 4.9 | 448.4 | ||||||||
Goodwill acquired through acquisition of Biomet, Inc. | 3,567.1 | 1,244.1 | 491.8 | 5,303.0 | ||||||||
Currency translation | — | 95.1 | 24.7 | 119.8 | ||||||||
Balance at May 31, 2008 (Successor) | $ | 3,567.1 | $ | 1,339.2 | $ | 516.5 | $ | 5,422.8 | ||||
Intangible assets consist of the following at May 31, 2008 and May 31, 2007:
| May 31, 2008 (Successor) | Net Carrying Amount | May 31, 2007 (Predecessor) | Net Carrying Amount | |||||||||||||||||||
| Gross Carrying Amount | Accumulated Amortization | Gross Carrying Amount | Accumulated Amortization | |||||||||||||||||||
Core Technology | $ | 2,080.6 | $ | (93.8 | ) | $ | 1,986.8 | — | — | — | ||||||||||||
Completed Technology | 720.4 | (47.5 | ) | 672.9 | $ | 53.7 | $ | (19.2 | ) | $ | 34.5 | |||||||||||
Product Trade Names | 178.0 | (8.5 | ) | 169.5 | 3.6 | (1.1 | ) | 2.5 | ||||||||||||||
Non-competes and other | — | — | — | 4.9 | (3.0 | ) | 1.9 | |||||||||||||||
Customer Relationships | 2,917.5 | (173.1 | ) | 2,744.4 | 16.5 | (8.3 | ) | 8.2 | ||||||||||||||
Sub-total | $ | 5,896.5 | $ | (322.9 | ) | $ | 5,573.6 | $ | 78.7 | $ | (31.6 | ) | $ | 47.1 | ||||||||
Corporate Trade Names | 408.0 | — | 408.0 | 27.5 | — | 27.5 | ||||||||||||||||
Currency translation | 233.0 | (6.4 | ) | 226.6 | — | — | — | |||||||||||||||
Total | $ | 6,537.5 | $ | (329.3 | ) | $ | 6,208.2 | $ | 106.2 | $ | (31.6 | ) | $ | 74.6 | ||||||||
F-14
Table of Contents
The weighted average useful life of the intangibles at May 31, 2008 is as follows:
| Weighted Average Useful Life | ||
Core Technology | 20 Years | |
Completed Technology | 14 Years | |
Product Trade Names | 18 Years | |
Customer Relationships | 19 Years | |
Corporate Trade Names | Indefinite life |
Expected amortization expense for the years ended May 31, 2009 through 2013 is $376.0 million, $373.9 million, $365.1 million, $357.2 million, and $348.9 million, respectively.
Bank Borrowing—In connection with the Merger, the Company entered into a credit agreement dated July 11, 2007 for a $6,165.0 million senior secured term loan facility, or the Tender Facility, maturing on June 6, 2008, and pursuant to which Purchaser borrowed $4,181.0 million to finance a portion of the Offer and pay related fees and expenses.
The Company refinanced all amounts borrowed under the Tender Facility at the closing of the Merger on September 25, 2007 (the “Closing Date”). On the Closing Date, the Company refinanced the Tender Facility with senior secured credit facilities (which included, term loan facilities, a cash flow revolving facility and an asset based revolving credit facility), senior notes, senior subordinated notes and unsecured bridge facilities. The senior secured cash flow facility and all of the notes are guaranteed by the Company subject to certain exceptions, and each of its existing and future wholly-owned domestic subsidiaries. The senior secured asset-based facility is guaranteed by the Company and secured, subject to certain exceptions by a first-priority security interest in substantially all of the Company’s assets and the assets of subsidiary borrowers that consist of all accounts receivable, inventory, cash, deposit accounts and certain related intangible assets. The facilities and notes bear interest at the rates set forth below. Interest is payable in cash, except with respect to the Company’s ability to elect to pay PIK (Payment-in-kind) interest, rather than cash interest, on the senior toggle notes through October 15, 2012 for any interest period other than the initial interest period. The terms and book value of each instrument at May 31, 2008 are:
| Maturity Date | Interest Rate | Currency | May 31, 2008 | Premium on Notes at May 31, 2008 | ||||||||
Debt Instruments | ||||||||||||
European facilities | Primarily | Euro | € | 29.9 | — | |||||||
| EuriBor + 1.40% | $ | 46.6 | ||||||||||
Term loan facility | March 25, 2015 | Libor + 3.00% | US Dollars | $ | 2,328.3 | — | ||||||
Term loan facility | March 25, 2015 | EuriBor + | Euro | € | 870.6 | — | ||||||
| 3.00% | $ | 1,355.2 | ||||||||||
Cash flow revolving credit facility | September 25, 2013 | Libor + 2.75% | US Dollars | — | — | |||||||
Cash flow revolving credit facility | September 25, 2013 | EuriBor + 2.75% | Euro | — | — | |||||||
Asset-based revolving credit facility | September 25, 2013 | Libor + 1.75% | US Dollars | — | — | |||||||
Senior cash pay notes | October 15, 2017 | 10% | US Dollars | $ | 775.0 | $ | 2.2 | |||||
Senior toggle notes | October 15, 2017 | 10 3/8%/11 1/8% | US Dollars | $ | 775.0 | $ | 1.2 | |||||
Senior subordinated notes | October 15, 2017 | 11 5/8% | US Dollars | $ | 1,015.0 | $ | 2.3 | |||||
A portion of the debt above is based on Libor and EuriBor rates which fluctuate regularly. As of May 31, 2008 the 3-month Libor and EuriBor were 2.68% and 4.86%, respectively. The term loan facilities require quarterly principal payments equal to one quarter percent of the original principal balance (equal payments each quarter) which commenced on the last business day of December 2007, and continue on the last business day of each calendar year quarter with the remaining outstanding principal due on the maturity date. On March 31, 2008 and December 31, 2007, the Company made required payments to both term loan facilities, $5.9 million for the U.S. denominated facility and $3.2 million for the Euro denominated facility. The cash flow and asset-based revolvers and the notes do not have terms for mandatory principal pay downs. To calculate the U.S. dollar equivalent for disclosure purposes, we used a currency conversion rate of 1 Euro to $1.5566, which represents the currency exchange rate from Euros to U.S. dollars on May 31, 2008 as published in The Wall Street Journal.
At May 31, 2008 and 2007, short-term borrowings consist of the following:
(in millions) | 2008 (Successor) | 2007 (Predecessor) | ||||||
Senior secured credit facilities | $ | 37.0 | — | |||||
Biomet Europe facilities | 38.4 | $ | 31.6 | |||||
Bank line of credit—Biomet Japan | — | 50.2 | ||||||
Total | $ | 75.4 | $ | 81.8 | ||||
F-15
Table of Contents
Summarized in the table below are our long-term obligations as of May 31, 2008:
| Total | 2009 | 2010 and 2011 | 2012 and 2013 | 2014 and thereafter | |||||||||||
| (in millions) | |||||||||||||||
Long-term debt (including current maturities) | $ | 6,300.8 | $ | 75.4 | $ | 74.0 | $ | 74.0 | $ | 6,077.4 | |||||
Note 8—Retirement and Pension Plans.
The Company has a defined contribution profit sharing plan which covers substantially all of the Team Members within the continental U.S. and allows participants to make contributions by salary reduction pursuant to Section 401(k) of the Internal Revenue Code. The Company currently matches up to 75% of the Team Member’s contribution up to a maximum of 5% of the Team Member’s compensation. The amounts expensed under this profit sharing plan for the periods June 1, 2007 to July 11, 2007 and July 12, 2007 to May 31, 2008 and for the years ended May 31, 2007, and 2006 were $0.9 million, $8.1 million, $4.9 million and $6.3 million, respectively.
The Company has an Employee Stock Bonus Plan for eligible Team Members of the Company and certain subsidiaries under which participants may elect to contribute up to 10% of their annual compensation and the Company may make discretionary contributions on behalf of participants in the form of cash or stock. The Company has historically contributed to the plan up to 3% of an eligible Team Member’s compensation in the form of stock. The amounts expensed under this plan for the years ended May 31, 2007, and 2006 were $5.8 million and $6.6 million, respectively. Amount expensed under the plan from June 1, 2007 to July 11, 2007 were nominal. Subsequent to the merger, the Company makes cash contributions to the plan rather than contributing stock. On March 31, 2007, the Company merged this plan into the existing 401(k) plan. This did not affect the funding of this plan.
The Company sponsors various retirement and pension plans, including defined benefit plans for some of its foreign operations. Many foreign employees are covered by government sponsored programs for which the direct cost to the Company is not significant. Retirement plan benefits are primarily based on the employee’s compensation during the last several years before retirement and the number of years of service. Some foreign subsidiaries have plans under which funds are deposited with trustees, annuities are purchased under group contracts or reserves are provided. The Company used May 31, 2008 for fiscal 2008 and April 30, 2007 for fiscal 2007 as the measurement date.
In September 2006, the FASB issued SFAS 158, Employers Accounting for Defined Benefit Pension and Other Postretirement Plans—an amendment of FASB Statements No. 87, 88, 106, and 132R, which requires an employer to fully recognize the over-funded or under-funded status of its pension and other postretirement benefit plans as an asset or liability in its financial statements. In addition, the Company is required to recognize as a component of other comprehensive income (loss) the actuarial gains or losses and the prior service costs and credits that arise during the period but are not immediately recognized as components of net periodic benefit costs. The Company adopted SFAS 158 effective May 31, 2007. The incremental effect of applying SFAS No. 158 was a $16.6 million reduction in shareholders’ equity, net of deferred taxes.
Net periodic benefit costs for the Company’s defined benefit plans for 2008, 2007 and 2006 include the following components:
(in millions) | July 12, 2007 to May 31, 2008 (Successor) | June 1, 2007 to July 11, 2007 (Predecessor) | Year Ended May 31, 2007 (Predecessor) | Year Ended May 31, 2006 (Predecessor) | ||||||||||||||
Net periodic benefit costs: | ||||||||||||||||||
Service costs | $ | 4.7 | $ | 0.6 | $ | 5.1 | $ | 3.7 | ||||||||||
Interest costs | 6.0 | 0.8 | 5.3 | 4.3 | ||||||||||||||
Expected return on plan assets | (4.8 | ) | (0.6 | ) | (4.5 | ) | (3.3 | ) | ||||||||||
Recognized actuarial losses | 1.2 | 0.1 | 1.3 | 1.4 | ||||||||||||||
Net periodic benefit costs | $ | 7.1 | $ | 0.9 | $ | 7.2 | $ | 6.1 | ||||||||||
The following table sets forth information related to the benefit obligation and the fair value of plan assets at May 31, 2008 and 2007 for the Company’s defined benefit retirement plans. The Company maintains no postretirement plans.
| May 31, 2008 (Successor) | May 31, 2007 (Predecessor) | |||||||||
Change in Benefit Obligation | ||||||||||
Projected benefit obligation—beginning of year | $ | 115.7 | $ | 98.9 | ||||||
Service costs | 5.3 | 5.1 | ||||||||
Interest costs | 6.8 | 5.3 | ||||||||
Plan participant contribution | 2.7 | 2.3 | ||||||||
Actuarial (gains)/losses | (10.0 | ) | (3.0 | ) | ||||||
Benefits paid from plan | (3.5 | ) | (2.6 | ) | ||||||
Effect of exchange rates | 0.7 | 9.7 | ||||||||
Projected benefit obligation—end of year | $ | 117.7 | $ | 115.7 | ||||||
Accumulated benefit obligation | $ | 77.0 | $ | 75.7 | ||||||
Change in Plan Assets | ||||||||||
Plan assets at fair value—beginning of year | $ | 74.1 | $ | 60.0 | ||||||
Actual return (loss) on plan assets | (1.1 | ) | 2.4 | |||||||
Company contribution | 6.7 | 5.9 | ||||||||
Plan participant contribution | 2.7 | 2.3 | ||||||||
Benefits paid from plan | (0.2 | ) | (2.6 | ) | ||||||
Effect of exchange rates | (3.7 | ) | 6.1 | |||||||
Plan assets at fair value—end of year | 78.5 | $ | 74.1 | |||||||
Funded status at end of year | 40.0 | $ | 41.6 | |||||||
F-16
Table of Contents
Amounts Recognized in the Company’s Balance Sheet consists of the following:
| May 31, 2008 (Successor) | May 31, 2007 (Predecessor) | |||||||||
Deferred income tax asset | $ | (8.9 | ) | $ | (8.4 | ) | ||||
Employee related obligations | 40.0 | 41.6 | ||||||||
Other comprehensive income (loss) | (1.6 | ) | (16.6 | ) | ||||||
| Year ended May 31, 2009 | |||
Amounts expected to be recognized in Net Periodic Cost in the coming year for the Company’s defined benefit retirement plans | |||
Amortization of net actuarial losses | $ | 1.0 | |
The weighted-average assumptions in the following table represent the rates used to develop the actuarial present value of the projected benefit obligation for periods presented and also the net periodic benefit cost for the following year.
| July 12, 2007 to May 31, 2008 (Successor) | June 1, 2007 to July 11, 2007 (Predecessor) | Year Ended | ||||||||||||
| May 31, 2007 (Predecessor) | May 31, 2006 (Predecessor) | |||||||||||||
Discount rate | 6.50 | % | 6.50 | % | 5.30 | % | 5.03 | % | ||||||
Expected long-term rate of return on plan assets | 6.55 | % | 6.55 | % | 6.72 | % | 7.17 | % | ||||||
Rate increase in compensation levels | 2.89 | % | 2.89 | % | 3.30 | % | 3.13 | % | ||||||
The projected future benefit payments from the Company’s defined benefit retirement plans are $3.1 million—2009, $3.2 million—2010, $3.3 million—2011, $3.5 million—2012, $3.6 million—2013 and $20.4 million—2014—2018. The Company expects to pay $7.1 million into the plans during fiscal year 2009. In certain countries, the funding of pension plans is not a common practice. Consequently, the Company has several pension plans which are not funded.
The Company’s retirement plan asset allocation at May 31, 2008 was 47% to equity securities, 45% to debt securities, 7% to real estate, and 1% to other. The Company’s retirement plan asset allocation at April 30, 2007 was 41% to equity securities, 41% to debt securities, 8% to real estate, and 10% to other.
Strategic asset allocations are determined by country, based on the nature of the liabilities and considering demographic composition of the plan participants (average age, years of service and active versus retiree status). The Company’s plans are considered non-mature plans and the long-term strategic asset allocations are consistent with these types of plans. Emphasis is placed on diversifying on a broad basis combined with currency matching the fixed income assets.
Note 9—Share-based Compensation and Stock Plans.
The Company adopted SFAS 123(R), Share-Based Payment, (SFAS 123R) to record share based payment expense on June 1, 2006 using the modified prospective method. SFAS 123(R) requires the fair value of all share-based payments to employees, including stock options, to be expensed based on their fair value over the required award service period. The Company’s share-based payments consist of stock options. For the Company’s non-employee distributors, share-based expense is recorded in accordance with Emerging Issues Task Force No. 96-18, Accounting for Equity Instruments That Are Issued to Other Than Employees for Acquisition, or in Conjunction with Selling, Goods or Services . Prior to the Merger, the Company’s Board of Directors modified certain stock options to change the exercise price to the fair market value on the date it was granted by adding a cash component paid in January 2008 for the difference from the original grant price to the amended grant price of $46.00 per share (related to predecessor options). In addition, on July 11, 2007, the Predecessor’s Company’s Board of Directors cancelled all outstanding stock options and paid the difference between the amended grant price and $46.00 per share (the offering price) in cash in conjunction with the Merger (see Note 1). The total amount expensed related to Predecessor company grants was $112.8 million, with amounts recorded as cost of sales, selling, general, and administrative, and research and development in our results of operations for the period June 1, 2007 to July 11, 2007.
Share-based compensation expense recognized for the period from June 1, 2007 to July 11, 2007 was $112.8 million and for the period from July 12, 2007 to May 31, 2008 was $25.8 million. Share-based compensation expense recognized for the year ended May 31, 2007 was $17.7 million.
The following table summarizes stock option activity for the periods July 12, 2007 to May 31, 2008, June 1, 2007 to July 11, 2007 and the year ended May 31, 2007.
| Stock Options | Weighted Average Exercise Price | |||||
Predecessor: | ||||||
Outstanding, June 1, 2006 | 9,162,956 | $ | 33.84 | |||
Granted | 2,832,903 | 33.49 | ||||
Exercised | (917,737 | ) | 26.13 | |||
Forfeitures/cancelled | (1,448,227 | ) | 34.75 | |||
Outstanding, May 31, 2007 | 9,629,895 | 34.34 | ||||
Granted | — | |||||
Exercised | (298,557 | ) | 35.99 | |||
Forfeitures/cancelled | (9,331,338 | ) | 34.00 | |||
Successor: | ||||||
Outstanding, July 12, 2007 | — | — | ||||
Granted | 31,855,000 | 10.00 | ||||
Forfeitures/cancelled | (59,000 | ) | 10.00 | |||
Outstanding, May 31, 2008 | 31,796,000 | $ | 10.00 | |||
F-17
Table of Contents
In November 2007, Parent authorized the issuance of approximately 37.5 million nonqualified stock options to key employees, directors, service providers, and consultants of Parent and its affiliates under the 2007 LVB Plan. Grants are consistent with the commitment to recognize and reward the recipients and to align their interests with its stakeholders. Stock options is granted with an exercise price equal to 100% of fair value of the underlying stock on the date of the grant and have 10-year terms. Vesting of these stock options are split into 3 categories: 1) Time Based Options: 50% of option grants generally vest ratably over 5 years, 2) Performance Based Options: 25% of stock option grants generally vest over 5 years, contingent upon the Company achieving certain EBITDA targets in each of those years, and 3) Accreting Exercise Price Options: 25% of stock options grants have exercise prices that will increase by 10% each year, and generally vest ratably over 5 years. The Company uses an attribution method to recognize compensation expense for stock options over the applicable vesting period.
The weighted average fair value of options granted during the period July 12, 2007 to May 31, 2008 and May 31, 2007 was $3.74 and $11.37, respectively. The Company estimates the fair value of each option primarily using the Black-Scholes option pricing model. Expected volatilities for fiscal 2008 grants are generally based on historical volatility of our competitors, stock. For stock options granted during the year ended May 31, 2007, expected volatility was derived based on historical volatility of the Company’s stock. The fiscal 2008 risk-free rate for periods within the expected life of the option is based on the U.S. Treasury yield curve in effect at the time of grant. The fiscal 2007 risk-free interest rate was determined using the implied yield currently available for zero-coupon U.S. Government issues with a remaining term equal to the expected life of the options. The Company did not declare dividends in fiscal 2008 and does not expect to declare dividends going forward, but rather invest excess cash in future operations. In fiscal 2007 a dividend yield is derived based on the historical dividend yield of the Company’s stock. In fiscal 2007 the expected term of the stock option was derived from historical employee exercise behavior.
The fair value estimates are based on the following weighted average assumptions:
| May 31, 2008 (Successor) | May 31, 2007 (Predecessor) | |||||||
Risk-free interest rate | 3.27 | % | 4.56 | % | ||||
Dividend yield | — | 0.90 | % | |||||
Expected volatility | 29.2 | % | 32.0 | % | ||||
Expected life in years | 6.21 | 5.41 |
The following table summarizes information about outstanding stock options as of May 31, 2008 and 2007, that were vested and those that were expected to vest, and that were currently exercisable:
| Outstanding Stock Options Already Vested and Expected to Vest | Options that are Exercisable | |||||||||||||||
| 2008 (Successor) | 2007 (Predecessor) | 2008 (Successor) | 2007 (Predecessor) | |||||||||||||
Number of outstanding options | 31,796,000 | 7,950,000 | — | 1,811,324 | ||||||||||||
Weighted average remaining contractual life | 6.4 years | 7.2 years | 5.2 years | 1 year | ||||||||||||
Weighted average exercise price per share | $ | 10.00 | $ | 34.34 | $ | 10.00 | $ | 34.02 | ||||||||
Intrinsic value | $ | — | $ | 73.8 | $ | — | $ | 17.4 | ||||||||
Options outstanding at May 31, 2008 and 2007, were exercisable at $10.00 and from $11.57 to $48.27, respectively, and had a weighted average remaining contractual life of 5.2 years and 7.2 years, respectively. At May 31, 2008 and 2007 there were 5,683,500 and 3,234,286 shares available for future option grants, respectively. The following table summarizes information about stock options outstanding at May 31, 2007.
Range of Exercise Price | Number Outstanding | Outstanding Weighted Average Remaining Contractual Life | Weighted Average Exercise Price | Number Exercisable | Weighted Average Exercise Price | |||||||
May 31, 2008 (Successor): | ||||||||||||
$ 10.00 | 31,796,000 | 5.2 years | $ | 10. 00 | — | $ | 10.00 | |||||
May 31, 2007: (Predecessor) | ||||||||||||
$ 11.57-20.00 | 18,346 | 1.0 year | 16.43 | 13,564 | 16.30 | |||||||
20.01-30.00 | 1,702,006 | 4.5 years | 26.05 | 610,763 | 25.62 | |||||||
30.01-40.00 | 6,209,455 | 8.1 years | 34.30 | 669,854 | 35.18 | |||||||
40.01-48.27 | 1,700,088 | 6.6 years | 42.97 | 517,143 | 42.89 | |||||||
| 9,629,895 | 1,811,324 | |||||||||||
Prior to adopting SFAS 123(R), Biomet classified all tax benefits of deductions resulting from the exercise of stock options as operating cash flows. SFAS 123(R) requires the cash flows resulting from excess tax benefits (i.e., tax deductions realized for stock options exercised in excess of the tax benefit recognized on the related share-based payment expense) to be classified as financing cash flows.
The predecessor Company had various stock option plans in place prior to the Merger date: the 1992 Employee and Non-Employee Director Stock Option Plan; the 1992 Distributor Stock Option Plan: the Biomet, Inc. 1998 Qualified and Non-Qualified Stock Option Plan, and the Biomet, Inc. 2006 Equity Incentive Plan. At May 31, 2007, the only plans with shares available for grant were the Biomet, Inc. 1998 Qualified and Non-Qualified Stock Option Plan and the Biomet, Inc. 2006 Equity Incentive Plan.
Under the stock option plans described above, options could be granted to key employees, non-employee directors and distributors, at the discretion of the Compensation Committee, and generally became exercisable in annual or biannual increments beginning one or two years after the date of grant in the case of employee options and in annual increments beginning at the date of grant for
F-18
Table of Contents
distributor options. In the case of options granted to an employee of the Company who was a 10% or more shareholder, the option price was an amount per share not less than 110% of the fair market value per share on the date of granting the option, as determined by the Compensation Committee. No options had been granted to employees who were 10% or more shareholders. The term of each option granted expired within the period prescribed by the Compensation Committee, but was not more than five years from the date the option was granted if the optionee was a 10% or more shareholder, and not more than ten years for all other optionees. All rights under the options automatically terminated upon the optionee’s separation from service with the Company, unless such separation results from retirement, disability or death. For the years ended May 31, 2007 and 2006, the amount of compensation expense applicable to options granted to distributors was not material to the consolidated financial statements. On the Merger date, all options granted under the plans described above were cancelled (See Note 1 above) and such plans no longer exist.
We also expect to grant LVB Options to our distributors, which are expected to be eligible to vest based on the achievement of specified sales targets. In 2008, the Board of Parent adopted an addendum to the 2007 LVB Plan, which provides for the grant of leveraged equity awards in Parent under the 2007 LVB Plan (the “LVB Leveraged Awards,” and together with the LVB Options, the “LVB Awards”) to certain of our European employees. LVB Leveraged Awards permit participants to purchase shares of LVB common stock using the proceeds of non recourse loans from Parent, which shares remain subject to forfeiture and other restrictions prior to the participant’s repayment of the loan.
Upon termination of a participant’s employment, the 2007 LVB Plan provides that any unvested portion of a participant’s LVB Award will be forfeited, and that the vested portion of his or her LVB Award will expire on the earlier of (1) the date participant’s employment is terminated for cause, (2) 30 days following the date the participant resigns without good reason, (3) 90 days after the date the participant’s employment is terminated either by us for any reason other than cause, death or disability or by the participant with good reason, (4) one year after the date the participant’s employment is terminated by reason of death or disability or (5) the tenth anniversary of the grant date of the LVB Award.
Prior to receiving shares of LVB common stock (whether pursuant to the exercise of LVB Options, purchased pursuant to an LVB Leveraged Award or otherwise), participants must execute a Management Stockholders’ Agreement , which provides that the shares are subject to certain transfer restrictions, put and call rights, and tag along and drag along rights (and, with respect to certain senior members of management, limited re offer registration and preemptive rights).
| Year Ended May 31, | |||||||||||||||||
| 2008 (Successor) | 2007 (Predecessor) | 2006 (Predecessor) | |||||||||||||||
| Number of Shares | Weighted Average Price Per Share | Number of Shares | Weighted Average Price Per Share | Number of Shares | Weighted Average Price Per Share | ||||||||||||
Options granted with an exercise price equal to fair value at date of grant | 31,855,000 | $ | 10.00 | 2,799,903 | $ | 33.49 | 1,100,845 | $ | 36.46 | ||||||||
Options granted with an exercise price greater than fair value at date of grant | — | — | — | — | 292,200 | $ | 34.85 | ||||||||||
Options granted with an exercise price less than fair value at date of grant | — | — | 33,000 | $ | 34.44 | 1,485,556 | $ | 34.02 | |||||||||
Note 10—Income Taxes (benefit).
The components of income (loss) before income taxes are as follows:
| Years Ended | ||||||||||||||||
(in millions) | July 12, 2007 - May 31, 2008 (Successor) | June 1, 2007 - July 11, 2007 (Predecessor) | May 31, 2007 (Predecessor) | May 31, 2007 (Predecessor) | ||||||||||||
Domestic | $ | (1,318.8 | ) | $ | (81.1 | ) | $ | 405.2 | $ | 531.3 | ||||||
Foreign | 124.5 | (0.8 | ) | 96.4 | 79.7 | |||||||||||
Total | $ | (1,194.3 | ) | $ | (81.9 | ) | $ | 501.6 | $ | 611.0 | ||||||
The provision for income taxes is summarized as follows:
(in millions) | July 12 - May 31, 2008 (Successor) | June 1 - July 11, 2007 (Predecessor) | May 31, 2007 (Predecessor) | May 31, 2006 (Predecessor) | ||||||||||||||
Current: | ||||||||||||||||||
Federal | $ | 0.2 | $ | (30.1 | ) | $ | 189.1 | $ | 170.6 | |||||||||
State | 0.3 | (4.2 | ) | 13.9 | 19.0 | |||||||||||||
Foreign | 45.1 | (0.4 | ) | 24.5 | 19.8 | |||||||||||||
Subtotal | 45.6 | (34.7 | ) | 227.5 | 209.4 | |||||||||||||
Deferred: | ||||||||||||||||||
Federal | (217.0 | ) | 6.5 | (52.1 | ) | (3.7 | ) | |||||||||||
State | (24.2 | ) | 0.9 | (7.5 | ) | (0.6 | ) | |||||||||||
Foreign | (34.5 | ) | — | (2.2 | ) | — | ||||||||||||
Subtotal | (275.7 | ) | 7.4 | (61.8 | ) | (4.3 | ) | |||||||||||
Total Income Tax Expense (Benefit) | $ | (230.1 | ) | $ | (27.3 | ) | $ | 165.7 | $ | 205.1 | ||||||||
F-19
Table of Contents
A reconciliation of the statutory federal income tax rate to the Company’s U.S. effective tax rate follows:
(in millions) | July 12 - May 31, 2008 (Successor) | June 1 - July 11, 2007 (Predecessor) | May 31, 2007 (Predecessor) | May 31, 2006 (Predecessor) | ||||||||||
U.S. statutory income tax rate | (35.0 | )% | (35.0 | )% | 35.0 | % | 35.0 | % | ||||||
State taxes, less effect of federal reduction | (1.8 | ) | (4.1 | ) | 1.1 | 1.7 | ||||||||
Foreign income taxes at rates different from the U.S. statutory rate | (0.2 | ) | 0.5 | (1.4 | ) | (0.9 | ) | |||||||
Tax benefit relating to operations in Puerto Rico | (0.2 | ) | (0.6 | ) | (1.1 | ) | (0.6 | ) | ||||||
Tax credits | (0.1 | ) | (0.1 | ) | (0.3 | ) | (0.3 | ) | ||||||
Tax benefit relating to U.S. export sales | — | — | (1.1 | ) | (1.2 | ) | ||||||||
In-process research and development | 14.0 | — | — | — | ||||||||||
Losses and other expenses not deductible for tax | 2.0 | 6.2 | — | — | ||||||||||
Tax on foreign earnings, net of foreign tax credits | 0.7 | — | — | — | ||||||||||
Other | 1.3 | (0.2 | ) | 0.8 | (0.1 | ) | ||||||||
Effective tax rate | (19.3 | )% | (33.3 | )% | 33.0 | % | 33.6 | % | ||||||
The components of the net deferred income tax assets and liabilities at May 31, 2008 and 2007 are as follows:
(in millions) | 2008 (Successor) | 2007 (Predecessor) | ||||||||
Deferred income tax assets: | ||||||||||
Accounts receivable | $ | 36.8 | $ | 72.5 | ||||||
Inventories | 56.7 | 37.4 | ||||||||
Accrued expenses | 60.3 | 26.9 | ||||||||
Tax benefit of net operating losses and tax credits | 52.3 | — | ||||||||
Future benefit of uncertain tax positions | 12.8 | — | ||||||||
Stock-based compensation | 9.5 | — | ||||||||
Other | 15.1 | — | ||||||||
Deferred income tax assets | 243.5 | 136.8 | ||||||||
Less: Valuation allowance | (6.0 | ) | — | |||||||
Total deferred income tax assets | $ | 237.5 | $ | 136.8 | ||||||
Deferred income tax liabilities: | ||||||||||
Property, plant, equipment and Intangibles | (2,242.9 | ) | (13.4 | ) | ||||||
Financial accounting basis of net assets of acquired companies different than tax basis | — | (11.2 | ) | |||||||
Other | (6.4 | ) | 3.4 | |||||||
Total deferred income tax liabilities | (2,249.3 | ) | (21.2 | ) | ||||||
Total net deferred income tax assets (liabilities) | $ | (2,011.8 | ) | $ | 115.6 | |||||
The Company’s deferred tax assets include federal, state, and foreign net operating loss carryforwards. The federal net operating loss carryforwards available are $97.4 million, which expire starting in 2028. The state and foreign net operating loss carryforwards are from various jurisdictions with various carryforward periods. As of May 31, 2008, the Company has a $6.0 million valuation allowance for a portion of its net deferred tax assets related to foreign net operating losses that management believes, more likely than not, will not be realized. All goodwill related to the merger is not tax deductible.
A deferred tax asset has been established for the foreign tax credit carryforwards in the amount of $7.6 million as of May 31, 2008. Federal foreign tax credits may be carried forward ten years. The Company believes that it is more like than not that it will be able to utilize the foreign tax credit carryforwards.
Deferred tax liabilities increased significantly from May 31, 2007 to May 31, 2008 due to the Merger. The intangibles, as well as the step-up in the fair value of the property, plant, and equipment for accounting purposes is not written up for tax, resulting in a temporary difference.
The Company has not provided for deferred taxes on certain of its excess of financial reporting over the tax basis of its investments in foreign subsidiaries that are essentially permanent in duration. Upon distribution of those earnings in the form of dividends or otherwise, the Company would be subject to U.S. income taxes (subject to an adjustment for foreign tax credits) and withholding taxes payable to the various foreign countries. Determination of the amount of any unrecognized deferred income tax liability on these undistributed earnings is not practical.
The Company has not recorded deferred taxes on its excess of financial reporting over the tax basis on certain of its investments in foreign subsidiaries related to current period earnings that are not considered to be indefinitely reinvested. The Company believes that there will not be a significant additional cost associated with the future repatriation of such foreign earnings.
Effective June 1, 2007, the Company adopted FIN 48. This interpretation prescribes a recognition threshold and measurement attribute for the financial statement recognition and measurement of tax contingencies and the tax position taken, or expected to be taken, in a tax return. Upon adoption of FIN 48, the Company had a liability of $41.2 million, $25.2 million of which would impact the Company’s effective tax rate, if recognized. The cumulative effect of the adoption of FIN 48 was recorded as a $9.2 million reduction to the beginning of the year retained earnings.
The Company recognizes accrued interest and penalties related to unrecognized tax benefits as a component of income tax expense. Tax expense for the year ending May 31, 2008 includes $1.4 million of interest and penalties. Interest and penalties of $4.8 million have been accrued at May 31, 2008. Interest and penalties for the period ended June 1, 2007 to July 11, 2007 was not material.
F-20
Table of Contents
The amount of unrecognized tax benefits at May 31, 2008 was $50.9 million, $38.7 million of which would impact the Company’s effective tax rate, if recognized. The Company does not anticipate a material change to the total amount of unrecognized tax benefits within the next 12 months.
A reconciliation of the beginning and ending amount of unrecognized tax benefits is as follows:
(in millions) | May 31, 2008 (Successor) | |||
Unrecognized tax benefits, July 11, 2007 | $ | 41.2 | ||
Gross increases—current-period tax positions | 23.2 | |||
Gross decreases—current-period tax positions | (1.4 | ) | ||
Gross increases—tax positions in prior period | 2.4 | |||
Gross decreases—tax positions in prior period | (6.6 | ) | ||
Settlements during the current period | (0.3 | ) | ||
Lapse of applicable statute of limitations | (7.6 | ) | ||
Unrecognized tax benefits, May 31, 2008 | $ | 50.9 | ||
The Company conducts business globally and, as a result, certain of its subsidiaries file income tax returns in the U.S. federal jurisdiction, and various state and foreign jurisdictions. In the normal course of business, the Company is subject to examinations by taxing authorities throughout the world, including major jurisdictions such as Australia, Canada, France, Germany, Japan, Netherlands, Spain, the United Kingdom and the United States. The Internal Revenue Service is currently auditing the predecessor Company’s federal tax returns for the years ended May 31, 2005 and 2006, and certain acquired entities for the years ended May 31, 2004 and 2005. In addition, certain state and foreign tax returns are under examination by various regulatory authorities. The statute of limitations for income tax examinations by the Internal Revenue Service has expired for the fiscal years prior to and including the year ended May 31, 2002. The Company regularly reviews issues that are raised from ongoing examinations and open tax years to evaluate the adequacy of its liabilities. As the various taxing authorities continue with their audit/examination programs, the Company will adjust its reserves accordingly to reflect these settlements.
Note 11—Segment Reporting.
The Company operates in one business segment, musculoskeletal products, which include the designing, manufacturing and marketing of reconstructive products, fixation devices, spinal products and other products. Other products consist primarily of softgoods and bracing products, sports medicine products, general instruments and operating room supplies. The Company manages its business segment primarily on a geographic basis. These geographic markets are comprised of the United States, Europe and International. Major markets included in the International geographic market are Canada, South America, Mexico and the Pacific Rim.
Net sales of musculoskeletal products by product category are as follows:
| July 12, 2007 -May 31, 2008 (Successor) | June 1, 2007 -July 11, 2007 (Predecessor) | Year Ended May 31, 2007 (Predecessor) | Year Ended May 31, 2006 (Predecessor) | |||||||||||
Net sales by product: | ||||||||||||||
Reconstructive | $ | 1,578.6 | $ | 178.1 | $ | 1,503.9 | $ | 1,379.4 | ||||||
Fixation | 203.2 | 27.1 | 224.7 | 251.4 | ||||||||||
Spinal | 183.1 | 24.9 | 205.8 | 221.9 | ||||||||||
Other | 169.6 | 18.7 | 173.0 | 173.0 | ||||||||||
Total | $ | 2,134.5 | $ | 248.8 | $ | 2,107.4 | $ | 2,025.7 | ||||||
| July 12, 2007-May 31, 2008 (Successor) | June 1, 2007-July 11, 2007 (Predecessor) | Year Ended May 31, 2007 (Predecessor) | Year Ended May 31, 2006 (Predecessor) | |||||||||||
Net sales by geographic segment: | ||||||||||||||
United States | $ | 1,251.4 | $ | 156.2 | $ | 1,306.5 | $ | 1,325.1 | ||||||
Europe | 663.7 | 70.8 | 595.9 | 520.7 | ||||||||||
International | 219.4 | 21.8 | 205.0 | 179.9 | ||||||||||
Total | $ | 2,134.5 | $ | 248.8 | $ | 2,107.4 | $ | 2,025.7 | ||||||
| May 31, 2008 (Successor) | May 31, 2007 (Predecessor) | |||||||
Long-term assets(1) by geographic segment: | ||||||||
United States | $ | 8,274.4 | $ | 526.4 | ||||
Europe | 2,995.4 | 391.0 | ||||||
International | 1,002.1 | 41.3 | ||||||
Total | $ | 12,271.9 | $ | 958.7 | ||||
| (1) | Defined as property, plant and equipment, intangibles and goodwill. |
Note 12—Guarantor and Non-guarantor Financial Statements.
Each of the Company’s existing wholly-owned domestic subsidiaries jointly, severally and unconditionally guarantee the senior cash pay and PIK toggle notes on a senior unsecured basis and the senior subordinated notes on a senior subordinated unsecured basis, in each case to the extent such subsidiaries guarantee our senior secured cash flow facilities.
F-21
Table of Contents
The following financial information illustrates the composition of the combined guarantor subsidiaries:
CONSOLIDATED BALANCE SHEETS
| May 31, 2008 (Successor) | ||||||||||||||||
| Parent | Guarantor | Non-guarantor | Eliminations | Total | ||||||||||||
Assets | ||||||||||||||||
Cash and cash equivalents | — | $ | 101.0 | $ | 25.4 | $ | 1.2 | $ | 127.6 | |||||||
Accounts receivable, net | — | 213.7 | 272.5 | — | 486.2 | |||||||||||
Inventories, net | — | 296.6 | 320.2 | (77.1 | ) | 539.7 | ||||||||||
Income tax receivable | — | 48.8 | — | — | 48.8 | |||||||||||
Deferred income taxes | — | 97.0 | 3.7 | — | 100.7 | |||||||||||
Prepaid expenses and other | — | 16.7 | 30.0 | — | 46.7 | |||||||||||
Total current assets | — | 773.8 | 651.8 | (75.9 | ) | 1,349.7 | ||||||||||
Property, plant and equipment, net | — | 407.6 | 233.3 | — | 640.9 | |||||||||||
Investments | — | 41.3 | — | — | 41.3 | |||||||||||
Investment in subsidiary | $ | 12,270.0 | — | — | (12,270.0 | ) | — | |||||||||
Goodwill | — | 4,677.5 | 1,847.7 | (1,102.4 | ) | 5,422.8 | ||||||||||
Intangible assets, net | — | 4,407.0 | 1,801.2 | — | 6,208.2 | |||||||||||
Other assets | — | 107.2 | 11.7 | — | 118.9 | |||||||||||
Total | $ | 12,270.0 | $ | 10,414.4 | $ | 4,545.7 | $ | (13,448.3 | ) | $ | 13,781.8 | |||||
Liabilities and Shareholders’ Equity | ||||||||||||||||
Short-term borrowings | $ | 37.0 | — | $ | 38.4 | — | $ | 75.4 | ||||||||
Accounts payable | — | $ | 53.0 | 38.6 | $ | (7.9 | ) | 83.7 | ||||||||
Accrued interest | 80.9 | — | — | — | 80.9 | |||||||||||
Accrued wages and commissions | — | 66.3 | 12.8 | — | 79.1 | |||||||||||
Other accrued expenses | — | 202.3 | 72.6 | (29.5 | ) | 245.4 | ||||||||||
Total current liabilities | 117.9 | 321.6 | 162.4 | (37.4 | ) | 564.5 | ||||||||||
Deferred income taxes | — | 1,438.0 | 725.3 | (50.8 | ) | 2,112.5 | ||||||||||
Employee related obligations | — | — | 40.0 | — | 40.0 | |||||||||||
Long-term debt | 6,225.7 | — | — | — | 6,225.7 | |||||||||||
Other long-term liabilities | — | — | 2.8 | — | 2.8 | |||||||||||
Shareholders’ equity | 5,926.4 | 8,654.8 | 3,615.2 | (13,360.1 | ) | 4,836.3 | ||||||||||
Total | $ | 12,270.0 | $ | 10,414.4 | $ | 4,545.7 | $ | (13,448.3 | ) | $ | 13,781.8 | |||||
F-22
Table of Contents
| May 31, 2007 (Predecessor) | |||||||||||||||||
| Parent | Guarantor | Non-guarantor | Eliminations | Total | |||||||||||||
Assets | |||||||||||||||||
Cash and cash equivalents | — | $ | 73.4 | $ | 21.4 | $ | 10.3 | $ | 105.1 | ||||||||
Investments | — | 125.8 | — | — | 125.8 | ||||||||||||
Accounts receivable, net | — | 312.4 | 222.5 | (36.2 | ) | 498.7 | |||||||||||
Inventories, net | — | 302.9 | 281.0 | (43.5 | ) | 540.4 | |||||||||||
Prepaid expense and other | — | 152.1 | 10.8 | 18.9 | 181.8 | ||||||||||||
Total current assets | — | 966.6 | 535.7 | (50.5 | ) | 1,451.8 | |||||||||||
Property, plant and equipment, net | — | 249.8 | 177.6 | — | 427.4 | ||||||||||||
Investments | — | 43.0 | — | — | 43.0 | ||||||||||||
Investment in subsidiaries | $ | 2,049.2 | — | — | (2,049.2 | ) | — | ||||||||||
Goodwill | — | 249.1 | 197.4 | 1.9 | 448.4 | ||||||||||||
Intangible assets, net | — | 35.1 | 39.5 | — | 74.6 | ||||||||||||
Other assets | — | 7.9 | 4.8 | — | 12.7 | ||||||||||||
Total | $ | 2,049.2 | $ | 1,551.5 | $ | 955.0 | $ | (2,097.8 | ) | $ | 2,457.9 | ||||||
Liabilities and Shareholders’ Equity | |||||||||||||||||
Short-term borrowings | — | — | $ | 81.8 | — | $ | 81.8 | ||||||||||
Accounts payable | — | $ | 30.2 | 39.2 | $ | (0.7 | ) | 68.7 | |||||||||
Accrued income taxes | — | 17.6 | (6.0 | ) | — | 11.6 | |||||||||||
Accrued wages and commissions | — | 51.4 | 28.9 | — | 80.3 | ||||||||||||
Other accrued expenses | — | 88.7 | 17.6 | (2.8 | ) | 103.5 | |||||||||||
Total current liabilities | — | 187.9 | 161.5 | (3.5 | ) | 345.9 | |||||||||||
Deferred income taxes | — | 9.9 | 11.3 | — | 21.2 | ||||||||||||
Long-term debt | — | 21.4 | 37.4 | (58.8 | ) | — | |||||||||||
Other long-term liabilities | — | — | 41.6 | — | 41.6 | ||||||||||||
Shareholders’ equity | 2,049.2 | 1,332.3 | 703.2 | (2,035.5 | ) | 2,049.2 | |||||||||||
Total | $ | 2,049.2 | $ | 1,551.5 | $ | 955.0 | $ | (2,097.8 | ) | $ | 2,457.9 | ||||||
CONSOLIDATED STATEMENTS OF OPERATIONS
| June 1, 2007 to July 11, 2007 (Predecessor) | ||||||||||||||||||||
| Parent | Guarantor | Non-guarantor | Eliminations | Total | ||||||||||||||||
Revenue | — | $ | 185.1 | $ | 82.5 | $ | (18.8 | ) | $ | 248.8 | ||||||||||
Cost of sales | ��� | 60.8 | 46.5 | (5.0 | ) | 102.3 | ||||||||||||||
Gross margin | — | 124.3 | 36.0 | (13.8 | ) | 146.5 | ||||||||||||||
Operating expenses | — | 179.2 | 49.3 | 0.2 | 228.7 | |||||||||||||||
Operating loss | — | (54.9 | ) | (13.3 | ) | (14.0 | ) | (82.2 | ) | |||||||||||
Other income (expense), net | — | (0.7 | ) | 1.0 | — | 0.3 | ||||||||||||||
Income (loss) before income taxes | — | (55.6 | ) | (12.3 | ) | (14.0 | ) | (81.9 | ) | |||||||||||
Tax provision (benefit) | — | (24.6 | ) | (2.5 | ) | (0.2 | ) | (27.3 | ) | |||||||||||
Equity in earnings of subsidiaries | $ | (40.8 | ) | — | — | 40.8 | — | |||||||||||||
Net income (loss) | $ | (40.8 | ) | $ | (31.0 | ) | $ | (9.8 | ) | $ | 27.0 | $ | (54.6 | ) | ||||||
| July 12, 2007 to May 31, 2008 (Successor) | ||||||||||||||||||||
| Parent | Guarantor | Non-guarantor | Eliminations | Total | ||||||||||||||||
Revenues | — | $ | 1,309.8 | $ | 1,060.0 | $ | (235.3 | ) | $ | 2,134.5 | ||||||||||
Cost of sales | — | 499.9 | 535.5 | (220.7 | ) | 814.7 | ||||||||||||||
Gross margin | — | 809.9 | 524.5 | (14.6 | ) | 1,319.8 | ||||||||||||||
Operating expenses | — | 1,220.0 | 768.1 | — | 1,988.1 | |||||||||||||||
Operating income (loss) | — | (410.1 | ) | (243.6 | ) | (14.6 | ) | (668.3 | ) | |||||||||||
Other income (expense), net | $ | (516.6 | ) | (10.4 | ) | — | 1.0 | (526.0 | ) | |||||||||||
Income (loss) before income taxes | (516.6 | ) | (420.5 | ) | (243.6 | ) | (13.6 | ) | (1,194.3 | ) | ||||||||||
Tax provision (benefit) | — | (141.2 | ) | (85.3 | ) | (3.6 | ) | (230.1 | ) | |||||||||||
Equity in earnings of subsidiaries | (437.6 | ) | — | — | 437.6 | — | ||||||||||||||
Net income (loss) | $ | (954.2 | ) | $ | (279.3 | ) | $ | (158.3 | ) | $ | 427.6 | $ | (964.2 | ) | ||||||
| Year Ended May 31, 2007 (Predecessor) | ||||||||||||||||||||
| Parent | Guarantor | Non-guarantor | Eliminations | Total | ||||||||||||||||
Revenues | — | $ | 1,501.2 | $ | 780.3 | $ | (174.1 | ) | $ | 2,107.4 | ||||||||||
Cost of sales | — | 429.3 | 382.9 | (169.9 | ) | 642.3 | ||||||||||||||
Gross margin | — | 1,071.9 | 397.4 | (4.2 | ) | 1,465.1 | ||||||||||||||
Operating expenses | — | 712.4 | 263.2 | (0.1 | ) | 975.5 | ||||||||||||||
Operating income (loss) | — | 359.5 | 134.2 | (4.1 | ) | 489.6 | ||||||||||||||
Other income (expense), net | — | 19.9 | (7.9 | ) | — | 12.0 | ||||||||||||||
Income (loss) before income taxes | — | 379.4 | 126.3 | (4.1 | ) | 501.6 | ||||||||||||||
Tax provision (benefit) | — | 132.6 | 35.1 | (2.0 | ) | 165.7 | ||||||||||||||
Equity in earnings (loss) of subsidiaries | $ | 338.0 | — | — | (338.0 | ) | — | |||||||||||||
Net income (loss) | $ | 338.0 | $ | 246.8 | $ | 91.2 | $ | (340.1 | ) | $ | 335.9 | |||||||||
F-23
Table of Contents
| Year Ended May 31, 2006 (Predecessor) | |||||||||||||||||
| Parent | Guarantor | Non-guarantor | Eliminations | Total | |||||||||||||
Revenue | — | $ | 1,487.2 | $ | 683.3 | $ | (144.8 | ) | $ | 2,025.7 | |||||||
Cost of sales | — | 390.0 | 324.6 | (132.5 | ) | 582.1 | |||||||||||
Gross margin | — | 1,097.2 | 358.7 | (12.3 | ) | 1,443.6 | |||||||||||
Operating expenses | — | 602.0 | 233.2 | — | 835.2 | ||||||||||||
Operating income (loss) | — | 495.2 | 125.5 | (12.3 | ) | 608.4 | |||||||||||
Other income (expense), net | — | 11.4 | (8.8 | ) | — | 2.6 | |||||||||||
Income (loss) before income taxes | — | 506.6 | 116.7 | (12.3 | ) | 611.0 | |||||||||||
Tax provision (benefit) | — | 179.6 | 30.0 | (4.5 | ) | 205.1 | |||||||||||
Equity in earnings (losses) of subsidiaries | $ | 413.7 | — | — | (413.7 | ) | — | ||||||||||
Net income (loss) | $ | 413.7 | $ | 327.0 | $ | 86.7 | $ | (421.5 | ) | $ | 405.9 | ||||||
CONSOLIDATED STATEMENTS OF CASH FLOWS
| June 1, 2007 to July 11, 2007 (Predecessor) | ||||||||||||||||||||
| Parent | Guarantor | Non-guarantor | Eliminations | Total | ||||||||||||||||
Cash flows from (used in) operating activities: | ||||||||||||||||||||
Net loss | $ | (40.8 | ) | $ | (31.0 | ) | $ | (9.8 | ) | $ | 27.0 | $ | (54.6 | ) | ||||||
Deferred taxes | — | 76.7 | — | — | 76.7 | |||||||||||||||
Prepaid expenses | — | (107.0 | ) | 14.9 | 19.2 | (72.9 | ) | |||||||||||||
Other | — | 75.0 | 25.2 | 10.0 | 110.2 | |||||||||||||||
Net cash from (used in) operating activities | (40.8 | ) | 13.7 | 30.3 | 56.2 | 59.4 | ||||||||||||||
Cash flows from (used in) investing activities: | ||||||||||||||||||||
Net proceeds (payments) for sale of investments | — | 42.8 | — | — | 42.8 | |||||||||||||||
Investment in and advances to subsidiaries | 39.5 | — | — | (39.5 | ) | — | ||||||||||||||
Other | — | (21.0 | ) | (7.8 | ) | (3.0 | ) | (31.8 | ) | |||||||||||
Net cash from (used in) investing activities | 39.5 | 21.8 | (7.8 | ) | (42.5 | ) | 11.0 | |||||||||||||
Cash flows from (used in) financing activities: | 1.3 | — | — | — | 1.3 | |||||||||||||||
Effect of exchange rate changes on cash | — | — | 0.1 | — | 0.1 | |||||||||||||||
Increase (decrease) in cash and cash equivalents | — | 35.5 | 22.6 | 13.7 | 71.8 | |||||||||||||||
Cash and cash equivalents, beginning of period | — | 73.4 | 21.4 | 10.3 | 105.1 | |||||||||||||||
Cash and cash equivalents, end of period | $ | — | $ | 108.9 | $ | 44.0 | $ | 24.0 | $ | 176.9 | ||||||||||
| July 12, 2007 to May 31, 2008 (Successor) | ||||||||||||||||||||
| Parent | Guarantor | Non-guarantor | Eliminations | Total | ||||||||||||||||
Cash flows from (used in) operating activities: | ||||||||||||||||||||
Net loss | $ | (954.2 | ) | $ | (279.3 | ) | $ | (158.3 | ) | $ | 427.6 | $ | (964.2 | ) | ||||||
Depreciation and amortization | — | 313.1 | 158.4 | — | 471.5 | |||||||||||||||
Non-cash in-process research and development charge | — | 328.0 | 151.0 | — | 479.0 | |||||||||||||||
Non-cash charges related to inventory step-up | — | 128.0 | 32.3 | — | 160.3 | |||||||||||||||
Non-cash stock compensation expense | — | 21.4 | 4.4 | — | 25.8 | |||||||||||||||
Deferred income taxes | — | (27.6 | ) | (17.3 | ) | — | (44.9 | ) | ||||||||||||
Accrued interest | 80.9 | — | — | — | 80.9 | |||||||||||||||
Other | 7.3 | (13.7 | ) | (13.1 | ) | — | (19.5 | ) | ||||||||||||
Net cash from (used in) operating activities | (866.0 | ) | 469.9 | 157.4 | 427.6 | 188.9 | ||||||||||||||
Cash flows from (used in) investing activities: | ||||||||||||||||||||
Net proceeds (payments) for sale of investments | — | 84.7 | — | — | 84.7 | |||||||||||||||
Investment in and advances to subsidiaries | 1,549.2 | (498.3 | ) | (97.9 | ) | (953.0 | ) | — | ||||||||||||
Capital expenditure | — | (80.2 | ) | (87.7 | ) | — | (167.9 | ) | ||||||||||||
Acquisition of Biomet, Inc. | (11,638.2 | ) | — | — | — | (11,638.2 | ) | |||||||||||||
Other | — | — | (0.4 | ) | — | (0.4 | ) | |||||||||||||
Net cash from (used in) investing activities | (10,089.0 | ) | (493.8 | ) | (186.0 | ) | (953.0 | ) | (11,721.8 | ) | ||||||||||
Cash flows from (used in) financing activities: | ||||||||||||||||||||
Proceeds (payments) on long-term debt | 6,065.1 | — | — | — | 6,065.1 | |||||||||||||||
Cash equity contributions | 5,521.9 | — | — | — | 5,521.9 | |||||||||||||||
Payment of deferred financing fees | (87.1 | ) | — | — | — | (87.1 | ) | |||||||||||||
Other | (18.3 | ) | — | — | — | (18.3 | ) | |||||||||||||
Net cash from (used in) financing activities | 11,481.6 | — | — | — | 11,481.6 | |||||||||||||||
Effect of exchange rate changes on cash | — | — | 2.0 | — | 2.0 | |||||||||||||||
Increase (decrease) in cash and cash equivalents | — | (23.9 | ) | (26.6 | ) | 1.2 | (49.3 | ) | ||||||||||||
Cash and cash equivalents, beginning of period | — | 108.9 | 44.0 | 24.0 | 176.9 | |||||||||||||||
Cash and cash equivalents, end of period | $ | — | $ | 85.0 | $ | 17.4 | $ | 25.2 | $ | 127.6 | ||||||||||
F-24
Table of Contents
| Year Ended May 31, 2007 (Predecessor) | ||||||||||||||||||||
| Parent | Guarantor | Non-guarantor | Eliminations | Total | ||||||||||||||||
Cash flows from (used in) operating activities: | ||||||||||||||||||||
Net income | $ | 338.0 | $ | 246.8 | $ | 91.2 | $ | (340.1 | ) | $ | 335.9 | |||||||||
Depreciation | — | 39.7 | 48.5 | — | 88.2 | |||||||||||||||
Deferred income taxes | — | (58.9 | ) | (0.9 | ) | (2.0 | ) | (61.8 | ) | |||||||||||
Other | — | 114.1 | (48.2 | ) | 11.6 | 77.5 | ||||||||||||||
Net cash from (used in) operating activities | 338.0 | 341.7 | 90.6 | (330.5 | ) | 439.8 | ||||||||||||||
Cash flows from (used in) investing activities: | ||||||||||||||||||||
Investment in and advances to subsidiaries | (101.3 | ) | (238.8 | ) | — | 340.1 | — | |||||||||||||
Capital expenditures | — | (72.1 | ) | (70.5 | ) | — | (142.5 | ) | ||||||||||||
Other | — | (66.8 | ) | (4.4 | ) | — | (71.2 | ) | ||||||||||||
Net cash from (used in) investing activities | (101.3 | ) | (377.7 | ) | (74.9 | ) | 340.1 | (213.7 | ) | |||||||||||
Cash flows from (used in) financing activities: | ||||||||||||||||||||
Payments on long-term debt | (196.8 | ) | — | — | — | (196.8 | ) | |||||||||||||
Dividends | (73.5 | ) | — | — | — | (73.5 | ) | |||||||||||||
Other | 33.6 | — | (14.4 | ) | — | 19.2 | ||||||||||||||
Net cash from (used in) financing activities | (236.7 | ) | — | (14.4 | ) | — | (251.1 | ) | ||||||||||||
Effect of exchange rate changes on cash | — | 0.6 | 3.0 | 0.6 | 4.2 | |||||||||||||||
Increase (decrease) in cash and cash equivalents | — | (35.4 | ) | 4.3 | 10.2 | (20.9 | ) | |||||||||||||
Cash and cash equivalents, beginning of period | — | 108.8 | 17.1 | 0.1 | 126.0 | |||||||||||||||
Cash and cash equivalents, end of period | $ | — | $ | 73.4 | $ | 21.4 | $ | 10.3 | $ | 105.1 | ||||||||||
| �� | ||||||||||||||||||||
| Year Ended May 31, 2006 (Predecessor) | ||||||||||||||||||||
| Parent | Guarantor | Non-guarantor | Eliminations | Total | ||||||||||||||||
Cash flows from (used in) operating activities: | ||||||||||||||||||||
Net income | $ | 413.7 | $ | 327.0 | $ | 86.7 | $ | (421.5 | ) | $ | 405.9 | |||||||||
Depreciation | — | 34.3 | 37.7 | — | 72.0 | |||||||||||||||
Inventories | — | (60.4 | ) | (22.2 | ) | 12.9 | (69.7 | ) | ||||||||||||
Other | — | 46.5 | (28.8 | ) | (12.5 | ) | 5.2 | |||||||||||||
Net cash from (used in) operating activities | 413.7 | 347.4 | 73.4 | (421.1 | ) | 413.4 | ||||||||||||||
Cash flows from (used in) investing activities: | ||||||||||||||||||||
Investment in and advances to subsidiaries | (158.8 | ) | (262.7 | ) | — | 421.5 | — | |||||||||||||
Capital expenditures | — | (49.0 | ) | (59.9 | ) | — | (108.9 | ) | ||||||||||||
Other | — | (6.9 | ) | (4.9 | ) | — | (11.8 | ) | ||||||||||||
Net cash from (used in) investing activities | (158.8 | ) | (318.6 | ) | (64.8 | ) | 421.5 | (120.7 | ) | |||||||||||
Cash flows from (used in) financing activities: | ||||||||||||||||||||
Purchase of common shares | (215.3 | ) | — | — | — | (215.3 | ) | |||||||||||||
Dividends | (62.5 | ) | — | — | — | (62.5 | ) | |||||||||||||
Other | 22.9 | — | (2.6 | ) | — | 20.3 | ||||||||||||||
Net cash from (used in) financing activities | (254.9 | ) | — | (2.6 | ) | — | (257.5 | ) | ||||||||||||
Effect of exchange rate changes on cash | — | 0.2 | (0.5 | ) | (0.3 | ) | (0.6 | ) | ||||||||||||
Increase (decrease) in cash and cash equivalents | — | 29.0 | 5.5 | 0.1 | 34.6 | |||||||||||||||
Cash and cash equivalents, beginning of period | — | 79.8 | 11.6 | — | 91.4 | |||||||||||||||
Cash and cash equivalents, end of period | $ | — | $ | 108.8 | $ | 17.1 | $ | 0.1 | $ | 126.0 | ||||||||||
Note 13—Contingencies.
U.S Department of Justice Consulting Agreement Investigation
On September 27, 2007, the Company entered into a Deferred Prosecution Agreement with the U.S. Attorney’s Office for the District of New Jersey. The agreement concludes the government’s investigation into whether consulting agreements between the largest orthopedic manufacturers and orthopedic surgeons who use joint reconstruction and replacement products may have violated the federal Anti-Kickback Statute.
Through the agreement, the U.S. Attorney’s Office agreed not to prosecute the Company in connection with this matter, provided that the Company satisfies its obligations under the agreement over the next 18 months. The agreement calls for the appointment of an independent monitor to review the Company’s compliance with the agreement, particularly in relation to its consulting agreements.
As part of the resolution of this matter, the Company also entered into a Corporate Integrity Agreement with the Office of the Inspector General of the U.S. Department of Health and Human Services.
U.S. Department of Justice EBI Products Investigation and Related Litigation
In May 2007, the Company received a subpoena from the U.S. Department of Justice through the U.S. Attorney for the Southern District of West Virginia requesting documents generally relating to a certain number of products manufactured, marketed and sold by the Company’s EBI subsidiary for the period from January 1999 through the date of this filing. In June 2007, the Company received a second administrative subpoena from the U.S. Attorney for the Southern District of West Virginia requesting documents relating to a specific physician’s assistant. The Company understands that the Department of Justice is conducting a civil investigation of EBI’s sales and marketing practices relating to certain spinal products. The Company is fully cooperating with the request of the Department of Justice. The Company can make no assurances as to the time or resources that will be needed to devote to this inquiry or its final outcome.
F-25
Table of Contents
U.S. Department of Justice Antitrust and Related Litigation
In June 2006, the Company received a federal grand jury subpoena issued at the request of the U.S. Department of Justice, Antitrust Division, requesting documents for the period from January 2001 through June 2006 regarding possible violations of federal criminal law, including possible violations of the antitrust laws, relating to the manufacture and sale of orthopedic implant devices. The Company is aware of similar subpoenas directed to other companies in the orthopedic industry. The Company has cooperated and intends to continue to fully cooperate with the Department of Justice investigation. The result of this investigation may not be known for several years. However, the scope of the June 2006 subpoena was narrowed to a specific geographic region and specific product lines. It is the belief that the other orthopedic companies that received similar subpoenas have received similar guidance. It is the belief that the investigation was prompted by an unsolicited e-mail sent by a representative of one of its competitors that proposed a common pricing strategy in connection with a particular hospital. This e-mail was received by an independent sales representative of an independent distributor for Biomet Orthopedics, but it was never transmitted to us. Biomet, Inc., the independent distributor, nor the independent sales representative took any action in response to the e-mail, and the company believes that no anticompetitive activity took place as a result of it. The Company requires compliance by its employees and its independent distributors with its Code of Business Conduct and Ethics and with applicable antitrust laws. On March 26, 2008, The company received a letter from a representative of the Department of Justice, Antitrust Division I advising that the Department has closed its grand jury investigation of antitrust and related offenses in the orthopedic implants industry.
The Company has received complaints in class action lawsuits alleging violations of the Sherman Antitrust Act that raise the same antitrust issues as the U.S. Department of Justice investigation described above. The complaints also named various other companies in the orthopedic industry as defendants. These cases were consolidated under the caption In Re Orthopedic Implant Device Antitrust Litigation, Case No. 1:07-ml-9831-JDT-WTL with the United States District Court Southern District Indianapolis, Indiana Division, and on October 18, 2007 were voluntarily dismissed without prejudice.
Litigation Relating to Past Stock Option Grant Practices
On September 21, 2006, two shareholder derivative complaints were filed against certain of the Company’s current and former officers and directors in Kosciusko Superior Court I in Kosciusko County, in the State of Indiana. The complaints, captioned Long v. Hann, et al. , and Thorson v. Hann, et al. , alleged violations of state law relating to the issuance of certain stock option awards by Biomet dating back to 1996. Both complaints sought unspecified money damages as well as other equitable and injunctive relief. These two cases were consolidated under the caption In re Biomet, Inc. Derivative Litigation , and on January 19, 2007, plaintiffs filed an amended complaint that made additional allegations based on the Company’s December 18, 2006 disclosures related to stock option awards, including allegations that the defendants sought to sell the Company in order to escape liability for their conduct, and that they did so at a devalued price, thus further breaching their fiduciary duties to shareholders. On February 16, 2007, defendants filed a motion to dismiss plaintiffs’ amended complaint. On October 11, 2007, after approval of the Company’s sale by its shareholders, the parties filed supplemental briefs on the issue of whether plaintiffs had standing to sue. On February 5, 2008, the court dismissed the case for lack of standing, and plaintiffs’ motion for leave to amend was denied. Plaintiffs appealed the district court’s decision, and on February 13, 2009, the Court of Appeals affirmed the dismissal of the case. Plaintiffs appealed the dismissal of the case to the Indiana Court.
On December 11, 2006, a third shareholder derivative complaint captioned International Brotherhood of Electrical Workers (IBEW) Local 98 Pension Fund v. Hann, et al. , No. 06 CV 14312, was filed in federal court in the Southern District of New York. The IBEW case makes allegations and claims similar to those made in the Indiana litigation, in addition to purporting to state three derivative claims for violations of the federal securities laws. On February 15, 2007, defendants filed a motion to dismiss the plaintiff’s complaint. On April 11, 2007, plaintiffs filed a motion for partial summary judgment claiming that the disclosures in the Company’s April 2, 2007 Form 8-K filing and press release regarding the Company’s historical stock options granting practices constitute admissions sufficient to establish defendants’ liability on certain of plaintiffs’ claims. On October 11, 2007, after approval of the Company’s sale by its shareholders, the parties filed supplemental briefs on the issue of whether plaintiff had standing to sue. On June 10, 2008, the motion to dismiss was granted without leave to amend due to plaintiff’s lack of standing. Plaintiffs have not filed an appeal. See Item 7. “Management’s Discussion and Analysis of Financial Condition and Results of Operations.”
Litigation Relating to the Merger
On December 20, 2006, a purported class-action lawsuit captioned Long, et al. v. Hann, et al., was filed in Indiana State court in the County of Kosciusko. The lawsuit names as defendants each member of the Company’s Board of Directors at the time, Dane Miller, Ph.D., and Blackstone Capital Partners V L.P., KKR 2006 Fund L.P., Goldman Sachs Investments Ltd. and TPG Partners V, L.P. The complaint alleges, among other things, that the defendants breached, or aided and abetted the breach of, fiduciary duties owed to the Company’s shareholders by its directors in connection with the Company’s entry into the Merger Agreement. Among the purported fiduciary breaches alleged in the complaint is that the Company’s director defendants “knew that the only way they could escape liability for their stock option granting improprieties would be to sell the Company, thus eliminating their liability.” The complaint seeks, among other relief, class certification of the lawsuit, a declaration that the Merger Agreement was entered into in breach of the fiduciary duties of the defendants, an injunction preventing the defendants from proceeding with the Merger unless and until the defendants implement procedures to obtain the highest possible sale price, an order directing the defendants to exercise their fiduciary duties to obtain a transaction which is in the best interests of the Company’s shareholders until the process for a sale of Biomet is completed and the highest price is obtained, an order directing the defendants to exercise their fiduciary duty to disclose all material information in their possession concerning the Merger prior to the shareholder vote, including fiscal 2007 second quarter financial results, imposition of a constructive trust upon any benefits improperly received by the defendants, an award of attorneys’ fees and expenses, and such other relief as the court might find just and proper. On March 29 and 30, 2007, the defendants filed motions to dismiss the plaintiffs’ complaint, and these motions are currently pending before the court.
On January 2, 2007, a purported class action lawsuit captioned Gervasio v. Biomet, Inc., et al., was filed in the Supreme Court for the State of New York, New York County. A virtually identical action was filed on January 9, 2007, captioned Corry v. Biomet, Inc., et al., in the same court. Both of these lawsuits named as defendants Biomet, Inc., each member of the Company’s Board of Directors at the time, Dane Miller, Ph.D., The Blackstone Group L.P., Kohlberg Kravis Roberts & Co., Goldman Sachs Capital Partners and Texas Pacific Group. The lawsuits made essentially the same claims and sought the same relief as in the Long action described above. On January 29, 2007, defendants filed a joint motion to dismiss Gervasio. On February 14, 2007, the plaintiff in Corry voluntarily discontinued his lawsuit and informed defendants that he intended to intervene in Gervasio. On March 26, 2007, the court granted defendants’ motion to dismiss Gervasio.
Pursuant to Indiana law and provisions of the Company’s articles of incorporation, the Company is advancing reasonable expenses, including attorneys’ fees, incurred by the Company’s current and former directors and officers in defending these lawsuits, with the exception of Dane Miller, Ph.D., whose status as a defendant does not arise from his status as a former director or officer.
Each of Biomet and the other defendants denies all of the allegations in these lawsuits, including any allegation that its current disclosures with regard to the pending Merger are false, misleading or incomplete in any way. Nevertheless, without admitting any liability or wrongdoing, the Company and other defendants in these cases have agreed in principle to settle them in order to avoid the potential cost and distraction of continued litigation and, at the time, to eliminate any risk of any delay to the closing of the Merger posed by these lawsuits.
On May 31, 2007, the Company entered into a memorandum of understanding regarding the settlement of class action lawsuits that were filed on behalf of the Company’s shareholders following the announcement of the proposed Merger. The parties to the memorandum of understanding executed a definitive settlement agreement dated as of April 17, 2008. This settlement is subject to court approval. On April 25, 2008, the parties moved the Indiana State court in the County of Kosciusko for approval of the settlement. If the settlement becomes effective the lawsuits will be dismissed with prejudice.
F-26
Table of Contents
Pursuant to the terms of the settlement, the Company agreed to make available meaningful additional information, including financial information, to its shareholders. Such additional information was contained in the Current Report on Form 8-K filed on May 31, 2007. In addition, the Sponsors have agreed to cause Biomet (or the Company’s Successors) to pay the legal fees and expenses of plaintiffs’ counsel, in an amount of $0.6 million in the aggregate, subject to approval by the court and other conditions. The settlement was entered into on April 17, 2008, and preliminary approval was granted by the court on May 12, 2008. On August 6, 2008, the Court gave final approval to the settlement and dismissed the litigation with prejudice.
U.S. Securities and Exchange Commission Informal Investigation
On September 25, 2007, the Company received a letter from the SEC informing the Company that it is conducting an informal investigation regarding possible violations of the Foreign Corrupt Practices Act in the sale of medical devices in certain foreign countries by companies in the medical devices industry. The Foreign Corrupt Practices Act prohibits U.S. companies and their officers, directors, employees, shareholders acting on their behalf and agents from offering, promising, authorizing or making payments to foreign officials for the purpose of obtaining or retaining business abroad or otherwise obtaining favorable treatment and this law requires companies to maintain records which fairly and accurately reflect transactions and to maintain internal accounting controls. In many countries, hospitals and clinics are government-owned and healthcare professionals employed by such hospitals and clinics, with whom the Company regularly interacts, may meet the definition of a foreign official for purposes of the Foreign Corrupt Practices Act. If the Company is found to have violated the Foreign Corrupt Practices Act, the Company may face sanctions including fines, criminal penalties, disgorgement of profits and suspension or debarment of the Company’s ability to contract with government agencies or receive export licenses. On November 9, 2007, the Company received a letter from the Department of Justice requesting any information provided to the SEC be provided to the Department of Justice on a voluntary basis. The Company intends to fully cooperate with both requests and the Company is in the process of conducting its own review relating to these matters in certain countries in which the Company and its distributors conduct business. As of August 28, 2008, the SEC and the DOJ have not taken, or advised that they intend to take, any specific action against the Company or any individuals currently or formerly affiliated with the Company in connection with their investigations.
Massachusetts AG
The Company received a Civil Investigative Demand (“CID”) issued by the Commonwealth of Massachusetts Office of the Attorney General (“Massachusetts AG”) on or about November 19, 2007. The CID requested documents for the period November 1, 2003 to the present concerning certain physicians and provider groups, including, among other things, documents concerning any contracts or agreements with, and any payments made to, those physicians or provider groups. The Company has produced documents in response to the CID, and intends to continue to cooperate with the Massachusetts AG. It is not possible at this time to predict the likely outcome of this inquiry or its financial impact should the outcome be adverse to the Company.
Other Matters
In February 2006, SDGI Holdings, Inc. and Medtronic Sofamor Danek, Inc. (collectively referred to herein as “Medtronic”) brought an action against EBI and the Company alleging infringement of seven patents. Specifically, Medtronic alleges that the patents are infringed by certain components of the Company’s Vuelock® Anterior Cervical Plate System, as well as instruments and surgical implantation methods associated with the Company’s Array® Spinal System. In Fall 2007, Medtronic included similar instruments used with EBI’s Biomet® Omega21™ , Polaris™ , and Synergy™ spinal fixation systems as accused products. Medtronic’s complaint does not seek a specific amount of damages, but does seek to enjoin the Company from manufacturing, selling and/or distributing the allegedly infringing products. The Company has filed a counterclaim seeking a finding of non-infringement of the patents at issue and a finding that certain of the patents are invalid and unenforceable. Discovery on the litigation continues. The Company is vigorously defending this matter and intends to continue to do so.
The Company and Biomet Orthopedics initiated legal proceedings against Zimmer US, Inc., or Zimmer, certain of the Company’s former distributors and David Montgomery, the Company’s former employee who currently works for Zimmer. The thirteen count lawsuit originally filed in Marion County, Indiana and refiled in Hamilton County, Indiana alleges, among other things, that Zimmer and Mr. Montgomery attempted to create an unfair market advantage by engaging in a campaign to misappropriate the Company’s confidential information, to interfere with the Company’s contractual relations with distributors and to attempt to buy the assets of most of the Company’s distributors (including the Company’s surgical instruments) throughout the United States. Further, the lawsuit alleges that the limited number of distributors who accepted Zimmer’s offer are in violation of their contractual obligations to Biomet. Although nearly all of the Company’s distributors rejected Zimmer’s offers and have remained with Biomet, and although no amount of money damages can completely compensate Biomet for the losses the Company has sustained as a result of defendants’ conduct, the Company is nonetheless seeking to recover compensatory damages that are attributable to financial and other resources spent on signing new agreements with the Company’s sales force. To the extent the Company sustained damages as a result of the Company’s former distributors agreeing to purportedly sell their assets to Zimmer, the Company is seeking to recover lost profits and other damages as well. In addition, the Company is seeking to recover punitive damages from the defendants. On November 9, 2007, defendants filed a motion to dismiss the Company’s complaint. On March 27, 2008, the court denied the motion in its entirety.
In a related matter, the Company brought suit against a former distributor for Biomet Orthopedics who, in violation of his contractual and other obligations to Biomet under agreements stretching back to 1994, sold the assets of his distributorship to Zimmer in an apparent effort to avoid his contractual obligations to the Company. The complaint, now pending in federal district court in Indiana, asserts five causes of action that include breach of contract, unjust enrichment and statutory wrongs. Among other things, the complaint seeks injunctive relief and compensatory and punitive damages. On July 16, 2007, a temporary restraining order was entered against this former distributor which subsequently lapsed ten days later. Prior to the filing of the suit described above, this former distributor sued one of his former employees who decided to continue to represent the Company’s products in the future as he has for nearly ten years. The suit brought against this employee by the Company’s former distributor who sold his assets to Zimmer claims, among other things, that the former employee is violating his non-competition agreement with the Company’s former distributor by continuing to sell the same Biomet products the former employee sold while employed by the Company’s former distributor. The suit also seeks, among other forms of relief, an injunction and compensatory and punitive damages.
In late 2004 and early 2005, approximately 120 plaintiffs sued Dr. John King in the Circuit Court of Putnam County, West Virginia. Plaintiffs alleged that Dr. King was professionally negligent when he performed surgery on the plaintiffs at Putnam General Hospital in Putnam County, West Virginia between November 2002 and June 2003. In 38 of these lawsuits, plaintiffs alleged that Dr. King had implanted a device manufactured by the Company’s EBI subsidiary and EBI was named a party in those 38 lawsuits. Plaintiffs have dismissed or have agreed to dismiss their claims against EBI in 11 cases, leaving EBI as a party in 27 pending lawsuits, all of which relate to EBI’s Ionic Spine Spacer System and its implanted bone stimulator devices, the SpF and OsteoGen. Plaintiffs allege that EBI entered into a joint venture and a civil conspiracy with Dr. King and/or his physician assistant, David McNair. The plaintiffs also allege that EBI failed to warn that its products were not safe for their intended use, that EBI knew that Dr. King was not properly trained or was performing surgeries inappropriately and claims based on strict liability, express and implied breach of warranty and negligent sale. Plaintiffs seek to recover lost income, medical expenses, future medical and life care expenses, damages relating to pain and suffering and punitive and other damages. Dr. King is uninsured in 25 of these 27 cases and has filed bankruptcy.
In July 2007, a Putnam County jury found that Putnam General Hospital had negligently credentialed Dr. King and that the hospital’s conduct in credentialing Dr. King was motivated by fraud, ill will, wantonness, oppressiveness, or by reckless or gross negligence, which allowed the plaintiffs to seek punitive damages against the hospital. In April, May and June of 2008, the hospital and its upstream affiliates and David McNair entered into a confidential settlement of all claims with all but one of the plaintiffs. EBI, Wright Medical Corporation, Wright Medical’s distributor’s employee, Robert Edwards, and Dr. King remain as defendants in the litigation.
F-27
Table of Contents
The Putnam County Circuit Court revised its case management order with respect to the remaining lawsuits on July 2, 2008 and scheduled a consolidated trial of six plaintiffs for June 1, 2009. The Company is vigorously defending these matters and intends to continue to do so.
There are various other claims, lawsuits, disputes with third parties, investigations and pending actions involving various allegations against the Company incident to the operation of its business, principally product liability and intellectual property cases. Each of these matters is subject to various uncertainties, and it is possible that some of these matters may be resolved unfavorably to Biomet. The Company accrues for losses that are deemed to be probable and subject to reasonable estimate. Based on the advice of the Company’s counsel in these matters, management believes that the ultimate outcome of these matters and any liabilities in excess of amounts provided will not have a material adverse impact on the Company’s consolidated financial statements taken as a whole.
Note 14—Related Parties.
Management Services Agreement
Upon completion of the Transactions, we entered into a management services agreement with certain affiliates of the Sponsors, pursuant to which such affiliates of the Sponsors or their Successors, assigns, affiliates, officers, employees and/or representatives and third parties (collectively, the “Managers”) provide management, advisory and consulting services to us. Pursuant to such agreement, the Managers will receive a transaction fee equal to 1% of total enterprise value of the Transactions for the services rendered by such entities related to the Transactions upon entering into the agreement, and an annual monitoring fee equal to 1% of our annual Adjusted EBITDA as compensation for the services rendered and reimbursement for out-of-pocket expenses incurred by the Managers in connection with the agreement and the Transactions. We are required to pay the sponsors a fee on a quarterly basis. In total we paid each of the above sponsors $1.5 million for a total of $6.1 million during fiscal 2008. As of May 31, 2008, the amount payable to the sponsors was $2.3 million. During the 2008 fiscal year we also entered into a consulting agreement with Capstone Consulting, a wholly owned subsidiary of Blackstone, LLC, to perform analysis related to our operational improvement initiative. We paid Capstone Consulting $1.2 million throughout fiscal 2008. We may also pay certain subsequent fees to the Managers for advice rendered in connection with financing or refinancing (equity or debt), acquisition, disposition, spin-off, split-off, dividend, recapitalization, initial underwritten public offering and change of control transactions involving us or any of our subsidiaries. The management services agreement includes customary exculpation and indemnification provisions in favor of the Managers and their affiliates.
The Company currently holds interest rate swaps with Goldman Sachs. As part of this relationship, we receive information from Goldman Sachs that allows us to run a regression on the swaps as part of our required effectiveness testing on a quarterly basis.
Capital Contributions
During the 2008 fiscal year, the Company received a capital contribution from its parent company by trusts affiliated with Dane A. Miller and Mary Louise Miller during the fourth quarter in the amount of $120.0 million. The Company also received an additional capital contribution of $14.4 million from its parent company from the participation of management under the LVB Acquisition Inc., Management Stockholders’ Agreement.
Biomet, Inc. and Subsidiaries Schedule II—Valuation and Qualifying Accounts
For the years ended May 31, 2008 and 2007 (in millions)
| Additions | |||||||||||||||||
Description | Balance at beginning of Period | (1) Charged to costs and expenses | (2) Charged to other accounts - describe | Deductions describe | Balance at end of year | ||||||||||||
Allowance for doubtful receivables: | |||||||||||||||||
For the year ended May 31, 2008 | $ | 84.1 | $ | 28.6 | $ | 1.0 | $ | (32.9 | )(A) | $ | 80.8 | ||||||
For the year ended May 31, 2007 | $ | 0.1 | (C) | ||||||||||||||
| $ | 69.1 | $ | 65.1 | 0.6 | (B) | $ | (50.8 | )(A) | $ | 84.1 | |||||||
For the year ended May 31, 2006 | $ | 59.5 | $ | 21.7 | $ | (0.3 | )(A) | $ | (11.8 | )(A) | $ | 69.1 | |||||
Excess and obsolete inventory reserves: | |||||||||||||||||
For the year ended May 31, 2008 | $ | 153.4 | $ | 56.2 | $ | (5.4 | )(B) | $ | 25.7 | (D) | $ | 178.5 | |||||
For the year ended May 31, 2007 | $ | 99.4 | $ | 67.4 | $ | 4.6 | (B) | $ | 18.0 | (D) | $ | 153.4 | |||||
For the year ended May 31, 2006 | $ | 93.0 | $ | 29.6 | $ | (1.3 | )(B) | $ | 21.9 | (D) | $ | 99.4 | |||||
Notes:
| (A) | Uncollectible accounts written off |
| (B) | Effect of foreign currency translation |
| (C) | Collection of previously written off accounts |
| (D) | Inventory written off |
F-28
Table of Contents
As a result of the Merger, as discussed within these financial statements in Note 1, the Predecessor and Successor companies are not comparable due to a new basis of accounting starting July 12, 2008.
(in millions) | June 1, 2007 to July 11, 2007 (Predecessor) | July 12, 2007 to August 31, 2008 (Successor) | 2nd Qtr. (Successor) | 3rd Qtr. (Successor) | 4th Qtr. (Successor) | Year | ||||||||||||||||||||
2008 | ||||||||||||||||||||||||||
Net sales | $ | 248.8 | $ | 288.6 | $ | 607.2 | $ | 603.1 | $ | 635.6 | $ | 2,383.3 | ||||||||||||||
Gross profit | 146.5 | 181.8 | 362.6 | 341.0 | 434.4 | 1,466.3 | ||||||||||||||||||||
Net loss | (54.6 | ) | (482.2 | ) | (302.0 | ) | (88.5 | ) | (91.5 | ) | (1,018.8 | ) | ||||||||||||||
| 1st Qtr. | 2nd Qtr. | 3rd Qtr. | 4th Qtr. | Year | ||||||||||||||||||||||
2007 | ||||||||||||||||||||||||||
Net sales | $ | 508.2 | $ | 520.3 | $ | 529.5 | $ | 549.4 | $ | 2,107.4 | ||||||||||||||||
Gross profit | 369.5 | 369.0 | 365.8 | 360.9 | 1,465.2 | |||||||||||||||||||||
Net income | 104.4 | 104.7 | 85.3 | 41.5 | 335.9 | |||||||||||||||||||||
Fiscal 2008
| • | Net loss for the period June 1, 2007 to July 11, 2007 was impacted by the merger. The primary charge was $112.8 million related to the payout of in-the-money stock options as a result of the merger. |
| • | Net loss for the period July 12, 2007 to May 31, 2008 was impacted by the merger. Charges related to IPRD, interest expense, inventory step-up, property, plant and equipment step-up, amortization of intangibles, and financing expenses, including accounting, legal, and financing fees on the new debt facilities provided for a total of $1,452.9 million of additional charges to our results of operations. |
Fiscal 2007
| • | Net income for the fourth quarter of fiscal 2007 was adversely impacted by pre-tax charges of $29.9 million related to the renewal and re-negotiation of distribution agreements with existing distributors; $46.3 million related to inventory write-downs and accounts receivable reserves related to its BTBS operations; $8.2 million in expenses related to the Merger Agreement, and retirement/employment costs associated with changes in executive management; and $2 million in legal and accounting fees related to the previously announced stock option investigation. |
| • | Net income for the third quarter of fiscal year 2007 was adversely impacted by pre-tax charges of $11 million related to inventory write-downs related to its BTBS Operations; $15.7 million in additional legal and distribution expenses; and $6.2 million in expenses related to the Merger Agreement. |
F-29
Table of Contents
Biomet, Inc. and Subsidiaries Condensed Consolidated Balance Sheets
(in millions)
| November 30, 2008 | May 31, 2008 | |||||||
| (Unaudited) | ||||||||
Assets | ||||||||
Current assets: | ||||||||
Cash and cash equivalents | $ | 229.3 | $ | 127.6 | ||||
Accounts receivable, net | 483.5 | 486.2 | ||||||
Income tax receivable | 48.8 | 48.8 | ||||||
Inventories | 510.3 | 539.7 | ||||||
Deferred income taxes | 97.1 | 100.7 | ||||||
Prepaid expenses and other | 45.5 | 46.7 | ||||||
Total current assets | 1,414.5 | 1,349.7 | ||||||
Property, plant and equipment, net | 611.9 | 640.9 | ||||||
Investments | 33.8 | 41.3 | ||||||
Intangible assets, net | 5,736.3 | 6,208.2 | ||||||
Goodwill | 5,103.4 | 5,422.8 | ||||||
Other assets | 111.4 | 118.9 | ||||||
Total assets | $ | 13,011.3 | $ | 13,781.8 | ||||
Liabilities & Shareholders’ Equity | ||||||||
Current liabilities: | ||||||||
Short-term borrowings | $ | 72.5 | $ | 75.4 | ||||
Accounts payable | 69.6 | 83.7 | ||||||
Accrued interest | 79.7 | 80.9 | ||||||
Accrued wages and commissions | 64.1 | 79.1 | ||||||
Other accrued expenses | 179.7 | 245.4 | ||||||
Total current liabilities | 465.6 | 564.5 | ||||||
Long-term liabilities: | ||||||||
Long-term debt | 6,124.4 | 6,225.4 | ||||||
Deferred income taxes | 1,902.7 | 2,112.5 | ||||||
Other long-term liabilities | 196.7 | 43.1 | ||||||
Total liabilities | 8,689.4 | 8,945.5 | ||||||
Shareholders’ equity: | ||||||||
Additional paid-in capital | 44.6 | 25.8 | ||||||
Contributed capital | 5,523.2 | 5,521.9 | ||||||
Accumulated deficit | (1,063.8 | ) | (964.2 | ) | ||||
Accumulated other comprehensive income (loss) | (182.1 | ) | 252.8 | |||||
Total shareholders’ equity | 4,321.9 | 4,836.3 | ||||||
Total liabilities and shareholders’ equity | $ | 13,011.3 | $ | 13,781.8 | ||||
The accompanying notes are a part of the condensed consolidated financial statements.
F-30
Table of Contents
Biomet, Inc. and Subsidiaries Condensed Consolidated Statements of Operations
(in millions)
| (Unaudited) Three Months Ended November 30, | (Unaudited) Six Months Ended November 30, 2008 | (Unaudited) July 12 - November 30, 2007 (Successor) | June 1 - July 11, 2007 (Predecessor) | |||||||||||||||||||
| 2008 | 2007 | |||||||||||||||||||||
Net sales | $ | 642.8 | $ | 607.2 | $ | 1,249.8 | $ | 895.8 | $ | 248.8 | ||||||||||||
Cost of sales | 194.9 | 244.6 | 376.4 | 351.4 | 102.3 | |||||||||||||||||
Gross margin | 447.9 | 362.6 | 873.4 | 544.4 | 146.5 | |||||||||||||||||
Selling, general and administrative expense | 254.7 | 413.2 | 508.2 | 600.5 | 194.2 | |||||||||||||||||
Research and development expense | 23.4 | 21.9 | 46.9 | 35.5 | 34.0 | |||||||||||||||||
In-process research and development | — | 86.2 | — | 479.0 | — | |||||||||||||||||
Amortization | 89.8 | 92.3 | 181.3 | 137.5 | 0.5 | |||||||||||||||||
Operating income (loss) | 80.0 | (251.0 | ) | 137.0 | (708.1 | ) | (82.2 | ) | ||||||||||||||
Interest expense, net | (139.2 | ) | (148.7 | ) | (280.3 | ) | (229.1 | ) | (0.3 | ) | ||||||||||||
Other income (expense) | (11.6 | ) | (4.9 | ) | (20.6 | ) | 0.5 | 0.6 | ||||||||||||||
Other income (expense), net | (150.8 | ) | (153.6 | ) | (300.9 | ) | (228.6 | ) | 0.3 | |||||||||||||
Loss before income taxes | (70.8 | ) | (404.6 | ) | (163.9 | ) | (936.7 | ) | (81.9 | ) | ||||||||||||
Benefit from income taxes | (31.1 | ) | (102.6 | ) | (64.3 | ) | (152.5 | ) | (27.3 | ) | ||||||||||||
Net loss | $ | (39.7 | ) | $ | (302.0 | ) | $ | (99.6 | ) | $ | (784.2 | ) | $ | (54.6 | ) | |||||||
The accompanying notes are a part of the condensed consolidated financial statements.
F-31
Table of Contents
Biomet, Inc. and Subsidiaries Condensed Consolidated Statements of Cash Flows
(in millions)
| (Unaudited) Six Months Ended November 30, 2008 | (Unaudited) July 12 - November 30, 2007 (Successor) | June 1 - July 11, 2007 (Predecessor) | ||||||||||||
Cash flows provided by (used in) operating activities: | ||||||||||||||
Net loss | $ | (99.6 | ) | $ | (784.2 | ) | $ | (54.6 | ) | |||||
Adjustments to reconcile net loss to net cash from operating activities: | ||||||||||||||
Depreciation and amortization | 261.4 | 195.2 | 9.3 | |||||||||||
Amortization of deferred financing costs | 5.7 | 4.7 | — | |||||||||||
In-process research and development | — | 479.0 | — | |||||||||||
Stock-based compensation expense | 18.8 | — | — | |||||||||||
Inventory step-up related to merger | — | 92.3 | — | |||||||||||
Allowance for doubtful accounts receivable | (3.5 | ) | — | — | ||||||||||
Loss (gain) and impairment on investments | 6.5 | — | (7.0 | ) | ||||||||||
Provision for inventory obsolescence | 0.4 | — | — | |||||||||||
Deferred income taxes | (69.7 | ) | (248.3 | ) | 76.7 | |||||||||
Excess tax benefit from exercise of stock options | — | — | (3.9 | ) | ||||||||||
Other | (0.1 | ) | (0.3 | ) | — | |||||||||
Changes in operating assets and liabilities, net of effects from acquisitions: | ||||||||||||||
Accounts receivable | (40.5 | ) | (29.7 | ) | 5.8 | |||||||||
Inventories | (25.5 | ) | 20.5 | (12.0 | ) | |||||||||
Prepaid expenses | (2.6 | ) | 35.8 | — | ||||||||||
Accounts payable | (6.7 | ) | (9.9 | ) | (1.6 | ) | ||||||||
Income taxes | (5.7 | ) | 27.9 | — | ||||||||||
Accrued interest | (0.8 | ) | 106.3 | — | ||||||||||
Share-based compensation accrual related to merger | — | — | 112.8 | |||||||||||
Other | 9.5 | 110.1 | (66.1 | ) | ||||||||||
Net cash provided by (used in) operating activities | 47.6 | (0.6 | ) | 59.4 | ||||||||||
Cash flows provided by (used in) investing activities: | ||||||||||||||
Net proceeds from investments | — | 95.8 | 42.8 | |||||||||||
Capital expenditures | (92.9 | ) | (76.7 | ) | (22.0 | ) | ||||||||
Acquisitions, net of cash acquired | (2.2 | ) | (0.4 | ) | (9.8 | ) | ||||||||
Acquisition of Biomet, Inc. | — | (11,638.2 | ) | — | ||||||||||
Net cash provided by (used in) investing activities | (95.1 | ) | (11,619.5 | ) | 11.0 | |||||||||
Cash flows provided by financing activities: | ||||||||||||||
Debt: | ||||||||||||||
Proceeds (payments) under amended revolving credit agreement | 8.5 | (40.4 | ) | 0.2 | ||||||||||
Proceeds (payments) under senior secured credit facility | (18.2 | ) | — | — | ||||||||||
Proceeds (payments) under asset based revolver | 165.4 | — | — | |||||||||||
Proceeds from long-term debt related to merger | — | 6,250.7 | — | |||||||||||
Payment of deferred financing costs | — | (87.1 | ) | — | ||||||||||
Equity: | ||||||||||||||
Capital contributions | 1.9 | 5,401.9 | — | |||||||||||
Repurchase of common shares | (0.6 | ) | — | (2.8 | ) | |||||||||
Excess tax benefit from exercise of stock options | — | — | 3.9 | |||||||||||
Net cash provided by financing activities | 157.0 | 11,525.1 | 1.3 | |||||||||||
Effect of exchange rate changes on cash | (7.8 | ) | 2.0 | 0.1 | ||||||||||
Increase (decrease) in cash and cash equivalents | 101.7 | (93.0 | ) | 71.8 | ||||||||||
Cash and cash equivalents, beginning of period | 127.6 | 176.9 | 105.1 | |||||||||||
Cash and cash equivalents, end of period | $ | 229.3 | $ | 83.9 | $ | 176.9 | ||||||||
Supplemental disclosures of cash flow information: | ||||||||||||||
Cash paid during the period for: | ||||||||||||||
Interest | $ | 277.1 | $ | 1.5 | $ | — | ||||||||
Income taxes | $ | 14.8 | $ | 21.0 | $ | — | ||||||||
The accompanying notes are a part of the condensed consolidated financial statements.
F-32
Table of Contents
Biomet, Inc. and Subsidiaries Notes to Condensed Consolidated Financial Statements (Unaudited)
Note 1—Merger.
On December 18, 2006, Biomet, Inc. entered into an Agreement and Plan of Merger with LVB Acquisition, LLC, a Delaware limited liability company (“LVB”), and LVB Acquisition Merger Sub, Inc., an Indiana corporation and a wholly-owned subsidiary of LVB (“Purchaser”), which agreement was amended and restated as of June 7, 2007 (the “Merger Agreement”). Pursuant to the Merger Agreement, on June 13, 2007, Purchaser commenced a cash tender offer (the “Offer” and together with the Merger, the “Transactions”), to purchase all of Biomet’s outstanding common shares, without par value. LVB is controlled by a consortium of private equity funds: Blackstone Capital Partners V L.P., GS Capital Partners VI Fund, L.P., KKR 2006 Fund L.P. and Texas Pacific Group (each a “Sponsor” and collectively, the “Sponsors”). The Sponsors, along with other investors, contributed $5,387.5 million of equity in connection with the Transactions. The remaining purchase price of $6,245.4 million included various proceeds from credit facilities. The unaudited condensed consolidated financial statements should be read in conjunction with Biomet’s Annual Report on Form 10-K for the fiscal year ended May 31, 2008, as amended.
The Merger was accounted for under the purchase method of accounting pursuant to Statements of Financial Accounting Standards (“SFAS”) No. 141, Business Combinations . Accordingly, the effect of the Merger has been included in the Company’s condensed consolidated statement of operations subsequent to July 11, 2007 (the “Merger Date”), and the respective assets and liabilities have been recorded at their estimated fair values in the Company’s condensed consolidated balance sheet as of the Merger Date, with the excess purchase price recorded as goodwill. As of July 12, 2007, the Successor Company began operating under a new basis of accounting for its financial statements. Because of the new basis of accounting, the Predecessor Company’s historical financial information is not comparable to the Successor Company’s financial information for periods after July 12, 2007. The term “Successor Company” refers to Biomet following its acquisition by Purchaser on July 12, 2007 and the term “Predecessor Company” refers to Biomet prior to its acquisition on July 12, 2007.
The Company has allocated the purchase price to the fair value of the assets and liabilities of Biomet based on estimated fair values utilizing generally accepted valuation methodologies. Both assets and liabilities were valued as of July 11, 2007. On July 12, 2007, 82.4% of the step-up was recorded and combined with 17.6% of the Predecessor Company. On September 25, 2007 (the “Closing Date”), the remaining fair value step-up of 17.6% was recorded. The additional step-up included an increase in the in-process research and development (“IPRD”) charge of $86.2 million, increase of the property plant and equipment fair value of $14.2 million, and an increase in the fair value of inventory of $28.2 million. Also, the Tender Facility (as defined in Note 8 below) starting on July 12, 2007 was refinanced on the Closing Date into various other credit facilities. See Note 8 – Debt below for a description of those facilities. See summary below of the allocation of the total purchase price:
| (in millions) | ||||
Cash | $ | 57.0 | ||
Short-term investments | 126.0 | |||
Accounts receivable | 494.0 | |||
Inventories | 714.3 | |||
Deferred tax assets | 60.6 | |||
Prepaids and other assets | 134.4 | |||
Property, plant and equipment | 608.0 | |||
In-process research and development | 479.0 | |||
Intangible assets | 6,304.5 | |||
Goodwill | 5,303.0 | |||
Deferred tax liabilities | (2,184.9 | ) | ||
Other liabilities | (463.0 | ) | ||
Purchase Price | $ | 11,632.9 | ||
The purchase price allocation was based on information then available to the Company, and expectations, assumptions, and valuation methodologies deemed reasonable by the Company’s management. No assurance can be given, however, that the underlying assumptions used to estimate expected technology based product revenues, development costs or profitability, or the events associated with such technology, will occur as projected. Goodwill recorded as a result of the Merger is not deductible for income tax purposes.
Note 2—Summary of Significant Accounting Policies and Nature of Operations.
General – The Company is one of the largest orthopedic medical device companies in the United States and worldwide with operations and offices in over 50 locations throughout the world and distribution in approximately 90 countries. The Company designs, manufactures and markets a comprehensive range of both surgical and non-surgical products used primarily by orthopedic surgeons and other musculoskeletal medical specialists. For approximately 30 years, the Company has applied advanced engineering and manufacturing technology to the development of highly durable joint replacement systems.
Basis of Presentation – The unaudited condensed consolidated financial statements include the accounts of Biomet, Inc. and its subsidiaries (individually and collectively referred to as “Biomet”, the “Company”, “we”, “us”, or “our”). The unaudited condensed consolidated financial statements include all accounts of Biomet and all of its wholly-owned subsidiaries. The unaudited condensed consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America for condensed financial information. In the opinion of management, all adjustments (consisting of normal recurring accruals) considered necessary for a fair presentation have been included. The Company’s results of operations for the six months ended November 30, 2008 are not comparative to the Company’s results of operations for the period June 1, 2007 to July 11, 2007 because of the new basis of accounting resulting from the Merger Date of July 11, 2007. The purchase price allocation included an IPRD charge of $479.0 million, and step-ups in fair value of inventory of $160.3 million and $80.4 million for fixed assets. The amounts were fully recorded as of the Closing Date of the Merger. Operating results for the period ended November 30, 2008 are not necessarily indicative of the results that may be expected for the fiscal year ending May 31, 2009. For further information, including the Company’s significant accounting policies, refer to the audited consolidated financial statements and notes thereto included in the Company’s Form 10-K for the fiscal year ended May 31, 2008, as amended.
Products – The Company operates in one business segment, musculoskeletal products, which includes the design, manufacture and marketing of products in four major categories: reconstructive products, fixation devices, spinal products and other products. The Company has three reportable geographic segments: United States, Europe and International.
Reconstructive – Orthopedic reconstructive implants are used to replace joints that have deteriorated as a result of disease (principally osteoarthritis) or injury. Reconstructive joint surgery involves the modification of the area surrounding the affected joint and the implantation of one or more manufactured components, and may involve the use of bone cement. The Company’s primary orthopedic reconstructive joints are knees, hips, and shoulders, but the Company manufactures other joints as well. The Company also produces
F-33
Table of Contents
the associated instruments required by orthopedic surgeons to implant the Company’s reconstructive products, as well as bone cements and cement delivery systems. In addition, dental reconstructive devices and associated instrumentation are used for oral rehabilitation through the replacement of teeth and repair of hard and soft tissues.
Fixation – Fixation devices are used for setting and stabilizing damaged bones to support and/or augment the body’s natural healing process. Electrical stimulation devices used in trauma indications offer implantable and non-invasive options to stimulate bone growth. Other products include internal fixation devices (such as nails, plates, screws, pins and wires used to stabilize traumatic bone injuries), external fixation devices (used to stabilize fractures when alternative methods of fixation are not suitable), craniomaxillofacial fixation systems and bone substitute materials.
Spinal – The Company’s spinal products include electrical stimulation devices for spinal applications, spinal fixation systems, bone substitute materials and motion preservation systems, as well as allograft services for spinal applications. These products and services are primarily marketed in the United States under the Biomet Spine trade name.
Other – The Company manufactures and distributes a number of other products, including sports medicine products (used in minimally-invasive orthopedic surgical procedures), orthopedic support products (also referred to as softgoods and bracing products), operating room supplies, casting materials, general surgical instruments, wound care products and other surgical products.
Effect of Foreign Currency – Assets and liabilities of foreign subsidiaries are translated at rates of exchange in effect at the close of their calendar month end. Revenues and expenses are translated at the weighted average exchange rates during the period. Translation gains and losses are accumulated within other comprehensive income (loss) as a separate component of shareholders’ equity. Foreign currency transaction gains and losses resulting from product transfer between subsidiaries are recorded in cost of goods sold. Other foreign currency exchange gains and losses that do not involve the movement of product are included in other income (expense), net.
Cash and Cash Equivalents – The Company considers all highly liquid investments with original maturities of three months or less to be cash equivalents.
Investments – The Company invests the majority of its excess cash in bank deposits and money market securities. The Company also holds municipal bonds, corporate and mortgage-backed securities, common stocks and auction-rate securities. The Company accounts for its investments in debt and equity securities under SFAS 115, Accounting for Certain Investments in Debt and Equity Securities , which requires certain securities to be categorized as trading, available-for-sale or held-to-maturity. The Company also accounts for its investments under SFAS 157, Fair Value Measurements , which establishes a framework for measuring fair value in accordance with generally accepted accounting principles, clarifies the definition of fair value within that framework, and expands disclosures about fair value measurements. Available-for-sale securities are carried at fair value with unrealized gains and losses, net of tax, recorded within other comprehensive income (loss) as a separate component of shareholders’ equity. Held-to-maturity securities are carried at amortized cost. The Company has no trading securities. The cost of investment securities sold is determined by the specific identification method. Dividend and interest income are accrued as earned. The Company reviews its investments quarterly for declines in fair value that are other-than-temporary. Investments that have declined in market value that are determined to be other-than-temporary are charged to other income (expense), net, by writing that investment down to fair value. Investments are classified as short-term for those expected to mature or be sold within twelve months and the remaining portion is classified in long-term investments.
Risk Management
Foreign Currency Instruments – Certain assets, liabilities and forecasted transactions are exposed to foreign currency risk, primarily the fluctuation of the U.S. Dollar against European currencies. The Company faces transactional currency exposures that arise when it or its foreign subsidiaries enter into transactions, primarily on an intercompany basis, denominated in currencies other than their functional currency. The Company also faces currency exposure that arises from translating the results of its global operations to the U.S. Dollar at exchange rates that have fluctuated from the beginning of the period. The Company has hedged a portion of its net investment in its European subsidiaries with the issuance of a €875.0 million principal amount term loan on September 25, 2007. The Company’s net investment in its European subsidiaries at the hedging date of September 25, 2007 was $1,690.0 million (€1,238.0 million). As of November 30, 2008, the Company’s net investment in European subsidiaries totaled €1,483.8 million ($1,884.0 million) and the outstanding principal balance was €866.3 million ($1,099.9 million). The difference of €617.5 million ($784.1 million) remained unhedged. Effectiveness is tested quarterly to determine whether hedge treatment is still appropriate. The Company tests effectiveness on this net investment hedge by determining if the net investment in its European subsidiaries is greater than the outstanding Euro denominated debt balance. Any ineffectiveness is recorded in the statement of operations.
Interest Rate Instruments – The Company entered into interest rate swap agreements (cash flow hedges) in both U.S. Dollars and Euros on September 25, 2007 and March 25, 2008 as a means of fixing the interest rate on portions of its floating-rate debt instruments. See the table below for existing contracts (U.S. Dollars and Euros in millions):
(in millions)
Structure | Currency | Notional Amount | Termination Date | Fair Value at November 30, 2008 Asset (Liability) | |||||||
1 year | Euro | € | 75.0 | September 25, 2009 | $ | (0.9 | ) | ||||
2 year | Euro | 75.0 | September 25, 2010 | (2.5 | ) | ||||||
3 year | Euro | 50.0 | March 25, 2011 | (1.3 | ) | ||||||
| Euro | 75.0 | September 25, 2011 | (3.6 | ) | |||||||
4 year | Euro | 40.0 | March 25, 2012 | (1.3 | ) | ||||||
| Euro | 230.0 | September 25, 2012 | (13.4 | ) | |||||||
5 year | Euro | 40.0 | March 25, 2013 | (1.5 | ) | ||||||
1 year | USD | $ | 195.0 | September 25, 2009 | (4.7 | ) | |||||
2 year | USD | 150.0 | March 25, 2010 | (0.6 | ) | ||||||
| USD | 195.0 | September 25, 2010 | (9.8 | ) | |||||||
3 year | USD | 110.0 | March 25, 2011 | (1.2 | ) | ||||||
| USD | 195.0 | September 25, 2011 | (15.3 | ) | |||||||
4 year | USD | 140.0 | March 25, 2012 | (2.4 | ) | ||||||
| USD | 585.0 | September 25, 2012 | (57.5 | ) | |||||||
5 year | USD | 190.0 | March 25, 2013 | (4.8 | ) | ||||||
Total | $ | (120.8 | ) | ||||||||
F-34
Table of Contents
The interest rate swaps were a liability of $120.8 million at November 30, 2008 and are included in other accrued expenses and other long term liabilities. As a result of cash flow hedge treatment being applied, all unrealized gains and losses related to the derivative instruments are included in other comprehensive income and are reclassified into operations in the same period in which the hedged transaction affects earnings. Effectiveness is tested quarterly to determine if hedge treatment is still appropriate. The amount of ineffectiveness recognized in operations was not material for any period presented. Subsequent to November 30, 2008, the Company entered into an additional swap contract to hedge the variable interest rate exposure of its U.S. Dollar term loan. The notional amount of the contract is $325.0 million and the maturity date is December 25, 2013. The Company did not enter into any derivative instruments prior to fiscal 2008.
As of November 30, 2008, the effective interest rate, including the applicable lending margin, on 76% ($1,760.0 million) of the outstanding principal of the Company’s U.S. Dollar term loan was fixed at 7.34% through the use of interest rate swaps. The effective interest rate on 68% (€585.0 million) of the outstanding principal of the Company’s Euro term loan was fixed at 7.31% through the use of interest rate swaps. The remaining unhedged balances of the U.S. Dollar and Euro term loans had effective interest rates of 6.76% and 8.14%, respectively.
Comprehensive Income – Total comprehensive income combines reported net loss and foreign currency translation adjustments, unrealized appreciation/depreciation of available-for-sale securities, unrealized gains and losses related to the net investment in the Euro term loan, and unrecognized actuarial loss on pension assets and interest rate swap derivatives. Amounts in accumulated other comprehensive income are presented net of the related tax impact. Foreign currency translation adjustments are not currently adjusted for income taxes, as they relate to permanent investments in international subsidiaries.
Other comprehensive income (loss) and the related components as included in total comprehensive income (loss) are included in the table below:
| Three Months Ended November 30, | Six Months Ended November 30, 2008 | July 12 - November 30, 2007 (Successor) | June 1, - July 11, 2007 (Predecessor) | |||||||||||||||||||
| (in millions) | 2008 | 2007 | ||||||||||||||||||||
Net loss | $ | (39.7 | ) | $ | (302.0 | ) | $ | (99.6 | ) | $ | (784.2 | ) | $ | (54.6 | ) | |||||||
Other comprehensive income (loss), net of tax: | ||||||||||||||||||||||
Foreign currency translation adjustments | (243.4 | ) | (5.1 | ) | (373.4 | ) | 0.5 | (6.6 | ) | |||||||||||||
Unrealized loss on interest rate swaps | (55.7 | ) | — | (62.2 | ) | — | — | |||||||||||||||
Unrealized gain (loss) on available-for-sale securities | (1.3 | ) | 0.2 | 0.7 | — | — | ||||||||||||||||
Total other comprehensive income (loss), net of tax | (300.4 | ) | (4.9 | ) | (434.9 | ) | 0.5 | (6.6 | ) | |||||||||||||
Total other comprehensive loss | $ | (340.1 | ) | $ | (306.9 | ) | $ | (534.5 | ) | $ | (783.7 | ) | $ | (61.2 | ) | |||||||
Concentrations of Credit Risk and Allowance for Doubtful Receivables – The Company provides credit, in the normal course of business, to hospitals, private and governmental institutions and healthcare agencies, insurance providers, dental practices and laboratories, and physicians. The Company maintains an allowance for doubtful receivables based on estimated collection rates and charges actual losses to the allowance when incurred. The estimated collection rates require management judgment.
Other Loss Contingencies – The Company has self-insured reserves against product liability claims with insurance coverage above the retention limits. There are various other claims, lawsuits, disputes with third parties, investigations and pending actions involving various allegations against it. Product liability claims are routinely reviewed by the Company’s insurance carrier and management routinely reviews all claims for purposes of establishing ultimate loss estimates. In addition, management must determine the estimated liability for claims incurred, but not reported. Such estimates and any subsequent changes in estimates may result in adjustments to the Company’s operating results in the future.
Revenue Recognition – The Company sells product through four principal channels: (1) direct to healthcare institutions, referred to as direct channel accounts, (2) through stocking distributors and healthcare dealers, (3) indirectly through insurance companies and (4) directly to dental practices and dental laboratories. Sales through the direct and distributor/dealer channels account for a majority of net sales. Through these channels, inventory is generally consigned to sales agents or customers so that products are available when needed for surgical procedures. Revenue is not recognized upon the placement of inventory into consignment as the Company retains title and maintains the inventory on the balance sheet; however, it is recognized upon implantation and receipt of proper purchase order and/or purchase requisition documentation. Pricing for products is generally predetermined by contracts with customers, agents acting on behalf of customer groups or by government regulatory bodies, depending on the market. Price discounts under group purchasing contracts are generally linked to volume of implant purchases by customer healthcare institutions within a specified group. At negotiated thresholds within a contract buying period, price discounts may increase. At certain locations the Company records a contractual allowance that is offset against revenue for each sale to a non-contracted payor so that revenue is recorded at the estimated determinable price at the time of the sale. At certain locations revenue is recognized on sales to stocking distributors, healthcare dealers, dental practices and dental laboratories when title to product passes to them, generally upon shipment. Certain subsidiaries allow customers to return product in the event that the Company terminates the relationship. Under those circumstances, the Company records an estimated sales return in the period in which constructive notice of termination is given to a distributor. Product returns were not significant for any period presented.
Research and Development – Research and development costs are charged to expense as incurred. IPRD is recognized in business combinations or asset acquisitions for the portion of the purchase price allocated to the appraised value of in-process technologies, defined as those technologies relating to products that have not received approval of the U.S Food and Drug Administration and have no alternative future use, consistent with SFAS 2, Accounting for Research and Development Costs , and Financial Accounting Standards Board Interpretation (“FIN”) 4, Applicability of SFAS 2 to Business Combinations.
Income Taxes – The Company records income tax estimates in accordance with SFAS 109, Accounting for Income Taxes, and FIN 48, Accounting for Uncertainty in Income Taxes – an interpretation of FASB Statement 109 (“FIN 48”); however, there are inherent risks that could create uncertainties related to the estimates. The Company adjusts estimates based on normal operating circumstances and conclusions related to tax audits. The Company does not believe any audit finding could materially affect its financial position; however there could be a material impact on the Company’s consolidated results of operations and cash flows of a given period.
Management’s Estimates and Assumptions – In preparing the financial statements in accordance with accounting principles generally accepted in the United States of America, management must often make estimates and assumptions that affect the reported amounts of assets, liabilities, revenues, expenses and related disclosures at the date of the financial statements and during the reporting period. Some of those judgments can be subjective and complex. Consequently, actual results could differ from those estimates.
Change in Accounting Principle – As of the Merger Date, the Company eliminated the one-month lag in reporting for certain subsidiaries in non-domestic locations. The elimination of the one-month lag is considered a change in accounting principle adopted in conjunction with the Merger and was applied prospectively. The effect of the elimination is not considered material to the condensed consolidated financial statements as of May 31, 2008, and for the period July 12, 2007 through November 30, 2007.
F-35
Table of Contents
Recent Accounting Pronouncements
SFAS 141R – In December 2007, the Financial Accounting Standards Board (“FASB”) issued SFAS 141R (revised 2007), Business Combinations. SFAS 141R establishes principles and requirements for how the acquirer in a business combination recognizes and measures in its financial statements, the identifiable assets acquired, the liabilities assumed and any noncontrolling interest in the acquiree at the acquisition date at fair value. SFAS 141R determines what information to disclose to enable users of the financial statements to evaluate the nature and financial effects of the business combination. SFAS 141R applies prospectively to business combinations for which the acquisition date is on or after the beginning of the first annual reporting period beginning on or after December 15, 2008. Early adoption is not permitted. The Company is currently evaluating the effect the adoption of FAS 141R will have on its unaudited condensed consolidated financial statements.
SFAS 157 – Effective June 1, 2008, the Company adopted FASB SFAS No. 157, Fair Value Measurements (“SFAS 157”). SFAS 157 establishes a framework for measuring fair value in accordance with generally accepted accounting principles, clarifies the definition of fair value within that framework, and expands disclosures about fair value measurements. SFAS 157 applies whenever other standards require (or permit) assets or liabilities to be measured at fair value, except for the measurement of share-based payments. SFAS 157 does not expand the use of fair value in any new circumstances. On February 12, 2008, the FASB issued FASB Staff Position (“FSP”) FAS 157-2, Effective Date of FASB Statement No. 157 (FSP FAS 157-2). FSP FAS 157-2 defers the implementation of SFAS 157 for certain nonfinancial assets and nonfinancial liabilities. Accordingly, the Company adopted the required provisions of SFAS 157 at the beginning of fiscal year 2009 and the remaining provisions will be adopted by the Company at the beginning of fiscal year 2010. The fiscal year 2009 adoption did not result in a material impact to the Company’s financial statements (see Note 6). The Company is currently evaluating the impact of adopting the remaining parts of SFAS 157 in fiscal year 2010 in accordance with FSP FAS No. 157-2. In October 2008, the FASB issued FASB Staff Position No. 157-3, Determining the Fair Value of a Financial Asset When the Market for That Asset is Not Active , which clarifies the application of SFAS 157 in a market that is not active and provides an example to illustrate key considerations in determining fair value of a financial asset when the market for that financial asset is not active.
SFAS 159 – In February 2007, the FASB issued SFAS 159, Establishing the Fair Value Option for Financial Assets and Liabilities, to permit all entities to choose to elect to measure eligible financial instruments at fair value. SFAS 159 applies to fiscal years beginning after November 15, 2007, with early adoption permitted for an entity that has also elected to apply the provisions of SFAS 157 . An entity is prohibited from retrospectively applying SFAS 159, unless it chooses early adoption. On June 1, 2008 the Company did not elect the fair value option for financial assets and liabilities held at June 1, 2008.
SFAS 160 – In December 2007, the FASB issued SFAS 160, Noncontrolling Interests in Consolidated Financial Statements – an amendment of ARB 51 . SFAS 160 establishes accounting and reporting standards that require noncontrolling interests to be reported as a component of equity, changes in a parent’s ownership interest while the parent retains its controlling interest be accounted for as equity transactions, and any retained noncontrolling equity investment upon the deconsolidation of a subsidiary be initially measured at fair value. This statement is effective for fiscal years, and interim periods within those fiscal years, beginning on or after December 15, 2008. Earlier adoption is prohibited. The Company does not expect the adoption of SFAS 160 to have a material impact on its consolidated financial statements.
SFAS 161 – In March 2008, the FASB issued SFAS 161, Disclosures about Derivative Instruments and Hedging Activities-an Amendment of FASB Statement No. 133 . This statement requires entities that utilize derivative instruments to provide qualitative disclosures about their objectives and strategies for using such instruments, as well as any details of credit-risk-related contingent features contained within derivatives. It also requires entities to disclose additional information about the amounts and location of derivatives located within the financial statements, how the provisions of SFAS No. 133 have been applied and the impact that hedges have on an entity’s financial position, financial performance and cash flows. This statement is effective for fiscal years, and interim periods within those fiscal years, beginning after November 15, 2008, with early adoption encouraged. The Company does not expect the adoption of SFAS 161 will have a material impact on its consolidated financial statements.
SFAS 162 – In May 2008, the FASB issued SFAS No. 162, The Hierarchy of Generally Accepted Accounting Principles. SFAS 162 identifies the sources of accounting principles and the framework for selecting the principles to be used in the preparation of financial statements of nongovernmental entities that are presented in conformity with generally accepted accounting principles in the United States of America. SFAS 162 is effective 60 days following the SEC’s approval of the Public Company Accounting Oversight Board amendments to AICPA Codification of Auditing Standards, AU Section 411, The Meaning of Present Fairly in Conformity with Generally Accepted Accounting Principles . The Company does not expect the adoption of SFAS 162 will have a material impact on its consolidated financial statements.
FASB Staff Position No. 140-4 and FIN 46(R)-8 – In December 2008, the FASB issued FASB Staff Position No. 140-4 and FIN 46(R)-8, Disclosures by Public Entities (Enterprises) about Transfers of Financial Assets and Interests in Variable Interest Entities . FAS 140-4 and FIN 46(R)-8 require additional disclosures about an entity’s involvement with variable interest entities and transfers of financial assets. FAS 140-4 and FIN 46(R)-8 will become effective for the Company’s fiscal year beginning June 1, 2009. The Company is currently evaluating the effect the adoption of FAS 140-4 and FIN 46(R)-8 will have on its consolidated financial statements.
FASB Staff Position No. 142-3 – In April 2008, the FASB issued FASB Staff Position No. 142-3, Determination of the Useful Life of Intangible Assets (FSP142-3). FSP 142-3 amends the factors that should be considered in developing renewal or extension assumptions that are used to determine the useful life of a recognized intangible asset under FASB Statement No. 142, Goodwill and Other Intangible Assets , and requires enhanced related disclosures. FSP 142-3 must be applied prospectively to all intangible assets acquired as of and subsequent to fiscal years beginning after December 15, 2008. The Company is in the process of determining the impact, if any, that the adoption of FSP 142-3 will have on its consolidated financial statements.
Emerging Issues Task Force (EITF) Issue No. 07-3 – In June 2007, the FASB Emerging Issues Task Force issued EITF-07-3, Accounting for Nonrefundable Advance Payments for Goods or Services Received for Use in Future Research and Development Activities . EITF 07-3 provides guidance for entities that may make nonrefundable advance payments for goods or services that will be used in future research and development activities and whether the advance payment should be expensed when the advance payment is made or when the research and development activity has been performed. EITF 07-3 is effective for financial statements issued for fiscal years beginning after December 15, 2007. On June 1, 2008 the Company adopted EITF 07-3 and the impact was immaterial to its unaudited condensed consolidated financial statements.
EITF Issue No. 07-1 – In December 2007, the FASB issued EITF 07-1, Accounting for Collaborative Agreements (EITF 07-1). EITF 07-1 provides guidance regarding financial statement presentation and disclosure of collaborative arrangements, as defined, which includes arrangements the Company has entered into regarding development and commercialization of products. EITF 07-1 is effective for the Company as of March 1, 2009. The Company has not yet completed its evaluation of EITF 07-1, but does not currently believe that adoption will have a material impact on its unaudited condensed consolidated financial statements.
F-36
Table of Contents
Note 3—Inventories.
Inventories are stated at lower of cost or market, with cost determined under the first-in, first-out method. The Company reviews inventory on hand and writes down excess and slow-moving inventory based on an assessment of future demand and historical experience. Inventories consisted of the following:
| (in millions) | November 30, 2008 | May 31, 2008 | ||||
Raw materials | $ | 91.9 | $ | 89.6 | ||
Work-in-process | 54.0 | 57.9 | ||||
Finished goods | 144.9 | 155.9 | ||||
Consigned distributor | 219.5 | 236.3 | ||||
Inventories | $ | 510.3 | $ | 539.7 | ||
Note 4—Property, Plant and Equipment.
Property, plant and equipment are carried at cost less accumulated depreciation. Depreciation is computed by the straight-line method over the estimated useful lives of 3 to 30 years. Related maintenance and repairs are expensed as incurred. In accordance with SFAS 144, Accounting for the Impairment or Disposal of Long-Lived Assets , the Company reviews property, plant and equipment for impairment whenever events or changes in circumstances indicate that the carrying value of an asset may not be recoverable. An impairment loss would be recognized when estimated undiscounted future cash flows relating to the asset are less than its carrying amount, with the amount of the loss equal to the excess of carrying cost of the asset over fair value. Depreciation on instruments is included within cost of sales. Property, plant and equipment consisted of the following:
| (in millions) | November 30, 2008 | May 31, 2008 | ||||||
Land and land improvements | $ | 45.4 | $ | 49.3 | ||||
Buildings and leasehold improvements | 121.7 | 125.5 | ||||||
Machinery and equipment | 232.6 | 246.6 | ||||||
Instruments | 311.0 | 323.9 | ||||||
Construction in progress | 20.5 | 13.5 | ||||||
Total property, plant and equipment | 731.2 | 758.8 | ||||||
Accumulated depreciation | (119.3 | ) | (117.9 | ) | ||||
Total property, plant and equipment, net | $ | 611.9 | $ | 640.9 | ||||
Note 5—Investments.
At November 30, 2008, the Company’s investment securities were classified as follows:
| (in millions) | Amortized Cost | Unrealized | Fair Value | ||||||||||
| Gains | Losses | ||||||||||||
Available-for-sale: | |||||||||||||
Debt securities | $ | 36.3 | $ | — | $ | (5.9 | ) | $ | 30.4 | ||||
Equity securities | 0.7 | — | — | 0.7 | |||||||||
Mortgage-backed securities | 0.7 | — | (0.2 | ) | 0.5 | ||||||||
Total available-for-sale | 37.7 | — | (6.1 | ) | 31.6 | ||||||||
Held-to-maturity: | |||||||||||||
Debt securities | 1.5 | — | — | 1.5 | |||||||||
Total held-to-maturity | 1.5 | — | — | 1.5 | |||||||||
Certificates of deposit | 0.7 | — | — | 0.7 | |||||||||
Total | $ | 39.9 | $ | — | $ | (6.1 | ) | $ | 33.8 | ||||
At May 31, 2008, the Company’s investment securities were classified as follows:
| (in millions) | Amortized Cost | Unrealized | Fair Value | ||||||||||
| Gains | Losses | ||||||||||||
Available-for-sale: | |||||||||||||
Debt securities | $ | 36.3 | $ | — | $ | (3.8 | ) | $ | 32.5 | ||||
Equity securities | 0.7 | 0.1 | — | 0.8 | |||||||||
Mortgage-backed securities | 5.9 | — | (0.1 | ) | 5.8 | ||||||||
Total available-for-sale | 42.9 | 0.1 | (3.9 | ) | 39.1 | ||||||||
Held-to-maturity: | |||||||||||||
Debt securities | 1.5 | — | — | 1.5 | |||||||||
Total held-to-maturity | 1.5 | — | — | 1.5 | |||||||||
Certificates of deposit | 0.7 | — | — | 0.7 | |||||||||
Total | $ | 45.1 | $ | 0.1 | $ | (3.9 | ) | $ | 41.3 | ||||
The net proceeds from sales of available-for-sale securities were $85.6 million and $42.8 million for the three months ended November 30, 2007 and for the period July 12, 2007 through November 30, 2007, respectively. There were no sales or purchases of available-for-sale securities for the three and six months ended November 30, 2008 or for the three months ended November 30, 2007. There were no sales of held-to-maturity securities for any period presented. The cost of marketable securities sold is determined by the specific identification method. For the period June 1, 2007 through July 11, 2007, net realized gains on sales of available-for-sale securities were $0.1 million. There were no net realized gains and (losses) on sales for available-for-sale securities for the three and six months ended November 30, 2008, for the three months ended November 30, 2007, or for the period July 12, 2007 through November 30, 2007.
As of November 30, 2008, the Company held auction-rate securities of $28.6 million. They are AAA rated securities with long-term nominal maturities secured by student loans, which are guaranteed by the U.S. Government. Each of these securities was subject to auction processes for which there were insufficient bidders on the scheduled rollover dates. The Company will not be able to liquidate any of its remaining auction-rate securities until a future auction is successful, a buyer is found outside of the auction process (a secondary market develops), a broker/dealer buys them back, or the notes are redeemed. These auction-rate securities have been classified as long-term available-for-sale securities as of November 30, 2008 because of the inability to predict when the market will stabilize. A significant portion of these auction-rate securities are held by the Company’s captive insurance company as part of
F-37
Table of Contents
required capital. The securities continue to earn and be paid interest at the maximum contractual rate. The Company has evaluated these securities for temporary or other-than-temporary impairment at November 30, 2008. In doing so, the Company has considered a variety of factors, including intent, liquidity factors, ability to generate alternative cash, other broker pricing, and internally-generated fair value analysis. The Company recorded an unrealized loss in comprehensive income of $3.2 million as of May 31, 2008 and an additional amount of $2.2 million as of November 30, 2008 related to these securities.
The Company reviews its impairments in accordance with SFAS 115, Accounting for Certain Investments in Debt and Equity Securities, Staff Accounting Bulletin Topic 5M, Miscellaneous Accounting and Financial Accounting Standards Board Staff Position, SFAS 115-1 and 124-1, The Meaning of Other-Than-Temporary Impairment and Its Application to Certain Investments , to determine if impairment is “temporary” or “other-than-temporary.” The Company reviews several factors to determine whether losses are other-than-temporary, including but not limited to (1) the length of time each security was in an unrealized loss position, (2) the extent to which fair value was less than cost, (3) the financial condition and near-term prospects of the issuer or insurer, and (4) the Company’s intent and ability to hold each security for a period of time sufficient to allow for any anticipated recovery in fair value.
Note 6—Fair Value Measurements.
As discussed in Note 2, the Company adopted SFAS 157 effective June 1, 2008, with respect to fair value measurements of (a) nonfinancial assets and liabilities that are recognized or disclosed at fair value in the Company’s financial statements on a recurring basis (at least annually) and (b) all financial assets and liabilities. SFAS 157 clarifies the definition of fair value, establishes a framework for measuring fair value, and expands the disclosures on fair value measurements.
Under SFAS 157, fair value is defined as the exit price, or the amount that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants as of the measurement date. SFAS 157 also establishes a hierarchy for inputs used in measuring fair value that maximizes the use of observable inputs and minimizes the use of unobservable inputs by requiring that the most observable inputs be used when available. Observable inputs are inputs market participants would use in valuing the asset or liability developed based on market data obtained from sources independent of the Company. Unobservable inputs are inputs that reflect the Company’s assumptions about the factors market participants would use in valuing the asset or liability developed based upon the best information available in the circumstances. The categorization of financial assets and financial liabilities within the valuation hierarchy is based upon the lowest level of input that is significant to the fair value measurement. The hierarchy is broken down into three levels defined as follows:
| • | Level 1 – Inputs are quoted prices in active markets for identical assets or liabilities. The Company’s Level 1 assets include money market funds, treasury bonds, and marketable equity securities. |
| • | Level 2 – Inputs include quoted prices for similar assets or liabilities in active markets, quoted prices for identical or similar assets or liabilities in markets that are not active, and inputs (other than quoted prices) that are observable for the asset or liability, either directly or indirectly. The Company’s Level 2 assets and liabilities primarily include agency bonds, corporate debt securities, asset-backed securities, certain mortgage-backed securities, and interest rate swaps whose value is determined using a pricing model with inputs that are observable in the market or can be derived principally from or corroborated by observable market data. |
| • | Level 3 – Inputs are unobservable for the asset or liability. The Company’s Level 3 assets include auction-rate securities and other equity investments. See the section below titled Level 3 Valuation Techniques for further discussion of how the Company determines fair value for investments classified as Level 3. |
Assets and Liabilities that are Measured at Fair Value on a Recurring Basis
For the Company, effective June 1, 2008, fair value under SFAS 157 is principally applied to financial assets and liabilities such as marketable equity securities and debt securities that are classified and accounted for as available-for-sale, investments in equity and other securities, and derivative instruments consisting of interest rate swaps. These items were previously and will continue to be marked-to-market at each reporting period; however, the definition of fair value used for mark-to-market accounting is now applied using SFAS 157. The information in the following paragraphs and tables primarily addresses matters relative to these financial assets and liabilities. Separately, there were no material fair value measurements with respect to nonfinancial assets or liabilities that are recognized or disclosed at fair value in the Company’s financial statements on a recurring basis subsequent to the effective date of SFAS 157.
The following table provides information by level for assets and liabilities that are measured at fair value, as defined by SFAS 157, on a recurring basis.
| Fair Value at November 30, 2008 | Fair Value Measurements Using Inputs Considered as | |||||||||||
| (in millions) | Level 1 | Level 2 | Level 3 | |||||||||
Assets: | ||||||||||||
Corporate debt securities | $ | 3.3 | $ | — | $ | 3.3 | $ | — | ||||
Auction-rate securities | 28.6 | — | — | 28.6 | ||||||||
Mortgage-backed securities | 0.5 | — | 0.5 | — | ||||||||
Government and agency securities | — | — | — | — | ||||||||
Certificates of deposit | 0.7 | 0.7 | — | — | ||||||||
Other equity securities | 0.7 | 0.2 | — | 0.5 | ||||||||
Total assets | $ | 33.8 | $ | 0.9 | $ | 3.8 | $ | 29.1 | ||||
Liabilities: | ||||||||||||
Interest rate swaps | $ | 120.8 | $ | — | $ | 120.8 | $ | — | ||||
Total liabilities | $ | 120.8 | $ | — | $ | 120.8 | $ | — | ||||
Level 3 Valuation Techniques
Financial assets are considered Level 3 when their fair values are determined using pricing models, discounted cash flow methodologies or similar techniques and at least one significant model assumption or input is unobservable. Level 3 financial assets also include certain investment securities for which there is limited market activity such that the determination of fair value requires significant judgment or estimation. Level 3 investment securities primarily include certain auction-rate securities and other equity investments for which there was a decrease in the observation of market pricing. At November 30, 2008, these securities were valued primarily using internal cash flow valuation that incorporates transaction details such as contractual terms, maturity, timing and amount of future cash flows, as well as assumptions about liquidity and credit valuation adjustments of marketplace participants at November 30, 2008.
F-38
Table of Contents
The following table provides a reconciliation of the beginning and ending balances of items measured at fair value on a recurring basis in the table above that used significant unobservable inputs (Level 3).
| (in millions) | ||||
Balance at May 31, 2008 | $ | 31.3 | ||
Total realized losses included in earnings | — | |||
Total unrealized losses included in other comprehensive income | (2.2 | ) | ||
Purchases, issuances, and settlements | — | |||
Net transfers in (out) of Level 3 | — | |||
Balance at November 30, 2008 | $ | 29.1 | ||
Realized gains or losses included in earnings are included in other income (expense), net in the consolidated statement of operations.
Assets and Liabilities that are Measured at Fair Value on a Nonrecurring Basis
During the six months ended November 30, 2008, the Company had no significant measurements of financial assets or liabilities at fair value on a nonrecurring basis subsequent to their initial recognition.
The aspects of SFAS 157 for which the effective date was deferred under FSP No. 157-2 until fiscal year 2010 relate to nonfinancial assets and liabilities that are measured at fair value, but are recognized or disclosed at fair value on a nonrecurring basis. This deferral applies to such items as nonfinancial assets and liabilities initially measured at fair value in a business combination (but not measured at fair value in subsequent periods) or nonfinancial long-lived asset groups measured at fair value for an impairment assessment.
Note 7—Goodwill and Other Intangible Assets.
The Company follows SFAS 142, Goodwill and Other Intangible Assets. Accordingly, goodwill and indefinite lived intangible assets are not amortized but are tested for impairment at least annually or more frequently if impairment indicators arise. The latest impairment assessment of goodwill and indefinite lived intangible assets was completed in the fourth quarter of fiscal 2007. Future impairment tests will be performed annually in the fiscal fourth quarter, or sooner if warranted.
The balance of goodwill as of November 30, 2008 and May 31, 2008 was $5,103.4 million and $5,422.8 million, respectively. The change in goodwill from May 31, 2008 to November 30, 2008 was a result of foreign currency fluctuations, primarily the weakening of the Euro against the U.S. Dollar.
The Company uses an accelerated method for amortizing customer relationship intangibles as the value for those relationships is greater at the beginning of their life. The change in intangible assets reflects foreign currency fluctuations, primarily the weakening of the Euro against the U.S. Dollar, as well as amortization.
Intangible assets consisted of the following at November 30, 2008 and May 31, 2008 (in millions):
| November 30, 2008 | May 31, 2008 | |||||||||||||||||||||
| Gross Carrying Amount | Accumulated Amortization | Net Carrying Amount | Gross Carrying Amount | Accumulated Amortization | Net Carrying Amount | |||||||||||||||||
Core technology | $ | 2,080.6 | $ | (148.0 | ) | $ | 1,932.6 | $ | 2,080.6 | $ | (93.8 | ) | $ | 1,986.8 | ||||||||
Completed technology | 720.4 | (74.9 | ) | 645.5 | 720.4 | (47.5 | ) | 672.9 | ||||||||||||||
Product trade names | 178.0 | (13.5 | ) | 164.5 | 178.0 | (8.5 | ) | 169.5 | ||||||||||||||
Customer relationships | 2,917.5 | (274.8 | ) | 2,642.7 | 2,917.5 | (173.1 | ) | 2,744.4 | ||||||||||||||
Sub-total | 5,896.5 | (511.2 | ) | 5,385.3 | 5,896.5 | (322.9 | ) | 5,573.6 | ||||||||||||||
Corporate trade names | 408.0 | — | 408.0 | 408.0 | — | 408.0 | ||||||||||||||||
Currency translation | (57.8 | ) | 0.8 | (57.0 | ) | 233.0 | (6.4 | ) | 226.6 | |||||||||||||
Total | $ | 6,246.7 | $ | (510.4 | ) | $ | 5,736.3 | $ | 6,537.5 | $ | (329.3 | ) | $ | 6,208.2 | ||||||||
The weighted average useful life of the intangibles at November 30, 2008 was as follows:
| Weighted Average Useful Life | ||
Core technology | 19 Years | |
Completed technology | 13 Years | |
Product trade names | 17 Years | |
Customer relationships | 18 Years | |
Corporate trade names | Indefinite life |
Note 8—Debt.
Bank Borrowing – In connection with the Merger, the Company entered into a credit agreement dated July 11, 2007 for a $6,165.0 million senior secured term loan facility, or the “Tender Facility”, pursuant to which Purchaser borrowed $4,181.0 million to finance a portion of the Offer and pay related fees and expenses.
F-39
Table of Contents
The Company refinanced all amounts borrowed under the Tender Facility at the Closing Date of the Merger. On the Closing Date, the Company refinanced the Tender Facility with senior secured credit facilities (which include term loan facilities, a cash flow revolving facility and an asset based revolving credit facility), senior notes, senior subordinated notes and unsecured bridge facilities. The senior secured cash flow facility and all of the notes are guaranteed by the Company subject to certain exceptions, and each of its existing and future wholly-owned domestic subsidiaries. The senior secured asset-based facility is guaranteed by the Company and secured, subject to certain exceptions, by a first-priority security interest in substantially all of the Company’s assets and the assets of subsidiary borrowers that consist of all accounts receivable, inventory, cash, deposit accounts, and certain related intangible assets. The facilities and notes bear interest at the rates set forth below. Interest is payable in cash, except with respect to the Company’s ability to elect to pay PIK (Payment-in-kind) interest, rather than cash interest, on the senior toggle notes through October 15, 2012 for any interest period other than the initial interest period. The Company has not made this election at November 30, 2008. The terms and book value of each instrument at November 30, 2008 are set forth below:
| (Dollars and Euros in millions) | Maturity Date | Interest Rate | Currency | November 30, 2008 | Premium on Notes at November 30, 2008 | |||||||
Debt Instruments | ||||||||||||
European facilities | Primarily | Euro | € | 35.2 | — | |||||||
| Euribor + 1.40% | $ | 44.7 | — | |||||||||
Term loan facility | March 25, 2015 | Libor + 3.00% | US Dollars | $ | 2,316.5 | — | ||||||
Term loan facility | March 25, 2015 | Libor + | Euro | € | 866.3 | — | ||||||
| 3.00% | $ | 1,099.9 | — | |||||||||
Cash flow revolving credit facility | September 25, 2013 | Libor + 2.75% | US Dollars | $ | — | — | ||||||
Cash flow revolving credit facility | September 25, 2013 | Libor + 2.75% | Euro | € | — | — | ||||||
Asset-based revolving credit facility | September 25, 2013 | Libor + 1.75% | US Dollars | $ | 165.4 | — | ||||||
Senior cash pay notes | October 15, 2017 | 10% | US Dollars | $ | 775.0 | $ | 2.1 | |||||
Senior toggle notes | October 15, 2017 | 103/8% / 111/8% | US Dollars | $ | 775.0 | $ | 1.1 | |||||
Senior subordinated notes | October 15, 2017 | 115/8% | US Dollars | $ | 1,015.0 | $ | 2.2 | |||||
The Company currently elects to use 3-month Libor for setting the interest rates on its U.S. Dollar and Euro term loans. The 3-month Libor rates for the U.S. Dollar and Euro in effect as of November 30, 2008 were 3.76% and 5.14%, respectively. The term loan facilities require quarterly principal payments equal to one quarter percent (0.25%) of the original principal balance (equal payments each quarter) which commenced on the last business day of December 2007, and continue on the last business day of each calendar year quarter with the remaining outstanding principal due on the maturity date. The Company made required payments of $5.9 million both June 30, 2008 and September 30, 2008 for the U.S. Dollar denominated term loan facility, and made required payments of $3.4 million and $3.0 million on June 30, 2008 and September 30, 2008, respectively, for the Euro denominated term loan facility. There were borrowings under the asset-based revolver of $165.4 million as of November 30, 2008. The cash flow and asset-based revolvers and the notes do not have terms for mandatory principal pay downs. To calculate the U.S. Dollar equivalent on outstanding balances for disclosure purposes, the Company used a currency conversion rate of 1 Euro to $1.2697, which represents the currency exchange rate from Euros to U.S. Dollars on November 30, 2008.
During the quarter ended November 30, 2008, Lehman Brothers Holdings Inc. (Lehman), whose subsidiaries have a $41.5 million credit commitment across the Company’s domestic revolving borrowing base, filed for bankruptcy. During the quarter, the Company submitted borrowing requests for $175.0 million from its senior secured asset-based revolving facility of which $165.4 million in net borrowing proceeds were received from the administration agent. The difference between the borrowed amount and the requested amount reflects Lehman’s election to not fund its pro rata share of the borrowing as required under its commitment to the facility. As a result, the Company does not expect that Lehman will fund its pro rata share of any future borrowing requests. Also, one of the Company’s subsidiaries has a bilateral revolving credit facility with Fortis Bank. The Company was informed during the quarter ended November 30, 2008 by the bank that due to the subsidiary’s limited usage of the facility, the size of the commitment was being reduced from €100.0 million to €50.0 million. Subsequent to November 30, 2008, the reorganized Fortis Bank initiated conversations to increase the facility to the original commitment of €100.0 million. The Company does not expect these reductions to impact liquidity or the Company’s business operations. Based on the above, the Company’s revolving borrowing base available under all debt facilities at November 30, 2008 was $560.0 million, which is net of the amount the Company believes will not be funded by Lehman and borrowing base limitations as it relates to the senior secured asset-based revolving facility.
Note 9—Share-based Compensation and Stock Plans.
The Company adopted SFAS 123(R), Share-Based Payment, (“SFAS 123(R)”) to record share-based payment expense on June 1, 2006 using the modified prospective method. SFAS 123(R) requires the fair value of all share-based payments to employees, including stock options, to be expensed based on their fair value over the required award service period. The Company’s share-based payments consist of stock options. For the Company’s non-employee distributors, share-based expense is recorded in accordance with EITF No. 96-18, Accounting for Equity Instruments That Are Issued to Other Than Employees for Acquisition, or in Conjunction with Selling, Goods or Services . Prior to the Merger, the Predecessor Company’s Board of Directors modified certain stock options to change the exercise price to the fair market value on the date it was granted by adding a cash component paid in January 2008 for the difference from the original grant price to the amended grant price of $46.00 per share (related to predecessor options). In addition, on July 11, 2007, the Predecessor Company’s Board of Directors cancelled all outstanding stock options and paid the difference between the amended grant price and $46.00 per share (the offering price) in cash in conjunction with the Merger. The total amount expensed related to Predecessor Company grants was $112.8 million, with amounts recorded as cost of sales, selling, general, and administrative, and research and development in the Company’s results of operations for the period June 1, 2007 to July 11, 2007. The first payment occurred on July 17, 2007 for $103.0 million, and the second payment was made on January 11, 2008 for $9.8 million.
Share-based compensation expense recognized was $11.6 million and $18.8 million for the three and six months ended November 30, 2008, respectively. Share-based compensation expense recognized for the period June 1, 2007 to July 11, 2007 was $112.8 million. There was no share-based compensation expense recognized for the three months ended November 30, 2007, or for the period July 12, 2007 to November 30, 2007.
Note 10—Income Taxes (Benefit).
Effective June 1, 2007, the Company adopted FIN 48. This interpretation prescribes a recognition threshold and measurement attribute for the financial statement recognition and measurement of tax contingencies and the tax position taken, or expected to be taken, in a tax return. The amount of unrecognized tax benefits at November 30, 2008 was $52.4 million, $39.1 million of which would impact the Company’s effective tax rate, if recognized. The Company continues to record the liability for unrecognized tax benefits as a long-term liability as it does not expect significant payments to occur or the total amount of unrecognized tax benefits to materially change over the next twelve months.
F-40
Table of Contents
The Company is currently under audit by the U.S. Internal Revenue Service (IRS) for fiscal years ended May 31, 2005 and 2006. However, based upon the status of the IRS field audit, the company cannot reasonably estimate the potential changes to its unrecognized tax benefits.
The effective income tax rate increased to 39.3% for the six months ended November 30, 2008 compared to 16.3% for the period of July 12, 2007 through November 30, 2007. This year-over-year increase was primarily due to the following items incurred in fiscal 2008 that are not deductible for tax purposes: (1) $479.0 million of in-process research and development expense related to the Merger, (2) a portion of the $26.9 million Department of Justice settlement, and (3) $51.5 million of Merger-related expenses. The effective income tax rate increased to 43.9% for the three months ended November 30, 2008 compared to 25.4% for the three months ended November 30, 2007. This increase was primarily due to the $86.2 million in process research and development expense related to the Merger being not deductible for tax purposes in the prior year and changes in the Company’s mix of profits and losses in certain international and domestic jurisdictions in the current year.
Note 11—Segment Reporting.
The Company operates in one business segment, musculoskeletal products, which includes the designing, manufacturing and marketing of reconstructive products, fixation devices, spinal products and other products. Other products consist primarily of softgoods and bracing products, sports medicine products, general instruments and operating room supplies. The Company manages its business segment primarily on a geographic basis. These geographic markets are comprised of the United States, Europe and International. Major markets included in the international geographic market are Canada, South America, and the Pacific Rim.
Net sales of musculoskeletal products by product category are as follows (in millions):
| Three Months Ended November 30, | Six Months Ended November 30, 2008 | July 12 - November 30, 2007 (Successor) | June 1 - July 11, 2007 (Predecessor) | ||||||||||||||
| 2008 | 2007 | ||||||||||||||||
Net sales by product: | |||||||||||||||||
Reconstructive | $ | 483.3 | $ | 454.1 | $ | 932.6 | $ | 662.5 | $ | 178.1 | |||||||
Fixation | 58.0 | 56.8 | 118.3 | 88.2 | 27.1 | ||||||||||||
Spinal | 55.3 | 51.1 | 106.5 | 79.7 | 24.9 | ||||||||||||
Other | 46.2 | 45.2 | 92.4 | 65.4 | 18.7 | ||||||||||||
Total | $ | 642.8 | $ | 607.2 | $ | 1,249.8 | $ | 895.8 | $ | 248.8 | |||||||
| Three Months Ended November 30, | Six Months Ended November 30, 2008 | July 12 - November 30, 2007 (Successor) | June 1 - July 11, 2007 (Predecessor) | ||||||||||||||
| 2008 | 2007 | ||||||||||||||||
Net sales by geographic segment: | |||||||||||||||||
United States | $ | 379.5 | $ | 347.0 | $ | 747.9 | $ | 527.7 | $ | 156.2 | |||||||
Europe | 195.4 | 196.5 | 364.8 | 268.3 | 70.8 | ||||||||||||
International | 67.9 | 63.7 | 137.1 | 99.8 | 21.8 | ||||||||||||
Total | $ | 642.8 | $ | 607.2 | $ | 1,249.8 | $ | 895.8 | $ | 248.8 | |||||||
| November 30, 2008 | May 31, 2008 | |||||
Long-term assets(1) by geographic segment: | ||||||
United States | $ | 7,776.2 | $ | 8,274.4 | ||
Europe | 2,698.9 | 2,995.4 | ||||
International | 976.5 | 1,002.1 | ||||
Total | $ | 11,451.6 | $ | 12,271.9 | ||
(1) | Defined as property, plant and equipment, intangibles and goodwill. |
F-41
Table of Contents
Note 12—Guarantor and Non-guarantor Financial Statements.
Each of the Company’s existing wholly-owned domestic subsidiaries (labeled as “Guarantors” below) has jointly, severally and unconditionally guaranteed the senior cash pay and PIK toggle notes on a senior unsecured basis and the senior subordinated notes on a senior subordinated unsecured basis, in each case to the extent such subsidiaries guarantee the Company’s senior secured cash flow facilities. Accordingly, this basis of presentation is not intended to present the Company’s financial condition, results of operations or cash flows for any purpose other than to comply with the specific requirements for subsidiary guarantor reporting.
The following unaudited condensed consolidating financial information illustrates the composition of the combined guarantor subsidiaries (in millions):
Unaudited Condensed Consolidating Balance Sheets
| November 30, 2008 | ||||||||||||||||
| Biomet, Inc. | Guarantors | Non- Guarantors | Eliminations | Total | ||||||||||||
Assets | ||||||||||||||||
Cash and cash equivalents | $ | — | $ | 193.7 | $ | 35.6 | $ | — | $ | 229.3 | ||||||
Accounts receivable, net | — | 231.0 | 252.5 | — | 483.5 | |||||||||||
Inventories | — | 314.6 | 266.1 | (70.4 | ) | 510.3 | ||||||||||
Deferred income taxes | — | 93.5 | 3.6 | — | 97.1 | |||||||||||
Prepaid expenses and other | — | 71.8 | 22.5 | — | 94.3 | |||||||||||
Total current assets | — | 904.6 | 580.3 | (70.4 | ) | 1,414.5 | ||||||||||
Property, plant and equipment, net | — | 403.2 | 212.1 | (3.4 | ) | 611.9 | ||||||||||
Investments | — | 33.8 | — | — | 33.8 | |||||||||||
Investment in subsidiaries | 13,032.0 | — | — | (13,032.0 | ) | — | ||||||||||
Goodwill | — | 3,322.2 | 1,728.1 | 53.1 | 5,103.4 | |||||||||||
Intangible assets, net | — | 4,054.3 | 1,682.0 | — | 5,736.3 | |||||||||||
Other assets | — | 80.0 | 31.4 | — | 111.4 | |||||||||||
Total | $ | 13,032.0 | $ | 8,798.1 | $ | 4,233.9 | $ | (13,052.7 | ) | $ | 13,011.3 | |||||
Liabilities & Shareholders’ Equity | ||||||||||||||||
Short-term borrowings | $ | 34.5 | $ | — | $ | 38.0 | $ | — | $ | 72.5 | ||||||
Accounts payable | — | 36.5 | 33.1 | — | 69.6 | |||||||||||
Accrued interest | 79.7 | — | — | — | 79.7 | |||||||||||
Accrued wages and commissions | — | 48.0 | 16.1 | — | 64.1 | |||||||||||
Other accrued expenses | — | 245.2 | 65.1 | 37.0 | 347.3 | |||||||||||
Total current liabilities | 114.2 | 329.7 | 152.3 | 37.0 | 633.2 | |||||||||||
Deferred income taxes | — | 1,958.2 | 5.8 | (61.3 | ) | 1,902.7 | ||||||||||
Employee related obligations | — | — | 28.5 | — | 28.5 | |||||||||||
Long-term debt | 6,117.6 | — | 6.8 | — | 6,124.4 | |||||||||||
Other long-term liabilities | — | — | 0.6 | — | 0.6 | |||||||||||
Shareholders’ equity | 6,800.2 | 6,510.2 | 4,039.9 | (13,028.4 | ) | 4,321.9 | ||||||||||
Total liabilities and shareholders’ equity | $ | 13,032.0 | $ | 8,798.1 | $ | 4,233.9 | $ | (13,052.7 | ) | $ | 13,011.3 | |||||
| May 31, 2008 | ||||||||||||||||
| Biomet, Inc. | Guarantors | Non- Guarantors | Eliminations | Total | ||||||||||||
Assets | ||||||||||||||||
Cash and cash equivalents | $ | — | $ | 101.0 | $ | 25.4 | $ | 1.2 | $ | 127.6 | ||||||
Accounts receivable, net | — | 213.7 | 272.5 | — | 486.2 | |||||||||||
Inventories | — | 296.6 | 320.2 | (77.1 | ) | 539.7 | ||||||||||
Deferred income taxes | — | 97.0 | 3.7 | — | 100.7 | |||||||||||
Prepaid expenses and other | — | 65.5 | 30.0 | — | 95.5 | |||||||||||
Total current assets | — | 773.8 | 651.8 | (75.9 | ) | 1,349.7 | ||||||||||
Property, plant and equipment, net | — | 407.6 | 233.3 | — | 640.9 | |||||||||||
Investments | — | 41.3 | — | — | 41.3 | |||||||||||
Investment in subsidiaries | 12,270.0 | — | — | (12,270.0 | ) | — | ||||||||||
Goodwill | — | 4,677.5 | 1,847.7 | (1,102.4 | ) | 5,422.8 | ||||||||||
Intangible assets, net | — | 4,407.0 | 1,801.2 | — | 6,208.2 | |||||||||||
Other assets | — | 107.2 | 11.7 | — | 118.9 | |||||||||||
Total | $ | 12,270.0 | $ | 10,414.4 | $ | 4,545.7 | $ | (13,448.3 | ) | $ | 13,781.8 | |||||
Liabilities & Shareholders’ Equity | ||||||||||||||||
Short-term borrowings | $ | 37.0 | $ | — | $ | 38.4 | $ | — | $ | 75.4 | ||||||
Accounts payable | — | 53.0 | 38.6 | (7.9 | ) | 83.7 | ||||||||||
Accrued interest | 80.9 | — | — | — | 80.9 | |||||||||||
Accrued wages and commissions | — | 66.3 | 12.8 | — | 79.1 | |||||||||||
Other accrued expenses | — | 202.3 | 72.6 | (29.5 | ) | 245.4 | ||||||||||
Total current liabilities | 117.9 | 321.6 | 162.4 | (37.4 | ) | 564.5 | ||||||||||
Deferred income taxes | — | 1,438.0 | 725.3 | (50.8 | ) | 2,112.5 | ||||||||||
Employee related obligations | — | — | 40.0 | — | 40.0 | |||||||||||
Long-term debt | 6,225.7 | — | — | — | 6,225.7 | |||||||||||
Other long-term liabilities | — | — | 2.8 | — | 2.8 | |||||||||||
Shareholders’ equity | 5,926.4 | 8,654.8 | 3,615.2 | (13,360.1 | ) | 4,836.3 | ||||||||||
Total liabilities and shareholders’ equity | $ | 12,270.0 | $ | 10,414.4 | $ | 4,545.7 | $ | (13,448.3 | ) | $ | 13,781.8 | |||||
F-42
Table of Contents
Unaudited Condensed Consolidating Statements of Operations
| The Three Months Ended November 30, 2008 | ||||||||||||||||||||
| Biomet, Inc. | Guarantors | Non- Guarantors | Eliminations | Total | ||||||||||||||||
Net sales | $ | — | $ | 397.7 | $ | 245.1 | $ | — | $ | 642.8 | ||||||||||
Cost of sales | — | 102.5 | 118.2 | (25.8 | ) | 194.9 | ||||||||||||||
Gross margin | — | 295.2 | 126.9 | 25.8 | 447.9 | |||||||||||||||
Operating expenses | — | 273.3 | 94.6 | — | 367.9 | |||||||||||||||
Operating income | — | 21.9 | 32.3 | 25.8 | 80.0 | |||||||||||||||
Other income (expense), net | (139.1 | ) | (1.4 | ) | (10.5 | ) | 0.2 | (150.8 | ) | |||||||||||
Income (loss) before income taxes | (139.1 | ) | 20.5 | 21.8 | 26.0 | (70.8 | ) | |||||||||||||
Tax expense (benefit) | (61.2 | ) | 9.1 | 9.5 | 11.5 | (31.1 | ) | |||||||||||||
Equity in earnings of subsidiaries | 23.6 | — | — | (23.6 | ) | — | ||||||||||||||
Net income (loss) | $ | (54.3 | ) | $ | 11.4 | $ | 12.3 | $ | (9.1 | ) | $ | (39.7 | ) | |||||||
| The Three Months Ended November 30, 2007 | ||||||||||||||||||||
| Biomet, Inc. | Guarantors | Non- Guarantors | Eliminations | Total | ||||||||||||||||
Net sales | $ | — | $ | 343.2 | $ | 265.5 | $ | (1.5 | ) | $ | 607.2 | |||||||||
Cost of sales | — | 156.9 | 104.7 | (17.0 | ) | 244.6 | ||||||||||||||
Gross margin | — | 186.3 | 160.8 | 15.5 | 362.6 | |||||||||||||||
Operating expenses | — | 622.8 | (9.7 | ) | 0.5 | 613.6 | ||||||||||||||
Operating income (loss) | — | (436.5 | ) | 170.5 | 15.0 | (251.0 | ) | |||||||||||||
Other income (expense), net | (187.6 | ) | 38.2 | (4.2 | ) | — | (153.6 | ) | ||||||||||||
Income (loss) before income taxes | (187.6 | ) | (398.3 | ) | 166.3 | 15.0 | (404.6 | ) | ||||||||||||
Tax expense (benefit) | (35.6 | ) | (96.1 | ) | 21.5 | 7.6 | (102.6 | ) | ||||||||||||
Equity in earnings of subsidiaries | (157.4 | ) | — | — | 157.4 | — | ||||||||||||||
Net income (loss) | $ | (309.4 | ) | $ | (302.2 | ) | $ | 144.8 | $ | 164.8 | $ | (302.0 | ) | |||||||
| The Six Months Ended November 30, 2008 | ||||||||||||||||||||
| Biomet, Inc. | Guarantors | Non- Guarantors | Eliminations | Total | ||||||||||||||||
Net sales | $ | — | $ | 781.1 | $ | 468.7 | $ | — | $ | 1,249.8 | ||||||||||
Cost of sales | — | 205.3 | 228.9 | (57.8 | ) | 376.4 | ||||||||||||||
Gross margin | — | 575.8 | 239.8 | 57.8 | 873.4 | |||||||||||||||
Operating expenses | — | 539.1 | 197.3 | — | 736.4 | |||||||||||||||
Operating loss | — | 36.7 | 42.5 | 57.8 | 137.0 | |||||||||||||||
Other expense, net | (279.5 | ) | (4.2 | ) | (12.3 | ) | (4.9 | ) | (300.9 | ) | ||||||||||
Income (loss) before income taxes | (279.5 | ) | 32.5 | 30.2 | 52.9 | (163.9 | ) | |||||||||||||
Tax expense (benefit) | (109.9 | ) | 12.9 | 11.9 | 20.8 | (64.3 | ) | |||||||||||||
Equity in earnings of subsidiaries | 38.0 | — | — | (38.0 | ) | — | ||||||||||||||
Net income (loss) | $ | (131.6 | ) | $ | 19.6 | $ | 18.3 | $ | (5.9 | ) | $ | (99.6 | ) | |||||||
| The Period From July 12, 2007 to November 30, 2007 (Successor) | ||||||||||||||||||||
| Biomet, Inc. | Guarantors | Non- Guarantors | Eliminations | Total | ||||||||||||||||
Net sales | $ | — | $ | 506.4 | $ | 372.9 | $ | 16.5 | $ | 895.8 | ||||||||||
Cost of sales | — | 243.5 | 154.7 | (46.8 | ) | 351.4 | ||||||||||||||
Gross margin | — | 262.9 | 218.2 | 63.3 | 544.4 | |||||||||||||||
Operating expenses | — | 1,080.2 | 171.5 | 0.8 | 1,252.5 | |||||||||||||||
Operating income (loss) | — | (817.3 | ) | 46.7 | 62.5 | (708.1 | ) | |||||||||||||
Other income (expense), net | (229.4 | ) | 9.5 | (8.7 | ) | — | (228.6 | ) | ||||||||||||
Income (loss) before income taxes | (229.4 | ) | (807.8 | ) | 38.0 | 62.5 | (936.7 | ) | ||||||||||||
Tax expense (benefit) | (40.6 | ) | (127.7 | ) | 7.0 | 8.8 | (152.5 | ) | ||||||||||||
Equity in earnings of subsidiaries | (649.1 | ) | — | — | 649.1 | — | ||||||||||||||
Net income (loss) | $ | (837.9 | ) | $ | (680.1 | ) | $ | 31.0 | $ | 702.8 | $ | (784.2 | ) | |||||||
| The Period From June 1, 2007 to July 11, 2007 (Predecessor) | ||||||||||||||||||||
| Biomet, Inc. | Guarantors | Non- Guarantors | Eliminations | Total | ||||||||||||||||
Net sales | $ | — | $ | 185.1 | $ | 82.5 | $ | (18.8 | ) | $ | 248.8 | |||||||||
Cost of sales | — | 60.8 | 46.5 | (5.0 | ) | 102.3 | ||||||||||||||
Gross margin | — | 124.3 | 36.0 | (13.8 | ) | 146.5 | ||||||||||||||
Operating expenses | — | 179.2 | 49.3 | 0.2 | 228.7 | |||||||||||||||
Operating loss | — | (54.9 | ) | (13.3 | ) | (14.0 | ) | (82.2 | ) | |||||||||||
Other income (expense), net | — | (0.7 | ) | 1.0 | — | 0.3 | ||||||||||||||
Income (loss) before income taxes | — | (55.6 | ) | (12.3 | ) | (14.0 | ) | (81.9 | ) | |||||||||||
Tax expense (benefit) | — | (24.6 | ) | (2.5 | ) | (0.2 | ) | (27.3 | ) | |||||||||||
Equity in earnings of subsidiaries | (40.8 | ) | — | — | 40.8 | — | ||||||||||||||
Net income (loss) | $ | (40.8 | ) | $ | (31.0 | ) | $ | (9.8 | ) | $ | 27.0 | $ | (54.6 | ) | ||||||
F-43
Table of Contents
Unaudited Condensed Consolidating Statements of Cash Flows
| The Six Months Ended November 30, 2008 | ||||||||||||||||||||
| Biomet, Inc. | Guarantor | Non- Guarantor | Eliminations | Total | ||||||||||||||||
Cash flows provided by (used in) operating activities | $ | (148.5 | ) | $ | 147.0 | $ | 59.9 | $ | (10.8 | ) | $ | 47.6 | ||||||||
Cash flows provided by (used in) investing activities | — | (46.0 | ) | (49.1 | ) | — | (95.1 | ) | ||||||||||||
Cash flows provided by (used in) financing activities | 148.5 | — | 8.5 | — | 157.0 | |||||||||||||||
Effect of exchange rate changes on cash | — | — | (7.8 | ) | — | (7.8 | ) | |||||||||||||
Increase (decrease) in cash and cash equivalents | — | 101.0 | 11.5 | (10.8 | ) | 101.7 | ||||||||||||||
Cash and cash equivalents, beginning of period | — | 101.0 | 26.6 | — | 127.6 | |||||||||||||||
Cash and cash equivalents, end of period | $ | — | $ | 202.0 | $ | 38.1 | $ | (10.8 | ) | $ | 229.3 | |||||||||
| The Period From July 12, 2007 to November 30, 2007 (Successor) | ||||||||||||||||||||
| Biomet, Inc. | Guarantor | Non- Guarantor | Eliminations | Total | ||||||||||||||||
Cash flows provided by (used in) operating activities | $ | 72.7 | $ | 23.4 | $ | (96.7 | ) | $ | — | $ | (0.6 | ) | ||||||||
Cash flows provided by (used in) investing activities | (11,638.2 | ) | 49.8 | (31.1 | ) | — | (11,619.5 | ) | ||||||||||||
Cash flows provided by (used in) financing activities | 11,565.5 | — | (40.4 | ) | — | 11,525.1 | ||||||||||||||
Effect of exchange rate changes on cash | — | — | 2.0 | — | 2.0 | |||||||||||||||
Increase (decrease) in cash and cash equivalents | — | 73.2 | (166.2 | ) | — | (93.0 | ) | |||||||||||||
Cash and cash equivalents, beginning of period | — | 124.9 | 52.0 | — | 176.9 | |||||||||||||||
Cash and cash equivalents, end of period | $ | — | $ | 198.1 | $ | (114.2 | ) | $ | — | $ | 83.9 | |||||||||
| The Period From June 1, 2007 to July 11, 2007 (Predecessor) | ||||||||||||||||||||
| Biomet, Inc. | Guarantor | Non- Guarantor | Eliminations | Total | ||||||||||||||||
Cash flows provided by (used in) operating activities | $ | (54.0 | ) | $ | 13.7 | $ | 30.3 | $ | 69.4 | $ | 59.4 | |||||||||
Cash flows provided by (used in) investing activities | 52.7 | 21.8 | (7.8 | ) | (55.7 | ) | 11.0 | |||||||||||||
Cash flows provided by (used in) financing activities | 1.3 | — | — | — | 1.3 | |||||||||||||||
Effect of exchange rate changes on cash | — | — | 0.1 | — | 0.1 | |||||||||||||||
Increase (decrease) in cash and cash equivalents | — | 35.5 | 22.6 | 13.7 | 71.8 | |||||||||||||||
Cash and cash equivalents, beginning of period | — | 95.7 | 9.4 | — | 105.1 | |||||||||||||||
Cash and cash equivalents, end of period | $ | — | $ | 131.2 | $ | 32.0 | $ | 13.7 | $ | 176.9 | ||||||||||
Note 13—Contingencies.
U.S Department of Justice Consulting Agreement Investigation
On September 27, 2007, the Company entered into a Deferred Prosecution Agreement with the U.S. Attorney’s Office for the District of New Jersey. The agreement concludes the government’s investigation into whether consulting agreements between the largest orthopedic manufacturers and orthopedic surgeons who use joint reconstruction and replacement products may have violated the federal Anti-Kickback Statute.
Through the agreement, the U.S. Attorney’s Office agreed not to prosecute the Company in connection with this matter, provided that the Company satisfies its obligations under the agreement over the 18 months following the date of the Deferred Prosecution Agreement. The agreement calls for the appointment of an independent monitor to review the Company’s compliance with the agreement, particularly in relation to its consulting agreements. The Company simultaneously entered into a settlement with the Department of Justice’s Civil Division pursuant to which it paid $26.9 million during the first quarter of fiscal 2008.
As part of the resolution of this matter, the Company also entered into a Corporate Integrity Agreement with the Office of the Inspector General of the U.S. Department of Health and Human Services. The agreement requires the Company for five years subsequent to September 27, 2007 to continue to adhere to its Code of Business Conducts and Ethics and certain other provisions, including reporting requirements.
U.S. Department of Justice EBI Products Investigation
In May 2007, the Company received a subpoena from the U.S. Department of Justice through the U.S. Attorney for the Southern District of West Virginia requesting documents generally relating to a certain number of products manufactured, marketed and sold by the Company’s EBI subsidiary for the period from January 1999 through the present. In June 2007, the Company received a second administrative subpoena from the U.S. Attorney for the Southern District of West Virginia requesting documents relating to a specific physician’s assistant. The Company understands that the Department of Justice is conducting a civil investigation of EBI’s sales and marketing practices relating to certain spinal products. The Company is fully cooperating with the request of the Department of Justice. The Company can make no assurances as to the time or resources that will be needed to devote to this inquiry or its final outcome.
Litigation Relating to Past Stock Option Grant Practices
On September 21, 2006, two shareholder derivative complaints were filed against certain of the Company’s current and former officers and directors in Kosciusko Superior Court I in Kosciusko County, in the State of Indiana. The complaints, captioned Long v. Hann, et al., and Thorson v. Hann, et al., alleged violations of state law relating to the issuance of certain stock option awards by Biomet dating back to 1996. Both complaints sought unspecified money damages as well as other equitable and injunctive relief. These two cases were consolidated under the caption In re Biomet, Inc. Derivative Litigation, and on January 19, 2007, plaintiffs filed an amended complaint that made additional allegations based on the Company’s December 18, 2006 disclosures related to stock option awards, including allegations that the defendants sought to sell the Company in order to escape liability for their conduct, and that they did so at a devalued price, thus further breaching their fiduciary duties to shareholders. On February 16, 2007, defendants filed a motion to dismiss plaintiffs’ amended complaint. On October 11, 2007, after approval of the Company’s sale by its shareholders, the parties filed supplemental briefs on the issue of whether plaintiffs had standing to sue. On February 5, 2008, the court dismissed the case for lack of standing, and plaintiffs’ motion for leave to amend was denied. Plaintiffs appealed the district court’s decision, and on February 13, 2009, the Court of Appeals affirmed the dismissal of the case. Plaintiffs may still seek review of the Court of Appeals’ decision by the Indiana Supreme Court.
F-44
Table of Contents
U.S. Securities and Exchange Commission Informal Investigation
On September 25, 2007, the Company received a letter from the SEC informing the Company that it is conducting an informal investigation regarding possible violations of the Foreign Corrupt Practices Act in the sale of medical devices in certain foreign countries by companies in the medical devices industry. The Foreign Corrupt Practices Act prohibits U.S. companies and their officers, directors, employees, shareholders acting on their behalf and agents from offering, promising, authorizing or making payments to foreign officials for the purpose of obtaining or retaining business abroad or otherwise obtaining favorable treatment and this law requires companies to maintain records which fairly and accurately reflect transactions and to maintain internal accounting controls. In many countries, hospitals and clinics are government-owned and healthcare professionals employed by such hospitals and clinics, with whom the Company regularly interacts, may meet the definition of a foreign official for purposes of the Foreign Corrupt Practices Act. If the Company is found to have violated the Foreign Corrupt Practices Act, the Company may face sanctions including fines, criminal penalties, disgorgement of profits and suspension or debarment of the Company’s ability to contract with government agencies or receive export licenses. On November 9, 2007, the Company received a letter from the Department of Justice requesting any information provided to the SEC be provided to the Department of Justice on a voluntary basis. The Company intends to fully cooperate with both requests and the Company is in the process of conducting its own review relating to these matters in certain countries in which the Company and its distributors conduct business.
Massachusetts AG
The Company received a Civil Investigative Demand (“CID”) issued by the Commonwealth of Massachusetts Office of the Attorney General (“Massachusetts AG”) on or about November 19, 2007. The CID requested documents for the period November 1, 2003 to the present concerning certain physicians and provider groups, including, among other things, documents concerning any contracts or agreements with, and any payments made to, those physicians or provider groups. The Company has produced documents in response to the CID, and intends to continue to cooperate with the Massachusetts AG. It is not possible at this time to predict the likely outcome of this inquiry or its financial impact should the outcome be adverse to the Company.
Other Matters
In February 2006, SDGI Holdings, Inc. and Medtronic Sofamor Danek, Inc. (collectively referred to herein as Medtronic) brought an action against EBI and the Company alleging infringement of seven patents. Specifically, Medtronic alleged that the patents were infringed by certain components of the Company’s Vuelock® Anterior Cervical Plate System, as well as instruments and surgical implantation methods associated with the Company’s Array® Spinal System. In Fall 2007, Medtronic included similar instruments used with EBI’s Biomet® Omega21™ , Polaris™ , and Synergy™ Spinal Fixation Systems as accused products. The Company filed a counterclaim seeking a finding of non-infringement of the patents at issue and a finding that certain of the patents are invalid and unenforceable. Effective on December 8, 2008, the parties entered into a confidential settlement agreement, without either party admitting liability, pursuant to which, among other things, the parties exchanged certain cross-licenses to various patents.
The Company and Biomet Orthopedics initiated legal proceedings on July 17, 2007 against Zimmer US, Inc., or Zimmer, certain of the Company’s former distributors and David Montgomery, the Company’s former employee who currently works for Zimmer. The thirteen count lawsuit originally filed in Marion County, Indiana and refiled in Hamilton County, Indiana alleges, among other things, that Zimmer and Mr. Montgomery attempted to create an unfair market advantage by engaging in a campaign to misappropriate the Company’s confidential information, to interfere with the Company’s contractual relations with distributors and to attempt to buy the assets of most of the Company’s distributors (including the Company’s surgical instruments) throughout the United States. Further, the lawsuit alleges that the limited number of distributors who accepted Zimmer’s offer are in violation of their contractual obligations to Biomet. Although nearly all of the Company’s distributors rejected Zimmer’s offers and have remained with Biomet, and although no amount of money damages can completely compensate Biomet for the losses the Company has sustained as a result of defendants’ conduct, the Company is nonetheless seeking to recover compensatory damages that are attributable to financial and other resources spent on signing new agreements with the Company’s sales force. To the extent the Company sustained damages as a result of the Company’s former distributors agreeing to purportedly sell their assets to Zimmer, the Company is seeking to recover lost profits and other damages as well. In addition, the Company is seeking to recover punitive damages from the defendants. On November 9, 2007, defendants filed a motion to dismiss the Company’s complaint. On March 27, 2008, the court denied the motion in its entirety.
In a related matter, the Company brought suit against a former distributor for Biomet Orthopedics who, in violation of his contractual and other obligations to Biomet under agreements stretching back to 1994, sold the assets of his distributorship to Zimmer in an apparent effort to avoid his contractual obligations to the Company. The complaint, now pending in federal district court in Indiana, asserts five causes of action that include breach of contract, unjust enrichment and statutory wrongs. Among other things, the complaint seeks injunctive relief and compensatory and punitive damages. On July 16, 2007, a temporary restraining order was entered against this former distributor which subsequently lapsed ten days later. Prior to the filing of the suit described above, this former distributor sued one of his former employees who decided to continue to represent the Company’s products in the future as he has for nearly ten years. The suit brought against this employee by the Company’s former distributor who sold his assets to Zimmer claims, among other things, that the former employee is violating his non-competition agreement with the Company’s former distributor by continuing to sell the same Biomet products the former employee sold while employed by the Company’s former distributor. The suit also seeks, among other forms of relief, an injunction and compensatory and punitive damages. In addition, on or about July 3, 2008, Zimmer U.S., Inc. and one of its distributors filed a five count complaint in Tennessee federal court against this same former employee seeking, among other things, injunctive relief, monetary damages, and punitive damages for alleged breach of contract, conspiracy, and other causes of action.
In late 2004 and early 2005, approximately 120 plaintiffs sued Dr. John King in the Circuit Court of Putnam County, West Virginia. Plaintiffs alleged that Dr. King was professionally negligent when he performed surgery on the plaintiffs at Putnam General Hospital in Putnam County, West Virginia between November 2002 and June 2003. In 38 of these lawsuits, plaintiffs alleged that Dr. King had implanted a device manufactured by the Company’s EBI subsidiary and EBI was named a party in those 38 lawsuits. Plaintiffs have dismissed or have agreed to dismiss their claims against EBI in 11 cases, leaving EBI as a party in 27 pending lawsuits, all of which relate to EBI’s Ionic Spine Spacer System and its implanted bone stimulator devices, the SpF and OsteoGen. Plaintiffs allege that EBI entered into a joint venture and a civil conspiracy with Dr. King and/or his physician assistant, David McNair. The plaintiffs also allege that EBI failed to warn that its products were not safe for their intended use, that EBI knew that Dr. King was not properly trained or was performing surgeries inappropriately and claims based on strict liability, express and implied breach of warranty and negligent sale. Plaintiffs seek to recover lost income, medical expenses, future medical and life care expenses, damages relating to pain and suffering and punitive and other damages. Dr. King is uninsured in 25 of these 27 cases and has filed bankruptcy.
In July 2007, a Putnam County jury found that Putnam General Hospital had negligently credentialed Dr. King and that the hospital’s conduct in credentialing Dr. King was motivated by fraud, ill will, wantonness, oppressiveness, or by reckless or gross negligence, which allowed the plaintiffs to seek punitive damages against the hospital. In April, May and June of 2008, the hospital and its upstream affiliates and David McNair entered into a confidential settlement of all claims with all but one of the plaintiffs. EBI, Wright Medical Corporation, Wright Medical’s distributor’s employee, Robert Edwards, and Dr. King remain as defendants in the litigation.
The Putnam County Circuit Court revised its case management order with respect to the remaining lawsuits on July 2, 2008 and scheduled a consolidated trial of six plaintiffs for June 1, 2009. The Company is vigorously defending these matters and intends to continue to do so. An unfavorable outcome in these matters could have a material adverse effect on the Company’s financial position, liquidity and results of operations.
There are various other claims, lawsuits, disputes with third parties, investigations, and pending actions involving various allegations against the Company incident al to the operation of its business, principally product liability and intellectual property cases. Each of these matters is subject to various uncertainties, and it is possible that some of these matters may be resolved unfavorably to Biomet.
F-45
Table of Contents
The Company accrues for losses that are deemed to be probable and subject to reasonable estimate. Based on the advice of the Company’s counsel in these matters, management believes that the ultimate outcome of these matters and any liabilities in excess of amounts provided, except in the immediately preceding paragraph above, will not have a material adverse impact on the Company’s unaudited condensed consolidated financial statements taken as a whole.
Note 14—Related Parties.
Management Services Agreement
Upon completion of the Transactions, the Company entered into a management services agreement with certain affiliates of the Sponsors, pursuant to which such affiliates of the Sponsors or their successors, assigns, affiliates, officers, employees, and/or representatives and third parties (collectively, the “Managers”) provide management, advisory, and consulting services to the Company. Pursuant to such agreement, the Managers received a transaction fee equal to 1% of total enterprise value of the Transactions for the services rendered by such entities related to the Transactions upon entering into the agreement, and the Sponsors receive an annual monitoring fee equal to 1% of the Company’s annual adjusted EBITDA as compensation for the services rendered and reimbursement for out-of-pocket expenses incurred by the Managers in connection with the agreement and the Transactions. The Company is required to pay the Sponsors the monitoring fee on a quarterly basis. The total amount of Sponsor fees was $2.8 million and $5.3 million for the three and six months ended November 30, 2008, respectively, $3.9 million for the three months ended November 30, 2007, and $5.1 million for the period July 12, 2007 through November 30, 2007. There were no Sponsor fees for the period June 1, 2007 through July 11, 2007. The Company may also pay certain subsequent fees to the Managers for advice rendered in connection with financing or refinancing (equity or debt), acquisition, disposition, spin-off, split-off, dividend, recapitalization, initial underwritten public offering and change of control transactions involving the Company or any of its subsidiaries. The management services agreement includes customary exculpation and indemnification provisions in favor of the Managers and their affiliates.
On May 8, 2006, Biomet, Inc. entered into a Separation, Release and Consultancy Agreement with Dane A. Miller, Ph.D. (the “Miller Agreement”). As previously disclosed in a Current Report on Form 8-K dated May 10, 2006, pursuant to the terms of the Miller Agreement, Dr. Miller received $4.0 million on October 1, 2006, $0.5 million on November 30, 2006 and has received or will receive $0.5 million on the last day of each quarter thereafter through the first quarter of fiscal year 2010 as compensation for his consulting services. Also pursuant to the Miller Agreement, Dr. Miller is reimbursed for any out-of-pocket fees and expenses relating to an off-site office and administrative support, in an amount not to exceed $0.1 million per year. The Miller Agreement contains certain restrictive covenants prohibiting Dr. Miller from competing with the Company and soliciting employees of the Company during the term of the Miller Agreement. As of November 30, 2008, the remaining amount accrued and payable to Dr. Miller was $1.5 million.
Other
The Company currently holds interest rate swaps with Goldman Sachs. As part of this relationship, the Company receives information from Goldman Sachs that allows it to perform a regression on the swaps as part of its required effectiveness testing on a quarterly basis.
During the six months ended November 30, 2008, the Company received an additional capital contribution of $1.3 million from its parent company from the participation of management under the LVB Acquisition, Inc. 2007 Management Equity Incentive Plan.
F-46
Table of Contents
Biomet, Inc.
10% Senior Notes due 2017;
10 3/8%/11 1/8% Senior Toggle Notes due 2017
and
11 5/8% Senior Subordinated Notes due 2017
PROSPECTUS
Table of Contents
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
| Item 13. | Other Expenses of Issuance and Distribution |
The registration rights agreements relating to the securities of the registrants being registered hereby provide that Biomet, Inc. will bear all expenses in connection with the performance of its obligations relating to the market-making activities of Goldman, Sachs & Co. and its affiliates. These estimated expenses are as follows:
Printing expenses | $ | 50,000 | |
Legal fees | 300,000 | ||
Accounting fees | 50,000 | ||
Miscellaneous | 20,000 | ||
Total | $ | 420,000 | |
| Item 14. | Indemnification of Directors and Officers. |
California Registrant: Interpore Cross International, LLC is a limited liability company organized under the laws of California.
Interpore Cross International, LLC (“Interpore Cross”) is organized under the laws of the State of California. Section 17155 of the California Beverly-Killea Limited Liability Company Act provides that, except for a breach of a manager’s fiduciary duties of loyalty and care owed to the limited liability company and to its members, the articles of organization or written operating agreement of a California limited liability company may provide for indemnification of any person, including, without limitation, any manager, member, officer, employee, or agent of the limited liability company, against judgments, settlements, penalties, fines, or expenses of any kind incurred as a result of acting in that capacity.
The Limited Liability Agreement of Interpore Cross (the “Interpore Cross LLC Agreement”) provides that to the fullest extent permitted by law, Interpore Cross shall indemnify and hold harmless each of Interpore Spine, Ltd., as the sole member of Interpore Cross, the managers, and any other officers, directors, shareholders, partners, employees, affiliates, representatives, or agents of any of the foregoing, or any officer, employee, representative, or agent of Interpore Cross (each, a “Covered Person”) from and against any and all losses, claims, demands, liabilities, expenses, judgments, fines, settlements, and other amounts arising from any and all claims, demands, actions, suits, or proceedings, civil, criminal, administrative, or investigative (“Claims”), in which each Covered Person may be involved, threatened to be involved, as a party or otherwise, by reason of its management of the affairs of Interpore Cross or which relates to or arises out of Interpore Cross or its property, business, or affairs. Pursuant to the Interpore Cross LLC Agreement, a Covered Person shall not be entitled to indemnification with respect to (i) any Claim with respect to which such Covered Person has engaged in fraud, willful misconduct, bad faith, or gross negligence or (ii) any Claim initiated by such Covered Person unless such Claim (or part thereof) (A) was brought to enforce such Covered Person’s rights to indemnification hereunder or (B) was authorized or consented to by the Board. Expenses incurred by a Covered Person in defending any Claim shall be paid by Interpore Cross in advance of the final disposition of such Claim upon receipt by Interpore Cross of an undertaking by or on behalf of such Covered Person to repay such amount if it shall be ultimately determined that such Covered Person is not entitled to be indemnified by Interpore Cross.
The Interpore Cross LLC Agreement also provides that, notwithstanding any other provisions of the Interpore Cross LLC Agreement, whether express or implied, or any obligation or duty at law or in equity, none of the Covered Persons shall be liable to Interpore Cross or any other person for any act or omission (in relation to Interpore Cross, its property, or the conduct of its business or affairs, the Interpore Cross LLC Agreement, any related document, or any transaction or investment contemplated thereby) taken or omitted by a Covered Person in the reasonable belief that such act or omission is in or is not contrary to the best interests of Interpore Cross and is within the scope of authority granted to such Covered Person by the Interpore Cross LLC Agreement, provided such act or omission does not constitute fraud, willful misconduct, bad faith, or gross negligence.
Delaware Registrants:
(a) Biolectron, Inc., Biomet Europe Ltd., Biomet Holdings Ltd., Biomet International Ltd., Interpore Spine Ltd. and Kirschner Medical Corporation are incorporated under the laws of Delaware.
Section 145 of the Delaware General Corporation Law (the “DGCL”) grants each corporation organized thereunder the power to indemnify any person who is or was a director, officer, employee or agent of a corporation or enterprise, against expenses, including attorneys’ fees, judgments, fines and amounts paid in settlement actually and reasonably incurred by him in connection with any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative, other than an action by or in the right of the corporation, by reason of being or having been in any such capacity, if he acted in good faith in a manner reasonably believed to be in, or not opposed to, the best interests of the corporation, and, with respect to any criminal action or proceeding, had no reasonable cause to believe his conduct was unlawful.
Section 102(b)(7) of the DGCL enables a corporation in its certificate of incorporation or an amendment thereto to eliminate or limit the personal liability of a director to the corporation or its shareholders for monetary damages for violations of the director’s fiduciary duty of care, except (i) for any breach of the director’s duty of loyalty to the corporation or its shareholders, (ii) for acts or omissions not in good faith or that involve intentional misconduct or a knowing violation of law, (iii) pursuant to Section 174 of the DGCL (providing for liability of directors for unlawful payment of dividends or unlawful stock purchases or redemptions) or (iv) for any transaction from which a director derived an improper personal benefit.
The certificate of incorporation and/or bylaws of the Delaware corporate registrants indemnify, to the fullest extent permitted by law, every director and officer made a party to a proceeding by reason of their position as a director or officer against all liability incurred by such individual in connection with the proceeding, except where the individual failed to meet the standard of conduct for indemnification specified by law. Such indemnification also extends to the payment for reasonable expenses incurred by the director or officer in connection with any proceeding in advance of final disposition thereof, but the bylaws of Interpore Spine, Ltd. and Kirschner Medical Corporation provide that such advancement of expenses is upon receipt of an undertaking by such director or officer to repay such amount if it is ultimately determined that he is not entitled to indemnification. Furthermore, a director or officer who is wholly successful, on the merits or otherwise, in the defense of any such proceeding is entitled to indemnification as a matter of right against reasonable expenses incurred by such individual in connection with such proceeding. The indemnification and advancement of expenses provided for by the certificate of incorporation and/or bylaws of the Delaware corporate registrants is not exclusive of any other rights, by contract or otherwise, relating to indemnification or advance of expenses that such individuals may have against the Delaware corporate registrants.
II-1
Table of Contents
Neither the certificate of incorporation nor the bylaws of Biomet Europe Ltd. provide for indemnification of directors or officers.
The certificates of incorporation of Biolectron, Inc., Intepore Spine, Ltd. and Kirschner Medical Corp. eliminate their directors’ personal liability to the corporation or its shareholders with respect to acts or omissions in the performance of their duties as director of the corporation, except for personal liability for (i) a breach of the directors’ duties of loyalty to the corporations or their shareholders, (ii) for acts or omissions not in good faith or that involve intentional misconduct or a knowing violation of law, (iii) pursuant to Section 174 of the DGCL or (iv) for any transaction from which a director derived an improper personal benefit.
(b) Cross Medical Products, LLC, EBI Holdings, LLC, EBI Medical Systems, LLC, and Electro-Biology, LLC and each organized as limited liability companies under the laws of Delaware.
Section 18-108 of the Delaware Limited Liability Company Act empowers a Delaware limited liability company to indemnify and hold harmless any member or manager of the limited liability company from and against any and all claims and demands whatsoever.
The operating agreements of each of the Delaware limited liability companies provide for the indemnification to the fullest extent permitted by law of the Members, Managers or any officers, directors, shareholders, partners, employees, affiliates, representatives or agents of any of the foregoing, as well as any officer, employee, representative or agent of the limited liability company (individually, a “Covered Person”, and collectively, “Covered Persons”). Each Covered Person is indemnified against any claims, liabilities, expenses, judgments, settlements or other amounts arising in any proceedings, whether civil, criminal, administrative or investigative in which the Covered Person is involved by reason of its management of the affairs of the limited liability company or which relates to or arises out of the limited liability company or its property, business or affairs. A Covered Person is not entitled to indemnification for such claim if such Covered Person engaged in fraud, willful misconduct, bad faith or gross negligence or if such claim was initiated by such Covered Person (unless the claim was brought to enforce such Covered Person’s right to indemnification or authorized by the Board). The limited liability company must pay expenses incurred by such Covered Person in defending against any claim in advance of the disposition of the claim upon receipt by the limited liability of an undertaking of the Covered Person to repay any amounts advanced if it is ultimately determined that Covered Person is not entitled to indemnification.
Florida Registrants: Biomet 3i, LLC, Biomet Microfixation, LLC and Biomet Florida Services, LLC are each organized as limited liability companies under the laws of Florida.
Section 608.4229(1) of the Florida Limited Liability Company Act provides that a limited liability company may, and shall have the power to, but shall not be required to, indemnify and hold harmless any member or manager or other person from and against any and all claims and demands whatsoever. Notwithstanding that provision, indemnification or advancement of expenses shall not be made to or on behalf of any member, manager, managing member, officer, employee, or agent if a judgment or other final adjudication establishes that the actions, or omissions to act, of such member, manager, managing member, officer, employee, or agent were material to the cause of action so adjudicated and constitute any of the following: (a) a violation of criminal law, unless the member, manager, managing member, officer, employee, or agent had no reasonable cause to believe such conduct was unlawful; (b) a transaction from which the member, manager, managing member, officer, employee, or agent derived an improper personal benefit; (c) in the case of a manager or managing member, a circumstance under which the liability provisions of Section 608.426 are applicable; or (d) willful misconduct or a conscious disregard for the best interests of the limited liability company in a proceeding by or in the right of the limited liability company to procure a judgment in its favor or in a proceeding by or in the right of a member.
Article VIII of the operating agreements of each of Biomet 3i, LLC, Biomet Microfixation, LLC and Biomet Florida Services, LLC provides for the limitation of personal liability of the managers and members thereof as follows:
To the fullest extent permitted by law, the Company shall indemnify and hold harmless each member and manager, and any officers, directors, shareholders, partners, employees, affiliates, representatives or agents thereof (“Covered Person”) from and against any and all losses, claims, demands, liabilities, expenses, judgments, fines, settlements and other amounts arising from any and all claims, demands, actions, suits or proceedings, civil, criminal, administrative or investigative (“Claims”), in which the Covered Person may be involved, or threatened to be involved, as a party or otherwise, by reason of its management of the affairs of the Company or which relates to or arises out of the Company or its property, business or affairs. A Covered Person shall not be entitled to indemnification under this Section 8.2 with respect to (i) any Claim with respect to which such Covered Person has engaged in fraud, willful misconduct, bad faith or gross negligence or (ii) any Claim initiated by such Covered Person unless such Claim (or part thereof) (A) was brought to enforce such Covered Person’s rights to indemnification hereunder or (B) was authorized or consented to by the Board. Expenses incurred by a Covered Person in defending any Claim shall be paid by the Company in advance of the final disposition of such Claim upon receipt by the Company of an undertaking by or on behalf of such Covered Person to repay such amount if it shall be ultimately determined that such Covered Person is not entitled to be indemnified by the Company as authorized by this Section 8.2.
Indiana Registrants:
(a) Biomet, Inc., Biomet Leasing Inc., Biomet Manufacturing Corporation, Biomet Travel, Inc., Blue Moon Diagnostics, Inc., Meridew Medical, Inc. and Thoramet, Inc. are incorporated under the laws of Indiana.
Chapter 37 of the Indiana Business Corporation Law provides that a corporation, unless limited by its Articles of Incorporation, is required to indemnify its directors and officers against reasonable expenses incurred in the wholly successful defense of any proceeding to which the director or officer was a party because of serving as a director or officer of the corporation.
Chapter 37 of the Indiana Business Corporation Law also provides that a corporation may voluntarily undertake to provide for indemnification of its directors and, unless otherwise provided in the articles of incorporation, its officers, employees and agents against any and all liability and reasonable expense that may be incurred by them, arising out of any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative and whether formal or informal, in which they may become involved by reason of being or having been a director or officer if (i) such persons acted in good faith, (ii) such persons reasonably believed their actions, in the case of their actions in their official capacity with the corporation, to be in the best interests of the corporation and, in all other cases, to be at least not opposed to its best interests, and (iii) in any criminal action, such persons had reasonable cause to believe their conduct was lawful, or had no reasonable cause to believe that their conduct was unlawful. A corporation may advance or reimburse reasonable expenses incurred by persons entitled to indemnification, in advance of final disposition, if such persons furnish the corporation with a written affirmation of their good faith belief that the applicable standard of conduct was observed, accompanied by a written undertaking to repay the advance if it is ultimately determined that the applicable standards were not met. Unless such persons have been successful in the defense of a proceeding, a corporation may not indemnify such persons unless authorized in the specific case after a determination has been made that indemnification of such persons is permissible in the circumstances because such persons met the standard of conduct set forth under the law.
The articles of incorporation and/or bylaws of all the Indiana corporate registrants indemnify any directors or officers made a party to a proceeding by reason of their position as director or officer against liability incurred by such persons in connection with the proceeding, except where the persons failed to meet the standard of conduct for indemnification specified by law. Such indemnification extends to the payment for or reimbursement of reasonable expenses incurred by the directors or officers in advance of final disposition of the proceeding. Such indemnification also extends, as a matter of right, to the payment of reasonable expenses incurred by directors or officers who are wholly successful, on the merits or otherwise, in defense of such proceeding.
II-2
Table of Contents
(b) Biomet Biologics, LLC, Biomet Fair Lawn, LLC, EBI, LLC, Biomet Orthopedics, LLC, Biomet Sports Medicine, LLC and Implant Innovations Holdings, LLC are each organized as limited liability companies under the laws of Indiana.
Chapter 2 of the Indiana Business Flexibility Act provides that, subject to any standards and restrictions set forth in a company’s operating agreement, a limited liability company may indemnify and hold harmless any member, manager, agent or employee from and against any and all claims and demands, unless the action or failure to act for which indemnification is sought constitutes willful misconduct or recklessness.
The operating agreements of all the Indiana limited liability company registrants indemnify, to the fullest extent permitted by law, any member, manager, officer, director, employee or agent of the company, and any officer, director, shareholder, partner, employee, affiliate, representative or agent of any member or manager of the company, from and against any and all losses, claims, demands, liabilities, expenses, judgments, fines, settlements and other amounts arising from any and all claims, demands, actions, suits or proceedings, civil, criminal, administrative or investigative, in which such persons may be involved, or threatened to be involved, as a party or otherwise, by reason of such persons’ management of the affairs of the company or which relates to or arises out of the company or its property, business or affairs. Such indemnification shall not be allowed with respect to (i) any claim with respect to which such persons have engaged in fraud, willful misconduct, bad faith or gross negligence or (ii) any claim initiated by such persons unless such claim (A) was brought to enforce such persons’ rights to indemnification under the operating agreements or (B) was authorized or consented to by the Board of the company. Expenses incurred by such persons in defending any claim shall be paid by the company in advance of the final disposition of such claim upon receipt by the company of an undertaking by or on behalf of such persons to repay such amount if it shall be ultimately determined that such persons were not entitled to be indemnified by the company as authorized by the operating agreements.
Certain Other Arrangements
Biomet, Inc. maintains a directors’ and officers’ liability insurance policy that covers the directors, officers, managers and members of each of the registrants in amounts that Biomet, Inc. believes are customary in its industry, including for liabilities in connection with the registration, offering and sale of the notes.
In addition, pursuant to the Management Services Agreement entered into with certain affiliates of the Sponsors, the Company has agreed to customary exculpation and indemnification provisions for the benefit of the managers and their affiliates. See “Certain Relationships and Related Party Transactions—Management Services Agreement” in the prospectus included in this registration statement.
Item 16. Exhibits and Financial Statement Schedules.
(a) Exhibits
See the Exhibit Index immediately following the signature pages included in this Registration Statement.
(b) Financial Statement Schedules
None.
| Item 17. | Undertakings. |
(a) Each of the undersigned registrants hereby undertakes:
(1) to file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
(i) to include any prospectus required by Section 10(a)(3) of the Securities Act;
(ii) to reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the SEC pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than a 20 % change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; and
(iii) to include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement.
(2) that, for the purpose of determining any liability under the Securities Act, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof;
(3) to remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering;
(b) Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrants have been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
II-3
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, Biomet, Inc. has duly caused this post-effective Amendment No. 2 to Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Warsaw, State of Indiana, on the 20th day of February, 2009.
| BIOMET, INC. | ||
| By: | /S/ JEFFREY R. BINDER | |
| Jeffrey R. Binder | ||
| President and Chief Executive Officer | ||
Pursuant to the requirements of the Securities Act of 1933, this post-effective Amendment No. 2 to Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
Signature | Capacity | Date | ||
/S/ JEFFREY R. BINDER | President (Principal Executive Officer) and Chief Executive Officer | February 20, 2009 | ||
| Jeffrey R. Binder | ||||
/S/ DANIEL P. FLORIN | Senior Vice President and Chief Financial Officer (Principal Financial Officer) | February 20, 2009 | ||
| Daniel P. Florin | ||||
/S/ KEVIN SIERKS | Vice President and Corporate Controller (Principal Accounting Officer) | February 20, 2009 | ||
| Kevin Sierks | ||||
* | Director | February 20, 2009 | ||
| David McVeigh | ||||
* | Director | February 20, 2009 | ||
| Jonathan J. Coslet | ||||
* | Director | February 20, 2009 | ||
| Michael Dal Bello | ||||
* | Director | February 20, 2009 | ||
| Adrian Jones | ||||
* | Director | February 20, 2009 | ||
| Michael Michelson | ||||
* | Director | February 20, 2009 | ||
| Dane A. Miller, Ph.D. | ||||
* | Director | February 20, 2009 | ||
| John Saer | ||||
* | Director | February 20, 2009 | ||
| Todd Sisitsky | ||||
/S/ GREGORY SUMME | Director | February 20, 2009 | ||
| Gregory Summe | ||||
| * | The undersigned, by signing his or her name hereto, does execute this post-effective Amendment No. 2 to Registration Statement on Form S-1 on behalf of the above-named officers and/or directors of the registrant pursuant to the Power of Attorney executed by such officers and/or directors on the signature pages to the Registration Statement previously filed on May 6, 2008. |
| By: | /S/ BRADLEY J. TANDY | |
| Bradley J. Tandy | ||
| Attorney-In-Fact |
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, Biolectron, Inc. has duly caused this post-effective Amendment No. 2 to Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Las Vegas, State of Nevada, on the 20th day of February, 2009.
| BIOLECTRON, INC. | ||
| By: | /S/ GLEN A. KASHUBA | |
| Glen A. Kashuba | ||
| President | ||
Pursuant to the requirements of the Securities Act of 1933, this post-effective Amendment No. 2 to Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
Signature | Capacity | Date | ||
/S/ GLEN A. KASHUBA | Director and President (Principal Executive Officer) | February 20, 2009 | ||
| Glen A. Kashuba | ||||
* | Director and Treasurer (Principal Financial Officer and Principal Accounting Officer) | February 20, 2009 | ||
| J. Pat Richardson | ||||
* | Director and Secretary | February 20, 2009 | ||
| Bradley J. Tandy | ||||
| * | The undersigned, by signing his or her name hereto, does execute this post-effective Amendment No. 2 to Registration Statement on Form S-1 on behalf of the above-named officers and/or directors of the registrant pursuant to the Power of Attorney executed by such officers and/or directors on the signature pages to the Registration Statement previously filed on May 6, 2008. |
| By: | /S/ GLEN A. KASHUBA | |
| Glen A. Kashuba | ||
| Attorney-In-Fact |
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, Biomet 3i, LLC has duly caused this post-effective Amendment No. 2 to Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Palm Beach Gardens, State of Florida, on the 20th day of February, 2009.
| BIOMET 3i, LLC | ||
| By: | /S/ JEFFREY R. BINDER | |
| Jeffrey R. Binder | ||
| Manager | ||
Pursuant to the requirements of the Securities Act of 1933, this post-effective Amendment No. 2 to Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
Signature | Capacity | Date | ||
* | President (Principal Executive Officer) | February 20, 2009 | ||
| Steven S. Schiess | ||||
/s/ Jeffrey R. Binder | Manager | February 20, 2009 | ||
* | Manager | February 20, 2009 | ||
| Bradley J. Tandy | ||||
* | Senior Vice President-Finance and Administration (Principal Financial Officer) | February 20, 2009 | ||
| Edward G. Sabin | ||||
/S/ J. PAT RICHARDSON | Manager and Vice President - Treasurer (Principal Accounting Officer) | February 20, 2009 | ||
| J. Pat Richardson | ||||
| * | The undersigned, by signing his or her name hereto, does execute this post-effective Amendment No. 2 to Registration Statement on Form S-1 on behalf of the above-named officers and/or managers of the registrant pursuant to the Power of Attorney executed by such officers and/or managers on the signature pages to the Registration Statement previously filed on May 6, 2008. |
| By: | /S/ J. PAT RICHARDSON | |
| J. Pat Richardson | ||
| Attorney-In-Fact |
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, Biomet Biologics, LLC has duly caused this post-effective Amendment No. 2 to Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Warsaw, State of Indiana, on the 20th day of February, 2009.
| BIOMET BIOLOGICS, LLC | ||
| By: | /s/ JEFFREY R. BINDER | |
| Jeffrey R. Binder | ||
| Manager | ||
Pursuant to the requirements of the Securities Act of 1933, this post-effective Amendment No. 2 to Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
Signature | Capacity | Date | ||
* | President (Principal Executive Officer) | February 20, 2009 | ||
| Stuart Kleopfer | ||||
/s/ Jeffrey R. Binder | Manager | February 20, 2009 | ||
/s/ BRADLEY J. TANDY | Manager | February 20, 2009 | ||
| Bradley J. Tandy | ||||
* | Manager and Treasurer (Principal Financial Officer and Principal Accounting Officer) | February 20, 2009 | ||
| J. Pat Richardson | ||||
| * | The undersigned, by signing his or her name hereto, does execute this post-effective Amendment No. 2 to Registration Statement on Form S-1 on behalf of the above-named officers and/or managers of the registrant pursuant to the Power of Attorney executed by such officers and/or managers on the signature pages to the Registration Statement previously filed on May 6, 2008. |
| By: | /S/ BRADLEY J. TANDY | |
| Bradley J. Tandy | ||
| Attorney-In-Fact |
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, Biomet Europe Ltd. has duly caused this post-effective Amendment No. 2 to Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Dordrecht, Country of The Netherlands, on the 20th day of February, 2009.
| BIOMET EUROPE LTD. | ||
| By: | /s/ GREGORY W. SASSO | |
| Gregory W. Sasso | ||
| Managing Director | ||
Pursuant to the requirements of the Securities Act of 1933, this post-effective Amendment No. 2 to Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
Signature | Capacity | Date | ||
/s/ GREGORY W. SASSO | Director and Managing Director (Principal Executive Officer) | February 20, 2009 | ||
| Gregory W. Sasso | ||||
* | Managing Director | February 20, 2009 | ||
| Roger P. Van Broeck | ||||
* | Director, Vice President and Treasurer (Principal Financial Officer and Principal Accounting Officer) | February 20, 2009 | ||
| J. Pat Richardson | ||||
* | Director and Secretary | February 20, 2009 | ||
| Bradley J. Tandy | ||||
| * | The undersigned, by signing his or her name hereto, does execute this post-effective Amendment No. 2 to Registration Statement on Form S-1 on behalf of the above-named officers and/or directors of the registrant pursuant to the Power of Attorney executed by such officers and/or directors on the signature pages to the Registration Statement previously filed on May 6, 2008. |
| By: | /S/ GREGORY W. SASSO | |
| Gregory W. Sasso | ||
| Attorney-In-Fact |
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, Biomet Fair Lawn, LLC has duly caused this post-effective Amendment No. 2 to Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Fairlawn, State of New Jersey, on the 20th day of February, 2009.
| BIOMET FAIR LAWN, LLC | ||
| By: | /s/ BRADLEY J. TANDY | |
| Bradley J. Tandy | ||
| Secretary | ||
Pursuant to the requirements of the Securities Act of 1933, this post-effective Amendment No. 2 to Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
Signature | Capacity | Date | ||
/s/ JEFFREY R. BINDER | President and Manager (Principal Executive Officer) | February 20, 2009 | ||
| Jeffrey R. Binder | ||||
* | Treasurer and Manager (Principal Financial Officer and Principal Accounting Officer) | February 20, 2009 | ||
| J. Pat Richardson | ||||
/s/ BRADLEY J. TANDY | Secretary and Manager | February 20, 2009 | ||
| Bradley J. Tandy | ||||
| * | The undersigned, by signing his or her name hereto, does execute this post-effective Amendment No. 2 to Registration Statement on Form S-1 on behalf of the above-named officers and/or managers of the registrant pursuant to the Power of Attorney executed by such officers and/or managers on the signature pages to the Registration Statement previously filed on May 6, 2008. |
| By: | /S/ JEFFREY R. BINDER | |
| Jeffrey R. Binder | ||
| Attorney-In-Fact |
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, Biomet Holdings Ltd. has duly caused this post-effective Amendment No. 2 to Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Warsaw, State of Indiana, on the 20th day of February, 2009.
| BIOMET HOLDINGS LTD. | ||
| By: | /s/ ROGER P. VAN BROECK | |
| Roger P. Van Broeck | ||
| President | ||
Pursuant to the requirements of the Securities Act of 1933, this post-effective Amendment No. 2 to Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
Signature | Capacity | Date | ||
/s/ ROGER P. VAN BROECK | President (Principal Executive Officer) | February 20, 2009 | ||
| Roger P. Van Broeck | ||||
/s/ J. PAT RICHARDSON | Director and Treasurer (Principal Financial Officer and Principal Accounting Officer) | February 20, 2009 | ||
| J. Pat Richardson | ||||
* | Director and Vice President | February 20, 2009 | ||
| Gregory W. Sasso | ||||
* | Director and Secretary | February 20, 2009 | ||
| Bradley J. Tandy | ||||
| * | The undersigned, by signing his or her name hereto, does execute this post-effective Amendment No. 2 to Registration Statement on Form S-1 on behalf of the above-named officers and/or directors of the registrant pursuant to the Power of Attorney executed by such officers and/or directors on the signature pages to the Registration Statement previously filed on May 6, 2008. |
| By: | /S/ J. PAT RICHARDSON | |
| J. Pat Richardson | ||
| Attorney-In-Fact |
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, Biomet International Ltd. has duly caused this post-effective Amendment No. 2 to Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Warsaw, State of Indiana, on the 20th day of February, 2009.
| BIOMET INTERNATIONAL LTD. | ||
| By: | /s/ WILBER C. BOREN, IV | |
| Wilber C. Boren, IV | ||
| President | ||
Pursuant to the requirements of the Securities Act of 1933, this post-effective Amendment No. 2 to Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
Signature | Capacity | Date | ||
/s/ WILBER C. BOREN, IV | President (Principal Executive Officer) | February 20, 2009 | ||
| Wilber C. Boren, IV | ||||
/s/ J. PAT RICHARDSON | Director and Treasurer (Principal Financial Officer and Principal Accounting Officer) | February 20, 2009 | ||
| J. Pat Richardson | ||||
* | Director and Secretary | February 20, 2009 | ||
| Bradley J. Tandy | ||||
* | Director | February 20, 2009 | ||
| Gregory W. Sasso | ||||
| * | The undersigned, by signing his or her name hereto, does execute this post-effective Amendment No. 2 to Registration Statement on Form S-1 on behalf of the above-named officers and/or directors of the registrant pursuant to the Power of Attorney executed by such officers and/or directors on the signature pages to the Registration Statement previously filed on May 6, 2008. |
| By: | /S/ J. PAT RICHARDSON | |
| J. Pat Richardson | ||
| Attorney-In-Fact |
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, Biomet Leasing, Inc. has duly caused this post-effective Amendment No. 2 to Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Warsaw, State of Indiana, on the 20th day of February, 2009.
| BIOMET LEASING, INC. | ||
| By: | /s/ BRADLEY J. TANDY | |
| Bradley J. Tandy | ||
| President | ||
Pursuant to the requirements of the Securities Act of 1933, this post-effective Amendment No. 2 to Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
Signature | Capacity | Date | ||
/s/ BRADLEY J. TANDY | Director and President (Principal Executive Officer) | February 20, 2009 | ||
| Bradley J. Tandy | ||||
/s/ J. PAT RICHARDSON | Director and Treasurer (Principal Financial Officer and Principal Accounting Officer) | February 20, 2009 | ||
| J. Pat Richardson | ||||
* | Secretary | February 20, 2009 | ||
| Elaine C. Piper | ||||
* | Director | February 20, 2009 | ||
| Jeffrey R. Binder | ||||
| * | The undersigned, by signing his or her name hereto, does execute this post-effective Amendment No. 2 to Registration Statement on Form S-1 on behalf of the above-named officers and/or directors of the registrant pursuant to the Power of Attorney executed by such officers and/or directors on the signature pages to the Registration Statement previously filed on May 6, 2008. |
| By: | /S/ J. PAT RICHARDSON | |
| J. Pat Richardson | ||
| Attorney-In-Fact |
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, Biomet Manufacturing Corporation has duly caused this post-effective Amendment No. 2 to Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Warsaw, State of Indiana, on the 20th day of February, 2009.
| BIOMET MANUFACTURING CORPORATION | ||
| By: | /s/ JEFFREY R. BINDER | |
| Jeffrey R. Binder | ||
| President | ||
Pursuant to the requirements of the Securities Act of 1933, this post-effective Amendment No. 2 to Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
Signature | Capacity | Date | ||
/s/ JEFFREY R. BINDER | Director and President (Principal Executive Officer) | February 20, 2009 | ||
| Jeffrey R. Binder | ||||
/s/ J. PAT RICHARDSON | Director and Treasurer (Principal Financial Officer and Principal Accounting Officer) | February 20, 2009 | ||
| J. Pat Richardson | ||||
* | Director and Secretary | February 20, 2009 | ||
| Bradley J. Tandy | ||||
| * | The undersigned, by signing his or her name hereto, does execute this post-effective Amendment No. 2 to Registration Statement on Form S-1 on behalf of the above-named officers and/or directors of the registrant pursuant to the Power of Attorney executed by such officers and/or directors on the signature pages to the Registration Statement previously filed on May 6, 2008. |
| By: | /S/ J. PAT RICHARDSON | |
| J. Pat Richardson | ||
| Attorney-In-Fact |
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, Biomet Microfixation, LLC has duly caused this post-effective Amendment No. 2 to Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Jacksonville, State of Florida, on the 20th day of February, 2009.
| BIOMET MICROFIXATION, LLC | ||
| By: | /s/ ADAM JOHNSON | |
| Adam Johnson | ||
| Manager | ||
Pursuant to the requirements of the Securities Act of 1933, this post-effective Amendment No. 2 to Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
Signature | Capacity | Date | ||
/s/ ADAM JOHNSON | President (Principal Executive Officer) | February 20, 2009 | ||
| Adam Johnson | ||||
* | Manager | |||
| Gregory W. Sasso | ||||
* | Manager | February 20, 2009 | ||
| Bradley J. Tandy | ||||
/s/ GARY BLACKALL | Vice President - Finance & Operations (Principal Financial Officer) | February 20, 2009 | ||
| Gary Blackall | ||||
/s/ J. PAT RICHARDSON | Manager and Treasurer (Principal Accounting Officer) | February 20, 2009 | ||
| J. Pat Richardson | ||||
| * | The undersigned, by signing his or her name hereto, does execute this post-effective Amendment No. 2 to Registration Statement on Form S-1 on behalf of the above-named officers and/or managers of the registrant pursuant to the Power of Attorney executed by such officers and/or managers on the signature pages to the Registration Statement previously filed on May 6, 2008. |
| By: | /S/ J. PAT RICHARDSON | |
| J. Pat Richardson | ||
| Attorney-In-Fact |
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, Biomet Orthopedics, LLC has duly caused this post-effective Amendment No. 2 to Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Warsaw, State of Indiana, on the 20th day of February, 2009.
| BIOMET ORTHOPEDICS, LLC | ||
| By: | /s/ JEFFREY R. BINDER | |
| Jeffrey R. Binder | ||
| Manager | ||
Pursuant to the requirements of the Securities Act of 1933, this post-effective Amendment No. 2 to Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
Signature | Capacity | Date | ||
/s/ JEFFREY R. BINDER | Manager and President (Principal Executive Officer) | February 20, 2009 | ||
| Jeffrey R. Binder | ||||
* | Manager | February 20, 2009 | ||
| Bradley J. Tandy | ||||
* | Vice President - Finance (Principal Financial Officer) | February 20, 2009 | ||
| Robert Vitoux | ||||
/s/ J. PAT RICHARDSON | Manager and Treasurer (Principal Accounting Officer) | February 20, 2009 | ||
| J. Pat Richardson | ||||
| * | The undersigned, by signing his or her name hereto, does execute this post-effective Amendment No. 2 to Registration Statement on Form S-1 on behalf of the above-named officers and/or managers of the registrant pursuant to the Power of Attorney executed by such officers and/or managers on the signature pages to the Registration Statement previously filed on May 6, 2008. |
| By: | /S/ J. PAT RICHARDSON | |
| J. Pat Richardson | ||
| Attorney-In-Fact |
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, Biomet Sports Medicine, LLC has duly caused this post-effective Amendment No. 2 to Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Warsaw, State of Indiana, on the 20th day of February, 2009.
| BIOMET SPORTS MEDICINE, LLC | ||
| By: | /s/ DAVID A. NOLAN, JR. | |
| David A. Nolan, Jr. | ||
| President | ||
Pursuant to the requirements of the Securities Act of 1933, this post-effective Amendment No. 2 to Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
Signature | Capacity | Date | ||
* | Manager | February 20, 2009 | ||
| Bradley J. Tandy | ||||
* | Manager | February 20, 2009 | ||
| Gregory W. Sasso | ||||
/s/ DAVID A. NOLAN, JR. | Manager and President (Principal Executive Officer) | February 20, 2009 | ||
| David A. Nolan, Jr. | ||||
/s/ J. PAT RICHARDSON | Treasurer (Principal Financial Officer and Principal Accounting Officer) | February 20, 2009 | ||
| J. Pat Richardson | ||||
| * | The undersigned, by signing his or her name hereto, does execute this post-effective Amendment No. 2 to Registration Statement on Form S-1 on behalf of the above-named officers and/or managers of the registrant pursuant to the Power of Attorney executed by such officers and/or managers on the signature pages to the Registration Statement previously filed on May 6, 2008. |
| By: | /S/ J. PAT RICHARDSON | |
| J. Pat Richardson | ||
| Attorney-In-Fact |
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, Biomet Travel, Inc. has duly caused this post-effective Amendment No. 2 to Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Warsaw, State of Indiana, on the 20th day of February, 2009.
| BIOMET TRAVEL, INC. | ||
| By: | /s/ JEFFREY R. BINDER | |
| Jeffrey R. Binder | ||
| President | ||
Pursuant to the requirements of the Securities Act of 1933, this post-effective Amendment No. 2 to Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
Signature | Capacity | Date | ||
/s/ JEFFREY R. BINDER | Director and President (Principal Executive Officer) | February 20, 2009 | ||
| Jeffrey R. Binder | ||||
/s/ J. PAT RICHARDSON | Director and Treasurer (Principal Financial Officer and Principal Accounting Officer) | February 20, 2009 | ||
| J. Pat Richardson | ||||
* | Director | February 20, 2009 | ||
| Daniel P. Florin | ||||
| * | The undersigned, by signing his or her name hereto, does execute this post-effective Amendment No. 2 to Registration Statement on Form S-1 on behalf of the above-named officers and/or directors of the registrant pursuant to the Power of Attorney executed by such officers and/or directors on the signature pages to the Registration Statement previously filed on May 6, 2008. |
| By: | /S/ J. PAT RICHARDSON | |
J. Pat Richardson Attorney-In-Fact |
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, Blue Moon Diagnostics, Inc. has duly caused this post-effective Amendment No. 2 to Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Warsaw, State of Indiana, on the 20th day of February, 2009.
| BLUE MOON DIAGNOSTICS, INC. | ||
| By: | /s/ JEFFREY R. BINDER | |
| Jeffrey R. Binder | ||
| President | ||
Pursuant to the requirements of the Securities Act of 1933, this post-effective Amendment No. 2 to Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
Signature | Capacity | Date | ||
/s/ JEFFREY R. BINDER | Director and President (Principal Executive Officer) | February 20 , 2009 | ||
| Jeffrey R. Binder | ||||
/s/ J. PAT RICHARDSON | Treasurer (Principal Financial Officer and Principal Accounting Officer) | February 20, 2009 | ||
| J. Pat Richardson | ||||
* | Director and Vice President | February 20, 2009 | ||
| Dave A. Nolan, Jr. | ||||
* | Director | February 20, 2009 | ||
| Bradley J. Tandy | ||||
| * | The undersigned, by signing his or her name hereto, does execute this post-effective Amendment No. 2 to Registration Statement on Form S-1 on behalf of the above-named officers and/or directors of the registrant pursuant to the Power of Attorney executed by such officers and/or directors on the signature pages to the Registration Statement previously filed on May 6, 2008. |
| By: | /S/ J. PAT RICHARDSON | |
J. Pat Richardson Attorney-In-Fact |
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, Cross Medical Products, LLC has duly caused this post-effective Amendment No. 2 to Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Irvine, State of California, on the 20th day of February, 2009.
| CROSS MEDICAL PRODUCTS, LLC | ||
| By: | /s/ JEFFREY R. BINDER | |
| Jeffrey R. Binder | ||
| Manager | ||
Pursuant to the requirements of the Securities Act of 1933, this post-effective Amendment No. 2 to Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
Signature | Capacity | Date | ||
* | Manager | February 20, 2009 | ||
| Jeffrey R. Binder | ||||
* | Manager | February 20, 2009 | ||
| Bradley J. Tandy | ||||
/s/ J. PAT RICHARDSON | Manager and Treasurer (Principal Financial Officer and Principal Accounting Officer) | February 20, 2009 | ||
| J. Pat Richardson | ||||
* | General Manager (Principal Executive Officer) | February 20, 2009 | ||
| Philip A. Mellinger | ||||
| * | The undersigned, by signing his or her name hereto, does execute this post-effective Amendment No. 2 to Registration Statement on Form S-1 on behalf of the above-named officers and/or managers of the registrant pursuant to the Power of Attorney executed by such officers and/or managers on the signature pages to the Registration Statement previously filed on May 6, 2008. |
| By: | /S/ J. PAT RICHARDSON | |
J. Pat Richardson Attorney-In-Fact |
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, EBI Holdings, LLC has duly caused this post-effective Amendment No. 2 to Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Parsippany, State of New Jersey, on the 20th day of February, 2009.
| EBI HOLDINGS, LLC | ||
| By: | /s/ JEFFREY R. BINDER | |
| Jeffrey R. Binder | ||
| Manager | ||
Pursuant to the requirements of the Securities Act of 1933, this post-effective Amendment No. 2 to Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
Signature | Capacity | Date | ||
/s/ JEFFREY R. BINDER | Manager | February 20, 2009 | ||
| Jeffrey R. Binder | ||||
* | Manager | February 20, 2009 | ||
| Bradley J. Tandy | ||||
* | Manager and President (Principal Executive Officer) | February 20, 2009 | ||
| Glen A. Kashuba | ||||
/s/ J. PAT RICHARDSON | Treasurer (Principal Financial Officer and Principal Accounting Officer) | February 20, 2009 | ||
| J. Pat Richardson | ||||
| * | The undersigned, by signing his or her name hereto, does execute this post-effective Amendment No. 2 to Registration Statement on Form S-1 on behalf of the above-named officers and/or managers of the registrant pursuant to the Power of Attorney executed by such officers and/or managers on the signature pages to the Registration Statement previously filed on May 6, 2008. |
| By: | /S/ J. PAT RICHARDSON | |
J. Pat Richardson Attorney-In-Fact |
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, EBI, LLC has duly caused this post-effective Amendment No. 2 to Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Parsippany, State of New Jersey, on the 20th day of February, 2009.
| EBI, LLC | ||
| By: | /s/ JEFFREY R. BINDER | |
| Jeffrey R. Binder | ||
| Manager | ||
Pursuant to the requirements of the Securities Act of 1933, this post-effective Amendment No. 2 to Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
Signature | Capacity | Date | ||
/s/ JEFFREY R. BINDER | Manager | February 20, 2009 | ||
| Jeffrey R. Binder | ||||
* | Manager | February 20, 2009 | ||
| Bradley J. Tandy | ||||
/s/ J. PAT RICHARDSON | Manager and Treasurer (Principal Financial Officer and Principal Accounting Officer) | February 20, 2009 | ||
| J. Pat Richardson | ||||
* | President (Principal Executive Officer) | February 20, 2009 | ||
| Glen A. Kashuba | ||||
| * | The undersigned, by signing his or her name hereto, does execute this post-effective Amendment No. 2 to Registration Statement on Form S-1 on behalf of the above-named officers and/or managers of the registrant pursuant to the Power of Attorney executed by such officers and/or managers on the signature pages to the Registration Statement previously filed on May 6, 2008. |
| By: | /S/ J. PAT RICHARDSON | |
J. Pat Richardson Attorney-In-Fact |
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, EBI Medical Systems, LLC has duly caused this post-effective Amendment No. 2 to Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Parsippany, State of New Jersey, on the 20th day of February, 2009.
| EBI MEDICAL SYSTEMS, LLC | ||
| By: | /s/ JEFFREY R. BINDER | |
| Jeffrey R. Binder | ||
| Manager | ||
Pursuant to the requirements of the Securities Act of 1933, this post-effective Amendment No. 2 to Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
Signature | Capacity | Date | ||
/s/ JEFFREY R. BINDER | Manager | February 20, 2009 | ||
| Jeffrey R. Binder | ||||
* | Manager | February 20, 2009 | ||
| Bradley J. Tandy | ||||
* | Manager and President (Principal Executive Officer) | February 20, 2009 | ||
| Glen A. Kashuba | ||||
/s/ J. PAT RICHARDSON | Treasurer (Principal Financial Officer and Principal Accounting Officer) | February 20, 2009 | ||
| J. Pat Richardson | ||||
| * | The undersigned, by signing his or her name hereto, does execute this post-effective Amendment No. 2 to Registration Statement on Form S-1 on behalf of the above-named officers and/or managers of the registrant pursuant to the Power of Attorney executed by such officers and/or managers on the signature pages to the Registration Statement previously filed on May 6, 2008. |
| By: | /S/ J. PAT RICHARDSON | |
J. Pat Richardson Attorney-In-Fact |
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, Electro-Biology, LLC has duly caused this post-effective Amendment No. 2 to Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Parsippany, State of New Jersey, on the 20th day of February, 2009.
| ELECTRO-BIOLOGY, LLC | ||
| By: | /s/ JEFFREY R. BINDER | |
| Jeffrey R. Binder | ||
| Manager | ||
Pursuant to the requirements of the Securities Act of 1933, this post-effective Amendment to Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
Signature | Capacity | Date | ||
/s/ JEFFREY R. BINDER | Manager | February 20, 2009 | ||
| Jeffrey R. Binder | ||||
* | Manager | February 20, 2009 | ||
| Bradley J. Tandy | ||||
* | Manager and President (Principal Executive Officer) | February 20, 2009 | ||
| Glen A. Kashuba | ||||
/s/ J. PAT RICHARDSON | Treasurer (Principal Financial Officer and Principal Accounting Officer) | February 20, 2009 | ||
| J. Pat Richardson | ||||
| * | The undersigned, by signing his or her name hereto, does execute this post-effective Amendment No. 2 to Registration Statement on Form S-1 on behalf of the above-named officers and/or managers of the registrant pursuant to the Power of Attorney executed by such officers and/or managers on the signature pages to the Registration Statement previously filed on May 6, 2008. |
| By: | /S/ J. PAT RICHARDSON | |
J. Pat Richardson Attorney-In-Fact |
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, Biomet Florida Services, LLC has duly caused this post-effective Amendment No. 2 to Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Palm Beach Gardens, State of Florida, on the 20th day of February, 2009.
| BIOMET FLORIDA SERVICES, LLC | ||
| By: | /s/ STEVEN SCHIESS | |
| Steven Schiess | ||
| Manager and President | ||
Pursuant to the requirements of the Securities Act of 1933, this post-effective Amendment No. 2 to Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
Signature | Capacity | Date | ||
/s/ STEVEN SCHIESS | Manager and President (Principal Executive Officer) | February 20, 2009 | ||
| Steven Schiess | ||||
* | Manager | February 20, 2009 | ||
| Bradley J. Tandy | ||||
/s/ J. PAT RICHARDSON | Manager and Treasurer (Principal Financial Officer) | February 20, 2009 | ||
| J. Pat Richardson | ||||
* | Assistant Treasurer (Principal Accounting Officer) | February 20, 2009 | ||
| Gary Blackall | ||||
* | Assistant Treasurer (Principal Accounting Officer) | February 20, 2009 | ||
| Scott Kanter | ||||
| * | The undersigned, by signing his or her name hereto, does execute this post-effective Amendment No. 2 to Registration Statement on Form S-1 on behalf of the above-named officers and/or managers of the registrant pursuant to the Power of Attorney executed by such officers and/or managers on the signature pages to the Registration Statement previously filed on May 6, 2008. |
| By: | /S/ J. PAT RICHARDSON | |
J. Pat Richardson Attorney-In-Fact |
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, Implant Innovations Holdings, LLC has duly caused this post-effective Amendment No. 2 to Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Warsaw, State of Indiana, on the 20th day of February, 2009.
| IMPLANT INNOVATIONS HOLDINGS, LLC | ||
| By: | /s/ JEFFREY R. BINDER | |
Jeffrey R. Binder Manager | ||
Pursuant to the requirements of the Securities Act of 1933, this post-effective Amendment No. 2 to Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
Signature | Capacity | Date | ||
/s/ STEVEN SCHIESS | President (Principal Executive Officer) | February 20, 2009 | ||
| Steven Schiess | ||||
* | Senior Vice President - Finance and Administration (Principal Financial Officer) | February 20, 2009 | ||
| Edward G. Sabin | ||||
/s/ J. PAT RICHARDSON | Treasurer (Principal Accounting Officer) | February 20, 2009 | ||
| J. Pat Richardson | ||||
* | Manager | February 20, 2009 | ||
| Jeffrey R. Binder | ||||
* | Manager | February 20, 2009 | ||
| Bradley J. Tandy | ||||
| * | The undersigned, by signing his or her name hereto, does execute this post-effective Amendment No. 2 to Registration Statement on Form S-1 on behalf of the above-named officers and/or managers of the registrant pursuant to the Power of Attorney executed by such officers and/or managers on the signature pages to the Registration Statement previously filed on May 6, 2008. |
| By: | /S/ J. PAT RICHARDSON | |
J. Pat Richardson Attorney-In-Fact |
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, Interpore Cross International, LLC has duly caused this post-effective Amendment No. 2 to Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Irvine, State of California, on the 20th day of February, 2009.
| INTERPORE CROSS INTERNATIONAL, LLC | ||
| By: | /s/ JEFFREY R. BINDER | |
Jeffrey R. Binder Manager | ||
Pursuant to the requirements of the Securities Act of 1933, this post-effective Amendment No. 2 to Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
Signature | Capacity | Date | ||
/s/ J. PAT RICHARDSON | Manager and Treasurer (Principal Financial Officer and Principal Accounting Officer) | February 20, 2009 | ||
| J. Pat Richardson | ||||
* | General Manager (Principal Executive Officer) | February 20, 2009 | ||
| Philip A. Mellinger | ||||
/s/ JEFFREY R. BINDER | Manager | February 20, 2009 | ||
| Jeffrey R. Binder | ||||
* | Manager | February 20, 2009 | ||
| Bradley J. Tandy | ||||
| * | The undersigned, by signing his or her name hereto, does execute this post-effective Amendment No. 2 to Registration Statement on Form S-1 on behalf of the above-named officers and/or managers of the registrant pursuant to the Power of Attorney executed by such officers and/or managers on the signature pages to the Registration Statement previously filed on May 6, 2008. |
| By: | /S/ J. PAT RICHARDSON | |
J. Pat Richardson Attorney-In-Fact |
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, Interpore Spine Ltd. has duly caused this post-effective Amendment No. 2 to Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Irvine, State of California, on the 20th day of February, 2009.
| INTERPORE SPINE LTD. | ||
| By: | /s/ PHILIP A. MELLINGER | |
Philip A. Mellinger General Manager | ||
Pursuant to the requirements of the Securities Act of 1933, this post-effective Amendment No. 2 to Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
Signature | Capacity | Date | ||
/s/ PHILIP A. MELLINGER | General Manager (Principal Executive Officer) | February 20, 2009 | ||
| Philip A. Mellinger | ||||
/s/ J. PAT RICHARDSON | Director and Treasurer (Principal Financial Officer and Principal Accounting Officer) | February 20, 2009 | ||
| J. Pat Richardson | ||||
* | Vice President | February 20, 2009 | ||
| Daniel A. Williamson | ||||
* | Director and Secretary | February 20, 2009 | ||
| Bradley J. Tandy | ||||
* | Director | February 20, 2009 | ||
| Jeffrey R. Binder | ||||
| * | The undersigned, by signing his or her name hereto, does execute this post-effective Amendment No. 2 to Registration Statement on Form S-1 on behalf of the above-named officers and/or directors of the registrant pursuant to the Power of Attorney executed by such officers and/or directors on the signature pages to the Registration Statement previously filed on May 6, 2008. |
| By: | /S/ J. PAT RICHARDSON | |
J. Pat Richardson Attorney-In-Fact |
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, Kirschner Medical Corporation has duly caused this post-effective Amendment No. 2 to Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Parsippany, State of New Jersey, on 20th day of February, 2009.
| KIRSCHNER MEDICAL CORPORATION | ||
| By: | /s/ GLEN A. KASHUBA | |
Glen A. Kashuba President | ||
Pursuant to the requirements of the Securities Act of 1933, this post-effective Amendment No. 2 to Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
Signature | Capacity | Date | ||
/s/ GLEN A. KASHUBA | Director and President (Principal Executive Officer) | February 20, 2009 | ||
| Glen A. Kashuba | ||||
/s/ J. PAT RICHARDSON | Director and Treasurer (Principal Financial Officer and Principal Accounting Officer) | February 20, 2009 | ||
| J. Pat Richardson | ||||
* | Director and Secretary | February 20, 2009 | ||
| Bradley J. Tandy | ||||
| * | The undersigned, by signing his or her name hereto, does execute this post-effective Amendment No. 2 to Registration Statement on Form S-1 on behalf of the above-named officers and/or directors of the registrant pursuant to the Power of Attorney executed by such officers and/or directors on the signature pages to the Registration Statement previously filed on May 6, 2008. |
| By: | /S/ J. PAT RICHARDSON | |
J. Pat Richardson Attorney-In-Fact |
Table of Contents
EXHIBIT INDEX
Exhibit No. | Exhibit | |
| 2.1 | Agreement and Plan of Merger, dated as of December 18, 2006, amended and restated as of June 7, 2007, among Biomet, Inc., LVB Acquisition, LLC and LVB Acquisition Merger Sub, Inc., incorporated herein by reference to the Company’s Current Report on Form 8-K filed on June 7, 2007. | |
| 3.1 | Amended and Restated Articles of Incorporation, incorporated herein by reference to Exhibit 3.1 to the Company’s Current Report on Form 8-K filed on September 25, 2007. | |
| 3.2 | Amended and Restated Bylaws, incorporated herein by reference to Exhibit 3.2 to the Company’s Current Report on Form 8-K filed on September 25, 2007. | |
| 3.3*** | Amended and Restated Certificate of Incorporation of Biolectron, Inc. | |
| 3.4*** | Bylaws of Biolectron, Inc. | |
| 3.5*** | Articles of Organization of Biomet 3i, LLC. | |
| 3.6*** | Limited Liability Company Agreement of Biomet 3i, LLC. | |
| 3.7*** | Articles of Entity Conversion of Biomet Biologics, LLC. | |
| 3.8*** | Limited Liability Company Agreement of Biomet Biologics, LLC. | |
| 3.9*** | Articles of Incorporation of Biomet Europe Ltd. (f/k/a OEC Ltd., Inc.), as amended. | |
| 3.10*** | Amended and Restated Bylaws of Biomet Europe, Ltd. (f/k/a OEC Ltd., Inc.). | |
| 3.11*** | Articles of Entity Conversion of Biomet Fair Lawn, LLC. | |
| 3.12*** | Limited Liability Company Agreement of Biomet Fair Lawn, LLC. | |
| 3.13*** | Certificate of Incorporation of Biomet Holdings Ltd. | |
| 3.14*** | Bylaws of Biomet Holdings Ltd. | |
| 3.15*** | Certificate of Incorporation of Biomet International Ltd. | |
| 3.16*** | Bylaws of Biomet International Ltd. | |
| 3.17*** | Articles of Incorporation of Biomet Leasing, Inc. | |
| 3.18*** | Bylaws of Biomet Leasing, Inc. | |
| 3.19*** | Articles of Incorporation of Biomet Manufacturing Corporation. | |
| 3.20*** | Bylaws of Biomet Manufacturing Corporation. | |
| 3.21*** | Articles of Organization of Biomet Microfixation, LLC. | |
| 3.22*** | Limited Liability Company Agreement of Biomet Microfixation, LLC. | |
| 3.23*** | Articles of Entity Conversion of Biomet Orthopedics, LLC. | |
| 3.24*** | Limited Liability Company Agreement of Biomet Orthopedics, LLC. | |
| 3.25*** | Articles of Entity Conversion of Biomet Sports Medicine, LLC. | |
| 3.26*** | Limited Liability Company Agreement of Biomet Sports Medicine, LLC. | |
| 3.27*** | Articles of Incorporation of Biomet Travel, Inc. | |
| 3.28*** | Bylaws of Biomet Travel, Inc. | |
| 3.29*** | Articles of Incorporation of Blue Moon Diagnostics, Inc. (f/k/a Surgical Ventures, Inc.), as amended. | |
| 3.30*** | Bylaws of Blue Moon Diagnostics, Inc. (f/k/a Surgical Ventures, Inc.). | |
| 3.31*** | Certificate of Formation of Cross Medical Products, LLC. | |
| 3.32*** | Limited Liability Company Agreement of Cross Medical Products, LLC. | |
| 3.33*** | Certificate of Formation of EBI Holdings, LLC. | |
| 3.34*** | Limited Liability Company Agreement of EBI Holdings, LLC. | |
| 3.35*** | Articles of Entity Conversion of EBI, LLC. | |
| 3.36*** | Limited Liability Company Agreement of EBI, LLC. | |
| 3.37*** | Certificate of Formation of EBI Medical Systems, LLC. | |
| 3.38*** | Limited Liability Company Agreement of EBI Medical Systems, LLC. | |
| 3.39*** | Certificate of Formation of Electro-Biology, LLC. | |
| 3.40*** | Limited Liability Company Agreement of Electro-Biology, LLC. | |
| 3.41*** | Articles of Organization of Biomet Florida Services, LLC. | |
| 3.42*** | Limited Liability Company Agreement of Biomet Florida Services, LLC. | |
| 3.43*** | Articles of Entity Conversion of Implant Innovations Holdings, LLC. | |
| 3.44*** | Limited Liability Company Agreement of Implant Innovations Holdings, LLC. | |
| 3.45*** | Articles of Organization - Conversion of Interpore Cross International, LLC. | |
| 3.46*** | Limited Liability Company Agreement of Interpore Cross International, LLC. | |
| 3.47*** | Amended and Restated Certificate of Incorporation of Interpore Spine Ltd. (f/k/a Interpore International, Inc.). | |
| 3.48*** | Amended and Restated Bylaws of Interpore Spine, Ltd. (f/k/a Interpore International, Inc.). | |
| 3.49*** | Certificate of Incorporation of Kirschner Medical Corporation (f/k/a Effner Biomet Corp., f/k/a Kirschner Acquisition Corp.), as amended. | |
| 3.50*** | Bylaws of Kirschner Medical Corporation. | |
| 4.1*** | Senior Notes Indenture, dated as of September 25, 2007, among LVB Acquisition Merger Sub, Inc., Biomet, Inc., the Guarantors listed therein and Wells Fargo Bank, National Association, as Trustee. | |
Table of Contents
| 4.2*** | First Supplemental Senior Notes Indenture, dated as of October 16, 2007, among Biomet, Inc., the Guarantors listed therein and Wells Fargo Bank, National Association, as Trustee. | |
| 4.3*** | Senior Subordinated Notes Indenture, dated as of September 25, 2007, among LVB Acquisition Merger Sub, Inc., Biomet, Inc., the Guarantors listed therein and Wells Fargo Bank, National Association, as Trustee. | |
| 4.4*** | First Supplemental Senior Subordinated Notes Indenture, dated as of October 16, 2007, among Biomet, Inc., the Guarantors listed therein and Wells Fargo Bank, National Association, as Trustee. | |
| 4.5 | Form of 10% Senior Notes due 2017 (included in Exhibit 4.1). | |
| 4.6 | Form of 10 3/8% / 11 1/8% Senior Toggle Notes due 2017 (included in Exhibit 4.1). | |
| 4.7 | Form of 11 5/8% Senior Subordinated Notes due 2017 (included in Exhibit 4.3). | |
| 4.8*** | Registration Rights Agreement, dated as of September 25, 2007, among LVB Acquisition Merger Sub, Inc., Biomet, Inc., the Guarantors listed therein, and Banc of America Securities LLC, Goldman, Sachs & Co., Lehman Brothers Inc., Merrill Lynch, Pierce, Fenner & Smith Incorporated, Wachovia Capital Markets, LLC and Bear, Stearns & Co. Inc. | |
| 4.9*** | Registration Rights Agreement, dated as of October 16, 2007, among Biomet, Inc., the Guarantors listed therein, and Banc of America Securities LLC, Goldman, Sachs & Co., Lehman Brothers Inc., Merrill Lynch, Pierce, Fenner & Smith Incorporated, Wachovia Capital Markets, LLC and Bear, Stearns & Co. Inc. | |
| 5.1** | Opinion of Cleary Gottlieb Steen & Hamilton LLP. | |
| 5.2** | Opinion of Kirkland & Ellis LLP. | |
| 5.3** | Opinion of Taft Stettinius & Hollister LLP. | |
| 5.4** | Opinion of Edwards Angell Palmer & Dodge LLP. | |
| 10.1*** | Credit Agreement, dated as of September 25, 2007, among Biomet, Inc., LVB Acquisition, Inc., Bank of America, N.A. and the Other Lenders party thereto. | |
| 10.2*** | Guaranty (Cash Flow), dated as of September 25, 2007, among LVB Acquisition, Inc., Certain Subsidiaries of Biomet, Inc. identified therein, and Bank of America, N.A. | |
| 10.3*** | Pledge and Security Agreement (Cash Flow), dated as of September 25, 2007, among Biomet, Inc., LVB Acquisition, Inc., Certain Subsidiaries of Biomet, Inc. identified therein, and Bank of America, N.A. | |
| 10.4*** | Intercreditor Agreement, dated as of September 25, 2007, by and among Bank of America, N.A., as ABL Collateral Agent, and Bank of America, N.A., as CF Collateral Agent. | |
| 10.5*** | Patent Security Agreement, dated as of September 25, 2007, among LVB Acquisition, Inc., Biomet, Inc., Certain Subsidiaries of Biomet, Inc. and Bank of America, N.A. | |
| 10.6*** | Trademark Security Agreement, dated as of September 25, 2007, among LVB Acquisition, Inc., Biomet, Inc., Certain Subsidiaries of Biomet, Inc. and Bank of America, N.A. | |
| 10.7*** | Credit Agreement, dated as of September 25, 2007, among Biomet, Inc., the Several Subsidiary Borrowers Party thereto, LVB Acquisition, Inc., Bank of America, N.A. and the Other Lenders Party thereto. | |
| 10.8*** | Guaranty (ABL), dated as of September 25, 2007 between LVB Acquisition, Inc. and Bank of America, N.A. | |
| 10.9*** | Pledge and Security Agreement (ABL), dated as of September 25, 2007 among Biomet, Inc., LVB Acquisition, Inc., Certain Subsidiaries of Biomet, Inc. identified therein and Bank of America, N.A. | |
| 10.10 | Joint Venture Agreement between Biomet, Inc. and Merck KgaA, dated as of November 24, 1997, incorporated herein by reference to Exhibit 2.01 to the Company’s Current Report on Form 8-K filed on February 17, 1998. | |
| 10.11 | Purchase and Substitution Agreement, dated March 19, 2004 by and among Merck KGaA, Biomet, Inc., BioHoldings UK Ltd. and Biomet Europe Ltd., incorporated herein by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on March 24, 2004. | |
| 10.12† | Executive Severance Pay Plan, dated as of September 22, 2006, incorporated herein by reference to Exhibit 10.4 to the Company’s Current Report on Form 8-K filed on September 26, 2006. | |
| 10.13† | Employment Agreement, dated as of February 26, 2007, by and among Biomet, Inc. and Jeffrey R. Binder, incorporated herein by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on February 27, 2007. | |
| 10.14† | Offer Letter, dated as of March 26, 2007, by and among Biomet, Inc. and J. Pat Richardson, incorporated herein by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on April 2, 2007. | |
| 10.15† | Retirement and Consulting Agreement, dated as of March 30, 2007, by and among Biomet, Inc. and Daniel P. Hann, incorporated herein by reference to Exhibit 10.2 to the Company’s Current Report on Form 8-K filed on April 23, 2007. | |
| 10.16† | Offer Letter, dated as of May 10, 2007, by and among Biomet, Inc. and Daniel P. Florin, incorporated herein by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on May 16, 2007. | |
| 10.17† | Employment Agreement, dated as of February 28, 2008, by and among Biomet, Inc. and Daniel P. Florin, incorporated herein by reference to Exhibit 10.16 to the Company’s Annual Report on Form 10-K filed on August 28, 2008. | |
| 10.18 | Limited Guarantee, dated June 7, 2007, by TPG Partners V L.P., incorporated herein by reference to Exhibit (d)(1)(I) of the Schedule TO filed by LVB Acquisition Merger Sub, Inc. and LVB Acquisition LLC on June 13, 2007. | |
| 10.19 | Limited Guarantee, dated June 7, 2007, by KKR 2006 Fund L.P., incorporated herein by reference to Exhibit (d)(1)(H) of the Schedule TO filed by LVB Acquisition Merger Sub, Inc. and LVB Acquisition LLC on June 13, 2007. | |
| 10.20 | Limited Guarantee, dated June 7, 2007, by GS Capital Partners VI Parallel, L.P., GS Capital Partners VI GmbH & Co. KG, GS Capital Partners VI Fund, L.P. and GS Capital Partners Offshore Fund, L.P., incorporated herein by reference to Exhibit (d)(1)(G) of the Schedule TO filed by LVB Acquisition Merger Sub, Inc. and LVB Acquisition LLC on June 13, 2007. | |
| 10.21 | Limited Guarantee, dated June 7, 2007, by Blackstone Capital Partners V L.P., incorporated herein by reference to Exhibit (d)(1)(F) of the Schedule TO filed by LVB Acquisition Merger Sub, Inc. and LVB Acquisition LLC on June 13, 2007. | |
Table of Contents
| 10.22†*** | LVB Acquisition, Inc. 2007 Management Equity Incentive Plan. | |
| 10.23†*** | Amendment to the Retirement and Consulting Agreement, dated as of March 30, 2008, by and between the Company and Daniel P. Hann. | |
| 10.24*** | Deferred Prosecution Agreement, dated as of September 27, 2007, by and between Biomet, Inc. and the United States Attorney’s Office for the District of New Jersey. | |
| 10.25*** | Corporate Integrity Agreement, dated as of September 27, 2007, by and between the Office of Inspector General of the Department of Health and Human Services and Biomet, Inc. | |
| 10.26*** | Settlement Agreement, dated as of September 27, 2007, by and between Biomet, Inc. and the Office of Inspector General of the Department of Health and Human Services. | |
| 10.27† | Biomet, Inc. Executive Annual Cash Incentive Plan, incorporated herein by reference to Exhibit 10.26 to the Company’s Annual Report on Form 10-K filed on August 28, 2008. | |
| 10.28† | Employment Agreement, dated as of February 28, 2008, by and between Biomet, Inc. and Glen A.Kashuba, incorporated herein by reference to Exhibit 10.27 to the Company’s Annual Report on Form 10-K filed on August 28, 2008. | |
| 10.29† | Employment Agreement, dated as of February 28, 2008, by and between Biomet, Inc. and Steven F. Schiess, incorporated herein by reference to Exhibit 10.28 to the Company’s Annual Report on Form 10-K filed on August 28, 2008. | |
| 10.30† | Employment Agreement, dated as of February 28, 2008, by and between Biomet, Inc. and Roger P. Van Broeck, incorporated herein by reference to Exhibit 10.29 to the Company’s Annual Report on Form 10-K filed on August 28, 2008. | |
| 10.31† | Biomet, inc. Deferred Compensation Plan (Post-409A Plan), incorporated herein by reference to Exhibit 10.2 to the Company’s Quarterly Report on Form 10-Q filed on January 14, 2009. | |
| 10.32† | First Amendment to Employment Agreement, dated as of December 31, 2008, by and between Biomet, Inc. and Jeffrey R. Binder, incorporated herein by reference to Exhibit 10.3 to the Company’s Quarterly Report on Form 10-Q filed on January 14, 2009. | |
| 10.33† | First Amendment to Employment Agreement, dated as of December 31, 2008, by and between Biomet, Inc. and Daniel P. Florin, incorporated herein by reference to Exhibit 10.4 to the Company’s Quarterly Report on Form 10-Q filed on January 14, 2009. | |
| 10.34† | First Amendment to Employment Agreement, dated as of December 31, 2008, by and between Biomet, Inc. and Glen A. Kashuba, incorporated herein by reference to Exhibit 10.5 to the Company’s Quarterly Report on Form 10-Q filed on January 14, 2009. | |
| 10.35† | First Amendment to Employment Agreement, dated as of December 31, 2008, by and between Biomet, Inc. and Steven F. Schiess, incorporated herein by reference to Exhibit 10.6 to the Company’s Quarterly Report on Form 10-Q filed on January 14, 2009. | |
| 10.36† | First Amendment to Employment Agreement, dated as of December 31, 2008, by and between Biomet, Inc. and Roger P. Van Broeck, incorporated herein by reference to Exhibit 10.7 to the Company’s Quarterly Report on Form 10-Q filed on January 14, 2009. | |
| 10.37† | Change in Control Agreement, dated as of September 20, 2006, by and between Biomet, Inc. and Steven F. Schiess, incorporated herein by reference to Exhibit 10.8 to the Company’s Quarterly Report on Form 10-Q filed on January 14, 2009. | |
| 10.38† | First Amendment to Change in Control Agreement, dated as of December 31, 2008, by and between Biomet, Inc. and Steven F. Schiess, incorporated herein by reference to Exhibit 10.9 to the Company’s Quarterly Report on Form 10-Q filed on January 14, 2009. | |
| 10.39† | Change in Control Agreement, dated as of September 20, 2006, by and between Biomet, Inc. and Roger P. Van Broeck, incorporated herein by reference to Exhibit 10.10 to the Company’s Quarterly Report on Form 10-Q filed on January 14, 2009. | |
| 10.40† | First Amendment to Change in Control Agreement, dated as of December 31, 2008, by and between Biomet, Inc. and Roger P. Van Broeck, incorporated herein by reference to Exhibit 10.11 to the Company’s Quarterly Report on Form 10-Q filed on January 14, 2009. | |
| 10.41† | Change in Control Agreement, dated as of February 6, 2007, by and between Biomet, Inc. and Jeffrey R. Binder, incorporated herein by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on February 27, 2007. | |
| 10.42† | Change in Control Agreement, dated as of March 29, 2007, by and between Biomet, Inc. and J. Pat Richardson, incorporated herein by reference to Exhibit 10.2 to the Company’s Current Report on Form 8-K filed on April 2, 2007. | |
| 10.43† | LVB Acquisition Management Stockholders’ Agreement, dated as of September 13, 2007, by and among LVB Acquisition, Inc. and the stockholders party thereto, incorporated herein by reference to Exhibit 10.30 to the Company’s Annual Report on Form 10-K filed on August 28, 2008. | |
| 10.44† | Separation, Release and Consultancy Agreement, dated May 8. 2006, by and among Biomet, Inc. and Dane A. Miller, Ph. D., incorporated herein by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on May 10, 2006. | |
| 12 | Computation of Ratio of Earnings to Fixed Charges incorporated herein by reference to Exhibit 12 to the Company’s Quarterly Report on Form 10-Q filed on January 14, 2009. | |
| 16** | Letter re Change in Certifying Accountant. | |
| 21.1* | Subsidiaries of Biomet, Inc. | |
| 23.1* | Consent of Deloitte & Touche LLP. | |
| 23.2* | Consent of Ernest & Young LLP. | |
| 23.3 | Consent of Cleary Gottlieb Steen & Hamilton LLP (included in the opinion filed as Exhibit 5.1). | |
| 23.4 | Consent of Kirkland & Ellis LLP (included in the opinion filed as Exhibit 5.2). | |
Table of Contents
| 23.5 | Consent of Taft Stettinius & Hollister LLP (included in the opinion filed as Exhibit 5.3). | |
| 23.6 | Consent of Edwards Angell Palmer & Dodge LLP (included in the opinion filed as Exhibit 5.4). | |
| 24 | Powers of Attorney (included on signature pages of the Company’s Registration Statement on Form S-1 filed on May 6, 2008). | |
| 25.1*** | Form T-1 statement of eligibility under the Trust Indenture Act of 1939, as amended, of Wells Fargo Bank, National Association, as Trustee with respect to the Indenture governing the 10% Senior Notes due 2017 and the 10 3/8%/11 1/8% Senior Toggle Notes due 2017. | |
| 25.2*** | Form T-1 statement of eligibility under the Trust Indenture Act of 1939, as amended, of Wells Fargo Bank, National Association, as Trustee with respect to the Indenture governing the 11 5/8% Senior Subordinated Notes due 2017. | |
| * | Filed herewith. |
| ** | Previously filed. |
| *** | Incorporated by reference to the Registration Statement on Form S-4 of Biomet, Inc. filed on April 30, 2008. |
| † | Management contract or compensatory plan or arrangement. |