Filed by Endologix, Inc. Pursuant to Rule 425 Under the Securities Act of 1933 and deemed filed pursuant to Rule 14d-2 of the Securities Exchange Act of 1934 Subject Company: TriVascular Technologies, Inc. Commission File No.: 001-36419 |
|
OCTOBER 26, 2015
Combining innovation leaders to revolutionize aortic treatments
Endologix Third Quarter 2015 Results and TriVascular Merger
|
Safe Harbor
Endologix and TriVascular will remain separate operational entities until the closing of the proposed merger transaction. Until closing,
Endologix will not offer TriVascular products and TriVascular will not offer Endologix products.
Forward-Looking Statements
This presentation includes statements that may be forward-looking statements. The words “believe,” “expect,” “anticipate,” “project” and similar expressions, among others, generally
identify forward-looking statements. Endologix and TriVascular caution that these forward-looking statements are subject to risks and uncertainties that may cause actual results to
differ materially from those indicated in the forward-looking statements. Such risks and uncertainties include, but are not limited to, the likelihood that the transaction is consummated
on a timely basis or at all, including whether the conditions required to complete the transaction will be met, realization of the expected benefits of the transaction, competition from
other products, changes to laws and regulations applicable to our industry, status of our ongoing clinical trials, clinical trial results, decisions and the timing of decisions of regulatory
authorities regarding our products and potential future products, risks relating to foreign currency fluctuations, and a variety of other risks. Additional information about the factors that
may affect the companies’ operations is set forth in Endologix’s and TriVascular’s annual and periodic reports filed with the Securities and Exchange Commission (the “SEC”). Neither
Endologix nor TriVascular undertakes any obligation to release publicly any revisions to forward-looking statements as a result of subsequent events or developments, except as required
by law.
Additional Information and Where to Find It
The transaction referenced in this presentation has not yet commenced, and no proxies are yet being solicited. Endologix plans to file a registration statement on Form S–4 (“S-4”) that
will serve as a prospectus for Endologix shares to be issued as consideration in the merger and as a proxy statement of TriVascular for the solicitation of votes of TriVascular stockholders
to approve the proposed transaction (the “Proxy Statement/Prospectus”). This presentation is for informational purposes only and is neither an offer to purchase nor a solicitation of an
offer to sell shares. It is also not a substitute for the S-4, the Proxy Statement/Prospectus or any other documents that Endologix or TriVascular may file with the SEC or send to
stockholders in connection with the proposed transaction. THE DEFINITIVE PROXY STATEMENT/PROSPECTUS WILL CONTAIN IMPORTANT INFORMATION ABOUT ENDOLOGIX,
TRIVASCULAR AND THE TRANSACTIONS. TRIVASCULAR STOCKHOLDERS ARE URGED TO READ THE PROXY STATEMENT/PROSPECTUS CAREFULLY AND IN ITS ENTIRETY WHEN IT BECOMES
AVAILABLE BEFORE MAKING ANY DECISION REGARDING VOTING ON THE PROPOSED TRANSACTION. In addition to the SEC filings made in connection with the transaction, each of
Endologix and TriVascular files annual, quarterly and current reports and other information with the SEC. Endologix ’s and TriVascular’s filings with the SEC, including the Proxy
Statement/Prospectus once it is filed, are available to the public free of charge at the website maintained by the SEC at http://www.sec.gov. Copies of documents filed with the SEC by
TriVascular will be made available free of charge on TriVascular’s website at http://investors.trivascular.com. Copies of documents filed with the SEC by Endologix will be made available
free of charge on Endologix’s website at http://investor.endologix.com.
Participants in the Solicitation
Endologix, TriVascular and their respective directors and executive officers may be deemed to be participants in any solicitation of proxies from TriVascular’s stockholders in connection
with the proposed transaction. Information regarding Endologix’s directors and executive officers is available in its proxy statement for its 2015 annual meeting of stockholders, which
was filed with the SEC on April 17, 2015; information regarding TriVascular’s directors and executive officers is available in its proxy statement for its 2015 annual meeting of
stockholders, which was filed with the SEC on April 14, 2015. Other information regarding the interests of such potential participants will be contained in the Proxy Statement/Prospectus
when it becomes available. You may obtain free copies of these documents as described in the preceding paragraph.
Combining innovation leaders to revolutionize aortic treatments | 10.26.15
2 |
|
|
Endologix 3Q 2015 Results
Combining innovation leaders to revolutionize aortic treatments | 10.26.15
3 |
|
|
Merger Rationale
Growth Technology Synergies
Expanded global sales force
Broad product offering for AAA
Multiple new product launches over next 24 months
An innovation leader with broad clinical indications
Significant clinical evidence
370 issued and pending patents
Leverages best-in-class technology with strong commercial capability
Over $30 million in annual synergies by 2017
EBITDA accretive
Combining innovation leaders to revolutionize aortic treatments | 10.26.15
4 |
|
|
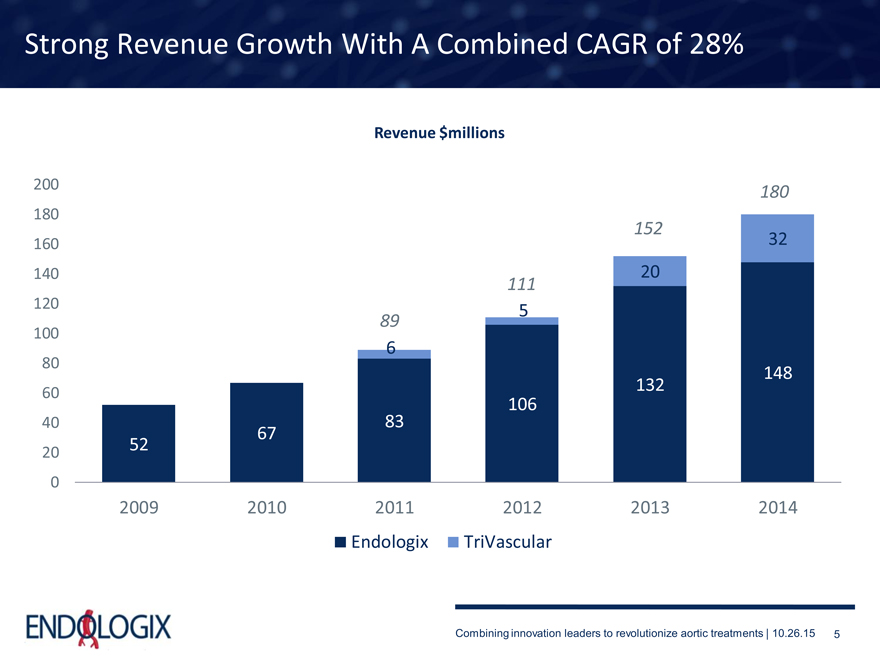
Strong Revenue Growth With A Combined CAGR of 28%
Revenue $millions
200
180
180 152
160 32 140 20
111
120 5
89
100
6 |
|
80
148 132
60
106
40 83
67 52
20
0
2009 2010 2011 2012 2013 2014
Endologix TriVascular
Combining innovation leaders to revolutionize aortic treatments | 10.26.15
5 |
|
|
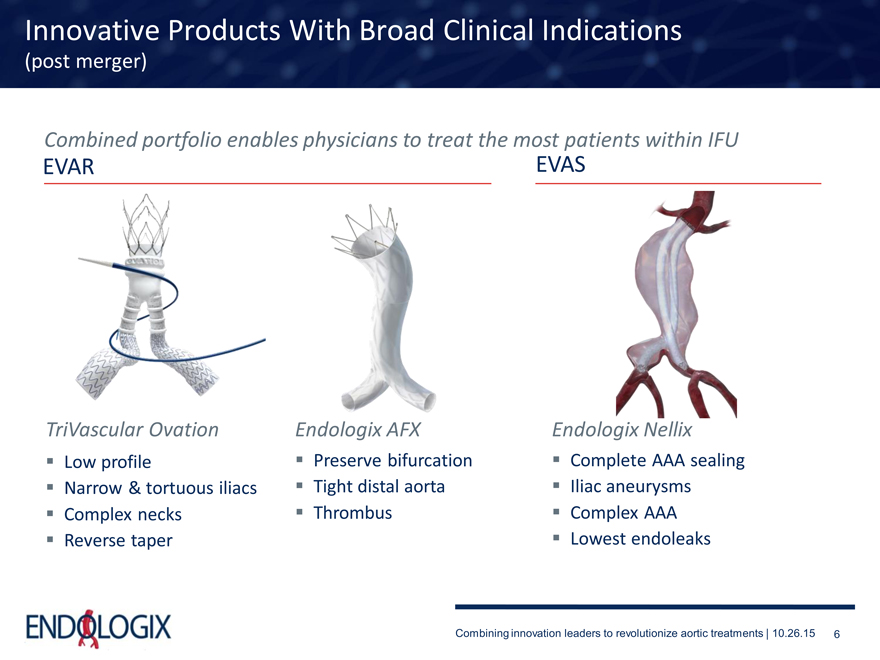
Innovative Products With Broad Clinical Indications
(post merger)
Combined portfolio enables physicians to treat the most patients within IFU
EVAR EVAS
TriVascular Ovation Endologix AFX Endologix Nellix
Low profile
Narrow & tortuous iliacs Complex necks Reverse taper
Preserve bifurcation Tight distal aorta Thrombus
Complete AAA sealing Iliac aneurysms Complex AAA Lowest endoleaks
Combining innovation leaders to revolutionize aortic treatments | 10.26.15
6 |
|
|
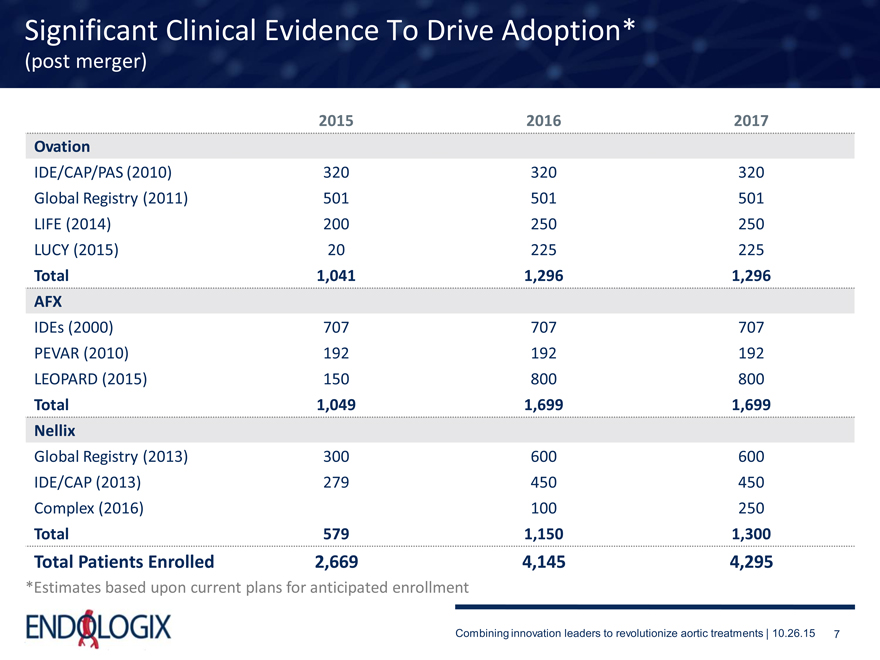
Significant Clinical Evidence To Drive Adoption*
(post merger)
2015 2016 2017 Ovation
IDE/CAP/PAS (2010) 320 320 320 Global Registry (2011) 501 501 501 LIFE (2014) 200 250 250 LUCY (2015) 20 225 225
Total 1,041 1,296 1,296 AFX
IDEs (2000) 707 707 707 PEVAR (2010) 192 192 192 LEOPARD (2015) 150 800 800
Total 1,049 1,699 1,699 Nellix
Global Registry (2013) 300 600 600 IDE/CAP (2013) 279 450 450 Complex (2016) 100 250
Total 579 1,150 1,300
Total Patients Enrolled 2,669 4,145 4,295
*Estimates based upon current plans for anticipated enrollment
Combining innovation leaders to revolutionize aortic treatments | 10.26.15
7 |
|
|
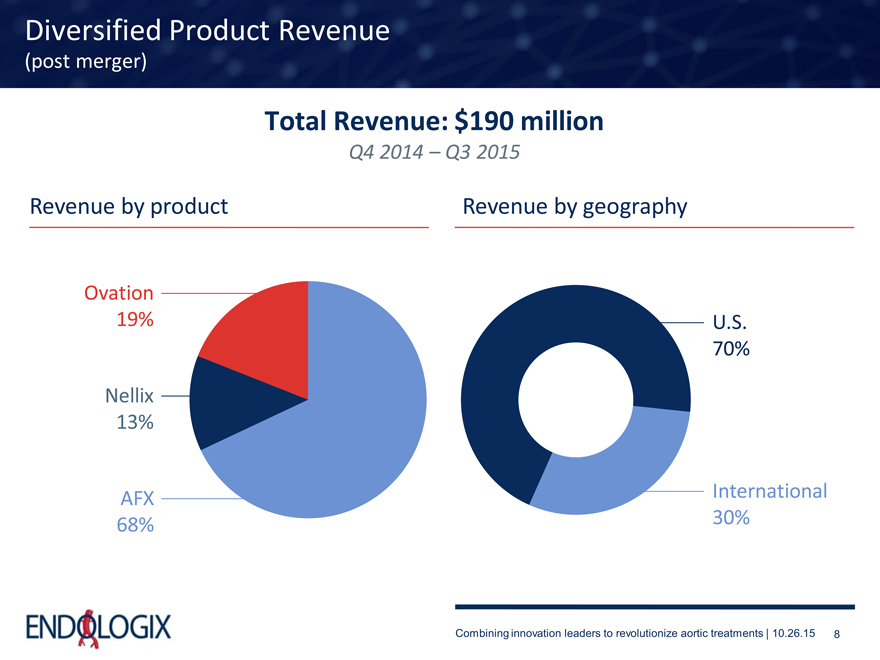
Diversified Product Revenue
(post merger)
Total Revenue: $190 million
Q4 2014 – Q3 2015
Revenue by product Revenue by geography
Ovation
19% U.S.
70%
Nellix
13%
AFX International 68% 30%
Combining innovation leaders to revolutionize aortic treatments | 10.26.15
8 |
|
|
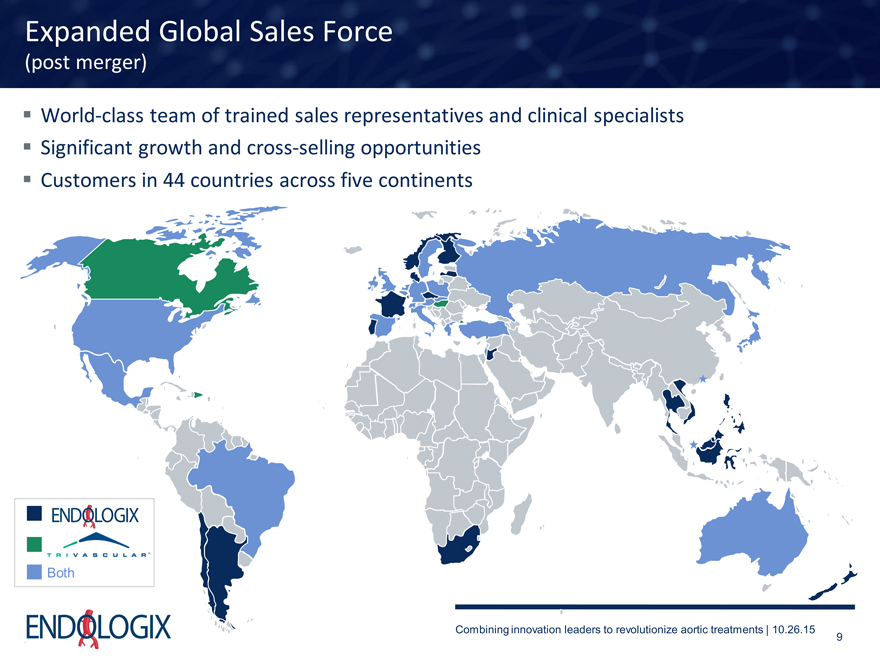
Expanded Global Sales Force
(post merger)
World-class team of trained sales representatives and clinical specialists
Significant growth and cross-selling opportunities Customers in 44 countries across five continents
Combining innovation leaders to revolutionize aortic treatments | 10.26.15
Both
9
|
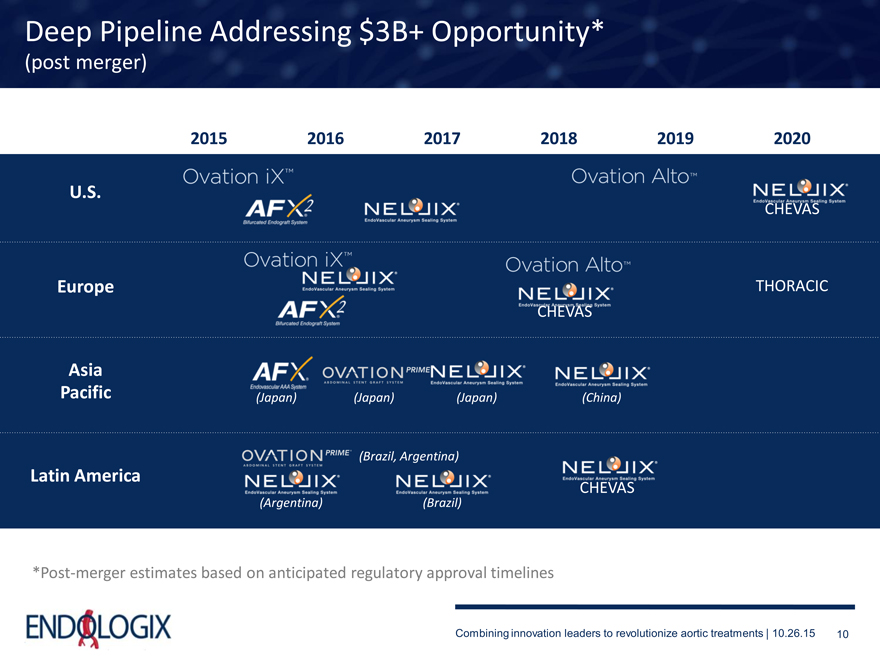
Deep Pipeline Addressing $3B+ Opportunity*
(post merger)
2015 2016 2017 2018 2019 2020
U.S.
CHEVAS
Europe THORACIC CHEVAS
Asia
Pacific (Japan) (Japan) (Japan) (China)
(Brazil, Argentina)
Latin America
CHEVAS
(Argentina) (Brazil)
*Post-merger estimates based on anticipated regulatory approval timelines
Combining innovation leaders to revolutionize aortic treatments | 10.26.15
10
|
Transaction Overview
$211 million of consideration consisting of Endologix stock and cash(1)
Transaction $9.10 per share
Terms 13.56 million Endologix shares, representing ~16.2% ownership
~$24 million in cash consideration
~$13 million for intrinsic value of outstanding equity awards(2)
~$11 million representing conversion value of TriVascular convertible debt(3)
Combined cash balance of $123 million as of June 30, 2015(4)
Balance Sheet
$150 million convertible debt offering to pay down higher cost TriVascular
existing debt and fund growth initiatives
Expected to close in the first quarter of 2016
Timing / Regulatory approvals
Next Steps Subject to customary closing conditions
Subject to TriVascular shareholder approval
1 As of October 23, 2015 and subject to change based upon Endologix stock price at closing. Assumes conversion of TriVascular convertible debt
2 Intrinsic value of TriVascular equity awards will be paid to shareholders and is based on 2.3M options, 0.8M restricted stock units, and 0.4M warrants
3 Assumes conversion of TriVascular convertible debt into 1.25M TriVascular shares and payment of conversion value to shareholders
4 Combined cash balance includes Endologix and TriVascular cash and cash equivalents and short-term marketable securities as of June 30, 2015 before the effect
of the issuance of the convertible notes and cash payments related to the acquisition of TriVascular
Combining innovation leaders to revolutionize aortic treatments | 10.26.15
11
|
Creating a Leader in Growth and Innovation
Increased revenue growth
Broad AAA product line Expanded global sales force Significant clinical evidence
$30M+ synergies in 2017 and EBITDA accretive in 2018
Combining innovation leaders to revolutionize aortic treatments | 10.26.15
12
|
Treat more patients more effectively