|
Filed by: TriVascular Technologies, Inc. Pursuant to Rule 425 under the Securities Act of 1933 and deemed filed pursuant to Rule 14a-12 of the Securities Exchange Act of 1934 Subject Company: TriVascular Technologies, Inc. Commission File No.: 001-36419 TriVascular Employee Presentation 
|
TriVascular Employee Presentation
All Employee Meeting
October 26, 2015
Confidential
Today’s announcement
Board has approved merger with Endologix
TriVascular shareholders will receive stock and cash consideration
After closing, new combined entity will continue to market Ovation and legacy products
Santa Rosa facility and core functions to remain intact
Deal is subject to regulatory and shareholder approval
No immediate changes; companies remain independent competitors until transaction closes 2
Merger rationale
Offers physicians three clinically proven platforms to customize the therapy for each unique anatomy
Dramatically expand global & functional capability
~$190+ million pro forma revenue base
Enables faster time to profitability
Accelerates growth for OVATION Platform
Increases value creation potential & reduces risk
Increase access to capital & reduces cost of capital
Creates powerful commercial & innovation potential with complementary technologies, capabilities & talent
Preserves small growth company entrepreneurial spirit
3
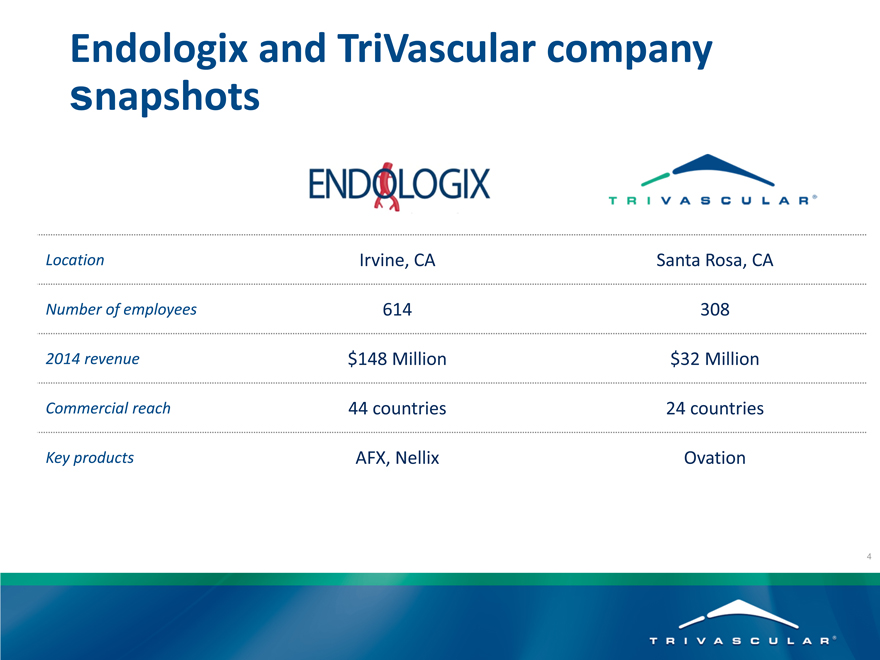
Endologix and TriVascular company snapshots
Location Irvine, CA Santa Rosa, CA
Number of employees 614 308
2014 revenue $148 Million $32 Million
Commercial reach 44 countries 24 countries
Key products AFX, Nellix Ovation
4
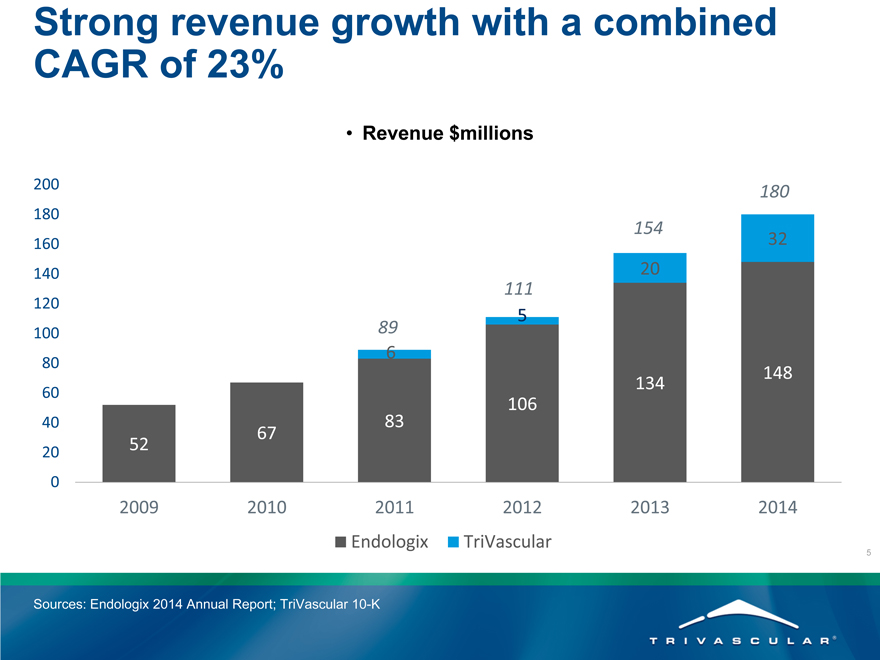
Strong revenue growth with a combined CAGR of 23%
Revenue $millions
200 180
180
154
160 32
140 20
111
120
5
100 89
6
80 148
60 134
106
40 83
67
20 52
0
2009 2010 2011 2012 2013 2014
Endologix TriVascular
Sources: Endologix 2014 Annual Report; TriVascular 10-K
5
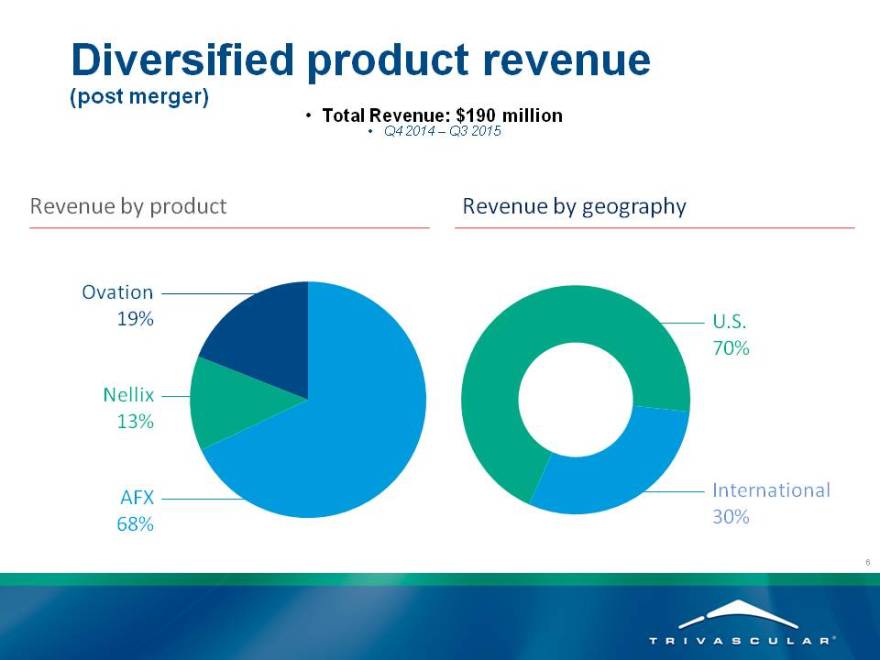
Diversified product revenue
(post merger)
Total Revenue: $190 million
Q4 2014 – Q3 2015
Revenue by product Revenue by geography
Ovation
19% U.S.
70%
Nellix
13%
AFX International
68% 30%
6
Founder’s Perspective
“If you make it clinically relevant, there will always be a path for the business”
John B. Simpson, MD
2002 TV1 “A” (pre-BSC)
[graphcies]
Is there a Better Way?
Can it be Made? Will it Work?
11
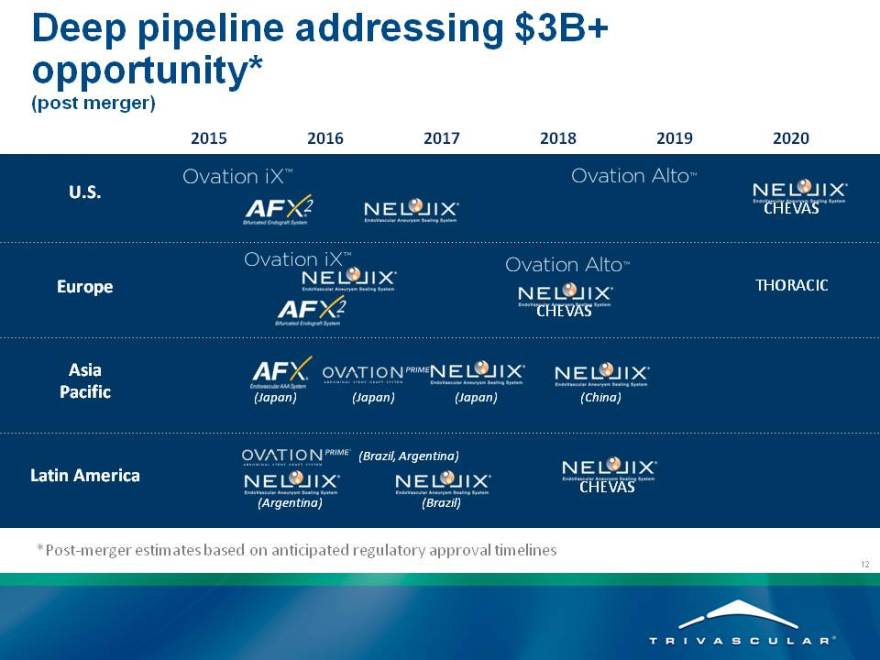
Deep pipeline addressing $3B+ opportunity*
(post merger)
2015 2016 2017 2018 2019 2020
U.S.
CHEVAS
Europe THORACIC
CHEVAS
Asia
Pacific (Japan) (Japan) (Japan) (China)
(Brazil, Argentina)
Latin America
CHEVAS
(Argentina) (Brazil)
*Post-merger estimates based on anticipated regulatory approval timelines
1 2
Clinical Perspective
The only company with multiple AAA treatment options
(post merger)
1 4
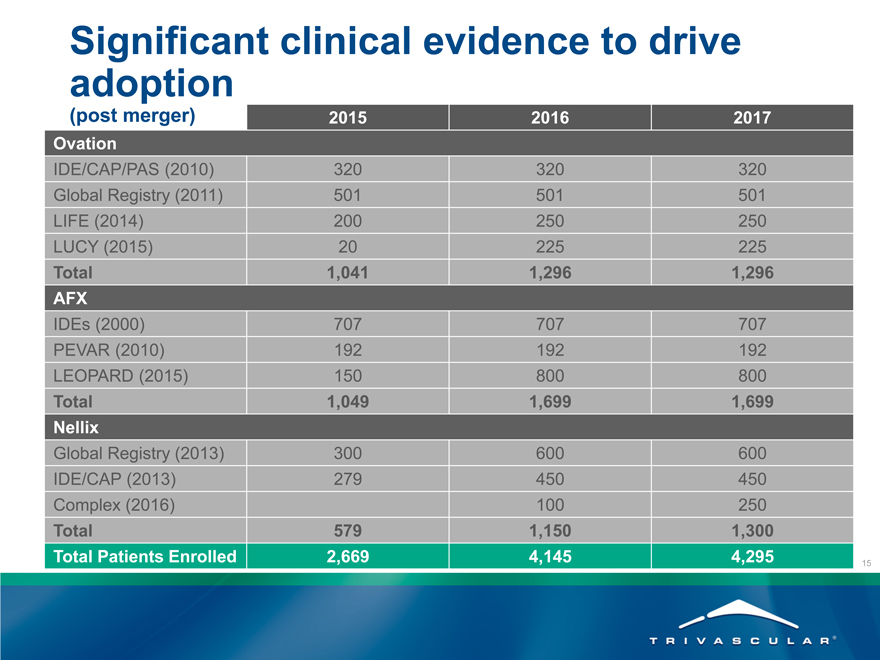
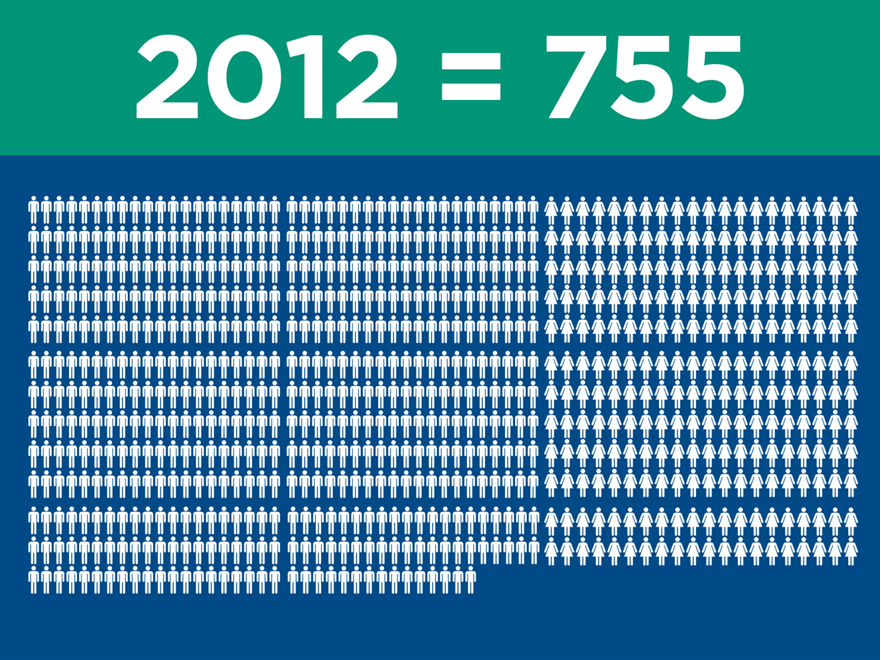
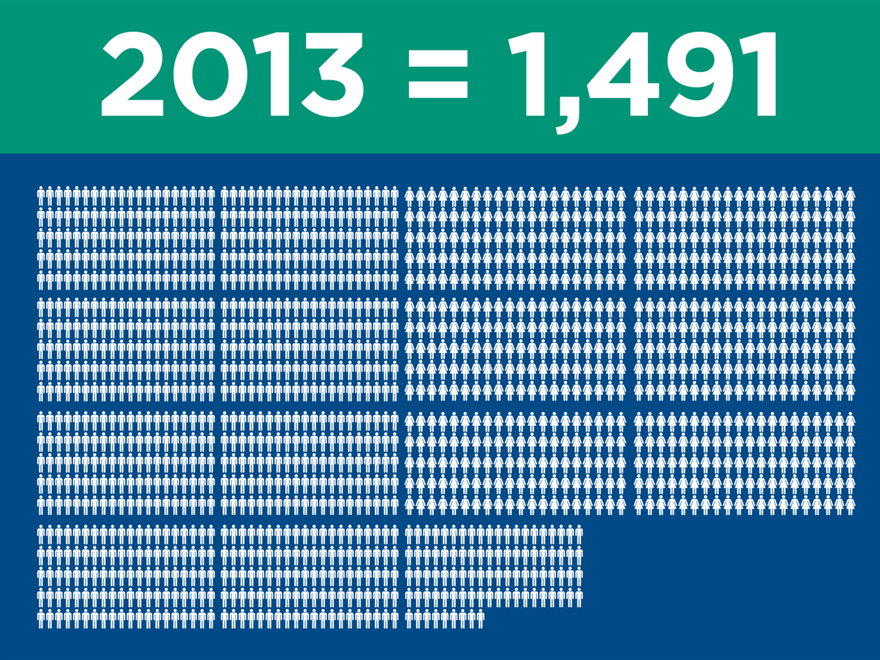
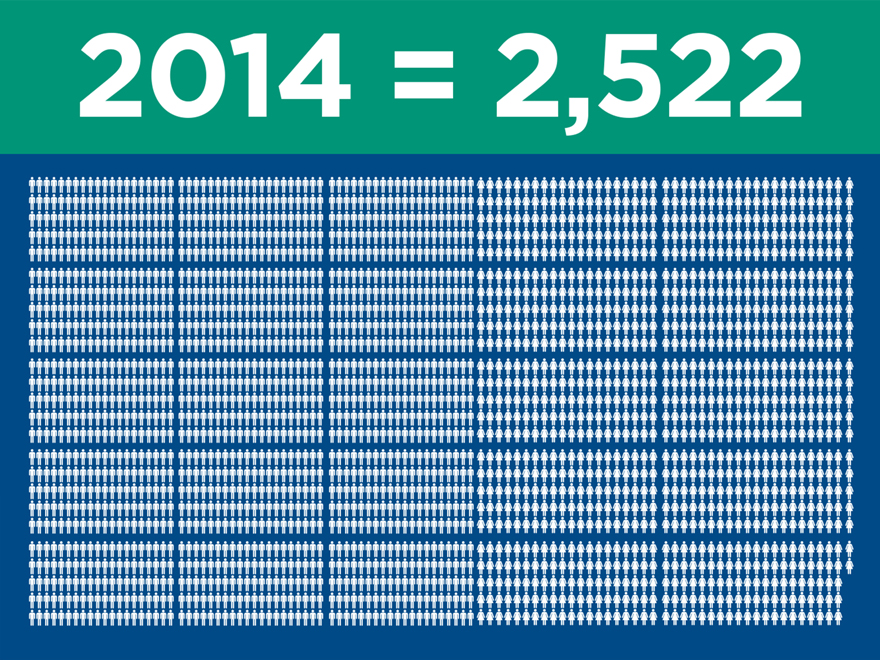
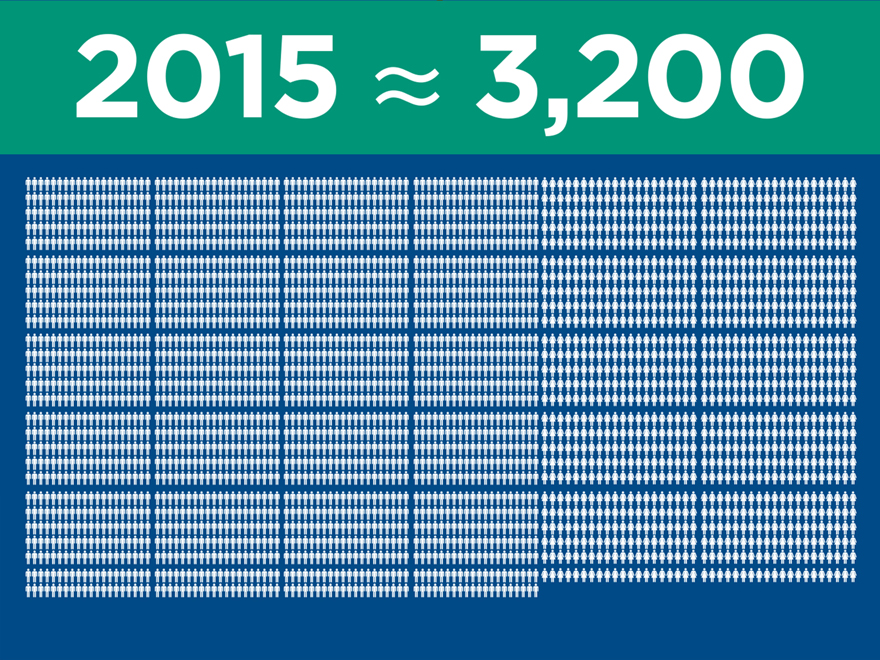
Significant clinical evidence to drive
adoption
(post merger) 2015 2016 2017
Ovation
IDE/CAP/PAS (2010) 320 320 320
Global Registry (2011) 501 501 501
LIFE (2014) 200 250 250
LUCY (2015) 20 225 225
Total 1,041 1,296 1,296
AFX
IDEs (2000) 707 707 707
PEVAR (2010) 192 192 192
LEOPARD (2015) 150 800 800
Total 1,049 1,699 1,699
Nellix
Global Registry (2013) 300 600 600
IDE/CAP (2013) 279 450 450
Complex (2016) 100 250
Total 579 1,150 1,300
Total Patients Enrolled 2,669 4,145 4,295
15
Commercial Perspective
16
[graphcies]
[graphcies]
[graphcies]
[graphcies]
21
22
24
Employee Issues
25
Organization and talent
No decisions on employment will be made or communicated until after the merger is completed (Q1 2016)
Antitrust laws require that we continue to function as independent competing companies until the merger is complete – Business as usual
Endologix has indicated that Santa Rosa will continue to be an important part of the future of the organization
After the merger is complete, efficiencies will be identified across the new company – core functions will remain intact
Significant number of people will be invited to be part of the new Company post closing
26
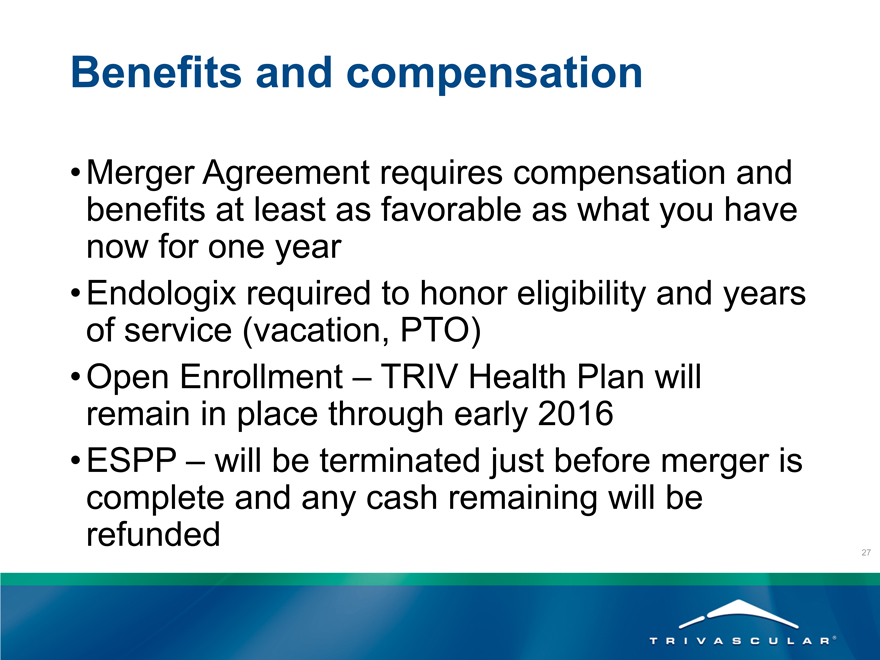
Benefits and compensation
Merger Agreement requires compensation and benefits at least as favorable as what you have now for one year
Endologix required to honor eligibility and years of service (vacation, PTO)
Open Enrollment – TRIV Health Plan will remain in place through early 2016
ESPP – will be terminated just before merger is complete and any cash remaining will be refunded
27
Benefits
Conversion to new benefits Q2 2016
Benefits at least as good as current plans to include Kaiser, Blue Shield PPO, HMO & HSA
Post merger, harmonization of PTO and
Vacation programs – all accrued & unused PTO will roll over
Generally Endologix PTO and Holiday plans are in line or slightly better than ours
401K will be terminated, roll over to Endologix or other tax deferred account
28
What to expect in coming weeks
Meeting with John McDermott, Endologix CEO, this Friday
Regular communications from Chris Chavez and others on the status of the upcoming merger
Communications and educational forums for stock option, RSU, ESPP and benefits questions and transition
Business as usual. Keep competing as individual competitors
29
Deal Structure/Overview
30
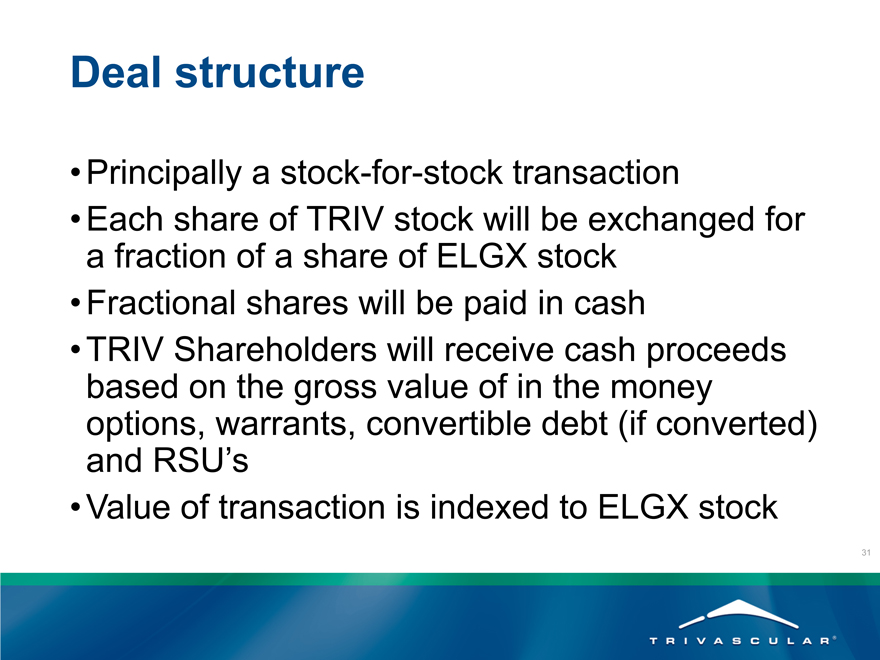
Deal structure
Principally a stock-for-stock transaction
Each share of TRIV stock will be exchanged for a fraction of a share of ELGX stock
Fractional shares will be paid in cash
TRIV Shareholders will receive cash proceeds based on the gross value of in the money options, warrants, convertible debt (if converted) and RSU’s
Value of transaction is indexed to ELGX stock
31
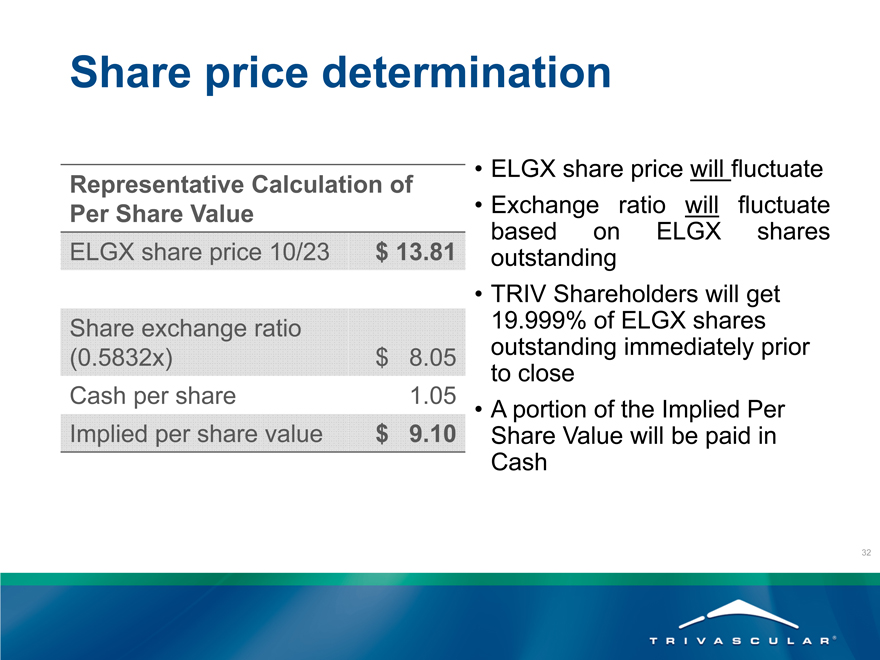
Share price determination
Representative Calculation of
Per Share Value
ELGX share price 10/23 $ 13.81
Share exchange ratio
(0.5832x) $ 8.05
Cash per share 1.05
Implied per share value $ 9.10
26
ELGX share price will fluctuate Exchange ratio will fluctuate based on ELGX shares outstanding TRIV Shareholders will get
19.999% of ELGX shares outstanding immediately prior to close A portion of the Implied Per Share Value will be paid in Cash
32
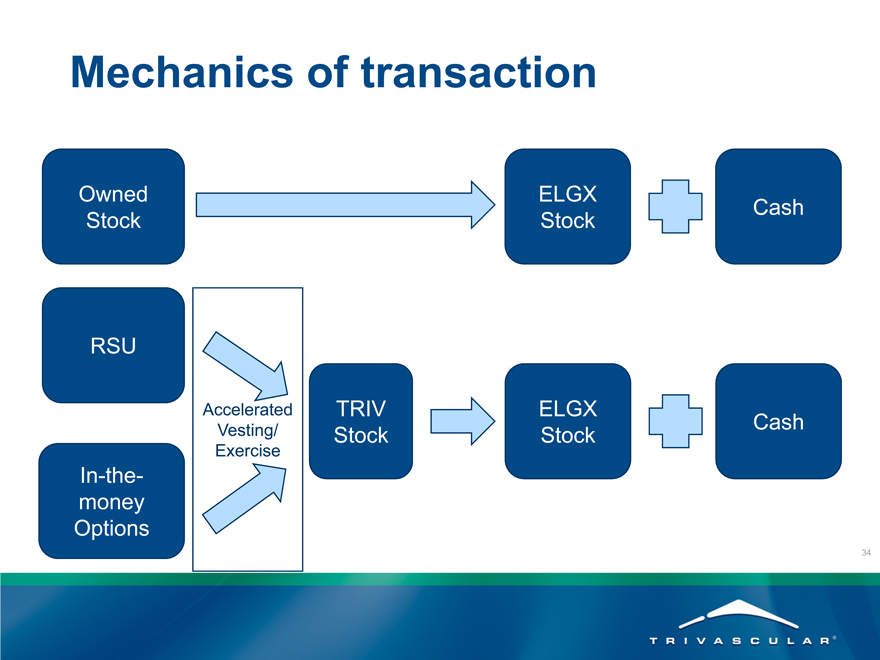
Options & RSU’s
All outstanding Options and RSU’s will vest prior to close
Employees will have a window to exercise “in-the-money” options
Exercised options and RSU’s will receive ELGX stock + cash consideration at close
Unexercised options will be canceled
33
Mechanics of transaction
Owned
Stock
RSU
In-the-money Options
Accelerated Vesting/ Exercise
TRIV Stock
ELGX
Stock
ELGX Stock
Cash
Cash
34
Wrap-up
35
Highlights of our future merged company: benefits to key stakeholders
Patients
Customers
Employees
Investors
Broadest indications for more on-label treatments Complex AAA and TAA solutions Enabling individualized AAA treatment
Aortic innovation leader with 370 patents and robust pipeline Broadest portfolio of solutions and clinical indications Exceptional clinical support and significant clinical evidence
Opportunity to save lives through advanced technology Global innovation leader Financial strength with strong revenue growth
Significant revenue growth powered by differentiated technology
Substantial synergies accretive in 2018
36
Timing/next steps
Now – Deal Close ? Continue to operate as independent competitors ? Integration planning (primarily by Endologix) ? Communications regarding intended organization
? Employees ? Customers ? Suppliers ? Partners
Post Deal Close ? Begin integration process
? Clarify new roles in organization (within 1 week)
37
|

|
Forward-Looking Statements: This communication includes statements that may be forward-looking statements. The words “believe,” “expect,” “anticipate,” “project” and similar expressions, among others, generally identify forward-looking statements. Endologix and TriVascular caution that these forward-looking statements are subject to risks and uncertainties that may cause actual results to differ materially from those indicated in the forward-looking statements. Such risks and uncertainties include, but are not limited to, the likelihood that the transaction is consummated on a timely basis or at all, including whether the conditions required to complete the transaction will be met, realization of the expected benefits of the transaction, competition from other products, changes to laws and regulations applicable to our industry, status of our ongoing clinical trials, clinical trial results, decisions and the timing of decisions of regulatory authorities regarding our products and potential future products, risks relating to foreign currency fluctuations, and a variety of other risks. Additional information about the factors that may affect the companies’ operations is set forth in Endologix’s and TriVascular’s annual and periodic reports filed with the Securities and Exchange Commission (the “SEC”). Neither Endologix nor TriVascular undertakes any obligation to release publicly any revisions to forward-looking statements as a result of subsequent events or developments, except as required by law.
38
Additional Information and Where to Find It: The transaction referenced in this communication has not yet commenced, and no proxies are yet being solicited. Endologix plans to file a registration statement on Form S–4 (“S-4”) that will serve as a prospectus for Endologix shares to be issued as consideration in the merger and as a proxy statement of TriVascular for the solicitation of votes of TriVascular stockholders to approve the proposed transaction (the “Proxy Statement/Prospectus”). This communication is for informational purposes only and is neither an offer to purchase nor a solicitation of an offer to sell shares. It is also not a substitute for the S-4, the Proxy Statement/Prospectus or any other documents that Endologix or TriVascular may file with the SEC or send to shareholders in connection with the proposed transaction. THE DEFINITIVE PROXY
STATEMENT/PROSPECTUS WILL CONTAIN IMPORTANT INFORMATION ABOUT ENDOLOGIX,
TRIVASCULAR AND THE TRANSACTIONS. TRIVSCULAR STOCKHOLDERS ARE URGED TO READ THE PROXY STATEMENT/PROSPECTUS CAREFULLY AND IN ITS ENTIRETY WHEN IT BECOMES AVAILABLE BEFORE MAKING ANY DECISION REGARDING VOTING ON THE PROPOSED TRANSACTION. In addition to the SEC filings made in connection with the transaction, each of Endologix and TriVascular files annual, quarterly and current reports and other information with the SEC. Endologix’s and TriVascular’s filings with the SEC, including the Proxy Statement/Prospectus once it is filed, are available to the public free of charge at the website maintained by the SEC at http://www.sec.gov. Copies of documents filed with the SEC by TriVascular will be made available free of charge on TriVascular’s website at http://investors.trivascular.com. Copies of documents filed with the SEC by Endologix will be made available free of charge on Endologix’s website at http.//investor.endologix.com.
Participants in the Solicitation: Endologix, TriVascular and their respective directors and executive officers may be deemed to be participants in any solicitation of proxies from TriVascular’s stockholders in connection with the proposed transaction. Information regarding Endologix’s directors and executive officers is available in its proxy statement for its 2015 annual meeting of stockholders, which was filed with the SEC on April 17, 2015; information regarding TriVascular’s directors and executive officers is available in its proxy statement for its 2015 annual meeting of stockholders, which was filed with the SEC on April 14, 2015. Other information regarding the interests of such potential participants will be contained in the Proxy Statement/Prospectus when it becomes available. You may obtain free copies of these documents as described in the preceding paragraph.
39