
MASTER Trial 12 Month Results Prof. Dariusz Dudek Hospital University of Krakow, Poland on behalf of the MASTER Trial Investigators

Disclosure Statement of Financial Interest • Grant/Research Support • Consulting Fees/Honoraria • Major Stock Shareholder/Equity • Royalty Income • Ownership/Founder • Intellectual Property Rights • Other Financial Benefit • InspireMD • No • No • No • No • No • No Within the past 12 months, I or my spouse/partner have had a financial interest/arrangement or affiliation with the organization(s) listed below. Affiliation/Financial Relationship Company
Background » Impaired myocardial perfusion after PCI in STEMI is common, and results in increased infarct size, heart failure and mortality. » PCI - induced distal embolization of thrombus and/or friable atheromatous debris contributes to impaired myocardial perfusion. » Efforts to improve myocardial perfusion with embolic protection devices have failed. » Recent results from the INFUSE - AMI and TASTE trials question whether aspiration results in any significant myocardial or clinical benefits.

MGuard stent: designed for embolic protection » MGuard is a thin - strut BMS wrapped with an expandable MicroNet mesh, designed to trap, exclude and secure the thrombus and friable atheromatous debris to prevent distal embolization during and post procedure. » The mesh is made of a 20 M Polyethylene Terephthalate (PET) fiber. » The pores expand to 180 m when deployed. » The mesh is expandable for side branch access. » The mesh is attached to the proximal and distal edges of the stent only to ensure high flexibility of the stent system.
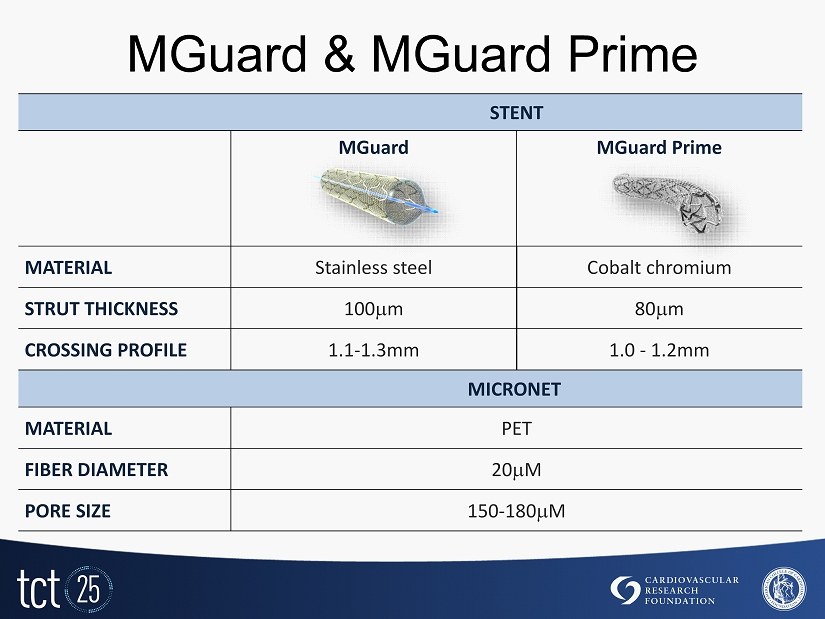
MGuard & MGuard Prime STENT MGuard MGuard Prime MATERIAL Stainless steel Cobalt chromium STRUT THICKNESS 100 m 80 m CROSSING PROFILE 1.1 - 1.3mm 1.0 - 1.2mm MICRONET MATERIAL PET FIBER DIAMETER 20 M PORE SIZE 150 - 180 M
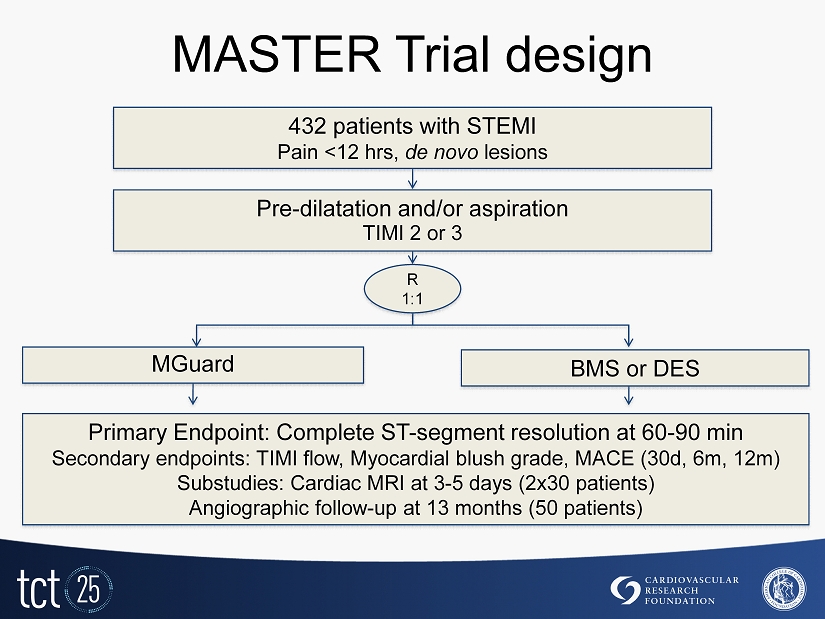
MASTER Trial design Primary Endpoint: Complete ST - segment resolution at 60 - 90 min Secondary endpoints: TIMI flow, Myocardial blush grade, MACE ( 30 d, 6 m, 12 m) Substudies: Cardiac MRI at 3 - 5 days ( 2 x 30 patients) Angiographic follow - up at 13 months ( 50 patients) 432 patients with STEMI P ain < 12 hrs, de novo lesions Pre - dilatation and/or aspiration TIMI 2 or 3 R 1 : 1 BMS or DES MGuard
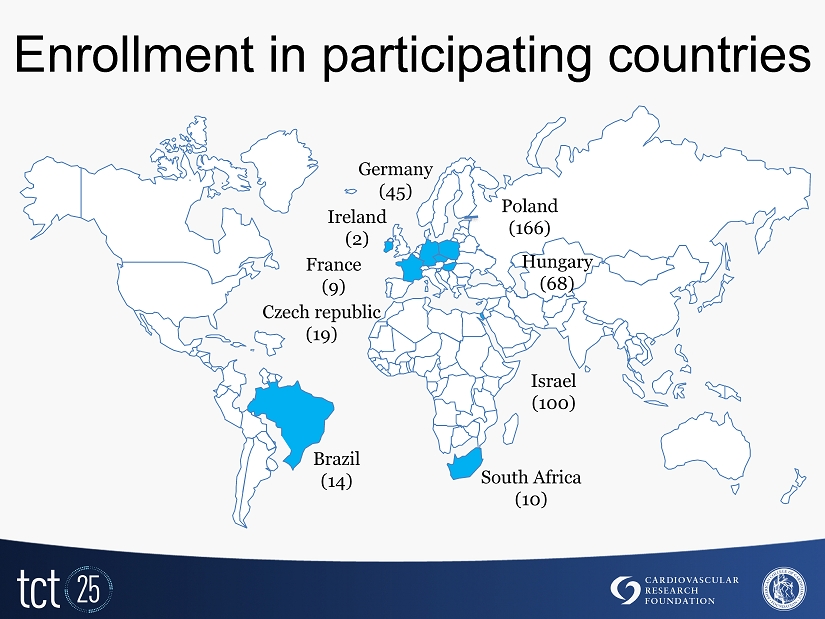
Enrollment in participating countries Czech republic (19) Poland ( 166 ) Hungary ( 68 ) Germany ( 45 ) Brazil ( 14 ) South Africa (10) France ( 9 ) Israel (100) Ireland (2)
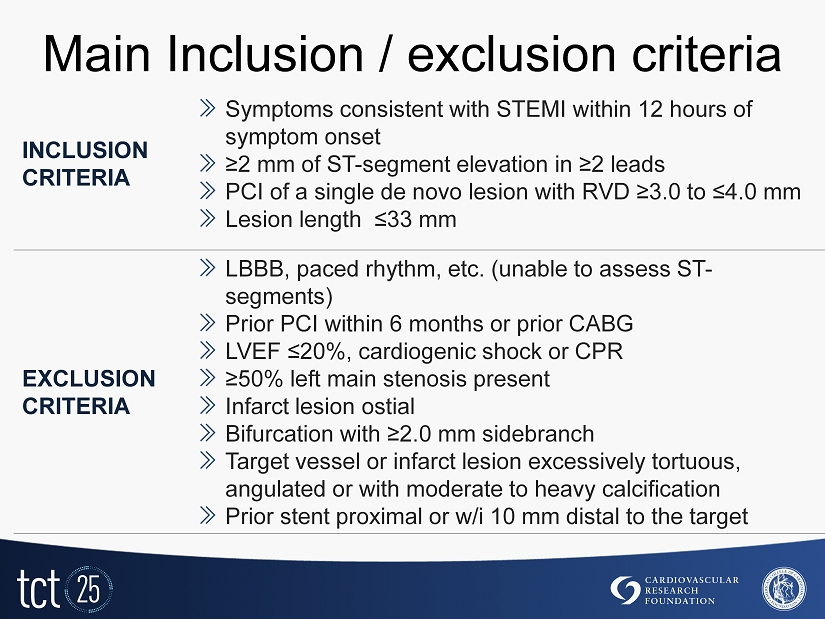
Main Inclusion / exclusion criteria INCLUSION CRITERIA » Symptoms consistent with STEMI within 12 hours of symptom onset » ≥2 mm of ST - segment elevation in ≥2 leads » PCI of a single de novo lesion with RVD ≥3.0 to ≤4.0 mm » Lesion length ≤33 mm EXCLUSION CRITERIA » LBBB, paced rhythm, etc. (unable to assess ST - segments) » Prior PCI within 6 months or prior CABG » LVEF ≤20%, cardiogenic shock or CPR » ≥50% left main stenosis present » Infarct lesion ostial » Bifurcation with ≥2.0 mm sidebranch » Target vessel or infarct lesion excessively tortuous, angulated or with moderate to heavy calcification » Prior stent proximal or w/i 10 mm distal to the target
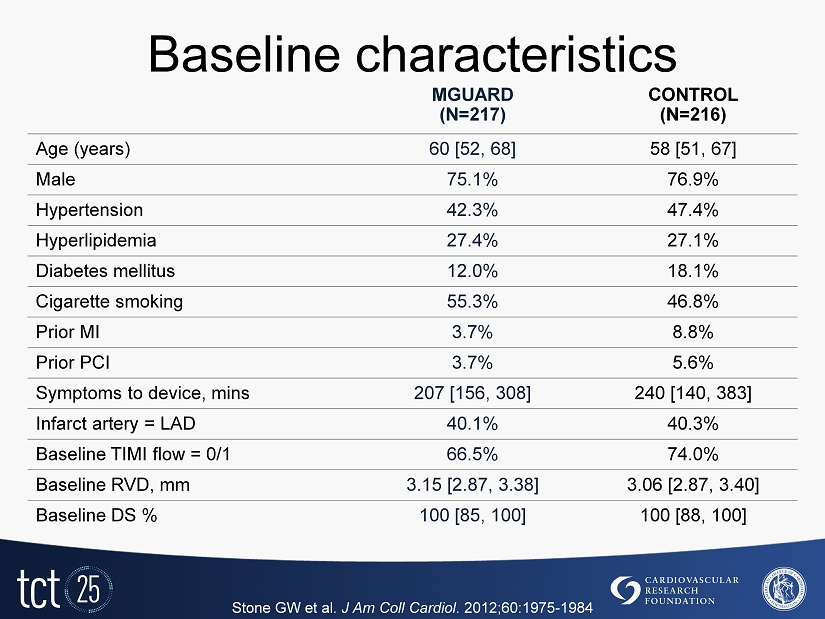
Baseline characteristics MGUARD (N=217) CONTROL (N=216) Age (years) 60 [52, 68] 58 [51, 67] Male 75.1% 76.9% Hypertension 42.3% 47.4% Hyperlipidemia 27.4% 27.1% Diabetes mellitus 12.0% 18.1% Cigarette smoking 55.3% 46.8% Prior MI 3.7% 8.8% Prior PCI 3.7% 5.6% Symptoms to device, mins 207 [156, 308] 240 [140, 383] Infarct artery = LAD 40.1% 40.3% Baseline TIMI flow = 0/1 66.5% 74.0% Baseline RVD, mm 3.15 [2.87, 3.38] 3.06 [2.87, 3.40] Baseline DS % 100 [85, 100] 100 [88, 100] Stone GW et al. J Am Coll Cardiol . 2012 ; 60 : 1975 - 1984

Procedural characteristics *Out of 217 patients, 27 were treated with MGuard Prime Stone GW et al. J Am Coll Cardiol . 2012;60:1975 - 1984 MGUARD (N=217) CONTROL (N=216) P Aspiration performed 65.9% 67.1% 0.79 Balloon pre - dilatation performed 50.2% 44.9% 0.27 Direct stenting 12.0% 10.6% 0.66 > 1 stent implanted 99.5% 100.0% 1.0 Stent type MGuard 96.3%* 0.5% <0.0001 Bare metal stent 1.4% 59.7% <0.0001 Drug - eluting stent 2.3% 39.8% <0.0001 Total stent length, mm 19 [15, 24] 20 [15, 24] 0.64 Post stent dilatation performed 36.4% 30.6% 0.20 Maximal device size, mm 3.5 [3.0, 3.5] 3.5 [3.0, 3.5] 0.78 Maximal dilatation pressure, atm 16 [14, 18] 16 [14, 18] 0.02
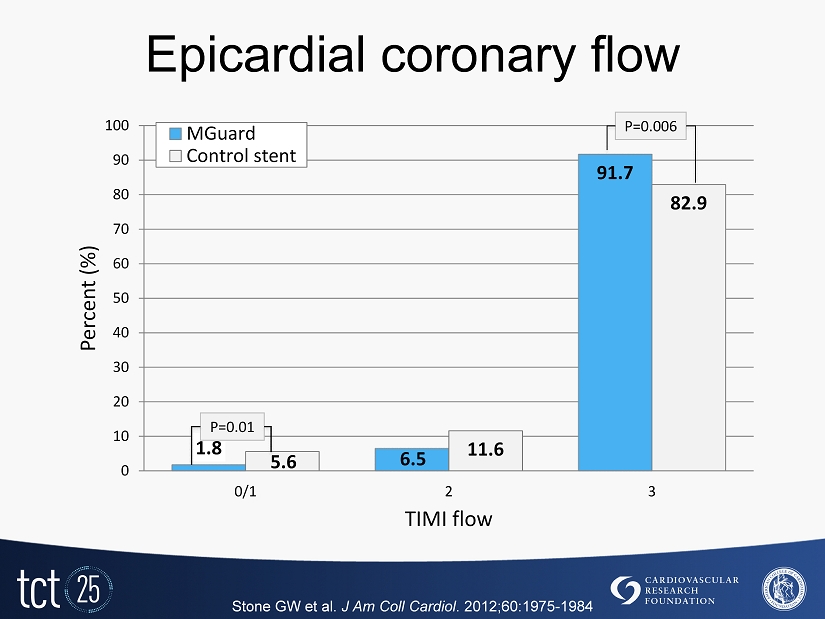
Epicardial coronary flow 1.8 6.5 91.7 5.6 11.6 82.9 0 10 20 30 40 50 60 70 80 90 100 0/1 2 3 Percent (%) TIMI flow MGuard Control stent P=0.006 P= 0.01 Stone GW et al. J Am Coll Cardiol . 2012 ; 60 : 1975 - 1984
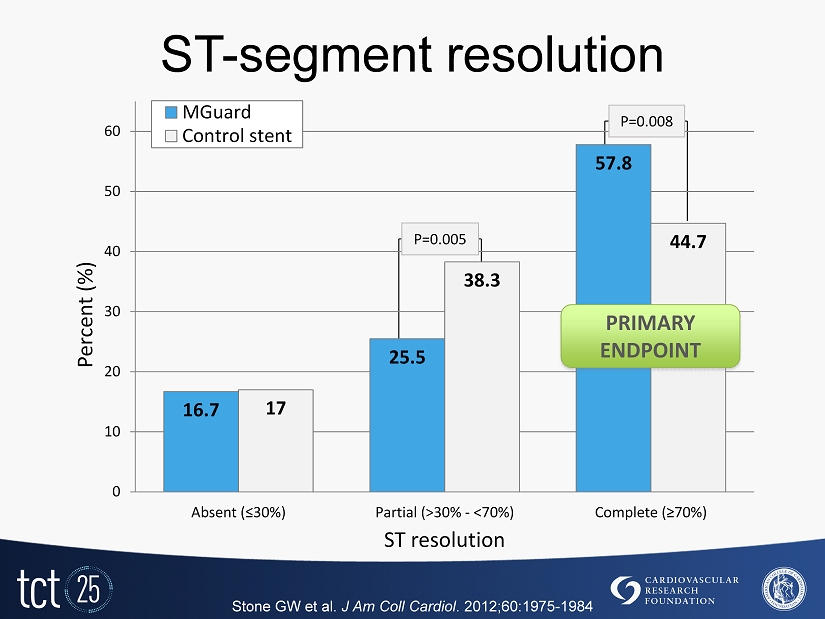
ST - segment resolution Stone GW et al. J Am Coll Cardiol . 2012;60:1975 - 1984 16.7 25.5 57.8 17 38.3 44.7 0 10 20 30 40 50 60 Absent (≤ 30 %) Partial (>30% - <70%) Complete (≥70%) Percent (%) ST resolution MGuard Control stent P= 0.005 P=0.008 PRIMARY ENDPOINT
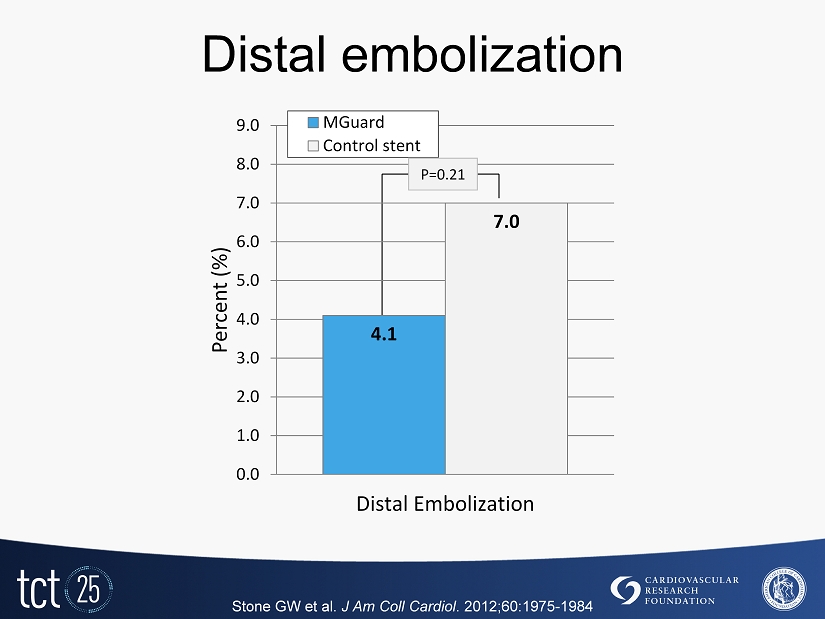
Distal embolization Stone GW et al. J Am Coll Cardiol . 2012 ; 60 : 1975 - 1984 4.1 7.0 0.0 1.0 2.0 3.0 4.0 5.0 6.0 7.0 8.0 9.0 Distal Embolization Percent (%) MGuard Control stent P= 0.21

Cardiac MRI sub - study at 5 days Stone GW et al. J Am Coll Cardiol . 2012;60:1975 - 1984 MGUARD (N=30) CONTROL BMS / DES (N=29) P Total LV myocardial mass, g 141 [117, 163] 147 [118, 174] 0.41 Infarct mass, g 17.1 [10.0, 30.0] 22.3 [15.7, 30.1] 0.27 Infarct mass (% total LV mass) 13.3 [7.9, 25.0] 16.6 [10.0, 22.6] 0.48 Total MVO, g 0.3 [0.0, 1.6] 1.0 [0.2, 2.8] 0.14 MVO (% total LV mass) 0.4 [0.0, 1.4] 0.8 [0.2, 1.9] 0.39 Abnormal wall motion score 22.5 [20.0, 26.0] 25.0 [21.0, 27.0] 0.48 LVEF (%) 48.3 [44.5, 52.3] 47.3 [42.0, 54.5] 0.79

30 - day clinical results Stone GW et al. J Am Coll Cardiol . 2012;60:1975 - 1984 MGUARD (N=217) CONTROL (N=214) P MACE 4 (1.8%) 5 (2.3%) 0.75 All cause mortality 0 (0.0%) 4 (1.9%) 0.06 Cardiac death 0 (0.0%) 4 (1.9%) 0.06 Reinfarction 3 (1.4%) 2 (0.9%) 1.00 TLR, ischemia - driven 4 (1.8%) 1 (0.5%) 0.37 TVR, ischemia - driven 5 (2.3%) 1 (0.5%) 0.10 Stent Thrombosis Definite or Probable 3 (1.4%) 2 (0.9%) 0.67 Definite 3 (1.4%) 1 (0.5%) 0.62 Stroke 1 (0.5%) 0 (0.0%) 1.00 TIMI Bleeding Major or Minor 4 (1.9%) 4 (1.9%) 1.00 Major 3 (1.4%) 2 (0.9%) 1.00
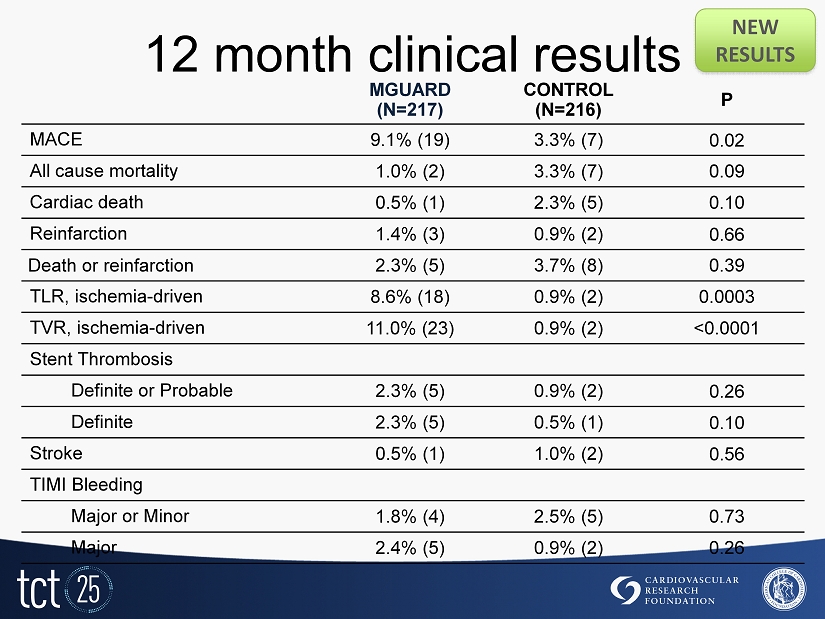
12 month clinical results NEW RESULTS MGUARD (N=217) CONTROL (N=216) P MACE 9.1% (19) 3.3% (7) 0.02 All cause mortality 1.0% (2) 3.3% (7) 0.09 Cardiac death 0.5% (1) 2.3% (5) 0.10 Reinfarction 1.4% (3) 0.9% (2) 0.66 Death or reinfarction 2.3% (5) 3.7% (8) 0.39 TLR, ischemia - driven 8.6% (18) 0.9% (2) 0.0003 TVR, ischemia - driven 11.0% (23) 0.9% (2) <0.0001 Stent Thrombosis Definite or Probable 2.3% (5) 0.9% (2) 0.26 Definite 2.3% (5) 0.5% (1) 0.10 Stroke 0.5% (1) 1.0% (2) 0.56 TIMI Bleeding Major or Minor 1.8% (4) 2.5% (5) 0.73 Major 2.4% (5) 0.9% (2) 0.26
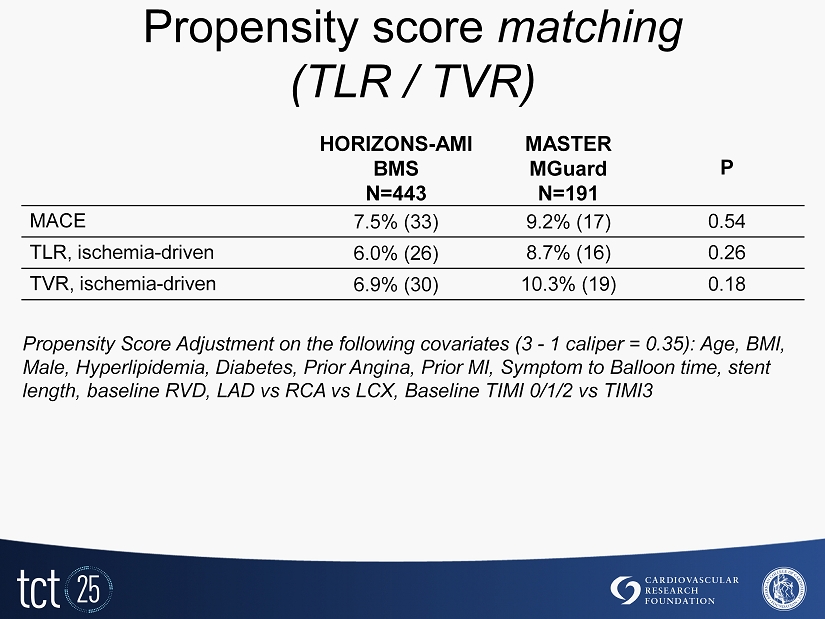
Propensity score matching ( TLR / TVR ) HORIZONS - AMI BMS N=443 MASTER MGuard N=191 P MACE 7.5% (33) 9.2% (17) 0.54 TLR, ischemia - driven 6.0% (26) 8.7% (16) 0.26 TVR, ischemia - driven 6.9% (30) 10.3% (19) 0.18 Propensity Score Adjustment on the following covariates (3 - 1 caliper = 0.35 ): Age, BMI, Male, Hyperlipidemia, Diabetes, Prior Angina, Prior MI, Symptom to Balloon time, stent length, baseline RVD, LAD vs RCA vs LCX, Baseline TIMI 0/1/2 vs TIMI3

Death Time - to - Event curve NEW RESULTS Death (%) 0 1 2 3 4 5 Months 0 1 2 3 4 5 6 7 8 9 10 11 12 217 214 209 206 126 216 210 209 207 123 Number at risk: MGuard BMS/DES P= 0.09 HR: 0.28 [ 95 % CI: 0.06 , 1.36 ] 1.0% 3.3% MGuard BMS/DES
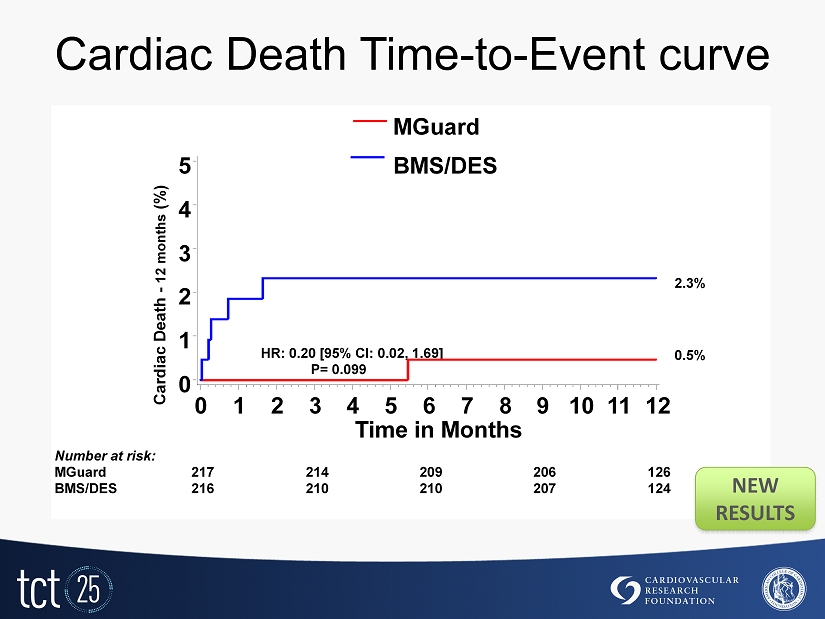
Cardiac Death Time - to - Event curve Cardiac Death - 12 months (%) 0 1 2 3 4 5 Time in Months 0 1 2 3 4 5 6 7 8 9 10 11 12 217 214 209 206 126 216 210 210 207 124 Number at risk: MGuard BMS/DES P= 0.099 HR: 0.20 [ 95 % CI: 0.02 , 1.69 ] 0.5 % 2.3% MGuard BMS/DES NEW RESULTS

Death or MI Time - to - Event curve DEATH/MI (%) 0 1 2 3 4 5 Months 0 1 2 3 4 5 6 7 8 9 10 11 12 217 212 207 205 125 216 209 208 206 123 Number at risk: MGuard BMS/DES 2.3% 3.7 % MGuard BMS/DES P= 0.39 HR: 0.62 [ 95 % CI: 0.20 , 1.89 ] NEW RESULTS
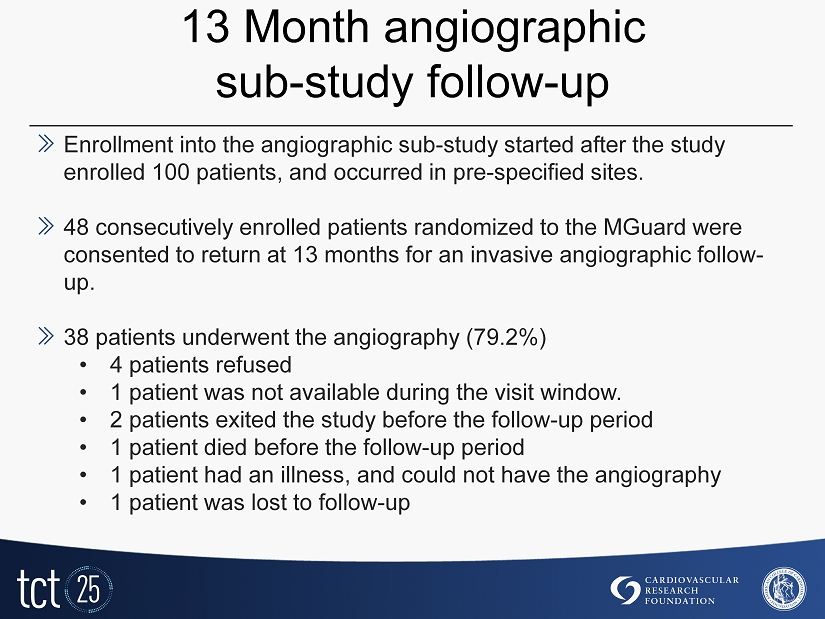
13 Month angiographic sub - study follow - up » Enrollment into the angiographic sub - study started after the study enrolled 100 patients, and occurred in pre - specified sites. » 48 consecutively enrolled patients randomized to the MGuard were consented to return at 13 months for an invasive angiographic follow - up. » 38 patients underwent the angiography (79.2%) • 4 patients refused • 1 patient was not available during the visit window. • 2 patients exited the study before the follow - up period • 1 patient died before the follow - up period • 1 patient had an illness, and could not have the angiography • 1 patient was lost to follow - up
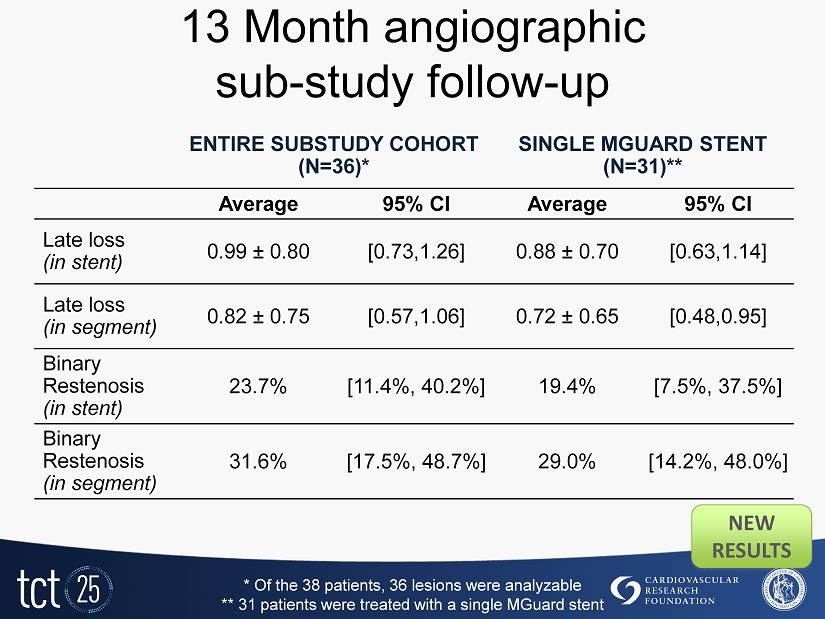
13 Month angiographic sub - study follow - up ENTIRE SUBSTUDY COHORT (N=36)* SINGLE MGUARD STENT (N=31)** Average 95% CI Average 95% CI Late loss (in stent) 0.99 ± 0.80 [0.73,1.26] 0.88 ± 0.70 [0.63,1.14] Late loss (in segment) 0.82 ± 0.75 [0.57,1.06] 0.72 ± 0.65 [0.48,0.95] Binary Restenosis (in stent) 23.7% [11.4%, 40.2%] 19.4% [7.5%, 37.5%] Binary Restenosis (in segment) 31.6% [17.5%, 48.7%] 29.0% [14.2%, 48.0%] * Of the 38 patients, 36 lesions were analyzable ** 31 patients were treated with a single MGuard stent NEW RESULTS
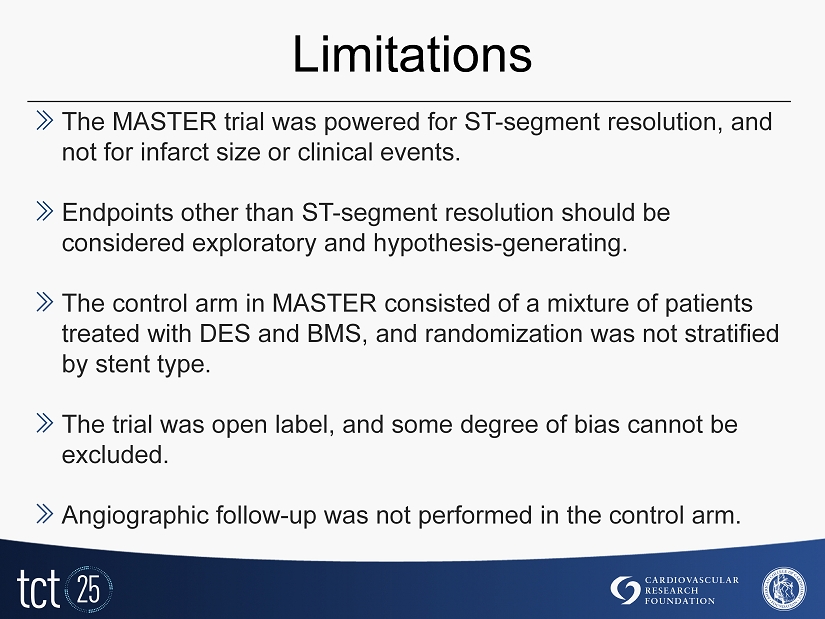
Limitations » The MASTER trial was powered for ST - segment resolution, and not for infarct size or clinical events. » Endpoints other than ST - segment resolution should be considered exploratory and hypothesis - generating. » The control arm in MASTER consisted of a mixture of patients treated with DES and BMS, and randomization was not stratified by stent type. » The trial was open label, and some degree of bias cannot be excluded. » Angiographic follow - up was not performed in the control arm.
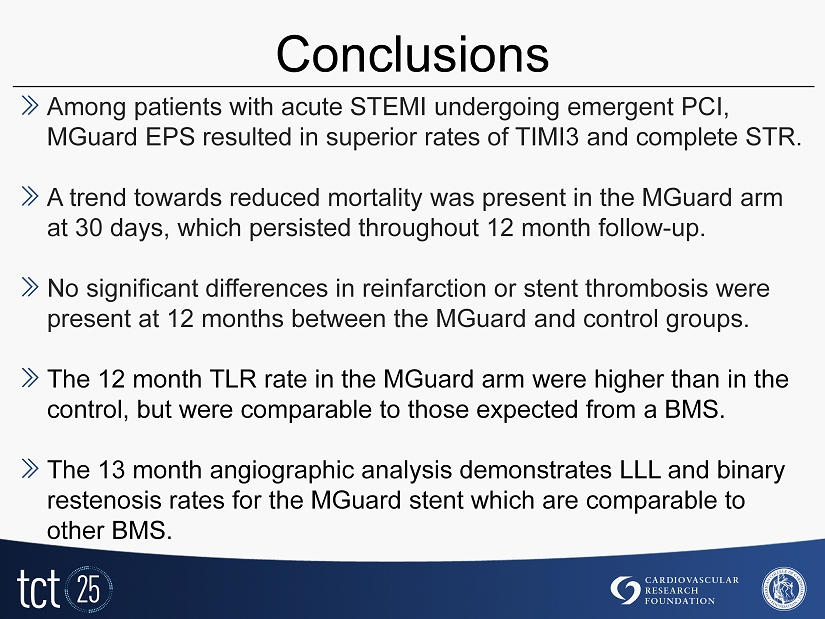
Conclusions » Among patients with acute STEMI undergoing emergent PCI, MGuard EPS resulted in superior rates of TIMI3 and complete STR. » A trend towards reduced mortality was present in the MGuard arm at 30 days, which persisted throughout 12 month follow - up. » No significant differences in reinfarction or stent thrombosis were present at 12 months between the MGuard and control groups. » The 12 month TLR rate in the MGuard arm were higher than in the control, but were comparable to those expected from a BMS. » The 13 month angiographic analysis demonstrates LLL and binary restenosis rates for the MGuard stent which are comparable to other BMS.
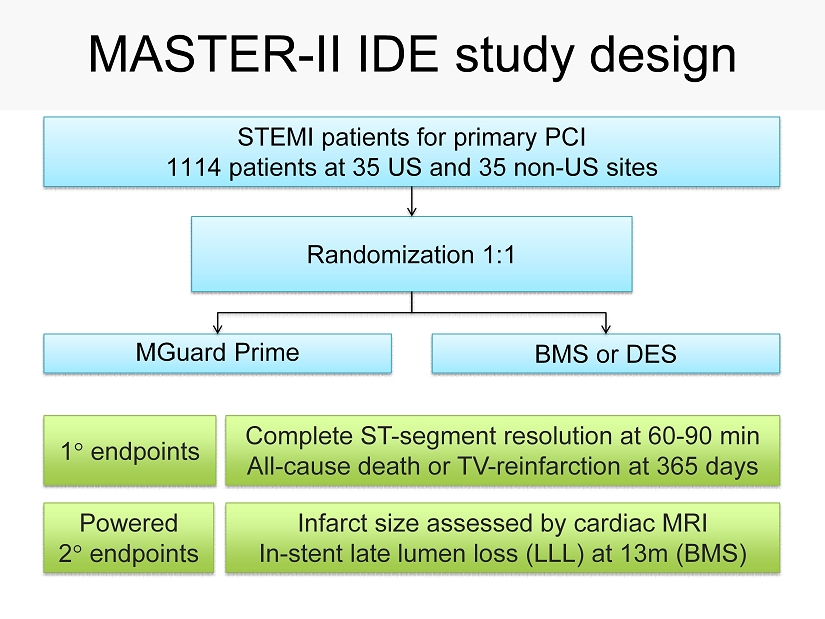
MASTER - II IDE study design BMS or DES MGuard Prime Randomization 1 : 1 STEMI patients for primary PCI 1114 patients at 35 US and 35 non - US sites Infarct size assessed by cardiac MRI In - stent late lumen loss (LLL) at 13m (BMS) Complete ST - segment resolution at 60 - 90 min All - cause death or TV - reinfarction at 365 days 1 endpoints Powered 2 endpoints
























