Free signup for more
- Track your favorite companies
- Receive email alerts for new filings
- Personalized dashboard of news and more
- Access all data and search results
Filing tables
Filing exhibits
NSPR similar filings
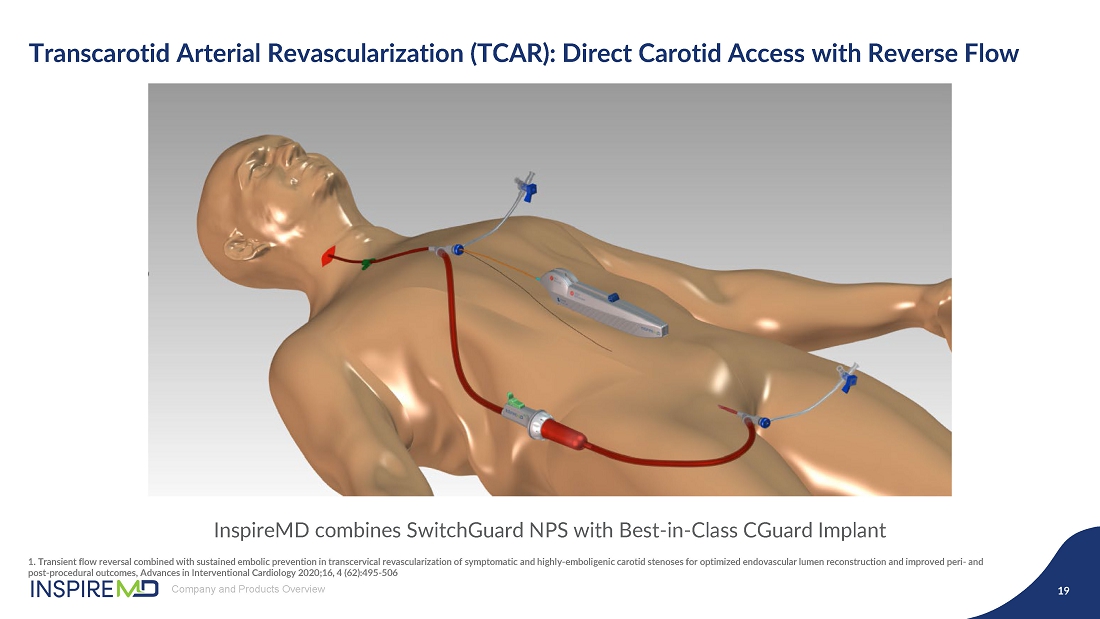
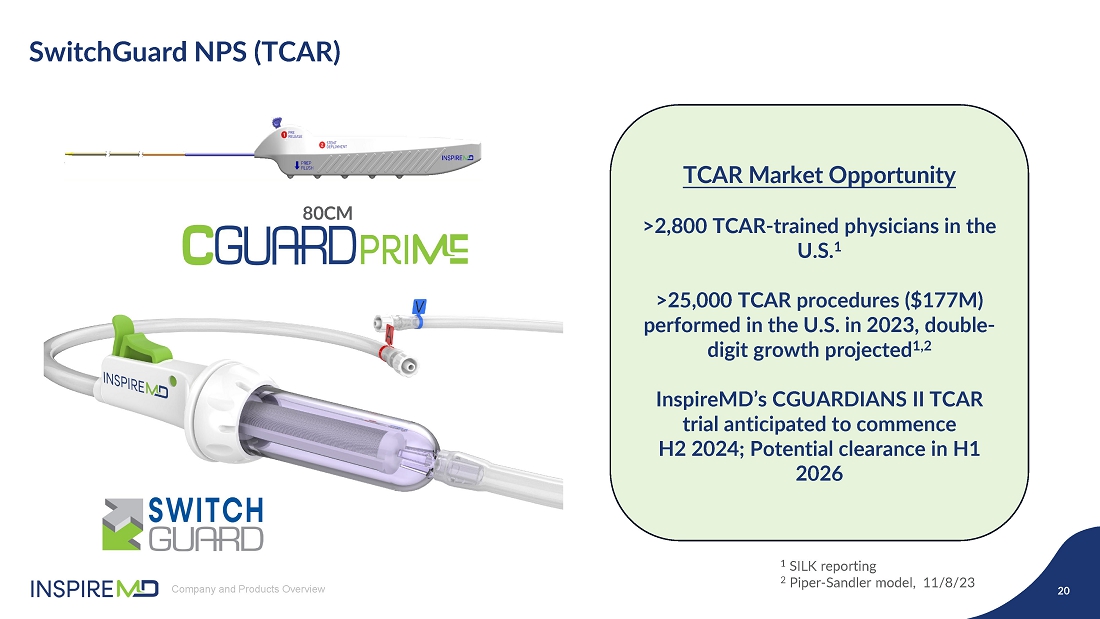
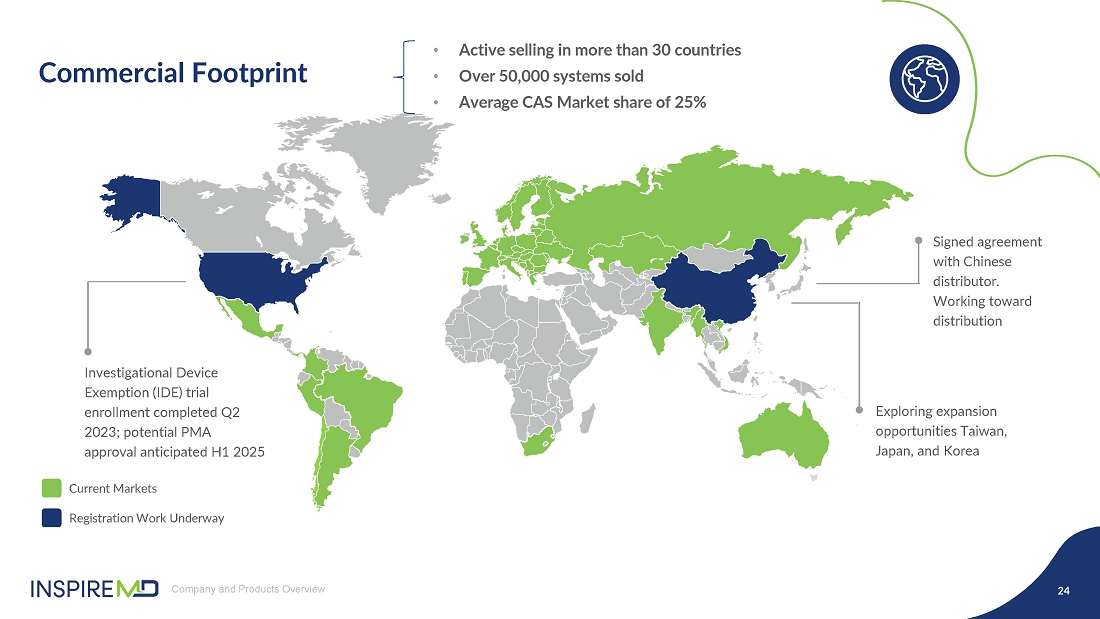
- 16 Sep 24 InspireMD Announces Submission of Premarket Approval Application to FDA Seeking U.S. Regulatory Approval of the CGuard™ Prime Carotid Stent System
- 6 Aug 24 InspireMD Reports Second Quarter 2024 Financial Results and Provides
- 1 Jul 24 InspireMD Announces Full Exercise of Series H Warrant Tranche for Gross Proceeds of $17.9 Million
- 13 Jun 24 Regulation FD Disclosure
- 12 Jun 24 Submission of Matters to a Vote of Security Holders
- 31 May 24 Entry into a Material Definitive Agreement
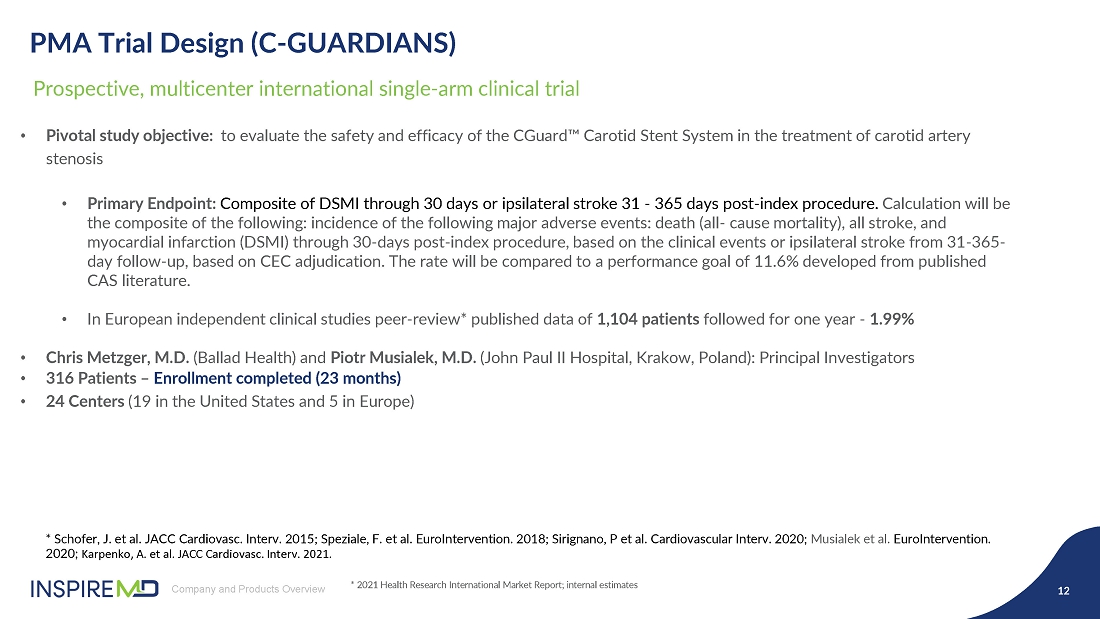
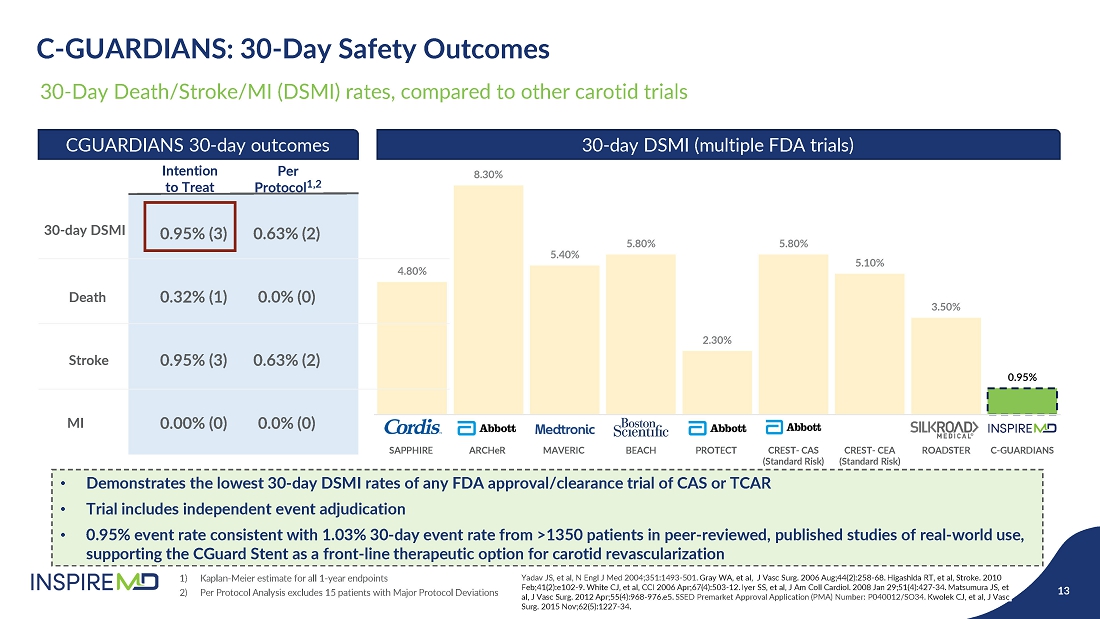
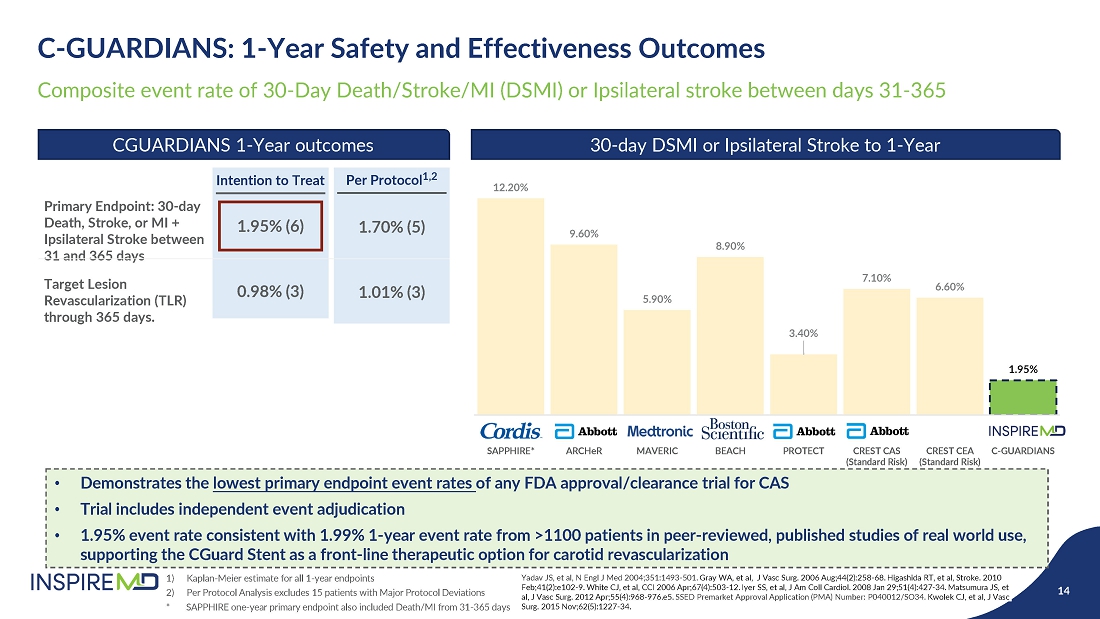
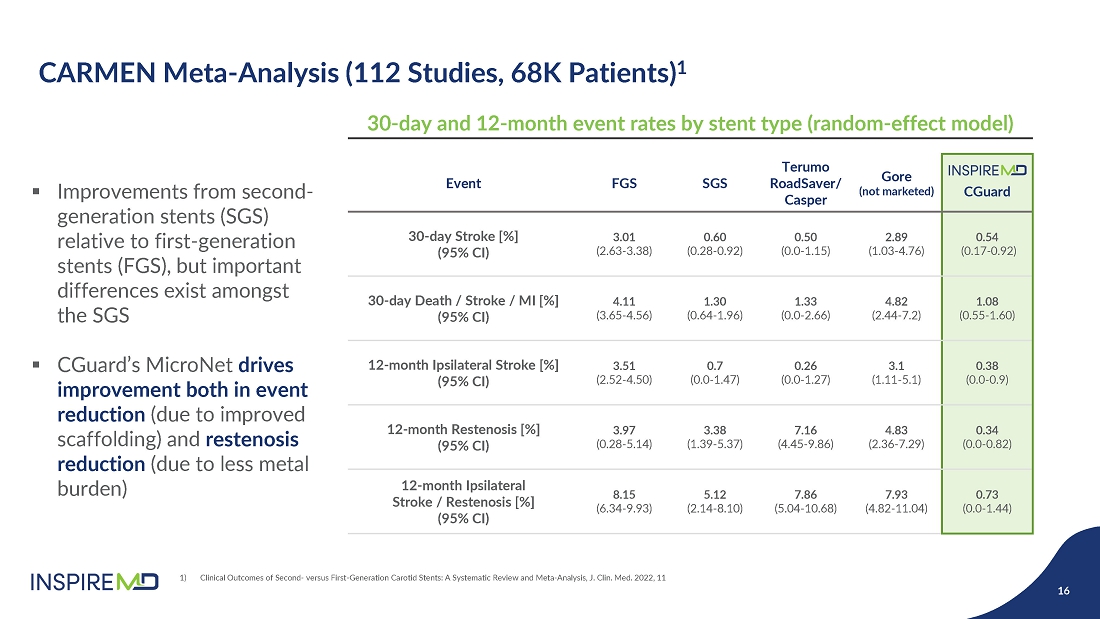
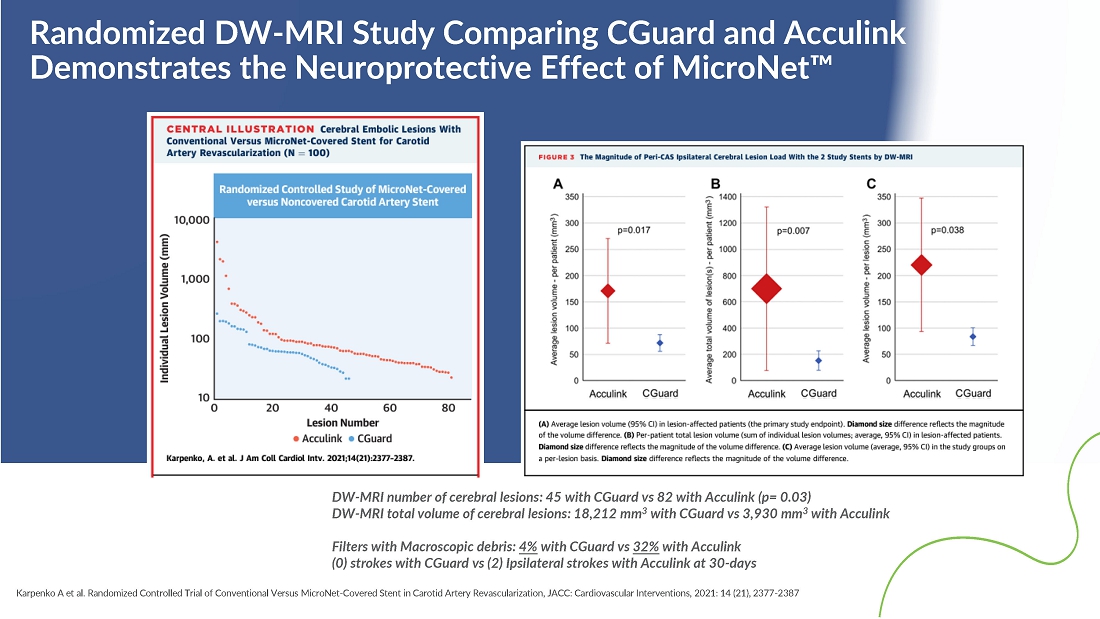
- 28 May 24 InspireMD Announces Presentation of Positive One-Year Follow-Up Results from the C-GUARDIANS U.S. Investigational Device Exemption (IDE) Clinical Trial of CGuard at Study results to support a Premarket Approval (PMA) application to FDA in H2 2024
Filing view
External links