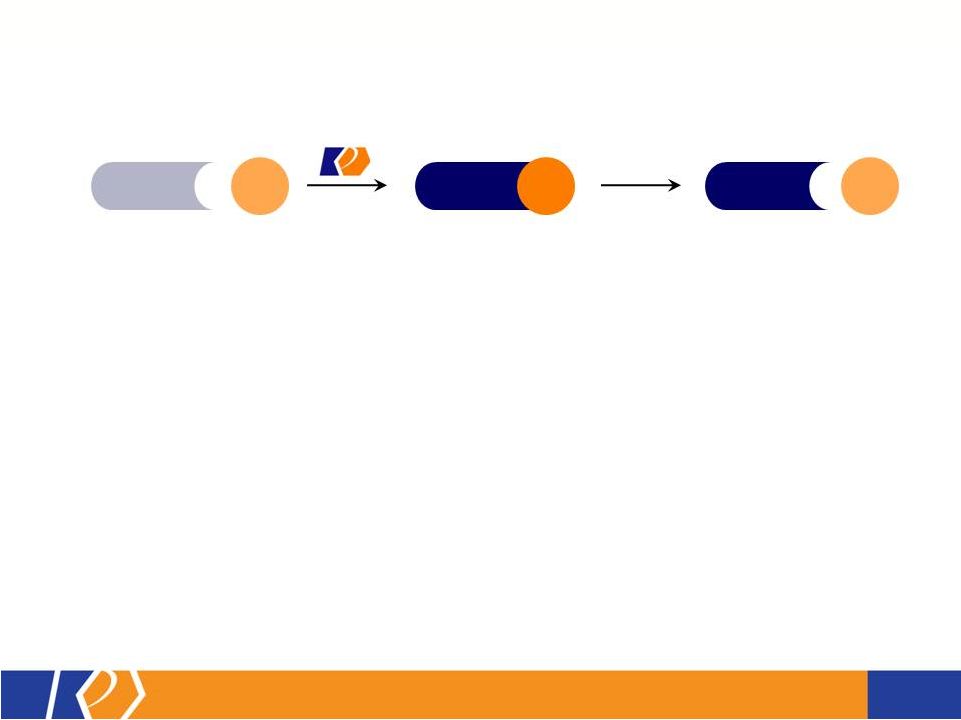
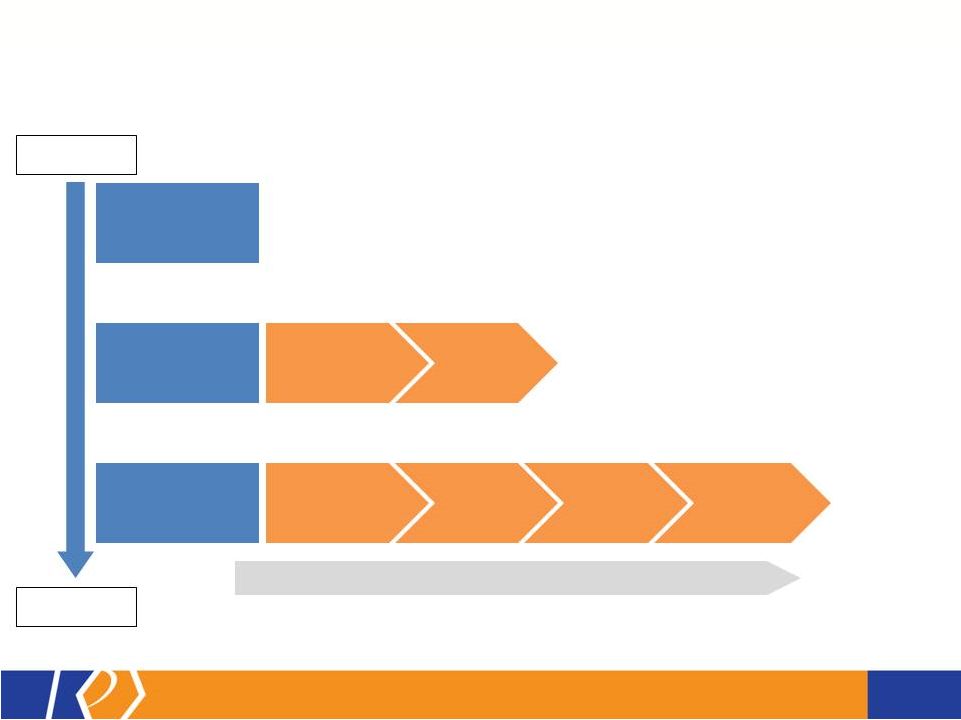
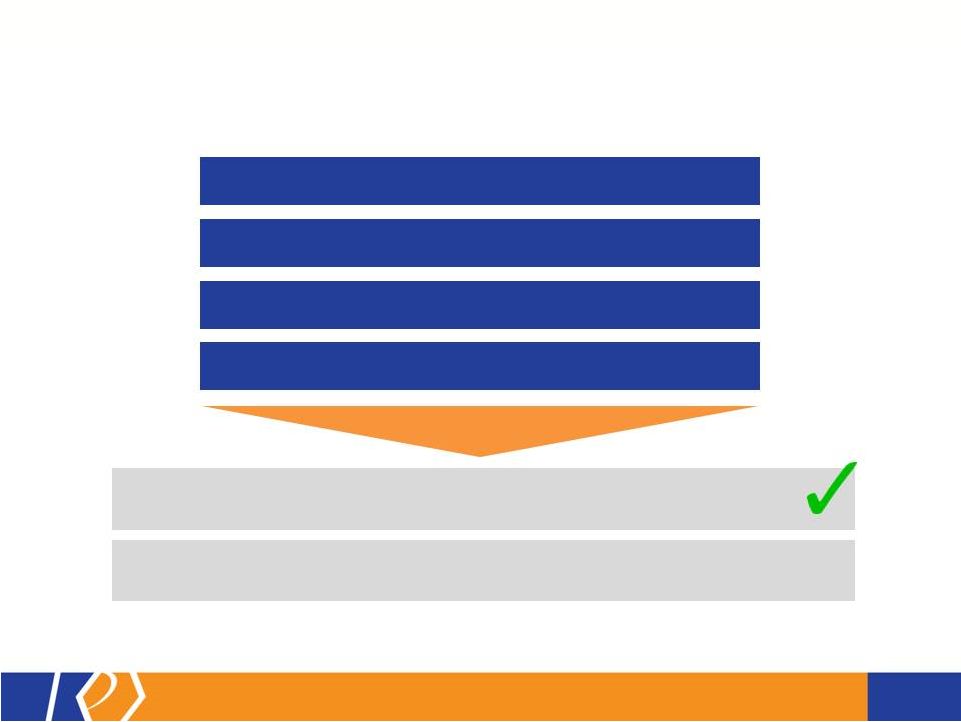
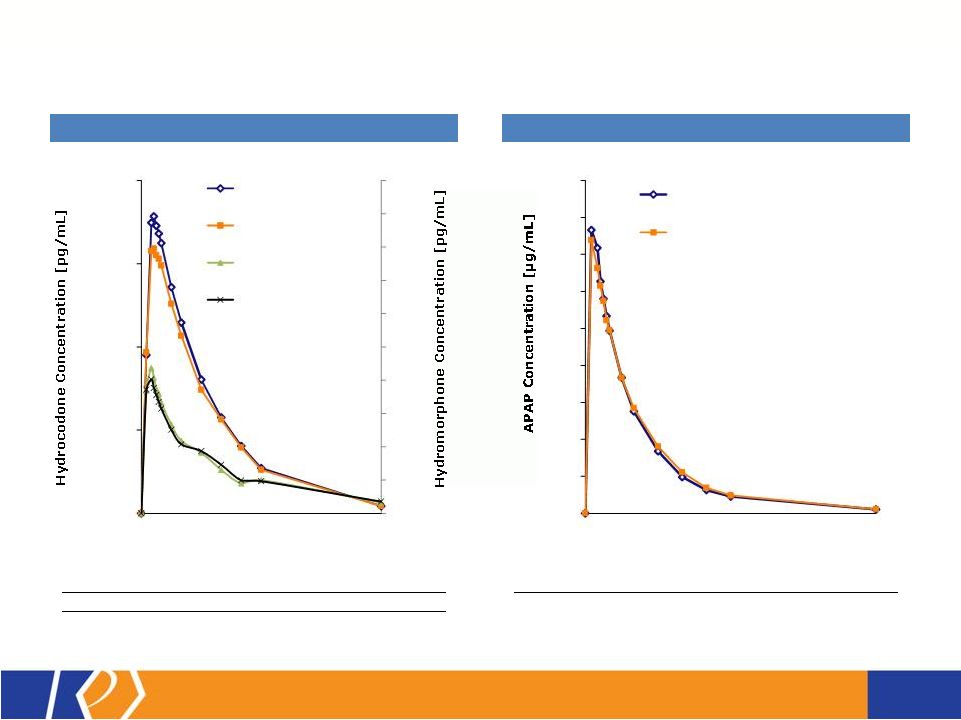
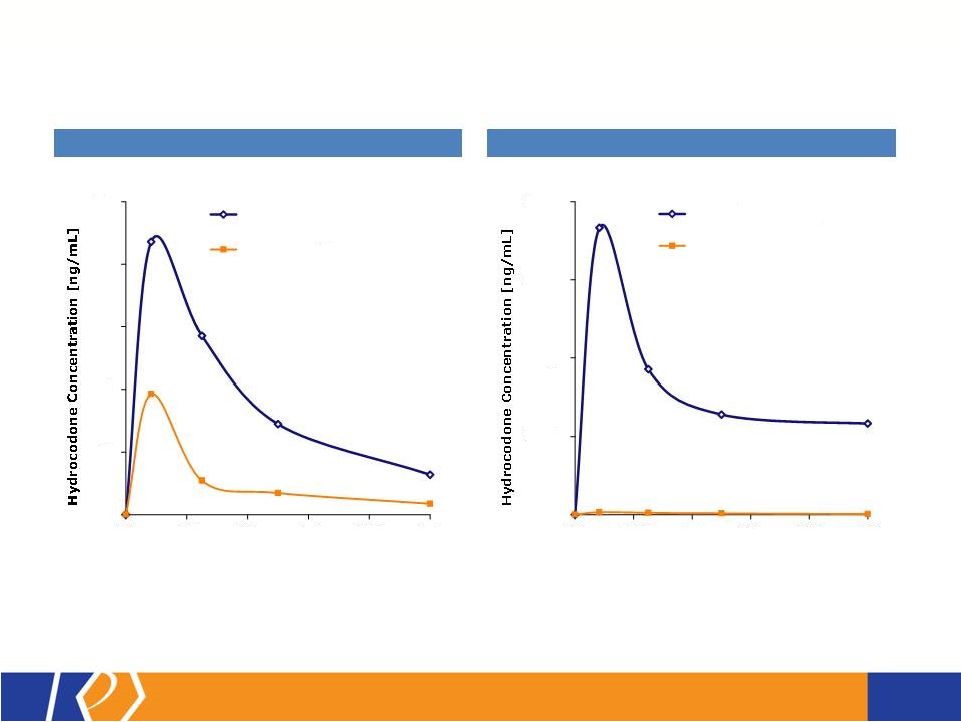
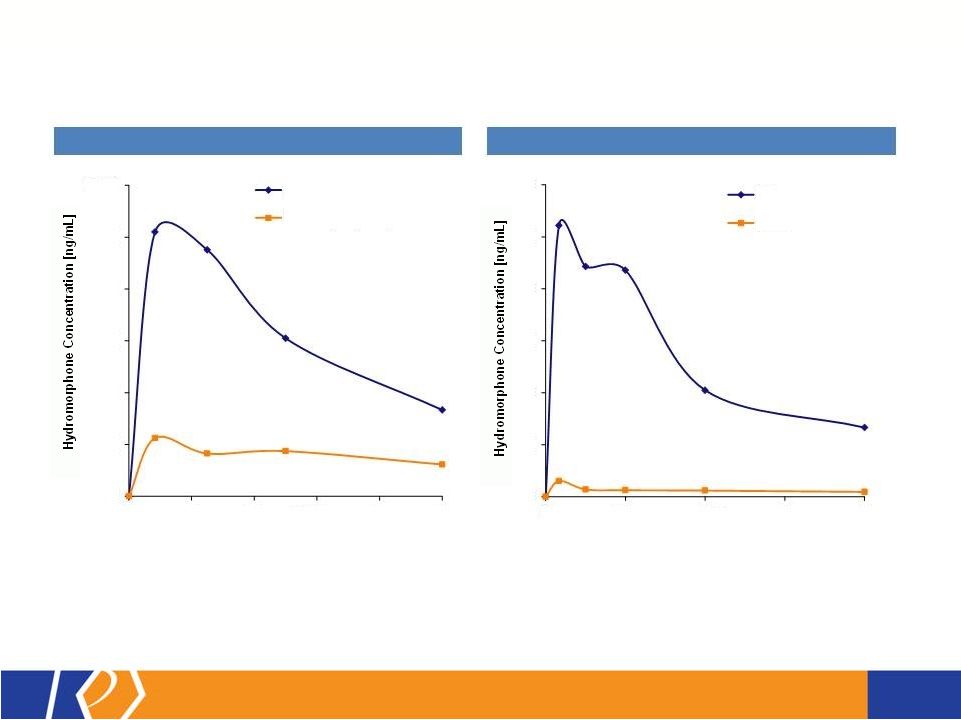
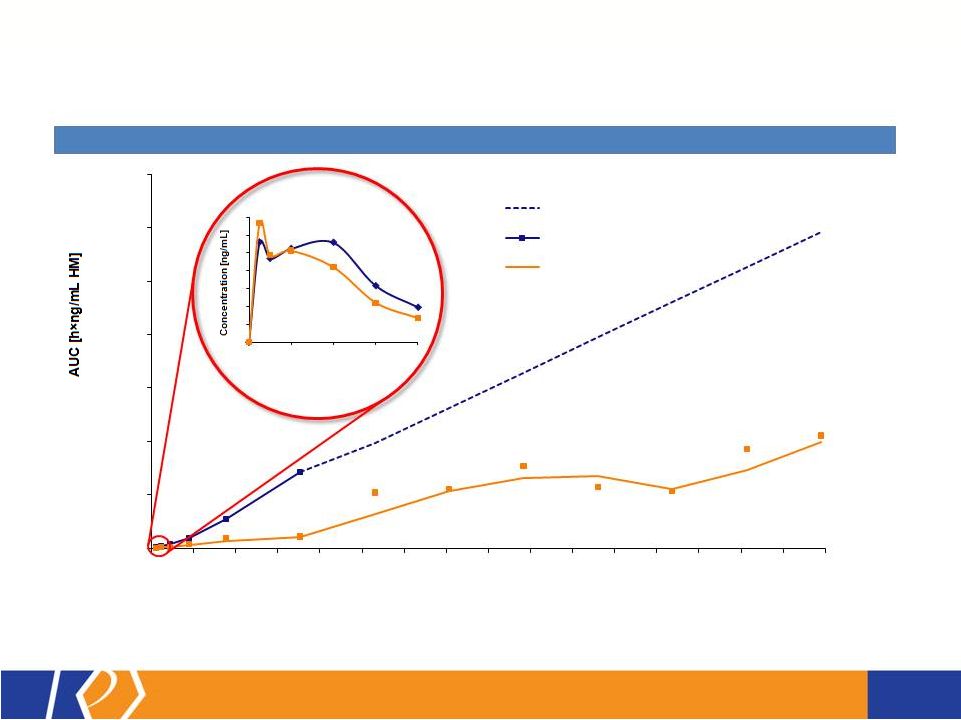
17 KP201/APAP Potential Abuse-Deterrent Label Claims • The FDA outlines 3 types of studies that may translate into 4 categories of label claims • KemPharm’s abuse liability program is consistent with the FDA Guidance: o Extraction/hydrolysis study o Oral abuse liability trial (with pharmacokinetics) o Intranasal abuse liability trial (with pharmacokinetics) • KP201/APAP will be compared with Norco ® in all studies • Studies will be completed before filing of NDA • If data is positive, label may include claims for up to 3 categories at approval FDA Guidance 1. Laboratory manipulation and extraction studies (Category 1) 2. Pharmacokinetic studies (Category 2) 3. Clinical abuse potential studies (Category 3) There are four general categories of claims available to describe the potential abuse-deterrent properties of a product. Depending on product and study data, a combination of categories can be included in the label claims. The FDA Guidance lists the following theoretical examples: • Category 1: In vitro data demonstrate the product has physical and chemical properties that are expected to deter intravenous abuse. 1 • Category 1 and 2: In vitro data demonstrate that the product has physical and chemical properties that are expected to deter oral, nasal and intravenous abuse. 1 • Category 2 and 3: Pharmacokinetic and clinical abuse potential studies indicate that the product has properties that are expected to deter abuse via the oral, intranasal and intravenous routes. 1 • Category 4: Data demonstrated a reduction in the abuse of the product in the community 1 1 Abuse of the product is still possible by other routes |