![]()
Media Contact:
Elliot Fox
W2O Group
212.257.6724
efox@w2ogroup.com
Investor Contact:
Audrey Gross
W2O Group
212.301.7214
agross@w2ogroup.com
Esperion Announces Positive Top-Line Results from First Pivotal Phase 3 Study of Bempedoic Acid
— Study 4 Met Primary Endpoint with 28% Additional LDL-C Lowering on Background Ezetimibe and Up to the Lowest Daily Dose of a Statin —
— hsCRP Reduction of 33% —
— Observed to be Safe and Well-Tolerated in This Study —
— Conference Call and Webcast on Wednesday, March 7, 2018, at 8:30 a.m. Eastern Standard Time —
Ann Arbor, Mich., — (Globe Newswire — March 7, 2018) — Esperion (NASDAQ: ESPR), the Lipid Management Company focused on developing and commercializing complementary, convenient, cost-effective, once-daily, oral therapies for the treatment of patients with elevated low density lipoprotein cholesterol (LDL-C), today announced positive top-line results from the first pivotal, Phase 3 study (Study 4 or 1002-048) of bempedoic acid 180 mg evaluating the LDL-C lowering efficacy and safety and tolerability of bempedoic acid versus placebo in patients with atherosclerotic cardiovascular disease (ASCVD) or at high risk for ASCVD with hypercholesterolemia inadequately treated with background ezetimibe 10 mg and up to the lowest daily starting dose of a statin.
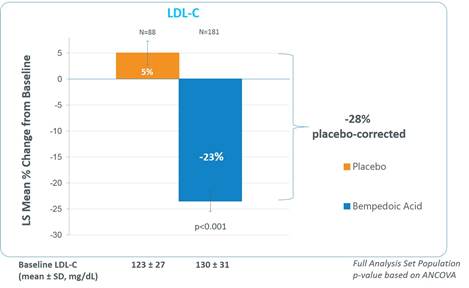
The 12-week study met its primary endpoint with LDL-C lowering totaling 28 percent (p<0.001). The LDL-C lowering for the bempedoic acid group was 23 percent from baseline, as compared to an LDL-C increase of five percent for the placebo group. Patients treated with bempedoic acid also achieved a significantly greater reduction of 33 percent in high-sensitivity C-reactive protein (hsCRP), an important marker of the underlying inflammation associated with cardiovascular disease, compared to the placebo group which had an increase of two percent (p<0.001).
“Many of my patients, including those considered statin intolerant, remain at risk for further cardiovascular disease, including heart attack and stroke, despite the availability of current LDL-C-lowering therapies,” said Christie M. Ballantyne, M.D., chairman of Esperion’s Phase 3 Executive Committee and Professor and Chief of Cardiology at Baylor College of Medicine in Houston. “These results suggest that bempedoic acid, with its targeted mechanism of action and convenient, oral, once-daily administration, could be an important new treatment option for a wide range of patients, including those unable to tolerate moderate or high doses of commonly-used statin therapy.”
In this study, bempedoic acid was observed to be safe and well-tolerated. There were no differences in the occurrence of adverse events (AEs), serious adverse events (SAEs) or muscle-related AEs; and no differences in discontinuations due to AEs or muscle-related AEs between the bempedoic acid group compared to the placebo group. Two patients (1.1 percent) treated with bempedoic acid had elevations in liver function tests (ALT/AST) of greater than three times the upper limit of normal, repeated and confirmed. The cumulative number of patients now treated with bempedoic acid in Phase 2 clinical trials and in Study 4 totals 919. Of these, six patients (0.65 percent) had elevations in liver function tests. This rate of elevations in liver function test is consistent with the rate observed in Phase 2 clinical trials and with all other previously approved oral LDL-C-lowering therapies, including statins and ezetimibe.

“Physicians are eagerly awaiting new, once-daily, oral therapies that complement existing oral drugs to provide the LDL-C lowering that their high-risk patients need, the value payers appreciate, and with the convenience and tolerability patients want and deserve,” said Tim M. Mayleben, president and chief executive officer of Esperion. “Looking ahead, we expect results from subsequent Phase 3 studies to further confirm that bempedoic acid and the bempedoic acid / ezetimibe combination pill will deliver consistent LDL-C lowering in safe, well-tolerated and convenient once-daily pills that are highly complementary to existing standard-of-care oral LDL-C lowering therapies.”
Esperion plans to present full results from this study at an upcoming medical conference and to publish in a major medical journal.
Design of Study 4 (1002-048)
The 12-week, global, pivotal, Phase 3 randomized, double-blind, placebo-controlled, multicenter study evaluated the efficacy and safety of bempedoic acid 180 mg/day versus placebo as add-on therapy in patients with atherosclerotic cardiovascular disease (ASCVD), or at a high risk for ASCVD, who are inadequately treated with current lipid-modifying therapies, including ezetimibe and up to the lowest approved daily starting dose of a statin. The study was conducted at 90 sites in the U.S., Canada and Europe. A total of 269 patients were randomized 2:1 to receive bempedoic acid or placebo. The primary objective was to assess the 12-week LDL-C-lowering efficacy of bempedoic acid versus placebo when added to ezetimibe and up to the lowest starting dose of a statin. Secondary objectives included evaluating the safety and tolerability of bempedoic acid versus placebo, and its effects on other risk markers, including hsCRP.
Conference Call and Webcast Information
Esperion will host a conference call and webcast today, Wednesday, March 7, 2018, at 8:30 a.m. Eastern Standard Time to discuss these Phase 3 study results. The call can be accessed by dialing (877) 312-7508 (domestic) or (253) 237-1184 (international) five minutes prior to the start of the call and providing access code 2389929. A live audio webcast can be accessed on the investors and media section of the Esperion website at investor.esperion.com. Access to the webcast replay will be available approximately two hours after completion of the call and will be archived on the Company’s website for approximately 90 days.
About Esperion’s Global Pivotal Phase 3 LDL-C Lowering Program
Esperion initiated its global, pivotal, Phase 3 clinical development program in January 2016 to evaluate the safety, tolerability and consistent, complementary LDL-C-lowering efficacy of bempedoic acid and the bempedoic acid / ezetimibe combination pill in patients with atherosclerotic cardiovascular disease (ASCVD), or who are at a high risk for ASCVD, with hypercholesterolemia who continue to have elevated levels of LDL-C despite the use of maximally-tolerated statins and ezetimibe, leaving them at high risk for cardiovascular events. The program includes five studies in approximately 4,000 patients, four for bempedoic acid and one for the bempedoic acid / ezetimibe combination pill.
· Two pivotal studies evaluating bempedoic acid (Studies 1 & 2) in 3,000 patients with ASCVD on maximally-tolerated statin therapy, with top-line results expected in early May and September 2018, respectively;
· Two pivotal studies evaluating bempedoic acid (Studies 3 & 4) in 600 patients with ASCVD, or at a high risk for ASCVD, considered statin intolerant, with top-line results expected in May 2018 and early March 2018, respectively;
· One pivotal study evaluating the bempedoic acid / ezetimibe combination pill (053 Study) in 350 patients with ASCVD, or at high risk for ASCVD, on maximally-tolerated statin therapy, with top-line results expected in August 2018.
Esperion plans to submit New Drug Applications (NDAs) to the U.S. Food and Drug Administration (FDA) for bempedoic acid and the bempedoic acid / ezetimibe combination pill for LDL-C-lowering indications by the first quarter of 2019. Additionally, Esperion plans to submit Marketing Authorization Applications (MAAs) to the European Medicines Agency (EMA) by the second quarter of 2019.
About Bempedoic Acid
With a targeted mechanism of action, bempedoic acid is a first-in-class, complementary, orally available, once-daily ATP Citrate Lyase (ACL) inhibitor that reduces cholesterol biosynthesis and lowers LDL-C by up-regulating the LDL receptor. Similar to statins, bempedoic acid also reduces hsCRP, a key marker of inflammation associated with cardiovascular disease. Completed Phase 1 and 2 studies conducted in approximately 1,300 patients, and more than 800 patients treated with bempedoic acid, have produced LDL-C lowering results of up to 30 percent as monotherapy, approximately 50 percent in combination with ezetimibe and an incremental 20+ percent when added to stable statin therapy.
The effect of bempedoic acid on cardiovascular morbidity and mortality has not yet been determined.
Esperion’s Commitment to Patients with Hypercholesterolemia
High levels of LDL-C can lead to a build-up of fat and cholesterol in and on artery walls (known as atherosclerosis), potentially leading to cardiovascular events, including heart attack or stroke. In the U.S., 78 million people, or more than 20 percent of the population, have elevated LDL-C; an additional 73 million people in Europe and 30 million people in Japan also live with elevated LDL-C. There are approximately 13 million people in the U.S. with atherosclerotic cardiovascular disease (ASCVD) who live with elevated levels of LDL-C despite taking maximally-tolerated lipid-modifying therapy — including individuals considered statin intolerant — leaving them at high risk for cardiovascular events. The vast majority of these patients, 9.5 million, require less than 30 percent additional LDL-C lowering to achieve treatment goals.
Esperion’s mission as the Lipid Management Company is to deliver once-daily, oral therapies that complement existing oral drugs to provide the additional LDL-C lowering that these patients need.
The Lipid Management Company
Esperion is the Lipid Management Company passionately committed to developing and commercializing convenient, complementary, cost-effective, once-daily, oral therapies for the treatment of patients with elevated LDL-C. Through scientific and clinical excellence, and a deep understanding of cholesterol biology, the experienced lipid management team at Esperion is committed to developing new LDL-C lowering therapies that will make a substantial impact on reducing global cardiovascular disease; the leading cause of death around the world. Bempedoic acid and the company’s lead product candidate, the bempedoic acid / ezetimibe combination pill, are targeted therapies that have been shown to significantly lower elevated LDL-C levels in patients with hypercholesterolemia, including patients inadequately treated with current lipid-
modifying therapies. For more information, please visit www.esperion.com and follow us on Twitter at https://twitter.com/EsperionInc.
Forward-Looking Statements
This press release contains forward-looking statements that are made pursuant to the safe harbor provisions of the federal securities laws, including statements regarding the regulatory approval pathway for the bempedoic acid / ezetimibe combination pill and bempedoic acid and the therapeutic potential of, clinical development plan for, the bempedoic acid / ezetimibe combination pill and bempedoic acid, including Esperion’s timing, designs, plans and announcement of results from studies in the global pivotal Phase 3 clinical development program for bempedoic acid and the bempedoic acid / ezetimibe combination pill, Esperion’s timing and plans for submission of NDAs to the FDA and MAAs to the EMA and Esperion’s expectations for the market for therapies to lower LDL-C. Any express or implied statements contained in this press release that are not statements of historical fact may be deemed to be forward-looking statements. Forward-looking statements involve risks and uncertainties that could cause Esperion’s actual results to differ significantly from those projected, including, without limitation, delays or failures in Esperion’s studies, including the risk that Esperion may need to change the design of its Phase 3 program, that existing cash resources may be used more quickly than anticipated, that the pivotal Phase 3 program may not produce sufficient safety or tolerability results or show meaningful change in LDL-C or other key lipid measures of patients, or the risk that other unanticipated developments or data could interfere with the scope of development, approval and commercialization of the bempedoic acid / ezetimibe combination pill and bempedoic acid, and the other risks detailed in Esperion’s filings with the Securities and Exchange Commission. Esperion disclaims any obligation or undertaking to update or revise any forward-looking statements contained in this press release, other than to the extent required by law.
# # #