hallmark gene set enrichments that are also observed in the glomerular transcriptome of IgAN patients. This study suggests an important role of the ETA receptor in mesangial cell activation, subsequent proteinuria and activation of pathogenic proliferative, inflammatory and fibrotic intra-renal transcriptional networks in MPGN. This further supports the therapeutic potential of atrasentan, a selective ETA receptor antagonist, to attenuate mesangial cell activation, proteinuria and pathogenic intra-renal signaling in MPGNs such as IgAN.
MO207 - A Phase 1/2, Multicenter Study to Investigate the Safety, Tolerability, Pharmacokinetics and Pharmacodynamics of BION-1301 in Healthy Volunteers and Adults with IgA Nephropathy
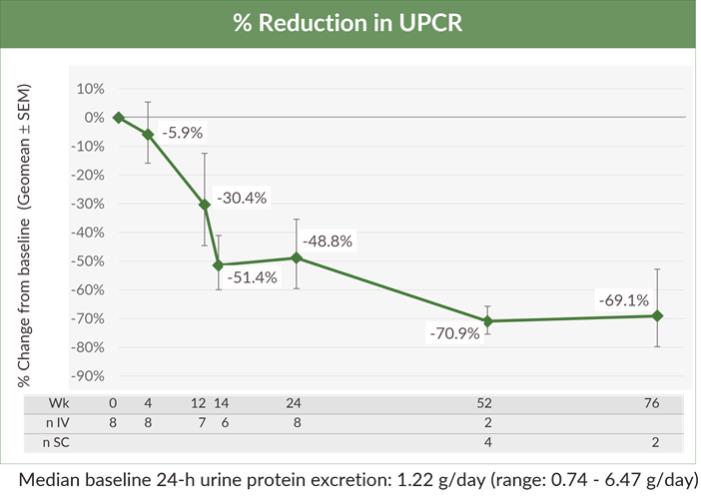
A trial-in-progress overview for the ongoing phase 1/2 study of BION-1301 was delivered as a mini-oral presentation at the 59th ERA Congress 2022.
All three presentations can be found in the Scientific Publications section of Chinook’s website.
Live Conference Call and Webcast
Chinook will host a live conference call and webcast on Friday, May 20, 2022 at 4:15 pm EDT to discuss the presentations at the 59th ERA Congress 2022 and provide program updates. Members of the Chinook executive team will be joined by Dr. Muh Geot Wong, associate professor of nephrology at Concord Repatriation General Hospital at University of Sydney in Sydney, Australia and Dr. Jonathan Barratt, Mayer Professor of Renal Medicine at University of Leicester in Leicester, UK.
Conference Call and Webcast Details
To access the call, please dial (844) 309-0604 (domestic) or (574) 990-9932 (international) and provide the Conference ID 9428716 to the operator.
To access the live webcast and subsequent archived recording of this and other company presentations, please visit the Investors section of Chinook’s website. The archived webcast will remain available for replay on Chinook’s website for 90 days.
About Chinook Therapeutics, Inc.
Chinook Therapeutics, Inc. is a clinical-stage biopharmaceutical company developing precision medicines for kidney diseases. Chinook’s product candidates are being investigated in rare, severe chronic kidney disorders with opportunities for well-defined clinical pathways. Chinook’s lead program is atrasentan, a phase 3 endothelin receptor antagonist for the treatment of IgA nephropathy and other proteinuric glomerular diseases. BION-1301, an anti-APRIL monoclonal antibody is being evaluated in a phase 1/2 trial for IgA nephropathy. CHK-336, an oral small molecule LDHA inhibitor for the treatment of hyperoxalurias, is being evaluated in a phase 1 healthy volunteer trial. In addition, Chinook is advancing research programs for other rare, severe chronic kidney diseases. Chinook is building its pipeline by leveraging insights in kidney single cell RNA sequencing, human-derived organoids and new translational models, to discover and develop therapeutics with differentiating mechanisms of action against key kidney disease pathways. To learn more, visit www.chinooktx.com.
Cautionary Note on Forward-Looking Statements
Certain of the statements made in this press release are forward looking, including those relating to Chinook’s business, future operations, advancement of its product candidates and product pipeline, clinical development of its product candidates, including expectations regarding timing of results of clinical trials and the readthrough to topline data. In some cases, you can identify these statements by forward-looking words such as “may,” “will,” “continue,” “anticipate,” “intend,” “could,” “project,” “expect” or the negative or plural of these words or similar expressions. Forward-looking