On September 21, 2023, Travere Therapeutics, Inc. (the “Company”) announced topline two-year confirmatory secondary endpoint results from the Company’s pivotal head-to-head Phase 3 PROTECT Study of FILSPARI® (sparsentan) in IgA nephropathy (IgAN) versus irbesartan. FILSPARI demonstrated long-term kidney function preservation and achieved a clinically meaningful difference in estimated glomerular filtration rate (eGFR) total and chronic slope versus irbesartan, narrowly missing statistical significance in eGFR total slope while achieving statistical significance in eGFR chronic slope for purposes of regulatory review in the EU. FILSPARI is currently available under accelerated approval in the U.S. The Company will engage with regulators and expects to submit a supplemental New Drug Application (sNDA) in the first half of 2024 for full approval in the U.S.
In the PROTECT Study, a total of 404 patients with persistent proteinuria despite active angiotensin-converting enzyme (ACE) inhibitor or angiotensin-receptor blocker (ARB) treatment, were randomized 1:1 to receive once daily oral doses of either FILSPARI or irbesartan, the active control. eGFR total and chronic slope are the secondary confirmatory endpoints for the U.S. and the EU, respectively. All topline efficacy endpoints favored FILSPARI as compared to irbesartan.
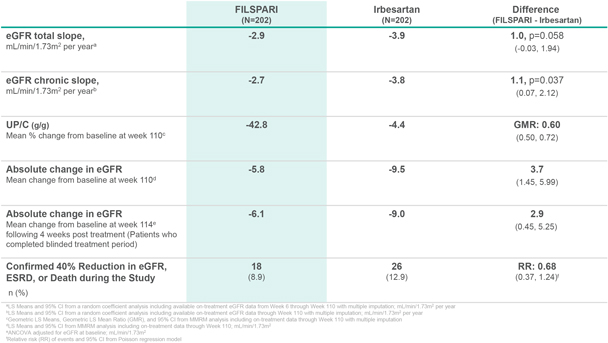
FILSPARI (N=202) Irbesartan (N=202) Difference (FILSPARI - Irbesartan) eGFR total slope, mL/min/1.73m2 per yeara -2.9 -3.9 1.0, p=0.058 (-0.03, 1.94) eGFR chronic slope, mL/min/1.73m2 per yearb -2.7 -3.8 1.1, p=0.037 (0.07, 2.12) UP/C (g/g) Mean % change from baseline at week 110c -42.8 -4.4 GMR: 0.60 (0.50, 0.72) Absolute change in eGFR Mean change from baseline at week 110d -5.8 -9.5 3.7 (1.45, 5.99) Absolute change in eGFR Mean change from baseline at week 114e following 4 weeks post treatment (Patients who completed blinded treatment period) -6.1 -9.0 2.9 (0.45, 5.25) Confirmed 40% Reduction in eGFR, ESRD, or Death during the Study n (%) 18 (8.9) 26 (12.9) RR: 0.68 (0.37, 1.24)f aLS Means and 95% CI from a random coefficient analysis including available on-treatment eGFR data from Week 6 through Week 110 with multiple imputation; mL/min/1.73m2 per year bLS Means and 95% CI from a random coefficient analysis including available on-treatment eGFR data through Week 110 with multiple imputation; mL/min/1.73m2 per year cGeometric LS Means, Geometric LS Mean Ratio (GMR), and 95% CI from MMRM analysis including on-treatment data through Week 110 with multiple imputation dLS Means and 95% CI from MMRM analysis including on-treatment data through Week 110; mL/min/1.73m2 eANCOVA adjusted for eGFR at baseline; mL/min/1.73m2 fRelative risk (RR) of events and 95% CI from Poisson regression model
A preliminary review of the safety results through 110 weeks of treatment indicates FILSPARI was generally well-tolerated and the overall safety profile in the study has been consistent between treatment groups.
The Company will complete a full evaluation of the data from the PROTECT Study and work with the study investigators on future presentations and publications of the results at an upcoming medical meeting and in a peer-reviewed publication.
In August 2022, the European Medicines Agency (EMA) accepted for review the Conditional Marketing Authorization (CMA) application of sparsentan for the treatment of IgAN. Together with its partner CSL Vifor, the Company anticipates a review opinion by the Committee for Medicinal Products for Human Use (CHMP) on the CMA application for sparsentan for the treatment of IgAN in the EU around the end of 2023.
Forward-Looking Statements
This report contains “forward-looking statements” as that term is defined in the Private Securities Litigation Reform Act of 1995. Without limiting the foregoing, these statements are often identified by the words “anticipate,” “believe,” “expect,” “intend,” “may,”
