Exhibit 99.1
What’s Possible at Agios
October 16, 2015
David Schenkein, M.D.
Chief Executive Officer
Cautionary Note Regarding Forward-Looking Statements
This “2015 Research & Development Day” presentation and various remarks we make during this presentation contain forward-looking statements of Agios Pharmaceuticals, Inc. within the meaning of The Private Securities Litigation Reform Act of 1995. Such forward-looking statements include those regarding the potential benefits of Agios’ product candidates targeting IDH1/IDH2 or pyruvate kinase-R mutations, including AG-221, AG-120, AG-881, AG-348 and AG-519; its plans and timelines for the clinical development of AG-221, AG-120, AG-881, AG-348 and AG-519; its plans regarding future data presentations; and the benefit of its strategic plans and focus. The words “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “plan,” “predict,” “project,” “potential,” “hope,” “could,” “would” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words.
Such statements are subject to numerous important factors, risks and uncertainties that may cause actual events or results to differ materially from Agios’ current expectations and beliefs. For example, there can be no guarantee that any product candidate Agios is developing will successfully commence or complete necessary preclinical and clinical development phases, or that development of any of Agios’ product candidates will successfully continue. There can be no guarantee that any positive developments in Agios’ business will result in stock price appreciation. Management’s expectations and, therefore, any forward-looking statements in this presentation or the various remarks made during this presentation could also be affected by risks and uncertainties relating to a number of other important factors, including: Agios’ results of clinical trials and preclinical studies, including subsequent analysis of existing data and new data received from ongoing and future studies; the content and timing of decisions made by the U.S. FDA and other regulatory authorities, investigational review boards at clinical trial sites and publication review bodies; Agios’ ability to obtain and maintain requisite regulatory approvals and to enroll patients in its planned clinical trials; unplanned cash requirements and expenditures; competitive factors; Agios’ ability to obtain, maintain and enforce patent and other intellectual property protection for any product candidates it is developing; Agios’ ability to maintain key collaborations, such as its agreement with Celgene; and general economic and market conditions. These and other risks are described in greater detail under the caption “Risk Factors” included in Agios’ Quarterly Report on Form 10-Q for the quarter ended June 30, 2015, and other filings that Agios may make with the Securities and Exchange Commission in the future.
Any forward-looking statements contained in this presentation or in remarks made during this presentation speak only as of the date hereof, and Agios expressly disclaims any obligation to update any forward-looking statements, whether as a result of new information, future events or, except as required by law.
We Are Driven By a Clear Vision and Values
VISION
Agios is passionately committed to the fundamental transformation of patients’ lives through scientific leadership in the field of cancer metabolism and rare genetic disorders of metabolism
Making a Difference for Patients & Building Long-Term Value
Vision Science
Team/Culture Execution
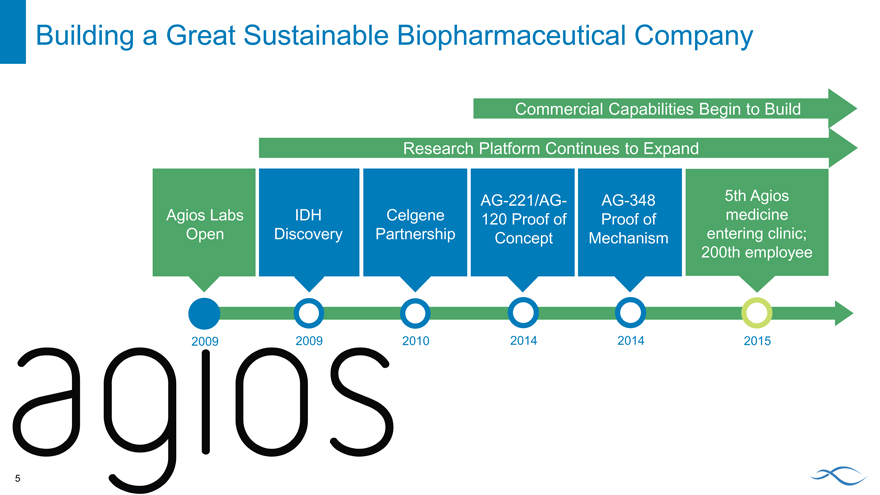
Building a Great Sustainable Biopharmaceutical Company
Commercial Capabilities Begin to Build
Research Platform Continues to Expand
AG-221/AG- AG-348 5th Agios
Agios Labs IDH Celgene 120 Proof of Proof of medicine
Open Discovery Partnership Concept Mechanism entering clinic;
200th employee
2009 2009 2010 2014 2014 2015
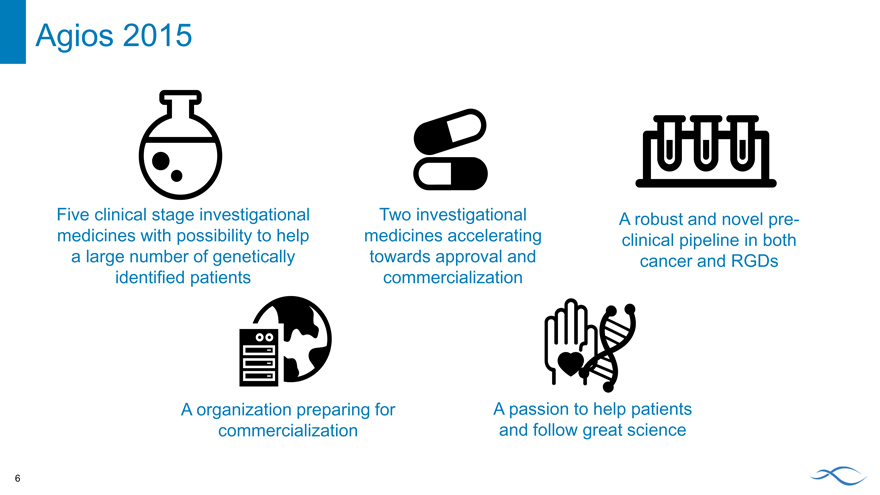
Agios 2015
Five clinical stage investigational medicines with possibility to help a large number of genetically identified patients
Two investigational medicines accelerating towards approval and commercialization
A robust and novel pre-clinical pipeline in both cancer and RGDs
A organization preparing for commercialization
A passion to help patients and follow great science
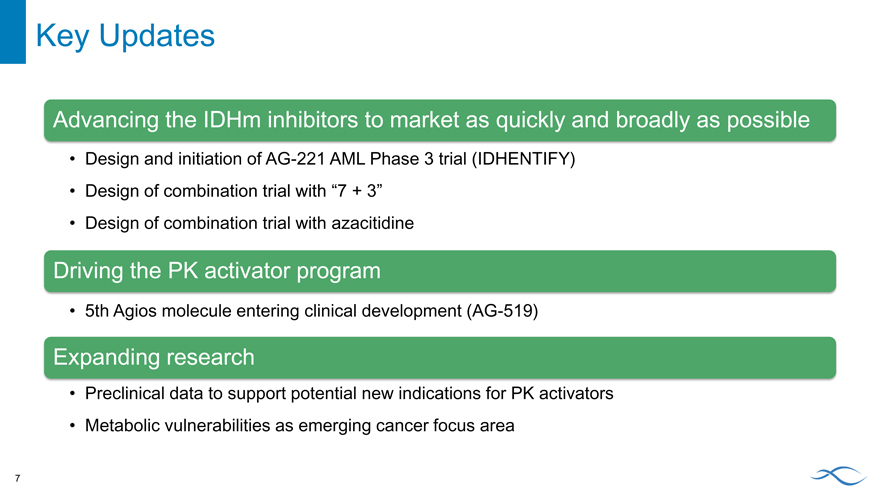
Key Updates
Advancing the IDHm inhibitors to market as quickly and broadly as possible
Design and initiation of AG-221 AML Phase 3 trial (IDHENTIFY)
Design of combination trial with “7 + 3”
Design of combination trial with azacitidine
Driving the PK activator program
5th Agios molecule entering clinical development (AG-519)
Expanding research
Preclinical data to support potential new indications for PK activators Metabolic vulnerabilities as emerging cancer focus area
What’s Possible: Our Science
Find the Source of the Problem and Correct It
Discovering novel unprecedented targets
Tackling tough chemistry with allosteric inhibitors and activators Requiring a predictive marker prior to development candidate selection Exploiting intersection of several key fields: metabolism, genomics, epigenetics and immunology Setting a high bar and rewarding answers
9
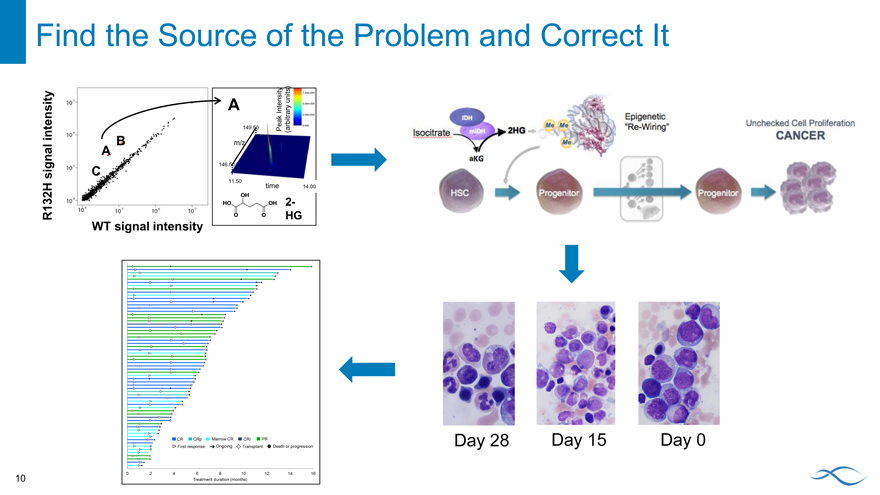
Find the Source of the Problem and Correct It
A Intensity units)
intensity 149.50 Peak (arbitrary
B m/z
A
146.50
signal C
11.50
time 14.00
2-
R132H HG
WT signal intensity
Day 28 Day 15 Day 0
10
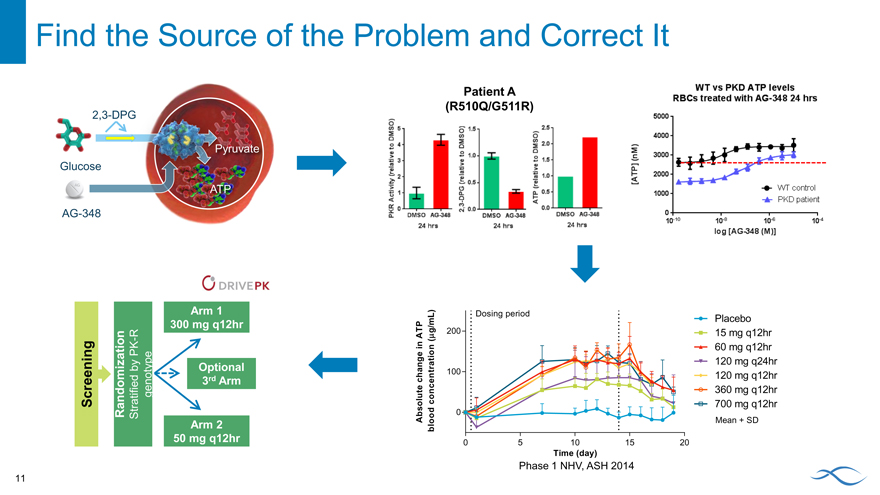
Find the Source of the Problem and Correct It
2,3-DPG
ruvate
Glucose
AG-348
Patient A (R510Q/G511R)
Arm 1
300 mg q12hr
R
-
PK
by Optional
Screening Randomization Stratified genotype 3rd Arm
Arm 2
L) Dosing period Placebo
m
g/
ATP m 200 15 mg q12hr
n (
i tion 60 mg q12hr
a 120 mg q24hr
100 120 mg q12hr
c hange entr
e c
lu t con 360 mg q12hr
o 700 mg q12hr
b s od 0
A lo Mean + SD
b
0 5 10 15 20
Time (day)
Phase 1 NHV, ASH 2014
11
What’s Possible: For Patients
What’s Possible for IDHm Patients
Next
Now
Relapsed/ Refractory AML
13
What’s Possible for IDHm Patients
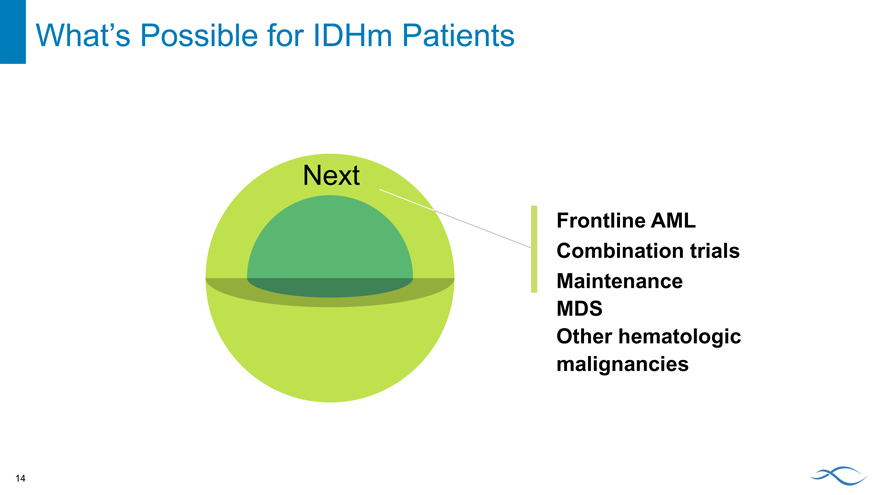
Next
Frontline AML Combination trials Maintenance MDS
Other hematologic malignancies
14
What’s Possible for IDHm Patients
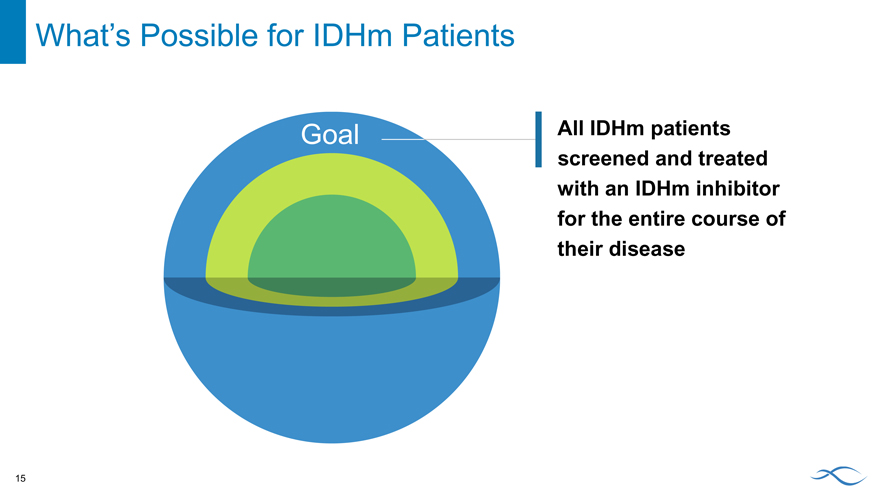
Goal
All IDHm patients screened and treated with an IDHm inhibitor for the entire course of their disease
15
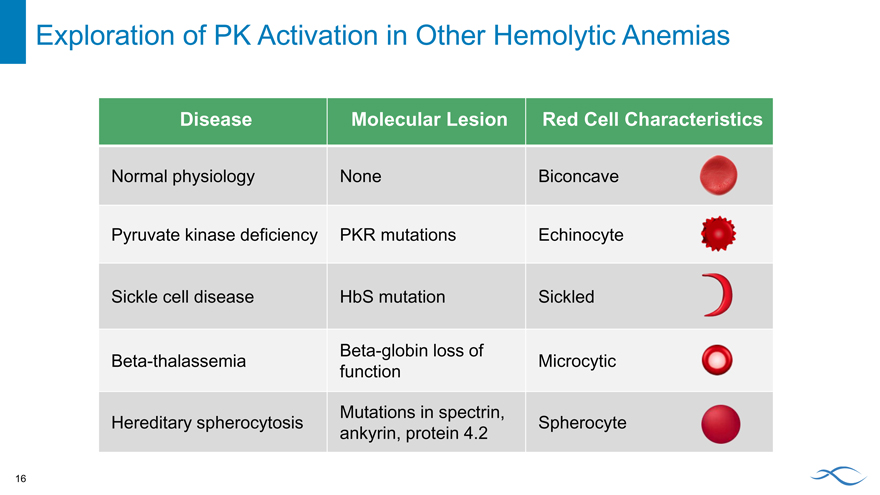
Exploration of PK Activation in Other Hemolytic Anemias
Disease Molecular Lesion Red Cell Characteristics
Normal physiology None Biconcave
Pyruvate kinase deficiency PKR mutations Echinocyte
Sickle cell disease HbS mutation Sickled
Beta-globin loss of
Beta-thalassemia Microcytic
function
Mutations in spectrin,
Hereditary spherocytosis Spherocyte
ankyrin, protein 4.2
16
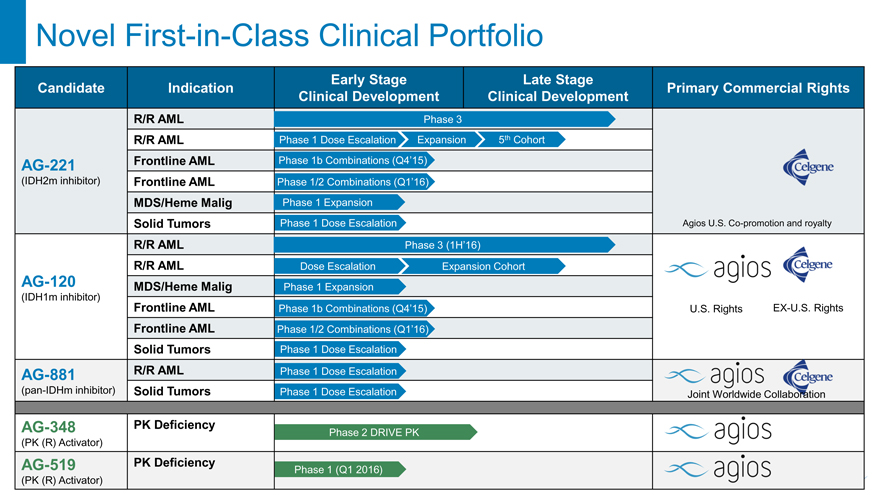
Novel First-in-Class Clinical Portfolio
Early Stage Late Stage
Candidate Indication Primary Commercial Rights
Clinical Development Clinical Development
R/R AML Phase 3
R/R AML Phase 1 Dose Escalation Expansion 5th Cohort
AG-221 Frontline AML Phase 1b Combinations (Q4’15)
(IDH2m inhibitor) Frontline AML Phase 1/2 Combinations (Q1’16)
MDS/Heme Malig Phase 1 Expansion
Solid Tumors Phase 1 Dose Escalation Agios U.S. Co-promotion and royalty
R/R AML Phase 3 (1H’16)
R/R AML Dose Escalation Expansion Cohort
AG-120 MDS/Heme Malig Phase 1 Expansion
(IDH1m inhibitor)
Frontline AML Phase 1b Combinations (Q4’15) U.S. Rights EX-U.S. Rights
Frontline AML Phase 1/2 Combinations (Q1’16)
Solid Tumors Phase 1 Dose Escalation
AG-881 R/R AML Phase 1 Dose Escalation
(pan-IDHm inhibitor) Solid Tumors Phase 1 Dose Escalation Joint Worldwide Collaboration
AG-348 PK Deficiency Phase 2 DRIVE PK
(PK (R) Activator)
AG-519 PK Deficiency Phase 1 (Q1 2016)
(PK (R) Activator)
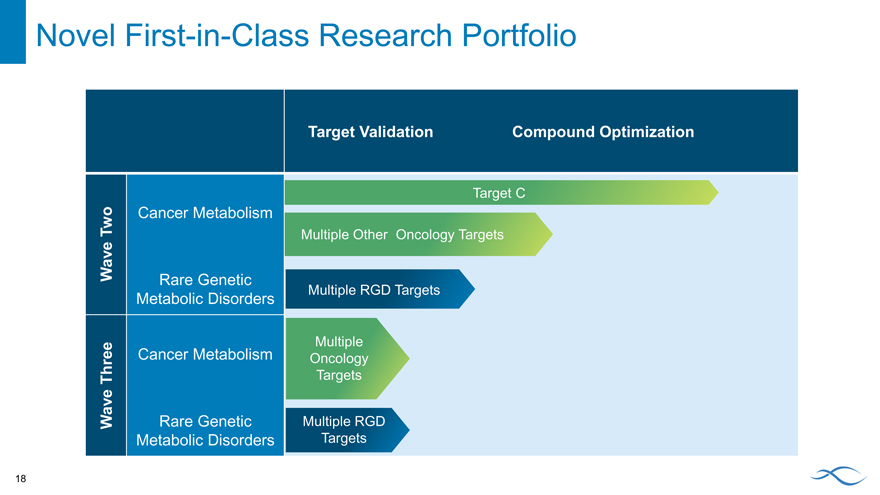
Novel First-in-Class Research Portfolio
Target Validation Compound Optimization
Target C
Two Cancer Metabolism
Multiple Other Oncology Targets
Wave Rare Genetic
Metabolic Disorders Multiple RGD Targets
Multiple
Cancer Metabolism Oncology
Three Targets
Wave Rare Genetic Multiple RGD
Metabolic Disorders Targets
18
Today’s Agenda
Time Speaker
8:30—9:00 am Registration and breakfast
9:00—9:20 am Opening Remarks – What’s Possible at Agios (David Schenkein)
IDH Program
9:20—9:40am AG-221/AG-120 and Hematologic Malignancies (Courtney DiNardo—MD Anderson)
9:40—10:00am Clinical and Regulatory Overview (Chris Bowden)
10:00—10:45am IDH-Mutant Solid Tumor Background (Sam Agresta and Tim Cloughesy—UCLA)
10:45—10:55am Q&A and Break
PKR & Research Programs
10:55—11:10am What We Know About PK Deficiency (Ann Barbier)
11:10—11:25am PKD Clinical and Regulatory Overview (Chris Bowden)
11:25– 11:50am Research Update (Scott Biller)
11:50am—12:00pm Q&A
12:00pm—1:00pm Buffet lunch
19
What You’ll Come Away with Today
Confident that we are leading the way in the disruptive field of dysregulated metabolism Clear that we are building world class clinical development and commercial capabilities on the foundation of our innovative research expertise Enthusiastic in your commitment to help us build a great biopharmaceutical company Passionate in our common mission of changing the lives of patients and creating significant shareholder value
20
IDHm Inhibitors and Hematologic Malignancies
October 16, 2015 Courtney DiNardo, M.D.
University of Texas MD Anderson Cancer Center,
Houston, TX, USA
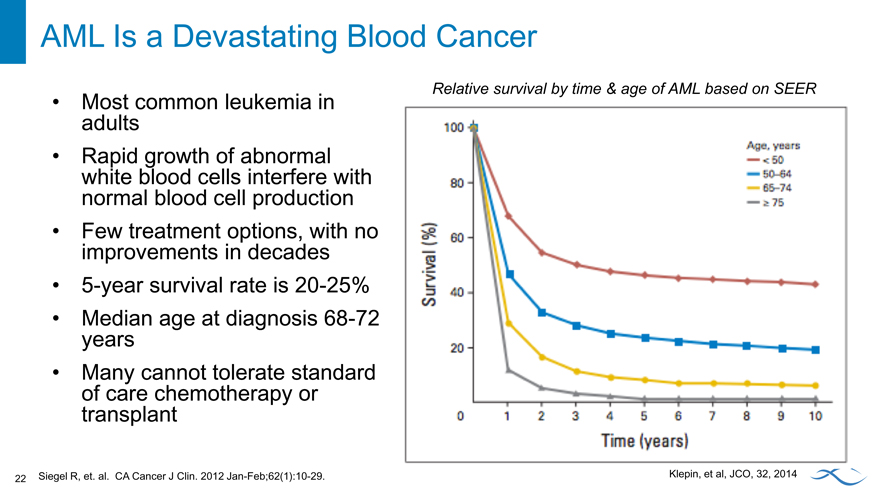
AML Is a Devastating Blood Cancer
Most common leukemia in adults Rapid growth of abnormal white blood cells interfere with normal blood cell production Few treatment options, with no improvements in decades 5-year survival rate is 20-25% Median age at diagnosis 68-72 years Many cannot tolerate standard of care chemotherapy or transplant
Relative survival by time & age of AML based on SEER.
Klepin, et al, JCO, 32, 2014
Siegel R, et. al. CA Cancer J Clin. 2012 Jan-Feb;62(1):10-29.
22
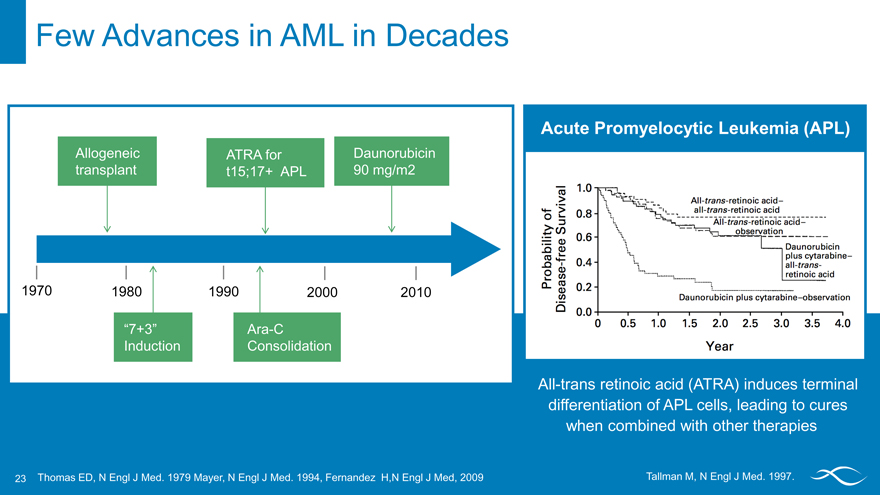
Few Advances in AML in Decades
Allogeneic ATRA for Daunorubicin
transplant t15;17+ APL 90 mg/m2
1970 1980 1990 2000 2010
“7+3” Ara-C
Induction Consolidation
Acute Promyelocytic Leukemia(APL)
All-trans retinoic acid (ATRA) induces terminal differentiation of APL cells, leading to cures when combined with other therapies
Thomas ED, N Engl J Med. 1979 Mayer, N Engl J Med. 1994, Fernandez H,N Engl J Med, 2009 Tallman M, N Engl J Med. 1997.
23
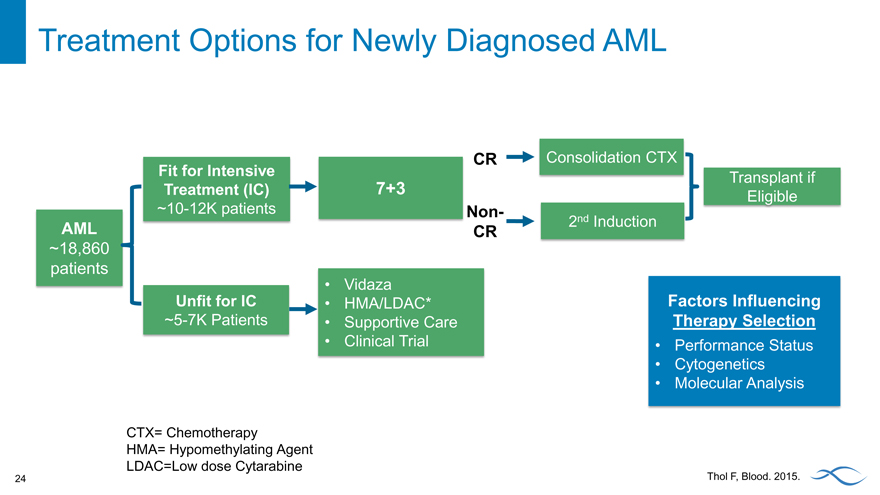
Treatment Options for Newly Diagnosed AML
CR Consolidation CTX
Fit for Intensive Transplant if
Treatment (IC) 7+3 Eligible
~10-12K patients Non-
AML CR 2nd Induction
~18,860
patients
Vidaza
Unfit for IC HMA/LDAC* Factors Influencing
~5-7K Patients Supportive Care Therapy Selection
Clinical Trial Performance Status
Cytogenetics
Molecular Analysis
CTX= Chemotherapy
HMA= Hypomethylating Agent LDAC=Low dose Cytarabine
Thol F, Blood. 2015.
24
Limited Treatment Options for Relapsed/Refractory (R/R) AML
Intensive Salvage
Fit for Intensive
CTX
Treatment (IC) Transplant
Clinical Trial Factors Influencing Therapy Selection
R/R AML
Performance Status
Cytogenetics
HMA/LDAC* Molecular Analysis
Unfit for IC
Supportive Care
Clinical Trial
CTX = Chemotherapy HMA = Hypomethylating Agent LDAC = Low Dose Cytarabine
25
Limited Treatment Options for Relapsed/Refractory (R/R) AML
Factors Influencing
Therapy Selection
R/R AML
Performance Status
Cytogenetics
Molecular Analysis
CTX = Chemotherapy
HMA = Hypomethylating Agent LDAC = Low Dose Cytarabine
Thol F, Blood. 2015
Mutations in IDH Occur Frequently in AML and MDS
IDH1m and IDH2m occur frequently in AML and MDS and are associated with a poor prognosis 2-HG acts as a competitive inhibitor of á-KG, which includes DNA and histone modifying enzymes and prolyl hydroxylases involved in collagen metabolism and hypoxia control
AML MDS
Bone marrow Bone marrow
Incidence
(cases/year US) 18K 15K
Prevalence (US) 25K >60K
IDH1m frequency 6-10% 3%
IDH2m frequency 9-13% 3-6%
Treatment Options Chemotherapy Chemotherapy
Transplant Transplant
5-year overall survival 20-25% ~30%
Images: David S. Rosenthal, M.D
Multiple sources including market research SEER
27
IDH Mutations Lead to Unchecked Proliferation
Mutations in IDH1 and IDH2 confer neomorphic activity
Increased levels of 2-HG affect epigenetic control of the cell
Inhibiting mutant IDH1 and IDH2 promotes maturation
IDH Epigenetic
“Re-Wiring” Unchecked Cell Proliferation
Isocitrate mIDH 2HG CANCER
aKG
HSC Progenitor Progenitor
28
First-in-Class, Oral, Potent, Selective, Reversible Inhibitors
AG-221 is an inhibitor of mutant IDH2
Study AG-221 Design
Single-arm, open-label dose escalation and expansion
Participants
Patients with IDH2 mutation-positive hematologic malignancies, including R/R AML, MDS and untreated AML
Treatment
Single agent AG-221 once (QD) or twice (BID) daily in continuous 28-day cycles
Primary Assessment
Objective responses are assessed by investigators using International Working Group AML and MDS criteria.
AG-120 is an inhibitor of mutant IDH1
Study AG-120 Design
Single-arm, open-label dose escalation and expansion
Participants
Patients with IDH1 mutation-positive hematologic malignancies, including R/R AML, MDS and untreated AML
Treatment
Single agent AG-120 QD or BID daily in continuous 28-day cycles
Primary Assessment
Objective responses are assessed by investigators using International Working Group AML and MDS criteria.
29
Patients Are Heavily Pre-Treated in Both Studies
AG-221 Demographics
All patients R/R AML
N=177 n=124
Age in years, median 69 67
(range)(22–90)(22–90)
ECOG performance status, n
(%)
0 43 (24) 30 (24)
No. of prior regimens, median 2 2
(range)(0-11)(1-11)
Prior Transplant, n 21 16
% 12% 13%
AG-120 Demographics
All patients
N=57
Age in years, median (range) 68 (38–89)
ECOG performance status, n
0 14
No. of prior chemotherapy regimens, 2
median (range)(0–5)
Prior Transplant, n 12
% 21%
Abnormal cytogenetics at study entry,% 78 %
Data Presented at EHA, 6/13/15
30
AG-221 and AG-120 Demonstrate Favorable Safety Profiles
AG-221 Safety Summary
Therapy has been well tolerated up to 450 mg QD
– MTD not reached
– Recommended dose for expansion is 100 mg PO QD
30-day all-cause mortality of 4.5%; 60-day, 11.3% Isolated increases in indirect bilirubin observed
AG-120 Safety Summary
Therapy has been well tolerated up to 800 mg QD
– MTD not reached
– Recommended dose for expansion is 500 mg PO QD
Two DLTs observed
– One grade 3 QT prolongation at 800 mg QD
– One grade 3 rash at 1200 mg QD
Grade 2-3 QT prolongation observed
Data Presented at EHA, 6/13/15
31
Traditional Response Criteria May Overlook Certain Aspects of Clinical Benefit
Modified IWG Absolute Neutrophil
Blasts Platelets
Criteria Count (ANC)
Marrow CR < 5% No recovery No recovery
CR < 5% > 100K >1,000
CRi/CRp <5% > 100K OR > 1,000
50% decrease
Partial Remission > 100K > 1,000
to 5-25%
Doesn’t meet PR
Stable Disease —
criteria
Cheson et al, J Clin Oncol 17:1244, 1999
32
AG-221 Single-Agent Response Rates of 30-50% in Phase 1
R/R AML Untreated MDS Other Total
n=111 AML n=14 n=10 N=157
n=22
CR, n (%) 20 (18.0) 3 (13.6) 2(14.3) 1 (10.0) 26 (16.6)
CRp, n 1 — 1 1 3
PR, n 16 2 — — 18
mCR, n 8 1 4 1 14
CRi, n 1 1 — — 2
SD, n 49 7 4 7 67
PD, n 7 5 2 — 14
NE, n 9 3 1 — 13
ORR, n (%) 46 (41.4) 7 (31.8) 7(50.0) 3 (30.0) 63 (40.1)
Data Presented at EHA, 6/13/15
33
Similar Magnitude of Response for AG-120
Cohorts 1 + 2 Cohort 3 Cohort 4 Cohort 5 Total
?300 mg/day 500 mg QD 800 mg QD 1200 mg QD N=52
n=8 n=22 n=15 n=7
CR, n 2 3 1 2 8
CRp, n — — — 1 1
PR, n — 1 3 — 4
Marrow CR, n — 3 — — 3
SD, n 5 13 7 2 27
PD, n 1 2 4 1 8
NE, n — — — 1 1
ORR, n(%) 2/8 (25) 7/22 (32) 4/15 (27) 3/7 (43) 16/52 (31)
Data Presented at EHA, 6/13/15
34
IDHm Inhibitor Response Fundamentally Different from Chemotherapy
D15 Marrow
Conventional Chemotherapy
Maturation of Biopsy post
mutated cells in conventional
response to chemotherapy
IDHm treatment shows an empty
through the first marrow
cycle
Image: Min Xu M.D.
Data Presented at AACR, 4/2014
35
Durable Responses Associated with Both AG-221 and AG-120
AG-221
Treatment duration: 3-month = 88.0% 6-month = 76.1% Median = Not estimable
AG-120
Treatment duration:
3-month = 79.1%
6-month = 50.2%
Data Presented at EHA, 6/13/15
36
Rethinking Clinical Benefit for IDHm Inhibitors in AML
Patient with Partial Response Shows Recovery of Platelet and Neutrophil Counts
Response to AG-221 appears to be fundamentally different compared to cytotoxic chemotherapy
Partial responses convert to complete remission and/or remain as best response over time
Screening 44% Blasts Cycle 2, Day 1 12% Blasts
Hgb Platelets ANC Hgb Platelets ANC
Data Presented at ASH 2014
37
Rethinking Clinical Benefit for IDHm Inhibitors in AML
Patients with Stable Disease Staying on Treatment Up to 10+ Months
AG-221
AG-120
Data Presented at EHA, 6/13/15
Treatment duration estimated using Kaplan-Meier method
38
Rethinking Clinical Benefit for IDHm Inhibitors in AML
Potential Benefit Associated with Stable Disease for a Patient Treated with AG-221
Day 1 AG-221 Platelets
Normal
Range
Day 1 AG-221 Absolute Neutrophil Count
Normal
Range
Blast count remains elevated with recovery of platelets and neutrophils for 4+ months of stable disease
Data from Clinical Investigator on AG-221 Study
39
Hematologic Recovery in Patient with High Risk Myelodysplastic Syndromes (MDS)
Improvement in hematologic parameters and platelet count on AG-221
Hematologic Parameters
9
Counts 4
0
Day Day Day Day Day Day Day Day Day Day Day Day Day Day Day Day
-4 1 4 11 18 25 32 46 62 75 92 120 148 175 204 232
WBC 1.1 1.6 1.8 2.2 4.5 3.5 2.5 1.9 1.8 3.4 4.7 8.2 4.2 5.9 5.7 4
Neutrophils 0.18 0.72 0.81 1.06 2.87 2.26 1.55 0.97 0.61 2.05 3.12 6.4 2.74 4.38 3.84 2.41
Hematocrit 29.2 33.1 33.3 33.4 35.6 37.5 36.8 36.4 39.1 38.9 40.4 40 39.7 40.9 40.1 44.5
Platelets
500
450
400
350
300
Counts 250
200
150
100
0
Day Day Day Day Day Day Day Day Day Day Day Day Day Day Day Day
-4 1 4 11 18 25 32 46 62 75 92 120 148 175 204 232
Platelet 52 46 51 81 116 173 197 207 274 286 324 375 437 327 322 334
Data from Clinical Investigator on AG-221 Study
40
Key Takeaways
IDHm inhibitors work by a unique mechanism entirely different from conventional chemotherapy Treatment with oral drugs AG-221 and AG-120 is well-tolerated AG-221 and AG-120 show clear activity as single agents
– Durable complete remissions
– Durable non-CR responses
– Prolonged stable disease
IDHm targeted differentiation treatment has demonstrated clinical benefit in AML
Phase 1 studies
41
Bringing AG-221 and AG-120 To Patients
Clinical and Regulatory Overview
October 16, 2015 Chris Bowden, M.D.
Chief Medical Officer
Phase 1 Program Rapidly Defined Single-Agent Profile for
IDHm Inhibitors in R/R AML
Phase 1 expansion cohorts designed to demonstrate compelling clinical benefit with registration quality data
Efficacy
Complete Remissions AG-120 AML
IDH1m Partial Remissions Expansion Cohort R/R AML
N~125
Safety
Adverse Events
Discontinuations
AG-221 AML IDH2m
Expansion Cohort Healthcare Utilization R/R AML
N~125 Transfusions
Infections
Our goal is to make AG-221 and AG-120 available as quickly as possible
Development Program Targets Multiple Lines of Treatment
from R/R to Frontline AML
Relapsed Newly Diagnosed (Untreated)
2nd+ Relapse Intensive Non-Intensive
Phase 1? 2 Phase 1 Phase 1 IDHm+ VIDAZA® AG-221 Expansion IDHm+ Induction (7+3) +
+ AG-221 or AG-120
AG-221 or AG-120 Ongoing Phase 1
Planned
AG-120 Expansion
Phase 3 AG-221 vs SOC
Phase 3 for AG-120 also planned for 1H16
“IDHENTIFY” Trial
A Phase 3, multicenter, open-label, randomized study comparing the efficacy and safety of AG-221 (CC-90007) vs. conventional care regimens in older patients with late stage Acute Myeloid Leukemia harboring an IDH2 mutation
IDHENTIFY: Global Phase 3 Study to Evaluate the Efficacy of AG-221 in R/R AML
KEY INCLUSION AG-221/CC-90007 1° ENDPOINT
> 60 years Starting 100 mg QD OS
IDH2+ 28 day cycles KEY 2° 280 patients nd rd Randomization
R/R AML after 2 /3 No crossover
ENDPOINTS
line
COMPARATOR EFS
STRATIFIED BY Best Supportive Care ORR
Duration of
Prior intensive therapy 1:1 (BSC) response
Prior refractory VIDAZA® + BSC
1-yr survival
Prior HSCT Low dose Ara-C + BSC CR rate
Intermediate Dose
Safety Ara -C + BSC
AG-221 + BSC
IDHENTIFY Is a Global Phase 3 Study
U.K. German y Russia Canada Belgium Denmark
France Austria
South Korea ~130 sites will
U.S. Spain Italy
participate
Brazil
Australia
What’s Possible for IDHm Patients
A Roadmap for Speed and Breadth
All IDHm patients screened and Goal treated with an IDHm inhibitor for the entire course of their disease
Next
Frontline AML Combination trials Now Maintenance MDS
Other hematologic malignancies
Relapsed/ Refractory AML
Frontline Therapy: Novel Clinical Development Strategies
Phase 1 Eligible for Intensive 7+3 *
Newly +
Chemotherapy
Diagnosed AG-221 or AG-120
Potential
AML
Registration
IDH1m+
Trials
or Phase 1/2 Not Eligible for
IDH2m+
Intensive VIDAZA®
+ Chemotherapy
AG-221 or AG-120
8 | | *7+3-Ara-C (Days 1-7), Daunorubicin or Idarubicin (D1-3) |
Intensive Eligible Patients: Adding IDHm Inhibitors to Standard
of Care
IDH1m (N~45)
AG-120 AG-120
+ + AG-120 (7+3) Ara-C*
Newly Phase 1 Trial Objectives:
Diagnosed AML
MAINTENANCE Safety IDHm INDUCTION CONSOLIDATION
(1 year) PK
Eligible for 7+3 PD
IDH2m (N~45) Preliminary efficacy
Not Eligible for
AG-221 Intensive AG-221
Chemotherapy
+ + AG-221 (7+3) Ara-C*
9 *Ara-C or Mitoxantrone/Etoposide
Non-Intensive Patients: Adding IDHm Inhibitors to Standard of
Care
PHASE 1 RANDOMIZED PHASE 2
Establish Safety for Compare Efficacy of Combination combinations versus VIDAZA® Newly VIDAZA®
Diagnosed IDH1m
AML AG120 + VIDAZA® IDH1m+
IDHm VIDAZA® +AG-120
Not Eligible
VIDAZA® for 7+3 IDH2m
AG-221+VIDAZA® IDH2m+
VIDAZA® +AG-221
Primary endpoint is ORR
10 Efficient transition to Phase 2
MDS Is a New Development Horizon
Clinical development strategies for MDS are being evaluated
VIDAZA® one of few approved agents
High-risk MDS after failure of hypomethylating agent remains an area of high unmet medical need
Also potential in low-risk population (role for oral drug)
11 Image: David S. Rosenthal, M.D.
What’s Next at ASH
Seven abstracts accepted (four for AG-221 and AG-120)
New data from dose escalation and expansion cohorts
New molecular data
12
Key Takeaways
R/R AML expansion cohorts in full operational mode
Broadening AML clinical development program into frontline
Novel trial design goals
– Bringing IDH1m and IDH2m patients into the same protocol
– Efficiently transition from Phase 1 to Phase 2
IDHm inhibitors have the potential for a paradigm shift in the treatment of AML
13
IDH Development in Solid Tumors
October 16, 2015 Sam Agresta, M.D., Agios
Tim Cloughesy, M.D., UCLA
Key Takeaways for Solid Tumors
Significant opportunity to develop targeted therapies across IDHm solid tumors
Uncharted development area with new biology and potential for differentiation treatment approach
High prevalence IDHm solid tumors are difficult-to-treat diseases with surgery as the only effective treatment
Three Phase 1 studies ongoing: AG-120, AG-221 and AG-881
– First AG-120 data to be presented in November at AACR-NCI-EORTC
Changing the Natural History of IDHm Solid Tumors
Our vision is to develop a foundational therapy used through multiple stages of
any IDHm tumor where POC is established
Post-resection to prevent Adjuvant recurrence ? potential cure
Relapsed/Refractory
Setting
Suppress driver mutation as Frontline monotherapy or in combination with other agents ? improve survival
Understanding the Role of Differentiation Therapy in Solid Tumors
Differentiation Restored Epigenetic
Inhibitor “Re-Wiring”
IDH
Isocitrate mIDH 2HG Normal Cell Death / Homeostasis
Differentiation aKG
HSC Progenitor Mature Cell
Goal is to Explore IDH Mutations AG-120 and AG-221 in IDHm occur across multiple solid tumor indications Solid Tumors
Intrahepatic
Glioma Chondrosarcoma
Cholangiocarcinoma (IHCC)
Low grade and 2ary GBM Bile ducts Cartilage
Incidence
5K 2K – 4K 700-1000
(cases/year U.S.)
Prevalence (U.S.) 24K 5K — IDH1m frequency 68-74% 11-24% 40-52% IDH2m frequency 3-5% 2-6% 6-11% Surgery, XRT Surgery, Chemotherapy Surgery, XRT Treatment options Chemotherapy Liver transplantation Chemotherapy 5-year O/S ~32– 68%* ~9% ~10-90%
Other solid tumor types include colon, melanoma, lung, ovarian.
Multiple sources, including market research and SEER. Estimates will continue to evolve with additional future data
Glioma
Tim Cloughesy M.D., UCLA
Glioma Is a Devastating CNS Neoplasm
Patient with an IDH1m right temporal lobe low-grade glioma (LGG)
34 year old 37 years old 39 years old 41 years old Treated with surgery followed by radiation Stable disease, observation Progressive disease. Attempts to control disease with multiple chemotherapies 2015 1 month before death
Glioma: Primary CNS Neoplasms
Multiple neuroglial cells (eg., astrocytes, oligodendrocytes)
Varying degrees of tumor aggressiveness
- Slower growing: LGG are WHO Grades 1 and 2
- Rapidly progressive: High-grade glioma (HGG) are WHO
Grades 3 and 4
About 23,000 cases of expected in U.S. (2015)
Common symptoms include memory disturbance, sensory impairment, neurologic deficits and seizures
Long-term prognosis is poor: 5-year survival rate of 33%
Median survival:12–15 months for glioblastoma and 2-5 years for anaplastic glioma
Source: UpToDate; Nature Reviews Cancer 10, 319-331 (May 2010) ; Cancer Manag Res. 2014 Mar 24;6:149-70.
Top Graphic © Mayo Foundation for Medical Education and Research. All rights reserved.
IDHm Glioma Is a Distinct Disease
Discovered in GBM samples in 2008
Different genetic/ epigenetic profile as compared to IDH wild type
The Cancer Genome Atlas Research Network. N Engl J Med 2015;372:2481-2498
9
IDHm Is Common in Low-Grade (II and III) Glioma
IDH1m highly prevalent:
– 5% primary GBM
– 83% secondary GBM
– 60-80% of low-grade diffuse glioma
– IDH2m are uncommon
Yan et al. N Engl J Med. (2009) 360(8): 765–773.
10
IDH1m GBMs Have Distinct MRI Appearance and Pathology
Conventional imaging and interpretation of pathology is
different for IDHm CNS disease
11 Lai et al. J Clin Oncol (2011) 29:4482-4490
Non-Invasive in vivo Detection of 2-HG by MR Spectroscopy
MR Spectroscopy may serve as an adjunct to conventional imaging modalities to understand response in IDHm glioma
12 Andronesi et al., Sci Transl Med (2012);4:116 Pope et al. J Neurooncol (2012) 107:197–205
IDHm Positive Glioma Manifests in Younger Patients with
Longer Survival
Lai et al. J Clin Oncol (2011) 29:4482-4490
13
Radiation and Chemotherapy Prolong Survival but with High Morbidity and Functional Decline
Patient with a IDH1m right frontal lobe Anaplastic Astrocytoma
45 year old (2003) 46 years old 50 year old 57 years old (2015)
Surgical Resection Is Mainstay of Treatment Followed by Radiation and/or Chemotherapy
Treatment Algorithm for Glioma Newly diagnosed
– Surgical intervention
– Treatment post-surgery depends on grade
Observation alone
Chemotherapy
Radiation (RT)
Chemotherapy plus RT
Limited effective treatment options for progressive disease
15 Nat Rev Neurol. 2013 Mar;9(3):141-51.
Key Takeaways: Glioma
IDHm glioma is a distinct disease where IDHm is an early event ? potential driver
Conventional imaging modalities are inadequate to assess treatment activity
Surgery is the mainstay of treatment for LGG
– Chemotherapy plus radiation for HGG
Limited treatment options for recurrent/progressive disease
IDHm inhibitors should be fully explored as potential new therapeutic option
16
Chondrosarcoma
October 16, 2015
Sam Agresta, M.D.
17
Chondrosarcoma (CS) Is a Rare and Potentially Deadly Disease
Central CS Peripheral CS
Heterogeneous group of cancers that arise from cartilage in the bone and joint
3rd most common type of bone cancer
– 700-1000 diagnosed per year in the U.S.
IDH1/2 mutations occur in 40-50%
of central chondrosarcomas radiopaedia.org rohan.sdsu.edu
Prognosis: Based on disease burden
– Curative potential with surgery, local disease
– Low 5-year survival for metastatic disease
18 http://www.nature.com/nrc/journal/v10/n7/images/nrc2869-f2.jpg
IDHm Occur Early in the Transformation Process
Proposed multistep genetic model for central
chondrosarcoma development and candidates for targeted
treatment
IDH 1/2
mutations Chondrosarcoma
19 Amary MF. Nature Genetics, 2011 Modified Bovée J,. Lancet Oncology 2006.
Surgery Is Only Curative Option for Chondrosarcoma
Surgery is the mainstay of treatment
– Complete surgical resection curative, but not possible in advanced disease
Radiation is not effective
Chemotherapy is of limited benefit
– Primarily used in neoadjuvant setting to convert nonresectable to resectable
Treatment for metastatic disease is mainly palliative
20
1st graph: Italiano A, Annals of Oncology 2013; 2nd graph: Wagner 2013 CTOS Presentation
Chondrosarcoma Typically Metastasizes to the Lungs
Surgery and radiation are palliative
Clinical trials preferred option
Chemotherapy resistant
– Single agent ifosphamide or methotrexate, or doxorubicin plus cisplatin are used
No therapies have demonstrated efficacy
21 Source: Emori et al. World Journal of Surgical Oncology 2011, 9:50 http://www.wjso.com/content/9/1/50 (16 May 2011)
Conventional Response Assessments Are Inadequate for
Chondrosarcoma
Residual calcification after chemotherapy poses challenges to conventional response assessment
22 Source: radiopaedia.org
Intrahepatic Cholangiocarcinoma
October 16, 2015
Sam Agresta, M.D.
23
Cholangiocarcinoma Is a Rare Cancer of the Bile Duct and Liver
2,000-4,000 new cases per year (U.S.)
50% of cases occur within the liver (intrahepatic cholangiocarcinoma, IHCC)
– Prognosis: Worse for IHCC than other biliary tract tumors
– Incidence of IHCC is increasing due to cirrhosis, alcoholic liver disease and hepatitis C
Typically presents with advanced disease
– Pain, weight loss, fever, elevated liver enzymes
Poor 5-year survival
– 15-30% for local disease
– 2% for metastatic disease
24
IDH Mutations Are Common in IHCC
IDH1/2 mutations present in approximately 25% of IHCCs
– Not present in extrahepatic CC or gallbladder cancers
– Majority of mutations are IDH1
IDH mutations do not affect prognosis
Zhu A, Oncologist, 2015
25
Options for Metastatic Cholangiocarcinoma Are Limited
Gemcitabine-Based Regimens
Surgery possibility curative, if not metastatic
– IHCC has the lowest resectability rates (due to late presentation)
– Five-year OS post-resection: 14-40%
– Majority of patients recur despite complete resection
Cisplatin plus gemcitabine standard of care for newly diagnosed metastatic disease
26
Randomized, Frontline Phase 2 study including 86 patients comparing cisplatin plus gemcitabine with gemcitabine alone in patients with previously untreated locally advanced or metastatic biliary tract cancer
Valle J. N Engl J Med. 2010.
Key Takeaways: Chondrosarcoma & IHCC
Current treatments are inadequate
Surgery is the only chance for cure for localized disease
Surgery, radiation and chemotherapy are palliative for metastatic disease
IDHm commonly occurs in these diseases
IDHm inhibitors should be fully explored as potential new therapeutic option
27
AG-120: Current Development Status in Advanced Solid Tumors
Phase 1 All IDH1m
Advanced hematologic malignancies
Advanced solid
tumors
IHCC
Chondrosarcoma
Glioma
Other advanced solid tumors
Phase 1 dose escalation study in advanced solid tumors
Study initiated in March 2014
First data expected at AACR-NCI-EORTC
Assess clinical activity, safety and tolerability of AG-120 as single agent
Administered orally in 28-day cycles
Assess 2-HG levels, differentiation & efficacy
28
AG-881: Brain Penetrant, Pan-IDHm Inhibitor
Now in Clinical Development, Two Phase 1 Studies Initiated
Phase 1
IDH1m or IDH2m
Advanced solid tumors
Advanced hematologic malignancies
Study initiated in June 2015
Assess safety, tolerability and clinical activity of AG-881 as a single agent
Administered orally in 28-day cycles
Assess 2-HG levels, differentiation & efficacy
Study initiated in August 2015
IDH1m or IDH2m patients whose cancer progressed on prior IDHm inhibitor therapy eligible
Purpose and dosing schedule same as above
29
Potential for IDHm Inhibitors in Solid Tumors
Committed to IDH exploring mutations both IDHm found in blood inhibition and solid cancer, in solid tumors No precedent clinical for development solid tumor programs differentiation and timelines are therapy exists
Different today
Realizing the full potential of targeting IDH mutations
LGG, IHCC is and a long-te chondrosarcoma m proposition have poor treatment options and limited drug development precedent Initial dose-escalation AG-120 data across multiple solid tumors to be presented at AACR-NCI-EORTC (Nov. 5-9)
30
What We Know About PK Deficiency
October 16, 2015 Ann Barbier, M.D., Ph.D.
VP, Clinical Development, Rare Genetic Diseases
Little Support for Patients and Caregivers
No formal, organized PK deficiency patient advocacy group to date
– Informal network of patients and caregivers are engaged online and via social media
Agios is organizing U.S. and EU patient ad boards for direct engagement and opportunity to listen/understand
If I say I’m tired, people think I need more sleep. . .I have fatigue so intense that it wakes me up at night.
How do you get someone to understand that?!
Think of a day when you are sick with a cold. That is me on my best day.
2
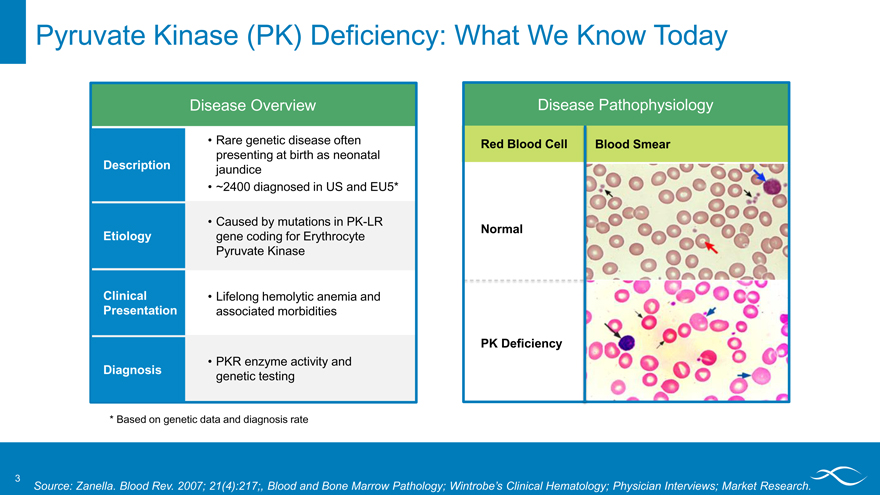
Pyruvate Kinase (PK) Deficiency: What We Know Today
Disease Overview
Description
Etiology
Clinical Presentation
Diagnosis
Rare genetic disease often presenting at birth as neonatal jaundice ~2400 diagnosed in US and EU5*
Caused by mutations in PK-LR gene coding for Erythrocyte Pyruvate Kinase
Lifelong hemolytic anemia and associated morbidities
PKR enzyme activity and genetic testing
Disease Pathophysiology
Red Blood Cell Blood Smear Normal PK Deficiency
* Based on genetic data and diagnosis rate
3
Source: Zanella. Blood Rev. 2007; 21(4):217;, Blood and Bone Marrow Pathology; Wintrobe’s Clinical Hematology; Physician Interviews; Market Research.
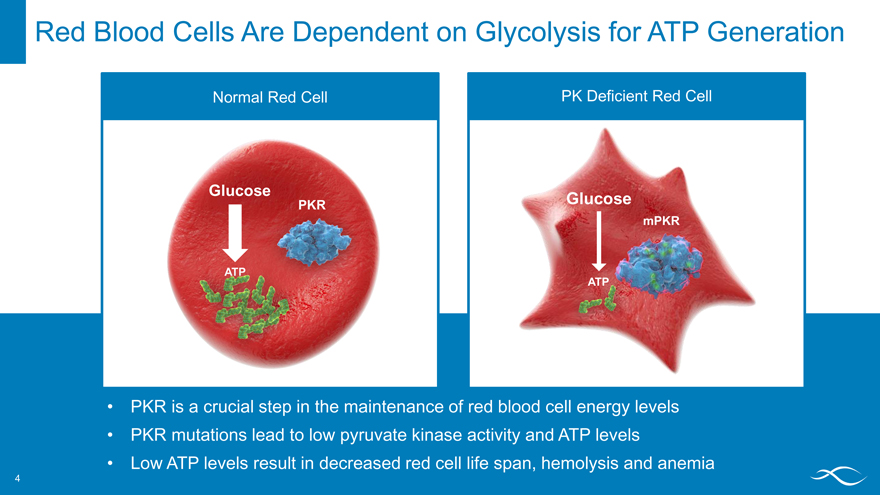
Red Blood Cells Are Dependent on Glycolysis for ATP Generation
Normal Red Cell PK Deficient Red Cell
PKR is a crucial step in the maintenance of red blood cell energy levels PKR mutations lead to low pyruvate kinase activity and ATP levels Low ATP levels result in decreased red cell life span, hemolysis and anemia
PKR ATP
Glucose
4
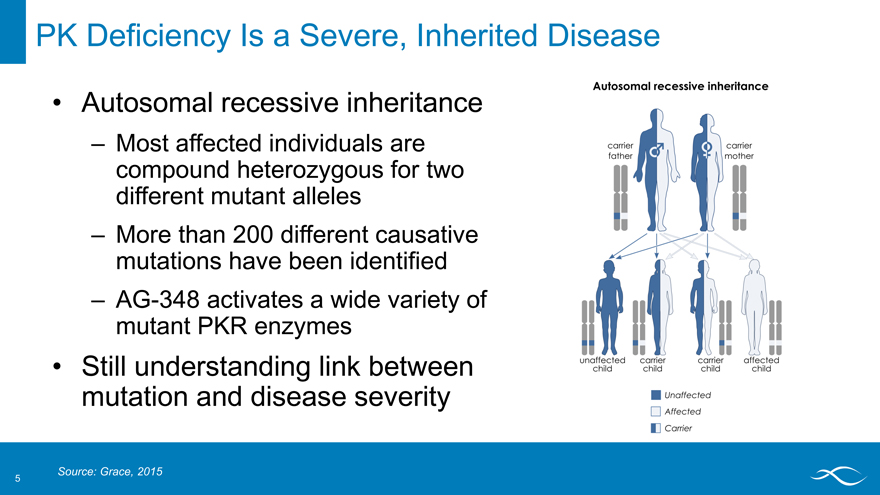
PK Deficiency Is a Severe, Inherited Disease
Autosomal recessive inheritance
– Most affected individuals are compound heterozygous for two different mutant alleles
– More than 200 different causative mutations have been identified
– AG-348 activates a wide variety of mutant PKR enzymes
Still understanding link between mutation and disease severity
Autosomal recessive Inheritance carrier father mother unaffected child
Source: Grace, 2015
5
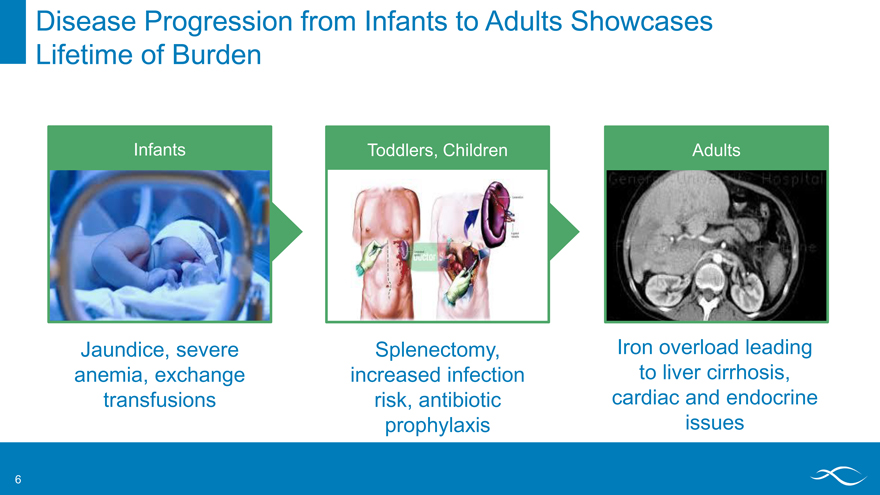
Disease Progression from Infants to Adults Showcases Lifetime of Burden
Infants Toddlers, Children Adults
Jaundice, severe anemia, exchange transfusions
Splenectomy, increased infection risk, antibiotic prophylaxis
Iron overload leading to liver cirrhosis, cardiac and endocrine issues
6
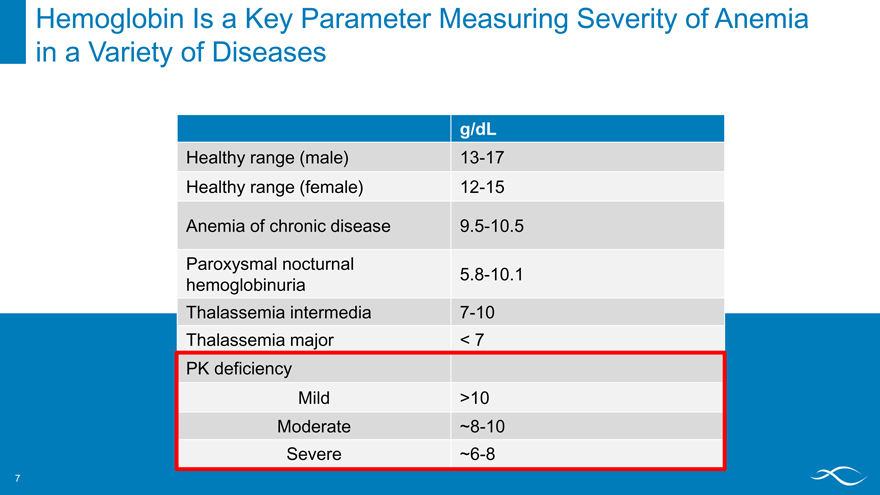
Hemoglobin Is a Key Parameter Measuring Severity of Anemia in a Variety of Diseases
g/dL
Healthy range (male) 13-17 Healthy range (female) 12-15
Anemia of chronic disease 9.5-10.5
Paroxysmal nocturnal
5.8-10.1 hemoglobinuria Thalassemia intermedia 7-10 Thalassemia major < 7 PK deficiency Mild >10 Moderate ~8-10 Severe ~6-8
7
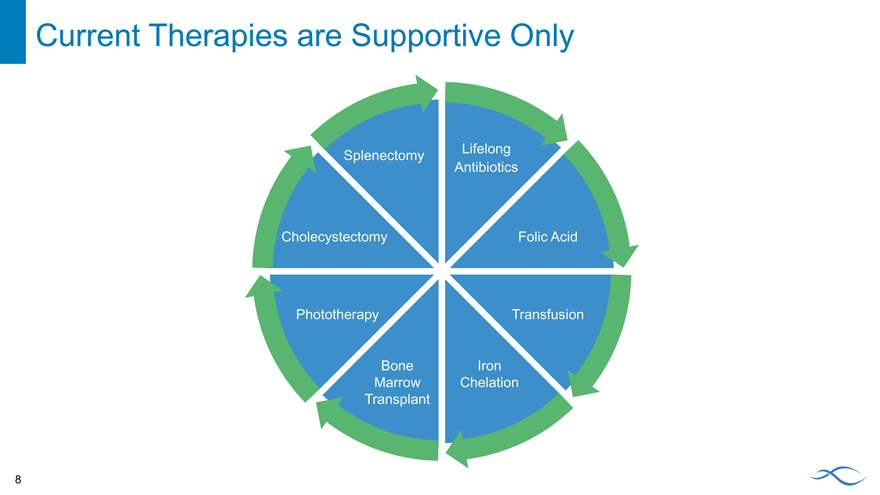
Current Therapies are Supportive Only
Lifelong Splenectomy Antibiotics
Cholecystectomy Folic Acid
Phototherapy Transfusion
Bone Iron Marrow Chelation Transplant
8
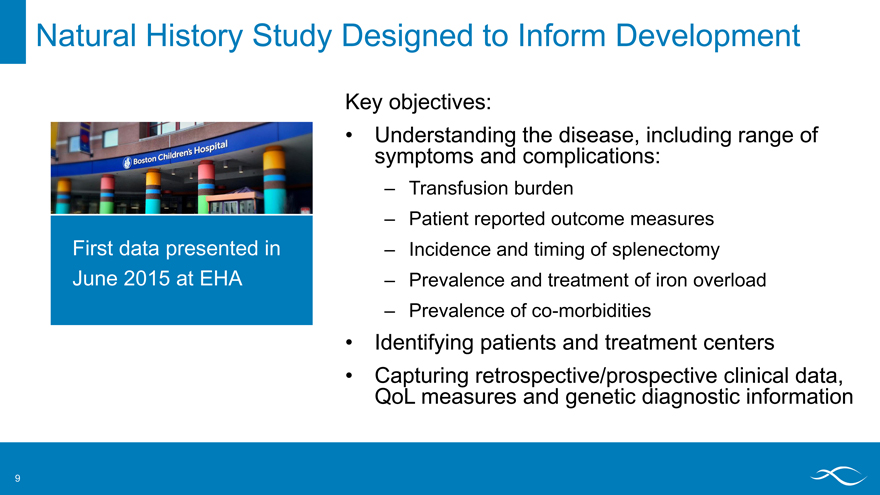
Natural History Study Designed to Inform Development
Key objectives:
Understanding the disease, including range of symptoms and complications:
– Transfusion burden
– Patient reported outcome measures
– Incidence and timing of splenectomy
– Prevalence and treatment of iron overload
– Prevalence of co-morbidities
Identifying patients and treatment centers
Capturing retrospective/prospective clinical data, QoL measures and genetic diagnostic information
9
Natural History Study Enrolling Patients at Treatment Centers Globally
Up to 250 participants of any age will be followed for at least two years As of September 2015: 189 patients in 27 sites
10
PK Deficiency is a Serious Disease with Varied, Frequent Complications
Baseline and retrospective data from 75 NHS patients Source : R. Grace, poster EHA 2015
Complications in PK Deficiency n=75
Gallstones and cholecystectomy frequent
Aplastic crises and thrombosis are rare, but potentially serious
% 80 60 40 20 0
splenectomy gallatones cholecystectomy aplastic crisis extramedullary hematopoiesis thrombosis chelation
11
Iron Overload Common in PK Deficiency Patients Across Severity Groups
Source : R. Grace, poster EHA 2015
Splenectomized
Upper Limit of Normal (~300 mcg/L)
12
Iron Overload Impacts Mortality
Symptoms of iron overload
Cardiac arrhythmias and cardiomyopathy Liver cirrhosis Testicular atrophy Diabetes Hepatomegaly Arthritis Bronze skin Memory loss Vertigo
Liver Pathology Normal vs. Iron Deposits
Normal Liver
Iron Deposition in the Liver
13
Takeaways for PK Deficiency
Key Learnings to Date
PK deficiency is a severe, rare hemolytic anemia Clinical spectrum ranges from mild to life-limiting Emerging picture of disease severity Current treatments are
Of limited efficacy
Often burdensome
Supportive only
Next data from the natural history study to be presented at ASH PK activation has the potential to be the first disease-altering therapy
Key Questions for the Future
What predicts severity? What is the long-term natural history of the disease? What insights can we get into the patient population?
What are the key complications and their impact on patients and caregivers?
14
Driving Forward Our PKR Activators:
Clinical and Regulatory Overview
October 16, 2015 Chris Bowden, M.D. Chief Medical Officer
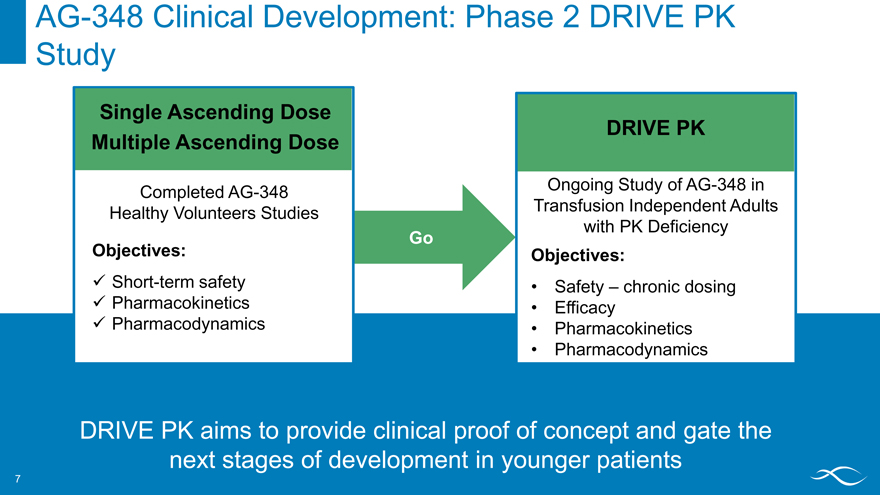
AG-348 Phase 1 Clinical Development in Healthy Volunteers
Completed Studies
Single Ascending Dose Multiple Ascending Dose
Objectives
Short-term safety Pharmacokinetics Pharmacodynamics Proof of mechanism
Healthy volunteer studies provide important guidance for longer trials in people with PK deficiency
2
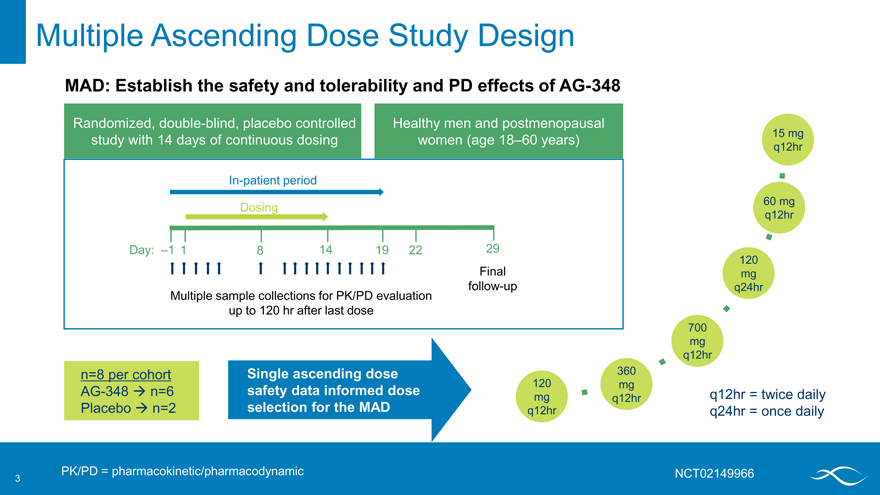
Multiple Ascending Dose Study Design
MAD: Establish the safety and tolerability and PD effects of AG-348
Randomized, double-blind, placebo controlled Healthy men and postmenopausal study with 14 days of continuous dosing women (age 18–60 years)
In-patient period
Dosing
Day: –1 1 8 14 19 22 29 Final follow-up Multiple sample collections for PK/PD evaluation up to 120 hr after last dose
n=8 per cohort Single ascending dose AG-348 ? n=6 safety data informed dose Placebo ? n=2 selection for the MAD
PK/PD = pharmacokinetic/pharmacodynamic
q12hr = twice daily q24hr = once daily
NCT02149966
15 mg q12hr
60 mg q12hr
120 mg q24hr
700 mg q12hr
360 mg q12hr
120 mg q12hr
3
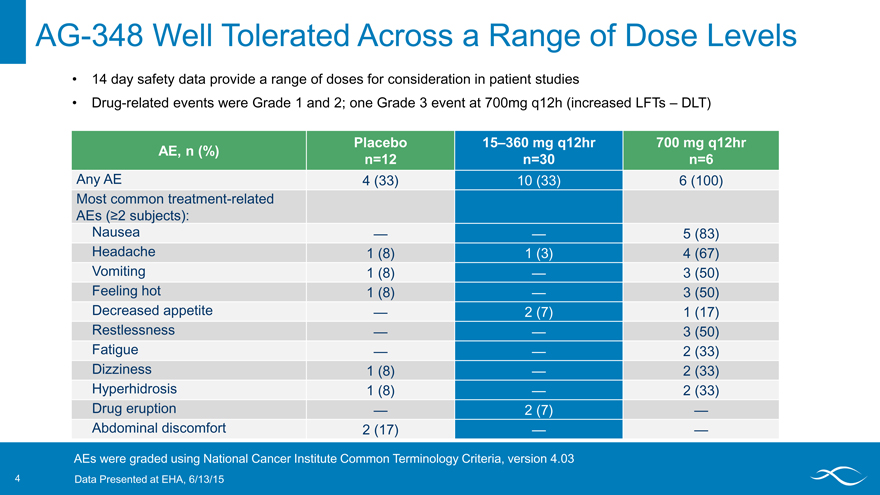
AG-348 Well Tolerated Across a Range of Dose Levels
14 day safety data provide a range of doses for consideration in patient studies
Drug-related events were Grade 1 and 2; one Grade 3 event at 700mg q12h (increased LFTs – DLT)
Placebo 15–360 mg q12hr 700 mg q12hr AE, n (%) n=12 n=30 n=6
Any AE 4 (33) 10 (33) 6 (100) Most common treatment-related
AEs (?2 subjects):
Nausea — — 5 (83) Headache 1 (8) 1 (3) 4 (67) Vomiting 1 (8) — 3 (50) Feeling hot 1 (8) — 3 (50) Decreased appetite — 2 (7) 1 (17) Restlessness — — 3 (50) Fatigue — — 2 (33) Dizziness 1 (8) — 2 (33) Hyperhidrosis 1 (8) — 2 (33) Drug eruption — 2 (7) — Abdominal discomfort 2 (17) — —
AEs were graded using National Cancer Institute Common Terminology Criteria, version 4.03
4 Data Presented at EHA, 6/13/15
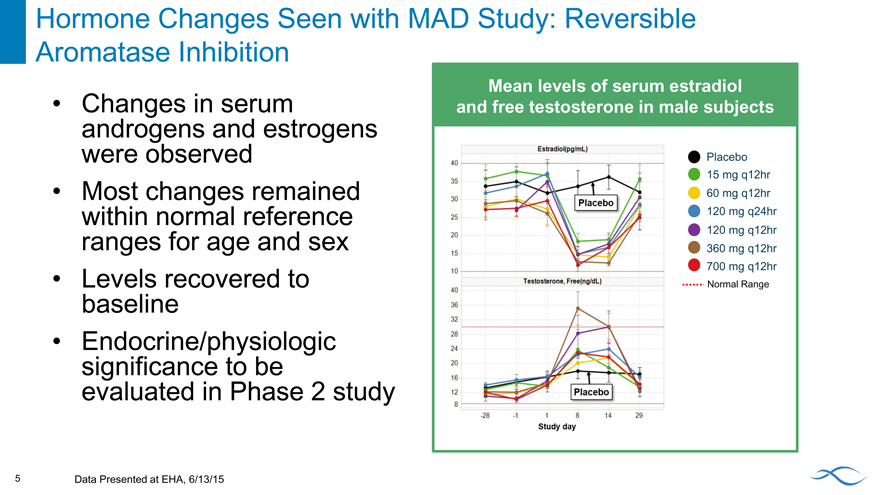
Hormone Changes Seen with MAD Study: Reversible Aromatase Inhibition
Mean levels of serum estradiol and free testosterone in male subjects
Changes in serum androgens and estrogens were observed Most changes remained within normal reference ranges for age and sex Levels recovered to baseline Endocrine/physiologic significance to be evaluated in Phase 2 study
Placebo 15 mg q12hr 60 mg q12hr 120 mg q24hr 120 mg q12hr 360 mg q12hr 700 mg q12hr
Normal Range
5 Data Presented at EHA, 6/13/15
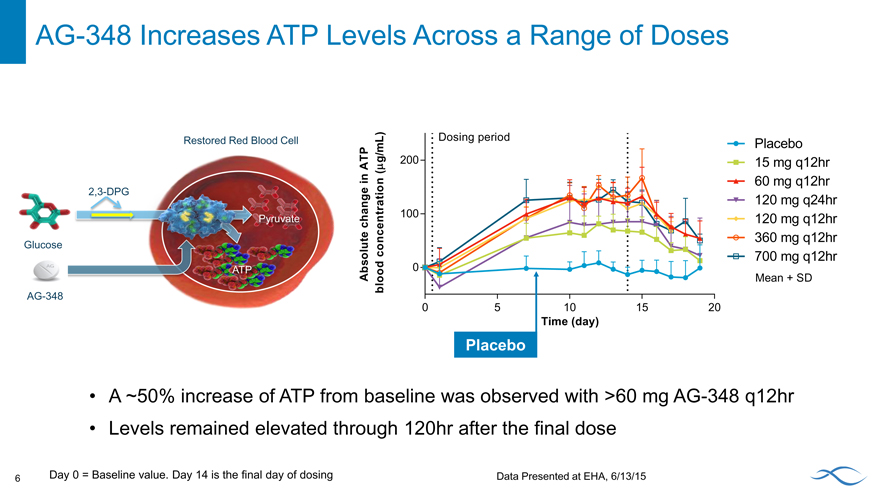
AG-348 Increases ATP Levels Across a Range of Doses
A ~50% increase of ATP from baseline was observed with >60 mg AG-348 q12hr Levels remained elevated through 120hr after the final dose
6 Day 0 = Baseline value. Day 14 is the final day of dosing Data Presented at EHA, 6/13/15
Placebo 15 mg q12hr 60 mg q12hr 120 mg q24hr 120 mg q12hr 360 mg q12hr 700 mg q12hr
Mean + SD
Restored Red Blood Cell
2,3-DPG
Gluco
AG-348
Absolute change in ATP blood concentration (mg/mL)
AG-348 Clinical Development: Phase 2 DRIVE PK
Study
Single Ascending Dose
DRIVE PK Multiple Ascending Dose
Completed AG-348 Ongoing Study of AG-348 in Healthy Volunteers Studies Transfusion Independent Adults with PK Deficiency
Go
Objectives: Objectives:
? Short-term safety • Safety – chronic dosing ? Pharmacokinetics • Efficacy ? Pharmacodynamics • Pharmacokinetics
Pharmacodynamics
DRIVE PK aims to provide clinical proof of concept and gate the next stages of development in younger patients
7
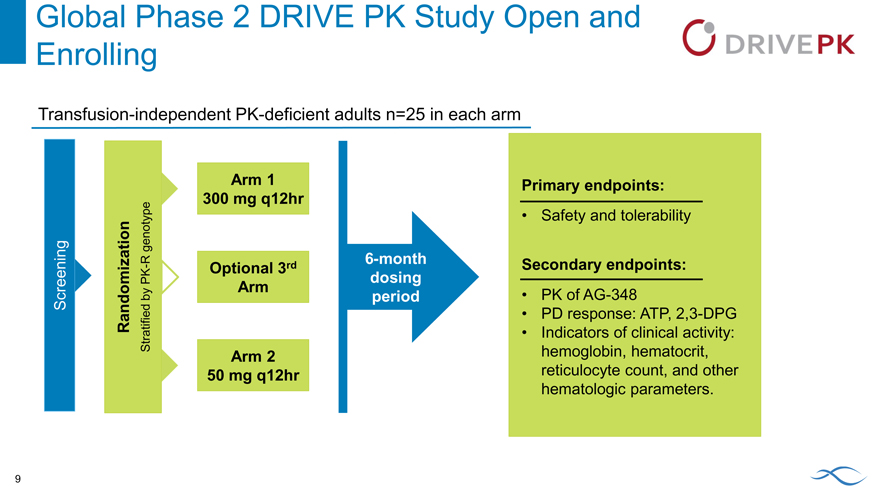
Global Phase 2 DRIVE PK Study Open and
Enrolling
Transfusion-independent PK-deficient adults n=25 in each arm
Arm 1 300 mg q12hr
genotype R
PK—Optional 3rd by Arm Screening Randomization Stratified Arm 2 50 mg q12hr
Global Phase 2 DRIVE PK Study Open and
Enrolling
Transfusion-independent PK-deficient adults n=25 in each arm
Arm 1 300 mg q12hr
genotype
R 6-month
- Optional 3rd
PK dosing by Arm period Screening Randomization Stratified Arm 2 50 mg q12hr
Primary endpoints:
Safety and tolerability
Secondary endpoints:
PK of AG-348
PD response: ATP, 2,3-DPG
Indicators of clinical activity: hemoglobin, hematocrit, reticulocyte count, and other hematologic parameters.
9
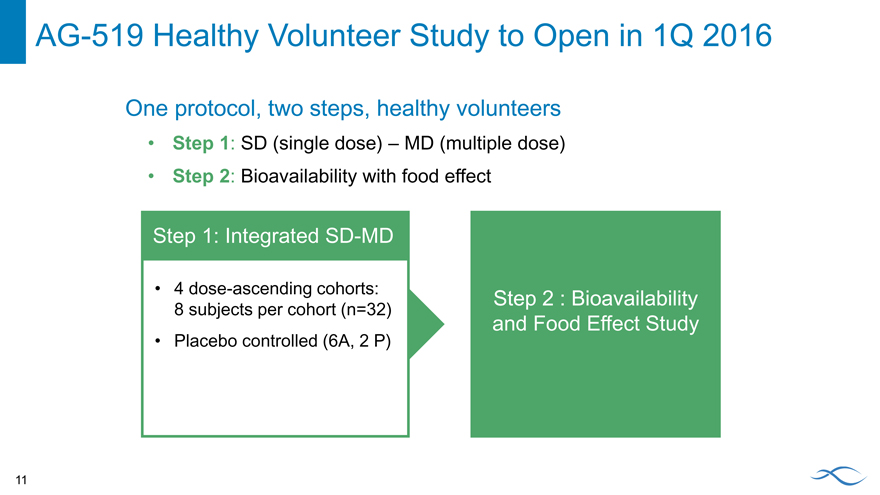
Follow-on PKR Activator AG-519
AG-519 is a potent, highly selective and orally bioavailable PKR activator Differentiated chemical structure versus AG-348 No activity against the aromatase enzyme AG-519 has similar activity in vitro, in vivo and ex vivo (patient samples) relative to AG-348 Clinical studies planned to initiate in 1Q 2016
10
AG-519 Healthy Volunteer Study to Open in 1Q 2016
One protocol, two steps, healthy volunteers
Step 1: SD (single dose) – MD (multiple dose)
Step 2: Bioavailability with food effect
Step 1: Integrated SD-MD
4 dose-ascending cohorts:
Step 2 : Bioavailability
8 subjects per cohort (n=32)
and Food Effect Study
Placebo controlled (6A, 2 P)
11
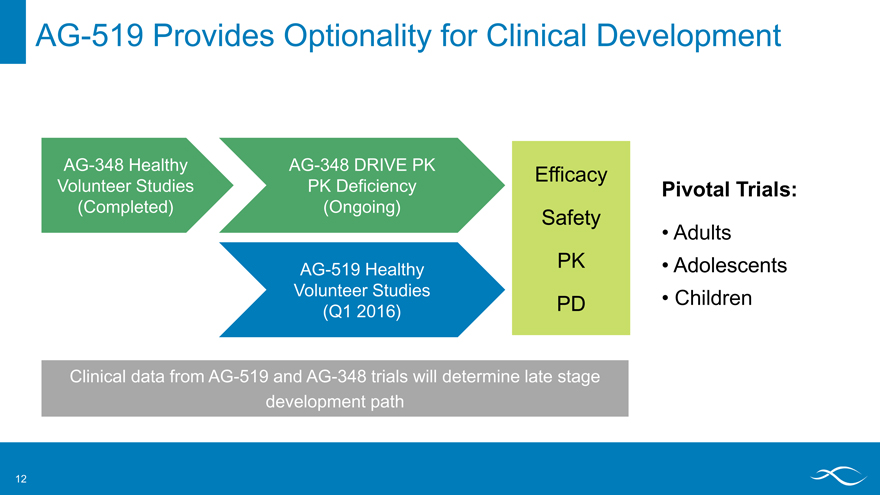
AG-519 Provides Optionality for Clinical Development
AG-348 Healthy AG-348 DRIVE PK
Efficacy
Volunteer Studies PK Deficiency Pivotal Trials: (Completed) (Ongoing)
Safety
Adults
AG-519 Healthy PK • Adolescents Volunteer Studies • Children (Q1 2016) PD
Clinical data from AG-519 and AG-348 trials will determine late stage development path
12
Takeaways: PK Deficiency Clinical Development
Key Learnings to Date
AG-348 activates PKR in humans
AG-348 set the path for DRIVE PK, which is on track and enrolling
AG-519 increases flexibility in the clinic with multiple assets with the potential to target multiple indications
Key Questions for the Future
How is long-term activation of PKR tolerated?
How do PKR mutations impact efficacy?
What are the effects of PKR activation on other measures of clinical benefit?
Iron deposition
Hemolysis
What are the best approaches to capture outcomes most important to patients?
13
Agios Discovery Strategies
October 16, 2015
Scott A. Biller, Ph.D.
Chief Scientific Officer
Leveraging Our Scientific Competencies: “The Engine” 2
Agios Core Capabilities
“Disruptive Science”
Cancer Metabolism
Untapped target space
Precision medicine strategy
Genetic- or metabolic-biomarkers for patient selection
Rare Genetic Metabolic Disorders
Clear causation by defined genetic lesions
Novel small molecule approach to correct the underlying dysfunction
Five first-in-class investigational medicines with deep pipeline
What You’ll Hear Today 3
New therapeutic opportunities for PK activators in other hemolytic anemias
A precision medicine strategy catalyzes progress in our oncology portfolio
Potential for extending of our platform to other therapeutic opportunities
What’s Possible for PK Activators 4
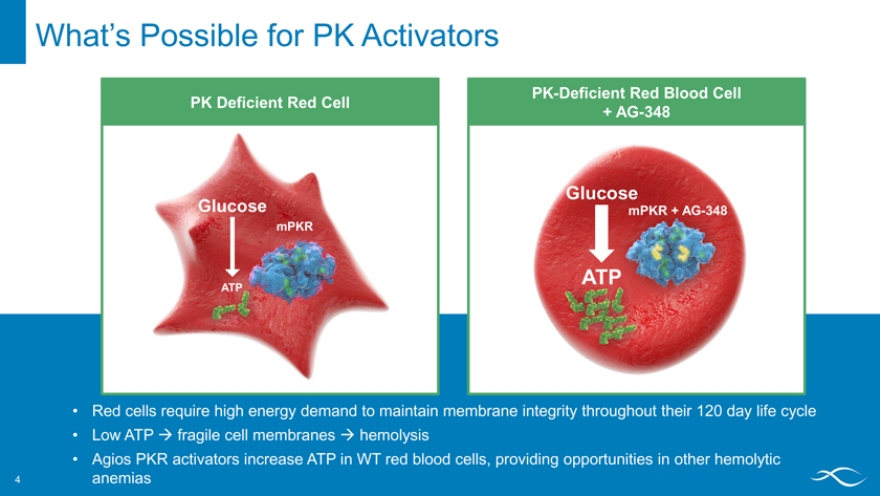
PK Deficient Red Cell
Glucose
mPKR
PK-Deficient Red Blood Cell
+ AG-348
Glucose
mPKR + AG-348
ATP
Red cells require high energy demand to maintain membrane integrity throughout their 120 day life cycle
Low ATP ? fragile cell membranes ? hemolysis
Agios PKR activators increase ATP in WT red blood cells, providing opportunities in other hemolytic anemias
What’s Possible for PK Activators 5
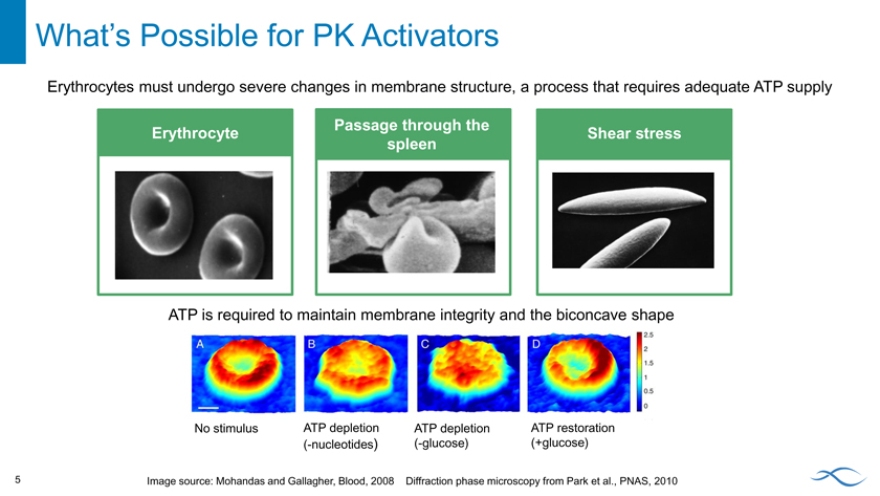
Erythrocytes must undergo severe changes in membrane structure, a process that requires adequate ATP supply
Erythrocyte
Passage through the spleen
Shear stress
ATP is required to maintain membrane integrity and the biconcave shape
No stimulus ATP depletion ATP depletion ATP restoration (-nucleotides) (-glucose) (+glucose)
Image source: Mohandas and Gallagher, Blood, 2008 Diffraction phase microscopy from Park et al., PNAS, 2010
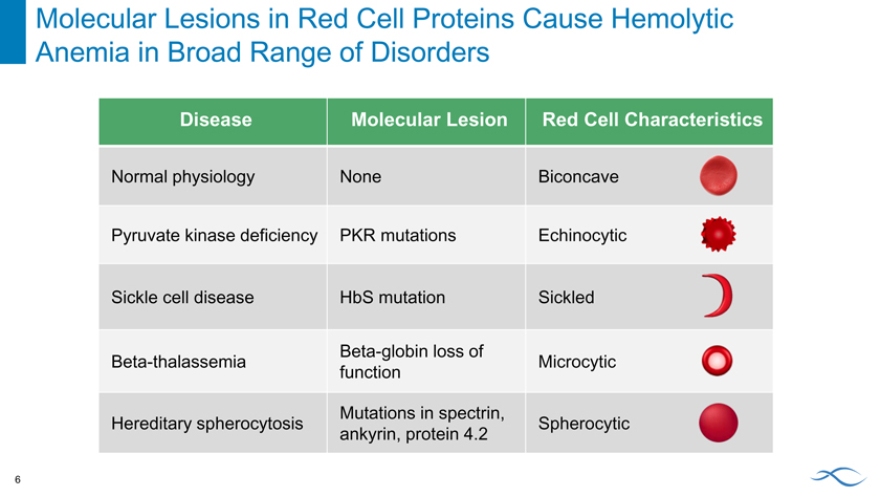
Molecular Lesions in Red Cell Proteins Cause Hemolytic Anemia in Broad Range of Disorders 6
Disease Molecular Lesion Red Cell Characteristics
Normal physiology None Biconcave Pyruvate kinase deficiency PKR mutations Echinocytic Sickle cell disease HbS mutation Sickled
Beta-globin loss of
Beta-thalassemia Microcytic function
Mutations in spectrin,
Hereditary spherocytosis Spherocytic ankyrin, protein 4.2
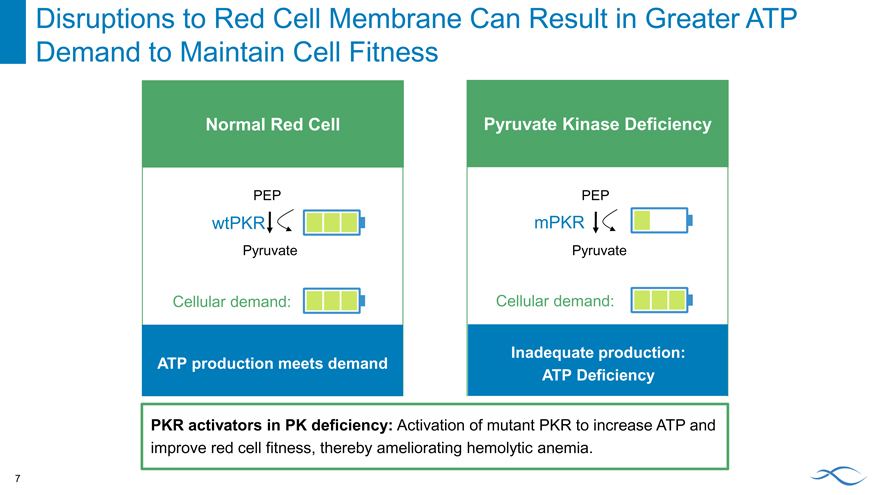
Disruptions to Red Cell Membrane Can Result in Greater ATP Demand to Maintain Cell Fitness 7
Normal Red Cell
PEP
wtPKR
Pyruvate
Cellular demand:
ATP production meets demand
Pyruvate Kinase Deficiency
PEP
mPKR
Pyruvate
Cellular demand:
Inadequate production: ATP Deficiency
PKR activators in PK deficiency: Activation of mutant PKR to increase ATP and improve red cell fitness, thereby ameliorating hemolytic anemia.
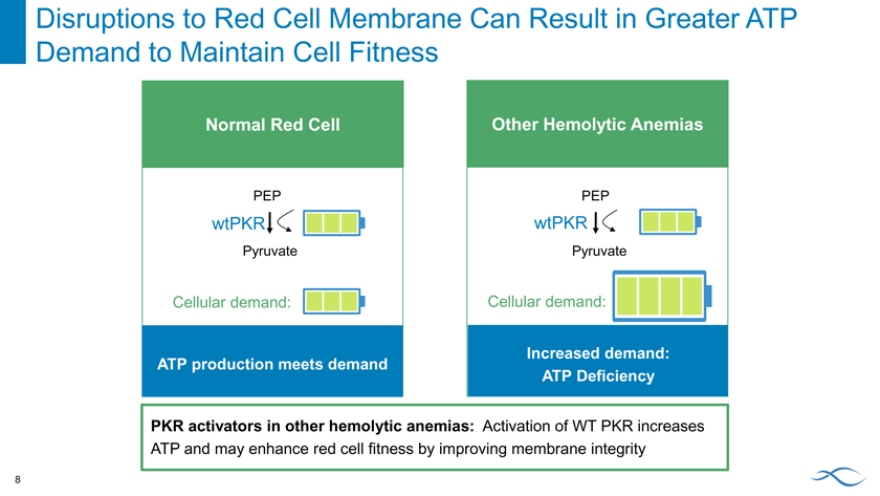
Disruptions to Red Cell Membrane Can Result in Greater ATP Demand to Maintain Cell Fitness 8
Normal Red Cell
PEP
wtPKR
Pyruvate
Cellular demand:
ATP production meets demand
Other Hemolytic Anemias
PEP
wtPKR
Pyruvate
Cellular demand:
Increased demand: ATP Deficiency
PKR activators in other hemolytic anemias: Activation of WT PKR increases
ATP and may enhance red cell fitness by improving membrane integrity
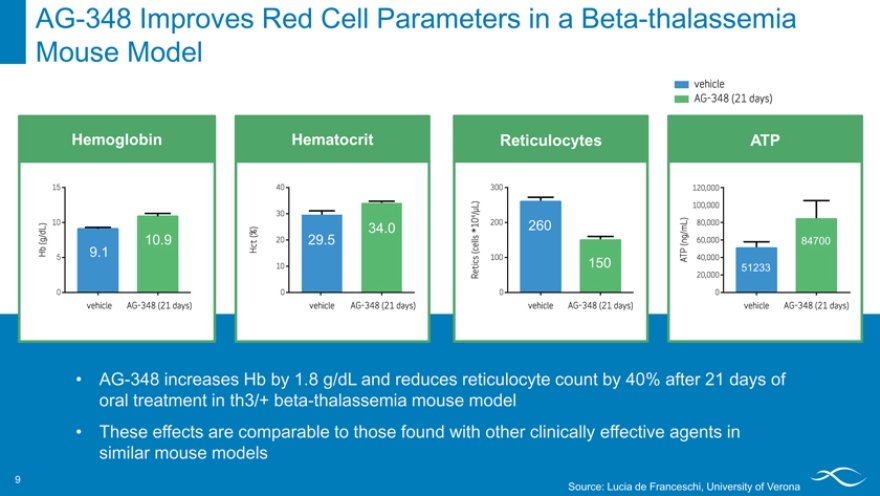
AG-348 Improves Red Cell Parameters in a Beta-thalassemia Mouse Model 9
Hemoglobin
9.1 10.9
Hematocrit
34.0 29.5
Reticulocytes
260
150
ATP
84700
51233
AG-348 increases Hb by 1.8 g/dL and reduces reticulocyte count by 40% after 21 days of oral treatment in th3/+ beta-thalassemia mouse model
These effects are comparable to those found with other clinically effective agents in similar mouse models
Source: Lucia de Franceschi, University of Verona
Evolving Portfolio in Cancer Metabolism
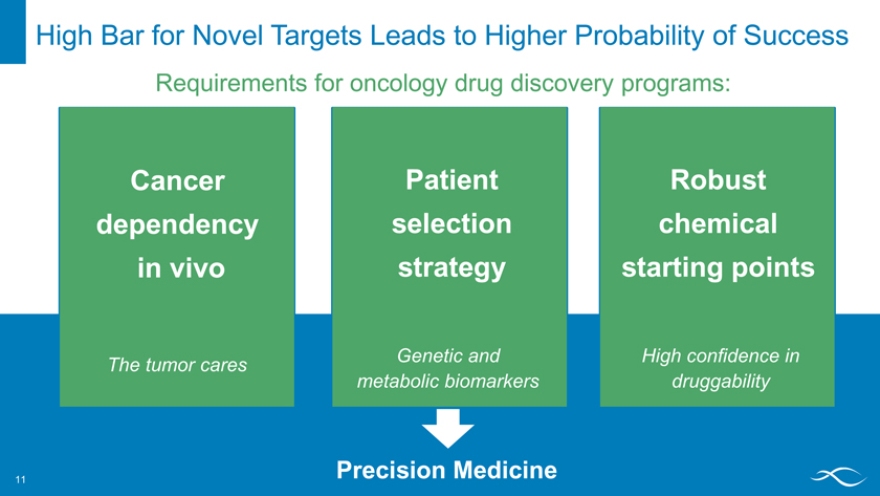
High Bar for Novel Targets Leads to Higher Probability of Success 11
Requirements for oncology drug discovery programs:
Cancer dependency in vivo
The tumor cares
Patient selection strategy
Genetic and metabolic biomarkers
Robust chemical starting points
High confidence in druggability
Precision Medicine
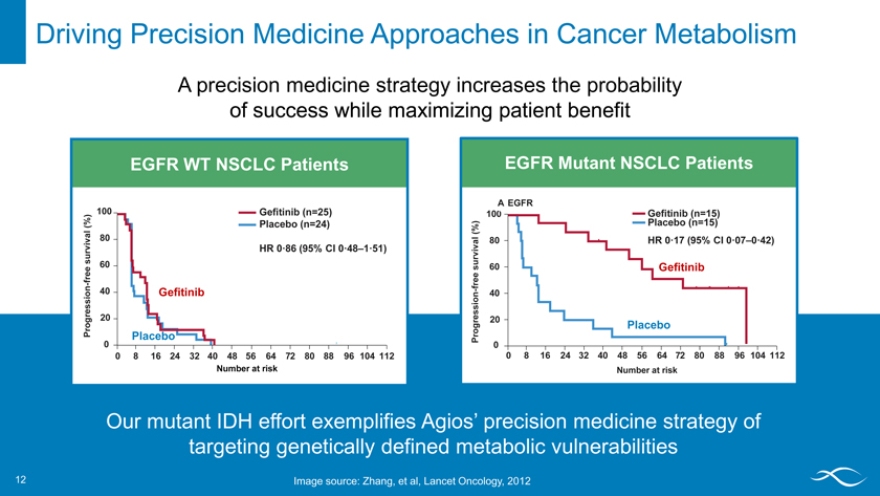
Driving Precision Medicine Approaches in Cancer Metabolism 12
A precision medicine strategy increases the probability of success while maximizing patient benefit
EGFR WT NSCLC Patients
100 Ge?tinib (n=25)
(%) Placebo (n=24) ival 80 v sur HR 0·86 (95% CI 0·48–1·51) e 60 fr e
n—40 Gefitinib
i o ess Progr 20
Placebo
0
0256248 16 32 40 48 64 7 80 88 96 104 112
Number at risk
EGFR Mutant NSCLC Patients
A EGFR
100 Ge?tinib (n=15)
% ) Placebo (n=15)
( al 80 HR 0·17 (95% CI 0·07–0·42) ur viv
s 60 Gefitinib
fre e
- 40 gression 20
o Placebo
Pr
0
0256248 16 32 40 48 64 7 80 88 96 104 112
Number at risk
Our mutant IDH effort exemplifies Agios’ precision medicine strategy of targeting genetically defined metabolic vulnerabilities
Image source: Zhang, et al, Lancet Oncology, 2012
The Metabolome: Untapped Opportunity for Novel Precision Medicines 13
Combining the metabolome and the genome provides insight into metabolic vulnerabilities
? Mutations in metabolic genes or metabolic regulators ? Tumor specific isoforms ? Deletions in metabolic genes
? Lineage dependence
? Fusions of metabolic genes
The Metabolome: Untapped Opportunity for Novel Precision Medicines 14
Combining the metabolome and the genome provides insight into metabolic vulnerabilities
? Mutations in metabolic genes or metabolic regulators ? Tumor specific isoforms
? Deletions in metabolic genes
? Lineage dependence
? Fusions of metabolic genes
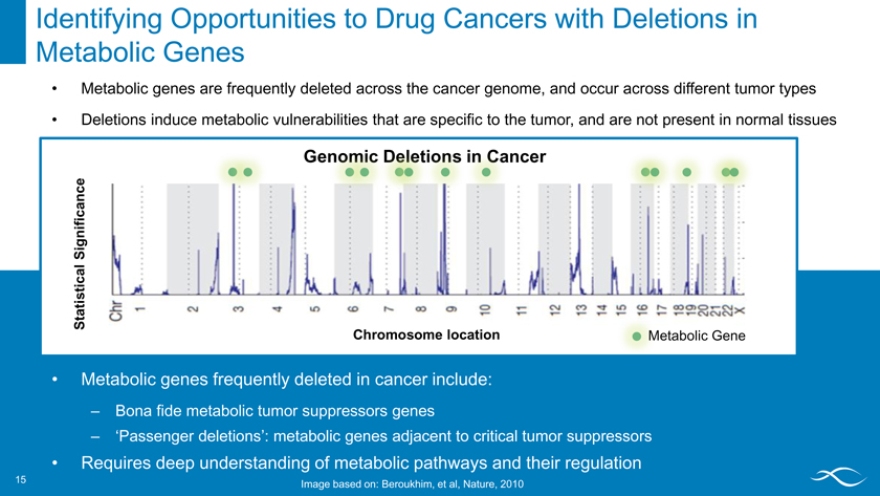
Identifying Opportunities to Drug Cancers with Deletions in Metabolic Genes 15
Metabolic genes are frequently deleted across the cancer genome, and occur across different tumor types
Deletions induce metabolic vulnerabilities that are specific to the tumor, and are not present in normal tissues
ncer
Significance Statistical
Chromosome location Metabolic Gene
Metabolic genes frequently deleted in cancer include:
– Bona fide metabolic tumor suppressors genes
– ‘Passenger deletions’: metabolic genes adjacent to critical tumor suppressors
Requires deep understanding of metabolic pathways and their regulation
15 Image based on: Beroukhim, et al, Nature, 2010
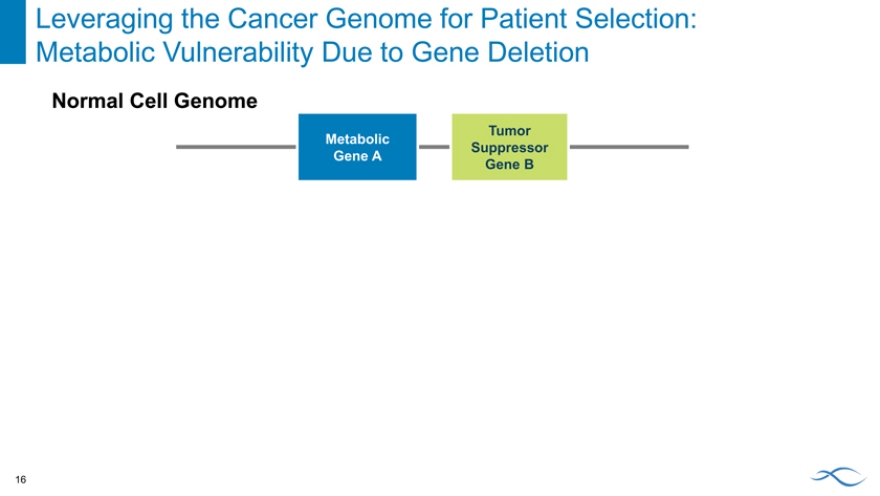
Leveraging the Cancer Genome for Patient Selection: Metabolic Vulnerability Due to Gene Deletion 16
Normal Cell Genome
Tumor Metabolic Suppressor Gene A
Gene B
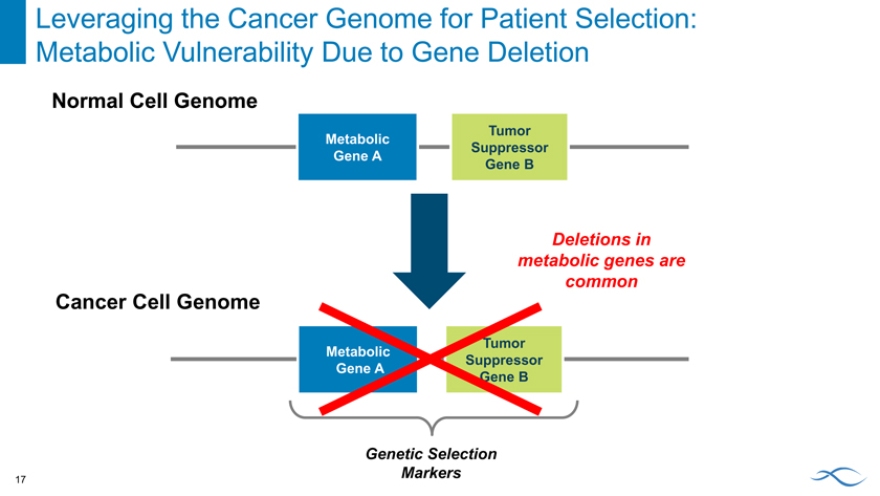
Leveraging the Cancer Genome for Patient Selection: Metabolic Vulnerability Due to Gene Deletion
Normal Cell Genome
Tumor Metabolic Suppressor Gene A
Gene B
Deletions in metabolic genes are common
Cancer Cell Genome
Tumor Metabolic Suppressor Gene A
Gene B
Genetic Selection Markers
17
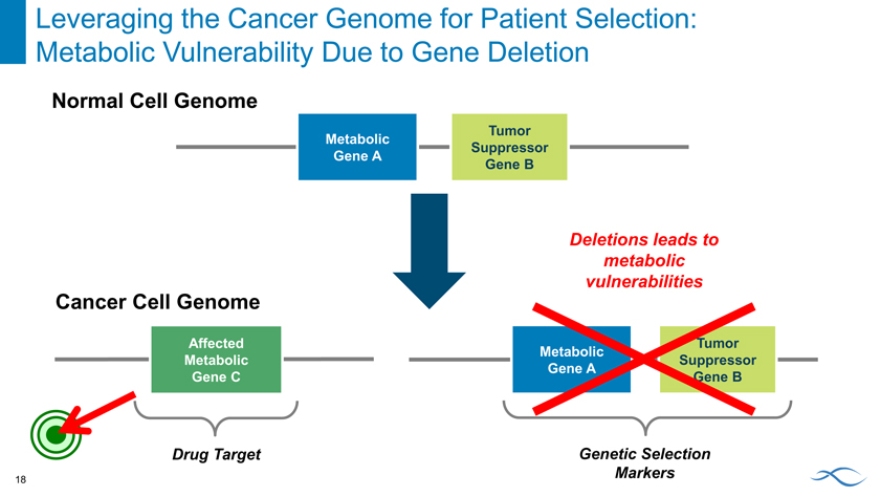
Leveraging the Cancer Genome for Patient Selection: Metabolic Vulnerability Due to Gene Deletion
Normal Cell Genome
Tumor Metabolic Suppressor Gene A
Gene B
Deletions leads to metabolic vulnerabilities
Cancer Cell Genome
Affected Tumor Metabolic Metabolic Suppressor Gene A
Gene C Gene B
Drug Target Genetic Selection Markers
18
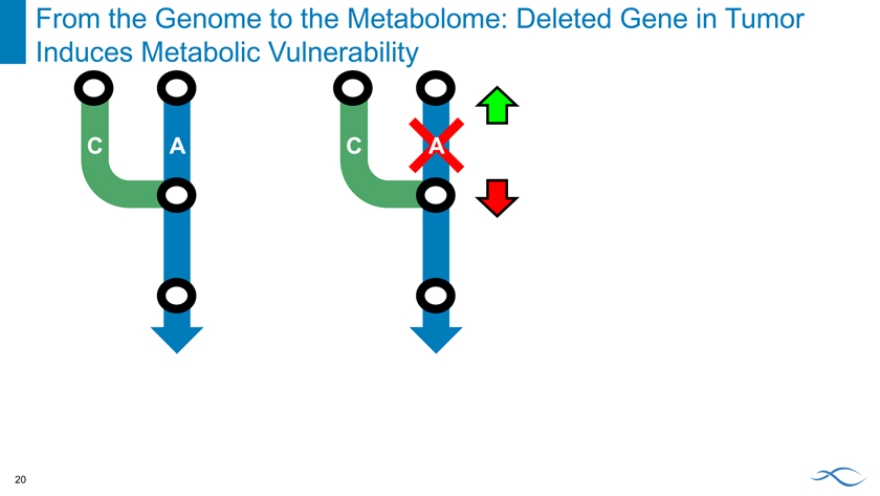
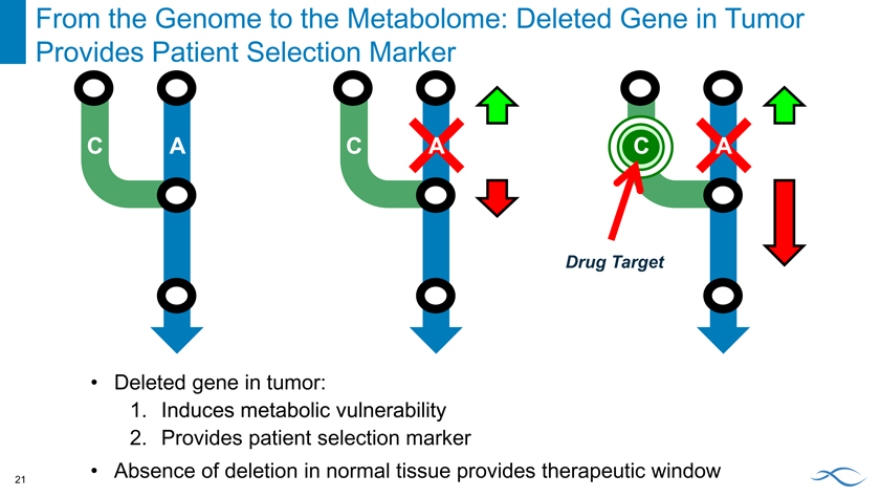
From the Genome to the Metabolome: Deleted Gene in Tumor Induces Metabolic Vulnerability
C A
From the Genome to the Metabolome: Deleted Gene in Tumor Induces Metabolic Vulnerability
C A C A
From the Genome to the Metabolome: Deleted Gene in Tumor Provides Patient Selection Marker
C A C A C A
Drug Target
Deleted gene in tumor:
1. Induces metabolic vulnerability
2. Provides patient selection marker
Absence of deletion in normal tissue provides therapeutic window
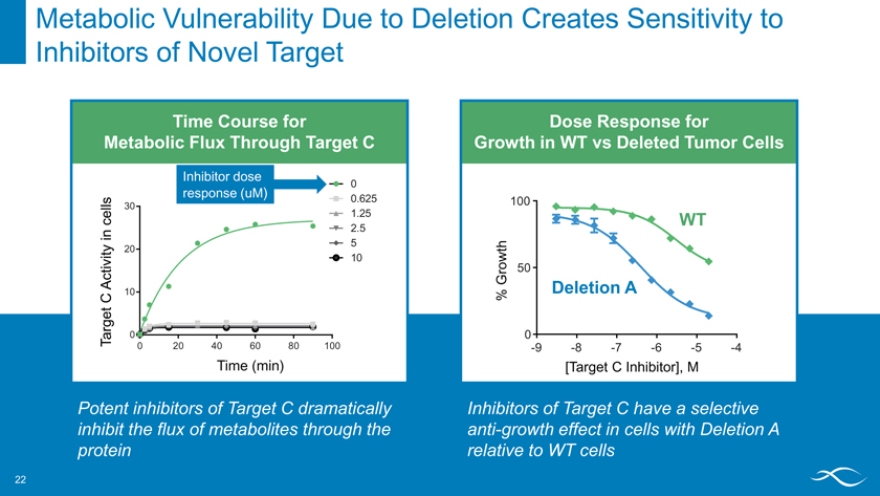
Metabolic Vulnerability Due to Deletion Creates Sensitivity to Inhibitors of Novel Target
Time Course for
Metabolic Flux Through Target C
Inhibitor dose
0
response (uM)
0.625
30
1.25 2.5 5
20 10 10
0
0 20406080 100
Time (min)
Potent inhibitors of Target C dramatically inhibit the flux of metabolites through the protein
Dose Response for
Growth in WT vs Deleted Tumor Cells
100
WT
t h r ow 50
G Deletion A
%
0
-9 -8 -7 -6 -5 -4
[Target C Inhibitor], M
Inhibitors of Target C have a selective anti-growth effect in cells with Deletion A relative to WT cells
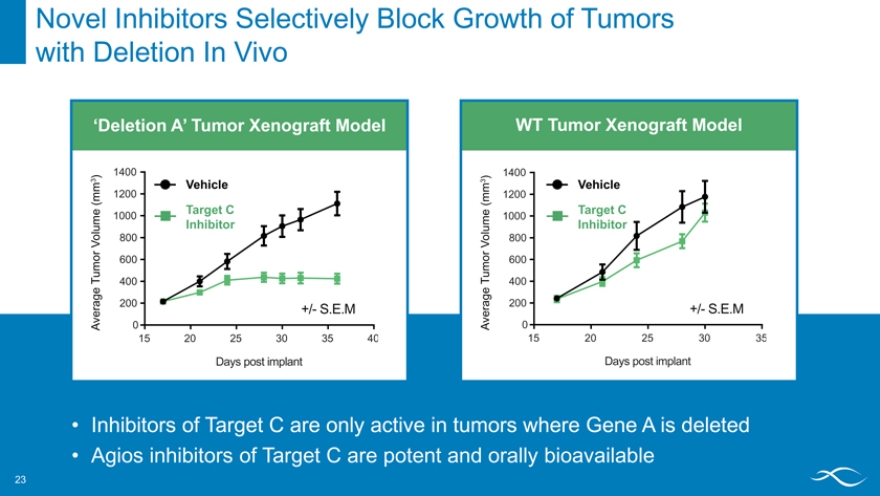
Novel Inhibitors Selectively Block Growth of Tumors with Deletion In Vivo
‘Deletion A’ Tumor HCT-116 Xenograft MTAP -/- Model
1400
) 3 Vehicle
(mm 1200
Target C
1000
Inhibitor
Volume 800 or
600 u m
T 400
erage 200 +/- S.E.M
A v 0
15 20 25 30 35 40
Days post implant
WT Tumor HCT-116 Xenograft MTAP +/+ Model
1400
) 3 m 1200 Vehicle
(m
Target C me 1000 u Inhibitor o l 800
V o r
600 u m
T 400
rage 200 +/- S.E.M
v e
A 0
15 20 25 30 35
Days post implant
Inhibitors of Target C are only active in tumors where Gene A is deleted
Agios inhibitors of Target C are potent and orally bioavailable
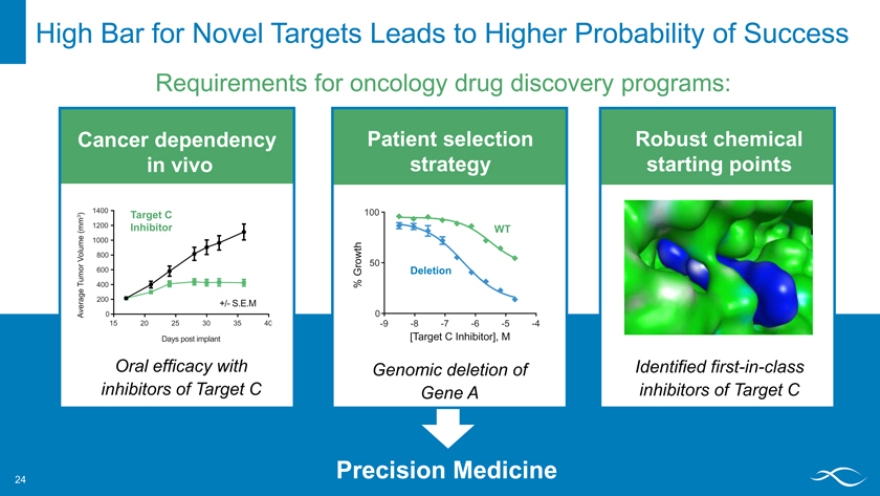
High Bar for Novel Targets Leads to Higher Probability of Success
Requirements for oncology drug discovery programs:
Cancer dependency in vivo
HCT-116 MTAP -/-
1400
) 3 Target C (mm 1200 Inhibitor
me 1000
V olu 800 or
600
Tum 400 ge a er 200 +/- S.E.M
Av 0
15 20 25 30 35 40
Days post implant
Oral efficacy with inhibitors of Target C
Patient selection strategy
100
WT wt h ro 50
G Deletion
%
0
-9 -8 -7 -6 -5 -4
[Target C Inhibitor], M
Genomic deletion of Gene A
Robust chemical starting points
Identified first-in-class inhibitors of Target C
Precision Medicine
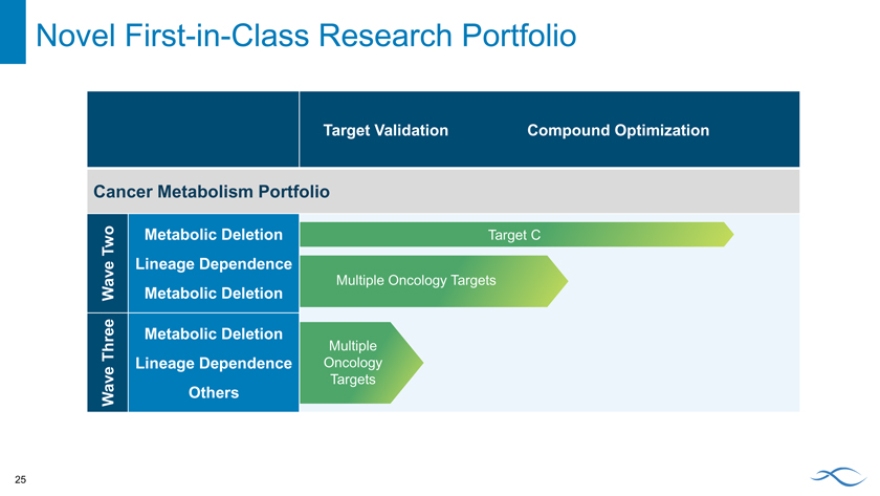
Novel First-in-Class Research Portfolio
Target Validation Compound Optimization
Cancer Metabolism Portfolio
Two Metabolic Deletion Target C
Lineage Dependence
Wave Metabolic Deletion Multiple Oncology Targets
Metabolic Deletion
Three Multiple
Lineage Dependence Oncology
Wave Others Targets
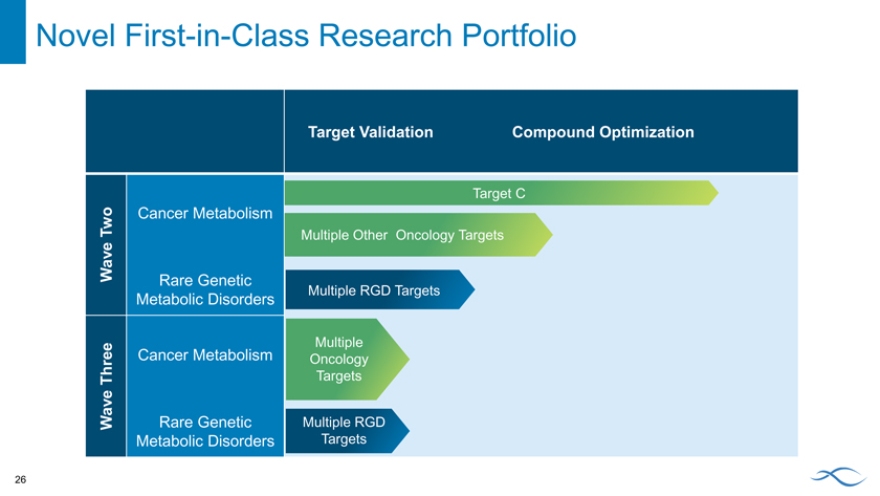
Novel First-in-Class Research Portfolio
Target Validation Compound Optimization
Target C
Two Cancer Metabolism
Multiple Other Oncology Targets
Wave
Rare Genetic
Multiple RGD Targets
Metabolic Disorders
Cancer Metabolism Multiple
Oncology Three Targets
Wave Rare Genetic Multiple RGD
Metabolic Disorders Targets
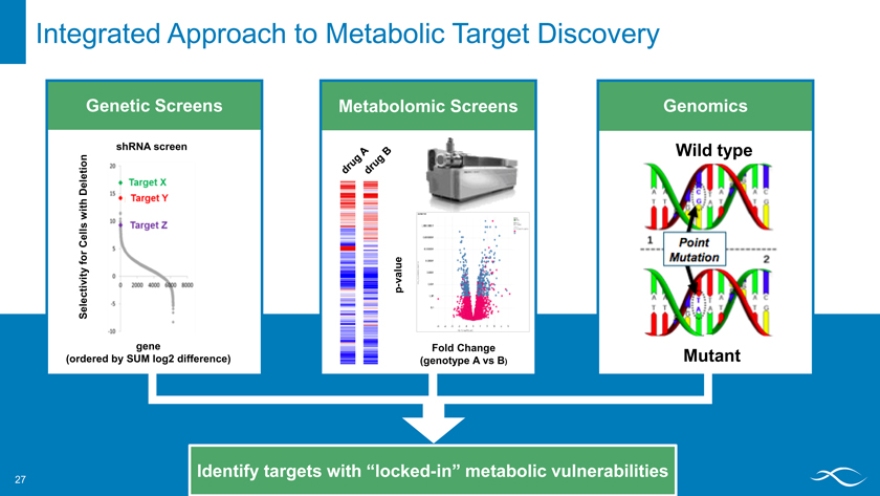
Integrated Approach to Metabolic Target Discovery
Genetic Screens Metabolomic Screens Genomics
shRNA screen
Deletion Wild type
with Cells for value ctivity p -Sele gene Fold Change
(ordered by SUM log2 difference) (genotype A vs B) Mutant
Identify targets with “locked-in” metabolic vulnerabilities
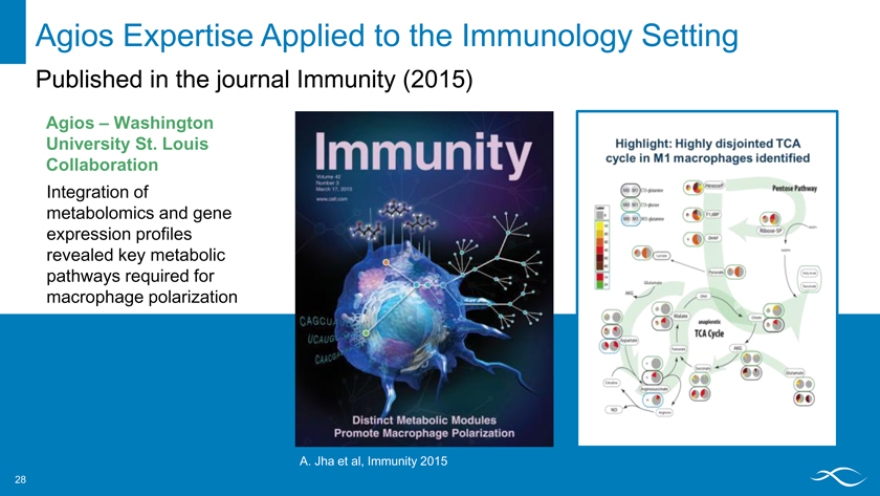
Agios Expertise Applied to the Immunology Setting
Published in the journal Immunity (2015)
Agios – Washington University St. Louis Collaboration
Integration of metabolomics and gene expression profiles revealed key metabolic pathways required for macrophage polarization
A. Jha et al, Immunity 2015
Key Takeaways: Drug Discovery Strategy
Precision medicine strategy increases the probability of technical success while maximizing patient benefit
Marrying the genome and metabolome enables a portfolio of novel precision medicine targets
PK activators may have utility in other hemolytic anemias, with promising results for beta-thalassemia intermedia
Deletions of metabolic genes in tumors provides exciting new therapeutic opportunities with high therapeutic windows
Science and toolkit provide opportunities in other disease areas
Closing Remarks
October 16, 2015 David Schenkein, M.D. Chief Executive Officer
Making a Difference for Patients & Building Long-Term Value
Vision Science
Team/Culture Execution
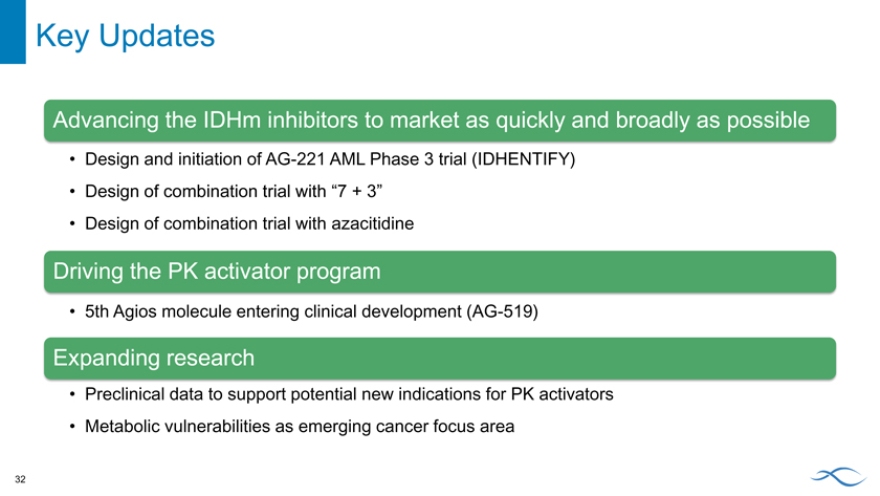
Key Updates
Advancing the IDHm inhibitors to market as quickly and broadly as possible
Design and initiation of AG-221 AML Phase 3 trial (IDHENTIFY)
Design of combination trial with “7 + 3”
Design of combination trial with azacitidine
Driving the PK activator program
5th Agios molecule entering clinical development (AG-519)
Expanding research
Preclinical data to support potential new indications for PK activators
Metabolic vulnerabilities as emerging cancer focus area
What We Hope You Came Away with Today
Confident that we are leading the way in the disruptive field of dysregulated metabolism
Clear that we are building world class clinical development and commercial capabilities on the foundation of our innovative research expertise
Enthusiastic in your commitment to help us build a great biopharmaceutical company
Passionate in our common mission of changing the lives of patients and creating significant shareholder value
Q&A