
J.P. Morgan Healthcare Conference Agios Pharmaceuticals Brian Goff, Chief Executive Officer January 15, 2025 Exhibit 99.2

Forward-Looking Statements This presentation and various remarks we make during this presentation contain forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. Such forward-looking statements include those regarding the potential benefits of PYRUKYND® (mitapivat), tebapivat (AG-946), AG-236 and AG-181, Agios’ PAH stabilizer; Agios’ plans, strategies and expectations for its preclinical, clinical and commercial advancement of its drug development, including PYRUKYND®, tebapivat, AG-181 and AG-236; the submission of PYRUKYND ® to regulators for approval in alpha-and-beta thalassemia; Agios’ strategic vision and goals, including its key milestones for 2025; and the potential benefits of Agios’ strategic plans and focus. The words “anticipate”, “expect”, “goal”, “hope”, “milestone”, “opportunity”, “plan”, “potential”, “possible”, “strategy”, “will”, “vision”, and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Such statements are subject to numerous important factors, risks and uncertainties that may cause actual events or results to differ materially from Agios’ current expectations and beliefs. For example, there can be no guarantee that any product candidate Agios is developing will successfully commence or complete necessary preclinical and clinical development phases, or that development of any of Agios’ product candidates will successfully continue. There can be no guarantee that any positive developments in Agios’ business will result in stock price appreciation. Management's expectations and, therefore, any forward-looking statements in this presentation and various remarks we make during this presentation could also be affected by risks and uncertainties relating to a number of other important factors, including, without limitation: risks and uncertainties related to the impact of pandemics or other public health emergencies to Agios’ business, operations, strategy, goals and anticipated milestones, including its ongoing and planned research activities, ability to conduct ongoing and planned clinical trials, clinical supply of current or future drug candidates, commercial supply of current or future approved products, and launching, marketing and selling current or future approved products; Agios’ results of clinical trials and preclinical studies, including subsequent analysis of existing data and new data received from ongoing and future studies; the content and timing of decisions made by the U.S. FDA, the EMA or other regulatory authorities, investigational review boards at clinical trial sites and publication review bodies; Agios’ ability to obtain and maintain requisite regulatory approvals and to enroll patients in its planned clinical trials; unplanned cash requirements and expenditures; competitive factors; Agios' ability to obtain, maintain and enforce patent and other intellectual property protection for any product candidates it is developing; Agios’ ability to establish and maintain key collaborations; uncertainty regarding any royalty payments related to the sale of its oncology business or any milestone or royalty payments related to its in-licensing of AG-236, and the uncertainty of the timing of any such payments; uncertainty of the results and effectiveness of the use of Agios’ cash and cash equivalents; and general economic and market conditions. These and other risks are described in greater detail under the caption "Risk Factors" included in Agios’ public filings with the Securities and Exchange Commission. Any forward-looking statements contained in this presentation and various remarks we make during this presentation speak only as of the date hereof, and Agios expressly disclaims any obligation to update any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law.

Fueled by Connections to Transform Rare Diseases All individuals featured are real patients who have been compensated for their time.
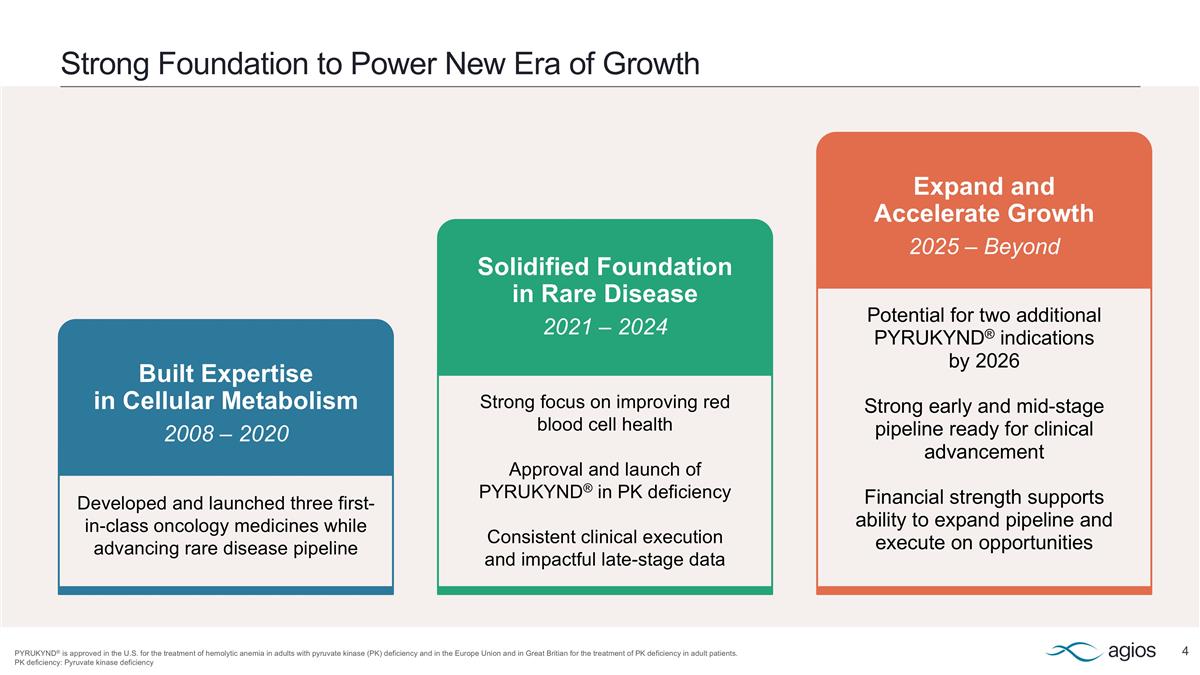
Strong Foundation to Power New Era of Growth PYRUKYND® is approved in the U.S. for the treatment of hemolytic anemia in adults with pyruvate kinase (PK) deficiency and in the Europe Union and in Great Britian for the treatment of PK deficiency in adult patients. PK deficiency: Pyruvate kinase deficiency Built Expertise in Cellular Metabolism 2008 – 2020 Developed and launched three first-in-class oncology medicines while advancing rare disease pipeline Solidified Foundation in Rare Disease 2021 – 2024 Strong focus on improving red blood cell health Approval and launch of PYRUKYND® in PK deficiency Consistent clinical execution and impactful late-stage data Expand and Accelerate Growth 2025 – Beyond Potential for two additional PYRUKYND® indications by 2026 Strong early and mid-stage pipeline ready for clinical advancement Financial strength supports ability to expand pipeline and execute on opportunities
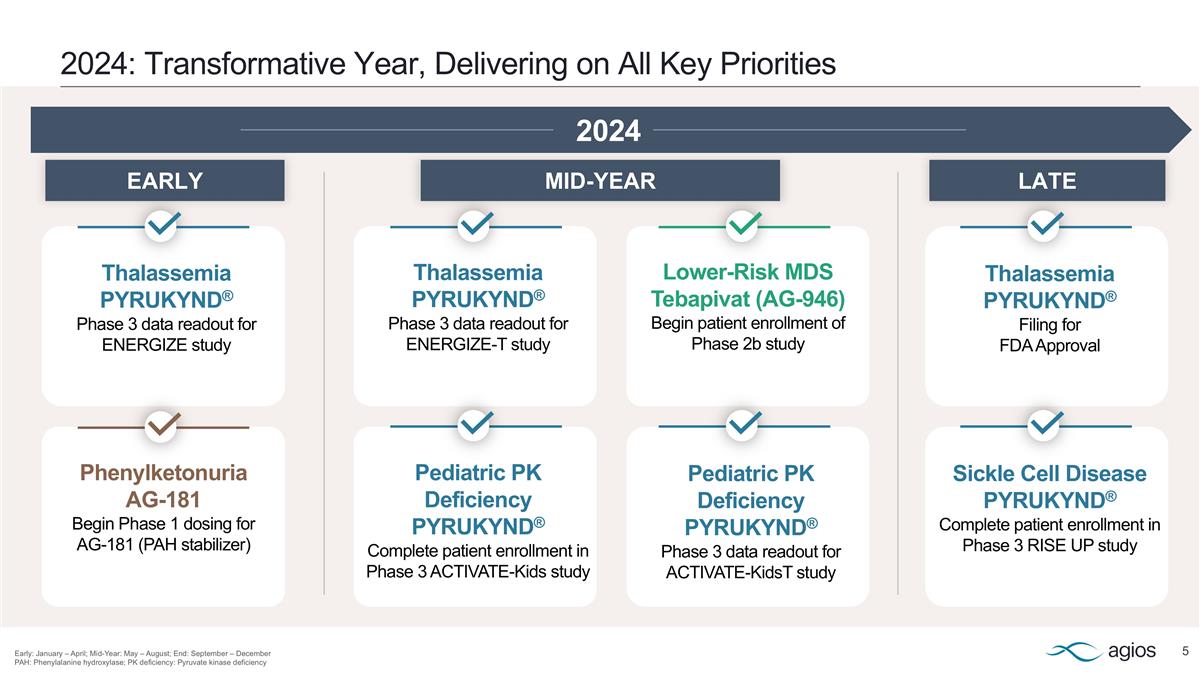
2024: Transformative Year, Delivering on All Key Priorities Early: January – April; Mid-Year: May – August; End: September – December PAH: Phenylalanine hydroxylase; PK deficiency: Pyruvate kinase deficiency Lower-Risk MDS Tebapivat (AG-946) Begin patient enrollment of Phase 2b study Thalassemia PYRUKYND® Filing for FDA Approval Thalassemia PYRUKYND® Phase 3 data readout for ENERGIZE-T study Thalassemia PYRUKYND® Phase 3 data readout for ENERGIZE study Pediatric PK Deficiency PYRUKYND® Complete patient enrollment in Phase 3 ACTIVATE-Kids study Phenylketonuria AG-181 Begin Phase 1 dosing for AG-181 (PAH stabilizer) Sickle Cell Disease PYRUKYND® Complete patient enrollment in Phase 3 RISE UP study Pediatric PK Deficiency PYRUKYND® Phase 3 data readout for ACTIVATE-KidsT study 2024 LATE MID-YEAR EARLY
A Rare Blueprint for Success Multi-Billion-Dollar Market Opportunity with PYRUKYND® Robust Pipeline, Rich with Near-Term Catalysts Proven Executional Excellence, Powered by Highly Experienced Team Strong Financial Position
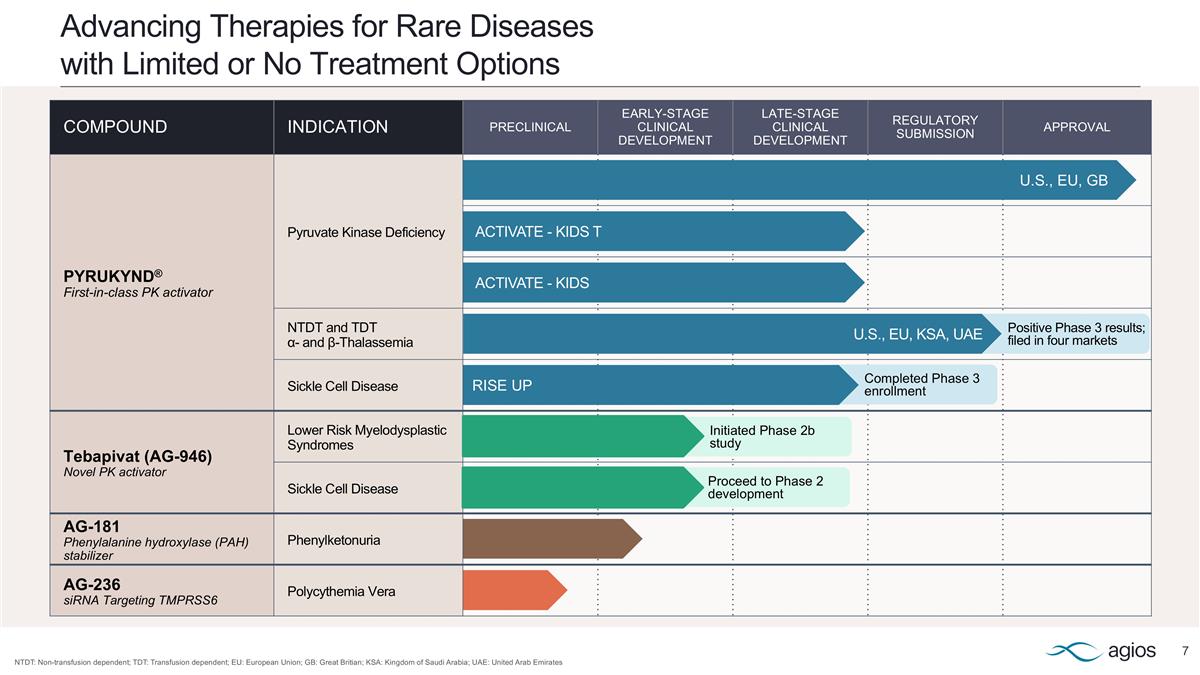
COMPOUND INDICATION PRECLINICAL EARLY-STAGE CLINICAL DEVELOPMENT LATE-STAGE CLINICAL DEVELOPMENT REGULATORY SUBMISSION APPROVAL PYRUKYND® First-in-class PK activator Pyruvate Kinase Deficiency NTDT and TDT α- and β-Thalassemia Sickle Cell Disease Tebapivat (AG-946) Novel PK activator Lower Risk Myelodysplastic Syndromes Sickle Cell Disease AG-181 Phenylalanine hydroxylase (PAH) stabilizer Phenylketonuria AG-236 siRNA Targeting TMPRSS6 Polycythemia Vera Advancing Therapies for Rare Diseases with Limited or No Treatment Options NTDT: Non-transfusion dependent; TDT: Transfusion dependent; EU: European Union; GB: Great Britian; KSA: Kingdom of Saudi Arabia; UAE: United Arab Emirates U.S., EU, GB ACTIVATE - KIDS T ACTIVATE - KIDS Positive Phase 3 results; filed in four markets Completed Phase 3 enrollment Initiated Phase 2b study Proceed to Phase 2 development U.S., EU, KSA, UAE RISE UP
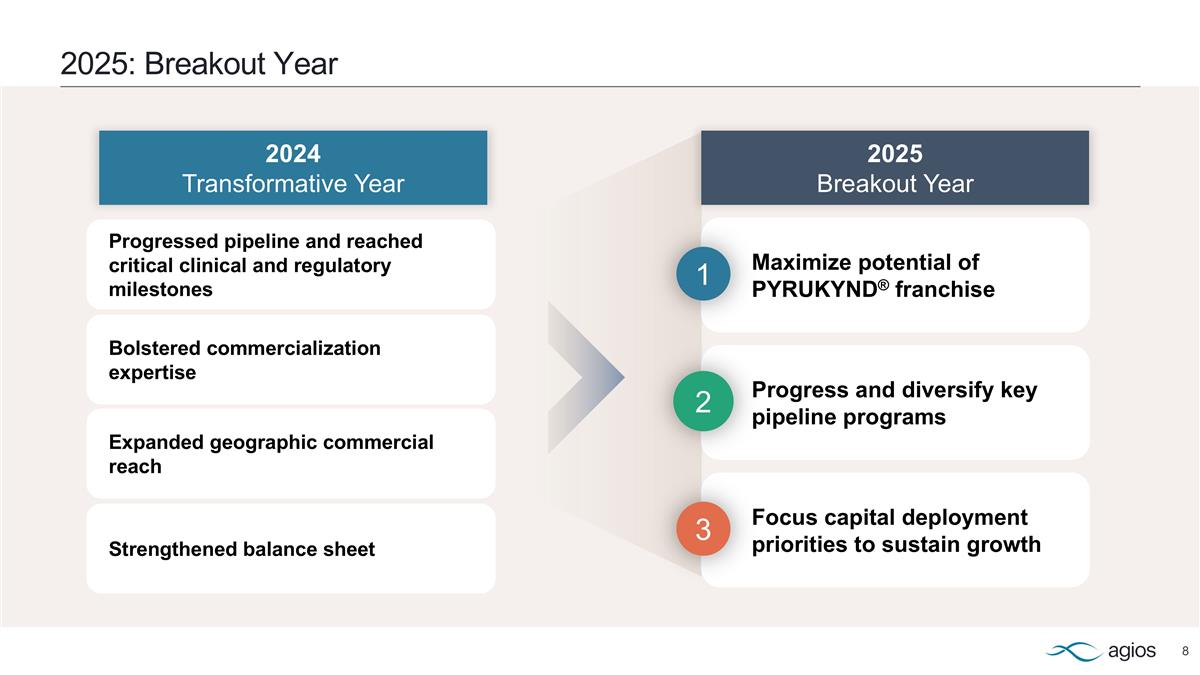
2025: Breakout Year Maximize potential of PYRUKYND® franchise Progress and diversify key pipeline programs Focus capital deployment priorities to sustain growth Progressed pipeline and reached critical clinical and regulatory milestones Expanded geographic commercial reach Bolstered commercialization expertise Strengthened balance sheet 2025 Breakout Year 2024 Transformative Year 1 2 3

Maximize Potential of PYRUKYND® Franchise Laurice Living with thalassemia, and her son, Ben 1
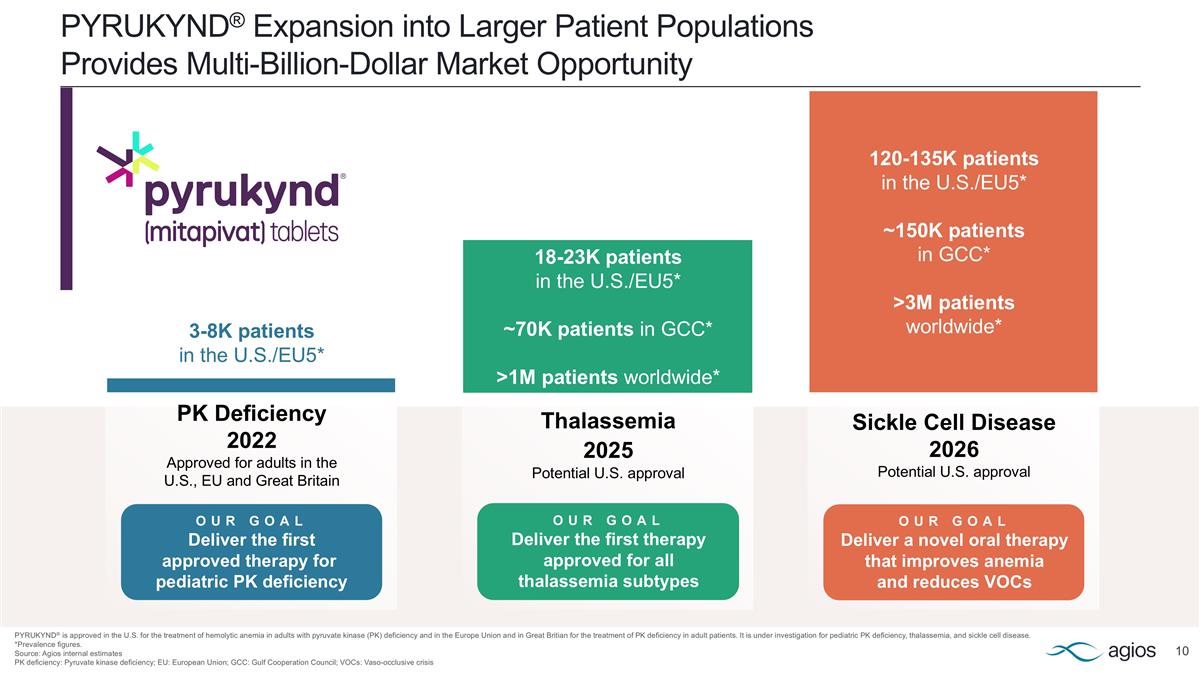
PYRUKYND® Expansion into Larger Patient Populations Provides Multi-Billion-Dollar Market Opportunity PYRUKYND® is approved in the U.S. for the treatment of hemolytic anemia in adults with pyruvate kinase (PK) deficiency and in the Europe Union and in Great Britian for the treatment of PK deficiency in adult patients. It is under investigation for pediatric PK deficiency, thalassemia, and sickle cell disease. *Prevalence figures. Source: Agios internal estimates PK deficiency: Pyruvate kinase deficiency; EU: European Union; GCC: Gulf Cooperation Council; VOCs: Vaso-occlusive crisis Sickle Cell Disease 2026 Potential U.S. approval 120-135K patients in the U.S./EU5* ~150K patients in GCC* >3M patients worldwide* OUR GOAL Deliver a novel oral therapy that improves anemia and reduces VOCs 18-23K patients in the U.S./EU5* ~70K patients in GCC* >1M patients worldwide* OUR GOAL Deliver the first therapy approved for all thalassemia subtypes Thalassemia 2025 Potential U.S. approval PK Deficiency 2022 Approved for adults in the U.S., EU and Great Britain OUR GOAL Deliver the first approved therapy for pediatric PK deficiency 3-8K patients in the U.S./EU5*
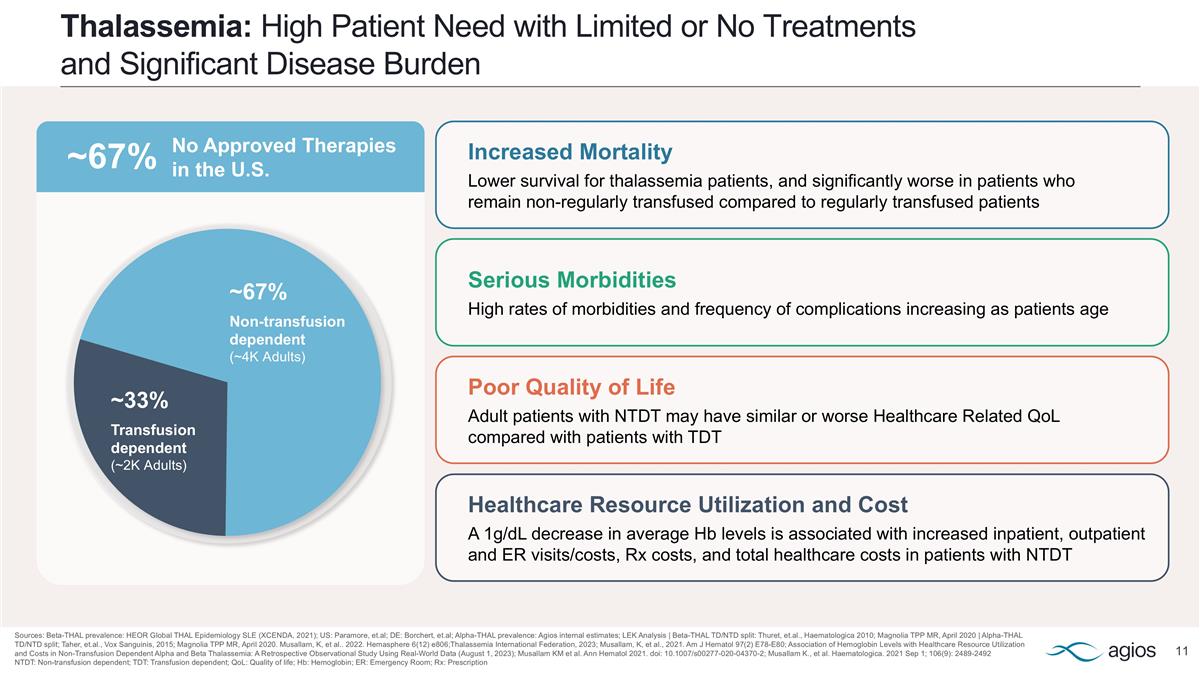
Thalassemia: High Patient Need with Limited or No Treatments and Significant Disease Burden Sources: Beta-THAL prevalence: HEOR Global THAL Epidemiology SLE (XCENDA, 2021); US: Paramore, et.al; DE: Borchert, et.al; Alpha-THAL prevalence: Agios internal estimates; LEK Analysis | Beta-THAL TD/NTD split: Thuret, et.al., Haematologica 2010; Magnolia TPP MR, April 2020 | Alpha-THAL TD/NTD split; Taher, et.al., Vox Sanguinis, 2015; Magnolia TPP MR, April 2020. Musallam, K, et al.. 2022. Hemasphere 6(12) e806;Thalassemia International Federation, 2023; Musallam, K, et al., 2021. Am J Hematol 97(2) E78-E80; Association of Hemoglobin Levels with Healthcare Resource Utilization and Costs in Non-Transfusion Dependent Alpha and Beta Thalassemia: A Retrospective Observational Study Using Real-World Data (August 1, 2023); Musallam KM et al. Ann Hematol 2021. doi: 10.1007/s00277-020-04370-2; Musallam K., et al. Haematologica. 2021 Sep 1; 106(9): 2489-2492 NTDT: Non-transfusion dependent; TDT: Transfusion dependent; QoL: Quality of life; Hb: Hemoglobin; ER: Emergency Room; Rx: Prescription Healthcare Resource Utilization and Cost A 1g/dL decrease in average Hb levels is associated with increased inpatient, outpatient and ER visits/costs, Rx costs, and total healthcare costs in patients with NTDT Serious Morbidities High rates of morbidities and frequency of complications increasing as patients age Poor Quality of Life Adult patients with NTDT may have similar or worse Healthcare Related QoL compared with patients with TDT Increased Mortality Lower survival for thalassemia patients, and significantly worse in patients who remain non-regularly transfused compared to regularly transfused patients ~67% Non-transfusion dependent (~4K Adults) ~33% Transfusion dependent (~2K Adults) ~67% No Approved Therapies in the U.S.
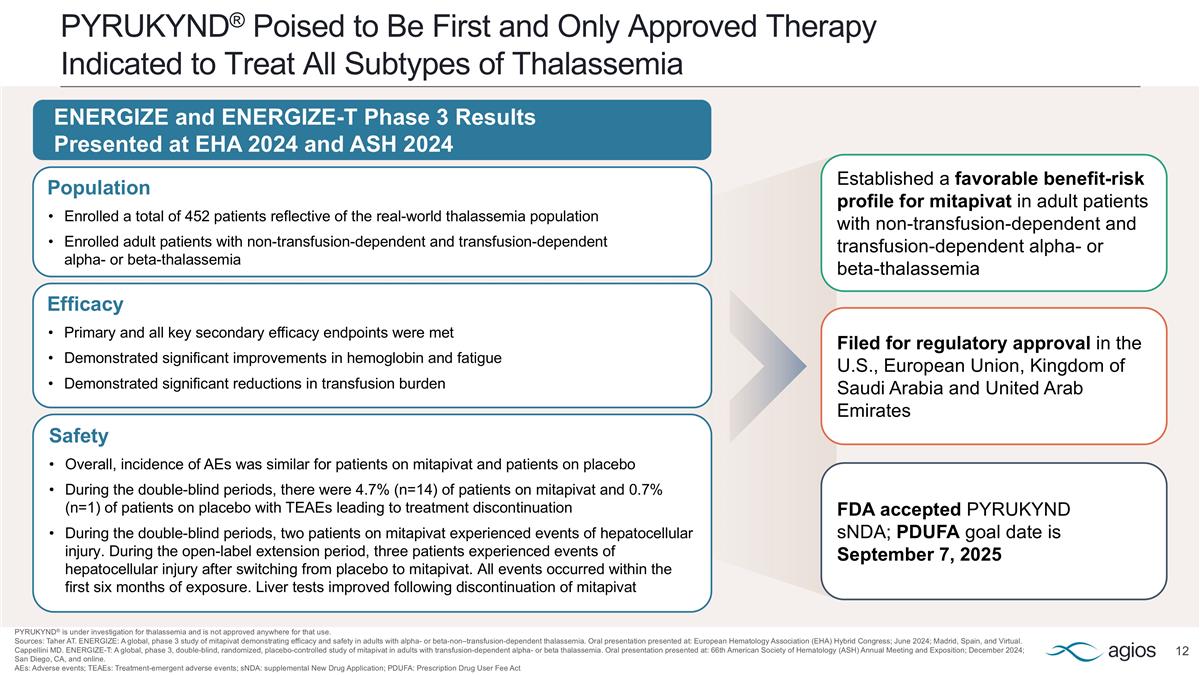
PYRUKYND® Poised to Be First and Only Approved Therapy Indicated to Treat All Subtypes of Thalassemia PYRUKYND® is under investigation for thalassemia and is not approved anywhere for that use. Sources: Taher AT. ENERGIZE: A global, phase 3 study of mitapivat demonstrating efficacy and safety in adults with alpha- or beta-non–transfusion-dependent thalassemia. Oral presentation presented at: European Hematology Association (EHA) Hybrid Congress; June 2024; Madrid, Spain, and Virtual. Cappellini MD. ENERGIZE-T: A global, phase 3, double-blind, randomized, placebo-controlled study of mitapivat in adults with transfusion-dependent alpha- or beta thalassemia. Oral presentation presented at: 66th American Society of Hematology (ASH) Annual Meeting and Exposition; December 2024; San Diego, CA, and online. AEs: Adverse events; TEAEs: Treatment-emergent adverse events; sNDA: supplemental New Drug Application; PDUFA: Prescription Drug User Fee Act Efficacy Primary and all key secondary efficacy endpoints were met Demonstrated significant improvements in hemoglobin and fatigue Demonstrated significant reductions in transfusion burden Safety Overall, incidence of AEs was similar for patients on mitapivat and patients on placebo During the double-blind periods, there were 4.7% (n=14) of patients on mitapivat and 0.7% (n=1) of patients on placebo with TEAEs leading to treatment discontinuation During the double-blind periods, two patients on mitapivat experienced events of hepatocellular injury. During the open-label extension period, three patients experienced events of hepatocellular injury after switching from placebo to mitapivat. All events occurred within the first six months of exposure. Liver tests improved following discontinuation of mitapivat Population Enrolled a total of 452 patients reflective of the real-world thalassemia population Enrolled adult patients with non-transfusion-dependent and transfusion-dependent alpha- or beta-thalassemia ENERGIZE and ENERGIZE-T Phase 3 Results Presented at EHA 2024 and ASH 2024 Established a favorable benefit-risk profile for mitapivat in adult patients with non-transfusion-dependent and transfusion-dependent alpha- or beta-thalassemia Filed for regulatory approval in the U.S., European Union, Kingdom of Saudi Arabia and United Arab Emirates FDA accepted PYRUKYND sNDA; PDUFA goal date is September 7, 2025
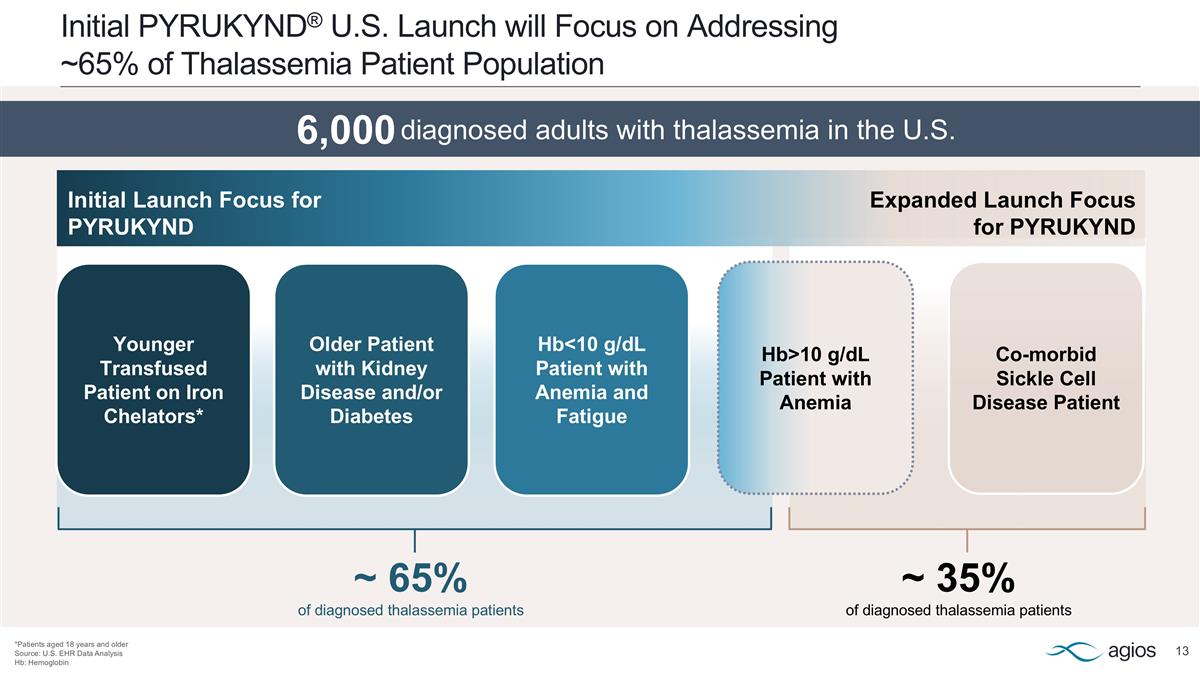
~ 65% of diagnosed thalassemia patients ~ 35% of diagnosed thalassemia patients Initial PYRUKYND® U.S. Launch will Focus on Addressing ~65% of Thalassemia Patient Population *Patients aged 18 years and older Source: U.S. EHR Data Analysis Hb: Hemoglobin Younger Transfused Patient on Iron Chelators* Older Patient with Kidney Disease and/or Diabetes Hb<10 g/dL Patient with Anemia and Fatigue Co-morbid Sickle Cell Disease Patient Hb>10 g/dL Patient with Anemia Initial Launch Focus for PYRUKYND Expanded Launch Focus for PYRUKYND 6,000 diagnosed adults with thalassemia in the U.S. diagnosed adults with thalassemia in the U.S.
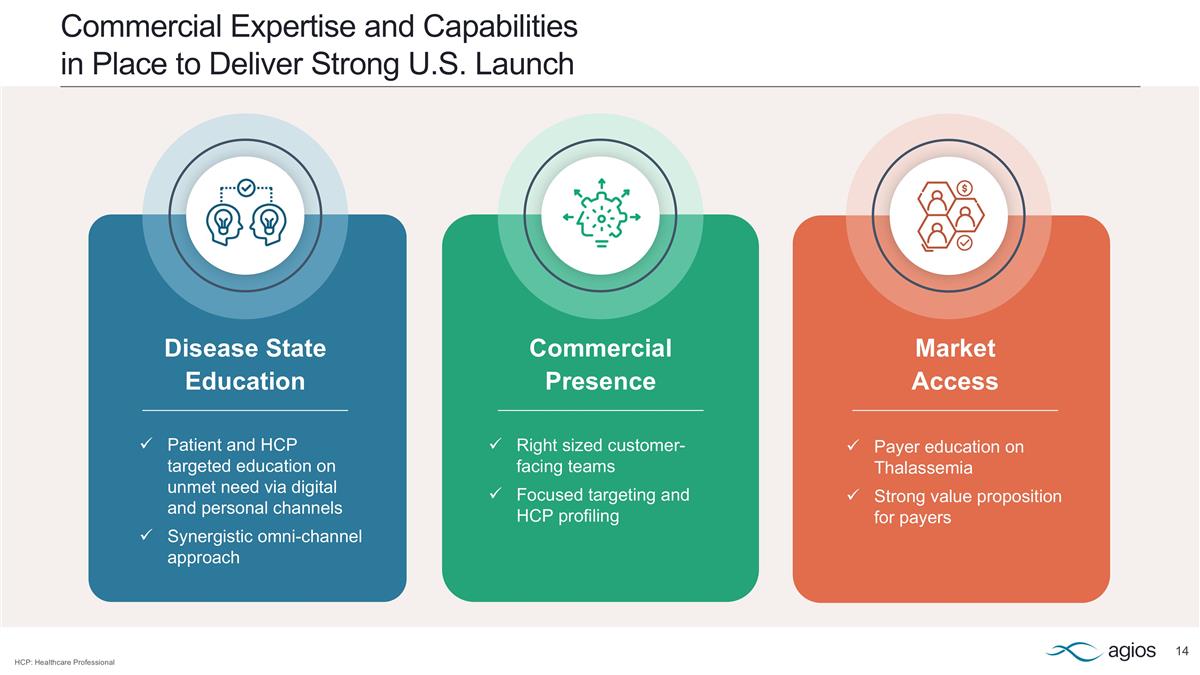
Commercial Expertise and Capabilities in Place to Deliver Strong U.S. Launch Disease State Education Patient and HCP targeted education on unmet need via digital and personal channels Synergistic omni-channel approach Commercial Presence Right sized customer-facing teams Focused targeting and HCP profiling Market Access Payer education on Thalassemia Strong value proposition for payers HCP: Healthcare Professional
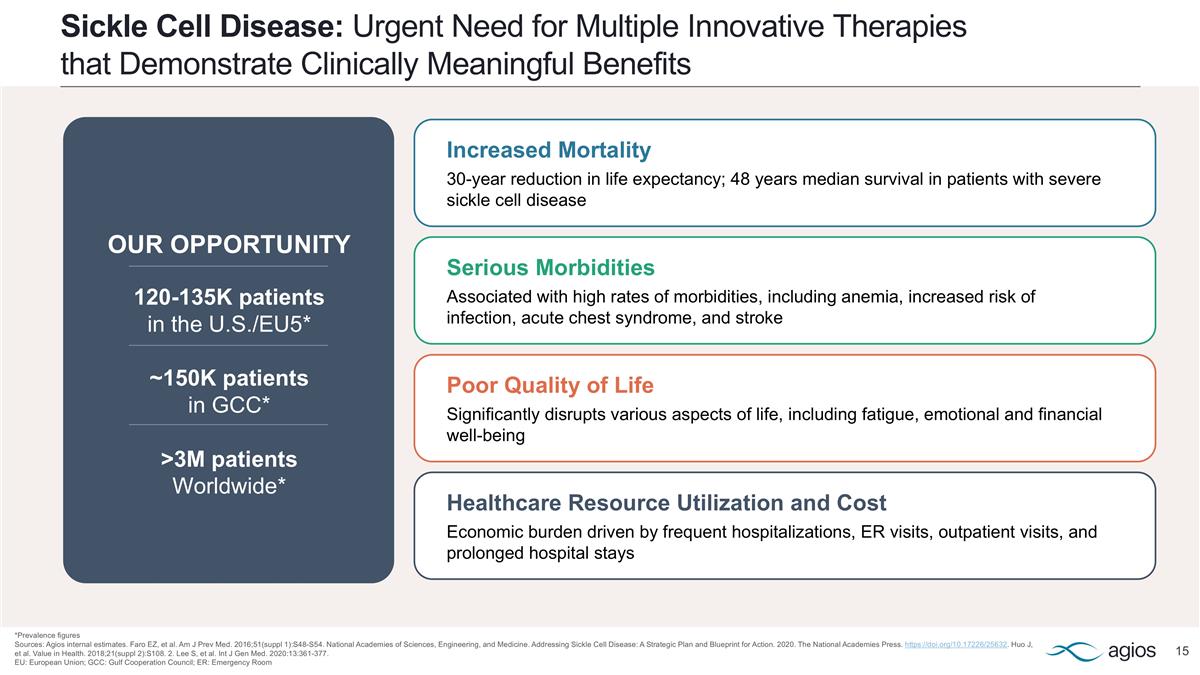
Sickle Cell Disease: Urgent Need for Multiple Innovative Therapies that Demonstrate Clinically Meaningful Benefits *Prevalence figures Sources: Agios internal estimates. Faro EZ, et al. Am J Prev Med. 2016;51(suppl 1):S48-S54. National Academies of Sciences, Engineering, and Medicine. Addressing Sickle Cell Disease: A Strategic Plan and Blueprint for Action. 2020. The National Academies Press. https://doi.org/10.17226/25632. Huo J, et al. Value in Health. 2018;21(suppl 2):S108. 2. Lee S, et al. Int J Gen Med. 2020:13:361-377. EU: European Union; GCC: Gulf Cooperation Council; ER: Emergency Room Healthcare Resource Utilization and Cost Economic burden driven by frequent hospitalizations, ER visits, outpatient visits, and prolonged hospital stays Serious Morbidities Associated with high rates of morbidities, including anemia, increased risk of infection, acute chest syndrome, and stroke Poor Quality of Life Significantly disrupts various aspects of life, including fatigue, emotional and financial well-being Increased Mortality 30-year reduction in life expectancy; 48 years median survival in patients with severe sickle cell disease OUR OPPORTUNITY 120-135K patients in the U.S./EU5* ~150K patients in GCC* >3M patients Worldwide*

PYRUKYND® Offers Best-in-Class Opportunity in Sickle Cell Disease with Potential to Improve Anemia, Reduce VOCs and Improve How Patients Feel and Function *Powering details in appendix PYRUKYND® is under investigation for sickle cell disease and is not approved anywhere for that use. Source: Andemariam B. Study design of the phase 3 portion of RISE UP: A phase 2/3, randomized, double-blind, placebo-controlled study of mitapivat in patients with sickle cell disease. Poster presentation presented at: 2024 European Hematology Association (EHA) Hybrid Congress; June 2024; Madrid, Spain, and Virtual. VOCs: Vaso-occlusive crisis; BID: Twice daily; Hb: Hemoglobin; SCPCs: sickle cell pain crises; 6MWT: 6 minute walking test Phase 3 RISE UP study topline readout in late 2025; Potential U.S. launch in 2026 STUDY POPULATION STUDY DESIGN TWO PRIMARY ENDPOINTS* SECONDARY ENDPOINTS Sickle cell disease patients 16 years of age or older 200+ patients enrolled worldwide (trial enrollment completed in October 2024) 52-week double blind period followed by 216-week open label extension 2:1 randomization (100 mg mitapivat or placebo, BID) Hb response defined as a ≥1.0 g/dL increase in average Hb concentration from Week 24 through Week 52 compared with baseline Annualized rate of SCPCs Additional clinical efficacy measures related to anemia, hemolysis, erythropoiesis, patient-reported fatigue and pain, annualized frequency of hospitalizations for SCPCs and 6MWT

Progress and Diversify Key Pipeline Programs Teonna Living with sickle cell disease 2
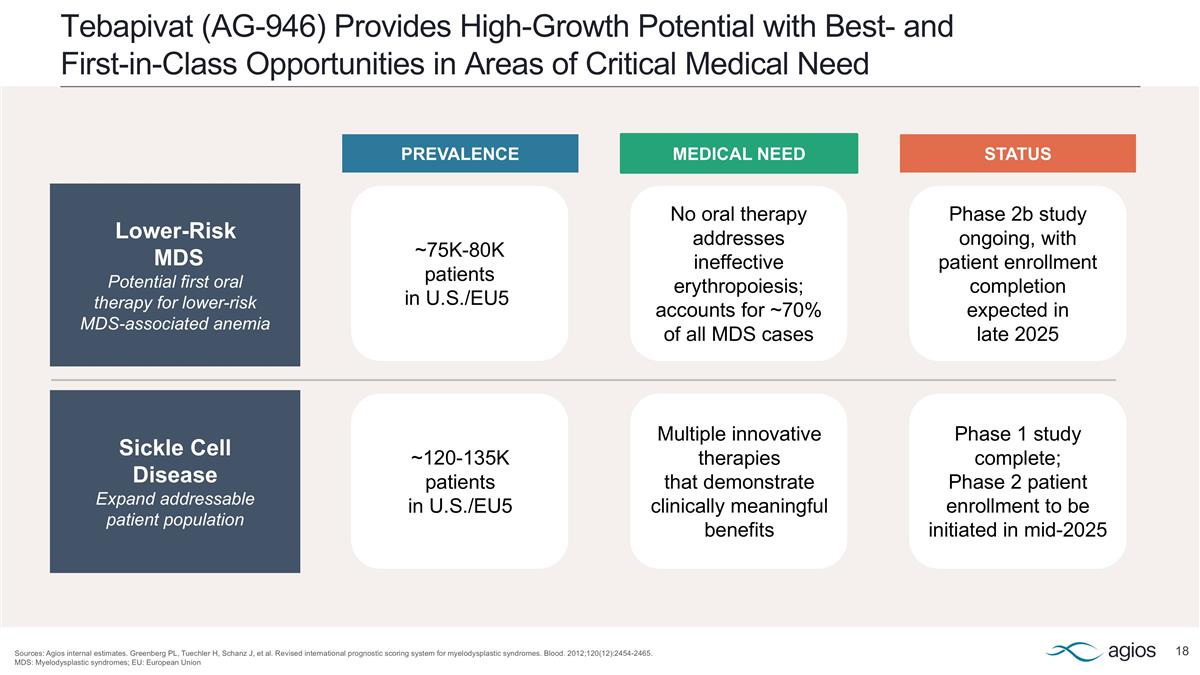
Tebapivat (AG-946) Provides High-Growth Potential with Best- and First-in-Class Opportunities in Areas of Critical Medical Need Sources: Agios internal estimates. Greenberg PL, Tuechler H, Schanz J, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120(12):2454-2465. MDS: Myelodysplastic syndromes; EU: European Union Lower-Risk MDS Potential first oral therapy for lower-risk MDS-associated anemia ~75K-80K patients in U.S./EU5 No oral therapy addresses ineffective erythropoiesis; accounts for ~70% of all MDS cases Phase 2b study ongoing, with patient enrollment completion expected in late 2025 Sickle Cell Disease Expand addressable patient population ~120-135K patients in U.S./EU5 Multiple innovative therapies that demonstrate clinically meaningful benefits Phase 1 study complete; Phase 2 patient enrollment to be initiated in mid-2025 PREVALENCE MEDICAL NEED STATUS
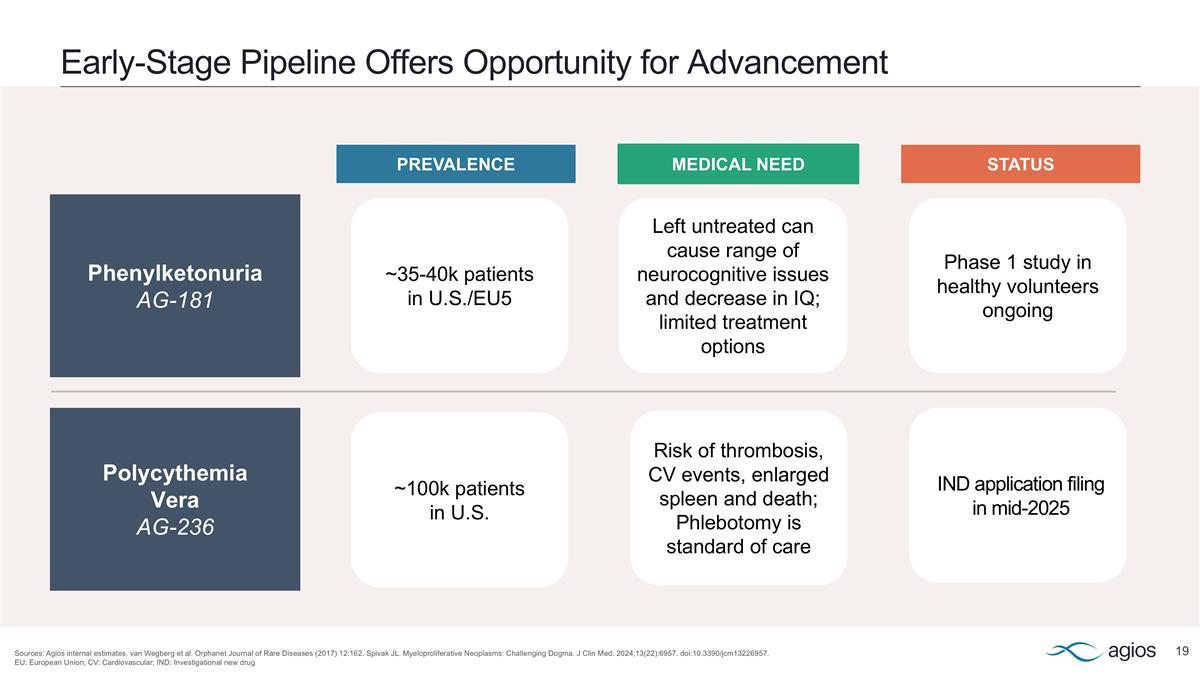
Early-Stage Pipeline Offers Opportunity for Advancement Sources: Agios internal estimates. van Wegberg et al. Orphanet Journal of Rare Diseases (2017) 12:162. Spivak JL. Myeloproliferative Neoplasms: Challenging Dogma. J Clin Med. 2024;13(22):6957. doi:10.3390/jcm13226957. EU: European Union; CV: Cardiovascular; IND: Investigational new drug Phenylketonuria AG-181 Polycythemia Vera AG-236 ~35-40k patients in U.S./EU5 ~100k patients in U.S. Left untreated can cause range of neurocognitive issues and decrease in IQ; limited treatment options Risk of thrombosis, CV events, enlarged spleen and death; Phlebotomy is standard of care PREVALENCE MEDICAL NEED STATUS IND application filing in mid-2025 Phase 1 study in healthy volunteers ongoing

Focus Capital Deployment Priorities to Sustain Growth Golie Living with sickle cell disease 3
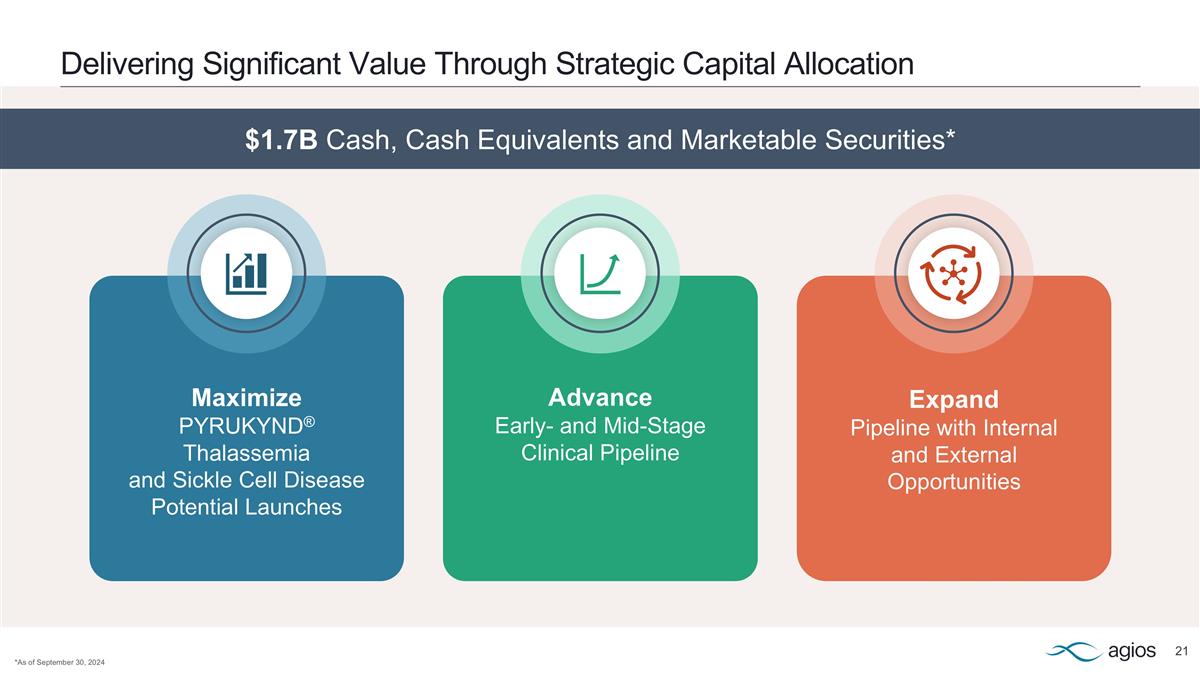
Maximize PYRUKYND® Thalassemia and Sickle Cell Disease Potential Launches Advance Early- and Mid-Stage Clinical Pipeline Expand Pipeline with Internal and External Opportunities Delivering Significant Value Through Strategic Capital Allocation *As of September 30, 2024 $1.7B Cash, Cash Equivalents and Marketable Securities*
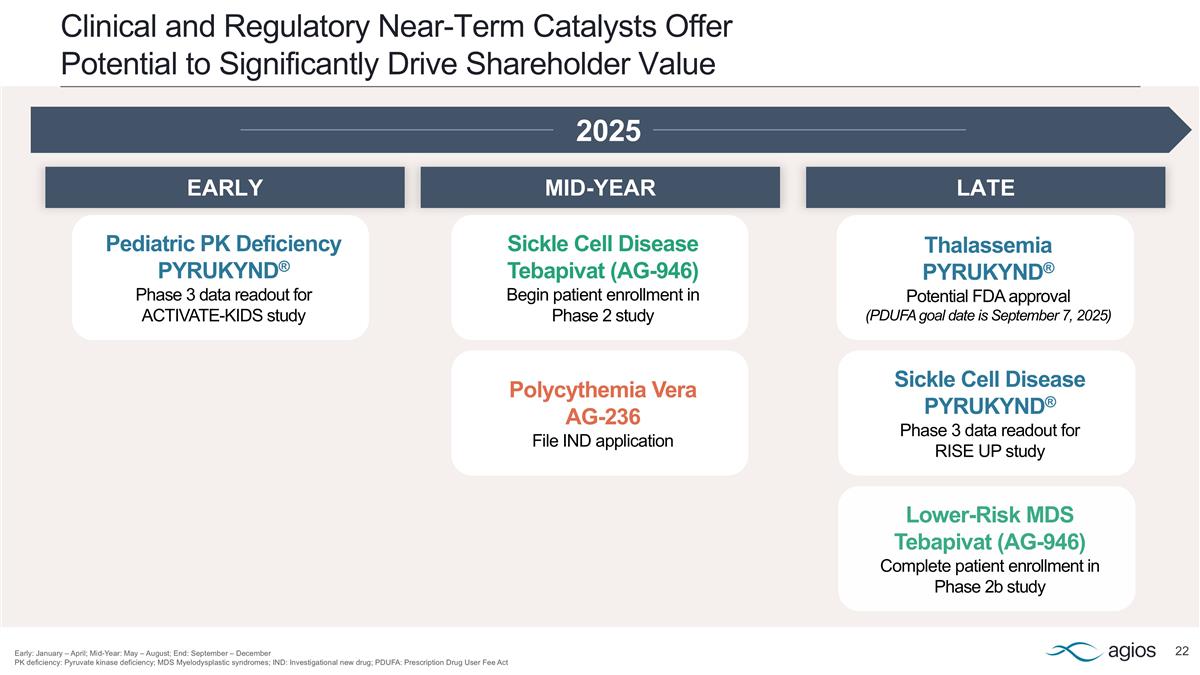
Clinical and Regulatory Near-Term Catalysts Offer Potential to Significantly Drive Shareholder Value Early: January – April; Mid-Year: May – August; End: September – December PK deficiency: Pyruvate kinase deficiency; MDS Myelodysplastic syndromes; IND: Investigational new drug; PDUFA: Prescription Drug User Fee Act 2025 Polycythemia Vera AG-236 File IND application Pediatric PK Deficiency PYRUKYND® Phase 3 data readout for ACTIVATE-KIDS study Lower-Risk MDS Tebapivat (AG-946) Complete patient enrollment in Phase 2b study Sickle Cell Disease Tebapivat (AG-946) Begin patient enrollment in Phase 2 study Thalassemia PYRUKYND® Potential FDA approval (PDUFA goal date is September 7, 2025) Sickle Cell Disease PYRUKYND® Phase 3 data readout for RISE UP study LATE MID-YEAR EARLY

Maximize Potential of PYRUKYND® Franchise Progress and Diversify Key Pipeline Programs Focus Capital Deployment Priorities to Sustain Growth 1 3 2 2025: Breakout Year

Thank you

Appendix
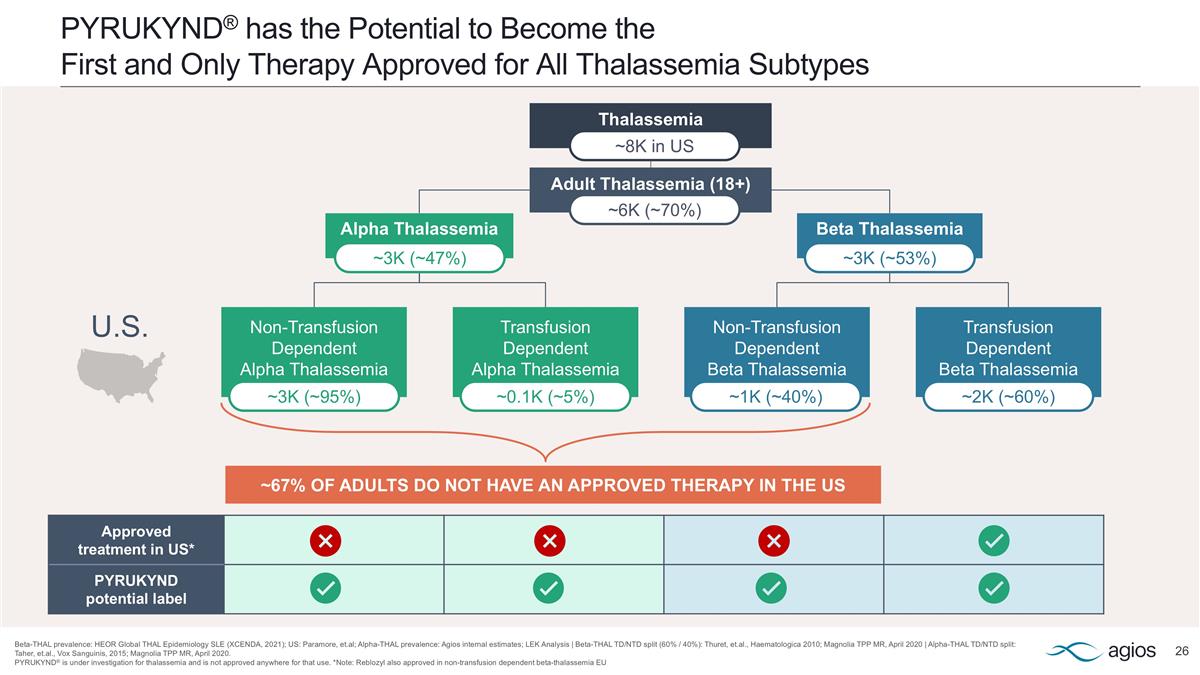
PYRUKYND® has the Potential to Become the First and Only Therapy Approved for All Thalassemia Subtypes Beta-THAL prevalence: HEOR Global THAL Epidemiology SLE (XCENDA, 2021); US: Paramore, et.al; Alpha-THAL prevalence: Agios internal estimates; LEK Analysis | Beta-THAL TD/NTD split (60% / 40%): Thuret, et.al., Haematologica 2010; Magnolia TPP MR, April 2020 | Alpha-THAL TD/NTD split: Taher, et.al., Vox Sanguinis, 2015; Magnolia TPP MR, April 2020. PYRUKYND® is under investigation for thalassemia and is not approved anywhere for that use. *Note: Reblozyl also approved in non-transfusion dependent beta-thalassemia EU Thalassemia Approved treatment in US* PYRUKYND potential label Alpha Thalassemia Beta Thalassemia Non-Transfusion Dependent Alpha Thalassemia Transfusion Dependent Alpha Thalassemia Non-Transfusion Dependent Beta Thalassemia Transfusion Dependent Beta Thalassemia ~3K (~95%) ~0.1K (~5%) ~1K (~40%) ~2K (~60%) Adult Thalassemia (18+) ~3K (~47%) ~3K (~53%) ~6K (~70%) ~8K in US ~67% OF ADULTS DO NOT HAVE AN APPROVED THERAPY IN THE US U.S.
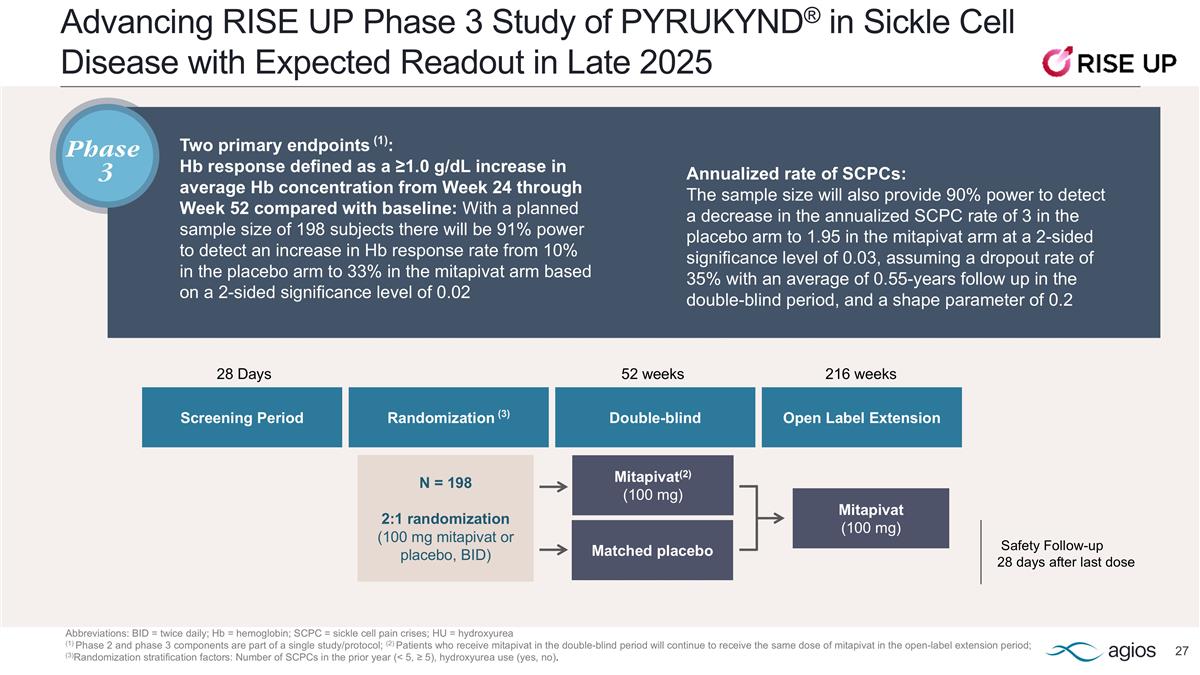
Advancing RISE UP Phase 3 Study of PYRUKYND® in Sickle Cell Disease with Expected Readout in Late 2025 Abbreviations: BID = twice daily; Hb = hemoglobin; SCPC = sickle cell pain crises; HU = hydroxyurea (1) Phase 2 and phase 3 components are part of a single study/protocol; (2) Patients who receive mitapivat in the double-blind period will continue to receive the same dose of mitapivat in the open-label extension period; (3)Randomization stratification factors: Number of SCPCs in the prior year (< 5, ≥ 5), hydroxyurea use (yes, no). Two primary endpoints (1): Hb response defined as a ≥1.0 g/dL increase in average Hb concentration from Week 24 through Week 52 compared with baseline: With a planned sample size of 198 subjects there will be 91% power to detect an increase in Hb response rate from 10% in the placebo arm to 33% in the mitapivat arm based on a 2-sided significance level of 0.02 Safety Follow-up 28 days after last dose Mitapivat(2) (100 mg) Mitapivat (100 mg) N = 198 2:1 randomization (100 mg mitapivat or placebo, BID) Screening Period 52 weeks Randomization (3) Double-blind 28 Days Open Label Extension 216 weeks Matched placebo Annualized rate of SCPCs: The sample size will also provide 90% power to detect a decrease in the annualized SCPC rate of 3 in the placebo arm to 1.95 in the mitapivat arm at a 2-sided significance level of 0.03, assuming a dropout rate of 35% with an average of 0.55-years follow up in the double-blind period, and a shape parameter of 0.2 Phase 3
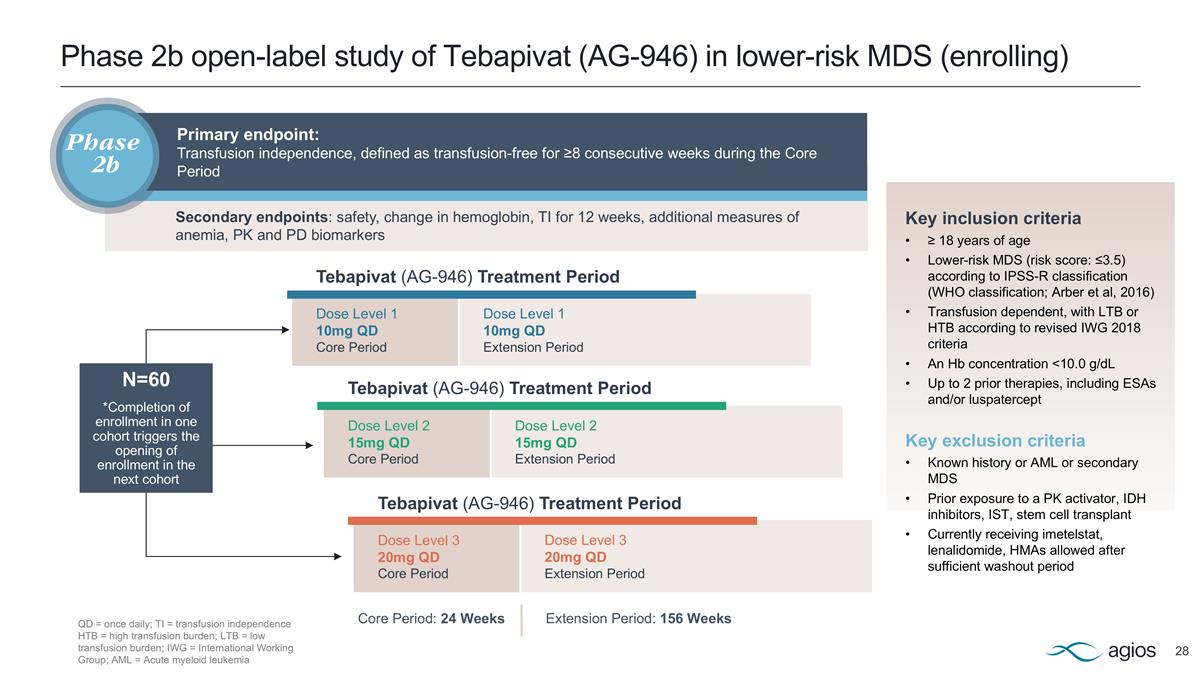
Phase 2b open-label study of Tebapivat (AG-946) in lower-risk MDS (enrolling) Dose Level 1 10mg QD Core Period Dose Level 2 15mg QD Core Period Dose Level 3 20mg QD Core Period Dose Level 1 10mg QD Extension Period Dose Level 2 15mg QD Extension Period Dose Level 3 20mg QD Extension Period Tebapivat (AG-946) Treatment Period Extension Period: 156 Weeks Tebapivat (AG-946) Treatment Period Tebapivat (AG-946) Treatment Period Core Period: 24 Weeks Key inclusion criteria ≥ 18 years of age Lower-risk MDS (risk score: ≤3.5) according to IPSS-R classification (WHO classification; Arber et al, 2016) Transfusion dependent, with LTB or HTB according to revised IWG 2018 criteria An Hb concentration <10.0 g/dL Up to 2 prior therapies, including ESAs and/or luspatercept Key exclusion criteria Known history or AML or secondary MDS Prior exposure to a PK activator, IDH inhibitors, IST, stem cell transplant Currently receiving imetelstat, lenalidomide, HMAs allowed after sufficient washout period N=60 *Completion of enrollment in one cohort triggers the opening of enrollment in the next cohort QD = once daily; TI = transfusion independence HTB = high transfusion burden; LTB = low transfusion burden; IWG = International Working Group; AML = Acute myeloid leukemia Primary endpoint: Transfusion independence, defined as transfusion-free for ≥8 consecutive weeks during the Core Period Phase 2b Secondary endpoints: safety, change in hemoglobin, TI for 12 weeks, additional measures of anemia, PK and PD biomarkers



























