Exhibit 99.2

Accelerating ADC Innovation …because patients are waiting Virtual Analyst & Investor Day January 5, 2021

Legal Disclaimer This presentation contains “forward - looking” statements within the meaning of federal securities laws . These forward - looking statements are not statements of historical facts and are based on management’s beliefs and assumptions and on information currently available to management . Forward - looking statements include information concerning Mersana Therapeutics, Inc . ’s (the “Company’s”) business strategy and the design, progression and timing of its clinical trials, the ability of the single - arm UPLIFT cohort to enable registration, expectations regarding future clinical trial results based on data achieved to date, and the sufficiency of the Company’s cash on hand . Forward - looking statements generally can be identified by terms such as “aims,” “anticipates,” “believes,” “contemplates,” “continues,” “could,” “estimates,” “expects,” “goal,” “intends,” “may,” “on track,” “opportunity,” “plans,” “poised for,” “possible,” “potential,” “predicts,” “projects,” “promises to be,” “seeks,” “should,” “target,” “will,” “would” or similar expressions and the negatives of those terms . Forward - looking statements represent management’s beliefs and assumptions only as of the date of this presentation . The Company’s operations involve risks and uncertainties, many of which are outside its control, and any one of which, or combination of which, could materially affect its results of operations and whether the forward - looking statements ultimately prove to be correct . Factors that may materially affect the Company’s results of operations and whether these forward - looking statements prove to be correct include, among other things, that preclinical testing or early clinical results may not be predictive of the results or success of ongoing or later clinical trials, regulatory changes, particularly with respect to the change in the U . S . presidential administration, the FDA’s review of the protocol for our study of the single - arm UPLIFT cohort, and that the development and testing of the Company’s product candidates will take longer and/or cost more than planned, as well as those listed in the Company’s Annual Report on Form 10 - K filed with the Securities and Exchange Commission (“SEC”) on February 28 , 2020 , the Company’s Quarterly Report on Form 10 - Q filed with the SEC on May 8 , 2020 and subsequent SEC filings . In addition, while we expect that the COVID - 19 pandemic might adversely affect the Company’s preclinical and clinical development efforts, business operations and financial results, the extent of the impact on the Company’s operations and the value of and market for the Company’s common stock will depend on future developments that are highly uncertain and cannot be predicted with confidence at this time, such as the ultimate duration of the pandemic, travel restrictions, quarantines, physical distancing and business closure requirements in the U . S . and in other countries, and the effectiveness of actions taken globally to contain and treat the disease . Except as required by law, the Company assumes no obligation to update these forward - looking statements publicly, or to update the reasons actual results could differ materially from those anticipated in the forward - looking statements, even if new information becomes available in the future . Copies of the Company’s Annual Report on Form 10 - K and our other SEC filings are available by visiting EDGAR on the SEC website at http : //www . sec . gov . 2
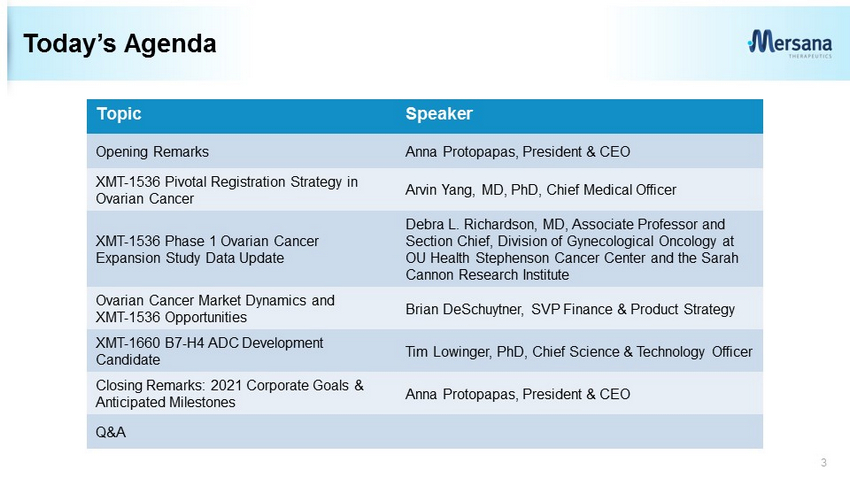
Today’s Agenda 3 Topic Speaker Opening Remarks Anna Protopapas, President & CEO XMT - 1536 Pivotal Registration Strategy in Ovarian Cancer Arvin Yang, MD, PhD, Chief Medical Officer XMT - 1536 Phase 1 Ovarian Cancer Expansion Study Data Update Debra L. Richardson, MD, Associate Professor and Section Chief, Division of Gynecological Oncology at OU Health Stephenson Cancer Center and the Sarah Cannon Research Institute Ovarian Cancer Market Dynamics and XMT - 1536 Opportunities Brian DeSchuytner, SVP Finance & Product Strategy XMT - 1660 B7 - H4 ADC Development Candidate Tim Lowinger, PhD, Chief Science & Technology Officer Closing Remarks: 2021 Corporate Goals & Anticipated Milestones Anna Protopapas, President & CEO Q&A
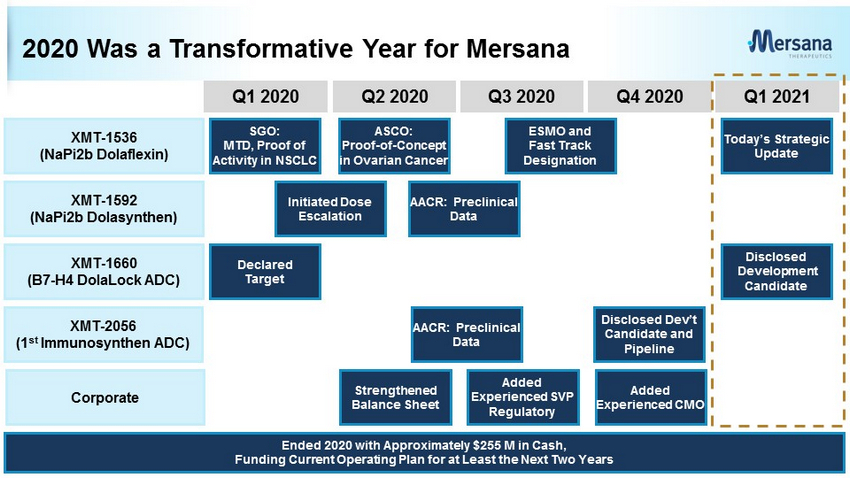
2020 Was a Transformative Year for Mersana 4 Corporate XMT - 1592 (NaPi2b Dolasynthen) XMT - 2056 (1 st Immunosynthen ADC) XMT - 1660 (B7 - H4 DolaLock ADC) XMT - 1536 (NaPi2b Dolaflexin) Q1 2020 Q2 2020 Q3 2020 Q4 2020 Q1 2021 Strengthened Balance Sheet Added Experienced CMO Added Experienced SVP Regulatory Initiated Dose Escalation AACR: Preclinical Data Disclosed Dev’t Candidate and Pipeline AACR: Preclinical Data Disclosed Development Candidate Declared Target SGO: MTD, Proof of Activity in NSCLC ASCO: Proof - of - Concept in Ovarian Cancer ESMO and Fast Track Designation Today’s Strategic Update Ended 2020 with Approximately $255 M in Cash, Funding Current Operating Plan for at Least the Next Two Years

Poised for Significant Value Inflection Points and Continued Momentum in 2021 5 1 XMT - 1536 in Ovarian IND Submission Q1 2022 5 XMT - 2056 (Immunosynthen) IND Submission Q1 2022 Select Lead in NSCLC Initiate Single - Arm Registration Strategy Initiate Lifecycle Management Studies / Combinations IND - Enabling Studies IND - Enabling Studies XMT - 1660 (B7 - H4) 4 3 2 XMT - 1592 XMT - 1536 In NSCLC Seek to Achieve Proof - of - Concept Complete Dose Escalation

XMT - 1536 Has a New Name 6 upifitamab rilsodotin or UpRi, for short

7 Earlier Lines of Therapy Single - Arm Registration Strategy in Platinum Resistant Disease New Combinations UpRi (XMT - 1536): An Opportunity to Deliver a Potentially Foundational Therapy for Ovarian Cancer • In a heavily - pretreated ovarian cancer population: – Proof of concept, >30% ORR in ovarian cancer with higher NaPi2b expression – Activity, including CRs, in patients failing platinum, bevacizumab, and/or PARPi – No severe neutropenia, peripheral neuropathy or ocular toxicity – Biomarker identification for improved patient outcomes INCREASING MARKET POTENTIAL .

UpRi (XMT - 1536): First - in - Class Dolaflexin ADC Targeting NaPi2b Single - Arm Registration Strategy in Ovarian Cancer Arvin Yang, MD, PhD Chief Medical Officer

Strategy Informed by End of Phase Meeting and Meeting Minutes UPLIFT Strategy: Key Areas Discussed with FDA • Population with high unmet medical need • Performance of current standard of care • Design of single - arm registration cohort • Primary and secondary endpoints • Biomarker validation strategy 9
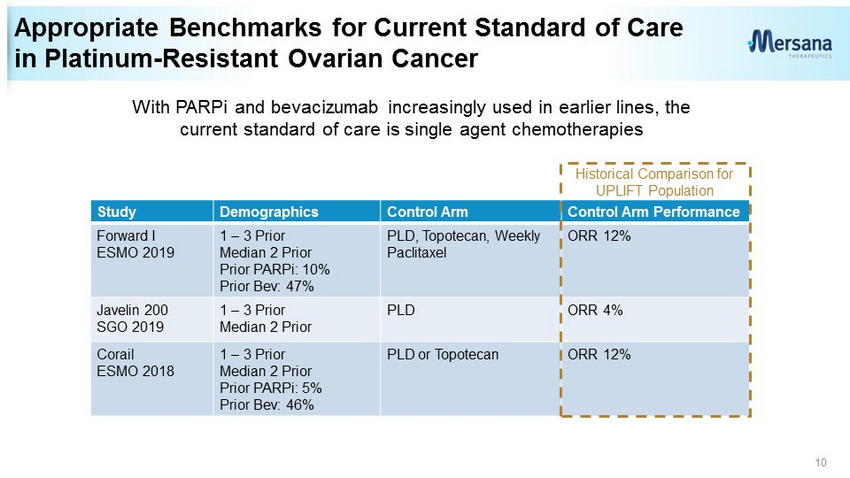
Appropriate Benchmarks for Current Standard of Care in Platinum - Resistant Ovarian Cancer Study Demographics Control Arm Control Arm Performance Forward I ESMO 2019 1 – 3 Prior Median 2 Prior Prior PARPi: 10% Prior Bev: 47% PLD, Topotecan, Weekly Paclitaxel ORR 12% Javelin 200 SGO 2019 1 – 3 Prior Median 2 Prior PLD ORR 4% Corail ESMO 2018 1 – 3 Prior Median 2 Prior Prior PARPi: 5% Prior Bev: 46% PLD or Topotecan ORR 12% 10 Historical Comparison for UPLIFT Population With PARPi and bevacizumab increasingly used in earlier lines, the current standard of care is single agent chemotherapies
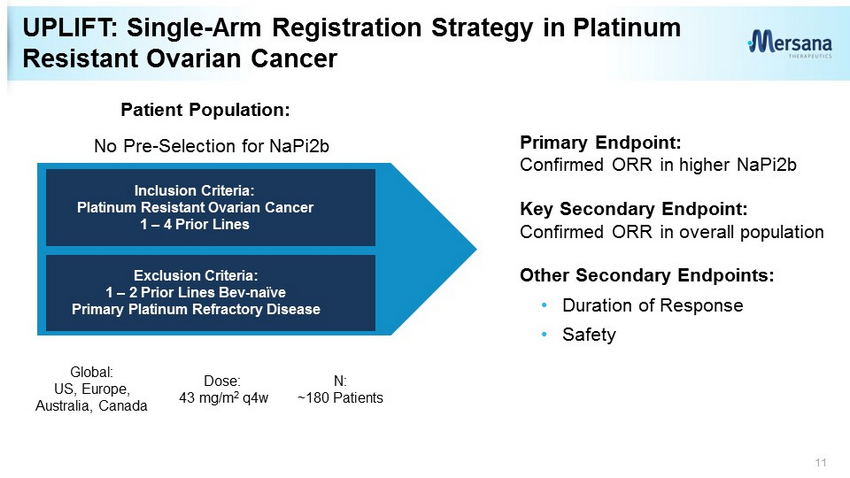
UPLIFT: Single - Arm Registration Strategy in Platinum Resistant Ovarian Cancer 11 Primary Endpoint: Confirmed ORR in higher NaPi2b Key Secondary Endpoint: Confirmed ORR in overall population Other Secondary Endpoints: • Duration of Response • Safety Inclusion Criteria: Platinum Resistant Ovarian Cancer 1 – 4 Prior Lines Exclusion Criteria: 1 – 2 Prior Lines Bev - naïve Primary Platinum Refractory Disease No Pre - Selection for NaPi2b Global: US, Europe, Australia, Canada Dose: 43 mg/m 2 q4w N: ~180 Patients Patient Population:
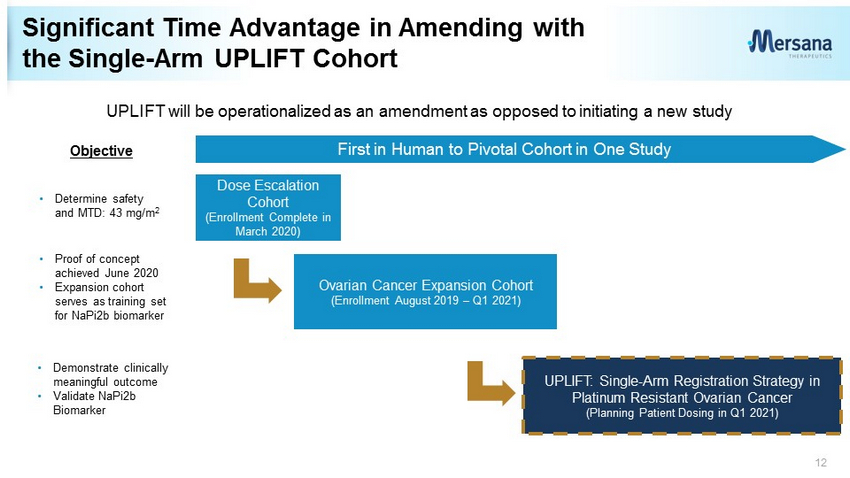
Significant Time Advantage in Amending with the Single - Arm UPLIFT Cohort 12 Dose Escalation Cohort (Enrollment Complete in March 2020) Ovarian Cancer Expansion Cohort (Enrollment August 2019 – Q1 2021) UPLIFT: Single - Arm Registration Strategy in Platinum Resistant Ovarian Cancer (Planning Patient Dosing in Q1 2021) First in Human to Pivotal Cohort in One Study UPLIFT will be operationalized as an amendment as opposed to initiating a new study • Determine safety and MTD: 43 mg/m 2 • Proof of concept achieved June 2020 • Expansion cohort serves as training set for NaPi2b biomarker • Demonstrate clinically meaningful outcome • Validate NaPi2b Biomarker Objective

Strategy to Deliver a Robust and Reproducible Commercial Diagnostic Assay 13 • NaPi2b expression assessed with clinical assay in >80 patients • “Train” proposed commercial assay – Repeat assessment on all samples – Ensures same read regardless of lab and pathologist • Determine cutoff for UPLIFT Pivotal Cohort based on full data set Ovarian Cancer Expansion Cohort and Relevant Doses from Escalation Cohort UPLIFT: Single - Arm Registration Strategy in Platinum - Resistant Ovarian Cancer • Prospectively - defined retrospective analysis validates NaPi2b biomarker cutoff with proposed commercial assay • Enroll without NaPi2b biomarker selection – Evaluate both NaPi2b biomarker higher and overall population – Optionality for either companion diagnostic or complementary diagnostic assay

UPLIFT Registration Strategy Creates Potential for Speed and Label Differentiation 14 • Streamlined Execution – Leverages expansion cohort enrollment momentum in high unmet need population for single - arm registration path • Broad Target Population – Includes patients with 4 prior lines of therapy, a broader population than historical studies in platinum - resistant ovarian cancer – Includes bevacizumab - naïve patients with 3 – 4 prior lines of therapy, accommodating differences in bevacizumab use in early disease – No pre - selection accelerates enrollment and provides potential upside opportunity for broad label regardless of NaPi2b expression level • Assay Validation Process – Training and validation method designed to support a commercial assay Planning to Initiate UPLIFT Patient Dosing in Q1 2021

UpRi (XMT - 1536): First - in - Class Dolaflexin ADC Targeting NaPi2b Phase 1 Ovarian Cancer Expansion Cohort Data Update Debra L. Richardson, MD Associate Professor and Section Chief, Division of Gynecological Oncology at OU Health Stephenson Cancer Center and the Sarah Cannon Research Institute The following information is from an ongoing study and based on December 3, 2020 data cut

We thank the patients, their families and caregivers for their contribution to this study UNTED STATES Allegheny Health Network, Pittsburgh, PA Arizona Oncology Associates, Tucson, AZ Billings Clinic, Billings, MT Dana Farber Cancer Institute, Boston, MA Emory University, Atlanta, GA Fox Chase Cancer Center, Philadelphia, PA H. Lee Moffitt Cancer Center, Tampa FL Henry Ford Medical Center, Detroit, MI Greenville Hospital System University Medical Center, Greenville, SC Lahey Clinic, Burlington, MA Levine Cancer Center, Charlotte, NC Mary Crowley Cancer Research Center, Dallas, TX Maryland Oncology and Hematology, Rockville, MD Massachusetts General Hospital, Boston, MA Mount Sinai, New York City, NY NEXT Oncology, San Antonio, TX Ohio State University Wexner Medical Center, Hilliard, OH Oncology and Hematology Assoc. of SW VA, Inc., Roanoke, VA QUEST Research Institute, Royal Oak, MI Rocky Mountain Cancer Centers, LLP, Denver, CO Sarah Cannon Research Institute, Nashville, TN START, San Antonio, TX UNITED STATES START Midwest, Grand Rapids, MI Stephenson Cancer Centre, Oklahoma City, OK Texas Oncology, Austin, TX Texas Oncology Fort Worth, Fort Worth, TX Texas Oncology, Tyler, TX University of Alabama at Birmingham, Birmingham, AL University of Colorado, Aurora, CO University of Florida, Gainesville, FL University of Miami, Miami, FL University of Pittsburgh Medical Center, Pittsburgh, PA University of Tennessee, Knoxville, TN University of Utah Huntsman Cancer Institute, Salt Lake City, UT Virginia Cancer Specialists, Fairfax, VA Virginia Commonwealth University Massey Cancer Center, Richmond, VA Washington University, St. Louis, MO Willamette Valley Cancer Institute, Eugene, OR CANADA McGill University (Glen - Cedars Cancer Center), Montreal British Columbia Cancer Agency, Vancouver AUSTRALIA Lifehouse Australia as trustee for the Lifehouse Australia Trust, Camperdown Peter MacCallum Center, Melbourne, Victoria Austin Health, Heidelberg, Victoria Acknowledgements 16

Design for the Ovarian Cancer Cohort of the XMT - 1536 (UpRi) Phase 1 Expansion Study 17 Ovarian Cancer Cohort • 1 - 3 prior lines in platinum resistant • 4 prior lines regardless of platinum status • High grade serous histology • Archived tumor and fresh biopsy (if medically feasible) for NaPi2b • Exclusion: primary platinum - resistant defined as lack of response or disease progression within 3 mos after completing front - line platinum containing therapy 1 Tolcher TW et al. J Clin Oncol 37, 2019 (suppl; abstr 3010) 2 Richardson DL et al. Presented at SGO Annual Meeting 2020; LBA8 3 Hamilton E et al. Presented during the 2020 European Society of Medical Oncology (ESMO) Virtual Congress Patient population : High grade serous ovarian cancer (including fallopian tube and primary peritoneal cancer) progressing after standard treatments • Measurable disease per RECIST v1.1 • ECOG Performance Status 0 or 1 Dosing : IV every 4 weeks until disease progression or unacceptable toxicity • 36 mg/m2 cohort initiated in August 2019 and enrollment closed • 43 mg/m2 cohort initiated in December 2019 and ongoing; current dose evaluated in EXP Primary Objectives: • Evaluate safety and tolerability of MTD • Assess preliminary efficacy (ORR, DCR) Secondary Objectives: • Association of tumor NaPi2b expression and objective tumor response using an immunohistochemistry (IHC) assay with a broad dynamic range to distinguish tumors with higher and lower NaPi2b expression (as previously reported 1,2,3 ) • Further assessment of preliminary anti - neoplastic activity (DOR) Assessments: • Tumor imaging (MRI or CT): baseline and every 2 nd cycle; response assessed per RECIST v1.1 Abbreviations: mos = months; EXP = expansion; RECIST = Response Evaluation Criteria in Solid Tumors; ECOG = Eastern Cooperative Oncology Group; MTD = maximum tolerated dose; ORR = objective response rate; DCR = disease control rate; DOR = duration of response
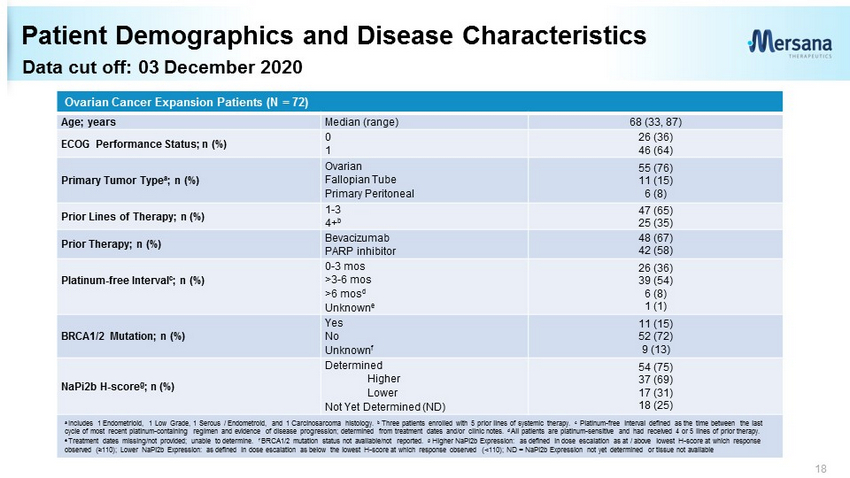
Data cut off: 03 December 2020 Patient Demographics and Disease Characteristics 18 Ovarian Cancer Expansion Patients (N = 72) Age; years Median (range) 68 (33, 87) ECOG Performance Status; n (%) 0 1 26 (36) 46 (64) Primary Tumor Type a ; n (%) Ovarian Fallopian Tube Primary Peritoneal 55 (76) 11 (15) 6 (8) Prior Lines of Therapy; n (%) 1 - 3 4+ b 47 (65) 25 (35) Prior Therapy; n (%) Bevacizumab PARP inhibitor 48 (67) 42 (58) Platinum - free Interval c ; n (%) 0 - 3 mos >3 - 6 mos >6 mos d Unknown e 26 (36) 39 (54) 6 (8) 1 (1) BRCA1/2 Mutation; n (%) Yes No Unknown f 11 (15) 52 (72) 9 (13) NaPi2b H - score g ; n (%) Determined Higher Lower Not Yet Determined (ND) 54 (75) 37 (69) 17 (31) 18 (25) a Includes 1 Endometrioid, 1 Low Grade, 1 Serous / Endometroid, and 1 Carcinosarcoma histology. b Three patients enrolled with 5 prior lines of systemic therapy. c Platinum - free interval d efined as the time between the last cycle of most recent platinum - containing regimen and evidence of disease progression ; determined from treatment dates and/or clinic notes. d All patients are platinum - sensitive and had received 4 or 5 lines of prior therapy. e Treatment dates missing/not provided; unable to determine. f BRCA1/2 mutation status not available/not reported. g Higher NaPi2b Expression: as defined in dose escalation as at / above lowest H - score at which response observed (≥110) ; Lower NaPi2b Expression: as defined in dose escalation as below the lowest H - score at which response observed (<110); ND = NaPi2b Expression not yet determined or tissue not available
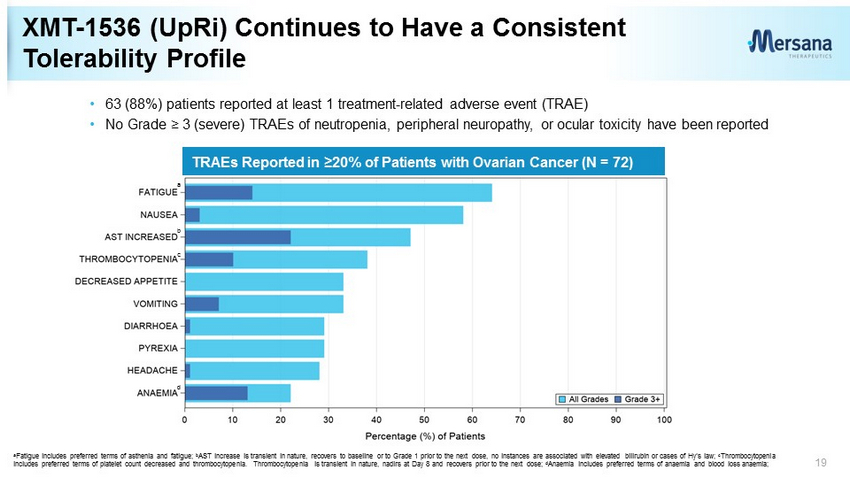
TRAEs Reported in ≥20% of Patients with Ovarian Cancer (N = 72) a b d c XMT - 1536 (UpRi) Continues to Have a Consistent Tolerability Profile 19 a Fatigue includes preferred terms of asthenia and fatigue; b AST increase is transient in nature, recovers to baseline or to Grade 1 prior to the next dose, no instances are associated w ith elevated bilirubin or cases of Hy’s law; c Thrombocytopenia includes preferred terms of platelet count decreased and thrombocytopenia. Thrombocytopenia is transient in nature, nadirs at Da y 8 and recovers prior to the next dose; d Anaemia includes preferred terms of anaemia and blood loss anaemia; • 63 (88%) patients reported at least 1 treatment - related adverse event (TRAE) • No Grade ≥ 3 (severe) TRAEs of neutropenia, peripheral neuropathy, or ocular toxicity have been reported

Safety Summary of XMT - 1536 (UpRi) in Patients with Ovarian Cancer (N = 72) 20 Dose Modifications Patients, n (%) Any dose reduction, delay, or discontinuation due to TRAE 22 (31%) Dose reductions due to TRAE 17 (24%) Dose delays due to TRAE 8 (11%) Discontinuations due to TRAE 5 (7%) * Includes both related and unrelated SAEs as assessed by the Investigator ** One grade 5 pneumonitis assessed by the Investigator as related to study drug Abbreviations: SAEs = serious adverse events; TRAE = treatment related adverse event SAEs Patients, n (%) Notes Any SAEs * 28 (39%) ▪ SAEs reported in ≥2 (3%) patients included: ▪ 5 patients: Gastrointestinal obstruction (0 related) ▪ 4 patients each: Abdominal pain (2 related), pyrexia (4 related), and vomiting (3 related) ▪ 2 patients each: Cerebrovascular accident/transient ischemic attack (0 related), pneumonitis (2 related, Grade 2 and Grade 5 ** ), pneumonia (0 related), r espiratory failure (0 related), renal impairment (1 related), fatigue (1 related), and atrial fibrillation (0 related) Treatment - Related SAEs 11 (15%)
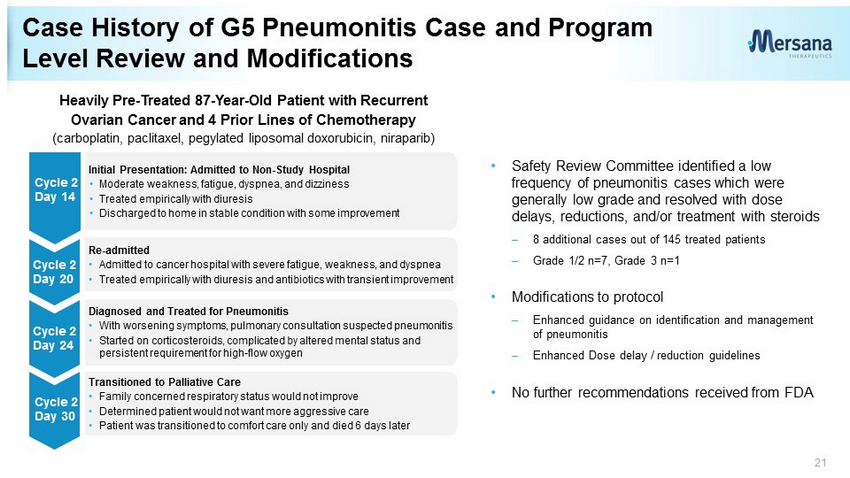
• Safety Review Committee identified a low frequency of pneumonitis cases which were generally low grade and resolved with dose delays, reductions, and/or treatment with steroids – 8 additional cases out of 145 treated patients – Grade 1/2 n=7, Grade 3 n=1 • Modifications to protocol – Enhanced guidance on identification and management of pneumonitis – Enhanced Dose delay / reduction guidelines • No further recommendations received from FDA Case History of G5 Pneumonitis Case and Program Level Review and Modifications 21 Diagnosed and Treated for Pneumonitis • With worsening symptoms, pulmonary consultation suspected pneumonitis • Started on corticosteroids, complicated by altered mental status and persistent requirement for high - flow oxygen Transitioned to Palliative Care • Family concerned respiratory status would not improve • Determined patient would not want more aggressive care • Patient was transitioned to comfort care only and died 6 days later Re - admitted • Admitted to cancer hospital with severe fatigue, weakness, and dyspnea • Treated empirically with diuresis and antibiotics with transient improvement Initial Presentation: Admitted to Non - Study Hospital • Moderate weakness, fatigue, dyspnea, and dizziness • Treated empirically with diuresis • Discharged to home in stable condition with some improvement Cycle 2 Day 14 Cycle 2 Day 24 Cycle 2 Day 30 Cycle 2 Day 20 Heavily Pre - Treated 87 - Year - Old Patient with Recurrent Ovarian Cancer and 4 Prior Lines of Chemotherapy (carboplatin, paclitaxel, pegylated liposomal doxorubicin, niraparib)
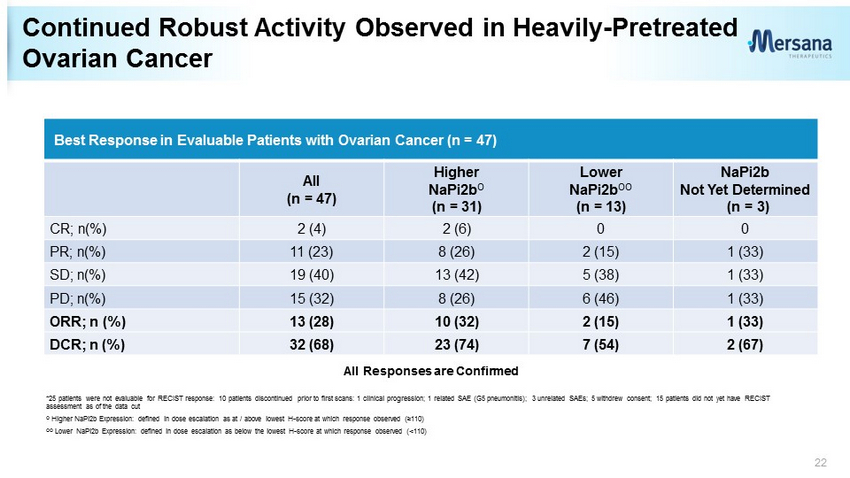
Continued Robust Activity Observed in Heavily - Pretreated Ovarian Cancer 22 Best Response in Evaluable Patients with Ovarian Cancer (n = 47) All (n = 47) Higher NaPi2b O (n = 31) Lower NaPi2b OO (n = 13) NaPi2b Not Yet Determined (n = 3) CR; n(%) 2 (4) 2 (6) 0 0 PR; n(%) 11 (23) 8 (26) 2 (15) 1 (33) SD; n(%) 19 (40) 13 (42) 5 (38) 1 (33) PD; n(%) 15 (32) 8 (26) 6 (46) 1 (33) ORR; n (%) 13 (28) 10 (32) 2 (15) 1 (33) DCR; n (%) 32 (68) 23 (74) 7 (54) 2 (67) *25 patients were not evaluable for RECIST response: 10 patients discontinued prior to first scans: 1 clinical progression; 1 re lated SAE (G5 pneumonitis); 3 unrelated SAEs; 5 withdrew consent; 15 patients did not yet have RECIST assessment as of the data cut O Higher NaPi2b Expression: defined in dose escalation as at / above lowest H - score at which response observed (≥110) OO Lower NaPi2b Expression: defined in dose escalation as below the lowest H - score at which response observed (<110) All Responses are Confirmed

Deep Responses Observed in Heavily - Pretreated Ovarian Cancer 23 ** ** *** Maximum % Change from Baseline in Target Lesions in Patients with Ovarian Cancer (n = 45*) * 2 patients not included in waterfall plot as tumor measurement data missing in the database as of data cut; both patients h ad BOR of PD due to new lesions ** Unconfirmed response, BOR per RECIST v1.1 is SD *** CR of target lesions and non - CR/non - PD of non - target lesions, BOR per RECIST v1.1 is PR H = Higher NaPi2b Expression; L = Lower NaPi2b Expression; ND = NaPi2b Expression not yet determined or tissue not available 30/45 (67%) had reductions in target tumor lesions
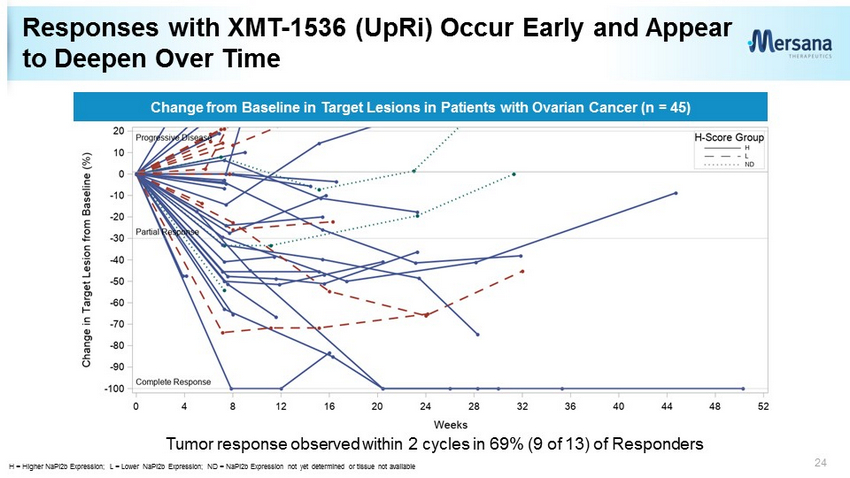
Responses with XMT - 1536 (UpRi) Occur Early and Appear to Deepen Over Time 24 Change from Baseline in Target Lesions in Patients with Ovarian Cancer (n = 45) H = Higher NaPi2b Expression; L = Lower NaPi2b Expression; ND = NaPi2b Expression not yet determined or tissue not available Tumor response observed within 2 cycles in 69% (9 of 13) of Responders
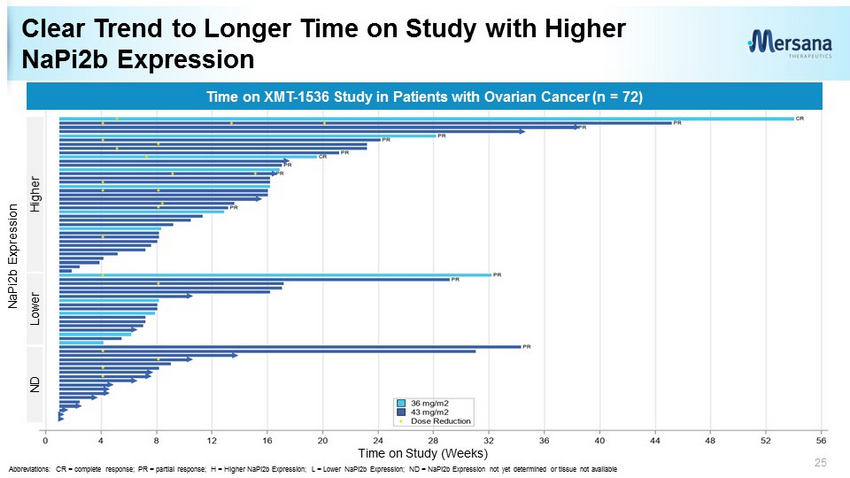
Clear Trend to Longer Time on Study with Higher NaPi2b Expression 25 Higher Lower ND Time on XMT - 1536 Study in Patients with Ovarian Cancer (n = 72) Time on Study (Weeks) NaPi2b Expression Abbreviations: CR = complete response; PR = partial response; H = Higher NaPi2b Expression ; L = Lower NaPi2b Expression; ND = NaPi2b Expression not yet determined or tissue not available
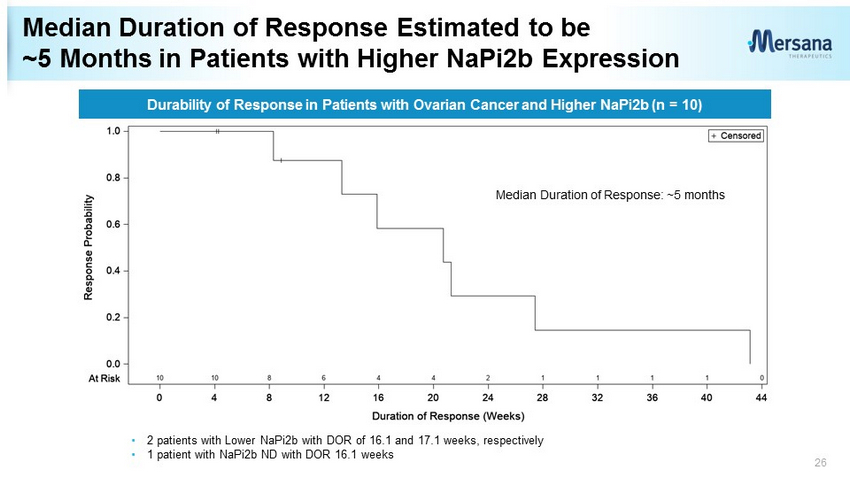
Median Duration of Response Estimated to be ~5 Months in Patients with Higher NaPi2b Expression 26 Durability of Response in Patients with Ovarian Cancer and Higher NaPi2b (n = 10) Median Duration of Response: ~5 months • 2 patients with Lower NaPi2b with DOR of 16.1 and 17.1 weeks, respectively • 1 patient with NaPi2b ND with DOR 16.1 weeks

Conclusions: UpRi (XMT - 1536) Expansion in Ovarian Cancer 27 • In this updated analysis of patients with ovarian cancer, UpRi (XMT - 1536) continued to be generally well - tolerated with a consistent profile – no severe neutropenia, peripheral neuropathy, or ocular toxicity • Consistent antitumor activity observed with UpRi (XMT - 1536) , including patients previously treated with bevacizumab and PARPi – Complete response observed in 2 patients with platinum - resistant ovarian cancer – Confirmed ORR of 32% and DCR of 74% in higher NaPi2b population – Median duration of response ~5 months in higher NaPi2b population • Trend toward higher response rate as well as deeper and more durable responses in patients with higher NaPi2b expression supports the continued development of NaPi2b diagnostic assay • These data support the continued development of UpRi (XMT - 1536) for the treatment of patients with platinum - resistant high - grade serous ovarian cancer who have received 1 to 4 prior lines of systemic therapy

UpRi (XMT - 1536): First - in - Class Dolaflexin ADC Targeting NaPi2b Ovarian Cancer Market Dynamics and UpRi Opportunities Brian DeSchuytner SVP Finance & Product Strategy

29 Frontline Therapy Surgery +/ - Neoadjuvant Platinum - Based Chemotherapy Platinum Sensitive Recurrence Platinum Doublet +/ - Bev +/ - PARP Maintenance +/ - Bev PARP Maintenance Platinum Doublet +/ - Bev +/ - PARP Maintenance PARP (if BRC/HRD 2 – 3 Prior) Platinum Resistant Recurrence PLD, Topotecan, Other Chemo Bev + Single Agent Chemo (1 – 2 prior) PARP (if BRCA and 2 – 3 prior) Prior Lines 0 1 - 3 1 - 4 Source: Product Labels, KOL interviews • Key approvals moving targeted therapy into the frontline – PAOLA - 1 (Bevacizumab + Olaparib maintenance vs Bevacizumab) – PRIMA (niraparib maintenance vs placebo) – GOG - 218 (Bevacizumab + platinum doublet vs platinum doublet) Early Use of Bevacizumab and PARP Inhibitors is Changing the Ovarian Cancer Landscape
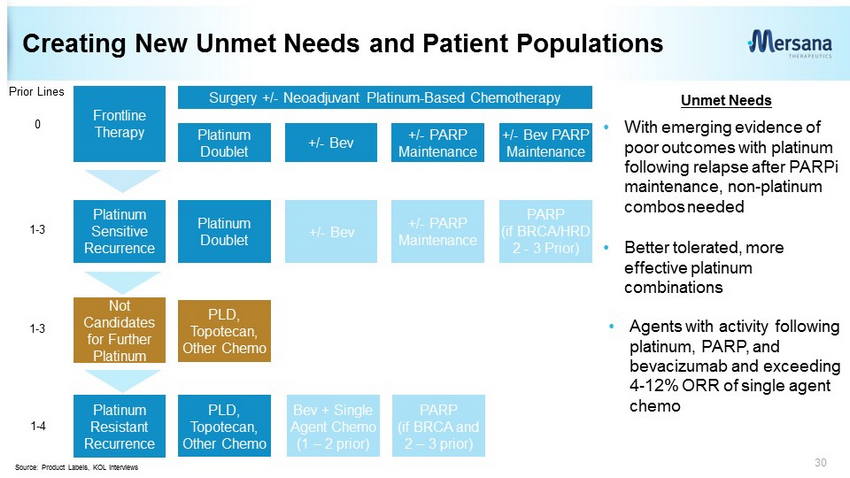
Creating New Unmet Needs and Patient Populations 30 Frontline Therapy Surgery +/ - Neoadjuvant Platinum - Based Chemotherapy Platinum Sensitive Recurrence Platinum Doublet +/ - Bev +/ - PARP Maintenance +/ - Bev PARP Maintenance Platinum Doublet +/ - Bev +/ - PARP Maintenance PARP (if BRCA/HRD 2 - 3 Prior) Platinum Resistant Recurrence PLD, Topotecan, Other Chemo Bev + Single Agent Chemo (1 – 2 prior) PARP (if BRCA and 2 – 3 prior) Prior Lines 0 1 - 3 1 - 4 Source: Product Labels, KOL interviews Not Candidates for Further Platinum 1 - 3 PLD, Topotecan, Other Chemo • With emerging evidence of poor outcomes with platinum following relapse after PARPi maintenance, non - platinum combos needed • Better tolerated, more effective platinum combinations • Agents with activity following platinum, PARP, and bevacizumab and exceeding 4 - 12% ORR of single agent chemo Unmet Needs
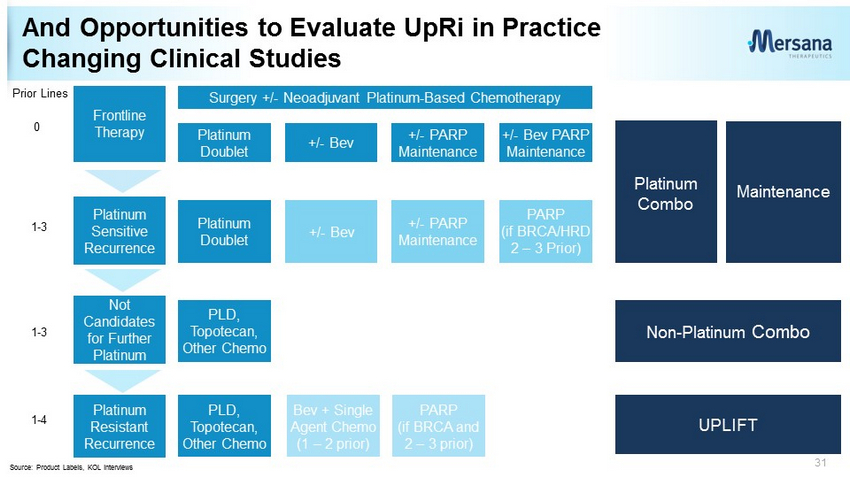
And Opportunities to Evaluate UpRi in Practice Changing Clinical Studies 31 Frontline Therapy Surgery +/ - Neoadjuvant Platinum - Based Chemotherapy Platinum Sensitive Recurrence Platinum Doublet +/ - Bev +/ - PARP Maintenance +/ - Bev PARP Maintenance Platinum Doublet +/ - Bev +/ - PARP Maintenance PARP (if BRCA/HRD 2 – 3 Prior) Platinum Resistant Recurrence PLD, Topotecan, Other Chemo Bev + Single Agent Chemo (1 – 2 prior) PARP (if BRCA and 2 – 3 prior) Prior Lines 0 1 - 3 1 - 4 Source: Product Labels, KOL interviews Not Candidates for Further Platinum 1 - 3 PLD, Topotecan, Other Chemo UPLIFT Non - Platinum Combo Platinum Combo Maintenance
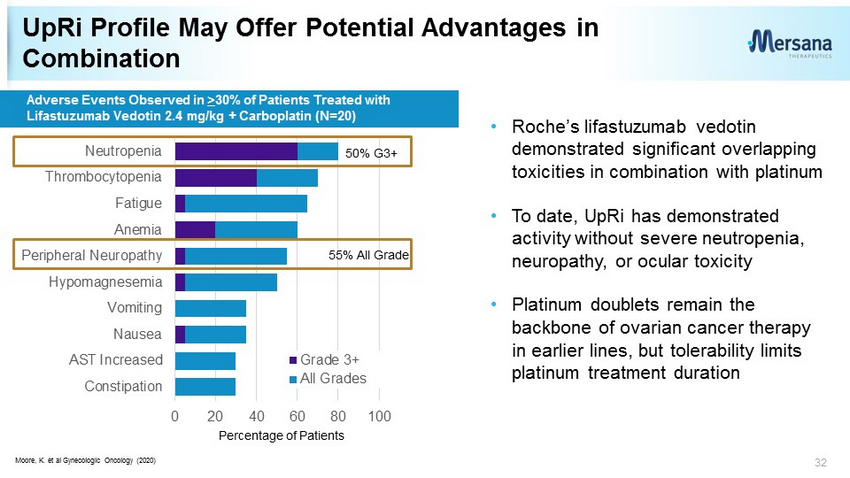
UpRi Profile May Offer Potential Advantages in Combination 32 0 20 40 60 80 100 Constipation AST Increased Nausea Vomiting Hypomagnesemia Peripheral Neuropathy Anemia Fatigue Thrombocytopenia Neutropenia Grade 3+ All Grades Adverse Events Observed in > 30% of Patients Treated with Lifastuzumab Vedotin 2.4 mg/kg + Carboplatin (N=20) Moore, K. et al Gynecologic Oncology (2020) 50% G3+ 55% All Grade Percentage of Patients • Roche’s lifastuzumab vedotin demonstrated significant overlapping toxicities in combination with platinum • To date, UpRi has demonstrated activity without severe neutropenia, neuropathy, or ocular toxicity • Platinum doublets remain the backbone of ovarian cancer therapy in earlier lines, but tolerability limits platinum treatment duration
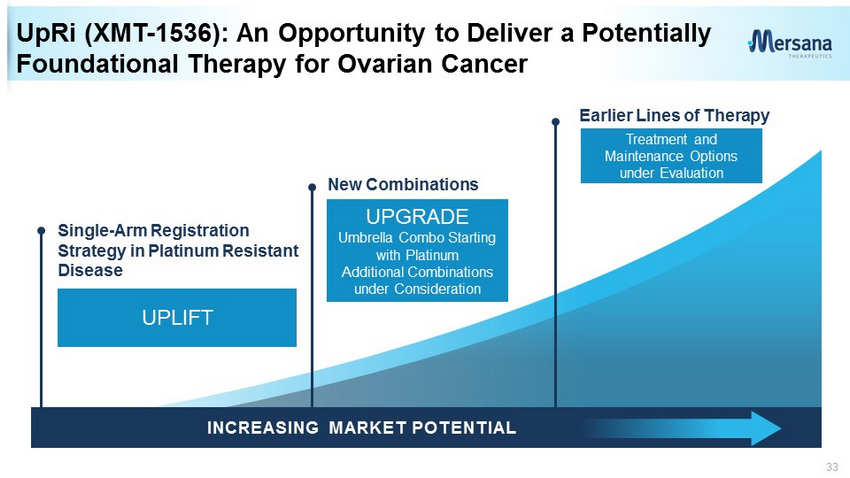
33 UpRi (XMT - 1536): An Opportunity to Deliver a Potentially Foundational Therapy for Ovarian Cancer INCREASING MARKET POTENTIAL . Earlier Lines of Therapy New Combinations UPGRADE Umbrella Combo Starting with Platinum Additional Combinations under Consideration Treatment and Maintenance Options under Evaluation UPLIFT Single - Arm Registration Strategy in Platinum Resistant Disease

XMT - 1660: First - in - Class B7 - H4 ADC Timothy B. Lowinger, PhD Chief Science & Technology Officer
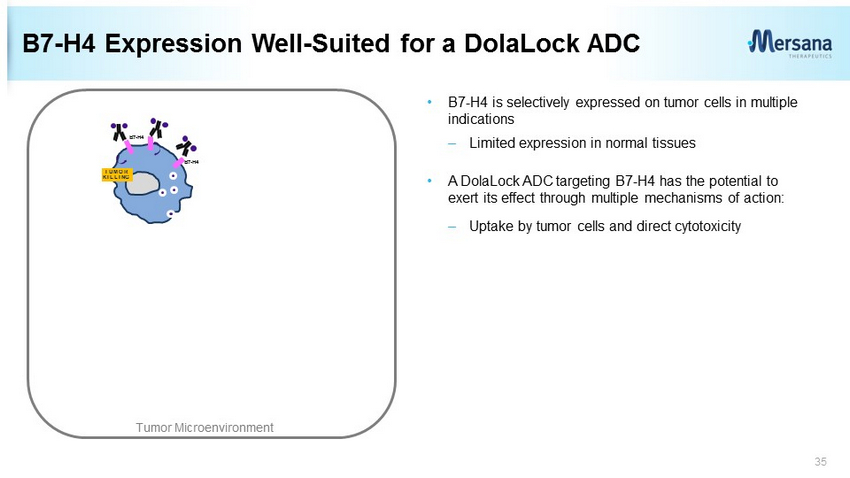
B7 - H4 Expression Well - Suited for a DolaLock ADC • B7 - H4 is selectively expressed on tumor cells in multiple indications – Limited expression in normal tissues • A DolaLock ADC targeting B7 - H4 has the potential to exert its effect through multiple mechanisms of action: – Uptake by tumor cells and direct cytotoxicity 35 B7 - H4 B7 - H4 TUMOR KILLING Tumor Microenvironment
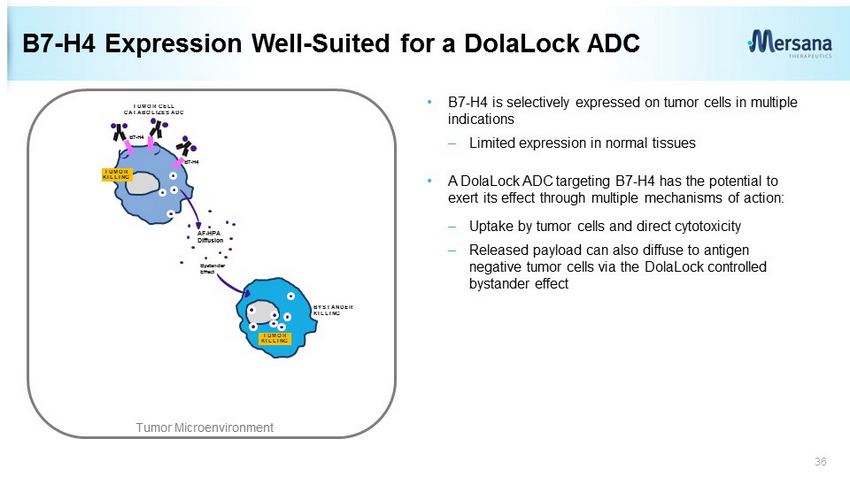
B7 - H4 Expression Well - Suited for a DolaLock ADC • B7 - H4 is selectively expressed on tumor cells in multiple indications – Limited expression in normal tissues • A DolaLock ADC targeting B7 - H4 has the potential to exert its effect through multiple mechanisms of action: – Uptake by tumor cells and direct cytotoxicity – Released payload can also diffuse to antigen negative tumor cells via the DolaLock controlled bystander effect 36 TUMOR CELL CATABOLIZES ADC AF - HPA Diffusion Bystander Effect BYSTANDER KILLING B7 - H4 B7 - H4 TUMOR KILLING TUMOR KILLING Tumor Microenvironment
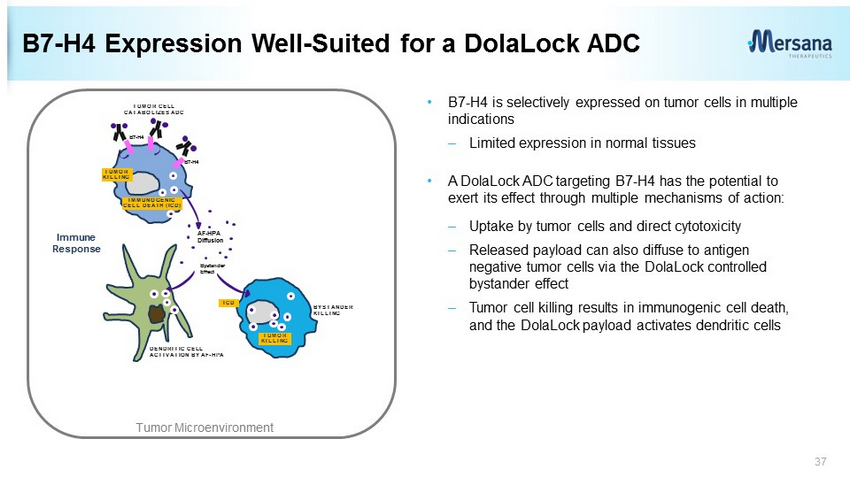
B7 - H4 Expression Well - Suited for a DolaLock ADC 37 TUMOR CELL CATABOLIZES ADC Immune Response AF - HPA Diffusion Bystander Effect DENDRITIC CELL ACTIVATION BY AF - HPA BYSTANDER KILLING IMMUNOGENIC CELL DEATH (ICD) ICD B7 - H4 B7 - H4 TUMOR KILLING TUMOR KILLING Tumor Microenvironment • B7 - H4 is selectively expressed on tumor cells in multiple indications – Limited expression in normal tissues • A DolaLock ADC targeting B7 - H4 has the potential to exert its effect through multiple mechanisms of action: – Uptake by tumor cells and direct cytotoxicity – Released payload can also diffuse to antigen negative tumor cells via the DolaLock controlled bystander effect – Tumor cell killing results in immunogenic cell death, and the DolaLock payload activates dendritic cells
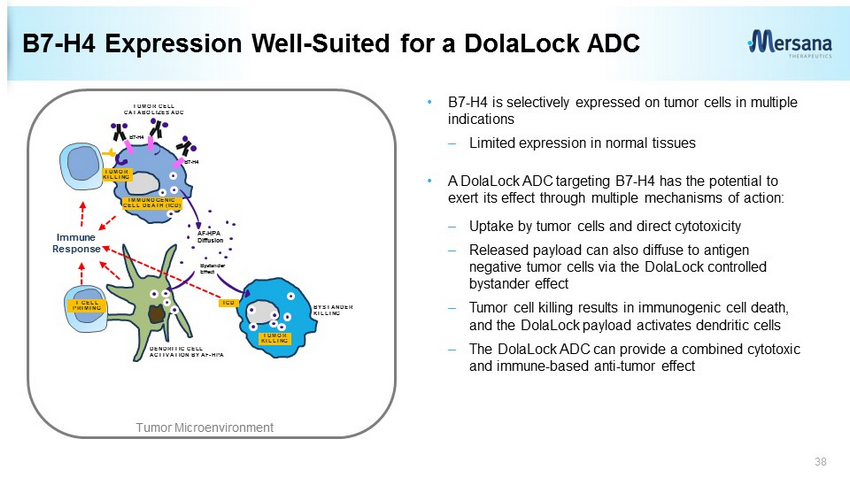
B7 - H4 Expression Well - Suited for a DolaLock ADC 38 TUMOR CELL CATABOLIZES ADC Immune Response AF - HPA Diffusion Bystander Effect DENDRITIC CELL ACTIVATION BY AF - HPA BYSTANDER KILLING IMMUNOGENIC CELL DEATH (ICD) ICD B7 - H4 B7 - H4 TUMOR KILLING TUMOR KILLING T CELL PRIMING Tumor Microenvironment • B7 - H4 is selectively expressed on tumor cells in multiple indications – Limited expression in normal tissues • A DolaLock ADC targeting B7 - H4 has the potential to exert its effect through multiple mechanisms of action: – Uptake by tumor cells and direct cytotoxicity – Released payload can also diffuse to antigen negative tumor cells via the DolaLock controlled bystander effect – Tumor cell killing results in immunogenic cell death, and the DolaLock payload activates dendritic cells – The DolaLock ADC can provide a combined cytotoxic and immune - based anti - tumor effect

B7 - H4 Expression Well - Suited for a DolaLock ADC 39 TUMOR CELL CATABOLIZES ADC Immune Response AF - HPA Diffusion Bystander Effect DENDRITIC CELL ACTIVATION BY AF - HPA BYSTANDER KILLING IMMUNOGENIC CELL DEATH (ICD) ICD B7 - H4 B7 - H4 B7 - H4 B7 - H4 TUMOR KILLING TUMOR KILLING TUMOR - ASSOCIATED MACROPHAGE CATABOLIZES ADC T CELL PRIMING Tumor Microenvironment • B7 - H4 is selectively expressed on tumor cells in multiple indications – Limited expression in normal tissues • A DolaLock ADC targeting B7 - H4 has the potential to exert its effect through multiple mechanisms of action: – Uptake by tumor cells and direct cytotoxicity – Released payload can also diffuse to antigen negative tumor cells via the DolaLock controlled bystander effect – Tumor cell killing results in immunogenic cell death, and the DolaLock payload activates dendritic cells – The DolaLock ADC can provide a combined cytotoxic and immune - based anti - tumor effect • B7 - H4 is also expressed on tumor - associated macrophages which can potentially further contribute to the effect

B7 - H4 Expression Well - Suited for a DolaLock ADC 40 TUMOR CELL CATABOLIZES ADC Immune Response AF - HPA Diffusion Bystander Effect DENDRITIC CELL ACTIVATION BY AF - HPA BYSTANDER KILLING IMMUNOGENIC CELL DEATH (ICD) ICD B7 - H4 B7 - H4 B7 - H4 B7 - H4 TUMOR KILLING TUMOR KILLING TUMOR - ASSOCIATED MACROPHAGE CATABOLIZES ADC T CELL PRIMING Tumor Microenvironment “The Perfect Storm” • B7 - H4 is selectively expressed on tumor cells in multiple indications – Limited expression in normal tissues • A DolaLock ADC targeting B7 - H4 has the potential to exert its effect through multiple mechanisms of action: – Uptake by tumor cells and direct cytotoxicity – Released payload can also diffuse to antigen negative tumor cells via the DolaLock controlled bystander effect – Tumor cell killing results in immunogenic cell death, and the DolaLock payload activates dendritic cells – The DolaLock ADC can provide a combined cytotoxic and immune - based anti - tumor effect • B7 - H4 is also expressed on tumor - associated macrophages which can potentially further contribute to the effect
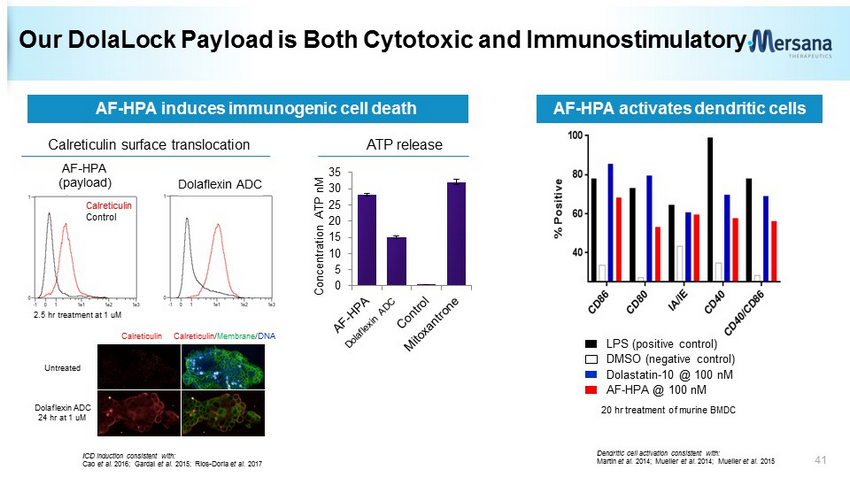
Our DolaLock Payload is Both Cytotoxic and Immunostimulatory Calreticulin surface translocation ATP release 0 5 10 15 20 25 30 35 Concentration ATP nM LPS (positive control) DMSO (negative control) Dolastatin - 10 @ 100 nM AF - HPA @ 100 nM AF - HPA induces immunogenic cell death Dolaflexin ADC AF - HPA (payload) Calreticulin Control AF - HPA activates dendritic cells 2.5 hr treatment at 1 uM 20 hr treatment of murine BMDC Dendritic cell activation consistent with: Martin et al. 2014; Mueller et al. 2014; Mueller et al. 2015 ICD induction consistent with: Cao et al. 2016; Gardai et al. 2015; Rios - Doria et al. 2017 Calreticulin Calreticulin / Membrane / DNA Untreated Dolaflexin ADC 24 hr at 1 uM 41
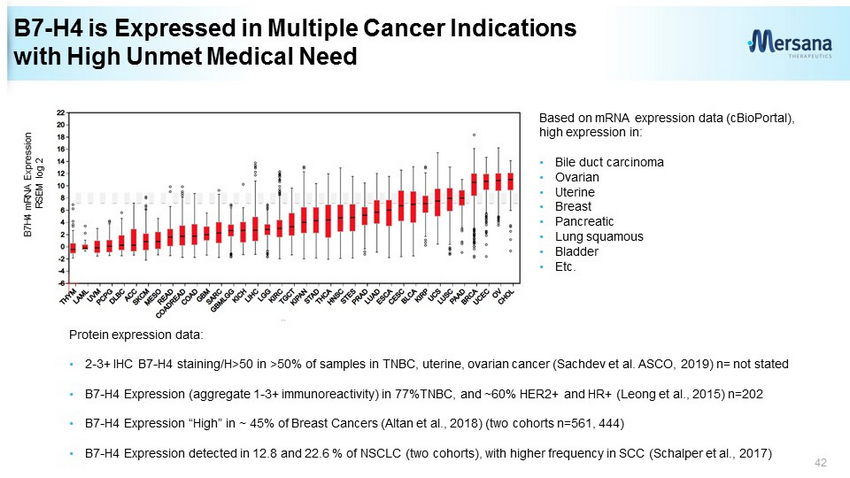
B7 - H4 is Expressed in Multiple Cancer Indications with High Unmet Medical Need 42 Protein expression data: • 2 - 3+ IHC B7 - H4 staining/H>50 in >50% of samples in TNBC, uterine, ovarian cancer (Sachdev et al. ASCO, 2019) n= not stated • B7 - H4 Expression (aggregate 1 - 3+ immunoreactivity) in 77%TNBC, and ~60% HER2+ and HR+ (Leong et al., 2015) n=202 • B7 - H4 Expression “High” in ~ 45% of Breast Cancers ( Altan et al., 2018) (two cohorts n=561, 444) • B7 - H4 Expression detected in 12.8 and 22.6 % of NSCLC (two cohorts), with higher frequency in SCC ( Schalper et al., 2017) Based on mRNA expression data ( cBioPortal ), high expression in: • Bile duct carcinoma • Ovarian • Uterine • Breast • Pancreatic • Lung squamous • Bladder • Etc. B7H4 mRNA Expression RSEM log 2
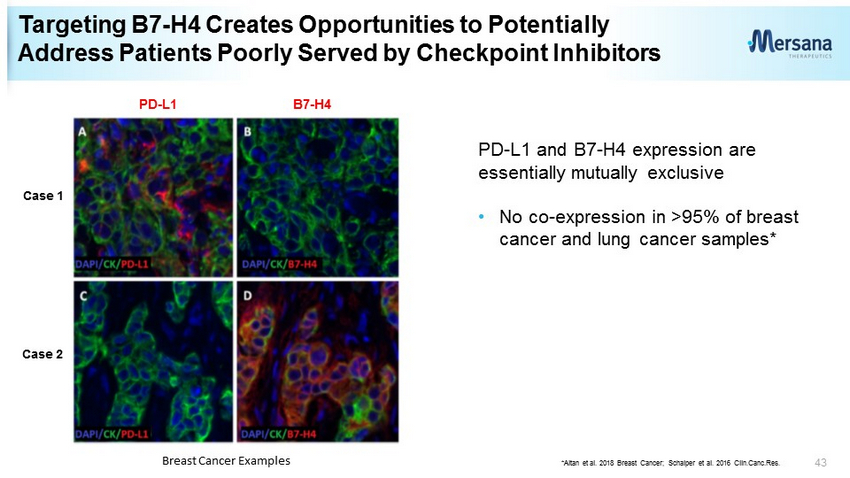
Targeting B7 - H4 Creates Opportunities to Potentially Address Patients Poorly Served by Checkpoint Inhibitors PD - L1 and B7 - H4 expression are essentially mutually exclusive • No co - expression in >95% of breast cancer and lung cancer samples* 43 Case 1 Case 2 Breast Cancer Examples PD - L1 B7 - H4 * Altan et al. 2018 Breast Cancer; Schalper et al. 2016 Clin.Canc.Res .
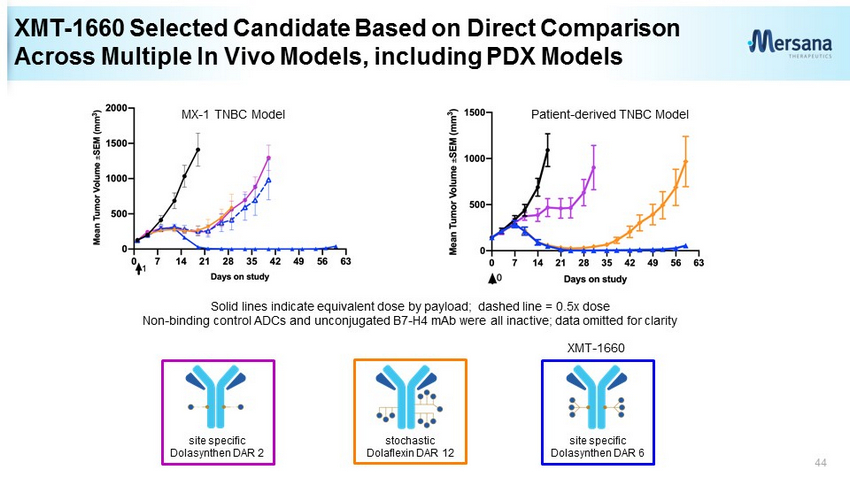
XMT - 1660 Selected Candidate Based on Direct Comparison Across Multiple In Vivo Models, including PDX Models 44 site specific Dolasynthen DAR 2 stochastic Dolaflexin DAR 12 site specific Dolasynthen DAR 6 XMT - 1660 Solid lines indicate equivalent dose by payload; dashed line = 0.5x dose Non - binding control ADCs and unconjugated B7 - H4 mAb were all inactive; data omitted for clarity MX - 1 TNBC Model Patient - derived TNBC Model
Preclinical Profile of XMT - 1660 Supports Advancement 45 • Pharmacokinetic profile displays long half life (~5 days in NHPs) and dose - dependent exposure – Highly stable with very low (<0.1%) free payload detected in circulation • Well tolerated in NHPs after multiple doses • Demonstrated therapeutic index based on well - tolerated exposure in NHPs and efficacious exposures in mouse

Summary of the Opportunity 46 • Potential first - in - class opportunity with compelling target biology and unique fit to DolaLock payload • Clinical candidate was optimized on multiple parameters – DAR, site specific bioconjugation, selection of optimal antibody – Dolasynthen DAR - 6 consistently outperformed stochastic Dolaflexin DAR - 12 and site specific Dolasynthen DAR - 2 across multiple tumor models • Expression in areas of high unmet medical need: TNBC, ER+ BC, Endometrial cancer and others – Opportunity for accelerated development path in key indications of interest • Expected to enter the clinic in Q1 2022

Corporate Update Anna Protopapas President & CEO
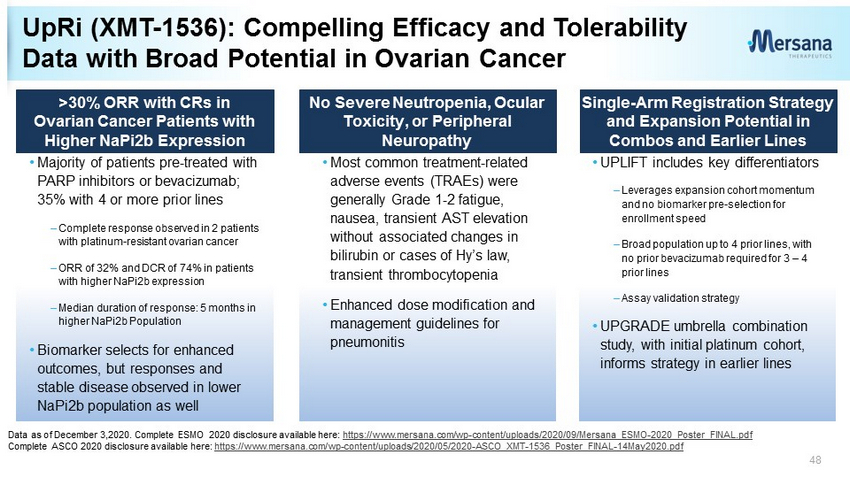
>30% ORR with CRs in Ovarian Cancer Patients with Higher NaPi2b Expression No Severe Neutropenia, Ocular T oxicity , or Peripheral N europathy • Majority of patients pre - treated with PARP inhibitors or bevacizumab; 35% with 4 or more prior lines – Complete response observed in 2 patients with platinum - resistant ovarian cancer – ORR of 32% and DCR of 74% in patients with higher NaPi2b expression – Median duration of response: 5 months in higher NaPi2b Population • Biomarker selects for enhanced outcomes, but responses and stable disease observed in lower NaPi2b population as well • Most common treatment - related adverse events (TRAEs) were generally Grade 1 - 2 fatigue, nausea, transient AST elevation without associated changes in bilirubin or cases of Hy’s law, transient thrombocytopenia • Enhanced dose modification and management guidelines for pneumonitis Single - Arm Registration Strategy and Expansion Potential in Combos and Earlier Lines • UPLIFT includes key differentiators – Leverages expansion cohort momentum and no biomarker pre - selection for enrollment speed – Broad population up to 4 prior lines, with no prior bevacizumab required for 3 – 4 prior lines – Assay validation strategy • UPGRADE umbrella combination study, with initial platinum cohort, informs strategy in earlier lines UpRi (XMT - 1536): Compelling Efficacy and Tolerability Data with Broad Potential in Ovarian Cancer Data as of December 3,2020. Complete ESMO 2020 disclosure available here: https://www.mersana.com/wp - content/uploads/2020/09/Mersana_ESMO - 2020_Poster_FINAL.pdf Complete ASCO 2020 disclosure available here: https://www.mersana.com/wp - content/uploads/2020/05/2020 - ASCO_XMT - 1536_Poster_FINAL - 14May2020.pdf 48
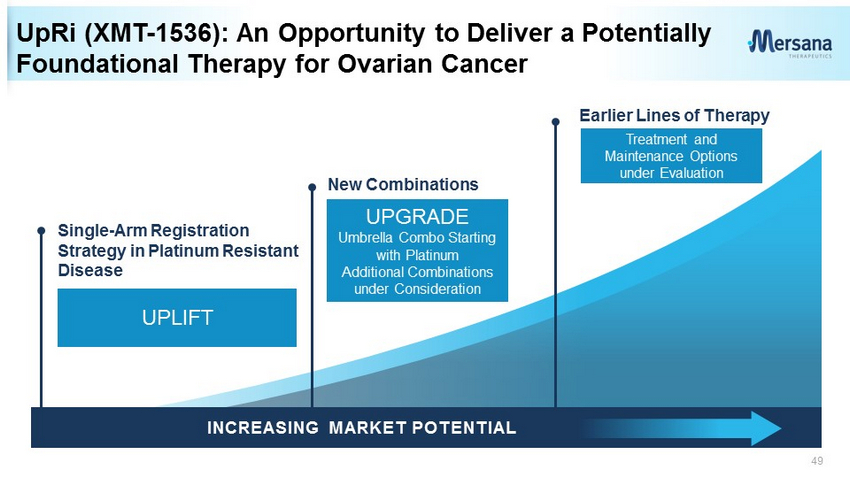
49 UpRi (XMT - 1536): An Opportunity to Deliver a Potentially Foundational Therapy for Ovarian Cancer INCREASING MARKET POTENTIAL . UPLIFT Earlier Lines of Therapy New Combinations Single - Arm Registration Strategy in Platinum Resistant Disease UPGRADE Umbrella Combo Starting with Platinum Additional Combinations under Consideration Treatment and Maintenance Options under Evaluation

Goals and Anticipated Milestones for 2021 50 Upifitamab Rilsodotin UpRi (XMT - 1536) • Q1 2021: Initiate UPLIFT single - arm registration strategy as amendment • Q3 2021: Initiate UPGRADE combination dose escalation umbrella study • 2H 2021: Report updated interim data from NSCLC expansion cohort XMT - 1592 • 2H 2021: Report dose escalation data • Q4 2021: Outline further development path XMT - 1660 • Q4 2021: Complete IND - enabling studies to initiate Phase I dose escalation in 2022 XMT - 2056 • Q4 2021: Complete IND - enabling studies to initiate Phase I dose escalation in 2022 • Q4 2021: Disclose target Corporate • Continue to leverage proprietary platforms to expand pipeline • Proactively evaluate potential for collaborations that maximize value

51 ADC Program Target Indication Platform Discovery Preclinical P1 Dose Escalation P1 Proof of Concept P2/Pivotal XMT - 1536* NaPi2b Ovarian Cancer Dolaflexin NSCLC Adenocarcinoma Dolaflexin XMT - 1592* NaPi2b Ovarian Cancer NSCLC Adenocarcinoma Dolasynthen XMT - 1660 B7 - H4 Multiple Solid Tumors Dolasynthen XMT - 2056 Undisclosed Undisclosed Immunosynthen Multiple Programs Undisclosed Undisclosed Immunosynthen Multiple Programs Undisclosed Undisclosed Dolasynthen or Dolaflexin Multiple Multiple Undisclosed Dolaflexin ASN004 5T4 Undisclosed Dolaflexin *NaPi2b antibody used in XMT - 1536 and XMT - 1592 is in - licensed from Recepta Biopharma. Recepta has rights to commercialize XMT - 1536 and XMT - 1592 in Brazil We are Leveraging our Novel ADC Platforms to Generate Differentiated Product Candidates
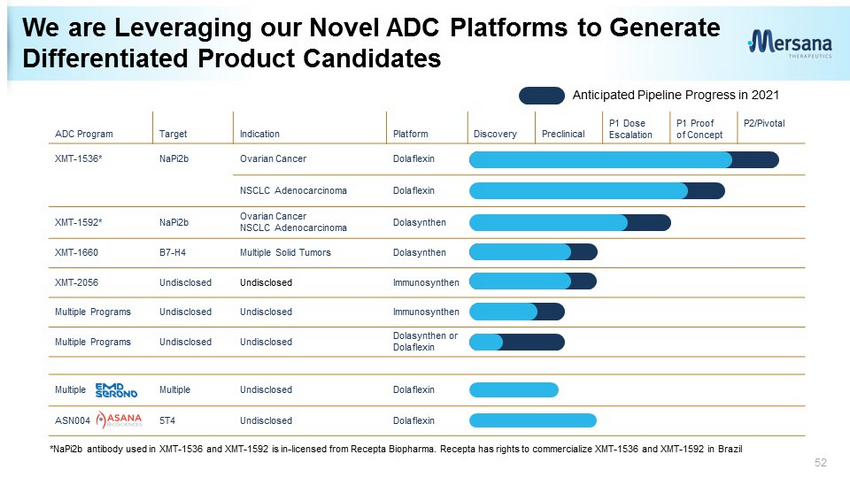
52 ADC Program Target Indication Platform Discovery Preclinical P1 Dose Escalation P1 Proof of Concept P2/Pivotal XMT - 1536* NaPi2b Ovarian Cancer Dolaflexin NSCLC Adenocarcinoma Dolaflexin XMT - 1592* NaPi2b Ovarian Cancer NSCLC Adenocarcinoma Dolasynthen XMT - 1660 B7 - H4 Multiple Solid Tumors Dolasynthen XMT - 2056 Undisclosed Undisclosed Immunosynthen Multiple Programs Undisclosed Undisclosed Immunosynthen Multiple Programs Undisclosed Undisclosed Dolasynthen or Dolaflexin Multiple Multiple Undisclosed Dolaflexin ASN004 5T4 Undisclosed Dolaflexin *NaPi2b antibody used in XMT - 1536 and XMT - 1592 is in - licensed from Recepta Biopharma. Recepta has rights to commercialize XMT - 1536 and XMT - 1592 in Brazil We are Leveraging our Novel ADC Platforms to Generate Differentiated Product Candidates Anticipated Pipeline Progress in 2021

Question & Answer Session




















































