Exhibit 99.3

Initial Phase 1 Dose Escalation Data for Emi - Le ( emiltatug ledadotin ; XMT - 1660) January 10, 2025

Legal Disclaimer 2 This presentation contains “forward - looking” statements and information within the meaning of the Private Securities Litigation Reform Act of 1995 . These statements may be identified by words such as “aims,” “anticipates,” “believes,” “could,” “estimates,” “expects,” “forecasts,” “goal,” “intends,” “may,” “plans,” “possible,” “potential,” “seeks,” “will” and variations of these words or similar expressions, although not all forward - looking statements contain these words . Forward - looking statements in this presentation include, but are not limited to, statements regarding Mersana Therapeutics, Inc . ’s (“Mersana”) business strategy, mission and vision ; the development and potential of Mersana’s product candidates and platforms, including Emi - Le (XMT - 1660 ) and Dolasynthen ; the potential clinical benefits of Emi - Le ; and the design, progression and timing of Mersana’s clinical trial of Emi - Le . Mersana may not actually achieve the plans, intentions or expectations disclosed in these forward - looking statements, and you should not place undue reliance on these forward - looking statements . Actual results or events could differ materially from the plans, intentions and expectations disclosed in these forward - looking statements as a result of various factors, including, among other things, uncertainties inherent in research and development, in the advancement, progression and completion of clinical trials and in the clinical development of Mersana's product candidates, including Emi - Le and XMT - 2056 ; the risk that Mersana may not realize the intended benefits of its platforms, technology and collaborations ; the risk that outcomes of preclinical studies may not be predictive of clinical trial results ; the risk that initial or interim results from a clinical trial may not be predictive of the final results of the trial or the results of future trials ; the risk that clinical trial data may not support regulatory applications or approvals ; the risk that Mersana may not realize the intended benefits of its platforms, technology and collaborations ; and other important factors, any of which could cause Mersana's actual results to differ from those contained in the forward - looking statements, that are described in greater detail in the section entitled “Risk Factors” in Mersana’s Quarterly Report on Form 10 - Q filed with the Securities and Exchange Commission (“SEC”) on November 13 , 2024 , as well as in other filings Mersana may make with the SEC in the future . New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties . Any forward - looking statements contained in this presentation speak only as of the date of this presentation, and Mersana expressly disclaims any obligation to update any forward - looking statements contained herein, whether because of any new information, future events, changed circumstances or otherwise, except as otherwise required by law . Mersana owns various trademark registrations and applications, and unregistered trademarks, including Mersana’s name and its corporate logo . All other trade names, trademarks and service marks of other companies appearing in this presentation are the property of their respective holders . Solely for convenience, the trademarks and trade names in this presentation may be referred to without the ®, or © symbols, but such references should not be construed as any indicator that their respective owners will not assert, to the fullest extent under applicable law, their rights thereto . Mersana does not intend to use or display other companies' trademarks and trade names to imply a relationship with, or endorsement or sponsorship of Mersana by, any other companies .

3 While today’s ADCs provide substantial benefits to some patients, significant platform and payload limitations remain. Mersana is focused on developing novel platforms and payloads that enable ADCs with meaningfully improved safety and efficacy. ADCs, antibody - drug conjugates
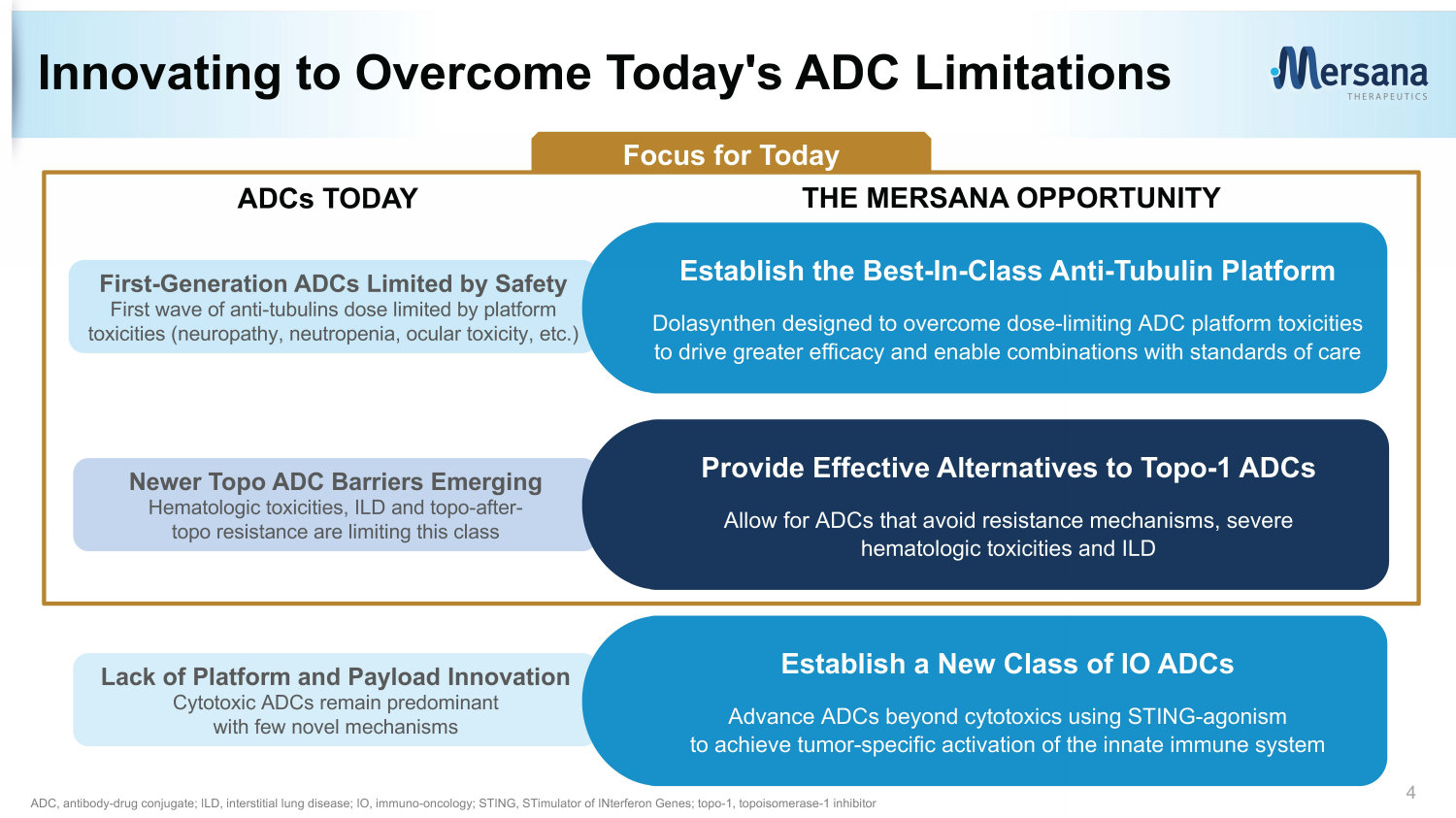
Lack of Platform and Payload Innovation Cytotoxic ADCs remain predominant with few novel mechanisms Innovating to Overcome Today's ADC Limitations 4 ADC, antibody - drug conjugate; ILD, interstitial lung disease; IO, immuno - oncology; STING, STimulator of INterferon Genes; topo - 1, topoisomerase - 1 inhibitor ADCs TODAY First - Generation ADCs Limited by Safety First wave of anti - tubulins dose limited by platform toxicities (neuropathy, neutropenia, ocular toxicity, etc.) Establish the Best - In - Class Anti - Tubulin Platform Dolasynthen designed to overcome dose - limiting ADC platform toxicities to drive greater efficacy and enable combinations with standards of care THE MERSANA OPPORTUNITY Newer Topo ADC Barriers Emerging Hematologic toxicities, ILD and topo - after - topo resistance are limiting this class Provide Effective Alternatives to Topo - 1 ADCs Allow for ADCs that avoid resistance mechanisms, severe hematologic toxicities and ILD Establish a New Class of IO ADCs Advance ADCs beyond cytotoxics using STING - agonism to achieve tumor - specific activation of the innate immune system Focus for Today
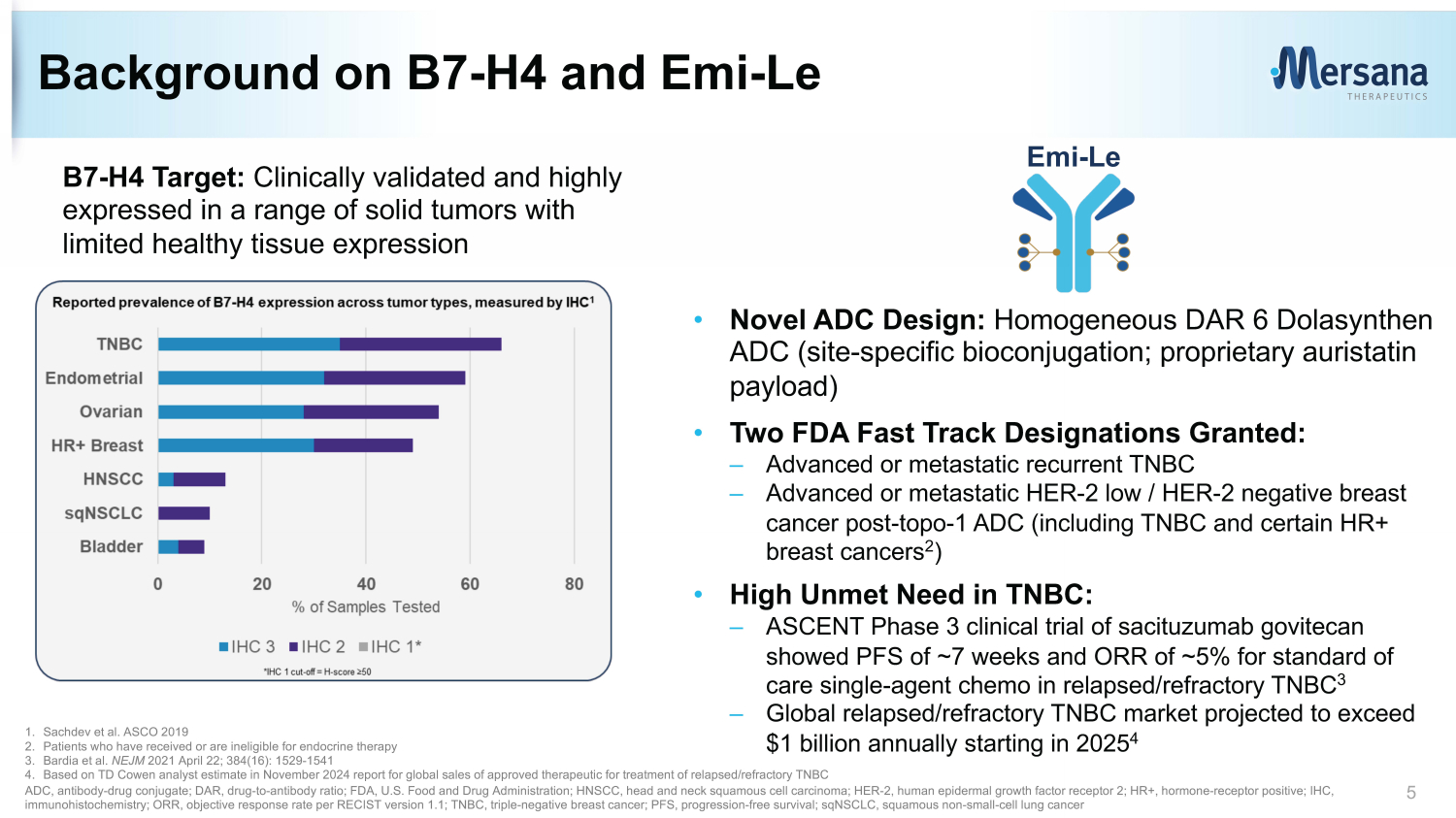
Background on B7 - H4 and Emi - Le B7 - H4 Target: Clinically validated and highly expressed in a range of solid tumors with limited healthy tissue expression • Novel ADC Design: Homogeneous DAR 6 Dolasynthen ADC (site - specific bioconjugation; proprietary auristatin payload) • Two FDA Fast Track Designations Granted: – Advanced or metastatic recurrent TNBC – Advanced or metastatic HER - 2 low / HER - 2 negative breast cancer post - topo - 1 ADC (including TNBC and certain HR+ breast cancers 2 ) • High Unmet Need in TNBC: – ASCENT Phase 3 clinical trial of sacituzumab govitecan showed PFS of ~7 weeks and ORR of ~5% for standard of care single - agent chemo in relapsed/refractory TNBC 3 – Global relapsed/refractory TNBC market projected to exceed $1 billion annually starting in 2025 4 5 Emi - Le 1. Sachdev et al. ASCO 2019 2. Patients who have received or are ineligible for endocrine therapy 3. Bardia et al. NEJM 2021 April 22; 384(16): 1529 - 1541 4. Based on TD Cowen analyst estimate in November 2024 report for global sales of approved therapeutic for treatment of relapsed /re fractory TNBC ADC, antibody - drug conjugate; DAR, drug - to - antibody ratio; FDA, U.S. Food and Drug Administration; HNSCC, head and neck squamous cell carcinoma; HER - 2, human e pidermal growth f actor r eceptor 2; HR+, hormone - receptor positive; IHC, immunohistochemistry; ORR, objective response rate per RECIST version 1.1; TNBC, triple - negative breast cancer; PFS, progression - free survival; sqNSCLC , squamous non - small - cell lung cancer

• Most common TRAEs: transient AST increase; low - grade nausea and fatigue; generally asymptomatic and reversible proteinuria • No Grade 4 or Grade 5 TRAEs reported; no dose - limiting treatment - related neutropenia, neuropathy, ocular toxicity, ILD or thrombocytopenia reported • Profile may enable combinations with platinum chemotherapy, other ADCs, PD - (L)1, etc. • 7 of 9 evaluable patients with B7 - H4 high tumors had ≥30% tumor reductions in target lesions; 2 confirmed responses • Implementing proteinuria mitigation efforts and continuing dose exploration in High Dose Range to identify a second expansion dose • Encouraging clinical activity and tolerability in Intermediate Dose Range (38.1 – 67.4 mg/m 2 ): – 23% confirmed ORR (6/26) in evaluable patients with B7 - H4 high tumors – 23% confirmed ORR (3/13) in evaluable patients with B7 - H4 high TNBC post - topo - 1 ADCs • Expansion underway at high end of Intermediate Dose Range (67.4 mg/m 2 Q4W) in TNBC post - topo - 1 ADC; high unmet need (~5% ORR and ~7 week PFS for standard of care 1 ) Emi - Le: A Potential Best - in - Class B7 - H4 ADC 6 Potential for Even Greater Clinical Activity in High Dose Range Encouraging Clinical Activity Observed in Post - Topo - 1 TNBC; Expansion Initiated Differentiated Safety and Tolerability Profile 1. Bardia et al. NEJM 2021 April 22; 384(16): 1529 - 1541 ADC, antibody - drug conjugate; mg/m 2 , milligrams per meter squared; ORR, objective response rate per RECIST version 1.1; PD - (L)1, programmed cell death ligand 1; PF S, progression - free survival; PRs, partial responses; Q4W, dosing every four weeks; TNBC, triple - negative breast cancer; topo - 1, topoisomerase - 1 inhibitor; TRAEs, treatment - related adverse event s; ILD, interstitial lung disease December 13, 2024 data cutoff

Trial Design and Demographics
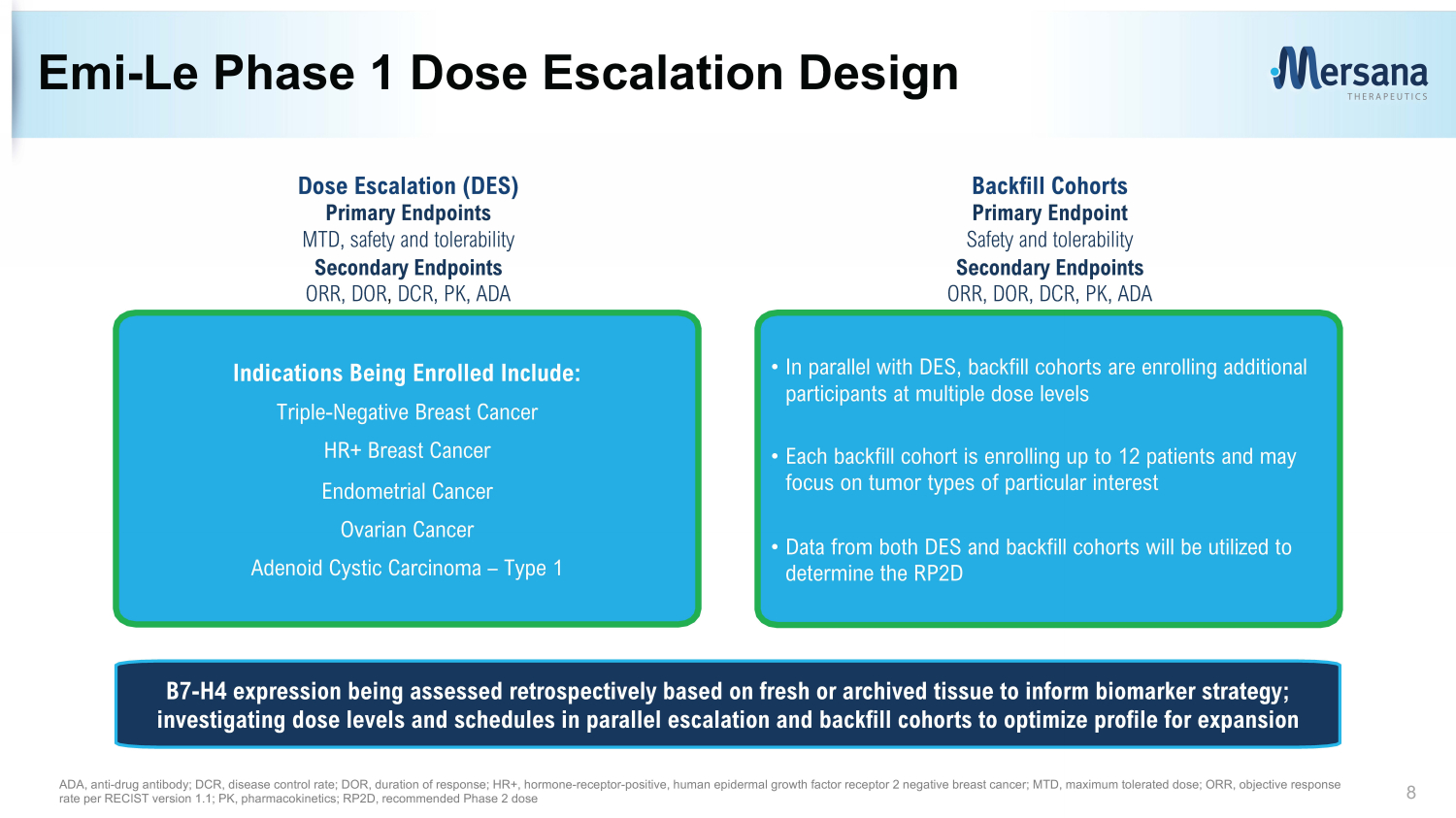
Emi - Le Phase 1 Dose Escalation Design 8 • In parallel with DES, backfill cohorts are enrolling additional participants at multiple dose levels • Each backfill cohort is enrolling up to 12 patients and may focus on tumor types of particular interest • Data from both DES and backfill cohorts will be utilized to determine the RP2D Indications Being Enrolled Include: Triple - Negative Breast Cancer HR+ Breast Cancer Endometrial Cancer Ovarian Cancer Adenoid Cystic Carcinoma – Type 1 Dose Escalation (DES) Primary Endpoints MTD , s afety and tolerability Secondary Endpoints ORR, DOR , DCR, PK, ADA Backfill Cohorts Primary Endpoint Safety and tolerability Secondary Endpoints ORR, DOR, DCR, PK, ADA ADA, anti - drug antibody; DCR, disease control rate; DOR, duration of response; HR+, hormone - receptor - positive, human e pidermal growth f actor r eceptor 2 negative breast cancer ; MTD, maximum tolerated dose; ORR, objective response rate per RECIST version 1.1; PK, pharmacokinetics; RP2D, recommended Phase 2 dose B7 - H4 expression being assessed retrospectively based on fresh or archived tissue to inform biomarker strategy; investigating dose levels and schedules in parallel escalation and backfill cohorts to optimize profile for expansion

Broad range of doses and multiple dosing schedules investigated Phase 1 Dose Escalation and Backfill Enrollment 9 Doses and Schedules Investigated (in mg/m 2 per cycle) 67.4 (n=4) 80.0 (n=11) 95.0 (n=5) 38.1x2 (n=9) 44.5x2 (n=10) 1 38.1 (n=12) 44.5 (n=8) 50.7 (n=14) 59.0 (n=14) 67.4 (n=15) 28.7x2 (n=3) 7.2 (n=1) 14.4 (n=3) 21.6 (n=3) 28.7 (n=6) 28.7 (n=3) 115.0 (n=3) Subtherapeutic Dose Range Q3W Q4W D1+8 Q4W Intermediate Dose Range High Dose Range 38.1 (n=6) 1. Includes four patients who were enrolled to receive this starting dose and a pre - specified modified dose following cycle 1 Q3W, dosing once every three weeks; Q4W, dosing once every four weeks; AST, aspartate aminotransferase; D1+8 Q4W, dosing on d ays one and eight every four weeks; DLT, dose - limiting toxicity; mg/kg, milligrams per kilogram; mg/m 2 , milligrams per meter squared; TNBC, triple - negative breast cancer; topo - 1, topoisomerase - 1 inhibitor Initial dose selected for expansion ~1 mg/kg per cycle ~2 mg/kg per cycle • 130 patients dosed as of December 13, 2024 data cutoff • 5 DLTs observed: – Three Grade 3 transient AST increases (one at 59 mg/m 2 Q4W and two at 115 mg/m 2 Q4W); 115 mg/m 2 deemed to be a non - tolerated dose – One reversible Grade 3 proteinuria accompanied by peripheral oedema at 80 mg/m 2 Q4W (event confounded by concurrent gout flare) – One Grade 3 pyrexia at 44.5 mg/m 2 D1+8 Q4W (self - reported and rapidly resolved) • Expansion initiated at 67.4 mg/m 2 Q4W in TNBC post - topo - 1 ADC; continuing dose exploration in High Dose Range
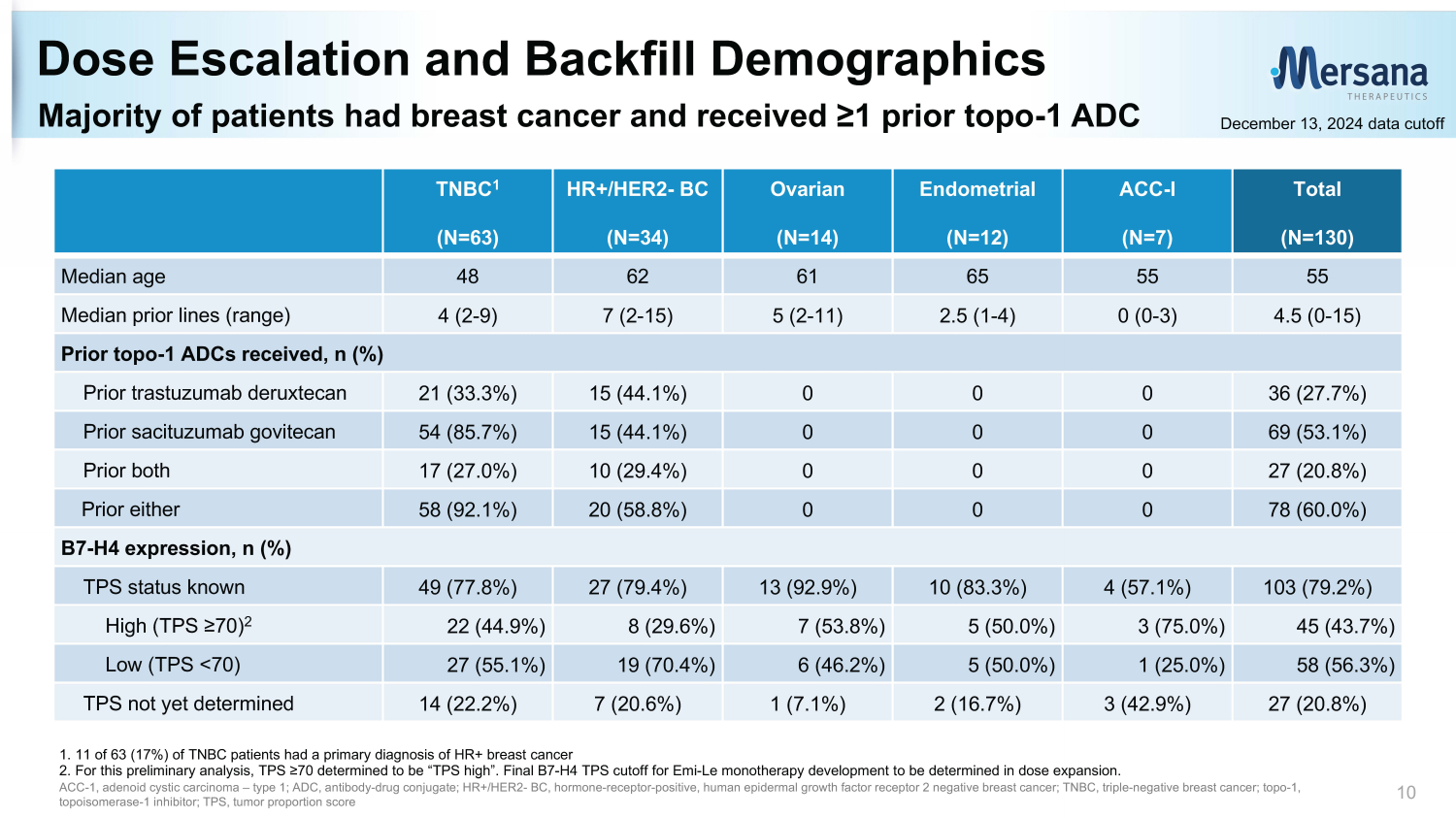
Majority of patients had breast cancer and received ≥1 prior topo - 1 ADC Dose Escalation and Backfill Demographics 10 1. 11 of 63 (17%) of TNBC patients had a primary diagnosis of HR+ breast cancer 2. For this preliminary analysis, TPS ≥70 determined to be “TPS high”. Final B7 - H4 TPS cutoff for Emi - Le monotherapy development to be determined in dose expansion. ACC - 1, a denoid cystic c arcinoma – type 1; ADC, antibody - drug conjugate; HR+/HER2 - BC, hormone - receptor - positive, human e pidermal growth f actor r eceptor 2 negative breast cancer; TNBC, triple - negative breast cancer; topo - 1, topoisomerase - 1 inhibitor; TPS, tumor proportion score Total (N=130) ACC - I (N=7) Endometrial (N=12) Ovarian (N=14) HR+/HER2 - BC (N=34) TNBC 1 (N=63) 55 55 65 61 62 48 Median age 4.5 (0 - 15) 0 (0 - 3) 2.5 (1 - 4) 5 (2 - 11) 7 (2 - 15) 4 (2 - 9) Median prior lines (range) Prior topo - 1 ADCs received, n (%) 36 (27.7%) 0 0 0 15 (44.1%) 21 (33.3%) Prior trastuzumab deruxtecan 69 (53.1%) 0 0 0 15 (44.1%) 54 (85.7%) Prior sacituzumab govitecan 27 (20.8%) 0 0 0 10 (29.4%) 17 (27.0%) Prior both 78 (60.0%) 0 0 0 20 (58.8%) 58 (92.1%) Prior either B7 - H4 expression, n (%) 103 (79.2%) 4 (57.1%) 10 (83.3%) 13 (92.9%) 27 (79.4%) 49 (77.8%) TPS status known 45 (43.7%) 3 (75.0%) 5 (50.0%) 7 (53.8%) 8 (29.6%) 22 (44.9%) High (TPS ≥70) 2 58 (56.3%) 1 (25.0%) 5 (50.0%) 6 (46.2%) 19 (70.4%) 27 (55.1%) Low (TPS <70) 27 (20.8%) 3 (42.9%) 2 (16.7%) 1 (7.1%) 7 (20.6%) 14 (22.2%) TPS not yet determined December 13, 2024 data cutoff

Safety and Tolerability
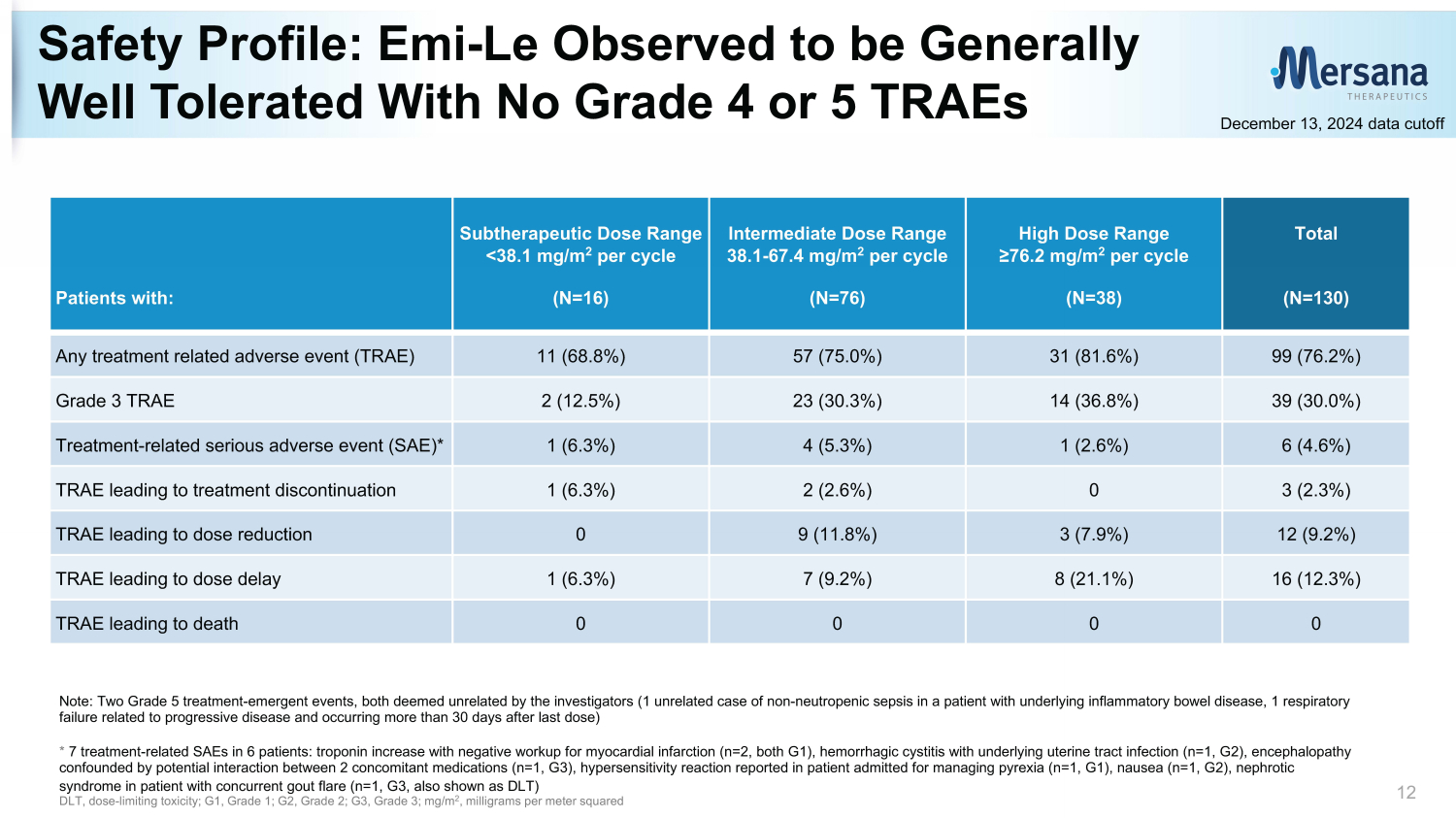
Safety Profile: Emi - Le Observed to be Generally Well Tolerated With No Grade 4 or 5 TRAEs 12 Note: Two Grade 5 treatment - emergent events, both deemed unrelated by the investigators (1 unrelated case of non - neutropenic sep sis in a patient with underlying inflammatory bowel disease, 1 respiratory failure related to progressive disease and occurring more than 30 days after last dose) * 7 treatment - related SAEs in 6 patients: troponin increase with negative workup for myocardial infarction (n=2, both G1), hemorrh agic cystitis with underlying uterine tract infection (n=1, G2), encephalopathy confounded by potential interaction between 2 concomitant medications (n=1, G3), hypersensitivity reaction reported in patien t a dmitted for managing pyrexia (n=1, G1), nausea (n=1, G2), nephrotic syndrome in patient with concurrent gout flare (n=1, G3, also shown as DLT) DLT, dose - limiting toxicity; G1, Grade 1; G2, Grade 2; G3, Grade 3; mg/m 2 , milligrams per meter squared Total (N=130) High Dose Range ≥76.2 mg/m 2 per cycle (N=38) Intermediate Dose Range 38.1 - 67.4 mg/m 2 per cycle (N=76) Subtherapeutic Dose Range <38.1 mg/m 2 per cycle (N=16) Patients with: 99 (76.2%) 31 (81.6%) 57 (75.0%) 11 (68.8%) Any treatment related adverse event (TRAE) 39 (30.0%) 14 (36.8%) 23 (30.3%) 2 (12.5%) Grade 3 TRAE 6 (4.6%) 1 (2.6%) 4 (5.3%) 1 (6.3%) Treatment - related serious adverse event (SAE)* 3 (2.3%) 0 2 (2.6%) 1 (6.3%) TRAE leading to treatment discontinuation 12 (9.2%) 3 (7.9%) 9 (11.8%) 0 TRAE leading to dose reduction 16 (12.3%) 8 (21.1%) 7 (9.2%) 1 (6.3%) TRAE leading to dose delay 0 0 0 0 TRAE leading to death December 13, 2024 data cutoff
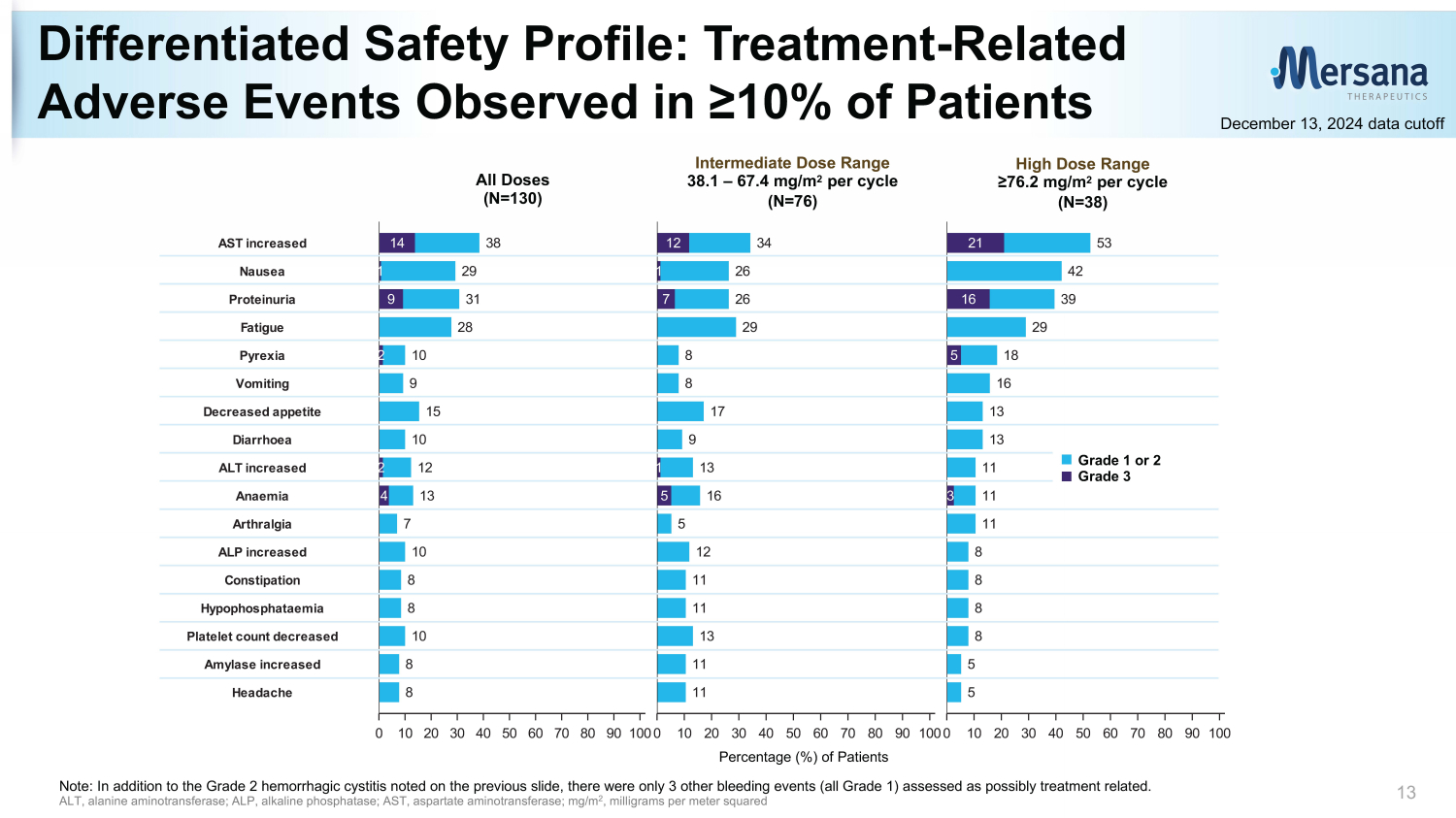
Differentiated Safety Profile: Treatment - Related Adverse Events Observed in ≥10% of Patients 13 December 13, 2024 data cutoff High Dose Range ≥76.2 mg/m 2 per cycle (N=38) Intermediate Dose Range 38.1 – 67.4 mg/m 2 per cycle (N=76) All Doses (N=130) Percentage (%) of Patients (N=130) (N=76) (N=38) All Doses 38.1 - 67.4 mg/m2 >76.2 mg/m2 53 42 39 29 18 16 13 13 11 11 11 8 8 8 8 5 5 0 10 20 30 40 50 60 70 80 90 100 Percentage (%) of Patients 21 16 5 3 G3 G1-2 34 26 26 29 8 8 17 9 13 16 5 12 11 11 13 11 11 0 10 20 30 40 50 60 70 80 90 100 Percentage (%) of Patients 12 1 7 1 5 38 29 31 28 10 9 15 10 12 13 7 10 8 8 10 8 8 0 10 20 30 40 50 60 70 80 90 100 Percentage (%) of Patients 14 1 9 2 2 4 Headache Amylase increased Platelet count decreased Hypophosphataemia Constipation ALP increased Arthralgia Anaemia ALT increased Diarrhoea Decreased appetite Vomiting Pyrexia Fatigue Proteinuria Nausea AST increased Note: In addition to the Grade 2 hemorrhagic cystitis noted on the previous slide, there were only 3 other bleeding events (a ll Grade 1) assessed as possibly treatment related. ALT, alanine aminotransferase; ALP, alkaline phosphatase; AST, aspartate aminotransferase; mg/m 2 , milligrams per meter squared Grade 1 or 2 Grade 3

Clinical Activity Data
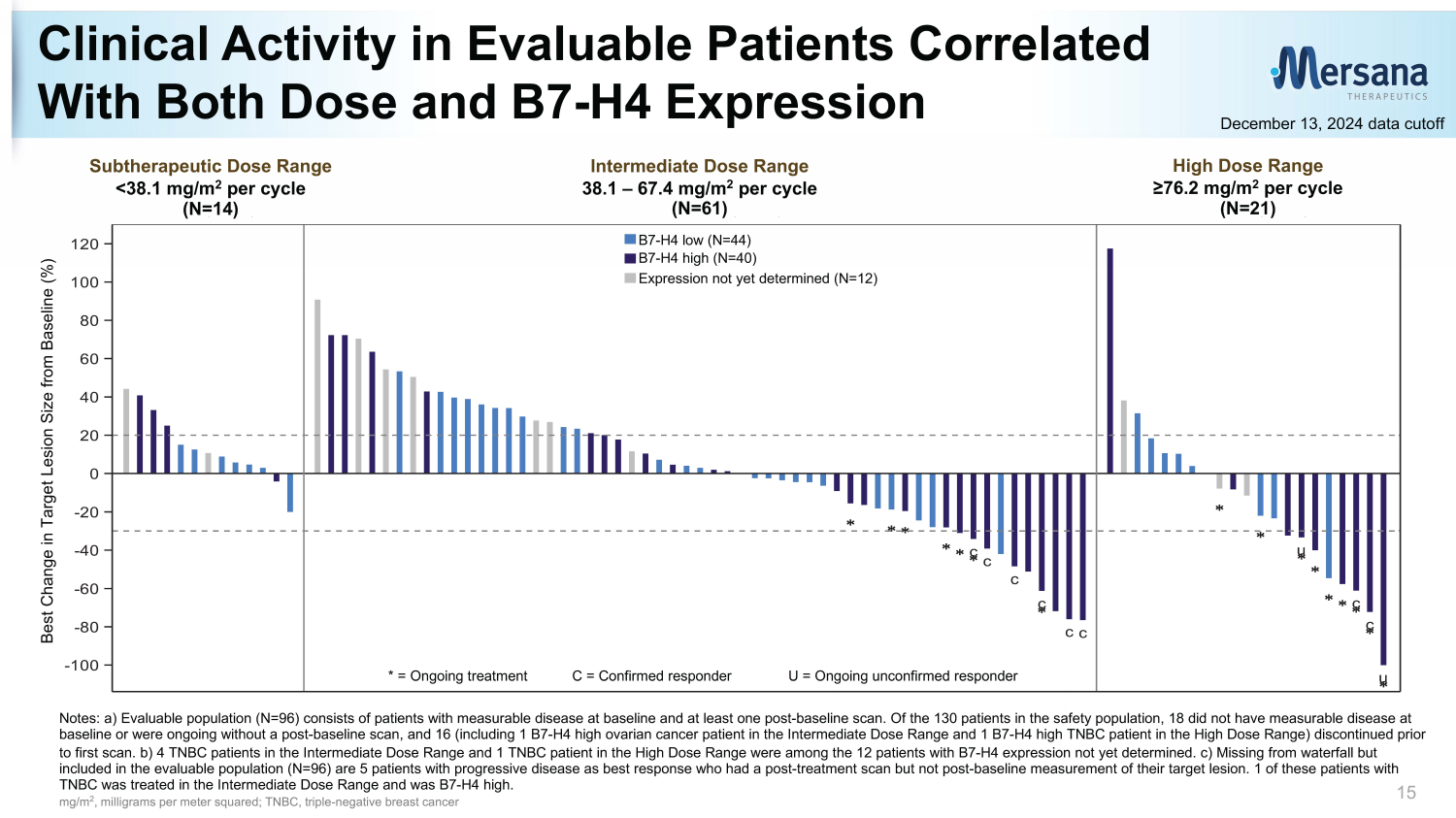
c c c c cc u c c u -100 -80 -60 -40 -20 0 20 40 60 80 100 120 B e s t C h a n g e i n T a r g e t L e s i o n S i z e f r o m B a s e l i n e ( % ) TPS Not Yet Determined (N=12) TPS >70 (N=40) TPS <70 (N=44) <38.1 mg/m2 38.1 - 67.4 mg/m2 >76.2 mg/m2 (N=14) (N=61) (N=21) Notes: a) Evaluable population (N=96) consists of patients with measurable disease at baseline and at least one post - baseline sc an. Of the 130 patients in the safety population, 18 did not have measurable disease at baseline or were ongoing without a post - baseline scan, and 16 (including 1 B7 - H4 high ovarian cancer patient in the Intermediate Dose Range and 1 B7 - H4 high TNBC patient in the High Dose Range) discontinued prior to first scan. b) 4 TNBC patients in the Intermediate Dose Range and 1 TNBC patient in the High Dose Range were among the 12 pat ients with B7 - H4 expression not yet determined. c) Missing from waterfall but included in the evaluable population (N=96) are 5 patients with progressive disease as best response who had a post - treatment sc an but not post - baseline measurement of their target lesion. 1 of these patients with TNBC was treated in the Intermediate Dose Range and was B7 - H4 high. mg/m 2 , milligrams per meter squared; TNBC, triple - negative breast cancer Clinical Activity in Evaluable Patients Correlated With Both Dose and B7 - H4 Expression 15 December 13, 2024 data cutoff High Dose Range ≥76.2 mg/m 2 per cycle (N=21) Intermediate Dose Range 38.1 – 67.4 mg/m 2 per cycle (N=61) Subtherapeutic Dose Range <38.1 mg/m 2 per cycle (N=14) * = Ongoing treatment C = Confirmed responder U = Ongoing unconfirmed responder Best Change in Target Lesion Size from Baseline (%) B7 - H4 low (N=44) B7 - H4 high (N=40) Expression not yet determined (N=12)
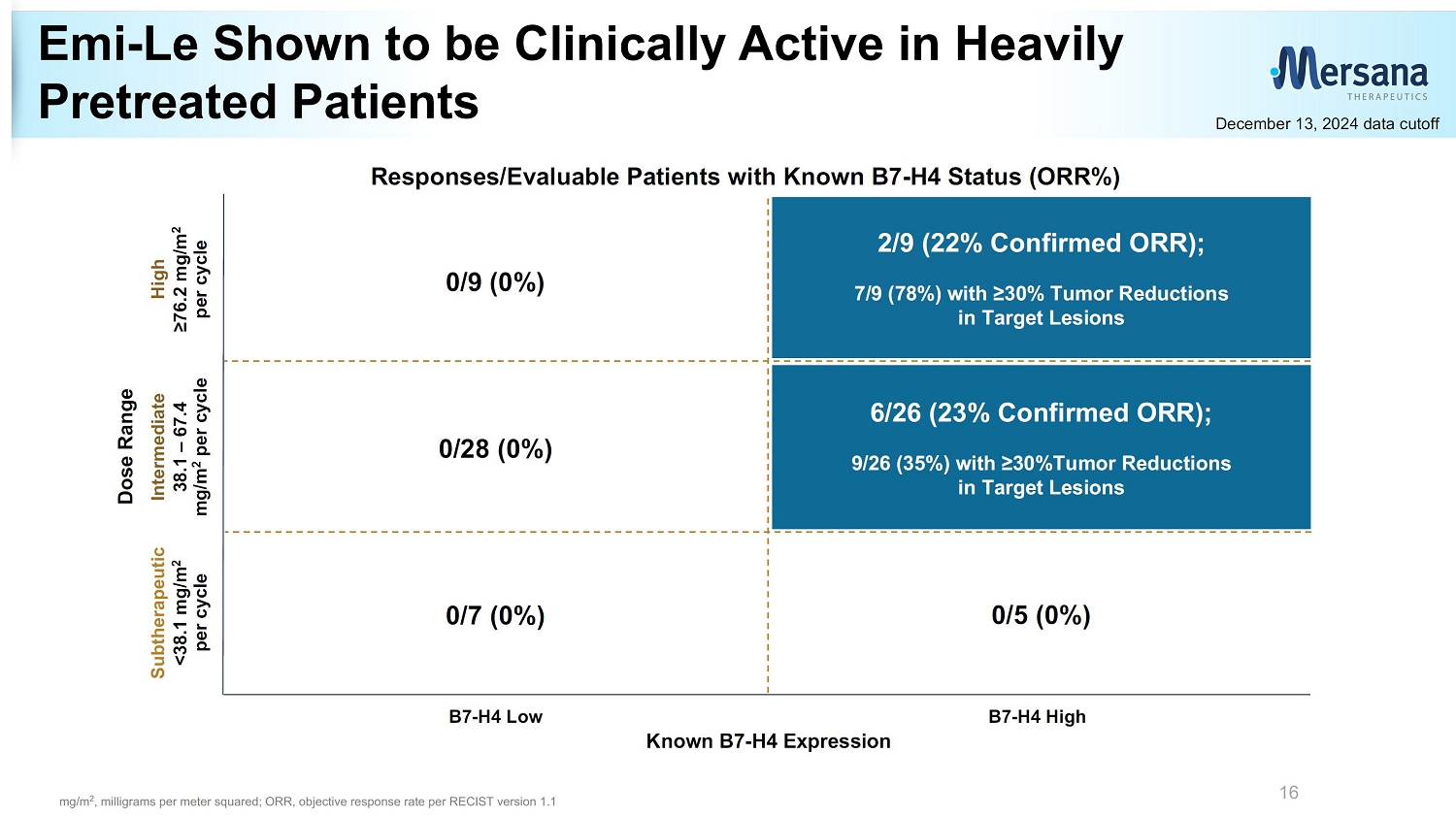
Emi - Le Shown to be Clinically Active in Heavily Pretreated Patients 16 Dose Range Known B7 - H4 Expression Subtherapeutic <38.1 mg/m 2 per cycle High ≥76.2 mg/m 2 per cycle Responses/Evaluable Patients with Known B7 - H4 Status (ORR%) Intermediate 38.1 – 67.4 mg/m 2 per cycle B7 - H4 Low B7 - H4 High 0/7 (0%) 0/5 (0%) 0/9 (0%) 0/28 (0%) 2/9 (22% Confirmed ORR); 7/9 (78%) with ≥30% Tumor Reductions in Target Lesions 6/26 (23% Confirmed ORR); 9/26 (35%) with ≥30%Tumor Reductions in Target Lesions December 13, 2024 data cutoff mg/m 2 , milligrams per meter squared; ORR, objective response rate per RECIST version 1.1
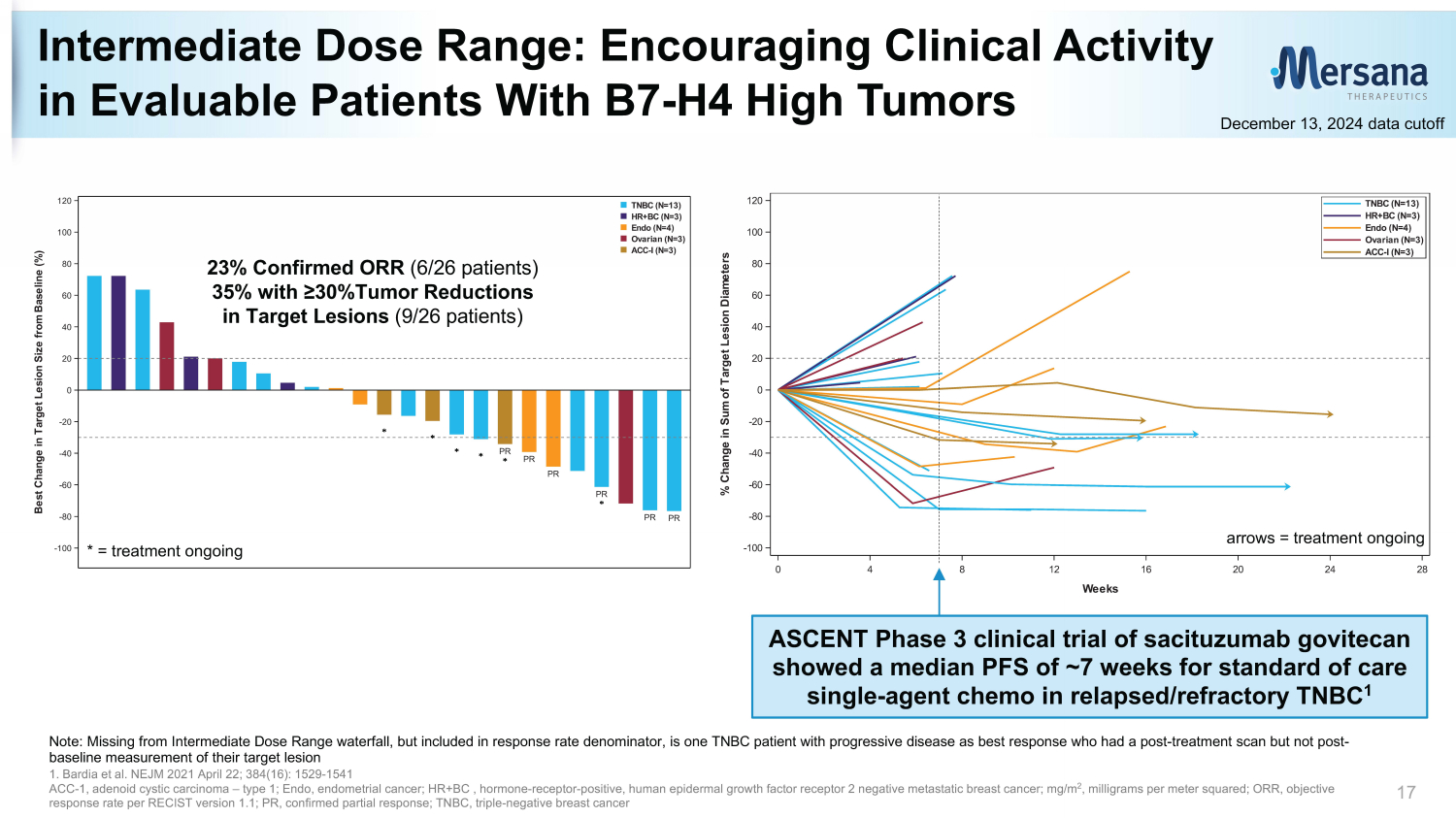
0 4 8 12 16 20 24 28 Weeks -100 -80 -60 -40 -20 0 20 40 60 80 100 120 % C h a n g e i n S u m o f T a r g e t L e s i o n D i a m e t e r s ACC-I (N=3) Ovarian (N=3) Endo (N=4) HR+BC (N=3) TNBC (N=13) Intermediate Dose Range: Encouraging Clinical Activity in Evaluable Patients With B7 - H4 High Tumors 17 December 13, 2024 data cutoff PR PR PR PR PR PR -100 -80 -60 -40 -20 0 20 40 60 80 100 120 B e s t C h a n g e i n T a r g e t L e s i o n S i z e f r o m B a s e l i n e ( % ) ACC-I (N=3) Ovarian (N=3) Endo (N=4) HR+BC (N=3) TNBC (N=13) * = treatment ongoing Note: Missing from Intermediate Dose Range waterfall, but included in response rate denominator, is one TNBC patient with pro gre ssive disease as best response who had a post - treatment scan but not post - baseline measurement of their target lesion 1. Bardia et al. NEJM 2021 April 22; 384(16): 1529 - 1541 ACC - 1, a denoid cystic c arcinoma – type 1; Endo, endometrial cancer; HR+BC , hormone - receptor - positive, human e pidermal growth f actor r eceptor 2 negative metastatic breast cancer; m g/m 2 , milligrams per meter squared; ORR, objective response rate per RECIST version 1.1; PR, confirmed partial response; TNBC, triple - negative breast cancer 23% Confirmed ORR (6/26 patients) 35% with ≥30%Tumor Reductions in Target Lesions (9/26 patients) ASCENT Phase 3 clinical trial of sacituzumab govitecan showed a median PFS of ~7 weeks for standard of care single - agent chemo in relapsed/refractory TNBC 1 arrows = treatment ongoing
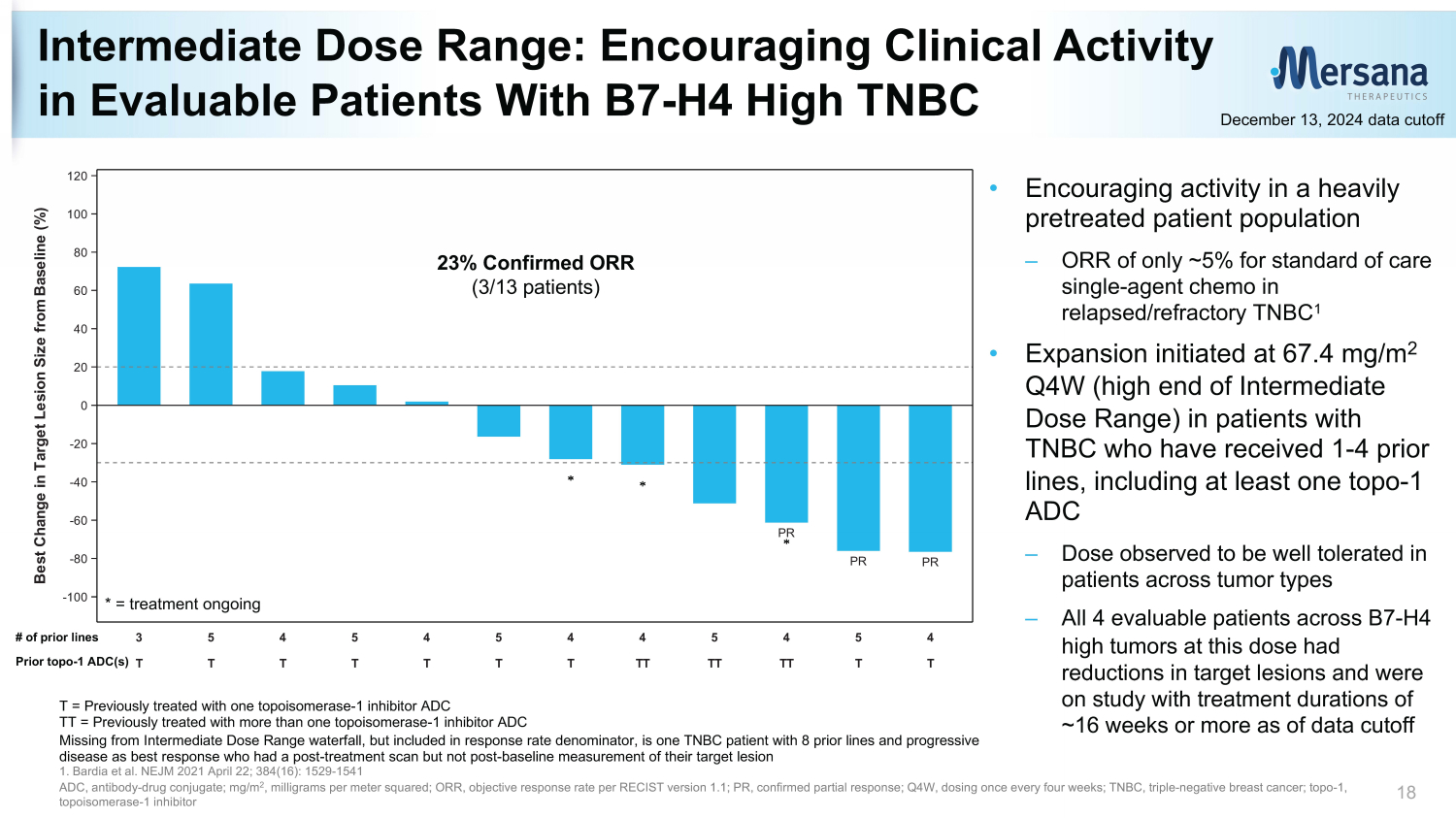
T T T T T T T TT TT TT T T 3 5 4 5 4 5 4 4 5 4 5 4 PR PR PR -100 -80 -60 -40 -20 0 20 40 60 80 100 120 B e s t C h a n g e i n T a r g e t L e s i o n S i z e f r o m B a s e l i n e ( % ) Intermediate Dose Range: Encouraging Clinical Activity in Evaluable Patients With B7 - H4 High TNBC 18 December 13, 2024 data cutoff 23% Confirmed ORR (3/13 patients) T = Previously treated with one topoisomerase - 1 inhibitor ADC TT = Previously treated with more than one topoisomerase - 1 inhibitor ADC Missing from Intermediate Dose Range waterfall, but included in response rate denominator, is one TNBC patient with 8 prior l ine s and progressive disease as best response who had a post - treatment scan but not post - baseline measurement of their target lesion 1. Bardia et al. NEJM 2021 April 22; 384(16): 1529 - 1541 ADC, antibody - drug conjugate; mg/m 2 , milligrams per meter squared; ORR, objective response rate per RECIST version 1.1 ; PR, confirmed partial response; Q4W, dosing once every four weeks; TNBC, triple - negative breast cancer; t opo - 1, topoisomerase - 1 inhibitor * = treatment ongoing • Encouraging activity in a heavily pretreated patient population – ORR of only ~5% for standard of care single - agent chemo in relapsed/refractory TNBC 1 • Expansion initiated at 67.4 mg/m 2 Q4W (high end of Intermediate Dose Range) in patients with TNBC who have received 1 - 4 prior lines, including at least one topo - 1 ADC – Dose observed to be well tolerated in patients across tumor types – All 4 evaluable patients across B7 - H4 high tumors at this dose had reductions in target lesions and were on study with treatment durations of ~16 weeks or more as of data cutoff # of prior lines Prior topo - 1 ADC(s)
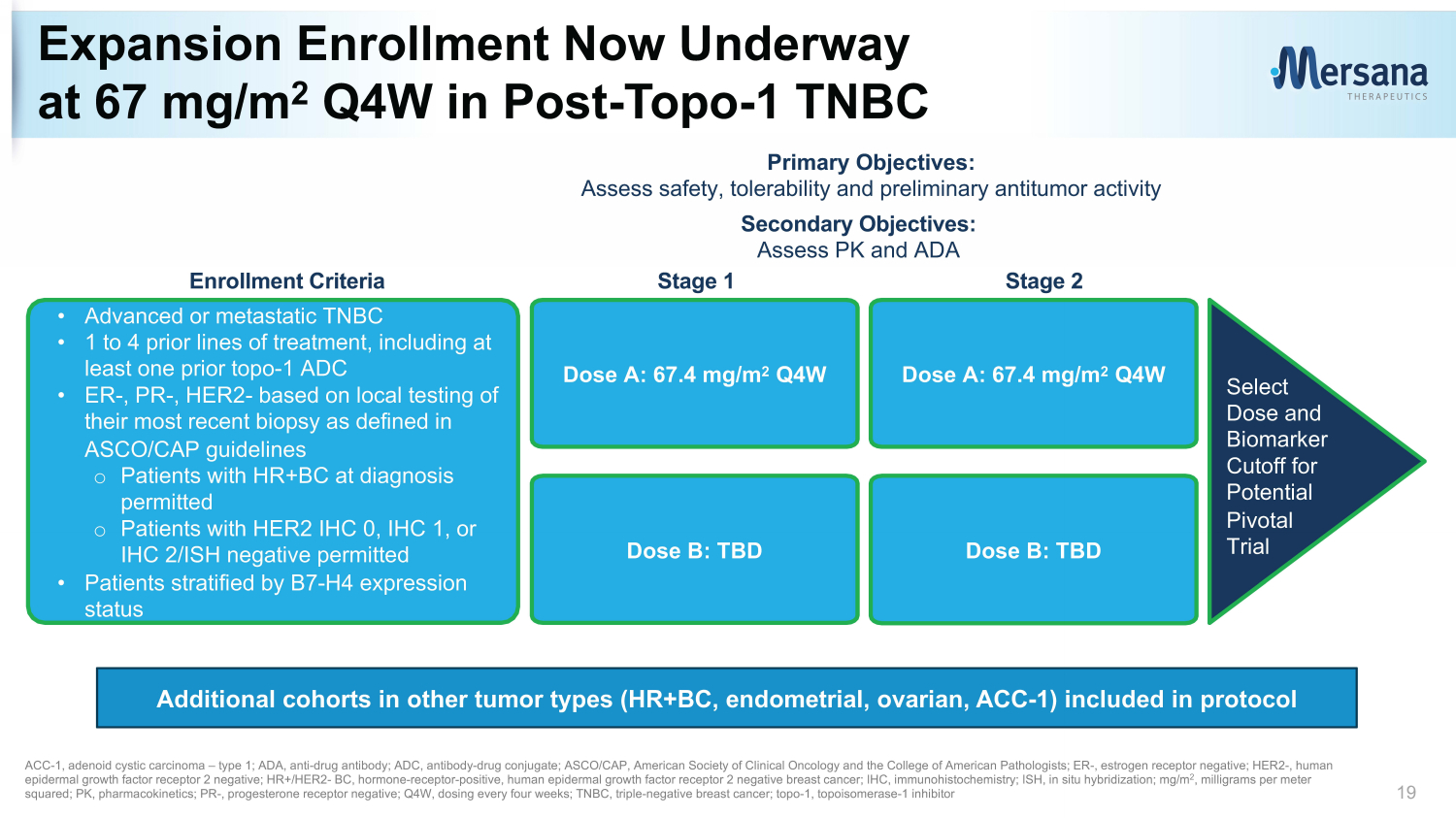
Expansion Enrollment Now Underway at 67 mg/m 2 Q4W in Post - Topo - 1 TNBC 19 Primary Objectives: Assess safety, tolerability and preliminary antitumor activity Secondary Objectives: Assess PK and ADA • Advanced or metastatic TNBC • 1 to 4 prior lines of treatment, including at least one prior topo - 1 ADC • ER - , PR - , HER2 - based on local testing of their most recent biopsy as defined in ASCO/CAP guidelines o Patients with HR+BC at diagnosis permitted o Patients with HER2 IHC 0, IHC 1, or IHC 2/ISH negative permitted • Patients stratified by B7 - H4 expression status Dose A: 67.4 mg/m 2 Q4W Dose B: TBD Dose A: 67.4 mg/m 2 Q4W Dose B: TBD Stage 1 Stage 2 Select Dose and Biomarker Cutoff for Potential Pivotal Trial ACC - 1, a denoid cystic c arcinoma – type 1; ADA, anti - drug antibody; ADC, antibody - drug conjugate; ASCO/CAP, American Society of Clinical Oncology and the College of American Pathologists; ER - , est rogen receptor negative; HER2 - , human e pidermal growth f actor r eceptor 2 negative; HR+/HER2 - BC, hormone - receptor - positive, human e pidermal growth f actor r eceptor 2 negative breast cancer; IHC, immunohistochemistry; ISH, in situ hybridization; mg/m 2 , milligrams per meter squared; PK, pharmacokinetics; PR - , progesterone receptor negative; Q4W, dosing every four weeks; TNBC, triple - negative breast c ancer; topo - 1, topoisomerase - 1 inhibitor Enrollment Criteria Additional cohorts in other tumor types (HR+BC, endometrial, ovarian, ACC - 1) included in protocol
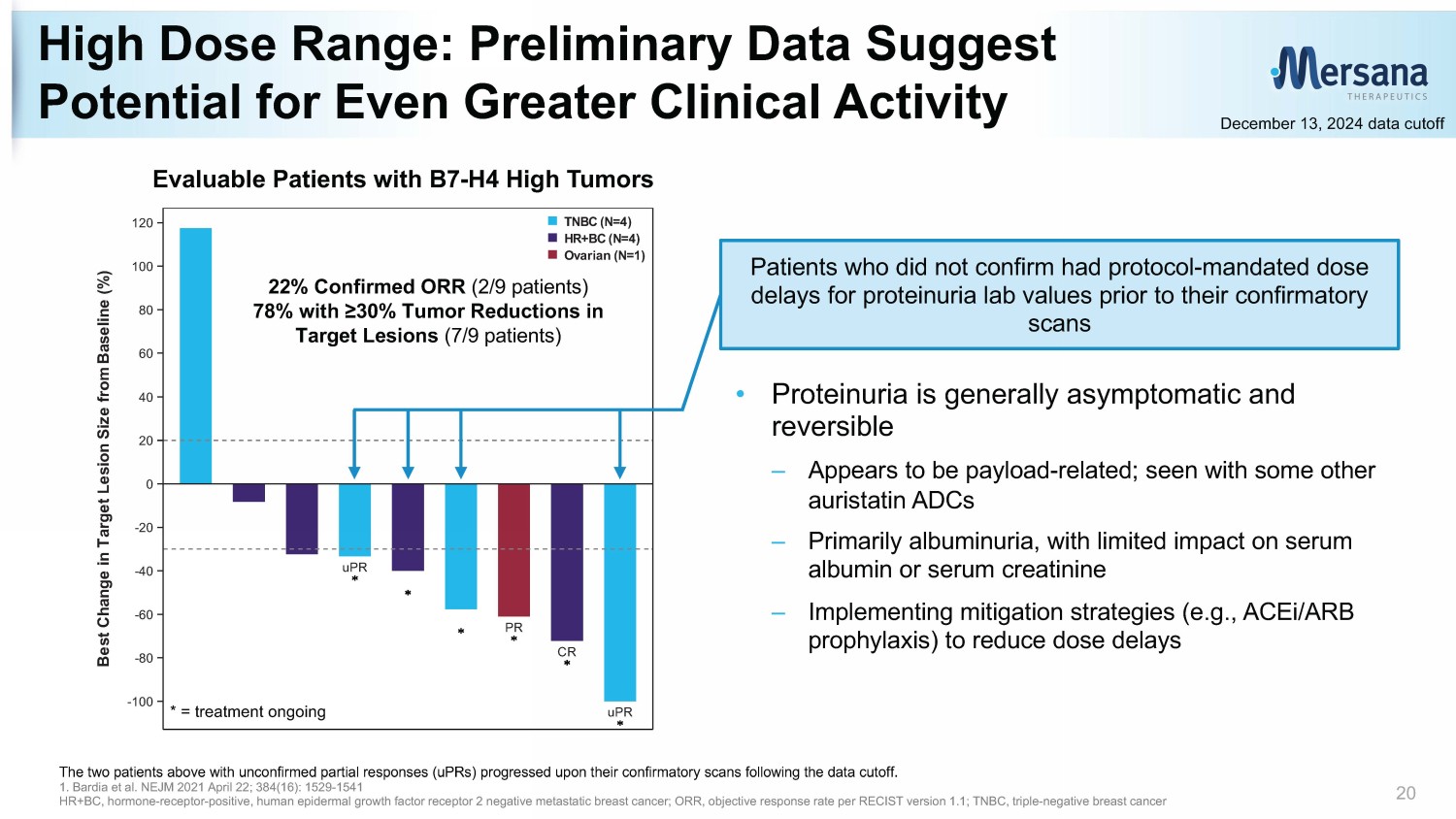
High Dose Range: Preliminary Data S uggest P otential for Even Greater Clinical Activity 20 December 13, 2024 data cutoff The two patients above with unconfirmed partial responses ( uPRs ) progressed upon their confirmatory scans following the data cutoff. 1. Bardia et al. NEJM 2021 April 22; 384(16): 1529 - 1541 HR+BC, hormone - receptor - positive, human e pidermal growth f actor r eceptor 2 negative metastatic breast cancer; ORR, objective response rate per RECIST version 1.1 ; TNBC, triple - negative breast cancer uPR PR CR uPR -100 -80 -60 -40 -20 0 20 40 60 80 100 120 B e s t C h a n g e i n T a r g e t L e s i o n S i z e f r o m B a s e l i n e ( % ) Ovarian (N=1) HR+BC (N=4) TNBC (N=4) >=76.2 mg/m2 (N=9) * = treatment ongoing 22% Confirmed ORR (2/9 patients) 78% with ≥30% Tumor Reductions in Target Lesions (7/9 patients) Patients who did not confirm had protocol - mandated dose delays for proteinuria lab values prior to their confirmatory scans • Proteinuria is generally asymptomatic and reversible – Appears to be payload - related; seen with other some auristatin ADCs – Primarily albuminuria, with limited impact on serum albumin or serum creatinine – Implementing mitigation strategies (e.g., ACEi /ARB prophylaxis) to reduce dose delays Evaluable Patients with B7 - H4 High Tumors

• 1H2025: Continue expansion enrollment at 67.4 mg/m 2 Q4W in TNBC patients who have previously received at least one topo - 1 ADC • 2025: Initiate expansion enrollment at second dose in post - topo - 1 TNBC • 2025: Present additional Phase 1 dose escalation and backfill cohort clinical data • Continue to support internal pipeline and existing collaborations with Johnson & Johnson and Merck KGaA, Darmstadt, Germany • 2025: Present initial clinical pharmacodynamic STING activation data Expected 2025 Milestones and Areas of Focus 21 XMT - 2056: Lead Immunosynthen Product Candidate Emi - Le: Lead Dolasynthen Product Candidate ADC, antibody - drug conjugate; mg/m 2 , milligrams per meter squared; Q4W, dosing every four weeks; TNBC, triple - negative breast cancer; topo - 1, topoisomerase - 1 inhib itor Pipeline

Q&A Session Thank you to the patients, families, caregivers and investigators who are participating in this clinical trial 22





















