Table of Contents
Post-Qualification Amendment No. 2
File No. 024-12202
June 9, 2023
An Offering Statement pursuant to Regulation A relating to these securities has been filed with the Securities and Exchange Commission. Information contained in this Preliminary Offering Circular is subject to completion or amendment. These securities may not be sold, nor may offers to buy be accepted before the Offering Statement filed with the Commission is qualified. This Preliminary Offering Circular shall not constitute an offer to sell or the solicitation of an offer to buy, nor may there be any sales of these securities in any state in which such offer, solicitation, or sale would be unlawful before registration or qualification under the laws of any such state. We may elect to satisfy our obligation to deliver a Final Offering Circular by sending you a notice within two business days after the completion of our sale to you that contains the URL where the Final Offering Circular or the Offering Statement, in which such Final Offering Circular was filed, may be obtained.

Wearable Health Solutions, Inc.
2901 W. Coast Highway
Suite 200
Newport Beach, CA 92663
(949) 270-7460
www.WearableHealthSolutions.com
$1,000,000
1,000,000,000 SHARES OF COMMON STOCK
Offering Price $0.001 per share
EXPLANATORY NOTE
The purpose of this Post Qualification Amendment is to decrease the total offering amount and price per share and to increase the number of our shares of common stock being offered. Unless otherwise defined in this Post-Qualification Amendment, capitalized terms used herein shall have the same meanings as set forth in the Offering Circular, including the disclosures incorporated by reference therein.
This is the public offering of securities of Wearable Health Solutions, Inc., a Nevada corporation. We are offering up to 1,000,000,000 shares of our Common Stock, par value $0.0001 ("Common Stock"), at an offering price of $0.001 per share (the “Offered Shares”) by the Company. This Offering (the “Offering”) will terminate at the earlier of (1) the date at which the maximum offering amount has been sold or (2) the date at which the Offering is earlier terminated by the Company at its sole discretion. At least every 12 months after this offering has been qualified by the United States Securities and Exchange Commission (the “Commission”), the Company will file a post-qualification amendment to include the Company’s recent financial statements. The minimum purchase requirement per investor is 10,000,000 Offered Shares ($10,000); however, we can waive the minimum purchase requirement on a case-by-case basis in our sole discretion.
These securities are speculative securities. Investment in the Company’s stock involves significant risk. You should purchase these securities only if you can afford a complete loss of your investment. See the “Risk Factors” section on page 6 of this Offering Circular.
No Escrow
The proceeds of this offering will not be placed into an escrow account. We will offer our Common Stock on a best efforts’ basis. As there is no minimum offering, upon the approval of any subscription to this Offering Circular, the Company shall immediately deposit said proceeds into the bank account of the Company and may dispose of the proceeds in accordance with the Use of Proceeds.
Subscriptions are irrevocable and the purchase price is non-refundable as expressly stated in this Offering Circular. The Company, by determination of the Board of Directors, in its sole discretion, may issue the Securities under this Offering for cash, promissory notes, services, and/or other consideration without notice to subscribers. All proceeds received by the Company from subscribers for this Offering will be available for use by the Company upon acceptance of subscriptions for the Securities by the Company.
Sale of these shares will commence within two calendar days of the qualification date, and it will be a continuous Offering pursuant to Rule 251(d)(3)(i)(F).
This Offering will be conducted on a “best-efforts” basis, which means our Officers will use their commercially reasonable best efforts in an attempt to offer and sell the Shares. Our Officers will not receive any commission or any other remuneration for these sales. In offering the securities on our behalf, the Officers will rely on the safe harbor from broker-dealer registration set out in Rule 3a4-1 under the Securities Exchange Act of 1934, as amended.
This Offering Circular shall not constitute an offer to sell or the solicitation of an offer to buy, nor shall there be any sales of these securities in any state or jurisdiction in which such offer, solicitation or sale would be unlawful, prior to registration or qualification under the laws of any such state.
The Company is using the Offering Circular format in its disclosure in this Offering Circular.
Our Common Stock is quoted on the OTC Markets Pink Open Market under the stock symbol “WHSI.”
Investing in our Common Stock involves a high degree of risk. See “Risk Factors” beginning on page 6 for a discussion of certain risks that you should consider in connection with an investment in our Common Stock.
| | | Per
Share | | | Total Maximum (4) | |
| Public Offering Price (1)(2) | | $ | 0.001 | | | $ | 1,000,000.00 | |
| Underwriting Discounts and Commissions (3) | | $ | 0.00 | | | $ | 0.00 | |
| Proceeds to Company(4) | | $ | 0.001 | | | $ | 1,000,000.00 | |
| (1) | We are offering shares on a continuous basis. See “Distribution – Continuous Offering. |
| | |
| (2) | This is a “best efforts” offering. The proceeds of this offering will not be placed into an escrow account. We will offer our Common Stock on a best efforts’ basis primarily through an online platform. As there is no minimum offering, upon the approval of any subscription to this Offering Circular, the Company shall immediately deposit said proceeds into the bank account of the Company and may dispose of the proceeds in accordance with the Use of Proceeds. See “How to Subscribe.” |
| | |
| (3) | We are offering these securities without an underwriter. |
| | |
| (4) | Excludes estimated total offering expenses that will be approximately $40,000 assuming the maximum offering amount is sold. |
Our Board of Directors used its business judgment in setting a value of $0.001 per share to the Company as consideration for the stock to be issued under the Offering. The sales price per share bears no relationship to our book value or any other measure of our current value or worth.
Generally, no sale may be made to you in this offering if the aggregate purchase price you pay is more than 10% of the greater of your annual income or net worth. Different rules apply to accredited investors and non-natural persons. Before making any representation that your investment does not exceed applicable thresholds, we encourage you to review Rule 251(d)(2)(i)(C) of Regulation A. For general information on investing, we encourage you to refer to www.investor.gov.
THE UNITED STATES SECURITIES AND EXCHANGE COMMISSION DOES NOT PASS UPON THE MERITS OF OR GIVE ITS APPROVAL TO ANY SECURITIES OFFERED OR THE TERMS OF THE OFFERING, NOR DOES IT PASS UPON THE ACCURACY OR COMPLETENESS OF ANY OFFERING CIRCULAR OR OTHER SOLICITATION MATERIALS. THESE SECURITIES ARE OFFERED PURSUANT TO AN EXEMPTION FROM REGISTRATION WITH THE COMMISSION; HOWEVER, THE COMMISSION HAS NOT MADE AN INDEPENDENT DETERMINATION THAT THE SECURITIES OFFERED ARE EXEMPT FROM REGISTRATION.
TABLE OF CONTENTS
STATE LAW EXEMPTION AND PURCHASE RESTRICTIONS
Our Common Stock is being offered and sold only to “qualified purchasers” (as defined in Regulation A). As a Tier 2 offering pursuant to Regulation A, this offering will be exempt from state law “Blue Sky” review, subject to meeting certain state filing requirements and complying with certain anti-fraud provisions, to the extent that our Common Stock offered hereby is offered and sold only to “qualified purchasers” or at a time when our Common Stock is listed on a national securities exchange. “Qualified purchasers” include: (i) “accredited investors” under Rule 501(a) of Regulation D under the Securities Act (“Regulation D”) and (ii) all other investors so long as their investment in our Common Stock does not represent more than 10% of the greater of their annual income or net worth (for natural persons), or 10% of the greater of annual revenue or net assets at fiscal year-end (for non-natural persons). However, our Common Stock is being offered and sold only to those investors that are within the latter category (i.e., investors whose investment in our Common Stock does not represent more than 10% of the applicable amount), regardless of an investor’s status as an “accredited investor.” Accordingly, we reserve the right to reject any investor’s subscription in whole or in part for any reason, including if we determine in our sole and absolute discretion that such investor is not a “qualified purchaser” for purposes of Regulation A.
To determine whether a potential investor is an “accredited investor” for purposes of satisfying one of the tests in the “qualified purchaser” definition, the investor must be a natural person who has:
| | 1. | an individual net worth, or joint net worth with the person’s spouse, that exceeds $1,000,000 at the time of the purchase, excluding the value of the primary residence of such person; or |
| | | |
| | 2. | earned income exceeding $200,000 in each of the two most recent years or joint income with a spouse exceeding $300,000 for those years and a reasonable expectation of the same income level in the current year. |
If the investor is not a natural person, different standards apply. See Rule 501 of Regulation D for more details.
For purposes of determining whether a potential investor is a “qualified purchaser,” annual income and net worth should be calculated as provided in the “accredited investor” definition under Rule 501 of Regulation D. In particular, net worth in all cases should be calculated excluding the value of an investor’s home, home furnishings and automobiles.
Continuous Offering
Under Rule 251(d)(3) to Regulation A, the following types of continuous or delayed Offerings are permitted, among others: (1) securities offered or sold by or on behalf of a person other than the issuer or its subsidiary or a person of which the issuer is a subsidiary; (2) securities issued upon conversion of other outstanding securities; or (3) securities that are part of an Offering which commences within two calendar days after the qualification date. These may be offered on a continuous basis and may continue to be offered for a period in excess of 30 days from the date of initial qualification. They may be offered in an amount that, at the time the Offering statement is qualified, is reasonably expected to be offered and sold within one year from the initial qualification date. No securities will be offered or sold “at the market.”
Sale of these shares will commence within two calendar days of the qualification date, and it will be a continuous Offering pursuant to Rule 251(d)(3)(i)(F).
Subscriptions are irrevocable and the purchase price is non-refundable as expressly stated in this Offering Circular. The Company, by determination of the Board of Directors, in its sole discretion, may issue the Securities under this Offering for cash, promissory notes, services, and/or other consideration without notice to subscribers. All proceeds received by the Company from subscribers for this Offering will be available for use by the Company upon acceptance of subscriptions for the Securities by the Company.
CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING STATEMENTS
______
Some of the statements under “Summary”, “Risk Factors”, “Management’s Discussion and Analysis of Financial Condition and Results of Operations”, “Our Business” and elsewhere in this Offering Circular constitute forward-looking statements. Forward-looking statements relate to expectations, beliefs, projections, future plans and strategies, anticipated events or trends and similar matters that are not historical facts. In some cases, you can identify forward-looking statements by terms such as “anticipate”, “believe”, “could”, “estimate”, “expect”, “intend”, “may”, “plan”, “potential”, “should”, “will” and “would” or the negatives of these terms or other comparable terminology.
You should not place undue reliance on forward looking statements. The cautionary statements set forth in this Offering Circular, including in “Risk Factors” beginning on page 6 and elsewhere, identify important factors which you should consider in evaluating our forward-looking statements. These factors include, among other things:
The speculative nature of the business;
Our reliance on suppliers and customers;
Our dependence upon external sources for the financing of our operations, particularly given that there are concerns about our ability to continue as a “going concern;”
Our ability to effectively execute our business plan;
Our ability to manage our expansion, growth and operating expenses;
Our ability to finance our businesses;
Our ability to promote our businesses;
Our ability to compete and succeed in highly competitive and evolving businesses;
Our ability to respond and adapt to changes in technology and customer behavior; and
Our ability to protect our intellectual property and to develop, maintain and enhance strong brands.
Although the forward-looking statements in this Offering Circular are based on our beliefs, assumptions and expectations, taking into account all information currently available to us, we cannot guarantee future transactions, results, performance, achievements or outcomes. No assurance can be made to any investor by anyone that the expectations reflected in our forward-looking statements will be attained, or that deviations from them will not be material and adverse. We undertake no obligation, other than as may be required by law, to re-issue this Offering Circular or otherwise make public statements updating our forward-looking statements.
SUMMARY
______
This summary highlights selected information contained elsewhere in this Offering Circular. This summary is not complete and does not contain all the information that you should consider before deciding whether to invest in our Common Stock. You should carefully read the entire Offering Circular, including the risks associated with an investment in the company discussed in the “Risk Factors” section of this Offering Circular, before making an investment decision. Some of the statements in this Offering Circular are forward-looking statements. See the section entitled “Cautionary Statement Regarding Forward-Looking Statements.”
Company Overview
Our common stock is not traded on any exchange. Our common stock is quoted on the Pink OTC Markets (“OTC Markets”) under the trading symbol “WHSI.” There is no established public trading market for our common stock. We cannot assure you that there will be a market in the future for our common stock.
Wearable Health Solutions
Wearable Health Solutions Inc. (the Company) was incorporated as Medical Alarm Concepts Holding, Inc. on June 4, 2008, under the laws of the State of Nevada. The Company was formed for the sole purpose of acquiring all of the membership units of Medical Alarm Concepts LLC, a Pennsylvania limited liability company (“Medical LLC”). On May 26, 2016, the Company filed an Amended and Restated Articles of Incorporation with the Secretary of State of the State of Nevada to change its name from “Medical Alarm Concepts, Inc.” to “Wearable Health Solutions Inc.”
Wearable Health Solutions, Inc. provides mobile health (mHealth) products and services to approximately 200 dealers and distributors throughout the globe (mostly Canada, United States and New Zealand). As a provider of personal emergency response devices, in the rapidly growing medical alarm device and eHealth sector, we provide innovative wearable healthcare products, tracking services, and turn-key solutions that enable our users to be proactive with their health, as well as safe and protected. According to the QYResearch report on Global PERS devices, MediPendant was the 17th largest global PERS company based on revenues in 2021 (“QYResearch Global PERs Devices Market Report, History and Forecast 2016-2027”). Our products and services are always state-of-the-art and cost effective. Through our culture, our drive, and the expertise of each individual employee, we are uniquely positioned to build shareholder value by setting the highest standards in service, reliability, and safety in our rapidly growing industry.
Our flagship products are the iHelp devices, MediPendant, the iHelp+ 3G™, iHelp Mini 4G™ and the next generation 4G iHelp MAX™ - personal emergency alarms that are used to summon help in the event of an emergency at home. As of 2021, approximately 60% of all medical alarms being sold globally, by sales revenue (or approx. 57% of volume by type), are either Landline type or Standalone type technologies that require the user to speak and listen through a central base station unit, according to the QYResearch report on PERS (“QYResearch Global PERs Devices Market Report, History and Forecast 2016-2027”). The MediPendant®, however, offers a product that, is always connected through a landline, and has the speaker in the pendant, enabling the user to simply speak and listen directly through the pendant in the event of an emergency.
The Company has not yet established an ongoing source of revenues sufficient to cover its operating costs for the next fiscal year and allow it to continue as a going concern. The ability of the Company to continue as a going concern is dependent on the Company obtaining adequate capital to fund operating losses until it becomes profitable. As of June 30, 2022, the Company has a net loss of $13,049,155, including non-cash expenses of $10,044,522 related to stock compensation. Iif the Company is unable to obtain adequate capital, it could be forced to cease operations.
During the quarter ended December 31, 2022, Company has net cash used in operating activities of $(1,055,780) as well as stock compensation non-cash expenses of $195,266 and a net loss of $1,288,558. The Company raised $1,040,191 from financing activities, net of repayments, in the quarter ended December 31, 2022, which resulted in a negative working capital of $(2,430,294) as of December 31, 2022. If the Company is unable to raise additional adequate capital, it could be forced to cease operations.
On August 2, 2021, the Company entered into an agreement with Voice of Things, Inc., in order to embed voice control into the devices. This agreement will provide the Company with cloud-to-cloud integration between Voice of Things, Inc. and iHelp without requiring any device-level touchpoints. The voice activation feature will be implemented at an estimated cost of $36,000 and has an approximate integration timeframe of two months, excluding certification time. This solution will also our products to integrate and function with Alexa/Google smart speakers and their respective apps. The voice control software has been completed as of February, 2022; however, the Company is currently in the design phase of the medical alert device that the software will be implemented in. The production of that medical device was supposed to be completed in early 2022, however, due to factory shutdown, supply chain issues as well as some minor changes to the medical alert device, we now expect the device to be completed in the third quarter of 2022.
On August 11, 2021, the Company entered into an Asset Purchase and Advisory Services Agreement (“Agreement”) with Anthony Chetta, owner of mHealth, whereby the Company acquired 100% ownership stake of mHealth.com, the user portal used by the subsidiary customers, all code and related operations, the domain name, and logos, data, storage and online operations for $50,000. The Company chose to forego the independent valuation of the asset. The Company also retained Mr. Chetta as the Chief Technology Advisor, by issuing 1,000,000 restricted shares as a stock signing bonus, valued at $10,500 or $0.0105 per share, issued on September 30, 2021, $8,000 per month service agreement, and 1,000,000 shares of common stock every 6 months for a 30 month period, valued at $52,500 or $0.0105 per share. By acquiring mHealth the Company now owns its backend portal system which integrates and controls how our dealers interact with our customers, invoicing, and device functionality, which should allow the Company to better control and improve on the services it provides.
All of our products are used in conjunction with our proprietary management and operation platform. The platform is a cloud-hosted service consisting of methods and automation tasks for accepting data transmission from personal safety and medical devices (“PS/M”) and storing, reformatting, and retransmitting this data to subscribers, monitoring centers, healthcare providers, front-end portal/user interfaces, and API controllers.
Our fiscal year end is June 30.
Our company is in the development stage and has generated minimal revenues.
We do not incorporate the information on or accessible through our websites into this Offering Circular, and you should not consider any information on, or that can be accessed through, our websites a part of this Offering Circular.
Section 15(g) of the Securities Exchange Act of 1934
Our shares are covered by section 15(g) of the Securities Exchange Act of 1934, as amended, that imposes additional sales practice requirements on broker/dealers who sell such securities to persons other than established customers and accredited investors (generally institutions with assets in excess of $5,000,000 or individuals with net worth in excess of $1,000,000 or annual income exceeding $200,000 or $300,000 jointly with their spouses). For transactions covered by the Rule, the broker/dealer must make a special suitability determination for the purchase and have received the purchaser’s written agreement to the transaction prior to the sale. Consequently, the Rule may affect the ability of broker/dealers to sell our securities and also may affect your ability to sell your shares in the secondary market.
Section 15(g) also imposes additional sales practice requirements on broker/dealers who sell penny securities. These rules require a one-page summary of certain essential items. The items include the risk of investing in penny stocks in both public offerings and secondary marketing; terms important to in understanding of the function of the penny stock market, such as bid and offer quotes, a dealers spread and broker/dealer compensation; the broker/dealer compensation, the broker/dealers’ duties to its customers, including the disclosures required by any other penny stock disclosure rules; the customers’ rights and remedies in cases of fraud in penny stock transactions; and, the FINRA’s toll free telephone number and the central number of the North American Securities Administrators Association, for information on the disciplinary history of broker/dealers and their associated persons.
We are a reporting company under Section 12(g).
We are subject to the reporting requirements under the Securities and Exchange Act of 1934. As a result, shareholders will have access to the information required to be reported by publicly held companies under the Exchange Act and the regulations thereunder. We intend to fully comply with all filing deadlines and rules regarding quarterly and annual reports.
Dividends
The Company has not declared or paid a cash dividend to stockholders since it was organized and does not intend to pay dividends in the foreseeable future. The board of directors presently intends to retain any earnings to finance our operations and does not expect to authorize cash dividends in the foreseeable future. Any payment of cash dividends in the future will depend upon the Company’s earnings, capital requirements and other factors.
Trading Market
Our Common Stock is quoted on the OTC Markets Pink Open Market Sheets under the symbol WHSI.
THE OFFERING
______
| Issuer: | | Wearable Health Solutions, Inc. |
| | | |
| Securities offered by the Company: | | A maximum of 1,000,000,000 shares of our common stock, par value $0.0001 (“Common Stock”) at an offering price of $0.001 per share (the “Offered Shares”). (See “Distribution.”) |
| | | |
| Number of shares of Common Stock outstanding before the offering | | 1,561,255,108 issued and outstanding as of June 6, 2023. |
| | | |
| Number of shares of Common Stock to be outstanding after the offering | | 2,561,255,108 shares, if the maximum amount of Offered Shares are sold. |
| | | |
| Price per share: | | $0.001 per share |
| | | |
| Maximum offering amount: | | 1,000,000,000 shares at $0.001 per share, or $1,000,000 (See “Distribution.”). |
| | | |
| Trading Market: | | Our Common Stock is quoted on the OTC Markets Pink Open Market Sheets division under the symbol “WHSI.” |
| | | |
| Use of proceeds: | | If we sell all of the shares being offered, our net proceeds (after our estimated offering expenses) will be $960,000. We will use these net proceeds for working capital and other general corporate purposes. |
| | | |
| Risk factors: | | Investing in our Common Stock involves a high degree of risk, including: Immediate and substantial dilution. Limited market for our stock. See “Risk Factors” beginning on page 6. |
RISK FACTORS
______
An investment in our common stock involves a high degree of risk. You should carefully consider the risks described below, together with all of the other information included in this report, before making an investment decision. If any of the following risks actually occurs, our business, financial condition or results of operations could suffer. In that case, the trading price of our common stock could decline, and you may lose all or part of your investment. You should read the section entitled “Special Note Regarding Forward Looking Statements” above for a discussion of what types of statements are forward-looking statements, as well as the significance of such statements in the context of this report.
RISKS RELATED TO OUR BUSINESS
Our business plan is speculative.
Our planned businesses are speculative and subject to numerous risks and uncertainties. The burden of government regulation on technology, medical, and commodities related industry participants, including manufacturers, distributors, retailers, suppliers and consumers, is uncertain and difficult to quantify. There is no assurance that we will ever earn enough revenue to make a net profit.
There are doubts about our ability to continue as a going concern.
The Company is an early-stage enterprise and has commenced principal operations. The Company had revenues of $608,244 and $819,601 for the nine months ended March 31, 2023 and 2022, respectively. These factors raise substantial doubt about the Company’s ability to continue as a going concern.
There can be no assurance that sufficient funds required during the next year or thereafter will be generated from operations or that funds will be available from external sources, such as debt or equity financings or other potential sources. The lack of additional capital resulting from the inability to generate cash flow from operations, or to raise capital from external sources would force the Company to substantially curtail or cease operations and would, therefore, have a material adverse effect on its business. Furthermore, there can be no assurance that any such required funds, if available, will be available on attractive terms or that they will not have a significant dilutive effect on the Company’s existing stockholders.
The Company intends to overcome the circumstances that impact its ability to remain a going concern through a combination of the growth of revenues, with interim cash flow deficiencies being addressed through additional equity and debt financing. The Company anticipates raising additional funds through public or private financing, strategic relationships, or other arrangements in the near future to support its business operations; however, the Company may not have commitments from third parties for a sufficient amount of additional capital. The Company cannot be certain that any such financing will be available on acceptable terms, or at all, and its failure to raise capital when needed could limit its ability to continue its operations. The Company’s ability to obtain additional funding will determine its ability to continue as a going concern. Failure to secure additional financing in a timely manner and on favorable terms would have a material adverse effect on the Company’s financial performance, results of operations and stock price and require it to curtail or cease operations, sell off its assets, seek protection from its creditors through bankruptcy proceedings, or otherwise. Furthermore, additional equity financing may be dilutive to the holders of the Company’s common stock, and debt financing, if available, may involve restrictive covenants, and strategic relationships, if necessary, to raise additional funds, and may require that the Company relinquish valuable rights. Please see Financial Statements – Note 3. Going Concern for further information.
We have limited existing brand identity and customer loyalty; if we fail to market our brand to promote our products and service offerings, our business could suffer.
We believe that establishing and maintaining brand identity and brand loyalty is critical to attracting customers. In order to attract customers to our Wearable Health Solutions products, we may be forced to spend substantial funds to create and maintain brand recognition among consumers. We believe that the cost of our sales campaigns could increase substantially in the future. If our branding efforts are not successful, our ability to earn revenues and sustain our operations will be harmed.
In the event we are not successful in reaching our revenue targets, additional funds may be required.
We may not be able to proceed with our business plan for the development and marketing of our core products and services. Should this occur, we may be forced to suspend or cease operations. Management intends to raise additional funds by way of a public or private offering. While we believe in the viability of our strategy to increase revenues and in our ability to raise additional funds, there can be no assurances to that effect. Our ability to continue as a going concern is dependent upon our ability to further implement our business plan, raise additional money, and generate sufficient revenues.
Due to shutdown of 3G networks in the United States and because our iHelp MAX™ is not expected to be available until the third quarter of 2023, there is a risk that our customers may purchase competitors products, instead of our iHelp MAX™ product.
Our current customers that are using the iHelp +3G devices in the United States will no longer be able to use these devices after the 3G networks have been shut down. As of this filing we have replaced most of our older 3G units in the United States with our iHelp Mini 4G™ units as the 3G networks have been shut down. We plan to have our iHelp MAX™ product ready for distribution during the third quarter of 2023 and we may lose U.S. customers to competitors’ products because we are not able to transfer them to our newer iHelp Mini 4G™ product before those customers choose to upgrade their devices. As such there is a material risk that we will not be able to retain our United States customers now that the 3G networks are shut down and we have not launched our iHelp MAX™ product, our current United States customers may chose a competitor’s product.
We have to keep up with rapid technological change to continue offering our client’s competitive products and services or we may lose clients and be unable to compete.
Our future success will depend on our ability to continue delivering to our medical alert and monitoring clients a better experience, with more technologically advanced products, that are easier to use, can be used more remotely and have longer battery lives. In order to do so, we will need to adapt to rapidly changing technologies, to adapt our services to evolving industry standards and to improve the performance of our services. Our failure to adapt to such changes would likely lead to a loss of clients or a substantial reduction in the fees we would be able to charge versus competitors who have more rapidly adopted improved technology. Any loss of clients or reduction of fees would adversely impact our revenue. In addition, the widespread adoption of new health monitoring technologies, Internet technologies or other technological changes could require substantial expenditures by us to modify or adapt our products, services or infrastructure. If we are unable to pass all or part of these costs on to our clients, our margins and, therefore, profitability will be reduced.
We may not be able to compete with other medical alert product and related software developers, almost all of whom have greater resources and experience than we do.
Mostly all of our competitors have significantly greater financial, marketing and other resources, broader product lines outside of medical and health monitoring, and larger customer bases than we have and are less financially leveraged than we are. As a result, these competitors may be able to: adapt to changes in customer requirements more quickly; introduce new and more innovative technological products more quickly; better adapt to downturns in the economy or other decreases in sales; better withstand pressure to accept customer concerns; take advantage of acquisition and other opportunities more readily; devote greater resources to the marketing and sale of their products and services; and adopt more aggressive pricing policies.
Our ability to adapt to industry changes in technology, or market circumstances, may drastically change the business environment in which we operate.
If we are unable to recognize these changes in good time, are late in adjusting our business model, or if circumstances arise such as pricing actions by competitors, then this could have a material adverse effect on our growth ambitions, financial condition and operating results.
The global increase in security threats and higher levels of professionalism in computer crime have increased the importance of effective IT security measures, including proper identity management processes to protect against unauthorized systems access.
Our systems, networks, products, solutions and services remain potentially vulnerable to attacks, which could potentially lead to the leakage of confidential information, improper use of our systems and networks, which could in turn materially adversely affect our financial condition and operating results.
We rely on the attraction and retention of talented employees.
Attracting and retaining talented employees in sales and marketing, research and development, finance and general management, as well as of specialized technical personnel, is critical to our success and could also result in business interruptions. There can be no assurance that we will be successful in attracting and retaining all the highly qualified employees and key personnel needed in the future.
We may from time to time be subject to warranty and product liability claims with regard to product performance and effects.
We could incur product liability losses as a result of repair and replacement costs in response to customer complaints or in connection with the resolution of contemplated legal proceedings relating to such claims. Successful claims for damages may be made that are in excess of our potential insurance coverage. Our insurance could become more expensive and there is no assurance that insurance will still be available on acceptable terms. In addition to potential losses arising from claims and related legal proceedings, product liability claims could affect our reputation and relationships with key customers. As a result, product liability claims could materially impact our financial condition and operating results.
In order to develop additional revenues and further our current products, we have plans to invest in product(s) that are synergistic with our current products.
Investing in these products’ adaptive technologies or business models may or may not be successful. They may not be timely nor cost-effective, and there is no assurance the desired results will be achieved. We may need to increase our inventory levels, increase our accounts receivables, and be exposed to bad debt and obsolete inventory, and this would negatively impact our operations and balance sheet.
We may not be able to successfully compete against companies with substantially greater resources.
The industries in which we operate in general are subject to intense and increasing competition. Some of our competitors may have greater capital resources, facilities, and diversity of product lines, which may enable them to compete more effectively in this market. Our competitors may devote their resources to developing and marketing products that will directly compete with our product lines. Due to this competition, there is no assurance that we will not encounter difficulties in obtaining revenues and market share or in the positioning of our products. There are no assurances that competition in our respective industries will not lead to reduced prices for our products. If we are unable to successfully compete with existing companies and new entrants to the market this will have a negative impact on our business and financial condition.
Certain of the Company’s products are dependent on consumer discretionary spending.
Certain of our medical and health products as well at technology platforms may be susceptible to unfavorable changes in economic conditions. Decreases in consumer discretionary spending could negatively affect the Company's business and result in a decline in sales and financial performance.
We face extensive government regulation both within and outside the U.S. relating to the development, manufacture, marketing, sale and distribution of our products, software and services.
Our operations are subject to numerous federal, state, and local laws and regulations, in areas such as consumer protection, labor and employment, tax, permitting, and other laws and regulations. Most jurisdictions in which we operate have licensing laws directed specifically toward the health monitoring industry. In certain jurisdictions, we must obtain licenses or permits in order to comply with standards governing employee selection, training, and business conduct. In addition, we are subject to certain administrative requirements and laws of the jurisdictions in which we operate. These laws and regulations may include restrictions on the manner in which we promote the sale of our monitoring services and may require us to provide most purchasers of our services with three-day or longer rescission rights.
Some local government authorities have adopted or are considering various measures aimed at reducing false alerts. Such measures include requiring permits for individual monitoring systems, revoking such permits following a specified number of false calls, imposing fines on customers or monitoring companies for false notifications, limiting the number of times ambulance or police personnel will respond to alerts at a particular location after a specified number of false alerts, requiring additional verification of an alert notice before the ambulance services or police respond, or providing no response to medical monitoring alerts.
Our business is dependent upon suppliers.
We plan on entering into supply agreements with manufacturers. Nevertheless, we remain dependent upon a limited number of suppliers for our products. Currently we have two manufacturers for our iHelp MAX™, iHelp Mini 4G™ and iHelp+ 3G™ products, if we were to lose either of those manufacturers, we anticipate that it would take us several months to replace them and have a new manufacturer that was capable of producing our products. Although we do not anticipate difficulty in obtaining adequate inventory at competitive prices, we can offer no assurance that such difficulties will not arise. The extent to which supply disruption will affect us remains uncertain. Our inability to obtain sufficient quantities of products at competitive prices would have a material adverse effect on our business, financial condition and results of operations. We also rely on third parties to monitor our devices, currently there are over a dozen companies that are capable of delivering this service to our customers and as such we do not believe that having to switch medical alert monitoring services will have a material adverse effect on our business or financial condition.
We cannot assure that we will earn a profit or that consumers will accept our products.
Our business is speculative and dependent upon acceptance of our products by consumers. Our operating performance will be heavily dependent on whether or not we are able to earn a profit on the sale of our products. We cannot assure that we will be successful or earn enough revenue to make a profit, or that investors will not lose their entire investment.
Inventories maintained by the Company, the manufacturers and its customers may fluctuate from time to time.
The Company relies in part on its dealer and customer relationships and predictions of the manufacturer and customer inventory levels in projecting future demand levels and financial results. These inventory levels may fluctuate, and may differ from the Company’s predictions, resulting in the Company’s projections of future results being different than expected. These changes may be influenced by changing relationships with the dealers and customers, economic conditions and customer preference for particular products. There can be no assurance that the Company’s manufacturers and customers will maintain levels of inventory in accordance with the Company’s predictions or past history, or that the timing of customers’ inventory build or liquidation will be in accordance with the Company’s predictions or past history.
New online store features could fail to attract new customers, retain existing customers, or generate revenue.
Our business strategy is partially dependent on our ability to develop online store features to attract new customers and retain existing ones. Staffing changes, changes in customer behavior or development of competing networks may cause customers to switch to competing brands or decrease our customer retention through our online store. To date, our online retail platform, is only in its early-stages and it has begun to generate some revenue for the Company. There is no guarantee that individual customers will use these features and as a result, we may fail to generate greater revenue. Additionally, any of the following events may cause decreased use of our online store:
| | · | Emergence of competing mPERS and competitors online retail stores; |
| | | |
| | · | Inability to convince potential customers to shop at our online store; |
| | | |
| | · | A decrease or perceived decrease in the quality of products at our online store(s); |
| | | |
| | · | An increase in content/products that are irrelevant to our users; |
| | | |
| | · | Technical issues on certain platforms or in the cross-compatibility of multiple platforms; |
| | | |
| | · | An increase in the level of advertisements by competitors may lower traffic acquisition rates; |
| | | |
| | · | A rise in safety or privacy concerns; and |
| | | |
| | · | An increase in the level of spam or undesired content on the sites. |
We have limited or no control over the manufacturing and quality of the products we sell.
We do not directly manufacture any of the products that we sell or we currently plan to sell. Consequently, we have limited or no control over manufacturing practices at the suppliers from whom we procure the products we sell. We put forth considerable efforts to ensure that the products we sell are safe and comply with all applicable regulations. In spite of these efforts, there is a risk that we could inadvertently resell products which fail to comply with applicable regulations or have other quality defects. If this were to occur, we could be forced to conduct a product recall, defend regulatory or civil claims, or take other actions, any of which could have a material adverse effect upon our business.
Increases in the cost of shipping, postage or credit card processing could harm our business.
We ship our products to customers by United States mail and other overnight delivery and surface services. We generally invoice the costs of delivery and parcel shipments directly to customers as separate shipping and handling charges. Any increases in shipping, postal or credit card processing rates could harm our operating results as we may not be able to effectively pass such increases on to our customers. Similarly, strikes or other service interruptions by these shippers could limit our ability to market or deliver our products on a timely basis.
We face an inherent risk of exposure to product liability claims in the event that the products we manufacture or sell allegedly cause personal injury.
We face an inherent risk of exposure to product liability claims in the event that the products we sell allegedly cause personal injury. Although we have not experienced any significant losses due to product liability claims, we may experience such losses in the future. While our suppliers maintain insurance against product liability claims but cannot be certain that such coverage will be adequate to cover any liabilities that we may incur, or that such insurance will continue to be available on acceptable terms. A successful claim brought against us in excess of available insurance coverage, or any claim that results in significant adverse publicity, could have a material adverse effect upon our business.
Capacity limits on some of our planned technology, monitoring and payment processing systems and network hardware and software may be difficult to project and we may not be able to expand by hiring additional employees and upgrade our systems in a timely manner to meet significant unexpected increased demand.
As the number of possible customers we potentially serve increases and if our customer base grows, the traffic on our planned monitoring and payment processing systems and network hardware and software will rise. In our capacity planning processes, we may be unable to accurately project the rate of increase in the use of our medical monitoring and payment processing systems and network hardware and software. In addition, we may not be able to expand our employee base and upgrade our systems and network hardware and software capabilities to accommodate significant unexpected increased or peak use. If we are unable to appropriately hire more employees, upgrade our systems and network hardware and software in a timely manner, our operations and processes may be temporarily disrupted.
We may be unable to keep pace with changes in the industries that we serve and advancements in technology as our business and market strategy evolves.
As changes in the industries we serve occur or macroeconomic conditions fluctuate we may need to adjust our business strategies or find it necessary to restructure our operations or businesses, which could lead to changes in our cost structure, the need to write down the value of assets, or impact our profitability. We will also make investments in existing or new businesses, including investments in technology and expansion of our business plans. These investments may have short-term returns that are negative or less than expected and the ultimate business prospects of the business may be uncertain.
As our business and market strategy evolves, we also will need to respond to technological advances and emerging industry standards in a cost-effective and timely manner in order to remain competitive, better and more interactive products and web accessibility standards. The need to respond to technological changes may require us to make substantial, unanticipated expenditures. There can be no assurance that we will be able to respond successfully to technological change.
Acquisitions, strategic investments, partnerships, or alliances could be difficult to identify, pose integration challenges, divert the attention of management, disrupt our business, dilute stockholder value, and adversely affect our business, financial condition, and results of operations.
We have in the past and may in the future seek to acquire or invest in businesses, joint ventures, and platform technologies that we believe could complement or expand our platform, enhance our technology, or otherwise offer growth opportunities. Further, our anticipated proceeds from future offerings increase the likelihood that we will devote resources to exploring larger and more complex acquisitions and investments than we have previously attempted. Any such acquisitions or investments may divert the attention of management and cause us to incur various expenses in identifying, investigating, and pursuing suitable opportunities, whether or not the transactions are completed, and may result in unforeseen operating difficulties and expenditures. In particular, we may encounter difficulties assimilating or integrating the businesses, technologies, products, personnel, or operations of any acquired companies, particularly if the key personnel of an acquired company choose not to work for us, their software is not easily adapted to work with our platform, or we have difficulty retaining the customers of any acquired business due to changes in ownership, management, or otherwise. Any such transactions that we are able to complete may not result in the synergies or other benefits we expect to achieve, which could result in substantial impairment charges. These transactions could also result in dilutive issuances of equity securities or the incurrence of debt, which could adversely affect our results of operations.
We are subject to anti-corruption, anti-bribery, anti-money laundering, and similar laws, and non-compliance with such laws can subject us to criminal or civil liability and harm our business, financial condition, and results of operations.
We are subject to the U.S. Foreign Corrupt Practices Act of 1977, as amended (the FCPA), U.S. domestic bribery laws, the UK Bribery Act 2010, and other anti-corruption and anti-money laundering laws in the countries in which we conduct business. Anti-corruption and anti-bribery laws have been enforced aggressively in recent years and are interpreted broadly to generally prohibit companies, their employees, and their third-party intermediaries from authorizing, offering, or providing, directly or indirectly, improper payments or benefits to recipients in the public or private sector. As we increase our international sales and business and sales to the public sector, potentially to hospitals, insurance companies, or medical providers, we may engage with business partners and third-party intermediaries to market our wearable health products and to obtain necessary permits, licenses, and other regulatory approvals, if necessary. In addition, we or our third-party intermediaries may have direct or indirect interactions with officials and employees of government agencies or state-owned or affiliated entities, such as hospitals, insurance companies, or medical providers. We can be held liable for the corrupt or other illegal activities of these third-party intermediaries, our employees, representatives, contractors, partners, and agents, even if we do not explicitly authorize such activities.
While we plan to have policies and procedures to address compliance with such laws, there is a risk that our employees and agents will take actions in violation of our policies and applicable law, for which we may be ultimately held responsible. As we expand internationally, our risks under these laws may increase.
Detecting, investigating, and resolving actual or alleged violations of anti-corruption laws can require a significant diversion of time, resources, and attention from senior management. In addition, noncompliance with anti-corruption, anti-bribery, or anti-money laundering laws could subject us to whistleblower complaints, investigations, sanctions, settlements, prosecution, enforcement actions, fines, damages, other civil or criminal penalties or injunctions, suspension or debarment from contracting with certain persons, reputational harm, adverse media coverage, and other collateral consequences. If any subpoenas or investigations are launched, or governmental or other sanctions are imposed, or if we do not prevail in any possible civil or criminal proceeding, our business, financial condition, and results of operations could be harmed.
Our intellectual property rights may not protect our business or provide us with a competitive advantage.
To be successful, we must protect our technology and brand in all jurisdictions through trademarks, trade secrets, patents, copyrights, service marks, invention assignments, contractual restrictions, and other intellectual property rights and confidentiality procedures. Despite our efforts to implement these protections, they may not protect our business or provide us with a competitive advantage for a variety of reasons, including:
| | · | the failure by us to obtain patents and other intellectual property rights for important innovations or maintain appropriate confidentiality and other protective measures to establish and maintain our trade secrets; |
| | · | uncertainty in, and evolution of, legal standards relating to the validity, enforceability, and scope of protection of intellectual property rights; |
| | · | potential invalidation of our intellectual property rights through administrative processes or litigation; |
| | · | any inability by us to detect infringement or other misappropriation of our intellectual property rights by third parties; and |
| | · | other practical, resource, or business limitations on our ability to enforce our rights. |
Further, the laws of certain foreign countries, particularly certain developing countries, do not provide the same level of protection of corporate proprietary information and assets, such as intellectual property, trademarks, trade secrets, know-how, and records, as the laws of the United States. As a result, we may encounter significant problems in protecting and defending our intellectual property or proprietary rights abroad. Additionally, we may also be exposed to material risks of theft or unauthorized reverse engineering of our proprietary information and other intellectual property, including technical data, data sets, or other sensitive information. Our efforts to enforce our intellectual property rights in such foreign countries may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop, which could have a material adverse effect on our business, financial condition, and results of operations. Moreover, if we are unable to prevent the disclosure of our trade secrets to third parties, or if our competitors independently develop any of our trade secrets, we may not be able to establish or maintain a competitive advantage in our market, which could seriously harm our business.
Litigation may be necessary to enforce our intellectual property or proprietary rights, protect our trade secrets, or determine the validity and scope of proprietary rights claimed by others. Any litigation, whether or not resolved in our favor, could result in significant expense to us, divert the efforts of our technical and management personnel, and result in counterclaims with respect to infringement of intellectual property rights by us. If we are unable to prevent third parties from infringing upon or misappropriating our intellectual property or are required to incur substantial expenses defending our intellectual property rights, our business, financial condition, and results of operations may be materially adversely affected.
We may become subject to intellectual property disputes, which are costly and may subject us to significant liability and increased costs of doing business.
We compete in markets where there are a large number of patents, copyrights, trademarks, trade secrets, and other intellectual and proprietary rights, as well as disputes regarding infringement of these rights. In addition, many of the holders of patents, copyrights, trademarks, trade secrets, and other intellectual and proprietary rights have extensive intellectual property portfolios and greater resources than we do to enforce their rights. As compared to our large competitors, our patent portfolio is currently non-existent, as we are just embarking on the phases of our iHelp MAX™ devices and patents may not provide a material deterrent to such assertions or provide us with a strong basis to counterclaim or negotiate settlements. Further, to the extent assertions are made against us by entities that hold patents but are not operating companies, our future patent portfolio may not provide deterrence because such entities are not concerned with counterclaims. We have currently trademarked or registered our following brands iHelp MAX™, MediPendant®, Wearable Health Solutions, iHelp Mini 4G™ and iHelp+ 3G™.
Any intellectual property litigation to which we become a party may require us to do one or more of the following:
| | · | cease selling, licensing, or using products or features that incorporate the intellectual property rights that we allegedly infringe, misappropriate, or violate; |
| | · | make substantial payments for legal fees, settlement payments, or other costs or damages, including indemnification of third parties; |
| | · | obtain a license or enter into a royalty agreement, either of which may not be available on reasonable terms or at all, in order to obtain the right to sell or use the relevant intellectual property; or |
| | · | redesign the allegedly infringing products to avoid infringement, misappropriation, or violation, which could be costly, time-consuming, or impossible. |
Intellectual property litigation is typically complex, time consuming, and expensive to resolve and would divert the time and attention of our management and technical personnel. It may also result in adverse publicity, which could harm our reputation and ability to attract or retain customers. As we grow, we may experience a heightened risk of allegations of intellectual property infringement. An adverse result in any litigation claims against us could have a material adverse effect on our business, financial condition, and results of operations.
Any future litigation against us could be costly and time-consuming to defend.
We may become subject to legal proceedings and claims that arise in the ordinary course of business, such as claims brought by our customers in connection with commercial disputes or employment claims made by our current or former employees. Litigation might result in substantial costs and may divert management’s attention and resources, which might seriously harm our business, financial condition, and results of operations. Insurance might not cover such claims, might not provide sufficient payments to cover all the costs to resolve one or more such claims, and might not continue to be available on terms acceptable to us (including premium increases or the imposition of large deductible or co-insurance requirements). A claim brought against us that is uninsured or underinsured could result in unanticipated costs, potentially harming our business, financial position, and results of operations. In addition, we cannot be sure that our existing insurance coverage and coverage for errors and omissions will continue to be available on acceptable terms or that our insurers will not deny coverage as to any future claim.
If we use open-source software inconsistent with our policies and procedures or the license terms applicable to such software, we could be subject to legal expenses, damages, or costly remediation or disruption to our business.
We plan to use some open-source software in our products and platform. While we plan to have policies and procedures in place governing the use of open-source software, there is a risk that we incorporate open-source software with onerous licensing terms, including the obligation to make our source code available for others to use or modify without compensation to us. If we receive an allegation that we have violated an open-source license, we may incur significant legal expenses, be subject to damages, be required to redesign our product to remove the open-source software, or be required to comply with onerous license restrictions, all of which could have a material impact on our business. Even in the absence of a claim, if we discover the use of open-source software inconsistent with our planned policies, we could expend significant time and resources to replace the open-source software or obtain a commercial license, if available. All of these risks are heightened by the fact that the ownership of open-source software can be uncertain, leading to litigation, and many of the licenses applicable to open-source software have not been interpreted by courts, and these licenses could be construed to impose unanticipated conditions or restrictions on our ability to commercialize our products. Any use of open-source software inconsistent with our future policies or licensing terms could harm our business and financial position.
Unfavorable conditions in our industry or the global economy, or reductions in cloud spending, could limit our ability to grow our business and negatively affect our results of operations.
Our results of operations may vary based on the impact of changes in our industry or the global economy on us or our customers and potential customers. Negative conditions in the general economy both in the United States and abroad, including conditions resulting from changes in gross domestic product growth, financial and credit market fluctuations, international trade relations, pandemic (such as the COVID-19 pandemic), political turmoil, natural catastrophes, warfare, and terrorist attacks on the United States, Europe, the Asia Pacific region, Japan, or elsewhere, could cause a decrease in business investments, including spending on cloud technologies, and negatively affect the growth of our business. Competitors, many of whom are larger and have greater financial resources than we do, may respond to challenging market conditions by lowering prices in an attempt to attract our customers. We cannot predict the timing, strength, or duration of any economic slowdown, instability, or recovery, generally or within any particular industry.
Our planned operations are international in scope, creating a variety of operational challenges.
A component of our growth strategy involves the expansion of our operations and customer base internationally. We expect that our international activities will grow as we expand our Medical Alarm Concept’s business and as we continue to pursue opportunities in existing and new international markets, which will require significant dedication of management attention and financial resources.
Our current and future international business and operations involve a variety of risks, including:
| | · | slower than anticipated public cloud adoption by international businesses; |
| | · | changes in a specific countries or region’s political, economic, or legal and regulatory environment, pandemics, tariffs, trade wars, or long-term environmental risks; |
| | · | the need to adapt and localize our platform for specific countries; |
| | · | greater difficulty collecting accounts receivable and longer payment cycles; |
| | · | unexpected changes in trade relations, regulations, or laws; |
| | · | new, evolving, and more stringent regulations relating to privacy and data security and the unauthorized use of, or access to, commercial and personal information, particularly in Asia; |
| | · | differing and potentially more onerous labor regulations; |
| | · | challenges inherent in efficiently managing, and the increased costs associated with, an increased number of employees over large geographic distances, including the need to implement appropriate systems, policies, benefits, and compliance programs that are specific to each jurisdiction; |
| | · | difficulties in managing a business in new markets with diverse cultures, languages, customs, legal systems, alternative dispute systems, and regulatory systems; |
| | · | increased travel, real estate, infrastructure, and legal compliance costs associated with international operations; |
| | · | currency exchange rate fluctuations and the resulting effect on our revenue and expenses, and the cost and risk of entering into hedging transactions if we chose to do so in the future; |
| | · | limitations on our ability to reinvest earnings from operations in one country to fund the capital needs of our operations in other countries; |
| | · | laws and business practices favoring local competitors or general market preferences for local vendors; |
| | · | limited or insufficient intellectual property protection or difficulties obtaining, maintaining, protecting, or enforcing our intellectual property rights, including our trademarks and patents; |
| | · | political instability or terrorist activities; |
| | · | COVID-19 or any other pandemics or epidemics that could result in decreased economic activity in certain markets, decreased use of our products and services, or in our decreased ability to import, export, or sell our products and services to existing or new customers in international markets; |
| | · | exposure to liabilities under anti-corruption and anti-money laundering laws, including the FCPA, U.S. bribery laws, the UK Bribery Act, and similar laws and regulations in other jurisdictions; |
| | · | burdens of complying with laws and regulations related to taxation; and |
| | · | regulations, adverse tax burdens, and foreign exchange controls that could make it difficult to repatriate earnings and cash. |
If we invest substantial time and resources to further expand our international operations and are unable to do so successfully and in a timely manner, our business and results of operations will suffer.
Changes in the economy could have a detrimental impact.
Changes in the general economic climate, both in the United States and internationally, could have a detrimental impact on consumer expenditure and therefore on the Company’s revenue. Recessionary pressures and other economic factors (such as declining incomes, future potential rising interest rates, higher unemployment, and tax increases) may decrease the disposable income that customers have available to spend on products and services like those of the Company and may adversely affect customers’ confidence and willingness to spend. Any of such events or occurrences could have a material adverse effect on the Company’s financial results and your investment.
We have a limited operating history, and we may not succeed.
The Company has a limited operating history and there can be no assurance that the Company’s proposed plan of business can be realized in the manner contemplated and, if it cannot be, investors may lose all or a substantial part of their investment. There is no guarantee that the Company will ever realize any significant operating revenues or that its operations will ever be profitable.
Management’s current estimates and expectations for its prospective business operations that are not historical facts are forward-looking statements that involve risks and uncertainties.
Sentences or phrases that use such words as “believes,” “anticipates,” “plans,” “may,” “hopes,” “can,” “will,” “expects,” “is, designed to,” “with the intent,” “potential” and others indicate forward-looking statements, but their absence does not mean that a statement is not forward-looking.
An investor should read this presentation with the understanding that actual future results may be materially different from what the Company expects.
Although such statements are based on the Management’s current estimates and expectations, and currently available competitive, financial, and economic data, forward-looking statements are inherently uncertain. A variety of factors could cause business conditions and results to differ materially from what is contained in any such forward-looking statements. Actual results from the operation of the Company may be different than the returns anticipated by the Management and/or that these returns may not be realized in the timeframe projected by Management, if at all.
You should further consider, among other factors, our prospects for success considering the risks and uncertainties encountered by companies that, like us, are in their early stages.
For example, unanticipated expenses, problems, and technical difficulties may occur and they may result in material delays in the operation of our business, concerning our new products. We may not successfully address these risks and uncertainties or successfully implement our operating strategies. If we fail to do so, it could materially harm our business to the point of having to cease operations where investors may lose their entire investment.
The success of the Company depends on management’s abilities.
The Company will be dependent upon the efforts, experience, contacts, and skills of its officers and certain members of the board of directors and officers. The loss of any such individuals could have a material, adverse effect on the Company, and such loss could occur at any time due to death, disability, resignation, or other reasons.
Conflicts of interest may arise between the Board of Directors and the Company.
Potential conflicts may exist between the Directors and the Company. These conflicts are not limited to the following: Our Board members, of which any or all may be involved in similar investments or have interests in similar entities, may raise capital for others, may serve on the board of other companies that may be a conflict of interest to the Company, may hire affiliates, contractors, vendors or suppliers to provide services to the Company on its behalf.
Further, there is a potential for a conflict of interest between BOAPIN.com, the Company, Mr. Mittler and Mr. Pizzino. Mr. Mittler and Mr. Pizzino are significant shareholders in BOAPIN.com and have the ability to effect decisions over BOAPIN.com’s operations through contractual arrangements and shareholder approvals. As, Mr. Mittler is also our Chief Executive Officer and Director, and Mr. Pizzino is also our President and Director, there is the potential for a conflict of interest between the Company, Mr. Mittler, Mr. Pizzino and BOAPIN.com.
Although dependent upon certain key personnel, the Company does not have any key person life insurance policies on any such people.
The Company is dependent upon management to conduct its operations and execute its business plan; however, the Company has not purchased any insurance policies for those individuals in the event of their death or disability. Therefore, should any of these key personnel, management, or founders die or become disabled, the Company will not receive any compensation that would assist with such a person’s absence. The loss of such a person could negatively affect the Company and its operations.
The Company has incurred and will likely incur additional debt.
The Company has incurred debt and will likely further incur debt (including secured debt) in the future with the continuing operations of its business. Complying with obligations under such indebtedness may have a material adverse effect on the Company and your investment.
The success of our new products and services is uncertain.
We have committed, and expect to continue to commit, significant resources and capital to develop and market existing product enhancements and medical alert products. We cannot assure you that we will achieve market acceptance for all our products, or of new products that we may offer in the future.
Moreover, these new products may be subject to significant competition with offerings by new and existing competitors. Also, new products and enhancements may pose a variety of challenges and require us to attract additional qualified employees. The failure to successfully develop and market these new products or enhancements could seriously harm our business, financial condition, and results of operations.
Consequently, our results of operations may decline quickly and significantly in response to changes in order patterns or rapid decreases in demand for our products.
We anticipate that fluctuations in operating results will continue in the future. The Company's operating results may vary. We may continue to incur net losses. The Company expects to experience variability in its revenues and net profit. While we fully intend to implement our business plan, we may experience net losses. Factors expected to contribute to this variability include, among other things:
| | · | The general economy |
| | · | The regulatory environment affecting our products. |
| | · | Consumer demand |
| | · | Transportation costs |
| | · | Competition in products |
The Company’s expenses could increase without a corresponding increase in revenues.
The Company’s operating and other expenses could increase without a corresponding increase in revenues, which could have a material adverse effect on the Company’s financial results and your investment. Factors which could increase operating and other expenses include, but are not limited to (1) increases in the rate of inflation, (2) increases in taxes and other statutory charges, (3) changes in laws, regulations or government policies which increase the costs of compliance with such laws, regulations or policies, (4) significant increases in insurance premiums, (5) increases in borrowing costs, and (6) unexpected increases in costs of supplies, goods, materials, construction, equipment or distribution.
Future acquisitions or strategic investments and partnerships could be difficult to identify and integrate with our business, disrupt our business, and adversely affect our financial condition and results of operations.
We may seek to acquire or invest in businesses and product lines that we believe could complement or expand our product offerings, or otherwise offer growth opportunities. The pursuit of potential acquisitions may divert the attention of management and cause us to incur various expenses in identifying, investigating, and pursuing suitable acquisitions, whether or not the acquisitions are completed. Future acquisitions could also result in dilutive issuances of equity securities or the incurrence of debt, which could adversely affect our financial position and results of operations. In addition, if an acquired business or product line fails to meet our expectations, our business, financial condition, and results of operations may be adversely affected.
Failure to successfully integrate acquired businesses and their products and other assets into our Company, or if integrated, failure to further our business strategy, may result in our inability to realize any benefit from such an acquisition.
We expect to grow by acquiring relevant businesses, including other PERS related businesses. The consummation and integration of any acquired business, product or other assets into our Company may be complex and time consuming and, if such businesses and assets are not successfully integrated, we may not achieve the anticipated benefits, cost-savings or growth opportunities. Furthermore, these acquisitions and other arrangements, even if successfully integrated, may fail to further our business strategy as anticipated, expose our Company to increased competition or other challenges with respect to our products or geographic markets, and expose us to additional liabilities associated with an acquired business, technology or other asset or arrangement.
Our business is dependent upon continued market acceptance by consumers.
We are substantially dependent on continued market acceptance of our services, and products by consumers. Although we believe that our services and products are gaining increasing consumer acceptance, we cannot predict that this trend will continue in the future.
The Company’s computers, website, or information system breakdown could affect the Company’s business.
The Company’s computers, website, and/or information system breakdowns, as well as cybersecurity attacks, could impair the Company’s ability to service its customers leading to reduced revenue from sales and/or reputational damage, which could have a material adverse effect on the Company’s financial results as well as your investment.
Our prior operating results may not be indicative of our future results.
You should not consider prior operating results concerning revenues, net income, or any other measure to be indicative of our future operating results. The timing and amount of future revenues will depend almost entirely on our ability to sell our services and products to new customers. Our future operating results will depend upon many other factors, including:
| | · | The level of product and price competition, |
| | · | Our success in expanding our distribution network and managing our growth, |
| | · | Our ability to develop and market product enhancements and new products, |
| | · | The timing of product enhancements, activities of and acquisitions by competitors, |
| | · | The ability to hire additional qualified employees, and |
| | · | The timing of such hiring and our ability to control costs. |
Risks Relating to Ownership of Our Securities
Because directors and officers currently and for the foreseeable future will continue to control Wearable Health Solutions, Inc., it is not likely that you will be able to elect directors or have any say in the policies of Wearable Health Solutions, Inc.
Our shareholders are not entitled to cumulative voting rights. Consequently, the election of directors and all other matters requiring shareholder approval will be decided by majority vote. The directors, officers and affiliates of Wearable Health Solutions, Inc., beneficially own a majority of our outstanding common stock voting rights through their control of 100% of the outstanding Series E Preferred Stock. Due to such significant ownership position held by our insiders, new investors may not be able to affect a change in our business or management, and therefore, shareholders would have no recourse as a result of decisions made by management.
In addition, sales of significant amounts of shares held by our directors, officers or affiliates, or the prospect of these sales, could adversely affect the market price of our common stock. Management may have conflicts of interest with the Company and its business, due to their significant shareholder position. Management’s stock ownership may discourage a potential acquirer from making a tender offer or otherwise attempting to obtain control of us, which in turn could reduce our stock price or prevent our stockholders from realizing a premium over our stock price.
Currently we are authorized to issue Three Billion, (3,000,000,000) shares of common stock with a par value of $0.0001 per share (the “Common Stock”) and Twenty-Five Million (25,000,000) shares of preferred stock (the “Preferred Stock"), of which 100,000 such shares have been designated as Series A Preferred Stock that convert into 2 shares of common stock for every 1 share of Series A Preferred Stock and have no voting rights. The Company has designated 62,500 shares as Series B Preferred Stock that convert into 2 shares of common stock for every 1 share of Series B Preferred Stock and have voting rights and vote on an “as converted” basis in actions required to have Series B Preferred Stockholder approval. The Company has designated 6,944,445 as Series C Preferred Stock that convert into 10 shares of common stock for every 1 share of Series C Preferred Stock and vote on an “as converted” basis on all matters submitted to our Stockholders for approval. The Company has designated 500,000 as Series D Preferred Stock that convert into 100 shares of common stock for each 1 share of Series D Preferred Stock and vote on actions required to have Series D Preferred Stockholder approval. The Company has designated 4,000,000 as Series E Preferred Stock which convert into 100 shares of common stock for each 1 share of Series E Preferred Stock and have voting rights equal to 10,000 votes of common stock per each 1 share of Series E Preferred Stock.
If additional shares are issued through employment agreements, common stock awards or our preferred shareholders decided to convert their shares of preferred shares into common stock there would be immediate dilution of the existing shareholders ownership percentages.
If our preferred shareholders converted their shares, without regard to any equity blocking restrictions there could be up to an additional 511,015,286 shares of common stock issued and outstanding. Due to these additional shares, our existing stockholders would suffer significant dilution to their share ownership percentages.
Our employment agreements grant periodic stock awards to our employees. Those periodic stock awards to our employees will result in dilution to the current shareholders of the Company.
The Company’s officers and directors will continue to exercise significant control over operations, which means as a minority shareholder, you would have no control over certain matters requiring shareholder approval that could affect your ability to ever resell any shares you purchase in this offering.
As of the date of this Registration Statement, Mr. Mittler and Mr. Pizzino, our CEO and President and directors will be able to vote up to 98% of the Company’s votes through their ownership of common and preferred shares. They will have a significant influence in determining the outcome of all corporate transactions, including the election of directors, approval of significant corporate transactions, changes in control of the Company or other matters that could affect your ability to ever resell your shares. Their interests may differ from the interests of the other shareholders and thus result in corporate decisions that are disadvantageous to other shareholders.
We expect to be a “controlled company” within the meaning of Nasdaq rules and, as a result, will qualify for exemptions from certain corporate governance requirements. As a result, you do not have the same protections afforded to stockholders of companies that are not exempt from such corporate governance requirements.
Upon completion of this Offering, our Management will continue to collectively hold more than 50% of the voting power for the election of directors of our company. As a result, we expect to be a controlled company within the meaning of Nasdaq corporate governance standards. Under Nasdaq rules, a company of which more than 50% of the voting power is held by an individual, company or group of persons acting together is a controlled company and will not to comply with certain Nasdaq corporate governance requirements, including the requirements that:
| | • | a majority of the Board consist of independent directors under Nasdaq rules; |
| | • | the nominating and governance committee be composed entirely of independent directors with a written charter addressing the committee’s purpose and responsibilities; and |
| | • | the compensation committee be composed entirely of independent directors with a written charter addressing the committee’s purpose and responsibilities. |
These requirements will not apply to us as long as we remain quoted on the Pink OTC Markets and as a controlled company. Accordingly, you may not have the same protections afforded to stockholders of companies that are subject to all of the corporate governance requirements of Nasdaq. See “Management— Controlled Company Status.”
Our stock price may be volatile, which may result in losses to our shareholders.
The stock markets have experienced significant price and trading volume fluctuations, and the market prices of companies quoted on the OTC Markets quotation system in which shares of our common stock are quoted, have been volatile in the past and have experienced sharp share price and trading volume changes. The trading price of our common stock is likely to be volatile and could fluctuate widely in response to many factors, including the following, some of which are beyond our control:
| · | | variations in our operating results; |
| · | | changes in expectations of our future financial performance, including financial estimates by securities analysts and investors; |
| · | | changes in operating and stock price performance of other companies in our industry; |
| · | | additions or departures of key personnel; and |
| · | | future sales of our common stock. |
Domestic and international stock markets often experience significant price and volume fluctuations. These fluctuations, as well as general economic and political conditions unrelated to our performance, may adversely affect the price of our common stock.
Our common shares may become thinly traded and you may be unable to sell at or near ask prices, or at all.
We cannot predict the extent to which an active public market for trading our common stock will be sustained. The trading volume of our common shares may be sporadically or “thinly-traded,” meaning that the number of persons interested in purchasing our common shares at or near bid prices at a certain given time may be relatively small or non-existent.
This situation is attributable to a number of factors, including the fact that we are a small company which is relatively unknown to stock analysts, stockbrokers, institutional investors and others in the investment community who generate or influence sales volume. Even if we came to the attention of such persons, those persons tend to be risk-averse and may be reluctant to follow, purchase, or recommend the purchase of shares of an unproven company such as ours until such time as we become more seasoned and viable. As a consequence, there may be periods of several days or more when trading activity in our shares is minimal or non-existent, as compared to a seasoned issuer which has a large and steady volume of trading activity that will generally support continuous sales without an adverse effect on share price. We cannot give you any assurance that a broader or more active public trading market for our common stock will develop or be sustained, or that current trading levels will be sustained.
Our common stock is currently deemed a “penny stock,” which makes it more difficult for our investors to sell their shares.
The SEC has adopted Rule 15g-9 which establishes the definition of a “penny stock,” for the purposes relevant to us, as any equity security that has a market price of less than $5.00 per share, subject to certain exceptions. For any transaction involving a penny stock, unless exempt, the rules require that a broker or dealer approve a person’s account for transactions in penny stocks, and the broker or dealer receive from the investor a written agreement to the transaction, setting forth the identity and quantity of the penny stock to be purchased.
In order to approve a person’s account for transactions in penny stocks, the broker or dealer must obtain financial information and investment experience objectives of the person and make a reasonable determination that the transactions in penny stocks are suitable for that person and the person has sufficient knowledge and experience in financial matters to be capable of evaluating the risks of transactions in penny stocks.
The broker or dealer must also deliver, prior to any transaction in a penny stock, a disclosure schedule prescribed by the SEC relating to the penny stock market, which, in highlight form sets forth the basis on which the broker or dealer made the suitability determination, and that the broker or dealer received a signed, written agreement from the investor prior to the transaction.
Generally, brokers may be less willing to execute transactions in securities subject to the “penny stock” rules. This may make it more difficult for investors to dispose of our common stock if and when such shares are eligible for sale and may cause a decline in the market value of its stock.
Disclosure also has to be made about the risks of investing in penny stocks in both public offerings and in secondary trading, and about the commission’s payable to both the broker-dealer and the registered representative, current quotations for the securities, and the rights and remedies available to an investor in cases of fraud in penny stock transactions. Finally, monthly statements have to be sent disclosing recent price information for the penny stock held in the account and information on the limited market in penny stock.
As an issuer of a “penny stock,” the protection provided by the federal securities laws relating to forward-looking statements does not apply to us.
Although federal securities laws provide a safe harbor for forward-looking statements made by a public company that files reports under the federal securities laws, this safe harbor is not available to issuers of penny stocks. As a result, we will not have the benefit of this safe harbor protection in the event of any legal action based upon a claim that the material provided by us contained a material misstatement of fact or was misleading in any material respect because of our failure to include any statements necessary to make the statements not misleading. Such an action could hurt our financial condition.
We do not anticipate paying any cash dividends to our common shareholders.
We presently do not anticipate that we will pay dividends on any of our common stock in the foreseeable future. If payment of dividends does occur at some point in the future, it would be contingent upon our revenues and earnings, if any, capital requirements, and general financial condition. The payment of any common stock dividends will be within the discretion of our Board of Directors. We presently intend to retain all earnings, if any, to implement our business plan; accordingly, we do not anticipate the declaration of any dividends for common stock in the foreseeable future.
Volatility in our common share price may subject us to securities litigation.
The market for our common stock may be characterized by significant price volatility as compared to seasoned issuers, and we expect that our share price will continue to be more volatile than a seasoned issuer for the indefinite future. In the past, plaintiffs have often initiated securities class action litigation against a company following periods of volatility in the market price of its securities. We may, in the future, be the target of similar litigation. Securities litigation could result in substantial costs and liabilities and could divert management's attention and resources.
The elimination of monetary liability against our directors, officers and employees under Nevada law and the existence of indemnification rights of our directors, officers and employees may result in substantial expenditures by our company and may discourage lawsuits against our directors, officers and employees.
Our Articles of Incorporation contain a specific provision that eliminates the liability of our directors and officers for monetary damages to our company and shareholders. Further, we are prepared to give such indemnification to our directors and officers to the extent provided for by Nevada law. We may also have contractual indemnification obligations under our employment agreements with our officers. The foregoing indemnification obligations could result in our company incurring substantial expenditures to cover the cost of settlement or damage awards against directors and officers, which we may be unable to recoup. These provisions and resultant costs may also discourage our company from bringing a lawsuit against directors and officers for breaches of their fiduciary duties and may similarly discourage the filing of derivative litigation by our shareholders against our directors and officers even though such actions, if successful, might otherwise benefit our company and shareholders.
Our business is subject to changing regulations related to corporate governance and public disclosure that have increased both our costs and the risk of noncompliance.
Because our common stock is publicly traded, we are subject to certain rules and regulations of federal, state and financial market exchange entities charged with the protection of investors and the oversight of companies whose securities are publicly traded. These entities, including the Public Company Accounting Oversight Board, the SEC and FINRA, have issued requirements and regulations and continue to develop additional regulations and requirements in response to corporate scandals and laws enacted by Congress, most notably the Sarbanes-Oxley Act of 2002. Our efforts to comply with these regulations have resulted in, and are likely to continue resulting in, increased general and administrative expenses and diversion of management time and attention from revenue-generating activities to compliance activities. Because new and modified laws, regulations and standards are subject to varying interpretations in many cases due to their lack of specificity, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies. This evolution may result in continuing uncertainty regarding compliance matters and additional costs necessitated by ongoing revisions to our disclosure and governance practices.
The market price for the common stock is particularly volatile given our status as a relatively unknown company with a thinly traded public float, limited operating history, and minimal revenues, which could lead to wide fluctuations in our share price. The price at which you purchase our shares may not be indicative of the price that will prevail in the trading market. You may be unable to sell your common shares at or above your purchase price, which may result in substantial losses to you.
The market for our shares of common stock is characterized by significant price volatility when compared to seasoned issuers, and we expect that our share price will continue to be more volatile than a seasoned issuer for the indefinite future. The volatility in our share price is attributable to a number of factors. First, as noted above, our shares are sporadically traded. As a consequence of this lack of liquidity, the trading of relatively small quantities of shares may disproportionately influence the price of those shares in either direction. The price for our shares could, for example, decline precipitously in the event that a large number of our shares is sold on the market without commensurate demand, as compared to a seasoned issuer which could better absorb those sales without adverse impact on its share price. Secondly, we are a speculative investment due to, among other matters, our limited operating history and small revenue or lack of profit to date, and the uncertainty of future market acceptance for our potential products. As a consequence of this enhanced risk, more risk-averse investors may, under the fear of losing all or most of their investment in the event of negative news or lack of progress, be more inclined to sell their shares on the market more quickly and at greater discounts than would be the case with the securities of a seasoned issuer. The following factors may add to the volatility in the price of our shares: actual or anticipated variations in our quarterly or annual operating results; acceptance of our inventory of games; government regulations, announcements of significant acquisitions, strategic partnerships or joint ventures; our capital commitments and additions or departures of our key personnel. Many of these factors are beyond our control and may decrease the market price of our shares regardless of our operating performance. We cannot make any predictions or projections as to what the prevailing market price for our shares will be at any time, including as to whether our shares will sustain their current market prices, or as to what effect the sale of shares or the availability of shares for sale at any time will have on the prevailing market price.
Shareholders should be aware that, according to SEC Release No. 34-29093, the market for penny stocks has suffered in recent years from patterns of fraud and abuse. Such patterns include (1) control of the market for the security by one or a few broker-dealers that are often related to the promoter or issuer; (2) manipulation of prices through prearranged matching of purchases and sales and false and misleading press releases; (3) boiler room practices involving high-pressure sales tactics and unrealistic price projections by inexperienced sales persons; (4) excessive and undisclosed bid-ask differential and markups by selling broker-dealers; and (5) the wholesale dumping of the same securities by promoters and broker-dealers after prices have been manipulated to a desired level, along with the resulting inevitable collapse of those prices and with consequent investor losses. Our management is aware of the abuses that have occurred historically in the penny stock market. Although we do not expect to be in a position to dictate the behavior of the market or of broker-dealers who participate in the market, management will strive within the confines of practical limitations to prevent the described patterns from being established with respect to our securities. The possible occurrence of these patterns or practices could increase the volatility of our share price.
A reverse stock split may decrease the liquidity of the shares of our common stock.
The liquidity of the shares of our common stock may be adversely affected by a reverse stock split given the reduced number of shares that will be outstanding following a reverse stock split, especially if the market price of our common stock does not increase as a result of the reverse stock split.
If we perform a reverse stock split, the resulting market price of our common stock may not attract new investors, including institutional investors, and may not satisfy the investing requirements of those investors. Consequently, the trading liquidity of our common stock may not improve.
Although we believe that a higher market price of our common stock may help generate greater or broader investor interest, we cannot assure you that a reverse stock split will result in a share price that will attract new investors.
Statements Regarding Forward-looking Statements
______
This Disclosure Statement contains various “forward-looking statements.” You can identify forward-looking statements by the use of forward-looking terminology such as “believes,” “expects,” “may,” “will,” “would,” “could,” “should,” “seeks,” “approximately,” “intends,” “plans,” “projects,” “estimates” or “anticipates” or the negative of these words and phrases or similar words or phrases. You can also identify forward-looking statements by discussions of strategy, plans or intentions. These statements may be impacted by a number of risks and uncertainties.
The forward-looking statements are based on our beliefs, assumptions and expectations of our future performance taking into account all information currently available to us. These beliefs, assumptions and expectations are subject to risks and uncertainties and can change as a result of many possible events or factors, not all of which are known to us. If a change occurs, our business, financial condition, liquidity and results of operations may vary materially from those expressed in our forward-looking statements. You should carefully consider these risks before you make an investment decision with respect to our Securities. For a further discussion of these and other factors that could impact our future results, performance or transactions, see the section entitled “Risk Factors.”
USE OF PROCEEDS
______
We will not receive any of the proceeds from the sale of the common stock by the Selling Shareholders. If we sell all of the shares being offered, our net proceeds (after our estimated offering expenses of $40,000) will be $960,000.
We will use these net proceeds for the following:
| Shares Offered (% Sold) | 1,000,000,000 Shares Sold (100%) | 750,000,000 Shares Sold (75%) | 500,000,000 Shares Sold (50%) | 250,000,000 Shares Sold (25%) |
| Gross Offering Proceeds | $1,000,000 | $750,000 | $500,000 | $250,000 |
| Approximate Offering Expenses (1) | | | | |
| Misc. Expenses | 10,000 | 10,000 | 10,000 | 10,000 |
| Legal and Accounting | 30,000 | 30,000 | 30,000 | 30,000 |
| Total Offering Expenses | 40,000 | 40,000 | 40,000 | 40,000 |
| Total Net Offering Proceeds | 960,000 | 710,000 | 460,000 | 210,000 |
| Principal Uses of Net Proceeds (2) | | | | |
| Purchase of Inventory | 470,000 | 350,000 | 200,000 | 150,000 |
| Marketing & Advertising Related to MAC Product Offering | 50,000 | 50,000 | 20,000 | 20,000 |
| Legal and Accounting Fees | 30,000 | 30,000 | 30,000 | 30,000 |
| Convertible Debt Repayment | 100,000 | 75,000 | 50,000 | - |
| Officers & Directors Compensation | 300,000 | 200,000 | 150,000 | - |
| Other | 10,000 | 5,000 | 10,000 | 10,000 |
| Total Principal Uses of Net Proceeds | 960,000 | 710,000 | 460,000 | 210,000 |
| Amount Unallocated | 0 | 0 | 0 | 0 |
(1) Offering expenses have been estimated to be $40,000.
(2) Any line-item amounts not expended completely shall be held in reserve as working capital and subject to reallocation to other line-item expenditures as required for ongoing operations.
The precise amounts that we will devote to each of the foregoing items, and the timing of expenditures, will vary depending on numerous factors.
As indicated in the table above, if we sell only 75%, or 50%, or 25% of the shares offered for sale in this offering, we would expect to use the resulting net proceeds for the same purposes as we would use the net proceeds from a sale of 100% of the shares, and in approximately the same proportions, until such time as such use of proceeds would leave us without working capital reserve. At that point we would expect to modify our use of proceeds by limiting our expansion.
The expected use of net proceeds from this offering represents our intentions based upon our current plans and business conditions, which could change in the future as our plans and business conditions evolve and change. The amounts and timing of our actual expenditures, specifically with respect to working capital, may vary significantly depending on numerous factors. The precise amounts that we will devote to each of the foregoing items, and the timing of expenditures, will vary depending on numerous factors. As a result, our management will retain broad discretion over the allocation of the net proceeds from this offering.
In the event we do not sell all of the shares being offered, we may seek additional financing from other sources in order to support the intended use of proceeds indicated above. If we secure additional equity funding, investors in this offering would be diluted. In all events, there can be no assurance that additional financing would be available to us when wanted or needed and, if available, on terms acceptable to us.
DILUTION
______
If you purchase shares in this offering, your ownership interest in our Common Stock will be diluted immediately, to the extent of the difference between the price to the public charged for each share in this offering and the net tangible book value per share of our Common Stock after this offering.
Our historical net tangible book value as of March 31, 2023, was $(2,953,764) or $(0.0019) per then-outstanding share of our Common Stock. Historical net tangible book value per share equals the amount of our total tangible assets less total liabilities, divided by the total number of shares of our Common Stock outstanding, all as of the date specified.
The following table illustrates the per share dilution to new investors discussed above, assuming the sale of, respectively, 100%, 75%, 50% and 25% of the shares offered for sale in this offering at a price per share of $.001 (without deducting estimated offering expenses of $40,000):
| Percentage of shares offered that are sold | | | 100% | | | | 75% | | | | 50% | | | | 25% | |
| Price to the public charged for each share in this offering | | $ | 0.001 | | | $ | 0.001 | | | $ | 0.001 | | | $ | 0.001 | |
| Historical net tangible book value per share as of March 31, 2023(1) | | | (.0019 | ) | | | (.0019 | ) | | | (.0019 | ) | | | (.0019 | ) |
| Increase in net tangible book value per share attributable to new investors in this offering (2) | | | (0.0011 | ) | | | (0.0009 | ) | | | (0.0007 | ) | | | (0.0004 | ) |
| Net tangible book value per share, after this offering | | | 0.0008 | | | | (0.0010 | ) | | | (0.0012 | ) | | | (0.0015 | ) |
| Dilution per share to new investors | | | 0.0018 | | | | 0.0020 | | | | 0.0022 | | | | 0.0025 | |
| (1) | Based on net tangible book value as of March 31, 2023, of $(2,963,764) and 1,561,255,108 outstanding shares of Common stock as of June 6, 2023. |
| (2) | With deducting estimated offering expenses from the Offering. |
DISTRIBUTION
______
This Offering Circular is part of an Offering Statement that we filed with the SEC, using a continuous offering process. Periodically, as we have material developments, we will provide an Offering Circular supplement that may add, update, or change information contained in this Offering Circular. Any statement that we make in this Offering Circular will be modified or superseded for any inconsistent statement made by us in a subsequent Offering Circular supplement. The Offering Statement we filed with the SEC includes exhibits that provide more detailed descriptions of the matters discussed in this Offering Circular. You should read this Offering Circular and the related exhibits filed with the SEC and any Offering Circular supplement, together with additional information contained in our annual reports, semi-annual reports and other reports and information statements that we will file periodically with the SEC. See the section entitled “Additional Information” below for more details.
Pricing of the Offering
Prior to the Offering, there has been a limited public market for the Offered Shares. The public offering price was determined by the Company. The principal factors considered in determining the public offering price include:
- the information set forth in this Offering Circular and otherwise available;
- our history and prospects and the history of and prospects for the industry in which we compete;
- our past and present financial performance;
- our prospects for future earnings and the present state of our development;
- the general condition of the securities markets at the time of this Offering;
- the recent market prices of, and demand for, publicly traded common stock of generally comparable companies; and
- other factors deemed relevant by us.
Offering Period and Expiration Date
This Offering will start on or after the Qualification Date will terminate at the earlier of (1) the date at which the maximum offering amount has been sold or (2) the date at which the Offering is earlier terminated by the Company at its sole discretion. At least every 12 months after this offering has been qualified by the United States Securities and Exchange Commission (the “Commission”), the Company will file a post-qualification amendment to include the Company’s recent financial statements.
Procedures for Subscribing
When you decide to subscribe for Offered Shares in this Offering, you should:
Contact us via phone or email.
| | 1. | Electronically receive, review, execute and deliver to us a subscription agreement; and |
| | | |
| | 2. | Deliver funds directly by check, wire or electronic funds transfer via ACH to the specified account maintained by us. |
Any potential investor will have ample time to review the subscription agreement, along with their counsel, prior to making any final investment decision. We shall only deliver such subscription agreement upon request after a potential investor has had ample opportunity to review this Offering Circular.
Right to Reject Subscriptions. After we receive your complete, executed subscription agreement and the funds required under the subscription agreement have been transferred to our account, we have the right to review and accept or reject your subscription in whole or in part, for any reason or for no reason. We will return all monies from rejected subscriptions immediately to you, without interest or deduction.
Acceptance of Subscriptions. Upon our acceptance of a subscription agreement, we will countersign the subscription agreement and issue the shares subscribed at closing. Once you submit the subscription agreement and it is accepted, you may not revoke or change your subscription or request your subscription funds. All accepted subscription agreements are irrevocable.
State Law Exemption and Offerings to “Qualified Purchasers”
Our Common Stock is being offered and sold only to “qualified purchasers” (as defined in Regulation A). As a Tier 2 offering pursuant to Regulation A, this offering will be exempt from state “Blue Sky” law review, subject to certain state filing requirements and anti-fraud provisions, to the extent that our Common Stock offered hereby is offered and sold only to “qualified purchasers” or at a time when our Common Stock is listed on a national securities exchange. “Qualified purchasers” include: (i) “accredited investors” under Rule 501(a) of Regulation D and (ii) all other investors so long as their investment in our Common Stock does not represent more than 10% of the greater of their annual income or net worth (for natural persons), or 10% of the greater of annual revenue or net assets at fiscal year-end (for non-natural persons). However, our Common Stock is being offered and sold only to those investors that are within the latter category (i.e., investors whose investment in our Common Stock does not represent more than 10% of the applicable amount), regardless of an investor’s status as an “accredited investor.” Accordingly, we reserve the right to reject any investor’s subscription in whole or in part for any reason, including if we determine in our sole and absolute discretion that such investor is not a “qualified purchaser” for purposes of Regulation A.
No Escrow
The proceeds of this offering will not be placed into an escrow account. We will offer our Common Stock on a best-efforts basis primarily through an online platform. As there is no minimum offering, upon the approval of any subscription to this Offering Circular, the Company shall immediately deposit said proceeds into the bank account of the Company and may dispose of the proceeds in accordance with the Use of Proceeds at Management’s discretion.
Other Selling Restrictions
Other than in the United States, no action has been taken by us that would permit a public offering of our Common Stock in any jurisdiction where action for that purpose is required. Our Common Stock may not be offered or sold, directly or indirectly, nor may this Offering Circular or any other offering material or advertisements in connection with the offer and sale of shares of our Common Stock be distributed or published in any jurisdiction, except under circumstances that will result in compliance with the applicable rules and regulations of that jurisdiction. Persons into whose possession this Offering Circular comes are advised to inform themselves about and to observe any restrictions relating to this offering and the distribution of this Offering Circular. This Offering Circular does not constitute an offer to sell or a solicitation of an offer to buy our Common Stock in any jurisdiction in which such an offer or solicitation would be unlawful.
Investment Limitations
Generally, no sale may be made to you in this Offering if the aggregate purchase price you pay is more than 10% of the greater of your annual income or net worth (please see below on how to calculate your net worth). Different rules apply to accredited investors and non-natural persons. Before making any representation that your investment does not exceed applicable thresholds, we encourage you to review Rule 251(d)(2)(i)(C) of Regulation A. For general information on investing, we encourage you to refer to www.investor.gov.
Because this is a Tier 2, Regulation A Offering, most investors must comply with the 10% limitation on investment in the Offering. The only investor in this Offering exempt from this limitation is an “accredited investor” as defined under Rule 501 of Regulation D under the Securities Act (an “Accredited Investor”). If you meet one of the following tests you should qualify as an Accredited Investor:
| (i) | You are a natural person who has had individual income in excess of $200,000 in each of the two most recent years, or joint income with your spouse in excess of $300,000 in each of these years, and have a reasonable expectation of reaching the same income level in the current year; |
| | |
| (ii) | You are a natural person and your individual net worth, or joint net worth with your spouse, exceeds $1,000,000 at the time you purchase Offered Shares (please see below on how to calculate your net worth); |
| | |
| (iii) | You are an executive officer or general partner of the issuer or a manager or executive officer of the general partner of the issuer; |
| | |
| (iv) | You are an organization described in Section 501(c)(3) of the Internal Revenue Code of 1986, as amended, or the Code, a corporation, a Massachusetts or similar business trust or a partnership, not formed for the specific purpose of acquiring the Offered Shares, with total assets in excess of $5,000,000; |
| | |
| (v) | You are a bank or a savings and loan association or other institution as defined in the Securities Act, a broker or dealer registered pursuant to Section 15 of the Exchange Act, an insurance company as defined by the Securities Act, an investment company registered under the Investment Company Act of 1940 (the “Investment Company Act”), or a business development company as defined in that act, any Small Business Investment Company licensed by the Small Business Investment Act of 1958 or a private business development company as defined in the Investment Advisers Act of 1940; |
| | |
| (vi) | You are an entity (including an Individual Retirement Account trust) in which each equity owner is an accredited investor; |
| (vii) | You are a trust with total assets in excess of $5,000,000, your purchase of Offered Shares is directed by a person who either alone or with his purchaser representative(s) (as defined in Regulation D promulgated under the Securities Act) has such knowledge and experience in financial and business matters that he is capable of evaluating the merits and risks of the prospective investment, and you were not formed for the specific purpose of investing in the Offered Shares; or |
| | |
| (viii) | You are a plan established and maintained by a state, its political subdivisions, or any agency or instrumentality of a state or its political subdivisions, for the benefit of its employees, if such plan has assets in excess of $5,000,000. |
MANAGEMENT’S DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
______
You should read the following discussion and analysis of our financial condition and results of our operations together with our financial statements and the notes thereto appearing elsewhere in this Offering Circular. This discussion contains forward-looking statements reflecting our current expectations, whose actual outcomes involve risks and uncertainties. Actual results and the timing of events may differ materially from those stated in or implied by these forward-looking statements due to a number of factors, including those discussed in the sections entitled “Risk Factors”, “Cautionary Statement regarding Forward-Looking Statements” and elsewhere in this Offering Circular. Please see the notes to our Financial Statements for information about our Critical Accounting Policies and Recently Issued Accounting Pronouncements.
Management’s Discussion and Analysis
The Company has had minimal revenues from operations in each of the last two fiscal years, and in the current fiscal year.
The Company has not yet established an ongoing source of revenues sufficient to cover its operating costs for the next fiscal year and allow it to continue as a going concern. The ability of the Company to continue as a going concern is dependent on the Company obtaining adequate capital to fund operating losses until it becomes profitable. As of June 30, 2022, the Company has a net loss of $13,049,155, and if the Company is unable to obtain adequate capital, it could be forced to cease operations.
During the twelve months ended June 30, 2022, Company has net cash used in operating activities of $(3,107,177) as well as stock compensation non-cash expenses of $10,044,522 and a net loss of $13,049,155. The Company raised $2,380,252 from financing activities, net of repayments, in the twelve months ended June 30, 2022, which resulted in a negative working capital of $(2,133,878) as of June 30, 2022. If the Company is unable to raise additional adequate capital, it could be forced to cease operations.
During the nine months ended March 31, 2023, the Company has net cash used in operating activities of $(1,161,977) and a net loss of $1,810,699. The Company had net cash flow of $1,117,255 from financing activities in the nine months ended March 31, 2023, which resulted in a working capital deficit of $2,953,764 as of March 31, 2023. If the Company is unable to raise additional adequate capital, it could be forced to cease operations.
Plan of Operation for the Next Twelve Months
Over the next 12 months we intend to carry on business to market, package, and distribute our wearable health-based products. We anticipate that we will incur the following operating expenses during this period:
| Advertising | | $ | 500,000 | |
| Compensation | | | 1800,000 | |
| G&A | | | 260,000 | |
| Total | | $ | 1,560,000 | |
We will require funds of approximately $1,560,000 over the next twelve months to operate our business. This capital will be used to build out infrastructure, purchase inventory, upgrade our products, upgrade our website, accounting, legal and marketing expenses.
These funds may be raised through equity financing, debt financing, or other sources, which may result in further dilution in the equity ownership of our shares. There is no assurance that we will be able to maintain operations at a level sufficient for an investor to obtain a return on their investment in our common stock. Further, we may continue to be unprofitable.
The Company believes that the proceeds of this Offering will satisfy its cash requirements for the next twelve months. To complete the Company’s entire deployment of its current and proposed products and its business plan, it may have to raise additional funds in the next twelve months. Contemporaneously we will work to recruit experts in the field as well as scout experienced firms to assist in the marketing and distribution of our wearable health products and services.
The Company expects to increase the number of employees at the corporate level.
Use of Estimates
The preparation of financial statements in accordance with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosures of contingent assets and liabilities at the date of the financial statements, and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates. Significant estimates include assumptions about collection of accounts and notes receivable, the valuation and recognition of stock-based compensation expense, the valuation and recognition of derivative liability, valuation allowance for deferred tax assets and useful life of fixed assets.
Company Overview
Wearable Health Solutions Inc. (the Company) was incorporated as Medical Alarm Concepts Holding, Inc. on June 4, 2008, under the laws of the State of Nevada. The Company was formed for the sole purpose of acquiring all of the membership units of Medical Alarm Concepts LLC, a Pennsylvania limited liability company (“Medical LLC”). On May 26, 2016, the Company filed an Amended and Restated Articles of Incorporation with the Secretary of State of the State of Nevada to change its name from “Medical Alarm Concepts, Inc.” to “Wearable Health Solutions Inc.”
Wearable Health Solutions provides mobile health (mHealth) products and services to be used by customers in case of an emergency. As a provider of personal emergency response devices, we provide innovative wearable healthcare products, tracking services, and turn-key solutions that enable our users to be proactive with their health, as well as safe and protected.
Our flagship products are the iHelp devices, the 3G, iHelp Mini 4G™ and the next generation iHelp MAX™ - personal emergency alarms that are used to summon help in the event of an emergency at home.
Results of Operations
Results for the Three Months Ended March 31, 2023, compared to the Three Months Ended March 31, 2022
| Working Capital | | March 31, 2023 $ | | | June 30, 2022 $ | |
| Cash | | | 16,341 | | | | 70,505 | |
| Current Assets | | | 52,434 | | | | 297,409 | |
| Current Liabilities | | | 3,006,198 | | | | 2,431,287 | |
| Working Capital (Deficit) | | | (2,953,764 | ) | | | (2,133,878 | ) |
| Cash Flows for the Nine Months Ended: | | March 31, 2023 $ | | | March 31, 2022 $ | |
| Cash Flows used in Operating Activities | | | (1,161,977 | ) | | | (2,671,141 | ) |
| Cash Flows used in Investing Activities | | | (9,442 | ) | | | (50,000 | ) |
| Cash Flows provided by Financing Activities | | | 1,117,255 | | | | 2,443,684 | |
| Net Decrease in Cash During Period | | | (54,164 | ) | | | (277,457 | ) |
Operating Revenues
The Company had revenues of $193,745 and $212,982 for the three months ended March 31, 2023 and 2022, respectively.
Cost of Revenues
The Company’s cost of revenues for the three months ended March 31, 2023 and 2022 was $135,307 and $111,434, respectively. Cost of revenues increased for the three months ended March 31, 203 primarily due to increased hardware revenues in the three months ended March 31, 2023 as compared to 2022.
Gross Profit
The Company’s gross profit for the three months ended March 31, 2023 was $58,538, compared to $101,548 for the three months ended March 31, 2022. The decrease in the Company’s gross profit was due primarily to the increase in hardware revenues in 2023 as compared to 2022.
Operating Expenses
Operating expenses consisted primarily of consulting fees, professional fees, and legal and accounting expenses. For the three months ended March 31, 2023, operating expenses were $538,180 compared to $7,483,638 for the three months ended March 31, 2022. The primary expenses for the three months ended March 31, 2023 were salaries and wages and consulting and professional fees totaling $377,914; the primary expenses for the three months ended March 31, 2022 were salaries and wages and consulting and professional fees totaling $7,081,234. The decrease in salaries and wages in the three months ended March 31, 2023 as compared to the three months ended March 31, 2022 is primarily attributable to lower stock-based compensation expense in the three months ended March 31, 2023 compared to the three months ended March 31, 2022.
Other (Income) Expense, net
The Company had other (income) expense, net for the three months ended March 31, 2023 and 2022 of $42,499 and $(14,507), respectively. Other (income) expense, net for the three months ended March 31, 2023 was all interest expense. Other (income) expense, net for the three months ended March 31, 2022 consisted of $23,000 in payments for settlement of the MCA Cure complaint and interest expense of $8,493.
Net loss
The net loss for the three months ended March 31, 2023, was $522,141 compared to $7,367,583 for the three months ended March 31, 2022.
Results for the Nine Months Ended March 31, 2023, compared to the Nine Months Ended March 31, 2022
Revenues
The Company had revenues of $608,244 and $819,601 for the nine months ended March 31, 2023 and 2022, respectively.
Cost of Revenues
The Company’s cost of revenues for the nine months ended March 31, 2023 and 2022 was $354,627 and $457,307, respectively.
Gross Profit
The Company’s gross profit for the nine months ended March 31, 2023 and 2022 was $253,617, and $362,294, respectively. The decrease in the Company’s gross profit in 2023 as compared to 2022 was due primarily to the decrease in revenues.
Operating Expenses
Operating expenses consisted primarily of consulting fees, professional fees, and legal and accounting expenses. For the nine months ended March 31, 2023, operating expenses were $2,012,711 compared to $12,448,296 for the nine months ended March 31, 2022. The primary expenses for 2023 were salaries and wages of $1,215,671; the primary expenses for 2022 were salaries and wages and consulting and professional services of $10,904,649 and $462,988, respectively. The decrease in salaries and wages in the nine months ended March 31, 2023 as compared to the nine months ended March 31, 2022 is primarily attributable to lower stock-based compensation expense in the nine months ended March 31, 2023 compared to the nine months ended March 31, 2022.
Other (Income) Expense, net
The Company had other (income) expense, net for the nine months ended March 31, 2023 and 2022 of $51,605 and $5,301, respectively. Other (income) expense, net for the nine months ended March 31, 2023 consisted of interest expense of $72,605 which was offset in part by $19,500 in settlement proceeds and interest income of $1,500. The Company had other income (expense), net for the nine months ended March 31, 2022 of $5,301. Other (income) expense, net for the nine months ended March 31, 2022 consisted of change in fair value of derivative instrument of $213,053, $(23,000) in payments for settlement of the MCA Cure complaint, gain on debt extinguishment of $(96,145), gain on settlement of accounts payable of $(156,616), and interest expense of $68,009.
Net loss
The net loss for the nine months ended March 31, 2023, was $1,810,699 compared to $12,091,303 for the comparable period ended March 31, 2022.
Liquidity and Capital Resources
The ability of the Company to continue as a going concern is dependent on the Company’s ability to raise additional capital and implement its business plan. Since its inception, the Company has been funded by related parties through capital investment and borrowing of funds and through the sale of equity.
At March 31, 2023, the Company had total current assets of $52,434. Current assets consisted primarily of cash and inventory. At March 31, 2023, the Company had total current liabilities of $3,006,198 compared to $2,431,287, at June 30, 2022. Current liabilities consisted primarily of accounts payable, accrued liabilities and accrued compensation, notes payable and convertible notes.
We had negative working capital of $2,953,764 as of March 31, 2023.
Cash flow from Operating Activities
During the nine months ended March 31, 2023, cash used in operating activities was $(1,161,977) compared to $(2,671,141) for the same period ended March 31, 2022. The decrease in the amount of cash used in operating activities was primarily due to the decrease in the net loss resulting from the lower salaries and wages incurred in the nine months ended March 31, 2023 as compared to the nine months ended March 31, 2022.
Cash flow from Financing Activities
For the nine months ended March 31, 2023, cash provided by financing activities was $1,117,255 compared to $2,443,684 provided during the nine months ended March 31, 2022.
Quarterly Developments
None.
Subsequent Developments
Resignation of Independent Registered Public Accounting Firm
On April 4, 2023, Assurance Dimensions, Inc. (the “Auditor) informed Wearable Health Solutions, Inc. (the “Company”) of their formal resignation as the Company’s independent registered public accounting firm.
The accounting reports of the Auditor on the Company’s consolidated financial statements for fiscal years (“FY”) ended June 30, 2021 (“2021”) and June 30, 2022 (“2022”) did not contain any adverse opinion or disclaimer of opinion and were not qualified or modified as to uncertainty, audit scope or accounting principle, except that each report on the Company’s consolidated financial statements contained an explanatory paragraph regarding the Company’s ability to continue as a going concern based on the Company’s significant working capital deficiency, significant losses and needs to raise additional funds in FY ended 2021 and 2022.
During FY ended 2021 and 2022 and the subsequent interim period through April 4, 2023, the effective date of the Auditors dismissal, there were (i) no disagreements (as that term is defined in Item 304(a)(1)(iv) of Regulation S-K and the related instructions) between the Company and the Auditor on any matter of accounting principles or practices, financial statement disclosure, or auditing scope or procedure, which, if not resolved to the satisfaction of the Auditors would have caused the Auditor to make reference thereto in its reports on the consolidated financial statements of the Company for such years, and (ii) no “reportable events” (as that term is defined in Item 304(a)(1)(v) of Regulation S-K).
The Company provided the Auditor a copy of this Report prior to its filing with the Securities and Exchange Commission (the “SEC”) and requested the Auditor to furnish the Company with a letter addressed to the SEC, stating whether or not it agrees with the statements made in this Item 4.01. A copy of the Auditor’s letter dated May 8, 2023, confirming its agreement with the disclosures in this Item 4.01 is attached as Exhibit 16.1 to our current report on Form 8-K that was filed on May 8, 2023, with the Securities and Exchange Commission.
New Independent Registered Public Accounting Firm
On April 27, 2023, the Company engaged RBSM, LLP (“RBSM”) as the Company’s independent registered public accounting firm, effective April, 27, 2022 (the “Engagement Date”). The Company’s Board of Directors approved the engagement with RBSM on April, 27, 2022.
During the two most recent fiscal years and through the Engagement Date, the Company did not consult with RBSM regarding either:
| | 1. | application of accounting principles to any specified transaction, either completed or proposed, or the type of audit opinion that might be rendered on the Company’s financial statements, and neither a written report was provided to the Company nor oral advice was provided that RBSM concluded was an important factor considered by the Company in reaching a decision as to the accounting, auditing or financial reporting issue; or |
| | 2. | any matter that was either the subject of a disagreement (as defined in Regulation S-K, Item 304(a)(1) (iv) and the related instructions) or reportable event (as defined in Regulation S-K, Item 304(a)(1)(v)). |
Other than the aforementioned the Company evaluated events that have occurred after the balance sheet date but before the financial statements are issued and determined that there are no material events that are required to be disclosed.
Results for the year ended June 30, 2022, compared to the year ended June 30, 2021
| Working Capital | | June 30, 2022 $ | | | June 30, 2021 $ | |
| Cash | | | 70,505 | | | | 847,430 | |
| Current Assets | | | 141,609 | | | | 907,806 | |
| Current Liabilities | | | 2,275,487 | | | | 4,258,106 | |
| Working Capital (Deficit) | | | (2,133,878 | ) | | | (3,350,300 | ) |
| Cash Flows | | June 30, 2022 $ | | | June 30, 2021 $ | |
| Cash Flows used in Operating Activities | | | (3,107,177 | ) | | | (1,173,370 | ) |
| Cash Flows provided by Financing Activities | | | 2,380,252 | | | | 2,020,800 | |
| Net (Decrease) increase in Cash During Period | | | (776,925 | ) | | | 847,430 | |
Operating Revenues
The Company had revenues of $1,045,890 and $1,394,149 for the years ended June 30, 2022 and 2021, respectively.
Cost of Revenues
The Company’s cost of revenues for the years ended June 30, 2022 and 2021 was $568,409 and $869,450, respectively.
Gross Profit
The Company’s gross profit for the years ended June 30, 2022 and 2021 was $477,481, and $524,699, respectively.
General and Administrative Expenses
General and administrative expenses consisted primarily of consulting fees, professional fees, and legal and accounting expenses. For the year ended June 30, 2022, general and administrative expenses were $13,522,061 compared to $3,686,379 for the year ended 2021. The primary expenses for 2022 were salaries and wages totaling $11,375,405; the primary expenses for 2021 were salaries and wages and professional services totaling $2,452,018.
Other Income (Expense)
The Company had other income (expense) for the years ended June 30, 2022 and 2021 of $(4,575) and $(151,326), respectively. Other income (expense) consisted primarily of a loss in fair value of derivative instrument of $213,053 and $11,003, gain on settlement of accounts payable of $156,616 and $-0-, and interest expense of $80,283 and $221,323, respectively.
Net loss
The net loss for the year ended June 30, 2022, was $13,049,155 compared to $3,316,006 for the year ended June 30, 2021.
Liquidity and Capital Resources
The ability of the Company to continue as a going concern is dependent on the Company’s ability to raise additional capital and implement its business plan. Since its inception, the Company has been funded by related parties through capital investment and borrowing of funds.
At June 30, 2022, the Company had total current assets of $141,609 compared to $907,806 at June 30, 2021. Current assets consisted primarily of cash and prepaid inventory in 2022, and in 2021. At June 30, 2022, the Company had total current liabilities of $2,275,487 compared to $4,258,106 at June 30, 2021. Current liabilities consisted primarily of accounts payable, accrued liabilities, notes payable, and convertible notes payable with derivative liabilities. The decrease in our current liabilities was primarily attributed to the decrease in accounts payable and notes payable.
We had negative working capital of $2,133,878 as of June 30, 2022.
Cash flow from Operating Activities
During the year ended June 30, 2022, cash used in operating activities was $(3,107,177) compared to $(1,173,370) for the year ended June 30, 2021. The increase in the amounts of cash used in operating activities was primarily due to increased operating expenses and the paydown of accounts payable in 2022 as compared to 2021.
Cash flow from Financing Activities
For the year ended June 30, 2022, cash provided by financing activity was $2,380,252 compared to $2,020,800 provided during the year ended June 30, 2021.
Going Concern
We have not attained profitable operations and are dependent upon obtaining financing to pursue any extensive business activities. For these reasons, we have included in our audited financial statements that there is substantial doubt that we will be able to continue as a going concern without further financing.
The Company is a going concern, which contemplates continuity of operations, realization of assets, and liquidation of liabilities in the normal course of business.
As reflected in the accompanying financial statements, the Company had a Stockholders’ Deficit at June 30, 2022, of $2,092,227 as its liabilities exceeded its assets. The Company has incurred recurring losses from operations and has an accumulated deficit of $41,234,081 as of March 31, 2023. These factors among others raise substantial doubt about the Company’s ability to continue as a going concern.
Wearable Health Solutions provides mobile health (mHealth) products and services to approximately 200 dealers and distributors throughout the globe (mostly Canada, United States and New Zealand). As a provider of personal emergency response devices in the rapidly growing medical alarm device and eHealth sector, we provide innovative wearable healthcare products, tracking services, and turn-key solutions that enable our users to be proactive with their health, as well as safe and protected. According to the QYResearch report on Global PERS devices, MediPendant was the 17th largest global PERS company based on revenues in 2022 (“QYResearch Global PERs Devices Market Report, History and Forecast 2016-2027”). Our products and services are always state-of-the-art and cost effective. Through our culture, our drive, and the expertise of each individual employee, we are uniquely positioned to build shareholder value by setting the highest standards in service, reliability, and safety in our rapidly growing industry.
The financial statements do not include any adjustments that might be necessary if the Company is unable to continue as a going concern.
The Company has not yet established an ongoing source of revenues sufficient to cover its operating costs for the next fiscal year and allow it to continue as a going concern. The ability of the Company to continue as a going concern is dependent on the Company obtaining adequate capital to fund operating losses until it becomes profitable. As of June 30, 2022, the Company has a net loss of $13,049,155, and if the Company is unable to obtain adequate capital, it could be forced to cease operations.
During the year ended June 30, 2022, Company has net cash used in operating activities of $3,107,177 as well as stock compensation non-cash expenses of $10,044,522 and a net loss of $13,049,155. The Company raised $2,622,118 from financing activities in the year ended June 30, 2022, which resulted in a negative working capital of $2,133,878 as of June 30, 2022. If the Company is unable to raise additional adequate capital, it could be forced to cease operations.
Future Financings.
We will continue to rely on equity sales of the Company’s common shares in order to continue to fund business operations. Issuances of additional shares will result in dilution to existing shareholders. There is no assurance that the Company will achieve any additional sales of the equity securities or arrange for debt or other financing to fund our business plan of selling the iHelp MAX®.
Since inception, we have financed our cash flow requirements through issuance of common stock and loans from third parties. As we expand our activities, we may, and most likely will, continue to experience net negative cash flows from operations, pending receipt of revenues. Additionally, we anticipate obtaining additional financing to fund operations through common stock offerings, to the extent available, or to obtain additional financing to the extent necessary to augment our working capital. In the future we will need to generate sufficient revenues from sales in order to eliminate or reduce the need to sell additional stock or obtain additional loans. There can be no assurance we will be successful in raising the necessary funds to execute our business plan.
We anticipate that we will incur operating losses in the next twelve months. Our minimal operating history makes predictions of future operating results difficult to ascertain. Our prospects must be considered in light of the risks, expenses and difficulties frequently encountered by companies in their early stage of development, particularly companies in new and rapidly evolving markets. Such risks for us include, but are not limited to, an evolving and unpredictable business model and the management of growth.
To address these risks, we must, among other things, obtain a customer base, implement and successfully execute our business and marketing strategy, continually develop and upgrade our products, business model and website, respond to competitive developments, and attract, retain and motivate qualified personnel. There can be no assurance that we will be successful in addressing such risks, and the failure to do so can have a material adverse effect on our business prospects, financial condition and results of operations.
Off-Balance Sheet Arrangements
We have no significant off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that are material to stockholders.
Recently Issued Accounting Pronouncements
In February 2016, the FASB issued ASU 2016-02, Leases (Topic 842), which supersedes the current accounting for leases and while retaining two distinct types of leases, finance and operating, (1) requires lessees to record a right of use asset and a related liability for the rights and obligations associated with a lease, regardless of lease classification, and recognize lease expense in a manner similar to current accounting, (2) eliminates most real estate specific lease provisions, and (3) aligns many of the underlying lessor model principles with those in the new revenue standard. Leases with a term of 12 months or less will be accounted for similar to existing guidance for operating leases today. For public companies, the new standard is effective for annual and interim periods in fiscal years beginning after December 15, 2018. For all other entities, including emerging growth companies, this standard is effective for annual reporting periods beginning after December 15, 2019, and interim periods within fiscal years beginning after December 2020. Earlier application is permitted. The Company evaluated the impact on the financial statements and implemented the provisions of ASU 2016-02 for the annual financial statements for the year ended June 30, 2019.
In December 2019, the FASB issued ASU No. 2019-12, Simplifying the Accounting for Income Taxes, as part of its initiative to reduce complexity in accounting standards. The amendments in the ASU are effective for fiscal years beginning after December 15, 2020, including interim periods therein. Early adoption of the standard is permitted, including adoption in interim or annual periods for which financial statements have not yet been issued. The Company is currently evaluating the effect, if any, that the ASU will have on its consolidated financial statements.
The Company reviewed all recent accounting pronouncements issued by the FASB (including its Emerging Issues Task Force), the AICPA and the SEC, and they did not or are not believed by management to have a material impact on the Company’s present or future financial statements.
The Company has implemented all new accounting pronouncements that are in effect. These pronouncements did not have any material impact on the financial statements unless otherwise disclosed, and the Company does not believe that there are any other new accounting pronouncements that have been issued that might have a material impact on its financial position or results of operations.
Quantitative and Qualitative Disclosures about Market Risk
In the ordinary course of our business, we are not exposed to market risk of the sort that may arise from changes in interest rates or foreign currency exchange rates, or that may otherwise arise from transactions in derivatives.
Contingencies
Certain conditions may exist as of the date the financial statements are issued, which may result in a loss to the Company, but which will only be resolved when one or more future events occur or fail to occur. The Company’s management, in consultation with its legal counsel as appropriate, assesses such contingent liabilities, and such assessment inherently involves an exercise of judgment. In assessing loss contingencies related to legal proceedings that are pending against the Company or unasserted claims that may result in such proceedings, the Company, in consultation with legal counsel, evaluates the perceived merits of any legal proceedings or unasserted claims, as well as the perceived merits of the amount of relief sought or expected to be sought therein. If the assessment of a contingency indicates it is probable that a material loss has been incurred and the amount of the liability can be estimated, then the estimated liability would be accrued in the Company’s financial statements. If the assessment indicates a potentially material loss contingency is not probable, but is reasonably possible, or is probable, but cannot be estimated, then the nature of the contingent liability, together with an estimate of the range of possible loss, if determinable and material, would be disclosed. Loss contingencies considered remote are generally not disclosed unless they involve guarantees, in which case the guarantees would be disclosed.
Contractual Obligations
As a “smaller reporting company,” we are not required to provide tabular disclosure obligations.
Inflation
Inflation and changing prices have not had a material effect on our business and we do not expect that inflation or changing prices will materially affect our business in the foreseeable future.
Seasonality
Our operating results and operating cash flows historically have not been subject to seasonal variations. This pattern may change, however, in the event that we succeed in bringing our planned products to market.
Critical Accounting Policies
Our financial statements and accompanying notes have been prepared in accordance with United States generally accepted accounting principles applied on a consistent basis. The preparation of financial statements in conformity with U.S. generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting periods.
We regularly evaluate the accounting policies and estimates that we use to prepare our financial statements. A complete summary of these policies is included in the notes to our financial statements. In general, management’s estimates are based on historical experience, on information from third party professionals, and on various other assumptions that are believed to be reasonable under the facts and circumstances. Actual results could differ from those estimates made by management.
Cash and Cash Equivalents
For purposes of reporting within the statement of cash flows, our company considers all cash on hand, cash accounts not subject to withdrawal restrictions or penalties, and all highly liquid debt instruments purchased with a maturity of three months or less to be cash and cash equivalents.
Revenue Recognition
In May 2014, the FASB issued ASU No. 2014-09, Revenue from Contracts with Customers (Topic 606). ASU 2014-09 establishes a single comprehensive model for entities to use in accounting for revenue arising from outside contracts with customers and supersedes most of the existing revenue recognition guidance and notes that lease contracts with customers are a scope exception. ASU 2014-09 requires an entity to recognize revenue when it transfers promised goods or services to customers in an amount that reflects the consideration to which an entity expects to be entitled in exchange for those goods or services and also requires certain additional disclosures. On August 12, 2015, the FASB issued ASU 2015-14 to defer the effective date of ASU 2014-09. Public business entities may elect to adopt the amendments as of the original effective date; however, adoption is required for annual reporting periods beginning after December 15, 2017. The Company has adopted this pronouncement.
The Company’s revenues are derived principally from utilizing new technology in the medical alarm industry to provide 24-hour personal response monitoring services and related products to subscribers with medical- or age-related conditions. The Company recognizes revenue when it is realized or realizable and earned. For hardware sales, the Company recognizes revenues when the product is shipped. Customers are billed on Net 30 terms. For service revenue, the Company recognizes revenues when the time period for service is current. For customers who pay several months at a time, the Company records revenues for the month’s services and the balance of funds to deferred revenues and records the balance of revenues as they become current.
Share-based Compensation
Our company follows the provisions of FASB Accounting Standards Codification (“ASC”) 718, “Share-Based Payment” which requires all share-based payments to employees, including grants of employee stock options, to be recognized in the income statement based on their fair values. Equity instruments issued to non-employees for goods or services are accounted for at either the fair market value of the goods and services rendered or on the instruments issued in exchange for such services, whichever is more readily determinable, using the measurement date guidelines enumerated in ASC 505-50-30.
Loss per Common Share
Basic loss per share is computed by dividing the net loss attributable to the common stockholders by the weighted average number of shares of common stock outstanding during the year. Fully diluted loss per share is computed similar to basic loss per share except that the denominator is increased to include the number of additional common shares that would have been outstanding if the potential common shares had been issued and if the additional common shares were dilutive. As our company has a loss for the years ended June 30, 2022, and 2021 the potentially dilutive shares are anti-dilutive and therefore they are not added into the earnings per share calculation.
Income Taxes
Our company accounts for income taxes pursuant to ASC 740. Deferred tax assets and liabilities are determined based on temporary differences between the bases of certain assets and liabilities for income tax and financial reporting purposes. The deferred tax assets and liabilities are classified according to the financial statement classification of the assets and liabilities generating the differences. Our company maintains a valuation allowance with respect to deferred tax assets. Our company establishes a valuation allowance based upon the potential likelihood of realizing the deferred tax asset and taking into consideration our financial position and results of operations for the current year. Future realization of the deferred tax benefit depends on the existence of sufficient taxable income within the carry forward year under the Federal tax laws. Changes in circumstances, such as our company generating taxable income, could cause a change in judgment about the realization of the related deferred tax asset. Any change in the valuation allowance will be included in income in the year of the change in estimate.
Fair Value of Financial Instruments
Financial Accounting Standards Board (FASB) Accounting Standards Codification (ASC) 820 “Fair Value Measurements and Disclosures” (ASC 820) defines fair value as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. ASC 820 also establishes a fair value hierarchy that distinguishes between (1) market participant assumptions developed based on market data obtained from independent sources (observable inputs) and (2) a reporting entity’s own assumptions about market participant assumptions developed based on the best information available in the circumstances (unobservable inputs). The fair value hierarchy consists of three broad levels, which gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (Level 1) and the lowest priority to unobservable inputs (Level 3). The three levels of the fair value hierarchy are described below:
Level 1 - Unadjusted quoted prices in active markets that are accessible at the measurement date for identical, unrestricted assets or liabilities.
Level 2 - Inputs other than quoted prices included within Level 1 that are observable for the asset or liability, either directly or indirectly, including quoted prices for similar assets or liabilities in active markets; quoted prices for identical or similar assets or liabilities in markets that are not active; inputs other than quoted prices that are observable for the asset or liability (e.g., interest rates); and inputs that are derived principally from or corroborated by observable market data by correlation or other means.
Level 3 - Inputs that are both significant to the fair value measurement and unobservable. Our company estimates the fair value of financial instruments using the available market information and valuation methods. Considerable judgment is required in estimating fair value. Accordingly, the estimates of fair value may not be indicative of the amounts our company could realize in a current market exchange. As of June 30, 2022, and 2021, the carrying value of accounts payable and loans that are required to be measured at fair value, approximated fair value due to the short-term nature and maturity of these instruments.
Patent and Intellectual Property
Our company expenses the costs associated with obtaining a patent or other intellectual property purchased for research and development and has no alternative future use. For the periods ended June 30, 2022, and 2021, no events or circumstances occurred for which an evaluation of the alternative future use of patent or intellectual property was required.
Impairment of Long-Lived Assets
Our company evaluates the recoverability of long-lived assets and the related estimated remaining lives when events or circumstances lead management to believe that the carrying value of an asset may not be recoverable. For the years ended June 30, 2022, and 2021, no events or circumstances occurred for which an evaluation of the recoverability of long-lived assets was required.
Estimates
The consolidated financial statements are prepared on the basis of accounting principles generally accepted in the United States (“U.S. GAAP”). The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities and expenses. Actual results could differ from those estimates made by management.
Contractual Obligations
We are a smaller reporting company as defined by Rule 12b-2 of the Securities Exchange Act of 1934 and are not required to provide the information under this item.
DESCRIPTION OF PROPERTIES
Our mailing address is 2901 W. Coast Highway, Suite 200, Newport Beach, CA 92663. Our main telephone number is (949) 270-7460. The Company currently pays $175 a month for its office space and the term is month to month, with a thirty-day written notice of termination. The Company’s subsidiary maintained a warehouse office in Pennsylvania to facilitate inventory arrival and product shipment. The three-year lease at $1,100 per month expired on September 30, 2021, and was renewed for 12 months at $1,300 per month beginning October 1, 2021 and expiring on September 30, 2022. The subsidiary subsequently entered into a month-to-month arrangement with this office warehouse and then terminated the arrangement and vacated the facility as of December 31, 2022. The Company entered into a new three-year lease agreement on September 9, 2022 for new warehouse space located in Mequon, Wisconsin. The monthly rent for this new warehouse space is currently $1,325 per month for the first twelve months of the lease agreement. Our website is www.wearablehealthsolutions.com and our email address is info@wearablehealthsolutions.com.
Wearable Health Solutions, Inc.
_______
DESCRIPTION OF BUSINESS
Wearable Health Solutions
Wearable Health Solutions Inc. (the Company) was incorporated as Medical Alarm Concepts Holding, Inc. on June 4, 2008, under the laws of the State of Nevada. The Company was formed for the sole purpose of acquiring all of the membership units of Medical Alarm Concepts LLC, a Pennsylvania limited liability company (“Medical LLC”). On May 26, 2016, the Company filed an Amended and Restated Articles of Incorporation with the Secretary of State of the State of Nevada to change its name from “Medical Alarm Concepts, Inc.” to “Wearable Health Solutions Inc.” Our common stock is not traded on any exchange. Our common stock is quoted on the Pink OTC Markets (“OTC Markets”) under the trading symbol “WHSI.” There is no established public trading market for our common stock. We cannot assure you that there will be a market in the future for our common stock.
Wearable Health Solutions provides mobile health (mHealth) products and services to approximately 200 dealers and distributors throughout the globe (mostly Canada, United States and New Zealand). As a provider of personal emergency response devices, in the rapidly growing medical alarm device and eHealth sector, we provide innovative wearable healthcare products, tracking services, and turn-key solutions that enable our users to be proactive with their health, as well as safe and protected. Our products and services are always state-of-the-art and cost effective. Through our culture, our drive, and the expertise of each individual employee, we are uniquely positioned to build shareholder value by setting the highest standards in service, reliability, and safety in our rapidly growing industry.
Our flagship products are the iHelp devices, MediPendant, the iHelp+ 3G™, iHelp Mini 4G™ and the next generation 4G iHelp MAX™ - personal emergency alarms that are used to summon help in the event of an emergency at home. As of 2021, approximately 60% of all medical alarms being sold globally, by sales revenue (or approx. 57% of volume by type), are either Landline type or Standalone type technologies that require the user to speak and listen through a central base station unit, according to the QYResearch report on PERS(“QYResearch Global PERs Devices Market Report, History and Forecast 2016-2027”). The MediPendant®, however, offers a product that, is always connected through a landline, and has the speaker in the pendant, enabling the user to simply speak and listen directly through the pendant in the event of an emergency.
On August 2, 2021, the Company entered into an agreement with Voice of Things, Inc., in order to embed voice control into the devices. This agreement will provide the Company with cloud-to-cloud integration between Voice of Things, Inc. and iHelp without requiring any device-level touchpoints. The voice activation feature will be implemented at an estimated cost of $36,000 and has an approximate integration timeframe of two months, excluding certification time. This solution will also our products to integrate and function with Alexa/Google smart speakers and their respective apps. The voice control software has been completed as of February, 2022; however, the Company is currently in the design phase of the medical alert device that the software will be implemented in. The production of that medical device was supposed to be completed in early 2022, however, due to factory shutdown, supply chain issues as well as some minor changes to the medical alert device, we now expect the device to be completed in the third quarter of 2022. The Company has now begun to receive the medical alert devices as of the latter part of September, 2022.
On August 11, 2021, the Company entered into an Asset Purchase and Advisory Services Agreement (“Agreement”) with Anthony Chetta, owner of mHealth, whereby the Company acquired ownership of mHealth.com, the user portal used by the subsidiary customers, all code and related operations, the domain name, and logos, data, storage and online operations for $50,000. The Company chose to forego the independent valuation of the asset. The Company also retained Mr. Chetta as the Chief Technology Advisor, by issuing 1,000,000 restricted shares as a stock signing bonus, valued at $10,500 or $0.0105 per share, issued on September 30, 2021, $8,000 per month service agreement, and 1,000,000 shares of common stock every 6 months for a 30 month period, valued at $52,500 or $0.0105 per share. By acquiring mHealth the Company now owns its backend portal system which integrates and controls how our dealers interact with our customers, invoicing, and device functionality, which should allow the Company to better control and improve on the services it provides.
All of our products are used in conjunction with our proprietary management and operation platform. The platform is a cloud-hosted service consisting of methods and automation tasks for accepting data transmission from personal safety and medical devices (“PS/M”) and storing, reformatting, and retransmitting this data to subscribers, monitoring centers, healthcare providers, front-end portal/user interfaces, and API controllers.
The front-end portal interface provides a friendly, intuitive, and seamless management and monitoring platform for all of the below listed integrations, coupled with PS/M device fulfillment, tracking, controlling, and remote reprogramming, along with portal user administration and role/privilege assignment, internal activity/audit trails, ordering and invoicing, support portal integration, and any other customizations needed based on solution requirements.
Back-end automation and integration with third-party providers and services include:
| | · | SMS, email, and smartphone messaging app push notifications for PS/M event/activity/location alerts and subscriber communication with PS/M devices (with locale specific SMS numbers where available) |
| | · | Programmatic voice dialing and routing (with locale specific voice numbers where available) |
| | · | Integration with SIM card providers for management (activation, suspension, and usage monitoring) of airtime |
| | · | Flexible signal relay and reformatting for alarm, activity and health event data to central stations and healthcare providers based on unique communication and transmission protocol, whether via API calls or data transmission to TCP/IP or other types of receivers |
| | · | Integration with customer CRM for account details and user activity |
| | · | Integration with billing systems for device/equipment ordering and recurring billing |
| | · | API endpoints can be exposed for data access and controlling feature-sets in third party environments |
The iHelp MAX™ device, will operate on 4G Networks, is anticipated along with our platform to be able to plug into multiple devices to enable remote monitoring and data collection of essential vital signs in real-time with historical data via Bluetooth, NFC, and Wi-Fi technology, thereby making our devices telehealth ready. WHSI is considering several wearable technology vendors to produce and implement body-mounted sensors, such as smart watches, heart monitors, blood pressure sensors that can monitor and transmit biological data for healthcare purposes. At this time, the Company has not entered into any agreements, is only in preliminary discussions and there is no guarantee that we will come to an agreement with any of these vendors. If we do not come to an agreement with these vendors, we do not foresee that as materially affecting our business as there are multiple vendors and manufacturers of these type of products already in the industry.
The company recently launched the iHelp Mini 4G™, a small, lightweight, 4G device that includes features and functions such as fall detection, Geo-fencing, medication reminders, GPS, and SOS alerts. Currently the company has sold over 600 devices to dealers and consumers and is expecting an additional 1000 devices delivered shortly.
We anticipate the launch of the iHelp MAX™ device in approximately the third fiscal quarter of 2023 which offers a customized lone worker program for use with the iHelp MAX™. The remaining steps for completing the launch of our iHelp MAX™ device are for the manufacturer to obtain CE and FCC certifications on the electronics and for the Company to review and test the second set of working samples, we have experienced some delays in the production of the samples due to the city lockdowns and holiday festivals in China, but the Company expects to complete the product reviews by the end of June 2023. The device features a multi-function button for check-in and SOS alerts to ensure that workers in the field can get help in the event their health or safety is at risk. The small, lightweight device is waterproof and durable enough for use indoors or outdoors. The loan worker device includes the following features and functions:
| | · | Two-Way Voice - Voice connection to an operator or when an SOS or fall is detected (and optionally missed check-in) |
| | · | One-Button Operation - One button to check-in or declare an SOS (monitored 24/7) |
| | · | Water-Resistant - IPX7: up to 3.3 ft for 30 min |
| | · | GPS Location - Location reporting within 65 ft |
| | · | Rechargeable Battery - 72 hours battery life, based on reporting location every 4 hours |
| | · | SOS Alerting - With 24/7 monitoring service |
| | · | Fall Detection - With three sensitivity settings and built-in cancellation timer to prevent false alarms |
| | · | Easy Check-In – With user-controlled start/stop of predefined check-in schedule |
| | · | Audible Alerts (can be disabled) For SOS, check-in, fall-detection, low battery, and cell signal status |
| | · | Protected Phone Number – Only designated parties can call your protected number |
Wearable Health Solutions Inc., through its wholly owned subsidiary Medical Alarm Concepts LLC,(MAC) is currently in operation and works with 15 central monitoring stations with trained EMT operators on a 24/7 stand-by basis serving approximately 200 dealers in the US, Canada, Barbados and New Zealand with over 7,000 active users providing recurring revenue for the company. During the year ended June 30, 2022, the Company sold 3,668 devices to new customers.
Asset Purchase Agreement - BOAPIN.com
On August 3, 2020, effective as of June 30, 2020, the Company entered into an Asset Purchase Agreement with Hypersoft Ventures, Inc. (“Asset Purchase Agreement”) where the Company purchased certain assets of Hypersoft Ventures and its international commodities trading portal referenced as BOAPIN.com. As part of the Asset Purchase Agreement, Hypersoft Ventures, Inc., sold 100% of its online commodities trading portal and certain other assets including but not limited to: its data, science and files, marketing material, logos, advertising data, subscriber lists, source code, intellectual property, trade secrets, trademarks, client lists, service markets, supplier data, banking documents, and website registrants derived from The Federation of Industries from the State of Rondônia (Brazil)(“FIERO”), in exchange for 6,700,003 shares of Series C Convertible Preferred Stock, a promissory note in the amount of $425,000 and performance-based fees payable to FIERO.
At this point of the Company’s business evolution, management has decided to focus its efforts on the Personal Emergency Response Systems (PERS) and Telehealth business model, including the launch of our new iHelp MAX™ a 4G enabled device and related products in approximately the middle of 2022. As such the Company is in the process of divesting itself of the BOAPIN.com asset and related business components. The Company is seeking to sell its BOAPIN.com assets to a third party but has not been actively searching for a buyer. As of this Offering, there is no pending purchaser, and the Company has had no offers for the purchase of BOAPIN.com and its assets.
As of June 30, 2020, the Company impaired the value of the BOAPIN portal to $-0- for lack of revenue generation, resulting in a loss of $800,200. Given the asset was acquired from a related party and considered to have no value, the cost was recorded as compensation expense, reflected in salaries and wages expense on the Statement of Operations.
Wearable Health Solutions, Inc. Products:
Products
The Company's efforts are on the sale of its medical alarm and safety alert devices, which are some of the most advanced systems on the market today as displayed below in schematic form.
 |  |  |
| Figure 1: iHelp MAX™ Monitor | Figure 2: iHelp MAX™ with Wristband | Figure 3: iHelp MAX™ on Charging Station |
iHelp MAX™ Personal Emergency Response Unit

iHelp+ 3G™ Unit and Charging base
a) MediPendant®
MediPendant® is the Company's flagship medical alarm product and the world’s first monitored two-way voice speakerphone pendant for the Personal Emergency Response System (PERS) industry. It allows the user to speak and listen to the operator directly through the pendant. Wearable Health Solutions' alarm pendant also offers superior range of radio frequency capabilities and an enhanced communication range that enables the user to move freely in and about the home up to an extended range that is evolutionary in the PERS industry. Specifically, the MediPendant® system, which is connected through a landline telephone base station, enables the user to move up to 600+ feet (line of sight) away from the main base station, a distance that far exceeds competitors’ offerings on the market today. Competitor offerings instead require the user to be within a much closer proximity to and speaking distance from the base station box, a situation that may not be possible in an emergency event. As part of the MediPendant® product offering, users receive Wearable Health Solutions' two- way communication pendant, base station unit and a subscription to the Company's around- the-clock personal response service monitoring center. Our MediPendant® will not be affected by the discontinued use of the 3G networks as it operates using landline telephone service.
Emergency calls made through the Company's MediPendant® device are always handled by certified operators at a call center who are available 24-hours a day and guaranteed to remain on the call with MediPendant® subscribers until the problem is resolved and/or help arrives. Operators are trained to immediately assess the situation and can either connect the caller to a loved-one, or dispatch medical personnel to the user's location. All emergency operators are prepared to bring calm, professional, knowledgeable insight to any situation. Additionally, the call center can also archive a list of personal information for all MediPendant® users that includes an updated list of medications, health information and the subscriber's contact information including home address for location and dispatch purposes. The personal information and medical history are securely stored by the monitoring center and can be provided to the dispatched authority and emergency responders, as necessary.
b) iHelp™
The Company also provides a medical alarm device called the iHelp™. The iHelp™ is designed to be easy to use, lightweight yet durable, but with multiple features. The company has invested time, labor, and money into the development and launch of this device which possesses enhanced features and functions including a GPS system, ability to remotely locate a loved one, voice prompts, and a dealer portal that enables dealers to manage their iHelp™ customer base. The iHelp™ dealers enjoy significant company benefits in; the ease of ordering our products, activating and deactivating customers, tracking customer usage, and creating and printing a variety of reports. The iHelp™ dealer program is “turn-key” and offers the dealer the opportunity to provide his/her customers with the latest products without altering "back end" systems. With the introduction of the new and improved iHelp+ 3G™ unit, the iHelp™ is no longer being produced; however, we are still servicing our customers who use our iHelp™ devices and have no plans of retiring that service.
c) iHelp+ 3G™
The iHelp+ 3G™ is currently our most complete mPERS compliant product and is similar to the iHelp™. However, the iHelp+ 3G™ has additional features and functions, including; the ability to detect falls by the wearer, for example in the shower, Geo-Fencing and tracking ability, operates on the "3G" networks; and can be telehealth enabled via blue tooth low energy 4.0. The unit has superior audio quality, an extended battery life, and operates on GSM networks for use with cell phone providers both domestically and internationally, therefore enabling extended coverage in most areas. With the impending shutdown of the 3G network in the United States, by AT&T, Verizon, and T-Mobile, approximately 20% of our customers (all U.S. based) who are on our iHelp+ 3G™ product will lose access to our services. We are preparing to work with those customers in order to transition them to our next generation iHelp MAX™ product, that will operate on 4G Networks and additional services. However, there is no guarantee that we will be able transition all or some of those customers to our new products. At this time none of our current devices have stopped functioning as there are still active 3G networks. We are not aware of any other countries where 3G networks are being eliminated. The following is a list of when the major cellular companies plan on shutting down their 3G service in the United States as provided by the Federal Communications Commission:
AT&T: 3G Shutdown date March 31, 2022
Verizon: 3G Shutdown date December 31, 2022
T-Mobile: 3G Shutdown date March 31, 2022
Sprint: 3G Shutdown date July 1, 2022
d) iHelp Mini™
The iHelp Mini™ is a 4G medical alarm device with next generation capabilities. It is small, lightweight, waterproof, and durable. It offers users the ability to summon help in the event of an emergency. Its features and functions include SOS alarm, fall detection, geo-fencing, medication reminders, alerts to loved ones, crystal clear audio, and low battery alarm. The offering of a small, lightweight 4G medical alarm device in the industry is a huge benefit to our dealers and distributors. The iHelp Mini™ dealers enjoy significant company benefits in; the ease of ordering our products, activating and deactivating customers, tracking customer usage, and creating and printing a variety of reports. The iHelp™ dealer program is “turn-key” and offers the dealer the opportunity to provide his/her customers with the latest products without altering "back end" systems:
| · | Small size – 2.4” x 1.732” x 0.63” |
| · | Battery: Rechargeable, 3.7V, 800mhAh |
| · | 4 locating technologies: GPS, BLE, WiFi, LBS |
e) iHelp MAX™
The iHelp MAX™ our next generation of devices, which will operate on 4G Networks, is anticipated to be telehealth ready along with the platform that should be able to plug into multiple devices, such as smart watches, to enable remote monitoring and data collection of essential vital signs in real-time with historical data via Bluetooth, NFC, and Wi-Fi technology. In order to complete iHelp MAX™ and get it telehealth ready, the iHelp MAX™ device will need to be Bluetooth enabled, and we will need to add the device to our HIPAA compliant servers and backend of our mHealth operating system. Being telehealth ready means that our devices will be able to connect to other devices such as smart watches, and body sensors through the use of Bluetooth technology, this will allow our iHelp MAX™ device to link to our customers’ existing devices and use those sensors to help our iHelp MAX™ build a more complete picture of the customers health. Our customers will be able to add other products to their iHelp MAX™ such as our remote patient monitoring and medication reminder features at an additional cost to the basic services we provide. We anticipate the launch of the iHelp MAX™ device in approximately the third quarter 2022 which will offer a customized “Lone Worker” program for use with the iHelp MAX™. The iHelp MAX™ devices will run on local mobile networks, such as AT&T, Verizon, Bell Canada, T-Mobile, depending on where the customer is located. The device will feature a multi-function button for check-in and SOS alerts to ensure workers in the field can obtain help in the event their health or safety is at risk. The small, lightweight device is waterproof and durable enough for use indoors or outdoors. The Loan Worker device includes the following features and functions:
| | · | Two-Way Voice - Voice connection to an operator or when an SOS or fall is detected (and optionally missed check-in) |
| | · | One-Button Operation - One button to check-in or declare an SOS (monitored 24/7) |
| | · | Water-Resistant - IPX7: up to 3.3 ft for 30 min |
| | · | GPS Location - Location reporting within 65 ft |
| | · | Rechargeable Battery - 72 hours battery life, based on reporting location every 4 hours |
| | · | SOS Alerting - With 24/7 monitoring service |
| | · | Fall Detection - With three sensitivity settings and built-in cancellation timer to prevent false alarms |
| | · | Easy Check-In - With user-controlled start/stop of predefined check-in schedule |
| | · | Audible Alerts (can be disabled) For SOS, check-in, fall-detection, low battery, and cell signal status |
| | · | Protected Phone Number - Only designated parties can call your protected number |
As a result of the recent emergence of smart mobile wireless and geographic location solutions, these systems will integrate via Bluetooth low energy 4.0 and other FDA approved medical device technologies including biosensors. The Company may collect data on vital signs, send data to HIPAA compliant servers in the cloud, and allow access by caregivers, nurses, doctors, hospitals, and other health organizations. This process can facilitate a low-monthly-cost environment for the implementation of all day user monitoring for emergency, health, and activity status changes. This evolution will change the face of the traditional PERS devices into a Wearable Health & Alarm Monitoring (“WHAM”) market.
In order for iHelp MAX™ to become telehealth ready the Company will need to complete the backend software, including engaging HIPPA compliant servers that are equipped for data collection and storage. The Company does not foresee this being a problem as it already has engaged servers for its current products and as such the Company is prepared to extend its server coverage to the new products when ready. As of February 8, 2022, the Company has received samples of the proposed iHelp MAX™ devices and has requested some upgrades to the speaker quality in the devices, unfortunately the factory had been placed in a citywide lockdown and our product production has been delayed several months. We now expect first delivery of the products to occur during the third quarter of 2022.
Wearable Health Solutions, Inc.
Market Background
Living arrangements have changed greatly in the United States among older people and other potentially vulnerable segments of the population, including those with physical disabilities and/or medical conditions. During the 20th century, one of the most dramatic changes in the lives of the aging in the United States was the rise of the number of aging people living at home alone. In 1910, for example, only 12% of widows aged 65 or older lived alone. In 1970, this figure was 70% and today it is estimated to be much higher.
In the 21st century, this trend has gained momentum with more of the aging and medically at-risk population living alone than at any other time in the past, especially with the rise of the aging Baby Boomer population. The Baby Boomers, those born between 1946 and 1964, started turning 65 years old in 2011, with the number of older people set to increase dramatically during 2010 to 2030. According to the U.S. Current Population Survey data, "between 2010 and 2030”, the number of people aged 65 and older is projected to grow by 31.7 million or 79.2%." Thus, the older population in 2030 is projected to be twice as large as in 2000, growing from 35 million to 71.5 million, representing 20% of the total U.S. population in year 2030.
This social dynamic of a rising older population is true in both the United States as well as in many developed nations worldwide. Social change, technological advancements, and general lifestyle choices have promoted increased independence and the ability to live alone among other potentially vulnerable segments of the population such as those with physical disabilities or medical conditions. These groups can be especially susceptible to health problems and concerns for their physical wellbeing. Industry experts agree that in order to help facilitate independence and safety, more help is needed to provide these people with contact in case of emergency, or the benefit of support in a time of need. It was in response to this situation that the personal emergency response systems (PERS) industry emerged in the United States and developed the first personal medical alarm. The common use for personal medical alarms is as a safeguard for the aged and persons with certain medical conditions, in case of an age or health related incident that requires immediate attention, where the victim is unable to reach out for assistance via traditional means, including the ability to make a telephone call.
Effective PERS, with their emergency alert capabilities, is a key technology solution that can greatly help the vulnerable segment of the population live a more free and active life while maintaining the security of being able to access immediate assistance as needed. In fact, there has been a boom in the PERS market in recent years due to the growing aging population worldwide.
While the PERS industry has been in existence for some time, much of the technology within the industry has remained stagnant, with the landline and standalone PERS types remaining a majority of sales. Many of the original PERS solutions are still designed today to provide alerts whereby a push of a button simply triggers a call center operator to respond by calling the device user at home. (“QYResearch Global PERs Devices Market Report, History and Forecast 2016-2027”). The communication is conducted with two-way voice communication done through a centralized speaker box and not the actual device itself. Thus, traditional PERS solutions currently on the market offer communication between user and a call center only through a speaker box. This greatly inhibits the users’ freedom and limits their mobility to an area near the speaker box. (“QYResearch Global PERs Devices Market Report, History and Forecast 2016-2027”)
Mobile medical alert devices, such as smart watches, have recently been introduced to the market. They are designed for the younger and more active person with medical issues, and active elderly adults. With the emergence of telehealth and biosensor technology, the market is changing again to an even younger age group with medical issues which require tracking on a regular basis. These new medical alert devices, such as some smart watches, can track blood pressure, heart rate, oxygen saturation and can even sense when a person falls, then these devices are potentially able to reach out to medical personnel on behalf of the user without any interaction from that user. Wearable Health Solutions currently offers a wide range of solutions for users from a simple at home medical alarm to a mobile device that enables users to get help in most areas and is working on adding to its function ability through the use of these other features.
The iHelp MAX™ device will be telehealth-ready, and we plan to have multiple additional devices that can be added on and enabled in remote monitoring, data collection, and threshold settings of essential vital signs, including blood pressure, oxygen levels, temperature, and more, in real-time and with historical data via Bluetooth, NFC, and Wi-Fi technology.
Our Software Platform
Our platform is a cloud-hosted service consisting of methods and automation tasks for accepting data transmission from personal safety and medical devices ("PS/M") and storing, reformatting, and retransmitting this data to subscribers, monitoring centers, healthcare providers, front-end portal/user interfaces, and API controllers.
The front-end portal interface provides a user-friendly management and monitoring platform for all of the integrations listed below, coupled with PS/M device fulfillment, tracking, controlling, and remote reprogramming, along with portal user administration and role/privilege assignment, internal activity/audit trails, ordering and invoicing, support portal integration, and any other customizations needed based on solution requirements.
Back-end automation and integration with third-party providers and services include:
| | · | SMS, email, and smartphone messaging app push notifications for PS/M event/activity/location alerts and subscriber communication with PS/M devices (with locale specific SMS numbers where available); |
| | · | Programmatic voice dialing and routing (with locale specific voice numbers where available); |
| | · | Integration with SIM card providers for management (activation, suspension, and usage monitoring) of airtime; |
| | · | Flexible signal relay and reformatting for alarm, activity and health event data to central stations and healthcare providers based on unique communication and transmission protocol, whether via API calls or data transmission to TCP/IP or other types of receivers; |
| | · | Integration with customer CRM for account details and user activity; |
| | · | Integration with billing systems for device/equipment ordering and recurring billing; |
| | · | API endpoints can be exposed for data access and controlling feature-sets in third party environments. |
This technology may also be marketed for future commercial applications on the Internet of Things (IoT) or Machine-to-Machine (M2M) devices in the ecosystem of remote patient monitoring. Such commercial applications will provide for additional corporate revenue streams and profits. Management believes significant revenues may be derived from such related industry opportunities.
The Company is taking a further step into the remote patient monitoring and biosensing space. Our commitment as a Telehealth ready company is demonstrated by the engagement of Product Development Corporation (“MIDI”), to produce our Next Generation Platform, that operates on 4G Networks, which will add updates and upgrades to the iHelp MAX™ device and platform. Features, functionalities and tools to be included and currently under development are; adding the capability of collection and routine measurement of a user’s vital signs such as monitoring of blood pressures, oxygen and glucose levels. The additional features contemplated will allow the iHelp MAX™ device to compile health data and transmit it to the cloud for easy storage, access and retrieval. As a result we will expand our services and offerings to customers in such areas as Remote Patient Monitoring and Medication Reminders for additional fees and revenues.
Remote Patient Monitoring
We will routinely add and embed upgrades and updates to include Remote Patient Monitoring (“RPM”) systems and relays into our iHelp MAX device. RPM technology is estimated to be approximately an $8.5 billion market according to LEX Consulting and is only in the early stages of adoption. We plan on upgrading the iHelp MAX™ to connect other blue tooth enabled devices in order to provide further assistance to our users by compiling and providing health data in an accessible manner. This RPM health data may help our users in obtaining increased support for chronic care management and allowing for early intervention for health care issues.
Medication Reminders
The iHelp MAX™ will eventually embed an update module that will allow users to input prescription medication schedules as a reminder to users for consumption based on a doctor’s recommendations. This system will track users progress in maintaining medication consumption schedules as advised by physicians where necessary prescription intake may be adjusted based on monitored results.
Market Opportunity
The healthcare industry is the largest in the world, with the home healthcare market in developed countries in particular growing rapidly, driven in part by aging baby boomers and a growing shift toward moving some types of healthcare from the hospital and into the home.
These trends make the home healthcare sector an increasingly attractive market for successful companies that offer effective solutions in the PERS industry space. The common use for personal medical alarms is as a safeguard for the aged and persons with certain medical conditions who are unable to reach out for assistance via traditional means such as a telephone call. Medical alarms mitigate the potential harm and expensive hospital stays caused by falls or other accidents where tracking devices, like the iHelp+3G™ with wearable biosensors will monitor users’ conditions.
There has been a boom in the PERS market in recent years due to the growing aging population worldwide. According to the U.S. Census Bureau, the number of people over 65 in the United States is set to jump from approximately 34 million to approximately 65 million in 2025. By 2050, this statistic is projected to reach 86.7 million, with many individuals living at home or in an alternative home-type environment. Worldwide, this number is expected to double from some 550 million currently at age 65 years old to over 1.2 billion seniors by 2025.
Experts in the health care industry expect that many of these seniors will prefer to continue living independently at home for as long as possible. This population is expected to be more technology-savvy as consumers of healthcare services and are very interested in playing an active role in personally managing their health and well-being. They may consider technologies that help them gain access to medical care while remaining independent and outside a hospital environment.
Effective PERS with their emergency alert capabilities, are a key technology solution that may help the vulnerable segment of the population live a more free and active lifestyle while maintaining the security of immediate assistance access as needed. According to industry statistics users of PERS are typically individuals over the age of 75 who are predominantly female and live alone. The buyers of PERS are frequently the end users’ children.
Lifeline Systems, Inc., the founder of the PERS industry in the U.S. approximately 35 years ago, served 250,000 users in the United States and Canada in 1992. Today, Philips Medical Systems' acquisition of Lifeline Medical Alarm has positioned it as the largest provider of traditional PERS systems with over 700,000 monitored accounts, implying that the total market size of users is likely much larger.
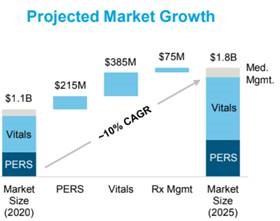
source : Lek Consultings
Sales and Marketing
The Company's marketing efforts are focused in three principal areas:
| | i. | Internet sales & marketing, |
| | | |
| | ii. | Wholesale distribution and |
| | | |
| | iii. | International markets. |
1) Internet Sales & Marketing
The Company markets the MediPendant® through its website at www.MediPendant.com and its iHelp™ mobile medical alarm to dealers at www.ihelpalarm.com. The sales process for medical alarms can be complex which often require several phone calls among the end user or customer's family members before a decision is reached. As a result the MediPendant® and iHelp™ websites are used mainly for informational purposes with the actual sale typically taking place over the phone with one of our customer service representatives or one of our many dealers. The company uses a variety of techniques, such as Internet paid ad campaigns and social media, in order to drive web traffic to the websites, and initiate potential customer sales calls.
2) Wholesale Distribution
The Company currently has several relationships with wholesalers which resell the MediPendant® and the iHelp™ in conjunction with their own monitoring services. The company believes its relationships with its strategic partners is good. The company is currently in discussions with several other wholesale groups interested in distributing our products through their independent channels. With the introduction of the iHelp+ 3G™, iHelp Mini™, iHelp MAX™, Wearable Health Solutions will be marketing its offerings only through Wholesale Distribution Centers in International Markets.
3) International Markets
The Company also distributes its products on a wholesale basis to selected international markets. To date, the company has completed sales in Denmark, Ireland, Bermuda, Brazil, the Caribbean, and the People's Republic of China. Considerable International interest has been received in the company's new iHelp+ 3G™, iHelp Mini™, iHelp MAX™ offerings and as a result distribution was initiated in Canada, and Europe. Expansion initiatives are also under consideration for global distribution.
Manufacturing and Raw Materials
The Company relies on third parties and suppliers to provide product raw materials, components and to build and package the finished goods. Third parties also provide order fulfillment, warehousing, distribution, cloud-hosting and monitoring services. The Company plans to purchase its products in larger production batches thereby increasing production efficiency for the outsourced suppliers, which will reduce costs.
The Company uses contracted third parties to manufacture its products and to monitor our customers. The third-party manufacturers are responsible for receipt and storage of raw material, production and packaging and labeling of finished goods. At present, the Company is dependent upon manufacturers for the production (manufacturing) of all of its products. To the extent the manufacturer should discontinue the relationship with the Company; the Company’s sales could be adversely impacted. The Company believes at the present time it will be able to obtain the quantity of products and supplies it will need to meet orders.
The Company purchases all of the products from T&W and Martin, Inc., third party supplier and manufacturer pursuant to purchase orders without any long-term agreements. In the event that the current manufacturers are unable to meet supply or manufacturing requirements at some time in the future, the Company may suffer short-term interruptions of delivery of certain products while it establishes an alternative source. While management believes alternative sources are in most cases available and it plans to have also established working relationships with several third-party suppliers and manufacturers, none of these agreements are long-term. The Company also relies on third party carriers for product shipments, including shipments to and from distribution facilities. The Company also relies on third parties to provide the monitoring of the Company’s customers and devices. It is therefore subject to the risks, including employee strikes and inclement weather, associated with the third parties ability to provide those services to meet the Company’s needs. Failure to deliver products or monitor our customers in a timely and accurate matter would harm the Company’s reputation, business and results of operations.
Patents, Trademarks and Licenses
We may rely on a combination of patent, trademark, copyright, and trade secret laws in the United States as well as confidentiality procedures and contractual provisions to protect our proprietary technology, databases, and our brand.
The trademarks currently owned by the Company, and for which it intends to seek federal transaction registration are the marks, Wearable Health Solutions, iHelp+ 3G™, iHelp Mini™, iHelp MAX™ and MediPendant®. The Company may federally register other trademarks in the future as the need arises. The Company intends to patent the processes and designs as the need arises; however, the Company currently does not have any federally registered patents.
Competition
The market for Personal Emergency Response Systems (PERS) is highly fragmented with limited information about competitors available.
The majority of competitors market first generation PERS systems that rely on a centralized base station (Landline or Standalone type PERS) for communication between the user and the monitoring center; however, the market for mobile based PERS systems has been increasing, with the ability of mobile devices to replace the central base station models. The largest market participant is Philips Lifeline, which makes up approximately 32% of all the revenues in the PERS market, which several years ago purchased Lifeline Medical Alarms. The next two largest market participants, based on revenues, according to the QYR research is believed to be ADT and Turnstall which have 3.4% and 3.3% of the revenues in the PERS Market, respectively. (“QYResearch Global PERs Devices Market Report, History and Forecast 2016-2027”). ADT’s product is a modern medical alert bracelet which connects to a monitoring platform. Additionally, there are dozens of smaller organizations marketing PERS devices and monitoring services. The fragmentation of the market means that there are a lot of opportunities to gain market share; however, most of the companies that are in the PERS market are much larger and better funded than we are, which means that they may be able to come out with new products and features faster than we can and better integrate those new features into their existing products and networks than we can.
Mobile Medical Alerts and health monitoring devices, have recently been introduced into the market and are designed for the adult and younger more active individuals with medical issues. This market is currently dominated by companies that are currently in the health and fitness markets as well as the cell phone markets, such as Apple, Samsung, Garmin, and Fitbit.
Mostly all of our competitors have significantly greater financial, marketing and other resources, broader product lines outside of medical and health monitoring, and larger customer bases than we have and are less financially leveraged than we are. As a result, these competitors may be able to: adapt to changes in customer requirements more quickly; introduce new and more innovative technological products more quickly; better adapt to downturns in the economy or other decreases in sales; better withstand pressure to accept customer concerns; take advantage of acquisition and other opportunities more readily; devote greater resources to the marketing and sale of their services; and adopt more aggressive pricing policies.
Employment Agreements
On May 11, 2020, and effective as of December 21, 2019, the Company entered into an Employment Agreement (“Mittler Agreement”) with its Chief Executive Officer, Chief Financial Officer (now former CFO) and Director, Harrysen Mittler; wherein, Mr. Mittler agreed to serve as an employee of the Company in consideration of $17,000 and 25,000 restricted common shares per month, modified to $20,000 and 200,000 shares per month plus cash and stock bonus, effective December 22, 2021. His duties as an Employee of the Company will be mainly to provide executive management and corporate finance duties related to the operating and financial reporting for the Company. The term of the contract is for an initial period of three years, and a renewal option for an additional two-year period.
On May 11, 2020, and effective as of December 21, 2019, the Company entered into an Employment Agreement (“Pizzino Agreement”) with its President and Director, Peter Pizzino; wherein, Mr. Pizzino agreed to serve as an employee of the Company in consideration of $17,000 and 25,000 restricted common shares per month, modified to $20,000 and 200,000 shares per month plus cash and stock bonus, effective December 22, 2021. His duties as an Employee of the Company will be mainly to provide executive management expertise for the Company. The term of the contract is for an initial period of three years, and then there is a renewal option for an additional two-year period as per section 2 of the Pizzino Agreement.
On August 9, 2021, the Company entered into an agreement with Mr. Anthony Chetta (Chetta Advisory Agreement”) that was effective as of August 15, 2021. Mr. Chetta will serve as our Chief Technical Advisor, where he will provide the customary services as a comparable person in that position. The term of the Chetta Advisory Agreement is for an initial period of three (3) years, with a renewal option for an additional two (2) year period. The Company will pay Mr. Chetta $8,000 per month, and a signing bonus of 1,000,000 shares of restricted common stock and 1,000,000 shares of Company common stock every six (6) months thereafter. The Company will also pay Mr. Chetta a bonus upon completion of the 4G software integration and launch into the market.
On May 16, 2022, the Company entered into an Employment Agreement (“Miceli Agreement”) with Vincent S. Miceli, that was effective as of May 18, 2022. Mr. Miceli is employed by WHSI as its Chief Financial Officer. The term of employment is for a period of three (3) years and contains a renewal option for an additional two (2) year period. Mr. Miceli’s base salary will be Eighteen Thousand Dollars ($18,000) per month. As per the Miceli Agreement, Mr. Miceli, was awarded Five Million (5,000,000) shares of restricted stock upon signing the Miceli Agreement, One Hundred Seventy-Five Thousand (175,000) restricted shares per month, and Seven Million (7,000,000) shares of restricted stock as an annual bonus, per year, thereafter. The Company will also pay for Mr. Miceli’s health insurance.
We plan to have a policy of requiring key employees and consultants to execute confidentiality agreements upon the commencement of an employment or consulting relationship with us. Our employee agreements also require relevant employees to assign to us all rights to any inventions made or conceived during their employment with us. In addition, in the future we will have a policy requiring individuals and entities with which we discuss potential business relationships to sign non-disclosure agreements. Our agreements with clients include confidentiality and non-disclosure provisions.
Regulation
We face extensive government regulation both within and outside the U.S. relating to the development, manufacture, marketing, sale and distribution of our products, software and services. Our operations are subject to numerous federal, state, and local laws and regulations, in areas such as consumer protection, labor and employment, tax, permitting, and other laws and regulations. Most jurisdictions in which we operate have licensing laws directed specifically toward the health monitoring industry. In certain jurisdictions, we must obtain licenses or permits in order to comply with standards governing employee selection, training, and business conduct. In addition, we are subject to certain administrative requirements and laws of the jurisdictions in which we operate. These laws and regulations may include restrictions on the manner in which we promote the sale of our monitoring services and may require us to provide most purchasers of our services with three-day or longer rescission rights.
Some local government authorities have adopted or are considering various measures aimed at reducing false alerts. Such measures include requiring permits for individual monitoring systems, revoking such permits following a specified number of false calls, imposing fines on customers or monitoring companies for false notifications, limiting the number of times ambulance or police personnel will respond to alerts at a particular location after a specified number of false alerts, requiring additional verification of an alert notice before the ambulance services or police respond, or providing no response to medical monitoring alerts.
Regulation in regard to Confidential Information
Along with our own confidential data and information in the normal course of our business, we or our partners may collect and retain significant volumes of certain types of data, some of which are subject to certain laws and regulations. Our ability to analyze this data to present the subscriber with an improved user experience is a valuable component of our services, but we cannot assure you that the data we require will be available from these sources in the future or that the cost of such data will not increase. If the data that we require is not available to us on commercially reasonable terms or at all, we may not be able to provide certain parts of our current or planned products and services, and our business and financial condition could be materially adversely affected.
In addition, we may also collect and retain other sensitive types of data, including audio recordings of telephone calls and video images of customer information. We must comply with applicable federal and state laws and regulations governing the collection, retention, processing, storage, disclosure, access, use, security, and privacy of such information in addition to our own posted information security and privacy policies and applicable industry standards. The legal, regulatory, and contractual environment surrounding the foregoing continues to evolve, and there has been an increasing amount of focus on privacy and data security issues with the potential to affect our business. These privacy and data security laws and regulations, as well as contractual requirements, could increase our cost of doing business, and failure to comply with these laws, regulations, and contractual requirements could result in government enforcement actions (which could include civil or criminal penalties), private litigation, and/or adverse publicity. In the event of a breach of personal information that we hold or that is held by third parties on our behalf, we may be subject to governmental fines, individual and class action claims, remediation expenses, and/or harm to our reputation.
Further, if we fail to comply with applicable privacy and security laws, regulations, policies, and standards; properly protect the integrity and security of our facilities and systems and the data located within them; or defend against cybersecurity attacks; or if our third-party service providers, partners, or vendors fail to do any of the foregoing with respect to data and information assessed, used, stored, or collected on our behalf, our business, reputation, results of operations, and cash flows could be materially adversely affected.
For example, the data that we collect and retain in the future may include personally identifiable information related to our customers and employees and may be protected health information subject to certain requirements under the Health Insurance Portability Accountability Act (“HIPAA”) and its implementing regulations, which regulate the use, storage, and disclosure of personally identifiable health information. We may change our processes or modify our product and service offerings in a manner that requires us to adopt additional or different policies and procedures to meet our obligations under HIPAA. Becoming fully HIPAA-compliant involves adopting and implementing privacy and security policies and procedures as well as administrative, physical, and technical safeguards. Additionally, HIPAA compliance requires certain agreements with contracting partners to be in place. Endeavoring to become fully HIPAA-compliant may be costly both financially and in terms of administrative resources. It may take substantial time and require the assistance of external resources, such as attorneys, information technology, and/or other consultants. We would have to be HIPAA-compliant to provide services pursuant to which we are required to collect or manage patient information for or on behalf of a health care provider or health plan. Thus, if we do not become fully HIPAA-compliant, our expansion opportunities may be limited. Furthermore, it is possible that HIPAA may be expanded in the future to apply to certain of our current products or services.
The California Consumer Privacy Act (“CCPA”), which will become enforceable in 2020, gives California residents certain rights in relation to their personal information, requires that companies take certain actions, and will apply to activities regarding personal information that is collected by us, directly or indirectly, from California residents. As interpretation and enforcement of the CCPA evolves, it will create a range of new compliance obligations, which could cause us to change our business practices, with the possibility for significant financial penalties for noncompliance that may materially adversely affect our business, reputation, results of operations, and cash flows.
The General Data Protection Regulation (“GDPR”) applies to activities regarding personal data that are conducted by us, directly or indirectly through vendors and subcontractors, from an establishment in the European Union. As interpretation and enforcement of the GDPR evolves, it will create a range of new compliance obligations, which could cause us to change our business practices, with the possibility for significant financial penalties for noncompliance. The European Commission in July 2016 and the Swiss Government in January 2017 approved the EU-U.S. and the Swiss-U.S. Privacy Shield frameworks, respectively, which are designed to allow U.S. companies that self-certify to the U.S. Department of Commerce and publicly commit to comply with the Privacy Shield requirements to freely import personal data from the EU and Switzerland. However, these frameworks face a number of legal challenges and their validity remains subject to legal, regulatory, and political developments in both Europe and the U.S. This has resulted in some uncertainty, and compliance obligations could cause us to incur costs or require us to change our business practices in a manner adverse to our business and failure to comply could result in significant penalties that may materially adversely affect our business, reputation, results of operations, and cash flows.
The Company has received all necessary approvals to operate in the jurisdictions it is operating. The Company does not expect the costs and effects of compliance with environmental laws to be a significant issue.
The following sections describe certain significant regulations that we are subject to. These are not the only regulations that our businesses must comply with. For a description of risks related to the regulations that our businesses are subject to, please refer to the section entitled “Risk Factors—Risks Related to Our Businesses.”
Our operations are potentially subject to a complex web of Federal and state regulations that are evolving at a rapid rate. The USDA and FDA may change rules or enforcement proceedings at any time.
Seasonality
We do not expect any seasonality in our business.
Property
Our mailing address is 2901 W. Coast Highway, Suite 200, Newport Beach, CA 92663. Our main telephone number is (949) 270-7460. The Company currently pays $175 a month for its office space and the term is month to month, with a thirty-day written notice of termination. The Company’s subsidiary maintained a warehouse office in Pennsylvania to facilitate inventory arrival and product shipment. The three-year lease at $1,100 per month expired on September 30, 2021, and was renewed for 12 months at $1,300 per month beginning October 1, 2021 and expiring on September 30, 2022. The subsidiary subsequently entered into a month-to-month arrangement with this office warehouse and then terminated the arrangement and vacated the facility as of December 31, 2022. The Company entered into a new three-year lease agreement on September 9, 2022 for new warehouse space located in Mequon, Wisconsin. The monthly rent for this new warehouse space is currently $1,325 per month for the first twelve months of the lease agreement. Our website is www.wearablehealthsolutions.com and our email address is info@wearablehealthsolutions.com.
Employees
Including our Officers and Directors we have 7 full-time employees of our business or operations who are employed at will by Wearable Health Solutions, Inc. We anticipate adding additional employees in the next 12 months, as needed. We do not feel that we would have any unmanageable difficulty in locating needed staff in large part because of our corporate structure that allows for location and time flexibility.
Legal Proceedings
We may from time to time be involved in various claims and legal proceedings of a nature we believe are normal and incidental to our business. These matters may include product liability, intellectual property, employment, personal injury cause by our employees, and other general claims.
| | 1) | Wearable Health Solutions, Inc. v. Barry Honig, GRQ Consultants Inc., Benza Pharma LLC and John Does 1-10, Supreme Court of the State of New York County of New York, July 22, 2021. Company is disputing the validity of Notes from 3/2016 and seeking damages, reparations, and related costs. |
| | | |
| | 2) | GRQ Consultants, Inc. v. Wearable Health Solutions, Inc., Supreme Court of the State of New York, County of New York, August 26, 2021, Parties are seeking summary judgment of $50,000 plus accrued interest in response to lawsuit by Company regarding $50,000 loan from 11/2016. |
| | | |
| | 3) | Benza Pharma LLC, Sandor Capital, LP, and John Lemak v. Wearable Health Solutions, Inc., District Court, Clark County, Nevada, Parties are seeking summary judgment of $3,000,000 plus accrued interest in regards to convertible notes payable from March, 2016. The Company believes that there is a very low probability that it will pay this amount and as a result has not accrued for it on the Company’s balance sheet. (See Note 15) |
| | | |
| | 4) | Medical Alarm Concepts LLC v. MCA Cure, LLC, Superior Court of New Jersey, Law Division, Morris County. Company is seeking return of payments for non-performance plus attorney fees and court costs. A settlement was reached between the parties whereby MCA Cure will pay an initial $10,000 and $6,500 each month until debt is satisfied in 2023 (See Note 14). |
On February 3, 2023, Sandor Capital, LP and John Lemak, the plaintiffs in the case of Benza Pharma LLC, Sandor Capital, LP, and John Lemak v. Wearable Health Solutions, Inc., informed the Company that they would be discontinuing their legal action against the Company.
Other than the aforementioned, we are not presently a party to any legal proceedings that, in the opinion of our management, are likely to have a material adverse effect on our business. Regardless of outcome, litigation can have an adverse impact on us because of defense and settlement costs, diversion of management resources and other factors.
MANAGEMENT
______
DIRECTORS, EXECUTIVE OFFICERS AND SIGNIFICANT EMPLOYEES
Directors and Executive Officers
The following table sets forth information regarding our executive officers, directors and significant employees, including their ages as of June 6, 2023:
As of June 6, 2023, Wearable Health Solutions, Inc. had seven-full-time employees, and no part-time employees. The directors and executive officers of the Company as of June 6, 2023, are as follows:
| Name | | Position | | Age | | Term of Office |
| Harrysen Mittler | | CEO, Director | | 70 | | 12/06/2019 |
| Peter Pizzino | | President, Director | | 49 | | 12/06/2019 |
| Vincent S. Miceli | | CFO | | 65 | | 05/16/2022 |
Harrysen Mittler, Age 70: Harrysen Mittler has over thirty years of experience in corporate finance, mergers and acquisitions, business administration and commerce. He served in the audit division of Deloitte Haskins and Sells, the predecessor to Deloitte & Touche LLP. Mr. Mittler from 2016 to 2020, served as Chairman, CEO and CFO of Pacific Software Inc. a public company, (symbol PFSF) as a designer, developer and acquirer of enterprise solutions in the emerging e-commerce technology sectors. He served as director and CFO of Nortia Capital Partners Inc., a publicly traded merchant banking company, as well as for Autoworks International Ltd. a company quoted on the Frankfurt Stock Exchange.
Peter Pizzino, Age 49: Peter Pizzino had a career in the securities and investment industry with financial experience spanning over 25 years. Since 2015, Mr. Pizzino held positions in Capital Markets at Spartan Capital, then with J. Streicher Capital on the New York Stock Exchange Floor where he financed both public and private client companies. He later, in 2018, served as president of Pacific Software Inc, PFSF on the OTC Markets, which is a software development and acquisition company, where he helped develop a commodities trade platform for the international markets. Mr. Pizzino extensive international business and Non-Profit experience. He studied Finance and held several securities licenses.
Vincent S. Miceli, Age 65: Mr. Miceli, is our Chief Financial Officer and joined us in May 2022. Vincent, from the years 2014 to 2021, was the CFO and in 2019 was also appointed CEO, of Nxt-ID, Inc., a healthcare application technology company that is focused on the biometric and identity verification technologies, where he was responsible for duties required of the CEO and CFO. While with Nxt-ID, Inc., Mr. Miceli, also completed three stock and warrant offerings for that company and $14.5 million in a term loan facility was repaid during his tenure. Vincent was also able to prepare and submit a GSA application with the US Government where the company was subsequently awarded a GSA contract in June 2021.
Mr. Miceli holds an M.B.A. with a concentration in finance from the University of Hartford in 1987 and a B.S. in Accounting from Quinnipiac College in1980. Mr. Miceli is also an affiliate member of both the American Institute of Certified Public Accountants and the Connecticut Society of Certified Public Accountants.
None of our officers or directors in the last five years has been the subject of any conviction in a criminal proceeding or named as a defendant in a pending criminal proceeding (excluding traffic violations and other minor offenses), the entry of an order, judgment, or decree, not subsequently reversed, suspended or vacated, by a court of competent jurisdiction that permanently or temporarily enjoined, barred, suspended or otherwise limited such person’s involvement in any type of business, securities, commodities, or banking activities; a finding or judgment by a court of competent jurisdiction (in a civil action), the Securities and Exchange Commission, the Commodity Futures Trading Commission, or a state securities regulator of a violation of federal or state securities or commodities law, which finding or judgment has not been reversed, suspended, or vacated; or the entry of an order by a self-regulatory organization that permanently or temporarily barred, suspended or otherwise limited such person’s involvement in any type of business or securities activities.
There are no family relationships among and between our directors, officers, persons nominated or chosen by the Company to become directors or officers, or beneficial owners of more than five percent (5%) of the any class of the Company’s equity securities.
Significant Employees
Other than the foregoing named officers and directors, we have seven full-time employees whose services are not materially significant to our business and operations who are employed at will by Wearable Health Solutions, Inc.
Family Relationships
There are no family relationships between any of our directors, executive officers and proposed directors or executive officers.
COMPENSATION OF DIRECTORS AND EXECUTIVE OFFICERS
______
The following summary compensation table sets forth all compensation awarded to, earned by, or paid to our named executive Officer paid by us during the year ended June 30, 2022 and 2021, in all capacities for the accounts of our executives, including the Chief Executive Officer (CEO) and Chief Financial Officer (CFO):
SUMMARY COMPENSATION TABLE
| Name and Principal | | | | Salary | | Bonus | | Stock Awards | | Option Awards | | Non-Equity Incentive Plan Compensation | | Non-Qualified Deferred Compensation Earnings | | All Other Compensation | | Totals | |
| Position | | Year | | ($) | | ($) | | ($) | | ($) | | ($) | | ($) | | ($)1 | | ($) | |
| | | | | | | | | | | | | | | | | | | | |
| Harrysen Mittler, | | 2022 | | 315,000 | | 120,000 | | | | 0 | | 0 | | 0 | | 30,350 | | 5,211,448 | |
| CEO, Director | | 2021 | | 294,000 | | 90,000 | | 858,004 | | 0 | | 0 | | 0 | | 0 | | 1,242,004 | |
| Peter Pizzino, | | 2022 | | 315,000 | | 120,000 | | 4,746,098 | | 0 | | 0 | | 0 | | 14,000 | | 5,195,098 | |
| President, Director | | 2021 | | 294,000 | | 90,000 | | 858,004 | | 0 | | 0 | | 0 | | 0 | | 1,242,004 | |
| Ronald Adams, | | 2022 | | 127,023 | | 0 | | 30,000 | | 0 | | 0 | | 0 | | 0 | | 157,023 | |
| Former, EVP Sales, President MAC | | 2021 | | 98,900 | | 0 | | 0 | | 0 | | 0 | | 0 | | 0 | | 98,900 | |
| Jennifer Loria, | | 2022 | | 172,082 | | 0 | | 61,500 | | 0 | | 0 | | 0 | | 0 | | 233,582 | |
| Former - COO, MAC | | 2021 | | 114,400 | | 0 | | 0 | | 0 | | 0 | | 0 | | 0 | | 114,400 | |
| Vincent S. Miceli, | | 2022 | | 27,000 | | 0 | | 70,000 | | 0 | | 0 | | 0 | | 1,881 | | 98,881 | |
| CFO | | 2021 | | 0 | | 0 | | 0 | | 0 | | 0 | | 0 | | 0 | | 0 | |
| Gail Rosenthal | | 2022 | | 143,000 | | 0 | | 5,050 | | 0 | | 0 | | 0 | | 10,001 | | 158,051 | |
| Former - CFO | | 2021 | | 117,000 | | 0 | | 4,011 | | 0 | | 0 | | 0 | | 0 | | 121,011 | |
| 1. | This column includes such things as medical reimbursements, and auto allowances. |
There are no compensatory plans or arrangements, including payments to be received from the Company with respect to any executive Officer, that would result in payments to such person because of his or her resignation, retirement or other termination of employment with the Company, or its subsidiaries, any change in control, or a change in the person’s responsibilities following a change in control of the Company.
Outstanding Equity Awards at Fiscal Year-End
No executive Officer received any equity incentive awards, or holds exercisable or unexercisable options, as of the year ended June 30, 2022.
| OPTION AWARDS | | STOCK AWARDS | |
| Name | | Number of Securities Underlying Unexercised Options (#) Exercisable | | | Number of Securities Underlying Unexercised Options (#) Unexercisable | | | Equity Incentive Plan Awards: Number of Securities Underlying Unexercised Unearned Options
(#) | | | Option Exercise Price ($) | | | Option Expiration Date | | | Number of Shares or Units of Stock that have not Vested (#) | | | Market Value of Shares or Units of Stock that have not Vested
($) | | | Equity Incentive Plan Awards: Number of Unearned Shares, Units or Other Rights that have not Vested
($) | | | Equity Incentive Plan Awards: Market or Payout Value of Unearned Shares, Units or Other Rights that have not Vested
($) | |
| (a) | | (b) | | | (c) | | | (d) | | | (e) | | | (f) | | | (g) | | | (h) | | | (i) | | | (j) | |
| None | | | 0 | | | | 0 | | | | 0 | | | | 0 | | | | 0 | | | | 0 | | | | 0 | | | | 0 | | | | 0 | |
Long-Term Incentive Plans
There are no arrangements or plans in which the Company would provide pension, retirement or similar benefits for our Director or executive Officer.
Compensation Committee
The Company currently does not have a compensation committee of the Board of Directors. The Board of Directors as a whole determines executive compensation.
Security Holders Recommendations to Board of Directors
The Company welcomes comments and questions from the shareholders. Shareholders can direct communications to the Chief Executive Officer, Harrysen Mittler, at our executive offices. However, while the Company appreciates all comments from shareholders, it may not be able to individually respond to all communications. Management attempts to address shareholder questions and concerns in press releases and documents filed with the SEC so that all shareholders have access to information about the Company at the same time. Harrysen Mittler collects and evaluates all shareholder communications. All communications addressed to the Director and executive Officer will be reviewed by Harrysen Mittler unless the communication is clearly frivolous.
Compensation of Directors
Directors are permitted to receive fixed fees and other compensation for their services as Directors. The Board of Directors has the authority to fix the compensation of Directors.
Director Independence
The Board of Directors is currently composed of 2 members. Harrysen Mittler, and Peter Pizzino, who do not qualify as independent Directors in accordance with the published listing requirements of the NASDAQ Global Market. The NASDAQ independence definition includes a series of objective tests, such as that the Director is not, and has not been for at least three years, one of the Company’s employees and that neither the Director, nor any of his family members has engaged in various types of business dealings with us. In addition, the Board of Directors has not made a subjective determination as to each Director that no relationships exist which, in the opinion of the Board of Directors, would interfere with the exercise of independent judgment in carrying out the responsibilities of a Director, though such subjective determination is required by the NASDAQ rules. Had the Board of Directors made these determinations, the Board of Directors would have reviewed and discussed information provided by the Directors and the Company with regard to each Director’s business and personal activities and relationships as they may relate to the Company and its management.
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
______
Related Party Transactions
Note payable – BOAPIN purchase
In August, 2020, effective as of June 30, 2020, the Company purchased the BOAPIN portal, including all software, licensing, and ownership rights from Hypersoft Ventures, Inc., a previously related party through its common ownership, for $800,200, which includes six million seven hundred thousand three (6,700,003) shares of Series C Convertible Preferred stock, valued at $375,200 or $0.056 per share, based on the conversion of one share of Series C Preferred stock for 10 shares of common stock and the stock price on the date of the transaction, and a note payable for $425,000, bearing twelve percent (12%) interest with no prepayment or delinquency clauses.
As of June 30, 2022 and 2021, the Company recorded Note payable-BOAPIN of $170,000 and $425,000, accrued interest of $49,415 and $28,225, and expensed $21,190 and $28,225 in interest, respectively.
As of March 31, 2023 and June 30, 2022, the Company has recorded a Note payable-BOAPIN of $170,000 and $170,000, respectively. During the three and nine months ended March 31, 2023, the Company recorded interest expense of $3,353 and $10,209, respectively. During the three and nine months ended March 31, 2022, the Company recorded interest expense of $6,521 and $15,090, respectively. The accrued interest balance at March 31, 2023 and June 30, 2022 was $59,624 and $49,415, respectively.
Related party debt, net
From time to time, the Company received funds from related parties for day-to-day operations. These are short-term loans which bear no interest, and the Company expects to repay these loans by the end of the fiscal year following the year in which the short-term loan was made. The following table reflects the composition of the related party debt, net balance at March 31, 2023 and June 30, 2022. The Company had a receivable from certain management employees totaling $155,800 at June 30, 2022. The total receivable balance was subsequently collected by the Company on September 27, 2022.
| | | | | | | |
| | | March 31, | | | June 30, | |
| | | 2023 | | | 2022 | |
| Related parties – subsidiary | | $ | 177,320 | | | $ | 202,875 | |
| Due from related parties | | | – | | | | (155,800 | ) |
| Accrued salaries, bonus, fees | | | 330,932 | | | | 10,965 | |
| Total loans from related parties, net | | $ | 508,252 | | | $ | 58,040 | |
Other than the aforementioned related party transactions, during the last two full fiscal years and the current fiscal year or any currently proposed transaction, there are no other transactions involving the Company, in which the amount involved exceeds the lesser of $120,000 or one percent of the average of the Company’s total assets at year-end for its last three fiscal years.
Stock Options
We have not issued and do not have outstanding any options to purchase shares of our Common Stock. We do not have any stock option plans.
Share Purchase Warrants
We have not issued and do not have outstanding any share purchase warrants to purchase shares of our Common Stock.
Controlled Company Status
Upon completion of this Registration Statement, our Management will continue to collectively hold more than 50% of the voting power for the election of directors of our company. As a result, we expect to be a controlled company within the meaning of Nasdaq corporate governance standards. Under Nasdaq rules, a company of which more than 50% of the voting power is held by an individual, company or group of persons acting together is a controlled company and will not to comply with certain Nasdaq corporate governance requirements, including the requirements that:
| | • | a majority of the Board consist of independent directors under Nasdaq rules; |
| | | |
| | • | the nominating and governance committee be composed entirely of independent directors with a written charter addressing the committee’s purpose and responsibilities; and |
| | | |
| | • | the compensation committee be composed entirely of independent directors with a written charter addressing the committee’s purpose and responsibilities. |
These requirements will not apply to us as long as we remain quoted on the Pink OTC Markets and as a controlled company. Accordingly, you may not have the same protections afforded to stockholders of companies that are subject to all of the corporate governance requirements of Nasdaq. See “Management— Controlled Company Status.”
Conflict of Interest
There is a potential for a conflict of interest between BOAPIN.com., and the Company, and Mr. Mittler and Mr. Pizzino. Mr. Mittler and Mr. Pizzino are significant shareholders in BOAPIN.com, and have the ability to effect decisions over the BOAPIN.com’s operations through contractual arrangements and shareholder approvals. As, Mr. Mittler is also our Chief Executive Officer and Director, and Mr. Pizzino is also our President and Director, there is the potential for a conflict of interest between the Company, Mr. Mittler, Mr. Pizzino and BOAPIN.com.
Except as described above, there are no conflicts of interest between the Company and any of its officers or directors.
INDEMNIFICATION OF DIRECTORS AND OFFICERS
Section 78.138 of the NRS provides that a director or officer will not be individually liable unless it is proven that (i) the director's or officer's acts or omissions constituted a breach of his or her fiduciary duties, and (ii) such breach involved intentional misconduct, fraud or a knowing violation of the law.
Section 78.7502 of NRS permits a company to indemnify its directors and officers against expenses, judgments, fines and amounts paid in settlement actually and reasonably incurred in connection with a threatened, pending or completed action, suit or proceeding if the officer or director (i) is not liable pursuant to NRS 78.138 or (ii) acted in good faith and in a manner the officer or director reasonably believed to be in or not opposed to the best interests of the corporation and, if a criminal action or proceeding, had no reasonable cause to believe the conduct of the officer or director was unlawful.
Section 78.751 of NRS permits a Nevada company to indemnify its officers and directors against expenses incurred by them in defending a civil or criminal action, suit or proceeding as they are incurred and in advance of final disposition thereof, upon receipt of an undertaking by or on behalf of the officer or director to repay the amount if it is ultimately determined by a court of competent jurisdiction that such officer or director is not entitled to be indemnified by the company. Section 78.751 of NRS further permits the company to grant its directors and officers additional rights of indemnification under its articles of incorporation or bylaws or otherwise.
Section 78.752 of NRS provides that a Nevada company may purchase and maintain insurance or make other financial arrangements on behalf of any person who is or was a director, officer, employee or agent of the company, or is or was serving at the request of the company as a director, officer, employee or agent of another company, partnership, joint venture, trust or other enterprise, for any liability asserted against him and liability and expenses incurred by him in his capacity as a director, officer, employee or agent, or arising out of his status as such, whether or not the company has the authority to indemnify him against such liability and expenses.
Our Articles of Incorporation provide that no director or officer of the Company will be personally liable to the Company or any of its stockholders for damages for breach of fiduciary duty as a director or officer; provided, however, that the foregoing provision shall not eliminate or limit the liability of a director or officer (i) for acts or omissions which involve intentional misconduct, fraud or knowing violation of law, or (ii) the payment of dividends in violation of Section 78.300 of NRS. In addition, our bylaws permit for the indemnification and insurance provisions in Chapter 78 of the NRS.
Insofar as indemnification by us for liabilities arising under the Securities Act may be permitted to our directors, officers or persons controlling the company pursuant to provisions of our articles of incorporation and bylaws, or otherwise, we have been advised that in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable. In the event that a claim for indemnification by such director, officer or controlling person of us in the successful defense of any action, suit or proceeding is asserted by such director, officer or controlling person in connection with the securities being offered, we will, unless in the opinion of our counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by us is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
At the present time, there is no pending litigation or proceeding involving a director, officer, employee or other agent of ours in which indemnification would be required or permitted. We are not aware of any threatened litigation or proceeding, which may result in a claim for such indemnification.
Further, in the normal course of business, we have in our contract’s indemnification clauses, written as either mutual where each party will indemnify, defend, and hold each other harmless against losses arising from a breach of representations or covenants, or out of intellectual property infringement or other claims made against certain parties; or single where we have agreed to hold certain parties harmless against losses etc. We have entered into indemnification agreements with two of our officers and all directors, and our bylaws contain similar indemnification obligations to our agents. Remaining officers will be required to signed indemnification agreements in the near future.
Review, Approval or Ratification of Transactions with Related Parties
We have adopted a related-party transactions policy under which our executive officers, directors, nominees for election as a director, beneficial owners of more than 5% of any class of our Common Stock, and any members of the immediate family of any of the foregoing persons are not permitted to enter into a related-party transaction with us without the consent of our audit committee. If the related party is, or is associated with, a member of our audit committee, the transaction must be reviewed and approved by another independent body of our Board of Directors, such as our governance committee. Any request for us to enter into a transaction with a related party in which the amount involved exceeds $120,000 and such party would have a direct or indirect interest must first be presented to our audit committee for review, consideration and approval. If advance approval of a related-party transaction was not feasible or was not obtained, the related-party transaction must be submitted to the audit committee as soon as reasonably practicable, at which time the audit committee shall consider whether to ratify and continue, amend and ratify, or terminate or rescind such related-party transaction. All of the transactions described above were reviewed and considered by, and were entered into with the approval of, or ratification by, our Board of Directors.
During the last two full fiscal years and the current fiscal year or any currently proposed transaction, there are transactions involving the issuer, in which the amount involved exceeds the lesser of $120,000 or one percent of the average of the issuer’s total assets at year-end for its last three fiscal years, except compensation awarded to executives.
Legal/Disciplinary History
None of Wearable Health Solutions, Inc.’s Officers or Directors have been the subject of any criminal proceeding or named as a defendant in a pending criminal proceeding (excluding traffic violations and other minor offenses);
None of Wearable Health Solutions, Inc.’s Officers or Directors have been the subject of any entry of an order, judgment, or decree, not subsequently reversed, suspended or vacated, by a court of competent jurisdiction that permanently or temporarily enjoined, barred, suspended or otherwise limited such person’s involvement in any type of business, securities, commodities, or banking activities;
None of Wearable Health Solutions, Inc.’s Officers or Directors have been the subject of any finding or judgment by a court of competent jurisdiction (in a civil action), the Securities and Exchange Commission, the Commodity Futures Trading Commission, or a state securities regulator of a violation of federal or state securities or commodities law, which finding or judgment has not been reversed, suspended, or vacated; or
None of Wearable Health Solutions, Inc.’s Officers or Directors has been the subject of any entry of an order by a self-regulatory organization that permanently or temporarily barred, suspended or otherwise limited such person’s involvement in any type of business or securities activities.
Board Composition
Our board of directors currently consists of two members. Each director of the Company serves until the next annual meeting of stockholders and until his successor is elected and duly qualified, or until his earlier death, resignation or removal. Our board is authorized to appoint persons to the offices of Chairman of the Board of Directors, President, Chief Executive Officer, one or more vice presidents, a Treasurer or Chief Financial Officer and a Secretary and such other offices as may be determined by the board.
We have no formal policy regarding board diversity. In selecting board candidates, we seek individuals who will further the interests of our stockholders through an established record of professional accomplishment, the ability to contribute positively to our collaborative culture, knowledge of our business and understanding of our prospective markets.
Board Leadership Structure and Risk Oversight
The board of directors oversees our business and considers the risks associated with our business strategy and decisions. The board currently implements its risk oversight function as a whole. Each of the board committees when established will also provide risk oversight in respect of its areas of concentration and reports material risks to the board for further consideration.
Code of Business Conduct and Ethics
Prior to one year from the date of this Offering’s qualification, we plan on adopting a written code of business conduct and ethics that applies to our directors, officers and employees, including our principal executive officer, principal financial officer and principal accounting officer or controller, or persons performing similar functions. We will post on our website a current copy of the code and all disclosures that are required by law or market rules in regard to any amendments to, or waivers from, any provision of the code.
SECURITY OWNERSHIP OF MANAGEMENT AND CERTAIN SECURITYHOLDERS
______
The following table sets forth as of June 6, 2023, certain information regarding beneficial ownership of our common stock by:
| | · | Each executive officer who in this proxy statement are collectively referred to as the “Named Executive Officers;” |
| | · | Each person known to us to beneficially own 5% or more of our common stock; |
| | · | Each of our directors; and |
| | · | All of our executive officers (as that term is defined under the rules and regulations of the SEC) and directors as a group |
We have determined beneficial ownership in accordance with Rule 13d-3 under the Exchange Act. Beneficial ownership generally means having sole or shared voting or investment power with respect to securities. Unless otherwise indicated in the footnotes to the table, each shareholder named in the table has sole voting and investment power with respect to the shares of common stock set forth opposite the shareholder’s name. We have based our calculation of the percentage of beneficial ownership on 1,561,255,108 shares outstanding as of June 6, 2023.
| Name of Beneficial Owner | | Amount and Nature of Beneficial Ownership(1) | | Percentage of Class Ownership | | Percentage of Beneficial Ownership of Common Stock Assuming Conversion of all Preferred Shares | | Percentage of Voting Power Based on Voting Privileges of Common and Preferred Shares without Conversions |
| Directors and Officers: | | | | | | | | |
| Harrysen Mittler | | 303,175,000 Common Stock | | 19.4% Common Stock | | 526,437,500 shares of common stock | | 20,326,437,500 Total Votes |
| | | 2,326,250 Series C Preferred | | 34.0% Series C Preferred | | | | |
| | | 2,000,000 Series E Preferred(4) | | 50% Series E Preferred | | | | |
| | | | | | | | | |
| Peter Pizzino | | 303,175,000 Common Stock | | 19.4% Common Stock | | 514,651,250 shares of common stock | | 20,314,651,250 Total Votes |
| | | 1,147,625 Series C Preferred | | 16.8% Series C Preferred | | | | |
| | | 2,000,000 Series E Preferred(4) | | 50% Series E Preferred | | | | |
| | | | | | | | | |
| Vincent S. Miceli | | 6,312,500 Common Stock | | >1% Common Stock | | | | 6,312,500 Total Votes |
| | | | | | | | | |
Gail Rosenthal Former CFO | | 10,875,000 Common Stock | | >1% Common Stock | | | | 10,875,000 Total Votes |
| | | | | | | | | |
| Aqualaro Corp.(3) | | 56,000,000 Common Stock | | 3.59% Common Stock | | | | 56,000,000 Total Votes |
| | | | | | | | | |
Pacific Software, Inc.
Harrysen Mittler | | 1,250,000 Series C Preferred | | 18.3% Series C Preferred | | 11,250,000 shares of Common Stock | | 1,250,000 Total Votes |
| | | | | | | | | |
Hypersoft Ventures, Inc.
Robert Johnson | | 1,162,500 Series C Preferred | | 17% Series C Preferred | | 11,625,000 shares of Common Stock | | 1,162,500 Total Votes |
| | | | | | | | | |
| Sandor Capital, Master Fund | | 400,000 Series D Preferred | | 94% Series D Preferred | | 40,000,000 shares of Common Stock | | 400,000 Total Votes |
| | | | | | | | | |
| JEBL Holdings LP | | 25,000 Series D Preferred | | 6% Series D Preferred | | 2,500,000 shares of Common Stock | | 25,000 Total Votes |
| | | | | | | | | |
| Venture Group Capital | | 100,000,000 Common stock | | 6.41% Common Stock | | | | 100,000,000 Total Votes |
| | | | | | | | | |
| All executive officers and directors | | Common Stock | | 39% Common Stock(2) | | | | |
| as a group (3 persons) | | Series C Preferred | | 69% Series C Preferred | | | | |
| | | Series E Preferred | | 100% Series E Preferred | | | | |
| | | Total Common Vote | | 98%(5) | | | | |
| 1) | The number and percentage of shares beneficially owned is determined under rules of the SEC and the information is not necessarily indicative of beneficial ownership for any other purpose. Under such rules, beneficial ownership includes any shares as to which the individual has sole or shared voting power or investment power and also any shares which the individual has the right to acquire within 60 days through the exercise of any stock option or other right. The persons named in the table have sole voting and investment power with respect to all shares of common stock shown as beneficially owned by them, subject to community property laws where applicable and the information contained in the footnotes to this table. |
| (2) | Based upon 1,561,255,108 shares outstanding as of June 6, 2023. |
| (3) | Company Aqualaro Corp. is controlled by Miro Zecevic. |
(4) | Reflects Series E Preferred Stock. Series E Preferred Stock converts at a ratio of 1 Series E Preferred Share into 100 Shares of Common Shares. Series E Preferred Stock has voting rights of 10,000 votes of common stock per share of Series E Preferred. |
| (5) | Based upon the voting preferences included in all of the classes of stock of the Company, which amounts to 41,633,915,250 votes. |
Changes in Control
We do not currently have any arrangements which if consummated may result in a change of control of our company.
INTEREST OF MANAGEMENT AND OTHERS IN CERTAIN TRANSACTIONS
______
Other than as reported herein, during the last two full fiscal years and the current fiscal year or any currently proposed transaction, there is no transaction involving the Company, in which the amount involved exceeds the lesser of $120,000 or one percent of the average of the Company’s total assets at year-end for its last three fiscal years.
DESCRIPTION OF SECURITIES
______
Common Stock
We are authorized to issue Three Billion, (3,000,000,000) shares of common stock with a par value of $0.0001 per share (the “Common Stock”) and Twenty Five Million (25,000,000) shares of preferred stock (the “Preferred Stock"), of which 100,000 such shares have been designated as Series A Preferred Stock, 62,500 such shares have been designated as Series B Preferred Stock, 6,944,445 such shares have been designated as Series C Preferred Stock, 500,000 such shares have been designated as Series D Preferred Stock, 4,000,000 such shares have been designated as Series E Preferred Stock. As of June 6, 2023, there were 1,561,255,108 shares of our Common Stock issued and outstanding.
Voting Rights. All shares of common stock shall be identical with each other in every respect and the holders of common shares shall be entitled to have unlimited voting rights on all shares and be entitled to one vote for each share on all matters on which Shareholders have the right to vote.
Liquidation. In the event of a liquidation, dissolution, or winding up of the Company, the holders of our Common Stock are entitled to share pro-rata all net assets remaining after payment in full of all liabilities, subject to prior distribution rights of preferred stock, if any, then outstanding.
Preemptive Rights. No holder of shares of stock of any class shall have any preemptive right to subscribe to or purchase any additional shares of any class, provided however that the Board of Directors may, in authorizing the issuance of shares of stock of any class, confer any right of first refusal that the Board of Directors may deem advisable in connection with such issuance.
Conversion Rights. The Board of Directors may authorize the issuance from time to time of shares of its stock of any class, whether now or hereafter authorized, or securities convertible into shares of its stock of any class, whether now or hereafter authorized, for such consideration as the Board of Directors may deem advisable, subject to such restrictions or limitations, if any, as may be outlined in the By-laws of the corporation.
The holders of shares of our common stock are entitled to dividends out of funds legally available when and as declared by our board of directors. Our board of directors has never declared a dividend and does not anticipate declaring a dividend in the foreseeable future. Should we decide in the future to pay dividends, as a holding company, our ability to do so and meet other obligations depends upon the receipt of dividends or other payments from our operating subsidiary and other holdings and investments. In the event of our liquidation, dissolution or winding up, holders of our common stock are entitled to receive, ratably, the net assets available to stockholders after payment of all creditors.
All of the issued and outstanding shares of our common stock are duly authorized, validly issued, fully paid and non-assessable. To the extent that additional shares of our common stock are issued, the relative interests of existing stockholders will be diluted.
Preferred Stock
Series A Convertible Preferred Stock: The Company is currently authorized to issue up to 100,000 shares of Series A Convertible Preferred Stock, par value $0.0001 per share, convertible at a ratio of 1 share of Series A Convertible Preferred Stock for 2 shares of common stock. These shares have no voting rights. As of June 6, 2023, 688 shares of Series A Convertible Preferred Stock were issued and outstanding.
Series B Convertible Preferred Stock: The Company is currently authorized to issue up to 62,500 shares of Series B Convertible Preferred Stock, par value $0.0001 per share, convertible at a ratio of 1 share of Series B Convertible Preferred Stock for 2 shares of common stock. These shares have voting rights and vote on an “as converted” basis in actions required to have Series B Preferred Stockholder approval. As of June 6, 2023, 9,938 shares of Series B Convertible Preferred Stock were issued and outstanding.
Series C Convertible Preferred Stock: The Company is currently authorized to issue up to 6,944,445 shares of Series C Convertible Preferred Stock, par value $0.0001 per share, convertible at a ratio of 1 share of Series C Convertible Preferred Stock for 10 shares common stock. These shares have voting rights and vote on an “as converted” basis on all matters submitted to our Stockholders for approval. As of June 6, 2023, 6,838,891 shares of Series C Convertible Preferred Stock were issued and outstanding.
Series D Convertible Preferred Stock: The Company is currently authorized to issue up to 500,000 shares of Series D Convertible Preferred Stock, par value $0.0001 per share, convertible at a ratio of 1 share of Series D Convertible Preferred stock for 100 shares of common stock. These shares have voting rights and vote on actions required to have Series D Preferred Stockholder approval. As of June 6, 2023, 425,000 shares of Series D Convertible Preferred Stock were issued and outstanding.
Series E Convertible Preferred Stock: The Company is currently authorized to issue up to 4,000,000 shares of Series E Convertible Preferred Stock, par value $0.0001 per share, convertible at a ratio of 1 share of Series E Convertible Preferred Stock for 100 shares of common stock. Each of these shares carries a voting right equivalent to 10,000 shares of common stock. The Company may not issue any other shares with extended voting rights. As of June 6, 2023, 4,000,000 shares of Series E Convertible Preferred Stock were issued and outstanding.
None of the Preferred Stock Certificates of Designation contains any event; wherein, the conversion of high-vote and /or low-vote shares is mandatory. The Preferred Stock may be converted into common stock by the holders thereof at their pleasure. If the Preferred Stockholder convert their shares there will be dilution into the common stock of the Company. If all of the currently outstanding shares of Preferred Stock were to convert, without regard to any equity blocking restrictions, those shares would currently convert into 511,015,286 shares of our common stock; thereby, causing substantial dilution to the Company’s common shares. There are no expiration provisions related to any of the Preferred Stock Certificates of Designations.
Preferred Stock
The powers, preferences, rights, qualifications, limitations and restrictions pertaining to the Preferred Stock, or any series thereof, shall be such as may be fixed, from time to time, by the Board in its sole discretion. Authority to do so being hereby expressly vested in the Board. The authority of the Board with respect to each such series of Preferred Stock will include, without limiting the generality of the foregoing, the determination of any or all of the following:
The number of shares of any series and the designation to distinguish the shares of such series from the shares of all other series: (1) the voting powers, if any, of the shares of such series and whether such voting powers are full or limited: (2) the redemption provisions, if any, applicable to such series, including the redemption price or prices to be paid; (3) whether dividends, if any, will be cumulative or noncumulative, the dividend rate or rates of such series and the dates and preferences of dividends on such series: (4) the rights of such series upon the voluntary or involuntary dissolution of, or upon any distribution of the assets of. the Corporation: (5) the provisions, if any, pursuant to which the shares of such series are convertible into, or exchangeable for, shares of any other class or classes of any other series of the same other any other class or classes of stock or any other security, of the Corporation or any other corporation or entity, and the rates or other determinants of conversion or exchange applicable thereto; (6) the right, if any, to subscribe for or to purchase any securities of the Corporation or any other corporation or other entity; (7) the provisions, if any. of a sinking fund applicable to such series: and (8) any other relative, participating, optional or other powers, preferences or rights, and any qualifications, limitations or restrictions thereof. of such series.
DIVIDEND POLICY
______
We have never declared or paid cash dividends on our capital stock. We currently intend to retain any future earnings for use in the operation of our business and do not intend to declare or pay any cash dividends in the foreseeable future. Any further determination to pay dividends on our capital stock will be at the discretion of our Board of Directors, subject to applicable laws, and will depend on our financial condition, results of operations, capital requirements, general business conditions, and other factors that our Board of Directors considers relevant.
Anti-takeover Effects of Our Articles of Incorporation and By-laws
Our amended and restated articles of incorporation and bylaws contain certain provisions that may have anti-takeover effects, making it more difficult for or preventing a third party from acquiring control of the Company or changing its board of directors and management. According to our bylaws and articles of incorporation, neither the holders of the Company’s common stock nor the holders of the Company's preferred stock have cumulative voting rights in the election of our directors. The combination of the present ownership by a few stockholders of a significant portion of the Company's issued and outstanding common stock and lack of cumulative voting makes it more difficult for other stockholders to replace the Company's board of directors or for a third party to obtain control of the Company by replacing its board of directors.
Anti-takeover Effects of Nevada Law
Business Combinations
The “business combination” provisions of Sections 78.411 to 78.444, inclusive, of the Nevada Revised Statutes, or NRS, prohibit a Nevada corporation with at least 200 stockholders from engaging in various “combination” transactions with any interested stockholder: for a period of three years after the date of the transaction in which the person became an interested stockholder, unless the transaction is approved by the board of directors prior to the date the interested stockholder obtained such status; or after the expiration of the three-year period, unless:
| · | | the transaction is approved by the board of directors or a majority of the voting power held by disinterested stockholders, or |
| · | | if the consideration to be paid by the interested stockholder is at least equal to the highest of: (a) the highest price per share paid by the interested stockholder within the three years immediately preceding the date of the announcement of the combination or in the transaction in which it became an interested stockholder, whichever is higher, (b) the market value per share of common stock on the date of announcement of the combination and the date the interested stockholder acquired the shares, whichever is higher, or (c) for holders of preferred stock, the highest liquidation value of the preferred stock, if it is higher. |
A “combination” is defined to include mergers or consolidations or any sale, lease exchange, mortgage, pledge, transfer or other disposition, in one transaction or a series of transactions, with an "interested stockholder" having: (a) an aggregate market value equal to 5% or more of the aggregate market value of the assets of the corporation, (b) an aggregate market value equal to 5% or more of the aggregate market value of all outstanding shares of the corporation, or (c) 10% or more of the earning power or net income of the corporation.
In general, an “interested stockholder” is a person who, together with affiliates and associates, owns (or within three years, did own) 10% or more of a corporation's voting stock. The statute could prohibit or delay mergers or other takeover or change in control attempts and, accordingly, may discourage attempts to acquire our company even though such a transaction may offer our stockholders the opportunity to sell their stock at a price above the prevailing market price.
Control Share Acquisitions
The “control share” provisions of Sections 78.378 to 78.3793, inclusive, of the NRS, which apply only to Nevada corporations with at least 200 stockholders, including at least 100 stockholders of record who are Nevada residents, and which conduct business directly or indirectly in Nevada, prohibit an acquirer, under certain circumstances, from voting its shares of a target corporation's stock after crossing certain ownership threshold percentages, unless the acquirer obtains approval of the target corporation's disinterested stockholders. The statute specifies three thresholds: one-fifth or more but less than one-third, one-third but less than a majority, and a majority or more, of the outstanding voting power. Once an acquirer crosses one of the above thresholds, those shares in an offer or acquisition and acquired within 90 days thereof become “control shares” and such control shares are deprived of the right to vote until disinterested stockholders restore the right. These provisions also provide that if control shares are accorded full voting rights and the acquiring person has acquired a majority or more of all voting power, all other stockholders who do not vote in favor of authorizing voting rights to the control shares are entitled to demand payment for the fair value of their shares in accordance with statutory procedures established for dissenters’ rights.
SECURITIES BEING OFFERED
______
Current Offering
Wearable Health Solutions, Inc. (“Wearable Health Solutions, Inc.,” “We,” or the “Company”) is offering up to $1,000,000 total of Securities, consisting of Common Stock, $0.0001 par value (the “Common Stock” or collectively the “Securities”).
Transfer Agent And Registrar
Our transfer agent is EQ Shareowner Services, whose address is 1110 Centre Point Curve, Suite 101, Mendota Heights MN 55120, telephone number is (800) 401-1957, and website is equiniti.com/us/.
The transfer agent is registered under the Exchange Act and operates under the regulatory authority of the SEC and FINRA.
SHARES ELIGIBLE FOR FUTURE SALE
_____
Prior to this Offering, there has been a limited market for our Common Stock. Future sales of substantial amounts of our Common Stock, or securities or instruments convertible into our Common Stock, in the public market, or the perception that such sales may occur, could adversely affect the market price of our Common Stock prevailing from time to time. Furthermore, because there will be limits on the number of shares available for resale shortly after this Offering due to contractual and legal restrictions described below, there may be resales of substantial amounts of our Common Stock in the public market after those restrictions lapse. This could adversely affect the market price of our Common Stock prevailing at that time.
Rule 144
In general, a person who has beneficially owned restricted shares of our Common Stock for at least twelve months, in the event we are a reporting company under Regulation A, or at least six months, in the event we have been a reporting company under the Exchange Act for at least 90 days before the sale, would be entitled to sell such securities, provided that such person is not deemed to be an affiliate of ours at the time of sale or to have been an affiliate of ours at any time during the 90 days preceding the sale. A person who is an affiliate of ours at such time would be subject to additional restrictions, by which such person would be entitled to sell within any three-month period only a number of shares that does not exceed the greater of the following:
| | - | 1% of the number of shares of our Common Stock then outstanding; or the average weekly trading volume of our Common Stock during the four calendar weeks preceding the filing by such person of a notice on Form 144 with respect to the sale; |
provided that, in each case, we are subject to the periodic reporting requirements of the Exchange Act for at least 90 days before the sale. Rule 144 trades must also comply with the manner of sale, notice and other provisions of Rule 144, to the extent applicable.
LEGAL MATTERS
_____
Certain legal matters with respect to the shares of common stock offered hereby will be passed upon by Jonathan Leinwand, Esq. of Aventura, FL.
EXPERTS
______
The below described expert has not been hired on a contingent basis and will not receive a direct or indirect interest in the Company.
The audited financial statements for the year ending June 30, 2022, that have been included in this Offering have been audited by Assurance Dimensions.
Included are the financial statements in reliance on their report, given upon their authority as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
______
We have filed with the SEC a Regulation A Offering Statement on Form 1-A under the Securities Act with respect to the shares of common stock offered hereby. This Offering Circular, which constitutes a part of the Offering Statement, does not contain all of the information set forth in the Offering Statement or the exhibits and schedules filed therewith. For further information about us and the common stock offered hereby, we refer you to the Offering Statement and the exhibits and schedules filed therewith. Statements contained in this Offering Circular regarding the contents of any contract or other document that is filed as an exhibit to the Offering Statement are not necessarily complete, and each such statement is qualified in all respects by reference to the full text of such contract or other document filed as an exhibit to the Offering Statement. Upon the completion of this Offering, we will be required to file periodic reports, proxy statements, and other information with the SEC pursuant to the Securities Exchange Act of 1934. You may read and copy this information at the SEC’s Public Reference Room, 100 F Street, N.E., Room 1580, Washington, D.C. 20549. You may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. The SEC also maintains an Internet website that contains reports, proxy statements and other information about issuers, including us, that file electronically with the SEC. The address of this site is www.sec.gov.

Wearable Health Solutions, Inc.
Index to Financial Statements

REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of Wearable Health Solutions, Inc. Opinion on the Financial Statements We have audited the accompanying consolidated balance sheets of Wearable Health Solutions, Inc. (the Company) as of June 30, 2022 and 2021, and the related statements of operations, stockholders’ deficit, and cash flows for each of the years in the two year period ended June 30, 2022, and the related notes (collectively referred to as the financial statements). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of June 30, 2022 and 2021, and the results of its operations and its cash flows for each of the two years in the ended June 30, 2022, in conformity with accounting principles generally accepted in the United States of America. Explanatory Paragraph- Going Concern The accompanying consolidated financial statements have been prepared assuming the Company will continue as a going concern. As discussed in Note 3 to the financial statements, the Company had a working capital deficit and stockholders' deficit of $2,133,878 and $2,092,227, as of June 30, 2022, respectively. The Company had net losses and net cash used in operations of $13,049,155 and $3,107,177 for the year ended June 30, 2022, respectively. These conditions raise substantial doubt about the Company's ability to continue as a going concern. Management's plans regarding these matters are also described in Note 3. The financial statements do not include any adjustments that might result from the outcome of this uncertainty. Basis for Opinion These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB. We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion. Critical Audit Matters The critical audit matters communicated below are matters arising from the current period audit of the financial statements that were communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing separate opinions on the critical audit matters or on the accounts or disclosures to which they relate. We did not identify any critical audit matters that need to be communicated. /s/ Assurance Dimensions We have served as the Company’s auditor since 2021. Margate, Florida October 13, 2022 |
Wearable Healthcare Solutions, Inc.
Consolidated Balance Sheets
As at June 30, 2022 and 2021
| | | | | | | |
| | | 2022 | | | 2021 | |
| ASSETS | | | | | | | | |
| Current Assets | | | | | | | | |
| Cash and cash equivalents | | $ | 70,505 | | | $ | 847,430 | |
| Accounts receivable, net | | | – | | | | 25,694 | |
| Due from related parties (See Footnote 9) | | | 155,800 | | | | – | |
| Accounts receivable, other | | | 2,000 | | | | 2,000 | |
| Inventory | | | 7,064 | | | | – | |
| Prepaid Inventory | | | 62,040 | | | | 22,682 | |
| Prepaid expenses | | | – | | | | 10,000 | |
| Total Current Assets | | | 297,409 | | | | 907,806 | |
| | | | | | | | | |
| Property, Plant and Equipment | | | | | | | | |
| Dealer Portal (net of $8,349 and $0 depreciation, as of June 30, 2022 and June 30, 2021) | | | 41,651 | | | | – | |
| Total Property, Plant and Equipment | | | 41,651 | | | | – | |
| | | | | | | | | |
| Total Assets | | $ | 339,060 | | | $ | 907,806 | |
| | | | | | | | | |
| LIABILITIES and SHAREHOLDERS' DEFICIT | | | | | | | | |
| | | | | | | | | |
| COMMITMENTS AND CONTINGENCIES (Note 10) | | | – | | | | – | |
| | | | | | | | | |
| Current liabilities | | | | | | | | |
| Accounts payable | | $ | 57,940 | | | $ | 331,876 | |
| Accrued expenses and other current liabilities | | | 374,278 | | | | 296,920 | |
| Accrued expenses - related party | | | 213,840 | | | | 579,673 | |
| Deferred revenue | | | 80,880 | | | | 108,298 | |
| Line of credit | | | 397,500 | | | | 397,500 | |
| Derivative liability | | | – | | | | 281,845 | |
| Notes payable | | | 413,099 | | | | 853,244 | |
| Note payable - other | | | 50,000 | | | | 50,000 | |
| Note payable - related party | | | 170,000 | | | | 425,000 | |
| Convertible notes - Leonite | | | – | | | | 260,000 | |
| Convertible notes - other | | | 673,750 | | | | 673,750 | |
| Total current liabilities | | | 2,431,287 | | | | 4,258,106 | |
| TOTAL LIABILITIES | | | 2,431,287 | | | | 4,258,106 | |
| | | | | | | | | |
| SHAREHOLDERS’ DEFICIT | | | | | | | | |
| Preferred stock | | | | | | | | |
| Series A Convertible Preferred Stock: $0.0001 par value; 100,000 shares authorized, 688 shares issued and outstanding as of June 30, 2022 and 2021, respectively | | $ | 1 | | | $ | 1 | |
| Series B Convertible Preferred Stock: $0.0001 par value; 62,500 shares authorized, 9,938 shares issued and outstanding as of June 30, 2022 and 2021, respectively | | | 1 | | | | 1 | |
| Series C Preferred Stock: $0.0001 par value; 6,944,445 authorized, 6,838,889 shares issued and outstanding as of June 30, 2022 and 2021, respectively | | | 684 | | | | 684 | |
| Series D Preferred Stock: $0.0001 par value; 500,000 shares authorized, 425,000 shares issued and outstanding as of June 30, 2022 and 2021, respectively | | | 43 | | | | 43 | |
| Series E Preferred Stock $0.0001 par value, 4,000,000 shares authorized, 4,000,000 and 1,900,000 shares issued and outstanding as of June 30, 2022 and 2021, respectively | | | 400 | | | | 190 | |
| Series E Preferred Stock to be issued (-0- and 100,000 shares as of June 30, 2022 and 2021, respectively) | | | – | | | | 57,000 | |
| Common stock | | | | | | | | |
| Common Stock: $0.0001 par value; 3,000,000,000 shares authorized, 1,493,142,608 and 647,074,177 shares issued and outstanding as of June 30, 2022 and 2021, respectively | | | 149,314 | | | | 64,708 | |
| Common stock to be issued (35,602,500 and 20,050,000 shares as of June 30, 2022 and 2021, respectively) | | | 407,677 | | | | 169,005 | |
| Additional paid in capital | | | 36,773,035 | | | | 22,732,295 | |
| Accumulated deficit | | | (39,423,382 | ) | | | (26,374,227 | ) |
| Total Shareholders’ Deficit | | | (2,092,227 | ) | | | (3,350,300 | ) |
| Total Liabilities and Shareholders’ Deficit | | $ | 339,060 | | | $ | 907,806 | |
The footnotes are an integral part of these consolidated financial statements.
Wearable Healthcare Solutions, Inc.
Consolidated Statements of Operations
For the years ended June 30, 2022 and 2021
| | | | | | | |
| | | 2022 | | | 2021 | |
| Revenue | | $ | 1,045,890 | | | $ | 1,394,149 | |
| | | | | | | | | |
| Cost of sales | | | (568,409 | ) | | | (869,450 | ) |
| Gross profit | | | 477,481 | | | | 524,699 | |
| | | | | | | | | |
| Operating expenses | | | | | | | | |
| Selling expense | | | 372,553 | | | | 104,288 | |
| Consulting and professional fees | | | 861,301 | | | | 245,786 | |
| Gain/loss on debt settlement | | | – | | | | (14,843 | ) |
| Insurance | | | 87,576 | | | | 50,683 | |
| Rent | | | 16,605 | | | | 15,325 | |
| Salaries and wages (including stock compensation expense) | | | 11,375,405 | | | | 2,425,018 | |
| Software expense | | | – | | | | 144,600 | |
| Depreciation | | | 8,349 | | | | – | |
| Research and development expense | | | 390,828 | | | | – | |
| General and administrative | | | 409,444 | | | | 715,522 | |
| Total Operating expenses | | | 13,522,061 | | | | 3,686,379 | |
| | | | | | | | | |
| Loss from operations | | | (13,044,580 | ) | | | (3,161,680 | ) |
| | | | | | | | | |
| Other Income / (expense), net | | | | | | | | |
| Other income | | | 36,000 | | | | – | |
| Gain on debt extinguishment | | | 96,145 | | | | – | |
| Change in fair value of derivative instrument | | | (213,053 | ) | | | (11,003 | ) |
| Gain on debt forgiveness | | | – | | | | 81,000 | |
| Gain on settlement of accounts payable | | | 156,616 | | | | – | |
| Interest expense | | | (80,283 | ) | | | (221,323 | ) |
| Total other (expense), net | | | (4,575 | ) | | | (151,326 | ) |
| Net loss before taxes | | | (13,049,155 | ) | | | (3,313,006 | ) |
| Income tax | | | – | | | | – | |
| Net loss | | $ | (13,049,155 | ) | | $ | (3,313,006 | ) |
| | | | | | | | | |
| Net loss per common share - Basic and Diluted | | $ | (0.01202 | ) | | $ | (0.00629 | ) |
| | | | | | | | | |
| Weighted average common shares outstanding - Basic & Diluted | | | 1,086,059,256 | | | | 526,577,492 | |
The footnotes are an integral part of these consolidated financial statements.
Wearable Healthcare Solutions, Inc.
Consolidated Statements of Changes in Shareholders’ Deficit
June 30, 2022 and 2021
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Series A | | Series B | | Series C | | Series C to be issued | | Series D | | Series E | |
| | | Shares | | Amount | | Shares | | Amount | | Shares | | Amount | | Shares | | Amount | | Shares | | Amount | | Shares | | Amount | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| As at June 30, 2020 | | 688 | | $ | 1 | | 9,938 | | $ | 1 | | 138,886 | | $ | 14 | | 6,700,003 | | $ | 375,200 | | 425,000 | | $ | 43 | | 4,000,000 | | $ | 400 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Loss for the period | | – | | | – | | – | | | – | | – | | | – | | – | | | – | | – | | | – | | – | | | – | |
| 3a10 Stock Issuance | | – | | | – | | – | | | – | | – | | | – | | – | | | – | | – | | | – | | – | | | – | |
| Cancellation of 3a10 agreement | | – | | | – | | – | | | – | | – | | | – | | – | | | – | | – | | | – | | – | | | – | |
| Issuance of Series C Preferred shares | | – | | | – | | – | | | – | | 6,700,003 | | | 670 | | (6,700,000 | ) | | (375,200 | ) | – | | | – | | – | | | – | |
| Return of Preferred Stock | | – | | | – | | – | | | – | | – | | | – | | – | | | – | | – | | | – | | (4,000,000 | ) | | (400 | ) |
| Preferred stock issued/officer comp | | – | | | – | | – | | | – | | – | | | – | | – | | | – | | – | | | – | | 1,900,000 | | | 190 | |
| Common stock issued/officer comp | | – | | | – | | – | | | – | | – | | | – | | – | | | – | | – | | | – | | – | | | – | |
| Stock for services | | – | | | – | | – | | | – | | – | | | – | | – | | | – | | – | | | – | | – | | | – | |
| Shares sold for cash | | – | | | – | | – | | | – | | – | | | – | | – | | | – | | – | | | – | | – | | | – | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| As at June 30, 2021 | | 688 | | $ | 1 | | 9,938 | | $ | 1 | | 6,838,889 | | $ | 684 | | – | | $ | – | | 425,000 | | $ | 43 | | 1,900,000 | | $ | 190 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Loss for the period | | – | | | – | | – | | | – | | – | | | – | | – | | | – | | – | | | – | | – | | | – | |
| Common stock issued for debt conversions | | – | | | – | | – | | | – | | – | | | – | | – | | | – | | – | | | – | | – | | | – | |
| Issuance of shares for cash | | – | | | – | | – | | | – | | – | | | – | | – | | | – | | – | | | – | | – | | | – | |
| Common stock for compensation | | – | | | – | | – | | | – | | – | | | – | | – | | | – | | – | | | – | | – | | | – | |
| Common stock for officer compensation | | – | | | – | | – | | | – | | – | | | – | | – | | | – | | – | | | – | | – | | | – | |
| Preferred stock issued/officer compensation | | – | | | – | | – | | | – | | – | | | – | | – | | | – | | – | | | – | | 2,100,000 | | | 210 | |
| Stock for services | | – | | | – | | – | | | – | | – | | | – | | – | | | – | | – | | | – | | – | | | – | |
| Shares sold for cash | | – | | | – | | – | | | – | | – | | | – | | – | | | – | | – | | | – | | – | | | – | |
| Subscriptions receivable | | – | | | – | | – | | | – | | – | | | – | | – | | | – | | – | | | – | | – | | | – | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| As at June 30, 2022 | | 688 | | $ | 1 | | 9,938 | | $ | 1 | | 6,838,889 | | $ | 684 | | – | | $ | – | | 425,000 | | $ | 43 | | 4,000,000 | | $ | 400 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Series E to be issued | | | Common Stock | | Common Stock
to be issued | | Additional Paid in | | Accumulated | | | |
| | | Shares | | Amount | | | Shares | | Amount | | Shares | | Amount | | Amount | | Profit/Deficit | | Total | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
| As at June 30, 2020 (Unaudited) | | 1,000,000 | | $ | 650,000 | | | 297,399,177 | | $ | 29,740 | | 10,350,000 | | $ | 71,415 | | $ | 18,578,122 | | $ | (23,061,221 | ) | $ | (3,356,285 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Loss for the period | | – | | | – | | | – | | | – | | – | | | – | | | – | | | (3,313,006 | ) | | (3,313,006 | ) |
| 3a10 stock issuance | | – | | | – | | | 48,989,000 | | | 4,899 | | – | | | – | | | – | | | – | | | 4,899 | |
| Cancellation of 3a10 agreement | | – | | | – | | | (48,989,000 | ) | | (4,899 | ) | – | | | – | | | – | | | – | | | (4,899 | ) |
| Issuance of Series C Preferred shares | | – | | | – | | | – | | | – | | – | | | – | | | 374,530 | | | – | | | – | |
| Return of preferred stock | | – | | | – | | | – | | | – | | – | | | – | | | – | | | – | | | (400 | ) |
| Preferred stock issued/officer comp | | (900,000 | ) | | (593,000 | ) | | – | | | – | | – | | | – | | | 1,162,810 | | | – | | | 570,000 | |
| Common stock issued/officer comp | | – | | | – | | | 201,275,000 | | | 20,128 | | (300,000 | ) | | (2,410 | ) | | 1,129,373 | | | – | | | 1,147,090 | |
| Stock for services | | – | | | – | | | 3,000,000 | | | 300 | | – | | | – | | | 48,000 | | | – | | | 48,300 | |
| Shares sold for cash | | – | | | – | | | 145,400,000 | | | 14,540 | | 10,000,000 | | | 100,000 | | | 1,439,460 | | | – | | | 1,554,000 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
| As at June 30, 2021 | | 100,000 | | $ | 57,000 | | | 647,074,177 | | $ | 64,708 | | 20,050,000 | | $ | 169,005 | | $ | 22,732,295 | | $ | (26,374,227 | ) | $ | (3,350,300 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Loss for the period | | – | | | – | | | – | | | – | | – | | | – | | | – | | | (13,049,155 | ) | | (13,049,155 | ) |
| Common stock issued for debt conversions | | – | | | – | | | 66,418,431 | | | 6,641 | | – | | | – | | | 671,066 | | | – | | | 677,707 | |
| Issuance of shares for cash | | – | | | – | | | 350,000,000 | | | 35,000 | | (10,050,000 | ) | | (100,005 | ) | | 3,465,004 | | | – | | | 3,399,999 | |
| Common stock for compensation | | – | | | – | | | 409,650,000 | | | 40,965 | | 7,262,500 | | | 88,551 | | | 6,515,880 | | | – | | | 6,645,396 | |
| Common stock for officer compensation | | – | | | – | | | – | | | – | | 6,840,000 | | | 104,126 | | | – | | | – | | | 104,126 | |
| Preferred stock issued/officer compensation | | (100,000 | ) | | (57,000 | ) | | – | | | – | | – | | | – | | | 3,056,790 | | | – | | | 3,000,000 | |
| Stock for services | | – | | | – | | | 20,000,000 | | | 2,000 | | (10,000,000 | ) | | (69,000 | ) | | 362,000 | | | – | | | 295,000 | |
| Shares sold for cash | | – | | | – | | | – | | | – | | 21,500,000 | | | 215,000 | | | – | | | – | | | 215,000 | |
| Subscriptions receivable | | – | | | – | | | – | | | – | | – | | | – | | | (30,000 | ) | | – | | | (30,000 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
| As at June 30, 2022 | | – | | $ | – | | | 1,493,142,608 | | $ | 149,314 | | 35,602,500 | | $ | 407,677 | | $ | 36,773,035 | | $ | (39,423,382 | ) | $ | (2,092,227 | ) |
The footnotes are an integral part of these consolidated financial statements.
Wearable Healthcare Solutions, Inc.
Consolidated Statement of Cash Flows
For the years ended June 30, 2022 and 2021
| | | | | | | | | |
| | | 2022 | | | 2021 | |
| Cash flow from operating activities | | | | | | | | |
| Net loss | | $ | (13,049,155 | ) | | $ | (3,313,006 | ) |
| Adjustment for non-cash charges and other items: | | | | | | | | |
| Depreciation | | | 8,349 | | | | – | |
| Default penalty on debt | | | – | | | | 110,000 | |
| Common stock issued for services | | | – | | | | 48,300 | |
| Stock compensation expense | | | 10,044,522 | | | | 1,816,702 | |
| Change in fair value of derivative instrument | | | 213,053 | | | | 11,003 | |
| Amortization of debt discount and original issue discount | | | – | | | | 4,167 | |
| Gain on settlement of accounts payable | | | (156,616 | ) | | | – | |
| Gain on debt extinguishment | | | (96,145 | ) | | | (81,000 | ) |
| Total adjustments | | | (3,035,992 | ) | | | (1,403,834 | ) |
| Changes in working capital | | | | | | | | |
| Decrease / (increase) in accounts receivables | | $ | 25,694 | | | $ | 12,596 | |
| Decrease / (increase) in inventory | | | (7,064 | ) | | | – | |
| Decrease / (increase) in prepaid inventory | | | (39,358 | ) | | | 85,129 | |
| Decrease / (increase) in prepaid expenses | | | 10,000 | | | | (10,000 | ) |
| Decrease / (increase) in other assets | | | – | | | | (2,000 | ) |
| (Decrease) / increase in trade and other payables | | | (117,320 | ) | | | 105,356 | |
| (Decrease) / increase in accrued expenses | | | 84,281 | | | | 103,192 | |
| (Decrease) / increase in deferred revenue | | | (27,418 | ) | | | (63,809 | ) |
| Total changes in working capital | | | (71,185 | ) | | | 230,464 | |
| Cash flow used in operating activities | | $ | (3,107,177 | ) | | $ | (1,173,370 | ) |
| | | | | | | | | |
| Cash flow used in investing activities | | | | | | | | |
| Purchase of property, plant & equipment | | | (50,000 | ) | | | – | |
| Cash flow used in investing activities | | | (50,000 | ) | | | – | |
| | | | | | | | | |
| Cash flow provided by financing activities | | | | | | | | |
| Proceeds from issuance of stock (net of subscriptions receivable) | | | 3,584,399 | | | | 1,454,000 | |
| Payments to related parties | | | (509,002 | ) | | | – | |
| Proceeds from notes payable | | | 25,000 | | | | 615,000 | |
| Repayments of note payable | | | (720,145 | ) | | | (48,200 | ) |
| Cash flow from financing activities | | | 2,380,252 | | | | 2,020,800 | |
| Increase/(decrease) in cash and cash equivalents | | | (776,925 | ) | | | 847,430 | |
| Cash and cash equivalents at beginning of the year | | | 847,430 | | | | – | |
| Cash and cash equivalents at end of the year | | $ | 70,505 | | | $ | 847,430 | |
| | | | | | | | | |
Supplemental Disclosures of Cash Flow Information: | | | | | | | | |
| Cash paid during the periods for: | | | | | | | | |
| Interest | | $ | – | | | $ | – | |
| Taxes | | $ | – | | | $ | – | |
The footnotes are an integral part of these consolidated financial statements.
WEARABLE HEALTHCARE SOLUTIONS INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
June 30, 2022 and 2021
Note 1 – Nature and Continuance of Operations
Wearable Healthcare Solutions Inc. (the “Company”) was incorporated as Medical Alarm Concepts Holding, Inc. on June 4, 2008 under the laws of the State of Nevada. The Company was formed for the sole purpose of acquiring all of the membership units of Medical Alarm Concepts LLC, a Pennsylvania limited liability company (“Medical LLC”). On May 26, 2016, the Company filed its Amended and Restated Articles of Incorporation with the Secretary of State of the State of Nevada to change its name from “Medical Alarm Concepts, Inc.” to “Wearable Health Solutions Inc.”
The Company provides mobile health (mHealth) products and services to be used by customers in case of an emergency. As a provider of personal emergency devices, the Company provides innovative wearable healthcare products, tracking services, and turn-key solutions that enable our users to be proactive with their health, as well as safe and protected.
The Company’s flagship products are the iHelp devices, the 3G and the next generation iHelp MAX™ – personal emergency alarm that are used to summon help in the event of an emergency at home.
Note 2 – Summary of Significant Accounting Policies
Basis of Presentation and Use of Estimates – The preparation of consolidated financial statements in conformity with accounting principles generally accepted in the United States of America (“GAAP”) requires management to make estimates and assumptions that affect reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenue and expenses during the reporting period. Future events and their effects cannot be determined with absolute certainty. Therefore, the determination of management’s estimates requires the exercise of judgment. The Company’s management evaluates these significant estimates and assumptions including those related to the fair value of acquired assets and liabilities, stock-based compensation, income taxes, allowance for doubtful accounts, long-lived assets, and inventories, and other matters that affect the consolidated financial statements and disclosures. Actual results could differ from those estimates.
The Company believes the following critical accounting policies affect its more significant judgments and estimates used in the preparation of financial statements.
Principles of Consolidation – The consolidated financial statements include the accounts of the Company and its wholly-owned or controlled operating subsidiary: Medical Alarm Concepts, LLC. All intercompany accounts and transactions have been eliminated.
Cash and Cash Equivalents – For purposes of the Statement of Cash Flows, the Company considers highly liquid investments purchased with an original maturity of three months or less to be cash equivalents.
Accounts Receivable – We estimate credit loss reserves for accounts receivable on an individual receivable basis. A specific impairment allowance reserve is established based on expected future cash flows and the financial condition of the debtor. We charge off customer balances in part or in full when it is more likely than not that we will not collect that amount of the balance due. We consider any balance unpaid after the contract payment period to be past due. There are $-0- and $25,694 in accounts receivables net of allowances of $-0- and $23,705 at June 30, 2022 and 2021, respectively.
Software Development for Internal Use – The Company accounts for software development costs in accordance with applicable guidelines. Software development costs include payroll, employee benefits, stock-based compensation expense, and other headcount-related expenses associated with product development. Software development costs also include third-party development and programming costs, localization costs incurred to translate software for international markets, and the amortization of purchased software code and services content. Such costs related to software development are included in software development expense until the point that technological feasibility is reached. Once technological feasibility is reached, such costs are capitalized and depreciated over the useful estimated lives of the software. For software modifications or developments, the Company expenses the costs.
Concentration of Credit Risk – Financial instruments that potentially subject us to concentrations of credit risk consist primarily of cash and cash equivalents and accounts receivable. The Company performs ongoing credit evaluations of its customers and maintains allowances for potential credit losses.
Recognition of Revenues – In May 2014, the FASB issued ASU No. 2014-09, Revenue from Contracts with Customers (Topic 606). ASU 2014-09 establishes a single comprehensive model for entities to use in accounting for revenue arising from outside contracts with customers and supersedes most of the existing revenue recognition guidance and notes that lease contracts with customers are a scope exception. ASU 2014-09 requires an entity to recognize revenue when it transfers promised goods or services to customers in an amount that reflects the consideration to which an entity expects to be entitled in exchange for those goods or services and also requires certain additional disclosures. On August 12, 2015, the FASB issued ASU 2015-14 to defer the effective date of ASU 2014-09. Public business entities may elect to adopt the amendments as of the original effective date; however, adoption is required for annual reporting periods beginning after December 15, 2017. The Company has adopted this pronouncement.
The Company’s revenues are derived principally from utilizing new technology in the medical alarm industry to provide 24-hour personal response monitoring services and related products to subscribers with medical- or age-related conditions. The Company recognizes revenue when it is realized or realizable and earned. For hardware sales, the Company recognizes revenues at a point in time when the product is shipped. Customers are billed on Net 30 terms. For service revenue, the Company recognizes revenues over the term of the service contract and when the services are rendered. For customers who pay several months at a time, the Company records revenues for the month’s services and the balance of funds to deferred revenues, and records the balance of revenues as they become current.
| Schedule of revenues | | | | | | | | |
| REVENUES | | 2022 | | | 2021 | |
| Hardware revenue | | $ | 134,045 | | | $ | 399,602 | |
| Service revenue | | | 911,845 | | | | 994,547 | |
| TOTAL REVENUES | | $ | 1,045,890 | | | $ | 1,394,149 | |
Deferred revenue activity for the years ended June 30, 2022 and 2021 was as follows:
| Schedule of deferred revenue | | | | | | |
| DEFERRED REVENUE | | 2022 | | | 2021 | |
| Balance at beginning of period | | $ | 108,298 | | | $ | 127,107 | |
| Deferred revenue recognized upon shipments to customers | | | 229,127 | | | | 285,285 | |
| Revenues recognized to end customers | | | (256,545 | ) | | | (304,094 | ) |
| Balance at end of period | | $ | 80,880 | | | $ | 108,298 | |
Deferred Taxes – The Company accounts for income taxes under Section 740-10-30 of the FASB Accounting Standards Codification. Deferred income tax assets and liabilities are determined based upon differences between the financial reporting and tax bases of assets and liabilities and are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse. Deferred tax assets are reduced by a valuation allowance to the extent management concludes it is more likely than not that the assets will not be realized. Deferred tax assets and liabilities are measured using enacted rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in the statements of operations in the period that includes the enactment date.
ASC 740, Income Taxes, requires a company to first determine whether it is more likely than not (which is defined as a likelihood of more than fifty percent) that a tax position will be sustained based on its technical merits as of the reporting date, assuming that taxing authorities will examine the position and have full knowledge of all relevant information. A tax position that meets this more-likely-than-not threshold is then measured and recognized at the largest amount of benefit that is greater than fifty percent likely to be realized upon effective settlement with a taxing authority.
The Federal and state income tax returns of the Company for 2022, 2021, and 2020 are subject to examination by the Internal Revenue Service and state taxing authorities for three (3) years from the date filed.
Related Party Transactions. The Company follows subtopic 850-10 of the FASB Accounting Standards Codification for the identification of related parties and disclosure of related party transactions. Pursuant to Section 850-10-20 the related parties include:
| | a. | Affiliates of the Company; |
| | b. | Entities for which investments in their equity securities would be required, absent the election of the fair value option under the Fair Value Option Subsection of Section 825-10-15, to be accounted for by the equity method by the investing entity; |
| | c. | Trusts for the benefit of employees, such as pension and profit sharing trusts that are managed by or under the trusteeship of management; |
| | d. | Principal owners of the Company; |
| | e. | Management of the Company; |
| | f. | Other parties with which the Company may deal if one party controls or can significantly influence the management or operating policies of the other to an extent that one of the transacting parties might be prevented from fully pursuing its own separate interests; and |
| | g. | Other parties that can significantly influence the management or operating policies of the transacting parties or that have an ownership interest in one of the transacting parties and can significantly influence the other to an extent that one or more of the transacting parties might be prevented from fully pursuing its own separate interests. |
The financial statements include disclosures of material related party transactions, other than compensation arrangements, expense allowances and other similar items in the ordinary course of business. However, disclosure of transactions that are eliminated in the preparation of financial statements is not required in those statements. The disclosures shall include:
| | a. | The nature of the relationship involved; |
| | b. | A description of the transactions, including transactions to which no amounts or nominal amounts were ascribed for each of the periods for which income statements are presented, and such other information deemed necessary to an understanding of the effects of the transactions on the financial statements; |
| | c. | The dollar amount of transactions for each of the periods for which income statements are presented and the effects of any change in the method of establishing the terms from that used in the preceding period; and |
| | d. | Amounts due from or to related parties as of the date of each balance sheet presented and, if not otherwise apparent, the terms and manner of settlement. |
Fair Value of Financial Instruments. The Company measures its financial and non-financial assets and liabilities, as well as makes related disclosures, in accordance with FASB Accounting Standards Codification No. 820, Fair Value Measurement (“ASC 820”), which provides guidance with respect to valuation techniques to be utilized in the determination of fair value of assets and liabilities. Approaches include, (i) the market approach (comparable market prices), (ii) the income approach (present value of future income or cash flow), and (iii) the cost approach (cost to replace the service capacity of an asset or replacement cost). ASC 820 utilizes a fair value hierarchy that prioritizes the inputs to valuation techniques used to measure fair value into three broad levels. The following is a brief description of those three levels:
Level 1: Observable inputs such as quoted prices (unadjusted) in active markets for identical assets or liabilities.
Level 2: Inputs other than quoted prices that are observable, either directly or indirectly. These include quoted prices for similar assets or liabilities in active markets and quoted prices for identical or similar assets or liabilities in markets that are not active.
Level 3: Unobservable inputs in which little or no market data exists, therefore requiring an entity to develop its own assumptions, such as valuations derived from valuation techniques in which one more significant inputs or significant value drivers are unobservable.
From time to time, our financial instruments include cash, accounts payable and accrued expenses, convertible notes, lines of credit, and credit cards.
Research and Development - Research and development costs are charged to operations as they are incurred. Legal fees and other direct costs incurred in obtaining and protecting patents are also expensed as incurred, due to the uncertainty with respect to future cash flows resulting from the patents. The Company incurred research and development costs of $390,828 and $-0- during the fiscal years ended June 30, 2022 and 2021, respectively.
Basic and Diluted Loss per Common Share - Basic loss per common share excludes dilution and is computed by dividing loss available to common stockholders by the weighted average number of common shares outstanding during the period of computation. Diluted loss per share gives effect to all potential dilutive common shares outstanding during the period of compensation. Diluted income (loss) per share reflects the potential dilution that could occur if securities or other contracts to issue common stock were exercised or converted into common stock or resulted in the issuance of common stock that would then share in the net income of the Company, subject to anti-dilution limitations.
| Schedule of anti-dilutive shares | | | | | | | | | |
| | | Basis of conversion | | Dilution | | 2022 | | 2021 | |
| Series A Convertible | | 688 shares outstanding | | 1 share A: 2 shares | | 1,376 | | 1,376 | |
| Series B Convertible | | 9,938 shares outstanding | | 1 share B: 2 shares | | 19,876 | | 19,876 | |
| Series C Convertible | | 6,838,889 shares outstanding | | 1 share C: 10 shares | | 68,388,890 | | 68,388,890 | |
| Series D Convertible | | 425,000 shares outstanding | | 1 share D: 10 shares | | 4,250,000 | | 4,250,000 | |
| Series E Convertible | | 4,000,000 and 1,900,000 shares outstanding in 2022 and 2021, respectively | | 1 share E: 100 shares | | 400,000,000 | | 190,000,000 | |
| Shares to be issued | | -0- and 100,000 Series E shares to be issued in 2022 and 2021, respectively | | 1 share E: 100 shares | | – | | 10,000,000 | |
| | | -0- and 20,050,000 shares of common stock to be issued in 2022 and 2021, respectively | | | | – | | 20,050,000 | |
| | | | | | | 472,660,142 | | 292,710,142 | |
Because the Company incurred losses for the past two years, the basic and diluted share bases will be presented as the same. For the years ended June 30, 2022 and 2021, the Company incurred losses of ($0.01202) and ($0.00629) per basic share and diluted share, respectively.
Recent Accounting Pronouncements
In February 2016, the Financial Accounting Standards Board (FASB) published the new standard for leasing accounting according to US GAAP (ASU 2016-02 “Leases”; ASC Topic 842). Depending on the company, the new standard is to be applied for the first time for the financial years beginning after December 15, 2018 or after December 15, 2019. According to ASC Topic 842, the lessee has to record a right of use and a leasing liability in his financial statements at the beginning of the transfer of use. The Company adopted the new lease standard during the first quarter of fiscal year 2020. The Company has no leases which meet the criteria.
In December 2019, the FASB issued ASU 2019-12, Income Taxes (Topic 740) – Simplifying the Accounting for Income Taxes. The update is intended to simplify the current rules regarding the accounting for income taxes and addresses several technical topics including accounting for franchise taxes, allocating income taxes between a loss in continuing operations and in other categories such as discontinued operations, reporting income taxes for legal entities that are not subject to income taxes, and interim accounting for enacted changes in tax laws. The new standard is effective for fiscal years beginning after December 15, 2020; however, early adoption is permitted. The Company does not expect the adoption of this standard have a material impact on the consolidated financial statements.
In August 2020, the FASB issued ASU No. 2020-06, Accounting for Convertible Instruments and Contracts in an Entity’s Own Equity (ASU 2020-06), which simplifies the accounting for certain financial instruments with characteristics of liabilities and equity, including convertible instruments and contracts in an entity’s own equity. Among other changes, ASU 2020-06 removes from U.S. GAAP the liability and equity separation model for convertible instruments with a cash conversion feature, and as a result, after adoption, entities will no longer separately present in equity an embedded conversion feature for such debt. Similarly, the embedded conversion feature will no longer be amortized into income as interest expense over the life of the instrument. Instead, entities will account for a convertible debt instrument wholly as debt unless (1) a convertible instrument contains features that require bifurcation as a derivative under ASC Topic 815, Derivatives and Hedging, or (2) a convertible debt instrument was issued at a substantial premium. Among other potential impacts, this change is expected to reduce reported interest expense, increase reported net income, and result in a reclassification of certain conversion feature balance sheet amounts from shareholders’ equity to liabilities as it relates to the Company’s convertible senior notes. Additionally, ASU 2020-06 requires the application of the if-converted method to calculate the impact of convertible instruments on diluted earnings per share (EPS), which is consistent with the Company’s accounting treatment under the current standard. ASU 2020-06 is effective for fiscal years beginning after December 15, 2021, with early adoption permitted for fiscal years beginning after December 15, 2020, and can be adopted on either a fully retrospective or modified retrospective basis. The Company is currently evaluating the timing, method of adoption and overall impact of this standard on its consolidated financial statements.
The Company does not believe other recently issued but not yet effective accounting standards, if currently adopted, would have a material effect on the consolidated financial position, statements of operations and cash flows.
Note 3 – Going Concern
The accompanying consolidated financial statements for the years ended June 30, 2022 and 2021 have been prepared on a going concern basis, which contemplates the realization of assets and the settlement of liabilities and commitments in the normal course of business. As of June 30, 2022, the Company has incurred losses for the last two years, and has an accumulated deficit of $(39,423,382) and ($26,374,227) as of June 30, 2022 and 2021, respectively.
During the year ended June 30, 2022, Company has net cash used in operating activities of $3,107,177 as well as stock compensation non-cash expenses of $10,044,522 and a net loss of $13,049,155. The Company had net cash flow of $2,380,252 from financing activities in the year ended June 30, 2022, which resulted in a negative working capital of $2,133,878 as of June 30, 2022. If the Company is unable to raise additional adequate capital, it could be forced to cease operations.
Management believes that the Company’s capital requirements will depend on many factors, including the success of the Company’s development efforts and its efforts to raise capital. Management also believes the Company needs to raise additional capital for working capital purposes. There can be no assurance that the Company will be able to obtain the additional capital resources necessary to implement its business plan or that any assumptions relating to its business plan will prove accurate.
These factors raise substantial doubt about the Company’s ability to continue as a going concern. The financial statements of the Company do not include any adjustments relating to the recoverability and classification of recorded assets, or the amounts and classifications of liabilities that might be necessary should the Company be unable to continue as a going concern.
Note 4 – Inventory, Prepaid Inventory and Prepaid Expenses
The Company maintains some inventories in house and purchases some of its inventory overseas. Inventories, except for stock in transit are stated at the lower of cost and net realizable value. Stock in transit is valued at cost comprising invoice value plus other charges thereon. Net realizable is the estimated selling price in ordinary course of business less estimated costs of completion and selling expenses. The quantity of inventory may vary from time to time depending on the delivery schedule of overseas shipments. Inventory and prepaid inventory are valued at lower of cost or net realizable value, comprising invoice value plus other charges thereon. Net realizable value is the estimated selling price in the ordinary course of business, less estimated costs of completion and selling expenses. Prepaid expenses are valued at cost.
| Schedule of inventory | | | | | | |
| | | 2022 | | | 2021 | |
| Inventory | | $ | 7,064 | | | $ | – | |
| | | 2022 | | | 2021 | |
| Prepaid inventory | | $ | 62,040 | | | $ | 22,682 | |
| | | 2022 | | | 2021 | |
| Prepaid expenses | | $ | – | | | $ | 10,000 | |
Note 5 – Property, Plant, and Equipment and Boapin
The Company has $20,000 in furnishings, $19,689 in office computers and equipment, and capitalized software development costs of $45,900 which are fully depreciated. On August 30, 2021, the Company purchased its dealer portal for $50,000 for internal use, amortized over 60 months.
As of June 30, 2022 and June 30, 2021, the Company recorded $41,651 and $-0- in net Property, Plant, and Equipment, respectively:
| Schedule of property, plant and equipment | | | | | | | | |
| | | 2022 | | | 2021 | |
| Furniture | | $ | 20,000 | | | $ | 20,000 | |
| Office computers, equipment, software | | | 19,689 | | | | 19,689 | |
| Software development costs | | | 45,900 | | | | 45,900 | |
| Dealer portal | | | 50,000 | | | | – | |
| Property, plant, and equipment | | | 135,589 | | | | 85,589 | |
| Less accumulated depreciation | | | (93,938 | ) | | | (85,589 | ) |
| Net property, plant, and equipment | | $ | 41,651 | | | $ | – | |
Note 6 – Accounts payable and accrued expenses and liabilities
The Company recorded Accounts Payable of $57,940 and $331,876 directly related to operating costs, including credit cards used in operations, as of June 30, 2022 and 2021, respectively.
Accrued expenses and other current liabilities are expenses that have been incurred but not yet paid and primarily include legal fees, audit fees, and other professional fees, as well as interest accrued in connection with credit lines and notes payable. The Company recorded $374,278 and $296,920 in accrued expenses and other current liabilities as of June 30, 2022 and 2021, respectively.
3a10 agreement
On August 17, 2020, the Company entered into a settlement agreement and stipulation (“Settlement Agreement”) with Trillium Partners LP (“Trillium”) in connection with the settlement of $310,494 of bona fide obligations the Company owed to certain of its creditors. The Settlement Agreement was subject to a fairness hearing, and on September 15, 2020, a Federal court in the District of Maryland held a fairness hearing and granted approval of the Settlement Agreement. In October 2020, the Company issued 48,989,000 shares of common stock to Trillium Partners LP to facilitate the 3a10 agreement.
In October 2020, the Company issued 48,989,000 shares of WHSI common stock, to be valued at 60% of the lowest bid price for the common stock on the date of the stock issuance. Subsequently, in April 2021, the agreement was voided for non-participation, and all 48,989,000 shares were returned to treasury.
Note 7 – Notes Payable and Notes Payable - Other
Notes payable consists of line of credit, notes payable incurred by our subsidiary, notes payable-other, convertible notes payable, notes payable for stock purchases under Reg A, and short-term bridge loans, as follows:
Short term bridge loan - COHEN
On July 31, 2020, the Company secured a $500,000 short term bridge loan from an unaffiliated individual (“COHEN”), 12% interest, due and payable October 20, 2020. The loan is currently in default and continues to accrue interest at 12%.
At June 30, 2021, the Company had recorded short term note payable of $500,000, expensed $55,027 in interest and accrued the same in interest liability for the year ended June 30, 2021.
On August 19, 2021, the Company repaid $300,000 of principal. In November 2021, the Company repaid an additional $100,000 in principal.
At June 30, 2022, the Company recorded a short term note payable of $100,000 and expensed $21,649 in interest. The accrued interest payable at June 30, 2022 was $76,677. These notes are included in the Notes payable total on the Company’s balance sheet.
Note payable – stock purchases under Reg A
In March 2021 and June 2021, the Company accepted payments of $115,000 for stock purchases under the Reg A filing from two unaffiliated investors, pending blue sky registrations in two states. The notes mature in one year and bear interest at 5%. The full amount of the note plus interest is convertible at the Reg A fixed price of $0.01, when possible.
At June 30, 2022 and 2021, the Company has recorded $115,000 and $115,000 in notes payable for stock purchases under Reg A, accrued interest of $1,539 and expensed $1,539 in interest, respectively. These notes are included in the Notes payable total on the Company’s balance sheet.
Note Payable – Other
In November 2016, the Company secured a $50,000 loan from a party related to a former CEO of the company, the note bearing 4% interest, maturing after a successful money raise of $1,000,000 through the acquisition of convertible notes payable (See BENZA, D2CF). The $1,000,000 fundraising was never completed, and the Company has been accruing interest on the original principal amount at 4% since inception. The Company has filed suit for damages resulting from the party, and the party has filed a countersuit. There has been no resolution to this situation, and we continue to accrue interest at the face amount.
As of June 30, 2022 and 2021, the Company expensed $2,000 and $2,000 in interest fees and has accrued $11,283 and $9,283 in interest payable, respectively.
Convertible note payable – BENZA, D2CF
On March 1, 2016 and March 3, 2016, the Company closed a private placement of debt and received an aggregate of $612,500 by issuing $13,750 (“B2CF”) and $660,000 (“BENZA”) unsecured convertible notes (“convertible notes”) and warrants to two investors, net of original issue discount of $61,250 per the subscription agreements, maturity at March 1, 2017 and March 3, 2017, respectively, bearing 0% interest and 18% default interest. The notes are currently in default, and all outstanding warrants have expired.
The Company is currently in negotiations to settle the $660,000 BENZA loan with principles in the company, although there has been no settlement to date.
As of June 30, 2022 and 2021, the Company reported $673,750 and $673,750 in convertible notes payable, respectively.
Convertible note payable: Leonite Capital, LLC:
On November 19, 2019, the Company, together with Hypersoft Ventures (collectively, the “Borrower”), received $135,000 on issuing the first tranche of $150,000 (prorated original issue discount of $15,000) of a $250,000 unsecured convertible note (“Leonite Convertible Note”) from Leonite Capital, LLC, a Delaware limited liability company (“Leonite”), net of an aggregate original issue discount of up to $77,778. The Leonite Convertible Note bears annual interest at the Prime Rate plus eight percent (8%), not to exceed twelve percent (12%) per annum, computed on a 365/360 basis, and is due nine months from the date of issuance. The Leonite Convertible Note is convertible into shares of the Company’s common stock at a conversion price equal to $0.02 per share with anti-dilution features. In connection with its purchase of the Leonite Convertible Note, the Company issued to Leonite 2,700,000 shares of common stock, prorated for the initial tranche. On June 4, 2021, the Company and Leonite amended the convertible note to $260,000 which included interest and penalties and extended the due date to June 4, 2023.
The Company has determined that the conversion feature embedded in the Leonite Convertible Note constitutes a derivative and has been bifurcated from the Leonite Convertible Note and recorded as a derivative liability, with a corresponding discount recorded to the associated debt, on the accompanying balance sheet, and revalued to fair market value at each reporting period. The initial issuance yielded a derivative liability of $94,225, with a discount of $150,000 to be amortized over the 9-month life of the Leonite Convertible Note.
Significant assumptions used in calculating fair value of conversion feature of Leonite Convertible Note at issuance date are as follows:
Notes Payable - Schedule of assumptions
Expected Dividends | | Expected volatility | | Risk-free rate of interest | | Expected term (year) | | Exercise (Conversion) price | | Common stock price per share |
| 0.00% | | 809.71% | | 0.0154% | | 0.75 | | $0.02 | | $0.01300 |
On June 4, 2021, the Company and Leonite renegotiated the convertible note for two years, face value of $260,000. At June 30, 2021, the Company recorded $281,845 in derivative liabilities.
On July 29, 2021, Leonite converted $42,750 in debt plus $2,250 in fees to 15,000,000 shares, and on August 27, 2021, Leonite converted $44,475 and $2,250 in fees to 10,269,253 shares.
On October 6, 2021, Leonite converted $57,952 in debt and interest plus $2,250 in fees to 13,231,209 shares, and on October 26, 2021, Leonite converted the remaining balance of $125,000 in debt and interest, and $2,250 in fees, to 27,917,969 shares, collectively resulting in a $96,145 gain on debt extinguishment.
| Schedule of extinguishment of debt | | | |
| Balance at June 30, 2021 | | $ | 260,000 | |
| Accrued interest | | | 9,954 | |
| Leonite Convertible Note converted | | | (269,954 | ) |
| Total | | | – | |
| Less: debt discount | | | – | |
| Balance at June 30, 2022 | | $ | – | |
The resulting derivative valuation is calculated as follows:
| Schedule of derivative liabilities at fair value | | | |
| Derivative as of June 30, 2021 | | $ | 281,845 | |
| Change in fair value | | | 213,053 | |
| | | | 494,898 | |
| Write off due to conversions | | | (398,753 | ) |
| | | | 96,145 | |
| Gain on extinguishment | | | (96,145 | ) |
| Derivative as of June 30, 2022 | | $ | – | |
Significant assumptions used in calculating fair value of conversion feature of Leonite convertible Note as of June 30, 2022 are as follows.
Note Payable - Schedule of assumptions
Expected Dividends | | Expected volatility | | Risk-free rate of interest | | Expected term (year) | | Exercise (Conversion) price | | Common stock price per share |
| 0.00% | | 269.75% | | 0.0007% | | 1.9288 | | $0.00550 | | $0.010 |
As of June 30, 2022 and June 30, 2021, the Company has recorded $-0- and $260,000 in Convertible Note: Leonite, respectively.
Credit line – MediPendant New York Inc.
On September 30, 2014, the subsidiary received a line of credit with Medi Pendant New York, Inc. (“MNY). Under the original terms of the line of credit agreement, the Company was able to borrow up to $500,000 with the rate of interest of 6.5% per annum. The maturity date of the line of credit was September 30, 2017 with a one-year extension to September 30, 2018. On January 31, 2015, the limit on the line of credit was increased to $500,000 with the same interest rate and due date, in consideration of the Company’s issuance of 200,000 shares of common stock to one of the owners of MNY, which was memorialized on October 19, 2015. Interest accrued of $25,653 and $8,436 were accrued as of June 30, 2016 and 2015, respectively. As of June 30, 2022 and 2021, the balance due on the line of credit was of $397,500 and $397,500, respectively.
Notes Payable - subsidiary
The Company has various loans and credit lines outstanding. The credit line carries an interest rate of 16.24%. The bank loans carry interest rates varying between 9.24% – 10.90%.
| Schedule of notes payable | | | | | | | | |
| | | 2022 | | | 2021 | |
| Wells Fargo Loan | | $ | 8,770 | | | $ | 12,454 | |
| On Deck Loan | | | 139,569 | | | | 139,569 | |
| Susquehanna Salt Loan | | | 10,500 | | | | 52,500 | |
| Prosper Loans | | | 9,994 | | | | 17,771 | |
| MARCUS Loan | | | 5,410 | | | | 15,950 | |
| Notes payable - subsidiary | | | 174,243 | | | | 238,244 | |
| Loans – See Stock purchases under Reg A and short-term bridge loans | | | 238,856 | | | | 615,000 | |
| TOTAL LOANS | | $ | 413,099 | | | $ | 853,244 | |
As of June 30, 2022 and 2021, the Company has outstanding $413,099 and $853,244 in bank loans and credit lines payable, respectively.
Notes Payable - SBA
The Company obtained SBA loans through pandemic offerings in 2020. As of March 2, 2021, the loans were forgiven, and the Company recorded gain on debt forgiveness of $81,000.
Debt settlement – On Deck, Susquehanna, MCA Cure
In 2019, our subsidiary engaged MCA CURE to negotiate settlements with two creditors: On Deck and Susquehanna Salt, noted in the table above. The Company ceased paying the loan payments and paid to MCA Cure $43,875 in 2019 and $47,000 in 2020, at which point the Company was contacted and MCA Cure assured they had enough funds to negotiate with the creditors. In 2020, the Company discovered MCA Cure had not performed when bank accounts were levied for $33,705 by the creditors. $18,705 was subsequently refunded by the collection firm. On September 30, 2020, the bank accounts were again levied for additional funds. Currently the Company has a settlement agreement in place with Susquehanna Salt Loan, and has booked a reserve against the $90,875 funds paid to MCA Cure. The Company has hired an attorney and is making every effort to recover funds and damages from MCA Cure. To date, there has been no resolution to the situation. As of June 30, 2022 and 2021, the Company recorded $-0- and $-0- in prepaid fund to MCA Cure, and $139,569 and $139,569 in indebtedness to On Deck. The Company negotiated a settlement with Susquehanna Salt for the loan balance, and as of June 30, 2022 and 2021, the Company recorded indebtedness to Susquehanna Salt of $10,500 and $52,500, respectively.
Note 8 – Shareholders’ (Deficit)
Preferred Stock:
Series A Convertible Preferred Stock: The Company is currently authorized to issue up to 100,000 shares of Series A Convertible Preferred Stock, par value $0.0001 per share, convertible at a ratio of 1 share of Series A Convertible Preferred Stock for 2 shares of common stock. These shares have no voting rights. As of June 30, 2022 and 2021, 688 shares of Series A Convertible Preferred Stock were issued and outstanding, respectively.
Series B Convertible Preferred Stock: The Company is currently authorized to issue up to 62,500 shares of Series B Convertible Preferred Stock, par value $0.0001 per share, convertible at a ratio of 1 share of Series B Convertible Preferred Stock for 2 shares of common stock. These shares have voting rights and vote on an “as converted” basis in actions required to have Series B Preferred Stockholder approval. As of June 30, 2022 and 2021, 9,938 shares of Series B Convertible Preferred Stock were issued and outstanding, respectively.
Series C Convertible Preferred Stock: The Company is currently authorized to issue up to 6,944,445 shares of Series C Convertible Preferred Stock, par value $0.0001 per share, convertible at a ratio of 1 share of Series C Convertible Preferred Stock for 10 shares common stock. These shares have voting rights and vote on an “as converted” basis on all matters submitted to our Stockholders for approval.
The Company issued 6,700,003 shares for the BOAPIN asset purchase; these shares were issued on September 1, 2020. As of June 30, 2022 and 2021, 6,838,889 shares of Series C Convertible Preferred Stock were issued and outstanding, respectively.
Series D Convertible Preferred Stock: The Company is currently authorized to issue up to 500,000 shares of Series D Convertible Preferred Stock, par value $0.0001 per share, convertible at a ratio of 1 share of Series D Convertible Preferred stock for 10 shares of common stock. These shares have voting rights and vote on an “as converted” basis in actions required to have Series D Preferred Stockholder approval. As of June 30, 2022 and 2021, 425,000 shares of Series D Convertible Preferred Stock were issued and outstanding, respectively.
Series E Convertible Preferred Stock: The Company is currently authorized to issue up to 4,000,000 shares of Series E Convertible Preferred Stock, par value $0.0001 per share, convertible at a ratio of 1 share of Series E Convertible Preferred Stock for 100 shares of common stock. Each of these shares carries a voting right equivalent to 10,000 shares of common stock. The Company may not issue any other shares with extended voting rights.
In November 2019, 4,000,000 shares were sold in a private transaction, along with 200,000,000 shares of common stock and $53,000 in notes payable which were forgiven, affecting a change in control. The Company accrued 1,000,000 shares of Series E Convertible Preferred Stock to be issued to directors and expensed $650,000 or $.65 per share, based on the conversion of one share of Series E Convertible Preferred Stock for 100 shares of common stock and the price of the stock on the date of award.
During the year ended June 30, 2021, as part of the change in control, 4,000,000 shares were returned to treasury to be canceled. In December 2020 the Company issued 1,000,000 shares of Series E Convertible Preferred Stock accrued in the prior year and issued 450,000 shares of Series E Convertible Preferred Stock to each of its two directors, 900,000 shares total, valued at $513,000 or $0.57 per share, accrued 100,000 shares of Series E Preferred stock to be issued to directors for services, valued at $57,000 or $.57 per share, all pricing based on the conversion of one share of Series E Convertible Preferred Stock for 100 shares of common stock and the price of the common stock on the date of accrual. During the year ended June 30, 2022, the Company issued 1,000,000 shares of Series E Convertible Preferred shares to each of its two directors for services, valued at $3,000,000 or $1.50 per share, and issued 50,000 shares to each of its two directors, previously accrued for at $57,000 or $0.57 per share. All shares were recorded at the quoted common stock price of the date of agreement or grant on an as-converted basis.
As of June 30, 2022 and 2021, 4,000,000 and 1,900,000 shares of Series E Convertible Preferred Stock were issued and outstanding, respectively.
Common Stock:
The Company is currently authorized to issue 3,000,000,000 shares of common stock, par value of $0.0001 per share, and 25,000,000 shares of preferred stock, par value of $0.0001 per share.
During the period ended June 30, 2021, the Company
| · | | issued 200,000,000 shares of common stock to its directors for services, valued at $1,140,000 or $0.0057 per share, |
| · | | accrued 150,000 shares of common stock for officer bonuses, valued at $1,500 or $0.01 per share, |
| · | | issued 1,275,000 shares to its officers for compensation and bonus, valued at $8,518, |
| · | | issued 3,000,000 shares of common stock for services, valued at $48,300 or $0.0161 per share, |
| · | | sold 145,400,000 shares of common stock to unaffiliated individuals and groups at $0.01 under the Reg A filing, and |
| · | | accrued 10,000,000 shares of common stock sold to an unaffiliated party for $100,000 or $.01 per share, under the Reg A offering. |
All shares were recorded at the stock price of the date of agreement or grant.
During the period ended June 30, 2022, the Company
| · | | issued a total of 409,650,000 shares of common stock for a total value of $6,556,845 resulting in an average price of $0.016 per share, |
| · | | issued 66,418,431 for $260,000 in debt, $9,954 in interest, and $9,000 in fees, valued at $677,707, which represents the fair value of shares issued, |
| · | | issued 10,000,000 shares of common stock for services, valued at $48,300 or $0.0161 per share, |
| · | | sold 360,000,000 shares of common stock to unaffiliated individuals and groups at $0.01 under the Reg A filing, of which 10,000,000 are yet to be issued, |
| · | | accrued 7,262,500 shares of common stock for officer’s compensation and bonuses, valued at $104,126 or $0.0143 shares, and |
| · | | accrued 6,840,000 shares of common stock for employee compensation and bonuses, valued at $88,551 or $0.0129 shares. |
All shares were recorded at the quoted stock price of the date of agreement or grant.
As of June 30, 2022 and 2021, the Company had 1,493,142,608 and 647,074,177 shares of common stock issued and outstanding, respectively.
Note 9 – Related Party Transactions
Note payable – BOAPIN purchase
In August 2020, and effective as of June 30, 2020, the Company purchased the BOAPIN portal, including all software, licensing, and ownership rights from Hypersoft Ventures, Inc., a related party through common ownership, for $800,200, which includes six million seven hundred thousand three (6,700,003) shares of Series C Convertible Preferred stock, valued at $375,200 or $0.056 per share, based on the conversion of one share of Series C Preferred stock for 10 shares of common stock and the stock price on the date of the transaction, and a note payable for $425,000, bearing eight percent (8%) interest with no prepayment or delinquency clauses.
As of June 30, 2022 and 2021, the Company recorded Note payable-BOAPIN of $170,000 and $425,000, accrued interest of $49,415 and $28,225, and expensed $21,190 and $28,225 in interest, respectively.
Related party debt, net
From time to time, the Company received funds from related parties for day-to-day operations. These are short-term loans which bear no interest, and the Company expects to repay these loans by the end of the fiscal year following the year in which the short-term loan was made. At June 30, 2022, the Company had a receivable from certain management employees totaling $155,800. The total receivable balance was subsequently collected by the Company on September 27, 2022.
| Schedule of related party transactions | | | | | | | | |
| | | 2022 | | | 2021 | |
| Related parties – subsidiary | | $ | 202,875 | | | $ | 263,473 | |
| Due from related parties | | | (155,800 | ) | | | – | |
| Accrued salaries, bonus, fees | | | 10,965 | | | | 316,200 | |
| Total loans from related parties, net | | $ | 58,040 | | | $ | 579,673 | |
Note 10 – Commitments and contingencies
Legal Matters
From time to time, we may become involved in various lawsuits and legal proceedings which arise in the ordinary course of business. Litigation is subject to inherent uncertainties, and an adverse result in these or other matters may arise from time to time that may harm our business.
| | 1) | Wearable Health Solutions, Inc. v. Barry Honig, GRQ Consultants Inc., Benza Pharma LLC and John Does 1-10, Supreme Court of the State of New York County of New York, July 22, 2021. Company is disputing the validity of Notes from 3/2016 and seeking damages, reparations, and related costs. |
| | 2) | GRQ Consultants, Inc. v. Wearable Health Solutions, Inc., Supreme Court of the State of New York, County of New York, August 26, 2021, Parties are seeking summary judgment of $50,000 plus accrued interest in response to lawsuit by Company regarding $50,000 loan from 11/2016. |
| | 3) | Benza Pharma LLC, Sandor Capital, LP, and John Lema v. Wearable Health Solutions, Inc., District Court, Clark County, Nevada, Parties are seeking summary judgment of $3,000,000plus accrued interest in regards to convertible notes payable from March, 2016. |
| | 4) | Medical Alarm Concepts LLC v. MCA Cure, LLC, Superior Court of New Jersey, Law Division, Morris County. Company is seeking return of payments for non-performance plus attorney fees and court costs. A settlement was reached between the parties whereby MCA Cure will pay an initial $10,000 and $6,500 each month until debt is satisfied in 2023 (See Note 11). |
Commitments and Contingencies. The Company follows subtopic 450-20 of the FASB Accounting Standards Codification to report accounting for contingencies. Certain conditions may exist as of the date the financial statements are issued, which may result in a loss to the Company but which will only be resolved when one or more future events occur or fail to occur. The Company assesses such contingent liabilities, and such assessment inherently involves an exercise of judgment. In assessing loss contingencies related to legal proceedings that are pending against the Company or unasserted claims that may result in such proceedings, the Company evaluates the perceived merits of any legal proceedings or unasserted claims as well as the perceived merits of the amount of relief sought or expected to be sought therein.
If the assessment of a contingency indicates that it is probable that a material loss has been incurred and the amount of the liability can be estimated, then the estimated liability would be accrued in the Company’s financial statements. If the assessment indicates that a potentially material loss contingency is not probable but is reasonably possible, or is probable but cannot be estimated, then the nature of the contingent liability, and an estimate of the range of possible losses, if determinable and material, would be disclosed. Loss contingencies considered remote are generally not disclosed unless they involve guarantees, in which case the guarantees would be disclosed. Management does not believe, based upon information available at this time that these matters will have a material adverse effect on the Company’s financial position, results of operations or cash flows. However, there is no assurance that such matters will not materially and adversely affect the Company’s business, financial position, and results of operations or cash flows.
Office lease
The Company maintains its corporate office at 2901 W. Coast Highway, Suite 200, Newport Beach, CA 92663. The Company currently pays $175 a month for its office space and the term is month-to-month. The Company’s subsidiary maintains a warehouse office in Pennsylvania to facilitate inventory arrival and product shipment. The 3 year lease at $1,100 per month expired on September 30, 2021, and was renewed for 12 months at $1,300 per month beginning October 1, 2021 and expiring on September 30, 2022. The subsidiary is currently in a month-to-month arrangement with this office warehouse. Expenditures for the years ending June 30, 2022 and 2021 are as follows:
| Schedule lease cost | | | | | | |
| | | 2022 | | | 2021 | |
| Rent expense | | $ | 15,700 | | | $ | 15,325 | |
Note 11 – Other income - settlement
Settlement
In 2019, the Company engaged MCA Cure to negotiate settlements with two note holders, and paid MCA Cure a total of $97,625. In 2020, the Company discovered MCA Cure had not performed when bank accounts were levied for $33,705 and $18,705, being subsequently refunded, and engaged an attorney to recover funds. Currently the Company has a settlement agreement in place with Susquehanna Salt Loan and has hired an attorney to recover funds and damages from MCA Cure. In February 2022, a settlement was reached with MCA Cure for fees and attorney costs of $105,125, amortized at 1.5%, by which the Company would receive an initial payment of $10,000, and $6,500 monthly until the debt is satisfied in May, 2023, with stipulations for any potential default.
As of June 30, 2022 and 2021, the Company has recorded $36,000 and $-0- to other income, respectively.
Note 12 – Income Taxes
Deferred income tax assets and liabilities are computed annually for differences between financial statement and tax bases of assets and liabilities that will result in taxable or deductible amounts in the future based on enacted tax laws and rates applicable to the periods in which the differences are expected to affect taxable income. Valuation allowances are established when necessary to reduce deferred tax assets to the amount expected to be realized. Income tax expense is the tax payable or refundable for the period plus or minus the change during the period in deferred tax assets and liabilities.
The effective tax rate on the net loss before income taxes differs from the U.S. statutory rate as follows:
| Schedule of effective tax rate | | | | | | | | |
| | | June 30, 2022 | | | June 30, 2021 | |
| U.S. statutory rate | | | 21.00% | | | | 21.00% | |
| PA state corporate tax | | | 9.99% | | | | 9.99% | |
| Less valuation allowance | | | (30.99% | ) | | | (30.99% | ) |
| Effective tax rate | | | 0% | | | | 0% | |
The significant components of deferred tax assets and liabilities are as follows, expiring in 2023 and 2024, on net operating losses of $39,423,382 and $26,903,013 for fiscal years ended June 30, 2022 and 2021, respectively:
| Schedule of deferred tax asset | | | | | | | | |
| | | 2022 | | | 2021 | |
| Net deferred tax assets | | $ | 12,217,306 | | | $ | 8,337,244 | |
| Less valuation allowance | | | (12,217,306 | ) | | | (8,337,244 | ) |
| Deferred tax asset - net valuation allowance | | $ | – | | | $ | – | |
Uncertain Tax Positions. The Company did not take any uncertain tax positions and had no adjustments to unrecognized income tax liabilities or benefits pursuant to the provisions of Section 740-10-25 for the years ended June 30, 2022 or 2021.
Note 13 – Subsequent Events
Common Stock
During the period July 1, 2022 through September 30, 2022, the Company issued a total of 38,450,000 shares of common stock. The Company issued 11,950,000 shares for compensation to employees and officers valued at $167,263; 5,000,000 shares for services valued at $89,000; and, 21,500,000 shares for cash valued at $215,000. At September 30, 2022, the Company had 1,531,592,608 shares of common stock issued and outstanding. Shares for services and compensation were valued using the stock price on the date of the transactions.
During August and September 2022, the Company received proceeds of $604,500 in connection with the issuance of 67,950,000 shares of common stock.
On September 27, 2022, the Company received payments totaling $155,800 from certain management employees and as a result, the Company no longer has a receivable balance from any of its management employees as of the filing date of this Annual Report on Form 10-K.
WEARABLE HEALTH SOLUTIONS, INC.
TABLE OF CONTENTS
Wearable Healthcare Solutions, Inc.
Consolidated Balance Sheets
As at March 31, 2023 and June 30, 2022
| | | | | | | |
| | | March 31, 2023 | | | June 30, 2022 | |
| | | (unaudited) | | | | |
| ASSETS | | | | | | | | |
| Current Assets | | | | | | | | |
| Cash and cash equivalents | | $ | 16,341 | | | $ | 70,505 | |
| Accounts receivable, net | | | 812 | | | | – | |
| Due from related parties (See Footnote 11) | | | – | | | | 155,800 | |
| Accounts receivable, other | | | 2,000 | | | | 2,000 | |
| Inventory | | | 33,281 | | | | 7,064 | |
| Prepaid inventory | | | – | | | | 62,040 | |
| Total Current Assets | | | 52,434 | | | | 297,409 | |
| | | | | | | | | |
| Property and Equipment | | | | | | | | |
| Property and equipment (net of $17,466 and $8,349 depreciation, as of March 31, 2023 and June 30, 2022, respectively) | | | 41,976 | | | | 41,651 | |
| Total Property and Equipment | | | 41,976 | | | | 41,651 | |
| Right-of-use assets | | | 35,505 | | | | – | |
| | | | | | | | | |
| Total Assets | | $ | 129,915 | | | $ | 339,060 | |
| | | | | | | | | |
| LIABILITIES and SHAREHOLDERS' DEFICIT | | | | | | | | |
| | | | | | | | | |
| COMMITMENTS AND CONTINGENCIES (Note 12) | | | – | | | | – | |
| | | | | | | | | |
| Current liabilities | | | | | | | | |
| Accounts payable | | $ | 66,081 | | | $ | 57,940 | |
| Accrued expenses and other current liabilities | | | 446,650 | | | | 374,278 | |
| Short-term lease liability | | | 13,643 | | | | – | |
| Related party debt, net | | | 508,252 | | | | 213,840 | |
| Deferred revenue | | | 61,473 | | | | 80,880 | |
| Line of credit | | | 397,500 | | | | 397,500 | |
| Notes payable | | | 397,189 | | | | 413,099 | |
| Note payable - other | | | 50,000 | | | | 50,000 | |
| Note payable - related party | | | 170,000 | | | | 170,000 | |
| Convertible notes – Leonite, net of debt discount of $46,371 and $-0-, respectively, and deferred debt issuance costs of $44,469 and $-0-, respectively | | | 221,660 | | | | – | |
| Convertible notes - other | | | 673,750 | | | | 673,750 | |
| Total current liabilities | | | 3,006,198 | | | | 2,431,287 | |
| Long-term lease liability | | | 22,107 | | | | – | |
| | | | | | | | | |
| TOTAL LIABILITIES | | | 3,028,305 | | | | 2,431,287 | |
| | | | | | | | | |
| SHAREHOLDERS’ DEFICIT | | | | | | | | |
| Preferred stock - 25,000,000 Shares Authorized, par value $0.0001 | | | | | | | | |
| Series A Convertible Preferred Stock: $0.0001 par value; 100,000 shares authorized, 688 shares issued and outstanding as of March 31, 2023 and June 30, 2022, respectively | | $ | 1 | | | $ | 1 | |
| Series B Convertible Preferred Stock: $0.0001 par value; 62,500 shares authorized, 9,938 shares issued and outstanding as of March 31, 2023 and June 30, 2022, respectively | | | 1 | | | | 1 | |
| Series C Preferred Stock: $0.0001 par value; 6,944,445 authorized, 6,838,889 shares issued and outstanding as of March 31, 2023 and June 30, 2022, respectively | | | 684 | | | | 684 | |
| Series D Preferred Stock: $0.0001 par value; 500,000 shares authorized, 425,000 shares issued and outstanding as of March 31, 2023 and June 30, 2022, respectively | | | 43 | | | | 43 | |
Series E Preferred Stock $0.0001 par value, 4,000,000 shares designated, 4,000,000 shares issued and outstanding as of March 31, 2023 and June 30, 2022, respectively
| | | 400 | | | | 400 | |
| | | | | | | | | |
| Common stock | | | | | | | | |
| Common Stock: $0.0001 par value; 3,000,000,000 shares authorized,1,549,255,108 and 1,493,142,608 shares issued and outstanding as of March 31, 2023 and June 30, 2022, respectively | | | 154,926 | | | | 149,314 | |
| Common stock to be issued (98,242,500 and 35,602,500 shares as of March 31, 2023 and June 30, 2022, respectively) | | | 814,555 | | | | 407,677 | |
| Additional paid-in capital | | | 37,365,081 | | | | 36,773,035 | |
| Accumulated deficit | | | (41,234,081 | ) | | | (39,423,382 | ) |
| Total Shareholders’ Deficit | | | (2,898,390 | ) | | | (2,092,227 | ) |
| Total Liabilities and Shareholders’ Deficit | | $ | 129,915 | | | $ | 339,060 | |
The accompanying footnotes are an integral part of these unaudited consolidated financial statements.
Wearable Healthcare Solutions, Inc.
Consolidated Statements of Operations
For the Three and Nine Months Ended March 31, 2023 and 2022 (unaudited)
| | | | | | | | | | | | | |
| | | For the Three Months Ended | | | For the Nine Months Ended | |
| | | March 31, 2023 | | | March 31, 2022 | | | March 31, 2023 | | | March 31, 2022 | |
| Revenue | | $ | 193,745 | | | $ | 212,982 | | | $ | 608,244 | | | $ | 819,601 | |
| Cost of sales | | | (135,207 | ) | | | (111,434 | ) | | | (354,627 | ) | | | (457,307 | ) |
| Gross profit | | | 58,538 | | | | 101,548 | | | | 253,617 | | | | 362,294 | |
| | | | | | | | | | | | | | | | | |
| Operating expenses | | | | | | | | | | | | | | | | |
| Selling expense | | | 33,784 | | | | 44,346 | | | | 304,066 | | | | 318,708 | |
| Depreciation | | | 3,286 | | | | 2,500 | | | | 9,117 | | | | 5,849 | |
| Research and development expense | | | – | | | | 209,800 | | | | 2,180 | | | | 374,484 | |
| Consulting and professional fees | | | 19,528 | | | | 106,624 | | | | 127,347 | | | | 462,998 | |
| Insurance | | | 35,557 | | | | 28,124 | | | | 92,075 | | | | 50,507 | |
| Rent | | | 4,080 | | | | 4,050 | | | | 16,270 | | | | 12,555 | |
| Salaries and wages | | | 358,386 | | | | 6,974,610 | | | | 1,215,671 | | | | 10,904,649 | |
| Software expense | | | – | | | | 24,500 | | | | – | | | | 22,887 | |
| General and administrative | | | 83,559 | | | | 89,084 | | | | 245,985 | | | | 295,659 | |
| Total Operating expenses | | | 538,180 | | | | 7,483,638 | | | | 2,012,711 | | | | 12,448,296 | |
| | | | | | | | | | | | | | | | | |
| Loss from operations | | | (479,642 | ) | | | (7,382,090 | ) | | | (1,759,094 | ) | | | (12,086,002 | ) |
| | | | | | | | | | | | | | | | | |
| Other income / (expense), net | | | | | | | | | | | | | | | | |
| Change in fair value of derivative instrument | | | – | | | | – | | | | – | | | | (213,053 | ) |
| Gain on debt extinguishment | | | – | | | | – | | | | – | | | | 96,145 | |
| Gain on settlement of accounts payable | | | – | | | | – | | | | – | | | | 156,616 | |
| Other income | | | – | | | | 23,000 | | | | 19,500 | | | | 23,000 | |
| Interest income | | | – | | | | – | | | | 1,500 | | | | – | |
| Interest expense | | | (42,499 | ) | | | (8,493 | ) | | | (72,605 | ) | | | (68,009 | ) |
| Total other income (expense), net | | | (42,499 | ) | | | 14,507 | | | | (51,605 | ) | | | (5,301 | ) |
| Net loss before taxes | | | (522,141 | ) | | | (7,367,583 | ) | | | (1,810,699 | ) | | | (12,091,303 | ) |
| Income tax | | | – | | | | – | | | | – | | | | – | |
| Net loss | | $ | (522,141 | ) | | $ | (7,367,583 | ) | | $ | (1,810,699 | ) | | $ | (12,091,303 | ) |
| | | | | | | | | | | | | | | | | |
| Net loss per common share - Basic and Diluted | | $ | (0.00034 | ) | | $ | (0.00731 | ) | | $ | (0.00118 | ) | | $ | (0.01487 | ) |
| | | | | | | | | | | | | | | | | |
| Weighted average common shares outstanding - Basic & Diluted | | | 1,536,378,441 | | | | 1,008,459,767 | | | | 1,528,530,473 | | | | 813,299,239 | |
The accompanying footnotes are an integral part of these unaudited consolidated financial statements.
Wearable Healthcare Solutions, Inc.
Consolidated Statements of Shareholders’ Deficit
For the Three and Nine Months Ended March 31, 2023 and 2022 (unaudited)
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Series A | | | Series B | | | Series C | | | Series C to be issued | |
| | | Shares | | | Amount | | | Shares | | | Amount | | | Shares | | | Amount | | | Shares | | | Amount | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| As at June 30, 2021 | | | 688 | | | $ | 1 | | | | 9,938 | | | $ | 1 | | | | 6,838,889 | | | $ | 684 | | | | – | | | $ | – | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Loss for the period | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Common stock for compensation | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Common stock for debt conversion | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Common stock for officer compensation | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Preferred stock for compensation | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Shares sold for cash | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| | �� | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| As at September 30, 2021 | | | 688 | | | $ | 1 | | | | 9,938 | | | $ | 1 | | | | 6,838,889 | | | $ | 684 | | | | – | | | $ | – | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Loss for the period | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Common stock for services | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Common stock for compensation | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Common stock for debt conversion | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Preferred stock for compensation | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Subscription receivable | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Shares sold for cash | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| As at December 31, 2021 | | | 688 | | | $ | 1 | | | | 9,938 | | | $ | 1 | | | | 6,838,889 | | | $ | 684 | | | | – | | | $ | – | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Loss for the period | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Common stock for services | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Common stock for compensation | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Common stock for debt conversion | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Preferred stock for compensation | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Subscription receivable | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Shares sold for cash | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| As at March 31, 2022 | | | 688 | | | $ | 1 | | | | 9,938 | | | $ | 1 | | | | 6,838,889 | | | $ | 684 | | | | – | | | $ | – | |
The accompanying footnotes are an integral part of these unaudited consolidated financial statements.
Wearable Healthcare Solutions, Inc.
Consolidated Statements of Shareholders’ Deficit
For the Three and Nine Months Ended March 31, 2023 and 2022 (unaudited)
(continued)
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Series A | | | Series B | | | Series C | | | Series C to be issued | |
| | | Shares | | | Amount | | | Shares | | | Amount | | | Shares | | | Amount | | | Shares | | | Amount | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| As at June 30, 2022 | | | 688 | | | $ | 1 | | | | 9,938 | | | $ | 1 | | | | 6,838,889 | | | $ | 684 | | | | – | | | $ | – | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Loss for the period | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Common stock for compensation | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
Common stock to be issued for
compensation | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Common stock for officer compensation | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
Common stock to be issued for
officer compensation | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Shares issued for services | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Shares sold for cash – prior quarter | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Shares sold for cash – current quarter | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Payment of subscription receivable | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| As at September 30, 2022 | | | 688 | | | $ | 1 | | | | 9,938 | | | $ | 1 | | | | 6,838,889 | | | $ | 684 | | | | – | | | $ | – | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Loss for the period | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Common stock for compensation | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
Common stock to be issued for
compensation | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Common stock for officer compensation | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
Common stock to be issued for
officer compensation | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Shares issued for services | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Shares sold for cash – prior quarter | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Shares sold for cash – current quarter | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Payment of subscription receivable | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| As at December 31, 2022 | | | 688 | | | $ | 1 | | | | 9,938 | | | $ | 1 | | | | 6,838,889 | | | $ | 684 | | | | – | | | $ | – | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Loss for the period | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Common stock for compensation | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Common stock to be issued compensation | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Common stock for officer compensation | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Common stock to be issued for officer compensation | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Shares issued for services | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Shares sold for cash – prior quarter | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Shares sold for cash – current quarter | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Payment of subscription receivable | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| As at March 31, 2023 | | | 688 | | | $ | 1 | | | | 9,938 | | | $ | 1 | | | | 6,838,889 | | | $ | 684 | | | | – | | | $ | – | |
The accompanying footnotes are an integral part of these unaudited consolidated financial statements.
Wearable Healthcare Solutions, Inc.
Consolidated Statements of Shareholders’ Deficit
For the Three and Nine Months Ended March 31, 2023 and 2022 (unaudited)
(continued)
| | | | | | | | | | | | | | | | | | | |
| | | Series D | | | Series E | | | Series E to be issued | |
| | | Shares | | | Amount | | | Shares | | | Amount | | | Shares | | | Amount | |
| | | | | | | | | | | | | | | | | | | |
| As at June 30, 2021 | | | 425,000 | | | $ | 43 | | | | 1,900,000 | | | $ | 190 | | | | 100,000 | | | $ | 57,000 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Loss for the period | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Common stock for compensation | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Common stock for debt conversion | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Common stock for officer compensation | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Preferred stock for compensation | | | – | | | | – | | | | 2,000,000 | | | | 200 | | | | – | | | | – | |
| Shares sold for cash | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| As at September 30, 2021 | | | 425,000 | | | $ | 43 | | | | 3,900,000 | | | $ | 390 | | | | 100,000 | | | $ | 57,000 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Loss for the period | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Common stock for services | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Common stock for compensation | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Common stock for debt conversion | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Preferred stock for compensation | | | – | | | | – | | | | 100,000 | | | | 10 | | | | (100,000 | ) | | | (57,000 | ) |
| Subscriptions receivable | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Shares sold for cash | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| As at December 31, 2021 | | | 425,000 | | | $ | 43 | | | | 4,000,000 | | | $ | 400 | | | | – | | | $ | – | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Loss for the period | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Common stock for services | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Common stock for compensation | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Common stock for debt conversion | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Preferred stock for compensation | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Subscriptions receivable | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Shares sold for cash | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| As at March 31, 2022 | | | 425,000 | | | $ | 43 | | | | 4,000,000 | | | $ | 400 | | | | – | | | $ | – | |
The accompanying footnotes are an integral part of these unaudited consolidated financial statements.
Wearable Healthcare Solutions, Inc.
Consolidated Statements of Shareholders’ Deficit
For the Three and Nine Months Ended March 31, 2023 and 2022 (unaudited)
(continued)
| | | | | | | | | | | | | | | | | | | |
| | | Series D | | | Series E | | | Series E to be issued | |
| | | Shares | | | Amount | | | Shares | | | Amount | | | Shares | | | Amount | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| As at June 30, 2022 | | | 425,000 | | | $ | 43 | | | | 4,000,000 | | | $ | 400 | | | | – | | | $ | – | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Loss for the period | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Common stock for compensation | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Common stock to be issued for compensation | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Common stock for officer compensation | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Common stock to be issued for officer compensation | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Shares issued for services | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Shares sold for cash – prior quarter | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Shares sold for cash – current quarter | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Payment of subscription receivable | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| As at September 30, 2022 | | | 425,000 | | | $ | 43 | | | | 4,000,000 | | | $ | 400 | | | | – | | | $ | – | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Loss for the period | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Common stock for compensation | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Common stock to be issued for compensation | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Common stock for officer compensation | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Common stock to be issued for officer compensation | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Shares issued for services | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Commitment shares – Leonite convertible note | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Shares sold for cash – prior quarter | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Shares sold for cash – current quarter | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Payment of subscription receivable | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| As at December 31, 2022 | | | 425,000 | | | $ | 43 | | | | 4,000,000 | | | $ | 400 | | | | – | | | $ | – | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Loss for the period | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Common stock for compensation | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Common stock to be issued for compensation | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Common stock for officer compensation | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Common stock to be issued for officer compensation | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Shares issued for services | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Commitment shares – Leonite convertible note | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Shares sold for cash – prior quarter | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Shares sold for cash – current quarter | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Payment of subscription receivable | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| As at March 31, 2023 | | | 425,000 | | | $ | 43 | | | | 4,000,000 | | | $ | 400 | | | | – | | | $ | – | |
The accompanying footnotes are an integral part of these unaudited consolidated financial statements.
Wearable Healthcare Solutions, Inc.
Consolidated Statements of Shareholders’ Deficit
For the Three and Nine Months Ended March 31, 2023 and 2022 (unaudited)
(continued)
| | | | | | | | | | | | | | | | | | | | | | |
| | | Common Stock | | | Common Stock to be issued | | | Additional Paid in Capital | | | Accumulated Profit/Deficit | | | Total | |
| | | Shares | | | Amount | | | Shares | | | Amount | | | Amount | | | Shares | | | Amount | |
| | | | | | | | | | | | | | | | | | | | | | |
| As at June 30, 2021 | | | 647,074,177 | | | $ | 64,708 | | | | 20,050,000 | | | $ | 169,005 | | | $ | 22,732,295 | | | $ | (26,374,227 | ) | | $ | (3,350,300 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Loss for the period | | | – | | | | – | | | | – | | | | – | | | | – | | | | (4,354,735 | ) | | | (4,354,735 | ) |
| Common stock for compensation | | | 7,000,000 | | | | 700 | | | | 5,000,000 | | | | 60,000 | | | | 74,300 | | | | – | | | | 135,000 | |
| Common stock for debt conversion | | | 25,269,253 | | | | 2,527 | | | | – | | | | – | | | | 250,166 | | | | – | | | | 252,693 | |
| Common stock for officer compensation | | | – | | | | – | | | | 225,000 | | | | 2,498 | | | | | | | | – | | | | 2,498 | |
| Preferred stock for compensation | | | – | | | | – | | | | – | | | | – | | | | 2,999,800 | | | | – | | | | 3,000,000 | |
| Shares sold for cash | | | 232,500,000 | | | | 23,250 | | | | (10,000,000 | ) | | | (100,000 | ) | | | 2,301,750 | | | | – | | | | 2,225,000 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| As at September 30, 2021 | | | 911,843,430 | | | $ | 91,185 | | | | 15,275,000 | | | $ | 131,503 | | | $ | 28,358,311 | | | $ | (30,728,962 | ) | | $ | (2,089,845 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Loss for the period | | | – | | | | – | | | | – | | | | – | | | | – | | | | (368,985 | ) | | | (368,985 | ) |
| Common stock for services | | | 10,000,000 | | | | 1,000 | | | | (10,000,000 | ) | | | (69,000 | ) | | | 68,000 | | | | – | | | | – | |
| Common stock for compensation | | | – | | | | – | | | | 225,000 | | | | 2,422 | | | | – | | | | – | | | | 2,422 | |
| Common stock for debt conversion | | | 41,149,178 | | | | 4,114 | | | | – | | | | – | | | | 420,900 | | | | – | | | | 425,014 | |
| Preferred stock for compensation | | | – | | | | – | | | | – | | | | – | | | | 56,990 | | | | – | | | | – | |
| Subscriptions receivable | | | – | | | | – | | | | – | | | | – | | | | (100,000 | ) | | | – | | | | (100,000 | ) |
| Shares sold for cash | | | 97,500,000 | | | | 9,750 | | | | 20,000,000 | | | | 200,000 | | | | 965,250 | | | | – | | | | 1,175,000 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| As at December 31, 2021 | | | 1,060,492,608 | | | $ | 106,049 | | | | 25,500,000 | | | $ | 264,925 | | | $ | 29,769,450 | | | $ | (31,097,947 | ) | | $ | (956,394 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Loss for the period | | | – | | | | – | | | | – | | | | – | | | | – | | | | (7,367,583 | ) | | | (7,367,583 | ) |
| Common stock for services | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Common stock for compensation | | | – | | | | – | | | | 402,132,500 | | | | 6,470,634 | | | | – | | | | – | | | | 6,470,634 | |
| Common stock for debt conversion | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Preferred stock for compensation | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Subscriptions receivable | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Shares sold for cash | | | 20,000,000 | | | | 2,000 | | | | (20,000,000 | ) | | | (200,000 | ) | | | 198,000 | | | | – | | | | – | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| As at March 31, 2022 | | | 1,080,492,608 | | | $ | 108,049 | | | | 407,632,500 | | | $ | 6,535,559 | | | $ | 29,967,450 | | | $ | (38,465,530 | ) | | $ | (1,853,343 | ) |
The accompanying footnotes are an integral part of these unaudited consolidated financial statements.
Consolidated Statements of Shareholders’ Deficit
For the Three and Nine Months Ended March 31, 2023 and 2022 (unaudited)
(continued)
| | | | | | | | | | | | | | | | | | | | | | |
| | | Common Stock | | | Common Stock to be issued | | | Additional Paid in Capital | | | Accumulated Profit/Deficit | | | Total | |
| | | Shares | | | Amount | | | Shares | | | Amount | | | Amount | | | Shares | | | Amount | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| As at June 30, 2022 | | | 1,493,142,608 | | | $ | 149,314 | | | | 35,602,500 | | | $ | 407,677 | | | $ | 36,773,035 | | | $ | (39,423,382 | ) | | $ | (2,092,227 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Loss for the period | | | – | | | | – | | | | – | | | | – | | | | – | | | | (748,767 | ) | | | (748,767 | ) |
| Common stock for compensation | | | 5,000,000 | | | | 500 | | | | (2,000,000 | ) | | | (30,000 | ) | | | 65,500 | | | | – | | | | 36,000 | |
| Common stock to be issued for compensation | | | – | �� | | | – | | | | 32,500 | | | | 441 | | | | – | | | | – | | | | 441 | |
| Common stock for officer compensation | | | 6,950,000 | | | | 695 | | | | (6,950,000 | ) | | | (101,262 | ) | | | 100,567 | | | | – | | | | – | |
| Common stock to be issued for officer compensation | | | – | | | | – | | | | 3,475,000 | | | | 47,144 | | | | – | | | | – | | | | 47,144 | |
| Shares issued for services | | | 5,000,000 | | | | 500 | | | | – | | | | – | | | | 88,500 | | | | – | | | | 89,000 | |
| Shares sold for cash – prior quarter | | | 21,500,000 | | | | 2,150 | | | | (21,500,000 | ) | | | (215,000 | ) | | | 212,850 | | | | – | | | | – | |
| Shares sold for cash – current quarter | | | – | | | | – | | | | 67,950,000 | | | | 604,500 | | | | – | | | | – | | | | 604,500 | |
| Fees incurred in connection with equity offering | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Payment of subscription receivable | | | – | | | | – | | | | – | | | | – | | | | 30,000 | | | | – | | | | 30,000 | |
| Other | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| As at September 30, 2022 | | | 1,531,592,608 | | | $ | 153,159 | | | | 76,610,000 | | | $ | 713,500 | | | $ | 37,270,452 | | | $ | (40,172,149 | ) | | $ | (2,033,909 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Loss for the period | | | – | | | | – | | | | – | | | | – | | | | – | | | | (539,791 | ) | | | (539,791 | ) |
| Common stock for compensation | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Common stock to be issued for compensation | | | – | | | | – | | | | 32,500 | | | | 210 | | | | – | | | | – | | | | 210 | |
| Common stock for officer compensation | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Common stock to be issued for officer compensation | | | – | | | | – | | | | 3,475,000 | | | | 22,422 | | | | 49 | | | | – | | | | 22,471 | |
| Shares issued for services | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Commitment shares – Leonite convertible note | | | – | | | | – | | | | 15,000,000 | | | | 49,936 | | | | – | | | | – | | | | 49,936 | |
| Shares sold for cash – prior quarter | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Shares sold for cash – current quarter | | | – | | | | – | | | | 312,500 | | | | 25,000 | | | | – | | | | – | | | | 25,000 | |
| Fees incurred in connection with equity offerings | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Payment of subscription receivable | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Other | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| As at December 31, 2022 | | | 1,531,592,608 | | | $ | 153,159 | | | | 95,430,000 | | | $ | 811,068 | | | $ | 37,270,501 | | | $ | (40,711,940 | ) | | $ | (2,476,083 | ) |
| | | | | | | | | | | | | | | | | | | | | | |
| Loss for the period | | | – | | | | – | | | | – | | | | – | | | | – | | | | (522,141 | ) | | | (522,141 | ) |
| Common stock for compensation | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Common stock to be issued for compensation | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Common stock for officer compensation | | | 2,662,500 | | | | 266 | | | | (2,662,500 | ) | | | (22,846 | ) | | | 22,580 | | | | – | | | | – | |
| Common stock to be issued for officer compensation | | | – | | | | – | | | | 3,475,000 | | | | 16,333 | | | | – | | | | – | | | | 16,333 | |
| Shares issued for services | | | 15,000,000 | | | | 1,500 | | | | – | | | | – | | | | 73,500 | | | | – | | | | 75,000 | |
| Commitment shares – Leonite convertible note | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Shares sold for cash – prior quarter | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Shares sold for cash – current quarter | | | – | | | | – | | | | 2,000,000 | | | | 10,000 | | | | – | | | | – | | | | 10,000 | |
| Fees incurred in connection with equity offering | | | – | | | | – | | | | – | | | | – | | | | (1,500 | ) | | | – | | | | (1,500 | ) |
| Payment of subscription receivable | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | |
| Other | | | – | | | | 1 | | | | – | | | | – | | | | – | | | | – | | | | 1 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| As at March 31, 2023 | | | 1,549,255,108 | | | $ | 154,926 | | | | 98,242,500 | | | $ | 814,555 | | | $ | 37,365,081 | | | $ | (41,234,081 | ) | | $ | (2,898,390 | ) |
The accompanying footnotes are an integral part of these unaudited consolidated financial statements.
Wearable Healthcare Solutions, Inc.
Consolidated Statements of Cash Flows
For the Nine Months Ended March 31, 2023 and 2022 (unaudited)
| | | | | | | |
| | | 2023 | | | 2022 | |
| Cash flow from operating activities | | | | | | | | |
| Net loss | | $ | (1,810,699 | ) | | $ | (12,091,303 | ) |
| Adjustment for non-cash charges and other items: | | | | | | | | |
| Depreciation | | | 9,117 | | | | 5,849 | |
| Amortization of deferred debt issuance costs | | | 15,467 | | | | – | |
| Stock-based compensation expense | | | 286,600 | | | | 9,610,554 | |
| Change in fair value of derivative instrument | | | – | | | | 213,053 | |
| Gain on debt extinguishment | | | – | | | | (96,145 | ) |
| Gain on settlement of accounts payable | | | – | | | | (156,616 | ) |
| Amortization of debt discount | | | 16,129 | | | | – | |
| Total adjustments | | | (1,483,386 | ) | | | (2,514,608 | ) |
| Changes in working capital | | | | | | | | |
| Decrease / (increase) in accounts receivable | | | (812 | ) | | | 25,694 | |
| Decrease / (increase) in inventory | | | (26,217 | ) | | | – | |
| Decrease / (increase) in prepaid inventory | | | 62,040 | | | | – | |
| Decrease / (increase) in prepaid expenses | | | – | | | | 16,485 | |
| (Decrease) / increase in trade and other payables | | | 8,141 | | | | (67,992 | ) |
| (Decrease) / increase in accrued expenses | | | 72,617 | | | | 99,393 | |
| (Decrease) / increase in accrued expenses - related party | | | 225,047 | | | | (204,121 | ) |
| (Decrease) / increase in deferred revenue | | | (19,407 | ) | | | (25,992 | ) |
| Total changes in working capital | | | 321,409 | | | | (156,533 | ) |
| Cash flow used in operating activities | | | (1,161,977 | ) | | | (2,671,141 | ) |
| | | | | | | | | |
| Cash flow from investing activities | | | | | | | | |
| Purchase of property and equipment | | | (9,442 | ) | | | (50,000 | ) |
| Cash flow used in investing activities | | | (9,442 | ) | | | (50,000 | ) |
| | | | | | | | | |
| Cash flow from financing activities | | | | | | | | |
| Proceeds from note payable | | | – | | | | 25,000 | |
| Proceeds received from related parties | | | 225,165 | | | | – | |
| Proceeds from issuance of convertible notes | | | 240,000 | | | | – | |
| Proceeds from issuance of stock for cash | | | 669,500 | | | | 3,300,000 | |
| Repayments of note payable | | | (15,910 | ) | | | (825,864 | ) |
| Fees paid in connection with equity offerings | | | (1,500 | ) | | | – | |
| Payments to related parties | | | – | | | | (55,452 | ) |
| Cash flow provided by financing activities | | | 1,117,255 | | | | 2,443,684 | |
| Decrease in cash and cash equivalents | | | (54,164 | ) | | | (277,457 | ) |
| Cash and cash equivalents at the beginning of the period | | | 70,505 | | | | 847,430 | |
| Cash and cash equivalents at end of the period | | $ | 16,341 | | | $ | 569,973 | |
| | | | | | | | | |
| Non-Cash Investing and Financial Activities: | | | | | | | | |
| Conversions of notes and accrued interest | | $ | – | | | $ | 269,954 | |
| | | | | | | | | |
| Cash paid for interest and taxes | | | | | | | | |
| Interest | | $ | 8,382 | | | $ | – | |
| Taxes | | $ | – | | | $ | – | |
The accompanying footnotes are an integral part of these unaudited consolidated financial statements.
WEARABLE HEALTHCARE SOLUTIONS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
March 31, 2023 (unaudited) and June 30, 2022
Note 1 – Nature and Continuance of Operations
Wearable Healthcare Solutions Inc. (the Company) was incorporated as Medical Alarm Concepts Holding, Inc. on June 4, 2008, under the laws of the State of Nevada. The Company was formed for the sole purpose of acquiring all of the membership units of Medical Alarm Concepts LLC, a Pennsylvania limited liability company (“Medical LLC”). On May 26, 2016, the Company filed an Amended and Restated Articles of Incorporation with the Secretary of State of the State of Nevada to change its name from “Medical Alarm Concepts, Inc.” to “Wearable Health Solutions, Inc.”
The Company provides mobile health (mHealth) products and services to be used by customers in case of an emergency. As a provider of personal emergency devices, the Company provides innovative wearable healthcare products, tracking services, and turn-key solutions that enable our users to be proactive with their health, as well as safe and protected.
The Company’s flagship products are the iHelp devices, the 3G and the next generation iHelp MAX™ – personal emergency alarm that are used to summon help in the event of an emergency at home.
Basis of presentation
The accompanying interim consolidated financial statements are unaudited, but in the opinion of management of Wearable Healthcare Solutions, Inc. (the Company), contain all adjustments, which include normal recurring adjustments, necessary to present fairly the financial position at March 31, 2023, and the results of operations and changes in shareholders’ deficit for the three and nine months ended March 31, 2023 and cash flows for the nine months ended March 31, 2023. The balance sheet as of June 30, 2022, is derived from the Company’s audited financial statements.
Certain information and footnote disclosures normally included in financial statements that have been prepared in accordance with generally accepted accounting principles have been condensed or omitted pursuant to the rules and regulations of the Securities and Exchange Commission, although management of the Company believes that the disclosures contained in these financial statements are adequate to make the information presented therein not misleading. For further information, refer to the financial statements and the notes thereto included in the Company’s Annual Report on this Form 10-K for the fiscal year ended June 30, 2022.
The results of operations for the three and nine months ended March 31, 2023, are not necessarily indicative of the results of operations to be expected for the full fiscal year ending June 30, 2023.
Note 2 – Summary of Significant Accounting Policies
Principles of Consolidation – The consolidated financial statements include the accounts of the Company and its wholly-owned operating subsidiary: Medical Alarm Concepts, LLC. All intercompany accounts and transactions have been eliminated in consolidation.
Use of Estimates – The preparation of consolidated financial statements in conformity with accounting principles generally accepted in the United States of America (“GAAP”) requires management to make estimates and assumptions that affect reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenue and expenses during the reporting period. Future events and their effects cannot be determined with absolute certainty. Therefore, the determination of management’s estimates requires the exercise of judgment. The Company’s management evaluates these significant estimates and assumptions including those related to the fair value of acquired assets and liabilities, stock-based compensation, income taxes, allowance for doubtful accounts, long-lived assets, and inventories, and other matters that affect the consolidated financial statements and disclosures. Actual results could differ from those estimates.
Cash and Cash Equivalents – For purposes of the Statement of Cash Flows, the Company considers highly liquid investments purchased with an original maturity of three months or less to be cash equivalents.
Accounts Receivable – The Company estimates credit loss reserves for accounts receivable on an individual receivable basis. A specific impairment allowance reserve is established based on expected future cash flows and the financial condition of the debtor. The Company charges off customer balances in part or in full when it is more likely than not that we will not collect that amount of the balance due. The Company considers any balance unpaid after the contract payment period to be past due. There are $24,517 and $-0- in accounts receivable net of allowances of $23,705 and $-0- at March 31, 2023 and June 30, 2022, respectively.
Software Development for internal use - The Company accounts for software development costs in accordance with applicable guidelines. Software development costs include payroll, employee benefits, stock-based compensation expense, and other headcount-related expenses associated with product development. Software development costs also include third-party development and programming costs, localization costs incurred to translate software for international markets, and the amortization of purchased software code and services content. Such costs related to software development are included in software development expense until the point that technological feasibility is reached. Once technological feasibility is reached, such costs are capitalized and depreciated over the useful estimated lives of the software. For software modifications or developments, the Company expenses the costs. The Company purchased its dealer portal for $50,000 on August 30, 2021 which is being depreciated over 5 years.
Concentration of Credit Risk - Financial instruments that potentially subject us to concentrations of credit risk consist primarily of cash and cash equivalents and accounts receivable. The Company performs ongoing credit evaluations of its customers and maintains allowances for potential credit losses.
Recognition of Revenues – Recognition of Revenues – In May 2014, the FASB issued ASU No. 2014-09, Revenue from Contracts with Customers (Topic 606). ASU 2014-09 establishes a single comprehensive model for entities to use in accounting for revenue arising from outside contracts with customers and supersedes most of the existing revenue recognition guidance and notes that lease contracts with customers are a scope exception. ASU 2014-09 requires an entity to recognize revenue when it transfers promised goods or services to customers in an amount that reflects the consideration to which an entity expects to be entitled in exchange for those goods or services and also requires certain additional disclosures. On August 12, 2015, the FASB issued ASU 2015-14 to defer the effective date of ASU 2014-09. Public business entities may elect to adopt the amendments as of the original effective date; however, adoption is required for annual reporting periods beginning after December 15, 2017. The Company has adopted this pronouncement.
The Company’s revenues are derived principally from utilizing new technology in the medical alarm industry to provide 24-hour personal response monitoring services and related products to subscribers with medical or age-related conditions. The Company recognizes revenue when it is realized or realizable and earned. For hardware sales, the Company recognizes revenues at a point in time when the product is shipped. Customers are billed on Net 30 terms. For service revenue, the Company recognizes revenues over the term of the service contract and when the services are rendered. For customers who pay several months at a time, the Company records revenues for the month’s services and the balance of funds to deferred revenues, and records the balance of revenues as they become current.
| Schedule of revenues | | | | | | | | | | | | |
| | | 3 months ended March 31, | | | 9 months ended March 31, | |
| REVENUES | | 2023 | | | 2022 | | | 2023 | | | 2022 | |
| Hardware revenue | | $ | 31,476 | | | $ | 5,659 | | | $ | 82,201 | | | $ | 82,644 | |
| Service revenue | | | 162,269 | | | | 207,323 | | | | 526,043 | | | | 736,957 | |
| TOTAL REVENUES | | $ | 193,745 | | | $ | 212,982 | | | $ | 608,244 | | | $ | 819,601 | |
The following table discloses changes in unearned revenue for the nine months ended March 31, 2023 and 2022:
| Schedule of unearned revenue | | | | | | |
| | | 2023 | | | 2022 | |
| Balance at beginning of period - June 30, | | $ | 80,880 | | | $ | 108,298 | |
| Deferred revenue | | | 120,688 | | | | 168,823 | |
| Recognition of unearned revenue | | | (140,095 | ) | | | (194,815 | ) |
| Balance at the end of the period - March 31, | | $ | 61,473 | | | $ | 82,306 | |
Deferral of revenues at March 31, 2023 and March 31, 2022 was $61,473 and $82,306, respectively. The deferred revenue represents quarterly and annual prepaid service fees, which were invoiced and paid at the onset of customer service agreements and which pertain to service obligations not realized at March 31, 2023 and March 31, 2022, respectively. We have no agreements longer than 12 months.
Deferred Taxes – The Company accounts for income taxes under Section 740-10-30 of the FASB Accounting Standards Codification. Deferred income tax assets and liabilities are determined based upon differences between the financial reporting and tax bases of assets and liabilities and are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse. Deferred tax assets are reduced by a valuation allowance to the extent management concludes it is more likely than not that the assets will not be realized. Deferred tax assets and liabilities are measured using enacted rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in the statements of operations in the period that includes the enactment date.
ASC 740, Income Taxes, requires a company to first determine whether it is more likely than not (which is defined as a likelihood of more than fifty percent) that a tax position will be sustained based on its technical merits as of the reporting date, assuming that taxing authorities will examine the position and have full knowledge of all relevant information. A tax position that meets this more-likely-than-not threshold is then measured and recognized at the largest amount of benefit that is greater than fifty percent likely to be realized upon effective settlement with a taxing authority.
The Federal and state income tax returns of the Company for 2022, 2021, and 2020 are subject to examination by the Internal Revenue Service and state taxing authorities for three (3) years from the date filed.
Fair value of financial instruments. The Company measures its financial and non-financial assets and liabilities, as well as makes related disclosures, in accordance with FASB Accounting Standards Codification No. 820, Fair Value Measurement (“ASC 820”), which provides guidance with respect to valuation techniques to be utilized in the determination of fair value of assets and liabilities. Approaches include, (i) the market approach (comparable market prices), (ii) the income approach (present value of future income or cash flow), and (iii) the cost approach (cost to replace the service capacity of an asset or replacement cost). ASC 820 utilizes a fair value hierarchy that prioritizes the inputs to valuation techniques used to measure fair value into three broad levels. The following is a brief description of those three levels:
Level 1: Observable inputs such as quoted prices (unadjusted) in active markets for identical assets or liabilities.
Level 2: Inputs other than quoted prices that are observable, either directly or indirectly. These include quoted prices for similar assets or liabilities in active markets and quoted prices for identical or similar assets or liabilities in markets that are not active.
Level 3: Unobservable inputs in which little or no market data exists, therefore requiring an entity to develop its own assumptions, such as valuations derived from valuation techniques in which one more significant inputs or significant value drivers are unobservable.
From time to time, our financial instruments include cash, accounts payable and accrued expenses, convertible notes, lines of credit, and credit cards.
Research and Development - Research and development costs are charged to operations as they are incurred. Legal fees and other direct costs incurred in obtaining and protecting patents are also expensed as incurred, due to the uncertainty with respect to future cash flows resulting from the patents. For the three and nine months ended March 31, 2023 and 2022, the Company recorded $-0- and $2,180 and $209,800 and $374,484 in research and development costs, respectively.
Basic and Diluted Loss per Common Share - Basic loss per common share excludes dilution and is computed by dividing loss available to common stockholders by the weighted average number of common shares outstanding during the period of computation. Diluted loss per share gives effect to all potential dilutive common shares outstanding during the period of compensation. Diluted income (loss) per share reflects the potential dilution that could occur if securities or other contracts to issue common stock were exercised or converted into common stock or resulted in the issuance of common stock that would then share in the net income of the Company, subject to anti-dilution limitations.
| Schedule of anti-dilutive shares | | | | | | | | | | | | |
| | | Basis of conversion | | Dilution | | 2023 | | | 2022 | |
| Series A Convertible | | 688 shares outstanding | | 1 share A: 2 shares | | | 1,376 | | | | 1,376 | |
| Series B Convertible | | 9,938 shares outstanding | | 1 share B: 2 shares | | | 19,876 | | | | 19,876 | |
| Series C Convertible | | 6,838,889 shares outstanding | | 1 share C: 10 shares | | | 68,388,890 | | | | 68,388,890 | |
| Series D Convertible | | 425,000 shares outstanding | | 1 share D: 10 shares | | | 4,250,000 | | | | 4,250,000 | |
| Series E Convertible | | 4,000,000 shares outstanding | | 1 share E: 100 shares | | | 400,000,000 | | | | 400,000,000 | |
| | | | | | | | 472,660,142 | | | | 472,660,142 | |
The Company has incurred losses for the past two years, as a result, the basic and diluted share bases will be presented as the same. For the three-month periods ended March 31, 2023 and 2022, the Company incurred losses of ($0.00034) and ($0.00731) per basic share and diluted share, respectively. For the nine months ended March 31, 2023 and 2022, the Company incurred losses of ($0.00118) and ($0.01487) per basic share and diluted share, respectively.
Recent Accounting Pronouncements
In December 2019, the FASB issued ASU 2019-12, Income Taxes (Topic 740) – Simplifying the Accounting for Income Taxes. The update is intended to simplify the current rules regarding the accounting for income taxes and addresses several technical topics including accounting for franchise taxes, allocating income taxes between a loss in continuing operations and in other categories such as discontinued operations, reporting income taxes for legal entities that are not subject to income taxes, and interim accounting for enacted changes in tax laws. The new standard is effective for fiscal years beginning after December 15, 2020; however, early adoption is permitted. The Company does not expect the adoption of this standard have a material impact on the consolidated financial statements.
In August 2020, the FASB issued ASU No. 2020-06, Accounting for Convertible Instruments and Contracts in an Entity’s Own Equity (ASU 2020-06), which simplifies the accounting for certain financial instruments with characteristics of liabilities and equity, including convertible instruments and contracts in an entity’s own equity. Among other changes, ASU 2020-06 removes from U.S. GAAP the liability and equity separation model for convertible instruments with a cash conversion feature, and as a result, after adoption, entities will no longer separately present in equity an embedded conversion feature for such debt. Similarly, the embedded conversion feature will no longer be amortized into income as interest expense over the life of the instrument. Instead, entities will account for a convertible debt instrument wholly as debt unless (1) a convertible instrument contains features that require bifurcation as a derivative under ASC Topic 815, Derivatives and Hedging, or (2) a convertible debt instrument was issued at a substantial premium. Among other potential impacts, this change is expected to reduce reported interest expense, increase reported net income, and result in a reclassification of certain conversion feature balance sheet amounts from shareholders’ equity to liabilities as it relates to the Company’s convertible senior notes. Additionally, ASU 2020-06 requires the application of the if-converted method to calculate the impact of convertible instruments on diluted earnings per share (EPS), which is consistent with the Company’s accounting treatment under the current standard. ASU 2020-06 is effective for fiscal years beginning after December 15, 2021, with early adoption permitted for fiscal years beginning after December 15, 2020, and can be adopted on either a fully retrospective or modified retrospective basis. The Company early adopted the ASU on July 1, 2022, the beginning of its fiscal year.
The Company reviewed all recent accounting pronouncements issued by the FASB (including its Emerging Issues Task Force), the AICPA and the SEC, and they did not or are not believed by management to have a material impact on the Company’s present or future financial statements.
Note 3 – Going Concern
The accompanying consolidated financial statements for the three and nine months ended March 31, 2023 and 2022 have been prepared on a going concern basis, which contemplates the realization of assets and the settlement of liabilities and commitments in the normal course of business. As at March 31, 2023 and June 30, 2022, the Company has shown losses for the last two years and has an accumulated deficit of ($41,234,081) and ($39,423,382), respectively.
During the nine months ended March 31, 2023, the Company has net cash used in operating activities of $1,161,977 as well as stock compensation non-cash expense of $286,600 and a net loss of $1,810,699. The Company had net cash flow of $1,117,255 from financing activities in the nine months ended March 31, 2023, which resulted in a working capital deficit of $2,953,764 as of March 31, 2023. If the Company is unable to raise additional adequate capital, it could be forced to cease operations.
Management believes that the Company’s capital requirements will depend on many factors including the success of the Company’s development efforts and its efforts to raise capital. Management also believes the Company needs to raise additional capital for working capital purposes. There can be no assurance that the Company will be able to obtain the additional capital resources necessary to implement its business plan or that any assumptions relating to its business plan will prove accurate.
These factors raise substantial doubt about our ability to continue as a going concern for a period of 12 months from the issue date of this report. The consolidated financial statements of the Company do not include any adjustments relating to the recoverability and classification of recorded assets, or the amounts and classifications of liabilities that might be necessary should the Company be unable to continue as a going concern.
Note 4 – Inventory, Prepaid Inventory, and Prepaid Expenses
The Company maintains some inventory in its warehouse and purchases some of its inventory overseas. Inventories, except for stock in transit, are stated at lower of cost and net realizable value. Stock in transit is valued at cost comprising invoice value plus other charges thereon. Net realizable value is the estimated selling price in ordinary course of business less estimated costs of completion and selling expenses. The quantity of inventory may vary from time to time depending on the delivery schedule of overseas shipments.
As of March 31, 2023 and June 30, 2022, the Company had $33,281 and $7,064 in inventory, respectively, as well as $-0- and $62,040 in prepaid inventory, respectively.
As of March 31, 2023 and June 30, 2022, the Company had $-0- and $-0- in prepaid expenses, respectively.
Note 5 – Property and Equipment
The Company has $20,000 in furnishings, $19,689 in office computers and equipment, and capitalized software development costs of $45,900 which are fully depreciated. On August 30, 2021, the Company purchased its dealer portal for $50,000 for internal use, amortized over 60 months. On September 26, 2022, the Company purchased used furniture for $9,442 for its office warehouse located in Mequon, WI and the Company is depreciating the used furniture over 36 months.
As of March 31, 2023 and June 30, 2022, the Company recorded $41,976 and $41,651 in net Property and Equipment, respectively:
| Schedule of property, plant and equipment | | | | | | |
| | | March 31, 2023 | | | June 30, 2022 | |
| Furniture | | $ | 29,442 | | | $ | 20,000 | |
| Office computers, equipment, software | | | 19,689 | | | | 19,689 | |
| Software development costs | | | 45,900 | | | | 45,900 | |
| Dealer Portal | | | 50,000 | | | | 50,000 | |
| Property, plant, and equipment | | | 145,031 | | | | 135,589 | |
| Less accumulated depreciation | | | (103,055 | ) | | | (93,938 | ) |
| Net property, plant, and equipment | | $ | 41,976 | | | $ | 41,651 | |
Note 6 – Accounts payable and accrued expenses and liabilities
The Company recorded Accounts Payable of $66,081 and $57,940, directly related to operating costs, as of March 31, 2023 and June 30, 2022, respectively.
Accrued expenses and other current liabilities are expenses that have been incurred but not yet paid, and mainly include legal fees, audit fees and other professional fees as well as accrued interest in connection with the credit line and notes payable. The Company recorded $446,650 and $374,278 in accrued expenses and other current liabilities as of March 31, 2023 and June 30, 2022, respectively.
Note 7 – Notes Payable and Note payable-other
Notes payable consists of notes payable from our subsidiary, notes payable-other, convertible notes payable, notes payable for stock purchases under Reg A, short term notes payable, and notes payable-BOAPIN portal, as follows:
| Schedule of short term notes payable | | | | | | |
| | | March 31, 2023 | | | June 30, 2022 | |
| Notes from subsidiary | | $ | 158,333 | | | $ | 174,243 | |
| Notes payable – Reg A deposits | | | 138,856 | | | | 138,856 | |
| Short term bridge loan | | | 100,000 | | | | 100,000 | |
| Total Notes Payable | | $ | 397,189 | | | $ | 413,099 | |
Notes Payable - subsidiary
The Company has various loans and credit lines outstanding. The credit line carries an interest rate of 6.24%. The bank loans carry interest rates varying between 9.24% – 10.90%.
| Schedule of notes payable | | | | | | |
| | | March 31, 2023 | | | June 30, 2022 | |
| Wells Fargo Loan | | $ | 8,770 | | | $ | 8,770 | |
| On Deck Loan | | | 139,569 | | | | 139,569 | |
| Susquehanna Salt Loan | | | – | | | | 10,500 | |
| Prosper Loans | | | 9,994 | | | | 9,994 | |
| Marcus Loan | | | – | | | | 5,410 | |
| Total Notes from Subsidiary (See Table Above) | | $ | 158,333 | | | $ | 174,243 | |
Short term bridge loan - COHEN
On July 31, 2020, the Company secured a $500,000 short term bridge loan from an unaffiliated individual (“COHEN”), 12% interest, due and payable October 20, 2020. The loan is currently in default and continues to accrue interest at 12%.
On August 19, 2021, the Company repaid $300,000 of principal and in November 2021, the Company repaid an additional $100,000 in principal.
At March 31, 2023 and June 30, 2022, the Company recorded a short term note payable of $100,000, respectively. During the three and nine months ended March 31, 2023, the Company expensed $3,000 and $9,000 in interest expense, respectively. During the three and nine months ended March 31, 2022, the Company expensed $3,000 and $18,649, respectively. The accrued interest payable at March 31, 2023 and June 30, 2022 was $85,677 and $76,677, respectively. These notes are included in the Notes payable total on the Company’s balance sheet.
Note payable – stock purchases under Reg A
In March 2021 and June 2021, the Company accepted payments of $115,000 for stock purchases under the Reg A filing from two unaffiliated investors, pending blue sky registrations in two states. In July 2021, the Company accepted loans totaling $20,000 from two unaffiliated investors pending blue sky registrations in two additional states. The notes mature in one year and bear interest at 5%. The full amount of the note plus interest is convertible at the Reg A fixed price of $0.01, when possible.
In October 2021, the Company accepted a loan of $5,000 from two unaffiliated investors pending blue sky registrations in two additional states.
At March 31, 2023 and June 30, 2022, the Company has recorded $138,856 and $138,856 in notes payable for stock purchases under Reg A. During the three and nine months ended March 31, 2023, the Company recorded interest expense of $2,700 and $8,100, respectively. During the three and nine months ended March 31, 2022, the Company recorded interest expense of $2,700 and $8,736, respectively. The accrued interest payable at March 31, 2023 and June 30, 2022 was $20,407 and $12,307, respectively. These notes are included in the Notes payable total on the Company’s balance sheet.
Note Payable – Other
In November, 2016, the Company secured a $50,000 loan from a party related to a previous CEO, bearing 4% interest, the loan maturing after a successful money raise of $1,000,000 through the acquisition of convertible notes payable (See BENZA, D2CF). The $1,000,000 fundraising was never completed, and the Company has been accruing interest on the original principal amount at 4% since inception. On July 22, 2021, the Company filed suit for damages and the party filed a countersuit on August 26, 2021. There has been no resolution to this situation, and we continue to accrue interest at the face amount.
During the three and nine months ended March 31, 2023, the Company recorded interest expense of $500 and $1,500, respectively. During the three and nine months ended March 31, 2022, the Company recorded interest expense of $500 and $1,000, respectively. The accrued interest payable at March 31, 2023 and June 30, 2022 was $12,782 and $11,282, respectively. These notes are included in the Notes payable total on the Company’s balance sheet.
Convertible note payable – BENZA, D2CF
On March 1, 2016 and March 3, 2016, the Company closed a private placement of debt and received an aggregate of $612,500 by issuing $13,750 (“B2CF”) and $660,000 (“BENZA”) unsecured convertible notes (“convertible notes”) and warrants to two investors, net of original issue discount of $61,250 per the subscription agreements, maturity at March 1, 2017 and March 3, 2017, respectively, bearing 0% interest and 18% default interest. The notes are currently in default, and all outstanding warrants have expired.
The Company is currently in negotiations to settle the $660,000 BENZA loan with principles in the company, although there has been no settlement to date.
As of March 31, 2023 and June 30, 2022, the Company reported $673,750 and $673,750 in convertible notes payable, respectively.
Convertible Note – Leonite Capital, LLC
On December 5, 2022, the Company, (the “Borrower”), received $250,000 on issuing the first tranche of $1,000,000 senior secured convertible note (“Leonite Convertible Note”) from Leonite Capital, LLC, a Delaware limited liability company (“Leonite”), net of an original issue discount of $62,500. The term of the convertible note is fifteen months from the date of closing and matures on March 5, 2024. The Company is required to only pay interest expense on a monthly basis for the first six months of the term. During the three and nine months ended March 31, 2023, the Company accrued $10,839 of interest expense related to the convertible notes. The Company will begin making nine equal amortization payments of $34,722 commencing in the month of July 2023. The Company is required to issue 15,000,000 commitment shares valued at $78,000 to Leonite of which $28,064 was charged to common stock to be issued. In addition, the Company also paid Leonite $10,000 for legal fees incurred by Leonite related to this transaction. The commitment shares and the legal fees have been recorded as deferred debt issuance costs totaling $59,936. The Company amortized $11,987 and $15,467 of the deferred debt issuance costs during the three and nine months ended March 31, 2023, respectively, and the Company also amortized $12,500 and $16,129 of the original issue discount during the three and nine months ended March 31, 2023, respectively. The Leonite Convertible Note bears annual interest at the greater of 10% or the Prime Rate plus three percent (3%). The Leonite Convertible Note is convertible into shares of the Company’s common stock at a conversion price equal to $0.007 per share with anti-dilution features.
Credit line – MediPendant New York Inc.
On September 30, 2014, our subsidiary entered into a line of credit with Medi Pendant New York, Inc. (“MNY”), which is partially owned by a principal of its subsidiary. Under the line of credit agreement, the Company will be able to borrow up to $500,000 with the rate of interest of 6.5% per annum. The maturity date of the credit line is September 30, 2017, with a one-year extension to September 30, 2018. On January 31, 2015, the limit on the line of credit was increased to $500,000 with same interest rate and due date. The company issued 200,000 shares of common stock to one of the owners of MNY as consideration for the increase of line of credit. These shares were issued on October 19, 2015 and value at $28,000 which was the fair market value at the grant date.
As of March 31, 2023 and June 30, 2022, the Company has recorded $397,500 and $397,500 in outstanding line of credit balance, respectively.
Debt settlement – On Deck, Susquehanna, MCA Cure
In 2019, our subsidiary engaged MCA CURE to negotiate settlements with two creditors: On Deck and Susquehanna Salt, noted in the table above. The Company ceased paying the loan payments and paid to MCA Cure $43,875 in 2019 and $47,000 in 2020, at which point the Company was contacted and MCA Cure assured they had enough funds to negotiate with the creditors. In 2020, the Company discovered MCA Cure had not performed when bank accounts were levied for $33,705 by the creditors. $18,705 was subsequently refunded by the collection firm. On September 30, 2020, the bank accounts were again levied for additional funds. Currently the Company has a settlement agreement in place with Susquehanna Salt Loan, and has booked a reserve against the $90,875 funds paid to MCA Cure. The Company has hired an attorney and is making every effort to recover funds and damages from MCA Cure. To date, there has been no resolution to the situation. As of March 31, 2023 and June 30, 2022, the Company recorded $-0- and $-0- in prepaid fund to MCA Cure, and $139,569 and $139,569 in indebtedness to On Deck. The Company negotiated a settlement with Susquehanna Salt for the loan balance, and as of March 31, 2023 and June 30, 2022, the Company recorded indebtedness to Susquehanna Salt of $-0- and $10,500, respectively.
Note 10 – Shareholders’ Deficit
Preferred Stock:
The Company is currently authorized to issue 25,000,000 shares of preferred stock, par value of $0.0001.
Series A Convertible Preferred Stock: The Company is currently authorized to issue up to 100,000 shares of Series A Convertible Preferred Stock, par value $0.0001 per share, convertible at a ratio of 1 share of Series A Convertible Preferred Stock for 2 shares of common stock. These shares have no voting rights. As of March 31, 2023 and June 30, 2022, 688 shares of Series A Convertible Preferred Stock were issued and outstanding, respectively.
Series B Convertible Preferred Stock: The Company is currently authorized to issue up to 62,500 shares of Series B Convertible Preferred Stock, par value $0.0001 per share, convertible at a ratio of 1 share of Series B Convertible Preferred Stock for 2 shares of common stock. These shares have voting rights and vote on an “as converted” basis in actions required to have Series B Preferred Stockholder approval. As of March 31, 2023 and June 30, 2022, 9,938 shares of Series B Convertible Preferred Stock were issued and outstanding, respectively.
Series C Convertible Preferred Stock: The Company is currently authorized to issue up to 6,944,445 shares of Series C Convertible Preferred Stock, par value $0.0001 per share, convertible at a ratio of 1 share of Series C Convertible Preferred Stock for 10 shares common stock. These shares have voting rights and vote on an “as converted” basis on all matters submitted to our Stockholders for approval.
The Company issued 6,700,003 shares for the BOAPIN asset purchase; these shares were issued on September 1, 2020. As of March 31, 2023 and June 30, 2022, 6,838,889 shares of Series C Convertible Preferred Stock were issued and outstanding, respectively.
Series D Convertible Preferred Stock: The Company is currently authorized to issue up to 500,000 shares of Series D Convertible Preferred Stock, par value $0.0001 per share, convertible at a ratio of 1 share of Series D Convertible Preferred stock for 10 shares of common stock. These shares have voting rights and vote on an “as converted” basis in actions required to have Series D Preferred Stockholder approval. As of March 31, 2023 and June 30, 2022, 425,000 shares of Series D Convertible Preferred Stock were issued and outstanding, respectively.
Series E Convertible Preferred Stock: The Company is currently authorized to issue up to 4,000,000 shares of Series E Convertible Preferred Stock, par value $0.0001 per share, convertible at a ratio of 1 share of Series E Convertible Preferred Stock for 100 shares of common stock. Each of these shares carries a voting right equivalent to 10,000 shares of common stock. The Company may not issue any other shares with extended voting rights.
During the year ended June 30, 2021, as part of the change in control, 4,000,000 shares were returned to treasury to be canceled. In December 2020 the Company issued 1,000,000 shares of Series E Convertible Preferred Stock accrued in the prior year and issued 450,000 shares of Series E Convertible Preferred Stock to each of its two directors, 900,000 shares total, valued at $513,000 or $0.57 per share, accrued 100,000 shares of Series E Preferred stock to be issued to directors for services, valued at $57,000 or $.57 per share, all pricing based on the conversion of one share of Series E Convertible Preferred Stock for 100 shares of common stock and the price of the common stock on the date of accrual. During the year ended June 30, 2022, the Company issued 1,000,000 shares of Series E Convertible Preferred shares to each of its two directors for services, valued at $3,000,000 or $1.50 per share, and issued 50,000 shares to each of its two directors, previously accrued for at $57,000 or $0.57 per share. All shares were recorded at the quoted common stock price of the date of agreement or grant on an as-converted basis.
As of March 31, 2023 and June 30, 2022, 4,000,000 and 4,000,000 shares of Series E Convertible Preferred Stock were issued and outstanding, respectively.
Common Stock:
The Company is currently authorized to issue 3,000,000,000 shares of common stock, par value of $0.0001 per share.
During the nine months ended March 31, 2023, the Company issued 9,612,500 shares to its officers as compensation (of which 8,825,000 shares were granted in a prior period), valued at $124,108 or $.0129 per share; 5,000,000 shares to an employee as compensation, valued at $66,000 or $0.0132 per share; 21,500,000 shares issued to investors, valued at $215,000 or $0.01 per share; and 20,000,000 shares issued for services, valued at $164,000 or $0.0082 per share. All shares were recorded at the stock price of the date of agreement or grant.
During the nine months ended March 31, 2023, the Company also recorded shares to be issued of 10,425,000 to its officers as compensation, valued at $85,949 or $.0082 per share, and shares to be issued of 65,000 to an employee as compensation, valued at $651 or $0.0100 per share. In addition, the Company recorded commitment shares to be issued of 15,000,000 valued at $78,000 or $0.0052 per share to Leonite Capital LLC in connection with the issuance of convertible notes on December 5, 2022. All shares were recorded at the stock price of the date of agreement or grant.
During the nine months ended March 31, 2023, the Company received proceeds totaling $639,500 in connection with the issuance of 70,262,500 shares of common stock. Of the total proceeds received, $325,000 in proceeds was received from the issuance of 37,812,500 common shares that were issued under the terms of subscription agreements at the contract price of $0.008. Proceeds of $304,500 were received from the issuance of 30,450,000 common shares that were issued under the terms of subscription agreements at the contract price of $0.01, and proceeds of $10,000 was received from the issuance of 2,000,000 common shares that were issued under the terms of a subscription agreement at the contract price of $0.005. These shares are yet to be issued as of March 31, 2023.
For the nine months ended March 31, 2022, the Company issued 7,000,000 common shares to employees and contractors for contractual bonuses, valued at $75,000 or $.0107 per share, accrued 5,000,000 shares in bonuses to be paid, valued at $52,500 or $.0105 per share, issued 66,418,431 for $260,000 in debt, $9,954 in interest, and $9,000 in fees, valued at $677,707, accrued 450,000 to be issued for management compensation, valued at $4,920 an average of $.0109 per share, and sold 340,000,000 shares under the Reg A at $3,400,000 or $.01 per share, 10,000,000 of which were accrued to be issued as of March 31, 2022. All shares were recorded at the quoted stock price of the date of agreement or grant.
As of March 31, 2023 and June 30, 2022, the Company has 1,549,255,108 and 1,493,142,608 shares of common stock issued and outstanding, respectively.
Note 11 – Related Party Transactions
Note payable – BOAPIN purchase
In August 2020, and effective as of June 30, 2020, the Company purchased the BOAPIN portal, including all software, licensing, and ownership rights from Hypersoft Ventures, Inc., a related party through common ownership, for $800,200, which includes six million seven hundred thousand three (6,700,003) shares of Series C Convertible Preferred stock, valued at $375,200 or $0.056 per share, based on the conversion of one share of Series C Preferred stock for 10 shares of common stock and the stock price on the date of the transaction, and a note payable for $425,000, bearing eight percent (8%) interest with no prepayment or delinquency clauses.
As of March 31, 2023 and June 30, 2022, the Company has recorded a Note payable-BOAPIN of $170,000 and $170,000, respectively. During the three and nine months ended March 31, 2023, the Company recorded interest expense of $3,353 and $10,209, respectively. During the three and nine months ended March 31, 2022, the Company recorded interest expense of $6,521 and $15,090, respectively. The accrued interest balance at March 31, 2023 and June 30, 2022 was $59,624 and $49,415, respectively.
Related party debt, net
From time to time, the Company received funds from related parties for day-to-day operations. These are short-term loans which bear no interest, and the Company expects to repay these loans by the end of the fiscal year following the year in which the short-term loan was made. The following table reflects the composition of the related party debt, net balance at March 31, 2023 and June 30, 2022. The Company had a receivable from certain management employees totaling $155,800 at June 30, 2022. The total receivable balance was subsequently collected by the Company on September 27, 2022.
| Schedule of related party transactions | | | | | | |
| | | March 31, | | | June 30, | |
| | | 2023 | | | 2022 | |
| Related parties – subsidiary | | $ | 177,320 | | | $ | 202,875 | |
| Due from related parties | | | – | | | | (155,800 | ) |
| Accrued salaries, bonus, fees | | | 330,932 | | | | 10,965 | |
| Total loans from related parties, net | | $ | 508,252 | | | $ | 58,040 | |
Note 12 – Commitments and contingencies
Legal Matters
From time to time, we may become involved in various lawsuits and legal proceedings which arise in the ordinary course of business. Litigation is subject to inherent uncertainties, and an adverse result in these or other matters may arise from time to time that may harm our business.
| | 1) | Wearable Health Solutions, Inc. v. Barry Honig, GRQ Consultants Inc., Benza Pharma LLC and John Does 1-10, Supreme Court of the State of New York County of New York, July 22, 2021. Company is disputing the validity of Notes from 3/2016 and seeking damages, reparations, and related costs. |
| | | |
| | 2) | GRQ Consultants, Inc. v. Wearable Health Solutions, Inc., Supreme Court of the State of New York, County of New York, August 26, 2021, Parties are seeking summary judgment of $50,000 plus accrued interest in response to lawsuit by Company regarding $50,000 loan from 11/2016. |
| | | |
| | 3) | Benza Pharma LLC, Sandor Capital, LP, and John Lemak v. Wearable Health Solutions, Inc., District Court, Clark County, Nevada, Parties are seeking summary judgment of $3,000,000 plus accrued interest in regards to convertible notes payable from March, 2016. The Company believes that there is a very low probability that it will pay this amount and as a result has not accrued for it on the Company’s balance sheet. On February 3, 2023, the plaintiffs in the case of Sandor Capital, LP and John Lemak v. Wearable Health Solutions, Inc., informed the Company that they would be discontinuing their legal action against the Company. |
| | | |
| | 4) | Medical Alarm Concepts LLC v. MCA Cure, LLC, Superior Court of New Jersey, Law Division, Morris County. Company is seeking return of payments for non-performance plus attorney fees and court costs. A settlement was reached between the parties whereby MCA Cure will pay an initial $10,000 and $6,500 each month until debt is satisfied in 2023 (See Note 14). |
Commitments and Contingencies. The Company follows subtopic 450-20 of the FASB Accounting Standards Codification to report accounting for contingencies. Certain conditions may exist as of the date the financial statements are issued, which may result in a loss to the Company but which will only be resolved when one or more future events occur or fail to occur. The Company assesses such contingent liabilities, and such assessment inherently involves an exercise of judgment. In assessing loss contingencies related to legal proceedings that are pending against the Company or unasserted claims that may result in such proceedings, the Company evaluates the perceived merits of any legal proceedings or unasserted claims as well as the perceived merits of the amount of relief sought or expected to be sought therein.
If the assessment of a contingency indicates that it is probable that a material loss has been incurred and the amount of the liability can be estimated, then the estimated liability would be accrued in the Company’s financial statements. If the assessment indicates that a potentially material loss contingency is not probable but is reasonably possible, or is probable but cannot be estimated, then the nature of the contingent liability, and an estimate of the range of possible losses, if determinable and material, would be disclosed. Loss contingencies considered remote are generally not disclosed unless they involve guarantees, in which case the guarantees would be disclosed. Management does not believe, based upon information available at this time that these matters will have a material adverse effect on the Company’s financial position, results of operations or cash flows. However, there is no assurance that such matters will not materially and adversely affect the Company’s business, financial position, and results of operations or cash flows.
Note 13 – Office/Warehouse lease
In February 2016, the FASB issued ASU 2016-02, Leases (Topic 842), which supersedes the current accounting for leases and while retaining two distinct types of leases, finance and operating, (1) requires lessees to record a right of use asset and a related liability for the rights and obligations associated with a lease, regardless of lease classification, and recognize lease expense in a manner similar to current accounting, (2) eliminates most real estate specific lease provisions, and (3) aligns many of the underlying lessor model principles with those in the new revenue standard. Leases with a term of 12 months or less will be accounted for similar to existing guidance for operating leases today. For public companies, the new standard is effective for annual and interim periods in fiscal years beginning after December 15, 2018. For all other entities, including emerging growth companies, this standard is effective for annual reporting periods beginning after December 15, 2019, and interim periods within fiscal years beginning after December 2020.
The Company maintains its corporate office at 2901 W. Coast Highway, Suite 200, Newport Beach, CA 92663. The Company currently pays $175 a month for its office space and the term is month-to-month. The Company’s subsidiary maintained a warehouse office in Pennsylvania to facilitate inventory arrival and product shipment. The three-year lease at $1,100 per month expired on September 30, 2021, and was renewed for 12 months at $1,300 per month beginning October 1, 2021 and expiring on September 30, 2022. The subsidiary subsequently entered into a month-to-month arrangement with this office warehouse and then terminated the arrangement and vacated the facility as of December 31, 2022. The Company entered into a new three-year lease agreement on September 9, 2022 for new warehouse space located in Mequon, Wisconsin. The monthly rent for this new warehouse space is currently $1,325 per month for the first twelve months of the lease agreement. Expenditures for the nine months ending March 31, 2023 and March 31, 2022 are as follows:
| Schedule lease cost | | | | | | |
| | | 2023 | | | 2022 | |
| Rent expense | | $ | 16,270 | | | $ | 12,555 | |
The Company leased a fulfillment center in the U.S., which was classified as an operating lease which subsequently expired on September 30, 2022. The Company determines if an arrangement qualifies as a lease at the lease inception. Operating lease liabilities are recorded based on the present value of the future lease payments over the lease term, assessed as of the commencement date. The Company’s real estate leases, which are for a fulfillment center, generally have a lease term between 3 and 5 years. The Company’s leases are comprised of fixed lease payments and also include executory costs such as common area maintenance, as well as property insurance and property taxes. The Company has elected to account for the lease and non-lease components as a single lease component for its real estate leases. Lease payments, which may include lease components and non-lease components, are included in the measurement of the Company’s lease liabilities to the extent that such payments are either fixed amounts or variable amounts based on a rate or index (fixed in substance) as stipulated in the lease contract. Any actual costs in excess of such amounts are expensed as incurred as variable lease cost.
The Company’s lease agreements generally do not specify an implicit borrowing rate, and as such, the Company utilizes its incremental borrowing rate by lease term, in order to calculate the present value of the future lease payments. The discount rate represents a risk-adjusted rate on a secured basis, and is the rate at which the Company would borrow funds to satisfy the scheduled lease liability payment streams commensurate with the lease term. The Company’s lease agreement for its warehouse space located in King of Prussia, Pennsylvania expired on September 30, 2022. The Company has terminated the month-to-month arrangement and has vacated the warehouse located in King of Prussia, Pennsylvania as of December 31, 2022. As a result, the Company entered into a new three-year lease agreement on September 9, 2022 for new warehouse space located in Mequon, Wisconsin. The monthly rent which commenced in September 2022 is $1,325 per month and increases approximately 3% annually thereafter. The discount rate used was determined based on the available data as of the lease commencement date. The Right-of-use (“ROU”) asset value added as a result of this new lease agreement was $43,058. The Company’s ROU asset and lease liability accounts reflect the inclusion of this new lease agreement on the Company’s consolidated balance sheet as of March 31, 2023.
Certain of the Company’s lease agreements, primarily related to real estate, include options for the Company to either renew (extend) or early terminate the lease. Leases with renewal options allow the Company to extend the lease term typically between 1 and 3 years. Renewal options are reviewed at lease commencement to determine if such options are reasonably certain of being exercised, which could impact the lease term. When determining if a renewal option is reasonably certain of being exercised, the Company considers several factors, including but not limited to, significance of leasehold improvements incurred on the property, whether the asset is difficult to replace, or specific characteristics unique to the particular lease that would make it reasonably certain that the Company would exercise such option. In most cases, the Company has concluded that renewal and early termination options are not reasonably certain of being exercised by the Company (and thus not included in the Company’s ROU asset and lease liability) unless there is an economic, financial or business reason to do so.
For the nine months ended March 31, 2023, total operating lease cost was $16,270 and is recorded in general and administrative expenses, dependent on the nature of the leased asset. The operating lease cost is recognized on a straight-line basis over the lease term. The following summarizes (i) the future minimum undiscounted lease payments under non-cancelable lease for each of the next four years and thereafter, incorporating the practical expedient to account for lease and non-lease components as a single lease component for our existing real estate leases, (ii) a reconciliation of the undiscounted lease payments to the present value of the lease liabilities recognized, and (iii) the lease-related account balances on the Company’s consolidated balance sheet, as of March 31, 2023:
| Schedule of future lease payments | | | |
| Fiscal Year Ending June 30, | | | |
| | | | |
| 2023 | | $ | 3,975 | |
| 2024 | | | 16,250 | |
| 2025 | | | 16,670 | |
| July & August 2025 | | | 2,790 | |
| Total future minimum lease payments | | | 39,685 | |
| Less imputed interest | | | (3,935 | ) |
| Total present value of future minimum lease payments | | $ | 35,750 | |
| Schedule of balance sheet related leases | | | |
| As of March 31, 2023 | | | |
| | | | |
| Operating lease right-of-use assets | | $ | 35,505 | |
| | | | | |
| Accrued lease liability | | | 13,643 | |
| Long-term lease liability | | | 22,107 | |
| | | $ | 35,750 | |
| | | | | |
| As of March 31, 2023 | | | | |
| | | | | |
| Weighted Average Remaining Lease Term | | | 2.42 years | |
| Weighted Average Discount Rate | | | 8.44% | |
Note 14 – Other income - settlement
Settlement
In 2019, the Company engaged MCA Cure to negotiate settlements with two note holders, and paid MCA Cure a total of $97,625. In 2020, the Company discovered MCA Cure had not performed when bank accounts were levied for $33,705 and $18,705, being subsequently refunded, and engaged an attorney to recover funds. Currently the Company has a settlement agreement in place with Susquehanna Salt Loan and has hired an attorney to recover funds and damages from MCA Cure. In February 2022, a settlement was reached with MCA Cure for fees and attorney costs of $105,125, amortized at 1.5%, by which the Company would receive an initial payment of $10,000, and $6,500 monthly until the debt is satisfied in May 2023, with stipulations for any potential default. MCA Cure ceased making payments to the Company in the three-month periods ended December 2022 and March 31, 2023, and as a result the Company is reopening its legal case against MCA Cure and an arbitration hearing has been scheduled for June 22, 2023.
For the nine months ended March 31, 2023 and 2022, the Company recorded $19,500 and $-0- in other income related to this settlement agreement, respectively.
Note 15 – Subsequent Events
The Company evaluated events that have occurred after the balance sheet date but before the financial statements are issued and determined that there are no material events that are required to be disclosed.
On April 4, 2023, the Company issued 2,000,000 shares valued at $24,000 or $0.012 per share to a computer consultant. These shares were previously accrued for by the Company in a prior period and are included in the Company’s balance sheet line item labeled “Common stock to be issued” at March 31, 2023. These shares were recorded at the stock price of the date of agreement or grant.
On May 5, 2023, the Company received $50,000 in proceeds from the issuance of 10,000,000 shares at $0.005 pursuant to the Reg A offering terms and conditions. The Company incurred $8,000 in legal fees related to this transaction.
On May 10, 2023, the Company entered into a severance and settlement agreement with Jennifer Loria, the Company’s former Chief Operating Officer of its wholly-owned subsidiary Medical Alarm Concepts LLC. The severance and settlement agreement includes payments totaling $35,000 for accrued bonus which will be paid in seven (7) monthly installments at $5,000 per installment. In addition, the Company will also pay severance of $15,000 over a three (3) month period of $5,000 per month and $15,000 in legal fees also payable in three monthly installments of $5,000. Lastly, the Company will reimburse Ms. Loria on a monthly basis for her medical insurance premiums for a period of twelve months which approximates $14,000 in aggregate for the twelve months.
On October 24, 2022 the Company and its transfer agent were named in an action in the Supreme Court of the State of New York by Acquarlaro Corp. claiming monetary damages and seeking an injunction ordering the Company’s transfer agent to transfer 56 million shares of its common stock allegedly belonging to Acqualaro Corp. to an individual. On October 26, 2022 an amended complaint was filed and on December 5, 2022 the Company filed a motion to dismiss the amended complaint. On April 13, 2023 the Court granted the Company’s motion and dismissed the amended complaint with leave for the plaintiff to refile. On April 26, 2023 the plaintiff filed a second amended complaint against the Company and adding Mr. Pizzino as a defendant. On May 8, 2023 Acqualaro filed a notice of appeal of the decision dismissing the first amended complaint.
PART III—EXHIBITS
Index to Exhibits
| Number | | Exhibit Description |
| | | |
| 2.1*** | | Amended Articles of Incorporation and Amendments Thereto (as filed on Form 10-12G with the SEC on March 23, 2022) |
| 2.2*** | | Bylaws (as filed on Form 10-12G with the SEC on March 23, 2022) |
| 3.1* | | Specimen Stock Certificate (as filed on Form 1-A with the SEC on September 11, 2020) |
| 4.1***** | | Subscription Agreement |
| 6.1* | | Asset Purchase Agreement, by and between the Company and Hypersoft Ventures, Inc., dated August 3, 2020 (as filed on Form 1-A with the SEC on September 11, 2020) |
| 6.2* | | Employment Agreement by and between the Company and Harrysen Mittler, dated May 11, 2020 (as filed on Form 1-A with the SEC on September 11, 2020) |
| 6.3* | | Employment Agreement by and between the Company and Peter Pizzino, dated May 11, 2020 (as filed on Form 1-A with the SEC on September 11, 2020) |
| 6.4* | | Promissory Note by and between the Company and Jason Cohen, dated July 31, 2020 (as filed on Form 1-A with the SEC on September 11, 2020) |
| 6.5* | | Promissory Note by and between the Company and Hypersoft Ventures, Inc., dated August 3, 2020 (as filed on Form 1-A with the SEC on September 11, 2020) |
| 6.6** | | Settlement Agreement by and between the Company and Trillium Partners LP, dated August 17, 2020 |
| 6.7** | | Court Order Approving Settlement Agreement, dated September 15, 2020 |
| 6.8*** | | Asset Purchase and Advisory Services Agreement, by and between the Company and Anthony Chetta, dated August 11, 2021. |
| 6.9*** | | Employment Agreement by and between the Company and Anthony Chetta, dated August 9, 2021 |
| 6.10*** | | Employment Agreement by and between the Company and Gail Rosenthal, dated October 10, 2021 |
| 6.11**** | | Employment Agreement by and between the Company and Vincent S. Miceli, dated May 13, 2022 |
| 11.1^ | | Auditors Consent |
| 12.1^ | | Legal Opinion |
_______________
^ Filed Herewith.
* Previously Filed by the Company on Form 1A with the SEC on September 11, 2020.
** Previously Filed by the Company on Form 1A with the SEC on October 14, 2020.
*** Previously Filed by the Company on Form 10-12G with the SEC on March 23, 2022
**** Previously Filed by the Company on Form 8-K with the SEC on May 24, 2022
***** Previously Filed by the Company on Form 1A with the SEC on March 30, 2023.
SIGNATURES
Pursuant to the requirements of Regulation A, the issuer certifies that it has reasonable grounds to believe that it meets all of the requirements for filing on Form 1-A and has duly caused this Offering Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Newport Beach, California on June 9, 2023.
| (Exact name of issuer as specified in its charter): | Wearable Health Solutions, Inc. |
| By: | /s/ Harrysen Mittler |
| | Title: Harrysen Mittler, Chief Executive Officer (Principal Executive Officer) |
(Date): | June 9, 2023 |
| | /s/ Vincent S. Miceli |
| | Title: Vincent S. Miceli, Chief Financial Officer (Principal Financial Officer, Principal Accounting Officer) |
(Date): | June 9, 2023 |
SIGNATURES OF DIRECTORS: | | |
/s/ Harrysen Mittler | | June 9, 2023 |
| | | Date |
/s/ Peter Pizzino | | June 9, 2023 |
| | | Date |






