UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-Q
| x | QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the quarterly period ended December 31, 2012
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission File Number: 000-53401
Bohai Pharmaceuticals Group, Inc.
(Exact name of registrant as specified in its charter)
Nevada (State or other jurisdiction of incorporation or organization) | 98-0697405 (I.R.S. Employer Identification No.) |
c/o YantaiBohai Pharmaceuticals Group Co. Ltd. No. 9 Daxin Road, Zhifu District Yantai, Shandong Province, China | 264000 |
| (Address of principal executive offices) | (Zip Code) |
Registrant’s telephone number (including area code): +86(535)-685-7928
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yesx No¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, or a non-accelerated filer or a smaller reporting company. See definition of “large accelerated filer”, “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer¨ | | Accelerated filer¨ |
| Non-accelerated filer¨ | | Smaller reporting companyx |
| (Do not check if a smaller reporting company) | | |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes¨Nox
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Website, if any, every Interactive Date File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files).x
As of February 14, 2013, there were 17,861,085 shares of company common stock issued and outstanding.
Bohai Pharmaceuticals Group, Inc.
Quarterly Report on Form 10-Q
TABLE OF CONTENTS
| PART I – FINANCIAL INFORMATION | |
| | |
| Cautionary Note Regarding Forward-Looking Statements | 3 |
| | |
| Item 1. | Financial Statements (unaudited) | 4 |
| | | |
| | Condensed Consolidated Balance Sheets as of December 31, 2012 and June 30, 2012 | 4 |
| | | |
| | Condensed Consolidated Statements of Income and Comprehensive Income for the Three and Six Months ended December 31, 2012 and 2011 | 5 |
| | | |
| | Condensed Consolidated Statements of Changes in Stockholders’ Equity for the Six Months Ended December, 2012 | 6 |
| | | |
| | Condensed Consolidated Statements of Cash Flows for the Six Months ended December 31, 2012 and 2011 | 7 |
| | | |
| | Notes to Condensed Consolidated Financial Statements | 8 |
| | | |
| Item 2. | Management’s Discussion and Analysis of Financial Condition and Results of Operations | 26 |
| | | |
| Item 3. | Quantitative and Qualitative Disclosures About Market Risk | 38 |
| | | |
| Item 4. | Controls and Procedures | 38 |
| | |
| PART II – OTHER INFORMATION | |
| | |
| Item 1. | Legal Proceedings | 39 |
| Item 2. | Unregistered Sales of Equity Securities and Use of Proceeds | 40 |
| Item 3. | Defaults Upon Senior Securities | 40 |
| Item 4. | Mine Safety Disclosures | 40 |
| Item 5. | Other Information | 40 |
| Item 6. | Exhibits | 40 |
| | |
| SIGNATURES | 41 |
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
In addition to historical information, this Quarterly Report on Form 10-Q contains forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These forward-looking statements are subject to certain risks and uncertainties that could cause actual results to differ materially from those reflected in such forward-looking statements. We cannot give any guarantee that the plans, intentions or expectations described in the forward looking statements will be achieved. All forward-looking statements involve significant risks and uncertainties, and actual results may differ materially from those discussed in the forward-looking statements as a result of various factors, including those factors described in the “Risk Factors” section of our Annual Report for the fiscal year ended June 30, 2012. Readers should carefully review such risk factors as well as factors described in other documents that we file from time to time with the Securities and Exchange Commission.
In some cases, you can identify forward-looking statements by terminology such as “guidance,” “may,” “will,” “should,” “expects,” “plans,” “anticipates,” “believes,” “estimates,” “predicts,” “projects,” “potential,” “proposed,” “intended,” or “continue” or the negative of these terms or other comparable terminology. You should read statements that contain these words carefully, because they discuss our expectations about our future operating results or our future financial condition or state other “forward-looking” information. There may be events in the future that we are not able to accurately predict or control. You should be aware that the occurrence of any of the events described in our risk factors and other disclosures could substantially harm our business, results of operations and financial condition, and that upon the occurrence of any of these events, the trading price of our securities could decline. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, growth rates, and levels of activity, performance or achievements. Factors that may cause actual results, our performance or achievements, or industry results, to differ materially from those contemplated by such forward-looking statements include, without limitation:
| | § | our ability to generate or obtain through financing sufficient working capital to (i) fund the acquisition of Yantai Tianzheng (a total of $12,827,185 is currently due); (ii) satisfy our obligations under our convertible notes due April 5, 2013 (currently $8,464,500 is due), (iii) satisfy an approximately $12,680,000 payable in 2013 for a new land use right we acquired in November 2012 (due in two equal installments at June 30 and December 31, 2013) or (iii) otherwise to support our business plans; |
| | | |
| | § | our ability to integrate the business of Yantai Tianzheng and any future acquisitions into our business; |
| | § | our ability to expand or adjust the mix of our product offerings and maintain the quality of our products; |
| | § | the availability of government granted rights to exclusively manufacture or co-manufacture our products; |
| | § | the availability of national healthcare reimbursement of our products in the PRC; |
| | § | our ability to manage our expanding operations and continue to fill customers’ orders on time; |
| | § | our ability to maintain adequate control of our expenses allowing us to realize anticipated revenue growth; |
| | § | our ability to maintain or protect our intellectual property; |
| | § | our ability to maintain our proprietary technology; |
| | § | the impact of government regulation in China and elsewhere, including the support provided by the Chinese government to the Traditional Chinese Medicine and healthcare sectors in China; |
| | § | our ability to implement product development, marketing, sales and acquisition strategies and adapt and modify them as needed; |
| | § | our implementation of required financial, accounting and disclosure controls and procedures and related corporate governance policies; and |
| | § | our ability to anticipate and adapt to changing conditions in the Traditional Chinese Medicine and healthcare industries resulting from changes in government regulations, mergers and acquisitions involving our competitors, technological developments and other significant competitive and market dynamics. |
We cannot give any guarantee that our plans, intentions or expectations will be achieved. All forward-looking statements involve significant risks and uncertainties, and actual results may differ materially from those discussed in the forward-looking statements as a result of various factors, including those factors listed above and described in the “Risk Factors” section of our Annual Report on Form 10-K for the fiscal year ended June 30, 2012. Except as required by applicable law and including the securities laws of the United States, we do not intend to update any of the forward-looking statements to conform these statements to actual results.
BOHAI PHARMACEUTICALS GROUP, INC. AND SUBSIDIARIES
CONDENSED CONSOLIDATED BALANCE SHEETS
| | | December 31, | | | June 30, | |
| | | 2012
(UNAUDITED) | | | 2012 | |
| ASSETS | | | | | | | | |
| Current assets: | | | | | | | | |
| Cash | | $ | 3,154,584 | | | $ | 18,386,288 | |
| Restricted cash | | | 8,407,565 | | | | 9,449,905 | |
| Accounts receivable | | | 42,866,521 | | | | 29,670,552 | |
| Inventories | | | 3,280,053 | | | | 3,795,915 | |
| Prepaid expenses and other current assets | | | 487,141 | | | | 879,696 | |
| | | | | | | | | |
| Total current assets | | | 58,195,864 | | | | 62,182,356 | |
| | | | | | | | | |
| Non - current assets: | | | | | | | | |
| Property, plant and equipment, net | | | 11,840,729 | | | | 11,681,272 | |
| Prepayment for property, plant and equipment | | | 2,626,567 | | | | 594,508 | |
| Intangible assets - pharmaceutical formulas | | | 13,823,121 | | | | 25,610,557 | |
| Long term prepayments - land use right, net | | | 37,388,468 | | | | 18,739,297 | |
| Other intangible assets, net | | | 29,762,154 | | | | 21,497,890 | |
| Goodwill | | | 5,096,740 | | | | 5,092,139 | |
| Total Non - current assets | | | 100,537,779 | | | | 83,215,663 | |
| | | | | | | | | |
| TOTAL ASSETS | | $ | 158,733,643 | | | $ | 145,398,019 | |
| | | | | | | | | |
| LIABILITIES AND STOCKHOLDERS’ EQUITY | | | | | | | | |
| | | | | | | | | |
| Current liabilities: | | | | | | | | |
| Convertible notes, due April 5, 2013 | | $ | 8,464,500 | | | $ | 10,036,000 | |
| Accounts payable | | | 3,612,366 | | | | 3,334,101 | |
| Accrued expenses | | | 12,126,265 | | | | 8,478,054 | |
| Land use right payable | | | 12,681,102 | | | | 0 | |
| Income taxes payable | | | 2,772,175 | | | | 2,338,825 | |
| Acquisition purchase price payable - current portion | | | 0 | | | | 5,000,000 | |
| Derivative liabilities - investor and agent warrants | | | 0 | | | | 1,211,236 | |
| Due to Related Party | | | 44,044 | | | | 36,002 | |
| | | | | | | | | |
| Total current liabilities | | | 39,700,452 | | | | 30,434,218 | |
| | | | | | | | | |
| Non - current liabilities: | | | | | | | | |
| Acquisition purchase price payable - non-current portion | | | 12,827,185 | | | | 20,300,000 | |
| Deferred tax liability | | | 7,845,142 | | | | 8,161,269 | |
| Total Non - current liabilities | | | 20,672,327 | | | | 28,461,269 | |
| | | | | | | | | |
| TOTAL LIABILITIES | | | 60,372,779 | | | | 58,895,487 | |
| | | | | | | | | |
| COMMITMENTS, CONTINGENCIES, AND OTHER MATTERS | | | | | | | | |
| | | | | | | | | |
| STOCKHOLDERS' EQUITY | | | | | | | | |
| Common stock, $0.001 par value, 150,000,000 shares authorized, 17,861,085 shares issued and outstanding as of December 31, 2012 and June 30, 2012, respectively | | | 17,861 | | | | 17,861 | |
| Additional paid-in capital | | | 24,615,353 | | | | 24,615,353 | |
| Accumulated other comprehensive income | | | 6,440,159 | | | | 6,236,076 | |
| Statutory reserves | | | 2,201,817 | | | | 2,201,817 | |
| Retained earnings | | | 65,085,674 | | | | 53,431,425 | |
| | | | | | | | | |
| Total stockholders’ equity | | | 98,360,864 | | | | 86,502,532 | |
| | | | | | | | | |
| TOTAL LIABILITIES AND STOCKHOLDERS' EQUITY | | $ | 158,733,643 | | | $ | 145,398,019 | |
See accompanying notes to the condensed consolidated financial statements
BOHAI PHARMACEUTICALS GROUP, INC. AND SUBSIDIARIES
CONDENSED CONSOLIDATED STATEMENTS OF
INCOME AND COMPREHENSIVE INCOME
(UNAUDITED)
| | | For the Three Months Ended December 31, | | | For the Six Months Ended December 31, | |
| | | 2012 | | | 2011 | | | 2012 | | | 2011 | |
| Net revenues | | $ | 40,932,506 | | | $ | 34,836,886 | | | $ | 76,281,326 | | | $ | 64,764,742 | |
| Cost of revenues | | | 8,632,261 | | | | 7,982,397 | | | | 17,224,365 | | | | 14,926,117 | |
| | | | | | | | | | | | | | | | | |
| Gross profit | | | 32,300,245 | | | | 26,854,489 | | | | 59,056,961 | | | | 49,838,625 | |
| | | | | | | | | | | | | | | | | |
| Operating expenses: | | | | | | | | | | | | | | | | |
| Selling, general and administrative expenses | | | 22,546,807 | | | | 18,108,529 | | | | 39,753,923 | | | | 33,713,420 | |
Impairment Charge - drug formula | | | 1,688,486 | | | | 0 | | | | 1,688,486 | | | | 0 | |
| Depreciation and amortization | | | 1,343,204 | | | | 617,463 | | | | 2,302,094 | | | | 1,247,061 | |
| | | | | | | | | | | | | | | | | |
| Total Operating expenses | | | 25,578,497 | | | | 18,725,992 | | | | 43,744,503 | | | | 34,960,481 | |
| Income from operations | | | 6,721,748 | | | | 8,128,497 | | | | 15,312,458 | | | | 14,878,144 | |
| | | | | | | | | | | | | | | | | |
| Other income (expenses): | | | | | | | | | | | | | | | | |
| Interest income | | | 8,541 | | | | 16,924 | | | | 26,326 | | | | 38,166 | |
| Interest expenses | | | (426,323 | ) | | | (6,951,562 | ) | | | (939,356 | ) | | | (9,574,073 | ) |
| Other (expenses) income, net | | | 115 | | | | (1,322 | ) | | | (16,591 | ) | | | (8,402 | ) |
| Change in fair value of derivative liabilities | | | 713,234 | | | | (191,747 | ) | | | 1,211,236 | | | | 140,738 | |
| Total other income (expenses) | | | 295,567 | | | | (7,127,707 | ) | | | 281,615 | | | | (9,403,211 | ) |
| Income before provision for income taxes | | | 7,017,315 | | | | 1,000,790 | | | | 15,594,073 | | | | 5,474,933 | |
| Provision for income taxes | | | (1,758,578 | ) | | | (2,296,292 | ) | | | (3,939,824 | ) | | | (4,114,651 | ) |
| Net income (loss) | | $ | 5,258,737 | | | $ | (1,295,502 | ) | | $ | 11,654,249 | | | $ | 1,360,282 | |
| | | | | | | | | | | | | | | | | |
| Comprehensive income (loss): | | | | | | | | | | | | | | | | |
| Net income (loss) | | $ | 5,258,737 | | | $ | (1,295,502 | ) | | $ | 11,654,249 | | | $ | 1,360,282 | |
| Unrealized foreign currency translation gain | | | 453,030 | | | | 1,040,980 | | | | 204,083 | | | | 1,655,580 | |
| Comprehensive income (loss) | | $ | 5,711,767 | | | $ | (254,522 | ) | | $ | 11,858,332 | | | $ | 3,015,862 | |
| | | | | | | | | | | | | | | | | |
| Net income (loss) per common share | | | | | | | | | | | | | | | | |
| Basic | | $ | 0.29 | | | $ | (0.07 | ) | | $ | 0.65 | | | $ | 0.08 | |
| Diluted | | $ | 0.25 | | | $ | (0.07 | ) | | $ | 0.55 | | | $ | 0.08 | |
| Weighted average common shares outstanding | | | | | | | | | | | | | | | | |
| Basic | | | 17,861,085 | | | | 17,861,085 | | | | 17,861,085 | | | | 17,861,085 | |
| Diluted | | | 22,093,335 | | | | 17,861,085 | | | | 22,093,335 | | | | 17,861,085 | |
See accompanying notes to the condensed consolidated financial statements
BOHAI PHARMACEUTICALS GROUP, INC. AND SUBSIDIARIES
CONDENSED CONSOLIDATED STATEMENT OF CHANGES IN STOCKHOLDERS’ EQUITY
FOR THE SIX MONTHS ENDED DECEMBER 31, 2012
(UNAUDITED)
| | | Common stock | | | Additional | | | | | | | | | | | | Total | |
| | | Shares
outstanding | | | Amount | | | paid-in
capital | | | Accumulated other
comprehensive income | | | Statutory
Reserves | | | Retained Earnings | | | Stockholders’
Equity | |
| | | | | | | | | | | | | | | | | | | | | | |
| Balance at June 30, 2012 | | | 17,861,085 | | | $ | 17,861 | | | $ | 24,615,353 | | | $ | 6,236,076 | | | $ | 2,201,817 | | | $ | 53,431,425 | | | $ | 86,502,532 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Foreign currency translation adjustment | | | 0 | | | | 0 | | | | 0 | | | | 204,083 | | | | 0 | | | | 0 | | | | 204,083 | |
| Net income | | | 0 | | | | 0 | | | | 0 | | | | 0 | | | | 0 | | | | 11,654,249 | | | | 11,654,249 | |
| Balance at December 31, 2012 | | | 17,861,085 | | | $ | 17,861 | | | $ | 24,615,353 | | | $ | 6,440,159 | | | $ | 2,201,817 | | | $ | 65,085,674 | | | $ | 98,360,864 | |
See accompanying notes to the condensed consolidated financial statements
BOHAI PHARMACEUTICALS GROUP, INC. AND SUBSIDIARIES
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
(UNAUDITED)
| | | For the Six Months Ended | |
| | | December 31, | |
| | | 2012 | | | 2011 | |
| | | | | | | |
| Cash flows from operating activities: | | | | | | | | |
| | | | | | | | | |
| Net income | | $ | 11,654,249 | | | $ | 1,360,282 | |
| Adjustments to reconcile net income to net cash provided by operating activities: | | | | | | | | |
| | | | | | | | | |
| Depreciation and amortization | | | 2,553,839 | | | | 1,452,600 | |
| Impairment of intangible assets - pharmaceutical formulas | | | 1,688,486 | | | | 0 | |
| Amortization of debt issue costs | | | 0 | | | | 472,140 | |
| Non-cash interest-convertible notes | | | 0 | | | | 8,597,701 | |
| Change in fair value of warrants | | | (1,211,236 | ) | | | (140,738 | ) |
| Stock based compensation | | | 0 | | | | 44,000 | |
| Deferred income taxes | | | (323,476 | ) | | | 460,185 | |
| | | | | | | | | |
| Changes in operating assets and liabilities: | | | | | | | | |
| Accounts receivable | | | (13,168,118 | ) | | | (3,034,163 | ) |
| Prepaid expenses and other assets | | | 393,515 | | | | 887,328 | |
| Inventories | | | 519,251 | | | | (99,530 | ) |
| Accounts payable | | | 275,229 | | | | (405,731 | ) |
| Accrued liabilities | | | 3,637,723 | | | | 1,115,297 | |
| Income taxes payable | | | 431,203 | | | | 1,048,516 | |
| Advance from customers | | | 0 | | | | (78,257 | ) |
| Restricted cash | | | 0 | | | | 10,814 | |
| | | | | | | | | |
| Net cash provided by operating activities | | | 6,450,665 | | | | 11,690,444 | |
| | | | | | | | | |
| Cash flows used in investing activities: | | | | | | | | |
| Purchases of property, plant and equipment | | | (433,131 | ) | | | (1,220,889 | ) |
| Proceeds from disposal of property, plant and equipment | | | 0 | | | | 101,734 | |
| Land use rights payments | | | (6,340,048 | ) | | | (143,471 | ) |
| Property, plant and equipment deposits | | | (2,031,361 | ) | | | 0 | |
| Cash received in acquisition of business | | | 0 | | | | 1,358,078 | |
| Cash paid for acquisition of business | | | (12,472,815 | ) | | | (9,700,000 | ) |
| | | | | | | | | |
| Deposit of restricted cash-convertible note escrow deposit | | | 1,885,695 | | | | 407,186 | |
| Release of restricted cash- convertible note escrow deposit | | | (834,988 | ) | | | (407,186 | ) |
| | | | | | | | | |
| Net cash used in investing activities | | | (20,226,648 | ) | | | (9,604,548 | ) |
| | | | | | | | | |
| Cash flows from financing activities: | | | | | | | | |
| Repayment of short term borrowings | | | 0 | | | | (1,479,058 | ) |
| Borrowing from related party | | | 8,020 | | | | 0 | |
Repayment of receivable due from related party | | | 0 | | | | 9,497 | |
| Repayment of convertible notes | | | (1,571,500 | ) | | | 0 | |
| Capital contribution from shareholder | | | 0 | | | | 6,260,565 | |
| | | | | | | | | |
| Net cash flows (used in) provided by financing activities | | | (1,563,480 | ) | | | 4,791,004 | |
| | | | | | | | | |
| Effect of foreign currency translation on cash and cash equivalents | | | 107,759 | | | | 212,634 | |
| | | | | | | | | |
| Net (decrease) increase in cash and cash equivalents | | | (15,231,704 | ) | | | 7,089,534 | |
| | | | | | | | | |
| Cash and cash equivalents at beginning of period | | | 18,386,288 | | | | 13,344,426 | |
| | | | | | | | | |
| Cash and cash equivalents at end of period | | $ | 3,154,584 | | | $ | 20,433,960 | |
| | | | | | | | | |
| Cash paid during the period for: | | | | | | | | |
| Interest | | $ | 282,150 | | | $ | 506,046 | |
| Income taxes | | $ | 3,832,103 | | | $ | 2,656,241 | |
| | | | | | | | | |
| Supplemental cash flow information | | | | | | | | |
| | | | | | | | | |
| Non-cash investing and financing activities: | | | | | | | | |
| Land use right increase by land use right payable | | | 12,681,102 | | | | 0 | |
| Acquisition liability | | $ | | | | $ | 25,300,000 | |
See accompanying notes to the condensed consolidated financial statements
BOHAI PHARMACEUTICALS GROUP, INC. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
FOR THE SIX MONTHS ENDED DECEMBER 31, 2012
| 1. | ORGANIZATION AND PRINCIPAL ACTIVITIES |
The Company’s Operations
Bohai Pharmaceuticals Group, Inc. (“BPGI”) was incorporated under the laws of the State of Nevada on January 9, 2008 under the name of Link Resources, Inc. Prior to January 5, 2010, BPGI was a public “shell” company. BPGI became a public operating company on January 5, 2010 pursuant to a Share Exchange Transaction completed on January 5, 2010.
BPGI is engaged in the production, manufacturing and distribution of herbal pharmaceuticals based on traditional Chinese medicine (“TCM”) in the People’s Republic of China (“China” or the “PRC”) through the following two operating subsidiaries:
(i) Yantai Bohai Pharmaceuticals Group Co., Ltd., (“Bohai”) a PRC company and the Company’s original operating subsidiary BPGI controls Bohai through a variable interest entity arrangement (“VIE”) described below; and
(ii) Yantai Tianzheng Pharmaceuticals Company, Ltd., a PRC company (“Yantai Tianzheng”) which BPGI acquired effective July 1, 2011 through a newly formed PRC wholly-foreign owned enterprise subsidiary, Yantai Nirui Pharmaceuticals, Ltd. (“WFOE II”).
BPGI owns 100% of Chance High International Limited, a British Virgin Islands company (“Chance High”). Chance High owns 100% of the issued and outstanding shares of capital stock of a Chinese wholly-foreign owned enterprise known as Yantai Shencaojishi Pharmaceuticals Co., Ltd. (the “WFOE”). On December 7, 2009 (prior to the date of the Share Exchange Transaction), the WFOE entered into a series of variable interest entity contractual agreements (the “VIE Agreements”) with Bohai and its three shareholders, including Mr. Hongwei Qu, the Company’s current Chairman and Chief Executive Officer (“Mr. Qu”). Mr. Qu currently owns 96.7% of the outstanding equity interests of Bohai and two other shareholders who collectively own the remaining 3.3% of Bohai.
The VIE Agreements include (i) a Consulting Services Agreement, (ii) an Operating Agreement, and (iii) a Proxy Agreement, through which the WFOE has the right to advise, consult, manage and operate Bohai for an annual fee equal to all of Bohai’s yearly net profits after tax. Pursuant to these agreements, the WFOE indirectly owns but has 100% managerial and economic control of the business activities of Bohai including the right to appoint all executives and senior management and members of the board of directors of Bohai. Additionally, Bohai’s shareholders pledged their rights, titles and equity interest in Bohai as security for the WFOE to collect consulting and services fees provided to Bohai pursuant to an equity pledge agreement. In order to further reinforce the WFOE’s rights to control and operate Bohai, Bohai’s shareholders granted the WFOE an exclusive right and option to acquire all of their equity interests in Bohai through an option agreement. The VIE Agreements have perpetual terms unless otherwise determined by PRC law, and can (particularly in the case of the Consulting Services Agreement (which is the principal VIE Agreement) be terminated by the parties under certain circumstances, including material breach, the termination of Bohai’s business or a liquidation of Bohai. The WFOE (which is controlled indirectly by BPGI through Chance High) can also terminate the Consulting Services Agreement at will.
BPGI, its wholly owned subsidiary Chance High, WFOE, WFOE II, Bohai and Yantai Tianzheng are referred to herein collectively and as a consolidated basis as the “Company” or “we”, “us” or “our” or similar terminology. Mr. Qu currently serves the Company’s Chairman, Chief Executive Officer and President. As used herein, the term “Common Stock” means the common stock of BPGI, $0.001 par value per share.
BPGI is headquartered and maintains its principal operations in the city of Yantai, Shandong Province, China, and conducts business operations exclusively in the PRC.
Recent Developments
During the three months ended December 31, 2012, management performed an evaluation of the Company’s product portfolio based on a confluence of factors that have emerged within China’s pharmaceutical industry and consumer markets. These factors include, among others, (i) current economic conditions in China and management’s expectations of future economic trends, (ii) changes in China’s Essential Drug Laws, (iii) the PRC Central Government’s policy designating lists of specific drugs eligible for reimbursement, (iv) consumers’ preference for drug delivery systems in the forms of pills, tablets and ingestible liquids, and (v) competition from other drug manufacturing companies within China. Based on these factors, management determined that the Company will streamline its operations to focus on the continued distribution of Lung Nourishing Syrup, Tongbi Capsules, Tongbi Tablets, Fangfengtongsheng Granule, and Zhengxintai Capsules and certain other products. As a consequence, management determined that it would be in the best interests of the Company and its stockholders to commit to a plan of (i) fully abandoning plans to create new products from a limited number of formulas purchase in 2005, and (ii) indefinitely suspending plans to develop and produce a more diversified portfolio that would be derived from a series of other approved formulas that the Company purchased in 2010 and 2005.
As a result, the Company (i) recorded an impairment charge of $1,688,486 for the aggregate carrying amount of certain product formulas that the Company will abandoned in their entirety, and (ii) reclassified $10,121,956 for the carrying amount of certain other product formulas that the Company will hold as defensive assets. Formulas to be held as defensive assets will be amortized over a period of 8 years (Note 6).
| 2. | LIQUIDITY AND FINANCIAL CONDITION |
The Company’s net income amounted to $5,258,737 and $11,654,249 for the three and six-month periods ended December 31, 2012. The Company’s cash flows from operations amounted to $6,450,665 for the six months ended December 31, 2012. The Company had working capital of approximately $18,495,412 as of December 31, 2012, including an $8,464,500 convertible note obligation, which following a series of amendments thereto is set to mature on April 5, 2013 (see below and Note 10). The Company has historically financed its operations principally from cash flows generated from operating activities and external financing raised in the private placement of convertible notes described above.
On August 8, 2011, the Company, through WFOE II, signed a share transfer agreement with the shareholders of Yantai Tianzheng to acquire 100% of Yantai Tianzheng for total purchase consideration of US$35,000,000 (paid in its RMB equivalent, of which US$6,000,000 was paid as of the Execution Date of the acquisition and the remaining $29,000,000 was payable in series of installments which the Company at its discretion, could elect to defer (Note 11). The Company paid $19,700,000 to date (of which $10,000,000 was paid during the three months ended December 31. 2012) and elected to defer $15,300,000. The Company also made $2,472,815 of individual income tax payments to tax authorities on behalf of the former Yantai Tianzheng shareholders concurrent with the $10,000,000 of principal payments. The tax payments made on behalf of the former Yantai Tianzheng shareholders was applied as a reduction of the remaining principal balance of the acquisition purchase price payable. As of December 31, 2012, the balance of $12,827,185 is payable as follows; $7,827,185 is due on August 8, 2014, and the remaining $5,000,000 is due on February 8, 2015.
The Company is expecting to gain the benefits of the economies of scale that management believes could be realized by having combined and streamlining the cost structures of the historical Bohai and the acquired Yantai Tianzheng businesses. As described above, the Company has committed to a plan of streamlining the combined around the more focused portfolio of products that include non-prescription drug products acquired as part of the YantaiTianzheng’s product portfolio.
On June 8, 2010, Yantai Tianzheng signed an agreement with Yantai Huanghai Construction Co. to construct certain portions of a factory The total contract price amounted to approximately $3.07 million (RMB 19.5 million). Construction is estimated to be completed in April 2013. The remaining commitment of the contract was approximately $1.25 million (RMB 7.9 million) as of December 31, 2012.
On November 5, 2012, the Company acquired a new land use right of 266,668 square meters located in the high-tech development district of Laishan. The Company was granted the right to use the land for a period of 50 years at a total cost of approximately $19 million (RMB 120,000,000). As of December 31, 2012, the Company paid $6,340,550. The Company is obligated to make two remaining installment payments of $6,340,551 each by June 30, 2013 and December 31, 2013 (see Note 7).
Management believes, based on the Company’s historical ability to fund operations using internally generated cash flow and the progress made towards integrating the business of Yantai Tianzheng, and subsequent commitment to focus on a more streamlined higher margin product portfolio, that the Company’s currently available cash and funds it expects to generate from operations will enable it to operate the business and satisfy short term obligations through at least January 1, 2014. Notwithstanding, the Company still has substantial obligations described herein and there is no assurance that unforeseen circumstances would not have a material adverse effect on the Company’s financial condition.
As described above, the Company has ongoing obligations with respect to the Yantai Tianzheng acquisition, whether or not the intended benefits of the acquisition are realized. The Company also has amounts due under its 2012 acquisition of land use rights. The Company is also required to repay the remaining $8,464,500 convertible notes balance due as of December 31, 2012, which pursuant to a series of amendments to the original notes, is currently due on an extended maturity date of April 5, 2013. As described in Note 10, the Company has been unable to convert a sufficient number of RMBs into US dollars due to certain currency restrictions in China. As a result, the Company was unable to repay the notes upon their original maturity of January 5, 2012.
As described whereas herein, the Company has at times, been in temporary default of its obligation to repay the convertible notes at previously extended maturity dates. Should the Company be unable to repay the notes on April 5, 2013 in the absence of a further extension of the maturity date, this circumstance would constitute an event of default under the terms of loan agreement. The Company cannot predict what the implications of the non-payment of the loan would be other than it would continue to experience difficulty converting sufficiency currency and will maintain an escrow account of restricted funds intended to secure their repayment. The non-payment of the notes could have a material adverse effect on the Company should the note holders pursue further action.
The Company will require significant additional capital in order to fund these obligations and execute its longer term business plan. If the Company is unable to generate sufficient operating cash flows or raise additional capital, or encounters unforeseen circumstances that place constraints on its capital resources, management will be required to take various measures to conserve liquidity. Such measures could include, but not necessarily be limited to, curtailing the Company’s business development activities (as was done recently), suspending the pursuit of one or more elements of its business plan, and controlling overhead expenses. There is a material risk, and management cannot provide any assurances, that the Company will raise additional capital if needed. The Company has not received any commitments for new financing, and cannot provide any assurance that new financing will be available to the Company on acceptable terms, if at all. The failure of the Company to fund its obligations when needed would have a material adverse effect on its business and results of operations.
| 3. | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES |
Principles of Consolidation
The accompanying consolidated financial statements include the accounts of BPGI, its wholly-owned subsidiary Chance High, WFOE, WFOE II, Yantai Tianzheng and Bohai. All significant intercompany accounts and transactions have been eliminated in consolidation.
The Company, in determining whether it is required to consolidate investee businesses, considers both the voting and variable interest models of consolidation as required under applicable GAAP. The Company adopted FAS Accounting Standards Codification (“ASC”) 810-10-15-14 and also ASC 810-10-05-8, which requires that a VIE be consolidated if that company is entitled to receive a majority of the VIE’s residual returns and has direct ability to make decisions on all operating activities of the VIE. The Company controls Bohai through the VIE Agreements described in Note 1, under the following series of agreements entered into on December 7, 2009.
Under the Operating Agreement entered into between WFOE and Bohai, the WFOE has the direct ability to make decisions on all the operating activities and exercise all voting rights of Bohai. Under the Consulting Services Agreement entered into between WFOE and Bohai, Bohai agreed to pay all of its net income to WFOE quarterly as a consulting fee. Accordingly, WFOE has the right to receive the expected residual returns of Bohai. As such, the Company is the primary beneficiary of and maintains controlling managerial and financial interest in, Bohai in accordance with ASC 810-10-15-14. Accordingly, Bohai’s financial position and results of operations are consolidated with those of the Company for all periods presented.
We initially measured the assets, liabilities, and non-controlling interests of Bohai at their carrying amounts as of the date of the Share Exchange. We have subsequently accounted for the assets, liabilities, and non-controlling interest of Bohai as if it was consolidated based on voting interests. The usual accounting rules for which the VIE operates are applied as they would to a consolidated subsidiary as follows:
| · | Carrying amounts of the VIE are consolidated into the financial statements of the Company as the primary beneficiary, or Primary Beneficiary (“PB”); and |
| · | Inter-company transactions and balances, such as revenues and costs, receivables and payables between or among the PB and the VIE(s) are eliminated in their entirety. |
The carrying amount and classification of Bohai’s assets and liabilities included in the condensed consolidated balance sheets are as follows:
| | | December 31, | | | June 30, | |
| | | 2012
(unaudited) | | | 2012 | |
| | | | | | | |
| Total current assets* | | $ | 59,807,268 | | | $ | 51,470,381 | |
| Total assets* | | | 127,235,857 | | | | 99,899,826 | |
| Total current liabilities** | | | 29,839,063 | | | | 11,689,137 | |
| Total liabilities** | | $ | 33,416,087 | | | $ | 15,277,230 | |
* Includes intercompany accounts in the amounts of $25,319,663 and $20,338,295 in current assets as of December 31, 2012 and June 30, 2012, respectively, that were eliminated in consolidation.
** Includes intercompany accounts in the amounts of $3,443,860 and $2,490,528 in current liabilities as of December 31, 2012 and June 30, 2012, respectively, that were eliminated in consolidation.
Basis of Presentation
The condensed consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America for interim financial statements and with the instructions to Form 10-Q and Article 8 of Regulation S-X of the United States Securities and Exchange Commission (“SEC”). Accordingly, they do not contain all information and footnotes required by GAAP for annual financial statements. In the opinion of the Company’s management, the accompanying unaudited condensed consolidated financial statements contain all adjustments necessary (consisting only of normal recurring accruals) to present the financial position of the Company as of December 31, 2012 and the results of operations and cash flows for the periods presented. The results of operations for the six months ended December 31, 2012 are not necessarily indicative of the operating results for the full fiscal year or any future period. These condensed consolidated financial statements should be read in conjunction with the consolidated financial statements and related notes thereto included in the Company’s Annual Report on Form 10-K for the year ended June 30, 2012. The Company’s accounting policies are described in the Notes to Consolidated Financial Statements in its Annual Report on Form 10-K for the year ended June 30, 2012, filed on September 28, 2012, and updated, as necessary, in this Quarterly Report on Form 10-Q.
Business Segments
The Company’s operates its business through a single reporting segment.
Use of Estimates
The preparation of the condensed consolidated financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the dates of the financial statements and the reported amounts of revenues and expenses during the reporting periods. Management makes these estimates using the best information available at the time the estimates are made; however, actual results could differ materially from those results.
Significant estimates and assumptions include allocating purchase consideration issued in business combinations, valuing equity securities and derivative financial instruments issued in financing transactions and in share-based payment arrangements, accounts receivable reserves, inventory reserves, and evaluating the carrying amounts and useful lives of intangible assets. Certain estimates, including accounts receivable and inventory reserves and the carrying amounts of intangible assets (including present value of future cash flow estimates for the Company’s pharmaceutical formulas) could be affected by external conditions including those unique to the Company’s industry and general economic conditions. It is reasonably possible that these external factors could have an effect on management’s estimates that could cause actual results to differ from management’s estimates.
Company management re-evaluates all of accounting estimates at least quarterly based on these conditions and records adjustments, when necessary.
Cash and Cash Equivalents
We consider all highly liquid investments purchased with original maturities of three months or less to be cash equivalents. We maintain bank accounts in the PRC and a checking account in the United States of America that principally consist of demand deposits. We also have restricted cash accounts in the United States that include funds designated for interest payments due to convertible note holders and for use in investor relations programs pursuant to a securities purchase agreement.
Restricted Cash
The Company is required by its Note holders to maintain deposits in escrow accounts to fund the principal and interest payments under convertible Notes obligation. Escrow account balances amounted to $8,407,565 and $9,449,905 as of December 31, 2012 and June 30, 2012, respectively. As of December 31, 2012, the Company had one escrow account in China amounting to $8,401,230 and one escrow account in US amounting to $6,335. As of June 30, 2012, the Company had one escrow account in China amounting to $9,343,870 and one escrow account in US amounting to $106,035.
Accounts Receivable
Accounts receivable consists of amounts due from customers. We extend unsecured credit to our customers in the ordinary course of business but mitigate the associated risks by performing credit checks and actively pursuing past due accounts. Company’s credit terms generally range from 90 to 180 days. The Company has one customer with payment terms of up to 360 days with an outstanding balance of $2,532,337 as of December 31, 2012. The Company’s policy with respect accounts receivable reserves is to establish an allowance for doubtful accounts based on management’s assessment of known requirements, aging of receivables, payment history, specific customer’s current credit worthiness, and the economic environment. The Company has a significantly low history of credit losses and no historical pattern of making any price or collection concessions with respect of its accounts receivable balances. Accordingly, an allowance for doubtful accounts is not considered necessary based on management’s assessment.
Inventories
Inventories are valued at the lower of cost, determined using the weighted average method, or market. Finished goods inventories include the costs of raw materials, direct labor and overhead associated with the manufacturing process. In assessing the ultimate realization of inventories, management makes judgments as to future demand requirements compared to current or committed inventory levels. Our reserve requirements generally increase/decrease due to management’s projected demand requirements, market conditions and product life cycle changes. As of December 31, 2012 and June 30, 2012, management does not believe that any inventory reserves are necessary.
Property, Plant and Equipment
Property, plant and equipment are carried at cost and are depreciated on a straight-line basis over the estimated useful lives of the assets that range from 5 to 10 years for office equipment, machinery, and vehicles and 30 to 40 years for buildings. The cost of repairs and maintenance is charged to expense as incurred; major replacements and improvements are capitalized. When assets are retired or disposed of, the cost and accumulated depreciation are removed from the accounts, and any resulting gains or losses are included in income in the year of disposition. We examine the possibility of impairment in the value of fixed assets when events or changes in circumstances reflect the fact that their recorded value may not be recoverable.
Intangible Asset – Pharmaceutical Formulas
The Company has purchased pharmaceutical formulas that were approved by the State Food and Drug Administration of China (“SFDA”). These formulas can be renewed every 5 years without limitation for a minimum fee and are subject to certain protections under PRC drug regulations for an indefinite period of time. These regulations mitigate competition and the ability of other suppliers to replicate the Company’s products or produce comparable substitutes. These intangible assets are measured initially at cost not subject to amortization and are tested for impairment annually or in interim reporting periods if events or changes in circumstances indicate that the carrying amounts of these intangible assets might not be recoverable.
During the second quarter of our fiscal year ended June 30, 2013, we determined that we will no longer manufacture or seek to develop a market for ten of our products due to a change in our business strategy as more fully described in notes 1 and 2. As a result of this decision, we recorded an impairment charge in the amount of $1,668,486 during the quarter ended December 31, 2012. In addition to the above, we reclassified certain other formulas with an aggregate carrying amount of $10,121,956 to other intangible assets. The Company has suspended plans to develop and manufacture products to be derived from these formulas but intends to retain them to mitigate competition and maintain the option of using these formulas should they be useful in the future. Accordingly, the Company has determined these formulas, which are approved by the State Food and Drug Administration, should be held as defensive assets. The Company determined that there formulas have an estimated useful life of 9 years as defensive assets.
Fair Value Measurements and Fair Value of Financial Instruments
We adopted the guidance of ASC 820 for fair value measurements, which clarifies the definition of fair value, prescribes methods for measuring fair value, and establishes a fair value hierarchy to classify the inputs used in measuring fair value as follows:
Level 1 - Inputs are unadjusted quoted prices in active markets for identical assets or liabilities available at the measurement date.
Level 2 - Inputs are unadjusted quoted prices for similar assets and liabilities in active markets, quoted prices for identical or similar assets and liabilities in markets that are not active, inputs other then quoted prices that are observable, and inputs derived from or corroborated by observable market data.
Level 3 - Inputs are unobservable inputs which reflect the reporting entity’s own assumptions on what assumptions the market participants would use in pricing the asset or liability based on the best available information.
The carrying amounts reported in the condensed consolidated balance sheets for cash, accounts receivable, other receivables, short-term borrowings, accounts payable and accrued expenses, customer advances, and amounts due from related parties approximate their fair market value based on the short-term maturity of these instruments.
ASC 825-10 “Financial Instruments,” allows entities to voluntarily choose to measure certain financial assets and liabilities at fair value (fair value option). The fair value option may be elected on an instrument-by-instrument basis and is irrevocable, unless a new election date occurs. If the fair value option is elected for an instrument, unrealized gains and losses for that instrument should be reported in earnings at each subsequent reporting date. We use Level 3 inputs to value the Company’s derivative liabilities.
The following table reflects gains and losses for the three and six months ended December 31, 2012 for all financial assets and liabilities categorized as Level 3.
| Liabilities: | | Three
months
(unaudited) | |
| | | | |
| Balance of warrant liabilities as of September 30, 2012 | | $ | 713,233 | |
| Change in the fair value of warrant liabilities | | | (713,233 | ) |
| Balance of warrant liabilities as of December 31, 2012 | | $ | - | |
| Liabilities: | | Six
months
(unaudited) | |
| | | | |
| Balance of warrant liabilities as of June 30, 2012 | | $ | 1,211,236 | |
| Change in the fair value of warrant liabilities | | | (1,211,236 | ) |
| Balance of warrant liabilities as of December 31, 2012 | | $ | - | |
Estimating the fair value of derivative financial instruments require the development of significant and subjective estimates that may, and are likely to, change over the duration of the instrument with related changes in internal and external market factors. The assumptions used to value the Company’s derivatives, which had a direct effect on the fair values described above are more fully described in Note 11. In addition, valuation techniques are sensitive to changes in the trading market price of the our Common Stock and its estimated volatility interest rate changes and other variables or market conditions not within the Company’s control that can significantly affect management’s estimates of fair value and changes in fair value. Because derivative financial instruments are initially and subsequently carried at fair value, the Company’s net income may include significant charges or credits as these estimates and assumptions change.
Foreign Currency Translation
The Company’s reporting currency is the U.S. dollar. The functional currency of the Company’s operating business based in the PRC is the RMB. For the Company’s subsidiaries and affiliates whose functional currencies are the RMB, results of operations and cash flows are translated at average exchange rates during the period, assets and liabilities are translated at the exchange rate in effect as of the end of the period, and equity is translated at historical exchange rates. Translation adjustments resulting from the process of translating the functional currency financial statements into U.S. dollars are included in comprehensive income.
Exchange gains or losses arising from foreign currency transactions are included in the determination of net income for the respective periods. All of the Company’s revenue transactions are transacted in the functional currency. The Company has not entered into any material transactions that are either originated, or to be settled, in currencies other than the RMB. Accordingly, transaction gains or losses have not had, and are not expected to have a material effect on the Company’s results of operations.
Period end exchange rates used to translate assets and liabilities and average exchange rates used to translate results of operations in each of the reporting periods are as follows:
| | | Six Months
ended
December 31, 2012 | | | Six Months
ended
December 31, 2011 | |
| Period end US$: RMB exchange rate | | | 6.3086 | | | | 6.3585 | |
| Average periodic US$: RMB exchange rate | | | 6.3091 | | | | 6.3892 | |
Period end US$ : RMB exchange rate as of June 30, 2012 is 6.3143. The RMB is not freely convertible into any other currencies. In addition, all foreign exchange transactions in the PRC must be conducted through authorized institutions. Accordingly, management cannot provide any assurance that the RMB underlying the condensed consolidated financial statement amounts could have been, or could be, converted into US dollars at the exchange rates used to translate the functional currency into the reporting currency.
Revenue Recognition
Revenue represents the invoiced value of goods sold recognized upon the delivery of goods to distributors. Pursuant to the guidance of ASC Topic 605 and ASC Topic 36, revenue is recognized when all of the following criteria are met:
| · | Persuasive evidence of an arrangement exists; |
| · | Delivery has occurred or services have been rendered; |
| · | The seller’s price to the buyer is fixed or determinable; and |
| · | Collectability is reasonably assured. |
We account for sales returns by establishing an accrual in an amount equal to management’s estimate of sales recorded for which the related products are expected to be returned. We determine the estimate of the sales return accrual primarily based on the Company’s historical experience regarding sales returns, but also by considering other factors that could impact sales returns. These factors include levels of inventory in the distribution channel, estimated shelf life, product discontinuances, and price changes of competitive products, introductions of generic products and introductions of competitive new products. For the three and six months ended December 31, 2012 and 2011, the Company’s sales return rate is low and deemed immaterial and accordingly, no provision for sales returns was recorded.
Shipping costs
Shipping costs are included in selling, general and administrative expense. Shipping costs amounted to $261,338 and $337,553 for the three months ended December 31, 2012 and 2011, respectively. Shipping costs amounted to $553,981 and $633,729 for the six months ended December 31, 2012 and 2011, respectively.
Advertising
Advertising and promotion costs are charged to expense as incurred. Advertising expense included in selling, general and administrative expenses amounted to $0and $1,261,285 for the three months ended December 31, 2012 and 2011, respectively. Advertising expenses included in selling, general and administrative expenses amounted to $0and $3,121,494 for the six months ended December 31, 2012 and 2011, respectively.
Income Taxes
We are governed by the PRC’s Income Tax Laws and the Internal Revenue Code of the United States. Income taxes are accounted for under the asset and liability method. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement and income tax base of assets and liabilities and operating loss and tax credit carry-forwards. Deferred tax assets are reduced by a valuation allowance to the extent that management concludes it is more likely than not that the benefit of such tax assets will not be realized in future periods. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in the periods that include the enactment date.
We account for certain tax positions based upon authoritative guidance that prescribes a recognition threshold and measurement processes for financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. The guidance also provides direction on recognition, classification, interest and penalties, accounting in interim periods and related disclosure.
Our policy is to classify assessments, if any, for tax related to interest as interest expense and penalties as general and administrative expense.
Earnings per Share
We report earnings per share in accordance with ASC Topic 260, “Earnings Per Share”. Basic earnings (loss) per share is computed by dividing net income (loss) by the weighted average number of shares of common stock outstanding during the period. Diluted earnings per share is computed by dividing net income by the weighted average number of shares of common stock, common stock equivalents and potentially dilutive securities outstanding during the period. Common equivalent shares are excluded from the computation of diluted shares in periods for which they have an anti-dilutive effect. Diluted shares underlying stock options and common stock purchase warrants are included in the determination of diluted earnings per share using the treasury stock method. Diluted shares underlying convertible debt obligations are included in the determination of diluted loss per share using the “if converted” method (Note 13).
Inventories consist of the following:
| | | December 31, | | | June 30, | |
| | | 2012
(unaudited) | | | 2012 | |
| | | | | | | |
| Raw materials | | $ | 2,129,554 | | | $ | 2,079,480 | |
| Work in progress | | | 665,887 | | | | 882,005 | |
| Finished goods | | | 484,612 | | | | 834,430 | |
| Total inventories | | $ | 3,280,053 | | | $ | 3,795,915 | |
| 5. | PROPERTY, PLANT AND EQUIPMENT, NET |
Property, plant and equipment consist of the following:
| | | December 31, | | | June 30, | |
| | | 2012
(unaudited) | | | 2012 | |
| | | | | | | |
| Buildings | | $ | 8,936,612 | | | $ | 8,928,545 | |
| Plant equipment | | | 2,878,432 | | | | 2,490,764 | |
| Office equipment | | | 221,026 | | | | 208,539 | |
| Motor vehicles | | | 285,868 | | | | 285,610 | |
| Total | | | 12,321,938 | | | | 11,913,458 | |
| | | | | | | | | |
| Less: accumulated depreciation | | | (2,477,830 | ) | | | (2,188,340 | ) |
| Construction in progress | | | 1,996,621 | | | | 1,956,154 | |
| | | | | | | | | |
| Property, plant and equipment, net | | $ | 11,840,729 | | | $ | 11,681,272 | |
Depreciation expense for property, plant and equipment for the three months ended December 31, 2012 and 2011 amounted to $144,367 and $99,734, respectively. Depreciation expense for property, plant and equipment for the six months ended December 31, 2012 and 2011 amounted to $287,490 and $ 236,391, respectively.
On June 8, 2010, Yantai Huanghai Construction Co. signed an agreement with Yantai Tianzheng to perform certain portions of a factory construction located at the premises of Yantai Tianzheng. The total contract price is approximately $3.07 million (RMB 19.5 million) and the construction is estimated to be completed by the end of April 2013. The remaining commitment of the contract was approximately $1.25 million (RMB 7.9 million) as of December 31, 2012.
| 6. | INDEFINITE LIVED INTANGIBLE ASSETS – PHARMACEUTICAL FORMULAS |
The Company purchased, and currently owns exclusive rights to, a series of pharmaceutical formulas that were approved by the State Food and Drug Administration of China (“SFDA”). This asset includes 14 formulas that are included in the Chinese government’s Essential Drug List (“EDL”) and an additional 5 medicines included in the National Drug Reimbursement List (“NDRL”). The intellectual property underlying these formulas can be renewed every 5 years without limitation for a nominal fee and are subject to certain protections under PRC drug regulations for an indefinite period of time. These regulations mitigate competition and the ability of other suppliers to replicate the Company’s products or produce comparable substitutes. These intangible assets are measured initially at cost not subject to amortization and are tested for impairment annually or in interim reporting periods if events or changes in circumstances indicate that the carrying amounts of these intangible asset might not be recoverable.
Pharmaceutical formulas with indefinite lives consist of the following:
| | | December 31, 2012 (unaudited) | | | June 30, 2012 | |
| | | | | | | |
| Pharmaceutical formulas, without amortization, at cost | | $ | 13,823,121 | | | $ | 25,610,557 | |
During the three months period ended December 31,2012, the Company recorded an impairment charge of $1,688,486,for a limited number of products formulas purchased in 2005 that will no longer be used to develop products. The Company also reclassified $10,121,956 for the cost of certain other SFDA approved drug formulas that the Company will retain as defensive assets, to other intangible assets (Notes 1 and 2). The Company has made a determination that that is in the Company best interests to retain these formulas to mitigate competition and provide the Company with the option of using them in future development efforts should it be advantageous to do so.
| 7. | LONG TERM PREPAYMENTS - LAND USE RIGHTS, NET |
| | | December 31, | | | June 30, | |
| | | 2012
(unaudited) | | | 2012 | |
| | | | | | | |
| Land use rights, at cost | | $ | 39,280,564 | | | $ | 20,240,623 | |
| Less: Accumulated amortization | | | (1,892,096 | ) | | | (1,501,326 | ) |
| Intangible assets – land use rights, net | | $ | 37,388,468 | | | $ | 18,739,297 | |
The Company acquired a new land use right for 266,668 square meters on November 5, 2012. The Company was granted the right to use the land for a period of 50 years at a cost paid of approximately $19m (RMB 120,000,000). As of December 31, 2012, the Company has made a payment of $6,340,550. The Company is obligated to make two remaining installment payments of $6,340,551 each by June 30, 2013 and December 31, 2013.
There is no private ownership of land in the PRC. All land is owned by the government, which grants land use rights for specified periods of time. Amortization expense for land use rights amounted to $226,819 and $123,915 for the three months ended December 31, 2012 and 2011, respectively. Amortization expense for land use rights amounted to $389,382 and $272,881 for the six months ended December 31, 2012 and 2011, respectively. Amortization is calculated over a period of 30-50 years. Amortization of land use rights for fiscal years ending subsequent to December 31, 2012 is as follows:
| | | Amortization | |
| Remainder of FY2013 | | $ | 516,183 | |
| 2014 | | | 1,032,366 | |
| 2015 | | | 1,032,366 | |
| 2016 | | | 1,032,366 | |
| 2017 | | | 1,032,366 | |
| Thereafter | | | 32,742,821 | |
| Total | | $ | 37,388,468 | |
| 8. | OTHER INTANGIBLE ASSETS, NET |
Other Intangible assets, net includes customer relationships and certain prescription drug product formulas. The Company acquired these assets in its business combination with Yantai Tianzheng. Customer relationships are amortized on a straight line basis over periods of 5 and 8 years. Pharmaceutical formulas are amortized on a straight line basis over periods of 8 years.
$10,121,956 for the carrying amount of certain other product formulas that the Company will hold as defensive assets reclassified from indefinite life drug formulas that will be amortized over a period of 8 years
Other intangible assets – net at December 31, 2012 as follow:
| | | Customer | | | YTP Drug | | | Defensive | | | | |
| | | Relationships | | | Formulas | | | Drug formulas | | | Total | |
| | | (unaudited) | | | (unaudited) | | | (unaudited) | | | (unaudited) | |
| Cost | | $ | 14,498,621 | | | $ | 10,130,774 | | | $ | 10,121,956 | | | $ | 34,751,351 | |
| | | | | | | | | | | | | | | | | |
| Accumulated Amortization | | | (2,768,602 | ) | | | (1,904,284 | ) | | | (316,311 | ) | | | (4,989,197 | ) |
| | | | | | | | | | | | | | | | | |
| Net carrying amount | | $ | 11,730,019 | | | $ | 8,226,490 | | | $ | 9,805,645 | | | $ | 29,762,154 | |
Amortization expense for customer relationships amounted to $462,608 and $489,422 for the three months ended December 31, 2012 and 2011, respectively. Amortization expense for customer relationships amounted to $922,794 and $943,328 for the six months ended December 31, 2012 and 2011, respectively.
Amortization expense for YTP drug formulas amounted to $319, 781 and $0 for the three months ended December 31, 2012 and 2011, respectively. Amortization expense for YTP drug formulas amounted to $637, 887 and $0 for the six months ended December 31, 2012 and 2011, respectively.
Amortization expense for defensive drug formulas amounted to $316,286 and $0 for the three months and six months ended December 31, 2012 and 2011, respectively. Amortization expenses are recorded in general and administrative expenses.
Amortization expense for fiscal years ending subsequent to December 31, 2012 is as follows:
| | | Amortization | |
| Remainder of FY2013 | | $ | 2,197,350 | |
| 2014 | | | 4,394,700 | |
| 2015 | | | 4,394,700 | |
| 2016 | | | 4,394,700 | |
| 2017 | | | 4,394,700 | |
| Thereafter | | | 9,986,004 | |
| Total | | $ | 29,762,154 | |
Accrued expense consists of the following:
| | | December 31, | | | June 30, | |
| | | 2012
(unaudited) | | | 2012 | |
| | | | | | | |
| Other accrued expense | | $ | 1,259,257 | | | $ | 1,557,472 | |
| Sales representatives commission and expenses | | | 7,059,280 | | | | 3,778,996 | |
| Other taxes payable | | | 2,362,928 | | | | 1,879,334 | |
| | | | | | | | | |
| Compensation and related cost | | | 334,323 | | | | 357,975 | |
| Interest | | | 850,197 | | | | 516,269 | |
| Advertising expense | | | 260,280 | | | | 388,008 | |
| Total | | $ | 12,126,265 | | | $ | 8,478,054 | |
| 10. | CONVERTIBLE PROMISSORY NOTES IN DEFAULT AND DUE ON DEMAND |
Convertible Notes
On January 5, 2010, pursuant to a Securities Purchase Agreement (the “Securities Purchase Agreement”) with 128 accredited investors (the “Investors”), BPGI sold 6,000,000 units for aggregate gross proceeds of $12,000,000, each unit consisting of an 8% senior convertible promissory note in the principal amount of $2 and one Common Stock purchase warrant (collectively, the “Investor Warrants”). By agreement with the Investors, each investor received: (i) A single Note representing the aggregate number of Notes purchased by them as part of the units (each, a “Note” and collectively, the “Notes”) and (ii) a single Investor Warrant exercisable at $2.40 per share subject to certain anti-dilution provisions. The majority of this debt is guaranteed by third-parties and our CEO, Mr. Qu, and a portion is secured by our inventories and fixed assets.
The Notes bear interest at 8% per annum, payable quarterly in arrears on the last day of each fiscal quarter of the Company. Principal is due on January 5, 2012. Each Note, plus all accrued but unpaid interest thereon, is convertible, in whole but not in part, at any time at the option of the holder, into shares of Common Stock at a conversion price of $2.00 per share, subject to adjustments for certain anti-dilution provisions.
The Convertible Notes were initially recorded at a discounted carrying amount of zero as a result of having allocated a portion of the proceeds to (i) the fair value of the warrants, which were recorded as liabilities stated at fair value, and (ii) a beneficial conversion feature that was not bifurcated as a free standing derivative at the time of issuance or at subsequent reporting dates based on periodic classification assessments. Accretion of the note discount amounted to $0 and $6,478,314 for the three months ended December 31, 2012 and 2011, respectively. Accretion of the note discount amounted to $0 and $8,597,701 for the six months ended December 31, 2012 and 2011, respectively. Accretion of the discount was recorded as a component of interest expense in the accompanying statements of income and comprehensive income. Contractual interest expense amounted to $0 and $6,687,314 for the three months ended December 31, 2012 and 2011, respectively. Contractual interest expense amounted to $0 and $9,015,701 for the six months ended December 31, 2012 and 2011, respectively. There is an aggregate of 4,232,250 shares of Common Stock issuable under all remaining convertible notes as of December 31, 2012.
The Notes contain certain events of default, including non-payment of interest or principal when due, bankruptcy, failure to maintain a listing of the Common Stock or to make required filings on a timely basis. No premium is payable by us if an event of default occurs. However, upon an Event of Default, and provided no more than 50% of the aggregate face amount of the Notes have been converted, the Investors holding Notes have the right to receive a portion, based on their pro-rata participation in the transaction, of 1,000,000 shares of our Common Stock that have been placed in escrow by our principal shareholder. The shares in escrow will be returned to our principal shareholder when 50% of the aggregate face amount of the Notes has been converted or, if later, when the Notes are repaid.
On December 31, 2011, the Company’s Chinese operating subsidiary determined it was unable to convert a sufficient number of RMB’s needed to repay the notes on their original maturity date of January 5, 2012. As a result, the Company entered into a series of amendments to the Notes with Euro Pacific as representative of the Investors to extend to the maturity date and increase the interest rate on the Notes. Pursuant to the most recent amendment, the maturity date of the notes was extended April 5, 2013 and the interest rate was increased to 12% per annum.
On June 27, 2012, Euro Pacific also agreed to release us from certain restrictions on our ability to incur debt, to incur liens or to make capital expenditures as stipulated in the note agreement. The purpose of the Third Amendment is to provide us with enhanced flexibility to seek potential sources of financing.
The Company has been in temporary default of this obligation at previously extended maturity dates. Should the Company be unable to repay the notes on the current extended maturity date of April 5, 2013 in the absence of a further extension of the maturity date, this circumstance would constitute an event of default under the terms of loan agreement. The Company cannot predict what the implications of the non-payment of the loan would be other than it would continue to experience difficulty converting sufficiency currency and will maintain an escrow account of restricted funds intended to secure their repayment. The non-payment of the notes could have a material adverse effect on the Company should the note holders pursue further action.
As of December 31, 2012 and June 30, 2012, the Company’s principal shareholder, Mr. Qu, is obligated to deliver 1,000,000 shares of Common Stock to the Investors if certain Events of Default occur.
| 11. | ACQUISITION PURCHASE PRICE PAYABLE |
On August 8, 2011, the Company, through WFOE II, acquired 100% of Yantai Tianzheng’s equity interests for total purchase consideration of US$35,000,000 (paid in its RMB equivalent) of which US$6,000,000 was paid as of the Execution Date of the acquisition the remaining $29,000,000 was due in a series of contractual installments.
Certain provisions in the acquisition agreement provided the Company with the ability to elect, at its own discretion, to automatically convert any portion or all of the installment payments due into a two-year term loan, with interest accruing at the rate of six percent (6%) per annum.
As of December 31, 2012, $19,700,000 was paid. The Company also made$2,472,815 of individual withholding tax payments on behalf of the former Yantai Tianzheng shareholders including $10,000,000 of principal paid during the three months ended December 31, 2012. The $2,472,815 of withholding tax payments were applied as a reduction of the remaining balance of the acquisition purchase price payable. As of December 31, 2012, the balance of $12,827,185 is payable as follows; $7,827,185 is due on August 8, 2014, and the remaining $5,000,000 is due on February 8, 2015.
| 12. | COMMITMENTS, CONTINGENCIES AND OTHER MATTERS |
| (a) | Contract Research and Development Arrangement |
On May 2009, the Company entered into a contract with Yantai Tianzheng Medicine Research and Development Co. to perform research and development on two new pharmaceutical products, namely Fern Injection and Forsythia Capsule. The total contract price is approximately $2,345,509 (RMB 15,000,000). Yantai Tianzheng Medicine Research and Development Co. committed to complete all research work required for the clinical trial within 3 years. As of December 31, 2012, the Company has paid $1,305,339 (RMB 8,300,000) and the remaining contract amount will be paid as the research services are performed. All payments of $1,305,339 (RMB 8,300,000) have been expensed. The Company extended the term of the contract to May 10, 2017 due to certain changes in government regulations that affected this research project. Research and development costs associated with this contract amounted to $0 for the six months ended December 31, 2012 and 2011.
| (b) | Supplier Concentrations |
We have the following concentrations of business with each supplier constituting greater than 10% of the Company’s purchases of raw materials or other supplies:
| | | Three
months | | | Three
months | | | Six months | | | | |
| | | ended | | | ended | | | ended | | | Six months | |
| | | December
31, | | | December
31, | | | December
31, | | | ended
December | |
| | | 2012
(unaudited) | | | 2011
(unaudited) | | | 2012
(unaudited) | | | 31, 2011
(unaudited) | |
| | | | | | | | | | | | | |
| Shandong Yantai Medicine Procurement and Supply Station | | | 25.9 | % | | | 10.0 | % | | | 26.4 | % | | | 11.9 | % |
| AnguoJinkangdi Chinese Herbal Medicine Co. Ltd | | | * | % | | | 11.1 | % | | | * | % | | | 10.5 | % |
| Anhui DeChang Pharmaceutical Co. Ltd. | | | * | % | | | 16.3 | % | | | * | % | | | 17.1 | % |
* Constitutes less than 10% of the Company’s purchases.
We had a commitment to purchase certain raw materials totaling $2,988,738 as of December 31, 2012 that was fulfilled upon the delivery of the goods in January 2013.
| (c) | Sales Product Concentrations |
Five of the Company’s products, namely Tongbi Capsules, Tongbi Tablets, Lung Nourishing Syrup, Zhengxintai Capsules and Fangfengtongsheng Tablets represented approximately 37.2 %, 14.1%, 17.1%, 11.0% and 15.3%, respectively, of total sales for the three months ended December 31, 2012.
Five of the Company’s products, namely Tongbi Capsules, Tongbi Tablets, Lung Nourishing Syrup, Zhengxintai Capsules and Fangfengtongsheng Tablets represented approximately 31.9 %, 12.0%, 16.1%, 11.3% and 16.8%, respectively, of total sales for the six months ended December 31, 2012.
Five of our products, namely Tongbi Capsules, Tongbi Tablets, Lung Nourishing Syrup, Zhengxintai Capsules and Fangfengtongsheng Tablets represented approximately 23.2%, 9.3%, 16.8%, 10.1% and 17.5%, respectively, of total sales for the three months ended December 31, 2011.
Five of our products, namely Tongbi Capsules, Tongbi Tablets, Lung Nourishing Syrup, Zhengxintai Capsules and Fangfengtongsheng Tablets represented approximately 22.8%, 9.3%, 17.2%, 10.0% and 17.1%, respectively, of total sales for the six months ended December 31, 2011.
| (d) | Economic and Political Risks |
The Company’s operations are conducted solely in the PRC. There are significant risks associated with doing business in the PRC, which include, among others, political, economic, legal and foreign currency exchange risks. The Company’s results may be adversely affected by changes in political and social conditions in the PRC, and by changes in governmental policies with respect to laws and regulations, anti-inflationary measures, currency conversion, remittances abroad, and rates and methods of taxation, among other things.
| (e) | Concentrations of Credit Risk |
Financial instruments which potentially subject us to concentrations of credit risk consist principally of cash and trade accounts receivable. Substantially all of the Company’s cash is deposited in state-owned banks within the PRC, and no deposits are covered by insurance. We have not experienced any losses in such accounts and believe that the Company’s loss exposure is insignificant due to the fact that banks in the PRC are state owned and are generally high credit quality financial institutions. A significant portion of the Company’s sales are credit sales which are made primarily to customers whose ability to pay are dependent upon the industry economics prevailing in these areas. We continually monitor the credit worthiness of the Company’s customers in an effort to reduce credit risk.
At December 31, 2012 and June 30, 2012, the Company’s cash balances by geographic area were as follows:
| | | December 31, | | | June 30, | |
| | | 2012
(unaudited) | | | 2012 | |
| Country: | | | | | | | | | | | | | | | | |
| United States | | $ | 69,166 | | | | 2.19 | % | | $ | 23,406 | | | | 0.13 | % |
| China | | | 3,085,418 | | | | 97.81 | % | | | 18,362,882 | | | | 99.87 | % |
| Total cash and cash equivalents | | $ | 3,154,584 | | | | 100 | % | | $ | 18,386,288 | | | | 100 | % |
| (f) | Certificate of land use right |
The Company’s corporate headquarters is located at No. 9 Daxin Road, Zhifu District, Yantai, Shandong Province in China. Under the current PRC laws, land is owned by the state, and parcels of land in rural areas which are known as collective land are owned by the rural collective economic organization. “Land use rights” are granted to an individual or entity after payment of a land use right fee is made to the applicable state or rural collective economic organization. Land use rights allow the holder of the right to use the land for a specified long-term period.
We have 5 land use rights, for a total of approximately 3,675,364 square meters of land on which the Company maintains its manufacturing facility.
The Company has not obtained a land use right certificate for two piece of land located in the high-tech development district of Laishan district, including an area of 266,668 square meters expiring in 2052 and an area of 3,333,335 square meters expiring in expiring in 2051. In the process of the planning of Yantai City, the usage of the aforesaid land use right has been changed from “industrial use” to “commercial use” and therefore, the approval process for the land use right certificates on five relevant parcels of land including the land occupied by us is suspended until the completion of the planning. We cannot provide any assurance that the Company will eventually obtain the land use right certificate for this land. If the Company is asked by the local government to relocate the Company’s facility, management believes that estimated relocation and other costs will be reimbursed by the local government. The Company does not believe that a requirement to relocate operations would have a material adverse effect on the business.
Business insurance is not readily available in the PRC. To the extent that the Company suffers a loss of a type which would normally be covered by insurance in the United States, such as product liability and general liability insurance, the Company would incur significant expenses in both defending any action and in paying any claims that could result from a settlement or judgment.
Basic earnings per share is computed on the basis of the weighted average number of shares of Common Stock outstanding during the period. Diluted earnings per share is computed on the basis of the weighted average number of shares of Common Stock plus the effect of potentially dilutive common shares outstanding during the period using the if-converted method for the convertible debt and equity securities and the treasury stock method for stock options and common stock purchase warrants. The following table sets forth the computation of basic and diluted net income per common share:
| | | Three
months
ended | | | Three
months
ended | | | Six months
ended | | | Six months
ended | |
| | | December
31, 2012
(unaudited) | | | December
31, 2011
(unaudited) | | | December 31,
2012
(unaudited) | | | December 31,
2011
(unaudited) | |
| | | | | | | | | | | | | |
| Net income available to common stockholders-basic | | $ | 5,258,737 | | | $ | (1,295,502 | ) | | $ | 11,654,249 | | | $ | 1,360,282 | |
| Effective interest on convertible notes and amortization of debt issue costs | | | 268,043 | | | | - | | | | 550,193 | | | | - | |
| Net income available for common shareholders – diluted | | $ | 5,526,780 | | | $ | (1,295,502 | ) | | $ | 12,204,442 | | | $ | 1,360,282 | |
| | | Three months
ended | | | Three months
ended | | | Six months
ended | | | Six months
ended | |
| | | December
31, 2012
(unaudited) | | | December
31, 2011
(unaudited) | | | December
31, 2012
(unaudited) | | | December
31, 2011
(unaudited) | |
| Weighted average number of common shares outstanding - basic | | | 17,861,085 | | | | 17,861,085 | | | | 17,861,085 | | | | 17,861,085 | |
| Common shares if converted from Convertible Debt | | | 4,232,250 | | | | - | | | | 4,232,250 | | | | - | |
| Weighted average number of common shares outstanding - diluted | | | 22,093,335 | | | | 17,861,085 | | | | 22,093,335 | | | | 17,861,085 | |
| | | | | | | | | | | | | | | | | |
| Earnings (loss) per share: | | | | | | | | | | | | | | | | |
| Basic | | $ | 0.29 | | | $ | (0.07 | ) | | $ | 0.65 | | | $ | 0.08 | |
| Diluted | | $ | 0.25 | | | $ | (0.07 | ) | | $ | 0.55 | | | $ | 0.08 | |
Warrants to purchase 6,600,000 shares of Common Stock and stock options to purchase 32,000 shares of Common Stock were outstanding as of December 31, 2012 and 2011 but both 6,600,000 shares of Common Stock and 32,000 shares of options were excluded from the computation of diluted earnings per share where applicable as the exercise prices of these securities exceeded the average stock prices for the three months ended December 31, 2012 and 2011 and for the six months ended December 31, 2012 and December 31, 2011.
For the three and six months ended December 31, 2012 and 2011, operating expenses consisted of the following:
| | | Three months
ended | | | Three months
ended | | | Six months
ended | | | Six months
ended | |
| | | December 31,
2012
(unaudited) | | | December 31,
2011
(unaudited) | | | December 31,
2012
(unaudited) | | | December 31,
2011
(unaudited) | |
| | | | | | | | | | | | | |
| Sales Commissions | | $ | 20,609,780 | | | $ | 14,145,939 | | | $ | 36,157,254 | | | $ | 26,667,477 | |
| Advertising expense | | | - | | | | 1,261,285 | | | | - | | | | 3,121,494 | |
| Audit fees and consulting expenses | | | 112,683 | | | | 13,075 | | | | 182,276 | | | | 66,250 | |
| Depreciation and amortization | | | 1,343,204 | | | | 617,463 | | | | 2,302,094 | | | | 1,247,061 | |
| Staff costs (salary & welfare) | | | 508,160 | | | | 543,218 | | | | 1,006,526 | | | | 1,094,933 | |
| Other operating expenses | | | 1,316,184 | | | | 2,145,012 | | | | 2,407,867 | | | | 2,763,266 | |
| Impairment of drug formula | | | 1,688,486 | | | | - | | | | 1,688,486 | | | | - | |
| | | | | | | | | | | | | | | | | |
| Total Operating expenses | | $ | 25,578,497 | | | $ | 18,725,992 | | | $ | 43,744,503 | | | $ | 34,960,481 | |
The Company is incorporated under the laws of State of Nevada in the United States of America and has legal subsidiaries in the British Virgin Islands (“BVI”) and the PRC. The Company does not have any employees or assets nor or is it engaged in any income producing activities in the Unites States and in the BVI. The Company is currently filing Federal income tax returns in the United States and applicable franchise tax returns in the state of Nevada. The Company’s net operating losses that may be available to offset future taxable income in the United States, if any, amount to approximately $9,000,000 and expire through 2031. The Company has fully reserved for these and all other deferred tax assets generated in the Company’s US operations since it currently more likely than not that such assets will not be realized in future periods.
The Company’s only income producing activities are in the PRC. The statutory corporation income tax rate in the PRC is 25%, which is approximately equal to the effective income tax rate that the Company expects to use when recording income tax expense for financial reporting purposes for the year ending June 30, 2013. Accordingly, the Company is recording a tax provision at interim reporting dates for taxable income earned in the PRC using the effective rate expected to be in effect for the year ending June 30, 2013.
To satisfy PRC laws and regulations, the Company conducts certain business in the PRC through the VIEs.
As a result of the VIE Agreements signed between Yantai Shencaojishi Pharmaceuticals Co., Ltd (“WFOE”) and Yantai Bohai Pharmaceuticals Group Co. Ltd (“Bohai”), the Company includes the assets, liabilities, revenues and expenses of Bohai(the “VIE”) in its consolidated financial statements.
Substantially all of the Company’s assets, including those of Bohai which is considered the VIE and Yantai Tianzheng, which is an acquired subsidiary of the Company are accessible to Bohai through WFOE II creditors irrespective of the VIE arrangement.
The VIE Agreements include:
Equity Interest Pledge Agreement.The WFOE and Bohai Shareholders have entered into Equity Interest Pledge Agreements, pursuant to which each Bohai Shareholder has pledged all of his shares of Bohai to the WFOE in order to guarantee cash-flow payments under the applicable Consulting Services Agreement. The Equity Pledge Agreement further entitles the WFOE to collect dividends from Bohai during the term of the pledge.
Consulting Service Agreement.Bohai and the WFOE has entered into a Consulting Services Agreement, which provides that the WFOE will be the exclusive provider of technology services to Bohai and Bohai will pay all of its net income based on the quarterly financial statements to the WFOE for such services. Any such payment from the WFOE to the Company would need to comply with applicable Chinese laws affecting payments from Chinese companies to non-Chinese companies. See “Risk Factors – Risks Associated With Doing Business in China.”
Operating Agreement.Pursuant to the operating agreement among the WFOE, Bohai and each of Bohai Shareholder, the WFOE provides guidance and instructions on Bohai’s daily operations and financial affairs. The Bohai Shareholders must designate the candidates recommended by the WFOE as their representatives on their respective boards of directors. The WFOE has the right to appoint senior executives of Bohai. In addition, the WFOE agrees to guarantee Bohai’s performance under any agreements or arrangements relating to Bohai’s business arrangements with any third party. Bohai, in return, agrees to pledge its accounts receivable and all of its assets to the WFOE. Moreover, Bohai agrees that without the prior consent of the WFOE, Bohai will not engage in any transactions that could materially affect its assets, liabilities, rights or operations, including, without limitation, incurrence or assumption of any indebtedness, sale or purchase of any assets or rights, incurrence of any encumbrance on any of its assets or intellectual property rights in favor of a third party or transfer of any agreements relating to its business operation to any third party.
These contractual arrangements may not be as effective in providing the Company with control over the VIEs as direct ownership. Due to its VIEs structure, the Company has to rely on contract right to effect control and management of the VIEs, which exposes it to the risk of potential breach of contract by the shareholders of Yantai Bohai Pharmaceuticals Group Co. Ltd. Our VIE Agreements are subject to significant risks as set forth in the following risk factors.
| ¨ | The PRC government may determine that the VIE Agreements which we utilize to control our operating subsidiary Bohai are not in compliance with applicable PRC laws, rules and regulations and that they are therefore unenforceable. |
| £ | There are risks involved with the operation of Bohai under the VIE Agreements. We have been advised by PRC legal counsel that if the PRC government determines the VIE Agreement used to control the operating company to be unenforceable as they circumvent the PRC restrictions relating to foreign investment restrictions, the relevant regulatory authorities would have broad discretion in dealing with such breach and could have a material adverse impact on our business, financial condition and results of operations. |
| £ | We depend upon the VIE Agreements in conducting our production, manufacturing, and distribution of traditional Chinese herbal medicines in the PRC, which may not be as effective as direct ownership. |
| £ | We conduct our production, manufacturing and distribution of traditional Chinese herbal medicines in the PRC and generate the revenues from our Bohai business through the VIE Agreements. The VIE Agreements may not be as effective in providing us with control over Bohai as direct ownership. The VIE Agreements are governed by PRC laws and provide for the resolution of disputes through arbitration proceedings pursuant to PRC laws. Accordingly, the VIE Agreements will be interpreted in accordance with PRC laws. If Bohai or its Shareholders fail to perform the obligations under the VIE Agreements, we may have to rely on legal remedies under PRC laws, including seeking specific performance or injunctive relief, and claiming damages, and there is a risk that we may be unable to obtain these remedies. The legal environment in China is not as developed as in other jurisdictions. As a result, uncertainties in the PRC legal system could limit our ability to enforce the VIE Agreements. |
| ¨ | The pricing arrangement under the VIE Agreements may be challenged by the PRC tax authorities. |
| £ | We could face adverse tax consequences if the PRC tax authorities determine that the VIE Agreements were not entered into based on arm’s length negotiations. If the PRC tax authorities determine that the VIE Agreements were not entered into on an arm’s length basis, they may adjust the income and expenses of our Company for PRC tax purposes which could result in higher tax liability |
In preparing these financial statements, the Company has evaluated events and transactions for potential recognition or disclosure through the reporting date, the date the financial statements were available to be issued.
Item 2. Management’s Discussion and Analysis of Financial Conditions of Operations.
The following discussion and analysis of financial condition and results of operations relates to the operations and financial condition reported in our unaudited condensed consolidated financial statements for the six months ended December 31, 2012, and should be read in conjunction with such financial statements and related notes included in this report. Those statements in the following discussion that are not historical in nature should be considered to be forward looking statements that are inherently uncertain. Actual results and the timing of the events may differ materially from those contained in these forward looking statements due to a number of factors, including those discussed in the “Cautionary Note on Forward Looking Statements” set forth elsewhere in this Report.
Overview
We are engaged in the production, manufacturing and distribution in China of herbal pharmaceuticals based on traditional Chinese medicine, which we refer to herein as Traditional Chinese Medicine, or TCM. We are based in the city of Yantai, Shandong Province, China and our operations are exclusively in China.
Our medicines address rheumatoid arthritis, viral infections, gynecological diseases, cardio vascular issues and respiratory diseases. Our initial operating subsidiary Bohai obtained Drug Approval Numbers (or DANs) for 29 varieties of traditional Chinese herbal medicines in 2004, an additional 14 varieties in December 2010. Through our acquisition of Yantai Tianzheng in August 2011, we obtained DANs for another 5 varieties in August 2011. We currently produce 7varieties of approved traditional Chinese herbal medicines in seven delivery systems: tablets, granules, capsules, formulations, concentrated powder, tincture and medicinal wine. Of these 7 products, 5 of which are prescription drugs and 2 of which are over the counter (or OTC) products.
Three of Bohai’s lead products, Tongbi Capsules and Tablets and Lung Nourishing Syrup, are eligible for reimbursement under China’s National Medical Insurance Program (or NRDL), which we believe significantly increases the marketability of these products. In addition to these lead products, three of our current products and five of our formulas we acquired in 2010 are eligible for NRDL reimbursement. In addition, one of our current products and four of our newly acquired formulas are currently included on the Chinese government's Essential Drug List (or EDL). Inclusion on either the EDL or NRDL allows for up to 100% insurance coverage by the Chinese government. Yantai Tianzheng owns five prescription products approved by the State Food and Drug Administration of China (which we refer to herein as the SFDA) and currently manufactures two of such products. Among Yantai Tianzheng’s products, Fangfengtongsheng Granule has an exclusive status and is on the EDL and NDRL, and Zhengxintai Capsule is in the process of renewal for its protective status and is currently under the NDRL.
Prior to January 5, 2010, we were a public “shell” company operating under the name “Link Resources, Inc.” On January 5, 2010, we consummated a share exchange transaction (the “Share Exchange”) pursuant to which we acquired Chance High, the indirect parent company of Bohai, our principal operating subsidiary, which is a Chinese variable interest entity that we (through a Chinese wholly-owned foreign enterprise subsidiary) control through certain contractual arrangements. On August 8, 2011, WFOE II, a PRC company and a newly formed subsidiary of Chance High, entered into a Share Purchase Agreement pursuant to which we acquired, from the three individual holders thereof, one hundred percent (100%) of the outstanding shares of Yantai Tianzheng, which became our second operating subsidiary effective as of July 1, 2011. Our current organizational structure is summarized below:
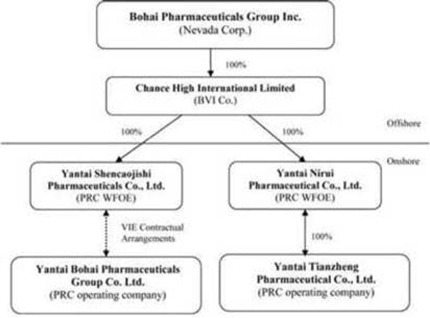
Recent Developments
During the three months ended December 31, 2012, management performed an evaluation of the Company’s product portfolio based on a confluence of factors that have emerged within China’s pharmaceutical industry and consumer markets. These factors principally include, but are not necessarily limited to (i) current economic conditions in China and management’s expectations of future economic trends, (ii) changes in China’s Essential Drug Laws, (iii) the PRC Central Government’s policy designating lists of specific drugs eligible for reimbursement, (iv) consumers’ preference for drug delivery systems in the forms of pills, tablets and ingestible liquids, and (v) competition from other drug manufacturing companies within China. Based on these factors, management has determined that the Company will streamline its operations to focus on the continued distribution of Lung Nourishing Syrup, Tongbi Capsules, Tongbi Tablets, Fangfengtongsheng Granule, and Zhengxintai Capsules and certain other products. As a consequence, management determined that it would be in the best interests of the company and its stockholders to commit to (i) a plan of fully abandon or suspend indefinitely plans to develop and produce a more diversified portfolio that would be derived from a series of approved formulas that the Company purchased in 2010.
As a result, the Company (i) recorded an impairment charge of $1,688,486 for the aggregate carrying amount of certain product formulas that the Company will abandoned in their entirety, and (ii) reclassified $10,121,956 for the carrying amount of certain other product formulas that the Company will hold as defensive assets. Management believes it is the best interests of the Company and its stockholders to retain ownership of these formulas, which are approved by the SFDA, to mitigate the risks of competition and provide the Company with the option of using the formulas to develop products in the future should it be advantageous to do so.
On November 5, 2012, the Company acquired a new land use right of 266,668 square meters located in high-tech development district of Laishan district. The land use right grants to the Company the right to use the land for a period of 50 years at a total cost of approximately $19 million (RMB 120,000,000). As of December 31, 2012, the Company paid $6,340,550. The Company is obligated to make two installment payments of $6,340,551 by June 30, 2013 and $6,340,551 by December 31, 2013. The Company plans to build workshops, logistic system and R&D centre on the land to better prepare for the future development.
Principal Factors Affecting Our Financial Performance
We believe that the following factors will continue to affect our financial performance:
Sales of Key Products
Our top selling products as a percentage of total net revenue consist of the following:
| | | For the three months ended | | | For the six months ended | |
| | | December 31, | | | December 31, | |
| | | 2012
(unaudited) | | | 2011
(unaudited) | | | 2012
(unaudited) | | | 2011
(unaudited) | |
| Lung Nourishing Syrup | | | 17.1 | % | | | 16.8 | % | | | 16.1 | % | | | 17.2 | % |
| Tongbi Capsules | | | 37.2 | % | | | 23.2 | % | | | 31.9 | % | | | 22.8 | % |
| TongbiTablets | | | 14.1 | % | | | 9.3 | % | | | 12.0 | % | | | 9.3 | % |
| Zhengxintai Capsule | | | 11.0 | % | | | 10.1 | % | | | 11.3 | % | | | 10 | % |
| Fangfengtongsheng Granule | | | 15.3 | % | | | 17.5 | % | | | 16.8 | % | | | 17.1 | % |
| Other Products | | | 5.3 | % | | | 23.1 | % | | | 11.9 | % | | | 23.6 | % |
| Total Sales | | | 100 | % | | | 100 | % | | | 100 | % | | | 100 | % |
We expect that a significant portion of our future revenue will continue to be derived from sales of our top five products, and we recently shifted our corporate strategy to focus exclusively on such seven products.
We held Certificates for a Protected Variety of Traditional Chinese Medicine (Grade Two) issued by the SFDA for Tongbi Capsules and Anti-flu Granules which gave the Company exclusive or near-exclusive rights to manufacture and distribute these two medicines. Tongbi Capsules’ certificates expired in September 2009. We filed an application for extending the protection period on March 12, 2009 and received certification extension until September 13, 2016. Lung Nourishing Syrup received a patent with duration of 20 years from the State Intellectual Property Office of the PRC and the patent will expire on September 12, 2027.
Experienced Management
Management’s marketing strategies and business relationships gives us the ability to expand our product market areas, which provides us with leverage to acquire less sophisticated operators, increase production volumes, and implement quality standards. Our future prospects depend substantially on the continued services of our senior management team, especially our President, Chief Executive Officer and Chairman of the Board, Mr. Qu.
Price Control of Drugs by PRC Government and SDRC
The State Development and Reform Commission of the PRC (“SDRC”) and the price administration bureaus of the relevant provinces of the PRC in which the pharmaceutical products are manufactured are responsible for retail price controls over our pharmaceutical products. The SDRC sets the price ceilings for certain pharmaceutical products in the PRC. All of our products except those under the protection periods are subject to such price controls and could affect our future revenue growth. However, due to the direct support of TCM by the Chinese government, China’s immense market, and our protected drugs, management believes that the continuous growth potential for TCM in China.
Operating Results
Comparison of the three months ended December 31, 2012 and 2011
Net Revenues
Net revenues are comprised of sales of 15 traditional Chinese medicines (corresponding to 11 formulas) in China during the three months ended December 31, 2012 (we currently sell 15 medicines following our acquisition of Yantai Tianzheng on August 8, 2011, but have recently made the strategic choice to narrow our focus to just our top seven products). Net revenues for the three months ended December 31, 2012 increased by $6,095,620, or 17.5%, to $40,932,506 as compared to $34,836,886 for the three months ended December 31, 2011. Net revenues were $29,979,509 and $10,952,997 for Bohai and Yantai Tianzheng, respectively, for the three months ended December 31, 2012. Net revenues were $24,055,719 and $10,781,167 for Bohai and Yantai Tianzheng, respectively, for the three months ended December 31, 2011. The increase in Bohai’s revenue was primarily due to a net increase in revenues of 44.1% from our four lead products in Bohai: Lung Nourishing Syrup, Tongbi Capsules, Tongbi Tablets, and Shangtongning Tablets, which together accounted for over 95.8% of our total net revenues for Bohai. All of our lead products from Bohai are listed for coverage and reimbursement under national medical insurance program starting in December 2009. The increase in Tianzheng’s revenue was primarily due to a net increase in revenue of 10.7% of our two lead products in Tianzheng: Zhengxintai Capsule, Fangfengtongsheng Granule. The sale of our prescription drug products for the three months ended December 31, 2012 represented 80% of total net revenue compared to 73% for the same period in last year. The increase in prescription sales was primary due to increases in sales volume of our two prescription drugs, Tongbi Capsules and Tongbi Tablets as well as prescription product sales from Yantai Tianzheng. The increase of the Company’s revenue is because of the increase of the market demand for the company’s key products that are included in the government’s essential drug list. The government encourages hospital and doctors to prescribe essential drugs to the patients. The increase of the revenue is also a result of the increased sales caused by the new commission policy that increased the percentage of the commission of sales.
Cost of Revenues
Cost of revenues is comprised of raw material costs, labor cost, overhead costs associated with the manufacturing processes and related expenses which are directly attributable to our revenues. Our cost of revenues for the three months ended December 31, 2012 was $8,632,261 as compared to $7,982,397 for the three months ended December 31, 2011, representing an increase of $649,864, or 8.1%. Cost of revenues were $6,006,897 and $2,625,364 for Bohai and Yantai Tianzheng, respectively, for the three months ended December 31, 2012. Cost of revenues were $5,031,124 and $2,951,273 for Bohai and Yantai Tianzheng, respectively, for the three months ended December 31, 2011. The increase in cost of revenues for the three months ended December 31, 2012, compared to the same period in last year, was mainly due to an increase in total costs of raw materials, labor, and overhead as a result of an increase in overall sales.
Gross Profit
Gross profit represents the difference between net revenues and cost of revenues. We achieved gross profit of $32,300,245 for the three months ended December 31, 2012, as compared to $26,854,489 for the same period in 2011, representing an increase of $5,445,756, or 20.3%, over the same period in 2011. The increase of the gross profit is mainly due to increased revenues.
Our overall gross profit margins as a percentage of net revenues increased by approximately 1.8% from 77.1% to 78.9% this fiscal quarter at December 31, 2012, as compared to the same period in 2011.The increase of the gross profit margin is attributable to the company's effort to improve its profit control.
Operating Expenses
Our operating expenses increased by $6,852,505 or 36.6% to $25,578,497, for the three months ended December 31, 2012, as compared to $18,725,992 for the same fiscal period in 2011. The overall increase in selling, general, and administrative expenses was related to increased selling expenses due to services supporting an overall increase in sales activities and increased amortization expenses. The percentage of operating expenses to net revenues was 62.5% and 53.8% for the three months ended December 31, 2012 and 2011, respectively, representing an increase of 16.3% as a percentage of net revenues. The increase of percentage of net revenue is because operating expense increased faster than the increase of net revenue this quarter compared to the same period last year.
Sales commissions amounted to $20,609,780 and $14,145,939 for the three months ended December 31, 2012 and 2011, respectively. Sales commissions represent 50.4% and 40.6% of total revenue for the three months ended December 31, 2012 and 2011, respectively. The Company increased commission rates for key products as part of its strategy to streamline operation around a more focused product portfolio. Management believes that the increase in commission rates was necessary to provide sales representatives with incentives to increase the sales of volume of its smaller more focused portfolio of products and that doing so is a critical part of the Company’s efforts to increase its market share. The company also increased commission rates on sales of any remaining goods in inventory certain for products lines that are that are being discontinued in order to clear such inventories from stock.
Depreciation and amortization expenses amounted to $1,343,204and $617,463for the three months ended December 31, 2012 and 2011, respectively. The Company reclassified $10,121,956 for the carrying amount of certain other product formulas that the Company will hold as defensive assets that will be amortized over a period of8 years commence from October 1, 2012. Amortization expense for defensive drug formulas amounted to $316,286 and $0 for the three months ended December 31, 2012 and 2011, respectively. The increase is also due to increased intangible assets including increased land use right of $19,021,652 acquired at November 5, 2012. Amortization expense for land use rights amounted to $226,819 and $123,915 for the three months ended December 31, 2012 and 2011, respectively.
Shipping costs are included in selling, general and administrative expense. Shipping costs amounted to $261,338 and $337,553 for the three months ended December 31, 2012 and 2011, respectively. Shipping costs decreased during the three months ended December 21, 2012 compared to the same period last year because Company streamlined our products that decreased the shipping quantity during the three months ended December 31, 2012 compared to the same period last year.
Advertising and promotion costs are charged to expense as incurred. Advertising expenses included in selling, general and administrative expenses amounted to $0and $1,261,285 for the three months ended December 31, 2012 and 2011, respectively. Although there is no advertising expenses this year, but Company signed a new advertising contract to advertising in year 2013. The contract commits to start from January 1, 2013 and ends December 31, 2013; the contract amount is $326,855.
Other operating expenses amounted to $1,316,184 and $2,145,014 for the three months ended December 31, 2012 and 2011, respectively. The decrease is mainly because decreased conference-hosting expenses. The Company sponsored and hosted a conference for Rheumatism branch of the Chinese Medical Association during the three months ended December 31, 2011 and spent about $300,000 for the conference.
Total Other Income (Expenses)
Total other income (expenses) are comprised of interest income (expenses), changes in fair value of derivative instruments, other income (expenses), and amortization of deferred financing fees. Total other income were $295,567 for the three months ended December 31, 2012 compared to total other expenses of $7,127,707 for the period ended December 31, 2011, a decrease of total other expenses of $7,423,274. The decrease in total other expenses were principally due to a net decrease of $6,525,239 for interest expenses and a net decrease in non-cash expense in fair value of warrants for $904,981 for convertible notes in connection with our private placement on January 5, 2010. The effective interest expense for convertible notes is calculated using a constant effective interest rate, applied to the carrying value of the notes each month. As the carrying value increases, so does the interest expense.
On December 31, 2011, the Company’s Chinese operating subsidiary determined it was unable to convert a sufficient number of RMB’s needed to repay the notes on their original maturity date of January 5, 2012. As a result, the Company entered into a series of amendments to the Notes with Euro Pacific as representative of the Investors to extend to the maturity date and increase the interest rate on the Notes. Pursuant to the most recent amendment, the maturity date of the notes was extended April 5, 2013 and the interest rate was increased to 12% per annum. The interest expenses related to convertible notes is $268,043 and $6,687,314 for the three months ended December 31, 2012.As of December 31, 2012, there were 4,232,250 shares of the Company’s Common Stock issuable upon conversion of the outstanding convertible notes (see Note 10).
Provision for Income Tax
Our provision for income taxes for the three months ended December 31, 2012 and 2011 were $1,758,578 and $2,296,292, a decrease of $537,714, or 23.4%, from this fiscal quarter to date over the same period last year. The decrease in provision for income tax was principally due to a decrease in taxable income under the PRC law from Bohai and from Yantai Tianzheng. The effective income tax rates were 25% and 229% for the three months ended December 31, 2012 and 2011, respectively.
Net Income
We had a net income of $5,258,737 for the three months ended December 31, 2012, as compared to net loss of $1,295,502 for the three months ended December 31, 2011, an increase in net income of $6,554,239, or 505.9%. This translates into basic earnings (loss) per common share of $0.29 and $(0.07), and diluted earnings per common share of $0.25 and $(0.07), for the three months ended December 31, 2012 and 2011, respectively. The increase in net income was primarily attributable to an increase in total gross profit of $5,445,756 and a decrease in total other expenses (resulting from mostly effective interest charges) of $7,423,274, offset by an increase in operating expenses of $6,852,505 for this fiscal quarter as compared to the same period of prior year.
Net income margin was 12.8% for the three months ended December 31, 2012 as compared to net loss margin 3.7% for the same period last year, an increase of 16.5%. The increase was mainly due to the net decrease in certain non-cash activities such as effective interest charges from convertible notes and increase in gross profit, offset by increased selling and general and administrative expenses.
Total other income included a non-cash charge in effective interest expenses of $0 for the three months ended December 31, 2012 compared to $6,687,314 for the same period in 2011.
Total other income for the three months ended December 31, 2012 also comprised of a non-cash credit for fair value of warrants of $713,234.
Comparison of the six months ended December 31, 2012 and 2011
Net Revenues
Net revenues are comprised of sales of 20 traditional Chinese medicines (corresponding to 16 drug formulas) in China during the six months ended December 31, 2012.We currently sell 20 medicines (corresponding to 16 drug formulas) following our acquisition of Yantai Tianzheng on August 8, 2011, but have recently made the strategic choice to narrow our focus to just our top 11 products(corresponding to 7 drug formulas).Net revenues for the six months ended December 31, 2012 increased by $11,516,584, or 17.8%, to $76,281,326 as compared to $64,764,742 for the six months ended December 31, 2011. Net revenues were $53,529,782 and $22,751,544 for Bohai and Yantai Tianzheng, respectively, for the six months ended December 31, 2012. Net revenues were $45,217,907 and $19,546,835 for Bohai and Yantai Tianzheng, respectively, for the six months ended December 31, 2011. The increase in Bohai’s revenue was primarily due to a net increase in revenues of 33.3% from our four lead products in Bohai: Lung Nourishing Syrup, Tongbi Capsules, Tongbi Tablets, and Shangtongning Tablets, which together accounted for over 91.5% of our total net revenues for Bohai. All of our lead products from Bohai are listed for coverage and reimbursement under national medical insurance program starting in December 2009. The increase in Tianzheng’s revenue was primarily due to a net increase in revenue of 20.8% of our two lead products in Tianzheng: Zhengxintai Capsule, Fangfengtongsheng Granule. The sale of our prescription drug products for the six months ended December 31, 2012 represented 80% of total net revenue compared to 73 % for the same period in last year. The increase in prescription sales was primary due to increases in sales volume of our two prescription drugs, Tongbi Capsules and Tongbi Tablets as well as prescription product sales from Yantai Tianzheng.The increase of the Company’s revenue is because of the increase of the market demand for the company’s key products that are included in the government’s essential drug list. The government encourages hospital and doctors to prescribe essential drugs to the patients. The increase of the revenue is also a result of the increased sales caused by the new commission policy that increased the percentage of the commission of sales.
Cost of Revenues
Cost of revenues is comprised of raw material costs, labor cost, overhead costs associated with the manufacturing processes and related expenses which are directly attributable to our revenues. Our cost of revenues for the six months ended December 31, 2012 was $17,224,365 as compared to $14,926,117 for the six months ended December 31, 2011, representing an increase of $2,298,248, or 15.4%. Cost of revenues were $11,321,530 and $5,902,835 for Bohai and Yantai Tianzheng, respectively, for the six months ended December 31, 2012. Cost of revenues were $9,688,876 and $5,237,240 for Bohai and Yantai Tianzheng, respectively, for the six months ended December 31, 2011. The increase in cost of revenues for the six months ended December 31, 2012, compared to the same period in last year, was mainly due to an increase in total costs of raw materials, labor, and overhead as a result of an increase in overall sales.
Gross Profit
Gross profit represents the difference between net revenues and cost of revenues. We achieved gross profit of $59,056,961 for the six months ended December 31, 2012, as compared to $49,838,625 for the same period in 2011, representing an increase of $9,218,336, or 18.5%, over the same period in 2011. The increase of the gross profit is mainly due to increased revenues.
Our overall gross profit margins as a percentage of net revenues increased by approximately 0.4% from 77.0% to 77.4% this first two fiscal quarters at December 31, 2012, as compared to the same period in 2011. The increase of the gross profit margin is attributable to the company’s effort to improve its profit control by monitoring the pricing to keep a stable profit margin.
Operating Expenses
Our operating expenses increased by $8,784,022 or 25.1% to $43,744,503, for the six months ended December 31, 2012, as compared to $34,960,481 for the same fiscal period in 2011. The overall increase in selling, general, and administrative expenses was related to increase selling expenses due to services supporting an overall increase in sales activities and increased amortization expenses. The percentage of operating expenses to net revenues was 57.3% and 54.0% for the six months ended December 31, 2012 and 2011, respectively, representing an increase of 6.2% as a percentage of net revenues. The increase of percentage of net revenue is because selling expenses increased this period compared to the same period last year.
Sales commissions amounted to $36,157,254 and $26,667,477for the six months ended December 31, 2012 and 2011, respectively. Sales commissions accounted for 47.4% and 41,2% of the total revenue for the six months ended December 31, 2012 and 2011, respectively. Management believes that the increase in commission rates was necessary to provide sales representatives with incentives to increase the sales of volume of its smaller more focused portfolio of products and that doing so is a critical part of the Company’s efforts to increase its market share. The company also increased commission rates on sales of any remaining goods in inventory certain for products lines that are that are being discontinued in order to clear such inventories from stock.
Depreciation and amortization expenses amounted to $2,302,094and $1,247,061for the six months ended December 31, 2012 and 2011, respectively. The Company reclassified $10,121,956 for the carrying amount of certain other product formulas that the Company will hold as defensive assets that will be amortized over a period of 8 years commence from October 1, 2012. Amortization expense for defensive drug formulas amounted to $316,286 and $0 for the six months ended December 31, 2012 and 2011, respectively. The increase is also due to increased intangible assets including increased land use right of $19,021,652 acquired at November 5, 2012. Amortization expense for land use rights amounted to $389,382 and $272,881 for the six months ended December 31, 2012 and 2011, respectively.
Shipping costs amounted to $553,981 and $633,729 for the six months ended December 31, 2012 and 2011, respectively. Shipping costs decreased during the six months ended December 21, 2012 compared to the same period last year because Company streamlined our products that decreased the shipping quantity during the six months ended December 31, 2012 compared to the same period last year.
Advertising and promotion costs are charged to expense as incurred. Advertising expenses included in selling, general and administrative expenses amounted to $0and $3,121,494 for the six months ended December 31, 2012 and 2011, respectively. Although there is no advertising expenses this year, but Company signed a new advertising contract to advertising in year 2013. The contract commits to start from January 1, 2013 and ends December 31, 2013; the contract amount is $326,855.
Other operating expenses amounted to $2,407,867 and $2,763,266 for the six months ended December 31, 2012 and 2011, respectively. The decrease is mainly because decreased conference-hosting expenses. The Company sponsored and hosted a conference for Rheumatism branch of the Chinese Medical Association during the six months ended December 31, 2011 and spent about $300,000 for the conference.
Total Other Income (Expenses)
Total other income (expenses) are comprised of interest income (expenses), changes in fair value of derivative instruments, other income (expenses), and amortization of deferred financing fees. Total other income were $281,615 for the six months ended December 31, 2012 compared to total other expenses of $9,403,211 for the period ended December 31, 2011, a decrease of total other expenses of $9,684,826. The decrease in total other expenses were principally due to a net decrease of $8,634,717 for interest expenses and a net increase in non-cash income in fair value of warrants for $1,070,498 for convertible notes in connection with our private placement on January 5, 2010. The effective interest expense for convertible notes is calculated using a constant effective interest rate, applied to the carrying value of the notes each month. As the carrying value increases, so does the interest expense.
On December 31, 2011, the Company’s Chinese operating subsidiary determined it was unable to convert a sufficient number of RMB’s needed to repay the notes on their original maturity date of January 5, 2012. As a result, the Company entered into a series of amendments to the Notes with Euro Pacific as representative of the Investors to extend to the maturity date and increase the interest rate on the Notes. Pursuant to the most recent amendment, the maturity date of the notes was extended April 5, 2013 and the interest rate was increased to 12% per annum. The interest expenses related to convertible notes is $550,193 and $9,015,701 for the three months ended December 31, 2012. As of December 31, 2012, there were 4,232,250 shares of the Company’s Common Stock issuable upon conversion of the outstanding convertible notes (see Note 10).
Provision for Income Tax
Our provision for income taxes for the six months ended December 31, 2012 and 2011 were $3,939,824 and $4,114,651, a decrease of $174,827, or 4.2 %, from this fiscal quarter to date over the same period last year. The decrease in provision for income tax was principally due to a decrease in taxable income under the PRC law from Bohai and from Yantai Tianzheng. The effective income tax rates were 25% and 75% for the six months ended December 31, 2012 and 2011, respectively.
Net Income
We had a net income of $11,654,249 for the six months ended December 31, 2012, as compared to net income of $1,360,282 for the six months ended December 31, 2011, an increase in net income of $10,293,967, or 756.8%. This translates into basic earnings per common share of $0.65 and $0.08, and diluted earnings per common share of $0.55 and $0.08, for the six months ended December 31, 2012 and 2011, respectively. The increase in net income was primarily attributable to an increase in total gross profit of $9,218,336 and a decrease in total other expenses (resulting from mostly effective interest charges) of $9,684,826, offset by an increase in operating expenses of $8,784,022 for this first two fiscal quarters as compared to the same period of prior year.
Net income margin was 15.3% for the six months ended December 31, 2012 as compared to net income margin 2.1% for the same period last year, an increase of 13.2%. The increase was mainly due to the net decrease in certain non-cash activities such as effective interest charges from convertible notes and increase in gross profit, offset by increased selling and general and administrative expenses.
Total other income included a non-cash charge in effective interest expenses of $0 for the six months ended December 31, 2012 compared to $9,015,701 for the same period in 2011.
Total other income for the six months ended December 31, 2012 also comprised of a non-cash credit for fair value of warrants of $1,211,236.
Liquidity and Capital Resources
Liquidity is the ability of a company to generate adequate amounts of cash to meet its needs for cash. As of December 31, 2012, we had cash and cash equivalents of $6,324,860 and restricted cash of $8,407,565, substantially almost all of which is located in financial institutions in China. The following table provides detailed information about our net cash flow for financial statement periods presented in this report:
Summary of Cash Flow Statements
| | | For the six months ended | |
| | | December 31 | |
| | | 2012
(unaudited) | | | 2011
(unaudited) | |
| Net cash provided by operating activities | | $ | 6,450,665 | | | $ | 11,690,444 | |
| Net cash used in investing activities | | | (20,226,648 | ) | | | (9,604,548 | ) |
| Net cash provided by financing activities | | | (1,563,480 | ) | | | 4,791,004 | |
| Effect of foreign currency translation on cash and cash equivalents | | | 107,759 | | | | 212,634 | |
| Net increase in cash and cash equivalents | | $ | (15,231,704 | ) | | $ | 7,089,534 | |
Net Cash Provided by Operating Activities
Net cash provided by operating activities totaled $6,450,665 for the six months ended December 31, 2012 as compared to net cash provided by operating activities of $11,690,444 for the six months ended December 31, 2011. The decrease in net cash provided by operating activities, for the six months ended December 31, 2012 compared to the same period 2011, was primarily due to increased net income of $10.3 million, increased accrued liabilities of $2.5 million, increased impairment of amortization $1.7 million, increased depreciation and amortization of $1.1 million, offset by a decrease of $8.6 million in the effective interest on convertible notes, an increase of $10.1 million in accounts receivables, and an increase in prepaid expenses and other assets of $0.5 million. We expect our cash flow from operating activities to maintain a positive flow due to our continuous cash flow management.
Net Cash Used In Investing Activities
Net cash used in investing activities was $20,226,648 for the six months ended December 31, 2012 and $9,604,548 for the six months ended December 31, 2011. The net increase in cash used in investing activities was due to a cash payment of approximately $12,472,875 for our Yantai Tianzheng acquisitions and a cash payment of $6.3 million from land use rights for the quarter ended December 31, 2012.
Net Cash Provided by Financing Activities
Net cash used in financing activities totaled $1,563,480 for the six months ended December 31, 2012 as compared to net cash provided by financing activities of $4,791,004 for the same period in 2011. The reason for the decrease in cash provided by financing activities was due to a cash receipt of $6.3 million from an equity shareholder, Mr. Qu (our Chairman and CEO) the six months ended December 31, 2011. Mr. Qu made a permanent equity capital contribution of approximately $6.3 million (RMB 40,000,000) into Bohai on August 3, 2011 to support its future capital needs.
Cash Position
As of December 31, 2012, we had cash of $3,154,584 as compared to $18,386,288 as of June 30, 2012, a decrease of $15,231,704. This decrease was due primarily to an increase in net income of approximately $10.3 million, an increase of accrued liabilities of $2.5 million, offset by increased accounts receivable of $10.1 million, purchase of land use right of $6.2 million, a decrease of $8.6 million in the effective interest on convertible notes, and decreased capital contribution by shareholder of $6.3 million.
We believe that we can meet our liquidity and capital requirements for our ongoing operations from our currently available working capital and maintain our operations at our current levels.
However, during the current fiscal year and thereafter, we will be required to fund two significant obligations (as well as others described under “Obligations under Material Contracts” below):
| | (i) | the completion of the acquisition of Yantai Tianzheng ($5,000,000 million is due within 12 months as of December 31, 2012. The installment balances has be turned into loans with a two-years term at 6% annual interest rate); |
| | (ii) | the repayment our convertible promissory notes due April 5, 2013 ($8.465 million due as of December 31, 2012); and |
| | (iii) | the payment of approximately $12,680,000 payable in 2013 for a new land use right we acquired in November 2012 (due in two equal installments at June 30 and December 31, 2013). |
As such, we will be required to raise substantial additional capital to fund these obligations, either through the issuance of debt or equity securities, bank loans or other methods. Readers are cautioned that additional funding, capital or loans may be unavailable to us on favorable terms, if at all. If adequate funds are not available, we would likely have to renegotiate the terms of these obligations, which we may be unable to do on favorable terms. We may thus be required to agree to unfavorable terms that could have a material adverse effect on us, our financial condition and our results of operations in 2013 and beyond. Moreover, to the extent that additional capital is raised through the sale of equity or convertible debt securities, the issuance of such securities would result in ownership and potentially economic dilution to existing shareholders.
In addition, if we are faced with worldwide financial and credit crises as occurred in recent years, it may make the future cost of raising funds through the debt or equity markets more expensive or make financial markets unavailable to us at times when we require additional financings.
Finally, it has been very difficult over the past several years for U.S.-listed Chinese companies to raise financing from investors. As a result, our ability to raise needed financing may be particularly compromised.
Critical Accounting Policies and Estimates
As discussed in Part II, Item 7, Management’s Discussion and Analysis of Financial Condition and Results of Operations, of our Annual Report on Form 10-K for the fiscal year ended June 30, 2012, we consider our use of estimates, accounts receivable, revenue recognition, inventories, property plant and equipment, intangible assets and income taxes to be the most critical accounting policies in understanding the judgments that are involved in preparing our condensed consolidated financial statements. There have been no significant changes to these estimates in the six months ended December 31, 2012.
Recent Accounting Pronouncements Adopted
See Note 3 to the condensed consolidated unaudited financial statements included in Item 1, Financial Information, of this Quarterly Report on Form 10-Q.
Recent Accounting Pronouncements Not Yet Adopted
See Note 3 to the condensed consolidated unaudited financial statements included in Item 1, Financial Information, of this Quarterly Report on Form 10-Q.
Obligations under Material Contracts
The following table summarizes our contractual obligations as of December 31, 2012, and the effect these obligations are expected to have on our liquidity and cash flows in future periods.
| | | Payments Due by Period
(unaudited) | |
| | | Less than | | | 1-3 | | | 4-5 | | | 5 | | | | |
| | | Total | | | 1 year | | | Years | | | years | | | Years+ | |
| Contractual Obligations: | | | | | | | | | | | | | | | | | | | | |
| Convertible notes | | $ | 8,464,500 | | | $ | 8,464,500 | | | $ | - | | | $ | - | | | $ | - | |
| | | | | | | | | | | | | | | | | | | | | |
| Construction In Process | | | 1,248,716 | | | | 1,248,716 | | | | - | | | | - | | | | - | |
| | | | | | | | | | | | | | | | | | | | | |
| Yantai Tianzheng acquisition | | | 12,827,185 | | | | 12,827,185 | | | | - | | | | - | | | | - | |
| | | | | | | | | | | | | | | | | | | | | |
| Land use right payable | | | 12,681,102 | | | | 12,681,102 | | | | - | | | | - | | | | - | |
| | | | | | | | | | | | | | | | | | | | | |
| Advertising | | | 326,855 | | | | 326,855 | | | | - | | | | - | | | | - | |
| | | | | | | | | | | | | | | | | | | | | |
| Contract Research and Development Arrangement | | | 1,040,170 | | | | - | | | | - | | | | - | | | | 1,040,170 | |
| | | | | | | | | | | | - | | | | - | | | | | |
| Total Contractual Obligations: | | $ | 36,588,528 | | | $ | 35,548,358 | | | $ | - | | | $ | - | | | $ | 1,040,170 | |
Other than discussed above, there are no other foreseeable material commitments or contingencies as of December 31, 2012.
Off-Balance Sheet Arrangements
We did not engage in any “off-balance sheet arrangements” (as that term is defined in Item 303(a)(4)(ii) of Regulation S-K) during the six months ended December 31, 2012. We have not entered into any financial guarantees or other commitments to guarantee the payment obligations of any third parties. We have not entered into any derivative contracts that are indexed to our shares and classified as shareholder’s equity or that are not reflected in our condensed consolidated financial statements. Furthermore, we do not have any retained or contingent interest in assets transferred to an unconsolidated entity that serves as credit, liquidity or market risk support to such entity. We do not have any variable interest in any unconsolidated entity that provides financing, liquidity, market risk, or credit support to us or engages in leasing, hedging, or research and development services with us.
Currency Exchange Risk
Our reporting currency is the U.S. dollar and our operations in China use their local currency as their functional currencies. Substantially all of our revenue and expenses are in the Chinese currency, the Renminbi. We are subject to the effects of exchange rate fluctuations with respect to any of these currencies. For example, the value of the Renminbi depends to a large extent on Chinese government policies and China’s domestic and international economic and political developments, as well as supply and demand in the local market. Since 1994, the official exchange rate for the conversion of the Renminbi to the U.S. dollar had generally been stable and the Renminbi had appreciated slightly against the U.S. dollar. In July 2005, the Chinese government changed its policy of pegging the value of the Renminbi to the U.S. dollar. Under this policy, which was halted in 2008 due to the worldwide financial crisis, the Renminbi was permitted to fluctuate within a narrow and managed band against a basket of certain foreign currencies. In June 2010, the Chinese government announced its intention to again allow the Renminbi to fluctuate within the 2005 parameters. It is possible that the Chinese government could adopt an even more flexible currency policy, which could result in more significant fluctuation of Renminbi against the U.S. dollar, or it could adopt a more restrictive policy. We can offer no assurance that the Renminbi will be stable against the U.S. dollar or any other foreign currency.
Our consolidated financial statements are translated into U.S. dollars at the average exchange rates in each applicable period. To the extent the U.S. dollar strengthens against foreign currencies, the translation of these foreign currencies denominated transactions results in reduced revenue, operating expenses and net income for our international operations. Similarly, to the extent the U.S. dollar weakens against foreign currencies, the translation of these foreign currency denominated transactions results in increased revenue, operating expenses and net income for our international operations. We are also exposed to foreign exchange rate fluctuations as we convert the financial statements of our foreign consolidated subsidiaries into U.S. dollars in consolidation. If there is a change in foreign currency exchange rates, the conversion of the foreign consolidated subsidiaries’ financial statements into U.S. dollars will lead to a translation gain or loss which is recorded as a component of other comprehensive income. In addition, we have certain assets and liabilities that are denominated in currencies other than the relevant entity’s functional currency. Changes in the functional currency value of these assets and liabilities create fluctuations that will lead to a transaction gain or loss. We have not entered into agreements or purchased instruments to hedge our exchange rate risks, although we may do so in the future. The availability and effectiveness of any hedging transaction may be limited and we may not be able to hedge our exchange rate risks. Most of our transactions are settled in Renminbi and U.S. dollars. We are not exposed to significant foreign currency risk.
Credit Risk
Our potential credit risk is mainly attributable to its debtors and bank balances. In respect of debtors, we have policies in place to ensure that it will only accept customers from countries which are politically stable and customers with an appropriate credit history. In addition, all the bank balances were made with financial institutions with high-credit quality. Thus, we are not considered to be subject to significant credit risk.
Interest Rate Risk
Our interest rate risk is primarily attributable to its short-term borrowings, loan to a third party and loan to equity holders. Our borrowings carry interest at fixed rate. Our management has not used any interest rate swaps to hedge its exposure to interest rate risk.
Inflation
In recent years, the Chinese economy has experienced periods of rapid expansion and high rates of inflation. During the past ten years, the rate of inflation in China has been as high as 20.7% and as low as -2.2%. These factors have led to the adoption by Chinese government, from time to time, of various corrective measures designed to restrict the availability of credit or regulate growth and contain inflation. While inflation has been more moderate since 1995, high inflation may in the future cause Chinese government to impose controls on credit and/or prices, or to take other action, which could inhibit economic activity in China, and thereby harm the market for our products.
Item 3. Quantitative and Qualitative Disclosures about Market Risk
Not applicable
Item 4. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
As of December 31, 2012, the quarterly period covered by this report, the management conducted evaluations of the Company’s disclosure controls and procedures. As defined under Sections 13a-15(e) and 15d-15(e) of the Securities Exchange Act of 1934, as amended (the Exchange Act”), the term disclosure controls and procedures” means controls and other procedures of an issuer that are designed to ensure that information required to be disclosed by the issuer in the reports that it files or submits under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures include without limitation, controls and procedures designed to ensure that information required to be disclosed by an issuer in the reports that it files or submits under the Exchange Act is accumulated and communicated to the issuer’s management, to allow timely decisions regarding required disclosures.
Based on this evaluation, the management have concluded that our disclosure controls and procedures were, due to certain material weaknesses in internal control and significant deficiencies on financial reporting discussed below, not effective to ensure that material information is recorded, processed, summarized and reported by our management on a timely basis in order to comply with our disclosure obligations under the Exchange Act and the rules and regulations promulgated there under.
Material weakness
We identified two material weaknesses in the design and operation of our internal controls. The material weakness is related to (i) the failure to retain sufficient qualified accounting personnel to prepare financial statements in accordance with accounting principles generally accepted in the US (including a qualified Chief Financial Officer); and (ii) the Company’s accounting department personnel have limited knowledge and experience in US GAAP.
To remediate the material weaknesses identified in internal control over financial reporting of the Company, we have commenced to: (i) hire additional personnel with sufficient knowledge and experience in US GAAP; and (ii) provide ongoing training courses in US GAAP to existing personnel, including our Financial Controller in China.
These new remediation initiatives were to be put into place in the fourth quarter of 2012. We will continue to monitor and assess our remediation initiatives to ensure that the aforementioned material weakness is remediated.
Significant deficiency
A significant deficiency is a deficiency, or a combination of deficiencies, in internal control over financial reporting that is less severe than a material weakness(within the meaning of PCAOB Auditing Standard No. 5) yet important enough to merit attention by those responsible for oversight of a company’s financial reporting.
The significant deficiencies identified by the management continue to be as described in our Annual Report on Form 10-K for the fiscal year ended June 30, 2012, which was filed with the SEC on September 28, 2012. As a result, the Certifying Officers and our board of directors are continuing to evaluate on our internal control over financial reporting and our disclosure controls and procedures in an attempt to address such significant deficiencies.
Changes in Internal Control over Financial Reporting
The Company currently lacks an audit committee. Thus there is no independent supervision from an audit committee to select and oversee the Company's independent accountant; monitor the procedures for handling complaints regarding the Company's accounting practices; engage advisors; and ensure funding for the independent auditor and any outside advisors engaged by the audit committee.
Subject to the foregoing disclosure, there were no other changes in our internal control over financial reporting during the six months ended December 31, 2012 that materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Limitations on the Effectiveness of Internal Controls
Readers are cautioned that our management does not expect that our disclosure controls and procedures or our internal control over financial reporting will necessarily prevent all fraud and material error. An internal control system, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, within the Company have been detected. The design of any system of controls also is based in part upon certain assumptions about the likelihood of future events, and there can be no assurance that any control design will succeed in achieving its stated goals under all potential future conditions. Over time, control may become inadequate because of changes in conditions, or the degree of compliance with the policies or procedures may deteriorate.
PART II - OTHER INFORMATION
Item 1. Legal Proceedings
From time to time, we may become involved in various lawsuits and legal proceedings, which arise in the ordinary course of business. However, litigation is subject to inherent uncertainties, and an adverse result in these or other matters may arise from time to time that may harm business. We are currently not aware of any such legal proceedings or claims that will have, individually or in the aggregate, a material adverse affect on our business, financial condition or operating results.
Item 1A. Risk Factors
1. The Company hereby adds the following risk factors to its disclosures:
We have significant payments that we are required to make in 2013 in connection with our acquisition of land use rights.
On November 5, 2012, we acquired a new land use right of 266,668 square meters located in high-tech development district of Laishan district. The land use right grants to us the right to use the land for a period of 50 years with a total acquisition cost of approximately $19 million (RMB 120,000,000). As of December 31, 2012, wemade two payments totaling of $6,340,550.. We are obligated to make the rest of payments by two installment payments as follows: $6,340,551 by June 30, 2013 and $6,340,551 by December 31, 2013
There is a significant risk that we may not generate sufficient cash flow from operations or otherwise have access to financing on favorable terms, or at all to meet this obligation (together with our other significant obligations). Failure to make these payments could result in the loss of our land use right, which could have a material adverse effect on our business, financial condition or operating results.
We have recently materially shifted our business focus, and there is a risk that we may not achieve the benefits of our new strategy.
During the quarter ending December 31, 2012, we determined to streamline our operations to focus on the continued distribution of our top selling products, namely, Lung Nourishing Syrup, Tongbi Capsules, Tongbi Tablets, Fangfengtongsheng Granule, and Zhengxintai Capsules and certain other products, and we fully abandoned or suspended indefinitely plans to develop and produce a more diversified portfolio that would be derived from a series of approved formulas that we currently own. While we are hopeful that this shift in strategy will help our operating results, there is a risk that we may be unable to obtain the desired results of this new strategy and a further risk that the new strategy will lead to lower revenues and profits.
2. The Company hereby amends the following risk factor:
We have significant payment obligations under the Notes due April 5, 2013, and we continue to face difficulties in converting our cash on hand from RMB to US Dollars in order to make payment under the Notes. Any non-payment of the Notes when due in the absence of an extension of the maturity date would constitute event of default under the Notes, and our financial condition may be adversely affected.
As of the date of this Report, we have outstanding Notes in the aggregate amount of approximately $8,464,500, all of which are due April 5, 2013. We had previously established an RMB denominated escrow account in China and deposited into such escrow account the remaining outstanding amount of the Notes. As of the date of this Report, the escrow account remains in place and we continue to work on converting RMB to Dollars in order to make payments under the Notes. However, there is a risk that we may be unable to pay the Notes by this current maturity date. We will continue to work with Euro Pacific on this matter and will continue our efforts in order to meet our obligations to the Note holders. However, there is no assurance that an additional Note amendment will be entered into to extend the due date again, and any non-payment of the Notes when due would constitute an event of default under the Notes. In such event, we might be subject to, among other things, non-payment claims of the Noteholders, and our financial condition may be adversely affected.
Item 2. Unregistered Sales of Equity Securities and Use of Proceeds
None.
Item 3. Defaults Upon Senior Securities
None.
Item 4. Mine Safety Disclosures
Not applicable.
Item 5. Other Information
None.
Item 6. Exhibits
(a) Exhibits
Exhibit
Number | | Description of Exhibit |
| 31.1* | | Certification of Principal Executive and Financial Officer pursuant to Rule 13a-14 and Rule 15d-14(a), promulgated under the Securities and Exchange Act of 1934, as amended. |
| 32.1* | | Certification pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 (Chief Executive Officer and principal financial officer). |
| | | |
101.INS* | | XBRL Instance Document |
| | | |
101.SCH* | | XBRL Taxonomy Extension Schema Document |
| | | |
101.CAL* | | XBRL Taxonomy Extension Calculation Linkbase Document |
| | | |
101.DEF* | | XBRL Taxonomy Extension Definition Linkbase Document |
| | | |
101.LAB* | | XBRL Taxonomy Extension Label Linkbase Document |
| | | |
101.PRE* | | XBRL Taxonomy Extension Presentation Linkbase Document |
* XBRL (eXtensible Business Reporting Language) information is furnished and not filed or a part of a registration statement or prospectus for purposes of Sections 11 or 12 of the Securities Act of 1933, as amended, is deemed not filed for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, and otherwise is not subject to liability under these section.
_________________
* Filed herewith
SIGNATURES
In accordance with the requirements of the Exchange Act, the registrant caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | Bohai Pharmaceuticals Group, Inc. |
| | | |
| February 14, 2013 | By: | /s/ HongweiQu |
| | | HongweiQu Chief Executive Officer (Principal Executive Officer, Principal Financial Officer and Principal Accounting Officer) |
