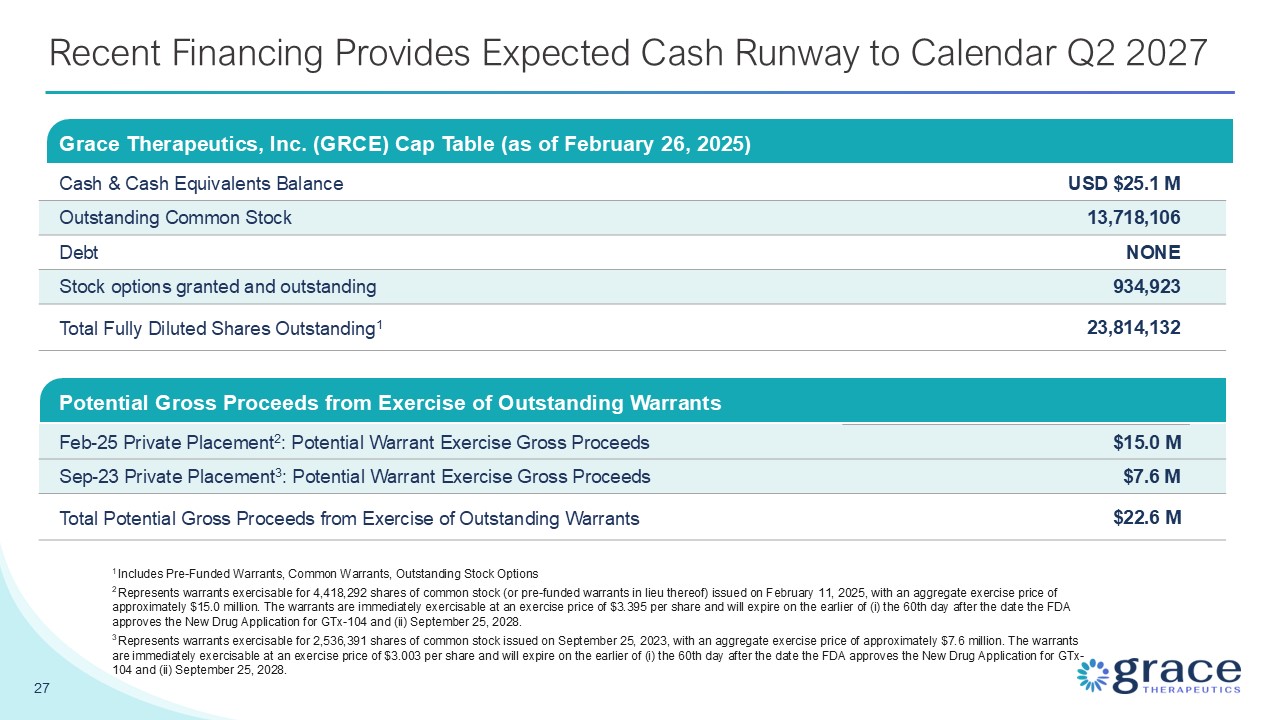
Summary 2 Forward Looking Statements Statements in this presentation that are not statements of historical or current fact constitute "forward-looking statements" within the meaning of the U.S. Private Securities Litigation Reform Act of 1995, as amended, Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended, and "forward-looking information" within the meaning of Canadian securities laws (collectively, "forward-looking statements"). Such forward looking statements involve known and unknown risks, uncertainties, and other factors that could cause the actual results of Grace Therapeutics, Inc. to be materially different from historical results or from any future results expressed or implied by such forward-looking statements. In addition to statements which explicitly describe such risks and uncertainties, readers are urged to consider statements containing the terms "believes," "belief," "expects," "intends," "anticipates," "estimates", "potential," "should," "may," "will," "plans," "continue", "targeted" or other similar expressions to be uncertain and forward-looking. Readers are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date of this presentation. The forward-looking statements in this presentation, including, but not limited to, statements regarding the Company’s expected cash runway, preliminary estimates of the Company’s cash and cash equivalents, the future prospects of the Company’s GTx-104 drug candidate, the timing of the Company's anticipated NDA submission for GTx-104 with the FDA, GTx-104's potential to bring enhanced treatment options to patients suffering from aneurysmal subarachnoid hemorrhage (“aSAH”), GTx-104’s potential to be administered to improve the management of hypotension in patients with aSAH, the ability of GTx-104 to achieve a pharmacokinetic and safety profile similar to the oral form of nimodipine, GTx-104’s potential to provide improved bioavailability and the potential for reduced use of rescue therapies, GTx-104’s potential to achieve pharmacoeconomic benefit over the oral form of nimodipine, GTx-104’s commercial prospects, the Company’s pre-commercial launch strategy for GTx104, the future prospects of the Company’s GTx-102 drug candidate, GTx-102’s potential to provide clinical benefits to decrease symptoms associated with Ataxia Telangiectasia, GTx-102’s potential ease of drug administration, the timing and outcomes of a Phase 3 efficacy and safety study for GTx-102, the timing of an NDA filing for GTx-102, the size of the addressable market for GTx-104 and GTx 102, and any future patent and other intellectual property filings made by the Company for new developments are based upon Grace Therapeutics, Inc.’s current expectations and involve assumptions that may never materialize or may prove to be incorrect. Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties, including, without limitation: (i) the success and timing of regulatory submissions of the STRIVE-ON Phase 3 safety trial for GTx-104; (ii) regulatory requirements or developments and the outcome and timing of the proposed NDA application for GTx-104; (iii) changes to clinical trial designs and regulatory pathways; (iv) legislative, regulatory, political and economic developments; and, (v) actual costs associated with Grace Therapeutics clinical trials as compared to management's current expectations. The foregoing list of important factors that could cause actual events to differ from expectations should not be construed as exhaustive and should be read in conjunction with statements that are included herein and elsewhere, including the risk factors detailed in the "Special Note Regarding Forward-Looking Statements," "Risk Factors" and "Management's Discussion and Analysis of Financial Condition and Results of Operations" sections of the Company's Annual Report on Form 10-K for the fiscal year ended March 31, 2024, Quarterly Report on Form 10-Q for the quarterly period ended June 30, 2024, the Quarterly Report on Form 10-Q for the quarterly period ended September 30, 2024, the Quarterly Reports on Form 10-Q and Form 10-Q/A for the quarterly period ended December 31, 2024 and other documents that have been and will be filed by Grace Therapeutics, Inc. from time to time with the Securities and Exchange Commission and Canadian securities regulators. All forward-looking statements contained in this presentation speak only as of the date on which they were made. Grace Therapeutics undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made, except as required by applicable securities laws.