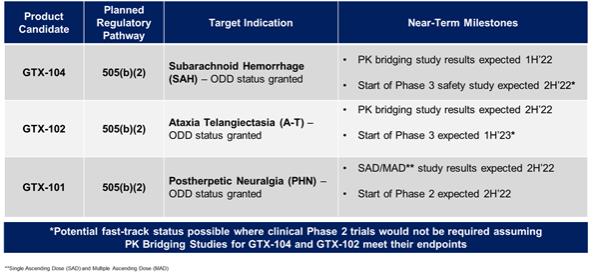
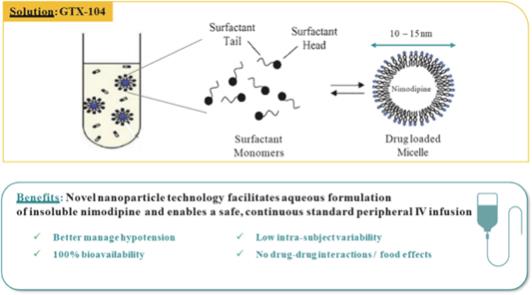
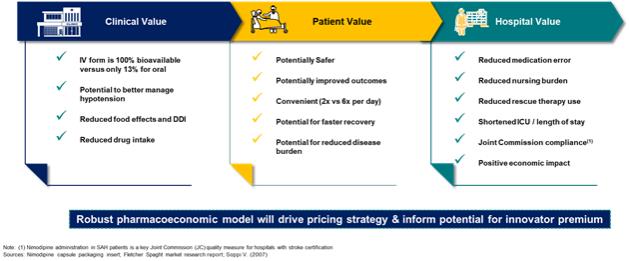
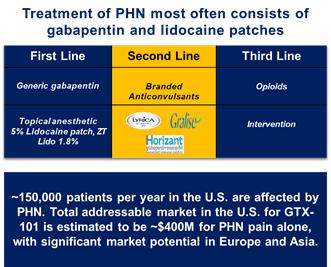
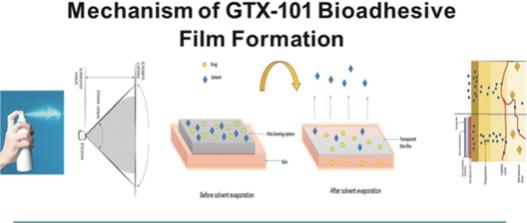
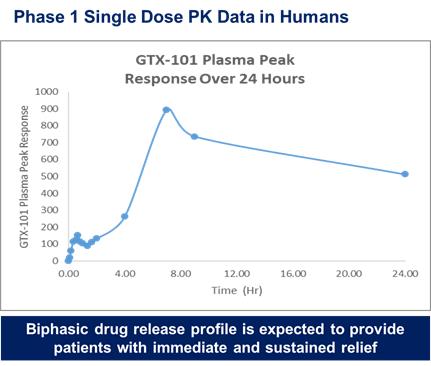
of President Biden’s Build Back Better program to lower prescription drug prices by allowing the Medicare program to negotiate drug prices.
The Health Reform Laws also are the subject of ongoing litigation. A collection of 20 state governors and state attorneys general (subsequently two states have dropped out) filed a lawsuit against the federal government in the Northern District of Texas seeking to enjoin the entire ACA following the elimination of the individual mandate penalty in 2019. In an opinion issued in June 2021, the United States Supreme Court held that plaintiffs did have standing to raise the constitutional challenges they asserted and remanded the case with instructions to dismiss.
Federal and state governments will likely continue to review and assess alternative healthcare delivery systems and payment methodologies, and public debate regarding these issues will continue in the future. Changes in the law or new interpretations of existing laws can have a substantial effect on permissible activities, the relative costs associated with doing business in the healthcare industry, and the amount of reimbursement available from government and other third-party payors. If the Health Reform Laws are repealed or modified, or if implementation of certain aspects of the Health Reform Laws continues to be delayed, such repeal, modification, or delay may have a material and adverse impact on our business, financial condition, results of operations, cash flow, capital resources and liquidity.
Foreign Regulation
To market any product outside of the United States, it is necessary to comply with numerous and varying regulatory requirements of other countries and jurisdictions regarding quality, safety and efficacy and governing, among other things, clinical trials, marketing authorization, commercial sales and distribution of its products. Regardless of FDA approval for a product, the necessary approvals by comparable foreign regulatory authorities are required before commencing clinical trials or marketing any product in foreign countries and jurisdictions. Although many of the issues discussed above with respect to the United States may apply similarly in the context of foreign countries, the approval process varies between countries and jurisdictions and can involve additional product testing and additional administrative review periods. The time required to obtain approval in other countries and jurisdictions might differ from and be longer than that required to obtain FDA approval. Regulatory approval in one country or jurisdiction does not ensure regulatory approval in another, but a failure or delay in obtaining regulatory approval in one country or jurisdiction may negatively impact the regulatory process in others.
Other Regulatory Requirements
We are also subject to various laws and regulations regarding laboratory practices, the experimental use of animals, and the use and disposal of hazardous or potentially hazardous substances in connection with our research. In each of these areas, the FDA and other government agencies have broad regulatory and enforcement powers, including the ability to levy fines and civil penalties, suspend or delay issuance of approvals, seize or recall products, and withdraw approvals, any of which could have a material adverse effect.
Should operations be found to be in violation of any of such laws or any other applicable governmental regulations, we may be subject to penalties, including, without limitation, administrative, civil and criminal penalties, damages, fines, disgorgement, contractual damages, reputational harm, diminished profits and future earnings, the curtailment or restructuring of our operations, exclusion from participation in federal and state healthcare programs and individual imprisonment, any of which could adversely affect our ability to operate our business and achieve financial results.
Recent Developments
Management and Operations
Post-merger, Acasti continues to be led by Jan D’Alvise as President and CEO, and we continue to maintain our corporate headquarters in Laval, Quebec, Canada and a quality laboratory in Sherbrooke, Quebec, Canada. We will also maintain the Acasti Pharma U.S. research and development laboratory and commercial presence in North Brunswick, New Jersey.
About the Merger
In connection with our acquisition of Grace, Grace merged with Acasti Pharma US, a new wholly owned subsidiary of Acasti. Grace was the surviving company in the merger, was renamed Acasti Pharma US Inc., and is now a wholly