|
Exhibit 99.1 
|
Exhibit 99.1
Proteostasis Therapeutics, Inc. (Nasdaq: PTI)
Investor Presentation December 2016
Safe Harbor and Disclaimer
To the extent that statements in this presentation are not historical facts, they are forward-looking statements reflecting the current beliefs and expectations of management made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. Words such as “may,” “will,” “expect,” “anticipate,” “estimate,” “intend,” and similar expressions (as well as other words or expressions referencing future events, conditions or circumstances) are intended to identify forward-looking statements. Examples of forward-looking statements made in this presentation include, without limitation, statements regarding the status of, and our expected timelines for, our ongoing and expected pre-clinical and clinical development programs. Forward-looking statements made in this presentation involve substantial risks and uncertainties that could cause actual results to differ materially from those expressed or implied by the forward-looking statements, and we therefore cannot assure you that our plans, intentions, expectations or strategies will be attained or achieved. Such risks and uncertainties include, without limitation, uncertainties inherent in the execution and completion of clinical trials, in the timing of availability of trial data, in the actions of regulatory agencies, and those set forth in our Form 10-Q for the quarter ended September 30, 2016, and our other SEC filings. We assume no obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise.
This presentation also contains estimates and other statistical data made by independent parties and by us relating to, among other items, disease incidence, market size and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. Projections, assumptions and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of risk and uncertainty. New risks emerge from time to time, and neither we nor any other person makes any representation as to the accuracy or completeness of such data or undertakes any obligation to update such date after the date of this presentation. By attending or receiving this presentation you acknowledge you are solely responsible for your own assessment of the market and our market position and that you will conduct your own analysis and are solely responsible for forming your own view of the potential future performance of our business. The trademarks included in this presentation are the property of the owners thereof and are used for reference purposes only. Such use should not be construed as an endorsement of the Company or its securities.
2
Investment Highlights
Proprietary platform to develop novel therapeutics for diseases caused by dysfunctional protein processing
Initial focus on increasing CFTR activity in patients with CF
Developing a novel class of CFTR modulators (amplifiers) that increase CFTR protein levels
- Significantly increase the activity of correctors and potentiators in standard HBE cell assays—No safety or tolerability issues noted in initial Phase I studies to date
Developing proprietary triple combination therapy including PTI-428 for the treatment of CF—cellular assays suggest full restoration of CFTR activity
Additional upside from Astellas collaboration for other protein processing diseases
Q3 16 Ending cash of $100M after successful follow-on financing
3
Key Updates Post-NACFC
PTI-428 CFTR Amplifier Advancing to POC
Completed dosing of SAD up to 300 mg in healthy volunteers
Completed dosing of MAD cohorts up to 150 mg in healthy volunteers
Completed dosing of SAD up to 100mg in CF subjects
Demonstrated dose proportional increase in exposure across all dose levels
Showed comparable PK in healthy volunteers and CF subjects
No safety concerns observed to date
POC study on target to be initiated in December
Expanded scope of clinical footprint, increased number of sites actively recruiting to 13 with
additional 2 sites in the queue
PTI Corrector (PTI-801) and Potentiator (PTI-808)
IND submissions for novel corrector PTI-801 and novel potentiator PTI-808 planned for Q1
2017
4
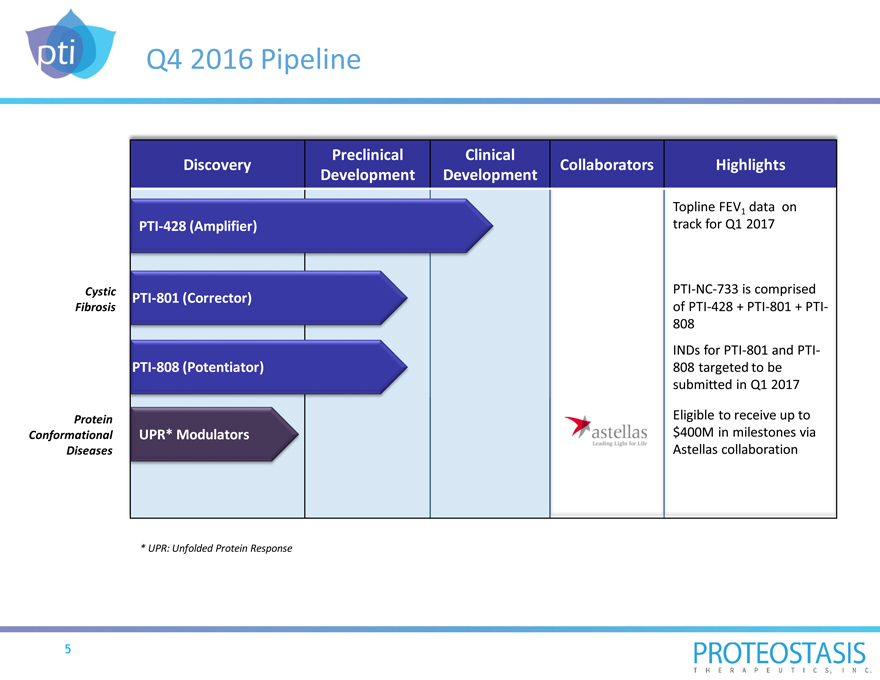
Q4 2016 Pipeline
Preclinical Clinical
Discovery Collaborators Highlights
Development Development
Topline FEV1 data on
PTI-428 (Amplifier) track for Q1 2017
Cystic PTI-NC-733 is comprised
PTI-801 (Corrector)
Fibrosis of PTI-428 + PTI-801 + PTI-
808
INDs for PTI-801 and PTI-
PTI-808 (Potentiator) 808 targeted to be
submitted in Q1 2017
Protein Eligible to receive up to
Conformational UPR* Modulators $400M in milestones via
Diseases Astellas collaboration
* UPR: Unfolded Protein Response
5
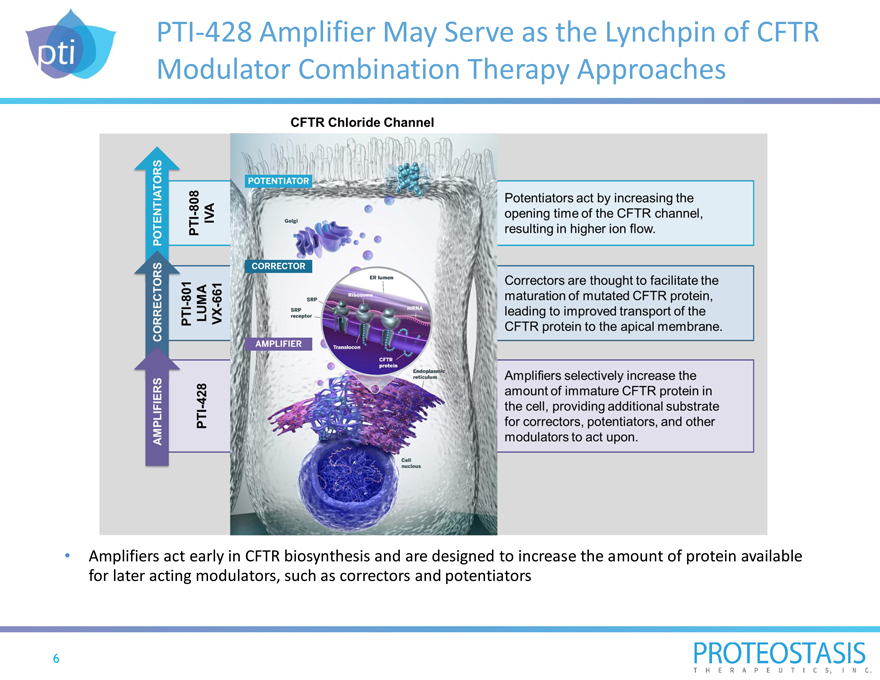
PTI-428 Amplifier May Serve as the Lynchpin of CFTR Modulator Combination Therapy Approaches
CFTR Chloride Channel AMPLIFIERS CORRECTORS POTENTIATORS PTI-428 PTI-801 LUMA VX-661 PTI-808 IVA POTENTIATOR CORRECTOR AMPLIFIER
Potentiators act by increasing the opening time of the CFTR channel, resulting in higher ion flow.
Correctors are thought to facilitate the maturation of mutated CFTR protein, leading to improved transport of the CFTR protein to the apical membrane.
Amplifiers selectively increase the amount of immature CFTR protein in the cell, providing additional substrate for correctors, potentiators, and other modulators to act upon.
Amplifiers act early in CFTR biosynthesis and are designed to increase the amount of protein available for later acting modulators, such as correctors and potentiators
6
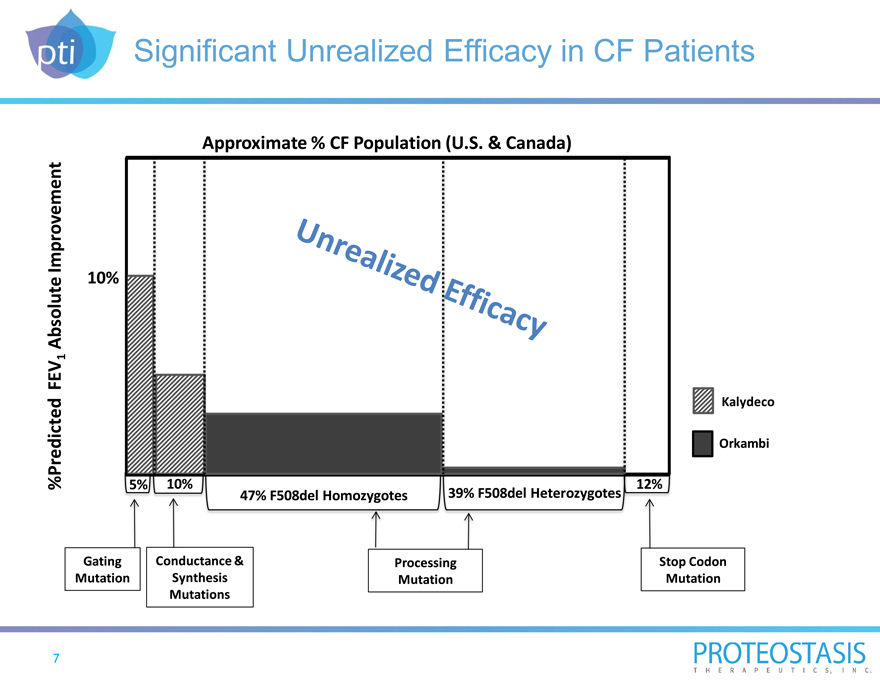
Significant Unrealized Efficacy in CF Patients
Approximate % CF Population (U.S. & Canada)
Unrealized Efficacy
%Predicted FEV1 Absolute Improvement
10%
Kalydeco
Orkambi
47% F508del Homozygotes 39% F508del Heterozygotes 12% Gating Conductance & Processing Stop Codon Mutation Synthesis Mutation Mutation Mutations
7
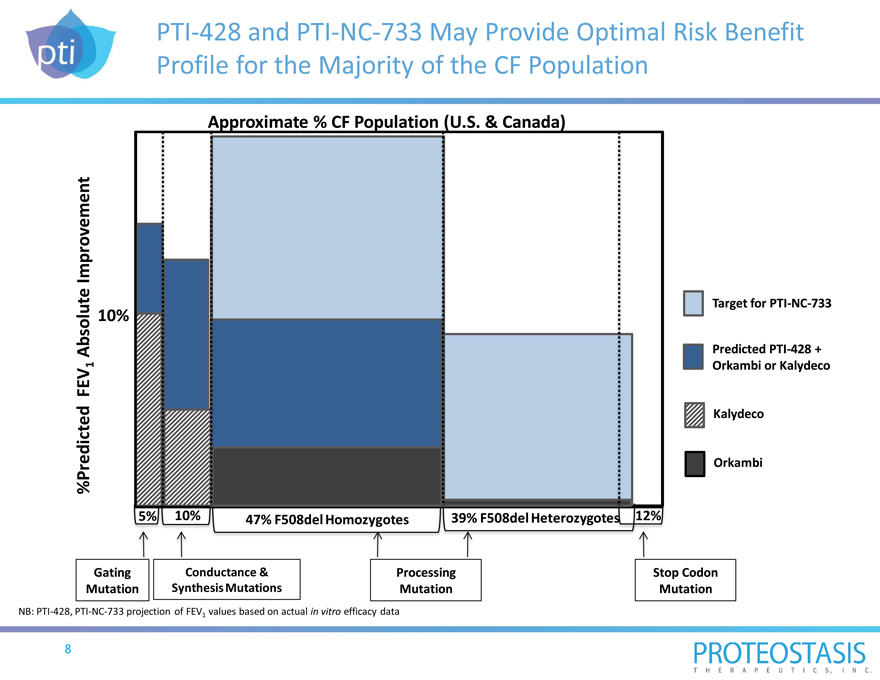
PTI-428 and PTI-NC-733 May Provide Optimal Risk Benefit Profile for the Majority of the CF Population
Approximate % CF Population (U.S. & Canada)
%Predicted FEV1 Absolute Improvement
Target for PTI-NC-733
Predicted PTI-428 + Orkambi or Kalydeco
Kalydeco
Orkambi
10% 5% 10% 47% F508del Homozygotes 39% F508del Heterozygotes 12% Gating Conductance & Processing Stop Codon Mutation Synthesis Mutations Mutation Mutation
NB: PTI-428, PTI-NC-733 projection of FEV1 values based on actual in vitro efficacy data
8
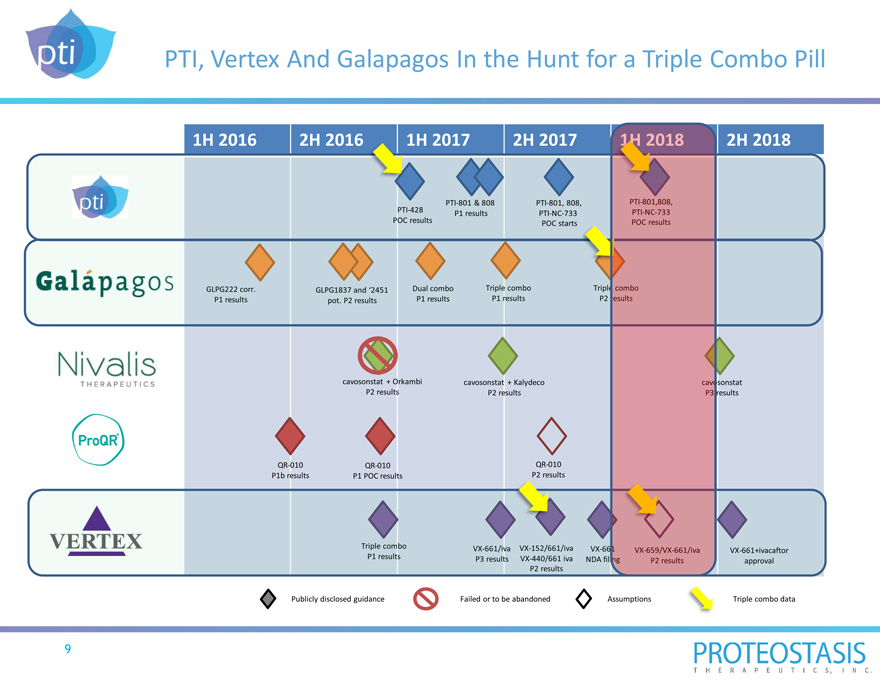
PTI, Vertex And Galapagos In the Hunt for a Triple Combo Pill
1H 2016 2H 2016 1H 2017 2H 2017 2018 2H 2018
PTI-801 & 808 PTI-801, 808, PTI-801,808, PTI-428 PTI-NC-733 P1 results PTI-NC-733 POC results POC starts POC results
GLPG222 corr. GLPG1837 and ‘2451 Dual combo Triple combo Triple combo P1 results pot. P2 results P1 results P1 results P2 results
cavosonstat + Orkambi cavosonstat + Kalydeco cavosonstat P2 results P2 results P3 results
QR-010 QR-010 QR-010 P1b results P1 POC results P2 results
Triple combo VX-661/iva VX-152/661/iva VX-661
P1 results VX-659/VX-661/iva VX-661+ivacaftor P3 results VX-440/661 iva NDA filing P2 results approval P2 results
Publicly disclosed guidance Failed or to be abandoned Assumptions Triple combo data
9
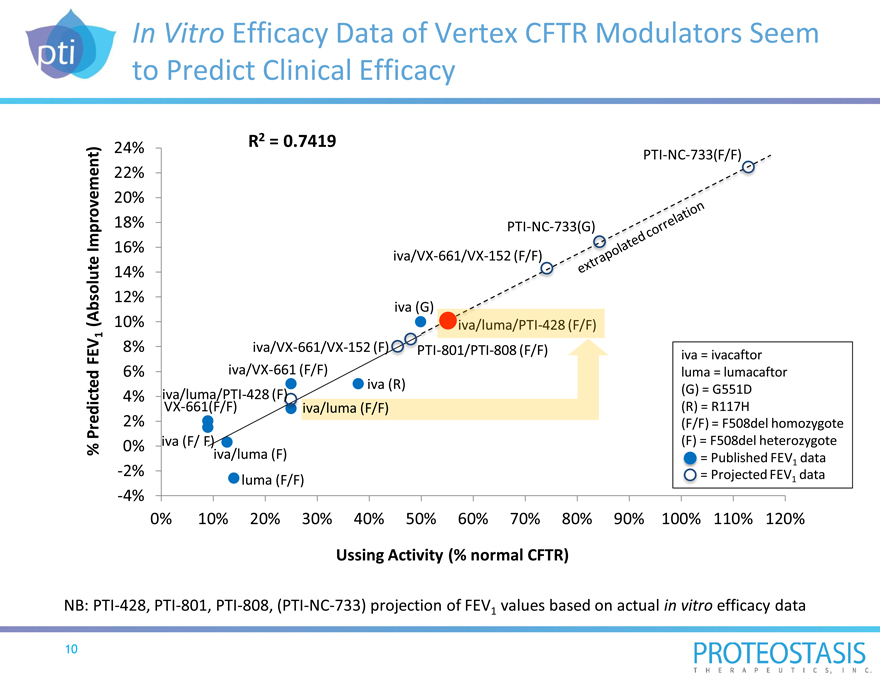
In Vitro Efficacy Data of Vertex CFTR Modulators Seem to Predict Clinical Efficacy
24% R2 = 0.7419
PTI-NC-733(F/F)
22%
20%
18% PTI-NC-733(G)
16% iva/VX-661/VX-152 (F/F)
14%
12%
iva
10%
8% iva/VX-661/VX-152 (F) PTI-801/PTI-808 (F/F) iva = ivacaftor
6% iva/VX-661 (F/F) luma = lumacaftor
iva (R)
4% iva/luma/PTI-428 (F) (G) = G551D
VX-661(F/F) iva/luma (F/F) (R) = R117H
2% (F/F) = F508del homozygote
0% iva (F/ F) (F) = F508del heterozygote
iva/luma (F) = Published FEV1 data
-2% luma (F/F) = Projected FEV1 data
-4%
0% 10% 20% 30% 40% 50% 60% 70% 80% 90% 100% 110% 120%
Ussing Activity (% normal CFTR)
% Predicted FEV1 (Absolute Improvement)
NB: PTI-428, PTI-801, PTI-808, (PTI-NC-733) projection of FEV1 values based on actual in vitro efficacy data
10
Measurement of CFTR Protein Activity In Vitro is Highly Correlated with Clinical Efficacy
Severity of CF progression is measured by FEV1 (forced expiratory volume in one second) in patients
CFTR modulators are evaluated in Ussing Chamber Assay
Potentiators and correctors show a strong correlation between their effect in vitro measured by the Ussing Chamber Assay and lung function improvement
FEV1 is industry-standard efficacy endpoint in CF clinical trials Rate of FEV1 decline correlates with life expectancy and is predictive of mortality
In vitro CFTR protein activity is measured in human bronchial epithelial (HBE) cells derived from the lungs of CF patients
Ussing Chamber Assay invented in 1946 and well-established in CF basic research
In vitro activity of CFTR protein expressed as 50% of normal CFTR function correlates with an absolute FEV1 improvement of approximately 10% observed in clinical trials
11
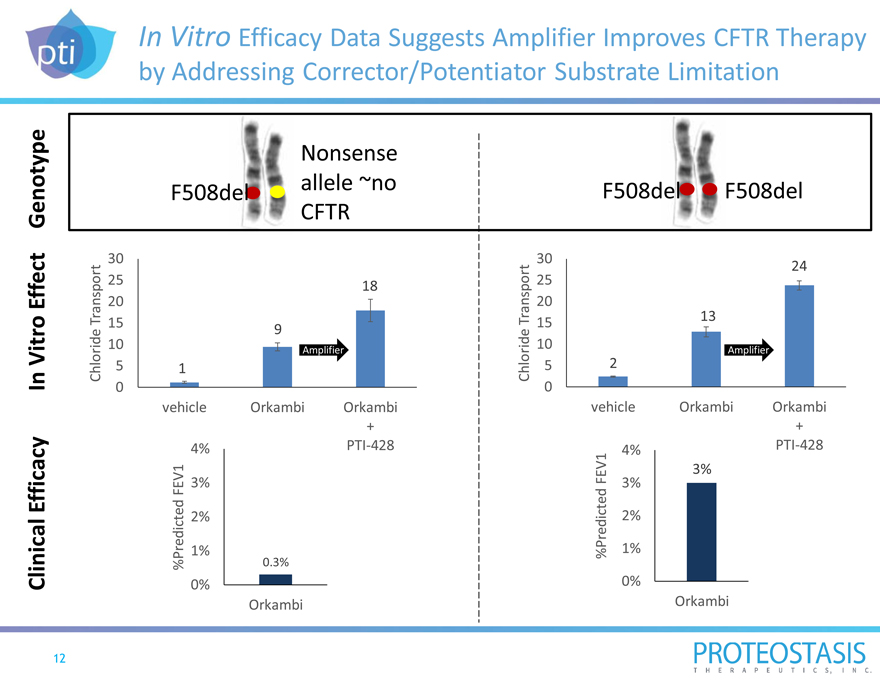
In Vitro Efficacy Data Suggests Amplifier Improves CFTR Therapy by Addressing Corrector/Potentiator Substrate Limitation
Clinical Efficacy In Vitro Effect Genotype
Chloride Transport
%Predicted FEV1
Nonsense allele ~no F508del CFTR
30 25
18 20 15 9 10
Amplifier
5 1 0 vehicle Orkambi Orkambi +
4% PTI-428
3%
2%
1%
0.3%
0%
Orkambi
F508del F508del
30
24 25 20 13 15 10
Amplifier
5 2 0 vehicle Orkambi Orkambi
+
4% PTI-428 3% 3%
2%
1%
0%
Orkambi
Chloride Transport
%Predicted FEV1
12
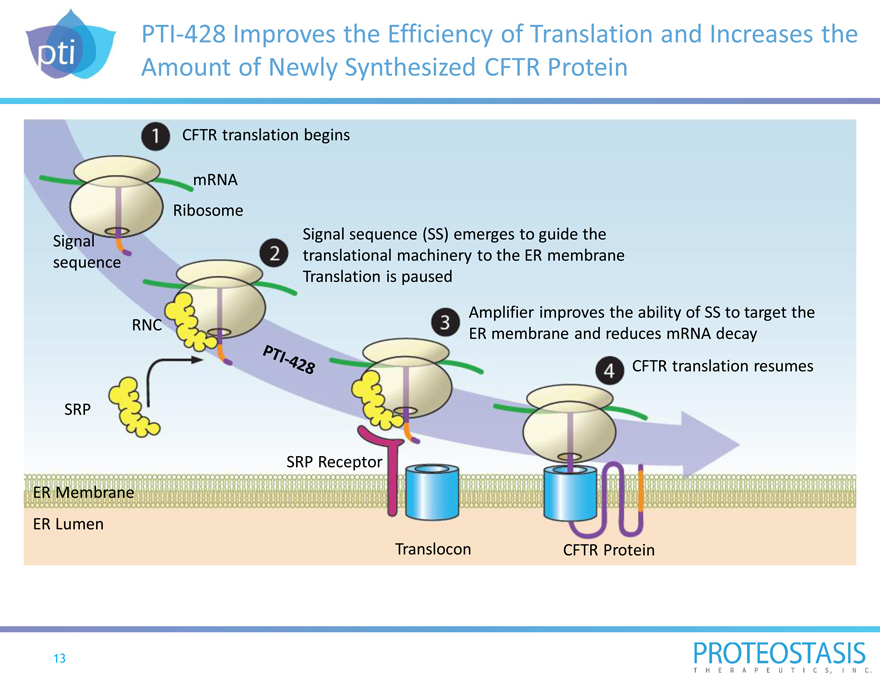
PTI-428 Improves the Efficiency of Translation and Increases the Amount of Newly Synthesized CFTR Protein
CFTR translation begins
mRNA
Ribosome
Signal sequence (SS) emerges to guide the Signal translational machinery to the ER membrane sequence Translation is paused
RNC Amplifier improves the ability of SS to target the ER membrane and reduces mRNA decay
CFTR translation resumes
SRP
SRP Receptor
ER Membrane
ER Lumen
Translocon CFTR Protein
13
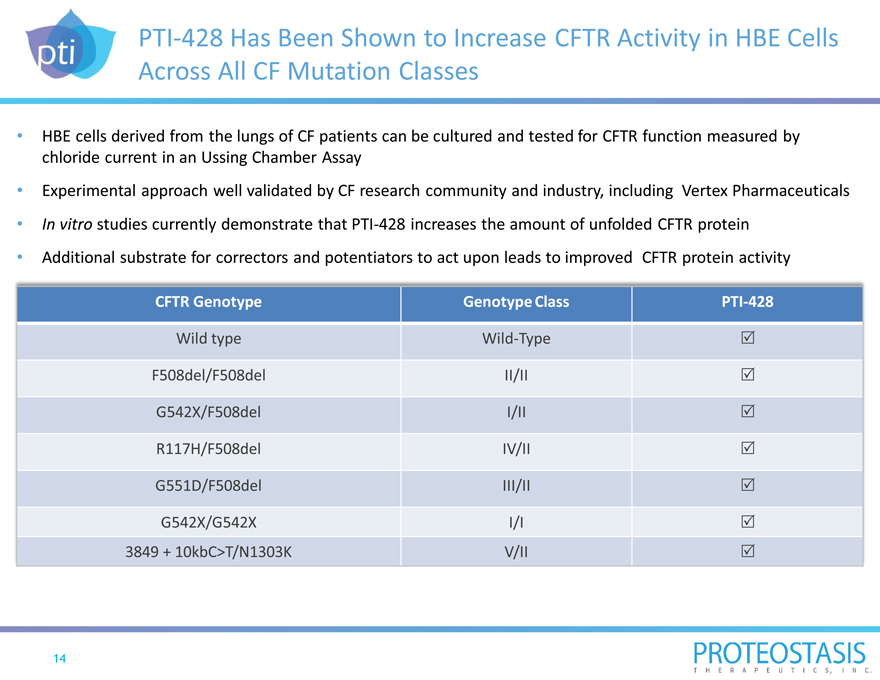
PTI-428 Has Been Shown to Increase CFTR Activity in HBE Cells Across All CF Mutation Classes
HBE cells derived from the lungs of CF patients can be cultured and tested for CFTR function measured by chloride current in an Ussing Chamber Assay
Experimental approach well validated by CF research community and industry, including Vertex Pharmaceuticals In vitro studies currently demonstrate that PTI-428 increases the amount of unfolded CFTR protein Additional substrate for correctors and potentiators to act upon leads to improved CFTR protein activity
CFTR Genotype Genotype Class PTI-428
Wild type Wild-Type
F508del/F508del II/II
G542X/F508del I/II
R117H/F508del IV/II
G551D/F508del III/II
G542X/G542X I/I
3849 + 10kbC>T/N1303K V/II
14
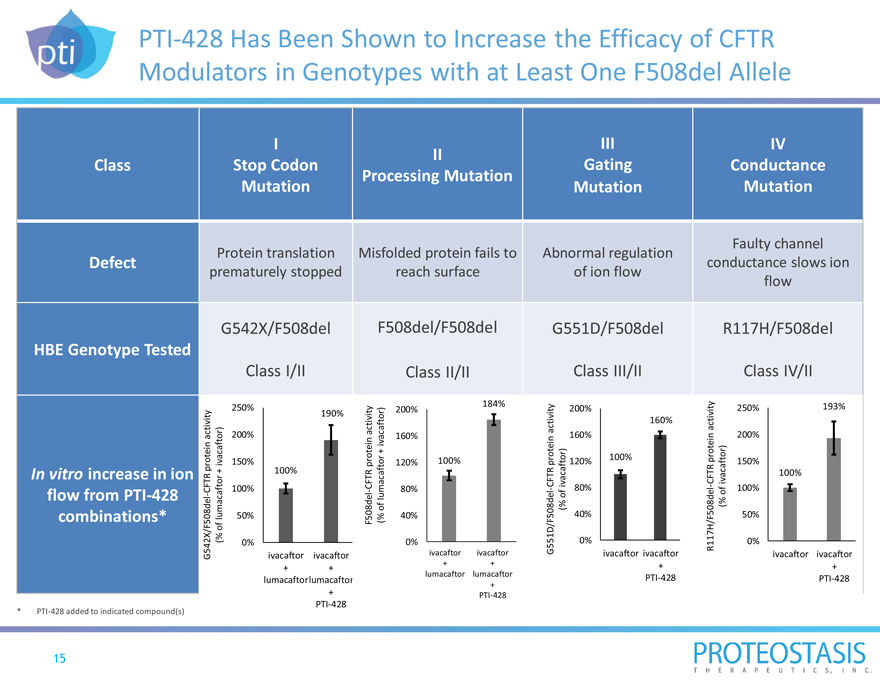
PTI-428 Has Been Shown to Increase the Efficacy of CFTR Modulators in Genotypes with at Least One F508del Allele
Class Defect HBE Genotype Tested
In vitro increase in ion flow from PTI-428 combinations*
* PTI-428 added to indicated compound(s)
I III IV II
Stop Codon Gating Conductance Processing Mutation Mutation Mutation Mutation
Faulty channel Protein translation Misfolded protein fails to Abnormal regulation conductance slows ion prematurely stopped reach surface of ion flow flow
G542X/F508del F508del/F508del G551D/F508del R117H/F508del
Class I/II Class II/II Class III/II Class IV/II
250%
190%
200%
150%
100%
100%
50%
0% ivacaftor ivacaftor
+ + lumacaftorlumacaftor + PTI-428
184% 200%
160%
120% 100% 80% 40%
0% ivacaftor ivacaftor
+ + lumacaftor lumacaftor + PTI-428
200%
160% 160%
100% 120%
80%
40%
0% ivacaftor ivacaftor + PTI-428
250% 193%
200%
150%
100% 100%
50%
0% ivacaftor ivacaftor + PTI-428
R117H/F508del-CFTR protein activity (% of ivacaftor)
G551D/F508del-CFTR protein activity (% of ivacaftor)
F508del-CFTR protein activity (% of lumacaftor + ivacaftor)
G542X/F508del-CFTR protein activity (% of lumacaftor + ivacaftor)
15
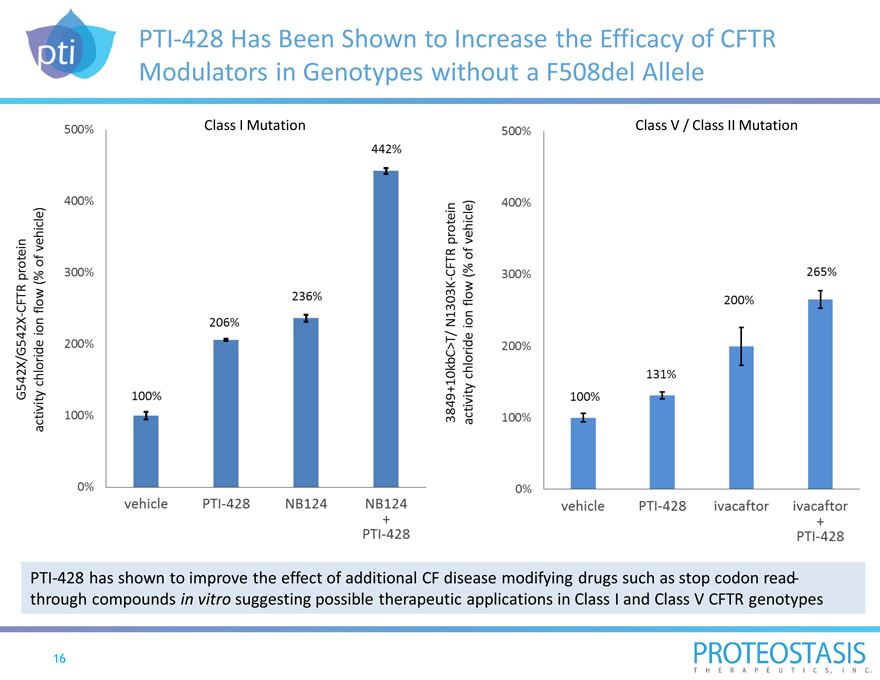
PTI-428 Has Been Shown to Increase the Efficacy of CFTR Modulators in Genotypes without a F508del Allele
Class I Mutation 0% 100% 200% 300% 400% 500% 100% 206% 236% 442%
G542X/G542X-CFTR protein activity chloride ion flow (% of vehicle)
Vehicle PTI-428 NB124 NB124+PTI-428
3849+10KBC>T/ N1303K-CFTR protein activity chloride ion flow (% of vehicle)
0% 100% 200% 300% 400% 500% 100%
131% 200% 265%
Vehicle PTI-428 PTI-428 ivacaftor ivacaftor+
PTI-428 has shown to improve the effect of additional CF disease modifying drugs such as stop codon read-through compounds in vitro suggesting possible therapeutic applications in Class I and Class V CFTR genotypes
16
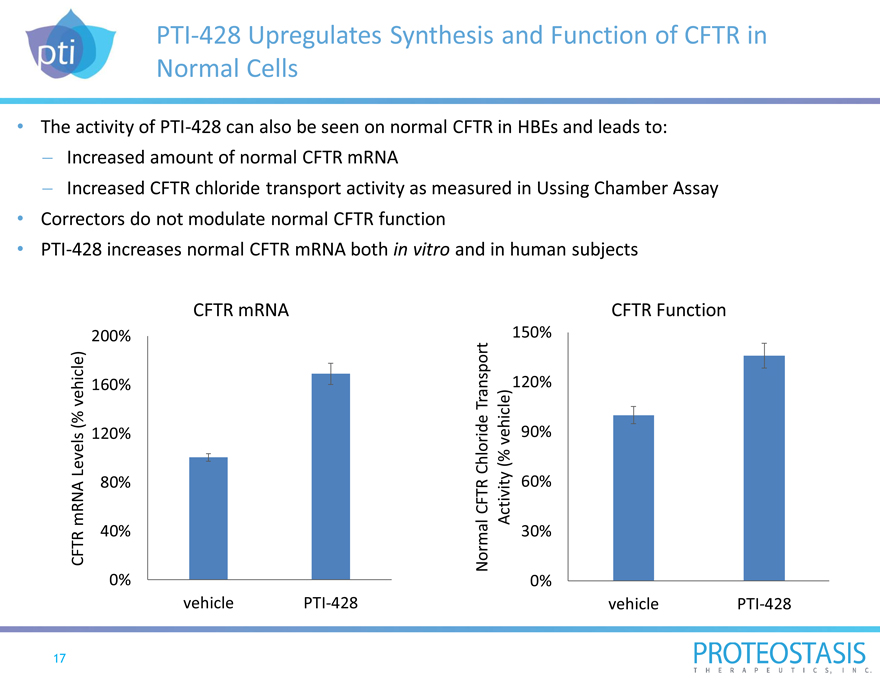
PTI-428 Upregulates Synthesis and Function of CFTR in
Normal Cells
The activity of PTI-428 can also be seen on normal CFTR in HBEs and leads to:
- Increased amount of normal CFTR mRNA
- Increased CFTR chloride transport activity as measured in Ussing Chamber Assay
Correctors do not modulate normal CFTR function
PTI-428 increases normal CFTR mRNA both in vitro and in human subjects
CFTR mRNA
200% 160% 120% 80% 40%
0% vehicle PTI-428
CFTR Function
150% 120% 90% 60% 30%
0% vehicle PTI-428Normal CFTR Chloride Transport Activity (% vehicle)
CFTR mRNA Levels (% vehicle)
17
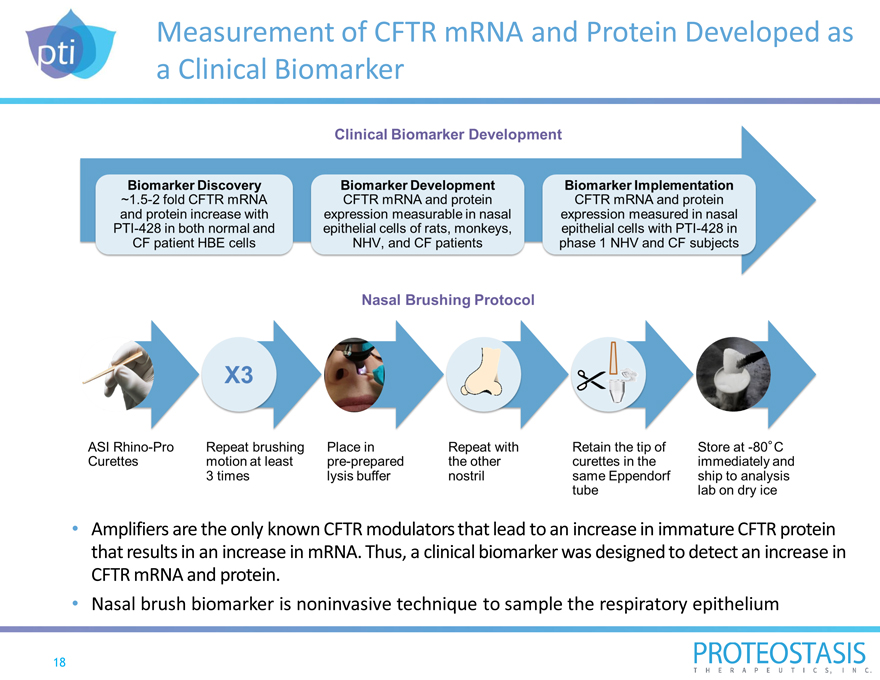
Measurement of CFTR mRNA and Protein Developed as a Clinical Biomarker
Amplifiers are the only known CFTR modulators that lead to an increase in immature CFTR protein that results in an increase in mRNA. Thus, a clinical biomarker was designed to detect an increase in
CFTR mRNA and protein.
Nasal brush biomarker is noninvasive technique to sample the respiratory epithelium
Biomarker discovery `1.5-2 fold CFTR mRNA and protein increase with PTI-428 in both normal and CF patient HBE cells biomarker development CFTR mRNA and protein expression measurable in nasal epithelial cells of rats, monkeys, NHV, and CF patients biomarker implementation CFTR mRNA and protein expression measured in nasal epithelial cells with PTI-428 in phase 1 NHV and CF subjects
ASI Rhino-Pro Curettes repeat brushing motion at least 3 times place in pre-prepared lysis buffer repeat with the other nostril retain the tip of curettes in the same Eppendorf tube store at -80c immediately and ship to analysis lab on dry ice
18
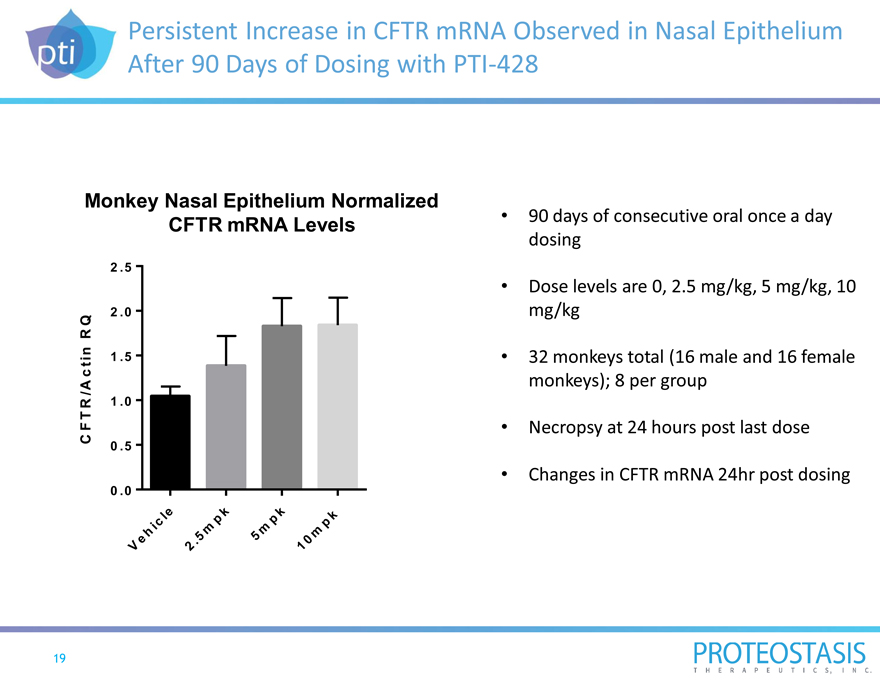
Persistent Increase in CFTR mRNA Observed in Nasal Epithelium After 90 Days of Dosing with PTI-428
C F T R /A c tin R Q
Monkey Nasal Epithelium Normalized
CFTR mRNA Levels
2 .5
2 .0
1 .5
1 .0
0 .5
0 .0
Vehicle 2.5mpk 5mpk 10mpk90 days of consecutive oral once a day dosing
Dose levels are 0, 2.5 mg/kg, 5 mg/kg, 10 mg/kg
32 monkeys total (16 male and 16 female monkeys); 8 per group
Necropsy at 24 hours post last dose
Changes in CFTR mRNA 24hr post dosing
19
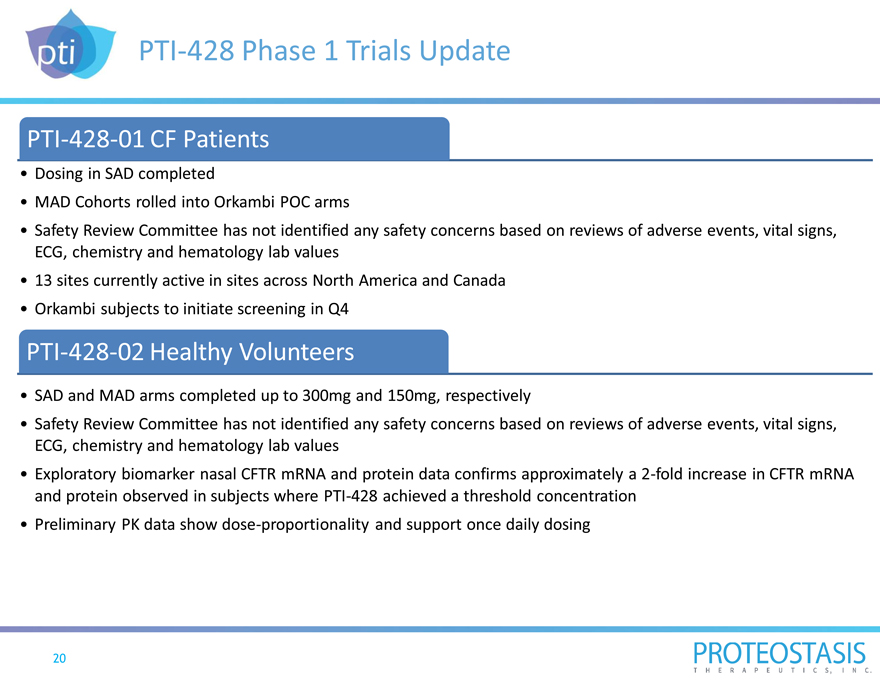
PTI-428 Phase 1 Trials Update
PTI-428-01 CF Patients
Dosing in SAD completed
MAD Cohorts rolled into Orkambi POC arms
Safety Review Committee has not identified any safety concerns based on reviews of adverse events, vital signs, ECG, chemistry and hematology lab values
13 sites currently active in sites across North America and Canada
Orkambi subjects to initiate screening in Q4
PTI-428-02 Healthy Volunteers
SAD and MAD arms completed up to 300mg and 150mg, respectively
Safety Review Committee has not identified any safety concerns based on reviews of adverse events, vital signs, ECG, chemistry and hematology lab values
Exploratory biomarker nasal CFTR mRNA and protein data confirms approximately a 2-fold increase in CFTR mRNA and protein observed in subjects where PTI-428 achieved a threshold concentration
Preliminary PK data show dose-proportionality and support once daily dosing
20
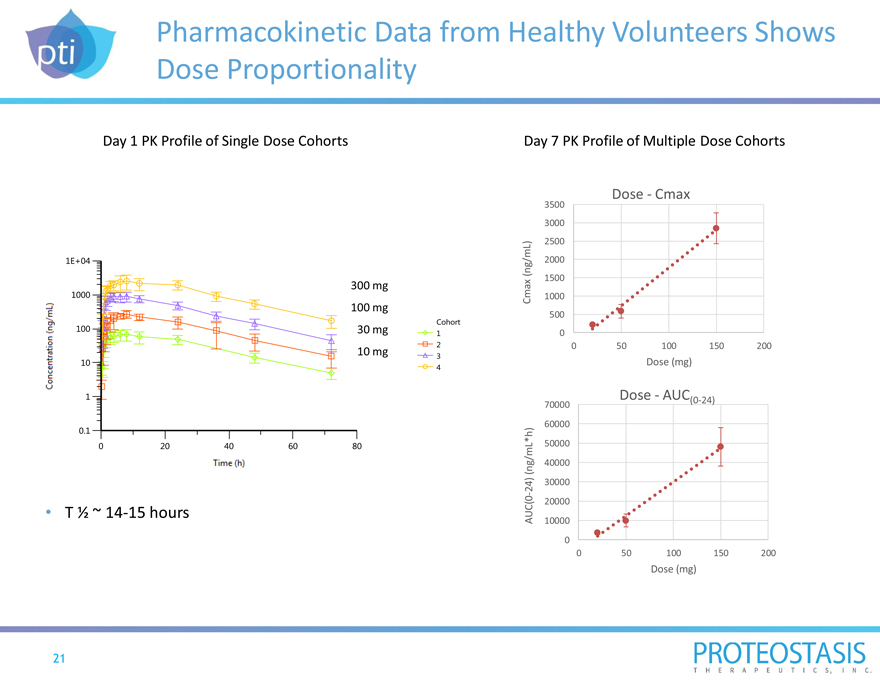
Pharmacokinetic Data from Healthy Volunteers Shows Dose Proportionality
Day 1 PK Profile of Single Dose Cohorts Day 7 PK Profile of Multiple Dose Cohorts
300 mg 100 mg 30 mg 10 mg
Dose—Cmax
3500
3000
2500
(ng/mL) 2000
1500
Cmax 1000 500
0
0 50 100 150 200
Dose (mg)
70000 Dose—AUC(0-24)
60000
50000
(ng/mL*h) 40000
24) 30000
-
20000
AUC(0 10000 0
0 50 100 150 200
Dose (mg)
T 1/2 ~ 14-15 hours
Concentration (ng/ml) cmax (ng/ml)
0.1 10 100 1 1000 1E+04 0 20 40 60 80 1 2 3 4 cohort Time (h) 21
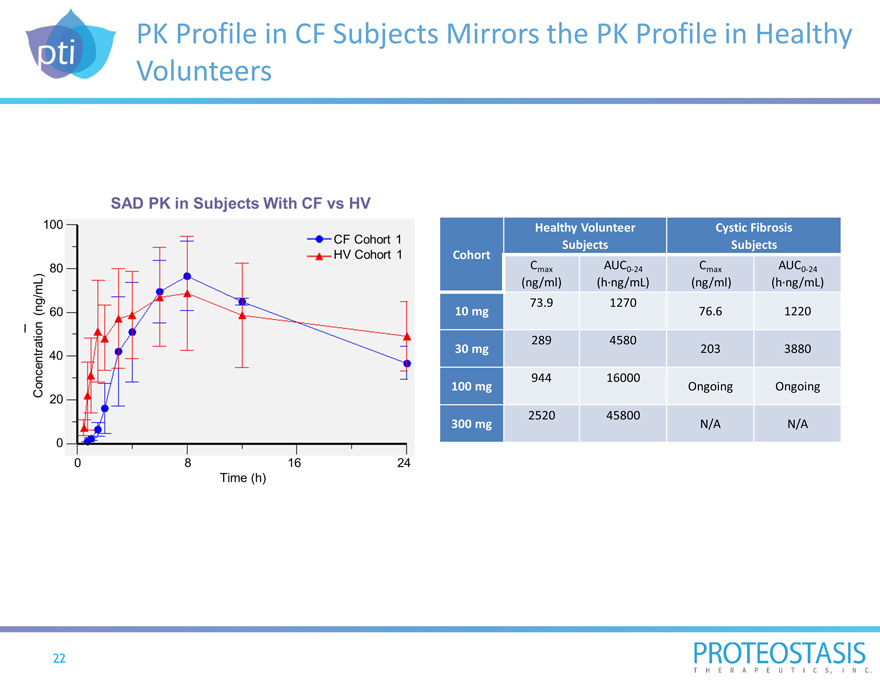
PK Profile in CF Subjects Mirrors the PK Profile in Healthy Volunteers
Concentration (ng/ml) 0 20 40 60 80 100 0 8 16 24 Time(h) SAD PK in Subjects with CF vs HV CF Cohort 1 HV Cohort 1 Healthy Volunteer Cystic Fibrosis Cohort Subjects Subjects
Cmax AUC0-24 Cmax AUC0-24
(ng/ml) (h?ng/mL) (ng/ml) (h?ng/mL) 73.9 1270 10 mg 76.6 1220
289 4580
30 mg 203 3880
944 16000
100 mg Ongoing Ongoing
2520 45800
300 mg N/A N/A
22
Nasal Epithelia Sample Processing For Biomarker Quantification
Real Time PCR Assay ELISA Assay mRNA Quantification Protein Quantification
Nasal mucosa sample
PTI approach to biomarker measurement allows for combined analysis of CFTR mRNA and total protein changes in subjects
23
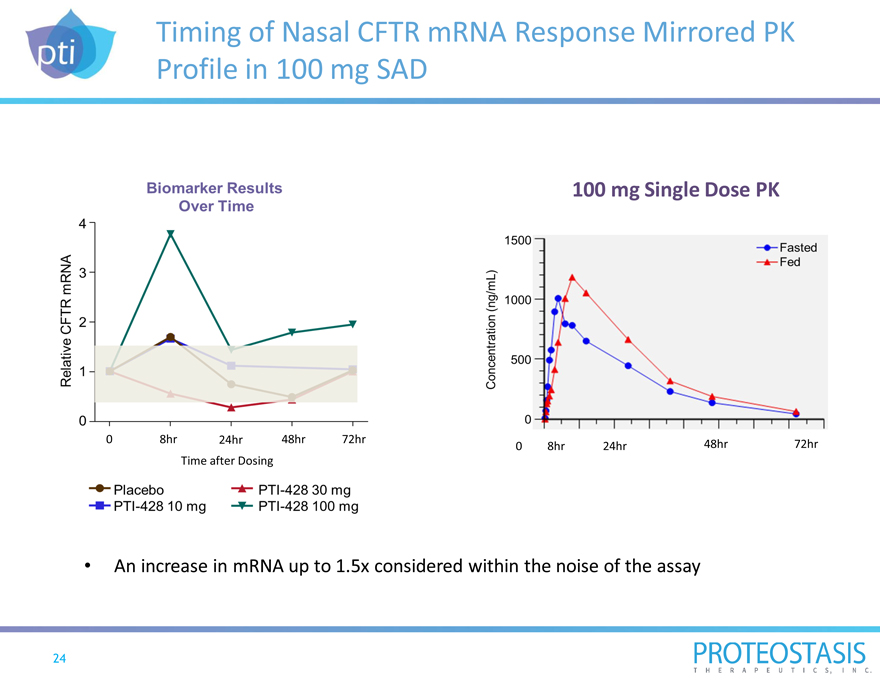
Timing of Nasal CFTR mRNA Response Mirrored PK Profile in 100 mg SAD
0 8hr 24hr 48hr 72hr Time after Dosing
0 relative CFTR mRNA biomarker results over time placebo PTI-428 10 mg PTI-428 100 mg
PTI-428 3 100 mg Single Dose PK
0 8hr 24hr 48hr 72hr
0 mg
0 500 1000 1500 fasted fed concentration (ng/ml) An increase in mRNA up to 1.5x considered within the noise of the assay
24
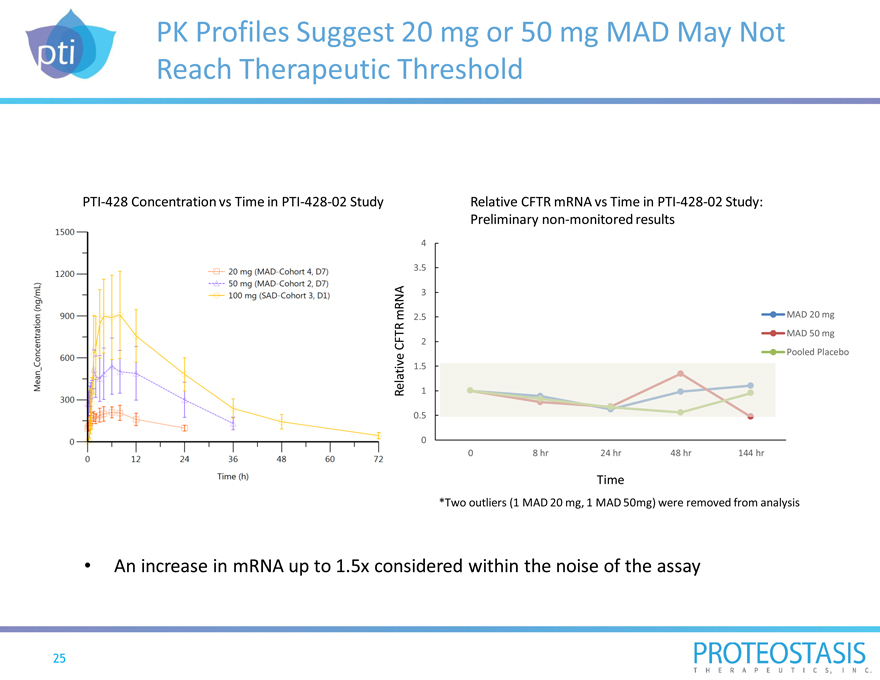
PK Profiles Suggest 20 mg or 50 mg MAD May Not Reach Therapeutic Threshold
PTI-428 Concentration vs Time in PTI-428-02 Study
20mg (MAD-Cohort 4, D7) 50mg (MAD-Cohort 2, D7) 100mg (SAD-Cohort 3, D1) 0 12 24 36 48 60 72 time (h) 0 300 600 900 1200 1500 *Two outliers (1 MAD 20 mg, 1 MAD 50mg) were removed from analysis
An increase in mRNA up to 1.5x considered within the noise of the assay
Mean concentration (ng/ml)
25
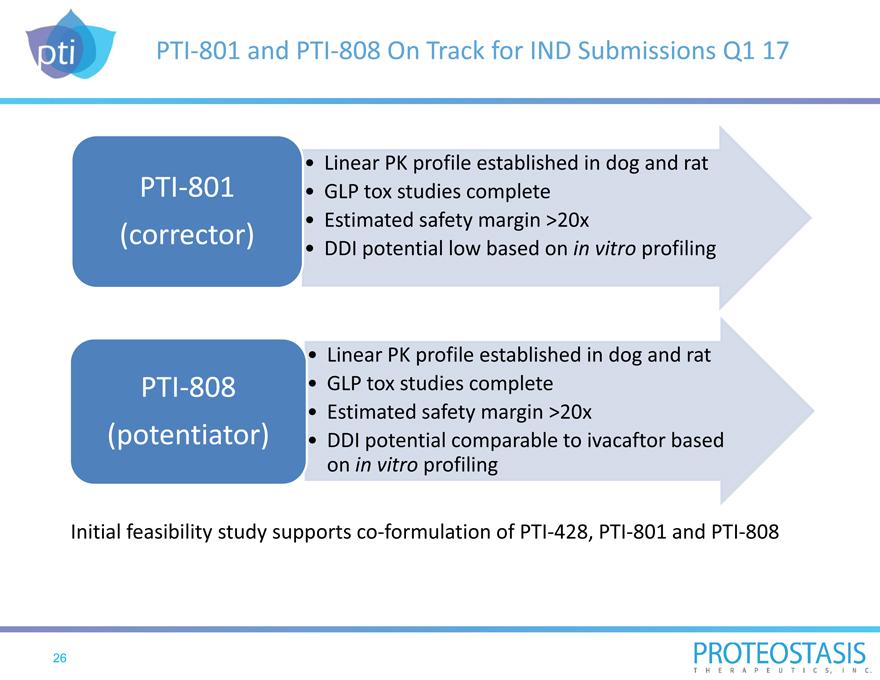
PTI-801 and PTI-808 On Track for IND Submissions Q1 17
Linear PK profile established in dog and rat
GLP tox studies complete PTI-801 (corrector) • Estimated safety margin >20x
DDI potential low based on in vitro profiling
Linear PK profile established in dog and rat
GLP tox studies complete PTI-808 (potentiator) • Estimated safety margin >20x
DDI potential comparable to ivacaftor based on in vitro profiling
Initial feasibility study supports co-formulation of PTI-428, PTI-801 and PTI-808
26
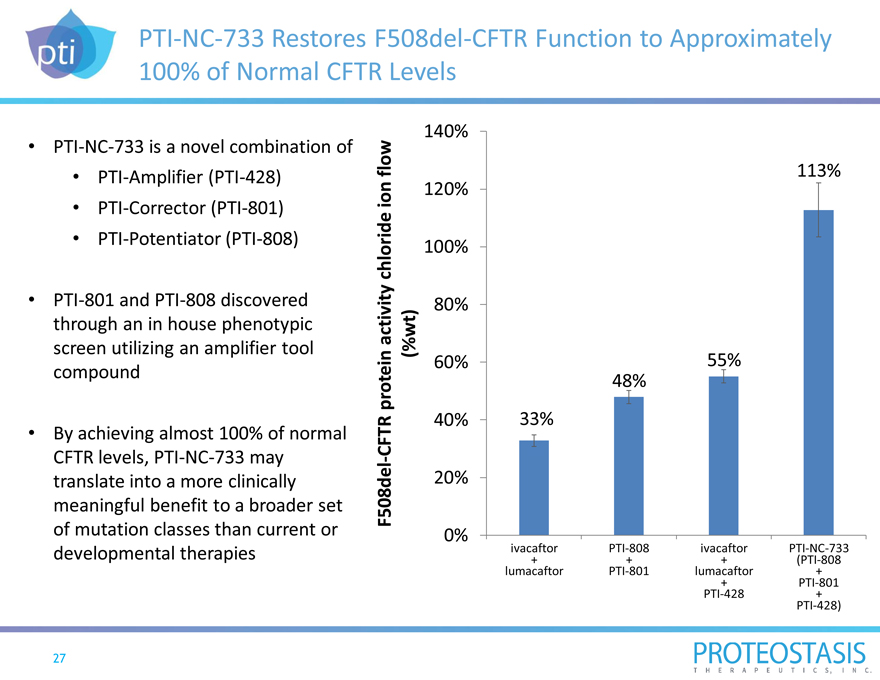
PTI-NC-733 Restores F508del-CFTR Function to Approximately 100% of Normal CFTR Levels
PTI-NC-733 is a novel combination of
PTI-Amplifier (PTI-428)
PTI-Corrector (PTI-801)
PTI-Potentiator (PTI-808)
PTI-801 and PTI-808 discovered through an in house phenotypic screen utilizing an amplifier tool compound
By achieving almost 100% of normal
CFTR levels, PTI-NC-733 may translate into a more clinically meaningful benefit to a broader set of mutation classes than current or developmental therapies
F508del-CFTR protein activity chloride ion flow
(%wt)
140%
113% 120%
100%
80%
60% 55% 48%
40% 33%
20%
0%
ivacaftor PTI-808 ivacaftor PTI-NC-733
+ + + (PTI-808 lumacaftor PTI-801 lumacaftor +
+ PTI-801 PTI-428 + PTI-428)
27
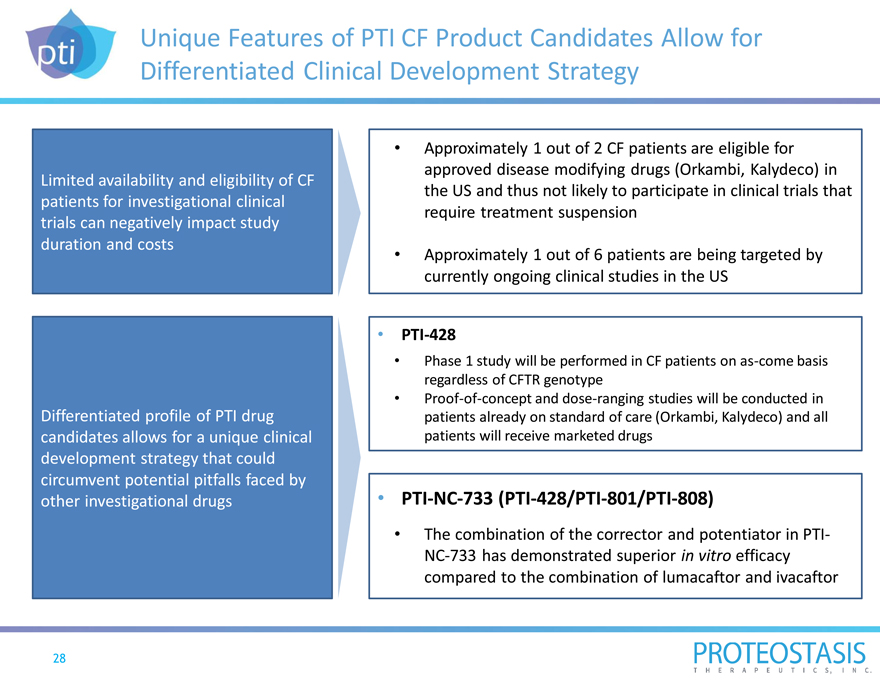
Unique Features of PTI CF Product Candidates Allow for Differentiated Clinical Development Strategy
Limited availability and eligibility of CF patients for investigational clinical trials can negatively impact study duration and costs
Differentiated profile of PTI drug candidates allows for a unique clinical development strategy that could circumvent potential pitfalls faced by other investigational drugs
Approximately 1 out of 2 CF patients are eligible for approved disease modifying drugs (Orkambi, Kalydeco) in the US and thus not likely to participate in clinical trials that require treatment suspension
Approximately 1 out of 6 patients are being targeted by currently ongoing clinical studies in the US
PTI-428
Phase 1 study will be performed in CF patients on as-come basis regardless of CFTR genotype
Proof-of-concept and dose-ranging studies will be conducted in patients already on standard of care (Orkambi, Kalydeco) and all patients will receive marketed drugs
PTI-NC-733 (PTI-428/PTI-801/PTI-808)
The combination of the corrector and potentiator in PTI-NC-733 has demonstrated superior in vitro efficacy compared to the combination of lumacaftor and ivacaftor
28
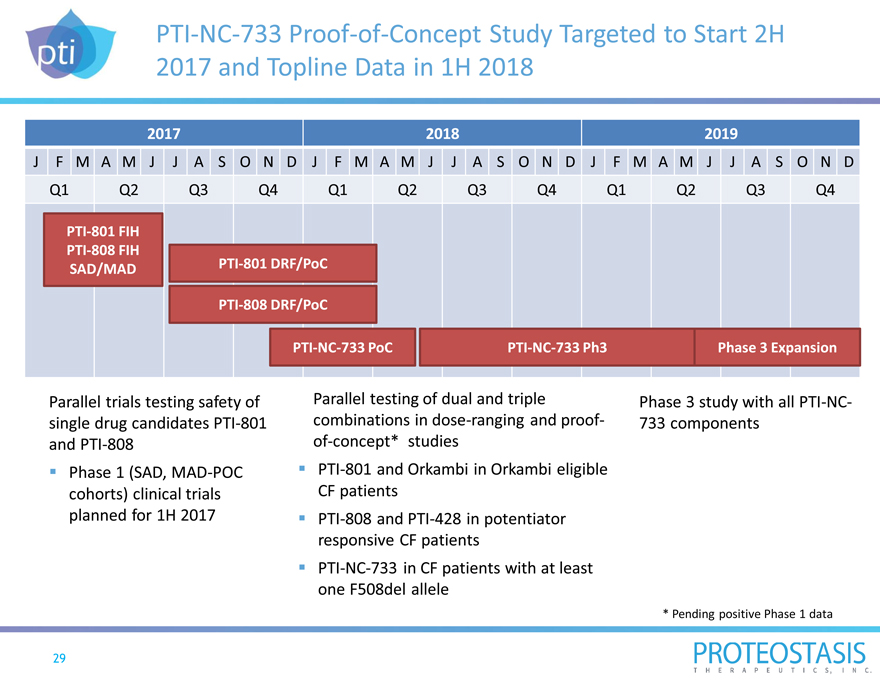
PTI-NC-733 Proof-of-Concept Study Targeted to Start 2H
2017 and Topline Data in 1H 2018
2017 2018 2019
J F M A M J J A S O N D J F M A M J J A S O N D J F M A M J J A S O N D
Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4
PTI-801 FIH
PTI-808 FIH
SAD/MAD PTI-801 DRF/PoC
PTI-808 DRF/PoC
PTI-NC-733 PoC PTI-NC-733 Ph3 Phase 3 Expansion
Parallel trials testing safety of Parallel testing of dual and triple Phase 3 study with all PTI-NC-
single drug candidates PTI-801 combinations in dose-ranging and proof- 733 components
and PTI-808 of-concept* studies
Phase 1 (SAD, MAD-POC PTI-801 and Orkambi in Orkambi eligible
cohorts) clinical trials CF patients
planned for 1H 2017 PTI-808 and PTI-428 in potentiator
responsive CF patients
PTI-NC-733 in CF patients with at least
one F508del allele
* Pending positive Phase 1 data
29
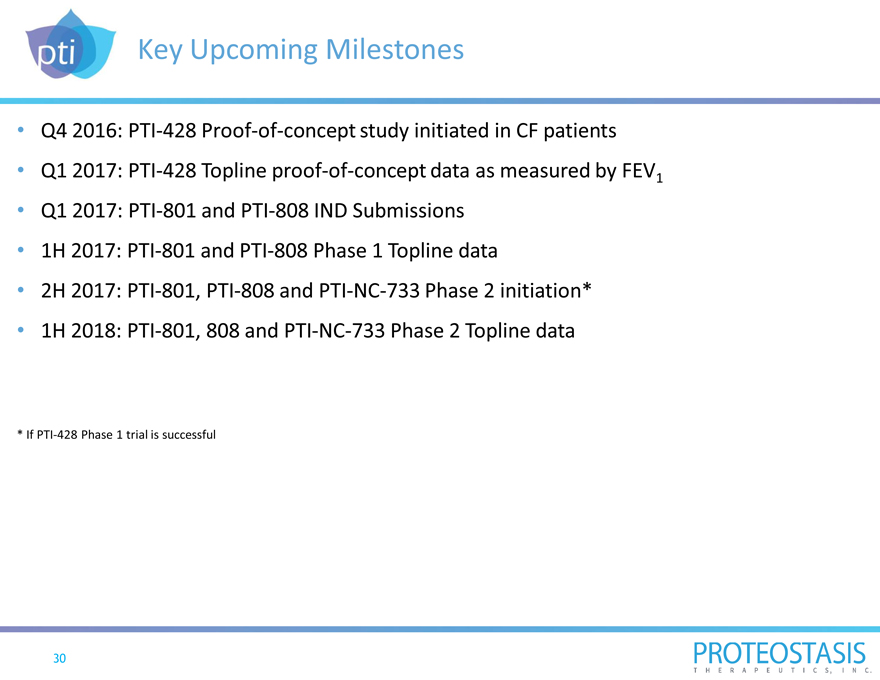
Key Upcoming Milestones
Q4 2016: PTI-428 Proof-of-concept study initiated in CF patients
Q1 2017: PTI-428 Topline proof-of-concept data as measured by FEV1
Q1 2017: PTI-801 and PTI-808 IND Submissions
1H 2017: PTI-801 and PTI-808 Phase 1 Topline data
2H 2017: PTI-801, PTI-808 and PTI-NC-733 Phase 2 initiation*
1H 2018: PTI-801, 808 and PTI-NC-733 Phase 2 Topline data
* If PTI-428 Phase 1 trial is successful
30