
Proteostasis Therapeutics, Inc. (PTI) March 2018 Investor Deck – Cowen Annual Healthcare Conference Exhibit 99.1

Safe Harbor and Disclaimer To the extent that statements in this presentation are not historical facts, they are forward-looking statements reflecting the current beliefs and expectations of management made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. Words such as “aim,” “may,” “will,” “expect,” “anticipate,” “estimate,” “intend,” and similar expressions (as well as other words or expressions referencing future events, conditions or circumstances) are intended to identify forward-looking statements. Examples of forward-looking statements made in this presentation include, without limitation, statements regarding the expected timing of the initiation of, patient enrollment in, data from, and our completion of, our clinical studies and cohorts for PTI-428, PTI-801, PTI-808 and our combination therapy candidates as well as cash guidance. Forward-looking statements made in this presentation involve substantial risks and uncertainties that could cause actual results to differ materially from those expressed or implied by the forward-looking statements, and we therefore cannot assure you that our plans, intentions, expectations or strategies will be attained or achieved. Such risks and uncertainties include, without limitation, the possibility final or future results from our drug candidate trials (including, without limitation, longer duration studies) do not achieve positive results or are materially and negatively different from or not indicative of the preliminary results reported in this presentation (noting that these results are on a small number of patients and small data set), uncertainties inherent in the execution and completion of clinical trials (including, without limitation, the possibility FDA requires us to run cohorts sequentially or conduct additional cohorts or pre-clinical or clinical studies), in the enrollment of CF patients in our clinical trials, in the timing of availability of trial data, in the results of the clinical trials, in possible adverse events from our trials, in the actions of regulatory agencies, in endorsement, if any, by therapeutic development arms of CF patient advocacy groups, and those set forth in our Annual Report on Form 10-K for the year ended December 31, 2017, and our other SEC filings. We assume no obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise. This presentation also contains estimates and other statistical data made by independent parties and by us relating to, among other items, disease incidence, market size and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. Projections, assumptions and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of risk and uncertainty. New risks emerge from time to time, and neither we nor any other person makes any representation as to the accuracy or completeness of such data or undertakes any obligation to update such date after the date of this presentation. By attending or receiving this presentation you acknowledge you are solely responsible for your own assessment of the market and our market position and that you will conduct your own analysis and are solely responsible for forming your own view of the potential future performance of our business. The trademarks included in this presentation are the property of the owners thereof and are used for reference purposes only. Such use should not be construed as an endorsement of the Company or its securities.
PTI Overview Clinical stage biopharma developing novel therapeutics for cystic fibrosis (CF) and other diseases caused by dysfunctional protein processing Focus on increasing CFTR activity in patients with CF Developing novel small molecules for CF combination therapy PTI-808: Novel Potentiator PTI-801: Third Generation Corrector that is additive in vitro to first and second generation correctors PTI-428: Novel Class of CFTR Modulator: Amplifiers Leveraging potential therapeutic benefit of multiple stand-alone combination options including PTI-801 and PTI-808 as a doublet and PTI-808, PTI-801, and PTI-428 as a triplet PTI-801 and PTI-428 can potentially be developed as add-on therapies to current and future standard of care CFTR modulator therapies
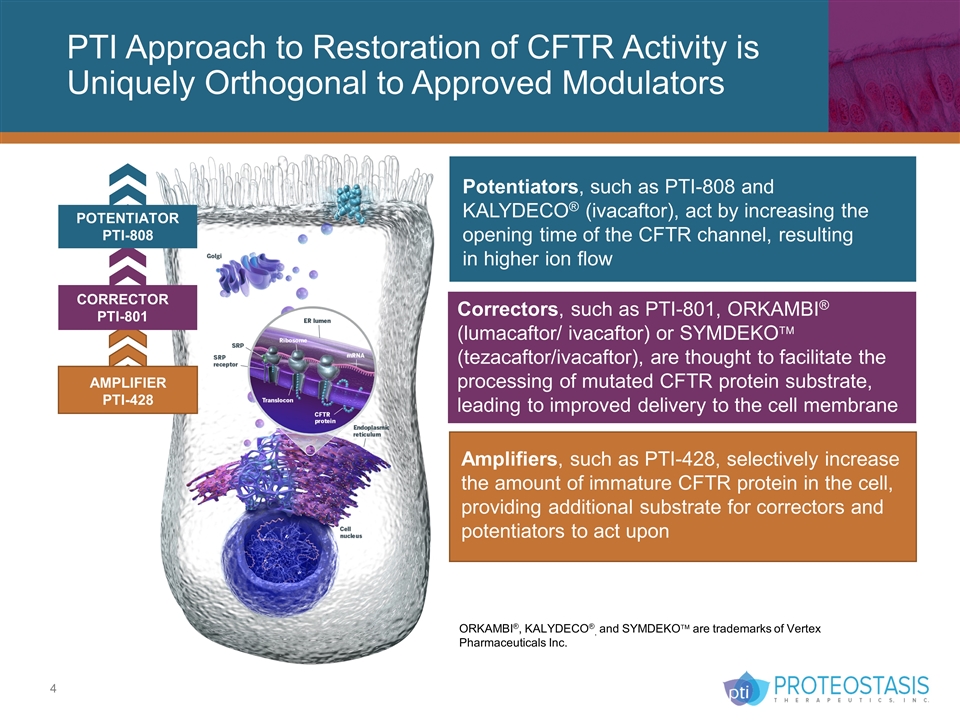
CORRECTOR PTI-801 PTI Approach to Restoration of CFTR Activity is Uniquely Orthogonal to Approved Modulators Amplifiers, such as PTI-428, selectively increase the amount of immature CFTR protein in the cell, providing additional substrate for correctors and potentiators to act upon Potentiators, such as PTI-808 and KALYDECO® (ivacaftor), act by increasing the opening time of the CFTR channel, resulting in higher ion flow Correctors, such as PTI-801, ORKAMBI® (lumacaftor/ ivacaftor) or SYMDEKOä (tezacaftor/ivacaftor), are thought to facilitate the processing of mutated CFTR protein substrate, leading to improved delivery to the cell membrane POTENTIATOR PTI-808 AMPLIFIER PTI-428 ORKAMBI®, KALYDECO®, and SYMDEKOä are trademarks of Vertex Pharmaceuticals Inc.
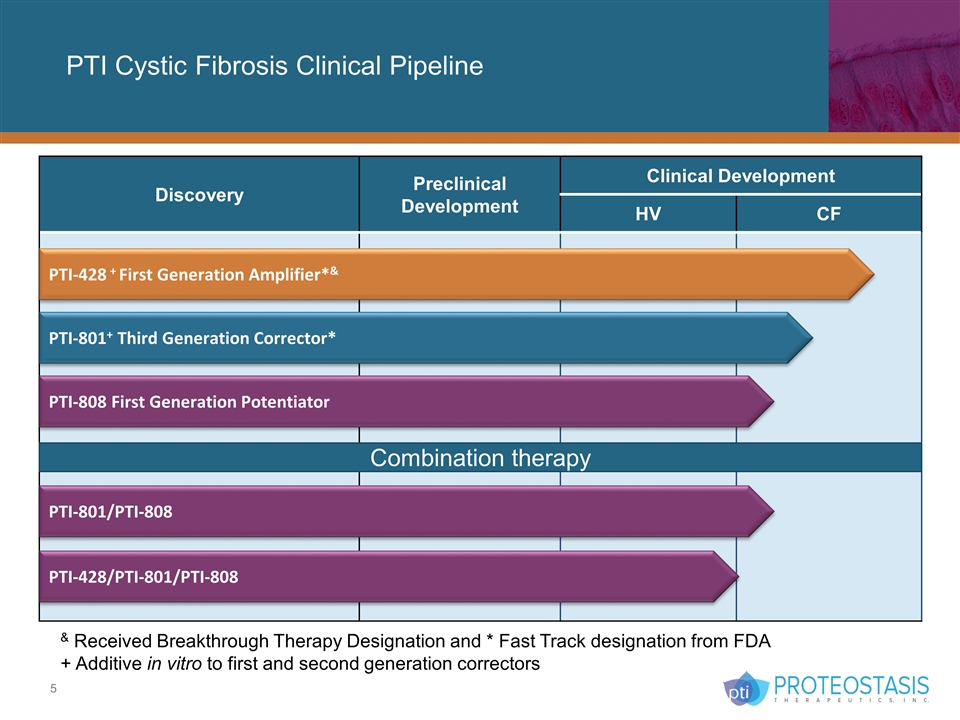
PTI Cystic Fibrosis Clinical Pipeline Discovery Preclinical Development Clinical Development HV CF PTI-428 + First Generation Amplifier*& PTI-801+ Third Generation Corrector* PTI-808 First Generation Potentiator & Received Breakthrough Therapy Designation and * Fast Track designation from FDA + Additive in vitro to first and second generation correctors Combination therapy PTI-428/PTI-801/PTI-808 PTI-801/PTI-808
2017 Key Accomplishments PTI-801 IND submission PTI-428 preliminary data PTI-808 IND submission PTI-808 Phase 1 initiation PTI-801 Phase 1 initial data PTI-428 28 day dosing, preliminary data in CF subjects on Orkambi® PTI-801 14 day dosing, initial data in CF subjects on Orkambi® PTI-808 SAD and MAD clinical data in HV PTI-808/PTI-801/PTI-428 combination clinical safety data in HV Orkambi® is trademark of Vertex Pharmaceuticals, Inc. 2017
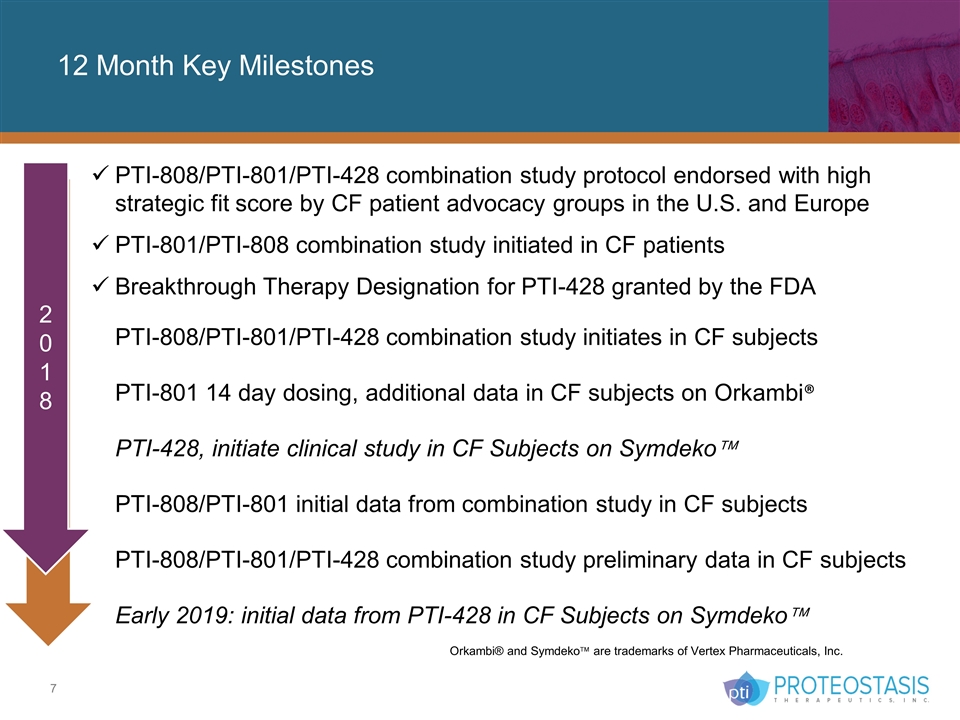
12 Month Key Milestones PTI-808/PTI-801/PTI-428 combination study protocol endorsed with high strategic fit score by CF patient advocacy groups in the U.S. and Europe PTI-801/PTI-808 combination study initiated in CF patients Breakthrough Therapy Designation for PTI-428 granted by the FDA PTI-808/PTI-801/PTI-428 combination study initiates in CF subjects PTI-801 14 day dosing, additional data in CF subjects on Orkambi® PTI-428, initiate clinical study in CF Subjects on Symdekoä PTI-808/PTI-801 initial data from combination study in CF subjects PTI-808/PTI-801/PTI-428 combination study preliminary data in CF subjects Early 2019: initial data from PTI-428 in CF Subjects on Symdekoä Orkambi® and Symdekoä are trademarks of Vertex Pharmaceuticals, Inc. 2018 2018
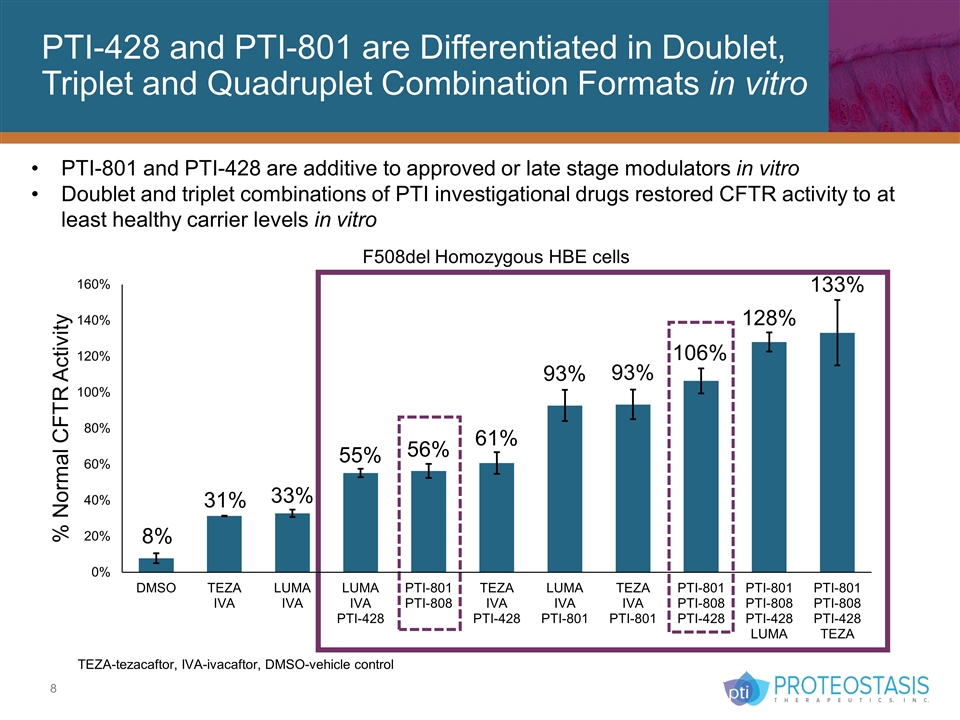
PTI-428 and PTI-801 are Differentiated in Doublet, Triplet and Quadruplet Combination Formats in vitro TEZA-tezacaftor, IVA-ivacaftor, DMSO-vehicle control PTI-801 and PTI-428 are additive to approved or late stage modulators in vitro Doublet and triplet combinations of PTI investigational drugs restored CFTR activity to at least healthy carrier levels in vitro F508del Homozygous HBE cells
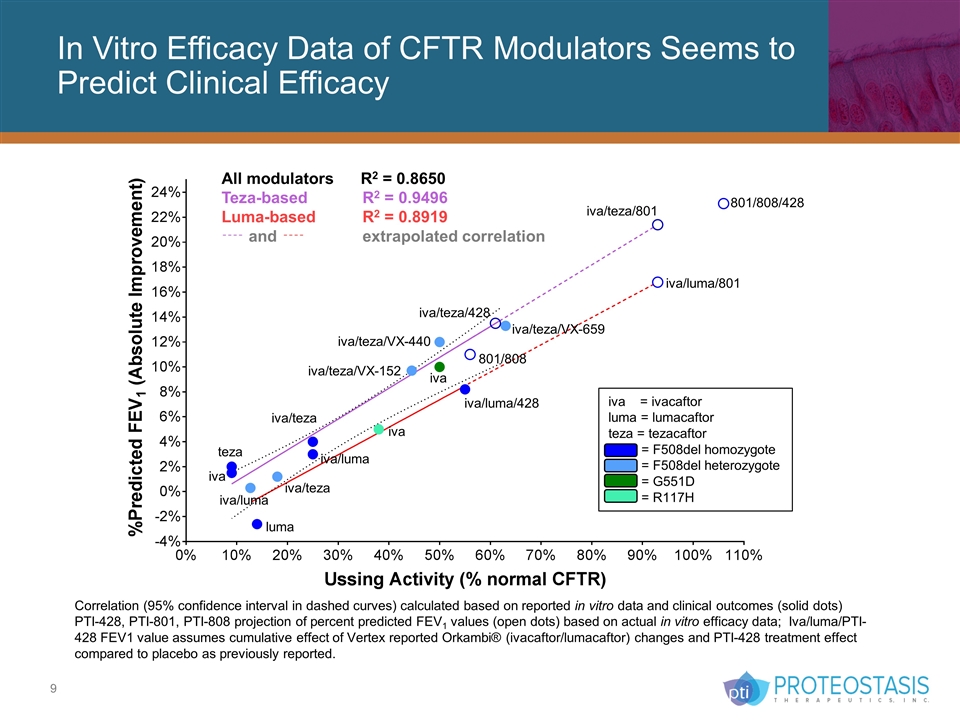
In Vitro Efficacy Data of CFTR Modulators Seems to Predict Clinical Efficacy iva iva/luma teza iva luma iva iva/luma iva/teza/VX-152 iva/teza/VX-659 iva/teza/VX-440 iva/teza iva/luma/428 iva = ivacaftor luma = lumacaftor teza = tezacaftor = F508del homozygote = F508del heterozygote = G551D = R117H Correlation (95% confidence interval in dashed curves) calculated based on reported in vitro data and clinical outcomes (solid dots) PTI-428, PTI-801, PTI-808 projection of percent predicted FEV1 values (open dots) based on actual in vitro efficacy data; Iva/luma/PTI-428 FEV1 value assumes cumulative effect of Vertex reported Orkambi® (ivacaftor/lumacaftor) changes and PTI-428 treatment effect compared to placebo as previously reported. 801/808/428 801/808 iva/luma/801 iva/teza/801 iva/teza All modulators R2 = 0.8650 Teza-based R2 = 0.9496 Luma-based R2 = 0.8919 and extrapolated correlation iva/teza/428
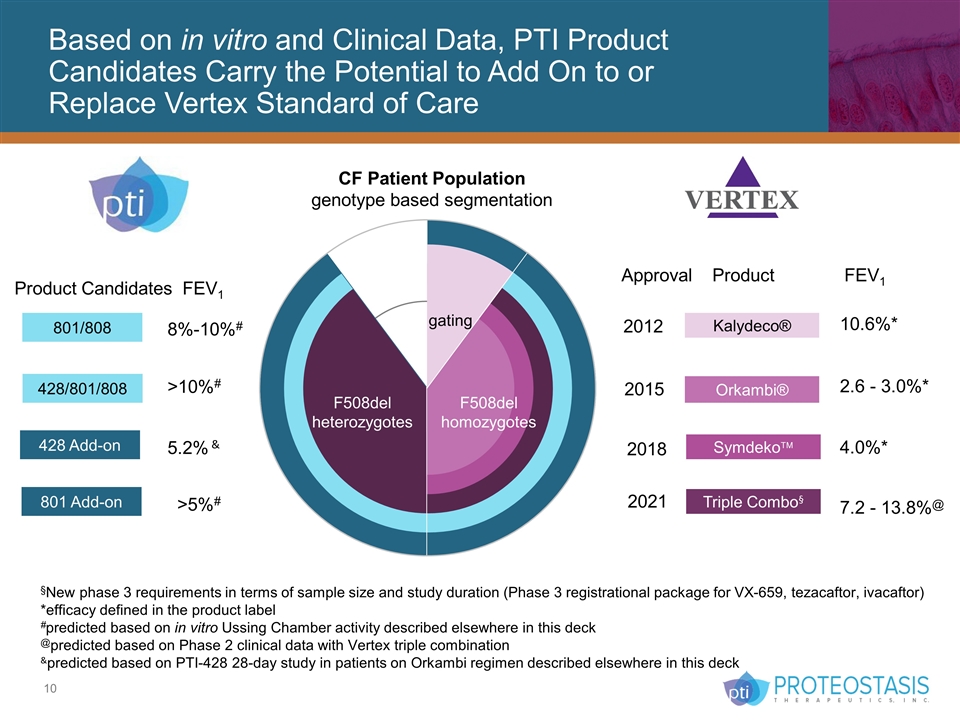
Based on in vitro and Clinical Data, PTI Product Candidates Carry the Potential to Add On to or Replace Vertex Standard of Care F508del homozygotes F508del heterozygotes gating 801/808 428 Add-on Product Candidates FEV1 8%-10%# >5%# 428/801/808 801 Add-on >10%# 5.2% & 2015 2018 2021 Kalydeco® Orkambi® Symdekoä Triple Combo§ Approval Product FEV1 10.6%* 4.0%* 7.2 - 13.8%@ 2012 2.6 - 3.0%* CF Patient Population genotype based segmentation §New phase 3 requirements in terms of sample size and study duration (Phase 3 registrational package for VX-659, tezacaftor, ivacaftor) *efficacy defined in the product label #predicted based on in vitro Ussing Chamber activity described elsewhere in this deck @predicted based on Phase 2 clinical data with Vertex triple combination &predicted based on PTI-428 28-day study in patients on Orkambi regimen described elsewhere in this deck

PTI CFTR Modulators Healthy Volunteer Data Summary
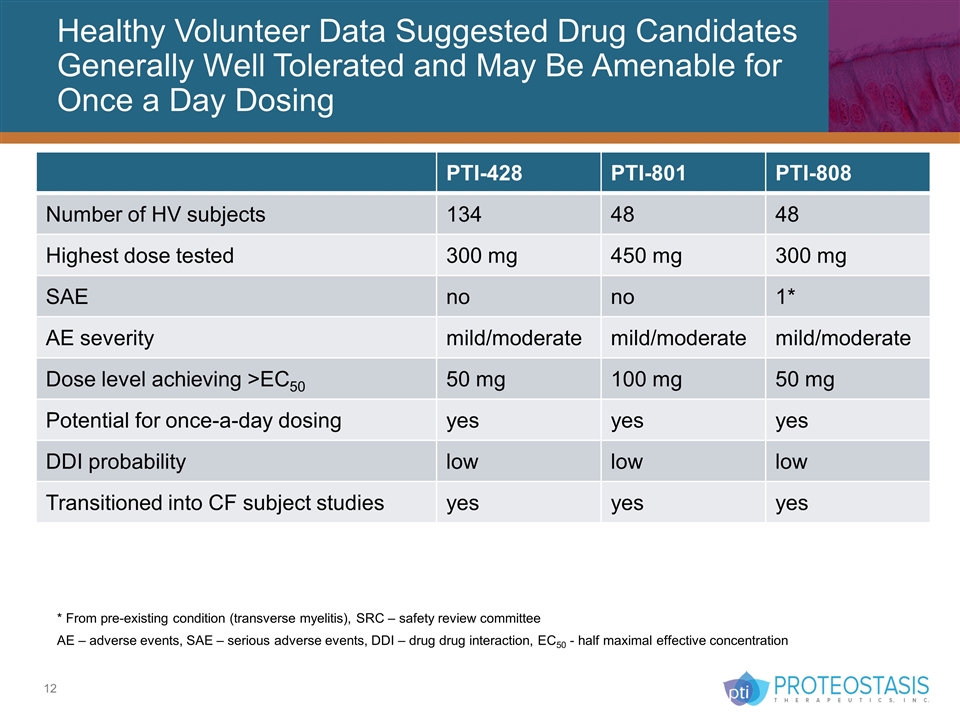
Healthy Volunteer Data Suggested Drug Candidates Generally Well Tolerated and May Be Amenable for Once a Day Dosing * From pre-existing condition (transverse myelitis), SRC – safety review committee AE – adverse events, SAE – serious adverse events, DDI – drug drug interaction, EC50 - half maximal effective concentration PTI-428 PTI-801 PTI-808 Number of HV subjects 134 48 48 Highest dose tested 300 mg 450 mg 300 mg SAE no no 1* AE severity mild/moderate mild/moderate mild/moderate Dose level achieving >EC50 50 mg 100 mg 50 mg Potential for once-a-day dosing yes yes yes DDI probability low low low Transitioned into CF subject studies yes yes yes
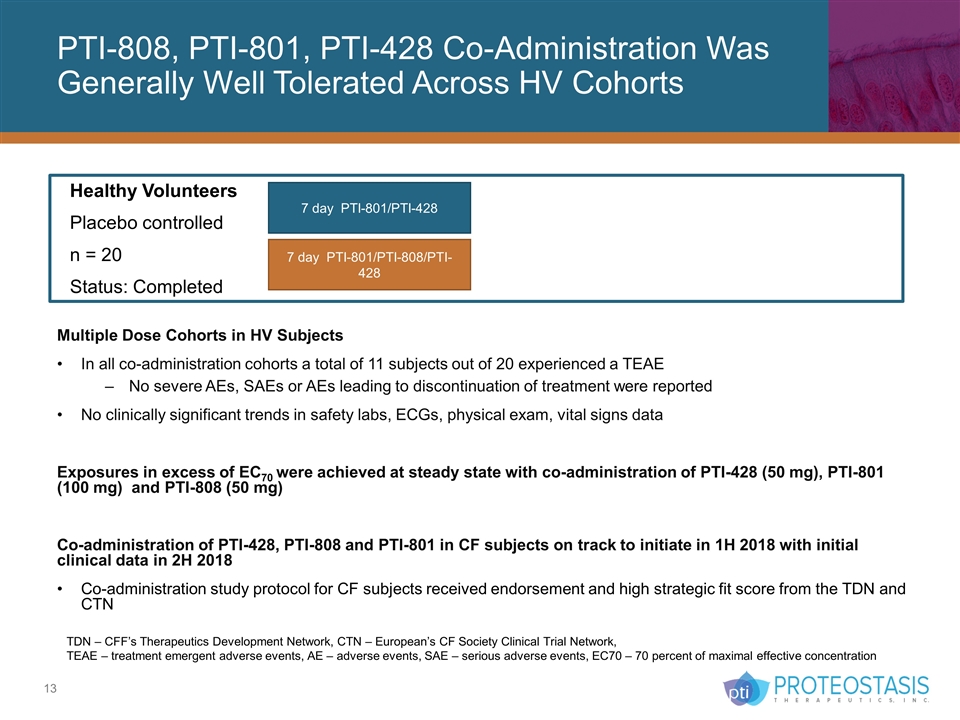
Healthy Volunteers Placebo controlled n = 20 Status: Completed 7 day PTI-801/PTI-428 7 day PTI-801/PTI-808/PTI-428 PTI-808, PTI-801, PTI-428 Co-Administration Was Generally Well Tolerated Across HV Cohorts Multiple Dose Cohorts in HV Subjects In all co-administration cohorts a total of 11 subjects out of 20 experienced a TEAE No severe AEs, SAEs or AEs leading to discontinuation of treatment were reported No clinically significant trends in safety labs, ECGs, physical exam, vital signs data Exposures in excess of EC70 were achieved at steady state with co-administration of PTI-428 (50 mg), PTI-801 (100 mg) and PTI-808 (50 mg) Co-administration of PTI-428, PTI-808 and PTI-801 in CF subjects on track to initiate in 1H 2018 with initial clinical data in 2H 2018 Co-administration study protocol for CF subjects received endorsement and high strategic fit score from the TDN and CTN TDN – CFF’s Therapeutics Development Network, CTN – European’s CF Society Clinical Trial Network, TEAE – treatment emergent adverse events, AE – adverse events, SAE – serious adverse events, EC70 – 70 percent of maximal effective concentration

PTI-428 CF Clinical Data Summary
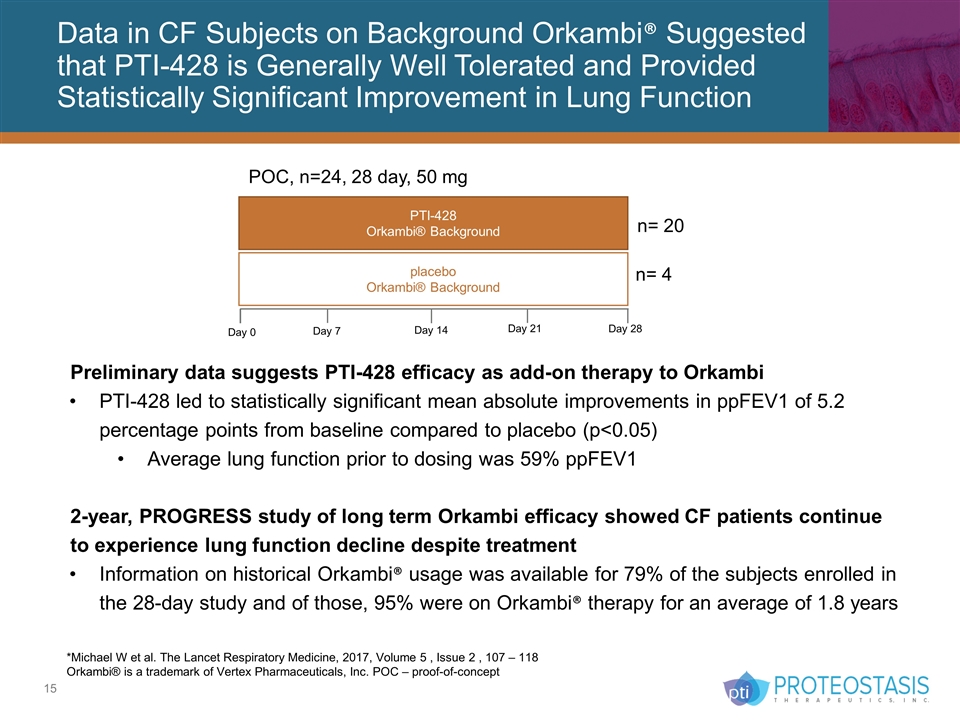
Data in CF Subjects on Background Orkambi® Suggested that PTI-428 is Generally Well Tolerated and Provided Statistically Significant Improvement in Lung Function *Michael W et al. The Lancet Respiratory Medicine, 2017, Volume 5 , Issue 2 , 107 – 118 Orkambi® is a trademark of Vertex Pharmaceuticals, Inc. POC – proof-of-concept Preliminary data suggests PTI-428 efficacy as add-on therapy to Orkambi PTI-428 led to statistically significant mean absolute improvements in ppFEV1 of 5.2 percentage points from baseline compared to placebo (p<0.05) Average lung function prior to dosing was 59% ppFEV1 2-year, PROGRESS study of long term Orkambi efficacy showed CF patients continue to experience lung function decline despite treatment Information on historical Orkambi® usage was available for 79% of the subjects enrolled in the 28-day study and of those, 95% were on Orkambi® therapy for an average of 1.8 years PTI-428 Orkambi® Background POC, n=24, 28 day, 50 mg placebo Orkambi® Background n= 4 n= 20 Day 0 Day 7 Day 14 Day 21 Day 28
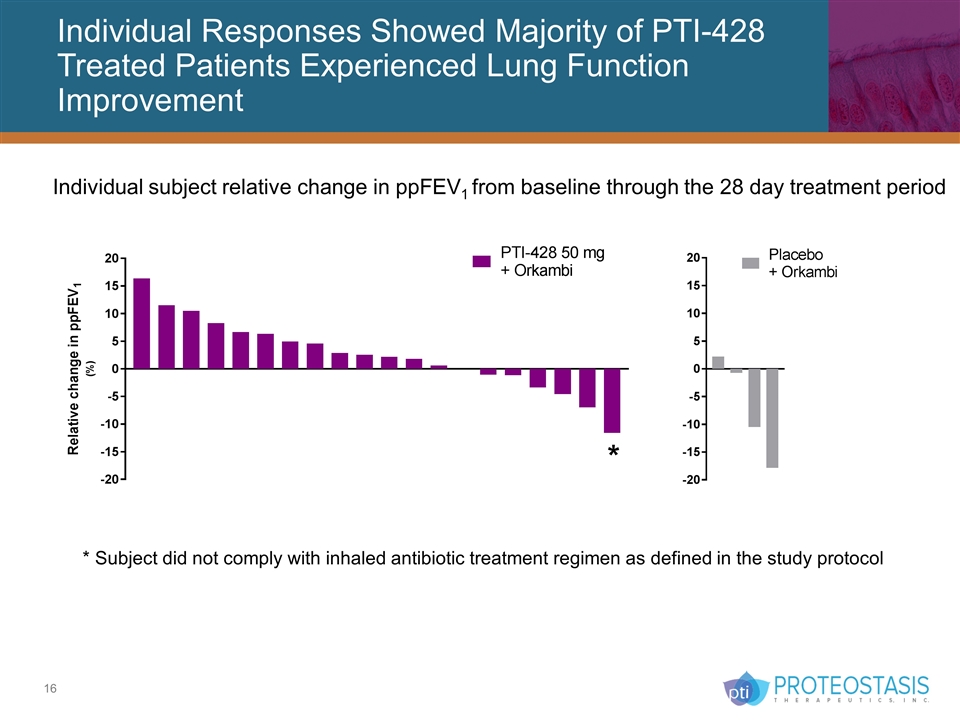
Individual Responses Showed Majority of PTI-428 Treated Patients Experienced Lung Function Improvement Individual subject relative change in ppFEV1 from baseline through the 28 day treatment period * Subject did not comply with inhaled antibiotic treatment regimen as defined in the study protocol
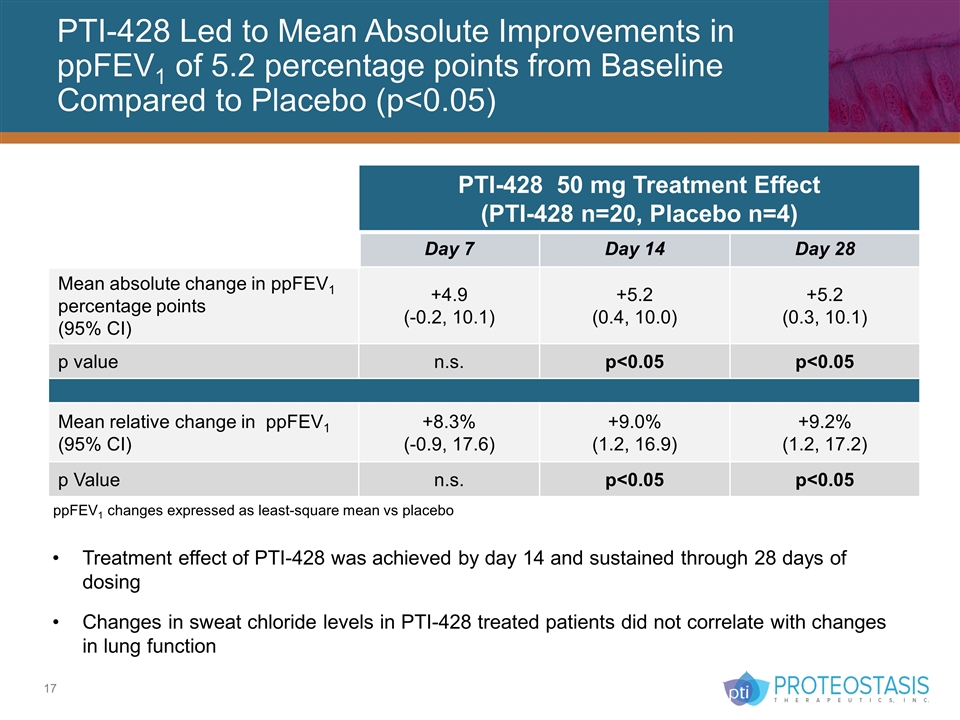
PTI-428 Led to Mean Absolute Improvements in ppFEV1 of 5.2 percentage points from Baseline Compared to Placebo (p<0.05) Treatment effect of PTI-428 was achieved by day 14 and sustained through 28 days of dosing Changes in sweat chloride levels in PTI-428 treated patients did not correlate with changes in lung function PTI-428 50 mg Treatment Effect (PTI-428 n=20, Placebo n=4) Day 7 Day 14 Day 28 Mean absolute change in ppFEV1 percentage points (95% CI) +4.9 (-0.2, 10.1) +5.2 (0.4, 10.0) +5.2 (0.3, 10.1) p value n.s. p<0.05 p<0.05 Mean relative change in ppFEV1 (95% CI) +8.3% (-0.9, 17.6) +9.0% (1.2, 16.9) +9.2% (1.2, 17.2) p Value n.s. p<0.05 p<0.05 ppFEV1 changes expressed as least-square mean vs placebo
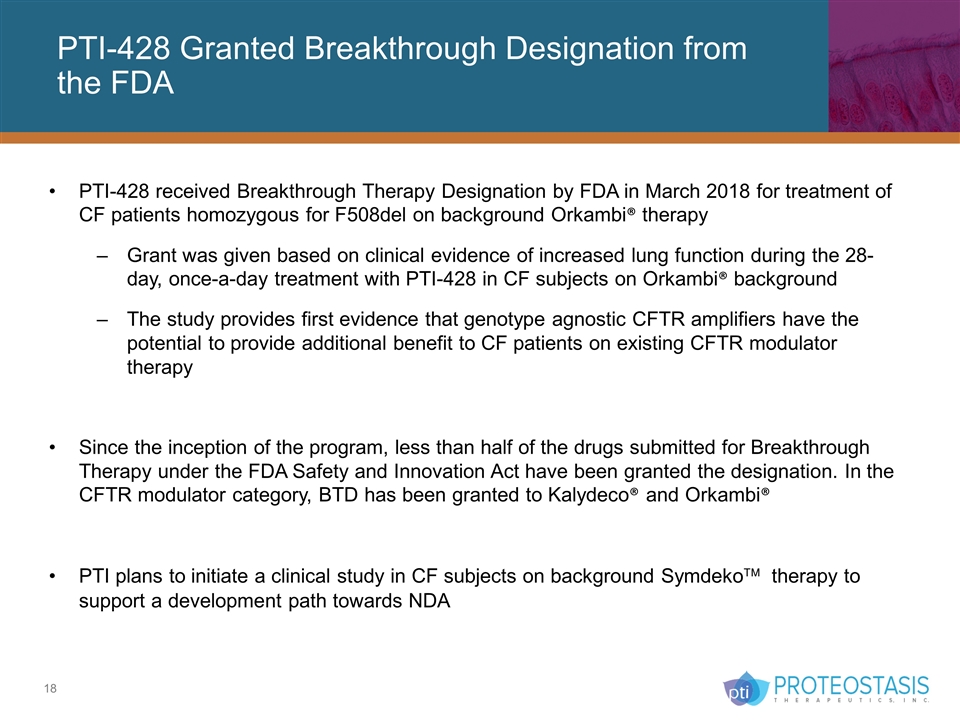
PTI-428 Granted Breakthrough Designation from the FDA PTI-428 received Breakthrough Therapy Designation by FDA in March 2018 for treatment of CF patients homozygous for F508del on background Orkambi® therapy Grant was given based on clinical evidence of increased lung function during the 28-day, once-a-day treatment with PTI-428 in CF subjects on Orkambi® background The study provides first evidence that genotype agnostic CFTR amplifiers have the potential to provide additional benefit to CF patients on existing CFTR modulator therapy Since the inception of the program, less than half of the drugs submitted for Breakthrough Therapy under the FDA Safety and Innovation Act have been granted the designation. In the CFTR modulator category, BTD has been granted to Kalydeco® and Orkambi® PTI plans to initiate a clinical study in CF subjects on background Symdekoä therapy to support a development path towards NDA

PTI-801 CF Clinical Data Summary and Enrollment Update
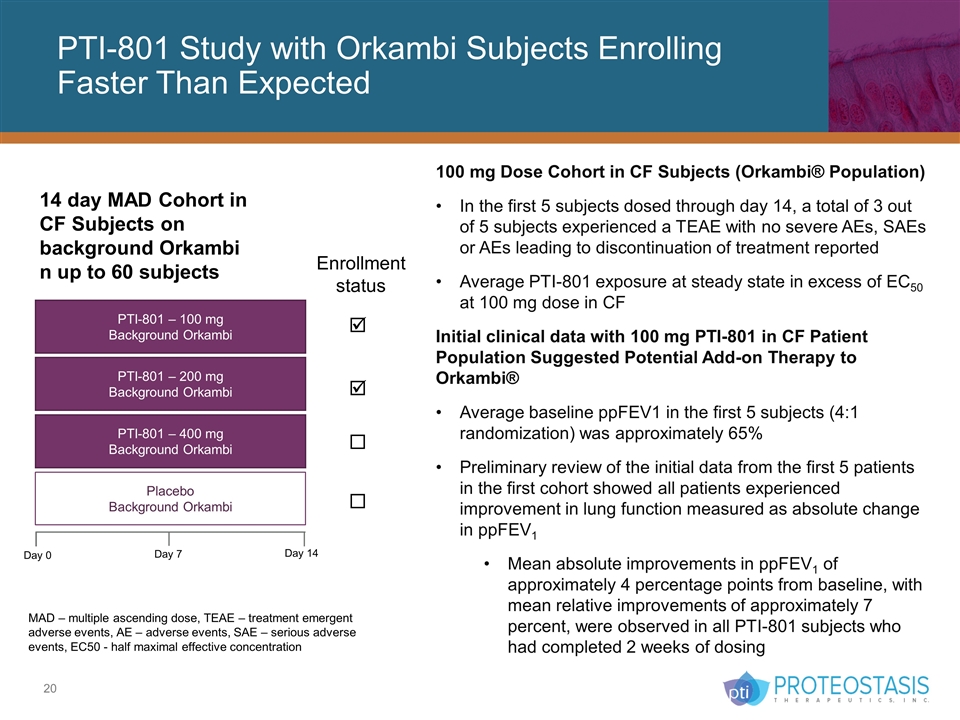
PTI-801 Study with Orkambi Subjects Enrolling Faster Than Expected MAD – multiple ascending dose, TEAE – treatment emergent adverse events, AE – adverse events, SAE – serious adverse events, EC50 - half maximal effective concentration 100 mg Dose Cohort in CF Subjects (Orkambi® Population) In the first 5 subjects dosed through day 14, a total of 3 out of 5 subjects experienced a TEAE with no severe AEs, SAEs or AEs leading to discontinuation of treatment reported Average PTI-801 exposure at steady state in excess of EC50 at 100 mg dose in CF Initial clinical data with 100 mg PTI-801 in CF Patient Population Suggested Potential Add-on Therapy to Orkambi® Average baseline ppFEV1 in the first 5 subjects (4:1 randomization) was approximately 65% Preliminary review of the initial data from the first 5 patients in the first cohort showed all patients experienced improvement in lung function measured as absolute change in ppFEV1 Mean absolute improvements in ppFEV1 of approximately 4 percentage points from baseline, with mean relative improvements of approximately 7 percent, were observed in all PTI-801 subjects who had completed 2 weeks of dosing PTI-801 – 100 mg Background Orkambi 14 day MAD Cohort in CF Subjects on background Orkambi n up to 60 subjects PTI-801 – 200 mg Background Orkambi PTI-801 – 400 mg Background Orkambi Placebo Background Orkambi Day 0 Day 7 Day 14 Enrollment status þ þ ¨ ¨

PTI Proprietary Combination Development Path in CF
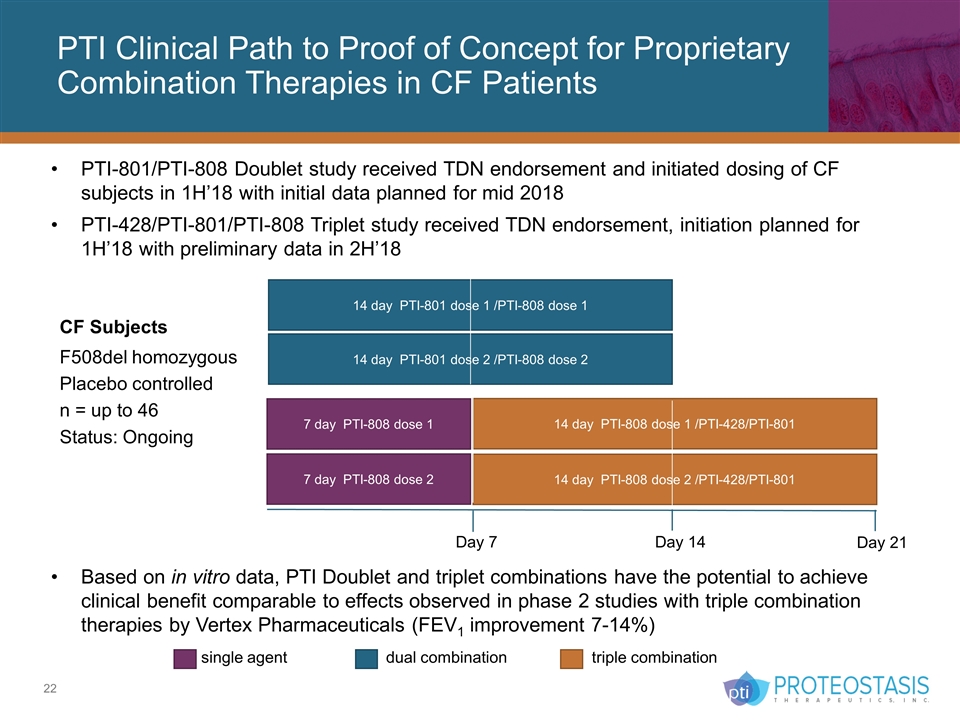
PTI Clinical Path to Proof of Concept for Proprietary Combination Therapies in CF Patients single agent dual combination triple combination CF Subjects F508del homozygous Placebo controlled n = up to 46 Status: Ongoing 14 day PTI-801 dose 1 /PTI-808 dose 1 14 day PTI-801 dose 2 /PTI-808 dose 2 7 day PTI-808 dose 2 14 day PTI-808 dose 2 /PTI-428/PTI-801 7 day PTI-808 dose 1 14 day PTI-808 dose 1 /PTI-428/PTI-801 Day 7 Day 14 Day 21 PTI-801/PTI-808 Doublet study received TDN endorsement and initiated dosing of CF subjects in 1H’18 with initial data planned for mid 2018 PTI-428/PTI-801/PTI-808 Triplet study received TDN endorsement, initiation planned for 1H’18 with preliminary data in 2H’18 Based on in vitro data, PTI Doublet and triplet combinations have the potential to achieve clinical benefit comparable to effects observed in phase 2 studies with triple combination therapies by Vertex Pharmaceuticals (FEV1 improvement 7-14%)

Financial Status $74.5 million as of December 31, 2017 in cash, cash equivalents and short-term investments Cash position estimated to be sufficient to fund operations into early 2019
12 Month Key Milestones PTI-808/PTI-801/PTI-428 combination study protocol endorsed with high strategic fit score by CF patient advocacy groups in the U.S. and Europe PTI-801/PTI-808 combination study initiated in CF patients Breakthrough Therapy Designation for PTI-428 granted by the FDA PTI-808/PTI-801/PTI-428 combination study initiates in CF subjects PTI-801 14 day dosing, additional data in CF subjects on Orkambi® PTI-428, initiate clinical study in CF Subjects on Symdekoä PTI-808/PTI-801 initial data from combination study in CF subjects PTI-808/PTI-801/PTI-428 combination study preliminary data in CF subjects Early 2019: initial data from PTI-428 in CF Subjects on Symdekoä Orkambi® and Symdekoä are trademarks of Vertex Pharmaceuticals, Inc. 2018 2018

Thank You
























