
Proteostasis Therapeutics, Inc. (PTI) July 2018 Investor Deck Exhibit 99.1

Safe Harbor and Disclaimer To the extent that statements in this presentation are not historical facts, they are forward-looking statements reflecting the current beliefs and expectations of management made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. Words such as “aim,” “may,” “will,” “expect,” “anticipate,” “estimate,” “intend,” and similar expressions (as well as other words or expressions referencing future events, conditions or circumstances) are intended to identify forward-looking statements. Examples of forward-looking statements made in this presentation include, without limitation, statements regarding the expected timing of the initiation of, patient enrollment in, data from, and our completion of, our clinical studies and cohorts for PTI-428, PTI-801, PTI-808 and our combination therapy candidates as well as cash guidance. Forward-looking statements made in this presentation involve substantial risks and uncertainties that could cause actual results to differ materially from those expressed or implied by the forward-looking statements, and we therefore cannot assure you that our plans, intentions, expectations or strategies will be attained or achieved. Such risks and uncertainties include, without limitation, the possibility final or future results from our drug candidate trials (including, without limitation, longer duration studies) do not achieve positive results or are materially and negatively different from or not indicative of the preliminary results reported in this presentation (noting that these results are on a small number of patients and small data set), uncertainties inherent in the execution and completion of clinical trials (including, without limitation, the possibility FDA requires us to run cohorts sequentially or conduct additional cohorts or pre-clinical or clinical studies), in the enrollment of CF patients in our clinical trials, in the timing of availability of trial data, in the results of the clinical trials, in possible adverse events from our trials, in the actions of regulatory agencies, in endorsement, if any, by therapeutic development arms of CF patient advocacy groups, and those set forth in our Quarterly Report on Form 10-Q for the quarter ended March 31, 2018, and our other SEC filings. We assume no obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise. This presentation also contains estimates and other statistical data made by independent parties and by us relating to, among other items, disease incidence, market size and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. Projections, assumptions and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of risk and uncertainty. New risks emerge from time to time, and neither we nor any other person makes any representation as to the accuracy or completeness of such data or undertakes any obligation to update such date after the date of this presentation. By attending or receiving this presentation you acknowledge you are solely responsible for your own assessment of the market and our market position and that you will conduct your own analysis and are solely responsible for forming your own view of the potential future performance of our business. The trademarks included in this presentation are the property of the owners thereof and are used for reference purposes only. Such use should not be construed as an endorsement of the Company or its securities.

PTI Overview Clinical stage biopharma developing novel therapeutics for cystic fibrosis (CF) and other diseases caused by dysfunctional protein processing Developing novel small molecules for CF combination therapy PTI-808: Novel Potentiator PTI-801: Third Generation Corrector that is additive in vitro to first and second generation correctors PTI-428: Novel Class of CFTR Modulator: Amplifiers Leveraging potential therapeutic benefit of multiple stand-alone combination options including PTI-801 and PTI-808 as a doublet and PTI-808, PTI-801, and PTI-428 as a triplet PTI-801 and PTI-428 can potentially be developed as add-on therapies to current and future standard of care CFTR modulator therapies $63.3 million as of March 31, 2018 in cash, cash equivalents and short-term investments As of May 3, 2018, net proceeds from ATM were $7.8 million in addition to cash balance above Cash position estimated to be sufficient to fund operations into early 2019
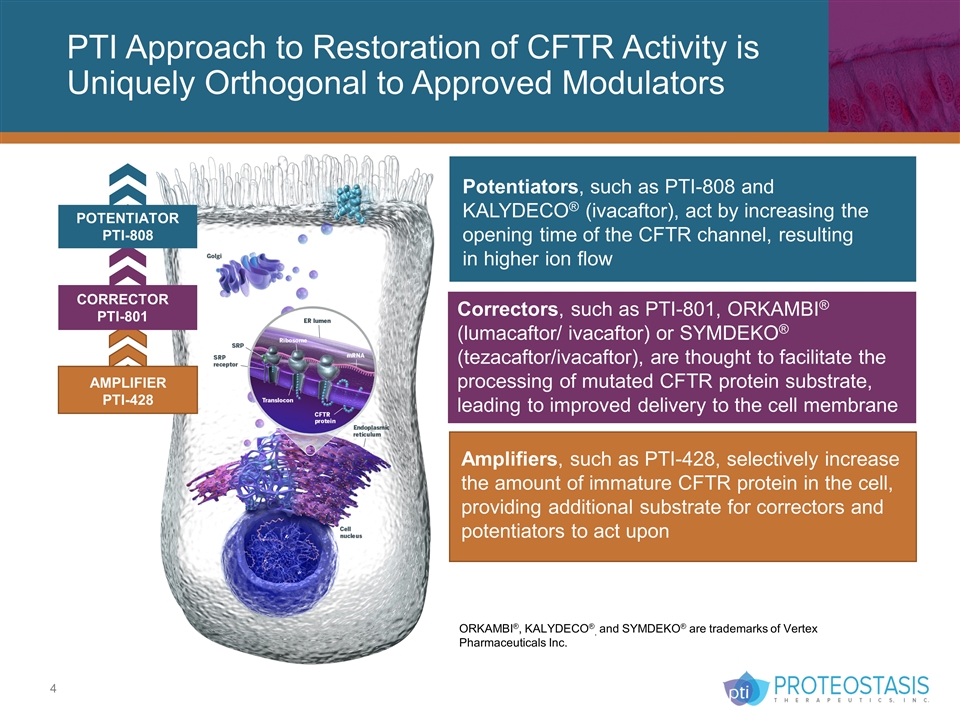
CORRECTOR PTI-801 PTI Approach to Restoration of CFTR Activity is Uniquely Orthogonal to Approved Modulators Amplifiers, such as PTI-428, selectively increase the amount of immature CFTR protein in the cell, providing additional substrate for correctors and potentiators to act upon Potentiators, such as PTI-808 and KALYDECO® (ivacaftor), act by increasing the opening time of the CFTR channel, resulting in higher ion flow Correctors, such as PTI-801, ORKAMBI® (lumacaftor/ ivacaftor) or SYMDEKO® (tezacaftor/ivacaftor), are thought to facilitate the processing of mutated CFTR protein substrate, leading to improved delivery to the cell membrane POTENTIATOR PTI-808 AMPLIFIER PTI-428 ORKAMBI®, KALYDECO®, and SYMDEKO® are trademarks of Vertex Pharmaceuticals Inc.
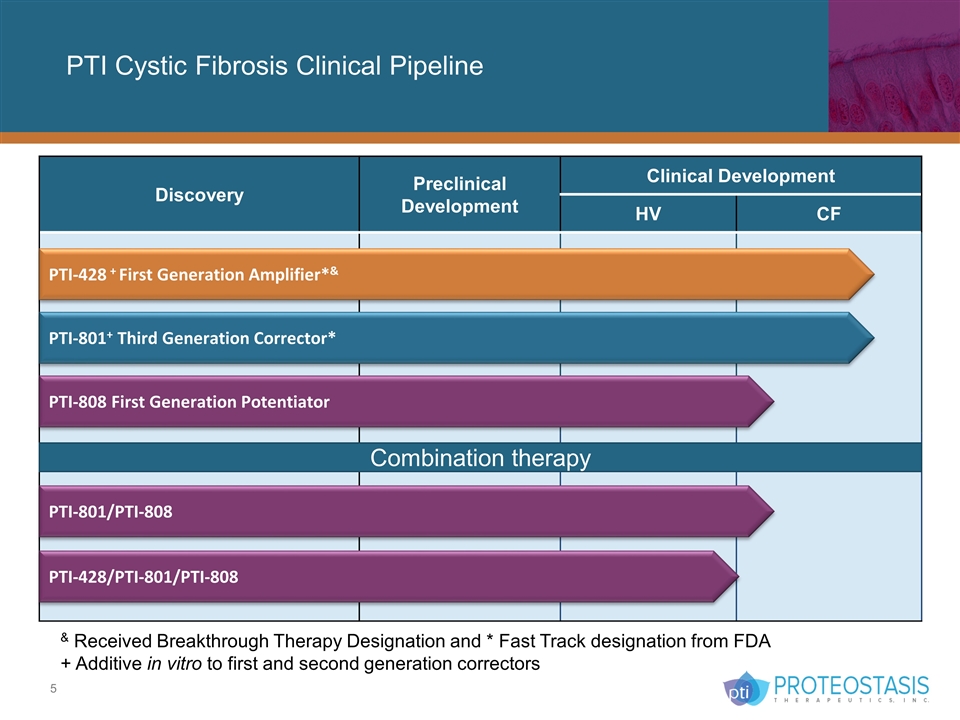
PTI Cystic Fibrosis Clinical Pipeline Discovery Preclinical Development Clinical Development HV CF PTI-428 + First Generation Amplifier*& PTI-801+ Third Generation Corrector* PTI-808 First Generation Potentiator & Received Breakthrough Therapy Designation and * Fast Track designation from FDA + Additive in vitro to first and second generation correctors Combination therapy PTI-428/PTI-801/PTI-808 PTI-801/PTI-808
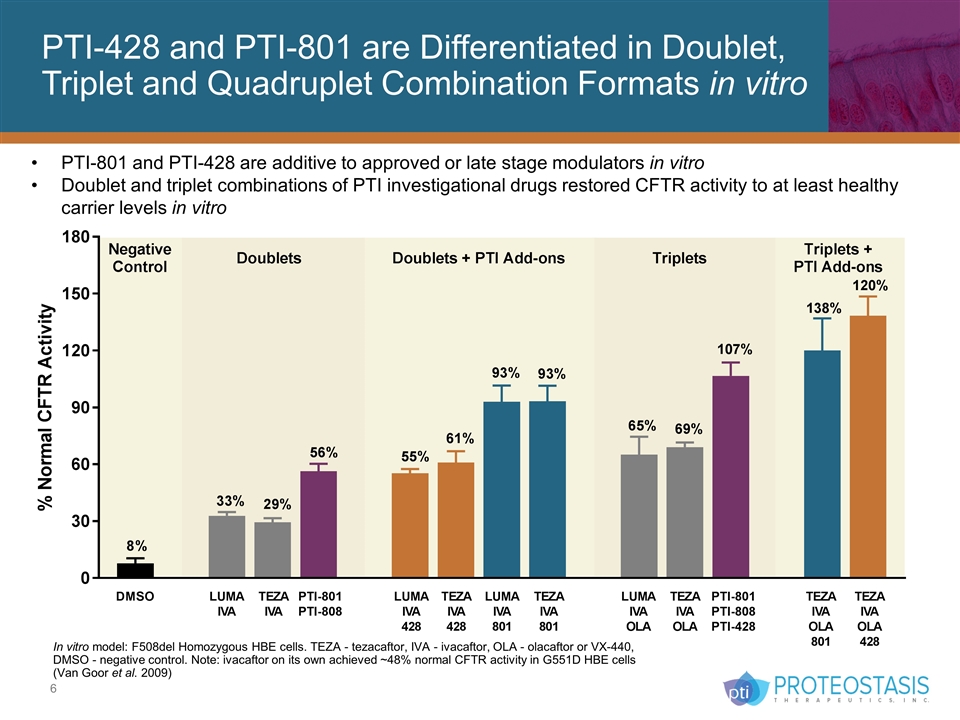
PTI-428 and PTI-801 are Differentiated in Doublet, Triplet and Quadruplet Combination Formats in vitro PTI-801 and PTI-428 are additive to approved or late stage modulators in vitro Doublet and triplet combinations of PTI investigational drugs restored CFTR activity to at least healthy carrier levels in vitro In vitro model: F508del Homozygous HBE cells. TEZA - tezacaftor, IVA - ivacaftor, OLA - olacaftor or VX-440, DMSO - negative control. Note: ivacaftor on its own achieved ~48% normal CFTR activity in G551D HBE cells (Van Goor et al. 2009)
12 Month Key Milestones PTI-808/PTI-801/PTI-428 combination study protocol endorsed with high strategic fit score by CF patient advocacy groups in the U.S. and Europe PTI-801/PTI-808 combination study initiated in CF patients Breakthrough Therapy Designation for PTI-428 granted by the FDA PTI-801 14 day dosing, additional data in CF subjects on ORKAMBI® PTI-808/PTI-801/PTI-428 combination study initiates in CF subjects PTI-428 starts dosing in CF Subjects on SYMDEKO® clinical study PTI-808/PTI-801 initial data from combination study in CF subjects PTI-808/PTI-801/PTI-428 combination study preliminary data in CF subjects Early 2019 initial data from PTI-428 in CF Subjects on SYMDEKO® ORKAMBI® and SYMDEKO® are trademarks of Vertex Pharmaceuticals, Inc. 2018 2018

PTI-801-01 CF Study Summary and Updates
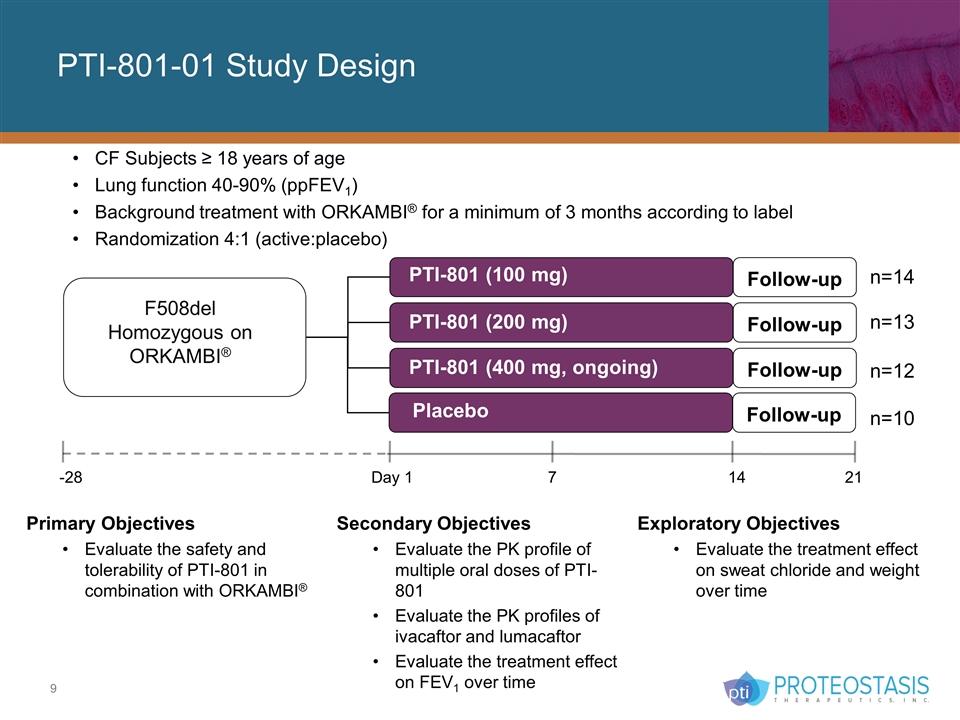
PTI-801-01 Study Design CF Subjects ≥ 18 years of age Lung function 40-90% (ppFEV1) Background treatment with ORKAMBI® for a minimum of 3 months according to label Randomization 4:1 (active:placebo) Follow-up Placebo F508del Homozygous on ORKAMBI® -28 Day 1 7 14 21 Follow-up Follow-up Follow-up PTI-801 (400 mg, ongoing) PTI-801 (200 mg) PTI-801 (100 mg) n=14 n=13 n=12 n=10 Primary Objectives Evaluate the safety and tolerability of PTI-801 in combination with ORKAMBI® Exploratory Objectives Evaluate the treatment effect on sweat chloride and weight over time Secondary Objectives Evaluate the PK profile of multiple oral doses of PTI-801 Evaluate the PK profiles of ivacaftor and lumacaftor Evaluate the treatment effect on FEV1 over time
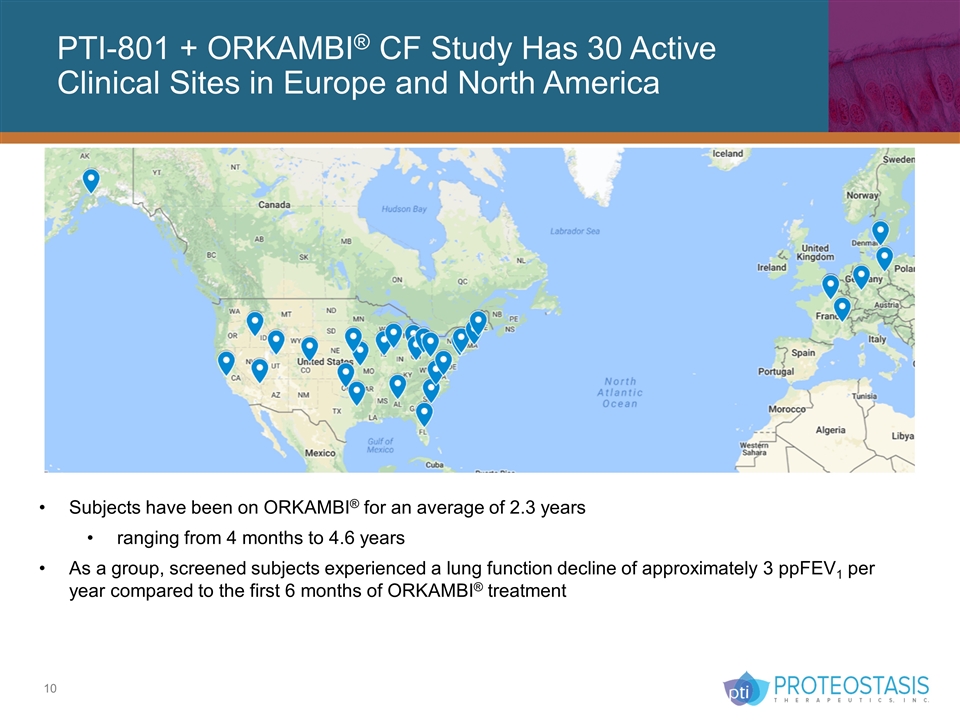
PTI-801 + ORKAMBI® CF Study Has 30 Active Clinical Sites in Europe and North America Subjects have been on ORKAMBI® for an average of 2.3 years ranging from 4 months to 4.6 years As a group, screened subjects experienced a lung function decline of approximately 3 ppFEV1 per year compared to the first 6 months of ORKAMBI® treatment
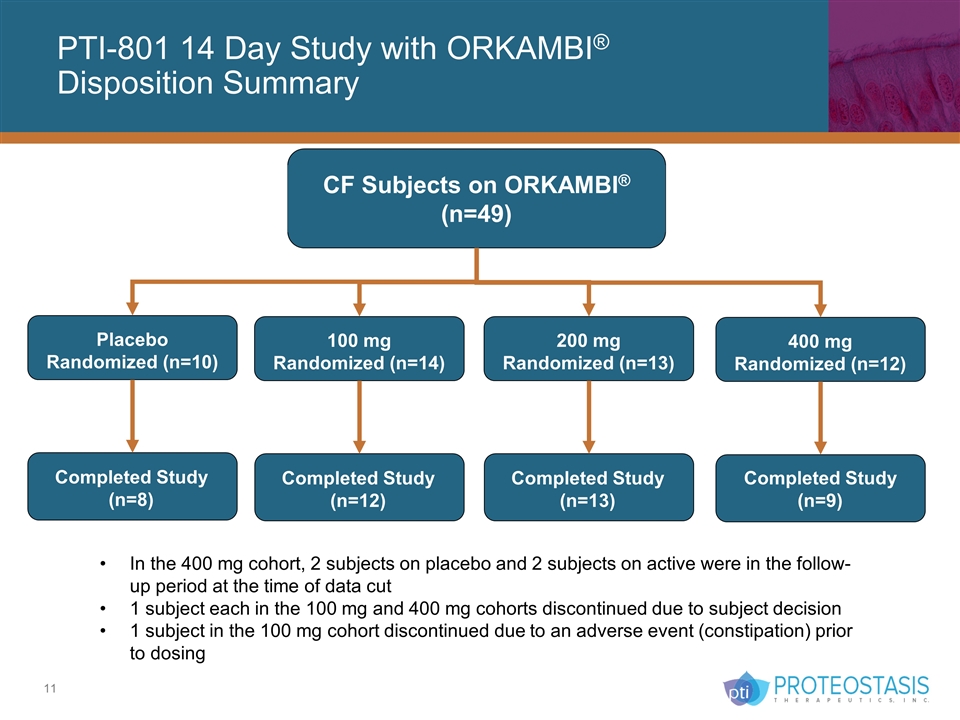
PTI-801 14 Day Study with ORKAMBI® Disposition Summary Placebo Randomized (n=10) Completed Study (n=8) CF Subjects on ORKAMBI® (n=49) 100 mg Randomized (n=14) Completed Study (n=12) 200 mg Randomized (n=13) Completed Study (n=13) 400 mg Randomized (n=12) Completed Study (n=9) In the 400 mg cohort, 2 subjects on placebo and 2 subjects on active were in the follow-up period at the time of data cut 1 subject each in the 100 mg and 400 mg cohorts discontinued due to subject decision 1 subject in the 100 mg cohort discontinued due to an adverse event (constipation) prior to dosing
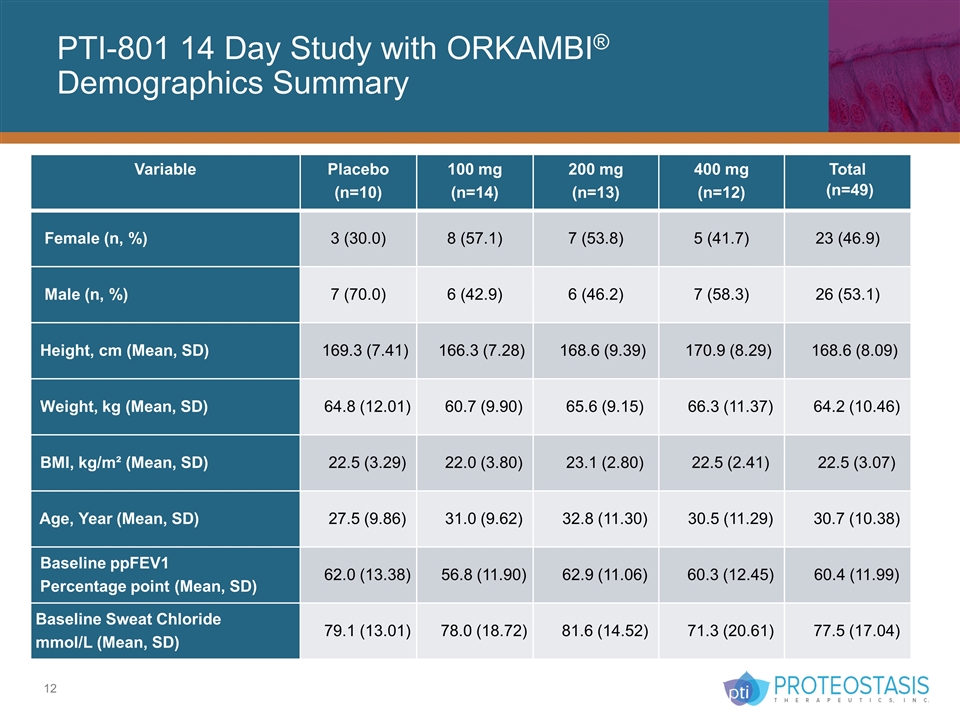
PTI-801 14 Day Study with ORKAMBI® Demographics Summary Variable Placebo (n=10) 100 mg (n=14) 200 mg (n=13) 400 mg (n=12) Total (n=49) Female (n, %) 3 (30.0) 8 (57.1) 7 (53.8) 5 (41.7) 23 (46.9) Male (n, %) 7 (70.0) 6 (42.9) 6 (46.2) 7 (58.3) 26 (53.1) Height, cm (Mean, SD) 169.3 (7.41) 166.3 (7.28) 168.6 (9.39) 170.9 (8.29) 168.6 (8.09) Weight, kg (Mean, SD) 64.8 (12.01) 60.7 (9.90) 65.6 (9.15) 66.3 (11.37) 64.2 (10.46) BMI, kg/m² (Mean, SD) 22.5 (3.29) 22.0 (3.80) 23.1 (2.80) 22.5 (2.41) 22.5 (3.07) Age, Year (Mean, SD) 27.5 (9.86) 31.0 (9.62) 32.8 (11.30) 30.5 (11.29) 30.7 (10.38) Baseline ppFEV1 Percentage point (Mean, SD) 62.0 (13.38) 56.8 (11.90) 62.9 (11.06) 60.3 (12.45) 60.4 (11.99) Baseline Sweat Chloride mmol/L (Mean, SD) 79.1 (13.01) 78.0 (18.72) 81.6 (14.52) 71.3 (20.61) 77.5 (17.04)
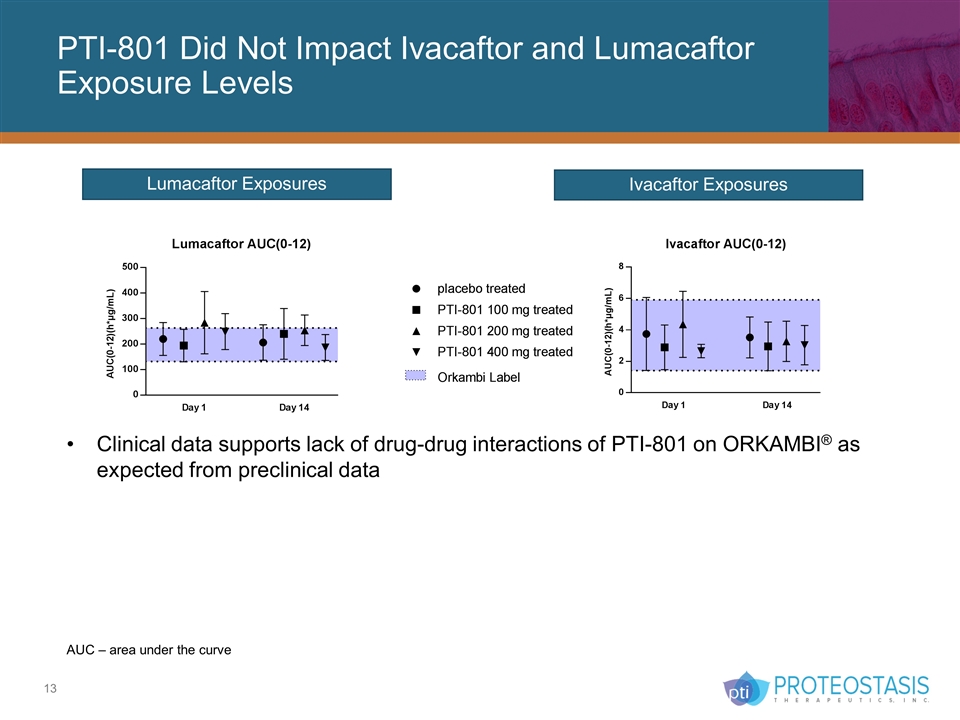
Ivacaftor Exposures Lumacaftor Exposures Clinical data supports lack of drug-drug interactions of PTI-801 on ORKAMBI® as expected from preclinical data PTI-801 Did Not Impact Ivacaftor and Lumacaftor Exposure Levels AUC – area under the curve
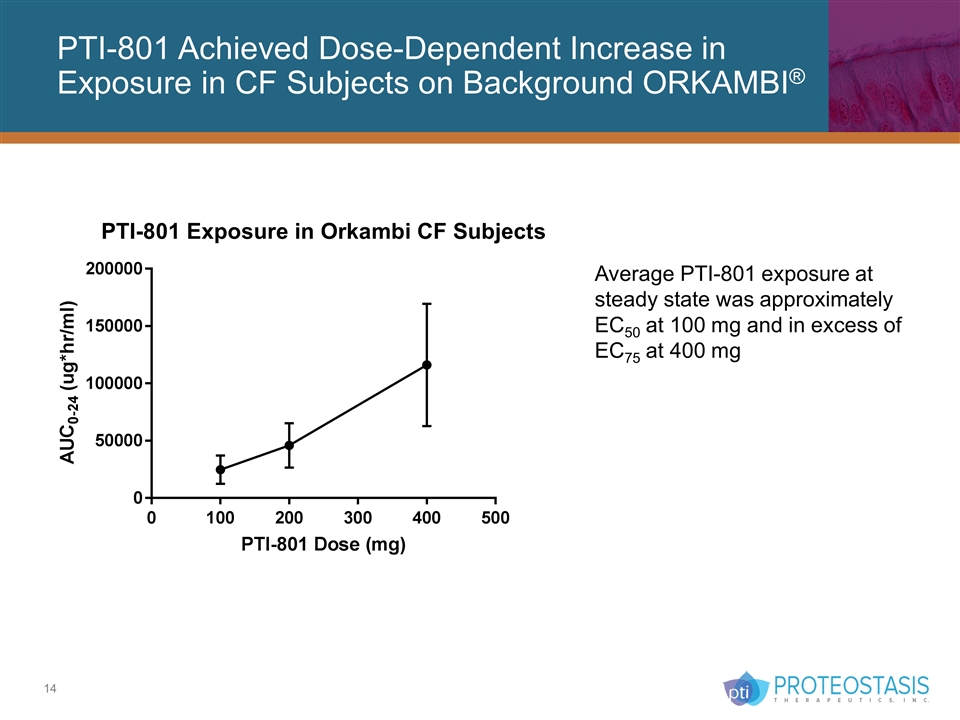
PTI-801 Achieved Dose-Dependent Increase in Exposure in CF Subjects on Background ORKAMBI® Average PTI-801 exposure at steady state was approximately EC50 at 100 mg and in excess of EC75 at 400 mg

PTI-801 14 Day Study with ORKAMBI® Safety Summary PTI-801 was generally well tolerated No SAE, no AE leading to discontinuation and mostly mild-moderate AE Only severe AEs were neck pain and poor sleep quality (unlikely related to study drug) No clinically significant safety signals in chemistry, hematology lab, ECG Category Placebo (n=10) 100 mg (n=13) 200 mg (n=13) 400 mg (n=12) Total (n=48) At Least One AE (n, %) 5 (50.0) 9 (69.2) 8 (61.5) 11 (91.7) 33 (68.8) Respiratory-related adverse events n (%) Infective pulmonary exacerbation of cystic fibrosis n (%) 1 (10.0) 2 (15.4) 1 ( 7.7) 1 ( 8.3) 5 (10.4) Cough n (%) 0 ( 0.0) 1 ( 7.7) 2 (15.4) 1 ( 8.3) 4 ( 8.3) Chest discomfort n (%) 0 (0.0) 1 (7.7) 0 (0.0) 1 (8.3) 2 (4.2) Dyspnoea exertional n (%) 0 (0.0) 0 (0.0) 1 (7.7) 0 (0.0) 1 (2.1) Other adverse events n (%) Headache n (%) 0 ( 0.0) 2 (15.4) 1 ( 7.7) 1 ( 8.3) 4 ( 8.3)
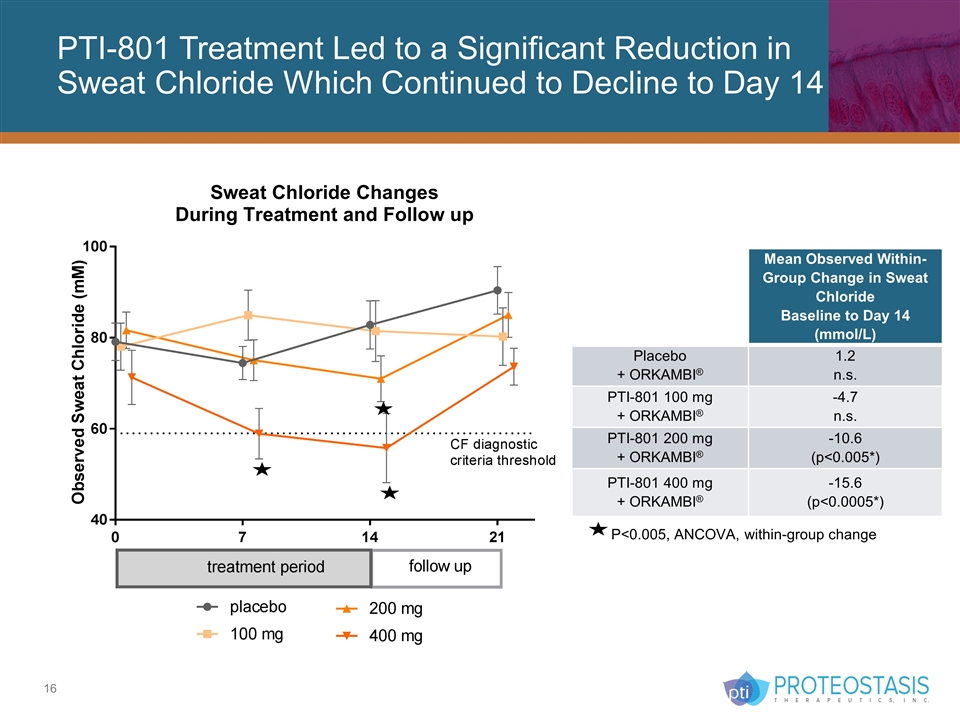
PTI-801 Treatment Led to a Significant Reduction in Sweat Chloride Which Continued to Decline to Day 14 P<0.005, ANCOVA, within-group change Within-Group Change From Baseline to Day 14 Mean Observed Within-Group Change in Sweat Chloride Baseline to Day 14 (mmol/L) Placebo + ORKAMBI® 1.2 n.s. PTI-801 100 mg + ORKAMBI® -4.7 n.s. PTI-801 200 mg + ORKAMBI® -10.6 (p<0.005*) PTI-801 400 mg + ORKAMBI® -15.6 (p<0.0005*)
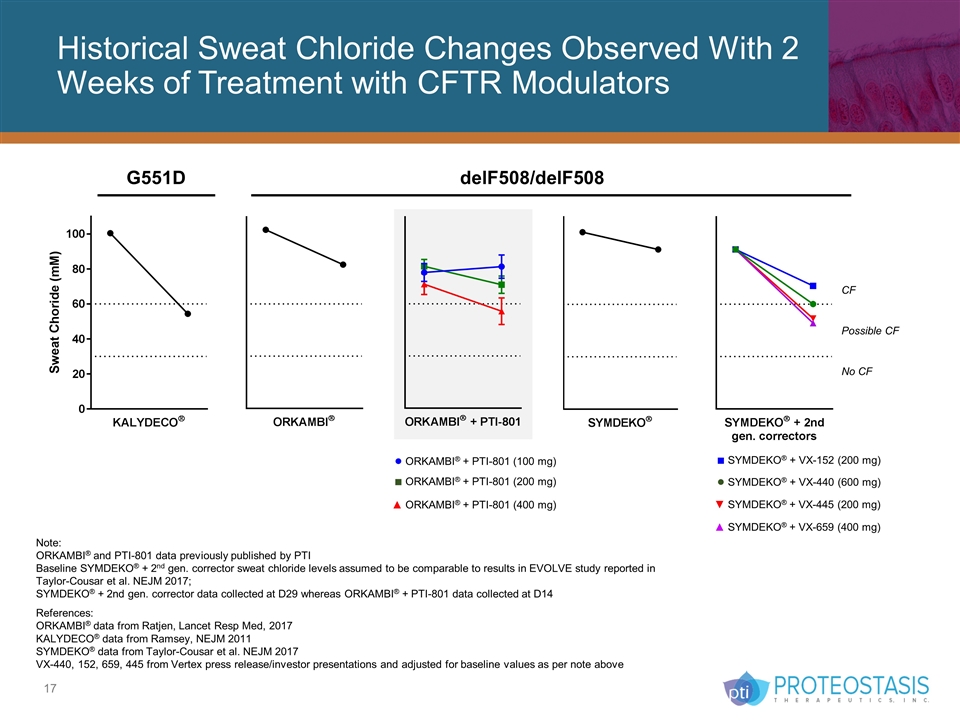
Historical Sweat Chloride Changes Observed With 2 Weeks of Treatment with CFTR Modulators G551D delF508/delF508 ■ SYMDEKO® + VX-152 (200 mg) ● SYMDEKO® + VX-440 (600 mg) ▼ SYMDEKO® + VX-445 (200 mg) ▲ SYMDEKO® + VX-659 (400 mg) Note: ORKAMBI® and PTI-801 data previously published by PTI Baseline SYMDEKO® + 2nd gen. corrector sweat chloride levels assumed to be comparable to results in EVOLVE study reported in Taylor-Cousar et al. NEJM 2017; SYMDEKO® + 2nd gen. corrector data collected at D29 whereas ORKAMBI® + PTI-801 data collected at D14 References: ORKAMBI® data from Ratjen, Lancet Resp Med, 2017 KALYDECO® data from Ramsey, NEJM 2011 SYMDEKO® data from Taylor-Cousar et al. NEJM 2017 VX-440, 152, 659, 445 from Vertex press release/investor presentations and adjusted for baseline values as per note above ● ORKAMBI® + PTI-801 (100 mg) ■ ORKAMBI® + PTI-801 (200 mg) ▲ ORKAMBI® + PTI-801 (400 mg) CF Possible CF No CF
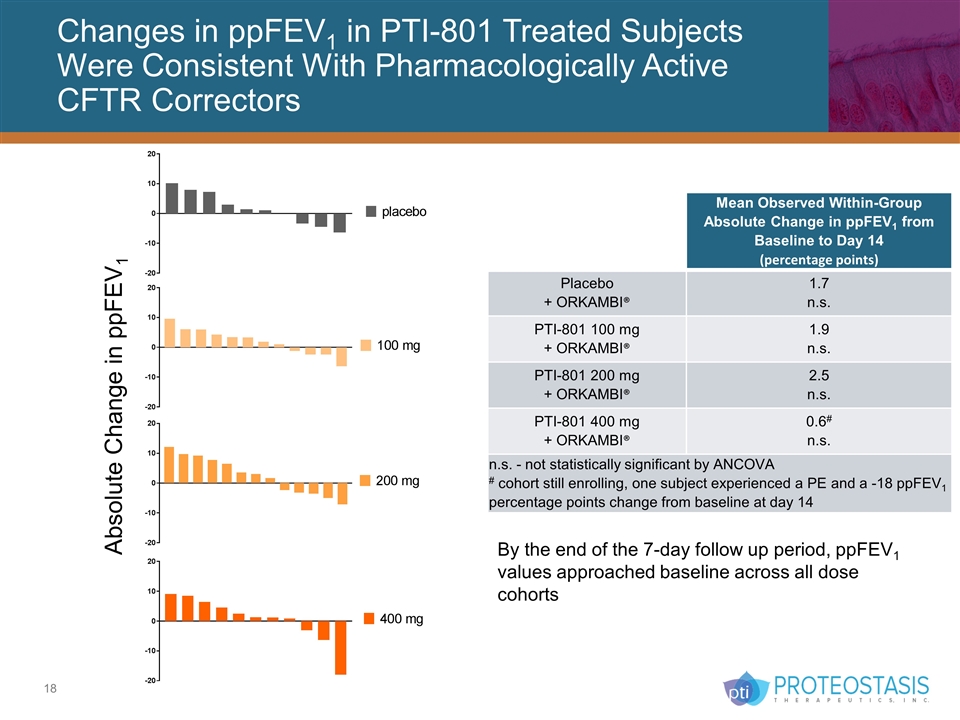
Changes in ppFEV1 in PTI-801 Treated Subjects Were Consistent With Pharmacologically Active CFTR Correctors Mean Observed Within-Group Absolute Change in ppFEV1 from Baseline to Day 14 (percentage points) Placebo + ORKAMBI® 1.7 n.s. PTI-801 100 mg + ORKAMBI® 1.9 n.s. PTI-801 200 mg + ORKAMBI® 2.5 n.s. PTI-801 400 mg + ORKAMBI® 0.6# n.s. n.s. - not statistically significant by ANCOVA # cohort still enrolling, one subject experienced a PE and a -18 ppFEV1 percentage points change from baseline at day 14 By the end of the 7-day follow up period, ppFEV1 values approached baseline across all dose cohorts Absolute Change in ppFEV1
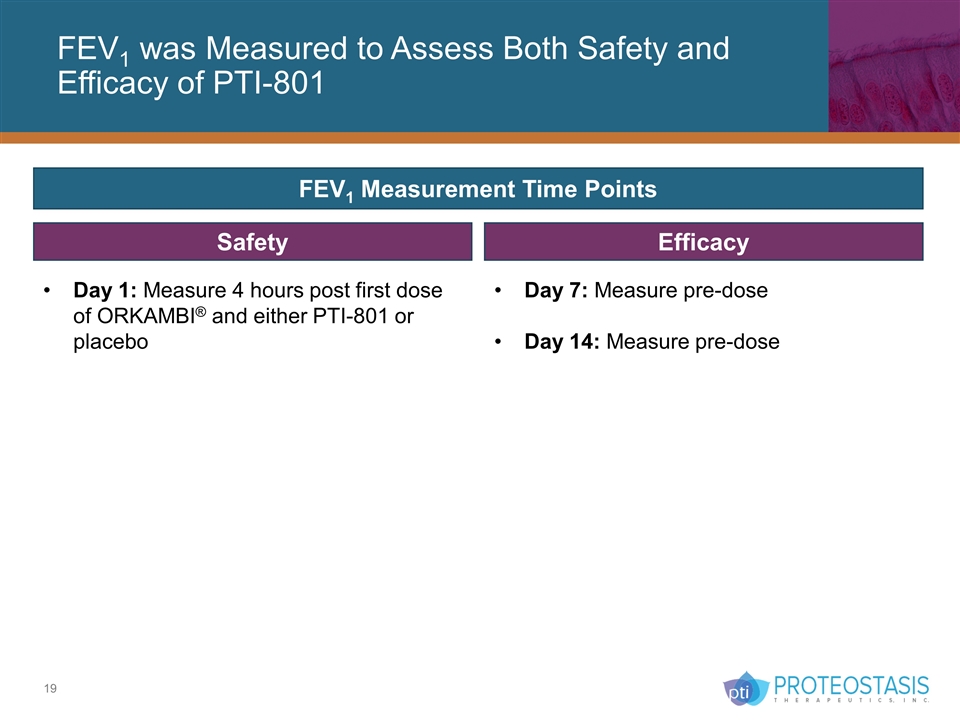
FEV1 was Measured to Assess Both Safety and Efficacy of PTI-801 Efficacy FEV1 Measurement Time Points Day 1: Measure 4 hours post first dose of ORKAMBI® and either PTI-801 or placebo Day 7: Measure pre-dose Day 14: Measure pre-dose Safety
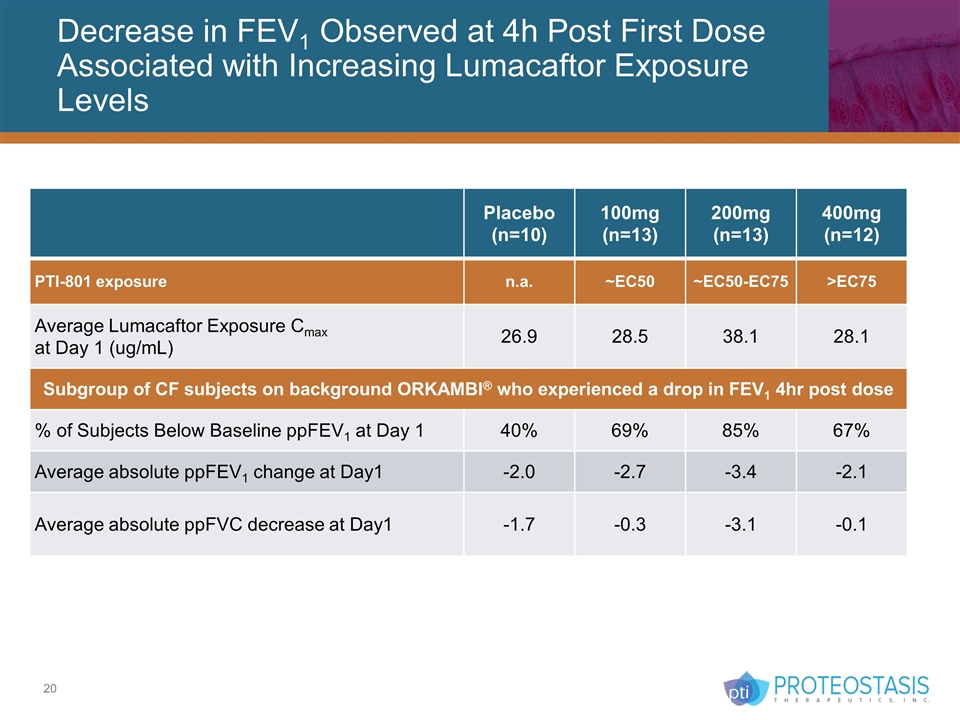
Decrease in FEV1 Observed at 4h Post First Dose Associated with Increasing Lumacaftor Exposure Levels Placebo (n=10) 100mg (n=13) 200mg (n=13) 400mg (n=12) PTI-801 exposure n.a. ~EC50 ~EC50-EC75 >EC75 Average Lumacaftor Exposure Cmax at Day 1 (ug/mL) 26.9 28.5 38.1 28.1 Subgroup of CF subjects on background ORKAMBI® who experienced a drop in FEV1 4hr post dose % of Subjects Below Baseline ppFEV1 at Day 1 40% 69% 85% 67% Average absolute ppFEV1 change at Day1 -2.0 -2.7 -3.4 -2.1 Average absolute ppFVC decrease at Day1 -1.7 -0.3 -3.1 -0.1
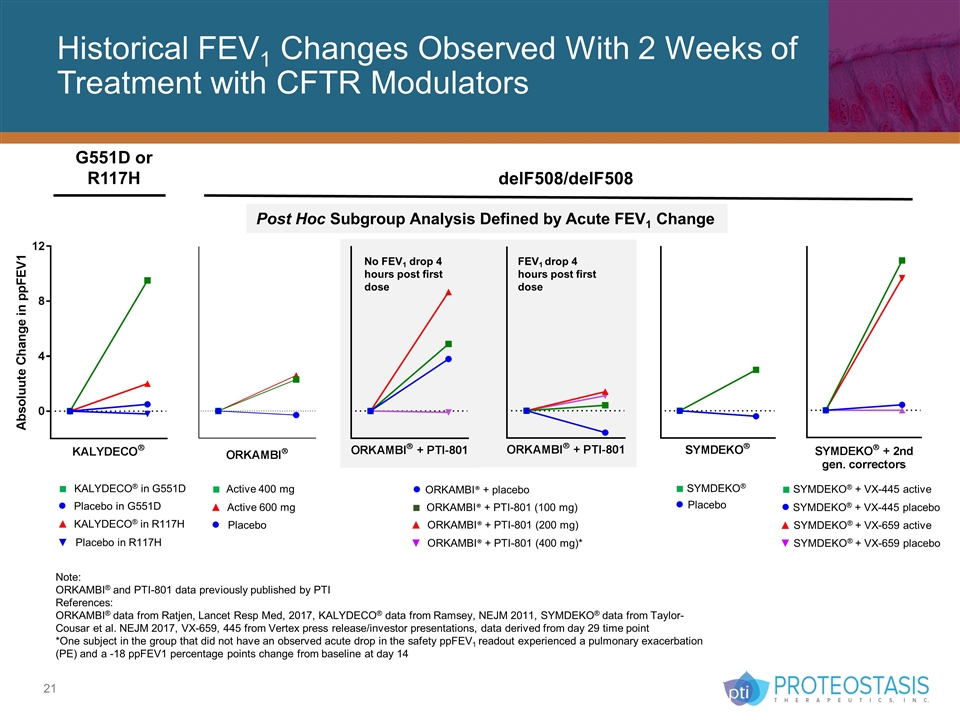
Historical FEV1 Changes Observed With 2 Weeks of Treatment with CFTR Modulators G551D or R117H delF508/delF508 Note: ORKAMBI® and PTI-801 data previously published by PTI References: ORKAMBI® data from Ratjen, Lancet Resp Med, 2017, KALYDECO® data from Ramsey, NEJM 2011, SYMDEKO® data from Taylor-Cousar et al. NEJM 2017, VX-659, 445 from Vertex press release/investor presentations, data derived from day 29 time point *One subject in the group that did not have an observed acute drop in the safety ppFEV1 readout experienced a pulmonary exacerbation (PE) and a -18 ppFEV1 percentage points change from baseline at day 14 ■ KALYDECO® in G551D ● Placebo in G551D ■ Active 400 mg ● Placebo ▲ Active 600 mg ■ SYMDEKO® + VX-445 active ● SYMDEKO® + VX-445 placebo ▼ SYMDEKO® + VX-659 placebo ▲ SYMDEKO® + VX-659 active ■ SYMDEKO® ● Placebo ▲ KALYDECO® in R117H Placebo in R117H ● ORKAMBI® + placebo ■ ORKAMBI® + PTI-801 (100 mg) ▲ ORKAMBI® + PTI-801 (200 mg) ▼ ORKAMBI® + PTI-801 (400 mg)* No FEV1 drop 4 hours post first dose FEV1 drop 4 hours post first dose Post Hoc Subgroup Analysis Defined by Acute FEV1 Change
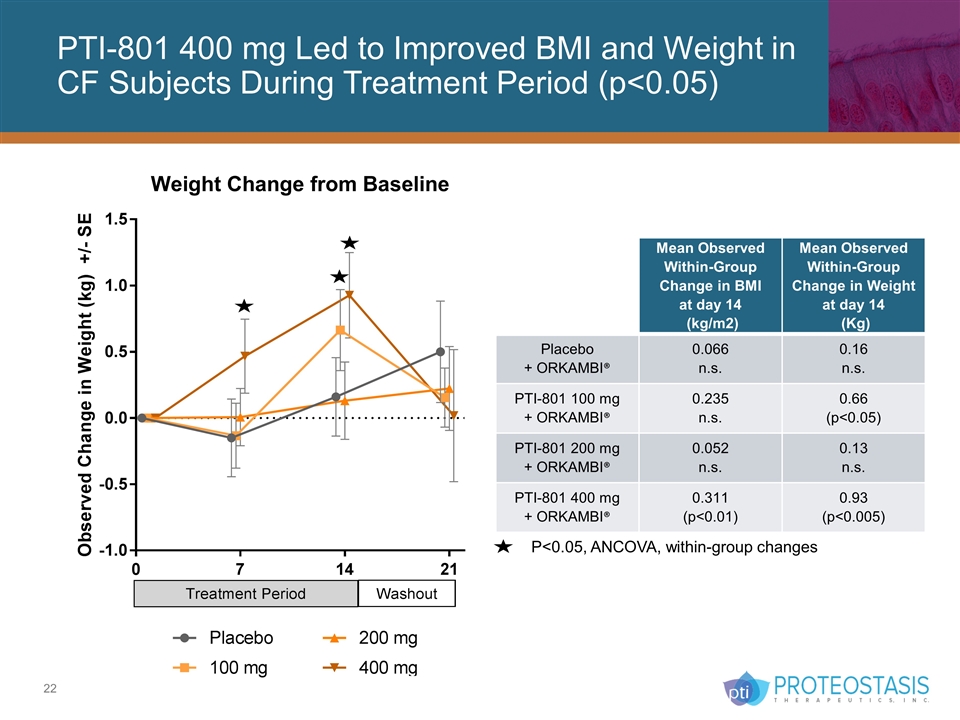
PTI-801 400 mg Led to Improved BMI and Weight in CF Subjects During Treatment Period (p<0.05) P<0.05, ANCOVA, within-group changes Mean Observed Within-Group Change in BMI at day 14 (kg/m2) Mean Observed Within-Group Change in Weight at day 14 (Kg) Placebo + ORKAMBI® 0.066 n.s. 0.16 n.s. PTI-801 100 mg + ORKAMBI® 0.235 n.s. 0.66 (p<0.05) PTI-801 200 mg + ORKAMBI® 0.052 n.s. 0.13 n.s. PTI-801 400 mg + ORKAMBI® 0.311 (p<0.01) 0.93 (p<0.005)
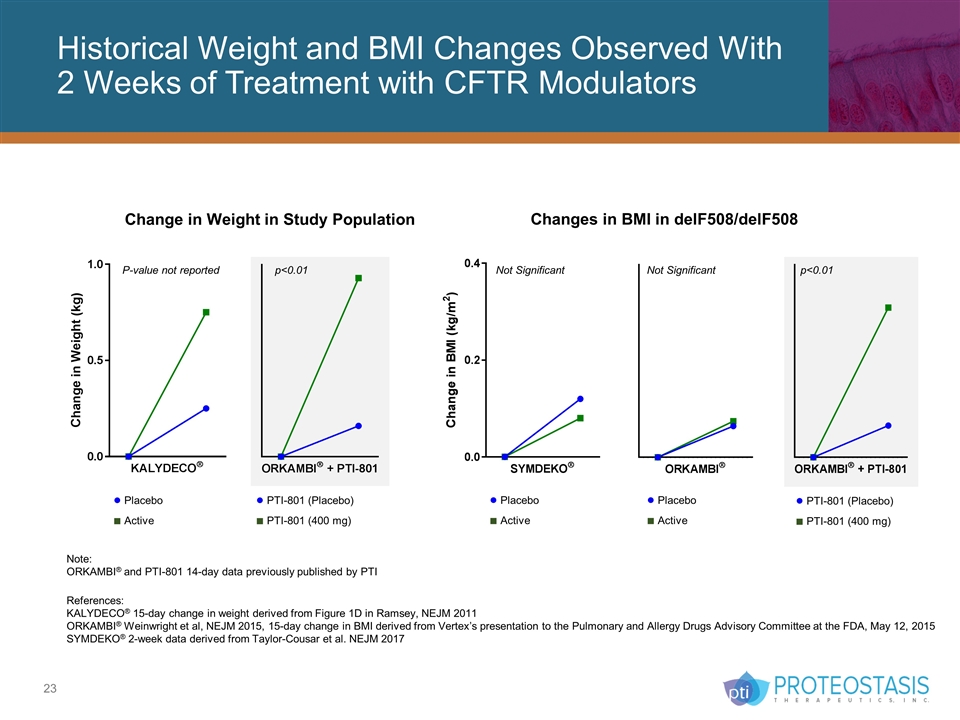
Historical Weight and BMI Changes Observed With 2 Weeks of Treatment with CFTR Modulators Change in Weight in Study Population Changes in BMI in delF508/delF508 Note: ORKAMBI® and PTI-801 14-day data previously published by PTI References: KALYDECO® 15-day change in weight derived from Figure 1D in Ramsey, NEJM 2011 ORKAMBI® Weinwright et al, NEJM 2015, 15-day change in BMI derived from Vertex’s presentation to the Pulmonary and Allergy Drugs Advisory Committee at the FDA, May 12, 2015 SYMDEKO® 2-week data derived from Taylor-Cousar et al. NEJM 2017 Not Significant Not Significant p<0.01 ● PTI-801 (Placebo) ■ PTI-801 (400 mg) p<0.01 P-value not reported ● Placebo ■ Active ● Placebo ■ Active ● Placebo ■ Active ● PTI-801 (Placebo) ■ PTI-801 (400 mg)
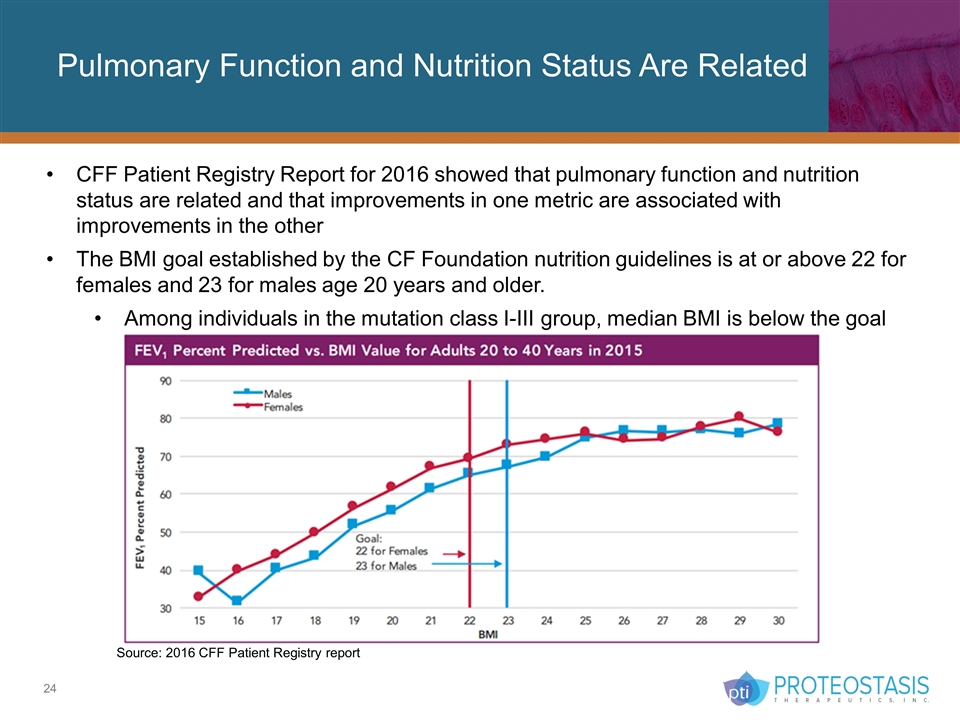
Pulmonary Function and Nutrition Status Are Related CFF Patient Registry Report for 2016 showed that pulmonary function and nutrition status are related and that improvements in one metric are associated with improvements in the other The BMI goal established by the CF Foundation nutrition guidelines is at or above 22 for females and 23 for males age 20 years and older. Among individuals in the mutation class I-III group, median BMI is below the goal Source: 2016 CFF Patient Registry report
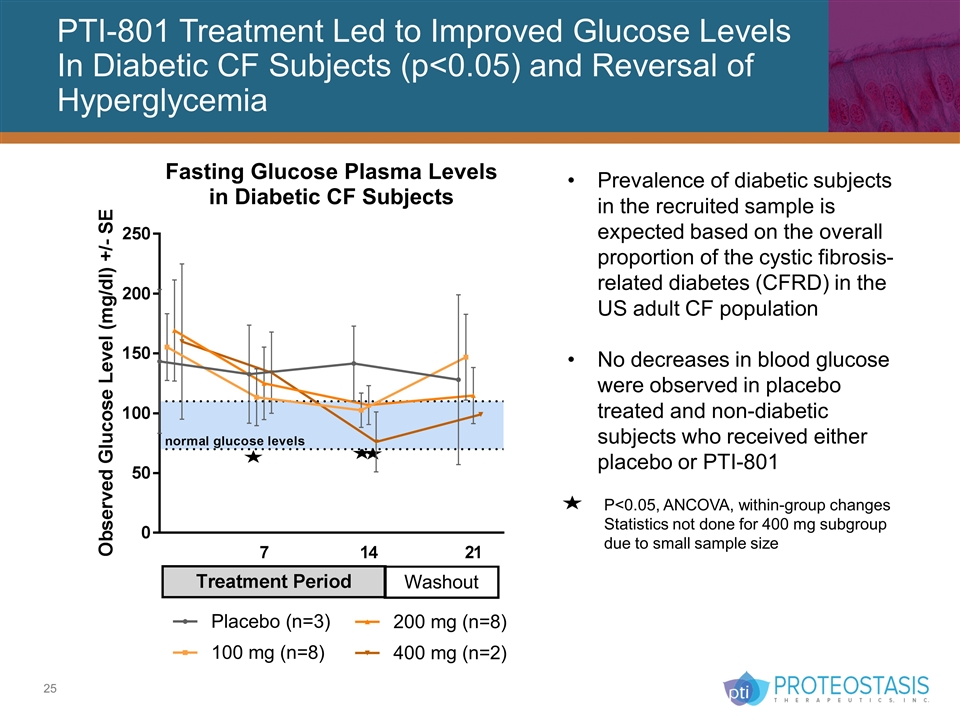
PTI-801 Treatment Led to Improved Glucose Levels In Diabetic CF Subjects (p<0.05) and Reversal of Hyperglycemia P<0.05, ANCOVA, within-group changes Statistics not done for 400 mg subgroup due to small sample size Prevalence of diabetic subjects in the recruited sample is expected based on the overall proportion of the cystic fibrosis-related diabetes (CFRD) in the US adult CF population No decreases in blood glucose were observed in placebo treated and non-diabetic subjects who received either placebo or PTI-801
Cystic fibrosis-related diabetes (CFRD) is a unique type of diabetes that is common in people with CF CFRD shares some features with both type 1 (insulin deficiency) and type 2 (lack of insulin response) diabetes Mouse models suggest CFTR (cystic fibrosis transmembrane regulator) may have a more direct role in insulin secreting, β-cell dysfunction In CF patients with gating mutations, small pilot studies and case reports demonstrated that ivacaftor ameliorated impaired insulin secretion, in some cases resulting in resolution of CFRD In Thomassen et al study with F508del homozygous CF patients, ORKAMBI® did not show improvement in CFRD related endpoints In Small Studies, Ivacaftor Improved Diabetes Related Outcomes in CF Patients References: M.S. Stalvey, et al. Cystic fibrosis transmembrane conductance regulator deficiency exacerbates islet cell dysfunction after beta-cell injury, Diabetes, 2006 Hayes D Jr, et al. Resolution of cystic fibrosis-related diabetes with ivacaftor therapy. American Journal of Respiratory and Critical Care Medicine, 2014 Bellin MD, et al. Insulin secretion improves in cystic fibrosis following ivacaftor correction of CFTR: a small pilot study. Pediatr Diabetes, 2013 JC Thomassen et al, Effect of Lumacaftor/Ivacaftor on glucose metabolism and insulin secretion in Phe508del homozygous cystic fibrosis patients, Journal of CF 2018

PTI-428-01 CF Study Summary
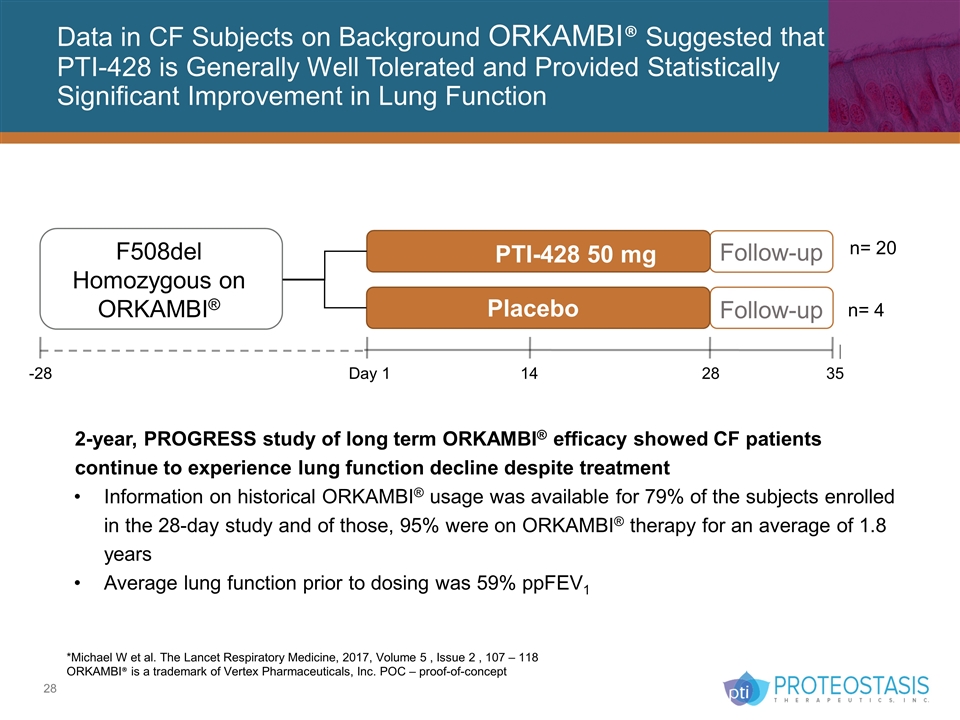
Data in CF Subjects on Background ORKAMBI® Suggested that PTI-428 is Generally Well Tolerated and Provided Statistically Significant Improvement in Lung Function *Michael W et al. The Lancet Respiratory Medicine, 2017, Volume 5 , Issue 2 , 107 – 118 ORKAMBI® is a trademark of Vertex Pharmaceuticals, Inc. POC – proof-of-concept 2-year, PROGRESS study of long term ORKAMBI® efficacy showed CF patients continue to experience lung function decline despite treatment Information on historical ORKAMBI® usage was available for 79% of the subjects enrolled in the 28-day study and of those, 95% were on ORKAMBI® therapy for an average of 1.8 years Average lung function prior to dosing was 59% ppFEV1 Follow-up Follow-up PTI-428 50 mg Placebo F508del Homozygous on ORKAMBI® -28 Day 1 14 28 35 n= 4 n= 20
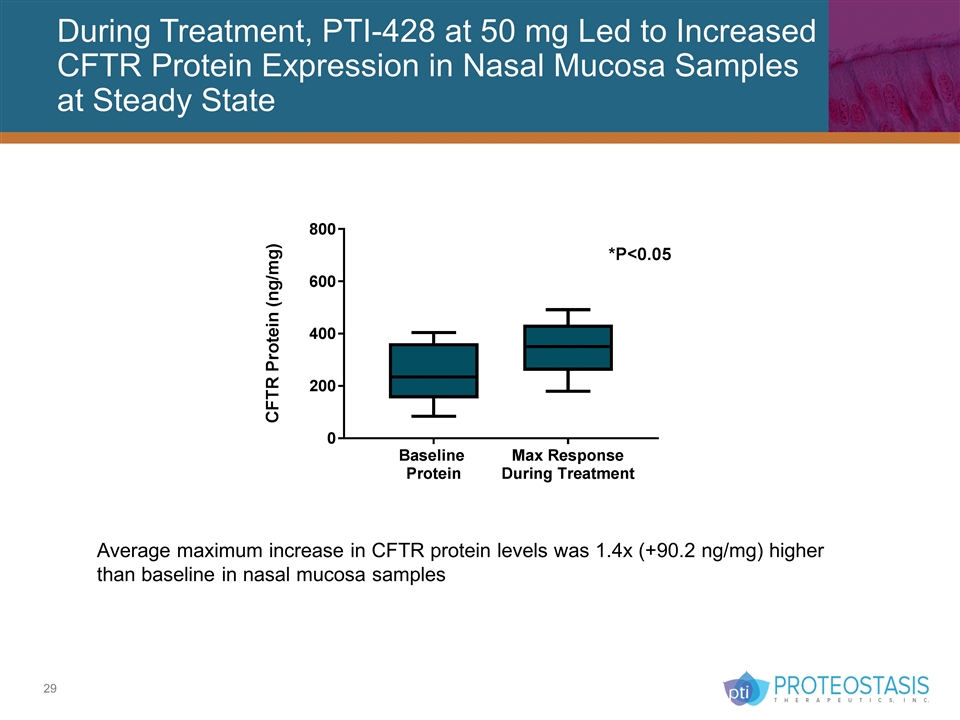
During Treatment, PTI-428 at 50 mg Led to Increased CFTR Protein Expression in Nasal Mucosa Samples at Steady State Average maximum increase in CFTR protein levels was 1.4x (+90.2 ng/mg) higher than baseline in nasal mucosa samples
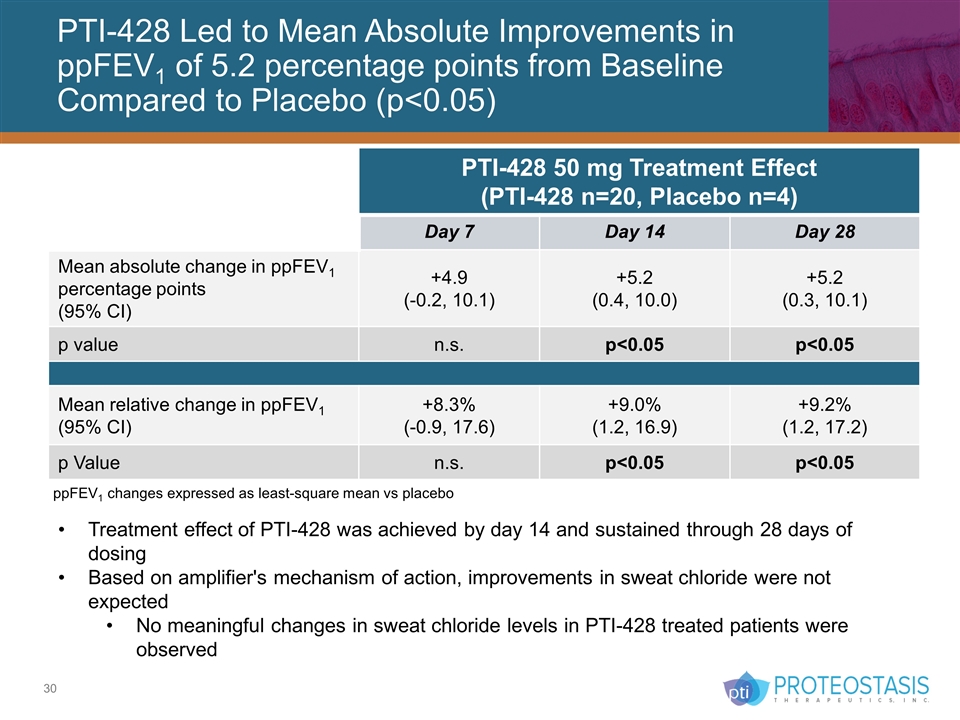
PTI-428 Led to Mean Absolute Improvements in ppFEV1 of 5.2 percentage points from Baseline Compared to Placebo (p<0.05) Treatment effect of PTI-428 was achieved by day 14 and sustained through 28 days of dosing Based on amplifier's mechanism of action, improvements in sweat chloride were not expected No meaningful changes in sweat chloride levels in PTI-428 treated patients were observed PTI-428 50 mg Treatment Effect (PTI-428 n=20, Placebo n=4) Day 7 Day 14 Day 28 Mean absolute change in ppFEV1 percentage points (95% CI) +4.9 (-0.2, 10.1) +5.2 (0.4, 10.0) +5.2 (0.3, 10.1) p value n.s. p<0.05 p<0.05 Mean relative change in ppFEV1 (95% CI) +8.3% (-0.9, 17.6) +9.0% (1.2, 16.9) +9.2% (1.2, 17.2) p Value n.s. p<0.05 p<0.05 ppFEV1 changes expressed as least-square mean vs placebo
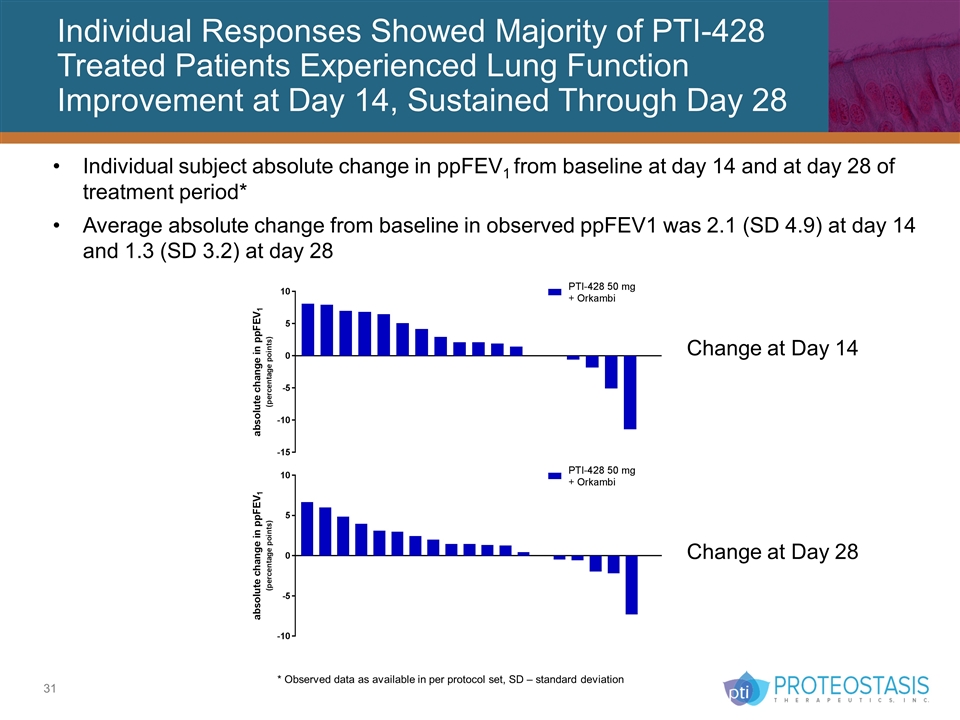
Individual Responses Showed Majority of PTI-428 Treated Patients Experienced Lung Function Improvement at Day 14, Sustained Through Day 28 Individual subject absolute change in ppFEV1 from baseline at day 14 and at day 28 of treatment period* Average absolute change from baseline in observed ppFEV1 was 2.1 (SD 4.9) at day 14 and 1.3 (SD 3.2) at day 28 * Observed data as available in per protocol set, SD – standard deviation Change at Day 14 Change at Day 28
PTI-428 Granted Breakthrough Designation from the FDA PTI-428 received Breakthrough Therapy Designation by FDA in March 2018 for treatment of CF patients homozygous for F508del on background ORKAMBI® therapy Grant was given based on clinical evidence of increased lung function during the 28-day, once-a-day treatment with PTI-428 in CF subjects on ORKAMBI® background The study provides first evidence that genotype agnostic CFTR amplifiers have the potential to provide additional benefit to CF patients on existing CFTR modulator therapy Since the inception of the program, less than half of the drugs submitted for Breakthrough Therapy under the FDA Safety and Innovation Act have been granted the designation. In the CFTR modulator category, BTD has been granted to KALYDECO®, ORKAMBI® and SYMDEKO® PTI has started a clinical study in CF subjects on background SYMDEKO® therapy with initial data expected in early 2019, to support a development path towards NDA
12 Month Key Milestones PTI-808/PTI-801/PTI-428 combination study protocol endorsed with high strategic fit score by CF patient advocacy groups in the U.S. and Europe PTI-801/PTI-808 combination study initiated in CF patients Breakthrough Therapy Designation for PTI-428 granted by the FDA PTI-801 14 day dosing, additional data in CF subjects on ORKAMBI® PTI-808/PTI-801/PTI-428 combination study initiates in CF subjects PTI-428 starts dosing in CF Subjects on SYMDEKO® clinical study PTI-808/PTI-801 initial data from combination study in CF subjects PTI-808/PTI-801/PTI-428 combination study preliminary data in CF subjects Early 2019 initial data from PTI-428 in CF Subjects on SYMDEKO® ORKAMBI® and SYMDEKO® are trademarks of Vertex Pharmaceuticals, Inc. 2018 2018

Thank You