
Proteostasis Therapeutics, Inc. (PTI) November 28, 2018 Exhibit 99.1

Safe Harbor and Disclaimer To the extent that statements in this presentation are not historical facts, they are forward-looking statements reflecting the current beliefs and expectations of management made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. Words such as “aim,” “may,” “will,” “expect,” “anticipate,” “estimate,” “intend,” and similar expressions (as well as other words or expressions referencing future events, conditions or circumstances) are intended to identify forward-looking statements. Examples of forward-looking statements made in this presentation include, without limitation, statements regarding the potential of our proprietary double combination therapy for the treatment of CF, the potential benefit to patients of our proprietary double combination therapy, the expected timing of the initiation of, patient enrollment in, data from, and our completion of, our clinical studies and cohorts for PTI-428, PTI-801, PTI-808 and our combination therapy candidates as well as cash guidance. Forward-looking statements made in this presentation involve substantial risks and uncertainties that could cause actual results to differ materially from those expressed or implied by the forward-looking statements, and we therefore cannot assure you that our plans, intentions, expectations or strategies will be attained or achieved. Such risks and uncertainties include, without limitation, the possibility final or future results from our drug candidate trials (including, without limitation, longer duration studies) do not achieve positive results or are materially and negatively different from or not indicative of the preliminary results reported in this presentation (noting that these results are on a small number of patients and small data set), uncertainties inherent in the execution and completion of clinical trials (including, without limitation, the possibility FDA requires us to run cohorts sequentially or conduct additional cohorts or pre-clinical or clinical studies), in the enrollment of CF patients in our clinical trials, in the timing of availability of trial data, in the results of the clinical trials, in possible adverse events from our trials, in the actions of regulatory agencies, in endorsement, if any, by therapeutic development arms of CF patient advocacy groups, and those set forth in our Annual Report on Form 10-K for the year ended December 31, 2017, our Quarterly Report on Form 10-Q for the quarter ended September 30, 2018, and our other SEC filings. We assume no obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise. This presentation also contains estimates and other statistical data made by independent parties and by us relating to, among other items, disease incidence, market size and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. Projections, assumptions and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of risk and uncertainty. New risks emerge from time to time, and neither we nor any other person makes any representation as to the accuracy or completeness of such data or undertakes any obligation to update such date after the date of this presentation. By attending or receiving this presentation you acknowledge you are solely responsible for your own assessment of the market and our market position and that you will conduct your own analysis and are solely responsible for forming your own view of the potential future performance of our business. The trademarks included in this presentation are the property of the owners thereof and are used for reference purposes only. Such use should not be construed as an endorsement of the Company or its securities.
Introduction to Proteostasis Therapeutics (PTI) Clinical stage biopharmaceutical company developing novel small molecule CFTR modulators for Cystic Fibrosis (CF) PTI-428: Amplifier (increases unfolded CFTR protein) achieved POC as ORKAMBI® add on (Q4’17); add on to Symdeko® POC results expected in Q1’19 PTI-801: Corrector (increases folded CFTR protein) achieved POC as ORKAMBI® add on (Q2’18); add on to Symdeko® POC results expected in Q1’19 PTI-808: Potentiator (increases ion transport across the CFTR protein) Now, CFTR modulator portfolio has begun to demonstrate value of proprietary combinations PTI-801 and PTI-808 as a stand-alone doublet achieved POC (Q4’18) PTI-428, PTI-801, and PTI-808 as a stand-alone triplet POC results expected later this quarter Company has cash to support operations into 2020 ORKAMBI® and SYMDEKO® are registered trademarks of Vertex Pharmaceuticals Inc. POC is proof of concept
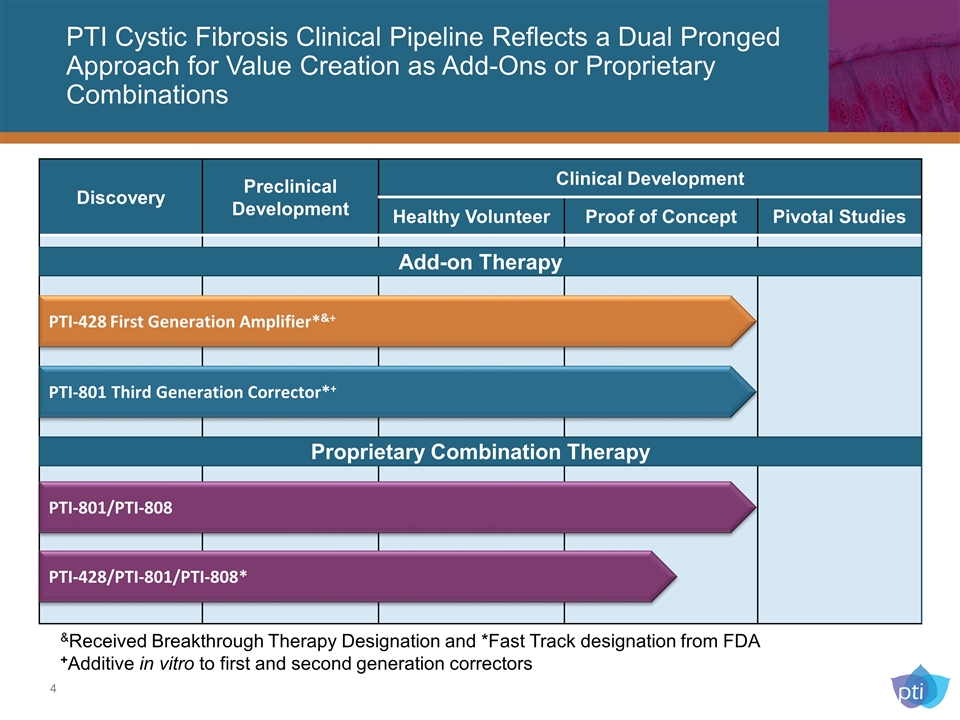
PTI Cystic Fibrosis Clinical Pipeline Reflects a Dual Pronged Approach for Value Creation as Add-Ons or Proprietary Combinations Discovery Preclinical Development Clinical Development Healthy Volunteer Proof of Concept Pivotal Studies PTI-428 First Generation Amplifier*&+ PTI-801 Third Generation Corrector*+ &Received Breakthrough Therapy Designation and *Fast Track designation from FDA +Additive in vitro to first and second generation correctors Proprietary Combination Therapy PTI-428/PTI-801/PTI-808* PTI-801/PTI-808 Add-on Therapy
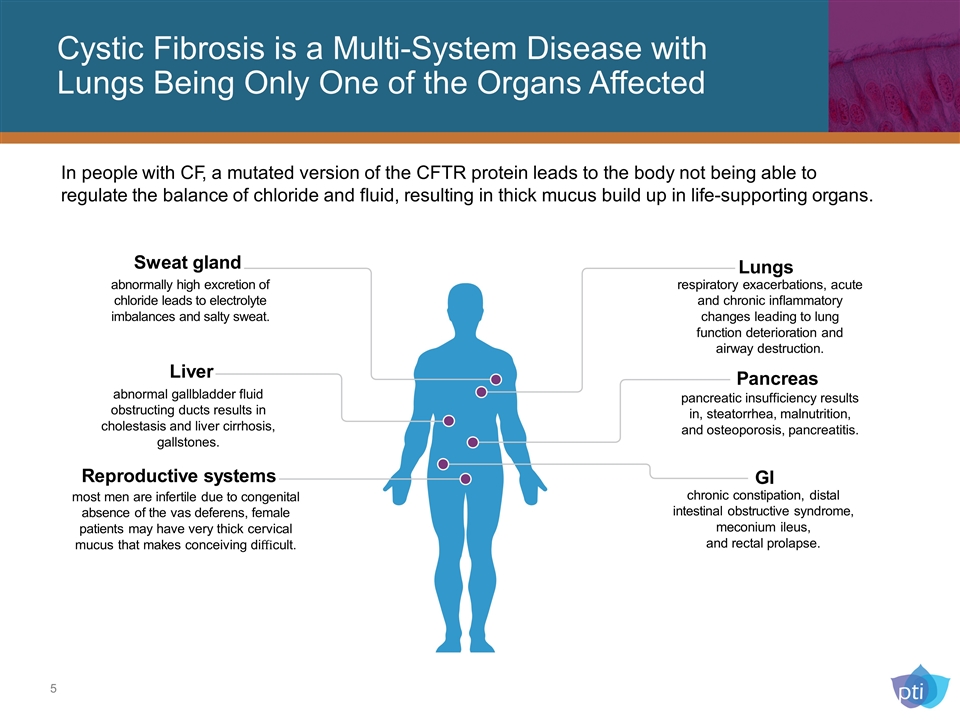
Cystic Fibrosis is a Multi-System Disease with Lungs Being Only One of the Organs Affected Sweat gland Liver GI chronic constipation, distal intestinal obstructive syndrome, meconium ileus, and rectal prolapse. Lungs respiratory exacerbations, acute and chronic inflammatory changes leading to lung function deterioration and airway destruction. Pancreas abnormal gallbladder fluid obstructing ducts results in cholestasis and liver cirrhosis, gallstones. Reproductive systems most men are infertile due to congenital absence of the vas deferens, female patients may have very thick cervical mucus that makes conceiving difficult. In people with CF, a mutated version of the CFTR protein leads to the body not being able to regulate the balance of chloride and fluid, resulting in thick mucus build up in life-supporting organs. abnormally high excretion of chloride leads to electrolyte imbalances and salty sweat. pancreatic insufficiency results in, steatorrhea, malnutrition, and osteoporosis, pancreatitis.
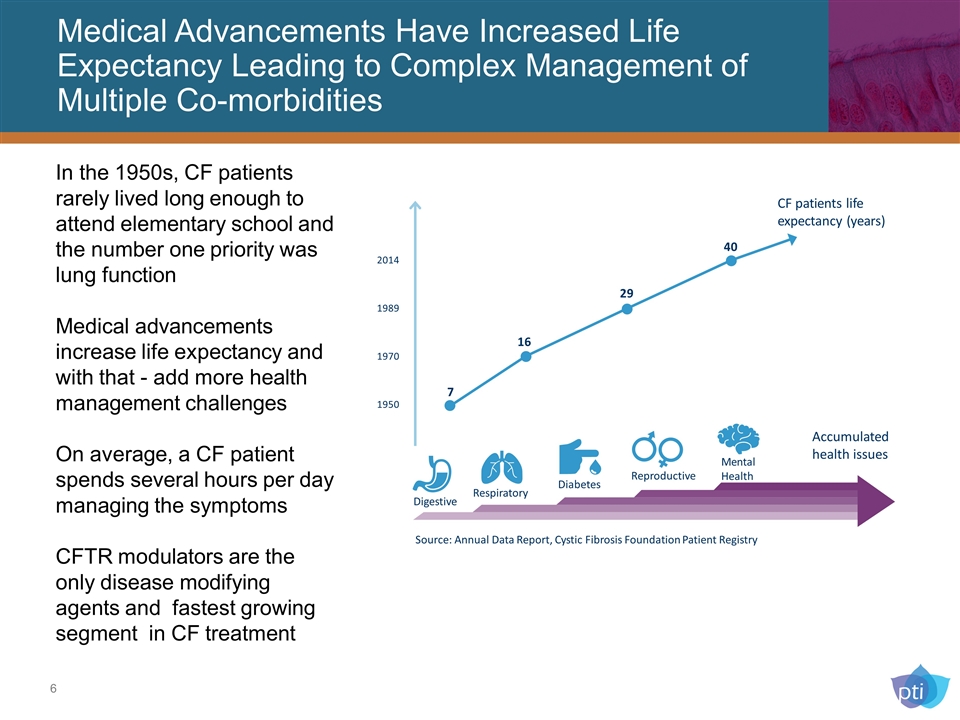
Medical Advancements Have Increased Life Expectancy Leading to Complex Management of Multiple Co-morbidities 1950 CF patients life expectancy (years) Accumulated health issues 16 29 40 1970 1989 2014 Respiratory Mental Health Digestive Diabetes Reproductive 7 In the 1950s, CF patients rarely lived long enough to attend elementary school and the number one priority was lung function Medical advancements increase life expectancy and with that - add more health management challenges On average, a CF patient spends several hours per day managing the symptoms CFTR modulators are the only disease modifying agents and fastest growing segment in CF treatment Source: Annual Data Report, Cystic Fibrosis Foundation Patient Registry
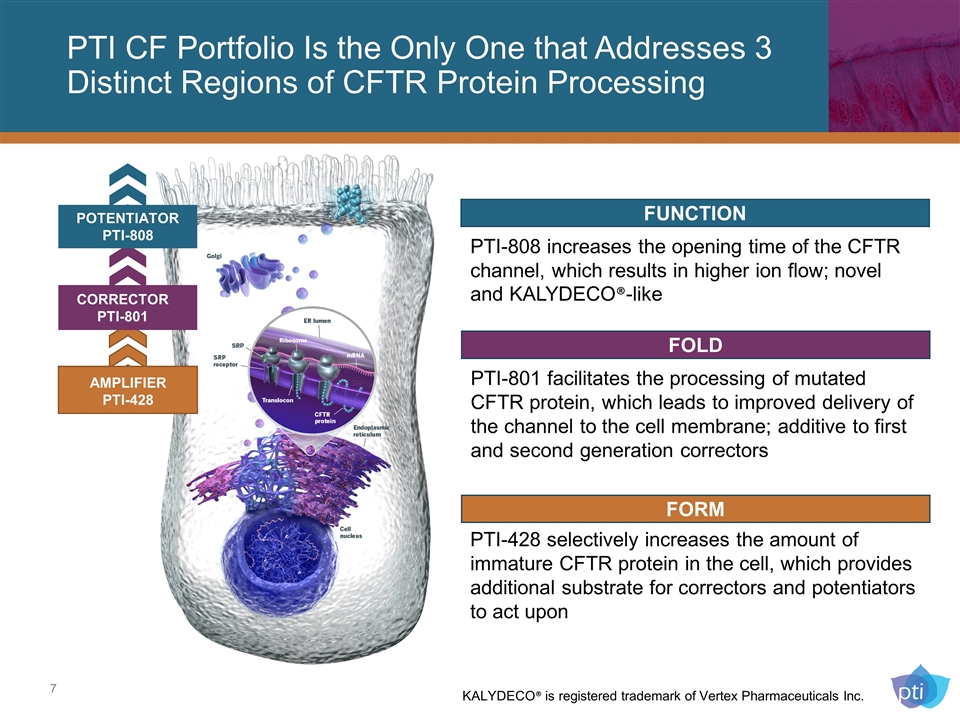
CORRECTOR PTI-801 PTI CF Portfolio Is the Only One that Addresses 3 Distinct Regions of CFTR Protein Processing PTI-808 increases the opening time of the CFTR channel, which results in higher ion flow; novel and KALYDECO®-like POTENTIATOR PTI-808 AMPLIFIER PTI-428 FUNCTION PTI-801 facilitates the processing of mutated CFTR protein, which leads to improved delivery of the channel to the cell membrane; additive to first and second generation correctors FOLD FORM PTI-428 selectively increases the amount of immature CFTR protein in the cell, which provides additional substrate for correctors and potentiators to act upon KALYDECO® is registered trademark of Vertex Pharmaceuticals Inc.
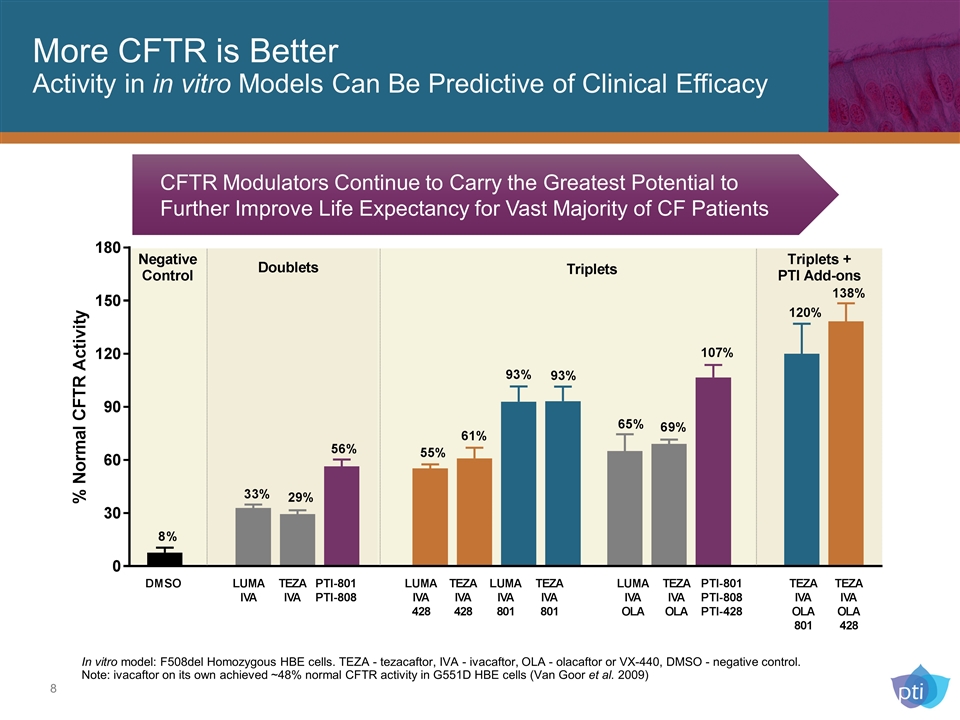
More CFTR is Better Activity in in vitro Models Can Be Predictive of Clinical Efficacy In vitro model: F508del Homozygous HBE cells. TEZA - tezacaftor, IVA - ivacaftor, OLA - olacaftor or VX-440, DMSO - negative control. Note: ivacaftor on its own achieved ~48% normal CFTR activity in G551D HBE cells (Van Goor et al. 2009) CFTR Modulators Continue to Carry the Greatest Potential to Further Improve Life Expectancy for Vast Majority of CF Patients
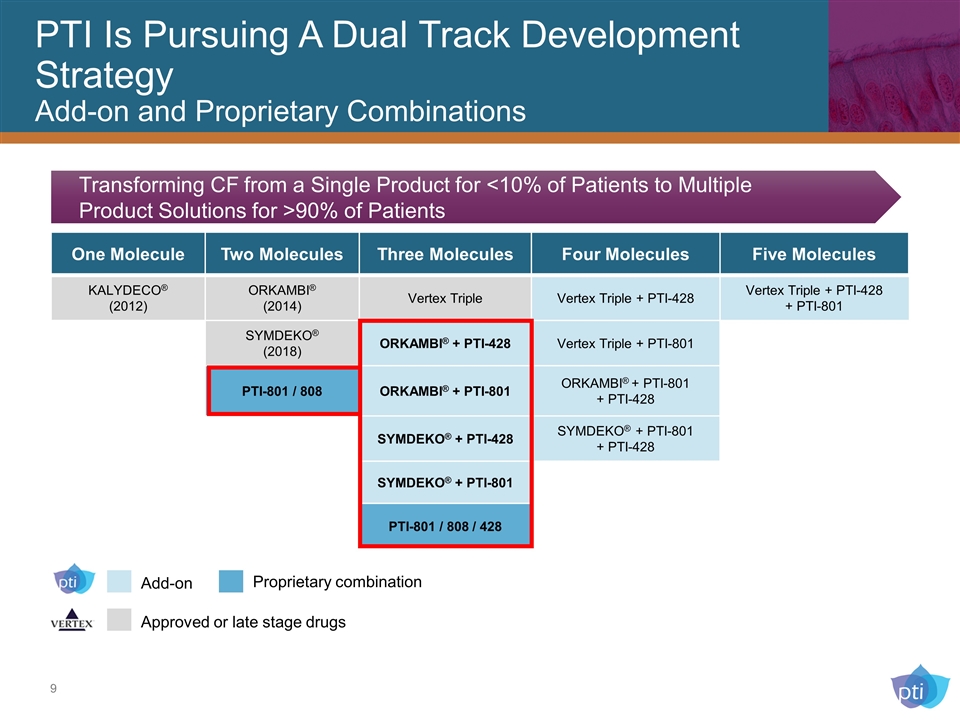
PTI Is Pursuing A Dual Track Development Strategy Add-on and Proprietary Combinations One Molecule Two Molecules Three Molecules Four Molecules Five Molecules KALYDECO® (2012) ORKAMBI® (2014) Vertex Triple Vertex Triple + PTI-428 Vertex Triple + PTI-428 + PTI-801 SYMDEKO® (2018) ORKAMBI® + PTI-428 Vertex Triple + PTI-801 PTI-801 / 808 ORKAMBI® + PTI-801 ORKAMBI® + PTI-801 + PTI-428 SYMDEKO® + PTI-428 SYMDEKO® + PTI-801 + PTI-428 SYMDEKO® + PTI-801 PTI-801 / 808 / 428 Transforming CF from a Single Product for <10% of Patients to Multiple Product Solutions for >90% of Patients Add-on Proprietary combination Approved or late stage drugs
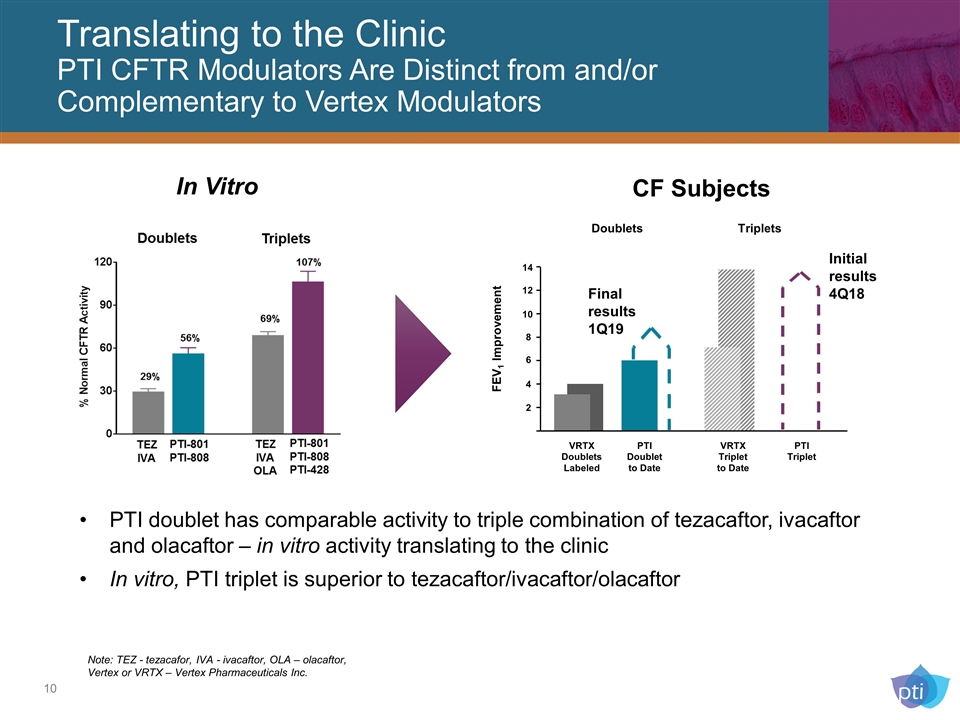
Translating to the Clinic PTI CFTR Modulators Are Distinct from and/or Complementary to Vertex Modulators PTI doublet has comparable activity to triple combination of tezacaftor, ivacaftor and olacaftor – in vitro activity translating to the clinic In vitro, PTI triplet is superior to tezacaftor/ivacaftor/olacaftor 4 2 14 6 8 12 10 VRTX Doublets Labeled PTI Doublet to Date VRTX Triplet to Date PTI Triplet FEV1 Improvement Doublets Triplets Initial results 4Q18 Final results 1Q19 In Vitro CF Subjects Note: TEZ - tezacafor, IVA - ivacaftor, OLA – olacaftor, Vertex or VRTX – Vertex Pharmaceuticals Inc.

Two Prong Strategy Add-on and Proprietary Combination POC Expected in Q1’19 Add-on PTI-428 + luma/iva PTI-428 + teza/iva PTI-801 + luma/iva PTI-801 + teza/iva PTI-801 + PTI-808 PTI-801 + PTI-808 + PTI-428 Proprietary combinations Pivotal trials POC expected in Q4’18 to Q1’19
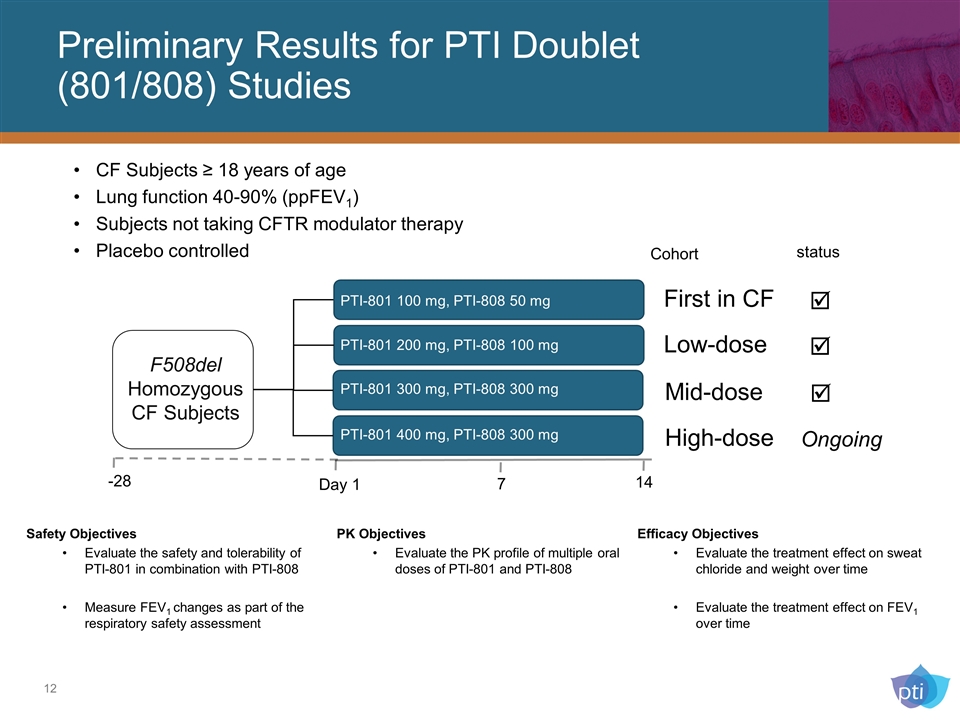
Preliminary Results for PTI Doublet (801/808) Studies F508del Homozygous CF Subjects -28 Day 1 7 14 placebo PTI-801 100 mg, PTI-808 50 mg PTI-801 200 mg, PTI-808 100 mg Cohort First in CF Low-dose Mid-dose High-dose PTI-801 300 mg, PTI-808 300 mg PTI-801 400 mg, PTI-808 300 mg CF Subjects ≥ 18 years of age Lung function 40-90% (ppFEV1) Subjects not taking CFTR modulator therapy Placebo controlled Safety Objectives Evaluate the safety and tolerability of PTI-801 in combination with PTI-808 Measure FEV1 changes as part of the respiratory safety assessment Efficacy Objectives Evaluate the treatment effect on sweat chloride and weight over time Evaluate the treatment effect on FEV1 over time PK Objectives Evaluate the PK profile of multiple oral doses of PTI-801 and PTI-808 þ þ status þ Ongoing
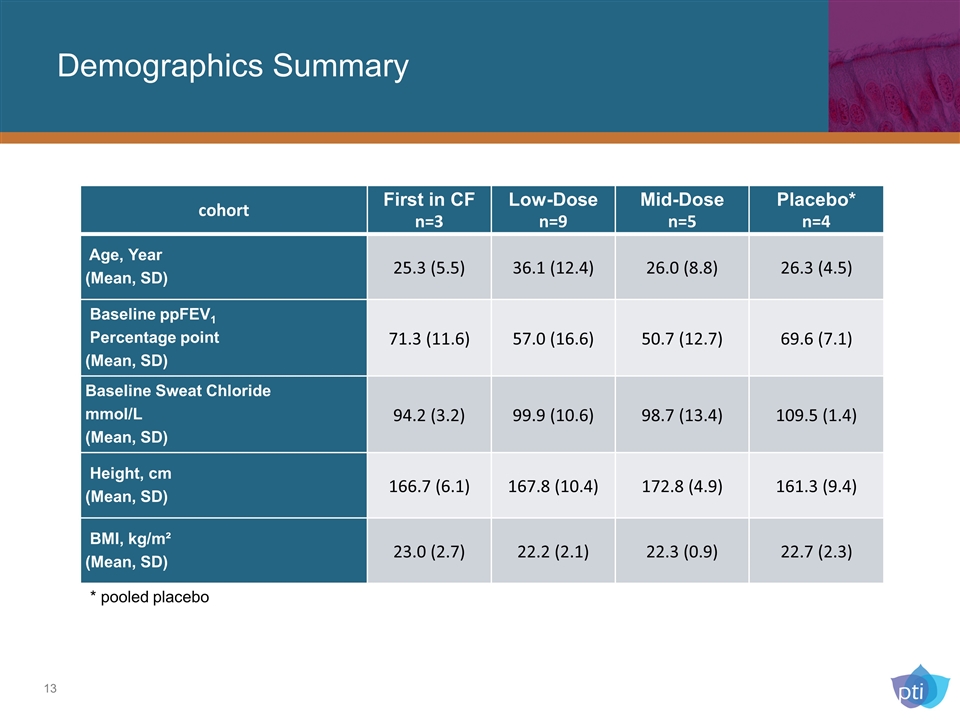
Demographics Summary cohort First in CF n=3 Low-Dose n=9 Mid-Dose n=5 Placebo* n=4 Age, Year (Mean, SD) 25.3 (5.5) 36.1 (12.4) 26.0 (8.8) 26.3 (4.5) Baseline ppFEV1 Percentage point (Mean, SD) 71.3 (11.6) 57.0 (16.6) 50.7 (12.7) 69.6 (7.1) Baseline Sweat Chloride mmol/L (Mean, SD) 94.2 (3.2) 99.9 (10.6) 98.7 (13.4) 109.5 (1.4) Height, cm (Mean, SD) 166.7 (6.1) 167.8 (10.4) 172.8 (4.9) 161.3 (9.4) BMI, kg/m² (Mean, SD) 23.0 (2.7) 22.2 (2.1) 22.3 (0.9) 22.7 (2.3) * pooled placebo
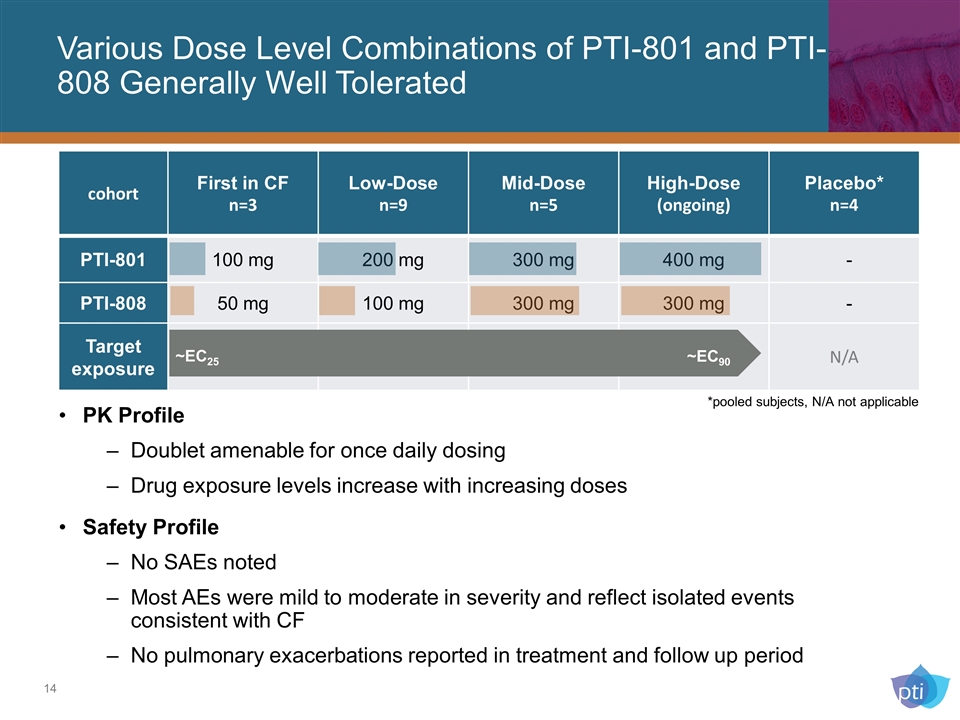
Various Dose Level Combinations of PTI-801 and PTI-808 Generally Well Tolerated cohort First in CF n=3 Low-Dose n=9 Mid-Dose n=5 High-Dose (ongoing) Placebo* n=4 PTI-801 100 mg 200 mg 300 mg 400 mg - PTI-808 50 mg 100 mg 300 mg 300 mg - Target exposure N/A *pooled subjects, N/A not applicable ~EC25 ~EC90 PK Profile Doublet amenable for once daily dosing Drug exposure levels increase with increasing doses Safety Profile No SAEs noted Most AEs were mild to moderate in severity and reflect isolated events consistent with CF No pulmonary exacerbations reported in treatment and follow up period
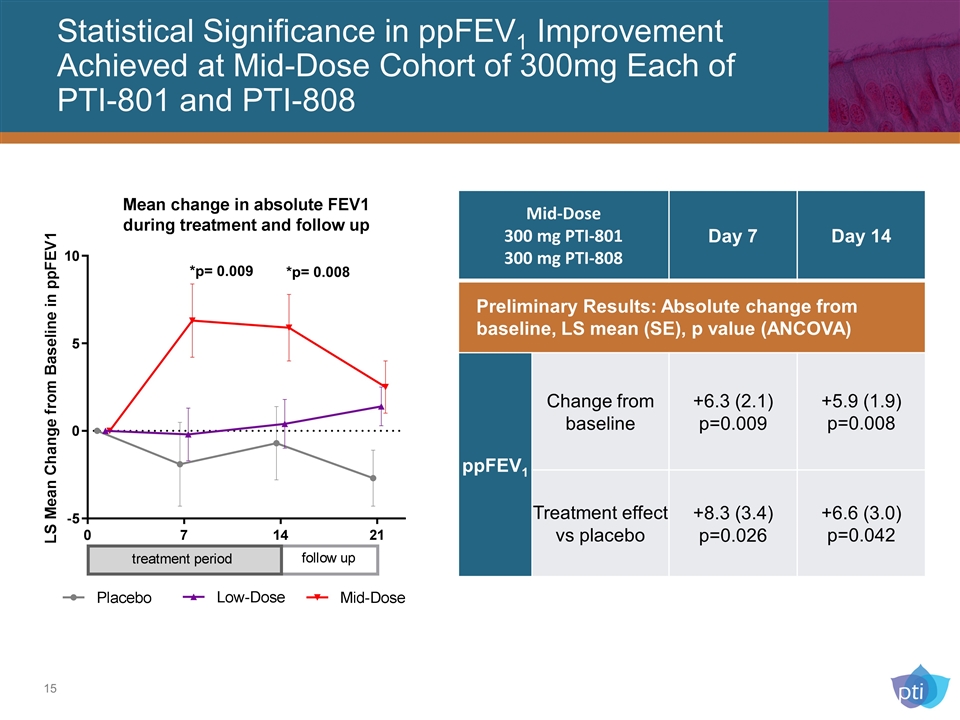
Statistical Significance in ppFEV1 Improvement Achieved at Mid-Dose Cohort of 300mg Each of PTI-801 and PTI-808 *p= 0.009 *p= 0.008 Mid-Dose 300 mg PTI-801 300 mg PTI-808 Day 7 Day 14 Preliminary Results: Absolute change from baseline, LS mean (SE), p value (ANCOVA) ppFEV1 Change from baseline +6.3 (2.1) p=0.009 +5.9 (1.9) p=0.008 Treatment effect vs placebo +8.3 (3.4) p=0.026 +6.6 (3.0) p=0.042
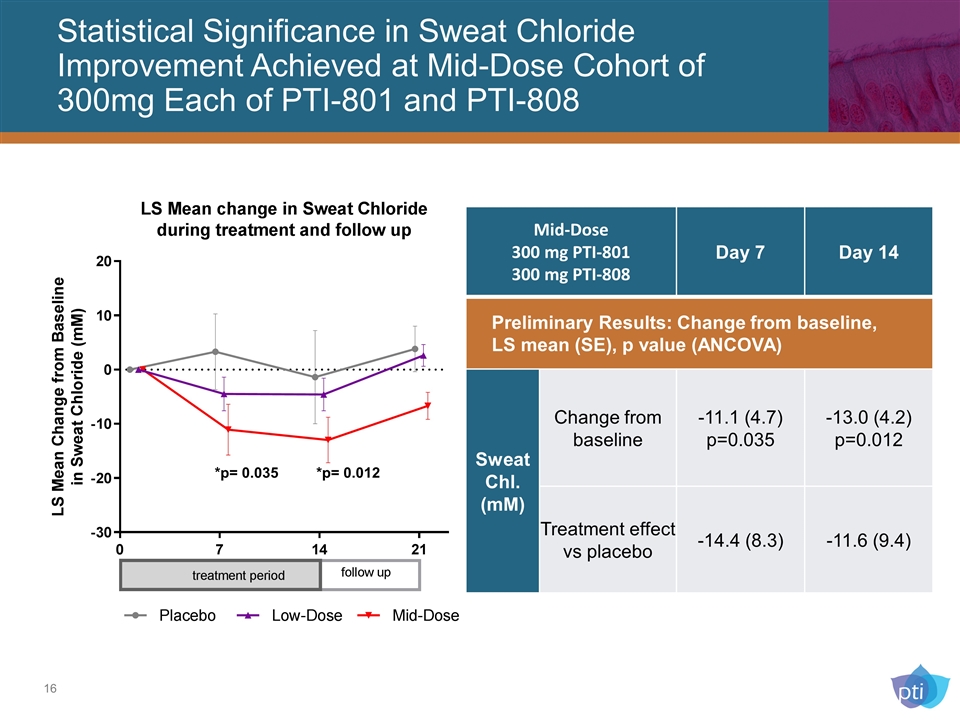
Statistical Significance in Sweat Chloride Improvement Achieved at Mid-Dose Cohort of 300mg Each of PTI-801 and PTI-808 *p= 0.035 *p= 0.012 Mid-Dose 300 mg PTI-801 300 mg PTI-808 Day 7 Day 14 Preliminary Results: Change from baseline, LS mean (SE), p value (ANCOVA) Sweat Chl. (mM) Change from baseline -11.1 (4.7) p=0.035 -13.0 (4.2) p=0.012 Treatment effect vs placebo -14.4 (8.3) -11.6 (9.4)

Dose Response Achieved in ppFEV1 and Sweat Chloride Across Cohorts ongoing * p<0.05 Day 14 ppFEV1 Day 14 Sweat Chloride ongoing Highest dose cohort dosing underway; initial data expected in Q1’19
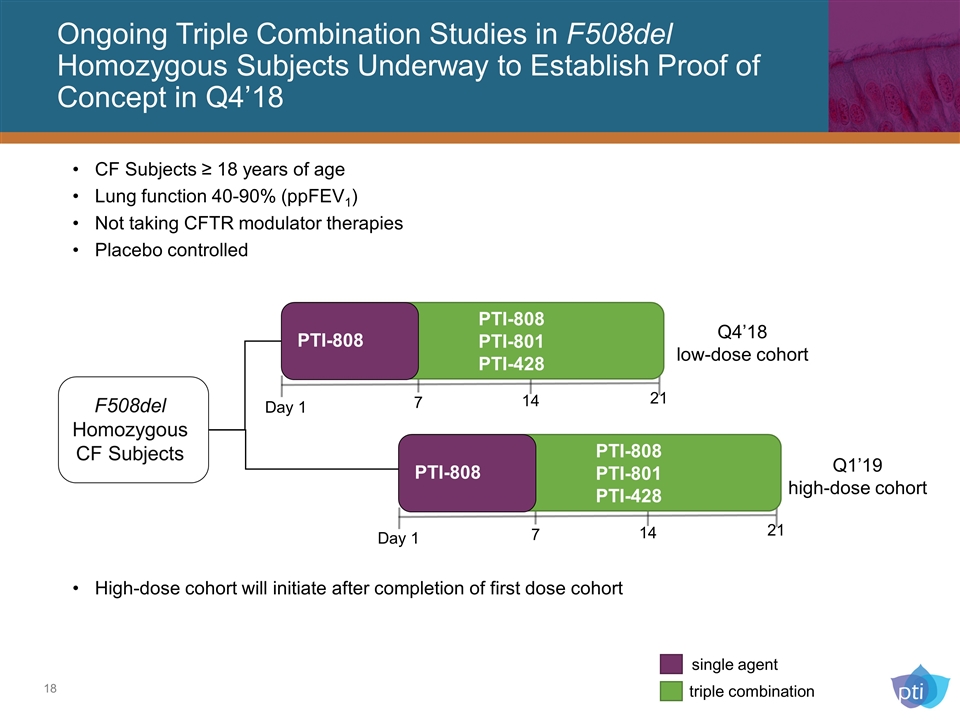
Ongoing Triple Combination Studies in F508del Homozygous Subjects Underway to Establish Proof of Concept in Q4’18 Placebo F508del Homozygous CF Subjects PTI-808 PTI-808 PTI-801 PTI-428 single agent triple combination CF Subjects ≥ 18 years of age Lung function 40-90% (ppFEV1) Not taking CFTR modulator therapies Placebo controlled Day 1 7 14 21 High-dose cohort will initiate after completion of first dose cohort Placebo PTI-808 PTI-808 PTI-801 PTI-428 7 14 21 Day 1 Q4’18 low-dose cohort Q1’19 high-dose cohort
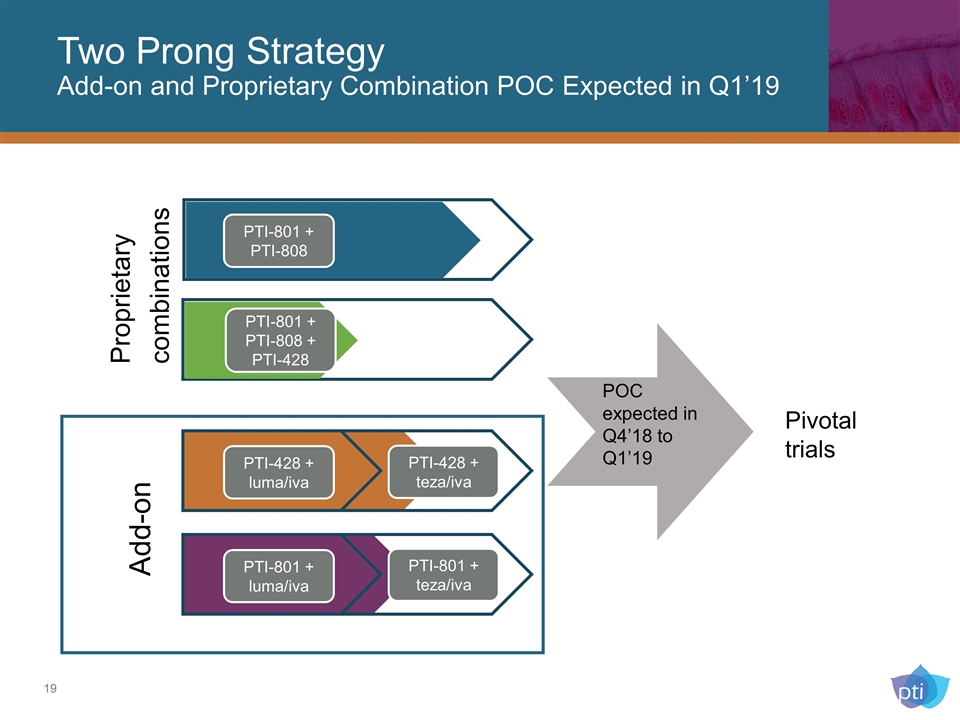
Two Prong Strategy Add-on and Proprietary Combination POC Expected in Q1’19 Add-on PTI-428 + luma/iva PTI-428 + teza/iva PTI-801 + luma/iva PTI-801 + teza/iva PTI-801 + PTI-808 PTI-801 + PTI-808 + PTI-428 Proprietary combinations Pivotal trials POC expected in Q4’18 to Q1’19
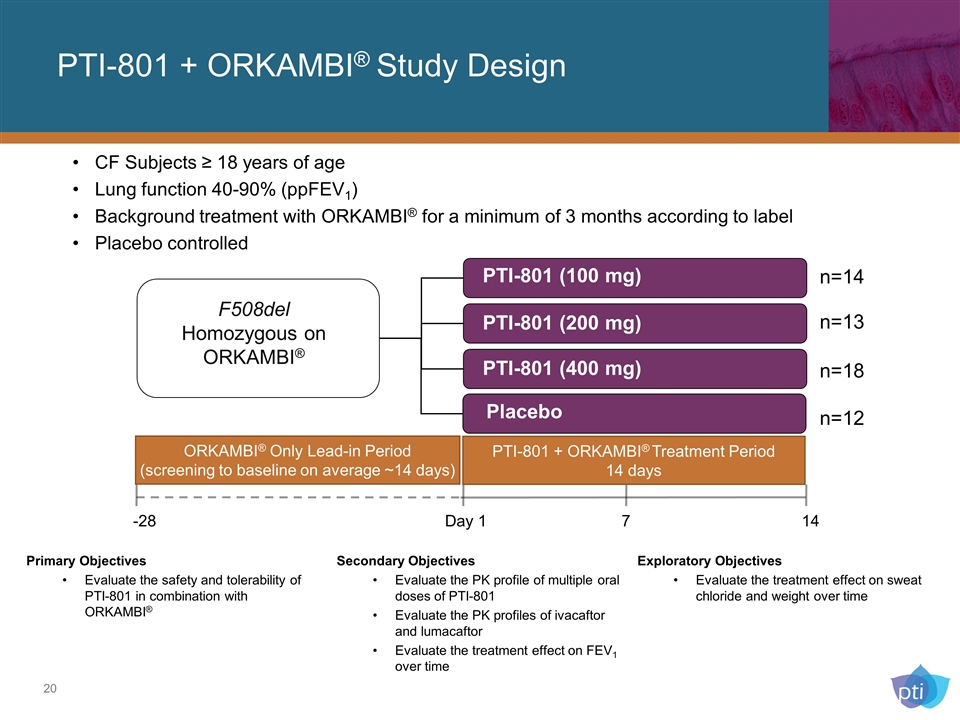
CF Subjects ≥ 18 years of age Lung function 40-90% (ppFEV1) Background treatment with ORKAMBI® for a minimum of 3 months according to label Placebo controlled Placebo F508del Homozygous on ORKAMBI® -28 Day 1 7 14 PTI-801 (400 mg) PTI-801 (200 mg) PTI-801 (100 mg) n=14 n=13 n=18 n=12 Primary Objectives Evaluate the safety and tolerability of PTI-801 in combination with ORKAMBI® Exploratory Objectives Evaluate the treatment effect on sweat chloride and weight over time Secondary Objectives Evaluate the PK profile of multiple oral doses of PTI-801 Evaluate the PK profiles of ivacaftor and lumacaftor Evaluate the treatment effect on FEV1 over time PTI-801 + ORKAMBI® Study Design ORKAMBI® Only Lead-in Period (screening to baseline on average ~14 days) PTI-801 + ORKAMBI® Treatment Period 14 days
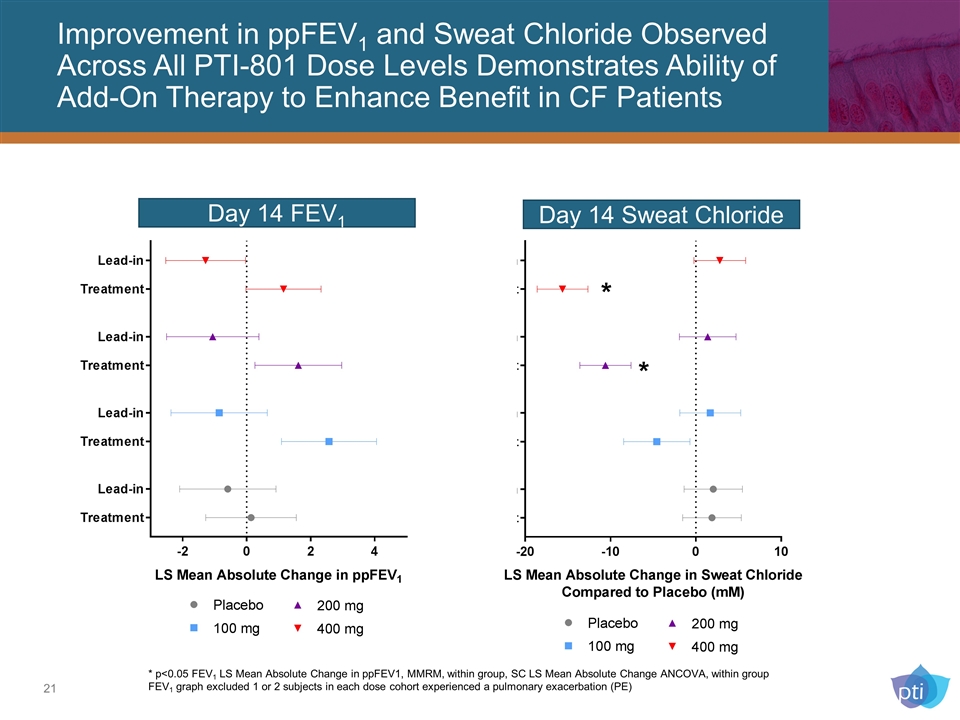
Improvement in ppFEV1 and Sweat Chloride Observed Across All PTI-801 Dose Levels Demonstrates Ability of Add-On Therapy to Enhance Benefit in CF Patients Day 14 FEV1 * p<0.05 FEV1 LS Mean Absolute Change in ppFEV1, MMRM, within group, SC LS Mean Absolute Change ANCOVA, within group FEV1 graph excluded 1 or 2 subjects in each dose cohort experienced a pulmonary exacerbation (PE) Day 14 Sweat Chloride * *
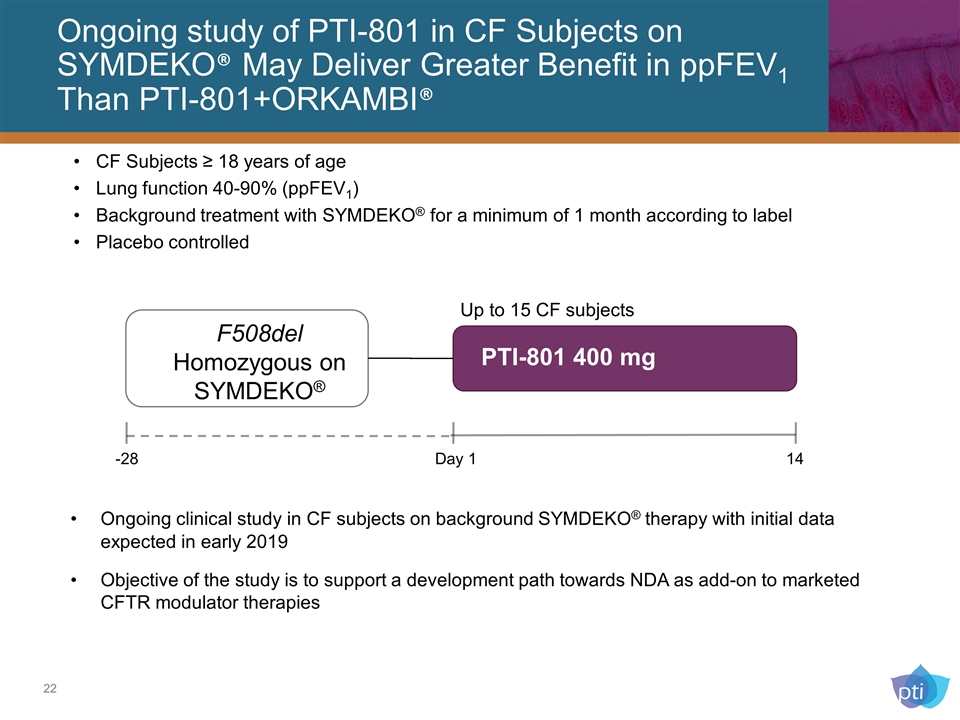
Ongoing study of PTI-801 in CF Subjects on SYMDEKO® May Deliver Greater Benefit in ppFEV1 Than PTI-801+ORKAMBI® PTI-801 400 mg F508del Homozygous on SYMDEKO® -28 Day 1 14 Ongoing clinical study in CF subjects on background SYMDEKO® therapy with initial data expected in early 2019 Objective of the study is to support a development path towards NDA as add-on to marketed CFTR modulator therapies Up to 15 CF subjects CF Subjects ≥ 18 years of age Lung function 40-90% (ppFEV1) Background treatment with SYMDEKO® for a minimum of 1 month according to label Placebo controlled
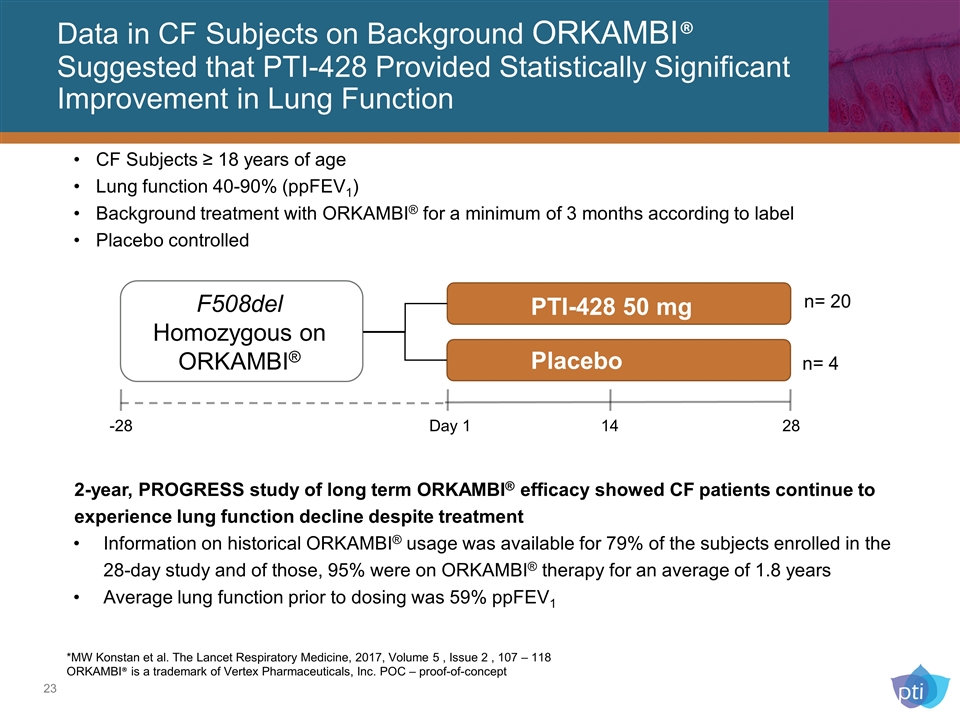
Data in CF Subjects on Background ORKAMBI® Suggested that PTI-428 Provided Statistically Significant Improvement in Lung Function *MW Konstan et al. The Lancet Respiratory Medicine, 2017, Volume 5 , Issue 2 , 107 – 118 ORKAMBI® is a trademark of Vertex Pharmaceuticals, Inc. POC – proof-of-concept 2-year, PROGRESS study of long term ORKAMBI® efficacy showed CF patients continue to experience lung function decline despite treatment Information on historical ORKAMBI® usage was available for 79% of the subjects enrolled in the 28-day study and of those, 95% were on ORKAMBI® therapy for an average of 1.8 years Average lung function prior to dosing was 59% ppFEV1 PTI-428 50 mg Placebo F508del Homozygous on ORKAMBI® -28 Day 1 14 28 n= 4 n= 20 CF Subjects ≥ 18 years of age Lung function 40-90% (ppFEV1) Background treatment with ORKAMBI® for a minimum of 3 months according to label Placebo controlled
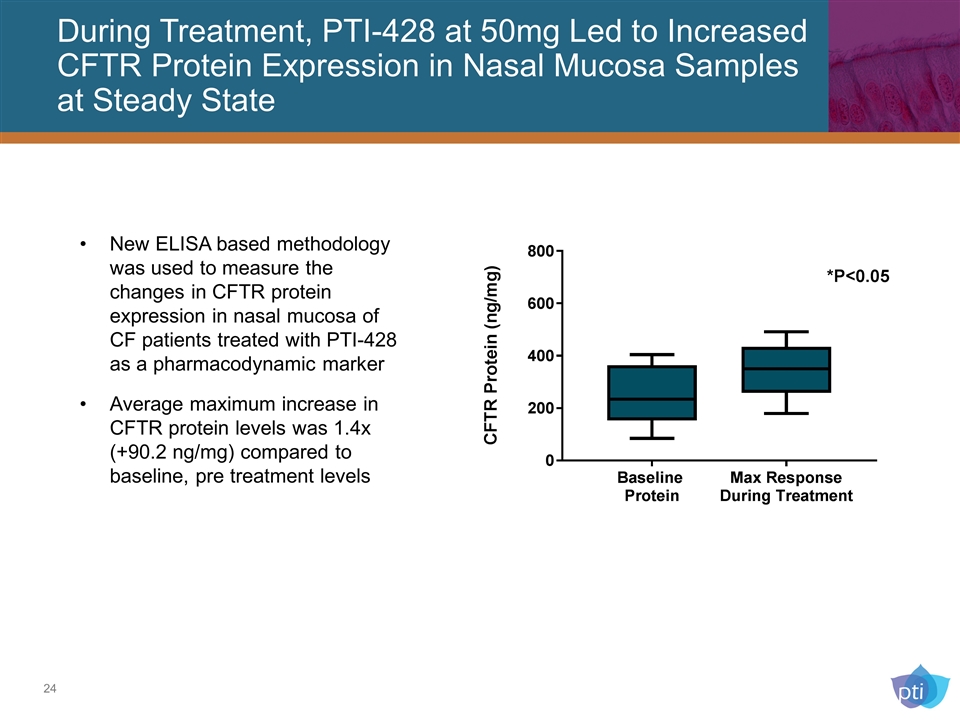
During Treatment, PTI-428 at 50mg Led to Increased CFTR Protein Expression in Nasal Mucosa Samples at Steady State New ELISA based methodology was used to measure the changes in CFTR protein expression in nasal mucosa of CF patients treated with PTI-428 as a pharmacodynamic marker Average maximum increase in CFTR protein levels was 1.4x (+90.2 ng/mg) compared to baseline, pre treatment levels
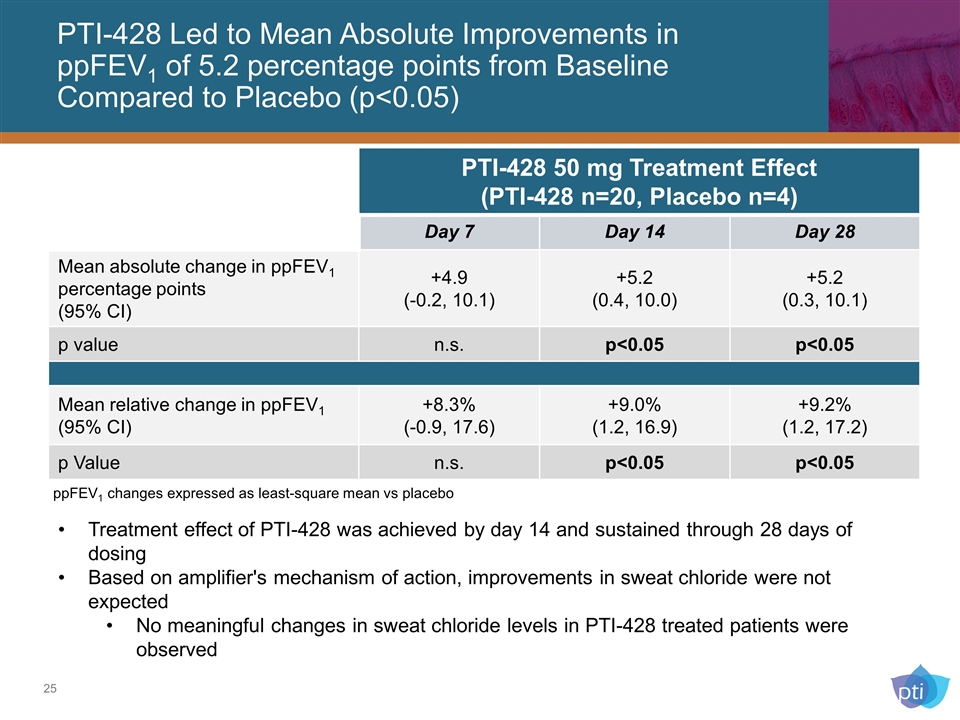
PTI-428 Led to Mean Absolute Improvements in ppFEV1 of 5.2 percentage points from Baseline Compared to Placebo (p<0.05) Treatment effect of PTI-428 was achieved by day 14 and sustained through 28 days of dosing Based on amplifier's mechanism of action, improvements in sweat chloride were not expected No meaningful changes in sweat chloride levels in PTI-428 treated patients were observed PTI-428 50 mg Treatment Effect (PTI-428 n=20, Placebo n=4) Day 7 Day 14 Day 28 Mean absolute change in ppFEV1 percentage points (95% CI) +4.9 (-0.2, 10.1) +5.2 (0.4, 10.0) +5.2 (0.3, 10.1) p value n.s. p<0.05 p<0.05 Mean relative change in ppFEV1 (95% CI) +8.3% (-0.9, 17.6) +9.0% (1.2, 16.9) +9.2% (1.2, 17.2) p Value n.s. p<0.05 p<0.05 ppFEV1 changes expressed as least-square mean vs placebo

Ongoing Study PTI-428 in CF Subjects on SYMDEKO® May Deliver Greater Benefit in FEV1 Than PTI-428+ ORKAMBI® PTI-428 dose 1 F508del Homozygous on SYMDEKO® -28 Day 1 14 28 PTI-428 dose 2 Ongoing clinical study in CF subjects on background SYMDEKO® therapy with initial data expected in early 2019 Objective of the study is to support a development path towards NDA as add-on to marketed CFTR modulator therapies CF Subjects ≥ 18 years of age Lung function 40-90% (ppFEV1) Background treatment with SYMDEKO® for a minimum of 1 month according to label Placebo controlled Up to 40 CF subjects across all cohorts
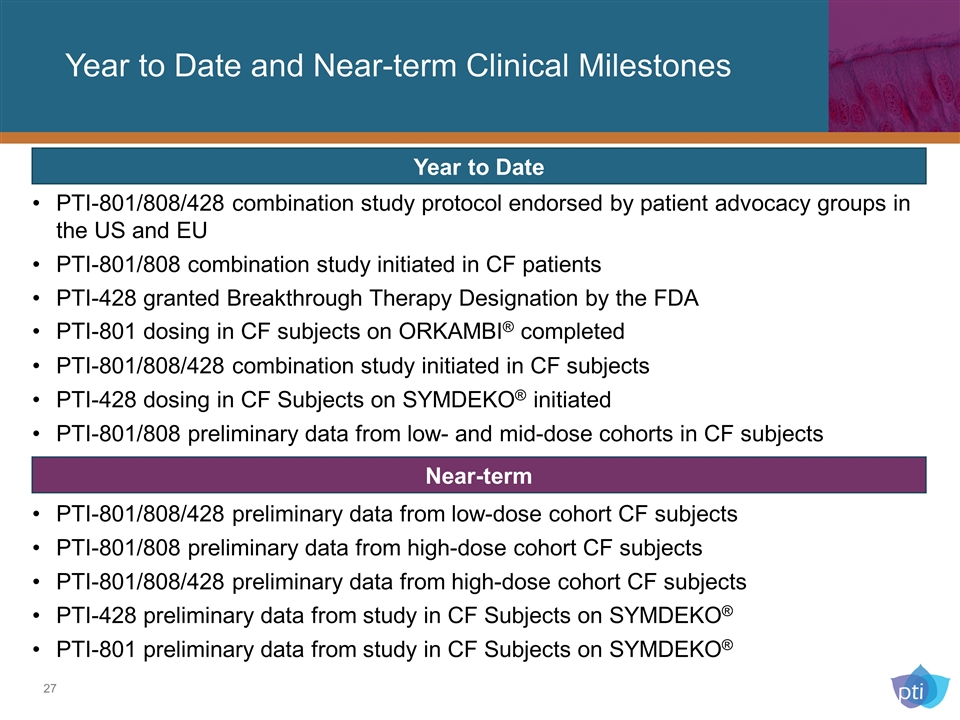
PTI-801/808/428 combination study protocol endorsed by patient advocacy groups in the US and EU PTI-801/808 combination study initiated in CF patients PTI-428 granted Breakthrough Therapy Designation by the FDA PTI-801 dosing in CF subjects on ORKAMBI® completed PTI-801/808/428 combination study initiated in CF subjects PTI-428 dosing in CF Subjects on SYMDEKO® initiated PTI-801/808 preliminary data from low- and mid-dose cohorts in CF subjects Year to Date and Near-term Clinical Milestones Year to Date Near-term PTI-801/808/428 preliminary data from low-dose cohort CF subjects PTI-801/808 preliminary data from high-dose cohort CF subjects PTI-801/808/428 preliminary data from high-dose cohort CF subjects PTI-428 preliminary data from study in CF Subjects on SYMDEKO® PTI-801 preliminary data from study in CF Subjects on SYMDEKO®

Thank You