
RESHAPING CYSTIC FIBROSIS TREATMENT: NOVEL CFTR MODULATOR COMBINATIONS #CForward Corporate Presentation May 13, 2019 Exhibit 99.1

Safe Harbor and Disclaimer To the extent that statements in this presentation are not historical facts, they are forward-looking statements reflecting the current beliefs and expectations of management made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. Words such as “aim,” “may,” “will,” “expect,” “anticipate,” “estimate,” “intend,” and simiforward-looking statements made in this presentation include, without limitation, statements regarding the potential of our proprietary combination therapies for the treatment of CF, the potential benefit to patients of our proprietary combination therapies, the expected timing of the initiation of, patient enrollment in, data from, and our completion of, our clinical studies and cohorts for PTI-428, PTI-801, PTI-808 and our combination therapy candidates, and cash guidance. Forward-looking statements made in this presentation involve substantial risks and uncertainties that could cause actual results to differ materially from those expressed or implied by the forward-looking statements, and we therefore cannot assure you that our plans, intentions, expectations or strategies will be attained or achieved. Such risks and uncertainties include, without limitation, the possibility that final or future results from our drug candidate trials (including, without limitation, longer duration studies) do not achieve positive results or are materially and negatively different from or not indicative of the preliminary results reported in this presentation (noting that these results are on a small number of patients and small data set), uncertainties inherent in the execution and completion of clinical trials (including, without limitation, the possibility FDA requires us to run cohorts sequentially or conduct additional cohorts or pre-clinical or clinical studies), in the enrollment of CF patients in our clinical trials, in the timing of availability of trial data, in the results of the clinical trials, in possible adverse events from our trials, in the actions of regulatory agencies, in endorsement, if any, by CF patient advocacy groups, and those set forth in our Annual Report on Form 10-K for the year ended December 31, 2018, our Quarterly Report on Form 10-Q for the period ended March 31, 2019, and our other SEC filings. We assume no obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise. This presentation also contains estimates and other statistical data made by independent parties and by us relating to, among other items, disease incidence, market size and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. Projections, assumptions and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of risk and uncertainty. New risks emerge from time to time, and neither we nor any other person makes any representation as to the accuracy or completeness of such data or undertakes any obligation to update such date after the date of this presentation. By attending or receiving this presentation you acknowledge you are solely responsible for your own assessment of the market and our market position and that you will conduct your own analysis and are solely responsible for forming your own view of the potential future performance of our business. The trademarks included in this presentation are the property of the owners thereof and are used for reference purposes only. Such use should not be construed as an endorsement of the Company or its securities. 2

Highlights May 2019 Expanded leadership team Appointment of Badrul A. Chowdhury, M.D., Ph.D., to Board of Directors. Dr. Chowdhury is former Director of the Division of Pulmonary, Allergy, and Rheumatology Products at the U.S. FDA’s Center for Drug Evaluation and Research (CDER) and has presided over approvals of all current CFTR modulators Clinical Progress 3 novel CFTR modulators with clinical proof of efficacy data across multiple studies in over 240 CF patients Potential best-in-class corrector/potentiator double combination identified On track to complete double and triple combinations in 28-day studies Phase 3 initiation remains on track for mid-2020 Financially solid $105M at the end of Q1, sufficient to fund operations into 2021 3

Cystic Fibrosis Market Opportunity Cystic Fibrosis is a rare and life limiting genetic disease Over 80,000 individuals live with CF in US, Canada, Europe and Australia with many more in regions that do not have established patient registries CFTR modulators are the only validated disease modifying treatment options but approved drugs still leave significant unaddressed medical need: Limited access due to lack of reimbursement Individual variability in response and tolerability to available products Neglected genotypes CFTR modulator market projected to grow from $3B (2018) to over $6B (2023) driven by approval of new therapies, premium pricing and monopoly 3
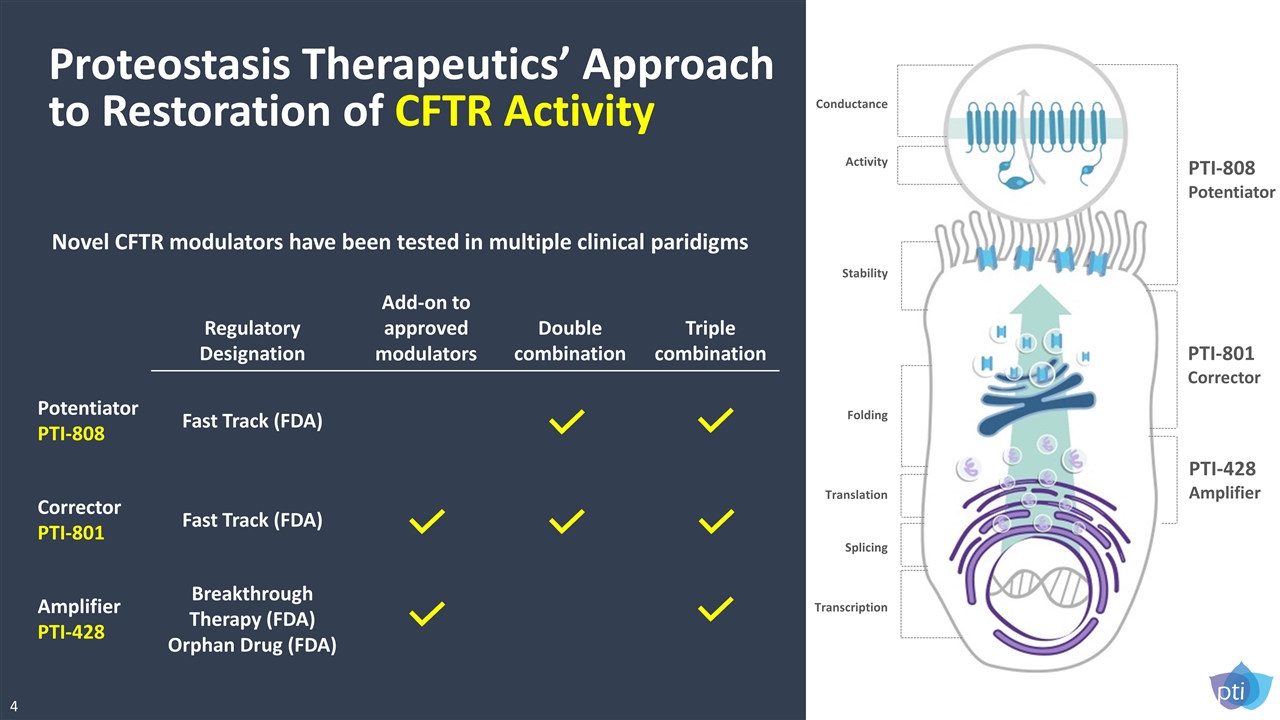
Proteostasis Therapeutics’ Approach to Restoration of CFTR Activity Novel CFTR modulators have been tested in multiple clinical paridigms Regulatory Designation Add-on to approved modulators Double combination Triple combination Potentiator PTI-808 Fast Track (FDA) Corrector PTI-801 Fast Track (FDA) Amplifier PTI-428 Breakthrough Therapy (FDA) Orphan Drug (FDA) Conductance Activity Stability Folding Translation Splicing Transcription PTI-808 Potentiator PTI-801 Corrector PTI-428 Amplifier 4
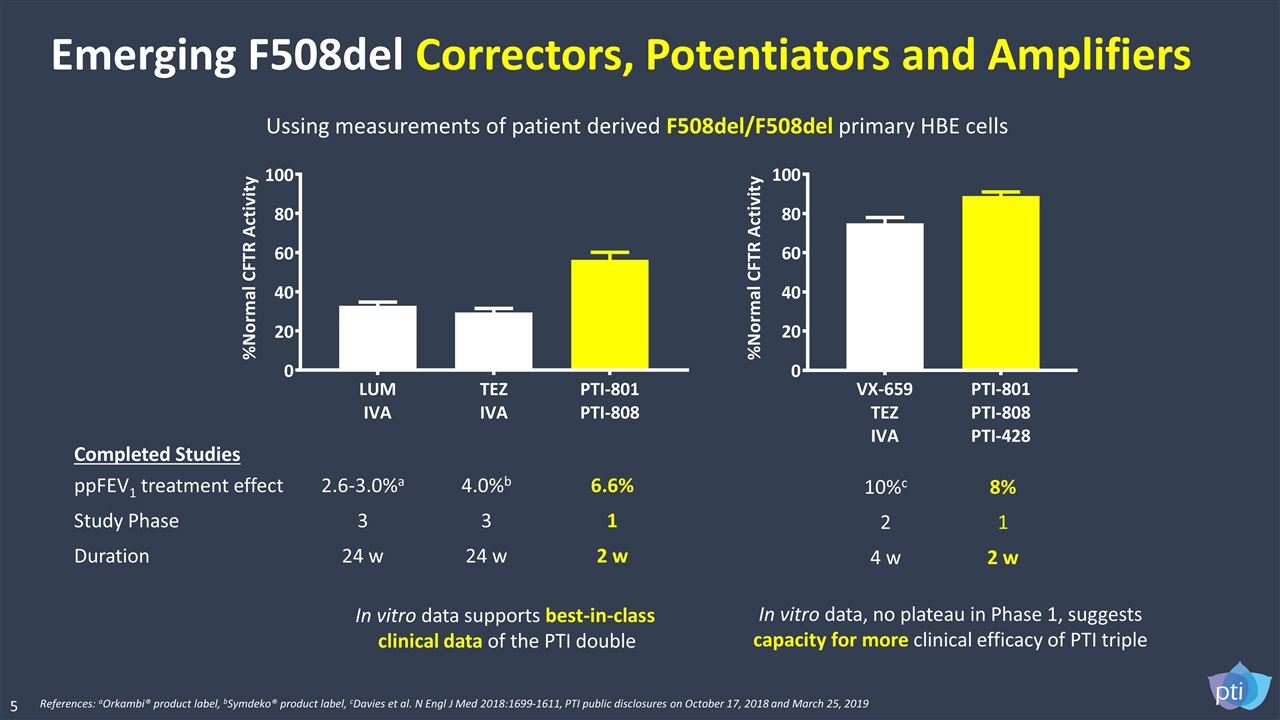
Completed Studies In vitro data supports best-in-class clinical data of the PTI double In vitro data, no plateau in Phase 1, suggests capacity for more clinical efficacy of PTI triple ppFEV1 treatment effect 2.6-3.0%a 4.0%b 6.6% Study Phase 3 3 1 Duration 24 w 24 w 2 w 10%c 8% 2 1 4 w 2 w Ussing measurements of patient derived F508del/F508del primary HBE cells References: aOrkambi® product label, bSymdeko® product label, cDavies et al. N Engl J Med 2018:1699-1611, PTI public disclosures on October 17, 2018 and March 25, 2019 Emerging F508del Correctors, Potentiators and Amplifiers 5
Over 50 Subjects Completed Combination Studies in North America and in Europe 6
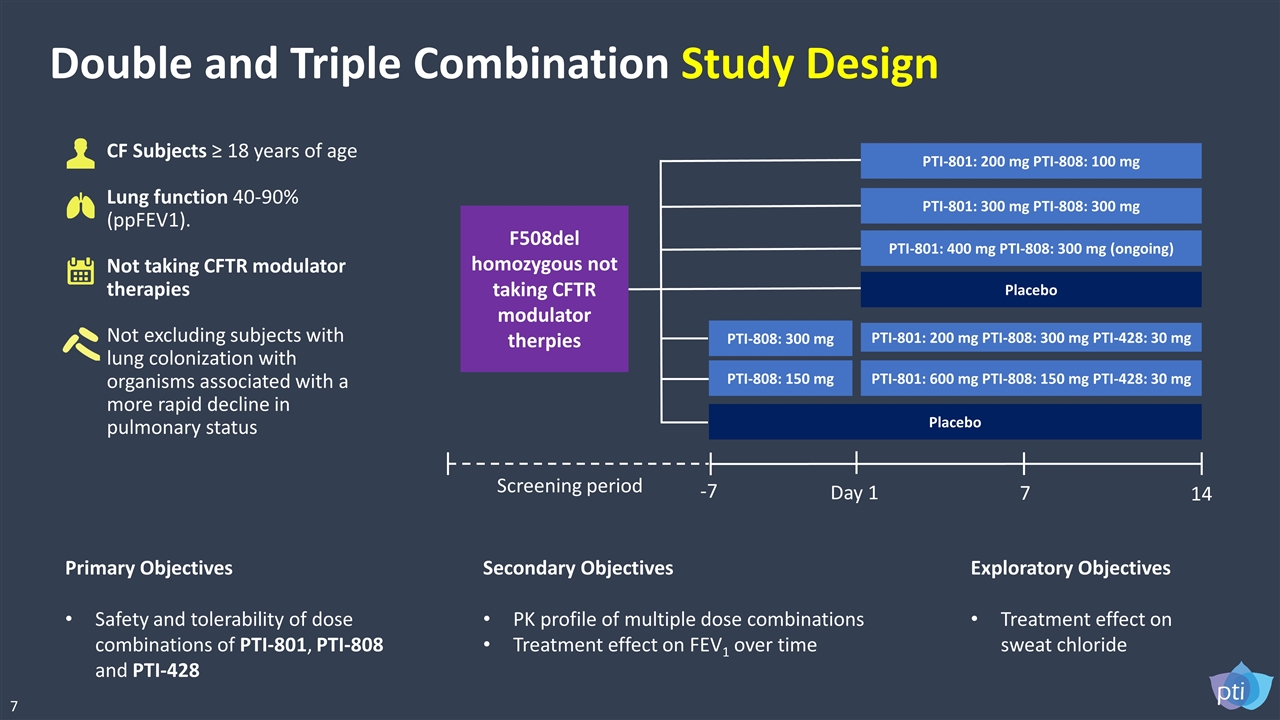
Double and Triple Combination Study Design CF Subjects ≥ 18 years of age Lung function 40-90% (ppFEV1). Not taking CFTR modulator therapies Not excluding subjects with lung colonization with organisms associated with a more rapid decline in pulmonary status Primary Objectives Safety and tolerability of dose combinations of PTI-801, PTI-808 and PTI-428 Secondary Objectives PK profile of multiple dose combinations Treatment effect on FEV1 over time Exploratory Objectives Treatment effect on sweat chloride PTI-801: 600 mg PTI-808: 150 mg PTI-428: 30 mg Placebo PTI-801: 400 mg PTI-808: 300 mg (ongoing) Placebo -7 Day 1 14 7 Screening period PTI-801: 200 mg PTI-808: 300 mg PTI-428: 30 mg PTI-808: 300 mg PTI-808: 150 mg PTI-801: 300 mg PTI-808: 300 mg PTI-801: 200 mg PTI-808: 100 mg F508del homozygous not taking CFTR modulator therpies 7
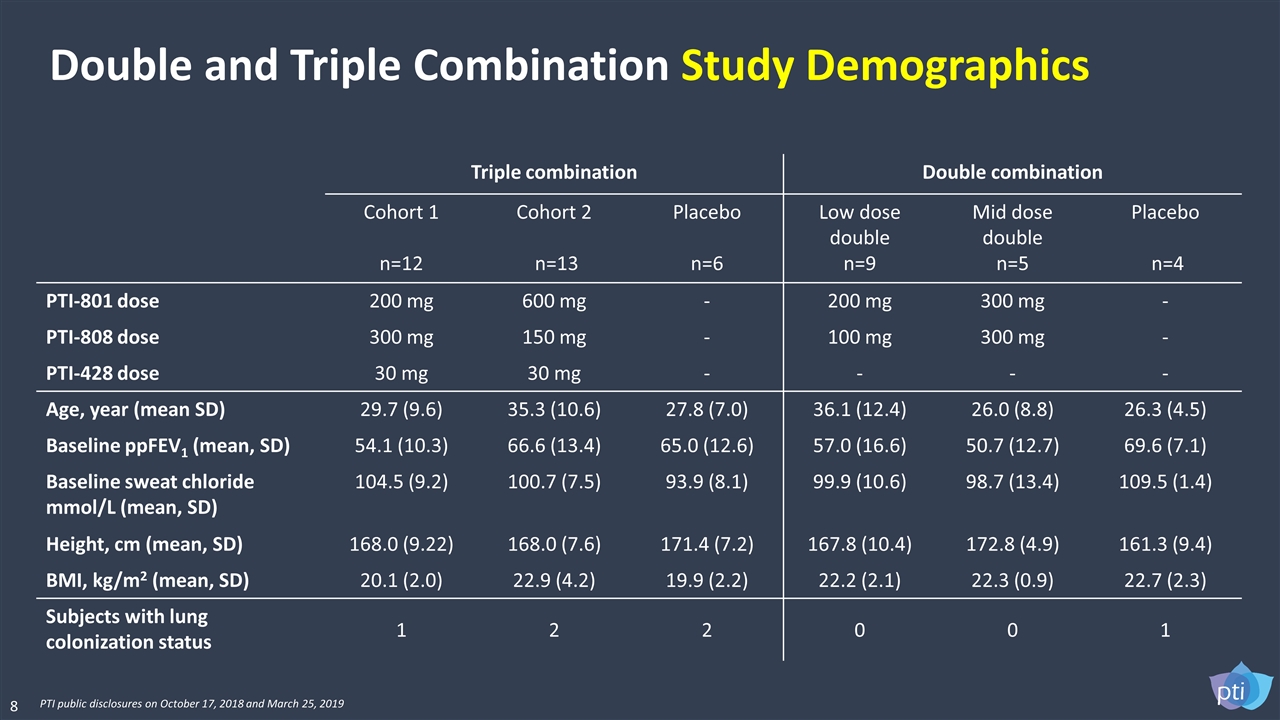
Triple combination Double combination Cohort 1 n=12 Cohort 2 n=13 Placebo n=6 Low dose double n=9 Mid dose double n=5 Placebo n=4 PTI-801 dose 200 mg 600 mg - 200 mg 300 mg - PTI-808 dose 300 mg 150 mg - 100 mg 300 mg - PTI-428 dose 30 mg 30 mg - - - - Age, year (mean SD) 29.7 (9.6) 35.3 (10.6) 27.8 (7.0) 36.1 (12.4) 26.0 (8.8) 26.3 (4.5) Baseline ppFEV1 (mean, SD) 54.1 (10.3) 66.6 (13.4) 65.0 (12.6) 57.0 (16.6) 50.7 (12.7) 69.6 (7.1) Baseline sweat chloride mmol/L (mean, SD) 104.5 (9.2) 100.7 (7.5) 93.9 (8.1) 99.9 (10.6) 98.7 (13.4) 109.5 (1.4) Height, cm (mean, SD) 168.0 (9.22) 168.0 (7.6) 171.4 (7.2) 167.8 (10.4) 172.8 (4.9) 161.3 (9.4) BMI, kg/m2 (mean, SD) 20.1 (2.0) 22.9 (4.2) 19.9 (2.2) 22.2 (2.1) 22.3 (0.9) 22.7 (2.3) Subjects with lung colonization status 1 2 2 0 0 1 Double and Triple Combination Study Demographics 8 PTI public disclosures on October 17, 2018 and March 25, 2019
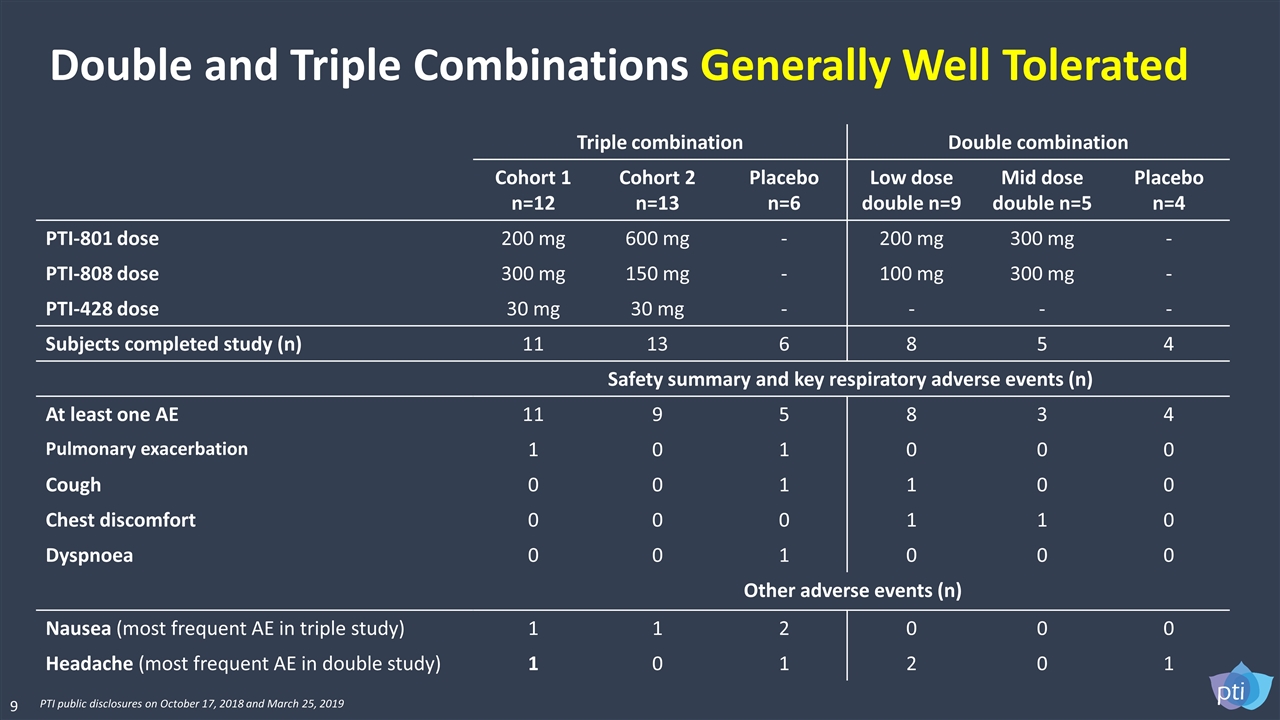
Triple combination Double combination Cohort 1 n=12 Cohort 2 n=13 Placebo n=6 Low dose double n=9 Mid dose double n=5 Placebo n=4 PTI-801 dose 200 mg 600 mg - 200 mg 300 mg - PTI-808 dose 300 mg 150 mg - 100 mg 300 mg - PTI-428 dose 30 mg 30 mg - - - - Subjects completed study (n) 11 13 6 8 5 4 Safety summary and key respiratory adverse events (n) At least one AE 11 9 5 8 3 4 Pulmonary exacerbation 1 0 1 0 0 0 Cough 0 0 1 1 0 0 Chest discomfort 0 0 0 1 1 0 Dyspnoea 0 0 1 0 0 0 Other adverse events (n) Nausea (most frequent AE in triple study) 1 1 2 0 0 0 Headache (most frequent AE in double study) 1 0 1 2 0 1 Double and Triple Combinations Generally Well Tolerated 9 PTI public disclosures on October 17, 2018 and March 25, 2019
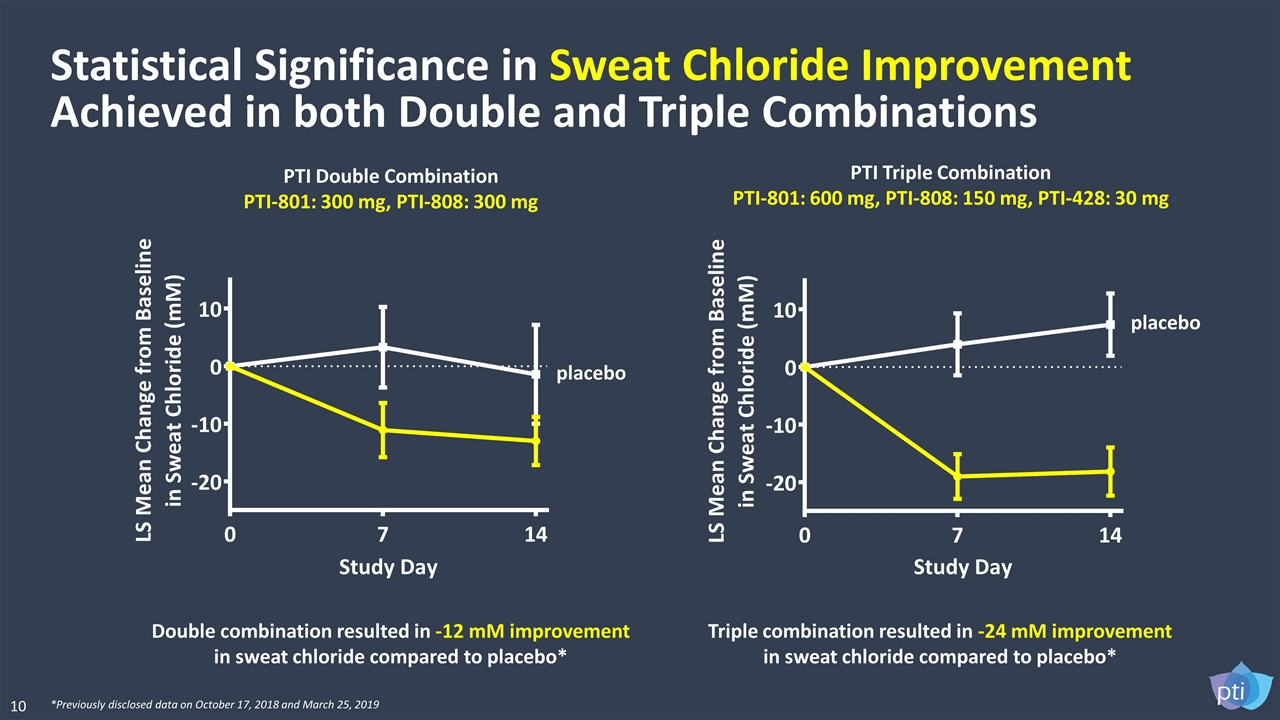
Double combination resulted in -12 mM improvement in sweat chloride compared to placebo* Triple combination resulted in -24 mM improvement in sweat chloride compared to placebo* Statistical Significance in Sweat Chloride Improvement Achieved in both Double and Triple Combinations PTI Triple Combination PTI-801: 600 mg, PTI-808: 150 mg, PTI-428: 30 mg PTI Double Combination PTI-801: 300 mg, PTI-808: 300 mg *Previously disclosed data on October 17, 2018 and March 25, 2019 placebo placebo 10
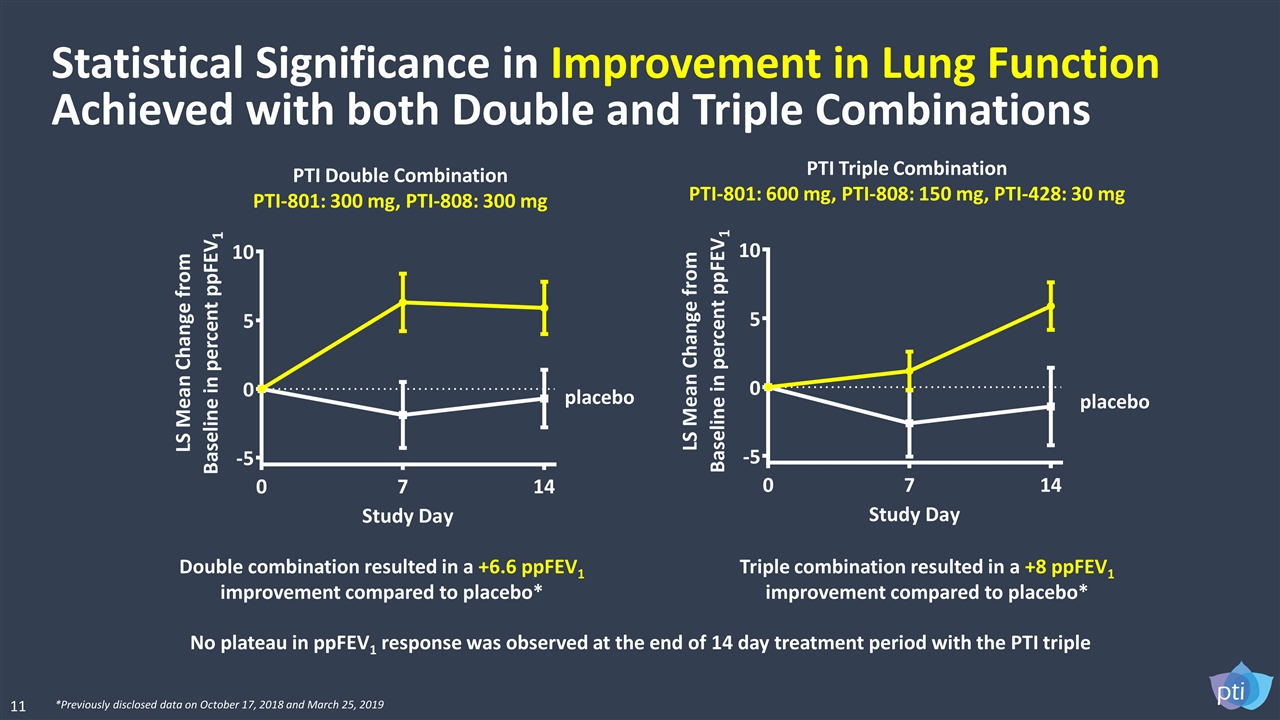
Double combination resulted in a +6.6 ppFEV1 improvement compared to placebo* Triple combination resulted in a +8 ppFEV1 improvement compared to placebo* Statistical Significance in Improvement in Lung Function Achieved with both Double and Triple Combinations No plateau in ppFEV1 response was observed at the end of 14 day treatment period with the PTI triple PTI Triple Combination PTI-801: 600 mg, PTI-808: 150 mg, PTI-428: 30 mg PTI Double Combination PTI-801: 300 mg, PTI-808: 300 mg *Previously disclosed data on October 17, 2018 and March 25, 2019 placebo placebo 11
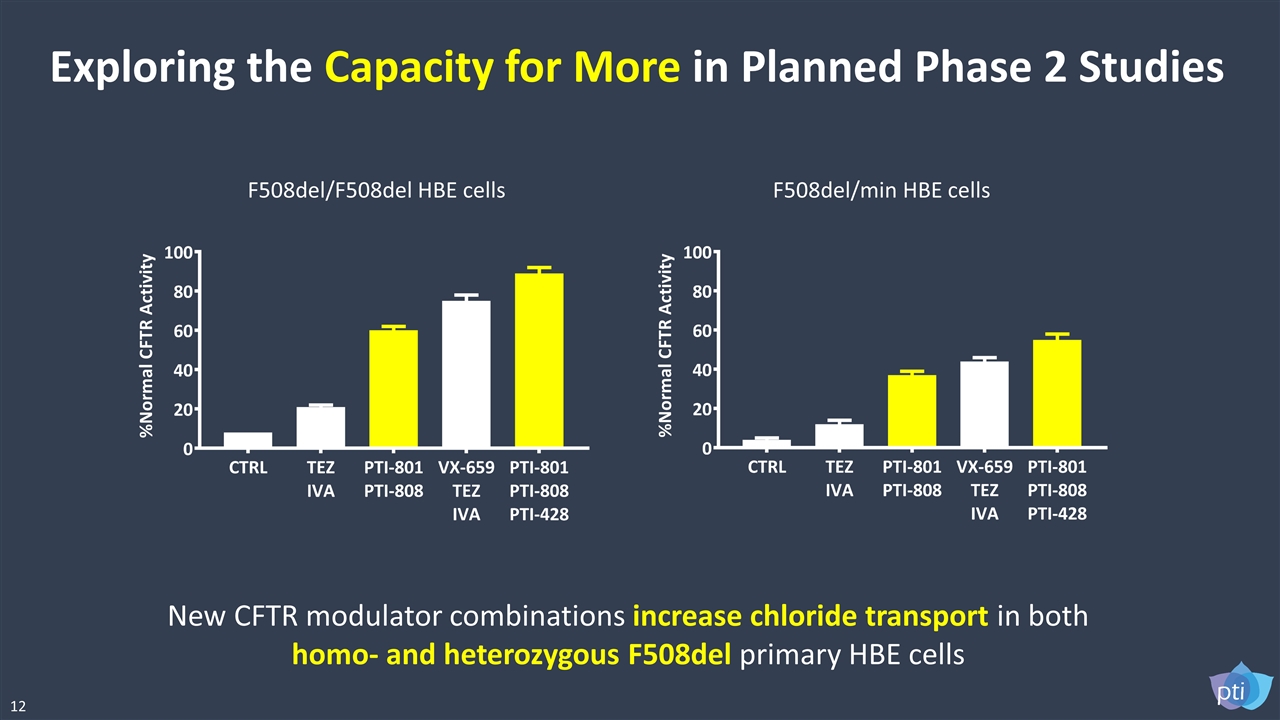
Exploring the Capacity for More in Planned Phase 2 Studies F508del/F508del HBE cells F508del/min HBE cells New CFTR modulator combinations increase chloride transport in both homo- and heterozygous F508del primary HBE cells 12

Potential Best-in-Class Double Backbone Serves as a Next Step in a Global Phase 2 Leading into Phase 3 Study Program 13
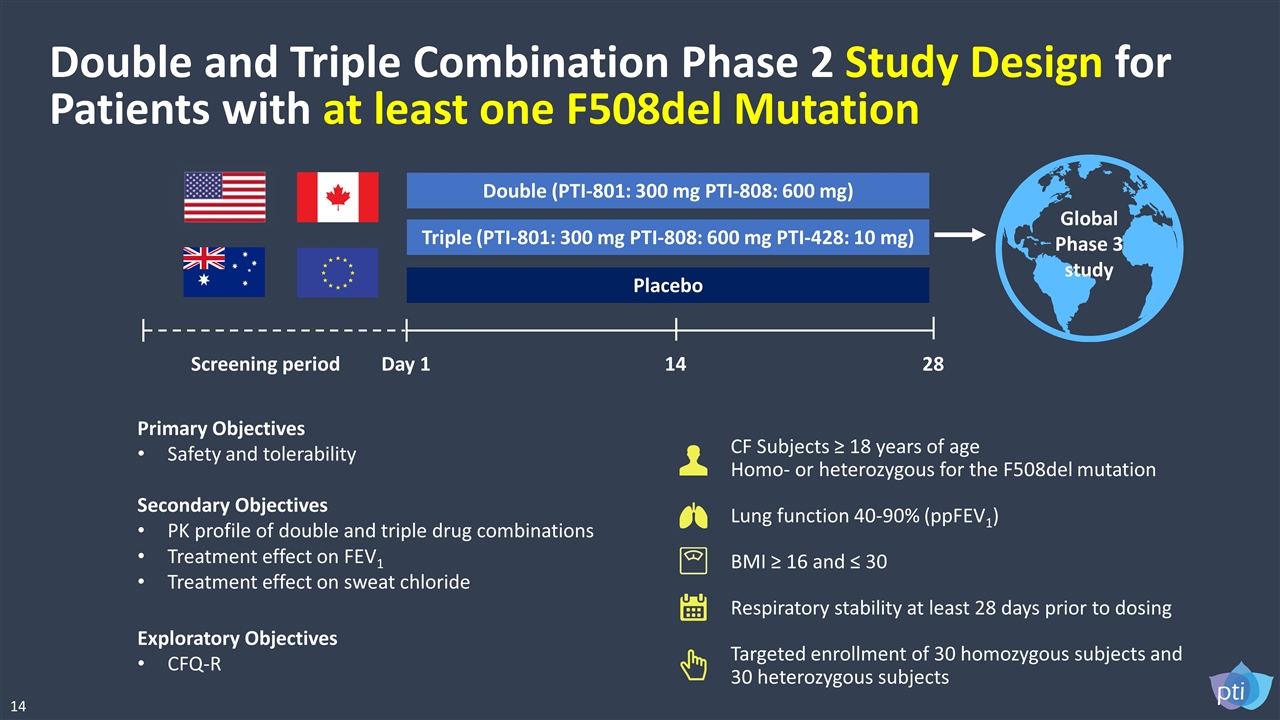
Double and Triple Combination Phase 2 Study Design for Patients with at least one F508del Mutation CF Subjects ≥ 18 years of age Homo- or heterozygous for the F508del mutation Lung function 40-90% (ppFEV1) BMI ≥ 16 and ≤ 30 Respiratory stability at least 28 days prior to dosing Targeted enrollment of 30 homozygous subjects and 30 heterozygous subjects Double (PTI-801: 300 mg PTI-808: 600 mg) Triple (PTI-801: 300 mg PTI-808: 600 mg PTI-428: 10 mg) Placebo Day 1 14 28 Primary Objectives Safety and tolerability Secondary Objectives PK profile of double and triple drug combinations Treatment effect on FEV1 Treatment effect on sweat chloride Exploratory Objectives CFQ-R Global Phase 3 study Screening period 14

ADD-ON STUDY RESULTS
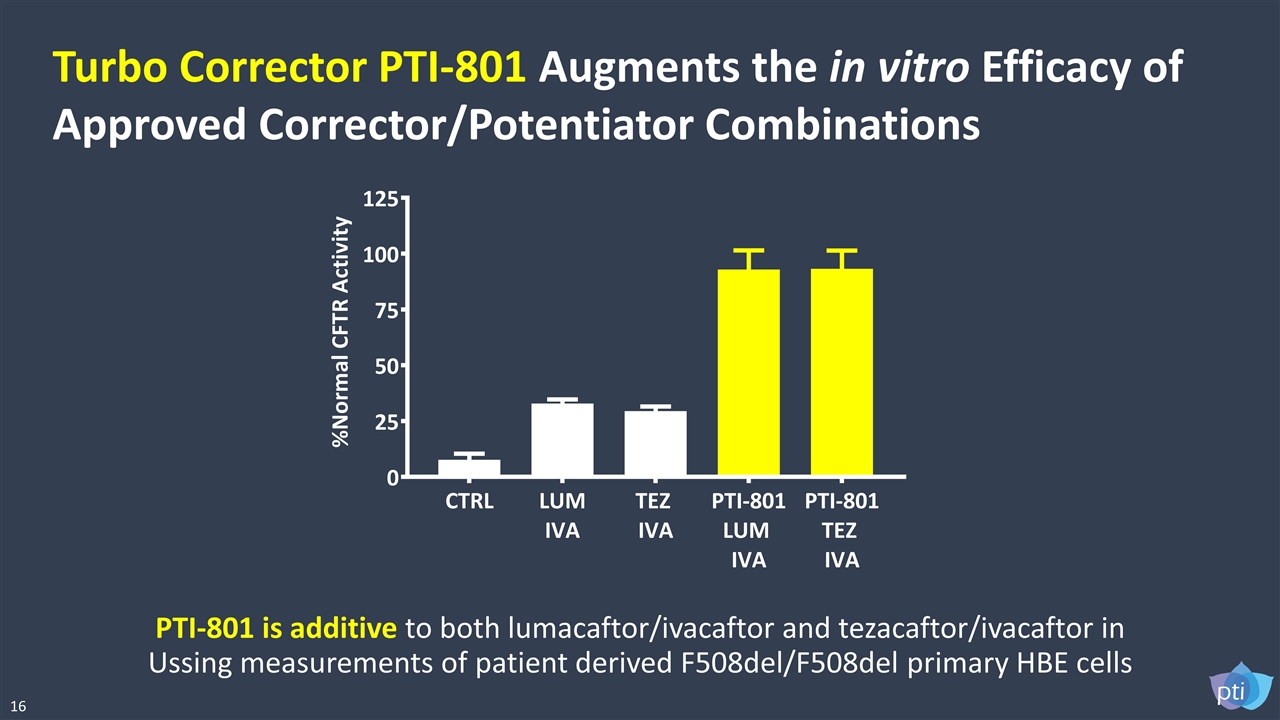
PTI-801 is additive to both lumacaftor/ivacaftor and tezacaftor/ivacaftor in Ussing measurements of patient derived F508del/F508del primary HBE cells Turbo Corrector PTI-801 Augments the in vitro Efficacy of Approved Corrector/Potentiator Combinations 16
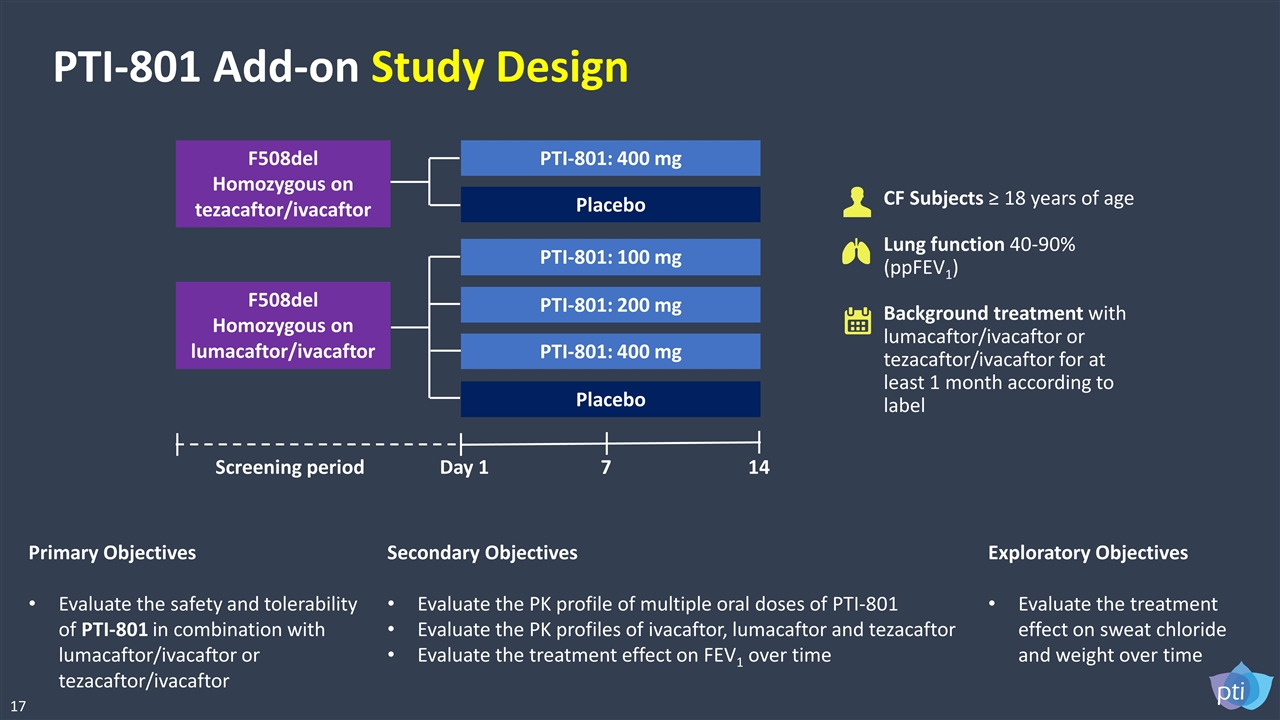
PTI-801 Add-on Study Design CF Subjects ≥ 18 years of age Lung function 40-90% (ppFEV1) Background treatment with lumacaftor/ivacaftor or tezacaftor/ivacaftor for at least 1 month according to label Primary Objectives Evaluate the safety and tolerability of PTI-801 in combination with lumacaftor/ivacaftor or tezacaftor/ivacaftor Secondary Objectives Evaluate the PK profile of multiple oral doses of PTI-801 Evaluate the PK profiles of ivacaftor, lumacaftor and tezacaftor Evaluate the treatment effect on FEV1 over time Exploratory Objectives Evaluate the treatment effect on sweat chloride and weight over time F508del Homozygous on tezacaftor/ivacaftor F508del Homozygous on lumacaftor/ivacaftor PTI-801: 400 mg Placebo PTI-801: 100 mg PTI-801: 200 mg PTI-801: 400 mg Placebo Day 1 7 14 Screening period 17
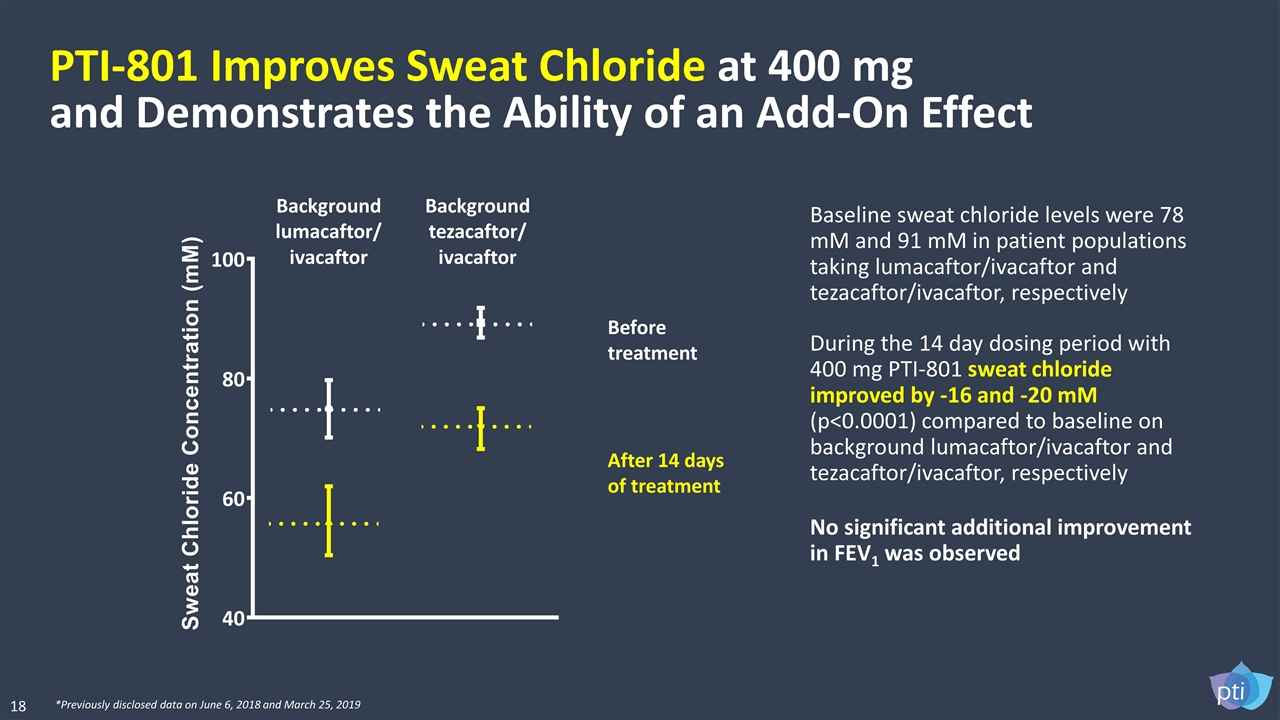
PTI-801 Improves Sweat Chloride at 400 mg and Demonstrates the Ability of an Add-On Effect Baseline sweat chloride levels were 78 mM and 91 mM in patient populations taking lumacaftor/ivacaftor and tezacaftor/ivacaftor, respectively During the 14 day dosing period with 400 mg PTI-801 sweat chloride improved by -16 and -20 mM (p<0.0001) compared to baseline on background lumacaftor/ivacaftor and tezacaftor/ivacaftor, respectively No significant additional improvement in FEV1 was observed Background lumacaftor/ ivacaftor Before treatment After 14 days of treatment Background tezacaftor/ ivacaftor 18 *Previously disclosed data on June 6, 2018 and March 25, 2019
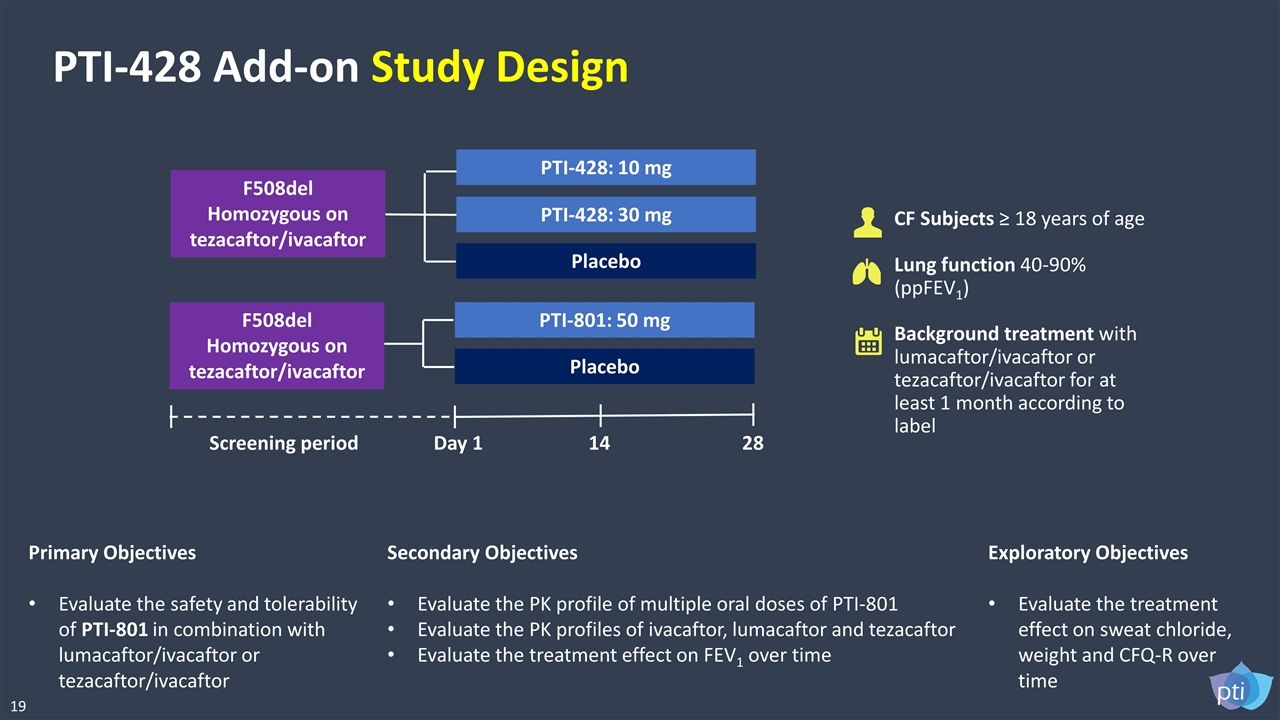
PTI-428 Add-on Study Design CF Subjects ≥ 18 years of age Lung function 40-90% (ppFEV1) Background treatment with lumacaftor/ivacaftor or tezacaftor/ivacaftor for at least 1 month according to label Primary Objectives Evaluate the safety and tolerability of PTI-801 in combination with lumacaftor/ivacaftor or tezacaftor/ivacaftor Secondary Objectives Evaluate the PK profile of multiple oral doses of PTI-801 Evaluate the PK profiles of ivacaftor, lumacaftor and tezacaftor Evaluate the treatment effect on FEV1 over time Exploratory Objectives Evaluate the treatment effect on sweat chloride, weight and CFQ-R over time F508del Homozygous on tezacaftor/ivacaftor PTI-801: 50 mg Placebo Day 1 14 28 Screening period F508del Homozygous on tezacaftor/ivacaftor PTI-428: 30 mg Placebo PTI-428: 10 mg 19
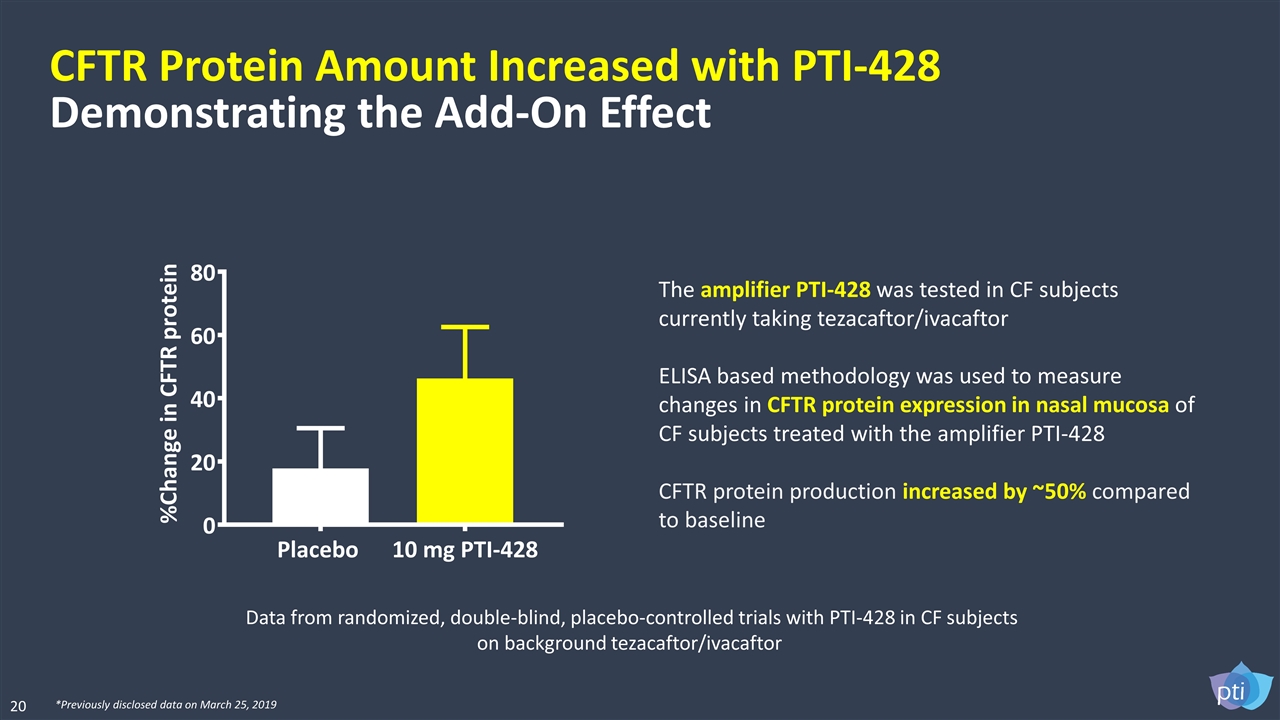
CFTR Protein Amount Increased with PTI-428 Demonstrating the Add-On Effect The amplifier PTI-428 was tested in CF subjects currently taking tezacaftor/ivacaftor ELISA based methodology was used to measure changes in CFTR protein expression in nasal mucosa of CF subjects treated with the amplifier PTI-428 CFTR protein production increased by ~50% compared to baseline 20 *Previously disclosed data on March 25, 2019 Data from randomized, double-blind, placebo-controlled trials with PTI-428 in CF subjects on background tezacaftor/ivacaftor

2019 Milestones Build Foundation for Phase 3 Study Initiation in Mid-2020 Achieved 2019 milestones Completed 14-day PTI-801 and 28-day PTI-428 Add on studies to tezacaftor/ivacaftor Completed 14-day PTI-808/801/428 triple combination study in F508del homozygous subjects PK/PD model developed for effective dose selection Additional 2019 Milestones Complete 28-day double PTI-808/801 and triple PTI-808/801/428 combination studies in F508del homozygous subjects Complete 28-day double PTI-808/801 and triple PTI-808/801/428 combination studies in F508del heterozygous subjects 21

THANK YOU #CForward “Proteostasis is striving to shake up the CF community in ways better than the vest ever could.” Brad Dell, a person with CF