
Patrick A. Flume, MD, on behalf of the study team Medical University of South Carolina Charleston, SC, United States Workshop 17: New & Emerging Therapies to Correct the Basic Defect EVALUATION OF NOVEL CFTR MODULATOR COMBINATIONS OF CORRECTOR PTI-801, POTENTIATOR PTI-808, AND AMPLIFIER PTI-428 IN CF SUBJECTS 33° NORTH AMERICAN CYSTIC FIBROSIS CONFERENCE October 31 – November 2, 2019 Nashville, TN Exhibit 99.1

Speaker’s Disclosures Grant support Bayer Healthcare AG Corbus Pharmaceuticals Cystic Fibrosis Foundation Therapeutics Galapagos Insmed National Institutes of Health Novartis Novoteris Proteostasis Therapeutics, Inc Savara Sound Pharmaceuticals, Inc Vertex Pharmaceuticals, Inc Consultant Corbus Pharmaceuticals Eloxx Pharmaceuticals Horizon Pharma Insmed Ionis Pharmaceuticals McKesson Novartis Proteostasis Therapeutics, Inc Savara Vertex Pharmaceuticals, Inc
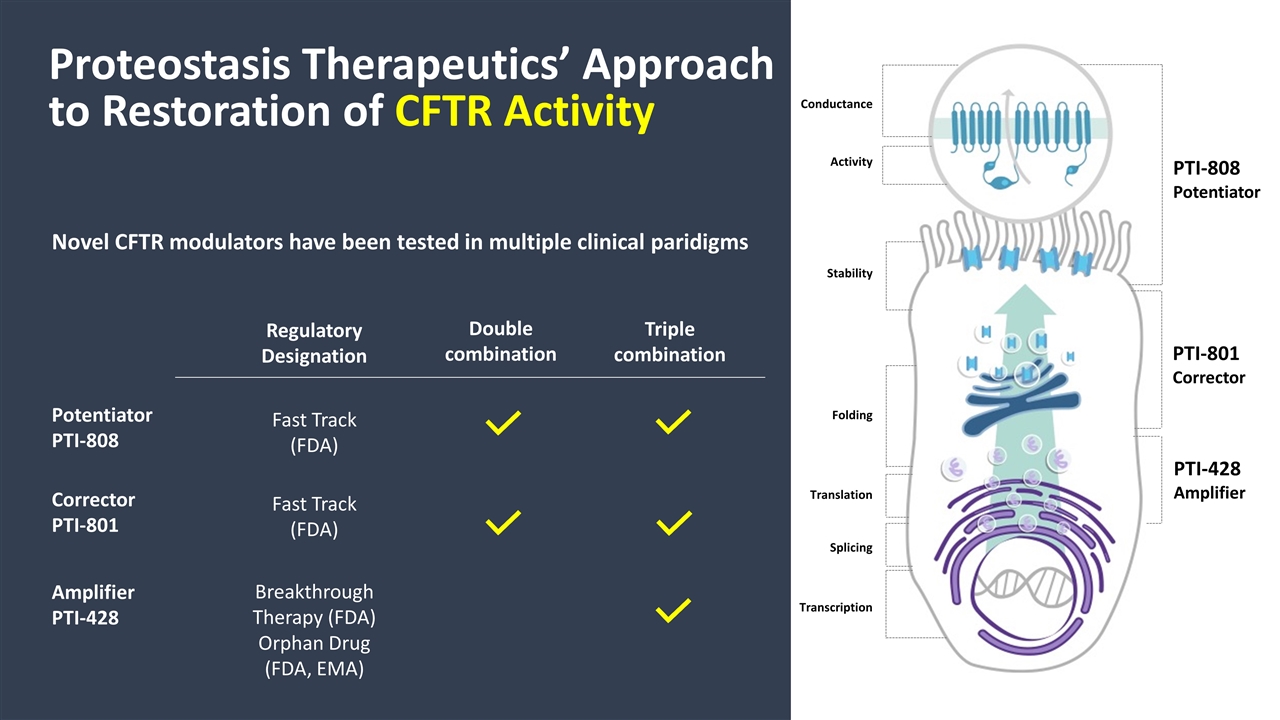
Proteostasis Therapeutics’ Approach to Restoration of CFTR Activity Novel CFTR modulators have been tested in multiple clinical paridigms Double combination Triple combination Conductance Activity Stability Folding Translation Splicing Transcription Corrector PTI-801 Fast Track (FDA) PTI-801 Corrector Amplifier PTI-428 Breakthrough Therapy (FDA) Orphan Drug (FDA, EMA) PTI-428 Amplifier Potentiator PTI-808 Fast Track (FDA) PTI-808 Potentiator Regulatory Designation
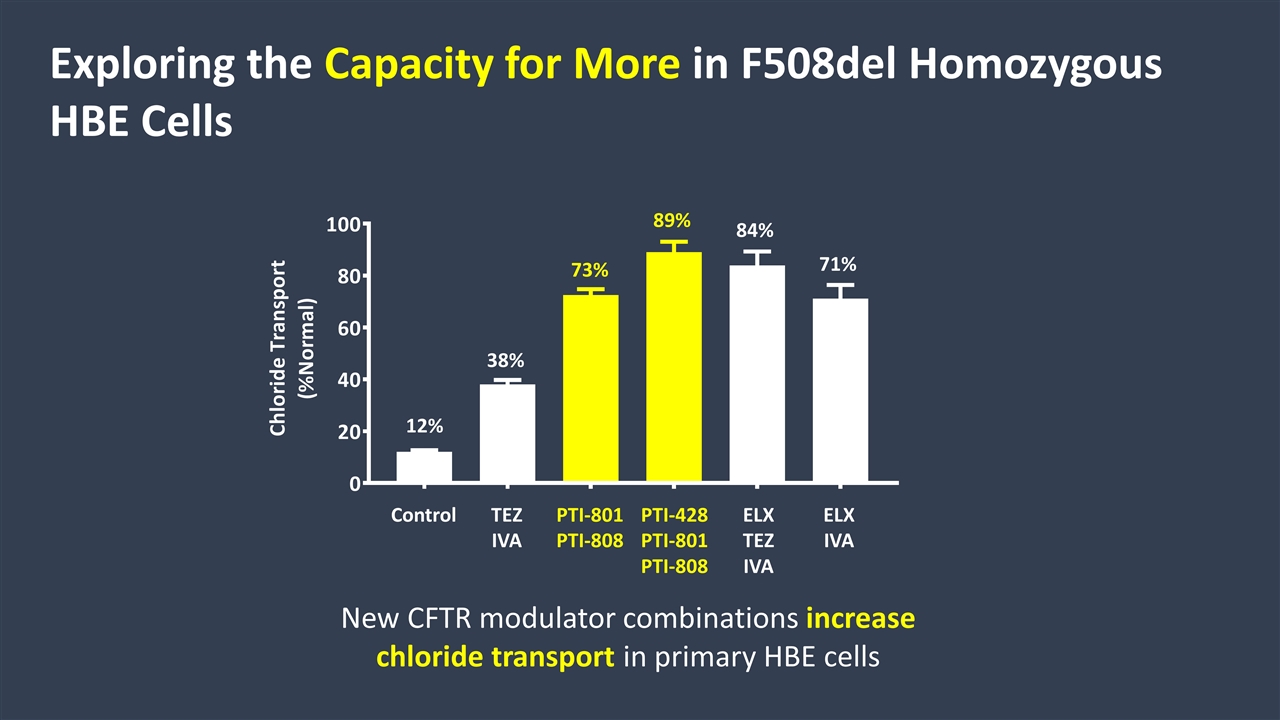
Exploring the Capacity for More in F508del Homozygous HBE Cells Control TEZ IVA PTI-801 PTI-808 PTI-428 PTI-801 PTI-808 ELX TEZ IVA ELX IVA New CFTR modulator combinations increase chloride transport in primary HBE cells 71% 84% 89% 73% 38% 12%
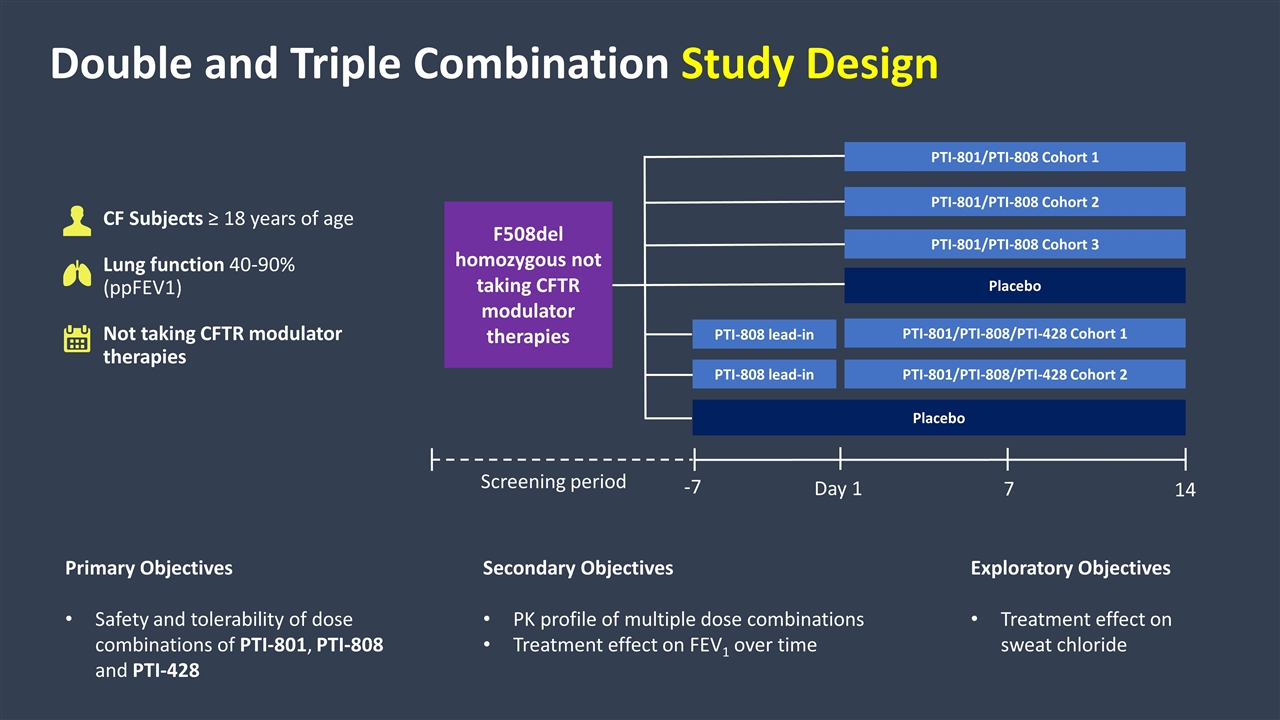
Double and Triple Combination Study Design CF Subjects ≥ 18 years of age Lung function 40-90% (ppFEV1) Not taking CFTR modulator therapies Primary Objectives Safety and tolerability of dose combinations of PTI-801, PTI-808 and PTI-428 Secondary Objectives PK profile of multiple dose combinations Treatment effect on FEV1 over time Exploratory Objectives Treatment effect on sweat chloride PTI-801/PTI-808/PTI-428 Cohort 2 Placebo PTI-801/PTI-808 Cohort 3 Placebo -7 Day 1 14 7 Screening period PTI-801/PTI-808/PTI-428 Cohort 1 PTI-808 lead-in PTI-808 lead-in PTI-801/PTI-808 Cohort 2 PTI-801/PTI-808 Cohort 1 F508del homozygous not taking CFTR modulator therapies
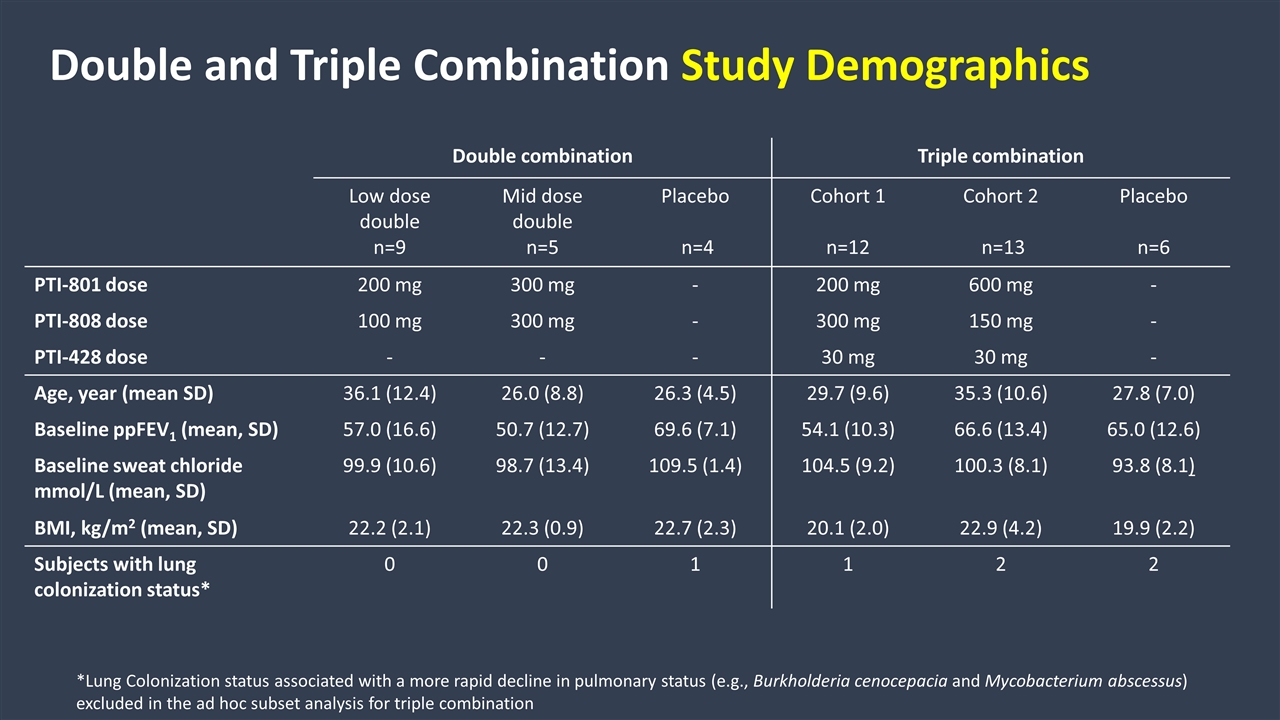
Double combination Triple combination Low dose double n=9 Mid dose double n=5 Placebo n=4 Cohort 1 n=12 Cohort 2 n=13 Placebo n=6 PTI-801 dose 200 mg 300 mg - 200 mg 600 mg - PTI-808 dose 100 mg 300 mg - 300 mg 150 mg - PTI-428 dose - - - 30 mg 30 mg - Age, year (mean SD) 36.1 (12.4) 26.0 (8.8) 26.3 (4.5) 29.7 (9.6) 35.3 (10.6) 27.8 (7.0) Baseline ppFEV1 (mean, SD) 57.0 (16.6) 50.7 (12.7) 69.6 (7.1) 54.1 (10.3) 66.6 (13.4) 65.0 (12.6) Baseline sweat chloride mmol/L (mean, SD) 99.9 (10.6) 98.7 (13.4) 109.5 (1.4) 104.5 (9.2) 100.3 (8.1) 93.8 (8.1) BMI, kg/m2 (mean, SD) 22.2 (2.1) 22.3 (0.9) 22.7 (2.3) 20.1 (2.0) 22.9 (4.2) 19.9 (2.2) Subjects with lung colonization status* 0 0 1 1 2 2 Double and Triple Combination Study Demographics *Lung Colonization status associated with a more rapid decline in pulmonary status (e.g., Burkholderia cenocepacia and Mycobacterium abscessus) excluded in the ad hoc subset analysis for triple combination
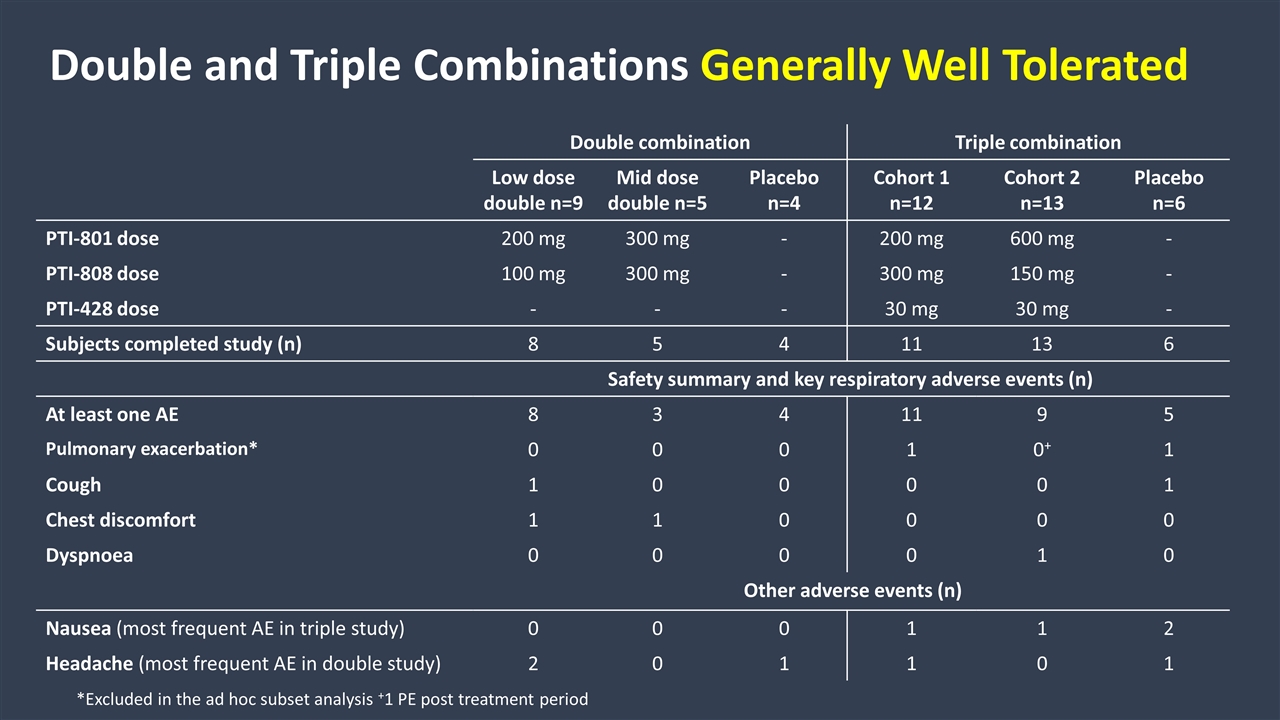
Double combination Triple combination Low dose double n=9 Mid dose double n=5 Placebo n=4 Cohort 1 n=12 Cohort 2 n=13 Placebo n=6 PTI-801 dose 200 mg 300 mg - 200 mg 600 mg - PTI-808 dose 100 mg 300 mg - 300 mg 150 mg - PTI-428 dose - - - 30 mg 30 mg - Subjects completed study (n) 8 5 4 11 13 6 Safety summary and key respiratory adverse events (n) At least one AE 8 3 4 11 9 5 Pulmonary exacerbation* 0 0 0 1 0+ 1 Cough 1 0 0 0 0 1 Chest discomfort 1 1 0 0 0 0 Dyspnoea 0 0 0 0 1 0 Other adverse events (n) Nausea (most frequent AE in triple study) 0 0 0 1 1 2 Headache (most frequent AE in double study) 2 0 1 1 0 1 Double and Triple Combinations Generally Well Tolerated *Excluded in the ad hoc subset analysis +1 PE post treatment period
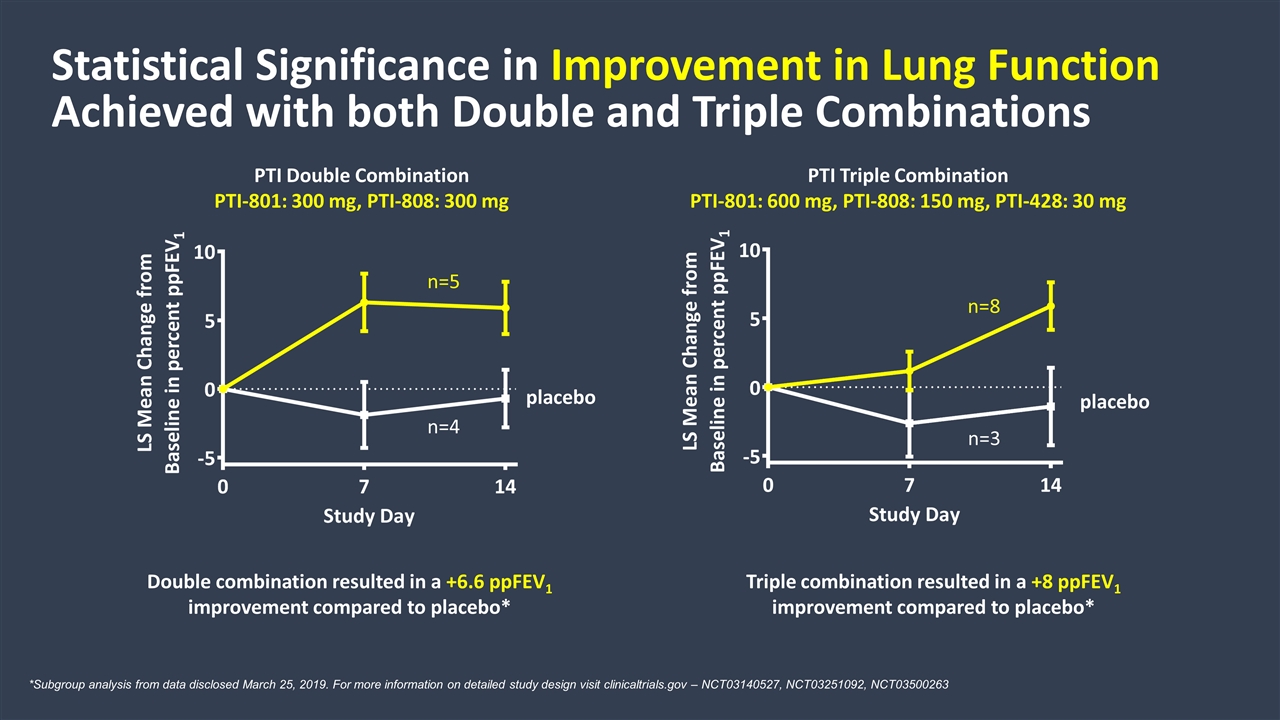
Double combination resulted in a +6.6 ppFEV1 improvement compared to placebo* Triple combination resulted in a +8 ppFEV1 improvement compared to placebo* Statistical Significance in Improvement in Lung Function Achieved with both Double and Triple Combinations PTI Triple Combination PTI-801: 600 mg, PTI-808: 150 mg, PTI-428: 30 mg PTI Double Combination PTI-801: 300 mg, PTI-808: 300 mg placebo placebo *Subgroup analysis from data disclosed March 25, 2019. For more information on detailed study design visit clinicaltrials.gov – NCT03140527, NCT03251092, NCT03500263 n=5 n=8 n=4 n=3
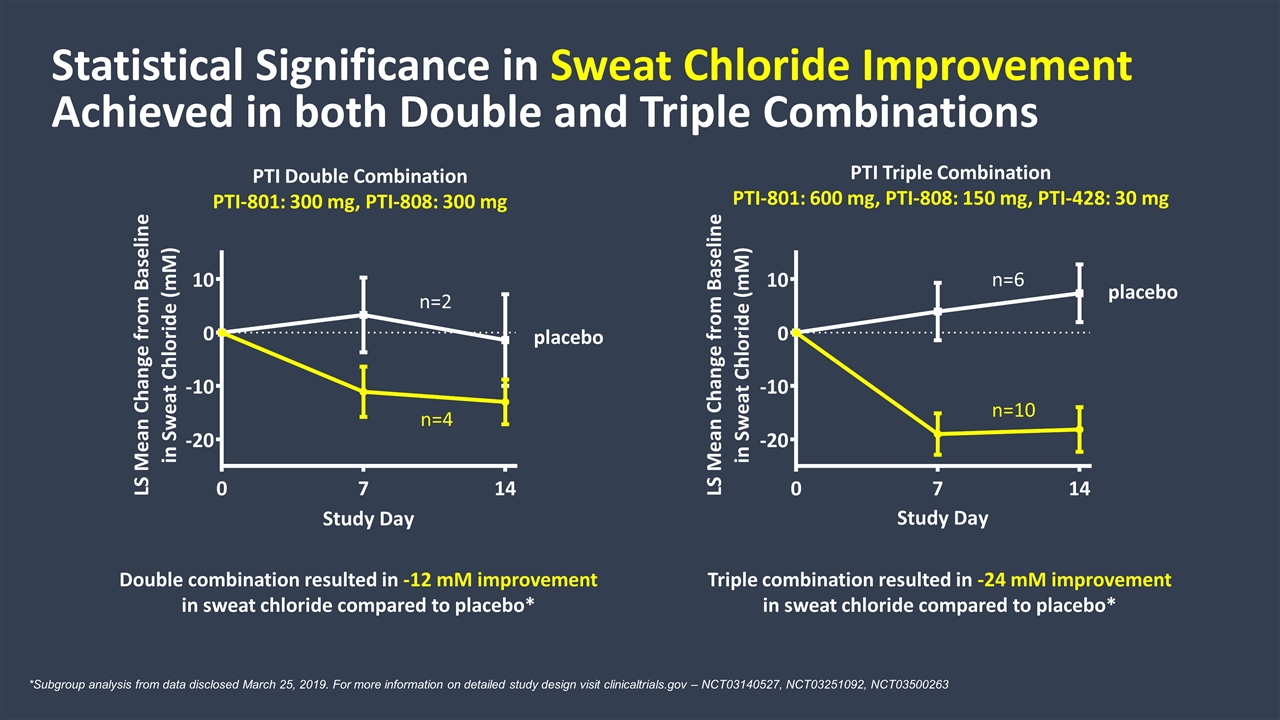
Triple combination resulted in -24 mM improvement in sweat chloride compared to placebo* Statistical Significance in Sweat Chloride Improvement Achieved in both Double and Triple Combinations placebo placebo PTI Triple Combination PTI-801: 600 mg, PTI-808: 150 mg, PTI-428: 30 mg PTI Double Combination PTI-801: 300 mg, PTI-808: 300 mg Double combination resulted in -12 mM improvement in sweat chloride compared to placebo* *Subgroup analysis from data disclosed March 25, 2019. For more information on detailed study design visit clinicaltrials.gov – NCT03140527, NCT03251092, NCT03500263 n=4 n=2 n=6 n=10
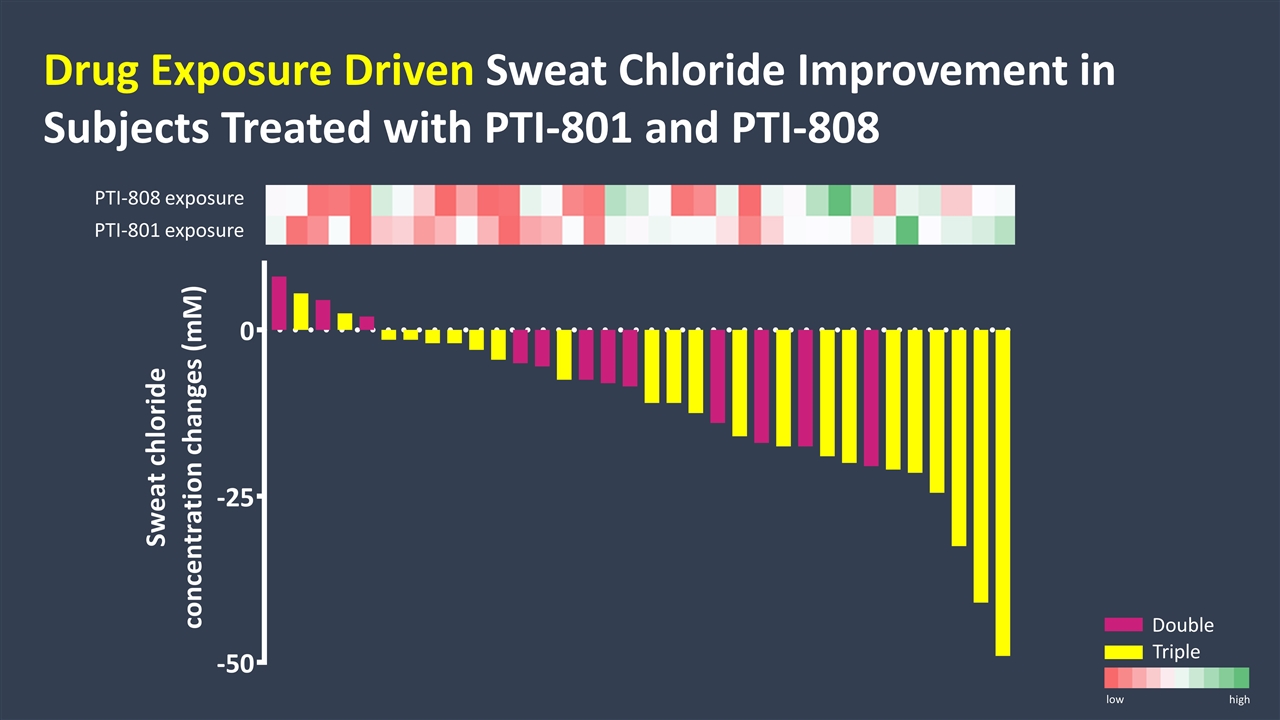
PTI-808 exposure PTI-801 exposure low high Double Triple Drug Exposure Driven Sweat Chloride Improvement in Subjects Treated with PTI-801 and PTI-808
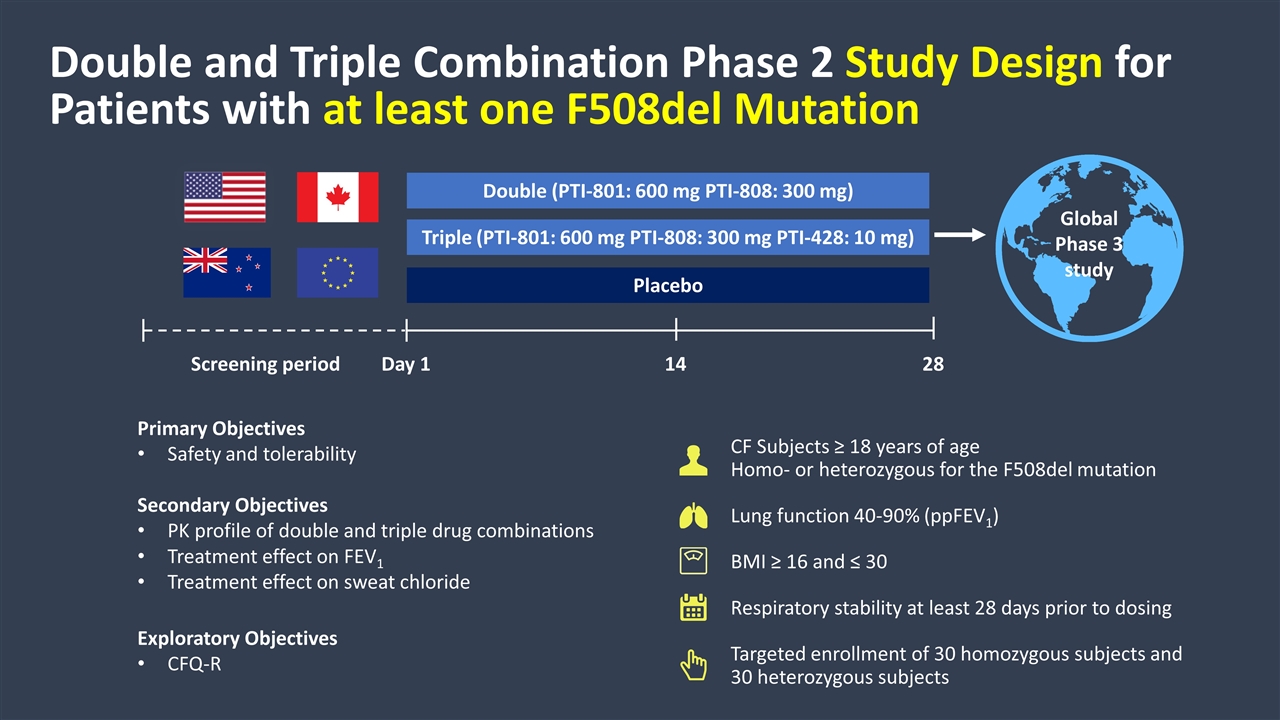
Double and Triple Combination Phase 2 Study Design for Patients with at least one F508del Mutation CF Subjects ≥ 18 years of age Homo- or heterozygous for the F508del mutation Lung function 40-90% (ppFEV1) BMI ≥ 16 and ≤ 30 Respiratory stability at least 28 days prior to dosing Targeted enrollment of 30 homozygous subjects and 30 heterozygous subjects Double (PTI-801: 600 mg PTI-808: 300 mg) Triple (PTI-801: 600 mg PTI-808: 300 mg PTI-428: 10 mg) Placebo Day 1 14 28 Primary Objectives Safety and tolerability Secondary Objectives PK profile of double and triple drug combinations Treatment effect on FEV1 Treatment effect on sweat chloride Exploratory Objectives CFQ-R Global Phase 3 study Screening period

THANK YOU Lead investigators Patrick A. Flume, MD, Damian Downey, MD, Manu Jain, MD, global study teams and all participating people with Cystic Fibrosis and their families 33° NORTH AMERICAN CYSTIC FIBROSIS CONFERENCE October 31 – November 2, 2019 Nashville, TN











