
MORE CHOICES IN CYSTIC FIBROSIS TREATMENT: NOVEL CFTR MODULATOR COMBINATIONS #CForward Corporate Presentation January 2020 Exhibit 99.2

Safe Harbor and Disclaimer To the extent that statements in this presentation are not historical facts, they are forward-looking statements reflecting the current beliefs and expectations of management made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. Words such as “aim,” “may,” “will,” “expect,” “anticipate,” “estimate,” “intend,” and similar expressions (as well as other words or expressions referencing future events, conditions or circumstances) are intended to identify forward-looking statements. Examples of forward-looking statements made in this presentation include, without limitation, statements regarding the potential of our proprietary combination therapies for the treatment of CF, the potential benefit to patients, including those with extremely rare genotypes, of our proprietary combination therapies, the expected timing of the initiation of, patient enrollment in, data from, and our completion of, our clinical studies and cohorts for nesolicaftor (PTI-428), posenacaftor (PTI-801), dirocaftor (PTI-808) and our combination therapy candidates, cash guidance, and HIT CF consortium subject recruitment, and in vitro testing and conduct of clinical trials with our drug candidates. Forward-looking statements made in this presentation involve substantial risks and uncertainties that could cause actual results to differ materially from those expressed or implied by the forward-looking statements, and we therefore cannot assure you that our plans, intentions, expectations or strategies will be attained or achieved. Such risks and uncertainties include, without limitation, the possibility that final or future results from our drug candidate trials (including, without limitation, longer duration studies) do not achieve positive results or are materially and negatively different from or not indicative of the preliminary results reported in this presentation (noting that these results are on a small number of patients and small data set), uncertainties inherent in the execution and completion of clinical trials (including, without limitation, the possibility FDA requires us to run cohorts sequentially or conduct additional cohorts or pre-clinical or clinical studies), in the enrollment of CF patients in our clinical trials, in the timing of availability of trial data, in the results of the clinical trials, in possible adverse events from our trials, in the actions of regulatory agencies, in endorsement, if any, by CF patient advocacy groups, the potential activity of our drug candidates in organoids and in personalized clinical trials in extremely rare genetic mutations may not be realized, and those set forth in our Annual Report on Form 10-K for the year ended December 31, 2018, our Quarterly Report on Form 10-Q for the period ended September 30, 2019, and our other SEC filings. We assume no obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise. This presentation also contains estimates and other statistical data made by independent parties and by us relating to, among other items, disease incidence, market size and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. Projections, assumptions and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of risk and uncertainty. New risks emerge from time to time, and neither we nor any other person makes any representation as to the accuracy or completeness of such data or undertakes any obligation to update such data after the date of this presentation. By attending, viewing, or receiving this presentation you acknowledge you are solely responsible for your own assessment of the market and our market position and that you will conduct your own analysis and are solely responsible for forming your own view of the potential future performance of our business. The trademarks included in this presentation are the property of the owners thereof and are used for reference purposes only. Such use should not be construed as an endorsement of the Company or its securities.
Highlights 2019 3 3 novel CFTR modulators ready for registrational studies in 2020 Proof-of-concept established across 14 clinical studies spanning 9 countries with over 300 subjects dosed Proprietary potentiator/corrector/amplifier combination of DIR/POS/NESa delivers ppFEV1 improvement of +8 ppFEV1 compared to placebo in F508del homozygous patients. $77.8M at the end of Q3; sufficient to fund operations and Phase 3 programs into 2021 Financially solid Phase 3 Planning Underway (MORE Trial, CHOICES Trial) Based on Positive Phase 2 Data On track to initiate registrational studies in CF subjects with both common (MORE) and rare genotypes (CHOICES) 28 day clinical Phase 2 data is consistent with predicted benefit based on independent in vitro testing performed by the Cystic Fibrosis Foundation in HBE cells from individuals with CF Phase 2 dose range finding complete aDIR – dirocaftor; POS – posenacaftor; NES – nesolicaftor; ELX – elexacaftor; TEZ – tezacaftor; IVA – ivacaftor
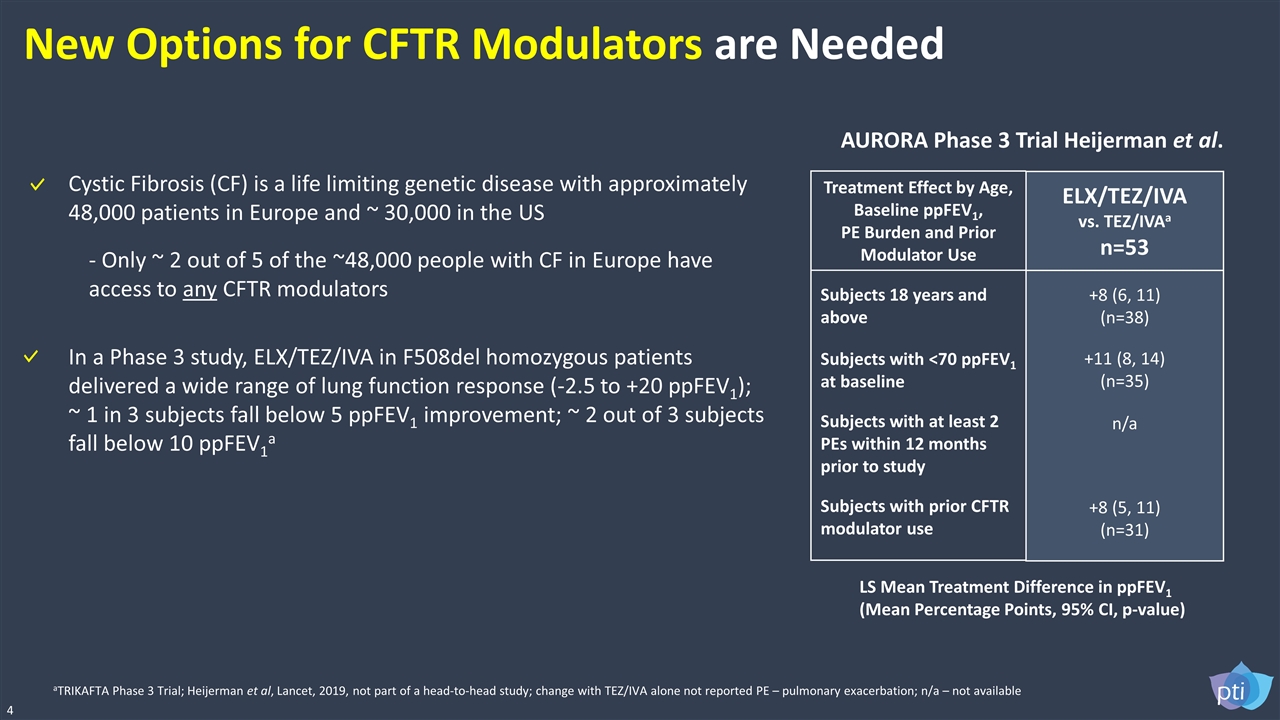
New Options for CFTR Modulators are Needed 4 aTRIKAFTA Phase 3 Trial; Heijerman et al, Lancet, 2019, not part of a head-to-head study; change with TEZ/IVA alone not reported PE – pulmonary exacerbation; n/a – not available LS Mean Treatment Difference in ppFEV1 (Mean Percentage Points, 95% CI, p-value) Treatment Effect by Age, Baseline ppFEV1, PE Burden and Prior Modulator Use Subjects 18 years and above Subjects with <70 ppFEV1 at baseline Subjects with at least 2 PEs within 12 months prior to study Subjects with prior CFTR modulator use ELX/TEZ/IVA vs. TEZ/IVAa n=53 +8 (6, 11) (n=38) +11 (8, 14) (n=35) n/a +8 (5, 11) (n=31) In a Phase 3 study, ELX/TEZ/IVA in F508del homozygous patients delivered a wide range of lung function response (-2.5 to +20 ppFEV1); ~ 1 in 3 subjects fall below 5 ppFEV1 improvement; ~ 2 out of 3 subjects fall below 10 ppFEV1a AURORA Phase 3 Trial Heijerman et al. Cystic Fibrosis (CF) is a life limiting genetic disease with approximately 48,000 patients in Europe and ~ 30,000 in the US - Only ~ 2 out of 5 of the ~48,000 people with CF in Europe have access to any CFTR modulators
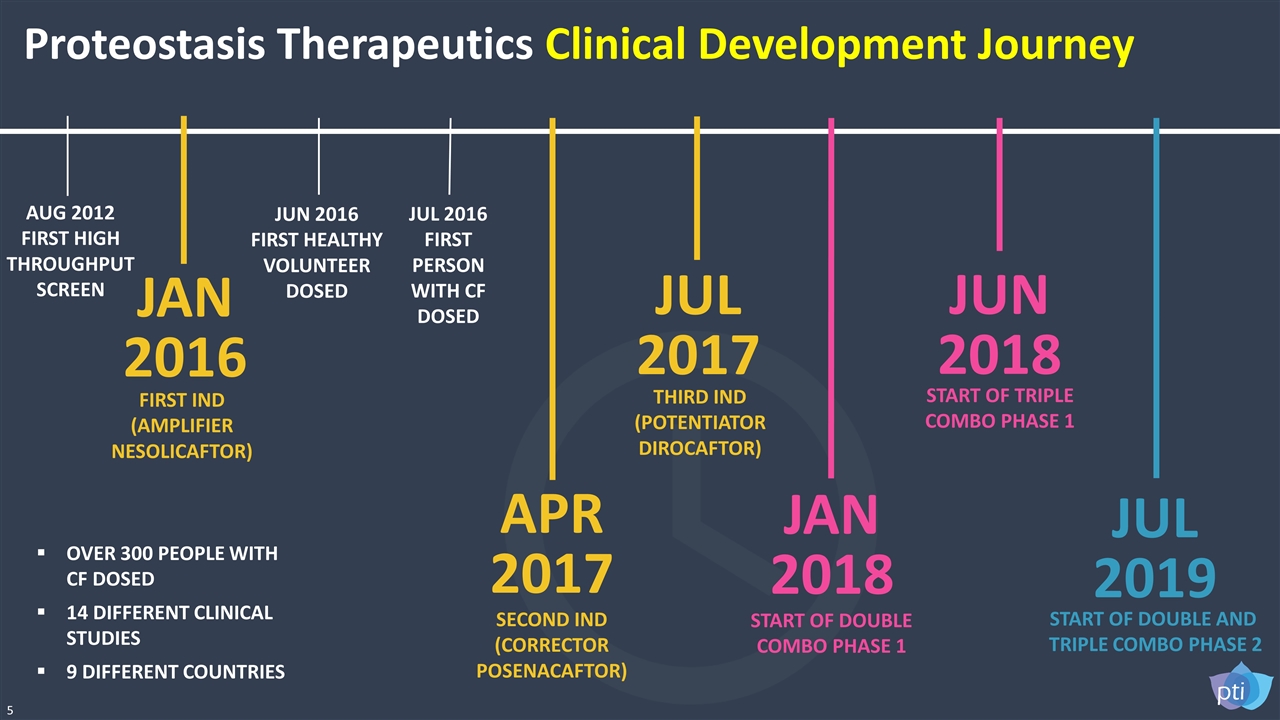
5 AUG 2012 FIRST HIGH THROUGHPUT SCREEN JUN 2016 FIRST HEALTHY VOLUNTEER DOSED JUL 2016 FIRST PERSON WITH CF DOSED APR 2017 SECOND IND (CORRECTOR POSENACAFTOR) JUL 2017 THIRD IND (POTENTIATOR DIROCAFTOR) JUN 2018 START OF TRIPLE COMBO PHASE 1 JAN 2018 START OF DOUBLE COMBO PHASE 1 JUL 2019 START OF DOUBLE AND TRIPLE COMBO PHASE 2 JAN 2016 FIRST IND (AMPLIFIER NESOLICAFTOR) OVER 300 PEOPLE WITH CF DOSED 14 DIFFERENT CLINICAL STUDIES 9 DIFFERENT COUNTRIES Proteostasis Therapeutics Clinical Development Journey
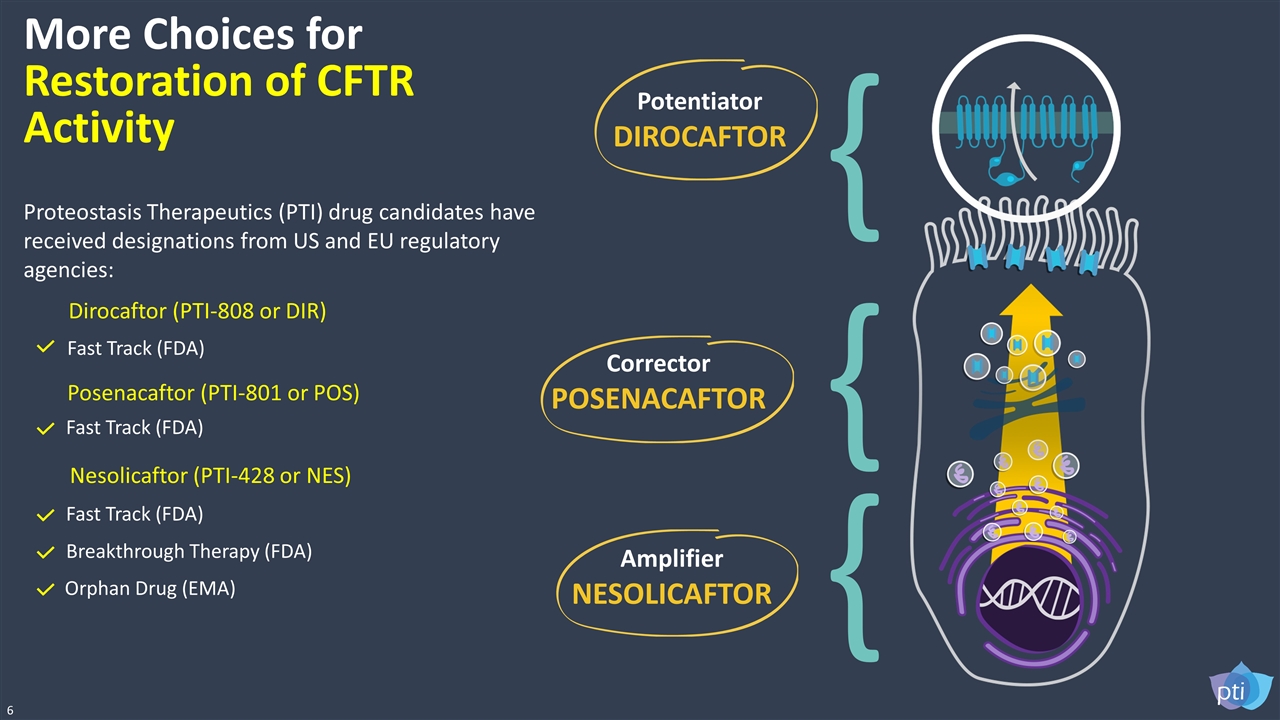
Amplifier NESOLICAFTOR { { Corrector POSENACAFTOR Potentiator DIROCAFTOR { More Choices for Restoration of CFTR Activity Dirocaftor (PTI-808 or DIR) Fast Track (FDA) Posenacaftor (PTI-801 or POS) Fast Track (FDA) Nesolicaftor (PTI-428 or NES) Breakthrough Therapy (FDA) Orphan Drug (EMA) Fast Track (FDA) 6 Proteostasis Therapeutics (PTI) drug candidates have received designations from US and EU regulatory agencies:
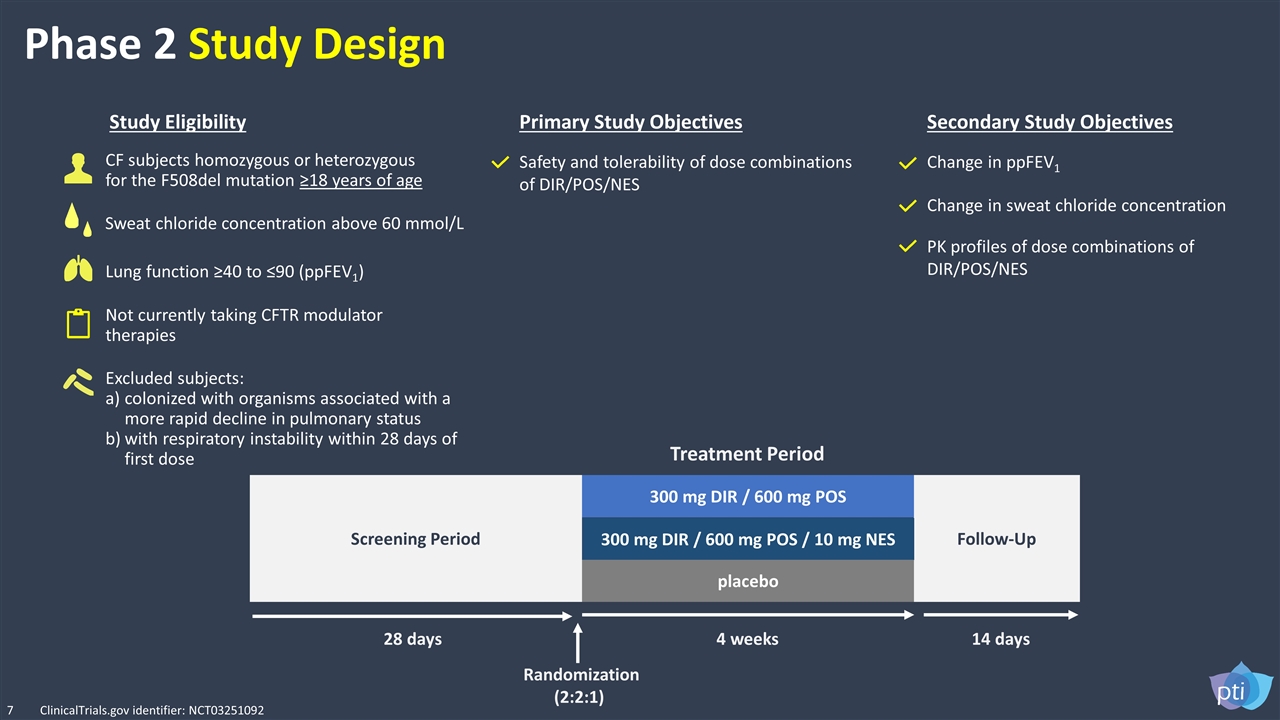
Phase 2 Study Design Not currently taking CFTR modulator therapies Safety and tolerability of dose combinations of DIR/POS/NES Change in ppFEV1 CF subjects homozygous or heterozygous for the F508del mutation ≥18 years of age Lung function ≥40 to ≤90 (ppFEV1) Excluded subjects: colonized with organisms associated with a more rapid decline in pulmonary status with respiratory instability within 28 days of first dose Study Eligibility Primary Study Objectives Secondary Study Objectives PK profiles of dose combinations of DIR/POS/NES Change in sweat chloride concentration Follow-Up Screening Period 300 mg DIR / 600 mg POS 300 mg DIR / 600 mg POS / 10 mg NES placebo 28 days 4 weeks 14 days Randomization (2:2:1) Treatment Period 7 Sweat chloride concentration above 60 mmol/L ClinicalTrials.gov identifier: NCT03251092
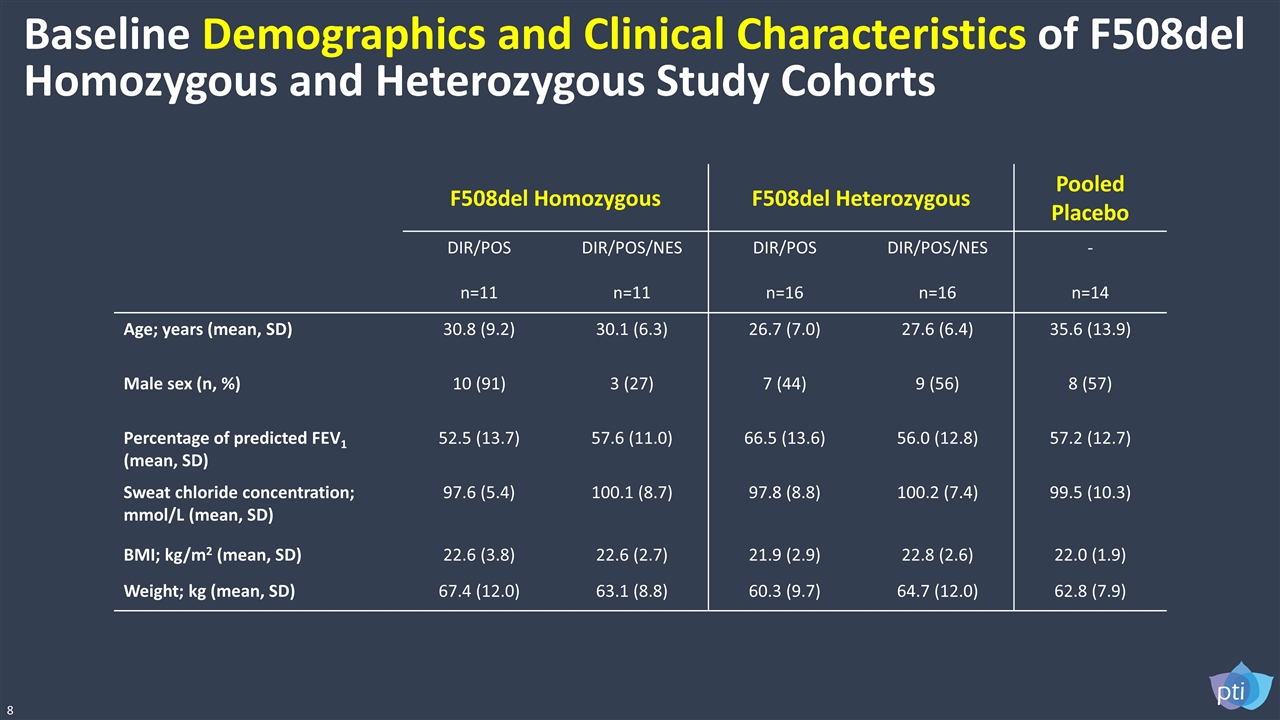
F508del Homozygous F508del Heterozygous Pooled Placebo DIR/POS n=11 DIR/POS/NES n=11 DIR/POS n=16 DIR/POS/NES n=16 - n=14 Age; years (mean, SD) 30.8 (9.2) 30.1 (6.3) 26.7 (7.0) 27.6 (6.4) 35.6 (13.9) Male sex (n, %) 10 (91) 3 (27) 7 (44) 9 (56) 8 (57) Percentage of predicted FEV1 (mean, SD) 52.5 (13.7) 57.6 (11.0) 66.5 (13.6) 56.0 (12.8) 57.2 (12.7) Sweat chloride concentration; mmol/L (mean, SD) 97.6 (5.4) 100.1 (8.7) 97.8 (8.8) 100.2 (7.4) 99.5 (10.3) BMI; kg/m2 (mean, SD) 22.6 (3.8) 22.6 (2.7) 21.9 (2.9) 22.8 (2.6) 22.0 (1.9) Weight; kg (mean, SD) 67.4 (12.0) 63.1 (8.8) 60.3 (9.7) 64.7 (12.0) 62.8 (7.9) Baseline Demographics and Clinical Characteristics of F508del Homozygous and Heterozygous Study Cohorts 8
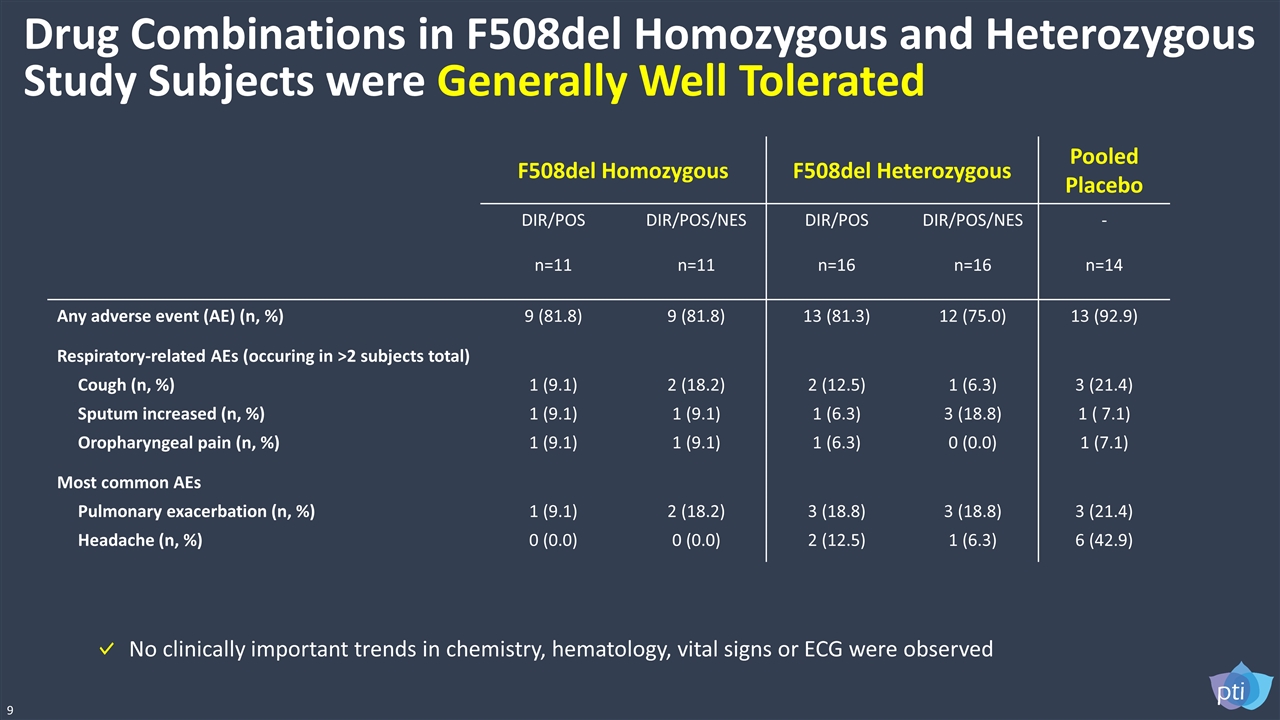
F508del Homozygous F508del Heterozygous Pooled Placebo DIR/POS n=11 DIR/POS/NES n=11 DIR/POS n=16 DIR/POS/NES n=16 - n=14 Any adverse event (AE) (n, %) 9 (81.8) 9 (81.8) 13 (81.3) 12 (75.0) 13 (92.9) Respiratory-related AEs (occuring in >2 subjects total) Cough (n, %) Sputum increased (n, %) Oropharyngeal pain (n, %) 1 (9.1) 1 (9.1) 1 (9.1) 2 (18.2) 1 (9.1) 1 (9.1) 2 (12.5) 1 (6.3) 1 (6.3) 1 (6.3) 3 (18.8) 0 (0.0) 3 (21.4) 1 ( 7.1) 1 (7.1) Most common AEs Pulmonary exacerbation (n, %) Headache (n, %) 1 (9.1) 0 (0.0) 2 (18.2) 0 (0.0) 3 (18.8) 2 (12.5) 3 (18.8) 1 (6.3) 3 (21.4) 6 (42.9) Drug Combinations in F508del Homozygous and Heterozygous Study Subjects were Generally Well Tolerated 9 No clinically important trends in chemistry, hematology, vital signs or ECG were observed
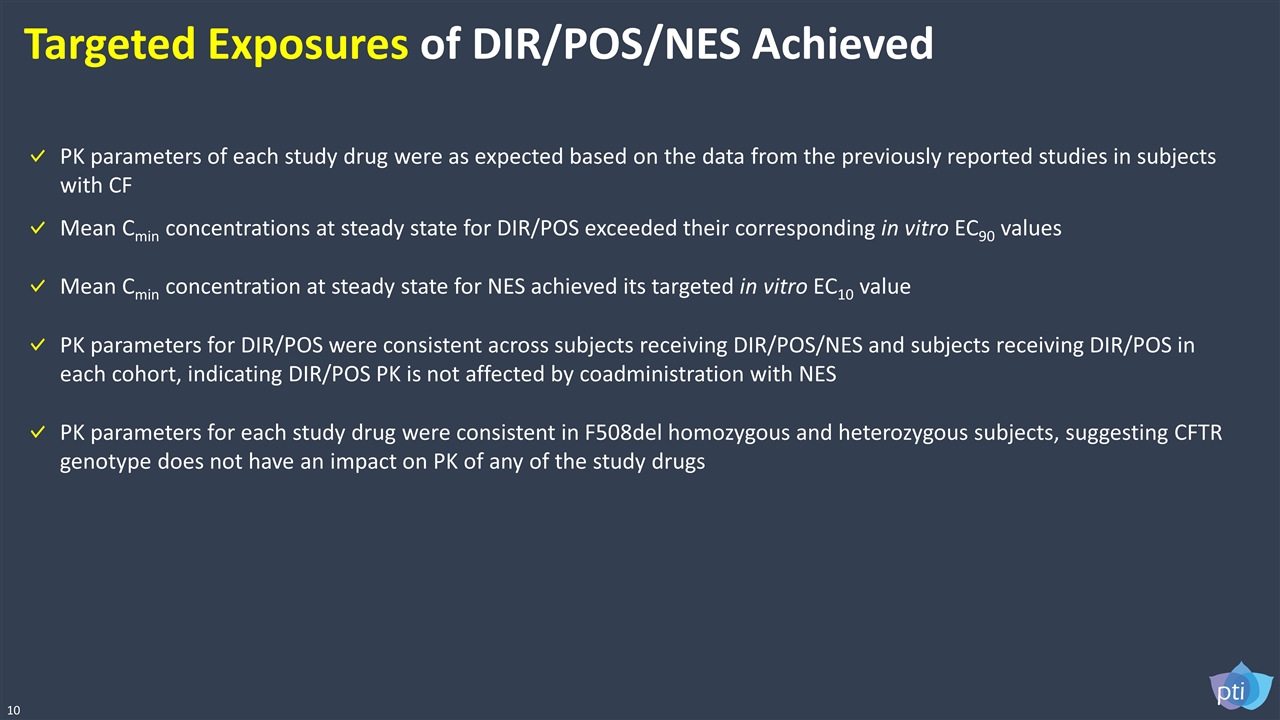
10 Targeted Exposures of DIR/POS/NES Achieved PK parameters of each study drug were as expected based on the data from the previously reported studies in subjects with CF Mean Cmin concentrations at steady state for DIR/POS exceeded their corresponding in vitro EC90 values PK parameters for DIR/POS were consistent across subjects receiving DIR/POS/NES and subjects receiving DIR/POS in each cohort, indicating DIR/POS PK is not affected by coadministration with NES PK parameters for each study drug were consistent in F508del homozygous and heterozygous subjects, suggesting CFTR genotype does not have an impact on PK of any of the study drugs Mean Cmin concentration at steady state for NES achieved its targeted in vitro EC10 value
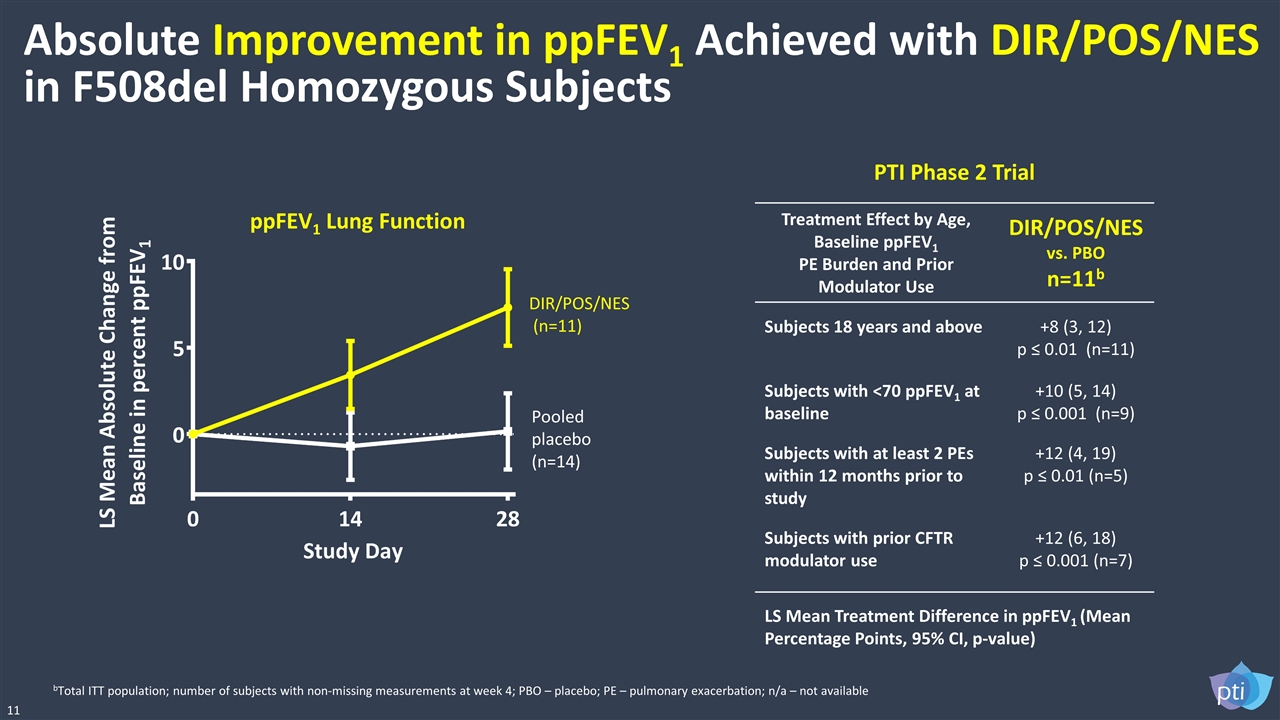
Absolute Improvement in ppFEV1 Achieved with DIR/POS/NES in F508del Homozygous Subjects 11 ppFEV1 Lung Function Pooled placebo (n=14) DIR/POS/NES (n=11) bTotal ITT population; number of subjects with non-missing measurements at week 4; PBO – placebo; PE – pulmonary exacerbation; n/a – not available LS Mean Treatment Difference in ppFEV1 (Mean Percentage Points, 95% CI, p-value) PTI Phase 2 Trial Treatment Effect by Age, Baseline ppFEV1 PE Burden and Prior Modulator Use DIR/POS/NES vs. PBO n=11b Subjects 18 years and above +8 (3, 12) p ≤ 0.01 (n=11) Subjects with <70 ppFEV1 at baseline +10 (5, 14) p ≤ 0.001 (n=9) Subjects with at least 2 PEs within 12 months prior to study +12 (4, 19) p ≤ 0.01 (n=5) Subjects with prior CFTR modulator use +12 (6, 18) p ≤ 0.001 (n=7)
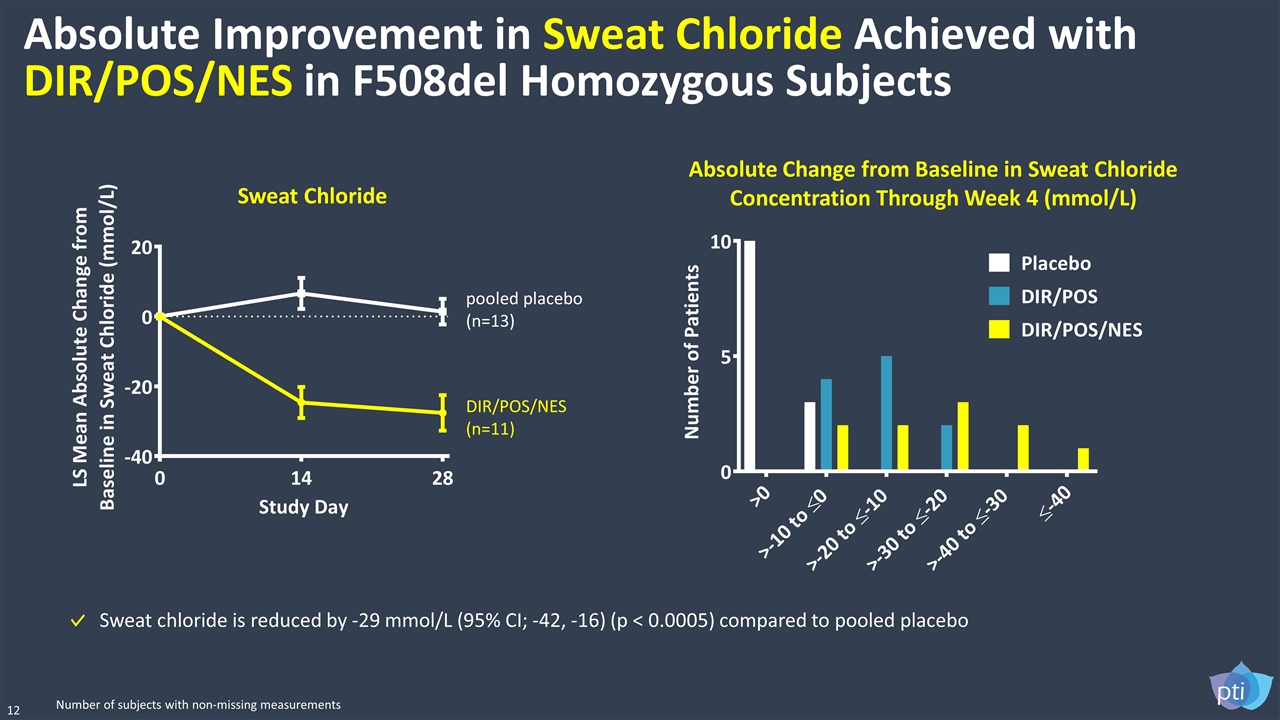
Absolute Improvement in Sweat Chloride Achieved with DIR/POS/NES in F508del Homozygous Subjects 12 Sweat Chloride Sweat chloride is reduced by -29 mmol/L (95% CI; -42, -16) (p < 0.0005) compared to pooled placebo pooled placebo (n=13) DIR/POS/NES (n=11) Number of subjects with non-missing measurements Absolute Change from Baseline in Sweat Chloride Concentration Through Week 4 (mmol/L)
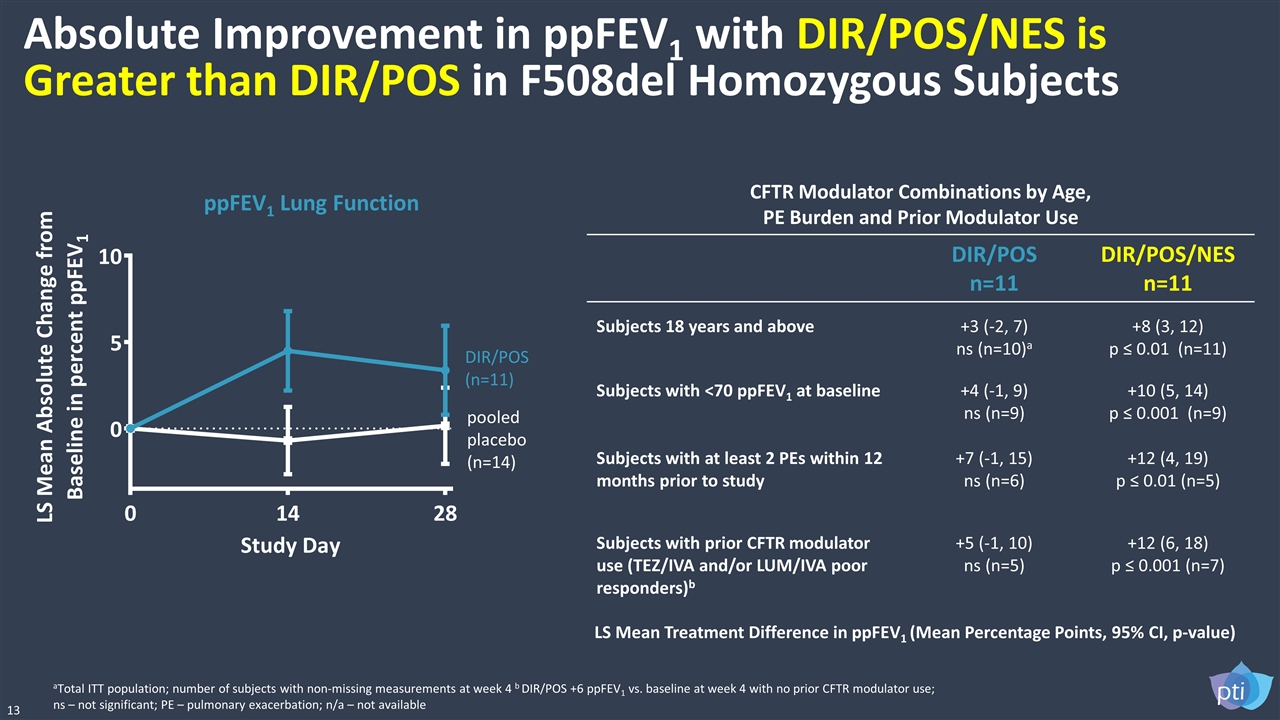
Absolute Improvement in ppFEV1 with DIR/POS/NES is Greater than DIR/POS in F508del Homozygous Subjects 13 CFTR Modulator Combinations by Age, PE Burden and Prior Modulator Use DIR/POS n=11 DIR/POS/NES n=11 Subjects 18 years and above +3 (-2, 7) ns (n=10)a +8 (3, 12) p ≤ 0.01 (n=11) Subjects with <70 ppFEV1 at baseline +4 (-1, 9) ns (n=9) +10 (5, 14) p ≤ 0.001 (n=9) Subjects with at least 2 PEs within 12 months prior to study +7 (-1, 15) ns (n=6) +12 (4, 19) p ≤ 0.01 (n=5) Subjects with prior CFTR modulator use (TEZ/IVA and/or LUM/IVA poor responders)b +5 (-1, 10) ns (n=5) +12 (6, 18) p ≤ 0.001 (n=7) ppFEV1 Lung Function pooled placebo (n=14) DIR/POS (n=11) aTotal ITT population; number of subjects with non-missing measurements at week 4 b DIR/POS +6 ppFEV1 vs. baseline at week 4 with no prior CFTR modulator use; ns – not significant; PE – pulmonary exacerbation; n/a – not available LS Mean Treatment Difference in ppFEV1 (Mean Percentage Points, 95% CI, p-value)
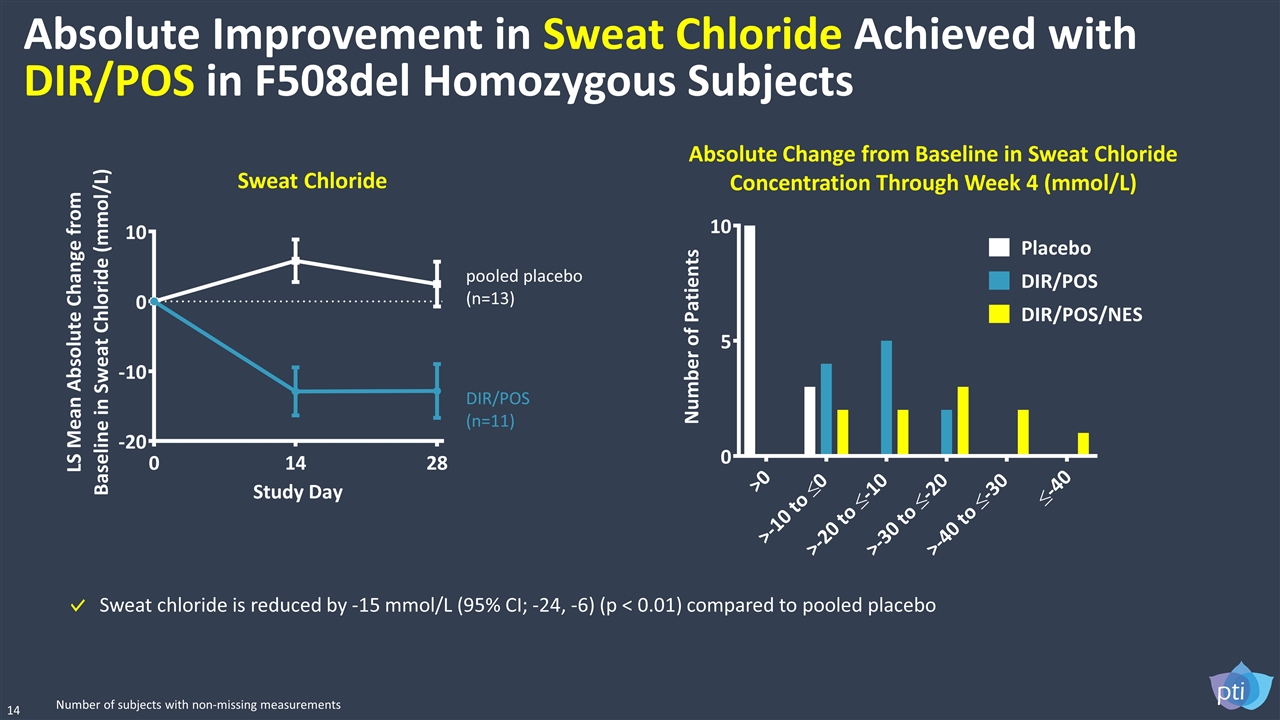
Absolute Improvement in Sweat Chloride Achieved with DIR/POS in F508del Homozygous Subjects pooled placebo (n=13) DIR/POS (n=11) 14 Sweat chloride is reduced by -15 mmol/L (95% CI; -24, -6) (p < 0.01) compared to pooled placebo Sweat Chloride Absolute Change from Baseline in Sweat Chloride Concentration Through Week 4 (mmol/L) Number of subjects with non-missing measurements
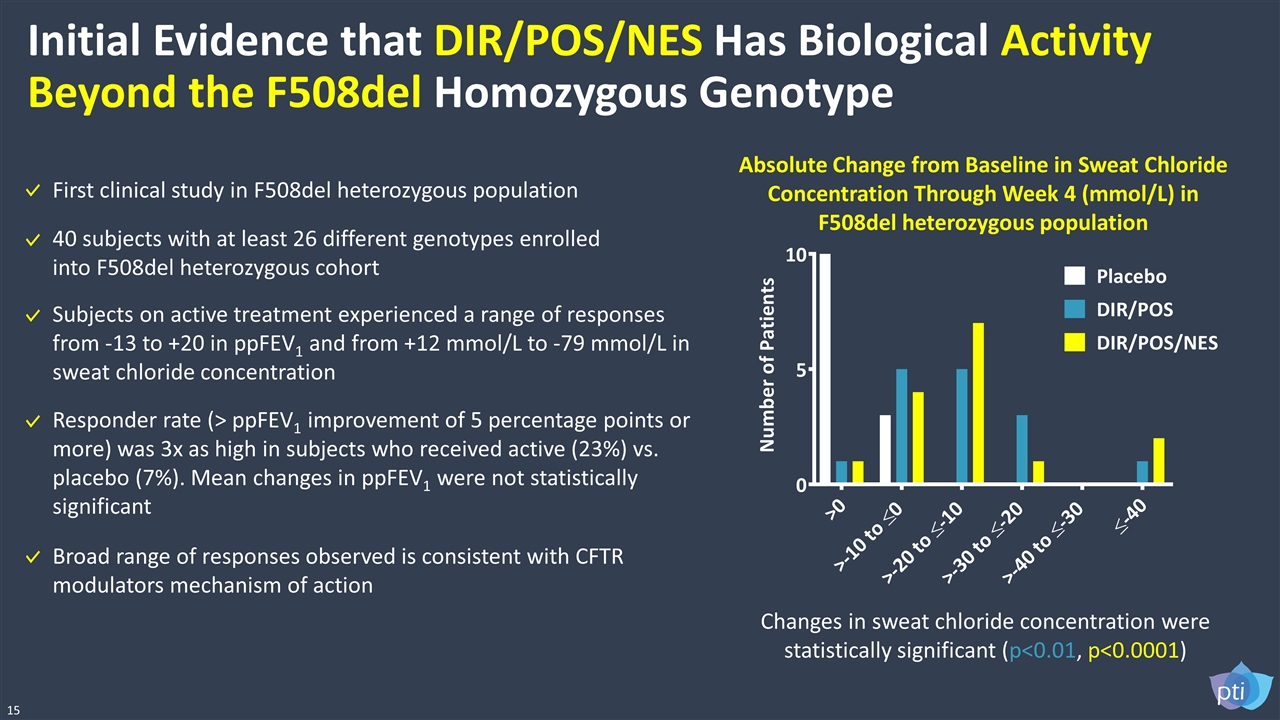
Initial Evidence that DIR/POS/NES Has Biological Activity Beyond the F508del Homozygous Genotype Responder rate (> ppFEV1 improvement of 5 percentage points or more) was 3x as high in subjects who received active (23%) vs. placebo (7%). Mean changes in ppFEV1 were not statistically significant Absolute Change from Baseline in Sweat Chloride Concentration Through Week 4 (mmol/L) in F508del heterozygous population Changes in sweat chloride concentration were statistically significant (p<0.01, p<0.0001) 15 First clinical study in F508del heterozygous population Subjects on active treatment experienced a range of responses from -13 to +20 in ppFEV1 and from +12 mmol/L to -79 mmol/L in sweat chloride concentration Broad range of responses observed is consistent with CFTR modulators mechanism of action 40 subjects with at least 26 different genotypes enrolled into F508del heterozygous cohort
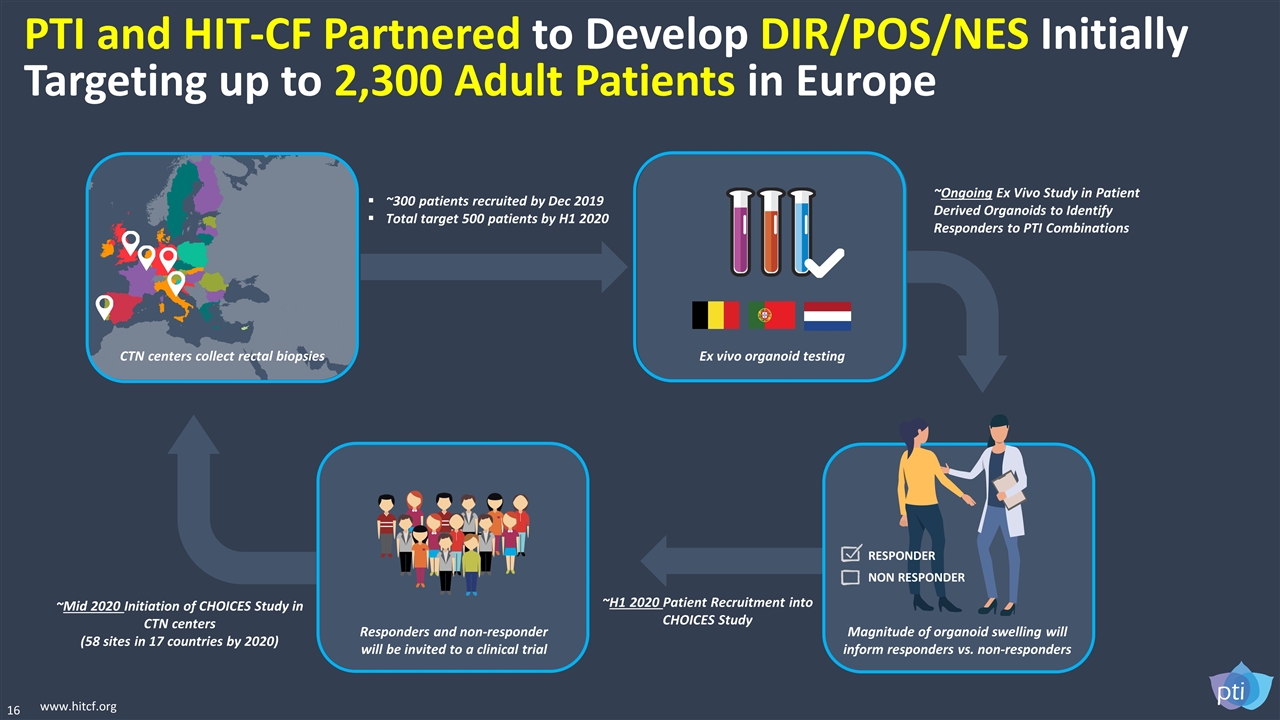
16 www.hitcf.org PTI and HIT-CF Partnered to Develop DIR/POS/NES Initially Targeting up to 2,300 Adult Patients in Europe Ex vivo organoid testing Magnitude of organoid swelling will inform responders vs. non-responders Responders and non-responder will be invited to a clinical trial CTN centers collect rectal biopsies RESPONDER NON RESPONDER ~300 patients recruited by Dec 2019 Total target 500 patients by H1 2020 ~Ongoing Ex Vivo Study in Patient Derived Organoids to Identify Responders to PTI Combinations ~H1 2020 Patient Recruitment into CHOICES Study ~Mid 2020 Initiation of CHOICES Study in CTN centers (58 sites in 17 countries by 2020)
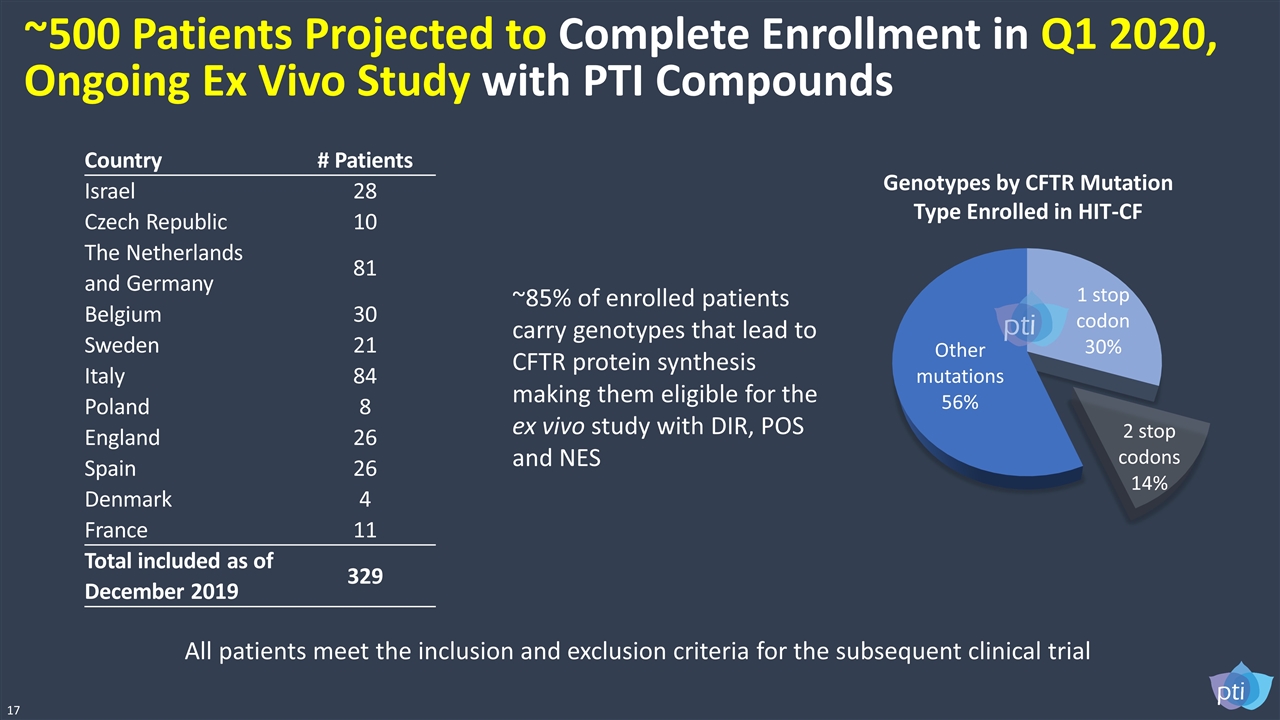
All patients meet the inclusion and exclusion criteria for the subsequent clinical trial Country # Patients Israel 28 Czech Republic 10 The Netherlands and Germany 81 Belgium 30 Sweden 21 Italy 84 Poland 8 England 26 Spain 26 Denmark 4 France 11 Total included as of December 2019 329 ~85% of enrolled patients carry genotypes that lead to CFTR protein synthesis making them eligible for the ex vivo study with DIR, POS and NES 17 Genotypes by CFTR Mutation Type Enrolled in HIT-CF ~500 Patients Projected to Complete Enrollment in Q1 2020, Ongoing Ex Vivo Study with PTI Compounds

THANK YOU #CForward “Proteostasis is striving to shake up the CF community in ways better than the vest ever could.” Brad Dell, a person with CF