
Exhibit 99.1 Developing next generation immunotherapies for cancer patients July 2022 1

Disclaimers and other information This presentation contains forward-looking statements based upon the current expectations of Yumanity Therapeutics, Inc. (“Yumanity”) and Kineta, Inc. (“Kineta”). Forward-looking statements involve risks and uncertainties and include, but are not limited to, statements about the structure, timing and completion of the proposed transaction; the listing of the combined company on Nasdaq after the closing of the proposed merger; expectations regarding the ownership structure of the combined company after the closing of the proposed merger; the expected executive officers and directors of the combined company; the expected cash position of each of Yumanity and Kineta and the combined company at the closing of the proposed merger; the future operations of the combined company; the nature, strategy and focus of the combined company; the development and commercial potential and potential benefits of any product candidates of the combined company; the executive and board structure of the combined company; the location of the combined company’s corporate headquarters; anticipated preclinical and clinical drug development activities and related timelines, including the expected timing for data and other clinical and preclinical results; Kineta having sufficient resources to advance its pipeline; and other statements that are not historical fact. Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of these risks and uncertainties, which include, without limitation: (i) the risk that the conditions to the closing of the proposed transaction are not satisfied, including the failure to timely obtain stockholder approval for the transaction, if at all; (ii) uncertainties as to the timing of the consummation of the proposed transaction and the ability of each of Yumanity and Kineta to consummate the proposed merger; (iii) risks related to Yumanity’s ability to manage its operating expenses and its expenses associated with the proposed transaction pending closing; (iv) risks related to the failure or delay in obtaining required approvals from any governmental or quasi-governmental entity necessary to consummate the proposed transaction; (v) the risk that as a result of adjustments to the exchange ratio, Yumanity stockholders and Kineta shareholders could own more or less of the combined company than is currently anticipated; (vi) risks related to the market price of Yumanity’s common stock relative to the exchange ratio; (vii) unexpected costs, charges or expenses resulting from either or both of the proposed transaction; (viii) potential adverse reactions or changes to business relationships resulting from the announcement or completion of the proposed transaction; (ix) the risk that the amount of the dividend distributed to Yumanity stockholders in connection with Yumanity’s previously announced asset sale, if any, may be lower than currently anticipated; (x) risks related to the inability of the combined company to obtain sufficient additional capital to continue to advance these product candidates and its preclinical programs; (xi) uncertainties in obtaining successful clinical results for product candidates and unexpected costs that may result therefrom; (xii) risks related to the failure to realize any value from product candidates and preclinical programs being developed and anticipated to be developed in light of inherent risks and difficulties involved in successfully bringing product candidates to market; and (xiii) risks associated with the possible failure to realize certain anticipated benefits of the proposed transaction, including with respect to future financial and operating results. Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of these risks and uncertainties. These and other risks and uncertainties are more fully described in periodic filings with the U.S. Securities and Exchange Commission (the SEC ), including the factors described in the section titled “Risk Factors” in Yumanity’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2022 filed with the SEC, and in other filings that Yumanity makes and will make with the SEC in connection with the proposed transaction, including the proxy statement/prospectus described under “Additional Information and Where to Find It.” You should not place undue reliance on these forward-looking statements, which are made only as of the date hereof or as of the dates indicated in the forward-looking statements. Except as required by law, Yumanity expressly disclaims any obligation or undertaking to update or revise any forward-looking statements contained herein to reflect any change in its expectations with regard thereto or any change in events, conditions or circumstances on which any such statements are based. 2

Disclaimers and other information Additional Information and Where to Find It This presentation may be deemed to be solicitation material with respect to the proposed transaction between Yumanity and Kineta. In connection with the proposed transaction, Yumanity intends to file relevant materials with the SEC, including a registration statement on Form S-4 that will contain a prospectus and a proxy statement. Yumanity will mail the proxy statement/prospectus to the Yumanity stockholders, and the securities may not be sold or exchanged until the registration statement becomes effective. Investors and securityholders of Yumanity and Kineta are urged to read these materials when they become available because they will contain important information about Yumanity, Kineta and the proposed transaction. This presentation is not a substitute for the registration statement, definitive proxy statement/prospectus or any other documents that Yumanity may file with the SEC or send to securityholders in connection with the proposed transaction. Investors and securityholders may obtain free copies of the documents filed with the SEC, once available, on Yumanity’s website at www.yumanity.com, on the SEC’s website at www.sec.gov or by directing a request to Yumanity’s Investor Relations at (212) 213-0006 ext. 331. This presentation shall not constitute an offer to sell or the solicitation of an offer to sell or the solicitation of an offer to buy any securities, nor shall there be any sale of securities in any jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such jurisdiction. No offering of securities shall be made except by means of a prospectus meeting the requirements of Section 10 of the Securities Act of 1933, as amended. Participants in the Solicitation Each of Yumanity, Kineta and their respective directors and executive officers may be deemed to be participants in the solicitation of proxies from the stockholders of Yumanity in connection with the proposed transaction. Information about the executive officers and directors of Yumanity is set forth in Yumanity’s Definitive Proxy Statement on Schedule 14A relating to the 2022 Annual Meeting of Stockholders, filed with the SEC on April 25, 2022. Other information regarding the interests of such individuals, who may be deemed to be participants in the solicitation of proxies for the stockholders of Yumanity, will be set forth in the proxy statement/prospectus, which will be included in Yumanity’s registration statement on Form S-4 when it is filed with the SEC. You may obtain free copies of these documents as described above. 3

Company highlights Strategic Value driving Immuno-oncology PiiONEER™ Strong financial partnerships catalysts platform pipeline position Multiple anticipated near- Proprietary innate immunity KVA12.1: best-in-class anti- Cash runway expected to term milestones to drive focused platform developing VISTA mAb initiating Phase fund operations for 6+ company valuation fully human mAbs 1/2 study in advanced solid quarters tumors in Q4 αCD27 mAb for solid tumors initiating IND enabling studies αCD24 mAb in discovery 4
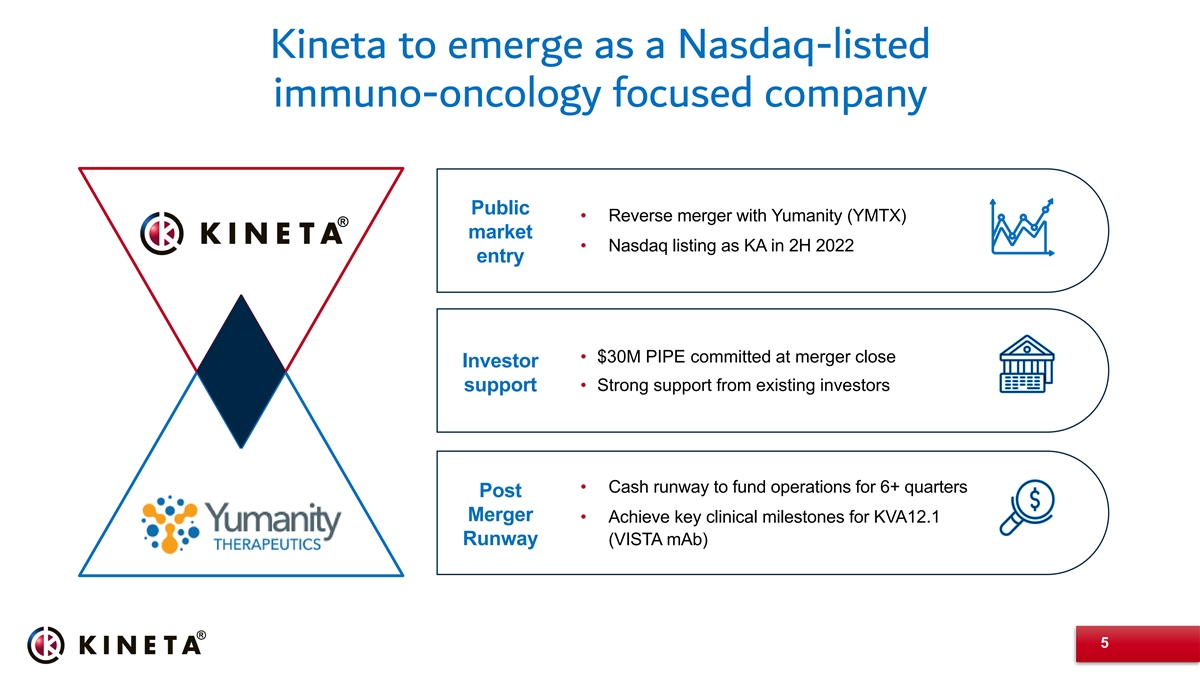
Kineta to emerge as a Nasdaq-listed immuno-oncology focused company Public • Reverse merger with Yumanity (YMTX) market • Nasdaq listing as KA in 2H 2022 entry • $30M PIPE committed at merger close Investor support• Strong support from existing investors • Cash runway to fund operations for 6+ quarters Post Merger • Achieve key clinical milestones for KVA12.1 Runway (VISTA mAb) 5

Experienced leadership team Shawn Craig Pauline Thierry Molly Jacques Chanya Sang-Seon Iadonato, PhD Philips Kenny Guillaudeux, PhD Dorr Bouchy Swartz Yun, PhD Chief Executive President General Chief Scientific Head of Human SVP Business Dev & Sr. Director, Finance & Head of Officer Counsel Officer Resources & Operations Communications Corporate Controller Scientific Affairs 6
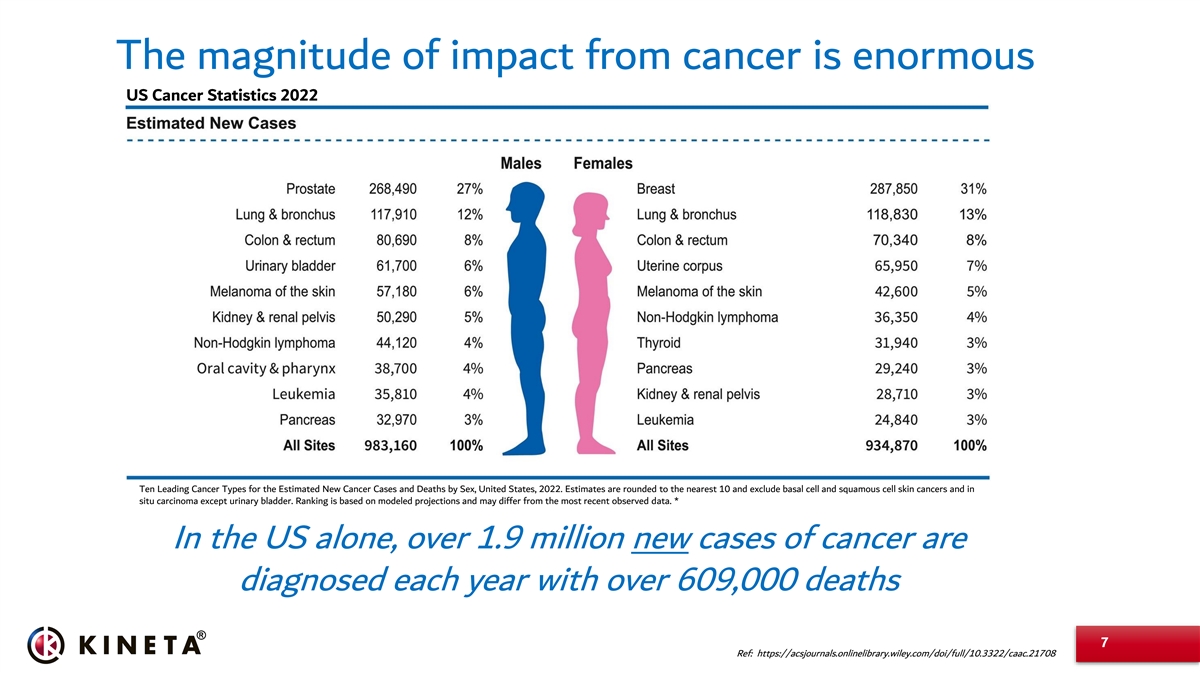
The magnitude of impact from cancer is enormous US Cancer Statistics 2022 Ten Leading Cancer Types for the Estimated New Cancer Cases and Deaths by Sex, United States, 2022. Estimates are rounded to the nearest 10 and exclude basal cell and squamous cell skin cancers and in situ carcinoma except urinary bladder. Ranking is based on modeled projections and may differ from the most recent observed data. * In the US alone, over 1.9 million new cases of cancer are diagnosed each year with over 609,000 deaths 7 Ref: https://acsjournals.onlinelibrary.wiley.com/doi/full/10.3322/caac.21708

Kineta is developing innate immune therapies to address the major challenges with current cancer therapy Next-generation cancer Blockade and down-regulation of immune response treatments require: • Improving survival for CPI non-responders (70-80%) T cells lose cancer fighting function • Reprogramming the immune system to attack cancer Tumor cells are invisible to • Integrating the innate and immune system adaptive immune response 8
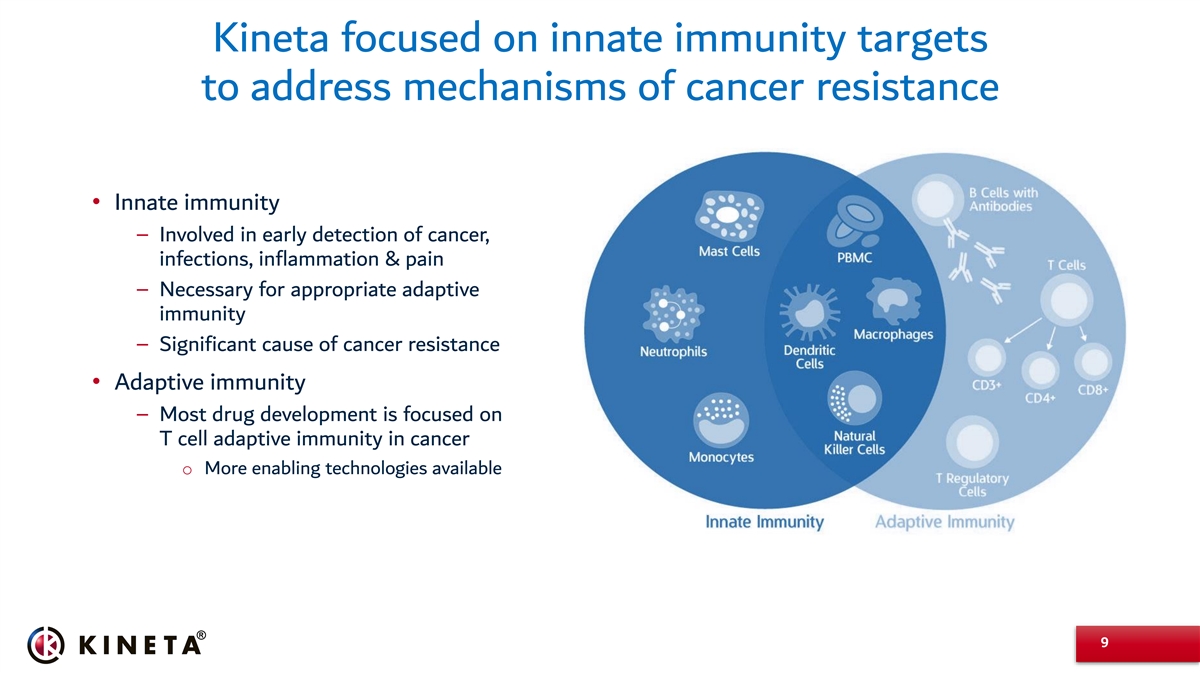
Kineta focused on innate immunity targets to address mechanisms of cancer resistance • Innate immunity – Involved in early detection of cancer, infections, inflammation & pain – Necessary for appropriate adaptive immunity – Significant cause of cancer resistance • Adaptive immunity – Most drug development is focused on T cell adaptive immunity in cancer o More enabling technologies available 9
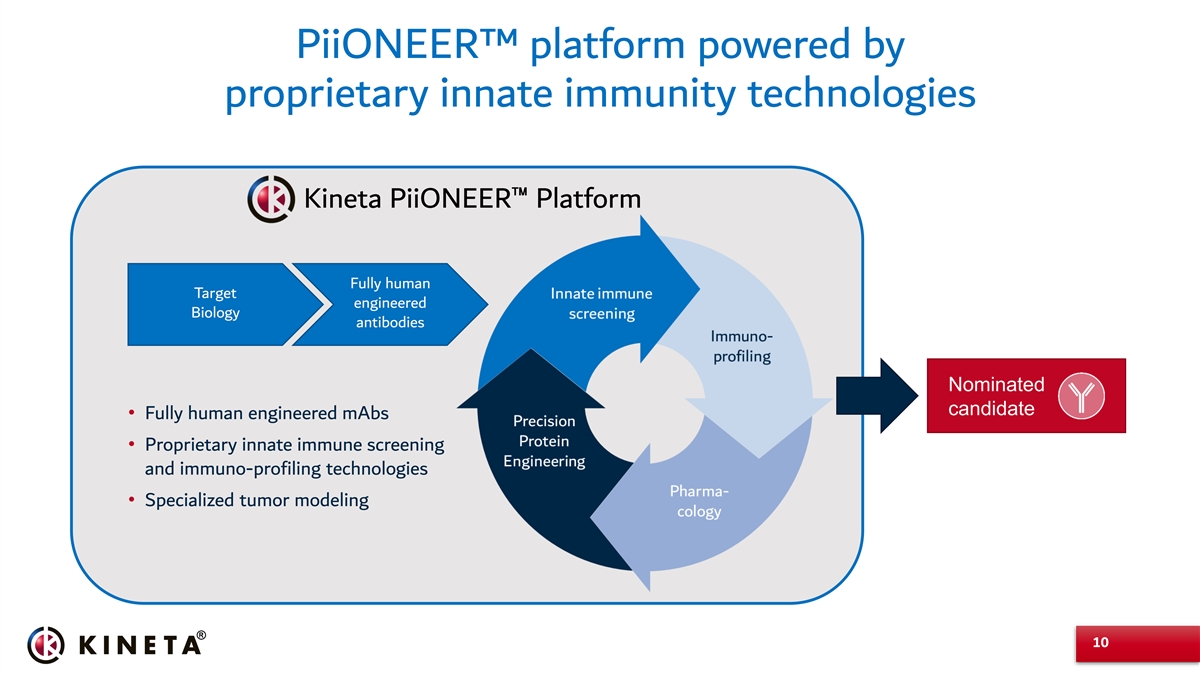
PiiONEER™ platform powered by proprietary innate immunity technologies Kineta PiiONEER™ Platform Fully human Target engineered Biology antibodies Nominated candidate • Fully human engineered mAbs • Proprietary innate immune screening and immuno-profiling technologies • Specialized tumor modeling 10

Immuno-oncology pipeline addressing the mechanisms of cancer resistance Lead IND- Commercial Anticipated Milestones Program/Target Indications Discovery selection enabling Phase 1 Phase 2/3 Rights Advanced solid tumors• Q3 2022: IND filing KVA12.1 NSCLC, CRC, OC, RCC & • Q4 2022: Initiate Phase 1 clinical study αVISTA mAb • Q4 2023: Initial Phase 1 data readout SCCHN Hematologic tumors • 2H 2023: IND filing αCD27 mAb • 2H 2023: Start Phase 1 clinical study Advanced solid tumors • 2H 2023: Nominate candidate / start IND- Solid tumors αCD24 mAb enabling studies 11

Strategic partnerships validate company science and provide potential revenue to Kineta Ongoing collaborations Licensed with no research obligations by Kineta KCP506 for chronic Neuromuscular diseases- Oncology Cystic fibrosis neuropathic pain ALS Discovery Discovery Program Preclinical Discovery Partner • Up to $359M in milestones• Up to $500M in milestones• Up to $96M in milestones• Up to $965M in commercial only milestones • Received $21M in development • Royalties on net sales• Royalties on net sales funding since inception • Royalties on net sales Key deal • Research collaboration & license • Worldwide license agreement • Single to double-digit royalties agreement• Revenue share on sub-license terms payments • Research collaboration, option & license agreement• Worldwide license agreement 12

KVA12.1 Potential best-in-class VISTA blocking immunotherapy 13

KVA12.1: Potential best-in-class VISTA blocking immunotherapy – Highly expressed in NSCLC, ovarian, colon, pancreatic and gastric cancers – Correlates with poor outcomes in cancer patients VISTA – Up-regulated after CPI therapy; associated with treatment failure – Engineered IgG1 mAb that binds to a unique epitope – Demonstrated single agent efficacy and in combination with PD-1 KVA12.1 differentiation – Binds preferentially at physiologic and acidic pH – Well-tolerated with no CRS-associated cytokine secretion – Dose escalation study: KVA12.1 single agent and in combination with pembrolizumab Clinical trial – Assess safety, tolerability, PK, immunogenicity and tumor responses 14
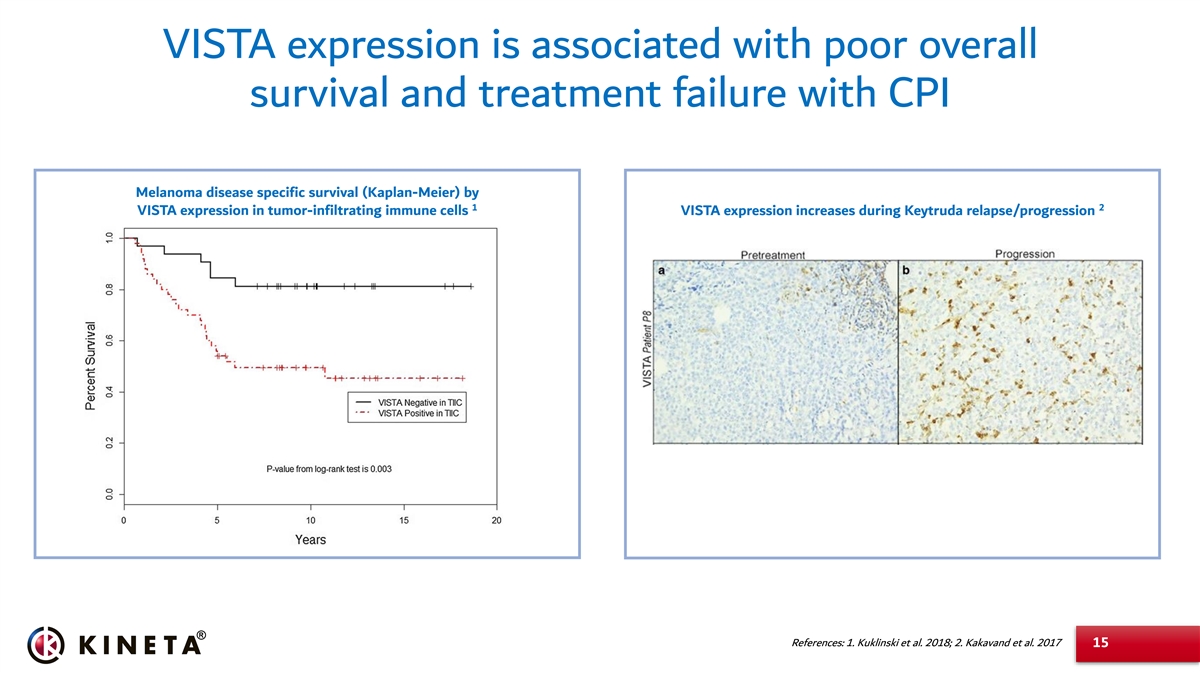
VISTA expression is associated with poor overall survival and treatment failure with CPI Melanoma disease specific survival (Kaplan-Meier) by 1 2 VISTA expression in tumor-infiltrating immune cells VISTA expression increases during Keytruda relapse/progression References: 1. Kuklinski et al. 2018; 2. Kakavand et al. 2017 15

Blocking VISTA drives an efficient polyfunctional immune response to turn cold tumors hot ✓ Inhibits MDSC (myeloid-derived suppressor cells) ✓ Reprograms M2 to M1 macrophages ✓ Promotes T function eff ✓ Enhances NK cell activation ✓ Enhances dendritic cell activity 16 Kineta Graphic – all rights reserved

KVA12.1 reverses immunosuppression in the TME Increases monocyte Reduces MDSC-mediated Increases HLA-dependent differentiation and activation T cell suppression T cell activation HLA-DR, CD80, CD86, CXCL10 (CD56+) HLA-DR, Donor #4058, 24hr Gated NK-Cell Expressing CD137, Donor #4058, 24hr 6000 VSTB174 (CI-8993) 1000 VSTB174 (CI-8993) KVA12104 KVA12104 KVA12.1 750 KVA12.1 4000 Enhances IgG1 IgG1 500 NK cell 2000 250 activation 0 0 -depleted PBMC + Monocytes NK PBMC + Monocytes PBMC + Monocytes Kineta data on file 17 VSTB174 3ug/ml VSTB174 0.3ug/ml VSTB174 0.03ug/ml KVA12104 3ug/ml KVA12104 0.3ug/ml KVA12104 0.03ug/ml KVA12123 3ug/ml KVA12123 0.3ug/ml KVA12123 0.03ug/ml IgG1 3ug/ml VSTB174 3ug/ml VSTB174 0.3ug/ml VSTB174 0.03ug/ml KVA12104 3ug/ml KVA12104 0.3ug/ml KVA12104 0.03ug/ml KVA12123 3ug/ml KVA12123 0.3ug/ml KVA12123 0.03ug/ml IgG1 3ug/ml VSTB174 3ug/ml VSTB174 0.3ug/ml VSTB174 0.03ug/ml KVA12104 3ug/ml KVA12104 0.3ug/ml KVA12104 0.03ug/ml KVA12123 3ug/ml KVA12123 0.3ug/ml KVA12123 0.03ug/ml IgG1 3ug/ml HLA-DR MFI CD137 MFI

KVA12.1 demonstrates strong efficacy as a monotherapy in cold tumors and in combination with CPIs Monotherapy Monotherapy Combination Therapy Combination Therapy Bladder Cancer Model MB49 T Cell Lymphoma Model EG7 Colon Carcinoma Model MC38* Bladder Cancer Model MB49* hVISTA KI mice hVISTA KI mice hVISTA KI mice hVISTA KI mice Mean Tumor Volume Mean Tumor Volume Mean Tumor Volume Mean Tumor Volume 2000 1500 2000 Control ms IgG2a Control ms IgG2a Human IgG1 3000 IgG Control KVA-12.2a KVA12.1 KVA-12.2a KVA12.2a Anti-mPD1 Anti-mPD1 1500 1500 KVA12.1/anti-mPD1 Combo KVA12.2a / Anti-mPD-1 1000 2000 1000 1000 * 500 1000 500 500 0 0 0 0 0 2 4 6 8 10 12 14 16 18 20 22 24 26 0 2 4 6 8 10 12 14 16 18 20 22 24 26 0 5 10 15 20 25 0 10 20 30 40 Days Post Implantation Days Post Implantation Days Post Implantation Days Post Implantation Tumor Growth Inhibition Tumor Growth Inhibition Tumor Growth Inhibition Tumor Growth Inhibition Anti-VISTA: 75% Anti-VISTA: 66% Anti-VISTA: 35-42% Anti-VISTA: 40% Anti-PD1: 42-60% Anti-PD1: 67% Combo: 68% Combo: 85% *Combination therapy studies used sub-optimal doses of each agent Kineta data on file 18 3 Avg. Tumor Volume (mm ) 3 Avg. Tumor Volume (mm ) 3 Tumor Volume (mm ) 3 Avg. tumor volume (mm )
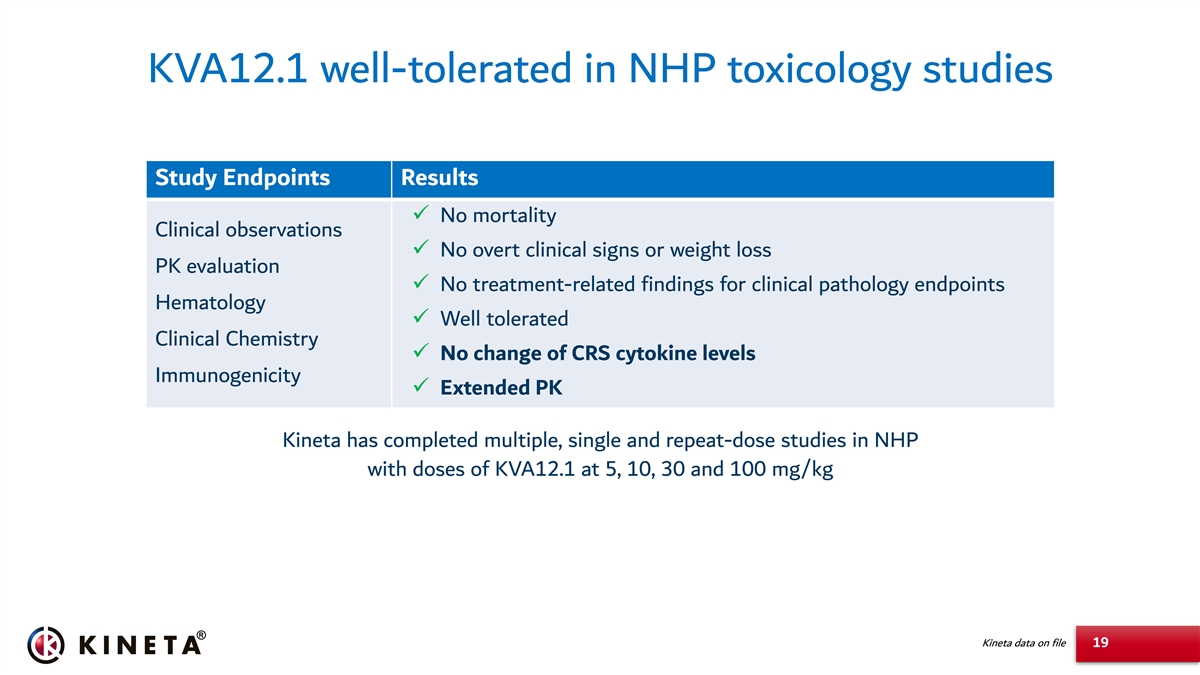
KVA12.1 well-tolerated in NHP toxicology studies Study Endpoints Results ✓ No mortality Clinical observations ✓ No overt clinical signs or weight loss PK evaluation ✓ No treatment-related findings for clinical pathology endpoints Hematology ✓ Well tolerated Clinical Chemistry ✓ No change of CRS cytokine levels Immunogenicity ✓ Extended PK Kineta has completed multiple, single and repeat-dose studies in NHP with doses of KVA12.1 at 5, 10, 30 and 100 mg/kg Kineta data on file 19

VISTA is highly expressed in lung, colon and ovarian cancers Human tumors 1 1 2 Lung Colon Ovarian Normal Normal Normal 3 Gastric Cancer NSCLC Adeno Ovarian Carcinoma Cancer 10x 20x 10x 20x 10x 20x References: 1. Kineta data on file 20 2. Mulati, K., et al. Br J Cancer 120, 115–127 (2019)
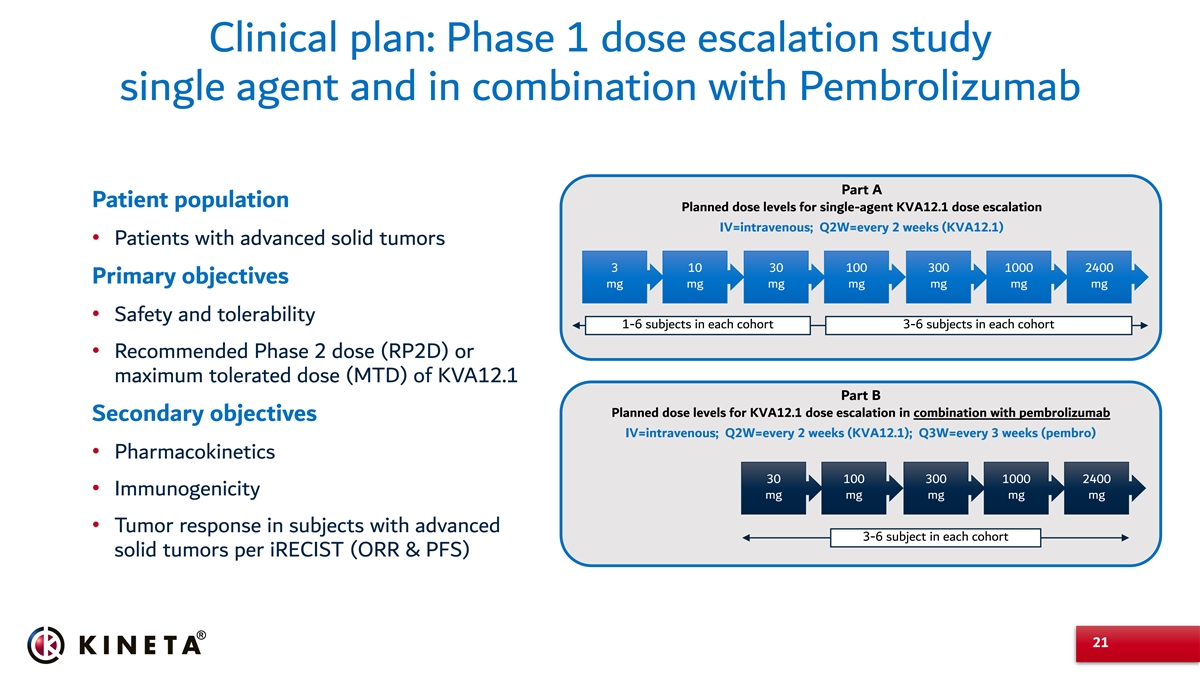
Clinical plan: Phase 1 dose escalation study single agent and in combination with Pembrolizumab Part A Patient population Planned dose levels for single-agent KVA12.1 dose escalation IV=intravenous; Q2W=every 2 weeks (KVA12.1) • Patients with advanced solid tumors 3 10 30 100 300 1000 2400 Primary objectives mg mg mg mg mg mg mg • Safety and tolerability 1-6 subjects in each cohort 3-6 subjects in each cohort • Recommended Phase 2 dose (RP2D) or maximum tolerated dose (MTD) of KVA12.1 Part B Planned dose levels for KVA12.1 dose escalation in combination with pembrolizumab Secondary objectives IV=intravenous; Q2W=every 2 weeks (KVA12.1); Q3W=every 3 weeks (pembro) • Pharmacokinetics 30 100 300 1000 2400 • Immunogenicity mg mg mg mg mg • Tumor response in subjects with advanced 3-6 subject in each cohort solid tumors per iRECIST (ORR & PFS) 21
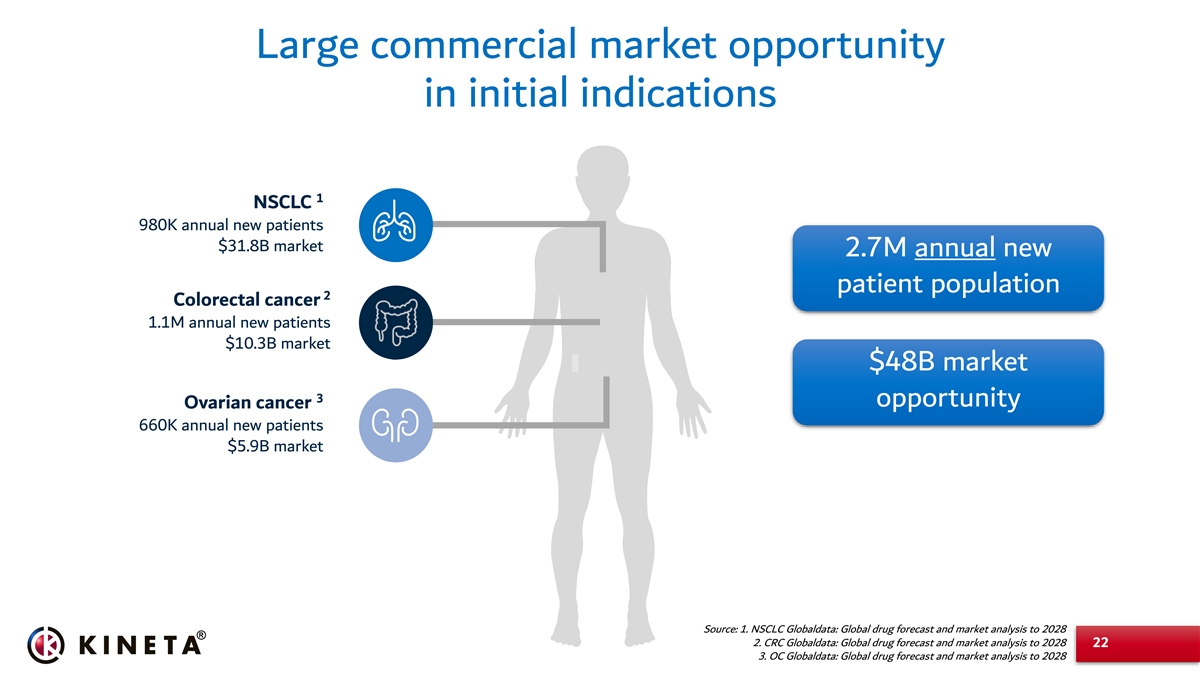
Large commercial market opportunity in initial indications 1 NSCLC 980K annual new patients $31.8B market 2.7M annual new patient population 2 Colorectal cancer 1.1M annual new patients $10.3B market $48B market 3 opportunity Ovarian cancer 660K annual new patients $5.9B market Source: 1. NSCLC Globaldata: Global drug forecast and market analysis to 2028 2. CRC Globaldata: Global drug forecast and market analysis to 2028 22 3. OC Globaldata: Global drug forecast and market analysis to 2028
Anti-CD27 agonist mAb immunotherapy 23

Anti-CD27 agonists generates new populations of functional anti-tumor immune cells ✓ Generates new tumor infiltrating T cells ✓ Broader repertoire of antigen- reactive T cells ✓ Enhances NK cell activation ✓ Activates low affinity antigens 24 Kineta Graphic – all rights reserved

Activating CD27 demonstrates strong efficacy as a monotherapy and in combination with CPIs Monotherapy Combination Therapy Combination Therapy 1 2 3 CT26 Colorectal Cancer BCL-1 B cell lymphoma B16-BL6 Melanoma References: 1. He et al. J. Immunol 2013 2. Turaj et al. Cancer Cell 2017 25 3. Buchan et al. Clin. Cancer Research 2018
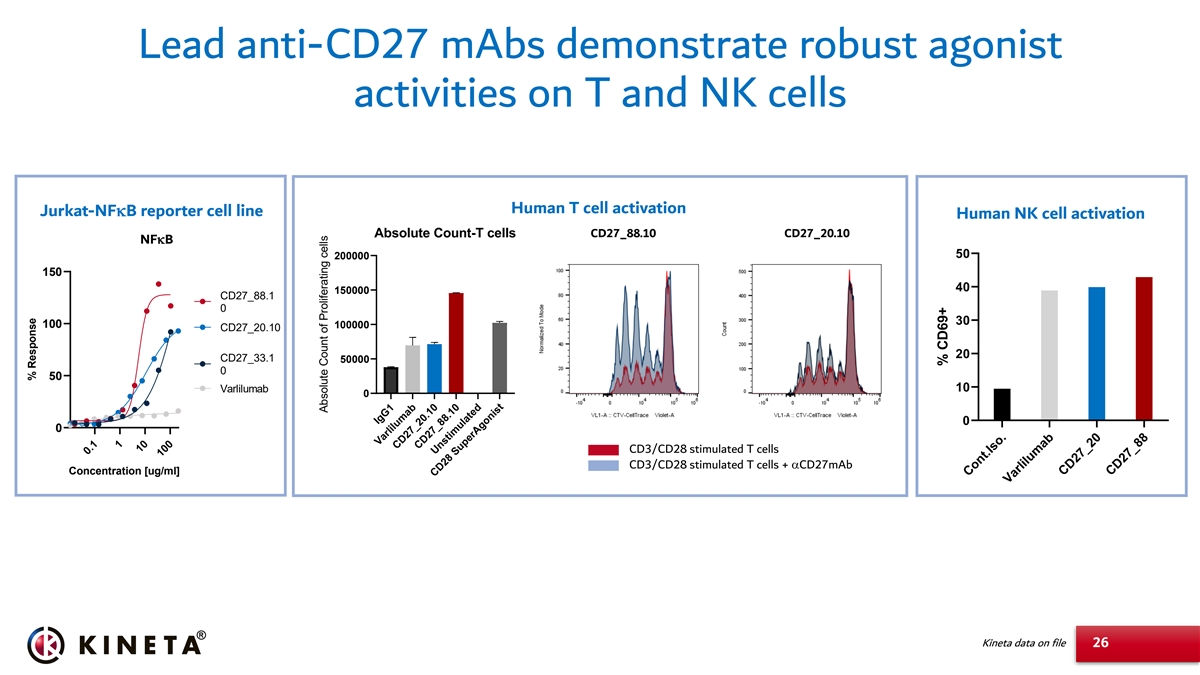
Lead anti-CD27 mAbs demonstrate robust agonist activities on T and NK cells Human T cell activation Jurkat-NFkB reporter cell line Human NK cell activation Absolute Count-T cells CD27_88.10 CD27_20.10 NFkB 50 200000 150 40 150000 CD27_88.1 0 30 100 100000 CD27_20.10 20 CD27_33.1 50000 0 50 10 Varlilumab 0 0 0 CD3/CD28 stimulated T cells CD3/CD28 stimulated T cells + aCD27mAb Concentration [ug/ml] Kineta data on file 26 0.1 1 10 100 IgG1 Varlilumab CD27_20.10 CD27_88.10 Unstimulated CD28 SuperAgonist Cont.Iso. Varlilumab CD27_20 CD27_88 % Response Absolute Count of Proliferating cells % CD69+

Significant catalysts anticipated over next 18 months • Q3 2022: IND filing • Q4 2022: Initiate Phase 1 clinical study KVA12.1 • Q4 2023: Initial Phase 1 data readout • 2H 2023: IND filing αCD27 mAb • 2H 2023: Start Phase 1 clinical study • 2H 2023: Nominate candidate / start IND-enabling studies αCD24 mAb • Q4 2022: KCP506 Phase 1 SAD/MAD/Pilot HEMP studies • 2H 2023: Genentech option Strategic Partnerships • Q4 2022: Preclinical research milestone • Q3 2023: Preclinical research milestone 27

Company highlights Strategic Value driving Immuno-oncology PiiONEER™ Strong financial partnerships catalysts platform pipeline position Multiple anticipated near- Proprietary innate immunity KVA12.1: best-in-class anti- Cash runway expected to term milestones to drive focused platform developing VISTA mAb initiating Phase fund operations for 6+ company valuation fully human mAbs 1/2 study in advanced solid quarters tumors in Q4 αCD27 mAb for solid tumors initiating IND enabling studies αCD24 mAb in discovery 28

Developing next generation immunotherapies for cancer patients www.kinetabio.com ir@kineta.us 29




























