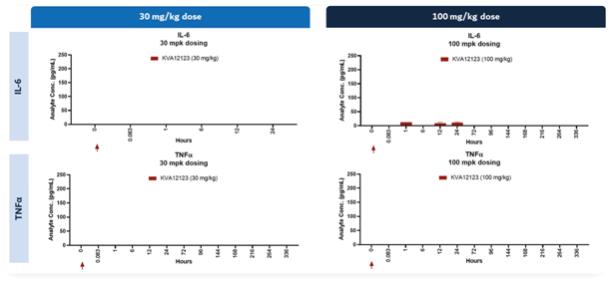
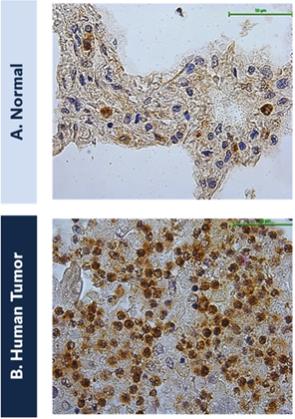
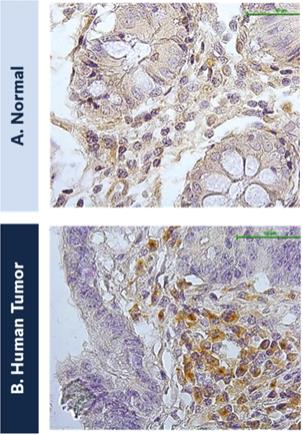
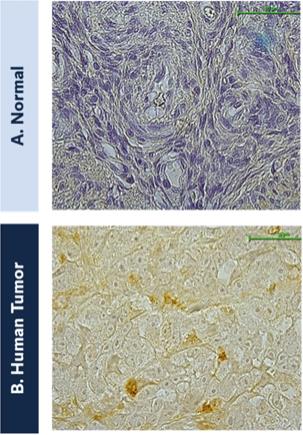
The federal civil and criminal false claims laws and civil monetary penalties laws, including the civil False Claims Act, prohibit, among other things, any individual or entity from knowingly presenting, or causing to be presented, a false claim for payment to the federal government or knowingly making, using or causing to be made or used a false record or statement material to a false or fraudulent claim to the federal government.
The federal Health Insurance Portability and Accountability Act of 1996 (“HIPAA”) created additional federal civil and criminal statutes that prohibit, among other things, knowingly and willfully executing a scheme to defraud any healthcare benefit program. In addition, HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, and their implementing regulations, imposes certain requirements relating to the privacy, security and transmission of protected health information on HIPAA covered entities, which include certain healthcare providers, health plans and healthcare clearinghouses, and their business associates who conduct certain activities for or on their behalf involving protected health information on their behalf as well as their covered subcontractors.
The federal Physician Payments Sunshine Act requires applicable group purchasing organizations and applicable manufacturers of covered drugs, devices, biologics and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program, with specific exceptions, to report annually to CMS information related to certain payments or other transfers of value made to covered recipients, including physicians licensed to practice in the U.S. (defined to include doctors of medicine and osteopathy, dentists, podiatrists, optometrists and licensed chiropractors), and teaching hospitals, in the previous year, including ownership and investment interests held by covered physicians and their immediate family members. Effective January 1, 2021, for data collected in 2021 and submitted to CMS in 2022, such reporting obligations with respect to covered recipients have been extended to include new provider types: physician assistants, nurse practitioners, clinical nurse specialists, certified registered nurse anesthetists and anesthesiologist assistants and certified nurse-midwives.
Similar state and local laws and regulations may also restrict business practices in the pharmaceutical industry, such as state anti-kickback and false claims laws, which may apply to business practices, including but not limited to, research, distribution, sales and marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third-party payors, including private insurers, or by patients themselves; state laws that require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government, or otherwise restrict payments or transfers of value that may be made to healthcare providers and other potential referral sources; state laws and regulations that require drug manufacturers to file reports relating to pricing and marketing information or which require tracking gifts and other remuneration and items of value provided to physicians, other healthcare providers and entities; state and local laws that require the registration of pharmaceutical sales representatives; and state and local laws governing the privacy and security of health information in some circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts.
Efforts to ensure compliance with applicable healthcare laws and regulations can involve substantial costs. Violations of healthcare laws can result in significant penalties, including the imposition of significant civil, criminal and administrative penalties, damages, monetary fines, disgorgement, individual imprisonment, possible exclusion from participation in Medicare, Medicaid and other U.S. healthcare programs, integrity oversight and reporting obligations, contractual damages, reputational harm, diminished profits and future earnings, and curtailment or restructuring of operations.
Foreign Regulation
In order to market any product outside of the U.S., Kineta would need to comply with numerous and varying regulatory requirements of other countries and jurisdictions regarding quality, safety and efficacy and governing, among other things, clinical trials, marketing authorization, commercial sales and distribution of Kineta’s products. Whether or not Kineta obtains FDA approval for a product, Kineta would need to obtain the necessary approvals by the comparable foreign regulatory authorities before Kineta can commence clinical trials or marketing of the product in foreign countries and jurisdictions.
Although many of the issues discussed above with respect to the U.S. apply similarly in the context of the EU, the approval process varies between countries and jurisdictions and can involve additional product testing and additional administrative review periods. The time required to obtain approval in other countries or jurisdictions might differ from and be longer than that required to obtain FDA approval. Regulatory approval in one country or jurisdiction does not ensure regulatory approval in another, but a failure or delay in obtaining regulatory approval in one country or jurisdiction may negatively impact the regulatory process in others.
40